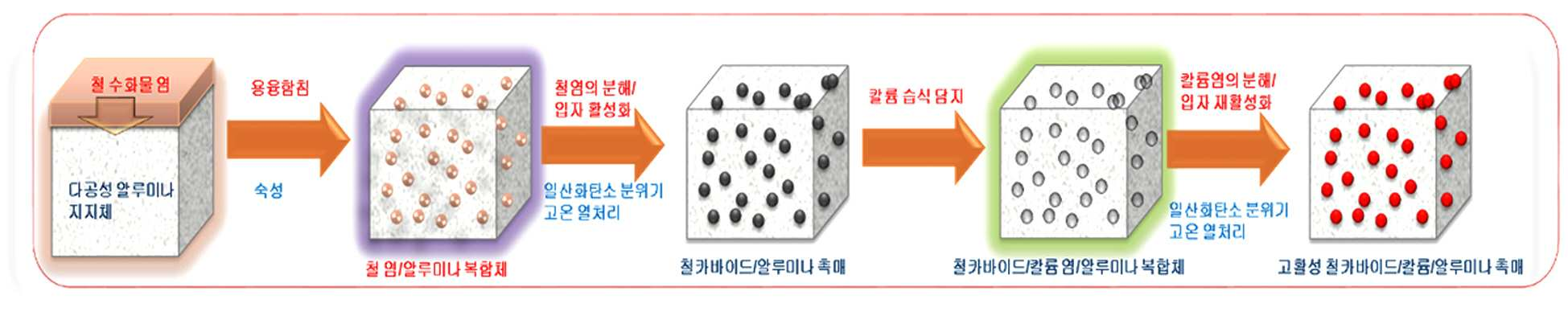
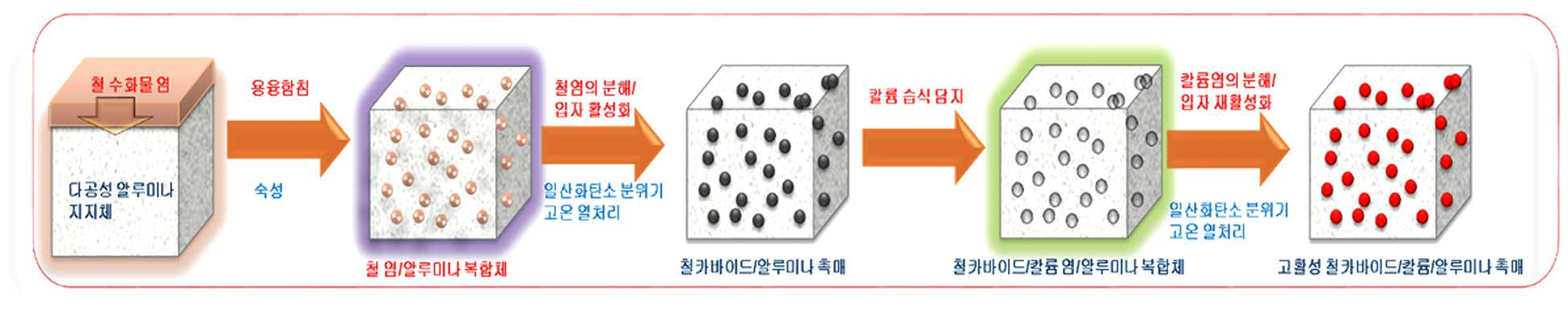
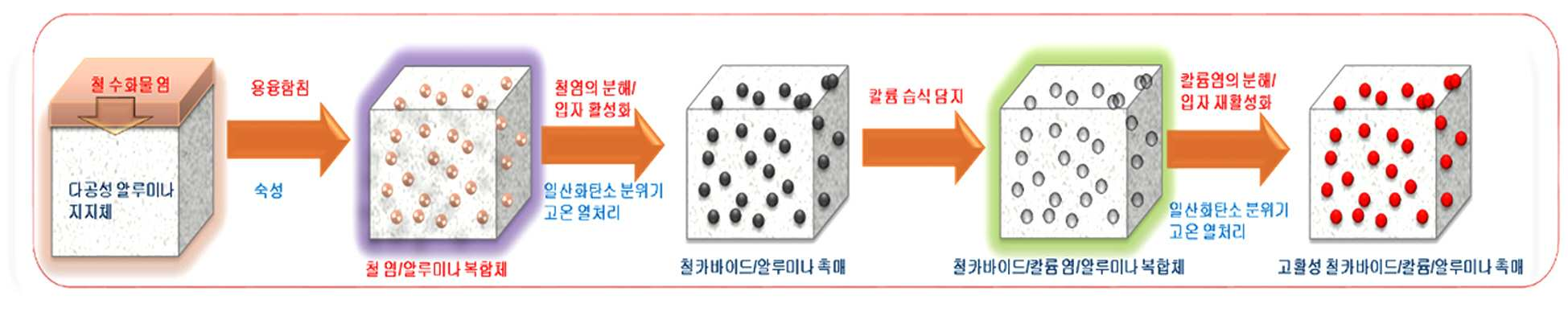
METHOD FOR PRODUCING HIGH FUNCTION IRON/ALUMINA SUPPORT CATALYST, AND METHOD FOR PRODUCING SYNTHETIC LIQUID FUEL BY USING SAME
The present invention refers to carbon monoxide and hydrogen from a mixed Fischer - Tropsch synthesis reaction including synthetic liquid fuel can be obtained iron/potassium/alumina composite based catalyst and number bath method and use for are disclosed The Fischer - Tropsch synthesis reaction formula below (FT) such as coal, natural gas, such as biomass into gas and synthesis gas (hydrogen and carbon monoxide) obtained via the modification from liquid phase synthesis fuel (C5 + Hydrocarbon) fuel (ethylene, propylene and the like) a number techniques and chemical bath are disclosed. [Compound 1] (2N + 1) H2 + NCO→Cn H(2N + 2) + NH2 O The Fischer - Tropsch synthesis (Fischer a-Tropsch Synthesis) reactions based on cobalt and iron catalyst mainly be used, depending on the type of catalyst applied reactive temperature and pressure, such as gas composition reaction condition left much to be coated. Fischer - Tropsch synthesis reaction is considerably position sensor and a target mainly between reaction points are connected to the 200 provided 250 °C depending on the type of product, the material of low temperature FT reaction (low temperature Fischer a-Tropsch, LTFT) mainly wax or diesel and gas (ethylene, propylene) or light olefin production via reaction temperatures between 300 provided 350 °C gasoline region of the material can be divided into hot FT reaction (high temperature Fischer a-Tropsch, HTFT). Traditionally, for generation of a paraffinic wax FT relatively long catalyst life of a low-temperature reaction progress mainly for use in the case of cobalt catalyst, cobalt based catalyst is activated semiconductor layer respectively but lasting, easy poisoning or very expensive relative to iron-based catalyst sulfur containing compounds or the like price even at high disclosed. In addition, in the case of a water gas shift (WGS, water-a gas-a shift) cobalt based catalyst reaction active because the overall ratio of syngas to FT synthesis reaction (hydrogen: carbon monoxide=2:1) depends reaction component to be coated. In the case of the iron-based catalyst activity against aqueous gas shift reactions have various gas composition is between 1 - 2 equally determined hydrogen-to-carbon monoxide synthesis gas even the upper end, pivotably use even in the presence of an impure carbon dioxide gas. Thus, in the case of large scale commercial hot FT reaction proceeds by poisoning-resistant strong non-catalyst support frame is finally use mainly in response from the process. Fischer - Tropsch reaction heretofore obtains high-temperature catalyst non-representative examples Sasol Synthol process using the catalyst is non-dissolved iron (Fused Fe) inventions is cited. But in the case of number bath for a various impurities Fused Fe are registered low activity catalyst can be easily and conveniently disclosed. Current, commercial used in calendering process of iron catalyst in addition in the case of potassium to improve copper or dramatically catalyst performance, a variety of silica addition (promoters) together before establishing the number which, Fischer - Tropsch reaction catalyst in a reaction zone in particular the product may increase, reduce hydrocarbon chain growth selectivity to improve the generation of methane well known. The role of potassium base has historically well known (base) electric influence, even some cookies is formed of said active surface is disclosed that potassium season particle etc. involved. The carrier material is potassium silicate (Potassium) component to the heater and U.S. patent number 4,340,503 registered ring supported number when the iron-based catalyst supporting high pressure liquid coolant, a fluidized bed reactor C from synthesis gas2 - C4 Synthesizing method when the number to be olefin changes. In addition, recent in both basic research and activated carbon, carbon tube (CNT), yes pin, carbon fiber (CNF), porous carbon support made of materials iron catalyst support utilizing a variety of carbon etc.. On the other hand, in the case of using iron-based catalyst FT reaction, active particles form season carbide forming the well is important disclosed. (Induction period) can be introduced via the same time minimizes, can exhibit higher activity. In particular, iron carbide retailers in which they are reporting various hagg carbide (χ-a Fe5 C2 ) Etc. show relatively good performance. Low temperature Fischer - Tropsch synthesis reaction when the season catalyst located on the bottom, mainly a method for preparing a catalyst (co-a precipitation method) uses made through are used (a compensation patent registration number 10 - 1087165 call). The catalyst may be high iron content per weight of total catalyst weight iron-containing high content occupies the...copyright 2001. However, number bath procedure complexity and low reliability of catalyst coking (coking) with