INDWELLING DEVICE FOR EMBOLIZATION
The present invention relates to an indwelling device for embolization, and particularly to an indwelling device for embolization used for embolization of a lesioned part such as an aneurysm. Currently, an endovascular treatment method using a catheter or the like is known as a less invasive treatment method for an intravascular lesion such as an aneurysm. In the endovascular treatment, there is a method of, for example, inserting an indwelling device for embolization (hereinafter simply referred to as an “indwelling device”) into an aneurysm through a catheter, placing a part of the indwelling device by cutting off at a detachable portion, and emboli zing the aneurysm. A part of the indwelling device placed in the aneurysm becomes a physical obstacle to a blood flow and a thrombus is formed around the part of the indwelling device, whereby a risk of rupture of the aneurysm can be reduced. An indwelling device has a structure in which a coil portion to be placed in a body and a pusher portion for delivering the coil portion to a predetermined site are connected via a detachable portion. The coil portion is placed at a predetermined site such as an aneurysm, followed by cutting the detachable portion by mechanical, thermal or electrical operation. The indwelling device is required to have a function so that the coil portion is prevented or suppressed from stretching unlimitedly, in order to reliably perform a repositioning operation that corrects an indwelling state by recovering the coil portion into a catheter after the coil portion pushed out from the catheter is placed at a predetermined site and before cutting. As a means for preventing or suppressing stretch of the coil portion, there is a method of disposing a stretch-resistant member, which can suppress the stretch of the coil portion in its axial direction, inside the coil portion. The stretch-resistant member is required to have high strength for the reason described above. Accordingly, in order to enhance safety more during the operation, the stretch-resistant member is preferably made of a material having relatively high strength such as, for example, a precious metal and a resin polymer (e.g., polypropylene or polyethylene). Patent Literature 1 discloses an embolization coil in which an extension-preventing wire and partially or entirely pitch-wound coil are fixed to each other at least two points different from each other. Patent Literature 1 also discloses that the extension-preventing wire has a region having a stretchable shape in a region placed between the two points different from each other. However, there is room for examining the size of the stretchable shape of the extension-preventing wire. Patent Literature 2 discloses an embolization device for positioning in a blood vessel, comprising an elongated wire body which in its unloaded condition has a predetermined shape and has an elongated shape with a substantially straight center line during its insertion through a catheter to a placement site in the blood vessel and after its release from the catheter assumes a complexly curved shape which depends on the predetermined shape and on the blood vessel impact on the wire body, which wire body preferably has a coupling means at its back end. Patent Literature 2 also discloses a measurement value of a spring constant of the wire body. However, since the embolization device of Patent Literature 2 does not have a stretch-resistant member, the coil portion cannot be prevented from unlimitedly stretching and operability of the coil portion may be likely to be deteriorated. Japanese Unexamined Laid-open Patent Application Publication No. 2015-128677 Japanese Unexamined Laid-open Patent Application Publication No. 2000-517222 In manufacturing the indwelling device, a sterilization treatment with heating is applied, and at the time, when the stretch-resistant member having a small spring constant is disposed inside the coil portion and sterilized, the size of the coil portion may change. In this case, it becomes difficult to manufacture the indwelling device including the coil portion having an appropriate size. An object of the present invention is to provide an indwelling device for embolization in which a change in size of a coil portion is suppressed at a time when a stretch-resistant member is disposed and sterilized. As a result of intensive studies for solving the above-mentioned problems, the present inventor has completed the present invention. That is, the present invention relates to the following indwelling device for embolization. [1] An indwelling device for embolization comprising a coil portion having a proximal side and a distal side and having a lumen extending in a longitudinal direction, and a stretch-resistant member disposed in the lumen, wherein a spring constant of the coil portion is 1.0 N/mm or less, the stretch-resistant member has a waveform, and a wave height, which is a distance between peaks on an inner side of the waveform of the stretch-resistant member, is 35 μm or larger and smaller than an inner diameter of the coil portion. [2] The indwelling device for embolization according to, wherein the spring constant is 0.6 N/mm or less and the wave height is larger than 35 μm.
a spring constant of the coil portion is 0.2 N/mm or less. the stretch-resistant member has a waveform, and a wave height, which is a distance between peaks on an inner side of the waveform of the stretch-resistant member, is 50 μm or larger and smaller than an inner diameter of the coil portion. [5] The indwelling device for embolization according to, wherein the wave height is larger than 50 μm.
a spring constant of the coil portion is 0.1 N/mm or less, the stretch-resistant member has a waveform, and a wave height, which is a distance between peaks on an inner side of the waveform of the stretch-resistant member, is 65 μm or larger and smaller than an inner diameter of the coil portion. [8] The indwelling device for embolization according to, wherein the wave height is larger than 65 μm.
