MIXED HYDROPHILIC/HYDROPHOBIC FIBER MEDIA FOR LIQUID-LIQUID COALESCENCE
This patent application is a divisional application of, and claims the benefit and priority date of, U.S. patent application Ser. No. 12/655,823, filed Jan. 7, 2010, which claims the benefit and priority of U.S. provisional application 61/144,226, filed Jan. 13, 2009, which are both hereby fully incorporated by reference. The present invention relates to the wettability of an immiscible liquid (e.g. emulsion) such as water in a continuous phase liquid such as oil utilizing a filter that has significant influence on the water removal efficiency. Wettability is an important parameter in designing such filter media. The wettability of the filter media is mainly governed by surface properties of fiber material and porosity of filter. The surface properties of filter can be expressed in terms of hydrophilic or hydrophobic nature of the filter. The wettability of the filter can be characterized using the concept of Lipophilic to Hydrophilic ratio (L/H) by using a modified Washburn equation that is based on capillary rise phenomena. Oil and water are used as reference liquids in the wettability characterization. In liquid-liquid coalescence filtration, separation efficiency depends on various factors including face velocity, fiber structures, fiber geometry, fiber orientations, etc., and also wettability of filter especially when interfacial tension between liquid phases is low. The hydrophilic and hydrophobic fibers used in the filter capture the immiscible liquid and form drops on the hydrophilic material that stay on the fiber surface for extended periods of time. Fibers having varying hydrophilic and hydrophobic properties can be mixed into filter media, so that the hydrophobic fibers will aid in drop migration towards the hydrophilic fibers and the formation of large drops on the hydrophilic surface. Large drops are desired for coalescence and drainage. The hydrophilic/hydrophobic filters can be utilized in the petrochemical industry as well as for fuels for vehicles including automobiles, planes, trains, and