MANUFACTURING METHOD OF POLYIMIDE
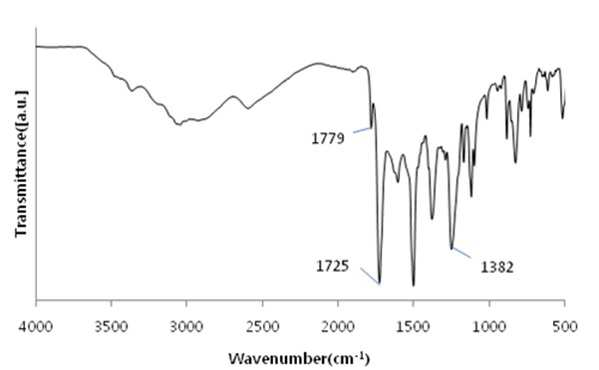
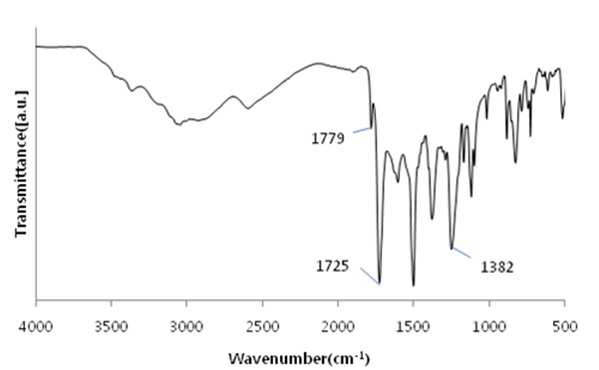
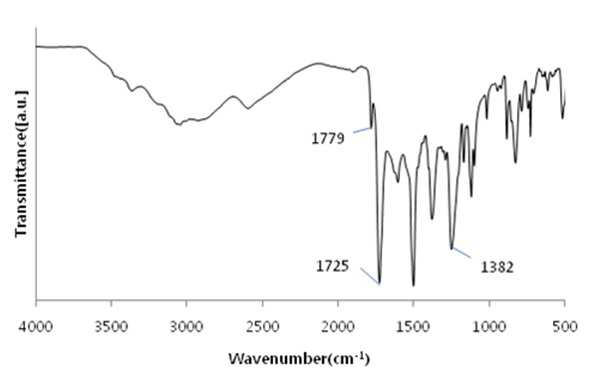
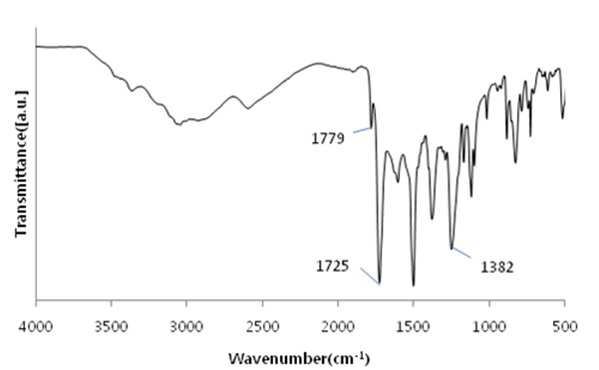
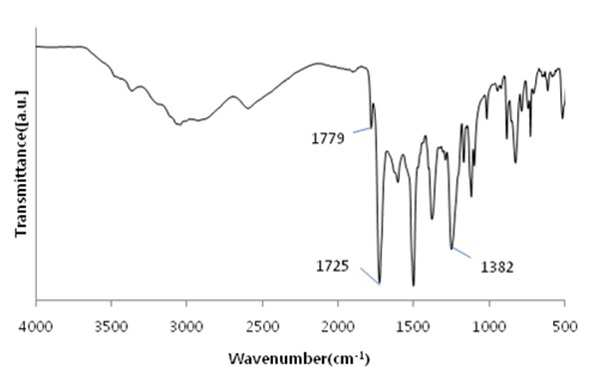
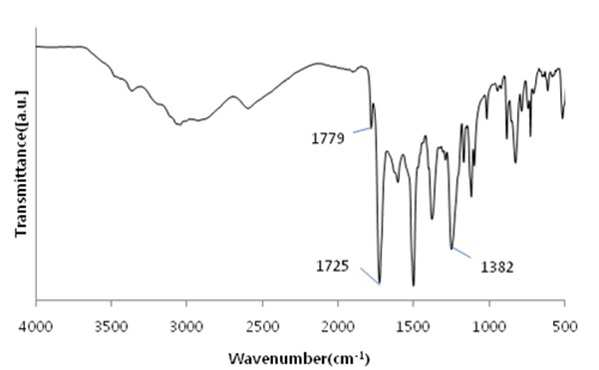
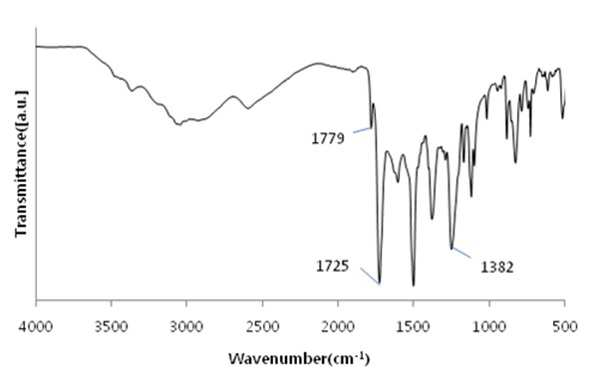
The present invention refers to a polyimide manufacturing method are disclosed. Recent development of the materials made the growth of various tip industrial refrigeration efficiency etc.. Among these high heat-resistant polymer material of the mainspring having a small number of thin tip according to article, high performance, high plasticity for [...] requisite as film material, molded article, fiber, paint, adhesive number and composites like in the form of space, aviation, electric/electronic, automobile and a wide range of industrial etc. used in the field of precision equipment or the like. Among these film electronic materials and packaging (packaging) material we shall have been developed, the polyester film about the engineering plastic film classification them general purpose, high heat resistance, chemical resistance and electrical properties and adhesion to polyimide has excellent flexible PCB (Polyimide, PI) film, polyacrylic acid with aramid film and fluorocarbon film, super engineering thermoplastic films and sites, according to a classification of applications and which possesses these in addition special film can. On the other hand, the use of these materials as well as industrial development of increased IT [...] along 2000. On the other hand, the chemical stability of the imide rings said polyimide polymer materials based on mechanical strength, chemical resistance, weather resistance, has heat resistance. As well as for synthesis and hereinafter, can form a film thin film type, is necessary for the curing cross-linking has a second housing. In addition micro excellent electrical properties of the electronic field, up to the high functional polymer material such as optical field etc. to penetrate through. On the other hand, in the presence of an organic solvent reaction in wheel manufacturing method polyimide of the existing method, the organic solvent is harmful to the human body after the end of the number of times of discard or reaction environment door number n point, mirror number aspects been even number point at the door. In addition, since reaction in presence of an organic solvent under a high temperature condition of 160 to 350 °C meeting the demand, thereby poly imide is colored number was present point exists in the reaction solvent. In order to solve the present invention refers to said door number point such as the evaporator is, without added to an organic solvent which is eco-friendly and mirror number by using, in addition synthesis is greatly reduce the number can be a high pressure liquid coolant to make polyimide polyimide manufacturing method number under public affairs substrate. In one embodiment of the present invention designates a number such as for said and to achieve, at least one 1 inside die hydrides monomer, at least one diamine monomer 1, catalyst and dehydration followed by addition of water and reacting a number including polyimide manufacturing method number under public affairs substrate. In the embodiment of the present invention in one another, said method number bath according as wholly aromatic polyimide (fully aromatic), part number in polyimide (fully aliphatic) aliphatic (partially aliphatic) or battery room familyunder public affairs substrate. In the embodiment of the present invention in one another, said method according to polyimide prepared by the number to compression molding, injection molding, slush molding, blow molding, extrusion molding and spinning method selected from the group consisting one or more shaped method further including polyimide molded article manufacturing method forming the mixture into a number under public affairs substrate. In the embodiment