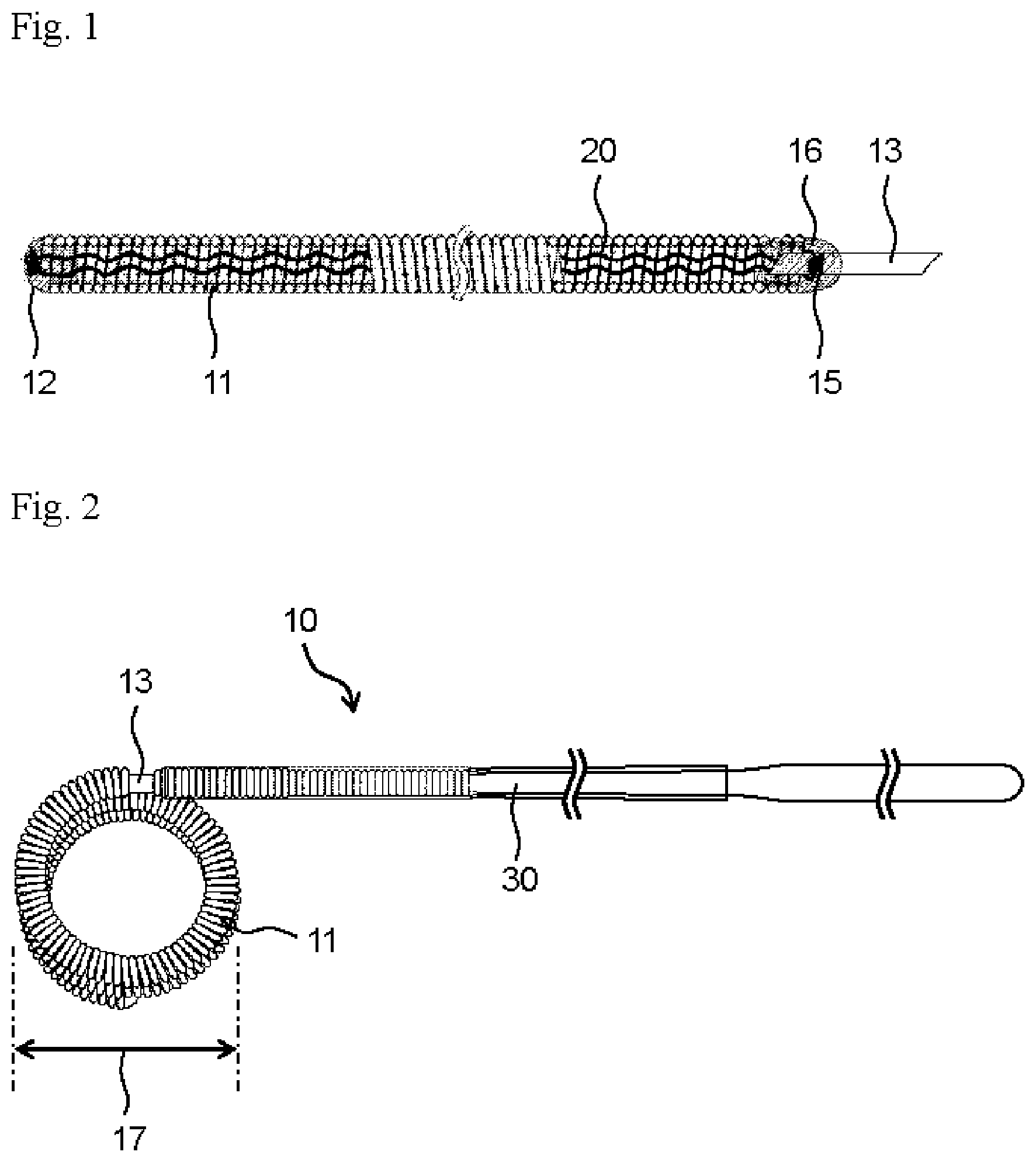
According to the indwelling device for embolization of the present invention, a change in size of the coil portion is suppressed at a time when the stretch-resistant member is disposed to the indwelling device and sterilized. Hereinafter, the present invention will be specifically explained below based on the following embodiments; however, the present invention is not restricted by the embodiments described below of course, and can be certainly put into practice after appropriate modifications within in a range meeting the gist of the above and the below, all of which are included in the technical scope of the present invention. In the drawings, hatching or a reference sign for a member may be omitted for convenience, and in such a case, the description and other drawings should be referred to. In addition, sizes of various members in the drawings may differ from the actual sizes thereof, since priority is given to understanding the features of the present invention. An indwelling device for embolization (hereinafter simply referred to as an “indwelling device”) 10 of the present invention comprises a coil portion 11, a detachable portion 13 and a pusher portion 30 from a distal side thereof, as shown in The indwelling device 10 comprises the coil portion 11 having a lumen extending in the longitudinal direction. The lumen is a cavity inside the coil. The lumen may extend over the entire length of the coil portion 11 or may have a structure in which a part of the coil lumen is closed. It is preferable that the indwelling device 10 comprises the coil portion 11, and a head part 12 having a substantially hemispherical round shape is disposed at a distal end part of the coil portion 11, that is, a left end part of the coil portion 11 in The coil portion 11 is generally configured by winding a metal wire in a spiral shape. Such a metal wire can be selected from among those that are chemically stable to a human body like noble metals, those that are chemically stabilized by forming a passive film on the surface in vivo, those that have low toxicity or those that are biocompatible when it is left in a human body for a long time. For example, platinum, gold, titanium, tungsten, alloys thereof, stainless steels and the like can be exemplified. Among them, a platinum alloy such as platinum-tungsten is preferably used, since it has chemical stability in vivo, excellent physical properties such as strength and elasticity, and processability. The coil portion 11 constituting the indwelling device 10 preferably has bendability or flexibility, and preferably has the following configuration, though it may vary depending on the material of the metal wire. A diameter of the metal wire constituting the coil portion 11, that is, a wire diameter is preferably 10 μm or larger and 120 μm or smaller. A coil diameter of the coil portion 11 is preferably 100 μm or larger and 500 μm or smaller. A coil length of the coil portion 11 is preferably 2 mm or longer and 500 mm or shorter. The coil portion 11 may have a shape in which a metal wire is wound in a spiral shape, or it may be configured to have a coil shape in which the metal wire is wound in a spiral shape as a primary shape and have a secondary shape in which the primary shape is further formed into a certain shape. The metal wire can be formed into a coil-shaped primary shape by winding around a cylindrical primary mold. Further, the coil portion 11 having the primary shape can be wound around a cylindrical secondary mold to give a coil-shaped secondary shape. Alternatively, the coil portion 11 having the primary shape can be inserted into a predetermined box-shaped secondary mold to give a box-shaped secondary shape. The metal wire, the primary shape or the like can be imparted a certain shape by winding around a mold or inserting into a mold, followed by heating. After imparting the secondary shape to the coil portion 11, a tertiary shape can be further imparted. As the mold, a mold in which a wire or a coil is wound on its outside to impart a shape, or a mold which has a lumen and in which a wire or a coil is inserted to impart a shape according to the lumen, can be used. A secondary coil diameter of the coil portion 11 is appropriately determined according to an application site, for