Irradiated Cortical Bone Sheet Allografts and Method of Forming Irradiated Cortical Bone Sheet Allografts
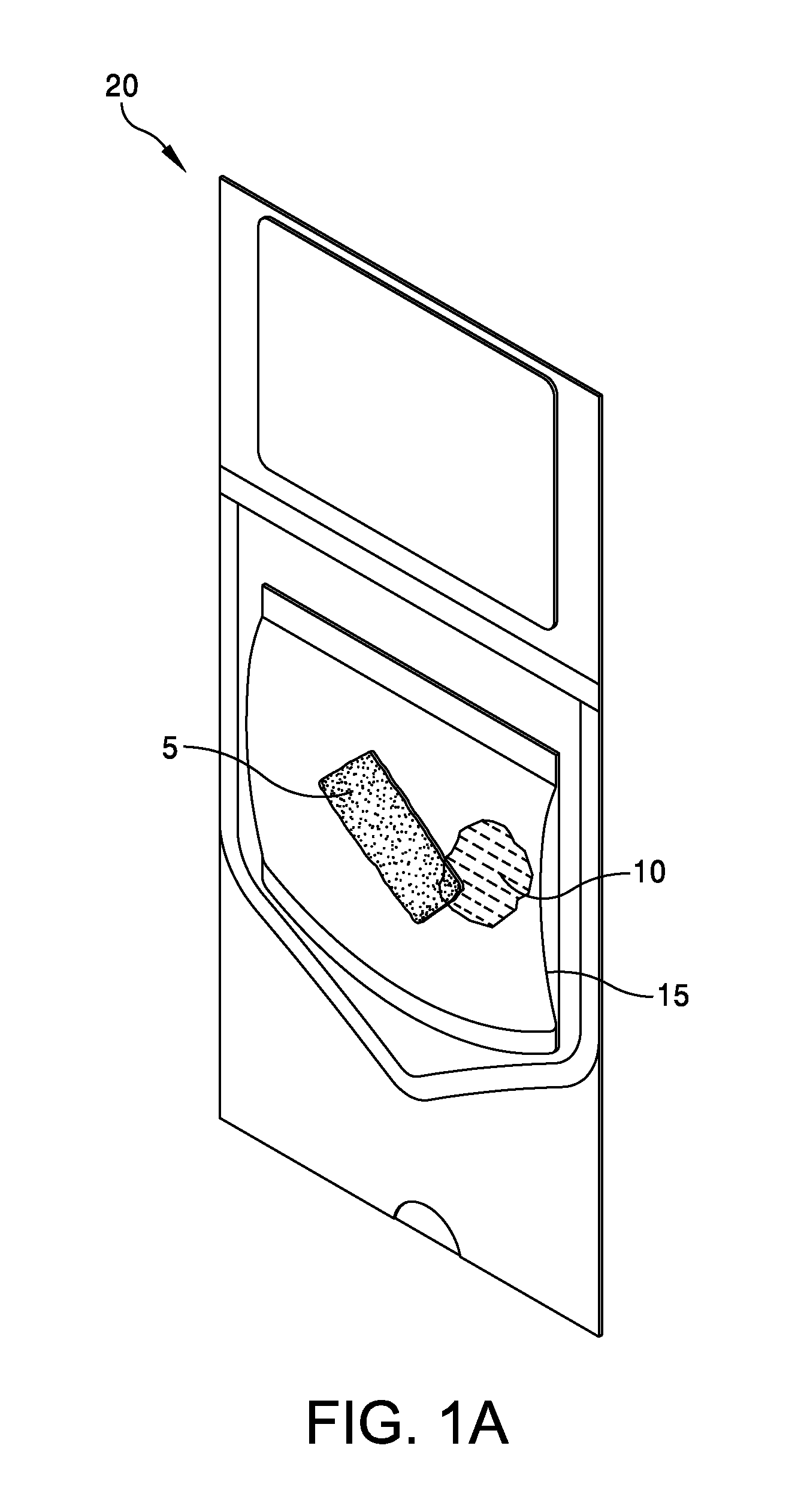
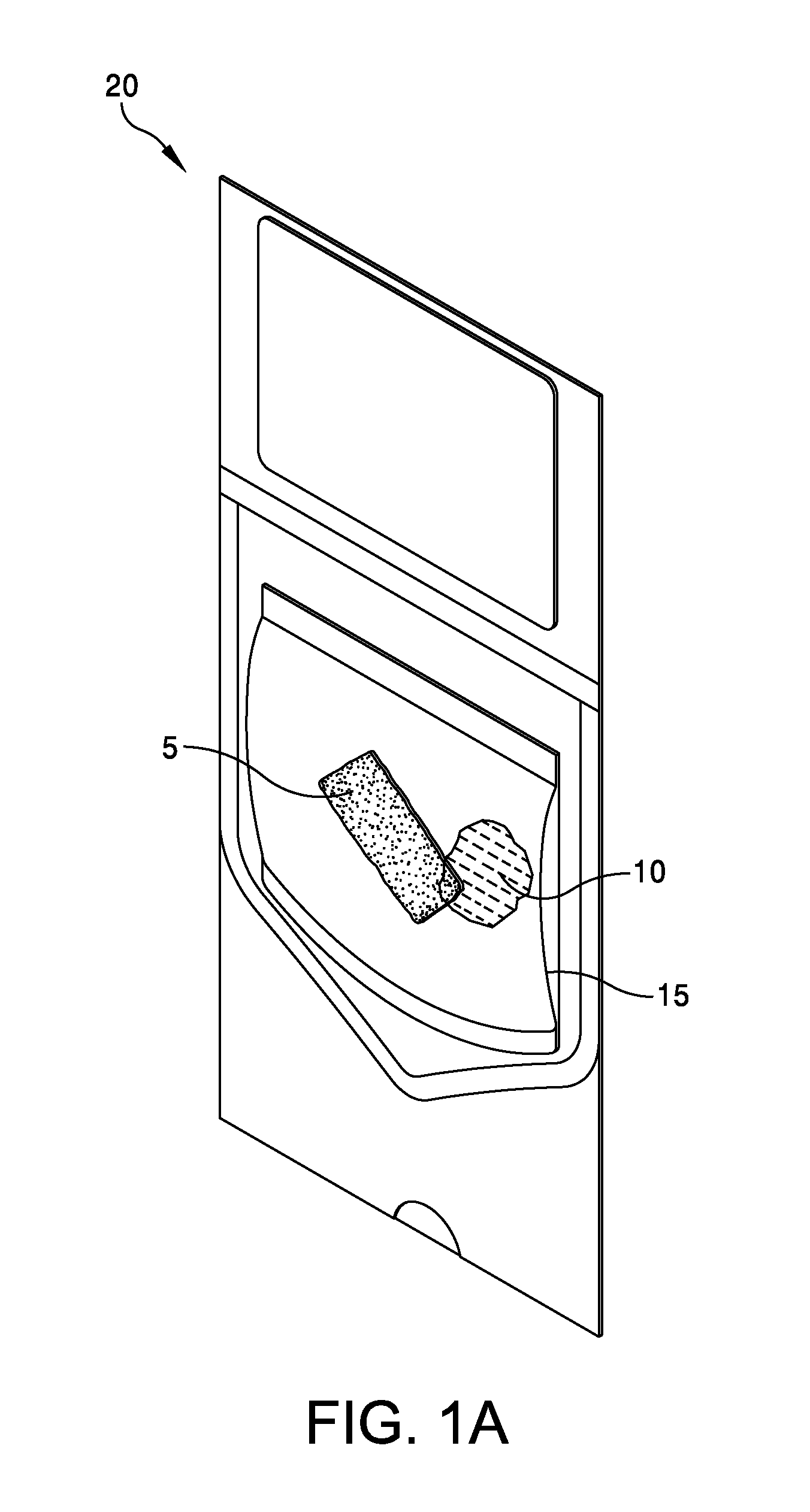
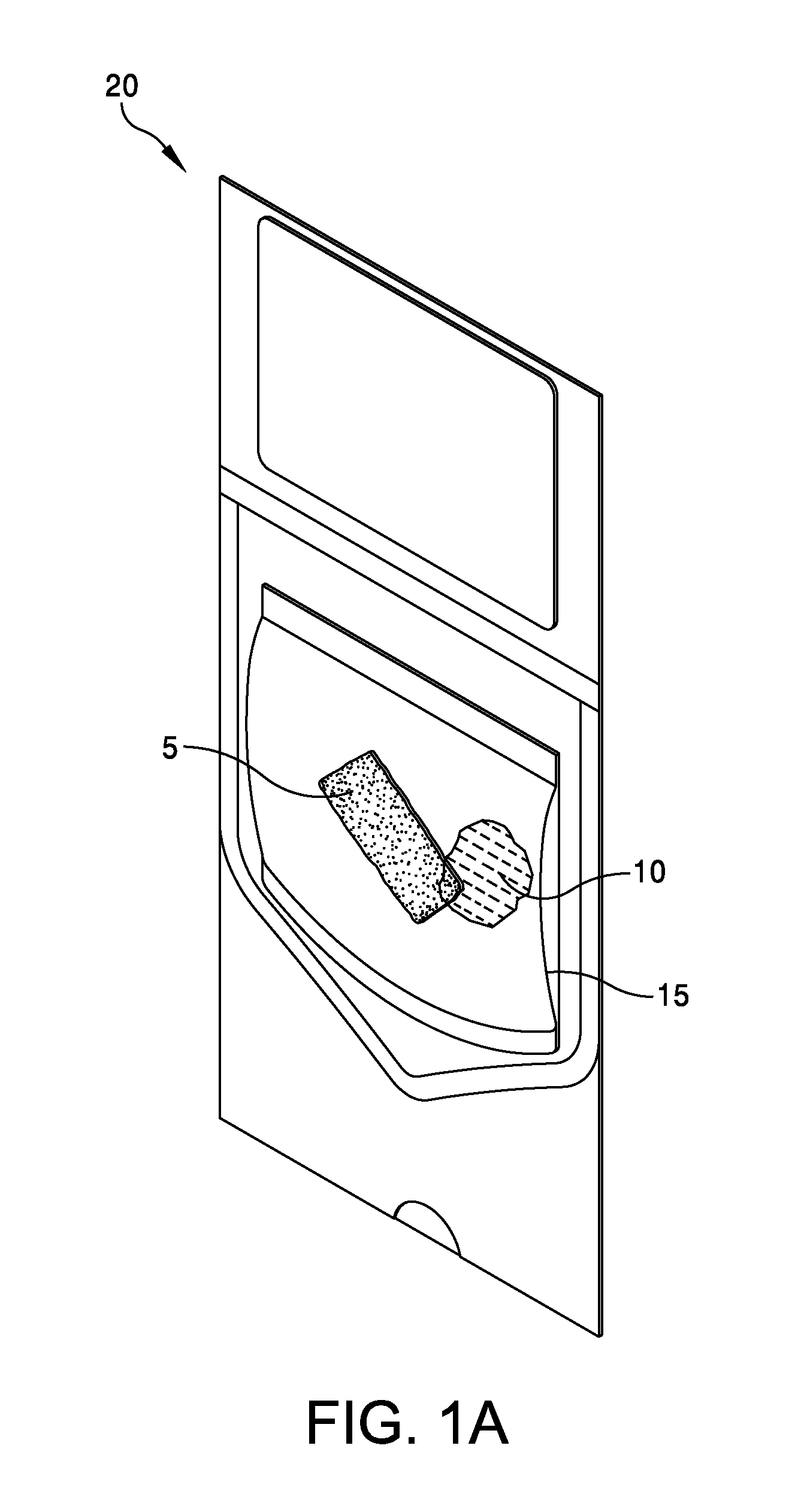
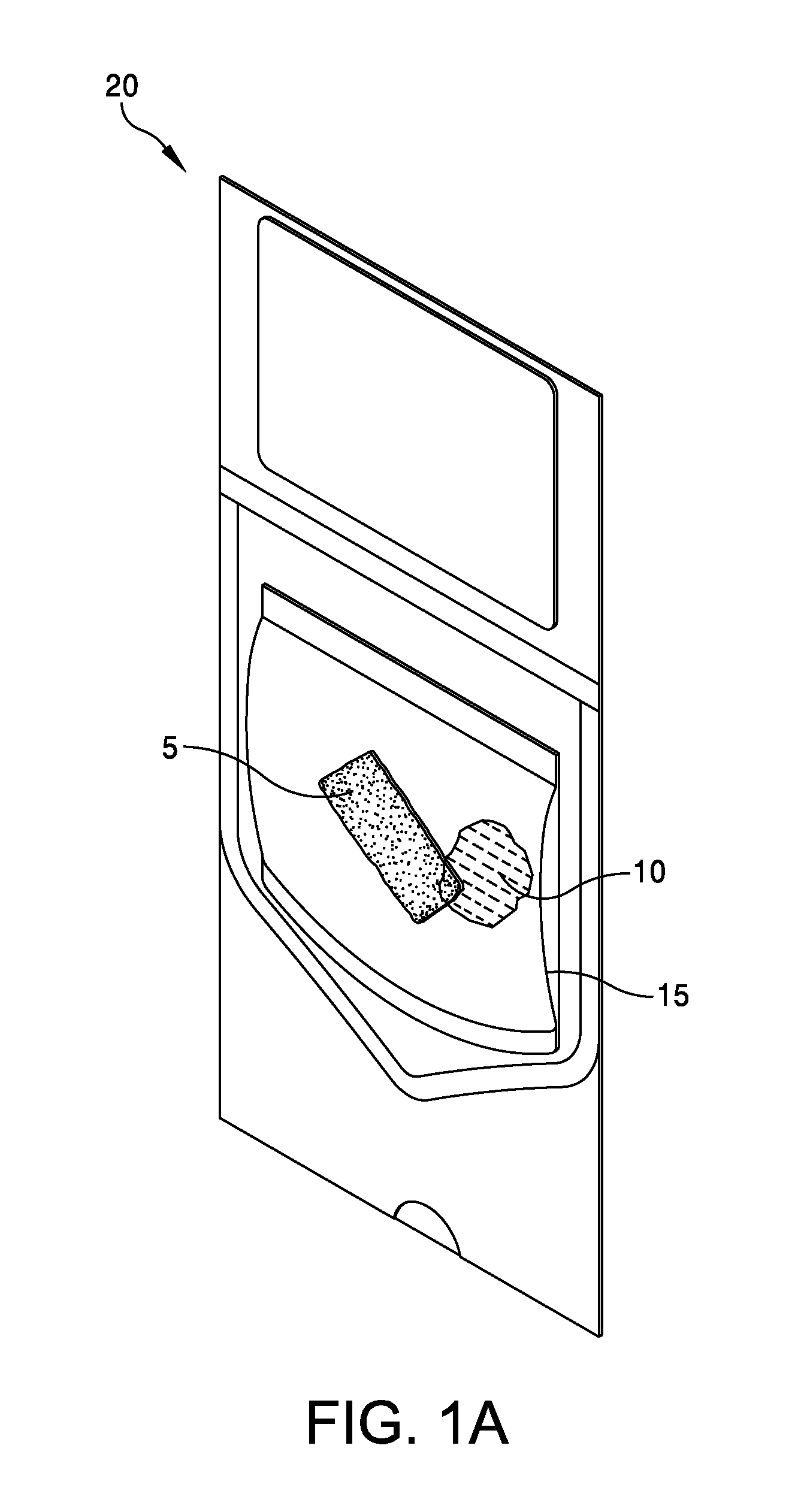
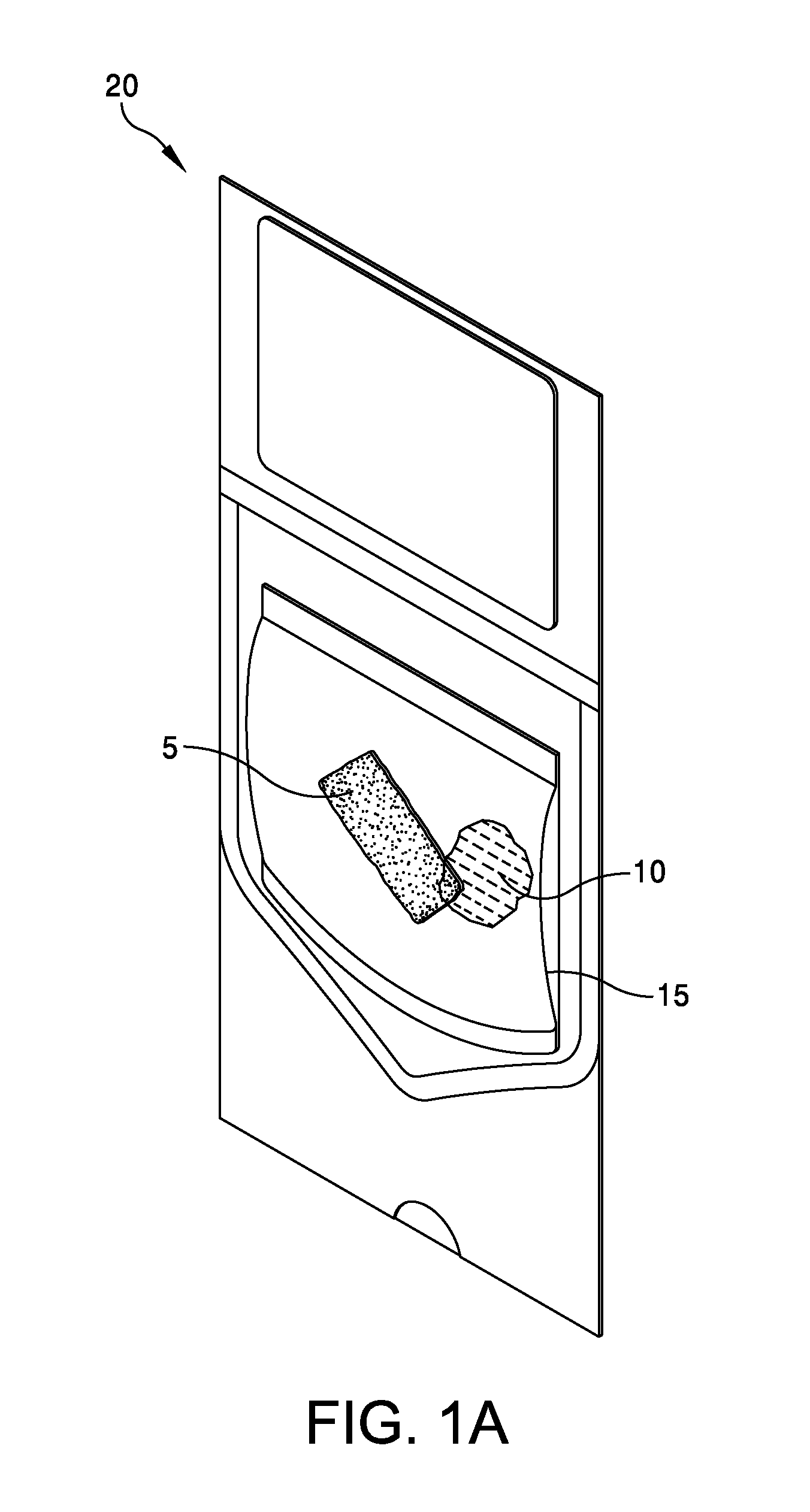
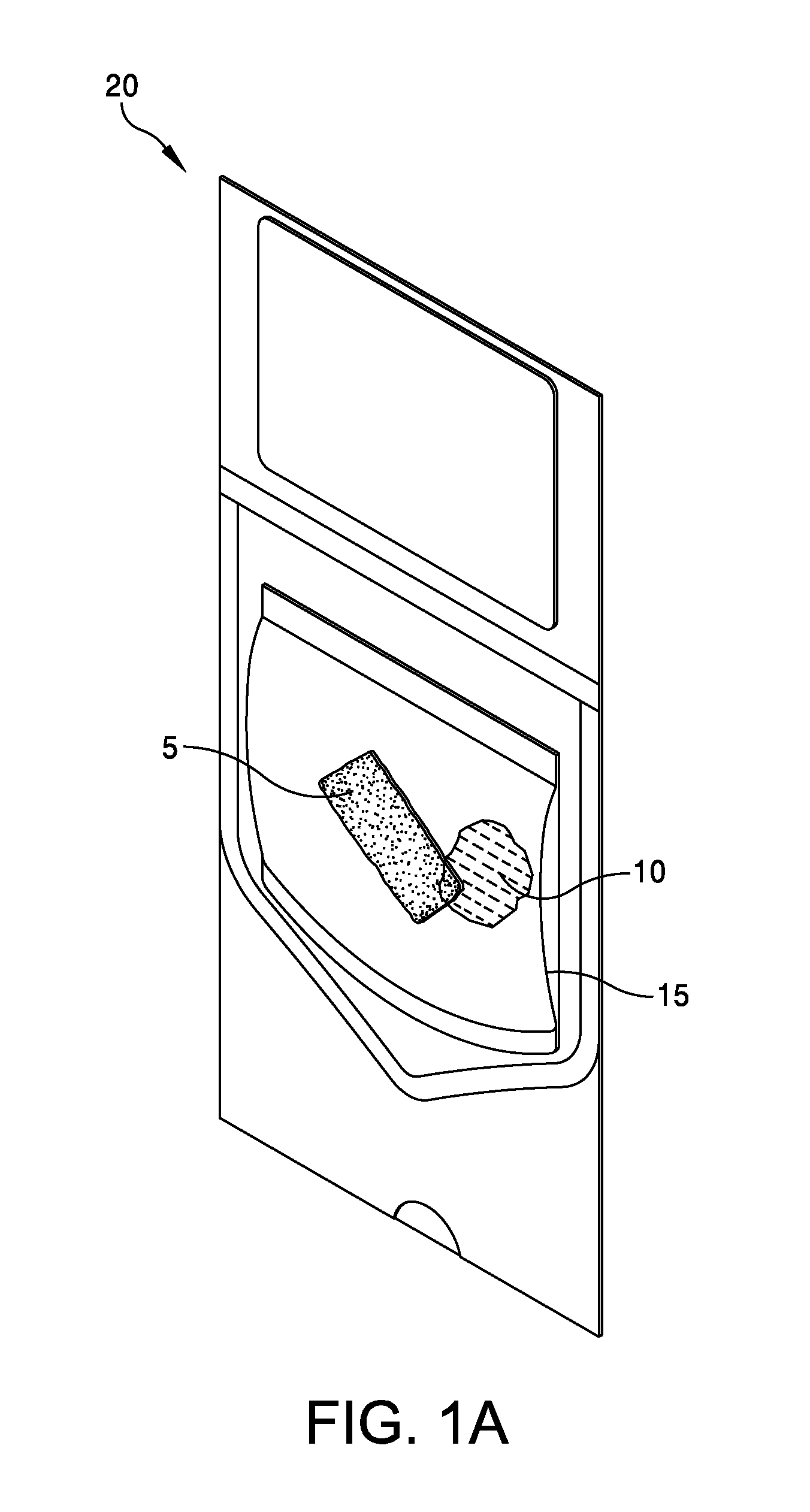
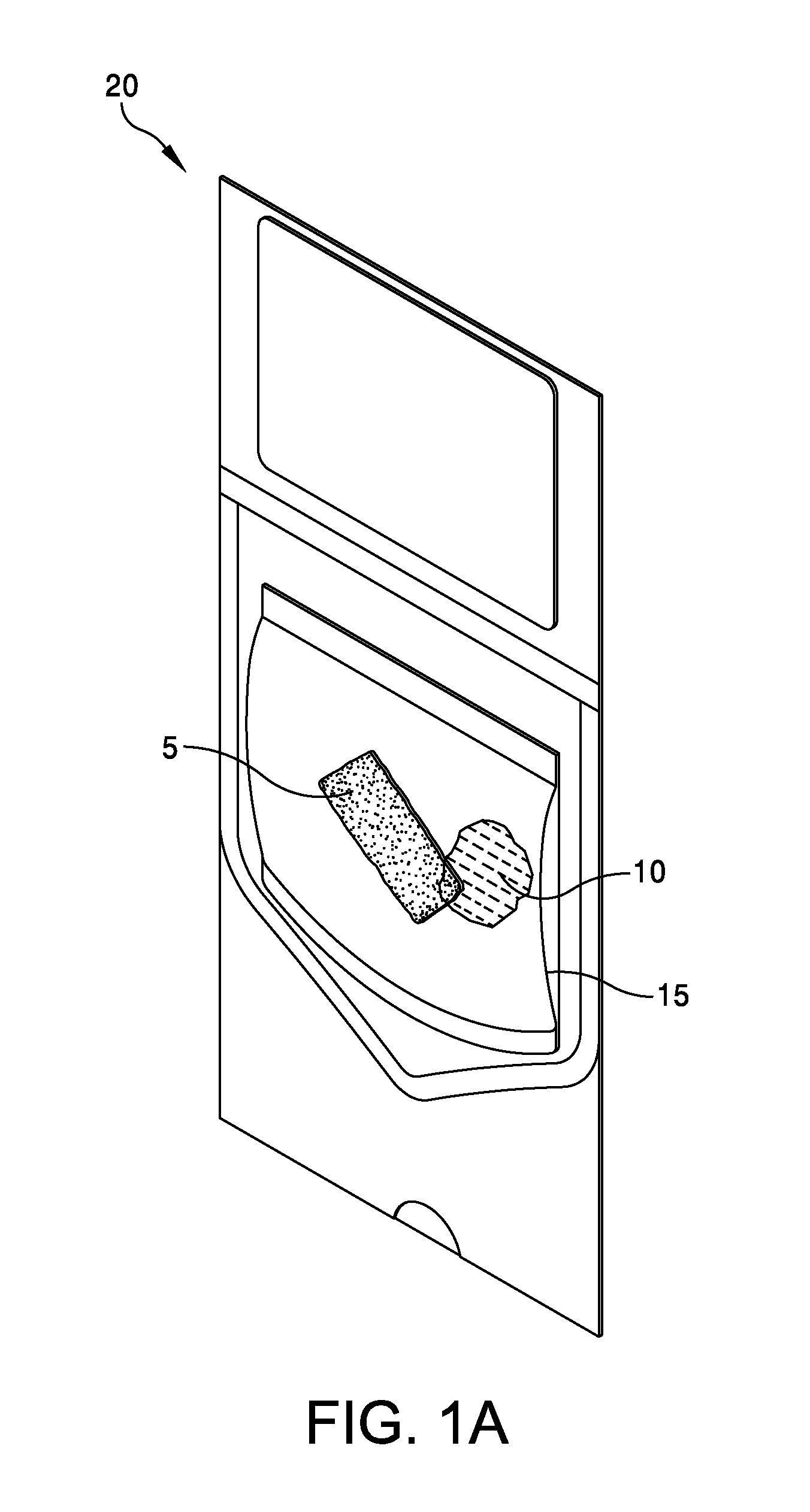
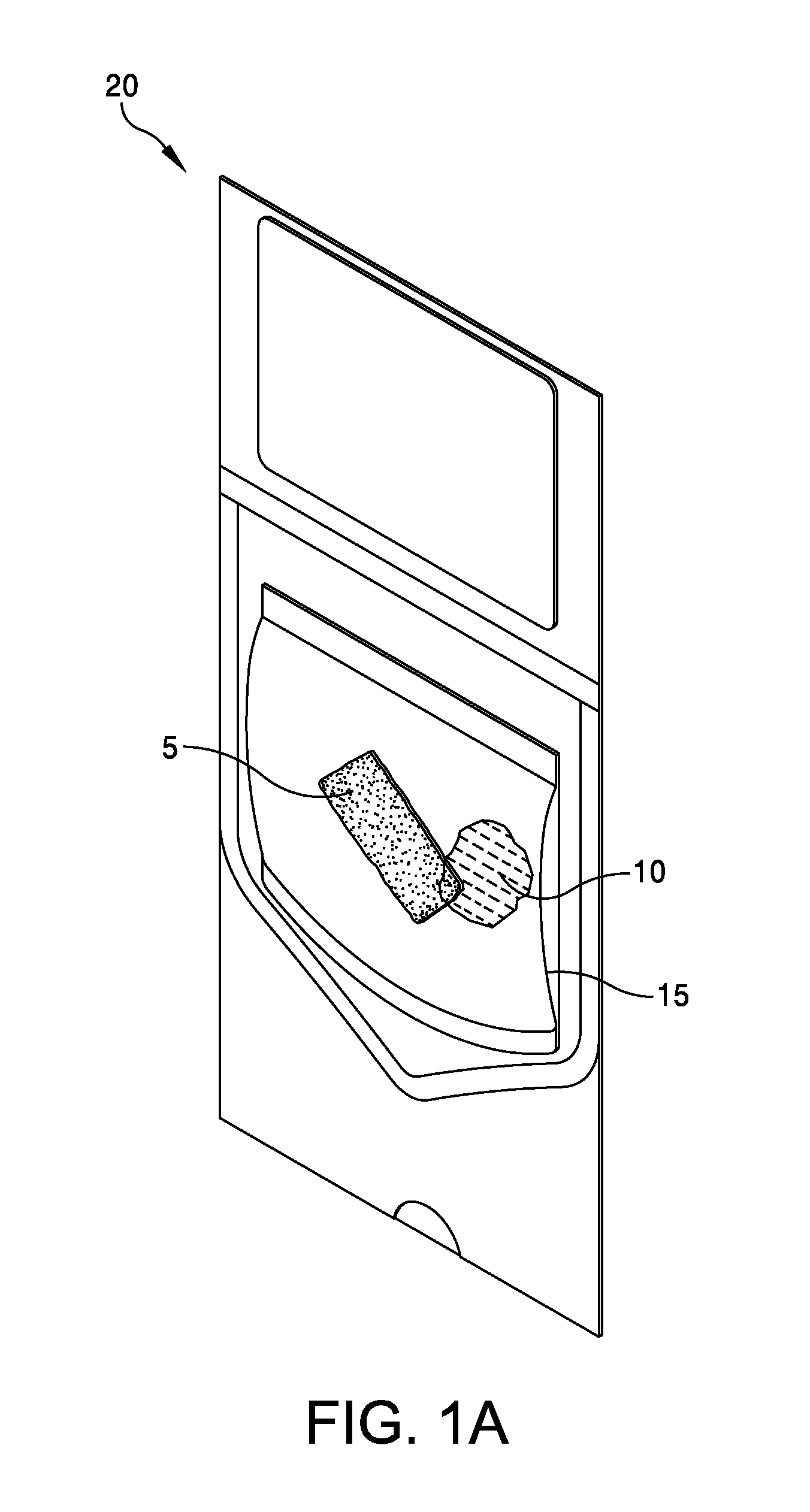
This application claims the benefit under 35 U.S.C. 119 (e) of U.S. Provisional Patent Application No. 61/539,781, filed Sep. 27, 2011 by Deborah Marie Spillman for “IRRADIATED CORTICAL BONE SHEET ALLOGRAFTS AND METHOD OF FORMING IRRADIATED CORTICAL BONE SHEET ALLOGRAFTS,” which patent application is hereby incorporated herein by reference. Generally, allograft bone materials are demineralized and either lyophilized or dehydrated. The allograft bone materials may be processed with a demineralization process, a dehydration process, or both. These processes change the grafts at a molecular level. This Summary is provided to introduce a selection of concepts in a simplified form that are further described below in the Detailed Description. This Summary is not intended to identify key aspects or essential aspects of the claimed subject matter. Moreover, this Summary is not intended for use as an aid in determining the scope of the claimed subject matter. In an embodiment, there is provided an irradiated cortical bone sheet allograft, the allograft comprising a unitary sheet of at least partially demineralized, irradiated cortical bone having a given thickness, a given width, and a given length, wherein the given thickness of the unitary sheet of at least partially demineralized, irradiated cortical bone is less than each one of the given width