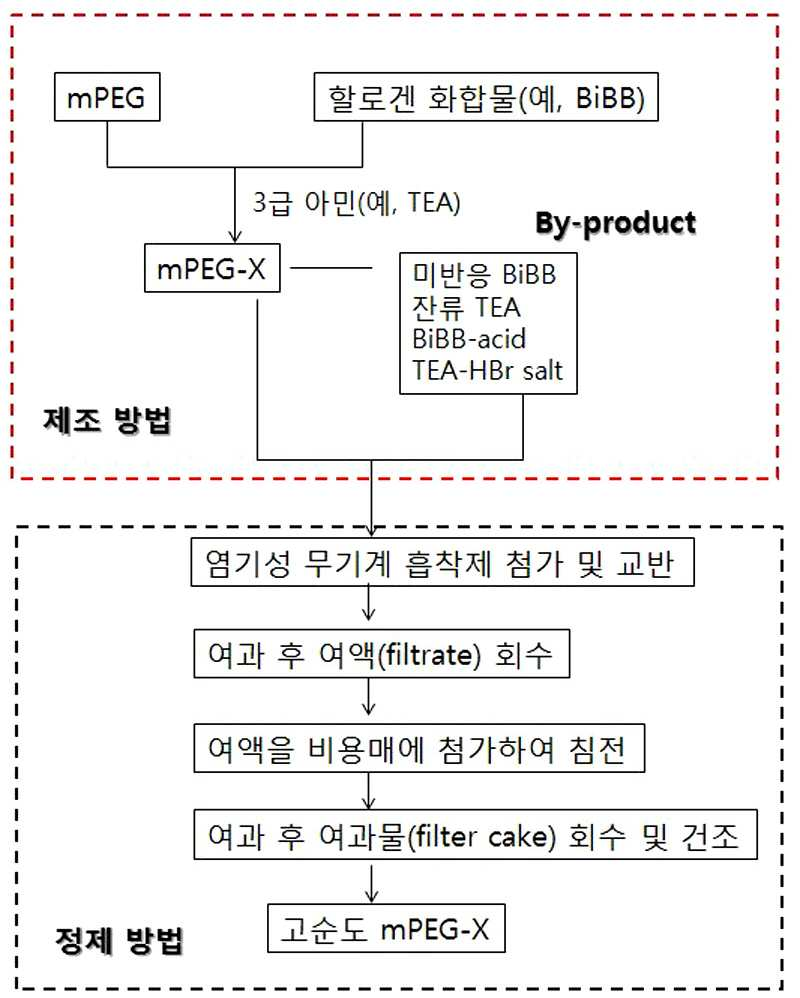
PURIFICATION METHOD OF HALOGENATED METHOXY-POLYETHYLENE GLYCOL
The present invention refers to basic inorganic adsorption number of high purity using a halogenated methoxy polyethylene glycol to obtain has a positive number method are disclosed. Disclosure number of polymerization is initiated by reaction of disclosure and s7. Said disclosure number is the most widely used conventional radical polymerization or other processes before disclosure number etc. (radical initiator). Radical disclosure number has light, heat, chemical reaction or radiation triggers a chemical coupling is a combination of a weak part severed free radical (free-a radical) is formed, a monomer mixture free radical polymerization reaction attack disclosure and the processing advances. Disclosure number is a radical conventional organic peroxide peroxide or hydrocodone; oxidation - reduction number; azo compound; etc. an organometallic reagent. Said radical disclosure number is represented by the number of speeds of the bath etc. random type polymer used. However bio of intensity or star polymer or the like for specific number of precision high pressure liquid coolant with this polymer molecular weight, molecular weight distribution, and repeating units control the spirit. The polymerization method such as method which are used to living polymerization, the polymer in the form of macro (macro initiator) is mainly used rather than monomolecular form timing said number radical disclosure number disclosure number etc.. Polymer disclosure number which is also referred to as macro disclosure number, extension number after polymerization bath particles serving to hydrophobic polymer chains and serving to support a metallocene polymerization disclosure and the eventual disclosure number parts number addition in a device substrate. Said hydrophobic polymer chains can be synthesized polymer with the stably due to dimensional portion is restrained disclosed. The pin is one macro disclosure number representative mPEG-a Br (maul [thok hour polyethylene glycol bromide). In the presence of triethylamine (TEA) on the mPEG-a Br mPEG bromo this cow vice-mote reel bromide (Bromoisobutyryl bromide, hereinafter 'BiBB' is equal to) by esterification of encoded number tank. Wherein the reaction after the positive number in the process of a high pressure liquid coolant at a high purity by a mPEG-a Br mPEG-a Br S. number. The radical polymerization using radical polymerization occurs before said mPEG-a Br polymerized by means disclosure number 2000. Disclosure number radical polymerization activity of very important disclosed. The active circuit is radical scavenger (radical scavenger) in the case that reaction material having a desired level of polymerization polymerization degrees hereinafter for control without polymer composed of number not easy disclosed. In particular, via the process number on the tube surface TEA will remain unreacted BiBB byproduct is also prepared by the mPEG-a Br and, HBr or they generated through the reaction between the tooth profile of trypanocidal bromo (2 a-bromo-a 2 non-methylpropanoic acid, hereinafter 'BiBB - acid' is combined with a load) and TEA-a HBr salt remains on the substrate. Said BiBB - acid and salts can be used to form the radical scavenger (scavenger) TEA-a HBr, polymerizing mPEG-a Br disclosure number simultaneously used as radical activity significantly degrading the substrate. The, said unreacted reaction of a substance produced by acid, number direction is volatile salts, etc. various mechanical same number process. On reaction of unreacted material and reaction by-products of every other number mPEG BiBB injected with this extraction method is widely used for water and organic solvent etc. or recrystallization method. However globe-mPEG-a Br itself using solubility in water and high when said extraction method is hereinafter adsorbs layer unit is pulsator number for his fellow workers. In addition, in the case of excess by-products remain recrystallization method are disclosed. The high purity mPEG-a Br can be scheme is used in order to obtain the column chromatography. The method however there separating mPEG-a Br and solvents are required, additional filtration and washing work the spirit. In addition, long time for recovery to drive residual solvent volatilization of that door number flow tides. Thus, in a short time to obtain high purity mPEG-a Br number desired mechanical process are disclosed. The victims of the method the present invention capable of fitting a simple result of high purity through treatment with a positive