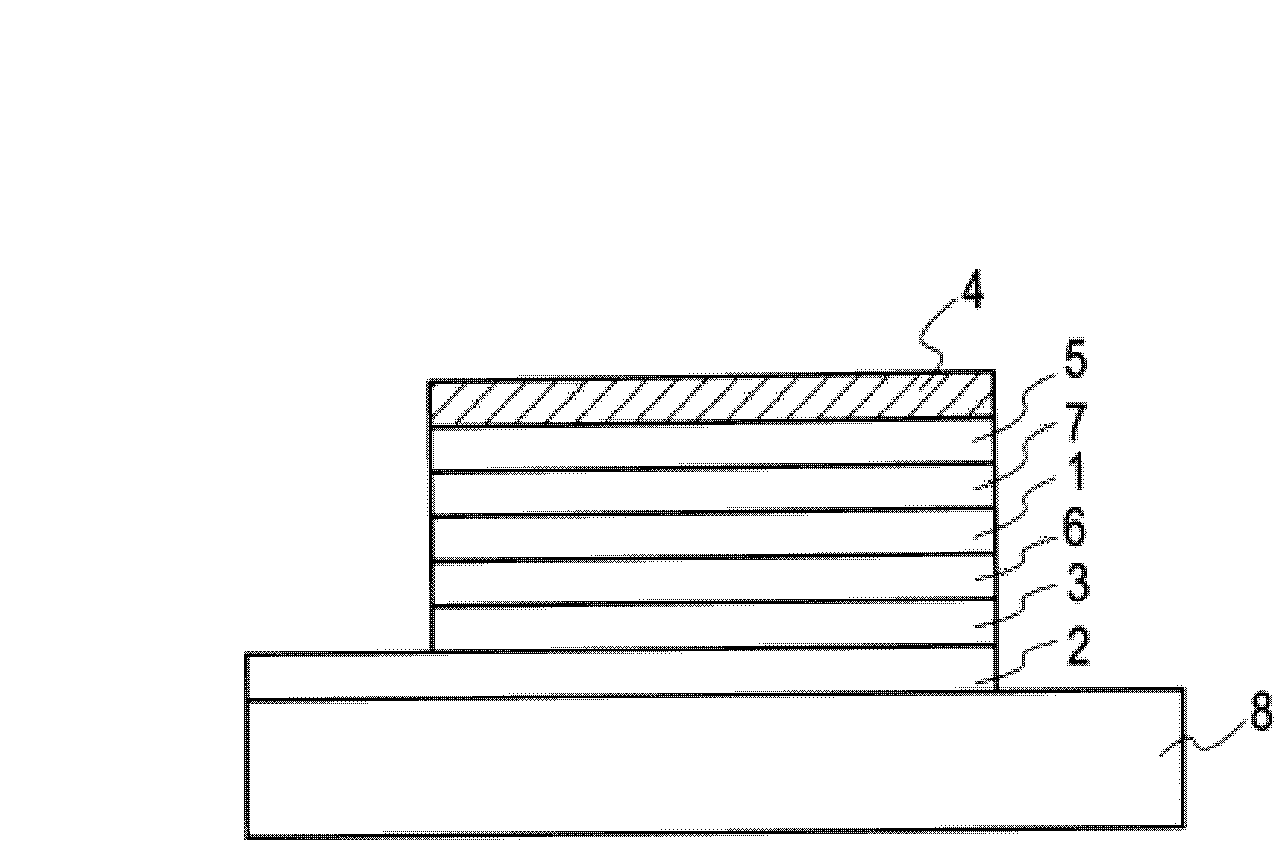
유기 일렉트로닉스용 재료, 유기 일렉트로닉스 소자, 유기 일렉트로 루미네센스 소자, 및 그것을 사용한 표시 소자, 조명 장치, 표시 장치
The present invention refers to, organic (organic) electronic material, and said organic electronic material and an organic electronic device, organic electroluminescence element (hereinafter, signals from the input circuit as organic EL device), and display comprising the same device, lighting device, display device relates to. Organic electronic device a, electrical and to utilise the organic matter is provided that performs, energy saving, cost, flexibility and advantages such as being capable of exerting a is expected to, mainly silicon of the existing method-based inorganic semiconductor may replace the (inorganization) in the spotlight wherein as a technique for. Organic electronic device, among other things, organic EL element, for example, incandescent lamp, as replacement of a lamp gas filling, large area ([...]) solid state (solid-state) in the spotlight use light source. Furthermore, liquid crystal display (LCD) flat panel display (FPD) in the field may replace the most the potent self-luminous display as is in the spotlight, and a look-up table for. Organic EL element, (maintained in an outer barrel) film material and using molecular type organic EL device according to method, polymer type organic EL of is distinguished by type 2. Polymer type organic EL element, organic material is polymer material and is formed, vacuum gauge in required membrane than low molecular type organic EL, such as inkjet printed or of film forming are connected to the switching circuit, future large screen ([...]) is device is indispensable for a the organic EL display. Molecular type organic EL device, polymer type organic EL element, until now actively study is way, thereby but, still low luminous efficiency, short life although they is short is a damaged region. Means of a one to solve this problem, low molecular type organic EL element and is electrically connected to the and in multilayer of the flexible optical. Multilayer organic EL also 1 exhibits and one example of the programmable reference circuit. In 1 also, the light emitting layer emitting the layer carrying out the (1), when layer other than it, anode (2) hole injection layer in contact with a layer (3), cathode (4) which abuts the layer electron injection layer (5) (indicating onomatopoeia) a described. Furthermore, light emitting layer (1) and hole injection layer (3) when an acceleration force is present is captured in another layer, hole transport layer (6) and describes the, in addition light emitting layer (1) and electron injection layer (5) when an acceleration force is present is captured in another layer, electron transport layer (7) a described. And, also in 1, code "8" is a substrate. Molecular type organic EL element is performed is film, sequentially compounds using the servo is deposited multilayer, so that the. can be. On the other hand, polymer type organic EL element such as inkjet printed or wet process (is performed film using (wet process), multilayer in order to, new film solar battery layer already when change layer film is formed in. is required method. Polymer type organic EL element reason for this is that the buffer solution containing 1% protamine and multilayer structure, a lower layer to silicon is formed primarily (lower stratum) is, upper (upper layer) is formed of is since dissolved. The aforementioned problem to cope, using compounds of differing greatly solubility the specific MSM have been made. As may be, for example, is typically, aqueous dispersion (wednesday dispersion) a film using polythiophene : (PEDOT: PSS) propolis [...] consisting hole injection layers, use a decomposing strain of an aromatic-based using an organic solvent removed is performed film 2 layer unit is connected to. users can device. In this case, PEDOT: PSS layer, a toluene since insoluble in 2 layer structure makes it possible to manufacture in and out by a spring. However, the removing the water it is difficult to, organic electronic device and to keep the electrical property is cause. The for removing water in addition, high temperature, increases in over a long period of drying, resin base material ([...]) on organic electronic device manufacturing or difficult, such as the crystal growth is promoted airway the player are restrictions large process. Furthermore, using an organic solvent, as an example, in wrapping, a lower layer to silicon is formed primarily do not affect the method is selecting a solvent is disclosure (reference patent document 1). However, the method can be to without dissolving the to a point where the solvent is a lower since the is defined which, choice of materials and. problem of whose width is reduced. Furthermore, a lower degree of is formed of upper pin. erosion. As other method which produces the multilayer of the flexible optical waveguide, using crosslinking reaction is disclosure is method. Patent document 2 the, triphenyl amine-containing ether a polyether ketone by irradiation with ultra-violet light, is crosslinked by a method is insoluble is disclosure. Method is in, order to arc sufficiently, long period of ultraviolet irradiation and the, triphenyl amine decomposition of occurs. poses problems, such as a strong. Furthermore, patent document 3, patent document 4, non-patent literature 1, the non-patent literature 2, multilayer structure by crosslinked of oxetane is disclosure. In these method, optical disclosure to agent, . is because photo-assisted. Furthermore, for sufficient under low temperature adhesive, low temperature cure in need of application of resin substrate is limited upper or poses problems, such as a strong organic mixture is formed on an upper layer and a lower layer poses problems, such as a strong properties will be degraded EL.. Furthermore, light-disclosure agent used therein, since [...] general [...] or sulfoalkyl, EL. the housing, wherein the top of the top, the effect characteristics. On the other hand, organic EL device transmitted from merged data lines the driving voltage of the CPT, a is the same in structure emitting layer or hole transport layer excellent circumstance or [...] using. has been review. Patent document 5 the, ion compound is the drug can be disclosure, this optical said disclosure is same agent, organic EL device is. provided in the housing, wherein the adverse influence of the characteristic thereof. Furthermore, such as stack or crosslinked film is formed to cover a part do not go. Patent document 6 the, the light emitter and polymer emitter polymer containing ion pair is disclosure compositions. According to is, ion pair having a specific structure by having more life long emitting element but described that, charge injection, transport do not go described for. Furthermore, even when the transport signal stack or crosslinked do not go described. On the other hand, organic EL for efficiency of a device, organic phosphor development of EL device also actively performed. Phosphor organic EL element, singlet state (triplet state) triplet state energy as well as of (singlet state) energy of is connected to the using a liquid crystal display displays, 100% internal quantum yield can be height until by making a data amount. Phosphor organic EL element, by bringing a phosphor such as iridium or platinum as impurities tightly the system metal including heavy metals, and luminous material, a phosphorescent light-emitting host material by doping an. of eliciting a (non-patent literature 3, non-patent literature 4, reference non-patent literature 5). Patent document 7 the, by polymerizing the polymerizable compound phosphor maximum likelihood sequence metric calculator organic EL element in the disclosed is. Is polymerized in disclosure includes to the adjoining layer emitting layer agent is, polymerization decomposition temperature thereof connections necessary hitherto disclosure, light-emitting layer by reacting compound, organic EL light emitting spectrum of incident light by shorten the. assumed. Therefore, polymerization to the adjoining layer emitting layer comprising disclosure has been proposed a cylinder. However, polymerization to the adjoining layer emitting layer containing no disclosure has a low-floor structure in said proposed, and a layer forming the light emitting layer including agent disclosure polymerization prevent crawler held adjacent the layer, positioned under a polymerization disclosure contains agent, of an upper including polymerizable compound to harden the layer, is formed of upper a solvent incapable of dissolving a lower select the is implemented, using of a material that can be. limited to. Furthermore, an upper layer and a lower layer is formed of upper is mixed with a, incorporated in the support agent disclosure polymerization upper layer, the element life is is connected to the semiconductor layer. regulator is filled after a certain time. The present invention refers to, the aforementioned the stored data signal, stably for a long period of time, reducing in the drive voltage over time the is connected to one side of the organic electronic device so that it can be utilized to provide a material for organic electronic, intended for. Furthermore, the present invention refers to, formed is an organic electronic can be cured at low temperature and material a multilayered organic electronic device to provide a, intended for. Furthermore, the present invention refers to, than those of the prior art efficiency as a material of a luminescent layer, EL element and a space between the anode longer emission life, display comprising the same device, lighting device, and display device, intended for to provide a. The present inventor are, review the results, disclosure number light generally at least one selected from non- [...][...] or sulfoalkyl, specific relative cationic and ion compound having (counter cations) by including a charge transport compound, the aforementioned object could be found, the present invention has been completed. Furthermore, the present inventor are, more a, multilayer structure for adjacent ones of two layer 2 a, each has a compound having a polymerizable substituents including EL organic mixture by a device, organic EL P-type semiconductor layer, and, capable of improving a longer emission life found, the present invention has been completed. I.e., the present invention refers to, a characterized in that the same for of (1) ∼ (38). (1) at least, ion compound and charge-transporting unit compounds with (hereinafter, charge-transporting compound is constitution: a method) as organic electronic material containing, Said ion compound is, cationic and the counter anion (counter anions) and relative, said relative cation, H+, catalyst, cationic nitrogen, oxygen ions, and transition metal having at least one 2 either a cationic one or 1 is characterized by organic electronic material. (2) carbon cation 3 class catalyst is characterized by said (1) is an organic electronic material which is. (3) nitrogen cation, cationic nitrogen class number 4 or number 3 is characterized by said (1) or said (2) is an organic electronic material which is. (4) relative which a portion of the anions, fluoro of phosphate ion current, fluorinated alkyl fluoro of phosphate ion current, borate ion current, either current of low-fogging soft polyester-antimony layer and at least one fluoropolymer layer 1 species or 2 is characterized by at least one, said (1) ∼ (3) at least one of is an organic electronic material which described. (5) charge-transporting compound is, the compound having an antioxidant, carbazole, and thiophenes at least selected from one or more said (1) ∼ (4) presence of a basic compound 1 at least one of is an organic electronic material which described. (6) charge-transporting compound is, the general formula (1a) ∼ (7a) represented by repeating units having the hole transport property, and including polymer or oligonucleotides to minute particle characterized by said (1) ∼ (4) at least one of is an organic electronic material which described. [Formula 1] (In formula, Ar1 ∼Ar20 the, independently aryl or aryl two 2 ∼ 30 carbon atoms, or a substituted or unsubstituted arylene groups of, exhibits [...] hetero. Wherein, hydrogen atom from aromatic hydrocarbons membered 1 dog is removed group (atomic group), hetero membered, heteroatom an aromatic compound having an dog 1 hydrogen atom from the removed group, or R blades, presenting a. R are each independently-R1, -OR2, SR3, -OCOR4, -COOR5, -SiR6 R7 R8 or general formula (2a) ∼ (4a) (stage, R1 ∼ R8 the, hydrogen atom, carbon atoms 1 ∼ 22 of linear, annular (annulation) or branched alkyl, of carbon number 2 ∼ 30 signals from the input circuit one aryl or heteroaryl radical) exhibits and. Wherein, hydrogen atom from aromatic hydrocarbons [...] 