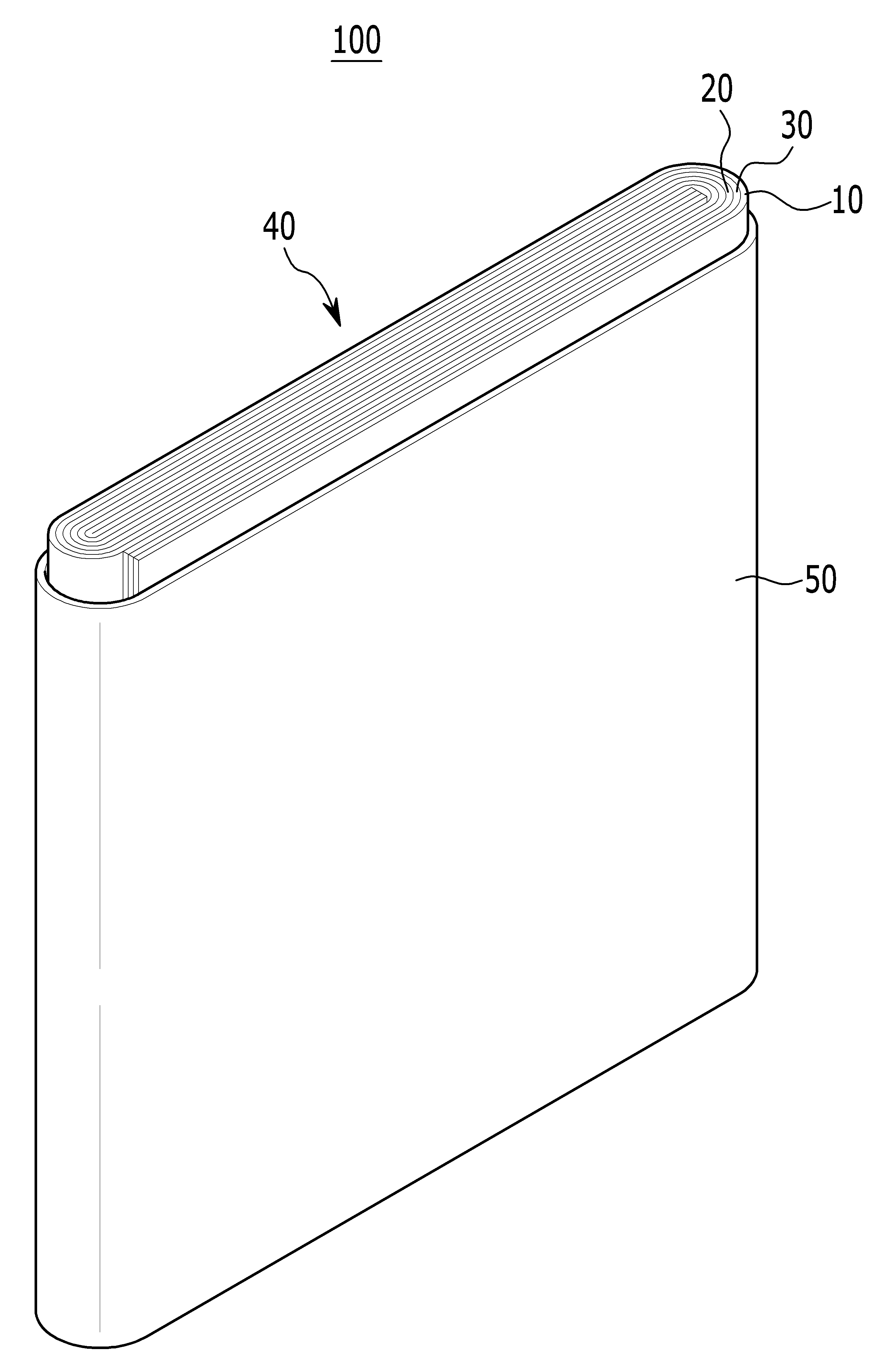
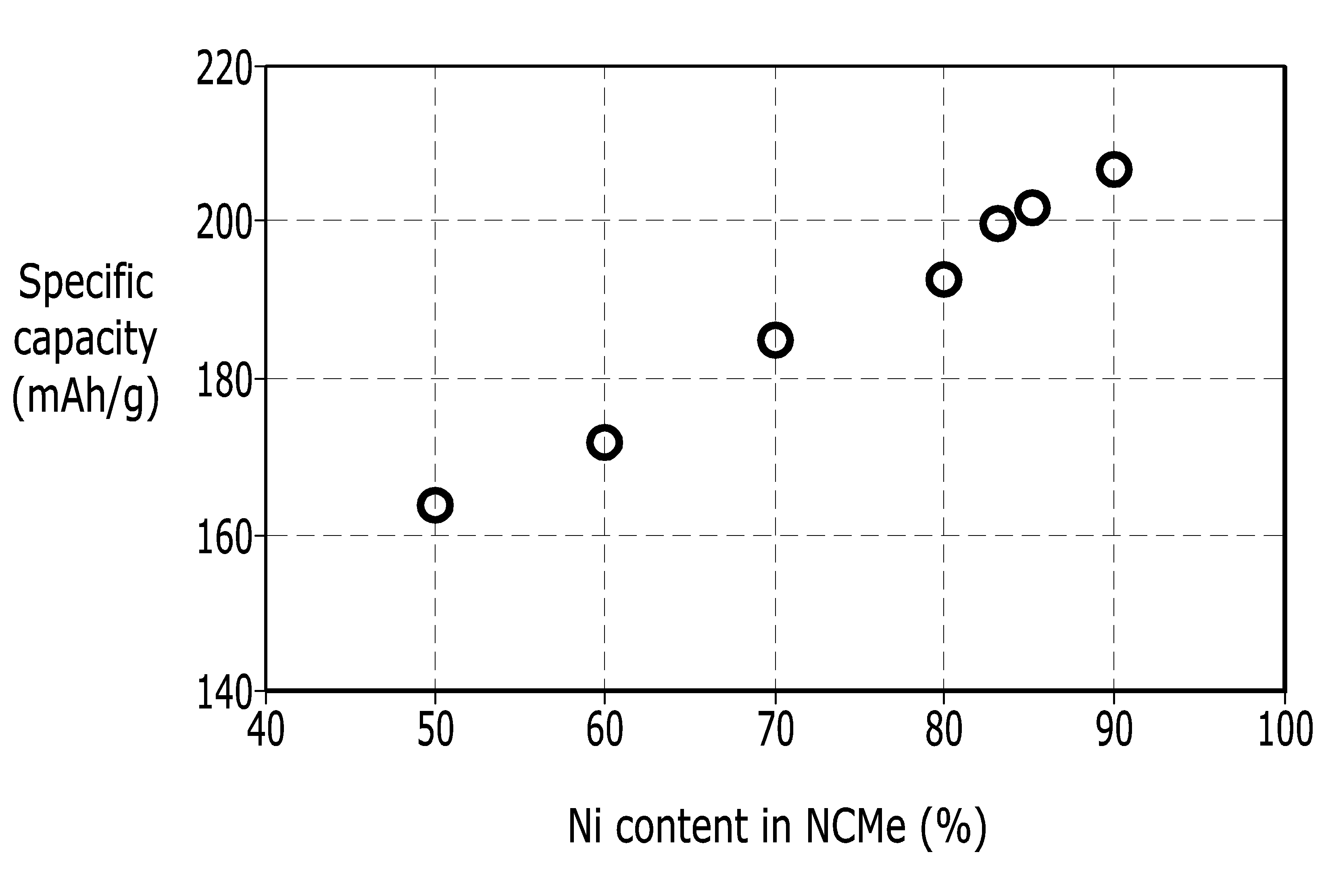
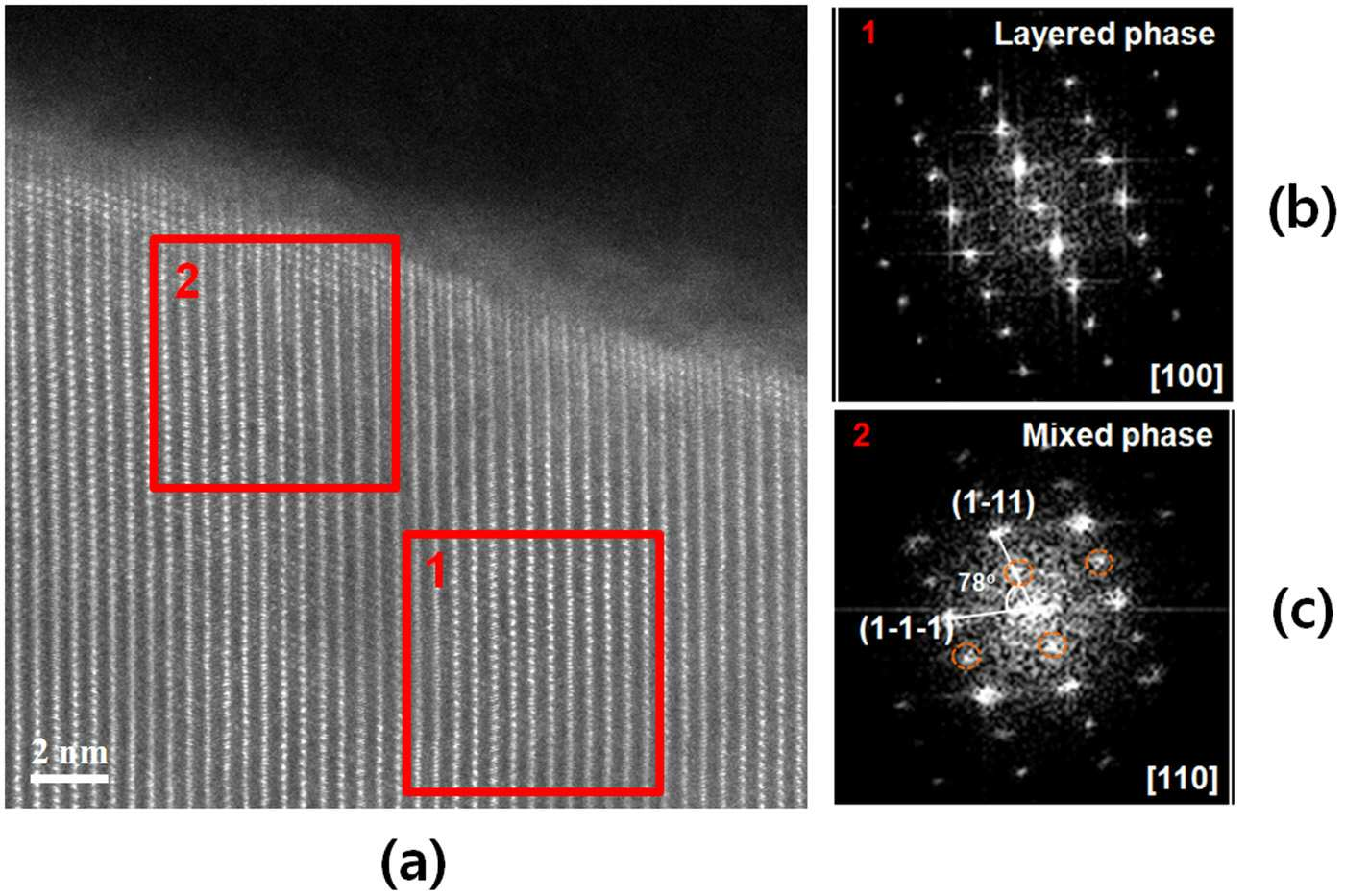
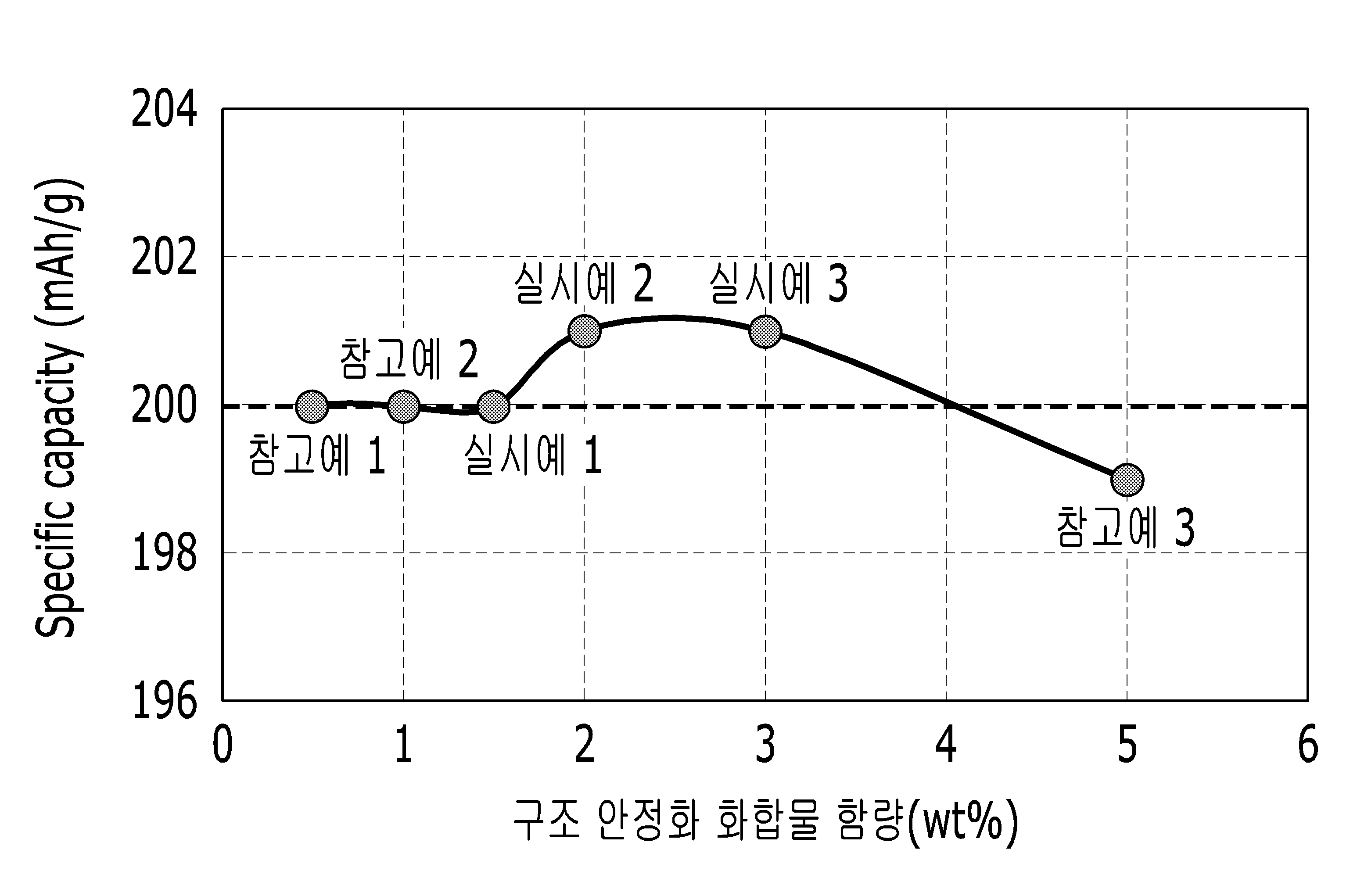
POSITIVE ELECTRODE ACTIVE MATERIAL FOR LITHIUM SECONDARY BATTERY AND LITHIUM SECONDARY BATTERY COMPRISING SAME
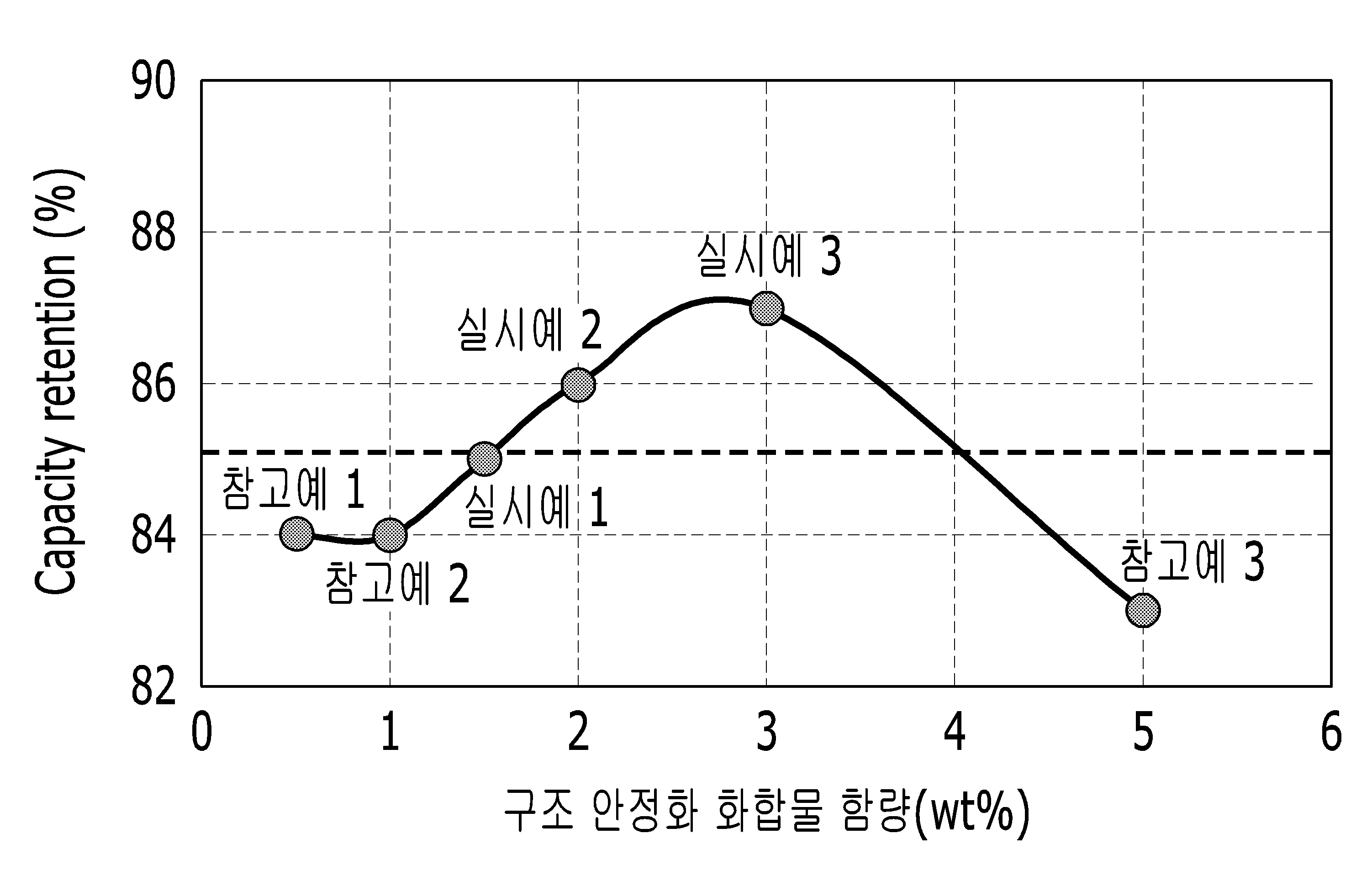
For lithium secondary cell battery and a lithium secondary battery including are disclosed. Cellular phone, notebook, smart phone etc. it mainly used lithium secondary cell such as a drive source for the mobile information terminal. Said lithium secondary cell includes an anode, a cathode and an electrolyte consists of. The, include LiCoO positive electrode active material for positive electrode2 , LiMn2 O4 , LiNi1-x Cox O2 (0 < x < 1) having a structure capable of intercalation of lithium ions such as lithium transition metal oxide mainly are used. As the negative electrode active material is a lithium secondary battery and be able/artificial, natural graphite, hard carbon including various forms of carbon-based material can be a thin plate. Recent miniaturization of mobile information terminal is abruptly and lightweight and clear, etc. of a lithium secondary battery capacity than required reduced in power for driving the same. In addition, lithium rechargeable battery power supply or power storage for power for driving hybrid automobiles cell vehicle for use, has good rapid rate characteristics and discharges the battery with excellent cycle characteristics extended development set inside progressing disclosed. Certain embodiments include capacity and good cycle life characteristics an anode active material for a lithium secondary battery [...] number are disclosed. Said another implementation relates an anode active material including lithium rechargeable battery [...] number are disclosed. Formula 1 of the present invention to certain embodiments include compounds represented including core; and said core is located at the surface, including an anode active material for a lithium secondary battery including Co Al compound or compound stability compound number [...] substrate. [Formula 1] Lia Nix Coy Mez M1k O2 a-p Tp (In said formula 1, 0. 9 ≤ a ≤ 1. 1, 0. 7 ≤ x ≤ 0. 93, 0 < y ≤ 0. 3, 0 < z ≤ 0. 3, 0 ≤ k ≤ 0. 005, x + y + z + k=1, 0 ≤ p ≤ 0. 005 and, Me is Mn or Al and, M1 The Mg, Ba, B, La, Y, Ti, Zr, Mn, Si, V, P, Mo, and W or combinations thereof, T is about F.) Said compound Al stability2 O3 , Co3 O4 , Lix CoO2 (A x 0. 9 to 1. 1) can be or a combination of these. Said compound can be Co compound stability. Said compound can be layered or island form said core surface stability. The content of 1% by weight with respect to 100 said structure said core stabilizing compound. 5 to 3% by weight. 0 implementation being % by weight. Said positive active material surface is layered crystal structure (layered phase) may be disclosed. In said formula 1, 0. 8 ≤ x ≤ 0. Wednesday 9 2000. Said anode including an anode active material of the present invention another implementation relates; including cathode active material of a cathode; and an electrolyte including a lithium rechargeable battery number [...] substrate. Other embodiments of the present invention specific of the operating requirements described hereinafter included in the nanometer range. The positive electrode active material for a lithium secondary battery excellent in capacity according to implementation, battery cycle life characteristics can be [...] number. Figure 1 shows a structure of the lithium secondary battery according to implementation of the present invention also indicating to determine. Figure 2 example experiments 1 to 8 according to using half cell discharge capacity graph representing the result of anode. Figure 3 in the embodiment 1 according to positive active material prepared by the number SEM photograph. Figure 4 in the embodiment 1 according to photograph pictures and SAD TEM positive active material prepared by the number (selected area diffraction). Figure 5 in the embodiment 1 to 3 and reference example 1 to 3 using half cell discharge capacity graph representing the result of the anode. Figure 6 in the embodiment 1 to 3 and reference example 1 to 3 using half cell normal positive cycle life characteristics graph representing the result. Hereinafter, one embodiment of the present invention detailed the on-sensors other. But, this is by way of example number so that when, the present invention is defined by the present invention refers to one category of claim number [...] 802.11a packets not only disclosed. The positive electrode active material for a lithium secondary battery of the present invention in one embodiment according to formula 1 represented compound for including core; and a surface of said core and, Co Al compound or compounds including structure comprises a stabilizing compound. [Formula 1] Lia Nix Coy Mez M1k O2 a-p Tp In said formula 1, 0. 9 ≤ a ≤ 1. 1, 0. 7 ≤ x ≤ 0. 93, 0 < y ≤ 0. 3, 0 < z ≤ 0. 3, 0 ≤ k ≤ 0. 005, x + y + z + k=1, 0 ≤ p ≤ 0. 005 and, Me is Mn or Al and, M1 The Mg, Ba, B, La, Y, Ti, Zr, Mn, Si, V, P, Mo, and W or combinations thereof, The F T are disclosed. Said positive electrode active material i.e. high nickel content, x is 0. 7 to 0. 93 in lithium secondary battery among others. In particular, in said formula 1, 0. 8 ≤ x ≤ 0. Wednesday 9 2000. The nickel high, i.e. x is 0. 7 to 0. 93 in compounds of said formula 1 compounds that exhibit capacity are disclosed. I.e., x is 0. 7 less than, as compared to compounds that demonstrate very high capacity low nickel content compounds are disclosed. In addition, in said formula 1, M1 An anode active material of formula 1 constituting the main component of Ni, and Me Co substituted as part of doping elements (doping element), and vertically moving the Mg, Ba, B, La, Y, Ti, Zr, Mn, Si, V, P, W or Mo implementation being. In addition, T of formula 1 is substituted as positive electrode active material for a portion of the oxygen doping elements, and vertically moving the implementation being F. Said compound Al stability2 O3 , Co3 O4 , Lix CoO2 (A x 0. 9 to 1. 1) can be or a combination of these. This construction allows the stabilizing compound solvent layer structure (layered structure) can be develops movement of negative ions increased capacity, core structure stabilizing initial positive active material can be capable of efficiency and a long life cycle. In addition compound which is highly core nickel content, i.e., x value 0. 7 to 0. 93 phosphorus compound core x value 0. 