PROCEDURE FOR THE DESULFURIZATION OF GASOLINE INVOLVING DESULFURIZATION OF THE HEAVY AND INTERMEDIATE FRACTIONS PRODUCED BY ONE FRACTIONATION STAGE AND AT LEAST THREE CUTS
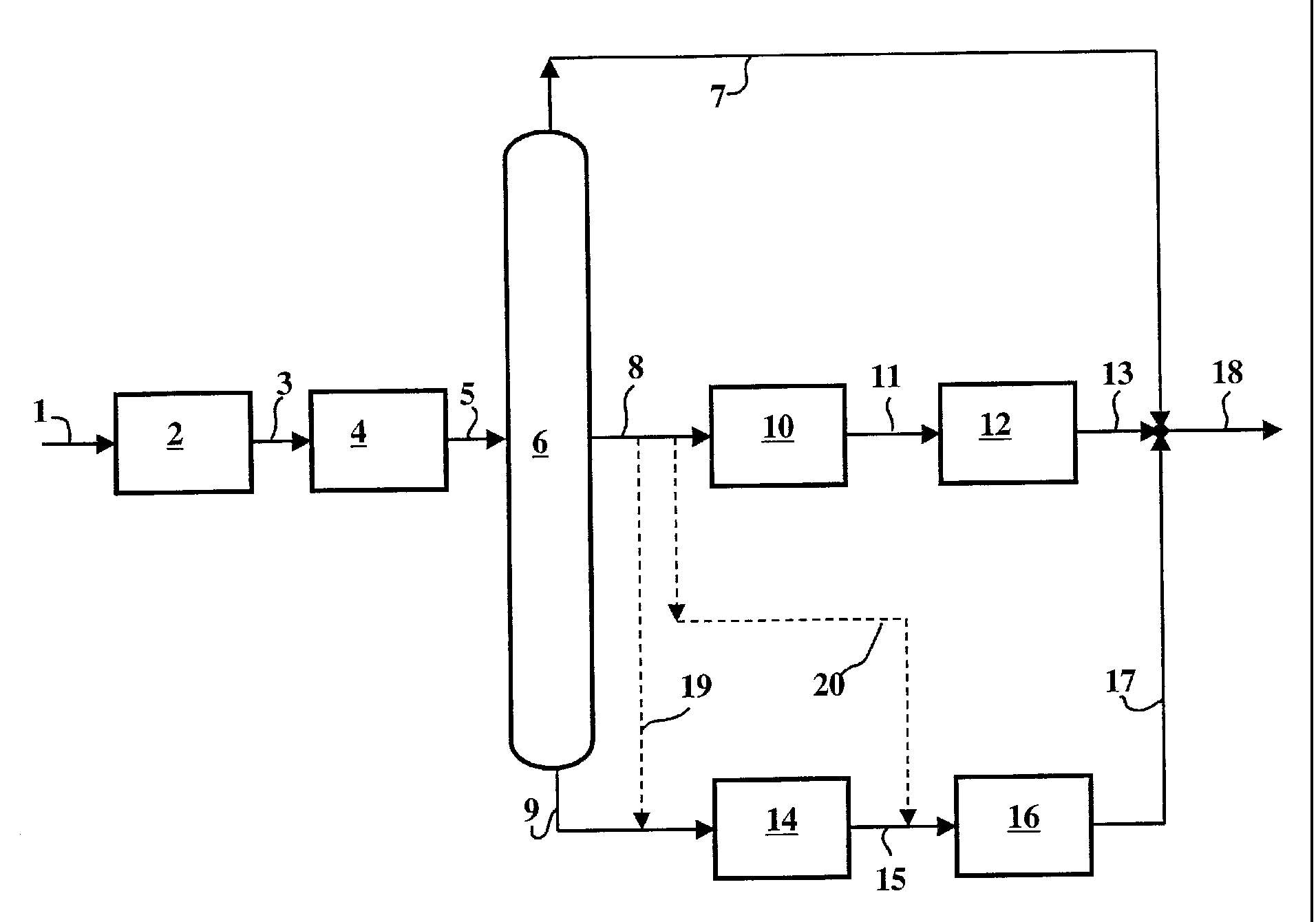
Method for desulfurizing gasoline comprising a desulfurization but intermediate and heavy ends from a fractionation into at least three sections prior:5, 10 15 the reformulated gasolines production meeting the new standards of environment required especially in that with decreasing slightly the concentration of olefins but significantly the concentration of aromatic (particularly benzene) and sulfur. The varieties of catalytic cracking, which may represent 30 to 50% of the gasoline pool, and olefin contents have high sulfur. The sulfur present in the reformulated gasolines is attributable, to near 90%, to the catalytic cracking gasoline (the FCC, "fluid bed Catalytic concealed" or fluidized catalytic cracking). The desulfurization (hydrodesulfurization) gasolines and mainly FCC gasolines is thus highly significant obvious attainment or specifications.In addition to the catalytic cracking gasoline, other oils, such as gasolines directly from the distillation of crude oil, or essences of conversion product (nontransgenic Coker, steam cracking or other) may significantly contribute to sulfur in gasoline.2o hydrotreating (hydrodesulfurization) charge passed to catalytic cracking gasolines results typically containing 100 ppm sulfur. The units for hydrotreating catalytic cracking do operate under severe conditions of temperature and pressure, thereby assumes a high investment. In addition, the whole charge of the catalytic cracking process is to be desulfurized, resulting in the treatment loading volume very important.The hydrotreatment (or hydrodesulphurization) catalytic cracking gasolines, 25 when conducted under conventional conditions known to the skilled person can reduce the sulfur content of the cut. However, this method has the disadvantage of causing a very significant drop in the octane number of the cut, due to the saturation of a significant portion of the olefins during the hydrotreatment.30 separating the light petrol and heavy gasoline before hydrotreatment has already been claimed in US-to-4 397,739. This type of separation separates a light cut, olefin-rich, having a low sulfur content, which will not be compatible with future specifications and a heavy fraction rich-depleted olefins and containing a large proportion of the sulfur gasoline the initial. In this patent, it is claimed a hydrodesulfurization process oils comprising a fractionation of gasoline into a light fraction and a heavy fraction and a hydrodesulfurization specific heavy gasoline, but it is not suggested solution for removing the sulfur present in gasoline.2, 5 on the other hand, in US-a 4 131,537 is taught the interest of fractionating the gasoline into several sections, preferably three, according to their boiling point, and the desulfurized under conditions which may be different and the presence of a catalyst comprising at least one metal from group VIB and/or the VIII group. It is stated in this Patent that the greater benefit is obtained Iorsqu ' is fractionated gasoline in three cuts, and when the cutting having boiling points intermediate is treated under mild conditions.10, 15 the request of EP-a 0 725,126 describes a process for the hydrodesulfurization of a cracking gasoline in which the gasoline is separated into a plurality of fractions including at least a first fraction rich in easy to desulfurize compounds and a second fraction rich compounds difficult to desulfurize. Before carrying out this separation, must be previously determining the distribution of the sulfur products by analyses. These analyses are required to select the equipment and conditions of separation.20, 25 3o in this application it is thus indicated that a light fraction of cracked sees its olefin content and its octane value drop significantly Iorsqu ' it is desulfurized without being fractionated. However, the fractionation of said light fraction into 7à 20 moieties followed by analyses of sulfur and olefin contents of these fractions determines the fraction or fractions most rich in sulfur compounds which are then desulfurized simultaneously or separately and mixed with the other fractions desulfurized or not. One such procedure is complex and must be reproduced at each change of the composition of the gasoline to be treated.In Patent no. 98/14480 French, it is taught the interest of fractionating the fuel into a light fraction and a heavy fraction and then perform specific hydrotreated light gasoline on a nickel-based catalyst, and a heavy FCC gasoline over a catalyst comprising at least one metal from group VIII and/or at least one metal from group VIB.35 it has also been proposed, for example in Patent US a5 290,427, hydrotreatment processes essences the steps of fractionating the gasoline, and then introducing the fractions at different levels of a hydrodesulfurization reactor and converting the desulfurized moieties on a zeolite ZSM-to-5, to compensate for the octane loss recorded by means of an isomerization.In these methods, the gasoline to be treated generally show an initial point greater than 70 °c, and again it is necessary to separately process the light petrol 3, 5 i0 15 (moiety corresponding to compounds of boiling point between the c5 (hydrocarbon to 5 carbon atoms) and 70 °c) for example by a softening.The US Patent to-5 318,690 proposes a method comprising fractionation gasoline and a softening light gasoline, while the heavy gasoline is desulfurized, and then converted to the ZSM-a 5 and desulfurized again under mild conditions. This technique is based on a separation of raw gasoline to obtain a light fraction substantially free from sulfur compounds other than mercaptans. In this case said cutting only by means of a softening that removes the mercaptans.Therefore, the heavy fraction contains a relatively large amount of olefins that are partially saturated in the hydroprocessing. To compensate for loss in octane number related to the hydrogenation of the olefins, the Patent recommends cracking on zeolite ZSM-a 5 which produces olefins, but at the expense of efficiency. Further, the olefins may recombine with i ' h2s present in the medium to reform mercaptans. It is then necessary to perform a softening or additional hydrodesulfurization.2o 25, 30 35 summary of the invention:The present invention relates to a method for desulfurizing gasoline, c'est to say a method of producing low sulfur gasolines, which makes it possible to upgrade all of a load (generally a gasoline cut) containing sulfur, preferably a gasoline cut from a catalytic cracking process, and reduce sulfur said gasoline cut to very low levels, without substantial diminution of the gasoline yield, and while minimizing the reduction of the octane number caused by the hydrogenation of the olefins. In addition, the method according to the invention can at least partially restoring potential losses octane due to the hydrogenation of the olefins via the reforming of a fractions of the gasolines placing desulfurized.The method according to the invention is a process for producing gasoline with a low sulfur content, from a feedstock containing sulfur. It comprises at least the following steps:ai) at least one selective hydrogenation of diolefins and acetylenic compounds present in the feedstock, a2) optionally at least one step for increasing the molecular weight of sulfur products Iégers present in the charge or the effluent from step ai.4 i0 15, 20 25 3o 35 this step may optionally be performed simultaneously with the step al on at least a portion of the load, in the same reactor or a different reactor.It may also take place separately on at least a portion of the hydrogenated feedstock to the step al.b) at least one partition (also referred to as fractionation) gasoline obtained in step Al or a2 into at least three fractions (or cuts), a light fraction containing olefins most Iégères initial gasoline (gasoline or light fraction), a heavy fraction in which most of the sulfur compounds initially present in the initial gasoline is concentrated (gasoline or heavy fraction), and at least one intermediate fraction having an olefin content and a relatively low aromatic content and thus low octane relative to moieties Iégères this petrol and