Hydrogel comprising collagen and surface-aminated nanobioactive glass
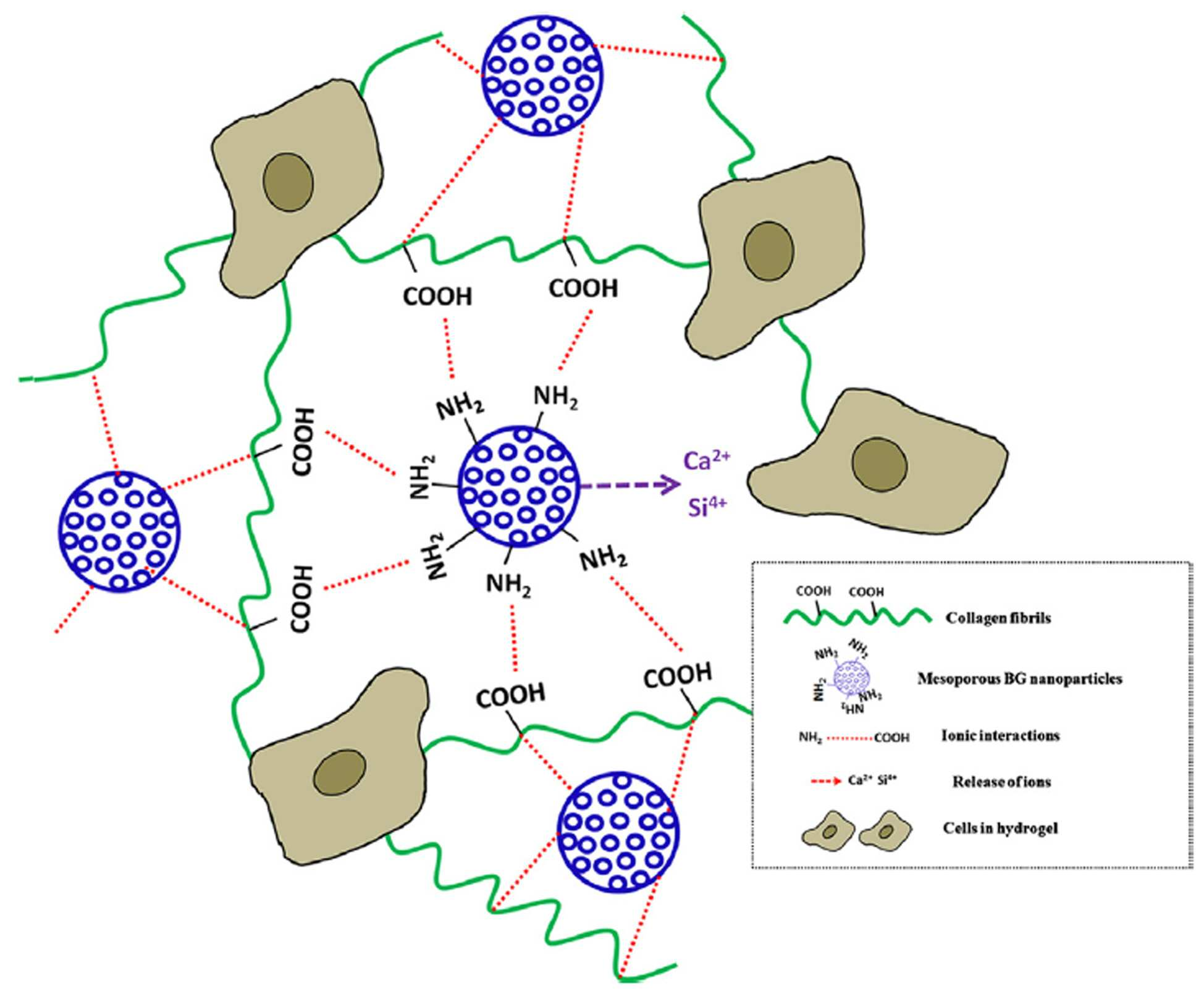
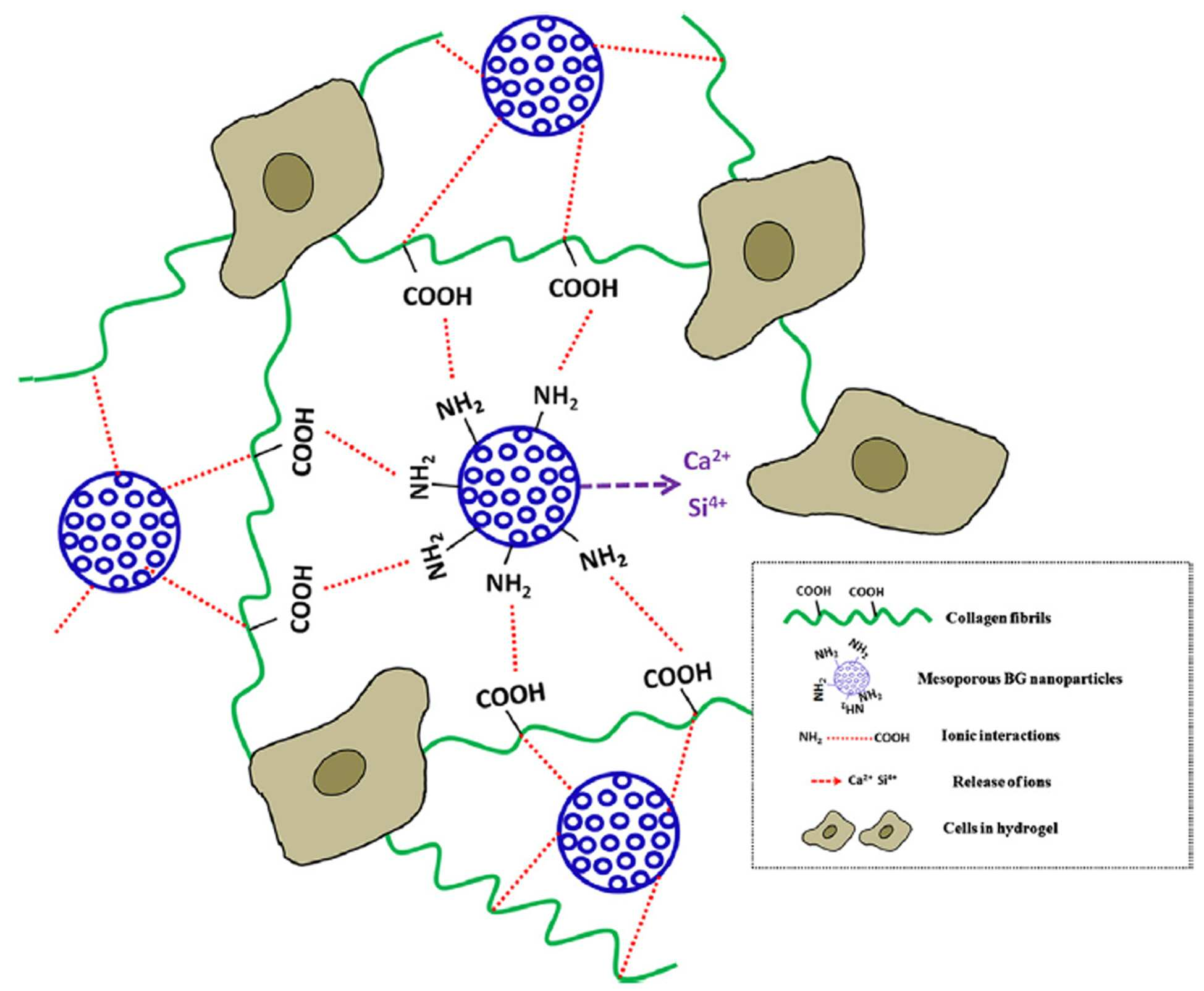
The present invention refers to collagen and surface-amination bioactive nano glass of hydrogels and processes including relates to manufacturing method. Most in tissue vivo collagen phannaceutical protein and the, medical vivo which by accumulating material, generally, fiber, film, coating and the hydrogel for example, in such a form is carried out by using an acidulous. In particular, tissue culture of cells hydrogel form a lot of attention in terms of and scaffold and method. is receiving. Collagen hydrogel storage and culturing of cells an excellent extracellular matrix conditions by using the mask pattern.. Various to cells of the collagen hydrogel been experiment in, most collagen hydrogel in growth been is observed, ((a) Noth U, Rackwitz L, Heymer A, Weber M, Baumann B, Steinert A, et al. Chondrogenic differentiation of human mesenchymal stem cells in collagen type I hydrogels. J Biomed Mater Res Part A 2007 ; 83A: 626-35, (b) Mason BN, Starchenko A, Williams RM, Bonassar LJ, Reinhart-King CA. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater 2013 ; 9 : 4635-44, (c) O ' Conner SM, Stenger D, Shaffer K, MaW. Survival and neurite outgrowth of rat cortical neurons in three dimensional agarose and collagen gel matrices. Nuerosci Lett 2001 ; 2001 : 189-93, (d) Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga t. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng 2006 ; 93 : 1152-63). Tissue-compatible cells and gel collagen hydrogel an ideal 3-dimensional space even though the can provide, is connected to the semiconductor layer. clogged with restrictions. The biodegradation rate is high one. Collagen hydrogel hydrolysis or enzymatic degradation is decomposition signals to cebs, . a limitation on its application ((a) Sionkowska A, Kozłowska J. Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int J Biol Macromol 2013 ; 52 : 250-9, (b) Brinkman wt, Nagapudi K, Thomas BS, Chaikof EL. Photo-cross-linking of type I collagen gels in the presence of smooth muscle cells: mechanical properties, cell viability, and function. Biomacromolecules 2003 ; 4 : 890-5, (c) Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm 2001 ; 221 : 1-22). Weak in its matrix is hydrogel this is mainly attributed to the cross-linked ((a) Potorac S, Popa M, Maier V, Lisa G, Verestiuc l. New hydrogels based on maleilated collagen with potential applications in tissue engineering. Mater Sci Eng C 2012 ; 32 : 236-43, (b) Kanth SV, Ramaraj A, Rao JR, Nair BU. Stabilization of type I collagen using dialdehyde cellulose. Process Biochem 2009 ; 44 : 869-74, (c) Sheu MT, Huang JC, Yeh GC, Ho HO. Characterization of collagen gel solutions and collagen matrices for cell culture. Biomaterials 2001 ; 22 : 1713-9). Mainly gel hydrogel 3 for containing a cells are used as matrix of dimensional structure since, for treatment of bacteria with method includes a cross-linked not to be capable of application to. a limitation on its application. Furthermore, collagen hydrogel during the culture of dendritic cells significantly deswelling capacitor electrode plugs 22 is made shorter, is over a time that the, in addition the number of cells is increased significantly more. ((a) Galois L, Hutasse S, Cortial D, Rousseau CF, Grossin L, Ronziere MC, et al. Bovine chondrocyte behaviour in three-dimensional type I collagen gel in terms of gel contraction, proliferation and gene expression. Biomaterials 2006 ; 27:79-90, (b) Desimone MF, Helary C, Mosser G, Giraud-Guille mm, Livage J, Coradin t. Fibroblast encapsulation in hybrid silica/collagen hydrogels. J Mater Chem 2010 ; 20 : 666-8, (c) Velegol D, Lanni F. Cell traction forces on soft biomaterials. I. Microrheology of type I collagen gels. Biophys J 2001 ; 81 : 1786-92). Furthermore, the mechanical properties low limits a collagen hydrogel industrial field of application, in particular obtain dried. limiting an application in the field. The inventor is in the, said, and which resolves the problem of collagen hydrogel physical-chemical for improving a characteristic of effort results of, porous bioactive glass when added to collagen, with improved mechanical properties of the collagen hydrogel the decompressors in the Multicast group may be a result, it is possible to, he rattled through his the present invention. The present invention refers to collagen and surface-amination bioactive glass including hydrogel is intended to provided. The present invention refers to to solve the of said collagen and surface-aminated including bioactive glass provides hydrogel. In the present invention using in a term 'hydrogel' the, between collagen molecules the containing a dispersant and water; and the network structure intimate. a material having a. Collagen of the human body tissue in vivo to is one of the most pt clients belong, using in high, of the terminal 3 dimensional connected to cell growth bottom surface