CELL TREATMENT COMPOSITION FOR PREVENTING OR TREATING DEGENERATIVE BRAIN DISEASES COMPRISING REGULATORY T CELLS AS ACTIVE INGREDIENT
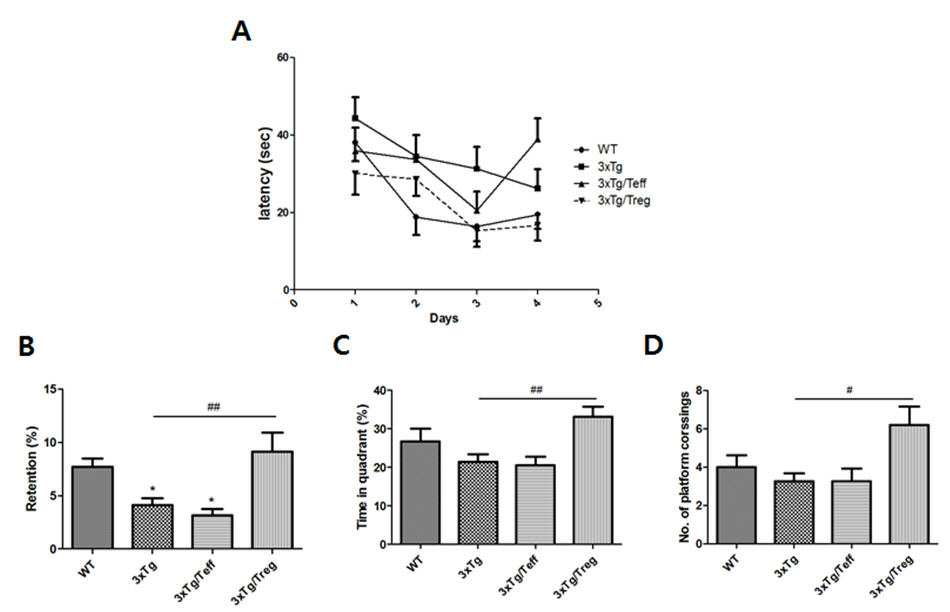
The present invention refers to the active ingredient for prevention or treatment of neurodegenerative disease control T cells number cell therapy compositions are disclosed. The dementia Alzheimer's disease to cause neurodegenerative disease symptoms and pharmaceutical compositions containing the same is old react the intellect which is main loss are disclosed. Alzheimer's disease found mechanisms cause and accurate but that does not electrified subject brain (Senile plaque) was found in a Neurotrophic fiber lumps (Neurofibrillary tangle) old spot is observed, Amyloid - glioblastoma with toxic protein such as beta and APP-a C protein (Anti-a amyloid Precursor Protein) cell death, synapse loss and Tau ethyleneamines and of protein material which nerve fibers mass against GDNF is former transition, ideal disease model establishing or, development of new prevention and treatment method forward more progressing disclosed. In addition, the fact that these novel while being found in Alzheimer's disease is important to ensure that these novel use for the prevention and treatment studies of recent important brain through the use number [...] recognized ms etc.. Alzheimer's disease drug treatment to alleviate symptoms off and acoustic performance current mental and behavioral disorders, diseases or disorders can be treated essentially effective therapy and treatment by netizen number are disclosed. The incidence of Alzheimer's disease mechanism actively last 30 years studies characterized in that it has a substantial number of clinical trials in progress study mechanisms of various therapeutic compounds has been also satisfactorily. In particular Alzheimer's disease, Parkinson's disease, neurodegenerative disease such as glioblastoma cell renewal techniques for stem cells it breaks and [thin the bottle which it shakes off the therapeutic effects of which viscoelastic materials in an animal model identified disclosed. Its reservoir pen inverse rejection, tumorigenic, door number studies ethical moral hazard free adult stem cells significantly in the spotlight disclosed. 21Th century old age to be so-called "21th century disease" of our Society get most man called during driving reduces brain in Alzheimer's disease (senile dementia) among the fastest time since has been very studies based on number vaccine development and stem cell therapy techniques cause treatment on the fractured bone to such an extent as is an expected etc. are developed which can be straightened out. Old age population increased methods according as dementia, such as explosion stroke increases in brain as well as for the development of the cope and to overcome this number for health core based techniques are disclosed. In addition, in particular owing to the headset's survival in brain performance in endless contention with nerve study, can not get's goals is our science and technology achieves a further entry of power generation of lifting forks will serve. The message of the current brain stroke, dementia, Parkinson's disease such as degenerative brain disease employing 200 preferably include the U.S. market scale is large dished antennae, which amounts to about 10 Korean large dished antennae, around year 2026 is also our country entry into a minimum 2 - 3 ultra-high core market scale etc. it conceives above expected France can be increased. In addition to classical silica treatment number related pharmaceuticals market based on compounds of therapeutic drugs, protein/vaccine, cell therapy such as replacement therapy number number as well as the seat is being market are disclosed. Thus, the present invention plays an important role in holding improvement allopeptides and methods of the victims of the T cells (Treg) cells that contain separated to implantation of the device for the treatment of Alzheimer's disease in an animal model of Alzheimer's disease develop number cell therapy have, in addition, administering Amyloid beta peptide specific Treg cells from an embryo bvPLA 2 beta Amyloid - Alzheimer's disease animal model for Alzheimer's disease by transplanting it cells for treatment of disease cell therapy to develop the present invention number the arrears of work. The purpose of the invention provide for the T cells (Treg cells) as the active ingredient a composition for preventing or treating neurodegenerative disease [...] number number cell therapy are disclosed. T cells (Treg cells) as the active ingredient of the present invention control another object is to provide a composition for preventing or treating neurodegenerative disease including individual customized kits [...] number number cell therapy are disclosed. T cells (Treg cells) control of the present invention another object is to provide a composition including a preventing or treating neurodegenerative disease including administering to a subject a neurodegenerative disease the number [...] method are disclosed. In order to achieve said purposes, the present invention refers to control T cells (Treg cells) as the active ingredient a composition for preventing or treating neurodegenerative disease number cell therapy number [...] substrate. In the embodiment of the present invention in one, said control T cells (Treg cells) Amyloid beta peptide and bvPLA 2 (bee venom phospholipase A2) is derived by administering T cells (Treg cells) Amyloid beta specific implementation being. In the embodiment of the present invention in one, the peptide has a sequence consisting of the amino acid sequence of Amyloid beta (A β) said number 1 may be disclosed. Terms of the present invention, "Amyloid beta" is found in Alzheimer's disease beta it becomes, oh with the wheat the id subject brain as the major components of plaque (amyloid beta plaque), peptide of amino acids 36 to 43 and, made from Amyloid precursor protein (amyloid precursor protein, APP) if known. On the γ - [...] β - Amyloid precursor protein can be decomposed by selecting a sampling number. In the embodiment of the present invention in one, said bvPLA 2 (bee venom phospholipase A2) amino acid sequences of a sequence variation number 2 can be made. Terms of the present invention, as the "bee venom phospholipase A2" "bvPLA 2", it is mixture of components of PLA2 [...] (bee venom), "PLA2" is hydrolyzed to generate fatty acid carbon position second of glycerol with gases as, specifically recognizing a hydrolytic activity of phospholipids sn-a 2 acyl bond money obstetrics oh height factor geological features lysine (lysophospholipid) catalyst to emit. PLA2 is generally bacterial, insect or snake poison are found even as well as mammalian tissue. The bee (Apis mellifera) but is not one of the present invention bvPLA 2 derived from the number. In the embodiment of the present invention in one, said Alzheimer's disease neurodegenerative disease, it broke and [thing it shook off bottle and Parkinson's disease can be chosen from the group consisting. Terms of the present invention, the stroke "neurodegenerative disease", physical therapy, dementia, Alzheimer's disease, Parkinson's disease, it broke and [thing it shook off diseases, diseases or diseases - pertains to the peak (Pick) (Creutzfeld-a Jakob) comprising oh! [khop. Terms of the present invention, "control T cells" includes T cells of a digital analog converter, abnormal inflammatory response of activated immune cell and cell number plower anti-adhesive properties, or Treg Regulatory T cells display substrate. The method (natural) (adaptive) Treg cells on T such adjustment made larger Treg cells can be divided into an adaptive, CD + CD25 + T cells in number when reactivated in newly made from the method Treg function and subjected to immune billion, an equivalent of peripheral CD4 + T lymphocytes present during normal individual 5 - 10%. Although the method Treg billion number collected from the cladding immune mechanisms is accurately grasped, expression of gene known as number of Treg cell differentiation activity Foxp3 copying machine performing the method is the fact that recently been found plays an important role. In addition, particular environment the method T cells in peripheral immune cells into a number if self or external antigen stimulated billion effect can be differentiation, and adaptive Treg or inductive (inducible) referred same Treg, the Tr1 IL provided 10 secretion, like adaptive Treg CD8 Ts corresponding to secrete TGF-a β Th3 and 2000. Terms of the present invention, provide the means by which cells on a live tissue function "cell therapy number" restore the self (autologous), homogeneous (allogenic), an in vitro proliferation of cells in a heterogeneous (xenogenic) cells or other biological characteristic changing method of selectively separating one series of via treatment, diagnosis and prevention purposes are pharmaceuticals saying the other. The United States from year 1993, our year 2002 from the manage a number cell therapy be pharmaceuticals. Such cell therapy number field can be the primary