and the given length, and the unitary sheet of at least partially demineralized, irradiated cortical bone being packaged in a sterile environment. In another embodiment, there is provided an irradiated cortical bone sheet allograft, the allograft comprising a unitary sheet of at least partially demineralized, irradiated cortical bone packaged with a liquid carrier in a sterile environment at room temperature. In yet another embodiment, there is provided a method of forming an irradiated cortical bone sheet allograft, the method comprising obtaining a natural bone from a donor different than a recipient, the natural bone containing a layer of a cortical bone; cleaning the natural bone to produce a unitary sheet of cortical bone; a least partially demineralizing the unitary sheet of cortical bone; freezing the unitary sheet of cortical bone into a frozen state within a sealed package; and irradiating the unitary sheet of cortical bone in the frozen state within the sealed package to sterilize the unitary sheet of cortical bone and producing the irradiated cortical bone sheet for implantation in the recipient other than the donor. Other embodiments are also disclosed. Additional objects, advantages and novel features of the technology will be set forth in part in the description which follows, and in part will become more apparent to those skilled in the art upon examination of the following, or may be learned from practice of the technology. Non-limiting and non-exhaustive embodiments of the present invention, including the preferred embodiment, are described with reference to the following figures, wherein like reference numerals refer to like parts throughout the various views unless otherwise specified. Illustrative embodiments of the invention are illustrated in the