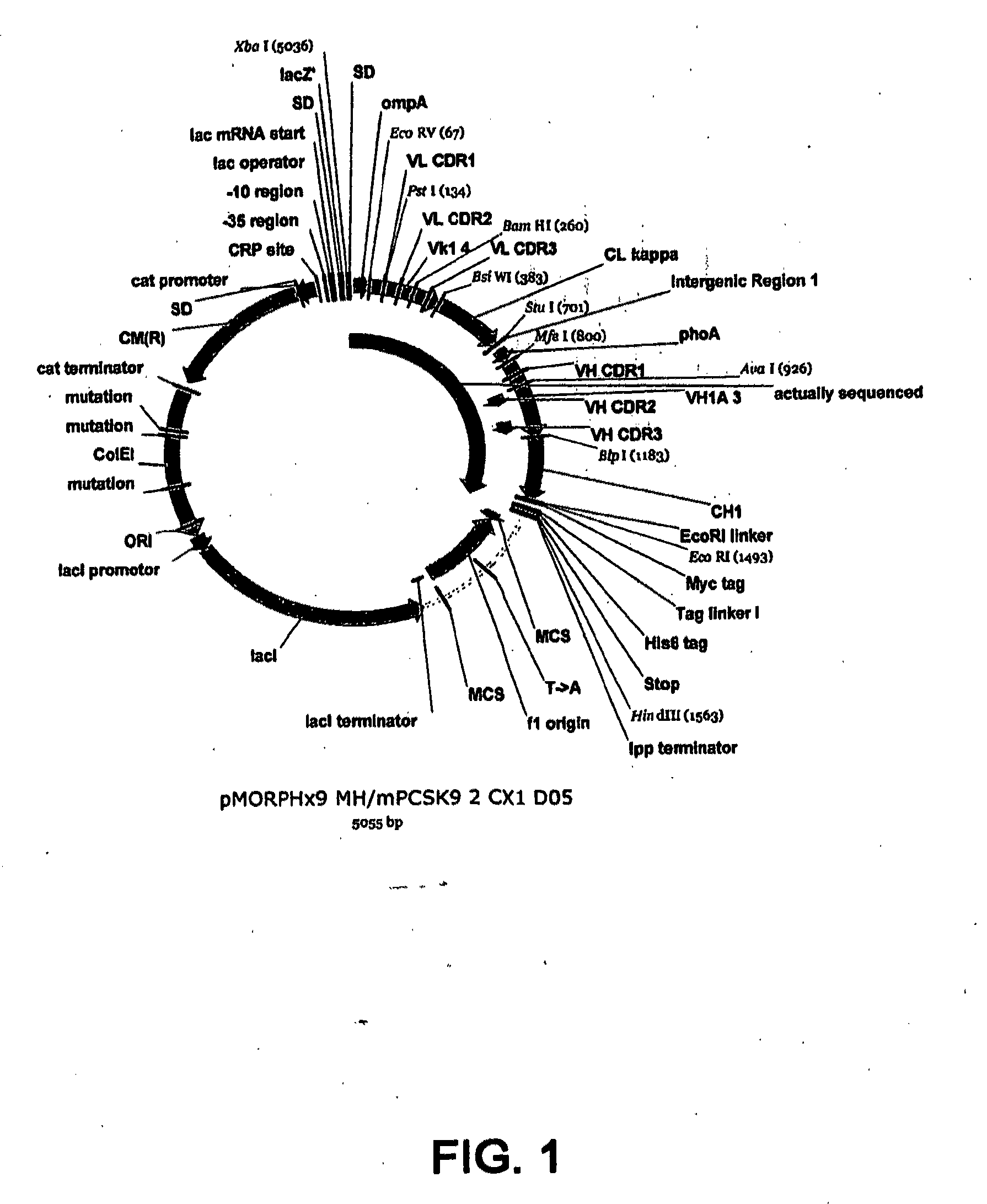
1D05 PCSK9 ANTAGONISTS
This application is a divisional of U.S. patent application Ser. No. 12/322,867; filed Feb. 6, 2009; which is herein incorporated by reference in its entirety; and which claims the benefit of U.S. Provisional Application No. 61/063,949, filed on Feb. 7, 2008, and 61/066,577, filed Feb. 21, 2008. Not Applicable. Not Applicable. Proprotein convertase subtilisin-kexin type 9 (hereinafter called “PCSK9”), also known as neural apoptosis-regulated convertase 1 (“NARC-1”), is a proteinase K-like subtilase identified as the 9thmember of the secretory subtilase family; see Seidah et al., 2003 Original synthesis of PCSK9 is in the form of an inactive enzyme precursor, or zymogen, of ˜72-kDa which undergoes autocatalytic, intramolecular processing in the endoplasmic reticulum (“ER”) to activate its functionality. This internal processing event has been reported to occur at the SSVFAQ ↓ SIPWNL158motif (SEQ ID NOs: 19 and 20, respectively); Benjannet et al., 2004 The sequence for human PCSK9 (˜22-kb long with 12 exons encoding a 692 amino acid protein) can be found in one instance at Deposit No. NP—777596.2. Human, mouse and rat PCSK9 nucleic acid sequences have been deposited; see, e.g., GenBank Accession Nos.: AX127530 (also AX207686), NP—705793 (also Q80W65), and P59996, respectively. PCSK9 possesses several domains found in other proprotein convertases, including an N-terminal signal sequence, a pro domain, a catalytic domain and a cysteine-rich C terminal domain. The PCSK9 catalytic domain shares high sequence similarity with the proteinase K family of subtilases and, notably, a catalytic triad of D186, H226 and S386. PCSK9 is disclosed and/or claimed in several patent publications including, but not limited to the following: PCT Publication Nos. WO 01/31007, WO 01/57081, WO 02/14358, WO 01/98468, WO 02/102993, WO 02/102994, WO 02/46383, WO 02/90526, WO 01/77137, and WO 01/34768; US Publication Nos. US 2004/0009553 and US 2003/0119038, and European Publication Nos. EP 1 440 981, EP 1 067 182, and EP 1 471 152. PCSK9 has been ascribed a role in the differentiation of hepatic and neuronal cells (Seidah et al., supra.), is highly expressed in embryonic liver, and has been strongly implicated in cholesterol homeostasis. Studies have suggested a specific role for PCSK9 in cholesterol biosynthesis or uptake. In a study of cholesterol-fed rats, Maxwell et al. found that PCSK9 was downregulated in a similar manner to three other genes involved in cholesterol biosynthesis, Maxwell et al., 2003 Several lines of evidence demonstrate that PCSK9, in particular, lowers the amount of hepatic LDLR protein and thus compromises the liver's ability to remove LDL cholesterol from the circulation. Adenovirus-mediated overexpression of PCSK9 in the livers of mice results in the accumulation of circulating LDL-C due to a dramatic loss of hepatic LDLR protein, with no effect on LDLR mRNA levels; Benjannet et al., 2004 A number of mutations in the gene PCSK9 have also been conclusively associated with autosomal dominant hypercholesterolemia (“ADH”), an inherited metabolism disorder characterized by marked elevations of low density lipoprotein (“LDL”) particles in the plasma which can lead to premature cardiovascular failure; see Abifadel et al., 2003 Accordingly, there can be no doubt that PCSK9 plays a role in the regulation of LDL. The expression or upregulation of PCSK9 is associated with increased plasma levels of LDL cholesterol, and the corresponding inhibition or lack of expression of PCSK9 is associated with reduced LDL cholesterol plasma levels. Decreased levels of LDL cholesterol associated with sequence variations in PCSK9 have been found to confer protection against coronary heart disease; Cohen, 2006 The identification of compounds and/or agents effective in the treatment of cardiovascular affliction is highly desirable. In clinical trials, reductions in LDL cholesterol levels have been directly related to the rate of coronary events; Law et al., 2003 The present invention advances these interests by providing antagonists of PCSK9 of use for inhibiting the activities of PCSK9 and the corresponding role PCSK9 plays in various therapeutic conditions. The present invention relates to antagonists of PCSK9 and, in particular embodiments, those antagonists that inhibit both human and murine PCSK9 and those exhibiting preferential targeting of processed PCSK9. Broadly, protein-specific antagonists of PCSK9 (or “PCSK9-specific antagonists” as referred to herein) are PCSK9 protein binding molecules or molecules effective in the selective binding of PCSK9 and inhibition of PCSK9 function. These molecules are of import in the treatment of conditions associated with or impacted by PCSK9 function, including, but not limited to hypercholesterolemia, coronary heart disease, metabolic syndrome, acute coronary syndrome and related conditions. PCSK9-specific antagonists are characterized by selective recognition and binding to PCSK9. PCSK9-specific antagonists do not show significant binding to proteins other than PCSK9, other than in those specific instances where the antagonist is supplemented or designed to confer an additional, distinct specificity to the PCSK9-specific binding component. PCSK9-specific antagonists forming particular embodiments hereof comprise (a) a heavy chain variable region comprising a CDR3 domain comprising SEQ ID NO: 17 or an equivalent of SEQ ID NO: 17, said equivalent characterized as having one or more conservative amino acid substitutions in the CDR3 domain; and/or (b) a light chain variable region comprising a CDR3 domain comprising SEQ ID NO: 7 or an equivalent of SEQ ID NO: 7, said equivalent characterized as having one or more conservative amino acid substitutions in the CDR3 domain. In specific embodiments, PCSK9-specific antagonists bind to human and/or murine PCSK9 with a KDof 1.2×10−6M or less. In more specific embodiments, PCSK9-specific antagonists bind to human and/or murine PCSK9 with a KDof 1×10−7M or less. In additional embodiments, PCSK9-specific antagonists bind to human and/or murine PCSK9 with a KDof 1×10−8M or less. In further embodiments, PCSK9-specific antagonists bind to human and/or murine PCSK9 with a KDof 5×10−9M or less, or of 1×10−9M or less. In select embodiments, PCSK9-specific antagonists bind to human and/or murine PCSK9 with a KDof 1×10−10M or less, a KDof 1×10−11M or less, or a KDof 1×10−12M or less. In specific embodiments, PCSK9-specific antagonists do not bind proteins other than PCSK9 at the above levels indicated for binding to PCSK9. Particular embodiments of the present invention include PCSK9-specific antagonists which exhibit binding to PCSK9 at one of the above prescribed levels and compete for binding to PCSK9 with 1D05 antibody molecules. 1D05 antibody molecules form important PCSK9-specific antagonists hereof. 1D05 antibody molecules are characterized as comprising a (i) heavy chain variable region (“VH”) comprising SEQ ID NO: 11; and (ii) a light chain variable region (“VL”) comprising SEQ ID NO: 27. Said VH and VL regions comprise the full complement of disclosed CDRs 1, 2 and 3 for the VH (SEQ ID NOs: 13, 15 and 17) and VL regions (SEQ ID NOs: 3, 5 and 7), respectively. Examples of 1D05 antibody molecules include without limitation: (i) a Fab which comprises a light chain comprising SEQ ID NO: 1 and an Fd chain comprising amino acids 1-233 of SEQ ID NO: 9 (or SEQ ID NO: 9); and (ii) a full length antibody molecule which comprises a light chain comprising SEQ ID NO: 26 and a heavy chain comprising SEQ ID NO: 25. PCSK9-specific antagonists are effective in counteracting PCSK9-dependent inhibition of cellular LDL-uptake, and particularly human and/or murine PCSK9-dependent inhibition of cellular LDL uptake. Repeatedly, PCSK9-specific antagonist 1D05 has demonstrated dose-dependent inhibition of the effects of PCSK9 on LDL uptake. Accordingly, the disclosed PCSK9-specific antagonists are of import for lowering plasma LDL cholesterol levels. The disclosed antagonists also have utility for various diagnostic purposes, including the detection and quantification of PCSK9. Select 1D05 antagonists are, in particular, useful because of their cross-reactivity with both human and murine PCSK9. This quality enables particular 1D05 antagonists to be studied pharmacologically in murine models without having to ensure that the mice express human PCSK9. In such experiments, the murine model is sufficiently representative of the native activity of the targeted protein and the antagonist's inhibition thereof. In specific embodiments, the present invention encompasses PCSK9-specific antagonists. In particular embodiments, the present invention encompasses antibody molecules comprising disclosed heavy and/or light chain variable regions, equivalents of said regions having one or more conservative amino acid substitutions, and homologs thereof. Select embodiments comprise isolated PCSK9-specific antagonists that comprise the disclosed CDR domains or sets of the heavy and/or light chain CDR domains, and equivalents of such domains characterized as having one or more conservative amino acid substitutions. As will be appreciated by those skilled in the art, fragments of PCSK9-specific antagonists that retain the ability to antagonize PCSK9 may be inserted into various frameworks; see, e.g., U.S. Pat. No. 6,818,418 and references contained therein, the collective disclosures of which are incorporated herein by reference, which discuss various scaffolds which may be used to display antibody loops previously selected on the basis of antigen binding. In the alternative, genes encoding for VL and VH may be joined, using recombinant methods, for example using a synthetic linker that enables them to be made as a single protein chain in which the VL and VH regions pair to form monovalent molecules, otherwise known as single chain Fvs (“ScFVs”); see, e.g., Bird et al., 1988 PCSK-9 specific antagonists and fragments may be in the form of various non-antibody-based scaffolds, including but not limited to avimers (Avidia); DARPins (Molecular Partners); Adnectins (Adnexus), Anticalins (Pieris) and Affibodies (Affibody). The use of alternative scaffolds for protein binding is well appreciated in the scientific literature, see, e.g., Binz & Plückthun, 2005 In another aspect, the present invention provides nucleic acid encoding the disclosed PCSK9-specific antagonists and, in particular embodiments, PCSK9-specific antagonists which comprise the disclosed heavy and light chains, the disclosed variable heavy and light regions and select components thereof (including CDRs 1, 2 and/or 3), particularly the disclosed respective CDR3 regions. In another aspect, the present invention provides vectors comprising said nucleic acid. The present invention, additionally, provides isolated cell(s) comprising nucleic acid encoding disclosed PCSK9-specific antagonists. In another aspect, the present invention provides isolated cell(s) comprising a polypeptide or vector of the present invention. The present invention provides methods for making PCSK9-specific antagonists disclosed herein including but not limited to antibodies, antigen binding fragments, derivatives, chimeric molecules, fusions of any of the foregoing with another polypeptide, or alternative structures/compositions capable of specifically binding PCSK9 which comprise the disclosed sequences. The methods comprise: (i) incubating a cell comprising nucleic acid encoding the PCSK9-specific antagonist(s), or which comprises individual nucleic acids encoding one or more components thereof, said nucleic acids which, when expressed, collectively produce the antagonist(s), under conditions that allow for the expression and/or assembly of the PCSK9-specific antagonist(s), and (ii) isolating said antagonist(s) from the cell. One of skill in the art can obtain PCSK9-specific antagonists disclosed herein using standard recombinant DNA techniques as well. The present invention provides a method for antagonizing the activity or function of PCSK9 or a noted effect of PCSK9 which comprises contacting a cell, population of cells, or tissue sample of interest expressing PCSK9 (or treated with or having therein human or murine PCSK9) with a PCSK9-specific antagonist disclosed herein under conditions that allow said antagonist to bind to PCSK9. Specific embodiments of the present invention include such methods wherein the cell is a human or murine cell. Additional embodiments are wherein the cell expresses human or murine-derived PCSK9. In another aspect, the present invention provides a method for antagonizing the activity or function of PCSK9 or a noted effect of PCSK9 in a subject exhibiting a condition associated with PCSK9 activity, or a condition where the functioning of PCSK9 is contraindicated for a particular subject, which comprises administering to the subject a therapeutically effective amount of a PCSK9-specific antagonist of the present invention in a pharmaceutical or other composition. The present invention, thus, encompasses a method of treating a condition associated with PCSK9 activity, or a condition wherein the functioning of PCSK9 is contraindicated for a particular subject, which comprises administering to the subject a therapeutically effective amount of a PCSK9-specific antagonist of the present invention in a pharmaceutical or other composition. In select embodiments, the condition is hypercholesterolemia, coronary heart disease, metabolic syndrome, acute coronary syndrome or related conditions. In specific embodiments, the present invention encompasses a method of administering a disclosed PCSK9-specific antagonist to a subject which comprises delivering a therapeutically effective amount of a pharmaceutical or other composition comprising a PCSK9-specific antagonist as disclosed herein. In another aspect, the present invention provides a pharmaceutical composition or other composition comprising a PCSK9-specific antagonist of the invention characterized as comprising a pharmaceutically acceptable carrier including but not limited to an excipient, diluent, stabilizer, buffer, or alternative designed to facilitate administration of the antagonist in the desired amount to the treated individual. The following table offers a generalized outline of the sequences discussed in the present application. The Sequence Listing including all notations, sequences and features forms as express part of the disclosure hereof: The present invention relates to antagonists of PCSK9 and, in particular embodiments, those antagonists that inhibit both human and murine PCSK9 and those that preferentially target processed PCSK9. Protein-specific antagonists of PCSK9 (or “PCSK9-specific antagonists”) in accordance herewith are effective in the selective binding to and inhibition of PCSK9 function and, thus, are of import in the treatment of conditions associated with or impacted by PCSK9 function, including, but not limited to, hypercholesterolemia, coronary heart disease, metabolic syndrome, acute coronary syndrome and related conditions. Use of the term “antagonist” refers to the fact that the subject molecule can antagonize the functioning of PCSK9. Use of the term “antagonizing” or derivatives thereof refers to the act of opposing, counteracting, inhibiting, neutralizing or curtailing one or more functions of PCSK9. Reference herein to PCSK9 function or PCSK9 activity refers to any function or activity that is driven by, requires, or is exacerbated or enhanced by PCSK9. PCSK9-specific antagonists as described herein have proven to be effective for counteracting human and/or murine PCSK9-dependent inhibition of cellular LDL-uptake. One important embodiment hereof relates to 1D05 antibody molecules. Such 1D05 antibody molecules are characterized as comprising a (i) heavy chain variable region (“VH”) comprising SEQ ID NO: 11; and (ii) a light chain variable region (“VL”) comprising SEQ ID NO: 27. Said VH and VL regions comprise the full complement of disclosed CDRs 1, 2 and 3 for the VH (SEQ ID NOs: 13, 15 and 17) and VL regions (SEQ ID NOs: 3, 5 and 7), respectively. Examples of 1D05 antibody molecules include without limitation: (i) a Fab which comprises a light chain comprising SEQ ID NO: 1 and an Fd chain comprising amino acids 1-233 of SEQ ID NO: 9 (or SEQ ID NO: 9); and (ii) a full length antibody molecule which comprises a light chain comprising SEQ ID NO: 26 and a heavy chain comprising SEQ ID NO: 25. The select group of 1D05 antibodies demonstrate that PCSK9-specific antagonists as disclosed herein effectively inhibit both human and murine PCSK9 and may be studied pharmacologically in murine models absent the expression of human PCSK9. The CDR definitions arrived at and disclosed herein were defined using the Morphosys software program Sequence Analysis Software (“SAS”). Applicants wish to note, however, that various other methods are available to delineate and define the start and end points of the CDR sequences, including but not limited to Kabat, 1991 PCSK9-specific molecules also have utility for various diagnostic purposes in the detection and quantification of PCSK9. Disclosed PCSK9-specific antagonists are, furthermore, unique in that select embodiments have demonstrated a preferential recognition of processed PCSK9, the active form of PCSK9. PCSK9-specific antagonists as disclosed herein are desirable molecules for lowering plasma LDL cholesterol levels and are of utility for any primate, mammal or vertebrate of commercial or domestic veterinary importance. PCSK9-specific antagonists are of utility as well to inhibit the activity of PCSK9 in any population of cells or tissues possessing the LDL receptor. The utility of the disclosed antagonists is directly measurable by assays readily available to the skilled artisan. Means for measuring LDL uptake are described in the literature; see, e.g., Barak & Webb, 1981 Any type of cell bearing the LDL receptor can be employed in the above methods including, but not limited to HEK cells, HepG2 cells, and CHO cells. LDL particles derived from any source are of use in the above-described assays. In particular assays, the LDL particles are fresh particles derived from blood. This can be accomplished by any method available to the skilled artisan including, but not limited to, the method of Havel et al., 1955 Broadly, PCSK9-specific antagonists as defined herein selectively recognize and specifically bind to PCSK9. An antibody is typically said to specifically bind an antigen when