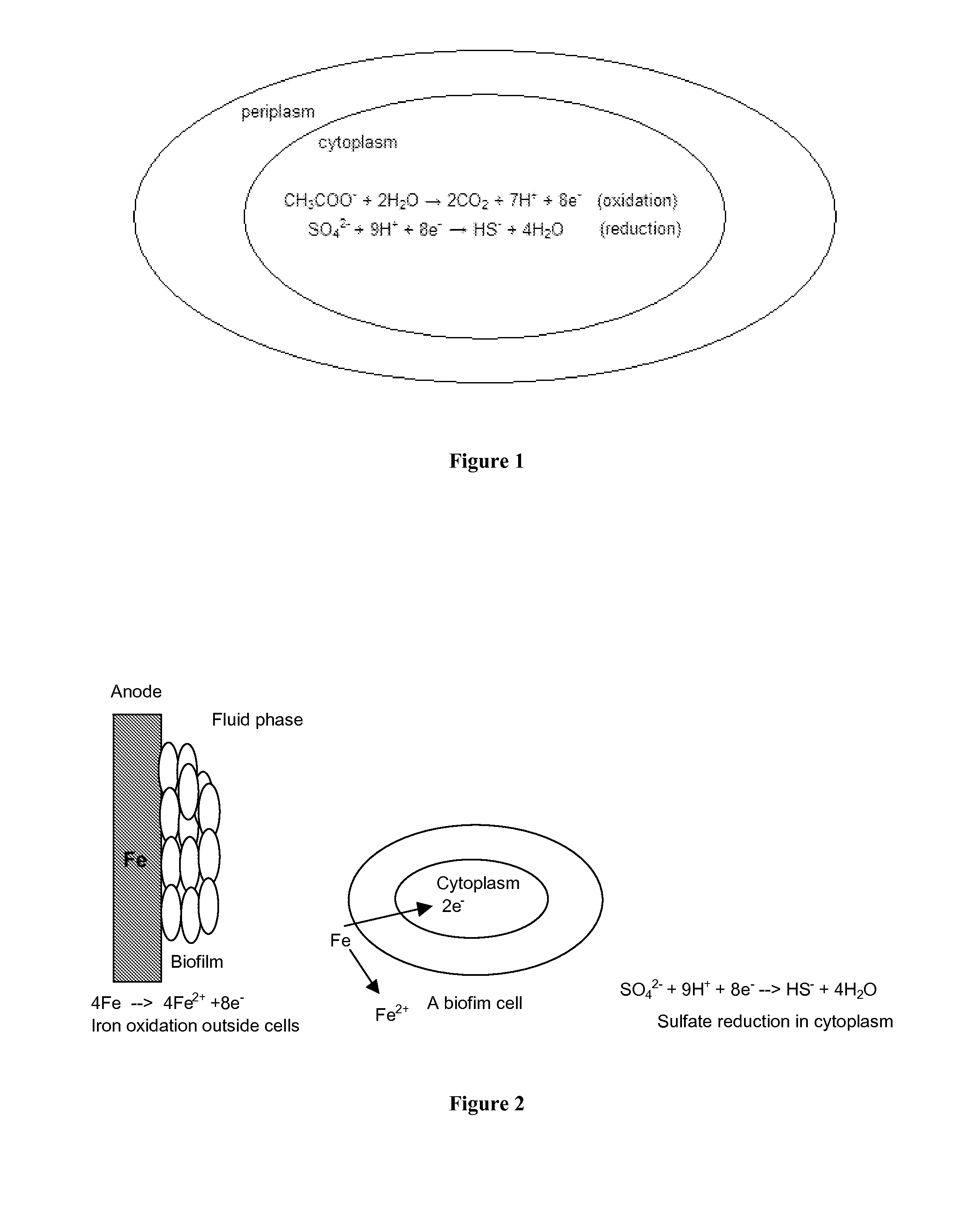
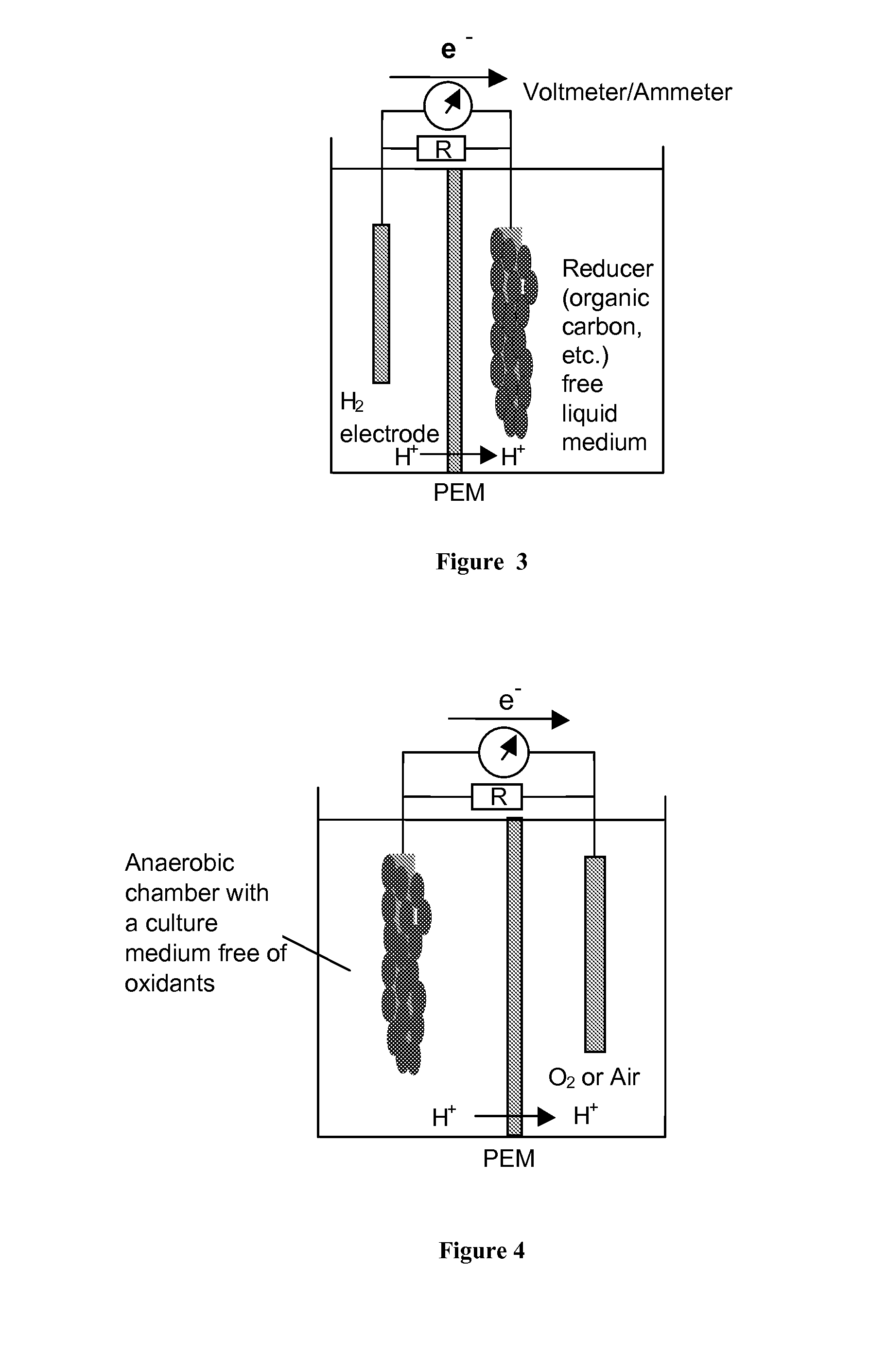
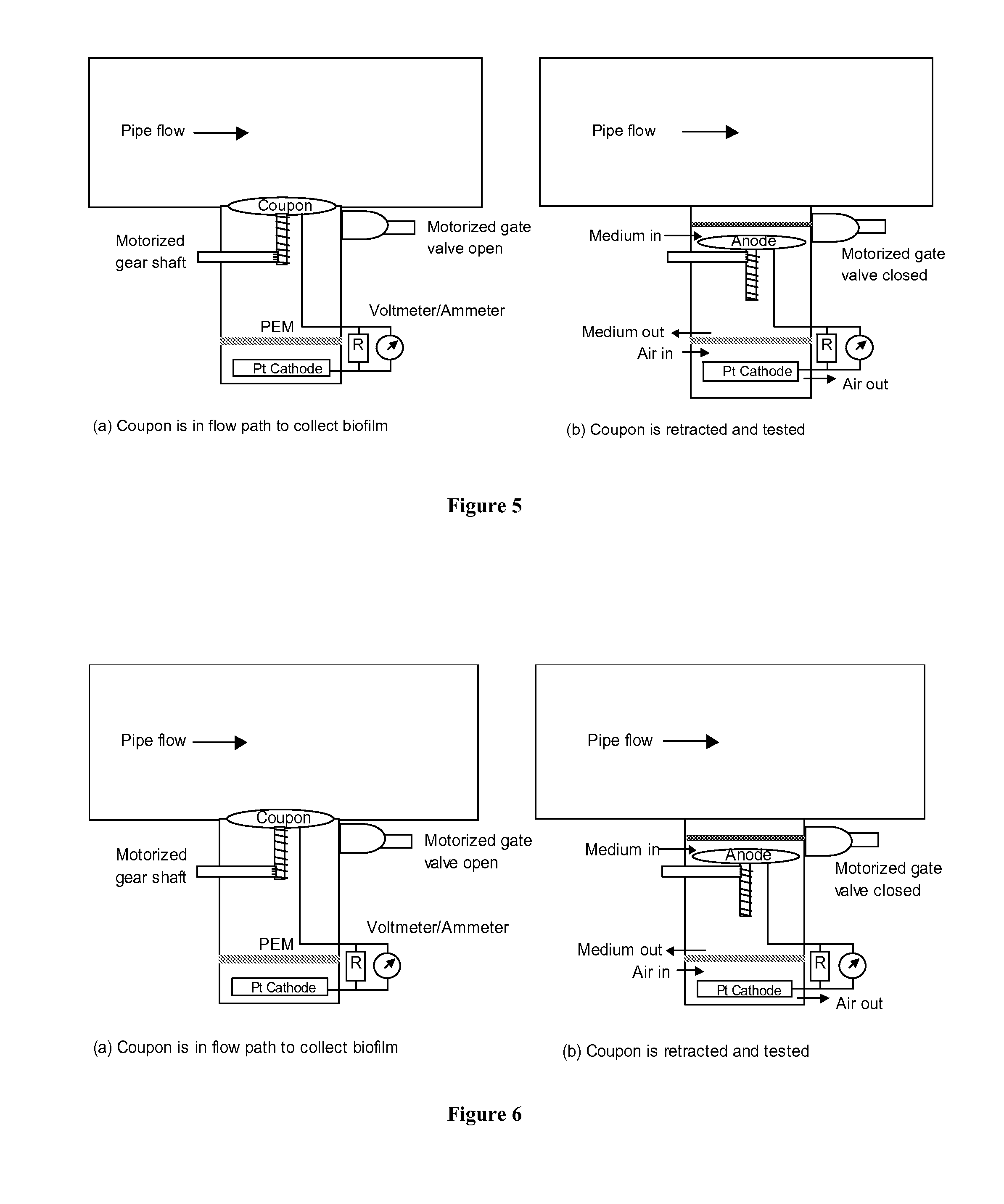
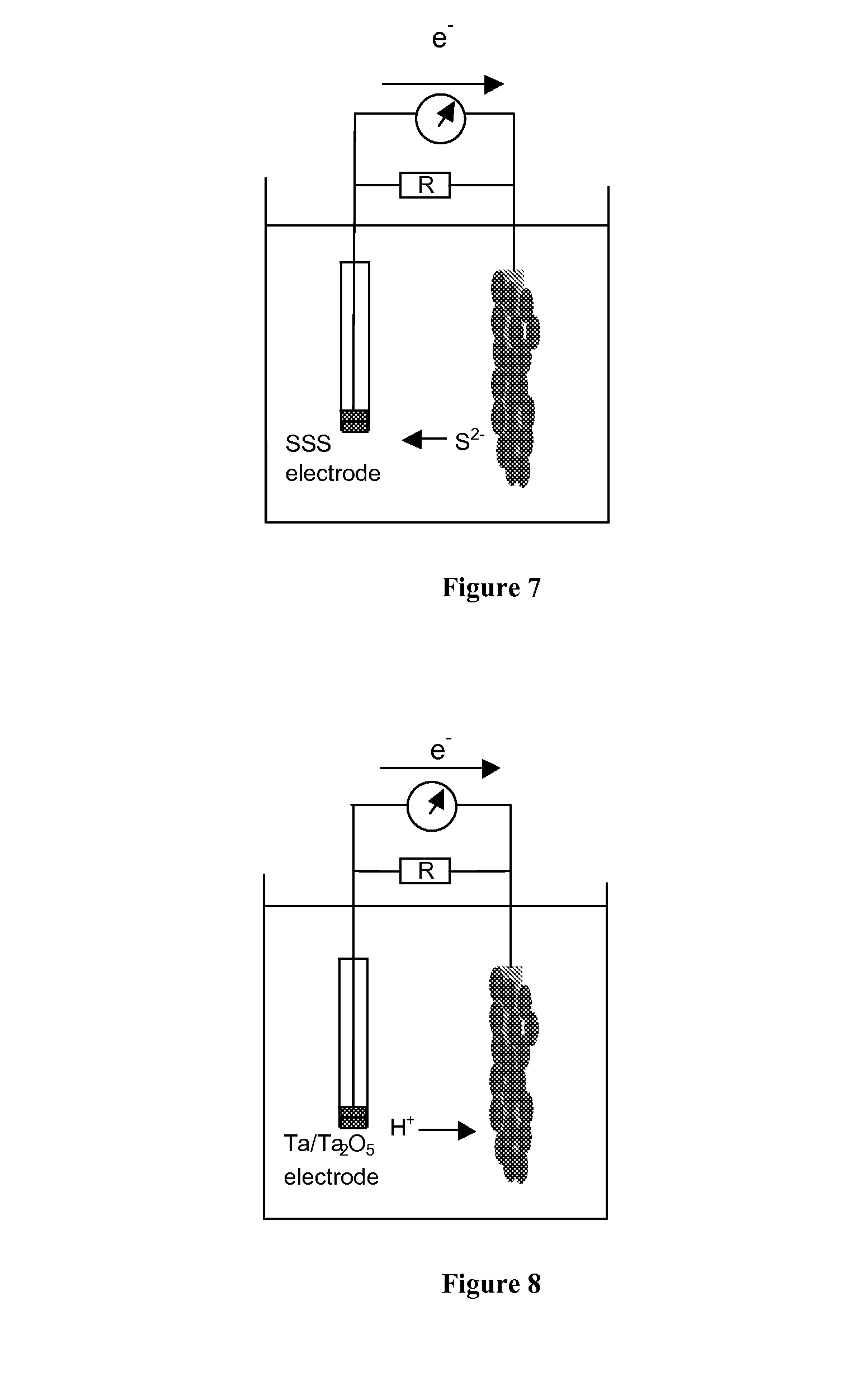
METHODS AND DEVICES FOR THE DETECTION OF BIOFILMS
This application claims priority to and any other benefit of U.S. Provisional Patent Application Ser. No. 61/479,635, filed on Apr. 27, 2011, the content of which is hereby incorporated by reference. The present disclosure relates to the field of biofilm detection, and more particularly to methods and devices for the detection of biofilms that are electrogenic, and thus corrosive. The present disclosure also relates to the measurement of how corrosive a biofilm can be against a metal such as carbon steel and stainless steel. Microbiologically influenced corrosion (MIC), also known as biocorrosion, causes billions of dollars in damage each year to various industries, including food processing, manufacturing, chemical processing, water utilities, and particularly, the oil and gas industry, just to name a few. Typically, MIC is a long-term process ranging from months to years. Mitigating MIC is costly, not only in terms of the cost of chemical treatments, but also in terms of lost production due to maintenance shutdowns. MIC is also known to adversely affect aging infrastructure, including piers, bridges, factories, shipyards, water towers, heat exchangers, fluid transfer pipes, and water treatment facilities. According to some sources, MIC accounts for approximately 20% of all corrosion of metals and building materials. MIC was the primary suspect in the 2006 Prudhoe Bay, Ak. pipeline leak (¼″ pinhole). MIC is becoming more problematic because infrastructures are aging and enhanced oil recovery is practiced more often than ever. Due to depleting reserves, and high oil and gas prices, previously unproductive or non-cost effective reservoirs remain in production by utilizing an enhanced oil recovery process known as flooding. The flooding process involves the use of water or carbon dioxide (CO2) to increase well pressure to push out residual oil from the reservoir. Most often, seawater, which introduces bacteria and nutrients to the system, is used in the flooding process. Seawater contains nutrients for microbial growth and bacteria such as sulfate reducing bacteria (SRB). In addition, bacteria from geological times may already be present in the reservoir. Microbial activities in reservoirs frequently cause souring due to sulfate reduction to form H2S gas. Oil pipelines are prone to MIC because microbes, water and nutrients are all present. Moreover, gas pipelines are not immune to MIC because a trace amount of moisture is unavoidable due to condensation. In contrast to general corrosion, MIC tends to be very localized. Biofilms, the major cause of MIC, are generally composed of microbial cells and their extracellular products (extracellular polymers), which confer to them a very porous structure, in agreement with the amount of water contained (>95% w/w). The distribution of microorganisms in a biofilm is not uniform. In multi-species biofilm consortia, highly complex structures containing voids, connecting channels between these voids, and microbial clusters or layers have been found. When a biofilm forms on a substrate, it may create a nodule and a pit may form beneath the nodule. The nodule may have an outer portion comprising aerobic bacteria that consumes oxygen, and an inner portion that experiences reduced oxygen levels that allow anaerobic bacteria to thrive. Once established, MIC is extremely difficult to eliminate and may develop into a chronic maintenance and operating problem for many years. The failure to totally remove MIC from crevices and the furthermost branches and dead legs of a piping system will generally result in reinfection by the same microorganisms within a short period of time after biocide and/or pigging treatments. In many cases, the primary concern with respect to MIC is anaerobic corrosion as opposed to aerobic corrosion because anaerobic biofilms live beneath aerobic biofilms. Anaerobic corrosion is due to dissolution of iron from the iron oxidation reaction below: where Fe2+ dissolves in the bulk fluid. The Fe2+may also react with other chemical species and precipitate. When sulfate and SRB are present, the SRB produce sulfide that can form iron sulfide (FeS), which has limited solubility in water. After reaching super saturation, FeS will precipitate, producing a black ink color. Generally, FeS precipitation along with the rotten egg-like smell of H2S indicates the presence of SRB activity. The electrons released by the iron oxidation reaction must be removed to drive Reaction 1 forward. Microbes in a biofilm on an iron-containing substrate, such as a carbon steel surface, may utilize the electrons for reduction reactions such as sulfate reduction by SRB and nitrate reduction by nitrate reducing bacteria (NRB), as shown below: Reactions 2 and 3 do not move forward without biocatalysis by a biofilm. Microbes such as SRB typically require organic carbons for growth. Oxidation of the organic carbon provides electrons and carbon building blocks for organic synthesis. The electrons are used for reduction reactions as seen in Reactions 2 and 3. The redox reaction produces energy for cellular metabolism and maintenance, which forms the basics of anaerobic respiration. Volatile fatty acids such as acetate and lactate are often used by SRB as a source of organic carbon. Acetate is usually more readily available than lactate in pipeline systems, but lactate is often used in laboratory tests because lactate is a better nutrient. As an example, the lactate oxidation reaction is shown below. The organic carbon oxidation reaction (Reaction 4) also requires biocatalysis by microbes such as SRB. When lactate oxidation is coupled with Reaction 2 or 3, the redox reaction produces energy. Organic carbon oxidation and oxidant (e.g., sulfate, nitrate, and nitrite) reduction occur in the cell's cytoplasm as shown in If Reactions 2 and 4 both occur in the cytoplasm, no external electrons are involved. Thus, direct electrochemical corrosion due to utilization of the electrons released by iron oxidation cannot occur. However, when a SRB biofilm forms on an iron substrate as shown in Unlike lactate, insoluble elemental iron cannot directly enter the cytoplasm to donate electrons. Therefore, the iron oxidation reaction (Reaction 1) occurs outside of the microbial cells. However, the electrons released by the iron oxidation reaction must enter the cell's cytoplasm to be utilized for sulfate reduction, as shown in There are two primary methods for electron transfer between a fluid and the cytoplasm of a cell: (a) direct electron transfer (DET); and (b) mediated electron transfer (MET). DET relies on special proteins and other molecules in the cell wall and inside the cell to pass electrons. For DET, direct contact with the substrate (e.g., iron substrate) is needed unless cells form pili to bridge the cell and the substrate. Typically a monolayer of