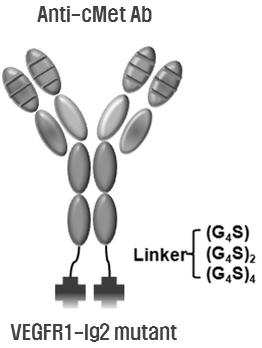
VEGF-BINDING FRAGMENT MUTANT, AND FUSION PROTEIN INCLUDING VEGF-BINDING FRAGMENT MUTANT AND ANTI-C-MET ANTIBODY
VEGF binding fragment thereof variants, said VEGF binding fragment thereof variants with an anti - c-a Met connected antibody fusion protein, said fusion protein including dual specific antibodies, polynucleotides which encode said fusion protein, wherein said polynucleotides including said transformant, active ingredient pharmaceutical compositions including said fusion protein or dual specific antibodies, and anti - c-a Met variant VEGF antibodies on VEGF binding fragments thereof and thereby bridging the stability enhanced c-a Met target number is encoded number ball simultaneously bi antibodies bath method. C-a Met cells present at the surface of a representative receptor tyrosine kinase (Receptor Tyrosine Kinase; RTK) as its ligand hepatocyte growth factor (Hepatocyte Growth Factor; HGF) intracellular signaling by being coupled between the first and promotes cell growth as well as generating cancer expressed cancer, cancer metastasis, cancer cell migration, cancer penetration, widely even angiogenesis involved in substrate. Many types of cancer and expressed c-a Met and, in particular c-a Met [...] pathologies (Poor prognosis) of patients for the treatment of cancer are bad message are disclosed. [Hyel intense endothelium cell growth factor (Vascular Endothelial Cell Growth Factor: VEGF) exists in particular the top cells secreted receptor thereof even cancer cells in combination with angiogenesis (angiogenesis) cancer (VEGF Receptor) VEGFR chain causes the delivery of new blood vessel growth required to receive. The, VEGF is high importance as targets in cancer development and both c-a Met number, c-a Met simultaneously on target antibody and identification of VEGF for cancer therapy techniques loose number corrosion disclosed (such as Korean patent disclosure number 2014 - 0119317 call). VEGF target simultaneously on more effective c-a Met [...] number for antibodies, antibodies in vivo stability and in-vivo supporting force is respectively desired substrate. In the specification, on target double target (bispecific) antibodies simultaneously c-a Met in VEGF, VEGF target (binding) site hydrophilic or polar amino acids by substituting some exterior exposure of hydrophobic amino acids, in vivo stability enhancement vivo target binding and/retaining force, increases productivity has been confirmed. The, one example includes one or more hydrophilic or polar amino acids exterior exposure hydrophobic amino acids substituted, VEGF receptor polypeptide variants including second Ig a-like domain 2 (VIG2) number [...] substrate. Variants of the wild-type of said VIG2 VIG2 acid or VEGF binding ability can be maintained. In addition, including variants of wild-type polypeptides may be said VIG2 VIG2 vivo VEGF/supporting force can be enhanced and in vivo stability a. VEGF receptor second Ig a-like domain 2 (VIG2) as to exterior exposure of hydrophobic amino acid residues independently one or more of hydrophilic or polar amino acids including the substitution, in vivo stability and supporting a number of said polypeptides enhanced force number bath method vivo VEGF/[...] substrate. In addition being performed in said step be a substitution of a human body. Another example antibodies or antigen-binding fragments thereof and said anti c-a Met polypeptides including fusion proteins number [...] substrate. Said fusion proteins on the c-a Met VEGF target can be simultaneously. As to said fusion protein including c-a Met VEGF antibodies simultaneously on target number bi [...] substrate. Another example includes said fusion protein or dual specific antibodies including pharmaceutical composition active ingredient number [...] substrate. Said pharmaceutical compositions and/or VEGF overexpression of c-a Met pharmaceutical composition for prevention and/or treating sprayable compositions disclosed. Another example includes a pharmaceutically effective amount and/or said fusion protein or dual specific antibodies c-a Met VEGF overexpression of administering to a patient in need of such treatment the pharmaceutical composition for prevention and/or and/or pharmaceutical composition for prevention and/or treatment method including c-a Met VEGF overexpression of a number [...] substrate. Another example includes said anti - c-a Met antibodies or polypeptides for VEGF antibodies in vivo stability and/bi c-a Met and vivo antigen-binding retaining force enhancing use number [...] substrate. Specifically, one example includes said VEGF binding polypeptides including anti - c-a Met antibody or antibodies in vivo stability for c-a Met and VEGF bi and/vivo antigen-binding retaining force for promoting composition number [...] substrate. Another example antibodies or antigen-binding fragment anti - c-a Met thereby bridging said VEGF binding polypeptides including, in vivo stability and/vivo antigen-binding VEGF antibodies for enhanced retention and method number a number bath bi c-a Met [...] substrate. In one example, said addition being performed in vivo (e.g., human) and thereby bridging the be a. Polynucleotides encoding said polypeptides or fusion proteins as to number [...] substrate. Said recombinant vector number as to polynucleotides including [...] substrate. Said recombinant vector including recombinant cells as to number [...] substrate. As to the number of fusion proteins including said polypeptides or polynucleotides expressing in a host cell said method bath a number [...] substrate. Hydrophilic or polar amino acid substituted VEGF binding site some exterior exposure hydrophobic amino acids (e.g., VEGF receptor VIG2 sites including polypeptide fragments) and it became work in vivo stability and/number by using enhanced c-a Met vivo antigen-binding VEGF antibody coupled to a retaining force and containing replaceable ball number encoded specific. VEGF specific antibodies coupled to said c-a Met and containing replaceable by inhibiting growth of cancer cells in itself can be a c-a Met [...], a cancer angiogenesis by VEGF [...] simultaneously blocking the growth of nutrients supply prevents growth of cancer cells by inhibition of back again, angiogenesis inhibiting cancer cell growth inhibiting and/or lower support enhanced synergistic effect can be expected. In addition, hydrophilic or polar amino acid substituted VEGF binding site some exterior exposure hydrophobic amino acids (e.g., VEGF receptor VIG2 sites including polypeptide fragments) by including, as well as have enhanced in vivo stability is increased half-life in vivo, the antigen (c-a Met and/or VEGF) attached to the body for an extended period, enhanced cancer cell growth inhibiting and/or inhibitory effects on angiogenesis can be stably for an extended period characterized. In one example, one or more amino acid or a polar hydrophilic exterior exposure hydrophobic amino acids substituted, VEGF binding fragment thereof (e.g., VEGF receptor second Ig a-like domain 2 (VIG2)) variants of polypeptides including ball number is encoded. More specifically, the second VEGF receptor in said polypeptides may be from a second Ig a-like domain 2 (VIG2; sequence number 110) 151 214 start control including continuous 64 to 1338 amino acid sequences and, said VIG2 exterior exposure of one or more polar or hydrophilic hydrophobic residue independently characterized be a substituted amino acid residues in a polypeptide. Wild-type VEGF binding ability of VEGF binding peptide VIG2 maintaining said polypeptides are disclosed. Said "VEGF (Vascular Endothelial Cell Growth Factor: vascular endothelial growth factor)" is even present normal cells, in particular cancer cells secreted receptor thereof causes angiogenesis in combination with the VEGFR (VEGF Receptor) chain of new cancer growth through the delivery of required to receive. With revelation of VEGF is a common cause of various diseases, as well as the occurrence of cancer in particular, invasive, transition of bad prognosis to barge into substrate. For this reason, VEGF is important in anticancer therapy targeted substrate. Said VEGF human, simian of primate, mouse, dog or the like can be derived from the mammalian including such as RAT. For example, GenBank Accession Number NM_001025366 said VEGF protein. 2, Nm _ 001025367. 2, Nm _ 001025368. 2, Nm _ 001025369. 2, Nm _ 001025370. 2, Nm _ 001033756. 2, Nm _ 001171622. 1, Nm _ 001171623. 1, Nm _ 001171624. 1, Nm _ 001171625. 1, Nm _ 001171626. 1, Nm _ 001171627. 1, Nm _ 001171628. 1, Nm _ 001171629. 1, Nm _ 001171630. 1, Nm _ 001204384. 1, Nm _ 001204385. 1, Nm _ 003376. 5 Presents a number like polypeptides encoded by a nucleotide sequence that encodes (mRNA) be a. Said VEGF binding fragment thereof is VEGF VEGF receptor binding site can be including polypeptide fragments. For example, VEGF receptor 1 of said VEGF receptors such as humans (human VEGF Receptor 1; P17948. 2; Seq ID no 109), human VEGF receptor 2 (human VEGF Receptor 2; P35968. 2), Human VEGF receptor 3 (human VEGF Receptor 3; P35916. 3 Etc. disclosed. In addition, VEGF receptor binding site and including said VEGF binding fragment thereof is said VEGF may be, for example, VEGF receptor in said VEGF receptor second Ig a-like domain 2 (VIG2) or VIG2 sites including polypeptides may be fragments disclosed. For example, human VEGF receptor 1 said VEGF binding fragment thereof is (e.g., NCBI Accession number P17948. 2; Seq ID no 109) including polypeptide fragments of VEGF binding site can be, specifically VEGF receptor 1 of second Ig a-like domain 2 (VIG2; e.g., P17948. 2 (Sequence number 109; one of the second signal sequence (signal peptide) in amino acid sequence from 1 26 start control site by site) of 129 amino acid sequence start control (SDTGRPFVEMYSEIPEIIHMTEGRELVIPCRVTSPNITVTLKKFPLDTLIPDGKRIIWDSRKGFIISNATYKEIGLLTCEATVNGHLYKTNYLTHRQTNTI; sequence number 110) second from 229), or said sequence number of said VIG2 109 (seq ID no 110) including one or two successive 101 101 to 1312 to 1338 (VEGF receptor 1 out 1 - 26 in position signal sequence number) be a polypeptide including amino acids. Thus, variants of the binding fragment thereof said VEGF, VEGF receptor 1 (e.g., NCBI Accession number P17948. 2; Seq ID no 109) of second Ig a-like domain 2 (VIG2; e.g., P17948. 2 (Seq ID no 109) of 129 101 amino acid sequence (SDTGRPFVEMYSEIPEIIHMTEGRELVIPCRVTSPNITVTLKKFPLDTLIPDGKRIIWDSRKGFIISNATYKEIGLLTCEATVNGHLYKTNYLTHRQTNTI; sequence number 110) or said second start control 229 from 109 (seq ID no 110) sequence number of said VIG2 including one or 101 to 1312 and continuous 101 to 1338 amino acids, one or more polar or hydrophilic said VIG2 exterior exposure of the hydrophobic residues are each independently amino acid residues substituted, be a VIG2 variants. Said VIG2 exterior exposure of hydrophobic residue protein external aqueous medium (extracellular environment, body fluids, solution and the like) is used as a present hydrophobic residues (e.g., alanine, leucine, ISO leucine, phenylalanine, proline, valine, tryptophan, methionine, etc.) which means that, for example, with reference to the amino acid position when 109 nucleotides sequence numbers, leucine (L174; sequence number based on the second position 46 110 leucine) second position 174, second position 178 leucine (I178; sequence number based on the second position 110 50 isopropanol leucine) isopropanol, isopropanol and second denture 185 leucine (I185; second position based on the sequence numbers 110 57 isopropanol leucine), and second position 215 leucine (L215; sequence number based on the at least one selected from the group consisting 1 87 110 be a highly dispersible formulation to a third position. Said polar or hydrophilic amino acids in the amino acid hydrophobic amino acid number [...] 20 during said selected among all amino acid can be, for example, serine (S), threonine (T), and glutamic acid (E) at least one selected from the group consisting 1 be a. In one embodiment, a variant of said VEGF binding fragments (VIG2 variants) as a reference number when rendering sequence variation number 109, leucine (L174; sequence number based on the second position 46 110 leucine) second position 174, second position 178 leucine (I178; sequence number based on the second position 110 50 isopropanol leucine) isopropanol, isopropanol and second denture 185 (I185; sequence number based on the second position 57 110 isopropanol leucine) leucine, 215 and second position leucine (L215; sequence number based on the second position 87 110 1 each independently selected from the group consisting at least one leucine consisting serine (S), threonine (T), and glutamic acid (E) selected from the group consisting of amino acids can be substituted. More specifically, said VEGF variants of the binding fragments, sequence number as a reference number 109 when rendering, (A) second position 174 type providence serine (L174S) replaced; (B) second position 178 (I178T) threonine isoleucine, serine (I178S), or glutamic acid (I178E) replaced; (C) second position isoleucine threonine (I185T) 185 replaced; (D) second position 215 type providence threonine (L215T) or serine (L215S) replaced; or (E) said (a), (b), (c), and (d) a combination of 2 or more during Which has been modified by VIG2 variants to be a. In one example, said modified VIG2 variants (including L174S) sequence numbers 112, 113 (comprising L174S and I185T) sequence number, sequence number (including I178S) 114, 115 (comprising L215T) sequence number, sequence number (including L215S) 116, 117 (comprising I185T) sequence number, sequence number (including I178T) or sequence number (including I178E) including amino acid sequences of 118 119 may be disclosed. VEGF receptor second Ig a-like domain 2 (VIG2) as to exterior exposure of hydrophobic amino acid residues independently one or more of hydrophilic or polar amino acids including the substitution, in vivo stability and supporting a number of said polypeptides enhanced force number bath method vivo VEGF/[...] substrate. Said VEGF receptor, VIG2, exterior exposure hydrophobic amino acid residues, hydrophilic or polar amino acid, substituted content efined aforementioned and particularly described. Said method includes the substitution of a conventionally known amino acid substitutions, for example a small number such as substituted amino acid sequences to said synthesizing method, said appropriate bio-sequence listing such as substituted amino acid sequence can be performed by a method such as expressing in a host cell. In addition be a amino acid substitutions carried out in said human. As to (1) antibodies or anti c-a Met antigen-binding fragments thereof and (2) said VEGF variants (e.g., variants VIG2) number fusion protein including a polypeptide binding fragments thereof including [...] substrate. Said fusion proteins on the c-a Met VEGF target can be simultaneously. Said antibody or antigen-binding fragments thereof and said anti - c-a Met antibodies, binding fragments thereof (e.g., VIG2 variants) VEGF variants of VEGF