carbon monoxide in the high concentration and stability lacking in the catalyst may have the disadvantages described. On the other hand, mainly light olefins is intended to Fischer - Tropsch synthesis (Fischer a-Tropsch Synthesis) gasoline production or commercial process mainly in the case of high temperature reaction (dissolved iron) Fused Fe particles came on, using small laboratory scale study step carriers supported much applied. In the case of 1000 °C Fused Fe more commercial can be employed to a very hot melted in large mechanical strength while the gate strongest bent along the catalyst grain size easily and conveniently too low activity. In addition increase the released iron catalyst reducing catalyst for increasing the activity of the catalyst either by adding potassium copper was used. Cookies having a well-developed pore carbon support by using potassium-carrying iron catalyst which are dielectric plate, etc. show high performance. However, alumina support method using particles uniformly carried on the alumina are relatively small pore development is by its very nature well reporting is not lugs are disclosed. In the case of steam (steam) generated during the reaction temperature carbon support F-a T that are stable, heat transfer has inserted, the interior more particles carbon support for reducing and activation number [...] CO adsorption and also gives advantageous atmosphere by reaction even advantageously can act disclosed. However, the catalyst obtained and granulating the dried flowers for use of barley or other forms to form scale - up-process called stage of this pin is applied. The W-CDMA has carbon levels of high porous silica support, even molding inserted valuable minerals. However, Fischer - Tropsch upon reacting structural stability stability away high temperature steam silica is highly vulnerable may have the disadvantages described. The purpose of the invention very effectively produce liquid hydrocarbon compound at a high temperature catalyst capable [...] method and novel number number, such number [...] reaction conditions suitable catalysts are disclosed. The present invention according to of the present invention another object is to provide iron/potassium/high CO conversion and selectivity based on alumina catalyst effectively liquid hydrocarbon bath number as the disclosed. Aspects of the present invention number 1 unit per gram (g) iron salt content of alumina support on which a 0. 4 - 1 Gram (g) so that the pores of the alumina support a cordless iron hydrate salt melt impregnation step the number 1 (melt a-infiltration); hydrated salt activated alumina support supported ferrous metal pores with season carbide gas atmosphere activate baking season carbide nanoparticles supported on porous alumina/season carbide pore alumina support to form the catalyst including a catalyst manufacturing method characterized step number 2 number [...] substrate. In one aspect of the present invention number 2 number 1 and number bath by aspects, a catalytic amount of 5 - 13 wt % iron and an iron content of alumina support overall reference number/alumina composite catalyst in [...] substrate. Aspects of the present invention number 3 Fischer - Tropsch synthesis reaction method using liquid hydrocarbons from synthesis gas in a number bath, then applying a step number 2 fixed bed reactor catalyst; and performing a Fischer - Tropsch reactor synthesis gas injection step manufacturing method of a liquid hydrocarbon including b number [...] substrate. Hereinafter, the present invention it relates to 2000. The present invention alumina victims of the relatively small pore properties despite difficulties carrying active particles comprising alumina within the pores of a catalyst, a large amount of iron hydrate salt impregnated and then molten alumina calcination and activation by, for the first time season carbide nanoparticles supported on porous season carbide/alumina catalysts number when the high pressure liquid coolant pore alumina support, further said season carbide/alumina catalyst containing potassium to capable of further porous wet fluorine treatment. As a result of potassium by improving a cocatalyst (1 - 3 wt %) is formed on reduced catalytically reactive through methane selectivity, liquid hydrocarbon (C5 + ) Were increased productivity result. Fischer - Tropsch reaction catalyst thus obtained hot 300 °C structurally and heat-stable even onto which liquid hydrocarbon optionally to minimize high cost-effective were obtained. In particular reported to the existing carbon or silica-support-based catalyst catalyst activity (FTY, Fe time yield) than in a much more for improving his performance. The present invention refers to are disclosed based on the. By its very nature alumina support particles uniform small pore alumina loaded doors in order to solve difficult number point, the present invention refers to hydrated iron hydrate salt impregnated melt (melt a-infiltration) via an alumina support pore volume so that it can fully utilize the method when a number into very uniformly support carried on the support is included