ships. In recent years, separation of water-in-oil emulsions has become industrially important. Water present in liquid fuels can combine with chemicals in fuels, such as sulfur, to form corrosive compounds which can damage sensitive engine parts. Corrosion is a major cause of reduction of engine life and efficiency. In addition, surfactants present in liquid fuels lower the interfacial tension between water and fuel and the problem of separation becomes more insidious. This can also cause a product to be off-specification due to haze and color [1, 2]. The water is present in fuels as primary emulsions with drop sizes greater than 100 μm and as secondary emulsions with drop sizes less than 100 μm. The separation of primary emulsions is often accomplished by gravity settling or mechanical means. However, coalescence filtration using fibrous filters is an efficient and economical way to separate secondary dispersions. The coalescence process occurs in three main steps. First, the fibrous bed captures water droplets. Second, the collected water phase migrates through the bed and coalesces. Third, the enlarged droplets are released from the fiber surfaces. Coalescer performance is generally characterized by separation efficiency and pressure drop. The separation efficiency is highly dependent on the characteristic properties of the dispersions (e.g. composition, density, viscosity drop size) and the fiber bed (material, diameter, surface structure, porosity). Flow rate is an important factor in water-in-oil dispersion flow, as it controls the capture mechanism and capture probability of droplets and the distribution of the dispersed phase.. Shin shows that wettability of the surface has effect on drop attachment on silane coated glass rods. It is known that critical surface tension of a solid to the liquid surface tension determines the character of solid wettability. Wettability of filter medium can be represented by its hydrophobic or hydrophilic behavior. Moorthy performed the coalescence experiments with surface functionalized filter media and showed