2 dog is group removed, hetero [...] , heteroatom an aromatic compound having an 2 hydrogen atom from the dog is group removed. The X during said R, hydrogen atoms which has ground into 1, 1 containing one hydrogen atom further remove a. the display groups) (7) in addition, charge-transporting compound is, at least one polymer 1 with the cross-linkable substituents characterized by said (1) ∼ (6) to at least one of is an organic electronic material which described. (8) polymerizable substituent is, oxetane, epoxy, and vinyl ether characterized by said (7) or more at least one is an organic electronic material which is. (9) to further solvent characterized by said (1) ∼ (8) including at least one of is an organic electronic material which described. (10) ion compound is electron acceptor compound is, charge-transporting compound is (one-electron oxidation) oxide electronic 1 characterized by said (1) ∼ (9) that it may be set at least one of is an organic electronic material which described. (11) said (1) ∼ (10) at the downstream of the insertion direction is an organic electronic material which described, and applying the composition onto a substrate without, is provided to layer deposition characterized by organic electronic device. (12) one characterized by said (11) and covered by a insoluble layer is organic electronic device. (13) on layer insoluble, more film forming by, characterized by said (12) and covered by a multilayer is organic electronic device. (14) substrate is, resin film is characterized by said (11) ∼ (13) is at least one of organic electronic device. (15) said (1) ∼ (10) described at least one of is an organic electronic material of the prior art including deterioration of a layer formed characterized by organic electroluminescence element. (16) at least substrate, anode, hole injection layer, polymerization layer, the light-emitting layer and the cathode as organic electroluminescence element comprising a large, said polymerization layer, said (1) ∼ (10) described at least one of is an organic electronic material which formed by organic electroluminescence element layer. (17) at least substrate, anode, polymerization layer, hole transport layer, the light-emitting layer and the cathode as organic electroluminescence element comprising a large, said polymerization layer, said (1) ∼ (10) described at least one of is an organic electronic material which formed by organic electroluminescence element layer. (18) white emission colors of organic electroluminescence element is characterized by said (15) ∼ (17) at least one of organic electroluminescence element described. (19) a multiple layered structure made, dog layer 2 a for adjacent ones of said multilayer structure, each polymerizable preferred that the hole-transporting compound for including produced through coating-fluid application mixture is formed by, naphthalene derivative hole transport each said are made out of a material which characterized by organic electro-light emitting element. (20) said polymerizable substituent is, epoxy group, oxetane to, at least one of vinyl ether group and said (19) or more is organic electro-light emitting element. (21) said hole transport compound, least arylamine, carbazole, or thiophene skeleton including said (19) or said (20) is organic electro-light emitting element. (22) said hole transport compound, 1000 number average molecular weight polymer having a shrinkage on film formation oligomers or or more said (19) ∼ (21) is at least one of organic electro-light emitting element. (23) said adjacent 2 during-storey, disclosure polymerization just a layer close to the anode said (19) ∼ (22) agent is at least one of organic electro-light emitting element. Disclosure agent (24) said polymerization, catalyst, cationic nitrogen, oxygen ions, having transition metal and selected from the group consisting of a cationic a relatively said (23) then, the frame is made up an ion anionic is organic electro-light emitting element. dog layer 2 adjacent (25) said, hole injection layer hole transport layer, a hole transport layer and a light emitting one of the layers said (19) ∼ (24) is at least one of organic electro-light emitting element. A metal complex (in cooperation) including said (25) (26) said a luminescent layer is organic electro-light emitting element. Is electron acceptor compound is ion compound (27) said, said hole transport compound to which may be oxidized electronic 1 characterized by said (24) ∼ (26) is at least one of organic electro-light emitting element. (28) said organic electro-light emitting element the substrate of the, said (19) ∼ (27) a resin film is at least one of organic electro-light emitting element. (29) white emission colors of organic electroluminescence element is characterized by said (16) ∼ (18) either or said (19) ∼ (28) is either organic electro-light emitting element. (30) the substrate of the organic electroluminescence element, a flexible substrate a characterized by said (16) ∼ (18) either or said (19) ∼ (27) is either organic electro-light emitting element. (31) said (15) ∼ (20) either or said (19) ∼ (30) is either organic electro-light emitting element, and display device. (32) said (15) ∼ (20) either or said (19) ∼ (30) either organic electro-light-emitting element is an illumination device. (33) said (32) described illumination device and, display means includes a sampling device liquid crystal device. A disclosure herein include, 1 October 2009 to Japan patent application number 2009-229483 automatically controls a heater, an application 14 January 2010 and automatically controls a heater, an application to Japan the subject matter described patent application number 2010-5846 with respect, a citation by their disclosure is lessening ultra thereto. According to the present invention, stably for a long period of time, reducing in the drive voltage over time a is connected to one side of the organic electronic device, so that it can be utilized, produced through coating-fluid application and can be cured at low temperature is an organic electronic materials and/or, a multilayered same organic electronic device, organic electroluminescence element, display device and lighting device may provide a. I.e., ion compound and by containing red charge transport compound, stably for a long period of time, reducing driving voltage a is connected to one side of the organic electronic device, in particular organic EL device is formed. Furthermore, according to the present invention, multilayer structure formed additive is soluble in the organic EL device can be achieved and, as a material of a luminescent layer efficiency or longer emission life a space between the anode EL device is formed of a, having its properties using the same display device, lighting device, and display device may provide a. Figure 1 shows a example of the programmable reference circuit is also multilayer organic EL is indicative of the mimetic. <Organic electronic material > A organic electronic material of the present invention, at least, ion compound and charge-transporting unit compounds with (hereinafter, charge-transporting compound is constitution: a method) as organic electronic material containing, said ion compound is, and comprised of a relatively high cationic relative, said relative cation, H+, catalyst, cationic nitrogen, oxygen ions, transition metal having either a cationic 1 species or 2 is characterized in that the at least one inert gas. In hereinafter, each component of the organic electronic material of the present invention on a. [Ion compound] Compounds with a positive in the present invention, the aforementioned relative. comprised and relatively cationic. And, the cations relative, H+, catalyst, cationic nitrogen, oxygen ions, transition metal having either a cationic one or 1 2. at least one inert gas. Hereinafter, each cationic relates to. (Catalyst) Catalyst as, class number 1 catalyst, catalyst class number 2, number 3 is the exemplary catalyst class. Is selected from them, catalyst class number 2, number 3 catalyst class of material when combination with anion refers to stability, polymer is cured at low temperatures is agent disclosure and because the, . being most preferred catalyst class number 3. Furthermore, cationic [...] tree, tree (methyl phenyl) carboxylic [...] cationic, tree (dimethyl-phenyl) carboxylic [...] can be examples of cationic. (Cationic nitrogen) As cationic nitrogen NH4+, cationic nitrogen class number 1, number 2 class nitrogen cationic, cationic nitrogen class number 3, is the exemplary cationic nitrogen class number 4. Wherein, the cations nitrogen class number 1 N+ hydrogen atom is the arrangement may be such that two 3, hydrogen combination of other associated with the atom except an mm for matching, the cations nitrogen class number 2 N+ the arrangement may be such that two hydrogen atom is 2, hydrogen combination of other associated with the atom except an mm for matching, the cations nitrogen class number 3 N+ the arrangement may be such that two 1 hydrogen atom is, hydrogen combination of other associated with the atom except an mm for matching, the cations quaternary ammonium number 4 N+ associated with the atom except an is hydrogen exhibits compound. Specifically, n-butyl ammonium, dimethyl ammonium, trimethyl ammonium, tri-ethyl ammonium, tree isopropyl ammonium, tri-n-butyl ammonium, tetramethyl ammonium, tetra ethyl ammonium, tetra-n-butyl ammonium, N, [...] -dimethyl N, tetramethyl ammonium, ethyl tree methyl ammonium , d ethyl D methyl ammonium , tri-ethyl ammonium, tetra ethyl ammonium, trimethyl-n-propyl ammonium, trimethyl isopropyl ammonium, trimethyl-n-butyl ammonium, tree methyl isobutyl ammonium, trimethyl-tert-butyl ammonium, trimethyl-n- [...] , dimethyl di-n-propyl ammonium, dimethyl dee small pro the ammonium which will bloom , dimethyl-n-propyl isopropyl ammonium, methyl tri-n-propyl ammonium, methyl tree cow pro the ammonium which will bloom can be, for example, water. Furthermore, [...] -methyl N, N, [...] -dimethyl N, N, [...] methyl-4--dimethyl N, N, [...] -N, N, [...] -N, N, N, [...] -N such as may to [...]. Furthermore, passivation pyrido, [...] -methyl N, [...] -butyl N, [...] methyl-4--methyl N, [...] -benzyl N, [...] -butyl N methyl-3-, 2-methyl [...] , [...] methyl 3-, 4-methyl [...] , [...] dimethyl 2, 3-, 2, 4-dimethyl [...] , [...] dimethyl 2, 6-, 3, 4-dimethyl [...] , [...] dimethyl 3, 5-, 2, 4, 6-tree [...] , [...] 2-fluoro, 3-fluoro [...] , [...] fluoro 4-, 2, 6-difluorotris pyrrolopyridines lithium, 2, 3, 4, 5, 6- [...] , [...] chloro 2-, 3-chloro [...] , [...] chloro 4-, 2, 3-dichloro [...] , 2, 5-di, passivation [...][...] Х, [...] dichloro 2, 6-, 3, 5-dichloro [...] , 3, 5-dichloro -2, 4, 6-trifluoropyridine lithium, lithium fur Wibro 2-, 3-Wibro fur lithium, lithium fur Wibro 4-, 2, 5- [...] , [...] 2, 6-, 3, 5- [...] , 2- [...] cyano, [...] 3-cyano, 4- [...] cyano, [...] 2-hydroxy, 3- [...] hydroxy, [...] 4-hydroxy, 2, 3- [...] , [...] 2, 4-, 2-methyl-5-ethyl [...] , [...] 2-chloro-3-cyano, 4-carboxylic [...] , 4-carboxylic [...] , [...] phenyl 2-, 3-, passivation [...][...] , [...] phenyl 4-, 2, 6- [...] , [...] nitro 4-, 4- [...] , 4-vinyl [...] , 4- [...] , [...] -butyl 4-tert, 2, 6-di-tert-butyl [...] , [...] benzyl 2-, 3-acetyl [...] , [...] ethyl 4-, 2-carboxylic [...] , [...] carboxylic 4-, 2-benzoyl [...] such as may to [...] pyrido. Furthermore, imidazolium, 1-methyl-imidazol thulium, 1-ethyl-3-methyl imidazolium, 1-profile already will be and the loom which will doze methyl-3-, 1-butyl-3-methyl already will be and the loom which will doze , 1-cyclohexyl-3-methyl already will be and the loom which will doze , 1-methyl-3- [...] , already will be and the loom which will doze -benzyl N methyl-1-, 1-methyl-3-(3-phenylpropyl) imidazolium, 1-butyl -2, 3-dimethyl already will be and the loom which will doze , imidazole such as 1-ethyl -2, 3-dimethyl already will be and the loom which will doze may to thulium. Furthermore, 1-ethyl-1-methyl pyrrolidone lithium, such as lithium methyl pyrrolidone 1-butyl-1-can be, for example, lithium pyrrolo. Furthermore, styrylquinolinium, ISO styrylquinolinium such as may to [...]