example, the size and inner diameter of an aneurysm, and is preferably 1 mm or larger, more preferably 1.5 mm or larger, and preferably 40 mm or smaller, more preferably 20 mm or smaller. The secondary coil diameter of the coil portion 11 means a length indicated by the reference numeral 17 in The shape of the detachable portion 13 is a rod shape, and can be a columnar shape, a prismatic shape, or a combination thereof. An outer diameter of the detachable portion 13 varies depending on specific configurations of the pusher portion 30 and the coil portion 11 and is not restricted as long as the target coil portion 11 can be connected to the pusher portion 30 by an appropriate means. The detachable portion 13 preferably has an outer diameter of, for example, 0.05 mm or larger and 2.0 mm or smaller, and preferably has a length of 1.0 mm or longer and 10 mm or shorter. A material of the detachable portion 13 is preferably those that do not adversely affect a living body and can be deformed or molten by a thermal, mechanical or electrical method, thereby separating the coil portion 11 from the pusher portion 30. Specifically, it is preferable that the detachable portion 13 contains a heat-melt material such as a polyvinyl alcohol polymer that is molten and cut by heating. The material of the detachable portion 13 is not limited to those, and a material which transforms by heating, such as a shape memory alloy and shape memory resin, for example, can be also used. Thereby, the coil portion 11 can be separated from the pusher portion 30 by a thermal operation. A stretch-resistant member 20 is disposed in the lumen of the coil portion 11. The stretch-resistant member 20 has a linear body, and is disposed so as to be long in the longitudinal direction of the lumen of the coil portion 11. The stretch-resistant member 20 is preferably disposed in the state where the entire stretch-resistant member 20 extends in the longitudinal direction in the lumen of the coil portion 11. A proximal end part of the stretch-resistant member 20 is preferably anchored to the detachable portion 13. A distal end part of the stretch-resistant member 20 is preferably anchored to a distal part of the coil portion 11 and may be anchored to the head part 12. The stretch-resistant member 20 prevents the coil portion 11 from stretching more than necessary in a catheter or in a living body when the indwelling device 10 passes through the catheter and is delivered into the body. The stretch-resistant member 20 has a waveform. That is, as viewed from a lateral side of the indwelling device 10, the stretch-resistant member 20 is formed in a waveform. Examples of the waveform include a substantially sine wave shape, a substantially rectangular wave shape, and a substantially triangular wave shape, and among them, a substantially sine wave shape is preferable. The stretch-resistant member 20 is preferably imparted a substantially sine wave shape over the entire distal and proximal direction, as shown in The stretch-resistant member 20 can be composed of a single monofiber or a stranded wire thereof. Alternatively, the stretch-resistant member 20 can be composed of, for example, a metal wire of a platinum alloy such as platinum-tungsten in the same manner as the coil portion 11. In the case where the stretch-resistant member 20 is composed of a metal wire, there is a possibility that the stretch-resistant member 20 may be ruptured by metal fatigue when the coil portion 11 is repeatedly put in and out of a catheter in placing the coil portion 11 at an appropriate site in a body. In order to prevent this, the stretch-resistant member 20 can be composed of, for example, a metal material which is resistant to metal fatigue or a resin material which does not cause metal fatigue. Examples of a constituent material of a resin wire that can be used for the stretch-resistant member 20 include synthetic resins such as polyethylene, polypropylene, polyethylene terephthalate, polyamide, polyester, polylactic acid, polyglycolic acid, poly (lactic acid-glycolic acid) copolymer, polyhydroxybutyric acid, polyhydroxybutyrate valeric acid and a copolyester of 3-hydroxybutyric acid and 3-hydroxyhexanoic acid copolyester; polymers derived from a biodegradable polymer such as cellulose, polydioxanone, protein and vinyl polymer; and