sessile cells directly on a substrate (e.g., an iron substrate) is capable of accepting electrons from iron oxidation. However, it is likely that cells that form pili may link several layers of sessile cells with the iron substrate, thus causing more severe MIC. On the other hand, MET relies on electron mediators that are redox active electron carriers to shuttle electrons between the substrate and the cells. Mediators are soluble molecules capable of catching and releasing electrons. Mediator diffusion in the fluid results in the transfer of electrons and these carriers may also cross cell walls and membranes. When mediators or electron carriers are present, more than one layer of cells may contribute to the corrosion process. Apart from externally added mediators, some microbial cells are capable of secreting mediators to facilitate electron transfer. As a result, more electrons may be harvested from the iron oxidation reaction, resulting in more severe MIC due to an increase in the number of available electrons utilized by cells for reduction of an oxidant, such as sulfate, in the cytoplasm. The above discussion indicates that certain microbial cells have the ability to transfer and accept electrons, which is also known as electrogenicity. To cause direct electrochemical MIC, the sessile biofilm cells must be electrogenic. Thus, the corrosiveness of a biofilm is directly related to the electrogenicity of the biofilm. In some cases, non-electrogenic biofilm cells may also be considered electrogenic biofilm cells if they are capable of electron transfer between a metal substrate and cells with the help of electron mediators, such as mediators secreted by other microbes in the same biofilm community or mediators that are externally added. The ability to detect corrosive biofilms in places such as the inner surface of pipe walls is a long-standing problem. Currently, there are no reliable means for detecting corrosive biofilms due to a lack of understanding of exactly how biofilms attack metals. Some current methods and devices for biofilm detection use the linear polarization resistance (LPR) sweep technology. The assumption behind this technology is that the presence of a biofilm will correlate to a LPR response. Theoretically, the assumption behind this technology is inconsistent with basic biofilm bio-electrochemistry. It has been determined that biofilms are typically poor electron conductors, and in most cases, biofilm behavior has been found to be inductive, rather than resistive in nature. The LPR technology is intended for resistive films rather than inductive films. As a consequence, methods and devices utilizing LPR technology are likely to provide false positives. For example, LPR technology cannot distinguish between the presence of a mineral film, which is sometimes resistive, and a biofilm. Moreover, imposing an external voltage across a biofilm (e.g., as required by LPR) may interfere with microbial metabolism. When an external voltage is applied, the biofilm may shut down its native corrosion process because it can use the “free” electrons supplied by the externally applied voltage without consuming resources needed to get the external electrons. In fact, during the investigation of impressed current cathodic protection (ICCP) against MIC, researchers found that an externally imposed voltage actually attracted SRB biofilm growth. Furthermore, current sensors cannot differentiate between a corrosive biofilm (i.e., an electrogenic biofilm) and a non-corrosive biofilm (i.e., a non-electrogenic biofilm), even if the sensor can detect the presence of a biofilm. Another major problem associated with LPR technology is the cost derived from the requirement for an expensive potentiostat and the appropriate software. The LPR sweep technology requires a potentiostat that may be programmed to gradually increase the externally imposed voltage across a system containing a biofilm and measures the current density and calculates the polarization resistance. The hardware and software required to run such LPR sensor systems currently costs up to several thousands of dollars. Another reported method relies on the presence of iron sulfide (FeS) to detect biofilms. However, this is not reliable in most cases because abiotic FeS is typically present in most systems. Furthermore, when MIC is caused by other microbes such as methanogens or NRB, the presence of FeS is irrelevant for biofilm detection. Moreover, other methods for the detection of biofilms and MIC rely upon the nutritional and microbiological environment from which a sample is collected. Often times, the sample merely contains planktonic microbes, and not the biofilm microbes known to cause MIC pitting. Thus, there remains a need in the art for a method and device that can accurately detect the presence of a corrosive biofilm while doing so in a passive manner so as to not interfere with the biofilm's intrinsic corrosion processes. There is also a need to measure how corrosive a biofilm is against a certain metal substrate. In addition, there remains a need in the art for a method and device for accurately detecting the presence of a corrosive biofilm that is not cost prohibitive. The present disclosure relates to methods and devices for passively detecting an electrogenic, and thus corrosive biofilm. In an exemplary embodiment, a method for passively detecting a corrosive biofilm includes the steps of: a) exposing a first electrode to at least one medium containing microbes capable of forming a biofilm; b) allowing a biofilm to form on at least a portion of the first electrode; c) electrically connecting the first electrode having the biofilm formed on a portion thereof to a second electrode; and d) measuring an electrical characteristic generated by the electrically connected first electrode and second electrode to determine whether the biofilm is electrogenic. In another embodiment, a sensor for passively detecting a corrosive biofilm is provided. The sensor may include at least one first electrode, at least one second electrode, and an external circuit for electrically connecting the first electrode to the second electrode. At least one of the first electrode and the second electrode is capable of being at least partially coated by a biofilm. A sustainable electrical characteristic, such as a voltage or a current, generated when the first electrode and the second electrode are electrically connected and exposed to at least one medium indicates that the biofilm partially coating at least one of the first electrode and the second electrode is electrogenic, and thus corrosive. Additional features and advantages will be set forth in part in the description that follows, and in part will be obvious from the description, or may be learned by practice of the disclosed embodiments. The objects and advantages of the disclosed embodiments will be realized and attained by means of the elements and combinations particularly pointed out in any appended claims. It is to be understood that both the foregoing general