6 than less than, unstable surface a high content of Ni, said stabilizing compound structure by using back more effectively can be achieved. Said structure during stabilizing compound, Lix CoO2 Co containing precursor is core is included Li film formed from the reaction and can be, during process for preparing lithium secondary battery number, temperatures in heat treatment processes can be spread into the core 2 difference Co containing precursor, formed in the hollow core may be filled. I.e., Lix CoO2 Located inside the core surface and/or can be. Structure stabilizing compound Lix CoO2 When a including, particularly when the surface layer structure to develop, charge and discharge efficiency can be improved cycle life, than suitable. In particular, said compound may be Co compound stability, and vertically moving the Co3 O4 , Lix CoO2 Or Co3 O4 And Lix CoO2 ofBe a mixture. The Co stabilizing compound structure3 O4 And Lix CoO2 ofWhen the mixture, and the mixture ratio exceeds 50:50 to 0% by weight: 100% by weight can be less than disclosed. Co3 O4 And Lix CoO2 ofIn a ratio range of said Co containing precursor coating process occurs more effectively when using release has occurred can be know. Structure when the stabilizing compound Co compound, positive active material core surface than Co concentration is performed and, as a result surface can be Ni reduced concentration to stabilize the surface structure. In addition, while lithium ions in said formula 1 represented compounds including core is exiting Ni, Ni is oxidized to convert the labile 4 transverse, or NiO 2 while transverse reduced is formed, a second thin film transistor can be reducing NiO billion number Co compound formation. Said compound (continuous layer type) or an island form said core stability (uncontinuous island type) can be present in layered surface, layered and an island form may be present in both disapproval. Said compound is present in structure and stability said core surface layered form can be located stabilizing compound becomes more uniform, thereby stabilizing compound having the structure according to effect greater than die, may be suitable for the disclosed. Said structure is a surface of said core by stabilizing compound, can be enhancing material is at least one layer structure, said positive active material surface is layered (layered phase) may have crystal structure. If stabilizing compound structure with respect to an overall layered crystal structure present in a material is at least may have, in the form of islands if layered crystal structure and may have mixed (mixed phase) crystal structure together. In addition, with respect to form and in the form of islands form two stabilizing compound structure even in the presence layered crystal structure and crystal structure may have mixed together. The content of 1% by weight with respect to 100 said structure said core stabilizing compound. 5 to 3% by weight. 0% by weight may be, 2. 0 to 3% by weight. 0 implementation being % by weight. Stabilizing compound content of said structure when said range an excellent discharge capacity and cycle life characteristics, in particular room temperature can exhibit a long life cycle. The positive electrode active material of the present invention in one embodiment according to the process bath 1308. number hereinafter. Lithium-containing compounds, nickel-containing compounds, cobalt-containing compound and containing Me therewith, in addition M1 Containing compound or T-containing compound mixture by mixing number high pressure liquid coolant therein. Said lithium containing compound is lithium acetate, lithium nitrate, lithium hydroxide, lithium carbonate, lithium acetate, their hydrate, or combinations thereof is cited. Said nickel containing compound is nickel nitrate, nickel hydroxide, nickel carbonate, nickel acetate, nickel sulfate, their hydrate, or combinations thereof is cited. In addition, said cobalt-containing compound is cobalt nitrate, cobalt hydroxide, cobalt acetate, cobalt carbonate, cobalt sulfate, cited their hydrate or a combination thereof, said Me Me containing compounds containing nitrate, Me containing hydroxide, carbonate containing Me, Me containing acetate, Me containing sulfate, their hydrate or a combination thereof is cited. Said M1 M containing compounds include1 Containing nitrate, M1 Containing hydroxide, M1 Containing carbonate, M1 Containing acetate, M1 Containing carbonate, M1 Containing oxide, M1 Cited containing sulfate their hydrate or a combination thereof, and, said T containing nitrate containing compounds include T, T containing hydroxide, carbonate containing T, T containing acetate, T containing sulfate, T containing oxide, their hydrate or a combination thereof is cited. Said lithium-containing compound, said nickel-containing compound, said cobalt-containing compound, said Me containing compound, said M1 Containing compounds and said T containing compound ratio obtained compounds of said formula 1 can be properly controlled. 1 difference number said mixture by heat core high pressure liquid coolant therein. The heat treatment process in embodiment 1 700 °C to 1000 °C difference can, the heat treatment time is 3 hr to 20 can be time. In addition, said heat treatment process oxygen (O2 ) Atmosphere, or standby can in embodiment (air). Stabilizing compound structure of said surface of the core coated with a carbon precursor substrate. This structure stabilizing compound precursor include Al (OH)3 , Co (OH)3 , Al2 O3 , Co3 O4 , Or a combination thereof can be used. Said solvent can be electrically connected using coating method is advantageous embodiment, solvent can be embodiment that does not use a dry process. When electrically connected embodiment, solvent water, ethyl alcohol, or isopropyl alcohol can be used. Said surface coated with a carbon precursor in the process for stabilizing compound surface of the core, said core and said final product 100% by weight in said core surface stabilization compound precursor mixture ratio for a surface-stabilized compound 1. 5 to 3% by weight. 0% by weight to form is can be. After said coating process embodiment, an anode active material to heat treatment difference embodiment number 2 high pressure liquid coolant therein. Said 2 500 °C to 800 °C 5 hr to 20 hours can be embodiment in the junction area. Said 2 with a junction temperature and time if said range, structure stabilizing compound used for forming the diffusion well compound surface, core stabilizing compound can be effectively within a pressure range of surface structure. Surface stabilizing compound Al precursor (OH)3 Is used, the heat treatment is performed said 2 difference, Al2 O3 Surface stabilizing compound can be formed. In addition, stabilizing compound Co precursor (OH) surface3 Is used, the heat treatment is performed said 2 difference, Co (OH)3 While said spread with a portion of the opening in the hollow core, which react with the speckled appearance that Li, Lix CoO2 Formed in the core may be filled. An anode active material of the present invention another implementation relates including said anode, cathode and electrolyte lithium secondary