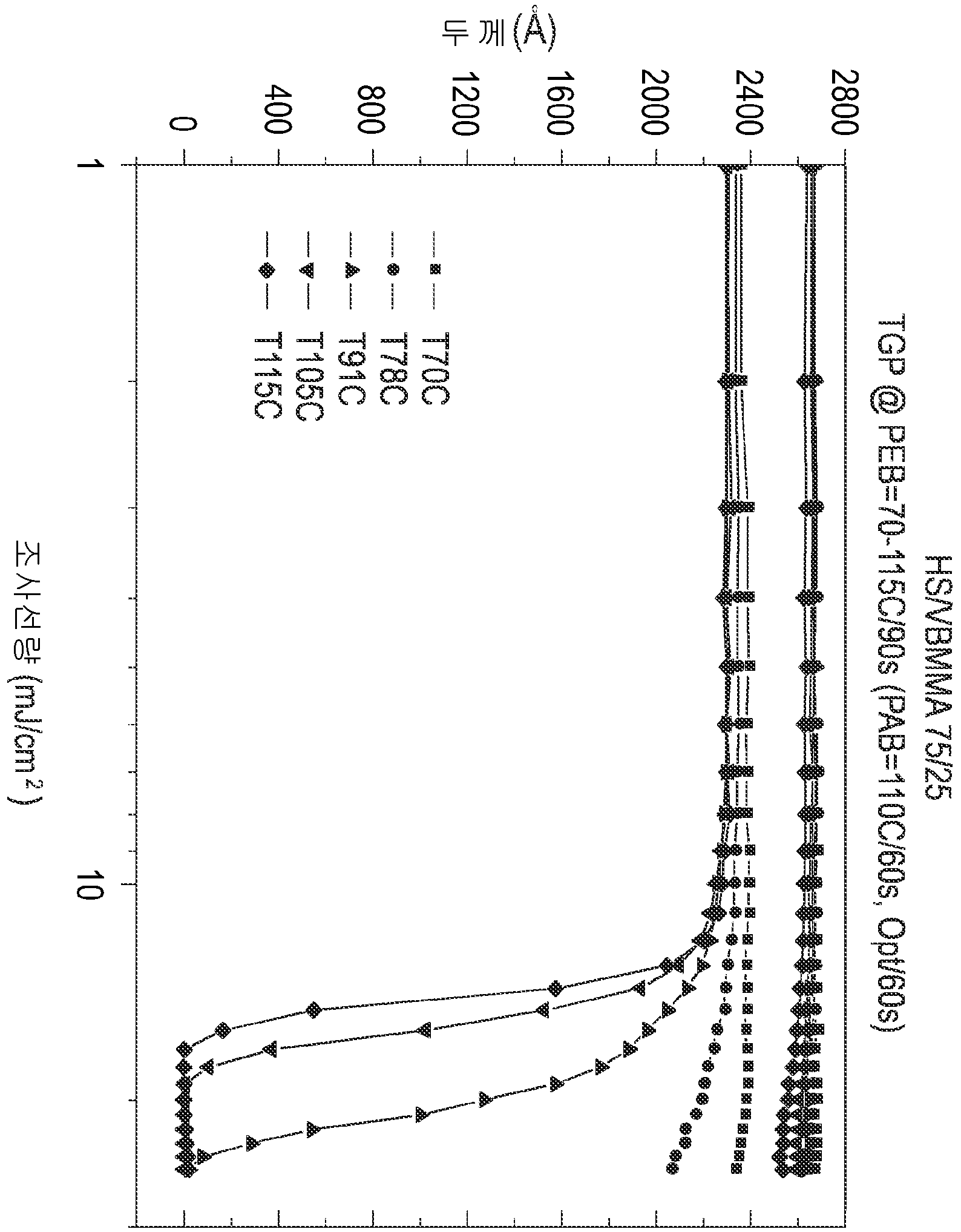
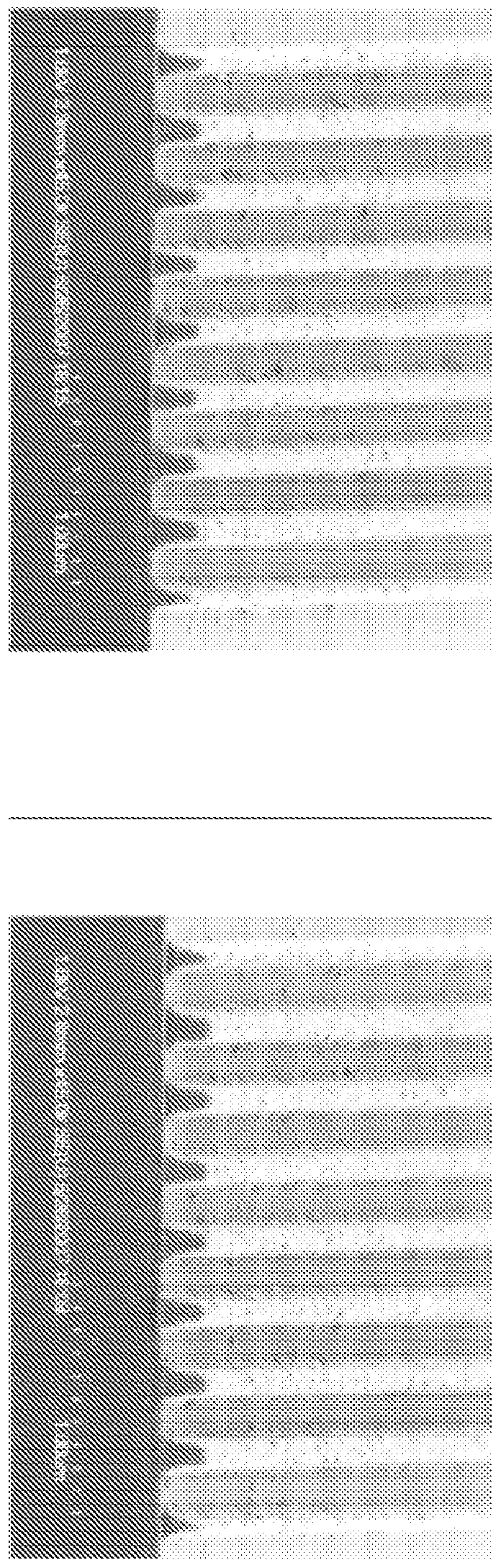
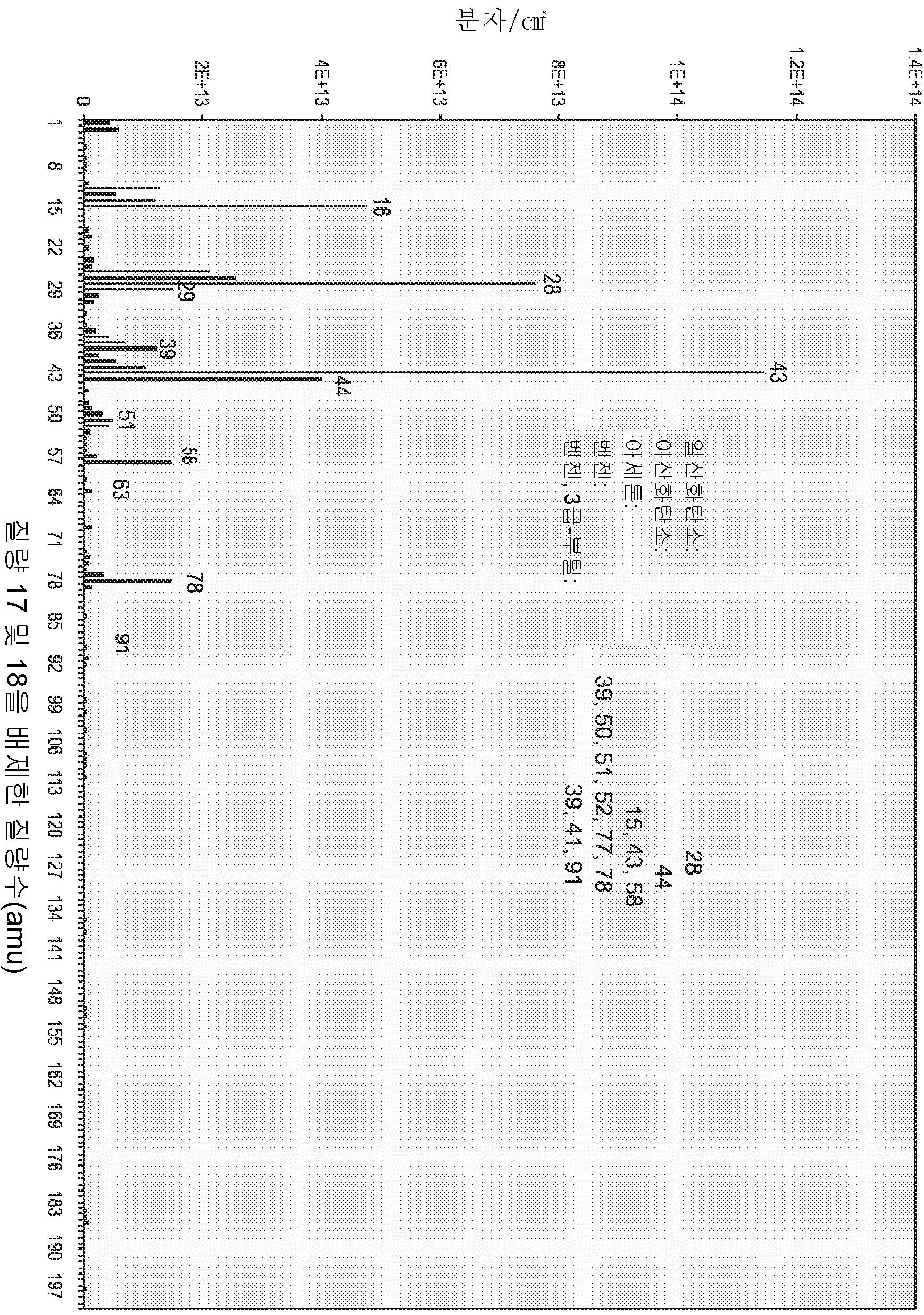
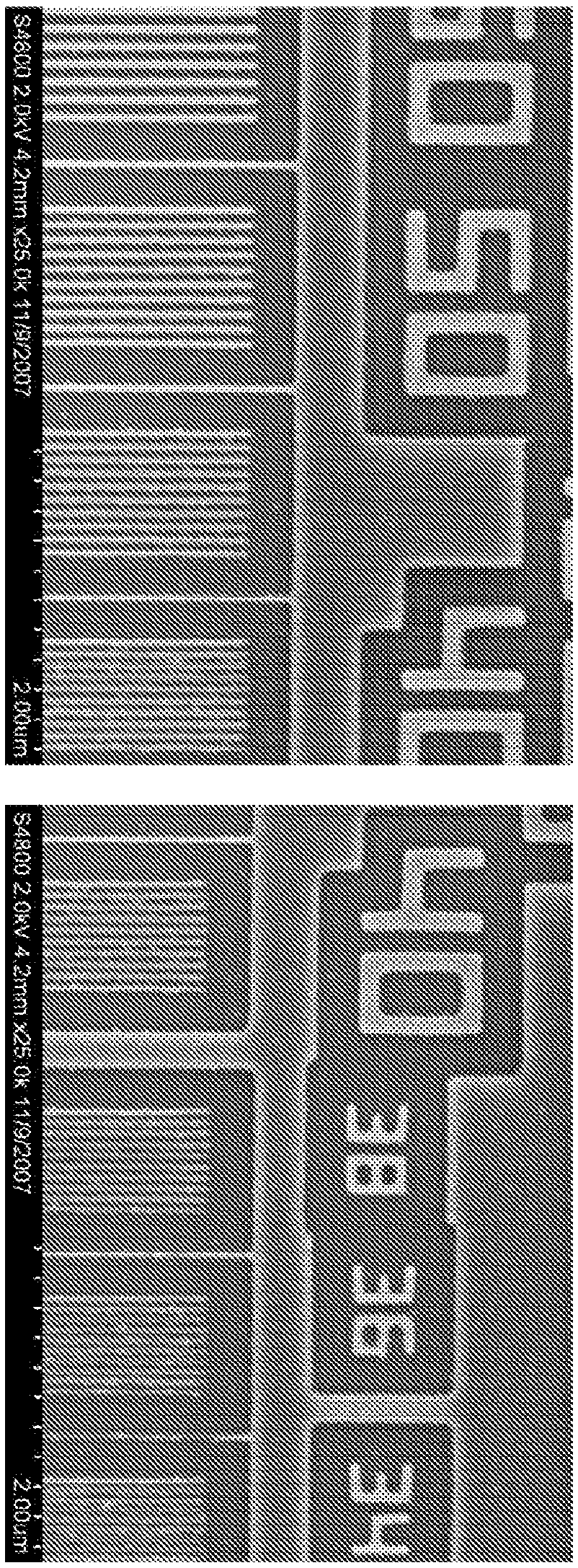
LOW OUTGASSING PHOTORESIST COMPOSITIONS
The present invention refers to photoresist polymer and is directed to including photoresist composition. In particular, the present invention refers to grippers polymers suitable and photoresist compositions and, the polymer the drum which it will carry on shoulder acid (Meldrum ' s acid) (i.e., 2,2-die methyl -1,3-dioxane -4,6-dione) based on derivatives. comprising the repeating unit comprising. Technology Roadmap semiconductor International according to (International Technology Roadmap for Semiconductor, ITRS), EUV [...] 32 nm semi-pitch (half-pitch). candidate potentials manufacturing device. Current of equipment and resist availability in progress a shortage the problematic but mention, said is to remain in a has EUV lithographic generation is judged whether the selected pixel is deemed one of. One of the problems of resist EUV, said resist in a vacuum a and energetic optical articles when irradiating the EUV radiation contamination a gas-release (outgassing) is. The-release gas EUV, 157 nm 193 nm and a specific gas pressure in the-release thereof is higher than have been shown to (literature [W. D. Domke et al. , Proc. SPIE 5753, 1066, 2005]). The (SEMATECH) deck semaphores, EUV equipment presently available prototypes that may be used in the photoresist layer, filling the openings 6.5E+14 molecules/cm2/s, to an gas-release threshold a (literature [K. R. Dean et al. , Proc. SPIE, 6519,65191P-1,2006]). Mass production (HVM) equipment is the, said threshold downwards and by way of which the forward to adjusted. Furthermore, conventional according study, a chemical-amplification-type resist most species-release gas in available photo acid generators (photo-acid generator, PAG) degradation products and number of. the pixels include derived from decomposition-acid-catalyzed (literature [m. M. Chauhan and P. J. Nealey, J. Vac. Sci. Technol. B, 18 (6), 3402,2000]). However, according to recent report, gas-emitted use in connection between an LCD light hydrocarbons and to demetallize the feed (less than about 100 amu) is trivial product optical EUV mention of suggesting a risk of wet liquid to flow down (literature [J. Hollenshead et al. , J. Vac. Sci. Technol. B 24 (1), 64,2006]). Therefore, resist gas-release requirements photoresist polymer order to meet number (PAG) photoacid generator as well as gas-it is desirable to control the.. In a chemical-amplification-type resist, in 50 nm hereinafter and to achieve relatively high resolution low activation energy (low Ea) preferably is (literature [g. M. Wallraff et al. , J. Vac. Sci. Technol. B 22 (6), 3479,2004]). However, a low activation energy generally more during exposure a gas-quantity of a fuel to be discharged provided. is believed to scavenge. Therefore, low activation energy protecting group in which the above method is implemented in addition polyacetal and/or ketal is a gas-quantity of a fuel to be discharged more forward to provide. However, in the absence of moisture, polyacetal and/or ketal a photoacid the presence of. unless the protection shaft part. The deprotected of the kinetic-of and water by irradiation electron beam and 248 nm exposure perfluoropolyethers made at gas-release study been found (literature [g. M. Wallraff et al. , J. Vac. Sci. Technol. B 22 (6), 3479,2004]). Polyacetal and/or ketal resist most the same flow of shortage of storage stability the current-oscillating is do not go where a new file does not exist. According to the present invention, derivatives acid the drum which it will carry on shoulder based on the repeating unit including polymer the prior art by providing additional to overcome weak points of the provides benefits. Various embodiment in one aspect, EUV, VUV E-beam such as an advanced such and apparatus photoresist composition for lithographic applications polymers suitable for use in a displayed by the following structural formula the drum which it will carry on shoulder acid (i.e., 2,2-die methyl -1,3-dioxane -4,6-dione) based on derivatives. comprising the repeating unit comprising: Ketal of polylactones acids are the drum which it will carry on shoulder includes functional groups. the drum which it will carry on shoulder acid derivatives based on the repeating unit including polymer is a chemical-amplification-type resist formulations provided that methanolate is used as an, the drum which it will carry on shoulder acids are switching (switching) will acts as. At elevated temperatures, photoacid (exposure created in the) in the presence of water and, the drum which it will carry on shoulder the dicarboxylic acid is will generate and acetone. The resist film (initial being insoluble in a) is soluble in basic developer (developer) is. so that. However, the derivatives of acid the drum which it will carry on shoulder on typical using conventional resist formulations acetals of poly-and/or ketal than since more stable and are hydrolysis, therefore longer is excellent in storage stability over a forward to have. Furthermore, a predetermined protective response of acid derivatives the drum which it will carry on shoulder equivalent of water is to make it possible to produce, EUV and E-beam equipment during exposure in the vacuum conditions in reaction does not involve to 2000. Diarrhea due vapour at temperatures residual into the chamber even if the reaction thereof, glass unique is acetone in combination with very small amounts of material. In one aspect one embodiment, polymer for chemically amplified photoresist containing repeating unit having the structural formula: Said in formula, The Z exhibits recurring structural units of polymer