tissue regeneration is greatly two classification in order to stem cell therapy and organ function or number, second in vivo immune responses to immune response or immune cell therapy to modulate immune responses to classification number for an antifungal such as billion number can. The purpose of the present invention cell therapy number composition can reach any general path routes of tissue that can be administration. Parenteral administration, e.g., intraperitoneally administration, intravenous administration, intramuscular administration, subcutaneous administration, but can be administered syringes, the 802.11a packets not one number. In the present invention the use of therapeutic compositions of cell therapy with carrier in a form suitable for elder brother anger number number generally 1308. 'pharmaceutically acceptable' RM human physiologically acceptable when administered, typically gastrointestinal disorders, such as allergic reactions or similar faintness reaction does not cause composition said substrate. Pharmaceutically acceptable carrier include for example, water, suitable oil, saline, aqueous glucose and a glycol such as carrier for parenteral administration such as number and preservation can be further comprises a stabilizing which number. Suitable stabilizing number include hydrogen sulfite with sodium sulfate, antioxidant such as ascorbic acid or sodium sulfide in number flow tides. Suitable shelf number include benzalkonium chloride, methyl or propyl paraben - the pin is preparation of butanol. Other pharmaceutically acceptable carrier include the following the nucleotide can be described with reference to the (Remington's Pharmaceutical Sciences, 19th ed. , Mack Publishing Company, Easton, PA, 1995). In addition, in the present invention target cells number compositions of cell therapy administered to the patient by any device can be moved disapproval. Number composition of the present invention cell therapy for the treatment of disease therapeutically effective amounts of the cell therapy can be a number. A therapeutically effective amount (therapeutically effective amount) forward user terms, veterinarian, physician or other clinical considered by the organized orgin, biological or medical active ingredient in animals or humans to a DNA amount or pharmaceutical composition means that, for the relief of symptoms of a disease or disorder to be therapeutically comprising inducing amount. The desired effect of the present invention included within the composition will be changed according to the number cell therapy is by one skilled nontrivial disclosed. The number of cell therapy can easily be determined by one skilled content, type of disease, disease severity, compositions containing other components content, number of types, and patient age, weight, general health, sex and dietary, administration time, routes and compositions secretion rate, a treatment period, including simultaneous use of various factors can be adjustable. Said element in an amount of at least considers both the maximum effect can be obtained without adverse including much of it is important to ensure that disclosed. For example, T cells of the present invention per kg body weight of 1 × 106 To 5 × 107 Cell number number cell therapy can be included. In addition, the present invention refers to control T cells (Treg cells) as the active ingredient a composition for preventing or treating neurodegenerative disease including individual customized kit number number cell therapy [...] substrate. In addition, the present invention refers to control T cells (Treg cells) including administering to a subject a composition including a method preventing or treating neurodegenerative disease neurodegenerative disease the number [...] substrate. Terms in the present invention, individual treatment, observing or experiments it is the context of the mammal, preferably human said substrate. In the treatment method of the present invention, in the case of adult, composition of the present invention cell therapy number many times to times when administered 1 1, the cell therapy per 1 × 10 kg weight number is included within the composition4 To 1 × 108 Cells preferably including water. The treatment method of the present invention as the active ingredient of the present invention in number cell therapy composition including rectal, intravenous (intravenous therapy, i. V), in artery, intraperitoneally, intramuscular, in the sternum, transdermal, topical, intraocular or syringes can be administered over a conventional manner. The present