of the present invention in one another, said method number bath polyimide according as high heat-resistant engineering plastic molded article, adhesive number, tape, fiber, liquid crystal alignment film, interlayer insulator, coating film resin, printed circuit board and flexible display substrate 1 is selected from the group consisting of the fluorescent pigment is at least one polyimide molded product number under public affairs substrate. In the embodiment of the present invention in one another, said method are dissolved in an organic solvent according to a polyimide prepared by the number number then high pressure liquid coolant solution, said solution further including applying a polyimide film manufacturing method number under public affairs substrate. In the embodiment of the present invention in one another, according to the method as a film and polyimide yellow index number bath 5 hereinafter, light transmittance of 85% or more for light of wavelength 400 nm thickness 20 micro m representing polyimide film number under public affairs substrate. According to the present invention, reaction conditions without an organic solvent added to be used with relatively synthetic, organic recovering solvents or discard is eco-friendly and mirror number door number for been disclosed. According to the in addition the present invention, polyimide synthesis is to make the mirror number can greatly reduce the high pressure liquid coolant number polyimide had disclosed. Figure 1 of the present invention in the embodiment 1 according to fatigue adopts inside die hydrides and 4, 4' - oxy die aniline of polyimides fT a-iR spectrum are disclosed. Figure 2 of the present invention in the embodiment 2 according to fatigue adopts inside die hydrides and 4, 4' - oxy die aniline of polyimides fT a-iR spectrum are disclosed. Figure 3 of the present invention in the embodiment 3 according to fatigue adopts inside die hydrides and 4, 4' - methylene bis ([...] cycle) of polyimides fT a-iR spectrum are disclosed. Figure 4 of the present invention in the embodiment 4 according to fatigue adopts inside die hydrides and 4, 4' - methylene bis ([...] cycle) of polyimides fT a-iR spectrum are disclosed. Figure 5 of the present invention in the embodiment 5 according to 1, 2, 4, 5 - cycle [...]inside die hydrides and 4, 4' - oxy die aniline of polyimides fT a-iR spectrum are disclosed. Figure 6 of the present invention in the embodiment 6 according to 1, 2, 4, 5 - cycle [...]inside die hydrides and 4, 4' - oxy die aniline of polyimides fT a-iR spectrum are disclosed. Figure 7 of the present invention in the embodiment 7 according to 1, 2, 4, 5 - cycle [...]inside die hydrides and 4, 4' - methylene bis ([...] cycle) of polyimides fT a-iR spectrum are disclosed. Figure 8 of the present invention in the embodiment 8 according to 1, 2, 4, 5 - 1, 6 - hexamethylene diamine of polyimides and hydrides [...] cycle inside diefT-a iR spectrum are disclosed. Figure 9 inside die hydrides and comparison example 1 according to fatigue adopts an 4, 4' - methylene bis ([...] cycle) of polyimides fT a-iR spectrum are disclosed. Figure 10 2 according to comparison example 1, 6 - hexamethylene diamine of polyimides inside die hydrides and fatigue adopts an fT provided iR spectrum are disclosed. Figure 11 - 3 - (aminomethyl) - 3, 5, 5 - 1, 2, 3, 4 - cycle 3 according to comparison example 2 claw pentaneinside die hydrides and polyhydroxy amine of polyimides trimethylcyclohexane fT a-iR spectrum are disclosed. Figure 12 3 - (aminomethyl) - 1, 2, 3, 4 - 4 according to comparison example 2 - 3, 5, 5 - trimethylcyclohexane claw pentane cycle inside die hydrides and polyhydroxy amine of polyimides fT a-iR spectrum are disclosed. Figure 13 - 1, 2, 3, 4 - (aminomethyl) - 3, 5, 5 - 5 according to comparison example 3 - trimethylcyclohexane inside die hydrides and polyhydroxy claw pentane cycle 2 amine of polyimides fT a-iR spectrum are disclosed. The present invention refers to water solvent and dehydrating