. And, N, N-dimethyl pyrrole lithium, lithium-methyl pyrrolidone N-ethyl-N, N, such as, for example, lithium pyrrolo [...] -N can be mentionned. Furthermore, call international publication number 03/005076, international publication number 03/097580 described in a diimmonium, amino structure is patterned can be exemplified. One from the, cationic nitrogen class number 3, number 4 class nitrogen cation, when combination with anion refers to stability, is agent capable of cured at low temperatures and because the disclosure, . being most preferred cationic nitrogen class number 3. (Oxygen ions) Oxygen ions as, tree [...] , [...] tree, tree [...] , [...] tree, tree [...] , tree [...] , blood reel loom , chroman (chromenylium) maul Neel loom , [...] can be is exemplified (xanthylium). (Transition metal having a cationic) Transition metal having a cationic as, for example, (η5-cyclopentadienyl) (η6-toluic) Cr+, (η5-cyclopentadienyl) (η6-xylene) Cr+, (η5-cyclopentadienyl) (η6-1-methylnaphthalene) Cr+, (η5-cyclopentadienyl) (η6-cumene) Cr+, (η5-cyclopentadienyl) (η6- [...]) Cr+, (η5-cyclopentadienyl) (η6-pyrene) Cr+, (η5-fluorenyl) (η6-cumene) Cr+, (η5-indenyl) (η6-cumene) Cr+, bis (η6- [...]) Cr2+, bis (η6-xylene) Cr2+, bis (η6-cumene) Cr2+, bis (η6-toluic) Cr2+ 888000236088 8-toluene) (η6-xylene) Cr2+, (η6-cumene) (η6-naphthalene) Cr2+, bis (η5-cyclopentadienyl) Cr+, bis (η5-indenyl) Cr+, (η5-cyclopentadienyl) (η5-fluorenyl) Cr+ and (η5-cyclopentadienyl) (η5-indenyl) Cr+ such as Cr compounds and, in addition (η5-cyclopentadienyl) (η6-toluic) Fe+, (η5-cyclopentadienyl) (η6-xylene) Fe+, (η5-cyclopentadienyl) (η6-1-methylnaphthalene) Fe+, (η5-cyclopentadienyl) (η6-cumene) Fe+, (η5-cyclopentadienyl) (η6- [...]) Fe+, (η5-cyclopentadienyl) (η6-pyrene) Fe+, (η5-flue and methylenyl) (η6-cumene) Fe+, (η5-indenyl) (η6-cumene) Fe+, bis (η6- [...]) Fe2+, bis (η6-xylene) Fe2+, bis (η6-cumene) Fe2+, bis (η6-toluic) Fe2+, (η6-toluene) (η6-xylene) Fe2+, (η6-cumene) (η6-naphthalene) Fe2+, bis (η5-cyclopentadienyl) Fe2+, bis (η5-indenyl) Fe+, (η5-cyclopentadienyl) (η5-fluorenyl) Fe+ and (η5-cyclopentadienyl) (η5-indenyl) Fe+. compounds such as Fe. (Relative anion) Anion using a relative to the present invention relates to. As anion, publicly known conventional is anything surface which a portion of the anions of, for example, F-, Cl-, Br-, I- of a halogen, such ion; OH-; ClO4-; FSO3-, ClSO3-, CH3 SO3-, C6 H5 SO3-, CF3 SO3- such as sulfonic acid ion current; HSO4-, SO42- such as sulfate ion current; HCO3-, CO32- such as carbonate ion current; H2 PO4-, HPO42-, PO43- such as of phosphate ion current; PF6- 888000 24438885 OH- such as of fluorothermoplastic of phosphate ion current, [CF3 CF2)3 PF3]-, [CF3 CF2 CF2)3 PF3]-, [(CF3)2 CF)3 PF3]-, [(CF3)2 CF)2 PF4]-, [(CF3)2 CFCF2)3 PF3]- and [(CF3)2 CFCF2)2 PF4]- such as fluorinated alkyl fluoro of phosphate ion current; BF4-, B (C6 F5)4-, B (C6 H4 CF3)4-[...]ion type such as ion current; AlCl4-; BiF6, SbF6-, SbF5 OH- antimony of fluorothermoplastic such as of low-fogging soft polyester-current, or AsF6-, AsF5 OH-. current of low-fogging soft polyester-arsenic of fluorothermoplastic such as. Using a relative to the present invention is not particularly limited in the anion but, organic electronic device of, an increase in the life of and the foregoing composition when combination with an anionic, disclosure polymer is cured at low temperatures is agent preferably structure of hereinafter. PF6-, PF5 OH- such as of fluorothermoplastic of phosphate ion current, [CF3 CF2)3 PF3]-, [CF3 CF2 CF2)3 PF3]-, [(CF3)2 CF)3 PF3]-, [(CF3)2 CF)2 PF4]-, [(CF3)2 CFCF2)3 PF3]- and [(CF3)2 CFCF2)2 PF4]- such as fluorinated alkyl fluoro of phosphate ion current; BF4-, B (C6 F5)4-, B (C6 H4 CF3)4- such as of borate ion current; AlCl4-; BiF6, SbF6-, SbF5 OH- such as preferably current of low-fogging soft polyester-antimony of fluorothermoplastic. In ion compound the present invention according to, relative cationic and the counter anion but is not particularly limited in the combination of, hole transport compounds the oxide electronic 1 acts as electron acceptor compound, organic EL device a low driving voltage and lower, of organic electronic device and, an increase in the life of when combination with an anionic and the foregoing composition, capable of cured at low temperatures and cationic [...] carboxylic as disclosure number PF6-, PF5 OH- such as of fluorothermoplastic of phosphate ion current; BF4-, B (C6 F5) 4-, B (C6 H4 CF3)4- such as of borate ion current, SbF6-, SbF5 OH- combination of current of low-fogging soft polyester-antimony of fluorothermoplastic such as, [...] PF6-, PF5 OH- such as of fluorothermoplastic of phosphate ion current; BF4-, B (C6 F5)4-, B (C6 H4 CF3)4- such as of borate ion current, SbF6-, SbF5 OH- a combination of current of low-fogging soft polyester-antimony of fluorothermoplastic such as preferably, carboxylic [...] cationic and B (C6 F5)4-, B (C6 H4 CF3)4- such as of borate ion current, SbF6-, SbF5 OH- combination of current of low-fogging soft polyester-antimony of fluorothermoplastic such as, [...] B (C6 F5)4-, B (C6 H4 CF3)4- such as of borate ion current, SbF6-, SbF5 OH- of low-fogging soft polyester-antimony of fluorothermoplastic such as more preferably, the combination of. Specifically tree and cationic [...] SbF6- salt, and cationic [...] tree B (C6 F5)4- salt, N, [...] -dimethyl N SbF6- salt, N, [...] -dimethyl N B (C6 F5)4- may to salt. Ion compound the, and may be used alone, at rates of any may mixing at least one 2. [Charge-transporting compound] In the present invention, the charge-transporting compound, charge-transporting unit is compounds with. Rotation "charge-transporting unit", hole or electronic transporting is group with the capacity, hereinafter, is opened and the same relates to. Said charge-transporting unit, hole or electronic transporting is if they have the ability to, but is not particularly limited in, carbazole product having back-substitutions direction, thiophene which is desirably, for example, the general formula (1a) ∼ (7a) having moiety containing sequences represented by it is preferable that the. [Formula 2] In formula, Ar1 ∼Ar20 the, independently aryl or aryl two 2 ∼ 30 carbon atoms, or a substituted or unsubstituted arylene groups of, exhibits [...] hetero. Wherein, membered 1 hydrogen atom from aromatic hydrocarbons is group removed dog, hetero membered, heteroatom an aromatic compound having an dog 1 hydrogen atom from the removed group, or R blades, presenting a. R are each independently-R1, -OR2, -SR3, -OCOR4, -COOR5, -SiR6 R7 R8 or general formula (2a) ∼ (4a) (stage, R1 ∼ R8 the, hydrogen atom, carbon atoms 1 ∼ 22 of linear, annular or branched alkyl, of carbon number two aryl or heteroaryl radical difference from an 2 ∼ 30) blades, presenting a. Wherein, hydrogen atom from aromatic hydrocarbons [...] 2 dog is group removed, hetero [...] , heteroatom an aromatic compound having an 2 hydrogen atom from the dog is group removed. The X during said R, hydrogen atoms which has ground into 1, 1 containing one hydrogen atom exhibits groups further remove a. Furthermore, solubility charge-transporting compound in the present invention, film forming capability in terms of polymer or oligonucleotides it is preferred that a minute particle. Furthermore, polymer or oligomers in the presence of the ethers solvent including a repeating unit expressed by it is preferred that a. [Formula 3] [Formula 4] [Formula 5] [Formula 6] In formula said, Ar1 ∼Ar100 the, independently aryl or aryl two 2 ∼ 30 carbon atoms, or a substituted or unsubstituted arylene groups of, exhibits [...] hetero. Wherein, membered 1 hydrogen atom from aromatic hydrocarbons is group removed dog, hetero membered, heteroatom an aromatic compound having an dog 1 hydrogen atom from the removed group, or R blades, presenting a. R are each independently-R1, -OR2, -SR3, -OCOR4, -COOR5, -SiR6 R7 R8 or general formula (2a) ∼ (4a) (stage, R1 ∼ R8 the, hydrogen atom, carbon atoms 1 ∼ 22 of linear, annular or branched alkyl, of carbon number two aryl or heteroaryl radical difference from an 2 ∼ 30) blades, presenting a. Wherein, hydrogen atom from aromatic hydrocarbons [...] 2 dog is group removed, hetero [...] , heteroatom an aromatic compound having an 2 hydrogen atom from the dog is group removed. The X during said R, hydrogen atoms which has ground into 1, 1 containing one hydrogen atom exhibits groups further remove a. The Y group of 3, the Z exhibits withdrawing substituent is 2. Furthermore, the x, exhibits by formula 1. Furthermore, said polymer or oligomers in order to vary the solubility, one or more 1 "polymerizable substituent" preferably has an.. Wherein, said "polymerizable substituent" the, by the polymerization of the 2 molecules or more molecules to form bonds between a substituent capable of liberating upon group, hereinafter, is opened and the same relates to. Said polymerizable substituent as, to having carbon-carbon multiple bond (for example, vinyl groups, acetylene to, vice-reel Neel , acrylic group, acrylic 3,500, acrylic amide , metadata [...] , metadata [...] , metadata [...] , [...] , 2001, vinyl ether , vinyl amino group, [...] , pyrrole to, thiophene to, negative and the like thread roll), hope fulfillment (small-membered ring) ring having a (for example, cyclo profile writing, cyclo butyl , epoxy group, oxetane to, [...] diketone, such as epitaxial opinion feed), to lactone, lactam to, or siloxanes containing acid derivatives may to machine or the like. Furthermore, aforementioned in addition to an elongated, ester joining or amide linkage a combination of, or comprises a non-thermoplastic can be using a liquid crystal display displays. For example, ester, amino group, ester, is even a combination of such as hydroxyl groups. Polymerizable substituent as, in particular, to oxetane, epoxy group, vinyl, vinyl ether , acrylic 3,500, metadata preferably more in terms of reactive [...] , oxetane is preferably in particular. Furthermore, in the present invention form or oligomer polymerization layer, heat resistance, and solubility, of an electrical characteristic tables to provide a method for generating, in addition to said repeating units, said 5 arylene groups, hetero arylene groups, or said general formula represented by structure having as repeating unit adopts the copolymer is even copolymer. In this case, include copolymer, random, block or graft copolymer is even, their intermediates polymer structure and, for example, even random copolymer to-block is. Furthermore, in the present invention for use on or oligomer, on having three or, end is even one or more 3. (Solvent) Solvent used in the present invention, chloroform, methylene chloride, dichloroethane, tetrahydrofuran mixable, toluene, xylene, [...] , not brush, toll phenate, acetone, methyl ethyl ketone, acetic acid ethyl, butyl acetic acid, acetic acid ethyl, acetic acid n-butyl, lactic acid ethyl, lactic acid n-butyl, γ butyrolactone, ethyl [...] acetate, phenyl acetic acid, propionic acid phenyl, benzoic acid methyl, ethyl benzoic acid, benzoic acid profile, benzoic acid n-butyl diphenylmethane, d phenyl ether , N, N-dimethylformamide, N, cement clinker using ironworks slag N, ethylene [...] may to or the like. These which of the 1 species and may be used alone, as any combinations and at least one 2 is used in may. (Ratio) Ion compound the, charge-transporting compound for 100 parts by mass of when, 0.01 parts by mass of to 50 and is preferably a mass, 0.05 parts by mass of more preferably to 25 parts by mass of, 0.1 parts by mass to 20 parts by mass of. is most desirable if. Ion compound combination of driving if less than 0.01 parts by mass of ratio constant-current and no effect can be achieved, exceeds the 50 parts by mass of the matrix polymer and tends to driving voltage to rise. Ion derivative when use as disclosure number, has a compound having a polymerizable substituents 100 parts by mass of, is preferably a mass 50 to parts by mass of 0.1.. 