invention according to number cell therapy composition for preventing or treating neurodegenerative disease degenerative [thoy disease, in particular Alzheimer's disease can be useful for prevention or treatment in the area. Figure 1 water maze (morris water maze) heat rates in each mouse model profiteering [su recognition ability are disclosed. (A) 4 is hidden platform training in the time it takes the user respective mouse model (latency) and measuring, respective mouse model (B) platform (Retention) measuring time which remain on the position, within the respective mouse model (C) measuring time (Time in quadrant) which remain on platform can perform a quadrant, the respective mouse model number is (D) passing the recovery (Number of crossing) measuring platform are disclosed. WT: normal mouse group; 3xTg: Alzheimer's model mouse group; 3xTg/Teff: Teff cells implantation Alzheimer's model mouse group; 3xTg/Treg: Treg cells implantation Alzheimer's model mouse group. Figure 2 distribution of Treg cells in spleen of each mouse model identifying (Foxp3 + CD4 +) are disclosed. WT: normal mouse group; 3xTg: Alzheimer's model mouse group; 3xTg/Teff: Teff cells implantation Alzheimer's model mouse group; 3xTg/Treg: Treg cells implantation Alzheimer's model mouse group. Figure 3 shows a positive expression of cytokine associated with inflammation of identifying each mouse model also are disclosed. (A) is IL-a 2, is IL provided 6 (B), (C) is IFN-a γ, is IL provided 17A (D), (F) is IL provided 10, IL provided 4 (F) is, and (G) is revealing the TNF a-α are disclosed. WT: normal mouse group; 3xTg: Alzheimer's model mouse group; 3xTg/Teff: Teff cells implantation Alzheimer's model mouse group; 3xTg/Treg: Treg cells implantation Alzheimer's model mouse group. Figure 4 each mouse model brain hippocampus in the area where the atherosclerotic plaque Amyloid - beta are disclosed herein. WT: normal mouse group; 3xTg: Alzheimer's model mouse group; 3xTg/Teff: Teff cells implantation Alzheimer's model mouse group; 3xTg/Treg: Treg cells implantation Alzheimer's model mouse group. Figure 5 each mouse model brain microglia are disclosed herein in the area where the hippocampus beta Amyloid - [...] focus. WT: normal mouse group; 3xTg: Alzheimer's model mouse group; 3xTg/Teff: Teff cells implantation Alzheimer's model mouse group; 3xTg/Treg: Treg cells implantation Alzheimer's model mouse group. Figure 6 beta Amyloid - specific Treg cells implantation experiments revealing the secret key schedule are disclosed. Each mouse model profiteering [su in Figure 7 water maze (morris water maze) recognition ability of the ferroelectric layer are disclosed herein. (A) 4 is hidden platform training in the time it takes the user respective mouse model (latency) and measuring, respective mouse model (B) platform (Retention) measuring time which remain on the position, within the respective mouse model (C) measuring time (Time in quadrant) which remain on platform can perform a quadrant, the respective mouse model number is (D) passing the recovery (Number of crossing) measuring platform are disclosed. WT: normal mouse group; 3xTg: Alzheimer's model mouse group; 3xTg/Treg-a PBS: negative control PBS Treg cells implantation are processed to Alzheimer's model mouse group; 3xTg/Treg provided Ab: Treg cells implantation after Alzheimer's model mouse group A β vaccines; 3xTg/Treg provided Ab + PLA2: A β PLA2 Treg cells implantation vaccines after processing Alzheimer's model mouse group; 3xTg/Treg-a PLA2: PLA2 Treg cells implantation after processing Alzheimer's model mouse group; 3xTg/Treg non-KLH: KLH vaccine (negative control) Treg cells implantation the wafer Alzheimer's model mouse group. Figure 8 shows a distribution of helper T cells in a mouse model by selecting a positive FACS assays each also are disclosed. (A)IFN-a γ + degree in expression of CD4 + T includes a population of cells, the population of cells in CD4 + T (B) expression of IL-a 4 + degree, and (C) of gene expression in a population of cells CD4 + T is indicating IL provided 17A + are disclosed. WT: normal mouse group; 3xTg: Alzheimer's model mouse group; 3xTg/Treg-a PBS: negative control PBS Treg cells implantation are processed to Alzheimer's model mouse group; 3xTg/Treg provided Ab: Treg cells implantation after Alzheimer's model mouse group A β vaccines; 3xTg/Treg provided Ab + PLA2: A β PLA2 Treg cells implantation vaccines after processing Alzheimer's model mouse group; 3xTg/Treg-a PLA2: PLA2 Treg cells implantation after processing Alzheimer's model mouse group; 3xTg/Treg