the dissociation constant is ≦1 μM, preferably ≦100 nM and most preferably ≦10 nM. Use of the terms “selective” or “specific” herein, further, refers to the fact that the disclosed antagonists do not show significant binding to proteins other than PSCK9, except in those specific instances where the antagonist is supplemented or designed to confer an additional, distinct specificity to the PCSK9-specific binding portion (as, for example, in bispecific or bifunctional molecules where the molecule is designed to bind two molecules or effect two functions, at least one of which is to specifically bind PCSK9). In specific embodiments, PCSK9-specific antagonists bind to human and/or murine PCSK9 with a KDof 1.2×10−6M or less. In more specific embodiments, PCSK9-specific antagonists bind to human and/or murine PCSK9 with a KDof 5×10−7M or less, of 2×10−7M or less, or of 1×10−7M or less. In additional embodiments, PCSK9-specific antagonists bind to human and/or murine PCSK9 with a KDof 1×10−8M or less. In further embodiments, PCSK9-specific antagonists bind to human and/or murine PCSK9 with a KDof 5×10−9M or less, or of 1×10−9M or less. In select embodiments, PCSK9-specific antagonists bind to human and/or murine PCSK9 with a KDof 1×10−10M or less, a KDof 1×10−11M or less, or a KDof 1×10−12M or less. In specific embodiments, PCSK9-specific antagonists do not bind proteins other than PCSK9 at the above KDs. KDrefers to the dissociation constant obtained from the ratio of Kd(the dissociation rate of a particular binding molecule-target protein interaction) to Ka(the association rate of the particular binding molecule-target protein interaction), or Kd/Kawhich is expressed as a molar concentration (M). KDvalues can be determined using methods well established in the art. A preferred method for determining the KDof a binding molecule is by using surface plasmon resonance, for example employing a biosensor system such as a Biacore™ (GE Healthcare Life Sciences) system. PCSK9-specific antagonists disclosed herein have been shown to dose-dependently inhibit human and/or murine PCSK9 dependent effects on LDL uptake. Accordingly, PCSK9-specific antagonists as disclosed herein are characterized by their ability to counteract PCSK9-dependent inhibition of LDL uptake into cells. This uptake of LDL into cells by the LDL receptor is referred to herein as “cellular LDL uptake”. In specific embodiments, PCSK9-specific antagonists counteract or antagonize human and/or murine PCSK9-dependent inhibition of LDL uptake into cells, exhibiting an IC50of less than 1.0×10−6M, or, in order of preference, less than 1×10−7M, 1×10−8M, 1×10−9M, 1×10−10M, 1×10−11M and 1×10−12M. The extent of inhibition by any PCSK9-specific antagonist may be measured quantitatively in statistical comparison to a control, or via any alternative method available in the art for assessing a negative effect on, or inhibition of, PCSK9 function (i.e., any method capable of assessing antagonism of PCSK9 function). In specific embodiments, the inhibition is at least about 10% inhibition. In other embodiments, the inhibition is at least 20%, 30%, 40%, 50%, 60%, 70,%, 80%, 90%, or 95%. Accordingly, PCSK9-specific antagonists capable of effecting these levels of inhibition of PCSK9 function form particular embodiments hereof. A PCSK9-specific antagonist in accordance herewith can be any binding molecule that specifically binds human and/or murine PCSK9 protein including, but not limited to, antibody molecules as defined below, any PCSK9-specific binding structure, any polypeptide or nucleic acid structure that specifically binds PCSK9, and any of the foregoing incorporated into various protein scaffolds; including but not limited to, various non-antibody-based scaffolds, and various structures capable of affording or allowing for selective binding to PCSK9 including but not limited to small modular immunopharmaceuticals (or “SMIPs”; see, Haan & Maggos, 2004 Importantly, the binding site (or epitope) for 1D05 on PCSK9 was identified through limited proteolysis and mass spectrometry (“LP-MS”). The limited proteolysis mass spectrometry analysis involved incubating wild-type PCSK9 (“wt-PCSK9”) and a complex of wt-PCSK9 and 1D05 with endoproteinase enzymes of different specificity in carefully controlled conditions. Under such conditions, the endoproteases cleaved only exposed primary cleavage sites. The experiment was designed so that the binding of 1D05 to wt-hPCSK9 masked surface residues normally exposed on wt-hPCSK9 not bound to the antibody. Such masked residues provided insight into the binding domain of 1D05. Through such experiments, a novel neutralizing epitope conformational in nature and represented by peptides RYRAD (SEQ ID NO: 42) AND REIEGR (SEQ ID NO: 37) was identified. This epitope falls within PCSK9's catalytic domain and provides a novel target epitope for which to identify additional effective PCSK9 antagonists. Identification of additional PCSK9-specific antagonists binding this epitope is of significant interest given 1D05's PCSK9-neutralizing activity. One means of identifying antagonists and particularly antibodies that bind to the identified 1D05 epitope or an overlapping epitope is through a competition or similar assay where the candidate antibody or binding molecule would have to out-compete 1D05 for the epitope. Competitive antagonists encompassed herein are molecules that inhibit (i.e., prevent or interfere with in comparison to a control) or reduce 1D05 binding by at least 50%, 60%, 70%, and 80% in order of increasing preference (even more preferably, at least 90% and, most preferably, at least 95%) at 1 μM or less with 1D05 at or below its KD, and in particular those molecules that antagonize (i) PCSK9 binding to the LDL receptor, (ii) PCSK9 internalization into cells, or (iii) both PCSK9 binding to the LDL receptor and PCSK9 internalization into cells. Competition between binding members may be readily assayed in vitro for example using ELISA and/or by monitoring the interaction of the antibodies with PCSK9 in solution. The exact means for conducting the analysis is not critical. PCSK9 may be immobilized to a 96-well plate or may be placed in a homogenous solution. In specific embodiments, the ability of unlabeled candidate antibody(ies) to block the binding of labeled 1D05 can be measured using radioactive, enzyme or other labels. In the reverse assay, the ability of unlabeled antibodies to interfere with the interaction of labeled 1D05 with PCSK9 wherein said 1D05 and PCSK9 are already bound is determined. In specific embodiments, (i) PCSK9 is contacted with labeled 1D05 (an antibody molecule which comprises a VL comprising SEQ ID NO: 27 and a VH comprising SEQ ID NO: 11); (ii) PCSK9 is contacted with the candidate antibody or pool of antibodies; and (iii) antibodies capable of interrupting or preventing complexes between PCSK9 and 1D05 are identified. The readout in such an example is through measurement of bound label. 1D05 and the candidate antibody(ies) may be added in any order or at the same time. A specific assay that may be run is that of Example 13 where the activity of an antibody found to bind to the same epitope domain as 1D05 is illustrated. Antibodies identified as 1D05 competitors in the above or other suitable assays may be tested for the ability to antagonize or neutralize (i) PCSK9 binding to the LDL receptor; and/or (ii) PCSK9 internalization into cells. These parameters may be measured through the use of assays similar to that employed or described in the current specification. In specific embodiments, the inhibition demonstrated by the competing antibody is at least about 10% inhibition. In other embodiments, the inhibition is at least 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90% or 95%. The present invention specifically encompasses PCSK9-specific antagonists and particularly monoclonal antibody molecules (and their corresponding amino acid and nucleic acid sequences) that selectively bind to the epitope identified for 1D05 or an overlapping epitope interfering with 1D05's binding to PCSK9. Critical residues for 1D05 binding that were identified on the epitope of PCSK9 are those residues corresponding to residues Arg194, Glu197 and Arg199 of human PCSK9. The narrow epitope comprising these amino acid residues is represented by SEQ ID NO: 37 and falls within the area of SEQ ID NO: 39 of human PCSK9 and SEQ ID NO: 41 of murine PCSK9. A secondary footprint of the antibody is represented by SEQ ID NO: 42. Monoclonal antibodies that specifically bind to the conformational epitope represented by SEQ ID NO: 37 and SEQ ID NO:42 or an overlapping epitope antagonize or neutralize (i) PCSK9 binding to the LDL receptor; (ii) PCSK9 internalization into cells, or (iii) both. Accordingly, monoclonal antibodies that bind to an epitope on PCSK9 which comprises and/or consists of: SEQ ID NO: 37, SEQ ID NO: 39 or SEQ ID NO: 41 form important embodiments of the present invention. Specific embodiments of the present invention relate to monoclonal antibodies that recognize the following epitopes on PCSK9: SEQ ID NO: 37 and SEQ ID NO: 42. A monoclonal antibody molecule in accordance herewith may be an intact (complete or full length) antibody, a substantially intact antibody, or a portion or fragment of an antibody comprising an antigen-binding portion, e.g., a Fab fragment, Fab′ fragment or F(ab′)2fragment of a murine antibody or of a chimeric antibody or of a humanized antibody or of a human antibody. Monoclonal, as used herein, refers to a homogeneous or substantially homogeneous (or pure) antibody population (i.e., at least about 90%, 91%, 92%, 93%, 94%, 95%, 96%, more preferably at least about 97% or 98%, or most preferably at least 99% of the antibodies in the population are identical and would compete in an ELISA assay for the same antigen or epitope. In specific embodiments of the present invention, the present invention provides monoclonal antibodies that (i) compete for binding to PCSK9 with a 1D05 antibody molecule, reducing 1D05 binding by at least 50% at 1 μM or less with 1D05 at or below its KD, (ii) block PCSK9 binding to the LDL receptor, (iii) inhibit PCSK9 internalization into the cell, and (iv) comprise a specific antigen-binding region, VH, VL, set of CDRs or heavy CDR3, heavy and/or light chain or any variant of these components described herein. Additional embodiments provide PCSK9-specific antagonists including but not limited to monoclonal antibodies that recognize/bind to SEQ ID NO: 37, SEQ ID NO: 39 or SEQ ID NO: 41, wherein the PCSK9-specific antagonists bind to human and/or murine PCSK9 with a KDof 1.2×10−6M or less, and wherein the PCSK9-specific antagonist competes with 1D05 for binding to PCSK9. In specific embodiments hereof, the PCSK9-specific antagonists are further defined by one or more of the following qualities: they (i) reduce 1D05 binding by at least 50% at 1 μM or less with 1D05 at or below its KD, (ii) block PCSK9 binding to the LDL receptor, (iii) inhibit PCSK9 internalization into the cell, and/or (iv) comprise a specific antigen-binding region, VH, VL, set of CDRs or heavy CDR3, heavy and/or light chain or any variant of these components described herein. In specific embodiments, the PCSK9-specific antagonists in accordance with the above comprise (i) the disclosed heavy and/or light chain variable region CDR3 sequences (SEQ ID NOs: 17 and 7, respectively), (ii) the disclosed heavy and/or light chain variable regions CDR1 (SEQ ID NOs: 13 and 3, respectively), CDR2 (SEQ ID NOs: 15 and 5, respectively) and CDR3 (SEQ ID NOs; 17 and 7, respectively, (iii) the full complement (SEQ ID NOs; 13, 15, 17, 3, 5 and 7) of disclosed heavy and light chain CDRs within a variable region framework of a human heavy and/or light chain sequence; (iv) the disclosed VL and/or VH regions (SEQ ID NOs: 27 and 11, respectively); (v) the disclosed light and/or Fd chains (SEQ ID NO: 1 and amino acids 1-233 of SEQ ID NO: 9 (or SEQ ID NO: 9)), or (vi) the disclosed light and/or heavy chains (SEQ ID NOs: 26 and 25). In specific embodiments, the PCSK9-specific antagonists bind to/recognize both SEQ ID NOs: 37 and SEQ ID NO: 42. In any of the above assays for identifying antibodies binding the same or overlapping epitope region as 1D05, binding of the known binder (i.e., 1D05 antibody molecule known to bind residues Arg194, Glu197 and Arg199 of SEQ ID NO: 37) as compared to the binding of the candidate binder should be distinguishable. This can (but need not) be accomplished through the use of labels on either or both molecules as will be readily appreciated by the skilled artisan. Labels, as used herein, refer to another molecule or agent incorporated into/affixed to the antibody molecule. In one embodiment, the label is a detectable marker, e.g., a radiolabeled amino acid or attachment to a polypeptide of biotinyl moieties that can be detected by marked avidin (e.g., streptavidin containing a fluorescent marker or enzymatic activity that can be detected by optical or colorimetric methods). Various methods of labeling polypeptides and glycoproteins are known in the art and may be used. Examples of labels for polypeptides include, but are not limited to, the following: radioisotopes or radionuclides (e.g.,3H,14C,15N,35S,90Y,99Tc,111In,125I,131I), fluorescent labels (e.g., FITC, rhodamine, lanthanide phosphors), enzymatic labels (e.g., horseradish peroxidase, β-galactosidase, luciferase, alkaline phosphatase), chemiluminescent markers, biotinyl groups, predetermined polypeptide epitopes recognized by a secondary reporter (e.g., leucine zipper pair sequences, binding sites for secondary antibodies, metal binding domains, epitope tags), magnetic agents, such as gadolinium chelates, toxins such as pertussis toxin, taxol, cytochalasin B, gramicidin D, ethidium bromide, emetine, mitomycin, etoposide, tenoposide, vincristine, vinblastine, colchicin, doxorubicin, daunorubicin, dihydroxy anthracin dione, mitoxantrone, mithramycin, actinomycin D, 1-dehydrotestosterone, glucocorticoids, procaine, tetracaine, lidocaine, propranolol, and puromycin and analogs or homologs thereof. In some embodiments, labels are attached by spacer arms of various lengths to reduce potential steric hindrance. A 1D05 antibody used for the competition assays may be any antibody molecule which is of the 1D05 description provided herein (i.e. any antibody molecule selective for PCSK9 which comprises a VL comprising SEQ ID NO: 27 and a VH comprising SEQ ID NO: 11). Examples of such antibodies include without limitation (i) a Fab which comprises a light chain comprising SEQ ID NO: 1 and an Fd chain comprising amino acids 1-233 of SEQ ID NO: 9 (or SEQ ID NO: 9); (ii) a full length antibody molecule which comprises a light chain comprising SEQ ID NO: 26 and a heavy chain comprising SEQ ID NO: 25. Peptides or peptidomimetics based on the regions corresponding to SEQ ID NO: 39 or SEQ ID NO: 41 (and in select embodiments the areas corresponding to SEQ ID NO: 37 and SEQ ID NO: 42) should have antagonistic properties by preventing the interaction of PCSK9 with LDLR. Importantly, peptides that comprise SEQ ID NO: 37 and SEQ ID NO: 42 should generate neutralizing antibodies able to inhibit PCSK9 binding to LDLR and/or inhibit PCSK9 internalization into cells. In specific embodiments, peptides encompassed herein comprise SEQ ID NO: 39 OR SEQ ID NO: 41. In select embodiments, the peptides comprise SEQ ID NO: 37 and are less than 50 amino acids. In certain embodiments, the peptides comprise both SEQ ID NO: 37 and SEQ ID NO: 42 and are 40 amino acids or less. In more specific embodiments, the peptides comprise SEQ ID NO: 37 and are less than 40 amino acids, less than 30 amino acids, less than 20 amino acids, or less than 10 amino acids. Screening of peptides of the invention may be carried out utilizing competition assays as described above. If the peptide being tested competes with a 1D05 antibody molecule (i.e. any antibody molecule selective for PCSK9 which comprises a VL comprising SEQ ID NO: 27 and a VH comprising SEQ ID NO: 11) as shown by a decrease in binding of such 1D05 antibody molecule then it is likely that the peptide and 1D05 bind to the same, or a closely related, epitope. Still another way to determine whether a peptide has the specificity of the 1D05 antibody molecule is to pre-incubate the 1D05 antibody molecule with PCSK9 with which it is normally reactive, and then add the peptide being tested with demonstrated specificity for PCSK9 to determine whether the peptide is inhibited in its ability to bind PCSK9. If the peptide being tested is inhibited then, in all likelihood, it has the same, or a functionally equivalent, epitope and specificity as the 1D05 antibody molecule. Using routine procedures as outlined throughout the instant specification and well known to those of ordinary skill in the art, one can then determine whether a peptide which binds to PCSK9 is useful by determining whether the peptide is blocks PCSK9 from binding to the LDL receptor and/or prevents PCSK9 internalization into cells. Expression and selection of any of the PCSK9-specific antagonists described in the present application may be achieved using suitable technologies including, but not limited to phage display (see, e.g., International Application Number WO 92/01047, Kay et al., 1996 Particular PCSK9-specific antagonists forming part of the present invention are antibody molecules or antibodies. “Antibody molecule” or “Antibody” as described herein refers to an immunoglobulin-derived structure with selective binding to human and/or murine PCSK9 including, but not limited to, a full length or whole antibody, an antigen binding fragment (a fragment derived, physically or conceptually, from an antibody structure), a derivative of any of the foregoing, a fusion of any of the foregoing with another polypeptide, or any alternative structure/composition which incorporates any of the foregoing for purposes of selectively binding to/inhibiting the function of PCSK9. “Whole” antibodies or “full length” antibodies refer to proteins that comprise two heavy (H) and two light (L) chains inter-connected by disulfide bonds which comprise: (1) in terms of the heavy chains, a variable region (abbreviated herein as “VH”) and a heavy chain constant region which comprises three domains, CH1, CH2, and CH3; and (2) in terms of the light chains, a light chain variable region (abbreviated herein as “VL”) and a light chain constant region which comprises one domain, CL. Antibody fragments and, more specifically, antigen