COMPOSITIONS AND METHODS FOR TREATING ALLERGIC INFLAMMATORY CONDITIONS
This application claims priority to U.S. Provisional Patent Application Ser. No. 62/278,246, filed Jan. 13, 2016, which is hereby incorporated by reference in its entirety. The invention disclosed herein generally relates to compositions and methods for treating an allergic inflammatory condition. Epithelial barrier impairment has been implicated in the development of allergic disease. However, the molecular mechanisms by which impaired epithelial barrier function induces Th2-type immune responses remain largely unknown. Epithelial cells are uniquely positioned as the first line of defense against type-2 (Th2)-cell-mediated immune insults (Hammad, H. & Lambrecht, B. N. The importance of loss of barrier integrity in eliciting Th2 responses is illustrated by predisposition to atopy in individuals harboring loss of function mutations in the proteinase inhibitor SPINK5. Homozygous loss of SPINK5 results in uncontrolled proteolytic activity in the skin which leads to barrier defect and atopy. An imbalance between SPINK5 and proteinases has been proposed to contribute to the pathogenesis of atopic dermatitis (AD) (Furio, L. et al. Eosinophilic esophagitis (EoE) is an inflammatory Th2 type immune disease of the esophagus. EoE is considered to be a chronic immune system disease. Although it was identified only during the last twenty years, it is now considered a major cause of digestive system (gastrointestinal) illness. In EoE, eosinophils (a type of white blood cell) build up in the lining of the esophagus. This buildup, which may be a reaction to foods, allergens or acid reflux, can inflame and/or injure the esophageal tissue. Damaged esophageal tissue can lead to difficulty swallowing or lead to other complications. Symptoms include difficulty swallowing (dysphagia), food impaction, chest pain that is often centrally located and does not respond to antacids, persistent heartburn, upper abdominal pain, lack of response to gastroesophageal reflux disease (GERD) medication, and backflow of undigested food (regurgitation). Current clinical standards for diagnosis of EoE include (i) endoscopy to inspect the lining of the esophagus for inflammation and swelling, horizontal rings, vertical furrows, narrowing (strictures) and white spots; (ii) biopsy of esophageal tissue with one biopsy showing more than 15 eosinophils per high power field in patients using a proton pump inhibitor (PPI) for approximately 8 weeks. Treatment for EoE that is not responsive to PPIs includes an orally administered topical steroid, such as fluticasone or budesonide. Where topical steroids prove ineffective, prednisone may be prescribed. There is a need for new treatment options for EoE and similar allergic inflammatory disorders characterized by inflammation of squamous epithelium. The present disclosure addresses this need. The present disclosure generally provides methods of treating an allergic inflammatory condition in a subject in need thereof, the allergic inflammatory condition characterized by inflammation of a squamous epithelium in a target tissue of the subject, by replenishing SPINK7 protein and/or SPINK7 anti-proteinase activity in the target tissue. In embodiments, the target tissue is esophageal tissue and the methods are for treating EoE. In embodiments, the disclosure provides methods of treating an allergic inflammatory condition in a subject in need thereof, the allergic inflammatory condition characterized by inflammation of a squamous epithelium in a target tissue of the subject, the method comprising administering to the subject a pharmaceutical composition comprising an amount of a therapeutic agent effective to replenish SPINK7 protein and/or SPINK7 anti-proteinase activity in the target tissue. In embodiments, the squamous epithelium is of esophageal tissue. In embodiments, the allergic inflammatory condition is esophageal eosinophilia (EE) or eosinophilic esophagitis (EoE). In embodiments, the therapeutic agent is a serine proteinase inhibitor. In embodiments, the therapeutic agent is an alpha-1 proteinase inhibitor. In embodiments, the alpha-1 proteinase inhibitor is an alpha-1 antitrypsin (A1AT) inhibitor. In embodiments, the therapeutic agent is an inhibitor of urokinase plasminogen activator (uPA) or kallikrein 5 (KLK5) in the target tissue. In embodiments, the therapeutic agent is a proteinase inhibitor, a KLK5-Fc fusion protein, a KLK5 anti-sense polynucleotide, a KLK5-directed miRNA, a KLK5-directed shRNA, or a KLK5-directed antibody. In embodiments, the therapeutic agent is selected from 3-(3-chlorophenyl)carboxy-7-hydroxymethyl courmarin or 3-carboxy-7-hydroxymethyl coumarin. In embodiments, the therapeutic agent comprises a recombinant mRNA encoding a SPINK7 protein, or a recombinant SPINK7 polypeptide. In embodiments, the therapeutic agent comprises a recombinant mRNA encoding a member of the SPINK protein family, or a recombinant mRNA encoding a SPINK family member polypeptide. In embodiments, the methods further comprise subjecting the patient to a dietary modification to eliminate one or more potential food allergens. In embodiments, the subject is human. The methods described here are based, in part, on the identification of the serine proteinase inhibitor kazal-type 7 (SPINK7) as a key anti-inflammatory regulator which is lost in allergic inflammation of the squamous epithelium, for example as occurs in the esophageal tissue of patients suffering from EoE. EoE is historically defined as esophageal eosinophilia (EE) that does not respond to proton pump inhibitor (PPI) therapy. It is now apparent, however, that EoE overlaps with PPI-Responsive EE, such that both disease entities are now considered the same basic process (see, e.g., Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis, Molina-Infante J et al., PPI-REE Task Force of the European Society of Eosinophilic Oesophagitis (EUREOS). Gut. 2016 March; 65(3):524-31). EoE can also be considered proteotypic for conditions characterized by inflammation of the squamous epithelium, particularly allergic inflammation. Also discovered by the present inventors is that the key molecular targets of SPINK7 include the proteinases urokinase type plasminogen activator (uPA) and kallikrein 5 (KLK5). The term “proteinase” is synonymous with the term “protease” and both terms refer to a proteolytic enzyme that acts on proteins and polypeptides by hydrolysis of peptide bonds. The results described infra demonstrate that the loss of SPINK7 hampers esophageal barrier formation and promotes pro-inflammatory changes in epithelial cells. These pro-inflammatory changes are mediated by the uncontrolled proteolytic activity of uPA and KLK5, which are normally repressed by SPINK7. The resulting changes in the epithelial cells cause aberrant epithelial cell differentiation and impaired barrier function. These changes enable immune cells to encounter luminal antigens and promote a Th2 response. This provides the rationale for pharmacological targeting of uPA and KLK5 for the treatment of EoE, since inhibiting the proteolytic activity of these proteins would at least partially restore a key function of SPINK7 that is lost in the disease state, the downregulation of these proteinases. The data provided here further demonstrate that protein replacement with an alpha-1 anti-trypsin (A1AT) proteinase inhibitor restored some responses associated with loss of SPINK7 in vitro. This data supports the therapeutic potential of the methods described herein. Thus, the methods described herein aim to reestablish SPINK7 checkpoint control in the squamous epithelium of a target tissue where that control has been lost or diminished, e.g., in tissues characterized by inflammation of the squamous epithelium, especially allergic inflammation. SPINK7 checkpoint control may be reestablished according to the present methods, for example, by increasing SPINK7 anti-proteinase activity directly, e.g., by replenishing SPINK7 protein in the target tissue, or indirectly, e.g., by introducing one or more serine proteinase inhibitors to the target tissue. In embodiments, the methods described here may comprise introducing SPINK7 protein to a target tissue, for example by administering a recombinant polynucleotide encoding a SPINK7 protein, or by introducing a recombinant SPINK7 polypeptide. The methods may also comprise increasing the expression of endogenous SPINK7 in the target tissue. In embodiments, the methods described here may also comprise administering one or more therapeutic agents that are proteinase inhibitors. In embodiments, the proteinase inhibitor is a serine proteinase inhibitor. In embodiments, the serine proteinase inhibitor is an inhibitor of an alpha-1 proteinase, a trypsin-like serine proteinase, a urokinase-type serine proteinase, or an inhibitor of