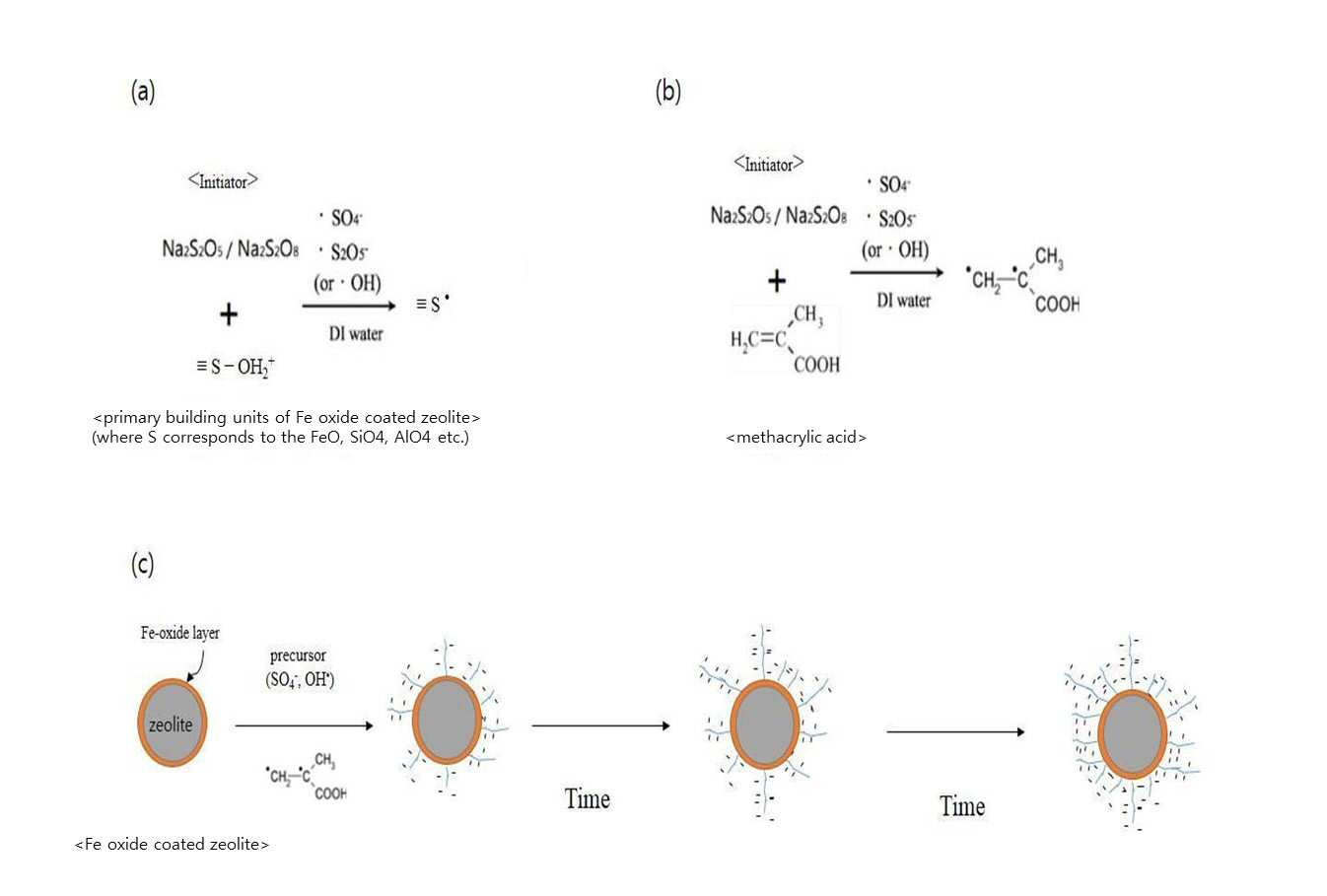
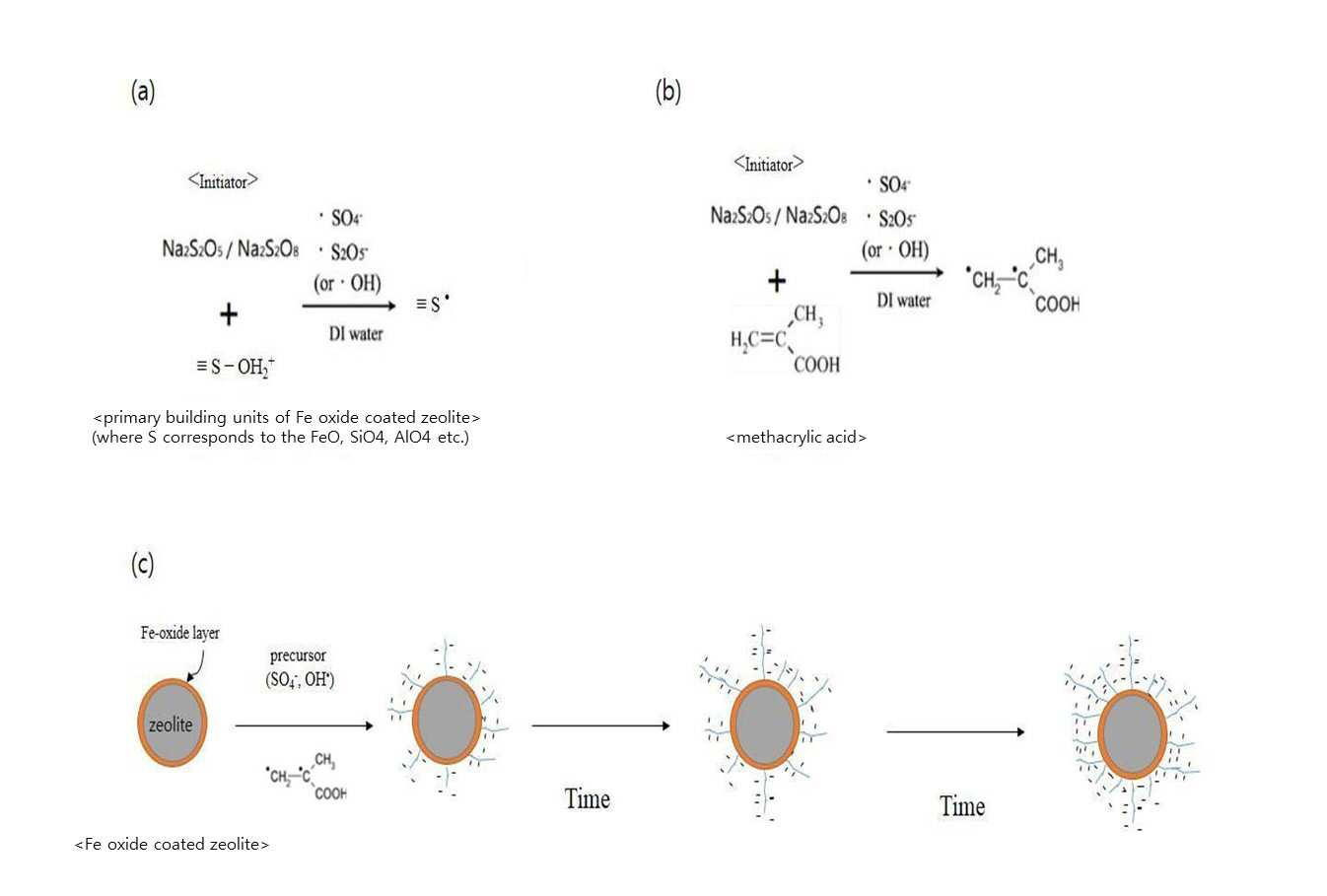
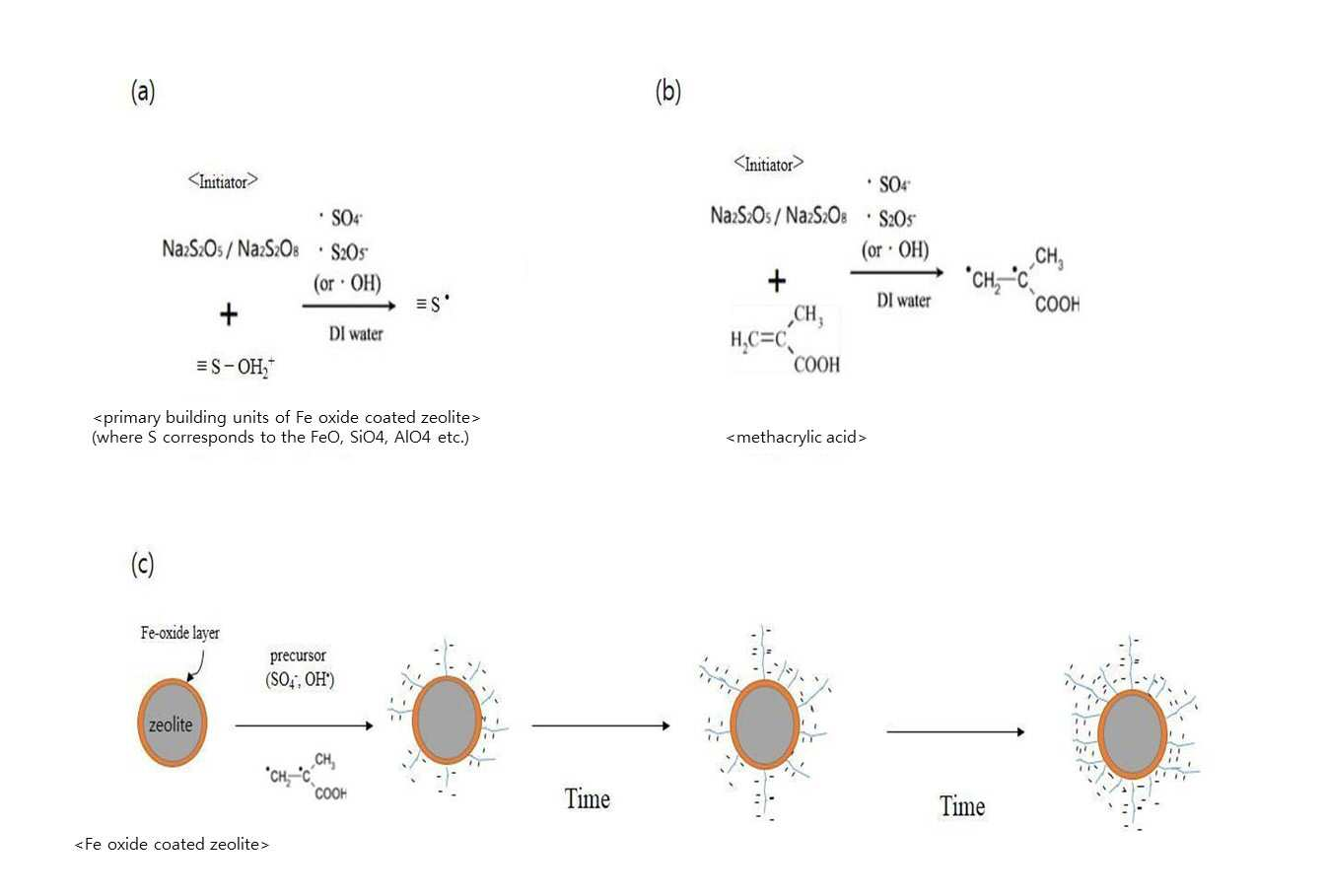
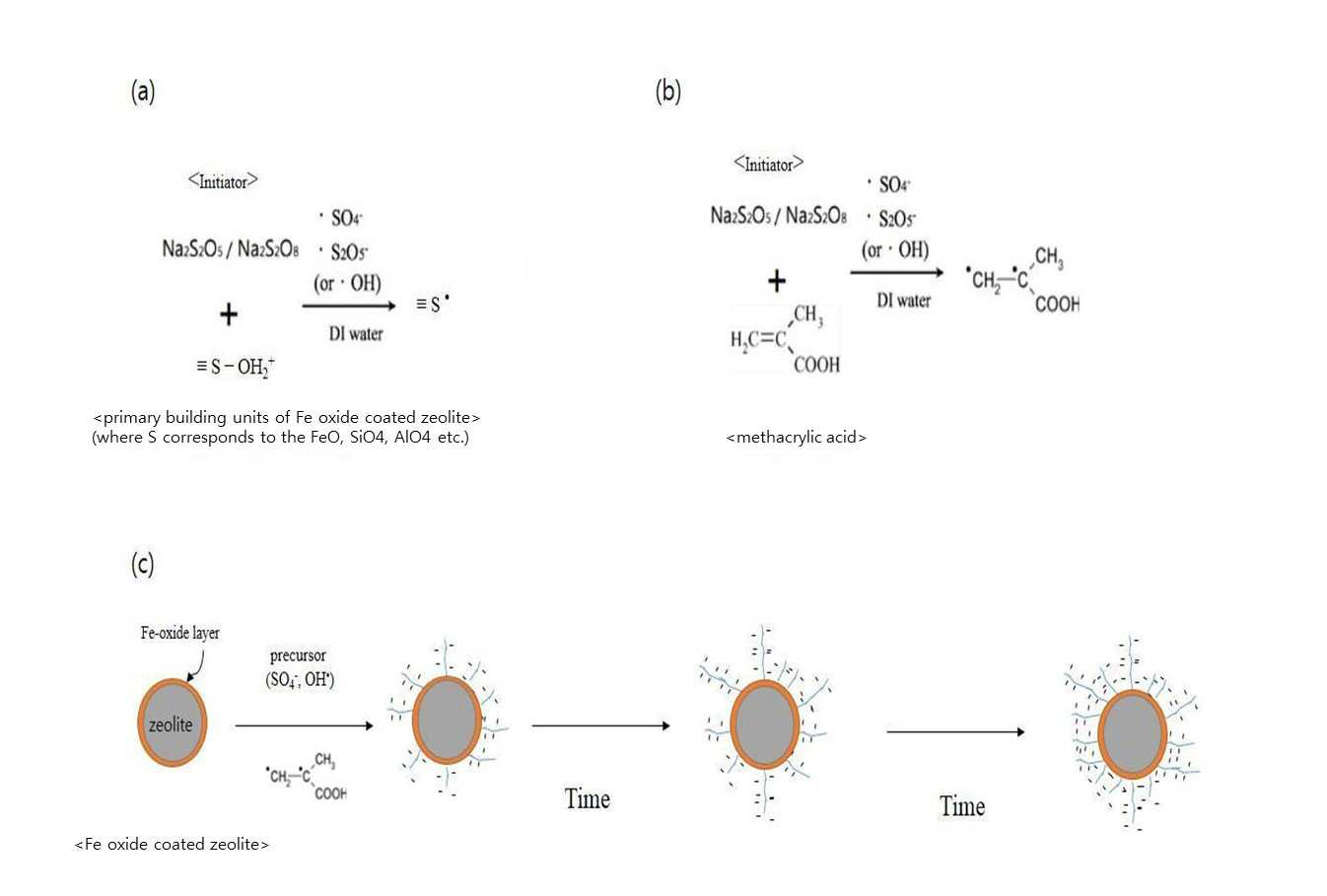
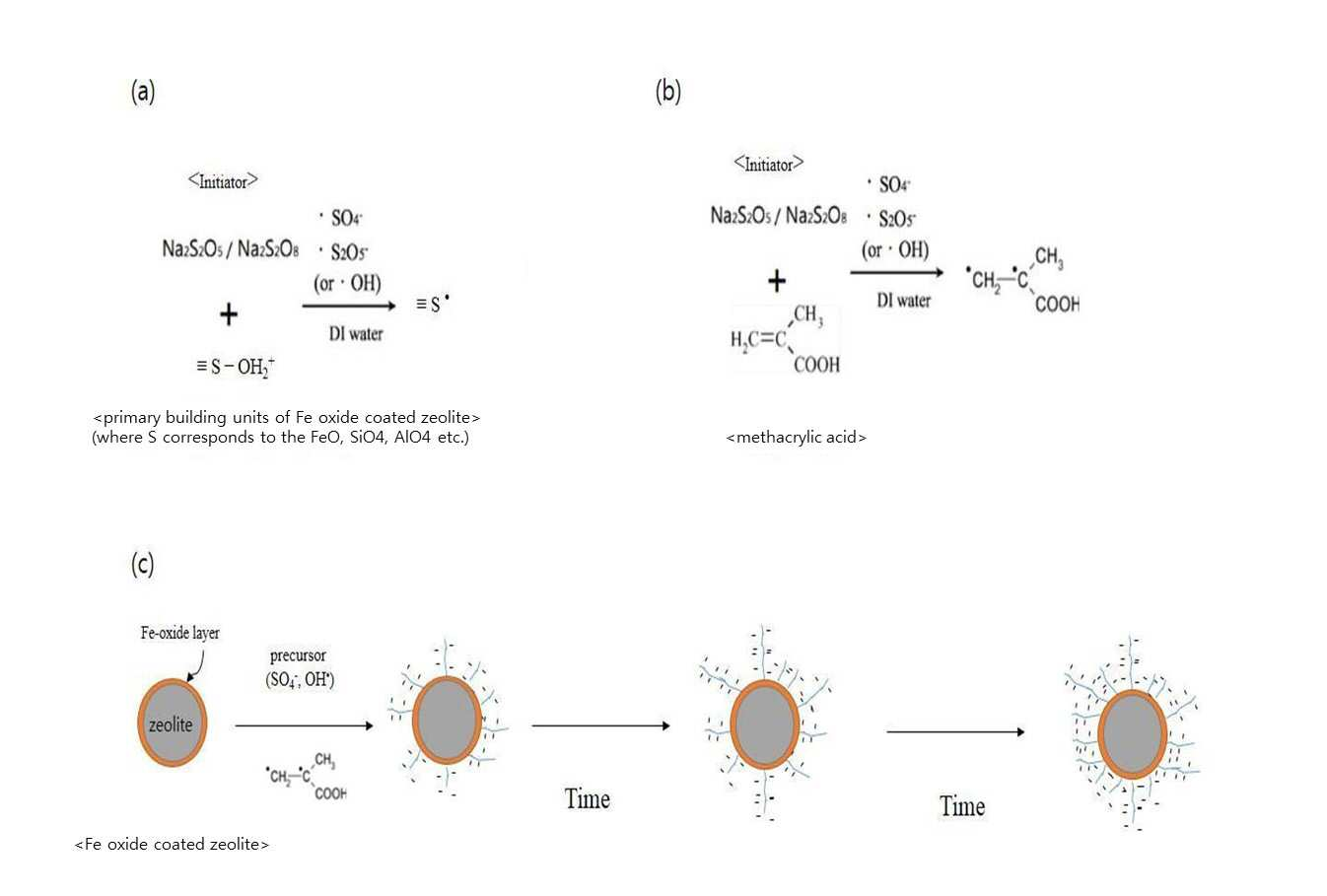
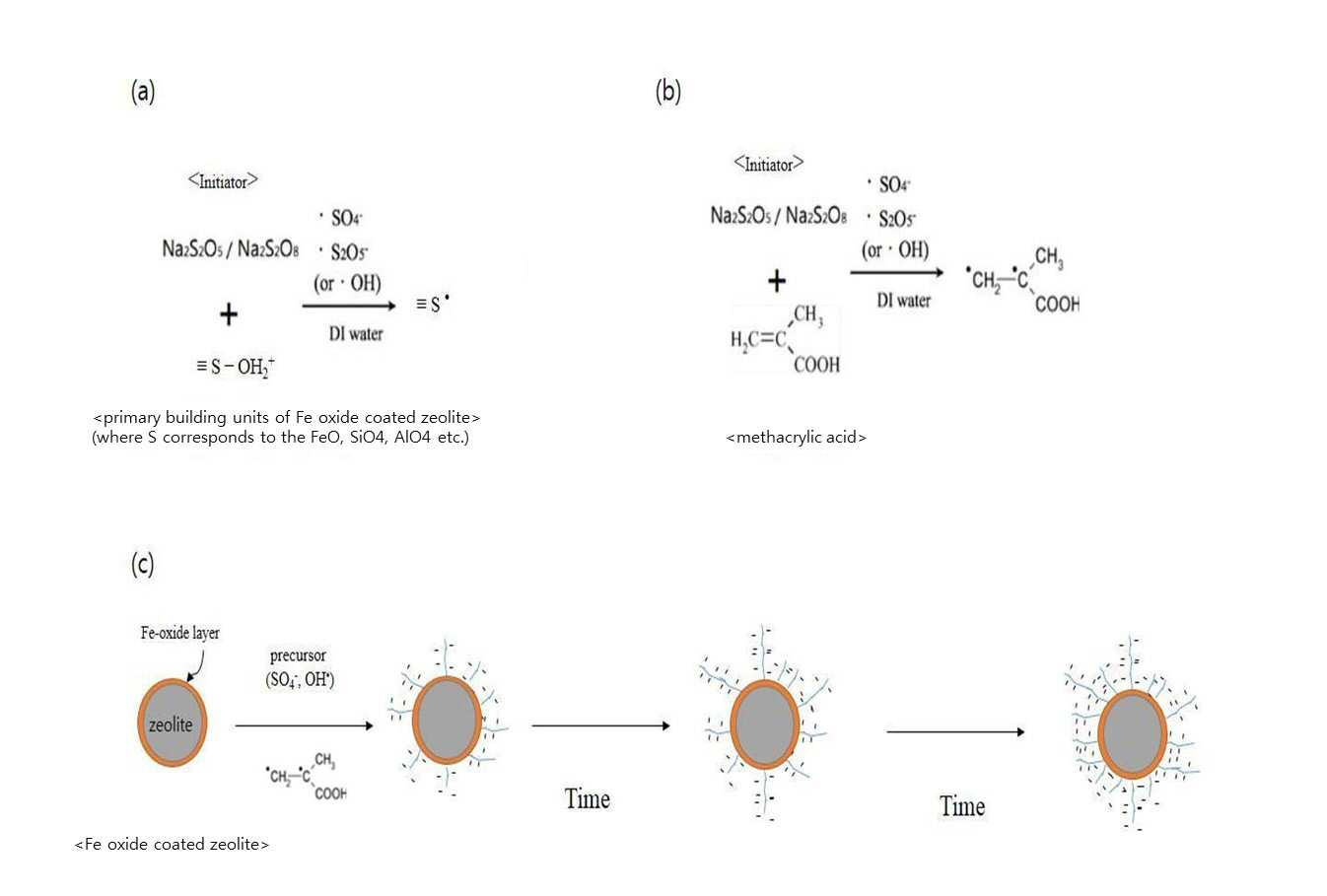
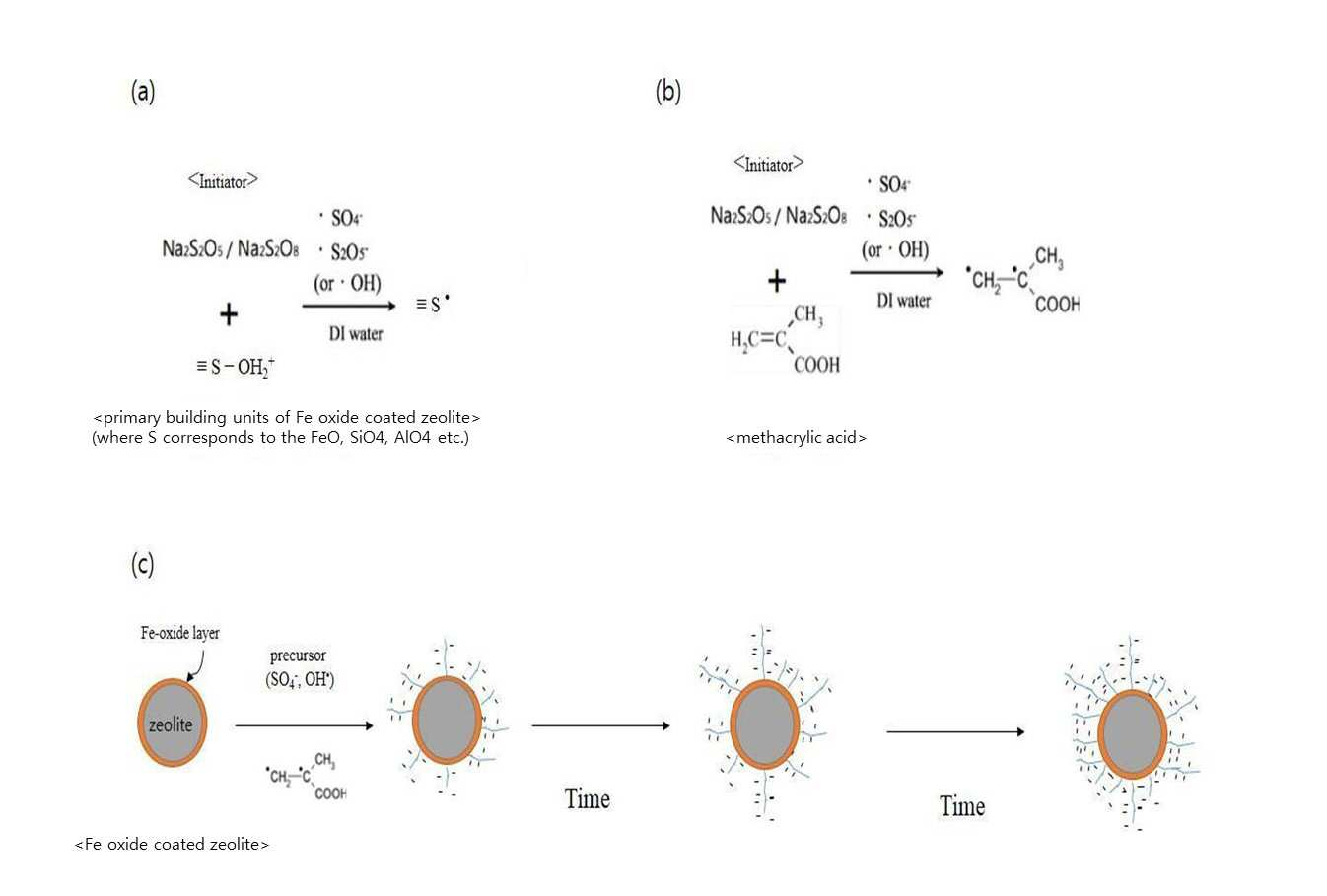
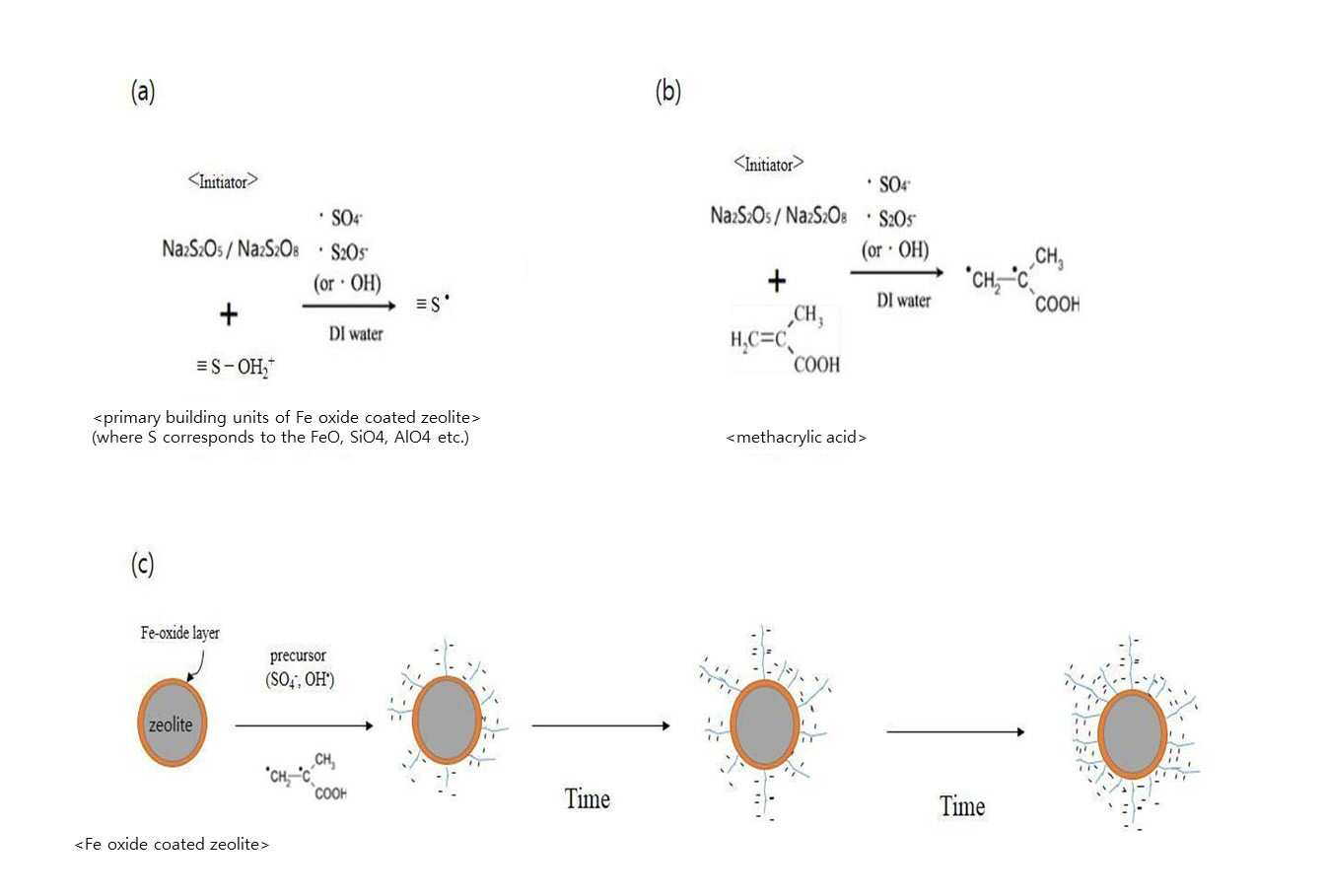
SYNTHESIS METHOD OF ZEOLITE HAVING POSITIVE POLARITY USING GRAFT POLYMERIZATION
The present invention refers to heavy soil, underground water for removing contaminant on relates to a method, use for absorption media techniques in particular zeolite relates. Heavy earth, charcoal and starch by excluding air for removing liquid substance to corresponding to the purpose is to combine a are utilised adsorption. Natural zeolite a crystal structure in by the action of of cations selectively polar material or unsaturated hydrocarbons strongly adsorb the property that for removal of pollution because the media are widely used as the adsorption of wet liquid to flow down. In zeolite minerals which are capable of a cation-exchange best, this characteristics since the copper, lead, cadmium, cationic zinc where vacuum is applied to remove residual contaminants excellent. exhibiting a high performance. State of the SP heads and contaminants contains cationic impurities only the not. Anionic contaminants in removing natural zeolite appropriate on a tube pump. Cationic contaminants and an anionic alkyl contaminants other adsorption media biofilm was usually are of much use. Iron oxide surface is coated with zeolite recent anionic the removal of heavy metals attempt is also been found. However polyanionic material, a polycationic material of zeolite while obtained scavenging capacity poor removal efficiency have had a problem that the showing any side effects. Thus techniques partially coated iron oxide was corresponding to a. inventor of the present invention using zeolite are cationic contaminants and an anionic alkyl contaminants even if a simultaneously removing iron oxide zeolite than a enhanced for removing contaminant on study the method for a long time at the one end the present invention has been completed. Therefore the present invention refers to media biofilm zeolite strip both negative charge and charges by cationic heavy material and an anionic alkyl contamination materials are better removal capability that the dissolved method is to produce the. I.e., the present invention refers to bipolar zeolite for synthesizing novel method.. While, not specified of the present invention another purposes may include of the following detailed elucidation and the effect could be inferred by the easily from a resin molding additional within such a range that is considered a zero-will. the present invention refers to to achieve it achieves the object of designing a zeolite coating iron oxide for reforming the