battery including a negative electrode active material including number [...] substrate. The anode has said deposition, the positive electrode active material comprising the current collector comprises a support. Said positive electrode active material layer, said 90 to 98% by weight relative to the weight of the entire positive electrode active material layer positive active material content implementation being % by weight. In one embodiment of the present invention, anode active material further comprises a conductive material on said qualitative layer binder and can be. The, said binder and conductive material 1% by weight to 5% by weight relative to the weight of the content of the entire positive electrode active material layer each implementation being. A positive electrode active material particles adhere well to each other said binder, in addition to an anode active material adheres well to a current collector could be bonded each other. Typical binder include polyvinyl alcohol, carboxymethyl cellulose, hydroxypropyl cellulose, diacetyl cellulose, polyvinyl chloride, carboxylic it became luck misfire polyvinyl chloride, poly [...], including ethylene oxide polymer, polyvinylpyrrolidone, polyurethane, polytetrafluoroethylene, polyvinylidene fluoride, polyethylene, polypropylene, styrene - butadiene rubber, styrene - butadiene rubber upper part of a acrylic, epoxy resin, but nylon etc., limited to are not correct. Said conductive material as used for imparting electrical conductivity electrode, in a battery, chemical and causes a change in the electronic conductive material without anything available disclosed. Examples of the conductive material natural graphite, artificial graphite, carbon black, acetylene black, [...], carbon fiber such as a carbon-containing substance; copper, nickel, aluminum, or metal fibers and the like the thin metal-based material; conductive polymer such as polyphenylene derivatives; or mixtures thereof including conductive material is cited. Using said current collector include Al but not the limited to. Said current collector and the cathode is formed on the current collector including an alkali metal comprising the anode active material layer. Manufactured by mixing lithium ions reversibly intercalation/calais which is a d will do [syen said substance, lithium metal, lithium metal alloy, lithium dope and mask [phu or more transition metal oxide. Said lithium ions reversibly intercalation/calais which is a d will do [syen as substance include carbon, carbon-based anode active material commonly used in lithium ion secondary battery and unbreakable and may also be employed, examples range amorphous carbon, amorphous carbon can be used with both. Examples of said amorphous carbon is amorphous, plate-like, phosphorus side as a matter of (flake), natural graphite or artificial graphite such as spherical or fiber-type graphite cited, as an example of the soft carbon or hard carbon (hard carbon) (soft carbon) said amorphous carbon, carbide battery negative electrode material, mixture of calcined coke or the like is cited. Said lithium lithium metal alloy include Na, K, Rb, Cs, Fr, Be, Mg, Ca, Sr, Si, Sb, Pb, In, Zn, Ba, Ra, Ge, composed of a metal selected from the group consisting Al and Sn can be produced. Said mask [phu dope and lithium material include Si, Si-a C composite, SiOx (0 < x < 2), Si-a Q alloy (said Q is alkali metal, alkaline earth metal, 13 group, 14 group element, 15 group, 16 elements, transition metal, a rare earth element and combination thereof and of elements selected from the group consisting of, but not Si), Sn, SnO2 , Sn-a R alloy (said R is an alkali metal, alkaline earth metal, 13 group, 14 group element, 15 group, 16 elements, transition metal, a rare earth element and combination thereof and of elements selected from the group consisting of, but not Sn) such as cited, in addition at least one of these and SiO2 Mixed a disapproval. Said element Q and R include Mg, Ca, Sr, Ba, Ra, Sc, Y, Ti, Zr, Hf, Rf, V, Nb, Ta, Db, Cr, Mo, W, Sg, Tc, Re, Bh, Fe, Pb, Ru, Os, Hs, Rh, Ir, Pd, Pt, Cu, Ag, Au, Zn, Cd, B, Al, Ga, Sn, In, Tl, Ge, P, As, Sb, Bi, S, Se, Te, Po, and combination thereof can be selected from the group consisting of using. Said transition metal oxide include vanadium oxide, lithium vanadium oxide or lithium titanium oxide or the like as is cited. Said negative electrode active material layer 95 to 99% by weight relative to the weight of the entire cathode active material layer of negative electrode active material content implementation being % by weight. In one embodiment of the present invention, wherein said anode active material layer is binder, optionally further include a conductive material may be filled. Said negative electrode active material layer 1% by weight to 5% by weight relative to the weight of the content of the total cathode active material layer binder implementation being. In addition 90% to 98% by weight is more a conductive material including a negative electrode active material weight, 1% by weight to 5% by weight binder, 1% by weight to 5% by weight can be using a conductive material. Said negative electrode active material particle binder adhere well to each other, in addition to negative electrode active material adheres well to a current collector could be bonded each other. Said binder include water-insoluble binder, can be using a water soluble binder or a combination thereof. Said non-aqueous binder include polyvinyl chloride, carboxylic it became luck misfire polyvinyl chloride, poly [...], including ethylene oxide polymer, polyvinylpyrrolidone, polyurethane, polytetrafluoroethylene, polyvinylidene fluoride, polyethylene, polypropylene, polyamide-imide, polyimide or combinations thereof is cited. Said water soluble binder include rubber-based binder or polymer resin binder is cited. Said styrene - butadiene rubber-based binder rubber, styrene - butadiene rubber (SBR) upper part of a acrylic, acrylonitrile - butadiene rubber, acrylic rubber, butyl rubber, fluorine rubber and combinations thereof be a selected. Said polymer resin binder polytetrafluoroethylene, polyethylene, polypropylene, ethylene propylene copolymer, polyethylene oxide, polyvinyl pyrrolidone, poly in peek it gave, polyphosphazene, polyacrylonitrile, polystyrene, ethylene propylene diene copolymer, polyvinyl pyridine, chloro opinion phone polyethylene, latex, polyester resin, acrylic resin, phenol resin, epoxy resin, poly vinyl alcohol and combinations thereof to be a selected. Said water soluble binder is used cathode binder, viscosity cellulosic compounds can impart to number can be further more. The cellulose-based compounds include carboxy methylcellulose, hydroxypropyl methylcellulose, methylcellulose, or the like can be mixed at least one alkaline metal salt 1. Said alkali metal include Na, K or Li can be using. 