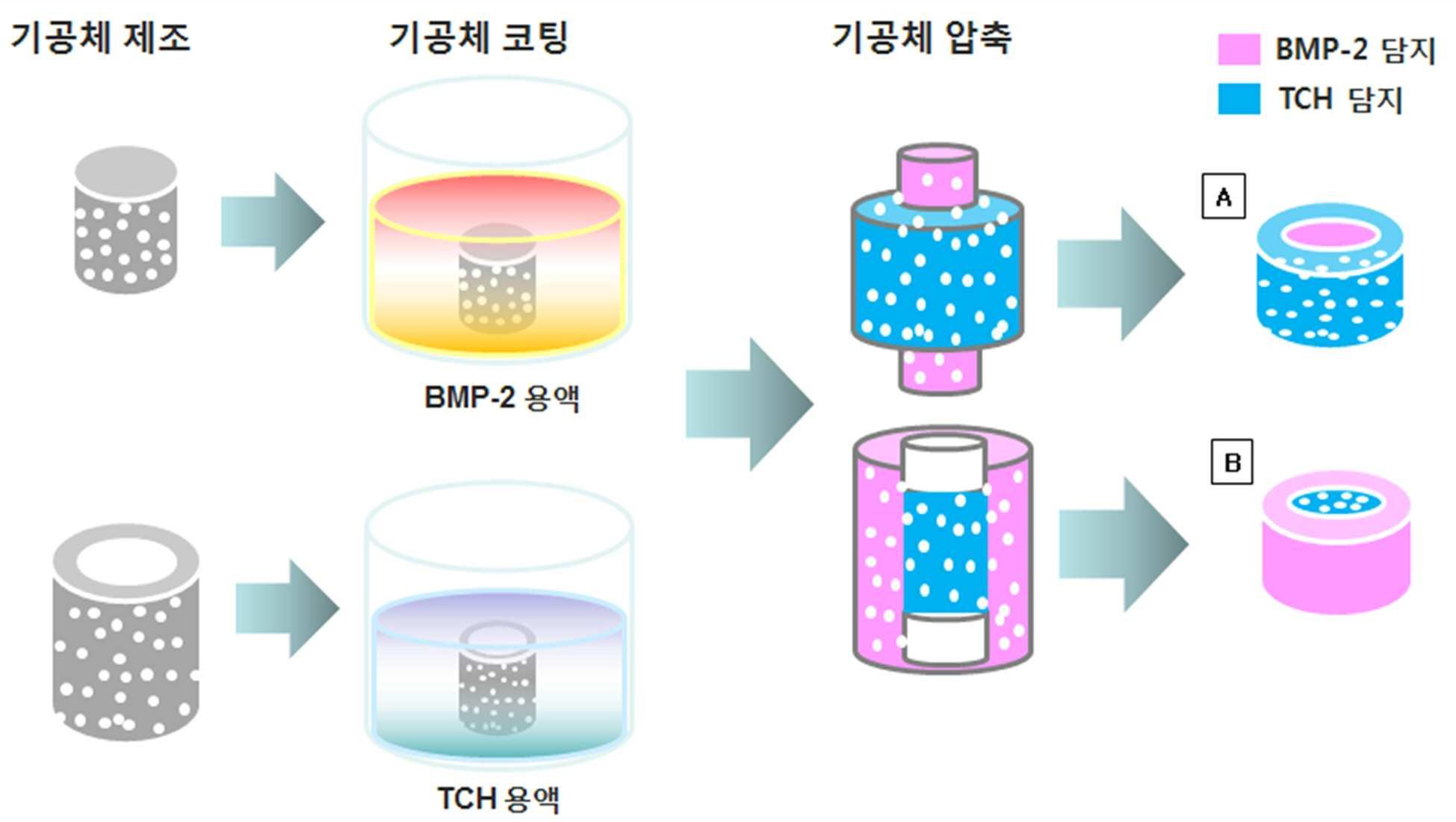
METAL IMPLANT CAPABLE OF MULTIPLE RELEASES OF DRUG, AND MANUFACTURING METHOD THEREOF
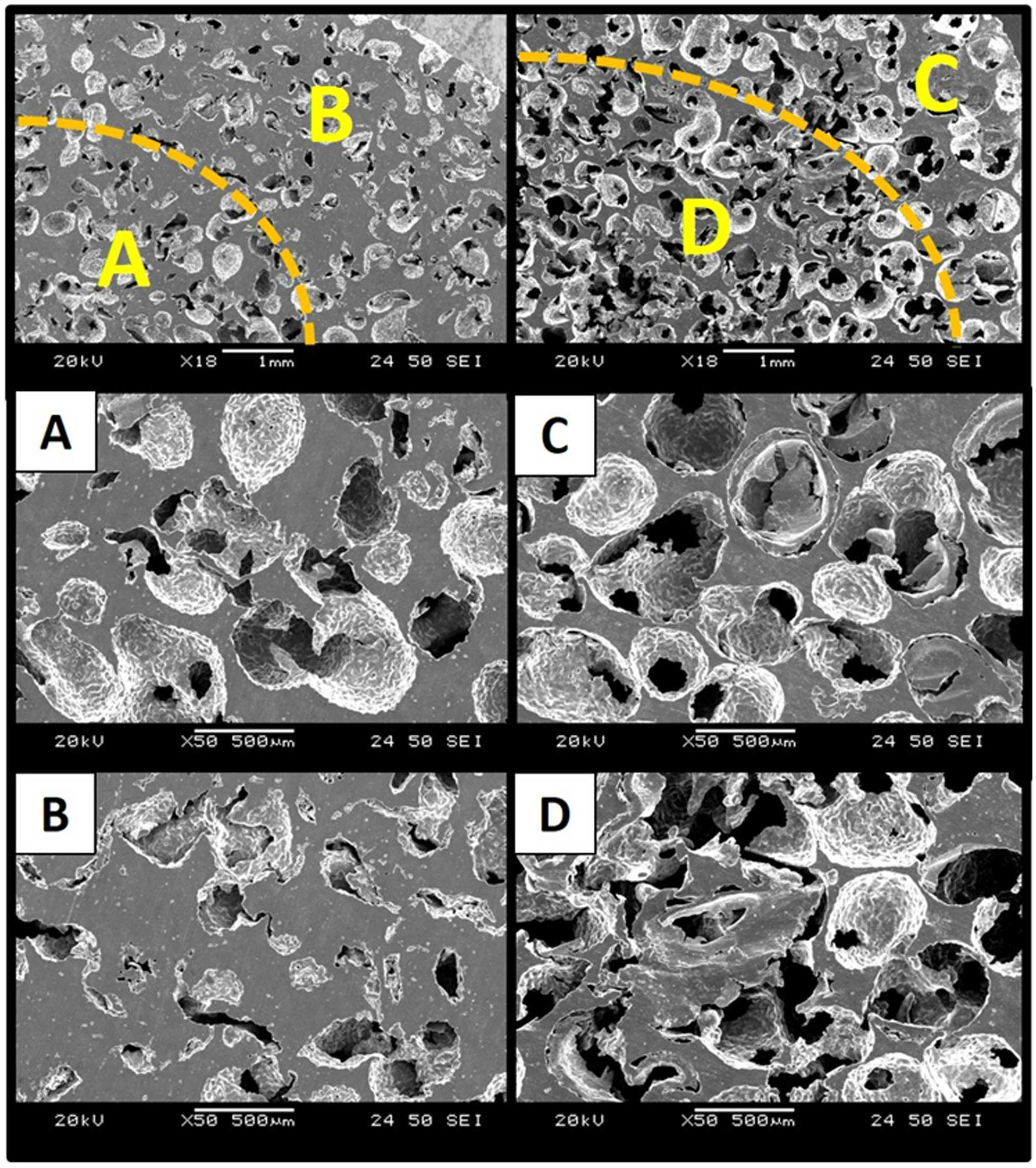
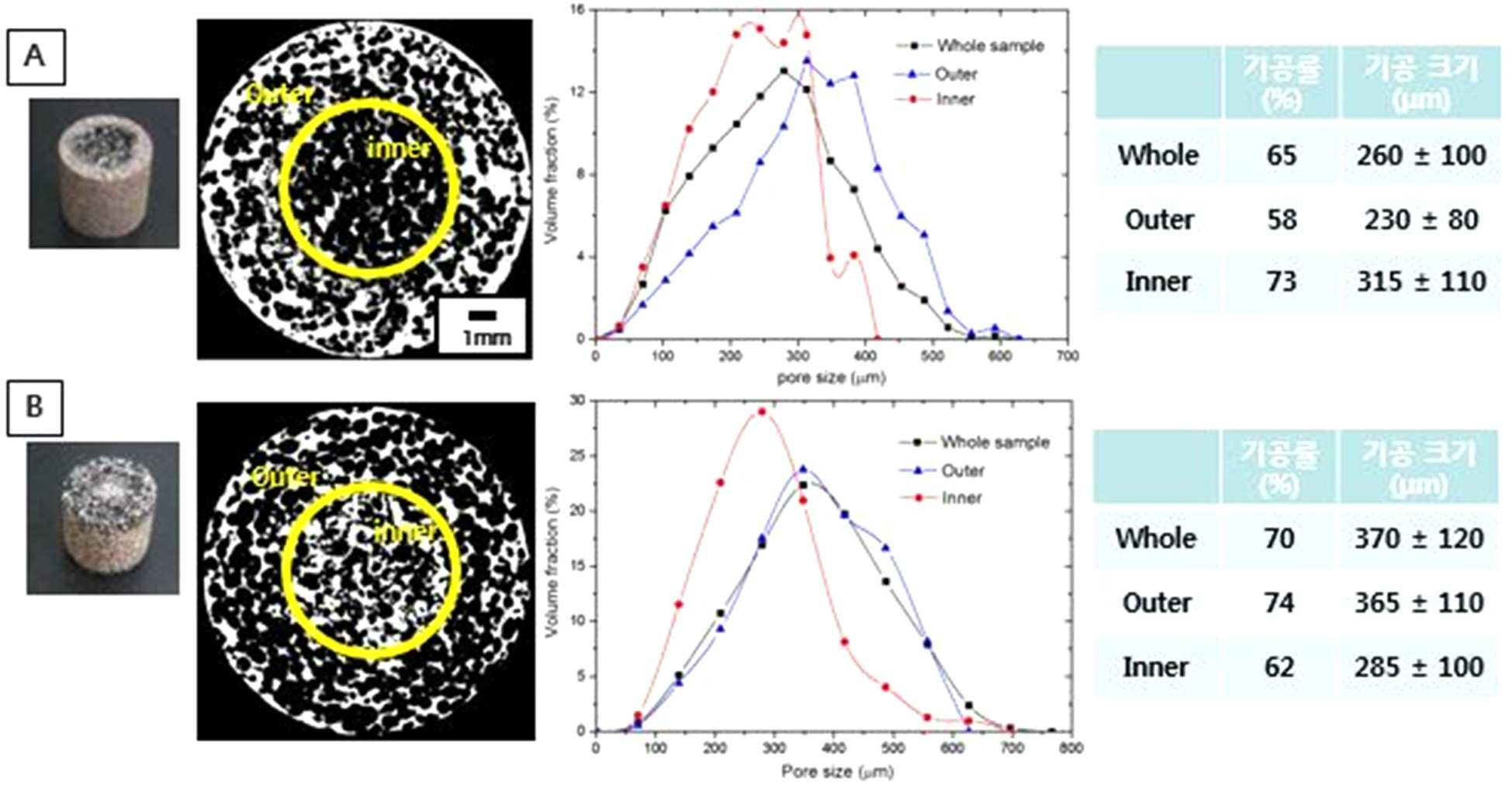
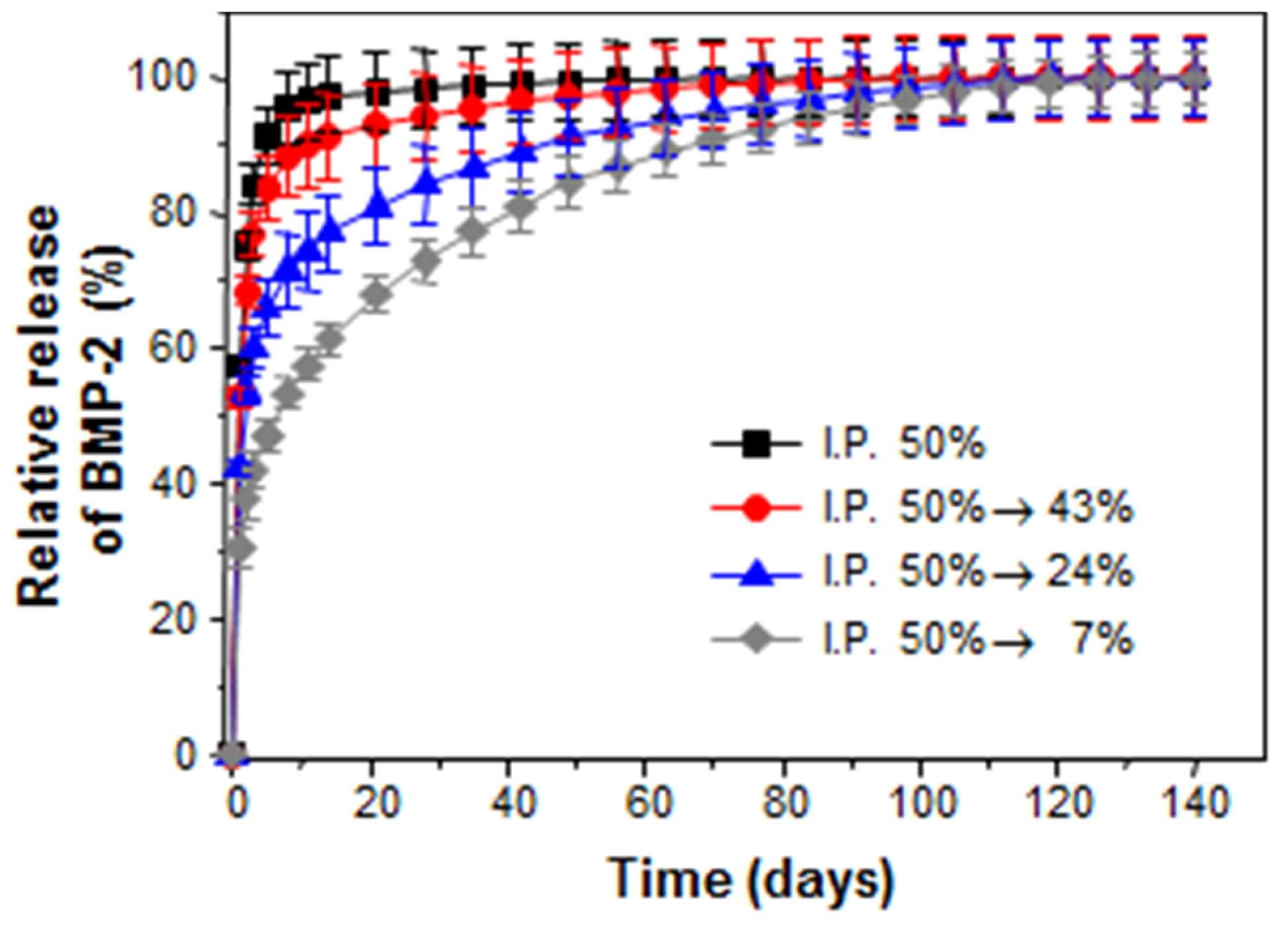
The present invention refers to a metal implant is capable of releasing a multiple of drugs and relates to manufacturing method. Medical implant, for the use of artificial hip joint, such as bio-implant implant as the material of the photosensitive drum and charges the photosensitive Golgi to lysosome, (i) titanium, stainless steel alloy, cobalt - chromium metal, such as metal material, (ii) alumina, and an inert material, and the such as and zirconia (iii) a bioactive such apatite and hydroxide a message of a typical message KIPO & ceramic material. Such bio-implant of the implant high are biocompatible titanium material, strong and mechanical properties, which a high corrosion resistant, in particular when compared to other metallic materials, has a significantly lower modulus of the implant bio-implant are used for manufacturing material of wet liquid to flow down. While, bio-implant implant for application of a the case of implantation via the, bio-implant implant is implanted in the recovery is in both damage when and the bio-implant implant is bone or tissue that a period of time for a given size on a circuit plate the restraining unit, after procedures to prevent acute inflammatory and. it is important to ensure that the. For this purpose,, the bio-implant implant enhance or biocompatible properties and is surface-treated with, antibiotic number such as the pharmaceutical drug or growth factor or bio-implant of a protein, such as insulin, inflammatory coating implant engagement with bone to prevent unit controls the constant speed compressor and a scheme is actively studied of wet liquid to flow down (25th which publicized patent number 10 - 2008 - 0016780 call). However, bio-implant implant thereafter or protein in a non-infectious, or therapeutic substance, and the 1) coating a therapeutic drugs or protein, the types of extremely amount which not limited to, number, 2) drug treatment of expensive thereafter being quickly with a possible time delay in initial drug to flowing into the in vivo time is operated by a global word too quickly extracorporeal to loss cost, and aspects and treatment of a specific highly inefficient which, 3) bio-implant implant coat ed onto the surface of rate of release of the anti-or protein of-glue agent including a number [...] performance of or protein, or therapeutic substance, and, meanwhile, could not exhibiting effective door not conformable, is connected to the semiconductor layer. point number. While, implant treatment of a body fluid is portions is formed on a to - 20 Ps/nm/km., procedure site drug delivery systems suitable to try to building a is, in addition to be administration also according to the type of a medicament should changed aspects of the release of the drug. For example, in the case of antibiotic number after operating implant within week to prevent acute inflammatory and release accessible from the whereas, in the case of a protein, such as an growth factor between tissue and implant as the degree order to increase the preferably at the ends of order reception preferably short for sustained release until whose radiation is it is required that a. However conventional inside of the pipe and the header are each these drug until now by so that it is released for a period of time which may desired multiple emission implant is to be electrically, fully exhibited performance of drug in a coolant pipe according door not conformable, is connected to the semiconductor layer. point number. Is suitable for procedure site thus, administering a medicament has properly emitted adjusted so as to development of implants is the regularized data in a database. the present invention the point number door such as in order to solve one implant even in multiple emission of drugs a metal implants for developing the laboratory results for, having a controllable rate of drug release a porosity and/or pore dimensions the metal support the center axis of the laminated body after each drug-supported interface of metal support and compressed by a slider so that engaged by, various drug release implant one metal between biological systems which exhibit oscillatory aspect was under trillion number. In order to solve number and said, the present invention refers to number 1 number 1 containing