drawings, in which: Embodiments are described more fully below in sufficient detail to enable those skilled in the art to practice the allograft and method. However, embodiments may be implemented in many different forms and should not be construed as being limited to the embodiments set forth herein. The following detailed description is, therefore, not to be taken in a limiting sense. In an embodiment, and with references to The allograft bone sheet may be produced more cost effectively by eliminating or reducing the cost of demineralization, dehydration, or both demineralization and dehydration. This allograft eliminates the need for a surgeon to harvest a sheet of bone from the patient to produce an autograft bone sheet. In one embodiment, donor bone material is processed into a desired size of an allograft bone sheet 5. For example, a bone may be thinly sliced to produce one or more thinly sliced sheets of cortical bone. This bone may be a long bone or an iliac bone. Examples of long bones include, but are not limited to, the femur, tibia, fibula, humerus, radius, and ulna. Next, the allograft bone sheet 5 may be packaged in a liquid carrier 10 within a plastic pouch 15. In an embodiment, the allograft bone sheet 5 is hydrated with, and packaged within, sterile water as the liquid carrier 10. The packaged allograft bone sheet 5 may be frozen after packaging and prior to irradiation. In one embodiment, the packaged allograft bone sheet may be frozen to approximately −70 degrees Celsius. The frozen, packaged allograft bone sheet 5 may be irradiated while frozen. An adequate irradiation procedure is used for sterilization of the allograft bone sheet 5. A Cobalt 60 source may be used to apply irradiation in a range of 2.5 to 3.8 mRads (25 to 38 kGy) of irradiation. In