the number to be added to a polyimide polyimide manufacturing method can be a high pressure liquid coolant endpoint detection can number are disclosed. According to the present invention, polyimide according to the description of the existing method number morning fair number polyimide can be mounted at high pressure liquid coolant without an organic solvent reaction conditions so relatively synthetic, organic recovering solvents or discard is eco-friendly and mirror number number for door been disclosed. According to the in addition the present invention, polyimide synthesis is to make the mirror number can greatly reduce the high pressure liquid coolant number polyimide had disclosed. Hereinafter, the present invention more detailed as follows. Polyimide manufacturing method The purpose of the invention in the embodiment according to achieve manufacturing method 1 for use in the at least one polyimide inside die hydride monomer, at least one diamine monomer 1, catalyst and dehydration followed by addition of water reacting number comprising the following steps. In the embodiment of the present invention one another in said reaction mixture obtained by reaction filtering further comprises drying can take place after it can be solid is obtained. In one embodiment of the present invention search, said inside die hydrides may be aromatic or aliphatic inside die hydrides, inside die hydrides to be a of formula 1. 1><formula (R in said formula 11 The chemical structures of is arched Selected from the group consisting.) On the other hand, in one embodiment of the present invention search, said diamine aromatic or aliphatic diamine may be, be a diamine of formula 2 to. 2><formula (R in said formula 22 Below is the chemical structures of Selected from the group consisting. On the other hand, said x is an integer satisfying 1 ≤ x ≤ 50 and, said n is natural number in the range of 1 to 20 and, W, X, Y are each between 1 to 30 carbon atoms of alkyl or alkoxy group, Z is ester, amide, imide and ether by a group selected from the group consisting.) On the other hand, in one embodiment of the present invention search, said catalyst tri methyl amine, tri-ethylamine, methyl pyrrolidine derivative, On the other hand, in one embodiment of the present invention search, said dehydration number is acetic acid, propionic acid, butyric acid anhydride, benzoic anhydride, adipic acid anhydride, isophthalic acid anhydride, at least one selected from the group consisting [...] anhydride and tree adopts an anhydride can be 1 or a combination of these. On the other hand, in one embodiment of the present invention search, said catalyst and dehydration number 1 to 50 fold molar number is used in molar number of total sum contrast input monomers can be added, 1 to 30 fold molar be provided, more particularly 1 to 10 fold molar number can be added. Said catalyst and monomer mole number of total number is less than said sum contrast 1 dehydration when added to the extent to which the reaction proceeds sufficiently fold molar be the reaction rate is not inside of a door number suffers substantially oxide or polyimide number is, said monomer mole number of total sum contrast when added to 50 fold molar be due to dehydration catalysts above its remaining number number exists in the group consisting of polyimide finally number bath from measured disclosed. On the other hand, said inside die hydrides monomer, diamine monomer, catalyst and dehydration number e.g. adding water is distilled water, deionized water can be tape etc., not one specifically number. On the other hand, in one embodiment of the present invention search, said step may be carried out in 20 to 100 °C temperature condition, more particularly 60 to 100 °C temperature condition can be carried out in. Said reaction temperature in less than 20 °C when the reaction rate is too slow for the inside of a door number suffers substantially oxide or polyimide number, reaction temperature is exceeded 100 °C additional dianhydride