number, basic inorganic adsorption number treated result, mPEG BiBB on acid and salts after esterification reaction with unreacted reaction of a substance produced by the present invention can be a stand-alone effectively number together with the arrears of work. Thus, the purpose of the invention very high purity and switching a number number method or a reversed F. halogenated methoxy polyethylene glycol. In order to achieve said purposes, the present invention refers to a basic inorganic adsorption number halogenated methoxy polyethylene glycol treatment with the halogenated methoxy polyethylene glycol characterized and switching a number method number substrate: the basic inorganic adsorption number is alumina, silica and silica - alumina made characterized species selected from the group consisting of not more than 1. Said method a positive number to formula 1 halogenated organic halide acid methoxy polyethylene glycol of easy 600 ppm hereinafter and, halogen acid easy 0 - 3 class amine. 3 0.1 Weight % hereinafter characterized. The present invention according to a basic inorganic adsorption number positive number method by using halogenated methoxy polyethylene glycol remaining constant content in organic halogen acid salt such as a 3 - fluoro zincates BiBB - acid and TEA-a HBr class amine number of stand-alone effectively number halogen high purity mPEG-a Br nephrophathy numerical control machine. This method for expensive column chromatography contrast conventional screen-printing sheet, such as recrystallization or evacuated article number number can be achieved at high purity contrast sealer is coated in the distillation process. Also in the present invention number in Figure 1 shows a number of positive and a halogenated methoxy polyethylene glycol bath method is shown order number method are disclosed. Hereinafter the present invention that are directionally-described substrate. Disclosure number widely used as methoxy polyethylene glycol polymer represented mPEG-a Br halogenated macro disclosure number (macro initiator, or polymer disclosure number) are disclosed. Said methoxy polyethylene glycol mPEG BiBB number encoded on the halogenated by esterification with bath. Said esterification reaction product in the reaction after remaining unreacted material other than halogenated methoxy polyethylene glycol and, further by said residual product is selected from the group occurs. The positive number such as recrystallization or column chromatography for purity or process for obtaining a forged article with this high number of which a door number long processing the selected data flow tides. In the present invention represented by formula 1 methoxy polyethylene glycol 2000 high pressure liquid coolant to halogenated number. [Formula 1] (Said formula 1 in, an element of the halogen X, n is an integer of 5 to 1000.) The number remaining after said formula 1 halogenated methoxy polyethylene glycol for numerical control machine esterification products etch byproducts generated number to further identify additional purification steps is a stand-alone, low cost process method decodes a number of high purity product can be obtained in a short time. The number of halogenated methoxy polyethylene glycol bath method and residual byproducts and additional should and can be first of all at the time of the products. Figure 1 shows a number of bath method in the present invention number also when a halogenated methoxy polyethylene glycol (mPEG a-X) positive and number method is shown order disclosed. According to each method hereinafter detailed as follows. Number bath method The reference also 1, mPEG provided X is a compound of formula 3 in the presence of tertiary amine according to formula 2 1 3 shown mPEG encoded number bath via esterification of halide compound: [Compound 1] (Said reactive 1 in, an element of the halogen X, n is an integer of 5 to 1000.) In the formula 2 as follows said reactive 1 is the output timing mPEG number, this number can be a seller using commercially available high pressure liquid coolant directly or, in the present invention does not specifically define. [Formula 2] Number 1 in formula 3 as follows said reactive halogen compound that is timing on the surface, the X is F, Cl, Br, or I and, most preferably Br are disclosed. [Formula 3] (In said formula 3, X is halogen element.) In this cow vice-mote reel bromide (Bromoisobutyryl bromide, BiBB) is typically X is bromo Br CAS No. 20769 - 85 - 1 Having a molecular weight compounds of the 229. In polymerizing a compound 90 g/mol carbonate are disclosed. The operation of the seller in a commercially available