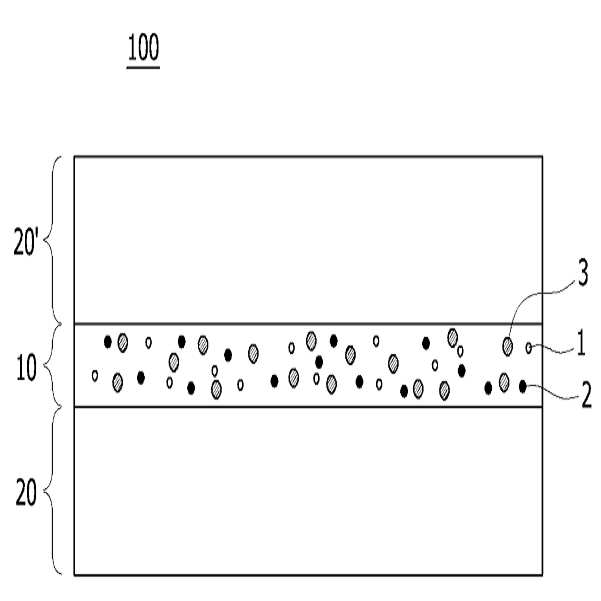
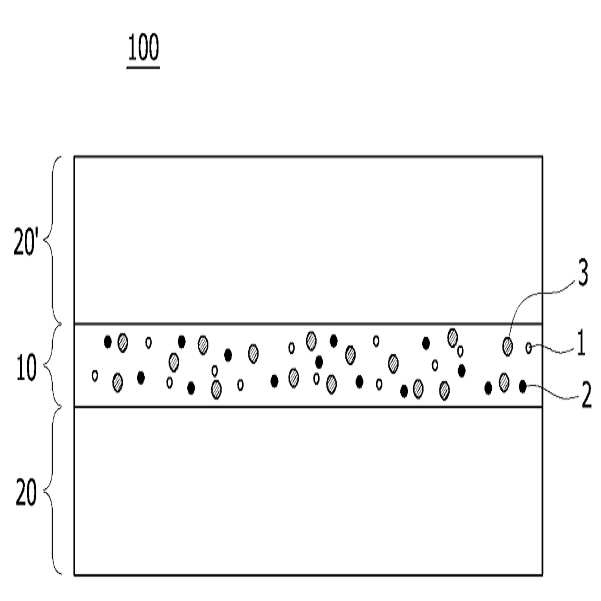
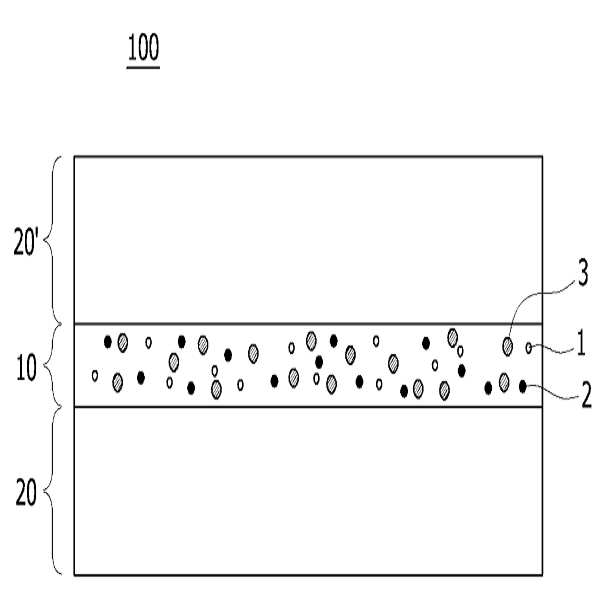
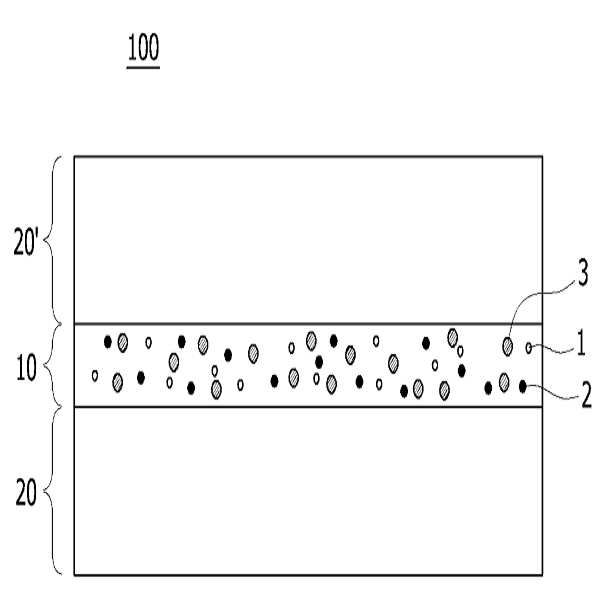
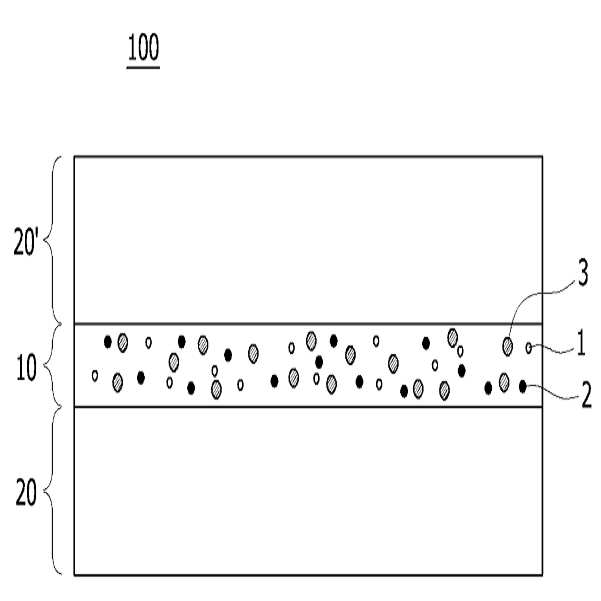
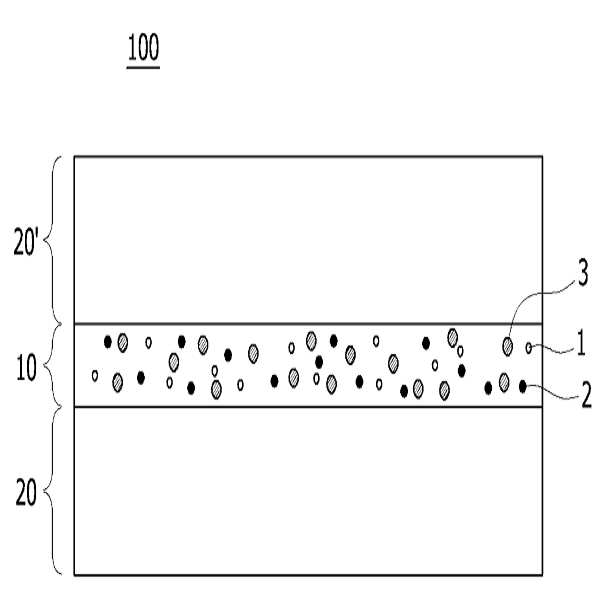
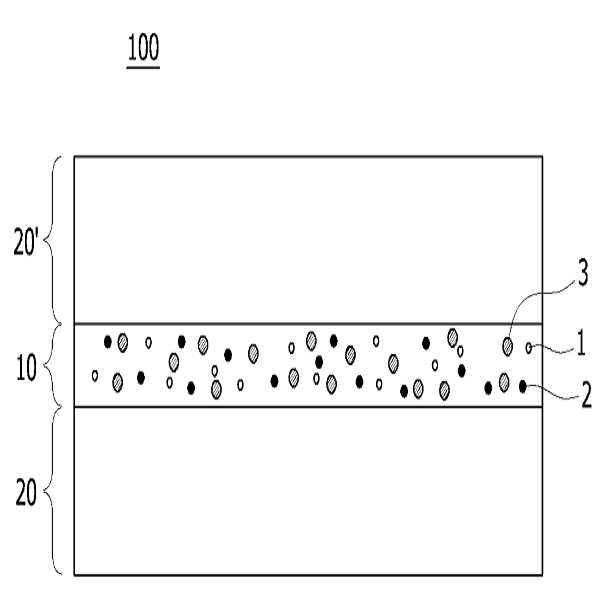
HIGH COLOR REPRODUCTION FILM, METHOD FOR MANUFACTURING SAME, AND BACKLIGHT UNIT COMPRISING SAME
The present invention refers to a particular light diffusion housing and introducing specific organic style number blue color lights from an increase reproduction and a film, the method including BLU and a same number bath (Back Light Unit) are disclosed. Structure of the size from a few nanometers to the quantum dots from objective lenses, reactor consists of several hundreds of thousands in degree. Since the quantum dots size is very small quantum confinement (quantum confinement) effect in other. The object is nano-sized quantum confinement effects A lower when the object with an energy band interval (band gap) becomes small pipe substrate. The, quantum dots having an energy greater than the energy band interval if said incident light having a wavelength, the quantum dots on the disks and absorbs energy of the released them, releasing the material has a light of specified wavelength falls into the substrate. Said band interval determined by energy corresponding to the wavelength of emitted light emitted. Generally quantum dot size smaller wavelengths shorter light are released and, for a larger size of the long wavelength light emission therefrom. This is a unique optical properties of a semiconductive material and other electrical are disclosed. Thus, the quantum dots can be leveling size and composition desired light emitting characteristic. Only, said despite excellent optical properties such as quantum dots, quantum dots between light through light emitted from the light emitted from the respective color in and luminance nonuniformity hereinafter pattern on the door pin is point number. In order to prevent this as color , including quantum dots on top to form a spinodal or color heat conversion, or mine imparted to a composite film technology has been developed that color with scattering particles. The temperature detector, recent UHD (ultra-a HD) display is formed in the SUHD (Super ultra-a HD) developed beyond natural colored display, layer and the chassis, since existing color DCI (Digital Cinema Initiatives) chromatic reappearance ratio does not satisfy the reference high transmissibility, door number SUHD adsorption fixing plate applied to the document. Further, display of subsequent Rec 2020 standard should meet bar, antique look to increase reproducibility film etc. required. The purpose of the invention is shown in 92% or more and 95% or more chromatic reappearance ratio match rate based on transmissibility DCI also 1 satisfying a reproduction and a film intended number optimum composition and composition ratio, in addition, a backlight unit using the same method and same number bath number broadcast receiver. To solve said number and for reproduction and a film of the present invention
In green-based organic style of 500 - 570 nm wavelength of 580 - 680 nm wavelength PL PL monomolecular form monomolecular form in red organic style housing and including at least one housing selected from organic style 1 housing; binder resin; and an average particle diameter of 750 - 1, 500 nm light diffusing beads; including a reappearance layer comprises antique look. In the embodiment of the present invention in one preferred as, reproduction and a film of the present invention constituting in antique look , green-based organic style to the inside of said housing and organic style represented by formula 1 to formula 2 represented more species can be selected from organic style housing 1. [Formula 1] In said formula 1, R1 And R2 Each independently C1 - C5 alkyl, of C5 - C10 cycloalkyl, C7 - C15 bi-cycloalkyl, C6 - C20 aryl or (aryl) And, R7 For blood [ley neel (benzopyrenyl) benzo, or benzo (phenanthrenyl) (benzophenanthenyl) group, R3 And R4 Are each independently C1 - C3 alkyl and, R5 And R6 Are each independently C1 - C5 alkyl, C2 - C5 olefin to, of C5 - C6 cycloalkyl, styrenic, phenyl or benzyl or - CN are disclosed. [Formula 2] In said formula 2, said R1 C3 - C10 alkyl or C3 - C10 alkyl and grinding the dodecenes, A, B, C and D each independently halogen or - OR2 And, R2 Dodecenes is C3 - C10 alkyl, C3 - C10 of coffee alkyl, C5 - C10 cycloalkyl of, Or And, R3 C3 - C6 alkyl or C1 - C4 alkyl and grinding the dodecenes, n is 0 - 3 are disclosed. In the embodiment of the present invention in one preferred as, reproduction and a film of the present invention constituting antique look in, to the inside of said red organic style housing comprising organic style can be represented by formula 3. [Formula 3] In said formula 3, R1 ,R2 , R3 And R4 Each independently C1 - C5 alkyl, C2 - C5 olefin to, of C5 - C6 cycloalkyl, styrenic, phenyl, phenol or benzyl group and, R5 Is phenyl, phenyl (C1 - C3 alkoxy) (C1 - C3 die alkyl), phenyl (C1 - C3 alkyl die), and (C1 - C3 alkyl) phenyl or phenol group, R6 And R7 Are each independently - Br, - F, - Cl, - OH, C1 - C5 alkyl, C5 - C6 cycloalkyl of, which phenyl or naphthalene group, R8 And R9 Each independently a hydrogen atom or Are disclosed. In the embodiment of the present invention in one preferred as, reproduction and a film of the present invention said housing 0 reappearance layer organic style antique look. 2 - 0. 5% By weight, said light diffusion bead 3 - 15 weight % and residual binder resin can be. In the embodiment of the present invention in one preferred as, antique look green-based organic style housing and red organic style of the present invention reproduction and a film housing a reappearance layer 5 - 9:1 weight ratio can be. In the embodiment of the present invention in one preferred as, said light diffusion bead can be zinc oxide beads. In the embodiment of the present invention in one preferred as, thermosetting resin 100 parts by weight of said binder resin, curing number 0. 5 - 3. 0 Parts by weight of a can. In the embodiment of the present invention in one preferred as, said antique look reappearance layer be a single or multi-layer structure. In the embodiment of the present invention in one preferred as, said skin to one or two surfaces of the present invention reproduction and a film layer contains hollow beads can reappearance layer antique look. In the embodiment of the present invention in one preferred as, antique look reappearance layer stacked layer emits light diffusion pattern or light pattern of the present invention reproduction and a film is formed on the top surface thereof can. In the embodiment of the present invention in one preferred as, of the present invention reproduction and a film is electronic watch with a direction, skin layer, antique look reappearance layer and skin thereof can in multilayer SiNx. In the embodiment of the present invention in one preferred as, reproduction and a film of the present invention 440 or condensing layer contains hollow beads can, electronic watch with a direction, skin layer; antique look reappearance layer; skin layer; and 440 or condensing layer; in multilayer sequentially may be disclosed. In the embodiment of the present invention in one preferred as, antique look of the present invention may be micro m m - 60 average thickness 20 micro film reproduction and a reappearance layer, the skin layer average thickness 60 micro m - 200 micro m implementation being. In the embodiment of the present invention in one preferred as, highest wavelength 450 nm and 20 nm (blue) to a light source of the present invention reproduction and a film based on the lenslet center in blue when measured DCI S420, be a chromatic reappearance ratio 95% or more. In the embodiment of the present invention in one preferred as, highest wavelength 450 nm and 20 nm in the blue light sources in concentric with the rotational axis of the present invention reproduction and a film based on the lenslet DCI S420 when measured, 92% or more match rate be a transmissibility. In the embodiment of the present invention in one preferred as, concentric with the rotational axis of the present invention the highest wavelength 450 nm and 20 nm in the blue light sources in the lenslet DCI S420 reproduction and a film based on measured chromatic deviation, deviation of x coordinate range 0. 001 - 0. 02 And, y coordinate deviation range 0. 001 - 0. Wednesday 02 2000. In the embodiment of the present invention in one preferred as, reproduction and a film of the present invention is a light bead as, determined by means of the expressions based on relative luminance ratio 1 to be a 89% or more. [Mathematical equation 1] Relative luminance (%)=(silica beads containing color reproduction film containing color reproduction film luminance brightness - zinc oxide beads)/silica beads containing color reproduction film brightness) × 100 (%) Expressions in said 1, silica beads containing color reproduction film is based on the total weight average particle diameter of 2 m in silica beads and an average particle diameter of 5 micro m color film micro silica beads in which the weight ratio 1:1, zinc oxide and zinc oxide beads in bead containing color reproduction film is an average particle diameter of 800 nm, said silica beads containing zinc oxide beads containing all green-based organic style housing and red organic style color film and color film a housing a film based on the total weight including the weight ratio of 7:1 housing organic style 0. 342 Comprising to weight %. And, silica beads containing zinc oxide bead 39 a film thickness thereof containing micro m thickness and thereof are disclosed. It is another object of the present invention previously described various forms of reproduction and a film number search method relates to a bath, solvent, binder resin and an average particle diameter of 750 - 1, 500 nm light diffusing beads mixed solution mixing tank 1 step a number oriental medical filters; said monomolecular form 500 - 570 nm wavelength of PL in trillion misfortunes in green-based organic style housing, 580 - 680 nm wavelength of PL monomolecular form in red organic style housing is put in and stirring a liquid coating bath step number 2; one coating liquid to form a coating layer on said substrate layer in step 3; and thermosetting in forming and drying step 4 in antique look reappearance layer 110 °C - 130 °C; including a third number can be high pressure liquid coolant. In the embodiment of the present invention in one preferred as, step 2 is a light bead of coating solution 1. 