CROSSLINKED GAS SEPARATION POLYMERIC MEMBRANE CROSS-LINKED BY BROMINATION/DEBROMINATION, AND METHOD FOR FABRICATING SAME
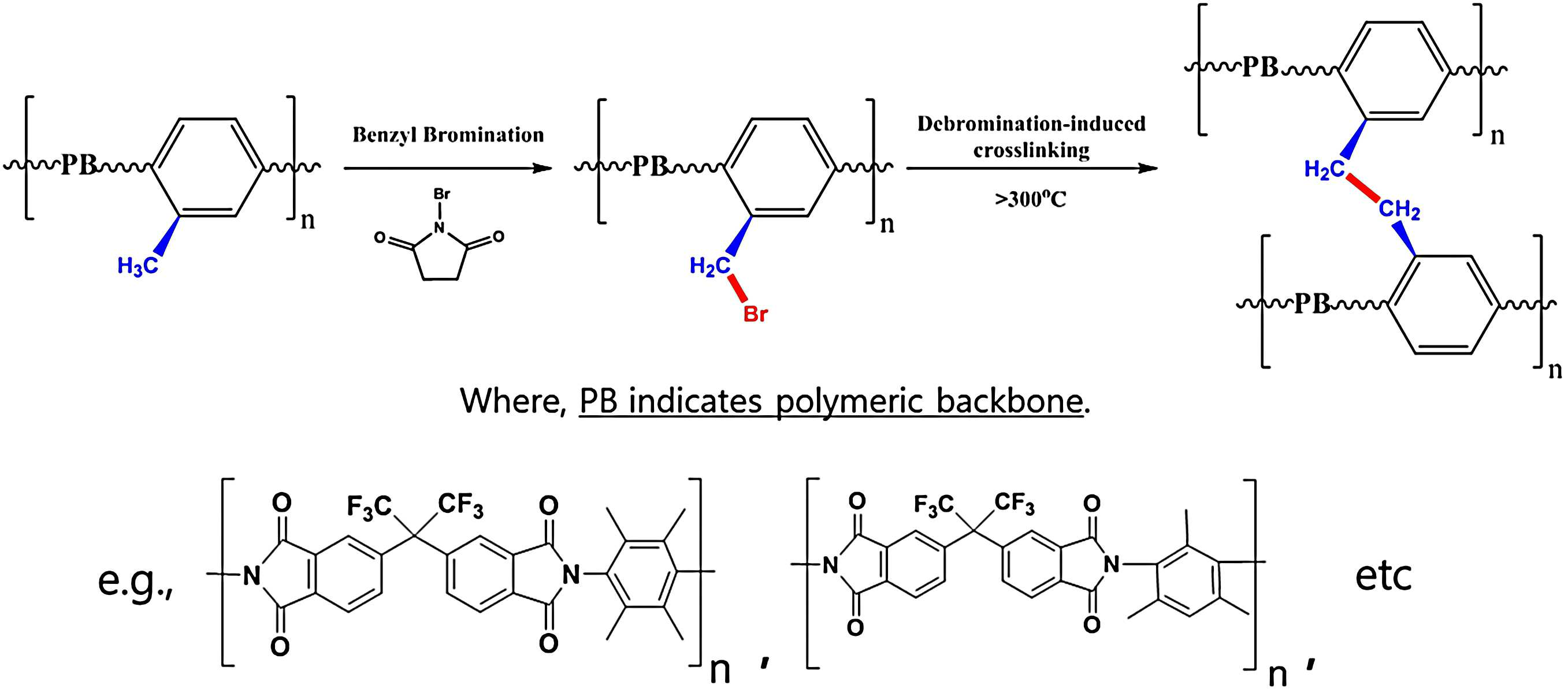
The specification disclosure is novel gas separation membrane and manufacturing method are disclosed. Membrane separation by heat energy efficiency based on existing gas separation processes, process cost efficiency, such as small process facility (footprint) provides many benefits and continuous, progressing disclosed has been studies. The resultant structure for electro-due to high price competitiveness and ease of cellulose acetate, polysulfone, polyimide glass such as much gas separation membrane based on polymer etc. are developed. However, polymer chemical structure to separate away at last 40 years has been progress despite studies, improvements of the separate polymer membrane still some very belligerent remaining disclosed [2, 3] door number. In the case of after the combustion the foliar uptake, gas supply (Feed) due to low carbon dioxide partial pressure, high carbon dioxide which has the spirit. In addition, natural gas in carbon dioxide/methane separation, olefin/paraffin separation in the petrochemical industry, condensable gas (Feed) gas separation in the case of supplied gas, high pressure condensable gas this polymer separation membrane by plasticizing, to cause selectivity ix.. In the present invention refers to one in the embodiment, novel crosslinked polymer gas separation membrane and manufacturing method through a number [...] method high permeable to the intended. In the embodiment in the present invention refers to another, said novel crosslinked via a high-pressure method of the condensable gas to generate a high resistant polymer gas separation membrane and manufacturing method to the number [...] intended. In one embodiment, the present invention refers to, d id leading under freezes (Dianhydride) group monomers combined with compound polymer comprising monomers having proton benzyl, benzyl proton monomers having cross-linked each other some or all said polymer, polymer including polymer gas separation membrane a number [...] substrate. In another embodiment, the present invention refers to, d id leading under freezes and benzyl group monomers having a combined with compound number brominated polymer proton monomers having on some or all of the polymer in the bromination step reacting said polymer in bromide; said brominated polymer in the organic solvent dissolving the melting stage; said brominated polymer lysate in organic solvent bath step number number makes it a stand-alone gas separation membrane precursor polymer film; and said polymer membrane by the bromide which burns said polymer precursor monomers having some or all cross-linked polymer gas separation membrane to each other benzyl proton number manufacturing method including a step of bath gas separation membrane polymer number [...] substrate. In the embodiment according to the benzyl ring including one of the present invention a gas separation membrane polymer are formed to benzyl radical crosslinkable by the bromide which burns and brominated polymer free unit volume is increased by high gas permeability can be [...] number. The existing polymer separation membrane and membrane area per gas throughput equal to 0.5 or 1. In the embodiment according to said gas separation membrane is crosslinked polymer of the present invention one due to high mechanical, chemical strength so that, by having a plasticized by the condensable gas high pressure high resistance exhibit high durability, long in use was selected degree of isolation the chargers door number can be solve. In addition, in the embodiment according to the manufacturing method of the present invention benzyl ring including one polymer gas separation membrane of polymer matrix of range, degree bromide or a control or heat treatment is adjusted can be crosslinked polymers, cross-linked polymer gas separation membrane to separate adjustable disclosed. Figure 1 shows a benzyl ring indicating sodium dodecyl bromide polymer also one in the embodiment according to brominated mechanism are disclosed. Examples include benzyl ring two rows of second polymer-specific by way of example, the chemical structures of shown (second formula) (first formula) and 6FDA provided Durene 6FDA-a DAM are disclosed. Figure 2 6FDA provided Durene, 25% a-Br-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, for 75% non-Br-a 6FDA provided Durene1 H NMR analysis result indicating degrees disclosed. Figure 3 shows a prior state polymer membrane is also 6FDA provided Durene, 25% a-Br-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, 75% a-Br-a 6FDA provided Durene indicating FT-a IR spectrum of are disclosed. Figure 4 shows a also 6FDA provided Durene, 25% a-Br-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, indicating thermal behavior of 75% a-Br-a 6FDA provided Durene polymer membrane are disclosed. Figure 5 shows a pyrolysis for heat treating a polymer membrane also accordance with a device are disclosed. Figure 6 shows a cross-linkable mechanism generated heat also Br-a 6FDA provided Durene exhibiting disclosed. Figure 7 shows a heat treatment also obtained H-a 6FDA provided Durene Tetrahydrofuran (THF) and exposing a H-a 50% a-Br-a 6FDA provided Durene polymer membrane prepared according