an embodiment, the packaged allograft product 20 may be allowed to thaw after irradiation to room temperature. The packaged allograft product 20 may be stored at room temperature after irradiation until use by a surgeon. In the various embodiments disclosed herein, the irradiated cortical bone sheet allograft 5 is not demineralized or dehydrated prior to use at a surgical site. In some embodiments, the irradiated cortical bone sheet allograft is at least partially demineralized prior to irradiation. This demineralization may occur within, or apart from, the plastic pouch 15 or other packaging. With reference to The amount of mineral removed from the bone may be adjusted to create the desired amount of flexibility. This demineralization conventionally uses an organic acid such as hydrochloric, nitric, or citric acid. In an embodiment, the demineralization solution comprises 0.1 to 1.0 N HCl, most preferably 0.3 N HCl. Once the sheet has been machined and partially demineralized, it may be stored prior to insertion. In various embodiments, irradiated cortical bone sheet allografts 5 may be sized from about 0.5 mm to 1.5 mm in thickness, 10 mm to 40 mm in width, and 10 mm to 40 mm in length. The irradiated cortical bone sheet allograft 5 is designed as a barrier, scaffolding matrix, or both, when utilized in a guided bone regeneration surgical application. For example, within a surgical site in the body, a surgeon may place an irradiated cortical bone sheet allograft 5 together with other osteo-integration material at a graft site. The irradiated cortical bone sheet allograft will guide the osteo-integration material by slowing the regeneration process of bone, and stop the ingrowth of soft tissue into the graft site. In one embodiment, the irradiated cortical bone