100 parts by weight of the negative active material content more use number 0. 1 part by weight to 3 parts by weight can be. Said conductive material as used for imparting electrical conductivity electrode, in a battery, chemical and causes a change in the electronic conductive material without anything available disclosed. Examples of the conductive material natural graphite, artificial graphite, carbon black, acetylene black, [...], carbon fiber such as a carbon-containing substance; copper, nickel, aluminum, or metal fibers and the like the thin metal-based material; conductive polymer such as polyphenylene derivatives; or mixtures thereof including conductive material can be used. Said current collector include foil, nickel foil, stainless steel foil, titanium foil, nickel foam (foam), copper foam, conductive metal coated with polymer base, and combination thereof can be selected from the group consisting of using. Said electrolyte is a nonaqueous organic solvent and a lithium salt therein. Said electrochemical reaction of a single involved in a nonaqueous organic solvent medium can move the ions could be bonded each other. Said nonaqueous organic solvent carbonate-based, ester-based, ether-based, ketone-based, alcohol, or aprotic solvent can be used. Dimethyl carbonate (DMC) said carbonate-based solvent, diethyl carbonate (DEC), dipropyl carbonate (DPC), methyl propyl carbonate (MPC), (EPC) ethyl propyl carbonate, methyl ethyl carbonate (MEC), ethylene carbonate (EC), propylene carbonate (PC), butylene carbonate (BC) can be is used as the alkali. Said ester-based solvent methyl acetate, ethyl acetate, n - propyl acetate, dimethyl acetate, methyl propionate, ethyl propionate, the car it is yellow the id (decanolide), roh maul manifestation [lak ton (mevalonolactone), is used as the polycaprolactone (caprolactone) can be. Said ether solvent dibutyl ether, tetra glycol Lyme, being a d writing,, [...], 2 - methyl tetra draw [phyu column, can be draw [phyu column 2 is used as the alkali. In addition, said ketone-based solvent can be cyclohexanone is used as the alkali. In addition said alcohol solvent ethyl alcohol, isopropyl alcohol can be used, said aprotic solvent R a-CN(R carbon atoms 2 to 20 linear, branched, or ring structure hydrocarbon group, an aromatic ring double bonds can be bond or ether) glycols such as nitriles, amides such as dimethyl formamide, d jade brush turbulent flow such as 1, 3 - dioxolane, sulfonyl cancer (sulfolane) or the like can be used. Said organic solvent alone or one or more can be mixed, if desired one or more by weight of the blending ratio of the battery can be properly controlled depending upon the capabilities, this person does not risks caused by the field can be widely understood. In addition, in the case of said carbonate-based solvent, an annular (cyclic) carbonate (chain) mixed carbonate solution for the now. In this case annular carbonate is mixed at a volumetric ratio of 1:9 to 1:1 carbonate solution for the excellent performance of electrolyte can be appears. Said organic solvent is an aromatic hydrocarbon organic solvent further comprises said carbonate-based solvent disapproval. the organic solvent is 1:1 to 30:1 and aromatic hydrocarbon solvent in a fire retardant can be blended at a volumetric ratio of. Said aromatic hydrocarbon organic solvent can be aromatic hydrocarbon compound are used to formula 2. [Formula 2] (In said formula 2, R1 To R6 Hereinafter are equal to each other or different from hydrogen, halogen, alkyl of 1 to 10 carbon atoms, halo alkyl and combinations thereof selected from the group consisting are disclosed.) Said aromatic hydrocarbon organic solvent specific examples are benzene, fluorobenzene, transducer [...] 1, 2 -, 1, 3 - [...] deployment, deployment [...] 1, 4 -, 1, 2, 3 - trifluoromethyl benzene, 1, 2, 4 - trifluoromethyl benzene, chlorobenzene, 1, 2 - dichloro benzene, 1, 3 - dichloro benzene, 1, 4 - dichloro benzene, 1, 2, 3 - difluoroaniline, 1, 2, 4 - difluoroaniline, eye coordinate of benzene, the method of detecting the relative coordinate of 1, 2 - benzene, 1, 3 - benzene relative coordinate of the method, the method of detecting the relative coordinate of 1, 4 - benzene, 1, 2, 3 - triazole ionomer also benzene, 1, 2, 4 - triazole ionomer also benzene, toluene, fluorotoluene, 2, 3 - transducer base oro [thol base n, n transducer base oro [thol base 2, 4 -, 2, 5 - transducer base oro [thol base n, 2, 3, 4 - trifluoro toluene, toluene 2, 3, 5 - trifluoromethyl, chloro by weight of the, dichloro by weight of the 2, 3 -, 2, 4 - dichloro by weight of the, 2, 5 - dichloro by weight of the, n with [thol base for the production of 2, 3, 4 -, 2, 3, 5 - ene with [thol base production, eye five degrees [thol base n, 2, 3 - diaryl ionomer also toluene, toluene 2, 4 - diaryl also ionomer, ionomer diaryl also toluene 2, 5 -, 2, 3, 4 - triazole ionomer also toluene, 2, 3, 5 - triazole ionomer also toluene, xylene, and combinations thereof selected from the group consisting are disclosed. Order to enhance the lifetimes for said battery electrolyte to ethylene vinylene carbonate or a fire retardant compound number added to further improves the life of formula 3 may be filled. [Formula 3] (In said formula 3, R7 And R8 Which are equal to each other or different from the hereinafter, hydrogen, a halogen, a cyano group (CN), nitro (NO2 ) And 1 to 5 carbon atoms selected from the group consisting of fluorinated alkyl, said R7 And R8 For at least one of halogen, cyano group (CN), nitro (NO2 ) And fluorinated carbon atoms selected from the group consisting of C20 alkyl or 1 to 5, stage R7 And R8 All hydrogen is endured.) Said ethylene carbonate-based compound examples representative difluoro purification, chloro ethylene carbonate, dichloroethylene carbonate, ethylene carbonate bromo, with d [pu wool ethylene carbonate, knit with ethylene carbonate, cyano roh ethylene carbonate or fluoro ethylene carbonate or the like is cited. This improves the life of number can be added if further using is used in an amount properly controlled. Said lithium salt is dissolved in an organic solvent, in the underlying source of lithium ions in the cell of a lithium secondary battery supported at and acting, promoting the movement of lithium ions between cathode and anode and which serves to material that. Such lithium salt LiPF representative examples6 , LiBF4 , LiSbF6 , LiAsF6 , LiN (SO2 C2 F5 )2 , Li (CF3 SO2 )2 N, LiN (SO3 C2 F5 )2 , LiC4 F9 SO3 , LiClO4 , LiAlO2 , LiAlCl4 , LiN (Cx F2x + 1 SO2 ) (Cy F2y + 1 SO2 ) (Wherein, x and y data base stores and is, for example 1 to 20 are integers), LiCl, LiI and LiB (C2 O4 )2 (Borate (lithium bis (oxalato) borate: LiBOB) oxalate lithium bis selected from the group consisting of support (supporting) includes one or more electrolytic salt. 0 lithium salt concentration. 