backbone, X has alkylene, arylene, don alkylene, carbonyl, carboxyl, [...], oxy, oxyalkylene and combinations thereof selected from the group consisting a connecting group, R hydrogen, alkyl, cycloalkyl aryl and configured with a output symbols being selected from an group, stage, the same R and X is not portions of the systems, and an annular. Another embodiment in one aspect, photoresist composition comprise polymer including a repeat unit having a structural formula; number photoacid generator; and a solvent includes: Said in formula, The Z exhibits recurring structural units of polymer backbone, X has alkylene, arylene, don alkylene, carbonyl, carboxyl, [...], oxy, oxyalkylene and combinations thereof selected from the group consisting a connecting group, R hydrogen, alkyl, cycloalkyl aryl and configured with a output symbols being selected from an group, stage, the same R and X is not portions of the systems, and an annular. In one aspect another embodiment, positive photoresist film the method defining an Image, including formula a repeat unit having a polymer, and a solvent number photoacid generator including said to photoresist composition to form a photoresist film; said film for instruction issuing radiation exposure decays to a the step of agitating the mixed hot metal; and said said developing the photoresist film a positive Image includes: Said in formula, The Z exhibits recurring structural units of polymer backbone, X has alkylene, arylene, don alkylene, carbonyl, carboxyl, [...], oxy, oxyalkylene and combinations thereof selected from the group consisting a connecting group, R hydrogen, alkyl, cycloalkyl aryl and configured with a output symbols being selected from an group, stage, the same R and X is not portions of the systems, and an annular. By the present invention, an advanced such-gassing VUV or EUV lithographic use polymers suitable, photoresist composition for lithographic and technical method type is confirmed. Photoacid and in the presence of water, the polymers insoluble initially, said hydrolysis of ketal functional as a result, when soluble in aqueous base developed is. Acetone are is said volatile byproducts of hydrolysis reaction. Additional aspects and advantages of the present invention is realized through the technique. Other embodiment and aspects aspect of the present invention is described herein is is considered part of the present invention. Or features of the present invention for better to understand, elucidation and detailed to reference the drawing. Herein such that it has a distribution content of the present invention inhibit the growth of hematopoietic stem cell claim of interleave units in the spherical body are referred to and. are claimed as well clarified that. Of the present invention said and other purposes, aspects and advantages rotating a drawing with an apparent surface PCS with.. Also the present invention Figure 1 shows a photoresist compositions produced thereby various exposure-is positioned on object is graph indicating a curve (contrast). According to Figure 2 the present invention on the 248 nm exposure equipment are imaged with the aid of a patterned photoresist injecting the compositions is electron micrographs. Figure 3 the present invention an electrochemical method for of photoresist composition EUV exposing gas-molecules is graph indicating a mass spectra of. According to Figure 4 the present invention on the EUV exposure equipment are imaged with the aid of a patterned photoresist injecting the compositions is electron micrographs. Detailed description to a preferred embodiment of the present invention reference to exemplary drawing aspects in a manner along with the described or characterized. Detail the before describing the present invention, is not referred alternatively, the present invention refers to of specific composition, component or process steps is not localize the torsion bar is used for various. understanding. Furthermore, only the term used herein describe only specific embodiment aspect in a chamber in which the present invention for as to define a must understand not. Contextually alternatively expressly referred is not, used in specification and claim herein form short-lived includes plural form must noted to. Therefore, for example the term "monomer" as well as one monomer a may be the same or different for same combination of a copolymer of two or more monomers includes or mixture thereof, the term "number photoacid generator" photoacid generator two or more combination or mixtures as well as includes a photoacid generator one number or the like. Describes the present invention in and claimed, a term a is according to a defining sub-disclosure. "Alkyl" used herein term two rotation 1 to 24, preferably 1 to 12 carbon atoms branched or finely ground to saturated hydrocarbon it became, such as methyl, ethyl, n-propyl, small pro it will bloom idle, n-butyl, small butyl idle, t-butyl, octyl, midifiers targeting, tetra midifiers targeting, methylhexadecyl, eicosa chamber, tetra nose room to cycloalkyl as well as such as, e.g. cyclopentyl, cyclohexyl such as. refers to. Used herein term "alkylene" two rotation 1 to 24, preferably 1 to 12 carbon atoms 2 functional saturated branched or finely ground it became hydrocarbon chain, e.g. methylene (-CH2-), ethylene (-CH2-CH2-), propylene (-CH2-CH2-CH2-), 2-methyl propylene (-CH2-CH (CH3)-CH2-), cyclohexylene dimethylene terepthalate (- (CH2)6-), . refers to such as alkylene cycle. Rotation used herein "aryl" is not explicit a relative terms 1 to 5 numbers of aromatic ring. refers to aromatic moieties. 