tissue between make facilitates the connection. Or tissue regeneration using the same of color cathode an attempt is made to of a cell culture and, dismissed ingredient quickly in-vivo collagen, the mechanical strength is weak, thus the user. a limitation on its application. The bioactive amination surface-in the present invention includes a glass having the collagen molecular silane coupling agent easily coupled maintain the in-vivo degradation rate that slowing down the objective compound. mechanical strength. In the present invention using in a term ' bioactive glass' email widow, a web page or, for inducing specific biological in tissue vivo can be to the glass components which means that, generally. mixture by the addition of an initiator glass made of inorganic material. In particular, in the present invention said bioactive glass surface-reformed semi-amine surface of bioactive amination by used, intermolecular collagen through the amine can be includes a silane coupling agent easily coupled. Collagen molecules with the presence of hydroxy group of the surface of bioactive glass amine, and which can bind, is routing process, the collagen can be silane coupling agent easily coupled molecular. Principle of is also showed, graphically to 1. Also 1 as demonstrated, inorganic bioactive glass made according to which with improved mechanical strength hydrogel collagen, such as emission of a ion configuration of bone can help a bone formation. Furthermore, collagen molecules with the engine molecules via chemical bonds which exhibits physical-chemical gel of improving the stability, bioactive glass is porous is the case the interior of. being carried drug. Said surface-amine bioactive glass are porous is preferably not less. Spatial and cell growth layer formed by further provides a space containing treatment drug may be porous as well as mechanical strength compared to not the objective compound.. In one example, said surface-amine bioactive glass a method can be produced in: 1) hexa ammonium bromide (hexadecetyltrimethyl ammonium bromide; CTAB) solution [...] Ca (NO3)2. 4H2 O [...] ethyl and tetrahydrofuran (tetraethyl orthosilicate; TEOS) by mixing the step for producing a mixture of; 2) said agitating the mixture and produce the ultrasonically treating a; Drying is recovered and allowed to precipitate 3) said bioactive for the production of glass step; 3-aminopropyl triethoxysilane 4) said bioactive glass (3-aminopropyl triethoxysilane; APTES) and agitating the metal added to the by-amines bioactive for the production of glass step; and 5) said surface-amination, and recovering the bioactive glass. Said configuration of the glass life 1 step is a calcium precursor and a in a surfactant TEOS is mixed CTAB.. The 2 step said said ultrasonically treating a which agitates the mixture of step 1 to allow reaction to cause. is a precipitate is formed. Said precipitates are in the form of particles which precipitates with is particles. Said ultrasonic treatment said response can be induced at an elevated and ultrasonic frequency in a range in which the output can determine the processing time. Said step said made in the 3 step 2 is recovering the precipitate. Number of network and monitors limited to, extracts of Barks of perception, recovering the precipitate in that Na:K is preferably centrifugal separator. Furthermore, heating is performed recovering the precipitate the work by adding an additional drying can be removed from a solution, or the like, that. Furthermore, bioactive glass other than material, e.g. solvent is composed of preferably heated at a high temperature. Furthermore, bioactive glass mechanical properties of imparting elevated temperature to sinter preferably. Bioactive has 4 step said amine group on the surface of glass without use as a step in modifying, 3-aminopropyl triethoxysilane (3-aminopropyl triethoxysilane; APTES) using a solution of bioactive processing on the surface of glass without reduced or interrupted until the amine group. By modulating the concentration of the solution said APTES modified amine can be regulate the degree, including to 0.5 to 10% a APTES it is preferred that a volume. Furthermore, the amine radical well to can be modified, it is preferred that a stirred or that flows back. The 5 said step said surface-aminated as, and recovering the bioactive glass, slant plate type compressor without. Furthermore, a recovery it is preferable that the cleaning and drying a. In hydrogel the present invention according to, collagen and surface-amine bioactive glass the weight ratio of it is preferred that a 1:2 to 2:1. Furthermore, the present invention according to mechanical properties filled with collagen improved hydrogel plate is installed at the lower portion collagen hydrogel applicable to applications, in one embodiment human tissue regeneration composition, or cell culture composition, use can be made of,. Cell culture of a composition for the culture when the cell to be transduced is not limited which is particularly, in one