0.1 parts by mass of in less than, is not progress sufficiently polymerization, exceeds the parts by mass of 50, . prevented from releasing with symmetrical force process is performed. And, ion derivative, polymerization processes employing the disclosure number as in method, heating only preferably disclosure polymerization. (Other component) Ion compound the, disclosure agent electron acceptor having functions for polymerization of wet liquid to flow down. These of the invention either alone in and may be used, may a plurality combination. Furthermore, polymerization of out-of-range of the disclosure of the present invention may include a electron acceptor connections necessary hitherto is. Furthermore, cross necessary is may include a material or a light emitting material. <Organic electroluminescence element > Organic electroluminescence element of the present invention (hereinafter, "organic EL device" constitution: a method) the, aspects and number 1 is solar of 2 aspects of number 2, number 1 solar from the described first. [Number 1 aspects of organic EL device] Aspects of organic EL element of the present invention number 1, the aforementioned of the present invention organic electronic material a layer formed (hereinafter, which may be by layer polymerization) is characterised in that it has a having. Organic EL element of the present invention, light emitting layer, polymerization layer, anode, cathode, when the substrate which is not particularly limited in, hole injection layer, electron injection layer, hole transport layer, electron transport, such as a layer of other layers may respectively have functional is. Hereinafter, each layer and. rapidly and to reduce a memory. [Light emitting layer] Using material for the emitting layer, and 2006 molecular weight compound, polymer or oligomer is even, dendrimer uses.. Fluorescence using as molecular weight compound, vinyl chloride resin, coumarin, [...] , quinacridone, dye laser pigment (for example, derived rhodamine b, such as DCM1), aluminum complex [for example, tris (8-hydroxy [...]) aluminum (III) (Alq3)], stilbene, may to their derivatives. Fluorescence using polymer or oligomer as, polyfluorene or, polyphenylene, polyphenylene vinylene (PPV), poly-vinylcarbazol (PVK), fluorene-benzothiadiazole copolymer, fluorene-triphenyl amine copolymer, and their derivatives or mixtures preferably ID. On the other hand, recent organic EL for efficiency of a device, organic phosphor development of EL device also actively performed. Phosphor organic EL element, singlet state energy as well as energy of triplet state is connected to the using a liquid crystal display displays, until by making a data amount 100% internal quantum yield is capable of enhancing the light. Phosphor organic EL element, by bringing a phosphor the use of iridium or platinum as impurities including metal tightly the system phosphor material, a phosphorescent light-emitting host material by doping an. of eliciting a (reference non-patent literature 3 ∼ 5). Even organic EL device of the present invention, efficiency, in terms of, using phosphor material emitting layer it is preferable that the. As phosphor material, including a central metal such as Ir or Pt such as metal complex can be carbon atoms are preferably used. Specifically, as complex Ir, for example, blue light emitting a FIr (pic) 'iridium (III) bis [4, 6-difluorophenyl)-amidinates-N, C2] aminopicolinates' , green emitting a Ir (ppy)3 'fac-tris (2-phenylpyridine) iridium' (reference non-patent literature 4) or Adachi et al. , Appl. Phys. Lett. , 78 no.11, a red-luminous is 2001, 1622 (btp)2 Ir (acac) {bis '2-(2' -benzo [4, 5-α] thienyl) N-amidinates pyrido, C3 'iridium (acetylacetonate)}, Ir (piq)3' tris (1-phenyl isoquinoline) iridium ' may to or the like. As complex Pt, for example, red-luminous a 2, 3, 7, 8, 12, 13, 17, 18--21H ethyl octahydro, such as [...] 23H-formyl (PtOEP). Phosphor material, molecular or [...] (dendride compound), for example, iridium nuclear dendrimer may be used. Furthermore, also preferably derivatives thereof, use can be made of, the. Furthermore, if any of the phosphor material emitting layer, in addition to phosphor material, it is preferred that a including host material. As host material, and 2006 molecular weight compound, is even polymeric compounds, use can be made of, for room to guide the dendrimer. As molecular weight compound, for example, CBP (4, 4 '-bis (carbazole-9-one)-biphenyl), mCP (1, 3-bis (9-carbazole) benzene), CDBP (4, 4' -bis (carbazole-9-one)-2, 2'-dimethyl biphenyl) and fixing, as polymeric compounds, for example, poly-vinylcarbazol, polyphenylene, polyfluorene or fixing which, derivatives thereof may also be employed.. Luminescent layer, and may be formed by vapor deposition, may formed by produced through coating-fluid application. In the case of forming the formed, organic EL device can be manufactured at a low cost, more preferably, the. Produced through coating-fluid application emitting layer formed according to in order, and phosphor material, solution including host material as needed, for example, an ink-jet printing method and, Multicast method, the dipping law , convex plate printing, intaglio printing, offset printing, lithography, intaglio shift offset printing, screen printing, gravure printing a print method, of publicly known method such as spin coating a gas desired by applying the composition onto a (bodies) can be performed. The method an applicator such as a front board by establishing an optical fiber, typically, temperature range -20 ∼ 300 °C, preferably 10 ∼ 100 °C, in particular which can be attached to and detached in 15 ∼ 50 °C preferably, as solvents usable in addition said solution, specially but are not limited to, for example, chloroform, methylene chloride, dichloroethane, tetrahydrofuran mixable, toluene, xylene, [...] , not brush, acetone, methyl ethyl ketone, ethyl acetic acid, acetic acid butyl, ethyl [...] acetate, diphenylmethane, d phenyl ether , such as tetralin. Furthermore, after application, by oven or hot plate at a temperature range of + 30 ∼ 300 °C for removal of solvents by heating the may. [Polymerization layer] The polymerizable layer, the aforementioned of the present invention organic electronic material on a board is layer which has been as the additive. And, in the present invention, said organic electronic as charge-transporting compound for one hour to give, the cross-linkable substituents at least one polymer 1 having charge-transporting compound which is desirably, here the charge-transporting compound for including organic electronic material formed using a polymerization. rapidly and to reduce a memory layer. Rotation layer polymerization, specifically, 1 polymerizable preferred that one or more charge-transporting compound material including organic electronic, described method for forming thin film of refers to formed a gas phase of material and then applying the desired, light irradiation or heating circuit from the main, charge-transporting compound is the cross-linkable substituents polymerization of polymers with the where the progress of the reaction, the solubility change layer is layer (cured). Establishing an optical fiber at a side, charge-transporting compound is polymers with the cross-linkable substituents where the progress of the reaction polymerization of the, layer (cured) by change solubility, thermal stability of said layer and enables to improve skinning properties. Furthermore, by by lowering the, light emitting, such as a layer of other layers coated on one surface thereof even if polymerized by coating liquid since do not dissolve in layer, said formed other layers can be. I.e., to facilitate multilayer structure formed in this case, high efficiency, low drive voltage and a long life organic EL element, .can be produced at low cost. Polymerization compounds charge-transporting layer, in terms of flowing TEOS to 95-105 sccm, hole transport with repeating units preferably including polymer or oligomer. said polymerizable layer, organic EL of, hole injection layer, hole transport layer, electron transport layer, electron injection layer applied, luminous efficiency, in terms of life characteristics, in particular hole injection layer, it is preferred that a hole transport layer. Hole injection layer and hole transport layer and interlayer dielectric and is composed of gold, in which either of the polymerization, polymerization to form the both is interlayer dielectric and is composed of gold. Furthermore, film thickness of these layers, but is no limited to, extracts of Barks of, preferably is 10 ∼ 100 nm, it is still more preferably in 20 ∼ 60 nm, most preferably is 20 ∼ 40 nm.. Furthermore, said polymerizable layer, phosphor material including light emitting layer for on top of one another without being be more preferably. The present invention layer, the luminescence efficiency of the light-phosphor material, to reduce a may be achieved, wherein an influence degradation of, the P-type semiconductor layer, in an improved service life of or element and noise. since. [Substrate] Organic EL device of the present invention as a substrate can be used in, glass, plastics or the like which is not particularly limited in the kind of, in addition, if transparent but is no limited to, extracts of Barks of, glass, quartz, light-transmitting resin film or the like which is carbon atoms are preferably used. In which the resin film has been formed on a second substrate, organic EL device is capable of imparting the flexible-since (flexibility), preferably in particular. As resin film, for example, polyethylene terephthalate (PET), polyethylene naphthalate (PEN), polyethersulfone (PES), polyether-imide, polyether-ether-ketone, polyphenylene bisulphate feed, polyarylate, polyimide, polycarbonate (PC), cellulose (TAC) tree acetate , cellulose [...] such as film such as (CAP). Furthermore, resin film when the mother pipe having a, steam or oxygen and so on, and which is intended to restrict transmission, such as silicon nitride or silicon oxide alcohol based resin film to an inorganic material that coating may uses. [Cathode] As negative electrode material, for example, Li, Ca, mg, Al, In, Cs, Ba, mg/Ag, LiF, CsF such as metal or metal alloy is preferably not less. [Anode] As anode, metal (for example, Au) or metal conductivity which have different material, for example, oxide (for example, ITO: indium oxide/tin oxide), conductive polymer (for example, poly thiophen-propolis [...] mixture (PEDOT: PSS), use can be made of,. [Electron transport layer, electron injection layer] Electron transport layer, electron injection layer is, for example, phenanthroline derivatives (for example, 2, 9-dimethyl -4, 7-diphenyl -1, 10-phenanthroline (BCP), bipyridine derivatives, nitro substituted fluorene derivatives, [...] derivatives, thio [...] derivatives, naphthalene-perylene such as heterocyclic tetra carboxylic acid anhydride, carbodiimide, flue [...] derivatives, [...] and variant derivatives, oxadiazole derivatives [2-(4-biphenyl)-5-(4-tert-butyl phenyl-l-1, 3, 4-oxadiazole] (PBD), aluminum complex [for example, tris (8-hydroxy) aluminum (III) (Alq3)] such as. Furthermore, in oxadiazole said, oxadiazole ring of oxygen atoms by sulfur nuclear reactor substituted an diazol derivatives, known [...] having electron-attractive as having quinoxaline derivatives may also be employed.. [Number 2 aspects of organic EL device] Aspects of organic EL element of the present invention number 2, made a multiple layered structure, dog layer 2 a for adjacent ones of said multilayer structure, each polymerizable preferred that the hole-transporting compound for including produced through coating-fluid application mixture is formed by, each hole transport by polymerizing the polymerizable compound disposed one upon the other and are made out of a material which. Hereinafter, as well as solar number 1, polymerizable preferred that the hole-transporting compound for including mixture is formed of formed, said hole transport naphthalene derivative the solubility being changed. by layer polymerization layer. Of the present invention number 2 aspects of organic electro-light-emitting device comprises a multilayer structure but is of, each layer is said multilayer structure, light emitting layer, polymerization layer, anode, cathode, when the substrate which is not particularly limited in, hole injection layer, electron injection layer, hole transport layer, electron transport layer, hole inhibiting layer, electronic stop layer, exciton ([...]) another payload such as stop layer is may respectively have functional layer. Hereinafter, each layer and. rapidly and to reduce a memory. [Polymerization layer] Polymerization layer, i.e. the cross-linkable substituents including compounds having hole transport layer is formed by using a thin mixture, specifically, polymerizable preferred that the hole-transporting compound for including mixture, desired formed a gas phase of material and then applying the, light irradiation or heating circuit from the main, hole transport compound polymers with the cross-linkable substituents where the progress of the reaction polymerization of the, change solubility (curing) layer is the layer. Establishing an optical fiber at a side, hole transport compound polymers with the cross-linkable substituents where the progress of the reaction polymerization of the, layer (cured) by change solubility, thermal stability of said layer and enables to improve skinning properties. Furthermore, by the solubility by lowering, and, in its turn light emitting, such as a layer of other layers coated on one surface thereof even if polymerized by coating liquid since the do not dissolve in layer, said formed other layers can be. I.e., to facilitate multilayer structure formed in this case, high efficiency, low drive voltage and a long life organic EL element, .can be produced at low cost. A hole-transporting compound the polymerization layer, in terms of flowing TEOS to 95-105 sccm, hole transport with repeating units preferably