description and the following detailed description are exemplary and explanatory only and are not restrictive of the disclosed embodiments, as may be claimed. The accompanying drawings, which are incorporated in and constitute a part of this specification, illustrate exemplary embodiments of the disclosed methods and devices, and together with the description, serve to explain principles of the methods and devices disclosed herein. Unless otherwise defined, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which the invention belongs. The terminology used in the description of the invention herein is for describing particular embodiments only and is not intended to be limiting of the invention. The present invention may be embodied in different forms and should not be construed as limited to the embodiments set forth herein. Rather, these embodiments are provided so that this disclosure will be thorough and complete, and will fully convey the scope of the invention to those skilled in the art. As used in the description of the invention and the appended claims, the singular forms “a,” “an,” and “the” are intended to include the plural forms as well, unless the context clearly indicates otherwise. Unless otherwise indicated (e.g., by the use of the term “precisely”), all numbers expressing quantities of ingredients, reaction conditions, and so forth as used in the specification and claims are to be understood as being modified in all instances by the term “about.” Accordingly, unless indicated to the contrary, the numerical parameters set forth in the following specification and attached claims are approximations that may vary depending upon the desired properties sought to be obtained in embodiments of the present invention. At the very least, and not as an attempt to limit the application of the doctrine of equivalents to the scope of the claims, each numerical parameter should be construed in light of the number of significant digits and ordinary rounding approaches. Notwithstanding that the numerical ranges and parameters setting forth the broad scope of the inventions are approximations, the numerical values set forth in the specific examples are reported as precisely as possible. Any numerical value, however, inherently contains certain errors necessarily resulting from the standard deviation found in their respective testing measurements. Every numerical range given throughout this specification will include every narrower numerical range that falls within such broader numerical range, as if such narrower numerical ranges were all expressly written herein. “Microbiologically influenced corrosion (MIC)” shall refer to processes in which any element of a system is structurally compromised due to the action of at least one member of a microbial population. The expressions “microbial fuel cell (MFC),” “biofilm sensor,” and “biological fuel cell” shall mean any bio-electrochemical system that drives a current by mimicking microbial interactions found in nature. “MFC” may also be defined as any biological device that evaluates the non-nutritional, electrochemical environment in which a sample is located and/or contained. “Partially coated,” shall mean that any portion of the surface of an electrode that is covered by a biofilm. In some embodiments the percentage of the coverage of the surface consists of 0.1, 0.25. 0.5, 0.75, 1, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95, and 100 percent. The expression “biofilm” refers to an aggregate of microorganisms in which cells adhere to adjacent cells and/or to a surface. These adjacent cells are frequently embedded within a self-produced extracellular matrix of polymeric substances often composed of proteins and polysaccharides. Microbial cells in a biofilm are physiologically distinct from planktonic cells of the same organism, which, by contrast are single cells that may swim or float through a fluid. The expression “aqueous solution,” when used to describe a composition, refers to a solution in which the solvent is water, including water containing salts, such as: magnesium sulfate (MgSO4), sodium citrate, calcium sulfate (CaSO4), ammonium chloride (NH4Cl), dipotassium phosphate (K2HPO4), sodium lactate (NaC3H5O3), and ammonium iron (II) sulfate Fe(NH4)2(SO4)2; water containing volatile fatty acids, salts of volatile fatty acids, alcohols, hexoses, and hydrogen; ocean or seawater; brackish water; sources of freshwater, including lakes, rivers, stream, bogs, ponds, marshes, runoff from the thawing of snow or ice; springs, groundwater, and aquifers; and precipitation. The expression “oil,” when used to describe a material in a method, refers to any substance that is a liquid at ambient temperature and is hydrophobic but soluble in organic solvents, including but not limited to hexanes, benzene, toluene, chloroform, and diethyl ether. Classes of compounds included within the context of the above definition include vegetable oils, petrochemical oils (e.g., crude and refined petrochemical products), and volatile essential oils (i.e., aroma compounds from plants). The expression “fuel,” when used to describe a material in a method, refers to any substance that stores energy, including fossil fuels, gasoline, mixtures of hydrocarbons, jet and rocket fuels, and biofuels. The expressions “metal,” “metallic,” and “metal alloy,” when used to describe a substance in a method, refer to any elemental metal or alloy comprised of elemental metals (e.g., brass, bronze, and steel). Examples of metal and metal alloy products include but are not limited to pipes, infrastructure, beams, sheeting, prefabricated structures, underwater structures, retaining structures (e.g., water towers), military installations and structures, military equipment (e.g., submarines and ships), and munitions. “Microbes” shall mean any and all microorganisms capable of colonizing and/or causing MIC, either directly or indirectly. Examples of microbes that generally colonize and cause damage to pipelines in the gas and oil industries are The expression “electrochemical,” when used to describe a property, refers to the study of chemical or biochemical reactions which take place in solution at the interface of an electron conductor and an ionic conductor, and which involves electron transfer between the electrode and the electrolyte species in solution. The terms “electrogenic” or “electrogenicity” shall herein refer to the property of a living organism to produce electrical activity or an electric response related to the transfer or utilization of electrons in biological processes. The term “electrical characteristic,” as used herein, refers to an electrical quantity, such as voltage, electric current, electrical resistance, open circuit voltage, etc. The term “medium,” as used herein, refers to a fluid to which an electrode of the present invention is exposed. The fluid may be a liquid and/or a gas, and includes, but is not limited to, MgSO4solution, NaCl solution, buffer solutions such as phosphate buffered saline (PBS), air, oxygen, hydrogen, water, and