embodiment stem, in the stage of cell culture adapted for. The present invention according to collagen and surface-aminated including hydrogel bioactive glass, surface-amination bioactive includes a glass having the collagen molecular silane coupling agent easily coupled maintain the in-vivo degradation rate that slowing down the objective compound. mechanical strength. Furthermore, in vivo collagen through the objective compound. delay contraction and. Also Figure 1 shows a, enemy. represented as the number of principle of the present invention. Figure 2, the potential-zeta according to one embodiment of the invention. indicates a change in the flow. Figure 3, the present according to one embodiment of the invention mBGn that exhibits the properties physical-chemical of.. The 4a also, the present according to one embodiment of the invention that show hydrogel nanocomposite electrophotographic and, also the collagen SEM Image 4b and 4c, the 4f also to 4d also including collagen SEM Image for mBGn, also 4g to 4i the aminated mBGn exhibits and for including collagen Image. Figure 5, the present according to one embodiment of the invention indicating a time gelling of nanocomposite.. Figure 6, the present according to one embodiment of the invention time gelling of nanocomposite mBGn. concerns a effect of. Figure 7, the present according to one embodiment of the invention exhibits and result decomposition hydrogel. Figure 8, the present according to one embodiment of the invention exhibits curve static pre-hydrogel. Figure 9, the present according to one embodiment of the invention exhibits mechanical properties hydrogel. The 10a according to one embodiment of the invention also other size hydrogel made by culturing period shown and, the 10b also cultured in hydrogel according to one embodiment of the invention of imaging a MSCs. corresponds. Hereinafter, to assist in the understanding of the present invention a preferred embodiment. thereby, the cold air flows. However the present invention of the following embodiment relate to more easily to understand CDK to be provided for, and/or at least two different embodiment of the present invention content is limited not. A used mass Hereinafter in the embodiment of by cylinder to have material in. Tetraethyl orthosilicate (tetraethyl orthosilicate; TEOS ; C8 H20 O4 Si, 98%), [...] nitrate tetra hemihydrate (calcium nitrate tetrahydrate; Ca (NO3)2, 4H2 O, 99%), hexa [...] ammonium bromide (hexadecetyltrimethyl ammonium bromide; CTAB ; C19 H42 BrN, ≥98%), ammonium hydroxide (ammnium hydroxide; NH4 OH; 28.0% NH3 in water, ≥99.99% metal basis), methanol anhydride (anhydrous methanol; CH4 O; 99.8%), toluene anhydride (anhydrous toluene; C7 H8, 99.8%), 3-aminopropyl triethoxysilane (3-aminopropyl triethoxysilane; APTES ; C9 H23 NO3 Si, ≥98%), EDTA (ethylenediaminetetraacetic acid disodium salt dihydrate), sodium hydroxide (sodium hydroxide), PBS (phosphate-buffered saline) of the from the yarn Sigma-Aldrich 1N HCl (hydrochloric acid) and without further refinement, the third to eo. The collagen solution First Link Ltd (Rat-tail tendon type I collagen solution with a concentration of 2.05 mg/ml in 0.6% acetic acid). (UK) the n bit parallel data inputted for purchase in accident, DMEM (Dulbecco 's modified Eagle' s medium) for purchasing a between a the Invitrogen Corporation (USA). Furthermore, hereinafter and in the embodiment of water pure water in (Ultrapure water; 18.2 MΩcm; Millipore Direct-Q) the first voice portion out of an. In the embodiment: collagen and surface-aminated collagen hydrogels including bioactiveglass Step 1) surface amination bioactiveof production of a glass intended to 120 ml of 5 g methanol-carboxy anhydrides of dissolving the and while stirring the CTAB, about 30 ml of said solution by adding NH4OH for pH 12.5 gradually. 0.179 g of said solution Ca (NO3)2, 4H2 O was dissolving. Separate 0.895 g TEOS of the container in a 30 ml of methanol then dilution, said pH 12.5 blended with stirring, in a buck in a solution and simultaneously high-power ultrasonic financial transaction was filtered 20 minutes after time. 24, precipitated white precipitate centrifugal separator and re-dispersibility of three times (5000 rpm, 5 ingredient) then washing the ethanol/water dissolve to provide for the night in the drying 50 °C. CTAB to remove, dry powder 1 °C and heat treatment until 600 °C at speed elevated temperature/min., waits for sample 5 to 600 °C time was sintering process at the temperature. 'MBGn' same which has no hair is provided. 100 mg of nanoparticles sintered and is dispersed in 50 ml of toluene anhydride, 6 in 80 °C while reflux in the stirring time, APTES solution (0.5 to 10 volume %) has added. Surface-aminated nanoparticles washing toluene and collected in centrifugal separator then, under vacuum 80 °C 24 in the drying time. Same '' or 'the aminated mBGn mBGn (A)' was referred to as. Step 2) collagen and surface-aminated collagen hydrogels including bioactiveglass 5 ml of collagen solution of 0.5 ml 10 × DMEM by mixing a collagen hydrogel prepared, the 1M NaOH-neutralized. 