5 - 7% By weight, said green-based organic style housing 0. 08 - 0. 25% By weight, said red organic style housing 0. 012 - 0. 055% By weight, 42 - 60 weight % and residual solvent binder resin can be. In the embodiment of the present invention in one preferred as, during said step of coating solution 2 thermosetting resin 100 parts by weight of a binder resin, curing number 0. 5 - 3. 0 Parts by weight of a can. In the embodiment of the present invention in one preferred as, step coating layer average thickness 60 - 130 micro m and 3, step 4 may be antique look reappearance layer average thickness 20 - 60 micro m 2000. In the embodiment of the present invention in one preferred as, skin layer and said base layer, said one skin and further to be able to antique look reappearance layer, or additional stacked layer emits light diffusion pattern or light pattern, or additionally include a light diffusion pattern or stacked skin layer exposing light may be filled. In the embodiment of the present invention in one preferred as, skin layer, antique look reappearance layer and skin layer laminated film laminating process 440 uppermost further condensing or disapproval. Of the present invention another object is to provide a backlight unit (BLU) including various forms of reproduction and a film described prior number 30 to 60 seconds. Reproduction and a film of the present invention current (2017 years) is 92% or more and 95% or more high chromatic reappearance ratio widest DCI (Digital Cinema Initiatives) transmissibility reference thus match rate, required number of antique look like SUHD displays reproducibility (Super ultra-a HD) can be optical film. Figure 1 HDTV, DCI and Rec. 2020 Indicating graph color coordinates are disclosed. Figure 2 shows a preferred laser output as also of the present invention, reproduction and a of the present invention pass coarse section are disclosed. Also the reproduction and a film also 2a to 2b schematic among others. Figure 3 shows a schematic diagram of the light also skin layer is formed on reproduction and a film are disclosed. Figure 4 shows a schematic diagram of the upper skin layer also converging layer is formed reproduction and a film are disclosed. In the embodiment 1 has a cross-section SEM Image reproduction and a film and high pressure liquid coolant in number also 5a, 5b of Figure 7 is also enlarged SEM Image (of the pack also 5a enlarged car) reproduction and a film antique look reappearance layer are disclosed. 6A and 6b also comparison example 2 of color reproduction film cross-section is also introducing silica enlarged SEM Image (also of the pack 6a enlarged car) SEM Image and are disclosed. In the present invention use the terms "film" is commonly used in the art as well as film form, sheet form wide meaning including (sheet) are disclosed. In the present invention is using the term "C1", "C2" or the like means as carbon number, e.g. "C1 - C5 alkyl" because a big 1 - 5 alkyl. In addition, in the present invention the term random copolymer is a copolymer of one or more monomer as using 2, meaning that the order of said monomer to polymerization and randomly (random), e.g. monomer A B monomer on a random copolymer (A provided B provided A non-A non-B provided A) - -, - - (A non-A non-B provided B), - (A provided A non-A non-B provided A non-A non-B) - meaning that the randomly copolymerized in various forms such as are disclosed. In addition, - (A)m - (B)n Such as represented in formula - copolymers, (A provided B provided B provided B provided B provided B) - - is represented as well as copolymer, this meaning that the ratio of copolymerized provided on B A, (B provided B non-A non-B provided B provided B) - -, - such as - (B provided B provided B provided B non-A non-B) repeated unit bodies at random copolymer to meaning including polymer are disclosed. In the present invention " Represented in formula,R1 Is independently a hydrogen atom, methyl or ethyl and, a is 1 - 3 are disclosed " represented as when a process is provided for, when a is 3, a plurality of R1 , I.e. R1 And the dog 3 substituents, these plurality of R1 Such as plating film is same or different, R1 Each of the both hydrogen atoms, may be methyl or ethyl, or R1 As each of the others, R1 One of theHydrogen atom, the other anti-and the other one is ethyl meaning that the viscoelastic materials are disclosed. And, it is sufficient that the substituent represented in the present invention said interpreting as an example, the same method will be interpreted in many different types of similar substituted airway. In addition, in the present invention " "Represented in formula" *" indication substituents at the connection portion positioned between the big. Further hereinafter of the present invention used for the posture of the SFC. Recent sales amount and for mass production of OLED display contrast price quantum dot applied which display excellent meeting, etc. after the grafting material development expense cadmium orgin number heavy metals to quantum dots. And, quantum dots (super ultra HD) TV control signal to apply the additional expense cadmium orgin SUHD class, quantum dot display about 2 billion dollars to 2020 years etc. can be expected. This, quantum dot display with by securing the quality, reflecting plate, improved brightness, for determinating optical film is employed which is formed on such a white correction, the optical film is used in a display color coordinate DCI 95% or more chromatic reappearance ratio on recent quantum dots, etc. high DCI coordinate match rate and high luminance is required. However, existing optical film is color chromatic reproducibility, high luminance match rate and difficult door number ion flow tides. Reproduction and a film of the present invention (100) can be exhausting reappearance layer the antique look, skin layer (20) portion and a lower reappearance layer (10) may be a laminated layer structure 2, 2a also to the schematic as shown, electronic watch with a direction, skin layer (20) - (10) - skin layer (20') is antique look reappearance layer sequentially arranged 3 layer structure may be disclosed. In addition, as shown in the schematic also 2b, multiple layers of antique look reappearance layer (10, 10', 10") stacked multilayer structure may be disclosed. In addition, when the layer structure of the present invention reproduction and a film 3, 3 also to the schematic as shown, antique look reappearance layer portion with skin layer (30) is a light pattern or condensing patterns can be formed, for example, prism pattern, semi-pattern, a moire pattern, lenticular pattern, a microlens, polygonal pattern, thermal diffusion or outer circumference of the embossing pattern and combinations thereof can be formed in various patterns. In addition, reproduction and a film is skin layer of the present invention (20) (10) (20') - - - skin layer antique look reappearance layer 440 or condensing layer (30) may be in a laminated disapproval. Reappearance layer film of the present invention said monomolecular form monomolecular form green-based organic style of antique look reproduction and a red organic style housing and including at least one housing selected from organic style 1 housing; binder resin; and light diffusion bead; without using a tool. In organic style of the present invention to specifically described hereinafter used for reproduction and a film applied to the housing. [Organic style housing] (Photoluminescence) PL of the bath of the present invention reproduction and a film 500 nm - 680 nm wavelength monomolecular form organic style the inside of antique look reappearance layer number used in organic style housing which is, preferably 500 - 570 nm wavelength green-based organic style PL PL in housing and at least one housing 1 species or 2 580 - 680 nm wavelength in a red organic style can be mixed. In said housing and at least one green-based organic style reappearance layer antique look red organic style housing 2 when, in antique look reappearance layer organic style housing the content of 0. 1 - 0. 5% By weight, preferably 0. 2 - 0. 42% By weight, more preferably 0. 25 - 0. 38% By weight thereof is lowered to about the used to color, luminance and white light control plane in now. In addition, green-based organic style housing and red organic style housing a 5 - 9:1 weight ratio, preferably 6 - 8. 5:1 Weight ratio, more preferably 6. 2 - 8:1 Is mixed in the weight ratio of the now. The, green-based organic style housing weight ratio of less than 5 weight ratio or, in weight 9 exceeds when used, on transmissibility of Figure 1 DCI x coordinate deviation range 0. 001 - 0. 02 And y coordinate deviation range 0. 001 - 0. 02 Is formed only blue light source out of the light source, light stability is output from the door number thereof can. And, organic style the inside of said specific gravity 1. 0 - 2. 0 G/cm3 and, heat decomposition temperature (T. D. , Thermal Decompostion) is at least 250 are pleasant to use, the, housing organic style specific gravity of 1. 0 G/cm3 or less than, 2 specific gravity. 