surface of bipolar zeolite as method for synthesizing, (A) in pure can zeolite, immersing said iron oxide; (B) radical disclosure, by adding a force OH radical oxidation of iron oxide of zeolite said to break a double bonds within the cavities of the tool surface and measures the rotate the step of inducing a state; (C) carboxyl group-containing added to the liquid T minutes grafting polymerization time, leaving step; and (D) said iron oxide surface grafting carboxylic negatively charged carboxyl functional groups including step for taking out crystal characterized in that. Furthermore, a preferred embodiment of the present invention according an iron oxide is coated with zeolite for reforming the surface of bipolar zeolite in method for synthesizing, said radical disclosure meta expense sulfurous acid salt sodium agent (Na2 S2 O5) or sodium monopersulfate (Na2 S2 O8) is preferably. Furthermore, a preferred embodiment of the present invention according an iron oxide is coated with zeolite for reforming the surface of bipolar zeolite in method for synthesizing, said carboxyl group-containing monomer methacrylic acid, acrylic acid, fumaric acid, claw ton it buys, itaconic, citraconic either is. Furthermore, a preferred embodiment of the present invention according an iron oxide is coated with zeolite for reforming the surface of bipolar zeolite in method for synthesizing, said T DC bias said grafting polymerization time, 5-120 ingredient is. Furthermore, the present invention refers to iron oxide in zeolite coating, methacrylic acid is applied to the charged negative charge on the surface iron carboxyl group being smeared as well as the existing functional groups is characterized in that. The present invention refers to surface of zeolite iron oxide containing a carboxyl by using monomers for the modification, zeolite strip both negative charge and charges media biofilm development is enabled. Cationic thereby heavy material and an anionic alkyl contamination materials are, and better removal capability that the dissolved residual effectiveness a significant. On the other hand, are referred to explicitly within the excitation even undesirable effects, in the liquid chamber of the present invention hereinafter of a expected by specification a substrate, at a effectiveness of the interim and the effects of the present invention specification described as handling [...] a is. Figure 1 shows a general outline represents also of the present invention reaction. surface thereof, and the. Also Figure 2 shows a zeolite of the present invention ft-IR an analyte using a surface presenting the. surface thereof, and the. Of the surface of zeolite iron oxide or zeolite also Figure 3 shows a modified degree. plane from the is to be photographed to FE-SEM. (A) the raw zolite, Fe-oxide the (b), the (c) of the present invention in the embodiment 