the previously defined terms “aqueous solution,” “oil,” and “fuel.” The medium must contain ionic species for electrical conduction. The terms “passive” or “passively,” as used herein, refers to the absence of an externally applied voltage or electric field. The term “sustainable,” as used herein to describe an electrical characteristic, means that the electrical characteristic being measured does not dissipate in less than about thirty minutes. Disclosed herein are methods and devices for passively detecting corrosive biofilms based upon the electrogenicity of the biofilm. As previously noted, direct electrochemical MIC process requires an electrogenic biofilm on a metal surface. In an exemplary embodiment, an electrogenic biofilm is capable of transporting electrons from a metal oxidation reaction (e.g., iron oxidation, Reaction 1) to the cytoplasm of biofilm cells where a reduction reaction utilizes the electrons. The biofilm's electron “uptake” drives Reaction 1 forward, causing iron dissolution. The loss of iron is stoichiometrically related to the loss of electrons from iron oxidation. For every two electrons lost, one iron (Fe) atom becomes soluble ferrous ion (Fe2+). Thus, directly measuring the number of electrons transferred between an iron surface and a biofilm may indicate biofilm corrosiveness, and the presence or rate of MIC. However, no sensor has been disclosed that can be placed between a metal surface and a biofilm for measuring electron transfer. Such a sensor is unlikely possible because it would interfere with the MIC process itself. Disclosed herein are passive sensors that detect whether a biofilm is electrogenic, and thus corrosive. In an exemplary embodiment, the sensor includes at least one first electrode, at least one second electrode, and an external circuit for electrically connecting the first electrode to the second electrode. At least one of the first electrode and the second electrode is capable of being at least partially coated by a biofilm. A sustainable electrical characteristic generated when the first electrode and the second electrode are electrically connected and exposed to at least one medium indicates that the biofilm partially coating at least one of the first electrode and the second electrode is electrogenic, and thus corrosive. In one embodiment, the sensor may be formed as a microbial fuel cell (MFC). In this embodiment, the first electrode may operate as an anode, while the second electrode may operate as a cathode. Alternatively, the first electrode may operate as a cathode, and the second electrode may operate as an anode. The sensor may include a chamber having a first electrode compartment and a second electrode compartment. A proton exchange membrane (PEM) may be utilized to separate the first electrode compartment and the second electrode compartment. The first electrode compartment includes the first electrode and a first medium, and the second electrode compartment includes the second electrode and a second medium. The sensor also includes an external circuit for electrically connecting the first electrode to the second electrode. Referring now to The oxidation and reduction reactions are split into two separate half cells so that the electron flow from the anodic chamber to the cathodic chamber can be measured. The coupling of Reactions 5 and 6 releases energy. In some embodiments, a sustainable electrical characteristic (e.g., voltage and current) may be generated, but only if the biofilm is capable of transferring electrons from the second electrode to the cytoplasm of the SRB for sulfate reduction. In some embodiments, the sensor is designed so that the limiting step is the electron transfer between the second electrode and the biofilm. To make this happen, electron transfer resistance between dissolved hydrogen (H2) and the anode is minimized via a first electrode having a sufficiently large area. The internal resistance is also minimized by using a Nafion™ membrane with a sufficiently large surface area. In some embodiments, the sustainable electrical characteristic is detected by a voltmeter, a zero resistance ammeter (ZRA), a picoammeter, or a standard multimeter. Preferably, an ammeter utilized herein also has the ability to measure voltage. Both open circuit voltage and closed circuit voltage are useful electrical characteristics. In order to measure the closed circuit voltage, an external resistor (represented by “R” in In some embodiments, the sensor shown in For example, a common corrosive biofilm such as In some embodiments, the second electrode (i.e., cathode) of the sensor shown in Referring now to Taking lactate as an exemplary organic carbon source, the anodic reaction and the cathodic reaction occurring in the sensor of In this embodiment, electrons released from lactate oxidation in the cytoplasm are donated by the biofilm to the first electrode (i.e., the anode). As a result of electron release and donation of the released electrons to the first electrode by the electrogenic microbes in the biofilm coating or partially coating the first electrode, electrons may flow through the external circuit to the second electrode to participate in oxygen reduction. The flow of electrons is opposite from the electron flow from a metal surface to a biofilm in an actual MIC process. However, the two directions of electron flow are closely related, and therefore if a biofilm donates electrons efficiently, it may also be able to accept electrons efficiently. This means that a sustainable electrical characteristic detected by the sensor shown in In one exemplary embodiment, the anodic biofilm sensor as shown in In one embodiment, an electrode may comprise a dual plate electrode comprising a first metal plate (preferably steel or the same metal as the structure) in direct contact with a second inert plate (preferably a graphite plate, or other inert, conductive material). The first metal plate may further include a porous membrane or porous coating that covers any surface of the first metal plate that is not in direct contact with the second inert plate to prevent direct attachment of a biofilm and to allow ionic species to diffuse into the medium. The first metal plate serves as an electron donor to the biofilm that forms of the second inert plate. This electrode design allows the biofilm to access electrons from metal oxidation through the conductive second inert plate. Accordingly, this electrode design will encourage the biofilm to attach to the second inert plate more closely in order to promote electrogenicity of the biofilm through more direct contact, or by forming pili between the sessile cells and the surface of the second inert plate. During sensor measurement of the electrogenicity of the biofilm, the first metal plate may be removed to eliminate any interference. In some embodiments, an electrode may comprise carbon foam, carbon paper, reticulated vitrified carbon, carbon cloth, molybdenum carbide, carbon nanotubes, conductive polymers, platinum, a platinized metal, cobalt complexes, manganese