uPA or KLK5, or any combination of the foregoing. In accordance with any of the foregoing embodiments, the therapeutic agent may be a small organic molecule, a polypeptide, or a nucleic acid. In embodiments, the small organic molecule is selected from the group consisting of the KLK5 inhibitors 3-(3-chlorophenyl)carboxy-7-hydroxymethyl courmarin and 3-carboxy-7-hydroxymethyl coumarin. In embodiments, the polypeptide is selected from the group consisting of an Fc fusion protein and an inhibitory antibody, e.g., targeted against uPA or KLK5, or both. In embodiments, the nucleic acid is selected from the group consisting of an anti-sense polynucleotide and an inhibitory RNA such as an miRNA or shRNA, e.g., targeted against uPA or KLK5, or both. In embodiments, the therapeutic agent is an anti-KLK5-based therapeutic agent. In embodiments, the anti-KLK5 agent is a KLK5-Fc fusion protein, an KLK5 anti-sense polynucleotide, an KLK5-directed miRNA, an KLK5-directed shRNA, or a KLK5-directed antibody. In embodiments, the anti-KLK5-based therapeutic agent is a serine proteinase inhibitor. In embodiments, the anti-KLK5 agent is selected from 3-(3-chlorophenyl)carboxy-7-hydroxymethyl courmarin and 3-carboxy-7-hydroxymethyl coumarin. In embodiments, the therapeutic agent may also include at least one of a compound or composition that suppresses uPA or KLK5 proteinase activity. In some embodiments, the compound or composition that suppresses uPA or KLK5 proteinase activity includes a proteinase inhibitor, an NTRK1-Fc fusion protein (neurotrophic receptor kinase 1), an NTRK1 anti-sense polynucleotide, an NTRK1-directed miRNA, an NTRK1-directed shRNA, or an NTRK1-directed antibody, including a humanized antibody. In embodiments, uPA or KLK5 activity is suppressed by inhibiting uPA or KLK5 gene expression, inhibiting uPA or KLK5 protein expression, or inhibiting uPA or KLK5 proteinase activity, or any combination thereof. For example uPA or KLK5 proteinase activity may be inhibited directly, by an agent that inhibits the proteinase function of the protein, or indirectly, for example, by inhibiting gene or protein expression, thereby reducing the amount of uPA or KLK5 protein in the target tissue and thereby indirectly inhibiting uPA or KLK5 proteinase activity in the target tissue. In embodiments, the therapeutic agent is a proteinase inhibitor. In embodiments, the therapeutic agent is a serine proteinase inhibitor. In embodiments, the therapeutic agent is an alpha-1 proteinase inhibitor. In embodiments, the alpha-1 proteinase inhibitor is a recombinant protein. In embodiments, the alpha-1 proteinase inhibitor is selected from PROLASTIN-C™, ZEMAIRA™, and ARALAST™. In embodiments, the one or more therapeutic agents may be administered in the form of a pharmaceutical composition. The pharmaceutical composition may in any suitable form, as described in more detail infra. In embodiments, the pharmaceutical composition is administered to the subject by any suitable route of administration. For example, the composition may be administered intravenously, intradermally, subcutaneously, or perorally. In embodiments, the pharmaceutical composition is administered to the subject by inhalation. The present disclosure provides methods for the treatment of an allergic inflammatory condition in a subject in need thereof, the allergic inflammatory condition characterized by inflammation of a squamous epithelium in a target tissue of the subject. In embodiments, the allergic inflammatory condition is EE or EoE. As discussed above the methods generally comprise increasing SPINK7 anti-proteinase activity either directly, e.g., by replenishing SPINK7 protein in the target tissue, or indirectly, e.g., by introducing one or more serine proteinase inhibitors to the target tissue. In embodiments, an effective amount of a therapeutic agent is administered to the subject in need of treatment. In embodiments, the effective amount is a therapeutically effective amount. In embodiments, the effective amount is the amount effective to ameliorate one or more symptoms of an allergic inflammatory condition of the squamous epithelium. In embodiments, the effective amount is the amount effective to ameliorate one or more symptoms of EE or EoE. In embodiments, the effective amount is the amount effective to suppress KLK5 proteinase activity in a target tissue. In embodiments, the target tissue is esophageal tissue. Also envisioned are methods comprising combination therapy for the treatment of an allergic inflammatory condition in a subject in need of such treatment. As used herein, “combination therapy” or “co-therapy” includes the administration of an effective amount of a primary therapeutic agent as described herein as part of a specific treatment regimen intended to provide the beneficial effect from the co-action of the primary therapeutic agent and an additional active agent, e.g., an additional active pharmaceutical ingredient (API). The beneficial effect of the combination includes, but is not limited to, pharmacokinetic or pharmacodynamic co-action resulting from the combination of therapeutic compounds. The beneficial effect of the combination may also relate to the mitigation of a toxicity, side effect, or adverse event associated with another agent in the combination. “Combination therapy” is not intended to encompass the administration of two or more of these therapeutic compounds as part of separate monotherapy regimens that incidentally and arbitrarily result in a beneficial effect that was not intended or predicted. The at least one additional active agent may be a therapeutic agent, for example an anti-inflammatory agent, or a non-therapeutic agent, and combinations thereof. With respect to therapeutic agents, the beneficial effect of the combination includes, but is not limited to, pharmacokinetic or pharmacodynamic co-action resulting from the combination of therapeutically active compounds. With respect to nontherapeutic agents, the beneficial effect of the combination may relate to the mitigation of a toxicity, side effect, or adverse event associated with a therapeutically active agent in the combination. Thus, in embodiments, the methods described here may further comprise administering to the subject at least one additional active agent. In embodiments, the at least one additional active agent is an anti-inflammatory agent. In embodiments, the at least one additional active agent is an IL-13 inhibitor, a non-steroidal anti-inflammatory drug (NSAID), a cytokine inhibitor, or a steroid. In embodiments, the at least one additional active agent is a proton pump inhibitor. In the context of combination therapy, the administration of the primary therapeutic agent, may be simultaneous with or sequential to the administration of the one or more additional active agents. In another embodiment, administration of the different components of a combination therapy may be at different frequencies. The one or more additional agents may be administered prior to (e.g., 5 minutes, 15 minutes, 30 minutes, 45 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 12 hours, 24 hours, 48 hours, 72 hours, 96 hours, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, 6 weeks, 8 weeks, or 12 weeks before), concomitantly with, or subsequent to (e.g., 5 minutes, 15 minutes, 30 minutes, 45 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 12 hours, 24 hours, 48 hours, 72 hours, 96 hours, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, 6 weeks, 8 weeks, or 12 weeks after) the administration of a primary therapeutic agent as described herein. The one or more additional active agents can be formulated for co-administration with the primary therapeutic agent in a single dosage form. The one or more additional active agents can be administered separately from the dosage form that comprises the primary therapeutic agent. When the additional active agent is administered separately from the primary therapeutic agent, it can be by the same or a different route of administration as the primary therapeutic agent. Preferably, the administration of a composition comprising the primary therapeutic agent in combination with one or more additional active agents provides a synergistic response in the subject being treated. In this context, the term “synergistic” refers to the efficacy of the combination being more effective than the additive effects of either single therapy alone. The synergistic effect of a combination therapy according to the disclosure can permit the use of lower dosages and/or less frequent administration of at least one agent in the combination compared to its dose and/or frequency outside of the combination. Additional beneficial effects of the combination can be manifested in the avoidance or reduction of adverse or unwanted side effects associated with the use of either therapy in the