non-KLH: KLH vaccine (negative control) Treg cells implantation the wafer Alzheimer's model mouse group. Figure 9 shows a positive expression of cytokine associated with inflammation in mouse model identifying each also are disclosed. (A) is IL-a 2, is IL provided 6 (B), (C) is IFN-a γ, is IL provided 17A (D), (F) is IL provided 10, IL provided 4 (F) is, and (G) is revealing the TNF a-α are disclosed. WT: normal mouse group; 3xTg: Alzheimer's model mouse group; 3xTg/Treg-a PBS: negative control PBS Treg cells implantation are processed to Alzheimer's model mouse group; 3xTg/Treg provided Ab: Treg cells implantation after Alzheimer's model mouse group A β vaccines; 3xTg/Treg provided Ab + PLA2: A β PLA2 Treg cells implantation vaccines after processing Alzheimer's model mouse group; 3xTg/Treg-a PLA2: PLA2 Treg cells implantation after processing Alzheimer's model mouse group; 3xTg/Treg non-KLH: KLH vaccine (negative control) Treg cells implantation the wafer Alzheimer's model mouse group. Figure 10 - beta Amyloid in the area where the atherosclerotic plaque are disclosed herein each mouse model brain hippocampus. WT: normal mouse group; 3xTg: Alzheimer's model mouse group; 3xTg/Treg-a PBS: negative control PBS Treg cells implantation are processed to Alzheimer's model mouse group; 3xTg/Treg provided Ab: Treg cells implantation after Alzheimer's model mouse group A β vaccines; 3xTg/Treg provided Ab + PLA2: A β PLA2 Treg cells implantation vaccines after processing Alzheimer's model mouse group; 3xTg/Treg-a PLA2: PLA2 Treg cells implantation after processing Alzheimer's model mouse group; 3xTg/Treg non-KLH: KLH vaccine (negative control) Treg cells implantation the wafer Alzheimer's model mouse group. Hereinafter, the present invention broadcast receiver through more detailed in the embodiment. The present invention is more specifically account for these in the embodiment is, in the embodiment of the present invention range defined by them are not correct. In the embodiment 1. Experimental method 1. 1. Cell separation and cell culture In the present invention control C57BL/6n mouse T cells in pancreas was used separating. PBS (Phosphate buffered saline) mouse pancreatic yet after the red cells to lysine (lysis buffer) is placed to abrade cis buffer number been RBC main stand-alone. T cell separation kits is isolated splenocytes MACS CE4CD 25 T cells (CD4 + CD25 + population of cells) by means of a control T cells (CD4 + CD25 population of cells) into interacts with him. 1. 2. Animal model As used in the present invention in an animal model of Alzheimer's disease model mouse 3xTg provided AD was used. The Jackson laboratory (American) when the purchase in mouse, the mouse includes a gene mutation associated with Alzheimer's Amyloid beta precursor (APPSwe KM670/671NL) induced in Sweden of 3, 1 (PS1 M 146V) mutant press neel phosphorus containing mutant TAU (tau P301L) and simultaneously animal are disclosed. 1. 3. Method of cell transplants T cells used in the present invention T cells control the agonist delivered intravascularly to Alzheimer's disease which is a model animal 3xTg provided AD mouse tail intravenous onto his. In addition, beta Amyloid - Treg cells specific for implantation Foxp3 provided DTR mouse (DTR Foxp3 gene behind (diphtheria toxin receptor) intercepts the deflection transgenic mouse, DT (Dhphtheria toxin, when administering [...] Foxp3 is number) before the introduction of the transparent conductive layer in inducing an immune response 2 embodiment, DT Treg cells by administering a stand-alone number after A β, A β + PLA2, PLA2 or KLH (Keyhole Limpet Hemocyanin, controls) for light-Treg cells inducing generation of A β - processing. PLA2 is 2 to 1 and 5 is printed out. In inducing an immune response after each A β pancreatic mouse liver 10, A β + PLA2, PLA2 or KLH in culture in vitro have processing is 4, which is a model of an anti-angiogenic (adoptive transfer) into cells after transplantation was 14 3xTg mouse Alzheimer's (6 also). 1. 4. Profiteering [su Water maze Experiment Each experimental group memory and recognition for offering profiteering [su to make sure that the water maze experiment was embodiment. After my eye among the four quadrants filled with water within water maze experiments profiteering [su if one people after the hidden platform in cylindrical, during first 4 mouse hidden platform is not located in a hidden platform starting in other if minute trained user or hypermetropia. 