porous metal support; and number 2 number 2 containing porous metal support; including a a metal implant is capable of releasing a multiple of drugs as, porous metal support and said number 1 number 2 porous metal support engaged it is compressed, a surface to which the imaging light inputs. under public affairs a metal implant a number. In addition, the present invention refers to number 1 number 1 containing one or more porous metal support; and number 2 containing porous metal support and at least one number 2; is which is joined to and integrated with a metal implant as, number 1 number 2 porous metal support and porous metal support interface (interface) engageable to engage to (interlocking) uneven-shaped which is used, number 1 containing porous metal support and number 1 number 2 number 2 containing porous metal of the support is compressed after interworking with a gear mechanism and coupled physical on an interface at re-growing one of a. under public affairs a metal implant a number. Further, the present invention refers to 1) number 1 number 1 porous metal support which the film is immersed in a solution including drug step; 2) drug number 2 including porous metal support step in which the film is immersed in solution number 2; 3) carried with drug within the pores said each metal support step for heating a substrate; and 4) said number 1 number 2 porous metal support and porous metal support after pencil case with the metal support including the engaged, a metal implant is capable of releasing a multiple of drugs for manufacturing method of. under public affairs number. Hereinafter, the present invention described a detail the. The present invention refers to when compressing the adjacent metal support to make interface that can be engaged and-purpose control board with the can, each porous metal of the support containing or drug carrier angiodependent number porous metal support in engagement with the mouth of by compression of interface between the porosity and/or pore size prints an which exhibit various drug release aspect, a monolithic metal implant. under public affairs number. In addition in the present invention 2 compresses the metal support or more unit by combining the one bio-implant implant the under trillion number, each unit of pressing against the pressure metal support or residential an elastic other, height of metal support or each, surface area or the like on a hereinafter applies pressure beginning, middle, and end, the compression is changed, the porosity of such that each metal co-polyester within the supporting body/of pore sizes and a hereinafter be changed in various forms and in addition on, release of the drug supported further aspect determines masks has been confirmed in addition. Specifically, compressed metal support the porosity of metal support increases applied to produce large-sized then, in addition of pore sizes and a which small, the rate of release of the drug by lower. As a result, one bio-implant implant drug release of several the implant by allowing a emitted in which the aspect of implantation of the device within the drug delivery to an the third step is to exhibiting as to form a mass a metal implants, as an the device. In the present invention "metal support" has a metal implant the basic framework of construction in the as, said implant into the body via the drug after determining an performs a function, or the like. Thus biodegradable metal as said metal support, in the predetermined time, the diagnosis of a human body since a breakdown in-it is preferable that the. On the other hand, said metal which porous, porous metal in the pores of the support member can be system in which drugs are supported onto. The porosity of said metal support 60 to 80%, more specifically 70% can be. The metal support said porous, include titanium, magnesium, iron, aluminum, copper, tantalum, or alloys thereof, and the can be, the number one are not. In the present invention 2 at least one unit of metallic supporting body is pressed out of engaged by has shape. Thus a metal implants of the present invention unit or more metal support 2 may comprise an. Each unit metallic supporting body includes the same metal and may be, may be different metal. I.e., in the present invention number 1 number 2 porous metal support and porous metal support groups may be the same. may be different. 