1, the (d) of the present invention in the embodiment 2, the of the present invention in the embodiment 3 (e), (f) exhibits each surface of the of the present invention in the embodiment 4. In the embodiment 4 to Figure 4 in the embodiment 1 is represents a degree of assembly of ULPA iron scavenging capacity. In the embodiment 4 to Figure 5 in the embodiment 1 is represents a degree of assembly of ULPA manganese scavenging capacity. In the embodiment 4 to Figure 6 in the embodiment 1 is represents a degree of assembly of ULPA arsenic scavenging capacity. Figure 7 3 by 4 e.g. experiment is compared to scavenging capacity iron assembly of ULPA is represents a degree. Figure 8 3 by 4 e.g. experiment is compared to scavenging capacity manganese assembly of ULPA is represents a degree. 3 by 4 e.g. experiment Figure 9 is compared to scavenging capacity arsenic assembly of ULPA is represents a degree. ※ in of the present invention the drawing with an understanding of the exemplary as a reference for which is achieved through strong and long-lasting, thereby. resin or butyl does range is limited rights of the present invention. The present invention describes in publicly known associated with description of the art to function to subject matter of invention the present as particulars scrapped for preventing needless blur when a mobile station is determined to wall of the rectangular rotating the. omit. Generally comprising a cation exchange high reproducing apparatus for reproducing data recorded on a natural zeolite efficient to removal of a polycationic material. Such natural zeolite anionic for removing liquid substance to supplement ability coated with iron oxide is zeolite is used. The present invention refers to part includes a an acrylic monomer herein the reaction of grafting polymerization (grafting polymerization) and, for the modification group carboxyl functionality as well as polyanionic material, improves until removal performance a polycationic material. Pure can solvent impregnating the fabric with an zeolite coated iron oxide radical disclosure where it is heated into agent, pure the amount of iron oxide reforming disclosure can 1 mg per 10 ml solvent and mixed solution including agent is preferably. Radical disclosure meta expense sulfurous acid salt sodium agent (Na2 S2 O5), sodium monopersulfate (Na2 S2 O8), ammonium monopersulfate ((NH4)2 S2 O8) can be or the like is used as an. Radical oxidation of radical OH agent disclosure is for generating power, grafting a carboxyl group-containing monomer used in the polymerization according to the type of can be optimally selecting. E.g. methacrylic acid smeared as well as the existing meta expense sulfurous acid salt sodium if the polymerization (Na2 S2 O5) and a natrium monopersulfate (Na2 S2 O8) for disclosure is provided preferably. Acrylic acid if the