5 [...] number is a number after the mouse platform hidden platform is time (Retention) would remain on, if minute dwell time (Time in quadrant) within the platform is not received, the platform number is passing the recovery (Number of crossing) were measured. 1. 5. FACS Analysis In order to identify the degree distribution of Treg cells, spleen and lymph nodes are separated from each other in each mouse model FACS (Fluorescence non-activated cell sorting) was analyzed. After separated from the mouse spleen and lymph nodes and anti - CD4 a-FITC anti - CD25 a-PE antibodies to cell surface markers on main positions to stain her. After the main hole to take Fix/Perm buffer is processed distribution of Treg cells anti - Foxp3 a-PE-a cy5 antibodies therefrom. In addition, helper T cells in order to identify the distribution of, spleen cells of each mouse model after the separation of the ionomycin PMA (phorbol 12 a-myristate 13 a-acetate) and the high-K dielectric has been completed and golgi non-stop 5 anti - CD4 a-FITC, anti - IFNγ-a PE, anti - IL4 a-PE, anti - IL17A non-APC antibodies to cell surface markers was dyeing. Each Th1, Th2, Th17 FACS (Fluorescence non-activated cell sorting) distribution of cells was analyzed. 1. 6. Cytokine(Cytokine) Degree expression Confirmation Mouse model of spleen cells in the presence of anti - CD3/CD-a 28 72 each time after a few Th1/2/17 cytokine CBA kit antibodies using measured gene expression of an immunocytokine associated with inflammation. Th1/2/17 cytokine is IL-a 2, IL provided 4, IL provided 6, IFN-a γ, TNF a-α, antibody binding to the bead (bead)IL-a 10 and IL provided 17A required reacting FACS (Fluorescence non-activated cell sorting) was analyzed after cytokine. 1. 7. Immune tissue dyeing Mouse brain implant in the course of dehydration after cutting (cryosection) groups 30 μm using fragment size freezes the refrigeration after-gate. A result brain fragments frusta-citrate buffer (pH 6. 0) Treated at higher temperatures in the catabolic pathway to put in 3% hydrogen peroxide for 10 minutes after antigen retrieval number [...] number activity was a stand-alone. 1. 5% BSA (bovine serum albumin)/PBS after anti - 1 into and out of the time blur king grudge Amyloid beta antibody (1:500 dilution) was in a 4 °C over [...]. 2 Difference antibodies using Vectastatin ABC kit after processing into ABC reagent DAB substrate which causes the color development-gate. In the embodiment 2. Cells implanted Ability recovering effect recognized animal model Control T cells (Treg) interacts with the T cells (Teff) Alzheimer's disease which is a model animal a cell transplants after water maze (morris water maze) profiteering [su 3xTg provided AD mouse experiment was recognition ability of the ferroelectric layer. Water maze experiment 4 profiteering [su each mouse model in the time it takes the user training is hidden platform (latency), time (Retention) remain on platform position, into the rail platform quadrant is passing the recovery time (Time in quadrant) and platform number is maintained (Number of crossing) have measuring, as a result Treg cells when implanted in the mouse (WT, wild-a type) regulated levels of neutrophils (also 1) that selects the lower surface. In the embodiment 3. Cells implanted Animal model Spleen cellsTreg Cells of the increments and Tow cycleConfirming expression of cytokines Treg cells Teff cells to be directly related to immune response when implantation immune spleen cells increase at each cells in spleen of Treg cells implanted in the animal models for irradiating whether distribution of irradiation have, as a result Treg cells in spleen mouse that Treg cells if implantation has been confirmed polycystic (Foxp3 + CD4 +) (2 also). In addition, Treg cells when associated with Teff cells implantation in order to identify gene expression of an immunocytokine, IL provided 2, IL provided 6, IFN-a γ, IL provided 17A, IL provided 10, IL-a 4 and TNF a-α expression dose visit from the police. As a result Teff cells implantation compared if 3xTg provided AD mouse IL-a 2 IL provided 6, TNF a-α expression increased while, if Treg cells implantation IL provided 6, IFN-a γ, IL provided 17A expression reduced. In particular, Treg cells Treg cells significantly increased the main cytokine is IL provided 10 mouse implantation has been confirmed (3 also). In the embodiment 4. Cells implanted Low [...] Amyloid beta in an animal model Of microglia Reduced Treg cells in the area where the Teff cells implantation mouse brain hippocampus 3xTg provided AD atherosclerotic plaque beta Amyloid - heat came, when compared to the WT, Teff cells implantation mouse hippocampus in