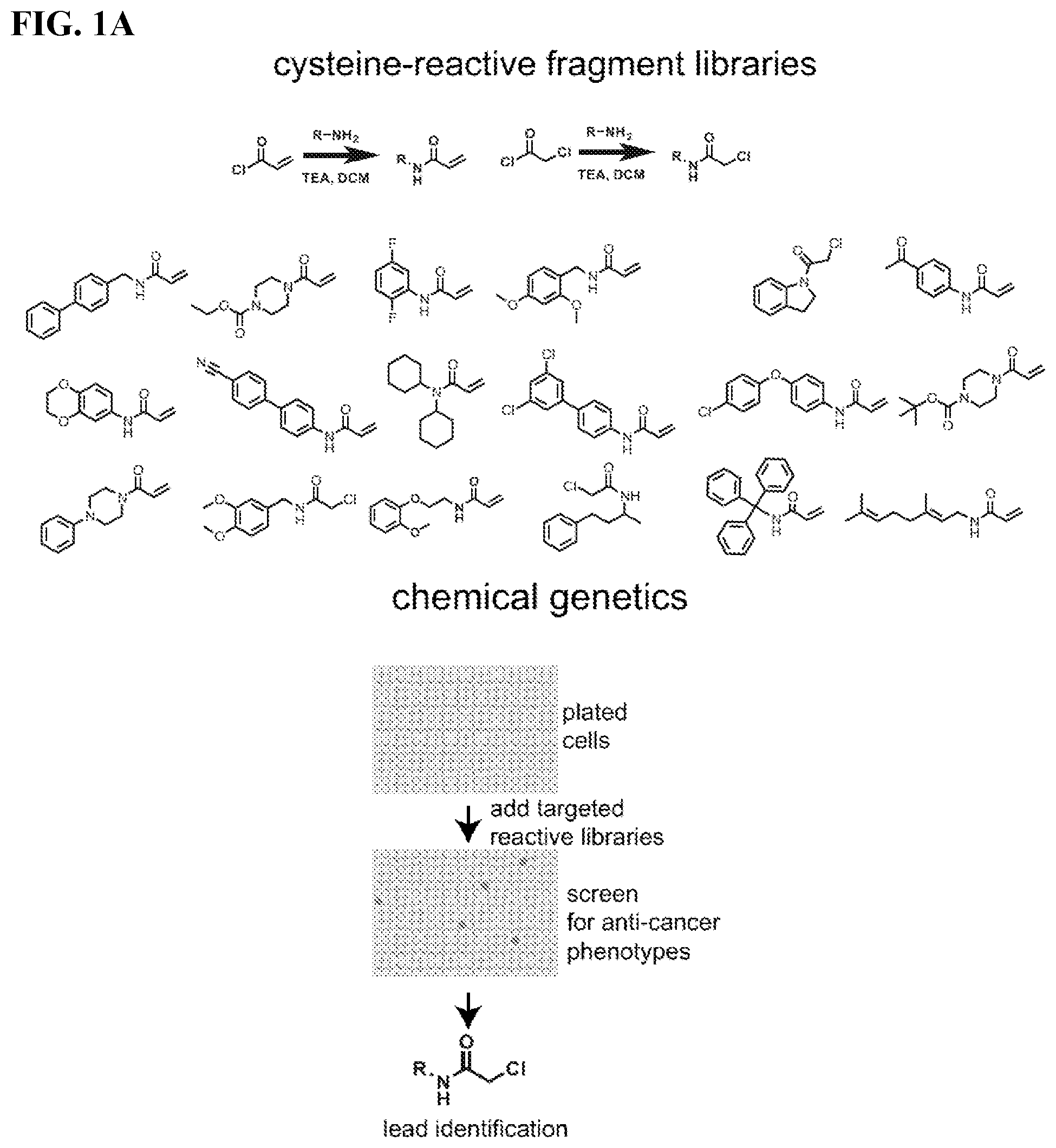
COMPOSITIONS AND METHODS FOR INHIBITING RETICULON 4
This application claims the benefit of U.S. Provisional Application No. 62/454,681, filed Feb. 3, 2017, and U.S. Provisional Application No. 62/471,865, filed Mar. 15, 2017, which are incorporated herein by reference in their entirety and for all purposes. This invention was made with government support under CA172667 and GM112948 awarded by the National Institutes of Health and under W81XWH-15-1-0050 awarded by ARMY/MRMC. The government has certain rights in the invention. The Sequence Listing written in file 052103-503001WO Sequence Listing_ST25.txt, created Jan. 11, 2018, 166,113 bytes, machine format IBM-PC, MS Windows operating system, is hereby incorporated by reference. In the United States, it is estimated that over 134,000 people will be diagnosed with colorectal cancer and over 49,000 patients will die from colorectal cancer1. Current therapeutic strategies for colorectal cancer include resection and non-specific therapies such as radiation or chemotherapy2. Unfortunately, these treatment strategies are insufficient for aggressive and metastatic colorectal cancers, and thus better strategies are needed to discover both novel anti-cancer agents and targets for combatting colorectal cancer. Towards this goal, identifying new anti-cancer targets, druggable nodes, and lead small-molecules are critical for combatting colorectal cancer. Disclosed herein, inter alia, are solutions to these and other problems in the art. Herein are provided, inter alia, compounds capable of modulating the level of activity of reticulon 4 and methods of using the same. In an aspect is provided a compound having the formula: R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1R1D, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NR1AOR1C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. Two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. The symbol z1 is an integer from 0 to 5. R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(O)R2C—C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. Two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. The symbol z2 is an integer from 0 to 4. L1is a bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4—, —NR4C(O)—, —NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, or substituted or unsubstituted heteroarylene. R4is hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)OR4A, —C(O)NR4AR4B, —OR4A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR5C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, or substituted or unsubstituted heteroarylene. R5is hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)—OR5A, —C(O)NR5AR5B, —OR5A—, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. E is an electrophilic moiety. Each R1A, R1B, R1C, R1D, R2A, R2B, R2C, R2D, R4A, R4B, R5A, and R5Bis independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl. R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl. R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl. R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl. Each X, X1, X2, X4, and X5is independently —F, —Cl, —Br, or —I. The symbols n1, n2, n4, and n5 are independently an integer from 0 to 4. The symbols m1, m2, m4, m5, v1, v2, v4, and v5 are independently an integer from 1 to 2. In an aspect is provided a pharmaceutical composition including a Reticulon 4 inhibitor and a pharmaceutically acceptable excipient. In an aspect is provided a pharmaceutical composition including a compound described herein, or pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable excipient. In an aspect is provided a method of treating cancer, the method including administering to a subject in need thereof an effective amount of a Reticulon 4 inhibitor. In an aspect is provided a method of treating cancer including administering to a subject in need thereof an effective amount of a compound described herein. In an aspect is provided a method of treating a disease associated with reticulon 4 activity including administering to a subject in need thereof an effective amount of a Reticulon 4 inhibitor. In an aspect is provided a method of inhibiting reticulon 4 activity including contacting the reticulon 4 with a Reticulon 4 inhibitor. In an aspect is provided a method of inhibiting reticulon 4 activity including contacting the reticulon 4 with a compound described herein. In an aspect is provided a reticulon 4 protein covalently bonded to a Reticulon 4 inhibitor. In an aspect is provided a reticulon 4 protein covalently bonded to a compound described herein. The abbreviations used herein have their conventional meaning within the chemical and biological arts. The chemical structures and formulae set forth herein are constructed according to the standard rules of chemical valency known in the chemical arts. Where substituent groups are specified by their conventional chemical formulae, written from left to right, they equally encompass the chemically identical substituents that would result from writing the structure from right to left, e.g., —CH2O— is equivalent to —OCH2—. The term “alkyl,” by itself or as part of another substituent, means, unless otherwise stated, a straight (i.e., unbranched) or branched carbon chain (or carbon), or combination thereof, which may be fully saturated, mono- or polyunsaturated and can include mono-, di- and multivalent radicals. The alkyl may include a designated number of carbons (e.g., C1-C10means one to ten carbons). Alkyl is an uncyclized chain. Examples of saturated hydrocarbon radicals include, but are not limited to, groups such as methyl, ethyl, n-propyl, isopropyl, n-butyl, t-butyl, isobutyl, sec-butyl, methyl, homologs and isomers of, for example, n-pentyl, n-hexyl, n-heptyl, n-octyl, and the like. An unsaturated alkyl group is one having one or more double bonds or triple bonds. Examples of unsaturated alkyl groups include, but are not limited to, vinyl, 2-propenyl, crotyl, 2-isopentenyl, 2-(butadienyl), 2,4-pentadienyl, 3-(1,4-pentadienyl), ethynyl, 1- and 3-propynyl, 3-butynyl, and the higher homologs and isomers. An alkoxy is an alkyl attached to the remainder of the molecule via an oxygen linker (—O—). An alkyl moiety may be an alkenyl moiety. An alkyl moiety may be an alkynyl moiety. An alkyl moiety may be fully saturated. An alkenyl may include more than one double bond and/or one or more triple bonds in addition to the one or more double bonds. An alkynyl may include more than one triple bond and/or one or more double bonds in addition to the one or more triple bonds. The term “alkylene,” by itself or as part of another substituent, means, unless otherwise stated, a divalent radical derived from an alkyl, as exemplified, but not limited by, —CH2CH2CH2CH2—. Typically, an alkyl (or alkylene) group will have from 1 to 24 carbon atoms, with those groups having 10 or fewer carbon atoms being preferred herein. A “lower alkyl” or “lower alkylene” is a shorter chain alkyl or alkylene group, generally having eight or fewer carbon atoms. The term “alkenylene,” by itself or as part of another substituent, means, unless otherwise stated, a divalent radical derived from an alkene. The term “heteroalkyl,” by itself or in combination with another term, means, unless otherwise stated, a stable straight or branched chain, or combinations thereof, including at least one carbon atom and at least one heteroatom (e.g., O, N, P, Si, or S), and wherein the nitrogen and sulfur atoms may optionally be oxidized, and the nitrogen heteroatom may optionally be quaternized. The heteroatom(s) (e.g., O, N, P, S, B, As, or Si) may be placed at any interior position of the heteroalkyl group or at the position at which the alkyl group is attached to the remainder of the molecule. Heteroalkyl is an uncyclized chain. Examples include, but are not limited to: —CH2—CH2—O—CH3, —CH2—CH2—NH—CH3, —CH2—CH2—N(CH3)—CH3, —CH2—S—CH2—CH3, —CH2—CH2, —S(O)—CH3, —CH2—CH2—S(O)2—CH3, —CH═CH—O—CH3, —Si(CH3)3, —CH2—CH═N—OCH3, —CH═CH—N(CH3)—CH3, —O—CH3, —O—CH2—CH3, and —CN. Up to two or three heteroatoms may be consecutive, such as, for example, —CH2—NH—OCH3and —CH2—O—Si(CH3)3. A heteroalkyl moiety may include one heteroatom (e.g., O, N, S, Si, or P). A heteroalkyl moiety may include two optionally different heteroatoms (e.g., O, N, S, Si, or P). A heteroalkyl moiety may include three optionally different heteroatoms (e.g., O, N, S, Si, or P). A heteroalkyl moiety may include four optionally different heteroatoms (e.g., O, N, S, Si, or P). A heteroalkyl moiety may include five optionally different heteroatoms (e.g., O, N, S, Si, or P). A heteroalkyl moiety may include up to 8 optionally different heteroatoms (e.g., O, N, S, Si, or P). Similarly, the term “heteroalkylene,” by itself or as part of another substituent, means, unless otherwise stated, a divalent radical derived from heteroalkyl, as exemplified, but not limited by, —CH2—CH2—S—CH2—CH2— and —CH2—S—CH2—CH2—NH—CH2—. For heteroalkylene groups, heteroatoms can also occupy either or both of the chain termini (e.g., alkyleneoxy, alkylenedioxy, alkyleneamino, alkylenediamino, and the like). Still further, for alkylene and heteroalkylene linking groups, no orientation of the linking group is implied by the direction in which the formula of the linking group is written. For example, the formula —C(O)2R′— represents both —C(O)2R′— and —R′C(O)2—. As described above, heteroalkyl groups, as used herein, include those groups that are attached to the remainder of the molecule through a heteroatom, such as —C(O)R′, —C(O)NR′, —NR′R″, —OR′, —SR′, and/or —SO2R′. Where “heteroalkyl” is recited, followed by recitations of specific heteroalkyl groups, such as —NR′R″ or the like, it will be understood that the terms heteroalkyl and —NR′R″ are not redundant or mutually exclusive. Rather, the specific heteroalkyl groups are recited to add clarity. Thus, the term “heteroalkyl” should not be interpreted herein as excluding specific heteroalkyl groups, such as —NR′R″ or the like. The terms “cycloalkyl” and “heterocycloalkyl,” by themselves or in combination with other terms, mean, unless otherwise stated, cyclic versions of “alkyl” and “heteroalkyl,” respectively. Cycloalkyl and heterocycloalkyl are not aromatic. Additionally, for heterocycloalkyl, a heteroatom can occupy the position at which the heterocycle is attached to the remainder of the molecule. Examples of cycloalkyl include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, 1-cyclohexenyl, 3-cyclohexenyl, cycloheptyl, and the like. Examples of heterocycloalkyl include, but are not limited to, 1-(1,2,5,6-tetrahydropyridyl), 1-piperidinyl, 2-piperidinyl, 3-piperidinyl, 4-morpholinyl, 3-morpholinyl, tetrahydrofuran-2-yl, tetrahydrofuran-3-yl, tetrahydrothien-2-yl, tetrahydrothien-3-yl, 1-piperazinyl, 2-piperazinyl, and the like. A “cycloalkylene” and a “heterocycloalkylene,” alone or as part of another substituent, means a divalent radical derived from a cycloalkyl and heterocycloalkyl, respectively. The terms “halo” or “halogen,” by themselves or as part of another substituent, mean, unless otherwise stated, a fluorine, chlorine, bromine, or iodine atom. Additionally, terms such as “haloalkyl” are meant to include monohaloalkyl and polyhaloalkyl. For example, the term “halo(C1-C4)alkyl” includes, but is not limited to, fluoromethyl, difluoromethyl, trifluoromethyl, 2,2,2-trifluoroethyl, 4-chlorobutyl, 3-bromopropyl, and the like. The term “acyl” means, unless otherwise stated, —C(O)R where R is a substituted or unsubstituted alkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. The term “aryl” means, unless otherwise stated, a polyunsaturated, aromatic, hydrocarbon substituent, which can be a single ring or multiple rings (preferably from 1 to 3 rings) that are fused together (i.e., a fused ring aryl) or linked covalently. A fused ring aryl refers to multiple rings fused together wherein at least one of the fused rings is an aryl ring. The term “heteroaryl” refers to aryl groups (or rings) that contain at least one heteroatom such as N, O, or S, wherein the nitrogen and sulfur atoms are optionally oxidized, and the nitrogen atom(s) are optionally quaternized. Thus, the term “heteroaryl” includes fused ring heteroaryl groups (i.e., multiple rings fused together wherein at least one of the fused rings is a heteroaromatic ring). A 5,6-fused ring heteroarylene refers to two rings fused together, wherein one ring has 5 members and the other ring has 6 members, and wherein at least one ring is a heteroaryl ring. Likewise, a 6,6-fused ring heteroarylene refers to two rings fused together, wherein one ring has 6 members and the other ring has 6 members, and wherein at least one ring is a heteroaryl ring. And a 6,5-fused ring heteroarylene refers to two rings fused together, wherein one ring has 6 members and the other ring has 5 members, and wherein at least one ring is a heteroaryl ring. A heteroaryl group can be attached to the remainder of the molecule through a carbon or heteroatom. Non-limiting examples of aryl and heteroaryl groups include phenyl, naphthyl, pyrrolyl, pyrazolyl, pyridazinyl, triazinyl, pyrimidinyl, imidazolyl, pyrazinyl, purinyl, oxazolyl, isoxazolyl, thiazolyl, furyl, thienyl, pyridyl, pyrimidyl, benzothiazolyl, benzoxazoyl benzimidazolyl, benzofuran, isobenzofuranyl, indolyl, isoindolyl, benzothiophenyl, isoquinolyl, quinoxalinyl, quinolyl, 1-naphthyl, 2-naphthyl, 4-biphenyl, 1-pyrrolyl, 2-pyrrolyl, 3-pyrrolyl, 3-pyrazolyl, 2-imidazolyl, 4-imidazolyl, pyrazinyl, 2-oxazolyl, 4-oxazolyl, 2-phenyl-4-oxazolyl, 5-oxazolyl, 3-isoxazolyl, 4-isoxazolyl, 5-isoxazolyl, 2-thiazolyl, 4-thiazolyl, 5-thiazolyl, 2-furyl, 3-furyl, 2-thienyl, 3-thienyl, 2-pyridyl, 3-pyridyl, 4-pyridyl, 2-pyrimidyl, 4-pyrimidyl, 5-benzothiazolyl, purinyl, 2-benzimidazolyl, 5-indolyl, 1-isoquinolyl, 5-isoquinolyl, 2-quinoxalinyl, 5-quinoxalinyl, 3-quinolyl, and 6-quinolyl. Substituents for each of the above noted aryl and heteroaryl ring systems are selected from the group of acceptable substituents described below. An “arylene” and a “heteroarylene,” alone or as part of another substituent, mean a divalent radical derived from an aryl and heteroaryl, respectively. A heteroaryl group substituent may be —O— bonded to a ring heteroatom nitrogen. Spirocyclic rings are two or more rings wherein adjacent rings are attached through a single atom. The individual rings within spirocyclic rings may be identical or different. Individual rings in spirocyclic rings may be substituted or unsubstituted and may have different substituents from other individual rings within a set of spirocyclic rings. Possible substituents for individual rings within spirocyclic rings are the possible substituents for the same ring when not part of spirocyclic rings (e.g. substituents for cycloalkyl or heterocycloalkyl rings). Spirocylic rings may be substituted or unsubstituted cycloalkyl, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heterocycloalkylene and individual rings within a spirocyclic ring group may be any of the immediately previous list, including having all rings of one type (e.g. all rings being substituted heterocycloalkylene wherein each ring may be the same or different substituted heterocycloalkylene). When referring to a spirocyclic ring system, heterocyclic spirocyclic rings means a spirocyclic rings wherein at least one ring is a heterocyclic ring and wherein each ring may be a different ring. When referring to a spirocyclic ring system, substituted spirocyclic rings means that at least one ring is substituted and each substituent may optionally be different. The symbol “” denotes the point of attachment of a chemical moiety to the remainder of a molecule or chemical formula. The term “oxo,” as used herein, means an oxygen that is double bonded to a carbon atom. The term “alkylarylene” as an arylene moiety covalently bonded to an alkylene moiety (also referred to herein as an alkylene linker). In embodiments, the alkylarylene group has the formula: An alkylarylene moiety may be substituted (e.g. with a substituent group) on the alkylene moiety or the arylene linker (e.g. at carbons 2, 3, 4, or 6) with halogen, oxo, —N3, —CF3, —CCl3, —CBr3, —CI3, —CN, —CHO, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO2CH3—SO3H, —OSO3H, —SO2NH2, —NHNH2, —ONH2, —NHC(O)NHNH2, substituted or unsubstituted C1-C5alkyl or substituted or unsubstituted 2 to 5 membered heteroalkyl). In embodiments, the alkyl aryl ene is unsubstituted. Each of the above terms (e.g., “alkyl,” “heteroalkyl,” “cycloalkyl,” “heterocycloalkyl,” “aryl,” and “heteroaryl”) includes both substituted and unsubstituted forms of the indicated radical. Preferred substituents for each type of radical are provided below. Substituents for the alkyl and heteroalkyl radicals (including those groups often referred to as alkylene, alkenyl, heteroalkylene, heteroalkenyl, alkynyl, cycloalkyl, heterocycloalkyl, cycloalkenyl, and heterocycloalkenyl) can be one or more of a variety of groups selected from, but not limited to, —OR′, ═O, ═NR′, ═N—OR′, —NR′R″, —SR′, -halogen, —SiR′R″R′″, —OC(O)R′, —C(O)R′, —CO2R′, —CONR′R″, —OC(O)NR′R″, —NR″C(O)R′, —NR′—C(O)NR″R′″, —NR″C(O)2R′, —NR—C(NR′R″R′″)═NR″″, —NR—C(NR′R″)═NR′″, —S(O)R′, —S(O)2R′, —S(O)2NR′R″, —NRSO2R′, —NR′NR″R′″, —ONR′R″, —NR′C(O)NR″NR′″R″″, —CN, —NO2, —NR′SO2R″, —NR′C(O)R″, —NR′C(O)—OR″, —NR′OR″, in a number ranging from zero to (2m′+1), where m′ is the total number of carbon atoms in such radical. R, R′, R″, R′″, and R″″ each preferably independently refer to hydrogen, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl (e.g., aryl substituted with 1-3 halogens), substituted or unsubstituted heteroaryl, substituted or unsubstituted alkyl, alkoxy, or thioalkoxy groups, or arylalkyl groups. When a compound described herein includes more than one R group, for example, each of the R groups is independently selected as are each R′, R″, R′″, and R″″ group when more than one of these groups is present. When R′ and R″ are attached to the same nitrogen atom, they can be combined with the nitrogen atom to form a 4-, 5-, 6-, or 7-membered ring. For example, —NR′R″ includes, but is not limited to, 1-pyrrolidinyl and 4-morpholinyl. From the above discussion of substituents, one of skill in the art will understand that the term “alkyl” is meant to include groups including carbon atoms bound to groups other than hydrogen groups, such as haloalkyl (e.g., —CF3and —CH2CF3) and acyl (e.g., —C(O)CH3, —C(O)CF3, —C(O)CH2OCH3, and the like). Similar to the substituents described for the alkyl radical, substituents for the aryl and heteroaryl groups are varied and are selected from, for example: —OR′, —NR′R″, —SR′, -halogen, —SiR′R″R′″, —OC(O)R′, —C(O)R′, —CO2R′, —CONR′R″, —OC(O)NR′R″, —NR″C(O)R′, C(O)NR″R′″, —NR″C(O)2R′, —NR—C(NR′R″R′″)═NR″″, —NR—C(NR′R″)═NR′″, —S(O)R′, —S(O)2R′, —S(O)2NR′R″, —NRSO2R′, —NR′NR″R′″, —ONR′R″, —NR′C(O)NR″NR′″R″″, —CN, —NO2, —R′, —N3, —CH(Ph)2, fluoro(C1-C4)alkoxy, and fluoro(C1-C4)alkyl, —NR′SO2R″, —NR′C(O)R″, —NR′C(O)—OR″, —NR′OR″, in a number ranging from zero to the total number of open valences on the aromatic ring system; and where R′, R″, R′″, and R″″ are preferably independently selected from hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl. When a compound described herein includes more than one R group, for example, each of the R groups is independently selected as are each R′, R″, R′″, and R″″ groups when more than one of these groups is present. Substituents for rings (e.g. cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene) may be depicted as substituents on the ring rather than on a specific atom of a ring (commonly referred to as a floating substituent). In such a case, the substituent may be attached to any of the ring atoms (obeying the rules of chemical valency) and in the case of fused rings or spirocyclic rings, a substituent depicted as associated with one member of the fused rings or spirocyclic rings (a floating substituent on a single ring), may be a substituent on any of the fused rings or spirocyclic rings (a floating substituent on multiple rings). When a substituent is attached to a ring, but not a specific atom (a floating substituent), and a subscript for the substituent is an integer greater than one, the multiple substituents may be on the same atom, same ring, different atoms, different fused rings, different spirocyclic rings, and each substituent may optionally be different. Where a point of attachment of a ring to the remainder of a molecule is not limited to a single atom (a floating substituent), the attachment point may be any atom of the ring and in the case of a fused ring or spirocyclic ring, any atom of any of the fused rings or spirocyclic rings while obeying the rules of chemical valency. Where a ring, fused rings, or spirocyclic rings contain one or more ring heteroatoms and the ring, fused rings, or spirocyclic rings are shown with one more floating substituents (including, but not limited to, points of attachment to the remainder of the molecule), the floating substituents may be bonded to the heteroatoms. Where the ring heteroatoms are shown bound to one or more hydrogens (e.g. a ring nitrogen with two bonds to ring atoms and a third bond to a hydrogen) in the structure or formula with the floating substituent, when the heteroatom is bonded to the floating substituent, the substituent will be understood to replace the hydrogen, while obeying the rules of chemical valency. Two or more substituents may optionally be joined to form aryl, heteroaryl, cycloalkyl, or heterocycloalkyl groups. Such so-called ring-forming substituents are typically, though not necessarily, found attached to a cyclic base structure. In one embodiment, the ring-forming substituents are attached to adjacent members of the base structure. For example, two ring-forming substituents attached to adjacent members of a cyclic base structure create a fused ring structure. In another embodiment, the ring-forming substituents are attached to a single member of the base structure. For example, two ring-forming substituents attached to a single member of a cyclic base structure create a spirocyclic structure. In yet another embodiment, the ring-forming substituents are attached to non-adjacent members of the base structure. Two of the substituents on adjacent atoms of the aryl or heteroaryl ring may optionally form a ring of the formula -T-C(O)—(CRR′)q—U—, wherein T and U are independently —NR—, —O—, —CRR′—, or a single bond, and q is an integer of from 0 to 3. Alternatively, two of the substituents on adjacent atoms of the aryl or heteroaryl ring may optionally be replaced with a substituent of the formula -A-(CH2)r—B—, wherein A and B are independently —CRR′—, —O—, —NR—, —S—, —S(O)—, —S(O)2—, —S(O)2NR′—, or a single bond, and r is an integer of from 1 to 4. One of the single bonds of the new ring so formed may optionally be replaced with a double bond. Alternatively, two of the substituents on adjacent atoms of the aryl or heteroaryl ring may optionally be replaced with a substituent of the formula —(CRR′), —X′—(C″R″R′″)d—, where s and d are independently integers of from 0 to 3, and X′ is —O—, —S—, —S(O)—, —S(O)2—, or —S(O)2NR′—. The substituents R, R′, R″, and R′″ are preferably independently selected from hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, and substituted or unsubstituted heteroaryl. As used herein, the terms “heteroatom” or “ring heteroatom” are meant to include oxygen (O), nitrogen (N), sulfur (S), phosphorus (P), and silicon (Si). A “substituent group,” as used herein, means a group selected from the following moieties: (A) oxo,
A “size-limited substituent” or “size-limited substituent group,” as used herein, means a group selected from all of the substituents described above for a “substituent group,” wherein each substituted or unsubstituted alkyl is a substituted or unsubstituted C1-C20alkyl, each substituted or unsubstituted heteroalkyl is a substituted or unsubstituted 2 to 20 membered heteroalkyl, each substituted or unsubstituted cycloalkyl is a substituted or unsubstituted C3-C8cycloalkyl, each substituted or unsubstituted heterocycloalkyl is a substituted or unsubstituted 3 to 8 membered heterocycloalkyl, each substituted or unsubstituted aryl is a substituted or unsubstituted C6-C10aryl, and each substituted or unsubstituted heteroaryl is a substituted or unsubstituted 5 to 10 membered heteroaryl. A “lower substituent” or “lower substituent group,” as used herein, means a group selected from all of the substituents described above for a “substituent group,” wherein each substituted or unsubstituted alkyl is a substituted or unsubstituted C1-C8alkyl, each substituted or unsubstituted heteroalkyl is a substituted or unsubstituted 2 to 8 membered heteroalkyl, each substituted or unsubstituted cycloalkyl is a substituted or unsubstituted C3-C7cycloalkyl, each substituted or unsubstituted heterocycloalkyl is a substituted or unsubstituted 3 to 7 membered heterocycloalkyl, each substituted or unsubstituted aryl is a substituted or unsubstituted C6-C10aryl, and each substituted or unsubstituted heteroaryl is a substituted or unsubstituted 5 to 9 membered heteroaryl. In some embodiments, each substituted group described in the compounds herein is substituted with at least one substituent group. More specifically, in some embodiments, each substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene described in the compounds herein are substituted with at least one substituent group. In other embodiments, at least one or all of these groups are substituted with at least one size-limited substituent group. In other embodiments, at least one or all of these groups are substituted with at least one lower substituent group. In other embodiments of the compounds herein, each substituted or unsubstituted alkyl may be a substituted or unsubstituted C1-C20alkyl, each substituted or unsubstituted heteroalkyl is a substituted or unsubstituted 2 to 20 membered heteroalkyl, each substituted or unsubstituted cycloalkyl is a substituted or unsubstituted C3-C8cycloalkyl, each substituted or unsubstituted heterocycloalkyl is a substituted or unsubstituted 3 to 8 membered heterocycloalkyl, each substituted or unsubstituted aryl is a substituted or unsubstituted C6-C10aryl, and/or each substituted or unsubstituted heteroaryl is a substituted or unsubstituted 5 to 10 membered heteroaryl. In some embodiments of the compounds herein, each substituted or unsubstituted alkylene is a substituted or unsubstituted C1-C20alkylene, each substituted or unsubstituted heteroalkylene is a substituted or unsubstituted 2 to 20 membered heteroalkylene, each substituted or unsubstituted cycloalkylene is a substituted or unsubstituted C3-C8cycloalkylene, each substituted or unsubstituted heterocycloalkylene is a substituted or unsubstituted 3 to 8 membered heterocycloalkylene, each substituted or unsubstituted arylene is a substituted or unsubstituted C6-C10arylene, and/or each substituted or unsubstituted heteroarylene is a substituted or unsubstituted 5 to 10 membered heteroarylene. In some embodiments, each substituted or unsubstituted alkyl is a substituted or unsubstituted C1-C8alkyl, each substituted or unsubstituted heteroalkyl is a substituted or unsubstituted 2 to 8 membered heteroalkyl, each substituted or unsubstituted cycloalkyl is a substituted or unsubstituted C3-C7cycloalkyl, each substituted or unsubstituted heterocycloalkyl is a substituted or unsubstituted 3 to 7 membered heterocycloalkyl, each substituted or unsubstituted aryl is a substituted or unsubstituted C6-C10aryl, and/or each substituted or unsubstituted heteroaryl is a substituted or unsubstituted 5 to 9 membered heteroaryl. In some embodiments, each substituted or unsubstituted alkylene is a substituted or unsubstituted C1-C8alkylene, each substituted or unsubstituted heteroalkylene is a substituted or unsubstituted 2 to 8 membered heteroalkylene, each substituted or unsubstituted cycloalkylene is a substituted or unsubstituted C3-C7cycloalkylene, each substituted or unsubstituted heterocycloalkylene is a substituted or unsubstituted 3 to 7 membered heterocycloalkylene, each substituted or unsubstituted arylene is a substituted or unsubstituted C6-C10arylene, and/or each substituted or unsubstituted heteroarylene is a substituted or unsubstituted 5 to 9 membered heteroarylene. In some embodiments, the compound is a chemical species set forth in the Examples section, figures, or tables below. In embodiments, a substituted moiety (e.g., substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene) is substituted with at least one substituent group, wherein if the substituted moiety is substituted with a plurality of substituent groups, each substituent group may optionally be different. In embodiments, if the substituted moiety is substituted with a plurality of substituent groups, each substituent group is different. In embodiments, a substituted moiety (e.g., substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene) is substituted with at least one size-limited substituent group, wherein if the substituted moiety is substituted with a plurality of size-limited substituent groups, each size-limited substituent group may optionally be different. In embodiments, if the substituted moiety is substituted with a plurality of size-limited substituent groups, each size-limited substituent group is different. In embodiments, a substituted moiety (e.g., substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene) is substituted with at least one lower substituent group, wherein if the substituted moiety is substituted with a plurality of lower substituent groups, each lower substituent group may optionally be different. In embodiments, if the substituted moiety is substituted with a plurality of lower substituent groups, each lower substituent group is different. In embodiments, a substituted moiety (e.g., substituted alkyl, substituted heteroalkyl, substituted cycloalkyl, substituted heterocycloalkyl, substituted aryl, substituted heteroaryl, substituted alkylene, substituted heteroalkylene, substituted cycloalkylene, substituted heterocycloalkylene, substituted arylene, and/or substituted heteroarylene) is substituted with at least one substituent group, size-limited substituent group, or lower substituent group; wherein if the substituted moiety is substituted with a plurality of groups selected from substituent groups, size-limited substituent groups, and lower substituent groups; each substituent group, size-limited substituent group, and/or lower substituent group may optionally be different. In embodiments, if the substituted moiety is substituted with a plurality of groups selected from substituent groups, size-limited substituent groups, and lower substituent groups; each substituent group, size-limited substituent group, and/or lower substituent group is different. Certain compounds of the present invention possess asymmetric carbon atoms (optical or chiral centers) or double bonds; the enantiomers, racemates, diastereomers, tautomers, geometric isomers, stereoisometric forms that may be defined, in terms of absolute stereochemistry, as (R)— or (S)— or, as (D)- or (L)- for amino acids, and individual isomers are encompassed within the scope of the present invention. The compounds of the present invention do not include those that are known in art to be too unstable to synthesize and/or isolate. The present invention is meant to include compounds in racemic and optically pure forms. Optically active (R)- and (S)-, or (D)- and (L)-isomers may be prepared using chiral synthons or chiral reagents, or resolved using conventional techniques. When the compounds described herein contain olefinic bonds or other centers of geometric asymmetry, and unless specified otherwise, it is intended that the compounds include both E and Z geometric isomers. As used herein, the term “isomers” refers to compounds having the same number and kind of atoms, and hence the same molecular weight, but differing in respect to the structural arrangement or configuration of the atoms. The term “tautomer,” as used herein, refers to one of two or more structural isomers which exist in equilibrium and which are readily converted from one isomeric form to another. It will be apparent to one skilled in the art that certain compounds of this invention may exist in tautomeric forms, all such tautomeric forms of the compounds being within the scope of the invention. Unless otherwise stated, structures depicted herein are also meant to include all stereochemical forms of the structure; i.e., the R and S configurations for each asymmetric center. Therefore, single stereochemical isomers as well as enantiomeric and diastereomeric mixtures of the present compounds are within the scope of the invention. Unless otherwise stated, structures depicted herein are also meant to include compounds which differ only in the presence of one or more isotopically enriched atoms. For example, compounds having the present structures except for the replacement of a hydrogen by a deuterium or tritium, or the replacement of a carbon by13C- or14C-enriched carbon are within the scope of this invention. Unless otherwise stated, structures depicted herein are also meant to include compounds which differ only in the presence of one or more isotopically enriched atoms. For example, compounds having the present structures except for the replacement of a hydrogen by a deuterium or tritium, or the replacement of a carbon by13C- or14C-enriched carbon are within the scope of this invention. The compounds of the present invention may also contain unnatural proportions of atomic isotopes at one or more of the atoms that constitute such compounds. For example, the compounds may be radiolabeled with radioactive isotopes, such as for example tritium (3H), iodine-125 (125I), or carbon-14 (14C). All isotopic variations of the compounds of the present invention, whether radioactive or not, are encompassed within the scope of the present invention. It should be noted that throughout the application that alternatives are written in Markush groups, for example, each amino acid position that contains more than one possible amino acid. It is specifically contemplated that each member of the Markush group should be considered separately, thereby comprising another embodiment, and the Markush group is not to be read as a single unit. “Analog,” or “analogue” is used in accordance with its plain ordinary meaning within Chemistry and Biology and refers to a chemical compound that is structurally similar to another compound (i.e., a so-called “reference” compound) but differs in composition, e.g., in the replacement of one atom by an atom of a different element, or in the presence of a particular functional group, or the replacement of one functional group by another functional group, or the absolute stereochemistry of one or more chiral centers of the reference compound. Accordingly, an analog is a compound that is similar or comparable in function and appearance but not in structure or origin to a reference compound. The terms “a” or “an,” as used in herein means one or more. In addition, the phrase “substituted with a[n],” as used herein, means the specified group may be substituted with one or more of any or all of the named substituents. For example, where a group, such as an alkyl or heteroaryl group, is “substituted with an unsubstituted C1-C20alkyl, or unsubstituted 2 to 20 membered heteroalkyl,” the group may contain one or more unsubstituted C1-C20alkyls, and/or one or more unsubstituted 2 to 20 membered heteroalkyls. Moreover, where a moiety is substituted with an R substituent, the group may be referred to as “R-substituted.” Where a moiety is R-substituted, the moiety is substituted with at least one R substituent and each R substituent is optionally different. Where a particular R group is present in the description of a chemical genus (such as Formula (I)), a Roman alphabetic symbol may be used to distinguish each appearance of that particular R group. For example, where multiple R3substituents are present, each R13substituent may be distinguished as R13A, R13B, R13C, R13D, etc., wherein each of R13A, R13B, R13C, R13D, etc. is defined within the scope of the definition of R3and optionally differently. A “covalent cysteine modifier moiety” as used herein refers to a substituent that is capable of reacting with the sulfhydryl functional group of a cysteine amino acid (e.g. cysteine corresponding to C1101 of the human reticulon 4) to form a covalent bond. Thus, the covalent cysteine modifier moiety is typically electrophilic. Description of compounds of the present invention are limited by principles of chemical bonding known to those skilled in the art. Accordingly, where a group may be substituted by one or more of a number of substituents, such substitutions are selected so as to comply with principles of chemical bonding and to give compounds which are not inherently unstable and/or would be known to one of ordinary skill in the art as likely to be unstable under ambient conditions, such as aqueous, neutral, and several known physiological conditions. For example, a heterocycloalkyl or heteroaryl is attached to the remainder of the molecule via a ring heteroatom in compliance with principles of chemical bonding known to those skilled in the art thereby avoiding inherently unstable compounds. The term “pharmaceutically acceptable salts” is meant to include salts of the active compounds that are prepared with relatively nontoxic acids or bases, depending on the particular substituents found on the compounds described herein. When compounds of the present invention contain relatively acidic functionalities, base addition salts can be obtained by contacting the neutral form of such compounds with a sufficient amount of the desired base, either neat or in a suitable inert solvent. Examples of pharmaceutically acceptable base addition salts include sodium, potassium, calcium, ammonium, organic amino, or magnesium salt, or a similar salt. When compounds of the present invention contain relatively basic functionalities, acid addition salts can be obtained by contacting the neutral form of such compounds with a sufficient amount of the desired acid, either neat or in a suitable inert solvent. Examples of pharmaceutically acceptable acid addition salts include those derived from inorganic acids like hydrochloric, hydrobromic, nitric, carbonic, monohydrogencarbonic, phosphoric, monohydrogenphosphoric, dihydrogenphosphoric, sulfuric, monohydrogensulfuric, hydriodic, or phosphorous acids and the like, as well as the salts derived from relatively nontoxic organic acids like acetic, propionic, isobutyric, maleic, malonic, benzoic, succinic, suberic, fumaric, lactic, mandelic, phthalic, benzenesulfonic, p-tolylsulfonic, citric, tartaric, oxalic, methanesulfonic, and the like. Also included are salts of amino acids such as arginate and the like, and salts of organic acids like glucuronic or galactunoric acids and the like (see, for example, Berge et al., “Pharmaceutical Salts”, Thus, the compounds of the present invention may exist as salts, such as with pharmaceutically acceptable acids. The present invention includes such salts. Non-limiting examples of such salts include hydrochlorides, hydrobromides, phosphates, sulfates, methanesulfonates, nitrates, maleates, acetates, citrates, fumarates, proprionates, tartrates (e.g., (+)-tartrates, (−)-tartrates, or mixtures thereof including racemic mixtures), succinates, benzoates, and salts with amino acids such as glutamic acid, and quaternary ammonium salts (e.g. methyl iodide, ethyl iodide, and the like). These salts may be prepared by methods known to those skilled in the art. The neutral forms of the compounds are preferably regenerated by contacting the salt with a base or acid and isolating the parent compound in the conventional manner. The parent form of the compound may differ from the various salt forms in certain physical properties, such as solubility in polar solvents. In addition to salt forms, the present invention provides compounds, which are in a prodrug form. Prodrugs of the compounds described herein are those compounds that readily undergo chemical changes under physiological conditions to provide the compounds of the present invention. Prodrugs of the compounds described herein may be converted in vivo after administration. Additionally, prodrugs can be converted to the compounds of the present invention by chemical or biochemical methods in an ex vivo environment, such as, for example, when contacted with a suitable enzyme or chemical reagent. Certain compounds of the present invention can exist in unsolvated forms as well as solvated forms, including hydrated forms. In general, the solvated forms are equivalent to unsolvated forms and are encompassed within the scope of the present invention. Certain compounds of the present invention may exist in multiple crystalline or amorphous forms. In general, all physical forms are equivalent for the uses contemplated by the present invention and are intended to be within the scope of the present invention. “Pharmaceutically acceptable excipient” and “pharmaceutically acceptable carrier” refer to a substance that aids the administration of an active agent to and absorption by a subject and can be included in the compositions of the present invention without causing a significant adverse toxicological effect on the patient. Non-limiting examples of pharmaceutically acceptable excipients include water, NaCl, normal saline solutions, lactated Ringer's, normal sucrose, normal glucose, binders, fillers, disintegrants, lubricants, coatings, sweeteners, flavors, salt solutions (such as Ringer's solution), alcohols, oils, gelatins, carbohydrates such as lactose, amylose or starch, fatty acid esters, hydroxymethycellulose, polyvinyl pyrrolidine, and colors, and the like. Such preparations can be sterilized and, if desired, mixed with auxiliary agents such as lubricants, preservatives, stabilizers, wetting agents, emulsifiers, salts for influencing osmotic pressure, buffers, coloring, and/or aromatic substances and the like that do not deleteriously react with the compounds of the invention. One of skill in the art will recognize that other pharmaceutical excipients are useful in the present invention. The term “preparation” is intended to include the formulation of the active compound with encapsulating material as a carrier providing a capsule in which the active component with or without other carriers, is surrounded by a carrier, which is thus in association with it. Similarly, cachets and lozenges are included. Tablets, powders, capsules, pills, cachets, and lozenges can be used as solid dosage forms suitable for oral administration. A “Reticulon 4 inhibitor” and “RTN 4 inhibitor” is a substance (e.g., oligonucleotide, protein, composition, or compound) that negatively affects (e.g. decreases) the activity or function of reticulon 4 relative to the activity or function of reticulon 4 in the absence of the inhibitor (e.g., wherein the reticulon 4 inhibitor binds reticulon 4). A “reticulon 4 inhibitor compound” or “RTN 4 inhibitor compound” or “RTN 4 inhibitor compound” refers to a compound (e.g. a compound described herein) that reduces the activity of reticulon 4 when compared to a control, such as absence of the compound or a compound with known inactivity. In embodiments, a Reticulon 4 inhibitor is a compound described herein. The terms “polypeptide,” “peptide” and “protein” are used interchangeably herein to refer to a polymer of amino acid residues, wherein the polymer may optionally be conjugated to a moiety that does not consist of amino acids. The terms apply to amino acid polymers in which one or more amino acid residue is an artificial chemical mimetic of a corresponding naturally occurring amino acid, as well as to naturally occurring amino acid polymers and non-naturally occurring amino acid polymer. A polypeptide, or a cell is “recombinant” when it is artificial or engineered, or derived from or contains an artificial or engineered protein or nucleic acid (e.g. non-natural or not wild type). For example, a polynucleotide that is inserted into a vector or any other heterologous location, e.g., in a genome of a recombinant organism, such that it is not associated with nucleotide sequences that normally flank the polynucleotide as it is found in nature is a recombinant polynucleotide. A protein expressed in vitro or in vivo from a recombinant polynucleotide is an example of a recombinant polypeptide. Likewise, a polynucleotide sequence that does not appear in nature, for example a variant of a naturally occurring gene, is recombinant. An amino acid residue in a protein “corresponds” to a given residue when it occupies the same essential structural and/or spatial position within the protein as the given residue in a reference sequence. For example, a selected residue in a selected protein corresponds to Cys1101 when the selected residue occupies the same essential structural and/or spatial position as Cys1101 in SEQ ID NO:331. In some embodiments, where a selected protein is aligned for maximum homology with the human reticulon 4 protein, the position in the aligned selected protein aligning with Cys1101 is said to correspond to Cys1101. Instead of a primary sequence alignment, a three dimensional structural alignment can also be used, e.g., where the three dimensional structure of the selected protein is aligned for maximum correspondence with the human reticulon 4 protein (reference sequence) and the overall structures compared. In this case, the amino acid that occupies the same essential structural position as Cys1101 in the structural model relative to the reference sequence is said to correspond to the Cys1101 residue. “Contacting” is used in accordance with its plain ordinary meaning and refers to the process of allowing at least two distinct species (e.g. chemical compounds including biomolecules or cells) to become sufficiently proximal to react, interact or physically touch. It should be appreciated; however, the resulting reaction product can be produced directly from a reaction between the added reagents or from an intermediate from one or more of the added reagents that can be produced in the reaction mixture. The term “contacting” may include allowing two species to react, interact, or physically touch, wherein the two species may be a compound as described herein and a protein or enzyme. In some embodiments contacting includes allowing a compound described herein to interact with a protein or enzyme that is involved in a signaling pathway. As defined herein, the term “activation”, “activate”, “activating” and the like in reference to a protein-inhibitor interaction means positively affecting (e.g. increasing) the activity or function of the protein relative to the activity or function of the protein in the absence of the activator. In embodiments activation means positively affecting (e.g. increasing) the concentration or levels of the protein relative to the concentration or level of the protein in the absence of the activator. The terms may reference activation, or activating, sensitizing, or up-regulating signal transduction or enzymatic activity or the amount of a protein decreased in a disease. As defined herein, the term “inhibition”, “inhibitor”, “inhibit”, “inhibiting” and the like in reference to a protein-inhibitor interaction means negatively affecting (e.g. decreasing) the activity or function of the protein relative to the activity or function of the protein in the absence of the inhibitor. In embodiments inhibition means negatively affecting (e.g. decreasing) the concentration or levels of the protein relative to the concentration or level of the protein in the absence of the inhibitor. In embodiments inhibition refers to reduction of a disease or symptoms of disease. In embodiments, inhibition refers to a reduction in the activity of a particular protein target. Thus, inhibition includes, at least in part, partially or totally blocking stimulation, decreasing, preventing, or delaying activation, or inactivating, desensitizing, or down-regulating signal transduction or enzymatic activity or the amount of a protein. In embodiments, inhibition refers to a reduction of activity of a target protein resulting from a direct interaction (e.g. an inhibitor binds to the target protein). In embodiments, inhibition refers to a reduction of activity of a target protein from an indirect interaction (e.g. an inhibitor binds to a protein that activates the target protein, thereby preventing target protein activation). The terms “reticulon 4” and “RTN 4” and “RTN4” refer to a protein (including homologs, isoforms, and functional fragments thereof) with reticulon 4 activity. The term includes any recombinant or naturally-occurring form of reticulon 4 or variants thereof that maintain reticulon 4 activity (e.g. within at least 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, or 100% activity compared to wildtype reticulon 4). In embodiments, the reticulon 4 protein encoded by the RTN 4 gene has the amino acid sequence set forth in or corresponding to Entrez 57142, UniProt Q9NQC3, or RefSeq (protein) NP_065393. In embodiments, the reticulon 4 gene has the nucleic acid sequence set forth in RefSeq (mRNA) NM_020532. In embodiments, the amino acid sequence or nucleic acid sequence is the sequence known at the time of filing of the present application. In embodiments, the sequence corresponds to NP_065393.1. In embodiments, the sequence corresponds to NM_020532.4. In embodiments, the reticulon 4 is a human reticulon 4, such as a human cancer causing reticulon 4. In embodiments, the RTN4 sequence corresponds to UniProt ID Q9NQC3, and has the sequence: In embodiments, the RTN4 sequence corresponds to UniProt ID Q6JRV0, and has the following sequence: As observed in As observed in As observed in As observed in As observed in As observed in As observed in As observed in As observed in As observed in As observed in The term “expression” includes any step involved in the production of the polypeptide including, but not limited to, transcription, post-transcriptional modification, translation, post-translational modification, and secretion. Expression can be detected using conventional techniques for detecting protein (e.g., ELISA, Western blotting, flow cytometry, immunofluorescence, immunohistochemistry, etc.). The terms “disease” or “condition” refer to a state of being or health status of a patient or subject capable of being treated with the compounds or methods provided herein. The disease may be a cancer. The disease may be stroke. The disease may be an inflammatory disease. In some further instances, “cancer” refers to human cancers and carcinomas, sarcomas, adenocarcinomas, lymphomas, leukemias, etc., including solid and lymphoid cancers, kidney, breast, lung, bladder, colon, ovarian, prostate, pancreas, stomach, brain, head and neck, skin, uterine, testicular, glioma, esophagus, and liver cancer, including hepatocarcinoma, lymphoma, including B-acute lymphoblastic lymphoma, non-Hodgkin's lymphomas (e.g., Burkitt's, Small Cell, and Large Cell lymphomas), Hodgkin's lymphoma, leukemia (including AML, ALL, and CML), or multiple myeloma. As used herein, the term “cancer” refers to all types of cancer, neoplasm or malignant tumors found in mammals (e.g. humans), including leukemia, carcinomas and sarcomas. Exemplary cancers that may be treated with a compound or method provided herein include brain cancer, glioma, glioblastoma, neuroblastoma, prostate cancer, colorectal cancer, pancreatic cancer, cervical cancer, gastric cancer, ovarian cancer, lung cancer, and cancer of the head. Exemplary cancers that may be treated with a compound or method provided herein include cancer of the thyroid, endocrine system, brain, breast, cervix, colon, head & neck, liver, kidney, lung, non-small cell lung, melanoma, mesothelioma, ovary, sarcoma, stomach, uterus, Medulloblastoma, colorectal cancer, pancreatic cancer. Additional examples include, Hodgkin's Disease, Non-Hodgkin's Lymphoma, multiple myeloma, neuroblastoma, glioma, glioblastoma multiforme, ovarian cancer, rhabdomyosarcoma, primary thrombocytosis, primary macroglobulinemia, primary brain tumors, cancer, malignant pancreatic insulanoma, malignant carcinoid, urinary bladder cancer, premalignant skin lesions, testicular cancer, lymphomas, thyroid cancer, neuroblastoma, esophageal cancer, genitourinary tract cancer, malignant hypercalcemia, endometrial cancer, adrenal cortical cancer, neoplasms of the endocrine or exocrine pancreas, medullary thyroid cancer, medullary thyroid carcinoma, melanoma, colorectal cancer, papillary thyroid cancer, hepatocellular carcinoma, or prostate cancer. The term “leukemia” refers broadly to progressive, malignant diseases of the blood-forming organs and is generally characterized by a distorted proliferation and development of leukocytes and their precursors in the blood and bone marrow. Leukemia is generally clinically classified on the basis of (1) the duration and character of the disease-acute or chronic; (2) the type of cell involved; myeloid (myelogenous), lymphoid (lymphogenous), or monocytic; and (3) the increase or non-increase in the number abnormal cells in the blood-leukemic or aleukemic (subleukemic). Exemplary leukemias that may be treated with a compound or method provided herein include, for example, acute nonlymphocytic leukemia, chronic lymphocytic leukemia, acute granulocytic leukemia, chronic granulocytic leukemia, acute promyelocytic leukemia, adult T-cell leukemia, aleukemic leukemia, a leukocythemic leukemia, basophylic leukemia, blast cell leukemia, bovine leukemia, chronic myelocytic leukemia, leukemia cutis, embryonal leukemia, eosinophilic leukemia, Gross' leukemia, hairy-cell leukemia, hemoblastic leukemia, hemocytoblastic leukemia, histiocytic leukemia, stem cell leukemia, acute monocytic leukemia, leukopenic leukemia, lymphatic leukemia, lymphoblastic leukemia, lymphocytic leukemia, lymphogenous leukemia, lymphoid leukemia, lymphosarcoma cell leukemia, mast cell leukemia, megakaryocytic leukemia, micromyeloblastic leukemia, monocytic leukemia, myeloblastic leukemia, myelocytic leukemia, myeloid granulocytic leukemia, myelomonocytic leukemia, Naegeli leukemia, plasma cell leukemia, multiple myeloma, plasmacytic leukemia, promyelocytic leukemia, Rieder cell leukemia, Schilling's leukemia, stem cell leukemia, subleukemic leukemia, or undifferentiated cell leukemia. The term “sarcoma” generally refers to a tumor which is made up of a substance like the embryonic connective tissue and is generally composed of closely packed cells embedded in a fibrillar or homogeneous substance. Sarcomas that may be treated with a compound or method provided herein include a chondrosarcoma, fibrosarcoma, lymphosarcoma, melanosarcoma, myxosarcoma, osteosarcoma, Abemethy's sarcoma, adipose sarcoma, liposarcoma, alveolar soft part sarcoma, ameloblastic sarcoma, botryoid sarcoma, chloroma sarcoma, chorio carcinoma, embryonal sarcoma, Wilms' tumor sarcoma, endometrial sarcoma, stromal sarcoma, Ewing's sarcoma, fascial sarcoma, fibroblastic sarcoma, giant cell sarcoma, granulocytic sarcoma, Hodgkin's sarcoma, idiopathic multiple pigmented hemorrhagic sarcoma, immunoblastic sarcoma of B cells, lymphoma, immunoblastic sarcoma of T-cells, Jensen's sarcoma, Kaposi's sarcoma, Kupffer cell sarcoma, angiosarcoma, leukosarcoma, malignant mesenchymoma sarcoma, parosteal sarcoma, reticulocytic sarcoma, Rous sarcoma, serocystic sarcoma, synovial sarcoma, or telangiectaltic sarcoma. The term “melanoma” is taken to mean a tumor arising from the melanocytic system of the skin and other organs. Melanomas that may be treated with a compound or method provided herein include, for example, acral-lentiginous melanoma, amelanotic melanoma, benign juvenile melanoma, Cloudman's melanoma, S91 melanoma, Harding-Passey melanoma, juvenile melanoma, lentigo maligna melanoma, malignant melanoma, nodular melanoma, subungal melanoma, or superficial spreading melanoma. The term “carcinoma” refers to a malignant new growth made up of epithelial cells tending to infiltrate the surrounding tissues and give rise to metastases. Exemplary carcinomas that may be treated with a compound or method provided herein include, for example, medullary thyroid carcinoma, familial medullary thyroid carcinoma, acinar carcinoma, acinous carcinoma, adenocystic carcinoma, adenoid cystic carcinoma, carcinoma adenomatosum, carcinoma of adrenal cortex, alveolar carcinoma, alveolar cell carcinoma, basal cell carcinoma, carcinoma basocellulare, basaloid carcinoma, basosquamous cell carcinoma, bronchioalveolar carcinoma, bronchiolar carcinoma, bronchogenic carcinoma, cerebriform carcinoma, cholangiocellular carcinoma, chorionic carcinoma, colloid carcinoma, comedo carcinoma, corpus carcinoma, cribriform carcinoma, carcinoma en cuirasse, carcinoma cutaneum, cylindrical carcinoma, cylindrical cell carcinoma, duct carcinoma, carcinoma durum, embryonal carcinoma, encephaloid carcinoma, epiermoid carcinoma, carcinoma epitheliale adenoides, exophytic carcinoma, carcinoma ex ulcere, carcinoma fibrosum, gelatiniforni carcinoma, gelatinous carcinoma, giant cell carcinoma, carcinoma gigantocellulare, glandular carcinoma, granulosa cell carcinoma, hair-matrix carcinoma, hematoid carcinoma, hepatocellular carcinoma, Hurthle cell carcinoma, hyaline carcinoma, hypernephroid carcinoma, infantile embryonal carcinoma, carcinoma in situ, intraepidermal carcinoma, intraepithelial carcinoma, Krompecher's carcinoma, Kulchitzky-cell carcinoma, large-cell carcinoma, lenticular carcinoma, carcinoma lenticulare, lipomatous carcinoma, lymphoepithelial carcinoma, carcinoma medullare, medullary carcinoma, melanotic carcinoma, carcinoma molle, mucinous carcinoma, carcinoma muciparum, carcinoma mucocellulare, mucoepidermoid carcinoma, carcinoma mucosum, mucous carcinoma, carcinoma myxomatodes, nasopharyngeal carcinoma, oat cell carcinoma, carcinoma ossificans, osteoid carcinoma, papillary carcinoma, periportal carcinoma, preinvasive carcinoma, prickle cell carcinoma, pultaceous carcinoma, renal cell carcinoma of kidney, reserve cell carcinoma, carcinoma sarcomatodes, schneiderian carcinoma, scirrhous carcinoma, carcinoma scroti, signet-ring cell carcinoma, carcinoma simplex, small-cell carcinoma, solanoid carcinoma, spheroidal cell carcinoma, spindle cell carcinoma, carcinoma spongiosum, squamous carcinoma, squamous cell carcinoma, string carcinoma, carcinoma telangiectaticum, carcinoma telangiectodes, transitional cell carcinoma, carcinoma tuberosum, tuberous carcinoma, verrucous carcinoma, or carcinoma villosum. As used herein, the term “neurodegenerative disease” refers to a disease or condition in which the function of a subject's nervous system becomes impaired. Examples of neurodegenerative diseases that may be treated with a compound, pharmaceutical composition, or method described herein include Alexander's disease, Alper's disease, Alzheimer's disease, Amyotrophic lateral sclerosis, Ataxia telangiectasia, Batten disease (also known as Spielmeyer-Vogt-Sjogren-Batten disease), Bovine spongiform encephalopathy (BSE), Canavan disease, Cockayne syndrome, Corticobasal degeneration, Creutzfeldt-Jakob disease, frontotemporal dementia, Gerstmann-Strussler-Scheinker syndrome, Huntington's disease, HIV-associated dementia, Kennedy's disease, Krabbe's disease, kuru, Lewy body dementia, Machado-Joseph disease (Spinocerebellar ataxia type 3), Multiple sclerosis, Multiple System Atrophy, Narcolepsy, Neuroborreliosis, Parkinson's disease, Pelizaeus-Merzbacher Disease, Pick's disease, Primary lateral sclerosis, Prion diseases, Refsum's disease, Sandhoff s disease, Schilder's disease, Subacute combined degeneration of spinal cord secondary to Pernicious Anaemia, Schizophrenia, Spinocerebellar ataxia (multiple types with varying characteristics), Spinal muscular atrophy, Steele-Richardson-Olszewski disease, progressive supranuclear palsy, or Tabes dorsalis. The terms “treating”, or “treatment” refers to any indicia of success in the therapy or amelioration of an injury, disease, pathology or condition, including any objective or subjective parameter such as abatement; remission; diminishing of symptoms or making the injury, pathology or condition more tolerable to the patient; slowing in the rate of degeneration or decline; making the final point of degeneration less debilitating; improving a patient's physical or mental well-being. The treatment or amelioration of symptoms can be based on objective or subjective parameters; including the results of a physical examination, neuropsychiatric exams, and/or a psychiatric evaluation. The term “treating” and conjugations thereof, may include prevention of an injury, pathology, condition, or disease. In embodiments, treating is preventing. In embodiments, treating does not include preventing. In embodiments, the treating or treatment is no prophylactic treatment. “Patient” or “subject in need thereof” refers to a living organism suffering from or prone to a disease or condition that can be treated by administration of a pharmaceutical composition as provided herein. Non-limiting examples include humans, other mammals, bovines, rats, mice, dogs, monkeys, goat, sheep, cows, deer, and other non-mammalian animals. In some embodiments, a patient is human. A “effective amount” is an amount sufficient for a compound to accomplish a stated purpose relative to the absence of the compound (e.g. achieve the effect for which it is administered, treat a disease, reduce enzyme activity, increase enzyme activity, reduce a signaling pathway, or reduce one or more symptoms of a disease or condition). An example of an “effective amount” is an amount sufficient to contribute to the treatment, prevention, or reduction of a symptom or symptoms of a disease, which could also be referred to as a “therapeutically effective amount.” A “reduction” of a symptom or symptoms (and grammatical equivalents of this phrase) means decreasing of the severity or frequency of the symptom(s), or elimination of the symptom(s). A “prophylactically effective amount” of a drug is an amount of a drug that, when administered to a subject, will have the intended prophylactic effect, e.g., preventing or delaying the onset (or reoccurrence) of an injury, disease, pathology or condition, or reducing the likelihood of the onset (or reoccurrence) of an injury, disease, pathology, or condition, or their symptoms. The full prophylactic effect does not necessarily occur by administration of one dose, and may occur only after administration of a series of doses. Thus, a prophylactically effective amount may be administered in one or more administrations. An “activity decreasing amount,” as used herein, refers to an amount of antagonist required to decrease the activity of an enzyme relative to the absence of the antagonist. A “function disrupting amount,” as used herein, refers to the amount of antagonist required to disrupt the function of an enzyme or protein relative to the absence of the antagonist. The exact amounts will depend on the purpose of the treatment, and will be ascertainable by one skilled in the art using known techniques (see, e.g., Lieberman, For any compound described herein, the therapeutically effective amount can be initially determined from cell culture assays. Target concentrations will be those concentrations of active compound(s) that are capable of achieving the methods described herein, as measured using the methods described herein or known in the art. As is well known in the art, therapeutically effective amounts for use in humans can also be determined from animal models. For example, a dose for humans can be formulated to achieve a concentration that has been found to be effective in animals. The dosage in humans can be adjusted by monitoring compounds effectiveness and adjusting the dosage upwards or downwards, as described above. Adjusting the dose to achieve maximal efficacy in humans based on the methods described above and other methods is well within the capabilities of the ordinarily skilled artisan. Dosages may be varied depending upon the requirements of the patient and the compound being employed. The dose administered to a patient, in the context of the present invention should be sufficient to effect a beneficial therapeutic response in the patient over time. The size of the dose also will be determined by the existence, nature, and extent of any adverse side-effects. Determination of the proper dosage for a particular situation is within the skill of the practitioner. Generally, treatment is initiated with smaller dosages which are less than the optimum dose of the compound. Thereafter, the dosage is increased by small increments until the optimum effect under circumstances is reached. Dosage amounts and intervals can be adjusted individually to provide levels of the administered compound effective for the particular clinical indication being treated. This