2 of metal implants of the present invention unit or more metal support engaged by compression the objective compound.. Of intermetallic is, as an union of physical, for example, attached, metal atoms or metal co-polyester within the supporting body of pores is mechanically engaged is disposed KIPO &. Thus said and a compression of the connector joined by support or more metal 2 may be removed again. After implantation of the retained in a seat of disease for a metal implants of the present invention, the motion triggers mirus transit TKO number from each unit metal support the according may be authored in the first client by a number. In addition, metal support each unit size of the entire implant is small in relation to the transducer part surgical when, as it is hereby possible to,, proactive occurs the unit part in the body additional drug loading detected by the surgical site for minimal permits replacement of unit. Debondable metallic unit support the separation that needed for the bonding of a support can be adjusted over a range. Must not be separation between support when the junction range is opened so that the washing water the required pressure (force) and capable of increasing a, separation one between the gate carriers if needed a smaller pressure drop gates of a and b are opened range (force) connection is set up between the separation even number can be under trillion. In addition, said compressed metal support when engaged is applied to, each unit metallic supporting to compression rate can be (the extent of compression) and then in contrary, the metal co-polyester within the supporting body of porosity and/or of pore sizes and a can be changed. Specifically, metal e.g. experiment of the present invention support co-polyester within the supporting body metal the higher the compression rate then the porosity of, of pore sizes and a in addition the torsion bar has been confirmed (experiment example 1). In addition, porous metal support to the shape of the present invention without being one number but can be used, preferably a hollow porous metal support said number 1 the, said number 1 number 2 porous metal support porous metal support hollow insertable form release of the drug supported by having the divided more specifically aspect may to enable the (also 1). "Drug" in the present invention the delivery into the body via the implant of the present invention the material to be is. Implant of the present invention may be included in the type being specifically of a medicament include immune response change factor, anti - number proliferation, anti - mitotic number, anti - platelet number, platinum coordination complex from, hormone, anticoagulated number, fibrin-number, anti - secretion number, anti - number-mediated, immune and for circuit controlling isolation gates of number number, angiogenesis drug, angiotensin receptor blocking material, nitric oxide donor, antisense oligonucleotide and their composite, cell inhibitors material, corticosteroid, hemostatic steroid, parasiticidal number, anti - glaucoma drug, antibiotic number, differentiation regulators, antiviral number, number anticancer, anti - inflammatory number and growth factor from the group consisting of can be selected, the not one number. In the case of "growth factor" during drug said a living body in a implanted implant is bone or tissue which helps to line coupling member using a transparent material can be function. Thus long after implantation in vivo of the implant (e.g., order reception composed of sugars,) is slowly released adjusted so as to preferably. Said a specific example of growth factors, bone formation-inducing protein, (Bone morphogenetic proteins, BMPs), border cells growth factor (Epidermal growth factor, EGF), erythropoietin (Erythropoietin, EPO), fibroblast growth factor (Fibroblast growth factor, FGF), hepatocyte growth factor (Hepatocytegrowth factor, HGF), insulin like growth factor (insulin-like growth factor, IGF), myostatin (Myostatin, gDF-8), nerve growth factor (Nerve growth factor, NGF), neurotrophin (neurotrophins), platelet derived growth factor (platelet-derived growth factor, PDGF), thrombopoietin (Thrombopoietin, TPO), transforming growth factor (Transforming growth factor beta, tGF-β), vascular endothelial growth factor (Vascular endothelial growth factor, VEGF), placental growth factor (placental growth factor, PlGF), (Adrenomedullin, AM) oh the dignity Roh maul it was surrounded, self secretion motion factor (Autocrine motility factor), granulocyte colony magnetic pole factor (granulocyte-colony stimulating factor, g-CSF), granulocyte main meal nine colony magnetic pole factor (granulocyte-macrophage