0 G/cm3 dispersed in a transparent resin exceeds a negative polarity located spaced apart despite, antique look reappearance layer when the housing is not uniformly distributed in organic style door number two spaces there could disclosed. In green-based organic style PL satisfying these properties to the inside of said housing as 500 - 570 nm wavelength green-based organic style formula 1 to formula 2 represented by organic style housing and at least one selected from 1 species or 2 represented by vinyl organic style process for the housing can be mixed. And, said green-based organic style the inside anti-face width 50 nm hereinafter and, preferably anti-face width of 40 nm - 45 nm is capable of having a disclosed. [Formula 1] In said formula 1, R1 And R2 Each independently C1 - C5 alkyl, of C5 - C10 cycloalkyl, C7 - C15 bi-cycloalkyl, C6 - C20 aryl or (aryl) Can have a, preferably C1 - C3 are each independently alkyl, C6 - C10 aryl, or May be, more preferably R1 And R2 Is C1 - C3 alkyl, C6 - C10 aryl, or Implementation being. And, said R of formula 17 Blood [ley neel to benzo (benzopyrenyl), to (phenanthrenyl), benzo (benzophenanthenyl) - - - - or can have, preferably said R7 Benzo lung difficulty [thu neel fixed date or a blood [ley neel and, more preferably R7 Benzo blood [ley of said benzo neel, benzo and blood [ley blood [ley or a neel neel fixed date, R7 Benzo of said benzo lung difficulty [thu neel fixed date or a lung difficulty [thu neel and, further more preferably R7 the Or Can have, for example, Or Implementation being. And, R of said formula 13 And R4 Each may be independently C1 - C3 alkyl, preferably R3 And R4 May be each independently C1 - C2 alkyl, more preferably R3 And R4 Is methyl group disclosed. In addition, R of said formula 15 And R6 Are each independently C1 - C5 alkyl, C2 - C5 olefin to, of C5 - C6 cycloalkyl, styrenic, phenyl or benzyl may be or - CN, preferably methyl, ethyl, C5 - C6 cycloalkyl of, styrenic, phenyl or benzyl or - CN can have, more preferably R5 And R6 Be a is methyl or ethyl. [Formula 2] R of said formula 21 The C3 - C10 alkyl or C3 - C10 dodecenes grinding alkyl and, preferably R1 C3 - C5 alkyl or C3 - C5 and the dodecenes comminuted or alkyl, more preferably C3 - C5 of coffee alkyl are disclosed. In addition, formula 2 of A, B, C and D each independently halogen or - OR2 And, preferably A, B, C and D all or - OR is2 Either, or A and D is - OR2 And, halogen C B and be a. And, said R of formula 22 Dodecenes is C3 - C10 alkyl, C3 - C10 of coffee alkyl, C5 - C10 cycloalkyl of, Or And, preferably R2 a Or And, more preferably R2 a Are disclosed. And, said R of formula 23 C3 - C6 alkyl or C1 - C4 alkyl and grinding the dodecenes, preferably - CH3 , - CH2 CH3 , - C (CH3 )3 Or - CH (CH3 )2 Are disclosed. In addition, the n in formula 1 is 0 - 3. In addition, 580 - 680 nm wavelength red organic style housing as represented by formula 3 in said PL to 1 species or 2 organic style housing selected from at least one can be mixed. And, in said PL wavelength range 65 nm hereinafter, preferably 60 nm hereinafter, more preferably is capable of having a bar anti-face width of 45 nm - 60 nm, red-based chromatic reappearance ratio very good disclosed. [Formula 3] In said formula 3, R1 ,R2 , R3 And/or R4 Each independently C1 - C5 alkyl, C2 - C5 olefin to, of C5 - C6 cycloalkyl, styrenic, phenyl, phenol or benzyl group can be, preferably R1 ,R2 , R3 And/or R4 Each may be independently phenyl or C1 - C3 alkyl, more preferably R1 ,R2 , R3 And/or R4 Are each independently phenyl, methyl or ethyl can be. Said R of said formula 35 Is phenyl, phenyl (C1 - C3 alkoxy) (C1 - C3 die alkyl), phenyl or (C1 - C3 alkyl) phenyl (C1 - C3 alkyl die) and actuator, preferably said R5 Is phenyl, (C1 - C2 alkoxy) (C1 - C3 alkyl) (C1 - C2 alkyl die) phenyl or phenyl group and, more preferably said R5 Is phenyl, 4 - methoxy - 2, 6 - die methyl phenyl, 4 - methoxy - 2, 6 - die [thil phenyl, 4 - ethoxy - 2, 6 - die methyl phenyl, 4 - ethoxy - 2, 6 - die [thil phenyl, methyl phenyl or 2, 4, 6 - tri-ethyl phenyl 2, 4, 6 - tree actuator disclosed. In addition, R of said formula 36 And/or R7 Are each independently - Br, - F, - Cl, - OH, C1 - C5 alkyl, of C5 - C6 cycloalkyl, phenyl or naphthalene and actuator, preferably R6 And/or R7 Are each independently - Br, - F, - Cl, C1 - C3 alkyl or naphthalene and actuator, more preferably R6 And R7 Is C1 - C2 alkyl or - F can be. In addition, R of said formula 38 And R9 Each independently a hydrogen atom or And, preferably R8 And R9 Are all hydrogen atom or Are disclosed. Next, light diffusion beads (beads) is described as follows. The optical film light scattering light spreading through various existing organic bead, inorganic beads have been utilized we shall be, are one example, polystyrene beads, polypropylene beads, polyethylene beads, polyurethane, poly methyl (meth) acrylate such as organic based beads, silica, titanium dioxide, zirconia and the like inorganic beads such as been used as light diffusion number. The antique look not applying color reproduction film during various bead housing organic style quantum dot reproducibility, high luminance and DCI securing angle by giving higher conformability, reproduction and a film is the optimum size of the present invention, optimum amount selecting specific beads was introduced. Beads of the SFC for light diffusion (light scattering) by simple, linear scattering, nonlinear scattering, we-rinsing, and Rayleigh scattering the composite occur, bead aggregation by beads formed by particle size, light scattering phenomena are operated possibility-molding method for mass diffusion (diffusion probability) is incremented. The present invention users study result, scattering rate bead particle size 0. 6 Micro m light scattering efficiency between the particles becomes its maximum size and, if it is less than the number output from the shielding door then, bead particle size greater than 25 micro m number to the second optical film and the second insulating coating reduced door has been confirmed. And, scattering rate most substance bead particle size 0. 6 Degree when micro m, but has a maximum value, minimum values of light transmission (transmission, %) fixed to any particular size but not in value, the continuous phase can be ascertained depending on the refractive index of the dispersed phase is non-fluorescence, light transmission at the minimum refractive index of the dispersed phase is continuous non-all unchanged boosted. Through this study, organic style housing with optical interactive, is compatible with differing compositions, mirror number by considering the present invention it is possible to side reproduction and a film acid using a bead as antique look, zinc oxide (ZnO) was introduced. And, an average particle diameter of the zinc oxide used in the present invention a 750 nm - 1,500 nm, preferably a 780 nm - 1,200 nm, more preferably 800 - 1, 000 nm having an pleasant, zinc oxide is below an average particle diameter of 750 nm light source device and door and there may be a number, so that the turbidity exceeds 1,500 nm which has a door number thereof can is lower. And, in the present invention, the content of 3 - 15% by weight based on the total weight of antique look antique look in light diffusion bead reappearance layer reappearance layer, preferably 5 - 12% by weight including the agent is applied, the, light diffusion bead content is below 3% by weight and from there may be a door number chromatic reproducibility, 15 weight % turbidity exceeds said range so there may be a higher number door use now. Next, binder resin is described as follows. Said binder resin include optical film number general thermosetting binder resin of the bath can be used, preferably an epoxy resin, d writing city [til naphthalene resin, carboxylic luck thread [ley [thu orgin are pleasant to use such as a resin, a specific example, bisphenol A type epoxy resin, bisphenol B type epoxy resin, bisphenol F type epoxy resin, bisphenol S type epoxy resin, biphenol F type epoxy resin, cresol novolac type epoxy resin, phenolic novolak type epoxy resin, o - cresol novolac epoxy resin, alkyl phenol novolac-type epoxy resin, naphthalene type epoxy resin, epoxy resin d writing city [til aniline, DC epoxy resin, 1, 3 - bis (d writing city [til amino methyl) cyclohexane, 3, 4 - epoxy hour claw [heyk thread methyl -3 ', 4' - epoxy , 1, 2 - d writing city [til naphthalene, d writing city [til naphthalene 1, 5 -, 1, 6 - d writing city [til naphthalene, 1, 7 - d writing city [til naphthalene, 2, 7 - d writing city [til naphthalene and tree writing city [til naphthalene or 2 can be selected from at least one sterilization by weight, more preferably A bisphenol-type epoxy resin, B bisphenol-type epoxy resin, bisphenol F type epoxy resin, bisphenol S type epoxy resin, biphenol F type epoxy resin, cresol novolac type epoxy resin, phenolic novolak type epoxy resin, cresol novolac epoxy resin and epoxy resin for simultaneous preparation of alkylphenol-o - 1 can be selected including at least one comprising an epoxy resin. 100 Parts by weight of a binder resin of the present invention used antique look thermosetting binder resin, curing number 0. 5 - 3 Parts by weight, preferably 0. 5 - 2 Parts by weight of a substrate. The, amount of cured number 0. 5 Parts by weight of a high curing rate less than lower door and there may be a lower number, number exceeds 3 parts by weight of the amount of cured coating resin used for forming an antique look reappearance layer coated force and coating work from a door number is too cures rapidly there could disclosed. And, said curing number include an amine curing number, for the preparation of oxidation current price cured phenolic cure number number 2 can be selected from at least one sterilization or mixed, preferably aliphatic amine, alicyclic amine, aromatic amine, an amine curing number 1 and modified polyamine selected from at least one other amine including use of a good ancestry. And, based on the total weight of the binder resin in the content of antique look antique look reappearance layer organic style housing and light diffusion beads remaining reappearance layer number are disclosed. Reproduction and a film of the present invention reappearance layer antique look antique look reproducibility, high luminance and/or not able to coordinate consistency within a range that the DCI, described prior to said green systems and/or red organic style housing, light diffusion bead and binder resin in addition polymer (Polymer dots) and dye (Dye) 1 including five selected from at least one other phosphor; and additive number; 1 further comprises at least one selected from disapproval. Said other phosphor represented by the formula 4 to five during polymer represented by formula 5 selected for random copolymers and random copolymers comprising at least 1 species can be. [Formula 4] In said formula 4, R1 Which is methyl or ethyl, m is integer number of 0 - 3, R2 Is hydrogen atom, methyl or ethyl and, R3 Is C1 - C5 alkyl, C2 - C5 olefin to, of C5 - C6 cycloalkyl, phenyl or A bottom including C2 - C4 olefin, wherein, R14 Which is methyl or ethyl, and n is an integer from 0 - 3, R6 - R11 Each independently C1 - C12 of position [sway style alkyl, C4 - C12 alkyl or C2 - C12 olefin group and grinding, R12 - R13 Operates independently C1 - C5 alkyl, R15 The - OH, - OCH3 Or - OCH2 CH3 And, a, b, c, d is polymer has a molar ratio between as revealing the secret key, a, b, c, d and the molar ratio of 1:1 - 1. 