oxides, and lead dioxide, just to name a few. In some embodiments, a corrosive biofilm may be harvested online, inside a pipeline or from other locations where biofilms may prove to be problematic. In some embodiments, the biofilm may then be grown in either a cathodic or anodic biofilm sensor to measure the electrogenicity of the biofilm. To grow a biofilm (with an inoculum collected from a suspected biofilm contaminated site) in an offline cathodic biofilm sensor, some limited amount of organic carbon (e.g., lactate) or dissolved H2nutrient (for methanogens) is added to the cathodic chamber. The added amount should be limited such that as the biofilm grows, it consumes most if not all of the added nutrient. This will allow an established biofilm on the cathode to start accepting electrons from the cathode that are supplied by the anode via the external circuit. This will generate a sustainable electrical characteristic, such as a voltage and a current, that can be measured by a voltmeter/ammeter. Similarly, to grow a biofilm in an offline anodic biofilm sensor, some limited amount of oxidant such as sulfate, nitrate, nitrite or carbon dioxide is added to the anodic chamber. When the biofilm is established on the anode, it should consume most if not all of the oxidant. Subsequently, the biofilm will use the anode as an electron acceptor instead of the oxidant and the biofilm will donate the electrons from organic carbon oxidation to the anode. This will generate a sustainable electrical characteristic, such as a voltage and a current, in the external circuit that can be measured by an electrical measuring device, such as a voltmeter/ammeter. For online anodic biofilm sensors, an artificial medium free of oxidants (e.g., oxygen, sulfate, nitrate, nitrite, etc.) may be utilized for an anodic biofilm sensor. In other embodiments, the online cathodic biofilm sensor may require an artificial medium free of organic carbon and dissolved hydrogen. In other embodiments, fluid from the biofilm's native environment may be used as the medium for testing or sensing procedures. In some embodiments, oxidants in the native medium may be removed allowing time for the biofilm to consume them while the coupon (i.e., electrode) withdrawn from a pipeline or a flow system is offline or the oxidant may be removed by precipitation. The native medium can be replaced with a medium free of oxidants to maximize sustainable electrical characteristics for the anodic biofilm sensor ( In some embodiments, the novel sensors may be used for the online detection of corrosive biofilms. In some embodiments, the sensors may be strategically placed in high-risk locations such as dead-legs in a pipeline where water and nutrients tend to accumulate. In another embodiment, sensors may be placed in high-risk locations including, storage tanks and water cooling towers. In an exemplary embodiment, a biofilm may be collected on a metal coupon (i.e., electrode) from a pipeline. Preferably, the metal coupon comprises the same material as the pipeline. Subsequently, the metal coupon is withdrawn from the pipeline, and then is treated as a cathode electrode in a sensor containing a hydrogen anode at pH 7 that provides a potential of −414 mV, as seen in With reference to In another exemplary embodiment, an online anodic biofilm sensor may be used to collect a biofilm on a metal coupon (i.e., electrode). Preferably, the metal coupon comprises the same material as the pipeline. Subsequently, the coupon is withdrawn from the pipeline and is used as an anode electrode in the sensor, as seen in With continued reference to Both cathodic and anodic biofilm sensors possess distinct advantages, especially when original pipeline fluids are used after removal of oxidants or reducers. The removal of oxidants (for an anodic biofilm sensor) may be easier than the removal of reducers (for a cathodic biofilm sensor) in some cases, and vice versa. As a result of the difficulty to selectively remove oxidants and reducers, an anodic or cathodic biofilm sensor may be more convenient than the other based upon the medium being utilized. In some embodiments, anodic and cathodic biofilm sensors may use any variety of electrogenic microbes that may form a biofilm, including SRB such as Examples of microbes that generally colonize and cause damage to pipelines in other industries are: In some embodiments, the new sensors may detect both the presence of a corrosive biofilm, and the aggressiveness of the biofilm toward a metal surface by comparing sensor output data to biofilm standard data that has been collected as a result of MIC pitting studies, such as studies conducted in anaerobic vials. In some embodiments, the sensors may use either a zero resistance ammeter (ZRA) or a picoammeter as opposed to the expensive potentiostats associated with LPR technologies. In some embodiments, a standard multimeter or voltmeter/ammeter combo meter may be used in place of a ZRA for preliminary sensing. In another embodiment, a visual sensor will provide a visual signal at a predetermined output current and/or voltage threshold. In another embodiment, the visual signal will be the illumination of a light, such as a light emitting diode (LED) to notify an inspector. Phototransistors may be provided in the external circuit to amplify the sustainable electrical characteristic, such as the voltage and/or current, to trigger the visual signal to alert an inspector. The inspector may go to the site where the sensor is located and use an electrical measuring device to confirm the presence of a sustainable electrical characteristic. Additionally, the inspector may retrieve the electrode for offline analysis. In other embodiments, the sensor may be configured to send a signal to a GPS, GSM, or WiFi device as part of a signaling system to notify the inspector of possible corrosive biofilm buildup or MIC. A more expensive and precise voltmeter/ammeter such as a potentiostat may be brought on site for more precise measurements of voltage and current output. In some embodiments, the sensor may be miniaturized to produce a biofilm microsensor (BMS) for online or offline uses. In other embodiments, a biofilm microsensor may be placed upon a chip or integrated into microcircuits to yield miniaturized devices for measurements in systems of reduced diameter or size. In addition to the exemplary embodiments of sensors as shown in Contemplated herein are a number of structurally diverse sensors that may be formed as, but not limited to, channels with removable plates, online plug sensors (e.g., threaded, tension-fit, clip-fit, washer-fit, collar-fit, and mechanically immobilized), multiport biofilm sensors, rack and plate sensors for batch sampling, and ball or disk sensors. Contemplated herein is a sensor comprising a flow cell or channel, a removable coupon or plate, and a fastening device to secure the plate to the flow chamber. The plate may be manually removed from the flow channel for offline sampling by insertion into an external system. The electrogenicity