sheet allograft may be formed in a tunnel shape to create a matrix, and the irradiated cortical bone sheet allograft used as a barrier to stop soft tissue growth into the graft site. In one embodiment, the irradiated cortical bone sheet allograft 5 may be positioned at a surgical site to create a package into which platelet rich plasma (PRP) is positioned within the barrier formed by the irradiated cortical bone sheet allograft 5. In another embodiment, the irradiated cortical bone sheet allograft 5 may be positioned at a surgical site near a native bone that has been de-corticalized. Removal of the cortical portion of the native bone causes blood and other substances to well up into an enclosed region formed by the irradiated cortical bone sheet allograft 5. This may provide better soft tissue healing at the surgical site. A fully demineralized sheet of allograft bone may resorb at a surgical site faster than a partially demineralized sheet of bone (i.e., the irradiated cortical bone sheet allograft 5.) For some surgical applications, slower resorbing of the bone is a positive aspect to allow enough time for ingrowth of other implanted particulate material. The partially demineralized physical properties and mechanical aspects of the irradiated cortical bone sheet allograft are also very different from those of a fully demineralized sheet of allograft bone. As the sheets are not fully demineralized, there is little or no shrinkage and the initially cut sheets provide a more predictable dimensional tailoring of the final bone sheet. The bone sheets are described hereinabove as generally square or rectangular faces forming a cuboid. However, other three dimensional “sheets” may be constructed with a substantially thin thickness in comparison to larger widths and