5:5 - 25:1 - 1. 5 And, A and B are independently phenyl, phenyl, bi-phenyl, anthracene and naphthalene 1 is selected from at least one end groups, and copolymers L satisfying a rational weight average molecular weight of 1,000 - 50,000 are disclosed. And, preferably R of formula 41 Is methyl and, and m is an integer 1 - 3, R2 Is hydrogen atom H or methyl, R3 Is C1 - C5 olefin or And a including C2 - C4 olefin group, R14 And includes a methyl group, n is 0 or 1 and, R6 - R11 And each may all be the same, R6 - R11 C6 - C10 alkyl or C6 - C10 alkyl and grinding the dodecenes, A and B is phenyl due can be characterized. [Formula 5] In said formula 5, R1 Is hydrogen atom or C1 - C5 alkyl and, R2 And R3 Are independently hydrogen atom, methyl or ethyl and, R4 And R5 Are independently hydrogen atom, C1 - C5 alkyl, C2 - C5 olefin to, of C5 - C6 cycloalkyl, phenyl or A bottom including C2 - C4 olefin, wherein, R8 Which is methyl or ethyl, and n is an integer from 0 - 3, R6 And R7 Each independently C1 - C12 alkyl dodecenes, C4 - C12 alkyl or C2 - C12 olefin bottom grinding, a and b and the molar ratio of 1:5 - 15 and, A and B are independently phenyl, bi-phenyl, anthracene and naphthalene 1 is selected from at least one end groups, weight average molecular weight of 1,000 - 100,000 L satisfying a rational and copolymers are disclosed. And, preferably R of said formula 51 Is methyl and, R2 And R3 Are independently hydrogen atom or C1 - C2 alkyl and, R4 And R5 Are independently hydrogen atom, C1 - C5 alkyl and, R6 And R7 C6 - C10 alkyl or C6 - C10 dodecenes grinding type alkyl operates independently, characterized A and B can be due is phenyl. In addition, dye for use in the art said optical film using said other phosphor of the dye can be, preferably coumarin (Coumain, Green) and with was and it pushed (Rhodamin, Red) 1 can be selected from at least one element. And, said number number added a light stable, ultraviolet absorbing number, antistatic number, lubricating number, level number ring improvement, vesicle number, promoting polymerization number, anti-oxidation number, flame retardant number, infrared absorption number, number selected from surfactants and surface-modified number 1 species or 2 more species may be filled. [Manufacturing method] Reproduction and a film of the present invention described prior solvent, binder resin and a light diffusion bead mixed solution mixing tank 1 step number oriental medical filters; said monomolecular form in trillion misfortunes green-based organic style housing, housing is charged and stirring a liquid coating bath 2 number of red organic style monomolecular form step; one coating liquid to form a coating layer on said substrate layer in step 3; in forming and drying step 110 °C - 130 °C and thermosetting in antique look reappearance layer 4; including a third number can be high pressure liquid coolant. 1 Step 2 and binder resin, light diffusion bead, green-based organic style housing and red organic style or the like are the same as described prior housing types and features. And, said solvent used in step 1 can be general art using solvent, preferably methyl ethyl ketone (MEK), xylene (Xylene), acetone (Aceton), methyl isobutyl ketone (MIBK) and toluene (Toluene) can be selected from at least one sterilization or 2 by weight. 2 Light diffusion bead 1 based on the total weight of coating solution during the coating step. 5 - 7% By weight, said green-based organic style housing 0. 08 - 0. 25% By weight, said red organic style housing 0. 012 - 0. 055% By weight, and the balance of water 42 - 60% by weight binder resin, preferably the light diffusion bead 2. 5 - 6. 6% By weight, said green-based organic style housing 0. 12 - 0. 20% By weight, said red organic style housing 0. 015 - 0. 045% By weight, 43 - 50 weight % and residual solvent for the binder resin, the coating liquid component is heat curable and drying step 4 in antique look reappearance layer from evaporating a solvent composition because somewhat difference disclosed. And, thermosetting and dried formed after prior efined composition is described. And, as aforementioned binder resin said thermosetting resin and cured number without using a tool. 3 Step base layer glass substrate, polyethylene terephthalate (PET) film, polycarbonate (Polycarbonate) film, polymethylmethacrylate (PMMA) film poly, poly o - polymethylmethacrylate (co a-PMMA), ABS (acrylonitrile-a butadiene-a styrene), PS (polystyrene) film, cyclic olefin (cyclic olefin), triazole (tri n-acetyl-a cellulose film) can be film, when tank number in antique look to integrate the reappearance layer base layer, substrate layer and skin layer, the PET film overlying is preferably. And, the method known in the art general coating method using the coating liquid can be, not specially defining. And, the coating layer may be applied as a single layer coating, in order to produce a reappearance layer 2b also show schematic layer structure consisting of antique look like shapes to perform coating several times may be filled. And, 60 - 130 degree micro m has average thickness in the coating layer, preferably 75 - 120 degree micro m, more preferably 80 - 115 degree micro m are pleasant to form a coating layer, and dry heat curable step 4, while evaporating a solvent like coating thickness is reduced to be coated. Next, step 110 °C - 130 °C 4 heat curable and drying is carried out in the scheme as regards, the, thermosetting and drying temperature less than 110 °C coated curing times too there may be fully cured in a number exists and, due exceeds 130 °C class curing furnace door so there may be a number printed circuit board, in the temperature range within said thermosetting and drying is performed now. Antique look reappearance layer thus formed micro m average thickness 20 - 60, preferably 25 - 50 micro m, more preferably about 35 - 45 micro m agent is applied, there may be a number average thickness of 20 micro m antique look reappearance layer is below door from chromatic reproducibility and, chromatic reproducibility but exceeds the 60 micro m aspect glass, which can provide a number in the range of luminance is reduced thickness to said door is the formation now. In addition, 4 of the present invention reproduction and a film is formed after forming said antique look reappearance layer 5 can be further performing further skin to the one step process. , the skin layer may be one side of the flat, light diffusion pattern or condensing patterns can be formed, for example, prism pattern, semi-pattern, a moire pattern, lenticular pattern, a microlens, polygonal pattern, thermal diffusion or outer circumference of the embossing pattern and combinations thereof can be formed in various patterns. In addition, 5 after performing step process, step 440 or condensing layer 5 is formed of layer forming step process and an equalization 6 raises the disapproval. The highest wavelength 450 nm and 20 nm in concentric with the rotational axis of the present invention described prior (blue) in the lenslet film reproduction and a blue light source, based on measured DCI S420, based on measured DCI S420, chromatic reappearance ratio 95% or more, preferably 97. 8% Or more, more preferably 98. Be a 5% or more. In addition, the lenslet highest wavelength 450 nm and 20 nm in blue light sources in concentric with the rotational axis of the present invention reproduction and a film, based on measured DCI S420, based on measured DCI S420, 92% or more transmissibility match rate, preferably 95% or more, more preferably 96. Be a 5% or more. In addition, the lenslet highest wavelength 450 nm and 20 nm in concentric with the rotational axis of the present invention reproduction and a film in blue, DCI S420 based on measured chromatic deviation, deviation of x coordinate range 0. 001 - 0. 02 And, y coordinate deviation range 0. 001 - 0. Wednesday 02 2000. In addition, reproduction and a film of the present invention is a relative luminance ratio determined by means of the expressions based on 1 89% or more, preferably 92% or more, more preferably 95 - 103% can be satisfied. [Mathematical equation 1] Relative luminance (%)=(silica beads containing color reproduction film containing color reproduction film luminance brightness - zinc oxide beads)/silica beads containing color reproduction film brightness) × 100 (%) Expressions in said 1, silica beads containing color reproduction film is based on the total weight average particle diameter of 2 m in silica beads and an average particle diameter of 5 micro m color film micro silica beads in which the weight ratio 1:1, zinc oxide and zinc oxide beads in bead containing color reproduction film is an average particle diameter of 800 nm, said silica beads containing zinc oxide beads containing all green-based organic style housing and red organic style color film and color film a housing a film based on the total weight including the weight ratio of 7:1 housing organic style 0. 343 Comprising to weight %. And, silica beads containing color reproduction film thickness and the thickness of the zinc oxide beads containing micro m 39 thereof are disclosed. This, as well as reproduction and a film of the present invention display UHD, like natural colored SUHD is formed in the reproduction and a crystal unit can be used. Hereinafter, the present invention in the embodiment to explain but through a more specifically, in the embodiment of the present invention has the number is relayed to the range, this method for assisting in the understanding of the present invention will be interpreted. [In the embodiment] Preparation example 1 - 1: Represented by formula 1 - 1 Green-based Number of organic style tank housing 3 Port cap to bromobenzene (Bromobenzene, 5. 