COMPOUNDS HAVING ANTIBACTERIAL ACTIVITY AND METHODS OF USE
The present application is a divisional application of U.S. patent application Ser. No. 16/052,212 filed Aug. 1, 2018 which claims priority pursuant to 35 U.S.C. § 119(e) to U.S. Provisional Patent Application Ser. No. 62/539,886 filed Aug. 1, 2017, which is incorporated herein by reference in its entirety. The present invention relates to antibacterial compounds and, in particular, to antibacterial compounds inhibiting antibacterial resistance via multiple modes of action. The discovery of penicillin in 1929 ushered in the ‘Golden Age’ of antibiotic discovery and with it, over the next three decades, more than twenty unique classes of antibiotics. The discovery and development of these life-saving molecules has been in serious decline. Since the end of the ‘Golden Age’ in 1962 only two orally available antibiotics with completely novel targets, linezolid and a daptomycin, have been brought to the market. Declining rates of antibiotic discovery would be unalarming if it were not for evolution's perpetual offensive, constantly selecting antibiotic resistant bacteria through horizontal gene transfer and spontaneous mutation. In the United States alone, this manifests in a record 2 million antibiotic resistant infections, which annually kill 23,000 people. Moreover, such infections have been estimated to cost our health system as much as $35 billion annually. Other than better antibiotic stewardship, which has been shown to decrease the rate of hospital acquired infections, the only way to combat bacterial infections is to continuously develop antibiotics and other therapeutics with novel mechanisms of action (MOA), which have yet to slip into obsolescence. In one aspect, methods of treating bacterial infections are described herein. In some embodiments, for example, a method comprises administering to a patient having a bacterial infection a therapeutically effective amount of one or more compounds of Formula (I) and/or salt(s) thereof: wherein R1-R4 are independently selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkyl-aryl, alkyl-heteroaryl, amide, sulfonamide, and urea, wherein the alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkyl-aryl, alkyl-heteroaryl, amide and sulfonamide are optionally substituted with one or more substituents selected from the group consisting of (C1-C10)-alkyl, (C1C10)-alkenyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkoxy, amide, sulfonamide, urea, halo, hydroxy, C(O)OR5, and C(O)R6, wherein R5 is selected from the group consisting of hydrogen, alkyl and alkenyl and R6 is selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl and NR7R8, wherein R7 and R8 are independently selected from the group consisting of hydrogen, alkyl, alkenyl, aryl and heteroaryl; and wherein X and Z are independently selected from the group consisting of C, N and O; and wherein Y is selected from the group consisting of OH and NR9R10, wherein R9 and R10 are independently selected from the group consisting of hydrogen, alkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, alkenyl, aryl, heteroaryl, amide, sulfonamide, urea and C(O)R11 wherein R11 is selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl and heteroaryl and wherein R9 and R10may optionally form a ring structure; and n is an integer from 0 to 5. In another aspect, a method comprises administering to a patient having a bacterial infection a therapeutically effective amount of one or more compounds of Formula (II) and/or salt(s) thereof: wherein R1-R4 are independently selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkyl-aryl, alkyl-heteroaryl, amide, sulfonamide, and urea, wherein the alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkyl-aryl, alkyl-heteroaryl, amide and sulfonamide are optionally substituted with one or more substituents selected from the group consisting of (C1-C10)-alkyl, (C1C10)-alkenyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkoxy, amide, sulfonamide, urea, halo, hydroxy, C(O)OR5, and C(O)R6, wherein R5 is selected from the group consisting of hydrogen, alkyl and alkenyl and R6 is selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl and NR7R8, wherein R7 and R8 are independently selected from the group consisting of hydrogen, alkyl, alkenyl, aryl and heteroaryl; and wherein X is selected from the group consisting of CR9R10, O, S, SO2, and NR11R12, and wherein Y is selected from the group consisting of OH and NR13R14, wherein R9-R154 are independently selected from the group consisting of hydrogen, alkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, alkenyl, aryl, heteroaryl, amide, sulfonamide, urea and C(O)R15 wherein R15 is selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl and heteroaryl and wherein R13 and R14 may optionally form a ring structure; and n is an integer from 0 to 5; and wherein Z is independently selected from the group consisting of C and N; and n is an integer from 0 to 5. In another aspect, pharmaceutical compositions are described herein. In some embodiments, the pharmaceutical compositions are operable for treating bacterial infections and/or cancerous tissue. A pharmaceutical composition, in some embodiments, comprises a compound of Formula (I) and/or salt thereof: wherein R1-R4 are independently selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkyl-aryl, alkyl-heteroaryl, amide, sulfonamide, and urea, wherein the alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkyl-aryl, alkyl-heteroaryl, amide and sulfonamide are optionally substituted with one or more substituents selected from the group consisting of (C1-C10)-alkyl, (C1-C10)-alkenyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkoxy, amide, sulfonamide, urea, halo, hydroxy, C(O)OR5, and C(O)R6, wherein R5 is selected from the group consisting of hydrogen, alkyl and alkenyl and R6 is selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl and NR7R8, wherein R7 and R8 are independently selected from the group consisting of hydrogen, alkyl, alkenyl, aryl and heteroaryl; and wherein X and Z are independently selected from the group consisting of C, N and O; and wherein Y is selected from the group consisting of OH and NR9R10, wherein R9 and R10 are independently selected from the group consisting of hydrogen, alkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, alkenyl, aryl, heteroaryl, amide, sulfonamide, urea and C(O)R11 wherein R11 is selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl and heteroaryl and wherein R9 and R10 may optionally form a ring structure; and n is an integer from 0 to 5. In other embodiments, a pharmaceutical composition comprises a compound of Formula (II) and/or salt thereof: wherein R1R4 are independently selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkyl-aryl, alkyl-heteroaryl, amide, sulfonamide, and urea, wherein the alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkyl-aryl, alkyl-heteroaryl, amide and sulfonamide are optionally substituted with one or more substituents selected from the group consisting of (C1-C10)-alkyl, (C1-C10)-alkenyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkoxy, amide, sulfonamide, urea, halo, hydroxy, C(O)OR5, and C(O)R6, wherein R5 is selected from the group consisting of hydrogen, alkyl and alkenyl and R6 is selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl and NR13R14, wherein R7 and R8 are independently selected from the group consisting of hydrogen, alkyl, alkenyl, aryl and heteroaryl; andwherein X is selected from the group consisting of CR9R10, O, S, SO2, and NR11R12, and wherein Y is selected from the group consisting of OH and NR13R14, wherein R9-R14 are independently selected from the group consisting of hydrogen, alkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, alkenyl, aryl, heteroaryl, amide, sulfonamide, urea