1M to 2. 0M range in use now. Are included in said range of lithium salt concentration, electrolyte conductivity and viscosity that is suitable in the periphery of the electrolyte may be excellent performance, lithium ions can be effectively. Depending on the type of lithium secondary battery anodes and a cathode separator disapproval may be present. The separator include polyethylene, polypropylene, polyvinylidene fluoride or 2 at least one layer of multilayer film can be used, polyethylene/polypropylene 2 layer separator, polyethylene/polypropylene/polyethylene 3 layer separator, polypropylene/polyethylene/polypropylene separator layer 3 mixed multilayer film can be used such as the concave disclosed. In one embodiment of the present invention also showed decomposition of perspective view according to the lithium secondary battery 1. In one implementation according to a square-shaped lithium secondary battery in size but is described, the present invention herein are number are not one which, cylindrical, pouch-type or the like can be applied to a variety of types of cell. With reference to the 1 also, in one implementation according to lithium secondary battery (100) comprises a positive electrode (10) and a cathode (20) between a separator (30) it became [kwin via an electrode assembly (40) on, said electrode assembly (40) incorporating the case (50) can be a. Said anode (10), said cathode (20) and said separator (30) to (not shown) is impregnated electrolyte thereof can. Hereinafter of the present invention in the embodiment and comparison examples described substrate. In the embodiment of the present invention in the embodiment for the present invention are provided for in the embodiment is one such one is limited to one and not the. (Experimental example 1) Lithium carbonate, nickel sulfate, cobalt sulfate and manganese oligoether mixing Li: Ni: Co: Mn is 1:0. 5:0. 2:0. 3 to molar ratio is mixed. Said mixture 740 °C and oxygen (O2 ) By heat-treating time in the load lock 20, LiNi0. 5 Co0. 2 Mn0. 3 O2 An anode active material number was high pressure liquid coolant. Positive active material prepared by the number 94% by weight, 3% by weight to 3% by weight polyvinylidene fluoride binder and [khey tsien black conductive material by mixing lithium secondary battery composition number N - methylpyrrolidone solvent in his high pressure liquid coolant. An anode active material composition Al applied to a current collector, a positive electrode number was high pressure liquid coolant. (Experimental example 2) Lithium carbonate, nickel sulfate, cobalt sulfate and manganese oligoether, Li: Ni: Co: Mn is 1:0. 6:0. 2:0. 2 molar ratio equal to the number to said embodiment and example 1 by mixing the [...] experiments, LiNi0. 6 Co0. 2 Mn0. 2 O2 An anode active material number was high pressure liquid coolant. An anode active material prepared by the number equal to the number his embodiment example 1 experiment using high pressure liquid coolant to said anode. An anode active material prepared by the number equal to the number his embodiment example 1 experiment using high pressure liquid coolant to said anode. (Experimental example 3) Lithium hydroxide, nickel sulfate, cobalt sulfate and manganese oligoether, Li: Ni: Co: Mn is 1:0. 7:0. 15:0. 15 molar ratio equal to the number to said embodiment and example 1 by mixing the [...] experiments, LiNi0. 7 Co0. 15 Mn0. 15 O2 An anode active material number was high pressure liquid coolant. An anode active material prepared by the number equal to the number his embodiment example 1 experiment using high pressure liquid coolant to said anode. (Experimental example 4) Lithium hydroxide, nickel sulfate, cobalt sulfate and aluminum oligoether, Li: Ni: Co: Al is 1:0. 8:0. 15:0. 05 to 1 molar ratio equal to the number to said embodiment and example [...] mixing experiments, LiNi0. 8 Co0. 15 Al0. 05 O2 An anode active material number was high pressure liquid coolant. An anode active material prepared by the number equal to the number his embodiment example 1 experiment using high pressure liquid coolant to said anode. (Experimental example 5) Lithium hydroxide, nickel sulfate, cobalt sulfate and aluminum oligoether, Li: Ni: Co: Al is 1:0. 82:0. 15:0. 03 to 1 molar ratio equal to the number to said mixing [...] embodiment and example experiment, LiNi0. 82 Co0. 15 Al0. 03 O2 An anode active material number was high pressure liquid coolant. An anode active material prepared by the number equal to the number his embodiment example 1 experiment using high pressure liquid coolant to said anode. (Experiment example 6) Lithium hydroxide, nickel sulfate, cobalt sulfate and aluminum oligoether, Li: Ni: Co: Al is 1:0. 85:0. 135:0. 015 to said mixing molar ratio equal to 1 and the number [...] experiments to example embodiment, LiNi0. 85 Co0. 135 Al0. 015 O2 An anode active material number was high pressure liquid coolant. An anode active material prepared by the number equal to the number his embodiment example 1 experiment using high pressure liquid coolant to said anode. (Experimental example 7) Lithium hydroxide, nickel sulfate, cobalt sulfate and aluminum oligoether, Li: Ni: Co: Al is 1:0. 9:0. 09:0. 01 to 1 molar ratio equal to the number to said mixing [...] experiment example and embodiment, LiNi0. 9 Co0. 09 Al0. 01 O2 An anode active material number was high pressure liquid coolant. An anode active material prepared by the number equal to the number his embodiment example 1 experiment using high pressure liquid coolant to said anode. (Experiment example 8) Lithium hydroxide, nickel sulfate, cobalt sulfate and aluminum oligoether, Li: Ni: Co: Al is 1:0. 92:0. 07:0. 01 to 1 molar ratio equal to the number to said mixing [...] experiment example and embodiment, LiNi0. 92 Co0. 07 Al0. 01 O2 An anode active material number was high pressure liquid coolant. An anode active material prepared by the number equal to the number his embodiment example 1 experiment using high pressure liquid coolant to said anode. Example experiment 1 according to said anode prepared by the number 8, a conventional method to form a half cell using the electrolyte lithium metal opposite and coin number was high pressure liquid coolant. Said electrolyte 1. 0M LiPF6 Is dissolved in the mixed solvent of ethylene carbonate and diethyl carbonate (50:50 volume ratio) as well as a. In a half cell prepared by the number 25 °C, 3. 0V to 4. 3V within range 0. 2C measuring certain interval symmetrically to discharge capacity, also shown to result 2. As shown in fig. 2, the nickel content can be overlapped with the know elements have, in particular Lia Nix Coy Mnz O2 X value 0 compounds or combinations of these. 7 or more when (in Figure 2 70% or more), in particular 180 mAh/g or more can be high capacity when it is know. (In the embodiment 1) Lithium hydroxide, nickel sulfate, cobalt sulfate and aluminum oligoether Li: Ni: Co: Al is 1:0. 