of microbes may be tested in the external system, or physical analysis of the coupon may be completed, including scanning electron microscopy (SEM) and atomic force microscopy (AFM), energy dispersive spectrometry (EDS) and X-Ray diffraction (XRD). Contemplated herein are a series of threaded plug sensors that are configured to engage a threaded opening of a structure, such as a pipeline, containing at least one medium. In some embodiments, the plug sensor may be secured via tension, or by devices including, clips, washers, nuts, screws, o-rings, pins, collars, or other means of mechanical immobilization. Also contemplated herein, is a multiport sensor in which the device contains two or more individual ports over which a sealing device (e.g., plate, coupon, plug, ball, disk, or other metallic object) may be placed so that a portion of the surface of the metallic object is in contact with a medium that may be prone to biofilm growth. In other embodiments, the sealing device may be held in place via a previously described method and any number of sealing devices from one to all devices on said multiport sensor may be removed for testing either at once or at different time points. Also contemplated herein, are ball or disk devices in which a portion of the ball or disk may be exposed to a fluid medium for sample collection of a biofilm before alteration of the position of the ball or disk provides access to the sample for either online or offline testing to establish either the presence and/or aggressiveness of an electrogenic biofilm. In some embodiments, the ball or disk may be supported by a rod along an axis. In other embodiments, tension may be used to secure the ball or disk in place for either sample collection or for data collection experiments. In one embodiment, a series of biofilm cell or planktonic cell samples are isolated from the field. Each sample is tested individually in a sensor in the lab using a medium that reflects the field condition best. In some embodiments, the culture medium may be local fluid (e.g., seawater, pipeline fluid, etc.) collected from the field. After a biofilm is established on the anode surface or the cathode surface, the growth medium is replaced with a new medium and the system is allowed to equilibrate. The new medium may be free of oxidants (if an anodic biofilm sensor is used) or reducers (if a cathodic biofilm sensor is used) which may allow voltage and current responses. In another embodiment, a vial with a Nafion™ membrane sealed bottom is used to sample field biofilm or planktonic cells. The vial contains a medium to promote biofilm growth on reusable platinized, stainless steel, or other metals in the vial. An oxygen scavenger such as cysteine may be added to the medium to remove oxygen in order to promote the growth of an anaerobic biofilm on the metal surface. After hours or a few days, a biofilm may form on the metal surface. The medium may be replaced with a new medium, such as an electrolytic solution, that is free of organic carbon and hydrogen. The vial is then used as the cathodic chamber in a microbial fuel cell with a tantalum/tantalum pentoxide anode electrode in the new medium at pH 7. The electrical characteristics, such as the voltage and the current, are measured to indicate whether the biofilm is electrogenic, and thus corrosive. In some embodiments, the new medium may comprise a MgSO4 solution (0.1% to 2% w/w), a NaCl solution (0.1% to 2% w/w) or a PBS buffer, just to name a few. In yet another embodiment, a vial with a Nafion™ membrane sealed bottom or side is used to sample field biofilm or planktonic cells. The vial contains a medium to promote biofilm growth on a reusable platinized, stainless steel, or other metal electrode in the vial. An oxygen scavenger such as cysteine may be added to the medium to remove oxygen in order to promote the growth of an anaerobic biofilm on the electrode surface. After hours or a few days, a biofilm may form on the electrode surface. The medium is replaced with a new medium comprising organic carbon, but free of oxidants such as sulfate, nitrate and carbon dioxide. The vial is then used as the anodic chamber in a microbial fuel cell with an oxygen half cell. The electrical characteristics, such as the voltage and the current, are measured to indicate whether the biofilm is electrogenic, and thus corrosive. Referring now to Utilizing a solid state silver/silver sulfide (Ag/Ag2S or SSS) electrode as the first electrode (i.e., anode) provides several advantages. First, the SSS electrode is an inexpensive and rigid electrode that comprises a wire linked to a silver disk coated with solid silver sulfide. The half cell reactions for the sensor utilizing a silver/silver sulfide electrode are as follows: Electrons generated by the anodic reaction (9) are utilized by the biofilm for sulfate reduction (10). The arrows shown in A silver/silver sulfide electrode may be made inexpensively by dipping a silver disk or rod into an alkaline sodium sulfide solution. An external voltage and a stainless steel electrode may be used to deposit the sodium sulfide onto the silver surface at a faster rate. Additionally, silver/silver sulfide electrodes are commercially available as a reference electrode. In addition to the silver/silver sulfide electrode, the first electrode (i.e., anode) may be a solid-state electrode selected from the group consisting of a tantalum/tantalum pentoxide (Ta/Ta2O5) electrode, an ion selective electrode (ISE), and an ion-selective field effect transistor. The important feature of such an electrode is that the electrode should be able to provide a standard reduction potential that is substantially more negative than the standard reduction potential of ferrous ion/iron (Fe2+/Fe), which is −0.447 V. A very negative standard reduction potential means that the oxidation reaction is more favorable and likely to occur. This is important because an electrode with a very negative standard reduction potential, such as that for the SSS electrode (−0.71 V), suppresses iron oxidation that can locally supply electrons to the biofilm, which can cause the sensor to produce no voltage or a faulty voltage. When this type of first electrode (i.e., anode) is utilized, the second electrode may comprise an iron-based metal instead of having to use a graphite or other inert electrode material. Using an iron-based metal has a distinct advantage in that the second electrode may comprise the same material as a structure in the field (e.g., a pipeline, a storage tank, a heat exchanger, etc.). Accordingly, the biofilm would be formed on a second electrode comprising the same material as the structure in the field, as opposed to a different material such as a graphite. Another advantage of using a first electrode (i.e., anode) with a very negative standard reduction potential is that there is no need to remove organic carbon from the medium. For example, organic carbon oxidation may be suppressed on the surface of the second electrode (i.e., cathode) because electrons are readily