binding fragments are molecules possessing an antibody variable region or segment thereof (which comprises one or more of the disclosed CDR 3 domains, heavy and/or light within framework regions of heavy and/or light chains, as appropriate), which confers selective binding to PCSK9, and particularly human and/or murine PCSK9. Antibody fragments containing such an antibody variable region include, but are not limited to the following antibody molecules: a Fab, a F(ab′)2, a Fd, a Fv, a scFv, bispecific antibody molecules (antibody molecules comprising a PCSK9-specific antibody or antigen binding fragment as disclosed herein linked to a second functional moiety having a different binding specificity than the antibody, including, without limitation, another peptide or protein such as an antibody, or receptor ligand), a bispecific single chain Fv dimer, an isolated CDR3, a minibody, a ‘scAb’, a dAb fragment, a diabody, a triabody, a tetrabody, a minibody, and artificial antibodies based upon protein scaffolds, including but not limited to fibronectin type III polypeptide antibodies (see, e.g., U.S. Pat. No. 6,703,199 and International Application Numbers WO 02/32925 and WO 00/34784) or cytochrome B; see, e.g., Nygren et al., 1997 The term “isolated” as used herein in reference to antibody molecules, PCSK9-specific antagonists in general, encoding nucleic acid or other describes a property as it pertains to the disclosed PCSK9-specific antagonists, nucleic acid or other that makes them different from that found in nature. The difference can be, for example, that they are of a different purity than that found in nature, or that they are of a different structure or form part of a different structure than that found in nature. A structure not found in nature, for example, includes recombinant human immunoglobulin structures including, but not limited to, recombinant human immunoglobulin structures with optimized CDRs. Other examples of structures not found in nature are PCSK9-specific antagonists or nucleic acid substantially free of other cellular material. Isolated PCSK9-specific antagonists are generally free of other protein-specific antagonists having different protein specificities (i.e., possess an affinity for other than PCSK9). In one particular aspect, the present invention provides isolated PCSK9-specific antagonists which antagonize PCSK9 function. In particular embodiments, said PCSK9-specific antagonists inhibit human and/or murine PCSK9's antagonism of cellular LDL uptake by interfering with PCSK9 binding to the LDL receptor and resultant PCSK9 cell internalization. Disclosed PCSK9-specific antagonists, thus, form desirable molecules for lowering plasma LDL-cholesterol levels; see, e.g., Cohen et al., 2005 Through repeat experiments, 1D05 antibody molecules as disclosed herein dose-dependently inhibited the effects of both human and/or murine PCSK9 on LDL uptake. In specific embodiments, the present invention, thus, encompasses PCSK9-specific antagonists and, in more specific embodiments, antibody molecules comprising the heavy and/or light chain variable regions (SEQ ID NO: 11 and 27, respectively) contained within these 1D05 antibody molecules or the heavy and/or light chains, e.g., amino acids 1-233 of SEQ ID NO: 9 (or SEQ ID NO: 9) and SEQ ID NO: 1, respectively, or SEQ ID NOs: 25 and 26, respectively, as well as equivalents (characterized as having one or more conservative amino acid substitutions that do not degrade the PCSK9-selective property of 1D05) or homologs thereof. Particular embodiments comprise isolated PCSK9-specific antagonists that comprise the CDR domains disclosed herein or sets of heavy and/or light chain CDR domains disclosed herein, or equivalents thereof, characterized as having one or more conservative amino acid substitutions. Use of the terms “domain” or “region” herein simply refers to the respective portion of the antibody molecule wherein the sequence or segment at issue will reside or, in the alternative, currently resides. In specific embodiments, the present invention provides isolated PCSK9-specific antagonists and, in more specific embodiments, antibody molecules comprising a heavy chain variable region which comprises SEQ ID NO: 11; equivalents thereof characterized as having one or more conservative amino acid substitutions, and homologs thereof. The disclosed antagonists should counteract or inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake. In specific embodiments, the present invention provides homologs of the disclosed antagonists characterized as being at least 90% identical over the heavy chain variable region to SEQ ID NO: 11; said antagonists which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. In specific embodiments, the present invention provides isolated PCSK9-specific antagonists and, in more specific embodiments, antibody molecules comprising a light chain variable region which comprises SEQ ID NO: 27; equivalents thereof characterized as having one or more conservative amino acid substitutions, and homologs thereof. The disclosed antagonists should counteract or inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake. In specific embodiments, the present invention provides homologs of the disclosed antagonists characterized as being at least 90% identical over the light chain variable region to SEQ ID NO: 27; said antagonists which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. In specific embodiments, the present invention provides isolated PCSK9-specific antibody molecules which comprise a heavy chain variable region comprising SEQ ID NO: 11 and a light chain variable region comprising SEQ ID NO: 27; or equivalents thereof characterized as having one or more conservative amino acid substitutions in the prescribed sequences. Specific embodiments are said antagonists which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. In specific embodiments, the present invention provides homologs of the disclosed antagonists characterized as being at least 90% identical over the heavy and light chain variable regions to SEQ ID NOs: 11 and 27, respectively; said antagonists which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. In particular embodiments, the present invention provides isolated PCSK9-specific antagonists and, in more specific embodiments, PCSK9 antibody molecules that comprise variable heavy CDR3 sequence SEQ ID NO: 17; and equivalents thereof characterized as having one or more conservative amino acid substitutions; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. Specific embodiments provide isolated antagonists which additionally comprise in the heavy chain variable region CDR1 and/or CDR2 sequences comprising SEQ ID NO: 13 and/or SEQ ID NO: 15, respectively; or equivalents thereof characterized as having one or more conservative amino acid substitutions in any one or more of the CDR sequences. In specific embodiments, the present invention provides homologs of the disclosed antagonists characterized as being at least 90% identical over the CDR3 sequences or within each of the CDR1, CDR2 and CDR3 sequences to SEQ ID NO: 17 or SEQ ID NOs: 13, 15 and 17, respectively, as appropriate; said antagonists which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. In particular embodiments, the present invention provides isolated PCSK9-specific antagonists and, in more specific embodiments, antibody molecules which comprise variable light CDR3 sequence which comprises SEQ ID NO: 7; and equivalents thereof characterized as having one or more conservative amino acid substitutions; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. Specific embodiments provide isolated antagonists which additionally comprise in the light chain variable region CDR1 and/or CDR2 sequences comprising SEQ ID NO: 3 and/or SEQ ID NO: 5, respectively; or an equivalent thereof characterized as having one or more conservative amino acid substitutions in any one or more of the CDR sequences. In specific embodiments, the present invention provides homologs of the disclosed antagonists characterized as being at least 90% identical over the CDR3 sequences or within each of the CDR1, CDR2 and CDR3 sequences to SEQ ID NO: 7 or SEQ ID NOs: 3, 5 and 7, respectively, as appropriate; said antagonists which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. In particular embodiments, the present invention provides isolated PCSK9-specific antagonists and, in more specific embodiments, antibody molecules which comprise heavy chain variable region CDR3 sequence and light chain variable region CDR3 sequence comprising SEQ ID NOs: 17 and 7, respectively; or equivalents thereof characterized as having one or more conservative amino acid substitutions in any one or more of the CDR3 sequences; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. In specific embodiments, the present invention provides homologs of the disclosed antagonists characterized as being at least 90% identical over the heavy and light chain variable region CDR3 sequences to SEQ ID NOs: 17 and 7, respectively; said antagonists which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. Specific embodiments provide isolated PCSK9-specific antagonists and, in more specific embodiments, antibody molecules which comprise heavy chain variable region CDR1, CDR2, and CDR3 sequences and light chain variable region CDR1, CDR2, and CDR3 sequences comprising SEQ ID NOs: 13, 15, 17, 3, 5 and 7, respectively; and equivalents thereof characterized as having one or more conservative amino acid substitutions in any one or more of the CDR sequences; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. In specific embodiments, the present invention provides homologs of the disclosed antagonists characterized as being at least 90% identical over the heavy and light chain variable region CDR1, CDR2 and CDR3 sequences to SEQ ID NOs: 13, 15, 17, 3, 5 and 7, respectively; said antagonists which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. One particular aspect of the present invention encompasses isolated PCSK9-specific antagonists and, in more specific embodiments, antibody molecules which are variants of that disclosed above which comprise a heavy chain variable region CDR3 sequence of SEQ ID NO: 45 wherein the CDR3 sequence is not SEQ ID NO: 17; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. Further embodiments hereof additionally comprise heavy chain variable region CDR1 sequence of SEQ ID NO: 43 wherein the variant sequence is not SEQ ID NO: 13 and/or heavy chain variable region CDR2 sequence of SEQ ID NO: 44 wherein the variant sequence is not SEQ ID NO: 15; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. In other embodiments, the present invention encompasses heavy chain variable region sequence comprising CDR1, CDR2, and CDR3 sequence which, respectively, comprises SEQ ID NOs: 43, 44 and 45 in the respective regions, which are, respectively, not SEQ ID NOs:13, 15 and 17; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. Another aspect of the present invention encompasses isolated PCSK9-specific antagonists and, in more specific embodiments, antibody molecules which are variants of that disclosed above which comprise a light chain variable region CDR3 sequence of SEQ ID NO: 48 wherein the CDR3 sequence is not SEQ ID NO: 7; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. Further embodiments hereof additionally comprise light chain variable region CDR1 sequence of SEQ ID NO: 46 wherein the variant sequence is not SEQ ID NO: 3 and/or light chain variable region CDR2 sequence of SEQ ID NO: 47 wherein the variant sequence is not SEQ ID NO: 5; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. In other embodiments, the present invention encompasses light chain variable region sequence comprising CDR1, CDR2 and CDR3 sequence which, respectively, comprises SEQ ID NOs: 46, 47 and 48 in the respective regions, which are, respectively, not SEQ ID NOs: 3, 5 and 7; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. Additional distinct embodiments encompass isolated PCSK9-specific antagonists which comprise: (a) a heavy chain variable region comprising CDR1, CDR2 and CDR3 sequence, wherein (i) the CDR1 sequence comprises SEQ ID NO: 13 or SEQ ID NO: 43; SEQ ID NO: 43 being different in sequence from SEQ ID NO: 13; (ii) the CDR2 sequence comprises SEQ ID NO: 15 or SEQ ID NO: 44; SEQ ID NO: 44 being different in sequence from SEQ ID NO: 15; and (iii) the CDR3 sequence comprises SEQ ID NO: 17 or SEQ ID NO: 45; SEQ ID NO: 45 being different in sequence from SEQ ID NO: 17; and/or (b) a light chain variable region comprising CDR1, CDR2 and CDR3 sequence, wherein (i) the CDR1 sequence comprises SEQ ID NO: 3 or SEQ ID NO: 46; SEQ ID NO: 46 being different in sequence from SEQ ID NO: 3; (ii) the CDR2 sequence comprises SEQ ID NO: 5 or SEQ ID NO: 47; SEQ ID NO: 47 being different in sequence from SEQ ID NO: 5; and (iii) the CDR3 sequence comprises SEQ ID NO: 7 or SEQ ID NO: 48; SEQ ID NO: 48 being different in sequence from SEQ ID NO: 7; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. Other aspects of the present invention encompass isolated PCSK9-specific antagonists and, in more specific embodiments, antibody molecules which are variants of that disclosed above which comprise (i) a heavy chain variable region sequence comprising CDR1, CDR2, and CDR3 sequence which, respectively, comprises SEQ ID NOs: 43, 44 and 45 in the respective regions, which are, respectively, not SEQ ID NOs:13, 15 and 17; and (ii) a light chain variable region sequence comprising CDR1, CDR2 and CDR3 sequence which, respectively, comprises SEQ ID NOs: 46, 47 and 48 in the respective regions, which are, respectively, not SEQ ID NOs: 3, 5 and 7; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. In specific embodiments herein the CDRs are in place of the corresponding regions of 1D05 with out without conservative amino acid substitutions; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. In particular embodiments, the present invention encompasses isolated PCSK9-specific antagonists and, in more specific embodiments, antibody molecules comprising heavy and/or light chain variable regions comprising SEQ ID NOs: 50 and 49, respectively; said variants SEQ ID NOs which are not SEQ ID NOs: 11 and 27, respectively; specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. Specific embodiments include any isolated PCSK9-specific antagonist and, in more specific embodiments, antibody molecules which comprise heavy chain variable region sequence found in any of SEQ ID NOs: 51-56, optionally comprising a light chain variable region sequence disclosed herein (e.g., SEQ ID NO: 27); specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. Other embodiments include any isolated PCSK9-specific antagonist and, in more specific embodiments, antibody molecules which comprise light chain variable region sequence found in any of SEQ ID NOs: 57-60, optionally comprising a heavy chain variable region sequence disclosed herein (e.g., SEQ ID NO: 11); specific embodiments of which inhibit human and/or murine PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. Particular embodiments are isolated PCSK9-specific antagonists which comprise the above-described VH and VL regions in a full length antibody. Specific embodiments herein further comprise a series of amino acids selected from the group consisting of: SEQ ID NO: 21 (IgG1), SEQ ID NO: 22 (IgG2), SEQ ID NO: 23 (IgG4) and SEQ ID NO: 24 (IgG2 m4). Conservative amino acid substitutions, as one of ordinary skill in the art will appreciate, are substitutions that replace an amino acid residue with one imparting similar or better (for the intended purpose) functional and/or chemical characteristics. Antagonists bearing such conservative amino acid substitutions can be tested for retained or better activity using functional assays available in the art or described herein. PCSK9-specific antagonists possessing one or more conservative amino acid substitutions which retain the ability to selectively bind to human PCSK9 and antagonize PCSK9 functioning at a level the same or better than 1D05 antibody molecules as described herein are referred to herein as “functional equivalents” of the disclosed antagonists and form specific embodiments of the present invention. Conservative amino acid substitutions are often ones in which the amino acid residue is replaced with an amino acid residue having a similar side chain. Families of amino acid residues having similar side chains have been defined in the art. These families include amino acids with basic side chains (e.g., lysine, arginine, histidine), acidic side chains (e.g., aspartic acid, glutamic acid), uncharged polar side chains (e.g., glycine, asparagine, glutamine, serine, threonine, tyrosine, cysteine, tryptophan), nonpolar side chains (e.g., alanine, valine, leucine, isoleucine, proline, phenylalanine, methionine), beta-branched side chains (e.g., threonine, valine, isoleucine) and aromatic side chains (e.g., tyrosine, phenylalanine, tryptophan, histidine). Such modifications are not designed to significantly reduce or alter the binding or functional inhibition characteristics of the PCSK9-specific antagonist, albeit they may improve such properties. The purpose for making a substitution is not significant and can include, but is by no means limited to, replacing a residue with one better able to maintain or enhance the structure of the molecule, the charge or hydrophobicity of the molecule, or the size of the molecule. For instance, one may desire simply to substitute a less desired residue with one of the same polarity or charge. Such modifications can be introduced by standard techniques known in the art, such as site-directed mutagenesis and PCR-mediated mutagenesis. One specific means by which those of skill in the art accomplish conservative amino acid substitutions is alanine scanning mutagenesis as discussed in, for example, MacLennan et al., 1998 In another aspect, the present invention provides isolated PCSK9-specific antagonists and, in more specific embodiments, antibody molecules which comprise heavy and/or light chain variable regions comprising amino acid sequences that are homologous to the corresponding amino acid sequences of the disclosed antibodies, wherein the antibody molecules inhibit PCSK9-dependent inhibition of cellular LDL uptake. Specific embodiments are antagonists which comprise heavy and/or light chain variable regions which are at least 90% identical to disclosed heavy and/or light chain variable