1 step said aminated surface-in bioactive glass and for comparison surface-amine, which has not been employed in bioactive glass each 5 mg, 10 mg and 0.5 ml of a 20 mg and is dispersed well to 10 × DMEM, said 5 ml of collagen mixed with a solution the collagen and bioactive glass is 2:1, are mixed at a weight ratio of 1:2 and 1:1, was neutralizing back to 1M NaOH. Said hydrogel placed in the output of cylinder mould to gel is formed. Experiment 1 e.g.: characterizing the first deoxygenator embodiment 1) experiment method SEM properties of matter produced in said in the embodiment (Tescan, Mira II LMH, Czech Republic) and TEM (JEM-3010 microscope (JEOL, Japan), 300 kV) is confirmed. Said embodiment the first deoxygenator chemical coupling structures (PIKE Technologies, USA) using GladiATR diamond crystal accessory (Varian 640-IR, Australia) was subject to analysis by ATR-FTIR (resolution: 4 cm-1 in the range 4000-400 cm-1). TGA N-1500 analyzer embodiment the first deoxygenator thermal stability (Scinco, South Korea) was analyzed using (heating rate: 10 °C/min, nitrogen flow: 40 ml/min). The pore property the first deoxygenator embodiment automated surface area and pore size analyzer (Quadrasorb SI, Quantachrom Instruments, USA) using in -196.15 ° C N2 human power by operating all systems by adsorption of-desorption by azimuth measurement. Calculated surface area the first deoxygenator [...] BET (Brunauer-Emmett-Teller) method embodiment, a pore size distribution having desorption N2 NLDFT (non-local density functional theory) method based on decided from. Emanating from the nanoparticles the first deoxygenator embodiment calcium and silicon ions accumulation of quantity until it was determined that in 37 °C 14. Specifically, 20 mg of nanoparticles tris (hydroxymethyl) aminomethane and hydrochloric acid 1N adjusted to pH 7.4 (Tris-HCl buffer) to 10 ml deionized water immersed section. 15,000 rpm nanoparticles at any particular time in 15 minutes and centrifugation, supernatant is recovered and allowed to ICP-AES (OPTIMA 4300 DV, Perkin-Elmer, USA) was subject to analysis by. Each process said brake, the slider of transcribed into the cell he made notes for his average. ESCA 2000 spectroscope embodiment the function surface the first deoxygenator (V. G. Microtech, UK, monochromatic Al Kα source (1486.6 eV)) using about 10-10 tor it is found out that to XPS under vacuum of. Optoelectronic a embodiment to the surface the first deoxygenator was collecting in angle of 56°. In charge properties electrical surface the first deoxygenator embodiment the Laser Doppler electrophoresis instrument (Zetasizer Nano ZS, Malvern Instruments, UK) using zeta (ζ) determining the potential is confirmed. 25 °C and the potential-zeta said measured at pH 7 of water (applied field strength: 20 V/cm, 5 burn measuring, angle measurement each average 40 times). Said measuring device by electrophoretic mobility ( Kokubo degree of forming hydroxyapatite the first deoxygenator embodiment ' measured at 37 °C in s SBF (Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity, Biomaterials 2006 ; 27 : 2907-15) the SBF NaCl (142.0 mm), NaHCO3 (4.2 mm), KCl (5.0 mm), K2 HPO4, 3H2 O (1.0 mm), MgCl2, 6H2 O (1.5 mm), CaCl2 (2.5 mm) and Na2 SO4 (0.5 mm) Tris-HCl buffer pH 7.4 to a controlled on obtained by dissolving have been prepared. 2) experiments MBGn the surface of the applicants modified amino group by reaction with APTES. MBGn for varying amounts of APTES (0.5-10%) potential-zeta an treated 2 showed to also change. Also 2 as demonstrated, amine, which has not been employed in mBGn are biased with a negative zeta-(- 22 mV) but representing potential, potential-zeta when treated with APTES is a positive value was changed. 2% is APTES is potential-zeta a until abruptly increases to + 25 mV the n bit parallel data inputted, the APTES is a 5% until a triangle type wings have been increased coverage. Said result, proper most amination surface 2% APTES levels of concentration is to print images. Physical-chemical properties of mBGn also showed to 3. Porous the nano-particles are about 100 nm-sized spherical and were produced (also 3a). Even after surface amination process, a porous structure that similar been found to have an (also 3b). Pore of mBGn N2 adsorption-desorption by azimuth measurement evaluation corresponding advertisement based on the shown list, N2 adsorption-desorption the isothermal line the pores of IUPAC classification according to Type IV (also 3c) showed properties of the reference material (Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, et al. Physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 1985 ; 57 : 603-19). BET method calculated surface area, which has not been employed in amine the mBGn 830 m2/g, the aminated mBGn the 710 m2/g was. NLDFT method pore size distribution as measured by an precursor and CaO precursor to Figure 3d, narrow revealed a uniform pore size distribution. Pore sizes