lengths. Irradiation preserves the irradiated cortical bone sheet allograft 5. This allows storage of the irradiated cortical bone sheet allograft 5 in ambient air (within a sealed pouch 15) rather than in a controlled temperature environment. The irradiated cortical bone sheet allograft 5 may be produced more cost effectively than other types of cortical, cortical cancellous, or cancellous bone implants. The irradiated cortical bone sheet allograft are less expensive to produce because of the elimination or reduction of the cost of demineralization, dehydration, or a combination of each of these processes. The irradiated cortical bone sheet allograft 5 also eliminates the cost of a surgeon having to harvest a sheet of bone (i.e., an irradiated cortical bone sheet allograft) from a patient. The advantages of allografts include ready availability, elimination of the need for a patient donor site, reduced anesthesia and surgical time, decreased blood loss, and fewer complications. The disadvantages of allografts are primarily associated with the antigenicity of tissues harvested from another individual. Irradiated cancellous bone (Rocky Mountain Tissue Bank, Denver, Colo., USA) has also been used as substitute graft material to autogenous bone. Allogeneic bone behaves similar to autologous bone in terms of creeping substitution and osseointegration of implants. Irradiation also releases growth factors for soft tissue formation. Although the above embodiments have been described in language that is specific to certain structures, elements, compositions, and methodological steps, it is to be understood that the technology defined in the appended claims is not necessarily limited to the specific structures, elements, compositions and/or steps described. Rather, the specific