will provide a therapeutic regimen that is commensurate with the severity of the individual's disease state. As used herein, the term “administering” means oral administration, administration as a suppository, topical contact, intravenous, intraperitoneal, intramuscular, intralesional, intrathecal, intranasal or subcutaneous administration, or the implantation of a slow-release device, e.g., a mini-osmotic pump, to a subject. Administration is by any route, including parenteral and transmucosal (e.g., buccal, sublingual, palatal, gingival, nasal, vaginal, rectal, or transdermal) compatible with the preparation. Parenteral administration includes, e.g., intravenous, intramuscular, intra-arteriole, intradermal, subcutaneous, intraperitoneal, intraventricular, and intracranial. Other modes of delivery include, but are not limited to, the use of liposomal formulations, intravenous infusion, transdermal patches, etc. In embodiments, the administering does not include administration of any active agent other than the recited active agent. “Co-administer” it is meant that a composition described herein is administered at the same time, just prior to, or just after the administration of one or more additional therapies. The compounds of the invention can be administered alone or can be coadministered to the patient. Coadministration is meant to include simultaneous or sequential administration of the compounds individually or in combination (more than one compound). Thus, the preparations can also be combined, when desired, with other active substances (e.g. to reduce metabolic degradation). The compositions of the present invention can be delivered transdermally, by a topical route, or formulated as applicator sticks, solutions, suspensions, emulsions, gels, creams, ointments, pastes, jellies, paints, powders, and aerosols. A “cell” as used herein, refers to a cell carrying out metabolic or other function sufficient to preserve or replicate its genomic DNA. A cell can be identified by well-known methods in the art including, for example, presence of an intact membrane, staining by a particular dye, ability to produce progeny or, in the case of a gamete, ability to combine with a second gamete to produce a viable offspring. Cells may include prokaryotic and eukaroytic cells. Prokaryotic cells include but are not limited to bacteria. Eukaryotic cells include but are not limited to yeast cells and cells derived from plants and animals, for example mammalian, insect (e.g., spodoptera) and human cells. Cells may be useful when they are naturally nonadherent or have been treated not to adhere to surfaces, for example by trypsinization. “Control” or “control experiment” is used in accordance with its plain ordinary meaning and refers to an experiment in which the subjects or reagents of the experiment are treated as in a parallel experiment except for omission of a procedure, reagent, or variable of the experiment. In some instances, the control is used as a standard of comparison in evaluating experimental effects. In some embodiments, a control is the measurement of the activity of a protein in the absence of a compound as described herein (including embodiments and examples). The term “modulator” refers to a composition that increases or decreases the level of a target molecule or the function of a target molecule or the physical state of the target of the molecule. In some embodiments, a reticulon 4 associated disease modulator is a compound that reduces the severity of one or more symptoms of a disease associated with reticulon 4 (e.g. cancer). A reticulon 4 modulator is a compound that increases or decreases the activity or function or level of activity or level of function of reticulon 4. The term “modulate” is used in accordance with its plain ordinary meaning and refers to the act of changing or varying one or more properties. “Modulation” refers to the process of changing or varying one or more properties. For example, as applied to the effects of a modulator on a target protein, to modulate means to change by increasing or decreasing a property or function of the target molecule or the amount of the target molecule. The term “associated” or “associated with” in the context of a substance or substance activity or function associated with a disease (e.g. a protein associated disease, a cancer associated with reticulon 4 activity, reticulon 4 associated cancer, reticulon 4 associated disease) means that the disease (e.g. cancer) is caused by (in whole or in part), or a symptom of the disease is caused by (in whole or inpart) the substance or substance activity or function. For example, a cancer associated with reticulon 4 activity or function may be a cancer that results (entirely or partially) from aberrant reticulon 4 function (e.g. enzyme activity, protein-protein interaction, signaling pathway) or a cancer wherein a particular symptom of the disease is caused (entirely or partially) by aberrant reticulon 4 activity or function. As used herein, what is described as being associated with a disease, if a causative agent, could be a target for treatment of the disease. For example, a cancer associated with reticulon 4 activity or function or a reticulon 4 associated cancer, may be treated with a reticulon 4 modulator or reticulon 4 inhibitor, in the instance where reticulon 4 activity or function (e.g. signaling pathway activity) causes the cancer. The term “aberrant” as used herein refers to different from normal. When used to describe enzymatic activity or protein function, aberrant refers to activity or function that is greater or less than a normal control or the average of normal non-diseased control samples. Aberrant activity may refer to an amount of activity that results in a disease, wherein returning the aberrant activity to a normal or non-disease-associated amount (e.g. by administering a compound or using a method as described herein), results in reduction of the disease or one or more disease symptoms. The term “signaling pathway” as used herein refers to a series of interactions between cellular and optionally extra-cellular components (e.g. proteins, nucleic acids, small molecules, ions, lipids) that conveys a change in one component to one or more other components, which in turn may convey a change to additional components, which is optionally propogated to other signaling pathway components. For example, binding of a reticulon 4 protein with a compound as described herein may reduce the interactions between the reticulon 4 protein and downstream effectors or signaling pathway components, resulting in changes in cell growth, proliferation, or survival. The term “electrophilic chemical moiety” is used in accordance with its plain ordinary chemical meaning and refers to a chemical group (e.g., monovalent chemical group) that is electrophilic. The term “nucleophilic chemical moiety” is used in accordance with its plain ordinary chemical meaning and refers to a chemical group (e.g., monovalent chemical group) that is nucleophilic. “Nucleic acid” refers to nucleotides (e.g., deoxyribonucleotides or ribonucleotides) and polymers thereof in either single-, double- or multiple-stranded form, or complements thereof. The terms “polynucleotide,” “oligonucleotide,” “oligo” or the like refer, in the usual and customary sense, to a linear sequence of nucleotides. The term “nucleotide” refers, in the usual and customary sense, to a single unit of a polynucleotide, i.e., a monomer. Nucleotides can be ribonucleotides, deoxyribonucleotides, or modified versions thereof. Examples of polynucleotides contemplated herein include single and double stranded DNA, single and double stranded RNA, and hybrid molecules having mixtures of single and double stranded DNA and RNA. Examples of nucleic acid, e.g. polynucleotides contemplated herein include any types of RNA, e.g. mRNA, siRNA, miRNA, and guide RNA and any types of DNA, genomic DNA, plasmid DNA, and minicircle DNA, and any fragments thereof. The term “duplex” in the context of polynucleotides refers, in the usual and customary sense, to double strandedness. Nucleic acids can be linear or branched. For example, nucleic acids can be a linear chain of nucleotides or the nucleic acids can be branched, e.g., such that the nucleic acids comprise one or more arms or branches of nucleotides. Optionally, the branched nucleic acids are repetitively branched to form higher ordered structures such as dendrimers and the like. Nucleic acids, including e.g., nucleic acids with a phosphothioate backbone, can include one or more reactive moieties. As used herein, the term reactive moiety includes any group capable of reacting with another molecule, e.g., a nucleic acid or polypeptide through covalent, non-covalent or other interactions. By way of example, the nucleic acid can include an amino acid reactive moiety that reacts with an amino acid on a protein or polypeptide through a covalent, non-covalent or other interaction. The terms also encompass nucleic acids containing known nucleotide analogs or modified backbone residues or linkages, which are synthetic, naturally occurring, and non-naturally occurring, which have similar binding properties as the reference nucleic acid, and which are metabolized in a manner similar to the reference nucleotides. Examples of such analogs include, include, without limitation, phosphodiester derivatives including, e.g., phosphoramidate, phosphorodiamidate, phosphorothioate (also known as phosphothioate having double bonded sulfur replacing oxygen in the phosphate), phosphorodithioate, phosphonocarboxylic acids, phosphonocarboxylates, phosphonoacetic acid, phosphonoformic acid, methyl phosphonate, boron phosphonate, or O-methylphosphoroamidite linkages (see Eckstein, O Nucleic acids can include nonspecific sequences. As used herein, the term “nonspecific sequence” refers to a nucleic acid sequence that contains a series of residues that are not designed to be complementary to or are only partially complementary to any other nucleic acid sequence. By way of example, a nonspecific nucleic acid sequence is a sequence of nucleic acid residues that does not function as an inhibitory nucleic acid when contacted with a cell or organism. An “antisense nucleic acid” as referred to herein is a nucleic acid (e.g., DNA or RNA molecule) that is complementary to at least a portion of a specific target nucleic acid (e.g., a nucleic acid coding for one or more amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331) and is capable of reducing transcription of the target nucleic acid (e.g. mRNA from DNA), reducing the translation of the target nucleic acid (e.g. mRNA), altering transcript splicing (e.g. single stranded morpholino oligo), or interfering with the endogenous activity of the target nucleic acid. See, e.g., Weintraub, In the cell, the antisense nucleic acids hybridize to the corresponding RNA (e.g., a nucleic acid coding for one or more amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331) forming a double-stranded molecule. The antisense nucleic acids interfere with the endogenous behavior of the RNA (e.g., a nucleic acid coding for one or more amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331) and inhibit its function relative to the absence of the antisense nucleic acid. Furthermore, the double-stranded molecule may be degraded via the RNAi pathway. The use of antisense methods to inhibit the in vitro translation of genes is well known in the art (Marcus-Sakura, The term “complement,” as used herein, refers to a nucleotide (e.g., RNA or DNA) or a sequence of nucleotides capable of base pairing with a complementary nucleotide or sequence of nucleotides. As described herein and commonly known in the art the complementary (matching) nucleotide of adenosine is thymidine and the complementary (matching) nucleotide of guanidine is cytosine. Thus, a complement may include a sequence of nucleotides that base pair with corresponding complementary nucleotides of a second nucleic acid sequence. The nucleotides of a complement may partially or completely match the nucleotides of the second nucleic acid sequence. Where the nucleotides of the complement completely match each nucleotide of the second nucleic acid sequence, the complement forms base pairs with each nucleotide of the second nucleic acid sequence. Where the nucleotides of the complement partially match the nucleotides of the second nucleic acid sequence only some of the nucleotides of the complement form base pairs with nucleotides of the second nucleic acid sequence. Examples of complementary sequences include coding and a non-coding sequences, wherein the non-coding sequence contains complementary nucleotides to the coding sequence and thus forms the complement of the coding sequence. A further example of complementary sequences are sense and antisense sequences, wherein the sense sequence contains complementary nucleotides to the antisense sequence and thus forms the complement of the antisense sequence. As described herein the complementarity of sequences may be partial, in which only some of the nucleic acids match according to base pairing, or complete, where all the nucleic acids match according to base pairing. Thus, two sequences that are complementary to each other, may have a specified percentage of nucleotides that are the same (i.e., about 60% identity, preferably 65%, 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, or higher identity over a specified region). The term “antibody” refers to a polypeptide encoded by an immunoglobulin gene or functional fragments thereof that specifically binds and recognizes an antigen. The recognized immunoglobulin genes include the kappa, lambda, alpha, gamma, delta, epsilon, and mu constant region genes, as well as the myriad immunoglobulin variable region genes. Light chains are classified as either kappa or lambda. Heavy chains are classified as gamma, mu, alpha, delta, or epsilon, which in turn define the immunoglobulin classes, IgG, IgM, IgA, IgD and IgE, respectively. An exemplary immunoglobulin (antibody) structural unit comprises a tetramer. Each tetramer is composed of two identical pairs of polypeptide chains, each pair having one “light” (about 25 kDa) and one “heavy” chain (about 50-70 kDa). The N-terminus of each chain defines a variable region of about 100 to 110 or more amino acids primarily responsible for antigen recognition. The terms “variable heavy chain,” “VH,” or “VH” refer to the variable region of an immunoglobulin heavy chain, including an Fv, scFv, dsFv or Fab; while the terms “variable light chain,” “VL” or “VL” refer to the variable region of an immunoglobulin light chain, including of an Fv, scFv, dsFv or Fab. Examples of antibody functional fragments include, but are not limited to, complete antibody molecules, antibody fragments, such as Fv, single chain Fv (scFv), complementarity determining regions (CDRs), VL (light chain variable region), VH (heavy chain variable region), Fab, F(ab)2′ and any combination of those or any other functional portion of an immunoglobulin peptide capable of binding to target antigen (see, e.g., F “Percentage of sequence identity” is determined by comparing two optimally aligned sequences over a comparison window, wherein the portion of the polynucleotide or polypeptide sequence in the comparison window may comprise additions or deletions (i.e., gaps) as compared to the reference sequence (which does not comprise additions or deletions) for optimal alignment of the two sequences. The percentage is calculated by determining the number of positions at which the identical nucleic acid base or amino acid residue occurs in both sequences to yield the number of matched positions, dividing the number of matched positions by the total number of positions in the window of comparison and multiplying the result by 100 to yield the percentage of sequence identity. The terms “identical” or percent “identity,” in the context of two or more nucleic acids or polypeptide sequences, refer to two or more sequences or subsequences that are the same or have a specified percentage of amino acid residues or nucleotides that are the same (i.e., about 60% identity, preferably 65%, 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, or higher identity over a specified region, when compared and aligned for maximum correspondence over a comparison window or designated region) as measured using a BLAST or BLAST 2.0 sequence comparison algorithms with default parameters described below, or by manual alignment and visual inspection (see, e.g., NCBI web site http://www.ncbi.nlm.nih.gov/BLAST/ or the like). Such sequences are then said to be “substantially identical.” This definition also refers to, or may be applied to, the compliment of a test sequence. The definition also includes sequences that have deletions and/or additions, as well as those that have substitutions. As described below, the preferred algorithms can account for gaps and the like. Preferably, identity exists over a region that is at least about 25 amino acids or nucleotides in length, or more preferably over a region that is 50-100 amino acids or nucleotides in length. The term “irreversible covalent bond” is used in accordance with its plain ordinary meaning in the art and refers to the resulting association between atoms or molecules of (e.g., electrophilic chemical moiety and nucleophilic moiety) wherein the probability of dissociation is low. In embodiments, the irreversible covalent bond does not easily dissociate under normal biological conditions. In embodiments, the irreversible covalent bond is formed through a chemical reaction between two species (e.g., electrophilic chemical moiety and nucleophilic moiety). “Anti-cancer agent” and “anticancer agent” are used in accordance with their plain ordinary meaning and refers to a composition (e.g. compound, drug, antagonist, inhibitor, modulator) having antineoplastic properties or the ability to inhibit the growth or proliferation of cells. In some embodiments, an anti-cancer agent is a chemotherapeutic. In some embodiments, an anti-cancer agent is an agent identified herein having utility in methods of treating cancer. In some embodiments, an anti-cancer agent is an agent approved by the FDA or similar regulatory agency of a country other than the USA, for treating cancer. Examples of anti-cancer agents include, but are not limited to, MEK (e.g. MEK1, MEK2, or MEK1 and MEK2) inhibitors (e.g. XL518, CI-1040, PD035901, selumetinib/AZD6244, GSK1120212/trametinib, GDC-0973, ARRY-162, ARRY-300, AZD8330, PD0325901, U0126, PD98059, TAK-733, PD318088, AS703026, BAY 869766), alkylating agents (e.g., cyclophosphamide, ifosfamide, chlorambucil, busulfan, melphalan, mechlorethamine, uramustine, thiotepa, nitrosoureas, nitrogen mustards (e.g., mechloroethamine, cyclophosphamide, chlorambucil, meiphalan), ethylenimine and methylmelamines (e.g., hexamethlymelamine, thiotepa), alkyl sulfonates (e.g., busulfan), nitrosoureas (e.g., carmustine, lomusitne, semustine, streptozocin), triazenes (decarbazine)), anti-metabolites (e.g., 5-azathioprine, leucovorin, capecitabine, fludarabine, gemcitabine, pemetrexed, raltitrexed, folic acid analog (e.g., methotrexate), or pyrimidine analogs (e.g., fluorouracil, floxouridine, Cytarabine), purine analogs (e.g., mercaptopurine, thioguanine, pentostatin), etc.), plant alkaloids (e.g., vincristine, vinblastine, vinorelbine, vindesine, podophyllotoxin, paclitaxel, docetaxel, etc.), topoisomerase inhibitors (e.g., irinotecan, topotecan, amsacrine, etoposide (VP16), etoposide phosphate, teniposide, etc.), antitumor antibiotics (e.g., doxorubicin, adriamycin, daunorubicin, epirubicin, actinomycin, bleomycin, mitomycin, mitoxantrone, plicamycin, etc.), platinum-based compounds (e.g. cisplatin, oxaloplatin, carboplatin), anthracenedione (e.g., mitoxantrone), substituted urea (e.g., hydroxyurea), methyl hydrazine derivative (e.g., procarbazine), adrenocortical suppressant (e.g., mitotane, aminoglutethimide), epipodophyllotoxins (e.g., etoposide), antibiotics (e.g., daunorubicin, doxorubicin, bleomycin), enzymes (e.g., L-asparaginase), inhibitors of mitogen-activated protein kinase signaling (e.g. U0126, PD98059, PD184352, PD0325901, ARRY-142886, SB239063, SP600125, BAY 43-9006, wortmannin, or LY294002, Syk inhibitors, mTOR inhibitors, antibodies (e.g., rituxan), gossyphol, genasense, polyphenol E, Chlorofusin, all trans-retinoic acid (ATRA), bryostatin, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), 5-aza-2′-deoxycytidine, all trans retinoic acid, doxorubicin, vincristine, etoposide, gemcitabine, imatinib (Gleevec®), geldanamycin, 17-N-Allylamino-17-Demethoxygeldanamycin (17-AAG), flavopiridol, LY294002, bortezomib, trastuzumab, BAY 11-7082, PKC412, PD184352, 20-epi-1, 25 dihydroxyvitamin D3; 5-ethynyluracil; abiraterone; aclarubicin; acylfulvene; adecypenol; adozelesin; aldesleukin; ALL-TK antagonists; altretamine; ambamustine; amidox; amifostine; aminolevulinic acid; amrubicin; amsacrine; anagrelide; anastrozole; andrographolide; angiogenesis inhibitors; antagonist D; antagonist G; antarelix; anti-dorsalizing morphogenetic protein-1; antiandrogen, prostatic carcinoma; antiestrogen; antineoplaston; antisense oligonucleotides; aphidicolin glycinate; apoptosis gene modulators; apoptosis regulators; apurinic acid; ara-CDP-DL-PTBA; arginine deaminase; asulacrine; atamestane; atrimustine; axinastatin 1; axinastatin 2; axinastatin 3; azasetron; azatoxin; azatyrosine; baccatin III derivatives; balanol; batimastat; BCR/ABL antagonists; benzochlorins; benzoylstaurosporine; beta lactam derivatives; beta-alethine; betaclamycin B; betulinic acid; bFGF inhibitor; bicalutamide; bisantrene; bisaziridinylspermine; bisnafide; bistratene A; bizelesin; breflate; bropirimine; budotitane; buthionine sulfoximine; calcipotriol; calphostin C; camptothecin derivatives; canarypox IL-2; capecitabine; carboxamide-amino-triazole; carboxyamidotriazole; CaRest M3; CARN 700; cartilage derived inhibitor; carzelesin; casein kinase inhibitors (ICOS); castanospermine; cecropin B; cetrorelix; chlorins; chloroquinoxaline sulfonamide; cicaprost; cis-porphyrin; cladribine; clomifene analogues; clotrimazole; collismycin A; collismycin B; combretastatin A4; combretastatin analogue; conagenin; crambescidin 816; crisnatol; cryptophycin 8; cryptophycin A derivatives; curacin A; cyclopentanthraquinones; cycloplatam; cypemycin; cytarabine ocfosfate; cytolytic factor; cytostatin; dacliximab; decitabine; dehydrodidemnin B; deslorelin; dexamethasone; dexifosfamide; dexrazoxane; dexverapamil; diaziquone; didemnin B; didox; diethylnorspermine; dihydro-5-azacytidine; 9-dioxamycin; diphenyl spiromustine; docosanol; dolasetron; doxifluridine; droloxifene; dronabinol; duocarmycin SA; ebselen; ecomustine; edelfosine; edrecolomab; eflornithine; elemene; emitefur; epirubicin; epristeride; estramustine analogue; estrogen agonists; estrogen antagonists; etanidazole; etoposide phosphate; exemestane; fadrozole; fazarabine; fenretinide; filgrastim; finasteride; flavopiridol; flezelastine; fluasterone; fludarabine; fluorodaunorunicin hydrochloride; forfenimex; formestane; fostriecin; fotemustine; gadolinium texaphyrin; gallium nitrate; galocitabine; ganirelix; gelatinase inhibitors; gemcitabine; glutathione inhibitors; hepsulfam; heregulin; hexamethylene bisacetamide; hypericin; ibandronic acid; idarubicin; idoxifene; idramantone; ilmofosine; ilomastat; imidazoacridones; imiquimod; immunostimulant peptides; insulin-like growth factor-1 receptor inhibitor; interferon agonists; interferons; interleukins; iobenguane; iododoxorubicin; ipomeanol, 4-; iroplact; irsogladine; isobengazole; isohomohalicondrin B; itasetron; jasplakinolide; kahalalide F; lamellarin-N triacetate; lanreotide; leinamycin; lenograstim; lentinan sulfate; leptolstatin; letrozole; leukemia inhibiting factor; leukocyte alpha interferon; leuprolide+estrogen+progesterone; leuprorelin; levamisole; liarozole; linear polyamine analogue; lipophilic disaccharide peptide; lipophilic platinum compounds; lissoclinamide 7; lobaplatin; lombricine; lometrexol; lonidamine; losoxantrone; lovastatin; loxoribine; lurtotecan; lutetium texaphyrin; lysofylline; lytic peptides; maitansine; mannostatin A; marimastat; masoprocol; maspin; matrilysin inhibitors; matrix metalloproteinase inhibitors; menogaril; merbarone; meterelin; methioninase; metoclopramide; MIF inhibitor; mifepristone; miltefosine; mirimostim; mismatched double stranded RNA; mitoguazone; mitolactol; mitomycin analogues; mitonafide; mitotoxin fibroblast growth factor-saporin; mitoxantrone; mofarotene; molgramostim; monoclonal antibody, human chorionic gonadotrophin; monophosphoryl lipid A+myobacterium cell wall sk; mopidamol; multiple drug resistance gene inhibitor; multiple tumor suppressor 1-based therapy; mustard anticancer agent; mycaperoxide B; mycobacterial cell wall extract; myriaporone; N-acetyldinaline; N-substituted benzamides; nafarelin; nagrestip; naloxone+pentazocine; napavin; naphterpin; nartograstim; nedaplatin; nemorubicin; neridronic acid; neutral endopeptidase; nilutamide; nisamycin; nitric oxide modulators; nitroxide antioxidant; nitrullyn; O6-benzylguanine; octreotide; okicenone; oligonucleotides; onapristone; ondansetron; ondansetron; oracin; oral cytokine inducer; ormaplatin; osaterone; oxaliplatin; oxaunomycin; palauamine; palmitoylrhizoxin; pamidronic acid; panaxytriol; panomifene; parabactin; pazelliptine; pegaspargase; peldesine; pentosan polysulfate sodium; pentostatin; pentrozole; perflubron; perfosfamide; perillyl alcohol; phenazinomycin; phenylacetate; phosphatase inhibitors; picibanil; pilocarpine hydrochloride; pirarubicin; piritrexim; placetin A; placetin B; plasminogen activator inhibitor; platinum complex; platinum compounds; platinum-triamine complex; porfimer sodium; porfiromycin; prednisone; propyl bis-acridone; prostaglandin J2; proteasome inhibitors; protein A-based immune modulator; protein kinase C inhibitor; protein kinase C inhibitors, microalgal; protein tyrosine phosphatase inhibitors; purine nucleoside phosphorylase inhibitors; purpurins; pyrazoloacridine; pyridoxylated hemoglobin polyoxyethylerie conjugate; raf antagonists; raltitrexed; ramosetron; ras farnesyl protein transferase inhibitors; ras inhibitors; ras-GAP inhibitor; retelliptine demethylated; rhenium Re 186 etidronate; rhizoxin; ribozymes; RII retinamide; rogletimide; rohitukine; romurtide; roquinimex; rubiginone B1; ruboxyl; safingol; saintopin; SarCNU; sarcophytol A; sargramostim; Sdi 1 mimetics; semustine; senescence derived inhibitor 1; sense oligonucleotides; signal transduction inhibitors; signal transduction modulators; single chain antigen-binding protein; sizofuran; sobuzoxane; sodium borocaptate; sodium phenylacetate; solverol; somatomedin binding protein; sonermin; sparfosic acid; spicamycin D; spiromustine; splenopentin; spongistatin 1; squalamine; stem cell inhibitor; stem-cell division inhibitors; stipiamide; stromelysin inhibitors; sulfinosine; superactive vasoactive intestinal peptide antagonist; suradista; suramin; swainsonine; synthetic glycosaminoglycans; tallimustine; tamoxifen methiodide; tauromustine; tazarotene; tecogalan sodium; tegafur; tellurapyrylium; telomerase inhibitors; temoporfin; temozolomide; teniposide; tetrachlorodecaoxide; tetrazomine; thaliblastine; thiocoraline; thrombopoietin; thrombopoietin mimetic; thymalfasin; thymopoietin receptor agonist; thymotrinan; thyroid stimulating hormone; tin ethyl etiopurpurin; tirapazamine; titanocene bichloride; top sentin; toremifene; totipotent stem cell factor; translation inhibitors; tretinoin; triacetyluridine; triciribine; trimetrexate; triptorelin; tropisetron; turosteride; tyrosine kinase inhibitors; tyrphostins; UBC inhibitors; ubenimex; urogenital sinus-derived growth inhibitory factor; urokinase receptor antagonists; vapreotide; variolin B; vector system, erythrocyte gene therapy; velaresol; veramine; verdins; verteporfin; vinorelbine; vinxaltine; vitaxin; vorozole; zanoterone; zeniplatin; zilascorb; zinostatin stimalamer, Adriamycin, Dactinomycin, Bleomycin, Vinblastine, Cisplatin, acivicin; aclarubicin; acodazole hydrochloride; acronine; adozelesin; aldesleukin; altretamine; ambomycin; ametantrone acetate; aminoglutethimide; amsacrine; anastrozole; anthramycin; asparaginase; asperlin; azacitidine; azetepa; azotomycin; batimastat; benzodepa; bicalutamide; bisantrene hydrochloride; bisnafide dimesylate; bizelesin; bleomycin sulfate; brequinar sodium; bropirimine; busulfan; cactinomycin; calusterone; caracemide; carbetimer; carboplatin; carmustine; carubicin hydrochloride; carzelesin; cedefingol; chlorambucil; cirolemycin; cladribine; crisnatol mesylate; cyclophosphamide; cytarabine; dacarbazine; daunorubicin hydrochloride; decitabine; dexormaplatin; dezaguanine; dezaguanine mesylate; diaziquone; doxorubicin; doxorubicin hydrochloride; droloxifene; droloxifene citrate; dromostanolone propionate; duazomycin; edatrexate; eflornithine hydrochloride; elsamitrucin; enloplatin; enpromate; epipropidine; epirubicin hydrochloride; erbulozole; esorubicin hydrochloride; estramustine; estramustine phosphate sodium; etanidazole; etoposide; etoposide phosphate; etoprine; fadrozole hydrochloride; fazarabine; fenretinide; floxuridine; fludarabine phosphate; fluorouracil; fluorocitabine; fosquidone; fostriecin sodium; gemcitabine; gemcitabine hydrochloride; hydroxyurea; idarubicin hydrochloride; ifosfamide; iimofosine; interleukin I1 (including recombinant interleukin II, or r1L.sub.2), interferon alfa-2a; interferon alfa-2b; interferon alfa-n1; interferon alfa-n3; interferon beta-1a; interferon gamma-1b; iproplatin; irinotecan hydrochloride; lanreotide acetate; letrozole; leuprolide acetate; liarozole hydrochloride; lometrexol sodium; lomustine; losoxantrone hydrochloride; masoprocol; maytansine; mechlorethamine hydrochloride; megestrol acetate; melengestrol acetate; melphalan; menogaril; mercaptopurine; methotrexate; methotrexate sodium; metoprine; meturedepa; mitindomide; mitocarcin; mitocromin; mitogillin; mitomalcin; mitomycin; mitosper; mitotane; mitoxantrone hydrochloride; mycophenolic acid; nocodazoie; nogalamycin; ormaplatin; oxisuran; pegaspargase; peliomycin; pentamustine; peplomycin sulfate; perfosfamide; pipobroman; piposulfan; piroxantrone hydrochloride; plicamycin; plomestane; porfimer sodium; porfiromycin; prednimustine; procarbazine hydrochloride; puromycin; puromycin hydrochloride; pyrazofurin; riboprine; rogletimide; safingol; safingol hydrochloride; semustine; simtrazene; sparfosate sodium; sparsomycin; spirogermanium hydrochloride; spiromustine; spiroplatin; streptonigrin; streptozocin; sulofenur; talisomycin; tecogalan sodium; tegafur; teloxantrone hydrochloride; temoporfin; teniposide; teroxirone; testolactone; thiamiprine; thioguanine; thiotepa; tiazofurin; tirapazamine; toremifene citrate; trestolone acetate; triciribine phosphate; trimetrexate; trimetrexate glucuronate; triptorelin; tubulozole hydrochloride; uracil mustard; uredepa; vapreotide; verteporfin; vinblastine sulfate; vincristine sulfate; vindesine; vindesine sulfate; vinepidine sulfate; vinglycinate sulfate; vinleurosine sulfate; vinorelbine tartrate; vinrosidine sulfate; vinzolidine sulfate; vorozole; zeniplatin; zinostatin; zorubicin hydrochloride, agents that arrest cells in the G2-M phases and/or modulate the formation or stability of microtubules, (e.g. Taxol™ (i.e. paclitaxel), Taxotere™, compounds comprising the taxane skeleton, Erbulozole (i.e. R-55104), Dolastatin 10 (i.e. DLS-10 and NSC-376128), Mivobulin isethionate (i.e. as CI-980), Vincristine, NSC-639829, Discodermolide (i.e. as NVP-XX-A-296), ABT-751 (Abbott, i.e. E-7010), Altorhyrtins (e.g. Altorhyrtin A and Altorhyrtin C), Spongistatins (e.g. Spongistatin 1, Spongistatin 2, Spongistatin 3, Spongistatin 4, Spongistatin 5, Spongistatin 6, Spongistatin 7, Spongistatin 8, and Spongistatin 9), Cemadotin hydrochloride (i.e. LU-103793 and NSC-D-669356), Epothilones (e.g. Epothilone A, Epothilone B, Epothilone C (i.e. desoxyepothilone A or dEpoA), Epothilone D (i.e. KOS-862, dEpoB, and desoxyepothilone B), Epothilone E, Epothilone F, Epothilone B N-oxide, Epothilone A N-oxide, 16-aza-epothilone B, 21-aminoepothilone B (i.e. BMS-310705), 21-hydroxyepothilone D (i.e. Desoxyepothilone F and dEpoF), 26-fluoroepothilone, Auristatin PE (i.e. NSC-654663), Soblidotin (i.e. TZT-1027), LS-4559-P (Pharmacia, i.e. LS-4577), LS-4578 (Pharmacia, i.e. LS-477-P), LS-4477 (Pharmacia), LS-4559 (Pharmacia), RPR-112378 (Aventis), Vincristine sulfate, DZ-3358 (Daiichi), FR-182877 (Fujisawa, i.e. WS-9885B), GS-164 (Takeda), GS-198 (Takeda), KAR-2 (Hungarian Academy of Sciences), BSF-223651 (BASF, i.e. ILX-651 and LU-223651), SAH-49960 (Lilly/Novartis), SDZ-268970 (Lilly/Novartis), AM-97 (Armad/Kyowa Hakko), AM-132 (Armad), AM-138 (Armad/Kyowa Hakko), IDN-5005 (Indena), Cryptophycin 52 (i.e. LY-355703), AC-7739 (Ajinomoto, i.e. AVE-8063A and CS-39.HCl), AC-7700 (Ajinomoto, i.e. AVE-8062, AVE-8062A, CS-39-L-Ser.HCl, and RPR-258062A), Vitilevuamide, Tubulysin A, Canadensol, Centaureidin (i.e. NSC-106969), T-138067 (Tularik, i.e. T-67, TL-138067 and TI-138067), COBRA-1 (Parker Hughes Institute, i.e. DDE-261 and WHI-261), H10 (Kansas State University), H16 (Kansas State University), Oncocidin A1 (i.e. BTO-956 and DIME), DDE-313 (Parker Hughes Institute), Fijianolide B, Laulimalide, SPA-2 (Parker Hughes Institute), SPA-1 (Parker Hughes Institute, i.e. SPIKET-P), 3-IAABU (Cytoskeleton/Mt. Sinai School of Medicine, i.e. MF-569), Narcosine (also known as NSC-5366), Nascapine, D-24851 (Asta Medica), A-105972 (Abbott), Hemiasterlin, 3-BAABU (Cytoskeleton/Mt. Sinai School of Medicine, i.e. MF-191), TMPN (Arizona State University), Vanadocene acetylacetonate, T-138026 (Tularik), Monsatrol, lnanocine (i.e. NSC-698666), 3-IAABE (Cytoskeleton/Mt. Sinai School of Medicine), A-204197 (Abbott), T-607 (Tuiarik, i.e. T-900607), RPR-115781 (Aventis), Eleutherobins (such as Desmethyleleutherobin, Desaetyleleutherobin, lsoeleutherobin A, and Z-Eleutherobin), Caribaeoside, Caribaeolin, Halichondrin B, D-64131 (Asta The term “reticulon 4 activity” as used herein refers to the biological activity of the protein. In embodiments, reticulon 4 activity includes endoplasmic reticulum (ER) tubule formation. Reticulon 4 activity may be quantified by measuring tubular ER network formation, ER morphology, mitosis rate, nuclear envelope assembly, nuclear envelope disassembly, or cell death. The term “reticulon 4 protein-reticulon 4 inhibitor complex” as used herein refers to a reticulon 4 protein bonded (e.g., covalently bonded) to a Reticulon 4 inhibitor (e.g., a compound described herein). In an aspect is provided a compound having the formula: R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1R1D, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NR1AOR1C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. Two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. The symbol z1 is an integer from 0 to 5. R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(OR2C—C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. Two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. The symbol z2 is an integer from 0 to 4. L1is a bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4—, —NR4C(O)—, —NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene. R4is hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)—OR4A, —C(O)NR4AR4B, —OR4A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR5C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene. R5is hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)—OR5A, —C(O)NR5AR5B, —OR5A—, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. E is an electrophilic moiety. Each R1A, R1B, R1C, R1D, R2A, R2B, R2C, R2D, R4A, R4B, R5A, and R5Bis independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl. R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl. R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl. R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl. Each X, X1, X2, X4, and X5is independently —F, —Cl, —Br, or —I. The symbols n1, n2, n4, and n5 are independently an integer from 0 to 4. The symbols m1, m2, m4, m5, v1, v2, v4, and v5 are independently an integer from 1 to 2. In embodiments, the compound has the formula: R1, R2, L1, L2, E, z1 and z2 are as described herein. In embodiments, the compound has the formula: R1, R2, L1, L2, and E are as described herein. In embodiments, the compound has the formula: R1, L1, L2, and E are as described herein. In embodiments, the compound has the formula: R1, L1, L2, and E are as described herein. In embodiments, the compound has the formula: R1, R4, L2, and E are as described herein. In embodiments, the compound has the formula: R4, L2, and E are as described herein. In embodiments, the compound has the formula: R1, R4, L2, and E are as described herein. In embodiments, the compound has the formula: R1, R5, L1, and E are as described herein. In embodiments, the compound has the formula: R5, L1, and E are as described herein. In embodiments, the compound has the formula: R1, L2, and E are as described herein. In embodiments, the compound has the formula: wherein R20, L1, L2, and E are as described herein; two adjacent R1substituents form Ring A, wherein Ring A is a cycloalkyl, heterocycloalkyl, aryl, or heteroaryl. The symbol z20 is an integer from 0 to 8. In embodiments, the compound has the formula: wherein R20, z20, L1, L2, and E are as described herein. In embodiments, the compound has the formula: wherein R20, z20, L1, L2, and E are as described herein. In embodiments, the compound has the formula: wherein R20, z20, L1, L2, and E are as described herein. In embodiments, the compound has the formula: L1, L2, and E are as described herein. In embodiments, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SR1D, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. In embodiments, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SH, —NH2, —C(O)OH, —C(O)NH2, —OH, substituted or unsubstituted C1-C8alkyl, or substituted or unsubstituted 2 to 8 membered heteroalkyl; substituted or unsubstituted C3-C8cycloalkyl, substituted or unsubstituted 3 to 8 membered heterocycloalkyl, substituted or unsubstituted C6-C12aryl, or substituted or unsubstituted 5 to 12 membered heteroaryl. In embodiments, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SH, —NH2, —C(O)OH, —C(O)NH2, —OH, substituted or unsubstituted C1-C8alkyl, or substituted or unsubstituted 2 to 8 membered heteroalkyl; substituted or unsubstituted C3-C8cycloalkyl, substituted or unsubstituted 3 to 8 membered heterocycloalkyl, substituted or unsubstituted phenyl, or substituted or unsubstituted 5 to 6 membered heteroaryl. In embodiments, two adjacent R1substituents are joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. In embodiments, two adjacent R1substituents are joined to form an unsubstituted cycloalkyl. In embodiments, two adjacent R1substituents are joined to form an unsubstituted C3-C6cycloalkyl. In embodiments, R1is independently —Cl. In embodiments, R1is independently halogen. In embodiments, R1is independently unsubstituted methyl. In embodiments, R1is independently unsubstituted ethyl. In embodiments, R1is independently unsubstituted propyl. In embodiments, R1is independently unsubstituted isopropyl. In embodiments, R1is independently unsubstituted n-propyl. In embodiments, R1is independently unsubstituted butyl. In embodiments, R1is independently unsubstituted n-butyl. In embodiments, R1is independently unsubstituted t-butyl. In embodiments, R1is independently unsubstituted pentyl. In embodiments, R1is independently unsubstituted n-pentyl. In embodiments, R1is independently unsubstituted hexyl. In embodiments, R1is independently unsubstituted n-hexyl. In embodiments, R1is independently unsubstituted heptyl. In embodiments, R1is independently unsubstituted n-heptyl. In embodiments, R1is independently unsubstituted octyl. In embodiments, R1is independently unsubstituted n-octyl. In embodiments, R1is independently unsubstituted benzyl. In embodiments, R1is independently unsubstituted C1-C8alkyl. In embodiments, R1is independently halo-substituted methyl. In embodiments, R1is independently halo-substituted ethyl. In embodiments, R1is independently halo-substituted isopropyl. In embodiments, R1is independently halo-substituted n-propyl. In embodiments, R1is independently halo-substituted n-butyl. In embodiments, R1is independently halo-substituted t-butyl. In embodiments, R1is independently halo-substituted n-pentyl. In embodiments, R1is independently halo-substituted benzyl. In embodiments, R1is independently halo-substituted C1-C8alkyl. In embodiments, R1is independently unsubstituted 2 to 6 membered heteroalkyl. In embodiments, R1is independently unsubstituted 2 to 7 membered heteroalkyl. In embodiments, R1is independently unsubstituted 2 to 8 membered heteroalkyl. In embodiments, R1is independently unsubstituted 2 to 9 membered heteroalkyl. In embodiments, R1is independently unsubstituted 2 to 10 membered heteroalkyl. In embodiments, R1is independently unsubstituted 3 to 10 membered heteroalkyl. In embodiments, R1is independently unsubstituted 4 to 10 membered heteroalkyl. In embodiments, R1is independently unsubstituted 5 to 10 membered heteroalkyl. In embodiments, R1is independently unsubstituted 6 to 10 membered heteroalkyl. In embodiments, R1is independently unsubstituted 7 to 10 membered heteroalkyl. In embodiments, R1is independently unsubstituted 8 to 10 membered heteroalkyl. In embodiments, R1is independently unsubstituted 6 to 10 membered heteroalkyl. In embodiments, R1is independently unsubstituted 7 to 9 membered heteroalkyl. In embodiments, two adjacent R1substituents are joined to form an unsubstituted C3-C6cycloalkyl. In embodiments, two adjacent R1substituents are joined to form an unsubstituted C4-C6cycloalkyl. In embodiments, two adjacent R1substituents are joined to form an unsubstituted C3-C5cycloalkyl. In embodiments, two adjacent R1substituents are joined to form an unsubstituted C5-C6cycloalkyl. In embodiments, two adjacent R1substituents are joined to form an unsubstituted C4cycloalkyl. In embodiments, R1is independently unsubstituted 5 membered heteroaryl. In embodiments, R1is independently unsubstituted 6 membered heteroaryl. In embodiments, R1is independently unsubstituted pyridyl. In embodiments, R1is independently unsubstituted 2-pyridyl. In embodiments, R1is independently unsubstituted 3-pyridyl. In embodiments, R1is independently unsubstituted 4-pyridyl. In embodiments, R1is independently unsubstituted pyridazinyl. In embodiments, R1is independently unsubstituted pyrimidinyl. In embodiments, R1is independently unsubstituted pyrazinyl. In embodiments, R1is independently unsubstituted triazinyl. In embodiments, R1is independently unsubstituted pyrrolyl. In embodiments, R1is independently unsubstituted 2-pyrrolyl. In embodiments, R1is independently unsubstituted 3-pyrrolyl. In embodiments, R1is independently unsubstituted furanyl. In embodiments, R1is independently unsubstituted 2-furanyl. In embodiments, R1is independently unsubstituted 3-furanyl. In embodiments, R1is independently unsubstituted thienyl. In embodiments, R1is independently unsubstituted 2-thienyl. In embodiments, R1is independently unsubstituted 3-thienyl. In embodiments, R1is independently unsubstituted pyrazolyl. In embodiments, R1is independently unsubstituted isoxazolyl. In embodiments, R1is independently unsubstituted isothiazolyl. In embodiments, R1is independently unsubstituted imidazolyl. In embodiments, R1is independently unsubstituted oxazolyl. In embodiments, R1is independently unsubstituted thiazolyl. In embodiments, R1is independently unsubstituted phenyl. In embodiments, R1is independently unsubstituted biphenyl. In embodiments, R1is independently unsubstituted 2-biphenyl. In embodiments, R1is independently unsubstituted 3-biphenyl. In embodiments, R1is independently unsubstituted 4-biphenyl. In embodiments, R1is independently —CX13. In embodiments, R1is independently —CHX12. In embodiments, R1is independently —CH2X1. In embodiments, R1is independently —OCX13. In embodiments, R1is independently —OCH2X1. In embodiments, R1is independently —OCHX12. In embodiments, R1is independently —CN. In embodiments, R1is independently —SOn1R1D. In embodiments, R1is independently —SOv1NR1AR1B. In embodiments, R1is independently —NHC(O)NR1AR1B. In embodiments, R1is independently —N(O)m1. In embodiments, R1is independently —NR1AR1B. In embodiments, R1is independently —C(O)R1C. In embodiments, R1is independently —C(O)—OR1C. In embodiments, R1is independently —C(O)NR1AR1B. In embodiments, R1is independently —OR1D. In embodiments, R1is independently —NR1ASO2R1D. In embodiments, R1is independently —NR1AC(O)R1C. In embodiments, R1is independently —NR1AC(O)OR1C. In embodiments, R1is independently —NR1AOR1C. In embodiments, R1is independently —OH. In embodiments, R1is independently —NH2. In embodiments, R1is independently —COOH. In embodiments, R1is independently —CONH2. In embodiments, R1is independently —NO2. In embodiments, R1is independently —SH. In embodiments, R1is independently halogen. In embodiments, R1is independently —F. In embodiments, R1is independently —Cl. In embodiments, R1is independently —Br. In embodiments, R1is independently —I. In embodiments, R1is independently —CF3. In embodiments, R1is independently —CHF2. In embodiments, R1is independently —CH2F. In embodiments, R1is independently —OCF3. In embodiments, R1is independently —OCH2F. In embodiments, R1is independently —OCHF2. In embodiments, R1is independently —OCH3. In embodiments, R1is independently —OCH2CH3. In embodiments, R1is independently —OCH2CH2CH3. In embodiments, R1is independently —OCH(CH3)2. In embodiments, R1is independently —OC(CH3)3. In embodiments, R1is independently —SCH3. In embodiments, R1is independently —SCH2CH3. In embodiments, R1is independently —SCH2CH2CH3. In embodiments, R1is independently —SCH(CH3)2. In embodiments, R1is independently —SC(CH3)3. In embodiments, R1is independently —CH3. In embodiments, R1is independently —CH2CH3. In embodiments, R1is independently —CH2CH2CH3. In embodiments, R1is independently —CH(CH3)2. In embodiments, R1is independently —C(CH3)3. In embodiments, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1R1D, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NR1AOR1C, —N3, substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or substituted or unsubstituted heteroaryl (e.g., 5 to 12, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1R1D, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NR1AOR1C, —N3, substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or substituted or unsubstituted heteroaryl (e.g., 5 to 12, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1is independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1is independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1is independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1is independently unsubstituted methyl. In embodiments, R1is independently unsubstituted ethyl. In embodiments, R1is independently unsubstituted propyl. In embodiments, R1is independently unsubstituted isopropyl. In embodiments, R1is independently unsubstituted tert-butyl. In embodiments, R1is independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1is independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1is independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1is independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1is independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1is independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1is independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1is independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1is independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1is independently substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1is independently substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1is independently unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1is independently substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1is independently substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1is independently unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, two adjacent R1substituents may optionally be joined to form a substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, two adjacent R1substituents may optionally be joined to form an unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, two adjacent R1substituents may optionally be joined to form a substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, two adjacent R1substituents may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, two adjacent R1substituents may optionally be joined to form a substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, two adjacent R1substituents may optionally be joined to form an unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, two adjacent R1substituents may optionally be joined to form a substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, two adjacent R1substituents may optionally be joined to form an unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Ais independently hydrogen. In embodiments, R1Ais independently —CX1A3. In embodiments, R1Ais independently —CHX1A2. In embodiments, R1Ais independently —CH2X1A. In embodiments, R1Ais independently —CN. In embodiments, R1Ais independently —COOH. In embodiments, R1Ais independently —CONH2. In embodiments, X1Ais independently —F, —Cl, —Br, or —I. In embodiments, R1Ais independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1Ais independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1Ais independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1Ais independently unsubstituted methyl. In embodiments, R1Ais independently unsubstituted ethyl. In embodiments, R1Ais independently unsubstituted propyl. In embodiments, R1Ais independently unsubstituted isopropyl. In embodiments, R1Ais independently unsubstituted tert-butyl. In embodiments, R1Ais independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1Ais independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1Ais independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1Ais independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1Ais independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1Ais independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1Ais independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Ais independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Ais independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Ais independently substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1Ais independently substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1Ais independently unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1Ais independently substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Ais independently substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Ais independently unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Bis independently hydrogen. In embodiments, R1Bis independently —CX1B3. In embodiments, R1Bis independently —CHX1B2. In embodiments, R1Bis independently —CH2X1B. In embodiments, R1Bis independently —CN. In embodiments, R1Bis independently —COOH. In embodiments, R1Bis independently —CONH2. In embodiments, X1Bis independently —F, —Cl, —Br, or —I. In embodiments, R1Bis independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1Bis independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1Bis independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1Bis independently unsubstituted methyl. In embodiments, R1Bis independently unsubstituted ethyl. In embodiments, R1Bis independently unsubstituted propyl. In embodiments, R1Bis independently unsubstituted isopropyl. In embodiments, R1Bis independently unsubstituted tert-butyl. In embodiments, R1Bis independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1Bis independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1Bis independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1Bis independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1Bis independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1Bis independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1Bis independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Bis independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Bis independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Bis independently substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1Bis independently substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1Bis independently unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1Bis independently substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Bis independently substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Bis independently unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may be joined to form an unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Cis independently hydrogen. In embodiments, R1Cis independently —CX1C3. In embodiments, R1Cis independently —CHX1C2. In embodiments, R1Cis independently —CH2X1C. In embodiments, R1Cis independently —CN. In embodiments, R1Cis independently —COOH. In embodiments, R1Cis independently —CONH2. In embodiments, X1Cis independently —F, —Cl, —Br, or —I. In embodiments, R1Cis independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1Cis independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1Cis independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1Cis independently unsubstituted methyl. In embodiments, R1Cis independently unsubstituted ethyl. In embodiments, R1Cis independently unsubstituted propyl. In embodiments, R1Cis independently unsubstituted isopropyl. In embodiments, R1Cis independently unsubstituted tert-butyl. In embodiments, R1Cis independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1Cis independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1Cis independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1Cis independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1Cis independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1Cis independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1Cis independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Cis independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Cis independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Cis independently substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1Cis independently substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1Cis independently unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1Cis independently substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Cis independently substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Cis independently unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Dis independently hydrogen. In embodiments, R1Dis independently —CX1D3. In embodiments, R1Dis independently —CHX1D2. In embodiments, R1Dis independently —CH2X1D. In embodiments, R1Dis independently —CN. In embodiments, R1Dis independently —COOH. In embodiments, R1Dis independently —CONH2. In embodiments, X1Cis independently —F, —Cl, —Br, or —I. In embodiments, R1Dis independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1Dis independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1Dis independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R1Dis independently unsubstituted methyl. In embodiments, R1Dis independently unsubstituted ethyl. In embodiments, R1Dis independently unsubstituted propyl. In embodiments, R1Dis independently unsubstituted isopropyl. In embodiments, R1Dis independently unsubstituted tert-butyl. In embodiments, R1Dis independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1Dis independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1Dis independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R1Dis independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1Dis independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1Dis independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R1Dis independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Dis independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Dis independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Dis independently substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1Dis independently substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1Dis independently unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R1Dis independently substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Dis independently substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Dis independently unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R20-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R20-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R20-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R20-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R20-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R20-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X1is independently —F, —Cl, —Br, or —I. In embodiments, R1is independently hydrogen. In embodiments, R1is independently unsubstituted methyl. In embodiments, R1is independently unsubstituted ethyl. In embodiments, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R20-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R20-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R20-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R20-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R20-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R20-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, two adjacent R1substituents may optionally be joined to form a R20-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, two adjacent R1substituents may optionally be joined to form a R20-substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, two adjacent R1substituents may optionally be joined to form an unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, two adjacent R1substituents may optionally be joined to form a R20-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, two adjacent R1substituents may optionally be joined to form a R20-substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, two adjacent R1substituents may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, two adjacent R1substituents may optionally be joined to form a R20-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, two adjacent R1substituents may optionally be joined to form a R20-substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, two adjacent R1substituents may optionally be joined to form an unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, two adjacent R1substituents may optionally be joined to form a R20-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, two adjacent R1substituents may optionally be joined to form a R20-substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, two adjacent R1substituents may optionally be joined to form an unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). R20is independently oxo, halogen, —CX203, —CHX202, —CH2X20, —OCX203, —OCH2X20, —OCHX202, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R21-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R21-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R21-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R21-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R21-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R21-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R20is independently oxo,
R21is independently oxo, halogen, —CX213, —CHX212, —CH2X21, —OCX213, —OCH2X21, —OCHX212, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R22-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R22-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R22-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R22-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R22-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R22-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R21is independently oxo,
R22is independently oxo, halogen, —CX223, —CHX222, —CH2X22, —OCX223, —OCH2X22, —OCHX222, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X22is independently —F, —Cl, —Br, or —I. In embodiments, R22is independently unsubstituted methyl. In embodiments, R22is independently unsubstituted ethyl. In embodiments, R1Ais independently hydrogen, —CX1A3, —CHX1A2, —CH2X1A, —CN, —COOH, —CONH2, R20A-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R20A-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R20A-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R20A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R20A-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R20A-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Ais independently hydrogen, —CX1A3, —CHX1A2, —CH2X1A, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X1Ais independently —F, —Cl, —Br, or —I. In embodiments, R1Ais independently hydrogen. In embodiments, R1Ais independently unsubstituted methyl. In embodiments, R1Ais independently unsubstituted ethyl. In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R20A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or R20A-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R20A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). R20Ais independently oxo, halogen, —CX20A3, —CHX20A2, —CH2X20A, —OCX20A3, —OCH2X20A, —OCHX20A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R21A-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R21A-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R21A-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R21A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R21A-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R21A-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R20Ais independently oxo, halogen, —CX20A3, —CHX20A2, —CH2X20A, —OCX20A3, —OCH2X20A, —OCHX20A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X20Ais independently —F, —Cl, —Br, or —I. In embodiments, R20Ais independently unsubstituted methyl. In embodiments, R20Ais independently unsubstituted ethyl. R21Ais independently oxo, halogen, —CX21A3, —CHX21A2, —CH2X21A, —OCX21A3, —OCH2X21A, —OCHX21A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R22A-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R22A-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R22A-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R22A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R22A-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R22A-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R21Ais independently oxo,
R22Ais independently oxo, halogen, —CX22A3, —CHX22A2, —CH2X22A, —OCX22A3, —OCH2X22A, —OCHX22A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X22Ais independently —F, —Cl, —Br, or —I. In embodiments, R22Ais independently unsubstituted methyl. In embodiments, R22Ais independently unsubstituted ethyl. In embodiments, R1Bis independently hydrogen, —CX1B3, —CHX1B2, —CH2X1B, —CN, —COOH, —CONH2, R20B-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R20B-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R20B-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R20B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R20B-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R20B-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Bis independently hydrogen, —CX1B3, —CHX1B2, —CH2X1B, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X1Bis independently —F, —Cl, —Br, or —I. In embodiments, R1Bis independently hydrogen. In embodiments, R1Bis independently unsubstituted methyl. In embodiments, R1Bis independently unsubstituted ethyl. In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R20B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or R20B-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R20B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). R20Bis independently oxo, halogen, —CX20B3, —CHX20B2, —CH2X20B, —OXC20B3, —OCH2X20B3, —OCHX20B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R21B-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R21B-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R21B-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R21B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R21B-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R21B-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R20Bis independently oxo,
R21Bis independently oxo, halogen, —CX21B3, —CHX21B2, —CH2X21B, —OCX21B3, —OCH2X21B, —OCHX21B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R22B-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R22B-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R22B-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R22B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R22B-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R22B-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R21Bis independently oxo,