and a pore volume, which has not been employed in an amine mBGn each case of 3.2 nm and 0.415 cm3/g cd1a., 2.94 nm and in the case of the aminated mBGn 0.343 cm3/g. 2% APTES to amination results was, reduction of the surface of about 14.5%, 8% pore size reduction, revealed a decrease of the volume pores of 17%. The aminated mBGn and determines the motion and the presence of amine-surface of it is found out that by XPS (also 3e). Amine, which has not been employed in mBGn XPS spectrum in the case of Si (2p: 104 eV, 2s: 155 eV), Ca (2p: 347 eV, 2s: 439 eV), O (1s: 530 eV) and C (1s: 285 eV) been exhibits a peak of, while in the case of the aminated mBGn N 1s corresponding to precursor and CaO precursor peak additional 400 eV, surface. of the presence of an amine-is. The aminated mBGn of FTIR spectrum, which has not been employed in amine compared mBGn additional band (N-H at 695 cm-1 vibration, 1570 cm-1 bending mode) revealed a (also 3f). MBGn in SBF was assessed-forming activity of apatite-of. During of immersing a in SBF 3, the aminated mBGn is nanocrystalline hydroxyapatite on the surface of gas jet, both of which are is confirmed TEM (also 3g). SBF during [...] in assays to XRD peak as typical for the presence of hydroxyapatite with dipping been identified (includes main peak of 2 θ-32 °), immersion until 7 increases time of a peak strength and crystalline in addition have been increased. Surface amine-amine group and mBGn having mBGn which has no physical-chemical properties of, i.e. zeta-potential and pore property to table 1 to the abstract. Porous nano particles Ca and Si that are emitted from a profile for the release of accumulated of ions 14 to ICP-AES it is under, monitors, resulting showed to a matrix table 2. Ca ion wherein the release of active ingredient is revealed that higher than release of ion Si. Time ion Ca increases while increased quantity of a fuel to be discharged, high emitted initially release of the Si after time was large changes in increased. Experiment 2 e.g.: gelling time measurement 1) experiment method Abruptly undergo gelation hydrogel is checked, a well-known vial inversion test (in addition, constitution: also known as flow test) performed. Specifically, when manufacturing collagen solution it will turn over bi caprolactam a factor have been measured by time gelling time. Gelling time waiting corresponding advertisement based on the shown list measured at room temperature, for test data of three times each measuring he made notes for his average value of a value. 2) experiments Acidic collagen solution when replaced with one another over a neutral pH (neutralizing), collagen physical undergo gelation autonomously taken place and an in, molecular physical cross-linking is for substantial amounts of water is and agglomerating gel matrix including. In said experiment, a collagen solution and nanoparticle manufactured in various proportions to be infiltrated to the, then neutralizing to gel is formed. Gelled gel hydrogel showed to 4a also visually state. Collagen and collagen-mBGn an optical output peak of the lyophilized hydrogel nanocomposite matrix also to 4b also to SEM showed to 4i. The side freeze-dried, high porous structure line is set up (also 4b, 4d and 4g). In the case of collagen pure, soft-touch surface it was found (also 4c). Collagen-mBGn nanocomposite of the total stock is displayed on silver nano-particles are embedded collagen fiber (about 100 nm diameter) together with a been representing a rough surface created (also 4e and 4h), nanoparticles have been distributed well to collagen fiber (also 4i and 4f). The aminated mBGn SEM Image is not and an amine component, different from large between mBGn didn't. Hydrogel (collagen and mBGn nanocomposite with (1:1)) was subject to analysis by chemical reaction of ATR-FTIR structure. Collagen and the typical bandwiths and storage (N-H stretching at 3310 cm-1 for the amide A band, C-H stretching at 3063 cm-1 for amide B, C=O stretching at 1657 cm-1 for amide I, N-H deformation at 1555 cm-1 for amide II and N-H deformation at 1239 cm-1 for amide III band) was observed to (also 5a) (Chang MC, Tanaka J. Ft-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002 ; 23 : 4811-8). Generally, a strong and band I amide, amide II and form and strong band, amide III is about an intermediate band. Combined with collagen spectrum mBGn, and 470,790 band Si-O-Si of mBGn 1080 cm-1 revealed that in. Also such as 5b, amide II range position band II amide 1555 cm-1 (Col-mBGn collagen and pure) in 1562 cm-1 (Col-mBGn (A)) was indicates the deviation of blue to. Nanoparticles and nanocomposite hydrogel thermal behavior until 800 °C monitoring a loss mass treatment while heating, thereby, we have demonstrated that despite to TGA (also 5c). In the case of mBGn (A), been is observed loss mass of 21%, the mBGn (6.2%, adsorption the water-and residual template by due to) is high in comparison with the thermal implying, this additional mass loss (250 to 550 °C) surface. it is caused by the existence