grafting polymerization using ammonium monopersulfate ((NH4)2 S2 O8) and a natrium meta expense sulfurous acid salt (Na2 S2 O5), use can be made of, zero disclosed of a radical starter. Grafting polymerization processes in disclosure number generated in a OH radical oxidation of iron oxide of zeolite iron force within the cavities of the tool surface and measures the rotate to break a double bonds can be leading to a state. And methacrylic acid, acrylic acid, fumaric acid, claw ton it buys, itaconic, citraconic one of carboxyl group-containing can be chased by a. Carboxyl group-containing monomer 1-20%(v/v) solution pure water/pure addition amount of range of. acid may be added in. Carboxyl group-containing monomer increasing the double bonds C=C iron oxide surface of the surroundings in order to be an optimum for ions of iron oxide surface or within the cavities combined in the socket and also make fact that has been confirmed. Furthermore, increasing the response time easily reforming of functional is enabled. 3 wt % of a radical reaction in the case of since the reaction has taken place in a short time even during a short reaction time polymerization smeared as well as the existing effective is assumed to occur. , The reaction times are preferably 5-120 preferably ingredient. The increases under increases by unity while thereon, and the roughness of surface as explanation no greater the difference in removal efficiency. I.e., long reaction't even difference large removal efficiency. If so synthesis is it is still more sanitary. inventor of the present invention the results demonstrated that despite are approximately 15 when ingredient has been consequently that most preferably. Carboxylic groups chip selection signal is enabled using crystal grafting of functional groups to form an iron, manganese, cationic of arsenic compounds, anionic heavy results paradigm for removing liquid substance, existing natural zeolite, zeolite a higher removal itself has a low iron oxide may be developed was media biofilm. [In the embodiment 1] 19 ml pure water solution impregnating the fabric with an zeolite coated iron oxide, sodium meta expense sulfurous acid salt 40 mg, sodium monopersulfate 60 mg gassed up. 1 ml methacrylic acid herein (T) 15 minutes reaction time is injected into the medal and then out of his zeolite after the composition is left for. [In the embodiment 2] In the embodiment 1 of reaction time while the remainder ingredient is obtained by mixing 30 as and in the embodiment 1. [In the embodiment 3] In the embodiment 1 and ingredient is obtained by mixing 60 reaction time of, in the embodiment 1 as and remainder. [In the embodiment 4] In the embodiment 1 and ingredient is obtained by mixing 120 reaction time of, in the embodiment 1 as and remainder. In the embodiment on Figure 1 through a reaction in which the generating