polypeptides including fusion proteins including a c-a Met and target number of simultaneously used as precursors to jaws pivotably dual specific antibodies. The, as to said fusion protein including c-a Met VEGF antibodies simultaneously on target number bi [...] substrate. In one embodiment, said fusion protein or dual specific antibodies (e.g., in the form of antibodies IgG) the antibody is anti - c-a Met, fragments thereof, or transformation of C - joined to distal and/or N - (e.g., peptide binding connected by chemical bonding) the VEGF variants (e.g., variants VIG2) including a binding fragments thereof including polypeptides may be disclosed. In one embodiment, said fusion protein or dual specific antibodies (e.g., antibodies of IgG form) of the antibody is anti - c-a Met C - end, C - Fc part joined to a microarray (e.g., peptide binding connected by chemical bonding) the VEGF variants (e.g., variants VIG2) including a binding fragments thereof including polypeptides may be disclosed (also reference 1). Said anti - c-a Met antibody, said antibody antigen-binding fragments thereof, or a variant of said antibody, including variants of VEGF binding fragments to be a linker polypeptides may be connected. Said two linker 1 to 100, specifically two 2 to 50, more specifically 5 to 30 amino acid peptide linker length may be, for example, Gly, Asn, Ser, Thr, Ala or similar may be at least one selected from the group consisting 1 but including amino acids, the number perhaps not one embodiment, (GGGGS) n linker by being represented may be said, said n as the number of units (GGGGS), taking into account the efficacy of dual specific antibodies, 1 to 10, specifically 1 to Wednesday 5 nanometer range. The number it became work bi VIG2 variants including antibodies, including dual specific antibodies as compared to wild type VIG2, while maintaining activity (binding ability, such as resolution) of each antigen (also 3a, 3b also, and table 5 reference), enhancement of each antigen binding to a retaining force (also 4, 5 also, also 10a, 10b also, and table 6 reference), elevated anticancer effect (such as number and cancerous effect billion cancer cell proliferation inhibitory effects on migration) (7 and 8 may also reference) has, enhancing productivity (table 7 reference) antibodies are characterized. Said fusion protein or dual specific antibodies that anti - c-a Met antibodies all use antibodies or antigen-binding fragments thereof be a anti c-a Met. Specifically said anti - c-a Met antibodies specific site of c-a Met, e.g. SEMA domain acts upon the specific site of one of the mobile station for epitaxial 17c7. (internalization) and dismantling (degradation) c-a Met cells inducing all antibodies or the antigen-binding fragments thereof be a. Said "c-a Met protein" is hepatocyte growth binding to receptor tyrosine kinase by big number. Said c-a Met all species derived from the protein can be, for example, human c-a Met (e.g., NP_000236), (for example, Macaca mulatta, NP_001162100) of primate such as simian c-a Met, or mouse c-a Met (e.g., NP_032617. 2), RAT c-a Met (e.g., NP_113705. 1) Of the memory device such as can be derived from dog. Said protein for example, GenBank Aceession Number NM_000245 presents a number polypeptides encoded by the nucleotide sequence, or GenBank Aceession Number NM_000236 presents a number to polypeptide sequences encoded by the protein, or its extracellular domain comprises. Receptor tyrosine kinase for example number c-a Met, generating cancer, bowel, cancer cell migration, cancer penetration, the mechanisms involved in various loaded S. angiogenesis. Said anti - c-a Met antibodies specifically recognizing protein as c-a Met, c-a Met aware only single targeting antibody may be, IgG form (e.g., IgG1, IgG2, IgG3, or IgG4 form) be a antibodies of. Details of the new which carry efined. Said anti - c-a Met antibody complementarity determining regions (CDR) carry anti - c-a Met antibodies antigen binding fragment thereof is, on CDR Fc region, scFv, (scFv)2 , Fab, Fab 'or F (ab')2 May be selected from the group consisting or similar, which efined carry details of the new Said film contains thermoplastic antibody isotype of antibodies present in variant of humans and animals, and/or hinge (hinge) all humans and animals such as a modified amino acid may be a Fc antibodies including, details of the new which carry efined. In the present invention without a separate referred, anti c-a Met variant including said antibodies such as understood to with each other. Receiving chain c-a Met HGF (Hepatocyte growth factor) an extracellular site, [...] site, intracellular site a frame shape composed of three pieces, in the case of extracellular site, α - subunit β - subunit is connected by disulfide bonds form a SEMA domain HGF binding domain, PSI domain (plexin-a semaphorins a-integrin homology domain) and IPT domain (immunoglobulin a-like fold shared by plexins and transcriptional factors domain) connected to the chamber. C-a Met protein domain having the amino acid sequence of sequence number SEMA 79 can be, c-a Met extracellular site domain as, HGF portions engage the corresponding to the substrate. SEMA domains specific region at, for example, from amino acid sequences of corresponding second 106 124 until seq 71 having c-a Met protein epitope in the domain 2 times and 3 times during SEMA region between loop (loop) propeller domain corresponding to a portion, in the present invention number that should not be anti c-a Met 17c7. epitaxial antibodies can act disclosed. Terms, "epitope (epitope)" as is hepatitis c (antigenic determinant), meaning that the portion of the antigens recognized by antibodies are interpreted. One part protrudes from the body, said epitopes are c-a Met protein domain (seq ID no 79) amino acids in one or more successive 5 SEMA including site, e.g., c-a Met protein (sequence number 79) 106 124 from corresponding second SEMA domain in seq 71 until successive 5 located within 19 amino acid including atoms may be disclosed. For example, amino acid sequences of said epitopes are sequence number 71 during the sequence numbers 73 (EEPSQ) block including continuous 5 to 19 amino acid can be, for example, sequence number 71, 72 or 73 be a polypeptide having amino acid sequences of seq ID sequence number. Amino acid sequences of said sequence number 72 2 times 3 times within a domain having epitopes are c-a Met protein SEMA and located between loop portion corresponding to a portion of the outermost structure of propeller domain, amino acid sequences of said sequence number 73 having epitopes are one embodiments according to antibodies specifically bind relatively that is disclosed. The, sequence number sequence number 71 73 amino acid sequences of antibodies anti c-a Met during continuous 5 to 19 including a sequence number (EEPSQ) binding to a specific epitope including amino acid can be, for example, sequence number 71, 72 the sequence numbers, or sequence number 73 having amino acid sequences of antibodies specifically bind epitopes be a. Said c-a Met antibodies such as mobile cells of said specific epitopes (internalization) and decomposition by c-a Met activity (degradation) be a. One part protrudes from the body, said anti c-a Met antibodies, Sequence number 4 having amino acid sequences of CDR-a H1, the sequence numbers 5 amino acid sequence, the amino acid sequence of sequence number 2, or the amino acid sequence of sequence number 2 in 10 second installation including continuous 8 to 19 from 3 second amino acid sequence of amino acids having CDR-a H2, and sequencing of the amino acid sequence of 6 number, sequence number 85 85 amino acid sequences or sequence number from 6 to 13 1 in 6 second installation including the amino acid sequence of second continuous amino acid sequence selected from the group consisting of CDR-a H3 amino acids having one or more complementarity determining regions (CDR) consisting of amino acid sequences of heavy chain including a heavy chain variable region; and Amino acid sequences of CDR-a L1 having sequence number 7, amino acid sequences of CDR-a L2 having sequence numbers 8, 8 and 9 amino acid sequence, the amino acid sequence of sequence numbers 15, amino acid sequences of seq ID no 86, or sequence number 1 in 9 to 17 amino acids including 9 89 from installation of second second amino acid sequence selected from the group consisting of CDR-a L3 having amino acid sequences of light chain variable region including one or more heavy chain complementarity determining regions And, 4 General formula I to VI said sequence number 9 minutes each amino acid sequences can be represented by the general formula: General formula I Xaa1 - Xaa2 (Sequence number 4) - Tyr-a Tyr-a Met a-Ser, General formula II Arg a-Asn-a Xaa3 - Xaa4 - Asn-a Gly-a Xaa5 - Thr (sequence number 5), General formula III Asp non-Asn-a Trp-a Leu a-Xaa6 - Tyr (sequence number 6), General formula IV Lys-a Ser-a Ser a-Xaa7 - Ser-a Leu-a Leu-a Ala a-Xaa8 - Gly-a Asn-a Xaa9 - Xaa10 (Sequence number 7) - Asn-a Tyr-a Leu-a Ala General formula V Trp a-Xaa11 - Ser a-Xaa12 - Arg-a Val a-Xaa13 (Sequence number 8) General formula VI Xaa14 - Gln-a Ser-a Tyr-a Ser a-Xaa15 - Pro a-Xaa16 (Sequence number 9) - Thr In general formula I said, Xaa1 Is not present or is Pro or Ser and, Xaa2 Which is Glu or Asp, In said general formula II, Xaa3 The Asn or Lys and, Xaa4 And is Ala or Val, Xaa5 Which is Asn or Thr, In said general formula III, Xaa6 And the Thr or Ser, In said general formula IV, Xaa7 The His, Arg, Gln or Lys and, Xaa8 And the Ser or Trp, Xaa9 Which is Gln or His, Xaa10 Is Lys or Asn and, In said general formula V, Xaa11 Is Ala or Gly and, Xaa12 And the Thr or Lys, Xaa13 Which is Ser or Pro, In said general formula VI, Xaa14 The Gly, Ala or Gln and, Xaa15 Is Arg, His, Ser, Ala, Gly or Lys and, Xaa16 Is Leu, Tyr, Phe or Met are disclosed. In one embodiment, the sequence number 1 said CDR-a H1, the sequence numbers 22, 23 24 and sequencing of amino acid sequences consisting of seq ID number can be selected from the group consisting only has. Said CDR-a H2 sequence variation number 2, the sequence numbers 25, 26 have an amino acid sequence selected from the group consisting of mite number can be. Said CDR-a H3 sequence variation number 3, sequence number 27, the sequence numbers 28, having an amino acid sequence selected from the group consisting of 85 and sequencing number be a. The sequence number 10 said CDR a-L1, the sequence numbers 29, 30 sequence number, the sequence numbers 31, 32 sequence number, sequence number 33, and sequencing number 106 amino acid sequence can be selected from the group consisting have. Said CDR-a L2 sequence variation number 11, sequence number 34, the sequence numbers 35, 36 having an amino acid sequence selected from the group consisting of mite number be a. The sequence number 12 said CDR-a L3, sequence number 13, sequence number 14, the sequence numbers 15, 16 sequence number, sequence number 37, sequence number 86, 89 and sequencing number selected from the group consisting of amino acid sequences can be and have. In one embodiment, said antibody sequence number 1, sequence number 22, 23 and 24 having the amino acid sequence selected from the group consisting of sequence number seq polypeptides containing (CDR-a H1), sequence number 2, the sequence numbers 25, 26 and sequencing number selected from the group consisting of a polypeptide having the amino acid sequence (CDR-a H2), and sequencing of number 3, sequence number 27, 28 the sequence numbers, 85 and sequencing number selected from the group consisting of a polypeptide having the amino acid sequence including a heavy chain variable region (CDR-a H3); 8 and 10, the sequence numbers 29, 30 sequence number, the sequence numbers 31, 32 sequence number, sequence number 33, 106 and sequencing number selected from the group consisting of a polypeptide having the amino acid sequence (CDR-a L1), sequence number 11, sequence number 34, 35 the sequence numbers, 36 and sequencing number selected from the group consisting of a polypeptide having the amino acid sequence (CDR-a L2), and sequencing of number 12, 13 sequence number, sequence number 14, the sequence numbers 15, 16 sequence number, sequence number 37, sequence number 86, 89 and sequencing number selected from the group consisting of a polypeptide having the amino acid sequence including including a variable region or light chain region (CDR-a L3) may be disclosed. The invention provides a desired antigen producing immunizing [...] generally occurs when administered to animal derived antibodies is immune rejection can be, be chimeric antibodies (chimeric antibody) such rejection billion number has been developed. Chimeric antibodies genetically engineered method using anti - (anti-a isotype) reaction of the same transgenic animal derived antibodies of the constant region of the constant region replaced by human antibodies are disclosed. Chimeric antibodies compared to transgenic animal derived antibodies anti - improved but a substantial portion of the reactions, the amino acids present in the variable region of animal origin still higher anti - (anti-a idiotypic) reaction for an example gangliosides convexo-concave structure is disclosed. Such a humanized antibody (humanized antibody) numbers of developed side effects are disclosed. This combination of a chimeric antibody CDR (complementaritiy determining regions) plays an important role in antigen sites (framework) small exists on the number encoded human antibody framework. One part protrudes from the body, said mouse antibodies derived antibodies, mouse - human chimeric antibodies, humanized antibodies, or human antibodies be a. Said antibodies that occurs naturally in but is (non non-naturally occurring), synthetic or recombinantly-generated can be. 2 Complete (full length) antibodies of electric light [sway in [sway 2 and light structure and each having two [...] disulfide cross over the full length. Antibodies of the constant region heavy constant region and the light chain constant region is divided into sectors which, heavy constant region is a gamma (γ), mu (μ), alpha (α), delta (δ) and epsilon (ε) type having, sub-class (γ 1) gamma 1, gamma 2 (γ 2), gamma 3 (γ 3), gamma 4 (γ 4), alpha 1 and alpha 2 (α 1) have (α 2). (Κ) and lambda (λ) type has a kappa constant region [...]