and C(O)R15 wherein R15 is selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl and heteroaryl and wherein R13 and R14 may optionally form a ring structure; and n is an integer from 0 to 5; and wherein Z is independently selected from the group consisting of C and N; and n is an integer from 0 to 5. In another aspect, methods of treating bacterial infections are described herein employing compounds previously unknown to exhibit antibacterial activity. Identification and screening of prior compounds for novel MOAs can greatly facilitate the development of new treatments at a time when bacterial species are exhibiting greater recalcitrance to current treatment options. For example, method comprises administering to a patient having a bacterial infection a therapeutically effective amount of a composition including an antibacterial agent selected from the group consisting of DL-erythro-dihydrosphingosine, N3-Cyclopropyl-7-[[4-(1-methylethyl)phenyl]methyl]-7H-pyrrolo[3,2-F]quinazoline-1,3-diamine dihydrochloride (SCH 79797), 3-(3,5-dibromo-4-hydroxybenzyliden)-5-iodo-1,3-dihydroindol-2-one (GW5074), 2-(p-amylcinnamoyl)amino-4-chlorobenzoic acid (ONO-RS-82), 2[3-(1,3-dihydro-1,3,3-trimethyl-2H-indol-2-ylidene)-1-propenyl]-3-ethyl-benzothiazolium iodide (AC-93253 iodide), 1-[bis(4-Chlorophenyl)methyl]-3-[2-(2,4-dichlorophenyl)-2-(2,4-dichlorobenzyloxy)ethyl]-1H-imidazolium chloride (Calmidazolium chloride), N,N-dimethyl-3-[2-(trifluoromethyl)phenothiazin-10-yl]propan-1-amine (Triflupromazine), N-[2-(p-Bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H-89-2HCl), (S)-5-Chloro-N-((2-oxo-3-(4-(3-oxomorpholino)phenyl)oxazolidin-5-yl)methyl)thiophene-2-carboxamide (Rivaroxaban), 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione (AZT) and CP000294 and salts and derivatives thereof. In some embodiments, the antibacterial agent is present in the composition at a minimum inhibitory concentration (MIC) of less than 1 μg/ml. In other embodiments, the antibacterial agent is present at a MIC of 0.1 to 10 μg/ml. Moreover, in some embodiments, antibacterial activity of one or more compounds described herein can be associated with disruption of one or more bacterial metabolic pathways. For example, one or more compounds may interfere with or disrupt the folate biosynthetic pathway. In another aspect, methods of treating cancer are described herein. In some embodiments, a method comprises administering to a patient having cancer a therapeutically effective amount of a composition comprising a compound of Formula (I) and/or Formula (II) and/or salt(s) thereof. Anticancer activity of compounds of Formula (I) and/or Formula (II), in some embodiments, is associated with disruption of the folate biosynthetic pathway employed by the cancer cells. These and other embodiments are described further in the following detailed description. Embodiments described herein can be understood more readily by reference to the following detailed description and examples and their previous and following descriptions. Elements, apparatus and methods described herein, however, are not limited to the specific embodiments presented in the detailed description and examples. It should be recognized that these embodiments are merely illustrative of the principles of the present invention. Numerous modifications and adaptations will be readily apparent to those of skill in the art without departing from the spirit and scope of the invention. The term “alkyl” as used herein, alone or in combination, refers to a straight or branched saturated hydrocarbon group optionally substituted with one or more substituents. For example, an alkyl can be C1-C30 or C1 C18. The term “alkenyl” as used herein, alone or in combination, refers to a straight or branched chain hydrocarbon group having at least one carbon-carbon double bond and optionally