regions, respectively. Reference to “at least 90% identical” includes at least 90, 91, 92, 93, 94, 95, 96, 97, 98, 99 and 100% identical sequences along the full length of the molecule disclosed herein. PCSK9-specific antagonists with amino acid sequences homologous to the amino acid sequences of antagonists described herein are typically produced to improve one or more of the properties of the antagonist without negatively impacting its specificity for PCSK9. One method of obtaining such sequences, which is not the only method available to the skilled artisan, is to mutate sequence encoding the PCSK9-specific antagonist or specificity-determining region(s) thereof, express an antagonist comprising the mutated sequence(s), and test the encoded antagonist for retained function using available functional assays including those described herein. Mutation may be by site-directed or random mutagenesis. As one of skill in the art will appreciate, however, other methods of mutagenesis can readily bring about the same effect. For example, in certain methods, the spectrum of mutants are constrained by non-randomly targeting conservative substitutions based on either amino acid chemical or structural characteristics, or else by protein structural considerations. In affinity maturation experiments, several such mutations may be found in a single selected molecule, whether they are randomly or non-randomly selected. There are also various structure-based approaches toward affinity maturation as demonstrated in, e.g., U.S. Pat. No. 7,117,096, PCT Pub. Nos.: WO 02/084277 and WO 03/099999; the disclosures of which are incorporated herein by reference. As used herein, the percent homology between two amino acid or nucleic acid sequences is equivalent to the percent identity between the two sequences, and these two terms will be used interchangeably throughout. As used herein, % identity of two nucleic acid or amino acid sequences is determined using the algorithm of Karlin and Altschul (Proc. Natl. Acad. Sci. USA 90:5873-5877, 1993). Such an algorithm is incorporated into the NBLAST and XBLAST programs of Altschul et al., 1990 Utilization of components of one or more disclosed PCSK9-specific molecules to produce other binding molecules with similar or better specificity is well within the realm of one skilled in the art. This can be accomplished, for example, using techniques of recombinant DNA technology. One specific example of this involves the introduction of DNA encoding the immunoglobulin variable region, or one or more of the CDRs, of an antibody to the variable region, constant region, or constant region plus framework regions, as appropriate, of a different immunoglobulin. Such molecules form important aspects of the present invention. Specific immunoglobulins or the corresponding sequences, into which particular disclosed sequences may be inserted or, in the alternative, form the essential part of, include but are not limited to the following antibody molecules which form particular embodiments of the present invention: a Fab (monovalent fragment with variable light (VL), variable heavy (VH), constant light (CL) and constant heavy 1 (CH1) domains), a F(ab′)2(bivalent fragment comprising two Fab fragments linked by a disulfide bridge or alternative at the hinge region), a Fd (VH and CH1 domains), a Fv (VL and VH domains), a scFv (a single chain Fv where VL and VH are joined by a linker, e.g., a peptide linker, see, e.g., Bird et al., 1988 Variable domains, into which CDRs of interest are inserted, may be obtained from any germ-line or rearranged human variable domain. Variable domains may also be synthetically produced. The CDR regions can be introduced into the respective variable domains using recombinant DNA technology. One means by which this can be achieved is described in Marks et al., 1992 Specific embodiments provide the CDR(s) in germline framework regions. Framework regions, including but not limited to human framework regions, are known to those of skill in the art (e.g., a human or non-human framework). The framework regions may be naturally occurring or consensus framework regions. In one aspect, the framework region of an antibody of the invention is human (see, e.g., Clothia et al., 1998 The present invention encompasses antibody molecules that are human, humanized, deimmunized, chimeric and primatized. The invention also encompasses antibody molecules produced by the process of veneering; see, e.g., Mark et al., 1994 Handbook of Experimental Pharmacology, vol. 113: The pharmacology of monoclonal Antibodies, Springer-Verlag, pp. 105-134; the disclosure of which is incorporated herein by reference. “Human” in reference to the disclosed antibody molecules specifically refers to antibody molecules having variable and/or constant regions derived from human germline immunoglobulin sequences, wherein said sequences may, but need not, be modified/altered to have certain amino acid substitutions or residues that are not encoded by human germline immunoglobulin sequence. Such mutations can be introduced by methods including, but not limited to, random or site-specific mutagenesis in vitro, or by somatic mutation in vivo. Specific examples of mutation techniques discussed in the literature are that disclosed in Gram et al., 1992 Specific antibodies of the present invention are monoclonal antibodies and, in particular embodiments, are in one of the following antibody formats: IgD, IgA, IgE, IgM, IgG1, IgG2, IgG3, IgG4 or any derivative of any of the foregoing. The language “derivatives thereof” or “derivatives” in this respect includes, inter alia, (i) antibodies and antibody molecules with conservative modifications in one or both variable regions (i.e., VH and/or VL), (ii) antibodies and antibody molecules with manipulations in the constant regions of the heavy and/or light chains, and/or (iii) antibodies and antibody molecules that contain additional chemical moieties which are not normally a part of the immunoglobulin molecule (e.g., pegylation). Manipulations of the variable regions can be within one or more of the VH and/or VL CDR regions. Site-directed mutagenesis, random mutagenesis or other method for generating sequence or molecule diversity can be utilized to create mutants which can subsequently be tested for a particular functional property of interest in available in vitro or in vivo assays including those described herein. Antibodies of the present invention also include those in which modifications have been made to the framework residues within VH and/or VL to improve one or more properties of the antibody of interest. Typically, such framework modifications are made to decrease the immunogenicity of the antibody. For example, one approach is to “backmutate” one or more framework residues to the corresponding germline sequence. More specifically, an antibody that has undergone somatic mutation may contain framework residues that differ from the germline sequence from which the antibody is derived. Such residues can be identified by comparing the antibody framework sequences to the germline sequences from which the antibody is derived. Such “backmutated” antibodies are also intended to be encompassed by the invention. Another type of framework modification involves mutating one or more residues within the framework region, or even within one or more CDR regions, to remove T cell epitopes to thereby reduce the potential immunogenicity of the antibody. This approach is also referred to as “deimmunization” and is described in further detail in U.S. Patent Publication No. 20030153043 by Carr et al; the disclosure of which is incorporated herein by reference. In addition or alternative to modifications made within the framework or CDR regions, antibodies of the invention may be engineered to include modifications within the Fc or constant regions, where present, typically to alter one or more functional properties of the antibody, such as serum half-life, complement fixation, Fc receptor binding, and/or antigen-dependent cellular cytotoxicity. The concept of generating “hybrids” or “combinatorial” IgG forms comprising various antibody isotypes to hone in on desired effector functionality has generally been described; see, e.g., Tao et al., 1991 Specific PCSK9-specific antagonists may carry a detectable label, or may be conjugated to a toxin (e.g., a cytotoxin), a radioactive isotope, a radionuclide, a liposome, a targeting moiety, a biosensor, a cationic tail, or an enzyme (e.g., via a peptidyl bond or linker). Such PCSK9-specific antagonist compositions form an additional aspect of the present invention. In another aspect, the present invention provides isolated nucleic acid encoding disclosed PCSK9-specific antagonists. “Isolated” as mentioned prior refers to the property of the thing referred to that makes them different from that found in nature. The difference can be, for example, that they are of a different purity than that found in nature, or that they are of a different structure or form part of a different structure than that found in nature. An example of nucleic acid not found in nature is, for example, nucleic acid substantially free of other cellular material. The nucleic acid may be present in whole cells, in a cell lysate, or in a partially purified or substantially pure form. In specific instances, a nucleic acid may be isolated when purified away from other cellular components or other contaminants, e.g., other cellular nucleic acids or proteins, for example, using standard techniques, including without limitation, alkaline/SDS treatment, CsCl banding, column chromatography, agarose gel electrophoresis and other suitable methods known in the art. The nucleic acid may include DNA (inclusive of cDNA) and/or RNA. Nucleic acids of the present invention can be obtained using standard molecular biology techniques. For antibodies expressed by hybridomas (e.g., hybridomas prepared from transgenic mice carrying human immunoglobulin genes), cDNAs encoding the light and heavy chains of the antibody made by the hybridoma can be obtained by standard PCR amplification or cDNA cloning techniques. For antibodies obtained from an immunoglobulin gene library (e.g., using phage display techniques), nucleic acid encoding the antibody can be recovered from the library. The present invention encompasses isolated nucleic acid encoding disclosed variable heavy and/or light chains and select components thereof, particularly the disclosed variable or respective CDR regions and, in particular CDR3. In specific embodiments hereof, the CDR(s) are provided within antibody framework regions and, in particular embodiments, human framework regions. Specific embodiments provide isolated nucleic acid encoding the CDR(s) into germline framework regions including, but not limited to, human germline framework regions. Specific embodiments herein provide isolated nucleic acid encoding heavy chain CDR SEQ ID NO: 17 (in specific embodiments, said nucleic acid of which comprises SEQ ID NO: 18) into VH1A—3 in place of the nucleic acid encoding the relevant CDR. Specific embodiments herein provide nucleic acid encoding heavy chain variable CDR1, CDR2 and/or CDR3 sequences SEQ ID NOs: 13, 15 and 17, respectively (and, in particular embodiments, said nucleic acid of which comprises SEQ ID NOs: 14, 16 and 18, respectively) into VH1A—3 in place of the relevant CDRs. Specific embodiments herein provide isolated nucleic encoding light chain CDR SEQ ID NO: 7 (in specific embodiments, said nucleic acid of which comprises SEQ ID NO: 8) into VK1—4 in place of the nucleic acid encoding the relevant CDR. Specific embodiments herein provide nucleic acid encoding light chain variable CDR1, CDR2 and/or CDR3 sequences SEQ ID NOs: 3, 5 and 7, respectively (and, in particular embodiments, said nucleic acid of which comprises SEQ ID NOs: 4, 6 and 8, respectively) into VK1—4 in place of the relevant CDRs. Specific embodiments further provide heavy chain variable CDR3 SEQ ID NO: 17 (and, in particular embodiments, said nucleic acid of which comprises SEQ ID NO: 18) and light chain variable CDR3 SEQ ID NO: 7 (and, in particular embodiments, said nucleic acid of which comprises SEQ ID NO: 8) into VH1A3 and VK1—4 germline sequences, respectively. Further embodiments provide heavy chain variable CDR1, CDR2 and/or CDR3 sequences SEQ ID NOs: 13, 15 and 17, respectively (and, in particular embodiments, said nucleic acid of which comprises SEQ ID NOs: 14, 16 and 18, respectively) into VH1A—3 in place of the relevant CDRs; and light chain variable CDR1, CDR2 and/or CDR3 sequences SEQ ID NOs: 3, 5 and 7, respectively (and, in particular embodiments, said nucleic acid of which comprises SEQ ID NOs: 4, 6 and 8, respectively) into VK1—4 in place of the relevant CDRs. The isolated nucleic acid encoding the variable regions can be provided within any desired antibody molecule format including, but not limited to, the following: F(ab′)2, a Fab, a Fv, a scFv, bispecific antibody molecules (antibody molecules comprising a PCSK9-specific antibody or antigen binding fragment as disclosed herein linked to a second functional moiety having a different binding specificity than the antibody, including, without limitation, another peptide or protein such as an antibody, or receptor ligand), a bispecific single chain Fv dimer, a minibody, a dAb fragment, diabody, triabody or tetrabody, a minibody, IgG, IgG1, IgG2, IgG3, IgG4, IgM, IgD, IgA, IgE or any derivatives thereof. Specific embodiments provide isolated nucleic acid which encodes PCSK9-specific antagonists and, in more specific embodiments, antibody molecules comprising a heavy chain variable domain which comprises SEQ ID NO: 11; specific embodiments of which comprise nucleic acid sequence SEQ ID NO: 12. Specific embodiments of the present invention provide isolated nucleic acid encoding PCSK9-specific antagonists and, in more specific embodiments, antibody molecules, which additionally comprise: (i) nucleic acid encoding heavy chain CDR1 amino acid sequence SEQ ID NO: 13 (specific embodiments of which comprise nucleic acid SEQ ID NO: 14) and/or (ii) nucleic acid encoding heavy chain CDR2 amino acid sequence SEQ ID NO: 15 (specific embodiments of which comprise nucleic acid SEQ ID NO: 16). Specific embodiments provide isolated nucleic acid encoding PCSK9-specific antagonists and, in more specific embodiments, antibody molecules comprising a light chain variable domain which comprises SEQ ID NO: 27; specific embodiments of which comprise nucleic acid sequence SEQ ID NO: 28. Specific embodiments of the present invention provide isolated nucleic acid encoding PCSK9-specific antagonists and, in more specific embodiments, antibody molecules, which additionally comprise: (i) nucleic acid encoding light chain CDR1 amino acid sequence SEQ ID NO: 3 (specific embodiments of which comprise nucleic acid SEQ ID NO: 4) and/or (ii) nucleic acid encoding light chain CDR2 amino acid sequence SEQ ID NO: 5 (specific embodiments of which comprise nucleic acid SEQ ID NO: 6). Specific embodiments provide isolated nucleic acid encoding PCSK9-specific antagonists and, in more specific embodiments, antibody molecules which comprise a heavy chain variable domain which comprises SEQ ID NO: 11; specific embodiments of which comprise nucleic acid sequence SEQ ID NO: 12; and a light chain variable domain which comprises SEQ ID NO: 27; specific embodiments of which comprise nucleic acid sequence SEQ ID NO: 28. Specific embodiments provide isolated nucleic acid encoding (i) heavy chain CDR1, CDR2 and/or CDR3 sequences (SEQ ID NOs: 13, 15 and 17, respectively; specific embodiments of which comprise nucleic acid SEQ ID NOs: 14, 16 and/or 18, respectively) preferably in a framework region (including but not limited to a human framework region); and (ii) light chain CDR1, CDR2 and/or CDR3 sequences (SEQ ID NO: 3, 5 and 7, respectively; specific embodiments of which comprise nucleic acid SEQ ID NOs: 4, 6 and/or 8, respectively) preferably in a framework region (including but not limited to a human framework region). The present invention further provides in specific embodiments, homologs of the antagonists disclosed above, characterized as being at least 90% identical over the heavy and/or light chain variable regions, or the CDR regions, as appropriate, whichever is present to the corresponding sequences of 1D05. Additional embodiments provide isolated nucleic acid encoding PCSK9-specific antagonists and, in more specific embodiments, antibody molecules which comprise a light chain comprising SEQ ID NO: 1 (specific embodiments of which comprise nucleic acid SEQ ID NO: 2) and a heavy chain or Fd chain comprising amino acids 1-233 of SEQ ID NO: 9, or SEQ ID NO: 9 (specific embodiments of which comprise nucleic acid 1-699 of SEQ ID NO: 10, or SEQ ID NO: 10, respectively). Further embodiments provide isolated nucleic acid encoding PCSK9-specific antagonists and, in more specific embodiments, antibody molecules which comprise a light chain comprising SEQ ID NO: 26 (specific embodiments of which comprise SEQ ID NO: 30) and a heavy chain comprising SEQ ID NO: 25 (specific embodiments of which comprise SEQ ID NO: 29). The present invention further provides in specific embodiments, homologs of the antagonists disclosed above, characterized as being at least 90% identical over the heavy and/or light chains to the corresponding sequences of 1D05. Specific embodiments of the present invention encompass nucleic acid encoding antibody molecules that possess manipulations in the Fc region which result in reduced or altered binding to FcγR receptors, C1q, or FcRn on the part of the antibody. One specific embodiment of the present invention is isolated nucleic acid which encodes for antibody molecules comprising as part of their immunoglobulin structure SEQ ID NO: 24 and, in particular embodiments, residues 107-326 of SEQ ID NO: 24. In specific embodiments, synthetic PCSK9-specific antagonists can be produced by expression from nucleic acid generated from oligonucleotides synthesized and assembled within suitable expression vectors; see, e.g., Knappick et al., 2000 The present invention encompasses nucleic acid encoding antibody molecules which comprise: (i) nucleic acid encoding a light chain comprising SEQ ID NO: 1 (specific embodiments of which comprise nucleic acid SEQ ID NO: 2), and (ii) nucleic acid encoding a heavy chain comprising SEQ ID NO: 11 (specific embodiments of which comprise nucleic acid SEQ ID NO: 12) followed in sequence by (adjacent to) a set of nucleotides encoding for a set of amino