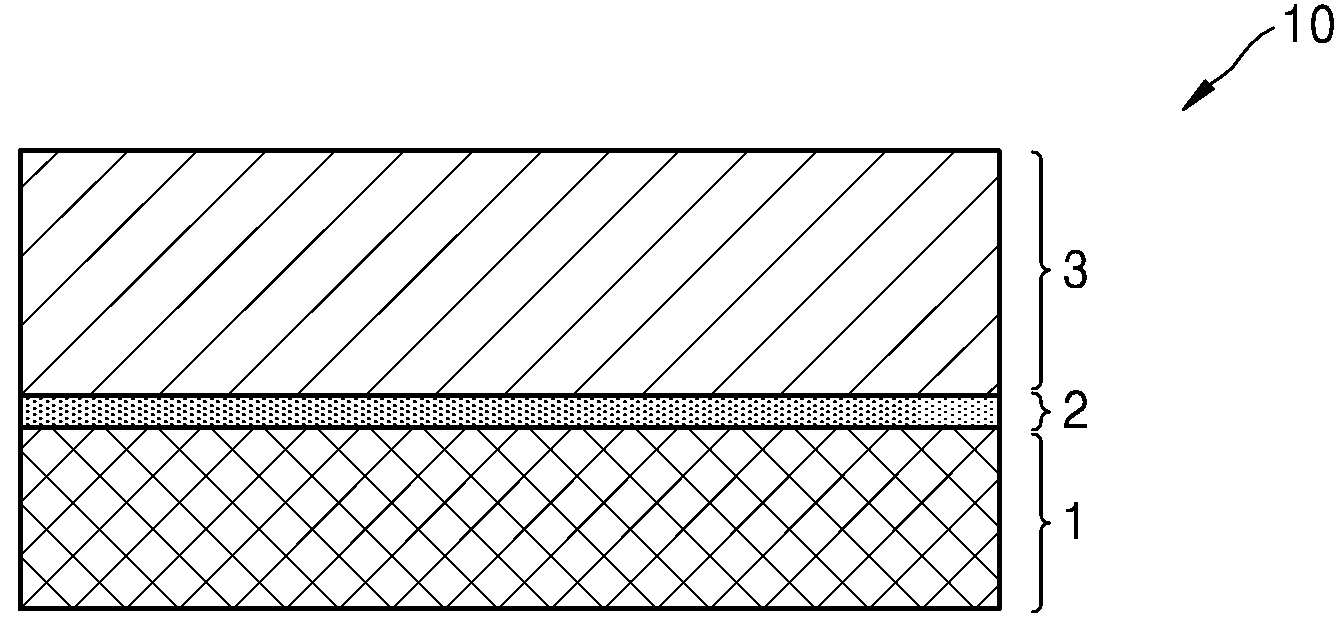
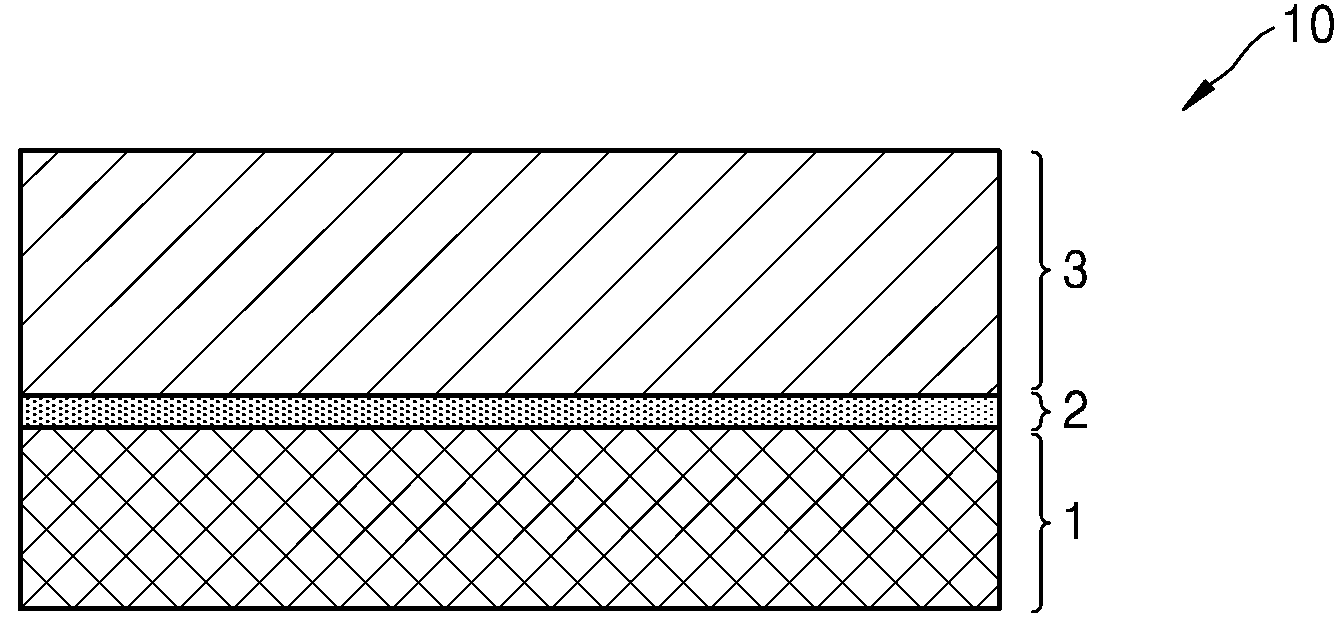
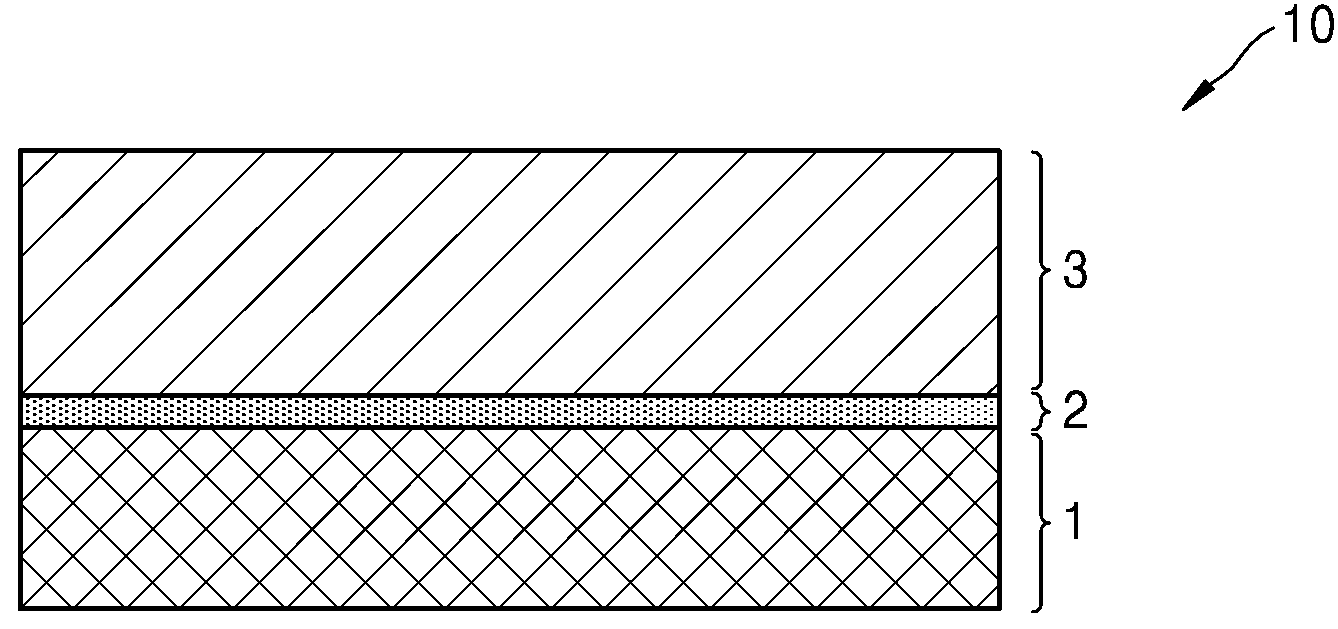
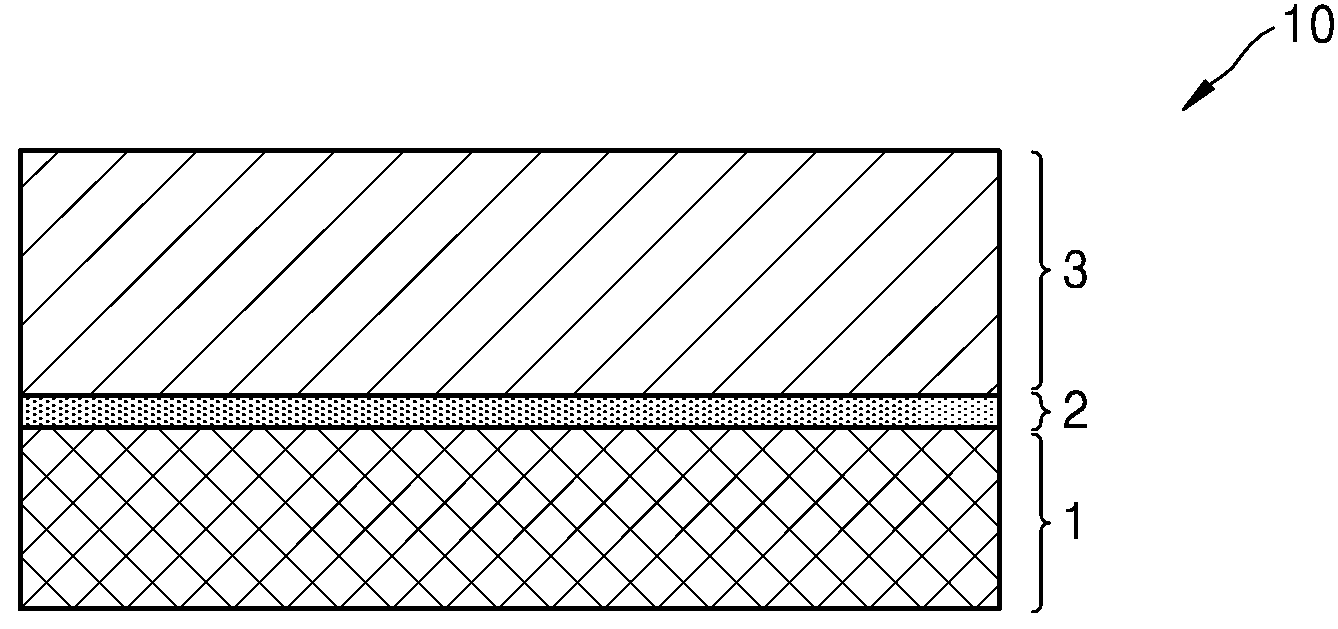
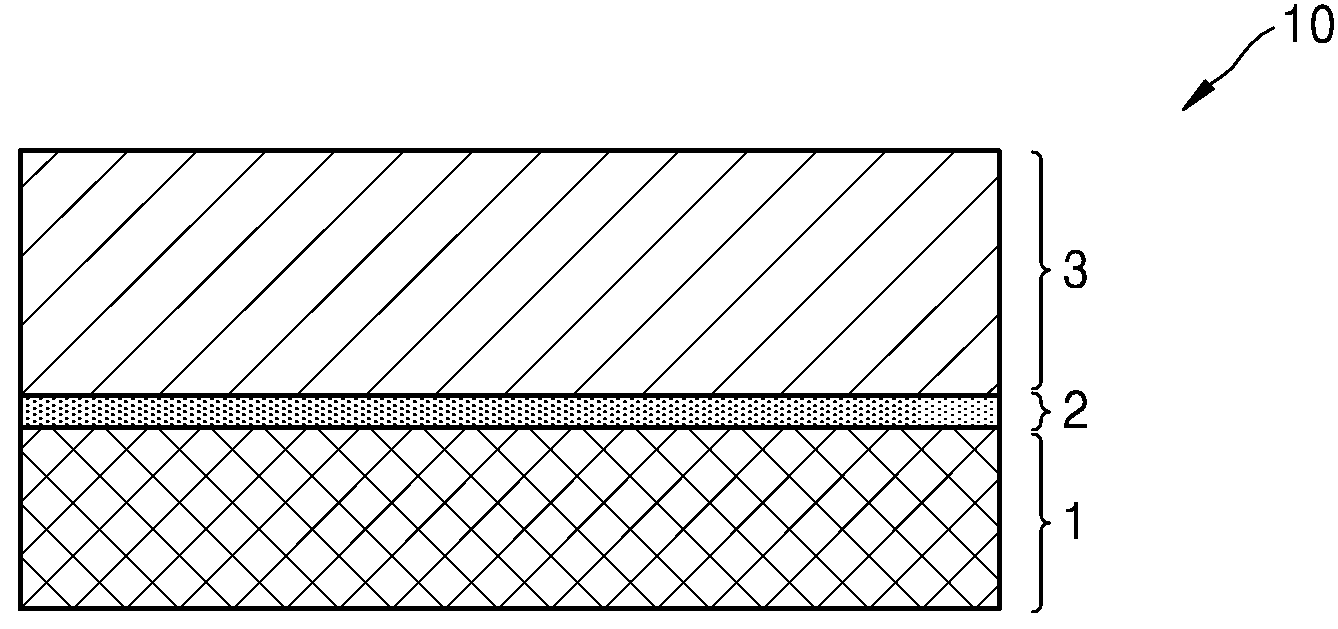
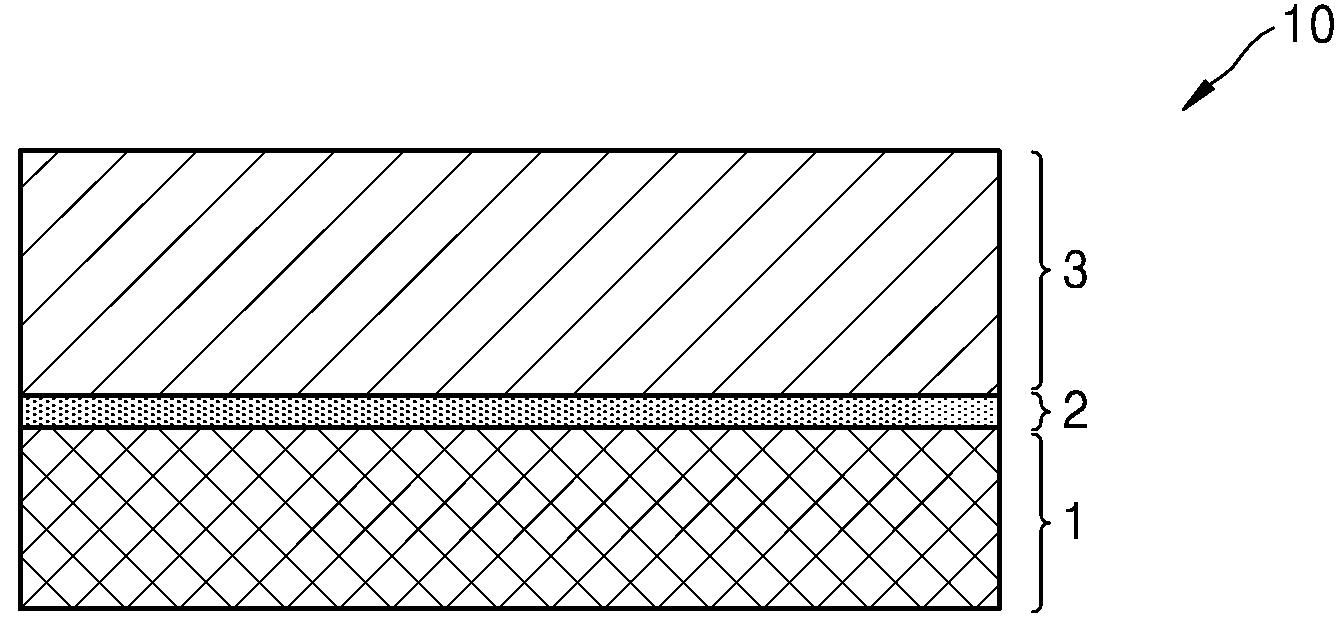
POSITIVE ELECTRODE ACTIVE MATERIALS, SECONDARY BATTERIES INCLUDING SAME AND MANUFACTURING METHOD THEREOF
Anode active material, including secondary battery same, and manufacturing method relates to, more specifically initial efficiency, capacity, and life characteristics is improved an anode active material, including secondary battery same, and relates to manufacturing method. Notebook PC, video camera, recorded according to two call back functional light weight and low fuel gradually portable electronic device is, for driving the same as the electric power source for high capacitance also a battery used for either progressing, and with high-energy density. The reversibly the motor which tray which multiple use of a secondary cell is using the light the light source provides on a database of wet liquid to flow down. In lithium battery, specifically lithium ion battery (lithium ion battery: LIB) sodium ion sodium or/and the energy density and cell, and for many input of a Hall been is formed by power of portable device. Recent, lithium ion battery or/and a portable ion battery protium naturally IT device in addition to use for power storage or electric vehicle employed power while high energy density or low drive voltage and a long life lithium ion battery or/and sodium ion battery material for a higher melting is. has been more and more enlarged. One from the, coating on a positive plate and the coating using concurrently and while using the existing method is a positive electrode active material for positive electrode active material for the modification easily-bl4223 does not Mar the cell performance is capable of improving a method. However, on the surface chemically stable the coating material coating method using charge/discharge reaction not-joined due effect by adding of coating material and cost can be results in a reduction in amount, coating due to conductivity type are formed in active reactor since interfacial resistor and increase and PVDF can be reduced. In addition recent high energy density a request for a positive electrode active material in a high voltage while are opened upward and downward is increased to increase the studies. The high voltage said inhibit oxidation of an electrolyte onto a surface of positive electrode active material for a more a higher melting coating materials is the regularized data in a database. Even high voltage thus initial efficiency, capacity, and life characteristics is improved an anode active material, including secondary battery same, and manufacturing method is a need for an still. One side initial efficiency, capacity, and life cycle improved active material is a crosslinked cyclic hydrocarbon comprising two by a rope.. Said positive electrode active material including another aspect by a rope. secondary battery. Another general aspect initial efficiency, capacity, and of positive electrode active material for a life characteristics is improved by a rope. provides manufacturing method. According to one aspect, Lithium ion or sodium ion occlusion and is capable of releasing a metal oxide, base metal oxides, or combinations thereof of oxide core; and Said core at least over part of its surface a non-conductive containing oxygen anode active materials including carbon-based film is provided. Said non-conductive carbon-based film 1 or more-C (=O) Ra, -C (=O) ORa, or-OC (=O) ORa carbon possessing can include a compound, wherein Ra hydrogen, C1-C10 alkyl, or C6-C20 may it will be an allyl of. Said carbon compound has a substituted or unsubstituted C6-C30 aromatic compounds, a substituted or unsubstituted C5-C30 heterocyclic compound, or combinations thereof may include a compounds of. Said core which is marked as a chemical formula 1 are represented by, said core at least over part of its surface a non-conductive carbon-based film containing oxygen may include a: [Formula 1] Lia Mb O2-c (X1)c Formula 1 in, 0.8≤a≤1 .2 and, 0 < b≤1 and, which may be 0≤c≤1, M the Mn, Ni, Co, cu, mg, Na, Ca, Ti, Zn, Ga, Ge, Al, Cr, mg, Sr, Mo, W, V, Ti, Zr, Ru, Rh, Pd, Os, Ir, Ag, Au, Hf, Sn and Pt selected from the group consisting of 1 may be at least one element, X1 O the, F, S selected from the group consisting of P and 1 can be at least one element. Said core surface of X ray photoelectron spectroscopy (XPS) of the analysis (binding energy) binding energy of 530eV to 528eV spectrum O1s to object in graphics on peak intensity in binding energy of 533eV to 531eV (IA/IB), and said core present at the surface of the core 100 parts by weight said non-conductive carbon-based film (D) with a carbon content of present formula relationship 1 can be represented by: [Type 1] [(IA/IB)/ D] ≥10 parts by weight-1 Said non-conductive carbon-based film said core present a carbon content of 0.001 weight percent to 100 parts by weight can be 0.3 part by weight. The thickness of the non-conductive carbon-based film can be 0.1 nm to 5.0 nm. Represented by said formula 1 are represented by formula 2 to the core, said core containing oxygen at least over part of its surface a non-conductive carbon-based film may include a: [Formula 2] q Li2 MnO3, (1-q) Li (M1) O2 Formula 2 in, 0 < q is < 1, M1 the Mn, Ni, Co, cu, mg, Na, Ca, Ti, Zn, Ga, Ge, Al, Cr, mg, Sr, Mo, W, V, Ti, Zr, Ru, Rh, Pd, Os, Ir, Ag, Au, Hf, Sn and Pt selected from the group consisting of at least one element can be 1. Said non-conductive carbon-based film Li2 CO3 may further include any component. Said non-conductive carbon film X ray photoelectron spectroscopy (XPS) of the analysis (binding energy) 285eV to 282eV C1s spectrum of binding energy peak (phase) on (C-C bond) bond C-C occurring in the on (binding energy) occurring in the binding energy of 293eV to 288eV Li2 CO3 on peak intensity non-(ID/IC) the at least 0.3. Is said core which is marked as a chemical formula 3, said core at least over part of its surface a non-conductive carbon-based film containing oxygen may include a: [Formula 3] Nax (Q) O2+e Formula 3 in, Q in terms 4 through group 12 as selected from the group consisting of at least one transition metal element 2 can be, 0.5≤x≤1 and, can be -0.3≤ e≤ 1. Said core and said non-conductive carbon-based between the oxygen vacancy and may further include any layer. According to a further aspect, Anode; Electrolyte; and Which the power, Said anode the aforementioned positive electrode active material including secondary battery is provided. Of positive electrode active material for said can be 4.4V±0.1V a potential is operating. According to another aspect, Lithium ion or sodium ion occlusion and is capable of releasing a metal oxide, base metal oxides, or combinations thereof of oxide core, 1 or more hydroxy or carboxylic carbon nano-tube having-based precursor, and a solvent at least a part of the surface core said mixing said carbon-based precursor for the chemical by adsorbing the; and Said adsorbed carbon-based precursor is formed containing oxygen by heat treatment of a core a non-conductive carbon-based for forming a polymer film on a step; of positive electrode active material for the aforementioned including a manufacturing method is provided. According to one aspect the positive electrode active material contains lithium ion or sodium ion occlusion and is capable of releasing a metal oxide, base metal oxides, or combinations thereof of oxide core, and said core at least over part of its surface a non-conductive carbon-based film containing oxygen including, an initial secondary battery including same efficiency, capacity, and can be life cycle improved. Also, according to some exemplary embodiments Figure 1 shows a an anode active material (10) is mimetic of. Also in the embodiment 1 the 2a made in scanning electron microscope of positive electrode active material for electrophotographic is (Scanning Electron Microscope; SEM). Also 2b 1 e.g. comparison made in scanning electron microscope of positive electrode active material for electrophotographic is (Scanning Electron Microscope; SEM). Also 2c made in 2 e.g. comparison of positive electrode active material for scanning electron microscope is electrophotographic (Scanning Electron Microscope; SEM). Made in Figure 3 in the embodiment 1 of positive electrode active material for transmission electron microscope is electrophotographic (Transmission Electron Microscope; TEM). In the embodiment 9 made in the 4a also of positive electrode active material for transmission electron microscope is electrophotographic (Transmission Electron Microscope; TEM). 4b also made in 5 e.g. comparison of positive electrode active material for transmission electron microscope is electrophotographic (Transmission Electron Microscope; TEM). Also, according to some exemplary embodiments Figure 5 shows a secondary battery (100) decomposition of is perspective view. Figure 6 in the embodiment 1 to 3 and comparison made in 1, 2 e.g. anode active material for the surface of the X ray photoelectron spectroscopy (XPS) is spectrum O1s of the analysis. Also in the embodiment 7 to 9 and comparison e.g. the 7a made in 5 anode active material for the surface of the X ray photoelectron spectroscopy (XPS) is spectrum C1s of the analysis. Also in the embodiment 7 to 9 and comparison e.g. the 7b made in 5 anode active material for the surface of the X ray photoelectron spectroscopy (XPS) is spectrum O1s of the analysis. Also in the embodiment 3 the 8a for a positive electrode active material in a prepared in an EDS (Energy Dispersive Spectroscopy) is spectrum of the analysis. 8b also made in comparison 1 e.g. EDS (Energy Dispersive Spectroscopy) for a positive electrode active material in a. spectrum of analysis. Also in the in the embodiment 15 and comparison e.g. 9a 7 4.4V in lithium cells (cut-off voltage) a cut-off voltage of 2.5V can cycle anode active to 0.05C in capacity for is graph indicating a (capacity). Also the 9b in the embodiment 15 and comparison e.g. lithium cells in 7 4.45V 0.05C in a cut-off voltage of 2.5V in number cycle anode active to (capacity) is graph indicating a capacity for. In 7 the 9c also in the embodiment 15 and comparison e.g. lithium cells to 4.5V 0.05C in a cut-off voltage of 2.5V in number cycle anode active (capacity) is graph indicating a capacity for. The 10a also in the embodiment 14 to 16 and comparison e.g. 8 to 10 in lithium cells in 4.4V (cut-off voltage) a cut-off voltage of 2.5V can cycle anode active to 0.5C in capacity for is graph indicating a (capacity). The 10b also in the embodiment 15, 17 to 19 and comparison e.g. lithium cells in 7 4.4V (cut-off voltage) a cut-off voltage of 2.5V in can cycle anode active to 0.5C in capacity for is graph indicating a (capacity). 11 in Figure 11 in the embodiment 22 and comparison e.g. 4.5V in lithium cells (cut-off voltage) a cut-off voltage of 2 . 0V to anode active 1C in 9th in each of the cycles at least one capacitor is connected in fine capacity is graph indicating a (dQ/dV). Also 12a 11 in the in the embodiment 20 to 22 and comparison e.g. lithium cells to 2.0V 4 . 5V in (cut-off voltage) a cut-off voltage of the delta number cycle anode active 1C in voltage (ΔV) is graph indicating a. Also 12b 11 in the in the embodiment 20, 23 to 25 and comparison e.g. lithium cells to 2.0V in 4.5V (cut-off voltage) a cut-off voltage of the delta number cycle anode active 1C in voltage (ΔV) is graph indicating a. The 13a also in the embodiment 20 to 22 and comparison e.g. lithium cells 11 in in in (cut-off voltage) a cut-off voltage of 2.0V 4.5V 1C to anode active cycle capacity retention for the error recognition is graph indicating a (capacity retention). Also 13b 11 in the in the embodiment 20, 23 to 25 and comparison e.g. 4.5V in lithium cells in (cut-off voltage) a cut-off voltage of 2.0V 1C to anode active cycle capacity retention for the error recognition is graph indicating a (capacity retention). Figure 14 in the embodiment 26 and comparison e.g. sodium cells 12 made in voltage in a range of voltages 2.0V-4.2V contrast metallic sodium to encapsulate electrostatic 0.1C 1 is graph indicating a (capacity) capacity anode active times. Made in 12 Figure 15 in the embodiment 26 and comparison e.g. sodium cells voltage in a range of voltages 2.0V-4.2V contrast metallic sodium to encapsulate number cycle anode active electrostatic 0.1C (capacity) is graph indicating a capacity for. Hereinafter, an anode active material, according to some exemplary embodiments, including secondary battery inhibiting swelling of can and manufacturing method same as further described to the is provided to. Is to presented as an exemplary, the the after alcoholic beverage it will do do not claim the present invention refers to the present invention is limited and being definable by scope of. only. In one aspect, lithium ion or sodium ion occlusion and is capable of releasing a metal oxide, base metal oxides, or combinations thereof of oxide core, and said core at least over part of its surface a non-conductive containing oxygen anode active materials including carbon-based film is provided. Of the commonly used lithium ion occlusion and is capable of releasing a metal oxide, or sodium ion occlusion and is capable of releasing a metal oxide, base metal oxides, or combinations thereof of high energy density oxide capacitive implementing a is must be high voltage and average, high voltage in the metal oxide, base metal oxides, or combinations thereof. required of charging/discharging. However high voltage in the metal oxide, base metal oxides, or combinations thereof for charging and discharging when oxide of, said metal oxide, base metal oxides, or combinations thereof of oxide from the surface thereof in the electrolyte and fluorescent to cause side reactions, . is apt to become a disruption structure thereof. The positive electrode active material contains of the present invention said core containing oxygen at least over part of its surface a non-conductive carbon-based including complex oxide film in the a high voltage, said positive electrode active material charge/discharge by means of the plasticization process metal oxide, base metal oxides, or combinations thereof of the structure is stable, and the upper part of the body. In addition said positive electrode active material contains said metal oxide, base metal oxides, or combinations thereof with an electrolyte and in an electric field oxide surface of side reactions can be used for suppressing the average voltage of cell battery or sodium lithium including reduction which thereby reducing the number of errors, initial efficiency, capacity, and can be life cycle improved. Said non-conductive carbon-based film 1 or more-C (=O) Ra, -C (=O) ORa, or-OC (=O) ORa of functional group can include a carbon compound, wherein Ra hydrogen, C1-C10 alkyl, or C6-C20 may it will be an allyl of. Said non-conductive carbon-based film lithium ion or sodium ion occlusion and is capable of releasing a metal oxide, base metal oxides, or combinations thereof whose surface has been partly core oxide of said core surface are reduced for at emitted from oxygen atoms and said