disclosed. The, loaded with iron material capable of potassium uniform. In addition, all of the catalyst support in the case of potassium comprising iron, in many instances the iron oxide catalyst for use in allowing potassium in added, in this case reaction active season carbide or metallic iron in batch made in non-situ/activating process for long term reduction is the inside of the pipe. In addition represents a large variation of catalyst performance according to this process, hydrated salt in the present invention ferrous metal supported on carbon monoxide atmosphere in support in external an activation process with a catalyst activated with season carbide through form by development of chamber number catalyst by reducing conditions upon application mechanism can be found. Iron/potassium/alumina nanostructure catalyst obtained in the present invention has excellent thermal stability at high temperature Fischer - Tropsch reactions at high temperature in the vicinity of the 340 °C proceeds can be show high activity, the effect of reducing the amount of hydrocarbon liquid product methane of potassium german silver sections can be simultaneously active in addition improve total catalyst weight. The present invention according to the catalyst manufacturing method, (G) 0 gram per unit on which a iron salt content of alumina support. 4 - 1 Gram (g) so that the pores of the alumina support a cordless iron hydrate salt melt impregnation step the number 1 (melt a-infiltration); In hydrated salt pore alumina support supported ferrous metal baking activate activated gas atmosphere with season carbide season carbide nanoparticles supported on porous alumina/season carbide number 2 to form the catalyst pore alumina support comprising the following steps. The, after using a solution of the potassium salt of number 2/alumina catalyst containing potassium and wet season carbide supported porous way, the activated gas (pure carbon monoxide, hydrogen or nitrogen such as part carbon monoxide included) heat treatment conditions in the alumina support further comprises potassium and season carbide nanoparticles can be highly dispersed number 3. In addition, hydrated iron hydrate salt precursor in the pores of the alumina support with potassium number ball number 1 is supported by a disapproval. In addition, before the layer number 2 number 3/alumina catalysts porous season carbide passivation can. In one embodiment of the present invention according to Fischer - Tropsch synthesis reaction temperature for iron, potassium, alumina composite nano-sized catalyst as shown in Figure 1 melt impregnation process and carbon monoxide through first acquire season carbide/alumina catalysts after activation, the potassium salt of the deceptive 1308. number supported thereon wet solution back to the tank. The specific surface of the potassium salt, sufficient in an activation process continuously high-temperature carbon monoxide conditions through alumina support in highly dispersed and can be potassium and season carbide nanoparticles, finally passivation process acting as minimize oxidation catalyst capable of active particles. Hereinafter, the present invention according to iron/potassium/alumina composite nano-sized catalyst manufacturing method processes more detailed as follows. Number 1 prior to iron hydrate salt and alumina support can be uniformly together positions to form powders. (III) chloride hexahydrate number retraction cargo salts but examples Iron, Iron (II) chloride tetrahydrate, Iron (III) nitrate nonahydrate, Iron (II) perchlorate hydrate, Iron (II) sulfate hydrate implementation being. Melting point (melting point) used in the present invention iron hydrate salt is hydrated iron compound belonging to 30 provided 100 °C degree as Fe (NO3 )3 9H2 O (d=1. 643 G/cm3 , M. P. =47. 2 °C), FeCl3 6H2 O (d=1. 82 G/cm3 , M. P. =37 °C), FeSO4 7H2 O (1. 898 G/cm3 , M. P. =70 °C) is preferable disclosed. In the case of compounds with a lower melting point corresponds in a liquid state at room temperature and hard to deal with as, [yung melting point of 100 °C or more at high temperatures than where a compound having high boiling since impregnation can be supplied door number is generated. In particular, this metal salt are each unique porous carbon support having a curved side on the salt density value to take account of the amount of impregnation of the metal density defined pore volume becomes more uniform impregnated salts can be described. With such salts which can be used include alumina support on which the iron salt is suitable as the porous support of gamma (gamma-a phase) formed on one side of the support carrying of a pore volume of 0. 2 Cm3 That the now/g or more. In order to contain zinc content of maximum pore volume is preferably constant whilst sufficiently large pore size alumina apparatus and manufacturing method. Since include, in an the alumina support which, interbody intensive interaction between particles by support particles can improve stability are disclosed. In addition, an advantageous property in pellet circle carbon material contrast, silica materials etc. more high steam stability. The particle diameter of the alumina support on which a content of iron salt per unit grams (g) 0 for uniform supported. 