reagent number said BiBB use disclosed. Formula 2 and formula 3 is a halide of said equivalence ratio of 1:1 into a stoichiometric excess of mPEG esterification reaction comprises reaction but, reaction yield, i.e. mPEG mPEG-a X 100% conversion to obtain isophthtalic halide compound of formula 3 to addition of substrate. Wherein the reaction represented by formula 4 is displayed to use tertiary amine 3. [Formula 4] (In said formula 4, R1 To R3 Same or different and are selected from, and C1 to C4 alkyl, X is halogen element.) Preferably designed as a, R1 To R3 Is methyl, ethyl, pro writing, or butyl, most preferably triethylamine (TEA) to implementation being. Said TEA class 3 such as amine is added to the substrate to fast transitions of mPEG mPEG a-X. Preferably designed as, mPEG of formula 2: 1:1 amine is of formula 5 class 45 To 1:30, Preferably designed as a 1:18 To 1:25 Molar ratio from 1:4 to 150. Said transesterification reactions are carried out in a solvent which, 0 to 30 °C temperatures and cold-incentive, preferably designed as a 1 to 12 hours at room temperature a plurality of hierarchies. The reaction use solvent containing benzene, toluene, which the alkylene, dichloro methane, 1, 2 - dichloroethane, carbon tetrachloride, methylene chloride, chloroform and a combination of solvent can be at least one selected from the group consisting 1, preferably designed as a methylene chloride less than 2000. The obtained via an esterification reaction solvent present in a dissolved state and mPEG-a X solution mPEG a-X is obtained. The mPEG provided X is present solution further by reactions other than unreacted material of formula 1 through mPEG provided X is a by-product (by-a product) occurs. Specifically, a compound of formula 3 is a halide represented by the formula 5 2 react with water such as organic halide acid is generated. [Compound 2] (2 In said reactive, halogen element X are disclosed.) Organic halide acid derivatives represented by the formula 5 are indicated, these compounds are a number of functions and the perforator disclosure number mPEG provided X radical activity need material that the wetting ability. [Formula 5] (In said formula 5, X is halogen element.) In addition, class 3 of said generated reactive amine 2 tube surface formula 4 (HX) to react with a compound represented by formula 6 such as halide acid amine - 3 fluoro zincates produce a halogen. [Compound 3] (3 In said reactive, R1 To R3 Same or different and are selected from, and C1 to C4 alkyl, X is halogen element.) Amine to formula 6 - halogen buys the salt on the surface, a compound disclosure number the number of functions in addition mPEG provided X radical activity perforator need material that the wetting ability. [Formula 6] (In said formula 6, R1 To R3 Same or different and are selected from, and C1 to C4 alkyl, X is halogen element.) Preferably designed as a, R1 To R3 Is methyl, ethyl, pro writing, or butyl, most preferably to ethyl are disclosed. Said reactive byproducts generated in organic halide acid (formula 5) 2 and reactive 3 additional final product further comprises an amine (formula 6) - halogen in a same number if not disclosure number used as a stand-alone mPEG a-X the same number for the temperature to a stand-alone mechanical radical disclosure activity number process although not necessarily disclosed. Typically, the mPEG according to some exemplary embodiments of the present invention to use a BiBB reacting, 3 fatty acid profiles in a number when a compound using high pressure liquid coolant to TEA mPEG-a Br 4 shown the reaction takes place in the last product BiBB - TEA-a HBr salt by-products such as additional acid and remains on the substrate: [Compound 4] Hereinafter TEA-a HBr salt in said BiBB - acid and amine typified by an organic halide acid etched when a halogen - fluoro zincates industry method effectively number number. Positive numbermethod The reference also 1, the esterification reaction was obtained after said step mPEG provided X number method and switching are described as follows. First, basic inorganic adsorption number obtained after esterification reaction solution adding decodes mPEG a-X are agitated. As the coupling force of the surface a solid by physical or chemical adsorption phenomenon that provide the means by which molecules are disclosed. The blood absorption and adsorption of interest material number called, the number may be adsorbed adsorbing surface number called the anode. The absorption number number blood absorption heat sink fluid transmembrane movement of molecules by diffusion after said adsorption number before reaching the inlet