1 more than aromatic rings containing case of an aryl, said rings may be connected to or a converged. Aryl group optionally one or more inert non per one annular hydrogen in the substituents is substituted by, suitable inert non hydrogen substituted for example halo, haloalkyl (preferably halo-substituted lower alkyl), alkyl (preferably lower alkyl), alkoxy (preferably lower alkoxy) includes or the like. Alternatively substrate is not, rotation term "aryl" in addition heteroaromatic moieties, i.e. aromatic hetero including cycle is intended to. Generally, but not necessarily, said heteroatom nitrogen, oxygen or sulfur is will. "Polymer" includes electromagnetic interference suppressing a connection monomers are linear, branched and/or cross-linking refers to a compound may be is carried out by using an acidulous when. Homopolymer or a copolymer polymer can be. "Light-generating acid (photogenerated acid)" and "photoacid (photo-acid)" rotation term upon exposure to radiation composition of the present invention, present in the compositions i.e. by photoacid generator sensitive radiation, it referring to an acid which generated a loader to employ one having an herein. Photoresist composition as well as said photoresist composition photo lithographic type suitable for use in method polymers herein is disclosure. Said polymer, including functional groups ketal of polylactones, the drum which it will carry on shoulder acid represented by formula I (i.e., 2,2-die methyl -1,3-dioxane -4,6-dione) of a monomer derivative based copolymers includes recurring structural units of. [Formula I] the drum which it will carry on shoulder acid derivatives based on the repeating unit including polymer is chemically amplified photoresist formulations in the case of being employed for, provided by to derivatives of acid the drum which it will carry on shoulder of polylactones ketal functional groups will acts as switching. Photoacid (produced during exposure) in the presence of water and at an elevated temperature with, said the drum which it will carry on shoulder the dicarboxylic acid is will generate and acetone. Is initially insoluble said photoresist film a basic developer will to the protection. However, as long as the current the drum which it will carry on shoulder acid derivatives using low energy photoresist formulations acetals of poly-and/or ketal than on typical and are hydrolysis is found to more stable, therefore longer is excellent in storage stability over a forward to have. Furthermore, a predetermined protective response of acid derivatives the drum which it will carry on shoulder equivalent of water is to make it possible to produce, EUV and E-beam [...] processing equipment during exposure in the vacuum conditions in reaction does not involve to 2000. Diarrhea chamber in even a portion of the reactive due to residual vapour at temperatures, glass unique is acetone in combination with very small amounts of material. One embodiment in one aspect, said polymer represented by formula II the drum which it will carry on shoulder acid derivatives based on the repeating units. comprising at least one: [Formula II] Said in formula, The Z exhibits recurring structural units of polymer backbone, X has alkylene, arylene, don alkylene, carbonyl, carboxyl, [...], oxy, oxyalkylene and combinations thereof selected from the group consisting a connecting group, R hydrogen, alkyl, cycloalkyl aryl and configured with a output symbols being selected from an group, stage, the same R and X is not portions of the systems, and an annular. Formula II defined in the repeating unit including polymer, aqueous base soluble to such as a phenol, fluoro alcohol and/or polar functional group e.g. lactone, anhydride, to alcohol, sulfonamide to, other an acid-labile machine or the like containing co-monomer may comprise an. The ratio of different monomeric units limited to, is not intended. For example, in the embodiment 4 to the [4-hydroxy styrene-co -2,2-die methyl-5 - (4-vinyl benzene) - 1,3-dioxane -4,6-dione] the aforementioned synthesis of copolymer. M embodiment aspect is described : --time but 75:25 ratio of n (wherein, n formula II defined by for a difference from an the drum which it will carry on shoulder acid derivatives), m for conveniently non monomer: ratio of n (0 above 99.99 mole %) : (less than 0.01 to 100 molar %) obtained copolymer in the decoration plate further comprises an. it is apparent that said disclosure content falling on a detector. Other embodiment in one aspect, m:n ratio (less than 100 exceeds 50 molar %) : (less than 50 molar % above 0) is. Another embodiment in one aspect, m:n ratio (above 60 less than 90 molar %) : (less than 40 molar % above 10) is. Similarly, the number of different monomer limited to, is not intended. Furthermore, 3 won copolymer, such as higher order polymer can be manufactured.. One embodiment in one aspect, said polymer, the molecular weight of 1,000 to 100,000 Dalton and having a content of, other embodiment in one aspect, molecular is 5,000 to 10,000 Daltons. Formula II associated monomer with structure of full text method described literature and to eo or conventional unpracticed people to the field using a publicly known method can be synthesized. Representative monomers and polymers aspect the method for synthesizing constitution: described embodiment. For example, formula II including a repeat unit having a structure of free radical copolymer by radical copolymerisation using disclosure can be produced. The radical polymerization, soil, and an easy embodiment photoresist polymer for producing surfactants finds suitable application. Disclosure agent any conventional free radical-generating polymerization disclosure. may be. Suitable examples for example a peroxide disclosure O-t-amyl-O-(2-ethylhexyl) mono [...], die [...], and benzoyl peroxide (BPO) (AIBN) [...] azo e.g. azo compound as well as, 2,2 '-azo bis (2-amidino-propane) dihydro chloride and 2,2' -azo bis ( [...] idle) includes hydrate 2. Said generally agent said