acids selected from the group consisting of: SEQ ID NO: 21 (IgG1), SEQ ID NO: 22 (IgG2), SEQ ID NO: 23 (IgG4) and SEQ ID NO: 24 (IgG2m4). Nucleotide sequences for mature, secreted anti-PCSK9 IgG2m4 heavy and light chains can be found as SEQ ID NOs: 29 and 30, respectively. Plasmid sequences comprising heavy and light chain 1D05 anti-PCSK9 IgG2m4 antibody molecules can be found as SEQ ID NOs: 31 and 32, respectively. Nucleic acid encoding such antibody molecules form important embodiments hereof. Also included within the present invention are isolated nucleic acids comprising nucleotide sequences which are at least about 90% identical and more preferably at least about 95% identical to the full length of the nucleotide sequences described herein, and which nucleotide sequences encode PCSK9-specific antagonists which inhibit PCSK9-dependent inhibition of cellular LDL uptake by at least 10%. Reference to “at least about 90% identical” throughout the application includes at least about 90, 91, 92, 93, 94, 95, 96, 97, 98, 99 or 100% identical. The invention further provides isolated nucleic acid at least a portion of which hybridizes to the complement of nucleic acid consisting of SEQ ID NO: 12 and/or SEQ ID NO: 28 under stringent hybridization conditions, said nucleic acid of which confers upon antibody molecules the ability to specifically bind PCSK9 and antagonize PCSK9 function, and PCSK9-specific antagonists expressed employing said nucleic acid. Methods for hybridizing nucleic acids are well-known in the art; see, e.g., Ausubel, In another aspect, the present invention provides vectors comprising the nucleic acid disclosed herein. Vectors in accordance with the present invention include, but are not limited to, plasmids and other expression constructs (e.g., phage or phagemid, as appropriate) suitable for the expression of the desired antibody molecule at the appropriate level for the intended purpose; see, e.g., Sambrook & Russell, In another aspect, the present invention provides isolated cell(s) comprising nucleic acid encoding disclosed PCSK9-specific antagonists. A variety of different cell lines are contemplated herein and can be used for the recombinant production of PCSK9-specific antagonists, including but not limited to those from prokaryotic organisms (e.g., In another aspect, the present invention provides isolated cell(s) comprising a polypeptide of the present invention. In another aspect, the present invention provides a method of making a PCSK9-specific antagonist of the present invention, which comprises incubating a cell comprising nucleic acid encoding the PCSK9-specific antagonist, or a heavy and/or light chain or a fragment thereof (e.g., VH and/or VL, or one or more of the disclosed heavy and/or light chain variable region CDRs) of a desired PCSK9-specific antagonist (dictated by the desired antagonist) with specificity for human and/or murine PCSK9 under conditions that allow the expression of the PCSK9-specific antagonist, or the expression and assembly of said heavy and/or light chains or fragment into a PCSK9-specific antagonist, and isolating said PCSK9-specific antagonist from the cell. One example by which to generate particular desired heavy and/or light chain sequence or fragment is to first amplify (and modify) the germline heavy and/or light chain variable sequences or fragment using PCR. Germline sequence for human heavy and/or light variable regions are readily available to the skilled artisan, see, e.g., the “Vbase” human germline sequence database, and Kabat, E. A. et al., 1991 Available techniques exist to recombinantly produce other antibody molecules which retain the specificity of an original antibody. A specific example of this is where DNA encoding the immunoglobulin variable region or the CDRs is introduced into the constant regions, or constant regions and framework regions, or simply the framework regions, of another antibody molecule; see, e.g., EP-184,187, GB 2188638, and EP-239400; the disclosures of which are incorporated herein by reference. Cloning and expression of antibody molecules, including chimeric antibodies, are described in the literature; see, e.g., EP 0120694 and EP 0125023; the disclosures of which are incorporated herein by reference. Antibody molecules in accordance with the present invention may, in one instance, be raised and then screened for characteristics identified herein using known techniques. Basic techniques for the preparation of monoclonal antibodies are described in the literature, see, e.g., Kohler and Milstein (1975 Alternatively, a library of PCSK9-specific antagonists in accordance with the present invention may be brought into contact with PCSK9, and ones able to demonstrate specific binding selected. Functional studies can then be carried out to ensure proper functionality, e.g., inhibition of PCSK9-dependent inhibition of cellular LDL uptake. There are various techniques available to the skilled artisan for the selection of protein-specific molecules from libraries using enrichment technologies including, but not limited to, phage display (e.g., see technology from Cambridge Antibody Technology (“CAT”) disclosed in U.S. Pat. Nos. 5,565,332; 5,733,743; 5,871,907; 5,872,215; 5,885,793; 5,962,255; 6,140,471; 6,225,447; 6,291,650; 6,492,160; 6,521,404; 6,544,731; 6,555,313; 6,582,915; 6,593,081, as well as other U.S. family members and/or applications which rely on priority filing GB 9206318, filed May 24, 1992; see also Vaughn et al., 1996 PCSK9-specific antagonists may be purified by techniques available to one of skill in the art. Titers of the relevant antagonist preparation, ascites, hybridoma culture fluids, or relevant sample may be determined by various serological or immunological assays which include, but are not limited to, precipitation, passive agglutination, enzyme-linked immunosorbent antibody (“ELISA”) techniques and radioimmunoassay (“RIA”) techniques. The present invention relates in part to methods employing PCSK9-specific antagonists described herein for antagonizing PCSK9 function; said methods of which are further described below. Use of the term “antagonizing” throughout the present application refers to the act of opposing, inhibiting, counteracting, neutralizing or curtailing one or more functions of PCSK9. Inhibition or antagonism of one or more of PCSK9-associated functional properties can be readily determined according to methodologies known to the art (see, e.g., Barak & Webb, 1981 In one aspect, the present invention provides a method for antagonizing the activity of PCSK9, which comprises contacting a cell, population of cells or tissue sample capable of being affected by PCSK9 (i.e., which expresses and/or comprises LDL receptors) with a PCSK9-specific antagonist disclosed herein under conditions that allow said antagonist to bind to PCSK9 when present and inhibit PCSK9's inhibition of cellular LDL uptake. Specific embodiments of the present invention include such methods wherein the cell is a human cell. Additional embodiments of the present invention include such methods wherein the cell is a murine cell. In another aspect, the present invention provides a method for antagonizing the activity of PCSK9 in a subject, which comprises administering to the subject a therapeutically effective amount of a PCSK9-specific antagonist of the present invention. In specific embodiments, the methods for antagonizing PCSK9 function are for the treatment of a PCSK9-associated disease, disorder or condition or, alternatively, a disease, disorder or condition that could benefit from the effects of a PCSK9 antagonist. The medicament would be useful in a subject(s) exhibiting a condition associated with PCSK9 activity, or a condition where the functioning of PCSK9 is contraindicated for a particular subject. In select embodiments, the condition may be hypercholesterolemia, coronary heart disease, metabolic syndrome, acute coronary syndrome or related conditions. The present invention, thus, contemplates the use of PCSK9-specific antagonists described herein in various methods of treatment where antagonizing PCSK9 function is desirable. The method of treatment can be prophylactic or therapeutic in nature. In specific embodiments, the present invention relates to a method of treatment for a condition associated with/attributed to PCSK9 activity, or a condition where the functioning of PCSK9 is contraindicated for a particular subject, which comprises administering to the subject a therapeutically effective amount of a PCSK9-specific antagonist of the present invention. In select embodiments, the condition may be hypercholesterolemia, coronary heart disease, metabolic syndrome, acute coronary syndrome or related conditions. Methods of treatment in accordance with the present invention comprise administering to an individual a therapeutically (or prophylactically) effective amount of a PCSK9-specific antagonist of the present invention. Use of the terms “therapeutically effective” or “prophylactically effective” in reference to an amount refers to the amount necessary at the intended dosage to achieve the desired therapeutic/prophylactic effect for the period of time desired. The desired effect may be, for example, amelioration of at least one symptom associated with the treated condition. These amounts will vary, as the skilled artisan will appreciate, according to various factors, including but not limited to the disease state, age, sex and weight of the individual, and the ability of the PCSK9-specific antagonist to elicit the desired effect in the individual. The response may be documented by in vitro assay, in vivo non-human animal studies, and/or further supported from clinical trials. The PCSK9-specific antagonist may be administered as a pharmaceutical composition. The present invention, thus, provides a pharmaceutically acceptable composition comprising a PCSK9-specific antagonist of the invention and a pharmaceutically acceptable carrier including but not limited to an excipient, diluent, stabilizer, buffer, or alternative designed to facilitate administration of the antagonist in the desired format and amount to the treated individual. The pharmaceutical composition may be formulated by any number of strategies known in the art, see, e.g., McGoff and Scher, 2000 The antagonist-based pharmaceutically acceptable composition may, in particular embodiments, be in liquid or solid form, or in the form of gas particles or aerosolized particles. Any technique for production of liquid or solid formulations may be utilized. Such techniques are well within the realm of the abilities of the skilled artisan. Solid formulations may be produced by any available method including, but not limited to, lyophilization, spray drying, or drying by supercritical fluid technology. Solid formulations for oral administration may be in any form rendering the antagonist accessible to the patient in the prescribed amount and within the prescribed period of time. The oral formulation can take the form of a number of solid formulations including, but not limited to, a tablet, capsule, or powder. Solid formulations may alternatively be lyophilized and brought into solution prior to administration for either single or multiple dosing according to methods well known to the skilled artisan. Antagonist compositions should generally be formulated within a biologically relevant pH range and may be buffered to maintain a proper pH range during storage. Both liquid and solid formulations generally require storage at lower temperatures (e.g., 2-8° C.) in order to retain stability for longer periods. Formulated antagonist compositions, especially liquid formulations, may contain a bacteriostat to prevent or minimize proteolysis during storage, including but not limited to effective concentrations (e.g., ≦1% w/v) of benzyl alcohol, phenol, m-cresol, chlorobutanol, methylparaben, and/or propylparaben. A bacteriostat may be contraindicated for some patients. Therefore, a lyophilized formulation may be reconstituted in a solution either containing or not containing such a component. Additional components may be added to either a buffered liquid or solid antagonist formulation, including but not limited to sugars as a cryoprotectant (including but not limited to polyhydroxy hydrocarbons such as sorbitol, mannitol, glycerol, and dulcitol and/or disaccharides such as sucrose, lactose, maltose, or trehalose) and, in some instances, a relevant salt (including but not limited to NaCl, KCl, or LiCl). Such antagonist formulations, especially liquid formulations slated for long term storage, will rely on a useful range of total osmolarity to both promote long term stability at temperatures of, for example, 2-8° C. or higher, while also making the formulation useful for parenteral injection. As appropriate, preservatives, stabilizers, buffers, antioxidants and/or other additives may be included. The formulations may contain a divalent cation (including but not limited to MgCl2, CaCl2, and MnCl2); and/or a non-ionic surfactant (including but not limited to Polysorbate-80 (Tween 80™), Polysorbate-60 (Tween 60™), Polysorbate-40 (Tween 40™), and Polysorbate-20 (Tween 20™), polyoxyethylene alkyl ethers, including but not limited to Brij 58™, Brij35™, as well as others such as Triton X-100™, Triton X-114™, NP40™, Span 85 and the Pluronic series of non-ionic surfactants (e.g., Pluronic 121)). Any combination of such components form specific embodiments of the present invention. Pharmaceutical compositions in liquid format may include a liquid carrier, e.g., water, petroleum, animal oil, vegetable oil, mineral oil, or synthetic oil. The liquid format may also include physiological saline solution, dextrose or other saccharide solution or glycols, such as ethylene glycol, propylene glycol or polyethylene glycol. Preferably, the pharmaceutical composition may be in the form of a parenterally acceptable aqueous solution that is pyrogen-free with suitable pH, tonicity, and stability. Pharmaceutical compositions may be formulated for administration after dilution in isotonic vehicles, for example, Sodium Chloride Injection, Ringer's Injection, or Lactated Ringer's Injection. One aspect of the present invention is a pharmaceutical composition which comprises: (i) about 50 to about 200 mg/mL of a PCSK9-specific antagonist described herein; (ii) a polyhydroxy hydrocarbon (including but not limited to sorbitol, mannitol, glycerol and dulcitol) and/or a disaccharide (including but not limited to sucrose, lactose, maltose and trehalose); the total of said polyhydroxy hydrocarbon and/or disaccharide being about 1% to about 6% weight per volume (“w/v”) of the formulation; (iii) about 5 mM to about 200 mM of histidine, imidazole, phosphate or acetic acid which serves as a buffering agent to prevent pH drift over the shelf life of the pharmaceutical composition and as a tonicity modifier; (iv) about 5 mM to about 200 mM of arginine, proline, phenylalanine, alanine, glycine, lysine, glutamic acid, aspartic acid or methionine to counteract aggregation; (v) about 0.01M to about 0.1M of hydrochloric acid (“HCl”) in an amount sufficient to achieve a pH in the range of about 5.5 to about 7.5; and (vi) a liquid carrier including but not limited to sterile water, petroleum, animal oil, vegetable oil, mineral oil, synthetic oil, physiological saline solution, dextrose or other saccharide solution or glycols, such as ethylene glycol, propylene glycol or polyethylene glycol; wherein said pharmaceutical composition has a pH in the range of about 5.5 to about 7.5; and wherein said pharmaceutical composition optionally comprises about 0.01% to about 1% w/v of the formulation of a non-ionic surfactant (including but not limited to Polysorbate-80 (Tween 80™), Polysorbate-60 (Tween 60™), Polysorbate-40 (Tween 40™), and Polysorbate-20 (Tween 20™), polyoxyethylene alkyl ethers, including but not limited to Brij 58™, Brij35™, as well as others such as Triton X-100™, Triton X-114™, NP40™, Span 85 and the Pluronic series of non-ionic surfactants (e.g., Pluronic 121)). HCl may be added as free acid, Histidine-HCl or Arginine-HCl. Where supplied as Histidine-HCl or Arginine-HCl, the total amounts of Histidine or Arginine in the HCl form should be that specified above. Accordingly, some or all of the HCl depending on the amounts of Histidine and/or Arginine may be supplied as Histidine-HCl and/or Arginine-HCl; as appropriate. Use of the term “about” with respect to amounts disclosed in the specification means within 10% of the specified numbers provided. A range provided as, for example” in “about 50 to about 200” expressly includes as distinct embodiments each number within said range. As such in the above example, embodiments including but not limited to those having 50, 100, 125, 150 and 200 form specific embodiments herein. Pharmaceutical compositions as disclosed herein have general applicability despite the mode of administration. In specific embodiments, the disclosed pharmaceutical compositions are useful for subcutaneous administration as a liquid or upon reconstitution of a lyophilized form. In specific embodiments, PCSK9-specific antagonists employed in the disclosed formulations may be pegylated or form part of fusion proteins. Specific aspects of the present invention relate to the above disclosed pharmaceutical compositions which comprise: (i) about 50 to about 200 mg/mL of a PCSK9-specific antagonist described herein; (ii) about 1% to about 6% (in particular embodiments from about 2% to about 6%) w/v mannitol, trehalose or sucrose; (iii) about 10 mM to about 100 mM of histidine; (iv) about 25 mM to about 100 mM of arginine or proline; (v) about 0.02 M to about 0.05M of hydrochloric acid (“HCl”) in an amount sufficient to achieve a pH in the range of about 5.8 to about 7; and (vi) a liquid carrier including but not limited to sterile water, petroleum, animal oil, vegetable oil, mineral oil, synthetic oil, physiological saline solution, dextrose or other saccharide solution or glycols, such as ethylene glycol, propylene glycol or polyethylene glycol; wherein said pharmaceutical composition has a pH in the range of about 5.8 to about 7; and wherein said pharmaceutical composition optionally comprising about 0.01% to about 1% w/v of the formulation of a non-ionic surfactant (including but not limited to Polysorbate-80 (Tween 80™), Polysorbate-60 (Tween 60™), Polysorbate-40 (Tween 40™), and Polysorbate-20 (Tween 20™), polyoxyethylene alkyl ethers, including but not limited to Brij 58™, Brij35™, as well as others such