colony stimulating factor, gm - CSF), growth differentiation factor (Growth differentiation factor-9, GDF9), il - 1, il - 2, 99900001 80999, il - 4, il - 5, il - 6, il - 7 as to the aromatic hydrocarbon but, the number one are not. In addition said drug during "antibiotic number" transplantation of metal implants of the present invention according to the in vivo initial inflammatory response due to rejection method and for circuit controlling isolation gates performs a function number. Is installed in the upper portion are produced at the beginning the inflammatory in particular such, and initial a drug such as a (e.g., within week) of or as released adjusted so as to preferably. Are carried on the metal support each of the present invention drug are identical to each other medicament has may be, may KIPO & different drugs. I.e., drugs and of the present invention number 1 number 2 the drug is an groups may be the same. may be different. A single drug in the present invention said can be a drug, is a mixture of 2 or more pharmaceuticals may be. In addition, said metal support metal according to (the extent of compression) compression rate of porosity and/or co-polyester within the supporting body according to of pore sizes and a can be changed aspects of the drug release. Specifically, support metal e.g. experiment of the present invention the compression is increased to thereby cause final pores supported with the lower rate of the drug release with the drug release speed is not made time it has been confirmed (e.g. experiment 2). Thus said metal support and/or a driving request receiver receives a drug in a solvent to form a slurry as well as, metal support and/or the same drug in a solvent to form a slurry to metallic supporting each unit even compression rate (the extent of compression) between emitter speed and release of the drug according to different since can be loaded, can be adjusting it. In addition metal support by controlling the position of a and the rate of release of the medicament containing period which may be loaded with. Preferably (i) vivo to release fast in the case of the immediate-release drug that are required, the porosity of the semiconductor device by lowering the compression rate obtain a great sufficient performing degree to the porosity and, in addition said drug carried with metal support implanting the for arranging optical elements related, and visa versa (ii) the extended release is desired when of the prolonged-release drug, for washing is opened so that the compression rate and the size of pores and porosity while, said drug carried with metal support of the implant positioned inside the drug release by. capable of modulating aspect. On the other hand, contact portion of metal support, i.e. engaged and in addition an interlace site such that each metal co-polyester within the supporting body and rate at size and pores of different pore size and pores for higher rate present interface said by-pored another number 3 the drug is an in a solvent to form a slurry can exhibit a KIPO & drug release aspect. Metal support (compression rate) according to the extent of compression of said temperature distribution of silicon melt using an thickness part of the horizontal frame, and SiO 2 3 in metal implants including support to derive aspect of drug release can be worked out. In addition, the present invention refers to number 1 number 1 containing one or more porous metal support; and number 2 containing porous metal support and at least one number 2; is which is joined to and integrated with a metal implant as, number 1 number 2 porous metal support and porous metal support interface (interface) engageable to engage to (interlocking) uneven-shaped which is used, number 1 containing porous metal support and number 1 number 2 number 2 containing porous metal of the support is compressed after interworking with a gear mechanism and coupled physical on an interface at re-growing one of a. under public affairs a metal implant a number. A metal implants of the present invention unit or more metal 2 which is joined to and integrated with support is and if a bit in the complimentary, metal support for than or bonding of one between the gate can be hereinafter may have uneven-shaped. In this case to be engaged with each other interface (contact surface) of aperture such as by the same pressure even in conditions where the stronger than that between the junction or binding that they can be unit is off. In addition, a retro-bonded through the uneven-shaped