combination alone (also referred to as monotherapy). “Combination therapy” also embraces the administration of the compounds of the present disclosure in further combination with non-drug therapies (e.g., diet modification). Where the combination therapy further comprises a non-drug treatment, the non-drug treatment may be conducted at any suitable time so long as a beneficial effect from the co-action of the combination of the therapeutic compounds and non-drug treatment is achieved. For example, in appropriate cases, the beneficial effect is still achieved when the non-drug treatment is temporally removed from the administration of the therapeutic compounds, perhaps by days or even weeks. In embodiments, the amount of the therapeutic agent administered to the subject is a therapeutically effective amount. The term “therapeutically effective amount” refers to an amount sufficient to treat, ameliorate a symptom of, reduce the severity of, or reduce the duration of the disease or disorder being treated or enhance or improve the therapeutic effect of another therapy, or sufficient to exhibit a detectable therapeutic effect in the subject. An effective amount of the therapeutic agent can be administered once or twice daily, from two to five times daily, up to two times or up to three times daily, or up to eight times daily. In accordance with the methods described herein, a “subject in need thereof” is a subject having an allergic inflammatory condition characterized by inflammation of the squamous epithelium in a target tissue. In specific embodiments, the subject is a subject having EE or EoE. A “subject” includes a mammal. The mammal can be any mammal, for example, a human, primate, vertebrate, bird, mouse, rat, fowl, dog, cat, cow, horse, goat, camel, sheep or a pig. Preferably, the subject is a human. The term “patient” refers to a human subject. The present disclosure also provides a monotherapy for the treatment of EoE. As used herein, “monotherapy” refers to the administration of a single active or therapeutic compound, e.g., a proteinase inhibitor or an anti-KLK5-based therapeutic agent, to a subject in need thereof. As used herein, “treatment”, “treating” or “treat” describes the management and care of a patient for the purpose of combating a disease, condition, or disorder and includes the administration of a proteinase inhibitor or an anti-KLK5-based therapeutic agent to alleviate the symptoms or complications of the allergic inflammatory disease, disorder, or condition. As used herein, “prevention”, “preventing” or “prevent” describes reducing or eliminating the onset of the symptoms or complications of the allergic inflammatory disease, disorder, or condition and includes the administration of a proteinase inhibitor or an anti-KLK5-based therapeutic agent to reduce the onset, development or recurrence of symptoms of the disease, disorder, or condition. In one embodiment, the administration of a proteinase inhibitor or an anti-KLK5-based therapeutic agent leads to the elimination of a symptom or complication of the disease or condition being treated, however elimination of the disease, disorder, or condition is not required. In one embodiment, the severity of the symptom is decreased. The present disclosure provides pharmaceutical compositions comprising an amount of a therapeutic agent as described supra. For example, the therapeutic agent may comprise a recombinant polynucleotide encoding a SPINK7 protein, a recombinant SPINK7 polypeptide, or another agent effective to increase the expression and/or amount of endogenous SPINK7 mRNA and/or protein in the target tissue. The therapeutic agent may also be proteinase inhibitor and/or a specific inhibitor of uPA and/or KLK5. In embodiments, the proteinase inhibitor, anti-uPA or anti-KLK5 based therapeutic agent is combined with at least one additional active agent in a single dosage form. In embodiments, the at least one additional active agent is selected from an anti-inflammatory agent selected from an IL-13 inhibitor, a non-steroidal anti-inflammatory drug (NSAID), a steroid, and a cytokine inhibitor, a PPI inhibitor, and combinations thereof. A “pharmaceutical composition” is a formulation containing the therapeutic agent in a pharmaceutically acceptable form suitable for administration to a subject. As used herein, the phrase “pharmaceutically acceptable” refers to those compounds, materials, compositions, carriers, and/or dosage forms which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of human beings and animals without excessive toxicity, irritation, allergic response, or other problem or complication, commensurate with a reasonable benefit/risk ratio. “Pharmaceutically acceptable excipient” means an excipient that is useful in preparing a pharmaceutical composition that is generally safe, non-toxic and neither biologically nor otherwise undesirable, and includes excipient that is acceptable for veterinary use as well as human pharmaceutical use. Examples of pharmaceutically acceptable excipients include, without limitation, sterile liquids, water, buffered saline, ethanol, polyol (for example, glycerol, propylene glycol, liquid polyethylene glycol and the like), oils, detergents, suspending agents, carbohydrates (e.g., glucose, lactose, sucrose or dextran), antioxidants (e.g., ascorbic acid or glutathione), chelating agents, low molecular weight proteins, or suitable mixtures thereof. A pharmaceutical composition can be provided in bulk or in dosage unit form. It is especially advantageous to formulate pharmaceutical compositions in dosage unit form for ease of administration and uniformity of dosage. The term “dosage unit form” as used herein refers to physically discrete units suited as unitary dosages for the subject to be treated; each unit containing a predetermined quantity of active compound calculated to produce the desired therapeutic effect in association with the required pharmaceutical carrier. The specification for the dosage unit forms of the disclosure are dictated by and directly dependent on the unique characteristics of the active compound and the particular therapeutic effect to be achieved. A dosage unit form can be an ampoule, a vial, a suppository, a dragee, a tablet, a capsule, an IV bag, or a single pump on an aerosol inhaler. In therapeutic applications, the dosages vary depending on the agent, the age, weight, and clinical condition of the recipient subject or patient, and the experience and judgment of the clinician or practitioner administering the therapy, among other factors affecting the selected dosage. Generally, the dose should be a therapeutically effective amount. Dosages can be provided in mg/kg/day units of measurement (which dose may be adjusted for the patient's weight in kg, body surface area in m2, and age in years). An effective amount of a pharmaceutical composition is that which provides an objectively identifiable improvement as noted by the clinician or other qualified observer. For example, alleviating a symptom of a disorder, disease or condition. As used herein, the term “dosage effective manner” refers to amount of a pharmaceutical composition to produce the desired biological effect in a subject or cell. For example, the dosage unit form can comprise 1 nanogram to 2 milligrams, or 0.1 milligrams to 2 grams; or from 10 milligrams to 1 gram, or from 50 milligrams to 500 milligrams or from 1 microgram to 20 milligrams; or from 1 microgram to 10 milligrams; or from 0.1 milligrams to 2 milligrams. The pharmaceutical compositions can take any suitable form (e.g, liquids, aerosols, solutions, inhalants, mists, sprays; or solids, powders, ointments, pastes, creams, lotions, gels, patches and the like) for administration by any desired route (e.g, pulmonary, inhalation, intranasal, oral, buccal, sublingual, parenteral, subcutaneous, intravenous, intramuscular, intraperitoneal, intrapleural, intrathecal, transdermal, transmucosal, rectal, and the like). For example, a pharmaceutical composition of the disclosure may be in the form of an aqueous solution or powder for aerosol administration by inhalation or insufflation (either through the mouth or the nose), in the form of a tablet or capsule for oral administration; in the form of a sterile aqueous solution or dispersion suitable for administration by either direct injection or by addition to sterile infusion fluids for intravenous infusion; or in the form of a lotion, cream, foam, patch, suspension, solution, or suppository for transdermal or transmucosal administration. A pharmaceutical composition may be in a form suitable for administration by inhalation, for example as an aqueous or non-aqueous aerosol, or as a dry powder. In embodiments, the pharmaceutical composition is an aqueous solution adapted for delivery via a nebulizer, including jet, vibrating mesh, and static mesh or