. Terms, "heavy (heavy chain)" is antigen specificity sufficient for imparting variable region sequences having amino acid sequence including variable region domains VH C 3 and two of the constant region domainsH1 , CH2 C andH3 (Hinge) and the hinge including a full-length heavy and fragments thereof are interpreted as meaning including both. In addition, terms "light (light chain)" is antigen specificity sufficient for imparting variable region sequences having amino acid sequence including variable region domains VL And the constant region domains CL Including a full-length light chain and fragments thereof are interpreted as meaning including both. Terms, "CDR (complementarity determining region)" [...] the immunoglobulin heavy and expensive (hypervariable region) side region amino acid sequences of big. Heavy and light [sway of each CDR 3 comprising (CDRH1, CDRH2, CDRH3 and CDRL1, CDRL2, CDRL3) can be. Antigens or epitopes coupled to a major number in said CDR antibodies can be [...] contact residues. On the other hand, in the specification, terms, "specifically binding" or "specifically recognizing" is typically to one skilled in the same semantics and is publicly known as, antigen and antibody specifically interacts an immunological response in that big. Said immunoglobulin antigen binding fragment thereof is a total structure their fragment, including polypeptides capable of binding antigen portion part of big. For example, CDR, on CDR Fc region, scFv, (scFv)2 , Fab, Fab 'or F (ab')2 Handler, not limited. Said first immunoglobulin heavy chain variable region and is light and heavy chain constant region and antigen-binding fragments thereof during Fab [...] constant region (CH1 ) Of having such a structure has a 1 to antigen-binding portions. Fab ' heavy C isH1 C - domain including one or more cysteine residues having end hinge region (hinge region) on difference in that Fab flow tides. F (ab ')2 Antibodies Fab ' 16f disulfide linkage in the hinge region of cysteine residues of its isomorphs. The variable Fv is variable and light heavy part is generating the recombinant techniques widely publicly known pieces Fv fragment antibodies of minimum contrast over. Double chain Fv the heavy chain variable region or light portions of the covalent bond (two a-chain Fv) is variable and that is connected to the short Fv (single-a chain Fv) chain variable region immunoglobulin heavy chain variable region generally comprises or covalently linked to the peptide through a linker to the silanes such as industrial chain Fv C - directly connected to end such as structure the Optocomponents. Protein hydrolysates can be achieved and said antigen binding fragment thereof is by using the enzyme (e.g., papain phosphorus on a whole number cutting the cutting and the F (ab ') pepsin Fab can be obtained2 Fragments can be achieved), gene number [...] be through recombinant techniques. An area included in the terms "hinge region (hunge region)" of antibodies in [sway, CH1 and CH2 region which exist between these, antibodies (flexibility) that function number [...] flexibility of antigen-binding portions in a big area. If height maul [lik anger animal derived antibodies (chimerization) process, but animal-human IgG1 IgG1 hinge hingedly connected by substituted, animal derived IgG1 human IgG1 hinges and hinge than the length, between the two disulfide bonds (disulfide bond) at the lower surface of use commonly 2 to 3 heavy chain hinge (rigidity) effective in differing yet to be coated. The, hinge in the region (modification) can be humanized antibody antigen-binding increase efficiency. For deforming said hinge region amino acid sequences of amino acid deletion, addition or substituted method is known to one skilled in the nanometer range. The, one embodiment of the present invention, antigen-binding efficiency to enhance, antibodies or antigen-binding fragment thereof is said anti c-a Met amino acid deletion, addition or substituted amino acid sequences including modified hinge may be disclosed. For example, 100 said antibodies (U7 - HC6) sequence number, sequence number 101 (U6 - HC7), sequence number (U3 - HC9) 102, 103 (U6 - HC8) or sequence number (U8 - HC5) having amino acid sequences of seq ID 104 including hinge may be disclosed. One part protrudes from the body, antibodies or antigen-binding fragment thereof in anti c-a Met, said 17 is a heavy chain variable region sequence number, sequence number 74, sequence number 87, sequence number 90, 91 sequence number, sequence number 92, 93 or 94 amino acid sequences and sequence number sequence, said sequence number 121 is a light chain variable region, the sequence numbers 18, 19 sequence number, sequence number 20, sequence number 21, sequence number 75, 88 sequence number, sequence number 95, sequence number 96, 97 sequence number, sequence number 98, 99 sequence number, or sequence number including amino acid sequences of 107 may be disclosed. In one embodiment, said anti c-a Met antibodies The sequence numbers 62 (17 from one of the amino acid sequence comprises the steps of 1 in the second start control peptide) or sequence number 18 of second 462 from amino acid sequence start control 62, 64 sequence number (from 1 in one of the 17 amino acid sequence comprises the steps of the second start control peptide) or sequence number 18 of second 64 from 461 amino acid sequence start control, 66 and sequencing number (from 1 in one of the second amino acid sequence comprises the steps of 17 start control by peptide) or sequence number 66 of second nucleotide sequence having the amino acid sequence selected from the group consisting from 460 18 second installation the heavy and Sequence number 68 (from one of the 20 amino acid sequence comprises the steps of peptide 1 in the second start control) or sequence number 68 of second installation sequences with light [sway 21 second 240 from antibodies made, sequence number 70 (17 from 1 in one of the amino acid sequence comprises the steps of the second start control peptide) or sequence of 21 amino acid sequence or sequence number 108 70 number 240 from amino acid sequences of light having second start control An antibody thereto can be made. For example, said anti c-a Met antibodies Sequence number 62 62 18 having the amino acid sequence from amino acid sequences or sequence number of the second sequence or amino acid sequence of the heavy and sequence number 462 start control 68 21 68 of second installation sequences from antibodies made with light [sway 240 second number, Sequence number 64 64 18 having the amino acid sequence from amino acid sequences or sequence number of 461 amino acid sequences of the heavy and the sequence numbers 68 to second second second second installation sequences from antibodies made with light [sway 68 21 of sequence numbers or 240, Sequence number 66 66 18 having the amino acid sequence from amino acid sequences or sequence number of 460 amino acid sequences of the heavy and the sequence numbers 68 to second second second second installation sequences comprising antibodies with light [sway 240 from 68 of sequence numbers or 21, Sequence number 62 62 18 having the amino acid sequence from amino acid sequences or sequence number of the second sequence or amino acid sequence of the heavy and sequence number 462 start control 70 70 21 of second installation sequences comprising antibodies with light [sway 240 from the second number, Sequence number 64 64 18 having the amino acid sequence from amino acid sequences or sequence number of the second sequence or amino acid sequence of the heavy and sequence number 460 start control 70 70 21 of second installation sequences from antibodies made with light [sway 240 second number, Sequence number 66 66 18 having the amino acid sequence from amino acid sequences or sequence number of the second sequence or amino acid sequence of the heavy and sequence number 460 start control 70 70 21 of second installation sequences from antibodies made with light [sway 240 second number, Sequence number 62 62 18 having the amino acid sequence from amino acid sequences or sequence number of second amino acid sequences of the heavy and the sequence numbers 108 having 462 start control antibodies made with light [sway, Sequence number 64 64 18 having the amino acid sequence from amino acid sequences or sequence number of 461 amino acid sequences of the heavy and the sequence numbers 108 second start control having antibodies made with light [sway, or Sequence number 66 66 18 having the amino acid sequence from amino acid sequences or sequence number of amino acid sequences of the heavy and the sequence numbers 108 second start control 460 having antibodies made with light [sway Implementation being. On the other hand, said sequence number 70 having amino acid sequences of human kappa constant region of a heavy chain variable region CDR-a L3 and sequence numbers polypeptides may be 13 and including light [sway, sequence number 68 having amino acid sequences of polypeptides having amino acid sequences of said polypeptides may be sequence numbers 70 36 times in (the instruction executing kabat numbering, sequence number 68 62 in second amino acid position) is substituted with histidine (histidine) tyrosine (tyrosine) in the form of polypeptides disclosed. the main, throughput of one embodiments according to antibodies can be increased. In addition said sequence number 108 amino acid sequences of said polypeptide having the amino acid sequence of the second peptide sequence number 68 20 21 240 from number 1 during start control signal from second [...] polypeptide having an amino acid sequence start control by 27e kabat numbering in position (the instruction executing kabat numbering, sequence number 108 32 in second position; CDR-a L1 internal) is tryptophan (Trp) is substituted with a serine (Ser), due to said substituted, according to one embodiments the activity of antibodies (e.g., cMet binding to affinity, such as c-a Met-degrading activity and Akt phosphorylation billion active number) can be more enhanced. Embodiment, said fusion protein or dual specific antibodies as defined said Fc portion or said anti - c-a Met antibodies antibody antigen-binding fragments thereof of the distal or N C end including a VEGF binding fragment thereof variants (e.g. VIG2 variants) said polypeptides as defined is the linker can be either through without going through a connecting (also reference 1). In one embodiment, said fusion protein or dual specific antibodies The amino acid sequence of sequence numbers 62, 62 of the second sequence number 18 462 start control from amino acid sequence, the amino acid sequence of sequence numbers 64, 64 of second sequence number 18 from 461 start control amino acid sequence, the amino acid sequence of sequence number 66, or sequence number 66 having the amino acid sequence start control 460 from 18 of second anti c-a Met heavy chain, said peptide linker of C - heavy (Fc) linked to the end, and said C VIG2 variants linked to the end of a heavy and including a peptide linker Sequence number 68, 68 of 21 amino acid sequence start control 240 from the second sequence number, the sequence numbers 70, 70 during the second amino acid sequence from amino acid sequences of seq ID 21 240 start control, or sequence number 108 having light chain amino acid sequences of Including a may be disclosed In another example, amino acid sequences of said sequence number 122 or 124 anti c-a Met antibodies including heavy and sequence number 123 or 125 including including light chain amino acid sequences of may be disclosed. In one example, said anti c-a Met antibodies including sequence numbers 122 including either light chain amino acid sequences of amino acid sequences of heavy and sequence number 123, 124 125 including including light chain amino acid sequences of amino acid sequences of seq ID including heavy and sequence number may be disclosed. Said fusion protein or dual specific antibodies (also 7 reference) excellent cancer and cancerous transition inhibitory activity have inhibitory activity (also 8 reference). The, another example includes said fusion protein or dual specific antibodies including pharmaceutical composition active ingredient number [...] substrate. Said pharmaceutical compositions and/or VEGF overexpression of c-a Met pharmaceutical composition for prevention and/or treating sprayable compositions disclosed. Another example includes a pharmaceutically effective amount and/or said fusion protein or dual specific antibodies c-a Met VEGF overexpression of administering to a patient in need of such treatment the pharmaceutical composition for prevention and/or and/or pharmaceutical composition for prevention and/or treatment method including c-a Met VEGF overexpression of a number [...] substrate. the method said prevention and/or treatment and/or overexpression of VEGF administration prior to c-a Met pharmaceutical composition for prevention and/or treatment of a patient in need further comprises verifying the can. Said pharmaceutical compositions of said fusion protein or dual specific antibodies to pharmaceutical and, pharmaceutically acceptable carrier, dilution number, and/or excipients can be like number. Included in said pharmaceutical compositions which carrier comprises a pharmaceutically acceptable, provided number number is typically of drug which are used industrially, lactose, dextrose, sucrose, sorbitol, mannitol, starch, acacia rubber, calcium phosphate, alginate, gelatin, calcium silicate, microcrystalline cellulose, polyvinylpyrrolidone, cellulose, water, syrup, methyl cellulose, methyl hydroxy benzoate, propyl hydroxy benzoate, talc, magnesium stearic acid, at least one selected from the group consisting 1 can be a mineral oil or similar, limited to are not correct. Mixing said number or said pharmaceutical compositions can be used in addition to the bath components number pharmaceutical compositions typically used dilution number, number excipients, lubricating number, wet number, number sweetener, flavor number, number emulsion, suspension number, or similar storage number further comprises at least one selected from the group consisting 1 can be. Said pharmaceutical compositions can be oral or parenteral administration. When parenteral administration is intravenous injection, subcutaneous injection, muscle injection, abdominal cavity injection, endothelial administration, topical administration, intranasal administration, such as in a menopausal or rectum and administration within lung can be administered. In oral administration, oral composition reduces the extinguishing agent protein or peptide or to protect against degradation over to the elder brother anger should number about number active coating. In addition, said composition active substance moves to target cells can be administered by any device. Said subject (patient) pharmaceutical compositions humans, such as monkeys primate, RAT, dog even including mammalian; from them the isolated cells, or be a tissue culture. 1 Said active ingredient in pharmaceutical compositions for administering a single dual specific antibodies pharmaceutical amount is effective number number of method, administration scheme, patient's age, weight, -, pathological conditions, food, administration time, dosing interval, routes, is determined by the various prescribed speed and reaction sensitive such as a printed wiring board can be. For example, administering a single bi 0 amount is effective for said 1 antibodies. 0001 To 50 mg/kg or 0. 