the metal support each unit according to junction can be varying the intensity. Uneven in the case of units having relatively small lower joining strength at the joint after bonding and a separation elements including, relatively large having groove is high in the joint strength between the units can be separately from a resulting in a reduced risk. Porous metal support and said number 1 number 2 material of porous metal support, pore size, porosity and specific surface or, same or the kind of drug drug and said number 1 number 2. may be different from the user. In addition, said number 1 porous metal support is not less than 2 each number 1 porous metal support contained in same or hereinafter or each other drug number 1, number 2 porous metal support is not less than 2 each number 2 porous metal support contained in same or each other drug number 2. may be different from the user. On the other hand, a metal implants said porous number 1 containing said number 1 and at least one metal support; and number 2 number 2 containing the grip. porous metal support and at least one material may have a form predetermined structure which. In Figure 5 such as a each other and metallic supporting unit several engaged by block (block) to a predetermined portion of a conformed to the apparatus of motor metal implant can be under public affairs number. under trillion said number implant metal such as the, unit number by binding a water compression and at may be under trillion, a metallic supporting unit several located under trillion made by compressing at a time may be number. In addition, the present invention refers to a number of steps of manufacturing method of metal implants including under public affairs.: 1) drug number 1 number 1 including porous metal support step in which the film is immersed in solution; 2) drug number 2 number 2 including porous metal support step in which the film is immersed in solution; 3) drug within the pores said carried with each metal support step for heating a substrate; and 4) said number 1 number 2 porous metal support and porous metal support after pencil case with the engaged the metal support. Said step 1 and step 2 each metal support as a step in which the film is immersed in a solution including drug, each metal support in the pores of the support member is a step for system in which drugs are supported onto. The order of said step 1 and step 2 may be reversed, both being. may be performed simultaneously. Said drug in pore 3 step carried with each metal support for heating a substrate wherein, of the pores in the metal support is maximum with a substance remaining inside the pores to supported bubbles of the bubble storing space is step number that triggers. Each metal support said step 4 after pencil case with the amount metal support engaged the wherein, drug carried with each metal support which intensifies the binding between, allowing them fit is formed in a portion required for the behavior of release of number [...] as a metal support is and compressing. 4 step said each metal support or residential an elastic other pressing against the pressure, height of metal support or each, surface area or the like hereinafter on the a, can be varied the compression is even not smaller than the width of the metal co-polyester within the supporting body/the porosity of of pore sizes, etc. can be differently controlling a. Metal support for modification of an maximum support is the pressure inside and outside the modified 5% a compression rate of compressed until impact and production costs can be applied. Specifically, a pressure can be added to metal support the range of 0. 1 to 2 GPa, the logical source including the device, the number one are not. In the case of titanium for example in 5% 70% porous when pressure is applied 2 GPa up. it can apply the compression. More compression may occur breakdown porous in pressure since the. precautions while one end of the table. The present invention refers to used and pores pore size of the metal support for rate adjustable hereinafter by bio-implant implant release of several drug can be released in which the aspect 360degree to, and the third step is to the implants to drug delivery to an period exhibiting as to form a mass: 1.. In addition, a left side of the base procedures that the duct shape is configured to fit the structure the multiplexer provides a path for bath number, the prior art technique shielding stress in an operating implant-precipitation method (Stress shielding) can be to reduce