substituted with one or more substituents The term “aryl” as used herein, alone or in combination, refers to an aromatic monocyclic or multicyclic ring system optionally substituted with one or more ring substituents. The term “heteroaryl” as used herein, alone or in combination, refers to an aromatic monocyclic or multicyclic ring system in which one or more of the ring atoms is an element other than carbon, such as nitrogen, oxygen and/or sulfur. The term “cycloalkyl” as used herein, alone or in combination, refers to a non-aromatic, mono- or multicyclic ring system optionally substituted with one or more ring substituents. The term “heterocycloalkyl” as used herein, alone or in combination, refers to a non-aromatic, mono- or multicyclic ring system in which one or more of the atoms in the ring system is an element other than carbon, such as nitrogen, oxygen or sulfur, alone or in combination, and wherein the ring system is optionally substituted with one or more ring substituents. The term “heteroalkyl” as used herein, alone or in combination, refers to an alkyl moiety as defined above, having one or more carbon atoms in the chain, for example one, two or three carbon atoms, replaced with one or more heteroatoms, which may be the same or different, where the point of attachment to the remainder of the molecule is through a carbon atom of the heteroalkyl radical. The term “alkoxy” as used herein, alone or in combination, refers to the moiety RO—, where R is alkyl or alkenyl defined above. The term “halo” as used herein, alone or in combination, refers to elements of Group VIIA of the Periodic Table (halogens). Depending on chemical environment, halo can be in a neutral or anionic state. In one aspect, methods of treating bacterial infections are described herein. In some embodiments, for example, a method comprises administering to a patient having a bacterial infection a therapeutically effective amount of one or more compounds of Formula (I) and/or salt(s) thereof: wherein R1-R4 are independently selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkyl-aryl, alkyl-heteroaryl, amide, sulfonamide, and urea, wherein the alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkyl-aryl, alkyl-heteroaryl, amide and sulfonamide are optionally substituted with one or more substituents selected from the group consisting of (C1-C10)-alkyl, (C1-C10)-alkenyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkoxy, amide, sulfonamide, urea, halo, hydroxy, C(O)OR5, and C(O)R6, wherein R5 is selected from the group consisting of hydrogen, alkyl and alkenyl and R6is selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl and NR7R8, wherein R7 and R8 are independently selected from the group consisting of hydrogen, alkyl, alkenyl, aryl and heteroaryl; and wherein X and Z are independently selected from the group consisting of C, N and O; and wherein Y is selected from the group consisting of OH and NR9R10, wherein R9 and R10 are independently selected from the group consisting of hydrogen. alkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, alkenyl, aryl, heteroaryl, amide, sulfonamide, urea and C(O)R11 wherein R11 is selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl and heteroaryl and wherein R9 and R610 may optionally form a ring structure; and n is an integer from 0 to 5. In some embodiments, one or more compounds of Formula (I) are of Formula (IA): wherein R3, R4, X, Y and Z and n are defined above and R12-R14 are independently selected from the group consisting of halo, alkyl, alkenyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkoxy, amide, sulfonamide, urea, hydroxy, C(O)OR15, and C(O)R16, wherein R15 is selected from the group consisting of hydrogen, alkyl and alkenyl and R16 is independently selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl and NR17R18, wherein R17 and R18 are independently selected from the group