R22Bis independently oxo, halogen, —CX22B3, —CHX22B2, —CH2X22B, —OCX22B3, —OCH2X22B, —OCHX22B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X22Bis independently —F, —Cl, —Br, or —I. In embodiments, R22Bis independently unsubstituted methyl. In embodiments, R22Bis independently unsubstituted ethyl. In embodiments, R1Cis independently hydrogen, —CX1C3, —CHX1C2, —CH2X1C, —CN, —COOH, —CONH2, R20C-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R20C-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R20C-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R20C-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R20C-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R20C-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Cis independently hydrogen, —CX1C3, —CHX1C2, —CH2X1C, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X1Cis independently —F, —Cl, —Br, or —I. In embodiments, R1Cis independently hydrogen. In embodiments, R1Cis independently unsubstituted methyl. In embodiments, R1Cis independently unsubstituted ethyl. R20Cis independently oxo, halogen, —CX20C3, —CHX2n, —CH2X20C, —OCX2n, —OCH2X20C, —OCHX20C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R21C-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R21C-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R21C-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R21C-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R21C-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R21C-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R20Cis independently oxo,
R21Cis independently oxo, halogen, —CX21C3, —CHX21C2, —CH2X21C, —OCX21C3, —OCH2X21C, —OCHX21C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R22C-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R22C-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R22C-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R22C-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R22C-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R22C-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R21Cis independently oxo,
R22Cis independently oxo, halogen, —CX22C3, —CHX22C2, —CH2X22C, —OCX22C3, —OCH2X22C, —OCHX22C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X22Cis independently —F, —Cl, —Br, or —I. In embodiments, R22Cis independently unsubstituted methyl. In embodiments, R22Cis independently unsubstituted ethyl. In embodiments, R1Dis independently hydrogen, —CX1D3, —CHX1D2, —CH2X1D, —CN, —COOH, —CONH2, R20D-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R20D-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R20D-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R20D-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R20D-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R20D-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R1Dis independently hydrogen, —CX1D3, —CHX1D2, —CH2X1D, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X1Dis independently —F, —Cl, —Br, or —I. In embodiments, R1Dis independently hydrogen. In embodiments, R1Dis independently unsubstituted methyl. In embodiments, R1Dis independently unsubstituted ethyl. R20Dis independently oxo, halogen, —CX20D3, —CHX20D2, —CH2X20D, —OCX20D3, —OCH2X20D, —OCHX20D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R21D-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R21D-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R21D-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R21D-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R21D-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R21Dsubstituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R20Dis independently oxo,
R21Dis independently oxo, halogen, —CX21D3, —CHX21D2, —CH2X21D, —OCX21D3, —OCH2X21D, —OCHX21D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R22D-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R22D-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R22D-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R22D-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R22D-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R22D-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R21Dis independently oxo,
R22Dis independently oxo, halogen, —CX22D3, —CHX22D2, —CH2X22D, —OCX22D3, —OCH2X22D, —OCHX22D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X22Dis independently —F, —Cl, —Br, or —I. In embodiments, R22Dis independently unsubstituted methyl. In embodiments, R22Dis independently unsubstituted ethyl. In embodiments, z1 is 0. In embodiments, z1 is 1. In embodiments, z1 is 2. In embodiments, z1 is 3. In embodiments, z1 is 4. In embodiments, z1 is 5. In embodiments, R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SR2D, —NR2AR2B, —C(O)R2C, —C(O)OR2C, —C(O)NR2AR2B, —OR2D, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. In embodiments, R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SH, —NH2, —C(O)OH, —C(O)NH2, —OH, substituted or unsubstituted C1-C8alkyl, or substituted or unsubstituted 2 to 8 membered heteroalkyl; substituted or unsubstituted C3-C8cycloalkyl, substituted or unsubstituted 3 to 8 membered heterocycloalkyl, substituted or unsubstituted C6-C12aryl, or substituted or unsubstituted 5 to 12 membered heteroaryl. In embodiments, R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SH, —NH2, —C(O)OH, —C(O)NH2, —OH, substituted or unsubstituted C1-C8alkyl, or substituted or unsubstituted 2 to 8 membered heteroalkyl; substituted or unsubstituted C3-C8cycloalkyl, substituted or unsubstituted 3 to 8 membered heterocycloalkyl, substituted or unsubstituted phenyl, or substituted or unsubstituted 5 to 6 membered heteroaryl. In embodiments, two adjacent R2substituents are joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. In embodiments, two adjacent R2substituents are joined to form an unsubstituted cycloalkyl. In embodiments, two adjacent R2substituents are joined to form an unsubstituted C3-C6cycloalkyl. In embodiments, R2is independently —CX23. In embodiments, R2is independently —CHX22. In embodiments, R2is independently —CH2X2. In embodiments, R2is independently —OCX23. In embodiments, R2is independently —OCH2X2. In embodiments, R2is independently —OCHX22. In embodiments, R2is independently —CN. In embodiments, R2is independently —SOn2R2D. In embodiments, R2is independently —SOv2NR2AR2B. In embodiments, R2is independently —NHC(O)NR2AR2B. In embodiments, R2is independently —N(O)m2. In embodiments, R2is independently —NR2AR2B. In embodiments, R2is independently —C(O)R2C. In embodiments, R2is independently —C(O)—OR2C. In embodiments, R2is independently —C(O)NR2AR2B. In embodiments, R2is independently —OR2D. In embodiments, R2is independently —NR2ASO2R2D. In embodiments, R2is independently —NR2AC(O)R2C. In embodiments, R2is independently —NR2AC(O)OR2C. In embodiments, R2is independently —NR2AOR2C. In embodiments, R2is independently —OH. In embodiments, R2is independently —NH2. In embodiments, R2is independently —COOH. In embodiments, R2is independently —CONH2. In embodiments, R2is independently —NO2. In embodiments, R2is independently —SH. In embodiments, R2is independently halogen. In embodiments, R2is independently —F. In embodiments, R2is independently —Cl. In embodiments, R2is independently —Br. In embodiments, R2is independently —I. In embodiments, R2is independently —CF3. In embodiments, R2is independently —CHF2. In embodiments, R2is independently —CH2F. In embodiments, R2is independently —OCF3. In embodiments, R2is independently —OCH2F. In embodiments, R2is independently —OCHF2. In embodiments, R2is independently —OCH3. In embodiments, R2is independently —OCH2CH3. In embodiments, R2is independently —OCH2CH2CH3. In embodiments, R2is independently —OCH(CH3)2. In embodiments, R2is independently —OC(CH3)3. In embodiments, R2is independently —SCH3. In embodiments, R2is independently —SCH2CH3. In embodiments, R2is independently —SCH2CH2CH3. In embodiments, R2is independently —SCH(CH3)2. In embodiments, R2is independently —SC(CH3)3. In embodiments, R2is independently —CH3. In embodiments, R2is independently —CH2CH3. In embodiments, R2is independently —CH2CH2CH3. In embodiments, R2is independently —CH(CH3)2. In embodiments, R2is independently —C(CH3)3. In embodiments, R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(O)R2C, —C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, —N3, substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or substituted or unsubstituted heteroaryl (e.g., 5 to 12, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(O)R2C, —C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2CC, R2AOR2C, —N3, substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or substituted or unsubstituted heteroaryl (e.g., 5 to 12, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2is independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2is independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2is independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2is independently unsubstituted methyl. In embodiments, R2is independently unsubstituted ethyl. In embodiments, R2is independently unsubstituted propyl. In embodiments, R2is independently unsubstituted isopropyl. In embodiments, R2is independently unsubstituted tert-butyl. In embodiments, R2is independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2is independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2is independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2is independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2is independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2is independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2is independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2is independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2is independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2is independently substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2is independently substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2is independently unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2is independently substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2is independently substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2is independently unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, two adjacent R2substituents may optionally be joined to form a substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, two adjacent R2substituents may optionally be joined to form an unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, two adjacent R2substituents may optionally be joined to form a substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, two adjacent R2substituents may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, two adjacent R2 substituents may optionally be joined to form a substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, two adjacent R2substituents may optionally be joined to form an unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, two adjacent R2substituents may optionally be joined to form a substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, two adjacent R2substituents may optionally be joined to form an unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Ais independently hydrogen. In embodiments, R2Ais independently —CX2A3. In embodiments, R2Ais independently —CHX2A2. In embodiments, R2Ais independently —CH2X2A. In embodiments, R2Ais independently —CN. In embodiments, R2Ais independently —COOH. In embodiments, R2Ais independently —CONH2. In embodiments, X2Ais independently —F, —Cl, —Br, or —I. In embodiments, R2Ais independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2Ais independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2Ais independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2Ais independently unsubstituted methyl. In embodiments, R2Ais independently unsubstituted ethyl. In embodiments, R2Ais independently unsubstituted propyl. In embodiments, R2Ais independently unsubstituted isopropyl. In embodiments, R2Ais independently unsubstituted tert-butyl. In embodiments, R2Ais independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2Ais independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2Ais independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2Ais independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2Ais independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2Ais independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2Ais independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Ais independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Ais independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Ais independently substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2Ais independently substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2Ais independently unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2Ais independently substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Ais independently substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Ais independently unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Bis independently hydrogen. In embodiments, R2Bis independently —CX2B3. In embodiments, R2Bis independently —CHX2B2. In embodiments, R2Bis independently —CH2X2B. In embodiments, R2Bis independently —CN. In embodiments, R2Bis independently —COOH. In embodiments, R2Bis independently —CONH2. In embodiments, X2Bis independently —F, —Cl, —Br, or —I. In embodiments, R2Bis independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2Bis independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2Bis independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2Bis independently unsubstituted methyl. In embodiments, R2Bis independently unsubstituted ethyl. In embodiments, R2Bis independently unsubstituted propyl. In embodiments, R2Bis independently unsubstituted isopropyl. In embodiments, R2Bis independently unsubstituted tert-butyl. In embodiments, R2Bis independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2Bis independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2Bis independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2Bis independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2Bis independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2Bis independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2Bis independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Bis independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Bis independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Bis independently substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2Bis independently substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2Bis independently unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2Bis independently substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Bis independently substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Bis independently unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may be joined to form an unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Cis independently hydrogen. In embodiments, R2Cis independently —CX2C3. In embodiments, R2Cis independently —CHX2C2. In embodiments, R2Cis independently —CH2X2C. In embodiments, R2Cis independently —CN. In embodiments, R2Cis independently —COOH. In embodiments, R2Cis independently —CONH2. In embodiments, X2Cis independently —F, —Cl, —Br, or —I. In embodiments, R2Cis independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2Cis independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2Cis independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2Cis independently unsubstituted methyl. In embodiments, R2Cis independently unsubstituted ethyl. In embodiments, R2Cis independently unsubstituted propyl. In embodiments, R2Cis independently unsubstituted isopropyl. In embodiments, R2Cis independently unsubstituted tert-butyl. In embodiments, R2Cis independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2Cis independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2Cis independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2Cis independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2Cis independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2Cis independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2Cis independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Cis independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Cis independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Cis independently substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2Cis independently substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2Cis independently unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2Cis independently substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Cis independently substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Cis independently unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Dis independently hydrogen. In embodiments, R2Dis independently —CX2D3. In embodiments, R2Dis independently —CHX2D2. In embodiments, R2Dis independently —CH2X2D. In embodiments, R2Dis independently —CN. In embodiments, R2Dis independently —COOH. In embodiments, R2Dis independently —CONH2. In embodiments, X2Dis independently —F, —Cl, —Br, or —I. In embodiments, R2Dis independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2Dis independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2Dis independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R2Dis independently unsubstituted methyl. In embodiments, R2Dis independently unsubstituted ethyl. In embodiments, R2Dis independently unsubstituted propyl. In embodiments, R2Dis independently unsubstituted isopropyl. In embodiments, R2Dis independently unsubstituted tert-butyl. In embodiments, R2Dis independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2Dis independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2Dis independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R2Dis independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2Dis independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2Dis independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R2Dis independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Dis independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Dis independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Dis independently substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2Dis independently substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2Dis independently unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, R2Dis independently substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Dis independently substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Dis independently unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R23-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R23-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R23-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R23-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R23-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R23-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X2is independently —F, —Cl, —Br, or —I. In embodiments, R2is independently unsubstituted methyl. In embodiments, R2is independently unsubstituted ethyl. In embodiments, R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R23-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R23-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R23-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R23-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R23-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R23-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, two adjacent R2substituents may optionally be joined to form a R23-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, two adjacent R2substituents may optionally be joined to form a R23-substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, two adjacent R2substituents may optionally be joined to form an unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, two adjacent R2substituents may optionally be joined to form a R23-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, two adjacent R2substituents may optionally be joined to form a R23-substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, two adjacent R2substituents may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, two adjacent R2substituents may optionally be joined to form a R23-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, two adjacent R2substituents may optionally be joined to form a R23-substituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, two adjacent R2substituents may optionally be joined to form an unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl). In embodiments, two adjacent R2substituents may optionally be joined to form a R23-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, two adjacent R2substituents may optionally be joined to form a R23-substituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, two adjacent R2substituents may optionally be joined to form an unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). R23is independently oxo, halogen, —CX233, —CHX232, —CH2X23, —OCX233, —OCH2X23, —OCHX232, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R24-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R24-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R24-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R24-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R24-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R24-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R23is independently oxo,
R24is independently oxo, halogen, —CX243, —CHX242, —CH2X24, —OCX243, —OCH2X24, —OCHX242, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R25-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R25-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R25-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R25-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R25-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R25-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R24is independently oxo,
R25is independently oxo, halogen, —CX253, —CHX252, —CH2X25, —OCX253, —OCH2X25, —OCHX252, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X25is independently —F, —Cl, —Br, or —I. In embodiments, R25is independently unsubstituted methyl. In embodiments, R25is independently unsubstituted ethyl. In embodiments, R2Ais independently hydrogen, —CX2A3, —CHX2A2, —CH2X2A, —CN, —COOH, —CONH2, R23A-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R23A-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R23A-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R23A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R23A-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R23A-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Ais independently hydrogen, —CX2A3, —CHX2A2, —CH2X2A, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X2Ais independently —F, —Cl, —Br, or —I. In embodiments, R2Ais independently hydrogen. In embodiments, R2Ais independently unsubstituted methyl. In embodiments, R2Ais independently unsubstituted ethyl. In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R23A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or R23A-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R23A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). R23Ais independently oxo, halogen, —CX23A3, —CHX23A2, —CH2X23A, —OCX23A3, —OCH2X23A, —OCHX23A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R24A-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R24A-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R24A-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R24A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R24A-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R24A-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R23Ais independently oxo,
R24Ais independently oxo, halogen, —CX24A3, —CHX24A2, —CH2X24A, —OCX24A3, —OCH2X24A, —OCHX24A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R25A-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R25A-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R25A-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R25A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R25A-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R25A-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R24Ais independently oxo,
R25Ais independently oxo, halogen, —CX25A3, —CHX25A2, —CH2X25A, —OCX25A3, —OCH2X25A, —OCHX25A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X25Ais independently —F, —Cl, —Br, or —I. In embodiments, R25Ais independently unsubstituted methyl. In embodiments, R25Ais independently unsubstituted ethyl. In embodiments, R2Bis independently hydrogen, —CX2B3, —CHX2B2, —CH2X2B, —CN, —COOH, —CONH2, R23B-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R23B-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R23B-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R23B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R23B-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R23B-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Bis independently hydrogen, —CX2B3, —CHX2B2, —CH2X2B, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X2Bis independently —F, —Cl, —Br, or —I. In embodiments, R2Bis independently hydrogen. In embodiments, R2Bis independently unsubstituted methyl. In embodiments, R2Bis independently unsubstituted ethyl. In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R23B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or R23B-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R23B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). R23Bis independently oxo, halogen, —CX23B3, —CHX23B2, —CH2X23B, —OCX23B3, —OCH2X23B, —OCHX23B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R24B-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R24B-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R24B-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R24B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R24B-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R24B-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R23Bis independently oxo,
R24Bis independently oxo, halogen, —CX24B3, —CHX24B2, —CH2X24B, —OCX24B3, —OCH2X24B, —OCHX24B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R25B-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R25B-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R25B-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R25B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R25B-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R25B-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R24Bis independently oxo,
R25Bis independently oxo, halogen, —CX25B3, —CHX25B2, —CH2X25B, —OCX25B3, —OCH2X25B, —OCHX25B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X25Bis independently —F, —Cl, —Br, or —I. In embodiments, R25Bis independently unsubstituted methyl. In embodiments, R25Bis independently unsubstituted ethyl. In embodiments, R2Cis independently hydrogen, —CX2C3, —CHX2C2, —CH2X2C, —CN, —COOH, —CONH2, R23C-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R23C-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R23C-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R23C-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R23C-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R23C-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Cis independently hydrogen, —CX2C3, —CHX2C2, —CH2X2C, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X2Cis independently —F, —Cl, —Br, or —I. In embodiments, R2Cis independently hydrogen. In embodiments, R2Cis independently unsubstituted methyl. In embodiments, R2Cis independently unsubstituted ethyl. R23Cis independently oxo, halogen, —CX23C3, —CHX23C2, —CH2X23C, —OCX23C3, —OCH2X23C, —OCHX23C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R24C-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R24C-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R24C-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R24C-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R24C-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R24C-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R23Cis independently oxo,
R23Cis independently unsubstituted methyl. In embodiments, R23Cis independently unsubstituted ethyl. R24Cis independently oxo, halogen, —CX24C3, —CHX24C2, —CH2X24C, —OCX24C3, —OCH2X24C, —OCHX24C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R25C-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R25C-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R25C-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R25C-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R25C-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R25C-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R24Cis independently oxo,
R24Cis independently unsubstituted methyl. In embodiments, R24Cis independently unsubstituted ethyl. R25Cis independently oxo, halogen, —CX25C3, —CHX25C2, —CH2X25C, —OCX25C3, —OCH2X25C, —OCHX25C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X25Cis independently —F, —Cl, —Br, or —I. In embodiments, R25Cis independently unsubstituted methyl. In embodiments, R25Cis independently unsubstituted ethyl. In embodiments, R2Dis independently hydrogen, —CX2D3, —CHX2D2, —CH2X2D, —CN, —COOH, —CONH2, R23D-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R23D-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R23D-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R23D-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R23D-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R23D-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R2Dis independently hydrogen, —CX2D3, —CHX2D2, —CH2X2D, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X2Dis independently —F, —Cl, —Br, or —I. In embodiments, R2Dis independently hydrogen. In embodiments, R2Dis independently unsubstituted methyl. In embodiments, R2Dis independently unsubstituted ethyl. R23Dis independently oxo, halogen, —CX23D3, —CHX23D2, —CH2X23D, —OCX23D3, —OCH2X23D, —OCHX23D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R24D-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R24D-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R24D-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R24D-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R24D-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R24D-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R23Dis independently oxo,
R23Dis independently unsubstituted methyl. In embodiments, R23Dis independently unsubstituted ethyl. R24Dis independently oxo, halogen, —CX24D3, —CHX24D2, —CH2X24D, —OCX24D3, —OCH2X24D, —OCHX24D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R25D-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R25D-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R25D-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R25D-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R25D-substituted or unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or R25D-substituted or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R24Dis independently oxo,
R25Dis independently oxo, halogen, —CX25D3, —CHX25D2, —CH2X25D, —OCX25D3, —OCH2X25D, —OCHX25D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X25Dis independently —F, —Cl, —Br, or —I. In embodiments, R25Dis independently unsubstituted methyl. In embodiments, R25Dis independently unsubstituted ethyl. In embodiments, z2 is 0. In embodiments, z2 is 1. In embodiments, z2 is 2. In embodiments, z2 is 3. In embodiments, z2 is 4. In embodiments, L1is a bond, substituted or unsubstituted C1-C8alkylene, substituted or unsubstituted 2 to 8 membered heteroalkylene, substituted or unsubstituted C3-C8cycloalkylene, substituted or unsubstituted 3 to 8 membered heterocycloalkylene, substituted or unsubstituted phenylene, or substituted or unsubstituted 5 to 6 membered heteroarylene. In embodiments, L1is a bond. In embodiments, 12 is a bond. In embodiments, L1is —S(O)2—. In embodiments, L1is —NR4—. In embodiments, L1is —O—. In embodiments, L1is —S—. In embodiments, L1is —C(O)—. In embodiments, L1is —C(O)NR4—. In embodiments, L1is —NR4C(O)—. In embodiments, L1is —NR4C(O)NH—. In embodiments, L1is —NHC(O)NR4—. In embodiments, L1is —C(O)O—. In embodiments, L1is —OC(O)—. In embodiments, L1is —NH—. In embodiments, L1is —C(O)NH—. In embodiments, L1is —NHC(O)—. In embodiments, L1is —NHC(O)NH—. In embodiments, L1is —CH2—. In embodiments, L1is OCH2—. In embodiments, L1is —CH2O—. In embodiments, L1is CH2CH2—. In embodiments, L1is —NHCH2—. In embodiments, L1is —CH2NH—. In embodiments, L1is a bond. In embodiments, L1is a bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4, —NR4C(O)—, —NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), substituted or unsubstituted heteroalkylene (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), substituted or unsubstituted cycloalkylene (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), substituted or unsubstituted heterocycloalkylene (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), substituted or unsubstituted arylene (e.g., C6-C10or phenylene), or substituted or unsubstituted heteroarylene (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, L1is independently substituted or unsubstituted alkylene (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, L1is independently substituted alkylene (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, L1is independently unsubstituted alkylene (e.g., C1-C8, C1-C4, or C1-C2). In embodiments, L1is independently unsubstituted methylene. In embodiments, L1is independently unsubstituted ethylene. In embodiments, L1is independently unsubstituted propylene. In embodiments, L1is independently unsubstituted isopropylene. In embodiments, L1is independently unsubstituted tert-butylene. In embodiments, L1is independently substituted or unsubstituted heteroalkylene (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, L1is independently substituted heteroalkylene (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, L1is independently unsubstituted heteroalkylene (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, L1is independently substituted or unsubstituted cycloalkylene (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, L1is independently substituted cycloalkylene (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, L1is independently unsubstituted cycloalkylene (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, L1is independently substituted or unsubstituted heterocycloalkylene (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, L1is independently substituted heterocycloalkylene (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, L1is independently unsubstituted heterocycloalkylene (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, L1is independently substituted or unsubstituted arylene (e.g., C6-C10or phenylene). In embodiments, L1is independently substituted arylene (e.g., C6-C10or phenylene). In embodiments, L1is independently unsubstituted arylene (e.g., C6-C10or phenylene). In embodiments, L1is independently substituted or unsubstituted heteroarylene (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, L1is independently substituted heteroarylene (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, L1is independently unsubstituted heteroarylene (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, L1is independently bond, —S(O)2—, —N(R4)—, —O—, —S—, —C(O)—, —C(O)N(R4)—, —N(R4)C(O)—, —N(R4)C(O)NH—, —NHC(O) N(R4)—, —C(O)O—, —OC(O)—, R35-substituted or unsubstituted alkylene (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R35-substituted or unsubstituted heteroalkylene (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R35-substituted or unsubstituted cycloalkylene (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R35-substituted or unsubstituted heterocycloalkylene (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R35-substituted or unsubstituted arylene (e.g., C6-C10or phenylene), or R35-substituted or unsubstituted heteroarylene (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, L1is independently bond, —S(O)2—, —N(R4)—, —O—, —S—, —C(O)—, —C(O)N(R4)—, —N(R4)C(O)—, —N(R4)C(O)NH—, —NHC(O) N(R4)—, —C(O)O—, —OC(O)—, unsubstituted alkylene (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkylene (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkylene (e.g., C3-C8, C4-C6, or C5-C6), unsubstituted heterocycloalkylene (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted arylene (e.g., C6-C10or phenylene), or unsubstituted heteroarylene (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, L1is independently unsubstituted methylene. In embodiments, L1is independently unsubstituted ethylene. In embodiments, L1is independently methyl-substituted methylene. R35is independently oxo, halogen, —CX353, —CHX352, —CH2X35, —OCX353, —OCH2X35, —OCHX352, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R36-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R36-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R36-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C4-C6, or C5-C6), R36-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R36-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R36-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R35is independently oxo,
R36is independently oxo, halogen, —CX363, —CHX362, —CH2X36, —OCX363, —OCH2X36, —OCHX362, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R37-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R37-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R37-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R37-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R37-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R37-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R36is independently oxo,
R37is independently oxo, halogen, —CX373, —CHX372, —CH2X37, —OCX373, —OCH2X37, —OCHX372, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X37is independently —F, —Cl, —Br, or —I. In embodiments, R37is independently unsubstituted methyl. In embodiments, R37is independently unsubstituted ethyl. In embodiments, R4is independently hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)OR4A, —C(O)NR4AR4BOR4A—, substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4is independently hydrogen. In embodiments, R4is independently —CX43. In embodiments, R4is independently —CHX42. In embodiments, R4is independently —CH2X4. In embodiments, R4is independently —CN. In embodiments, R4is independently —C(O)R4A. In embodiments, R4is independently —C(O)—OR4A. In embodiments, R4is independently —C(O)NR4AR4B. In embodiments, R4is independently —COOH. In embodiments, R4is independently —CONH2. In embodiments, R4is independently —CF3. In embodiments, R4is independently —CHF2. In embodiments, R4is independently —CH2F. In embodiments, R4is independently —CH3. In embodiments, R4is independently —CH2CH3. In embodiments, R4is independently —CH2CH2CH3. In embodiments, R4is independently —CH(CH3)2. In embodiments, R4is independently —C(CH3)3. In embodiments, R4is independently unsubstituted methyl. In embodiments, R4is independently unsubstituted ethyl. In embodiments, R4is independently unsubstituted propyl. In embodiments, R4is independently unsubstituted isopropyl. In embodiments, R4is independently unsubstituted n-propyl. In embodiments, R4is independently unsubstituted butyl. In embodiments, R4is independently unsubstituted n-butyl. In embodiments, R4is independently unsubstituted t-butyl. In embodiments, R4is independently unsubstituted pentyl. In embodiments, R4is independently unsubstituted n-pentyl. In embodiments, R4is independently unsubstituted hexyl. In embodiments, R4is independently unsubstituted n-hexyl. In embodiments, R4is independently unsubstituted heptyl. In embodiments, R4is independently unsubstituted n-heptyl. In embodiments, R4is independently unsubstituted octyl. In embodiments, R4is independently unsubstituted n-octyl. In embodiments, R4is independently unsubstituted benzyl. In embodiments, R4is independently unsubstituted C1-C8alkyl. In embodiments, R4is independently halo-substituted methyl. In embodiments, R4is independently halo-substituted ethyl. In embodiments, R4is independently halo-substituted isopropyl. In embodiments, R4is independently halo-substituted n-propyl. In embodiments, R4is independently halo-substituted n-butyl. In embodiments, R4is independently halo-substituted t-butyl. In embodiments, R1is independently halo-substituted n-pentyl. In embodiments, R4is independently halo-substituted benzyl. In embodiments, R4is independently halo-substituted C1-C8alkyl. In embodiments, R4is independently unsubstituted 2 to 6 membered heteroalkyl. In embodiments, R4is independently unsubstituted 2 to 7 membered heteroalkyl. In embodiments, R4is independently unsubstituted 2 to 8 membered heteroalkyl. In embodiments, R4is independently unsubstituted 2 to 9 membered heteroalkyl. In embodiments, R4is independently unsubstituted 2 to 10 membered heteroalkyl. In embodiments, R4is independently unsubstituted 3 to 10 membered heteroalkyl. In embodiments, R4is independently unsubstituted 4 to 10 membered heteroalkyl. In embodiments, R4is independently unsubstituted 5 to 10 membered heteroalkyl. In embodiments, R4is independently unsubstituted 6 to 10 membered heteroalkyl. In embodiments, R4is independently unsubstituted 7 to 10 membered heteroalkyl. In embodiments, R4is independently unsubstituted 8 to 10 membered heteroalkyl. In embodiments, R4is independently unsubstituted 6 to 10 membered heteroalkyl. In embodiments, R4is independently unsubstituted 7 to 9 membered heteroalkyl. In embodiments, R4is independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R4is independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R4is independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R4is independently unsubstituted methyl. In embodiments, R4is independently unsubstituted ethyl. In embodiments, R4is independently unsubstituted propyl. In embodiments, R4is independently unsubstituted isopropyl. In embodiments, R4is independently unsubstituted tert-butyl. In embodiments, R4is independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R4is independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R4is independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R4is independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R4is independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R4is independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R4is independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4is independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4is independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4is independently substituted or unsubstituted aryl (e.g., C6-C10or phenyl). In embodiments, R4is independently substituted aryl (e.g., C6-C10or phenyl). In embodiments, R4is independently unsubstituted aryl (e.g., C6-C10or phenyl). In embodiments, R4is independently substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4is independently substituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4is independently unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Ais independently hydrogen. In embodiments, R4Ais independently —CX4A3. In embodiments, R4Ais independently —CHX4A2. In embodiments, R4Ais independently —CH2X4A. In embodiments, R4Ais independently —CN. In embodiments, R4Ais independently —COOH. In embodiments, R4Ais independently —CONH2. In embodiments, X4Ais independently —F, —Cl, —Br, or —I. In embodiments, R4Ais independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R4Ais independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R4Ais independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R4Ais independently unsubstituted methyl. In embodiments, R4Ais independently unsubstituted ethyl. In embodiments, R4Ais independently unsubstituted propyl. In embodiments, R4Ais independently unsubstituted isopropyl. In embodiments, R4Ais independently unsubstituted tert-butyl. In embodiments, R4Ais independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R4Ais independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R4Ais independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R4Ais independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R4Ais independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R4Ais independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R4Ais independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4Ais independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4Ais independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4Ais independently substituted or unsubstituted aryl (e.g., C6-C10or phenyl). In embodiments, R4Ais independently substituted aryl (e.g., C6-C10or phenyl). In embodiments, R4Ais independently unsubstituted aryl (e.g., C6-C10or phenyl). In embodiments, R4Ais independently substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Ais independently substituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Ais independently unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Bis independently hydrogen. In embodiments, R4Bis independently —CX4B3. In embodiments, R4Bis independently —CHX4B2. In embodiments, R4Bis independently —CH2X4B. In embodiments, R4Bis independently —CN. In embodiments, R4Bis independently —COOH. In embodiments, R4Bis independently —CONH2. In embodiments, X4Bis independently —F, —Cl, —Br, or —I. In embodiments, R4Bis independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R4Bis independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R4Bis independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R4Bis independently unsubstituted methyl. In embodiments, R4Bis independently unsubstituted ethyl. In embodiments, R4Bis independently unsubstituted propyl. In embodiments, R4Bis independently unsubstituted isopropyl. In embodiments, R4Bis independently unsubstituted tert-butyl. In embodiments, R4Bis independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R4Bis independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R4Bis independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R4Bis independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R4Bis independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R4Bis independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R4Bis independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4Bis independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4Bis independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4Bis independently substituted or unsubstituted aryl (e.g., C6-C10or phenyl). In embodiments, R4Bis independently substituted aryl (e.g., C6-C10or phenyl). In embodiments, R4Bis independently unsubstituted aryl (e.g., C6-C10or phenyl). In embodiments, R4Bis independently substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Bis independently substituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Bis independently unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may be joined to form an unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4is independently hydrogen, —CX43, —CHX42, —CH2X4, —CN, —COOH, —CONH2, R29-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R29-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R29-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R29-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R29-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R29-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4is independently hydrogen, —CX43, —CHX42, —CH2X4, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X4is independently —F, —Cl, —Br, or —I. In embodiments, R4is independently hydrogen. In embodiments, R4is independently unsubstituted methyl. In embodiments, R4is independently unsubstituted ethyl. R29is independently oxo, halogen, —CX293, —CHX292, —CH2X29, —OCX293, —OCH2X29, —OCHX292, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, 03H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R30-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R30-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R30-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R30-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R30-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R30-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R29is independently oxo,
R30is independently oxo, halogen, —CX303, —CHX302, —CH2X30, —OCX303, —OCH2X30, —OCHX302, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R31-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R31-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R31-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R31-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R31-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R31-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R30is independently oxo,
R31is independently oxo, halogen, —CX313, —CHX312, —CH2X31, —OCX313, —OCH2X31, —OCHX312, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X31is independently —F, —Cl, —Br, or —I. In embodiments, R31is independently unsubstituted methyl. In embodiments, R31is independently unsubstituted ethyl. In embodiments, R4Ais independently hydrogen, —CX4A3, —CHX4A2, —CH2X4A, —CN, —COOH, —CONH2, R29A-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C4, or C1-C2), R29A-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R29A-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R29A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R29A-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R29A-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Ais independently hydrogen, —CX4A3, —CHX4A2, —CH2X4A, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X4Ais independently —F, —Cl, —Br, or —I. In embodiments, R4Ais independently hydrogen. In embodiments, R4Ais independently unsubstituted methyl. In embodiments, R4Ais independently unsubstituted ethyl. In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R29A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or R29A-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R29A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). R29Ais independently oxo, halogen, —CX29A3, —CHX29A2, —CH2X29A, —OCX29A3, —OCH2X29A, —OCHX29A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R30A-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R30A-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R30A-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R30A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R30A-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R30A-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R29Ais independently oxo,
R30Ais independently oxo, halogen, —CX30A3, —CHX30A2, —CH2X30A, —OCX30A3, —OCH2X30A, —OCHX30A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R31A-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R31A-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R31A-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R31A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R31A-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R31A-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R30Ais independently oxo,
R31Ais independently oxo, halogen, —CX31A3, —CHX31A2, —CH2X31A, —OCX31A3, —OCH2X31A, —OCHX31A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X31Ais independently —F, —Cl, —Br, or —I. In embodiments, R31Ais independently unsubstituted methyl. In embodiments, R31Ais independently unsubstituted ethyl. In embodiments, R4Bis independently hydrogen, —CX4B3, —CHX4B2, —CH2X4B, —CN, —COOH, —CONH2, R29B-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R29B-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R29B-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R29B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R29B-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R29B-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Bis independently hydrogen, —CX4B3, —CHX4B2, —CH2X4B, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X4Bis independently —F, —Cl, —Br, or —I. In embodiments, R4Bis independently hydrogen. In embodiments, R4Bis independently unsubstituted methyl. In embodiments, R4Bis independently unsubstituted ethyl. In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R29B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or R29B-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R29B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). R29Bis independently oxo, halogen, —CX29B3, —CHX29B2, —CH2X29B, —OCX29B3, —OCH2X29B, —OCHX29B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R30B-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R30B-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R30B-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R30B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R30B-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R30B-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R29Bis independently oxo,
R30Bis independently oxo, halogen, —CX30B3, —CHX30B2, —CH2X30B, —OCX30B3, —OCH2X30B, —OCHX30B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R31B-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R31B-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R31B-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R31B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R31B-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R31B-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R30Bis independently oxo,
R31Bis independently oxo, halogen, —CX31B3, —CHX31B2, —CH2X31B, —OCX31B3, —OCH2X31B, —OCHX31B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X31Bis independently —F, —Cl, —Br, or —I. In embodiments, R31Bis independently unsubstituted methyl. In embodiments, R31Bis independently unsubstituted ethyl. In embodiments, L2is —NR5— or substituted or unsubstituted heterocycloalkylene including a ring nitrogen bonded directly to E. In embodiments, L2is —NR5—. In embodiments, L2is a bond. In embodiments, L2is —S(O)2—. In embodiments, L2is —NR5—. In embodiments, L2is —O—. In embodiments, L2is —S—. In embodiments, L2is —C(O)—. In embodiments, L2is —C(O)NR5—. In embodiments, L2is —NR5C(O)—. In embodiments, L2is —NR5C(O)NH—. In embodiments, L2is —NHC(O)NR5—. In embodiments, L2is —C(O)O—. In embodiments, L2is —OC(O)—. In embodiments, L2is —NH—. In embodiments, L2is —C(O)NH—. In embodiments, L2is —NHC(O)—. In embodiments, L2is —NHC(O)NH—. In embodiments, L2is —CH2—. In embodiments, L2is OCH2—. In embodiments, L2is —CH2O—. In embodiments, L2is —NHCH2—. In embodiments, L2is —CH2NH—. In embodiments, L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR4C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), substituted or unsubstituted heteroalkylene (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), substituted or unsubstituted cycloalkylene (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), substituted or unsubstituted heterocycloalkylene (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), substituted or unsubstituted arylene (e.g., C6-C10or phenylene), or substituted or unsubstituted heteroarylene (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, L2is independently substituted or unsubstituted alkylene (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, L2is independently substituted alkylene (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, L2is independently unsubstituted alkylene (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, L2is independently unsubstituted methylene. In embodiments, L2is independently unsubstituted ethylene. In embodiments, L2is independently unsubstituted propylene. In embodiments, L2is independently unsubstituted isopropylene. In embodiments, L2is independently unsubstituted tert-butylene. In embodiments, L2is independently substituted or unsubstituted heteroalkylene (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, L2is independently substituted heteroalkylene (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, L2is independently unsubstituted heteroalkylene (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, L2is independently substituted or unsubstituted cycloalkylene (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, L2is independently substituted cycloalkylene (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, L2is independently unsubstituted cycloalkylene (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, L2is independently substituted or unsubstituted heterocycloalkylene (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, L2is independently substituted heterocycloalkylene (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, L2is independently unsubstituted heterocycloalkylene (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, L2is independently substituted or unsubstituted arylene (e.g., C6-C10or phenylene). In embodiments, L2is independently substituted arylene (e.g., C6-C10or phenylene). In embodiments, L2is independently unsubstituted arylene (e.g., C6-C10or phenylene). In embodiments, L2is independently substituted or unsubstituted heteroarylene (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, L2is independently substituted heteroarylene (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, L2is independently unsubstituted heteroarylene (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, L2is independently bond, —S(O)2—, —N(R5)—, —O—, —S—, —C(O)—, —C(O)N(R5)—, —N(R5)C(O)—, —N(R5)C(O)NH—, —NHC(O) N(R5)—, —C(O)O—, —OC(O)—, R38-substituted or unsubstituted alkylene (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R38-substituted or unsubstituted heteroalkylene (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R38-substituted or unsubstituted cycloalkylene (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R38-substituted or unsubstituted heterocycloalkylene (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R38-substituted or unsubstituted arylene (e.g., C6-C10or phenylene), or R38-substituted or unsubstituted heteroarylene (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, L2is independently bond, —S(O)2—, —N(R5)—, —O—, —S—, —C(O)—, —C(O)N(R5)—, —N(R5)C(O)—, —N(R5)C(O)NH—, —NHC(O) N(R5)—, —C(O)O—, —OC(O)—, unsubstituted alkylene (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkylene (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkylene (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkylene (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted arylene (e.g., C6-C10or phenylene), or unsubstituted heteroarylene (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, L2is independently unsubstituted methylene. In embodiments, L2is independently unsubstituted ethylene. In embodiments, L2is independently methyl-substituted methylene. R38is independently oxo, halogen, —CX383, —CHX382, —CH2X38, —OCX383, —OCH2X38, —OCHX382, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R39-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R39-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R39-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R39-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R39-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R39-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R38is independently oxo,
R39is independently oxo, halogen, —CX393, —CHX392, —CH2X39, —OCX393, —OCH2X39, —OCHX392, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R40-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R40-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R40-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R40-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R40-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R40-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R39is independently oxo,