a, drug is coated on the surface of an a metal implant than can be discharged therefrom drug long periods of time after initial since inflammatory can occur effectively. part. Also Figure 1 shows a number of metal implants the present invention according to, graphically trillion processes is indicative of the. Figure 2 of the present invention metal support with different height in a process bath number with a metallic support, a fluid consumption can be restrained simultaneously compressing pore scene of a photo through the lines are adjacent to each is. Figure 3 of the present invention metal support of a after compression, a fluid consumption can be restrained of pore sizes and a porosity is measurement of the. foil. Also advanced or delayed, respectively, Figure 4 shows a different number and compressed by a slider so that rate in an implant under trillionbMP-2 is a behaviour is discharged. Also uneven-shaped Figure 5 shows a a number of metal support distance is reduced with increasing the integration of construction one exemplary that forms the bottom of the is shown with codes. Hereinafter, the present invention and/or at least two different and in the embodiment to more rapidly and to reduce a memory.. However, in the embodiment and relate to examples of the present invention only these for not limited range of the present invention. In the embodiment 1: number bath of the implant In the embodiment 1 - 1: metal support test specimen number bath step Metal support about porosity as shown support titanium in 70%. Specifically, titanium metal powder (Alfa Aesar, Ward Hill, Ma, USA) 10g, freeze as the medium cam pin( C10H16) 11gand dispersion number polyester oligomer as (Hypermer kD-4, UniQema, Everburg, Belgium) 0. 21g solution which is prepared by mixing, 24 in a temperature range from about 60 °C ball milling time (ball milling) slurry shown. After, said mold of aluminium workpieces are implanted into the slurry rotational speed of 30 rpm which to rotate it a mold at, about 24 time held under temperature of about 44 °C freeze by the front/rear sides of the molded body. Freeze from a mold said molding for separating, from the resultant molding freeze number triggers 5 to freeze medium. 0 × 10-3 Torrvacuum the egg is from about?Lyophilized to 50 °C number medium maintains the temperature of the spring coefficient of the piston spring are porous by triggers. Said porous about 2 time about 1300 °C a heat treatment is performed on the porosity about 70% in titanium support was under trillion number. Said titanium as support body (i) diameter 8 mm, height 18 mm, weight 1. 2g, and diameter 8 mm, 14 mm height, weight for the inside of a specimen 1g in the n bit parallel data inputted under trillion number, (ii) 12 mm outer diameter, inner diameter 8 mm, height 18 mm, weight 1. 5g, and outer diameter 12 mm, inner diameter 8 mm, 14 mm height, weight 1. 2g in specimen was under trillion number for external use. In the embodiment 1 - 2: immersion (coating) and drying steps Always during specimen support titanium under trillion number in the embodiment 1 - 1 in 8 mm diameter, height 18 mm, weight 1. 2g in for the inside of a specimen of fused bMP-2 1 mg then immersed 10 ml PBS solution, about 20 minutes in degree of vacuum of about 70cmHg, which has the vacuum state. After solving the vacuum then two time 2 at a normal, 24 was very dry time. In addition, in said in the embodiment 1 - 1 during specimen support titanium under trillion number 12 mm diameter, inner diameter 9 mm, 14 mm height, weight 1. 2g TCH for external use in specimen a is dissolved 1 mg (tetracycline-hydrochloride) dipped in an 10 ml PBS solution, about 20 minutes, which has the degree of vacuum of about 70cmHg vacuum in. After, 2 at a normal solving the vacuum then two time, was very dry time 24. In the embodiment 1 - 3: compression-expander and molding steps In Figure 1 such as a, said in the embodiment 1 - 2 number prepared by the method in (i) bMP-2 support and titanium are supported (ii) TCH titanium are supported directly and indirectly sells products support about 1 GPa herein the coating powders by compacting the growth factor and drug delivery implant was under trillion number. bMP-2 titanium are supported within support height of specimen (18 mm) on the outside support titanium are supported TCH a height of specimen (14 mm) to relatively larger than bMP-2 titanium are supported up high compression rate of compressed support (A upper end of Figure 1). In addition, said two support disposed