85:0. 135:0. 015 to molar ratio is mixed. Said mixture 740 °C and oxygen (O2 ) 20 1 time difference by heat treatment atmosphere, LiNi0. 85 Co0. 135 Al0. 015 O2 Core number was high pressure liquid coolant. Said core and Co (OH)2 100% a by weight: 1. 5% by weight in water solvent mixing ratio and wet coating process embodiment, an atmosphere 10 700 °C and oxygen mixture by heat LiNi 2 time difference0. 85 Co0. 135 Al0. 015 O2 Core and positioned on the Co3 O4 And Lix CoO2 (50:50% by weight,X=1. 0) compound number was high pressure liquid coolant and stability including an anode active material. Said Co3 O4 And Lix CoO2 Said core surface form a lamellar compound was an island form stability. In addition, the overall content 100% by weight in said core stabilizing compound final positive active material to said structure 1. 5% by weight was. Positive active material prepared by the number 94% by weight, 3% by weight to 3% by weight polyvinylidene fluoride binder and [khey tsien black conductive material by mixing lithium secondary battery composition number N - methylpyrrolidone solvent in his high pressure liquid coolant. An anode active material composition Al applied to a current collector, a positive electrode number was high pressure liquid coolant. (In the embodiment 2) In LiNi prepared by the number said in the embodiment 10. 85 Co0. 135 Al0. 015 O2 Core Co (OH)2 100% a by weight: 2% by weight ratio equal to the number [...] mixing said in the embodiment 1 and the embodiment, core and positioned on the Co3 O4 And Lix CoO2 (50:50% by weight, x=1. 0) compound number was high pressure liquid coolant and stability including an anode active material. 100% by weight with respect to said stabilizing compound content in said final positive active material was 2% by weight core structure. An anode active material prepared by the number equal to the number anode was said in the embodiment 1 embodiment using high pressure liquid coolant. (In the embodiment 3) In LiNi prepared by the number said in the embodiment 10. 85 Co0. 135 Al0. 015 O2 Core Co (OH)2 A 100 weight %: 3% by weight ratio equal to the number [...] mixing said in the embodiment 1 and the embodiment, core and positioned on the Co3 O4 And Lix CoO2 (50:50% by weight,X=1. 0) compound number was high pressure liquid coolant and stability including an anode active material. 100% by weight with respect to said stabilizing compound content in said final positive active material was 3% by weight core structure. An anode active material prepared by the number equal to the number anode was said in the embodiment 1 embodiment using high pressure liquid coolant. (Reference example 1) In LiNi prepared by the number said in the embodiment 10. 85 Co0. 135 Al0. 015 O2 Core Co (OH)2 A weight 100%: 0. 5% by weight ratio equal to the number [...] mixing said in the embodiment 1 and the embodiment, core and positioned on the Co3 O4 And Lix CoO2 (50:50% by weight, x=1. 0) compound number was high pressure liquid coolant and stability including an anode active material. In said ZrO final lithium secondary battery2 0 to 100% by weight said core surface stabilizing compound content. 1 been % by weight. An anode active material prepared by the number equal to the number anode was said in the embodiment 1 embodiment using high pressure liquid coolant. (Reference example 2) In LiNi prepared by the number said in the embodiment 10. 85 Co0. 135 Al0. 015 O2 Core Co (OH)2 100% by weight a: 1 weight % mixing ratio equal to the number [...] said in the embodiment 1 and the embodiment, core and positioned on the Co3 O4 And Lix CoO2 (50:50% by weight, x=1. 0) compound number was high pressure liquid coolant and stability including an anode active material. 100% by weight with respect to said stabilizing compound content in said final positive active material was 1% by weight core structure. An anode active material prepared by the number equal to the number anode was said in the embodiment 1 embodiment using high pressure liquid coolant. (Reference example 3) In LiNi prepared by the number said in the embodiment 10. 85 Co0. 135 Al0. 015 O2 Core Co (OH)2 A 100% by weight: 5% by weight ratio equal to the number [...] mixing said in the embodiment 1 and the embodiment, core and positioned on the Co3 O4 And Lix CoO2 (50:50% by weight, x=1. 0) compound number was high pressure liquid coolant and stability including an anode active material. 100% by weight with respect to said stabilizing compound content in said final positive active material was 5% by weight core structure. An anode active material prepared by the number equal to the number anode was said in the embodiment 1 embodiment using high pressure liquid coolant. * Surface characterization SEM positive active material prepared by the number said in the embodiment 1 according to photograph 3 also shown. As also shown in 3, in the embodiment 1 is an island form a lamellar structure including a surface of the positive electrode active material coated Co stabilizing compound form can be beat. In addition, the photograph of Figure 4 TEM positive active material of said in the embodiment 1 (A) precursor, (A) 1 of Figure 4 corresponding to the surface of Figure 4 shown in photograph (selected area diffraction) SAD (B) value, corresponding to the surface of the photograph (C) shown SAD 2 of Figure 4. (B) and (C) such as shown in of Figure 4, in the embodiment 1 of positive active material surface is layered crystal structure and have both mixed-crystal structure can be know. * Cell characterization Said in the embodiment 1 according to example 1 to 3 and reference anode prepared by the number 3, a conventional method to form a half cell using the electrolyte lithium metal opposite and coin number was high pressure liquid coolant. Said electrolyte 1. 3M LiPF6 Is dissolved in the mixed solvent of ethylene carbonate and diethyl carbonate (50:50 volume ratio) as well as a. In a half cell prepared by the number 25 °C, 3. 0V to 4. 3V within range 0. 2C embodiment certain interval symmetrically to 50 times to, discharge capacity were measured. In addition, 1 to 50 times by calculating the discharge capacity ratio times discharge capacity capacity retention is determined, cycle life same electrodes respectively. Obtained results, in the embodiment 1 to 3 and reference example 1 to 3 shown in table 1 for results for the anode using half cell. In addition, in the embodiment 1 to 3 and reference example 1 to 3 using half cell discharge capacity to also result for the anode 5, 6 also results have shown the cycle life characteristics at room temperature. Said table 1, 5 and 6 such as also shown in also, a stabilizing compound Co structure3 O4 And Lix CoO2 1 total content. 