consisting of hydrogen and alkyl. In another aspect, a method comprises administering to a patient having a bacterial infection a therapeutically effective amount of one or more compounds of Formula (II) and/or salt(s) thereof: wherein R1-R4 are independently selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkyl-aryl, alkyl-heteroaryl, amide, sulfonamide, and urea, wherein the alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkyl-aryl, alkyl-heteroaryl are optionally substituted with one or more substituents selected from the group consisting of (C1-C10)-alkyl, (C1-C10)-alkenyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkoxy, amide, sulfonamide, urea, halo, hydroxy, C(O)OR5, and C(O)R6, wherein R5 is selected from the group consisting of hydrogen, alkyl and alkenyl and R6 is selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl and NR7R8, wherein R7 and R8 are independently selected from the group consisting of hydrogen, alkyl, alkenyl, aryl and heteroaryl; and wherein X is selected from the group consisting of CR9R10, O, S, SO2, and NR11R12, and wherein Y is selected from the group consisting of OH and NR13R14, wherein R9-R14 are independently selected from the group consisting of hydrogen, alkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, alkenyl, aryl, heteroaryl, amide, sulfonamide, urea and C(O)R15 wherein R15 is selected from the group consisting of hydrogen, alkyl, alkenyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl and heteroaryl and wherein R13 and R14 may optionally form a ring structure; and n is an integer from 0 to 5; and wherein Z is independently selected from the group consisting of C and N; and n is an integer from 0 to 5. Compounds and/or salt(s) of Formula (I), (IA) and Formula (II) can be administered in any amount consistent with treating bacterial infections. In some embodiments, one or more of the compounds are administered in an amount of 1 μg/ml to 1 μg/ml. In some embodiments, one or more of the compounds are administered in an amount of 1 μg/ml to 100 μg/ml or 1 μg/ml to 15 μg/ml. Additionally, compounds and/or salt(s) of Formula (I), (IA) and Formula (II) can be combined with any physiologically suitable carrier or excipient. In another aspect, pharmaceutical compositions are described herein. In some embodiments, the pharmaceutical compositions are operable for treating bacterial infections and/or cancerous tissue. A pharmaceutical composition, in some embodiments, comprises a compound of Formula (I), Formula (IA) and/or Formula (II) and/or salt(s) thereof. General structures of Formula (I), Formula (IA) and Formula (II) are provided hereinabove. Moreover, several non-limiting embodiments are illustrated in In another aspect, methods of treating bacterial infections are described herein employing compounds previously unknown to exhibit antibacterial activity. With the aim of finding antibiotics with novel MOAs, an unbiased whole-cell screening approach was applied. To include antibiotics that target either gram-negative and gram-positive bacteria, compounds were screened that inhibited growth of the To amplify the ‘growth’ signal compound-exposed To understand where in chemical space antibacterial compounds reside in comparison to all other molecules in the library, the water to octanol partitioning coefficient was calculated employing the proven XLOGP algorithm and, for every screened molecule, these values were plotted against the molecular weight of each compound (see From the 186 unique hits, 32 lead compounds were selected that either had not been identified as antibiotics or had unknown or ambiguous MOAs for further investigation. To understand the potency of these compounds, their minimum inhibitory concentration (MIC) was measured on the imp strain using the microdilution method in 96-well plates. The 32 leads were pared down to the 20 most potent plus the single remaining