interior and exterior of the tubular member a combination and thus other implant was under trillion number (B lower end of Figure 1). Experiment 1 e.g.: in of the implant, external porosity and pore size measuring In said in the embodiment 1 of an implant under trillion number?External porosity and pore-size observation by using scanning electron microscope it was determined that and. The B and a of Figure 2 A lower end of Figure 1, the upper end of Figure 1 and of Figure 2 C D efficacy as an anti-observation the reproductivity of the laser diode by structure, the compression is small, a portion of which is is carried TCH C and a A bMP-2 a compressed, a portion of which is is carried B D and a higher porosity structurally capable of being utilized in.. As demonstrated 3 also in addition, the compression is relatively small TCH the porosity of portion are supported is still while retaining to 70%, the compression is relatively large bMP-2 are supported about the porosity of portion it has been confirmed that reduced to 60%. In addition also of pore sizes and a relatively the compression is small TCH are supported portion comprises from about 340 μm while selection that exceeds a preset pore size of large, relatively the compression is large bMP-2 are supported portion comprises from about 260 μm by the electric a smaller pore size of about 80 - 100 μm size desired by a 80 M. has been confirmed. Experiment 2 e.g.: observed aspect behavior emission according to compression rate of compressed implant Different compression rate number and compressed by a slider so that said in the embodiment 1 a under trillionbMP-2 is discharged in implant beam to corresponding advertisement based on the shown list, result to the computer of the also showed to 4. As a result also 4 as demonstrated, the compression is increased to thereby cause final pore with the lower rate of the drug release with the drug release speed is not made time it has been confirmed. The carrier compression rate of compressed and to adjust the CDMA timing to (growth factor) bMP-2 TCH and the rate of release of the (drug) can be better compensation rate of release of the anti-of identifying improving. Or more from a description, the present invention is in the field of the present invention a those skilled in the technical idea or essentially changing a characteristic the first or the second embodiment form specifically can be database for each consumer 2000. In this regard, in the embodiment described or more exemplary in all of the limiting and there has as which was not. must understand. Rather than the description of the present invention said range meaning of claim refers to equivalent and and range modification or all derived from general outline of an altered form is included within the scope of the present invention should interpreted to. The present invention relates to a metal implant capable of multiple releases of a drug, and to a manufacturing method thereof. According to the present invention, a pore size and the porosity of a used metal support are easily adjustable, and the drug can be discharged from a single bio-implantable implant while exhibiting multiple release conditions. Thus, an effect of delivering the drug to a site to which the implant is transplanted within certain time is maximized. COPYRIGHT KIPO 2016 Number 1 number 1 containing porous metal support; and number 2 number 2 containing porous metal support; including a a metal implant is capable of releasing a multiple of drugs as, porous metal support and said number 1 number 2 porous metal support engaged it is compressed, a surface to which the imaging light inputs a metal implant. According to Claim 1, said number 1 porous metal support the a hollow, porous metal said number 1 number 2 porous metal support insertable hollow support is in the form of a, a metal implant is capable of releasing a multiple of drugs. According to Claim 1, said number 1 number 2 porous metal support and porous metal support different compression rate a compressed, a metal implant is capable of releasing a multiple of drugs. According to Claim 1, said number 1 number 2 porous metal support and porous metal support the porosity of a different each other, a metal implant is capable of releasing a multiple of drugs. According to Claim 1, porous metal support and said number 1 number 2 of pore sizes and a porous metal of the support characterized in that different, a metal implant is capable of releasing a multiple of drugs. According to Claim 1, porous metal support