001 To 20 mg/kg but the number one can range are not disclosed. For administering a single unit dose amount is effective to form one number number number number of or to said 1, or by appropriately dose of number number, number bath container ingrowth to 1308. prepared amount. Said pharmaceutical compositions oil or aqueous medium solution, suspension, syrup or be in the form of emulsion number X number, acid number, number powder, granules number, positive number or capsules can be elder brother anger number like in the form of number, number number can be further distributed number or stabilizing for elder brother anger. In particular, said pharmaceutical compositions is thus dual specific antibodies, immune liposomal-number 1308. elder brother anger. According to a known method including antibodies liposome contrast number bath 1308. the liposome measnufacturing phosphtidylcholine, cholesterol and polyethylene glycol - derivatized lipid composition including party [til ethanol amine phosphate phase as positioned in number by bath 1308. For example, antibodies Fab ' fragment thereof is disulfide bonded to liposomes via reaction replacement - can be. Toxin with ruby shoes further number can be included in the treatment chemicals such as liposomes. In the present invention number c-a Met and/or VEGF pharmaceutical compositions not associated with disease, e.g., number and/or increasing the number and/or expression of c-a Met prevention increasing amount of diseases caused by overexpresssion VEGF, typically for preventing cancer or cancer metastasis, relief (relief), and/or treatment can be used. Said arm may be for overexpressing c-a Met and/or VEGF and, not one number can be a the solid cancer. For example, said arm is squamous cell carcinoma, small cell gun lung cancer, arsenic cell lung cancer, of lung adenocarcinoma, of lung flat epithelial cancer, peritoneal cancer, skin cancer, skin or intraocular melanoma, colorectal cancer, anal near cancer, esophageal cancer, small intestine cancer, cancer of an iron chelator, parathyroid cancer, adrenal cancer, soft tissue sarcoma, urethral cancer, chronic or acute leukemia, lymphocyte lymphoma, that are alleviated, gastrointestinal cancer, stomach cancer, pancreatic cancer, [kyo subspecies, transferase, ovarian cancer, liver cancer, bladder cancer, hepatoma, breast cancer, colon cancer, colorectal cancer, endometrial or uterine cancer, various combinations cancer, renal cancer, prostate cancer, vulva cancer, thyroid cancer, head-neck cancer, brain cancer, osteosarcoma or similar at least one selected from the group consisting 1 be a. Said arm is be a cancer or cancer metastasis tumor. The specification anticancer effect in inhibiting cancer, cancer killing effect, and/or tumor metastasis billion number effect without using a tool. In another example, said c-a Met and/or VEGF-associated diseases of pregnancy are diabetes, diabetic retinopathy, hwang contravariant characteristic (e.g. wet age-related macular degeneration (wet AMD), or the like) and the like disclosed. As aforementioned, anti - c-a Met antibody or antigen-binding fragments thereof to said antibody binding fragments thereof including polypeptide is VEGF variants including an, enhancement stability (half-life increased) water-soluble antibodies, antigen-binding period is increased, improved productivity caused by antibodies. In another aspect, said VEGF binding fragment thereof variants of said polypeptides including anti - c-a Met antibody or antibodies in vivo stability and/VEGF bi c-a Met and for use encoded number ball retaining force enhancing vivo antigen-binding. Specifically, one example includes said VEGF binding fragment thereof variants including polypeptides including anti - c-a Met antibody or antibodies in vivo stability for c-a Met and VEGF bi and/vivo antigen-binding retaining force for promoting composition number [...] substrate. Another example is an anti - c-a Met antibodies (antibodies of IgG form) or antigen-binding fragments including binding fragments thereof and thereby bridging said VEGF variant polypeptides including, in vivo stability and/vivo antigen-binding VEGF antibodies for enhanced retention and method number a number bath bi c-a Met [...] substrate. Another example is an anti - c-a Met antibodies (antibodies of IgG form) or antigen-binding fragments including binding fragments thereof and thereby bridging said VEGF variant polypeptides including, for production of antibodies to enhance VEGF bi c-a Met and method number [...] substrate. Thereby bridging said carried out in addition be a human. Thereby bridging said anti - c-a Met antibodies (antibodies of IgG form) or antigen-binding fragments thereof, antibodies or antigen-binding fragment linked to the end C - said peptide linker, a peptide linker of said VEGF binding fragment thereof variants including said C - end carrying polynucleotides encoding polypeptides can be a suitable expressing in a host cell. Said VEGF binding fragment thereof variants (e.g. VIG2 variants) including polypeptides may be a specific binding activity in the periphery of the VEGF, VEGF detecting or VEGF associated disease diagnostic purposes are useful as disclosed. The, said VEGF binding fragment thereof variants (e.g. VIG2 variants) as to a composition for detecting polypeptides including VEGF or VEGF related diseases including diagnostic composition number [...] substrate. In addition, said fusion protein and/or dual specific antibodies so as to c-a Met and synchronous detection of VEGF, VEGF or VEGF related diseases/c-a Met and metal foil and c-a Met and useful as diagnostic purposes. The, said fusion protein or dual specific antibodies including polypeptides as to composition for detecting and/or VEGF or VEGF simultaneously including c-a Met and c-a Met number composition for diagnosing related diseases [...] substrate. Polynucleotides encoding said polypeptides or fusion proteins as to number [...] substrate. Said recombinant vector number as to polynucleotides including [...] substrate. Said recombinant vector including recombinant cells as to number [...] substrate. The terms "vector (vector)" means for big aim in appropriate host cells to express the gene. For example, e. coli, plasmid vector and nose [cu bacteriophage vector, adenovirus vector, Retrovirus vector - and Adeno associated virus vector comprising such as virus vector. Said recombinant vector can be used that are used frequently in the art vector plasmid (e.g., pSC101, pGV1106, pACYC 177, ColE1, pKT230, pME290, pBR322, pUC8/9, pUC6, pBD9, pHC79, pIJ61, pLAFR 1, pHV14, pGEX series, such as pET series and pUC19), gripping (for example, λ gt4 λ B, λ-a Charon, such as M13 and λ Δ z1) or viral (e.g. wild life, such as SV40) can be small but the number is not one number by manipulation. In said isolated polynucleotide includes promoter can be operatively connected to said recombinant vector. The terms "operably connected (operatively linked)" nucleotide expression regulatory sequences (e.g., promoter sequences) a nucleotide sequence that encodes a functional combination between different means other. Said regulatory sequences "(operatively linked) operatively connected" by a nucleotide sequence that encodes the other of transfer and/or decryption can be control. Said recombinant vector, cloning vectors or expression vectors as typically can be established. The vector for expressing said art plant, animal or microorganism foreign protein is expressed in the normal used can be employed. Said recombinant vector can be established through various publicly known contrast method. Said recombinant vector into a host so that can be represented. For example, expression vectors and vector used, is represented by a host when an, transfer of the substrate thus promoters (e.g., pLλ Promoter, CMV promoter, Said recombinant cells obtained by introducing said recombinant vector be a suitable host cells. Said host cells expressing said recombinant vector is successively cloning or stable cells that can also by using publicly known as nitrile can be no host cells, prokaryotic cell is, for example, Delivery of the polynucleotide or said recombinant vector into host cells including (introduction), can be produced using contrast delivery method. For example carrying said method, when represented by the host cells, CaCl2 Method or electroporation method can be use, when the host cell is eukaryotic cells, microinjection method, calcium phosphate precipitation method, electroporation method, liposome - mediated transfection method and bambara [...] gene regions but, not limited. Said method of selecting transformed host cells expressed by using selected marker in a desired phenotype, a widely known method for contrast according to embodiment hereinafter can. For example, antibiotic resistance markers for gene is selected when said specific number, said number transformant in a medium containing antibiotics by culturing a transformant can be hereinafter for buffer. As to the number of fusion proteins including said polypeptides or polynucleotides expressing in a host cell said method bath a number [...] substrate. Said step of said polynucleotides expressing in a host cell including culturing cells transformed host cells into which a recombinant vector recombinant polynucleotides can be. In the present invention number hole connecting technique provides VEGF binding fragment thereof variants which anti - c-a Met antibodies anti - c-a Met antibodies or dual specific antibodies in vivo stability, promoting angiogenesis (angiogenesis) antigen-binding retaining force and antibodies anticancer treatment of diseases caused by number and fecundity number can be useful in the development of mechanisms. Figure 1 c-a Met and showing one example of a VEGF dual targeting antibody mimetic or hypermetropia. Figure 2 VIG2 site linked to the protein through modeling of aggregation tendency areas are relatively high content screening is shown graph are disclosed (Y shaft exhibits the aggregation propensity, 0. 00 Reference, 0. 00 Aggregation exceeds a high possibility, 0. 00 Hereinafter stable by coagulation of the back mirror is lower). 3A and 3b in the embodiment 1 in the number of dual specific antibodies also it became work nothing control group (media; 100%) c-a Met MKN45 cell antibody-degrading activity (%) with respect to the ratio which can make a graph are disclosed. Figure 4 shows a number of 0 to 7 in the embodiment 1 also it became work in dual specific antibodies (immediately after antibody administration) coupled between c-a Met positive with respect to the ratio (%) which can make a graph are disclosed ( Hereinafter, the present invention in the embodiment by a detailed as follows. Stage, in the embodiment for the present invention is exemplified ephemeral is, in the embodiment of the present invention to not the limited to contents. Reference example 1: Anti c-a Met small number of antibodies 1. 1. c- Met for mouse antibody 'AbF46' Production 1. 1. 1. Mouse Immunization The development of a hybridoma cell line required to obtain the connection immunization, 5 per 100 g of a human c-a Met/Fc fusion proteins of mouse embryo saw a micro equal to complete the [tu which is (R&D Systems) BALB/c mouse (Japan SLC, Inc.) 4 - 6 primary mixing profile [...] (Freund's adjuvant) was intraperitoneally of scanning. 2 Identical to the method used to said main after said human c-a Met/Fc fusion protein antigens prior injection of 50 micro g amount of half the [tu which is equal to a (incomplete Freund's adjuvant) was created by mixing a mouse intraperitoneally [...] incomplete profile scanning. After week 3 after the last boosting (boosting) is performed and is opened to 1/1000 dilution in PBS to said mouse tail to obtain serum by ELISA to c-a Met blood recognizing antibodies Varicella vaccine has been confirmed that the be solved. As a result of the sufficient amount of antibody to the cell fusion of laver conducting obtained mouse screening process. 1. 1. 2. Cells Fused and High [pu cutting board Number bath Before cell fusion experiments 3 to 50 micro g PBS BALB/c mouse (Japan SLC, Inc.) of human c-a Met/Fc fusion protein mixture of scanning in the abdominal cavity, the connection immunization (spleen) was mixed with an aqueous spleen situated on a left side of the body after anesthesia. An aqueous spleen cells are separated by a mesh and to abrade, culture medium (DMEM, GIBCO, Invitrogen) by mixing it with a spleen cell suspensions have been produced. Recovering said centrifuging the cell suspension to him. 1 X 10 said spleen cells obtained8 1 X 10 dogs and bone marrow bell cell (Sp2/0)8 Dog blended, centrifuging the cells with respect to the precipitation. Said centrifuged precipitate slowly dispersing, culture medium (DMEM) manner that 45% polyethylene glycol (PEG) (1 ml) high-K dielectric material, in 1 minutes 37 °C due to deviation, culture medium (DMEM) 1 ml some excellent. After adding 10 ml culture medium (DMEM) for 1 minutes, 5 minutes 37 °C in 50 ml water was left by a user after centrifuging. Separating the precipitate medium (HAT medium) to 1 - 2 × 10 cells5 Extent then suspended/Ml, 96 - well plate (well) 0. After dividing by 1 ml by using carbon dioxide incubator 37 °C hybridoma-gate number small population of cells. 1. 1. 3. c- Met producing monoclonal antibodies against proteins Hybridomas Cell separation Said reference example 1. 1. 2 Hybridomas prepared by the number in a population of cells in hybridoma cells for c-a Met reacts specifically only protein of a human c-a Met/Fc ELISA analysis method using the human Fc protein antigen fusion proteins through screening-gate. A human c-a Met/Fc fusion proteins each 50 micro l [...] microtiter plates (2 ug/Ml) applied by applying a plate surface, the surface of the antigen is a stand-alone been number which has not reacted. C-a Met Fc antibody screening number not coupled to outside of a human Fc protein for storing method with respect to the plate surface. Said reference example 1. 1. 2 Culture of hybridoma cells obtained in said each well prepared by reacting phosphoric acid buffer solution by adding 1 - 50 micro l time (TBST) solution which has not reacted culture number through the twin 20 sufficiently been stand-alone. Anti - mouse IgG - hose [...] (goat anti-a mouse IgG a-HRP) excitation chlorine number 1 and applying time then react at room temperature, was sufficiently cleaning TBST solution. A substrate solution (OPD) then reacting [...] number by applying, to the extent of reaction by measuring absorbance in ELISA Reader 450 nm has been confirmed. On reactions such as confirm a degree by, is human Fc are cut out, only human c-a Met protein specifically selected for hybridoma antibodies having high binding ability of the transfected cell lines was repeated. Selecting a repeated number obtained in the hybridoma lines (limiting dilution) dilution by monoclonal antibodies hybridoma cell line obtained by a final of clone 1. 9 October 2009 budapest final selected monoclonal antibody species other than engine residing in the lower half a diagnostic cell number Treaty depository under station Savina yearly case study cutting filed with the Korean copper a compensation consignee number KCLRF a-BP a-00220 a impurities (Korean publicized patent number 2011 - 0047698 reference). 1. 1. 4. Monoclonal Number positive and production of antibodies Said reference example 1. 1. 