aspects and steps are described as forms of implementing the claimed technology. Since many embodiments of the technology can be practiced without departing from the spirit and scope of the invention, the invention resides in the claims hereinafter appended. There is disclosed an irradiated cortical bone sheet allograft. In an embodiment, the allograft includes a unitary sheet of at least partially demineralized, irradiated cortical bone having a thickness, a width, and a length. The thickness of the unitary sheet of irradiated cortical bone is less the width and the length. In another embodiment, a method of forming the allograft includes obtaining a natural bone from a donor different than a recipient. The natural bone contains a layer of a cortical bone. The method includes cleaning the natural bone to produce a unitary sheet of cortical bone. The method includes at least partially demineralizing the unitary sheet of cortical bone. The method includes freezing the unitary into a frozen state within a sealed package. The method includes irradiating the unitary sheet in the frozen state within the sealed package to sterilize the cortical bone and produce the irradiated cortical bone sheet for implantation in the recipient other than the donor. Other embodiments are also disclosed. 1. An irradiated cortical bone sheet allograft product, the allograft product comprising:
a unitary sheet of at least partially demineralized, irradiated cortical bone having a given thickness, a given width, and a given length, wherein the given thickness of the unitary sheet of at least partially demineralized, irradiated cortical bone is less than each one of the given width and the given length, and the unitary sheet of at least partially demineralized, irradiated cortical bone being packaged in a sterile environment. 