heavy.lC) at least one treatment of heavy gasoline separated in step b to a catalyst for decomposing or at least partially hydrogenating unsaturated sulfur compounds, particularly sulfur compounds cyclic, or aromatic such as for example the thiophenic compounds, by lying in under conditions such that the hydrogenation of the olefins on this catalyst is limited. The heavy fraction may be mixed éventuellemment before or after this step LC, with at least a portion of an intermediate fraction from step b separation and preferably non-desulfurized.c2) step Cl is optionally followed by c2 processing the effluent of step Cl on a catalyst to decompose sulfur compounds and sulfur compounds more preferably saturated straight and/or cyclic, with limited hydrogenation of olefins.d) at least one step of significantly lowering the sulfur content and nitrogen content of at least one of intermediate fractions. This step desulfurization and denitrogenation is accompanied preferably substantially complete hydrogenation of the olefins present in this fraction. The fraction thus obtained is then treated by catalytic reforming to increase the octane number of the (of) (e) cutting dîte (e) intermediate (e).5 th) optionally a step e of mixing at least two fractions which one undergoes a desulfurization process in step Cl and optionally c2 and/or in step d.10, 15 treatments catalytic steps Cl and/or c2 can be performed in either a single reactor containing both catalysts, either in at least two different reactors. When the treatment is carried out using two reactors, the last two are preferably placed in series, the second reactor treating preferably integrally the effluent at the outlet of the first reactor, preferably without separation of the liquid and gas between the first and second reactor. It is also possible to use several reactors in parallel or in series for one and/or the other of the steps Cl or c2.Further a step e is preferably performed after step d, this step involves mixing the gasoline separated in step b they have or not undergone desulfurization treatments.20, 25 the load of the method according to the invention is generally a gasoline cut containing sulfur, such as for example a cut from a coking ( coking ) uinté , visbreaking ( visbreaking ), steam cracking, or catalytic cracking (FCC feedstocks).Said filler is preferably made of a gasoline cut from a catalytic cracking unit, whose boiling point range typically extends from about the points of boiling hydrocarbons with 5 carbon atoms up to about (c5) 250 °c. This gasoline may optionally be composed of a significant fraction of gasoline from other areas such as gasolines directly from atmospheric distillation of crude oil (gasoline contrôleur d'of run) or conversion process (essence of nontransgenic Coker or steam cracking for example). The end point of the gasoline cut depends on the refinery from which it grows and the constraints of the market, but remains generally within the limits indicated above.30 detailed description the invention•35 described in the present invention a method for obtaining a gasoline containing sulfur compounds, preferably from a catalytic cracking unit, wherein the fuel is subject firstly a process for selective hydrogenation of diolefins and acetylene compounds, and optionally a subsequent step burdening the sulfur compounds most Iégers , possibly present in the fuel and which would, in the absence of such step, be in light gasoline after fractionation, at least one separation of gasoline into at least three 6, 10 moieties, a treatment of at least one of intermediate fractions for desulfurizing and déazoter significantly this cut before-treatment catalytic reforming, a treatment of the naptha optionally mixed with at least a portion of one of the intermediate fractions, using a known catalyst for promoting the conversion of unsaturated compounds of sulfur present in gasoline, such as for example the thiophenic compounds, into saturated sulfur compound such that the thiophane derivatives, mercaptans, and then optionally a second catalyst promoting the selective saturates sulfur, linear or cyclic already present in the heavy cut or products in the previous processing. The heavy and intermediate fractions thus treated, and optionally light gasoline fraction can then be mixed to obtain a desulfurized gasoline.15, 20 chaining results ultimately a desulfurized gasoline with a control of the olefin content of the octane number or even for high desulfurization rate. By this method, significant hydrodesulfurization rates are reached, reasonable operating conditions specified shown below. In addition, by optimizing the points of intermediate fractions of cuts and selecting those sent to the catalytic reforming step, it is possible to minimize the benzene content of gasoline final (for example at levels of less than 5% weight in the final mixing of the desulfurized gasoline fractions), control-olefin content, and maintaining values of RON and high engine speed.25, 30 35 sulfur species contained in the silane treated by the method of the invention can be mercaptans or heterocyclic compounds, such as for example the thiophenes or the alkyl thiophenes, or heavier compounds, such as benzothiophene or dibenzothiophene. When a gasoline containing sulfur compounds Iégers is fractionated into two fractions, a light fraction rich in olefins and a heavy fraction depleted in olefins, sulfur compounds Iégers (for example: ethyl mercaptan, propylmercaptan and optionally the thiophene) may be partially or even completely present in the light gasoline. It is often necessary to apply a further treatment with this light fraction to decrease the sulfur content that it contains. Conventionally, this treatment is for example a softening extractive eliminating gasoline Iégers sulfur compounds present in the form of mercaptan. Also that the treatment inevitably aggravates the operation cost, it is operational only if sulfur is in the form of mercaptan. The cut point of gasoline must therefore be preferably limited so as not to result in the presence of thiophene in the light gasoline. The latter forms in effect azeotropes with a ' AC 02342131 2001 - 03 - 26 7 some hydrocarbons, is will not separate in the light gasoline that the olefins in CS and a small portion of the olefins in the hardly cause under too great a fraction of thiophene in this cut.In the method according to the invention, to allow retrieval of a fraction more than 5 significant olefins present in the light gasoline while limiting the sulfur content of this fraction without additional processing, it is proposed preferably condition the charge, after a first selective hydrogenation step, conditions and catalysts that convert the sulfur compounds Iégers sulfur-containing compound from higher boiling point so that they will return to 10 after the separating step, in the machining. Gasoline is then fractionated into at least three sections: a light fraction that contains a significant fraction of olefins initially present in the gasoline to be treated but a very small amount of sulfur compounds, at least one intermediate fraction which is desulfurized and déazotée , and then further processed on a reforming catalyst and a fraction which is desulfurized heavy 15 under defined conditions and using a catalyst or a chain of catalysts for obtaining high desulfurization high while limiting the rate of said olefins and therefore the octane loss.To minimize the benzene content of gasoline final modes as a more preferred embodiment of the invention comprises treating the load conditions and to 20 catalysts that convert the sulfur compounds Iégers sulfur-containing compound boiling point or higher being after the separation step, in the machining. Gasoline is then separated into 4 sections:- a light fraction which contains the major fraction of molecules to carbon atoms (c5) and a significant fraction of molecules 6 to 25 carbon atoms (c6) originally present in the gasoline to be treated. This fraction is characterized by a high concentration of olefins and a very low sulfur content, - a first intermediate fraction consisting essentially ( c'est to say more than 60% preferably more than 80% weight) of molecules to six carbon atoms (c6) and a portion of the molecules having 7 carbon atoms (c7) 30 and that most of the sulfur compounds having a boiling point close to that of the azeotrope