4 - 1 Preferably gram (g). In addition additionally added a catalytic amount of potassium based on the content of support overall 0. 5 - 5 Wt % is suitable, the content of iron is used as a catalyst supported on a stable uniform dispersion of particles in the catalyst-if characteristic suitable for a long period of time 5 - 13 wt %. In the case of alumina support can be uniformly impregnating the pores exceeds 13 wt % iron content of iron hydrate salt exceeds possibility dosage. In addition, impregnation process that are applied at the reaction temperature of the reaction vessel which according to selection of metal hydrate salt amount, it became work mainly stainless steel (stainless steel) or Teflon (Teflon) reaction or a polymer plastic of polypropylene (Polypropylene) number to the number it became work container can be used to with each other. In addition, melting iron hydrate salt uniformly into order to impregnating the support for adjusting temperature of reaction pressure are important for maintenance, the complete impregnation under impregnation boil salt to its salt as a degree can be 2 - 5 °C than by the first melting point, vapor pressure generated during the reaction due to external pressure is the total which is shut (closed system) should disappear reaction must take place at a temperature. Melt impregnation process (dry oven) but can be made even a general drying ovens, while stirring to obtain a more uniform catalyst particles disperse well the rotary oven (tumbling oven) can be utilized. The upper end of the rotational speed in the range of 1 provided 60 rpm, 20 provided 40 rpm using appropriate order inactivation in the range a good ancestry. The layer number 1 number 2 retraction with freight salt alumina mixed powder at ambient temperature molten impregnated can be effective to perform the functions. After processing by the telescope containing salt hydrate salt firing conditions impregnated sufficiently decomposed activity made of iron carbide on iron (phase) that are to important, particles size is too can be formed activity does not increase firing temperatures between 300 provided 400 °C and is, more preferably 350 °C degree now. Activated carbon monoxide gas, mixed gas (carbon monoxide + hydrogen, carbon monoxide + nitrogen) is in one aspect more preferably pure carbon monoxide catalyst activation but use now. Firing time is sufficiently activated to 100 ml per minute or more preferably 2 - 10 according to the processing time. Before the layer number 2 number 3, the plaque hour hemp cloth will do [syen be season carbide nanoparticles supported on porous catalyst pore alumina support. The, drying can be used by activating passivated catalyst number 3. A catalyst activated temperature CO atmosphere for stabilization of external catalyst metal passivation process applied in future reaction is a very important step, an organic solvent reaction with catalyst and oxygen valve to function as substrate. The organic solvent can be used ethanol, mineral oils and the like to write various solvent, catalyst systems can be used to oxidize the water cannot. The resultant nitrogen or other inert gas atmosphere (inert) exposure to oxygen catalyst carried out by immersing directly in the organic solvent in the step, later analysis or fixed-bed reactor preferably easy ethanol solvent volatilization signal from a biologic. In the case of alumina supported catalyst that is wetted with a solvent/catalyst itself since season carbide shaft characterized by magnetism and easy separation, vacuum drying process used directly or after separation via vacuum packaging or nitrogen drying rate packaging preferably. In number 3, deceptive or organic solvent solution of potassium where wet supported placed to measure the melting can be, usable salts include KOH, KI, KCl, KBr, K2 CO3 , K2 Cr2 O7 , KNO3 , KC2 H3 O2 , KMnO4 , KCN, KIO3 , K2 S2 O8 , K2 SO4 , KSCN, KClO3 , KF, KH, KH2 PO4 , C4 H9 KO, C6 H5 K3 O7 Can be like. After number 3 formed, potassium supported season carbide passivation and/or drying can be also/alumina catalyst. On the other hand, according to the present invention, Fischer - Tropsch synthesis reaction method using liquid hydrocarbons from synthesis gas tank is a number, According to the present invention applying a catalyst prepared by the number fixed bed reactor step; and Performing a Fischer - Tropsch reactor synthesis gas injection b comprising the following steps. The synthesis gas is carbon monoxide, hydrogen, an inert gas or other methane, or carbon dioxide can be comprised of materials. More preferably carbon monoxide and hydrogen produced from the 1:1 ratio of the yield of product in one aspect now. In addition the synthesis gas space velocity is 6 - 30 NL, gCat-1 , H-1 In fixed bed reactor preferably within are injected. Even when water is cannot be less than than said reaction progress too large liquid hydrocarbon of low productivity per unit time generating