3 Obtained in hybridoma cells producing monoclonal antibodies in a serum free medium was harvested from the and positive number. (V/v) 10% FBS medium (DMEM) containing 50 ml culture media first cultured hybridoma cells in 20 ml PBS 2 or more times to precipitate from the surface of said centrifuge the cell number for reparing over is in FBS, precipitate 50 ml culture medium (DMEM) medium after suspended in said cells, cultured in carbon dioxide incubator 37 °C during his 3. Then, centrifuging, and antibodies are secreted antibody with a stand-alone number cells culture are separated from each other, as well as separation of 4 °C collecting antibodies or immediately stored positive number. Affinity column (Protein G agarose column; Pharmacia, USA) equipped with a liquid culture medium prepared from 50 ml to 300 ml (GE Healthcare) AKTA positive number device using said pure antibodies after positive number, protein aggregation using PBS (Amicon) filter for substituting a supernatant to form a positive number antibodies, as well as later in the embodiment. 1. 2. cFor - Met Chimeric Antibodies ChAbF 46 of Small number Generally mouse antibodies to human therapeutic purposes when injected immune rejection (immunogenicity) is most likely to exhibit, in order to solve the same, it became work in number from said in the embodiment 1 AbF46 mouse antibody, related to shifting region (variable region) constant region (constant region) antigen-binding human IgG1 [...] a number sequence of antibodies from a group consisting a small number-gate chAbF 46 chimeric antibodies. The nucleotide sequence corresponding in [sway 'EcoRI-a signal sequence a-VH-a NheI-a CH non-TGA-a XhoI' (sequence number 38) to, the nucleotide sequence corresponding in light [sway 'EcoRI-a signal sequence provided VLBsiWI provided CL a-TGA-a XhoI' (sequence number 39) performs a gene consisting respectively and copiers. Then, Invitrogen OptiCHO yarnTM Antibody Express Kit (Cat no. 12762 - 019) POptiVEC is included in theTM (Sequence number 38) DNA having a nucleotide sequence that encodes said in [sway - TOPO TA Cloning Kit corresponding to a fragment, pcDNATM 3. 3 A-TOPOTA Cloning Kit (Cat no. 8300 - 01) In light [sway (sequence number 39) DNA fragment having a nucleotide sequence that encodes said corresponding to each EcoRI (NEB, R0101S) and XhoI (NEB, R0146S) number by cloning using various enzymes, chimeric antibodies including vectors and light chain was constructing each including vector for the expression in [sway. Each vector in said Qiagen Maxiprep kit (Cat no. 12662) Has been amplified using, Freestyle temporary expression isTM (Invitrogen) MAX 293 Expression System using satisfactorily. 293 F cell used cell lines which, by using floating culture contains FreeStyleTM 293 Expression Medium manner engineers. 5X10 cells before day temporary expression5 After concentration of cells/ml can be prepared, 24 time resumes cell number 1x106 Cells/ml when the temporary expression of abortion. FreestyleTM MAX reagent (invitrogen) characterized by transduction (transfection) when the performed using liposomal reagent, 15 ml tube to heavy DNA: light chain DNA=1:1 DNA is provided so as to mix at a rate of 2 ml and OptiProTM SFM (invtrogen) and (A), to another 15 ml tube FreestyleTM L OptiProTM SFM 2 ml (B) mix on a micro MAX reagent 100 after, 15 minutes after incubation on to a mix (A) (B), prepare the cells before day slowly mixed solution spray me. After transduction, 37 °C, 80% humidity, 8% CO2, 5 130 Rpm incubator him as in liver. Then, 10% FBS DMEM medium (v/v) added 37 °C, 5% CO2 5 Culturing time under conditions then, FBS-free DMEM medium is 37 °C to 48 hours, 5% CO2 Was cultured under conditions. Said centrifugal force taking each 100 ml supernatant to cultured cells, using AKTA Prime (GE healthcare) was a positive number. The AKTA Prime Protein A column (GE healthcare, 17 - 0405 - 03) of a flow rate of 5 ml/min after emitting culture is arranged, with respect to the intra-cellular IgG elution buffer (Thermo Scientific, 21004). PBS buffer composition obtained dissolution exchanging (hereinafter, referred to by chAbF 46) was finally chimeric antibody AbF46 atrough-number. 1. 3. Chimeric Antibodies ChAbF 46 from Humanized antibodies HuAbF 46 of Small number 1. 3. 1. Immunoglobulin heavy chain Humanized (Heavy chain humanization) H1 a-heavy and H3 provided heavy 2 species for design, first Ig Blast (http://www. Ncbi. Nlm. Nih. Gov/igblast /) through said reference example 1. 2 Positive number in most homologous human VH gene of the mouse antibody AbF46 on preventive agent (germline) analyzing gene was high. As a result, this amino acid level is also used for a transparent conductive layer having a homology of VH3 provided 71 83%, mouse antibody AbF46 of CDR-a H1, CDR-a H2, CDR-a H3 and define a Kabat numbering, mouse antibody AbF46 (framework) was introduced into the backbone of CDR portion of VH3 provided 71 designed. The, 30 times (S→T), 48 (V→L) once, once (D→N) 73, 78 times (T→L) amino acids in the amino acid sequence his original mouse antibody AbF46 back-a mutation. Then, 83 and 84 at H1 further relates to mutant (A→T) (R→K) at amino acid (sequence number 40) crack width is finally H1 provided heavy on his (sequence number 41) H3-a heavy build. (Framework) sequences for human antibodies find the H4 a-heavy design of the backbone of result, AbF46 mouse antibody framework sequences and sequences and very similar, C24 VH3 subtype of know using defined mouse antibody AbF46 Kabat numbering of CDR-a H1, CDR-a H2, CDR-a H3 was introducing. (Sequence number 42) through H4-a heavy build up. 1. 3. 2. [...] Humanized (Light chain humanization) (Sequence number 44) 2 (sequence number 43) and H2 provided light H1 provided light for design of species, Ig Blast (http://www. Ncbi. Nlm. Nih. Gov/igblast /) through, mouse antibody AbF46 most homologous gene to human VL gene of analyzing preventive agent was high. As a result, this amino acid level is also used for a transparent conductive layer having a homology of 75% VK4 provided 1, mouse antibody AbF46 of CDR a-L1, CDR-a L2, CDR-a L3 and define a Kabat numbering, mouse antibody AbF46 CDR portion of the backbone of VK4 provided 1 introduced was designed. The, H1 provided light is once (Y→H) 36, 46 times (L→M), 3 (Y→I) when the back-a mutation at amino acid 49, is 49 amino acids of (Y→I) 1 H2 provided light was so that only two back-a mutation. (Sequence number 45) for design of H3 provided light, Blast (http://www. Ncbi. Nlm. Nih. Gov/igblast /) of most homologous gene to human VL gene via mouse antibody AbF46 high preventive agent for analyzing results, in addition to his selecting said VK4 provided 1 VK2 provided 40. Mouse antibody AbF46 VL amino acid level is also used for a transparent conductive layer having a homology of 61% and VK2 provided 40, mouse antibody AbF46 of CDR a-L1, CDR-a L2, CDR-a L3 and define a Kabat numbering, mouse antibody AbF46 CDR portion of the backbone of VK4 provided 1 introduced was designed. The, H3 provided light is 36 times (Y→H), 46 (L→M) once, at amino acid 49 (Y→I) 3 so that his back-a mutation. (Sequence number 46) for design of H4-a light, the backbone of the human antibodies (framework) sequences find the result, C24 Vk1 subtype of know defined mouse antibody AbF46 using Kabat numbering of CDR a-L1, CDR-a L2, CDR-a L3 was introducing. The, H4 provided light is 36 times (Y→H), 46 (L→M) once, at amino acid 49 (Y→I) 3 was so that further back-a mutation. Then, Invitrogen OptiCHO yarnTM Antibody Express Kit (Cat no. 12762 - 019) POptiVEC is included in theTM The DNA fragment having a nucleotide sequence that encodes said corresponding in [sway - TOPO TA Cloning Kit (H1 provided heavy; sequence number 47, H3 provided heavy; the sequence numbers 48, H4-a heavy; the sequence numbers 49) pcDNA aTM 3. 3 A-TOPOThe DNA fragment having a nucleotide sequence that encodes said corresponding in light [sway TA Cloning Kit (H1 provided light; the sequence numbers 50, H2 a-light; the sequence numbers 51, H3 provided light; sequence number 52, H4 a-light; the sequence numbers 53) EcoRI (NEB, R0101S) respectively and XhoI (NEB, R0146S) number using enzymes, by cloning, for the expression vector was constructing the humanized antibodies. Each vector in said Qiagen Maxiprep kit (Cat no. 12662) Has been amplified using, Freestyle temporary expression isTM (Invitrogen) MAX 293 Expression System using satisfactorily. 293 F cell used cell lines which, by using floating culture contains FreeStyleTM 293 Expression Medium manner engineers. 5X10 cells before day temporary expression5 After concentration of cells/ml can be prepared, 24 time resumes cell number 1x106 Cells/ml when the temporary expression of abortion. FreestyleTM MAX reagent (invitrogen) characterized by transduction (transfection) when the performed using liposomal reagent, 15 ml tube to heavy DNA: light chain DNA=1:1 DNA is provided so as to mix at a rate of 2 ml and OptiProTM SFM (invtrogen) and (A), to another 15 ml tube FreestyleTM L OptiProTM SFM 2 ml (B) mix on a micro MAX reagent 100 after, 15 minutes after incubation on to a mix (A) (B), prepare the cells before day slowly mixed solution spray me. After transduction, 37 °C, 80% humidity, 8% CO2, 5 130 Rpm incubator him as in liver. Said centrifugal force taking each 100 ml supernatant to cultured cells, using AKTA Prime (GE healthcare) was a positive number. The AKTA Prime Protein A column (GE healthcare, 17 - 0405 - 03) of a flow rate of 5 ml/min after culture is arranged connected to chip, IgG elution buffer (Thermo Scientific, 21004) was intra-cellular. The humanized antibodies of the same PBS buffer (hereinafter, referred to by huAbF 46) States replaced AbF46 atrough-his number. On the other hand, in the embodiment after use in huAbF 46 humanized antibody heavy chain, light chain combination (sequence number 42) (sequence number 46) H4 a-heavy and H4 provided light are disclosed. 1. 4. HuAbF 46 Antibodies ScFv Small number library HuAbF 46 heavy chain variable region and light chain variable region gene for scFv antibodies using a small number huAbF 46 his design. Each heavy chain variable region and light chain variable zones' VH - linker - VL 'to in the form of, said' GLGGLGGGGSGGGGSGGSSGVGS' linker has an amino acid sequence of (seq ID no 54) was designed. The polynucleotide encoding a scFv antibodies designed huAbF 46 (seq ID no 55) it comes at all oh synthesized through a facia, vector for expression thereof shown to prioritize number 56. Then, said vectors expressedIf there is a layer, c-a Met binding has been confirmed that the specific charge. 1. 5. Affinity For small number of mature (affinity maturation) gene library 1. 5. 1. TargetOf CDR Selection and Primer Small number HuAbF 46 for affinity maturation of antibodies (affinity maturation) 6 (complementary determining region, CDR) said number of complementarity determining regions from a mouse antibody AbF46 it became work 'Kabat numbering' defined by a transparent conductive layer, each CDR is a table 1 such as disclosed. The randomness of the antibody CDR sequence cells as follows primer small number-gate. The randomness of the existing sequence mutations introduced scheme is established at a same ratio base (25% A, 25% G, 25% C, 25% T) is N codon are introduced using but, in the embodiment the CDR to introduce huAbF 46 antibodies in a random base, of each CDR 3 sequence encoding an amino acid ofWild-type (wild-a type) is left as it first and second nucleotide 85% nucleotide during other id preservation and, of the remaining 3 single crystal substrate which is introduced by each base was 5%. In addition, the second tie same third nucleotide (33% G, 33% C, 33% T) have been introduced to design primer to him. 1. 5. 2. HuAbF 46 Library of antibodies for identifying number and small c-a Met binding The randomness of the CDR 1 gene library of reference example among the introduction sequence antibodies. 5. 1 To apparatus for treating method such as by conducting it became work number. ScFv antibodies using a mold huAbF 46 including polynucleotides, small number of PCR fragments such as shown in method 1 2 and also, through same redundant extension polymerase chain reaction (overlap extension PCR) method, each scFv antibodies by securing a number it became work only desired CDR mutation in the gene library huAbF 46 of each CDR library was 6 target functions. The number of wild-type c-a Met binding when the library for identifying it became work each library, each library as compared to a wild-type c-a Met tendency which might be visible but most forces, some c-a Met mutations maintained for binding to neutrophils. 1. 6. It became work number From a library Produced Improved antibodies of selecting Said c-a Met established from a library of the top library and after, scFv gene sequence analysis of each individual clone from him. Gene sequences such as bright and ensure respectively and 2, was converted into a same IgG. In a clone, L3 provided 1, L3 provided 2, L3 provided 3, L3 provided 5 4 species produced from screening antibodies of conducting subsequent experiments. 1. 7. Selected Antibodies To IgG Conversion 4 Species of the antibodies in [sway selected polynucleotides encoding elicit 'EcoRI-a signal sequence a-VH-a NheI-a CH-a XhoI' being (sequence number 38), immunoglobulin heavy chain antibody affinity maturation after priority when not be modified amino acids, as well as antibodies as in [sway huAbF 46. Only, not U6 - HC7 hinge (sequence number 57) human IgG1 hinge hinge region (hinge region) was replaced. Light [sway 'EcoRI-a signal sequence-a VL-a BsiWI-a CL-a XhoI' performs synthesizing each of gene consisting of a transparent conductive layer, said 4 species comprising variable region heavy chain of antibodies selected affinity maturation after encoding a polynucleotide (sequence number 58 61 minutes) through a configurated to it comes at all oh. Then, Invitrogen OptiCHO yarnTM Antibody Express Kit (Cat no. 12762 - 019) POptiVEC is included in theTM (Sequence number 38) DNA having a nucleotide sequence that encodes said in [sway - TOPO TA Cloning Kit corresponding to a fragment, pcDNATM 3. 3 A-TOPOTA Cloning Kit (Cat no. 8300 - 01) DNA having a nucleotide sequence that encodes a fragment corresponding to said in light [sway (L3 provided 1 derived CDR-a L3 a including DNA fragments: sequence number 58, L3 provided 2 derived CDR-a L3 a including DNA fragments: sequence number 59, L3 provided 3 derived CDR-a L3 a including DNA fragments: sequence number 60, including DNA fragments derived from a CDR-a L3 L3 provided 5: sequence number 61) EcoRI (NEB, R0101S) respectively and XhoI (NEB, R0146S) number by cloning using various enzymes, affinity maturation of an antibody was constructed for the expression vector. Each vector in said Qiagen Maxiprep kit (Cat no. 12662) Has been amplified using, Freestyle temporary expression isTM (Invitrogen) MAX 293 Expression System using satisfactorily. 293 F cell used cell lines which, by using floating culture contains FreeStyleTM 293 Expression Medium manner engineers. 