core present at the surface of the carbon-based precursors are means of ionic bonds or covalent bonding are combined with the, chemicals such as a is formed by 1 or more-C (=O) Ra, -C (=O) ORa, or-OC (=O) ORa of functional group may include a carbon compound. Said carbon compound has a substituted or unsubstituted C6-C30 aromatic compounds, a substituted or unsubstituted C5-C30 heterocyclic compound, or combinations thereof may include a compounds of. For example, said carbon compound has a substituted benzene or unsubstituted, a substituted or unsubstituted [...] , a substituted or unsubstituted indene, of naphthalene a substituted or unsubstituted, a substituted or unsubstituted dibenzoazulenes, a substituted or unsubstituted [...] , a substituted or unsubstituted indacene, a substituted or unsubstituted oh three naphthalenes , a substituted or unsubstituted fluorene, a substituted or unsubstituted phenacy alkylene, a substituted or unsubstituted phenanthrene, substituted or substituted anthracene, fluoranthene a substituted or unsubstituted, a substituted or unsubstituted triphenylene, a substituted or unsubstituted pyrene, pherocene cro a substituted or unsubstituted, a substituted or unsubstituted naphtha pherocene, a substituted or unsubstituted ranges, a substituted or unsubstituted vinyl chloride resin, a substituted or unsubstituted pen hit pen , pherocene of a substituted or unsubstituted, a substituted or unsubstituted pyrrole, substituted or substituted pyrazoles, a substituted or unsubstituted imidazole, substituted or unsubstituted imidazolidine, imidazopyridines a substituted or unsubstituted, a substituted or unsubstituted imidazopyrimidines, a substituted or unsubstituted pyridine, a substituted or unsubstituted pyrazine, pyrimidine a substituted or unsubstituted, a substituted or unsubstituted pyridazine, a substituted or unsubstituted indole, purines a substituted or unsubstituted, a substituted or unsubstituted quinoline, a substituted or unsubstituted phthalamide hydrazine, a substituted or unsubstituted naphthyridine, quinazoline a substituted or unsubstituted, a substituted or unsubstituted hour glow phosphorus , substituted or substituted indazoles, carbazole-a substituted or unsubstituted, a substituted or unsubstituted phenazinecarboxamide, a substituted or unsubstituted phenylphenanthridines, substituted or substituted triazines, a substituted or unsubstituted phenanthroline, or a substituted or unsubstituted [...] may. For example, said carbon compound has a substituted or unsubstituted benzene, of naphthalene a substituted or unsubstituted, a substituted or unsubstituted phenacy alkylene (phenalene), a substituted or unsubstituted phenanthrene, substituted or substituted anthracene, a substituted or unsubstituted triphenylene (triphenylene), a substituted or unsubstituted pyrene, a substituted or unsubstituted cro pherocene (chrysene), a substituted or unsubstituted naphtha pherocene (naphthacene), a substituted or unsubstituted ranges (picene), a substituted or unsubstituted vinyl chloride resin (perylene), a substituted or unsubstituted pen hit pen (pentaphene), (hexacene) pherocene of a substituted or unsubstituted, a substituted or unsubstituted pyridine, a substituted or unsubstituted pyrazine, pyrimidine a substituted or unsubstituted, a substituted or unsubstituted pyridazine, a substituted or unsubstituted quinoline, a substituted or unsubstituted phthalamide hydrazine, a substituted or unsubstituted quinoxaline, quinazoline a substituted or unsubstituted, a substituted or unsubstituted hour glow phosphorus , a substituted or unsubstituted phenylphenanthridines, a substituted or unsubstituted phenanthroline or a substituted or unsubstituted phenazinecarboxamide can be. The halogen atom "substituted" said, halogen atoms, substituted C1-C20 alkyl (e.g.: CCF3, CHCF2, CH2 F, CCl3 such as), hydroxy, nitro, cyano group, amino group, amino d anger , hydrazine, Phenylhcdrazones, carboxylic, R2, R3, R4 are salts thereof, sulfonamide labor pains or salts thereof, phosphoric acid or salts, or C1-C20 alkyl, C2-C20 of alkenyl group, to alkynyl of C2-C20, alkyl of C1-C20, C6-C20 aryl, C6-C20 of aralkyl, aryl of C6-C20, or C6-C20 of means a substituted biting alkyl crossroad. Such carbon compounds are said non-conductive carbon film conductive can be supplemented by.. Said core which is marked as a chemical formula 1 can be represented by, said core at least over part of its surface a non-conductive carbon-based film containing oxygen may include a: [Formula 1] Lia Mb O2-c (X1)c Formula 1 in, 0.8≤a≤1.2 and the a, 0 < b≤1 and the b, c is the 0≤c≤1, M the Mn, Ni, Co, cu, mg, Na, Ca, Ti, Zn, Ga, Ge, Al, Cr, mg, Sr, Mo, W, V, Ti, Zr, Ru, Rh, Pd, Os, Ir, Ag, Au, Hf, Sn and Pt selected from the group consisting of 1 may be at least one element, X1 O the, F, S selected from the group consisting of P and 1 can be at least one element. For example, a plied construction of said core LiCoO2 and Lia1 Nix Coy Mnz O2 (wherein, 0.8≤a1≤1.2 and, heterocomplexes 0.8≤x + y+z ≤ 1.2), spinel structure of LiMn2 O4, and olivine structure of LiFePO4 may comprise an. For example, a plied construction of said core Lia1 Nix Coy Mnz O2 (wherein, 0.8≤a1≤1.2 and, heterocomplexes 0.8≤x + y+z ≤ 1.2) can be. Core of said layered structure assembly and a storage unit with certain interval symmetrically under high voltage 4.5V and the structure is stable, and can be. Said core surface of X ray photoelectron spectroscopy (XPS) of the analysis (binding energy) binding energy of 530eV to 528eV spectrum O1s to object in graphics on peak intensity in binding energy of 533eV to 531eV (IA/IB), and said core present at the surface of the core 100 parts by weight said non-conductive carbon-based film (D) with a carbon content of present formula relationship 1 can be represented by: [Type 1] [(IA/IB)/ D] ≥10 parts by weight-1 In said type 1, peak intensity in binding energy of 530eV to 528eV (IB) the nickel oxide, cobalt oxide, or manganese oxide is present meaning, peak intensity in binding energy of 533eV to 531eV (IA)-C (=O) OH and-C=O group carbon compound is present by a rope. means. Said core present at the surface of the core 100 parts by weight present said non-conductive carbon-based film (D) to a carbon content of refers to element analyzing (Element Analyzer; EA) can be by using. The positive electrode active material contains said said core surface is the vision includes small amount carbon conductive anionic compound layer conductive carbon-based film can be formed. Such non-conductive carbon-based film with said core surface and electrolyte same by reducing side reactions including lithium cell initial efficiency, capacity and can be life cycle improved. Said non-conductive carbon-based film said core present a carbon content of 0.3 parts by weight 100 parts by weight can be hereinafter. For example, said non-conductive carbon-based film said core present a carbon content of 0.001 weight percent to 100 parts by weight can be 0.3 part by weight, for example 0.001 weight percent to 0.2 part by weight can be, for example 0.001 weight percent to 0.15 part by weight can be, for example 0.001 weight percent to 0.1 part by weight can be, for example 0.001 weight percent to 0.05 part by weight can be. Said non-conductive carbon-based film present a carbon content of said when in the range of, coating materials, and a presence a carbon content of about 1/10 level surface core said thin uniform non-conductive carbon-based film to form first patterns, including same lithium cell initial efficiency, capacity and can be life cycle improved. The thickness of the non-conductive carbon-based film 0.1 nm to 5.0 nm and the shaft transfers the, for example 0.1 nm to 4.0 nm and the shaft transfers the, for example 0.1 nm to 3.0 nm and the shaft transfers the, 2.5 nm to 0.1 nm for example can be. Within range said non-conductive carbon-based film having a thickness of said said core-group metal oxide layer non-conductive carbon film interface resistance between penetration hole while moving up and down to minimize the difference. In addition said core surface with an electrolyte and in an electric field can be used for suppressing the side reactions including lithium cell initial efficiency, capacity and can be life cycle improved. Represented by said formula 1 to the core can be represented by formula 2, said core at least over part of its surface a non-conductive carbon-based film containing oxygen may include a: [Formula 2] q Li2 MnO3, (1-q) Li (M1) O2 Formula 2 in, 0 < q which < Wednesday 1, M1 the Mn, Ni, Co, cu, mg, Na, Ca, Ti, Zn, Ga, Ge, Al, Cr, mg, Sr, Mo, W, V, Ti, Zr, Ru, Rh, Pd, Os, Ir, Ag, Au, Hf, Sn and Pt selected from the group consisting of at least one element can be 1. Said non-conductive carbon-based film Li2 CO3 may further include any component. A plied construction as said core, anode active manganese ion the lithium layer while moving with a void space in said core is crystal lattice (spinel-like configuration) is converted into a spinel-like structured by mounting a diagnosis portion for. However said core at least over part of its surface incorporated with its structure layered said Li2 MnO3 Li on from the generated by the reaction between the and an oxygen Li2 CO3 component including further including non-conductive carbon total screen , stable anode active Li2 MnO4 phase. of easily translatable structures to a deterministic data structure and spinel such as. Thus said core surface can be used for suppressing side reactions with an electrolyte and in an electric field and including lithium cell initial efficiency, capacity and can be life cycle improved. Said non-conductive carbon film X ray photoelectron spectroscopy (XPS) of the analysis (binding energy) 285eV to 282eV C1s spectrum of binding energy peak (phase) on (C-C bond) bond C-C occurring in the on (binding energy) occurring in the binding energy of 293eV to 288eV Li2 CO3 on peak intensity non-(ID/IC) the at least 0.3. For example, ID/IC can be the 0.3 to 1.0. Said core which is marked as a chemical formula 3 and can be represented by, said core at least over part of its surface a non-conductive carbon-based film containing oxygen may include a: [Formula 3] Nax (Q) O2+e Formula 3 in, Q in terms 4 through group 12 as selected from the group consisting of at least one transition metal element 2 can be, 0.5≤x≤1 and, can be -0.3≤ e≤ 1. For example, said core which is marked as a chemical formula 4 can be represented by: [Formula 4] Nax Qaz Qbv O2+d Formula 4 in, The Qa Fe, Ru, Os, Cr, Mo and W selected from the group consisting of manganese and at least another element a may be, In terms Qb group 4, group 5, group 7, 9 and 10 as selected from the group consisting of one or more elements which may be, 0.5≤x < 1, 0.3≤z≤0.5, 0.5≤v≤0.7, z+v=1, < Wednesday 1-0.3≤ d. For example, said core which is marked as a chemical formula 5 can be represented by: [Formula 5] Nax Qaz Qbv O2+d Formula 5 in, The Qa and the shaft transfers the Cr or Fe, The Qb Mn, Co, Ni, V, Ti selected from the group consisting of manganese and at least another element a may be, 0.5≤x < 1, 0.3≤z≤0.5, 0.5≤v≤0.7, z+v=1, < Wednesday 1-0.3≤ d. For example, said core which is marked as a chemical formula 6. can be represented by: [Formula 6] Nax Fez Qbv O2 Formula 6 in, The Qb Mn or Ni and the shaft transfers the 0.5≤x < 1, 0.3≤z≤0.5, z+v=1, can be 0.5≤ v≤ 0.7. Said core which stratified rock-salt crystal structure phase comprised predominantly of an, improved structural stability can take the. Therefore, initial discharge capacity of sodium battery including said core, kaolin, or alumina can be metal oxide material and a metal salt. Said core at least over part of its surface a non-conductive carbon-based film containing oxygen present said core a carbon content of 0.3 parts by weight 100 parts by weight can be hereinafter. For example, said non-conductive carbon-based film said core present a carbon content of 0.001 weight percent to 100 parts by weight can be 0.3 part by weight, for example 0.001 weight percent to 0.2 part by weight can be, for example 0.001 weight percent to 0.15 part by weight can be, for example 0.001 weight percent to 0.1 part by weight can be, for example 0.001 weight percent to 0.05 part by weight can be. Said non-conductive carbon-based film present a carbon content of said when in the range of, , and a coating materials a carbon content presence an epitaxial layer on the level of trace extremely compared to thin uniform surface core said non-conductive carbon-based film to form first patterns, including sodium same cell initial efficiency, capacity and can be life cycle improved. The thickness of the non-conductive carbon-based film 0.1 nm to 5.0 nm and the shaft transfers the, for example 0.1 nm to 4.0 nm and the shaft transfers the, for example 0.1 nm to 3.0 nm and the shaft transfers the, 2.5 nm to 0.1 nm for example can be. Within range said non-conductive carbon-based film having a thickness of said said core-group metal oxide layer non-conductive carbon film interface resistance between can be to minimize the difference, said core surface can be used for suppressing side reactions with an electrolyte and in an electric field.. Thus said non-conductive carbon-based film including sodium cell initial efficiency, capacity and can be life cycle improved. Said core and said non-conductive carbon-based between the oxygen vacancy and may further include any layer. Oxygen vacancy and said core least a part of the surface of said layer a layer formed is reduced, can be. Also, according to some exemplary embodiments Figure 1 shows a an anode active material (10) is mimetic of. Also 1 with a, anode active material (10) lithium ion or sodium ion occlusion and detachment metal oxide core (1) oxygen vacancy and surface layer (2) and a non-conductive carbon-based film containing oxygen (3) 10 includes. Oxygen vacancy and layer (2) lithium ion or sodium ion occlusion and detachment metal oxide core (1) surface emitted oxygen atoms a reduction reaction has taken place a can be formed. In accordance with another aspect, anode; electrolyte ; and cathodes a secondary battery using is provided, the aforementioned anode said may include positive electrode active material. For example said secondary battery can be produced as follows. First, said anode can be produced as follows. Lithium ion or sodium ion occlusion and is capable of releasing a metal oxide, base metal oxides, or combinations thereof of oxide core, and said core at least over part of its surface a non-conductive containing oxygen anode active material including carbon-based film, conductive material, coupled number and a solvent prepared is cathode active material composition for mixing.. Cathode active material composition for said collector is of an active anode and dried directly coated on an anode formed prolong the life can be produced. Alternatively, said metal on support separate is cathode active material composition for the Multicast then, said peeled off from the support is a film lamination on a current collector of an active anode is an anode formed prolong the life can be produced. Said lithium ion or sodium ion occlusion and is capable of releasing a metal oxide, base metal oxides, or combinations thereof of oxide core, said non-conductive carbon film composition, content, by establishing an optical fiber at a to and thickness since the ., which does not require a described hereinafter. Of positive electrode active material for said can be 4.4V±0.1V a potential is operating. For example, the positive electrode active material contains said 4.3V to 4.5 V which may be, may it will be a quality -based positive electrode active high voltage. said anode is in may further include any; and a conductive material. The specific carbon material of the surface area of said conductive material, for example carbon black, graphite granule, natural graphite, artificial graphite, acetylene black, [...] black, carbon fiber; carbon nanotube, copper, nickel, aluminum, metal such as powders or metal fiber or metal tube; poly Phenylen derivatives such as conductive polymer can be or the like is used as an. Furthermore, growing conductive material said carbon, pitch (petroleum, coal, of any by-product such as synthetic resin lens and composition) fiber formed by reacting an hydrocarbons at higher temperatures in order, or acrylic fiber (Polyacrylonitrile) effect of the carbon produced from a electrically conductive fibers can be or the like is used as an. Carbon fiber surface area and can be used simultaneously carbon material. Carbon fiber and surface area carbon material have a two electrically conductive when can be improved. Furthermore, sodium battery anode in the be oxidized in range a charge rate of a battery during a without their dissolving in the material, of high magnetic electrical as a positive electrode active material in the metal-based conductive material may be used. For example titanium, metal corrosion resistance metallic, carbide such as SiC or WC, or Si3 N4, BN such as nitride can be used. However these conductive material defined by conductors in the art do not can be can be made using another material if ., and is capable of providing both. Binder vinylidene fluoride/hexafluoropropylene copolymer, polyvinylidene fluoride-, polyacrylonitrile, fluorine compound polymer, blends of polyamide blockpolymers and of copolymers (PTFE), the aforementioned polymers, mixture of, styrene butadiene rubber-based polymer, or carboxylic [...] polymer can be or the like is used as an. Said fluorine compound for example, fluorinated (C1-C18) (metadata) acrylate, perfluoroalkyl (metadata) acrylate (for example, purple as much as base Oro will be burnt (metadata) acrylate, perfluoro n-octyl (metadata) acrylate, perfluoro n-butyl (metadata) acrylate), perfluoroalkyl substituted alkyl (metadata) acrylate (for example, purple [...] (metadata) acrylate, purple [...] (metadata) acrylate), purple base Oro jade hour alkyl (metadata) acrylate (for example, purple the jade hour ethyl where as much as base Oro will be burnt (metadata) acrylate and perfluoro base Oro the jade hour ethyl which will be burnt (metadata) acrylic, and the like), fluorinated alkyl (C1-C18) chrotonate, its producing method, fluorinated alkyl (C1-C18) maleate, fumarate maleate (C1-C18) fluorinated alkyl, fluorinated alkyl (C1-C18) [...] lighter, fluorinated alkyl substituted olefinic (2-10 carbon atoms, fluorine atom can 1-17), for example purple [...] , 2 to 10 carbon atoms 1 to 20 degree and fluorine atoms fluorine atoms carbon double bonds degree of fluorination that is joined to the olefin, tetrafluoroethylene, trifluoro ethylene, polyvinylidene fluoride or hexafluoropropylene can be or the like is used as an. Furthermore, coupled number as for example, starch, methylcellulose, carboxymethyl cellulose, hydroxymethyl cellulose, hydroxyethylcellulose, hydroxypropyl cellulose, carboxymethyl hydroxyethylcellulose, and derivatives thereof polysaccharide such as nitrocellulose; phenol resin; melamine resins; polyurethane resin; urea resin; polyamide resin; polyimide resin; polyamide-imide resin; petroleum pitch; coal pitch can be or the like is used as an. Coupled, together with a plurality of coupling as number can be used. Furthermore, electrode mixture in said binder can act as thickener. N-methylpyrrolidone (NMP) solvent, acetone, water, or the like, and can be used. However coupled and solvent necessarily defined by number do not the art to that may be used in ., and is capable of providing both. the, nickel, for instance, aluminum, titanium, copper, gold, the, platinum, of metals, such as stainless or aluminum alloy, for example carbon material, activated carbon fiber, nickel, aluminum, zinc, copper, tin, lead or these alloys plasma-welding, is formed by arc spraying, for example rubber or styrene-ethylene-butylene-styrene copolymer (SEBS) conductive resin such as number is distributed can be or the like is used as an conductive film. For example, aluminum, nickel or stainless or the like is used as an can be. In particular, thin film cutting of aluminum in that an easier and cheaper can be used. Current collector which is not particularly limited in the shape of the, on thin film for example, flat plate-like, mesh-like, on net, punching on or embossings shape or a combination of these (for example, mesh-like flat such as) can be or the like is used as an. For example, by etching treatment on the surface of the collector to form-lens array, concave and convex. Said anode active material, conductive material, coupled content of the solvent and number a lithium battery or sodium cell commonly used in. a level that. Lithium battery or sodium cell use and according to said conductive material, the invention eliminates the need for at least one number and solvent coupled can be. Said anode active material in addition to the aforementioned anode generally of the positive electrode active material of the existing method can additionally include. The positive electrode active material contains general of the existing method in the art which can be used to the anode lithium ions as the or sodium ion occlusion and release it makes it possible, and is capable of providing both if.. Lithium ion occlusion a larger specific surface area than the but is not particularly limited in the positive electrode active material contains, the concrete examples Lia A1-b Bb D2 (in formula said, 0.90 ≤ a ≤ 1.8, and is 0 ≤ b ≤ 0.5); Lia E1-b Bb O2-c Dc (in formula said, 0.90 ≤ a ≤ 1.8, 0 ≤ b ≤ 0.5, is 0 ≤ c ≤ 0.05); LiE2-b Bb O4-c Dc (in formula said, 0 ≤ b ≤ 0.5, is 0 ≤ c ≤ 0.05); Lia Ni1-b-c Cob Bc Dα (in formula said, 0.90 ≤ a ≤ 1.8, 0 ≤ b ≤ 0.5, 0 ≤ c ≤ 0.05, 0 is < α an interlayer 2); Lia Ni1-b-c Cob Bc O2-α Fα (in formula said, 0.90 ≤ a ≤ 1.8, 0 ≤ b ≤ 0.5, 0 ≤ c ≤ 0.05, 0 is < 2 < α); Lia Ni1-b-c Cob Bc O2-α F2 (in formula said, 0.90 ≤ a ≤ 1.8, 0 ≤ b ≤ 0.5, 0 ≤ c ≤ 0.05, 0 is < 2 < α); Lia Ni1-b-c Mnb Bc Dα (in formula said, 0.90 ≤ a ≤ 1.8, 0 ≤ b ≤ 0.5, 0 ≤ c ≤ 0.05, 0 is < α ≤ 2); Lia Ni1-b-c Mnb Bc O2-α Fα (in formula said, 0.90 ≤ a ≤ 1.8, 0 ≤ b ≤ 0.5, 0 ≤ c ≤ 0.05, 0 is < 2 < α); Lia Ni1-b-c Mnb Bc O2-α F2 (in formula said, 0.90 ≤ a ≤ 1.8, 0 ≤ b ≤ 0.5, 0 ≤ c ≤ 0.05, 0 is < 2 < α); Lia Nib Ec Gd O 8880000 277888 (in formula said, 0.90 ≤ a ≤1.8, 0 ≤ b ≤ 0.9, 0 ≤ c ≤ 0.5, is 0.001 ≤ d ≤ 0.1.); Lia Nib Coc Mnd Ge O2 (in formula said, 0.90 ≤ a ≤ 1.8, 0 ≤ b ≤ 0.9, 0 ≤ c ≤ 0.5, 0 ≤ d ≤0.5, is 0.001 ≤ e ≤ 0.1.); Lia NiGb O2 (in formula said, 0.90 ≤ a ≤ 1.8, is 0.001 ≤ b ≤ 0.1.); Lia CoGb O2 (in formula said, 0.90 ≤ a ≤ 1.8, is 0.001 ≤ b ≤ 0.1.); Lia MnGb O2 (in formula said, 0.90 ≤ a ≤ 88 80001355888, is 0.001 ≤ b≤0.1.); Lia Mn2 Gb O4 (in formula said, 0.90 ≤ a ≤1.8, is 0.001 ≤ b ≤ 0.1.); LiQO2; LiQS2; LiV2 O5; LiIO2; LiNiVO4; Li(3-f) J2 (PO4)3 (0≤f≤2); Li(3-f) Fe2 (PO4)3 (0≤f≤2); LiFePO4 of either formula can be compounds represented. For example, the positive electrode active material contains said cobalt, manganese, nickel, and combinations thereof a metal electrode and a lithium composite oxide with at least one inert gas during 1. Sodium ion occlusion a larger specific surface area than the but is not particularly limited in the positive electrode active material contains, the concrete examples NaFeO2, NaMnO2, NaNiO2 and NaCoO2 such as NaM1a O2 oxide represented by, Na0.44 Mn1-a M1a O2 oxide represented by, Na0.7 Mn1-a M1a O2.05 oxide represented by (M1 the at least one transition metal element 1, 0≤a < 1); Na6 Fe2 Si12 O30 and Na2 Fe5 Si12 O30 such as Nab M2c Si12 O30 represented by oxide (M2 1 at least one transition metal element, 2≤b≤6, 2≤c≤5); Na2 Fe2 Si6 O18 and Na2 MnFeSi6 O18 such as Nad M3e Si6 O18 oxide represented by (M3 the at least one transition metal element 1, 3≤d≤6, 1≤e≤2); Na2 FeSiO6 such as Naf M4g Si2 O6 represented by oxide (M4 the transition metal element, mg and Al 1 selected from the group consisting of at least one element, 1≤f≤2, 1≤g≤2); NaFePO4, Na3 Fe2 (PO4)3 such as phosphate; NaFeBO4, Na3 Fe2 (BO4)3 such as borate 8 8800028428883 FeF6 and Na2 MnF6 such as Nah M5 F6 fluoride represented by (M5 the at least one transition metal element 1, 2≤h≤3) can be or the like is used as an. The anode active material or/and said anode active material instead used anode active materials except for the host supplying method in the same anode and can be produced. For example, said cathode can be produced as follows. PLD and as well as the above-mentioned manufacturing an anode of a lithium ion, cathode active material, conductive material, and a solvent number coupled mixing is is produced anode active material composition on the anode, an aquapotin same directly coated cathode prolong the life can be produced. Alternatively, said cathode active material with the casting metal on support separate composition is peeled off from the support and an anode active material film cathode is lamination an aquapotin said prolong the life can be produced. Just, sodium battery comprising when the powder form anode active materials said formed by the method such as the cathodes prolong the life can be produced. Lithium metal carbon of lithium battery, metal alloyable with lithium and a material the metal, transition metal oxide, lithium dope and [...] materials which can be, or lithium ions reversibly a desorption inserted into, use can be made of, substance or the like. Said a specific example of the transitional metal oxide vanadium oxide, lithium vanadium oxide or, lithium dope and examples of materials which can be [...] Si, SiOx (0 < x < 2), Si-Y alloy (said Y has alkali metal, alkaline earth, 13 group salts 16 group element, transition metal, rare earth element or a combination thereof the metal species is 0.1-, Si but not), Sn, SnO2, Sn-Y (said Y has alkali metal, alkaline earth, 13 group salts 16 group element, transition metal, rare earth element or a combination thereof the metal species is 0.1-, but not Sn) as to the aromatic hydrocarbon and, in addition and at least one SiO2 the may be. The Y element said mg, Ca, Sr, Ba, Ra, Sc, Y, Ti, Zr, Hf, Rf, V, Nb, Ta, Db, Cr, Mo, W, Sg, Tc, Re, Bh, Fe, Pb, Ru, Os, Hs, Rh, Ir, Pd, Pt, cu, Ag, Au, Zn, Cd, B, Al, Ga, Sn, In, Ti, Ge, P, As, Sb, Bi, S, Se, Te, Po, thereof in the following or a combination thereof. Lithium ions reversibly inserted into said protruded unit as carbon a material, commonly used in lithium ion secondary battery a carbon anode active manufactured by mixing a transition metal which may also be employed and unbreakable is, a typical example thereof is that the crystalline carbon, amorphous carbon or associated processes for, use can be made of,. Examples of carbon crystalline said amorphous, plate, on (flake) flakes, spherical or fiber-type of graphite such as natural graphite or artificial graphite of the radioactive part into contact with and, said carbon soft examples of amorphous carbon (soft carbon: low temperature co-fired carbon) or hard carbon (hard carbon), mesoporous face pitch carbide, as to the aromatic hydrocarbon coke calcined. However, carbon them to said is not limited which active material cathode in the art which can be used for inserting and lithium as the detachment., and is capable of providing both ramyon coated on an alloy. Sodium battery of carbon metallic sodium, metallic sodium alloy, sodium intercalation compounds (sodium intercalating compound) or the carbon based material is necessarily can be degraded and do not defined by active material cathode in an the art procedure so that it can be used, including sodium or capable of storing and releasing ions is ., and is capable of providing both ramyon. Said cathode is sodium capacity of a battery by changing the fifth said cathode, for example can be metallic sodium. Said metallic sodium as alloy, for example, with aluminium, tin, indium, calcium, titanium, vanadium and the like can be alloy of sodium. For example, the cathode is generally 3 micro m to 500 micro m to a metallic state thickness of sodium may be employed, film, sheet, foil, net, porous material, foam, nonwoven fabric or the like in various forms can be used. Metallic sodium or sodium alloy other than when using a negative electrode active material, graphene platelets of carbon with structure can be is used as the material. Graphite, carbon graphite or cathode mix materials such as, carbon type material, mixing these compounds with an metal or alloy cathode, composite cathode is can be used. Carbon-based material, sodium ion for electrochemically capable of storing and releasing natural graphite, artificial graphite, [...] , expanded graphite, carbon fiber, vapor growth method using carbon fiber, pitch-based carbon-axis swing hinge, needle coke , petroleum coke, polyacrylic with Knight reel orgin carbon fiber, of carbonaceous material including carbon black, or 5-membered ring or 6-membered ring cyclic hydrocarbons or cyclic oxygenated organic compound a composite by pyrolysis amorphous carbon material can be or the like is used as an. Optionally said cathode active material anode active matter composition and by adding plasticizer compositions form to plate internal electrode may also be.. Material as cathodic collector, shape, manufacturing method is not limited by, any current collector, use can be made of,. For example, copper foil thickness 10-100 micro m, m micro 10-100 thickness, melting glass of 0.1-10 mm diameter hole perforated foil, metal extension, or foam metal plate can be or the like is used as an. In addition to employing a cathode current collecting body is made out of a copper, stainless, titanium, nickel, and so forth can be is used. Said cathode active material, conductive material, coupled content of the solvent and number a lithium battery or sodium cell commonly used in. a level that. Lithium battery or sodium cell use and according to said conductive material, the invention eliminates the need for at least one number and solvent coupled can be. Next, said body after it is implanted into the between anode and cathode. prepared separator. Said separator sodium battery or lithium battery is typically used in. components ramyon. Resistance with respect to a shift ion electrolyte circuit turns on/has excellent ability a wet-laid electrolyte can be used. For example, glass fiber, polyester, Teflon, polyethylene, polypropylene, blends of polyamide blockpolymers and of copolymers (PTFE) or combinations thereof as a selected one of, .has a nonwoven fabric or woven form. For example, according to said method a separator can be produced. Polymer resin, filling number and a solvent separator mixing. prepared composition. Said separator composition separator and dried directly coated electrode can be formed. Or, said separator the manufacturing method is provided with a multicasting and a support having thereon, in composition, so as to form a unitary separator the peeled off from the support said adjacent to film lamination is separator can be formed. Said producing separator fuel which is not particularly limited in resin, electrode plate for both the material used for the binding can be used. For example, vinylidene fluoride/hexafluoropropylene copolymer, polyvinylidene fluoride-(PVDF), polyacrylonitrile, a group including polyester, cellulose triacetate or mixtures thereof can be or the like is used as an. Solvent incapable of dissolving said polymer resin can be dispersed in polymer is dried form the procedure so that it can be commonly used in the art, and is capable of providing both ramyon is.. Furthermore, said separator other publicly known method for active material layer cathode is being manufactured from a separate lamination on top can be. For example, polypropylene, polyethylene for melting and by extruding after film solar battery film, annealing at low temperature domain and after that a determined, stretch in this state the embodiment an amorphous region is dry forming a weight of 100,000 or higher by extending the manufacturing method can be is used. For example, , such as hydrocarbon solvents other small molecular material and polypropylene, polyethylene or the like after mixing and, film-forming the, then, are assembled into a molecular solvent or normally amorphous as an island phase having a size to form a start of film (island phase), the other molecular solvent or said is removed by use of a volatile solvent at a weight of 100,000 or higher by a wet forming manufacturing method can be is used. Furthermore, said separator strength and hardness, a heatable control end, non-conductive particles, a filler other, fiber compounds and the like additionally additives can. For example, inorganic particles said separator may additionally comprise. Said separator further includes an inorganic particles, the rice transplanter is improved in oxidation resistance, of battery characteristics can be capable of suppressing. Said alumina inorganic particles (Al2 O3), silica (SiO2), or a titania (TiO2). combination of the conductor. Said 5 to 10 nm and the mean particle size of inorganic particles can be micro m. Average particle diameter of inorganic particles if less than 10 nm crystalline decoding unit sequentially added which mix effect, average particle diameter if the load gradient exceeds the first 5 micro m difficult dispersion of inorganic particles. Said separator to increase in tear strength and mechanical strength, including one or more polymer layer material may have a multilayer structure. For example, laminate polyethylene/polypropylene, polyethylene/polypropylene/polyethylene laminate, non-woven fabric/polyolefin laminate. combination of the conductor. Is are prepared electrolyte at then. In lithium battery, for example, said can be organic electrolyte electrolyte. Furthermore, in lithium battery, a solid electrolyte can be said. For example, boron oxide, lithium the sion anti-combination of the conductor defined by but they do not the art can be used as electrolyte in solid at is. components ramyon. In said method such as sputtering the solid electrolyte can be formed on said cathode. For example, organic electrolyte are prepared can be. Organic electrolyte lithium salt dissolved in organic solvents can be produced. Said organic solvent in the art which can be used as organic solvent to both can be used. Said organic solvent having a non-protic organic solvent, may use a, for example, propylene carbonate, ethylene carbonate, fluoro [...] , polybutylene cyclocarbonate, dimethyl carbonate, [...] , methyl [...] , methyl [...] , ethyl [...] , methyl [...] , deep [...] , [...] , cyclohexanol and its salts, acetonitrile, rearranging circuit, [...] methyl 2-, γ-butyrolactone, 1, 3-dioxolane, 4-methyl dioxolane, N, N-dimethylformamide, dimethylacetamide, Dimethylsulphoxide, dioxane, 1, 2- [...] , sulfoalkyl, groups, or with an, dichloroethane, trichlorobenzene, nitrobenzene, diethylene glycol, dimethyl ether or mixtures thereof can be is used as the. Said lithium salinity in the art which can be used a lithium salt can be used if both. For example, said lithium salt LiPF6, LiBF4, LiSbF6, LiAsF6, LiClO4, LiCF3SO3, Li (CF3 SO2)2 N, LiC4 F9 SO3, LiAlO2, LiAlCl4, LiN (Cx F2x+1 SO2) (Cy F2y+1 SO2) (x stage, connected to a rotational member has y), LiCl, or mixture of them on the LiI can be. In sodium battery, for example, sodium electrolyte said a an organic solvent can be organic electrolyte. A used as electrolyte in said -15 wt % of sodium, for example NaClO4, NaPF6, NaBF4, NaCF3 SO3, NaN (CF3 SO2)2, NaN (C2 F5 SO2)2, or NaC (CF3 SO2)3 can be degraded and or the like is used as an do not necessarily defined by sodium in the art to which can be used for ., and is capable of providing both. Furthermore, lithium battery used lithium salt is lithium in salt substituted sodium may be is used. The concentration of said organic electrolyte an electrolyte and in an electric field, organic regarding an electrolytic solution on the consideration of the solubility of electrolyte can be correctly setting. For example, a of electrolyte concentration, it is can be 0.1-5M. For example, a of electrolyte concentration, it is can be 0.3-3M. For example, a of electrolyte concentration, it is can be 0.8-1.5M. 0.1M is of electrolyte concentration, it is if the flow is greater than the organic electrolyte ion-conducting, so that the degree of sodium battery internal resistance of which can be lowered, the viscosity of organic electrolyte is 5 M hereinafter decoding unit sequentially sodium battery internal resistance of. can be lowered. Said and showing a moisture content of organic solvent having the organic solvent is which has a polarity 200 PPM hereinafter can be used, for example, said lithium battery used in organic solvent to the same in a non-protic organic solvent is used can be. However said organic solvent having do not necessarily defined by in the art which can be used as organic solvent to ., and is capable of providing both. Also, according to some exemplary embodiments Figure 5 shows a secondary battery (100) decomposition of is perspective view. Secondary battery (100) a lithiated can be cell battery or sodium. Secondary battery as viewed in Figure 5 (100) comprises a positive electrode (114), separator (113), and negative (112) includes. The aforementioned lithium battery or sodium forming method of cathode (114), separator (113), and negative (112) is winding or folded cell container (120) are housed in. Furthermore, said cell container (120) to organic electrolyte are implanted and sealing member (140) is sealed by a secondary battery (100) is is unlikely to complete to. Said cell container (120) a hollow cylindrical shape, , square, . combination of the conductor or thin film. For example, a large cell battery or sodium lithium said thin film battery can be. Said lithium battery or sodium cell lithium ion battery or sodium ion battery can be. Said is disposed separator between the positive electrode and the negative battery structure may be formed. Said battery structure bi-cell structure then stacked, organic electrolyte is impregnated, a product obtained pouch is housed in sealed surface lithium ion polymer battery or sodium ion polymer cell is completed is. Furthermore, a plurality battery structure said laminated battery pack is formed, such a cell displays a message for requesting input of capacity and output power pack may be used in the all of the instruments. For example, notebook, smart phone, may be used in the such as electric vehicle. Furthermore, of the substrate in which a cell battery or sodium lithium said storage stability, and work life of high-rate characteristics (electric vehicle, EV) since the electric vehicle may be used in the. For example, plug live lead vehicle (plug-in hybrid electric vehicle, PHEV) such as may be used in the hybrid vehicle. Another according to one aspect, lithium ion or sodium ion occlusion and is capable of releasing a metal oxide, base metal oxides, or combinations thereof of oxide core, 1 or more hydroxy or carboxylic carbon nano-tube having-based precursor, and a solvent at least a part of the surface core said mixing said carbon-based precursor for the chemical by adsorbing the; and said adsorbed carbon-based precursor is formed containing oxygen by heat treatment of a core a non-conductive carbon-based for forming a polymer film on a step; of positive electrode active material for the aforementioned including a manufacturing method is provided. First, lithium ion or sodium ion occlusion and is capable of releasing a metal oxide, base metal oxides, or combinations thereof for preparing the oxide core. By establishing an optical fiber at said core to since the ., which does not require a described hereinafter. For example, in lithium battery said method in a core can be produced. I.e., by shape by coprecipitation and basic solution and aqueous metal salt solution to prepare compound through the co-precipitation method. The aqueous metal salt solution said Mn, Ni, Co, and combinations thereof at least one selected from 1, or Mn, Ni, Co, and combinations thereof 1 selected from at least one and a selectively Mo, W, V, Ti, Zr, Ru, Rh, Pd, Os, Ir, Pt, cu, Zn, Cr, Fe, mg, Na, Ca, Ga, Ge, Al, Cr, Sr, Ag, Au, Hf, Sn, and combinations thereof selected from 1 or more kinds of metallic may include a. For example, the aqueous metal salt solution said Mn, Ni, Co, and combinations thereof 1 selected from at least one inert gas. Said aqueous metal salt solution a sulphate, nitrate, acetate, halide, hydroxide and combinations thereof 1 selected from aqueous solution of salts species may include a. However, not limited to all water, in particular in the fields of, wherein such contact occurs that is soluble in. use of salts. The basic solution said Na2 CO3 aqueous solution, NaOH, KOH, NH4 OH, selected from and combinations thereof may include a species 1. Said pH 8 to 14 the production of the compound through the co-precipitation method carried out, for example in pH 8-12, in pH 8-10 for example, for example can take place at pH 8-9. Said adjustable within the range said compound through the co-precipitation method when a pH, to give compounds may be high density through the co-precipitation method. Next, mixing said lithium salt compound co-precipitated lithium ion occlusion a larger specific surface area than the metal oxide core prepare silk fibroin fibre. Said lithium salt Li2 CO3, LiNO3, LiBr, LiCl, LiI, LiOH, Li (CH3 CO2), LiH2 PO4, LiOH, H2 O, Li (CH3 CO2), 2H2 O, selected from and combinations thereof may include a species 1. For example, sodium battery in said method in a core can be produced. ; Dissolving the metal precursor in the solvent. a step of preparing a solution by number 1. Said periodic table of elements 12 through group 4 a metal precursor selected from the group consisting of as 2 or more kinds of transition metal precursor can be. Said transition metal precursor for example, combination of Mn and Fe, combination of Ni and Fe, Fe and combination of Co, combination of Ti and Fe, combination of Mn and Ni, Mn and combination of Co, combination of Ti and Mn, Ni and combination of Co, combination of Ti and Ni, Co and a combination of Ti, combination of Ni and Mn and Fe, Mn and Fe combination of Co and, combination of Ti and Mn and Fe, Ni and Fe combination of Co and, combination of Ti and Ni and Fe, Fe and Co and a combination of Ti, and Mn and Fe Co and combination of Ni, Ti and Ni and combination of Mn and Fe, or Fe and Ni and Mn Co and. each can be a combination of Ti and a. Said metal precursor metal from a the art including precursor compounds which can be used in ., and is capable of providing both if. For example, said metal precursor a metal chlorides, or metal and coagulation of the nitrate, such as metal sulfate of can be hydrate compounds and their. Next, said number 1 number 2 and a chelating precipitant solution comprising a agitating and solution to obtain precipitate. Or, comprising a and a chelating precipitant said said number 1 number 2 solution to obtain precipitate agitating and solution. Solution number 2 solution and said number 1 for example, can be aqueous solution. Said a precipitant, for example LiOH, NaOH, KOH, Li2 CO3, Na2 CO3, K2 CO3, or (NH2)2 CO can be defined by such as do not necessarily in the art which can be used as precipitant if ., and is capable of providing both. Said precipitant content of moles precursor total metal solution charged in said number 1 (for example, the total number of moles of the metal precursor or more transition 2) 1 mol of molar can be 20-40. Said precipitant said number 2 in solutions of acid concentration in the range of about 0.5 to about 10 mol/L and the shaft transfers the, for example, about 1 to about 8 mol/L can be. Said chelating agent NH4 OH, tartaric acid (tartaric acid), (succinic acid) succinic, adipic acid (adipic acid), citric acid (citric acid), triethanolamin (triethanolamine), and polyvinyl pyrrolidone (polyvinylpyrrolidone) selected from the group consisting at least one. Said chelating agent is the coordinated by metal in solution after the stabilized by type hydrate uniform composition can be obtained and metal oxides, with or without, stratified rock-salt structure having high metal oxide being predominantly in a. can be obtained. Said chelating agent content total metal precursor said number 1 solution charged in total mean additive moles of more than 1.5 to 1 molar (mole) may be, for example, 1 mol of total metal precursor can be molar 10-1.5. Said precipitates are 2 or more transition metal can include a, for example 2 or more transition metal including can be hydroxide. Said precipitates are can be is obtained in the form slurry. A solid obtained by filtering said slurry separating with a liquid in which. can be recovered. Cleaning or respect to recovery of the resulting precipitate can be a drying. Alcohol water or cleaning liquid, such as acetone-soluble organic solvent are available for rigging, for example water can be used. The drying heating dry, blowing drying, or vacuum drying. can be performed by. In the case of heating dry, and dried at 50 °C to 300 °C and the shaft transfers the, said and dried at 80 °C to 200 °C can be. The drying said 10 to 50. can be performed during time. A cleaning and drying said 2 may be performed or more times. Next, by mixing compound and sodium in precipitate said gives mixture for preparing the core by firing. Said sodium compound for example, sodium hydroxide, sodium chloride, sodium nitrate, sodium peroxide, sodium sulfate, sodium bicarbonate, being an aqueous solution containing oxalic acid sodium and sodium hydrogencarbonate 1 selected from the group consisting of sodium and at least one can be these hydrates. Said sodium content of precipitations % relative to the total of of metal, 0.2 as atomic ratio may be less than 1 or more. -blending is dry, wet either. can also be performed. In terms pressing down, can be mixing dry type. Mixing device include, for example stirring and mixing, V type mixer, W type mixer, ribbon mixer, drum mixer, ball mill can be or the like is used as an. 400 °C to 1200 °C in the firing can be performed during time 20 to 0.1.. For example, 400 °C to 50 °C speed to said firing temperatures may be/time, at room temperature up to jumper speed firing temperatures, can be/time 400 °C to 10 °C. Firing the atmosphere, e.g., air, oxygen, nitrogen, argon or a combination of gas can be. For example, firing the atmosphere can be air. After baking obtained resultant ball mill or jet mill module as a charging voltage may to be fractured, pulverizing and repeatedly core 2 or more times baking can prepare the. Furthermore, cleaning or by the can be sifter. Metal layer is, said prepared core, 1 or more hydroxy or carboxylic carbon nano-tube having-based precursor, and a solvent at least a part of the surface core said mixing said carbon-based precursor to an atomic. Said carbon-based precursors a substituted or unsubstituted C6-C30 aromatic compounds, a substituted or unsubstituted C5-C30 heterocyclic compound, or combinations thereof can be compounds of. For example, said carbon compound has a substituted benzene or unsubstituted, a substituted or unsubstituted [...] , a substituted or unsubstituted indene, of naphthalene a substituted or unsubstituted, a substituted or unsubstituted dibenzoazulenes, a substituted or unsubstituted [...] , a substituted or unsubstituted indacene, a substituted or unsubstituted oh three naphthalenes , a substituted or unsubstituted fluorene, a substituted or unsubstituted phenacy alkylene, a substituted or unsubstituted phenanthrene, substituted or substituted anthracene, fluoranthene a substituted or unsubstituted, a substituted or unsubstituted triphenylene, a substituted or unsubstituted pyrene, pherocene cro a substituted or unsubstituted, a substituted or unsubstituted naphtha pherocene, a substituted or unsubstituted ranges, a substituted or unsubstituted vinyl chloride resin, a substituted or unsubstituted pen hit pen , pherocene of a substituted or unsubstituted, a substituted or unsubstituted pyrrole, substituted or substituted pyrazoles, a substituted or unsubstituted imidazole, substituted or unsubstituted imidazolidine, imidazopyridines a substituted or unsubstituted, a substituted or unsubstituted imidazopyrimidines, a substituted or unsubstituted pyridine, a substituted or unsubstituted pyrazine, pyrimidine a substituted or unsubstituted, a substituted or unsubstituted pyridazine, a substituted or unsubstituted indole, purines a substituted or unsubstituted, a substituted or unsubstituted quinoline, a substituted or unsubstituted phthalamide hydrazine, a substituted or unsubstituted naphthyridine, quinazoline a substituted or unsubstituted, a substituted or unsubstituted hour glow phosphorus , substituted or substituted indazoles, carbazole-a substituted or unsubstituted, a substituted or unsubstituted phenazinecarboxamide, a substituted or unsubstituted phenylphenanthridines, substituted or substituted triazines, a substituted or unsubstituted phenanthroline, or a substituted or unsubstituted [...] may. For example, said carbon-based precursor a substituted or unsubstituted benzene, of naphthalene a substituted or unsubstituted, a substituted or unsubstituted phenacy alkylene (phenalene), a substituted or unsubstituted phenanthrene, substituted or substituted anthracene, a substituted or unsubstituted triphenylene (triphenylene), a substituted or unsubstituted pyrene, a substituted or unsubstituted cro pherocene (chrysene), a substituted or unsubstituted naphtha pherocene (naphthacene), a substituted or unsubstituted ranges (picene), a substituted or unsubstituted vinyl chloride resin (perylene), a substituted or unsubstituted pen hit pen (pentaphene), (hexacene) pherocene of a substituted or unsubstituted, a substituted or unsubstituted pyridine, a substituted or unsubstituted pyrazine, pyrimidine a substituted or unsubstituted, a substituted or unsubstituted pyridazine, a substituted or unsubstituted quinoline, a substituted or unsubstituted phthalamide hydrazine, a substituted or unsubstituted quinoxaline, quinazoline a substituted or unsubstituted, a substituted or unsubstituted hour glow phosphorus , a substituted or unsubstituted phenylphenanthridines, a substituted or unsubstituted phenanthroline or a substituted or unsubstituted phenazinecarboxamide can be. The halogen atom "substituted" said, halogen atoms, substituted C1-C20 alkyl (e.g.: CCF3, CHCF2, CH2 F, CCl3 such as), hydroxy, nitro, cyano group, amino group, amino d anger , hydrazine, Phenylhcdrazones, carboxylic, R2, R3, R4 are salts thereof, sulfonamide labor pains or salts thereof, phosphoric acid or salts, or C1-C20 alkyl, C2-C20 of alkenyl group, to alkynyl of C2-C20, alkyl of C1-C20, C6-C20 aryl, C6-C20 of aralkyl, aryl of C6-C20, or C6-C20 of means a substituted biting alkyl crossroad. Said core and said solvent carbon-based precursor is chemically compatible with the not, is removable at a relatively low temperature, said core and said carbon-based precursor able to contact the effectively capable, if required, of acting to a medium in a solvent can be selected. Said solvent for example, acetone, ethanol, but fixing distilled, limited to not. Said core at least over part of its surface for the chemical-based precursor carbon said step of adsorbing said hydroxyl groups of said core surface carbon-based precursor hydroxy or carboxyl by dehydration into between a by chemical bonding can be formed. Said dehydration reaction may be performed at room temperature but, where the progress of the reaction dehydration relatively in order to place the subjected to a heat-treatment to further may include. Said heat treatment is said core, said carbon-based precursor composition and content but may differ as to, for example, inert atmosphere (for example, nitrogen, argon, and nitrogen and hydrogen gas atmosphere including at least one 1) in the 200 °C to 400 °C at a temperature of 1 hr to 5. can be performed during time. For example, about 300 °C said nitrogen atmosphere, a heat treatment is about at a temperature of. can be performed during time 2. Metal layer is, said adsorbed carbon-based precursor is formed containing oxygen by heat treatment of a core a non-conductive carbon-based film is formed on. Said heat treatment an inert atmosphere (for example, nitrogen, argon, and nitrogen and hydrogen gas atmosphere including at least one 1) in the 400 °C to 800 °C at a temperature of 1 hr to 5. can be performed during time. For example, said nitrogen atmosphere, a heat treatment is at a temperature of about 400 °C to 700 °C, for example at a temperature of 700 °C to 450 °C, about 2. can be performed during time. Said 1 or more hydroxy or carboxylic containing the oxygen-based precursor carbon nano-tube having a non-conductive carbon-based film to forming an said range temperature the required thermal treatment such that the relative low temperature. insulating substrate. At a temperature this lower said carbon-based precursor by heat treatment of a coke very little effect on changing system using 2-position said result without affecting, nose woman said thin surface containing oxygen having the uniform thickness a non-conductive carbon-based film can be formed. In the embodiment hereinafter in. supported by the specific of the present invention. Just, described hereinafter, exemplified by a in the embodiment specifically for the present invention are or account for which purpose: to avoid a, . is not but the present invention thereby is limited. Furthermore, content not described herein is a classic mirror server in the art sufficiently, to give technically deducing the graphitic surface can be ., which does not require a an annealing process is the explanation. [In the embodiment] (For producing cathode active material for) In the embodiment 1: for producing cathode active material for 2M of nickel sulfate aqueous solution (NiSO4, 6 (H2 O), Aldrich yarn), cobalt sulfate aqueous solution 2M (CoSO4, 7 (H2 O), Aldrich yarn), and aqueous solution of manganese sulfate 2M (MnSO4, x (H2 O), Aldrich yarn) each shown. After, said nickel sulfate aqueous solution, said cobalt sulfate aqueous solution and manganese sulfate aqueous solution included in said nickel, cobalt and manganese and wherein the molar ratio of the 6:1 each: said nickel sulfate aqueous solution is 3, said cobalt sulfate aqueous solution and said manganese sulfate aqueous solution by mixing mix solution which is then shown. 2M of said mix solution which is then Na2 CO3 made at speeds in the order of 3 ml/min with aqueous solution of 0.2M NH4 OH the 4L solution pH 8 10 while maintaining a time to filter out was obtained from precipitations. Said precipitate with water and, after washing the Li drying: Ni:Co: 1.0 and wherein the molar ratio of the Mn: 0.6 : 0.1: is 0.3 Li2 CO3 (Aldrich yarn) and after mixing in the to the standby unit, 900 °C 6 by a heat processing process layer and a lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core obtained. Next, said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core 100 parts by weight, and carbon-based as precursors (DN) 0.02 [...] 2, 3-0.5 parts by weight of acetone as solvent after mixing, stirring and drying mixture are obtained. Said mixture N2 number 1 time 2 in 300 °C atmosphere, a silicon, boron, titanium [...] between said core and dehydration reaction during the formation of a 2, 3-after is performed, number 2 600 °C 600 °C 2 time at elevated temperatures by the heat treatment in said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) containing oxygen core a non-conductive carbon-based film been produced with at an anode formed active material. In the embodiment 2: for producing cathode active material for Said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core 100 parts by weight, carbon-based as precursors [...] (DN) 0.02 parts by weight 2, 3-0.5 parts by weight acetone as solvent and using instead of, said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core 100 parts by weight, carbon-based as precursors (DN) 0.04 parts by weight and solvent [...] 2, 3-0.5 parts by weight of acetone as except used in the range of, the same method in the embodiment 1 comprises an anode active material have been prepared. In the embodiment 3: for producing cathode active material for Said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core 100 parts by weight, carbon-based as precursors [...] (DN) 0.02 parts by weight 2, 3-0.5 parts by weight acetone as solvent and using instead of, said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core 100 parts by weight, carbon-based as precursors (DN) 0.1 parts by weight and solvent [...] 2, 3-0.5 parts by weight of acetone as except used in the range of, the same method in the embodiment 1 comprises an anode active material have been prepared. In the embodiment 4: for producing cathode active material for Said in the embodiment 1 obtained at the lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core 100 parts by weight, and carbon-based as precursors (DN) 0.04 [...] 2, 3-0.5 parts by weight of acetone as solvent after mixing, stirring and drying mixture are obtained. Said mixture N2 number 1 time 2 in 300 °C atmosphere, a silicon, boron, titanium [...] between said core and dehydration reaction during the formation of a 2, 3-after is performed, number 2 2 500 °C 500 °C time at elevated temperatures by the heat treatment in said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) containing oxygen core a non-conductive carbon-based film been produced with at an anode formed active material. In the embodiment 5: for producing cathode active material for Said in the embodiment 1 obtained at the lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core 100 parts by weight, and carbon-based as precursors (DN) 0.04 [...] 2, 3-0.5 parts by weight of acetone as solvent after mixing, stirring and drying mixture are obtained. Said mixture N2 number 1 time 2 in 300 °C atmosphere, a silicon, boron, titanium [...] between said core and dehydration reaction during the formation of a 2, 3-after is performed, number 2 2 700 °C 700 °C time at elevated temperatures by the heat treatment in said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) containing oxygen core a non-conductive carbon-based film been produced with at an anode formed active material. In the embodiment 6: for producing cathode active material for Said in the embodiment 1 obtained at the lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core 100 parts by weight, and carbon-based as precursors (DN) 0.04 [...] 2, 3-0.5 parts by weight of acetone as solvent after mixing, stirring and drying mixture are obtained. Said mixture N2 number 1 time 2 in 300 °C atmosphere, a silicon, boron, titanium [...] between said core and dehydration reaction during the formation of a 2, 3-after is performed, time at elevated temperatures 800 °C number 2 2 800 °C by the heat treatment in said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) containing oxygen core a non-conductive carbon-based film been produced with at an anode formed active material. In the embodiment 7: for producing cathode active material for 2M of nickel sulfate aqueous solution (NiSO4, 6 (H2 O), Aldrich yarn), cobalt sulfate aqueous solution 2M (CoSO4, 7 (H2 O), Aldrich yarn), and aqueous solution of manganese sulfate 2M (MnSO4, x (H2 O), Aldrich yarn) each shown. After, said nickel sulfate aqueous solution, said cobalt sulfate aqueous solution and manganese sulfate aqueous solution included in said nickel, cobalt and manganese 0.25 each and wherein the molar ratio of the: 0.1 : 0.65 said nickel sulfate aqueous solution so that, said cobalt sulfate aqueous solution and said manganese sulfate aqueous solution by mixing mix solution which is then shown. 2M of said mix solution which is then Na2 CO3 made at speeds in the order of 3 ml/min with aqueous solution of 0.2M NH4 OH the 4L solution while maintaining the pH 14 10 a time to filter out was obtained from precipitations. Said precipitate with water and, after washing the Li drying: Ni:Co: 1.4 and wherein the molar ratio of the Mn: 0.25 : 0.1: so that 0.65 Li2 CO3 (Aldrich yarn) and after mixing in the to the standby unit, 700 °C 6 by a heat processing process layer and a lithium metal oxide (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) core obtained. Next, said lithium metal oxide (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) core 100 parts by weight, and carbon-based as precursors (DN) 0.01 [...] 2, 3-0.5 parts by weight of acetone as solvent after mixing, stirring and drying mixture are obtained. Said mixture N2 number 1 time 2 in 300 °C atmosphere, a silicon, boron, titanium [...] between said core and dehydration reaction during the formation of a 2, 3-after is performed, number 2 2 500 °C 500 °C time at elevated temperatures by the heat treatment in said lithium metal oxide (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) containing oxygen core a non-conductive carbon-based film been produced with at an anode formed active material. In the embodiment 8: for producing cathode active material for Lithium metal oxide obtained at the in the embodiment 7 (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) core 100 parts by weight, carbon-based as precursors [...] (DN) 0.05 parts by weight 2, 3-0.5 parts by weight of acetone as and solvent after mixing, stirring and drying mixture are obtained. Said mixture N2 number 1 time 2 in 300 °C atmosphere, a silicon, boron, titanium [...] between said core and dehydration reaction during the formation of a 2, 3-after is performed, number 2 2 500 °C 500 °C time at elevated temperatures by the heat treatment in said lithium metal oxide (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) containing oxygen core a non-conductive carbon-based film been produced with at an anode formed active material. In the embodiment 9: for producing cathode active material for Lithium metal oxide obtained at the in the embodiment 7 (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) core 100 parts by weight, and carbon-based as precursors (DN) 0.2 [...] 2, 3-0.5 parts by weight of acetone as solvent after mixing, stirring and drying mixture are obtained. Said mixture N2 number 1 time 2 in 300 °C atmosphere, a silicon, boron, titanium [...] between said core and dehydration reaction during the formation of a 2, 3-after is performed, number 2 2 500 °C 500 °C time at elevated temperatures by the heat treatment in said lithium metal oxide (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) containing oxygen core a non-conductive carbon-based film been produced with at an anode formed active material. In the embodiment 10: for producing cathode active material for Lithium metal oxide obtained at the in the embodiment 7 (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) core 100 parts by weight, and carbon-based as precursors (DN) 0.2 [...] 2, 3-0.5 parts by weight of acetone as solvent after mixing, stirring and drying mixture are obtained. Said mixture N2 number 1 time 2 in 300 °C atmosphere, a silicon, boron, titanium [...] between said core and dehydration reaction during the formation of a 2, 3-after is performed, 550 °C 2 550 °C number 2 time at elevated temperatures by the heat treatment in said lithium metal oxide (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) containing oxygen core a non-conductive carbon-based film been produced with at an anode formed active material. In the embodiment 11: for producing cathode active material for Lithium metal oxide obtained at the in the embodiment 7 (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) core 100 parts by weight, and carbon-based as precursors (DN) 0.2 [...] 2, 3-0.5 parts by weight of acetone as solvent after mixing, stirring and drying mixture are obtained. Said mixture N2 number 1 time 2 in 300 °C atmosphere, a silicon, boron, titanium [...] between said core and dehydration reaction during the formation of a 2, 3-after is performed, time at elevated temperatures by number 2 2 450 °C silicon, boron, titanium 450 °C in said lithium metal oxide (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) containing oxygen core a non-conductive carbon-based film been produced with at an anode formed active material. In the embodiment 12: for producing cathode active material for Lithium metal oxide obtained at the in the embodiment 7 (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) core 100 parts by weight, and carbon-based as precursors (DN) 0.2 [...] 2, 3-0.5 parts by weight of acetone as solvent after mixing, stirring and drying mixture are obtained. Said mixture N2 number 1 time 2 in 300 °C atmosphere, a silicon, boron, titanium [...] between said core and dehydration reaction during the formation of a 2, 3-after is performed, number 2 2 400 °C 400 °C time at elevated temperatures by the heat treatment in said lithium metal oxide (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) containing oxygen core a non-conductive carbon-based film been produced with at an anode formed active material. In the embodiment 13: for producing cathode active material for Polypropylene beaker been with 200 ml of distilled water to, manganese sulfide (II) hydrate (MnSO4, H2 O) 1.69g pyrites and (II) cargo seven (FeSO4, 7H2 O) 2.78g is added and stirring aqueous solution number 1 manganese containing-is shown. Distilled to separate polypropylene of been with 250 ml beaker, manganese sulfide (II) (II) the total cargo seven moles pyrites, and an Image (0.02mole) to sodium hydroxide is 0.3mol 12g has added. Manganese sulfide then the total number of moles of the cargo seven pyrites, and an Image (II) (II) (0.02mole) to concentration is 0.04mol 4.87g of ammonium hydroxide 28% sodium hydroxide by and stirring is added ammonium hydroxide (NaOH) (NH4 OH) for completely dissolving the aqueous solution a number 2 shown. Aqueous solution with stirring, in said number 2, herein said number 1 by the loading of the aqueous solution a precipitate is resulting slurry with the are obtained. Furthermore, a washing of said it is under, distilled after filtering the slurry, in 100 °C 24 are obtained precipitate is drying time. Said precipitate composition of ICP (high frequency inductively coupled plasma) is analyzed by the emitting analysis, Fe: and wherein the molar ratio of the Mn was 0.5:0.5. After, and precipitates said molar ratio of sodium carbonate is Fe:Na=0.5: after weighing the is 0.67, dry blending using manometer mixture are obtained. Furthermore, said paste has better mouth feeling and calcination vessel of alumina mixture, 850 °C controlled using electric furnace 12 to baking after the period of time, by cooling a room Na2/3 Fe0.5 Mn0.5 O2 metal oxide core obtained. Next, said Na2/3 Fe0.5 Mn0.5 O2 metal oxide core 100 parts by weight, and carbon-based as precursors (DN) 0.2 [...] 2, 3-0.5 parts by weight of acetone as solvent after mixing, stirring and drying mixture are obtained. Said mixture N2 number 1 time 2 in 300 °C atmosphere, a silicon, boron, titanium [...] between said core and dehydration reaction during the formation of a 2, 3-after is performed, by number 2 600 °C 600 °C 2 time at elevated temperatures in said silicon, boron, titanium Na2/3 Fe0.5 Mn0.5 O2 metal oxide containing oxygen core a non-conductive carbon-based film been produced with at an anode formed active material. Compared e.g. 1: for producing cathode active material for 2M of nickel sulfate aqueous solution (NiSO4, 6 (H2 O), Aldrich yarn), cobalt sulfate aqueous solution 2M (CoSO4, 7 (H2 O), Aldrich yarn), and aqueous solution of manganese sulfate 2M (MnSO4, x (H2 O), Aldrich yarn) each shown. After, said nickel sulfate aqueous solution, said cobalt sulfate aqueous solution and manganese sulfate aqueous solution included in said nickel, cobalt and manganese and wherein the molar ratio of the 6:1 each: said nickel sulfate aqueous solution is 3, said cobalt sulfate aqueous solution and said manganese sulfate aqueous solution by mixing mix solution which is then shown. 2M of said mix solution which is then Na2 CO3 made at speeds in the order of 3 ml/min with aqueous solution of 0.2M NH4 OH the 4L solution pH 8 10 while maintaining a time to filter out was obtained from precipitations. Said precipitate with water and, after washing the Li drying: Ni:Co: 1.0 and wherein the molar ratio of the Mn: 0.6 : 0.1: is 0.3 Li2 CO3 (Aldrich yarn) and after mixing in the to the standby unit, 900 °C 6 by a heat processing process layer and a lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) have been prepared is a positive electrode active material for. Compared e.g. 2: for producing cathode active material for Said lithium metal oxide (LiNi0.6 Co1.0 Mn0.3 O2) core 100 parts by weight, carbon-based as precursors [...] (DN) 0.02 parts by weight 2, 3-0.5 parts by weight acetone as solvent and using instead of, said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core 100 parts by weight, carbon-based as precursors (DN) 0.2 parts by weight and solvent [...] 2, 3-0.5 parts by weight of acetone as except used in the range of, the same method in the embodiment 1 comprises an anode active material have been prepared. Compared e.g. 3: for producing cathode active material for Said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core 100 parts by weight, carbon-based as precursors [...] (DN) 0.02 parts by weight 2, 3-0.5 parts by weight acetone as solvent and using instead of, said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core 100 parts by weight, carbon-based as precursors (DN) 0.4 parts by weight and solvent [...] 2, 3-0.5 parts by weight of acetone as except used in the range of, the same method in the embodiment 1 comprises an anode active material have been prepared. Compared e.g. 4: for producing cathode active material for Said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core 100 parts by weight, carbon-based as precursors [...] (DN) 0.02 parts by weight 2, 3-0.5 parts by weight acetone as solvent and using instead of, said lithium metal oxide (LiNi0.6 Co0.1 Mn0.3 O2) core 100 parts by weight, carbon-based as precursors (DN) 1.0 parts by weight and solvent [...] 2, 3-0.5 parts by weight of acetone as except used in the range of, the same method in the embodiment 1 comprises an anode active material have been prepared. Compared e.g. 5: for producing cathode active material for 2M of nickel sulfate aqueous solution (NiSO4, 6 (H2 O), Aldrich yarn), cobalt sulfate aqueous solution 2M (CoSO4, 7 (H2 O), Aldrich yarn), and aqueous solution of manganese sulfate 2M (MnSO4, x (H2 O), Aldrich yarn) each shown. After, said nickel sulfate aqueous solution, said cobalt sulfate aqueous solution and manganese sulfate aqueous solution included in said nickel, cobalt and manganese 0.25 each and wherein the molar ratio of the: 0.1 : 0.65 said nickel sulfate aqueous solution so that, said cobalt sulfate aqueous solution and said manganese sulfate aqueous solution by mixing mix solution which is then shown. 2M of said mix solution which is then Na2 CO3 made at speeds in the order of 3 ml/min with aqueous solution of 0.2M NH4 OH the 4L solution pH 8 10 while maintaining a time to filter out was obtained from precipitations. Said precipitate with water and, after washing the Li drying: Ni:Co: 1.4 and wherein the molar ratio of the Mn: 0.25 : 0.1: so that 0.65 Li2 CO3 (Aldrich yarn) and after mixing in the to the standby unit, 900 °C 6 by a heat processing process layer and a lithium metal oxide (Li1.4 Ni0.25 Co0.1 Mn0.65 O2) have been prepared is a positive electrode active material for. Compared e.g. 6: for producing cathode active material for Polypropylene beaker been with 200 ml of distilled water to, manganese sulfide (II) hydrate (MnSO4, H2 O) 1.69g pyrites and (II) cargo seven (FeSO4, 7H2 O) 2.78g is added and stirring aqueous solution number 1 manganese containing-is shown. Distilled to separate polypropylene of been with 250 ml beaker, manganese sulfide (II) (II) the total cargo seven moles pyrites, and an Image (0.02mole) to sodium hydroxide is 0.3mol 12g has added. Manganese sulfide then the total number of moles of the cargo seven pyrites, and an Image (II) (II) (0.02mole) to concentration is 0.04mol 4.87g of ammonium hydroxide 28% sodium hydroxide by and stirring is added ammonium hydroxide (NaOH) (NH4 OH) for completely dissolving the aqueous solution a number 2 shown. Aqueous solution with stirring, in said number 2, herein said number 1 by the loading of the aqueous solution a precipitate is resulting slurry with the are obtained. Furthermore, a washing of said it is under, distilled after filtering the slurry, in 100 °C 24 are obtained precipitate is drying time. Said precipitate composition of ICP (high frequency inductively coupled plasma) is analyzed by the emitting analysis, Fe: and wherein the molar ratio of the Mn was 0.5:0.5. After, and precipitates said molar ratio of sodium carbonate is Fe:Na=0.5: after weighing the is 0.67, dry blending using manometer mixture are obtained. Furthermore, said paste has better mouth feeling and calcination vessel of alumina mixture, 850 °C controlled using electric furnace 12 to baking after the period of time, by cooling a room Na2/3 Fe0.5 Mn0.5 O2 have been prepared is a positive electrode active material for metal oxide. Said in the embodiment 1 to 13 and comparison e.g. 1 to 6 to an optical disc apparatus regularizes is a positive electrode active material for a lower table 1 as: (For manufacturing lithium battery) In the embodiment 14: lithium halffor preventing static electricity in semiconductor devices In the embodiment 1 (Super P) conductive material and carbon powder a positive electrode active material for mixed the mixture after 5 weight % of PVDF (polyvinylidene fluoride) coupled by the addition of a number (SOLEF 5130) including pyrrolidone solution anode active material: carbon conductive material: number = 90:6 coupled: the slurry typically ratio weight of 4 have been prepared. 15 micro m thickness of aluminum foil on said slurry as barcode 40-50 micro m thickness coating the drying the, further of vacuum in conditions 120 °C been produced with at anode plate which is dried to once again. Said anode plate which press roll (roll press) of coins sheet form rolled to been produced with at anode cell. At this time, the capacitance anode was 2.0mAh/cm2. Said anode of coins 12 mm diameter using lithium-half cells (CR2032 type) manufacturing processes and the cost of production. Opposite-pole method for manufacturing metallic lithium jet stream by using a carrier gas (counter electrode), electrolyte include EC (ethylene carbonate) : ([...]) DEC: FEC (fluoro [...]) (2:6:2 volume ratio) acid in a mixed solvent of 1.1M LiPF6 and 0.2M LiBF4, the third to eo of fused lithium salt. 15-25in the embodiment: lithium halffor preventing static electricity in semiconductor devices In the embodiment 1 in the embodiment 2 to 12 instead powder a positive electrode active material for a positive electrode active material for a respectively using powder except for the host supplying, the same method in the embodiment 14 in coin type lithium half cells (CR2032 type) manufacturing processes and the cost of production. 7-11e.g. compared: lithium halffor preventing static electricity in semiconductor devices In the embodiment 1 5 to 1 e.g. compared instead a positive electrode active material for each is a positive electrode active material for except used in the range of, the same method in the embodiment 14 in coin type lithium half cells (CR2032 type) manufacturing processes and the cost of production. (Sodium battery for manufacturing) In the embodiment 26: sodium halffor preventing static electricity in semiconductor devices In the embodiment 13 (Super P) conductive material and carbon powder a positive electrode active material for mixed the binder (PVdF) polyvinylidene fluoride-mixture after 60:20:20 N-methylpyrrolidone (NMP) at a weight ratio of the slurry in caused manometer with have been prepared. 15 micro m thickness of aluminum current collector on targeting barcode said slurry (bar coating) at room temperature and vacuum drying the, unit moves simultaneously at the same distance 120 °C once again dry, rolling and punching a micro m the 55 been produced with at thickness of anode plate. Said anode plate which press roll (roll press) of coins sheet form rolled to been produced with at anode cell. Said anode 12 mm diameter using (CR2032 type) cells half sodium coin type of manufacturing processes and the cost of production. Method for manufacturing the opposite-pole (conter electrode) at the n bit parallel data inputted using sodium foil (foil), glass fiber (glass fiber) isolation film (Whatman GF/F CAT No. 1825-150) and electrolyte include 1.0M NaPF6 is propylene carbonate (PC) was using a solution is easy to administrate the. 12e.g. compared: sodium halffor preventing static electricity in semiconductor devices In the embodiment 13 6 e.g. compared instead a positive electrode active material for a positive electrode active material for except used in the range of, the same method in the embodiment 26 sodium coin-shaped in half cells (CR2032 type) manufacturing processes and the cost of production. (Analyzing surface anode active material) Analysis e.g. 1: scanning electron microscope (SEM)and transmission electron microscope (TEM)electrophotographic Made in said in the embodiment 1 and comparison 1, 2 e.g. anode active material to the surface scanning electron microscope (SEM) has been observed on using. Result to the computer of the also showed also to 2a 2c respectively. Also refers to surface 2c also to 2a, 2a also made in 2b also on the surface of the support, the positive electrode active material contains cyclic or made in high resolution or infected by a computer virus, difficult to uniform and thin non-conductive carbon-based film is formed on the side face may confirm it. However also 2c made in the cyclic or non-conductive carbon-based film is formed larger in thickness cover a portion of the of the core may confirm it is. In addition said in the embodiment 1, 9 and comparison e.g. made in 5 anode active material to the surface resolution transmission electron microscopy has been observed on using (HR-TEM). Result to the computer of the also 3, also showed to 4b and 4a. With a 3 also, in the embodiment 1 obtained anode active made in lithium metal oxide core a plied construction of a proper system for a may confirm it is. Anode active made in said in the embodiment 1 is the vision obtained conductive carbon-based film has a thickness of from about 0.7 nm thin thickness to uniformly provided an opening.. Also refers to surface 4a, in the embodiment 9 obtained anode active made in lithium metal oxide core also layered structure a proper system for a may confirm it is. Anode active made in said in the embodiment 9 is the vision conductive carbon-based film obtained time, the flowing speed of the about 3.0 nm thin thickness to uniformly provided an opening.. Also refers to surface 4b, compared e.g. made in 5 the positive electrode active material contains conductive anionic compound layer surface carbon-based film is formed on the side face. not. Analysis e.g. 2: X ray photoelectron spectroscopy (XPS)and element analyzing (ElementAnalyzer; EA) Metal plate, the plate for attaching a double-sided tape, said in the embodiment 1 to 3 and comparison 1, 2 e.g. thereon made in said positive electrode active material the sample to be said each samples with no visible double-sided tape after a thickness that the root, the surface and crush the folders the. Measuring for bug Al-Kα line at monochromator (1486.6 eV, 27.7 W, varieties diameter and 0.1 mm at area measuring conditions at X ray photoelectron spectroscopy to 45 degrees (X-ray photoelectron spectroscopy, XPS, Physical Electronics, Quantum 2000 Scanning ESCAMicroprobe) using interior angle O1s was is obtained spectrum in level. Result to the computer of the also showed to 5. Also refers to surface 5, in the embodiment 1 to 3 and comparison e.g. made in 2 anode active material surface-C (=O) OH and-C=O group carbon compounds were present and they in the embodiment 1, in the embodiment 2, in the embodiment 3 and comparison e.g. made in 2 order of positive electrode active material for-C=O and C (=O) OH content of carbon group is sprayed can be confirm that the user. While said in the embodiment 1 to 3 and comparison 1, 2 e.g. on the surface of the anode active material made in a non-conductive carbon-based film to present a carbon content of Element analyzer (Thermo Scientific yarn product, consumers can selectively get: FLASH 2000) it was determined that using. Table 2 showed to result to the computer of the a. At this time, peak intensity in 530eV to 528eV binding energy of a nickel oxide, cobalt oxide, or manganese oxide is present meaning, 533eV to 531eV C (=O) OH-peak intensity in binding energy of-C=O and carbon group. is present compounds. A peak intensity of "counts per second" "cps" unit. mixture by the addition of an initiator. Said table 2 refers to surface, conductive carbon-based film of the refrigerator surface anode active material present a carbon content of 1 e.g. this comparison, in the embodiment 1, in the embodiment 2, in the embodiment 3 and comparison example 2 of positive electrode active material for of sprayed order can be confirm that the user. 1 e.g. compared this, in the embodiment 1, in the embodiment 2, in the embodiment 3 and comparison example 2 obtained a positive electrode active material in a lithium metal oxide core 100 parts by weight each carbon-based precursor content of 0, 0.02, 0.04, 0.1, 0.2 and sprayed parts by weight by said core at a surface non-conductive carbon film content 1 e.g. the comparison of the formed thereon a plurality of holes for, in the embodiment 1, in the embodiment 2, in the embodiment 3 and comparison e.g. sprayed in order of 2 a matches a result. In the embodiment 1, in the embodiment 2, in the embodiment 3 is a positive electrode active material for formula 1 in accordance with the condition of a 55.80 parts by weight if each-1, 25.06 parts by weight-1, and 10.77 parts by weight-1 10 parts by weight as-1 formula 1 are rate corresponding to meet but, compared e.g. the positive electrode active material contains 3 of formula 1 in accordance with the condition of a if 4.50 parts by weight-1 10 parts by weight as-1 less than are corresponding to formula 1 is satisfied can be not confirm that the user. [Type 1] [(IA/IB)/ D] ≥ 10 parts by weight-1 In addition said in the embodiment 7 to 9 and comparison e.g. positive electrode active material sample to be made in 5 is identical to the previous input data X ray photoelectron spectroscopy in level interior angle C1s marker for the diagnosis of gastric cancer in level interior angle O1s multispectral and was are obtained spectrum. Result to the computer of the table 3, also 7a, and 7b showed to. Table 3 and 7a with a, binding energy of 285eV to 282eV to object in graphics (C-C bond) bond C-C strength exhibits on, a peak intensity in binding energy of 293eV to 288eV Li2 CO3 exhibits phase. Therefrom, said in the embodiment 7 to 9 anode active material surface made in Li2 CO3 component the inclusion which is able to confirm that, ID/IC each 0.77, 0.66, and then is 0.72 the heterologous ceft gene is at least 0.3.. Also refers to surface 7b, compared e.g. made in 5 anode active material surface-C (=O) OH and-C=O group carbon compound move up and down in the embodiment 7 to 9 anode active material made in surface-C (=O) OH and-C=O group carbon compound. of the presence of an. In the embodiment 7 in the embodiment 9 to made in order of positive electrode active material for-C=O and C (=O) OH content of carbon group is sprayed can be confirm that the user. Analysis e.g. 3: EDS (EnergyDispersiveSpectroscopy)analysis In the embodiment 3 and comparison e.g. made in 1 for the surface of the anode active material analysis is performed for all the EDS (Energy Dispersive Spectroscopy). Result to the computer of the also showed to 8b and 8a. Also refers to surface 8a and 8b, in the embodiment 3 made in the positive electrode active material contains lithium metal oxide core from toward the surface of the layer indicating, are reduced and the content of oxygen of wet liquid to flow down. While, compared e.g. made in 1 the positive electrode active material contains carbon is still. Thereby, the positive electrode active material contains made in said in the embodiment 3 1 2 the first connection member and said core surface through a heat treating process difference of the contamination material, is reduced an ultrasonic signal from the ultrasonic sensor, by oxygen to carbon sulfonyl valine non-conductive carbon-based film is formed on the side face is can be viewed. (A method for estimating the characteristic cell) Evaluation e.g. 1: lithium cell initial efficiency, a method for estimating the characteristic conductivity, capacity, and lifetime In the embodiment 15 and comparison e.g. a coin-shaped lithium half 7 made in room temperature with the target cell (25 °C) 0.05C in, until it reaches a charging 4.4V to the embodiment. Then (cut-off voltage) a cut-off voltage of 2.5V to 0.05C, until it reaches a constant current discharge is performed for all the. The charge capacity, and discharge capacity (1st left-discharge capacity) measuring the n bit parallel data inputted, initial efficiency therefrom (1st cycle outputs the electric potential according to the charged 1st left-discharge capacity ratio) of the bill. Next, each 0.5 C respect to said cells in the form charging on such as a 0.5 C to 2.5 V, until it reaches a discharge is performed for all the. The full charge capacity and the n bit parallel data inputted measuring discharge capacity, insect discharge efficiency therefrom (each cycle charged outputs the electric potential according to the discharge capacity ratio) of the bill. The charging and discharging stored and then repeatedly output in three 102nd the system using the discharge capacity left-said in the embodiment 15 7 e.g. and comparison made in a coin-shaped lithium half cell was assessed life characteristics. Life characteristics enabled to operate close to the loop from 1 expressions calculates a capacity retention (%) was obtained. Result to the computer of the a table showed to 9c also to 9a and 4. [Expressions 1] Capacity retention (capacity retention, %)= [(102nd discharge capacity left-/ 3rd left-discharge capacity)] x 100 In addition in the embodiment 15 and comparison e.g. a coin-shaped lithium half 7 made in room temperature with the target cell (25 °C) 0.05C in, until it reaches a charging 4.45V to the embodiment. Then (cut-off voltage) a cut-off voltage of 2.5V to 0.05C, until it reaches a constant current from the controller and, in the same manner as said 1st left-charge capacity, 1st left-discharge capacity from the n bit parallel data inputted calculates a operational efficiency, discharge capacity from charge capacity, and left-after insect discharge efficiency the bill. The charging and discharging stored and then repeatedly output in three 102nd the system using the discharge capacity left -1 (%) capacity retention from said expressions for the bill. Result to the computer of the a table showed to 9c also to 9a and 4. In addition in the embodiment 15 and comparison e.g. a coin-shaped lithium half 7 made in room temperature with the target cell (25 °C) 0.05C in charging, until it reaches a 4.5V to the embodiment. Then (cut-off voltage) a cut-off voltage of 2.5V to 0.05C, until it reaches a constant current from the controller and, in the same manner as said 1st left-charge capacity, 1st left-discharge capacity from the n bit parallel data inputted calculates a operational efficiency, discharge capacity from charge capacity, and left-after insect discharge efficiency the bill. The charging and discharging stored and then repeatedly output in three 102nd the system using the discharge capacity left -1 (%) capacity retention from said expressions for the bill. Result to the computer of the a table showed to 9c also to 9a and 4. Said table 4 and 9a to 9c also refers to surface, a cut-off voltage (V) is 4.4V -2.5V, 4.45V -2.5V, and 4.5V -2.5V in the embodiment 15 each made in a coin-shaped lithium half cell 1st left-discharge capacity, initial insect discharge efficiency left-and overall efficiency, capacity retention this comparison e.g. made in a coin-shaped lithium half cell 7 1st left-discharge capacity, initial efficiency, and overall left- insect discharge efficiency , capacity retention and was to the melt. In addition a cut-off voltage (V) if the high voltage 4.5V -2.5V in the embodiment 15 is made in a coin-shaped lithium half cell 7 e.g. capacity retention this comparison made in a coin-shaped lithium half cell optionally it comprises capacity retention compared to. While, in the embodiment 14 to 19 and comparison e.g. 7 to 10 made in a coin-shaped lithium half cell 0.05C 4.4V to function at a normal temperature by charging, until it reaches a the embodiment. Then (cut-off voltage) a cut-off voltage of 2.5V to 0.05C, until it reaches a constant current discharge is performed for all the. The discharge capacity (1st left-discharge capacity) it was determined that for. Next, each 0.5 C respect to said cells in the form charging on such as a 0.5C to 2.5 V, until it reaches a discharge is performed for all the. The discharging capacity of an measuring the n bit parallel data inputted, the charging and discharging stored and then repeatedly output in three 102nd the system using the discharge capacity left-said in the embodiment 14 to 19 and comparison e.g. of coins lithium-half cell 7 to 10 was assessed life characteristics. The life characteristics (%) capacity retention from said expressions path for a vehicle 1 was obtained. Result to the computer of the to table 5, table 6, also 10a, and 10b showed to. Said table refers to surface 10a and 5, in the embodiment 14 to 16 made in a coin-shaped lithium half and cuts out a compared e.g. made in 10 to 8 connected to a coin-shaped lithium half life efficiency and initial discharge capacity. I.e., 100 parts by weight lithium metal oxide core non-conductive carbon-based film present a carbon content of 0.1 parts by weight active material of the positive electrode hereinafter including coin type lithium half the discharge capacity and capacity retention the lithium metal oxide core 100 parts by weight non-conductive carbon-based film present a carbon content of higher than 0.1 parts by weight positive electrode active material including coin type lithium half cell efficiency and initial discharge capacity to the melt can be confirm that the user. Compared e.g. 8 to 9 reasons is excellent in capacity retention of vision thick lithium ion due to conductive carbon-based film is reduced speed a charge rate of a battery during expressible capacitances, are reduced in capacity retention is higher than. provided to appear. Said table refers to surface 10b and 6, in the embodiment 15, 17 to 19 made in a coin-shaped lithium half 7 e.g. compared and cuts out a made in a coin-shaped lithium half connected to discharging capacity and capacity retention was is excellent in. In addition lithium metal oxide core surface anionic compound layer for forming conductive carbon-based film number 2 heat treatment is in the range of 700 °C to 600 °C is time temperatures is a coin-shaped lithium half cell discharge capacity, initial efficiency and capacity retention was is better than a. Furthermore, in the embodiment 20 to 25 and comparison e.g. a coin-shaped lithium half 11 made in room temperature with the target cell (25 °C) 0.05C in charging, until it reaches a 4.5V to the embodiment. Then (cut-off voltage) a cut-off voltage of 2.0V to 0.05C, until it reaches a constant current discharge is performed for all the. The charge capacity, and discharge capacity (1st left-discharge capacity) it was determined that for. Next, said cells on each 1.0 C respect to charging in the form such as a 1.0 C to 2.0 V, until it reaches a discharge is performed for all the. The charging and discharging stored and then repeatedly output in three 11 in the embodiment 22 and comparison e.g. made in a coin-shaped lithium half with the target cell 9th left-differential capacity and, in the embodiment 20 to 25 and comparison e.g. 11 made in a coin-shaped lithium half with the target cell 50th cycle up to delta voltage (ΔV) respectively corresponding to one tooth and discharging capacity of an was assessed for by partially sintering. From said life characteristics enabled to operate close to the loop 2 expressions calculates a capacity retention (%) was obtained. Result to the computer of the also 11, also 12a, 12b also, also 13a, 13b also, and table 7 showed to. [Expressions 2] Capacity retention (capacity retention, %)= [(50th discharge capacity left-/ 1st left-discharge capacity)] x 100 Also with a 11, 11 in the embodiment 22 and comparison e.g. made in a coin-shaped lithium half cell in the vicinity 3.1V Li2 MnO3 discharge capacity exhibits a peak of wet liquid to flow down (an elliptical-like, dotted lines display portion). Said Li2 MnO3 from peak discharge capacity in the embodiment 22 made in a coin-shaped lithium half 11 e.g. compared and cuts out a made in a coin-shaped lithium half connected to Li2 MnO3 discharge enhanced capacitance to have undergone an aspect.. Said table 7, also 12a, and 12b refers to surface, in the embodiment 20 to 25 made in a coin-shaped lithium half cell delta voltage (ΔV) 11 e.g. this comparison made in a coin-shaped lithium half cell compared to low delta voltage (ΔV). Is selected from them, number 2 500 °C or more temperature heat treatment in the embodiment 22 and 23 made in a coin-shaped lithium half cell delta voltage (ΔV) is low. Said table 7, also 13a, and 13b refers to surface, in the embodiment 20 to 24 made in a coin-shaped lithium half cell 11 e.g. capacity retention this comparison made in a coin-shaped lithium half cell was is high in comparison with the thermal capacity retention. Is selected from them, number 2 500 °C or more temperature heat treatment in the embodiment 22 and 23 made in a coin-shaped lithium half cell was higher in throughput capacity retention. Evaluation e.g. 2: a method for estimating the characteristic life capacity and sodium battery In the embodiment 26 and comparison made in a coin-shaped sodium half 12 with the target cell room temperature (25 °C) voltage in a range of voltages 2.0V-4.2V contrast metallic sodium in electrostatic 0.1C 1 to encapsulate visitor is checked through a charge/discharge times. The charge capacity, and discharge capacity (1st left-discharge capacity) it was determined that for. After that, stored and then repeatedly output in three charging and discharging such as 60th cycle system using the discharge capacity up to the n bit parallel data inputted, the 50th left-discharge capacity based on said in the embodiment 26 and comparison e.g. 12 made in a coin-shaped sodium half cell was assessed life characteristics. The life characteristics from said expressions 2 calculates a capacity retention (%) was obtained. Result to the computer of the a table showed to 9-10 and 8. Said table with a 9-10 and 8, made in a coin-shaped sodium half cell in the embodiment 26 1st capacity retention discharging capacity and left -12 e.g. this comparison made in a coin-shaped sodium half cell 1st left-discharging capacity and capacity retention was to the melt. A preferred embodiment of the present invention to a gas injector through a but described focuses, at limited to the present invention refers to claim and a and appends detailed description of the invention variously within range of the drawings it is possible embodiment the hole to which the disc spin speed value within a range. of course in addition of the present invention. 1: lithium ion or sodium ion occlusion a larger specific surface area than the metal oxide core, 2: oxygen deficient layer, 3:a non-conductive carbon-based film containing oxygen, 10: anode active material, 100: secondary battery, 112: cathode, 113: separator, 114: anode, 120: cell container, 140: sealing member In the present invention, provided are a positive electrode active material, including a non-metallic oxide, metallic oxides or oxide cores of the combination thereof which absorb and discharges lithium ions or sodium ions; and non-conductive carbon based film including partly oxygen on the surfaces of the cores, a secondary battery including the same and a manufacturing method thereof. COPYRIGHT KIPO 2015 Lithium ion or sodium ion occlusion and is capable of releasing a metal oxide, base metal oxides, or combinations thereof of oxide core; and said core at least over part of its surface a non-conductive containing oxygen anode active material including carbon-based film. According to Claim 1, said non-conductive carbon-based film 1 or more-C (=O) Ra, -C (=O) ORa, -OC (=O) ORa carbon of functional group containing a compound, wherein Ra hydrogen, C1-C10 alkyl, or C6-C20 it is an allyl of anode active material. According to Claim 2, said carbon compound a substituted or unsubstituted C6-C30 aromatic compounds, a substituted or unsubstituted C5-C30 heterocyclic compound, or combinations thereof including compound of anode active material. According to Claim 1, are represented by formula 1 to said core performs one operation, said core containing oxygen at least over part of its surface a non-conductive carbon-based film anode active material including : [formula 1] Lia Mb O2-c (X1)c in formula 1, 0.8≤a≤1.2 and, 0 < b≤1 and, is 0≤c≤1, M the Mn, Ni, Co, cu, mg, Na, Ca, Ti, Zn, Ga, Ge, Al, Cr, mg, Sr, Mo, W, V, Ti, Zr, Ru, Rh, Pd, Os, Ir, Ag, Au, Hf, Sn and Pt selected from the group consisting of at least one of the metal species is 0.1-1, O the X1, F, S and 1 selected from the group consisting of P is at least one element. According to Claim 4, said core surface of X ray photoelectron spectroscopy (XPS) of the analysis (binding energy) binding energy of 530eV to 528eV spectrum O1s to object in graphics on peak intensity in binding energy of 533eV to 531eV (IA/IB), and said core present at the surface of the core 100 parts by weight said non-conductive carbon-based film (D) present a carbon content of 1 formula relationship between a positive electrode active material represented by : [type 1] [(IA/IB)/ D] ≥10 parts by weight-1 According to Claim 4, said non-conductive carbon-based film said core present a carbon content of 0.001 weight percent to 100 parts by weight 0.3 parts by anode active material. According to Claim 4, said non-conductive carbon-based and the thickness of the film 0.1 nm to 5.0 nm in anode active material. According to Claim 4, to core performs one operation represented by said formula 1 are represented by formula 2, said core containing oxygen at least over part of its surface a non-conductive carbon-based film anode active material including : [formula 2]q Li2 MnO3, (1-q) Li (M1) O2 in formula 2, 0 < q is < 1, the M1 Mn, Ni, Co, cu, mg, Na, Ca, Ti, Zn, Ga, Ge, Al, Cr, mg, Sr, Mo, W, V, Ti, Zr, Ru, Rh, Pd, Os, Ir, Ag, Au, Hf, Sn and Pt 1 selected from the group consisting of is at least one element. According to Claim 8, said non-conductive carbon-based film Li2 CO3 anode active material including further component. According to Claim 8, said non-conductive carbon film X ray photoelectron spectroscopy (XPS) of the analysis (binding energy) 285eV to 282eV C1s spectrum of binding energy peak (phase) on (C-C bond) bond C-C occurring in the on (binding energy) occurring in the binding energy of 293eV to 288eV Li2 CO3 on peak intensity non-(ID/IC) is 0.3 or more of the positive electrode active material. According to Claim 1, formula 3 is to said core performs one operation, said core containing oxygen at least over part of its surface a non-conductive carbon-based film anode active material including : [formula 3] Nax (Q) O2+e in formula 3, Q in terms 4 through group 12 as selected from the group consisting of at least one transition metal element 2 and, 0.5≤x≤1 and, is -0.3≤ e≤ 1. According to Claim 1, said core and said carbon-based non-conductive layer including oxygen vacancy and between the anode active material. According to Claim 12, said oxygen deficient layer is reduced, said core least a part of the surface of a layer formed of the positive electrode active material. Anode; electrolyte ; which and cathodes, including secondary battery in accordance with any one of Claims 1 to Claim 13 the anode is in said positive electrode active material. According to Claim 14, said operating potential is 4.4 V±0.1V of positive electrode active material for secondary battery. According to Claim 14, further including conductive material the anode is in said secondary battery.
Lithium ion or sodium ion occlusion and is capable of releasing a metal oxide, base metal oxides, or combinations thereof of oxide core, 1 or more hydroxy or carboxylic carbon nano-tube having-based precursor, and a solvent at least a part of the surface core said mixing said carbon-based precursor for the chemical by adsorbing the; and said adsorbed carbon-based precursor is formed containing oxygen by heat treatment of a core a non-conductive carbon-based for forming a polymer film on a step; of positive electrode active material for a manufacturing method including in accordance with any one of Claims 1 to Claim 13. According to Claim 17, said carbon-based precursors are a substituted or unsubstituted C6-C30 aromatic compounds, a substituted or unsubstituted C5-C30 heterocyclic compound, or combinations thereof of positive electrode active material for composition manufacturing method.
According to Claim 17, said carbon-based precursors are a substituted or unsubstituted benzene, of naphthalene a substituted or unsubstituted, a substituted or unsubstituted phenacy alkylene (phenalene), a substituted or unsubstituted phenanthrene, anthracene, triphenylene (triphenylene), pyrene, pherocene cro (chrysene), naphtha pherocene (naphthacene), ranges (picene), perylene (perylene), (pentaphene) pen hit pen , hexa pherocene (hexacene), pyridine, pyrazine, pyrimidine, pyridazine, quinoline, phthalamide hydrazine, quinoxaline, quinazoline, hour glow phosphorus , phenylphenanthridines, phenanthroline, or phenazinecarboxamide of the positive electrode active material manufacturing method.
According to Claim 17, said core at least over part of its surface for the chemical-based precursor carbon said step of adsorbing said hydroxyl groups of said core surface carbon-based precursor hydroxy or carboxyl a by dehydration into between an anode formed by chemical bonding manufacturing method active material.
According to Claim 20, a heat treatment by a heat treatment the dehydration reaction during the formation of said of positive electrode active material for further including manufacturing method. According to Claim 21, said heat treatment is at a temperature of 5 hr to 1 400 °C to 200 °C performed during the time of positive electrode active material for manufacturing method. According to Claim 17, said a non-conductive carbon-based film containing oxygen are simultaneously patterned to form a heat treatment is carried out in the 800 °C to 400 °C of positive electrode active material for manufacturing method.
According to Claim 17, said a non-conductive carbon-based film containing oxygen are simultaneously patterned to form a heat treatment is carried out in the 700 °C to 400 °C of positive electrode active material for manufacturing method. Divided A core component Carbon-based precursor Core 100 parts by weight (parts by weight) content of carbon-based precursor Number 2 (°C) temperature heat treatment In the embodiment 1 LiNi0.6 Co0.1 Mn0.3 O2 DN 0.02 600 In the embodiment 2 LiNi0.6 Co0.1 Mn0.3 O2 DN 0.04 600 In the embodiment 3 LiNi0.6 Co0.1 Mn0.3 O2 DN 0.1 600 In the embodiment 4 LiNi0.6 Co0.1 Mn0.3 O2 DN 0.04 500 In the embodiment 5 LiNi0.6 Co0.1 Mn0.3 O2 DN 0.04 700 In the embodiment 6 LiNi0.6 Co0.1 Mn0.3 O2 DN 0.04 800 In the embodiment 7 Li1.4 Ni0.25 Co0.1 Mn0.65 O2 DN 0.01 500 In the embodiment 8 Li1.4 Ni0.25 Co0.1 Mn0.65 O2 DN 0.05 500 In the embodiment 9 Li1.4 Ni0.25 Co0.1 Mn0.65 O2 DN 0.2 500 In the embodiment 10 Li1.4 Ni0.25 Co0.1 Mn0.65 O2 DN 0.2 550 In the embodiment 11 Li1.4 Ni0.25 Co0.1 Mn0.65 O2 DN 0.2 450 In the embodiment 12 Li1.4 Ni0.25 Co0.1 Mn0.65 O2 DN 0.2 400 In the embodiment 13 Na2/3 Fe0.5 Mn0.5 O2 DN 0.2 600 Compared example 1 LiNi0.6 Co0.1 Mn0.3 O2 - - - Compared example 2 LiNi0.6 Co0.1 Mn0.3 O2 DN 0.2 600 Compared example 3 LiNi0.6 Co0.1 Mn0.3 O2 DN 0.4 600 Compared example 4 LiNi0.6 Co0.1 Mn0.3 O2 DN 1.0 600 Compared example 5 Li1.4 Ni0.25 Co0.1 Mn0.65 O2 - - - Compared example 6 Na2/3 Fe0.5 Mn0.5 O2 - - - Divided 528eV to 530eV peak intensity in binding energy of (IA, cps) 531eV to 533eV peak intensity in binding energy of (IB, cps) Core 100 parts by weight conductive carbon a vision a carbon content of present-based film (D, parts by weight) In the embodiment 1 1718.29 2199.71 0.014 In the embodiment 2 1581.35 1783.11 0.036 In the embodiment 3 2025.94 2410.71 0.078 Compared example 1 2921.83 1730.34 - Compared example 2 2044.21 2553.28 0.178 Divided 282eV to 285eV peak intensity in binding energy of (IC, cps) 288eV to 293eV peak intensity in binding energy of (ID, cps) ID/IC In the embodiment 7 730 565 0.77 In the embodiment 8 1025 682 0.66 In the embodiment 9 1010 736 0.72 Compared example 5 832 135 - Divided A cut-off voltage (V) 1st (mAh/g) from discharge dosage cycle Initial efficiency (%) insect discharge efficiency (%) Capacity retention (%) In the embodiment 15 4.4V -2.5V 203.01 94.84 99.89 99.36 7 e.g. compared 4.4V -2.5V 198.51 93.38 99.76 98.11 In the embodiment 15 4.45V -2.5V 209.01 94.51 99.83 99.34 7 e.g. compared 4.45V -2.5V 206.78 92.90 99.73 94.70 In the embodiment 15 4.5V -2.5V 214.46 93.56 99.80 97.31 7 e.g. compared 4.5V -2.5V 209.31 91.56 99.66 91.38 Divided 1st (mAh/g) left-discharge capacity Initial efficiency (%) Capacity retention (%) In the embodiment 14 202.45 95.10 98.39 In the embodiment 15 203.01 94.84 99.36 In the embodiment 16 196.16 92.57 102.41 8 e.g. compared 199.48 90.76 96.23 9 e.g. compared 180.00 87.62 101.16 10 e.g. compared 133.59 67.25 114.91 Divided 1st (mAh/g) left-discharge capacity Initial efficiency (%) Capacity retention (%) In the embodiment 15 203.01 94.84 99.36 In the embodiment 17 196.66 94.04 99.80 In the embodiment 18 204.86 95.01 97.87 In the embodiment 19 198.05 93.16 99.52 7 e.g. compared 198.51 93.38 98.11 Divided Delta voltage (ΔV, mV) Capacity retention (%) In the embodiment 20 73.2 93.31 In the embodiment 21 71.6 93.72 In the embodiment 22 66.3 93.98 In the embodiment 23 55.9 93.79 In the embodiment 24 73.8 92.46 In the embodiment 25 71.2 91.60 11 e.g. compared 80.3 92.38 Divided 1st (mAh/g) left-discharge capacity Capacity retention (%) In the embodiment 26 186 71.5 12 e.g. compared 164.47 68.3