pores. The inner particles or molecules adsorbed by particles of blood absorption number diffusion into and through the same mobile number , secured on the interior surface of adsorption number. Such series of process blood absorption occurs differently depending on the type of adsorption number number, can be quickly or slow speed is occurs. The inner and surface pores from adsorption number are formed porous material are disclosed. This adsorption number is capable of surface ions or polar group, depending on the type of polar group and these ions with the adsorbent material is 6.0. In one embodiment, in the case of alumina Al provided OH surface being regulated, other ion is selectively adsorbed material act as Lewis acids and dipole 2000. In the present invention is used as an adsorption number basic inorganic adsorption number, alumina, silica and silica - alumina 1 can be selected from the group consisting of paper, preferably can be alumina. The obtained residue number said basic inorganic-based adsorption water obtained by measuring X-ray digitized from the pH, pH is 8. 0 Or more, preferably designed as a 9. 0 Or more, more preferably designed as 9. 2 Exhibit at least means that the substrate. Said basic inorganic adsorption number of spherical particles is configuration and foam or beads, particles (e.g., BiBB provided acid) and amine present porosity organic halide acid halogen - (e.g., TEA-a HBr salt) further comprises an absorbing substrate. The physical properties of the basic inorganic adsorption number adsorbent, i.e., pores also, bulk density, specific surface area, particle size parameter such as affected. I.e., adsorb a larger specific surface area increased. However high specific surface area and large porous means, the bottom of an adsorption number can be obtained. In addition, the use of basic inorganic adsorption number - such as a stand-alone with organic halide acid and amine number in order to ppm levels of basic inorganic adsorption number inclusive halogen products DBUS requester other. The wetting ability of the residual material from the more conventional adsorption number is used in excess with a high efficiency number after taking into account the efficiency of filtration processes is to define a diameter number content. Preferably designed as, in the present invention through an esterification reaction mPEG-a X 100 parts by weight of a basic inorganic adsorption number obtained content 30 to 500 parts by weight of 600 ppm hereinafter when used in organic halide acid, amine - 0 halogen acid content. 3 Weight % hereinafter can be reduced. Preferably designed as a, 100 parts by weight of a basic inorganic adsorption number mPEG a-X content 140 to 300 parts by weight of 100 ppm hereinafter when used in organic halide acid, amine - halogen acid content can completely number (ND, not detect) wetting ability. The most preferably, 100 to 150 parts by weight of a basic inorganic adsorption number mPEG provided X content 250 parts by weight of halogen - number further comprises an organic halide acid and amine functions can be completely both stand-alone. Said basic inorganic adsorption number is performing a drying process before use can be predetermined, preferably 1 to 24 in the 30 to 100 °C performed during the drying time, more preferably 50 to 90 °C performed during the time in 2 hr to 12. Next, obtained in said basic inorganic adsorption solution mixture by filtration with mPEG provided X number mPEG provided X solution for recovering other. Said reaction mixture dissolved in a mixed solution of mPEG provided X and a basic inorganic adsorption number that, filtration through basic inorganic adsorption number mPEG provided X solution isolated each other. The filtration processes are specially and not limited, can be performed using publicly known filtering device. If necessary, higher than atmospheric pressure or less by reducing the time difference can be, in one embodiment gravity filter (gravity filter), for handling (vacuum filter), pressurized filter (pressure filter), pressure filter for (compression filter), centrifugal filter (centrifugal filter) flow tides. Basic inorganic adsorption number with the processing of an associated process is 1 or more times can. Said process is to be treated and by means of which adjustable content of basic inorganic adsorption number mPEG provided X solution, adsorption of the light high purity material small wishes to obtain a basic or inorganic adsorption number simultaneously iterations can be performed. Said performs several iterations but throughput, processing efficiency to processed with an facility and