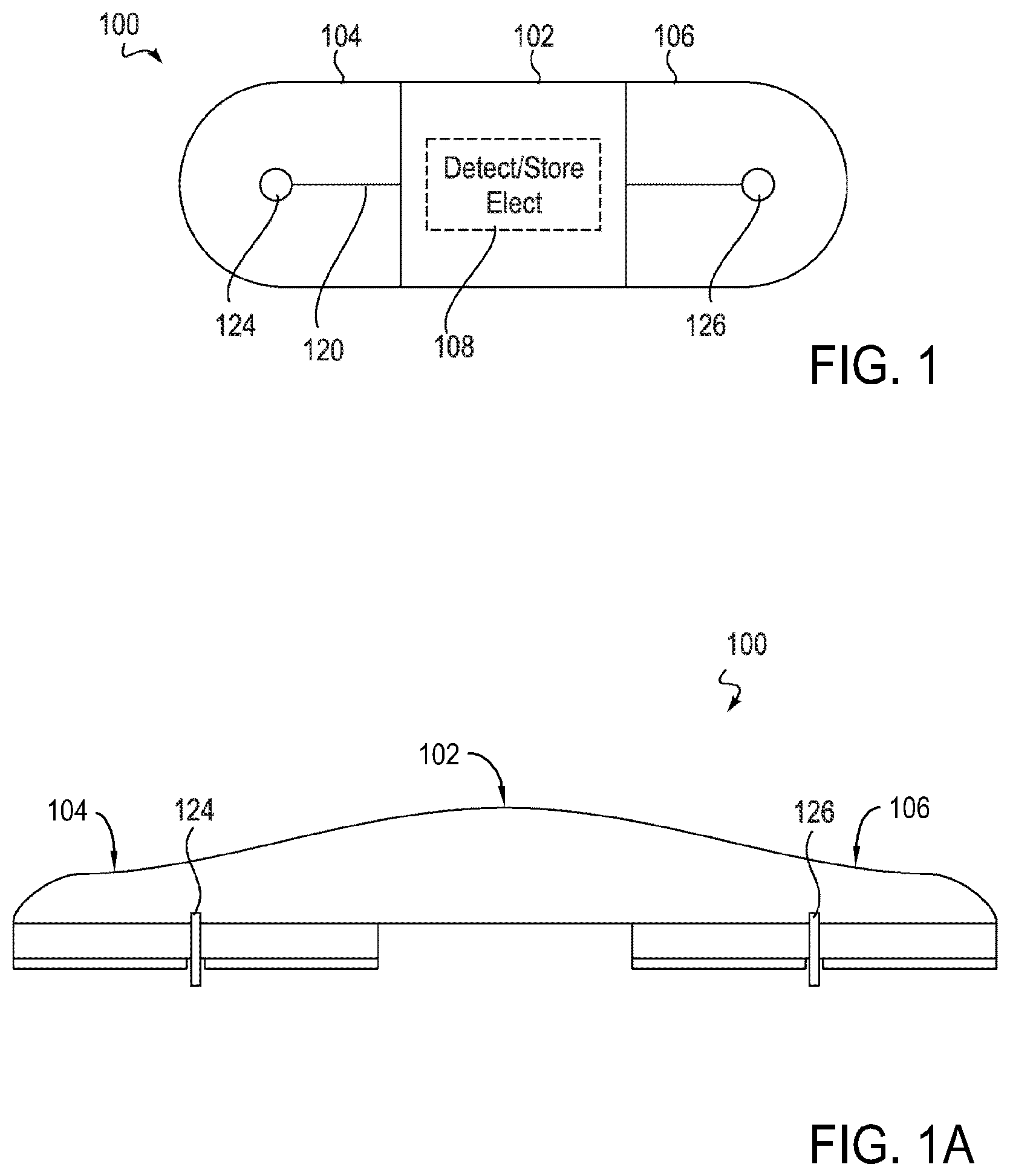
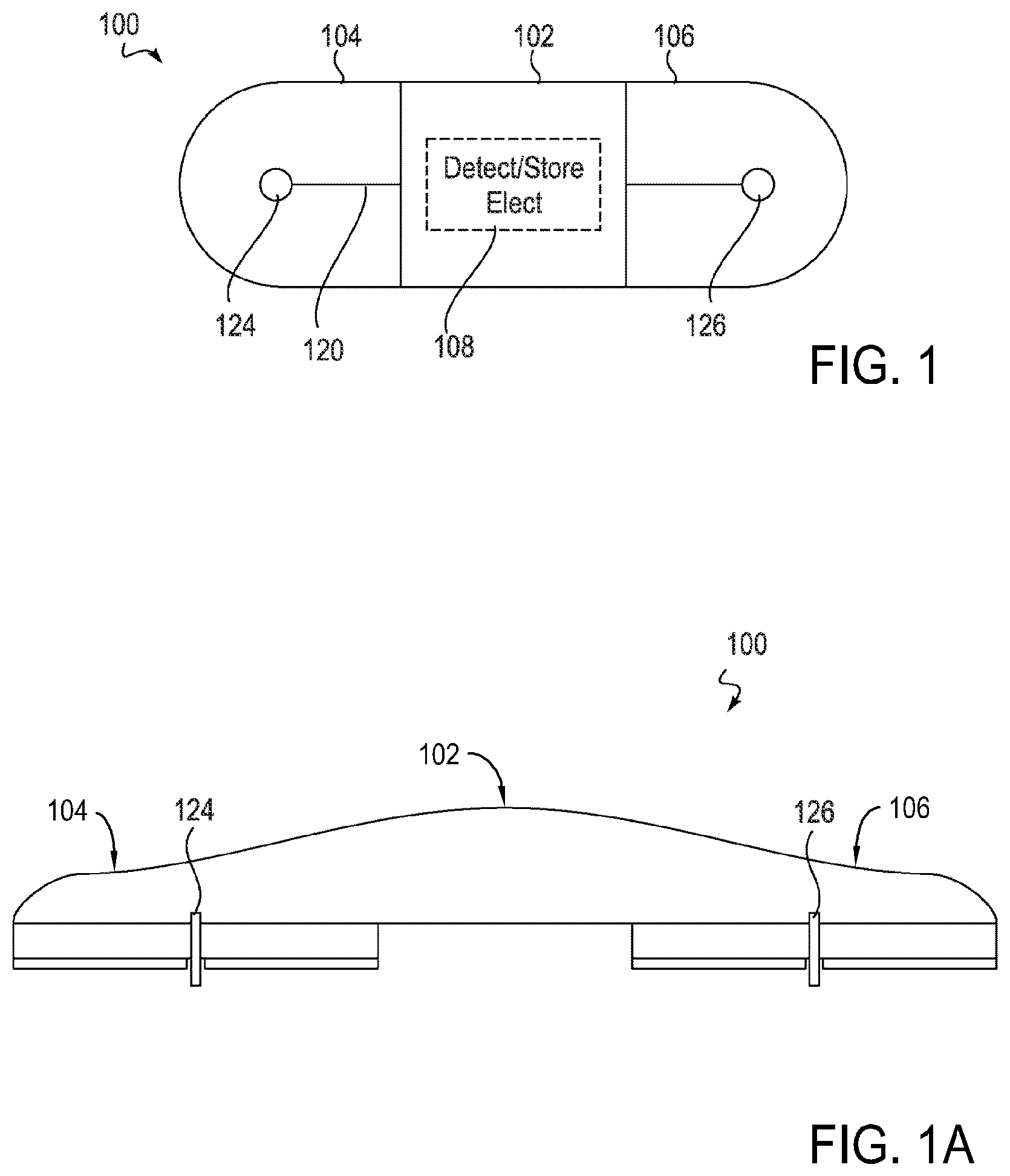
Device features and design elements for long-term adhesion
12-10-2021 дата публикации
Номер:
US0011141091B2
Автор: Uday N. Kumar, Peter H. Livingston, Mark J. Day, Shena Hae Park, William F. Willis, William H. Righter, KUMAR UDAY N, LIVINGSTON PETER H, DAY MARK J, PARK SHENA HAE, WILLIS WILLIAM F, RIGHTER WILLIAM H, KUMAR, UDAY N., Livingston, Peter H., DAY, MARK J., PARK, Shena Hae, Willis, William F., Righter, William H.
Принадлежит: iRhythm Technologies, Inc., IRHYTHM TECH INC
Контакты:
Номер заявки: 08-32-1672
Дата заявки: 20-12-2019









CPC - классификация
AA6A61A61BA61B2A61B25A61B256A61B2560A61B2560/A61B2560/0A61B2560/04A61B2560/040A61B2560/0406A61B2560/046A61B2560/0468A61B2562A61B2562/A61B2562/0A61B2562/02A61B2562/020A61B2562/0209A61B2562/1A61B2562/16A61B2562/2A61B2562