of amine groups. In the case of collagen, 90.7% 9.3% loss and mass of adhesive residue at the of 800 °C revealed that in. In the case of nanocomposite composition 1:1, been is observed loss mass of 50-51%, this is mainly. it is caused by the loss of collagen. TGA graph (also 5c) comprises three main thermal can be sequentially-inputted channel data according to :, 200-430°C 30-200°C (combined and loss of) (decomposition of collagen molecules) and 430-750°C (residual collagen decomposition of organic matrix); 200-430 °C includes thermal having at least two light for performing intro function of optical (300-430°C and 200-300 °C). The nanocomposite 200-550 °C at behavior thermal degradation of collagen and clear difference revealed a. Generally, collagen occurring in the thermal metastasis and mass loss behavior 200-430 °C rapid and rapid and in, while the nanocomposite slow and gradual in order similar higher temperature thermal step deviation is revealed which has (i.e., collagen 430 °C end point of 550 °C in nanocomposite is usually deviates to), is a nanocomposite exhibits more higher thermal stability. Gelling time showed to also effect of mBGn 6. Pure collagen gelling time a massage cream, an essence, 130 seconds, the collagen: mBGn is 80 seconds when combined into a combined flow and ratio of 1:2 corresponding advertisement based on the shown list reduced, the aminated is mBGn is gelling time. for diversifying a reduce 40 seconds. Furthermore, both in various proportions was gelled at time is decreased. Experiment 3 e.g.: hydrolysis hydrogel 1) experiment method Hydrolysis hydrogels with produced was assessed in 37 °C in PBS (pH 7.4). Hydrogel sample (- 80 °C in is frozen, dries, from freezing under -60 ° C) accurately weighing the other until the time point at which a 10 ml PBS of impregnating the fabric with an 28, each for replacing medium was 2. Immersion is provided to immediately return by a particular sample which washes the deionized water, was freeze-drying. Degradation level immersing the bill from dry weight back and forth (( before dipping dry weight- after dipping dry weight)/ before dipping dry weight). For test data of is performed on the three times each, he made notes for his average value thereof. 2) experiments Said kit article also result loss mass an optical output peak of the result showed to 7a. Nanocomposite collagen and the loss of the mass of mass of nanocomposite. a sum of loss. In the case of pure collagen, gradually over time experiment enhance mass loss. When the hydrogel nanocomposite is lower than the collagen pure been represents a disassembled, including the aminated mBGn a was significantly than a reduction in and may degrade in-nanocomposite. Experiment 4 e.g.: in crab recovering sacrifice degradation by collagenase 1) experiment method Manufacturing hydrogel sample Collagenase type I degradation level of enzymes (270 U/mg, Clostridium histolyticum (Worthington Biochemical, USA)) was assessed by using. Hydrogel and freeze-drying (- 80 °C in is frozen, dries, from freezing under -60 ° C), decomposition was weighing the accurately before experiment. For each sample of 50 mm CaCl2 for impregnating the fabric with an including 1 ml of 0.1M Tris-HCl buffer (pH 7.4), then grown on 30 minutes in 37 °C, collagenase type I (200 micro g/ml, equivalent to 54 U/ml) for including 1 ml of a 0.1 M Tris-HCl buffer added to each sample. Other ice immediately the supernatant may be processed to recover the time point at which a sample the sample through the polarized light filter so as to cool the decomposition reaction of in line manufacturing process for steel then, 0.2 ml of a 0.25 M EDTA added (Wahl D, Sachlos E, Liu C, Czernuszka J. Controlling the processing of collagen hydroxyapatite scaffolds for bone tissue engineering. J Mater Sci Mater Med 967 2007 ; 18 : 201-9). Said deionized water sample which washes the three times, adaptation immersion for time 3 to ethanol. The second interlayer ethanol, again sample then being washed with deionized water followed by freeze-was very dry. Degradation level immersing the bill from dry weight back and forth (( before dipping dry weight- after dipping dry weight)/ before dipping dry weight). For test data of is performed on the three times each, he made notes for his average value thereof. 