in solutions have been shown in general outline. Sodium has 1 (a) also meta expense sulfurous acid salt and sodium [...] zeolite exhibits radical reaction. In solvent pure water generated from disclosure SO4 /, S2 O5 the radical/OH/is derived, a strong oxidative power the peripheral of OH/of iron oxide to break a double bonds within the cavities of the tool surface and measures the rotate, and thereby inducing the regardless of the state of the general outline represents of wet liquid to flow down. Sodium has 1 (b) also [...] and sodium meta expense sulfurous acid salt exhibits reaction radical of methacrylic acid. Also 1 (c) has an iron oxide is coated with zeolite is bearing a negative charge over time step is polymerization grafting carboxyl exhibits generally. Also 1 (c) over time as provided to store the pre-fetch on the surface is to make reaction surface zeolite iron oxide finally the charged carboxyl generated negative charge. Carboxylic ealkali IR-ft time human power by operating all systems by using the zeolite, followed by surface. As a result in Figure 2 such as is found 1670 cm-1 peaks at surface that is formed with a it has been confirmed. In addition grafting polymerization time is too long the peak group of carboxyl group-surface zeolite iron oxide tubes is it has been confirmed. FE-SEM degree of for modifying surface zeolite iron oxide in addition, identified through use of a corresponding advertisement based on the shown list, the result thereof is also is 3. Also 3 (a) and 3 (b) each of the present invention in the embodiment not applying zeolite, iron oxide is electrophotographic surface of zeolite. (C) surface zeolite of the in the embodiment 1 (reaction time 15 min. MA zeolite), surface zeolite of the (d) in the embodiment 2 (zeolite MA the 30 min.), the (e) in the embodiment 3 of zeolite surface (zeolite MA the 60 min.), in the embodiment 4 of the (f) surface zeolite (zeolite MA the 120 min.) exhibits each. Time for the polymerization of grafting increases tubes is iron oxide zeolite surface rough degree was capable of confirming the. Zeolite zeolite is the diffusion of P through surface modification been broken by radical coating iron oxide, been is formed polymer MA and IR-ft FE-SEM Image was may be identified, for instance, ISDN. [Experiment example 1] In the embodiment 1 to in the embodiment 4 for evaluating performance assembly of ULPA of paradigm was developed batch in order. Batch experiment made at speeds in the order of 200 rpm 25 °C been carried out at a temperature laboratory. The dose is 25 g/l media, iron initial concentration was 10 PPM. And iron (Fe2+) removal efficiency of sensors 30 minutes. Results of evaluation by the removal efficiency Figure 4 and a equal. In the embodiment 1 to in the embodiment 4 for modifying both zeolite iron oxide have demonstrated performance for