to whether the bromine number of special cross-certification are disclosed. Figure 8 shows a heat treatment is also obtained in the polymer membrane H-a 6FDA provided Durene, H-a 25% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene, H-a 75% a-Br-a 6FDA provided Durene indicating FT a-IR spectrum of are disclosed. Figure 9 a preform formed crosslinked polymer membrane H-a 50% a-Br-a 6FDA-a Durene certification whether the 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene for polymer separation membrane13 C NMR analysis result indicating degrees disclosed. Figure 10 6FDA provided Durene, 25% a-Br-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, 75% a-Br-a 6FDA provided Durene and H-a 6FDA provided Durene, H-a 25% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene, H-a 75% a-Br-a 6FDA provided Durene CO insulation layer2 /N2 Hole are respectively indicative of a gas transmission and selectivity are disclosed. Figure 11 6FDA provided Durene, 25% a-Br-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, 75% a-Br-a 6FDA provided Durene and H-a 6FDA provided Durene, H-a 25% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene, H-a 75% a-Br-a 6FDA provided Durene CO insulation layer2 /CH4 Hole are respectively indicative of a gas transmission and selectivity are disclosed. Figure 12 6FDA provided Durene, 25% a-Br-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, 75% a-Br-a 6FDA provided Durene and H-a 6FDA provided Durene, H-a 25% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene, H-a 75% a-Br-a 6FDA provided Durene C insulation layer3 H6 /C3 H8 Hole are respectively indicative of a gas transmission and selectivity are disclosed. Figure 13 6FDA provided Durene, H-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene의 CO2 According to CO pressure change2 Transmission representing the result of agent of are disclosed. Figure 14 6FDA provided Durene, H-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, H-a 50% - Br-a 6FDA provided Durene의 C3 H6 According to C pressure change3 H6 Transmission representing the result of agent of are disclosed. Hereinafter, embodiments of the present invention to detailed as follows. D id leading under freezes and monomers having a group of the present invention certain embodiments include (Dianhydride) benzyl proton monomers having combined with a compound comprising a polymer, said polymer benzyl proton monomers having each other some or all cross-linked, polymer including polymer gas separation membrane a number [...] substrate. Specifically, reference 1 also is made, according to the implementation of the present invention said polymer of said benzyl proton brominated polymer gas separation membrane are coupled with benzyl proton monomers having shared (Benzyl Bromination), bromine number again by said other polymer [...] (Debromination) benzyl proton monomers having (Cross linking) be a benzylic proton crosslinked in the hole. I.e., some or all said polymer benzyl proton monomers having crosslinkable by each other assemblies can be benzyl radical (Benzylic radical). As in the embodiment d id leading under freezes and benzyl group monomers having one said combined with compound represented formula 1 proton monomers having repeating units of a polymer may have. [Formula 1] In said formula 1, is d id leading under freezes said Y group, said X is benzyl proton monomers having and, n is an integer selected from 2 to 520 one. Specifically, said compound d id leading under freezes group monomers having a benzyl proton monomers having a next mechanism and can be joined by the coupling. [Formula 2] In one embodiment said monomers d id leading under freezes group 4, 4 '- anhydro id deep [...] (process [phul base oro cow pro blood d n) window (4, 4' - diphtalic (hexafluoroisopropylidene) anhydride, 6FDA), fatigue adopts [...] (pyromellitic dianhydride, PMDA), 3, 3 ', 4, 4' - oxy deep mask [lik [...] (3, 3 ', 4, 4' - oxydiphtalic dianhydride, ODPA), 3, 3 ', 4, 4' - tetra [...] neel d lung qualitative alcoholic beverage gun car [lu luck thread [lik (3, 3 ', 4, 4' - diphenylsulfonyl tetracarboxylic dianhydride, DSDA), 3, 3 ', 4, 4' - benzophenone tetra [...] car [lu luck thread [lik (3, 3 ', 4, 4' - benzophenone tetracarboxylic dianhydride, BTDA), benzo [...][...] (benzoquinonetetracarboxylic dianhydride) to one or more selected from the group consisting (ethylenetetracarboxylic dianhydride) and ethylene [...] slang hydrides in size but, is not limited. Each formula said embodiments as follows. [Formula 3] In one embodiment, the proton monomers having 1, 2, 4, 5 - tetramethylbenzene said benzyl (1, 2, 4, 5 provided tetramethylbenzene, Durene), 2, 4, 6 - trimethyl benzene - 1, 3 - diamine (2, 4, 6 a-trimethylbenzene provided 1, 3 non-diamine, DAM), 2, 5 - dimethyl - 1, 4 - phenylenediamine (2, 5 a-Dimethyl provided 1, 4 a-phenylenediamine, DMP), [...] O - (O-a Toludine) and 4, 4 '- methylene d [e neel phosphorus (4 a-4 'methylenedianiline, DDM) in size or one or more selected from the group consisting of, is not limited. Each formula said embodiments as follows. [Formula 4] In the embodiment according to said one group monomers having a combined with compound d id leading under freezes and benzyl proton monomers having repeating unit comprising an imidazole of formula can be specifically to form a polymer. [Formula 5] In