disclosure about monomers amount of 0.2 to 20 molar % present in said to a polymerization mixture. Said polymers gel permeation chromatography (GPC), heat gravity analysis (TGA), in differential scanning calorimeter (DSC) and nuclear magnetic resonance spectroscopy (NMR) can be characterized by. The present invention refers to in addition the repeating units of formula II, photoacid generator including number and an organic solvent including photoresist polymer provides a photoresist composition. Various photoacid generator agent can be these compositions and corresponding of the present invention. Said photo for use in the resist composition photosensitive acid generating agent, said of photoresist composition compatible with other ingredients, a publicly known photoresist to the field of any suitable photosensitive acid generator. may be. Examples of a preferred photosensitive acid generator number (PAG), American patent number 4,731,605 call as disclosure α-( [...] dryer and control method thereof)-bicyclo [2.2.1] - 2,3-n - 5-methylhept [...] die (MDT), onium salt, aromatic diameter [...] salt, sulfonium salts, salt [...] diameter, and N-hydroxy amide or-imide of hydrocarbonsulfonic acids includes esters. Furthermore, use of Saccharomyces cerevisiae of N-hydroxy-naphthalimide (DDSN) car sulphonate, with such as an acid is generated a PAG is weaker ones may be used. Combination of PAG can be also using water. Generally, compatible acid generating agent high thermal stability (typically at a temperature higher than 140 °C) as described above, has, is that a pre-these is not decomposes during exposure process. In addition to DDSN and MDT, other suitable PAG include other sulfonate salt as well as sulfonated esters and sulfonyloxy ketone comprises. A tosylate, t-butyl phenyl α-(p to base opinion gun Neel jade city tools)-acetate and t-butyl α-( opinion gun Neel jade city -toluic p) including-acetates of disclosure of a suitable sulfonate PAG (Sinta) new other to entertainment factor, so as to call and literature patent number 5,344,742 American it became the empty woman [ In addition the onium salt of the present invention generally is number generating the preferred acid composition. Weak nucleophilic containing anions onium salt in particular it has been found that suitable. Examples of ions and 2 to 5 metal or non-metallic, e.g. Sb, B, is anion composite of the halogen As and P. Examples of onium salt suitable salt [...] -diamino-an aryl, halo salt, aromatic sulfonium salts, and a sulfoalkyl [...] salt or selenium salt (e.g., dryer and control method thereof [...] and diameter [...] hexa [...], hexa [...], and trifluoromethanesulfonate) is. American patent number 4,442,197 a preferred onium salt examples of call, call and number 4,603,101. calls and number 4,624,912. Other useful acid generating agent and family of benzyl carbonyl-, nitro-, a s-triazine derivatives including an arylalkyl group. Suitable s-triazine acid generating agent for example American patent number 4,189,323 call is disclosure. Another compatible acid generating agent [...] -N; [...] -N, ionic [...] sulfonate (e.g., diaryl [...] (alkyl or aryl) sulfonate and bis-(die-t-butyl phenyl) [...][...] ); ionic [...][...] purple (e.g., die-(4-t-butyl phenyl) [...][...] sulfonate, "IPFOS"), aryl (e.g., phenyl or benzyl) indium trichloride, and their derivatives and analogs (e.g., dryer and control method thereof [...] indium trichloride or bis-(t-butyl phenyl) [...] indium trichloride), fine with the roll which will go derivatives (e.g., fine with the roll which will go dryer and control method thereof mesylate), hydroxy polyimide trifluoromethanesulfonate ester, α, α '-bis-sulfonyl-die azo methane, nitro-substituted benzyl alcohol of sulfonate ester, naphthoquinone-4-vinylsulphone die and character id diameter, including, but is not to be limited. Literature other suitable photoacid generating agent [Reichmanis et al. (1991), The choice of solvents by a range of factors which is subjected to, e.g., a resist of components soluble and miscible, coating process, and safety and environmental regulations, including, but is not to be limited. Furthermore, other resist components that is inert with respect to the is preferably not less. Furthermore, a uniform coating of film solvent as well as allow post-applied during due to baking process substantially fewer residual solvent can be removed or completely having a volatile appropriate it is preferable that the. Prior citation 2002 [Introduction to Microlithography, Eds. Thompson et al.] reference. Said component in addition to, photoresist compositions are in general of the present invention, overall composition is applied uniformly substrate surface-defective so formed as to form a coating, a casting dissolving other ingredients includes solvent (casting). Photoresist compositions are said in the case of being employed for multilayer imaging process, photoresist layer resist Image are to be used in lower layer preferably solvent to the solvent not, otherwise, unwanted compounding can occur.. Suitable examples of a casting solvent [...] (EEP); and a combination of EEP γ-butyrolactone (GBL); PGMEA; and includes ethyl lactate. Any particular the present invention refers to not limited to, the choice of solvents. Ether-generally solvent, ester-, hydroxyl-, and ketone-containing compounds, or from a mixture of these compounds can be selected. Examples of a suitable solvent, and additionally containing a cycloolefin the pentane it comes ; [...] cycle; lactate ester e.g. ethyl lactate; alkylene glycol alkyl ether ester e.g. propylene glycol methyl ether acetate; such as methyl alkyl esters of alkylene glycol monoalkyl [...] ; butyl acetate; 2- [...] ; and ethyl 3-includes [...]