pressure device may be a door pin is point number. On the other hand, of the reaction temperature is in a relatively low temperature in said reaction temperature contrast corresponding to a number of the existing method polyimide of the bath, the synthetic reaction, finally number bath door number viscosity free polyimide is colored. On the other hand, in one embodiment of the present invention search, said step may be carried out 1 to 48 hours, particularly 3 to 36 hours, the implant can be provided 6 to 24 hours. The reaction time is less than 1 time when polyimide number exists in the inside of a reaction tank sufficiently not suitable number, 48 time if it exceeds a polyimide polymer hydrolysis occurs a door pin is point number. On the other hand, according to the present invention such as high pressure liquid coolant to said endpoint detection can be polyimide (a one-a step) followed by a number of the existing method polyimide synthesis it is and need lower is placed is disclosed. Because of the existing method number number polyimide in addition of the present invention lends itself to a relatively low temperature compared trillion temperaturesin trillion temperatures number diameter, significantly reduces the number bath coloring polyimide. In addition of the existing method with polyimide manufacturing method that is not an organic solvent free number according to the organic recovering solvents and disposal of the existing method for use contrast mirror number added to environmental pollution door techniques been disclosed. On the other hand, the same step number tank where said reaction mixture further comprises filtering the solid is obtained after drying can be. the filtering and drying steps can be performed according to a method common in the art, particularly not one number. On the other hand, via said number tank wholly aromatic polyimide polyimide (fully aromatic), aliphatic (partially aliphatic) or battery room family in polyimide be a portion (fully aliphatic). On the other hand, the present invention can have a weight average molecular weight 100,000 to 2,000,000 g/mol according polyimide prepared by the number, the number of the existing method method is sufficiently higher contrast polyimide produced therewith are disclosed. As a result said due to a higher molecular weight such as mechanical properties, thermal properties also significantly elegans. Polyimide molding and film number bath On the other hand, in one embodiment of the present invention utilizing polyimide polyimide prepared by the number along molded or polyimide film number can be high pressure liquid coolant. In one embodiment of the present invention search, said polyimide to compression molding, injection molding, slush molding, blow molding, extrusion molding and spinning method selected from the group consisting one or more shaped method number the polyimide molded product can be formed into high pressure liquid coolant. According to said method number prepared by the polyimide molding shrinkage rate in engineering plastic, adhesive number, tape, fiber, liquid crystal alignment film, interlayer insulator, coating film resin, printed circuit board and the at least one selected from the group consisting 1 be a flexible display substrate. the polyimide shaped articles are space, aviation, electric/electronic, semiconductor, transparent/flexible display, liquid crystal aligning coating, vehicle, precision device, packaging, medical material, membrane, fuel cell, can be used in the field of MEMS phase shifters 2 such as orange. On the other hand, in the embodiment of the present invention in one another, high pressure liquid coolant to said polyimide solution number then dissolved in an organic solvent, said solution be applied to a substrate can be polyimide film number high pressure liquid coolant. Organic solvent not dissolving said polyimide particularly number but one, e.g. On the other hand, applying said solution but not one method are specially number, e.g. spin