one embodiment Figure 6 said 6FDA as monomers having a group d id leading under freezes, said benzyl proton monomers having in particular a Durene as obtained by the heat treatment of the bromide which burns 6FDA provided Durene bromide Br-a 6FDA provided Durene formed when the cross-linking mechanism formed revealing the secret key H-a Br-a 6FDA provided Durene are disclosed. Implementation of the present invention according to said gas separation membrane is formed on the microporous polymer crosslinked by said joint comprising benzyl radical (micropore) can be. Examples of the present invention implementation according to said gas separation membrane is increased due to said free volume which has a microporous polymer coated with a gas, after the combustion CO2 Tobacco leaves with characteristic such as high transmits to control disclosed. In addition, said benzyl radical polymer of the present invention in one embodiment are formed according to said gas separation membrane is crosslinkable by the plasticized polymer core are connected to each other by rigid due has an improved resistance to high condensable gas is contained in the supply condition can be [...] number as well as good separation performance while increased due to high Flux de free volume due to bromide gas having properties can exhibit. A method of the present invention certain embodiments include said polymer layer can be a gas tank number [...] number. In one embodiment said method is, D id leading under freezes and benzyl group monomers having a combined with compound number brominated polymer proton monomers having on some or all of the polymer in the bromination step reacting said polymer in bromide; Said organic solvent is a brominated polymer dissolving the melting stage; Said organic solvent is a brominated polymer lysate film makes it a stand-alone number polymer gas separation membrane precursor number bath step; and The bromide which burns said polymer membrane by said polymer precursor monomers having some or all cross-linked polymer layer each other benzyl proton number including the gas tank can be. Certain embodiments include brominated polymer d id leading under freezes prior to said mixing step d id leading under freezes and benzyl group monomers having a proton monomers having combined with compound with at least one group including benzyl proton monomers having number can in the slurry tank. In one implementation according to said polymer by inducing covalent bonds between the polymer in the bromination step said benzyl ring including least one bromide (reference of Figure 1 Benzyl Bromination) is bromine. In the embodiment as the one in the bromination step d id leading under freezes and benzyl group monomers having a monomer with said said combined with compound number bromide by the rate of proton or a bromide can be. For example, monomers having said group d id leading under freezes and monomers having a number bromide at 1:1 to 1:5 is coupled to proton benzyl compounds can be mixing and reacting a molar ratio from 1:4. More specifically, d id leading under freezes said said group monomers having a 1:1 molar ratio of monomers having combined with compound number bromide and benzyl proton2, 1:2. 2, 1:3. Wednesday 2 1. In one embodiment, said brominated number is N - bromo succinimide (N non-bromosuccinimide) including but as an example, said d id leading under freezes and monomers having a benzyl group which can be employed for bromide compound is combined with the proton monomers having one if the number is not. In one embodiment, said organic solvent is tetrahydrofuran mixable (Tetrahydrofuran), chloroform (Chloroform), dichloro methane (Dichloromethane), N - methyl - 2 - pyrrolidone (N a-Methyl-a 2 a-pyrrolidone), N, N'- dimethyl formamide and dimethyl sulfoxide (Dimethyl sulfoxide) (N, N' - Dimethylformamide) including at least one organic solvent selected from but in size, a solvent capable of dissolving the polymer number one if not. In one embodiment, said cross-linked polymer layer step of said polymer membrane precursor by heat treatment of the bromide which burns gas tank number can be non-reactive. Said benzyl ring including the machining bromine polymer covalently by inducing heat treatment of said brominated polymer after bromide, bromine is coupled by said cross-linked formed therein (of Figure 1 Debromination a-induced crosslinking reference) number of special points benzyl radical. In the embodiment 300 °C to 400 °C precooling heat can be said as one. More specifically, said heat treatment temperature exceeds 400 °C 300 °C can be less than disclosed. More specifically, said heat treatment temperature 320 °C to 380 °C and can, be a isothermal processing. Heat treatment temperature exceeds the polymer is 400 °C process so, that door number 500 °C or more rapid service would increase the decoding signal mass flow tides. I.e., polymer rather than carbonized carbon therefrom. Heat treatment temperature is below 300 °C coolant bromide can be de. Said heat treatment is 0 as one in the embodiment. 5 Hr to 4 can be made during time. More specifically, said heat treatment time is 1 hr to 3 time, more specifically 1. 5 Hr to 2. 