others. Among them, from the viewpoint of biocompatibility, the resin wire of the stretch-resistant member 20 is preferably made of polyethylene, polypropylene, nylon, polyester, polydioxanone, polytetrafluoroethylene, polyglycolic acid, polylactic acid, silk, or a composite material composed by any combination thereof. As a method for anchoring the distal end part of the stretch-resistant member 20 to the distal part of the coil portion 11 or the head part 12 or a method for anchoring the proximal end part of the stretch-resistant member 20 to the detachable portion 13, adhesion using an adhesive, welding by including fusion or a inclusion, crimping such as mechanical caulking, physical connection, ligation, or other methods can be used, for example. Anchoring of the stretch-resistant member 20 to the distal part of the coil portion 11 may be performed by, for example, disposing a structure formed by winding one round in a small spiral shape at the head part 12 of the coil portion 11 and hanging the stretch-resistant member 20 on this structure. In this case, both ends of the stretch-resistant member 20 may be anchored to the rod-shaped detachable portion 13. The indwelling device 10 is sterilized with ethylene oxide gas after assembly. Since the sterilization treatment is accompanied by heating, the stretch-resistant member 20 made of a resin wire is heated in a contracted state, and may be solidified while maintaining the contracted shape after cooling. As a result, the natural length of the stretch-resistant member 20 after sterilization may become shorter than that before sterilization, and the secondary coil diameter of the coil portion 11 may change. The softer the coil portion 11 is, the greater the influence that the secondary coil diameter is affected by shortening of the stretch-resistant member 20 is. If the secondary coil diameter of the coil portion 11 changes greatly after disposing the stretch-resistant member 20 in the coil portion and sterilizing, it becomes difficult to adjust the secondary coil diameter of the coil portion 11 to a desired size in the indwelling device 10 after sterilization. In this case, it becomes difficult to manufacture the indwelling device 10 comprising the coil portion 11 having an appropriate specification suitable for the size of an aneurysm or the like. Softness of the coil portion 11 can be defined by a spring constant k of the coil. The spring constant k is expressed by the following equation, D1represents the wire diameter of the coil portion 11. G represents a shear modulus specific to the material of the coil portion 11, D2represents the primary coil diameter of the coil portion 11, and n represents a number of loops of the wire per 1 mm of the coil portion 11, that is, a number of loops of a metal wire. The coil portion 11 is assumed to be formed by densely winding a metal wire in a spiral shape; and in this case, the number n of loops of a wire per 1 mm of the coil portion 11 is the reciprocal of the wire diameter. As the value of the spring constant k is smaller, the coil portion 11 is softer and more susceptible to the shortening of the stretch-resistant member 20 after the sterilization treatment. The coil portion 11 preferably has a spring constant of 1.0 N/mm or less, and more preferably 0.6 N/mm or less. Thereby, breaking through an aneurysm due to the coil portion 11 being too hard is less occurred. From the same viewpoint, the wire diameter of the coil portion 11 is preferably 0.1 mm or smaller, and thereby, the coil portion 11 is prevented from being too hard. Meanwhile, the lower limit value of the spring constant of the coil portion 11 is preferably 0.01 N/mm or more, from the viewpoint of ensuring a shape retaining property of the coil portion 11. As shown in It is preferable that the stretch-resistant member 20 has a predetermined wave height. As shown in In the stretch-resistant member 20, it is preferable that the smaller the spring constant of the coil portion 11 is, the larger the wave height is. Thereby, even when the wave height of the stretch-resistant member 20 becomes smaller after the sterilization treatment, the influence on the coil portion 11 which has a small spring constant and is made soft can be suppressed to the minimum. For example, in the case where the spring constant of