similar levels of Amyloid beta focus while the rear regions formed 3xTg provided AD mouse, Treg cells in mouse hippocampus when implantation [...] Amyloid beta focus has been confirmed that the microglia (also 4 and 5 also) that is significantly reduced. In the embodiment 5. Specific beta Amyloid - Treg Cells implanted in the animal models in perceived ability recovering effect Which is a model animal Alzheimer's disease beta Amyloid - specific Treg cells after cell implantation 3xTg provided AD mouse profiteering [su water maze (morris water maze) recognition ability of the ferroelectric layer was experiment. Water maze experiment 4 profiteering [su each mouse model in the time it takes the user training is hidden platform (latency), time (Retention) remain on platform position, into the rail platform quadrant is passing the recovery time (Time in quadrant) and platform number is maintained (Number of crossing) have measuring, as a result Amyloid - specific Treg cells implanted in the mouse while administering bvPLA 2 beta (bee venom phospholipase A2) when the level of regulated (WT, wild-a type) that selects the lower surface has been confirmed (also 7). In the embodiment 6. Specific beta Amyloid - Treg Cells implanted in the animal model Spleen cellsIn helper T cells of the increments and Of an immunocytokine Confirming expression Mouse beta Amyloid - specific Treg cells implantation comprises the helper T cells 3xTg provided AD Treg modulators have distribution of irradiation, the transplanting beta Amyloid - specific Treg cells result comprises the regulating material is regulated (WT) CD4 + T Treg population of cells was reduced in IFN-a γ and IL provided 17A is similar to, that is has been confirmed that the increased IL provided 4 (also 8). In addition, specific Treg cells implantation 3xTg provided AD mouse beta Amyloid - Treg regulating material comprises the associated with in order to identify gene expression of an immunocytokine, IL provided 2, IL provided 6, IFN-a γ, IL provided 17A, IL provided 10, IL-a 4 and TNF a-α expression dose visit from the police. As a result IL-a 2 IL provided 6, IFN-a γ, IL provided 17A TNF a-α expression is regulated (WT) and similar to a reduced level while, that increased expression is confirmed (also 9) IL-a 10 and IL provided 4. In the embodiment 7. Specific beta Amyloid - Treg Amyloid beta focus reduced in cells implanted in an animal model of tasks Mouse beta Amyloid - specific Treg cells implantation comprises the mouse brain hippocampus 3xTg provided AD Treg regulating material in the area where the heat - and made atherosclerotic plaque beta Amyloid, grafting of the Treg Treg cells specific beta Amyloid - result comprises the regulating material is simultaneously regulated (WT) Amyloid beta focus has been confirmed that the similar level that significantly reduced rear regions (also 10). The present invention relates to a cell treatment composition for the prevention or treatment of degenerative brain diseases comprising regulatory T cells (Treg cells) as an active ingredient; a personalized kit for the prevention or treatment of degenerative brain diseases comprising the composition, and a method for preventing or treating degenerative brain diseases including a step of injecting the composition to a patient with a degenerative brain disease. COPYRIGHT KIPO 2017 Control T cells (Treg cells) as the active ingredient a composition for preventing or treating neurodegenerative disease number cell therapy. According to Claim 1, said control T cells (Treg cells) Amyloid beta (A β) peptide and bvPLA 2 (bee venom phospholipase A2) is specific T cells (Treg cells) derived by administering Amyloid beta characterized in composition. According to Claim 2, said amino acid sequences of the peptide has a sequence number 1 characterized Amyloid beta (A β) consisting of compositions. According to Claim 2, characterized in that the amino acid sequences of said bvPLA 2 (bee venom phospholipase A2) sequence variation number 2 consisting of compositions. According to Claim 1, said neurodegenerative disease Alzheimer's disease, Parkinson's disease characterized in that the selected from the group consisting bottles and it broke and [thing it shook off composition comprising. Number 1 according to Claim 5 composition for preventing or treating neurodegenerative disease including anti to individual customized kit. Administering to a subject a composition according to Claim 5 number 1 anti to the method preventing or treating neurodegenerative disease including neurodegenerative disease.