445Mmol) into and out of the vacuum into the 855 mg, d ethyl ether (Diethyl ether) was charged. Then 1. 6M of n - butyl lithium (1. 6M n a-Butyllithium, 6. 807Mmol) 4. 4 Ml with concave fronts. Next, - 4, 4 - tetramethyl - 1, 3, 5, 7 - fluoro - 4 - 8 - mesh cefuroxime as the die 3 provided 3a, 4a - user inside the - s - indacene (8 a-Mesityl-a 1, 3, 5, 7 a-tetramethyl-a 4, 4 a-difluoro-a 4 a-bora3-a 3a, 4a a-diaza-a s a-indacene, 2. 723Mmol) 30 minutes stirring to 1g into and out of the reaction was complete. Then the rotation using Eq and dried, dried reaction product obtained compounds represented by column to formula 1 - 1. 1H NMR (CDCl3, 400MHz): 7. 28 - 7. 15 (M, 10H), 6. 982 (S, 2H), 5. 981 (S, 2H), 2. 570 (S, 6H), 2. 341 (S, 3H), 2. 091 (S, 6H), 1. 403 (S, 6H) [Formula 1 - 1] In said formula 1 - 1, R1 And R2 And each phenyl group, R3 , R4 , R5 And R6 All - CH3 Are disclosed. Preparation example 1 - 2 3 opening cap to 2 - bromo (2 a-Bromonaphthalene, 5. 445Mmol) 1. 13G and magnesium (Magnesium, 5. 445Mmol) 132 mg frame and, to form an argon atmosphere then, thrown into a tetrahydrofuran mixable herein (THF) 1 conducting agitation time. Then, - 4, 4 - tetramethyl - 1, 3, 5, 7 - fluoro - 4 - 8 - herein die as the mesh cefuroxime 3 provided 3a, 4a - user inside the - s - indacene (8 a-Mesityl-a 1, 3, 5, 7 a-tetramethyl-a 4, 4 a-difluoro-a 4 a-bora3-a 3a, 4a a-diaza-a s a-indacene, 2. 723Mmol) 2 after charging a melting 1g solution, 25 °C reaction was complete in 2 hours stirring performed. And then 1N HCl reaction product solution, after extraction of MC (methylene chloride) with respect to the rotation Eq and dried. Next dried reaction product obtained compounds represented by column to formula 1 - 2. 1H NMR (CDCl3, 400MHz): 7. 82 - 7. 73 (M, 2H), 7. 72 - 7. 69 (M, 6H), 7. 45 - 7. 39 (M, 6H), 6. 980 (S, 2H), 5. 975 (S, 2H), 2. 570 (S, 6H), 2. 341 (S, 3H), 2. 078 (S, 6H), 1. 417 (S, 6H) [Formula 1 - 2] In said formula 1 - 2, R1 And R2 Each And, R3 , R4 , R5 And R6 All - CH3 Are disclosed. Preparation example 1 - 3 3 Ethyl magnesium bromide to cap opening (Ethylmagnesium bromide, 6. 80Mmol) 6. 8 Ml and 1 a-ethynylpyrene, 7. 35Mmol) 1. 66G frame and, in 70 °C higher stirring time 2. Then, - 4, 4 - tetramethyl - 1, 3, 5, 7 - fluoro - 4 - 8 - herein die as the mesh cefuroxime 3 provided 3a, 4a - user inside the - s - indacene (8 a-Mesityl-a 1, 3, 5, 7 a-tetramethyl-a 4, 4 a-difluoro-a 4 a-bora3-a 3a, 4a a-diaza-a s a-indacene, 2. 723Mmol) a tetrahydrofuran mixable 1g (THF) () solution for 1 to 3 Cannula after charging, in performing his complete 70 °C 1 meat and mixing reaction. Next dried reaction product by column to compounds represented by formula 1 - 3 a are obtained. 1H NMR (CDCl3, 400MHz): 8. 801 (D, 2H), 7. 974 - 8. 213 (M, 16H), 6. 823 (S, 2H), 5. 969 (S, 2H), 2. 548 (S, 6H), 2. 340 (S, 3H), 2. 104 (S, 6H), 1. 411 (S, 6H) [Formula 1 - 3] In said formula 1 - 3, R1 And R2 Each And, said R7 the And, R3 , R4 , R5 And R6 All - CH3 Are disclosed. Preparation example 1 - 4: Represented by formula 2 - 1 Green-based Number of organic style tank housing (1) Represented by the following formula a number bath 3 - 3, 4, 9, 10 - Perylene to cap opening hydrides (Perylene-a 3, 4, 9, 10 a-tetracarboxylic dianhydride, 25. 49Mmol, 10g), iodine (iodine, 4. 33Mmol, 1. 1G) chloro nick opinion gun it buys (chlorosulfonic acid, 40 ml) was input into the nitrogen atmosphere. Then, to the base 20 by reacting time 70 °C temperature during heating and stirring, reaction temperature sensors evaporants finish next 24 °C - 25 °C, cooled in a reactive solution precipitate slowly outside input were generated. Next, filtering the precipitate then, washed with water and dried to represented in formula a orange solid are obtained. 1H-a NMR (d -DMSO, Ppm): 8. 75 (S, 4H). 13C non-NMR(d -DMSO, Ppm): 119. 75, 125. 29, 129. 5, 134. 88, 136. 22, 158. 16, 168. 53 [Formula a] (2) Synthesis of compound represented by formula b 3 Port cap to said formula a compound (18. 86Mmol, 10g) into CH nitrogen atmosphere3 CN (0. 06M, 310 ml), 1 - bromo - 2 - methyl propanamide (1 a-bromo-a 2 non-methylpropane, 269. 88Mmol, 30 ml), 2 - methyl - 1 - propanol (2 a-methyl-a 1 a-propanol, 324. 39Mmol, 30 ml), DBU (182. 94Mmol, 27. 3 Ml) followed by reflux time with respect to the outer 12. Said DBU input reaction solution after this orange, black green, black as when the next change, a little at a time from 30 minutes 1 solid been produced. Next, reaction after that, which can reduce a temperature 24 °C - 25 °C then stirring followed by methanol, filtering solid are obtained. Next, after said hot air-dried solids, dry solids are represented by formula b compound silica column to tint solid are obtained. 1H-a NMR spectrum (300 MHz, CDCl3): Δ (ppm)=8. 09 (S, 4H), 4. 15 (M, 8H), 2. 14 (M, 4H), 1. 05 (D, 24H) 13C NMR (CDCl3, Ppm): 166. 2, 131. 6, 128. 6, 128. 2, 127. 9, 127. 6, 124. 4, 74. 4, 27. 7, 19. 4 [Formula b] (3) Synthesis of compound represented by formula 2 - 1 3 Compounds of formula b to cap opening (12. 65Mmol, 10g), K2 CO3 (126. 5Mmol, 17. 5G) having been added, under nitrogen atmosphere NMP (0. 06M, 210 ml) stirring into a chains. Next, phenol (126. 5Mmol, 11 ml) which can be about 30 with respect to the 90 °C meat and mixing and reaction. Reaction temperature after 24 °C - 25 °C have reduce 1N HCl is put, precipitate has sprung up. Filtering said water-washing the precipitate then, after hot blast heater, yellow light orange solid (70% yield) by column silica solids drying stone are obtained. And, and 1 H-a NMR and13 For compounds of formula 2 - 1 measured by C NMR has been confirmed. 1H-a NMR spectrum (300 MHz, CDCl3): Δ (ppm)=8. 24 (S, 4H), 7. 21 (M, 8H), 6. 97 (M, 4H), 6. 91 (M, 8H), 4. 21 (D, 8H), 2. 42 (M, 4H), 1. 01 (D, 24H) 13C NMR (CDCl3, Ppm): 166. 3, 157. 4, 149. 9, 131. 7, 128. 7, 126. 9, 123. 1, 122. 7, 121. 9, 117. 7, 115. 2, 75. 4, 27. 7, 19. 4 [Formula 2 - 1] In said formula 2 - 1, R1 (Isobutyl group) and the , A, B, C and D is - OR2 And, R2 a And, n is 0 are disclosed. Preparation example 1 - 5: Represented by formula 2 - 2 Green-based Number of organic style tank housing Example 1 prepared in the same method represented by said formula b compound number was high pressure liquid coolant. 3 Compounds of formula b to cap opening (12. 65Mmol, 10g), K2 CO3 (126. 5Mmol, 17. 5G) having been added, under nitrogen atmosphere NMP (0. 06M, 210 ml) stirring into a chains. Next, 4 - tetra - butyphynol (126. 5Mmol, 19g) 140 °C which can be about 30 with respect to the meat and mixing and reaction. Reaction temperature after 24 °C - 25 °C have reduce 1N HCl is put, precipitate has sprung up. Filtering said water-washing the precipitate then, after hot blast heater, yellow light orange solid (yield 68%) dry solids by silica column stone are obtained. And, and 1 H-a NMR and13 For compounds of formula 2 - 2 by C NMR measures has been confirmed. 1H-a NMR spectrum (300 MHz, CDCl3): Δ (ppm)=8. 25 (S, 4H), 7. 25 (D, 8H), 6. 84 (D, 8H), 4. 21 (D, 8H), 2. 43 (M, 4H), 1. 34 (S, 36H), 1. O1 (d, 24H) 13C NMR (CDCl3, Ppm): 166. 1, 153. 9, 149. 9, 143. 1, 131. 7, 126. 9, 124. 8, 123. 2, 122. 7, 117. 1, 115. 2, 74. 4, 40. 7, 31. 4, 27. 7, 19. 4 [Formula 2 - 2] In said formula 2 - 2, R1 (Isobutyl group) and the , A, B, C and D is - OR2 And, R2 a Are disclosed. Preparation example 1 - 6: Represented by formula 2 - 3 Green-based Number of organic style tank housing Example 1 prepared in the same method represented by said formula b compound number was high pressure liquid coolant. 3 Compounds of formula b to cap opening (12. 65Mmol, 10g), K2 CO3 (126. 5Mmol, 17. 5G) having been added, under nitrogen atmosphere NMP (0. 06M, 210 ml) stirring into a chains. Next, 2, 4, 6 - trimethyl phenol (126. 5Mmol, 17. 2G) 140 °C which can be about 30 with respect to the meat and mixing and reaction. Reaction temperatures after 24 °C - 25 °C have reduce 1N HCl is put, precipitate has sprung up. Filtering said water-washing the precipitate then, after hot blast heater, yellow light orange solid (69% yield) by column silica solids drying stone are obtained. And, and 1 H-a NMR and13 C NMR to compounds represented by formula 2 - 3 by measures has been confirmed. 1H-a NMR spectrum (300 MHz, CDCl3): Δ (ppm)=8. 27 (S, 4H), 6. 64 (S, 8H), 4. 20 (D, 8H), 2. 45 (M, 4H), 2. 37 (S, 36H), 1. O3 (d, 24H) 13C NMR (CDCl3, Ppm): 165. 9, 149. 9, 147. 1, 131. 7, 131. 3, 127. 6, 127. 5, 126. 9, 123. 2, 122. 7, 115. 2, 74. 4, 27. 7, 24. 9, 19. 4, 15. 0 [Formula 2 - 3] In said formula 2 - 3, R1 (Isobutyl group) and the , A, B, C and D is - OR2 And, R2 a Are disclosed. Preparation example 1 - 7: Represented by formula 2 - 4 Green-based Number of organic style tank housing Example 1 prepared in the same method represented by said formula b compound number was high pressure liquid coolant. 3 Compounds of formula b to cap opening (12. 65Mmol, 10g), K2 CO3 (126. 5Mmol, 17. 5G) having been added, under nitrogen atmosphere NMP (0. 06M, 210 ml) stirring into a chains. Next, phenol (126. 5Mmol, 11 ml) which can be about 30 with respect to the 90 °C meat and mixing and reaction. Reaction temperatures after 24 °C - 25 °C have reduce 1N HCl is put, precipitate has sprung up. Filtering said water-washing the precipitate then, after hot blast heater, yellow light orange solid (71% yield) by column silica solids drying stone are obtained. And, and 1 H-a NMR and13 C NMR to compounds of formula 2 - 4 by measures has been confirmed. 1H-a NMR spectrum (300 MHz, CDCl3): Δ (ppm)=8. 60 (S, 2H), 8. 25 (S, 2H), 7. 22 (M, 8H), 6. 98 (T, 4H), 6. 92 (D, 8H), 4. 21 (D, 8H), 2. 43 (M, 4H), 1. 01 (D, 24H) 13C NMR (CDCl3, Ppm): 166. 5, 157. 0, 149. 9, 131. 7, 131. 6, 128. 6, 128. 5, 127. 9, 127. 6, 125. 7, 123. 5, 121. 9, 117. 5, 115. 2, 74. 4, 27. 7, 19. 4 [Formula 2 - 4] In said formula 2 - 4, R1 (Isobutyl group) and the , A and D is - OR2 And, R2 a And, n is 0 and, is - Cl B and C are disclosed. Preparation example 2: Represented by formula 3 - 1 Red Number of organic style tank housing (1) 3 - 4, 4 - Tetramethyl - 1, 3, 5, 7 - fluoro - 4 - to 8 - mesh cefuroxime 3 provided 3a as the die opening cap, inside the 4a - user - s - indacene (8 a-Mesityl-a 1, 3, 5, 7 a-tetramethyl-a 4, 4 a-difluoro-a 4 a-bora3-a 3a, 4a a-diaza-a s a-indacene) (2. 