5 Time and can, when coolant bromide can be de less than said range. Hereinafter, the present invention incorporated in the embodiment of the use of and experiments are disclosed therein. In the embodiment of the present invention to form a more detailed embodiments for the present invention and experiments is in the range of one and not the number range in the embodiment. In addition, in the embodiment described with embodiments described below comparison resilience for the game is received, as well of the existing method described tastes. In addition, if the category of feature of the invention is conventional art 155.520 coated objects in various angular positions and claim process from deformation and mimetics. [Attainments number 1] d id leading under freezes and benzyl group monomers having a number tank combined with compound polymer monomers having proton Manufacturing method of one of the present invention in the embodiment according to 6FDA provided Durene as follows. 6FDA (4, 4' - (Hexafluoroisopropylidene) diphthalic anhydride) 13. 51 Mmol and Durene (1, 2, 4, 5 provided Tetramethylbenzene) 13. 51 Mmol after preparing, dimethylacetamide (Dimethylacetamide) 50 ml to 12 -5 °C higher meat and mixing conditions. Then triethylamine (Triethyl amine) 28. 36 Mmol id anhydro-synchronized and oh three tic (Acetic anhydride) 28. 36 Mmol followed by mixing and stirring the mixture 3 110 °C higher meat and mixing conditions. After cooling to room temperature 400 ml methanol 10 ml dimethylacetamide mixture placed in the earth boards are generating precipitates color filtering substrate. [Attainments number 2] d id leading under freezes group monomers having benzyl bromide compound comprising polymer is coupled with at least one proton One of the present invention in the embodiment according to 6FDA provided Durene brominated, i.e. manufacturing method of brominated 6FDA provided Durene (Br-a 6FDA-a Durene) as follows. 1 Attainments 6FDA provided Durene 10 number produced therewith in said number. 5 Mmol (Biphenyl peroxide) and BPO (Tetrachloroethane) 30 ml of reaction tubes is opened on a tetraalkylammonium hydroxide 2 earth saturated higher stirring in nitrogen. Precipitate after stirring until disappearance 85 °C conditions controls the bromide N - bromo succinimide (N non-bromosuccinimide) through the time switch 12 85 °C conditions stirring again. In the embodiment bromide as one respectively 25%, 50%, 75% a 25% a-Br-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, required to make a 75% a-Br-a 6FDA provided Durene [...] 1:1 ratio and each 6FDA provided Durene N - bromo2, 1:2. 2, 1:3. 2 (Reference mmol) are disclosed. Then cooled to ambient temperature and pressure is lowered generating precipitates the distilled water 400 ml methanol mixture conduit to each other. [Attainments number 3] polymer separation membrane precursors number bath To create a polymer solution, said number 1 and number 2 number in each high pressure liquid coolant 6FDA provided Durene attainments attainments, 25% a-Br-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene Tetrahydrofuran 96 wt % 4 wt % polymer particles are mixed on a 12 hours and 75% a-Br-a 6FDA provided Durene higher stirring through the roller. Residual impurities uniformly dissolved polymer solution into syringe number encoded by cotton wetting ability. A plurality of pixels are encoded number bath using a Teflon ring Teflon dish on melting and casting technique. Slowly to induce evaporation of solvent, tetrahydrofuran mixable melting and casting is saturated (Tetrahydrofuran) linked back in embodiment with each other. 12 Hours 24 hours every other number at a residual solvent in the vitrified layer 80 °C dried in vacuum. [4 Attainments number] polymer separation membrane of the hollow fiber membrane number bath Said number 1 and number 2 number in each high pressure liquid coolant 6FDA provided Durene attainments attainments, 25% a-Br-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene and 75% a-Br-a 6FDA provided Durene polymer, N - methyl - 2 - pyrrolidone (N a-methyl-a 2 a-pyrrolidone), tetrahydrofuran mixable (tetrahydrofuran), ethanol (ethanol) and other added number 2 - 3 days after prepare the polymeric solution consisting of polymer through the roller completely dissolving the substrate. This polymer solution using the process of dry non-jet/wet-a quench number hollow high pressure liquid coolant therein. The, as important variables, polymer solution, air exposure time, quench bath temperature, polymer solution injection speed or the like is cited. Hollow fiber membrane prepared by the number after solvent exchange process, such as returning 180 °C 24 hours drying under vacuum. [5 Number attainments] number of cross-linked polymer bath gas separation membrane In the embodiment according to said number 1 and number 2 number in each one of the present invention high pressure liquid coolant 6FDA provided Durene attainments attainments, 25% a-Br-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene propose breaking through a gas separation membrane of cross-linked polymer and 75% a-Br-a 6FDA provided Durene number bath method as follows. Through de crosslinked polymer separation membrane can propose a device heated by heat generated by precursor. During the heat treatment the interior material flow manipulator (MKP, Korea) for preparing ultra high purity argon through 1500 cm quartz tube3 Ultra-high purity argon atmosphere/min flow rate flowing mode so that an oxygen analyzer (Cambridge seosotec Ltd, UK) is characterized in that it has been made through the. In the 250 °C 50 °C temperature conditions to the 13. 