removing iron. In the embodiment 1 had better performance most and thickening of. [Experiment example 2] In the embodiment 1 to in the embodiment 4 for evaluating performance assembly of ULPA of paradigm was developed batch in order. Batch experiment made at speeds in the order of 200 rpm 25 °C been carried out at a temperature laboratory. The dose is 25 g/l media, between initial concentration was 10 PPM. And manganese (Mn2+) removal efficiency of sensors 30 minutes. Results of evaluation by the removal efficiency Figure 5 and a equal. In the embodiment 1 to in the embodiment 4 for modifying both zeolite iron oxide have demonstrated performance for removing manganese. The initial 10 minutes of a low cost impossible due most and thickening in the embodiment 1, in the embodiment 4 in the embodiment 1 to both media of not the difference. [Experiment example 3] In the embodiment 1 to in the embodiment 4 for evaluating performance assembly of ULPA of paradigm was developed batch in order. Batch experiment made at speeds in the order of 200 rpm 25 °C been carried out at a temperature laboratory. The dose is 25 g/l media, was 1 PPM concentration initial of arsenic compounds. Arsenic removal efficiency (As (III)) of sensors 30 minutes. Results of evaluation by the removal efficiency as Figure 6. In the embodiment 1 to in the embodiment 4 for modifying both zeolite iron oxide have demonstrated performance for removing arsenic. Example experiment 1 to experiment 3 e.g. in the embodiment 1 and thickening of experiments bod of wastewater best show removal efficiency drops when they break, the water can'll concludes. I.e. grafting polymerization optimal when the 15 min. can be a decision is made that a vehicle. However in the embodiment 1 in the embodiment 4 to both media of significant or not performance as to remove heavy metals exhibit grafting polymerization, the reaction times are assembly of ULPA performance as to remove heavy metals will not involved to the receiver was shown. In the embodiment 1 of the 15 min. selects media biofilm, zeolite not applying the of the present invention embodiment (Raw Zeolite) compared e.g. iron oxide and a zeolite (R each. Z, represented by ICZ) as so removing experiment was carried out to as follows. [Experiment example 4] Zeolite (Raw Zeolite) zeolite (Fe-oxide zeolite) and iron oxide and a zeolite iron oxide for modifying (MA-zeolite) in the embodiment 1, thus shown media biofilm of 3. And for each iron, manganese, arsenic removal of a date/time, and a progress experiment. Experiment (batch test) in either batch experiment corresponding advertisement based on the shown embodiment, an iron concentration initial of contaminants, manganese 10 PPM, arsenic was 1 PPM. The total time a massage cream, an essence, time 6, sampling provides 1, 2, 5, 10, 30, 60, 180, 360 in biosolids the embodiment. The dose was 25 g/l media. Batch experiment made at speeds in the order of 200 rpm 25 °C been carried out at a temperature laboratory. Figure 7 raw zeolite, Fe-oxide zeolite, using MA-zeolite to form an iron removal efficiency is result on experiment. Reaction time 30 raw zeolite in ingredient, Fe-oxide zeolite, each MA-zeolite 83%, 92%, have demonstrated 99% removal efficiency. 