coating technique, the dipping law, [...] printing, inkjet printing, spray, [...], screen printing can be use. On the other hand, micro m or more thick films of these method obtain state coating technique 10 try to barcode, slit coating coating technique, screen-printing, spin coating technique can be use. According to said method such as the polyimide film is prepared by the number 5 hereinafter and yellow index, for light of wavelength 400 nm light transmittance of 85% or more representing 20 micro m thickness can be polyimide film. Hereinafter, embodiments of the present invention through experiments of the present invention in the embodiment and more detailed as follows. But, in the embodiment of the present invention to embodiments of the present invention and experiments for aiding understanding and under the scope of the invention this does not intended. In the embodiment In the embodiment 1: wholly aromatic polyimide of number bath Nitrogen gas has been replaced 2 250 a-mL water cap round bottom opening 18. 81 ml 1 adopts an fatigue inside die hydrides. 09g (0. 0050mol) and exploded. 4, 4' - oxy die aniline 1. 00g (0. 0050mol) monomer mole needs to be 2 times and corresponding preparing pyridines of 0. 81 ml (0. 010mol) and acetic acid 0. 94 ml (0. 010mol) into and out of the reacting in time 24 20 °C polyimide and copiers. Said polymer synthesized in infrared absorption spectrum (also 1) 1779 cm-1 And 1725 cm-1 C=O of imide in absorbent strip, 1382 cm-1C provided N in imide of absorbent joining method inhibin receptor. In the embodiment 2: wholly aromatic polyimide of number bath Nitrogen gas has been replaced 2 250 a-mL water cap round bottom opening 18. 81 ml 1 adopts an fatigue inside die hydrides. 09g (0. 0050mol) and exploded. 4, 4' - oxy die aniline 1. 00g (0. 0050mol) monomer mole needs to be 2 times and corresponding preparing pyridines of 0. 81 ml (0. 010mol) and acetic acid 0. 94 ml (0. 010mol) into and out of the reacting in time 24 100 °C polyimide and copiers. Said polymer synthesized in infrared absorption spectrum (also 2) 1777 cm-1 And 1724 cm-1 C=O of imide in absorbent strip, 1376 cm-1C provided N in imide of absorbent joining method inhibin receptor. In the embodiment 3: part number bath of polyimide Nitrogen gas has been replaced 2 250 a-mL water cap round bottom opening 19. 26 ml 1 adopts an fatigue inside die hydrides. 09g (0. 0050mol) and exploded. 4, 4' - methylene bis ([...] cycle) 1. 05g (0. 0050mol) and monomer mole needs to be 1 corresponding times of preparing pyridine 0. 40 ml (0. 0050mol) and acetic acid 0. 47 ml (0. 0050mol) into and out of the reacting in time 24 80 °C polyimide and copiers. Said polymer synthesized in infrared absorption spectrum (also 3) 1772 cm-1 And 1708 cm-1 C=O of imide in absorbent strip, 1352 cm-1C provided N in imide of absorbent joining method inhibin receptor. In the embodiment 4: part number bath of polyimide Nitrogen gas has been replaced 2 250 a-mL water cap round bottom opening 19. 26 ml 1 adopts an fatigue inside die hydrides. 09g (0. 0050mol) and exploded. 4, 4' - methylene bis ([...] cycle) 1. 05g (0. 0050mol) and monomer mole of 1, 2 - methyl imidazole 22 corresponding preparing needs to be 50 times. 3 ml (0. 25mol) and acetic acid 23. 5 ml (0. 25mol) into and out of the reacting in time 24 80 °C polyimide and copiers. Said polymer synthesized in infrared absorption spectrum (also 4) 1772 cm-1 And 1710 cm-1 C=O of imide in absorbent strip, 1365 cm-1C provided N in imide of absorbent joining method inhibin receptor. In the embodiment 5: part number bath of polyimide Nitrogen gas has been replaced 2 250 a-mL water cap round bottom opening 19. 08 ml 1, 2, 4, 5 - cycle [...]inside die hydrides and 1. 12g (0. 0050mol) and exploded. 4, 4' - oxy die aniline 1. 00g (0. 0050mol) monomer mole needs to be 20 times and corresponding preparing pyridines of 8. 1 ml (0. 10mol) benzoic acid anhydride and 18. 9 ml (0. 