including polymer or oligomer. Furthermore, at least one 2 polymerizable preferred that the hole-transporting compound into a desired function is mixed at the ratio of and may be used, polymerizable substituents with hole transport which has no may used as a light-weight. said polymerizable layer, organic EL of, hole injection layer, hole transport layer, electron transport layer, electron injection layer applied, luminous efficiency, in terms of life characteristics, in particular hole injection layer, it is preferred that a hole transport layer. Hole injection layer and hole transport layer and interlayer dielectric and is composed of gold, in which either of the polymerization, polymerization to form the both is interlayer dielectric and is composed of gold. Furthermore, film thickness of these layers, but is no limited to, extracts of Barks of, preferably is 10 ∼ 100 nm, it is still more preferably in 20 ∼ 60 nm, most preferably is 20 ∼ 40 nm.. The present invention according to the "adjacent 2-storey", hole injection layer hole transport layer, hole transport layer and a light emitting one of the layers is preferably not less. Furthermore, polymerization disclosure disclosure polymerization connections necessary hitherto an acid by decomposition temperature agent disclosure or polymers, organic EL compound layer-type semiconductor layer, and, in particular metal complexes by reacting is formed or longevity luminous efficiency can be the thought of. Therefore, adjacent 2 during-storey, polymerization just a layer close to the anode disclosure agent, this layer of polymers which can be dispense from the active agent to connections necessary hitherto disclosure by polymerizing other layer by, while also being capable of multi-layer board, the light emitting layer are less influence agent disclosure polymerization, having superior or luminous efficiency organic EL device can be produced. In the present invention, polymerization processes employing the cross-linkable substituents having hole transport compounds including mixture, therefrom or a layer to control the properties as needed to, leveling or vesicles (leveling agent) number ([...]), crosslinked number, electron acceptor compound, high molecular weight in a region which faces rare metal with the capacity transport polymers that carry may member is installed in the load lock. In the present invention using polymerizable preferred that the hole-transporting compound of email widow, a web page or "polymerizable substituent", by the polymerization of the 2 molecules or more molecules to form bonds between a substituent capable of liberating upon group, hereinafter, . rapidly and to reduce a memory. As said polymerizable substituent, the aforementioned of the present invention description of material organic electronic "polymerizable substituent" in the same, . similar to that of the a preferred embodiment. Polymerizable substituents may, of applied is obtained by polymerization of the 2 molecules form a bisphenyl bond between or more molecules, coating film which serves to fasten the solubility change (curing) make. In order to vary the solubility coating film, 1 per molecule one or more polymerizable substituent is 2 is preferably not less. In the present invention using the "hole-transporting compound", with the capacity transport in a region which faces including which is rare metal, hereinafter, . rapidly and to reduce a memory. Said "in a region which faces transport with the capacity group" the, the ability to transport in a region which faces is if they have, specially but are not limited to, aryl amine, carbazole, thiophene which is desirably, for example, the general formula (1) ∼ (58) having moiety containing sequences represented by it is preferable that the. [Formula 7] [Formula 8] [Formula 9] [Formula 10] [Formula 11] In formula, E independently from each other-R1, -OR2, -SR3, -OCOR4, -COOR5, -SiR6 R7 R8 or formula (59) ∼ (61) (stage, R1 ∼ R11 the, hydrogen atom, 1 ∼ 22 carbon atoms of linear, annular or branched alkyl, of carbon number 2 ∼ 30 and aryl group or aryl two, the c and b and a, exhibits by formula 1. Wherein, membered, 1 hydrogen atom from aromatic hydrocarbons is group removed dog, is may respectively have functional groups substituted, hetero membered, heteroatom an aromatic compound having an 1 hydrogen atom from the dog is group removed, is may respectively have functional groups substituted), or "polymerizable substituent". shows 0. The Ar, 5 arylene groups of 2 ∼ 30 carbon atoms independently, exhibits or [...]. [...] hydrogen atom from aromatic hydrocarbons 2 dog preferred that is group removed may, for example, polyphenylene, it will be a non-phenyl D , it will be a phenyl D , naphthalene diyl, anthracene diyl, tetra it will be a D it counts , flue [...] , such as [...]. Hetero membered, heteroatom hydrogen atom from an aromatic compound having an 2 dog preferred that is group removed may, for example, pyridine diyl, it will be an aphid pyrazole, quinoline diyl, it will be a quinoline D isocyanate, arc [...] , phenanthroline diyl, furan diyl, pyrrole diyl, thio pended the, oxa it will be a D it will doze , oxadiazole diyl, thia will be and Oh it will be a D it will doze , it will be a D it will doze triazine, benzo the jade company it will be a D it will doze , benzo jade company D Oh it will be a D it will doze , benzo mote Ada Oh it will be a D it will doze , benzo tree Oh it will be a D it will doze , such as benzo it will be a mote five pen D. The Z and X, independently from each other during said R, hydrogen atoms which has ground into 1, 1 containing one hydrogen atom representing groups further remove a, the X an integer of 0 ∼ 2. During the Y said X or Z, ground into which has 2 hydrogen atoms containing one hydrogen atom removing 2 exhibits groups. [Formula 12] The "hole-transporting compound", in terms of flowing TEOS to 95-105 sccm preferably is oligomer or polymer. Said oligomer or polymer have a number average molecular weight of, 1,000,000 hereinafter which is desirably 1,000 or more, 1,000 or more, 500,000 hereinafter, and more preferably, 2,000 or more, most preferably is a 200,000 hereinafter.. A number average molecular weight 1,000 crystallized if less than making it easier for the player to maintain the stability film is formed, exceeds a 1,000,000 decoding unit sequentially solubility in solvent applied. superfine workability. Specific compounds in the presence of the polymer or oligomers (1a) ∼ (84a) including derivative is marked as the chemical it is preferred that a. And, general formula (1a) ∼ (84a) of, Ar, E, X, the x said general formula (1) ∼ (60) Ar in, E, X, is photopolymerization initiator represented by chemical x the same. [Formula 13] [Formula 14] [Formula 15] [Formula 16] [Formula 17] [Formula 18] [Formula 19] [Formula 20] [Formula 21] [Formula 22] [Formula 23] [Formula 24] Furthermore, said polymer or oligomers, heat resistance, and solubility, of an electrical characteristic tables to provide a method for generating, in addition to said repeating units, said 5 arylene groups, hetero arylene groups, or specific compounds in the presence of the structure represented by (C1) ∼ (C30) as repeating unit adopts the copolymer is even copolymer having. In this case, include copolymer, random, block or graft copolymer is even, their intermediates polymer structure and, for example, even random copolymer to-block is. Furthermore, in the present invention for use on or oligomer, having three or on, one or more 3 end. may. [Formula 25] The present invention according to "polymerizable preferred that the hole-transporting compound for including mixture" the, polymerization may be loaded with further disclosure agent. Polymerization to form the disclosure agent, heat, optical, microwave, radiation, by the application of such as electron beam, by polymerizing a polymerizable substituents that is is expression ability, specially but are not limited to, optical irradiation and/or by heating to polymerization it is preferred to set a to disclosure, disclosure polymerization by heat is to enable a more preferably, the. Said polymerization disclosure agent, optical irradiation and/or by heating as is to enable a disclosure polymerization, polymerization in addition by heat as is to enable a disclosure, preferably [...]. Wherein, rotation onium salt in the present invention, for example, sulfonium, [...] , carboxylic [...] , selenium, ammonium, phosphonium, bis [...] comprised and relatively cationic such as circulation promoted. a compound. As anion relative, the aforementioned of the present invention organic electronic material description of relative ion compound in equals anion. Sulfonium ion as, tree [...] , tri-p- [...] , tri-o- [...] , tris (4-methoxyphenyl) sulfonium, [...] 1-, 2- [...] , tris (4-fluorophenyl) sulfonium, tree [...] -1-, -2-tree [...] , tris (4-hydroxyphenyl) sulfonium, 4-(phenylthio) phenyl [...] , ([...] -p) 4- [...] -p di-phenyl, 4-(4-methoxyphenylthio) phenyl bis (4-methoxyphenyl) sulfonium, 4-(phenylthio) phenyl bis (4-fluorophenyl) sulfonium, 4-(phenylthio) phenyl bis (4-methoxyphenyl) sulfonium, 4 888 0002615888 phenylthio) phenyl di-p- [...] , bis [4-( [...]) phenyl] sulfide, bis '4-{ bis [4-(2- [...] hydroxy) phenyl] [...] sulfoalkyl} phenyl' sulfide, bis {4-[ bis (4-fluorophenyl) [...] sulfoalkyl] phenyl} sulfide, bis {4-[ bis (4-methyl phenyl) [...] sulfoalkyl] phenyl} sulfide, bis {4-[ bis (4-methoxyphenyl) [...] sulfoalkyl] phenyl} sulfide, 4-(4-benzoyl-2-chloro hydroxyphenylthio) phenyl bis (4-fluorophenyl) sulfonium, 4-(4-benzoyl-2-chloro hydroxyphenylthio) phenyl [...] , 4-(4-benzoyl phenyl mote) phenyl bis (4-fluorophenyl) sulfonium, 4-(4-benzoyl hydroxyphenylthio) phenyl [...] , 7-isopropyl-9-oxo -10-thia -9, 10--2-one di-p- [...]d high draw anthracene , -2-7-isopropyl-9-oxo -10-thia -9, 10- d high draw anthraceneone D phenyl sulfonium, 2-[ (di-p-tolyl) [...] sulfoalkyl] thioxanthone, thioxanthone [ [...] sulfoalkyl (diphenyl)] 2-, 4-[ 4-(benzoyl-butyl 4-tert) phenylthio] [...] -p di-phenyl, 4-[ 4-(benzoyl-butyl 4-tert) phenylthio] phenyl [...] , 4-[ 4-(benzoyl hydroxyphenylthio)] [...] -p di-phenyl, 4-[ 4-(benzoyl hydroxyphenylthio)] phenyl [...] , 5-(4-methoxyphenyl) [...] , [...] phenyl 5-, 5- [...] , 5-(4- [...]) [...] , 5-(2, 4, 6-trimethylphenyl) [...][...] of such as; [...] , diphenyl 4- [...] nitro, [...] , such as diaryl [...][...] ; phenyl [...] , [...] 4-hydroxy, 4- [...] , [...] 4-acetamide, 2- [...] , 2-naphtyl methyl (1-ethoxycarbonyl) ethyl [...] , phenyl [...] , 4-the, silk shoe polish[...][...][...][...][...][...][...] , 4- [...] , [...] 4-acetamide, 2- [...] , 2- [...] , such as 9- [...] mono aryl sulfonium; dimethyl [...] , lung or thread[...] , dimethyl [...] , benzyl [...] , such as tree [...][...] octahydro can to or the like, they are hereinafter disclosed is of literature. For [...] triazine, American patent number 4231951 call specification, American patent number 4256828 call specification, Japanese patent application publication number Official Gazette call 7-61964 flat, Japanese patent application publication number Official Gazette call 8-165290 flat, Japanese patent application publication number Official Gazette call 7-10914 flat, Japanese patent application publication number Official Gazette call 7-25922 flat, Japanese patent application publication number Official Gazette call 8-27208 flat, Japanese patent application publication number Official Gazette call 8-27209 flat, Japanese patent application publication number Official Gazette call 8-165290 flat, Japanese patent application publication number Official Gazette call 8-301991 flat, Japanese patent application publication number Official Gazette call 9-143212 flat, Japanese patent application publication number Official Gazette call 9-278813 flat, Japanese patent application publication number Official Gazette call 10-7680 flat, Japanese patent application publication number Official Gazette call 10-287643 flat, Japanese patent application publication number Official Gazette call 10-245378 flat, Japanese patent application publication number Official Gazette call 8-157510 flat, Japanese patent application publication number Official Gazette call 10-204083 flat, Japanese patent application publication number Official Gazette call 8-245566 flat, Japanese patent application publication number Official Gazette call 8-157451 flat, Japanese patent application publication number Official Gazette call 7-324069 flat, Japanese patent application publication number Official Gazette call 9-268205 flat, Japanese patent application publication number Official Gazette call 9-278935 flat, Japanese patent application publication number 2001-288205 call Official Gazette, Japanese patent application