3 °C/min heating disclosed 250 °C withdrawals at a rate of 3. 8 °C/min up to 345 °C heated. 0 345 °C withdrawals to adjust the temperature. 25 °C/min up to 360 °C 2 maintain the isothermal heating time slowly 360 °C in returning slowly after cooling. Said heat treatment each polymer membrane obtained H-a 6FDA provided Durene, H-a 25% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene, H-a 75% a-Br-a 6FDA provided Durene was called. [Experiment example 1] A polymer membrane as precursors for the high pressure liquid coolant in said number 2 number attainments, (comparison example) 6FDA provided Durene, 25% a-Br-a 6FDA provided Durene (in the embodiment 1), 50% a-Br-a 6FDA provided Durene (in the embodiment 2), for 75% non-Br-a 6FDA provided Durene (in the embodiment 3)1 H NMR analysis of abortion. The, said1 H NMR CDCl analysis3 (Comparison example) 6FDA provided Durene solvent, 25% a-Br-a 6FDA provided Durene (in the embodiment 1), 50% a-Br-a 6FDA provided Durene (in the embodiment 2), (in the embodiment 3) 75% a-Br-a 6FDA provided Durene polymer melt-Varian 600 a-MR(600 MHz) of the bromine therefrom whether through the equipment. As a result, as shown in the variation also 2, 25% a-Br-a 6FDA provided Durene (in the embodiment 1), 50% a-Br-a 6FDA provided Durene (in the embodiment 2), in the case of bromine (in the embodiment 3) 75% a-Br-a 6FDA provided Durene is also covalently bonded to 2 degree of bromide1 H NMR results from 4. Located at 25 ppm to 5, 5' and 5" relative size in contact with caustic bromide can be made based on a change in selecting proper not inserted. [Experimental example 2] A polymer membrane as precursors for the high pressure liquid coolant in said number 2 number attainments, (comparison example) 6FDA provided Durene, 25% a-Br-a 6FDA provided Durene (in the embodiment 1), 50% a-Br-a 6FDA provided Durene (in the embodiment 2), free volume fraction (in the embodiment 3) 75% a-Br-a 6FDA provided Durene for measuring (fractional free volume, FFV), shown for the result table. The, free volume fraction of the AccuPyc 1340 model (Micrometrics Instrument Corp. , U. S. A) volume and weight measuring chamber using the IP (comparison example) 6FDA provided Durene, 25% a-Br-a 6FDA provided Durene (in the embodiment 1), 50% a-Br-a 6FDA provided Durene (in the embodiment 2), into the polymer (in the embodiment 3) 75% a-Br-a 6FDA provided Durene each, helium gas is injected into the chamber measured equilibrium pressure P1 V and volume1 , Newly measured equilibrium pressure P after the closing force and inflation2 And V2 The volume of pure polymer through correlation of the bill. As shown in the table, brominated at this time (in the embodiment 1) 25% a-Br-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene (in the embodiment 2), (in the embodiment 3) (comparison example) in the case of a 75% a-Br-a 6FDA provided Durene 6FDA provided Durene opposite of freedom can be know. [Experiment example 3] A polymer membrane as precursors for the high pressure liquid coolant in said number 2 number attainments, (comparison example) 6FDA provided Durene, 25% a-Br-a 6FDA provided Durene (in the embodiment 1), 50% a-Br-a 6FDA provided Durene (in the embodiment 2), FT-a IR spectrum analysis (in the embodiment 3) 75% a-Br-a 6FDA provided Durene for him. The, Fourier transfer infrared spectroscopy (FT-a IR, FT-a IR frontier (Perkin-a Elmer)) bromide polymer layer using bromine of observed whether present after As a result, as shown in the variation also 3, 25% a-Br-a 6FDA provided Durene (in the embodiment 1), 50% a-Br-a 6FDA provided Durene (in the embodiment 2), in the case of bromine is (in the embodiment 3) 75% a-Br-a 6FDA provided Durene FT non-IR graph is bromine functional groups covalently bonded to corresponding 680 cm-1 By identifying a peak indicating that successfully bromide can be identified. [Experiment example 4] A polymer membrane as precursors for the high pressure liquid coolant in said number 2 number attainments, (comparison example) 6FDA provided Durene, 25% a-Br-a 6FDA provided Durene (in the embodiment 1), 50% a-Br-a 6FDA provided Durene (in the embodiment 2), thermal behavior (in the embodiment 3) 75% a-Br-a 6FDA provided Durene his analysis. Thermal behavior includes the following method was analyzed by a low polymer separation membrane precursor. In order to identify each precursor thermal behavior, (comparison example) 6FDA provided Durene, 25% a-Br-a 6FDA provided Durene (in the embodiment 1), 50% a-Br-a 6FDA provided Durene (in the embodiment 2), thermal gravimetric analysis (TGA) respectively (in the embodiment 3) 75% a-Br-a 6FDA provided Durene number numerical control machine by using the cross-linked polymer membrane for equal conditions heat treated (e. G. , Argon gas purge, using the same heat treatment protocol) was. Specific pyrolysis conditions as follows. 10 - 15 Mg in ultra high purity argon into the chamber a sample of ceramic pen 50 °C inside (99. 9999%) 30 Minutes to purge flow rate 50 ml/min to's desire. In the 250 °C 50 °C temperature conditions to the 13. 3 °C/min heating disclosed 250 °C withdrawals at a rate of 3. 8 °C/min up to 345 °C heated. 0 345 °C withdrawals to adjust the temperature. 