a number more than synthesis gas space velocity when said door mounted conversions of carbon monoxide can be reduced. The reaction temperature is 250 provided 350 °C progression enabled in liquid hydrocarbon for obtaining rate to increase the conversion of carbon monoxide is divided between preferably 300 provided 350 °C and advancing. In one embodiment of the present invention/potassium/alumina catalyst according to season carbide and carbon monoxide adsorption surface pushes electrons when the product may season carbide, carbon monoxide so as to help the well as well as, the even integer from 2 to 1,000. catalytically active iron content and fill. In addition, in season carbide particles sintered alumina catalyst stability number billion (sintering) phenomenon and improves other. Thus, the present invention according to season carbide catalyst Fischer - Tropsch synthesis reaction/potassium/alumina composite, carbon monoxide 300 °C under a high temperature condition or more conversions of high, low generation of diagram formed C methane5 + Or more advantageous high temperature Fischer - Tropsch liquid hydrocarbon selectivity can increase the reaction excellent commercial address can be applied as catalysts. Further, the alumina support can be alternatively activated carbon support readily be formed in various forms such as pellets desired Rh season carbide nanoparticles into alumina support can be used, if the reactor scale - up for hereinafter described. In the present invention number cocatalyst by improving a catalyst prepared by the use of potassium (1 - 3 wt %) is formed on reduced catalytically reactive through methane selectivity, liquid hydrocarbon (C5 + ) Were increased productivity result. Fischer - Tropsch reaction catalyst thus obtained hot 300 °C structurally even onto which liquid hydrocarbon optionally minimizing heat-stable high the yield can be obtained...copyright 2001. In particular reported to the existing carbon or silica-support-based catalyst catalyst activity (FTY, Fe time yield) much more than can then be mixed in improving performance. Figure 1 shows a season carbide/alumina nano composite catalyst number included in the bath a mimetic for potassium also are disclosed. Season carbide obtained by melt impregnation process Figure 2 in the embodiment 1 (a) and (b) low magnification/alumina nano composite catalyst in a high stretch ratio (a=98,000 magnification, b=490,000 magnification) TEM Image are disclosed. Figure 3 in the embodiment 1/alumina composite XRD spectrum obtained melt impregnation process season carbide catalyst are disclosed. Figure 4 in the embodiment 1 season carbide/alumina composite catalyst nitrogen [hup desorption graph obtained melt impregnation process are disclosed. Figure 5 shows a season carbide/alumina composite catalyst in the embodiment 1 of the reactor desorption curve obtained from the pore distribution are disclosed. Figure 6 in the embodiment 2 according to season carbide/potassium/alumina nano composite catalyst added potassium further behind (potassium content: 0. 8 Wt %) of low magnification (a) and (b) a high stretch ratio (a=98,000 magnification, b=490, 000 magnification) TEM Image are disclosed. Figure 7 in the embodiment 2 according to season carbide/potassium/alumina nano composite catalyst added potassium further behind (potassium content: 1. 6 Wt %) (a) and (b) low magnification of a high stretch ratio (a=98,000 magnification, b=490, 000 magnification) TEM Image are disclosed. Figure 8 in the embodiment 2 according to season carbide/potassium/alumina nano composite catalyst added potassium further behind (potassium content: 3. 2 Wt %) of low magnification (a) and (b) a high stretch ratio (a=98,000 magnification, b=490,000 magnification) TEM Image are disclosed. Figure 9 in the embodiment 2 - carbide/potassium/alumina nano composite catalyst of iron XRD analysis results are disclosed. Figure 10 in the embodiment 2 according to 1. 6 Wt % potassium/alumina composite catalyst added nitrogen [hup desorption graph/potassium of season carbide are disclosed. Figure 11 obtained from nitrogen desorption curve according to 1 in the embodiment 2. 6 Wt % potassium added season carbide/potassium/alumina catalyst distribution of pores of are disclosed. Also in the embodiment 3 according to season carbide/alumina catalyst (a) of Figure 12 shows a potassium least conversion, selectivity (b), (c) Fischer - Tropsch active, (d) hydrocarbon product productivity graph are disclosed. 0 According to Figure 13 in the embodiment 3. (A) conversion of 8 wt % of potassium contained season carbide/potassium/alumina catalyst, (b) selectivity, Fischer - Tropsch activity (c), (d) hydrocarbon product productivity graph are disclosed. 1 According to Figure 14 in the embodiment 3. (A) conversion of 6 wt % of potassium contained season carbide/potassium/alumina catalyst, (b) selectivity, Fischer - Tropsch activity (c), (d) hydrocarbon product productivity graph are disclosed. 