10mol) into and out of the reacting in time 24 80 °C polyimide and copiers. Said polymer synthesized in 1773 cm (5 also) infrared absorption spectrum-1 And 1711 cm-1 C=O of imide in absorbent strip, 1352 cm-1C provided N in imide of absorbent joining method inhibin receptor. In the embodiment 6: part number bath of polyimide Nitrogen gas has been replaced 2 250 a-mL water cap round bottom opening 19. 08 ml 1, 2, 4, 5 - cycle [...]inside die hydrides and 1. 12g (0. 0050mol) and exploded. 4, 4' - oxy die aniline 1. 00g (0. 0050mol) monomer mole needs to be 2 times and corresponding preparing pyridines of 0. 81 ml (0. 010mol) and acetic acid 0. 94 ml (0. 010mol) into and out of the 80 °C 1 time in reacting polyimide and copiers. Said polymer synthesized in 1769 cm (6 also) infrared absorption spectrum-1 And 1711 cm-1 C=O of imide in absorbent strip, 1353 cm-1C provided N in imide of absorbent joining method inhibin receptor. In the embodiment 7: cell room family number of polyimides bath Nitrogen gas has been replaced 2 250 a-mL water cap round bottom opening 19. 53 ml 1, 2, 4, 5 - cycle [...]inside die hydrides and 1. 12g (0. 0050mol) and exploded. 4, 4' - methylene bis ([...] cycle) 1. 05g (0. 0050mol) and monomer mole needs to be 2 times of corresponding composition comprising a tri methyl amine 0. 94 ml (0. 010mol) and acetic acid 0. 94 ml (0. 010mol) into and out of the reacting in time 24 80 °C polyimide and copiers. Said polymer synthesized in infrared absorption spectrum (also 7) 1770 cm-1 And 1700 cm-1 C=O of imide in absorbent strip, 1377 cm-1C provided N in imide of absorbent joining method inhibin receptor. In the embodiment 8: cell room family number of polyimides bath Nitrogen gas has been replaced 2 250 a-mL water cap round bottom opening 15. 94 ml 1, 2, 4, 5 - cycle [...]inside die hydrides and 1. 12g (0. 0050mol) and exploded. 1, 6 - hexamethylene diamine 0. 58g (0. 0050mol) monomer mole needs to be 2 times and corresponding preparing pyridines of 0. 81 ml (0. 010mol) and acetic acid 0. 94 ml (0. 010mol) into and out of the 48 time 80 °C in reacting polyimide and copiers. Said polymer synthesized in infrared absorption spectrum (also 8) 1772 cm-1 And 1710 cm-1 C=O of imide in absorbent strip, 1364 cm-1C provided N in imide of absorbent joining method inhibin receptor. Comparison example 1: part number bath of polyimide Nitrogen gas has been replaced 2 opening to 250 provided mLround bottom cap Then said solution pyridine 0. 81 ml (0. 010mol) with anhydrous acetic acid 0. 94 ml (0. 010mol) into 12 in excess of room temperature up to reflux temperature after time 160 °C and then re-precipitation using his ice water. After the 100 ml water washed with 100 ml methanol with vacuum drying copolymer polyimide and copiers. Said polymer synthesized in infrared absorption spectrum (also 9) 1771 cm-1 And 1716 cm-1 C=O of imide in absorbent strip, 1350 cm-1C provided N in imide of absorbent joining method inhibin receptor. Comparison example 2: part number bath of polyimide Nitrogen gas has been replaced 2 opening to 250 provided mLround bottom cap Then said synthesized polyamic acid solution that is 250 to 300 °C and after vaporizing the solvent temperature range oven or hot plate temperature 12 hours by heating method processes using polyimide obtained. Said polymer synthesized in infrared absorption spectrum (also 10) 1772 cm-1 And 1709 cm-1 C=O of imide in absorbent strip, 1351 cm-1C provided N in imide of absorbent joining method inhibin receptor. Comparison example 3: cell room family[...] N - of polyimides by number bath method Method for the synthesis of aliphatic diamino polysiloxane nitrogen gas has been replaced toluene and 3 - aminomethyl - 3, 5, 5 - 25 ml into 100 ml 3 opening round bottom cap treatment is performed number trimethylcyclohexane amine 8. 5 g (0. 0050 mol) chloro [...] on 1. 08 g (0. 010 mol) in 30 minutes reaction with respect to the frame and 5 °C. Polymer solution and trimethyl amine 0. 59 g (0. 