Chiromics library compound that still displayed activity. These compounds and their MICs are provided in Table I. To determine the MOAs of the lead compounds, a single-cell, high-content imaging methodology known as bacterial cytological profiling, BCP, was improved upon. First, a training set of compounds with known MOAs spanning 37 distinct antibiotic drug families was assembled for comparison to the present set of unknown leads. Other than increasing the throughput of the BCP assay by making 45 agarose pads per slide which gives the method a throughput of approximately 100 samples a day, the exact same set of dyes, relative antibiotic concentrations, and cell preparatory methods were used as previously described in Poochit Nonejuie et al., Bacterial cytological profiling rapidly identifies the cellular pathways targeted by antibacterial molecules, In total, 14 features were extracted from each single antibiotic treated cell, which were divided into three classes. The cell morphology class consisted of measurements of area, length, width and eccentricity. For the nucleoid morphology class, these same features were measured, but of the nucleoid, plus an additional feature which is the ratio of nucleoid area to cell area, termed nucleoid decondensation. Finally, the mean intensity of each of the dyes, Sytox Green, Dapi and FM4-64, for the membrane permeability class were measured. These data were then compared using three complimentary approaches: a simple principle component analysis (PCA) on the means of each feature for each antibiotic treatment group, a PCA on the means with an additional set of group features composing the variance of each feature for each antibiotic treatment class, and finally a neighborhood defining method that makes pairwise comparisons of the mahalanobis distances of each group to all other groups. The first method neglected the variance in cell death while the final two attempted to account for it. To make each feature comparable for the dimensional reduction analyses, each feature was log2-scaled and subsequently mean-centered and scaled by its variance. In both of these analyses it was decided to keep principle components until greater than 90% of the cumulative variance was accounted for. In the simple average feature scheme, this resulted in reducing the number of dimensions down to 4 from the original 14. For the second scheme where the averages and the variances were employed, which were also mean-centered, of each feature as dimension this requirement reduced the dimensions down to 6 from 28. The loadings for each of the principle components that were kept are shown in The final data analysis scheme took the heterogeneity observed in the antibiotic-treated cell groups into account without reducing the dimensions of the data, while also accounting for position of a single treatment group in the context of all other groups. For each treatment group, a neighborhood representation vector was populated with the one-way mahalanobis distance as measured from the single-cell feature mean in question to the distributions measurement of all other treatment groups. This distance was normalized by the covariance matrix of the antibiotic treatment group so that dimensions with large amounts of variance, for example in the case of triflupromazine mean Sytox stain intensity per cell (see IV. Triflupromazine and aminoglycoside Antibiotics both Activate the cpx Envelope Stress Response in Cytological profiling has been heralded as being capable of accurately pairing, not just the MOAs of antibiotics that inhibit the same pathway, but even differentiating between different molecular targets on the same molecule. It was sought to understand how closely families of cell-death states, as reported by the neighborhood analysis described herein, were in their overall response to the antibiotics in question. To this end, the clustered family