the coil portion 11 is 1.0 N/mm or less, the wave height of the stretch-resistant member 20 is preferably 35 μm or larger and smaller than the inner diameter of the coil portion 11. The wave height of the stretch-resistant member 20 may be larger than 35 μm. In this case, the spring constant of the coil portion 11 is preferably 0.6 N/mm or less and preferably more than 0.2 N/mm. In the case where the spring constant of the coil portion 11 is 0.2 N/mm or less, the wave height of the stretch-resistant member 20 is preferably 50 μM or larger and smaller than the inner diameter of the coil portion 11. The wave height of the stretch-resistant member 20 may be larger than 50 μm. In this case, the spring constant of the coil portion 11 is preferably more than 0.1 N/mm. In the case where the spring constant of the coil portion 11 is 0.1 N/mm or less, the wave height of the stretch-resistant member 20 is preferably 65 μm or larger and smaller than the inner diameter of the coil portion 11. The wave height of the stretch-resistant member 20 may be larger than 65 μm. In this case, the spring constant of the coil portion 11 is preferably 0.01 N/mm or more. This application claims priority to Japanese Patent Application No. 2017-174747, filed on Sep. 12, 2017. All of the contents of the Japanese Patent Application No. 2017-174747, filed on Sep. 12, 2017, are incorporated by reference herein. Hereinafter, examples performed for confirming effects of the indwelling device of the present invention are explained. Using a platinum-tungsten alloy wire having a wire diameter of 0.05 mm, a coil portion of a primary shape having a coil diameter of 0.35 mm was prepared. The obtained coil portion was wound around a cylindrical secondary mold having a predetermined diameter and heated to give a secondary shape to the coil portion. A stretch-resistant member was prepared by imparting a substantially sine wave shape having a wave height of 35 μm and a wavelength of 340 μm to a polypropylene resin wire and folding back, and the resultant was disposed inside the coil portion. The stretch-resistant member was arranged such that the folded portion of the resin wire was hanged on a distal end of the coil portion and both ends of the resin wire extended out from a proximal end of the coil portion, and a rod-shaped detachable portion was inserted into the proximal end of the coil portion. An adhesive was injected into a distal end part and a proximal end part of the coil portion, respectively, and the stretch-resistant member was fixed to the distal end part and the proximal end part of the coil portion. A proximal end part of the detachable portion, that extended proximally from the proximal end of the coil portion, was plugged into a pusher portion, and the detachable portion was attached to the pusher portion with an adhesive, thereby assembling an indwelling device. Thus obtained indwelling device was placed into the interior of a spiral tube and sterilized. The resultant was referred to as an “indwelling device 1-1”. An indwelling device was assembled and sterilized in the same manner as Preparation Example 1, except that a resin wire to which a substantially sine wave shape having a wave height of 50 μm and a wavelength of 340 μm was imparted was used for the resin wire constituting the stretch-resistant member. The resultant was referred to as an “indwelling device 1-2”, An indwelling device was assembled and sterilized in the same manner as Preparation Example 1, except that a secondary mold having a smaller diameter was used as the cylindrical secondary mold for imparting a secondary shape to the coil portion and a resin wire to which a substantially sine wave shape having a wave height of 65 μm and a wavelength of 340 μm was imparted was used for the resin wire constituting the stretch-resistant member. The resultant was referred to as an “indwelling device 1-3”. An indwelling device was assembled and sterilized in the same manner as Preparation Example 1, except that the coil portion was formed from a platinum-tungsten alloy wire having a strand wire diameter of 0.06 mm. The resultant was referred to as an “indwelling device 2-1”. An indwelling device was assembled and sterilized in the same manner as Preparation Example 4, except that a resin wire to which a substantially sine wave shape