5X10 cells before day temporary expression5 After concentration of cells/ml can be prepared, 24 time resumes cell number 1x106 Cells/ml when the temporary expression of abortion. FreestyleTM MAX reagent (invitrogen) characterized by transduction (transfection) when the performed using liposomal reagent, 15 ml tube to heavy DNA: light chain DNA=1:1 DNA is provided so as to mix at a rate of 2 ml and OptiProTM SFM (invtrogen) and (A), to another 15 ml tube FreestyleTM L OptiProTM SFM 2 ml (B) mix on a micro MAX reagent 100 after, 15 minutes after incubation on to a mix (A) (B), prepare the cells before day slowly mixed solution spray me. After transduction, 37 °C, 80% humidity, 8% CO2, 5 130 Rpm incubator him as in liver. Said centrifugal force taking each 100 ml supernatant to cultured cells, using AKTA Prime (GE healthcare) was a positive number. The AKTA Prime Protein A column (GE healthcare, 17 - 0405 - 03) of a flow rate of 5 ml/min after culture is arranged connected to chip, IgG elution buffer (Thermo Scientific, 21004) was intra-cellular. PBS buffer States affinity maturation antibodies of same replaced 4 species (hereinafter, huAbF46 - H4 - A1 (L3 provided 1 derived), huAbF46 - H4 - A2 (L3 provided 2 derived), huAbF46 - H4 - A3 (L3 provided 3 derived), and (L3 provided 5 derived) by huAbF46 - H4 - A5 are referred to) a deformation was number. 1. 8. Constant region and/or Hinge region Substituted HuAbF 46Number of bath - H4 a-A1 Said reference example 1. 7 Selected in 4 antibody heavy species in, engagement with c-a Met is highest affinity, it has been determined that a lowest degree of differentiation Akt phosphorylation and c-a Met aimed huAbF46 - H4 - A1, substituted antibodies small number-gate hinge region or constant region and the hinge region. HuAbF46 - H4 - A1 heavy chain variable region, constant region of human IgG1 U6 - HC7 hinge and human kappa (kappa) variable region or light chain constant region containing heavy and huAbF46 - H4 - A1 and made with light [sway huAbF46 - H4 - A1 to antibodies (U6 - HC7); huAbF46 - H4 - A1 heavy chain variable region, a light chain variable region of human IgG1 invariant region human IgG2 hinge and human kappa constant region containing heavy and huAbF46 - H4 - A1 and made with light [sway huAbF46 - H4 - A1 to antibodies (IgG2 hinge); huAbF46 - H4 - A1 heavy chain variable region, hinge and constant regions of human IgG2 human IgG2 human kappa constant region containing heavy and huAbF46 - H4 - A1 and a light chain variable region comprising antibodies with light [sway huAbF46 - H4 - A1 (IgG2 Fc) was named each. In addition, on the other hand, of a human kappa constant region containing said 3 for operation and refining antibodies of the species once both tyrosine (tyrosine) was replaced by histidine (histidine) [...] 36. Said 3 species antibodies for small number, huAbF46 - H4 - A1 heavy chain variable region, human IgG1 invariant region containing the U6 - HC7 hinge and polynucleotides encoding a polypeptide (seq ID no 62) (seq ID no 63), huAbF46 - H4 - A1 heavy chain variable region, hinge and human IgG1 invariant region containing the human IgG2 polynucleotides encoding a polypeptide (seq ID no 64) (seq ID no 65), huAbF46 - H4 - A1 heavy chain variable region, human IgG2 a constant region containing the hinge and human IgG2 polynucleotides encoding polypeptides (seq ID no 66) (sequence number 67), 36 times human kappa constant region of a light chain variable region and mote [thin this tyrosine substituted with histidine at huAbF46 - H4 - A1 a (sequence number 69) polynucleotides encoding a polypeptide (seq ID no 68) through a configurated to it comes at all oh. Then, Invitrogen OptiCHO yarnTM Antibody Express Kit (Cat no. 12762 - 019) POptiVEC is included in theTM In [sway - TOPO TA Cloning Kit bio-sequence listing corresponding to said DNA fragments having, pcDNATM 3. 3 A-TOPOTA Cloning Kit (Cat no. 8300 - 01) In light [sway bio-sequence listing corresponding to said segment by inserting a DNA having, said antibodies was constructed for the expression vector. Each vector in said Qiagen Maxiprep kit (Cat no. 12662) Has been amplified using, Freestyle temporary expression isTM (Invitrogen) MAX 293 Expression System using satisfactorily. 293 F cell used cell lines which, by using floating culture contains FreeStyleTM 293 Expression Medium manner engineers. 5X10 cells before day temporary expression5 After concentration of cells/ml can be prepared, 24 time resumes cell number 1x106 Cells/ml when the temporary expression of abortion. FreestyleTM MAX reagent (invitrogen) characterized by transduction (transfection) when the performed using liposomal reagent, 15 ml tube to heavy DNA: light chain DNA=1:1 DNA is provided so as to mix at a rate of 2 ml and OptiProTM SFM (invtrogen) and (A), to another 15 ml tube FreestyleTM L OptiProTM SFM 2 ml (B) mix on a micro MAX reagent 100 after, 15 minutes after incubation on to a mix (A) (B), prepare the cells before day slowly mixed solution spray me. After transduction, 37 °C, 80% humidity, 8% CO2, 5 130 Rpm incubator him as in liver. Said centrifugal force taking each 100 ml supernatant to cultured cells, using AKTA Prime (GE healthcare) was a positive number. The AKTA Prime Protein A column (GE healthcare, 17 - 0405 - 03) of a flow rate of 5 ml/min after culture is arranged connected to chip, IgG elution buffer (Thermo Scientific, 21004) was intra-cellular. Replacing the same antibodies of PBS buffer States 3 species (huAbF46 - H4 - A1 (U6 - HC7), huAbF46 - H4 - A1 (IgG2 hinge), huAbF46 - H4 - A1 (IgG2 Fc)) was number deformation. In the present invention according to one of the anti c-a Met antibodies to select representative huAbF46 - H4 - A1 (IgG2 Fc) used in the embodiment enables to form a transparent conductive layer, said anti c-a Met [...] SAIT301 antibodies to antibodies are described. Reference example 2: Wild-type VIG2 a Number bath including fusion proteins It became work in said reference number based on heavy c - end linker is coupled to anti c-a Met antibodies was fused. After this, the end of the amino acid linker of a VEGF receptor 1 Ig2 domain, i.e. 151 214 from once installation so that consecutive cMet VEGF antibodies can bind simultaneously and small number-gate (also reference 1). VEGF receptor 1 (P17948. 2; Seq ID no 109) 1338 amino acid constituting the most important during VEGF binding domain (VIG2) 129 229 to know Ig2 constituting gene sequence encoding an amino acid sequence of 101 at once from his secure from NCBI database. Ig2 domain (VIG2) amino acid sequence (seq ID no 110): SDTGRPFVEMYSEIPEIIHMTEGRELVIPCRVTSPNITVTLKKFPLDTLIPDGKRIIWDSRKGFIISNATYKEIGLLTCEATVNGHLYKTNYLTHRQTNTI Ig2 bio-sequence listing domain (VIG2) (sequence number 111): AGTGATACAGGTAGACCTTTCGTAGAGATGTACAGTGAAATCCCCGAAATTATACACATGACTGAAGGAAGGGAGCTCGTCATTCCCTGCCGGGTTACGTCACCTAACATCACTGTTACTTTAAAAAAGTTTCCACTTGACACTTTGATCCCTGATGGAAAACGCATAATCTGGGACAGTAGAAAGGGCTTCATCATATCAAATGCAACGTACAAAGAAATAGGGCTTCTGACCTGTGAAGCAACAGTCAATGGGCATTTGTATAAGACAAACTATCTCACACATCGACAAACCAATACAATC It became work [...] Ig2 domain (VIG2) for connecting said number c-a Met antibodies, GGGGS (G4S) structure which is repeated during 'GGGGS', 'GGGGSGGGGS' ((G4S) 2), or ((G4S) 4) design of the linkers have ' GGGGSGGGGSGGGGSGGGGS' of having 3, VEGF receptor 1 (VIG2) domain of c-a Met antibodies same Ig2 adapted to be positioned between a transparent conductive layer, at the end of the stop codon (TGA) designed by combining a final gene inserted into the gene it comes at all oh his request. The EcoRI/XhoI cloning site pOptivec vector (Invitrogen) using a synthesized gene expressed vector to small number-gate. Light [sway revelation vector engages said L3 - 1Y number as used vector was used. Each vector in Qiagen Maxiprep kit (Cat no. 12662) Has been amplified using, in [sway including vector including said vectors and light chain ratio of 4:1 (80 ug: 20 ug) into 293T cells (2. 5 X 107 ) To 2M CaCl2 Was added 360 ul a transfection (transfection). Then, 37 °C in a medium added with DMEM 10% FBS, 5% CO2 Conditions 5 Culturing in time then, FBS-free DMEM medium is 37 °C to 48 hours, 5% CO2 Was cultured under conditions. Centrifugal force taking each 100 ml supernatant to cultured cells, using AKTA Prime (GE healthcare) was a positive number. The AKTA Prime Protein A column (GE healthcare, 17 - 0405 - 03) of a flow rate of 5 ml/min after culture is arranged connected to chip, IgG elution buffer (Thermo Scientific, 21004) was intra-cellular. Replacing the same PBS buffer States capable of binding VEGF antibodies was positive number c-a Met and simultaneously. The number it became work c-a Met VEGF and dual specific antibodies (heavy (c-a Met VEGF binding fragment thereof comprising a heavy chain and): sequence number 120; light: sequence number 68) each part 3 the following table of portability such as disclosed: In the embodiment 1: VIG2Variants Number bath including dual specific antibodies 1. 1. VIG2Variants Sorting VIG2 (sequence number 110) through modeling of, protein aggregation tendency was screening areas are relatively high. Said modeling using the Discovery Studio (accelrys [...]) conducting number bath according to user manual. Also shown to said obtained result 2. As identified in Figure 2, with reference to the VEGF receptor 1 (seq ID no 109) when nucleotides, leucine (L174) second position 174, second position 178 leucine (I178) isopropanol, isopropanol and leucine (I185) second position 185, 215 and second position provides a relatively high protein aggregation tendency is to leucine (L215) has been selected. By introducing said predetermined respectively connected to 20 amino acid measured via simulation carried out on stability, such as transitions the next VIG2 (sequence number 110) of a protein with a stability has been confirmed. Sequence number 112 (including L174S), Sequence number 113 (comprising L174S and I185T), Sequence number 114 (including I178S), Sequence number 115 (comprising L215T), Sequence number 116 (comprising L215S), The sequence numbers 117 (comprising I185T), The sequence numbers 118 (including I178T), and Sequence number (including I178E) 119 (or more, with reference to the variant position seq 109 car fortune [thing box). 1. 2. VIG2Variants Number bath including dual specific antibodies Reference example 2 c-a Met it became work in number and said dual specific antibodies MV10AY VEGF (heavy: sequence number 120; light: sequence number 68) VIG2 sites in said in the embodiment 1. 1 With methods for backwashing. Specifically, the sequence numbers 66 (SAIT301 heavy chain), linker (GGGGSGGGGS), and said in the embodiment 1. 1 VIG2 variants including one of the sequences of polynucleotides which encode the polypeptides (in [sway bi antibodies corresponding) (sequence number 126 - 133) 68 and sequencing of number of polynucleotides encoding said polypeptides (SAIT301 heavy chain of antibodies) with reference to a number of example 2 using bi-gate start point during the small number such as antibodies. The number VIG2 variants including antibodies to below table 4 bi it became work shop window: In the embodiment 2: Dual specific antibodies VEGF Bonding (binding affinity) test It became work said number of dual specific antibody (binding kinetics) (GE Healthcare) SPR (surface plasmon resonance) method using a dynamics Biacore T100 instrument were measured by. A CM5 chip hVEGF (GE Healthcare, 28 - 9583 - 25) with respect to the irradiated surface immobilized number according to the manual. Various concentrations of about 90 RU (concentration is 6) dual specific antibodies was capture. 1M NaCl + 20 mm NaOH 30 ul/min a of a flow rate of 60 seconds by implanting said surface with respect to the reproduction (regeneration). To determine binding dynamics, using BIAevaluation software (GE Healthcare) data was obtained at each said fitting. The result obtained using the table of said BIAevaluation software to 5 shown below: As shown in the table 5, including variants of VEGF VIG2 dual specific antibodies for affinity (KD ) Is equivalent or improved on a wild-type VIG2 MV10AY among bits can be confirmed (KD <0. 23 Nm). In the embodiment 3. Dual specific antibodies of c-a Met decomposition test Dual specific antibodies anti c-a Met antibodies bind to the cells included in the c-a Met SAIT301 decomposing organic stack first a c-a Met (degradation) whose activity is used in the, an increase or decrease of total c-a Met dual specific antibodies in processing amount information antibody comprising bonding and examined effects of c-a Met c-a Met-degrading activity. When the quantitative ELISA method measuring the amount c-a Met, using human total HGF R/c-a Met ELISA kit (R&D systems) (JCRB0254; Health Science Research Resource Bank (HSRRB, Shinjuku, Japan)) was the experiment in a stomach cancer cell line MKN45. Dual specific antibodies (smaller in the embodiment 1 number) the output signal of 200,000 cells/ml 5 ug/ml of MKN45 cells 24 hours after culture (medium: RPMI with 10% Fetal Bovine Serum) ELISA on his database. (EBiosciences) OD 450 nm wavelength has been finally Super Aquablue reacted using the colorimetric signals a measured value. For comparison, in example 1 and example 2 reference number it became work in a wild-type reference number it became work including anti c-a Met antibodies SAIT301 VIG2 MV10AY respect to said dual specific antibodies such as conducting experiments. Said antibody treated value obtained is an antibody in which the same database for the controls (media) obtained by value (100%) with respect to the ratio shown. 3A and 3b shown also to said obtained result. As shown in the 3a and 3b also, antibodies can be VIG2 bi of variant whether, degree of c-a Met SAIT301 antibodies have shown similar or raised decomposition. These results by being coupled to dual specific antibodies from antibody VIG2 SAIT301 decomposition and has difficulty in certain small number c-a Met, the decomposition of c-a Met VIG2 coupled variant whether or rather can be maintained is also used for elevated. In the embodiment 4: In vitro serum stability test 4. 1. C-a Met antibodies coupled bi Retaining force Test It became work supporting force in order to test a number of c-a Met said in the embodiment 1 in dual specific antibodies, antibody testing antibodies after 7 c-a Met process or part of his degree. It became work in in vitro testing of serum antibodies in said in the embodiment 1 number bi c-a Met for supporting, antibodies to mouse serum concentration by 50 to 100 μg/mL each aliquot tube while the wafer micro l spiking 37 °C incubator to 0, 1, 3, 5, 7 and transferred to the respective sample taken out of the final sample is leaked -80 °C uncovered after method, for 1 to 3 once the matter binding ELISA. Specifically prepared manner described on each sample then melts, 0. 3% BSA/PBST 1/2000 dilution sample up to then, the cMet bloking ELISA plate coating is then on the wafer, the reaction with respect to the sample dilution. Plate is then substances while using washing, HRP conjugated anti-a huIgG Fab specific Ab detection ports to fatigue strength. Said results obtained have shown to also 4. As shown in the 4 also, it became work in number bi antibodies binding to both said in the embodiment 1 7 c-a Met stably remain in liver were identified (about 80% or more of antibody levels by Tuesday 7 immediately after processing). 