consisting of three well-known aminoglycosides (kanamycin, apramycin, and gentimicin) was selected along with an antipsychotic that was observed to bear antibacterial activity(triflupromazine) but whose MOA was in question. It has been suggested that aminoglycoside antibiotics (AGAs) lead to bacterial cell death by inducing mistranslation of cytoplasmic membrane-associated proteins which compromise the lipid bilayer. Thus, that the three AGAs fell into a distinct neighborhood not similar to the other ribosome-targeting antibiotics was unsurprising. Since the Cpx envelope stress response has been implicated in conferring AGA resistance to V. Triflupromazine Resistance does not Confer Resistance to AGAs Having confirmed that triupormazine and AGAs induce the same stress response, it was sought to see if they might bind to the same molecular targets. To do this, VI. Compounds with Novel MOAs have Broad-spectrum Activity The mahalanobis distance based analysis suggests that 8 of the 21 lead compounds cause phenotypic death-states unlike any other of the antibiotics tested (see In some embodiments, compounds described herein may exhibit antibacterial activity via disruption of one or more bacterial metabolic pathways. For example, one or more compounds may interfere with or disrupt the folate biosynthetic pathway. Notably, N3-Cyclopropyl-7-[[4-(1-methylethyl)phenyl]methyl]-7H-pyrrolo[3,2-F]quinazoline-1,3-diamine dihydrochloride (SCH 79797) displayed the ability to inhibit or otherwise disrupt the folate biosynthetic pathway, rending this compound effective against gram-negative and gram-positive bacteria without being prone to resistance. Details of folate metabolism disruption and antibacterial efficacy of SCH 79797 is provided in Example 1. To acquire bacterial mutants that were resistant to SCH79797, a culture of WT The MOA investigation was initiated by measuring the activity of SCH 79797 (SCH) against a broad-spectrum of both gram positive and negative organisms ( The classical method of identifying the molecular target of an antibiotic is by selecting for antibiotic resistant mutants. However, SCH resistant mutants were not easily identified. A potential reason for this may be that SCH has multiple targets. To probe this hypothesis, a To investigate what aspects of folate synthesis were being altered by SCH treatment, metabolomics and mass spectrometry were performed to measure the relative abundance of folate metabolites in NCM 3722 To further validate this hypothesis, thermal profiling was performed on lptd4213 Lastly, the in vitro enzymatic activity of purified FolA from Although SCH shares a target with Trimethoprim, these two antibiotics do not have similar phenotypes. Trimethoprim resistance is much more frequent than SCH. Additionally, previous literature has demonstrated that ΔthyA cells are resistant to antifolate drugs when supplemented with exogenous Thymine. However, ΔthyA 1ptD4213 cells are still susceptible SCH while resistant to high levels of Trimethoprim ( Various embodiments of the invention have been described in fulfillment of the various objects of the invention. It should be recognized that these embodiments are merely illustrative of the principles of the present invention. Numerous modifications and adaptations thereof will be readily apparent to those skilled in the art without departing from the spirit and scope of the invention. In one aspect, methods of treating bacterial infections are described herein employing compounds having more than one target for antibacterial activity. Additionally, pharmaceutical compositions comprising such compounds are also described. 