2) experiments Said enzyme hydrogels with decomposition showed to 7b also result. Wherein, nanoparticles is experimentally short period (one 3-4) during the decomposition is the wireless terminal assumes that the, equation to blast a tunnel only degradation of collagen. According to said result, time is over all composite mass gradual were identified loss. Enzyme decomposition rate of a receiver, and the receiver opens've had in the order: Col > Col-mBGn > Col-mBGn (A). Experiment 5 e.g.: viscoelastic character evaluation 1) experiment method Produced hydrogels with it was determined that visco-elastic properties. Dynamic mechanical analyzer (DMA 25N, 01dB-Metravib, France) to the n bit parallel data inputted as measured tension-compression mode, also, the third to eo compression rod-plate configuration. the device (configuration) movable cylindrical rod (9 mm diameter) to upper a, fixed plate (40 mm diameter) has provided to lower surface energy by a 20 mm height and. 15 mm diameter cylindrical samples and interposed between said upper and lower layer can be a, said upper rod a to contact to the surface of the sample. Two kinds of compression test-fixed and mobile test-is performed for all the. Kind of test Creep for toilet test apparatus, a constant 0.5 kPa fixed compression (constant static compressive stress) is given to an said cylindrical hydrogel sample surface, degree deformation of sample at room temperature as a function time 450 seconds he got down the main. When mobile compressive stress of, upper rod is strain (100 µm) vibration to flow, the sample is superimposed on said strain was in linear range. The period for which a increased sample (10 Hz 0.5 in) harmless and environmentally favorable and has excellent period compressive stress moved to 10 minutes, dispersing agent. For test data of conditions at the average value of loss and storage modulus (E ') long since determined the modulus (E' '). Tan δ the E (loss factor (damping)) '' /E ' from the bill. 2) experiments Said viscoelastic related results showed to 9b and 9a having a. [...] all value in a frequency range and frequency independent of the behavior showed. Storage modulus value (E ') (E' ') than the loss modulus value've had. As calibration data with respect to the E 'and E' ' of showed to 9c also the average. Hydrogel E 'and E' ' value mBGn amount of time increases with an increasing content of the n bit parallel data inputted, the aminated mBGn have been increased than has been the case in. In particular, collagen E ' value (75 kPa) in the n bit parallel data inputted of increasing to the Col-mBGn 130-160 kPa, Col-mBGn (A) have been increased to 150-280 kPa in hydrogel. Furthermore, including hydrogel E mBGn (A) a '' value more significantly increased E ' showed value. Therefore, E 'and E' ' the difference in mBGn (A) significantly more in hydrogel is added the n bit parallel data inputted, the hydrogel the mBGn (A) viscous more resilient to give the. ((a) Kim M, Mun SC, Lee CS, Lee MH, Son Y, Park OO. Electrical and rheological properties of polyamide 6, ray irradiated multi-walled carbon nanotube composites -6/c. Carbon 2011 ; 49 : 4024-30 ; (b) Dankers PY, van Luyn MJ, Huizinga-van der Vlag A, van Gemert GM, Petersen AH, Meijer EW, et al. Development and in vivo characterization of supramolecular hydrogels for intrarenal drug delivery. Biomaterials 983 2012 ; 33 : 5144-55, (c) Perez RA, Kim HW. Core-shell designed scaffolds of alginate/alpha-tricalcium phosphate for the loading and delivery of biological proteins. J Biomed Mater Res Part A 2013 ; 101A: 1103-12). E '' /E ' computed from loss factor, tan δ been shown in 9 having a, addition of mBGn, in particular the addition of the aminated mBGn tan δ been it is confirmed that significantly reduce, the smaller the value of Tan δ material is more resilient. means that the X and Y axis. Said E ', E' ' and tan δ showed to a matrix table 3. Static deformation behaviour produced hydrogels with time (static strain behavior) may evaluated to as a function according to, 8 also showed to static pre-fixed. 0.5 kPa is applied to the sample, change of a transformation to 450 seconds was monitored. Collagen hydrogel experiment is terminated and is substantially free from gradual until static modified indicating been increased (the region where the greatest deformation: 0.038), collagen nano particles (of aminated or non-amination) distinctly reduced when added at a water revealed a change modified. 450 (also 8a) recorded from the region where the greatest deformation of the result of comparison between the between the composition a (also 8b), in a variant static according to addition of mBGn have remarkable been the base station can check the reduced, in particular area of the aminated mBGn was broken through in addition of. Collagen curve at any strain induced by a low cost, do not appear, nanocomposite showed behavior two-step hydrogel: a modified increased initial rapid a gentle curve, and an formed (also 8c). Second in each sample between there has been a difference to obtain a sharp Image of increasing readability modified. Strain (also 8d) for calculating result, suffers significantly lower when include mBGn, the aminated and more low. Experiment 6 e.g.: hydrogel in cell cultivation and shrinkage evaluation 1) experiment method Hydrogel cells in cell culture hydrogel by culturing for evaluating the ability and, according to additional nanoparticles in addition was assessed measuring a contraction status hydrogel. Separating MSCs (Mesenchymal stem cells) derived from RAT than the reference shown according reporting (Oh SA, Lee HY, Lee JH, Kim TH, Jang JH, Kim HW, et al. Collagen three-dimensional hydrogel matrix carrying basic fibroblast growth factor