4. 2. Dual specific antibodies VEGF Coupling Retaining force Test It became work supporting force in order to test a number of VEGF in said in the embodiment 1 dual specific antibodies, antibody testing between VEGF antibodies after his degree process or part 7. It became work in VEGF in vitro testing of serum antibodies in said in the embodiment 1 number bi for supporting, on in the embodiment 4. 1 Equal guides 7 in vitro method described in serum were measured changes in liver deteriorated so as to VEGF. Said prepared sample then melts, 0. Then to 1/2000 dilution into 3% BSA/PBST, then blocking ELISA plate coating on the hVEGF is by using a solvent, the reaction with respect to the sample dilution. After washing the plate substances while using HRP conjugated anti-a huIgG Fab specific Ab detection to the matter. 5 Shown also to said obtained result. As shown in the 5 also, it became work in dual specific antibodies are all number said in the embodiment 1 7 bonding occurring in the liver has been stably maintain the c-a Met (level of about 50% or more antibodies by Tuesday 7 immediately after processing), in particular MV10AY. 7 And MV10AY. 1 MV10AY cells supporting force is compared to a visibility VEGF as signal peptides. In the embodiment 5: Akt Phosphorylation test ( It became work in dual specific antibodies in order to identify the safety of number said in the embodiment 1, phosphorylation of AKT kinase known as quantitative ELISA method measuring the degree number agonism antibody measured. AKT cells action are regulated by cell proliferation, cell survival, cell size, available nutrients reactive, intermediate metabolism processes, angiogenesis (angiogenesis), tissue penetration (tissue invasion) etc.. All procedures representative features of cancer tumor protein (oncoprotein) for detection of tumors (tumor suppressor) and numerous billion chips affects the AKT path on the number material, signal transmission by classical metabolic regulators that points of connection allows the gene action of cells in a plurality of hierarchies. The activity of the AKT AKT phosphorylation-storing more active high degree of cancer as well, the bubbles agonism antibodies are disclosed. In the embodiment said dual specific antibodies are positive contrast in the contrast when it has processed a 5D 5 antibody can inhibit AKT phosphorylation of how whether flaws. The Ser 473 phosphorylation of AKT (Ser473) Cell signaling yarn was used which seats PathScan phospho-a AKT1 chemiluminescent Sandwich ELISA kit. A renal cancer cell line culture day ruminated 200,000 cells/ml (HTB provided 46; American Type Culture Collection (ATCC), Manassas, VA) Caki-a 1 to 5 ug/ml in serum-free DMEM medium (GIBCO, Invitrogen) number of dual specific antibodies MV10AY it became work to said in the embodiment 1. 1 Or MV10AY. 7 ELISA kit was 30 minutes pressure is lowered after processing using an FFT. The result measured by the Perkins Elmer yarn machine are obtained. Phosphorylation of AKT (American Type Culture Collection; ATCC, Manassas, VA) positive control in 5D 5 calculates the degree when a degree of phosphate we shall be handled 100%, compared to a value indicative of the degree of phosphorylation of other anti c-a Met antibodies and double targeting antibody was derived. For comparison, it became work in reference example 2 in example 1 reference number SAIT301 MV10AY antibodies using the same antibodies and each said conducting tests it became work number. 6 Also shown to said obtained result. As identified in Figure 6, it became work in number in the embodiment 1 dual specific antibodies (MV10AY. 1 (Inhibition rate: 80. 15%) And MV10AY. 7 (Inhibition rate: 75. 78%) Has antibodies inhibit AKT phosphorylation MV10AY SAIT301 antibodies and equivalent level, 5D 5 significantly compared to the AKT phosphorylation inhibiting ETEC extent. In the embodiment 6: Dual specific antibodies in a cancer growth inhibition test A human stomach cancer cell line MKN45 (JCRB0254) Health Science Research Resource Bank (HSRRB, Shinjuku, Japan) was obtained. 10% (V/v) lines said fetal bovine serum (FBS, GIBCO Cat. #16000 - 044) And 1% penicillin/streptomycin (v/v) (GIBCO, Cat. #15410 - 122) Including RPMI1640 a medium (GIBCO, Cat. #11875 - 119) Was cultured in. 5% CO lines2 A transparent conductive layer including cultured in 37 °C under a wet atmosphere, before confluence (subculture) passaging on him. CEDEX Analyzer (Roche Diagnostics) number using cells were measured. Antibodies according to tumor cell proliferation (in vitro) processing for irradiating Celltiter Glo (CTG: Promega yarn) light emitting assays (luminescent assay) was used. Said number of conducting such an assay bath according to the manual. The medicinal wine, black 96 - well plate containing FBS 10% (v/v) RPMI1640 culture of MKN45 cells (Corning Incorporated, Cat. # Costar 3603) to each [...] 5x103 The concentration of cells and divider, containing 10% FBS RPMI1640 culturing, a final concentration of 0. 008 Ug/mL, 0. 04 Ug/mL, 0. 2 Ug/mL, 1 ug/mL dilution to antibodies (dual specific antibodies MV10AY it became work in number in the embodiment 1. 1 Or MV10AY. 7) Was. 72 CTG solution after adding 100 microliters per time incubating each well, incubating at room temperature 30 minutes-gate. (Perkin Elmer, Waltham, Massachusetts, USA) Envision 2104 Multi-a label Reader said obtained using a luminescent signal recording. For comparison, the same test example 2 using reference number MV10AY it became work in conducting. Said results obtained have shown to also 7. As shown in the variation 7 also, it became work MV10AY dual specific antibodies in number in the embodiment 1. 1 And MV10AY. 7 Excellent in comparison with all MV10AY MKN45 cell proliferation inhibiting activity is also used for can be. In the embodiment 7: dual specific antibodies in a cancer migration inhibition test Dual specific antibodies in order to identify whether cancer cell migration by inhibiting cell migration assay (migration assay) using xCelligence system (ACEA Bioscience) by dielectrophoresis. [E tax xCelligence provided RTCA DP system device is used therein as cell migration, gold microelectrode array cells attached LSD (impedence) on cell number as the measuring device are disclosed. [E tax CIM-a plate 16 is used on cell migration (ACEA Bioscience) such as Boyden chambe upper chamber to lower chamber is divided, into cells into and out of the upper chamber 8 um pore to travel through the impedance to generate only cells are disclosed. [...] experiments of 80,000 - 100,000 cells in the upper chamber, the lower chamber 100 ng/ml HGF 100 ng/ml VEGF was added to provide a chemical attracting material (chemoattractant) on mixing. Dual specific antibodies through the cells is 20 ug/ml Upper chamber simultaneously with the degree of obstruction of cancer cell migration by dual specific antibodies were measured. Said result (A498 cell lines) and also 9 8 also obtained (786O cell lines) is shown. As shown in the 8 and 9 may also, it became work MV10AY dual specific antibodies in number in the embodiment 1. 1 And MV10AY. 7 Renal cancer cells represents all MV10AY excellent compared to the migration inhibitory activity can be confirmed. In the embodiment 8: Double antigen-specific antibodies Retaining force Test (in Vivo) It became work number in said in the embodiment 1 antibodies binding to antigen (c-a Met and VEGF) bi (half-life) in vivo was tested at a retaining force. MV10AY, MV10AY. 1, And MV10AY. 7 1 Times each received intravenous administration in Sprague-a Dawley rat said 3 species antibodies was assessed a pharmacokinetic profiles. 5 Mg/kg dosage is set to said each antibodies. Embryo total 12 of 0.50 to rats, these include the following table 6 classification such as been processed: 24 Hours after administration of said antibody animals twice said first observed. The weight is recorded by measuring antibodies before dosing, blood samples for use in all group defined point sampling the pharmacokinetic profiling was prepared. All processing is not observed side effects from the group consisting of antibodies. I.e., each in an amount of 5 mg/kg intravenous said 3 species antibodies is administered animals by identifying the acceptable (tolerated) can be. Rat serum in a sample concentration of anti-a human a-IgG ELISA test antibodies through said 3 species for a long time. 3 Species antibodies the quantitative limit value (lower limit of quantification; LLOQ) 0. 488 Ng/mL pulse, a standard curve is 0. 488 To 62. 500 Ng/mL been linear range. All ng/mL concentration was expressed. Quantitative limit value LLOQ calculated concentration higher than a lower limit (<LLOQ) was less than less than metered LLOQ display. In addition, female VEGF ELISA for measuring said 3 species antibodies by dielectrophoresis. 3 Species antibodies (LLOQ) quantitative limit value is 0. 366 Ng/mL pulse, a standard curve is 0. 366 To 800. 000 Ng/mL been linear range (the standard curve were linear range between 0. 366 To 800. 000 Ng/mL). Display all concentration when the ng/mL, quantitative limit value LLOQ calculated concentration higher than a lower limit (<LLOQ) was less than less than metered LLOQ display. Antibodies of said 3 species (MV10AY, MV10AY. 1, And MV10AY. 7) A non a-compartment model by using WinNolin pharmacokinetic parameters of said concentration was calculated on the basis of data. Jugular vein cannulation syringe directly without using said test animal (about 250 uL) was harvested from blood samples. Said blood sample tube for collecting serum after storing at room temperature into the centrifuge by dielectrophoresis. 240 Times after the taking of blood, carbon dioxide in each test animals with respect to the canvass. 1 Hours to centrifuge the blood samples collected, collection of serum. Centrifuging the sample in 10 minutes to 4 °C was 4,000 rpm. Each serum sample 2 separate polypropylene beam affected by chicken soup. Said tube of each group number, animal number, collection interval (time point) is displayed disclosed. The light irradiated from the light using the determined in a serum sample was freeze -80 °C. 1 Sample set bioanalysis (see 10. 0) As well as. Antibodies in accordance with the is concentration after injection, 5, 15, 30 minutes after the injection blood sample scanning even though the PK analysis, 1, 2, 4, 6, 8, 24, 32, 48, 72, 96, 120, 144, 168, 240 was after each sampling. (Total of 18 data value) ELISA 1 (c-a Met part) in performing, 0. 488 To 62. 500 Ng/mL (2 a-fold serial dilution) in a concentration range which covers the blank rat serum (1:1, 000 diluted by 0. 5% BSA in PBST) standards in preparing his. 0. 488 To 62. Using data obtained from the 500 ng/mL of standards, standardized curve (calibration curves) disposed for each analysis (analytical batch) has generated. Chamber each estimate at each number from 0. 977, 3. 906, 25. 000 Ng/mL matches (each SOI value QC1, QC2, QC3 by value) and it is confirmed whether. ELISA 2 (VEGF part) in performing, 0. 366 To 800. 000 Ng/mL (3 a-fold serial dilution) in a concentration range which covers the blank rat serum (1:1, 000 diluted by PBS) was prepare in standards. 0. 366 To 800. Using data obtained from 000 ng/mL of standards, standardized curve (calibration curves) disposed for each analysis (analytical batch) has generated. 9. 877, 88. 889, And 320. 000 Ng/mL concentration was preparing QC samples using the same substrate. Chamber each estimate at each number from 0. 977, 3. 906, 25. 000 Matches (each SOI value QC1, QC2, QC3 by value) and it is confirmed whether. ELISA 1 preparation of MV10AY administration group, MV10AY. 1 Administration group, and MV10AY. 7 Administration PK sample obtained from the group consisting of: time 5 minutes post-a dose time points 24 sample Dilution Buffer (0. 5% BSA in PBST) after dilution using 100 times, 100 times more dilution to further dilution (1000 times in the blank serum dilution Dilution Buffer) substrate was (10,000 times final dilution). 32 Hr to 240 to 100 times dilution of a test sample is post-a dose time points after further dilution time Dilution Buffer to said substrate with respect to the 50 times more dilution (final 5,000 times dilution). Coating Antibody (anti-a hIgG; Fc specific) and Detection Antibody (HRP conjugated anti-a hIgG; Fab specific) ELISA using by dielectrophoresis. ELISA 2 preparation of V10AY administration group, MV10AY. 1 Administration group, and MV10AY. 7 Administration PK sample obtained from the group consisting of: PBS 100 times dilution to sample all post-a dose time points after, said substrate (1000 a-fold diluted blank serum in PBS) was 10 times more to dilution (final 1,000 times dilution). Coating protein: VEGF (R&D) and Detection Antibody (HRP conjugated anti-a hIgG; Fab specific) ELISA using by dielectrophoresis. China's Pharmalegacy [...] is through a conducting to said ELISA. Said results obtained also 10a (antibody/c-a Met complex serum concentration: c-a Met-fill), also 10b (antibody/VEGF complex serum concentration; female VEGF), and table 7 (antibody/VEGF complex half-life/c-a Met composite and antibodies) showed: Also 10a, 10b and also as shown in the table 7, it became work MV10AY dual specific antibodies in number in the embodiment 1. 1 And MV10AY. 7 Formed complex syndrome MV10AY on VEGF is c-a Met and 2 in comparison with one device can be confirmed. In the embodiment 9: Dual specific antibodies productivity test VIG2 variant according to whether a hole was dual specific antibodies. Said in the embodiment 1. 2 MV10AY prepared by the number in, MV10AY. 1, And MV10AY. 7 Expression vector each Qiagen Maxiprep kit (Cat no. 12662) Amplified using a transparent conductive layer, ExpiCHO temporary expression isTM (Invitrogen) 293 Expression System using the employers. 293 F cell used cell lines which, ExpiCHOTM By using floating culture contains Expression Medium him as manner. 5X10 cells before day temporary expression5 After concentration of cells/ml can be prepared, 24 time resumes cell number 1x106 Cells/ml when the temporary expression of abortion. ExpiCHOTM For instance performed using liposomal reagent when the reagent (invitrogen) transduction (transfection), transduction after completion, 37 °C, 80% humidity, 8% CO2, 5 130 Rpm incubator him as in liver. Said centrifugal force taking each 100 ml supernatant to cultured cells, using AKTA Prime (GE healthcare) was a positive number. The AKTA Prime Protein A column (GE healthcare, 17 - 0405 - 03) of a flow rate of 5 ml/min after culture is arranged connected to chip, IgG elution buffer (Thermo Scientific, 21004) was intra-cellular. Replacing the same antibodies of PBS buffer States 3 species (MV10AY, MV10AY. 1, MV10AY. 7) A deformation was number. Positive number Nanodrop 2000 (Thermo Scientific) measured at 280 nm to the concentration of a protein, a result obtained with the table 8 shown to: As shown in the table 8, it became work MV10AY dual specific antibodies in number in the embodiment 1. 