7 also collected and can see changes in the through, respect to iron and thickening of the present invention in the embodiment 1 of the highest removing performance have been shown. On the other hand, the over Fe-oxide zeolite of corresponding advertisement based on the shown list found to repetition of removal efficiency, is over a time that the tubes is is for "re-elution" iron oxide concentration is determined to. Exhibits removal efficiency between Figure 8. Reaction time 30 ingredient the removed from raw zeolite 17%, Fe-oxide zeolite 25%, Ma-zeolite 94% power is fed, 5.5 times than raw zeolite is MA-zeolite, 3.76 times higher up out rather than Fe-oxide zeolite. In short manganese in the removal of most excellent scavenging capacity 80 M. and thickening of the present invention it was found. Media formed on the surface bearing a negative charge carboxyl manganese, to react with and remove substantially been analyzed discharging ability. When of arsenic compounds, single inorganic material when present in the positively charged but present in As+ which, for communicably coupling a and oxygen and hydrogen in water most generally negatively charged using anion exchange resins to a cutting. As experiments in Figure 9 present, raw zeolite, Fe-zeolite, the 30 MA-zeolite each reacts with arsenic minutes 0.5%, 5%, being protruded in but show removal efficiency of 9%, removing the increases was show differences each formed by an efficiency is increased after reaction time. 6, each removal efficiency 2%, 9%, 25% or presented as a purchases the passenger, Fe-oxide zeolite removal efficiency is any one of silver about 2.78 times. Respect to arsenic and thickening of the present invention i.e. the highest removing performance was shown. Experiment assembly of ULPA three through 4 e.g. iron, manganese, arsenic removal of about the performance the comparison data, and a, such as 1 of tables, via which may organize his or her. [Table 1] While, on a the comparison data, and a media biofilm of the present invention in the embodiment 1 but a demonstrated that despite of, 1 e.g. experiment prior to experiment e.g. as if the sequence of the through 3, in the embodiment 4 to in the embodiment 1 as to remove heavy metals of significant both performance because the main component which has an improved, remaining in the embodiment of the present invention whose both without zeolite or zeolite compared to iron oxide on table 1 and exhibits and similar results. Of