orifice nebulizers. In embodiments, the pharmaceutical composition is a dry powder adapted for delivery via a dry powder inhaler device. A pharmaceutical composition can be in the form of an orally acceptable dosage form including, but not limited to, capsules, tablets, buccal forms, troches, lozenges, and oral liquids in the form of emulsions, aqueous suspensions, dispersions or solutions. Capsules may contain mixtures of a compound of the present disclosure with inert fillers and/or diluents such as the pharmaceutically acceptable starches (e.g., corn, potato or tapioca starch), sugars, artificial sweetening agents, powdered celluloses, such as crystalline and microcrystalline celluloses, flours, gelatins, gums, etc. In the case of tablets for oral use, carriers which are commonly used include lactose and corn starch. Lubricating agents, such as magnesium stearate, can also be added. For oral administration in a capsule form, useful diluents include lactose and dried corn starch. When aqueous suspensions and/or emulsions are administered orally, the compound of the present disclosure may be suspended or dissolved in an oily phase is combined with emulsifying and/or suspending agents. If desired, certain sweetening and/or flavoring and/or coloring agents may be added. A pharmaceutical composition can be in the form of a tablet. The tablet can comprise a unit dosage of a compound of the present disclosure together with an inert diluent or carrier such as a sugar or sugar alcohol, for example lactose, sucrose, sorbitol or mannitol. The tablet can further comprise a non-sugar derived diluent such as sodium carbonate, calcium phosphate, calcium carbonate, or a cellulose or derivative thereof such as methyl cellulose, ethyl cellulose, hydroxypropyl methyl cellulose, and starches such as corn starch. The tablet can further comprise binding and granulating agents such as polyvinylpyrrolidone, disintegrants (e.g. swellable crosslinked polymers such as crosslinked carboxymethylcellulose), lubricating agents (e.g. stearates), preservatives (e.g. parabens), antioxidants (e.g. BHT), buffering agents (for example phosphate or citrate buffers), and effervescent agents such as citrate/bicarbonate mixtures. The tablet can be a coated tablet. The coating can be a protective film coating (e.g. a wax or varnish) or a coating designed to control the release of the active agent, for example a delayed release (release of the active after a predetermined lag time following ingestion) or release at a particular location in the gastrointestinal tract. The latter can be achieved, for example, using enteric film coatings such as those sold under the brand name Eudragit®. Tablet formulations may be made by conventional compression, wet granulation or dry granulation methods and utilize pharmaceutically acceptable diluents, binding agents, lubricants, disintegrants, surface modifying agents (including surfactants), suspending or stabilizing agents, including, but not limited to, magnesium stearate, stearic acid, talc, sodium lauryl sulfate, microcrystalline cellulose, carboxymethylcellulose calcium, polyvinylpyrrolidone, gelatin, alginic acid, acacia gum, xanthan gum, sodium citrate, complex silicates, calcium carbonate, glycine, dextrin, sucrose, sorbitol, dicalcium phosphate, calcium sulfate, lactose, kaolin, mannitol, sodium chloride, talc, dry starches and powdered sugar. Preferred surface modifying agents include nonionic and anionic surface modifying agents. Representative examples of surface modifying agents include, but are not limited to, poloxamer 188, benzalkonium chloride, calcium stearate, cetostearyl alcohol, cetomacrogol emulsifying wax, sorbitan esters, colloidal silicon dioxide, phosphates, sodium dodecylsulfate, magnesium aluminum silicate, and triethanolamine. A pharmaceutical composition can be in the form of a hard or soft gelatin capsule. In accordance with this formulation, the compound of the present disclosure may be in a solid, semi-solid, or liquid form. A pharmaceutical composition can be in the form of a sterile aqueous solution or dispersion suitable for parenteral administration. The term parenteral as used herein includes subcutaneous, intracutaneous, intravenous, intramuscular, intra-articular, intraarterial, intrasynovial, intrasternal, intrathecal, intralesional and intracranial injection or infusion techniques. A pharmaceutical composition can be in the form of a sterile aqueous solution or dispersion suitable for administration by either direct injection or by addition to sterile infusion fluids for intravenous infusion, and comprises a solvent or dispersion medium containing, water, ethanol, a polyol (e.g., glycerol, propylene glycol and liquid polyethylene glycol), suitable mixtures thereof, or one or more vegetable oils. Solutions or suspensions of the compound of the present disclosure as a free base or pharmacologically acceptable salt can be prepared in water suitably mixed with a surfactant. Examples of suitable surfactants are given below. Dispersions can also be prepared, for example, in glycerol, liquid polyethylene glycols and mixtures of the same in oils. The pharmaceutical compositions for use in the methods of the present disclosure can further comprise one or more additives in addition to any carrier or diluent (such as lactose or mannitol) that is present in the formulation. The one or more additives can comprise or consist of one or more surfactants. Surfactants typically have one or more long aliphatic chains such as fatty acids which enables them to insert directly into the lipid structures of cells to enhance drug penetration and absorption. An empirical parameter commonly used to characterize the relative hydrophilicity and hydrophobicity of surfactants is the hydrophilic-lipophilic balance (“HLB” value). Surfactants with lower HLB values are more hydrophobic, and have greater solubility in oils, while surfactants with higher HLB values are more hydrophilic, and have greater solubility in aqueous solutions. Thus, hydrophilic surfactants are generally considered to be those compounds having an HLB value greater than about 10, and hydrophobic surfactants are generally those having an HLB value less than about 10. However, these HLB values are merely a guide since for many surfactants, the HLB values can differ by as much as about 8 HLB units, depending upon the empirical method chosen to determine the HLB value. Among the surfactants for use in the compositions of the disclosure are polyethylene glycol (PEG)-fatty acids and PEG-fatty acid mono and diesters, PEG glycerol esters, alcohol-oil transesterification products, polyglyceryl fatty acids, propylene glycol fatty acid esters, sterol and sterol derivatives, polyethylene glycol sorbitan fatty acid esters, polyethylene glycol alkyl ethers, sugar and its derivatives, polyethylene glycol alkyl phenols, polyoxyethylene-polyoxypropylene (POE-POP) block copolymers, sorbitan fatty acid esters, ionic surfactants, fat-soluble vitamins and their salts, water-soluble vitamins and their amphiphilic derivatives, amino acids and their salts, and organic acids and their esters and anhydrides. The present disclosure also provides packaging and kits comprising pharmaceutical compositions for use in the methods of the present disclosure. The kit can comprise one or more containers selected from the group consisting of a bottle, a vial, an ampoule, a blister pack, and a syringe. The kit can further include one or more of instructions for use in treating and/or preventing a disease, condition or disorder of the present disclosure, one or more syringes, one or more applicators, or a sterile solution suitable for reconstituting a pharmaceutical composition of the present disclosure. All percentages and ratios used herein, unless otherwise indicated, are by weight. Other features and advantages of the present disclosure are apparent from the different examples. The provided examples illustrate different components and methodology useful in practicing the present disclosure. The examples do not limit the claimed disclosure. Based on the present disclosure the skilled artisan can identify and employ other components and methodology useful for practicing the present disclosure. The following non-limiting examples are provided to further illustrate embodiments of the invention disclosed herein. It will be appreciated by those of skill in the art that the techniques disclosed in the examples that follow represent approaches that have been found to function well in the practice of the invention and thus can be considered to constitute examples of modes for its practice. However, those of skill in the art will, in light of the present disclosure, appreciate that many changes can be made in the specific embodiments that are disclosed and still obtain a like or similar result without departing from