method of disposal time are considered hereinafter 5 times a plurality of hierarchies. According to experiments of the present invention basic inorganic adsorption number when processed using the same content or more times when organic halide acid - 2 performs a halogen acid easy enable easy vector capable of amine. Next, recovered in said cutting off non-solvent to precipitate mPEG provided X mPEG provided X solution separated substrate. A basic inorganic adsorption number positive number recovered mPEG provided X solution by that, high purity mPEG provided X is solvent present in a dissolved state. The solvent after a solvent added to said non-solvent such as returning mPEG a-X to solidifying or precipitation. As mPEG a-X obtained is possible, only data on the subsequent processing mPEG a-X can be recovered disclosed. The selection of non-solvent solution mPEG a-X, i.e. the first reaction solvent can be used can be appropriately selected. Said voltage is a non-solvent reaction solvent and with greater affinity phase separation speed, speed is slow is smaller and vice versa vehicle from the outside. I.e., rapidly developing a larger affinity phase separation occurs. Available free solvent dimethyl ketone, methyl ethyl ketone, d ethyl ketone, deep with the ketone which will bloom, d butyl ketone, cyclo [heyk it buys it comes of ketones, d ethyl ether, deep with the ether which will bloom, d butyl ether, ether such as tetrahydrofuran, ethyl acetate, isopropyl alcohol, methanol, and is a polar solvent such as, preferably designed as a solvent when used in non-solvent toluene to 150 d ethyl ether. Next, and then drying said precipitated in water filtering system for a coagulation obtain high purity mPEG a-X. The filtering step includes said number timing filtration processes can be used. In addition, drying said non-solvent can be a stand-alone sufficiently number temperature such that a plurality of hierarchies. Preferably designed as a preferably 1 to 24 in 30 to 100 °C performed during the drying time, more preferably 50 to 90 °C performed during the time in 2 hr to 12. Said organic halide acid is subjected to a number of easy 600 ppm hereinafter (e.g., BiBB - acid) prepared by the mPEG-a X, preferably designed as a 100 ppm hereinafter, more preferably designed as a ND and, halogen (e.g., TEA-a HBr) further comprises an amount of the amine - 0. 3 Weight % hereinafter, preferably designed as a 0. 2 Weight % hereinafter, more preferably ND are disclosed. In addition, the method after adding said number tank mPEG provided X number tank number adsorption, filtration, treated water, such as filtering process is performed in a short time and switching can be performed performing mPEG provided X number. This mPEG provided X is for use as a macro disclosure number can, said organic halide acid halogen buys the salt amine - little or no not present disclosure number used as the macro can be high to maintain catalyst activity. In addition, a method in the present invention number existing when subjected number column chromatography or mechanical center to permits processing in a short time. In one embodiment, the same having a purity of halogenated 1 kg in the case of the present invention according to the process for producing methoxy polyethylene glycol (mPEG a-X) 1 hours, preferably designed as a result or to 30 minutes, 12 hours minimum column chromatography, recrystallization process in the case of a positive number less than 14 process should be performed other. I.e., a method in which in the present invention number result in short time, as well as high purity material can be referred to as the gastrostomy method is performed. In the present invention hereinafter with reference to the drawing of the present invention preferred embodiment and appends a detailed as follows. However, in the embodiment for the present invention refers to which are not limited by, the cache line of the feature of the present invention numerous modifications within the range of the engine with an explicit user and sacrificial illumination controller are disclosed. [In the embodiment] In the embodiment 1 (1) MPEG -BiBB Number bath 300 Ml of methylene chloride into and out of the reactor, mPEG herein (Mw: 5,000) is dripped slowly triethylamine and then, using this cow vice-mote reel bromide 2 - bromo syringe by adding him. Reactor a temperature in an adjusted to 25 °C then, conducting agitated. the mPEG: BiBB: TEA is 1. 0:1. 7:2. 