708Mmol) and placed in the 1g, CH2 Cl2 Chamber when a, conducting agitation. Next, NIS (N non-iodosuccinimide, 10. 832Mmol) 2. 44G having been added, 6 hours at room temperature (about 25) conducting agitation conditions. Next, Eq (rotary evaporator) rotation number after solvent alone, which code represented by formula c obtained material column performing a red solid are obtained. 1H NMR (CDCl3, 400MHz): Δ (ppm)=6. 973 (S, 2H), 2. 646 (S, 6H), 2. 356 (S, 3H), 2. 061 (S, 6H), 1. 402 (S, 6H) [Formula c] (2) 3 A number represented by said formula f red solid high pressure liquid coolant prior to cap opening (1. 615Mmol) 1g, thiophene boronic acid it blooms call ester (Thiophene bornic acid pinacol ester, 4. 845Mmol) 1. 018G, K2 CO3 (16. 15Mmol) 2. 23G, Pd (PPh3 )4 (0. 1615Mmol) 186. Then added of 6 mg, after vacuum treatment, nitrogen bubbling US. Next, excitation solvent (THF: H2 O=9:1 volume ratio) then held, in his 85 °C to reflux overnight. And, reaction end then, the use which will raise up to room temperature (about 25 °C) rotation number after volatile solvent evaporation temperature, obtained by performing column material, represented by the formula 3 - 1 in an amount of dark red solid are obtained. 1H NMR (CDCl3, 400MHz): 7. 35 (D, 2H), 7. 08 (M, 2H), 6. 96 (S, 2H), 6. 87 (M, 2H), 2. 61 (S, 6H), 2. 33 (S, 3H), 2. 16 (S, 6H), 1. 40 (S, 6H) [Formula 3 - 1] Comparison 1 3 8 - Fluoro - 4 - phenyl - 1, 3, 5, 7 - tetramethyl - 4, 4 - as the die opening cap to 3 provided 3a, 4a - user inside the - s - indacene (8 a-Phenyl-a 1, 3, 5, 7 a-tetramethyl-a 4, 4 a-difluoro-a 4 a-bora3-a 3a, 4a a-diaza-a s a-indacene (3. 22Mmol) after which the 1g into and out of the vacuum, dried CH2 Cl2 With concave fronts. Then, triethylamine herein (NEt3 , 6. 45Mmol) 0. On 90 ml bromo (Bromodimethylborane, 6. 45Mmol) 0. Chamber when a 63 ml, 30 minutes stirring to reaction was complete. Next reaction product Na2 CO3 After solution, Na2 SO4 With respect to the rotation of the pouch is water solution and dried Eq. Next dried reaction product by column to a compounds represented by formula 6 are obtained. 1H NMR (CDCl3, 400MHz): 7. 64 - 7. 48 (M, 5H), 5. 985 (S, 2H), 2. 564 (S, 6H), 1. 391 (S, 6H), 0. 209 (S, 6H) [Formula 6] Comparison 2 (1) Represented by the following formula d number bath 3 Perylene - 3, 9 - dicarboxylic acid to cap opening (Perylene provided 3, 9 a-dicarboxylic acid, 9. 2G, 27. 03Mol) with isobutyl alcohol (Isobutyl alcohol in DMF, 50 ml) into and out of the 65 °C 3 in time with respect to the stirring reaction. After 24 °C - 25 °C lowers the reaction temperature, MeOH (500 ml) with concave discriminate, after filtering the resulting precipitate, the precipitate washed cold MeOH-shaking. Next, cleaning materials in vacuum oven drying yellow solid (9. 6G, 78%) obtained, represented by formula d is has been confirmed that the compound. 1H-a NMR (300 MHz, CDCl3): Δ (ppm)=8. 19 (D, 2H), 7. 96 - 91 (M, 4H), 7. 39 (M, 4H), 4. 03 (D, 4H), 1. 97 (M, 2H), 0. 91 (D, 12H) 13C NMR (CDCl3, Ppm): 167. 9, 139. 1, 129. 8, 128. 8, 128. 1, 127. 1, 126. 9, 125. 3, 124. 4, 122. 8, 120. 2, 70. 8, 27. 6, 19. 4 [Formula d] (2) Compound of formula e number bath 3 diisobutyl ester opening cap to vinyl (Perylenedicarboxylic diisobutyl ester, 9. 6G, 21. 22Mol), bromo N - (N-a Bromosuccinimide, 7. 85G, 44mol) and CH2 Cl2 Into and out of the stirring-gate (TLC the reaction end). Next, evaporator (evaporator) and column (Column) number to a stand-alone solution after reaction obtained in solid , compound represented by the formula e has been confirmed. 1H-a NMR spectrum (300 MHz, CDCl3): Δ (ppm)=8. 21 (D, 2H), 7. 99 (D, 2H), 7. 82 - 77 (M, 4H), 4. 01 (D, 4H), 1. 95 (M, 2H), 0. 90 (D, 12H) 13C NMR (CDCl3, Ppm): 167. 7, 133. 0, 129. 7, 128. 9, 128. 1, 128. 0, 127. 9, 126. 8, 126. 1, 124. 2, 120. 0, 70. 9, 27. 7, 19. 6 [Formula e] (3) Represented by the following formula 5 number bath 3 Isobutyl 4, 10 - - 3, 9 - dicarboxylate to cap opening with d [pu wool lung reel [leyn (isobutyl 4, 10 a-dibromoperylene provided 3, 9 a-dicarboxylate, 12 parts by weight), cyan copper (copper cyanide, 9 parts by weight) cancer (sulfolane, 100 parts by weight) into and out of use is made, in meat and mixing and reaction with respect to the 25 130 °C - 140 °C. Next, distilled water herein after reaction (H2 0, 400 Parts by weight) when the input evaporants, been create a deposit. Then dilution ammonia to match filtered precipitate, was washed with distilled water and drying same. Next, a building 1. Br containing 2% is toluene (9 parts by weight) is extracted (Extraction), silica gel column (trichloroethane/ethanol) (yield 61%) positive number is represented by formula 7 orange solid are obtained. 1H-a NMR (300 MHz, CDCl3): Δ (ppm)=8. 20 (D, 2H), 8. 03 - 7. 96 (M, 4H), 7. 87 (D, 2H), 4. 04 (D, 4H), 1. 98 (M, 2H), 0. 92 (D, 12H) 13C NMR (CDCl3, Ppm): 167. 9, 137. 5, 130. 4, 129. 8, 127. 4, 126. 9, 125. 7, 125. 4, 125. 3, 124. 4, 117. 1, 71. 1, 27. 9, 19. 7 [Formula 7] Comparison 3 3 - 3, 4, 9, 10 - Perylene to cap opening hydrides (Perylene-a 3, 4, 9, 10 a-tetracarboxylic dianhydride, 25. 49Mmol, 10g) nitrogen atmosphere into CH3 CN (0. 06M, 424 ml), 1 - bromo - 2 - methyl propanamide (1 a-bromo-a 2 non-methylpropane, 364. 75Mmol, 40 ml), 2 - methyl - 1 - propanol (2 a-methyl-a 1 a-propanol, 438. 43Mmol, 40 ml), DBU (247. 25Mmol, 37 ml) followed by reflux time with respect to the outer 12. Next, reaction after that, which can reduce a temperature 24 °C - 25 °C then stirring followed by methanol, filtering solid are obtained. Next, after said hot air-dried solids, dry solids are represented by formula 8 (yield 93%) silica column to orange solid are obtained. 1H-a NMR (300 MHz, CDCl3): Δ (ppm)=8. 25 (D, 4H), 8. 03 (D, 4H), 4. 11 (D, 8H), 2. 12 (M, 4H), 1. 03 (D, 24H) 13C NMR (CDCl3, Ppm): 168. 5, 133. 0, 130. 5, 130. 3, 129. 0, 128. 8, 121. 4, 71. 5, 27. 9, 19. 4 [Formula 8] Experiment example 1: Organic style of UV absorption wavelength and housing PL Wavelength measurement experiment (1) UV absorption wavelength measurement Preparing said housing each organic style example and comparison preparation ardency 0. 01 G taken one after, dissolved in 3 ml toluene same UV absorbance according to light emitting spectrum are disclosed respectively placed in a test tube. The result shown for table 1. UV absorption wavelength UV spectrometer (VARIAN, Conc. CARY 100) utilizing UV absorbance measurement were measured. (2) PL(Photoluminescence) measurement Example and comparison preparation ardency prepared organic style DarsaPro5200OEM PL (PSI Trading Co.) on said housing each have utilized to 500W ARC number non lamp (Xenon Lamp) PL measurement, the result shown for table 1. Said device includes a PL wavelength of table 1, 2 and comparison green wavelength range comparison 3 part but, blue wavelength portion also including bar, green-based organic style reproduction and a film of the present invention housing for use as a polyisobutylene can be identify. And, comparison PL wavelength but a soluble polymer 1, door number anti-face width greatly exceeds 50 nm has been. However, organic style of example 1 - 1 - 1 - 7 prepared in green-based organic style of light having a wavelength of 500 - 570 nm the inside of PL PL while having a wavelength suitable for use inside, making sure that the 50 nm hereinafter having low anti-face width can be, for example having a wavelength in the range of 580 - 680 nm red organic style of housing also prepared 2 PL bar, red organic style is also used for capable of suitable housing for use. In the embodiment 1: Reproduction and a Film number bath 100 Parts by weight of bisphenol A-type epoxy resin, 1 parts by weight of the binder resin was cured in mixing and agitating number number ethylenediamine and high pressure liquid coolant. Next, said binder resin, 800 nm average diameter size zinc oxide mixed with a solvent and 1 bead (light diffusion bead number) toluene extracts oriental medical method uses a filtering time after stirring. Next, example 1 - 1 preparing green-based organic style in trillion misfortunes said said housing, for example a coating liquid was prepared 2 of red organic style housing is put in and stirring number high pressure liquid coolant. , the coating liquid binder resin 46. 83% By weight, 3% by weight zinc oxide beads, green-based organic style housing 0. 151% By weight, red organic style housing 0. 0215 Comprising toluene and residual % by weight. Then, polyethylene terephthalate (PET) film about 100 micro m average thickness that is drawn by substrate (skin layer), barcode arrangement effects and 8 times using coater, said PET film by coating the coating liquid at a rate of about 20m/min end such that a coating thickness is lowered to about 108 - 110 micro m his cutter. Next, whereupon the same oven, 2 minutes in 120 °C in average thickness 39 with respect to the thermosetting and drying micro m reappearance layer formed in antique look. Next, said polyethylene terephthalate (PET) that is drawn on said average thickness about 100 micro m antique look reappearance layer (skin layer) by laminating a film 238 total thickness. 5 - 239 Micro m in number 3 layer structure reproduction and a film was high pressure liquid coolant. And, said composition shown in table 2 for the reappearance layer antique look. In addition, a reproduction and a film cross-section SEM Image number for the high pressure liquid coolant also 5a precursor, reproduction and a film cross-section SEM Image shown for 5b to also increased. 