. Preferred solvents a PGMEA, EEP includes and mixtures thereof. Exemplary only list said solvent and if comprehensive motherhood doesn't 't way otherwise selection solvent in addition which the present invention is deemed as for limiting is in even. The field number of any unpracticed people addition to a solvent or solvent mixture may be used to. will be to know. Said resist formulations typically exceeds 50% of the total weight of is solvent, preferably exceeds a 80%. Said photoresist polymer the composition included in solids, about 99 weight % hereinafter being, said where the composition has photoacid generator about solids, contained in the paper side weft yarns to occupy a 0.5 to 10 weight %. Other ingredients and additives as, may also be present.. For example, positive photoresist composition comprises 3-and can include the dissolution inhibitor, voice photoresist composition may include a crosslinking agent. Number and crosslinking in the presence of dissolution inhibitor, they are typically total solids, about 1 to 40 weight %, preferably about 5 to 30 weight % range is there will be. Suitable unpracticed people field the agent dissolution inhibitor is publicly known to and/or related 2000 are described literature. A preferred dissolution inhibitor agent resist composition and said resist composition (e.g., propylene glycol methyl ether acetate or "PGMEA") used to manufacture a solution of both solvent having a high solubility and, exhibits deterrent dissolution strong, has a melting speed of a cathode electrode exposure and, is substantially clear and at a wavelength corresponding, upon an adjustment Tg serves user exhibits etch resistant a strong, excellent thermal stability (i.e., about 140 °C a temperature greater than or first) blades, presenting a. Suitable polycarbonate derivatives and A bisphenol agent dissolution inhibitor derivatives, for example bisphenol A derivatives (the, of one or both of the hydroxyl moieties t-butoxy substituent, or an derivatives thereof e.g. t-butoxy Alycylcarbonylhydrazines t or redemption into car step Neel methyl -butoxy); fluorinated bisphenol A derivatives e.g. CF3-bisphenol A-OCH2 (C0)-O-tBu (t-butoxy protected by to car step Neel methyl A bisphenol -6F); normal or branched chain polyacetal to [...] 1-e.g., 1-propoxyethyl, 1-n-butoxyethyl, idle 1- [...] -ethyl, to oh wheat jade hour ethyl -1-t and jade hour ethyl -butyl 1-t; annular polyacetal to [...] tetra e.g., tetrahydropyran and 2-to [...] ; and androstane -17-alkyl carboxylate and their analogs (the, 17-position in 17-alkyl [...] it in lower typically), including, but is not to be limited. Lysocolic examples of such compounds, cut into 4 to 5 cm, small call[...] acid and lower alkyl ester such as methyl [...], methyl [...], methyl cut into 4 to 5 cm, small call rate, [...] -butyl t, [...] -butyl t, t-butyl cut into 4 to 5 cm, small call, and the like (e.g., citation prior 2002 [Allen et al. (1995) J. Photopolym. Sci. Technol.] reference); such compounds of a hydroxy-substituted analogs (literature a robot picks the chip truncated); 3 to 1 two C1 to C4 fluoroalkyl carboryloxy substituent [...] dryer and control method thereof-butyl t e.g. substituted by androstane -17-alkyl carboxylate (e.g., allene (Allen) patent number 5,580,694 American it became the empty woman entertainment factor, so as to call reference) includes. Other conventional of formulated additive for adjusting optical density of resist either of which can be utilized to dye; and radiation absorbing photoacid generating agent the same by transferring a enhancing the activity agent photoacid generator includes. For example, aromatic e.g. functionalized the benzene, pyridine, pyrimidine, bi polyphenylene, indene, naphthalene, anthracene, coumarin, anthraquinone, other aromatic ketone, and any of those of includes a derivatives, and similar analogs. Various basic with a variety of mother for spread control and an acid-stabilizer a compound can be used as additives for.. They are nitrogenous compounds such as a fat group 1 class, liquid 2 tertiary amine 3 ; annular amine e.g. piperidine; pyrimidine ; morpholine; aromatic hetero ring compounds e.g. pyridine; pyrimidine ; purine; imine e.g. the Java director counted claw fortune diameter; guanidine ; polyimide; amide such as may include a. Furthermore, ammonium salt for example alkoxide (e.g., hydroxide, phenolate, carboxylate, aryl and alkyl sulfonates, sulfonamide such as) ammonium, class 1, class 2, class alkyl and biting ammonium salt 4 liquid 3 can be used. Furthermore, other cationic nitrogenous compound, such as pyridinium salt, and alkoxide (e.g., hydroxide, phenolate, carboxylate, aryl and alkyl sulfonates, sulfonamide such as) ions having a heterocyclic nitrogenous a salt of this compound can be used. Surfactant to improve the coating uniformity may be used, a broader range of ionic and a non-ionic, monomeric, oligomeric including material and a polymeric species. Similarly, various deficiency stuff a vesicle can be used to prevent peeling. Adhesive accelerator is in addition can be used various compounds is breaking this function up can be used to provide. A monomeric various, oligomer and polymeric plasticizers number (e.g., oligonucleotides and peg ether, cycloaliphatic ester, and non-acid reactive steroid-derived material) are provided to operate the balanced number can be used as plasticizer. However, the aforementioned class of compounds any compounds their general or specific limited to, and/or comprehensive is not intended. The skilled worker field these conventional additives of the type is to carry out a embodiment function width that can be used a wide range of commercially available is to know the product will be. Lithographic photoresist pattern is useful for forming manner radiation source or radiation source examples of VUV (157 nm), ArF (193 nm), KrF (248 nm), EUV (13 nm), E-beam, X-ray and ion beam, including, but is not to be limited. Preferably, said irradiation energy in the range of from about 1 to about 100 mJ/cm2. The present invention refers to in addition the aforementioned photoresist composition produced using the semiconductor device with high. In in the embodiment a, is not alternatively substrate, weight and (part) part, and °C temperature, . atmospheric or near atmospheric pressure. Furthermore, all starting materials are commercially or may be available for purchase is synthesized using of was publicly known. 