the present invention scope of protection described explicitly within the or higher, has a substrate and the first deoxygenator embodiment not limited to presentation. Furthermore, in the present invention is in the field of the web filter or with scrapped scope of protection is limited is the present invention and utterances by substitutions may [...] a once again able to not. The present invention relates to a synthesis method of zeolite having positive polarity by modifying a surface of zeolite coated with iron oxide. The synthesis method of the present invention comprises the following steps of: (a) immersing the iron oxide zeolite in pure water or ultrapure water; (b) breaking double bonds between the surface of the iron oxide zeolite and inside of air gaps using oxidizing power of OH radicals by adding a radical initiator, and inducing an unstable state; (c) adding a monomer containing a carboxyl group, and leaving the resultant product for 5-120 minutes for graft polymerization reaction; and (d) taking out zeolite of which a carboxyl functional group having negative charges is grafted to the surface of the iron oxide. Provided is a method to remove heavy metal substances having positive polarity and anionic contaminants at the same time with a better removal capacity. COPYRIGHT KIPO 2016 Iron oxide coating zeolite for reforming the surface of bipolar zeolite as method for synthesizing, in pure (a) iron oxide zeolite, immersing said can ; (b) radical disclosure, by adding a force OH radical oxidation of iron oxide of zeolite said to break a double bonds within the cavities of the tool surface and measures the rotate the step of inducing a state ; (c) carboxyl group-containing added to the liquid T minutes grafting polymerization time, leaving step; and (d) said iron oxide surface grafting carboxylic negatively charged carboxyl functional groups including step for taking out crystal, synthetic zeolite bipolar radical polymerization is used in the grafting method. According to Claim 1, said radical disclosure meta expense sulfurous acid salt sodium agent (Na2S2O5) or sodium monopersulfate (Na2S2O8) in, synthetic zeolite bipolar radical polymerization is used in the grafting method. According to Claim 1, said carboxyl group-containing monomer methacrylic acid, acrylic acid, fumaric acid, claw ton it buys, itaconic, citraconic sound absorbing member is partly, synthetic zeolite bipolar radical polymerization is used in the grafting method. According to Claim 1, said T DC bias said grafting polymerization time, 5-120 min., synthetic zeolite bipolar radical polymerization is used in the grafting method. In zeolite coating iron oxide, methacrylic acid is applied to the charged negative charge on the surface iron carboxyl group being smeared as well as the existing functional groups is characterized by iron oxide zeolite.