having a wave height of 50 μm and a wavelength of 340 μm was imparted was used for the resin wire constituting the stretch-resistant member. The resultant was referred to as an “indwelling device 2-2”. An indwelling device was assembled and sterilized in the same manner as Preparation Example 4, except that a resin wire to which a substantially sine wave shape having a wave height of 65 μm and a wavelength of 340 μm was imparted was used for the resin wire constituting the stretch-resistant member. The resultant was referred to as an “indwelling device 2-3”. An indwelling device was assembled and sterilized in the same manner as Preparation Example 4, except that a secondary mold having a larger diameter was used as the cylindrical secondary mold for imparting a secondary shape to the coil portion and a resin wire to which a substantially sine wave shape having a wave height of 50 μm and a wavelength of 340 μm was imparted was used for the resin wire constituting the stretch-resistant member. The resultant was s referred to as an “indwelling device 2-4”. An indwelling device was assembled and sterilized in the same manner as Preparation Example 1, except that a secondary mold having a larger diameter was used as the cylindrical secondary mold for imparting a secondary shape to the coil portion, the coil portion was formed from a platinum-tungsten alloy wire having a strand wire diameter of 0.07 mm, and a resin wire to which a wave shape was not imparted was used for the resin wire constituting the stretch-resistant member. The resultant was referred to as an “indwelling device 3-1”. An indwelling device was assembled and sterilized in the same manner as Preparation Example 8, except that a resin wire to which a substantially sine wave shape having a wave height of 35 μm and a wavelength of 340 μm was imparted was used for the resin wire constituting the stretch-resistant member. The resultant was referred to as an “indwelling device 3-2”. For each indwelling device prepared as described above, the secondary coil diameters of the coil portion before installing the stretch-resistant member and after the sterilization treatment were measured. The secondary coil diameter of the coil portion was determined by placing the coil portion or the indwelling device in an unloaded state under an ordinary temperature environment to form a secondary coil shape which the coil portion of the primary shape was wound (the winding number was 3) as shown in In the indwelling devices 1-1 to 1-3, stretch-resistant members having different wave heights to each other were disposed inside a coil portion having a spring constant k of 0.06 N/mm, that was 0.1 N/nm or less. Provided that the secondary coil diameter change rate of the coil portion was 5% or less was a non-defective product, the indwelling device 1-3 in which the stretch-resistant member had a wave height of 65 μm was resulted in having a secondary coil diameter change rate of 2%, that was 5% or less, and could be classified as a non-defective product. Therefore, for a coil portion having a spring constant k of 0.1 N/mm or less, the wave height of the stretch-resistant member is preferably 65 μm or larger. In the indwelling devices 2-1 to 2-4, stretch-resistant members having different wave heights to each other were disposed inside a coil portion having a spring constant k of 0.14 N/mm, that was 0.2 N/mm or less. Provided that the secondary coil diameter change rate of the coil portion was 5% or less was a non-defective product, the coils in which the stretch-resistant member had a wave height of 50 μm or 65 μm were resulted in having a secondary coil diameter change rate of 4%, 2% or 0%, that were 5% or less, and could be classified as non-defective products. Therefore, for a coil portion having a spring constant k of 0.2 N/mm or less, the wave height of the stretch-resistant member is preferably 50 μm or larger. In the indwelling devices 3-1 and 3-2, stretch-resistant members having different wave heights to each other were disposed inside a coil portion having a spring constant k of 0.27 N/mm, that was 1.0 N/mm or less. Provided that the secondary coil diameter change rate of the coil portion was 5% or less was a non-defective product, the coil in which the stretch-resistant member had a wave height of 35 μm was resulted