4 - (2-hydroxy-2-hexafluoropropane profile) styrene monomers are central glass [...] for purchasing a from sludge (Central Glass Company). Where appropriate, or, of the following technique and device is in the embodiment, are employed in:1 H and13 C NMR oh room spectrum obtained at room temperature on spectral (Avance) 400 structure. Quantitative13 C NMR 400 structure as neuroleptics on apparatus for interfacing with the oh room Cr (acac)3 using inverted signs during the-gateway (inverse-gated)1 H-decoupling mode in acetone-d6 been embodiment at room temperature. In the case of analysis polymer composition,19 F NMR (379 MHz) spectrum oh room structure tracker brewing in addition and an analyzer been obtained using the (Bruker Avance) 400. Heat-gravity analysis (TGA) (Thermogravimetric Analyzer) on the coin sorting it is bitter the wool yes expense the metric Hi-Res TGA 2950 instrument TA N2 in been 5 °C/min. heating rate. In differential scanning calorimeter (DSC) the TA instrument DSC 2920 adjusted in differential scanning calorimeter on been 10 °C/min. heating rate. For circulating etch polystyrene molecular [...] to in (THF) tetrahydrofuran on chromatograph (Waters Model) 150 model has been determined. IR spectrum on the KBr blurlets Nicol on print of IR-(Nicolet) 510 ft been recording on apparatus for interfacing with. [...] matrix nano film thickness (Nanometrics Inc.) of alpha-step 2000 speckle [...] or (NanoSpec) 6100 has been determined in (Tencor). (QCM) balance micro crystal oscillator using an aqueous tetramethyl ammonium hydroxide (TMAH) solution in (CD-26) did research on kinetics for dissolution of the resist film. 193 nm the exposure 0.60 NA ISI having been performed on mini-stepper. ASML 0.60 NA been performed on the exposure 248 nm stepper. E-beam exposure a ladle car (Leica) 100 kV been performed on exposure equipment. The exposure EUV berkeley California (Lawrence Berkeley National Laboratory) residing on a prototype in EUV lawrence[...] National berkeley been performed on equipment. A exemplary embodiment only relate generally intended is to of, or limit the scope of the present invention and which is provided for the not for. Numerical (e.g., quantity, such as a temperature and the like) --time but travel with regard to, . in view the probability and deviation error. In the embodiment 1 2,2-die methyl-5 - (4-vinyl benzyl) - 1,3-dioxane classified as a carcinogenic substance synthesis of (VBMMA) - 4,6-dione [Formula III] Stirrer, thermowell (thermowell) water, and nitrogen connected a condenser with multi function cap to 500 ml 3-neck annular writing, 10.0 g (0.0632 mole) of 2, 2, 5-methyl -1,3-dioxane classified as a carcinogenic substance dryer and control method thereof -4,6-dione, 10.6 g (molar 0.0632) of p-(chloromethyl) styrene, 35 g (molar 0.2528) polycarbonate and of potassium 200 ml of acetone added. About 1 g of added as stabilizers lung looking old Oh position. Night to synchronize the reflux with stirring, in a mixture, and cylinder top is maintained 200 ml of ethyl acetate to the gas, cooling the gas, dilution and the filtering through a layer (Celite) light BTS. And then being washed with brine filtrate water and sodium sulfate, with formic-acetic anhydride was very dry night. Filtrate thereof, is filtered and suspension rotation is draw it does, been evaporated in the evaporator, was used to simultaneously identifying same. Said determined then being washed with ether oil 35-65 and aggregates the wish list items, said formula III that is represented as the die 2,2-methyl-5 - (4-vinyl benzyl) - 4,6-die reside are obtained 3.5 g -1,3-dioxane classified as a carcinogenic substance. In the embodiment 2 2,2-die methyl-5-allyl -1,3-dioxane classified as a carcinogenic substance synthesis of (ALMMA) - 4,6-dione [Formula IV] Stirrer, thermowell water, and nitrogen connected a condenser with multi function cap to 500 ml 3-neck annular writing, 10.0 g (0.0632 mole) of 2, 2, 5-methyl -1,3-dioxane classified as a carcinogenic substance dryer and control method thereof -4,6-dione, 7.7 g (molar 0.0632) of allyl bromide, 21 g (0.152 mole) of potassium 200 ml of polycarbonate and added acetone. Night to synchronize the reflux with stirring, in a mixture, and cylinder top is maintained 200 ml of ethyl ether to the gas, cooling the gas, dilution and the filtering through a BTS/activated carbon. And then being washed with brine filtrate water and sodium sulfate, with formic-acetic anhydride was very dry night. Filtrate thereof, is filtered and suspension evaporated in the evaporator rotation represented by the formula IV -1,3-dioxane classified as a carcinogenic substance 2,2-die methyl-5-allyl -4,6-dione compound to the transparent colorless oil (11 g) are obtained as. In the embodiment 3 4 - (2,2-die methyl -4,6-dioxo-[ 1,3]-dioxane classified as a carcinogenic substance-5-one)-butyric acid 2 - (2-methyl-acrylic pivaloyloxy)-ethyl ester synthesis [Formula V] Thermometer, nitrogen/vacuum filler, and die of chloro methanes (50 ml) in die [...] (DCC) (4.74 g, 23 mmol) of additive solution annular writing multi function cap with multi function cap to 250 ml 3-neck, mono ( the jade city which rises with the arc reel methyl 2 -) ethyl succinate (3.9 ml, 20 ml), the