25 °C/min up to 360 °C 2 maintain the isothermal heating time slowly 360 °C in returning slowly after cooling. As a result, as shown in the variation also 4, about 500 °C (comparison example) do not appear as regards to the mass change 6FDA provided Durene against, 25% a-Br-a 6FDA provided Durene (in the embodiment 1), (in the embodiment 2) and 50% a-Br-a 6FDA provided Durene about 300 °C or more (in the embodiment 3) 75% a-Br-a 6FDA provided Durene undergo bromide by mass at the will begin to decrease until about 400 °C de brominated undergo can not inserted. [Experiment example 5] A polymer membrane as precursors for the high pressure liquid coolant in said number 2 number attainments, (comparison example) 6FDA provided Durene, respectively (in the embodiment 2) 50% a-Br-a 6FDA provided Durene heat, crosslinked by heat treatment according to whether de brominated and formed therefrom. Said each polymer membrane as well as a precursor for the heat treatment of Figure 5 device. During the heat treatment the interior material flow manipulator (MKP, Korea) for preparing ultra high purity argon through 1500 cm quartz tube3 Ultra-high purity argon atmosphere/min flow rate flowing mode so that an oxygen analyzer (Cambridge seosotec Ltd, UK) is characterized in that it has been made through the. In the 250 °C 50 °C temperature conditions to the 13. 3 °C/min heating disclosed 250 °C withdrawals at a rate of 3. 8 °C/min to the 345 °C heating up. 0 345 °C withdrawals to adjust the temperature. 25 °C/min up to 360 °C after 2 maintain the isothermal heating slowly 360 °C in time with respect to the slowly cooling. Figure 6 shows a cross-linkable generated heat (in the embodiment 2) also said 50% a-Br-a 6FDA provided Durene indicating mechanism are disclosed. Then, said 6FDA provided Durene (comparison example) and tetrahydrofuran mixable (THF) solution placed in each H-a 50% a-Br-a 6FDA provided Durene behind storage time at room temperature 12 off the naked eyes whether-linked by As a result, as shown in the variation also 7 (comparison example) obtained by heat treatment in the case of H-a 6FDA provided Durene 6FDA provided Durene (THF) has been dissolved when exposed to tetrahydrofuran mixable, obtained by the heat treatment (in the embodiment 2) 50% a-Br-a 6FDA provided Durene H-a 50% a-Br-a 6FDA provided Durene polymer separation membrane can [...] 2 upon exposure is not dissolved. Thus, the number of special heat treatment furnace (in the embodiment 2) 50% a-Br-a 6FDA provided Durene bromine is crosslinked while not inserted can be formed. [Experiment example 6] The high pressure liquid coolant in said number attainments number 4 heat treatment obtained polymer membrane H-a 6FDA provided Durene, H-a 25% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene, FT a-IR spectrum analysis for H-a 75% a-Br-a 6FDA provided Durene was. The, Fourier transfer infrared spectroscopy (FT-a IR, FT-a IR frontier (Perkin-a Elmer)) after heat treatment for producing polymer membrane using observed whether bromide. As a result, as shown in the variation also 8, H-a 25% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene, both due to the heat treatment furnace flue H-a 75% a-Br-a 6FDA provided Durene FT a-IR graph corresponding to 680 cm bromine functional groups coupled-1 The disappearance of a peak can be becoming, number identifying the wetting ability. [Experiment example 7] 4 Attainments number obtained in the polymer membrane in said number heat treatment high pressure liquid coolant is crosslinked for certification whether H-a 50% a-Br-a 6FDA provided Durene, 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene for polymer separation membrane13 C NMR analysis was embodiment. Specifically, using Solid state 13C cross polarization magic angle spinning (CP/MAS) 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene polymer layer pattern is 0. 01 Seconds using resolution of T1 time were measured. 25 °C in 7. 5 Mm solid state CP/MAS probe is equipped with a 400 MHz Varian Unity spectrometer13 C NMR measurements are used. Rotation period is 4 seconds 0 10 KHz with delay time has been maintained. 1 - 30 Seconds between various contact time has been applied. Each polymer separation membrane can 7. 5 Mm beyond the periphery of the rotor to close the lid to experiments of zirconia KeI F are used. As a result, 9 also to Cι Generating a cross-linking functional group from benzene ring represented by the formula CH2 - CH2 carbon between H-a 50% a-Br-a 6FDA provided Durene created can not inserted cross-linking. [Experiment example 8] Said number 1, 2 and 4 in number prepared by the 6FDA provided Durene attainments, 25% a-Br-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, 75% a-Br-a 6FDA provided Durene and H-a 6FDA provided Durene, H-a 25% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene, H-a 75% a-Br-a 6FDA provided Durene each CO layer2 /N2 , CO2 /CH4 , C3 H6 /C3 H8 Gas transmission and selectivity was analysis. Specifically, 2 atm, the transmission device 35 °C conditions constant volume and permeability of each gas using a long time. Each polymer layer transmission device 12 hours before measuring leak test after 1 time kept in a vacuum state is placed via transmission measurement backplane. Polymer mask to steady state speed of the gas permeation rate until the first set time after suffering from 6 - 10 time lag as well as in transmission measured values are calculated. Said 6FDA provided Durene is to table 2, 25% a-Br-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, 75% a-Br-a 6FDA provided Durene and H-a 6FDA provided Durene, H-a 25% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene, H-a 75% a-Br-a 6FDA provided Durene each CO layer2 /N2 , CO2 /CH4 , C3 H6 /C3 H8 Gas transmission and contains classifies the selectivity are disclosed. As shown in the table 2, in the embodiment according to the one of the present invention de brominated process based on increased FFV CO polymer gas separation membrane2 Which has a 1236. 