in having a secondary coil diameter change rate of 1%, that was 5% or less, and could be classified as a non-defective product. Therefore, for a coil portion having a spring constant k of 1.0 N/mm or less, the wave height of the stretch-resistant member is preferably 35 μm or larger. From the above results, the indwelling devices 1-3, 2-2 to 2-4, and 3-2 had the secondary coil diameter change rate of the coil portion of 5% or less and change in the secondary coil diameter of the coil portion after sterilization was small. As a result, it was confirmed that the indwelling device for embolization with a small dimensional change of the coil portion after placement of the stretch-resistant member can be provided. In an indwelling device for embolization comprising a coil portion (11) having a proximal side and a distal side and having a lumen extending in a longitudinal direction, and a stretch-resistant member (20) disposed in the lumen, a spring constant of the coil portion (11) is 1.0 N/mm or less, the stretch-resistant member (20) has a waveform, and a wave height, which is a distance between peaks on an inner side of the waveform of the stretch-resistant member (20), is 35 μm or larger and smaller than an inner diameter of the coil portion (11), in order to provide the indwelling device for embolization that suppresses changes in dimension of the coil portion (11) during sterilization. 1. An indwelling device for embolization comprising:
a coil portion having a proximal side and a distal side and having a lumen extending in a longitudinal direction; and a stretch-resistant member disposed in the lumen, wherein a spring constant of the coil portion is 1.0 N/mm or less, the stretch-resistant member has a waveform, and a wave height, which is a distance between peaks on an inner side of the waveform of the stretch-resistant member, is 35 μm or larger and smaller than an inner diameter of the coil portion. 2. The indwelling device for embolization according to the spring constant of the coil portion is 0.6 N/mm or less, and the wave height of the stretch-resistant member is larger than 35 μm. 3. The indwelling device for embolization according to the spring constant of the coil portion is more than 0.2 N/mm. 4. An indwelling device for embolization comprising:
a coil portion having a proximal side and a distal side and having a lumen extending in a longitudinal direction; and a stretch-resistant member disposed in the lumen, wherein a spring constant of the coil portion is 0.2 N/mm or less, the stretch-resistant member has a waveform, and a wave height, which is a distance between peaks on an inner side of the waveform of the stretch-resistant member, is 50 m or larger and smaller than an inner diameter of the coil portion. 5. The indwelling device for embolization according to the wave height of the stretch-resistant member is larger than 50 μm. 6. The indwelling device for embolization according to the spring constant of the coil portion is more than 0.1 N/mm. 7. An indwelling device for embolization comprising:
a coil portion having a proximal side and a distal side and having a lumen extending in a longitudinal direction; and a stretch-resistant member disposed in the lumen, wherein a spring constant of the coil portion is 0.1 N/mm or less, the stretch-resistant member has a waveform, and a wave height, which is a distance between peaks on an inner side of the waveform of the stretch-resistant member, is 65 μm or larger and smaller than an inner diameter of the coil portion. 8. The indwelling device for embolization according to the wave height of the stretch-resistant member is larger than 65 μm. 9. The indwelling device for embolization according to the spring constant of the coil portion is 0.01 N/mm or more. 10. The indwelling device for embolization according to a wavelength of the stretch-resistant member is 280 μm or longer and 400 μm or shorter. 11. The indwelling device for embolization according to a wire diameter of the coil portion is 0.1 mm or smaller. 12. The indwelling device for embolization according to the coil portion, the detachable portion, and the pusher portion are disposed in this order from a distal side of the indwelling device, and the coil portion and the pusher portion are connected to each other via the detachable portion. 