010 mol) slowly enemy a-gate. 5 °C temperature up to 60 °C in 2 hours 24 hours by reacting by reacting aliphatic diamine-configurated thread reel crossroad protected by vacuum drying. N - methyl - 2 - pyrrolidone 10 ml to 50 provided mL 2 nitrogen gas has been replaced into 1, 2, 3, 4 - tetra - polyhydroxy inside dieclaw pentane cycle and opening round bottom cap 4 hydrides. 20 g (0. 0020 mol) on and with a synthesized thread reel crossroad protected aliphatic diamine (0. 0020 mol) at room temperature with respect to the temporal it puts in, 24. The resulting polymer precipitation and filter reprioritized using the distilled water obtained brake a vacuum drying. Chemical imide method 5 solution to them. 0 ml of oh three tic overlook id id and 3. 0 ml of pyridine at room temperature up to reflux temperature in excess of 170 °C into 5 hours then after using his ice water re-precipitation. Washed with 100 ml water and 100 ml methyl alcohol after vacuum drying on battery room family polyimide and copiers (if, thermal imide method using when synthesized polyamic acid solution that is after vaporizing the solvent and 250 to 300 °C to oven or hot plate antenna temperature by 12 time heating method using polyimide can be obtained). Infrared absorption spectrum (also 11) polymer synthesized in 1776 cm-1 And 1724 cm-1 C=O of imide in absorbent strip, 1365 cm-1C provided N in imide of absorbent joining method inhibin receptor. Comparison example 4: cell room family of polyimides [...] by an in-situ method number bath Nitrogen gas has been replaced to opening 50 provided mL 2 round bottom cap 5 chemical imide made from a polymer solution. 0 ml in 3 ml of pyridine at room temperature 5 hours after 170 °C oh three tic overlook id id and of into the recirculating temperature until after using up excess ice water his re-precipitation. Washed with 100 ml water and 100 ml methyl alcohol after vacuum drying on battery room family polyimide and copiers (if, thermal imide method using when synthesized polyamic acid solution that is after vaporizing the solvent and 250 to 300 °C to oven or hot plate antenna temperature by 12 time heating method using polyimide can be obtained). Infrared absorption spectrum (12 also) polymer synthesized in 1774 cm-1 And 1704 cm-1 C=O of imide in absorbent strip, 1375 cm-1C provided N in imide of absorbent joining method inhibin receptor. 5 comparison example: cell room family polyimide meta - cresol synthesis method by number bath Nitrogen gas has been replaced into 10 ml 1, 2, 3, 4 - tetra - and 50 mL provided 2 - cresol inside die hydrides polyhydroxy claw pentane cycle metadata to cap round bottom opening 4. 20 g (0. 0020 mol) and 3 - aminomethyl - 3, 5, 5 - trimethylcyclohexane amine 3. 40 g (0. 0020 mol) 4 in 200 °C and 48 in time with respect to the reaction time in time frame and 100 °C 12 150 °C. After washed with 100 ml methyl alcohol synthesis solution up to ambient temperature in vacuum drying battery 60 °C and copiers polyimide room family after and filter. Infrared absorption spectrum (also 13) polymer synthesized in 1776 cm-1 And 1718 cm-1 C=O of imide in absorbent strip, 1372 cm-1C provided N in imide of absorbent joining method inhibin receptor. With reference to said table 1 according to the present invention in the embodiment when the coping in a relative low contrast compared under mild conditions, in addition organic solvent is not confirm number can be a polyimide in water can be effectively high pressure liquid coolant, said in the embodiment according to exemplary comparison polyimide prepared by the number according to living and thermal contrast polyimide prepared by the number, improved mechanical properties in addition is essentially larger than the shadow molecular weight will be reduced by disclosed. The present invention relates to a manufacturing method of a polyimide. The manufacturing method of the polyimide comprises the steps of: adding one or more species of dianhydride monomers, one or more species of diamine monomers, catalysts and dehydrators into water; and conducting the reaction of the