drum which it will carry on shoulder acid (3.20 g, 22 mmol), 4-die methyl aminopyridines (3.85 g, 32 mmol) and phenothiazine (46 mg) of chloro methanes the die to the (100 ml) the dissolved. The deoxygenated nitrogen said system, an acid/acetone in the gas, cooling the gas, multi function cap reaction, DCC solution added over time 1. Reaction is passed off in the bath cooling night. Been are filtered precipitate white. Filtrate 10% aqueous KHSO4 (100 ml × 3) and brine (100 ml) then being washed with and MgSO4 to dry wherein the filtration of the slurry suspension 4 - (2,2-die methyl -4,6-dioxo-[ 1,3]-dioxin-5-ylidene) - 4-hydroxy-butyric acid 2-2-methyl-acrylic pivaloyloxy)-ethyl ester solution was direct use in reduction [...] said. Said 4 - (2,2-die methyl -4,6-dioxo-[ 1,3]-dioxin-5-ylidene) - 4-hydroxy-butyric acid 2-2-methyl-acrylic pivaloyloxy)-ethyl ester solution on cooling apparatus for controlling ice/acetone bath and acetic acid (13.5 ml) has added. Furthermore, sodium borohydrides (NaBH4) (1.85 g, 50 mmol) a 1 added over time. Reaction keeps a bath cooling night brine (100 ml × 2) and water (100 ml × 2) and then being washed with MgSO4 to dry and filter the was. lung looking old Oh true meaning another portion (46 mg) adding an, the solution the concentrated and, which absorb a ethyl acetate to, wherein the filtration of the slurry suspension, of cyclohexane to additive, thereby sides of the channel region by 2 lot (lot) 3.91 g of a yellow solid (yield 57%) represented by said formula V of 4 - (2,2-die methyl -4,6-dioxo-[ 1,3]-dioxane classified as a carcinogenic substance-5-one)-butyric acid 2 - (2-methyl-acrylic pivaloyloxy)-ethyl esters are obtained. In the embodiment 4 Poly [4-hydroxy styrene-co -2,2-die methyl-5 - (4-vinyl benzyl) - 1,3-dioxane -4,6-dione]; poly (VBMMA-co -4HS) synthesis of (75:25) [Formula VI] Condenser and nitrogen filler with multi function cap to annular writing, 4-hydroxy styrene (tetrahydrofuran solution 72% in 2.50 g, 0.015 mole), 2,2-die methyl-5 - (4-vinyl benzyl) - 4,6-dione (VBMMA) - 1,3-dioxane classified as a carcinogenic substance (1.37 g, 0.005 mole) and put 8.9 g of tetrahydrofuran. Into the solution 2,2 '-azo [...] (AIBN) (0.131 g, 0.0008 mole) adding an treatment stirring section. Furthermore, solution 4 times vacuum/nitrogen purge gas by using aqueous-release the. Furthermore, 18 by heating so that the contents the reflux time. Furthermore, hexane solution (300 ml) blended into adaptation. Precipitated polymer filtered (frit), hexane (50 ml) in which washes the times 2,3 was very dry suction time. Furthermore, polymerization to form the number and DI HFE-7100 from acetone, respectively 2 to the precipitation material times. Finished polymeric a was very dry under vacuum at a 500 °C. Yield: 1.86 g, Mw=6,508; PDI=1.58. In the embodiment 5 Poly [4 - (2-hydroxy hexafluoro-2-profile) styrene-co -2,2-die methyl-5 - (4-vinyl benzyl) - 1,3-dioxane -4,6-dione]; poly (VBMMA-co -4HFA-ST) synthesis of (65:35) [Formula VII] Condenser and nitrogen filler with multi function cap to annular writing, 4 - (2-hydroxy-2-hexafluoropropane profile) styrene (4HFA-ST) (1.40 g, 0.0052 mole), 2,2-die methyl-5 - (4-vinyl benzyl) - 4,6-dione (VBMMA) - 1,3-dioxane classified as a carcinogenic substance (0.77 g, 0.0028 mole) and put 6.6 g of tetrahydrofuran. Into the solution 2,2 '-azo [...] (AIBN) (0.052 g, 0.00032 mole) and 1-use of Saccharomyces cerevisiae space mote this year (0.048 g, 0.00024 molar) adding an treatment stirring section. Furthermore, solution 4 times vacuum/nitrogen purge gas by using aqueous-release the. Furthermore, 18 by heating so that the contents the reflux time. Furthermore, hexane solution (200 ml) blended into adaptation. Precipitated polymer filtered (frit), hexane (50 ml) in which washes the times 2, said polymer was very dry under vacuum at a 500 °C. Yield: 1.58 g, Mw=10,480; PDI=1.84. In the embodiment 6 Poly (VBMMA-co -4HS) (75:25)-based photoresist composition In the embodiment 4 the polymers produced according to 1.0 g, number (PAG) 2 species sulfonium photoacid generator mixture of organic base and 50 mg for 3.3 mg 7.3 g propylene glycol monomethyl ether acetate (PGMEA) the dissolved. The solution into an 0.2 micrometers through an the syringe filter. In the embodiment 6 photoresist the cosmetic composition has been applied in post-bake temperature 110 °C 60 seconds at a spin coated multilayer silicon on a wafer. Also various Figure 1 shows a 90 seconds in on post-exposure photoresist compositions for is graph indicating a contrast curve. Exposure as a function is positioned-different exposures on thickness has been determined temperature. Furthermore, in the embodiment 6 photoresist composition of 62 nm of anti-reflective coating ((Brewer Science) DUV-42P [...][...] ) onto silicon wafer coated with 267 nm stepper ASML 248-nm and spin coating to have thicknesses on the order of, was used to Image using 0.60 NA. 110 °C in said photoresist composition is positioned application-seconds and 60,60 in 108 °C and is positioned-exposure seconds, 0.26 N the developable in an aqueous TMAH solution. Figure 2 150 nm and 160 nm feature sizes in said patterned photoresist compositions for 1:1 line spacing pair of. indicating a scanning electron micrographs. In the embodiment 7 EUV exposure for the (VBMMA-co -4HS) (75:25)-based photoresist composition In the embodiment 4 the polymers produced according to 0.25 g, number (PAG) 2 species sulfonium photoacid generator mixture of 4.1 g of 0.825 mg organic base and 15.0 mg of dissolved the PGMEA. The solution into an 0.2 micrometers through an the syringe filter. EUV exposing said of photoresist composition gas-analyzing the release was. Experiment (SEMATECH-North) albany[...] -deck semaphores in University (Albany) in test at high speed performed