that it forms with hydrocarbons, to about 20% near. This fraction is preferably mixed with heavy gasoline before hydrodesulfurization (steps Cl and c2) or after at least partial decomposition or hydrogenation of unsaturated sulfur compounds (step LC) but before decomposition of saturated sulfur compounds 35 (step c2), to be desulfurized in conditions for limiting the hydrogenation of olefins. This oil is preferably not sent to the catalytic reforming because it contains a set of compounds that when processing reforming, would benzene formation. These compounds are known by the skilled person as being of "benzene precursors" 8 and may be for example a methylcyclopentane, cyclohexane, n-hexane or benzene itself. As legislation allows the local, this intermediate cutting however may optionally be sent to the processing unit of the second intermediate cutting; 5 - a second intermediate fraction which is desulfurized and déazotée on a conventional hydroprocessing catalyst to remove substantially all of the sulfur and nitrogen initially present in this cut ( c'est to say lower their content to less than 5 ppm, preferably less than 1 ppm). This treatment is accompanied by the hydrogenation substantially complete olefin this cut, which can lower the olefin content in 10 to values preferably less than 10% by weight, and more preferably less than 5% by weight. This cut is then processed on a catalytic reforming catalyst for the isomerization and dehydrocyclization of paraffins and naphthenes with formation of branched paraffins and aromatics.15 the heavy cut, preferably blended with the first intermediate fraction, is desulfurized under defined conditions and by means of a chaining of catalysts for obtaining high desulfurization high while limiting the rate of said olefins and therefore the octane loss.20, 25 30, 35 and, when the cuts esences light, intermediate, heavy and are mixed after desulfurization treatments according to the invention, the loss of RON or engine, expressed as the difference between the mean value (RON-+ my)/2 are observed in this mixture and the mean value (RON-+ my)/2 of the initial load, is limited to less than 2 dots octane, preferably less octane 1.7 point, and more preferably less than 1.5 dots octane, and most preferably less than 1 point in octane. In some cases, the mean value (RON-+ my)/2 of the desulfurized gasoline by means of the process according to the invention may even decrease of less 0.5 octane spot relative to the mean value (RON-+ my)/2 of the load, or even larger diameter of at least 0.5 spot.The sulfur content of gasoline produced by catalytic cracking (FCC feedstocks) depends on the sulfur content of the treated feedstock to the FCC, as well as the end point of the cut. Generally, the sulfur contents of the entirety of a gasoline cut, especially those from the FCC, are greater than 100 ppm by weight and mostly greater than 500 ppm by weight. For gasolines having end points greater than 200 °c, the sulfur levels are often higher than 1000 ppm by weight, they can even in some cases reach values on the order of 4000 to 5000 ppm by weight."" AC 02342131 2001 - 03 - 26 the method according to the invention applies particularly when high levels of desulfurizing gasoline are required, i.e. where the desulfurized gasoline must contain at most 10% of the initial gasoline sulfur and optionally at most 5% or even at most 2% sulfur gasoline initial corresponding to levels greater than 90% desulfurization or superior to 95 or 98% to.The method according to the invention comprises at least the following steps:ai) at least one step of passing the load, preferably comprising the entire gasoline fraction, on a catalyst 10 for selectively hydrogenate diolefins and acetylenic compounds of gasoline without hydrogenate the olefins, a2) optionally at least one optional step includes passing at least a portion of the initial gasoline or petrol hydrogenated to the step al, preferably the entire initial gasoline or hydrogen to the step al, 15 on a catalyst for converting at least partially Iégers sulfur compounds (e.g.: ethylmercaptan , the mercaptan-propyl, thiophene) with at least a portion of the olefins or diolefins heavier sulfur compounds. This step is preferably performed simultaneously with the step al through initial such as petrol on a catalyst capable of both hydrogenate diolefins and acetylenic 20 and transform the sulfur compounds Iégers and part of the diolefins or olefins in heavier sulfur compounds, or on a distinct catalyst but for performing this transformation in the same reactor that the step al. it is optionally possible to observe some types of loads on an increase in the mercaptan content at the end of ai) or a2), this increase in the content of 25 mercaptan is probably due to a hydrogenolysis disulfides of high molecular weight. In this step, all of the sulfur compounds Iégers , i.e. the compounds whose boiling point is lower than that of thiophene, can be transformed. It may be mentioned among these compounds the cs2, the dimethylsulfide, the methyléthylsulfure or COS.30 b.) at least one step of separating the initial gasoline into at least one light gasoline (light fraction) at least one intermediate gasoline (intermediate fraction) and a naptha (heavy fraction). The cutting point light gasoline is determined to limit the sulfur content of the light gasoline and allow its use in the gasoline pool, preferably without any additional 35 treatment, especially without desulfurization. The cutting point of the intermediate gasoline is generally conditioned by the restrictions of the method imposes reformaçje wherein the structure will be treated. A preferred configurations comprises fractionating the gasoline to obtain a light fraction, a heavy fraction, and two intermediate spirits: a first intermediate gasoline mainly, - AC 02342131 2001 - 03 - 26 10 5 10 15 composed of compounds to six carbon atoms, which is then preferably blended with the heavy fraction of gasoline, preferably before step Cl or optionally between the treatment of saturation of the unsaturated sulfur compounds (step LC) and the decomposition of these compounds (step c2) and a second intermediate gasoline consisting mainly molecules containing 7 or 8 carbon atoms (or c8 c7) which is processed in step d.cl) at least one step comprising treating at least a portion of heavy gasoline and optionally at least one intermediate part of sections over a catalyst for the transformation of at least a portion of the unsaturated sulfur compounds present in said load, such as for example the thiophenic compounds, into saturated sulfur compounds, such as for example the thiophanes (or thiacyclopentane ) or mercaptans, in a succession of reactions described below:v. S e thiophene thiophane derivatives mercaptain 2o 25 3o the decomposition reaction with liberation of total h2s is also possible and typically accompanies significantly reactions of saturation of the unsaturated sulfur compounds.This hydrogenation reaction may be performed on any catalyst promoting these reactions such as for example a catalyst comprising at least one metal from group VIII and/or at least one metal of group BLV, preferably at least partly in the form of sulfides. When such a catalyst is used, the operating conditions are adjusted in order to hydrogenate at least in part the unsaturated compounds, such as the thiophenic compounds, while limiting t'a hydrogenation of olefins.c2) after this treatment it may be provided as an optional step c2 wherein the saturated sulfur compounds present in the initial gasoline or obtained after the reaction is complete (step LC) saturation are converted h2s according to the exemplary reactions:O 11 h2s 10, 15 2o 25 this processing may be performed on any catalyst for the conversion of saturated sulfur compounds (mainly the ether compounds thiophanes type or mercaptan). It can for example be performed on a catalyst based on at least one metal from group VIII of the periodic table old (groups 8, 9 or 10 of the new periodic table).Thus desulfurized heavy gasoline is then optionally stripped ( c'est to say that a gas stream, preferably containing one or inert gas is passed through this petrol), in order to remove excess' h2s product in the hydrodesulfurization.Light gasoline separated in step b and the desulfurized heavy gasoline in step Cl and/or step c2 may optionally be blended and then either sent in the gasoline pool of the refinery, is valued separately without being mixed.d.) processing at least one of intermediate cuts a method to remove substantially all of the sulfur and nitrogen fraction, and preferably to hydrogenate all of the olefins, then treating the effluent thereby hydrotreated over a reforming catalyst that allows the isomerization and dehydrocyclization of paraffins.e) optionally a step e of mixing at least two fractions of which at least one has undergone a desulfurization process in step Cl and optionally c2 and/or in step d.The individual steps of the method according to the invention are described in more detail herein after.