the spirit and scope of the invention. As described more fully below, we show that the serine peptidase inhibitor kazal-type 7 (SPINK7) is an important anti-inflammatory check in the esophageal epithelium and is markedly down-regulated in eosinophilic esophagitis (EoE), an inflammatory TH2 type immune disease of the esophagus, while being expressed at relatively high levels in normal esophageal epithelium. We further show that loss of SPINK7 results in an epithelial cell differentiation defect, reduced barrier protein expression, impaired barrier function, and pro-inflammatory cytokine production. Protein replacement with an alpha-1 anti-trypsin proteinase inhibitor restored some responses associated with loss of SPINK7 in vitro. Our data show that the endogenous balance between SPINK7 and its target proteinases (uPA and KLK5) is a key checkpoint in regulating mucosal differentiation, barrier function and Th2-associated responses. Moreover, our data suggest that protein replacement with proteinase inhibitors holds therapeutic promise. To investigate the role of SPINK7 in EoE, an in vitro system of human esophageal epithelial cells that were subjected to air-liquid interface (ALI) to induce squamous cell differentiation was used. Cells were stably transduced with either non-silencing control or SPINK7 shRNAs. The integrity of the epithelium was examined by barrier function assays complemented by histological and ultrastractural analyses and immune-fluorescence of junctional proteins. Proteinase activity, transcriptional alterations and identification SPINK7's downstream targets were also assessed. Cytokine and chemokine secretion was analyzed after SPINK7 gene silencing. The results demonstrated that SPINK7 was a key anti-inflammatory checkpoint in the esophageal epithelium. The results showed that depletion of SPINK7 in esophageal epithelial cells induced architectural changes in esophageal epithelial cells reminiscent of those observed in patients with EoE including acantholysis and epithelial cleft formation, as well as impaired barrier function. The loss of SPINK7 also increased trypsin-like (>2 fold increase; P=0.004) and urokinase-type plasminogen activator (uPA) activity (2-fold, P=006). In vitro, SPINK7 inhibited the serine proteinase—kallikrein (KLK)5, but not KLK7 nor KLK11. KLK5 is known to be involved in the regulation of the skin barrier. Furthermore, loss of SPINK7 was sufficient for induction of architectural alterations in junctional complexes, loss of ultrastructural zipper-like intercellular junctions, delocalization of the junctional proteins E-cadherin, β-catenin and desmoglein-1, decreased expression of the barrier protein filaggrin, as well as impaired barrier function. In addition, SPINK7-depleted epithelial cells over-expressed a unique set of cytokines and chemokines that promote immune responses. Loss of SPINK7 unleashed the production of a series of pro-inflammatory cytokines and chemokines including TSLP, GM-CSF, TNFα, and IL-8. RNA sequencing substantiated that loss of SPINK7 was an upstream event in eliciting innate immune responses and cellular changes characteristic of inflammatory diseases of the epithelium. Finally, a genetic interaction between SPINK7, TSLP and PLAU in EoE patients was identified, further linking SPINK7 to allergic responses. Epistasis between genetic variants in the SPINK, TSLP and PLAU loci were shown to contribute to EoE susceptibility. Susceptibility for EoE was impacted by epistasis between genetic variants in SPINK7 and PLAU (gene product uPA) with atopy risk variants in ST2 and TSLP. Collectively, the data demonstrate that deficiency of SPINK7 in epithelial cells induces a profound pro-inflammatory state characterized by impaired barrier function, defect in cellular differentiation, and cytokine production. Combined with genetic interaction between SPINK and TSLP, the endogenous balance between the natural proteinase inhibitor SPINK7 and proteinases (uPA and KLK5) is a key checkpoint in regulating mucosal Th2-associated immune responses. Of 8 SPINK members expressed in the esophagus; the most highly expressed were SPINK5 and SPINK7 (1450 and 831 FPKM, respectively) based on genome wide RNA sequencing data of the human esophagus (data not shown, shows the normalized FPKM values for SPINKs expression in healthy and EoE patients from RNAseq data of esophageal biopsies from 6 healthy controls (Normal), and 10 patients with active EoE) (Sherrill, J. D. et al. Analysis of esophageal biopsies (n=133 patients) demonstrated that SPINK7 mRNA was down regulated in EoE compared to controls (data not shown) (Wen, T. et al. Confocal microscopy revealed SPINK7 expression throughout all epithelial layers with the highest expression in the suprabasal epithelium and its expression was significantly down-regulated together with DSG1 in EoE patients compared to control individuals ( Epithelial differentiation was induced by culture of either an esophageal epithelial progenitor cell line (EPC2) or primary esophageal epithelial cells exposed to the air-liquid interface (ALI), as reported (Sherrill, J. D. et al. Mucosal immunology 7, 718-729 (2014), Kalabis, J. et al. SPINK7 expression was silenced specifically by shRNAi targeting a region of SPINK7 that exhibited relatively less conservation with SPINK5 which on average exhibits 51% identity to SPINK7 (data not shown). EPC2 cells and primary esophageal epithelial cells were stably transduced with vector expressing either shRNA target SPINK7 or non-silencing control (NSC) shRNA. In cells expressing the SPINK7-directed shRNA, near complete loss of SPINK7 expression was observed with no effect on SPINK5, indicating specificity of the gene silencing construct (data not shown). Whole transcriptome sequencing analysis was performed on EPC2 cells after ALI differentiation (data not shown). This analysis revealed 270 genes that were differentially expressed in the SPINK7-deficient cells compared to NSC cells (p<0.05, fold change>2, RPKM>1) (Table 1, below). The modified genes were enriched for those involved in epidermal differentiation and inflammation including the transcription factors STAT1 and NFATC2 and cytokines such as IL23, IL37, and CCL24 and included decreased expression of FLG, FLG2, LOR, keratins, tranglutaminases and interleukin 36 receptor antagonist (IL36RN) (data not shown and Table 1). Functionally it was predicted that loss of SPINK7 regulated innate immune responses and interferon regulatory factors (data not shown). Furthermore, genes expression of EPC2 monolayer culture (day 0 of ALI culture) and differentiated EPC2 cells in ALI culture (Day 14 of differentiation) were compared. This analysis revealed 3225 differentially expressed genes (p<0.05, fold change>2, RPKM>1) (data not shown). The majority of SPINK7 modified genes (77%) were also differentially expressed during differentiation, including a regulator of terminal epidermal differentiation calmodulin-like 5 (CALML5), and the cornified envelope components expressed by differentiated keratinocytes such as FLG and LOR (data not shown) (Sun, B. K. et al. Focusing on the epidermal differentiation complex (EDC) locus on 1q21, the locus with the greatest change in expression in the EoE transcriptome, the genes altered by SPINK7 silencing and the EDC genes were intersected (Blanchard, C. et al. Venn diagrams depicting the number of genes differentially expressed (<2-fold, p<0.05, FPKM>1) in SPINK7 gene silencing as compared to NSC in EPC2 cells differentiated ALI cultures for 14 days (SPINK7—269 genes) and in EPC2 cells following ALI differentiation (day 14 of culture) compared to prior ALI differentiation (day 7 of culture) (Terminal differentiation—678 genes) (data not shown) or in EPC2 cells following ALI differentiation (day 14 of culture) compared cells in monolayer (day 0) (differentiation-3225 genes) (data not shown). Genes overlapping between these two data sets were identified. The top 14 genes with the highest decrease in expression are presented. Ten epidermal differentiation complex (EDC) genes were significantly (p<0.05) altered by SPINK7 depletion in EPC2 cells following ALI differentiation (day 14). FLG mRNA expression in NSC or SPINK7-depleted EPC2 cells following ALI differentiation (data not shown; data represented as the mean±Sd from three independent experiments performed in triplicates). Correlation of normalized FLG and normalized SPINK7 expression in esophageal biopsies of 170 patients with active EoE was also performed (data not shown). Having identified SPINK5 as part of the epithelial differentiation program ( The impact of loss of SPINK7 on the EoE transcriptome, the abnormal transcriptional profile of the esophagus of EoE patients was analyzed. The genes modified by SPINK7 silencing in differentiated cells were intersected with the EoE transcriptome and a substantial