ATRAZINE-DEGRADING BACTERIUM AND METHOD OF UISNG THE SAME FOR SOIL AND PLANT REMEDIATION
Pursuant to 35 U.S.C. §119 and the Paris Convention Treaty, this application claims the foreign priority benefit of Chinese Patent Application No. 201410839729.8 filed Dec. 30, 2014, the contents of which, are incorporated herein by reference. 1. Field of the Invention The present invention relates to an atrazine-degrading bacterial strain that has the ability to colonize plant roots and application of the strain for bioremediation of atrazine-polluted soil. 2. Description of the Related Art Atrazine (2-chloro-4-(ethylamino)-6-(isopropylamino)-1,3,5-triazine) is the most widely and heavily used herbicide among the broad class of s-triazine compounds. Atrazine and its metabolites desethyl atrazine, desisopropyl atrazine and 2-hydroxyatrazine are commonly detected contaminants of soils, underground and surface streams and basins, where they persist for years and even decades. Conventionally, the only approach allowing the remediation of contaminated of atrazine soils on large areas was phytoremediation, which includes the growing of plants resistant to the herbicide in the contaminated soil without application of microorganisms. However, this method interferes with harvesting and has a low efficacy of atrazine degradation. In addition, the lack of bacteria capable of rapidly degrading atrazine results in the conversion of atrazine to its dealkylated metabolites, which are persistent in soil and which are toxic to plants. In view of the above-described problems, it is one objective of the invention to provide a novel root-colonizing atrazine-degrading bacterium and applications thereof. The novel atrazine-degrading bacterium can be used for remediation of soil polluted by atrazine or other s-triazine herbicides and for protecting plants from damage caused by application of atrazine or other s-triazine herbicides. The strain possesses a unique combination of properties, such as high atrazine-degrading activity, active motility, an ability to colonize plant roots after seed inoculation, a tolerance to drying and a long-term survival on the inoculated dry seeds, plant growth stimulating activity, an ability to mobilize insoluble phosphates. The bacterium possesses all of the identifying characteristics of The strain The strain was isolated from the rhizosphere of maize sampled from the field with a long history of atrazine application. The strain can be found in the bulk and rhizosphere soils, on plant roots. The strain can be isolated by direct spreading of serial dilutions of soil harboring the bacterium on a selective solid medium SM, containing (per liter of distilled water) 0.5 g K2HPO4, 0.2 g MgSO4×7H2O, 0.1 g NaCl, 0.02 g CaCl2, 2 g D-glucose, 10 mL atrazine stock solution, 5 mL of ZnFe-Cit solution and 13 g of bacteriological agar. The atrazine stock solution contains (per 100 mL of distilled water) 1 mL Tween 80 and 5 g atrazine powder. ZnFe-Cit stock solution contains (per 100 mL of distilled water) 0.04 g ZnSO4×7H2O, 0.4 g FeSO4×7H2O and 10 g trisodium citrate. A biologically pure culture of the bacterial strain can be obtained by repeated streaking on solid medium SMY, which is medium SM, amended with 0.1 g L−1 yeast extract. Pure culture of the bacterial strain liulou 1 can be maintained on the solid media SM, SMY (SM amended with 0.1 g/L yeast extract) or other suitable agar media, such as TY (10 g tryptone, 1 g yeast extract, and 0.02 g of CaCl2per liter of distilled water) or nutrient agar. As used herein, a “biologically pure culture” means a culture which contains primarily only bacteria from the liulou 1 strain and which is substantially free of other contaminating bacteria. The present invention also includes a biological agent for remediation of liquids and soils contaminated with atrazine or related s-triazine compounds and for plant protection from atrazine or related s-triazine herbicides. The biological agent can be in the form of a biologically pure culture of The present invention also includes a method of the strain The strain The method of lane R1, For further illustrating the invention, experiments detailing the atrazine-degrading bacterium and applications thereof are described below. It should be noted that the following examples are intended to describe and not to limit the invention. The strain liulou 1 was directly isolated on SM agar which contained atrazine as a sole nitrogen source and Tween 80 as a dispersant. The surfactant improved bioavailability of hydrophobic atrazine in SM agar and enabled direct selective isolation of atrazine-degrading bacteria from various sources, including industrial and agricultural soils. Eliminating the bias from routine enrichment procedures, direct plating on SM agar facilitates the isolation of soil- and rhizosphere-competent atrazine degraders. The strain liulou 1 was isolated from the rhizosphere of maize sampled from the field with a long history of atrazine application at Liulou Village, Binhe Subdistrict, Dingtao County, Heze Prefecture, Shandong Province. Soil cores 15×10×10 cm (length×width×depth) containing the root system of 1 maize plant were accurately cut out using a surface-sterilized shovel, to avoid disturbing the natural soil structure for the samples. Next day after sampling, the maize roots were carefully retrieved from the cores and the loose soil was removed. The soil associated with root surface was scrapped off and suspended in the buffer (SM medium salt solution). Decimal serial dilutions of the soil suspension were spread on SM agar. Colonies of atrazine degraders were distinguished on SM agar by production of clearing halos due to atrazine degradation after 3 days incubation at 28° C. (as shown in Method The gene for 16S rRNA were amplified with the primer pair 63KWf (5′CAKGCCTWACACATGCAAGTC3′) and 1387r (5′GGGCGGWGTGTACAAGGC3′). The PCRs were performed in a total volume of 50 μL containing 5 μL of TaKaRa 10× Ex Taq Buffer, (Takara Biotechnology (Dalian) Co., Ltd., China), 2.0 mM MgCl2, 250 μM of each dNTP, 0.5 μM of each primer, 0.5 U of TaKaRa Ex Taq polymerase (Takara Biotechnology (Dalian) Co., Ltd., China) and 1.25 μL of a bacterial lysate as a template. The PCRs were started by denaturation at 95° C. for 3 min. followed by 30 cycles: 94° C. for 1 min., 55° C. for 1 min., 72° C. for 2 min.; followed by extension at 72° C. for 5 min. The amplification products were analyzed by electrophoresis on an agarose gel in 0.5×TBE. The target fragment of about 1.3 kb was cut out from the gel, purified using a TaKaRa MiniBEST Agarose Gel DNA Extraction Kit Ver. 4.0 (Takara Biotechnology (Dalian) Co., Ltd., China) and used as templates in sequencing reactions with the primers 63KWf and 1387r. The automated sequencing was performed on a 3730xl DNA Analyzer (Applied Biosystems, United States). The resulting DNA traces and sequences were checked and corrected manually. The BLASTn similarity search was performed against GenBank Reference RNA sequences (refsec_rna) database. The phylogenetic analysis was carried out using MEGA5 software package (Tamura et al., 2011). 16S rRNA gene nucleotide sequences of the species type strains from the GenBank database shared more than 98% similarity with the studied one were included into the datasets. Multiple alignments were implemented using CLUSTALW aligner of MEGA5 and then refined by hand. Phylogenies were inferred using Neighbor-Joining algorithm with elimination of all positions containing gaps and missing data. Results: Partial (1255 bp) nucleotide sequence of 16S rRNA gene of liulou 1 is shown by the following: SEQ ID NO. 1. The BLASTn search results gave evidence that strain liulou 1 belonged to the genus Method Identification of the strain liulou 1 based on Rep-PCR method was conducted by using primers BOXA1R (5′ CTACGGCAAGGCGACGCTGACG 3′) and ERIC2 (5′ AAGTAAGTGACTGGGGTGAGCG 3′). The PCR mixture (20 μL) contained: 2 μL 10× Ex Taq Buffer from Takara Biotechnology (Dalian) Co., Ltd., 2.0 mM MgCl2, 250 μM dNTP, 0.5 μM primers, 0.5 U TaKaRa Ex Taq polymerase, and 0.5 μL of a bacterial lysate as a template. The PCR temperature cycling was as follows: 95° C. predenaturing for 3 min., followed by 4 cycles at 94° C. for 1 min., 40° C. (ERIC-PCR) or 55° C. (BOX-PCR) for 1 min., 68° C. for 8 min.; followed by 30 cycles: 94° C. for 1 min., 52° C. (ERIC-PCR) or 65° C. (BOX-PCR) for 1 min., 72° C. for 2 min. A final extension was performed at 72° C. for 5 min. Products of the amplification were separated by electrophoresis on a 2.0% agarose (Genview, China) gel in 0.5×TBE. The gel was supplemented with 50 μL/L−1 of GoldView Nucleic Acid Stain (Beijing Dingguochangsheng Biotechnology Co., Ltd., China) in order to visualize DNA bands. The Rep-PCR banding patterns were analyzed visually. Type strains Results: As shown in An ERIC-PCR genotyping detected no similarity between patterns of strain liulou 1 and type strain Method Identification of genes atzA, atzB, atzC and trzN in strain Results: As shown in Method: Twenty five mL of liquid medium SM25 (SM with concentration of atrazine reduced to 25 mg L−1) in 250 mL Erlenmeyer flask were inoculated by suspending one 3 days old colony of the strain The liquids were analyzed by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) on an UltiMate 3000 HPLC system in combination with TSQ Vantage Triple Quadrupole Mass Spectrometer (Thermo Fisher Scientific, USA). Atrazine and metabolites were separated on a column Syncronis HILIC (250 mm×4.6 mm, particle size 5 μm). A binary mobile phase gradient (20 mM ammonium formate and CH3CN) was used for the pesticide separation. Mass transitions 216.1→69.0, 216.1→104.0 and 216.1→174.0 were used for monitoring atrazine. Results: HPLC-MS/MS analysis of the stored sterile medium SM25 and incubated sterile medium SM25 revealed atrazine concentration 25 mg L−1 and 26 mg L−1 respectively. Slight increase in the concentration of atrazine in the incubated medium seemed to be caused by water evaporation from the medium during 1 week incubation. No atrazine was detected in the liquid inoculated with liulou 1 (the detection limit was 10 ng L−1), indicating complete degradation of atrazine and a high atrazine-degrading activity of the bacterium Method Motility of Results: As shown in Method The capacity of Germinated plant seeds with radicles <2 mm were inoculated by soaking in a suspension of To determine the inoculation density, bacteria were washed from the seeds. 10 wheat seeds or 20 alfalfa seeds were placed in a 2 mL polypropylene tube with 1.0 mL buffer. Maize seeds were washed in 5 mL polypropylene tubes (5 seeds with 2.0 mL buffer). The tubes with seeds were secured in a MO BIO Vortex Adapter assembled on Vortex-Genie® 2 Vortex (MO BIO Laboratories, Inc., USA) and vortexed at maximal speed for 10 min. The resulting suspension was considered a “0” dilution. Serial decimal dilutions were prepared and spread on agar SM. The colonies with clearing halos were counted after 4 days' incubation at 28° C. To evaluate densities of root-associated populations, the root systems were harvested 23 days post seeding. The tubes were accurately broken and the roots were cut under the soil. The root segments were placed in Petri dishes with 30 mL buffer for 20 minutes to detach adhered sand particles. Then, the root segments were placed on filter paper for 5 sec. and transferred to a 2 mL polypropylene tube with 1.0 mL buffer for wheat and alfalfa roots, or to a 5 mL polypropylene tube with 2.0 mL buffer for maize roots. The tubes with root sections were secured in a MO BIO Vortex Adapter assembled on Vortex-Genie® 2 Vortex (MO BIO Laboratories, Inc., USA) and vortexed at maximal speed for 10 min. The resulting suspensions were considered a “0” dilutions. Serial decimal dilutions were prepared and spread on SM agar. The colonies with clearing halos were counted after 4 days' incubation at 28° C. After washing, the root segments were blotted on filter paper and weight immediately. The densities of To demonstrate the presence and distribution of Results: The test results (as shown in Table 1) demonstrated that, after seed inoculation, the strain After inoculation of alfalfa seeds with the density of 105CFU per seed, the root-associated population of Method Dry seeds of wheat and alfalfa were inoculated by soaking in a suspension of Results: Amounts of bacteria survived on the dry seed are presented in Table 2. It was found that 1 day air-drying reduced seed population of Method The capacity of Results: The test result (as shown by Table 3) demonstrated that drying and storage of the inoculated seeds did not affect the capacity of Method The atrazine-degrading capacity of Atrazine content was determined in 4 replicate tubes per treatment. Atrazine was extracted with 30 mL of 80:20 (v/v) methanol/25 mM ammonium acetate adjusted to pH 8.0 from the whole volume of the soil-sand sample in a tube. The suspension was sonicated for 50 min in an ultrasonic bath and centrifuged at 8000 g for 15 min., and the supernatant was transferred to a 50-mL polypropylene tube. The extraction procedure was repeated, and the supernatants were combined. The supernatant was evaporated to <5 mL at 50° C. Atrazine was selectively extracted by solid phase extraction on a C18 column, preconditioned with 3 mL each of ethyl acetate, methanol and purified water. The column was dried under negative pressure for 90 min, and then atrazine was eluted with 5 mL ethyl acetate. The extract was concentrated to a final volume of 10 μL by gentle nitrogen stream evaporation. Volume of the extract was adjusted to 1 mL with methanol. The sample was analyzed by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) as it has been described in Example 5. Results: Atrazine injury symptoms became apparent on non-inoculated wheat seedlings during the last week of incubation. Severe leaf chlorosis and necrosis resulted in death of all the control seedlings by the end of incubation. No herbicide injury occurred in the plants from seeds inoculated by Injury symptoms from atrazine appeared on small alfalfa plants from 15 to 17 days post seeding and resulted in seedling death during the following week. Alfalfa plants treated with HPLC-MS/MS analysis of the soil-sand extracts gave evidence that Method Filter paper (15×60 cm, 2 layers) and wax paper (10×70 cm) were prepared. Seeds of alfalfa were inoculated with Results: As shown in Table 5, the root length of seedlings from dry inoculated seeds of alfalfa was significantly higher than that of the control seedlings. The root length of seedlings from the inoculated seeds stored for 7, 9, and 11 weeks was increased by 136.1%, 33.1%, and 50% respectively compared to the control groups. This example demonstrates that inoculation of alfalfa seed with Methods Phosphate solubilization test was conducted on a SMP solid medium plate. The SMP medium was SM agar without atrazine stock solution, and amended with 0.5 g/L NH4Cl and 0.1 g/L yeast extract. CaHPO4 was added to SMP agar as 10% powder suspension in 1% Tween 80 to a final concentration of 5 g/L. An agar concentration in SMP solid medium was 17 g/L. To detect phosphate solubilization, atrazine-degrading isolates were streaked on the SMP agar sectors, 9 sectors per plate. The plates were incubated at 28° C. for 1 week. After incubation, the bacteria were removed from the agar surface to observe zones of CaHPO4 solubilization. Results: As shown in Sequence Listing: Partial (1255 bp) nucleotide sequence of 16S rRNA gene of liulou 1 is shown by the following: SEQ ID NO. 1: Method To evaluate degradation of atrazine and its derivatives by The liquids were analyzed by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) on an UltiMate 3000 HPLC system interfaced to a TSQ Vantage Triple Quadrupole Mass Spectrometer (Thermo Fisher Scientific, USA). Atrazine and metabolites were separated on a Thermo Scientific™ Acclaim™ 120 C18 column (250 mm×4.6 mm, particle size 5 μm) with a flow rate of 0.6 mL/min. Methanol (A) and 0.1% aqueous formic acid solution (B) were used for HPLC gradient elution. The program of gradient elution was 0 to 6 min, 5% A; 20 to 25 min, 90% A; 25.1 to 30 min, 5% A. MS/MS analysis was performed in positive electrospray ionization mode (ESI+) and transitions were measured in multiple reaction monitoring (MRM). Parameters of MS/MS and limits of quantification for the analytes are specified in Table 6. Results HPLC-MS/MS analysis of the cultural liquids detected trace amounts of atrazine (<0.01 ng/mL), desethyl atrazine (<0.1 ng/mL) and desisopropyl atrazine (<0.5 ng/mL), indicating >99.99% degradation of the analytes. No significant degradation of the compounds was detected in the non-inoculated control liquid. This example demonstrates nearly complete degradation of atrazine, desethyl atrazine and desisopropyl atrazine in a solution at their initial concentration as low as 5 mg/L that is close to the ecologically relevant concentration. Method Capacity of Contents of atrazine and its metabolites were analyzed in soils from 4 replicate pots. Atrazine and its metabolites were extracted from the soil samples by vortexing method. For extraction, 1 g samples of freeze-dried homogenized soil were weighed into 2 mL tubes and mixed with 1 mL methanol-water solution (4:1 v/v). The tubes were secured horizontally in a MO BIO Vortex Adapter assembled on Vortex-Genie® 2 Vortex (MO BIO Laboratories, Inc., USA), vortexed at maximal speed for 1 h and leaved in the horizontal position for 16 h. After the incubation, tubes were vortexed for 5 min. The suspension was centrifuged at 8000 g for 3 min., and the supernatant was transferred to a 15 mL tube. Then 4 additional extractions were performed consisting of 30 min. vortexing and centrifugation at 8000 g for 3 min. All vortexing steps and the incubation after the first vortexing were performed at 30° C. The supernatants were combined and analyzed by HPLC-MS/MS in the manner described in Example 13. Parameters of MS/MS and limits of quantification for the analytes in soil are specified in Table 6. Recovery of the analytes from fortified soil samples was 90.5-101.2% for atrazine, 73.7-78.5% for 2-hydroxyatrazine, 89.6-104.7% for desethyl atrazine, 80.0-103.6% for desisopropyl atrazine, 91.4-104.1% for desethyl desisopropyl atrazine. Results HPLC-MS/MS analysis detected 5911.4±518.0 μg atrazine kg-1 soil from non-incubated Control 0 (Table 7), indicating that the extraction recovery was 101.3±8.9%. 2-hydroxyatrazine, desethyl atrazine, desisopropyl atrazine and desethyl desisopropyl atrazine were detected in the Control 0 soil as minor contaminants. Atrazine content in soil from the pots with bacterized plants was 125.1±8.4 μg kg-1, indicating that inoculation of wheat seeds with Degradation of atrazine in non-planted (Control 1) and planted (Control W) soils amounted to 42-46% (Table 7). The analysis of atrazine metabolites in the non-treated soils from planted (Control W) and non-planted (Control 1) pots revealed accumulation of desethyl atrazine as a major contaminant and 2-hydroxyatrazine, desisopropyl atrazine, and desethyl desisopropyl atrazine as minor ones (Table 7). Application of This example demonstrates that The bacterium Arthrobacter ureafaciens liulou 1 (CGMCC 9667) possesses a unique combination of high atrazine-degrading activity, a capability of colonizing plant roots after seed inoculation and traits of a plant growth promoting bacterium. Also disclosed is a method of A. ureafaciens liulou 1 (CGMCC 9667) application for remediation of the polluted soils and plant protection from atrazine. 1. An atrazine-degrading bacterium having all identifying characteristics of the strain 2. The bacterium of 3. The bacterium of 4. The bacterium of 5. The bacterium of 6. The bacterium of 7. A method of remediation of liquids and soils contaminated with atrazine or related s-triazine compounds and for plant protection from atrazine or related s-triazine compounds, wherein the related s-triazine compounds are desethyl atrazine, desisopropyl atrazine, simazine, cyanazine, propazine, terbuthylazine, prometryn, ametryn, terbutryn, simetone, the method comprising applying a biological agent comprising an effective amount of a biologically pure culture of the bacterium of 8. The method of a) inoculating plant seeds with the biological agent comprising an effective amount of a biologically pure culture of the bacterium; and b) sowing the inoculated seeds to contaminated soil and growing the plants. 9. The method of 10. The method of 11. The method of CROSS-REFERENCE TO RELATED APPLICATIONS
BACKGROUND OF THE INVENTION
SUMMARY OF THE INVENTION
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION OF THE EMBODIMENTS
Example 1
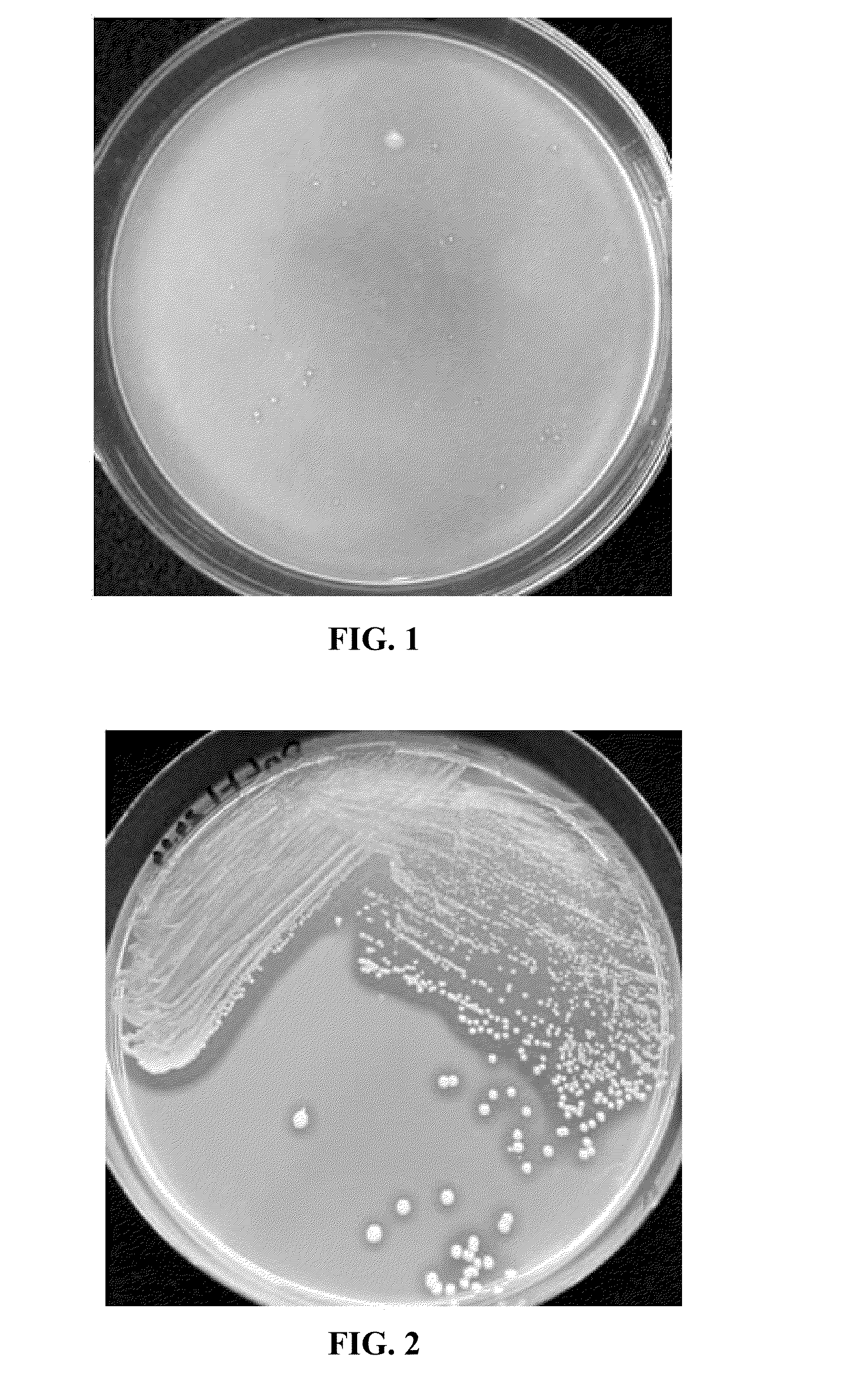
Isolation of Strain Liulou 1
Example 2
Phylogenetic Identification of Strain Liulou 1
Example 3
Taxonomic and Individual Identification of Strain Liulou 1 by Repetitive Elements Sequence-Based PCR (Rep-PCR)
Example 4
Identification of the Genes for Atrazine Degradation in Strain
Example 5
Degradation of Atrazine by
Example 6
Examination of Motility in
Example 7
Colonization of Plant Roots by
Colonization of plant roots by liulou 1 after Seed Inoculation Min-max root Atrazine Total populations concen- amount of Inoculation of tration atrazine density Plant (μg/mL) (μg/tube) (CFU/seed) (CFU/g fresh root) Corn 25.0 175.0 Non-inoculated <102 (7.5 ± 0.3) × 107 4.7 × 105-1.2 × 106 Wheat 25.0 175.0 Non-inoculated <102 (3.2 ± 0.1) × 107 0.7 × 106-1.9 × 106 (4.7 ± 0.2) × 106 0.7 × 106-3.9 × 106 (6.2 ± 0.3) × 103 0.9 × 106-2.4 × 106 Alfalfa 2.5 17.5 Non-inoculated <102 (5.3 ± 0.2) × 105 1.8 × 105-2.3 × 105 (1.2 ± 0.1) × 102 2.3 × 104-6.5 × 104 Example 8
Survival of
Dynamics of liulou 1 Populations on Dry Seed Seed populations of liulou 1 (CFU/seed) Storage time after inoculation Wheat Alfalfa 0 (wet seed after inoculation) (8.0 ± 0.3) × 105 (3.8 ± 0.2) × 105 1 day (6.3 ± 0.4) × 104 (5.3 ± 0.4) × 104 2 days (2.3 ± 0.1) × 105 Not analyzed 1 week (3.9 ± 0.1) × 105 (2.3 ± 0.2) × 105 2 weeks (3.6 ± 0.2) × 105 (1.4 ± 0.1) × 105 5 weeks (2.0 ± 0.1) × 105 (1.0 ± 0.1) × 105 7 weeks (1.1 ± 0.1) × 105 (7.0 ± 0.5) × 104 9 weeks (6.4 ± 0.2) × 104 (3.1 ± 0.2) × 104 11 weeks (3.1 ± 0.1) × 104 (1.4 ± 0.1) × 104 15 weeks (1.0 ± 0.1) × 104 (7.5 ± 0.4) × 103 Example 9
Colonization of Wheat Roots by
Colonization of Wheat Roots by liulou 1 after Seed Inoculation Min-max root populations of Atrazine dose Inoculum density (mg/pot) (CFU/seed) (CFU/g of fresh roots) None Non-inoculated <102 (6.4 ± 0.2) × 104 1.0 × 104-4.9 × 105 1.75 Non-inoculated <102 (6.4 ± 0.2) × 104 8.1 × 105-1.8 × 106 Example 10
Remediation Test
Degradation of Atrazine by liulou 1 in Association with Wheat or Alfalfa Atrazine Atrazine dose Test Inoculation density recovered Atrazine (μg/tube) tube Plant (CFU/seed) (μg/tube) degradation 175.0 Control None Non-inoculated 145.3 ± 11.2 0% W* W1 Non-inoculated 108.0 ± 11.4 25.7% W2 Wheat (4.7 ± 0.2) × 106 0.37 ± 0.14 99.7% W3 (6.2 ± 0.3) × 103 0.25 ± 0.06 99.8% 17.5 Control None Non-inoculated 12.4 ± 1.6 0% A* A1 Non-inoculated 7.9 ± 2.3 36.3% A2 Alfalfa (5.3 ± 0.2) × 105 3.0 ± 0.9 75.8% A3 (1.2 ± 0.1) × 102 3.7 ± 1.1 70.2% *The control tubes W and A were kept at −20° C. from the beginning of the experiments. Example 11
Promotion of Root Growth by
Growth Promotion of Alfalfa Radicles by Inoculation of Dry Seeds with Root length (mm) Percent Seedlings increase in Storage Inoculation from the root length period density inoculated Control due to the (weeks) (CFU/seed) seeds seedlings inoculation 7 (7.0 ± 0.5) × 104 49.1 ± 6.6 20.8 ± 5.5 136.1 9 (3.1 ± 0.2) × 104 43.1 ± 4.2 32.6 ± 5.0 33.1 11 (1.4 ± 0.1) × 104 48.9 ± 5.1 32.6 ± 4.8 50.0 Example 12
Phosphate Solubilization Test
1 TAGTGGCGAA CGGGTGAGTA ACACGTGAGT AACCTGCCCT TGACTCTGGG ATAAGCCTGG 61 GAAACTGGGT CTAATACCGG ATATGACTCC TCATCGCATG GTGGGGGGTG GAAAGCTTTT 121 TGTGGTTTTG GATGGACTCG CGGCCTATCA GCTTGTTGGT GGGGTAATGG CCTACCAAGG 181 CGACGACGGG TAGCCGGCCT GAGAGGGTGA CCGGCCACAC TGGGACTGAG ACACGGCCCA 241 GACTCCTACG GGAGGCAGCA GTGGGGAATA TTGCACAATG GGCGAAAGCC TGATGCAGCG 301 ACGCCGCGTG AGGGATGACG GCCTTCGGGT TGTAAACCTC TTTCAGTAGG GAAGAAGCCC 361 TCTTTGGGGG TGACGGTACT TGCAGAAGAA GCGCCGGCTA ACTACGTGCC AGCAGCCGCG 421 GTAATACGTA GGGCGCAAGC GTTATCCGGA ATTATTGGGC GTAAAGAGCT CGTAGGCGGT 481 TTGTCGCGTC TGCTGTGAAA GACCGGGGCT CAACTCCGGT TCTGCAGTGG GTACGGGCAG 541 ACTAGAGTGC AGTAGGGGAG ACTGGAATTC CTGGTGTAGC GGTGAAATGC GCAGATATCA 601 GGAGGAACAC CGATGGCGAA GGCAGGTCTC TGGGCTGTAA CTGACGCTGA GGAGCGAAAG 661 CATGGGGAGC GAACAGGATT AGATACCCTG GTAGTCCATG CCGTAAACGT TGGGCACTAG 721 GTGTGGGGGA CATTCCACGT TTTCCGCGCC GTAGCTAACG CATTAAGTGC CCCGCCTGGG 781 GAGTACGGCC GCAAGGCTAA AACTCAAAGG AATTGACGGG GGCCCGCACA AGCGGCGGAG 841 CATGCGGATT AATTCGATGC AACGCGAAGA ACCTTACCAA GGCTTGACAT GGACCGGAAA 901 GACCTGGAAA CAGGTGCCCC GCTTGCGGCC GGTTTACAGG TGGTGCATGG TTGTCGTCAG 961 CTCGTGTCGT GAGATGTTGG GTTAAGTCCC GCAACGAGCG CAACCCTCGT TCTATGTTGC 1021 CAGCGGTTCG GCCGGGGACT CATAGGAGAC TGCCGGGGTC AACTCGGAGG AAGGTGGGGA 1081 CGACGTCAAA TCATCATGCC CCTTATGTCT TGGGCTTCAC GCATGCTACA ATGGCCGGTA 1141 CAAAGGGTTG CGATACTGTG AGGTGGAGCT AATCCCAAAA AGCCGGTCTC AGTTCGGATT 1201 GGGGTCTGCA ACTCGACCCC ATGAAGTCGG AGTCGCTAGT AATCGCAGAT CAGCA Example 13
Degradation of Atrazine, Desethyl Atrazine and Desisopropyl Atrazine by
MS/MS parameters and limits of quantification (LOQ) Retention Product ions Analytical LOQ in time Precursor [confirmation Collision S-lens LOQ soil Analyte (min) ion (m/z) ions] (m/z) energies (eV) offset (ng/mL) (ng/g)* Atrazine 24.16 216 174, [104, 68] 18, [29, 34] 100 0.01 0.045 Atrazine-2- hydroxyl 18.02 198 156, [86, 69] 17, [23, 33] 92 0.01 0.045 Atrazine- desethyl 21.24 188 146, [104, 68] 17, [25, 30] 81 0.1 0.45 Atrazine- desisopropyl 19.46 174 132, [104, 68] 16, [22, 29] 96 0.5 2.25 Atrazine- desethyl- desisopropyl 14.27 146 110, [104, 68] 16, [18, 27] 59 0.2 0.90 *Indicated LOQ in soil could be improved by concentration of the extracts. Example 14
Remediation of Atrazine-Contaminated Soil by Using Seed Inoculation with
Soil remediation with Analyte contents in control and bacterized soils, ng/g dry soil Control 0 Control 1 (Atrazine dose (Atrazine dose (Atrazine dose 1.75 1.75 mg/pot 1.75 mg/pot Control W mg/pot (5833 ng/g (5833 ng/g dry (5833 ng/g dry (Atrazine dose 1.75 dry soil), wheat, soil), no plants, soil), no plants, mg/pot (5833 ng/g seed inoculation no inoculation, no inoculation, dry soil), wheat, no with Analyte no incubation) incubated) inoculation, incubated) liulou 1, incubated) Atrazine 5911.4 ± 518.0 3192.7 ± 387.5 3404.7 ± 272.7 125.1 ± 8.4 Atrazine-2- 67.7 ± 2.8 198.3 ± 15.0 226.1 ± 44.4 151.7 ± 8.6 hydroxy Atrazine- 63.2 ± 1.5 1029.2 ± 114.6 975.4 ± 60.5 17.2 ± 2.3 desethyl Atrazine- 20.6 ± 0.9 219.0 ± 18.5 234.3 ± 11.6 17.6 ± 1.6 desisopropyl Atrazine- 0.90 ± 0.03 186.8 ± 15.6 178.1 ± 6.9 41.3 ± 1.8 desethyl- desisopropyl