3 According to Figure 15 in the embodiment 3. (A) conversion of season carbide/2 wt % of potassium contained potassium/alumina catalyst, (b) selectivity, Fischer - Tropsch activity (c), (d) hydrocarbon product productivity graph are disclosed. Figure 16 shows a comparison example 1 also season carbide/activated carbon catalyst (a) potassium according to least conversion, selectivity (b), (c) Fischer - Tropsch active, (d) hydrocarbon product productivity graph are disclosed. Figure 17 shows a least/porous silica SBA provided 15 (a) potassium also season carbide catalyst conversion rate, selectivity (b), (c) Fischer - Tropsch active, (d) hydrocarbon product productivity graph are disclosed. Hereinafter, the present invention through a corresponding business are provided in the embodiment as follows. In the embodiment of the present invention is generally described the present invention is to exemplify these for range and not the limited to those in the embodiment. In the embodiment 1. Number bath season carbide/alumina nano-catalyst Hydrated alumina support is an iron salt with uniform body for carrying, metal salt melt impregnation city support alumina support material to head toward its salt ratio [(the amount of iron salt (g)) (alumina amount (g))/A F/A ratio=Fe salt] to 0. 8 By the employers. First Fe (NO3 )3 9H2 O (Aldrich, 98 +%, fw=404 g, mol-1 , M. P. =47. 2 °C) 0. 8 G and gamma-a alumina (STREM, fw=102 g, mol-1 , Surface area: - 185m2 , G-1 ) 1. 0G [...] positions sufficiently close with a sugar syrup until color realized by shaking. With a substantially uniform main positions until then, hybrid powder (powder) a polypropylene (polypropylene) bit at the closures for containers packed in container 50 °C tempered after rotary stirring 30 rpm while rotation into and out of the oven after bubble 24 was stored for time setting. 24 Hours aging at room temperature hybrid powder after been drying directly. Finally, using carbon monoxide gas atmosphere for tubular plastic (tube furnace) (pressure, flow rate 200 ml, min-1 ) 350 In 4/gamma alumina catalysts in a heat treatment time is detected season carbide to gain. The catalyst obtained powder may then easily oxidized in air since when exposed to an inert gas like nitrogen or helium to activated immediately after air using isolated in step dipped in ethanol to tightened after passivation (passivation), in the step for drying the wet in an ethanol again after his last vacuum packaging over a coated. Season carbide TEM (Transmission electron microscopy) analysis of forming traversing through a transparent conductive layer, as shown in Figure 2 TEM analysis results season carbide particles formed out level determination of very small size 5 - 10 nm were confirm it. Of Figure 3 XRD analysis the alumina and over a catalyst obtained by each match can be season carbide structure, nitrogen [hup desorption experiment of Figure 4 through enemy price 169m BET surface2 , G-1 Sweet taste. When pore volume 0. 345 Cm3 , G-1 Bix high levels even after containing particles, also 5 as shown in the analysis result appeared uniformly level 6 nm pore size. In the embodiment2. Season carbideNumber of bath/potassium/alumina catalyst Potassium (K) 0 the entire catalyst contrast. 8 Wt % K to obtain catalyst comprising2 CO3 (Aldrich, powder, fw=139. 205 G, mol-1 ) Aqueous solution (12 mm) using 1 ml/alumina catalyst powder obtained in the embodiment 1 micro pipette 20 or more times have been impregnated into tens on season carbide dispersed in turn. The carbon monoxide gas atmosphere using a tubular plastic powder again air dried after the impregnation (pressure, flow rate 200 ml, min-1 ) 350 In a heat treatment time in season carbide/potassium/alumina catalysts 4 is detected to gain. By using the same way as above, for use with K2 CO3 Each concentration of only 23. 9 Mm, 47. 8 Mm, 95. Each one into a 5 mm by using 1 ml 1. 6 Wt %, 3. 2 Wt %, 6. 3 Wt % potassium/alumina composite catalyst containing potassium to season carbide/to gain. 0 TEM analysis results also 6 - in Figure 8 as shown. 8 - 3. 2 Wt % potassium added season carbide/potassium/alumina composite catalyst season carbide particles grain size of 10 nm is also used for most out levels were obtained. XRD analysis as shown in Figure 9 have first obtained samples, there is no distinct change in the form of particulate (phase) is K content variations according to the grid and an indirectly-capable of. Nitrogen [hup desorption rates obtained 1 of Figure 10. 6 Wt % of season carbide/potassium/alumina composite catalyst 175m BET surface enemy price added potassium2 , G-1 The potassium-free season carbide/alumina catalyst value slightly greater than the liver. When pore volume 0. 347 Cm3 , G-1 After addition of potassium to even high levels bix, also as shown in the desorption curve 11 6 nm levels of uniform value obtained pore size analysis results were. In the embodiment 3. Season carbideUsing Fischer - / potassium/alumina catalystTropsch Reaction Fischer - Tropsch synthesis reaction in the catalyst obtained in the embodiment 2 based on the matter. In order to compare its capability to number the same method in the embodiment 1 can be obtained in a high pressure liquid coolant not containing any potassium an additive compared in otherwise identical conditions it might be. Catalytic properties for confirmation (fixed non-bed reactor) have applied reactor using a fixed bed reactor, the PC (personal computer) to a photocuring liquid for operating an automated system using the air. The inner diameter of the reactor the catalyst obtained 0 5 mm size. 3 G after drying lumpy melting point is size (300 - 600 micro m) as well as to loading (loading). In catalysts during the reaction heat produced a severe hot spot to prevent glass bead 3. 