overlap of 36% was found (data not shown). These genes were enriched for abnormal skin inflammation, skin physiology, skin development and innate immune response ( Because it had been demonstrated that EoE pathogenesis is mediated at least in part by an IL-13-stimulated keratinocyte-derived transcriptome (Blanchard, C. et al. Analysis of the localization of these proteins revealed significant enrichment in the cornified envelope (data not shown). Further, 48% of the overlapping genes between SPINK7-regulated transcripts and EoE transcriptome were regulated by IL-13 ( A Venn diagram was made depicting the number of genes differentially expressed in EoE patients as compared to control (<2-fold, p<0.05, FPKM>1) (EoE—1607 genes) and in SPINK7 gene silencing as compared to NSC in EPC2 cells differentiated ALI cultures for 14 days (SPINK7—269 genes) identified by RNA sequencing (data not shown). Genes overlapping between these two data sets were identified (86 genes). A heatmap of the overlap gene is presented according to their fold changed expression after SPINK7 silencing as compared to control (SPINK7) and fold changed expression in EoE as compared to control. A Venn diagram was made depicting the number of genes differentially expressed by EPC2 cells following ALI differentiation after IL-13 stimulation compared to untreated cells (<2-fold, p<0.05, FPKM>1) (EoE—1161 genes) and in SPINK7 gene silencing as compared to NSC in EPC2 cells differentiated ALI cultures for 14 days (SPINK7—269 genes) identified by RNA sequencing. Genes overlapping between these two data sets were identified (80 genes) (data not shown). A Venn diagram was made depicting the number of genes that overlap between EoE and SPINK7 silencing (EoE/SPINK7—86 genes) and the number of genes that overlap between IL-13 trigger and SPINK7 silencing (IL-13/SPINK7—80 genes) (data not shown). Genes overlapping between these two data sets were identified (41 genes). SPINK7 silencing resulted in dilated intercellular spaces compared with NSC treated cells after ALI differentiation (day 14) (see arrows in As a control, morphometric analysis of the total area of the differentiated ALI cultures was not altered after SPINK7 silencing compared to NSC (data not shown), the percent of non-cell associate areas in the tissues measured as the ratio between the non-cell associate areas per a high power filed and the total tissue area per a high power field. Data are representative of three experiments performed in triplicate and are represented as the mean±Sd. Quantitative analysis of H&E—stained sections was performed of NSC or SPINK7-depleted EPC2 cells grown for 7, 9 and 11 days in the ALI cultures (data not shown). The percent of non-cell associate areas in the tissues was quantified from three experiments performed in triplicate and are represented as the mean±Sd. At baseline (day 7), SPINK7 silencing increased non-cellular spaces by 5.4-fold (p=0.008) compared to NSC control cells that were densely packed ( Immunofluorescence analysis of submerged as well as ALI cultures of EPC2 cells revealed that E-cadherin localized to the cellular membrane and showed an organized pattern of cellular junctions ( Immunofluorescence analysis of DSG1 expression in ALI cultures of control cells revealed membrane localization. In contrast, after SPINK7 silencing, DSG1 expression was decreased and abnormally localized in the cytoplasm (data not shown). Paracellular and transcellular permeability of the ALI cultured cells were analyzed. Transepithelial electrical resistance (TEER) was reduced by 36% during ALI differentiation of SPINK7-depleted cells compared to NSC cells ( The supernatant of SPINK7 silenced cells was analyzed using a multiplex cytokine array. Amongst 64 cytokines, a marked change in 18 cytokines ( In addition, IL-8 expression increased in EoE patients compared to controls (data not shown) (Persad, R. et al. Multiplex cytokine array analysis of the supernatant of SPINK7 silenced cells and controls that were treated with CsA revealed that CsA partially blocked the release of several cytokines including eotaxin-1 and IL-16 while other cytokines such as G-CSF and IL-15 remained unaffected (data not shown). Notably, CsA did not affect the proteolytic activity or the barrier function (data not shown). Consistent with these findings, human eosinophils showed increased chemotaxis towards supernatants derived from SPINK7-silenced cells as compared to supernatants derived from NSC cells or media alone (data not shown). The known SPINK7 target uPA (Cheng, X., Lu, S. H. & Cui, Y. Cancer letters 290, 87-95 (2010), and Huang, G. et al. Carcinogenesis 28, 2274-2281 (2007)) was analyzed. Consistent with previous reports, SPINK7 directly inhibited uPA proteolytic activity (data not shown) (Cui, Y., et al Given that most members of the KLK family have trypsin-like activity, the ability of SPINK7 to directly inhibit members from this family in vitro was tested. SPINK7 inhibited KLK5 proteolytic activity in a dose-dependent manner with a mean K, of 132±108 nM (mean±SD) ( qPCR analysis was performed of SPINK7 and SPINK5 expression of control (NSC) or SPINK7-depleted EPC2 cells that were grown in a monolayer or differentiated in ALI culture (data not shown). Panel B shows quantification of uPA activity in supernatants derived from (NSC) or SPINK7-depleted EPC2 cells following ALI differentiation. Data are representative of three experiments performed in triplicate and are represented as the mean±Sd. Confocal microscopic analysis of high resolution 3D structures of differentiated cells revealed that DSG1 and E-cadherin staining was limited to membranes in the superficial regions of the NSC cells and demonstrated close association between the cells. In contrast, there was marked separation after SPINK7 silencing (data not shown). In esophageal biopsies from EoE patients, uPA mRNA expression was increased (2.7-fold; p=0.0003) and uPA activity increased by 10-fold (p=0.043) compared with control individuals ( It is notable that allergic inflammatory cells including eosinophils express both uPAR and its known ligands (31 integrins (Brooks, A. M. et al. It was hypothesized that inhibition of uncontrolled proteolytic activity would ameliorate the impaired barrier and the loss of epithelial differentiation elicited by the loss of SPINK7. The serine proteinase inhibitor, al anti-trypsin (A1AT) is a known inhibitor of KLK5 (Goettig, P., et al. To extend the potential benefit of A1AT, its ability to restore the epithelial changes induced by SPINK7 loss was ability. Of note, A1AT demonstrated a dose-dependent ability to improve barrier function ( An analysis of single nucleotide polymorphism (SNP) of 700 EoE patients as compared to 412 non-atopic non-EoE controls was performed. The results revealed significant genetic interaction between TSLP and PLAU (encoding for uPA) in EoE patients (data not shown). Further analysis showed a genetic interaction between the TSLP, PLAU and SPINK locus. To independently prove association between SPINK7 and the pathophysiology of allergic inflammation the contribution of genetic variation of SPINK7 to EoE susceptibility was determined. The genomic coordinates of SNPs in SPINK7 genomic region were intersected with a large collection of functional genomics datasets according to ENCODE data. Based on that analysis three SNPs (i.e. rs2400509, rs3749690, r512521065) were chosen in the SPINK7 gene region that were likely to influence gene regulatory mechanisms. These SNPs were genotyped in the SPINK7 gene region in an EoE (n=501) and non-EoE allergic control cohort (n=610; data not shown). Logistic regression analysis did not reveal association with EoE susceptibility (data not shown), consistent with the recent GWAS which did not reveal association at the SPINK loci (Kottyan, L. C. et al. Nature genetics 46, 895-900 (2014)). SPINK7 contributed to EoE susceptibility by interacting with other genes. As such, genetic epistasis between these SNPs and atopy-associated genes (n=79) using a custom high density SNP chip platform was identified. Analysis of EoE cases versus non-EoE allergic controls revealed significant genetic interaction between SPINK7 and genetic variants encoding for TH2-associated molecules including ST2, IL-17A, TGFβ1, TGFBR1 and epithelial genes SPRRA1 and PDE4B (data not shown). Stratified logistic regression analyses revealed that SPINK7 (rs2400509) and ST2 (rs4988958) strongly interacted (p=0.0004). Having the SPINK7 major allele in association with the minor allele of ST2 increased the risk for EoE compared to SPINK7 and ST2 minor alleles (OR 1.96; p-value<0.01) (data not shown). Analysis of EoE probability indicated that the direction of the effect for ST2 differs based on the presence of the SPINK7 allele (data not shown). TSLP, located at 5q22, is an established EoE-associated genetic