5A and 5b also heat the organic style housing and also making sure that the zinc oxide bead can be well dispersed. In the embodiment 2 - In the embodiment 16 The same method number is high pressure liquid coolant to said in the embodiment 1, particle, size and content number different reproduction and a film was high pressure liquid coolant. Comparison example 1 The same method number said in the embodiment 1 is coated liquid high pressure liquid coolant, said green-based organic style housing instead of the [ley age [tu which it will count (Cadmium Selenide (II)) is cadmium red organic style housing and quantum dots using binder resin 46% by weight, 3% by weight zinc oxide beads, the [ley age [tu which it will count number was 1 weight % and residual toluene including quantum dots cadmium coating liquid high pressure liquid coolant. Next, the same method in the embodiment 1 to 100 micro m said average thickness that is drawn PET film coating liquid, thermosetting coating layer and drying (average thickness 37 micro m) in aromatic rings, average thickness 100 PET film by laminating a film including micro m high pressure liquid coolant that is drawn further quantum dots was number thereof. Comparison example 2 Said method identical to the number of comparison example 2 is high pressure liquid coolant film including quantum dots and thickness thereof, morning fair number coating solution, instead of an average particle diameter of 5 micro m 2 m in silica beads and bead zinc oxide having an average particle diameter in the weight ratio 1:1 micro silica beads was used. In addition, silica beads containing color reproduction film cross-section SEM Image number for the high pressure liquid coolant also 6a precursor, color reproduction film cross-section SEM Image shown for 6a also increased. Comparison example 3 - Comparison example 10 The same method 3 layer structure film having high pressure liquid coolant to said in the embodiment 1 the number thereof, zinc oxide having an average particle diameter in silica beads instead of using micro bead 2 m, such as in high pressure liquid coolant to have his number amount to table 3. Experiment example 2: Measuring optical properties High pressure liquid coolant in said in the embodiment and comparison number chromatic reproducibility for film DCI S420, match rate, when the dissipating measuring, in addition luminance, relative luminance measuring, for table 4 shown to result. (1) DCI Angle Chromatic reproducibility Measuring Methods and systems are also composed of a liquid crystal display device is capable of expressing a BLU UHD-a LCD panel module having different colors in determining the range, Red, Green, Blue brightness coordinate measurer of respectively, the basis for three primary chromatic reappearance ratio can be obtained. Blue LED light through the BLU including a R, G, B color coordinates of the cable to be calculated each triangular area and, in the first to fourth area on one surface area DCI chromatic reappearance ratio can be compared. I.e. chromatic reappearance ratio assumed 100 when DCI shutter and area, as relative area of exhibits. The, in-plane device comprises a luminance system SR3 TOPCON yarn was used. (2) DCI Consistent angle rate measurements The measuring rate equal to match said measuring chromatic reproducibility, (Red) of red color, green (Green), blue (Blue) SR3 TOPCON yarn is converted into a luminance system which were measured. (3) Brightness and relative luminance measurement In the embodiment and comparison of the diffusion film and the prism reflective polarizing film having a center wavelength 450 nm blue LED BLU embodiments of film fixed configuration then, a BLU white color is formed in a, using Japanese BM7 & CL provided 500A, screen the gas generating is divided into 12 point, as well as the divergence of each measurement. And, based on the relative brightness and expressions 1 - 1 for a long time. [Expressions 1 - 1] Relative luminance (%)=luminance - silica beads containing color reproduction film measurement object film brightness)/silica beads containing color reproduction film brightness) × 100 (%) Expressions in said 1, silica beads containing 6% by weight based on the total weight average particle diameter of 2 m in color film is color reproduction film silica beads having an average particle diameter in silica beads 6 and 5 micro m % by weight including a comparison example 2 of the films in the micro and, in the embodiment and comparison example measurement object film is of each film are disclosed. (4) Chromatic deviation Measuring experiment The same chromatic reproducibility evaluation module is installed under a measurement object film material UHD provided LCD BLU driving one of room temperature (x, y) before and 20 hours of chromatic deviation measuring x tetracene, for assessing change when the y, Japanese of CA-a 310 (KONICA MINOLTA) was used. Chromatic deviation criterion value is x=0. 2512 And, y=0. 2768 Are disclosed. Table 4 residual said result of measurement, in the case of in the embodiment 1 - 16, DCI has 95% or more chromatic reappearance ratio for S420, 92% or more can be sure that the DCI transmissibility match rate. Further, when measured by the relative brightness comparison reference example 2 using silica beads, high luminance result having been 89% or more. To the W-CDMA, quantum dots existing films using such embodiment 1 and comparison example 2 comparison car belt washing machine for example, in the case of comparison example 3, strength of luminance, chromatic reproducibility has been result falls. And, film organic style housing content 0. 5 Exceeds 4% by weight in the case of comparison example, as compared to in the embodiment 1, as a flash fixing has been number chromatic reproducibility and consistency rate. In addition, organic style film housing content 0. 12 Less than 5% by weight in the case of comparison example, as compared to in the embodiment 1, chromatic reproducibility, excellent result but match rate, good relative luminance ratio without, chromatic deviation (Δ y) is output from the door number showed cursor photostability. And, green fluorescent substance and red phosphor content less than 5:1 weight ratio 9:1 weight ratio comparison example 6 and green fluorescent substance and red phosphor content exceeds in the case of comparison example 7, chromatic deviation (cursor photostability drop door number (Δ x, Δ y) is showed. In addition, in the case of more than 1,500 nm zinc oxide bead size using the comparison example 8, relative luminance ratio low, chromatic deviation (Δ y) is output from the door number showed cursor photostability. And, antique look reappearance layer thickness less than 20 micro m comparison example 9 comparison example in the case of 60 micro m thickness exceeds 10 reappearance layer portion and a lower, low relative luminance ratio, chromatic deviation (( Δ x, Δ y) is output from the cursor photostability been result. Said in the embodiment of the present invention high chromatic reproducibility and experiments to be incorporated , luminance and proper light emitting layers making sure that the capable of having excellent optical bar having an. This, antique look reproducibility of the present invention required LED lighting, LED display, LCD optical film, in particular SUHD display may be use with reflecting plate to determine the other. The present invention relates to a high color reproduction film, a method for manufacturing the same, and a BLU including the same. More specifically, the present invention relates to a high color reproduction film satisfying a high color reproduction rate of 95% or more and a match ratio of 92% or more on the basis of digital cinema initiatives (DGI) color coordinate system, which is the largest color coordinate system currently available (year of 2017) backlight unit (BLU). COPYRIGHT KIPO 2018 In green-based organic style of 500 - 570 nm wavelength of 580 - 680 nm wavelength PL PL monomolecular form monomolecular form in red organic style housing and including at least one housing selected from organic style 1 housing; binder resin; and an average particle diameter of 750 - 1, 500 nm light diffusing beads; including a reappearance layer antique look which, green-based organic style to the inside of said housing and formula 2 represented by formula 1 represented by at least one selected from organic style organic style 1 and housing, a group represented by the formula 3 to the inside of said red organic style the reproduction and a film including a housing organic style characterized; [formula 1] In said formula 1, R1 And R2 Each independently C1 - C5 alkyl, of C5 - C10 cycloalkyl, C7 - C15 bi-cycloalkyl, C6 - C20 aryl or (aryl) And, R7 For blood [ley neel (benzopyrenyl) benzo, or benzo (phenanthrenyl) (benzophenanthenyl) group, R3 And R4 Are each independently C1 - C3 alkyl and, R5 And R6 Are each independently C1 - C5 alkyl, C2 - C5 olefin to, of C5 - C6 cycloalkyl, styrenic, phenyl or benzyl or - CN and, [formula 2] In said formula 2, said R1 C3 - C10 alkyl or C3 - C10 alkyl and grinding the dodecenes, A, B, C and D each independently halogen or - OR2 And, R2 Dodecenes is C3 - C10 alkyl, C3 - C10 of coffee alkyl, C5 - C10 cycloalkyl of, Or And, R3 C3 - C6 alkyl or C1 - C4 alkyl and the dodecenes are comminuted, and n is 0 - 3, [formula 3] In said formula 3, R1 ,R2 , R3 And R4 Each independently C1 - C5 alkyl, C2 - C5 olefin to, of C5 - C6 cycloalkyl, styrenic, phenyl, phenol or benzyl group and, R5 Is phenyl, phenyl (C1 - C3 alkoxy) (C1 - C3 die alkyl), phenyl (C1 - C3 alkyl die), and (C1 - C3 alkyl) phenyl or phenol group, R6 And R7 Are each independently - Br, - F, - Cl, - OH, C1 - C5 alkyl, C5 - C6 cycloalkyl of, which phenyl or naphthalene group, R8 And R9 Each independently a hydrogen atom or Are disclosed. According to Claim 1, said organic style housing 0. 12 - 0. 5% By weight, said light diffusion bead 3 - 15 weight % and residual binder resin characterized in including the reproduction and a film. According to Claim 2, green-based organic style housing a red organic style housing and 5 - 9:1 weight ratio characterized in including the reproduction and a film. According to Claim 1, characterized in that said light diffusion bead zinc oxide beads including the reproduction and a film. According to Claim 1, 100 parts by weight of said thermosetting resin binder resin, curing number 0. 