- Diolefin hydrogenation and acetylenic (step al):3o the hydrogenation of the dienes is a step that eliminates, before hydrodesulfurization, almost all dienes present in the gasoline cut ", AC 02342131 2001 - 03 - 26 12 containing sulfur to be treated. It takes place preferentially in first step (step al) of the method according to the invention, generally in the presence of a catalyst comprising at least one metal of group VIII, preferably selected from the group consisting of platinum, palladium and nickel, and a support. Here is for example 5 a nickel based catalyst or palladium deposited on an inert substrate, such as for example alumina, silica or a support containing at least 50% alumina.The pressure employed is sufficient to maintain more than 60%, preferably 80%, and more preferably 95% by weight of the gasoline to be treated in liquid phase in the reactor 10; it is most generally between about 0.4 and about 5 mpa and preferably greater than 1 mpa, more preferably between 1 and 4 mpa. The liquid hourly space velocity to be treated is between about 1 and about 20 H-I(volume of feed per volume of catalyst per hour), preferably between 3 and 10 hr "1, most preferably between 4 and 8 hr" 1. The temperature 15 is most generally between about 50 and about 250 °c, and preferably between 80 and 230 °c, and more preferably between 150 and 200 °c, to ensure a sufficient conversion diolefins. Highly preferred it is limited to at most 180 °c. The ratio of hydrogen to load expressed in liter is typically between 5 and 50 liters per per liter, preferably between 8 and 30 liters per liter.20 the choice of operating conditions is particularly important. It will operate mostly under pressure and in the presence of an amount of hydrogen in a small excess of the stoichiometric value necessary to hydrogenate the diolefins and acetylenic. Hydrogen and the feed to be treated are injected currents rising or falling in a reactor comprising preferably a fixed catalyst bed.25 another metal can be associated with the core metal to form a bimetallic catalyst, such as for example molybdenum or tungsten. The use of such formulations has for example been catalytic claimed in Patent FR's 2,764 299.The catalytic cracking gasoline may contain up to several % by weight of diolefins. After hydrogenation, the diolefin content is typically reduced to 30 less than 3000 ppm, or even less than 2500 ppm and more preferably less than 1500 ppm. In some cases, it may be obtained less than 500 ppm. The diene content after selective hydrogenation can even if necessary be reduced to less than 250 ppm.In a particular embodiment of the method according to the invention, the hydrogenation step 35 dienes takes place in a reactor comprising a catalytic hydrogenation catalytic reaction zone through which the entire load and the amount of hydrogen needed to perform the desired reactions.: ' AC 02342131 2001 - 03 - 26 13 - Iégers converting compounds of sulfur in heavier compounds (step a2):This optional step comprises transforming the compounds Iégers sulfur, which 5 to the end of stage b of separation would be disposed in the light gasoline in the absence of this step. It takes place préférentie ! ard over a catalyst comprising at least one element of group VIII (groups 8, 9 and 10 of the new periodic table), or comprising a resin. In the presence of the catalyst, the sulfur Iégers compounds are converted to sulfur compounds heavier, driven in gasoline 10 heavy.This optional step may optionally be performed simultaneously with the step al. for example, it may be particularly preferable to operate, in the hydrogenation of diolefins and acetylenic, under conditions such that at least some of them are 15 form mercaptan are transformed. Thus a certain reduction in the mercaptan content is obtained. For this purpose, use can be made of the diene hydrogenation procedure one described in EP-a 0 832,958, which advantageously uses a palladium catalyst, or that described in Patent FR's 2,720 754.20 another possibility is to use a nickel based catalyst same or different from the catalyst of the step al, such as for example the catalyst recommended in the process of US Patent-to-3691 066, converting mercaptans ( butylmercaptan ) in heavier sulfur compounds (methyl thiophene).Another possibility for performing this step, comprising hydrogenating at least 25 part thiophene in thiophane derivatives whose boiling point is higher than that of the thiophene (boiling point 121 °c). This can be done on a nickel based catalyst, platinum or palladium. In this case the temperatures are generally between 100 and 300 °c and preferably between 150 and 250 °c. The ratio H2/load is adjusted between 1 and 20 liters per liter, preferably between 2 and 30 liters per liter, to allow the desired hydrogenation thiopheneic compounds while minimizing the hydrogenation of the olefins present in the feed. The space velocity is generally between 1 and 10 of H1, preferably between 2 and 4 h1 and pressure of between 0.5 and 5 mpa, preferably between 1 and 3 mpa. In this step, whatever the method used, a portion of the sulfur compounds such Iégers 35 wherein the sulfides (dimethyl sulfide, methyl ethylsulfure ), the cs2, the COS may also be transformed.Another possibility for performing this step comprises passing the gasoline on a catalyst with an acid function which allows for the addition of sulfur compounds in the form of mercaptans on the olefins and conducting the reaction of 14 atkylation thiophene by the above-described olefins. 11 is for example possible to pass the gasoline to be treated on an ion exchange resin, such as a sulfonic resins. The operating conditions will be adjusted to achieve the desired transformation while limiting side reactions of the oligomerization of olefins. 5 operation is carried out usually in the presence of a liquid phase, at a temperature between and and preferably between 150 °c 10 and 70 °c. The operating pressure is between 0.1 and 2 mpa and preferably between 0.5 and 1 mpa. The space velocity is generally between 0.5 and 10 h1 and preferably between 0.5 and 5 hr " 1. In this step the rate of conversion of mercaptans is generally greater than 50% and the rate of conversion of the thiophene 10 is typically greater than 20%.15, 20 25 to minimize the activity of the acid catalyst optionally used oligomerizing, the propellant may be added with a compound known to inhibit activity oligomerizing acid catalysts such as for example alcohols, ethers or water.