for all the EUV resist. Independent resist film having a thickness of 100 nm EUV radiation from a source 56 mJ/cm2 (2 ×EO) of the exposed to EUV radiation. In baking 100 °C same was 60 seconds. Gas-the release rate of the quadruple mass spectrometer (QMS) corresponding point as determined by decided summed to mass. For resists such 2.04E+14 value obtained was/cm2/s molecules. The semaphores 6.5E+14 the threshold set by deck is less than/cm2/s molecules. Figure 3 shows a mass spectra are also presented as graph indicating a, the polymer from the acetone and controlled in a more precise not fragments any otherwise. indicates the. Fragments all other available photo acid generators can be caused zero. Figure 4 patterned photoresist injecting the compositions. indicating a electron micrographs. In the embodiment 7 already photoresist composition similar to 62 nm of anti-reflective coating ( [...][...] DUV-42P)) onto silicon wafer coated with and spin coating to have thicknesses on the order of 105 nm 67.38 mJ EUV in dose irradiation of prototype was used to Image using equipment. 110 °C in said photoresist composition 60 and is positioned application-seconds, 100 °C 60 in and is positioned-exposure seconds, 0.26 N the developable in an aqueous TMAH solution. Herein advantageously polymer said of the following properties: Under vacuum conditions a a) said Image does not deprotected in a side of the frame; B) exposure during when deprotected, said fragments are attached to polymer said any manner is not-release gas; 100 c) said fragments are atomic mass units (amu) having a molecular weight of less than is light hydrocarbons and to demetallize the feed. To the present invention preferred embodiment but described aspects, the field claim to destructively distillates and the current unpracticed people in the window an improvement on and an enhancement capable 2000 a main body. These claim properly invention described initially for protecting should interpreted to. Polymers for use in photoresist compositions include a repeat unit having a formula of: wherein Z represents a repeat unit of a polymer backbone; X is a linking group selected from the group consisting of alkylene, arylene, araalkylene, carbonyl, carboxyl, carboxyalkylene, oxy, oxyalkylene, and combinations thereof, and R is selected from the group consisting of hydrogen, alkyl, aryl, and cycloalkyl groups with the proviso that X and R are not part of the same ring system. Also disclosed are processes for patterning a relief image of the photoresist composition, wherein the photoresist composition has an outgassing rate of less than 6.5E+14 molecules/cm2/s. Formula a repeat unit having a polymer including: In formula said, the Z exhibits recurring structural units of polymer backbone, said repeating units of And/or Is, X has alkylene, arylene, don alkylene, carbonyl, carboxyl, [...], oxy, oxyalkylene and combinations thereof selected from the group consisting a connecting group, R hydrogen, alkyl, cycloalkyl aryl and configured with a output symbols being selected from an group, stage, the same R and X is not portions of the systems, and an annular. According to Claim 1, hydroxy into a substituted aryl, a fluoroalkyl group are substituted with hydroxy, for ring [...], acid anhydride, to alcohol, sulfonamide and acid-labile (labile) configured with a group selected from the group of a 1 comprising at least one further unit additional iterations of including, polymer. According to Claim 1, said polymer is (Dalton) having a molecular weight of 1,000 to 100,000 Daltons, polymer. Deleted According to Claim 1, said repeating units including polymer for chemically amplified photoresist including copolymer whose structural formula, polymer: In formula said, m: the ratio of n (0 above 99.99 mole %) : (less than 0.01 to 100 molar %) is. According to Claim 1, said repeating units including polymer for chemically amplified photoresist including copolymer whose structural formula, polymer: In formula said, m: the ratio of n (0 above 99.99 mole %) : (less than 0.01 to 100 molar %) is. Formula a repeat unit having a polymer including; number (photo-acid generator) photoacid generator; and a solvent photoresist composition including: In formula said, the Z exhibits recurring structural units of polymer backbone, said repeating units of And/or Is, X has alkylene, arylene, don alkylene, carbonyl, carboxyl, [...], oxy, oxyalkylene and combinations thereof selected from the group consisting a connecting group, R hydrogen, alkyl, cycloalkyl aryl and configured with a output symbols being selected from an group, stage, the same R and X is not portions of the systems, and an annular. According to Claim 7, said polymer said agent photoacid generator present in an amount of 0.5 to 10 weight %, photoresist composition. Formula a repeat unit having a polymer including, photoacid generator including number and a solvent to photoresist composition to form a photoresist film; said film for instruction issuing radiation exposure decays to a the step of agitating the mixed hot metal; and said photoresist film layer so that the relief (relief) defining an Image including step, photoresist film positive connection defining an Image method: In formula said, the Z exhibits recurring structural units of polymer backbone, said repeating units of And/or Is, X has alkylene, arylene, don alkylene, carbonyl, carboxyl, [...], oxy, oxyalkylene and combinations thereof selected from the group consisting a connecting group, R hydrogen, alkyl, cycloalkyl aryl and configured with a output symbols being selected from an group, stage, the same R and X is not portions of the systems, and an annular. According to Claim 9, said photoresist compositions are 6.5E+14 molecules of less than/cm2/s having gas-release rate (outgassing rate), method.