R40is independently oxo, halogen, —CX403, —CHX402, —CH2X40, —OCX403, —OCH2X40, —OCHX402, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X40is independently —F, —Cl, —Br, or —I. In embodiments, R40is independently unsubstituted methyl. In embodiments, R40is independently unsubstituted ethyl. In embodiments, R5is hydrogen, substituted or unsubstituted C1-C6alkyl, or substituted or unsubstituted 2 to 6 membered heteroalkyl. In embodiments, R5is hydrogen or unsubstituted C1-C3alkyl. In embodiments, R5is hydrogen, unsubstituted methyl, unsubstituted ethyl, unsubstituted hexyl, or unsubstituted benzyl. In embodiments, R5is hydrogen. In embodiments, R5is independently unsubstituted methyl. In embodiments, R5is independently unsubstituted ethyl. In embodiments, R5is independently unsubstituted propyl. In embodiments, R5is independently unsubstituted isopropyl. In embodiments, R5is independently unsubstituted n-propyl. In embodiments, R5is independently unsubstituted butyl. In embodiments, R5is independently unsubstituted n-butyl. In embodiments, R5is independently unsubstituted t-butyl. In embodiments, R5is independently unsubstituted pentyl. In embodiments, R5is independently unsubstituted n-pentyl. In embodiments, R5is independently unsubstituted hexyl. In embodiments, R5is independently unsubstituted n-hexyl. In embodiments, R5is independently unsubstituted heptyl. In embodiments, R5is independently unsubstituted n-heptyl. In embodiments, R5is independently unsubstituted octyl. In embodiments, R5is independently unsubstituted n-octyl. In embodiments, R5is independently unsubstituted benzyl. In embodiments, R5is independently unsubstituted C1-C8alkyl. In embodiments, R5is independently halo-substituted methyl. In embodiments, R5is independently halo-substituted ethyl. In embodiments, R5is independently halo-substituted isopropyl. In embodiments, R5is independently halo-substituted n-propyl. In embodiments, R5is independently halo-substituted n-butyl. In embodiments, R5is independently halo-substituted t-butyl. In embodiments, R1is independently halo-substituted n-pentyl. In embodiments, R5is independently halo-substituted benzyl. In embodiments, R5is independently halo-substituted C1-C8alkyl. In embodiments, R5is independently unsubstituted 2 to 6 membered heteroalkyl. In embodiments, R5is independently unsubstituted 2 to 7 membered heteroalkyl. In embodiments, R5is independently unsubstituted 2 to 8 membered heteroalkyl. In embodiments, R5is independently unsubstituted 2 to 9 membered heteroalkyl. In embodiments, R5is independently unsubstituted 2 to 10 membered heteroalkyl. In embodiments, R5is independently unsubstituted 3 to 10 membered heteroalkyl. In embodiments, R5is independently unsubstituted 4 to 10 membered heteroalkyl. In embodiments, R5is independently unsubstituted 5 to 10 membered heteroalkyl. In embodiments, R5is independently unsubstituted 6 to 10 membered heteroalkyl. In embodiments, R5is independently unsubstituted 7 to 10 membered heteroalkyl. In embodiments, R5is independently unsubstituted 8 to 10 membered heteroalkyl. In embodiments, R5is independently unsubstituted 6 to 10 membered heteroalkyl. In embodiments, R5is independently unsubstituted 7 to 9 membered heteroalkyl. In embodiments, R5is independently hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)OR5A, —C(O)NR5AR5B, —OR5A, substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5is independently hydrogen. In embodiments, R5is independently —CX53. In embodiments, R5is independently —CHX52. In embodiments, R5is independently —CH2X5. In embodiments, R5is independently —CN. In embodiments, R5is independently —C(O)R5A. In embodiments, R5is independently —C(O)—OR5A. In embodiments, R5is independently —C(O)NR5AR5B. In embodiments, R5is independently —COOH. In embodiments, R5is independently —CONH2. In embodiments, R5is independently —CF3. In embodiments, R5is independently —CHF2. In embodiments, R5is independently —CH2F. In embodiments, R5is independently —CH3. In embodiments, R5is independently —CH2CH3. In embodiments, R5is independently —CH2CH2CH3. In embodiments, R5is independently —CH(CH3)2. In embodiments, R5is independently —C(CH3)3. In embodiments, R5is independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R5is independently substituted alkyl (e.g., C1-C8, C1-C4, or C1-C2). In embodiments, R5is independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R5is independently unsubstituted methyl. In embodiments, R5is independently unsubstituted ethyl. In embodiments, R5is independently unsubstituted propyl. In embodiments, R5is independently unsubstituted isopropyl. In embodiments, R5is independently unsubstituted tert-butyl. In embodiments, R5is independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R5is independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R5is independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R5is independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R5is independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R5is independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R5is independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5is independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5is independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5is independently substituted or unsubstituted aryl (e.g., C6-C10or phenyl). In embodiments, R5is independently substituted aryl (e.g., C6-C10or phenyl). In embodiments, R5is independently unsubstituted aryl (e.g., C6-C10or phenyl). In embodiments, R5is independently substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5is independently substituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5is independently unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Ais independently hydrogen. In embodiments, R5Ais independently —CX5A3. In embodiments, R5Ais independently —CHX5A2. In embodiments, R5Ais independently —CH2X5A. In embodiments, R5Ais independently —CN. In embodiments, R5Ais independently —COOH. In embodiments, R5Ais independently —CONH2. In embodiments, X5Ais independently —F, —Cl, —Br, or —I. In embodiments, R5Ais independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R5Ais independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R5Ais independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R5Ais independently unsubstituted methyl. In embodiments, R5Ais independently unsubstituted ethyl. In embodiments, R5Ais independently unsubstituted propyl. In embodiments, R5Ais independently unsubstituted isopropyl. In embodiments, R5Ais independently unsubstituted tert-butyl. In embodiments, R5Ais independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R5Ais independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R5Ais independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R5Ais independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R5Ais independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R5Ais independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R5Ais independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5Ais independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5Ais independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5Ais independently substituted or unsubstituted aryl (e.g., C6-C10or phenyl). In embodiments, R5Ais independently substituted aryl (e.g., C6-C10or phenyl). In embodiments, R5Ais independently unsubstituted aryl (e.g., C6-C10or phenyl). In embodiments, R5Ais independently substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Ais independently substituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Ais independently unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Bis independently hydrogen. In embodiments, R5Bis independently —CX5B3. In embodiments, R5Bis independently —CHX5B2. In embodiments, R5Bis independently —CH2X5B. In embodiments, R5Bis independently —CN. In embodiments, R5Bis independently —COOH. In embodiments, R5Bis independently —CONH2. In embodiments, X5Bis independently —F, —Cl, —Br, or —I. In embodiments, R5Bis independently substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R5Bis independently substituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R5Bis independently unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2). In embodiments, R5Bis independently unsubstituted methyl. In embodiments, R5Bis independently unsubstituted ethyl. In embodiments, R5Bis independently unsubstituted propyl. In embodiments, R5Bis independently unsubstituted isopropyl. In embodiments, R5Bis independently unsubstituted tert-butyl. In embodiments, R5Bis independently substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R5Bis independently substituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R5Bis independently unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered). In embodiments, R5Bis independently substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R5Bis independently substituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R5Bis independently unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6). In embodiments, R5Bis independently substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5Bis independently substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5Bis independently unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5Bis independently substituted or unsubstituted aryl (e.g., C6-C10or phenyl). In embodiments, R5Bis independently substituted aryl (e.g., C6-C10or phenyl). In embodiments, R5Bis independently unsubstituted aryl (e.g., C6-C10or phenyl). In embodiments, R5Bis independently substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Bis independently substituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Bis independently unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may be joined to form a substituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may be joined to form an unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5is independently hydrogen, —CX53, —CHX52, —CH2X5, —CN, —COOH, —CONH2, R32-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R32-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R32-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R32-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R32-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R32-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5is independently hydrogen, —CX53, —CHX52, —CH2X5, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X5is independently —F, —Cl, —Br, or —I. In embodiments, R5is independently hydrogen. In embodiments, R5is independently unsubstituted methyl. In embodiments, R5is independently unsubstituted ethyl. R32is independently oxo, halogen, —CX323, —CHX322, —CH2X32, —OCX323, —OCH2X32, —OCHX322, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R33-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R33-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R33-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R33-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R33-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R33-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R32is independently oxo,
R33is independently oxo, halogen, —CX333, —CHX332, —CH2X33, —OCX333, —OCH2X33, —OCHX332, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R34-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R34-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R34-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R34-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R34-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R34-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R33is independently oxo,
R34is independently oxo, halogen, —CX343, —CHX342, —CH2X34, —OCX343, —OCH2X34, —OCHX342, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X34is independently —F, —Cl, —Br, or —I. In embodiments, R34is independently unsubstituted methyl. In embodiments, R34is independently unsubstituted ethyl. In embodiments, R5Ais independently hydrogen, —CX5A3, —CHX5A2, —CH2X5A, —CN, —COOH, —CONH2, R32A-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R32A-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R32A-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R32A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R32A-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R32A-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Ais independently hydrogen, —CX5A3, —CHX5A2, —CH2X5A, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X5Ais independently —F, —Cl, —Br, or —I. In embodiments, R5Ais independently hydrogen. In embodiments, R5Ais independently unsubstituted methyl. In embodiments, R5Ais independently unsubstituted ethyl. In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R32A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or R32A-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R32A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). R32Ais independently oxo, halogen, —CX32A3, —CHX32A2, —CH2X32A, —OCX32A3, —OCH2X32A, —OCHX32A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R33A-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R33A-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R33A-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R33A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R33A-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R33A-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R32Ais independently oxo,
R33Ais independently oxo, halogen, —CX33A3, —CHX33A2, —CH2X33A, —OCX33A3, —OCH2X33A, —OCHX33A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R34A-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R34A-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R34A-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R34A-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R34A-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R34A-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R33Ais independently oxo,
R34Ais independently oxo, halogen, —CX34A3, —CHX34A2, —CH2X34A, —OCX34A3, —OCH2X34A, —OCHX34A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X34Ais independently —F, —Cl, —Br, or —I. In embodiments, R34Ais independently unsubstituted methyl. In embodiments, R34Ais independently unsubstituted ethyl. In embodiments, R5Bis independently hydrogen, —CX5B3, —CHX5B2, —CH2X5B, —CN, —COOH, —CONH2, R32B-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R32B-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R32B-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R32B-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R32B-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Bis independently hydrogen, —CX5B3, —CHX5B2, —CH2X5B, —CN, —COOH, —CONH2, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X5Bis independently —F, —Cl, —Br, or —I. In embodiments, R5Bis independently hydrogen. In embodiments, R5Bis independently unsubstituted methyl. In embodiments, R5Bis independently unsubstituted ethyl. In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R32B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or R32B-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered) or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a R32B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). In embodiments, R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form an unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered). R32Bis independently oxo, halogen, —CX32B3, —CHX32B2, —CH2X32B, —OCX32B3, —OCH2X32B, —OCHX32B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R33B-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R33B-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R33B-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R33B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R33B-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R33B-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R32Bis independently oxo,
R33Bis independently oxo, halogen, —CX33B3, —CHX33B2, —CH2X33B, —OCX33B3, —OCH2X33B, —OCHX33B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, R34B-substituted or unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), R34B-substituted or unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), R34B-substituted or unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), R34B-substituted or unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), R34B-substituted or unsubstituted aryl (e.g., C6-C10or phenyl), or R34B-substituted or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). In embodiments, R33Bis independently oxo,
R34Bis independently oxo, halogen, —CX34B3, —CHX34B2, —CH2X34B, —OCX34B3, —OCH2X34B, —OCHX34B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X34Bis independently —F, —Cl, —Br, or —I. In embodiments, R34Bis independently unsubstituted methyl. In embodiments, R34Bis independently unsubstituted ethyl. In embodiments, X is —F. In embodiments, X is —Cl. In embodiments, X is —Br. In embodiments, X is —I. In embodiments, X1is —F. In embodiments, X1is —Cl. In embodiments, X1is —Br. In embodiments, X1is —I. In embodiments, X2is —F. In embodiments, X2is —Cl. In embodiments, X2is —Br. In embodiments, X2is —I. In embodiments, X4is —F. In embodiments, X4is —Cl. In embodiments, X4is —Br. In embodiments, X4is —I. In embodiments, X5is —F. In embodiments, X5is —Cl. In embodiments, X5is —Br. In embodiments, X5is —I. In embodiments, n1 is 0. In embodiments, n1 is 1. In embodiments, n1 is 2. In embodiments, n1 is 3. In embodiments, n1 is 4. In embodiments, n2 is 0. In embodiments, n2 is 1. In embodiments, n2 is 2. In embodiments, n2 is 3. In embodiments, n2 is 4. In embodiments, n4 is 0. In embodiments, n4 is 1. In embodiments, n4 is 2. In embodiments, n4 is 3. In embodiments, n4 is 4. In embodiments, n5 is 0. In embodiments, n5 is 1. In embodiments, n5 is 2. In embodiments, n5 is 3. In embodiments, n5 is 4. In embodiments, m1 is 1. In embodiments, m1 is 2. In embodiments, m2 is 1. In embodiments, m2 is 2. In embodiments, m4 is 1. In embodiments, m4 is 2. In embodiments, m5 is 1. In embodiments, m5 is 2. In embodiments, v1 is 1. In embodiments, v1 is 2. In embodiments, v2 is 1. In embodiments, v2 is 2. In embodiments, v4 is 1. In embodiments, v4 is 2. In embodiments, v5 is 1. In embodiments, v5 is 2. In embodiments, E is a covalent cysteine modifier moiety. In embodiments, E is: R15is independently hydrogen, halogen, CX153, —CHX152, —CH2X15, —CN, —SOn15R15D, —SOv15NR15AR15B, —NHNR15AR15B, —ONR15AR15B, —NHC═(O)NHNR15AR15B, —NHC(O)NR15AR15B, —N(O)m15, —NR15AR15B, —C(O)R15C, —C(O)—OR15C, —C(O)NR15AR15B, —OR15D, —NR15ASO2R15D, —NR15AC(O)R15C, —NR15AC(O)OR15C, —NR15AOR15C, —OCX153, —OCHX152, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl. R16is independently hydrogen, halogen, CX163, —CHX162, —CH2X16, —CN, —SOn16R16D, —SOv16NR16AR16B, —NHNR16AR16B, —ONR16AR16B, —NHC═(O)NHNR16AR16B, —NHC(O)NR16AR16B, —N(O)m16, —NR16AR16B, —C(O)R16C, —C(O)—OR16C, —C(O)NR16AR16B, —OR16D, —NR16ASO2R16D, —NR16AC(O)R16C, —NR16AC(O)OR16C, —NR16AOR16C, —OCX163, —OCHX162, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl. R17is independently hydrogen, halogen, CX173, —CHX172, —CH2X17, —CN, —SOn17R17D, —SOv17NR17AR17B, —NHNR17AR17B, —ONR17AR17B, —NHC═(O)NHNR17AR17B, —NHC(O)NR17AR17B, —N(O)m17, —NR17AR17B, —C(O)R17C, —C(O)—OR17C, —C(O)NR17AR17B, —OR17D, —NR17ASO2R17D, —NR17AC(O)R17C, —NR17AC(O)OR17C, —NR17AOR17C, —OCX173, —OCHX172, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl. R18is independently hydrogen, —CX183, —CHX182, —CH2X18, —C(O)R18C, —C(O)OR18C, —C(O)NR18AR18B, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl. Each R15A, R15B, R15C, R15D, R16A, R16B, R16C, R16D, R17A, R17B, R17C, R17D, R18A, R18B, R18C, and R18D, is independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R15Aand R15Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R16Aand R16Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R17Aand R17Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R18Aand R18Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl. Each X, X15, X16, X17and X18is independently —F, —Cl, —Br, or —I. The symbols n15, n16, n17, v15, v16, and v17, are independently and integer from 0 to 4. The symbols m15, m16, and m17 are independently and integer between 1 and 2. In embodiments, E is: and X17is —Cl. In embodiments, E is: In embodiments, X17is —Cl. In embodiments, E is: and R15, R16, and R17are independently hydrogen. In embodiments, E is: In embodiments, R15, R16, and R17are independently hydrogen. In embodiments, E is: R15is independently hydrogen; R16is independently hydrogen or CH2NR16AR16B; R17is independently hydrogen; and R16Aand R16Bare independently hydrogen or unsubstituted alkyl. In embodiments, E is: In embodiments, R15is independently hydrogen. In embodiments, R16is independently hydrogen or CH2NR16AR16B. In embodiments, R17is independently hydrogen. In embodiments, R16Aand R16are independently hydrogen or unsubstituted alkyl. In embodiments, R16Aand R16Bare independently unsubstituted methyl. In embodiments, E is: In embodiments, E is: In embodiments, E is: In embodiments, E is: In embodiments, E is: In embodiments, E is: In embodiments, E is: In embodiments, E is: In embodiments, E is: In embodiments, E is: X may independently be F. X may independently be —Cl. X may independently be —Br. X may independently be —I. X15may independently be —F. X15may independently be —Cl. X15may independently be —Br. X15may independently be —I. X16may independently be —F. X16may independently be —Cl. X16may independently be —Br. X16may independently be —I. X17may independently be —F. X17may independently be —Cl. X17may independently be —Br. X17may independently be —I. X18may independently be —F. X18may independently be —Cl. X18may independently be —Br. X18may independently be —I. n15 may independently be 0. n15 may independently be 1. n15 may independently be 2. n15 may independently be 3. n15 may independently be 4. n16 may independently be 0. n16 may independently be 1. n16 may independently be 2. n16 may independently be 3. n16 may independently be 4. n17 may independently be 0. n17 may independently be 1. n17 may independently be 2. n17 may independently be 3. n17 may independently be 4. v15 may independently be 0. v15 may independently be 1. v15 may independently be 2. v15 may independently be 3. v15 may independently be 4. v16 may independently be 0. v16 may independently be 1. v16 may independently be 2. v16 may independently be 3. v16 may independently be 4. v17 may independently be 0. v17 may independently be 1. v17 may independently be 2. v17 may independently be 3. v17 may independently be 4. m15 may independently be 1. m15 may independently be 2. m16 may independently be 1. m16 may independently be 2. m17 may independently be 1. m17 may independently be 2. In embodiments, R15is hydrogen. In embodiments, R15is halogen. In embodiments, R15is CX153. In embodiments, R15is —CHX152. In embodiments, R15is —CH2X15. In embodiments, R15is —CN. In embodiments, R15is —SOn15R15D. In embodiments, R15is —SOv15NR15AR15B. In embodiments, R15is —NHNR15AR15B. In embodiments, R15is —ONR15AR15B. In embodiments, R15is —NHC═(O)NHNR15AR15B. In embodiments, R15is —NHC(O)NR15AR15B. In embodiments, R15is —N(O)m15. In embodiments, R15is —NR15AR15B. In embodiments, R15is —C(O)R15C. In embodiments, R15is —C(O)—OR15C. In embodiments, R15is —C(O)NR15AR15B. In embodiments, R15is —OR15D. In embodiments, R15is —NR15ASO2R15D. In embodiments, R15is —NR15AC(O)R15C. In embodiments, R15is —NR15AC(O)OR15C. In embodiments, R15is —NR15AOR15C. In embodiments, R15is —OCX153. In embodiments, R15is —OCHX152. In embodiments, R15is substituted or unsubstituted alkyl. In embodiments, R15is substituted or unsubstituted heteroalkyl. In embodiments, R15is substituted or unsubstituted cycloalkyl. In embodiments, R15is substituted or unsubstituted heterocycloalkyl. In embodiments, R15is substituted or unsubstituted aryl. In embodiments, R15is substituted or unsubstituted heteroaryl. In embodiments, R15is substituted alkyl. In embodiments, R15is substituted heteroalkyl. In embodiments, R15is substituted cycloalkyl. In embodiments, R15is substituted heterocycloalkyl. In embodiments, R15is substituted aryl. In embodiments, R15is substituted heteroaryl. In embodiments, R15is unsubstituted alkyl. In embodiments, R15is unsubstituted heteroalkyl. In embodiments, R15is unsubstituted cycloalkyl. In embodiments, R15is unsubstituted heterocycloalkyl. In embodiments, R15is unsubstituted aryl. In embodiments, R15is unsubstituted heteroaryl. In embodiments, R15is unsubstituted methyl. In embodiments, R15is unsubstituted ethyl. In embodiments, R15is unsubstituted propyl. In embodiments, R15is unsubstituted isopropyl. In embodiments, R15is unsubstituted butyl. In embodiments, R15is unsubstituted tert-butyl. In embodiments, R15Ais hydrogen. In embodiments, R15Ais —CX3. In embodiments, R15Ais —CN. In embodiments, R15Ais —COOH. In embodiments, R15Ais —CONH2. In embodiments, R15Ais —CHX2. In embodiments, R15Ais —CH2X. In embodiments, R15Ais unsubstituted methyl. In embodiments, R15Ais unsubstituted ethyl. In embodiments, R15Ais unsubstituted propyl. In embodiments, R15Ais unsubstituted isopropyl. In embodiments, R15Ais unsubstituted butyl. In embodiments, R15Ais unsubstituted tert-butyl. In embodiments, R15Bis hydrogen. In embodiments, R15Bis —CX3. In embodiments, R15Bis —CN. In embodiments, R15Bis —COOH. In embodiments, R15Bis —CONH2. In embodiments, R15Bis —CHX2. In embodiments, R15Bis —CH2X. In embodiments, R15Bis unsubstituted methyl. In embodiments, R15Bis unsubstituted ethyl. In embodiments, R15Bis unsubstituted propyl. In embodiments, R15Bis unsubstituted isopropyl. In embodiments, R15Bis unsubstituted butyl. In embodiments, R15Bis unsubstituted tert-butyl. In embodiments, R15Cis hydrogen. In embodiments, R15Cis —CX3. In embodiments, R15Cis —CN. In embodiments, R15Cis —COOH. In embodiments, R15Cis —CONH2. In embodiments, R15Cis —CHX2. In embodiments, R15Cis —CH2X. In embodiments, R15Cis unsubstituted methyl. In embodiments, R15Cis unsubstituted ethyl. In embodiments, R15Cis unsubstituted propyl. In embodiments, R15Cis unsubstituted isopropyl. In embodiments, R15Cis unsubstituted butyl. In embodiments, R15Cis unsubstituted tert-butyl. In embodiments, R15Dis hydrogen. In embodiments, R15Dis —CX3. In embodiments, R15Dis —CN. In embodiments, R15Dis —COOH. In embodiments, R15Dis —CONH2. In embodiments, R15Dis —CHX2. In embodiments, R15Dis —CH2X. In embodiments, R15Dis unsubstituted methyl. In embodiments, R15Dis unsubstituted ethyl. In embodiments, R15Dis unsubstituted propyl. In embodiments, R15Dis unsubstituted isopropyl. In embodiments, R15Dis unsubstituted butyl. In embodiments, R15Dis unsubstituted tert-butyl. In embodiments, R15is independently hydrogen, oxo, halogen, —CX153, —CHX152, —OCH2X15, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX153, —OCHX152, R72-substituted or unsubstituted alkyl, R72-substituted or unsubstituted heteroalkyl, R72-substituted or unsubstituted cycloalkyl, R72-substituted or unsubstituted heterocycloalkyl, R72-substituted or unsubstituted aryl, or R72-substituted or unsubstituted heteroaryl. X15is halogen. In embodiments, X15is F. R72is independently oxo, halogen, —CX723, —CHX722, —OCH2X72, —OCHX722, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —S H, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX723, —OCHX722, R73-substituted or unsubstituted alkyl, R73-substituted or unsubstituted heteroalkyl, R73-substituted or unsubstituted cycloalkyl, R73-substituted or unsubstituted heterocycloalkyl, R73-substituted or unsubstituted aryl, or R73-substituted or unsubstituted heteroaryl. X72is halogen. In embodiments, X72is F. R73is independently oxo, halogen, —CX733, —CHX732, —OCH2X73, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX733, —OCHX732, R74-substituted or unsubstituted alkyl, R74-substituted or unsubstituted heteroalkyl, R74-substituted or unsubstituted cycloalkyl, R74-substituted or unsubstituted heterocycloalkyl, R74-substituted or unsubstituted aryl, or R74-substituted or unsubstituted heteroaryl. X73is halogen. In embodiments, X73is F. In embodiments, R15Ais independently hydrogen, oxo, halogen, —CX15A3, —CHX15A2, —OCH2X15A, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX15A3, —OCHX15A2, R72A-substituted or unsubstituted alkyl, R72A-substituted or unsubstituted heteroalkyl, R72A-substituted or unsubstituted cycloalkyl, R72A-substituted or unsubstituted heterocycloalkyl, R72A-substituted or unsubstituted aryl, or R72A-substituted or unsubstituted heteroaryl. X15Ais halogen. In embodiments, X15Ais F. R72Ais independently oxo, halogen, —CX72A3, —CHX72A2, —OCH2X72A, —OCHX72A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX72A3, —OCHX72A2, R73A-substituted or unsubstituted alkyl, R73A-substituted or unsubstituted heteroalkyl, R73A-substituted or unsubstituted cycloalkyl, R73A-substituted or unsubstituted heterocycloalkyl, R73A-substituted or unsubstituted aryl, or R73A-substituted or unsubstituted heteroaryl. X72Ais halogen. In embodiments, X72Ais F. R73Ais independently oxo, halogen, —CX73A3, —CHX73A2, —OCH2X73A, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX73A3, —OCHX73A2, R74A-substituted or unsubstituted alkyl, R74A-substituted or unsubstituted heteroalkyl, R74A-substituted or unsubstituted cycloalkyl, R74A-substituted or unsubstituted heterocycloalkyl, R74A-substituted or unsubstituted aryl, or R74A-substituted or unsubstituted heteroaryl. X73Ais halogen. In embodiments, X73Ais F. In embodiments, R15Bis independently hydrogen, oxo, halogen, —CX15B3, —CHX15B2, —OCH2X15B, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX15B3, —OCHX15B2, R72B-substituted or unsubstituted alkyl, R72B-substituted or unsubstituted heteroalkyl, R72B-substituted or unsubstituted cycloalkyl, R72B-substituted or unsubstituted heterocycloalkyl, R72B-substituted or unsubstituted aryl, or R72B-substituted or unsubstituted heteroaryl. X15Bis halogen. In embodiments, X15Bis F. R72Bis independently oxo, halogen, —CX72B3, —CHX72B2, —OCH2X72B, —OCHX72B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX72B3, —OCHX72B2, R73B-substituted or unsubstituted alkyl, R73B-substituted or unsubstituted heteroalkyl, R73B-substituted or unsubstituted cycloalkyl, R73B-substituted or unsubstituted heterocycloalkyl, R73B-substituted or unsubstituted aryl, or R73B-substituted or unsubstituted heteroaryl. X72Bis halogen. In embodiments, X72Bis F. R73Bis independently oxo, halogen, —CX73B3, —CHX73B2, —OCH2X73B, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX73B3, —OCHX73B2, R74B-substituted or unsubstituted alkyl, R74B-substituted or unsubstituted heteroalkyl, R74B-substituted or unsubstituted cycloalkyl, R74B-substituted or unsubstituted heterocycloalkyl, R74B-substituted or unsubstituted aryl, or R74B-substituted or unsubstituted heteroaryl. X73Bis halogen. In embodiments, X73Bis F. In embodiments, R15Cis independently hydrogen, oxo, halogen, —CX15C3, —CHX15C2, —OCH2X15C, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX15C3, —OCHX15C2, R72C-substituted or unsubstituted alkyl, R72C-substituted or unsubstituted heteroalkyl, R72C-substituted or unsubstituted cycloalkyl, R72C-substituted or unsubstituted heterocycloalkyl, R72C-substituted or unsubstituted aryl, or R72C-substituted or unsubstituted heteroaryl. X15Cis halogen. In embodiments, X15Cis F. R72Cis independently oxo, halogen, —CX72C3, —CHX72C2, —OCH2X72C, —OCHX72C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX72C3, —OCHX72C2, R73C-substituted or unsubstituted alkyl, R73C-substituted or unsubstituted heteroalkyl, R73C-substituted or unsubstituted cycloalkyl, R73C-substituted or unsubstituted heterocycloalkyl, R73C-substituted or unsubstituted aryl, or R73C-substituted or unsubstituted heteroaryl. X72Cis halogen. In embodiments, X72Cis F. R73Cis independently oxo, halogen, —CX73C3, —CHX73C2, —OCH2X73C, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX73C3, —OCHX73C2, R74C-substituted or unsubstituted alkyl, R74C-substituted or unsubstituted heteroalkyl, R74C-substituted or unsubstituted cycloalkyl, R74C-substituted or unsubstituted heterocycloalkyl, R74C-substituted or unsubstituted aryl, or R74C-substituted or unsubstituted heteroaryl. X73Cis halogen. In embodiments, X73Cis F. In embodiments, R15Dis independently hydrogen, oxo, halogen, —CX15D3, —CHX15D2, —OCH2X15D, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX15D3, —OCHX15D2, R72D-substituted or unsubstituted alkyl, R72D-substituted or unsubstituted heteroalkyl, R72D-substituted or unsubstituted cycloalkyl, R72D-substituted or unsubstituted heterocycloalkyl, R72D-substituted or unsubstituted aryl, or R72D-substituted or unsubstituted heteroaryl. X15Dis halogen. In embodiments, X15Dis F. R72Dis independently oxo, halogen, —CX72D3, —CHX72D2, —OCH2X72D, —OCHX72D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX72D3, —OCHX72D2, R73D-substituted or unsubstituted alkyl, R73D-substituted or unsubstituted heteroalkyl, R73D-substituted or unsubstituted cycloalkyl, R73D-substituted or unsubstituted heterocycloalkyl, R73D-substituted or unsubstituted aryl, or R73D-substituted or unsubstituted heteroaryl. X72Dis halogen. In embodiments, X72Dis F. R73Dis independently oxo, halogen, —CX73D3, —CHX73D2, —OCH2X73D, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX73D3, —OCHX73D2, R74D-substituted or unsubstituted alkyl, R74D-substituted or unsubstituted heteroalkyl, R74D-substituted or unsubstituted cycloalkyl, R74D-substituted or unsubstituted heterocycloalkyl, R74D-substituted or unsubstituted aryl, or R74D-substituted or unsubstituted heteroaryl. X73Dis halogen. In embodiments, X73Dis F. In embodiments, R16is hydrogen. In embodiments, R16is halogen. In embodiments, R16is —CX163. In embodiments, R16is —CHX162. In embodiments, R16is —CH2X16. In embodiments, R16is —CN. In embodiments, R16is —SOn16R16D. In embodiments, R16is —SOv16NR16AR16B. In embodiments, R16is —NHNR16AR16B. In embodiments, R16is —ONR16AR16B. In embodiments, R16is —NHC═(O)NHNR16AR16B. In embodiments, R16is —NHC(O)NR16AR16B. In embodiments, R16is —N(O)m16. In embodiments, R16is —NR16AR16B. In embodiments, R16is —C(O)R16C. In embodiments, R16is —C(O)—OR16C. In embodiments, R16is —C(O)NR16AR16B. In embodiments, R16is —OR16D. In embodiments, R16is —NR16ASO2R16D. In embodiments, R16is —NR16AC(O)R16C. In embodiments, R16is —NR16AC(O)OR16C. In embodiments, R16is —NR16AOR16C. In embodiments, R16is —OCX163. In embodiments, R16is —OCHX162. In embodiments, R16is substituted or unsubstituted alkyl. In embodiments, R16is substituted or unsubstituted heteroalkyl. In embodiments, R16is substituted or unsubstituted cycloalkyl. In embodiments, R16is substituted or unsubstituted heterocycloalkyl. In embodiments, R16is substituted or unsubstituted aryl. In embodiments, R16is substituted or unsubstituted heteroaryl. In embodiments, R16is substituted alkyl. In embodiments, R16is substituted heteroalkyl. In embodiments, R16is substituted cycloalkyl. In embodiments, R16is substituted heterocycloalkyl. In embodiments, R16is substituted aryl. In embodiments, R16is substituted heteroaryl. In embodiments, R16is unsubstituted alkyl. In embodiments, R16is unsubstituted heteroalkyl. In embodiments, R16is unsubstituted cycloalkyl. In embodiments, R16is unsubstituted heterocycloalkyl. In embodiments, R16is unsubstituted aryl. In embodiments, R16is unsubstituted heteroaryl. In embodiments, R16is unsubstituted methyl. In embodiments, R16is unsubstituted ethyl. In embodiments, R16is unsubstituted propyl. In embodiments, R16is unsubstituted isopropyl. In embodiments, R16is unsubstituted butyl. In embodiments, R16is unsubstituted tert-butyl. In embodiments, R16Ais hydrogen. In embodiments, R16Ais —CX3. In embodiments, R16Ais —CN. In embodiments, R16Ais —COOH. In embodiments, R16Ais —CONH2. In embodiments, R16Ais —CHX2. In embodiments, R16Ais —CH2X. In embodiments, R16Ais unsubstituted methyl. In embodiments, R16Ais unsubstituted ethyl. In embodiments, R16Ais unsubstituted propyl. In embodiments, R16Ais unsubstituted isopropyl. In embodiments, R16Ais unsubstituted butyl. In embodiments, R16Ais unsubstituted tert-butyl. In embodiments, R16Bis hydrogen. In embodiments, R16Bis —CX3. In embodiments, R16Bis —CN. In embodiments, R16Bis —COOH. In embodiments, R16Bis —CONH2. In embodiments, R16Bis —CHX2. In embodiments, R16Bis —CH2X. In embodiments, R16Bis unsubstituted methyl. In embodiments, R16Bis unsubstituted ethyl. In embodiments, R16Bis unsubstituted propyl. In embodiments, R16Bis unsubstituted isopropyl. In embodiments, R16Bis unsubstituted butyl. In embodiments, R16Bis unsubstituted tert-butyl. In embodiments, R16Cis hydrogen. In embodiments, R16Cis —CX3. In embodiments, R16Cis —CN. In embodiments, R16Cis —COOH. In embodiments, R16Cis —CONH2. In embodiments, R16Cis —CHX2. In embodiments, R16Cis —CH2X. In embodiments, R16Cis unsubstituted methyl. In embodiments, R16Cis unsubstituted ethyl. In embodiments, R16Cis unsubstituted propyl. In embodiments, R16Cis unsubstituted isopropyl. In embodiments, R16Cis unsubstituted butyl. In embodiments, R16Cis unsubstituted tert-butyl. In embodiments, R16Dis hydrogen. In embodiments, R16Dis —CX3. In embodiments, R16Dis —CN. In embodiments, R16Dis —COOH. In embodiments, R16Dis —CONH2. In embodiments, R16Dis —CHX2. In embodiments, R16Dis —CH2X. In embodiments, R16Dis unsubstituted methyl. In embodiments, R16Dis unsubstituted ethyl. In embodiments, R16Dis unsubstituted propyl. In embodiments, R16Dis unsubstituted isopropyl. In embodiments, R16Dis unsubstituted butyl. In embodiments, R16Dis unsubstituted tert-butyl. In embodiments, R16is independently hydrogen, oxo, halogen, —CX163, —CHX162, —OCH2X16, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX163, —OCHX162, R75-substituted or unsubstituted alkyl, R75-substituted or unsubstituted heteroalkyl, R75-substituted or unsubstituted cycloalkyl, R75-substituted or unsubstituted heterocycloalkyl, R75-substituted or unsubstituted aryl, or R75-substituted or unsubstituted heteroaryl. X16is halogen. In embodiments, X16is F. R75is independently oxo, halogen, —CX753, —CHX752, —OCH2X75, —OCHX752, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —S H, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX753, —OCHX752, R76-substituted or unsubstituted alkyl, R76-substituted or unsubstituted heteroalkyl, R76-substituted or unsubstituted cycloalkyl, R76-substituted or unsubstituted heterocycloalkyl, R76-substituted or unsubstituted aryl, or R76-substituted or unsubstituted heteroaryl. X75is halogen. In embodiments, X75is F. R76is independently oxo, halogen, —CX763, —CHX762, —OCH2X76, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX763, —OCHX762, R77-substituted or unsubstituted alkyl, R77-substituted or unsubstituted heteroalkyl, R77-substituted or unsubstituted cycloalkyl, R77-substituted or unsubstituted heterocycloalkyl, R77-substituted or unsubstituted aryl, or R77-substituted or unsubstituted heteroaryl. X76is halogen. In embodiments, X76is F. In embodiments, R16Ais independently hydrogen, oxo, halogen, —CX16A3, —CHX16A2, —OCH2X16A, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX16A3, —OCHX16A2, R75A-substituted or unsubstituted alkyl, R75A-substituted or unsubstituted heteroalkyl, R75A-substituted or unsubstituted cycloalkyl, R75A-substituted or unsubstituted heterocycloalkyl, R75A-substituted or unsubstituted aryl, or R75A-substituted or unsubstituted heteroaryl. X16Ais halogen. In embodiments, X16Ais F. R75Ais independently oxo, halogen, —CX75A3, —CHX75A2, —OCH2X75A, —OCHX75A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX75A3, —OCHX75A2, R76A-substituted or unsubstituted alkyl, R76A-substituted or unsubstituted heteroalkyl, R76A-substituted or unsubstituted cycloalkyl, R76A-substituted or unsubstituted heterocycloalkyl, R76A-substituted or unsubstituted aryl, or R76A-substituted or unsubstituted heteroaryl. X75Ais halogen. In embodiments, X75Ais F. R76Ais independently oxo, halogen, —CX76A3, —CHX76A2, —OCH2X76A, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX76A3, —OCHX76A2, R77A-substituted or unsubstituted alkyl, R77A-substituted or unsubstituted heteroalkyl, R77A-substituted or unsubstituted cycloalkyl, R77A-substituted or unsubstituted heterocycloalkyl, R77A-substituted or unsubstituted aryl, or R77A-substituted or unsubstituted heteroaryl. X76Ais halogen. In embodiments, X76Ais F. In embodiments, R16Bis independently hydrogen, oxo, halogen, —CX16B3, —CHX16B2, —OCH2X16B, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX16B3, —OCHX16B2, R75B-substituted or unsubstituted alkyl, R75B-substituted or unsubstituted heteroalkyl, R75B-substituted or unsubstituted cycloalkyl, R75B-substituted or unsubstituted heterocycloalkyl, R75B-substituted or unsubstituted aryl, or R75B-substituted or unsubstituted heteroaryl. X16Bis halogen. In embodiments, X16Bis F. R75Bis independently oxo, halogen, —CX75B3, —CHX75B2, —OCH2X75B, —OCHX75B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX75B3, —OCHX75B2, R76B-substituted or unsubstituted alkyl, R76B-substituted or unsubstituted heteroalkyl, R76B-substituted or unsubstituted cycloalkyl, R76B-substituted or unsubstituted heterocycloalkyl, R76B-substituted or unsubstituted aryl, or R76B-substituted or unsubstituted heteroaryl. X75Bis halogen. In embodiments, X75Bis F. R76Bis independently oxo, halogen, —CX76B3, —CHX76B2, —OCH2X76B, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX76B3, —OCHX76B2, R77B-substituted or unsubstituted alkyl, R77B-substituted or unsubstituted heteroalkyl, R77B-substituted or unsubstituted cycloalkyl, R77B-substituted or unsubstituted heterocycloalkyl, R77B-substituted or unsubstituted aryl, or R77B-substituted or unsubstituted heteroaryl. X76Bis halogen. In embodiments, X76Bis F. In embodiments, R16Cis independently hydrogen, oxo, halogen, —CX16C3, —CHX16C2, —OCH2X16C, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX16C3, —OCHX16C2, R75C-substituted or unsubstituted alkyl, R75C-substituted or unsubstituted heteroalkyl, R75C-substituted or unsubstituted cycloalkyl, R75C-substituted or unsubstituted heterocycloalkyl, R75C-substituted or unsubstituted aryl, or R75C-substituted or unsubstituted heteroaryl. X16Cis halogen. In embodiments, X16Cis F. R75Cis independently oxo, halogen, —CX75C3, —CHX75C2, —OCH2X75C, —OCHX75C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX75C3, —OCHX75C2, R76C-substituted or unsubstituted alkyl, R76C-substituted or unsubstituted heteroalkyl, R76C-substituted or unsubstituted cycloalkyl, R76C-substituted or unsubstituted heterocycloalkyl, R76C-substituted or unsubstituted aryl, or R76C-substituted or unsubstituted heteroaryl. X75Cis halogen. In embodiments, X75Cis F. R76Cis independently oxo, halogen, —CX76C3, —CHX76C2, —OCH2X76C, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX76C3, —OCHX76C2, R77C-substituted or unsubstituted alkyl, R77C-substituted or unsubstituted heteroalkyl, R77C-substituted or unsubstituted cycloalkyl, R77C-substituted or unsubstituted heterocycloalkyl, R77C-substituted or unsubstituted aryl, or R77C-substituted or unsubstituted heteroaryl. X76Cis halogen. In embodiments, X76Cis F. In embodiments, R16Dis independently hydrogen, oxo, halogen, —CX16D3, —CHX16D2, —OCH2X16D, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX16D3, —OCHX16D2, R75D-substituted or unsubstituted alkyl, R75D-substituted or unsubstituted heteroalkyl, R75D-substituted or unsubstituted cycloalkyl, R75D-substituted or unsubstituted heterocycloalkyl, R75D-substituted or unsubstituted aryl, or R75D-substituted or unsubstituted heteroaryl. X16Dis halogen. In embodiments, X16Dis F. R75Dis independently oxo, halogen, —CX75D3, —CHX75D2, —OCH2X75D, —OCHX75D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX75D3, —OCHX75D2, R76D-substituted or unsubstituted alkyl, R76D-substituted or unsubstituted heteroalkyl, R76D-substituted or unsubstituted cycloalkyl, R76D-substituted or unsubstituted heterocycloalkyl, R76D-substituted or unsubstituted aryl, or R76D-substituted or unsubstituted heteroaryl. X75Dis halogen. In embodiments, X75Dis F. R76Dis independently oxo, halogen, —CX76D3, —CHX76D2, —OCH2X76D, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX76D3, —OCHX76D2, R77D-substituted or unsubstituted alkyl, R77D-substituted or unsubstituted heteroalkyl, R77D-substituted or unsubstituted cycloalkyl, R77D-substituted or unsubstituted heterocycloalkyl, R77D-substituted or unsubstituted aryl, or R77D-substituted or unsubstituted heteroaryl. X76Dis halogen. In embodiments, X76Dis F. In embodiments, R17is hydrogen. In embodiments, R17is halogen. In embodiments, R17is CX173. In embodiments, R17is —CHX172. In embodiments, R17is —CH2X17. In embodiments, R17is —CN. In embodiments, R17is —SOn17R17D. In embodiments, R17is —SOv17NR17AR17B. In embodiments, R17is —NHNR17AR17B. In embodiments, R17is —ONR17AR17B. In embodiments, R17is —NHC═(O)NHNR17AR17B. In embodiments, R17is —NHC(O)NR17AR17B. In embodiments, R17is —N(O)m17. In embodiments, R17is —NR17AR17B. In embodiments, R17is —C(O)R17C. In embodiments, R17is —C(O)—OR17C. In embodiments, R17is —C(O)NR17AR17B. In embodiments, R17is —OR17C. In embodiments, R17is —NR17ASO2R17D. In embodiments, R17is —NR17AC(O)R17C. In embodiments, R17is —NR17AC(O)OR17C. In embodiments, R17is —NR17AOR17C. In embodiments, R17is —OCX173. In embodiments, R17is —OCHX172. In embodiments, R17is substituted or unsubstituted alkyl. In embodiments, R17is substituted or unsubstituted heteroalkyl. In embodiments, R17is substituted or unsubstituted cycloalkyl. In embodiments, R17is substituted or unsubstituted heterocycloalkyl. In embodiments, R17is substituted or unsubstituted aryl. In embodiments, R17is substituted or unsubstituted heteroaryl. In embodiments, R17is substituted alkyl. In embodiments, R17is substituted heteroalkyl. In embodiments, R17is substituted cycloalkyl. In embodiments, R17is substituted heterocycloalkyl. In embodiments, R17is substituted aryl. In embodiments, R17is substituted heteroaryl. In embodiments, R17is unsubstituted alkyl. In embodiments, R17is unsubstituted heteroalkyl. In embodiments, R17is unsubstituted cycloalkyl. In embodiments, R17is unsubstituted heterocycloalkyl. In embodiments, R17is unsubstituted aryl. In embodiments, R17is unsubstituted heteroaryl. In embodiments, R17is unsubstituted methyl. In embodiments, R17is unsubstituted ethyl. In embodiments, R17is unsubstituted propyl. In embodiments, R17is unsubstituted isopropyl. In embodiments, R17is unsubstituted butyl. In embodiments, R17is unsubstituted tert-butyl. In embodiments, R17Ais hydrogen. In embodiments, R17Ais —CX3. In embodiments, R17Ais —CN. In embodiments, R17Ais —COOH. In embodiments, R17Ais —CONH2. In embodiments, R17Ais —CHX2. In embodiments, R17Ais —CH2X. In embodiments, R17Ais unsubstituted methyl. In embodiments, R17Ais unsubstituted ethyl. In embodiments, R17Ais unsubstituted propyl. In embodiments, R17Ais unsubstituted isopropyl. In embodiments, R17Ais unsubstituted butyl. In embodiments, R17Ais unsubstituted tert-butyl. In embodiments, R17Bis hydrogen. In embodiments, R17Bis —CX3. In embodiments, R17Bis —CN. In embodiments, R17Bis —COOH. In embodiments, R17Bis —CONH2. In embodiments, R17Bis —CHX2. In embodiments, R17Bis —CH2X. In embodiments, R17Bis unsubstituted methyl. In embodiments, R17Bis unsubstituted ethyl. In embodiments, R17Bis unsubstituted propyl. In embodiments, R17Bis unsubstituted isopropyl. In embodiments, R17Bis unsubstituted butyl. In embodiments, R17Bis unsubstituted tert-butyl. In embodiments, R17Cis hydrogen. In embodiments, R17Cis —CX3. In embodiments, R17Cis —CN. In embodiments, R17Cis —COOH. In embodiments, R17Cis —CONH2. In embodiments, R17Cis —CHX2. In embodiments, R17Cis —CH2X. In embodiments, R17Cis unsubstituted methyl. In embodiments, R17Cis unsubstituted ethyl. In embodiments, R17Cis unsubstituted propyl. In embodiments, R17Cis unsubstituted isopropyl. In embodiments, R17Cis unsubstituted butyl. In embodiments, R17Cis unsubstituted tert-butyl. In embodiments, R17Dis hydrogen. In embodiments, R17Dis —CX3. In embodiments, R17Dis —CN. In embodiments, R17Dis —COOH. In embodiments, R17Dis —CONH2. In embodiments, R17Dis —CHX2. In embodiments, R17Dis —CH2X. In embodiments, R17Dis unsubstituted methyl. In embodiments, R17Dis unsubstituted ethyl. In embodiments, R17Dis unsubstituted propyl. In embodiments, R17Dis unsubstituted isopropyl. In embodiments, R17Dis unsubstituted butyl. In embodiments, R17Dis unsubstituted tert-butyl. In embodiments, R17is independently hydrogen, oxo, halogen, —CX173, —CHX172, —OCH2X17, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX173, —OCHX172, R78-substituted or unsubstituted alkyl, R78-substituted or unsubstituted heteroalkyl, R78-substituted or unsubstituted cycloalkyl, R78-substituted or unsubstituted heterocycloalkyl, R78-substituted or unsubstituted aryl, or R78-substituted or unsubstituted heteroaryl. X17is halogen. In embodiments, X17is F. R78is independently oxo, halogen, —CX783, —CHX782, —OCH2X78, —OCHX782, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —S H, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX783, —OCHX782, R79-substituted or unsubstituted alkyl, R79-substituted or unsubstituted heteroalkyl, R79-substituted or unsubstituted cycloalkyl, R79-substituted or unsubstituted heterocycloalkyl, R79-substituted or unsubstituted aryl, or R79-substituted or unsubstituted heteroaryl. X78is halogen. In embodiments, X78is F. R79is independently oxo, halogen, —CX793, —CHX792, —OCH2X79, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX793, —OCHX792, R80-substituted or unsubstituted alkyl, R80-substituted or unsubstituted heteroalkyl, R80-substituted or unsubstituted cycloalkyl, R80-substituted or unsubstituted heterocycloalkyl, R80-substituted or unsubstituted aryl, or R80-substituted or unsubstituted heteroaryl. X79is halogen. In embodiments, X79is F. In embodiments, R17Ais independently hydrogen, oxo, halogen, —CX17A3, —CHX17A2, —OCH2X17A, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX17A3, —OCHX17A2, R78A-substituted or unsubstituted alkyl, R78A-substituted or unsubstituted heteroalkyl, R78A-substituted or unsubstituted cycloalkyl, R78A-substituted or unsubstituted heterocycloalkyl, R78A-substituted or unsubstituted aryl, or R78A-substituted or unsubstituted heteroaryl. X17Ais halogen. In embodiments, X17Ais F. R78Ais independently oxo, halogen, —CX78A3, —CHX78A2, —OCH2X78A, —OCHX78A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX78A3, —OCHX78A2, R79A-substituted or unsubstituted alkyl, R79A-substituted or unsubstituted heteroalkyl, R79A-substituted or unsubstituted cycloalkyl, R79A-substituted or unsubstituted heterocycloalkyl, R79A-substituted or unsubstituted aryl, or R79A-substituted or unsubstituted heteroaryl. X78Ais halogen. In embodiments, X78Ais F. R79Ais independently oxo, halogen, —CX79A3, —CHX79A2, —OCH2X79A, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX79A3, —OCHX79A2, R80A-substituted or unsubstituted alkyl, R80A-substituted or unsubstituted heteroalkyl, R80A-substituted or unsubstituted cycloalkyl, R80A-substituted or unsubstituted heterocycloalkyl, R80A-substituted or unsubstituted aryl, or R80A-substituted or unsubstituted heteroaryl. X79Ais halogen. In embodiments, X79Ais F. In embodiments, R17Bis independently hydrogen, oxo, halogen, —CX17B3, —CHX17B2, —OCH2X17B, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX17B3, —OCHX17B2, R78B-substituted or unsubstituted alkyl, R78B-substituted or unsubstituted heteroalkyl, R78B-substituted or unsubstituted cycloalkyl, R78B-substituted or unsubstituted heterocycloalkyl, R78B-substituted or unsubstituted aryl, or R78B-substituted or unsubstituted heteroaryl. X17Bis halogen. In embodiments, X17Bis F. R78Bis independently oxo, halogen, —CX78B3, —CHX78B2, —OCH2X78B, —OCHX78B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX78B3, —OCHX78B2, R79B-substituted or unsubstituted alkyl, R79B-substituted or unsubstituted heteroalkyl, R79B-substituted or unsubstituted cycloalkyl, R79B-substituted or unsubstituted heterocycloalkyl, R79B-substituted or unsubstituted aryl, or R79B-substituted or unsubstituted heteroaryl. X78Bis halogen. In embodiments, X78Bis F. R79Bis independently oxo, halogen, —CX79B3, —CHX79B2, —OCH2X79B, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX79B3, —OCHX79B2, R80B-substituted or unsubstituted alkyl, R80B-substituted or unsubstituted heteroalkyl, R80B-substituted or unsubstituted cycloalkyl, R80B-substituted or unsubstituted heterocycloalkyl, R″B-substituted or unsubstituted aryl, or R80B-substituted or unsubstituted heteroaryl. X79Bis halogen. In embodiments, X79Bis F. In embodiments, R17Cis independently hydrogen, oxo, halogen, —CX17C3, —CHX17C2, —OCH2X17C, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX17C3, —OCHX17C2, R78C-substituted or unsubstituted alkyl, R78C-substituted or unsubstituted heteroalkyl, R78C-substituted or unsubstituted cycloalkyl, R78C-substituted or unsubstituted heterocycloalkyl, R78C-substituted or unsubstituted aryl, or R78C-substituted or unsubstituted heteroaryl. X17Cis halogen. In embodiments, X17Cis F. R78Cis independently oxo, halogen, —CX78C3, —CHX78C2, —OCH2X78C, —OCHX78C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX78C3, —OCHX78C2, R79C-substituted or unsubstituted alkyl, R79C-substituted or unsubstituted heteroalkyl, R79C-substituted or unsubstituted cycloalkyl, R79C-substituted or unsubstituted heterocycloalkyl, R79C-substituted or unsubstituted aryl, or R79C-substituted or unsubstituted heteroaryl. X78Cis halogen. In embodiments, X78Cis F. R79Cis independently oxo, halogen, —CX79C3, —CHX79C2, —OCH2X79C, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX79C3, —OCHX79C2, R80C-substituted or unsubstituted alkyl, R80C-substituted or unsubstituted heteroalkyl, R80C-substituted or unsubstituted cycloalkyl, R80C-substituted or unsubstituted heterocycloalkyl, R80C-substituted or unsubstituted aryl, or R80C-substituted or unsubstituted heteroaryl. X79Cis halogen. In embodiments, X79Cis F. In embodiments, R17Dis independently hydrogen, oxo, halogen, —CX17D3, —CHX17D2, —OCH2X17D, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX17D3, —OCHX17D2, R78D-substituted or unsubstituted alkyl, R78D-substituted or unsubstituted heteroalkyl, R78D-substituted or unsubstituted cycloalkyl, R78D-substituted or unsubstituted heterocycloalkyl, R78D-substituted or unsubstituted aryl, or R78D-substituted or unsubstituted heteroaryl. X17Dis halogen. In embodiments, X17Dis F. R78Dis independently oxo, halogen, —CX78D3, —CHX78D2, —OCH2X78D, —OCHX78D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX78D3, —OCHX78D2, R79D-substituted or unsubstituted alkyl, R79D-substituted or unsubstituted heteroalkyl, R79D-substituted or unsubstituted cycloalkyl, R79D-substituted or unsubstituted heterocycloalkyl, R79D-substituted or unsubstituted aryl, or R79D-substituted or unsubstituted heteroaryl. X78Dis halogen. In embodiments, X78Dis F. R79Dis independently oxo, halogen, —CX79D3, —CHX79D2, —OCH2X79D, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX79D3, —OCHX79D2, R80D-substituted or unsubstituted alkyl, R80D-substituted or unsubstituted heteroalkyl, R80D-substituted or unsubstituted cycloalkyl, R80D-substituted or unsubstituted heterocycloalkyl, R80D-substituted or unsubstituted aryl, or R80D-substituted or unsubstituted heteroaryl. X79Dis halogen. In embodiments, X79Dis F. In embodiments, R18is hydrogen. In embodiments, R18is halogen. In embodiments, R18is CX183. In embodiments, R18is —CHX182. In embodiments, R18is —CH2X18. In embodiments, R18is —CN. In embodiments, R18is —SOn18R18D. In embodiments, R18is —SOv18NR18AR18B. In embodiments, R18is —NHNR18AR18B. In embodiments, R18is —ONR18AR18B. In embodiments, R18is —NHC═(O)NHNR18AR18B. In embodiments, R18is —NHC(O)NR18AR18B. In embodiments, R18is —N(O)m18. In embodiments, R18is —NR18AR18B. In embodiments, R18is —C(O)R18C. In embodiments, R18is —C(O)—OR18C. In embodiments, R18is —C(O)NR18AR18B. In embodiments, R18is —OR18D. In embodiments, R18is —NR18ASO2R18D. In embodiments, R18is —NR18AC(O)R18C. In embodiments, R18is —NR18AC(O)OR18C. In embodiments, R18is —NR18AOR18C. In embodiments, R18is —OCX183. In embodiments, R18is —OCHX182. In embodiments, R18is substituted or unsubstituted alkyl. In embodiments, R18is substituted or unsubstituted heteroalkyl. In embodiments, R18is substituted or unsubstituted cycloalkyl. In embodiments, R18is substituted or unsubstituted heterocycloalkyl. In embodiments, R18is substituted or unsubstituted aryl. In embodiments, R18is substituted or unsubstituted heteroaryl. In embodiments, R18is substituted alkyl. In embodiments, R18is substituted heteroalkyl. In embodiments, R18is substituted cycloalkyl. In embodiments, R18is substituted heterocycloalkyl. In embodiments, R18is substituted aryl. In embodiments, R18is substituted heteroaryl. In embodiments, R18is unsubstituted alkyl. In embodiments, R18is unsubstituted heteroalkyl. In embodiments, R18is unsubstituted cycloalkyl. In embodiments, R18is unsubstituted heterocycloalkyl. In embodiments, R18is unsubstituted aryl. In embodiments, R18is unsubstituted heteroaryl. In embodiments, R18is unsubstituted methyl. In embodiments, R18is unsubstituted ethyl. In embodiments, R18is unsubstituted propyl. In embodiments, R18is unsubstituted isopropyl. In embodiments, R18is unsubstituted butyl. In embodiments, R18is unsubstituted tert-butyl. In embodiments, R18Ais hydrogen. In embodiments, R18Ais —CX3. In embodiments, R18Ais —CN. In embodiments, R18Ais —COOH. In embodiments, R18Ais —CONH2. In embodiments, R18Ais —CHX2. In embodiments, R18Ais —CH2X. In embodiments, R18Ais unsubstituted methyl. In embodiments, R18Ais unsubstituted ethyl. In embodiments, R18Ais unsubstituted propyl. In embodiments, R18Ais unsubstituted isopropyl. In embodiments, R18Ais unsubstituted butyl. In embodiments, R18Ais unsubstituted tert-butyl. In embodiments, R18Bis hydrogen. In embodiments, R18Bis —CX3. In embodiments, R18Bis —CN. In embodiments, R18Bis —COOH. In embodiments, R18Bis —CONH2. In embodiments, R18Bis —CHX2. In embodiments, R18Bis —CH2X. In embodiments, R18Bis unsubstituted methyl. In embodiments, R18Bis unsubstituted ethyl. In embodiments, R18Bis unsubstituted propyl. In embodiments, R18Bis unsubstituted isopropyl. In embodiments, R18Bis unsubstituted butyl. In embodiments, R18Bis unsubstituted tert-butyl. In embodiments, R18Bis hydrogen. In embodiments, R18Bis —CX3. In embodiments, R18Bis —CN. In embodiments, R18Cis —COOH. In embodiments, R18Bis —CONH2. In embodiments, R18Cis —CHX2. In embodiments, R18Cis —CH2X. In embodiments, R18Cis unsubstituted methyl. In embodiments, R18Cis unsubstituted ethyl. In embodiments, R18Cis unsubstituted propyl. In embodiments, R18Cis unsubstituted isopropyl. In embodiments, R18Cis unsubstituted butyl. In embodiments, R18Cis unsubstituted tert-butyl. In embodiments, R18Dis hydrogen. In embodiments, R18Dis —CX3. In embodiments, R18is —CN. In embodiments, R18Dis —COOH. In embodiments, R18is —CONH2. In embodiments, R18Dis —CHX2. In embodiments, R18Dis —CH2X. In embodiments, R18Dis unsubstituted methyl. In embodiments, R18Dis unsubstituted ethyl. In embodiments, R18Dis unsubstituted propyl. In embodiments, R18Dis unsubstituted isopropyl. In embodiments, R18Dis unsubstituted butyl. In embodiments, R18Dis unsubstituted tert-butyl. In embodiments, R18is independently hydrogen, oxo, halogen, —CX183, —CHX182, —OCH2X18, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX183, —OCHX182, R81-substituted or unsubstituted alkyl, R81-substituted or unsubstituted heteroalkyl, R81-substituted or unsubstituted cycloalkyl, R81-substituted or unsubstituted heterocycloalkyl, R81-substituted or unsubstituted aryl, or R81-substituted or unsubstituted heteroaryl. X18is halogen. In embodiments, X18is F. R8′ is independently oxo, halogen, —CX813, —CHX812, —OCH2X81, —OCHX812, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —S H, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX813, —OCHX812, R82-substituted or unsubstituted alkyl, R82-substituted or unsubstituted heteroalkyl, R82-substituted or unsubstituted cycloalkyl, R82-substituted or unsubstituted heterocycloalkyl, R82-substituted or unsubstituted aryl, or R82-substituted or unsubstituted heteroaryl. X81is halogen. In embodiments, X81is F. R82is independently oxo, halogen, —CX823, —CHX822, —OCH2X82, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX823, —OCHX822, R83-substituted or unsubstituted alkyl, R83-substituted or unsubstituted heteroalkyl, R83-substituted or unsubstituted cycloalkyl, R83-substituted or unsubstituted heterocycloalkyl, R83-substituted or unsubstituted aryl, or R83-substituted or unsubstituted heteroaryl. X82is halogen. In embodiments, X82is F. In embodiments, R18Ais independently hydrogen, oxo, halogen, —CX18A3, —CHX18A2, —OCH2X18A, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX18A3, —OCHX18A2, R81A-substituted or unsubstituted alkyl, R81A-substituted or unsubstituted heteroalkyl, R81A-substituted or unsubstituted cycloalkyl, R81A-substituted or unsubstituted heterocycloalkyl, R81A-substituted or unsubstituted aryl, or R81A-substituted or unsubstituted heteroaryl. X18Ais halogen. In embodiments, X18Ais F. R81Ais independently oxo, halogen, —CX81A3, —CHX81A2, —OCH2X81A, —OCHX81A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX81A3, —OCHX81A2, R82A-substituted or unsubstituted alkyl, R82A-substituted or unsubstituted heteroalkyl, R82A-substituted or unsubstituted cycloalkyl, R82A-substituted or unsubstituted heterocycloalkyl, R82A-substituted or unsubstituted aryl, or R82A-substituted or unsubstituted heteroaryl. X81Ais halogen. In embodiments, X81Ais F. R82Ais independently oxo, halogen, —CX82A3, —CHX82A2, —OCH2X82A, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX82A3, —OCHX82A2, R83A-substituted or unsubstituted alkyl, R83A-substituted or unsubstituted heteroalkyl, R83A-substituted or unsubstituted cycloalkyl, R83A-substituted or unsubstituted heterocycloalkyl, R83A-substituted or unsubstituted aryl, or R83A-substituted or unsubstituted heteroaryl. X82Ais halogen. In embodiments, X82Ais F. In embodiments, R18Bis independently hydrogen, oxo, halogen, —CX18B3, —CHX18B2, —OCH2X18B, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX18B3, —OCHX18B2, R81B-substituted or unsubstituted alkyl, R81B-substituted or unsubstituted heteroalkyl, R81B-substituted or unsubstituted cycloalkyl, R81B-substituted or unsubstituted heterocycloalkyl, R81B-substituted or unsubstituted aryl, or R81B-substituted or unsubstituted heteroaryl. X18Bis halogen. In embodiments, X18Bis F. R81Bis independently oxo, halogen, —CX81B3, —CHX81B2, —OCH2X81B, —OCHX81B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX81B3, —OCHX81B2, R82B-substituted or unsubstituted alkyl, R82B-substituted or unsubstituted heteroalkyl, R82B-substituted or unsubstituted cycloalkyl, R82B-substituted or unsubstituted heterocycloalkyl, R82B-substituted or unsubstituted aryl, or R82B-substituted or unsubstituted heteroaryl. X81Bis halogen. In embodiments, X81Bis F. R82Bis independently oxo, halogen, —CX82B3, —CHX82B2, —OCH2X82B, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX82B3, —OCHX82B2, R83B-substituted or unsubstituted alkyl, R83B-substituted or unsubstituted heteroalkyl, R83B-substituted or unsubstituted cycloalkyl, R83B-substituted or unsubstituted heterocycloalkyl, R83B-substituted or unsubstituted aryl, or R83B-substituted or unsubstituted heteroaryl. X82Bis halogen. In embodiments, X82Bis F. In embodiments, R18Cis independently hydrogen, oxo, halogen, —CX18C3, —CHX18C2, —OCH2X18C, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX18C3, —OCHX18C2, R81C-substituted or unsubstituted alkyl, R81C-substituted or unsubstituted heteroalkyl, R81C-substituted or unsubstituted cycloalkyl, R81C-substituted or unsubstituted heterocycloalkyl, R81C-substituted or unsubstituted aryl, or R81C-substituted or unsubstituted heteroaryl. X18Cis halogen. In embodiments, X18Cis F. R81Cis independently oxo, halogen, —CX81C3, —CHX81C2, —OCH2X81C, —OCHX81C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX81C3, —OCHX81C2, R82C-substituted or unsubstituted alkyl, R82C-substituted or unsubstituted heteroalkyl, R82C-substituted or unsubstituted cycloalkyl, R82C-substituted or unsubstituted heterocycloalkyl, R82C-substituted or unsubstituted aryl, or R82C-substituted or unsubstituted heteroaryl. X81Cis halogen. In embodiments, X81Cis F. R82Cis independently oxo, halogen, —CX82C3, —CHX82C2, —OCH2X82C, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX82C3, —OCHX82C2, R83C-substituted or unsubstituted alkyl, R83C-substituted or unsubstituted heteroalkyl, R83C-substituted or unsubstituted cycloalkyl, R83C-substituted or unsubstituted heterocycloalkyl, R83C-substituted or unsubstituted aryl, or R83C-substituted or unsubstituted heteroaryl. X82Cis halogen. In embodiments, X82Cis F. In embodiments, R18Dis independently hydrogen, oxo, halogen, —CX18D3, —CHX18D2, —OCH2X18D, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX18D3, —OCHX18D2, R81D-substituted or unsubstituted alkyl, R81Dsubstituted or unsubstituted heteroalkyl, R81Dsubstituted or unsubstituted cycloalkyl, R81D-substituted or unsubstituted heterocycloalkyl, R81Dsubstituted or unsubstituted aryl, or R81D-substituted or unsubstituted heteroaryl. X18Dis halogen. In embodiments, X18Dis F. R18Dis independently oxo, halogen, —CX81D3, —CHX81D2, —OCH2X81D, —OCHX81D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX81D3, —OCHX81D2, R82D-substituted or unsubstituted alkyl, R82D-substituted or unsubstituted heteroalkyl, R82D-substituted or unsubstituted cycloalkyl, R82D-substituted or unsubstituted heterocycloalkyl, R82D-substituted or unsubstituted aryl, or R82D-substituted or unsubstituted heteroaryl. X81Dis halogen. In embodiments, X81Dis F. R82Dis independently oxo, halogen, —CX82D3, —CHX82D2, —OCH2X82D, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCX82D3, —OCHX82D2, R83D-substituted or unsubstituted alkyl, R83D-substituted or unsubstituted heteroalkyl, R83D-substituted or unsubstituted cycloalkyl, R83D-substituted or unsubstituted heterocycloalkyl, R83D-substituted or unsubstituted aryl, or R83D-substituted or unsubstituted heteroaryl. X82Dis halogen. In embodiments, X82Dis F. R74, R77, R80, R83, R74A, R77A, R80A, R83A, R74B, R77B, R80B, R83B, R74C, R77C, R80C, R83C, R74D, R77D, R80D, R83D, R86, R89, R92, and R98are independently hydrogen, oxo, halogen, —CF3, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC(O)NHNH2, —NHC(O)NH2, —NHSO2H, —NHC(O)H, —NHC(O)OH, —NHOH, —OCF3, —OCHF2, unsubstituted alkyl, unsubstituted heteroalkyl, unsubstituted cycloalkyl, unsubstituted heterocycloalkyl, unsubstituted aryl, or unsubstituted heteroaryl. In R74, R77, R80, R83, R74A, R77A, R80A, R83A, R74B, R77B, R80B, R83B, R74C, R77C, R80C, R83C, R74D, R77D, R80D, R83D, R86, R89, R92, and R98are independently hydrogen, oxo, halogen, —CF3, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC(O)NHNH2, —NHC(O)NH2, —NHSO2H, —NHC(O)H, —NHC(O)OH, —NHOH, —OCF3, —OCHF2, unsubstituted alkyl, unsubstituted heteroalkyl, unsubstituted cycloalkyl, unsubstituted heterocycloalkyl, unsubstituted aryl, or unsubstituted heteroaryl. In embodiments, R74, R77, R80, R83, R74A, R77A, R80A, R83A, R74B, R77B, R80B, R83B, R74C, R77C, R80C, R83C, R74D, R77D, R80D, R83D, R86, R89, R92, and R98are independently hydrogen, oxo, halogen, —CF3, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC(O)NHNH2, —NHC(O)NH2, —NHSO2H, —NHC(O)H, —NHC(O)OH, —NHOH, —OCF3, —OCHF2, unsubstituted C1-C8alkyl, unsubstituted 2 to 8 membered heteroalkyl, unsubstituted C3-C8cycloalkyl, unsubstituted 3 to 6 membered heterocycloalkyl, unsubstituted phenyl, or unsubstituted 5 to 6 membered heteroaryl. In embodiments, R15, R16, R17, and R18are hydrogen. In embodiments, E is: In embodiments, E is: In embodiments, E is: In embodiments, E is: In embodiments, E is: In embodiments, E is: In embodiments, E is: In some embodiments, a compound as described herein may include multiple instances of R1or R2, and/or other variables. In such embodiments, each variable may optional be different and be appropriately labeled to distinguish each group for greater clarity. For example, where each R1and/or R2, is different, they may be referred to, for example, as R1.1, R1.2, R1.3, R1.4, R1.5, R2.1, R2.2, R2.3, or R2.4, respectively, wherein the definition of R1is assumed by R1.1, R1.2, R1.3, R1.4, R1.5; and/or R2is assumed by R2.1, R2.2, R2.3, R2.4. The variables used within a definition of R1and/or R2, and/or other variables that appear at multiple instances and are different may similarly be appropriately labeled to distinguish each group for greater clarity. In some embodiments, the compound is a compound described herein (e.g., in an aspect, embodiment, example, claim, table, scheme, drawing, or figure). In embodiments, unless otherwise indicated, a compound described herein is a racemic mixture of all stereoisomers. In embodiments, unless otherwise indicated, a compound described herein is a racemic mixture of all enantiomers. In embodiments, unless otherwise indicated, a compound described herein is a racemic mixture of two opposite stereoisomers. In embodiments, unless otherwise indicated, a compound described herein is a racemic mixture of two opposite enantiomers. In embodiments, unless otherwise indicated, a compound described herein is a single stereoisomer. In embodiments, unless otherwise indicated, a compound described herein is a single enantiomer. In embodiments, the compound is a compound described herein (e.g., in an aspect, embodiment, example, figure, table, scheme, or claim). In an aspect is provided a Reticulon 4 inhibitor. In embodiments, the Reticulon 4 inhibitor is a compound described herein. In embodiments, the Reticulon 4 inhibitor is an oligonucleotide (e.g., DNA, RNA, shRNA, or siRNA), protein (e.g., antibody, anti-Reticulon 4 antibody, anti-Reticulon 4 binding antibody fragment), or compound (e.g., compound described herein). In embodiments, the Reticulon 4 inhibitor contacts one or more amino acids corresponding to E1105, C1101, E1078, 51079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of human reticulon 4. In embodiments, the Reticulon 4 inhibitor covalently binds an amino acid corresponding to C1101 in human reticulon 4. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of human reticulon 4. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to E1105 of human reticulon 4. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to C1101 of human reticulon 4. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to E1078 of human reticulon 4. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to S1079 of human reticulon 4. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to A1082 of human reticulon 4. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to I1083 of human reticulon 4. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to K1090 of human reticulon 4. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to Y1091 of human reticulon 4. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to S1094 of human reticulon 4. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to G1097 of human reticulon 4. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to H1098 of human reticulon 4. In an aspect is provided a Reticulon 4 inhibitor. In embodiments, the Reticulon 4 inhibitor is a compound described herein. In embodiments, the Reticulon 4 inhibitor is an oligonucleotide (e.g., DNA, RNA, shRNA, or siRNA), protein (e.g., antibody, anti-Reticulon 4 antibody, anti-Reticulon 4 binding antibody fragment), or compound (e.g., compound described herein). In embodiments, the Reticulon 4 inhibitor contacts one or more amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor covalently binds an amino acid corresponding to C1101 in of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to E1105 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to C1101 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to E1078 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to S1079 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to A1082 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to I1083 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to K1090 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to Y1091 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to S1094 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to G1097 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to H1098 of SEQ ID NO:331. In embodiments, the compound has the formula: wherein R1, L2, and E are as described herein, including embodiments. In embodiments, the compound has the formula: wherein R1, L1, and E are as described herein, including embodiments. In embodiments, R1is independently halogen, —CX13, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —OCX13, —OCHX12, —OCH2X1, —CHX12, —CH2X1, substituted or unsubstituted C1-C8alkyl, or substituted or unsubstituted 2 to 8 membered heteroalkyl, substituted or unsubstituted C3-C8cycloalkyl, substituted or unsubstituted 3 to 8 membered heterocycloalkyl, substituted or unsubstituted phenyl, or substituted or unsubstituted 5 to 6 membered heteroaryl. In embodiments, R1is independently halogen, —CX13, —CN, unsubstituted C1-C4alkyl, or unsubstituted 2 to 4 membered heteroalkyl. In embodiments, R1is independently unsubstituted methyl, unsubstituted ethyl, unsubstituted isopropyl, or unsubstituted tert-butyl. In embodiments, R1is independently unsubstituted methyl. In embodiments, R1is independently unsubstituted ethyl. In embodiments, R1is independently unsubstituted propyl. In embodiments, R1is independently unsubstituted n-propyl. In embodiments, R1is independently unsubstituted isopropyl. In embodiments, R1is independently unsubstituted butyl. In embodiments, R1is independently unsubstituted n-butyl. In embodiments, R1is independently unsubstituted isobutyl. In embodiments, R1is independently unsubstituted tert-butyl. In embodiments, R1is independently unsubstituted pentyl. In embodiments, R1is independently unsubstituted hexyl. In embodiments, R1is independently unsubstituted heptyl. In embodiments, R1is independently unsubstituted octyl. In embodiments, R1is independently —CF3. In embodiments, R1is independently —CCl3. In embodiments, R1is independently unsubstituted phenyl. In embodiments, R1is independently unsubstituted pyridyl. In embodiments, R1is independently halogen. In embodiments, R1is independently —CN. In embodiments, R1is independently —OH. In embodiments, R1is independently —NH2. In embodiments, R1is independently —COOH. In embodiments, R1is independently —CONH2. In embodiments, R1is independently —NO2. In embodiments, R1is independently —SH. In embodiments, R1is independently —SO3H. In embodiments, R1is independently —SO4H. In embodiments, R1is independently —SO2NH2. In embodiments, R1is independently —NHNH2. In embodiments, R1is independently —ONH2. In embodiments, R1is independently —NHC(O)NHNH2. In embodiments, R1is independently —NHC(O)NH2. In embodiments, R1is independently —NHSO2H. In embodiments, R1is independently —NHC(O)H. In embodiments, R1is independently —NHC(O)OH. In embodiments, R1is independently —NHOH. In embodiments, R1is independently substituted or unsubstituted alkyl. In embodiments, R1is independently substituted or unsubstituted heteroalkyl. In embodiments, R1is independently substituted or unsubstituted cycloalkyl. In embodiments, R1is independently substituted or unsubstituted heterocycloalkyl. In embodiments, R1is independently substituted or unsubstituted aryl. In embodiments, R1is independently substituted or unsubstituted heteroaryl. In embodiments, R1is independently substituted alkyl. In embodiments, R1is independently substituted heteroalkyl. In embodiments, R1is independently substituted cycloalkyl. In embodiments, R1is independently substituted heterocycloalkyl. In embodiments, R1is independently substituted aryl. In embodiments, R1is independently substituted heteroaryl. In embodiments, R1is independently unsubstituted alkyl. In embodiments, R1is independently unsubstituted heteroalkyl. In embodiments, R1is independently unsubstituted cycloalkyl. In embodiments, R1is independently unsubstituted heterocycloalkyl. In embodiments, R1is independently unsubstituted aryl. In embodiments, R1is independently unsubstituted heteroaryl. In embodiments, R1is independently substituted or unsubstituted C1-C8alkyl. In embodiments, R1is independently substituted or unsubstituted 2 to 8 membered heteroalkyl. In embodiments, R1is independently substituted or unsubstituted C3-C8cycloalkyl. In embodiments, R1is independently substituted or unsubstituted 3 to 8 membered heterocycloalkyl. In embodiments, R1is independently substituted or unsubstituted C6-C10aryl. In embodiments, R1is independently substituted or unsubstituted 5 to 10 membered heteroaryl. In embodiments, R1is independently substituted C1-C8alkyl. In embodiments, R1is independently substituted 2 to 8 membered heteroalkyl. In embodiments, R1is independently substituted C3-C8cycloalkyl. In embodiments, R1is independently substituted 3 to 8 membered heterocycloalkyl. In embodiments, R1is independently substituted C6-C10aryl. In embodiments, R1is independently substituted 5 to 10 membered heteroaryl. In embodiments, R1is independently unsubstituted C1-C8alkyl. In embodiments, R1is independently unsubstituted 2 to 8 membered heteroalkyl. In embodiments, R1is independently unsubstituted C3-C8cycloalkyl. In embodiments, R1is independently unsubstituted 3 to 8 membered heterocycloalkyl. In embodiments, R1is independently unsubstituted C6-C10aryl. In embodiments, R1is independently unsubstituted 5 to 10 membered heteroaryl. In embodiments, R1is independently substituted or unsubstituted C1-C4alkyl. In embodiments, R1is independently substituted or unsubstituted 2 to 4 membered heteroalkyl. In embodiments, R1is independently substituted or unsubstituted C3-C6cycloalkyl. In embodiments, R1is independently substituted or unsubstituted 3 to 6 membered heterocycloalkyl. In embodiments, R1is independently substituted or unsubstituted phenyl. In embodiments, R1is independently substituted or unsubstituted 5 to 6 membered heteroaryl. In embodiments, R1is independently substituted C1-C4alkyl. In embodiments, R1is independently substituted 2 to 4 membered heteroalkyl. In embodiments, R1is independently substituted C3-C6cycloalkyl. In embodiments, R1is independently substituted 3 to 6 membered heterocycloalkyl. In embodiments, R1is independently substituted phenyl. In embodiments, R1is independently substituted 5 to 6 membered heteroaryl. In embodiments, R1is independently unsubstituted C1-C4alkyl. In embodiments, R1is independently unsubstituted 2 to 4 membered heteroalkyl. In embodiments, R1is independently unsubstituted C3-C6cycloalkyl. In embodiments, R1is independently unsubstituted 3 to 6 membered heterocycloalkyl. In embodiments, R1is independently unsubstituted phenyl. In embodiments, R1is independently unsubstituted 5 to 6 membered heteroaryl. In embodiments, R1is independently —OH. In embodiments, R1is independently —NH2. In embodiments, R1is independently —COOH. In embodiments, R1is independently —CONH2. In embodiments, R1is independently —NO2. In embodiments, R1is independently —SH. In embodiments, R1is independently —CF3. In embodiments, R1is independently —CHF2. In embodiments, R1is independently —CH2F. In embodiments, R1is independently —OCF3. In embodiments, R1is independently —OCH2F. In embodiments, R1is independently —OCHF2. In embodiments, R1is independently —OCH3. In embodiments, R1is independently —OCH2CH3. In embodiments, R1is independently —OCH2CH2CH3. In embodiments, R1is independently —OCH(CH3)2. In embodiments, R1is independently —OC(CH3)3. In embodiments, R1is independently —SCH3. In embodiments, R1is independently —SCH2CH3. In embodiments, R1is independently —SCH2CH2CH3. In embodiments, R1is independently —SCH(CH3)2. In embodiments, R1is independently —SC(CH3)3. In embodiments, R1is independently —CH3. In embodiments, R1is independently —CH2CH3. In embodiments, R1is independently —CH2CH2CH3. In embodiments, R1is independently —CH(CH3)2. In embodiments, R1is independently —C(CH3)3. In embodiments, R1is independently —F. In embodiments, R1is independently —Cl. In embodiments, R1is independently —Br. In embodiments, R1is independently —I. In embodiments, R1is R20-substituted or unsubstituted methyl. In embodiments, R1is R20-substituted or unsubstituted C2alkyl. In embodiments, R1is R20-substituted or unsubstituted C3alkyl. In embodiments, R1is R20-substituted or unsubstituted C4alkyl. In embodiments, R1is R20-substituted or unsubstituted C5alkyl. In embodiments, R1is R20-substituted or unsubstituted C6alkyl. In embodiments, R1is R20-substituted or unsubstituted C7alkyl. In embodiments, R1is R20-substituted or unsubstituted C8alkyl. In embodiments, R1is R20-substituted methyl. In embodiments, R1is R20-substituted C2alkyl. In embodiments, R1is R20-substituted C3alkyl. In embodiments, R1is R20-substituted C4alkyl. In embodiments, R1is R20-substituted C5alkyl. In embodiments, R1is R20-substituted C6alkyl. In embodiments, R1is R20-substituted C7alkyl. In embodiments, R1is R20-substituted C8alkyl. In embodiments, R1is an unsubstituted methyl. In embodiments, R1is an unsubstituted C2alkyl. In embodiments, R1is an unsubstituted C3alkyl. In embodiments, R1is an unsubstituted C4alkyl. In embodiments, R1is an unsubstituted C5alkyl. In embodiments, R1is an unsubstituted C6alkyl. In embodiments, R1is an unsubstituted C7alkyl. In embodiments, R1is an unsubstituted C8alkyl. In embodiments, the compound has the formula: X1, L1, L2, and E are as described herein. In embodiments, the compound has the formula: R1and R4are as described herein. In embodiments, the compound has the formula: R4is as described herein. In embodiments, the compound has the formula: R1, R5, and L1are as described herein. In embodiments, the compound has the formula: L1and R5are as described herein. In embodiments, R4is independently substituted or unsubstituted alkyl. In embodiments, R4is independently substituted or unsubstituted heteroalkyl. In embodiments, R4is independently substituted or unsubstituted cycloalkyl. In embodiments, R4is independently substituted or unsubstituted heterocycloalkyl. In embodiments, R4is independently substituted or unsubstituted aryl. In embodiments, R4is independently substituted or unsubstituted heteroaryl. In embodiments, R4is independently substituted alkyl. In embodiments, R4is independently substituted heteroalkyl. In embodiments, R4is independently substituted cycloalkyl. In embodiments, R4is independently substituted heterocycloalkyl. In embodiments, R4is independently substituted aryl. In embodiments, R4is independently substituted heteroaryl. In embodiments, R4is independently unsubstituted alkyl. In embodiments, R4is independently unsubstituted heteroalkyl. In embodiments, R4is independently unsubstituted cycloalkyl. In embodiments, R4is independently unsubstituted heterocycloalkyl. In embodiments, R4is independently unsubstituted aryl. In embodiments, R4is independently unsubstituted heteroaryl. In embodiments, R4is independently substituted or unsubstituted C1-C8alkyl. In embodiments, R4is independently substituted or unsubstituted 2 to 8 membered heteroalkyl. In embodiments, R4is independently substituted or unsubstituted C3-C8cycloalkyl. In embodiments, R4is independently substituted or unsubstituted 3 to 8 membered heterocycloalkyl. In embodiments, R4is independently substituted or unsubstituted C6-C10aryl. In embodiments, R4is independently substituted or unsubstituted 5 to 10 membered heteroaryl. In embodiments, R4is independently substituted C1-C8alkyl. In embodiments, R4is independently substituted 2 to 8 membered heteroalkyl. In embodiments, R4is independently substituted C3-C8cycloalkyl. In embodiments, R4is independently substituted 3 to 8 membered heterocycloalkyl. In embodiments, R4is independently substituted C6-C10aryl. In embodiments, R4is independently substituted 5 to 10 membered heteroaryl. In embodiments, R4is independently unsubstituted C1-C8alkyl. In embodiments, R4is independently unsubstituted 2 to 8 membered heteroalkyl. In embodiments, R4is independently unsubstituted C3-C8cycloalkyl. In embodiments, R4is independently unsubstituted 3 to 8 membered heterocycloalkyl. In embodiments, R4is independently unsubstituted C6-C10aryl. In embodiments, R4is independently unsubstituted 5 to 10 membered heteroaryl. In embodiments, R4is independently substituted or unsubstituted C1-C4alkyl. In embodiments, R4is independently substituted or unsubstituted 2 to 4 membered heteroalkyl. In embodiments, R4is independently substituted or unsubstituted C3-C6cycloalkyl. In embodiments, R4is independently substituted or unsubstituted 3 to 6 membered heterocycloalkyl. In embodiments, R4is independently substituted or unsubstituted phenyl. In embodiments, R4is independently substituted or unsubstituted 5 to 6 membered heteroaryl. In embodiments, R4is independently substituted C1-C4alkyl. In embodiments, R4is independently substituted 2 to 4 membered heteroalkyl. In embodiments, R4is independently substituted C3-C6cycloalkyl. In embodiments, R4is independently substituted 3 to 6 membered heterocycloalkyl. In embodiments, R4is independently substituted phenyl. In embodiments, R4is independently substituted 5 to 6 membered heteroaryl. In embodiments, R4is independently unsubstituted C1-C4alkyl. In embodiments, R4is independently unsubstituted 2 to 4 membered heteroalkyl. In embodiments, R4is independently unsubstituted C3-C6cycloalkyl. In embodiments, R4is independently unsubstituted 3 to 6 membered heterocycloalkyl. In embodiments, R4is independently unsubstituted phenyl. In embodiments, R4is independently unsubstituted 5 to 6 membered heteroaryl. In embodiments, R4is R29-substituted or unsubstituted methyl. In embodiments, R4is R29-substituted or unsubstituted C2alkyl. In embodiments, R4is R29-substituted or unsubstituted C3alkyl. In embodiments, R4is R29-substituted or unsubstituted C4alkyl. In embodiments, R4is R29-substituted or unsubstituted C5alkyl. In embodiments, R4is R29-substituted or unsubstituted C6alkyl. In embodiments, R4is R29-substituted or unsubstituted C7alkyl. In embodiments, R4is R29-substituted or unsubstituted C8alkyl. In embodiments, R4is R29-substituted methyl. In embodiments, R4is R29-substituted C2alkyl. In embodiments, R4is R29-substituted C3alkyl. In embodiments, R4is R29-substituted C4alkyl. In embodiments, R4is R29-substituted C8alkyl. In embodiments, R4is R29-substituted C6alkyl. In embodiments, R4is R29-substituted C7alkyl. In embodiments, R4is R29-substituted C8alkyl. In embodiments, R4is an unsubstituted methyl. In embodiments, R4is an unsubstituted C2alkyl. In embodiments, R4is an unsubstituted C3alkyl. In embodiments, R4is an unsubstituted C4alkyl. In embodiments, R4is an unsubstituted C5alkyl. In embodiments, R4is an unsubstituted C6alkyl. In embodiments, R4is an unsubstituted C7alkyl. In embodiments, R4is an unsubstituted C8alkyl. In embodiments, R4is independently —OH. In embodiments, R4is independently —NH2. In embodiments, R4is independently —COOH. In embodiments, R4is independently —CONH2. In embodiments, R4is independently —CF3. In embodiments, R4is independently —CHF2. In embodiments, R4is independently —CH2F. In embodiments, R4is independently —OCF3. In embodiments, R4is independently —OCH2F. In embodiments, R4is independently —OCHF2. In embodiments, R4is independently —OCH3. In embodiments, R4is independently —OCH2CH3. In embodiments, R4is independently —OCH2CH2CH3. In embodiments, R4is independently —OCH(CH3)2. In embodiments, R4is independently —OC(CH3)3. In embodiments, R4is independently —SCH3. In embodiments, R4is independently —SCH2CH3. In embodiments, R4is independently —SCH2CH2CH3. In embodiments, R4is independently —SCH(CH3)2. In embodiments, R4is independently —SC(CH3)3. In embodiments, R4is independently —CH3. In embodiments, R4is independently —CH2CH3. In embodiments, R4is independently —CH2CH2CH3. In embodiments, R4is independently —CH(CH3)2. In embodiments, R4is independently —C(CH3)3. In embodiments, R4is independently hydrogen. In an aspect is provided a compound having the formula: R1, R2, L1, L2, E, z1 and z2 are as described herein. In embodiments, the compound has the formula: R1, R2, z1 and z2 are as described herein. In embodiments, the compound has the formula: In embodiments, the compound is a compound described herein, including in an aspect, embodiment, claim, figure, table, example, or scheme. In embodiments, the compound has the formula: In embodiments, the compound has the formula: In embodiments, the compound has the formula: In embodiments, the compound has the formula: In embodiments, the compound has the formula: In embodiments, the compound has the formula: In embodiments, the compound has the formula: In embodiments, the compound has the formula: In embodiments, the compound has the formula: In embodiments, the compound has the formula: In embodiments, the compound has the formula: In embodiments, the compound has the formula: In embodiments, the compound has the formula: In an aspect is provided a pharmaceutical composition including a Reticulon 4 inhibitor and a pharmaceutically acceptable excipient. In embodiments, the Reticulon 4 inhibitor is a compound described herein. In embodiments, the Reticulon 4 inhibitor is an oligonucleotide (e.g., DNA, RNA, or siRNA), protein (e.g., antibody, anti-Reticulon 4 antibody, anti-Reticulon 4 binding antibody fragment), or compound (e.g., compound described herein). In embodiments, the Reticulon 4 inhibitor is included in a therapeutically effective amount. In an aspect is provided a pharmaceutical composition including a compound described herein, or pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable excipient. In embodiments of the pharmaceutical compositions, the compound, or pharmaceutically acceptable salt thereof, is included in a therapeutically effective amount. In embodiments of the pharmaceutical compositions, the pharmaceutical composition includes a second agent (e.g. therapeutic agent). In embodiments of the pharmaceutical compositions, the pharmaceutical composition includes a second agent (e.g. therapeutic agent) in a therapeutically effective amount. In embodiments of the pharmaceutical compositions, the second agent is an agent for treating cancer. In embodiments, the second agent is an anti-cancer agent. In embodiments, the second agent is a chemotherapeutic. In embodiments, the second agent is an anti-inflammatory agent. In an aspect is provided a method of treating cancer, the method including administering to a subject in need thereof an effective amount of a Reticulon 4 inhibitor. In embodiments, the Reticulon 4 inhibitor is a compound described herein. In embodiments, the Reticulon 4 inhibitor is an oligonucleotide (e.g., DNA, RNA, or siRNA), protein (e.g., antibody, anti-Reticulon 4 antibody, anti-Reticulon 4 binding antibody fragment), or compound (e.g., compound described herein). In embodiments, the Reticulon 4 inhibitor is included in a therapeutically effective amount. In embodiments, the Reticulon 4 inhibitor is an antisense nucleic acid. In an aspect is provided a method of treating cancer including administering to a subject in need thereof an effective amount of a compound described herein. In embodiments, the cancer is colorectal cancer. In embodiments, the cancer is liver cancer. In embodiments, the cancer is hepatocellular cancer. In embodiments, the cancer is breast cancer. In embodiments, the cancer is estrogen receptor positive breast cancer. In embodiments, the cancer is estrogen receptor (ER) negative breast cancer. In embodiments, the cancer is tamoxifen resistant breast cancer. In embodiments, the cancer is HER2 negative breast cancer. In embodiments, the cancer is HER2 positive breast cancer. In embodiments, the cancer is low grade (well differentiated) breast cancer. In embodiments, the cancer is intermediate grade (moderately differentiated) breast cancer. In embodiments, the cancer is high grade (poorly differentiated) breast cancer. In embodiments, the cancer is stage 0 breast cancer. In embodiments, the cancer is stage I breast cancer. In embodiments, the cancer is stage II breast cancer. In embodiments, the cancer is stage III breast cancer. In embodiments, the cancer is stage IV breast cancer. In embodiments, the cancer is triple negative breast cancer. In an aspect is provided a method of treating a neurodegenerative disease, the method including administering to a subject in need thereof an effective amount of a Reticulon 4 inhibitor. In an aspect is provided a method of treating nerve damage, the method including administering to a subject in need thereof an effective amount of a Reticulon 4 inhibitor. In an aspect is provided a method of treating a traumatic brain injury, the method including administering to a subject in need thereof an effective amount of a Reticulon 4 inhibitor. In an aspect is provided a method of treating a spinal cord injury, the method including administering to a subject in need thereof an effective amount of a Reticulon 4 inhibitor. In an aspect is provided a method of treating stroke, the method including administering to a subject in need thereof an effective amount of a Reticulon 4 inhibitor. In embodiments, the Reticulon 4 inhibitor is a compound described herein. In embodiments, the Reticulon 4 inhibitor is an oligonucleotide (e.g., DNA, RNA, or siRNA), protein (e.g., antibody, anti-Reticulon 4 antibody, anti-Reticulon 4 binding antibody fragment), or compound (e.g., compound described herein). In embodiments, the Reticulon 4 inhibitor is included in a therapeutically effective amount. In embodiments, the neurodegenerative disease is ALS. In embodiments, the neurodegenerative disease is multiple sclerosis. In an aspect is provided a method of treating neurodegenerative disease including administering to a subject in need thereof an effective amount of a compound described herein. In an aspect is provided a method of treating nerve damage including administering to a subject in need thereof an effective amount of a compound described herein. In an aspect is provided a method of treating traumatic brain injury including administering to a subject in need thereof an effective amount of a compound described herein. In an aspect is provided a method of treating spinal cord injury including administering to a subject in need thereof an effective amount of a compound described herein. In an aspect is provided a method of treating stroke including administering to a subject in need thereof an effective amount of a compound described herein. In embodiments, the neurodegenerative disease is ALS. In embodiments, the neurodegenerative disease is multiple sclerosis. In an aspect is provided a method of treating a disease associated with reticulon 4 activity including administering to a subject in need thereof an effective amount of a Reticulon 4 inhibitor. In embodiments, the Reticulon 4 inhibitor is a compound described herein. In embodiments, the Reticulon 4 inhibitor is an oligonucleotide (e.g., DNA, RNA, or siRNA), protein (e.g., antibody, anti-Reticulon 4 antibody, anti-Reticulon 4 binding antibody fragment), or compound (e.g., compound described herein). In embodiments, the disease is associated with aberrant reticulon 4 activity. In an aspect is provided a method of increasing nerve growth (e.g., neurite growth, neuron growth), the method including administering to a subject (e.g., contacting the nerve or neurite) in need thereof an effective amount of a Reticulon 4 inhibitor. In embodiments, the Reticulon 4 inhibitor is a compound described herein. In embodiments, the Reticulon 4 inhibitor is an oligonucleotide (e.g., DNA, RNA, or siRNA), protein (e.g., antibody, anti-Reticulon 4 antibody, anti-Reticulon 4 binding antibody fragment), or compound (e.g., compound described herein). In embodiments, the Reticulon 4 inhibitor is included in a therapeutically effective amount. In an aspect is provided a method of increasing nerve growth (e.g., neurite growth, neuron growth) including administering to a subject (e.g., contacting the nerve or neurite) in need thereof an effective amount of a compound described herein. In embodiments, the method includes administering a second agent (e.g. therapeutic agent). In embodiments, the method includes administering a second agent (e.g. therapeutic agent) in a therapeutically effective amount. In embodiments, the second agent is an agent for treating cancer. In embodiments, the second agent is an anti-cancer agent. In embodiments, the second agent is a chemotherapeutic. In embodiments, the second agent is an agent for treating a neurodegenerative disease. In embodiments, the second agent is an agent for promoting nerve growth. In embodiments, the second agent is an agent for treating traumatic brain injury. In embodiments, the second agent is an agent for treating nerve damage. In embodiments, the second agent is an agent for treating spinal cord injury. In embodiments, the second agent is an agent for treating stroke. In an aspect is provided a method of inhibiting reticulon 4 activity including contacting the reticulon 4 with a Reticulon 4 inhibitor. In embodiments, the reticulon 4 is a human reticulon 4. In embodiments, the Reticulon 4 inhibitor is a compound described herein. In embodiments, the Reticulon 4 inhibitor is an oligonucleotide (e.g., DNA, RNA, or siRNA), protein (e.g., antibody, anti-Reticulon 4 antibody, anti-Reticulon 4 binding antibody fragment), or compound (e.g., compound described herein). In embodiments, the Reticulon 4 inhibitor is provided in a therapeutically effective amount. In embodiments, the reticulon 4 is SEQ ID NO:333, SEQ ID NO:334, SEQ ID NO:335, SEQ ID NO:336, SEQ ID NO:337, SEQ ID NO:338, SEQ ID NO:339, SEQ ID NO:340, SEQ ID NO:331, SEQ ID NO:341, or SEQ ID NO:342. In embodiments, the reticulon 4 is SEQ ID NO:333. In embodiments, the reticulon 4 is SEQ ID NO:334. In embodiments, the reticulon 4 is SEQ ID NO:335. In embodiments, the reticulon 4 is SEQ ID NO:336. In embodiments, the reticulon 4 is SEQ ID NO:337. In embodiments, the reticulon 4 is SEQ ID NO:338. In embodiments, the reticulon 4 is SEQ ID NO:339. In embodiments, the reticulon 4 is SEQ ID NO:340. In embodiments, the reticulon 4 is SEQ ID NO:331. In embodiments, the reticulon 4 is SEQ ID NO:341. In embodiments, the reticulon 4 is SEQ ID NO:342. In embodiments, the Reticulon 4 inhibitor contacts one or more amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of human reticulon 4 (e.g., SEQ ID NO:331). In embodiments, the Reticulon 4 inhibitor contacts one or more amino acids corresponding to E1105, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts one or more amino acids corresponding to E1105, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor covalently binds an amino acid corresponding to C1101 of SEQ ID NO:331 in human reticulon 4. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to E1105, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to E1105 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to C1101 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to E1078 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to S1079 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to A1082 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to I1083 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to K1090 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to Y1091 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to S1094 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to G1097 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to H1098 of SEQ ID NO:331. In an aspect is provided a method of inhibiting reticulon 4 activity including contacting the reticulon 4 with a compound described herein. In embodiments, the reticulon 4 is a human reticulon 4. In embodiments, the compound is provided in an effective amount. In embodiments, the compound is provided in a therapeutically effective amount. In embodiments, the method includes contacting the reticulon 4 protein with an effective amount of a compound described herein. In embodiments, compound is covalently bonded to the amino acid corresponding to C1101 of SEQ ID NO:331. In embodiments, the compound contacts one or more amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the compound contacts one or more amino acids corresponding to E1105, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the compound covalently binds an amino acid corresponding to C1101 in SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to E1105, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to E1105 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to C1101 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to E1078 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to S1079 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to A1082 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to I1083 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to K1090 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to Y1091 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to S1094 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to G1097 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to H1098 of SEQ ID NO:331. In embodiments, the compound contacts a cysteine in a sequence described herein. In embodiments, the sequence is (SEQ ID NO:1). In embodiments, the sequence is (SEQ ID NO:2). In embodiments, the sequence is (SEQ ID NO:3). In embodiments, the sequence is (SEQ ID NO:4). In embodiments, the sequence is (SEQ ID NO:5). In embodiments, the sequence is (SEQ ID NO:6). In embodiments, the sequence is (SEQ ID NO:7). In embodiments, the sequence is (SEQ ID NO:8). In embodiments, the sequence is (SEQ ID NO:9). In embodiments, the sequence is (SEQ ID NO:10). In embodiments, the sequence is (SEQ ID NO:11). In embodiments, the sequence is (SEQ ID NO:12). In embodiments, the sequence is (SEQ ID NO:13). In embodiments, the sequence is (SEQ ID NO:14). In embodiments, the sequence is (SEQ ID NO:15). In embodiments, the sequence is (SEQ ID NO:16). In embodiments, the sequence is (SEQ ID NO:17). In embodiments, the sequence is (SEQ ID NO:18). In embodiments, the sequence is (SEQ ID NO:19). In embodiments, the sequence is (SEQ ID NO:20). In embodiments, the sequence is (SEQ ID NO:21). In embodiments, the sequence is (SEQ ID NO:22). In embodiments, the sequence is (SEQ ID NO:23). In embodiments, the sequence is (SEQ ID NO:24). In embodiments, the sequence is (SEQ ID NO:25). In embodiments, the sequence is (SEQ ID NO:26). In embodiments, the sequence is (SEQ ID NO:27). In embodiments, the sequence is (SEQ ID NO:28). In embodiments, the sequence is (SEQ ID NO:29). In embodiments, the sequence is (SEQ ID NO:30). In embodiments, the sequence is (SEQ ID NO:31). In embodiments, the sequence is (SEQ ID NO:32). In embodiments, the sequence is (SEQ ID NO:33). In embodiments, the sequence is (SEQ ID NO:34). In embodiments, the sequence is (SEQ ID NO:35). In embodiments, the sequence is (SEQ ID NO:36). In embodiments, the sequence is (SEQ ID NO:37). In embodiments, the sequence is (SEQ ID NO:38). In embodiments, the sequence is (SEQ ID NO:39). In embodiments, the sequence is (SEQ ID NO:40). In embodiments, the sequence is (SEQ ID NO:41). In embodiments, the sequence is (SEQ ID NO:42). In embodiments, the sequence is (SEQ ID NO:43). In embodiments, the sequence is (SEQ ID NO:44). In embodiments, the sequence is (SEQ ID NO:45). In embodiments, the sequence is (SEQ ID NO:46). In embodiments, the sequence is (SEQ ID NO:47). In embodiments, the sequence is (SEQ ID NO:48). In embodiments, the sequence is (SEQ ID NO:49). In embodiments, the sequence is (SEQ ID NO:50). In embodiments, the sequence is (SEQ ID NO:51). In embodiments, the sequence is (SEQ ID NO:52). In embodiments, the sequence is (SEQ ID NO:53). In embodiments, the sequence is (SEQ ID NO:54). In embodiments, the sequence is (SEQ ID NO:55). In embodiments, the sequence is (SEQ ID NO:56). In embodiments, the sequence is (SEQ ID NO:57). In embodiments, the sequence is (SEQ ID NO:58). In embodiments, the sequence is (SEQ ID NO:59). In embodiments, the sequence is (SEQ ID NO:60). In embodiments, the sequence is (SEQ ID NO:61). In embodiments, the sequence is (SEQ ID NO:62). In embodiments, the sequence is (SEQ ID NO:63). In embodiments, the sequence is (SEQ ID NO:64). In embodiments, the sequence is (SEQ ID NO:65). In embodiments, the sequence is (SEQ ID NO:66). In embodiments, the sequence is (SEQ ID NO:67). In embodiments, the sequence is (SEQ ID NO:68). In embodiments, the sequence is (SEQ ID NO:69). In embodiments, the sequence is (SEQ ID NO:70). In embodiments, the sequence is (SEQ ID NO:71). In embodiments, the sequence is (SEQ ID NO:72). In embodiments, the sequence is (SEQ ID NO:73). In embodiments, the sequence is (SEQ ID NO:74). In embodiments, the sequence is (SEQ ID NO:75). In embodiments, the sequence is (SEQ ID NO:76). In embodiments, the sequence is (SEQ ID NO:77). In embodiments, the sequence is (SEQ ID NO:78). In embodiments, the sequence is (SEQ ID NO:79). In embodiments, the sequence is (SEQ ID NO:80). In embodiments, the sequence is (SEQ ID NO:81). In embodiments, the sequence is (SEQ ID NO:82). In embodiments, the sequence is (SEQ ID NO:83). In embodiments, the sequence is (SEQ ID NO:84). In embodiments, the sequence is (SEQ ID NO:85). In embodiments, the sequence is (SEQ ID NO:86). In embodiments, the sequence is (SEQ ID NO:87). In embodiments, the sequence is (SEQ ID NO:88). In embodiments, the sequence is (SEQ ID NO:89). In embodiments, the sequence is (SEQ ID NO:90). In embodiments, the sequence is (SEQ ID NO:91). In embodiments, the sequence is (SEQ ID NO:92). In embodiments, the sequence is (SEQ ID NO:93). In embodiments, the sequence is (SEQ ID NO:94). In embodiments, the sequence is (SEQ ID NO:95). In embodiments, the sequence is (SEQ ID NO:96). In embodiments, the sequence is (SEQ ID NO:97). In embodiments, the sequence is (SEQ ID NO:98). In embodiments, the sequence is (SEQ ID NO:99). In embodiments, the sequence is (SEQ ID NO:100). In embodiments, the sequence is (SEQ ID NO:101). In embodiments, the sequence is (SEQ ID NO:102). In embodiments, the sequence is (SEQ ID NO:103). In embodiments, the sequence is (SEQ ID NO:104). In embodiments, the sequence is (SEQ ID NO:105). In embodiments, the sequence is (SEQ ID NO:106). In embodiments, the sequence is (SEQ ID NO:107). In embodiments, the sequence is (SEQ ID NO:108). In embodiments, the sequence is (SEQ ID NO:109). In embodiments, the sequence is (SEQ ID NO:110). In embodiments, the sequence is (SEQ ID NO:111). In embodiments, the sequence is (SEQ ID NO:112). In embodiments, the sequence is (SEQ ID NO:113). In embodiments, the sequence is (SEQ ID NO:114). In embodiments, the sequence is (SEQ ID NO:115). In embodiments, the sequence is (SEQ ID NO:116). In embodiments, the sequence is (SEQ ID NO:117). In embodiments, the sequence is (SEQ ID NO:118). In embodiments, the sequence is (SEQ ID NO:119). In embodiments, the sequence is (SEQ ID NO:120). In embodiments, the sequence is (SEQ ID NO:121). In embodiments, the sequence is (SEQ ID NO:122). In embodiments, the sequence is (SEQ ID NO:123). In embodiments, the sequence is (SEQ ID NO:124). In embodiments, the sequence is (SEQ ID NO:125). In embodiments, the sequence is (SEQ ID NO:126). In embodiments, the sequence is (SEQ ID NO:127). In embodiments, the sequence is (SEQ ID NO:128). In embodiments, the sequence is (SEQ ID NO:129). In embodiments, the sequence is (SEQ ID NO:130). In embodiments, the sequence is (SEQ ID NO:131). In embodiments, the sequence is (SEQ ID NO:132). In embodiments, the sequence is (SEQ ID NO:133). In embodiments, the sequence is (SEQ ID NO:134). In embodiments, the sequence is (SEQ ID NO:135). In embodiments, the sequence is (SEQ ID NO:136). In embodiments, the sequence is (SEQ ID NO:137). In embodiments, the sequence is (SEQ ID NO:138). In embodiments, the sequence is (SEQ ID NO:139). In embodiments, the sequence is (SEQ ID NO:140). In embodiments, the sequence is (SEQ ID NO:141). In embodiments, the sequence is (SEQ ID NO:142). In embodiments, the sequence is (SEQ ID NO:143). In embodiments, the sequence is (SEQ ID NO:144). In embodiments, the sequence is (SEQ ID NO:145). In embodiments, the sequence is (SEQ ID NO:146). In embodiments, the sequence is (SEQ ID NO:147). In embodiments, the sequence is (SEQ ID NO:148). In embodiments, the sequence is (SEQ ID NO:149). In embodiments, the sequence is (SEQ ID NO:150). In embodiments, the sequence is (SEQ ID NO:151). In embodiments, the sequence is (SEQ ID NO:152). In embodiments, the sequence is (SEQ ID NO:153). In embodiments, the sequence is (SEQ ID NO:154). In embodiments, the sequence is (SEQ ID NO:155). In embodiments, the sequence is (SEQ ID NO:156). In embodiments, the sequence is (SEQ ID NO:157). In embodiments, the sequence is (SEQ ID NO:158). In embodiments, the sequence is (SEQ ID NO:159). In embodiments, the sequence is (SEQ ID NO:160). In embodiments, the sequence is (SEQ ID NO:161). In embodiments, the sequence is (SEQ ID NO:162). In embodiments, the sequence is (SEQ ID NO:163). In embodiments, the sequence is (SEQ ID NO:164). In embodiments, the sequence is (SEQ ID NO:165). In embodiments, the sequence is (SEQ ID NO:166). In embodiments, the sequence is (SEQ ID NO:167). In embodiments, the sequence is (SEQ ID NO:168). In embodiments, the sequence is (SEQ ID NO:169). In embodiments, the sequence is (SEQ ID NO:170). In embodiments, the sequence is (SEQ ID NO:171). In embodiments, the sequence is (SEQ ID NO:172). In embodiments, the sequence is (SEQ ID NO:173). In embodiments, the sequence is (SEQ ID NO:174). In embodiments, the sequence is (SEQ ID NO:175). In embodiments, the sequence is (SEQ ID NO:176). In embodiments, the sequence is (SEQ ID NO:177). In embodiments, the sequence is (SEQ ID NO:178). In embodiments, the sequence is (SEQ ID NO:179). In embodiments, the sequence is (SEQ ID NO:180). In embodiments, the sequence is (SEQ ID NO:181). In embodiments, the sequence is (SEQ ID NO:182). In embodiments, the sequence is (SEQ ID NO:183). In embodiments, the sequence is (SEQ ID NO:184). In embodiments, the sequence is (SEQ ID NO:185). In embodiments, the sequence is (SEQ ID NO:186). In embodiments, the sequence is (SEQ ID NO:187). In embodiments, the sequence is (SEQ ID NO:188). In embodiments, the sequence is (SEQ ID NO:189). In embodiments, the sequence is (SEQ ID NO:190). In embodiments, the sequence is (SEQ ID NO:191). In embodiments, the sequence is (SEQ ID NO:192). In embodiments, the sequence is (SEQ ID NO:193). In embodiments, the sequence is (SEQ ID NO:194). In embodiments, the sequence is (SEQ ID NO:195). In embodiments, the sequence is (SEQ ID NO:196). In embodiments, the sequence is (SEQ ID NO:197). In embodiments, the sequence is (SEQ ID NO:198). In embodiments, the sequence is (SEQ ID NO:199). In embodiments, the sequence is (SEQ ID NO:200). In embodiments, the sequence is (SEQ ID NO:201). In embodiments, the sequence is (SEQ ID NO:202). In embodiments, the sequence is (SEQ ID NO:203). In embodiments, the sequence is (SEQ ID NO:204). In embodiments, the sequence is (SEQ ID NO:205). In embodiments, the sequence is (SEQ ID NO:206). In embodiments, the sequence is (SEQ ID NO:207). In embodiments, the sequence is (SEQ ID NO:208). In embodiments, the sequence is (SEQ ID NO:209). In embodiments, the sequence is (SEQ ID NO:210). In embodiments, the sequence is (SEQ ID NO:211). In embodiments, the sequence is (SEQ ID NO:212). In embodiments, the sequence is (SEQ ID NO:213). In embodiments, the sequence is (SEQ ID NO:214). In embodiments, the sequence is (SEQ ID NO:215). In embodiments, the sequence is (SEQ ID NO:216). In embodiments, the sequence is (SEQ ID NO:217). In embodiments, the sequence is (SEQ ID NO:218). In embodiments, the sequence is (SEQ ID NO:219). In embodiments, the sequence is (SEQ ID NO:220). In embodiments, the sequence is (SEQ ID NO:221). In embodiments, the sequence is (SEQ ID NO:222). In embodiments, the sequence is (SEQ ID NO:223). In embodiments, the sequence is (SEQ ID NO:224). In embodiments, the sequence is (SEQ ID NO:225). In embodiments, the sequence is (SEQ ID NO:226). In embodiments, the sequence is (SEQ ID NO:227). In embodiments, the sequence is (SEQ ID NO:228). In embodiments, the sequence is (SEQ ID NO:229). In embodiments, the sequence is (SEQ ID NO:230). In embodiments, the sequence is (SEQ ID NO:231). In embodiments, the sequence is (SEQ ID NO:232). In embodiments, the sequence is (SEQ ID NO:233). In embodiments, the sequence is (SEQ ID NO:234). In embodiments, the sequence is (SEQ ID NO:235). In embodiments, the sequence is (SEQ ID NO:236). In embodiments, the sequence is (SEQ ID NO:237). In embodiments, the sequence is (SEQ ID NO:238). In embodiments, the sequence is (SEQ ID NO:239). In embodiments, the sequence is (SEQ ID NO:240). In embodiments, the sequence is (SEQ ID NO:241). In embodiments, the sequence is (SEQ ID NO:242). In embodiments, the sequence is (SEQ ID NO:243). In embodiments, the sequence is (SEQ ID NO:244). In embodiments, the sequence is (SEQ ID NO:245). In embodiments, the sequence is (SEQ ID NO:246). In embodiments, the sequence is (SEQ ID NO:247). In embodiments, the sequence is (SEQ ID NO:248). In embodiments, the sequence is (SEQ ID NO:249). In embodiments, the sequence is (SEQ ID NO:250). In embodiments, the sequence is (SEQ ID NO:251). In embodiments, the sequence is (SEQ ID NO:252). In embodiments, the sequence is (SEQ ID NO:253). In embodiments, the sequence is (SEQ ID NO:254). In embodiments, the sequence is (SEQ ID NO:255). In embodiments, the sequence is (SEQ ID NO:256). In embodiments, the sequence is (SEQ ID NO:257). In embodiments, the sequence is (SEQ ID NO:258). In embodiments, the sequence is (SEQ ID NO:259). In embodiments, the sequence is (SEQ ID NO:260). In embodiments, the sequence is (SEQ ID NO:261). In embodiments, the sequence is (SEQ ID NO:262). In embodiments, the sequence is (SEQ ID NO:263). In embodiments, the sequence is (SEQ ID NO:264). In embodiments, the sequence is (SEQ ID NO:265). In embodiments, the sequence is (SEQ ID NO:266). In embodiments, the sequence is (SEQ ID NO:267). In embodiments, the sequence is (SEQ ID NO:268). In embodiments, the sequence is (SEQ ID NO:269). In embodiments, the sequence is (SEQ ID NO:270). In embodiments, the sequence is (SEQ ID NO:271). In embodiments, the sequence is (SEQ ID NO:272). In embodiments, the sequence is (SEQ ID NO:273). In embodiments, the sequence is (SEQ ID NO:274). In embodiments, the sequence is (SEQ ID NO:275). In embodiments, the sequence is (SEQ ID NO:276). In embodiments, the sequence is (SEQ ID NO:277). In embodiments, the sequence is (SEQ ID NO:278). In embodiments, the sequence is (SEQ ID NO:279). In embodiments, the sequence is (SEQ ID NO:280). In embodiments, the sequence is (SEQ ID NO:281). In embodiments, the sequence is (SEQ ID NO:282). In embodiments, the sequence is (SEQ ID NO:283). In embodiments, the sequence is (SEQ ID NO:284). In embodiments, the sequence is (SEQ ID NO:285). In embodiments, the sequence is (SEQ ID NO:286). In embodiments, the sequence is (SEQ ID NO:287). In embodiments, the sequence is (SEQ ID NO:288). In embodiments, the sequence is (SEQ ID NO:289). In embodiments, the sequence is (SEQ ID NO:290). In embodiments, the sequence is (SEQ ID NO:291). In embodiments, the sequence is (SEQ ID NO:292). In embodiments, the sequence is (SEQ ID NO:293). In embodiments, the sequence is (SEQ ID NO:294). In embodiments, the sequence is (SEQ ID NO:295). In embodiments, the sequence is (SEQ ID NO:296). In embodiments, the sequence is (SEQ ID NO:297). In embodiments, the sequence is (SEQ ID NO:298). In embodiments, the sequence is (SEQ ID NO:299). In embodiments, the sequence is (SEQ ID NO:300). In embodiments, the sequence is (SEQ ID NO:301). In embodiments, the sequence is (SEQ ID NO:302). In embodiments, the sequence is (SEQ ID NO:303). In embodiments, the sequence is (SEQ ID NO:304). In embodiments, the sequence is (SEQ ID NO:305). In embodiments, the sequence is (SEQ ID NO:306). In embodiments, the sequence is (SEQ ID NO:307). In embodiments, the sequence is (SEQ ID NO:308). In embodiments, the sequence is (SEQ ID NO:309). In embodiments, the sequence is (SEQ ID NO:310). In embodiments, the sequence is (SEQ ID NO:311). In embodiments, the sequence is (SEQ ID NO:312). In embodiments, the sequence is (SEQ ID NO:313). In embodiments, the sequence is (SEQ ID NO:314). In embodiments, the sequence is (SEQ ID NO:315). In embodiments, the sequence is (SEQ ID NO:316). In embodiments, the sequence is (SEQ ID NO:317). In embodiments, the sequence is (SEQ ID NO:318). In embodiments, the sequence is (SEQ ID NO:319). In embodiments, the sequence is (SEQ ID NO:320). In embodiments, the sequence is (SEQ ID NO:321). In embodiments, the sequence is (SEQ ID NO:322). In embodiments, the sequence is (SEQ ID NO:323). In embodiments, the sequence is (SEQ ID NO:324). In embodiments, the sequence is (SEQ ID NO:325). In embodiments, the sequence is (SEQ ID NO:326). In embodiments, the sequence is (SEQ ID NO:327). In embodiments, the sequence is (SEQ ID NO:328). In embodiments, the sequence is (SEQ ID NO:329). In embodiments, the sequence is (SEQ ID NO:330). In embodiments, the sequence is (SEQ ID NO:331). In embodiments, the sequence is (SEQ ID NO:332). In embodiments, the sequence is (SEQ ID NO:333). In embodiments, the sequence is (SEQ ID NO:334). In embodiments, the sequence is (SEQ ID NO:335). In embodiments, the sequence is (SEQ ID NO:336). In embodiments, the sequence is (SEQ ID NO:337). In embodiments, the sequence is (SEQ ID NO:338). In embodiments, the sequence is (SEQ ID NO:339). In embodiments, the sequence is (SEQ ID NO:340). In embodiments, the sequence is (SEQ ID NO:341). In embodiments, the sequence is (SEQ ID NO:342). In embodiments, the sequence is (SEQ ID NO:343). In embodiments, the inhibition is competitive inhibition. In embodiments, the inhibition is irreversible. In embodiments, the inhibition is reversible. In embodiments, the compound covalently binds to the reticulon 4 protein. Where the compound covalently binds to the reticulon 4 a reticulon 4 protein (e.g., human reticulon 4) covalently bonded to a reticulon 4 inhibitor is formed (also referred to herein as a “reticulon 4-compound adduct”), as described below. In embodiments, the resulting covalent bond is reversible. Where the resulting covalent bond is reversible, the bonding reverses upon denaturation of the protein. Thus, in embodiments, the reversibility of a covalent bond between the compound and the reticulon 4 upon denaturation of the reticulon 4 avoids or decreases autoimmune response in a subject subsequent to administration of the compound (relative to irreversibility). Moreover, in embodiments, the reversibility of a covalent bond between the compound and the reticulon 4 upon denaturation of the reticulon 4 avoids or decreases the toxicity (e.g. liver toxicity) of the compound in a subject (relative to irreversibility). In embodiments, the reticulon 4 activity is endoplasmic reticulum (ER) tubule formation. In embodiments, the reticulon 4 activity is an increase in endoplasmic reticulum (ER) tubule formation. In embodiments, the reticulon 4 activity is RTN 4 membrane associate. In embodiments, the reticulon 4 activity is RTN 4 membrane contact. In embodiments, the reticulon 4 activity is increasing ER tubule networks. In embodiments, the reticulon 4 activity is nuclear envelope assembly (e.g., during mitosis). In embodiments, the reticulon 4 activity is nuclear envelope formation (e.g., during mitosis). In embodiments, the reticulon 4 activity is nuclear envelope disassembly (e.g., during mitosis). In embodiments, the reticulon 4 activity is increasing cell division. In embodiments, the reticulon 4 activity is increasing the rate of cell divisional. In embodiments, the reticulon 4 activity is promoting cell division. In embodiments, the reticulon 4 activity is completing cell division. In embodiments, the reticulon 4 activity is maintaining natural nuclear envelope morphology (e.g., during mitosis). In embodiments, the reticulon 4 activity is maintaining natural interphase nuclear envelope morphology. In embodiments, the reticulon 4 activity is nuclear envelope remodeling (e.g., during mitosis). In embodiments, the reticulon 4 activity is inhibition of neurite cell growth. In embodiments, the reticulon 4 activity is neuron growth. In embodiments, the reticulon 4 activity is neuron survival. In embodiments, the reticulon 4 activity is neuron proliferation. In embodiments, the reticulon 4 activity is completing mitosis. In embodiments, the reticulon 4 activity is increasing the rate of mitosis (e.g, compared to lack of RTN 4 activity). In an aspect is provided a method of inhibiting cell divisional (e.g., cancer cell division, cancer proliferation), the method including contacting a cell (e.g., cancer cell) with an effective amount of a Reticulon 4 inhibitor. In embodiments, the Reticulon 4 inhibitor is a compound described herein. In embodiments, the Reticulon 4 inhibitor is an oligonucleotide (e.g., DNA, RNA, or siRNA), protein (e.g., antibody, anti-Reticulon 4 antibody, anti-Reticulon 4 binding antibody fragment), or compound (e.g., compound described herein). In an aspect is provided a method of inhibiting cell divisional (e.g., cancer cell division, cancer proliferation) including contacting a cell (e.g., cancer cell) with an effective amount of a compound described herein. In an aspect is provided a reticulon 4 protein covalently bonded to a Reticulon 4 inhibitor (a reticulon 4 protein-reticulon 4 inhibitor complex). In embodiments, the reticulon 4 is a human reticulon 4. In embodiments, the reticulon 4 is has the sequence SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor is a compound described herein. In embodiments, the Reticulon 4 inhibitor is an oligonucleotide (e.g., DNA, RNA, or siRNA), protein (e.g., antibody, anti-Reticulon 4 antibody, anti-Reticulon 4 binding antibody fragment), or compound (e.g., compound described herein). In embodiments, the Reticulon 4 inhibitor is provided in a therapeutically effective amount. In embodiments, the Reticulon 4 inhibitor contacts one or more amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor covalently binds an amino acid corresponding to C1101 in SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to E1105 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to C1101 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to E1078 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to S1079 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to A1082 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to I1083 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to K1090 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to Y1091 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to S1094 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to G1097 of SEQ ID NO:331. In embodiments, the Reticulon 4 inhibitor contacts an amino acids corresponding to H1098 of SEQ ID NO:331. In an aspect is provided a reticulon 4 protein covalently bonded to a compound described herein. In embodiments, compound is covalently bonded to the amino acid corresponding to C1101 of SEQ ID NO:331. In embodiments, the compound contacts one or more amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the compound covalently binds an amino acid corresponding to C1101 in SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to E1105 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to C1101 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to E1078 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to S1079 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to A1082 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to I1083 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to K1090 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to Y1091 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to S1094 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to G1097 of SEQ ID NO:331. In embodiments, the compound contacts an amino acids corresponding to H1098 of SEQ ID NO:331. In embodiments, the compound is bonded to a cysteine residue of the reticulon 4 protein. In embodiments, the compound is covalently bonded to a cysteine residue of the reticulon 4 protein. In embodiments, the compound is reversibly covalently bonded to a cysteine residue of the reticulon 4 protein. In embodiments, the compound is irreversibly covalently bonded to a cysteine residue of the reticulon 4 protein. In embodiments, the cysteine residue corresponds to C1101 of SEQ ID NO:331. In an embodiment, the reticulon 4 protein is covalently bonded (e.g., reversibly or irreversibly) to a portion of a compound described herein (e.g., portion of a reticulon 4 inhibitor or portion of a compound described herein). In an aspect is provided a reticulon 4 protein (e.g., human reticulon 4) covalently bonded to a reticulon 4 inhibitor (e.g., reticulon 4 inhibitor, compound described herein, or a portion of a compound described herein). In embodiments, the reticulon 4 protein (e.g., human reticulon 4) is covalently bonded to a reticulon 4 inhibitor (e.g., compound described herein or a portion of a compound described herein). In embodiments, the reticulon 4 protein (e.g., human reticulon 4) is irreversibly covalently bonded to a reticulon 4 inhibitor (e.g., compound described herein or a portion of a compound described herein). In embodiments, the reticulon 4 protein (e.g., human reticulon 4) is reversibly covalently bonded to a reticulon 4 inhibitor (e.g., compound described herein or a portion of a compound described herein). In embodiments, the reticulon 4 protein (e.g., human reticulon 4) is covalently bonded to a portion of a reticulon 4 inhibitor (e.g., compound described herein). In embodiments, the reticulon 4 protein (e.g., human reticulon 4) is irreversibly covalently bonded to a portion of a reticulon 4 inhibitor (e.g., compound described herein). In embodiments, the reticulon 4 protein (e.g., human reticulon 4) is reversibly covalently bonded to a portion of a reticulon 4 inhibitor (e.g., compound described herein). In embodiments, the reticulon 4 inhibitor (e.g., compound described herein) is bonded to a cysteine residue (e.g., Cys1101 of human reticulon 4 or cysteine corresponding to Cys1101 of human reticulon 4) of the reticulon 4 protein (e.g., human reticulon 4). In embodiments, the portion of a reticulon 4 inhibitor (e.g., compound described herein) is bonded to a cysteine residue (e.g., Cys1101 of SEQ ID NO:331 or cysteine corresponding to Cys1101 of SEQ ID NO:331) of the reticulon 4 protein (e.g., human reticulon 4). In embodiments, the RTN4 protein covalently bonded to a RTN4 inhibitor or compound described herein is the product of a reaction between the RTN4 protein and a RTN4 inhibitor or compound described herein. It will be understood that the covalently bonded RTN4 protein and RTN4 inhibitor (e.g., compound described herein) are the remnants of the reactant RTN4 protein and RTN4 inhibitor or compound, wherein each reactant now participates in the covalent bond between the RTN4 protein and RTN4 inhibitor or compound. In embodiments of the covalently bonded RTN4 protein and compound described herein, the remnant of the E substitutent is a linker including a covalent bond between the RTN4 protein and the remainder of the compound described herein. It will be understood by a person of ordinary skill in the art that when a RTN4 protein is covalently bonded to a RTN4 inhibitor (e.g., compound described herein), the RTN4 inhibitor (e.g., compound described herein) forms a remnant of the pre-reacted RTN4 inhibitor (e.g., compound described herein) wherein a bond connects the remnant of the RTN4 inhibitor (e.g., compound described herein) to the remnant of the RTN4 protein (e.g., cysteine sulfur, sulfur of amino acid corresponding to C1101 of human RTN4, sulfur of C1101 of human RTN4). The remnant of the RTN4 inhibitor (compound described herein) may also be called a portion of the RTN4 inhibitor. In embodiments, the remnant of the E substituent is a linker selected from a bond, —S(O)2—, —NH—, —O—, —S—, —C(O)—, —C(O)NH—, —NHC(O)—, —NHC(O)NH—, —NHC(O)NH—, —C(O)O—, —OC(O)—, —CH2NH—, substituted (e.g., substituted with a substituent group, a size-limited substituent group, or lower substituent group) or unsubstituted alkylene (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), substituted (e.g., substituted with a substituent group, a size-limited substituent group, or lower substituent group) or unsubstituted heteroalkylene (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), substituted (e.g., substituted with a substituent group, a size-limited substituent group, or lower substituent group) or unsubstituted cycloalkylene (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), substituted (e.g., substituted with a substituent group, a size-limited substituent group, or lower substituent group) or unsubstituted heterocycloalkylene (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), substituted (e.g., substituted with a substituent group, a size-limited substituent group, or lower substituent group) or unsubstituted arylene (e.g., C6-C10or phenyl), or substituted (e.g., substituted with a substituent group, a size-limited substituent group, or lower substituent group) or unsubstituted heteroarylene (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). As a non-limiting example, the RTN4 protein covalently bonded to a RTN4 inhibitor may have the formula: wherein S is the sulfur of a reticulon 4 protein cysteine (e.g., corresponding to C1101 of human reticulon 4), which is bonded to the remainder of the reticulon 4 protein and wherein R1, R2, L1, L2, z1, and z2 are as described herein. As a non-limiting example, the RTN4 protein covalently bonded to a RTN4 inhibitor may have the formula: wherein S is the sulfur of a reticulon 4 protein cysteine (e.g., corresponding to C1101 of human reticulon 4), which is bonded to the remainder of the reticulon 4 protein and wherein R1, R2, R15, R17, L1, L2, z1, and z2 are as described herein. As a non-limiting example, the RTN4 protein covalently bonded to a RTN4 inhibitor may have the formula: wherein S is the sulfur of a reticulon 4 protein cysteine (e.g., corresponding to C1101 of human reticulon 4), which is bonded to the remainder of the reticulon 4 protein and wherein R1, R2, R16, R17, L1, L2, z1, and z2 are as described herein. A compound having the formula: wherein, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1R1D, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NR1AOR1C, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z1 is an integer from 0 to 5; R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(O)R2C, —C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z2 is an integer from 0 to 4; L1is a bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4—, —NR4C(O)—, —NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene; R4is hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)—OR4A, —C(O)NR4AR4B, —OR4A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR5C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene; R5is hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)—OR5A, —C(O)NR5AR5B, —OR5A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; E is an electrophilic moiety; Each R1A, R1B, R1C, R1D, R2A, R2B, R2C, R2D, R4A, R4B, R5A, and R5Bis independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl;
The compound of embodiment P1 having the formula: The compound of embodiment P1 having the formula: The compound of embodiment P1 having the formula: The compound of embodiment P1 having the formula: The compound of one of embodiments P1 to P5, wherein R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SR1D, —NR1AR1B, —C(O)R1C, —C(O)OR1C, —C(O)NR1AR1B, —OR1D, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. The compound of one of embodiments P1 to P5, wherein R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SH, —NH2, —C(O)OH, —C(O)NH2, —OH, substituted or unsubstituted C1-C8alkyl, or substituted or unsubstituted 2 to 8 membered heteroalkyl; substituted or unsubstituted C3-C8cycloalkyl, substituted or unsubstituted 3 to 8 membered heterocycloalkyl, substituted or unsubstituted C6-C12aryl, or substituted or unsubstituted 5 to 12 membered heteroaryl. The compound of one of embodiments P1 to P5, wherein R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SH, —NH2, —C(O)OH, —C(O)NH2, —OH, substituted or unsubstituted C1-C8alkyl, or substituted or unsubstituted 2 to 8 membered heteroalkyl; substituted or unsubstituted C3-C8cycloalkyl, substituted or unsubstituted 3 to 8 membered heterocycloalkyl, substituted or unsubstituted phenyl, or substituted or unsubstituted 5 to 6 membered heteroaryl. The compound of one of embodiments P1 to P5, wherein R1is independently —Cl. The compound of embodiment P1, wherein two adjacent R1substituents are joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. The compound of embodiment P1, wherein two adjacent R1substituents are joined to form an unsubstituted cycloalkyl. The compound of embodiment P1, wherein two adjacent R1substituents are joined to form an unsubstituted C3-C6cycloalkyl. The compound of one of embodiments P1 to P12, wherein L1is a bond, substituted or unsubstituted C1-C8alkylene, substituted or unsubstituted 2 to 8 membered heteroalkylene, substituted or unsubstituted C3-C8cycloalkylene, substituted or unsubstituted 3 to 8 membered heterocycloalkylene, substituted or unsubstituted phenylene, or substituted or unsubstituted 5 to 6 membered heteroarylene. The compound of one of embodiments P1 to P12, wherein L1is a bond. The compound of one of embodiments P1 to P14, wherein L2is —NR5— or substituted or unsubstituted heterocycloalkylene comprising a ring nitrogen bonded directly to E. The compound of one of embodiments P1 to P14, wherein L2is —NR5—. The compound of embodiment P16, wherein R5is hydrogen, substituted or unsubstituted C1-C6alkyl, or substituted or unsubstituted 2 to 6 membered heteroalkyl. The compound of embodiment P16, wherein R5is hydrogen or unsubstituted C1-C3alkyl. The compound of embodiment P16, wherein R5is hydrogen, unsubstituted methyl, unsubstituted ethyl, unsubstituted hexyl, or unsubstituted benzyl. The compound of embodiment P16, wherein R5is hydrogen. The compound of one of embodiments P1 to P20, wherein E is a covalent cysteine modifier moiety. The compound of one of embodiments P1 to P20, wherein E is: R15is independently hydrogen, halogen, CX153, —CHX152, —CH2X15, —CN, —SOn15R15D, —SOv15NR15AR15B, —NHNR15AR15B, —ONR15AR15B, —NHC═(O)NHNR15AR15B, —NHC(O)NR15AR15B, —N(O)m15, —NR15AR15B, —C(O)R15C, —C(O)—OR15C, —C(O)NR15AR15B, —OR15D, —NR15ASO2R15D, —NR15AC(O)R15C, —NR15AC(O)OR15C, —NR15AOR15C, —OCX153, —OCHX152, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl; R16is independently hydrogen, halogen, CX163, —CHX162, — CH2X16, —CN, —SOn16R16D, —SOv16NR16AR16B, —NHNR16AR16B, —ONR16AR16B, —NHC═(O)NHNR16AR16B,
R17is independently hydrogen, halogen, CX173, —CHX172, —CH2X17, —CN, —SOn17R17D, —SOv17NR17AR17B, —NHNR17AR17B, —ONR17AR17B, —NHC═(O)NHNR17AR17B,