as Triton X-100™, Triton X-114™, NP40™, Span 85 and the Pluronic series of non-ionic surfactants (e.g., Pluronic 121)). Specific embodiments provide pharmaceutical compositions which comprise: (i) 50 to 200 mg/mL of a PCSK9-specific antagonist described herein; (ii) about 1% to about 6% (in particular embodiments from about 2% to about 6%) w/v mannitol, trehalose or sucrose; (iii) about 10 mM to about 150 mM of histidine; (iv) about 10 mM to about 150 mM of arginine or proline; (v) about 0.03 M to about 0.05 M of hydrochloric acid (“HCl”) in an amount sufficient to achieve a pH in the range of about 5.8 to about 6.5; and (vi) a liquid carrier including but not limited to sterile water, petroleum, animal oil, vegetable oil, mineral oil, synthetic oil, physiological saline solution, dextrose or other saccharide solution or glycols, such as ethylene glycol, propylene glycol or polyethylene glycol; wherein said pharmaceutical composition has a pH in the range of about 5.8 to about 6.5; and wherein said pharmaceutical composition optionally comprising about 0.01% to about 1% w/v of Polysorbate-80 (Tween 80™) or Polysorbate-20 (Tween 20™). Specific embodiments herein provide pharmaceutical compositions which comprise: (i) 50 to 200 mg/mL of a PCSK9-specific antagonist described herein; (ii) about 1% to about 6% (in particular embodiments from about 2% to about 6%) w/v sucrose; (iii) about 25 mM to about 100 mM of histidine; (iv) about 25 mM to about 100 mM of arginine; (v) about 0.040 M to about 0.045 M of hydrochloric acid (“HCl”) in an amount sufficient to achieve a pH of about 6; and (vi) sterile water; wherein said pharmaceutical composition has a pH of about 6; and wherein said pharmaceutical composition optionally comprising about 0.01% to about 1% w/v of Polysorbate-80 (Tween 80™) or Polysorbate-20 (Tween 20™). In specific embodiments thereof, the levels of histidine and arginine are within 25 mM of each other and, in other embodiments are the same. Specific embodiments herein provide pharmaceutical compositions which comprise (i) 50 to 200 mg/mL of a PCSK9-specific antagonist described herein; (ii) sucrose, histidine and arginine in one of the following amounts: (a) about 1% w/v sucrose, about 10 mM histidine and about 25 mM arginine; (b) about 2% w/v sucrose, about 25 mM histidine and about 25 mM arginine; (c) about 3% w/v sucrose, about 50 mM histidine and about 50 mM arginine; or (d) about 6% w/v sucrose, about 100 mM histidine and about 100 mM arginine; (iii) about 0.04 mol or, alternatively, about 1.46 g of HCl; and (iv) sterile water; wherein said pharmaceutical composition has a pH of about 6; and wherein said pharmaceutical composition optionally comprising about 0.01% to about 1% w/v of Polysorbate-80 (Tween 80™) or Polysorbate-20 (Tween 20™). Specific embodiments herein are wherein the amounts of sucrose, histidine and arginine in (ii) above are that described in (c) or (d). Specific embodiments herein provide pharmaceutical compositions as described which comprise 50 to 200 mg/ml of any one of the various PCSK9-specific antagonists described herein. For purposes of exemplification of one distinct embodiment thereof, and not to be construed as a limitation, is the following: a pharmaceutical formulation as described above which comprises: a PCSK9-specific antagonist which comprises: (a) a light chain comprising SEQ ID NO: 26; and (b) a heavy chain comprising SEQ ID NO: 25; wherein said PCSK9-specific antagonist is an antibody molecule that antagonizes PCSK9's inhibition of cellular LDL uptake. Particular embodiments herein are pharmaceutical compositions according to the above description which are lyophilized and reconstituted. In specific embodiments, said protein concentration in said lyophilized and reconstituted solution is up to 2-fold higher than in the pre-lyophilized composition. In specific embodiments, the protein or PCSK9-specific antagonist concentration in the lyophilized and/or reconstituted pharmaceutical composition is in the range of about 50 mg/mL to about 300 mg/mL. Diluents useful for reconstituting the lyophilized pharmaceutical compositions include but are not limited to sterile water, bacteriostatic water for injection (“BWFI”), phosphate-buffered saline, a sterile saline solution, physiological saline solution, Ringer's solution or dextrose solution and may in specific embodiments contain 0.01-1% (w/v) of Polysorbate-80 (Tween 80™) or Polysorbate-20 (Tween 20™). In specific embodiments, lyophilized powder can be reconstituted with 1/60.2× original volume (or 0.167 mL) up to 1× (1 mL). Exemplary embodiments of the present invention are pharmaceutical compositions as described herein which are stable. Other embodiments of the present invention are pharmaceutical compositions as described herein which are stable to lyophilization and reconstitution. Various methods are available to the skilled artisan to prepare lyophilized compositions; see, e.g., Martin & Mo, 2007 “Stability Considerations for Lyophilized Biologics” Amer. Pharm. Rev. “Stable” as used herein refers to the property of the protein or PCSK9-specific antagonist to retain its physical or chemical stability, conformational integrity, or its ability to exhibit less denaturation, protein clipping, aggregation, fragmentation, acidic variant formation or loss of biological activity compared with a control sample at a temperature in the range of 4-37° C. for at least about 30 days. Other embodiments remain stable for up to 3 months, 6 months, 12 months, 2 years or longer periods at the above temperatures. In specific embodiments the formulation exhibits no significant changes at 2-8° C. for at least 6 months, and preferably 12 months, 2 years or longer, in order of preference. Specific embodiments experience less than 10% or, in particular embodiments, less than 5% of denaturation, protein clipping, aggregation, fragmentation, acidic variant formation or loss of biological activity compared with a control sample at a temperature in the range of 25-45° C. (or alternatively 2-8° C.) for at least about 30 days, 3 months, 6 months, 12 months, 2 years or longer. Stability of the formulations can be tested via several means known to the skilled artisan including, but not limited to Size Exclusion Chromatography (SEC-HPLC) to measure aggregation and fragmentation, Dynamic Light Scattering (DLS) to measure particle size of concentrated samples, capillary SDS-PAGE to measure fragmentation and capillary iso-electric focusing (cIEF) or cation exchange chromatography (“CEX”) to measure acidic variants formation. Techniques suitable for the analysis of protein stability are well understood by those of skill in the art: see review in Pharmaceutical compositions as described herein should be sterile. There are various techniques available to the skilled artisan to accomplish this including, but not limited to, filtration through sterile filtration membranes. In specific embodiments, employing lyophilized and reconstituted compositions, this may be done prior to or following lyophilization and reconstitution. Dosing of antagonist therapeutics is well within the realm of the skilled artisan, see, e.g., Lederman et al., 1991 Individuals (subjects) capable of treatment as described herein include primates, human and non-human, and include any non-human mammal or vertebrate of commercial or domestic veterinary importance. The PCSK9-specific antagonist may be administered to an individual by any route of administration appreciated in the art, including but not limited to oral administration, administration by injection (specific embodiments of which include intravenous, subcutaneous, intraperitoneal or intramuscular injection), administration by inhalation, intranasal, or topical administration, either alone or in combination with other agents designed to assist in the treatment of the individual. The PCSK9-specific antagonist may also be administered by injection devices, injector pens, needleless devices; and subcutaneous patch delivery systems. The route of administration should be determined based on a number of considerations appreciated by the skilled artisan including, but not limited to, the desired physiochemical characteristics of the treatment. Treatment may be provided on a daily, weekly, biweekly, or monthly basis, or any other regimen that delivers the appropriate amount of PCSK9-specific antagonist to the individual at the prescribed times such that the desired treatment is effected and maintained. The formulations may be administered in a single dose or in more than one dose at separate times. Also contemplated are methods of using the disclosed antagonists in the manufacture of a medicament for treatment of a PCSK9-associated disease, disorder or condition or, alternatively, a disease, disorder or condition that could benefit from the effects of a PCSK9 antagonist. The medicament would be useful in a subject(s) exhibiting a condition associated with PCSK9 activity, or a condition where the functioning of PCSK9 is contraindicated for a particular subject. In select embodiments, the condition may be hypercholesterolemia, coronary heart disease, metabolic syndrome, acute coronary syndrome or related conditions. PCSK9-specific antagonists disclosed herein may also be used as a method of diagnosis of PCSK9. In select embodiments, the present invention encompasses methods of identifying or quantifying the level of PCSK9 present in a sample (including but not limited to a biological sample, e.g., serum or blood) which comprises contacting the sample with a PCSK9-specific antagonist described herein and detecting or quantifying, respectively, binding to PCSK9. The PCSK9-specific antagonist may be used in various assay formats known to the skilled artisan and may form part of a kit (the general features of a kit of which are further described below). The present invention further provides for the administration of disclosed anti-PCSK9 antagonists for purposes of gene therapy. Through such methods, cells of a subject are transformed with nucleic acid encoding a PCSK9-specific antagonist of the invention. Subjects comprising the nucleic acids then produce the PCSK9-specific antagonists endogenously. Previously, Alvarez, et al, Nucleic acids encoding any PCSK9-specific antagonist may be introduced to a subject. The nucleic acids may be introduced to the cells of a subject by any means known in the art. In preferred embodiments, the nucleic acids are introduced as part of a viral vector. Examples of preferred viruses from which the vectors may be derived include lentiviruses, herpes viruses, adenoviruses, adeno-associated viruses, vaccinia virus, baculovirus, alphavirus, influenza virus, and other recombinant viruses with desirable cellular tropism. Various companies produce viral vectors commercially, including, but by no means limited to, Avigen, Inc. (Alameda, Calif.; AAV vectors), Cell Genesys (Foster City, Calif.; retroviral, adenoviral, AAV vectors, and lentiviral vectors), Clontech (retroviral and baculoviral vectors), Genovo, Inc. (Sharon Hill, Pa.; adenoviral and AAV vectors), Genvec (adenoviral vectors), IntroGene (Leiden, Netherlands; adenoviral vectors), Molecular Medicine (retroviral, adenoviral, AAV, and herpes viral vectors), Norgen (adenoviral vectors), Oxford BioMedica (Oxford, United Kingdom; lentiviral vectors), and Transgene (Strasbourg, France; adenoviral, vaccinia, retroviral, and lentiviral vectors). Methods for constructing and using viral vectors are known in the art (see, e.g., Miller, et al, Examples of vectors comprising attenuated or defective DNA virus sequences include, but are not limited to, a defective herpes virus vector (Karmo et al, Adenoviruses are eukaryotic DNA viruses that can be modified to efficiently deliver a nucleic acid of the invention to a variety of cell types. Attenuated adenovirus vectors, such as the vector described by Strafford-Perricaudet et al, The adeno-associated viruses (AAV) are DNA viruses of relatively small size which can integrate, in a stable and site-specific manner, into the genome of the cells which they infect. They are able to infect a wide spectrum of cells without inducing any effects on cellular growth, morphology or differentiation, and they do not appear to be involved in human pathologies. The use of vectors derived from the AAVs for transferring genes in vitro and in vivo has been described (see Daly, et al, In another embodiment, the gene can be introduced in a retroviral vector, e.g., as described in U.S. Pat. Nos. 5,399,346, 4,650,764, 4,980,289, and 5,124,263; Mann et al, Lentiviral vectors can be used as agents for the direct delivery and sustained expression of nucleic acids encoding a PCSK9-specific antagonist of the invention in several tissue types, including brain, retina, muscle, liver and blood. The vectors can efficiently transduce dividing and nondividing cells in these tissues, and maintain long-term expression of the PCSK9-specific antagonist. For a review, see Zufferey et al, Sindbis virus is a member of the alphavirus genus and has been studied extensively since its discovery in various parts of the world beginning in 1953. Gene transduction based on alphavirus, particularly Sindbis virus, has been well-studied in vitro (see Straus et al, In another embodiment, a vector can be introduced to cells by lipofection or with other transfection facilitating agents (peptides, polymers, etc.). Synthetic cationic lipids can be used to prepare liposomes for in vivo and in vitro transfection of a gene encoding a marker (Feigner et al, It is also possible to introduce the vector in vivo as a naked DNA plasmid. Naked DNA vectors for gene therapy can be introduced into desired host cells by methods known in the art, e.g., electroporation, microinjection, cell fusion, DEAE dextran, calcium phosphate precipitation, use of a gene gun, or use of a DNA vector transporter (see, e.g., Wilson, et al, Pharmaceutical compositions suitable for such gene therapy approaches and comprising nucleic acids encoding an anti-PCSK9 antagonist of the present invention are included within the scope of the present invention. In another aspect, the present invention provides a method for identifying, isolating, quantifying or antagonizing PCSK9 in a sample of interest using a PCSK9-specific antagonist of the present invention. The PCSK9-specific antagonists may be utilized as research tools in immunochemical assays, such as Western blots, ELISAs, radioimmunoassay, immunohistochemical assays, immunoprecipitations, or other immunochemical assays known in the art (see, e.g., Immunological Techniques Laboratory Manual, ed. Goers, J. 1993, Academic Press) or various purification protocols. The antagonists may have a label incorporated therein or affixed thereto to facilitate ready identification or measurement of the activities associated therewith. One skilled in the art is readily familiar with the various types of detectable labels (e.g., enzymes, dyes, or other suitable molecules which are either readily detectable or cause some activity/result that is readily detectable) which are or may be useful in the above protocols. An additional aspect of the present invention are kits comprising PCSK9-specific antagonists or pharmaceutical compositions disclosed herein and instructions for use. Kits typically but need not include a label indicating the intended use of the contents of the kit. The term label includes any writing, or recorded material supplied on or with the kit, or which otherwise accompanies the kit. In specific embodiments wherein the pharmaceutical composition is provided lyophilized, the kit may include sterile water or saline for reconstitution of the formulation into liquid form. In specific embodiments, the amount of water or saline is from about 0.1 ml to 1.0 ml. The following examples are provided to illustrate the present invention without limiting the same hereto: Recombinant Morphosys HuCAL Gold Fab phage display libraries (see, e.g., Knappik et al., 2000 For the first round of panning, each phage pool was bound independently to V5−, His-tagged PCSK9 protein immobilized in wells of Nunc Maxisorp plate. Immobilized phage-PCSK9 complexes were washed sequentially with (1) PBS/0.5% Tween™ 20 (Three quick washes); (2) PBS/0.5% Tween™ 20 (One 5 min. incubation with mild shaking); (3) PBS (Three quick washes); and (4) PBS (Two 5-min. incubations with mild shaking). Bound phages were eluted with 20 mM DTT and all three eluted phage suspensions were combined into one tube. For the second round of panning, phages from Round 1 were bound to immobilized, blocked V5−, His-tagged PCSK9 protein. Immobilized phage-PCSK9 complexes were washed sequentially with (1) PBS/0.05% Tween™ 20 (One quick wash); (2) PBS/0.05% Tween™ 20 (Four 5 min. incubations with mild shaking); (3) PBS (One quick wash); and (4) PBS (Four 5-min. incubations with mild shaking). Bound phages were eluted, For the third round of panning, phages from Round 2 were bound to immobilized, blocked V5-His-tagged PCSK9 protein. Immobilized phage-PCSK9 complexes were washed sequentially with (1) PBS/0.05% Tween™ 20 (Ten quick washes); (2) PBS/0.05% Tween™ 20 (Five 5 min. incubations with mild shaking); (3) PBS (Ten quick washes); and (4) PBS (Five 5-min. incubations with mild shaking). Bound phages were eluted and XbaI-EcoRI inserts from Round 3 phagemid DNA were subcloned into Morphosys Fab expression vector pMORPH_x9_MH to yield plasmid pMORPHx9 MH/mPCSK9 2 CX1D05 (see, e.g., Cultures of individual transformants were IPTG-induced and grown overnight for Fab expression. Culture supernatants (candidate Fabs) were incubated with purified V5−, His-tagged PCSK9 protein immobilized in wells of 96-well Nunc Maxisorp plates, washed with 0.1% Tween™ 20 in PBS using a plate washer, incubated with HRP-coupled anti-Fab antibody, and washed again with PBS/Tween™ 20. Bound HRP was detected by addition of TMP substrate, and A450 values of wells were read with a plate reader. Negative controls were included as follows: Controls for nonspecific Fab binding on each plate were incubated with parallel expressed preparations of anti-EsB, an irrelevant Fab.