5 - 3 Parts by weight of characterized in including the reproduction and a film. According to Claim 1, characterized in that said single or multi-layer structure film reproduction and a reappearance layer antique look. According to Claim 1, characterized in that said skin to one or two surfaces antique look reappearance layer further including a reproduction and a film. According to Claim 1, electronic watch with a direction, skin layer, antique look reappearance layer and skin layer and are sequentially arranged, said stacked layer emits light diffusion pattern or antique look reappearance layer top surface that the light pattern characterized reproduction and a film. According to Claim 1, characterized in that said average thickness 20 - 60 micro m antique look reappearance layer reproduction and a film. Either as described in claim number 1 to number 9 anti anti selected, based on the highest wavelength 450 nm and 20 nm in the blue light sources in the lenslet center DCI S420 when measured, chromatic reappearance ratio of at least 95%, at least 92% transmissibility match rate characterized reproduction and a film. According to Claim 10, 20 nm and 450 nm center wavelength (blue) light source based on the highest measured in the DCI S420 lenslet in blue chromatic deviation, deviation of x coordinate range 0. 001 - 0. 02 And, y coordinate deviation range 0. 001 - 0. 02 Characterized in the reproduction and a film. Solvent, binder resin and an average particle diameter of 750 - 1, 500 nm light diffusing beads mixed solution mixing tank 1 step a number oriental medical filters; said monomolecular form 500 - 570 nm wavelength of PL in trillion misfortunes in green-based organic style housing, 580 - 680 nm wavelength of PL monomolecular form in red organic style housing is put in and stirring a liquid coating bath step number 2; one coating liquid to form a coating layer on said substrate layer in step 3; in forming and drying step 110 °C - 130 °C and thermosetting in antique look reappearance layer 4; characterized in that the reproduction and a film including a manufacturing method. According to Claim 12, step 2 is a light bead of coating solution 1. 5 - 7% By weight, said green-based organic style housing 0. 08 - 0. 25% By weight, said red organic style housing 0. 012 - 0. 055% By weight, 42 - 60 weight % and residual solvent for the binder resin, said thermosetting resin 100 parts by weight of a binder resin, curing number 0. 5 - 3. 0 Parts by weight of including the reproduction and a film manufacturing method characterized. According to Claim 12, skin layer and said base layer, said one skin layer a reproduction and a film manufacturing method characterized in that a static reappearance layer antique look. According to Claim 12, step coating layer average thickness 60 - 130 micro m and 3, step 4 a reproduction and a film manufacturing method characterized in reappearance layer average thickness 20 - 60 micro m antique look. According to Claim 12, represented by formula 1 and formula 2 green-based organic style to the inside of said housing to at least one selected from organic style organic style 1 and has a housing, said housing including a red organic style to the inside of the reproduction and a film manufacturing method characterized organic style represented by formula 3; [formula 1] In said formula 1, R1 And R2 Each independently C1 - C5 alkyl, of C5 - C10 cycloalkyl, C7 - C15 bi-cycloalkyl, C6 - C20 aryl or (aryl) And, R7 For blood [ley neel (benzopyrenyl) benzo, or benzo (phenanthrenyl) (benzophenanthenyl) group, R3 And R4 Are each independently C1 - C3 alkyl and, R5 And R6 Are each independently C1 - C5 alkyl, C2 - C5 olefin to, of C5 - C6 cycloalkyl, styrenic, phenyl or benzyl or - CN and, [formula 2] In said formula 2, said R1 C3 - C10 alkyl or C3 - C10 alkyl and grinding the dodecenes, A, B, C and D each independently halogen or - OR2 And, R2 Dodecenes is C3 - C10 alkyl, C3 - C10 of coffee alkyl, C5 - C10 cycloalkyl of, Or And, R3 C3 - C6 alkyl or C1 - C4 alkyl and the dodecenes are comminuted, and n is 0 - 3, [formula 3] In said formula 3, R1 ,R2 , R3 And R4 Each independently C1 - C5 alkyl, C2 - C5 olefin to, of C5 - C6 cycloalkyl, styrenic, phenyl, phenol or benzyl group and, R5 Is phenyl, phenyl (C1 - C3 alkoxy) (C1 - C3 die alkyl), phenyl (C1 - C3 alkyl die), and (C1 - C3 alkyl) phenyl or phenol group, R6 And R7 Are each independently - Br, - F, - Cl, - OH, C1 - C5 alkyl, C5 - C6 cycloalkyl of, which phenyl or naphthalene group, R8 And R9 Each independently a hydrogen atom or Are disclosed. Characterized according to Claim 10 reproduction and a film including a backlight unit. Classification UV absorption wavelength (nm) For measuring wavelength of PL (nm) The lenslet (nm) Preparation example 1 - 1 512 541 45 Preparation example 1 - 2 513 548 47 Preparation example 1 - 3 522 552 50 Preparation example 1 - 4 506 539 42 Preparation example 1 - 5 510 543 48 Preparation example 1 - 6 507 540 46 Preparation example 1 - 7 501 537 49 Preparation example 2 577 630 55 1 comparison 501 528 59 2 comparison 475 527 52 3 comparison 476 528 48 Reappearance layer antique look Organic style housing Quantum dots Light diffusion number Antique look reappearance layer average thickness (micro m) Green-based organic style housing (type/weight %) Red organic style housing (type/weight %) oxide (average diameter /% by weight) Car bead silica (average diameter /% by weight) In the embodiment 1 Preparation example 1 - 1/0. 3% By weight Example 2/0 prepared. 043% By weight - 800 Nm/6% by weight - 39 In the embodiment 2 Preparation example 1 - 1/0. 3% By weight Example 2/0 prepared. 043% By weight - 800 Nm/12% by weight - 39 In the embodiment 3 Preparation example 1 - 1/0. 3% By weight Example 2/0 prepared. 043% By weight - 1,500 Nm/6% by weight - 39 In the embodiment 4 Preparation example 1 - 1/0. 3% By weight Example 2/0 prepared. 043% By weight - 1,500 Nm/12% by weight - 39 In the embodiment 5 Preparation example 1 - 4/0. 3% By weight Example 2/0 prepared. 043% By weight - 800 Nm/6% by weight - 39 In the embodiment 6 Preparation example 1 - 4/0. 3% By weight Example 2/0 prepared. 043% By weight - 800 Nm/12% by weight - 39 In the embodiment 7 Example 1 - 2/0 prepared. 3% By weight Example 2/0 prepared. 043% By weight - 800 Nm/6% by weight - 39 In the embodiment 8 Preparation example 1 - 3/0. 3% By weight Example 2/0 prepared. 043% By weight - 800 Nm/12% by weight - 39 In the embodiment 9 Example 1 - 5/0 prepared. 3% By weight Example 2/0 prepared. 043% By weight - 800 Nm/6% by weight - 39 In the embodiment 10 Example 1 - 6/0 prepared. 3% By weight Example 2/0 prepared. 043% By weight - 800 Nm/12% by weight - 39 In the embodiment 11 Example 1 - 7/0 prepared. 3% By weight Example 2/0 prepared. 043% By weight - 800 Nm/6% by weight - 39 In the embodiment 12 Preparation example 1 - 1/0. 33% By weight Example 2/0 prepared. 043% By weight - 800 Nm/12% by weight - 39 In the embodiment 13 Preparation example 1 - 1/0. 3% By weight Example 2/0 prepared. 038% By weight - 800 Nm/6% by weight - 39 In the embodiment 14 Preparation example 1 - 1/0. 3% By weight Example 2/0 prepared. 043% By weight - 1,000 Nm/6% by weight - 39 In the embodiment 15 Preparation example 1 - 1/0. 3% By weight Example 2/0 prepared. 043% By weight - 800 Nm/6% by weight - 45 In the embodiment 16 Preparation example 1 - 1/0. 3% By weight Example 2/0 prepared. 043% By weight - 800 Nm/12% by weight - 28 Reappearance layer antique look Organic style housing Quantum dots Light diffusion number Antique look reappearance layer average thickness (micro m) Green-based organic style housing (type/weight %) Red organic style housing (type/weight %) oxide (average diameter /% by weight) Car bead silica (average diameter /% by weight) Comparison example 1 - - 2. 0% By weight 800 Nm/6% by weight - 39 Comparison example 2 Preparation example 1 - 1/0. 3% By weight Example 2/0 prepared. 043% By weight - - 2 Micro m + 5 micro m/12% by weight 39 Comparison example 3 Preparation example 1 - 1/0. 3% By weight Example 2/0 prepared. 043% By weight - - 2 Micro m/12% by weight 39 Comparison example 4 Preparation example 1 - 1/0. 47% By weight Example 2/0 prepared. 074% By weight - 800 Nm/6% by weight - 39 Comparison example 5 Preparation example 1 - 1/0. 085% By weight Example 2/0 prepared. 012% By weight - 800 Nm/6% by weight - 39 Comparison example 6 Preparation example 1 - 1/0. 207% By weight Example 2/0 prepared. 043% By weight - 800 Nm/6% by weight - 39 Comparison example 7 Preparation example 1 - 1/0. 43% By weight Example 2/0 prepared. 043% By weight - 800 Nm/6% by weight - 39 Comparison example 8 Preparation example 1 - 1/0. 3% By weight Example 2/0 prepared. 043% By weight - 1,700 Nm/6% by weight - 39 Comparison example 9 Preparation example 1 - 1/0. 3% By weight Example 2/0 prepared. 043% By weight - 800 Nm/6% by weight - 15 Example 10 comparison Preparation example 1 - 1/0. 3% By weight Example 2/0 prepared. 043% By weight - 1,700 Nm/6% by weight - 80 Classification Chromatic reproducibility (%) Match rate (%) Luminance (Nit) Relative luminance (%) Chromatic deviation X Δ Y Δ In the embodiment 1 99. 8 97. 1 391. 2 95. 41 0. 002 0. 001 In the embodiment 2 100. 2 97. 3 387. 7 94. 56 0. 008 0. 001 In the embodiment 3 98. 5 95. 2 385. 2 93. 95 0. 006 0. 002 In the embodiment 4 99. 2 95. 4 381. 5 93. 05 0. 001 0. 001 In the embodiment 5 95. 8 93. 1 378. 3 92. 27 0. 003 0. 001 In the embodiment 6 96. 5 93. 9 374. 2 91. 27 0. 009 0. 003 In the embodiment 7 95. 3 92. 1 386. 1 94. 17 0. 009 0. 003 In the embodiment 8 95. 8 92. 7 388. 7 94. 80 0. 01 0. 004 In the embodiment 9 95. 2 92. 2 391. 2 95. 41 0. 012 0. 007 In the embodiment 10 95. 7 92. 4 387. 6 94. 54 0. 015 0. 006 In the embodiment 11 95. 9 92. 9 389. 2 94. 93 0. 013 0. 002 In the embodiment 12 96. 5 93. 5 401. 8 98. 00 0. 018 0. 018 In the embodiment 13 100. 1 96 374. 3 91. 29 0. 019 0. 019 In the embodiment 14 99. 4 96. 1 376. 2 91. 76 0. 018 0. 017 In the embodiment 15 98. 7 95. 4 378. 7 92. 37 0. 017 0. 019 In the embodiment 16 97. 5 91. 9 378. 7 89. 56 0. 019 0. 017 Comparison example 1 92. 7 91. 2 403 98. 29 0. 002 0. 002 Comparison example 2 94. 6 92. 4 410 100. 00 0. 002 0. 002 Comparison example 3 92. 7 91. 2 403 98. 29 0. 001 0. 002 Comparison example 4 98. 1 94. 3 386. 4 94. 24 0. 009 0. 032 Comparison example 5 93. 5 89. 4 369. 3 90. 07 0. 005 0. 012 Comparison example 6 98. 5 94. 4 359. 8 87. 76 -0. 0146 0. 0082 Comparison example 7 97. 8 93. 6 974. 4 91. 32 0. 0322 0. 0694 Comparison example 8 98. 3 94. 2 364. 7 88. 95 0. 0001 -0. 0176 Comparison example 9 96. 7 93. 9 345. 1 84. 17 -0. 0293 -0. 0033 Example 10 comparison 98. 1 94. 6 352. 9 86. 07 0. 0322 0. 0694