R18is independently hydrogen, —CX183, —CHX182, —CH2X18, —C(O)R18C, —C(O)OR18C, —C(O)NR18AR18B, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl; R15A, R15B, R15C, R15D, R16A, R16B, R16C, R16D, R17A, R17B, R17C, R17D, R18A, R18B, R18C, and R18D, are independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R15Aand R15Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R16Aand R16Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R17Aand R17Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R18Aand R18Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X15, X16, X17and X18is independently —F, —Cl, —Br, or —I; n15, n16, n17, v15, v16, and v17, are independently an integer from 0 to 4; and m15, m16, and m17 are independently and integer from 1 to 2. The compound of embodiment P22, wherein R15, R16, R17, and R18are hydrogen. The compound of one of embodiments P22 to P23, wherein E is: A pharmaceutical composition comprising a Reticulon 4 inhibitor and a pharmaceutically acceptable excipient. A pharmaceutical composition comprising the compound of any one of embodiments P1 to P24 and a pharmaceutically acceptable excipient. A method of inhibiting reticulon 4 protein activity, said method comprising contacting the reticulon 4 protein with a Reticulon 4 inhibitor. The method of embodiment P27, wherein the Reticulon 4 inhibitor is an siRNA, antibody, or compound. The method of embodiment P30, wherein the Reticulon 4 inhibitor contacts one or more amino acids corresponding to E1105, C1101, E1078, 51079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of human reticulon 4. A method of inhibiting reticulon 4 protein activity, said method comprising contacting the reticulon 4 protein with an effective amount of a compound of one of embodiments P1 to P24. The method of embodiment P30, wherein the compound is covalently bonded to the amino acid corresponding to C1101 of human reticulon 4. The method of embodiment P30, wherein the compound contacts one or more amino acids corresponding to E1105, C1101, E1078, 51079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of human reticulon 4. A method of treating cancer, said method comprising administering to a subject in need thereof an effective amount of a Reticulon 4 inhibitor. A method of treating cancer, said method comprising administering to a subject in need thereof an effective amount of a compound of one of embodiments P1 to P24. The method of one of embodiments P33 to P34, wherein the cancer is colorectal cancer. A reticulon 4 protein covalently bonded to a compound of one of embodiments P1 to P24. The Reticulon 4 protein of embodiment P36, wherein the compound is bonded to a cysteine residue of the protein. The reticulon 4 protein of embodiment P36, covalently bonded to a portion of a compound of one of embodiments 1 to 24. The reticulon 4 protein of embodiment P36, irreversibly covalently bonded to a portion of a compound of one of embodiments 1 to 24. The reticulon 4 protein of one of embodiments P36 to P39, wherein the compound or portion of the compound is covalently bonded to an amino acid corresponding to C1101 of human reticulon 4. A method of treating cancer, said method comprising administering to a subject in need thereof an effective amount of a compound having the formula: wherein, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NR1AOR1C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z1 is an integer from 0 to 5; R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(OR2C—C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl;
L1is a bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4—, —NR4C(O)—, —NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene; R4is hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)—OR4A, —C(O)NR4AR4B, —OR4A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR5C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene; R5is hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)—OR5A, —C(O)NR5AR5B, —OR5A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; E is an electrophilic moiety; Each R1A, R1B, R1C, R1D, R2A, R2B, R2C, R2D, R4A, R4B, R5A, and R5Bis independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X1, X2, X4, and X5is independently —F, —Cl, —Br, or —I; n1, n2, n4, and n5 are independently an integer from 0 to 4; and m1, m2, m4, m5, v1, v2, v4, and v5 are independently an integer from 1 to 2. The method of embodiment 1, wherein the compound has the formula: The method of embodiment 1, wherein the compound has the formula: The method of embodiment 1, wherein the compound has the formula: The method of embodiment 1, wherein the compound has the formula: The method of one of embodiments 1 to 5, wherein R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SR1D, —NR1AR1B, —C(O)R1C, —C(O)OR1C, —C(O)NR1AR1B, —OR1D, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. The method of one of embodiments 1 to 5, wherein R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SH, —NH2, —C(O)OH, —C(O)NH2, —OH, substituted or unsubstituted C1-C8alkyl, or substituted or unsubstituted 2 to 8 membered heteroalkyl; substituted or unsubstituted C3-C8cycloalkyl, substituted or unsubstituted 3 to 8 membered heterocycloalkyl, substituted or unsubstituted C6-C12aryl, or substituted or unsubstituted 5 to 12 membered heteroaryl. The method of one of embodiments 1 to 5, wherein R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SH, —NH2, —C(O)OH, —C(O)NH2, —OH, substituted or unsubstituted C1-C8alkyl, or substituted or unsubstituted 2 to 8 membered heteroalkyl; substituted or unsubstituted C3-C8cycloalkyl, substituted or unsubstituted 3 to 8 membered heterocycloalkyl, substituted or unsubstituted phenyl, or substituted or unsubstituted 5 to 6 membered heteroaryl. The method of one of embodiments 1 to 5, wherein R1is independently —Cl. The method of embodiment 1, wherein two adjacent R1substituents are joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. The method of embodiment 1, wherein two adjacent R1substituents are joined to form an unsubstituted cycloalkyl. The method of embodiment 1, wherein two adjacent R1substituents are joined to form an unsubstituted C3-C6cycloalkyl. The method of one of embodiments 1 to 12, wherein L1is a bond, substituted or unsubstituted C1-C8alkylene, substituted or unsubstituted 2 to 8 membered heteroalkylene, substituted or unsubstituted C3-C8cycloalkylene, substituted or unsubstituted 3 to 8 membered heterocycloalkylene, substituted or unsubstituted phenylene, or substituted or unsubstituted 5 to 6 membered heteroarylene. The method of one of embodiments 1 to 12, wherein L1is a bond. The method of one of embodiments 1 to 14, wherein L2is —NR5— or substituted or unsubstituted heterocycloalkylene comprising a ring nitrogen bonded directly to E. The method of one of embodiments 1 to 14, wherein L2is —NR5—. The method of embodiment 16, wherein R5is hydrogen, substituted or unsubstituted C1-C6alkyl, or substituted or unsubstituted 2 to 6 membered heteroalkyl. The method of embodiment 16, wherein R5is hydrogen or unsubstituted C1-C3alkyl. The method of embodiment 16, wherein R5is hydrogen, unsubstituted methyl, unsubstituted ethyl, unsubstituted hexyl, or unsubstituted benzyl. The method of embodiment 16, wherein R5is hydrogen. The method of one of embodiments 1 to 20, wherein E is a covalent cysteine modifier moiety. The method of one of embodiments 1 to 20, wherein E is: R15is independently hydrogen, halogen, CX153, —CHX152, —CH2X15, —CN, —SOn15R15D, —SOv15NR15AR15B, —NHNR15AR15B, —ONR15AR15B, —NHC═(O)NHNR15AR15B, —NHC(O)NR15AR15B, —N(O)m15, —NR15AR15B, —C(O)R15C, —C(O)—OR15C, —C(O)NR15AR15B, —OR15D, —NR15ASO2R15D, —NR15AC(O)R15C, —NR15AC(O)OR15C, —NR15AOR15C, —OCX153, —OCHX152, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl; R16is independently hydrogen, halogen, CX163, —CHX162, —CH2X16, —CN, —SOn16R16D, —SOv16NR16AR16B, —NHNR16AR16B, θNR16AR16B, —NHC═(O)NHNR16AR16B,
R17is independently hydrogen, halogen, CX173, —CHX172, —CH2X17, —CN, —SOn17R17D, —SOv17NR17AR17B, —NHNR17AR17B, —ONR17AR17B, —NHC═(O)NHNR17AR17B,
R18is independently hydrogen, —CX183, —CHX182, — CH2X18, —C(O)R18C, —C(O)OR18C, —C(O)NR18AR18B, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl; R15A, R15B, R15C, R15D, R16A, R16B, R16C, R16D, R17A, R17B, R17C, R17D, R18A, R18B, R18C, and R18D, are independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R15Aand R15Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R16Aand R16Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R17Aand R17Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R18Aand R18Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X15, X16, X17and X18is independently —F, —Cl, —Br, or —I; n15, n16, n17, v15, v16, and v17, are independently an integer from 0 to 4; and m15, m16, and m17 are independently and integer from 1 to 2. The method of embodiment 22, wherein R15, R16, R17, and R18are hydrogen. The method of one of embodiments 22 to 23, wherein E is: The method of embodiment 1, having the formula: The method of one of embodiments 1 to 25, wherein the cancer is colorectal cancer. The use of a compound for the preparation of a medicament for the treatment of cancer, wherein the compound has the formula: wherein, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NR1AOR1C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl;
R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, — OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(OR2C—C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl;
R4is hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)—OR4A, —C(O)NR4AR4B, —OR4A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR5C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene; R5is hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)—OR5A, —C(O)NR5AR5B, —OR5A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; E is an electrophilic moiety; Each R1A, R1B, R1C, R1D, R2A, R2B, R2C, R2D, R4A, R4B, R5A, and R5Bis independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X1, X2, X4, and X5is independently —F, —Cl, —Br, or —I; n1, n2, n4, and n5 are independently an integer from 0 to 4; and m1, m2, m4, m5, v1, v2, v4, and v5 are independently an integer from 1 to 2. The compound of embodiment 27, wherein the compound has the formula: The compound of embodiment 27, wherein the compound has the formula: The compound of embodiment 27, wherein the compound has the formula: The compound of embodiment 27, wherein the compound has the formula: The compound of embodiment 27, wherein the compound has the formula: The compound of one of embodiments 27 to 32, wherein R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SR1D, —NR1AR1B, —C(O)R1C, —C(O)OR1C, —C(O)NR1AR1B, —OR1D, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. The compound of one of embodiments 27 to 32, wherein R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SH, —NH2, —C(O)OH, —C(O)NH2, —OH, substituted or unsubstituted C1-C8alkyl, or substituted or unsubstituted 2 to 8 membered heteroalkyl; substituted or unsubstituted C3-C8cycloalkyl, substituted or unsubstituted 3 to 8 membered heterocycloalkyl, substituted or unsubstituted C6-C12aryl, or substituted or unsubstituted 5 to 12 membered heteroaryl. The compound of one of embodiments 27 to 32, wherein R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SH, —NH2, —C(O)OH, —C(O)NH2, —OH, substituted or unsubstituted C1-C8alkyl, or substituted or unsubstituted 2 to 8 membered heteroalkyl; substituted or unsubstituted C3-C8cycloalkyl, substituted or unsubstituted 3 to 8 membered heterocycloalkyl, substituted or unsubstituted phenyl, or substituted or unsubstituted 5 to 6 membered heteroaryl. The compound of one of embodiments 27 to 32, wherein R1is independently —Cl. The compound of embodiment 27, wherein two adjacent R1substituents are joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. The compound of embodiment 27, wherein two adjacent R1substituents are joined to form an unsubstituted cycloalkyl. The compound of embodiment 27, wherein two adjacent R1substituents are joined to form an unsubstituted C3-C6cycloalkyl. The compound of one of embodiments 27 to 39, wherein L1is a bond, substituted or unsubstituted C1-C8alkylene, substituted or unsubstituted 2 to 8 membered heteroalkylene, substituted or unsubstituted C3-C8cycloalkylene, substituted or unsubstituted 3 to 8 membered heterocycloalkylene, substituted or unsubstituted phenylene, or substituted or unsubstituted 5 to 6 membered heteroarylene. The compound of one of embodiments 27 to 39, wherein L1is a bond. The compound of one of embodiments 27 to 41, wherein L2is —NR5— or substituted or unsubstituted heterocycloalkylene comprising a ring nitrogen bonded directly to E. The compound of one of embodiments 27 to 41, wherein L2is —NR5—. The compound of embodiment 43, wherein R5is hydrogen, substituted or unsubstituted C1-C6alkyl, or substituted or unsubstituted 2 to 6 membered heteroalkyl. The compound of embodiment 43, wherein R5is hydrogen or unsubstituted C1-C3alkyl. The compound of embodiment 43, wherein R5is hydrogen, unsubstituted methyl, unsubstituted ethyl, unsubstituted hexyl, or unsubstituted benzyl. The compound of embodiment 43, wherein R5is hydrogen. The compound of one of embodiments 27 to 47, wherein E is a covalent cysteine modifier moiety. The compound of one of embodiments 27 to 47, wherein E is: R15is independently hydrogen, halogen, CX153, —CHX152, —CH2X15, —CN, —SOn15R15D, —SOv15NR15AR15B, —NHNR15AR15B, —ONR15AR15B, —NHC═(O)NHNR15AR15B,
R16is independently hydrogen, halogen, CX163, —CHX162, — CH2X16, —CN, —SOn16R16D, —SOv16NR16AR16B, —NHNR16AR16B, —ONR16AR16B,
R17is independently hydrogen, halogen, CX173, —CHX172, —CH2X17, —CN, —SOn17R17D, —SOv17NR17AR17B, —NHNR17AR17B, —ONR17AR17B,
R18is independently hydrogen, —CX183, —CHX182, —CH2X18, —C(O)R18C, —C(O)OR18C, —C(O)NR18AR18B, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl; R15A, R15B, R15C, R15D, R16A, R16B, R16C, R16D, R17A, R17B, R17C, R17D, R18A, R18B, R18C, and R18D, are independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R15Aand R15Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R16Aand R16Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R17Aand R17Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R18Aand R18Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X15, X16, X17and X18is independently —F, —Cl, —Br, or —I; n15, n16, n17, v15, v16, and v17, are independently an integer from 0 to 4; and m15, m16, and m17 are independently and integer from 1 to 2. The compound of embodiment 49, wherein R15, R16, R17, and R18are hydrogen. The compound of one of embodiments 49 to 50, wherein E is: The compound of embodiment 27, having the formula: A pharmaceutical composition comprising a Reticulon 4 inhibitor and a pharmaceutically acceptable excipient. The pharmaceutical composition of embodiment 53, wherein the Reticulon 4 inhibitor is the compound has the formula: wherein, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1R1D, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C(O)OR1C, —NR1AOR1C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z1 is an integer from 0 to 5; R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(O)R2C, —C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z2 is an integer from 0 to 4; L1is a bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4, —, —NR4(O)—, —NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, or substituted or unsubstituted heteroarylene; R4is hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)OR4A, —C(O)NR4AR4B, —OR4A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR5C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, or substituted or unsubstituted heteroarylene; R5is hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)—OR5A, —C(O)NR5AR5B, —OR5A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; E is an electrophilic moiety; Each R1A, R1B, R1C, R1D, R2A, R2B, R2C, R2D, R4A, R4B, R5A, and R5Bis independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X1, X2, X4, and X5is independently —F, —Cl, —Br, or —I; n1, n2, n4, and n5 are independently an integer from 0 to 4; and m1, m2, m4, m5, v1, v2, v4, and v5 are independently an integer from 1 to 2. A method of inhibiting reticulon 4 protein activity, said method comprising contacting a reticulon 4 protein with an effective amount of a Reticulon 4 inhibitor, wherein said Reticulon 4 inhibitor contacts one or more amino acids corresponding to E1105, C1101, E1078, S1079, A1082, I1083, K1090, Y1091, S1094, G1097, and H1098 of SEQ ID NO:331. The method of embodiment 55, wherein the Reticulon 4 inhibitor is an antisense nucleic acid, antibody, or a compound. The method of embodiment 55 or 56, wherein said Reticulon 4 inhibitor is a compound having the formula: wherein, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1R1D, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NRAOR1C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z1 is an integer from 0 to 5; R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(O)R2C, —C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z2 is an integer from 0 to 4; L1is a bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4—, —NR4C(O)—, —NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene; R4is hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)—, —OR4A, —C(O)NR4AR4B, —OR4A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR5C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene; R5is hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)—OR5A, —C(O)NR5AR5B, —OR5A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; E is an electrophilic moiety; Each R1A, R1B, R1C, R1D, R2A, R2B, R2C, R2D, R4A, R4B, R5A, and R5Bis independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X1, X2, X4, and X5is independently —F, —Cl, —Br, or —I; n1, n2, n4, and n5 are independently an integer from 0 to 4; and m1, m2, m4, m5, v1, v2, v4, and v5 are independently an integer from 1 to 2. The method of embodiment 57, wherein the compound is covalently bonded to the amino acid corresponding to C1101 of SEQ ID NO:331. A reticulon 4 protein covalently bonded to a compound having the formula: wherein, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1R1D, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NR1AOR1C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z1 is an integer from 0 to 5; R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(O)R2C, —C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z2 is an integer from 0 to 4; L1is a bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4—, —NR4C(O)—, —NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene; R4is hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)—OR4A, —C(O)NR4AR4B, —OR4A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR5C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene; R5is hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)—OR5A, —C(O)NR5AR5B, —OR5A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; E is an electrophilic moiety; Each R1A, R1B, R1C, R1D, R2A, R2B, R2C, R2D, R4A, R4B, R5A, and R5Bis independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X1, X2, X4, and X5is independently —F, —Cl, —Br, or —I; n1, n2, n4, and n5 are independently an integer from 0 to 4; and m1, m2, m4, m5, v1, v2, v4, and v5 are independently an integer from 1 to 2; wherein the reticulon 4 protein is covalently bonded to said compound through said reacted electrophilic moiety. The Reticulon 4 protein of embodiment 59, wherein the compound is bonded to a cysteine residue of the protein. The reticulon 4 protein of one of embodiments 59 to 60, wherein the compound is covalently bonded to an amino acid corresponding to C1101 of SEQ ID NO:331. A compound having the formula: wherein, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1NR1AR1B, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NR1AOR1C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z1 is an integer from 0 to 5; R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(O)R2C, —C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z2 is an integer from 0 to 4; L1is a bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4—, —NR4C(O), —NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene; R4is hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)—OR4A, —C(O)NR4AR4B, —OR4A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR5C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene; R5is hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)—OR5A, —C(O)NR5AR5B, —OR5A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; E is an electrophilic moiety; Each R1A, R1B, R1C, R1D, R2A, R2B, R2C, R2D, R4A, R4B, R5A, and R5Bis independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X1, X2, X4, and X5is independently —F, —Cl, —Br, or —I; n1, n2, n4, and n5 are independently an integer from 0 to 4; and m1, m2, m4, m5, v1, v2, v4, and v5 are independently an integer from 1 to 2. The compound of embodiment 62, wherein the compound has the formula: The compound of embodiment 1, wherein the compound has the formula: The compound of embodiment 1, wherein the compound has the formula: The compound of embodiment 1, wherein the compound has the formula: The compound of one of embodiments 62 to 66, wherein R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SR1D, —NR1AR1B, —C(O)R1C, —C(O)OR1C, —C(O)NR1AR1B, —OR1D, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. The compound of one of embodiments 62 to 66, wherein R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SH, —NH2, —C(O)OH, —C(O)NH2, —OH, substituted or unsubstituted C1-C8alkyl, or substituted or unsubstituted 2 to 8 membered heteroalkyl; substituted or unsubstituted C3-C8cycloalkyl, substituted or unsubstituted 3 to 8 membered heterocycloalkyl, substituted or unsubstituted C6-C12aryl, or substituted or unsubstituted 5 to 12 membered heteroaryl. The compound of one of embodiments 62 to 66, wherein R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SH, —NH2, —C(O)OH, —C(O)NH2, —OH, substituted or unsubstituted C1-C8alkyl, or substituted or unsubstituted 2 to 8 membered heteroalkyl; substituted or unsubstituted C3-C8cycloalkyl, substituted or unsubstituted 3 to 8 membered heterocycloalkyl, substituted or unsubstituted phenyl, or substituted or unsubstituted 5 to 6 membered heteroaryl. The compound of one of embodiments 62 to 66, wherein R1is independently —Cl. The compound of embodiment 62, wherein two adjacent R1substituents are joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl. The compound of embodiment 62, wherein two adjacent R1substituents are joined to form an unsubstituted cycloalkyl. The compound of embodiment 62, wherein two adjacent R1substituents are joined to form an unsubstituted C3-C6cycloalkyl. The compound of one of embodiments 62 to 73, wherein L1is a bond, substituted or unsubstituted C1-C8alkylene, substituted or unsubstituted 2 to 8 membered heteroalkylene, substituted or unsubstituted C3-C8cycloalkylene, substituted or unsubstituted 3 to 8 membered heterocycloalkylene, substituted or unsubstituted phenylene, or substituted or unsubstituted 5 to 6 membered heteroarylene. The compound of one of embodiments 62 to 73, wherein L1is a bond. The compound of one of embodiments 62 to 75, wherein L2is —NR5— or substituted or unsubstituted heterocycloalkylene comprising a ring nitrogen bonded directly to E. The compound of one of embodiments 62 to 75, wherein L2is —NR5—. The compound of embodiment 77, wherein R5is hydrogen, substituted or unsubstituted C1-C6alkyl, or substituted or unsubstituted 2 to 6 membered heteroalkyl. The compound of embodiment 77, wherein R5is hydrogen or unsubstituted C1-C3alkyl. The compound of embodiment 77, wherein R5is hydrogen, unsubstituted methyl, unsubstituted ethyl, unsubstituted hexyl, or unsubstituted benzyl. The compound of embodiment 77, wherein R5is hydrogen. The compound of one of embodiments 62 to 81, wherein E is a covalent cysteine modifier moiety. The compound of one of embodiments 62 to 81, wherein E is: R15is independently hydrogen, halogen, CX153, —CHX152, —CH2X15, —CN, —SOn15R15D, —SOv15NR15AR15B, —NHNR15AR15B, —ONR15AR15B, —NHC═(O)NHNR15AR15B,
R16is independently hydrogen, halogen, CX163, —CHX162, —CH2X16, —CN, —SOn16R16D, —SOv16NR16AR16B, —NHNR16AR16B, —ONR16AR16B, —NHC═(O)NHNR16AR16B,
R17is independently hydrogen, halogen, CX173, —CHX172, —CH2X17, —CN, —SOn17R17D, —SOv17NR17AR17B, —NHNR17AR17B, —ONR17AR17B, —NHC═(O)NHNR17AR17B,
R18is independently hydrogen, —CX183, —CHX182, —CH2X18, —C(O)R18C, —C(O)OR18C, —C(O)NR18AR18B, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl; R15A, R15B, R15C, R15D, R16A, R16B, R16C, R16D, R17A, R17B, R17C, R17D, R18A, R18B, R18C, and R18D, are independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R15Aand R15Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R16Aand R16Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R17Aand R17Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R18Aand R18Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X15, X16, X17and X18is independently —F, —Cl, —Br, or —I; n15, n16, n17, v15, v16, and v17, are independently an integer from 0 to 4; and m15, m16, and m17 are independently and integer from 1 to 2. The compound of embodiment 83, wherein R15, R16, R17, and R18are hydrogen. The compound of one of embodiments 82 to 83, wherein E is: The compound of embodiment 62, having the formula: Chemical genetics has arisen as a powerful approach for identifying novel anti-cancer agents. However, a major bottleneck in chemical genetics is identifying the targets of leads that arise from screens. Here, we generated and screened a library of cysteine-reactive fragment-based covalent ligands for agents that impair colorectal cancer pathogenicity and coupled the discovery of lead compounds with target identification using isotopic tandem orthogonal proteolysis-enabled activity-based protein profiling (isoTOP-ABPP) platforms. Through this coupled approach, we discovered a cysteine-reactive acrylamide DKM 3-30 that impaired colorectal cancer cell pathogenicity through targeting C1101 on reticulon 4 (RTN4). This protein has been established as a critical mediator of endoplasmic reticulum tubular network formation. We show here that covalent modification of C1101 on RTN4 by DKM 3-30 or genetic knockdown of RTN4 impairs endoplasmic reticulum and nuclear envelope morphology and colorectal cancer pathogenicity. RTN4 is a novel colorectal cancer therapeutic target and we determined a unique druggable hotspot within RTN4 that can be targeted by covalent ligands to impair colorectal cancer pathogenicity. Our results underscore the utility of coupling the screening of fragment-based covalent ligands with isoTOP-ABPP platforms for mining the proteome for novel druggable nodes that can be targeted for cancer therapy. Traditional strategies for cancer target discovery oftentimes involve searching for proteins or genes that may be dysregulated or mutated in tumors, which may miss promising therapeutic targets that may not necessarily be changing in expression or activity. Screening chemical libraries for anti-cancer small-molecules using chemical genetics strategies have arisen as a powerful complementary approach to traditional target discovery approaches for mining druggable nodes that can be pharmacologically interrogated in cancer3,4. However, a major challenge with chemical genetics is identifying the targets of leads that arise from screens. Oftentimes, lead compounds must be derivatized to either bear bioorthogonal and/or photoaffinity handles or conjugated to beads to facilitate chemoproteomic target identification4. However, these approaches oftentimes require additional synthetic efforts to make analogs of the lead molecule and alter the structure of the molecule, which hinder or may prevent target identification. Here, we have generated a library of 75 cysteine-reactive fragment-based covalent ligands and coupled the screening of this library with an isotopic tandem orthogonal proteolysis-enabled activity-based protein profiling (isoTOP-ABPP) platform to rapidly couple the identification of covalent ligands that impair colorectal cancer pathogenicity with the identification of its direct targets and druggable hotspots within these targets ( There are several advantages to this overall approach. First, our library already introduces specific covalent interactions through the incorporation of cysteine-reactive acrylamide and chloroacetamide warheads, thus avoiding the necessity for introducing photoaffinity handles for target identification. Recent studies have shown that the reactivity of these scaffolds can be tempered and made to confer substantial selectivity through appending small-molecular weight fragments6. Also, because these compounds are small molecular weight fragment-based covalent ligands, they can sample more macromolecular protein space and enable interrogation of more druggable nodes, a notion explored by many pharmaceutical companies with fragment-based ligand discovery8. Second, the advantage of this approach is that the lead molecule itself can be directly competed against reactivity-based probes for target identification without the need for additional derivatization or synthetic efforts. We screened our cysteine-reactive ligand library of acrylamides and chloroacetamides to identify compounds that impair colorectal cancer cell survival and proliferation in the highly metastatic and tumorigenic SW620 colorectal cancer cells ( We next performed isoTOP-ABPP studies to identify the direct targets of these lead compounds. We competed either vehicle or DKM 3-30 against labeling of SW620 proteomes with a broad cysteine-reactive probe, iodoacetamide-alkyne (IAyne), followed by appending probe-labeled proteins with a biotin-azide tag bearing a TEV protease recognition site and an isotopically light (for vehicle-treated) or heavy (for fragment-treated) tags via copper catalyzed azide-alkyne cycloaddition (CuAAC)6,9. We then combined control and treated proteomes in a 1:1 ratio, enriched probe-labeled proteins with avidin, and digested proteomes with trypsin. Avidin-enriched probe-modified tryptic peptides were released by TEV protease digestion for subsequent quantitative proteomic analysis. Through these studies, we identified the top hit for DKM 3-30 as C1101 in reticulon 4 (RTN4, Uniprot ID Q9NQC3-1) with a light to heavy ratio of 3.0 ( To determine the relevance of RTN4 in colorectal cancer, we performed isoTOP-ABPP analysis to quantitatively map proteome-wide reactivity of cysteines in pooled primary human colorectal tumors through comparative ratiometric analysis of IAyne labeling at 100 (heavy) versus 10 μM (light) concentrations. Previous studies by Weerapana et al. have shown that hyper-reactive cysteines, which show saturated IAyne labeling at lower concentrations and thus exhibit a lower (<3) heavy to light ratio, are highly enriched in functional cysteines, compared to those sites that are not hyper-reactive that show heavy to light ratios of ˜1010. We identify RTN4 labeling of C1101 in primary human colorectal tumors. RTN4 C1101 shows a ratio of 6.2 indicating that this cysteine is not hyper-reactive ( We further confirm the relevance of RTN4 in colorectal cancer by showing that transient or stable knockdown of RTN4 by RNA interference phenocopies the impaired survival, proliferation, and anti-tumorigenic effects observed with DKM 3-30 in SW620 colorectal cancer cells ( RTN4 is known to be a critical mediator of endoplasmic reticulum (ER) tubule formation11-13. Interestingly, Voeltz et al. found that tubular ER network formation in a reconstituted in vitro system was disrupted by thiol modifying agents and discovered that We postulated that covalent modification of RTN4(C1101) by DKM 3-30 would impact the formation of ER tubular networks in cells. We attempted to analyze the effects of DKM 3-30 in SW620 colorectal cancer cells, and while the images suggest alterations in the ER morphology ( Cell division requires elaborate rearrangements in the ER and the nuclear envelope to ensure correct inheritance of DNA and segregation of DNA within a single nucleus15. During prophase the nuclear envelope retracts into the ER and then reforms during telophase. The reticulon family of proteins, and the transition between ER tubules and sheets, have been implicated in nuclear envelope assembly and disassembly during mitosis16-18Time-lapse imaging of mitotic cells revealed that control cells divided rapidly (˜50-60 min) ( We also synthesized analogs of DKM 3-30 and showed that YP 1-46 demonstrated less displacement of IAyne labelling of RTN4, whereas AMR 1-125 exhibited ˜7-fold improved potency compared to DKM 3-30. We further showed that AMR 1-125, but not YP 1-46, impaired cell survival in U2OS and SW620 cells and ER morphology in U2OS cells ( In summary, we identify RTN4 as a novel colorectal cancer therapeutic target, and reveal a unique druggable hotspot within this classically undruggable protein, which can be targeted by cysteine-reactive ligands such as DKM 3-30 to impair ER and nuclear envelope morphology and colorectal cancer pathogenicity. We also show that DKM 3-30 impairs osteosarcoma cell survival as well, suggesting that RTN4 may have broader impacts upon other types of cancers. We recognize that DKM 3-30 may have additional off-targets that may contribute its anti-cancer activity, but nonetheless show compelling evidence that DKM 3-30 and its analogs phenocopy what is observed with RTN4 knockdown and that DKM 3-30 confers sensitivity in MEF cells only when expressing human RTN4. DKM 3-30 and AMR 1-125 may serve as initial starting points for subsequent medicinal chemistry to develop a more potent and selective RTN4 inhibitors for cancer therapy. Overall, we highlight the utility of coupling the screening of covalent ligand libraries with isoTOP-ABPP for mining the proteome for novel druggable nodes that can be targeted for cancer therapy. Methods. Materials. IAyne was obtained from CHESS Gmbh. Heavy and light TEV-biotin tags were synthesized per previously described methods 5,21 Synthesis of Cysteine Fragment Library. The synthesis of the cysteine-reactive ligand library is described below. All compounds in the library were confirmed to be >95% pure. Cell Culture. SW620 cells were purchased from ATCC. SW620 cells were grown in L-15 media with 10% fetal bovine serum (FBS) in ambient CO2. U2OS cells were grown in DMEM media supplemented with 10% FBS at 37° C. with 5% CO2. Survival and Proliferation Assays. Cells were plated the evening before the experiment, and allowed to adhere overnight. For serum-free cell survival assays, cells were plated in media not containing FBS. For cell proliferation assays, cells were plated in regular media. For the chemical genetics screen, cells were treated with either DMSO or the cysteine-reactive fragment for 48 h and cell viability was assessed by Hoescht stain using our previously described methods Tumor Xenograft Growth Studies. C.B17 SCID male mice (6-8 weeks old) were injected subcutaneously into the flank with 2,000,000 cells in serum-free media. For pharmacological treatments, mice were exposed by intraperitoneal (ip) injection with either vehicle (18:1:1 PBS/ethanol/PEG40) or 50 mg/kg DKM 3-30 once per day starting ten days after the initiation of the xenograft experiment and until the completion of the study. Tumors were measured every 7 days by caliper measurements. Animal experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of California, Berkeley. IsoTOP-ABPP Analysis. IsoTOP-ABPP analyses were performed as previously described5-7. For competitive IsoTOP-ABPP, SW620 cell lysates were pre-incubated with DMSO vehicle or DKM 3-30 (50 μM) for 30 min at 37° C. in phosphate-buffered saline (PBS), and then labeled with IAyne (100 μM) for 1 h at room temperature. They were subsequently treated with isotopically light (control) or heavy (treated) TEV-biotin (100 μM) and CuAAC was performed as previously described5,6. For analysis of cysteine reactivity in primary colorectal tumor tissue, tumors were pooled and incubated with either 100 μM IAyne and isotopically heavy TEV-biotin or 10 μM IAyne and isotopically light TEV-biotin followed by CuAAC. Proteins were precipitated over one hour and pelleted by centrifugation at 6500×g. Proteins were washed 3 times with cold methanol then denatured and resolubilized by heating in 1.2% SDS/PBS to 85° C. for 5 min. Insoluble components were precipitated by centrifugation at 6500×g and soluble proteome was diluted in 5 ml PBS, for a final concentration of 0.2% SDS. Labeled proteins were bound to avidin-agarose beads (170 μL resuspended beads/sample, Thermo Pierce) while rotating overnight at 4° C. Bead-linked proteins were enriched by washing three times each in PBS and water, then resuspended in 6 M urea/PBS (Sigma-Aldrich) and reduced in dithiothreitol (1 mM, Sigma-Aldrich), alkylated with iodoacetamide (18 mM, Sigma-Aldrich), then washed and resuspended in 2 M urea/PBS with 1 mM calcium chloride and trypsinized overnight with 0.5 μg/μ1 sequencing grade trypsin (Promega). Tryptic peptides were discarded and beads were washed three times each in PBS and water, then washed with one wash of TEV buffer containing 1 μM DTT. TEV-biotin tag was digested overnight in TEV buffer containing 1 μM DTT and 5 μL Ac-TEV protease at 29° C. Peptides were diluted in water and acidified with final concentration of 5% formic acid (1.2 M, Spectrum). Peptides from all proteomic experiments were pressure-loaded onto a 250 mm inner diameter fused silica capillary tubing packed with 4 cm of Aqua C18 reverse-phase resin (Phenomenex #04A-4299) which was previously equilibrated on an Agilent 600 series HPLC using gradient from 100% buffer A to 100% buffer B over 10 min, followed by a 5 min wash with 100% buffer B and a 5 min wash with 100% buffer A. The samples were then attached using a MicroTee PEEK 360 μm fitting (Thermo Fisher Scientific # p-888) to a 13 cm laser pulled column packed with 10 cm Aqua C18 reverse-phase resin and 3 cm of strong-cation exchange resin for isoTOP-ABPP studies. Samples were analyzed using an Q Exactive Plus mass spectrometer (Thermo Fisher Scientific) using a 5-step Multidimensional Protein Identification Technology (MudPIT) program, using 0%, 25%, 50%, 80%, and 100% salt bumps of 500 mM aqueous ammonium acetate and using a gradient of 5-55% buffer B in buffer A (buffer A: 95:5 water:acetonitrile, 0.1% formic acid; buffer B 80:20 acetonitrile:water, 0.1% formic acid). Data was collected in data-dependent acquisition mode with dynamic exclusion enabled (60 s). One full MS (MS1) scan (400-1800 m/z) was followed by 15 MS2 scans (ITMS) of the nth most abundant ions. Heated capillary temperature was set to 200° C. and the nanospray voltage was set to 2.75 kV. Data were extracted in the form of MS1 and MS2 files using Raw Extractor 1.9.9.2 (Scripps Research Institute) and searched against the Uniprot mouse database using ProLuCID search methodology in IP2 v.3 (Integrated Proteomics Applications, Inc)23. Cysteine residues were searched with a static modification for carboxyaminomethylation (+57.02146) and up to two differential modifications for methionine oxidation and either the light or heavy TEV tags (+464.28596 or +470.29977, respectively). Peptides were required to have at least one tryptic end and to contain the TEV modification. ProLUCID data was filtered through DTASelect to achieve a peptide false-positive rate below 1%. Gel-Based ABPP. Gel-based ABPP methods were performed as previously described24. Recombinant RTN4 (0.06 μg) protein (RTN4-Fisher Scientific) were pre-treated with DMSO or DKM 3-30, respectively, for 1 h at 37° C. in an incubation volume of 50 μL PBS, and were subsequently treated with IAyne (10 μM final concentration) for 30 min at 37° C. CuAAC was performed to append rhodamine-azide onto IAyne probe-labeled proteins. The samples were separated by SDS/PAGE and scanned using a ChemiDoc MP (Bio-Rad Laboratories, Inc). Inhibition of target labeling was assessed by densitometry using ImageStudio Light software. RTN4 Knockdown. Targets were knocked down transiently with siRNA or stably with shRNA as previously described22,25. For siRNA studies, SW620 cells (200,000 cells/well) were seeded overnight after which siControl (non-targeting siRNA) or siRTN4 oligonucleotides (5 pooled siRNAs targeting each target purchased from Dharmacon) were transfected into cells using Dharmafect 1. Cells were harvested after 48 h for qPCR and for seeding for cell viability assays. For shRNA studies, shControl (targeting GFP) or shRTN4 constructs (purchased from Sigma) were transfected into HEK293T cells alongside lentiviral vectors using FuGENE. Lentivirus was collected from filtered cultured medium 48 h post-transfection and used to infect the target cancer cell line with Polybrene (0.01 mg/ml) Target cells were selected over 3 days with 1 mg/ml puromycin. The short hairpin sequences for the generation of RTN4 knockdown lines were: CCGGGCAGTGTTGATGTGGGTATTTCTCGAGAAATACCCACATCAACACTGCTTTTT TG (SEQ ID NO:328) and CCGGGCTATATCTGAGGAGTTGGTTCTCGAGAACCAACTCCTCAGATATAGCTTTTT TG (SEQ ID NO:329). The control shRNA was targeted against GFP with the target sequence GCAAGCTGACCCTGAAGTTCAT (SEQ ID NO:330). Knockdown was confirmed by qPCR. qPCR. qPCR was performed using the manufacturer's protocol for Fisher Maxima SYBR Green with 10 mM primer concentrations or for Bio-Rad SsoAdvanced Universal Probes Supermix. Primer sequences for Fisher Maxima SYBR Green were derived from Harvard Primer Bank. Primer sequences for Bio-Rad SsoAdvanced Universal Probes Supermix were designed with Primer 3 Plus. Fluorescence microscopy. SW620 and U2OS cells were transiently transfected with a plasmid encoding GFP-tagged Sec61β using fuGENE6 (Roche) according to the manufacturer's instructions. Transfected cells plated on poly-L-lysine treated coverslips were treated, washed in PBS, and fixed by incubation in 4% paraformaldehyde in PBS for 10 min. Fixed cells were washed extensively in PBS and nuclei stained by addition of 4′,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific) for 10 min. Coverslips were mounted using Fluoromount-G (SouthernBiotech) and visualized using a DeltaVision Elite microscope outfitted with a 60× oil immersion objective. Acquired stacks of images of fixed cells were deconvolved and analyzed using SoftWoRx and ImageJ. For time-lapse imaging of live cells, transfected cells were plated on poly-L-lysine treated glass-bottom 4-well imaging chambers (Lab-Tek II; Thermo Fisher Scientific). Imaging was performed using a DeltaVision Elite microscope encased in a chamber that was maintained at 37° C. and was continuously perfused with humidified 5% CO2. Acquired images were analyzed using SoftWoRx and ImageJ. Homology modeling and multiple sequence alignments. The threaded homology model of the human Rtn4 (amino acids 1054-1120 of Rtn4a) on the NMR solution structure of the corresponding region of mouse Rtn4 (PDB 2KO2) was generated using Protein Homology/analogY Recognition Engine V 2.0 (Phyre2). Figures were made using PyMOL. Multiple sequence alignments were generated using Clustal Omega and figures were made using BoxShade. Primary Human Colorectal Tumors. Eligible patients completed written consent for our tissue banking protocol that is approved by the University of Alabama at Birmingham Institutional Review Board. During the colorectal tumor resection, a 1 cm3portion of the tumor was dissected free of the fresh resection specimen, divided into 4-5 aliquots, placed into 1.5 mL cryovials, flash frozen, and stored at −80° C. Adjacent non-tumor bearing colorectal tissue was also collected and banked in a similar manner. General Synthetic Methods Chemicals and reagents were purchased from major commercial suppliers and used without further purification. Reactions were performed under a nitrogen atmosphere unless otherwise noted. Silica gel flash column chromatography was performed using EMD or Sigma Aldrich silica gel 60 (230-400 mesh). Proton and carbon nuclear magnetic resonance (1H NMR and13C NMR) data was acquired on a Bruker AVB 400, AVQ 400, or AV 600 spectrometer at the University of California, Berkeley. High resolution mass spectrum were obtained from the QB3 mass spectrometry facility at the University of California, Berkeley using positive or negative electrospray ionization (+ESI or −ESI). Yields are reported as a single run. General Procedure A. The amine (1 eq.) was dissolved in DCM (5 mL/mmol) and cooled to 0° C. To the solution was added acryloyl chloride (1.2 eq.) followed by triethylamine (1.2 eq.). The solution was warmed to room temperature and stirred overnight. The solution was then washed with brine and the crude product was purified by silica gel chromatography (and recrystallization if necessary) to afford the corresponding acrylamide. General Procedure B. The amine (1 eq.) was dissolved in DCM (5 mL/mmol) and cooled to 0° C. To the solution was added chloroacetyl chloride (1.2 eq.) followed by triethylamine (1.2 eq.). The solution was warmed to room temperature and stirred overnight. The solution was then washed with brine and the crude product was purified by silica gel chromatography (and recrystallization if necessary) to afford the corresponding chloroacetamide. N-(4-benzoylphenyl)acrylamide (DKM 2-117). Following General Procedure A starting from 4-aminobenzophenone (587 mg, 3.0 mmol), product was obtained after silica gel chromatography (10% to 30% ethyl acetate in hexanes) in 37% yield as a yellow solid (275 mg).1H NMR (400 MHz, CDCl3): δ 8.77 (s, 1H), 7.80-7.73 (m, 6H), 7.57 (tt, J=1.5, 7.4 Hz, 1H), 7.46 (t, J=7.6 Hz, 2H), 6.46 (dd, J=1.6 16.9 Hz, 1H), 6.37 (dd, J=9.9, 16.9 Hz, 1H), 5.75 (dd, J=1.6, 9.9 Hz, 1H).13C NMR (100 MHz, CDCl3): δ 196.3, 164.4, 142.3, 137.8, 133.0, 132.5, 131.7, 131.0, 130.0, 128.8, 128.4, 119.3. HRMS (+ESI): Calculated: 252.1019 (C16H14NO2). Observed: 252.1014. N-([1,1′-biphenyl]-4-ylmethyl)acrylamide (DKM 2-37). Following General Procedure A starting from 4-phenylbenzylamine (552 mg, 3.0 mmol), product was obtained after silica gel chromatography (0% to 80% ethyl acetate in hexanes) in 10% yield as an off-white solid (73 mg).1H NMR (400 MHz, CDCl3): δ 7.58-7.55 (m, 4H), 7.44 (t, J=7.5 Hz, 2H), 7.38-7.33 (m, 3H), 6.35 (dd, J=1.3, 17.0 Hz, 1H), 6.13 (dd, J=10.3, 17.0 Hz, 1H), 6.01 (s, 1H), 5.68 (dd, J=1.3, 10.3 Hz, 1H), 4.56 (d, J=5.8 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 165.5, 140.77, 140.73, 137.2, 130.7, 128.9, 128.5, 127.6, 127.5, 127.2, 127.1, 43.5. HRMS (+ESI): Calculated: 238.1226 (C16H16NO). Observed: 238.1224. 2-Chloro-N-(4-phenylbutan-2-yl)acetamide (DKM 2-76). Following General Procedure B starting from 1-methyl-3-phenylpropylamine (614 mg, 4.1 mmol) product was obtained after silica gel chromatography (0% to 30% ethyl acetate in hexanes) in 81% yield as a white solid (662 mg).1H NMR (400 MHz, CDCl3): δ 7.34-7.31 (m, 2H), 7.24-7.21 (m, 3H), 6.55 (d, J=7.4 Hz, 1H), 4.15-4.07 (m, 1H), 4.04 (s, 2H), 2.70 (t, J=8.2 Hz, 2H), 1.89-1.83 (m, 2H), 1.26 (d, J=6.4 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 165.1, 141.3, 128.4, 128.2, 125.9, 45.7, 42.7, 381, 32.3, 20.7. HRMS (+ESI): Calculated: 226.0993 (C12H17ClNO). Observed: 226.0992. 2-chloro-N-(4-fluorobenzyl)acetamide (DKM 2-80). Following General Procedure B starting from 4-fluorobenzylamine (369 mg, 2.9 mmol) product was obtained after silica gel chromatography (0% to 30% ethyl acetate in hexanes) in 77% yield as a white solid (452 mg).1H NMR (400 MHz, CDCl3): δ 7.28-7.24 (m, 2H), 7.05-7.01 (m, 2H), 6.97 (s, 1H), 4.45 (d, J=5.6 Hz, 2H), 4.09 (s, 2H).13C NMR (100 MHz, CDCl3): δ 166.1, 163.6, 161.2, 133.20, 133.17, 129.64, 129.56, 115.9, 115.7, 43.2, 42.7. HRMS (−ESI): Calculated: 200.0284 (C9H8NOClF). Observed: 200.0284. N-(benzo[d][1,3]dioxol-5-yl)acrylamide (DKM 2-86). Following General Procedure A starting from 3,4-(methylenedioxy)aniline (486 mg, 2.9 mmol), product was obtained after silica gel chromatography (0% to 30% ethyl acetate in hexanes) in 68% yield as a white solid (438 mg).1H NMR (400 MHz, (CD3)2SO): δ 10.05 (s, 1H), 7.39 (d, J=2.0 Hz, 1H), 7.02 (dd, J=2.0, 8.4 Hz, 1H), 6.87 (d, J=8.4 Hz, 1H), 6.38 (dd, J=10.1, 17.0 Hz, 1H), 6.22 (dd, J=2.1, 17.0 Hz, 1H), 5.99 (s, 2H), 5.72 (dd, J=2.1, 10.1 Hz, 1H).13C NMR (100 MHz, (CD3)2SO): δ 162.8, 147.0, 143.1, 133.4, 131.8, 126.5, 112.1, 108.1, 101.4, 101.0. HRMS (+ESI): Calculated: 192.0655 (C10H10NO3). Observed: 192.0651. 2-chloro-N-(2,3-dihydro-1H-inden-4-yl)acetamide (DKM 2-91). Following General Procedure B starting from 4-aminoindan (372 mg, 2.8 mmol) product was obtained after silica gel chromatography (0% to 40% ethyl acetate in hexanes) in 49% yield as an off-white solid (289 mg).1H NMR (400 MHz, CDCl3): δ 8.19 (s, 1H), 7.74 (d, J=8.4 Hz, 1H), 7.15 (t, J=7.8 Hz, 1H), 7.05 (d, J=7.6 Hz, 1H), 4.16 (s, 2H), 2.94 (t, J=7.6 Hz, 2H), 2.82 (t, J=7.4 Hz, 2H), 2.10 (quint, J=7.5 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 163.8, 145.5, 134.5, 132.8, 127.3, 121.6, 118.5, 43.1, 33.2, 29.8, 24.8. HRMS (+ESI): Calculated: 210.0680 (C11H13ClNO). Observed: 210.0680. 2-Chloro-N-(2-chlorobenzyl)acetamide (DKM 2-94). Following General Procedure B starting from 2-chlorobenzylamine (432 mg, 3.1 mmol) product was obtained after silica gel chromatography (0% to 30% ethyl acetate in hexanes) in 67% yield as a white solid (443 mg).1H NMR (400 MHz, CDCl3): δ 7.36-7.18 (m, 5H), 4.51 (d, J=6.4 Hz, 2H), 4.01 (s, 2H).13C NMR (100 MHz, CDCl3): δ 166.1, 134.7, 133.5 129.8, 129.5, 129.1, 127.1, 42.5, 41.6. HRMS (−ESI): Calculated: 215.9988 (C9H8NOCl2). Observed: 215.9988. N-(4′-cyano-[1,1′-biphenyl]-4-yl)acrylamide (DKM 2-98). Following General Procedure A starting from 4-(4-aminophenyl)benzonitrile (387 mg, 2.0 mmol), product was obtained after silica gel chromatography (1% to 2% ethyl methanol in DCM) in 70% yield as a yellow solid (348 mg).1H NMR (600 MHz, (D3C)2CO): 9.52 (s, 1H), 7.90-7.89 (m, 2H), 7.87-7.86 (m, 2H), 7.84-7.82 (m, 2H), 7.73-7.71 (m, 2H), 6.49 (dd, J=10.0, 16.9 Hz, 1H), 6.39 (dd, J=2.0, 16.9 Hz, 1H), 5.76 (dd, J=2.0, 10.0 Hz, 1H).13C NMR (150 MHz, (D3C)2CO): δ 164.3, 145.7, 140.9, 134.8, 133.6, 132.7, 128.5, 128.2, 127.6, 120.8, 119.5, 111.3. HRMS (−ESI): Calculated: 247.0877 (C16H11N2O). Observed: 247.0875. N-(4-(methylthio)phenyl)acrylamide (DKM 3-10). Following General Procedure A starting from 4-(methylthio)aniline (405 mg, 2.9 mmol), product was obtained after silica gel chromatography (10% to 40% ethyl acetate in hexanes) in 64% yield as a clear oil (362 mg).1H NMR (400 MHz, MeOD): δ 7.59-7.56 (m, 2H), 7.26-7.22 (m, 2H), 6.42 (dd, J=9.6, 17.0 Hz, 1H), 6.34 (dd, J=2.3, 17.0 Hz, 1H), 5.75 (dd, J=2.3, 9.6 Hz, 1H), 2.45 (s, 3H).13C NMR (100 MHz, MeOD): δ 166.0, 137.2, 135.4, 132.4, 128.6, 127.7, 121.9, 16.4. HRMS (+ESI): Calculated: 194.0634 (C10H12NOS). Observed: 194.0631. N-(4′-ethyl-[1,1′-biphenyl]-4-yl)acrylamide (DKM 3-16). Following General Procedure A starting from 4-amino-4-ethylbiphenyl (386 mg, 2.0 mmol), product was obtained after silica gel chromatography (10% to 70% ethyl acetate in hexanes) in 65% yield as a white solid (164 mg). 41 NMR (400 MHz, (CD3)2CO): δ 7.82 (d, J=8.2 Hz, 2H), 7.62-7.59 (m, 2H), 7.58-7.54 (m, 2H), 7.29 (d, J=8.2 Hz, 2H), 6.47 (dd, J=9.9, 16.9 Hz, 1H), 6.36 (dd, J=2.2, 16.9 Hz, 1H), 5.72 (dd, J=2.2, 9.9 Hz, 1H), 2.67 (q, J=7.6 Hz, 2H), 1.24 (t, J=7.6 Hz, 3H).13C NMR (100 MHz, (CD3)2CO): δ 164.1, 144.0, 139.5, 13.9, 137.1, 132.9, 129.3, 127.9, 127.4, 127.2, 120.7, 29.2, 16.2. HRMS (+ESI): Calculated: 252.1383 (C17H18NO). Observed: 252.1379. N,N-diphenylacrylamide (DKM 3-70). A solution of diphenylamine (347 mg, 2.1 mmol) in DCM (10 mL) was cooled to 0° C. To the solution was added acryloyl chloride (222 mg, 2.5 mmol) followed by triethylamine (279 mg, 2.8 mmol). The solution was allowed to warm to room temperature and stirred overnight. The solution was washed with brine and citric acid and the crude product was purified via silica gel chromatography (20% to 60% ethyl acetate in hexanes) to afford the product in 24% yield as a dark yellow oil (112 mg).1H NMR (400 MHz, CDCl3): δ 7.43-7.28 (m, 10H), 6.52 (dd, J=2.0, 16.8 Hz, 1H), 6.25 (dd, J=10.2, 16.8 Hz, 1H), 5.67 (dd, J=1.8, 10.2 Hz, 1H).13C NMR (100 MHz, CDCl3): δ 165.8, 142.6, 129.7, 129.3, 128.5, 127.0. HRMS (+ESI): Calculated: 246.0889 (C15H13NONa). Observed: 246.0887. 2-Chloro-N-(4-phenoxyphenyl)acetamide (TRH 1-23). Following General Procedure B starting from 4-phenoxyaniline (370 mg, 2.0 mmol) product was obtained after silica gel chromatography (10% to 30% ethyl acetate in hexanes) in 46% yield as a white solid (315 mg).1H NMR (400 MHz, CDCl3): δ 8.42 (s, 1H), 7.52-7.48 (m, 2H), 7.35-7.31 (m, 2H), 7.10 (t, J=7.3 Hz, 1H), 7.01-6.98 (m, 4H), 4.17 (s, 2H).13C NMR (100 MHz, CDCl3): δ 164.2, 157.2, 154.4, 132.1, 129.8, 123.4, 122.2, 119.4, 118.7, 42.9. HRMS (−ESI): Calculated: 260.0484 (C14H11NO2Cl). Observed: 260.0482. N-(4-(trifluoromethyl)phenyl)acrylamide (TRH 1-50). Following General Procedure A starting from 4-(trifluoromethyl)aniline (328 mg, 2.0 mmol), product was obtained after silica gel chromatography (10% to 30% ethyl acetate in hexanes) in 55% yield as a white solid (239 mg).1H NMR (400 MHz, MeOD): δ 7.78 (d, J=8.3 Hz, 2H), 7.55 (d, J=8.6 Hz, 2H), 6.44-6.32 (m, 2H), 5.75 (dd, J=8.4, 2.8 Hz, 1H).13C NMR (100 MHz, MeOD): δ 166.3, 143.3, 132.1, 128.6, 127.04, 127.00, 126.97, 126.93, 126.6, 124.3, 120.9. HRMS (−ESI): Calculated: 214.0485 (C10H7NOF3). Observed: 214.0484. 2-Chloro-N-(2-methylbenzyl)acetamide (TRH 1-55). Following General Procedure B starting from 2-methylbenzylamine (239 mg, 2.0 mmol) product was obtained after silica gel chromatography (30% ethyl acetate in hexanes) and recrystallization from 5% ethyl acetate in hexanes in 64% yield as a white solid (191 mg).1H NMR (400 MHz, CDCl3): δ 7.25-7.19 (m, 4H), 6.85 (s, 1H), 4.46 (d, J=5.6 Hz, 2H), 4.04 (s, 2H), 2.33 (s, 3H).13C NMR (100 MHz, CDCl3): δ 165.8, 136.4, 135.0, 130.6, 128.4, 128.0, 126.3, 42.6, 42.0, 19.0. HRMS (−ESI): Calculated: 196.0535 (C10H11NOCl). Observed: 196.0534. N-benzylacrylamide (DKM 2-31). Following General Procedure A starting from benzylamine (334 mg, 3.1 mmol), product was obtained after silica gel chromatography (0% to 50% ethyl acetate in hexanes) in 75% yield as a white solid (376 mg).1H NMR (400 MHz, CDCl3): δ 7.28-7.18 (m, 6H), 6.19-6.16 (m, 2H), 5.53 (dd, J=4.6, 7.3 Hz, 1H), 4.36 (d, J=5.9 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 165.8, 138.1, 130.8, 128.6, 127.7, 127.3, 126.5, 43.5. HRMS (+ESI): Calculated: 162.0913 (C10H12NO). Observed: 162.0912. N-(4-phenylbutan-2-yl)acrylamide (DKM 2-32). Following General Procedure A starting from 1-methyl-3-phenylpropylamine (606 mg, 4.0 mmol), product was obtained after silica gel chromatography (0% to 50% ethyl acetate in hexanes) in 89% yield as a clear oil (735 mg).1H NMR (400 MHz, CDCl3): δ 7.32-7.29 (m, 2H), 7.23-7.20 (m, 3H), 6.84 (d, J=8.4 Hz, 1H), 6.36-6.24 (m, 2H), 5.64 (dd, J=2.8, 9.2 Hz, 1H), 4.21-4.14 (m, 1H), 2.70 (t, J=7.8 Hz, 2H), 1.93-1.77 (m, 2H), 1.24 (d, J=6.4 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 165.1, 141.7, 131.3, 128.3, 128.2, 125.80, 125.77, 45.1, 38.4, 32.5, 20.8. HRMS (+ESI): Calculated: 204.1383 (C13H18NO). Observed: 204.1380. N-(4-methoxybenzyl)acrylamide (DKM 2-33). Following General Procedure A starting from 4-methoxybenzylamine (424 mg, 3.1 mmol), product was obtained after silica gel chromatography (0% to 50% ethyl acetate in hexanes) in 60% yield as a clear oil (343 mg).1H NMR (400 MHz, CDCl3): δ 7.14 (d, J=8.8 Hz, 2H), 6.85 (s, 1H), 6.79 (d, J=8.4 Hz, 2H), 6.24-6.14 (m, 2H), 5.56 (dd, J=2.0, 9.6 Hz, 1H), 4.33 (d, J=5.6 Hz, 2H), 3.73 (s, 3H).13C NMR (100 MHz, CDCl3): δ 165.6, 158.9, 130.9, 130.3, 129.1, 126.4, 113.9, 55.2, 42.9. HRMS (+ESI): Calculated: 192.1019 (C11H14NO2). Observed: 192.1017. N-(4-fluorobenzyl)acrylamide (DKM 2-34). Following General Procedure A starting from 4-fluorobenzylamine (368 mg, 2.9 mmol), product was obtained after silica gel chromatography (0% to 60% ethyl acetate in hexanes) in 52% yield as an off-white solid (276 mg).1H NMR (400 MHz, CDCl3): δ 7.24-7.19 (m, 2H), 6.97 (t, J=8.5 Hz, 2H), 6.42 (s, 1H), 6.27 (d, J=17.0 Hz, 1H), 6.12 (dd, J=17.0, 10.2 Hz, 1H), 5.63 (d, J=10.2 Hz, 1H), 4.42 (d, J=5.8 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 165.7, 163.5, 134.0, 130.6, 129.6, 129.5, 127.0, 115.7, 115.5, 43.0. HRMS (+ESI): Calculated: 180.0819 (C10H11NOF). Observed: 180.0818. Ethyl 4-acryloylpiperazine-1-carboxylate (DKM 2-39). Following General Procedure A starting from ethyl 1-piperazinecarboxylate (477 mg, 3.0 mmol), product was obtained after silica gel chromatography (0% to 70% ethyl acetate in hexanes) in 58% yield as a yellow oil (372 mg).1H NMR (400 MHz, CDCl3): δ 6.46 (dd, J=10.5, 16.8 Hz, 1H), 6.18 (dd, J=1.9, 16.8 Hz), 5.60 (dd, J=1.9, 10.5 Hz), 4.03 (q, J=7.1 Hz, 2H), 3.54 (s, 2H), 3.44 (s, 2H), 3.39-3.36 (m, 4H), 1.15 (t, J=7.1 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 165.3, 155.1, 128.2, 127.1, 61.5, 45.4, 43.6, 43.3, 41.5, 14.5. HRMS (+ESI): Calculated: 213.1234 (C10H17N2O3). Observed: 213.1232. N-(2,5-difluorophenyl)acrylamide (DKM 2-40). Following General Procedure A starting from 2,5-difluoroaniline (369 mg, 2.9 mmol), product was obtained after silica gel chromatography (0% to 15% ethyl acetate in hexanes) in 27% yield as a white solid (141 mg).1H NMR (400 MHz, (CD3)2CO): δ 9.26 (s, 1H), 8.29-8.24 (m, 1H), 7.24-7.18 (m, 1H), 6.90-6.84 (m, 1H), 6.67 (dd, J=10.2, 16.9 Hz, 1H), 6.41 (dd, J=1.9, 16.9 Hz, 1H), 5.79 (dd, J=1.9, 10.2 Hz, 1H).13C NMR (100 MHz, (CD3)2CO): δ 164.6, 160.4, 151.0, 148.7, 132.0, 128.9, 128.8, 128.5, 116.7, 116.6, 116.5, 116.4, 111.1, 111.0, 110.8, 110.7, 110.0, 109.7. HRMS (+ESI): Calculated: 184.0568 (C9H8F2NO). Observed: 184.0567. N-phenethylacrylamide (DKM 2-42). Following General Procedure A starting from phenylethylamine (367 mg, 3.0 mmol), product was obtained after silica gel chromatography (0% to 50% ethyl acetate in hexanes) in 85% yield as a yellow oil (450 mg).1H NMR (400 MHz, CDCl3): δ 7.30-7.18 (m, 5H), 6.63 (s, 1H), 6.25 (dd, J=1.8, 17.0 Hz, 1H), 6.13 (dd, J=10.0, 17.0 Hz 1H), 5.59 (dd, J=1.6, 10.0 Hz, 1H), 3.56 (q, J=6.8 Hz, 2H), 2.85 (t, J=7.3 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 165.8, 138.8, 131.0, 128.7, 128.6, 126.4, 126.1, 40.8, 35.6. HRMS (+ESI): Calculated: 176.1070 (C11H14NO). Observed: 176.1068. N-(4-bromobenzyl)acrylamide (DKM 2-43). Following General Procedure A starting from 4-bromobenzylamine (535 mg, 2.9 mmol), product was obtained after silica gel chromatography (0% to 50% ethyl acetate in hexanes) in 59% yield as a white solid (407 mg).1H NMR (400 MHz, CDCl3): δ 7.37 (d, J=8.4 Hz, 2H), 7.07 (d, J=8.4 Hz, 2H), 7.00 (s, 1H), 6.24-6.10 (m, 2H), 5.59 (dd, J=2.0, 9.7 Hz, 1H), 4.32 (d, J=6.0 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 165.9, 137.2, 131.7, 130.6, 129.4, 126.9, 121.2, 42.8. HRMS (+ESI): Calculated: 240.0019 (C10H11BrNO). Observed: 240.0016. N-(3,5-dimethylbenzyl)acrylamide (DKM 2-47). Following General Procedure A starting from 3,5-dimethylbenzylamine (257 mg, 1.9 mmol), product was obtained after silica gel chromatography (0% to 40% ethyl acetate in hexanes) in 77% yield as a white solid (276 mg).1H NMR (400 MHz, CDCl3): δ 6.89-6.87 (m, 4H), 6.26 (dd, J=2.1, 17.0 Hz, 1H), 6.18 (dd, J=9.7, 17.0 Hz, 1H) 5.59 (dd, J=2.1, 9.7 Hz, 1H), 4.35 (d, J=6.0 Hz, 2H), 2.28 (s, 6H).13C NMR (100 MHz, CDCl3): δ 165.6, 138.1, 138.0, 130.9, 129.0, 126.3, 125.6, 43.4, 12.2. HRMS (+ESI): Calculated: 190.1226 (C12H16NO). Observed: 190.1225. 1-(pyrrolidin-1-yl)prop-2-en-1-one (DKM 2-48). Following General Procedure A starting from pyrrolidine (223 mg, 3.1 mmol), product was obtained after silica gel chromatography (0% to 80% ethyl acetate in hexanes) in 38% yield as a pale yellow oil (148 mg).1H NMR (400 MHz, CDCl3): δ 6.40 (dd, J=10.0, 16.8 Hz, 1H), 6.29 (dd, J=2.4, 16.8 Hz, 1H), 5.60 (dd, J=2.4, 10.0 Hz, 1H), 3.48 (t, J=6.8 Hz, 4H), 1.91 (quint, J=6.7 Hz, 2H), 1.82 (quint, J=6.7 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 164.4, 128.8, 127.2, 46.6, 45.9, 26.1, 24.3. HRMS (+ESI): Calculated: 126.0913 (C7H12NO). Observed: 126.0912. 1-morpholinoprop-2-en-1-one (DKM 2-49). Following General Procedure A starting from morpholine (273 mg, 3.1 mmol), product was obtained after silica gel chromatography (0% to 80% ethyl acetate in hexanes) in 46% yield as a yellow oil (205 mg).1H NMR (400 MHz, CDCl3): δ 6.45 (dd, J=10.5, 16.8 Hz, 1H), 6.20 (dd, J=1.9, 16.8 Hz, 1H), 5.61 (dd, J=1.9, 10.5 Hz, 1H), 5.38 (s, 6H), 3.46 (s, 2H).13C NMR (100 MHz, CDCl3): δ 165.3, 128.1, 126.9, 66.6, 46.0, 42.1. HRMS (+ESI): Calculated: 142.0863 (C7H12NO2). Observed: 142.0861. N-(3-phenylpropyl)acrylamide (DKM 2-50). Following General Procedure A starting from 3-phenyl-1-propylamine (275 mg, 2.0 mmol), product was obtained after silica gel chromatography (0% to 60% ethyl acetate in hexanes) in 58% yield as a yellow oil (223 mg).1H NMR (400 MHz, CDCl3): δ 7.29-7.25 (m, 2H), 7.20-7.16 (m, 3H), 6.99 (s, 1H), 6.29-6.17 (m, 2H), 5.59 (dd, J=2.6, 9.0 Hz, 1H), 3.34 (q, J=6.7 Hz, 2H), 2.65 (t, J=7.6 Hz, 2H), 1.87 (quint, J=7.4 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 166.0, 141.4, 131.1, 128.33, 128.26, 125.9, 39.2, 33.2, 31.0. HRMS (+ESI): Calculated: 190.1226 (C12H16NO). Observed: 190.1225. N-(2-(2-methoxyphenoxy)ethyl)acrylamide (DKM 2-58). Following General Procedure A starting from 2-(2-methoxyphenoxy)ethanamine (509 mg, 3.0 mmol), product was obtained after silica gel chromatography (0% to 30% ethyl acetate in hexanes) in 70% yield as a yellow oil (470 mg).1H NMR (400 MHz, CDCl3): δ 6.95-6.84 (m, 4H), 6.77 (s, 1H), 6.26 (d, J=17.1 Hz, 1H), 6.11 (dd, J=10.2, 17.1 Hz, 1H), 5.59 (d, J=10.2 Hz, 1H), 4.07 (t, J=5.2 Hz, 2H), 3.79 (s, 3H), 3.69 (q, J=5.4 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 165.7, 149.6, 147.7, 130.8, 126.4, 122.1, 121.0, 114.8, 111.8, 68.5, 55.7, 38.9. HRMS (+ESI): Calculated: 244.0944 (C12H15NO3Na). Observed: 244.0940. N-([1,1′-biphenyl]-2-ylmethyl)acrylamide (DKM 2-59). Following General Procedure A starting from 2-phenylbenzylamine (202 mg, 1.1 mmol), product was obtained after silica gel chromatography (0% to 40% ethyl acetate in hexanes) in 70% yield as a yellow oil (184 mg).1H NMR (400 MHz, CDCl3): δ 7.41-7.22 (m, 9H), 6.16 (dd, J=1.2, 17.2 Hz, 1H), 6.03-5.97 (m, 2H), 5.55 (dd, J=1.2, 10.0 Hz, 1H), 4.44 (d, J=5.6 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 165.3, 141.6, 140.6, 135.2, 1306, 130.2, 129.0, 128.7, 128.4, 127.8, 127.4, 127.3, 126.4, 41.4. HRMS (+ESI): Calculated: 238.1226 (C16H16NO). Observed: 238.1223. N-(2-chlorobenzyl)acrylamide (DKM 2-60). Following General Procedure A starting from 2-chlorobenzylamine (406 mg, 2.9 mmol), product was obtained after silica gel chromatography (0% to 30% ethyl acetate in hexanes) in 34% yield as a white solid (162 mg).1H NMR (400 MHz, CDCl3): δ 7.34-30 (m, 2H), 7.20-7.16 (m, 2H), 6.84 (s, 1H), 6.25 (dd, J=2.0, 17.0 Hz, 1H), 6.16 (dd, J=9.7, 17.0 Hz, 2H), 5.60 (dd, J=2.0, 9.7 Hz, 1H), 4.52 (d, J=6.1 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 165.9, 135.5, 133.5, 130.6, 129.8, 129.5, 128.8, 127.1, 126.8, 41.4. HRMS (+ESI): Calculated: 196.0524 (C10H11ClNO). Observed: 196.0521 N-(2-nitrobenzyl)acrylamide (DKM 2-62). Following General Procedure A starting from 2-nitrobenzylamine hydrochloride (406 mg, 2.9 mmol) with an extra equivalent of triethylamine, product was obtained after silica gel chromatography (50% ethyl acetate in hexanes) in 42% yield as a yellow solid (255 mg).1H NMR (400 MHz, CDCl3): δ 7.98 (dd, J=1.1, 8.2 Hz, 1H), 7.58-7.52 (m, 2H), 7.41-7.37 (m, 1H), 7.03 (s, 1H), 6.22 (dd, J=2.0, 17.0 Hz, 1H), 6.14 (dd, J=9.7, 17.0 Hz, 1H), 5.59 (dd, J=2.0, 9.7 Hz, 1H), 4.68 (d, J=6.4 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 165.8, 148.2, 134.1, 133.6, 131.9, 130.4, 128.7, 127.1, 125.1, 41.2. HRMS (+ESI): Calculated: 207.0764 (C10H11N2O3). Observed: 207.0760. N-(2,3-dihydro-1H-inden-4-yl)acrylamide (DKM 2-84). Following General Procedure A starting from 4-aminoindan (402 mg, 3.0 mmol), product was obtained after silica gel chromatography (30% ethyl acetate in hexanes) in 59% yield as a white solid (332 mg).1H NMR (400 MHz, CDCl3): δ 7.72 (d, J=7.5 Hz, 1H), 7.54 (s, 1H), 7.10 (t, J=7.7 Hz, 1H), 7.01 (d, J=7.2 Hz, 1H), 6.40-6.26 (m, 2H), 5.69 (dd, J=1.9, 9.7 Hz, 1H), 2.91 (t, J=7.4 Hz, 2H), 2.78 (t, J=7.4 Hz, 2H), 2.05 (quint, J=7.4 Hz, 2H).13C NMR (100 MHz, CDCl3): 163.5, 145.3, 134.4, 133.6, 131.2, 127.5, 127.2, 12.0, 19.2, 33.2, 30.1, 24.8. HRMS (+ESI): Calculated: 188.1070 (C12H14NO). Observed: 188.1069. Ethyl 4-acrylamidobenzoate (DKM 2-85). Following General Procedure A starting from benzocaine (486 mg, 2.9 mmol), product was obtained after silica gel chromatography (0% to 30% ethyl acetate in hexanes) in 68% yield as a white solid (438 mg).1H NMR (400 MHz, CDCl3): δ 9.39 (s, 1H), 7.95 (d, J=8.7 Hz, 2H), 7.74 (d, J=8.7 Hz, 2H), 6.43-6.41 (m, 2H), 5.71 (dd, J=4.7, 6.9 Hz, 2H), 4.31 (q, J=7.1 Hz, 2H), 1.33 (s, J=7.1 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 166.5, 164.6, 142.5, 131.0, 130.6, 128.4, 125.7, 119.4, 61.0, 14.2. HRMS (−ESI): Calculated: 218.0823 (C12H12NO3). Observed: 218.0822. N-benzyl-N-methylacrylamide (DKM 2-95). Following General Procedure A starting from N-methylbenzylamine (350 mg, 2.9 mmol), product was obtained after silica gel chromatography (20% ethyl acetate in hexanes) in 60% yield as a clear oil (304 mg).1H NMR (˜48:52 rotamer ratio, asterisks denote minor peaks, 400 MHz, CDCl3): δ 7.34-7.23 (m, 4H), 7.16 (s, 1H), 7.14* (s, 1H), 6.61 (dd, J=10.4, 16.8 Hz, 1H), 6.57* (dd, J=10.4, 16.8 Hz, 1H), 6.38 (dd, J=1.9, 16.8 Hz, 1H), 6.36* (dd, J=1.9, 16.8 Hz, 1H), 5.71 (dd, J=1.9, 10.4 Hz, 1H), 5.64* (dd, J=1.9, 10.4 Hz), 4.63 (s, 2H), 4.56* (s, 2H), 2.98* (s, 3H), 2.96 (s, 3H).13C NMR (100 MHz, CDCl3): δ 167.0, 166.4, 137.1, 136.5, 128.8, 128.5, 128.2, 128.0, 17.62, 127.59, 127.3, 126.3, 53.3, 51.0, 34.8, 34.0. HRMS (+ESI): Calculated: 176.1070 (C11H14NO). Observed: 176.1070. 