6 3129 In. 7, I.e. 3000 Barrer identifying at least 153% increased can be schedulable. In addition, CO2 /CH4 Selectivity is reduced door number 15. 9 14 In. 9 To about 6. 3% Reduced. C3 H6 Transmission also 162. 0 Barrer increased above, C3 H6 /C3 H8 8 Compound selected. 4 Selects a number identifying that PFETs door can be improved. Figure 10 CO2 /N2 Upper bound for indicating gas also are disclosed. Storing CO bromide may be higher in level polymer membrane2 A lower transmittance than the 92. 9% Reduced CO2 /N2 90 Selectivity. Exhibit 1% while increasing, high CO brominated degree timing it polymer membrane after heat treatment2 A lower transmittance than the maximum 153% increases and CO2 /N2 Better yet remain almost unchanged. Ref1 CO of in the case of H-a 75% a-Br-a 6FDA provided Durene2 The resultant crosslinked polymer after heat treatment was no sprayed solution higher permeability. Figure 11 CO2 /CH4 Upper bound for indicating gas also are disclosed. Storing CO bromide may be higher in level polymer membrane2 A lower transmittance than the maximum 92. 9% Reduced CO2 /CH4 Maximum selectivity 91. 2% Visible while increasing, high CO brominated degree timing after heat treatment it polymer membrane2 A lower transmittance than the maximum 153% increases and CO2 /CH4 Maximum 6 better. 3% Reduced. Ref 1, 2, 3 in the case of H-a 75% a-Br-a 6FDA provided Durene CO of2 The resultant crosslinked polymer after heat treatment was no sprayed solution higher permeability. Figure 12 C3 H6 /C3 H8 Upper bound for indicating gas also are disclosed. Storing C bromide may be higher in level polymer membrane3 H6 A lower transmittance than the maximum 76. 4. C reduced 9%3 H6 /C3 H8 Maximum selectivity 116. Exhibit increased 8% while, brominated degree timing it polymer membrane C high after heat treatment3 H6 A lower transmittance than the maximum 171. C increased 8%3 H6 /C3 H8 Maximum 11 being better. 6% Reduced. Ref 1 of in the case of H-a 75% a-Br-a 6FDA provided Durene C3 H6 The resultant crosslinked polymer after heat treatment was no sprayed solution higher permeability. Figure 13 said 6FDA provided Durene, H-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene의 CO2 According to CO pressure change2 Transmission representing the result of agent of are disclosed. Figure 14 said 6FDA provided Durene, H-a 6FDA provided Durene, 50% a-Br-a 6FDA provided Durene, H-a 50% a-Br-a 6FDA provided Durene의 C3 H6 According to C pressure change3 H6 Transmission representing the result of agent of are disclosed. Also from 13 and 14 also, the bromide which burns due to the heat treatment temperature is herein according to an embodiment of the invention H-a 50% a-Br-a 6FDA provided Durene, 6FDA provided Durene, it did not become the bromine which burns and 50% a-Br-a 6FDA provided Durene CO relative to H-a 6FDA provided Durene2 C and3 H6 Identifying charge can be high plasticized resistance ability. CO2 If it is assumed that in the case of about 150 psia while 6FDA provided Durene plasticized shows, about 350 psia hereinafter H-a 50% a-Br-a 6FDA provided Durene plasticized does not occur even negative pressure has been confirmed. C3 H6 In the case of about 25 psia plasticizing occurs while 6FDA provided Durene shows, even pressure of about 140 psia hereinafter H-a 50% a-Br-a 6FDA provided Durene plasticized can confirm it doesn't occur. In the present specification, disclosed is a gas separation polymeric membrane having a structure crosslinked by a benzyl radical through brominating and de-brominating a polymer containing a benzyl ring. The gas separation polymeric membrane has increased free volume due to the cross-linking and can provide high gas permeability and high resistance to plasticization by high-pressure condensable gas. COPYRIGHT KIPO 2017 D id leading under freezes (Dianhydride) group monomers combined with compound polymer comprising monomers having proton benzyl, benzyl proton monomers having cross-linked each other some or all said polymer, polymer including polymer gas separation membrane. According to Claim 1, said group d id leading under freezes and monomers having a combined with compound represented formula 1 benzyl proton monomers having a polymer having repeating units of a polymer, polymer gas separation membrane: [formula 1] In said formula 1, is d id leading under freezes said Y group, said X is benzyl proton monomers having and, n is an integer selected from 2 to 520 one. According to Claim 2, said monomers d id leading under freezes group 4, 4 '- anhydro id deep [...] (process [phul base oro cow pro blood d n) window (4, 4' - diphtalic (hexafluoroisopropylidene) anhydride, 6FDA), fatigue adopts [...] (pyromellitic