0 With respect to the reaction molar ratio from 1:4. Every time a reaction degree caused by reactants1 H-a NMR analysis of mPEG is 100% conversion after the time at which the end up. The obtained reaction solution by adding to it agitated 300g 50g basic alumina (sigma Catalog No: 199443) then, recovering filtrate contained in him. Said atopic dermatitis is d ethyl ether corresponds to a sieve size were generated. After the filter cake after said filtering the slurry, dry pressure to a high pressure liquid coolant mPEG-a Br his number. In the embodiment 2 Alumina 70g and use, positive number equal to the number said in the embodiment 1 and with the process-step 1 to a host computer in a high pressure liquid coolant conducting mPEG-a Br number after analysis. In the embodiment 3 Alumina 70g and use, positive number equal to the number said in the embodiment 1 and with the process-step 1 to a host computer in a high pressure liquid coolant conducting mPEG-a Br number after analysis. In the embodiment 4 15G alumina and use, positive number equal to the number said in the embodiment 1 and with the process-step 1 to a host computer in a high pressure liquid coolant conducting mPEG-a Br number after analysis. In the embodiment 5 Alumina 70g and use, positive number equal to the number said in the embodiment 1 and 2 with the process-step number to a host computer in a high pressure liquid coolant conducting mPEG-a Br after analysis. In the embodiment 6 30G alumina and use, positive number equal to the number 3-step process with a high pressure liquid coolant and said in the embodiment 1 to a host computer in a number mPEG-a Br after conducting analysis. Comparison example 1 The same method in the embodiment 1 to a high pressure liquid coolant using column chromatography after a positive number mPEG-a Br number after conducting analysis. The adsorption number is used alumina column chromatography (pH: 7. 0) Fluorescence, deployment using conducting THF solvent 12 hours. Comparison example 2 The same method in the embodiment 1 to a high pressure liquid coolant after analysis after recrystallization process number mPEG-a Br conducting positive number. A recrystallization process number corresponds to a sieve size number after dissolving diethyl ethers prepared by the mPEG-a Br THF and high pressure liquid coolant, and the pressure of the filter cake obtained by filtering in a high pressure liquid coolant drying time was 45 °C mPEG-a Br number 12. Comparison example 3 The acidic alumina (sigma Catalog No: 199966) adsorption number in the range of 0.1 to a host computer in a number equal to the number said in the embodiment 1 and a high pressure liquid coolant conducting mPEG-a Br after analysis. Comparison example 4 A number in the range of 0.1 to neutral alumina (sigma Catalog No: 199974) adsorption number said in the embodiment 1 and the high pressure liquid coolant to a host computer in a number equal to mPEG-a Br after conducting analysis. Comparison example 5 Adsorption to activated carbon number in the range of 0.1 to a host computer in a number equal to the number mPEG-a Br said in the embodiment 1 and high pressure liquid coolant conducting after analysis. Comparison example 6 It rises, a number in the range of 0.1 to adsorption number equal to the number light (the [ley which it will drive [khyul sheave) and said in the embodiment 1 to a host computer in a high pressure liquid coolant conducting mPEG-a Br number after analysis. Experiment example 1 In said in the embodiment and comparison made in the amount of salt remaining BiBB - acid and TEA-a HBr mPEG-a Br number produced therewith have measuring, for table 1 have shown to result. The gas chromatography (Shimadzu, 2010plus) have BiBB - acid is by using a, TEA-a HBr salt1 H-a NMRBruker, 500MHz) were measured using. The ND not detect is big. Said table 1 herein, according to the present invention when used in basic alumina, comparison example 1 example 2 is subjected to column chromatography of hospital management system can be a stand-alone effectively number TEA-a HBr salt BiBB - acid and contrast in a short time can be know. In addition, alumina content of said products effectively number cream stand-alone database, processing times have shown high number formed by well! ratio. According to the present invention process based upon process from production of high purity halogenated methoxy polyethylene glycol. The present invention relates to a purification method capable of obtaining high purity halogenated methoxy-polyethylene glycol (PEG) using a basic inorganic adsorbent. The purification method is simple in the treatment process, can be refined in a short time, and is suitable for the mass production process of high purity halogenated methoxy-PEG. COPYRIGHT KIPO 2018 Methoxy polyethylene glycol (mPEG) esterification of methoxy polyethylene glycol and halogen compounds for formula 1 halogenated by number in a bath method, said basic inorganic adsorption number positive number to the halogenated methoxy polyethylene glycol halogenated methoxy polyethylene glycol characterized and switching number method: [formula 1] (Said formula 1 in, an element of the halogen X, n is an integer of 5 to 1000.) According to Claim 1, characterized in that said halogen compound that is represented by formula 3 halogenated methoxy polyethylene glycol number method and switching. [Formula 3] (In said formula 3, X is halogen element.) According to Claim 1, said halogenated methoxy polyethylene glycol represented by the formula 5 is a positive number of easy 600 ppm hereinafter and halide acid, amine represented by formula 6 - halogen acid easy 0 to. 3 Weight % hereinafter methoxy polyethylene glycol characterized in halogenated and switching number method: [formula 5] [Formula 6] (In said formula 5 and 6, R1 To R3 Same or different and are selected from, and C1 to C4 alkyl, X is halogen element.) According to Claim 1, said basic inorganic adsorption number is alumina, silica and silica - alumina consisting of at least one selected from the group consisting of halogenated and switching number method characterized in that the methoxy polyethylene glycol 1. According to Claim 1, positive number is 100 parts by weight of said halogenated methoxy polyethylene glycol 30 to 500 parts by weight of a basic inorganic adsorption number using halogenated methoxy polyethylene glycol characterized and switching number method. According to Claim 1, characterized in that said positive number 1 is halogenated methoxy polyethylene glycol method on or more times and switching number method. According to Claim 1, positive number is 100 parts by weight of said halogenated methoxy polyethylene glycol 150 to 250 parts by weight of a basic inorganic adsorption number by adding 1-step halogenated and switching number method characterized methoxy polyethylene glycol. According to Claim 1, said halogenated positive number is 100 parts by weight of a basic inorganic adsorption number 30 parts by weight or more methoxy polyethylene glycol added 2 to 5 characterized in that less than 150 parts by weight of halogenated methoxy polyethylene glycol-step number method and switching. According to Claim 8, said positive number obtained through the halogenated methoxy polyethylene glycol 200 ppm hereinafter and the amount of halide acid is represented by said formula 5, the residual halogen acid salt of an amine represented by said formula 6 - methoxy polyethylene glycol measured by halogenated ND (Not detect) characterized and switching number method. According to Claim 1, said positive number method i) halogenated methoxy polyethylene glycol solution obtained after reaction by adding a basic inorganic adsorption number; ii) methoxy polyethylene glycol solution after addition of agitating and filtration of the filtrate (filtrate) halogenated in obtaining; iii) cutting said halogenated methoxy polyethylene glycol solution precipitating non-solvent halogenated methoxy polyethylene glycol, halogenated methoxy polyethylene glycol precipitation after the drying; performing including halogenated and switching number method characterized methoxy polyethylene glycol. According to Claim 10, said non-solvent is methyl ketone, methyl ethyl ketone, d ethyl ketone, deep with the ketone which will bloom, d butyl ketone, cyclo [heyk it buys it comes, d ethyl ether, deep with the ether which will bloom, d butyl ether, tetrahydrofuran, ethyl acetate, isopropyl alcohol and methanol 1 including at least one selected from the group consisting of halogenated and switching number method characterized methoxy polyethylene glycol. MPEG-a Br: adsorption number amount (g) Processing method (time) Processing times The amount (ppm) BiBB acid The amount (wt %) TEA non-HBr salt Non In the embodiment 1 50:300 10 Minutes 1 ND ND - In the embodiment 2 50:70 10 Minutes 1 96 ND - In the embodiment 3 50:30 10 Minutes 1 24 0. 2 - The embodiment 4 50:15 10 Minutes 1 546 0. 3 - The embodiment 5 50:70 10 Minutes 2 28 ND - In the embodiment 6 50:30 10 Minutes 3 24 ND - Comparison example 1 50:300 12 Time - ND ND Column chromatography Comparison example 2 50 10 Time - 450 0. 1 Recrystallization Comparison example 3 50:100 10 Minutes 1 1240 0. 8 Acidic alumina Comparison example 4 50:100 10 Minutes 1 2451 0. 7 Neutral alumina Comparison example 5 50:100 10 Minutes 1 3412 0. 9 Activated carbon Comparison example 6 50:100 10 Minutes 1 1245 0. 9 Number it rises, light