and said number 1 number 2 a that each emit from the support porous metal drugs and number 1 number 2 release of the drug a different aspects of the, a metal implant is capable of releasing a multiple of drugs. According to Claim 1, said porous metal titanium, magnesium, iron, aluminum, copper, tantalum or their alloys characterized in that, a metal implant is capable of releasing a multiple of drugs. According to Claim 1, said drug immune response change factor, anti - number proliferation, anti - mitotic number, anti - platelet number, platinum coordination complex from, hormone, anticoagulated number, fibrin-number, anti - secretion number, anti - number-mediated, immune and for circuit controlling isolation gates of number number, angiogenesis drug, angiotensin receptor blocking material, nitric oxide donor, antisense oligonucleotide and their composite, cell inhibitors material, corticosteroid, hemostatic steroid, parasiticidal number, anti - glaucoma drug, antibiotic number, differentiation regulators, antiviral number, growth factor, anticancer number and anti - inflammatory number selected from the group consisting of more than one characterized in that, a metal implant is capable of releasing a multiple of drugs. According to Claim 8, bone formation-inducing protein, said growth factor, border cells growth factor, erythropoietin, fibroblast growth factor, hepatocyte growth factor, insulin like growth factor, myostatin, nerve growth factor, neurotrophin, platelet derived growth factor, thrombopoietin, transforming growth factor, vascular endothelial growth factor, placental growth factor, oh the dignity Roh maul it was surrounded, self secretion motion factor, granulocyte colony magnetic pole factor, granulocyte main meal nine colony magnetic pole factor, growth differentiation factor, il - 1, il - 2, il - 3, il - 4, il - 5, il - 6 and il - 7 selected from the group consisting of more than one characterized in that, a metal implant is capable of releasing a multiple of drugs. According to Claim 1, said porous the porosity of the metal support-free bismuth glass characterized in that 60 to 80%, a metal implant is capable of releasing a multiple of drugs. Number 1 number 1 containing one or more porous metal support; and number 2 containing porous metal support and at least one number 2; is which is joined to and integrated with a metal implant as, number 1 number 2 porous metal support and porous metal support interface (interface) engageable to engage to (interlocking) uneven-shaped which is used, number 1 number 1 containing porous metal support and porous metal number 2 number 2 containing physical interworking with a gear mechanism and coupled on an interface at re-growing of the support are integrally compressed after having been a metal implant. According to Claim 11, number 1 number 2 porous metal support and porous metal support to translate material, pore size is at least one of rate and pores at the same or different each other characterized by metal implants. According to Claim 11, number 1 number 2 the drug is an same or different drug and, characterized by a metal implants. According to Claim 11, number 1 porous metal support is not less than 2 each number 1 porous metal support contained in same or hereinafter or each other drug number 1, number 2 porous metal support is not less than 2 each number 2 porous metal support contained in the drug number 2 or different is characterized metal implants. According to Claim 11, number 1 number 1 containing one or more porous metal support; and number 2 number 2 containing the grip. and at least one porous metal support the form predetermined structure which the inventive arrangement is characterized in that a metal implant. 1) drug number 1 number 1 porous metal support which the film is immersed in a solution including step; 2) drug number 2 including porous metal support step in which the film is immersed in solution number 2; 3) carried with drug within the pores said each metal support step for heating a substrate; and 4) said number 1 number 2 porous metal support and porous metal support after pencil case with the metal support including the engaged, according to Claim 1 a metal implant is capable of releasing a multiple of drugs of manufacturing method. According to Claim 16, said 4) step number 2 number 1 porous metal support and porous metal support compressed rate different pressures each characterized in that, a metal implant is capable of releasing a multiple of drugs of manufacturing method.