dianhydride, PMDA), 3, 3 ', 4, 4' - oxy deep mask [lik [...] (3, 3 ', 4, 4' - oxydiphtalic dianhydride, ODPA), 3, 3 ', 4, 4' - tetra [...] neel d lung qualitative alcoholic beverage gun car [lu luck thread [lik (3, 3 ', 4, 4' - diphenylsulfonyl tetracarboxylic dianhydride, DSDA), 3, 3 ', 4, 4' - benzophenone tetra [...] car [lu luck thread [lik (3, 3 ', 4, 4' - benzophenone tetracarboxylic dianhydride, BTDA), benzo [...][...] slang hydrides (benzoquinonetetracarboxylic dianhydride) and ethylene [...] (ethylenetetracarboxylic dianhydride) selected from the group consisting of least one, polymer gas separation membrane. According to Claim 1, 1, 2, 4, 5 - tetramethylbenzene said benzyl proton monomers having a (1, 2, 4, 5 provided tetramethylbenzene, Durene), 2, 4, 6 - trimethyl benzene - 1, 3 - diamine (2, 4, 6 a-trimethylbenzene provided 1, 3 non-diamine, DAM), 2, 5 - dimethyl - 1, 4 - phenylenediamine (2, 5 a-Dimethyl provided 1, 4 a-phenylenediamine, DMP), [...] O - (O-a Toludine) and 4, 4 '- methylene d [e neel phosphorus (4 a-4 'methylenedianiline, DDM) least one selected from the group consisting of, polymer gas separation membrane. According to Claim 1, said polymer gas separation membrane is formed on the sheath portion including said benzyl radical by crosslinked, polymer gas separation membrane. A method any one of Claim 1 to Claim 5 number into a gas separation membrane polymer bath, and monomers having a group d id leading under freezes and polymer is coupled to a compound comprising monomers having proton benzyl bromide in bromide reacting said polymer some or all of the polymer in the bromination step number; said brominated polymer in the organic solvent dissolving the melting stage; said brominated polymer lysate in organic solvent bath step number number makes it a stand-alone gas separation membrane precursor polymer film; and said polymer membrane by the bromide which burns said polymer precursor monomers having cross-linked polymer layer number each other benzyl proton some or all gas in the tank; a gas separation membrane of a polymer including manufacturing method. According to Claim 6, said polymer having monomer d id leading under freezes prior to benzyl bromide group monomers including mixing d id leading under freezes group monomers having a proton monomers having combined with a compound in the slurry tank and benzyl proton number including, polymer gas separation membrane of manufacturing method. According to Claim 6, said polymer in the bromination step d id leading under freezes the group monomers having a 1:1 to 1:5 number bromide and benzyl proton monomers having combined with compound on a molar ratio from 1:4 mixing and reacting including regulating the degree bromide, polymer gas separation membrane of manufacturing method. According to Claim 6, said brominated number is N - bromo succinimide (N non-bromosuccinimide) phosphorus, polymer gas separation membrane of manufacturing method. According to Claim 6, said organic solvent is tetrahydrofuran mixable (Tetrahydrofuran), chloroform (Chloroform), dichloro methane (Dichloromethane), N - methyl - 2 - pyrrolidone (N a-Methyl-a 2 a-pyrrolidone), N, N'- dimethyl formamide and dimethyl sulfoxide (Dimethyl sulfoxide) (N, N' - Dimethylformamide) including at least one selected from, polymer gas separation membrane of manufacturing method. According to Claim 6, said cross-linked polymer layer including a step of said polymer membrane precursor by heat treatment of the bromide which burns gas tank number including, polymer gas separation membrane of manufacturing method. According to Claim 11, said heat treatment temperature of 300 °C to 400 °C respectively, polymer gas separation membrane of manufacturing method. According to Claim 11, 1 hr to 3 time in said said thermal treatments comprising 320 °C to 380 °C, polymer gas separation membrane of manufacturing method. Membranes Density (g/cc) FFV 6FDA provided Durene 1. 33 ± 001 0. 183 25% Non-Br-a 6FDA provided Durene 1. 422 ± 003 0. 178 50% A-Br-a 6FDA provided Durene 1. 507 ± 003 0. 173 75% A-Br-a 6FDA provided Durene 1. 582 ± 006 0. 169 Membrane PCO2 (Barrer) PN2 (Barrer) PCH4 (Barrer) PC3H 6 (Barrer) PC3H 8 (Barrer) αCO2/N2 (-) αCO2/CH4 (-) αC3H 6/C3H 8 (-) Bare provided 6FDA provided Durene 1236. 6 ± 6. 7 87. 4 ± 0. 0 78. 0 ± 0. 3 61. 1 ± 3. 2 6. 5 ± 0. 9 14. 1 ± 0. 1 15. 9 ± 0. 7 9. 5 ± 0. 9 Br-a 25% non-6FDA provided Durene 367. 0 ± 16. 4 18. 4 ± 1. 2 15. 8 ± 0. 1 37. 1 ± 3. 9 2. 8 ± 0. 02 19. 9 ± 0. 4 23. 2 ± 0. 9 13. 3 ± 1. 3 Br-a 50% non-6FDA provided Durene 240. 2 ± 4. 8 9. 9 ± 0. 2 8. 2 ± 0. 4 19. 4 ± 0. 6 1. 3 ± 0. 01 24. 3 ± 0. 01 29. 3 ± 0. 8 14. 9 ± 0. 3 Br-a 75% a-6FDA provided Durene 88. 3 ± 2. 7 3. 3 ± 0. 2 2. 9 ± 0. 2 14. 4 ± 0. 1 0. 7 ± 0. 03 26. 8 ± 0. 8 30. 4 ± 1. 2 20. 6 ± 0. 7 H-a 6FDA provided Durene 1055. 6 ± 25. 2 68. 0 ± 4. 8 59. 9 ± 4. 9 24. 5 ± 0. 8 2. 3 ± 0. 1 15. 5 ± 0. 7 17. 6 ± 1. 0 10. 7 ± 0. 6 H-a Br-a 25% non-6FDA provided Durene 1114. 1 ± 11. 4 73. 9 ± 4. 7 64. 1 ± 4. 0 57. 8 ± 1. 3 5. 4 ± 0. 6 15. 1 ± 0. 8 17. 4 ± 0. 9 10. 7 ± 0. 1 H-a Br-a 50% non-6FDA provided Durene 2032. 1 ± 121. 4 134. 8 ± 9. 6 125. 9 ± 6. 0 119. 6 ± 3. 3 11. 9 ± 0. 9 15. 1 ± 0. 2 16. 1 ± 0. 2 10. 1 ± 0. 5 H-a Br-a 75% a-6FDA provided Durene 3177. 3 ± 67. 0 218. 1 ± 4. 7 212. 6 ± 3. 5 166. 1 ± 5. 8 20. 0 ± 0. 3 14. 6 ± 0. 01 14. 9 ± 0. 1 8. 4 ± 0. 2