available on the surface of the second electrode from the external circuit. The very negative standard reduction potential makes this possible, which is similar to the aforementioned iron oxidation suppression. This requires the biofilm present on the second electrode to be electrogenic, so that the biofilm is able to accept electrons from the second electrode. Still another advantage of utilizing an anode electrode with a very negative standard reduction potential, such as a Ta/Ta2O5electrode, is that the proton exchange membrane may be eliminated. The external circuit of the sensor only needs to be electrically connected to the first electrode and the second electrode periodically to measure an electrical characteristic such as a voltage or current. Most of the time, the external circuit is open, which allows the biofilm to form on and corrode the second electrode (i.e., cathode). In some embodiments, the second electrode, or cathode, may be harvested for MIC pitting examination offline. Referring now to The tantalum/tantalum pentoxide (Ta/Ta2O5) electrode has a standard reduction potential of −0.75 V at pH 7. The half cell reaction for a sensor utilizing a tantalum/tantalum pentoxide (Ta/Ta2O5) electrode is as follows: The oxidation reaction (11) has a potential of +0.75 V and may be coupled with a reduction reaction, such as a sulfate reduction reaction (−0.217 V) or a nitrate reduction reaction (+0.76 V) as shown below. Theoretically, the open-circuit potential of the sensor shown in As previously mentioned, the first electrode (i.e., anode) may comprise an ion-selective field effect transistor (ISFET) if the ISFET provides a sufficiently negative standard reduction potential. An ion-selective field effect transistor may allow miniature sensors to be produced, with the size being primarily dependent on how large a cathodic surface area is desired. With reference now to In the embodiments shown in In some embodiments, the presence of oxygen in contact with an electrode partially coated with a biofilm (i.e., a biocathode) may interfere with sensor output. Oxygen will cause an abnormally large voltage output because oxygen has a very large standard reduction potential (+0.818 V). If the electrode is completely coated with a biofilm, the electrode surface may still be anaerobic underneath the biofilm due to the consumption of oxygen by aerobic biofilm cells in the outer layer of the biofilm consortium. In one embodiment, an oxygen scavenger, such as cysteine, may be added to the medium to remove oxygen. In another embodiment, if a sensor is suspected of experiencing oxygen interference, the measurement of an electrical characteristic may be performed offline by using nitrogen sparging or an oxygen scavenger chemical to remove oxygen. In other embodiments, the anode electrode in a biocathode type sensor may become fouled by a biofilm buildup. In most cases, the fouling of the anode electrode will not substantially impact the sensor operation. However, the anode may be cleaned periodically for maintenance. In cases where anode electrode fouling is a problem, the anode electrode may be covered by a membrane, such as a 0.1 micron microfiltration membrane or a large-pore ultrafiltration membrane (e.g., UF 100,000), to block the microbes and their spores, while still allowing the ionic species to diffuse through. In some embodiments, an ion-exchange membrane may be used. Still further, placing the anode electrode surface downward can sometimes slow down biofilm buildup. Referring now to Referring now to With reference now to The embodiment shown in An exemplary method for passively detecting a corrosive biofilm includes the following general steps: a) exposing a first electrode to at least one medium containing microbes capable of forming a biofilm; b) allowing a biofilm to form on at least a portion of the first electrode; c) electrically connecting the first electrode having the biofilm formed on a portion thereof to a second electrode; and d) measuring an electrical characteristic generated by the electrically coupled first electrode and second electrode to determine whether the biofilm is electrogenic. As previously mentioned, the first electrode and the second electrode may function either as an anode or as a cathode. For purposes of discussing the steps in the exemplary method presented above, the first electrode will operate as a cathode, while the second electrode will operate as an anode. Thus, in one embodiment, the first electrode is selected from the group consisting of a graphite, a metal, and a metal alloy, and the second electrode is selected from the group consisting of a tantalum/tantalum pentoxide electrode, a silver/silver sulfide electrode, an ion selective electrode, and an ion-selective field effect transistor. The step of measuring an electrical characteristic may be accomplished by using an electrical measuring device such as a multimeter, a high impedance voltmeter, or a low (or zero) resistance ammeter, just to name a few. As previously discussed, the electrical characteristic may comprise at least one of a voltage and a current. In one particular embodiment, steps c) and d) of the exemplary method may be performed simultaneously by electrically connecting the first electrode having the biofilm formed on a portion thereof to the second electrode with a high impedance voltmeter. In another embodiment, steps c) and d) of the exemplary method may be performed simultaneously by electrically connecting the first electrode having the biofilm formed on a portion thereof to the second electrode with a zero resistance ammeter. In yet another embodiment, the method may further comprise the step of comparing a measured electrical characteristic to electrical characteristics associated with known corrosive biofilm compositions to determine the type of corrosive biofilm present. For example, the measured voltage may be compared to the voltages of know corrosive biofilm compositions, and a measured voltage similar to the voltage of a known biofilm provides an indication of the type of biofilm present. Similarly, a measured current may correlate to the aggressiveness of biofilm present, as discussed in detail above. In an additional embodiment, after step b) and before step c), the exemplary method may further comprise the steps of: i) removing the first electrode having the biofilm formed on a portion thereof from the at least one medium containing microbes capable of forming a biofilm; and ii) placing the first electrode having the biofilm formed on a portion thereof and the second electrode in a medium different from the at least one medium containing microbes capable of forming a biofilm. In this particular embodiment, a biofilm may form on the first electrode online, and subsequently the first electrode and its biofilm may be removed and tested for electrogenicity in an offline sensor containing a different medium, or in an online sensor, such as shown in This description is provided to describe the scope of contemplated methods and devices for passively