Positive controls for ELISA and Fab expression were included as follows: EsB antigen was bound to three wells of the plate and subsequently incubated with anti-EsB Fab. To control for Fabs reacting with the V5 or His tags of the recombinant PCSK9 antigen, parallel ELISAs were performed using V5−, His-tagged secreted alkaline phosphatase protein (SEAP) expressed in the same cells as the original PCSK9 antigen and similarly purified. Putative PCSK9-reactive Fabs were identified as yielding >3× background values when incubated with PCSK9 antigen but negative when incubated with SEAP. Clones scoring as PCSK9-reactive in the first round of screening were consolidated onto a single plate, re-grown in triplicate, re-induced with IPTG, and re-assayed in parallel ELISAs vs. PCSK9 and SEAP. Positive and negative controls were included as described above. Clones scoring positive in at least 2 of 3 replicates were carried forward into subsequent characterizations. In cases of known or suspected mixed preliminary clones, cultures were re-purified by streaking for single colonies on 2xYT plates with chloramphenicol, and liquid cultures from three or more separate colonies were assayed again by ELISAs in triplicate as described above. Bacterial culture for DNA preps was made by inoculating 1.2 ml 2xYT liquid media with chloramphenicol from master glycerol stocks of positive Fabs, and growing overnight. DNA was prepared from cell pellets centrifuged out of the overnight cultures using the Qiagen Turbo Mini preps performed on a BioRobot 9600. ABI Dye Terminator cycle sequencing was performed on the DNA with Morphosys defined sequencing primers and run on an ABI 3100 Genetic Analyzer, to obtain the DNA sequence of the Fab clones. DNA sequences were compared to each other to determine unique clone sequences and to determine light and heavy chain subtypes of the Fab clones. Fabs from ELISA-positive clone m2CX1D05 and the EsB (negative control) Fab were expressed by IPTG-induction in The DNA sequence encoding the m2CX1D05 light chain variable region was amplified by polymerase chain reaction from plasmid template pMORPHx9_MH/mPCSK9—2_CX1_D05, using primers: ACAGATGCCAGATGCGATATCCAGATGACCCAGA (SEQ ID NO: 33) and TGCAGCCACCGTACGTTTAATTTCAACTTTCGTACC (SEQ ID NO: 34). The product of this amplification was cloned into plasmid pV1JNSA-GS-FB-LCK that had been previously digested with FspI and BmtI, using the InFusion cloning system (Clontech). The resulting plasmid was verified by DNA sequencing across the variable region. Endotoxin-free plasmid preparations were made using the Qiagen Endo-Free plasmid maxiprep kit. The DNA sequence encoding the heavy chain variable region of pMORPHx9_MH/mPCSK9—2_CX1_D05 was amplified by polymerase chain reaction using primers: ACAGGTGTCCACTCGCAGGTGCAATTGGTTCAGTCT (SEQ ID NO: 35) and GCCCTTGGTGGATGCTGAGCTAACCGTCACCAGGGT (SEQ ID NO: 36), and the amplified product was cloned into plasmid pV1JNSA-BF-HCG2M4 that had been previously digested with FspI and BmtI. The resulting plasmid was verified by DNA sequencing across the variable region. Endotoxin-free plasmid preparations were made using the Qiagen Endo-Free plasmid maxiprep kit. Full-length IgG was obtained by co-transfection of HEK293 cells with the 1D05 light chain- and heavy-chain-encoding plasmids, following by Protein A purification of the expressed IgG. SPR measurements were performed using a Biacore™ (Pharmacia Biosensor AB, Uppsala, Sweden) 2000 system. Sensor chip CM5 and Amine Coupling Kit for immobilization were from Biacore™. Anti-Fab IgG (Human specific) (Sigma, catalog #I5260) was covalently coupled to surfaces 1 and 2 of a Sensor Chip CM5 via primary amine groups, using the immobilization wizard with the “Aim for immobilization” option using Biacore™ Amine Coupling Kit (cat #BR-1000-50. A target immobilization of 5000 RU was specified. The wizard uses a 7 minute activation with a 1:1 mixture of 100 mM NHS (N-Hydroxysuccinimide) and 400 mM EDC (1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide), injects the ligand in several pulses to achieve the desired level, then deactivates the remaining surface with a 7 minute pulse of ethanolamine. Anti-PCSK9 Fabs were captured on capture surface 2, and surface 1 was used as a reference for kinetic studies of Fab:PCSK9 interactions. Each Fab was captured by flowing a 500 ng/ml solution at 5 or 10 μl/min for 1-1.5 minutes to reach a target RLfor an Rmaxof 100-150 RU for the reaction. 5-10 concentrations of hPCSK9v5His or mPCSK9v5His antigens were flowed across the surface at 30 μl/minute for 3-4 minutes. 15-60 minutes dissociation time was allowed before regeneration of the Anti-Fab surface with a 30 second pulse of 10 mM glycine pH 2.0. BiaEvaluation Software was used to evaluate the sensograms from the multiple concentration of PCSK9 antigen analyzed with each Fab, to estimate the kinetics constants of the Fab:PCSK9 interactions. The kinetic constants were determined as follows: SPR measurements were performed using a Biacore™ (Pharmacia Biosensor AB, Uppsala, Sweden) 2000 system. Sensor chip CM5 and Amine Coupling Kit for immobilization were from Biacore™. A goat Anti-Human IgG (Caltag, catalog #H10700) was covalently coupled to surfaces 1 and 2 of a Sensor Chip CM5 via primary amine groups, using the immobilization wizard with the “Aim for immobilization” option using Biacore™ Amine Coupling Kit (cat #BR-1000-50. A target immobilization of 5000 RU was specified. The wizard uses a 7 minute activation with a 1:1 mixture of 100 mM NHS (N-Hydroxysuccinimide) and 400 mM EDC (1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide), injects the ligand in several pulses to achieve the desired level, then deactivates the remaining surface with a 7 minute pulse of ethanolamine. Anti-PCSK9 IgGs were captured on capture surface 2, and surface 1 was used as a reference for kinetic studies of IgG:PCSK9 interactions. IgG was captured by flowing a 10 nM solution at 10 μl/min for 1-1.5 minutes to reach a target RLfor an Rmaxof 100-150 RU for the reaction. 5-10 concentrations of hPCSK9v5His or mPCSK9v5His antigens were flowed across the surface at 30 or 60 μl/minute for 4 minutes. 15-60 minutes dissociation time was allowed before regeneration of the Anti-IgG surface with a 60 second pulse of 10 mM Glycine pH 1.7. BiaEvaluation Software was used to evaluate the sensograms from the multiple concentration of PCSK9 antigen analyzed with each IgG, to estimate the kinetics constants of the IgG:PCSK9 interactions. The kinetic constants were determined as follows: This assay is a variant of the one described in Fisher et al., 2007 On day 1, 30,000 HEK cells/well were plated in a 96 well polyD-lysine coated plate. On day 2, the media was switched to no-serum containing DMEM media. On day 3, the media was removed and the cells were washed with OptiMEM. Purified PCSK9 was added in 100 μl of DMEM media containing LPDS and dI-LDL. The plates were incubated at 37° C. for 6.5 hrs. The cells were washed quickly in TBS containing 2 mg/ml BSA; then washed in TBS-BSA for 2 minutes; and then washed twice (but quickly) with TBS. The cells were lysed in 100 μl RIPA buffer. Fluorescence was then measured in the plate using an Ex 520, Em 580 nm. The total cellular protein in each well was measured using a BCA Protein Assay and the fluorescence units were then normalized to total protein. The Exopolar Assay is effective for characterizing variant effects on LDL uptake; see Table 4 below illustrating how the potencies of PCSK9 mutants correlate with plasma LDL-cholesterol in the Exopolar Assay. Results: m2CX1D05, both Fab and IgG, dose-dependently inhibited the effects of both human and murine PCSK9 on LDL uptake; an effect which was reproducibly observed. The amount of PCSK9 added to the cells was ˜100-320 nM. m2CX1D05 (Fab) comprises a light chain of SEQ ID NO: 1 (comprising a VL of SEQ ID NO: 27) and a Fd chain of SEQ ID NO: 9 inclusive of linkers and tags (comprising a VH of SEQ ID NO: 11). M2CX1D05 (IgG) comprises a light chain of SEQ ID NO: 26, and a heavy chain comprising SEQ ID NO: 25. 1D05 clearly cross reacts with both human and mouse PCSK9. 1D05 can inhibit the effect of PCSK9 on cellular LDL uptake. IC50s for 1D05 (Fab) are 97 and 144 nM for mouse and human PCSK9 protein, respectively. ICH's for 1D05 (IgG) are 85 and 79 nM for mouse and human PCSK9 protein, respectively. The assay that follows was carried out according to the methods of Fisher et al., 2007 Cells treated with Alexa Fluor 647-labeled PCSK9 were imaged as follows. CHO cells were plated on poly-D-lysine-coated 96-well optical CVG sterile black plates (Nunc) at a density of 20,000 cells/well. Cells were plated in F-12K medium (nutrient mixture, Kaighn's modification (1×)) (Invitrogen) containing 100 units of penicillin and 100 μg/ml streptomycin sulfate and supplemented with 10% FBS. Plates were incubated overnight at 37° C. and 5% CO2. The following morning, the medium was removed and replaced with 100 μl of F-12K medium containing 100 units of penicillin and 100 μg/ml streptomycin sulfate. After 18 h, the medium was removed. Purified PCSK9 protein was labeled with Alexa Fluor 647 as described under “Experimental Procedures.” Alexa Fluor 647-labeled PCSK9 (1, 5, or 20 μg/ml) was added in 50 μl of F-12K medium containing 10% lipoprotein-deficient serum to the cells. The plates were incubated at 37° C. for 4 h, and the cells were washed quickly with Tris-buffered saline before imaging. To label cellular nuclei, Hoechst 33342 at a final concentration of 0.1 μg/ml was added to each well. The plates were run on an Opera imager (Evotec Technologies GmbH, Hamburg, Germany) with a x40 water immersion objective. Images were captured using excitation wavelengths of 405 nm for fluorescent nuclei and 635 nm for Alexa Fluor 647-labeled PCSK9. For each well, 11 individual fields containing >500 cells were captured for two emission wavelengths. The data were analyzed using a customized algorithm written using the Acapella language (Evotec Technologies GmbH). The algorithm identified and marked the nuclear and cytoplasmic areas of individual cells, followed by measurement of the total cytoplasmic intensity of the cell. The intensity was expressed in arbitrary fluorescent units. For testing the 1D05 Ab, the identical procedure was used, but with either HEK293 or HepG2 cells. For HepG2 cells, the plates would not have been poly-D-lysine coated. 5 μg/ml of AF647-labeled WT PCSK9 was added along with a titration of Fab ranging from 50 μg/ml down. Using this procedure, we obtained IC50values of roughly 80 nM for the Fab in both cell types. Results: Both Fab fragments and whole IgG of human 1D05 were tested in vivo in mice and changes in the level of LDL cholesterol were monitored. The mice used in these studies were (B6×B6-Tg(CETP) Ldlrtm1)F1 mice which are hemizygous for the transgenic (Tg) expression of human CETP (which mice lack) as well as the disruption of the LDL receptor (tm1). These mice are particularly useful because of their human-like lipid profiles and LDL-rich nature. Each mouse was bled twice, once at the beginning of the study to establish individual baseline levels of LDL cholesterol (“pre”) and a second time 3 hours later (“post”) to assess what changes took place in LDL levels after treatment. Each mouse received two IV doses of Dulbecco's PBS as a vehicle control, 1D05 IgG (0.5 mg), or 1D05 Fab fragments (0.5 mg) over the course of 3 hours. The 1D05 whole IgG was centrifuged at 230,000×g to remove aggregates immediately prior to injection. In The limited proteolysis mass spectrometry strategy consists in the incubation of wt-hPCSK9 and 1D05/wt-hPCSK9 complex (substrates) with endoproteinase enzymes of different specificity in carefully controlled conditions (i.e., low enzymes concentration and short digestion time). Under these conditions, the endoproteases will cleave only the primary cleavage sites of the protein substrate (i.e., sites that are on the surface of the protein substrate and exposed to the solvent). The binding of 1D05 Fab to wt-hPCSK9 will mask some surface residues normally exposed to the solvent in both proteins. Therefore the primary sites cleaved in wt-hPCSK9 and not in the 1D05/wt-hPCSK9 complex correspond to residues of PCSK9 protected by 1D05 in the complex. Some of these residues are likely to be directly involved in 1D05 binding. The proteolytic peptides generated by wt-hPCSK9 and 1D05/wt-hPCSK9 limited proteolysis are identified and characterized by analysis of the digest by Matrix Assisted Laser Desorption/Ionization Mass Spectrometry (MALDI-MS). Finally, the use of endoproteases with different specificity helps to more accurately define the residues involved in binding. The amount of proteolytic enzyme normally used in limited proteolysis experiments had to be considerably reduced to avoid excess hydrolysis of wt-hPCSK9 and loss of the primary binding sites (exposed residues). Incubation of wt-hPCSK9, 1D05 and 1D05/wt-hPCSK9 with endoproteases was done in 25 mM HEPES pH 7.5, 150 mM NaCl at room temperature. The endoproteases used were AspN added at a 2500/1 (w/w) excess of protein compared to proteolytic enzyme, and Trypsin and GluC added at a 1000/1 (w/w) protein to endoprotease ratio. At periods of time 5, 15 and 30 minutes after endoprotease addition, an aliquot of sample was deposited onto the MALDI target and subjected to direct MALDI-MS analysis in the presence of sinapinic acid (SA) as matrix. The fragment peptides originated from wt-PCSK9 after incubation with the proteolytic enzyme at various time were compared with those originated from wt-hPCSK9 in the 1D05/wt-hPCSK9 complex sample to identify the residues protected from proteolysis in the 1D05/wt-hPCSK9 interaction. The Figures and Tables provided herein report only the most relevant fragment peptides. Limited Proteolysis with Endoprotease AspN: The wt-hPCSK9 protein, 1D05/wt-hPCSK9 complex and 1D05 Fab were incubated for 5, 15 and 30 minutes in the presence of AspN, which cleaves N-terminally to Asp residues, at a 2500/1 (w/w) ratio between the protein and the proteolytic enzyme. MALDI-MS analysis of the digests revealed the primary wt-hPCSK9 and 1D05/wt-hPCSK9 complex cleavage sites (see The species at m/z 1969.1, originated from the cleavage at Asp169 and matching the theoretical mass of peptide 153-168 of the catalytic domain of wt-hPCSK9, was formed only in the wt-hPCSK9 sample indicating that this residue is protected from proteolysis by 1D05 Fab binding in the 1D05/wt-hPCSK9 complex. Several species were formed in both wt-hPCSK9 and 1D05/wt-hPCSK9 hydrolyses. In particular the ions at m/z 2222.0 and 4412.9 corresponding to peptides 31-49 of the prodomain and 698-737 of the catalytic domain of wt-hPCSK9 were originated from cleavage at Asp49 and Asp698. At longer endoprotease AspN incubation time (i.e., 15, 30 minutes) the peptide profile shown in the MALDI-MS spectra did not change significantly compared to the one at 5 minutes shown in It is important to note that the observed degree of agreement between the expected and measured mass values is within the norm for this type of experiment, since mass calibration must be made with an external standard. Limited Proteolysis with Endoprotease GluC: Endoprotease GluC cleaves C-terminally Glu residues. Incubation of GluC with wt-hPCSK9, 1D05/wt-hPCSK9 complex and 1D05 was conducted at a 1000/1 and 100/1 (w/w) ratio between protein and proteolytic enzyme. To detect the primary cleavage sites, the MALDI-MS analysis of the samples was conducted after 5, 15 and 30 minutes of incubation. The wt-hPCSK9 residues cleaved in the wt-hPCSK9 protein and protected in the 1D05/wt-hPCSK9 complex, and the corresponding peptides detected in the MS spectrum, are shown in With endoprotease GluC, protection is shown in the wt-hPCSK9 surface area including residues Glu170, Glu197 and Glu195. The specie at m/z 3357.4, corresponding to peptide 153-181, and obtained in the incubation with GluC of both wt-hPCSK9 and 1D05/wt-hPCSK9, indicates that Glu181 is not protected by the Fab 1D05 binding to wt-hPCSK9. Limited Proteolysis with Trypsin: Trypsin cleaves C-terminally Arg and Lys residues. The enzyme was added at 1000 (w/w) ratio to wt-PCSK9, 1D05/wt-PCSK9 complex and 1D05 for 5, 15 and 30 minutes. MALDI-MS analysis of the wt-PCSK9 and 1D05/wt-PCSK9 complex is shown in The primary cleavage sites at 5 minutes were Arg46 on the prodomain and Arg160, Arg165, Arg167, Arg199, Arg215, Arg218, Arg705 and Arg729 on the catalytic domain of wt-hPCSK9. The species at m/z 2279.3, 3909.9 and 4474.6 corresponding to peptides 200-218, 166-199 and 161-199 are detected only in the wt-hPCSK9 hydrolysis and indicate that residue Arg199 is protected by 1D05 binding. These peptide fragments together with the species at m/z 5410.5, 5729.7, 5850.8, 6170.2 correspond to peptides 168-215, 166-215, 168-218, 166-218, detected only in wt-PCSK9 thus indicating protection also on residues Arg165 and Arg167. At 15 minutes of Trypsin incubation, protection on residue Arg199 is confirmed. In fact the species at m/z 2280.0, 3591.4, 3910.9 and 4475.6, all originated from cleavage at Arg199, are present only in the wt-PCSK9 hydrolysis and become more abundant. In addition, protection at Arg194 is detected (as shown by the presence of the specie at m/z 3325.7 in the wt-PCSK9 spectrum). The species originated from cleavage at Arg165 and Arg167 (m/z 5411.5, 5730.9, 5851.8 and 6171.6) are present in the wt-hPCSK9 hydrolysis and start to appear with much lower intensity also in the 1D05/wt-hPCSK9 complex proteolysis. This may indicate that the protection from proteolysis on such residues is due to steric hindrance of the Fab rather than to primary contacts between 1D05 and wt-PCSK9 residues. Results: With LP-MS using three enzymes of different specificity, we identified the surface area of wt-hPCSK9 protected by the 1D05 Fab in the 1D05-wt-hPCSK9. Arg165, Arg167, Asp169, Glu170, Arg194, Glu197 and Arg199 are the residues of wt-hPCSK9 protected upon binding to the 1D05 Fab (see Residues in peptides R194-R199 are conserved in human and mouse PCSK9. 