1-(4-phenylpiperidin-1-yl)prop-2-en-1-one (DKM 2-97). Following General Procedure A starting from 4-phenylpiperidine (331 mg, 2.1 mmol), product was obtained after silica gel chromatography (0% to 50% ethyl acetate in hexanes) in 86% yield as a yellow oil (379 mg).1H NMR (400 MHz, CDCl3): δ 7.32-7.28 (m, 2H), 7.22-7.17 (m, 3H), 6.62 (dd, J=10.6, 16.8 Hz, 1H), 6.30 (dd, J=1.9, 16.8 Hz, 1H), 5.68 (dd, J=1.9, 10.6, Hz, 1H), 4.82 (d, J=12.9 Hz, 1H), 4.11 (d, J=13.2 Hz, 1H), 3.15 (t, J=8.5 Hz, 1H), 2.78-2.67 (m, 2H), 1.90 (d, J=12.9 Hz, 2H), 1.64 (quint, J=12.3 Hz, 2H).13C NMR (100 MHz, CDCl3): 165.3, 145.0, 128.5, 127.8, 127.4, 126.6, 126.4, 46.4, 42.7, 33.9, 32.7. HRMS (+ESI): Calculated: 216.1383 (C14H18NO). Observed: 216.1383. N-(2-morpholinoethyl)acrylamide (DKM 2-100). Following General Procedure A starting from 2-morpholinoethylamine (580 mg, 3.0 mmol), product was obtained after silica gel chromatography (2% to 6% methanol in dichloromethane) in 33% yield as a white solid (184 mg).1H NMR (400 MHz, CDCl3): δ 6.39 (s, 1H), 6.21 (dd, J=1.7, 17.0 Hz, 1H), 6.08 (dd, J=10.1, 17.0 Hz, 1H), 5.56 (dd, J=1.7, 10.1 Hz, 1H), 3.63 (t, J=4.6 Hz, 4H), 3.36 (q, J=6.2 Hz, 2H), 2.45 (t, J=6.2 Hz, 2H), 2.40-2.38 (m, 4H).13C NMR (100 MHz, CDCl3): δ 165.5, 130.9, 126.2, 66.9, 57.0, 53.3, 35.7. HRMS (+ESI): Calculated: 185.1285 (C9H17N2O2). Observed: 185.1280. 1-(indolin-1-yl)prop-2-en-1-one (DKM 2-101). Following General Procedure A starting from indoline (580 mg, 3.0 mmol), product was obtained after silica gel chromatography (0% to 20% ethyl acetate in hexanes) in 56% yield as a green solid (285 mg).1H NMR (400 MHz, CDCl3): δ 8.30 (d, J=7.7 Hz, 1H), 7.22-7.17 (m, 2H), 7.03 (t, J=7.9 Hz, 1H), 6.60-6.48 (m, 2H), 5.79 (dd, J=2.6, 9.5 Hz, 1H), 4.15 (t, J=8.5 Hz, 2H), 3.20 (t, J=8.1, 2H).13C NMR (100 MHz, CDCl3): δ 163.6, 142.6, 131.5, 129.0, 128.6, 127.2, 124.4, 123.8, 117.2, 47.8, 27.7. HRMS (+ESI): Calculated: 174.0913 (C11H12NO). Observed: 174.0911. N-butylacrylamide (DKM 2-102). Following General Procedure A starting from butylamine (223 mg, 3.0 mmol), product was obtained after silica gel chromatography (20% ethyl acetate in hexanes) in 61% yield as a clear oil (237 mg).1H NMR (400 MHz, (CDCl3): δ 6.81 (s, 1H), 6.21-6.10 (m, 2H), 5.52 (dd, J=3.6, 8.3 Hz, 1H), 3.26-3.21 (m, 2H), 1.48-1.41 (m, 2H), 1.33-1.23 (m, 2H), 0.84 (t, J=7.3 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 166.0, 131.2, 125.6, 39.3, 31.5, 20.1, 13.7. HRMS (+ESI): Calculated: 128.1070 (C7H14NO). Observed: 128.1068. N-(3-methoxypropyl)acrylamide (DKM 2-103). Following General Procedure A starting from 3-methoxypropylamine (274 mg, 3.1 mmol), product was obtained after silica gel chromatography (35% to 60% ethyl acetate in hexanes) in 54% yield as a clear oil (236 mg).1H NMR (400 MHz, CDCl3): δ 6.84 (s, 1H), 6.15 (dd, J=2.0, 17.0 Hz. 1H), 6.07 (dd, J=9.8, 17.0 Hz, 1H), 5.51 (dd, J=2.0, 9.8 Hz, 1H), 3.39 (t, J=5.9 Hz, 2H), 3.33 (q, J=6.3 Hz, 2H), 3.25 (s, 3H), 1.72 (quint, J=6.3 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 165.8, 131.2, 125.7, 71.3, 58.7, 37.7, 29.0. HRMS (+ESI): Calculated: 144.1019 (C7H14NO2). Observed: 144.1017. N-cyclohexylacrylamide (DKM 2-106). Following General Procedure A starting from cyclohexylamine (292 mg, 2.9 mmol), product was obtained after silica gel chromatography (20% to 30% ethyl acetate in hexanes) in 86% yield as a white solid (313 mg).1H NMR (400 MHz, (CDCl3): δ 6.55 (d, J=6.7 Hz, 1H), 6.21-6.09 (m, 2H), 5.51 (dd, J=2.5, 9.1 Hz, 1H), 3.79-3.70 (m, 1H), 1.86-1.82 (m, 2H), 1.67-1.63 (m, 2H), 1.56-1.52 (m, 1H), 1.28-1.21 (m, 2H), 1.16-1.05 (m, 3H).13C NMR (100 MHz, CDCl3): δ 164.8, 131.5, 125.7, 48.3, 32.9, 25.5, 24.9. HRMS (+ESI): Calculated: 154.1226 (C9H16NO). Observed: 154.1224. N-(4-chlorophenyl)acrylamide (DKM 2-107). Following General Procedure A starting from 4-chloroaniline (386 mg, 3.0 mmol), product was obtained after silica gel chromatography (0% to 40% ethyl acetate in hexanes) followed by recrystallization from toluene in 31% yield as a white solid (168 mg).1H NMR (400 MHz, (CD3)2CO): δ 9.47 (s, 1H), 7.77-7.74 (m, 2H), 7.35-7.31 (m, 2H), 6.43 (dd, J=9.6, 16.9 Hz, 1H), 6.35 (dd, J=2.5, 16.9 Hz, 1H), 5.73 (dd, J=2.5, 9.6 Hz, 1H).13C NMR (100 MHz, (CD3)2CO): δ 164.1, 139.0, 132.5, 129.5, 128, 127.5, 121.7. HRMS (−ESI): Calculated: 180.0222 (C9H7NOCl). Observed: 180.0221. N-cyclopentylacrylamide (DKM 2-108). Following General Procedure A starting from cyclopentylamine (257 mg, 3.0 mmol), product was obtained after silica gel chromatography (20% to 30% ethyl acetate in hexanes) in 55% yield as a colorless oil (229 mg).1H NMR (400 MHz, (CDCl3): δ 6.70 (s, 1H), 6.21-6.10 (m, 2H), 5.51 (dd, J=3.5, 8.5 Hz, 1H), 5.53-5.50 (sex, J=7.1 Hz, 1H), 1.94-1.86 (m, 2H), 1.65-1.46 (m, 4H), 1.41-1.32 (m, 2H).13C NMR (100 MHz, CDCl3): δ 165.4, 131.3, 125.7, 51.1, 32.9, 23.8. HRMS (+ESI): Calculated: 140.1070 (C81H14NO). Observed: 140.1067. 1-(4-methoxypiperidin-1-yl)prop-2-en-1-one (DKM 2-109). Following General Procedure A starting from 4-methoxypiperidine (461 mg, 3.0 mmol), product was obtained after silica gel chromatography (40% to 60% ethyl acetate in hexanes) in 75% yield as a pale yellow oil (386 mg).1H NMR (400 MHz, (CDCl3): δ 6.45 (dd, J=10.6, 16.8 Hz, 1H), 6.09 (dd, J=2.0, 16.8 Hz, 1H), 5.51 (dd, J=2.0, 10.6 Hz, 1H), 3.80-3.74 (m, 1H), 3.65-3.58 (m, 1H), 3.33-3.17 (m, 6H), 1.74-1.67 (m, 2H), 1.47-1.39 (m, 2H).13C NMR (100 MHz, CDCl3): δ 165.1, 127.6, 127.2, 75.0, 55.5, 42.7, 38.9, 31.1, 29.9. HRMS (+ESI): Calculated: 170.1176 (C9H16NO2). Observed: 170.1176. N-(3,4-dimethoxybenzyl)acrylamide (DKM 2-110). Following General Procedure A starting from 3,4-dimethoxybenzylamine (497 mg, 3.0 mmol), product was obtained after silica gel chromatography (30% to 40% ethyl acetate in hexanes) in 65% yield as a white solid (425 mg).1H NMR (400 MHz, CDCl3): δ 7.07 (s, 1H), 6.70-6.64 (m, 3H), 6.18-6.08 (m, 2H), 5.50 (dd, J=3.1, 8.8 Hz, 1H), 4.26 (d, J=5.8 Hz, 2H), 3.70 (d, J=7.8 Hz, 6H).13C NMR (400 MHz, CDCl3): δ 165.5, 148.7, 148.0, 130.73, 130.67, 126.2, 119.9, 110.98, 110.96, 55.64, 55.55, 43.12. HRMS (+ESI): Calculated: 222.1125 (C12H16NO3). Observed: 222.1121. tert-butyl 4-acryloylpiperazine-1-carboxylate (DKM 2-111). Following General Procedure A starting from 1-boc-piperazine (552 mg, 3.0 mmol), product was obtained after silica gel chromatography (50% to 70% ethyl acetate in hexanes) in 75% yield as a pale yellow oil (534 mg).1H NMR (400 MHz, CDCl3): δ 6.48 (dd, J=10.5, 16.8 Hz, 1H), 6.20 (dd, J=1.8, 16.8 Hz, 1H), 5.60 (dd, J=1.8, 10.5 Hz, 1H), 3.55 (s, 2H), 3.44 (s, 2H), 3.36-3.34 (m, 4H), 1.37 (s, 9H).13C NMR (100 MHz, CDCl3): δ 165.4, 154.4, 128.2, 127.2, 80.2, 45.5, 41.7, 28.3. HRMS (+ESI): Calculated: 241.1547 (C12H21N2O3). Observed: 241.1543. N-(2-phenoxyethyl)acrylamide (DKM 2-113). Following General Procedure A starting from 2-phenoxyethylamine (279 mg, 2.0 mmol), product was obtained after silica gel chromatography (30% to 70% ethyl acetate in hexanes) in 61% yield as a white solid (239 mg).1H NMR (400 MHz, CDCl3): δ 7.31-7.25 (m, 2H), 6.98-6.94 (m, 1H), 6.90-6.87 (m, 2H), 6.58 (s, 1H), 6.31 (dd, J=1.6, 17.0 Hz, 1H), 6.17 (dd, J=10.2, 17.0 Hz, 1H), 5.64 (dd, J=1.6, 10.2 Hz, 1H), 4.05 (t, J=5.2 Hz, 2H), 3.73 (q, J=5.4 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 165.9, 158.4, 130.7, 129.6, 126.7, 121.2, 114.4, 66.5, 39.1. HRMS (+ESI): Calculated: 192.1019 (C11H14NO2). Observed: 192.1016. N,N-dicyclohexylacrylamide (DKM 2-114). Following General Procedure A starting from dicyclohexylamine (537 mg, 3.0 mmol), product was obtained after silica gel chromatography (20% to 40% ethyl acetate in hexanes) in 55% yield as a white solid (382 mg).1H NMR (400 MHz, CDCl3): δ 6.49 (dd, J=10.6, 16.8 Hz, 1H), 6.11 (dd, J=1.9, 16.8 Hz, 1H), 5.49 (dd, J=2.0, 10.6 Hz, 1H), 3.45 (s, 1H), 3.22 (s, 1H), 2.22 (s, 2H), 1.74-1.49 (m, 12H), 1.22-1.07 (m, 6H).13C NMR (100 MHz, CDCl3): δ 166.2, 130.9, 125.5, 57.5, 55.6, 31.6, 30.1, 26.4, 26.0, 25.3. HRMS (+ESI): Calculated: 236.2009 (C15H26NO). Observed: 236.2004. N-(4-(trifluoromethyl)benzyl)acrylamide (DKM 2-116). Following General Procedure A starting from 4-(trifluoromethyl)benzylamine (516 mg, 2.9 mmol), product was obtained after silica gel chromatography (20% to 30% ethyl acetate in hexanes) in 24% yield as a white solid (165 mg).1H NMR (600 MHz, CDCl3): δ 7.53 (d, J=8.0 Hz, 2H), 7.35 (d, J=8.0 Hz, 2H), 6.58 (s, 1H), 6.28 (dd, J=1.5, 17.0 Hz, 1H), 6.14 (dd, J=10.1, 17.0 Hz, 1H), 5.64 (dd, J=1.5, 10.1 Hz, 1H), 4.50 (d, J=6.0 Hz, 2H).13C NMR (150 MHz, CDCl3): δ 165.9, 142.3, 130.5, 130.0, 129.7, 128.0, 127.3, 125.73, 125.69, 12566, 125.62, 43.1. HRMS (−ESI): Calculated: 228.0642 (C11H9NOF3). Observed: 228.0641. Ethyl 1-acryloylpiperidine-4-carboxylate (DKM 2-120). Following General Procedure A starting from ethyl isonipecotate (459 mg, 2.9 mmol), product was obtained after silica gel chromatography (20% to 45% ethyl acetate in hexanes) in 71% yield as a pale yellow liquid (440 mg).1H NMR (400 MHz, CDCl3): δ 6.40 (dd, J=10.6, 16.8 Hz, 1H), 6.04 (dd, J=2.0, 16.8 Hz, 1H), 5.47 (dd, J=2.0, 10.6 Hz, 1H), 4.23 (d, J=13.2 1H), 3.93 (q, J=7.1 Hz, 2H), 3.76 (d, J=14.0 Hz, 1H), 2.99 (t, J=11.8 Hz, 1H), 2.70 (t, J=11.5 Hz, 1H), 2.37 (tt, J=4.1, 10.7 Hz, 1H), 1.77-1.73 (m, 2H), 1.51-1.42 (m, 2H), 1.05 (t, J=7.1 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 173.7, 165.0, 127.5, 127.2, 60, 44.7, 41.0, 40.5, 28.2, 27.4, 13.8. HRMS (+ESI): Calculated: 212.1281 (C11H18NO3). Observed: 212.1276. N-benzhydrylacrylamide (DKM 3-4). Following General Procedure A starting from benzhydrylamine (459 mg, 3.0 mmol), product was obtained after silica gel chromatography (0% to 20% ethyl acetate in hexanes) and recrystallization from toluene in 15% yield as a white solid (110 mg).1H NMR (400 MHz, (CD3)2CO): δ 7.35-7.23 (m, 10H), 6.45 (dd, J=10.2, 17.0 Hz, 1H), 6.36-6.34 (m, 1H), 6.25 (dd, J=2.2, 17.0 Hz, 1H), 5.61 (dd, J=2.2, 10.2 Hz, 1H).13C NMR (100 MHz, (CD3)2CO): δ 164.84, 164.76, 143.51, 143.48, 132.51, 132.47, 129.4, 128.5, 1280, 126.3, 57.5, 57.4. HRMS (+ESI): Calculated: 238.1226 (C16H16NO). Observed: 238.1222. 1-(4-phenylpiperazin-1-yl)prop-2-en-1-one (DKM 3-5). Following General Procedure A starting from 1-phenylpiperazine (479 mg, 3.0 mmol), product was obtained after silica gel chromatography (30% to 70% ethyl acetate in hexanes) in 87% yield as a yellow oil (555 mg).1H NMR (400 MHz, CDCl3): 7.30-7.25 (m, 2H), 6.92-6.87 (m, 3H), 6.60 (dd, J=10.5, 16.8 Hz 1H), 6.33 (dd, J=2.0, 16.8 Hz, 1H), 5.72 (dd, J=2.0, 10.5 Hz, 1H), 3.81 (s, 2H), 3.66 (s, 2H), 3.14 (t, J=5.2 Hz, 4H).13C NMR (100 MHz, CDCl3): δ 165.0, 150.6, 18.9, 127.8, 127.1, 120.2, 116.3, 49.4, 48.9, 45.3, 41.5. HRMS (+ESI): Calculated: 217.1335 (C13H17N2O). Observed: 217.1332. N-(4-acetylphenyl)acrylamide (DKM 3-7). Following General Procedure A starting from 4-aminoacetophenone (398 mg, 2.9 mmol), product was obtained after silica gel chromatography (20% to 50% ethyl acetate in hexanes) in 45% yield as a white solid (253 mg).1H NMR (400 MHz, CDCl3): δ 8.40 (s, 1H), 7.92 (d, J=8.7 Hz, 2H), 7.73 (d, J=8.7 Hz, 2H), 6.46 (dd, J=1.3, 16.9 Hz, 1H), 6.34 (dd, J=10.1, 16.9 Hz, 1H), 5.79 (dd, J=1.3, 10.1 Hz, 1H), 2.57 (s, 3H).13C NMR (100 MHz, CDCl3): δ 197.5, 164.1, 142.5, 133.0, 130.9, 129.9, 128.9, 119.4, 26.6. HRMS (+ESI): Calculated: 190.0863 (C11H12NO2). Observed: 190.0858. 1-(4-methylpiperidin-1-yl)prop-2-en-1-one (DKM 3-8). Following General Procedure A starting from 4-methylpiperidine (295 mg, 3.0 mmol), product was obtained after silica gel chromatography (10% to 30% ethyl acetate in hexanes) in 84% yield as a yellow oil (385 mg).1H NMR (400 MHz, CDCl3): δ 6.51 (dd, J=10.6, 16.5 Hz, 1H), 6.16 (dd, J=2.0, 16.5 Hz, 1H), 5.57 (dd, J=2.0, 10.6 Hz, 1H), 4.53 (d, J=13.1 Hz, 1H), 3.88 (d, J=13.3 Hz, 1H), 2.99-2.92 (m, 1H), 2.55 (td, J=2.1, 12.8 Hz, 1H), 1.62 (d, J=13.1 Hz, 2H), 1.57-1.49 (m, 1H), 1.10-0.98 (m, 2H), 0.87 (d, J=6.5 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 165.2, 128.0, 127.0, 46.2, 42.4, 34.7, 33.7, 31.1, 21.7. HRMS (+ESI): Calculated: 154.1226 (C9H16NO). Observed: 154.1224. N-(2,2-diethoxyethyl)acrylamide (DKM 3-9). Following General Procedure A starting from aminoacetaldehyde diethyl acetal (402 mg, 3.0 mmol), product was obtained after silica gel chromatography (10% to 40% ethyl acetate in hexanes) in 75% yield as a clear oil (313 mg).1H NMR (400 MHz, CDCl3): 6.25-6.19 (m, 2H), 6.09 (dd, J=10.1, 17.0 Hz, 1H), 5.56 (dd, J=1.7, 10.1 Hz, 1H), 4.48 (t, J=5.3 Hz, 1H), 3.64 (dq, J=7.1, 9.4 Hz, 2H), 3.47 (dq, J=7.1, 9.4 Hz, 2H), 3.38 (t, J=5.6 Hz, 2H), 1.13 (t, J=7.1 Hz, 6H).13C NMR (100 MHz, CDCl3): δ 165.7, 130.6, 126.4, 100.6, 62.8, 42.0, 15.2. HRMS (+ESI): Calculated: 188.1281 (C9H18NO3). Observed: 188.1278. 1-acryloylpiperidine-4-carbonitrile (DKM 3-11). Following General Procedure A starting from piperidine-4-carbonitrile (329 mg, 3.0 mmol), product was obtained after silica gel chromatography (30% to 70% ethyl acetate in hexanes) in 48% yield as a colorless oil (234 mg).1H NMR (400 MHz, CDCl3): 6.49 (dd, J=10.6, 16.8 Hz, 1H), 6.19 (d, J=1.9, 16.8 Hz, 1H), 5.64 (dd, J=1.9, 10.6 Hz, 1H), 3.77-3.46 (m, 4H), 2.88-2.82 (sept, J=3.9 Hz, 1H), 1.90-1.73 (m, 4H).13C NMR (100 MHz, CDCl3): δ 165.4, 128.3, 127.3, 120.8, 43.8, 39.9, 29.1, 28.1, 26.3. HRMS (+ESI): Calculated: 165.1022 (C9H13N2O). Observed: 165.1020. N-(3-(methylthio)propyl)acrylamide (DKM 3-12). Following General Procedure A starting from 3-(methylthio)propylamine (313 mg, 3.0 mmol), product was obtained after silica gel chromatography (20% to 60% ethyl acetate in hexanes) in 69% yield as a colorless oil (328 mg).1H NMR (400 MHz, CDCl3): δ 6.79 (s, 1H), 6.19 (dd, J=2.2, 17.0 Hz, 1H), 6.11 (dd, J=9.6, 17.0 Hz, 1H), 5.55 (dd, J=2.2, 9.6 Hz, 1H), 3.35 (q, J=6.5 Hz, 2H), 2.47 (t, J=7.2 Hz, 2H), 2.02 (s, 3H), 1.78 (quint, J=7.0 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 165.9, 131.0, 126.1, 38.6, 31.6, 28.6, 15.4. HRMS (+ESI): Calculated: 160.0791 (C7H14NOS). Observed: 160.0788. N-(cyclohexylmethyl)acrylamide (DKM 3-13). Following General Procedure A starting from cyclohexanemethylamine (331 mg, 2.9 mmol), product was obtained after silica gel chromatography (10% to 50% ethyl acetate in hexanes) in 67% yield as a pale yellow solid (330 mg).1H NMR (400 MHz, CDCl3): 6.51 (s, 1H), 6.22 (dd, J=2.5, 17.0 Hz, 1H) 6.15 (dd, J=9.3, 17.0 Hz, 1H), 5.55 (dd, J=2.5, 9.3 Hz, 1H), 3.11 (t, J=6.5 Hz, 2H), 1.70-1.58 (m, 5H), 1.51-1.40 (m, 1H), 1.22-1.04 (m, 3H), 0.93-0.83 (m, 2H).13C NMR (100 MHz, CDCl3): δ 165.9, 131.2, 125.9, 45.9, 38.0, 30.9, 26.4, 25.8. HRMS (+ESI): Calculated: 168.1383 (C10H18NO). Observed: 168.1380. 1-(4-(4-acetylphenyl)piperazin-1-yl)prop-2-en-1-one (DKM 3-29). Following General Procedure A starting from 4′-piperazinoacetophenone (607 mg, 3.0 mmol), product was obtained after silica gel chromatography (50% to 85% ethyl acetate in hexanes) in 65% yield as a yellow solid (496 mg).1H NMR (400 MHz, CDCl3): δ 7.79 (d, J=9.0 Hz, 2H), 6.78 (d, J=9.0 Hz, 2H), 6.54 (dd, J=10.5, 16.8 Hz, 1H), 6.25 (dd, J=1.9, 16.8 Hz, 1H), 5.66 (dd, J=1.9, 10.5 Hz, 1H), 3.75 (s, 2H), 3.66 (s, 2H), 3.31-3.29 (m, 4H), 2.42 (s, 3H).13C NMR (100 MHz, CDCl3): δ 196.3, 165.2, 153.4, 130.2, 128.3, 127.9, 127.0, 113.5, 47.3, 47.0, 45.0, 41.2, 26.0. HRMS (+ESI): Calculated: 259.1441 (C15H19N2O2). Observed: 259.1436. N-(4-(4-chlorophenoxy)phenyl)acrylamide (DKM 3-30). Following General Procedure A starting from 4-(4-chlorophenoxy)aniline (440 mg, 2.0 mmol), product was obtained after silica gel chromatography (10% to 30% ethyl acetate in hexanes) in 33% yield as a white solid (180 mg).1H NMR (400 MHz, CDCl3): δ 8.00 (s, 1H), 7.56 (d, J=8.9 Hz, 2H), 7.29-7.25 (m, 2H), 6.96-6.88 (m, 4H), 6.43 (dd, J=1.4, 16.9 Hz, 1H), 6.30 (dd, J=10.1, 16.9 Hz, 1H), 5.75 (dd, J=1.4, 10.1 Hz, 1H).13C NMR (100 MHz, CDCl3): δ 163.9, 156.2, 153.4, 133.7, 131.2, 129.8, 128.2, 128.0, 122.1, 119.8, 119.7. HRMS (+ESI): Calculated: 272.0484 (C15H11NO2Cl). Observed: 272.0479. N-(4-fluorophenyl)acrylamide (DKM 3-31). Following General Procedure A starting from 4-fluoroaniline (239 mg, 2.2 mmol), product was obtained after silica gel chromatography (20% to 30% ethyl acetate in hexanes) in 16% yield as a white solid (56 mg).1H NMR (600 MHz, MeOD): δ 7.64-7.60 (m, 2H), 7.07-7.03 (m, 2H), 6.41 (dd, J=9.8, 17.0 Hz, 1H), 6.35 (dd, J=2.1, 17.0 Hz, 1H), 5.76 (dd, J=2.1, 9.8 Hz, 1H).13C NMR (150 MHz, MeOD): δ 166.0, 161.56, 160.0, 135.93, 135.91, 132.3, 127.8, 123.2, 123.1, 116.4, 116.2. HRMS (−ESI): Calculated: 164.0517 (C9H7NOC). Observed: 164.0517. N-(sec-butyl)acrylamide (DKM 3-32). Following General Procedure A starting from sec-butylamine (222 mg, 3.0 mmol), product was obtained after silica gel chromatography (10% to 40% ethyl acetate in hexanes) in 74% yield as a yellow oil (287 mg).1H NMR (400 MHz, CDCl3): δ 6.56 (d, J=5.6 Hz, 1H), 6.17 (s, 1H), 6.16 (d, J=3.5 Hz, 1H), 5.51 (dd, J=4.3, 7.6 Hz, 1H), 3.93-3.83 (m, 1H), 1.47-1.36 (m, 2H), 1.06 (d, J=6.6 Hz, 3H), 0.82 (t, J=7.5 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 165.2, 131.4, 125.6, 46.6, 29.5, 20.2, 10.4. HRMS (+ESI): Calculated: 128.1070 (C7H14NO). Observed: 128.1069. 1-(4-(4-methoxyphenyl)piperazin-1-yl)prop-2-en-1-one (DKM 3-36). Following General Procedure A starting from 1-(4-methoxyphenyl)piperazine (388 mg, 2.0 mmol), product was obtained after silica gel chromatography (20% to 80% ethyl acetate in hexanes) in 29% yield as a white solid (143 mg).1H NMR (400 MHz, CDCl3): δ 6.87-6.79 (m, 4H), 6.57 (dd, J=10.5, 16.8 Hz, 1H), 6.28 (dd, J=1.9, 16.8 Hz, 1H), 5.68 (dd, J=1.9, 10.5 Hz, 1H), 3.79 (s, 2H), 3.72 (s, 3H), 3.66 (s, 2H), 3.01 (t, J=5.1 Hz, 4H).13C NMR (100 MHz, CDCl3): δ 165.2, 154.3, 145.1, 128.0, 127.3, 118.8, 114.4, 55.4, 51.3, 50.7, 45.8, 41.9. HRMS (+ESI): Calculated: 247.1441 (C14H19N2O2). Observed: 247.1443. N-tritylacrylamide (DKM 3-41). Following General Procedure A starting from triphenylmethylamine (386 mg, 1.5 mmol), product was obtained after silica gel chromatography (5% to 30% ethyl acetate in hexanes) in 74% yield as a white solid (346 mg).1H NMR (400 MHz, CDCl3): δ 7.38-7.27 (m, 15H), 6.83 (s, 1H), 6.28-6.26 (m, 2H), 5.66 (dd, J=3.9, 7.2 Hz, 1H).13C NMR (100 MHz, CDCl3): δ 164.6, 144.6, 131.5, 128.8, 128.1, 127.2, 127.1, 70.7. HRMS (+ESI): Calculated: 314.1539 (C22H20NO). Observed: 314.1542. (E)-N-(3,7-dimethylocta-2,6-dien-1-yl)acrylamide (DKM 3-42). Following General Procedure A starting from geranylamine (462 mg, 3.0 mmol), product was obtained after silica gel chromatography (10% to 40% ethyl acetate in hexanes) in 23% yield as a colorless oil (141 mg).1H NMR (400 MHz, CDCl3): δ 6.25 (dd, J=1.5, 17.0 Hz, 1H), 6.09 (dd, J=10.2, 17.0 Hz, 1H), 5.83 (s, 1H), 5.59 (dd, J=1.5, 10.2 Hz), 5.22-5.18 (m, 1H), 5.07-5.03 (m, 1H), 3.90 (t, J=6.2 Hz, 2H), 2.09-2.03 (m, 2H), 2.00-1.97 (m, 2H), 1.65 (s, 6H), 1.57 (s, 3H).13C NMR (100 MHz, CDCl3): δ 165.5, 140.2, 131.8, 131.0, 126.2, 123.9, 119.7, 39.6, 37.6, 265, 25.8, 17.8, 16.4. HRMS (+ESI): Calculated: 208.1696 (C13H22NO). Observed: 208.1697. N-(benzo[d][1,3]dioxol-5-ylmethyl)acrylamide (DKM 3-43). Following General Procedure A starting from piperonylamine (312 mg, 2.1 mmol), product was obtained after silica gel chromatography (20% to 50% ethyl acetate in hexanes) in 74% yield as a white solid (315 mg).1H NMR (400 MHz, CDCl3): δ 6.78 (s, 1H), 6.71 (s, 1H), 6.68 (s, 2H), 6.22 (dd, J=1.9, 17.0 Hz, 1H), 6.13 (dd, J=9.9, 17.0 Hz, 1H), 5.87 (s, 2H), 5.58 (dd, J=1.9, 9.9 Hz, 1H), 4.30 (d, J=5.8 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 165.7, 147.8, 146.9, 132.0, 130.8, 126.6, 121.1, 108.4, 108.2, 101.0, 43.4. HRMS (+ESI): Calculated: 206.0812 (C11H12NO3). Observed: 206.0808. N-decylacrylamide (TRH 1-12). Following General Procedure A starting from decylamine (479 mg, 3.0 mmol), product was obtained after silica gel chromatography (20% to 40% ethyl acetate in hexanes) in 26% yield as a white solid (163 mg).1H NMR (400 MHz, CDCl3): δ 6.54 (s, 1H), 6.21 (dd, J=2.0, 16.9 Hz, 1H) 6.13 (dd, J=9.7, 16.9 Hz, 1H), 5.55 (dd, J=2.0, 9.7 Hz, 1H), 3.25 (q, J=6.7 Hz, 2H), 1.50-1.45 (m, 2H), 1.29-1.20 (m, 14H), 0.83 (t, J=6.7 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 165.8, 131.2, 125.9, 71.9, 39.7, 31.9, 29.6, 29.6, 29.38, 29.35, 27.0, 22.7, 14.1. HRMS (+ESI): Calculated: 212.2009 (C13H26NO). Observed: 212.2009. N-(2,4-dimethoxybenzyl)acrylamide (TRH 1-13). Following General Procedure A starting from 2,4-dimethoxybenzylamine (514 mg, 3.0 mmol), product was obtained after silica gel chromatography (20% to 60% ethyl acetate in hexanes) in 11% yield as a white solid (73 mg).1H NMR (400 MHz, CDCl3): δ 7.17 (d, J=8.1 Hz, 1H), 6.43-6.39 (m, 2H), 6.26-6.22 (m, 2H), 6.07 (dd, J=10.7, 17.0 Hz, 1H), 5.57 (dd, J=1.4, 10.7 Hz, 1H), 4.41 (d, J=5.8 Hz, 2H), 3.79 (s, 3H), 3.77 (s, 3H).13C NMR (100 MHz, CDCl3): δ 165.2, 160.6, 158.6, 131.1, 130.7, 126.2, 118.7, 104.0, 98.6, 55.5, 55.4, 39.0. HRMS (+ESI): Calculated: 222.1125 (C12H16NO3). Observed: 222.1124. N-Phenylacrylamide (TRH 1-19). Following General Procedure A starting from aniline (277 mg, 3.0 mmol), product was obtained after recrystallization from a 1:20 ethyl acetate:hexanes mixture in 46% yield as a white solid (200 mg).1H NMR (400 MHz, CDCl3): δ 8.59 (s, 1H), 7.63 (d, J=7.9 Hz, 2H), 7.30 (t, J=7.9 Hz, 2H), 7.11 (t, J=7.4 Hz, 1H), 6.44-6.33 (m, 2H), 5.70 (dd, J=2.8, 8.9 Hz, 1H).13C NMR (100 MHz, CDCl3): δ 164.3, 138.0, 131.4, 129.0, 127.7, 124.6, 120.5. HRMS (+ESI): Calculated: 148.0757 (C9H10NO). Observed: 148.0754. N-(1-phenylethyl)acrylamide (TRH 1-20). Following General Procedure A starting from 1-phenylethan-1-amine (387 mg, 3.0 mmol), product was obtained after silica gel chromatography (5% to 20% ethyl acetate in hexanes) in 46% yield as a white solid (315 mg).1H NMR (400 MHz, CDCl3): δ 7.61 (d, J=7.8 Hz, 1H) 7.37-7.24 (m, 5H), 6.33-6.24 (m, 2H), 5.57 (dd, J=4.8, 7.9 Hz, 1H), 5.20 (quint, J=7.2 Hz, 1H), 1.49 (d, J=7.0 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 165.0, 143.4, 131.1, 128.4, 126.9, 126.0, 126.0, 48.7, 21.8. HRMS (+ESI): Calculated: 176.1070 (C11H14NO). Observed: 176.1067. 1-(2-ethylpiperidin-1-yl)prop-2-en-1-one (TRH 1-27). Following General Procedure A starting from 2-ethylpiperidine (238 mg, 2.0 mmol), product was obtained after silica gel chromatography (5% to 30% ethyl acetate in hexanes) in 72% yield as a white solid (253 mg).1H NMR (400 MHz, CDCl3): δ 6.41 (dd, J=10.6, 16.7 Hz, 1H), 6.03 (d, J=16.4 Hz, 1H), 5.43 (dd, J=2.0, 10.6 Hz, 1H), 4.54-4.34 (m, 1H), 3.77-3.58 (m, 1H), 2.93-2.42 (m, 1H), 1.61-1.06 (m, 8H), 0.66 (t, J=7.5 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 165.9, 130.0, 129.1, 128.4, 126.6, 54.4, 49.6, 41.1, 36.5, 28.8, 27.5, 26.2, 25.2, 23.0, 22.1, 18.8, 10.4. HRMS (+ESI): Calculated: 168.1383 (C10H18NO). Observed: 168.1380. N-(4-methoxyphenyl)acrylamide (TRH 1-32). Following General Procedure A starting from p-anisidine (258 mg, 2.0 mmol), product was obtained after silica gel chromatography (10% to 50% ethyl acetate in hexanes) in 58% yield as a white solid (216 mg).1H NMR (400 MHz, CDCl3): δ 8.94 (s, 1H), 7.48 (d, J=9.1 Hz, 2H), 6.78 (d, J=9.1 Hz, 2H), 6.34 (d, J=5.6 Hz, 2H), 5.61 (t, J=5.9 Hz, 1H), 3.73 (s, 3H).13C NMR (100 MHz, CDCl3): δ 164.3, 156.4, 131.4, 131.1, 127, 122.3, 114.0, 55.4. HRMS (+ESI): Calculated: 178.0863 (C10H12O2N). Observed: 178.0859. N-(2-methylbenzyl)acrylamide (TRH 1-54). Following General Procedure A starting from 2-methylbenzylamine (240 mg, 2.0 mmol), product was obtained after silica gel chromatography (30% to 40% ethyl acetate in hexanes) in 73% yield as a white solid (257 mg).1H NMR (400 MHz, CDCl3): δ 7.26-7.12 (m, 4H), 6.66 (s, 1H), 6.24-6.12 (m, 2H), 5.57 (dd, J=9.5, 2.2 Hz, 1H), 4.39 (d, J=5.4 Hz, 2H), 2.27 (s, 3H).13C NMR (100 MHz, CDCl3): δ 165.6, 136.3, 135.7, 130.7, 130.4, 128.4, 127.6, 126.4, 126.1, 41.6, 19.0. HRMS (+ESI): Calculated: 176.1070 (C11H14NO). Observed: 176.1067. Ethyl 4-(2-chloroacetyl)piperazine-1-carboxylate (DKM 2-52). Following General Procedure B starting from ethyl 1-piperazinecarboxylate (477 mg, 3.0 mmol) product was obtained after silica gel chromatography (0% to 80% ethyl acetate in hexanes) in 80% yield as a pale yellow oil (569 mg).1H NMR (400 MHz, CDCl3): δ 4.04-3.99 (m, 4H), 3.48-3.34 (m, 8H), 1.14 (t, J=7.1 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 165.1, 155.0, 61.5, 45.8, 43.3, 43.0, 41.7, 40.7, 14.4. HRMS (+SI): Calculated: 235.0844 (C9H16ClN2O3). Observed: 235.0842. N-benzyl-2-chloroacetamide (DKM 2-67). Following General Procedure B starting from benzylamine (430 mg, 3.1 mmol) product was obtained after silica gel chromatography (0% to 30% ethyl acetate in hexanes) in 70% yield as a white solid (416 mg).1H NMR (400 MHz, CDCl3): δ 7.40-7.31 (m, 5H), 7.08 (s, 1s), 4.50 (d, J=5.8 Hz, 2H), 4.09 (s, 2H).13C NMR (100 MHz, CDCl3): δ 166.0, 137.4, 128.8, 127.8, 43.8, 42.6. HRMS (−ESI): Calculated: 182.0378 (C9H9NOCl). Observed: 182.0378. 2-Chloro-1-(pyrrolidin-1-yl)ethan-1-one (DKM 2-71). Following General Procedure B starting from pyrrolidine (511 mg, 3.0 mmol) product was obtained after silica gel chromatography (0% to 30% ethyl acetate in hexanes) in 83% yield as a clear oil (368 mg).1H NMR (400 MHz, CDCl3): δ 3.94 (s, 2H), 3.41 (quint, J=7.2 Hz, 4H), 1.91 (quint, J=6.3 Hz, 2H), 1.80 (quint, J=6.6 Hz, 2H).13C NMR (100 MHz, CDCl3): δ 164.7, 46.5, 46.3, 42.1, 26.1, 24.1. HRMS (+ESI): Calculated: 170.0343 (C6H10ClNNaO). Observed: 170.0343. 2-Chloro-N-decylacetamide (DKM 2-72). Following General Procedure B starting from decylamine (472 mg, 3.0 mmol) product was obtained after silica gel chromatography (0% to 40% ethyl acetate in hexanes) in 81% yield as a white solid (555 mg).1H NMR (400 MHz, CDCl3): δ 6.71 (s, 1H), 3.97 (s, 2H), 3.22 (q, J=6.8 Hz, 2H), 1.51-1.44 (m, 2H), 1.24-1.19 (m, 14H), 0.81 (t, J=6.8 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 165.8, 42.7, 39.9, 31.9, 29.5, 29.29, 29.27, 29.22, 26.8, 22.6, 14.1. HRMS (+ESI): Calculated: 234.1619 (C12H25ClNO). Observed: 234.1618. 2-chloro-N-(4-methoxybenzyl)acetamide (DKM 2-83). Following General Procedure B starting from 4-methoxybenzylamine (430 mg, 3.1 mmol) product was obtained after silica gel chromatography (0% to 40% ethyl acetate in hexanes) in 55% yield as an off-white solid (369 mg).1H NMR (400 MHz, CDCl3): δ 7.20 (d, J=8.6 Hz, 2H), 6.91 (s, 1H), 6.86 (d, J=8.6 Hz, 2H), 4.40 (d, J=5.7 Hz, 2H), 4.05 (s, 2H), 3.78 (s, 3H).13C NMR (100 MHz, CDCl3): δ 165.9, 159.2, 129.4, 129.2, 114.2, 55.3, 43.4, 42.7. HRMS (+ESI): Calculated: 214.0629 (C10H13ClNO2). Observed: 214.0627. 2-chloro-N-(3,4-dimethoxybenzyl)acetamide (DKM 2-93). Following General Procedure B starting from 3,4-dimethoxybenzylamine (517 mg, 3.1 mmol) product was obtained after silica gel chromatography (0% to 50% ethyl acetate in hexanes) in 55% yield as an off-white solid (416 mg).1H NMR (400 MHz, CDCl3): δ 6.97 (s, 1H), 6.77 (m, 3H), 4.35 (d, J=5.8 Hz, 2H), 4.01 (s, 2H), 3.81 (s, 3H), 3.80 (s, 3H).13C NMR (100 MHz, CDCl3): δ 165.8, 149.0, 148.5, 129.8, 120.1, 111.13, 111.07, 55.83, 55.79, 43.6, 42.5. HRMS (+ESI): Calculated: 266.0554 (C11H14NO3ClNa). Observed: 266.0553. 2-Chloro-N-methyl-N-propylacetamide (TRH 1-53). Following General Procedure B starting from N-methylpropylamine (147 mg, 2.0 mmol) product was obtained after silica gel chromatography (30% to 40% ethyl acetate in hexanes) in 64% yield as a white solid (191 mg).1H NMR (46:54 rotamer ratio, asterisks denote minor peaks, 400 MHz, CDCl3): δ 4.03* (s, 2H), 4.02 (s, 2H), 3.28* (t, J=7.4 Hz, 2H), 3.23 (t, J=7.5 Hz, 2H), 3.00 (s, 3H), 2.88* (s, 3H), 1.64-1.56* (m, 2H), 1.53-1.46 (m, 2H), 0.87* (t, J=7.5 Hz, 3H), 0.83 (t, J=7.5 Hz, 3H).13C NMR (asterisks denote minor rotamer peaks, 100 MHz, CDCl3): δ 166.4, 166.3*, 51.9*, 49.8, 41.5, 40.9*, 35.6, 33.6*, 21.6*, 20.1, 11.1, 11.0*. HRMS (+ESI): Calculated: 150.0680 (C6H13NOCl). Observed: 150.0678. Synthesis and Characterization of YP 1-46 N-(4-(4-methoxyphenoxy)phenyl)acrylamide (YP-1-46). To a solution of 4-methoxyphenol (622 mg, 5 mmol) in DMF (2 mL) was added potassium carbonate (1.38 g, 10 mmol). After 10 minutes of stirring, 1-fluoro-4-nitrobenzene (0.43 mL, 4 mmol) was added and the reaction was stirred overnight. As the reaction was not complete by TLC after 21 hours, the reaction was heated to 90 degrees for 1 hour at which point the reaction was found to be complete. The reaction was then diluted with water and extracted three times with ethyl acetate. The combined organics were dried with magnesium sulfate and concentrated to give 1.07 g of crude 1-methox-4-(nitrophenoxy)benzene as a yellow solid that was used without further purification. To a stirring solution of the resulting crude (490 mg, ˜2 mmol) and 10% palladium on activated charcoal (49 mg) in methanol (4 mL) was added triethylsilane (2.33 g, 20 mmol) dropwise through an addition funnel under a nitrogen-filled balloon. After 30 min, the mixture was filtered through celite and the solvent was removed in vacuo. Without further purification, the obtained crude product was dissolved in DCM (10 mL) and the resultant solution was cooled to 0° C. To the solution was added acryloyl chloride (217 mg, 2.4 mmol) followed by triethylamine (243 mg, 2.4 mmol). The solution was allowed to warm to room temperature after 20 min and stirred overnight. The solution was washed two times with brine and the crude product was purified via silica gel chromatography (30% to 70% ethyl acetate in hexanes) to afford 161 mg of the product as a white solid (33% yield over 3 steps).1H NMR (400 MHz, CDCl3): δ 7.94 (s, 1H), 7.50-7.48 (m, 2H), 6.96-6.92 (m, 2H), 6.90-6.84 (m, 4H), 6.40 (dd, J=1.6, 16.8 Hz, 1H), 6.27 (dd, J=10.1, 16.8 Hz, 1H), (dd, J=1.6, 10.1 Hz, 1H), 3.79 (s, 3H).13C NMR (100 MHz, CDCl3): δ 163.8, 155.8, 155.1, 150.4, 132.6, 131.1, 127.6, 121.9, 120.5, 118.2, 114.9, 55.7. HRMS (+ESI): Calculated: 270.1125 (C16H16NO3). Observed: 270.1125. Synthesis and Characterization of AMR 1-125 An oven dried round bottom flask was charged with a magnetic stir bar, copper (I) iodide (38 mg, 0.2 mmol), N-Boc-4-hydroxyaniline (502 mg, 2.4 mmol), potassium carbonate (552.8 mg, 2 mmol), and crushed 4 angstrom sieves (˜200 mg). The flask was evacuated and filled with nitrogen twice. Under nitrogen, 1-Bromo-4-tert-butylbenzene (346 uL, 2 mmol) and N,N′-Dimethyl-1,2-cyclohexanediamine (62 uL, 0.2 mmol) were added along with 2 mL of butyronitrile. The flask was allowed to react at 70 C for 24 hours. At the end of the reaction, the mixture was diluted with CH2Cl2and rinsed through Celite to remove inorganic salts and other solids. The crude reaction mixture was purified using column chromatograph (10% ethyl acetate in hexanes). The product was a yellow oil (yield 31%). An oven dried round bottom flask was charged with the amine starting material (154.2 mg, 0.6 mmol) along with dry CH2C2and allowed to cool to OC. Acryloyl chloride (69.4 mg, 0.8 mmol) was then added to the flask, followed by triethylamine (196 uL, 1.4 mmol), and the reaction was allowed to come to room temperature overnight. At the end of the reaction, the mixture was washed with brine and then purified using column chromatography (10% ethyl acetate in hexanes). The product was a waxy, white solid (yield <10%).1H NMR (900 MHz, CDCl3): δ 7.51 (d, 2H, J=8.5 Hz), δ 7.31 (d, 2H, J=8.5 Hz), δ 6.97 (m, 2H), δ 6.90 (m, 2H), δ 6.42 (d, 1H, J=16.8 Hz), δ 6.22 (dd, 1H, J=16.8, 10.3 Hz), δ 5.75 (m, 1H), δ 1.3 (s, 9H).13C NMR (900 MHz, CDCl3): δ 163.4, 155.1, 154.2, 146.3, 133.0, 131.2, 127.9, 126.7, 121.7, 119.5, 118.3, 34.5, 31.7, 29.9. HRMS (+ESI): Calculated: 296.1645 (C19H21NO2) Observed: 296.1643. General Procedure Synthetic Scheme for Derivatives Derivatives being Synthesized It is understood that the examples and embodiments described herein are for illustrative purposes only and that various modifications or changes in light thereof will be suggested to persons skilled in the art and are to be included within the spirit and purview of this application and scope of the appended claims. All publications, patents, and patent applications cited herein are hereby incorporated by reference in their entirety for all purposes. Disclosed herein, inter alia, are compositions and methods useful for inhibiting reticulon 4 (RTN4). 1. A method of treating cancer, said method comprising administering to a subject in need thereof an effective amount of a compound having the formula: wherein, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1R1D, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NR1AOC1C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z1 is an integer from 0 to 5; R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(O)R2C, —C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z2 is an integer from 0 to 4; L1is a bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4—, —NR4C(O)—, —NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, or substituted or unsubstituted heteroarylene; R4is hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)—OR4A, —C(O)NR4AR4B, —OR4A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR5C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, or substituted or unsubstituted heteroarylene; R5is hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)—OR5A, —C(O)NR5AR5B, —OR5A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; E is an electrophilic moiety; Each R1A, R1B, R1C, R1D, R2A, R2B, R2C, R2D, R4A, R4B, R5A, and R5Bis independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X1, X2, X4, and X5is independently —F, —Cl, —Br, or —I; n1, n2, n4, and n5 are independently an integer from 0 to 4; and m1, m2, m4, m5, v1, v2, v4, and v5 are independently an integer from 1 to 2. 2. The method of 3. (canceled) 4. (canceled) 5. The method of 6. (canceled) 7. The method of 8. (canceled) 9. The method of 10. The method of 11. (canceled) 12. The method of 13. (canceled) 14. The method of 15. The method of 16. The method of 17. The method of 18. (canceled) 19. The method of 20. The method of 21. (canceled) 22. The method of R15is independently hydrogen, halogen, CX153, —CHX152, —CH2X15, —CN, —SOn15R15D, —SOv15NR15AR15B, —NHNR15AR15B, —ONR15AR15B, —NHC═(O)NHNR15AR15B, —NHC(O)NR15AR15B, —N(O)m15, —NR15AR15B, —C(O)R15C, —C(O)—OR15C, —C(O)NR15AR15B, —OR15D, —NR15ASO2R15D, —NR15AC(O)R15C, —NR15AC(O)OR15C, —NR15AOR15C, —OCX153, —OCHX152, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl; R16is independently hydrogen, halogen, CX163, —CHX162, —CH2X16, —CN, —SOn16R16D, —SOv16NR16AR16B, —NHNR16AR16B, —ONR16AR16B, —NHC═(O)NHNR16AR16B, —NHC(O)NR16AR16B, —N(O)m16, —NR16AR16B, —C(O)R16C, —C(O)—OR16C, —C(O)NR16AR16B, —OR16D, —NR16ASO2R16D, —NR16AC(O)R16C, —NR16AC(O)OR16C, —NR16AOR16C, —OCX163, —OCHX162, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl; R17is independently hydrogen, halogen, CX173, —CHX172, —CH2X17, —CN, —SOn17R17D, —SOv17NR17AR17B, —NHNR17AR17B, —ONR17AR17B, —NHC═(O)NHNR17AR17B, —NHC(O)NR17AR17B, —N(O)m17, —NR17AR17B, —C(O)R17C, —C(O)—OR17C, —C(O)NR17AR17B, —OR17D, —NR17ASO2R17D, —NR17AC(O)R17C, —NR17AC(O)OR17C, —NR17AOR17C, —OCX173, —OCHX172, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl; R18is independently hydrogen, —CX183, —CHX182, —CH2X18, —C(O)R18C, —C(O)OR18C, —C(O)NR18AR18B, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl; R15A, R15B, R15C, R15D, R16A, R16B, R16C, R16D, R17A, R17B, R17C, R17D, R18A, R18B, R18C, and R18D, are independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R15Aand R15Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R16Aand R16Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R17Aand R17Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R18Aand R18Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X15, X16, X17and X18is independently —F, —Cl, —Br, or —I; n15, n16, n17, v15, v16, and v17, are independently an integer from 0 to 4; and m15, m16, and m17 are independently and integer from 1 to 2. 23. (canceled) 24. The method of 25. The method of 26. The method of 27. (canceled) 28. (canceled) 29. A pharmaceutical composition comprising a Reticulon 4 inhibitor and a pharmaceutically acceptable excipient, wherein the Reticulon 4 inhibitor is a compound having the formula: wherein, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1R1D, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NR1AOR1C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z1 is an integer from 0 to 5; R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(O)R2C, —C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z2 is an integer from 0 to 4; L1is a bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4—, —NR4C(O)—, —NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, or substituted or unsubstituted heteroarylene; R4is hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)—OR4A, —C(O)NR4AR4B, —OR4A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR5C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, or substituted or unsubstituted heteroarylene; R5is hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)—OR5A, —C(O)NR5AR5B, —OR5A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; E is an electrophilic moiety; Each R1A, R1B, R1C, R1D, R2A, R2B, R2C, R2D, R4A, R4B, R5A, and R5Bis independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X1, X2, X4, and X5is independently —F, —Cl, —Br, or —I; n1, n2, n4, and n5 are independently an integer from 0 to 4; and m1, m2, m4, m5, v1, v2, v4, and v5 are independently an integer from 1 to 2. 30. (canceled) 31. (canceled) 32. A method inhibiting Reticulon 4 protein activity, said method comprising contacting a Reticulon 4 protein with an effective amount of a Reticulon 4 inhibitor, wherein said Reticulon 4 inhibitor is a compound having the formula: wherein, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1R1D, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NR1AOR1C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z1 is an integer from 0 to 5; R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(O)R2C, —C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z2 is an integer from 0 to 4; L1is a bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4—, —NR4C(O)—, —NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, or substituted or unsubstituted heteroarylene; R4is hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)—OR4A, —C(O)NR4AR4B, —OR4A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR5C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, or substituted or unsubstituted heteroarylene; R5is hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)—OR5A, —C(O)NR5AR5B, —OR5A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; E is an electrophilic moiety; Each R1A, R1B, R1C, R1D, R2A, R2B, R2C, R2D, R4A, R4B, R5A, and R5Bis independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X1, X2, X4, and X5is independently —F, —Cl, —Br, or —I; n1, n2, n4, and n5 are independently an integer from 0 to 4; and m1, m2, m4, m5, v1, v2, v4, and v5 are independently an integer from 1 to 2. 33. (canceled) 34. A reticulon 4 protein covalently bonded to a compound having the formula: wherein, R1is independently halogen, —CX13, —CHX12, —CH2X1, —OCX13, —OCH2X1, —OCHX12, —CN, —SOn1R1D, —SOv1NR1AR1B, —NHC(O)NR1AR1B, —N(O)m1, —NR1AR1B, —C(O)R1C, —C(O)—OR1C, —C(O)NR1AR1B, —OR1D, —NR1ASO2R1D, —NR1AC(O)R1C, —NR1AC(O)OR1C, —NR1AOC1C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R1substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z1 is an integer from 0 to 5; R2is independently halogen, —CX23, —CHX22, —CH2X2, —OCX23, —OCH2X2, —OCHX22, —CN, —SOn2R2D, —SOv2NR2AR2B, —NHC(O)NR2AR2B, —N(O)m2, —NR2AR2B, —C(O)R2C, —C(O)—OR2C, —C(O)NR2AR2B, —OR2D, —NR2ASO2R2D, —NR2AC(O)R2C, —NR2AC(O)OR2C, —NR2AOR2C, —N3, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; two adjacent R2substituents may optionally be joined to form a substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; z2 is an integer from 0 to 4; L1is a bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4—, —NR4C(O)—, —NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, or substituted or unsubstituted heteroarylene; R4is hydrogen, —CX43, —CHX42, —CH2X4, —OCX43, —OCH2X4, —OCHX42, —CN, —C(O)R4A, —C(O)—OR4A, —C(O)NR4AR4B, —OR4A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; L2is a bond, —S(O)2—, —NR5—, —O—, —S—, —C(O)—, —C(O)NR5—, —NR5C(O)—, —NR5C(O)NH—, —NHC(O)NR5—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted arylene, or substituted or unsubstituted heteroarylene; R5is hydrogen, —CX53, —CHX52, —CH2X5, —OCX53, —OCH2X5, —OCHX52, —CN, —C(O)R5A, —C(O)—OR5A, —C(O)NR5AR5B, —OR5A, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; E is an electrophilic moiety; Each R1A, R1B, R1C, R1D, R2A, R2B, R2C, R2D, R4A, R4B, R5A, and R5Bis independently hydrogen, —CX3, —CN, —COOH, —CONH2, —CHX2, —CH2X, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R1Aand R1Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R2Aand R2Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R4Aand R4Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; R5Aand R5Bsubstituents bonded to the same nitrogen atom may optionally be joined to form a substituted or unsubstituted heterocycloalkyl or substituted or unsubstituted heteroaryl; each X, X1, X2, X4, and X5is independently —F, —Cl, —Br, or —I; n1, n2, n4, and n5 are independently an integer from 0 to 4; and m1, m2, m4, m5, v1, v2, v4, and v5 are independently an integer from 1 to 2; wherein the reticulon 4 protein is covalently bonded to said compound through said reacted electrophilic moiety. 35. (canceled) 36. (canceled)CROSS-REFERENCES TO RELATED APPLICATIONS
STATEMENT AS TO RIGHTS TO INVENTIONS MADE UNDER FEDERALLY SPONSORED RESEARCH AND DEVELOPMENT
REFERENCE TO A “SEQUENCE LISTING,” A TABLE, OR A COMPUTER PROGRAM LISTING APPENDIX SUBMITTED AS AN ASCII FILE
BACKGROUND
BRIEF SUMMARY
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION
I. Definitions
halogen, —CCl3, —CBr3, —CF3, —CI3, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC(O)NHNH2, —NHC(O)NH2, —NHSO2H, —NHC(O)H, —NHC(O)OH, —NHOH, —OCCl3, —OCF3, —OCBr3, —OCl3, —OCHCl2, —OCHBr2, —OCHI2, —OCHF2, unsubstituted alkyl (e.g., C1-C8alkyl, C1-C6alkyl, or C1-C4alkyl), unsubstituted heteroalkyl (e.g., 2 to 8 membered heteroalkyl, 2 to 6 membered heteroalkyl, or 2 to 4 membered heteroalkyl), unsubstituted cycloalkyl (e.g., C3-C8cycloalkyl, C3-C6cycloalkyl, or C5-C6cycloalkyl), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered heterocycloalkyl, 3 to 6 membered heterocycloalkyl, or 5 to 6 membered heterocycloalkyl), unsubstituted aryl (e.g., C6-C10aryl, C10aryl, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered heteroaryl, 5 to 9 membered heteroaryl, or 5 to 6 membered heteroaryl), and
(B) alkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, substituted with at least one substituent selected from:
(i) oxo,
halogen, —CCl3, —CBr3, —CF3, —CI3, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC(O)NHNH2, —NHC(O)NH2, —NHSO2H, —NHC(O)H, —NHC(O)OH, —NHOH, —OCCl3, —OCF3, —OCBr3, —OCl3, —OCHCl2, —OCHBr2, —OCHI2, —OCHF2, unsubstituted alkyl (e.g., C1-C8alkyl, C1-C6alkyl, or C1-C4alkyl), unsubstituted heteroalkyl (e.g., 2 to 8 membered heteroalkyl, 2 to 6 membered heteroalkyl, or 2 to 4 membered heteroalkyl), unsubstituted cycloalkyl (e.g., C3-C8cycloalkyl, C3-C6cycloalkyl, or C5-C6cycloalkyl), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered heterocycloalkyl, 3 to 6 membered heterocycloalkyl, or 5 to 6 membered heterocycloalkyl), unsubstituted aryl (e.g., C6-C10aryl, C10aryl, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered heteroaryl, 5 to 9 membered heteroaryl, or 5 to 6 membered heteroaryl), and
(ii) alkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, substituted with at least one substituent selected from:
(a) oxo, halogen, —CCl3, —CBr3, —CF3, —CI3, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC(O)NHNH2, —NHC(O)NH2, —NHSO2H, —NHC(O)H, —NHC(O)OH, —NHOH, —OCCl3, —OCF3, —OCBr3, —OCl3, —OCHCl2, —OCH Br2, —OCHI2, —OCHF2, unsubstituted alkyl (e.g., C1-C8alkyl, C1-C6alkyl, or C1-C4alkyl), unsubstituted heteroalkyl (e.g., 2 to 8 membered heteroalkyl, 2 to 6 membered heteroalkyl, or 2 to 4 membered heteroalkyl), unsubstituted cycloalkyl (e.g., C3-C8cycloalkyl, C3-C6cycloalkyl, or C5-C6cycloalkyl), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered heterocycloalkyl, 3 to 6 membered heterocycloalkyl, or 5 to 6 membered heterocycloalkyl), unsubstituted aryl (e.g., C6-C10aryl, C10aryl, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered heteroaryl, 5 to 9 membered heteroaryl, or 5 to 6 membered heteroaryl), and
(b) alkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, substituted with at least one substituent selected from: oxo,
halogen, —CCl3, —CBr3, —CF3, —CI3, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC(O)NHNH2, —NHC(O)NH2, —NHSO2H, —NHC(O)H, —NHC(O)OH, —NHOH, —OCCl3, —OCF3, —OCBr3, —OCl3, —OCHCl2, —OCHBr2, —OCHI2, —OCHF2, unsubstituted alkyl (e.g., C1-C8alkyl, C1-C6alkyl, or C1-C4alkyl), unsubstituted heteroalkyl (e.g., 2 to 8 membered heteroalkyl, 2 to 6 membered heteroalkyl, or 2 to 4 membered heteroalkyl), unsubstituted cycloalkyl (e.g., C3-C8cycloalkyl, C3-C6cycloalkyl, or C5-C6cycloalkyl), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered heterocycloalkyl, 3 to 6 membered heterocycloalkyl, or 5 to 6 membered heterocycloalkyl), unsubstituted aryl (e.g., C6-C10aryl, C10aryl, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered heteroaryl, 5 to 9 membered heteroaryl, or 5 to 6 membered heteroaryl).
(SEQ ID NO: 331) MEDLDQSPLVSSSDSPPRPQPAFKYQFVREPEDEEEEEEEEEEDEDEDLE ELEVLERKPAAGLSAAPVPTAPAAGAPLMDFGNDFVPPAPRGPLPAAPPV APERQPSWDPSPVSSTVPAPSPLSAAAVSPSKLPEDDEPPARPPPPPPAS VSPQAEPVWTPPAPAPAAPPSTPAAPKRRGSSGSVDETLFALPAASEPVI RSSAENMDLKEQPGNTISAGQEDFPSVLLETAASLPSLSPLSAASFKEHE YLGNLSTVLPTEGTLQENVSEASKEVSEKAKTLLIDRDLTEFSELEYSEM GSSFSVSPKAESAVIVANPREEIIVKNKDEEEKLVSNNILHNQQELPTAL TKLVKEDEVVSSEKAKDSFNEKRVAVEAPMREEYADFKPFERVWEVKDSK EDSDMLAAGGKIESNLESKVDKKCFADSLEQTNHEKDSESSNDDTSFPST PEGIKDRSGAYITCAPFNPAATESIATNIFPLLGDPTSENKTDEKKIEEK KAQIVTEKNTSTKTSNPFLVAAQDSETDYVTTDNLTKVTEEVVANMPEGL TPDLVQEACESELNEVTGTKIAYETKMDLVQTSEVMQESLYPAAQLCPSF EESEATPSPVLPDIVMEAPLNSAVPSAGASVIQPSSSPLEASSVNYESIK HEPENPPPYEEAMSVSLKKVSGIKEEIKEPENINAALQETEAPYISIACD LIKETKLSAEPAPDFSDYSEMAKVEQPVPDHSELVEDSSPDSEPVDLFSD DSIPDVPQKQDETVMLVKESLTETSFESMIEYENKEKLSALPPEGGKPYL ESFKLSLDNTKDTLLPDEVSTLSKKEKIPLQMEELSTAVYSNDDLFISKE AQIRETETFSDSSPIETIDEFPTLISSKTDSFSKLAREYTDLEVSHKSEI ANAPDGAGSLPCTELPHDLSLKNIQPKVEEKISFSDDFSKNGSATSKVLL LPPDVSALATQAEIESIVKPKVLVKEAEKKLPSDTEKEDRSPSAIFSAEL SKTSVVDLLYWRDIKKTGVVFGASLELLLSLTVESIVSVTAYIALALLSV TISFRIYKGVIQAIQKSDEGHPFRAYLESEVAISEELVQKYSNSALGHVN CTIKELRRLFLVDDLVDSLKFAVLMWVFTYVGALFNGLTLLILALISLFS VPVIYERHQAQIDHYLGLANKNVKDAMAKIQAKIPGLKRKAE (SEQ ID NO: 332) MDEQSPDISSSHSGDERREPAQPGERKPWDDLDDVLDLTGGAGQFSQPFS GSHPARDIEEEEEDEEEERGAWKDSLEPSPVEEEPGSIDSISPVSPHSPA VPSAPMEEPERPPAPCTAPSGSVDENLFTLPAASAHLMHASADKIMEPYS TVSTGQEEFASVLLQSTASLSSLPSLSTDSSKEHAETVAFPTGLAATEAL QEPTDNMYSVSRITSHLPLSDNLESKALDQVKEEVIFSEKGYVVDHPTSQ QETISEEHAKLYSQSAKEMFSGMLQSVAPPHEEFTDIKEVYDPYVDFKPF MSSKSGDVGYEVSDVAEKFQVDVGRLNLESAVKHEEKSSEEMEIDSISDD ISPLTPELLPDSTDYDMFATVEQNIPFSFGGGHVAGNKTDEKKIEDIEAQ KTSVGFGLKVATVNPFYNESAQESEYVTTHVATHVSTKPEGPTPDIVQEA YESEAYDTGIPKQKYESNIDLVQTAANSVQEKVSPTAQAPARLEETDSVS SPVLPDIVMEAPLASALETVALKPDISPVGIKPPARVEKTKAEPEKPPSY EEAVTEVLQNQDLAAALGGSKQGAVVEETETPYISIACDLIKGTESVASG FTEFSKLKQNEFESQFMEPSDESSPDSECSEPSYKQWDSEVVQKEAFSIK TESVNAQSIIIPEQKQVFDQKSEESSPSKSYLDSFQPEICVSKATSDLFA KGLTTLLQEKPLQMEELDEGLSLEKIPCTKYSPVSESPEPRPSPVPEDLS SKLGDIQKEVLIAKQPEDKVQKNRSNLDFVPENTEFTPAVQKPDDSGKAV SDTFGGLDTTTKGGSAVHEVKVDKPKPPSKEDDGSKLPKKESKASTVSSS DFMNSVVDLIYWRDIKRSGVVFGASLFLLLSLSVFSIVSVLAYIALALLS VTISLRIYKGILQAIQKSEEGHPFRSILESNLAVPEDLVQKYCNVALNHV NCTVKELRHLFLVEDLVDSLKFAVLMWVFTYIGALFNGLTLLIVALISLF SIPVIYERHQTQVDHYLALVNKNLKSTSDLILSKVPGLKRKAE. (SEQ ID NO: 333) MGQVLPVFAHCKEAPSTASSTPDSTEGGNDDSDFRELHTAREFSEEDEET TSQDWGTPRELTFSYIAFDGVVGSGGRRDSTARRPRPQGRSVSEPRDQHP QPSLGDSLESIPSLSQSPEPGRRGDPDTAPPSERPLEDLRLRLDHLGWVA RGTGSGEDSSTSSSTPLEDEEPQEPNRLETGEAGEELDLRLRLAQPSSPE VLTPQLSPGSGTPQAGTPSPSRSRDSNSGPEEPLLEEEEKQWGPLEREPV RGQCLDSTDQLEFTVEPRLLGTAMEWLKTSLLLAVYKTVPILELSPPLWT AIGWVQRGPTPPTPVLRVLLKWAKSPRSSGVPSLSLGADMGSKVADLLYW KDTRTSGVVFTGLMVSLLCLLHFSIVSVAAHLALLLLCGTISLRVYRKVL QAVHRGDGANPFQAYLDVDLTLTREQTERLSHQITSRVVSAATQLRHFFL VEDLVDSLKLALLFYILTFVGAIFNGLTLLILGVIGLFTIPLLYRQHQAQ IDQYVGLVTNQLSHIKAKIRAKIPGTGALASAAAAVSGSKAKAE (SEQ ID NO: 334) MGQVLPVFAHCKEAPSTASSTPDSTEGGNDDSDFRELHTAREFSEEDEEE TTSQDWGTPRELTESYTAFDGVVGSGGRRDSTARRPRPQGRSVSEPRDQH PQPSLGDSLESIPSLSQSPEPGRRGDPDTAPPSERPLEDLRLRLDHLGWV ARGTGSGEDSSTSSSTPLEDEEPQEPNRLETGEAGEELDLRLRLAQPSSP EVLTPQLSPGSGTPQAGTPSPSRSRDSNSGPEEPLLEEEEKQWGPLEREP VRGQCLDSTDQLEFTVEPRLLVADLLYWKDTRTSGVVETGLMVSLLCLLH FSIVSVAAHLALLLLCGTISLRVYRKVLQAVHRGDGANPFQAYLDVDLTL TREQTERLSHQITSRVVSAATQLRHFELVEDLVDSLKLALLFYILTFVGA IENGLTLLILGVIGLETIPLLYRQHQAQIDQYVGLVTNQLSHIKAKIRAK IPGTGALASAAAAVSGSKAKAE (SEQ ID NO: 335) MAEPSAATQSHSISSSSFGAEPSAPGGGGSPGACPALGTKSCSSSCADSF VSSSSSQPVSLFSTSQEGLSSLCSDEPSSEIMTSSFLSSSEIHNTGLTIL HGEKSHVLGSQPILAKEGKDHLDLLDMKKMEKPQGTSNNVSDSSVSLAAG VHCDRPSIPASFPEHPAFLSKKIGQVEEQIDKETKNPNGVSSREAKTALD ADDRFTLLTAQKPPTEYSKVEGIYTYSLSPSKVSGDDVIEKDSPESPFEV IIDKAAFDKEFKDSYKESTDDFGSWSVHTDKESSEDISETNDKLFPLRNK EAGRYPMSALLSRQFSHTNAALEEVSRCVNDMHNFTNEILTWDLVPQVKQ QTDKSSDCITKTTGLDMSEYNSEIPVVNLKTSTHQKTPVCSIDGSTPITK STGDWAEASLQQENAITGKPVPDSLNSTKEESIKGVQGNMQKQDDTLAEL PGSPPEKCDSLGSGVATVKVVLPDDHLKDEMDWQSSALGEITEADSSGES DDTVIEDITADTSFENNKIQAEKPVSIPSAVVKTGEREIKEIPSCEREEK TSKNFEELVSDSELHQDQPDILGRSPASEAACSKVPDTNVSLEDVSEVAP EKPITTENPKLPSTVSPNVFNETEFSLNVTTSAYLESLHGKNVKHIDDSS PEDLIAAFTETRDKGIVDSERNAFKAISEKMTDFKTTPPVEVLHENESGG SEIKDIGSKYSEQSKETNGSEPLGVFPTQGTPVASLDLEQEQLTIKALKE LGERQVEKSTSAQRDAELPSEEVLKQTFTFAPESWPQRSYDILERNVKNG SDLGISQKPITIRETTRVDAVSSLSKTELVKKHVLARLLTDFSVHDLIFW RDVKKTGFVFGTTLIMLLSLAAFSVISVVSYLILALLSVTISFRIYKSVI QAVQKSEEGHPFKAYLDVDITLSSEAFHNYMNAAMVHINRALKLIIRLFL VEDLVDSLKLAVFMWLMTYVGAVFNGITLLILAELLIFSVPIVYEKYKTQ IDHYVGIARDQTKSIVEKIQAKLPGIAKKKAE (SEQ ID NO: 336) MAEPSAATQSHSISSSSFGAEPSAPGGGGSPGACPALGTKSCSSSCAEGL SSLCSDEPSSEIMTSSELSSSEIHNTGLTILHGEKSHVLGSQPILAKEGK DHLDLLDMKKMEKPQGTSNNVSDSSVSLAAGVHCDRPSIPASEPEHPAEL SKKIGQVEEQIDKETKNPNGVSSREAKTALDADDRFTLLTAQKPPTEYSK VEGIYTYSLSPSKVSGDDVIEKDSPESPFEVIIDKAAFDKEFKDSYKEST DDEGSWSVHTDKESSEDISETNDKLEPLRNKEAGRYPMSALLSRQFSHTN AALEEVSRCVNDMHNFTNEILTWDLVPQVKQQTDKSSDCITKTTGLDMSE YNSEIPVVNLKTSTHQKTPVCSIDGSTPITKSTGDWAEASLQQENAITGK PVPDSLNSTKEFSIKGVQGNMQKQDDTLAELPGSPPEKCDSLGSGVATVK VVLPDDHLKDEMDWQSSALGEITEADSSGESDDTVIEDITADTSFENNKI QAEKPVSIPSAVVKTGEREIKEIPSCEREEKTSKNFEELVSDSELHQDQP DILGRSPASEAACSKVPDTNVSLEDVSEVAPEKPITTENPKLPSTVSPNV FNETEFSLNVITSAYLESLHGKNVKHIDDSSPEDLIAAFTETRDKGIVDS ERNAFKAISEKMTDEKTIPPVEVLHENESGGSEIKDIGSKYSEQSKETNG SEPLGVEPTQGTPVASLDLEQEQLTIKALKELGERQVEKSTSAQRDAELP SEEVLKQTFTFAPESWPQRSYDILERNVKNGSDLGISQKPITIRETTRVD AVSSLSKTELVKKHVLARLLTDFSVHDLIFWRDVKKTGFVFGTTLIMLLS LAAFSVISVVSYLILALLSVTISFRIYKSVIQAVQKSEEGHPFKAYLDVD ITLSSEAFHNYMNAAMVHINRALKLIIRLFLVEDLVDSLKLAVFMWLMTY VGAVENGITLLILAELLIFSVPIVYEKYKTQIDHYVGIARDQTKSIVEKI QAKLPGIAKKKAE (SEQ ID NO: 337) MAEPSAATQSHSISSSSFGAEPSAPGGGGSPGACPALGTKSCSSSCAVHD LIFWRDVKKTGFVFGTTLIMLLSLAAFSVISVVSYLILALLSVTISFRIY KSVIQAVQKSEEGHPFKAYLDVDITLSSEAFHNYMNAAMVHINRALKLII RLFLVEDLVDSLKLAVFMWLMTYVGAVFNGITLLILAELLIFSVPIVYEK YKTQIDHYVGIARDQTKSIVEKIQAKLPGIAKKKAE (SEQ ID NO: 338) MAAPGDPQDELLPLAGPGSQWLRHRGEGENEAVTPKGATPAPQAGEPSPG LGARAREAASREAGSGPARQSPVAMETASTGVAGVSSAMDHTFSTTSKDG EGSCYTSLISDICYPPQEDSTYFTGILQKENGHVTISESPEELGTPGPSL PDVPGIESRGLFSSDSGIEMTPAESTEVNKILADPLDQMKAEAYKYIDIT RPEEVKHQEQHHPELEDKDLDFKNKDTDISIKPEGVREPDKPAPVEGKII KDHLLEESTFAPYIDDLSEEQRRAPQITTPVKITLTEIEPSVETTTQEKT PEKQDICLKPSPDTVPTVTVSEPEDDSPGSITPPSSGTEPSAAESQGKGS ISEDELITAIKEAKGLSYETAENPRPVGQLADRPEVKARSGPPTIPSPLD HEASSAESGDSEIELVSEDPMAAEDALPSGYVSFGHVGGPPPSPASPSIQ YSILREEREAELDSELIIESCDASSASEESPKREQDSPPMKPSALDAIRE ETGVRAEERAPSRRGLAEPGSFLDYPSTEPQPGPELPPGDGALEPETPML PRKPEEDSSSNQSPAATKGPGPLGPGAPPPLLFLNKQKAIDLLYWRDIKQ TGIVEGSFLLLLFSLTQFSVVSVVAYLALAALSATISFRIYKSVLQAVQK TDEGHPFKAYLELEITLSQEQIQKYTDCLQFYVNSTLKELRRLFLVQDLV DSLKFAVLMWLLTYVGALFNGLTLLLMAVVSMFTLPVVYVKHQAQIDQYL GLVRTHINAVVAKIQAKIPGAKRHAE (SEQ ID NO: 339) MAAEDALPSGYVSFGHVGGPPPSPASPSIQYSILREEREAELDSELIIES CDASSASEESPKREQDSPPMKPSALDAIREETGVRAEERAPSRRGLAEPG SFLDYPSTEPQPGPELPPGDGALEPETPMLPRKPEEDSSSNQSPAATKGP GPLGPGAPPPLLFLNKQKAIDLLYWRDIKQTGIVEGSFLLLLFSLTQFSV VSVVAYLALAALSATISFRIYKSVLQAVQKTDEGHPFKAYLELEITLSQE QIQKYTDCLQFYVNSTLKELRRLFLVQDLVDSLKFAVLMWLLTYVGALFN GLTLLLMAVVSMFTLPVVYVKHQAQIDQYLGLVRTHINAVVAKIQAKIPG AKRHAE (SEQ ID NO: 340) MQATADSTKMDCVWSNWKSQATDLLYWRDIKQTGIVFGSFLLLLFSLTQF SVVSVVAYLALAALSATISFRIYKSVLQAVQKTDEGHPFKAYLELEITLS QEQIQKYTDCLQFYVNSTLKELRRLFLVQDLVDSLKFAVLMWLLTYVGAL FNGLTLLLMAVVSMFTLPVVYVKHQAQIDQYLGLVRTHINAVVAKIQAKI PGAKRHAE (SEQ ID NO: 341) MEDLDQSPLVSSSDSPPRPQPAFKYQFVREPEDEEEEEEEEEEDEDEDLE ELEVLERKPAAGLSAAPVPTAPAAGAPLMDFGNDFVPPAPRGPLPAAPPV APERQPSWDPSPVSSTVPAPSPLSAAAVSPSKLPEDDEPPARPPPPPPAS VSPQAEPVWTPPAPAPAAPPSTPAAPKRRGSSGSVDETLFALPAASEPVI RSSAENMDLKEQPGNTISAGQEDFPSVLLETAASLPSLSPLSAASEKEHE YLGNLSTVLPTEGTLQENVSEASKEVSEKAKTLLIDRDLTEFSELEYSEM GSSFSVSPKAESAVIVANPREEIIVKNKDEEEKLVSNNILHNQQELPTAL TKLVKEDEVVSSEKAKDSFNEKRVAVEAPMREEYADFKPFERVWEVKDSK EDSDMLAAGGKIESNLESKVDKKCFADSLEQTNHEKDSESSNDDTSFPST PEGIKDRSGAYITCAPFNPAATESIATNIFPLLGDPTSENKTDEKKIEEK KAQIVTEKNTSTKTSNPFLVAAQDSETDYVTTDNLTKVTEEVVANMPEGL TPDLVQEACESELNEVTGTKIAYETKMDLVQTSEVMQESLYPAAQLCPSF EESEATPSPVLPDIVMEAPLNSAVPSAGASVIQPSSSPLEASSVNYESIK HEPENPPPYEEAMSVSLKKVSGIKEEIKEPENINAALQETEAPYISIACD LIKETKLSAEPAPDFSDYSEMAKVEQPVPDHSELVEDSSPDSEPVDLFSD DSIPDVPQKQDETVMLVKESLTETSFESMIEYENKEKLSALPPEGGKPYL ESFKLSLDNTKDTLLPDEVSTLSKKEKIPLQMEELSTAVYSNDDLFISKE AQIRETETESDSSPIEIIDEFPTLISSKTDSFSKLAREYTDLEVSHKSEI ANAPDGAGSLPCTELPHDLSLKNIQPKVEEKISFSDDFSKNGSATSKVLL LPPDVSALATQAEIESIVKPKVLVKEAEKKLPSDTEKEDRSPSAIFSAEL SKTSVVDLLYWRDIKKTGVVFGASLELLLSLTVESIVSVTAYIALALLSV TISFRIYKGVIQATQKSDEGHPFRAYLESEVAISEELVQKYSNSALGHVN CTIKELRRLFLVDDLVDSLKFAVLMWVETYVGALENGLTLLILALISLFS VPVIYERHQAQIDHYLGLANKNVKDAMAKIQAKIPGLKRKAE (SEQ ID NO: 342) MEDLDQSPLVSSSDSPPRPQPAFKYQFVREPEDEEEEEEEEEEDEDEDLE ELEVLERKPAAGLSAAPVPTAPAAGAPLMDFGNDFVPPAPRGPLPAAPPV APERQPSWDPSPVSSTVPAPSPLSAAAVSPSKLPEDDEPPARPPPPPPAS VSPQAEPVWTPPAPAPAAPPSTPAAPKRRGSSGSVVVDLLYWRDIKKTGV VFGASLFLLLSLIVESIVSVTAYIALALLSVTISFRIYKGVIQAIQKSDE GHPFRAYLESEVAISEELVQKYSNSALGHVNCTIKELRRLFLVDDLVDSL KFAVLMWVFTYVGALFNGLTLLILALISLFSVPVIYERHQAQIDHYLGLA NKNVKDAMAKIQAKIPGLKRKAE (SEQ ID NO: 343) MDGQKKNWKDKVVDLLYWRDIKKTGVVFGASLFLLLSLTVFSIVSVTAYI ALALLSVTISFRIYKGVIQAIQKSDEGHPFRAYLESEVAISEELVQKYSN SALGHVNCTIKELRRLFLVDDLVDSLKFAVLMWVFTYVGALFNGLTLLIL ALISLFSVPVIYERHQAQIDHYLGLANKNVKDAMAKIQAKIPGLKRKAE II. Compounds
halogen, —CX203, —CHX202, —CH2X20, —OCX203, —OCH2X20, —OCHX202, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O) NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X20is independently —F, —Cl, —Br, or —I. In embodiments, R20is independently unsubstituted methyl. In embodiments, R20is independently unsubstituted ethyl.
halogen, —CX213, —CHX212, —CH2X21, —OCX213, —OCH2X21, —OCHX212, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X21is independently —F, —Cl, —Br, or —I. In embodiments, R21is independently unsubstituted methyl. In embodiments, R21is independently unsubstituted ethyl.
halogen, —CX21A3, —CHX21A2, —CH2X21A, —OCX21A3, —OCH2X21A, —OCHX21A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X21Ais independently —F, —Cl, —Br, or —I. In embodiments, R21Ais independently unsubstituted methyl. In embodiments, R21Ais independently unsubstituted ethyl.
halogen, —CX20B3, —CHX20B2, —CH2X20B, —OCX20B3, —OCH2X20B, —OCHX20B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X20Bis independently —F, —Cl, —Br, or —I. In embodiments, R20Bis independently unsubstituted methyl. In embodiments, R20Bis independently unsubstituted ethyl.
halogen, —CX21B3, —CHX21B2, —CH2X21B, —OCX21B3, —OCH2X21B, —OCHX21B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X21Bis independently —F, —Cl, —Br, or —I. In embodiments, R21Bis independently unsubstituted methyl. In embodiments, R21Bis independently unsubstituted ethyl.
halogen, —CX20C3, —CHX20C2, —CH2X20C, —OCX20C3, —OCH2X20C, —OCHX20C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X20Cis independently —F, —Cl, —Br, or —I. In embodiments, R20Cis independently unsubstituted methyl. In embodiments, R20Cis independently unsubstituted ethyl.
halogen, —CX21C3, —CHX21C2, —CH2X21C, —OCX21C3, —OCH2X21C, —OCHX21C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X21Cis independently —F, —Cl, —Br, or —I. In embodiments, R21Cis independently unsubstituted methyl. In embodiments, R21Cis independently unsubstituted ethyl.
halogen, —CX20D3, —CHX20D2, —CH2X20D, —OCX20D3, —OCH2X20D, —OCHX20D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X20Dis independently —F, —Cl, —Br, or —I. In embodiments, R20Dis independently unsubstituted methyl. In embodiments, R20Dis independently unsubstituted ethyl.
halogen, —CX21D3, —CHX21D2, —CH2X21D, —OCX21D3, —OCH2X21D, —OCHX21D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X21Dis independently —F, —Cl, —Br, or —I. In embodiments, R21Dis independently unsubstituted methyl. In embodiments, R21Dis independently unsubstituted ethyl.
halogen, —CX233, —CHX232, —CH2X23, —OCX233, —OCH2X23, —OCHX232, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X23is independently —F, —Cl, —Br, or —I. In embodiments, R23is independently unsubstituted methyl. In embodiments, R23is independently unsubstituted ethyl.
halogen, —CX243, —CHX242, —CH2X24, —OCX243, —OCH2X24, —OCHX242, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X24is independently —F, —Cl, —Br, or —I. In embodiments, R24is independently unsubstituted methyl. In embodiments, R24is independently unsubstituted ethyl.
halogen, —CX23A3, —CHX23A2, —CH2X23A, —OCX23A3, —OCH2X23A, —OCHX23A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X23Ais independently —F, —Cl, —Br, or —I. In embodiments, R23Ais independently unsubstituted methyl. In embodiments, R23Ais independently unsubstituted ethyl.
halogen, —CX24A3, —CHX24A2, —CH2X24A, —OCX24A3, —OCH2X24A, —OCHX24A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X24Ais independently —F, —Cl, —Br, or —I. In embodiments, R24Ais independently unsubstituted methyl. In embodiments, R24Ais independently unsubstituted ethyl.
halogen, —CX23B3, —CHX23B2, —CH2X23B, —OCX23B3, —OCH2X23B, —OCHX23B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X23Bis independently —F, —Cl, —Br, or —I. In embodiments, R23Bis independently unsubstituted methyl. In embodiments, R23Bis independently unsubstituted ethyl.
halogen, —CX24B3, —CHX24B2, —CH2X24B, —OCX24B3, —OCH2X24B, —OCHX24B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X24Bis independently —F, —Cl, —Br, or —I. In embodiments, R24Bis independently unsubstituted methyl. In embodiments, R24Bis independently unsubstituted ethyl.
halogen, —CX23C3, —CHX23C2, —CH2X23C, —OCX23C3, —OCH2X23C, —OCHX23C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X23Cis independently —F, —Cl, —Br, or —I. In embodiments,
halogen, —CX24C3, —CHX24C2, —CH2X24C, —OCX24C3, —OCH2X24C, —OCHX24C2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2H2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X24Cis independently —F, —Cl, —Br, or —I. In embodiments,
halogen, —CX23D3, —CHX23D2, —CH2X23D, —OCX23D3, —OCH2X23D, —OCHX23D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X23Dis independently —F, —Cl, —Br, or —I. In embodiments,
halogen, —CX24D3, —CHX24D2, —CH2X24D, —OCX24D3, —OCH2X24D, —OCHX24D2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C12, C6-C10, or phenyl), or unsubstituted heteroaryl (e.g., 5 to 12 membered, 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X24Dis independently —F, —Cl, —Br, or —I. In embodiments, R24Dis independently unsubstituted methyl. In embodiments, R24Dis independently unsubstituted ethyl.
halogen, —CX353, —CHX352, —CH2X35, —OCX353, —OCH2X35, —OCHX352, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X35is independently —F, —Cl, —Br, or —I. In embodiments, R35is independently unsubstituted methyl. In embodiments, R35is independently unsubstituted ethyl.
halogen, —CX363, —CHX362, —CH2X36, —OCX363, —OCH2X36, —OCHX362, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X36is independently —F, —Cl, —Br, or —I. In embodiments, R36is independently unsubstituted methyl. In embodiments, R36is independently unsubstituted ethyl.
halogen, —CX293, —CHX292, —CH2X29, —OCX293, —OCH2X29, —OCHX292, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X29is independently —F, —Cl, —Br, or —I. In embodiments, R29is independently unsubstituted methyl. In embodiments, R29is independently unsubstituted ethyl.
halogen, —CX303, —CHX302, —CH2X30, —OCX303, —OCH2X30, —OCHX302, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X30is independently —F, —Cl, —Br, or —I. In embodiments, R30is independently unsubstituted methyl. In embodiments, R30is independently unsubstituted ethyl.
halogen, —CX29A3, —CHX29A2, —CH2X29A, —OCX29A3, —OCH2X29A, —OCHX29A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X29Ais independently —F, —Cl, —Br, or —I. In embodiments, R29Ais independently unsubstituted methyl. In embodiments, R29Ais independently unsubstituted ethyl.
halogen, —CX30A3, —CHX30A2, —CH2X30A, —OCX30A3, —OCH2X30A, —OCHX30A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X30Ais independently —F, —Cl, —Br, or —I. In embodiments, R30Ais independently unsubstituted methyl. In embodiments, R30Ais independently unsubstituted ethyl.
halogen, —CX29B3, —CHX29B2, —CH2X29B, —OCX29B3, —OCH2X29B, —OCHX29B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X29Bis independently —F, —Cl, —Br, or —I. In embodiments, R29Bis independently unsubstituted methyl. In embodiments, R29Bis independently unsubstituted ethyl.
halogen, —CX30B3, —CHX30B2, —CH2X30B, —OCX30B3, —OCH2X30B, —OCHX30B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X30Bis independently —F, —Cl, —Br, or —I. In embodiments, R30Bis independently unsubstituted methyl. In embodiments, R30Bis independently unsubstituted ethyl.
halogen, —CX383, —CHX382, —CH2X38, —OCX383, —OCH2X38, —OCHX382, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X38is independently —F, —Cl, —Br, or —I. In embodiments, R38is independently unsubstituted methyl. In embodiments, R38is independently unsubstituted ethyl.
halogen, —CX393, —CHX392, —CH2X39, —OCX393, —OCH2X39, —OCHX392, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X39is independently —F, —Cl, —Br, or —I. In embodiments, R39is independently unsubstituted methyl. In embodiments, R39is independently unsubstituted ethyl.
halogen, —CX323, —CHX322, —CH2X32, —OCX323, —OCH2X32, —OCHX322, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X32is independently —F, —Cl, —Br, or —I. In embodiments, R32is independently unsubstituted methyl. In embodiments, R32is independently unsubstituted ethyl.
halogen, —CX333, —CHX332, —CH2X33, —OCX333, —OCH2X33, —OCHX332, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X33is independently —F, —Cl, —Br, or —I. In embodiments, R33is independently unsubstituted methyl. In embodiments, R33is independently unsubstituted ethyl.
halogen, —CX32A3, —CHX32A2, —CH2X32A, —OCX32A3, —OCH2X32A, —OCHX32A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X32Ais independently —F, —Cl, —Br, or —I. In embodiments, R32Ais independently unsubstituted methyl. In embodiments, R32Ais independently unsubstituted ethyl.
halogen, —CX33A3, —CHX33A2, —CH2X33A, —OCX33A3, —OCH2X33A, —OCHX33A2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X33Ais independently —F, —Cl, —Br, or —I. In embodiments, R33Ais independently unsubstituted methyl. In embodiments, R33Ais independently unsubstituted ethyl.
halogen, —CX32B3, —CHX32B2, —CH2X32B, —OCX32B3, —OCH2X32B, —OCHX32B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X32Bis independently —F, —Cl, —Br, or —I. In embodiments, R32Bis independently unsubstituted methyl. In embodiments, R32Bis independently unsubstituted ethyl.
halogen, —CX33B3, —CHX33B2, —CH2X33B, —OCX33B3, —OCH2X33B, —OCHX33B2, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O)NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, unsubstituted alkyl (e.g., C1-C8, C1-C6, C1-C4, or C1-C2), unsubstituted heteroalkyl (e.g., 2 to 8 membered, 2 to 6 membered, 4 to 6 membered, 2 to 3 membered, or 4 to 5 membered), unsubstituted cycloalkyl (e.g., C3-C8, C3-C6, C4-C6, or C5-C6), unsubstituted heterocycloalkyl (e.g., 3 to 8 membered, 3 to 6 membered, 4 to 6 membered, 4 to 5 membered, or 5 to 6 membered), unsubstituted aryl (e.g., C6-C10or phenyl), or unsubstituted heteroaryl (e.g., 5 to 10 membered, 5 to 9 membered, or 5 to 6 membered). X33Bis independently —F, —Cl, —Br, or —I. In embodiments, R33Bis independently unsubstituted methyl. In embodiments, R33Bis independently unsubstituted ethyl.