detecting an electrogenic, and thus corrosive biofilm. However, the previously described methods and devices may be embodied in different forms and such methods and devices should not be construed as limited to the previously stated embodiments. Rather, these embodiments are provided so that this disclosure will be thorough and complete, and will fully convey the scope of the methods and devices to those of ordinary skill in the art. For convenience, an existing dual-chamber microbiological fuel cell (MFC) with a Nafion™ proton exchange membrane (PEM) was used to form electrogenic biofilms, as seen in After three days, an established SRB biofilm formed on the graphite electrode. The biofilm utilized the electrons supplied by the anode electrode through the external circuit for the oxidation of sulfate in the cathodic chamber. Two identical graphite electrodes partially coated with biofilms were harvested from the MFC. The two graphite electrodes were rinsed with deoxygenated MgSO4solution (0.2% w/w, as in the ATCC 1249 medium) to remove loosely attached planktonic cells. One graphite cathode was treated with 4% (w/w) glutaraldehyde for 8 hours to kill the biofilm cells. These operations were conducted in a glovebox with a positive nitrogen gas pressure to prevent oxygen contamination. Referring now to Methods and devices for the detection of corrosive biofilms and microbiologically influenced (MIC) corrosion rates are based upon the electrogenicity of the biofilms. The device may comprise a passive sensor having at least one first electrode, at least one second electrode, and an external circuit for electrically connecting the first electrode to the second electrode. At least one of the first electrode and the second electrode is capable of being at least partially coated by a biofilm. A sustainable electrical characteristic, such as voltage and current, generated when the first electrode and the second electrode are electrically connected and exposed to at least one medium indicates that the biofilm partially coating at least one of the first electrode and the second electrode is electrogenic, and thus corrosive. Special electrode and sensor designs are needed for the implementation of online and offline biofilm sensors. 1. A method for passively detecting a corrosive biofilm, comprising the steps of:
a) exposing a first electrode to at least one medium containing microbes capable of forming a biofilm; b) allowing a biofilm to form on at least a portion of the first electrode; c) electrically connecting the first electrode having the biofilm formed on at least a portion thereof to a second electrode; and d) measuring an electrical characteristic generated by the electrically connected first electrode and second electrode to determine whether the biofilm is electrogenic. 2. The method according to 3. The method according to 4. The method according to 5. The method according to i) removing the first electrode having the biofilm formed on a portion thereof from the at least one medium containing microbes capable of forming a biofilm; and ii) placing the first electrode having the biofilm formed on a portion thereof and the second electrode in a medium different from the at least one medium containing microbes capable of forming a biofilm. 6. The method according to 7. The method according to 8. The method according to 9. A sensor for passively detecting a corrosive biofilm, the sensor comprising:
a) at least one first electrode; b) at least one second electrode; and c) an external circuit for electrically connecting the first electrode to the second electrode; wherein at least one of the first electrode and the second electrode is capable of being at least partially coated by a biofilm; and whereby a sustainable electrical characteristic generated when the first electrode and the second electrode are electrically connected and exposed to at least one medium indicates that the biofilm is electrogenic. 10. The sensor according to 11. The sensor according to 12. The sensor according to 13. The sensor according to 14. The sensor according to 15. The sensor according to a top plate having a plurality of top plate wells, wherein each of the plurality of top plate wells includes a first electrode having a first electrode lead wire, the first electrode comprising tantalum/tantalum pentoxide; a bottom plate having a plurality of bottom plate wells, wherein each of the plurality of bottom plate wells includes a second electrode having a second electrode lead wire, and each of the plurality of bottom plate wells contains at least one medium capable of forming a biofilm on the second electrode; the top plate communicating with the bottom plate such that the first electrode in one of the plurality of top plate wells is in contact with the at least one medium contained in one of the plurality of bottom plate wells; and whereby a sustainable electrical characteristic generated when a first electrode lead wire and a corresponding second electrode lead wire are electrically connected by an external circuit indicates that an electrogenic biofilm is present. 16. The sensor according to 17. The sensor according to 18. The sensor according to 19. A drop-in biofilm sensor module for placement within a structure containing at least one medium suspected of containing microbes capable of forming a corrosive biofilm, the sensor module comprising:
a chamber at least partially enclosing at least one first electrode in an interior of the chamber, the chamber configured such that the at least one medium enters the interior and contacts the at least one first electrode to allow a biofilm to form on at least a portion of the at least one first electrode; wherein the at least one first electrode having the biofilm formed on at least a portion thereof is subsequently electrically connected to at least one second electrode by an external circuit; and whereby a sustainable electrical characteristic generated when the at least one first electrode and the at least one second electrode are electrically connected and exposed to the at least one medium indicates that the biofilm is electrogenic.CROSS-REFERENCE TO RELATED APPLICATIONS
TECHNICAL FIELD
BACKGROUND
Fe→Fe2++2e− (iron oxidation reaction) (1)
SO42−+9H++8e−→HS−+4H2O (sulfate reduction) (2)
2NO3−+10e−+12H+→N2+6H2O (nitrate reduction) (3)
CH3CHOHCOO−+H2O→CH3COO−+CO2+4H++4e− (lactate oxidation) (4)BRIEF SUMMARY
BRIEF DESCRIPTION OF THE DRAWINGS
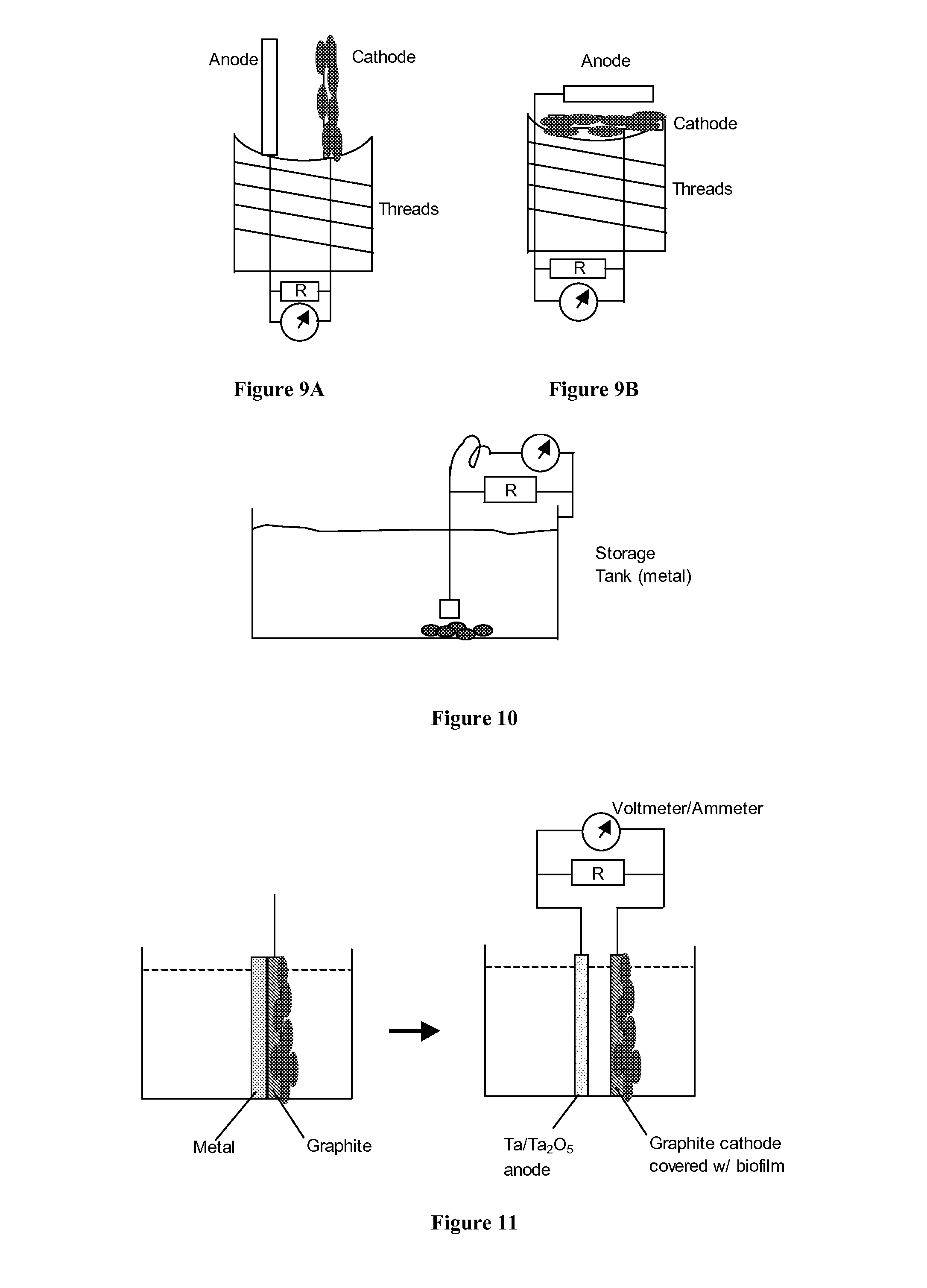
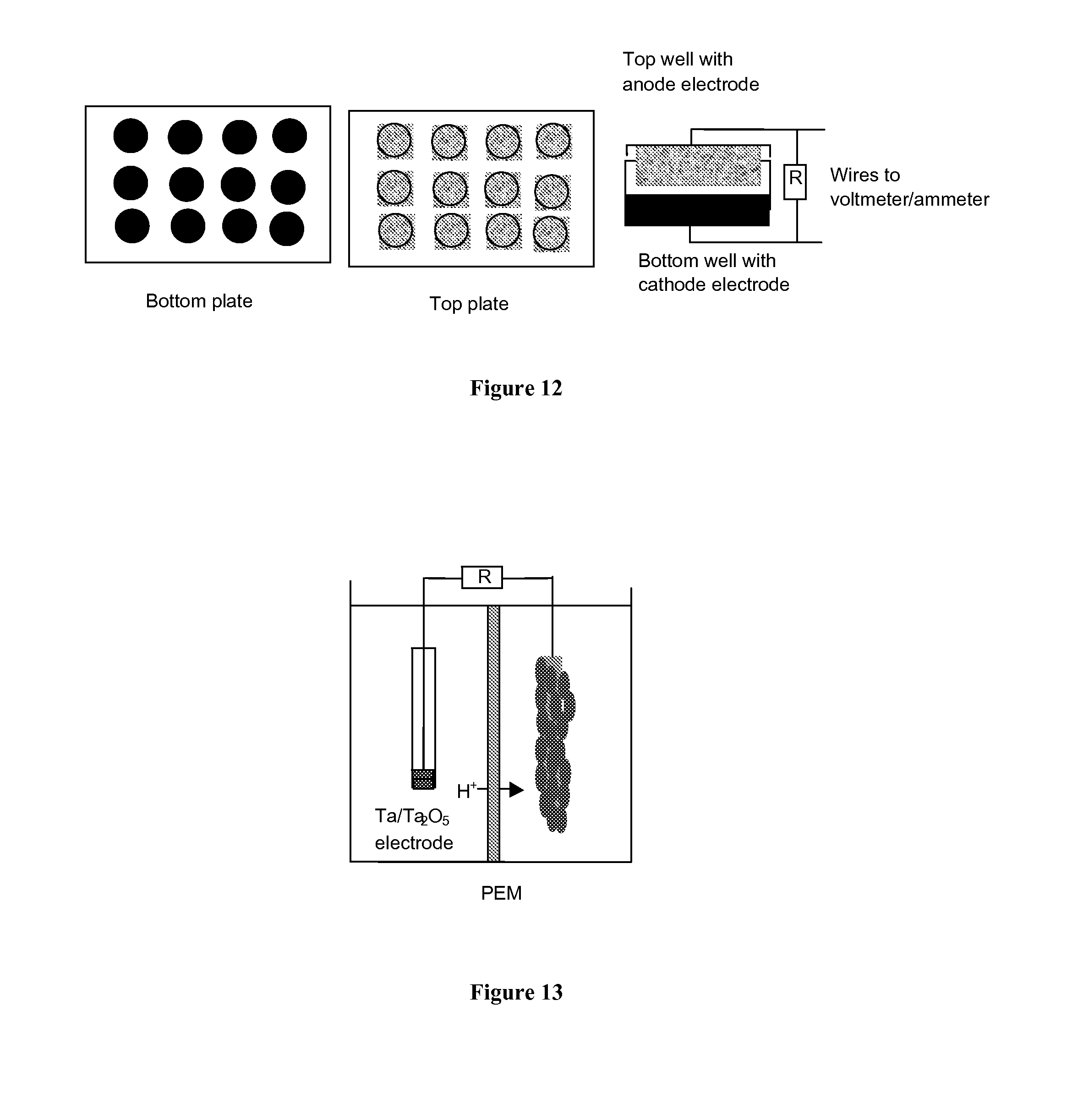
DETAILED DESCRIPTION
H2→2H++2e− Anodic reaction (5)
SO42−+9H++8e−→HS−+4H2O Cathodic reaction (6)
CH3CHOHCOO−+H2O→CH3COO−+CO2+4H++4e− Anodic reaction (7)
O2+4e−+4H+→2H2O Cathodic reaction (8)
2Ag+S2−→Ag2S+2e− Anodic reaction (9)
SO42−+9H++8e−→HS−+4H2O Cathodic reaction (10)
2Ta+5H2O→Ta2O5+10H++10e− Anodic reaction (11)
SO42−+9H++8e−→HS−+4H2O Sulfate reduction (12)
2NO3−+12H++10e−→N2+6H2O Nitrate reduction (13)EXAMPLE
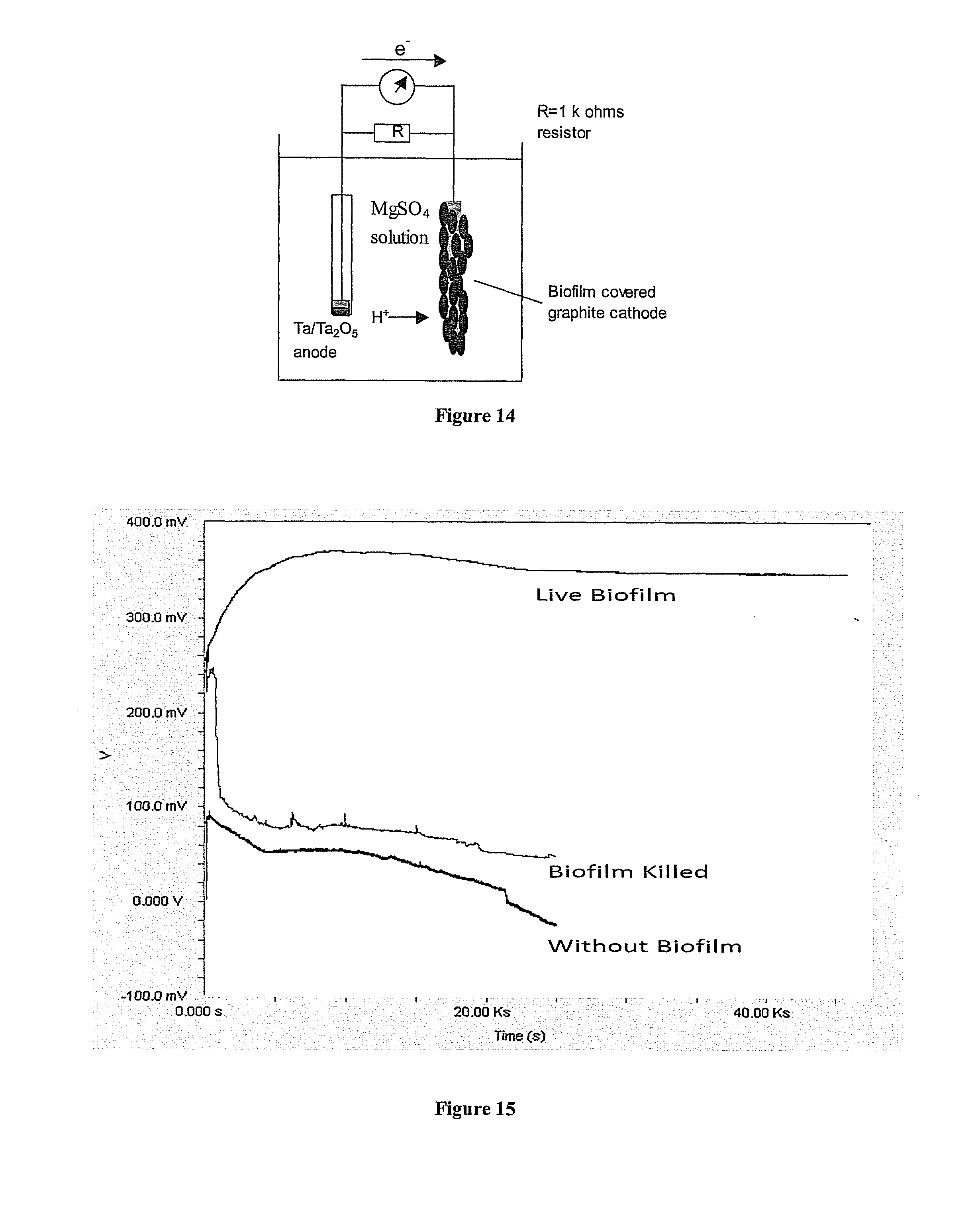
Formation of an Electrogenic Biofilm
Testing for Electrogenicity
Results