2. The allograft product of 3. The allograft product of 4. The allograft product of 5. The allograft product of 6. The allograft product of 7. The allograft product of 8. The allograft product of 9. The allograft product of 10. The allograft product of 11. The allograft product of 12. The allograft product of 13. The allograft product of 14. An irradiated cortical bone sheet allograft product, the allograft comprising:
a unitary sheet of at least partially demineralized, irradiated cortical bone packaged with a liquid carrier in a sterile environment at room temperature. 15. The allograft product of 16. The allograft product of 17. The allograft product of 18. The allograft product of 19. The allograft product of 20. The allograft product of 21. A method of forming an irradiated cortical bone sheet allograft, the method comprising:
obtaining a natural bone from a donor different than a recipient, the natural bone containing a layer of a cortical bone; cleaning the natural bone to produce a unitary sheet of cortical bone; at least partially demineralizing the unitary sheet of cortical bone; freezing the unitary sheet of cortical bone into a frozen state within a sealed package; and irradiating the unitary sheet of cortical bone in the frozen state within the sealed package to sterilize the unitary sheet of cortical bone and producing the irradiated cortical bone sheet for implantation in the recipient other than the donor. 22. The method of 23. The method of 24. The method of 25. The method of 26. The method of 27. The method of 28. The method of 29. The method of 30. The method of 31. The method of 32. The method of REFERENCE TO PENDING PRIOR PATENT APPLICATION
BACKGROUND
SUMMARY
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION