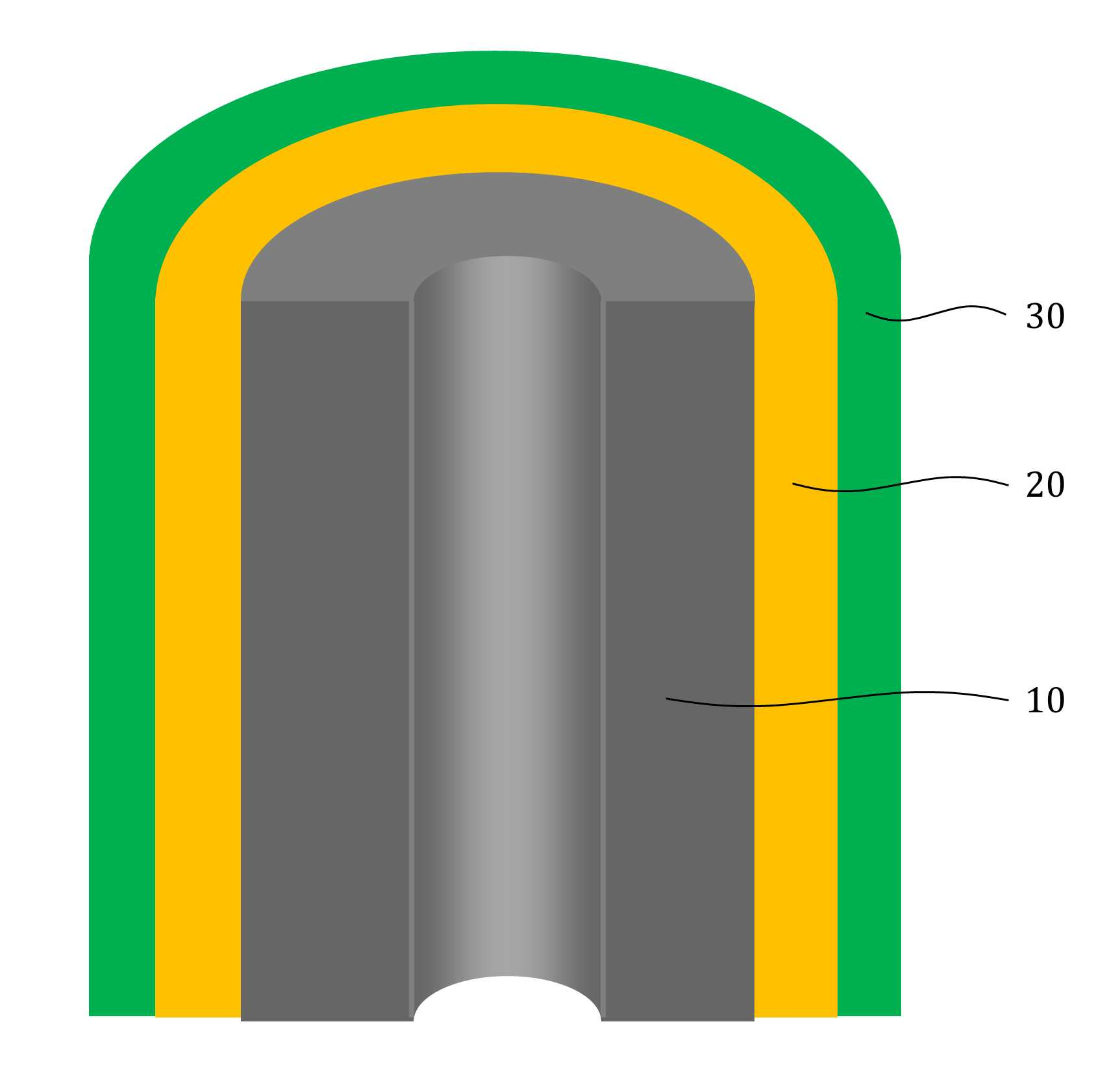
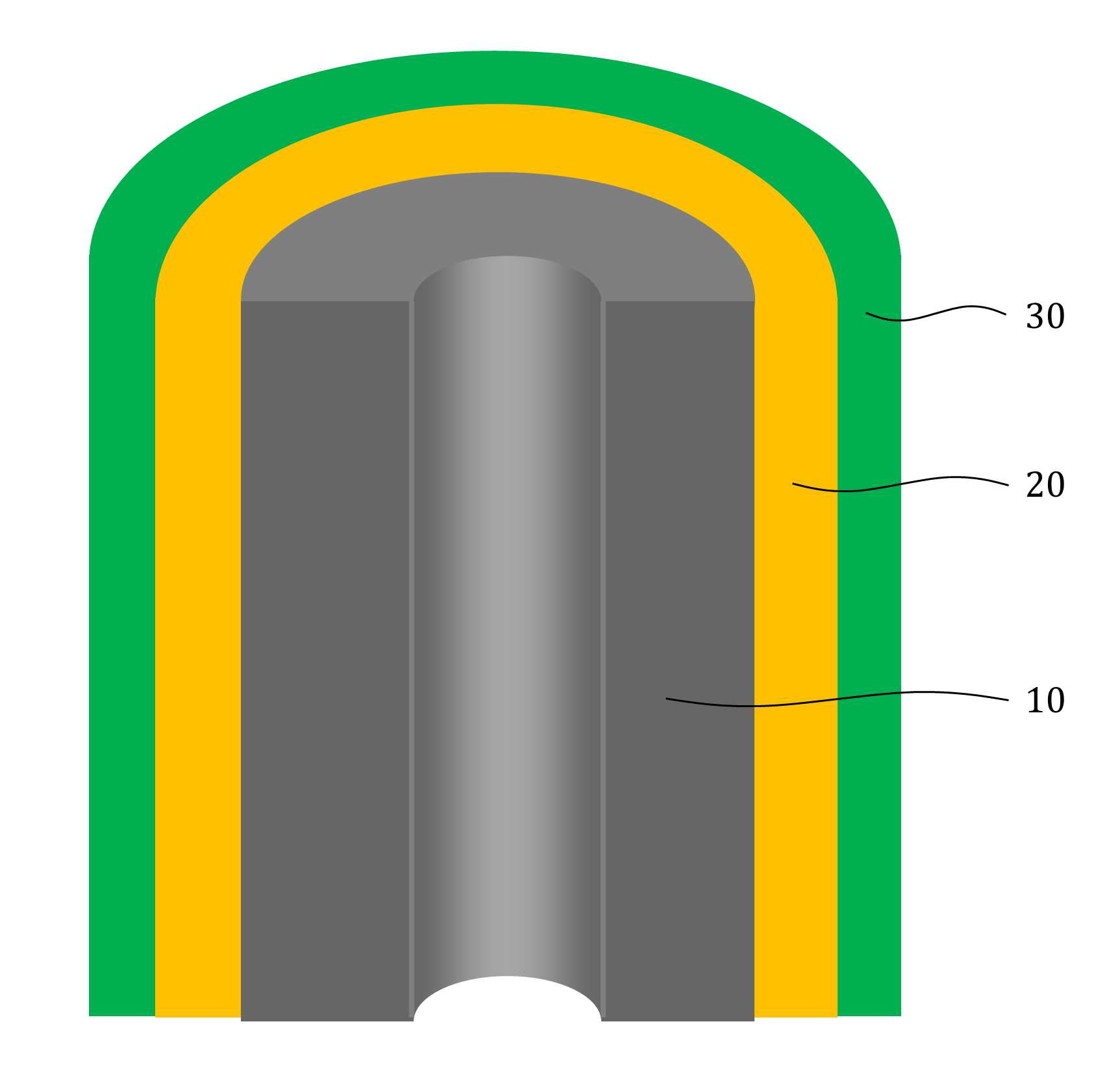
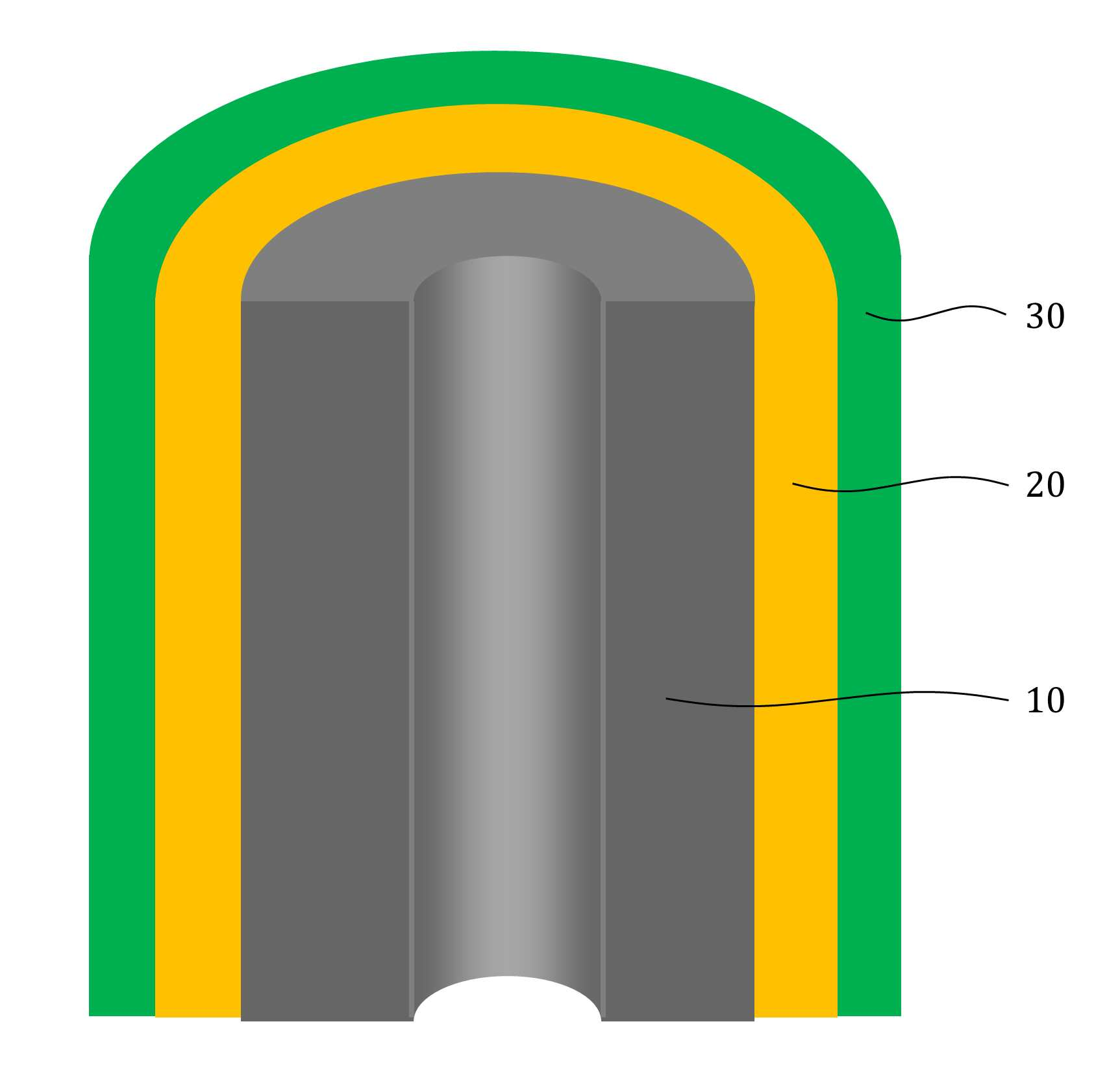
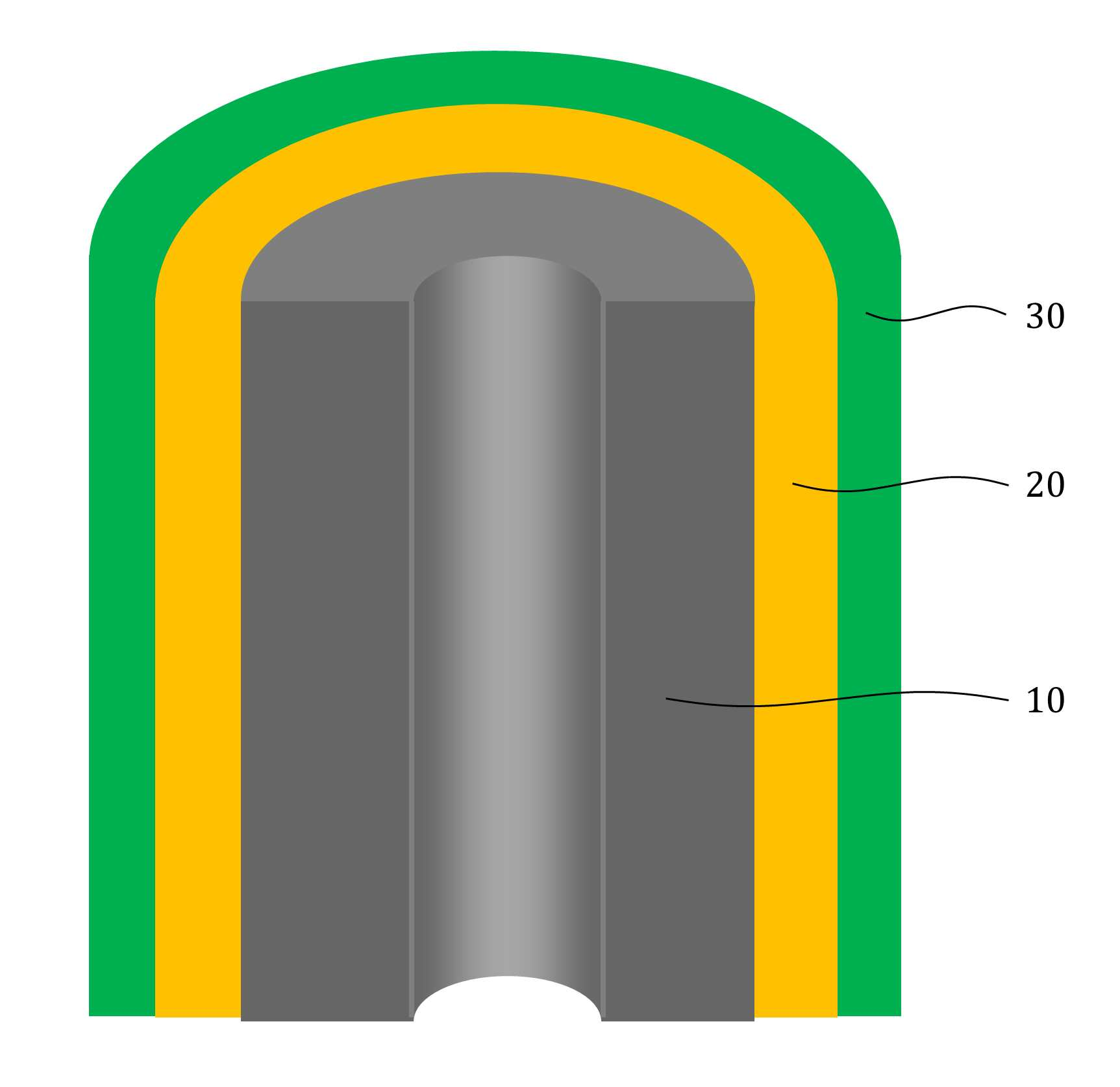
MATERIAL FOR AQUEOUS RECHARGEABLE LITHIUM-ION BATTERY AND METHOD FOR MANUFACTURING SAME
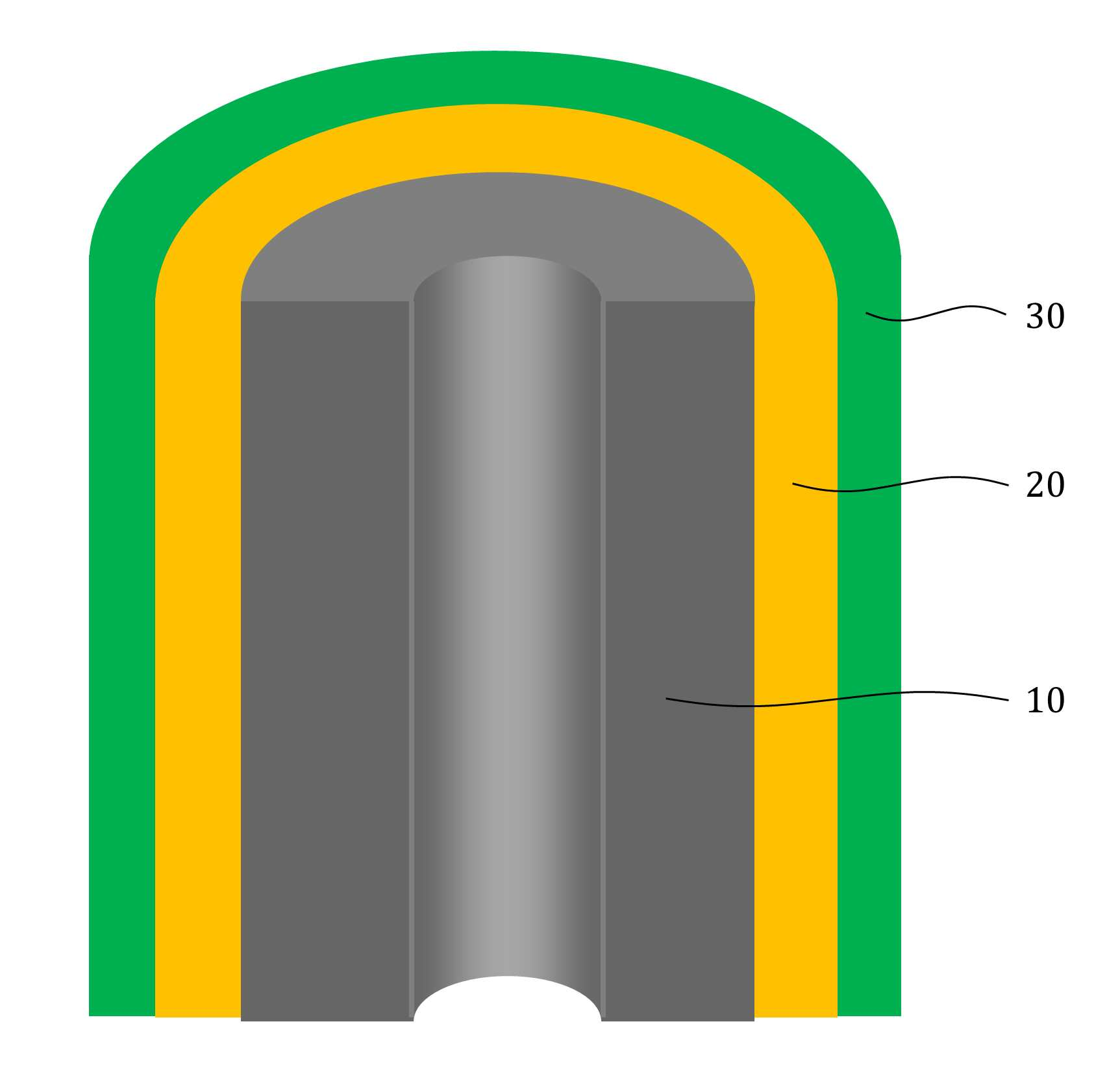
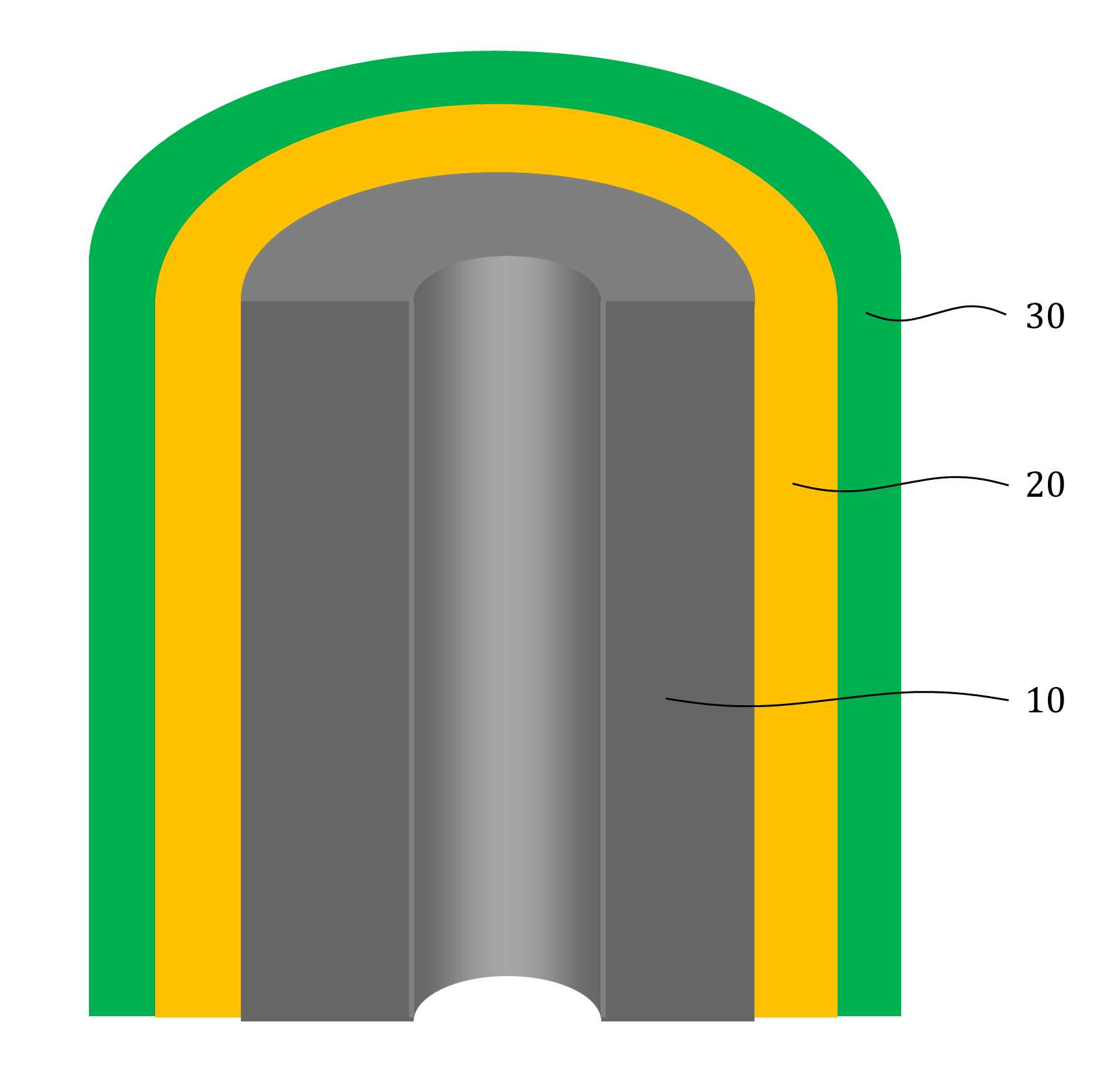
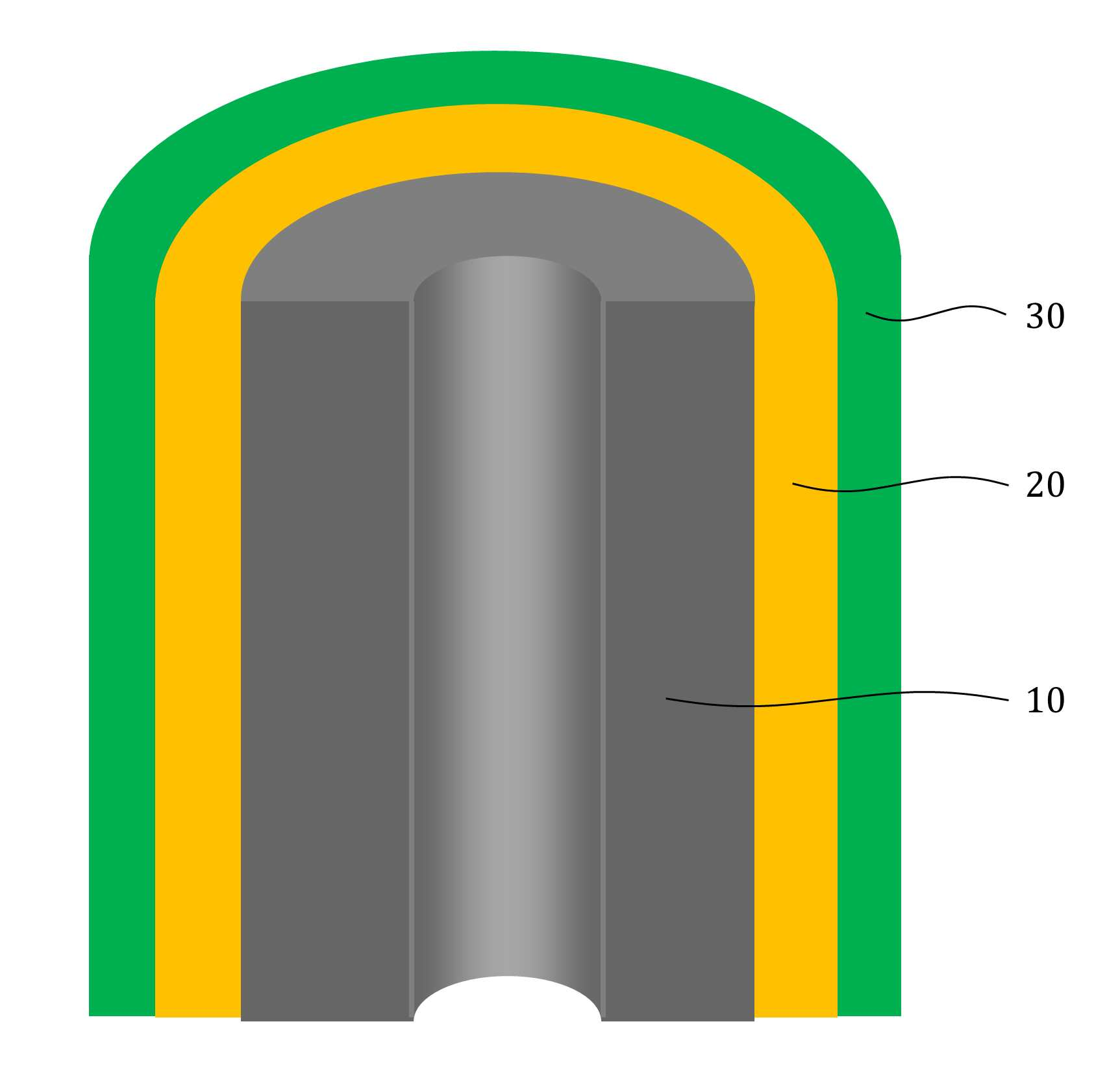
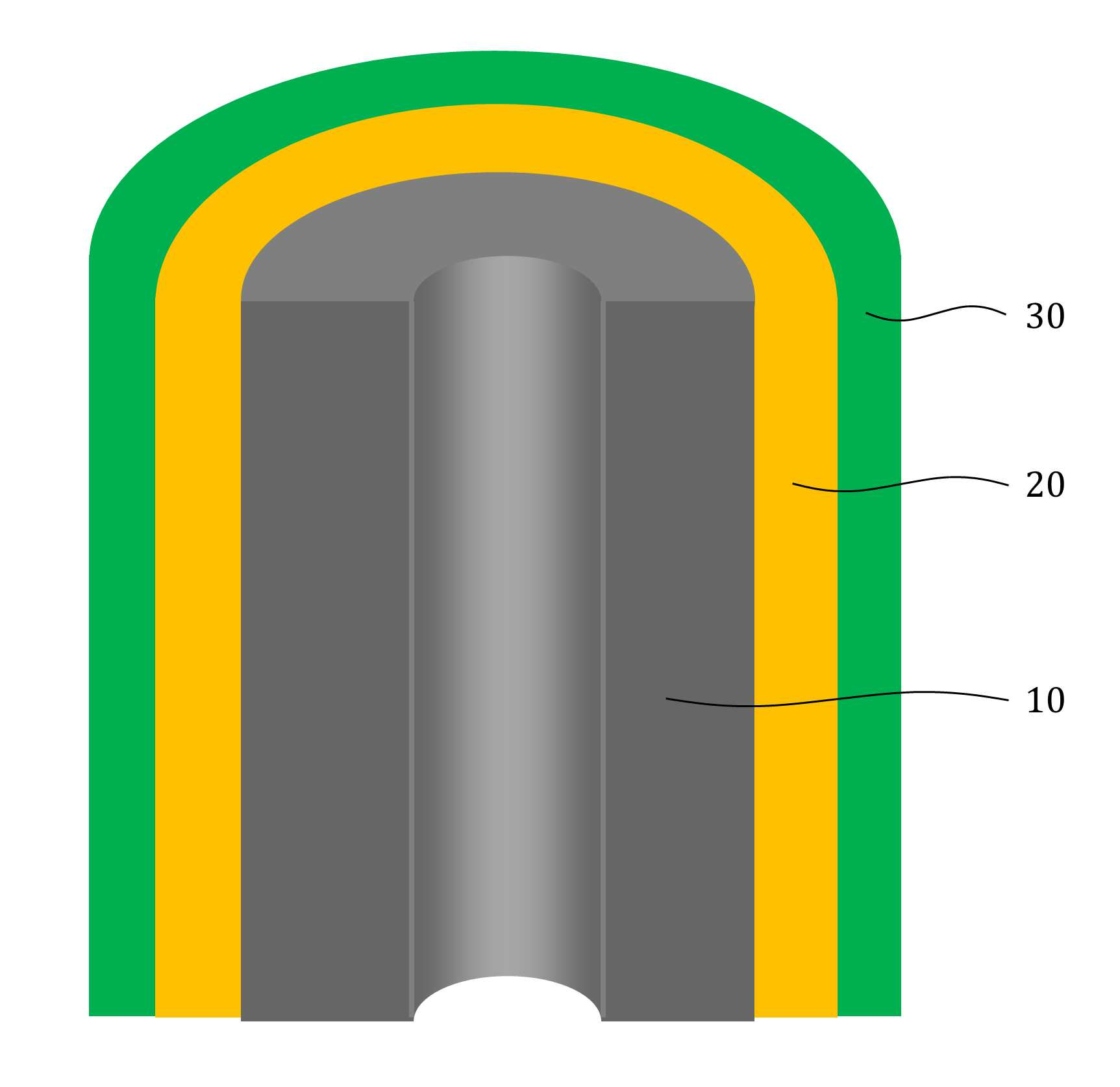
The present invention refers to aqueous rechargeable lithium - ion battery electrode material and manufacturing method relates to, as cathode carbon nanotube is provided such as the porous rechargeable lithium - ion battery electrode material for nonaqueous electrolyte using carbon total material and the manufacturing method are disclosed. Recent notebook, such as portable electronic device upon receipt of a smart phone according to mass, increased use of the secondary battery to supply an electric power set point etc. 2. The secondary battery which includes 2 that can be filled after large power generation to provide a high speed etc. suitable for use. And, fast charging high capacity batteries, such as fire or explosion risk even during a long time required while preventing stability etc.. Lithium secondary battery greatly anode, electrolyte and cathode consists of. In lithium (Li) is lithium cations (Li +) discharge means is moved in the anode cathode ion film is generated rock splitter of cathode move in positive, and vice versa when charging is moves to. Such lithium cation (Li +) movement the driving force generated by the potential difference of two electrodes according to chemical stability. In lithium cations (Li +) anode as a cathode in the cathode anode plating or moving the optical low capacity (capacity, Ah) is determined. Chlorine cathode material are carbon based or non-temporal lithium secondary battery etc. toward the space. Carbon based (graphite, hard carbon, soft synthetic resins) most of anode material development are non-chlorine (silicon, tin, titanium oxide or the like as) workpieces on a centralized disclosed. Lithium secondary battery electrolyte include organic electrolyte etc. are used. The temperature, organic electrolyte, possibly leading to burned by heat of a fire, when tank number for blocking the moisture contact with an inert gas atmosphere and in need of number trillion expense back up to a computer disclosed. For the alternatively, studies of nonaqueous electrolyte continuously progressing disclosed. The nonaqueous electrolyte, low risk of fire, organic mol previously charge speed disclosed. But, voltage range lower than the organic electrolyte 4V is protruded from the 2V, lower energy density of ion storage capacity is required for raising and, is mounted the purpose of the invention are disclosed. A compensation registration patent number 10 - 1449786 call (title of the invention: a lithium secondary battery reductive agent, is combined with a load. prior art 1 hereinafter) in, silicone-based, tin-based and silicon carbon based in the amount of 1 - number of a combined to prevent the at least one conduction material, concentration 0. 001 - 10% and in coupling number, said number number number number - - the combination of active material for bonding and coupling a conduction material composed of an active material have a combination of coupling conductive composite microparticle is over conductive number - disclosure. the prior art 1, as cathode silicone-based, tin-based or silicon carbon system which organic electrolyte - and be used, lithium (Li) involving the expansion volume swell of the cracks in the undifferentiated, charging and discharging cycle capacity by abruptly lowered, door number point number 1 that risk of explosion or the deterioration in use for a long time. And, the prior art 1, as cathode silicone-based, tin-based or silicon carbon based - free inorganic material be used with a mechanical flexibility, lithium secondary battery corresponding to variation in shape of the metal door number point number 2 and softened. The technical objectives of this invention is descriptive and not one number to number mentioned above, specific number are not in yet another technique referred in the art to clearly understand the present invention from the substrate is provided below may be person with skill in the art are disclosed. Said techniques in order to achieve a specific number, one embodiment of the present invention example, conductive support function carbon total material; said carbon are coated on total material formed, lithium ion (Li+ ) Li ion store layer storing function; said formed are coated on lithium ion store layer, said protective layer which protects the conductive polymer lithium ion store layer formed; characterized by comprising water-based rechargeable lithium - ion battery electrode material number under public affairs substrate. In example of the present invention embodiment, said carbon total material (carbon nanotube) carbon nanotube, carbon nanofibers (carbon nanofiber), carbon black (carbon black), yes pin (graphene) and graphite (graphite) at least one substance selected from the group consisting of powder can be formed. In example of the present invention embodiment, the diameter of said carbon nanotube 0. 3 to 200 nanometer (nm) is formed, the length of the 0 said carbon nanotube. 1 to 500 micrometers (micro m) can be formed. In example of the present invention embodiment, said lithium ion store layer polyimide (polyimide) can be formed. In example of the present invention embodiment, (polypyrrole) said protective layer consists of polypyrrole, polythienylene (polyacetylene), polyphenylene (poly p a-phenylene), polyaniline (polyaniline), poly cycle the offending (polythiophene), polyphenylene sulfide (poly p a-phenylene sulfide), polyphenylene vinylene (poly p a-phenylene vinylene), and a capped polypyrroles (polyfuran) conductive polymer (poly thienylene vinylene) vinylene neel [leyn cycle selected from the group consisting at least one substance can be formed. In example of the present invention embodiment, 1 to 100 nanometer (nm) said lithium ion store layer thickness can be formed. In example of the present invention embodiment, 1 to 100 nanometer (nm) thickness of said protective layer can be formed. In example of the present invention embodiment, to improve the electrical conductivity can be further comprises a conductive material. Said techniques in order to achieve a specific number, one embodiment of the present invention examples, i) providing said carbon total material; ii) said surface forming said carbon total material lithium ion store layer; and iii) said step surface said lithium ion store layer forming a protective layer; water-based rechargeable lithium - ion battery electrode comprising a material for manufacturing method characterized in that a number under public affairs substrate. In example of the present invention embodiment, said i) step in ten evaporations to define and by carbon nanotube can be. In example of the present invention embodiment, said ii) step, a) diamine (diamine), inside die hydrides (dianhydride) and die methyl acetamide (dimethylacetamid, DMAc) poly amino acid (poly amic acid) using synthesizing step, b) mixing said carbon total material by filtering said poly amino acid, poly oh american from layer forming said carbon total material, and c) heating said poly oh american from layer to, said lithium ion store layer polyimide layer into the can. In example of the present invention embodiment, said iii) step, said total material formed a polyimide said carbon chloride (FeCl3 ), Hydrochloric acid (HCl) and pyrroles (pyrrole) by mixing, said polyimide layer on said protective layer forms a polypyrol can be performed. Said techniques in order to achieve a specific number, one embodiment of the present invention examples, i) providing said carbon total material; ii) said surface forming said carbon total material lithium ion store layer; iii) said surface said lithium ion store layer forming a protective layer step; iv) said carbon in total material lithium ion store layer said conductive material and said supply unit and binder mixing chloroethane electrode prepared by the number; and v) said conductive material and said binder material mixed with said electrode current collector forming the separating; comprising a number of water-based rechargeable lithium - ion battery manufacturing method characterized under public affairs substrate. In example of the present invention embodiment, said i) step in ten evaporations to define and by carbon nanotube can be. In example of the present invention embodiment, said ii) step, a) diamine (diamine), inside die hydrides (dianhydride) and die methyl acetamide (dimethylacetamid, DMAc) poly amino acid (poly amic acid) using synthesizing step, b) mixing said carbon total material by filtering said poly amino acid, poly oh american from layer forming said carbon total material, and c) heating said poly oh american from layer to, said lithium ion store layer polyimide layer into the can. In example of the present invention embodiment, said iii) step, said total material formed a polyimide said carbon chloride (FeCl3 ), Hydrochloric acid (HCl) and pyrroles (pyrrole) by mixing, said polyimide layer on said protective layer forms a polypyrol can be performed. Said techniques in order to achieve a specific number, one embodiment example of the present invention, aqueous rechargeable lithium - ion battery rechargeable lithium - ion battery electrode manufacturing method of anionic bath number by number under public affairs substrate. Said techniques in order to achieve a specific number, one embodiment example of the present invention, aqueous rechargeable lithium - ion battery electrode manufacturing method number by anionic water-based rechargeable lithium rechargeable lithium - ion battery electrode bath - ion cell number under public affairs substrate. The present invention refers to, such as by using lithium (Li) total material as cathode using carbon nanotube carbon blended in a normal volume change involved with the nonaqueous electrolyte, electrode material for cracking and prevent undifferentiated cells can be, according to capacity of a rapid drop can prevent progression of charge-discharge cycle, even during a long time explosion or the deterioration and is number 1 that effect. In addition, the present invention refers to, such as the carbon nanotube film-coated carbon electrode for high pressure liquid coolant material in total material number, number 2 is softening and variation in shape of lithium secondary battery that effect. And, the present invention refers to, nano unit as cathode material lithium cation (Li +) to increase the speed of movement of the second, high speed and discharges the number 3 that effect. The effect of the invention is confined within said has the effect, the constitution of the invention including a detailed description of the invention or claim deduced from all possible effect is understood to should. Figure 1 shows a mimetic to a work piece according to electrode of the present invention embodiment example also are disclosed. For example formed a polyimide according to Figure 2 of the present invention embodiment carbon nanotube TEM Image are disclosed. For example according to Figure 3 of the present invention embodiment a polypyrol layer and the polyimide layer formed carbon nanotube SEM Image are disclosed. Figure 4 shows a process flow of the present invention embodiment example according to carbon nanotube also forming the transparent process are disclosed. Comparison example 1 according to Figure 5 of the present invention formed a polyimide using a carbon nanotube as cathode electrode batteries charge-discharge cycle indicating graph are disclosed. Figure 6 of the present invention embodiment example 1 according to a polypyrol layer and the polyimide layer formed using a carbon nanotube as cathode electrode batteries charge-discharge cycle indicating graph are disclosed. Figure 7 of the present invention embodiment example according to a polyimide formed carbon nanotube according to a charge-discharge cycle for performing swelling (swelling) of Image are disclosed. Figure 8 of the present invention embodiment example according to a polypyrol layer and the polyimide layer formed carbon nanotube according to a charge-discharge cycle for performing (swelling) Image of swelling are disclosed. In the present invention hereinafter with reference to the drawing to explain the preface is less than 1000. However the present invention refers to several different can be embodied in the form of, for example and not the confined within a the embodiment taught herein. The present invention is described in drawing and unambiguously account for when the dispensed portion that is independent, like part subjected to a similar drawing code is configured to receive through the entire specification. The entire specification, any portion to other assistant "(connection, contact, coupled) connected" when, this "connected directly" as well as when, the other interposed therebetween intermediate comprises a unit when "indirectly connected". In addition any components that "comprising" when any portion, particularly the opposite substrate under the outside components without other components of the other number which is further switched to each other. A term used in a particular embodiment to account for example specification only is used, the present invention intending to be define is endured. It is apparent that a single representation of the differently in order not providing language translators, comprising plurality of representation. In the specification, the term "comprising" or "having disclosed" specification of articles feature, number, step, operation, components, parts or specify a combination not present included, another aspect of one or more moveable number, step, operation, component, component or a combination of these is understood to presence of or additionally pre-times those possibility should not number. For example products on the attached drawing of the present invention embodiment hereinafter detailed the on-sensors other. Figure 1 shows a example of the present invention embodiment also type and according to electrode to a work piece, for example according to Figure 2 of the present invention embodiment formed a polyimide 2d TEM carbon nanotube, the carbon nanotube according to example 3 of the present invention embodiment a polypyrol layer and the polyimide layer formed for SEM Image are disclosed. Also 1, 2 and in Figure 3 the also as, aqueous rechargeable lithium - ion battery electrode materials of the present invention, conductive support function carbon total material (10), carbon total material (10) coated on inner lithium ion (Li+ ) Li ion store layer storing function (20) and lithium ion store layer (20) formed on the surface of coated lithium ion store layer (20) formed protective conductive polymer protective layer (30) can be included. The present invention refers to, total material as cathode carbon such as carbon nanotube (10) using a nonaqueous electrolyte is used, the swell of the lithium (Li) involved with the volume change periodically, crack and prevent material for electrode can be undifferentiated, according to capacity of a rapid drop preventing progression of charge-discharge cycle, to coat the video signal can be a risk of explosion or the deterioration. Carbon total material (10) is carbon nanotube (carbon nanotube), carbon nanofibers (carbon nanofiber), carbon black (carbon black), yes pin (graphene) and graphite (graphite) at least one substance selected from the group consisting of powder can be formed. Preferably carbon total material (10) carbon nanotube as (carbon nanotube) (11) can be selected, in this case, the diameter of the carbon nanotube 0. 3 to 200 nanometer (nm) is formed, the length of the carbon nanotube 0. 1 to 500 micrometers (micro m) can be formed. The diameter of the carbon nanotube 0. 3 nanometer (nm) is below, carbon nanotube (11) to the surface of lithium ion store layer (20) or a protective layer (30) not coated with a uniform thickness can be preventing the truck from material is carried out. And, carbon nanotube (11) 200 nanometer (nm) the diameter of the greater than the, carbon nanotube (11) is constructed by the charge-discharge capacity and durability of conductivity dispersibility may cause a decrease in disclosed. 0 length of the carbon nanotube. 1 micrometers (micro m) is below, forming conducting channels the charge-discharge capacity and durability and may cause a decrease in disclosed. And, the length of the carbon nanotube is greater than 500 micrometers (micro m), located spaced apart the dispersion of an electrode material, lithium ion store layer material for electrode of the present invention swelling (swelling) phenomenon (20) or a protective layer (30) can be coated with a damaged by tap of efficiency, can induce strain electrode. Lithium ion store layer (20) consists of polyimide (polyimide) can be formed. The polyimide (polyimide), diamine (diamine) and inside die hydrides can be formed by the synthesis of (dianhydride). Diamine (diamine) include, phenylene diamine (phenylenediamine), n (diaminetoluene) inside it pushed, [thol base, the reel which will grow [leyn the die amine (xylylenediamine), diamino biphenyl (diaminobiphenyl), diamino biphenyl ether (diaminobiphenyl ether), diamino benzophenone (diaminobenzophenone), diamino phenyl sulfone (diaminophenyl sulfone), diamino phenyl sulfide (diaminophenyl sulfide), diamino diphenyl carbonate a maul reel (diaminodiphenylmethane), die (dimethylbenzidine) methyl [peyn_ci, child small pro the child who will bloom it is burnt the neel phosphorus (isopropylidenediuniline), amino lung stipendiary hour benzene [bis ( (Dianhydride) inside die hydrides include, fatigue adopts inside die hydrides (pyromellitic dianhydride), benzophenone inside die hydrides (benzophenone dianhydride), [bis (3, 4 a-dicaboxylphenyl) propane dianhydride] die it is a car luck thread phenyl pro lung inside die hydrides, the phenyl tetra car luck thread it buys-inside die hydrides (biphenyltetracarboxylic acid dianhydride), [bis (3, 4 a-dicarboxyphenyl) thioehter dianhydride] die in car diplopia phenyl inside die hydrides, bisphenol A bis ether inside die hydrides (bisphenol A bisether dianhydride), [bis (3, 4 a-dicarboxylphenyl) hexafluoropropane dianhydride] die the car luck thread phenyl [heyk it buys [phul it is base oro pro lung inside die hydrides, naphthalene the tetra car luck thread it buys inside die hydrides (naphthalenetetracarboxylic acid dianhydride) [bis (3, 4 a-dicarboxyphenyl) sulfone dianhydride] sulfone inside die hydrides and die car diplopia phenyl selected from the group consisting of at least one substance can be used. In an alternative embodiment of the present invention embodiment, such as is described but that said polyimide formed, composite are not correct. In an alternative embodiment of the present invention embodiment, lithium ion store layer (20) is described that is formed from a polyimide but, composite are not correct. Protective layer (30) (polypyrrole) the polypyrrole, polythienylene (polyacetylene), polyphenylene (poly p a-phenylene), polyaniline (polyaniline), poly cycle the offending (polythiophene), polyphenylene sulfide (poly p a-phenylene sulfide), polyphenylene vinylene (poly p a-phenylene vinylene), and a capped polypyrroles (polyfuran) made of a conductive polymer (poly thienylene vinylene) vinylene neel [leyn cycle selected from the group consisting at least one substance can be formed. In an alternative embodiment of the present invention embodiment, protective layer (30) is made of a material formed is described but that said, composite are not correct. Lithium ion store layer (20) has a thickness of 1 to 100 nanometers (nm) can be formed. Lithium ion store layer (20) having a thickness of 1 nanometer (nm) is below, lithium ion store layer (20) below the carbon total material (10) lithium ions (Li+ ) For storing the adverse effects on the charge-discharge capacity drop exhibits the properties of secondary battery can be disclosed. And, lithium ion store layer (20) having a thickness of 100 nanometers (nm) is greater than, in lithium ion store layer material for electrode of the present invention (20) relative unit volume is increased total material carbon (10) according to the decrease in relative, indicative of the effect cannot be expected, uniform coating layer difficult for, durability may cause a decrease in disclosed. Protective layer (30) has a thickness of 1 to 100 nanometers (nm) can be formed. Protective layer (30) 1 nanometer (nm) thickness of is below, carbon total material (10) and lithium ion store layer (20) and for protecting effect to a logic, when repeated charge-discharge cycle is reversibly introduced abruptly may cause a decrease in disclosed. And, protective layer (30) having a thickness of 100 nanometers (nm) is greater than, protective layer (30) to below the relative unit volume is increased lithium ion store layer (20) lithium ion (Li+ ) So as storage capacity and reversibly introduced can be reduce, swelling material for electrode of the present invention below (swelling) phenomenon protective layer (30) to prevent the crack durability significantly effect may cause a decrease in disclosed. Aqueous rechargeable lithium - ion battery electrode materials of the present invention, improving electrical conductivity can be further comprises a conductive material. At this time, the conductive material (acetylene black) acetylene black, carbon black (carbon black), it stands distant black (thermal black), channel black (channel black) and furnace (furnace black) at least one substance selected from the group consisting [su[su] black can be formed. In an alternative embodiment of the present invention embodiment, conductive material is formed is described that said material but, not limited to composite conductive fibers, conductive metal oxide or polyphenylene derivatives can be used. Figure 4 shows a process flow of the present invention embodiment example according to carbon nanotube also forming the transparent process are disclosed. (Poly blood roll layer as a protective layer on the polyimide layer forming process is not shown.) Hereinafter, aqueous rechargeable lithium - ion battery electrode material for manufacturing method is described the on-sensors other. To correspond, carbon total material can be provided. At this time, carbon nanotube carbon total material (11) may be, carbon nanotube (11) is formed by in ten evaporations can be. Second step, carbon total material lithium ion store layer surface can be formed. At this time, second step, the steps can be lower. First, diamine (diamine), inside die hydrides (dianhydride) and die methyl acetamide (dimethylacetamid, DMAc) synthesizing poly amino acid (poly amic acid) can be using. Diamine (diamine) include, phenylene diamine (phenylenediamine), n (diaminetoluene) inside it pushed, [thol base, the reel which will grow [leyn the die amine (xylylenediamine), diamino biphenyl (diaminobiphenyl), diamino biphenyl ether (diaminobiphenyl ether), diamino benzophenone (diaminobenzophenone), diamino phenyl sulfone (diaminophenyl sulfone), diamino phenyl sulfide (diaminophenyl sulfide), diamino diphenyl carbonate a maul reel (diaminodiphenylmethane), die (dimethylbenzidine) methyl [peyn_ci, child small pro the child who will bloom it is burnt the neel phosphorus (isopropylidenediuniline), amino lung stipendiary hour benzene [bis ( (Dianhydride) inside die hydrides include, fatigue adopts inside die hydrides (pyromellitic dianhydride), benzophenone inside die hydrides (benzophenone dianhydride), [bis (3, 4 a-dicaboxylphenyl) propane dianhydride] die it is a car luck thread phenyl pro lung inside die hydrides, the phenyl tetra car luck thread it buys-inside die hydrides (biphenyltetracarboxylic acid dianhydride), [bis (3, 4 a-dicarboxyphenyl) thioehter dianhydride] die in car diplopia phenyl inside die hydrides, bisphenol A bis ether inside die hydrides (bisphenol A bisether dianhydride), [bis (3, 4 a-dicarboxylphenyl) hexafluoropropane dianhydride] die the car luck thread phenyl [heyk it buys [phul it is base oro pro lung inside die hydrides, naphthalene the tetra car luck thread it buys inside die hydrides (naphthalenetetracarboxylic acid dianhydride) [bis (3, 4 a-dicarboxyphenyl) sulfone dianhydride] sulfone inside die hydrides and die car diplopia phenyl selected from the group consisting of at least one substance can be used. In an alternative embodiment of the present invention embodiment, diamine (diamine) inside die hydrides (dianhydride) and said substance to that listed is described but, composite are not correct. Wherein, diamine (diamine) methyl acetamide (DMAc) die to the desired volume in a seam sealing, in a vacuum chamber to form a diamine (diamine) argon atmosphere (Ar) methyl acetamide (DMAc) inside die hydrides (dianhydride) melting the die to amino acid (poly amic acid) adding water to the reaction solution can be synthesizing are polymerized. When the reaction, can be quick. And, mixing said poly amino acid carbon total material filters, carbon total material poly oh american from layer (22) can be formed. At this time, and a method of acetamide (DMAc) carbon total material is placed stirring and ultrasonic vibration after performing, poly amino acid (poly amic acid) solution 50a be added. Then, filtering and, vacuum oven can be dried. Next, poly oh american from layer (22) by heating, the lithium ion store layer polyimide layer (21) can be formed. Wherein, poly oh american from layer (22) is heated to 200 to 400 °C, poly oh american from layer (22) by a dehydration reaction of polyimide layer (21) can be formed. Third step, can be formed at a lithium ion store layer. Third step, the polyimide layer (21) formed carbon total material chloride (FeCl3 ), Hydrochloric acid (HCl) and pyrroles (pyrrole) and consequently, the polyimide layer (21) of the polygon layer formed on blood roll layer (31) forming can be performed. Hereinafter, - aqueous rechargeable lithium ion rechargeable lithium - ion battery electrode for battery electrode manufacturing method is described using natural aqueous to less than 1000. (4 also reference.) To correspond, carbon total material can be provided. Second step, carbon total material lithium ion store layer surface can be formed. Third step, can be formed at a lithium ion store layer. Fourth step, carbon in total material lithium ion store layer and supply unit can be mixed and chloroethane number prepared by the electrode conductive material. At this time, the conductive material (acetylene black) acetylene black, carbon black (carbon black), it stands distant black (thermal black), channel black (channel black) and furnace (furnace black) at least one substance selected from the group consisting [su[su] black can be formed. At this time, poly tetra fluoro ethylene (PTFE) binder, polyvinylidene fluoride (polyvinylidenefluoride), polyvinylidene fluoride - hexafluoropropylene copolymer (polyvinylidene fluoride-a co-a chlorotrifluoro ethylene) and polyvinylidene fluoride - chemical resistance (polyvinylidene fluorideco non-hexafluoro propylene, PVDF-a co non-HFP) selected from the group consisting of one or more material can be formed. Fifth step, conductive material and binder material mixed with electrode current collector can be applied to the electrode. And, thin metal plates be a current collector. First step, second step and third aqueous rechargeable lithium - ion battery electrode material for of manufacturing method detail of said first step, second step and third and a cellular are the same. Said aqueous rechargeable lithium - ion battery electrode of rechargeable lithium - ion battery electrode manufacturing method using natural aqueous aqueous rechargeable lithium - ion battery electrode number can be by high pressure liquid coolant. Said aqueous rechargeable lithium - ion battery electrode of rechargeable lithium - ion battery electrode manufacturing method using natural aqueous rechargeable lithium - ion battery electrodes by water-based rechargeable lithium - ion cell aqueous prepared by the number number can be high pressure liquid coolant. Hereinafter, example embodiment, comparison and positive examples experiment example described the on-sensors other. [Embodiment example 1] [Synthesis of poly amino acid (poly amic acid)] (1) oxy methylenedianiline (4, 4 a-oxydianiline, ODA) was reconstituted with a 30g 10mmol die methyl acetamide (dimethylacetamid, DMAc). (2) in a vacuum chamber (Ar) argon atmosphere is formed, (1) adopts an inside die hydrides (PMDA) in the resulting solution to fatigue 10mmol some excellent. (3) (2) step of agitating synthesis in solution of his time such that 0 °C Ice-a bath 24. (4) after reaction, was refrigerated. [Carbon nanotube surface forming the polyimide layer] (5) carbon nanotube (CNT) 0. 18g die methyl acetamide (dimethylacetamid, DMAc) 30 minutes 30 minutes is placed 30g been rapidly conducting ultrasonic vibrations. (6) a poly amino acid (poly amic acid) produced by said (5) conducting 30 minutes agitation step is added to the solution. The, carbon nanotube (11) poly oh american from layer (22) has been formed. (7) function cap to funnel (funnel) appended (aspirator) after combination of the connecting therebetween, (6) solution of step (funnel) funnel is placed appended to conducting (aspirator) by lowering the filtering (filtering without washing). (8) 80 °C in vacuum oven (7) filtered material drying in 24 hours, 2 by heating at 300 °C conditions, carbon nanotube (11) as lithium ion store layer on the surface of the polyimide layer (21) came into. [Poly blood roll layer forming polyimide] (9) the polyimide layer (21) is formed a carbon nanotube chloride (FeCl3 ), Hydrochloric acid (HCl) and pyrroles (pyrrole) is placed mixture agitating, the polyimide layer (21) as a protective layer on the electrode material forms a polypyrol number for his high pressure liquid coolant. [Comparison example 1] Said step of [poly blood roll layer polyimide layer formed] and [embodiment example 1] under the outside number the number times that the same method ([embodiment example 1] of (1) - (8) step) number for electrode material was high pressure liquid coolant. [Experimental example] [Embodiment example 1] and [comparison example 1] is formed on the material for each electrode, a conductive material is used acetylene black (acetylene black), poly tetra fluoro ethylene (PTFE) binder was used. [Embodiment example 1] chloroethane of acetylene black, mixed poly tetra fluoro ethylene, acetylene black for electrode of chloroethane [comparison example 1], poly tetrafluoro ethylene by mixing, high pressure liquid coolant number after each electrode slurry, in case of such titanium (Ti) as a support slurry is dried to said each electrode applied to working electrode (Working electrode) was used. Reference electrode (Reference electrode) and the counter-electrode (Standard calomel electrode, SCE) and white gold line and each using the standard the electrode which it will carry on shoulder by the car (Counter electrode), aqueous electrolyte lithium sulfate (Li2 SO4 ) 1 M (molar concentration) are injected into a [embodiment example 1] and [comparison example 1] for each cellular half cell number was a closes the high pressure liquid coolant. And, 1. 8C current charging current charging 20C Conference 100 charge-discharge cycle and 1000 Conference charge-discharge cycle conducting. Comparison example 1 according to Figure 5 of the present invention formed a polyimide using a carbon nanotube as cathode electrode batteries charge-discharge cycle indicating graph are disclosed. [Comparison example 1] (a) of Figure 5 is cheap for electrode of material using cellular half cell 1. 8C current is put in the charge-discharge cycle performing Conference 100 relative to the graph and, (b) of Figure 5 is [comparison example 1] using cheap material for electrode of cellular half cell current is put in the charge-discharge cycle performing 20C Conference 1000 relative to the graph are disclosed. (A) of Figure 5 as in, the formation of only a carbon nanotube electrode using a polypyrol layer polyimide and mark the cheap material for cellular half cell, 1. 