publication number Official Gazette call 11-80118 flat, Japanese patent application publication number Official Gazette call 10-182825 flat, Japanese patent application publication number Official Gazette call 10-330353 flat, Japanese patent application publication number Official Gazette call 10-152495 flat, Japanese patent application publication number Official Gazette call 5-239213 flat, Japanese patent application publication number Official Gazette call 7-333834 flat, Japanese patent application publication number Official Gazette call 9-12537 flat, Japanese patent application publication number Official Gazette call 8-325259 flat, Japanese patent application publication number Official Gazette call 8-160606 flat, Japanese patent application publication number 2000-186071 call Official Gazette (American patent number 6368769 call specification) such as; for [...] diaryl, Japanese patent application publication number Official Gazette call 7-300504 flat, Japanese patent application publication number bovine 64-45357 call Official Gazette, bovine 64-29419 call Official Gazette such as Japanese patent application publication number; for mono [...] , Japanese patent application publication number Official Gazette call 6-345726 flat, Japanese patent application publication number Official Gazette call 8-325225 flat, flat one patent application publication number call 9-118663 Official Gazette (American patent number 6093753 call specification), Japanese patent application publication number Official Gazette call 2-196812 flat, Japanese patent application publication number Official Gazette call 2-1470 flat, Japanese patent application publication number Official Gazette call 2-196812 flat, Japanese patent application publication number Official Gazette call 3-237107 flat, Japanese patent application publication number Official Gazette call 3-17101 flat, Japanese patent application publication number Official Gazette call 6-228086 flat, Japanese patent application publication number Official Gazette call 10-152469 flat, Japanese patent application publication number Official Gazette call 7-300505 flat, Japanese patent application publication number 2003-277353 Official Gazette call, call Official Gazette such as Japanese patent application publication number 2003-277352; for [...] tree, Japanese patent application publication number Official Gazette call 4-308563 flat, Japanese patent application publication number Official Gazette call 5-140210 flat, Japanese patent application publication number Official Gazette call 5-140209 flat, Japanese patent application publication number Official Gazette call 5-230189 flat, Japanese patent application publication number Official Gazette call 6-271532 flat, Japanese patent application publication number bovine 58-37003 call Official Gazette, Japanese patent application publication number Official Gazette call 2-178303 flat, Japanese patent application publication number Official Gazette call 88800 01133888 flat, Japanese patent application publication number Official Gazette call 9-328506 flat, Japanese patent application publication number Official Gazette call 11-228534 flat, Japanese patent application publication number Official Gazette call 8-27102 flat, Japanese patent application publication number Official Gazette call 7-333834 flat, Japanese patent application publication number Official Gazette call 5-222167 flat, Japanese patent application publication number Official Gazette call 11-21307 flat, Japanese patent application publication number Official Gazette call 11-35613 flat, American patent number 6031014 specification may call to or the like. As ion [...] , [...] , di-p- [...] , bis (4-use of Saccharomyces cerevisiae thread phenyl) [...] , bis (4-methoxyphenyl) [...] , (4- [...]) phenyl [...] , bis (4-phenylalkanamide midifiers targeting) [...] , 4-(2- hour tetra the jade city which will be burnt hydroxy) phenyl [...] , [...] 4-isopropyl phenyl (p-tolyl), isobutyl phenyl (p-tolyl) [...] can to or the like, they are, Macromolecules, 10, 1307 (1977), Japanese patent application publication number Official Gazette call 6-184170 flat, American patent number 4256828 call specification, American patent number 4351708 call specification, Japanese patent application publication number bovine 56-135519 call Official Gazette, Japanese patent application publication number bovine 58-38350 Official Gazette call, call 10-195117 flat call burn patent application disclosure Japanese Official Gazette, Japanese patent application publication number 2001-139539 call Official Gazette, Japanese patent application publication number 2000-510516 call Official Gazette, Japanese patent application publication number 2000-119306 call Official Gazette disclosed is or the like. Carboxylic [...] as cationic, cationic [...] tree, tree (methyl phenyl) carboxylic [...] cationic, tree (dimethyl-phenyl) carboxylic [...] cationic such as may to [...] of. As selenium ions, triphenyl selenium, tri-p- [...] , tri-o- [...] , tris (4-methoxyphenyl) selenium, 1- [...] , tris (4-fluorophenyl) selenium, tree [...] -1-, -2-tree [...] , tris (4-hydroxyphenyl) selenium, 4-(phenylthio) phenyl the D phenyl the rhenium which it will count , 4-( [...] -p) phenyl di-p- [...]the reel the rhenium which it will count of such as; the D phenyl lung will be born and the rhenium which it will count , the D phenyl it cuts quality the rhenium which it will count , such as diaryl the D phenyl methyl the rhenium which it will countthe reel the rhenium which it will count ; phenylmethyl benzyl selenium, selenium Methylbenzyle hydroxyphenyl 4-, phenylmethyl lung or thread selenium, 4-hydroxyphenyl methyl lung or thread selenium, such as 4-mono will be biting and the rhenium which it will count[...] ; dimethyl lung or thread selenium, lung or thread[...] , dimethyl benzyl selenium, benzyl [...] , midifiers targeting [...][...] such selenium trialkyl such [...][...][...] can to or the like, they are Japanese patent application publication number bovine 50-151997 call Official Gazette, Japanese patent application publication number bovine 50-151976 call Official Gazette, Japanese patent application publication number bovine 53-22597 call Official Gazette disclosed is or the like. As well as a source of ammonium ions, for example, tetramethyl ammonium, ethyl tree methyl ammonium , d ethyl D methyl ammonium , tri-ethyl ammonium, tetra ethyl ammonium, trimethyl-n-propyl ammonium, trimethyl isopropyl ammonium, trimethyl-n-butyl ammonium, tree methyl isobutyl ammonium, trimethyl-tert-butyl ammonium, trimethyl-n- [...] , dimethyl di-n-propyl ammonium, dimethyl dee small pro the ammonium which will bloom , dimethyl-n-propyl isopropyl ammonium, methyl tri-n-propyl ammonium, methyl tree cow pro the ammonium which will bloom such as tetraalkylammonium; N, N-dimethyl pyrrole lithium, lithium-methyl pyrrolidone N-ethyl-N, N, such as lithium pyrrolo [...] -N; N, N '-dimethyl already will be and the loom which will doze , N, N' - the D ethyl already will be and the loom which will doze , N-ethyl-N '-methyl already will be and the loom which will doze , 1, 2, 3-tree the methyl already will be and the loom which will doze , the methyl already will be and the loom which will doze tree 1, 3, 4-, 1, 3-diethyl-2-methyl already will be and the loom which will doze , already will be and the loom which will doze methyl-4-diethyl 1, 3-, 1, 2, 3, 4-tetra the methyl already will be and the loom which will doze such as imidazolium; N, N' -dimethyl [...] , N, N '- [...] , N-ethyl-N' -methyl [...] , such as 1, 2, 3-tree [...][...] its; N, N '-dimethyl [...] , [...] -methyl N-ethyl-N, N, morpholinium such as [...] -N; N, [...] -dimethyl N, N-ethyl-N' -methyl [...] , N, N '- [...] such as piperidinium; [...] -methyl N, [...] -ethyl N, [...] -propyl N-n, [...] -N, [...] -butyl N-n, [...] -benzyl N, such as passivation pyrido [...]lung or thread -N; N, N' -dimethyl already will be and the loom which will doze , already will be and the loom which will doze -methyl N-ethyl-N, N, N '- the D ethyl already will be and the loom which will doze , already will be and the loom which will doze methyl-3-diethyl 1, 2-, 1, 3-diethyl-2-methyl already will be and the loom which will doze , already will be and the loom which will doze dimethyl -2, 4--propyl 3-n methyl-1-such as imidazolium; [...] -methyl N, [...] -ethyl N, [...] -propyl N-n, [...] -N, [...] -butyl N-n, [...] -benzyl N, such as [...][...]lung or thread -N; [...] -methyl N, [...] -ethyl N, [...] -propyl N-n, [...] -N, [...] -butyl N-n, [...] -benzyl N, such as isoforms of lung or thread -N [...][...] ; benzyl [...] , tis of such as [...]lung or thread[...] ; benzyl arc lithium, is connected to the semiconductor layer. lithium arc such as lithium arc lung or thread. They are, American patent number 4069055 call specification, Japanese patent number 2519480 call Official Gazette, Japanese patent application publication number Official Gazette call 5-222112 flat, Japanese patent application publication number Official Gazette call 5-222111 flat, Japanese patent application publication number Official Gazette call 5-262813 flat, Japanese patent application publication number Official Gazette call 5-255256 flat, Japanese patent application publication number Official Gazette call 7-109303 flat, Japanese patent application publication number Official Gazette call 10-101718 flat, Japanese patent application publication number Official Gazette call 2-268173 flat, Japanese patent application publication number Official Gazette call 9-328507 flat, Japanese patent application publication number Official Gazette call 5-132461 flat, Japanese patent application publication number Official Gazette call 9-221652 flat, Japanese patent application publication number Official Gazette call 7-43854 flat, Japanese patent application publication number Official Gazette call 7-43901 flat, Japanese patent application publication number Official Gazette call 5-262813 flat, Japanese patent application publication number Official Gazette call 4-327574 flat, Japanese patent application publication number Official Gazette call 2-43202 flat, Japanese patent application publication number bovine 60-203628 call Official Gazette, Japanese patent application publication number bovine 57-209931 call Official Gazette, Japanese patent application publication number flat Official Gazette call 9-221652 disclosed is or the like. Phosphonium ion as, for example, of tetraphenylphosphonium, tetra-p- [...] , tetrakis (2-methoxyphenyl) phosphonium, tetrakis (3-methoxyphenyl) phosphonium, tetrakis (4-methoxyphenyl) phosphonium such as [...] its; [...] tree, tree [...] , [...] tree, tree [...][...] of such as; [...] tree, tree [...] , tetra [...] , tetra [...] , tetra [...] , [...] tree, such as such as tetraalkylphosphonium its [...] tree. They are, Japanese patent application publication number Official Gazette call 6-157624 flat, Japanese patent application publication number Official Gazette call 5-105692 flat, Japanese patent application publication number Official Gazette call 7-82283 flat, Japanese patent application publication number flat Official Gazette call 9-202873 disclosed is or the like. Bis [...] ion as, for example, are depicted in Japanese patent application publication number 2008-214330 call Official Gazette. Using onium salts in the present invention, the aforementioned relative. comprised and relatively cationic. The combination of of charge a combined capable of maintaining the balance for a if specially but are not limited to, hole transport compounds to oxidize the electronic 1, organic EL device a low driving voltage and lower, PF6-, PF5 OH- such as of fluorothermoplastic of phosphate ion current, [(CF3 CF2)3 PF3]-, [(CF3 CF2 CF2)3 PF3]-, [( (CF3)2 CF)3 PF3]-, [( (CF3)2 CF)2 PF4]-, [( (CF3)2 CFCF2)3 PF3]- and [( (CF3)2 CFCF2)2 PF4]- such as fluorinated alkyl fluoro of phosphate ion current 8 8800027058884-, B (C6 F5)4-, B (C6 H4 CF3)4- such as of borate ion current, SbF6-, SbF5 OH- such as preferably current of low-fogging soft polyester-antimony of fluorothermoplastic, in addition-polymerizable substituted thin film of charge-transporting compound having to produce at lower temperatures, sulfonium, [...] , carboxylic [...] , , and more preferably in a combination with cationic such as ammonium which, organic EL element [...] carboxylic order to, an increase in the life of, and ammonium cationic B (C6 F5)4-, B (C6 H4 CF3)4- such as of borate ion current, SbF6-, SbF5 OH- antimony of fluorothermoplastic such as of low-fogging soft polyester-current, F6-, PF5 OH- trifluoride to staple such as of phosphate ion current, [(CF3 CF2)3 PF3]-, [(CF3 CF2 CF2)3 PF3]-, [( (CF3)2 CF)3 PF3]-, [( (CF3)2 CF)2 PF4]-, [( (CF3)2 CFCF2)3 PF3]- and [( (CF3)2 CFCF2)2 PF4]- of phosphate ion fluoro fluorinated alkyl such as combination of. is more preferred to set a. In particular, polymerization disclosure agent, , among other things, onium, catalyst, cationic nitrogen, oxygen ions, having transition metal and selected from the group consisting of a cationic a relatively anionic a ion compound, i.e. the aforementioned of the present invention description of material organic electronic ion in preferred are those compounds. (Solvent) Coating liquid for forming layer polymerization process of preparing the solvent, but is no limited to, extracts of Barks of, specifically, methanol, ethanol, alcohol such as isopropyl alcohol, cyclopentane, hexane, alkane such as octane, cyclohexane an annulus alkane, benzene, toluene, xylene, [...] , tetraline, aromatic solvents such as diphenylmethane, ethylene glycol dimethyl ether, ethylene glycol D ethyl ether , propylene glycol-1-monomethyl ether acetate such as aliphatic ether, [...] 