/22A61B2562/221A61B5A61B5/A61B5/2A61B5/25A61B5/257A61B5/259A61B5/27A61B5/273A61B5/28A61B5/282A61B5/29A61B5/291A61B5/3A61B5/38A61B5/389A61B5/6A61B5/68A61B5/683A61B5/6832A61B5/6833A61B5/68335YY1Y10Y10TY10T1Y10T15Y10T156Y10T156/Y10T156/1Y10T156/10IPC - классификация
AA6A61A61BA61B5A61B5/A61B5/0A61B5/00A61B5/2A61B5/25A61B5/259A61B5/28A61B5/282A61B5/29A61B5/291A61B5/3A61B5/38A61B5/389A61B5/6A61B5/68A61B5/683A61B5/6833BB3B31B31BB31B5B31B50B31B50/B31B50/6B31B50/62Цитирование НПИ
3M Corporation, “3M Surgical Tapes—Choose the Correct Tape” quicksheet (2004).600/382
600/382
600/386
600/386
600/391
600/391
600/391
600/392
600/508
600/508
604/180
604/20
607/60
Altini, et al., An ECG Patch Combining a Customized Ultra-Low-Power ECG SOC With Bluetooth Low Energy for Long Term Ambulatory Monitoring, Conference: Proceddings of Wireless Health 2011, WH 2011, Oct. 10-13, 2011.