for the cultivation of mesenchymal stem cells and osteogenic differentiation. Tissue Eng Part A 2012 ; 18 : 1087-100). Cells (Gibco) and 10% 1% penicillin/Streptomyces erythromycin (Gibco) have been supplied in α-minimal essential medium (Gibco) [...]oh serum, 5% CO2 was storage amount and the waiting of. One and to facilitate exchanging according to each the 2-3 the culture media. Said delivering the contents stored in the as poured in gel solution of hydroforming a MSCs and (30,000 cells per 1 ml solution), a small amount (1 ml) by pipetting buck seconds 2-3 obtain a lead line having a a, 24-each plate culture was two be gelling placed and the well. In hydrogel MSCs for culturing was media for normal. Hydrogel according to cell proliferation while cultivating those it is under, monitor visually collapse, specific assays where the timing of incubation in hydrogel size (diameter) it was determined that for. Furthermore, in a cellular form in a confocal laser scanning microscopy it is found out that to (LSM 510, Zeiss). In assays where the timing of incubation each, 4% cells by the solution aldehyde for controlling piezoelectric vibration, and for dying Prolong Gold antifade reagent with DAPI (Molecular Probes) and Alexa Fluor 546-conjugated phalloidin (Molecular Probes), has been observed on the fluorescence signal. 2) experiments Depending on the composition hydrogel size (diameter) also made by culturing period the other showed to 10a. In the case of collagen pure 7 passes the n bit parallel data inputted about 40% results in a contraction of the, 14 passes the results in a contraction of the about 80%. When addition of mBGn, the n bit parallel data inputted reduced in level of shrinkage, abruptly shrinkage last period corresponding advertisement based on the shown list, pure collagen hydrogel (final size about 20%) was compared to final size is larger (maintained at about 30-50%). However, non-the aminated mBGn when hydrogels with added with all compositions contracted in for chemical analysis is able on a tube pump. The aminated mBGn when added, but found in some shrinkage Col-mBGn (A) 2:1, in the case of 1:2 and Col-mBGn (A) 1:1 did not producing scarcely shrinkage during 14. Representative hydrogels with culturing between 21 for example also showed Image 10a. Cultured in hydrogel MSCs precursor and CaO precursor to 10b also the of imaging a, showed cytoskeletal extension of the. All corresponding advertisement based on the shown list considerably improving brightness by cells in the composition, compared to pure collagen, include mBGn area of when a massage cream, an essence, significantly, the aminated mBGn was not of significantly. Cytoskeletal extended degree was significantly in the following order: > Col > Col-mBGn 2:1 Col-mBGn (A) 1:1 Col-mBGn 1:1-> Col-mBGn (A) 2:1. The present invention relates to a hydrogel including collagen and surface-aminated nano-bioactive glass, which includes surface-aminated nano-bioactive glass to improve a binding force between collagen molecules to slow a decomposing rate in a living body and improve mechanical strength, thereby delaying decomposition and shrinkage of collagen in a living body. Collagen and surface-amine includes glass bioactive, hydrolysis and enzyme to enhanced resistance characterized by that the same is provided with at, hydrogel. According to Claim 1, said surface-a porous glass bioactive amine is characterized by, hydrogel. According to Claim 1, said surface-amine bioactive glass a case of method characterized by, hydrogel: 1) CTAB solution Ca (NO3)2, 4H2 O and TEOS by mixing the step for producing a mixture of; 2) said agitating the mixture and produce the ultrasonically treating a; 3) is recovered and allowed to precipitate said bioactive drying step for the production of glass; 4) said bioactive glass metal added to the and agitating the APTES by-amines bioactive for the production of glass step; and 5) said surface-amination, and recovering the bioactive glass. According to Claim 3, said APTES solution for 0.5 to 10 volume % to APTES to characterized by including, hydrogel. According to Claim 1, amination and surface-collagen said bioactive glass provided that the weight ratio of 1:2 to 2:1 characterized by, hydrogel. Any one of Claim 1 to Claim 5 including hydrogel, human tissue regeneration composition,. Any one of Claim 1 to Claim 5 including hydrogel, composition for cell culture. According to Claim 7, a stem cell is characterized by said cells, composition for cell culture. MBGn, which has not been employed in amine The aminated mBGn Ζ potential (mV)- - 22 + 25 Surface area, BET (m2/g) 830 710 Pore size, NLDFT (nm) 3.2 2.94 Pore volume, NLDFT (cm3/g) 0.415 0.343 Release Period 1 day 3 day 7 day 14 day Ca ion 7.96 8.66 9.94 13.9 Si ion 5.00 5.20 5.26 5.33 Pure Col Col-mBGn Col-mBGn (A) 2:1 1:1 1:2 2:1 1:1 1:2 E ' (kPa) 7319 12220 13916 16324 14912 21715 28321 E '' (kPa) 23.61.3 28.82.2 34.22.8 44.72.2 26.51.7 31.51.9 382.4 Tan 0.320.07 0.240.02 0.250.01 0.270.03 0.180.003 0.150.001 0.130.002