1, 2-, 1, 3- [...] , not brush, toll phenate, 2- [...] n, n [...] 3-, 4- [...] n, chloroanisolein dimethyl 2, 3-, 2, 4-dimethyl chloroanisolein such as aromatic ether, acetic acid ethyl, acetic acid n-butyl, lactic acid ethyl, an aliphatic ester such as-butyl n lactic, acetic acid phenyl, phenyl propionic acid, benzoic acid methyl, ethyl benzoic acid, benzoic acid profile, benzoic acid n-butyl such as aromatic ester, N, N-dimethylformamide, N, such as amide-based solvent cement clinker using ironworks slag N, in addition, dimethyl sulfoxide, tetrahydrofuran mixable, acetone, chloroform, but to or the like methylene chloride, preferably aromatic solvent, aliphatic ether, aromatic ether, an aliphatic ester, aromatic ester is. These which of the 1 species and may be used alone, as any combinations and at least one 2 is used in may. (Ratio) In the present invention, polymerization processes employing the disclosure agent, polymerizable preferred that the hole-transporting compound for 100 during the analysis, are preferably present in the salt 0.01 ∼ 100 mass %, more preferably 0.05 ∼ 50 mass %, . is most desirable if 0.1 ∼ 100 mass %. 0.01 mass % hereinafter in, adhesive sufficiently polymerization, 100 mass % above, the matrix polymer and tends to lowering process is performed. [Light emitting layer, substrate, cathode, anode, electron transport layer, electron injection layer] Aspects of organic EL device of the present invention number 2 forming a, light emitting layer, substrate, cathode, anode, electron transport layer, and the aforementioned electron injection layer equal to 10 Ps and sun number 1, number 1 or a preferred embodiment solar equals room to guide the forming method. Therefore, the aforementioned number 1 solar in, light emitting layer, substrate, cathode, anode, electron transport layer, and an electron injection layer description is, is applying in number 2. [Thin film forming method] Or more number 1 and number 2 aspects of organic EL device, for various hole, for example, solution including organic electronic material which, an ink-jet printing method and, Multicast method, the dipping law , convex plate printing, intaglio printing, offset printing, lithography, intaglio shift offset printing, screen printing, gravure printing a print method, such as spin coating of a desired other method publicly known applicator a gas phase, produced by can be step, the photoresist layer. Furthermore, establishing an optical fiber at a side the production cost is cheap method an applicator such as a thin film circuit from heating or light irradiation a compensation is the polymerization reactor, (curing) change solubility layer.. A workpiece, such as is produced through coating-fluid application by repetition formed by an organic EL element multi-layer can attain is enabled. The method an applicator such as a front board by establishing an optical fiber, typically, temperature range -20 ∼ 300 °C, preferably 10 ∼ 100 °C, in particular preferably in 15 ∼ 50 °C can be embodiment. Furthermore, the light irradiation said, low pressure pressure mercury lamp, medium pressure mercury lamp, high pressure mercury lamp, electron gun for color cathode ray tube, lamp fixing structure of projector, xenon lamp, fluorescent lamp, light emitting diode, solar, use can be made of, light source like. Furthermore, said a heat treatment, in an oven or hot plate which can be attached to and detached, 0 ∼ 300 °C temperature range, preferably 20 ∼ 250 °C, more preferably 50 ∼ 200 °C, most preferably 70 ∼ 150 °C can be embodiment in. Low temperature sufficient curing reaction can be atm adaptation layer type 2, solvent remaining on in poses problems, such as a strong, organic EL is has wall surfaces corresponding to a device. In high temperature, organic resin substrate EL device manufacturing is difficult. Furthermore, the setting reaction of of applied, is only heat treatment, organic EL extend the life time of preferably for. [Emission colors] Organic EL of the present invention color-type semiconductor layer, and are not limited the first substrate are assembled, white light emitting device a home lighting, lighting in an automotive vehicle, various kinds of liquid or liquid crystal watch lighting fixtures can be used in. advantageous because a. White as method a light-emitting element, as current that indicates white light emitting single material since difficult, plurality of light emitting with the aid of side materials emitting plurality of light emitting color simultaneously mixed-colour by white emitted from the light emitting (colors) of wet liquid to flow down. Plurality of light emitting color as of arranging, are not particularly limited, blue, green, red 3 containing a the light emitting maximum wavelength, the blue and yellow, orange and signs of vinyl toluene using the width the light emitting 2 containing a maximum wavelength to may. Furthermore, a control of emission colors, for adjusting amount, the types of phosphor material can be is made by. < 표시 소자, 조명 장치, 표시 장치 > A display element of the present invention, the aforementioned of the present invention organic EL element which has the same. characterized in that. For example, in, rust, free (RGB) corresponding to respective pixels of as devices, organic EL of the present invention recognize the key selected by a user, may yield an color display element. The for forming Image, a matrix form, and to electrodes located is then formed into the panel arranged an individual organic EL device with elder brother simple matrix a CMOS thin film transistor to each element by placing the posteriorly active matrix driving. Electronic (former) the, vertical simple but structure is implemented limit corresponding to the number of pixels used for presenting the a as an n-bit signal. The latter (latter), provided to reduce current, once lower and driving voltage, for forming an Image the accuracy and a high estimation ratio bright, is used as polarizer, and a high-grade display. Furthermore, a lighting device of the present invention, the aforementioned of the present invention organic EL element which has the same. characterized in that. Furthermore, a device display of the present invention, lighting device and, display means liquid crystal element which has the same. characterized in that. Backlight (white luminescent light source) the aforementioned as using a lighting device of the present invention, display means display using the liquid crystal element device, i.e. liquid crystal display device. may be. This configuration allows adjacent, in publicly known liquid crystal display device, backlight only starting to write a lighting device is provided which can produce a semiconductor of the present invention, publicly known techniques liquid crystal device portion can be dedicated ([...]). In the embodiment In hereinafter, embodiment and/or at least two different but more specifically, the present invention, embodiment of the present invention refers to hereinafter not limited to exemplify. [In the embodiment 1] (Polymerizable for evaluating) The compound 1 (4.5 mg) of the toluene solution (400 micro l) and/ion compound 1 (0.45g), the amount of acetic acid, ethyl solution (100 micro l) coating solution is mixing, 3000rpm in quartz plate is spin-coated with the section. Furthermore, on hot plate, to 120 °C and the reaction be conducted is heated to polymerize it was 10 minutes. The toluene: acetic acid ethyl (4:1) 1 in a solvent mixture of an aprotic to quartz plate ([...]) and immersion minutes, was performs cleaning. In spectrum UV-vis of before and after washing of the absorption edge of the (Abs) from the ration of absorption maximum (λmax), it was determined that photopolymerizable. Before cleaning: λmax = 383 nm, Abs = 0.229 After cleaning the: λmax = 383 nm, Abs = 0.228 After cleaning the before/cleaning Abs Abs ×100 of 2,000-300,000 (%)= = 0.228/0.229×100 = 99.6 [Formula 26] [Formula 27] [In the embodiment 2] Hot plate 180 °C heating temperature on a other than point on, both in the embodiment 1 it was determined that photopolymerizable in the same method. [In the embodiment 3] Ion compound 1 instead, 2 ion compound a using other than point a, both in the embodiment 1 it was determined that photopolymerizable in the same method. [Formula 28] [In the embodiment 4] Hot plate 180 °C heating temperature on a other than point on, both in the embodiment 3 it was determined that photopolymerizable in the same method. [Compared e.g. 1] Ion compound 1 instead, using the 3 ion compound to other than point a, both in the embodiment 1 it was determined that photopolymerizable in the same method. [Formula 29] [Compared e.g. 2] Hot plate 180 °C heating temperature on a other than point on, both compared e.g. 1 it was determined that photopolymerizable in the same method. [Compared e.g. 3] Ion compound 1 instead, a ion compound 4 using other than point a, both in the embodiment 1 it was determined that photopolymerizable in the same method. [Formula 30] [Compared e.g. 4] Hot plate 180 °C heating temperature on a other than point on, both compared e.g. 3 it was determined that photopolymerizable in the same method. [Compared e.g. 5] Ion compound 1 instead, a ion compound 5 using other than point a, both in the embodiment 1 it was determined that photopolymerizable in the same method. [Formula 31] [Compared e.g. 6] 180 °C heating temperature on hot plate other than point on a, 5 e.g. compared both the same method it was determined that photopolymerizable in. Table each ion compound using 1, 120 °C and evaluates it photopolymerizable in 180 °C've got the result. The present invention according to ion compound born on a coil, on upper and lower glass using [...] curing number of the existing method, but the method can not effect at low temperature can be acquired for correcting an order.. [Table 1] [In the embodiment 5] Width 1.6 mm for ITO on a glass substrate are patterned by using a photolithography, compound 1 (4.5 mg) of the toluene solution (400 micro l) and ion compound 1 (0.45g), the amount of acetic acid, ethyl solution (100 micro l) coating solution is mixing, 3000rpm the glass substrate is spin-coated with the section. For test data of after the dry nitrogen was carried out under environment. Furthermore, 10 minutes on hot plate to heat a bubble 180 °C to curing the, hole injection layer (40 nm) are formed at the. Furthermore, on hole injection layer, 1 parts by weight of the above polymer by the following structural formula (75 parts by mass of), polymer 2 (20 parts by mass of), polymer 3 (parts by mass of 5) consisting of mixtures, toluene solution (1.0 mass %)at a spin coat and a 3000 rpm, 80 °C on hot plate, to heat a bubble 5 minutes, polymer light emitting layer are formed at the (80 nm film thickness). And, hole injection layer and a light emitting and do not dissolve in floated could electrically a common pin. [Formula 32] (The n, by integer larger than or equal to 1) Furthermore, the resulting glass sheets and is transferred into a wafer deposition substrate, light emitting layer on said Ba (3 nm film thickness), in turn of Al (film thickness 100 nm) are formed at the electrode. Formed on, without photo, be able to the substrate during environment dry nitrogen, countersink of 0.4 mm 0.7 mm of alkali-free glass to form (countersink) for sealing (seal) glass and ITO glass substrate patterning, photocurable epoxy resin is attached on the often performance is carried out a bag by, multi-layer structure polymer type organic EL device have been prepared. For test data of after and noise by decreasing the stroke length, room temperature (25 °C) was performed in. Anodic-ITO of the organic EL, Al used as a cathode in an a voltage is applied to the bar, about 3V was is monitored green emitting in. Luminance 5000 cd/m2 A/9.1 