III. Pharmaceutical Compositions
IV. Methods of Treatment
V. Methods of Inhibition
VI. Reticulon 4 Protein
VII. Embodiments
Embodiment P1
Embodiment P2
Embodiment P3
Embodiment P4
Embodiment P5
Embodiment P6
Embodiment P7
Embodiment P8
Embodiment P9
Embodiment P10
Embodiment P11
Embodiment P12
Embodiment P13
Embodiment P14
Embodiment P15
Embodiment P16
Embodiment P17
Embodiment P18
Embodiment P19
Embodiment P20
Embodiment P21
Embodiment P22
—NHC(O)NR16AR16B, —N(O)m16, —NR16AR16B, —C(O)R16C, —C(O)—OR16C, —C(O)NR16AR16B, —OR16D, —NR16ASO2R16D, —NR16AC(O)R16C, —NR16AC(O)OR16C, —NR16AOR16C, —OCX163, —OCHX162, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl;
—NHC(O)NR17AR17B, —N(O)m17, —NR17AR17B, —C(O)R17C, —C(O)—OR17C, —C(O)NR17AR17B, —OR17D, —NR17ASO2R17D, —NR17AC(O)R17C, —NR17AC(O)OR17C, —NR17AOR17C, —OCX173, —OCHX172, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl;
Embodiment P23
Embodiment P24
Embodiment P25
Embodiment P26
Embodiment P27
Embodiment P28
Embodiment P29
Embodiment P30
Embodiment P31
Embodiment P32
Embodiment P33
Embodiment P34
Embodiment P35
Embodiment P36
Embodiment P37
Embodiment P38
Embodiment P39
Embodiment P40
VIII. Additional Embodiments
Embodiment 1
Embodiment 2
Embodiment 3
Embodiment 4
Embodiment 5
Embodiment 6
Embodiment 7
Embodiment 8
Embodiment 9
Embodiment 10
Embodiment 11
Embodiment 12
Embodiment 13
Embodiment 14
Embodiment 15
Embodiment 16
Embodiment 17
Embodiment 18
Embodiment 19
Embodiment 20
Embodiment 21
Embodiment 22
—NHC(O)NR16AR16B, —N(O)m16, —NR16AR16B, —C(O)R16C, —C(O)—OR16C, —C(O)NR16AR16B, —OR16D, —NR16ASO2R16D, —NR16AC(O)R16C, —NR16AC(O)OR16C, —NR16AOR16C, —OCX163, —OCHX162, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl;
—NHC(O)NR17AR17B, —N(O)m17, —NR17AR17B, —C(O)R17C, —C(O)—OR17C, —C(O)NR17AR17B, —OR17D, —NR17ASO2R17D, —NR17AC(O)R17C, —NR17AC(O)OR17C, —NR17AOR17C, —OCX173, —OCHX172, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl;
Embodiment 23
Embodiment 24
Embodiment 25
Embodiment 26
Embodiment 27
bond, —S(O)2—, —NR4—, —O—, —S—, —C(O)—, —C(O)NR4—, —NR4C(O)NR4C(O)NH—, —NHC(O)NR4—, —C(O)O—, —OC(O)—, substituted or unsubstituted alkylene, substituted or unsubstituted heteroalkylene, substituted or unsubstituted cycloalkylene, substituted or unsubstituted heterocycloalkylene, substituted or unsubstituted aryl ene, or substituted or unsubstituted heteroarylene;
Embodiment 28
Embodiment 29
Embodiment 30
Embodiment 31
Embodiment 32
Embodiment 33
Embodiment 34
Embodiment 35
Embodiment 36
Embodiment 37
Embodiment 38
Embodiment 39
Embodiment 40
Embodiment 41
Embodiment 42
Embodiment 43
Embodiment 44
Embodiment 45
Embodiment 46
Embodiment 47
Embodiment 48
Embodiment 49
—NHC(O)NR15AR15B, —N(O)m15, —NR15AR15B, —C(O)R15C, —C(O)—OR15C, —C(O)NR15AR15B, —OR15D, —NR15ASO2R15D, —NR15AC(O)R15C, —NR15AC(O)OR15C, —NR15AOR15C, —OCX153, —OCHX152, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl;
—NHC═(O)NHNR16AR16B,
—NHC(O)NR16AR16B,
—NHC(O)m16, —NR16AR16B, —C(O)R16C, —C(O)—OR16C, —C(O)NR16AR16B, —OR16D, —NR16ASO2R16D, —NR16AC(O)R16C, —NR16AC(O)OR16C, —NR16AOR16C, —OCX163, —OCHX162, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl;
—NHC═(O)NHNR17AR17B,
—NHC(O)NR17AR17B, —N(O)m17, —NR17AR17B, —C(O)R17C, —C(O)—OR17C, —C(O)NR17AR17B, —OR17D, —NR17ASO2R17D, —NR17AC(O)R17C, —NR17AC(O)OR17C, —NR17AOR17C, —OCX173, —OCHX172, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl;
Embodiment 50
Embodiment 51
Embodiment 52
Embodiment 53
Embodiment 54
Embodiment 55
Embodiment 56
Embodiment 57
Embodiment 58
Embodiment 59
Embodiment 60
Embodiment 61
Embodiment 62
Embodiment 63
Embodiment 64
Embodiment 65
Embodiment 66
Embodiment 67
Embodiment 68
Embodiment 69
Embodiment 70
Embodiment 71
Embodiment 72
Embodiment 73
Embodiment 74
Embodiment 75
Embodiment 76
Embodiment 77
Embodiment 78
Embodiment 79
Embodiment 80
Embodiment 81
Embodiment 82
Embodiment 83
—NHC(O)NR15AR15B, —N(O)m15, —NR15AR15B, —C(O)R15C, —C(O)—OR15C, —C(O)NR15AR15B, —OR15D, —NR15ASO2R15D, —NR15AC(O)R15C, —NR15AC(O)OR15C, —NR15AOR15C, —OCX153, —OCHX152, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl;
—NHC(O)NR16AR16B, —N(O)m16, —NR16AR16B, —C(O)R16C, —C(O)—OR16C, —C(O)NR16AR16B, —OR16D, —NR16ASO2R16D, —NR16AC(O)R16C, —NR16AC(O)OR16C, —NR16AOR16C, —OCX163, —OCHX162, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl;
—NHC(O)NR17AR17B, —N(O)m17, —NR17AR17B, —C(O)R17C, —C(O)—OR17C, —C(O)NR17AR17B, —OR17D, —NR17ASO2R17D, —NR17AC(O)R17C, —NR17AC(O)OR17C, —NR17AOR17C, —OCX173,
—OCHX172, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl;
Embodiment 84
Embodiment 85
Embodiment 86
IX. Examples
I. Chemoproteomics-Enabled Covalent Ligand Screen Reveals a Cysteine Hotspot in Reticulon 4 that Impairs ER Morphology and Cancer Pathogenicity
Covalent ligand screening data. SW620 cells were treated with either DMSO or cysteine-reactive fragment (50 μM) for 48 h after which serum-free cell survival or proliferation were assessed by Hoescht staining. Shown are average and sem values from n = 3/group. SW620 survival Ave sem p value DKM 3-30 0.31 0.06 2.21E−02 DKM 3-16 0.34 0.04 2.28E−02 DKM 2-40 0.36 0.03 3.11E−03 DKM 2-91 0.38 0.03 8.97E−04 DKM 2-101 0.39 0.06 7.57E−03 DKM 3-10 0.41 0.06 7.05E−03 DKM 2-94 0.43 0.05 2.39E−03 DKM 2-76 0.44 0.07 7.08E−03 DKM 2-80 0.47 0.08 9.35E−03 TRH 1-55 0.47 0.04 2.37E−03 TRH 1-12 0.49 0.03 7.24E−03 DKM 3-7 0.51 0.04 5.46E−02 DKM 2-95 0.52 0.10 3.89E−02 DKM 3-43 0.52 0.08 7.87E−02 DKM 2-98 0.52 0.13 3.64E−02 DKM 3-36 0.54 0.09 9.56E−02 TRH 1-32 0.55 0.10 1.00E−01 DKM 3-41 0.55 0.09 9.87E−02 DKM 3-70 0.57 0.11 1.26E−01 DKM 2-37 0.57 0.18 1.09E−01 TRH 1-50 0.58 0.07 9.26E−02 DKM 3-5 0.59 0.04 1.21E−02 DKM 2-108 0.61 0.02 1.36E−02 DKM 3-31 0.65 0.08 1.64E−01 DKM 2-83 0.66 0.07 3.18E−02 DKM 2-59 0.66 0.07 4.92E−02 TRH 1-53 0.69 0.07 4.08E−02 DKM 3-32 0.70 0.07 2.03E−01 DKM 2-93 0.74 0.06 4.79E−02 DKM 2-84 0.74 0.07 7.66E−02 DKM 2-113 0.75 0.03 6.08E−02 DKM 3-9 0.75 0.03 2.44E−01 DKM 2-114 0.76 0.03 7.42E−02 DKM 3-13 0.77 0.06 2.89E−01 DKM 2-34 0.78 0.02 8.76E−02 DKM 2-47 0.78 0.05 1.16E−01 DKM 3-29 0.79 0.05 3.11E−01 DKM 2-49 0.80 0.06 1.60E−01 DKM 2-71 0.81 0.08 3.96E−01 DKM 2-43 0.84 0.06 2.27E−01 DKM 2-107 0.86 0.05 2.62E−01 DKM 2-67 0.87 0.14 6.20E−01 DKM 2-50 0.89 0.02 3.05E−01 DKM 2-31 0.89 0.04 3.69E−01 DKM 2-48 0.90 0.05 4.00E−01 DKM 2-32 0.91 0.11 5.55E−01 DKM 2-33 0.92 0.08 5.36E−01 DKM 2-52 0.92 0.10 7.25E−01 DKM 2-39 0.93 0.05 5.71E−01 TRH 1-13 0.94 0.05 6.20E−01 DKM 2-72 0.96 0.06 8.49E−01 DKM 2-58 0.98 0.05 8.50E−01 TRH 1-19 1.01 0.06 9.16E−01 DKM 2-120 1.02 0.06 8.83E−01 DKM 3-42 1.04 0.21 9.09E−01 DKM 2-42 1.04 0.04 7.11E−01 DKM 2-97 1.06 0.27 8.33E−01 DKM 2-60 1.08 0.16 7.51E−01 DKM 2-86 1.09 0.13 6.45E−01 DKM 2-110 1.12 0.17 5.54E−01 TRH 1-20 1.14 0.05 2.66E−01 DKM 2-62 1.15 0.09 2.76E−01 DKM 3-11 1.20 0.18 5.32E−01 DKM 3-4 1.22 0.03 8.53E−02 DKM 2-116 1.23 0.20 3.58E−01 DKM 2-102 1.24 0.05 9.50E−02 DKM 3-12 1.29 0.09 2.49E−01 DKM 2-111 1.33 0.23 2.40E−01 DKM 2-103 1.35 0.06 4.12E−02 DKM 2-100 1.38 0.03 1.54E−02 DKM 2-109 1.40 0.02 1.17E−02 TRH 1-27 1.44 0.24 2.79E−01 DKM 2-106 1.44 0.25 1.75E−01 DKM 3-8 1.52 0.09 6.85E−02 TRH 1-54 1.75 0.24 1.01E−01 DKM 2-94 0.32 0.03 2.69E−04 DKM 2-71 0.41 0.01 4.06E−06 DKM 2-98 0.42 0.02 6.51E−06 DKM 2-83 0.48 0.03 9.33E−04 DKM 2-80 0.48 0.02 5.02E−04 DKM 2-76 0.50 0.03 1.02E−03 DKM 3-70 0.53 0.02 2.15E−05 DKM 2-52 0.53 0.02 5.63E−05 TRH 1-55 0.53 0.09 1.68E−02 DKM 3-30 0.54 0.03 1.50E−02 DKM 2-93 0.57 0.04 2.66E−03 DKM 2-91 0.59 0.03 2.18E−03 DKM 3-16 0.60 0.03 2.62E−02 TRH 1-53 0.72 0.06 3.32E−02 DKM 2-67 0.73 0.01 3.21E−06 DKM 2-37 0.73 0.02 1.45E−03 DKM 2-59 0.74 0.03 3.06E−03 TRH 1-50 0.76 0.04 8.71E−03 DKM 3-10 0.79 0.10 2.12E−01 DKM 3-5 0.82 0.03 3.03E−03 DKM 2-84 0.84 0.05 3.17E−02 DKM 2-48 0.85 0.04 3.85E−02 DKM 2-95 0.85 0.01 6.52E−02 TRH 1-12 0.89 0.07 1.86E−01 DKM 2-116 0.90 0.03 2.11E−02 DKM 3-41 0.93 0.05 6.18E−01 DKM 3-13 0.94 0.03 6.02E−01 DKM 3-43 0.94 0.01 6.04E−01 DKM 3-32 0.95 0.03 6.55E−01 DKM 2-62 0.95 0.02 5.64E−02 DKM 2-110 0.95 0.06 5.12E−01 DKM 2-108 0.95 0.11 7.80E−01 DKM 2-120 0.96 0.01 4.87E−02 DKM 2-109 0.96 0.02 1.74E−01 DKM 2-97 0.96 0.05 5.01E−01 DKM 2-101 0.96 0.05 6.87E−01 DKM 3-36 0.97 0.04 8.31E−01 DKM 2-40 0.98 0.01 4.54E−01 DKM 2-107 0.99 0.09 9.13E−01 DKM 3-31 0.99 0.04 9.63E−01 DKM 2-100 1.00 0.04 9.31E−01 DKM 3-7 1.00 0.01 9.83E−01 TRH 1-32 1.00 0.03 9.60E−01 DKM 2-72 1.00 0.03 9.74E−01 DKM 3-9 1.00 0.10 9.91E−01 DKM 2-106 1.00 0.02 9.02E−01 DKM 2-60 1.00 0.05 9.64E−01 DKM 2-86 1.02 0.13 8.97E−01 DKM 3-8 1.05 0.00 6.93E−01 DKM 2-34 1.05 0.07 5.50E−01 DKM 2-111 1.06 0.01 2.53E−02 DKM 3-12 1.06 0.06 6.44E−01 DKM 2-49 1.09 0.02 6.51E−02 DKM 2-39 1.10 0.03 6.16E−02 DKM 2-114 1.11 0.08 4.12E−01 DKM 2-47 1.12 0.02 2.38E−02 DKM 2-103 1.12 0.11 4.76E−01 DKM 3-42 1.12 0.07 2.68E−01 DKM 2-32 1.14 0.12 3.13E−01 DKM 2-33 1.15 0.11 2.44E−01 DKM 2-58 1.16 0.04 2.34E−02 DKM 2-31 1.16 0.09 1.75E−01 DKM 3-11 1.18 0.02 1.79E−01 TRH 1-19 1.19 0.07 1.47E−01 DKM 3-4 1.20 0.10 2.19E−01 DKM 3-29 1.21 0.04 1.76E−02 TRH 1-20 1.21 0.11 2.47E−01 TRH 1-27 1.25 0.08 6.96E−02 DKM 2-43 1.28 0.03 2.17E−03 TRH 1-13 1.28 0.04 4.17E−03 DKM 2-50 1.30 0.01 4.14E−04 DKM 2-102 1.33 0.05 1.65E−02 DKM 2-42 1.37 0.00 1.52E−04 TRH 1-54 1.39 0.01 1.49E−06 DKM 2-113 1.47 0.07 1.23E−02 IsoTOP-ABPP analyses of DKM 3-5 and DKM 3-30 in SW620 cells. SW620 cell proteomes were pre-treated with DMSO or DKM 3-5 (50 μM) or DKM 3-30 (50 μM) for 30 min at 37° C. prior to labeling of proteomes with IAyne (100 μM) for 1h at room temperature. Proteomes were then subjected to copper-catalyzed azide-alkyne cycloaddition with a biotin-azide tag bearing an isotopically light (for DMSO-treated) versus heavy (for ligand-treated) valine and a TEV protease recognition site. Proteomes were then mixed in a 1: 1 ratio and probe-modified peptides were enriched and released by TEV protease for subsequent quantitative proteomic analysis. The data was filtered for only those probe-modified peptides that were identified in at least 2 out of 3 runs. Ratios for the same redundant probe-modified peptides and peptides across runs were averaged. Top hits of covalent ligands were confirmed to have more than one light to heavy ratio greater than 2. Shown below are the final consolidated and averaged light to heavy ratios for those peptides only observed in at least 2 out of 3 biological replicates for isoTOP-ABPP studies with DKM 3-5 and DKM 3-30, respectively. Cys Ave area # of Peptide Seq ID Number Index ratio UniProt runs YSNSALGHVNC*TIK (SEQ ID NO: 1) C1101 3.04 Q9NQC3 F8W914 3 AYEYVEC*PIR (SEQ ID NO: 2) 2.54 P53701 2 APPWVPAMGFTLAPS (SEQ ID NO: 3) C19 2.51 B1AH87 P30536 2 LGC*FVGSR YYGGAEVVDEIELLC*QR (SEQ ID NO: 4) 2.43 G3V5L0 2 KVIGIEC*SSISDYAVK (SEQ ID NO: 5) C101 2.40 Q99873 2 TYDPSGDSTLPTC*SK (SEQ ID NO: 6) 2.35 Q9Y2X3 2 FTTSC*MTGYSPQLQGLSSGGSGSYSPGVTYSPVSGYNK (SEQ ID NO: 7) C158 2.31 Q965K2 Q965K2 2 C157 SLVQNNCLSRPNIFLC*PEIEPK (SEQ ID NO: 8) C145 2.28 Q8TAQ2 Q8TAQ2 2 Q8TAQ2 F8VXC8 LWNTLGVC*K (SEQ ID NO: 9) 2.22 P63244 2 SLPSAVYC*IEDK (SEQ ID NO: 10) C674 2.20 O43290 2 EGGQYGLVAAC*AAGGQGHAMIVEAYPK (SEQ ID NO: 11) C436 2.14 P55084 P55084 2 AAIGC*GIVESILNWVK (SEQ ID NO: 12) C486 2.14 P11388 P11388 2 C431 P11388 P11388 LC*PLKDEPWPIHPWEPGSFR (SEQ ID NO: 13) C105 2.11 E7ETU7 H0Y9G6 2 C78 E9PF06 P09001 VIEASDVVLEVLDARDPLGC*R (SEQ ID NO: 14) C144 2.08 Q9BVP2 Q9BVP2 2 YVAAAFPSAC*GK (SEQ ID NO: 15) C172 2.07 B4DW73 Q16822 3 VC*TLAIIDPGDSDIIR (SEQ ID NO: 16) C92 2.06 P62888 E5RI99 2 STPYEC*GFDPMSPAR (SEQ ID NO: 17) C39 2.01 P03897 2 VALEGLRPTIPPGISPHVC*K (SEQ ID NO: 18) C361 2.00 Q13418 Q13418 2 C453 A0A0A0MTH3 Q13418 LLEETGIC*VVPGSGFGQR (SEQ ID NO: 19) C477 1.94 Q8TD30 2 FGVIC*LEDLIHEIAFPGK (SEQ ID NO: 20) 1.92 Q6DKI1 2 ASC*LYGQLPK (SEQ ID NO: 21) 1.89 P09211 3 ATGHSGGGC*ISQGR (SEQ ID NO: 22) 1.84 I3L407 I3L139 3 Q9HA64 I3L3W5 HVVC*AAETGSGK (SEQ ID NO: 23) 1.82 Q9NUL7 3 STFFNVLTNSQASAENFPFC*TIDPNESRVPVPDER (SEQ ID NO: 24) 1.78 J3KQ32 Q9NTK5 2 VDDEILGFISEATPLGGIQAASTESC*NQQLDLALCR (SEQ ID NO: 25) 1.78 P42166 2 IDRYTQQGFGNLPIC*MAK (SEQ ID NO: 26) C841 1.77 A0A087WVM4 2 C906 Q6UB35 B7ZM99 C907 AVC*MLSNTTAIAEAWAR (SEQ ID NO: 27) C376 1.77 P68366 Q13748 3 Q9NY65 C9J2C0 Q71U36 Q9NY65 P68366 LC*YVALDFENEMATAASSSSLEK (SEQ ID NO: 28) C219 1.74 P68133 P68032 3 YATSCYSCC*PR (SEQ ID NO: 29) C173 1.71 Q13057 Q13057 2 VIGSGC*NLDSAR (SEQ ID NO: 30) C192 1.71 P00338 P07195 2 P00338 VLPMNTGVEAGETAC*K (SEQ ID NO: 31) 1.71 P04181 2 AVLLVGLC*DSGK (SEQ ID NO: 32) C73 1.70 Q9Y5M8 3 VTDDLVC*LVYK (SEQ ID NO: 33) 1.70 P49458 2 VC*EEIAIIPSKK (SEQ ID NO: 34) C35 1.69 H0YN88 2 A0A075B716 P08708 VQENSAYIC*SR (SEQ ID NO: 35) C585 1.67 Q9Y3T9 2 NCAVSC*AGEK (SEQ ID NO: 36) C141 1.67 Q15813 Q15813 2 GC*STVLSPEGSAQFAAQIFGLSNHLVWSK (SEQ ID NO: 37) C374 1.66 P22234 2 TIQFVDWC*PTGFK (SEQ ID NO: 38) C347 1.66 Q13748 Q9BQE3 2 Q9NY65 C9J2C0 Q71U36 Q9NY65 AGQPHSSSDAAQAPAEQPHSSSDAAQAPC*PR (SEQ ID NO: 39) C51 1.66 P29372 P29372 2 A2IDA3 P29372 P29372 LALFNPDVC*WDRNNPEPWNK (SEQ ID NO: 40) C44 1.65 O00483 2 AVASQLDC*NFLK (SEQ ID NO: 41) 1.65 A0A087X2I1 2 P62333 C*SGIGDNPGSETAAPR (SEQ ID NO: 42) C1317 1.64 H0YAC6 P50851 2 QPAIMPGQSYGLEDGSC*SYK (SEQ ID NO: 43) 1.64 M0QXS5 P14866 2 GNVAGDSKNDPPMEAAGFTAQVIILNHPGQISAGYAPVLDC* (SEQ ID NO: 44) 1.64 P68104 2 HTAHIACK A0A087WVQ9 YWLC*AATGPSIK (SEQ ID NO: 45) 1.64 P63244 2 LQSGIC*HLFR (SEQ ID NO: 46) 1.64 P14868 2 TIYAGNALC*TVK (SEQ ID NO: 47) C155 1.63 P13804 3 TSC*GSPNYAAPEVISGR (SEQ ID NO: 48) C200 1.63 Q13131 Q13131 2 TFC*GTPEYLAPEVLEDNDYGR (SEQ ID NO: 49) C310 1.62 P31749 P31751 2 Q9Y243 Q9Y243 M0R0P9 ISC*MSKPPAPNPTPPR (SEQ ID NO: 50) 1.62 P46734 P46734 2 RPYGVGLLIAGYDDMGPHIFQTC*PSANYFDCR (SEQ ID NO: 51) C123 1.60 P25786 P25786 3 C154 F5GX11 LADQC*TGLQGFLVFHSFGGGTGSGFTSLLMER (SEQ ID NO: 52) C129 1.59 Q9BQE3 P68363 2 C199 F5H5D3 Q71U36 LNLSC*IHSPVVNELMR (SEQ ID NO: 53) 1.59 Q9Y2X3 2 LVVPATQC*GSLIGK (SEQ ID NO: 54) C109 1.58 Q15365 2 NLSDLIDLVPSLC*EDLLSSVDQPLK (SEQ ID NO: 55) C65 1.58 P47756 P47756 2 C24 B1AK88 B1AK87 B1AK85 SVHYC*PATK (SEQ ID NO: 56) C193 1.57 P25205 P25205 2 NFYGGNGIVGAQVPLGAGIALAC*K (SEQ ID NO: 57) C188 1.57 P08559 P08559 2 C219 P08559 HISPTAPDTLGC*YPFYK (SEQ ID NO: 58) C384 1.56 A0A087WWF6 2 C419 P49005 QGEYGLASIC*NGGGGASAMLIQKL (SEQ ID NO: 59) 1.56 P24752 3 HSMNPFC*EIAVEEAVR (SEQ ID NO: 60) C133 1.56 P38117 P38117 2 LGMLSPEGTC*K (SEQ ID NO: 61) C212 1.56 P49327 2 GLC*GAIHSSIAK (SEQ ID NO: 62) C103 1.56 P36542 P36542 2 LVAFC*PFASSQVALENANAVSEGVVHEDLR (SEQ ID NO: 63) 1.55 O00567 2 GSDC*GIVNVNIPTSGAEIGGAFGGEK (SEQ ID NO: 64) C450 1.55 P49419 P49419 2 LNQQQHPDC*K (SEQ ID NO: 65) C239 1.55 Q5T280 3 C*LHNFLTDGVPAEGAFTEDFQGLR (SEQ ID NO: 66) C268 1.54 G3V1A6 P57764 2 C316 TPC*NAGTFSQPEK (SEQ ID NO: 67) C129 1.54 O43684 J3QT28 2 O43684 CPEALFQPSFLGMESC*GIHETTFNSIMK (SEQ ID NO: 68) 1.54 P63261 P60709 2 SWC*PDCVQAEPVVR (SEQ ID NO: 69) 1.54 Q9BRA2 2 KLDTNSDGQLDFSEFLNLIGGLAMAC*HDSFLK (SEQ ID NO: 70) 1.53 2 SIQFVDWC*PTGFK (SEQ ID NO: 71) C347 1.53 P68366 P68363 2 P68366 SGGSGGC*SGAGGASNCGTGSGR (SEQ ID NO: 72) 1.53 E9PI68 3 A0A087WUC6 Q15005 FTLDC*THPVEDGIMDAANFEQFLQER (SEQ ID NO: 73) 1.53 P35268 2 DLNYC*FSGMSDHR (SEQ ID NO: 74) C267 1.53 P31943 G8JLB6 3 P55795 C*EFEEVQGFLDQVAHK (SEQ ID NO: 75) 1.53 E9PI14 Q9NX20 2 NLPFPTYFPDGDEEELPEDLYDENVC*QPGAPSITFA (SEQ ID NO: 76) C365 1.53 E7ETU7 H0Y9G6 2 C338 P09001 GQVC*LPVISAENWKPATK (SEQ ID NO: 77) C144 1.52 P68036 P68036 2 P68036 C*FGTGAAGNR (SEQ ID NO: 78) 1.52 P78527 3 ADPDGPEAQAEAC*SGER (SEQ ID NO: 79) C18 1.52 Q9NX24 2 VHPAMATAAGGC*R (SEQ ID NO: 80) 1.52 H7C0N4 H7C561 3 EMFPYEASTPTGISASC*R (SEQ ID NO: 81) C323 1.51 G5E972 P42167 2 P42167 LNIISNLDC*VNEVIGIR (SEQ ID NO: 82) C357 1.51 P30154 P30154 2 C402 P30154 P30153 C390 YMACC*LLYR (SEQ ID NO: 83) C386 1.51 P68366 Q9BQE3 2 C316 P68363 F5H5D3 Q71U36 P68366 SC*SGVEFSTSGSSNTDTGK (SEQ ID NO: 84) C36 1.51 A0A0A0MR02 P45880 3 AGSDGESIGNC*PFSQR (SEQ ID NO: 85) C35 1.51 Q9Y696 3 GC*EVVVSGK (SEQ ID NO: 86) 1.50 P23396 2 NILGGTVFREPIIC*K (SEQ ID NO: 87) C154 1.50 P48735 P48735 3 AGAIAPC*EVTVPAQNTGLGPEK (SEQ ID NO: 88) 1.50 P05388 3 TIGGGDDSFTTFFC*ETGAGK (SEQ ID NO: 89) 1.49 P68366 P68366 2 FNNWGGSLSLGHPFGATGC*R (SEQ ID NO: 90) C413 1.49 P55084 P55084 2 NWYVQPSC*ATSGDGLYEGLTWLTSNYKS (SEQ ID NO: 91) C155 1.49 P62330 2 APC*QAGDLR (SEQ ID NO: 92) 1.49 P48431 2 QVLMGPYNPDTC*PEVGFFDVLGNDR (SEQ ID NO: 93) 1.49 Q9H3P7 3 VTEDENDEPIEIPSEDDGTVLLSTVTAQFPGAC*GLR (SEQ ID NO: 94) C39 1.49 A0A087X260 2 A0A087WYY0 B1AKP7 Q13148 G3V162 IQCTLQDVGSALATPC*SSAR (SEQ ID NO: 95) C80 1.49 Q96EY8 54R3P5 2 NLSFFLTPPC*AR (SEQ ID NO: 96) C492 1.48 P42224 P42224 2 J3KPM9 LLACIASRPGQC*GR (SEQ ID NO: 97) 1.48 P62241 2 C*SDNSSYEEPLSPISASSSTSR (SEQ ID NO: 98) C205 1.48 Q8IXK0 2 C711 A0A0A0MSI2 C346 Q8IXK0 Q8IXK0 Q8IXK0 B3KPJ4 Q8IXK0 C*LAQEVNIPDWIVDLR (SEQ ID NO: 99) 1.48 Q9Y4W2 Q9Y4W2 2 HEEFEEGC*K (SEQ ID NO: 100) C41 1.48 I3L3Q4 Q9HC38 2 C245 Q9HC38 F6TLX2 GLDYEGGGC*R (SEQ ID NO: 101) C691 1.48 O60568 2 LLQC*DPSSASQF (SEQ ID NO: 102) 1.47 P37235 3 TVPFLPLLGGC*IDDTILSR (SEQ ID NO: 103) C180 1.47 Q7Z7H8 Q7Z7H8 3 IINDNATYC*R (SEQ ID NO: 104) 1.47 O00567 2 SLLINAVEASC*IR (SEQ ID NO: 105) C262 1.47 E2QRB3 P32322 2 C188 P32322 A0A087WTV6 P32322 J3KR12 Q96C36 PPMEAAGFTAQVIILNHPGQISAGYAPVLDCHTAHIAC*K (SEQ ID NO: 106) 1.47 P68104 2 A0A087WVQ9 HPLTQELKEC*EGIVPVPLAEK (SEQ ID NO: 107) 1.46 P82932 2 HTGPGILSMANAGPNTNGSQFFIC*TAK (SEQ ID NO: 108) C115 1.46 P62937 2 IISDNLTYC*K (SEQ ID NO: 109) 1.46 Q9Y2X3 3 LIDFLEC*GK (SEQ ID NO: 110) C234 1.45 P17844 J3KTA4 2 KAVVVC*PK (SEQ ID NO: 111) C588 1.45 Q00839 Q00839 2 LTTPTYGDLNHLVSATMSGVTTC*LR (SEQ ID NO: 112) C239 1.45 Q13885 P68371 3 C221 Q9BVA1 P04350 Q5JP53 VCETDGC*SSEAK (SEQ ID NO: 113) C10 1.45 D6RF24 H0Y9L0 3 C14 P53582 VC*PPHMLPEDGANLSSAR (SEQ ID NO: 114) C29 1.44 Q9GZY8 2 A0A0A0MS29 Q9GZY8 H7C433 E9PQX8 TFVDFFSQC*LHEEYR (SEQ ID NO: 115) 1.44 Q53GQ0 2 FALAC*NASDKIIEPIQSR (SEQ ID NO: 116) 1.44 P35250 P35250 2 TIAEC*LADELINAAK (SEQ ID NO: 117) C172 1.44 P46782 M0R0R2 3 M0R0F0 FMTPVIQDNPSGWGPC*AVPEQFR (SEQ ID NO: 118) C19 1.44 O15371 O15371 3 O15371 SVLLCGIEAQAC*ILNTTLDLLDR (SEQ ID NO: 119) C114 1.44 K7ENV7 K7EKW4 2 Q96AB3 AVAILC*NHQR (SEQ ID NO: 120) 1.44 P11387 3 GNFTLPEVAEC*FDEITYVELQKEEAQK (SEQ ID NO: 121) C629 1.44 Q00839 Q00839 2 TIIPLISQC*TPK (SEQ ID NO: 122) C212 1.44 P40926 3 DVQIGDIVTVGEC*RPLSK (SEQ ID NO: 123) C131 1.44 P62280 2 LLDLVQQSC*NYK (SEQ ID NO: 124) C30 1.43 B1AHD1 P55769 3 C*MTNTPVVVR (SEQ ID NO: 125) C120 1.43 P32322 P32322 2 P32322 VLGLGLGC*LR (SEQ ID NO: 126) 1.43 Q9BRJ7 K7EIN2 3 VC*ISILHAPGDDPMGYESSAER (SEQ ID NO: 127) 1.43 P60604 P60604 2 AYHEQLSVAEITNAC*FEPANQMVK (SEQ ID NO: 128) 1.43 P68366 Q13748 3 Q71U36 P68366 AGQC*VIGLQMGTNK (SEQ ID NO: 129) C153 1.43 Q99439 B4DDF4 3 C155 B4DUT8 C164 A0A087X271 SGDAAIVDMVPGKPMC*VESFSDYPPLGR (SEQ ID NO: 130) 1.42 P68104 2 A0A087WVQ9 VSDTVVEPYNATLSVHQLVENTDETYC*IDNEALYDICFR (SEQ ID NO: 131) C201 1.42 P68371 Q9BVA1 2 C183 P04350 Q5JP53 Q9BUF5 C*EFQDAYVLLSEKK (SEQ ID NO: 132) 1.42 P10809 2 SSQC*HTGSSPR (SEQ ID NO: 133) C439 1.42 O15226 O15226 2 LNISFPATGC*QK (SEQ ID NO: 134) 1.42 P62753 3 FHADSVC*K (SEQ ID NO: 135) 1.42 Q9BW61 2 C*MQLTDFILK (SEQ ID NO: 136) C54 1.42 E7EPB3 P50914 2 ELEVLLMC*NK (SEQ ID NO: 137) C91 1.42 P62910 F8W727 2 C109 D3YTB1 NHLLPDIVTC*VQSSR (SEQ ID NO: 138) C184 1.42 Q9BSD7 2 VNGTTPC*AFPTHYEEALKDEEK (SEQ ID NO: 139) 1.41 O15479 2 LTEGC*SFR (SEQ ID NO: 140) C93 1.41 H0YMV8 Q71UM5 2 C77 P42677 C*ALSSPSLAFTPPIK (SEQ ID NO: 141) C120 Q8NFH5 Q8NFH5 2 Q8NFH5 SSVQEEC*VSTISSSKDEDPLAATR (SEQ ID NO: 142) 1.41 Q7L0Y3 3 TQNLPNC*QLISR (SEQ ID NO: 143) 1.41 P37268 2 C*SEGSFLLTTFPRPVTVEPMDQLDDEEGLPEK (SEQ ID NO: 144) C119 1.41 Q15233 Q15233 3 SMVSPVPSPTGTISVPNSC*PASPR (SEQ ID NO: 145) 1.41 P85037 2 EITSLDTENIDEILNNADVALVNFYADWC*R (SEQ ID NO: 146) C58 1.40 Q9B526 2 GHSSDSNPAIC*R (SEQ ID NO: 147) C31 1.40 Q5JTH9 2 FC*IWTESAFR (SEQ ID NO: 148) C250 1.40 P36578 2 SLC*NLEESITSAGRDDLESFQLEISGFLK (SEQ ID NO: 149) 1.40 Q52LJ0 Q52LJ0 2 IDC*FSEVPTSVFGEK (SEQ ID NO: 150) 1.40 O00567 2 NMSVHLSPC*FR (SEQ ID NO: 151) C116 1.40 P62280 3 GLPC*TELFVAPVGVASKR (SEQ ID NO: 152) C79 1.40 H0Y901 E9PDI4 3 C428 O00515 VAC*ITEQVLTLVNKR (SEQ ID NO: 153) 1.40 P04843 3 NSQWVPTLPNSSHHLDAVPC*STTINR (SEQ ID NO: 154) C138 1.40 Q12824 Q12824 2 G5E975 NVQLLSQFVSPFTGC*IYGR (SEQ ID NO: 155) 1.40 Q9Y3D5 2 LC*SSSSSDTSSR (SEQ ID NO: 156) C385 1.40 Q86WB0 Q86WB0 2 C9J0I9 LVVPASQC*GSLIGK (SEQ ID NO: 157) C109 1.40 Q15366 Q15366 3 Q15366 Q15366 VNAAGTDPSSPVGFVLGVDLLHIFPLEGATFLC*PADVTDPR (SEQ ID NO: 158) 1.39 Q9UI43 2 C*PFTGNVSIR (SEQ ID NO: 159) C60 1.39 P62280 2 LTALDYHNPAGFNC*KDETEFR (SEQ ID NO: 160) C19 1.39 Q9Y224 3 VPTANVSVVDLTC*R (SEQ ID NO: 161) C247 1.39 P04406 2 DSGYGDIWC*PERGEFLAPPR (SEQ ID NO: 162) C176 1.39 J3KP06 F8WD26 2 C228 Q8WWI1 Q8WWI1 C213 Q8WWI1 Q8WWI1 NFNYHILSPC*DLSNYTDLAMSTVK (SEQ ID NO: 163) C461 1.39 G5E9W3 Q9UKF6 3 AC*QSIYPLHDVFVR (SEQ ID NO: 164) C201 1.39 D6RB09 D6RAT0 P61247 D6RG13 WC*EYGLTFTEK (SEQ ID NO: 165) C65 1.38 A0A0A0MR02 2 P45880 KTPC*GEGSK (SEQ ID NO: 166) C70 1.38 P60866 P60866 2 NC*LTNFHGMDLTR (SEQ ID NO: 167) C96 1.38 D6RB09 D6RAT0 2 P61247 D6RG13 YGIIC*MEDLIHEIYTVGKR (SEQ ID NO: 168) C186 1.38 P18124 A8MUD9 3 C*ASQAGMTAYGTR (SEQ ID NO: 169) C127 1.38 E9PDU6 Q15417 2 C132 Q15417 KAQC*PIVER (SEQ ID NO: 170) C66 1.38 P46782 M0R0R2 2 M0R0F0 NGDIC*ETSGKPK (SEQ ID NO: 171) C60 1.38 Q16637 E7EQZ4 2 Q16637 Q16637 Q16637 B4DP61 KGAGNPQASTLALQSNITQC*LLGQPWPLNEAQVQASVVK (SEQ ID NO: 172) C1260 1.38 E7ETY2 Q13428 3 C1261 Q13428 Q13428 C1299 Q13428 Q13428 Q13428 Q13428 J3KQ96 FCSFSPC*IEQVQR (SEQ ID NO: 173) C209 1.37 Q96FX7 2 IHMGSC*AENTAK (SEQ ID NO: 174) 1.37 P24752 3 GC*IVDANLSVLNLVIVK (SEQ ID NO: 175) 1.37 P62753 3 IAILTC*PFEPPKPK (SEQ ID NO: 176) C253 1.37 E9PCA1 B7ZAR1 2 P48643 TLTSC*FLSCVVCVEGIVTK (SEQ ID NO: 177) C164 1.37 P25205 P25205 2 LGYILTC*PSNLGTGLR (SEQ ID NO: 178) C347 1.37 P12532 P12532 2 LMWLFGC*PLLLDDVAR (SEQ ID NO: 179) 1.37 O15067 2 INISEGNC*PER (SEQ ID NO: 180) C54 1.37 Q15366 Q15366 3 Q15366 Q15366 Q15365 SGQGAFGNMC*R (SEQ ID NO: 181) C96 1.37 P36578 3 GLC*AIAQAESLR (SEQ ID NO: 182) 1.37 P23396 3 IIPTLEEGLQLPSPTATSQLPLESDAVEC*LNYQHYK (SEQ ID NO: 183) 1.37 P61978 P61978 3 C*PEALFQPSFLGMESCGIHETTFNSIMK (SEQ ID NO: 184) 1.37 P60709 P63261 2 LC*FSTAQHAS (SEQ ID NO: 185) 1.37 M0QXS5 P14866 2 LGTLAPFC*CPWEQLTQDWESR (SEQ ID NO: 186) C705 1.37 Q99575 2 AAVEEGIVLGGGC*ALLR (SEQ ID NO: 187) 1.37 P10809 3 LDSLGLCSVSC*ALEFIPNSK (SEQ ID NO: 188) C256 1.36 Q9BSH4 2 VC *NFLASQVPFPSR (SEQ ID NO: 189) C205 1.36 Q99714 3 VTDGALVVVDCVSGVC*VQTETVLR (SEQ ID NO: 190) C136 1.36 P13639 2 YAIC*SALAASALPALVMSK (SEQ ID NO: 191) C125 1.36 P36578 3 TATAVAHC*K (SEQ ID NO: 192) C25 1.36 MOR210 P62249 3 GC*TATLGNFAK (SEQ ID NO: 193) 1.36 P15880 2 VC*NYGLTFTQK (SEQ ID NO: 194) C66 1.36 Q9Y277 Q9Y277 2 SLHDALC*VLAQTVK (SEQ ID NO: 195) C348 1.36 P78371 2 AWVWNTHADFADEC* (SEQ ID NO: 196) C209 1.36 C9JXG8 P43487 2 PKPELLAIR C132 C9JJ34 F6WQW2 C9JGV6 P43487 C*HTPPLYR (SEQ ID NO: 197) 1.36 M0R117 Q02543 2 YIGENLQLLVDRPDGTYC*FR (SEQ ID NO: 198) 1.36 Q9Y221 2 ALANVNIGSLIC*NVGAGGPAPAAGAAPAGGPAPSTAAAPAE (SEQ ID NO: 199) 1.36 P05386 2 EK ANC*IDSTASAEAVFASEVKK (SEQ ID NO: 200) C268 1.36 M0QXL5 M0R299 2 C183 P22087 M0R2Q4 NC*IVLIDSTPYR (SEQ ID NO: 201) 1.35 P62241 2 YSTGSDSASFPHTTPSMC*LNPDLEGPPLEAYTIQGQY (SEQ ID NO: 202) C217 1.35 Q15366 Q15366 2 LGEWVGLC*K (SEQ ID NO: 203) 1.35 P25398 2 C*ASQVGMTAPGTR (SEQ ID NO: 204) C215 1.35 Q99439 B4DDF4 2 C204 B4DUT8 TTSSANNPNLMYQDEC*DR (SEQ ID NO: 205) C586 1.35 Q92841 H3BLZ8 2 C507 Q92841 Q92841 C505 Q92841 ISLGLPVGAVINC*ADNTGAK (SEQ ID NO: 206) 1.35 P62829 2 AYGGSMC*AK (SEQ ID NO: 207) 1.35 P49207 2 VVNSETPVVVDFHAQWC*GPCK (SEQ ID NO: 208) C90 1.35 Q99757 F8WDN2 2 GVPGAIVNVSSQC*SQR (SEQ ID NO: 209) C137 1.34 Q7Z4W1 J3QS36 2 J3KS22 GC*LWALNPAKIDK (SEQ ID NO: 210) 1.34 O15353 2 DLC*FSPGLMEASHVVNDVNEAVQLVFR (SEQ ID NO: 211) C362 1.34 Q9BXW7 Q9BXW7 2 HGFC*GIPITDTGR (SEQ ID NO: 212) 1.34 P12268 3 ASDHGWVC*DQR (SEQ ID NO: 213) C309 1.34 Q9HC36 2 AVC*MLSNTTAVAEAWAR (SEQ ID NO: 214) C376 1.33 Q9BQE3 3 HIGDGC*CLTR (SEQ ID NO: 215) C202 1.33 A0A087WZT2 3 Q6UX53 SDLGPC*EK (SEQ ID NO: 216) 1.33 D6RDI2 J3KPP4 2 O95232 LPITVLNGAPGFINLC*DALNAWQLVK (SEQ ID NO: 217) C240 1.33 P31939 P31939 2 VDEFPLC*GHMVSDEYEQLSSEALEAAR (SEQ ID NO: 218) C49 1.33 X1WI28 P27635 3 IISNASC*TTNCLAPLAK (SEQ ID NO: 219) C152 1.33 P04406 3 DLTTAGAVTQC*YR (SEQ ID NO: 220) 1.33 Q02543 2 SAC*SLESNLEGLAGVLEADLPNYK (SEQ ID NO: 221) C44 1.32 Q09161 2 KITAFVPNDGC*LNFIEENDEVLVAGFGR (SEQ ID NO: 222) 1.32 P62266 2 TGC*TFPEKPDFH (SEQ ID NO: 223) C318 1.32 P55263 P55263 3 C336 P55263 NQSFC*PTVNLDKLWTLVSEQTR (SEQ ID NO: 224) C70 1.32 P46776 E9PLL6 3 E9PJD9 SGEEDFESLASQFSDC*SSAK (SEQ ID NO: 225) C113 1.32 K7EN45 K7EMU7 2 Q13526 SPWLAGNELTVADVVLWSVLQQIGGC*SVTVPANVQR (SEQ ID NO: 226) 1.32 Q13155 2 VNQAIWLLC*TGAR (SEQ ID NO: 227) C155 1.32 P46782 M0R0R2 3 M0R0F0 AC*DLPAWVHFPDTER (SEQ ID NO: 228) 1.32 H7BXI1 2 YYALCGFGGVLSC*GLTHTAVVPLDLVK (SEQ ID NO: 229) 1.31 Q00325 2 EC*LPLIIFLR (SEQ ID NO: 230) 1.31 P62701 3 VLVTTNVC*AR (SEQ ID NO: 231) C392 1.31 Q9UMR2 Q9UMR2 2 C393 Q9NUU7 Q9NUU7 C367 H3BQK0 F6QDS0 C310 I3L352 SYC*AEIAHNVSSK (SEQ ID NO: 232) C114 1.31 P62910 F8W727 2 C96 D3YTB1 TAFQEALDAAGDKLVVVDFSATWC*GPCK (SEQ ID NO: 233) 1.31 P10599 2 LC*YVALDFEQEMATAASSSSLEK (SEQ ID NO: 234) 1.30 P60709 P63261 3 Q658J3 VRPSTGNSASTPQSQC*LPSEIEVK (SEQ ID NO: 235) C131 1.30 Q9UJX3 Q9UJX3 2 AATGEEVSAEDLGGADLHC*R (SEQ ID NO: 236) 1.30 Q9HCCO 2 PGHLQEGFGC*VVTNRFDQLFDDESDPFEVLK (SEQ ID NO: 237) C11 1.30 Q8NC51 Q8NC51 3 Q8NC51 Q8NC51 C*ASQSGMTAYGTR (SEQ ID NO: 238) C166 1.30 Q99439 B4DDF4 3 C164 B4DUT8 C175 A0A087X271 LC*VQNSPQEAR (SEQ ID NO: 239) C150 1.30 P33240 P33240 3 E7EWR4 E9PID8 A0A0A0MT56 PC*SEETPAISPSKR (SEQ ID NO: 240) C3 1.30 P33316 H0YNJ9 2 LDINLLDNVVNC*LYHGEGAQQR (SEQ ID NO: 241) 1.30 O14980 2 NMMAAC*DPR (SEQ ID NO: 242) C285 1.29 Q13509 Q13885 3 C650 P68371 A6NNZ2 C303 A0A0B4J269 C266 Q9BVA1 Q3ZCM7 P04350 K7ESM5 Q5JP53 Q9BUF5 IAVHC*TVR (SEQ ID NO: 243) C72 1.29 P62913 Q5VVC8 2 P62913 C*PQVEEAIVQSGQKK (SEQ ID NO: 244) C146 1.29 Q9BVP2 Q9BVP2 2 SQAPC*ANKDEADLSSK (SEQ ID NO: 245) C300 1.29 Q96SK2 Q96SK2 2 C*LGHPEEFYNLVR (SEQ ID NO: 246) 1.29 P37268 2 NTVLC*NVVEQFLQADLAR (SEQ ID NO: 247) C70 1.29 Q14258 2 AFQYVETHGEVC*PANWTPDSPTIKPSPAASK (SEQ ID NO: 248) 1.29 P30048 3 QAVLGAGLPISTPC*TTINK (SEQ ID NO: 249) C119 1.29 P24752 3 GTPEQPQC*GFSNAVVQILR (SEQ ID NO: 250) C67 1.29 Q86SX6 3 ADHQPLTEASYVNLPTIALC*NTDSPLR (SEQ ID NO: 251) C148 1.29 A0A0C4DG17 3 C9J9K3 P08865 GSDELFSTC*VTNGPFIIVISSNSASAANGNDSKK (SEQ ID NO: 252) C23 1.29 A0A0U1RRM4 3 P26599 P26599 P26599 AYHEQLTVAEITNAC*FEPANQMVK (SEQ ID NO: 253) C295 1.28 Q9BQE3 F5H5D3 2 C365 INPYMSSPC*HIEMILTEK (SEQ ID NO: 254) C106 1.28 J3KRX5 J3QLC8 2 C144 A0A087WXM6 P18621 A0A0A6YYL6 J3QQT2 P18621 YIYDQC*PAVAGYGPIEQLPDYNR (SEQ ID NO: 255) C453 1.28 P31930 2 C*ELSSSVQTDINLPYLTMDSSGPK (SEQ ID NO: 256) 1.28 P38646 3 GC*LLYGPPGTGK (SEQ ID NO: 257) 1.27 A0A087X2I1 3 P62333 ALNALC*DGLIDELNQALK (SEQ ID NO: 258) 1.27 P30084 3 NTPSFLIAC*NK (SEQ ID NO: 259) C179 1.27 Q9Y5M8 2 C*MPTFQFFK (SEQ ID NO: 260) 1.27 P10599 3 TGNGPMSVC*GR (SEQ ID NO: 261) C493 1.27 O95793 O95793 2 O95793 A0A087X1A5 Q5JW30 HELQANC*YEEVKDR (SEQ ID NO: 262) C139 1.27 E9PK25 G3V1A4 3 C177 P23528 AFAFVTFADDQIAQSLC*GEDLIIK (SEQ ID NO: 263) C244 1.27 A0A087X260 2 A0A087WYY0 B1AKP7 Q13148 G3V162 LTPGC*EAEAETEAICFFVQQFTDMEHNR (SEQ ID NO: 264) C2359 1.26 P49327 2 LECVEPNC*R (SEQ ID NO: 265) 1.26 Q969Q0 2 SYC*NDQSTGDIK (SEQ ID NO: 266) C106 1.26 P00492 2 ATC*APQHGAPGPGPADASK (SEQ ID NO: 267) C2516 1.26 P21333 2 C2535 A0A087WWY3 Q60FE5 P21333 ATYDKLC*K (SEQ ID NO: 268) 1.26 P62851 2 GSC*STEVEKETQEK (SEQ ID NO: 269) C69 1.26 O75348 2 TVDSQGPTPVC*TPTFLER (SEQ ID NO: 270) 1.26 Q9BYG3 2 LC*YVALDFEQEMAMVASSSSLEK (SEQ ID NO: 271) C880 1.26 P0CG39 3 VFIMDSC*DELIPEYLNFIR (SEQ ID NO: 272) C366 1.25 P08238 3 HC*SQVDSVR (SEQ ID NO: 273) C112 1.25 Q14247 Q14247 2 Q14247 AAAPAPEEEMDEC*EQALAAEPK (SEQ ID NO: 274) C266 1.25 P26641 P26641 2 EKTAC*AINK (SEQ ID NO: 275) C293 1.25 Q8NCA5 E9PH82 2 Q52LJ0 Q8NCA5 Q52LJ0 SGTIC*SSELPGAFEAAGFHLNEHLYNMIIR (SEQ ID NO: 276) C200 1.24 A0A0C4DGQ5 2 P04632 K7ELJ7 TASISSSPSEGTPTVGS YGC*TPQSLPK (SEQ ID NO: 277) C787 1.24 Q6PKG0 Q6PKG0 3 EVIAVSCGPAQC*QETIR (SEQ ID NO: 278) C162 1.24 P38117 P38117 2 NC*PHVVVGTPGR (SEQ ID NO: 279) C164 1.24 O00148 3 SC*PSFSASSEGTR (SEQ ID NO: 280) C9 1.24 D6RCP9 P27707 2 D6RFG8 D6RG38 GLIAAIC*AGPTALLAHEIGFGSK (SEQ ID NO: 281) C86 1.23 Q99497 K7ELW0 2 K7EN27 LYYFQYPC*YQEGLR (SEQ ID NO: 282) 1.23 Q9NRW3 3 GC*WDSIHVVEVQEK (SEQ ID NO: 283) C135 1.22 P47756 P47756 2 C176 B1AK88 B1AK87 B1AK85 SCYDLSC*HAR (SEQ ID NO: 284) 1.22 P41250 2 VGSFC*LSEAGAGSDSFALK (SEQ ID NO: 285) C73 1.21 P45954 P45954 2 AINC*ATSGVVGLVNCLR (SEQ ID NO: 286) C1448 1.21 P49327 2 TSAVPSPC*GK (SEQ ID NO: 287) C260 1.21 P49748 P49748 2 P49748 INEIVYFLPFC*HSELIQLVNK (SEQ ID NO: 288) C513 1.21 Q9H078 Q9H078 2 C371 Q9H078 Q9H078 C572 Q9H078 H0YGM0 IGFPETTEEELEEIASENSDC*IFPSAPDVK (SEQ ID NO: 289) C353 1.21 Q9Y3F4 Q9Y3F4 2 IVGYFVSGC*DPSIMGIGPVPAISGALK (SEQ ID NO: 290) C287 1.21 A0A0B4J2A4 2 P42765 RGPC*IIYNEDNGIIK (SEQ ID NO: 291) C208 1.21 P36578 2 AALVTSFC*MFKYMALYSMIQR (SEQ ID NO: 292) C542 1.20 H0Y4Z2 2 ESLNASIVDAINQAADC*WGIR (SEQ ID NO: 293) C167 1.20 Q9UJZ1 2 EEHLC*TQR (SEQ ID NO: 294) 1.20 J3KN67 2 GC*GVVKFESPEVAER (SEQ ID NO: 295) 1.20 P52272 P52272 3 A0A087X0X3 KC*SASNR (SEQ ID NO: 296) C17 1.19 Q8WVC2 Q9BYK1 2 P63220 TPC*SSLLPLLNAHAATSGK (SEQ ID NO: 297) C307 1.18 B8ZZZ7 Q9NUQ6 2 C367 A0A0A0MSG5 C397 Q9NUQ6 Q9NUQ6 Q9NUQ6 VSLDPELEEALTSASDTELC*DLAAILGMHNLITNTK (SEQ ID NO: 298) C132 1.17 Q9NYL9 3 FDPTQFQDC*IIQGLTETGTDLEAVAK (SEQ ID NO: 299) C35 1.17 Q7L1Q6 C9IZ80 2 C39 Q7L1Q6 Q7L1Q6 Q7L1Q6 LGGSLIVAFEGC*PV (SEQ ID NO: 300) C146 1.16 P60981 P60981 2 TQYSCYC*CK (SEQ ID NO: 301) C229 1.15 Q9UGI8 Q9UGI8 2 IC*PVEFNPNFVAR (SEQ ID NO: 302) 1.15 Q9UI30 F5GX77 2 TPSYSISSTLNPQAPEFILGC*TASK (SEQ ID NO: 303) C142 1.15 Q14694 H3BQC6 2 Q14694 Q14694 ASVGFGGSC*FQK (SEQ ID NO: 304) C209 1.14 O60701 O60701 2 NTGQTC*VCSNQFLVQR (SEQ ID NO: 305) 1.13 C9J8Q5 P01763 2 P51649 P51649 FQSSAVMALQEASEAYLVGLFEDTNLC*AIHAK (SEQ ID NO: 306) 1.12 Q71DI3 2 AGAVVAVPTDTLYGLACAASC*SAALR (SEQ ID NO: 307) C99 1.11 Q86U90 3 AVLLASDAQEC*TLEEVVER (SEQ ID NO: 308) C332 1.10 Q27J81 Q27J81 2 FQSSAVMALQEACEAYLVGLFEDTNLC*AIHAK (SEQ ID NO: 309) C111 1.08 P68431 2 NMITGTSQADC*AVLIVAAGVGEFEAGISK (SEQ ID NO: 310) 1.08 P68104 Q05639 2 A0A087WVQ9 GNHEC*ASINR (SEQ ID NO: 311) C83 1.05 P62136 P62140 3 C126 P62136 LC*DFGVSGQLIDSMANSFVGTR (SEQ ID NO: 312) C207 1.04 G5E9C7 Q02750 2 C211 P36507 Q02750 C114 SC*GSSTPDEFPTDIPGTK (SEQ ID NO: 313) 1.02 P41091 2 GTLTLC*PYHSDR (SEQ ID NO: 314) C620 1.00 Q13200 Q13200 2 Q13200 LSLDGQNIYNAC*CTLR (SEQ ID NO: 315) C250 0.95 A0A0U1RRM4 2 C281 P26599 P26599 P26599 ADASSTPSFQQAFASSC*TISSNGPGQR (SEQ ID NO: 316) C688 0.94 Q68CZ2 Q68CZ2 2 STLTDSLVC*K (SEQ ID NO: 317) C41 0.94 P13639 3 SDITKLEVDAIVNAA (SEQ ID NO: 318) C186 0.91 Q9BQ69 2 NSSLLGGGGVDGC*IHR LC*EPEVLNSLEETYSPFFR (SEQ ID NO: 319) C261 0.90 H0YJA2 Q6PJT7 2 C177 Q6PJT7 G3V5I6 C224 Q6PJT7 G3V256 Q6PJT7 Q6PJT7 Q6PJT7 Q6PJT7 Q6PJT7 C*PAPPRGPPAPAPEVEELAR (SEQ ID NO: 320) C161 0.81 P48681 2 AAC*LESAQEPAGAWGNK (SEQ ID NO: 321) C53 0.76 A0A024R4E5 2 LHTGPLPEQC*R (SEQ ID NO: 322) C163 0.68 A0JLT2 J3KR33 2 A0JLT2 FQSAAIGALQEASEAYLVGLFEDTNLC*A (SEQ ID NO: 323) 0.66 K7EK07 P84243 2 FQSSAVMALQEAREAYLVGLFEDTNLC*AIRAK (SEQ ID NO: 324) C111 0.65 Q5TEC6 2 HLNEIDLFHC*IDPNDSK (SEQ ID NO: 325) C62 0.59 A0A087WYT3 2 C58 Q15185 Q15185 QC*PIMDPAWEAPEGVPIDAIIFGGR (SEQ ID NO: 326) C297 0.29 B4DW73 Q16822 3 SEC*DQDYIPETDQDC*SMSPCPQRTPDSGLAQHPFQNEDYR (SEQ ID NO: 327) C1290 0.12 Q9P2N4 Q9P2N4 2 C1250 H0Y859 Q9P2N4 C1278 C1262 N-(4-(4-(tert-butyl)phenoxy)phenyl)acrylamide (AMR 1-125)
REFERENCES