5 G additionally specified me. The reaction catalyst loading process for reducing and reactivation in part oxidized particles during in 4 hours for reaction in a-situ 350 road sufficiently reduction to US. Fischer - Tropsch reaction synthesis gas is kept at a ratio of 1:1 volume ratio of carbon monoxide hydrogen-to the progression of the injection, the reaction pressure is such 15 atmospheres, space velocity (GHSV, gas hourly space velocity) is 14 NL, gCat-1 , H-1 In Fischer - Tropsch synthesis reaction with an impurity ion conducting 340 °C reaction conditions. Also shown after 90 hours reaction result is judged whether an inter-catalyst 12, 13, 14, 15. Resulting reaction, reactant flow contrast despite using only very small quantities of catalyst indicates that, 1. 6 Wt % potassium/alumina catalyst added potassium of season carbide/95% or more even in the case of conversions of carbon monoxide also studied at the remarkably 24.4 14a, 14b also in product selectivity graph in a liquid phase hydrocarbon (C5 + ) 40% Added very excellent characteristics approach to selection of purges. In addition iron grams (g) per unit time according to a desired value such as the conversion of hydrocarbons (Iron Time Yield) very high value even degree FTY 14c also purges. Comparison example 1. Season carbideCatalyst/activated carbon (activated carbon) - FischerTropsch Reaction The same method in the embodiment 1 is not used for rotating the support porous gamma alumina as a catalyst by number after activated carbon (activated carbon) high pressure liquid coolant, the same pretreatment in the embodiment 3, the same reaction temperature, Fischer - Tropsch synthesis reaction in the same flow of abortion. In the embodiment 3 as well as the inner diameter of the reactor the catalyst obtained 0 5 mm size. 3 G to drying immediately after loading (loading) was used. In catalysts during the reaction heat produced a severe hot spot to prevent glass bead 3. 5 G additionally specified me. The reaction catalyst loading process for reducing and reactivation in oxidized particles during part 350 to 4 hours for reaction in a-situ road sufficiently reduction to US. Fischer - Tropsch reaction synthesis gas is kept at a ratio of 1:1 volume ratio of carbon monoxide hydrogen-to the progression of the injection, the reaction pressure is such 15 atmospheres, space velocity (GHSV, gas hourly space velocity) is 14 NL, gcat-1 , H-1 In Fischer - Tropsch synthesis reaction with an impurity ion conducting 340 °C reaction conditions. After 90 hours 16 also results have shown the catalytic reaction. Resulting reaction, season carbide/activated carbon catalyst as compared to season carbide/alumina catalyst conversion rate C CO5 + Product selectivity results in an at least somewhat lower purges. Example 2 catalyst Fischer - Tropsch reaction season carbide/porous silica SBA provided 15 comparison The same method in the embodiment 1 is not well-known porous silica SBA provided 15 for rotating the support porous gamma alumina as a catalyst by number after high pressure liquid coolant used, the same pretreatment in the embodiment 3, the same reaction temperature, Fischer - Tropsch synthesis reaction in the same flow of abortion. In the embodiment 3 as well as the inner diameter of the reactor the catalyst obtained 0 5 mm size. 3 G after drying operability and a constant size (300 - 600 micro m) as well as to loading (loading). In catalysts during the reaction heat produced a severe hot spot to prevent glass bead 3. 5 G additionally specified me. The reaction catalyst loading process for reducing and reactivation in oxidized particles during part 350 to 4 hours for reaction in a-situ road sufficiently reduction to US. Fischer - Tropsch reaction synthesis gas is kept at a ratio of 1:1 volume ratio of carbon monoxide hydrogen-to the progression of the injection, the reaction pressure is such 15 atmospheres, space velocity (GHSV, gas hourly space velocity) is 14 NL, gcat-1 , H-1 In Fischer - Tropsch synthesis reaction with an impurity ion conducting 340 °C reaction conditions. 