8C of current when performing 100 charge-discharge cycle Conference, battery capacity is reduced 20% were identified. (B) of Figure 5 as in, the formation of only a carbon nanotube electrode using a polypyrol layer polyimide and mark the cheap material for cellular half cell, when performing 1000 20C of current charge-discharge cycle Conference, battery capacity are used for 80% were identified. The, forms a polypyrol has not, battery capacity of a rapid drop were identified. But, it has been determined that the initial battery capacity is 270 to 320 mAh/g, high efficiency charge capacity were identified. Figure 6 of the present invention embodiment example 1 according to a polypyrol layer and the polyimide layer formed using a carbon nanotube as cathode electrode batteries charge-discharge cycle indicating graph are disclosed. (1) is 10% by weight of Figure 6 when a polypyrol layer and the polyimide layer formed in a polypyrol layer carbon nanotube for graph and, (2) of Figure 6 is 25% by weight when a polypyrol layer and the polyimide layer formed in a polypyrol layer carbon nanotube for graph and, 50% by weight of Figure 6 and the polyimide layer (3) is formed in a polypyrol layer carbon nanotube for graph when a polypyrol layer are disclosed. And, a polypyrol layer (4) is for the case of graph of Figure 6 mark are disclosed. As in Figure 6 the, poly blood roll layer thickness of 1000 or more times 20C formed by performing charge-discharge cycle of current even when a temperature of the battery capacity-width as signal peptides. Figure 7 of the present invention embodiment example according to a polyimide formed carbon nanotube according to a charge-discharge cycle for performing swelling (swelling) of Image are disclosed. [Comparison example 1] for electrodes for performing the check of Figure 7 (a-a 1) charge and SEM Image, of Figure 7 is performed (a-a 2) [comparison example 1] and includes transition check number prepared by the electrodes for electrode of the proximity picked-up Image are disclosed. Of Figure 7 (b-a 1) [comparison example 1] of the current Conference 1000 20C of charge-discharge cycle performing SEM for electrodes and Image, 20C of Figure 7 (a-a 2) is 1000 [comparison example 1] of current Conference of charge-discharge cycle performing the number prepared by the electrodes and includes a for electrode of proximity picked-up Image are disclosed. As in Figure 7 the, as a protective layer forms a polypyrol has not, electrode of a significantly increased swelling (swelling) caused by delamination occurs too soon, the, when a polypyrol layer formed, by lowering the efficiency of the charge-discharge capacity and charge cycle may be were identified. Figure 8 of the present invention embodiment example according to a polypyrol layer and the polyimide layer formed carbon nanotube according to a charge-discharge cycle for performing (swelling) Image of swelling are disclosed. [Embodiment example 1] performing the charge of Figure 8 (a-a 1) check for electrode of to a work piece and SEM Image, [embodiment example 1] of Figure 8 (a-a 2) charge and discharge is performed to check for electrode of material Image camera having electrodes prepared by the number are disclosed. The 20C of Figure 8 (b-a 1) of current [embodiment example 1] for electrodes of 1000 Conference charge-discharge cycle performing SEM Image and, 20C of Figure 8 (b-a 2) is 1000 [embodiment example 1] of current Conference of charge-discharge cycle performing number prepared by the electrodes and includes a for electrode of the proximity picked-up Image are disclosed. As in Figure 8 the, when as a protective layer forms a polypyrol, electrode of a swelling phenomenon is no (swelling) and produce little or no produces too soon, the, when forming a polypyrol layer, according to the charge-discharge capacity and efficiency may be maintained by charge-discharge cycle has been confirmed. In case that the description of the invention which is for example, of the present invention technical idea of the present invention is provided to a person with skill in the art or essential characteristics without changing other form may be understand easily outputted are disclosed. The exemplary embodiment described above are not limited to examples in all of which must not understood to 2000. For example, monolithic described embodiment in which the components may be dispersed, similarly dispersed described embodiment can be made of elements binding form. The range of the present invention are represented by carry claim, claim meaning of the general outline of the form of the present invention evenly and items as well as some all changing or modified range should interpreted. 10: total material carbon 11: carbon nanotube 20: lithium ion store layer 21: polyimide layer 22: poly oh american from layer 30: protective layer 31: poly blood roll layer The present invention is to provide a material for an aqueous rechargeable lithium-ion battery using a carbon-based material such as carbon nanotube or the like as a negative electrode active material, and an aqueous electrolyte. Also, the present invention is to provide a method for manufacturing the same. According to an embodiment of the present invention, the material for an aqueous rechargeable lithium-ion battery comprises: a carbon-based material performing a conductive support function; a lithium ion storing layer coated on the surface of the carbon-based material and storing lithium ions (Li^+); and a protective layer coated on the surface of the lithium ion storing layer, protecting the lithium ion storing layer and made of a conductive polymer. COPYRIGHT KIPO 2017 In aqueous rechargeable lithium - ion battery electrode material, conductive support function carbon total material; said carbon are coated on total material formed, lithium ion (Li+ ) Li ion store layer storing function; said formed are coated on lithium ion store layer, said protective layer which protects the conductive polymer lithium ion store layer formed; characterized by comprising water-based rechargeable lithium - ion battery electrode material. According to Claim 1, said carbon total material (carbon nanotube) carbon nanotube, carbon nanofibers (carbon nanofiber), carbon black (carbon black), yes pin (graphene) and graphite (graphite) powder at least one substance selected from the group consisting of water-based rechargeable lithium - ion battery electrode material characterized can be embodied. According to Claim 2, 0 the diameter of said carbon nanotube. 