13. The indwelling device for embolization according to a wavelength of the stretch-resistant member is 280 μm or longer and 400 μm or shorter. 14. The indwelling device for embolization according to a wire diameter of the coil portion is 0.1 mm or smaller. 15. The indwelling device for embolization according to the coil portion, the detachable portion, and the pusher portion are disposed in this order from a distal side of the indwelling device, and the coil portion and the pusher portion are connected to each other via the detachable portion. 16. The indwelling device for embolization according to a wavelength of the stretch-resistant member is 280 μm or longer and 400 μm or shorter. 17. The indwelling device for embolization according to a wire diameter of the coil portion is 0.1 mm or smaller. 18. The indwelling device for embolization according to the coil portion, the detachable portion, and the pusher portion are disposed in this order from a distal side of the indwelling device, and the coil portion and the pusher portion are connected to each other via the detachable portion. 19. The indwelling device for embolization according to one end of the stretch-resistant member is fixed at a distal portion of the coil portion, and another end of the stretch-resistant member is fixed at a proximal portion of the coil portion. 20. The indwelling device for embolization according to one end of the stretch-resistant member is fixed at a distal portion of the coil portion, and another end of the stretch-resistant member is fixed at a proximal portion of the coil portion. 21. The indwelling device for embolization according to one end of the stretch-resistant member is fixed at a distal portion of the coil portion, and another end of the stretch-resistant member is fixed at a proximal portion of the coil portion.TECHNICAL FIELD
BACKGROUND ART
CITATION LIST
Patent Literature
PATENT LITERATURE 1
PATENT LITERATURE 2
SUMMARY OF INVENTION
Technical Problem
Solution to Problem
[3] The indwelling device for embolization according to or, wherein the spring constant is more than 0.2 N/mm.
[4] An indwelling device for embolization comprising a coil portion having a proximal side and a distal side and having a lumen extending in a longitudinal direction, and a stretch-resistant member disposed in the lumen, wherein
[6] The indwelling device for embolization according to or, wherein the spring constant is more than 0.1 N/mm.
[7] An indwelling device for embolization comprising a coil portion having a proximal side and a distal side and having a lumen extending in a longitudinal direction, and a stretch-resistant member disposed in the lumen, wherein
[9] The indwelling device for embolization according to or, wherein the spring constant is 0.01 N/mm or more.
[10] The indwelling device for embolization according to any one of to, wherein a wavelength of the stretch-resistant member is 280 μm or longer and 400 μm or shorter.
[11] The indwelling device for embolization according to any one of to, wherein a wire diameter of the coil portion is 0.1 mm or smaller.
[12] The indwelling device for embolization according to any one of to, wherein the coil portion, a detachable portion and a pusher portion are disposed in this order from a distal side of the indwelling device, and the coil portion and the pusher portion are connected to each other via the detachable portion.
Advantageous Effects of Invention
BRIEF DESCRIPTION OF DRAWINGS
DESCRIPTION OF EMBODIMENTS
EXAMPLES
Preparation Example 1
Preparation Example 2
Preparation Example 3
Preparation Example 4
Preparation Example 5
Preparation Example 6
Preparation Example 7
Preparation Example 8
Preparation Example 9
Wire diameter: 0.05 0.06 0.07 D1(mm) Primary coil diameter: 0.35 0.15 0.36 D2(mm) Number of loops of wire 20 17 14 per 1 mm: n Shear modulus of Pt: 61 61 61 G (Gpa) Spring constant: 0.06 0.14 0.27 k (N/mm) Wave height of stretch- 35 50 65 35 50 65 50 0 35 resistant member (μm) Wavelength of stretch- 340 340 340 340 340 340 340 — 340 resistant member (μm) Secondary coil diameter 5.7 5.8 2.1 5.8 5.8 5.8 12.1 12.0 12.1 before placement of stretch-resistant member (mm) Secondary coil diameter 6.7 6.4 2.1 6.6 6.0 5.9 12.1 13.3 12.0 after sterilization (mm) Secondary coil diameter 18% 10% 2% 14% 4% 2% 0% 10% 1% change rate REFERENCE SIGNS LIST