1. A pharmaceutical composition comprising a compound of Formula (I) and/or salt thereof: wherein R1, R63, and R4 are independently selected from the group consisting of hydrogen and alkyl; wherein R2 is selected from the group consisting of aryl and heteroaryl; wherein X and Z are independently selected from the group consisting of C, N and O; and; wherein Y is selected from the group consisting of OH and NR9R10, wherein R9 and R10 are independently selected from the group consisting of hydrogen, alkyl, heteroalkyl, cycloalkyl, and heterocycloalkyl, wherein R9 and R10 may optionally form a ring structure; and n is an integer from 0 to 5; wherein the compound of Formula (I) is present in the pharmaceutical composition at a concentration of 0.1 μg/ml to 100 μg/ml to induce membrane depolarization and/or membrane permeability in cells of a disease and/or disrupt a folate metabolic pathway in the cells of the disease. 2. The pharmaceutical composition of 3. The pharmaceutical composition of 4. The pharmaceutical composition of 5. The pharmaceutical composition of 6. The pharmaceutical composition of 7. The pharmaceutical composition of 8. The pharmaceutical composition of 9. The pharmaceutical composition of 10. The pharmaceutical composition of 11. The pharmaceutical composition of 12. The pharmaceutical composition of 13. A method of treating a disease in a patient comprising:
inducing membrane depolarization and/or membrane permeability in cells of the disease; and disrupting a folate metabolic pathway of the cells of the disease, wherein inducing the membrane depolarization and/or membrane permeability and disrupting the folate metabolic pathway is effectuated by administering to the patient a compound of Formula (I) and/or a salt thereof at a concentration of 0.1 μg/ml to 100 μg/ml: wherein R1, R3, and R4 are independently selected from the group consisting of hydrogen and alkyl; wherein R2 is selected from the group consisting of aryl and heteroaryl; wherein X and Z are independently selected from the group consisting of C, N and O; and; wherein Y is selected from the group consisting of OH and NR9R10, wherein R9 and R10 are independently selected from the group consisting of hydrogen, alkyl, heteroalkyl, cycloalkyl, and heterocycloalkyl, wherein R9 and R10 may optionally form a ring structure; and n is an integer from 0 to 5. 14. The method of 15. The method of 16. The method of 17. The method of 18. The method of 19. The method of 20. The method of 21. The method of 22. The method of 23. The method of 24. The method of 25. The method of RELATED APPLICATION DATA
FIELD
BACKGROUND
SUMMARY
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION
Definitions
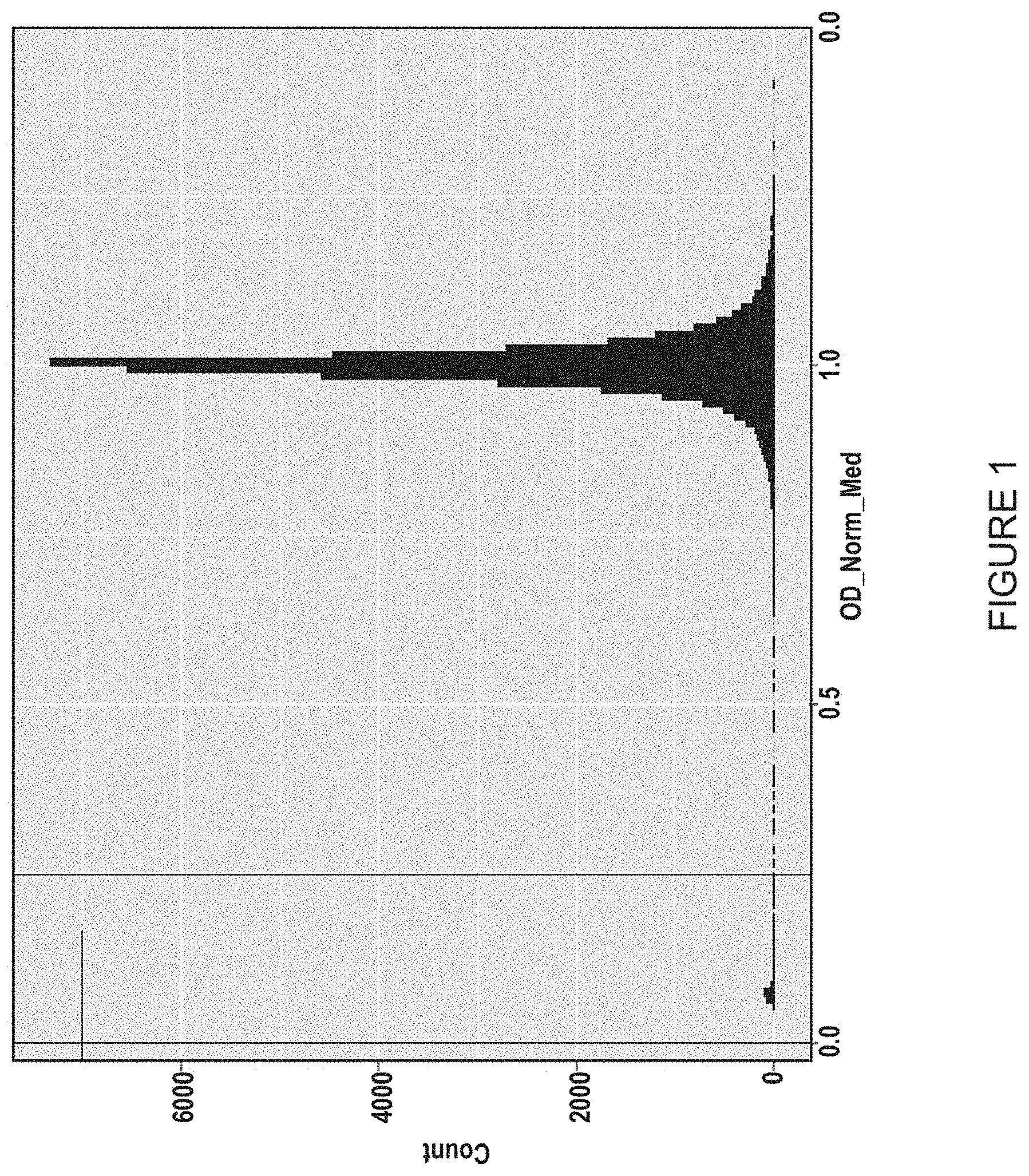
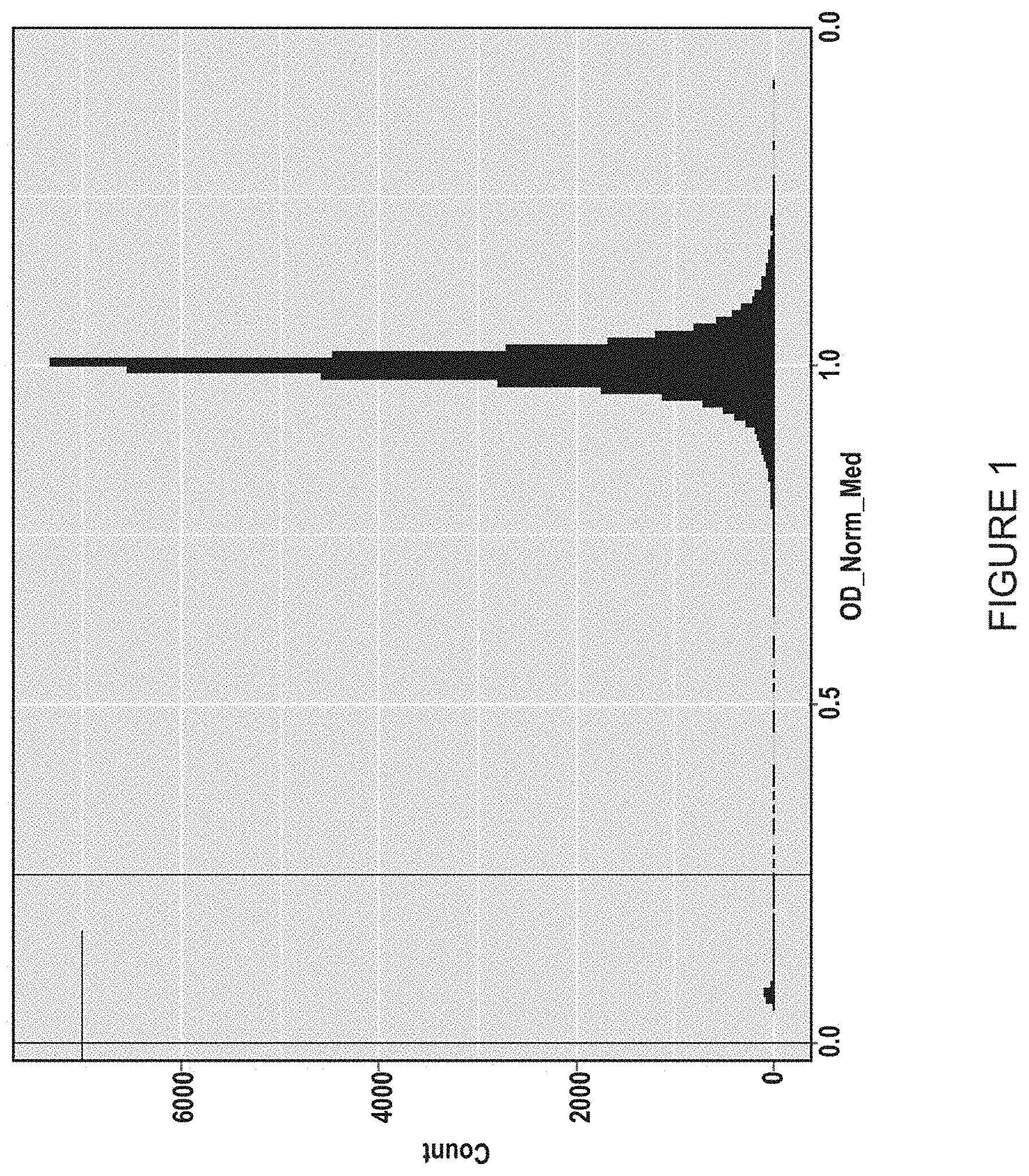
I. Primary Screen
II. MIC Determination of Hits
Lead Compound MICs Compound MIC (μM) Rivaroxaban 0.05 Bleomycin 0.1 AZT* 0.2 Calmidazolium chloride 3.125 ONO-RS-082 3.125 AZT* 3.125 Tramadol hydrochloride 6.25 AC-93253 iodide 6.25 Floxuridine 6.25 Auranofin 6.25 GW5074 6.25 Dichlorophen 12.5 DL-erythro-dihydrosphingosine 12.5 Alexidine hydrochloride 12.5 H 89 dihydrochloride 12.5 Homidium bromide 25 Bronopol 25 Idarubicin 25 Triflupromazine 25 SCH 79797 25 Chlormidazole 25 CP000294 125 *A span of AZT concentrations that inhibited growth of cells with an intervening region of growth was observed. III. High-throughput Bacterial Cytological Profiling
Strains used in MIC determination of lead compounds with novel MOAs Species Strain Description ATCC BAA-1875 Toxigenic ATCC29399 Human skin isolate ATCC BAA- 1710 Multi-drug resistant ATCC 25416 ATCC8090 NCTC 13461 CTX-M betalactamase positive ATCC35056 ATCCBAA-1705 KPC carbapenemase positive ATCC25830 CCUG57598 Cip-R, Cef-R ATCC29906 BCCM 27650 Multi-drug resistant ATCC13880 ATCC 13637 ATCC BAA-1143 ESBL ATCC BAA-2320 Vancomycin resistant ATCC 110 NARSA NRS384 Methicillin resistant NARSA VRS11b Vancomycin resistant NARSA NRS17 Intermediate vancomycin resistance ATCC 51625 Methicillin resistant NTU HospitalTM532 Multi-drug resistant EXAMPLE 1
Antibacterial Activity of SCH 79797
EXAMPLE 2
SCH79797 Mechanism of Action (MOA)