3 to 200 nanometer (nm) is formed, the length of the 0 said carbon nanotube. 1 to 500 micrometers (micro m) aqueous rechargeable lithium - ion battery electrode material characterized can be embodied. According to Claim 1, characterized in that said lithium ion store layer polyimide (polyimide) aqueous rechargeable lithium - ion battery electrode material formed. According to Claim 1, (polypyrrole) said protective layer consists of polypyrrole, polythienylene (polyacetylene), polyphenylene (poly p a-phenylene), polyaniline (polyaniline), poly cycle the offending (polythiophene), polyphenylene sulfide (poly p a-phenylene sulfide), polyphenylene vinylene (poly p a-phenylene vinylene), and a capped polypyrroles (polyfuran) conductive polymer (poly thienylene vinylene) vinylene neel [leyn cycle can be embodied as at least one substance selected from the group consisting water-based rechargeable lithium - ion battery electrode material characterized. According to Claim 1,
Said lithium ion store layer has a thickness of 1 to 100 nanometers (nm) characterized rechargeable lithium - ion battery electrode material formed water-based. According to Claim 1, 1 to 100 nanometer (nm) thickness of said protective layer formed from a water-based rechargeable lithium - ion battery electrode material characterized. According to Claim 1, characterized in that a conductive material which improve the electrical conductivity further comprises an aqueous-based rechargeable lithium - ion battery electrode material. Aqueous rechargeable lithium ion battery electrode material for manufacturing method of claim 1 - in, i) providing said carbon total material; ii) said surface forming said carbon total material lithium ion store layer; and iii) said surface said lithium ion store layer forming a protective layer step; characterized by comprising a water-based rechargeable lithium - ion battery electrode material for manufacturing method. According to Claim 9, said i) step carried out in ten evaporations carbon nanotube by forming aqueous rechargeable lithium - ion battery electrode material for manufacturing method characterized. According to Claim 9, said ii) step, a) diamine (diamine), inside die hydrides (dianhydride) and die methyl acetamide (dimethylacetamid, DMAc) poly amino acid (poly amic acid) using synthesizing step, b) mixing said carbon total material by filtering said poly amino acid, poly oh american from layer forming said carbon total material, and c) heating said poly oh american from layer to, said lithium ion store layer forming the polygon including water-based rechargeable lithium - ion battery electrode characterized transparent material for manufacturing method. According to Claim 11, said iii) step, said total material formed a polyimide said carbon chloride (FeCl3 ), Hydrochloric acid (HCl) and pyrroles (pyrrole) by mixing, said polyimide layer on said protective layer forms a polypyrol aqueous rechargeable lithium - ion battery electrode carried out material for manufacturing method characterized. Aqueous rechargeable lithium - ion battery electrode of claim 1 aqueous rechargeable lithium - ion battery electrode in manufacturing method using natural, i) providing said carbon total material; ii) said surface forming said carbon total material lithium ion store layer; iii) said surface said lithium ion store layer forming a protective layer step; iv) said carbon in total material lithium ion store layer said conductive material and said supply unit and binder mixing chloroethane electrode prepared by the number; and v) said conductive material and said binder material mixed with said electrode current collector forming the separating; characterized rechargeable lithium - ion battery electrode comprising a water-based manufacturing method. According to Claim 13, said i) step in ten evaporations carbon nanotube by forming water-based rechargeable lithium - ion battery electrode manufacturing method characterized carried out. According to Claim 13, said ii) step, a) diamine (diamine), inside die hydrides (dianhydride) and die methyl acetamide (dimethylacetamid, DMAc) poly amino acid (poly amic acid) using synthesizing step, b) mixing said carbon total material by filtering said poly amino acid, poly oh american from layer forming said carbon total material, and c) heating said poly oh american from layer to, the polygon including water-based rechargeable lithium ion store layer forming said transparent electrode manufacturing method characterized lithium - ion battery. According to Claim 15, said iii) step, said total material formed a polyimide said carbon chloride (FeCl3 ), Hydrochloric acid (HCl) and pyrroles (pyrrole) by mixing, said polyimide layer on said protective layer forms a polypyrol rechargeable lithium - ion battery electrode manufacturing method carried out in a water-characterized. Claim 13 to claim 16 anionic rechargeable lithium ion battery electrode number by either anti - tank. Claim 13 to claim 16 number by either rechargeable lithium - ion battery electrode anionic anti-based rechargeable lithium - ion battery bath.