British-Made Early Warning Monitor A “Game Changer”, healthcare-in-europe.com, Mar. 31, 2014.
Comstock, Proteus Digital Health Quietly Launches Consumer-Facing Wearable for Athletes, Mobile Health News, Oct. 29, 2014.
Coxworth, Small Adhesive Partch Outperforms Traditional Tech for Detecting Arrhythmia, Scripps, iRhythm Technologies, Jan. 3, 2014.
Del Mar et al.; The history of clinical holter monitoring; A.N.E.; vol. 10; No. 2; pp. 226-230; Apr. 2005.
Enseleit et al.; Long-term continuous external electrocardiographic recording: a review; Eurospace; vol. 8; pp. 255-266; 2006.
Hoefman et al.; Optimal duration of event recording for diagnosis of arrhythmias in patients with palpitations and light-headedness in the general practice; Family Practice; Dec. 7, 2006.
International Preliminary Reporton Patentability for International Application No. PCT/US2011/036335 dated Nov. 13, 2012 in 5 pages.
International Search Report and Written Opinion for International Application No. PCT/US2011/036335 dated Oct. 31, 2011 in 7 pages.
Kennedy et al.; The history, science, and innovation of holter technology; A.N.E.; vol. 11; No. 1; pp. 85-94; 2006.
Medtronic Launches SEEQ Wearable Cardiac Monitoring System in United States, Diagnostic and Interventional Cardiology, Oct. 7, 2014.
Mundt et al. “A Multiparameter Wearable Physiologic Monitoring System for Space and Terrestrial Applications” IEEE Transactions on Information Technology in Biomedicine, vol. 9, No. 3, pp. 382-384, Sep. 2005.
Prakash, New Patch-Based Wearable Sensor Combines Advanced Skin Adhesives and Sensor Technologies, Advantage Business Marketing, Jul. 17, 2012.
Reiffel et al.; Comparison of autotriggered memory loop recorders versus standard loop recorders versus 24-hour holter monitors for arrhythmia detection; Am. J. Cardiology; vol. 95; pp. 1055-1059; May 1, 2005.
Request for Reexamination of U.S. Pat. No. 7,020,508 under 35 U.S.C. §§ 311-318 and 37 C.F.R. § 1.913 as submitted Sep. 14, 2012 in 78 pages.
Scapa Medical product listing and descriptions (2008) available at http://www.caapana.com/productlist.jsp and http://www.metplus.co.rs/pdf/prospekti/Samolepljivemedicinsketrake.pdf; retrieved via WayBack Machine Sep. 24, 2012.
Strong, Wearable Technologies Conference 2013 Europe—Notes and Roundup, Wearable Technologies Conference, Feb. 8, 2013.
Sumner, Stanford Engineers Monitor Heart Health Using Paper—Thin Flexible ‘Skin’, Stanford Report, May 14, 2013.
U.S. Appl. No. 15/005,854, filed Jan. 25, 2016, Kumar et al.
US 8,750,980 B2, 06/2014, Katra et al. (withdrawn)
Ward et al.; Assessment of the diagnostic value of 24-hour ambulatory electrocardiographic monitoring; Biotelemetry Patient monitoring; vol. 7; 1980.
Ziegler et al.; Comparison of continuous versus intermittent monitoring of atrial arrhythmias; Heart Rhythm; vol. 3; No. 12; pp. 1445-1452; Dec. 2006.
Zimetbaum et al.; The evolving role of ambulatory arrhythmia monitoring in general clinic practice; Ann. Intern. Med.; vol. 130; pp. 846-8556; 1999.
Zimetbaum et al.; Utility of patient-activated cardiac event recorders in general clinical practice; The Amer. J. of Cardiology; vol. 79; Feb. 1, 1997.
“Mayo Alumni”, Mayo Clinic, Rochester, MN, Spring 2011, in 24 pages.