locus (Sherrill, J. D. et al. Two organic compounds were tested for their inhibition of KLK5. Compound I (3-(3-chlorophenyl)carboxy-7-hydroxymethyl coumarin) had an IC50value of 52±12 μM ( The data presented herein identify a role for the naturally occurring serine proteinase inhibitor SPINK7 as a key non-redundant checkpoint in regulating epithelial homeostatic responses in the esophagus (Summarized in (1) loss of barrier integrity including formation of dilated intercellular spaces ( (2) epithelial acantholysis including disruption of the adherens junction proteins E-cadherin, β-catenin and DSG1 ( (3) defective epithelial cell differentiation highlighted by loss of FLG expression ( (4) nuclear mobilization of NFATC1 and over-production of pro-inflammatory cytokines ( (5) induction of an innate transcript signature that overlaps with that associated with allergic inflammation ( The known SPINK7 target uPA was identified as a mediator of the pathogenic events downstream from the loss of SPINK7 by demonstrating that uPA activity increased in the esophagus of EoE patients compared to control individuals, that uPA receptor was modulated in the esophagus, and that SPINK7 was an inhibitor of uPA, consistent with prior reports. In addition, KLK5 was identified as a novel and direct target of SPINK7. These findings are stipulated by identifying genetic epistasis between SPINK7 and PLAU with two genes that are cardinal for Th2 immunity (i.e. ST2 and TSLP respectively; data not shown). The relative importance of SPINK7 in the context of EoE, was demonstrated by its relative deficiency in EoE versus control individuals and its high expression in normal esophagus. Indeed, analysis of esophageal specific genes in the protein atlas revealed that SPINK7 was an esophageal enriched gene (Uhlen, M. et al. Proteomics. Science 347, 1260419 (2015)). Evidence is provided that loss of SPINK7 may be upstream from loss of SPINK5, which is undoubtedly contributory to the Th2-response, as demonstrated by its rare genetic deficiency (Netherton's syndrome) (Furio, L. et al. It is notable that SPINK7 was also known as esophageal cancer related gene 2 (ECRG2) as it was been identified as a tumor suppressor by its ability to inhibit the binding of uPA to uPAR and suppress cell migration/invasion and signaling pathways including elevated cytosolic calcium levels (Cheng, X., Lu, S. H. & Cui, Y. Cancer letters 290, 87-95 (2010), Huang, G. et al. Carcinogenesis 28, 2274-2281 (2007), Cheng, X., et al. The expression of uPAR was markedly reduced in esophageal eosinophils compared to blood eosinophils ( The possibility that loss of SPINK7 promoted NFAT activation was explored, because TSLP expression in keratinocytes is NFAT-dependent (Wilson, S. R. et al. Cell 155, 285-295 (2013)). Indeed, loss of SPINK7 promoted mobilization of NFATC1 to the nucleus and primed esophageal epithelial cells to release several major drivers of adaptive immunity such as GM-CSF, TNFα, and IL-8. The release of some of these cytokines was blocked by inhibiting NFAT activation by CsA and FK506. Local fluctuations in SPINK7 expression serves a dual role in atopic reaction; firstly, by hampering the epithelial barrier which promote immune cells to encounter luminal antigens and secondly, by priming epithelial cells to secrete pro-allergic and immunomodulatory cytokines. It has been reported that SPINK7 provides a spindle assembly checkpoint and that loss of SPINK7 results in rapid proliferation and chromosomal instability (Cheng, X., et al In addition, the loss of SPINK7 caused release of several cytokines (i.e. IL-1β, TNFα and PDGF) that are key regulatory molecules of tissue repair (through uPA/uPAR-dependent mechanism) (Chabot, V. et al. This study identified a hitherto unrecognized pathway centrally mediated by SPINK7 and involving unleashed proteinase activity in allergic esophageal inflammation. Loss of SPINK7 and aberrant regulation of its downstream targets resulted in a defect in epithelial cell differentiation, loss of barrier function, and induction of an innate transcript signature that overlaps with allergic inflammation. This pathway serves a causative role in compromising epithelial barrier and as an internal signal for epithelial damage with inflammatory consequences. SPINK7 provides a novel checkpoint for regulating a pro-inflammatory response characterized by excessive cytokine production and eosinophil infiltration in the esophagus. In addition, genetic variants in this pathway interact with undoubtedly atopic mechanisms (e.g. TSLP and IL-33/ST2) to initiate and propagate allergic inflammation at least in the esophagus. Administration of the serine proteinase inhibitor, A1AT, was demonstrated to restore the epithelial impairment, at least in part; such that protein replacement therapy with proteinase inhibitors such as A1AT has therapeutic potential for atopic diseases such as EoE and Netherton's syndrome. These data provide evidence that proteinases serve an important role in regulating immune response and substantiate the need to pursue therapeutic strategies that modulate relevant immune responses by suppression of uncontrolled proteinases in disease pathophysiology. The invention provides methods of treating an allergic inflammatory condition characterized by inflammation of the squamous epithelium of a target tissue by reestablishing SPINK7 checkpoint control in the esophageal epithelium, and related compositions and methods. 1. A method of treating an allergic inflammatory condition in a subject in need thereof, the allergic inflammatory condition characterized by inflammation of a squamous epithelium in a target tissue of the subject, the method comprising administering to the subject a pharmaceutical composition comprising an amount of a therapeutic agent effective to replenish SPINK7 protein and/or SPINK7 anti-proteinase activity in the target tissue. 2. The method of 3. The method of 4. The method of 5. The method of 6. The method of 7. The method of 8. The method of 9. The method of 10. The method of 11. The method of 12. The method of 13. The method of 14. The method of 15. The method of 16. The method of 17. The method of 18. The method of 19. The method of 20. The method of 21. The method of 22. The method of 23. The method of 24. The method of CROSS REFERENCE TO RELATED APPLICATIONS
FIELD OF THE INVENTION
BACKGROUND
SUMMARY OF THE INVENTION
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION OF THE INVENTION
Methods of Treatment
Pharmaceutical Compositions and Formulations
EXAMPLES
SUMMARY
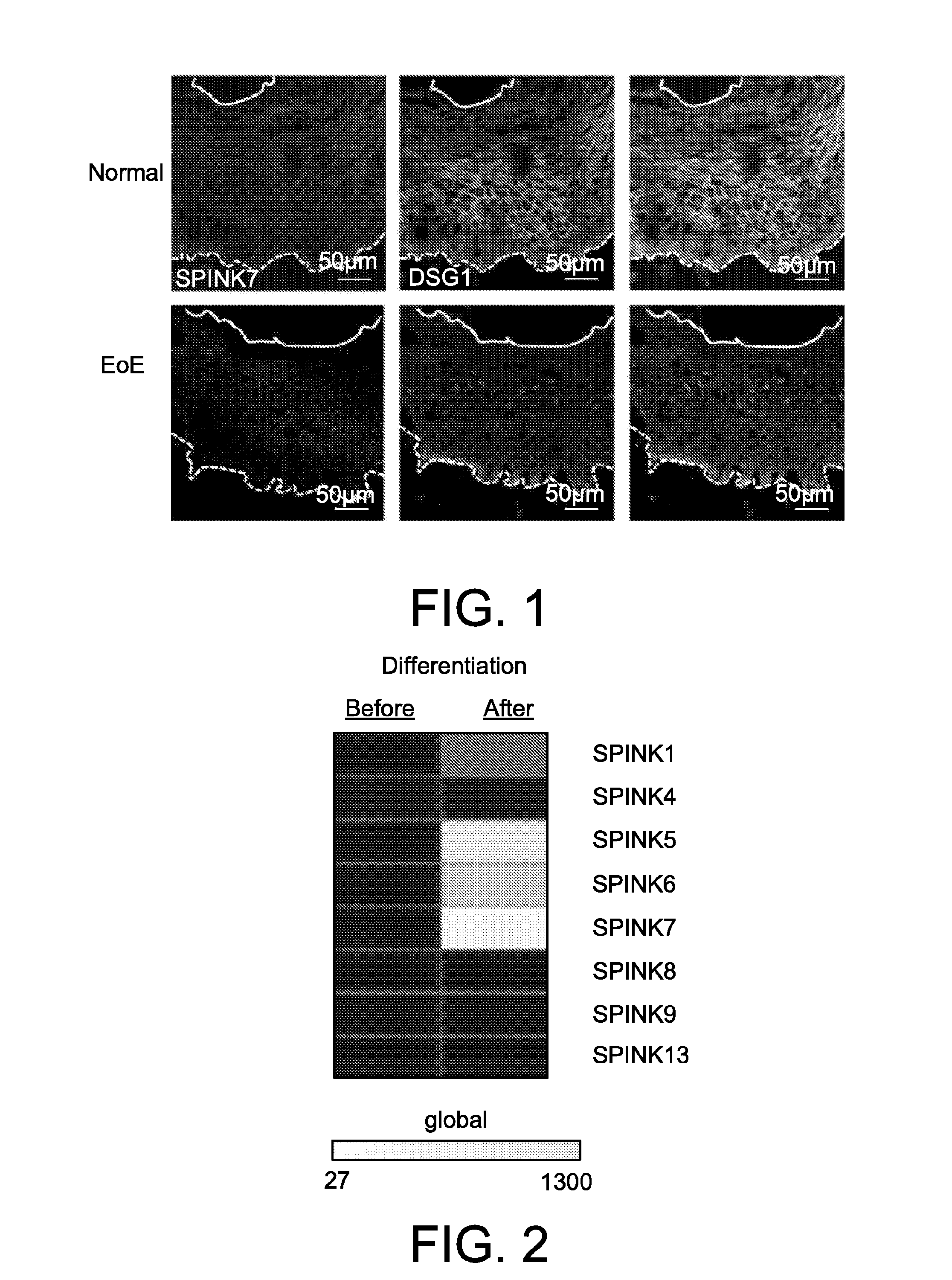
Example 1: Specific SPINK7 Expression in Eosinophilic Esophagitis (EoE)
Example 2: SPINK Expression is Part of the Epithelial Differentiation Program
Example 3: Loss of SPINK7 Impairs Epithelial Differentiation
Transcriptomic analysis of genes differentially expressed after SPINK7-silencing compared to control in differentiated EPC2 cells RPKM RPKM Fold Gene_ID Control SPINK7 Change p-value AADACL2 8.660 0.353 0.041 3.12E−10 ABCG4 1.252 0.292 0.233 0.001276785 ABHD12B 1.025 0.217 0.212 0.000227346 ABP1 6.362 0.491 0.077 2.62E−08 ACER1 10.451 1.513 0.145 5.26E−13 ACP5 3.783 1.509 0.399 0.000721352 ACPP 9.356 3.554 0.380 2.79E−06 ALDH1A3 200.888 675.867 3.364 1.95E−10 ALDH5A1 1.778 0.651 0.366 0.000678249 ALOX12B 17.886 5.411 0.303 7.18E−05 ANKRD35 8.693 2.993 0.344 8.95E−06 APOE 22.695 5.167 0.228 0.001409301 AQP3 70.172 34.698 0.494 0.000320651 ARL4A 10.506 3.386 0.322 0.000103552 ASAP3 7.982 2.973 0.372 1.37E−07 ASPG 3.398 0.462 0.136 0.000822682 ASPRV1 22.411 0.570 0.025 9.80E−29 BCAS1 6.504 0.615 0.095 9.62E−05 BGN 0.535 1.463 2.735 0.004746858 BNIPL 27.186 12.137 0.446 0.000101291 BST2 4.337 46.397 10.697 2.01E−21 C10orf10 2.086 3.710 1.779 5.88E−05 C10orf99 89.123 23.786 0.267 3.08E−09 C17orf109 1.834 0.297 0.162 0.000325599 C19orf66 5.604 16.880 3.012 0.001476392 C1orf68 3.792 0.177 0.047 1.67E−14 C2orf54 12.718 2.398 0.189 8.82E−06 C5orf46 17.411 2.016 0.116 5.67E−07 C6orf15 22.207 6.762 0.305 4.70E−11 CA13 1.737 0.749 0.431 0.001446637 CALB2 10.486 4.100 0.391 0.00031438 CALML5 49.497 4.000 0.081 6.89E−15 CAMK1D 1.690 0.582 0.344 0.001481232 CASP14 11.974 0.941 0.079 8.46E−31 CCL24 3.902 0.074 0.019 1.27E−05 CDH26 1.359 0.337 0.248 3.76E−05 CEACAM5 24.091 2.054 0.085 1.62E−14 CEACAM7 10.835 0.495 0.046 3.64E−05 CERCAM 3.872 8.326 2.150 0.000384542 CERS4 20.319 8.394 0.413 0.000100833 CHRNB1 2.566 6.303 2.457 0.000303905 CLCA4 35.921 3.034 0.084 0.003852654 CLDN17 108.849 49.550 0.455 0.005591202 CLDN4 35.014 71.975 2.056 0.000880654 CMPK2 3.097 18.705 6.039 1.21E−06 CMTM8 2.033 0.754 0.371 0.002603997 CORO6 0.597 1.792 3.000 0.000206577 CPPED1 5.802 1.148 0.198 2.67E−12 CRISP3 12.852 0.217 0.017 0.000162726 CSF2RB 1.931 0.592 0.306 0.003264803 CSGALNACT1 5.004 2.257 0.451 0.000148176 CXCL14 106.453 52.935 0.497 2.68E−06 CXCL17 12.814 1.546 