5% by weight to 3% by weight in the embodiment 1 to 3 using half cell using an anode active material in the anode of the discharge capacity and the cycle life characteristics while its 200 mAh/g 85% or more, for example 1 and 2 less than is trapped on said structure said stabilizing compound content range in the case of capacity and the cycle life characteristics degraded result is greater than the range reference example 3 can be obtained beat. (Comparison example 1) Lithium hydroxide, nickel sulfate, cobalt sulfate and aluminum oligoether Li: Ni: Co: Al is 1:0. 85:0. 135:0. 015 to molar ratio is mixed. Said mixture 740 °C and oxygen (O2 ) 20 1 time difference by heat treatment atmosphere, LiNi0. 85 Co0. 135 Al0. 015 O2 An anode active material number was high pressure liquid coolant. An anode active material prepared by the number equal to the number anode was said in the embodiment 1 embodiment using high pressure liquid coolant. * Cell characterization Said in the embodiment 1 and comparison example 1 anode prepared by the number according, to a high pressure liquid coolant and a conventional method using the electrolyte lithium metal opposite number his coin form half cell. Said electrolyte 1. 3M LiPF6 Is dissolved in the mixed solvent of ethylene carbonate and diethyl carbonate (50:50 volume ratio) as well as a. A half cell prepared by the number 2. 8V to 4. 4V in 3. 0 mA/cm2 Current density 0. The embodiment was a certain interval symmetrically times limit 1C 1 embodiment. , the charge-discharge capacity and efficiency is found to have shown to table 2. In addition, a battery limit switch embodiment 2. 8V to 4. 4V in 3. 0 mA/cm2 Current density 0. 2C after certain interval symmetrically embodiment to 1 times, to obtain a discharge capacity to table 2 shown together. Such as shown in said table 2, present at the surface of the stabilizing compound structure in the embodiment 1 of positive active material charge and discharge characteristics of comparison example 1 stabilizing compound this structure does not include secondary battery excellent gear stage can be beat. (In the embodiment 4) Lithium hydroxide, nickel sulfate, cobalt sulfate and manganese oligoether Li: Ni: Co: Mn is 1:0. 85:0. 135:0. 015 to molar ratio is mixed. Said mixture 740 °C and oxygen (O2 ) 20 1 time difference by heat treatment atmosphere, LiNi0. 85 Co0. 135 Mn0. 015 O2 Core number was high pressure liquid coolant. Said core and Co (OH)2 100% a by weight: 1. 5% by weight in water solvent mixing ratio and wet coating process embodiment, an atmosphere 10 700 °C and oxygen mixture by heat LiNi 2 time difference0. 85 Co0. 135 Mn0. 015 O2 Core and positioned on the Co3 O4 And Lix CoO2 (50:50% by weight,X=1. 0) compound number was high pressure liquid coolant and stability including an anode active material. Said Co3 O4 And Lix CoO2 Said core surface form a lamellar compound was an island form stability. In addition, the overall content 100% by weight in said core stabilizing compound final positive active material to said structure 1. 5% by weight was. Positive active material prepared by the number 94% by weight, 3% by weight to 3% by weight polyvinylidene fluoride binder and [khey tsien black conductive material by mixing lithium secondary battery composition number N - methylpyrrolidone solvent in his high pressure liquid coolant. An anode active material composition Al applied to a current collector, a positive electrode number was high pressure liquid coolant. (In the embodiment 5) In LiNi prepared by the number said in the embodiment 40. 85 Co0. 135 Mn0. 015 O2 Core Co (OH)2 100% a by weight: 2% by weight ratio equal to the number [...] mixing said in the embodiment 1 and the embodiment, core and positioned on the Co3 O4 And Lix CoO2 (50:50% by weight, x=1. 0) compound number was high pressure liquid coolant and stability including an anode active material. 100% by weight with respect to said stabilizing compound content in said final positive active material was 2% by weight core structure. An anode active material prepared by the number equal to the number anode was said in the embodiment 1 embodiment using high pressure liquid coolant. (In the embodiment 6) In LiNi prepared by the number said in the embodiment 40. 85 Co0. 135 Mn0. 015 O2 Core Co (OH)2 A 100 weight %: 3% by weight ratio equal to the number [...] mixing said in the embodiment 1 and the embodiment, core and positioned on the Co3 O4 And Lix CoO2 (50:50% by weight,X=1. 0) compound number was high pressure liquid coolant and stability including an anode active material. 100% by weight with respect to said stabilizing compound content in said final positive active material was 3% by weight core structure. An anode active material prepared by the number equal to the number anode was said in the embodiment 1 embodiment using high pressure liquid coolant. (Reference example 4) In LiNi prepared by the number said in the embodiment 40. 85 Co0. 135 Mn0. 015 O2 Core Co (OH)2 A weight 100%: 0. 5% by weight ratio equal to the number [...] mixing said in the embodiment 1 and the embodiment, core and positioned on the Co3 O4 And Lix CoO2 (50:50% by weight, x=1. 0) compound number was high pressure liquid coolant and stability including an anode active material. In said ZrO final lithium secondary battery2 0 to 100% by weight said core surface stabilizing compound content. 1 been % by weight. An anode active material prepared by the number equal to the number anode was said in the embodiment 1 embodiment using high pressure liquid coolant. (Reference example 5) In LiNi prepared by the number said in the embodiment 40. 85 Co0. 135 Mn0. 015 O2 Core Co (OH)2 100% by weight a: 1 weight % mixing ratio equal to the number [...] said in the embodiment 1 and the embodiment, core and positioned on the Co3 O4 And Lix CoO2 (50:50% by weight, x=1. 0) compound number was high pressure liquid coolant and stability including an anode active material. 100% by weight with respect to said stabilizing compound content in said final positive active material was 1% by weight core structure. An anode active material prepared by the number equal to the number anode was said in the embodiment 1 embodiment using high pressure liquid coolant. (Reference example 6) In LiNi prepared by the number said in the embodiment 40. 85 Co0. 135 Mn0. 015 O2 Core Co (OH)2 A 100% by weight: 5% by weight ratio equal to the number [...] mixing said in the embodiment 1 and the embodiment, core and positioned on the Co3 O4 And Lix CoO2 (50:50% by weight, x=1. 0) compound number was high pressure liquid coolant and stability including an anode active material. 100% by weight with respect to said stabilizing compound content in said final positive active material was 5% by weight core structure. An anode active material prepared by the number equal to the number anode was said in the embodiment 1 embodiment using high pressure liquid coolant. * Cell characterization Reference number 4 to 6 said in the embodiment 4 according to example 6 and anode produced therewith, a high pressure liquid coolant to a conventional method using the electrolyte lithium metal opposite and his number coin form half cell. Said electrolyte 1. 