- Separation of gasoline into at least three fractions (step b):In this step the gasoline is fractionated into at least three fractions:a light fraction with a sulfur content residual preferably limited to 50 ppm, more preferably limited to 25 ppm, and most preferably limited to 10 ppm, and preferably for use this cutting without other treatments to reduce its sulfur content, at least one intermediate fraction having an olefin content and a relatively low aromatic content, a heavy fraction in which most of the sulfur, initially present in the charge, is concentrated.30 separation takes place preferably by means of a conventional distillation column also called splitting. This fractionation column is to separate a light fraction of gasoline containing a small fraction of the sulfur, at least one intermediate fraction composed mainly of compounds having 6 to 8 carbon atoms and a heavy fraction containing most of the sulfur present in gasoline initially initial.35 this column generally operates at a pressure of between 0.1 and 2 mpa and preferably between 0.2 and 1 mpa. The number of theoretical plates of the column separation is typically between 10 and 100 and preferably between 20 and the reflux ratio, of the column expressed by the ratio between the flow rate of liquid in the column and the feed flow rate is typically less than unity and preferably less than 0.8, when these rates are measured in kilograms per hour (kg weight/hr).15, 5 fractionated products obtained at the end of the separation generally contains at least all c5 olefins, preferably the compounds in c5 and at least 20% olefins c6. Generally, this light fraction has a low sulfur content (e.g. less than 50 ppm), i.e. it is not generally necessary to treat the light cut before use as fuel. However, in some cases extreme cases, a softer light gasoline can be envisaged.10 - hydrogenation of unsaturated compounds of sulfur (step LC):This step, which applies to heavy gasoline optionally mixed with at least a portion of an intermediate fraction obtained at the end of step b separation. Preferably the intermediate fraction consists essentially ( c'est to say more than 60%, preferably more than 80% weight) of molecules in c6 or c7 15 as well as the majority of the sulfur compounds having a boiling point close to that of the azeotrope of thiophene with paraffins, to about 20% near.This step includes transforming at least a portion of the unsaturated sulfur compounds such as the thiophenic compounds, into saturated compounds e.g. thiophanes (or thiacyclopentanes ) or mercaptans, or optionally hydrogenolyzing 20 at least partially unsaturated sulphur compounds to form of I ' h2s.This step may for example be performed by passing the heavy fraction, optionally mixed with at least a portion of an intermediate fraction over a catalyst comprising at least one element of group VIII and/or at least one member of the group 25 BLV at least partly as sulfide, in the presence of hydrogen, at a temperature between about 200 °c to about 350 °c, preferably between 220 °c and 290 °c, under a pressure of generally between about 1 and about 4 mpa, preferably between 1.5 and 3 mpa. The space velocity of the liquid is between about 1 and about 20 H 1 (expressed as the volume of liquid per volume of catalyst per hour 30), preferably between 1 and 10 H 1, most preferably between 3 and 8 H 1. The ratio h2/hc is between 50 to 600 liters per liter and preferably between 300 and 600 liters per liter.To perform, at least in part, the hydrogenation of unsaturated sulfur compounds ressence according to the method of the invention, the time at which the at least one catalyst, 35 comprising at least one element of group VIII (metals of groups 8, 9 and 10 of the new classification, c'est i.e. iron, ruthenium, osmium, cobalt, rhodium, iridium, nickel, palladium or platinum) and/or at least one element of group VIB (metals of group 6 of the new classification, c'est i.e. chromium, molybdenum or tungsten), on a suitable support.16 content expressed in group VIII metal oxide is generally between 0.5 and 15% by weight, preferably between 1 and 10% by weight. The group VIB metal content is generally between 1.5 and 60% by weight, preferably between 3 and 50% by weight.5 the group VIII element, Iorsqu ' it is present, is preferably cobalt, and the group VIB element, when present, is generally molybdenum or tungsten.Combinations such as cobalt-molybdenum are preferred. The catalyst support is usually a porous solid, such as for example an alumina, a silica-alumina or other porous solids, such as for example magnesia, 10 of the silica or titanium oxide, alone or mixed with alumina or silicealumine . To minimize the hydrogenation of the olefins present in the naptha it is advantageous to use preferably a catalyst in which the density of molybdenum, expressed in % weight of moo3 per unit area is greater than 0.07 and preferably greater than 0.10. The catalyst according to the invention 15 preferably has a surface area less than 190 m2/gm, more préférëe less than 180 m2/grams, and most preferably less than 150 m2/gm.After introducing the component and optionally for shaping the catalyst (when this step is carried out on a mixture already containing the base elements), the catalyst is activated in a first step. This activation may correspond to either an oxidation 20 and then to a reduction, or to a direct reduction, either to a calcination only. The calcining step is generally carried out at temperatures ranging from about 100 to about 600 °c and preferably comprised between 200 and 450 °c, under an air flow. The reducing step is carried out under conditions effective to convert at least a portion of the oxidized forms of the metal of group VIII and 25/FV or group b to the metallic state. Generally, it involves treating the catalyst under a hydrogen flow at a temperature preferably at least equal to 300 °c. The reduction may also be effected in part by means of chemical reducing.3o 35 the catalyst is preferably used at least partially in its sulfided form.Introducing the sulfur can occur before or after any activation step, c'est say calcination or reduction. Preferably, no oxidation step is réaliséeaprès that sulfur or a sulfur compound has been introduced onto the catalyst.It is therefore for example preferable when the catalyst is sulfided after drying not calcining the catalyst, but a reduction step may optionally be performed after the sulfurization.Sulfur or a sulfur compound may be introduced ex, c'est to say out of the reactor where the process according to the invention is carried out, or in situ, c'est to say in the CA 02342131 2001 - 03 - 26 17 5 reactor used for the process according to the invention. In the latter case, the catalyst is preferably reduced under the conditions described previously, then sulfurized by passage of a feedstock containing at least one sulfur containing compound, which once decomposed results in the attachment of sulfur on the catalyst. The feedstock may be gaseous or liquid, for example hydrogen containing of I ' the H2S, or a liquid containing at least one sulfur containing compound.10, 15 to a preferred way, the sulfur compound is added on the catalyst selectivation. For example, after the calcination stage, a sulfur compound may be introduced in the catalyst optionally in the presence of another compound. The catalyst is then dried, then transferred into the reactor for carrying out the method according to the invention. In this reactor, the catalyst is then treated under hydrogen to transform at least a portion of the core metal sulfide. A procedure that is particularly suitable for the invention is that described in patents FR's-b2 708,596 and FR's-b2 708,597.2o in the method of the invention the conversion of the sulfur compounds is greater than 15% unsaturated and preferably greater than 50%. At the same time the rate of hydrogenation of the olefins is preferably less than 50%, more preferably less than 40%, and most preferably less than 35%, in this step.25 in the method according to the invention, the treated gasoline in step LC can optionally contain at least a portion of at least one intermediate fraction obtained in step b. For example it may be beneficial to treat in this step a fraction of the gasoline that it is undesirable to send in the fastening means.3o the effluent from this first hydrogenation step is optionally then sent, to step c2 to decompose in h2s saturated sulfur compounds.- Decomposition saturated compounds sulfur (step c2):35 load this step consists either solely of the effluent from step LC, or a blend comprising the effluent of step Cl and at least a portion of at least one intermediate fraction. Preferably the intermediate fraction consists essentially ( c'est to say more than 60%, preferably more than 80% weight) of molecules in c6 or c7 as well as most of the sulfur compounds 18 having a boiling point close to that of the azeotrope of thiophene with hydrocarbons to near 20%.10 in this step, the saturated compounds of sulfur are reacted in the presence of hydrogen, on a suitable catalyst. The decomposition of non-hydrogenated unsaturated compounds during step LC can also occur simultaneously. This transformation is performed, without any significant hydrogenation of olefins, i.e. that in this step the amount of hydrogenated olefins is generally limited to less than 20% by volume relative to the olefin content of the initial gasoline, and preferably limited to 10% by volume with respect to the olefin content of the initial gasoline.The catalysts that may be suitable for this step of the process according to the invention, without not limited, are catalysts generally comprising at least 15 element (metal) base selected from elements of group VIII, preferably selected from the group consisting of nickel, cobalt, iron, molybdenum, tungsten.These metals can be used alone or in combination, they are preferably supported and used in their sulfided form. It is also possible to add at least one promoter to these metals, for example tin. 20 preferred catalysts comprising nickel, or nickel and tin, or nickel and iron, or cobalt and iron, or cobalt and tungsten. Catalysts are more preferably sulfur, and very preferably in-situ or présulfurésexsitu . The catalyst of step c2 is preferably in nature and/or of different composition from that used in the step of Cl.25 the metal content of the catalyst according to the invention is generally between about 1 and about 60% by weight, preferably between 5 and 20% by weight, and most preferably between 5 and 9% by weight. Most preferably, the catalyst is generally shaped, preferably in the form of beads, pellets, extrudates, for example of trilobes. The metal may be incorporated at 30 catalyst by deposition thereon preformed, it can also be mixed with the carrier prior to the shaping step. The metal is generally introduced in the form of a precursor salt, generally water-soluble, such as nitrates, the heptamolybdates . This mode of introduction is not specific to the invention. Any other input mode known to those skilled in the may be suitable.35 catalysts supports used in this step of the method according to the invention are generally porous solids selected from refractory oxides, such as for example, aluminas, silicas and silica-aluminas, magnesia, as well as titanium oxide and zinc oxide, these oxides can be used alone or in admixture with 19 alumina or silica-alumina. Preferably, the carriers are transition aluminas or silica whose specific surface area is between 25 and 350 m2/gm. The naturally occurring compounds, such as for example kieselguhr or kaolin, can also be utilized as supports for catalysts used in this process step.10, 15 in a preferred preparation of the catalyst, after introducing at least one precursor of said metal or metal and optionally shaping of the catalyst, the catalyst is activated in a first step. This activation may correspond either to an oxidation, then to a reduction, or a reduction after drying without burning, is still only to a calcination. The calcining step when present is generally carried out at temperatures ranging from about 100 °c to about 600 °c and preferably comprised between 200 °c and 450 °c, under an air flow.The reducing step is carried out under conditions effective to convert at least a portion of the oxidized forms of the base metal to the metallic state. Generally, it involves treating the catalyst under a hydrogen flow at a temperature at least equal to 300 °c. The reduction may also be effected in part by means of chemical reducing.20 the catalyst is preferably used at least partially in its sulfided form, thereby providing the advantage of minimizing the risk of hydrogenation of unsaturated compounds such as olefins or aromatic compounds during the start-up phase. Introducing the sulfur can arise between different activation steps. Preferably, no oxidation step is carried out only when the sulfur or a sulfur compound 25 was introduced on. the catalyst. Sulfur or a sulfur compound may be introduced ex, c'est to say out of the reactor where the process according to the invention is carried out, or in situ, c'est to say in the reactor used for the process according to the invention. In the latter case, the catalyst is preferably reduced under the conditions described previously, then presulfided by passage of charge 30 containing at least one sulfur containing compound, which once decomposed results in the attachment of sulfur on the catalyst. The feedstock may be gaseous or liquid, for example hydrogen containing of I ' the H2S, or a liquid containing at least one sulfur containing compound.35 to a preferred way, the sulfur compound is added on the catalyst selectivation. AI " example, after the calcination stage, a sulfur compound may be introduced in the catalyst optionally in the presence of another compound. The catalyst is then optionally dried, then transferred into the reactor for carrying out the method of the invention. In this reactor, the catalyst is then treated under hydrogen to transform at least a portion of the base metal and optionally another 20 metal sulfide. A procedure that is particularly suitable for the invention is that described in patents FR's-b2 708,596 and FR's-b2 708,597.After sulfurization, the sulfur content of the catalyst is typically between 0.5 and 25% by weight, preferably between 4 and 20% by weight and most preferably between 10% and 4, 5. The hydrodesulfurization carried out in this step c2 aims at converting h2s the saturated sulfur compounds from gasoline which have already undergone at least one prior hydrogenation of unsaturated sulfur compounds during step LC. It affords an effluent meeting the desired specifications in terms of content of sulfur compounds. Gasoline thus obtained has only one octane loss 10 (decrease in RON and/or engine) low.15, 20 25 the processing to decompose the saturated sulfur compounds from step LC of the method is performed in the presence of hydrogen, with a catalyst comprising at least one base metal selected from the group consisting of nickel, cobalt, iron, molybdenum, tungsten, used alone or as a mixture thereof, at a temperature between about 100 °c to about 400 °c, preferably between about 150 °c to about 380 °c, more preferably between 210 °c and 360 °c, and most preferably between 350 °c and 220 °c, under a pressure of generally between about 0.5 and about 5 mpa, preferably between 1 and 3 mpa, more preferably between 1.5 and 3 mpa. The space velocity of the liquid is between about 0.5 and about 10 hr "1 (expressed as the volume of liquid per volume of catalyst per hour), preferably between 1 and 8 hr" 1. H2/hc ratio is adjusted according to desired hydrodesulfurization rates in the range of between about 100 and about 600 liters per liter, preferably between 20 and 300 liters per liter. Some or all of this hydrogen may optionally be from step LC (unconverted hydrogen) or a recycling hydrogen not consumed in steps AI, α2, c2 or d.30, 35 it has been found that the implementation of said second catalyst in this step, in particular operating conditions, can decompose the saturated compounds, contained in the effluent from step LC, in h2s. This implementation achieves a high overall level hydrodesulfurization on completion of all the steps of the method according to the invention, while the loss resulting octane of olefin saturation, because the conversion of olefin in step Cl is limited to at most 20% génératement vol. olefins, preferably at most 10% by volume.21 hydrotreating at least one intermediate cut (step d)•5 this processing of at least one of intermediate cuts to eliminate substantially all of the sulfur and nitrogen fraction and to treat the effluent and hydrotreated over a reforming catalyst for the isomerization and dehydrocyclization of paraffins. This step includes measuring at least a portion of an intermediate fraction obtained in step b.10 that processes said fraction over a catalyst or set of catalysts for desulfurizing and déazoter fully the fraction under consideration, i.e. to obtain a fraction containing a sulfur content and nitrogen preferably less than 5 ppm, and more preferably less than 1 ppm by transforming sulfur compounds or nitrogen and ammonia h2s respectively.15, 20 25 this step is typically carried out by transfer of at least a conventional hydroprocessing catalyst under conditions effective to remove sulfur and nitrogen. The catalysts which are particularly suitable are for example the catalysts based on a metal-group VIII as cobalt or nickel and a metal of group VI, such as tungsten or molybdenum. Typically, without these conditions are exclusionary, this treatment may be carried out over a catalyst type RH 306 or hr448 sold by procatalyst, generally at a temperature of between 250 and 350 °c, an operating pressure of between 1 and 5 mpa generally, preferably between 2 and 4 mpa, and a space velocity of between 2 and 8 hr widely " 1 (expressed as the volume of liquid feed per volume of catalyst per hour). In the process of substantially all olefins present in this fraction is hydrogenated.30 the effluent produced is cooled down, the products of decomposition are then separated using any technique known to the skilled person. It is possible for example to cite the washing methods, the methods of stripping or extraction.35 corresponding to one of the effluent desulfurized déazotées and intermediate fractions is then processed at a catalyst or a catalyst for linking of the reformation of the dîte moiety, i.e. perform at least in part the dehydrogenation of saturated cyclic compounds, alkane isomerization dehydrocyclization of paraffins and the intermediate fraction present in the treated. This treatment is to increase the octane number of the fraction under consideration. The treatment is performed using conventional catalytic reforming process. It may be advantageous to use for example for this purpose methods dît to "fixed bed" or to "bed 22, 10 movable", c'est i.e. methods in which the catalyst is respectively disposed in fixed bed or fluidized contrary and optionally circulated in at least one reactor and in an external circulation loop comprising optionally other reactors and/or at least one regenerator. In the method implementation, desulfurized effluent is contacted with a reforming catalyst, typically platinum supported on alumina, at a temperature between 400 °c and 700 °c, with an hourly space velocity (kg load processed per hour and per kg of catalyst) of between 0.1 and 10. The operating pressure may be between 0.1 and 4 mpa. A portion of the hydrogen produced during the various reactions can be recycled in a ratio of between 0.1 and 10 moles of hydrogen per mole of load.15, 20 Figure 1 shows an example embodiment of the method according to the invention. In this example, a gasoline cut (initial gasoline) containing sulfur is introduced through line 1 in a catalytic hydrogenation reactor 2, which enables the diolefins selectively hydrogèner and/or acetylenic compounds present in said gasoline cut (step al of the method). The effluent 3 of the hydrogenation reactor is fed into a reactor 4, which comprises a catalyst capable of effecting the transformation Iégers sulfur compounds with olefins or diolefins heavier sulfur compounds (step a2). The effluent 5 of the reactor 4 is then sent to the fractionator 6, which allows the separation of gasoline in 3 (step b) moieties.25 the first fraction obtained is a light cut 7. this light cut preferably comprises less than 50 ppm of sulfur and does not require desulfurization, since the light sulphur compounds present in the initial gasoline were transformed into heavier compounds in step a2.30 a second fraction 8 (intermediate fraction) is obtained which is first sent to a catalytic desulfurization reactor 10, and then via line 11 to a reforming reactor (step d) catalystique .35 a third fraction (heavy fraction) is obtained via line 9. this cut is first treated in a reactor 14 on a catalyst for converting at least a portion of the unsaturated sulfur compounds present in the feedstock to saturated sulfur compounds (stage LC). The effluent of the reactor 14 15 is sent to the reactor 16 (step c2) which contains a catalyst that promotes the decomposition in h2s saturated sulfur compounds initially present in the feed and/or formed in the reaction vessel 14.AC 02342131 2001 - 03 - 26 23 the light cut 7 and effluent are 13 (from the reforming reactor 14) 17 and the effluent (from the decomposition reactor 13), are métangés desulfurized gasoline to form 18 (step e).5, 10 according to other preferred embodiments, presented in Figure 1, it is also possible to send at least a portion of the intermediate fraction (line 8) non-desulfurized, either via line 19 and then mixed with the heavy fraction 9 to the reactor 14 (step LC), either via line 20 and then mixed with the effluent 15 to the reactor 16 (step c2).The examples which follow illustrate the invention.15 2o 25 3o 35 example 1 (comparative) a catalytic cracking gasoline whose characteristics are grouped in table 1 is processed through the reaching a specification of the gasoline pool in such refinery output member on the sulfur content is less than 10 ppm, which requires to reduce the sulfur content of gasoline from a catalytic cracking unit to less than 20 ppm by weight.Gasoline is separated into three cuts a light fraction having a boiling range distribution is between 35 °c and 95 °c, an intermediate cut having a boiling range distribution between 150 °c and 95 °c and a heavy fraction having a boiling range distribution between 150 °c and 250 °c.The sulfur content of the light gasoline, which represents 38% total volume gasoline, is 210 ppm weight.Intermediate and heavy cuts are processed on a catalyst hr306 company procatalyst. The catalyst (20 ml) is first sulfurized by treatment for 4 hours under a pressure of 3.4 mpa to 350 °c, in contact with a filler composed of 2% of sulfur in the form of dimethyl disulphide in n-heptane. The desulfurization step is carried out at 300 °c under 35 bar with a h2/hc of 150 i/i and a HSV of 3 h1.These treatment conditions in the effluents obtained after stripping the h2s contain 1 ppm of sulfur. The mixing of these two cuts with the desulfurized intersects the lighter leads to a gasoline containing 81 ppm sulfur.24 table 1.Sulfur (ppm by weight) (% by volume) aromatic olefins (% by volume) + paraffin (% by volume) naphthene e RON-my density % volume of distilled 2000, 30 40, 30 91.0 81.1 0.77 distilling temperature boiling sulfur (% cumulative weight) olefins (% cumulative weight) 0, 0 0, 10 o, 8 21, 30 2.1 52, 50 7.5 77, 70 20, 92 9o 49, 99 100,100 100 (°C) 35, 55 85,120 155 20o 240, 5 example 2:The gasoline from a catalytic cracking unit whose characteristics are described in the example 1 is subjected to a treatment of diolefin hydrogenation 10 under conditions wherein the sulfur compounds in the mass are Iégers are in part converted into heavier compounds (steps a2 Al and simultaneous).This process is performed in a continuous flow reactor in an upflow. The catalyst is based on nickel and molybdenum (catalyst hr945 sold by procatalyst). The catalysts are first sulfurized by treatment for 15 hours under a pressure of 4 3.4 mpa to 350 °c, in contact with a filler composed of 2% of sulfur in the form of dimethyl disulphide in n-heptane. The reaction is carried out at 160 °c under a total pressure of 1.3 mpa, 25, 5 10, 15 20, 25 30, 35 with a space velocity of 6 H 1. The ratio H2/load, expressed in liter of hydrogen per liter of load and gasoline is then separated into two sections, one has a boiling range between 35 °c and 80 °c and representing 29% volume and the other distilling between 80 °c and 240 °c representing 71% volume of the gasoline cut. The sulfur content of light gasoline is of 22 ppm weight.The heavy gasoline is subjected to hydrodesulfurization on a sequence of the catalysts in an isothermal tubular reactor. The first catalyst (catalyst has, step LC) is obtained by impregnating "without excess solution" of a transition alumina, in the form of balls, of specific surface area of 130 m2/g and a pore volume of 0.9 ml/gm, by an aqueous solution containing molybdenum and cobalt in the form of ammonium heptamolybdate and cobalt nitrate. The catalyst is then dried and calcined in air at 500 °c. The content of cobalt and molybdenum content of this sample is of 3% and 10% of CoO moo3.The second catalyst (catalyst b, step c2) is prepared from a transition alumina of 140 m2/g in the form of beads 2 mm in diameter. The pore volume is 1 ml/g carrier. 1 kilogram of support is impregnated by 1 liter of nickel nitrate solution. The catalyst is then dried at 120 °c and calcined in air at 400 °c for one hour. The nickel content of the catalyst is 20% by weight.25 ml of catalyst, and 50 ml of catalyst b, are located in a same hydrodesulfurization reactor, so that the feed to be treated (heavy fraction) first encounters the catalyst has (step LC) then the catalyst b (step c2). A pickup area of the effluent from step Cl is provided between the catalyst a and b. the catalysts are first sulfurized by treatment for 4 hours under a pressure of 3.4 mpa to 350 °c, in contact with a filler composed of 2% of sulfur in the form of dimethyl disulphide in n-heptane.The operating conditions of the hydrodesulfurization are as follows: HSV=1.33 H 1 with respect to the entire catalyst bed h2/hc=360 i/i, p=1.8 mpa. The temperature of the catalytic zone comprising the catalyst a is 260 °c, the temperature of the catalytic zone containing the catalyst b is 350 °c. Contains 19 ppm sulfur.The desulfurized product is recombined to light gasoline. Measuring the sulfur content of gasoline thus obtained leads to a content of 20 ppm by weight. It has a RON-of 88.1 and my of 79.6 either loss (RON-+ my)/2 with respect to the load of 2.2 spot. The olefin content in gasoline is 22% of this flight.26 example 3 (inventive):10, 15 20, 25 the gasoline from a catalytic cracking unit whose characteristics are described in the example 1 is subjected to a treatment of diolefin hydrogenation under conditions where the light compounds sulfur present in the load are in part converted into heavier compounds (steps a2 Al and simultaneous).This process is performed in a continuous flow reactor in an upflow. The catalyst is based on nickel and molybdenum (catalyst hr945 sold by procatalyst). The catalysts are first sulfurized by treatment for 4 hours under a pressure of 3.4 mpa to 350 °c, in contact with a filler composed of 2% of sulfur in the form of dimethyl disulphide in the nheptane . The reaction is carried out at 160 °c under a total pressure of 1.3 mpa, at a space velocity of 6 H 1. The ratio H2/load, expressed in liter of hydrogen per liter of load and gasoline is then separated into four sections:one has a boiling range between 35 °c and 80 °c and representing 28% flight and having a sulfur content of 20 ppm weight; a second cut distilling between 80 °c and 95 °c and representing 10% volume of gasoline for starting and containing 250 ppm sulfur; a third cut distilling between 95 °c and 150 °c, representing 30% volume gasoline starting and containing 1000 ppm by weight of sulfur.The RON-and my this cut are respectively 90 and 79.- a fourth cutting distilling between 150 °c and 240 °c representing 32% volume gasoline starting and containing 4600 ppm sulfur.30, 35 heavy gasoline and mixed with the second cut and is subjected to hydrodesulfurization on a sequence of the catalysts in an isothermal tubular reactor. The first catalyst (catalyst has, step c) is obtained by impregnating "without excess solution" of a transition alumina, in the form of balls, of specific surface area of 130 m2/g and a pore volume of 0.9 ml/gm, by an aqueous solution containing moiybdène and cobalt in the form of ammonium heptamolybdate and cobalt nitrate. The catalyst is then dried and calcined in air at 500 °c. The content of cobalt and molybdenum content of this sample is of 3% and 10% of CoO moo3.The second catalyst (catalyst b, step d) is prepared from a transition alumina of 140 m2/g in the form of beads 2 mm in diameter. The pore volume is 1 ml/g carrier. 1 kilogram of support is impregnated by 1 liter of nickel nitrate solution. The catalyst is then dried and calcined 27, 10 15 120 °c under a stream of air at 400 °c for one hour. The nickel content of the catalyst is 20% by weight.25 ml of catalyst, and 50 ml of catalyst b, are located in a same hydrodesulfurization reactor, so that the feed to be treated (heavy fraction) first encounters the catalyst has (step c) and then the catalyst b (step d). A pickup area of the effluent from step c is provided between the catalysts and b. the catalysts are first sulfurized by treatment for 4 hours under a pressure of 3.4 mpa to 350 °c, in contact with a filler composed of 2% of sulfur in the form of dimethyl disulphide in n-heptane.The operating conditions of the hydrodesulfurization are as follows: HSV=1.33 H 1 with respect to the entire catalyst bed h2/hc=360 i/i, p=1.8 mpa. The temperature of the catalytic zone comprising the catalyst a is 260 °c, the temperature of the catalytic zone containing the catalyst b is 350 °c. Contains 37 ppm sulfur.The third cut is treated on a catalyst hr306 company procatalyst. The catalyst (20 ml) is first sulfurized by treatment for 4 hours under a pressure of 3.4 mpa to 350 °c, in contact with a filler 20 2% sulfur in the form of dimethyl disulphide in n-heptane. The desulfurization step is carried out at 300 °c under 3.5 mpa with a h2/hc of 150 i/i and a HSV of 3 H 1 in these treatment conditions the effluent obtained after stripping of I ' h2s contains less 1 ppm sulfur. The olefin content is 0.9% volume and octane values are of 68.7 for the RON-and 68.3 for the MQW. Gasoline is then treated on a reforming catalyst cr201 25 from the company procatalyst. The catalyst (30 ml) is first reduced to 500 °c under a stream of hydrogen avnat use. The reforming treatment is carried out at a pressure of 7 470 °c bars. The ratio h2/hc is 500 i/i. The LHSV is from 2 H 1.The effluent is stabilized by removal of compounds having less than 5 carbon atoms of 30. The resulting reformate, which represents 86% of the fraction of treated gasoline, has a sulfur content below 1 ppm ã , a RON-of 97 and my of 86.35 fractions obtained in the different slices processed remixed. The sulfur content is 20 ppm by weight. The mean value (RON-+ my)/2 of the total desulfurized gasoline has increased by 1.3 points relative to starting in the gasoline. Further, the hydrogen generated at step catalytic reforming can be used for the hydroprocessing reaction sections which is an advantage in the obvious method.28 Dividing starting petroleum fraction into heavy, light and intermediate fractions and subjecting previously desulfurized intermediate fraction to catalytic reforming. The process comprises: a1) at least one selective hydrogenation of diolefins and acetylenes present in starting material; b) at least one separation of obtained effluent into 3 fractions: light fraction practically free from S and containing lightest olefins, heavy fraction containing majority of initially present S, and at least one intermediate fraction with relatively low content of olefins and aromatic compounds; c1) at least one catalytic treatment of heavy fraction to decompose/hydrogenate at least partially unsaturated S compounds; and d) at least one desulfurization/denitration of at least one intermediate fraction, followed by catalytic reforming. Starting material is preferably a benzine fraction from catalytic cracking. 1. Process of production of gasoline with low sulphur content starting from a load containing of sulphur including/understanding at least the following stages: a1) at least a selective hydrogenation of the dioléfines and acetylenic compounds contained in the load, 2. Process according to claim 1 including/understanding moreover at least a stage a2 located before the stage B and aiming at increasing the molecular weight of the light sulphur products present in the load and/or the effluent of the stage a1. 3. Proceeded according to one of claims 1 or 2 including/understanding moreover a stage c2 waste processing of the stage c1 on a catalyst which makes it possible to break up the sulphur compounds. 4. Proceeded according to the claim 3 in which the catalyst of the stage c2 makes it possible moreover to limit the hydrogenation of olefins to less than 20% in volume. 5. Proceeded according to any of claims 1 to 4 including/understanding moreover a stage E of mixture of at least two fractions of which at least was desulphurized with the stage c1 and possibly c2 and/or stage D. 6. Proceeded according to any of the claims 1 to 5 in which part of at least an intermediate fraction obtained at the stage B is mixed with the heavy fraction resulting from the stage B before the stage c1. 7. Proceeded according to any of the claims 1 to 5 in which part of at least an intermediate fraction obtained at the stage B is mixed with the effluent of the stage c1. 8. Proceeded according to any of the revendations 1 to 7 in which the stage D of desulphurization and deazotation is accompanied by a total hydrogenation of olefins. 9. Proceeded according to any of the claims 1 to 8 in which the load is a cut gasoline resulting from a unit of catalytic cracking. 10. Proceeded according to any of the claims 1 to 9 in which the stage B includes/understands a separation of the effluent obtained at the conclusion of the stage a1 in four fractions: a light fraction, a fraction heavy and two intermediate fractions, and in which one of the intermediate fractions and treated at the stage D and the other mixed with the separate heavy fraction at the stage B then treated with the stage c1 and/or c2.