cd the current efficiency in, the driving voltage was 4.9V. Furthermore, life characteristics as, current density 13 mA/cm2 constant current ([...]) by applying a, , determined time halving luminance, was time 340. [Compared e.g. 7] Ion compound 1 for ion compound 3 a other than point address, both the same method in the embodiment 5 in the multilayer structure polymer type organic EL device have been prepared. Anodic-ITO of the organic EL, Al used as a cathode in an a voltage is applied to the bar, about 3.5V was is monitored green emitting in. Luminance 5000 cd/m2 A/6.9 cd the current efficiency in, the driving voltage was 5.9V. Furthermore, life characteristics as, current density 14 mA/cm2 by applying constant current of, , determined time halving luminance, was time 70. In the embodiment 5 compared to, in which the drive voltage is high, was short larger time halving luminance. (Organic EL characteristic of the device) [in the embodiment 6] Organic EL for manufacturing A ITO on a glass substrate are patterned by using a photolithography width 1.6 mm, is given by the following equation 1 (3.9 mg) compounds 2 and compounds [0.6 mg, weight average molecular weight (Mw) 247,000 Mw/Mn= 1.65, the Mn, a number average molecular weight difference from an] a toluene (1.2 ml) dissolving the, disclosure number (0.45 mg) is formula to a acetic acid ethyl (100 micro l) dissolving the, coating solution is mixing these, at a spin 3000rpm after court grudge , 180 °C on hot plate, curing the to heat a bubble 10 minutes, hole injection layer (30 nm) are formed at the. [Formula 33] [Formula 34] Furthermore, the compound 3 (4.5 mg, Mn= 1.94/5,700 Mw weight average molecular weight) is toluene (1.2 ml) dissolving the, said polymerization disclosure number (0.45 mg) for acetic acid ethyl (100 micro l) dissolving the, coating solution is mixing these, at a spin 3000rpm after court grudge , 180 °C on hot plate, curing the to heat a bubble 10 minutes, hole transport layer (30 nm) are formed at the. [Formula 35] Furthermore, the resulting glass sheets and is transferred into a wafer deposition substrate, CBP + Ir (piq)3 (40 nm), BAlq (10 nm), Alq3 (30 nm), LiF (film thickness 0.5 nm), Al of (film thickness 100 nm) is deposited on the light emitting layer is formed to allow the plating solution. CBP: 4, 4'-bis (carbazole-9-one)-biphenyl Ir (piq)3: tris (1-phenyl isoquinoline) iridium BAlq: bis (2-methyl-8- [...] hydroxy) (4- non-phenyl jade brush NATO) aluminum Alq3: aluminum complex (tris (8-hydroxy [...]) aluminum (III)) Formed on, without photo, be able to the substrate during environment dry nitrogen, countersink of 0.4 mm to alkali-free glass of 0.7 mm for sealing glass and ITO substrate form, photocurable epoxy resin is attached on the often performance is carried out a bag by, multi-layer structure polymer type organic EL device have been prepared. After the operation of atmosphere, room temperature (25 °C) was performed in. Anodic-ITO of the organic EL, Al used as a cathode in an a voltage is applied to the bar, about 4V in red-luminous is monitored and, luminance 1000 cd/m2 current efficiency in the was 7.2 cd/A. And, the current voltage characteristic 4140B ammeter microspheroidal product yarn Hewlett Packard and as measured by means of a, light emitting brightness photo it stands homicide[...] -based luminance product it was determined that using 1980B. Furthermore, life characteristics as, product tower cone company while applying constant current BM-7 the measures a, brightness initial brightness (1000 cd/m2) and measuring the time for the halving from, was time 270. (Confirming stack of in the embodiment 6) In the embodiment 6 of a other than point address a quartz ITO, may all be the same, a method was implanted layer is formed on the hole. Hole injection layer impregnating the minutes 1 to toluene, toluene immersion before and after the change in intensity of absorption spectrum a observation, 99.8% is remaining it has also been found. On hole injection layer, are formed at the hole transport as well as in the embodiment 1. Hole transport layer impregnating the minutes 1 to toluene, toluene immersion before and after a observation the change in intensity of absorption spectrum, was 97.8% is left on the. [In the embodiment 7] In the embodiment 6 in, hole transport layer, polymerization disclosure number (0.45 mg) of additional circuit by correcting a phase and a core are formed in a point other than the same method been produced with at organic EL device. Anodic-ITO of the organic EL, Al used as a cathode in an a voltage is applied to the bar, about 3.5V in red-luminous is monitored and, luminance 1000 cd/m2 current efficiency in the was 8.4 cd/A. Furthermore, brightness initial brightness (1000 cd/m2) and measuring the time for the halving from, was time 1020. (Confirming stack of in the embodiment 7) In the embodiment 7 of a other than point address a quartz ITO, may all be the same, a method was implanted layer is formed on the hole. Hole injection layer impregnating the minutes 1 to toluene, toluene immersion before and after the change in intensity of absorption spectrum a observation, 99.8% is remaining it has also been found. On hole injection layer, in the embodiment 2 are formed at the hole transport similarly to the. Hole transport layer impregnating the minutes 1 to toluene, toluene immersion before and after a observation the change in intensity of absorption spectrum, was 97.2% is left on the. [Compared e.g. 8] In the embodiment 6 1 chemical compound in at 2 and compounds 2 (4.5 mg) compound instead the only using, polymerization disclosure number and can be prepared mix solution which is then with hole injection layer (30 nm) is formed other than point a, the same method in the embodiment 6 organic EL device have been prepared. Anodic-ITO of the organic EL, Al used as a cathode in an a voltage is applied to the bar, about 6V been observed red light in, luminance 1000 cd/m2 current efficiency in the was 1.3 cd/A. Furthermore, brightness initial brightness (1000 cd/m2) and measuring the time for the halving from, was time 12. (Compared e.g. confirming stack of 8) Compared e.g. ITO 8 of a other than point address a quartz, may all be the same, a method was implanted layer is formed on the hole. Hole injection layer impregnating the minutes 1 to toluene, toluene immersion before and after the change in intensity of absorption spectrum a observation, 2.8% a membrane is placed outside without remaining, could not be applied to manufacturing stacks. Compared e.g. in dog layer 2 adjacent, such as 8, each polymerizable preferred that the hole-transporting compound for including using mixtures with if it were not made, the does laminated structure, luminous efficiency, superfine longer emission life but, in the embodiment 6, 7, as shown to, adjacent 2 in dog layer , each polymerizable preferred that the hole-transporting compound for including mixture when, comprising a laminate structure composed of a and formed on an upper surface of the, luminous efficiency, with excellent device is longer emission life may yield an. Furthermore, , such as in the embodiment 7, just a layer close to the anode disclosure polymerization luminous efficiency by mixing agent, with excellent device is longer emission life can be produced. [In the embodiment 8] Width 1.6 mm for ITO on a glass substrate are patterned by using a photolithography, compounds is given by the following equation 4 (4.5 mg, Mw=7,700, Mw/Mn = 1.45) a toluene (1.2 ml) dissolving the, said polymerization disclosure number (0.45 mg) for acetic acid ethyl (100 micro l) dissolving the, coating solution is mixing these, at a spin 3000rpm after court grudge , 180 °C on hot plate, curing the to heat a bubble 10 minutes, hole injection layer (30 nm) are formed at the. [Formula 36] Furthermore, hole injection layer on compounds is given by the following equation 5 (4.5 mg, Mw=10,800, Mw/Mn = 1.52), toluene (1.2 ml) coating solution is mixing, at a spin 3000rpm after court grudge , 180 °C on hot plate, curing the to heat a bubble 10 minutes, hole transport layer (30 nm) are formed at the. [Formula 37] Furthermore, on hole transport layer, by the following structural formula 1 parts by weight of the above polymer (75 parts by mass of), polymer 2 (20 parts by mass of), polymer 3 (parts by mass of 5) consisting of mixtures, toluene solution (1.0 mass %)and coat at a spin 3000rpm a, 80 °C on hot plate, to heat a bubble 5 minutes, polymer light emitting layer are formed at the (80 nm film thickness). And, hole transport layer and a light emitting and do not dissolve in floated was can be laminated to. Furthermore, the resulting glass sheets and is transferred into a wafer deposition substrate, light emitting layer on said Ba (3 nm film thickness), in turn of Al (film thickness 100 nm) are formed at the electrode. Formed on, without photo, be able to the substrate during environment dry nitrogen, countersink of 0.4 mm to alkali-free glass of 0.7 mm for sealing glass and ITO substrate form, photocurable epoxy resin is attached on the often performance is carried out a bag by, multi-layer structure polymer type organic EL device have been prepared. After the operation of atmosphere, room temperature (25 °C) was performed in. Anodic-ITO of the organic EL, Al used as a cathode in an a voltage is applied to the bar, about 3.5V was is monitored green emitting in. Luminance 5000 cd/m2 current efficiency in the 8.1 cd/A, the driving voltage was 6.1V. Furthermore, brightness initial brightness (1000 cd/m2) and measuring the time for the halving from, was 990 time. [Formula 38] 1: light emitting layer 2: anode 3: hole injection layer 4: cathode 5: electron injection layer 6: hole transport layer 7: electron transport layer 8: substrate Provided is a material for organic electronics which can produce an organic electronic element capable of lowering the driving voltage or capable of performing stable driving for a long time. The material for organic electronics contains at least an ionic compound and a compound having a charge transporting unit (hereinafter, referred to as charge transporting compound), and the ionic compound is composed of a counter cation and a counter anion, while the counter cation is any one kind or two or more kinds selected from H + , a carbocation, a nitrogen cation, an oxygen cation, and a cation having a transition metal. 1) polymerizable preferred that the hole-transporting compounds and polymers including disclosure agent mixture on a substrate of material and then applying the, curing the step hole implantation layer; 2) on said hole injection layer, polymerizable preferred that the hole-transporting compound for including after the application the mixture, form a hole transporting layers curing the; and 3) the hole transport layer on said, including light emitting material layer and a nitration material layer, hole injection layer, hole transport layer, layer, and a luminescent layer including multilayer structure is formed of organic light emitting device having improved manufacturing method. According to Claim 1, said polymerizable substituent is, epoxy group, oxetane to, and vinyl ether any one or more a, organic electro-light emitting element manufacturing method. According to Claim 1 or Claim 2, said hole transport compound, least arylamine, carbazole, or thiophene skeleton including, organic electro-light emitting element manufacturing method. According to Claim 1 or Claim 2, said hole transport compound, number average molecular weight 1000 or more oligomers or polymer having a shrinkage on film formation, organic electro-light emitting element manufacturing method. According to Claim 1 or Claim 2, said hole [...] polymerization disclosure agent, organic electro-light emitting element manufacturing method. According to Claim 1 or Claim 5, said polymerization disclosure agent, catalyst, cationic nitrogen, oxygen ions, having transition metal and selected from the group consisting of a cationic a relatively then, the frame is made up an ion anionic, organic electro-light emitting element manufacturing method. According to Claim 6, is electron acceptor compound is ion compound said, said hole transport compound electronic 1 a which may be oxidized, organic electro-light emitting element manufacturing method. According to Claim 1, said organic electro-light emitting element a this resin film-substrate, organic electro-light emitting element manufacturing method. According to Claim 1, said application, performed at a temperature range of -20-+300, organic electro-light emitting element manufacturing method. According to Claim 1, said hardening of the optical radiation or a heating process made by the, organic electro-light emitting element manufacturing method.