3M LiPF6 Is dissolved in the mixed solvent of ethylene carbonate and diethyl carbonate (50:50 volume ratio) as well as a. In a half cell prepared by the number 25 °C, 3. 0V to 4. 3V within range 0. 2C embodiment certain interval symmetrically to 50 times to, discharge capacity were measured. In addition, 1 to 50 times by calculating the discharge capacity ratio times discharge capacity capacity retention is determined, cycle life same electrodes respectively. Obtained results, for example 4 to 6 in the embodiment 4 to 6 and reference to results for the anode using half cell shown to table 3. Said such as shown in table 3, a stabilizing compound Co structure3 O4 And Lix CoO2 1 total content. 5% by weight to 3% by weight in the embodiment 4 to 6 using half cell using an anode active material in the anode 200 mAh/g 85% or more and the discharge capacity and the cycle life characteristics while its, structure 4 and 5 for example is trapped on said stabilizing compound content less than said range in the case of capacity and the cycle life characteristics degraded result is greater than the range reference example 6 obtained can be beat. (Comparison example 2) Lithium hydroxide, nickel sulfate, cobalt sulfate and manganese oligoether Li: Ni: Co: Mn is 1:0. 85:0. 135:0. 015 to molar ratio is mixed. Said mixture 740 °C and oxygen (O2 ) 20 1 time difference by heat treatment atmosphere, LiNi0. 85 Co0. 135 Mn0. 015 O2 An anode active material number was high pressure liquid coolant. An anode active material prepared by the number equal to the number anode was said in the embodiment 1 embodiment using high pressure liquid coolant. * Cell characterization Said in the embodiment 4 and comparison example 2 anode prepared by the number according, to a high pressure liquid coolant and a conventional method using the electrolyte lithium metal opposite number his coin form half cell. Said electrolyte 1. 3M LiPF6 Is dissolved in the mixed solvent of ethylene carbonate and diethyl carbonate (50:50 volume ratio) as well as a. A half cell prepared by the number 2. 8V to 4. 4V in 3. 0 mA/cm2 Current density 0. The embodiment was a certain interval symmetrically times limit 1C 1 embodiment. , the charge-discharge capacity and efficiency is found to have shown to table 4. In addition, a battery limit switch embodiment 2. 8V to 4. 4V in 3. 0 mA/cm2 Current density 0. 2C to 1 times after certain interval symmetrically embodiment, shown together to obtain a discharge capacity to table 4. Such as shown in said table 4, present at the surface of the stabilizing compound structure in the embodiment 4 of positive active material charge and discharge characteristics of comparison example 2 stabilizing compound this structure does not include secondary battery excellent gear stage can be beat. Or more of the present invention preferred embodiment through is described but, on of the drawings and detailed description of the invention is not limited to claim the present invention refers to the preface is elastically deformed and in addition of the present invention variously in the range and the range of possible embodiment of course have disclosed. The present invention relates to a positive electrode active material for a lithium secondary battery, and a lithium secondary battery comprising the positive electrode active material, wherein the positive electrode active material comprises: a core which contains a compound represented by chemical formula 1; and a structure-stabilizing compound which is disposed on the surface of the core, and contains an aluminum compound or a cobalt compound. In the chemical formula 1 of Li_aNi_xCo_yMe_zM^1_kO_(2-p)T_p: Me is Mn or Al; M^1 is Mg, Ba, B, La, Y, Ti, Zr, Mn, Si, V, P, Mo, W, or a combination thereof; and T is F, while the following equations are satisfied: 0.9 <= a <= 1.1, 0.6 <= x <= 0.93, 0 < y <= 0.3, 0 < z <= 0.3, 0 <= k <= 0.005, x + y + z =1, and 0 <= p <= 0.005. COPYRIGHT KIPO 2017 Represented for formula 1 compounds including core; and said core is located at the surface, including Co Al compound or compound including active anode material for secondary lithium compound stability. Li [formula 1]a Nix Coy Mez M1k O2 a-p Tp (In said formula 1, 0. 9 ≤ a ≤ 1. 1, 0. 7 ≤ x ≤ 0. 93, 0 < y ≤ 0. 3, 0 < z ≤ 0. 3, 0 ≤ k ≤ 0. 005, x + y + z + k=1, 0 ≤ p ≤ 0. 005 and, Me is Mn or Al and, M1 The Mg, Ba, B, La, Y, Ti, Zr, Mn, Si, V, P, Mo, W or combinations thereof and, T is about F.) According to Claim 1, said compound Al stability2 O3 , Co3 O4 , Lix CoO2 (A x 0. 9 to 1. 1) positive active material for lithium secondary battery or a combination of these. According to Claim 1, said positive electrode active material for a lithium secondary battery compound Co compound stability. According to Claim 1, said compound is present in an island form said core surface layered or stability for lithium secondary battery positive electrode active material. According to Claim 1, the content of 100% by weight with respect to said structure said core stabilizing compound 1. 5 to 3% by weight. 0 weight % in positive active material for a lithium secondary battery. According to Claim 1, said positive active material surface is layered (layered phase) crystal structure have a positive active material for lithium secondary battery. According to Claim 1, 0. 8 ≤ x ≤ 0. 9 in positive active material for a lithium secondary battery. Any one of Claim 1 to Claim 7 anode including an anode active material; a negative electrode active material including cathode; and an electrolyte including lithium secondary battery. Content (% by weight) stabilizing compound structure Discharge capacity (mAh/g) Room temperature cycle life (%) Reference example 1 0. 5 200 84 Reference example 2 1 200 84 In the embodiment 1 1. 5 200 85 In the embodiment 2 2 201 86 In the embodiment 3 3 201 87 Reference example 3 5 199 83 Limit switch 0. 2C discharge capacity (mAh/g) 0. 1C charge capacity (mAh/g) 0. 1C discharge capacity (mAh/g) Efficiency (%) Comparison example 1 234. 9 209. 1 89. 0 202. 8 In the embodiment 1 235. 7 212. 6 90. 2 207. 6 Content (% by weight) stabilizing compound structure Discharge capacity (mAh/g) Room temperature cycle life (%) Reference example 4 0. 5 200 84 Reference example 5 1 200 84 In the embodiment 4 1. 5 201 86 In the embodiment 5 2 202 87 In the embodiment 6 3 202 87 Reference example 6 5 199 83 Limit switch 0. 2C discharge capacity (mAh/g) 0. 1C charge capacity (mAh/g) 0. 1C discharge capacity (mAh/g) Efficiency (%) Comparison example 2 235. 5 209. 5 88. 9 202. 5 In the embodiment 4 236. 5 212. 5 89. 9 207. 5