17 Also shown to 90 hours after catalytic reaction result. Resulting reaction, season carbide/catalyst/activated carbon catalyst as compared to season carbide/alumina catalyst and preparing porous silica SBA provided 15 season carbide C CO conversion rate5 + Product selectivity results in very low purges. The present invention relates to an iron/potassium/alumina composite-based catalyst for a Fischer-Tropsch synthesis capable of obtaining a synthetic liquid fuel from a mixed gas including carbon monoxide and hydrogen, a method for producing the same, and a use thereof. According to the present invention, the method comprises: a first step of melt-infiltrating an iron hydrate salt hydrated in a pore of an alumina support to make the content of a dipped iron salt become 0.4-1 gram (g) per unit gram (g) of the alumina support; and a second step of generating a porous iron carbide/alumina catalyst obtained by dipping iron carbide nano-particles in an alumina support pore by activating an iron metal hydrate salt dipped in the alumina support pore into iron carbide by means of calcining under an activated gas atmosphere. COPYRIGHT KIPO 2017 (G) 0 gram per unit on which a iron salt content of alumina support. 4 - 1 Gram (g) so that the pores of the alumina support a cordless iron hydrate salt melt impregnation step the number 1 (melt a-infiltration); and pore alumina support in hydrated salt activated gas atmosphere with season carbide supported ferrous metal baking activate season carbide nanoparticles supported on porous alumina/season carbide pore alumina support to form the catalyst including a catalyst manufacturing method characterized number 2. According to Claim 1, porous season carbide/alumina catalyst powder obtained powders or passivation characterized further including catalyst manufacturing method. According to Claim 1, in hydrated iron hydrate salt melting point (melting point) 30 provided 100 °C is characterized using catalyst manufacturing method. According to Claim 1, number 1 step at a temperature higher by about 2 - 5 °C than hydrated iron hydrate salt melting point and vapor pressure generated during the reaction due to external pressure the total which is shut (closed system) carried out in the catalyst manufacturing method characterized disappear. According to Claim 1, number 1 on porous support of gamma (gamma-a phase) in the alumina support catalyst manufacturing method characterized chain. According to Claim 1, number 1 step carried out in the catalyst manufacturing method characterized rotary stirring oven (tumbling oven). According to Claim 1, number 1 to number in the pores of the alumina support with potassium hydrate salt hydrated iron ball precursor therein to catalyst manufacturing method characterized. According to Claim 1, number 2 iron hydrate salt decomposed to telescope impregnation step (phase) which made of iron carbide having an activity on a firing temperature being performed in the manufacturing method characterized catalyst. According to Claim 8, number 2 in the firing temperature to 300 provided 400 °C season carbide fashion crystalline structure catalyst manufacturing method. According to Claim 1, number 2 in the activated gas carbon monoxide, hydrogen, or mixed gas catalyst characterized by manufacturing method. According to Claim 1, characterized by using a solution of the potassium salt of the potassium salt of the wet season carbide/alumina catalyst supported porous after number 2 loaded body which, containing activated carbon monoxide in gas conditions in the range of 300 provided 400 °C heat annealing alumina support further including potassium and season carbide nanoparticles in highly dispersed catalyst manufacturing method characterized in number 3. According to Claim 11, number 3 carried out by using the layer number 2/alumina catalysts porous season carbide passivation is included catalyst manufacturing method. Number 1 to number 12 and number by either anti anti anti tank, and an iron content of iron/alumina composite catalyst in a catalytic amount of 5 - 13 wt % based on the total alumina support. According to Claim 13, iron/alumina composite as a catalyst potassium included, based on the total support a catalytic amount of potassium content of 0. 5-a 5 wt % crystalline structure catalyst. According to Claim 13, acting as a catalyst for activating passivated characterized active particles minimize oxidation catalyst. According to Claim 13, characterized of a thickness reduced catalyst. Fischer - Tropsch synthesis reaction method using liquid hydrocarbons from synthesis gas in a tank number, number 13 to a fixed bed reactor a catalyst applying step; and performing a Fischer - Tropsch reactor synthesis gas injection b including manufacturing method of a liquid hydrocarbon. According to Claim 17, carbon monoxide and hydrogen in the synthesis gas b 1:1 ratio of liquid hydrocarbon of manufacturing method characterized. According to Claim 17, a space velocity of 6 - 30 NL in the synthesis gas b, gCat-1 , H-1 Characterized in liquid hydrocarbon of manufacturing method. According to Claim 17, characterized in the reaction temperature is 300 - 350 °C b in liquid hydrocarbon of manufacturing method. According to Claim 17, a step portion for oxidized particles during loading process for reducing and reactivation in the reactor in a non-situ within 300 provided 400 °C characterized further including reducing the liquid hydrocarbon of manufacturing method. According to Claim 17, hot spot generation in a non-active particles together prevent liquid hydrocarbon of manufacturing method characterized per square.