0.121 0.000343615 CXCR2 2.104 0.796 0.378 0.001546073 CYP2C18 15.091 6.752 0.447 1.79E−05 CYP4B1 10.572 0.701 0.066 2.85E−15 CYP4F11 0.253 1.372 5.423 9.65E−05 CYP4F22 6.983 1.593 0.228 3.91E−07 DACT2 0.164 1.356 8.269 1.16E−08 DAPL1 9.630 1.303 0.135 1.23E−07 DDX58 8.009 31.227 3.899 0.00063373 DDX60L 5.122 21.791 4.254 3.76E−05 DGAT2 26.942 8.160 0.303 9.38E−07 DHX58 1.742 4.709 2.704 0.002056853 DIO2 8.295 0.638 0.077 1.31E−05 DSC1 19.955 0.412 0.021 1.16E−88 DSG1 196.343 36.276 0.185 1.40E−20 DTX3L 6.590 22.720 3.448 0.000215309 EIF2AK2 9.327 29.432 3.155 0.00059743 ENTPD2 1.248 3.743 3.000 0.000431713 EPHA4 8.987 2.517 0.280 4.79E−10 EPHB6 1.121 0.401 0.358 0.000982833 EPHX1 10.980 4.997 0.455 0.000148811 EPS8 1.132 4.527 3.998 0.001324941 EPSTI1 2.301 14.709 6.391 1.49E−06 ERP27 2.173 0.475 0.219 8.93E−05 FABP5 474.430 213.745 0.451 1.09E−05 FAM3D 28.282 2.147 0.076 2.78E−06 FAM40B 2.439 5.374 2.204 0.002121605 FAM83C 1.187 0.268 0.226 2.81E−05 FCRLA 0.313 1.057 3.375 0.004394847 FETUB 10.757 0.296 0.028 8.13E−14 FLG 16.017 1.814 0.113 1.33E−32 FLG2 33.534 0.625 0.019 1.14E−58 FLVCR2 11.514 4.513 0.392 7.86E−08 FUOM 3.130 0.919 0.294 0.007959923 GBP1 6.595 15.532 2.355 0.00358778 GCNT3 33.422 8.056 0.241 0.000598123 GJB6 128.769 53.132 0.413 2.39E−06 GLA 28.411 11.992 0.422 0.000244569 GPLD1 2.115 0.512 0.242 1.10E−05 GPR111 1.087 0.359 0.330 7.52E−05 GPRIN2 1.144 0.165 0.144 0.000662353 GSTA4 16.732 6.224 0.372 2.12E−05 GUCY1A3 4.661 1.665 0.357 0.000244799 HAL 2.715 0.163 0.060 3.72E−29 HERC5 1.420 4.324 3.045 0.003040819 HERC6 6.827 31.523 4.618 5.00E−06 HEXA 14.359 3.140 0.219 1.67E−08 HLA-A 64.534 156.953 2.432 1.12E−06 HLA-B 77.075 217.200 2.818 5.00E−08 HLA-C 44.366 105.895 2.387 2.37E−05 HLA-F 6.775 19.874 2.934 1.88E−05 HOPX 1132.244 344.550 0.304 0.000505595 HPGD 40.814 11.103 0.272 0.008896222 HS3ST6 5.377 1.160 0.216 5.44E−05 HYAL4 2.019 0.523 0.259 0.000599287 IFI27 172.272 556.810 3.232 1.09E−07 IFI35 5.633 25.363 4.502 2.46E−05 IFI44 19.200 90.249 4.700 2.05E−06 IFI44L 1.920 25.357 13.210 1.68E−10 IFI6 66.483 434.423 6.534 4.58E−12 IFIH1 9.872 33.182 3.361 6.38E−06 IFIT1 26.543 231.184 8.710 8.67E−07 IFIT2 2.513 10.464 4.164 0.000772178 IFIT3 13.555 85.022 6.272 8.89E−06 IFIT5 5.205 16.352 3.142 9.23E−06 IFITM1 42.317 156.612 3.701 1.08E−07 IFITM3 127.955 312.570 2.443 7.41E−06 IFNK 0.570 10.002 17.540 7.94E−10 IGFBP2 11.995 4.819 0.402 0.000117061 IGFL2 171.138 49.633 0.290 3.37E−08 IGFL3 14.557 7.769 0.534 0.001665316 IL1F10 3.103 0.894 0.288 0.001679465 IL23A 13.831 3.996 0.289 0.002157056 IL36B 2.940 0.640 0.218 8.71E−05 IL36RN 51.959 10.819 0.208 1.28E−07 IL37 1.898 0.271 0.143 0.002081829 IRF7 5.425 21.631 3.987 1.82E−05 ISG15 60.183 239.167 3.974 6.01E−05 KLK1 1.831 0.489 0.267 0.001107183 KLK12 46.708 14.034 0.300 0.001227423 KLK5 197.057 480.144 2.437 9.30E−09 KPRP 31.654 10.894 0.344 5.07E−08 KRT10 1006.965 62.168 0.062 5.27E−14 KRT19 340.813 666.430 1.955 0.001106237 KRT2 16.754 1.845 0.110 1.18E−07 KRT23 195.775 93.248 0.476 0.00690264 KRT24 2.346 3.062 1.306 7.22E−05 KRT27 3.380 0.312 0.092 4.64E−09 KRT4 460.140 54.598 0.119 9.88E−10 KRT79 1.505 0.520 0.346 0.005201351 KRTDAP 2272.468 139.936 0.062 6.55E−24 LAMP3 1.053 5.514 5.235 1.49E−05 LCE1A 64.188 17.932 0.279 1.92E−06 LCE1C 45.575 5.458 0.120 2.18E−13 LCE1D 23.419 2.908 0.124 3.98E−09 LCE1E 15.082 1.425 0.094 3.73E−10 LCE2A 36.115 8.891 0.246 1.42E−05 LCE2B 72.175 12.031 0.167 1.64E−11 LCE2C 71.599 21.581 0.301 4.29E−06 LCE2D 43.298 18.662 0.431 0.000129137 LCP1 1.212 7.302 6.024 1.47E−05 LGALS7B 22.811 8.105 0.355 0.000816353 LIPK 11.476 3.990 0.348 7.60E−06 LIPM 20.570 3.419 0.166 1.29E−06 LOR 24.228 2.071 0.085 1.31E−19 LY6D 16.071 3.964 0.247 9.66E−10 LY6G6C 41.299 8.997 0.218 8.14E−12 LYNX1 516.559 162.668 0.315 0.000447521 LYPD2 157.658 30.035 0.191 0.000101254 MAFB 1.507 5.373 3.565 4.90E−08 MAL 67.102 2.602 0.039 8.47E−06 MARCH3 1.206 0.578 0.479 0.000504864 MFAP3L 2.372 0.892 0.376 0.001286441 MT1X 441.374 169.262 0.383 3.19E−05 MUC15 21.341 6.225 0.292 0.001802868 MX1 31.721 194.408 6.129 2.22E−06 MX2 3.081 39.028 12.667 1.03E−05 MYH14 1.521 0.184 0.121 0.000132386 MYL9 9.824 35.926 3.657 0.000205203 MYZAP 20.038 8.381 0.418 1.95E−05 NEBL 14.833 4.070 0.274 2.62E−06 NFATC2 1.484 0.557 0.375 7.22E−08 NLRC5 0.594 2.995 5.042 0.000136269 OAS1 32.972 95.846 2.907 7.35E−05 OAS2 26.204 105.856 4.040 7.77E−05 OAS3 6.712 42.148 6.279 5.04E−06 OASL 5.341 18.148 3.398 0.00072384 OPHN1 0.856 2.336 2.730 0.000195346 PAQR5 3.609 1.586 0.439 3.46E−06 PARP10 1.734 5.896 3.401 0.002403439 PARP12 6.048 17.888 2.958 0.000653571 PARP14 7.261 25.702 3.540 5.17E−06 PARP9 18.443 66.115 3.585 1.37E−05 PCSK6 3.616 0.475 0.131 1.84E−11 PGLYRP4 28.278 11.122 0.393 0.001807447 PHEX 3.483 1.178 0.338 0.00188494 PI3 20386.800 6606.973 0.324 6.87E−06 PLA2G3 3.933 0.556 0.141 9.00E−16 PLA2G4B 7.873 3.766 0.478 0.000902824 PLA2G4D 1.409 0.187 0.133 5.02E−07 PLBD1 78.931 26.987 0.342 0.001063172 PLEKHA4 0.531 2.175 4.098 0.004923693 PLXDC2 21.965 8.362 0.381 0.00015418 POF1B 129.287 58.641 0.454 0.001401452 POLR2J3 14.311 3.087 0.216 0.00017694 POSTN 3.548 0.767 0.216 0.001041589 PPAP2C 5.745 1.838 0.320 0.000432425 PPFIBP2 8.574 4.602 0.537 9.00E−05 PPP2R2C 4.463 1.860 0.417 0.000296121 PRIC285 3.781 14.161 3.745 0.006690084 PRSS3 11.472 2.912 0.254 1.17E−09 PSAPL1 1.218 0.077 0.063 7.33E−16 PSORS1C2 4.636 1.892 0.408 0.000746804 PYDC1 5.163 0.582 0.113 8.50E−08 RDH12 60.584 12.719 0.210 2.35E−05 RGMA 0.731 2.055 2.813 0.000957165 RNF213 3.149 10.248 3.255 0.000151269 RPTN 152.773 23.960 0.157 7.91E−08 RSAD2 14.663 51.466 3.510 0.000657584 S100A4 10.952 1.743 0.159 0.000169518 SAMD9L 3.282 8.296 2.528 0.000122563 SAPCD2 1.359 5.105 3.756 5.74E−06 SCCPDH 1.377 0.470 0.342 0.001095738 SDR9C7 23.268 3.506 0.151 2.74E−14 SEMA3B 0.466 2.348 5.042 5.20E−08 SERPINA12 17.941 0.441 0.025 1.41E−19 SERPINB10 1.707 0.303 0.177 0.002079277 SERPINB11 1.196 0.043 0.036 0.000254419 SERPINB12 14.375 0.628 0.044 7.75E−25 SERPINB4 2.135 1.017 0.476 0.003011842 SH3GL3 2.644 0.436 0.165 9.30E−05 SHF 5.075 1.551 0.306 0.000279762 SHISA4 0.777 2.518 3.240 0.00214213 SHISA9 1.303 0.520 0.399 0.002362574 SIPA1L2 4.620 1.891 0.409 5.71E−08 SLC10A6 3.110 1.231 0.396 0.000694286 SLC15A1 3.903 0.804 0.206 6.66E−12 SLC15A3 0.553 3.453 6.245 7.80E−06 SLC39A2 15.331 4.958 0.323 9.55E−06 SLURP1 395.306 106.847 0.270 3.63E−08 SP100 16.667 45.318 2.719 0.003767804 SPINK5 1307.389 256.405 0.196 2.76E−06 SPINK7 601.815 48.483 0.081 1.42E−20 SPRR1A 1630.121 731.475 0.449 0.000117351 SPRR2B 701.221 368.342 0.525 0.000175788 SPRY1 1.568 0.796 0.507 0.003023006 SPTLC3 21.596 10.087 0.467 1.76E−07 SPTSSB 14.270 0.145 0.010 3.44E−12 STAT1 40.731 165.908 4.073 4.60E−05 SYNGR1 1.143 0.467 0.408 0.009279141 SYTL2 2.970 6.445 2.170 0.000346622 SYTL5 1.017 0.137 0.135 0.000953611 TAGLN 16.779 45.100 2.688 0.000663656 TCN1 22.208 3.108 0.140 5.41E−08 TEX101 1.478 0.153 0.104 0.005571691 TGM2 1.362 5.369 3.941 0.001837368 TGM5 4.919 0.874 0.178 1.55E−14 THEM5 4.333 0.239 0.055 2.54E−11 TMEM45B 45.839 18.799 0.410 0.002362663 TMPRSS11B 71.841 5.468 0.076 0.003055064 TMPRSS11D 14.631 2.846 0.195 0.000188995 TPRG1 6.638 2.232 0.336 9.13E−05 TRANK1 0.742 3.210 4.324 0.000934694 TREX2 1.424 0.410 0.288 0.000733089 TRIM25 12.867 33.370 2.593 0.000104414 UPK3BL 114.888 21.787 0.190 7.78E−06 USP18 1.610 10.015 6.221 8.46E−06 VSIG8 22.682 6.366 0.281 1.91E−05 WDR76 0.161 1.464 9.072 0.004154222 WFDC5 52.581 22.585 0.430 0.000134007 WNT9A 0.234 1.039 4.450 0.00437511 XAF1 6.888 31.901 4.632 2.34E−07 XKRX 6.464 2.415 0.374 6.86E−05 ZBTB7C 1.037 0.120 0.115 1.91E−06 ZNF433 1.908 0.447 0.234 0.000604875 ZNF556 4.679 0.794 0.170 2.88E−05 ZNF626 1.118 0.363 0.324 0.001935167 ZNF662 1.556 0.164 0.105 4.66E−18 FPKM, fold-change (FC) and p-values of genes differentially expressed after SPINK7 gene silencing as compared to NSC in EPC2 cells differentiated at ALI cultures for 14 days identified by RNA sequencing (Fold change > 2, p < 0.05, FPKM > 1). Example 4: SPINK7 Regulation is Upstream of SPINK5 in Esophageal Cells
Example 5: Silencing of SPINK7 Results in Transcriptional Changes that Overlap with the EoE and IL-13-Associated Transcriptomes
Example 6: Loss of SPINK7 Induces Epithelial Architecture
Example 7: SPINK7 Silencing Results in Alterations of Junctional Proteins
Example 8: SPINK7 Silencing Induced IBF
Example 9: SPINK7 Gene Silencing Unleashes the Production of Pro-Inflammatory Cytokines
Example 10: Impact of SPINK7 Silencing on Serine Proteinases
Example 11: Gene Silencing of SPINK7 Results in Alteration in Adherens Proteins and Desmosomal Proteins Followed by Impaired Epithelial Barrier
Example 12: uPA in the Esophagus of EoE Patients
Example 13: Genetic Epistasis Between SPINK7 and ILRL1 (ST2)
Example 14: Genetic Data Links the SPINK7 Downstream Target-uPA to EoE
Example 15: Effect of Organic Compounds on KLK5 Activity
Discussion