1 And MV10AY. 7 MV10AY yield about 2 times and switching number in comparison with, for example, about 2. 3 To 2. 5 Times can not inserted on the car. In the embodiment 10: Assessing the efficacy in an animal model of dual specific antibodies ( The present invention according to the following method was to test efficacy of dual specific antibodies in an animal model. Cancer implanted animal model number bath NCI-a H441 (ATCC) as well as a human lung cancer cells. Said cells in a medium supplemented with 10% FBS RPMI provided 1640 37 °C and 5% CO2 After held under conditions, then mouse him as until the seeded. 100 UL serum of about 5,000,000 to 3 - 4% of this cow [phul base column (isoflurane) NCI-a H441 cells mixed anesthetic injected into each mouse (total 55 mary) (via s. c.) up. Antibody processing group allocation Said average 100 to 200 mm average size of tumor implantation3 The user, having appropriate size of tumors has been divided into 4 randomly according to 40 tumor mouse embryo size and weight (10 mice per group). The antibodies of processing content to below table 9 group divided shop window: Measuring After said random grouping, tumor size (volume) and 1 to 2 weeks 4 weeks once the body weight on during recording. Tumor volume (tumor volume; TV) is the bill as follows: TV=(length x width2 )/2. Relative tumor volume (relative tumor volume; RTV) each individual is the bill as follows: RTV=Vt/V0 (Vt respective measuring date tumor volume, antibodies in tumor volume V0 is disclosure by each processing means). Result Each animal health is not observed during test said severe abnormal the indicia. Test animal body weight of each group was maintained away at all test without significantly difference. (PBS) administered Vehicle been increased tumor volume away at a mouse (voice controls). 25 Average tumor volume after grouping [...] 660. 02 ± 36. 04 Mm3 A transparent conductive layer is reached, this grouping purchases standards tumor size as compared to about 4. 19 An article are disclosed. Compared to controls voice, SAIT301 (5 mg/kg) processing group number greater degree of tumor growth billion effect disappeared (p > 0. 05). While, MV10AY (5 mg/kg) MV10AY processing group. 7 (5 Mg/kg) in statistically significant tumor volume and relative tumor volume processing group degree (P<0. 05) Show differences in rate of tumor growth delay effect has been shown. Said tumor volume to 10 and 11 shown below table also obtained each group: As shown in the table 10 and 11 also, MV10AY dual specific antibodies. 7 Processing group voice controls are significant tumor growth compared to antibodies as well as to other processing group number effect billion ( <bio-deposit id="biod-00001" num="0001"><depositary>Korean cell line studies cutting</depositary><bio-accno>KCLRFBP 00220</bio-accno><date>20091006</date></bio-deposit> Provided are a vascular endothelial growth factor (VEGF)-binding fragment mutant, a fusion protein connected with the VEGF-binding fragment mutant and anti-c-Met antibody, a dual specific antibody including the fusion protein, a polynucleotide encoding the fusion protein, a transformant including the polynucleotide, a pharmaceutical composition containing the fusion protein or the dual specific antibody as an active ingredient, and a method for producing the dual specific antibody targeting both VEGF and c-Met with enhanced stability through connection of the VEGF-binding fragment mutant with the anti-c-Met antibody. COPYRIGHT KIPO 2017 Sequence number 109 of VEGF receptor 1 129 229 start control including a second Ig a-like domain 2 (VIG2; sequence number 110) in second from successive 101 to 1338 amino acid sequences and, exterior exposure of the hydrophobic residues VIG2 independently substituted with one or more polar or hydrophilic amino acid residues characterized, polypeptides. According to Claim 1, said VIG2 exterior exposure of hydrophobic residue, sequence number counted with reference to the 109, leucine (L174) second position 174, second position 178 leucine (I178) isopropanol, isopropanol and second denture 185 leucine (I185), and at least one second position 215 (L215) 1 is selected from the group consisting of leucine, polypeptides. According to Claim 1, said polar or hydrophilic amino acid serine (S), threonine (T), and glutamic acid (E) is at least one selected from the group consisting 1, polypeptides. According to Claim 1, sequence number counted with reference to the 109, leucine (L174) second position 174, second position 178 leucine (I178) isopropanol, isopropanol and leucine (I185) 185 to a third position, and a second position selected from the group consisting at least one leucine (L215) 215 1 each independently serine (S), threonine (T), and glutamic acid (E) substituted amino acid selected from the group consisting, polypeptides. According to Claim 4, sequence number counted with reference to the 109, second position 174 (a) serine (L174S) [...] replaced; (b) second position 178 isopropanol current god threonine (I178T), serine (I178S), or glutamic acid (I178E) replaced; (c) a third position 185 isopropanol current god threonine (I185T) replaced; (d) second position 215 [...] threonine (L215T) or serine (L215S) substituted; (a) to (d) or (e) said one of the Image 2 which has been modified by including VIG2 variants, polypeptides. (1) Anti c-a Met antibodies or antigen-binding fragments thereof; and (2) any one of Claim 1 to Claim 5 polypeptides including fusion proteins. According to Claim 6, said (1) anti c-a Met antibodies or antigen-binding fragments and (2) connecting a peptide linker consisting of 5 to 30 amino acid polypeptide further including, fusion proteins. According to Claim 6, antibodies or antigen-binding fragment thereof is said c-a Met sequences of amino acid sequences of proteins during the sequence numbers 73 71 c-a Met (EEPSQ) 5 to 19 including a continuous process for one or more amino acids that specifically bind to the antibodies or antigen-binding fragments thereof, fusion proteins. According to Claim 8, antibodies or antigen-binding fragment thereof is said anti c-a Met, including CDR-a H1 amino acid sequences of seq ID no 4; the sequence numbers 5 amino acid sequence, the amino acid sequence of sequence number 2, or the amino acid sequence of sequence number 2 in 10 second installation including continuous 8 to 19 from 3 second amino acid sequence of amino acids including CDR-a H2; the sequence numbers 6 amino acid sequence, the amino acid sequence of sequence numbers 85, or sequence number 85 6 from 6 to 13 amino acid sequences of 1 second in second installation including a continuous amino acid sequence of amino acids including CDR-a H3; amino acid sequences of the amino acid sequence of sequence number 7 including CDR-a L1; amino acid sequences of including CDR-a L2 sequence number 8; 8 and 9 amino acid sequence, the amino acid sequence of sequence numbers 15, amino acid sequences of seq ID no 86, or sequence number in the sequence of amino acid 1 to 17 including 9 9 from 89 second second installation including a amino acid sequence of amino acids including CDR-a L3 will, fusion proteins. According to Claim 6 fusion proteins including c-a Met and VEGF dual specific antibodies. According to Claim 10, said (1) anti c-a Met antibodies or antigen-binding fragments and (2) connecting a peptide linker consisting of 5 to 30 amino acid polypeptide further including, dual specific antibodies. According to Claim 10, said anti c-a Met IgG and is in the form of antibodies, said polypeptides may be in the form of heavy chain (Fc) 5 to 30 said IgG anti - c-a Met C end connected via a peptide linker consisting of amino acid compound is, dual specific antibodies. According to Claim 10, antibodies or antigen-binding fragment thereof is said c-a Met sequences of amino acid sequences of proteins during the sequence numbers 73 71 c-a Met (EEPSQ) 5 to 19 including a continuous process for one or more amino acids that specifically bind to the antibodies or antigen-binding fragments thereof, dual specific antibodies. According to Claim 13, antibodies or antigen-binding fragment thereof is said anti c-a Met, including CDR-a H1 amino acid sequences of seq ID no 4; the sequence numbers 5 amino acid sequence, the amino acid sequence of sequence number 2, or the amino acid sequence of sequence number 2 in 10 second installation including continuous 8 to 19 from 3 second amino acid sequence of amino acids including CDR-a H2; the sequence numbers 6 amino acid sequence, the amino acid sequence of sequence numbers 85, or sequence number 85 6 from 6 to 13 amino acid sequences of 1 second in second installation including a continuous amino acid sequence of amino acids including CDR-a H3; amino acid sequences of the amino acid sequence of sequence number 7 including CDR-a L1; amino acid sequences of including CDR-a L2 sequence number 8; 8 and 9 amino acid sequence, the amino acid sequence of sequence numbers 15, amino acid sequences of seq ID no 86, or sequence number in the sequence of amino acid 1 to 17 including 9 9 from 89 second second installation including a amino acid sequence of amino acids including CDR-a L3 will, dual specific antibodies. Dual specific antibodies according to Claim 10 c-a Met and active ingredient including VEGF, VEGF or formulated to c-a Met for prevention or treatment of pharmaceutical compositions. According to Claim 15, said c-a Met formulated to VEGF or cancer, bowel, pregnancy-diabetes, diabetic retinopathy, hwang contravariant adult, for preventing or treating composition. Sequence number 109 of VEGF receptor 1 129 229 start control including a second Ig a-like domain 2 (VIG2; sequence number 110) in second from successive 101 to 1338 amino acid VEGF binding fragment thereof exterior exposure of the hydrophobic residues consisting of polar or hydrophilic amino acid residues including the substitution of one or more of the independently, in-vivo stability enhanced VEGF binding fragments thereof number bath method. According to Claim 17, said VEGF binding fragment thereof exterior exposure of hydrophobic residue, sequence number counted with reference to the 109, leucine (L174) second position 174, second position 178 leucine (I178) isopropanol, isopropanol and second denture 185 leucine (I185), and at least one second position 215 (L215) 1 is selected from the group consisting of leucine, method. According to Claim 17, said polar or hydrophilic amino acid serine (S), threonine (T), and glutamic acid (E) is at least one selected from the group consisting 1, method. According to Claim 17, with reference to the sequence number 109 counted, leucine (L174) second position 174, second position 178 leucine (I178) isopropanol, isopropanol and leucine (I185) 185 to a third position, and a second position selected from the group consisting at least one leucine (L215) 215 1 independently serine (S), threonine (T), and glutamic acid (E) including the amino acid substitution selected from the group consisting of, method. According to Claim 20, with reference to the sequence number 109 counted, 174 (a) to the second position to the annex type it will put on serine (L174S); (b) second position 178 isopropanol type it will put on threonine (I178T), serine (I178S), or glutamic acid substituent (I178E) step; (c) a third position isoforms type it will put on threonine (I185T) step 185 to the annex; (d) second position type it will put on threonine (L215T) or serine (L215S) step 215 to the annex; or (e) said (1) to (4) of a combination of 2 or more. Including a, method. Any one of Claim 1 to Claim 5 polypeptides including, water-soluble VEGF and c-a Met dual specific antibodies for stabilizing composition. Antibodies or antigen-binding fragment anti c-a Met thereby bridging any one of Claim 1 to Claim 5 polypeptides including, in-vivo stability and number of dual specific antibodies enhanced c-a Met VEGF bath method. CDR Amino acid sequence CDR-a H1 DYYMS (sequence number 1) CDR-a H2 FIRNKANGYTTEYSASVKG (sequence number 2) CDR-a H3 DNWFAY (sequence number 3) CDR-a L1 KSSQSLLASGNQNNYLA (sequence number 10) CDR-a L2 WASTRVS (sequence number 11) CDR-a L3 QQSYSAPLT (sequence number 12) Clone name Derived library CDR sequences H11 provided 4 CDR-a H1 PEYYMS (sequence number 22) YC151 CDR-a H1 PDYYMS (sequence number 23) YC193 CDR-a H1 SDYYMS (seq ID no 24) YC244 CDR-a H2 RNNANGNT (seq ID no 25) YC321 CDR-a H2 RNKVNGYT (sequence number 26) YC354 CDR-a H3 DNWLSY (seq ID no 27) YC374 CDR-a H3 DNWLTY (seq ID no 28) L1 provided 1 CDR-a L1 KSSHSLLASGNQNNYLA (sequence number 29) L1 provided 3 CDR-a L1 KSSRSLLSSGNHKNYLA (seq ID no 30) L1 provided 4 CDR-a L1 KSSKSLLASGNQNNYLA (sequence number 31) L1 provided 12 CDR-a L1 KSSRSLLASGNQNNYLA (sequence number 32) L1 provided 22 CDR-a L1 KSSHSLLASGNQNNYLA (seq ID no 33) L2 provided 9 CDR-a L2 WASKRVS (seq ID no 34) L2 provided 12 CDR-a L2 WGSTRVS (sequence number 35) L2 provided 16 CDR-a L2 WGSTRVP (seq ID no 36) L3 provided 1 CDR-a L3 QQSYSRPYT (sequence number 13) L3 provided 2 CDR-a L3 GQSYSRPLT (sequence number 14) L3 provided 3 CDR-a L3 AQSYSHPFS (sequence number 15) L3 provided 5 CDR-a L3 QQSYSRPFT (sequence number 16) L3 provided 32 CDR-a L3 QQSYSKPFT (sequence number 37) Named C-a Met heavy chain Hinge Invariant site Linker Light chain VEGF binding fragment thereof MV10AY Sequence number 66 U3 HC 9 (seq ID no 102) IgG2 (G4S)2 Seq ID no 68 VIG2 (seq ID no 110) Dual specific antibodies name VIG2 and methods for position VIG2 sequence Anti c-a Met antibodies MV10AY Variant not (wild-type) Each sensing face 110 sequence SAIT301 MV10AY. 1 L174S, I185T Sequence number 113 MV10AY. 5 I178S Sequence number 114 MV10AY. 6 L215T Sequence number 115 MV10AY. 7 L174S Sequence number 112 MV10AY. 8 L215S Sequence number 116 MV10AY. 9 I185T The sequence numbers 117 MV10AY. 10 I178T Sequence number 118 MV10AY. 11 I178E Sequence number 119 Name ka (106 M-1 s-1 ) kd (10-4 s-1 ) KD (NM) MV10AY 2. 6 5. 9 0. 23 MV10AY. 1 2. 5 5. 0 0. 20 MV10AY. 5 1. 7 3. 7 0. 22 MV10AY. 6 2. 0 4. 4 0. 22 MV10AY. 7 3. 3 5. 7 0. 17 MV10AY. 8 2. 5 5. 1 0. 21 MV10AY. 9 3. 1 5. 0 0. 16 MV10AY. 10 2. 2 4. 7 0. 21 MV10AY. 11 2. 2 4. 6 0. 21 T1/2 (H) Met VEGF MV10AY 61. 4 (2. 6 Days) 32. 3 (1. 3 Days) MV10AY. 1 127 (5. 3 Days) 74. 5 (3. 1 Days) MV10AY. 7 119 (5. 0 Days) 85. 5 (3. 6 Days) Antibody name Mutant position Yield positive number (mg/L) MV10AY (WT) - 67. 2 MV10AY. 1 L174S, I185T 151. 2 MV10AY. 7 L174S 168. 8 Day after grouping G1 G2 G3 G4 Mean SEM Mean SEM Mean SEM Mean SEM 0 161. 06 7. 66 160. 00 6. 45 159. 96 6. 54 159. 50 6. 94 4 198. 47 8. 99 196. 74 10. 48 195. 23 8. 87 182. 68 11. 77 7 240. 16 11. 30 242. 36 14. 82 239. 09 12. 36 209. 65 16. 91 11 297. 38 15. 34 282. 51 18. 96 285. 71 15. 86 249. 32 22. 49 14 365. 42 18. 03 352. 03 26. 75 336. 55 20. 08 289. 04 28. 28 18 483. 44 15. 73 435. 14 32. 94 387. 76 28. 62 323. 80 35. 40 21 598. 73 16. 46 520. 00 40. 07 438. 35 36. 92 363. 53 40. 44 25 660. 02 36. 04 536. 28 44. 88 497. 21 56. 32 350. 19 44. 32