1D05 Fab recognizes human and mouse protein. As illustrated by the sequence alignment between human and mouse PCSK9 (see Anti-V5 antibody (QED Biosciences) was labeled and purified as described previously (see Fisher et al., 2007 TR-FRET assays were carried out in black Microfluor 2 96 well plates (Dynex Technologies) in 10 mM Hepes pH 7.4, 150 mM NaCl, 100 uM CaCl2and 0.05% BSA. To 25 μL of 20 nM each AF647 labeled anti-V5 antibody and V5/His-PCSK9 was added a serial dilution of the unlabeled candidate antibody (i.e., 1D05 and 1B20), either Fab or IgG. Reagents were equilibrated for ˜15 minutes at room temperature and then Eu(W8044)-1D05 IgG was added to give a final concentration of 1.5 nM Eu labeled antibody (˜18000 counts at Fl620nm; S/B=12) and a total volume of 50 uL. After, equilibration assays were read in a BMG LabTech Rubystar Reader as described previously (Fisher et al., Id.). Data are reported as Fl665/Fl620×10000. IC50s were determined using data fitted to a sigmoidal dose response curve using non-linear regression analysis (Kaleidagraph 4.03, Synergy Software). To characterize pharmacokinetics, pharmacodynamics and target engagement of 1D05, a single dose IV study was conducted in male Rhesus monkeys at 3 mg/kg (7.0-9.0 kg, n=3). All Rhesus monkeys used in the study were naïve to biologics. Monkeys were given an IV bolus dose of 1D05 via the cephalic vein. Blood samples were collected from the saphenous/femoral vessel at designated time points post dosing and the resulting plasma/serum was stored at −70° C. until analysis. The dosing solutions of 1D05 were prepared at 47.2 mg/mL in 100 mM Histidine, 100 mM Arginine, 6% sucrose, pH 6.0. The dosing solutions were stored at 4° C. and kept on wet ice during dosing. The lipoprotein analysis of the serum samples were carried out as described below. An anti-human IgG ELISA using commercially available reagents was used to quantify 1D05 levels. As shown in To generate lipoprotein profiles, plasma or serum was fractionated by chromatography over Superose-6 size exclusion column (GE LifeSciences, Inc.). Total cholesterol levels in the column effluent were continuously measured via in-line mixture with a commercially available enzymatic colorimetric cholesterol detection reagent (Total Cholesterol E, Wako USA) followed by downstream spectrophotometric detection of the reaction products at 600 nm absorbance. The first peak of cholesterol eluted from the column was attributed to VLDL, the second peak to LDL and the third to HDL; the area under each peak was calculated using software provided with the HPLC. To calculate the cholesterol concentration for each lipoprotein fraction, the ratio of the corresponding peak area to total peak area was multiplied by the total cholesterol concentration measured in the sample. Monoclonal antibodies comprising a light chain comprising SEQ ID NO: 26 and a heavy chain comprising SEQ ID NO: 25) were dialyzed into the appropriate formulations and concentrated. Solutions were then dispensed into 3 mL glass vials for stability studies. Studies carried out in liquid form were immediately placed on stability at 2-8° C. or 25° C. Analytical methods included Size Exclusion Chromatography (SEC-HPLC) to measure aggregation and fragmentation. Below is a table of Time 0 and 6M SEC data. The formulations containing 3/50/50 or 6/100/100 (sucrose/His/Arg) form fewer aggregates and fragments after storage for 6 months at 2-8° C. or 25° C. All formulations are at 6.0 except for the standard—that is frozen at 1 mg/mL in Phosphate buffered saline (pH ˜7). Site-directed mutant variants of 1D05 were generated and are disclosed herein as SEQ ID NOs: 51-60. Kds of site-directed mutant variants of 1D05 Fabs were determined using a Bio-Rad ProteOn; with affinity being measured against human PCSK9-V5-His. The methodologies for measuring Fab affinities are essentially the same as previously described for Biacore®. Antagonists of human proprotein convertase subtilisin-kexin type 9 (PCSK9) are disclosed. The disclosed antagonists are effective in the inhibition of PCSK9 function and, accordingly, present desirable antagonists for use in the treatment of conditions associated with PCSK9 activity. The present invention also discloses nucleic acid encoding said antagonists, vectors, host cells, and compositions comprising the antagonists. Methods of making PCSK9-specific antagonists as well as methods of using the antagonists for inhibiting or antagonizing PCSK9 function are also disclosed and form important additional aspects of the present disclosure. 1. An isolated PCSK9-specific antagonist which comprises:
(a) a heavy chain variable region comprising a CDR3 domain comprising SEQ ID NO: 17 or an equivalent thereof, said equivalent characterized as having one or more conservative amino acid substitutions in the CDR3 domain; and/or (b) a light chain variable region comprising a CDR3 domain comprising SEQ ID NO: 7 or an equivalent thereof, said equivalent characterized as having one or more conservative amino acid substitutions in the CDR3 domain; wherein said PCSK9-specific antagonist antagonizes PCSK9's inhibition of cellular LDL uptake. 2. The PCSK9-specific antagonist of 3. The PCSK9-specific antagonist of 4. The PCSK9-specific antagonist of 5. The PCSK9-specific antagonist of 6. The PCSK9-specific antagonist of 7. The PCSK9-specific antagonist of 8. The PCSK9-specific antagonist of 9. The PCSK9-specific antagonist of 10. The PCSK9-specific antagonist of 11. The PCSK9-specific antagonist of 12. The PCSK9-specific antagonist of (a) a heavy chain variable CDR1 sequence comprising SEQ ID NO: 13; (b) a heavy chain variable CDR2 sequence comprising SEQ ID NO: 15; (c) a light chain variable CDR1 sequence comprising SEQ ID NO: 3; and/or (d) a light chain variable CDR2 sequence comprising SEQ ID NO: 5. 13. The PCSK9-specific antagonist of 14. The PCSK9-specific antagonist of (a) a heavy chain variable region comprising a CDR3 domain comprising SEQ ID NO: 17; (b) a light chain variable region comprising a CDR3 domain comprising SEQ ID NO: 7; (c) a heavy chain variable CDR1 sequence comprising SEQ ID NO: 13; (d) a light chain variable CDR1 sequence comprising SEQ ID NO: 3; (e) a heavy chain variable CDR2 sequence comprising SEQ ID NO: 15; and (f) a light chain variable CDR2 sequence comprising SEQ ID NO: 5. 15. The PCSK9-specific antagonist of 16. The PCSK9-specific antagonist of 17. The PCSK9-specific antagonist of 18. The PCSK9-specific antagonist of 19. A PCSK9-specific antagonist which comprises:
(a) a light chain comprising SEQ ID NO: 1; and (b) a heavy chain comprising SEQ ID NO: 11; wherein said PCSK9-specific antagonist is an antibody molecule that antagonizes PCSK9's inhibition of cellular LDL uptake. 20. A PCSK9-specific antagonist of 21. An isolated PCSK9-specific antagonist which comprises:
(a) a light chain comprising SEQ ID NO: 26; and (b) a heavy chain comprising SEQ ID NO: 25; wherein said PCSK9-specific antagonist is an antibody molecule that antagonizes PCSK9's inhibition of cellular LDL uptake. 22. An isolated PCSK9-specific antagonist that:
(a) inhibits the binding of a 1D05 Fab to PCSK9 by at least 50%; said 1D05 Fab characterized as comprising a light chain comprising SEQ ID NO: 1 and an Fd chain comprising amino acids 1-233 of SEQ ID NO: 9; and (b) antagonizes (i) PCSK9 binding to the LDL receptor and/or (ii) PCSK9 internalization into cells. 23. A PCSK9-specific antagonist that:
(a) inhibits the binding of a 1D05 IgG to PCSK9 by at least 50%; said 1D05 IgG characterized as comprising (i) a light chain comprising SEQ ID NO: 1 and (ii) a heavy chain comprising SEQ ID NO: 11; and (b) antagonizes (i) PCSK9 binding to the LDL receptor and/or (ii) PCSK9 internalization into cells. 24. A PCSK9-specific antagonist of 25. An isolated PCSK9-specific antagonist which comprises:
(a) heavy chain variable region CDR3 sequence of SEQ ID NO: 45; (b) heavy chain variable region CDR3 sequence of SEQ ID NO: 45; heavy chain CDR1 sequence of SEQ ID NO: 43 and heavy chain CDR2 sequence of SEQ ID NO: 44; (c) light chain variable region CDR3 sequence of SEQ ID NO: 48; (d) light chain variable region CDR3 sequence of SEQ ID NO: 48; light chain CDR1 sequence of SEQ ID NO: 46 and light chain CDR2 sequence of SEQ ID NO: 47; (e) both (a) and (c); (f) both (b) and (d); (g) heavy and/or light chain variable regions comprising SEQ ID NOs: 50 and 49, respectively; (h) a heavy chain variable region comprising any one of SEQ ID NOs: 51-56 and optionally a light chain variable region comprising SEQ ID NO: 27; or (i) a light chain variable region comprising any one of SEQ ID NOs: 57-60 and optionally a heavy chain variable region comprising SEQ ID NO: 11; wherein said PCSK9-specific antagonist is an antibody molecule that antagonizes PCSK9's inhibition of cellular LDL uptake. 26. A composition comprising the PCSK9-specific antagonist of 27. A composition in accordance with (a) about 50 mg/mL to about 200 mg/mL of the PCSK9-specific antagonist; (b) a polyhydroxy hydrocarbon (including but not limited to sorbitol, mannitol, glycerol and dulcitol) and/or a disaccharide (including but not limited to sucrose, lactose, maltose and trehalose); the total of said polyhydroxy hydrocarbon and/or disaccharide being about 1% to about 6% w/v of the formulation; (c) about 5 mM to about 200 mM of histidine, imidazole, phosphate or acetic acid; (d) about 5 mM to about 200 mM of arginine, proline, phenylalanine, alanine, glycine, lysine, glutamic acid, aspartic acid or methionine; (e) about 0.01 M to about 0.1 M of hydrochloric acid (“HCl”) in an amount sufficient to achieve a pH in the range of about 5.5 to about 7.5; and (f) a liquid carrier including but not limited to sterile water, petroleum, animal oil, vegetable oil, mineral oil, synthetic oil, physiological saline solution, dextrose or other saccharide solution or glycols, such as ethylene glycol, propylene glycol or polyethylene glycol; wherein said pharmaceutical composition has a pH in the range of about 5.5 to about 7.5; and wherein said pharmaceutical composition optionally comprises about 0.01% to about 1% w/v of the formulation of a non-ionic surfactant (including but not limited to Polysorbate-80 (Tween 80™), Polysorbate-60 (Tween 60™), Polysorbate-40 (Tween 40™), and Polysorbate-20 (Tween 20™), polyoxyethylene alkyl ethers, including but not limited to Brij 58™, Brij35™, as well as others such as Triton X-100™, Triton X-114™, NP40™, Span 85 and the Pluronic series of non-ionic surfactants (e.g., Pluronic 121)). 28. The composition of (a) about 50 mg/mL to about 200 mg/mL of the PCSK9-specific antagonist; (b) about 1% to about 6% w/v of mannitol, trehalose or sucrose; (c) about 10 mM to about 150 mM of histidine; (d) about 10 mM to about 150 mM of arginine or proline; (e) about 0.003 M to about 0.005 M of hydrochloric acid (“HCl”) in an amount sufficient to achieve a pH in the range of about 5.8 to about 6.5; and (f) a liquid carrier including but not limited to sterile water; petroleum, animal oil, vegetable oil, mineral oil, synthetic oil, physiological saline solution dextrose, or other saccharide solution or glycols, such as ethylene glycol, propylene glycol or polyethylene glycol; wherein said pharmaceutical composition has a pH in the range of about 5.8 to about 6.5; and wherein said pharmaceutical composition optionally comprises about 0.01% to about 1% w/v of Polysorbate-80 (Tween 80™) or Polysorbate-20 (Tween 20™). 29. The composition of (a) about 50 mg/mL to about 200 mg/mL of the PCSK9-specific antagonist; (b) about 2% to about 6% w/v of sucrose; (c) about 25 mM to about 100 mM of histidine; (d) about 25 mM to about 100 mM of arginine; (e) about 0.0040 M to about 0.0045 M of hydrochloric acid (“HCl”) in an amount sufficient to achieve a pH in the range of about 6; and (f) sterile water; wherein said pharmaceutical composition has a pH in the range of about 6; and wherein said pharmaceutical composition optionally comprises about 0.01% to about 1% w/v of Polysorbate-80 (Tween 80™) or Polysorbate-20 (Tween 20™). 30. The composition of (a) about 50 mg/mL to about 200 mg/mL of the PCSK9-specific antagonist; (b) sucrose, histidine and arginine in one of the following amounts: (i) about 3% w/v sucrose, about 50 mM histidine and about 50 mM arginine; or (ii) about 6% w/v sucrose, about 100 mM histidine and about 100 mM arginine; (c) about 0.0040 M to about 0.0045 M of hydrochloric acid (“HCl”) in an amount sufficient to achieve a pH in the range of about 6; and (d) sterile water; wherein said pharmaceutical composition has a pH in the range of about 6; and wherein said pharmaceutical composition optionally comprises about 0.01% to about 1% w/v of Polysorbate-80 (Tween 80™) or Polysorbate-20 (Tween 20™). 31. A method for antagonizing PCSK9 function which comprises administering a PCSK9-specific antagonist of 32. Use of a PCSK9-specific antagonist of 33. Isolated nucleic acid encoding a PCSK9-specific antagonist of 34. Isolated nucleic acid which encodes a PCSK9-specific antagonist of 35. The isolated nucleic acid of (a) CDR1 and/or CDR2 domains in the heavy chain variable region that are encoded, respectively, by a nucleotide sequence comprising SEQ ID NO: 14 and SEQ ID NO: 16; and/or (b) CDR1 and/or CDR2 domains in the light chain variable region that are encoded, respectively, by a nucleotide sequence comprising SEQ ID NO: 4 and SEQ ID NO: 6. 36. Isolated nucleic acid which encodes a PCSK9-specific antagonist of (a) a heavy chain variable region encoded by a nucleotide sequence comprising SEQ ID NO: 12; and/or (b) a light chain variable region encoded by a nucleotide sequence comprising SEQ ID NO: 28. 37. Isolated nucleic acid which encodes a PCSK9-specific antagonist of (a) a heavy chain region encoded by a nucleotide sequence comprising SEQ ID NO: 29; and/or (b) a light chain region encoded by a nucleotide sequence comprising SEQ ID NO: 30. 38. A vector comprising nucleic acid of 39. An isolated host cell or population of host cells in vitro or in situ comprising nucleic acid of 40. A method for producing a PCSK9-specific antagonist which comprises:
(a) culturing the cell(s) of (b) isolating the PCSK9-specific antagonist produced. 41. An isolated host cell or population of host cells in vitro or in situ comprising a PCSK9-specific antagonist of CROSS-REFERENCE TO RELATED APPLICATIONS
STATEMENT REGARDING FEDERALLY-SPONSORED R&D
REFERENCE TO MICROFICHE APPENDIX
BACKGROUND OF THE INVENTION
SUMMARY OF THE INVENTION
SEQ ID NO: 1 LIGHT CHAIN (“LC”); 1D05 SEQ ID NO: 2 LIGHT CHAIN (“LC”) NUCLEIC ACID; 1D05 SEQ ID NO: 3 VL CDR1; 1D05 SEQ ID NO: 4 VL CDR1 NUCLEIC ACID; 1D05 SEQ ID NO: 5 VL CDR2; 1D05 SEQ ID NO: 6 VL CDR2 NUCLEIC ACID; 1D05 SEQ ID NO: 7 VL CDR3; 1D05 SEQ ID NO: 8 VL CDR3 NUCLEIC ACID; 1D05 SEQ ID NO: 9 Fd CHAIN inclusive of linkers and tags; 1D05 SEQ ID NO: 10 Fd CHAIN NUCLEIC ACID; 1D05 SEQ ID NO: 11 VH; 1D05 SEQ ID NO: 12 VH NUCLEIC ACID; 1D05 SEQ ID NO: 13 VH CDR1; 1D05 SEQ ID NO: 14 VH CDR1 NUCLEIC ACID; 1D05 SEQ ID NO: 15 VH CDR2; 1D05 SEQ ID NO: 16 VH CDR2 NUCLEIC ACID; 1D05 SEQ ID NO: 17 VH CDR3; 1D05 SEQ ID NO: 18 VH CDR3 NUCLEIC ACID; 1D05 SEQ ID NO: 19 FRAGMENT OF PROCESSING SITE SEQ ID NO: 20 FRAGMENT OF PROCESSING SITE SEQ ID NO: 21 Constant domain of IgG1 SEQ ID NO: 22 Constant domain of IgG2 SEQ ID NO: 23 Constant domain of IgG4 SEQ ID NO: 24 Constant domain of IgG2m4 SEQ ID NO: 25 1D05 IgG2m4 Heavy Chain (“HC”) SEQ ID NO: 26 1D05 IgG Light (Kappa) Chain SEQ ID NO: 27 VL; 1D05 SEQ ID NO: 28 VL NUCLEIC ACID; 1D05 SEQ ID NO: 29 1D05 IgG2m4 HC NUCLEIC ACID SEQ ID NO: 30 1D05 IgG LC NUCLEIC ACID SEQ ID NO: 31 1D05 IgG2m4 HC PLASMID SEQ ID NO: 32 1D05 IgG LC PLASMID SEQ ID NO: 33 PRIMER SEQ ID NO: 34 PRIMER SEQ ID NO: 35 PRIMER SEQ ID NO: 36 PRIMER SEQ ID NO: 37 1D05 EPITOPE DOMAIN SEQ ID NO: 38 PORTION OF PCSK9 SEQUENCE IN FIGURE SEQ ID NO: 39 HUMAN EPITOPE AREA SEQ ID NO: 40 CONSENSUS SEQUENCE SEQ ID NO: 41 MURINE EPITOPE AREA SEQ ID NO: 42 SECONDARY FOOTPRINT EPITOPE SEQ ID NO: 43 1D05 Variant VH CDR1 Sequence SEQ ID NO: 44 1D05 Variant VH CDR2 Sequence SEQ ID NO: 45 1D05 Variant VH CDR3 Sequence SEQ ID NO: 46 1D05 Variant VL CDR1 Sequence SEQ ID NO: 47 1D05 Variant VL CDR2 Sequence SEQ ID NO: 48 1D05 Variant VL CDR3 Sequence SEQ ID NO: 49 VL; 1D05 Variant Sequence SEQ ID NO: 50 VH; 1D05 Variant Sequence SEQ ID NO: 51 VH; 1D05 Variant Sequence H32Y SEQ ID NO: 52 VH; 1D05 Variant Sequence M48A SEQ ID NO: 53 VH; 1D05 Variant Sequence M48L SEQ ID NO: 54 VH; 1D05 Variant SequenceH99Y SEQ ID NO: 55 VH; 1D05 Variant Sequence M48L/M109L/M115L SEQ ID NO: 56 VH; 1D05 Variant SequenceM48V SEQ ID NO: 57 V1; 1D05 Variant Sequence N50D SEQ ID NO: 58 V1; 1D05 Variant Sequence N50Q SEQ ID NO: 59 V1; 1D05 Variant SequenceN50T SEQ ID NO: 60 V1; 1D05 Variant SequenceN50Y BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION OF THE INVENTION
Example 1
Isolation of recombinant Fab display phage
Example 2
ELISA Screening of Bacterially Expressed Fabs
Growth medium only.
Example 3
DNA Sequence Determination of PCSK9 ELISA-Positive Fab Clones
Example 4
Expression and Purification of Fabs from Unique PCSK9 ELISA-Positive Clone
Example 5
Conversion of m2CX1D05 Fab to Full Length IgG
Example 6
Kinetic Evaluation of Fab:PCSK9 Interactions with Surface Plasmon Resonance (“SPR”)
m2CX1D05 Fab Human 0.22 ± 0.01 2.47 ± 0.05 11.5 ± 0.75 mean (N = 3) PCSK9 m2CX1D05 Fab Murine 0.86 ± 0.02 2.57 ± 0.19 3.35 ± 0.39 mean (N = 3) PCSK9 Example 7
Kinetic Evaluation of IgG:PCSK9 Interactions with Surface Plasmon Resonance (“SPR”)
1D05 IgG2m4 hPCSK9 0.88 ± 0.01 3.16 ± 0.27 3.6 ± 0.33 mean (N = 2) 1D05 IgG2m4 mPCSK9 0.67 ± 0.06 2.15 ± 0.16 3.2 ± 0.06 mean (N = 2) Example 8
PCSK9-LDLR TR-Fret Assay for 1D05
Example 9
Exopolar Assay: Effects of Exogenous PCSK9 on Cellular LDL Uptake
S127R Gain 277 14 D374Y Gain 388 1.3 Wild-type 140 51 R46L Loss 116 78 Example 10
PCSK9 Cellular Uptake
Example 11
In Vivo Assay
Example 12
Limited Proteolysis
2222.0 2222.0 31-49 Asp49 4412.9 4411.2 698-737 Asp698 3357.4 3357.7 153-181 Glu181 1877.9 1878.0 31-46 Arg46 2581.9 2581.9 706-729 Arg705 Arg729 3562.9 3562.9 706-737 Arg705 7290.7 7291.0 153-215 Arg215 7731.5 7731.5 153-218 Arg218 Example 13
PCSK9/1D05 TR-Fret Assay
ka(1/Ms) 6.6E+04 ± 6.1E+03 1.41E+05 ± 1.2E+04 kd(1/s) 4.8E−05 ± 7.4E−06 7.18E−05 ± 2.9E−06 KA(1/M) 1.5E+09 ± 3.0E+08 2.0E+09 ± 1.5E+08 KD(M) 7.4E−10 ± 1.6E−10 5.1E−10 ± 3.8E−11 Example 14
1D05 Rhesus PK/PD Study
Example 15
Lipoprotein Analysis of Plasma/Serum Samples from 1D05 Rhesus PK/Pd Study
Example 16
Formulation
1D05 standard T0 0.32% 1.59% 98.09% 0.00% 10His/150 NaCl Time 0 0.26% 1.59% 98.15% 0.00% 3/50/50 Time 0 0.30% 1.59% 98.11% 0.00% 1D05 standard −70 C. 6 M 0.60% 2.79% 96.61% 0.00% His/NaCl 4 C. 6 M 0.98% 3.18% 95.83% 0.01% 6/100/100 4 C. 6 M 0.95% 2.90% 96.13% 0.02% 3/50/50 4 C. 6 M 0.92% 3.00% 96.07% 0.01% 100 mg/mL 4 C. 6 M 0.97% 3.13% 95.85% 0.05% His/NaCl 25 C. 6 M 1.71% 4.45% 93.45% 0.40% 6/100/100 25 C. 6 M 1.16% 3.69% 94.74% 0.39% 3/50/50 25 C. 6 M 1.31% 3.70% 94.62% 0.37% Example 17
Variants
H32Y HEAVY SEQ ID NO: 51 2.01 M48AQ HEAVY SEQ ID NO: 52 2.06 M48L HEAVY SEQ ID NO: 53 1.52 H99Y HEAVY SEQ ID NO: 54 1.45 M48L/M109L/M115L HEAVY SEQ ID NO: 55 1.13 M48V HEAVY SEQ ID NO: 56 1.95 N50D LIGHT SEQ ID NO: 57 3.42 N50Q LIGHT SEQ ID NO: 58 0.615 N50T LIGHT SEQ ID NO: 59 2.13 N50Y LIGHT SEQ ID NO: 60 2.58 * Amino acid numbering begins with the first residue of FR1, immediately following signal peptide.