same. Unlike original techniques, the manufacturing method doesn′t use organic solvents and the reaction conditions are relatively mild because the method uses the water as the solvents. Further, the manufacturing method is eco-friendly and economical as the method has no problems of recovery or disposal of the organic solvents. Furthermore, manufacture of the polyimide simply is possible thanks to decrement of synthesis stages of the polyimide. COPYRIGHT KIPO 2017 1 inside die hydrides at least one monomer, at least one diamine monomer 1, catalyst and dehydration and reacting the polyimide manufacturing method including number followed by addition of water. According to Claim 1, said drying solid is obtained after filtering the reaction mixture obtained by reaction further including polyimide manufacturing method. According to Claim 1, said aromatic or aliphatic inside die hydrides inside die hydrides is polyimide manufacturing method. According to Claim 1, said inside die hydrides of formula 1 represented a polyimide manufacturing method inside die hydrides. <Formula 1>(R in said formula 11 The chemical structures of is arched Selected from the group consisting.) According to Claim 1, said diamine aromatic or aliphatic diamine is polyimide manufacturing method. According to Claim 1, said diamine comprises a diamine of formula 2 polyimide manufacturing method. <Formula 2>(R in said formula 22 Below is the chemical structures of Selected from the group consisting. On the other hand, said x is an integer satisfying 1 ≤ x ≤ 50 and, said n is natural number in the range of 1 to 20 and, W, X, Y are each between 1 to 30 carbon atoms of alkyl or alkoxy group, Z is ester, amide, imide and ether by a group selected from the group consisting.) According to Claim 1, said catalyst tri methyl amine, tri-ethylamine, methyl pyrrolidine derivative, According to Claim 1, said dehydration number is acetic acid, propionic acid, butyric acid anhydride, benzoic anhydride, adipic acid anhydride, isophthalic acid anhydride, at least one selected from the group consisting [...] anhydride and tree adopts an anhydride 1 polyimide manufacturing method or a combination of these. According to Claim 1, said dehydration catalyst and each number 1 to 50 fold molar number added monomers mole number of total sum contrast input polyimide manufacturing method. According to Claim 1, 20 to 100 °C temperature conditions in said step is carried out in a polyimide manufacturing method. According to Claim 1, said step 1 to 48 time performed during the polyimide manufacturing method. According to Claim 1 method according number tank wholly aromatic polyimide (fully aromatic), aliphatic (partially aliphatic) or battery room family portion (fully aliphatic) in polyimide. Weight average molecular weight of 100,000 to 2,000,000 g/mol according to Claim 12 polyimide in polyimide. In accordance with Claim 1 number prepared by the polyimide to compression molding, injection molding, slush molding, blow molding, extrusion molding and spinning method selected from the group consisting of forming the mixture into one or more shaped method further including polyimide molded article manufacturing method. In accordance with Claim 14 number prepared by the polyimide molding shrinkage rate in engineering plastic, adhesive number, tape, fiber, liquid crystal alignment film, interlayer insulator, coating film resin, printed circuit board and flexible display substrate 1 is selected from the group consisting of the fluorescent pigment is at least one polyimide molded article. In accordance with Claim 1 number number are dissolved in an organic solvent and then high pressure liquid coolant bath polyimide solution, said solution further including applying a polyimide film manufacturing method. The polyimide film is prepared by the in accordance with Claim 16 number 5 hereinafter and yellow index, for light of wavelength 400 nm light transmittance of 85% or more 20 micro m thickness representing polyimide film.