that intermediate wettability gives better performance. Research results and common experience in industry show that coalescing filters work best with an intermediate wettability. If the medium is too wetting it tends to load up with liquid drops which restricts the air flow and results in an undesired high pressure drop. If the medium is too non-wetting the droplets captured on the fibers quickly move along the fibers or leave the fibers before they have a chance to coalesce and hence the separation is not efficient. Common practice to control the wettability is by selecting a material from which the fibers are made that has an intermediate wettability, or by applying a coating, such as silanes, that gives the surface of the fiber structures the intermediate wettability. The difficulty here is finding the right material or coating that has the best wettability for a given application. This approach does not allow for incremental changes in wettability. The above-noted references as well as others are as follows:
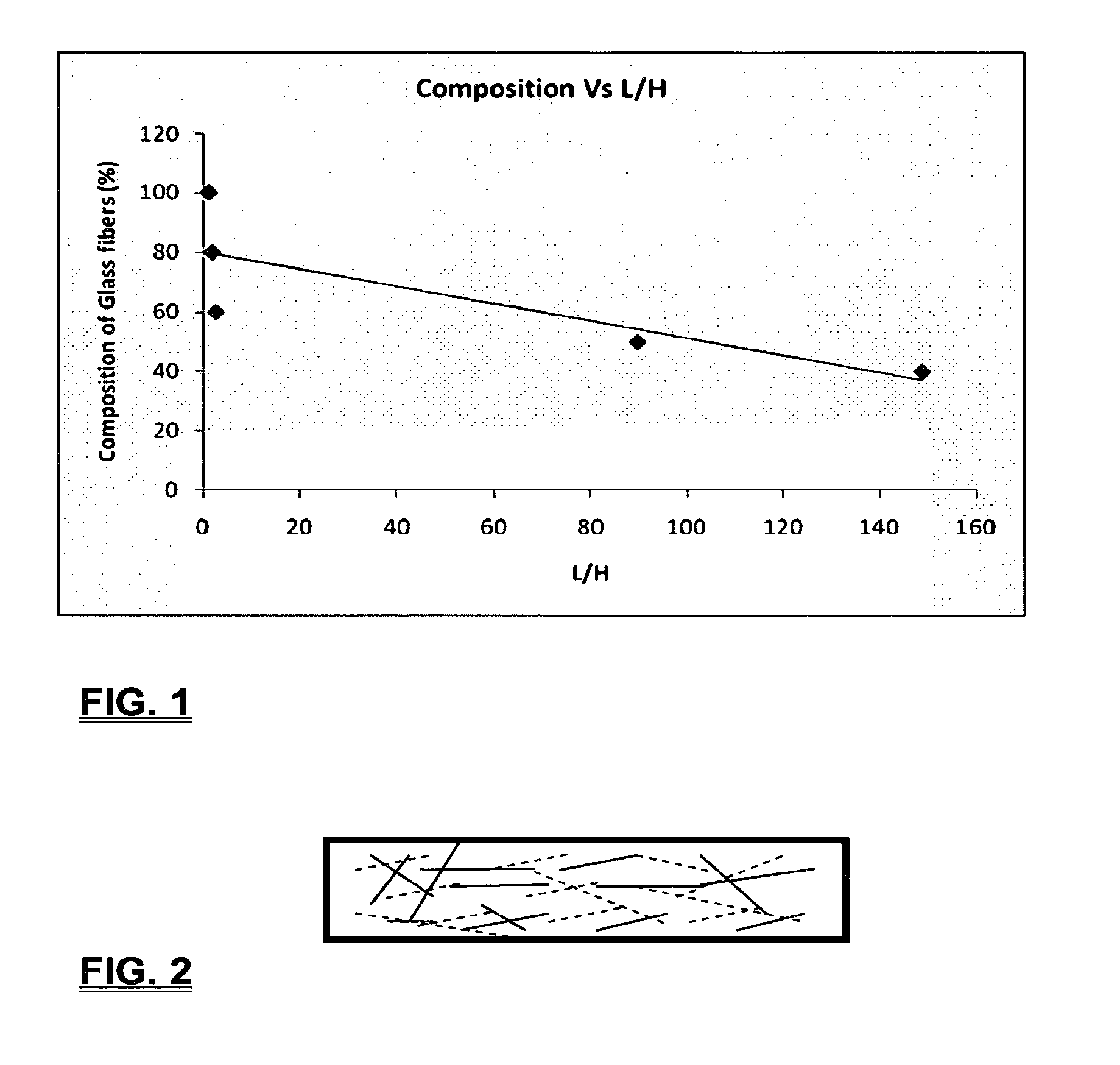
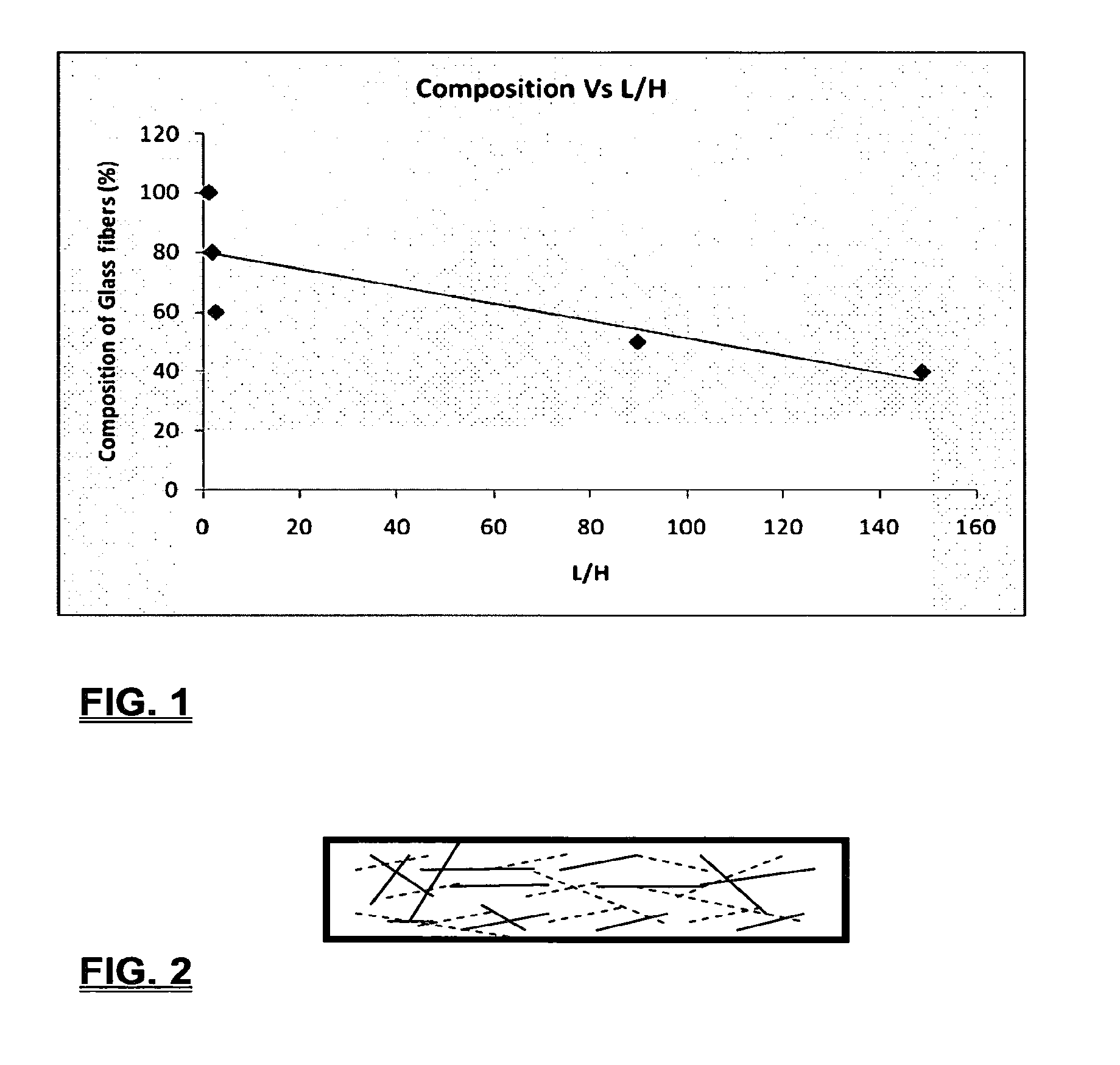
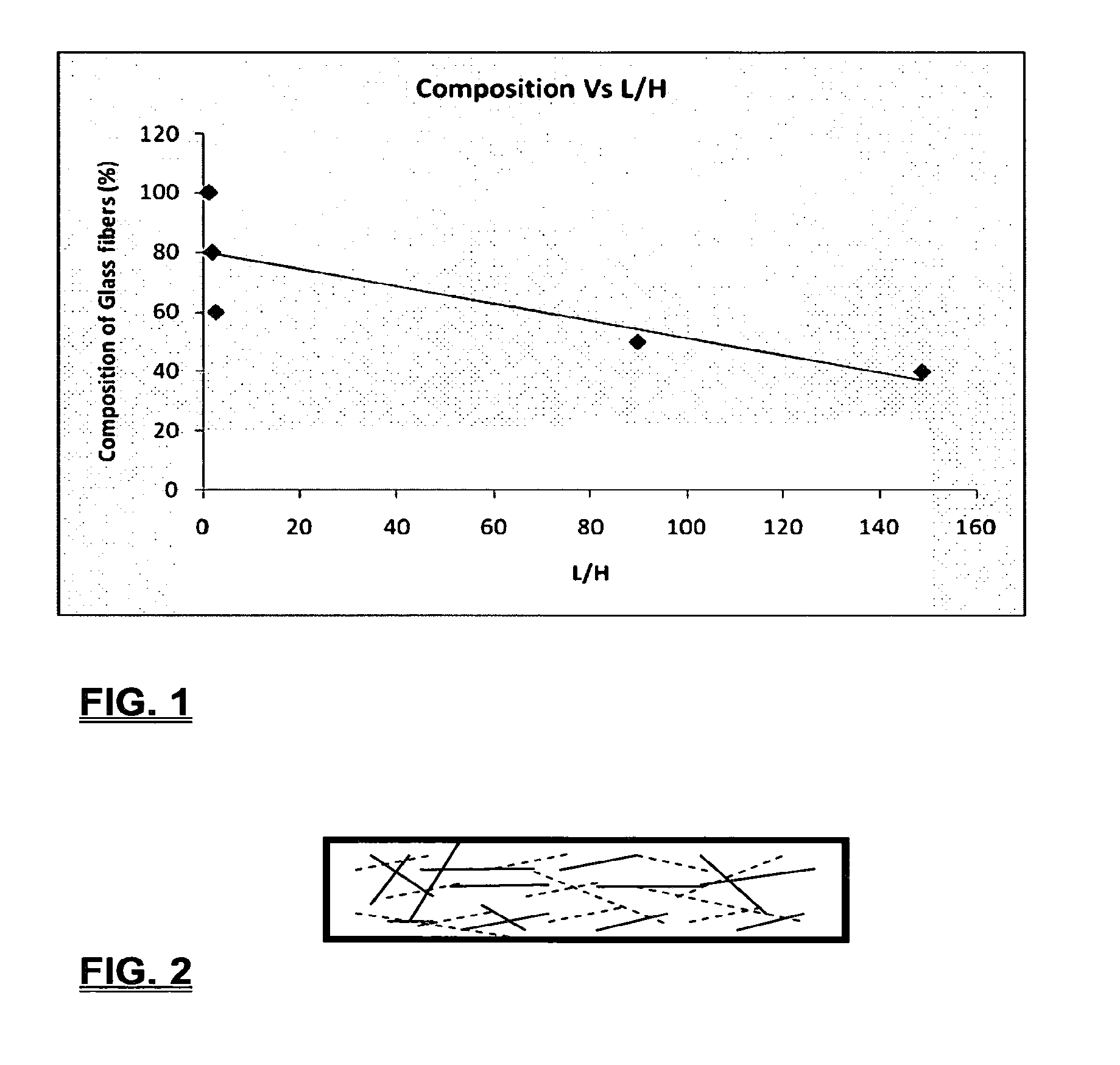
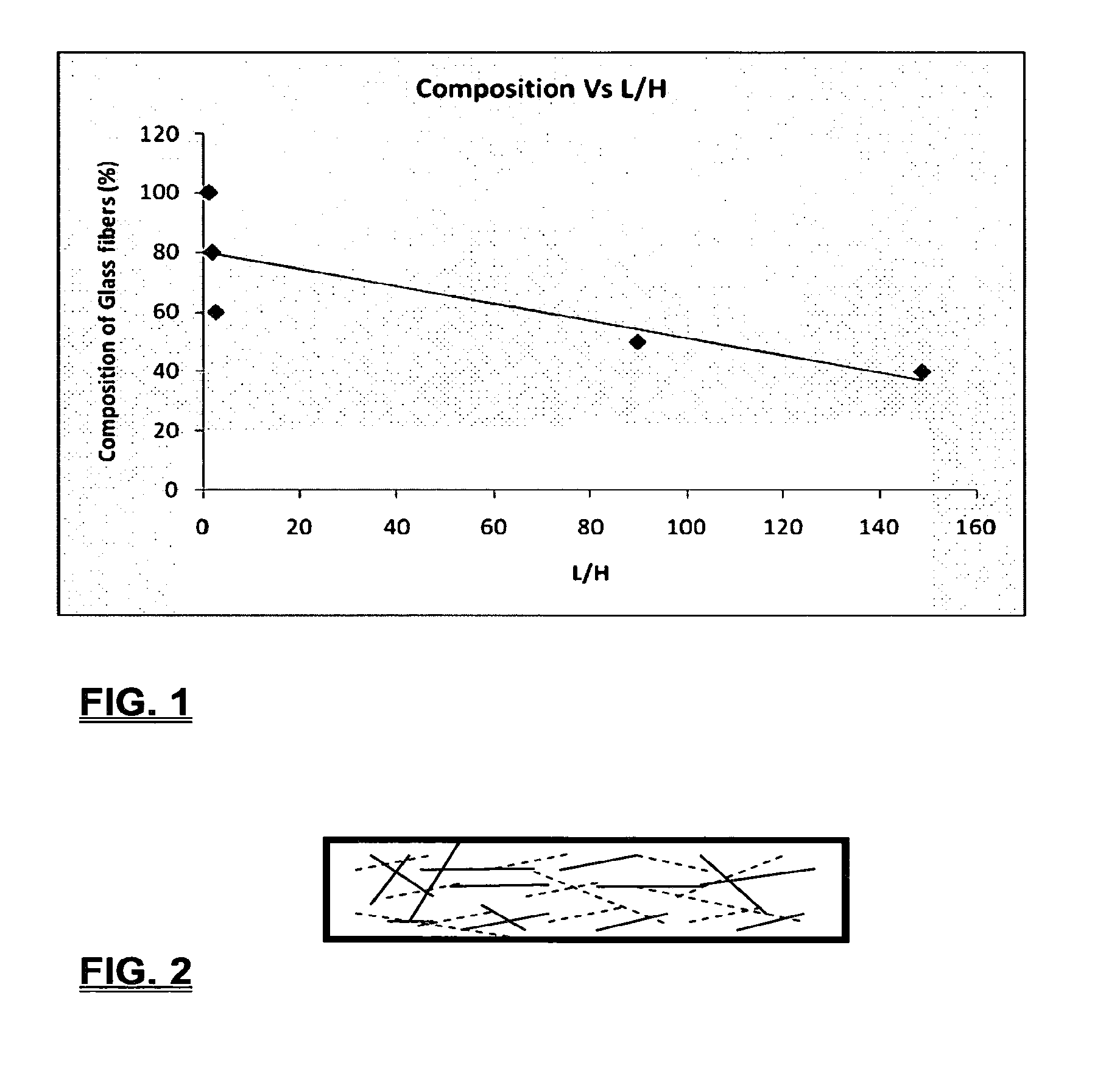
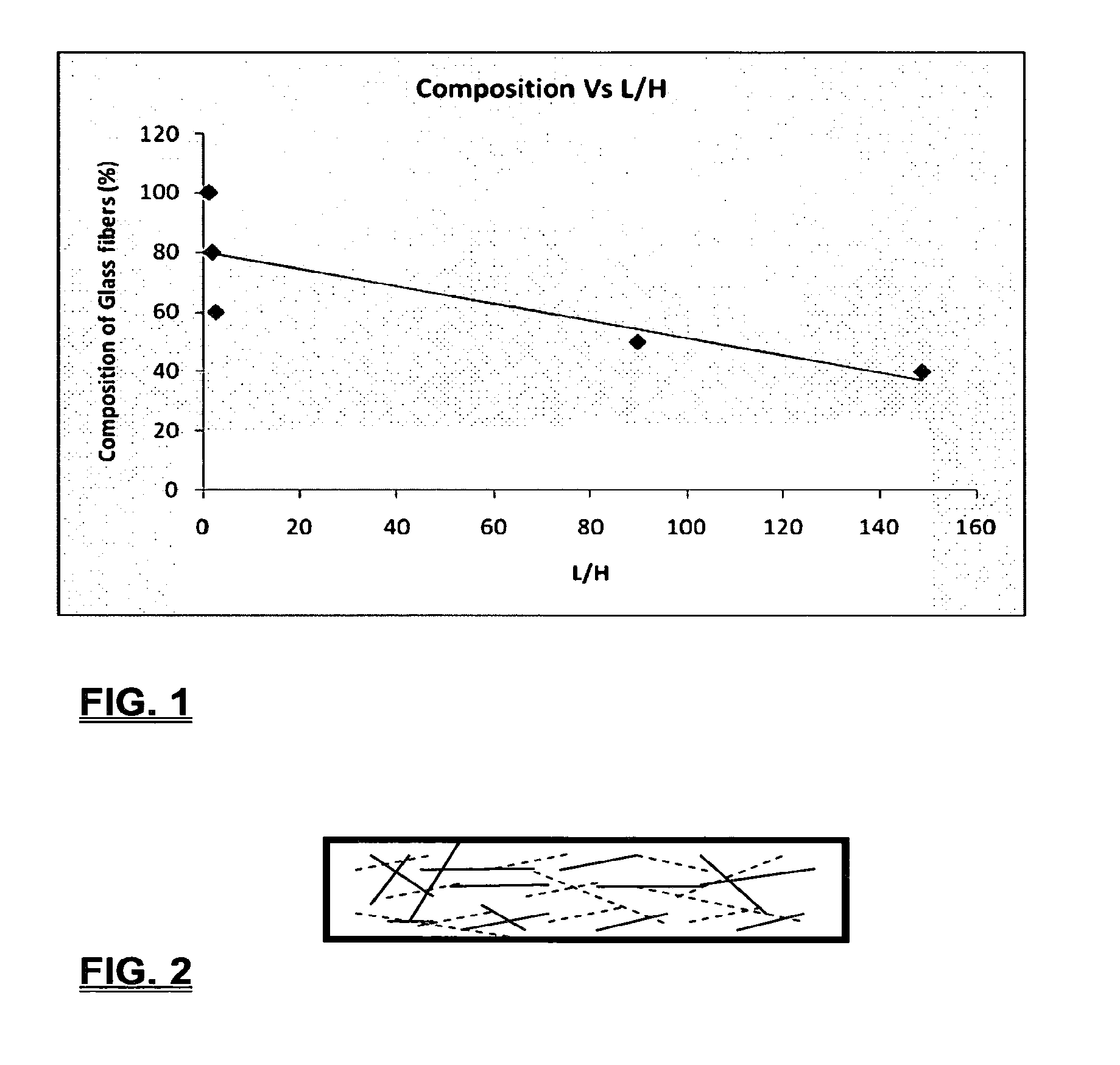
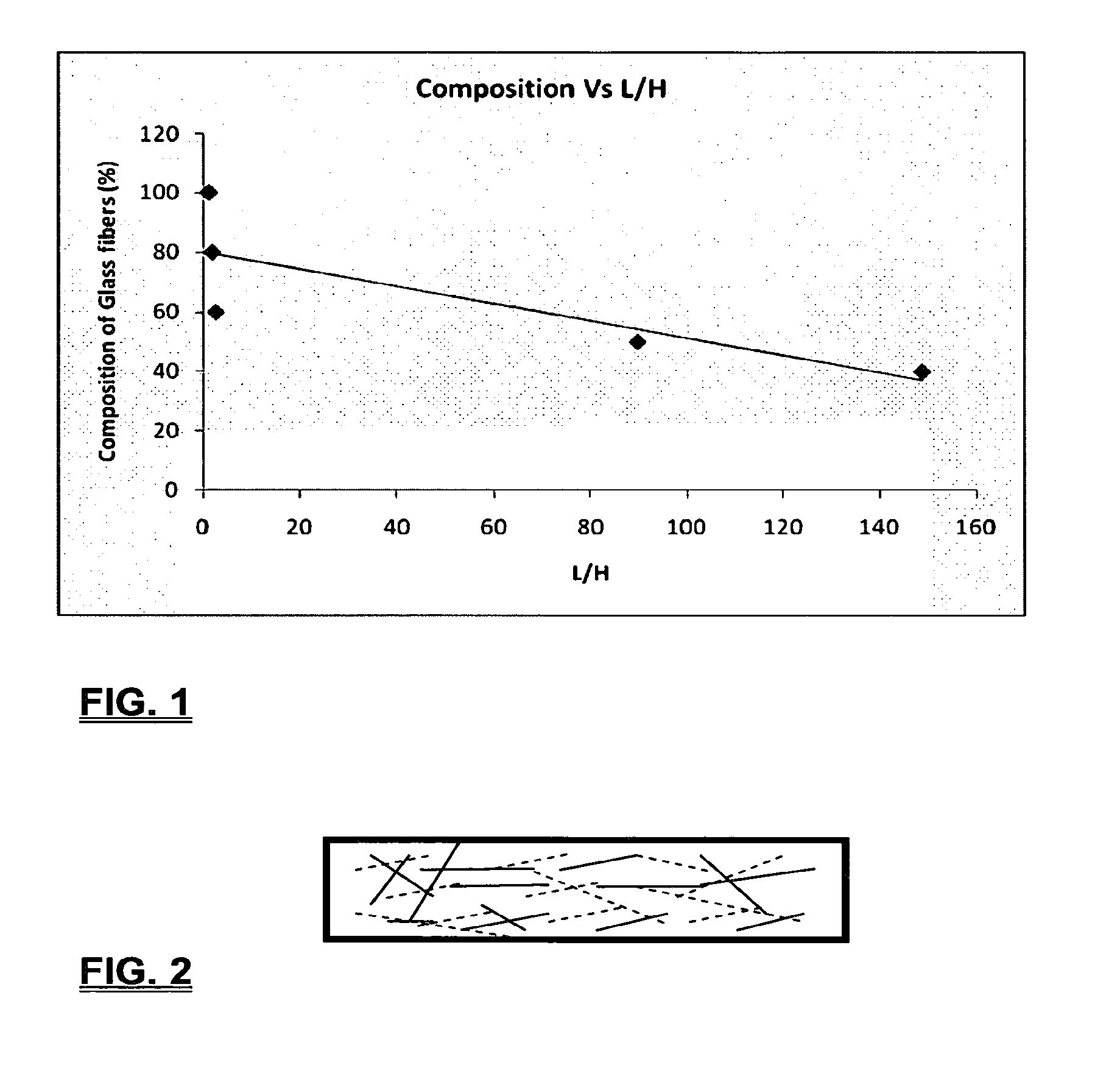
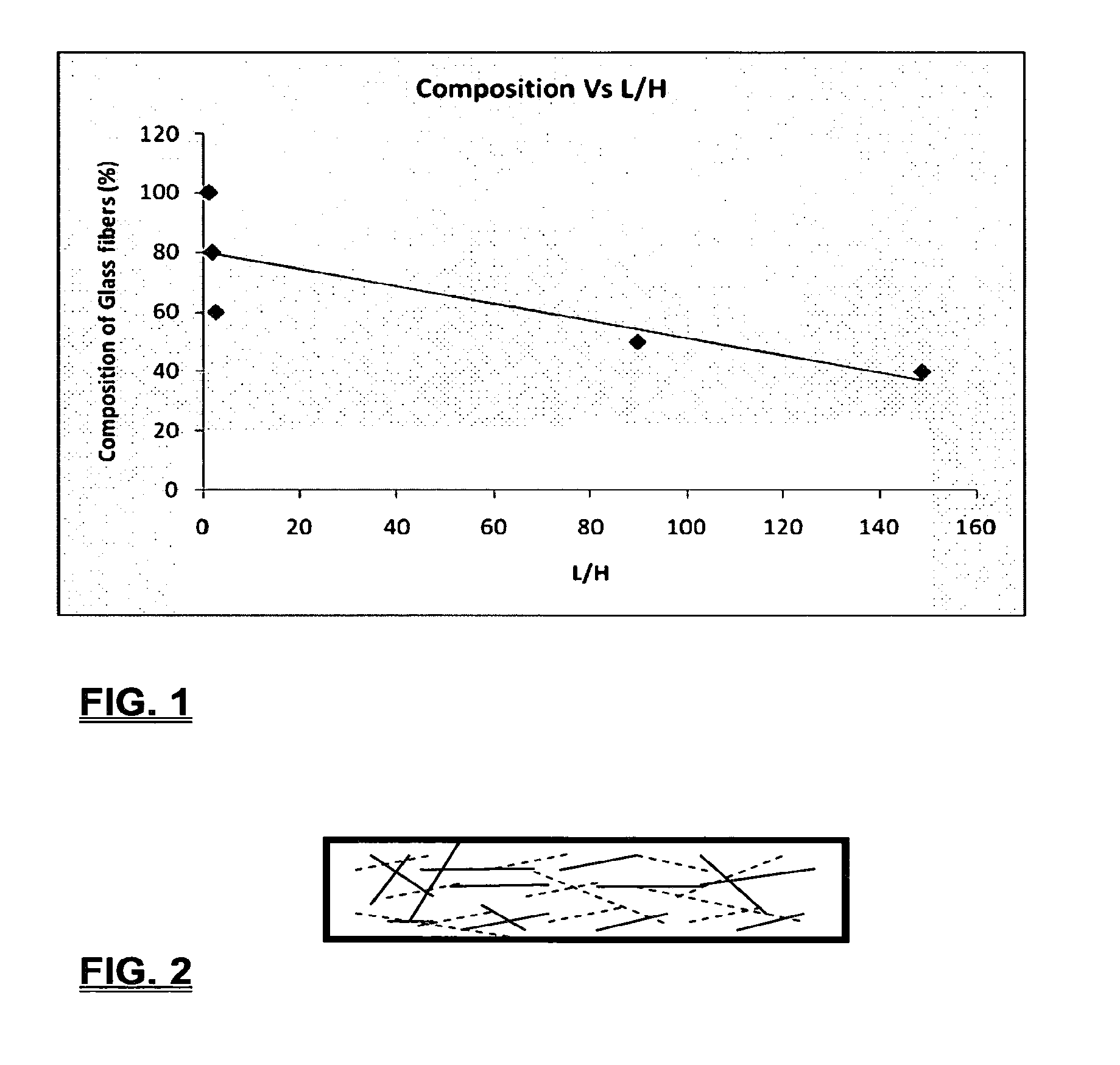
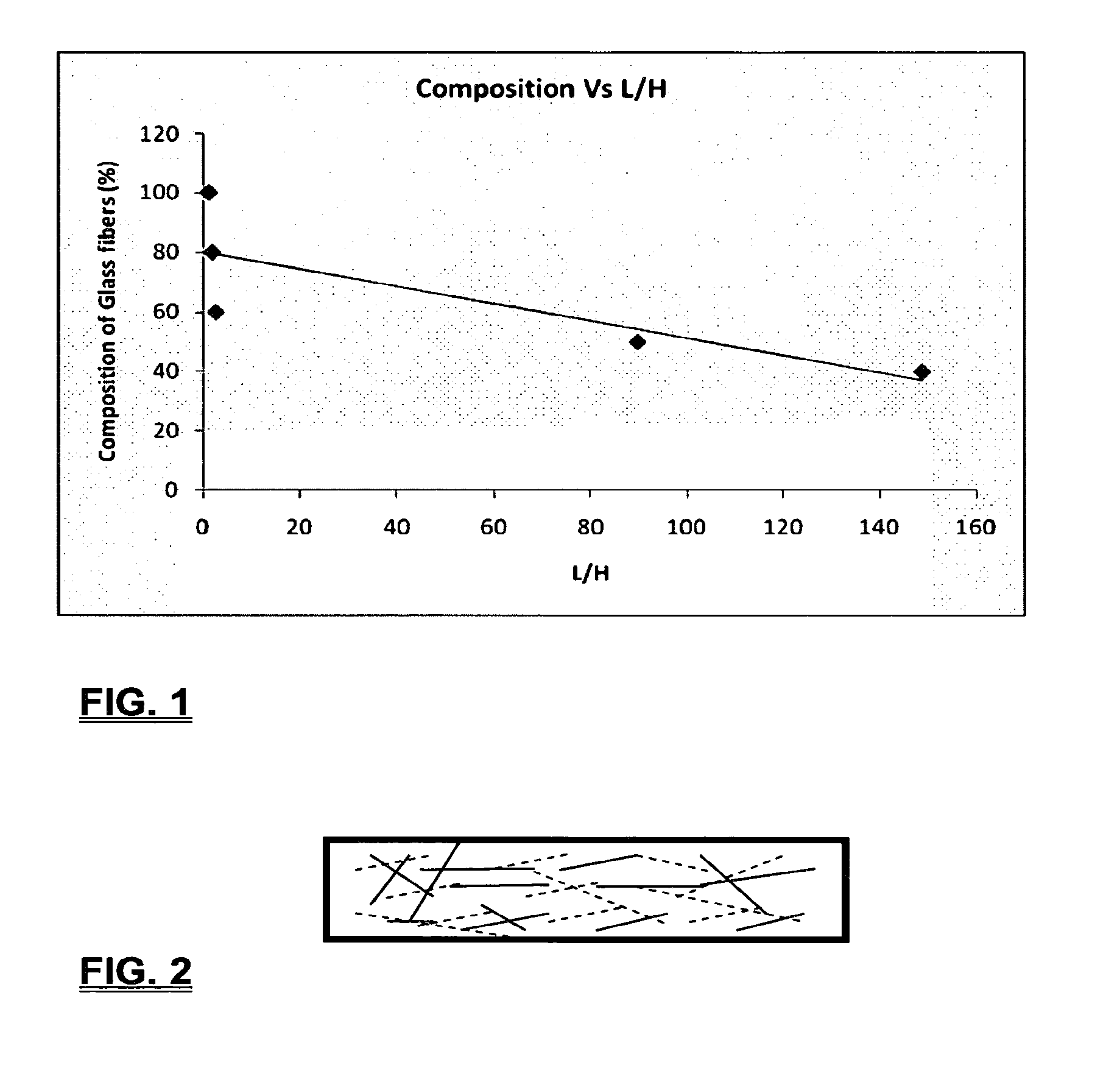
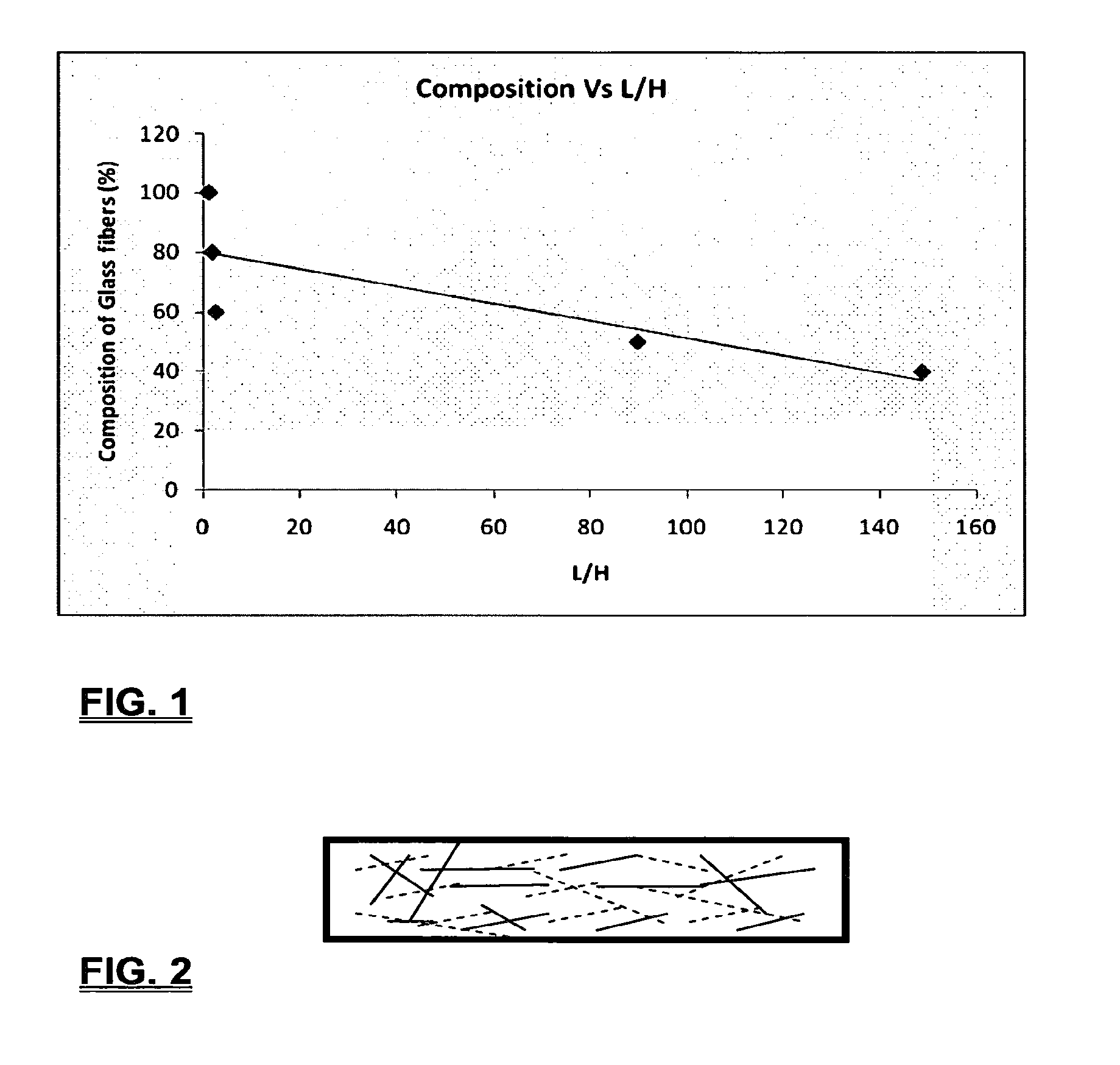
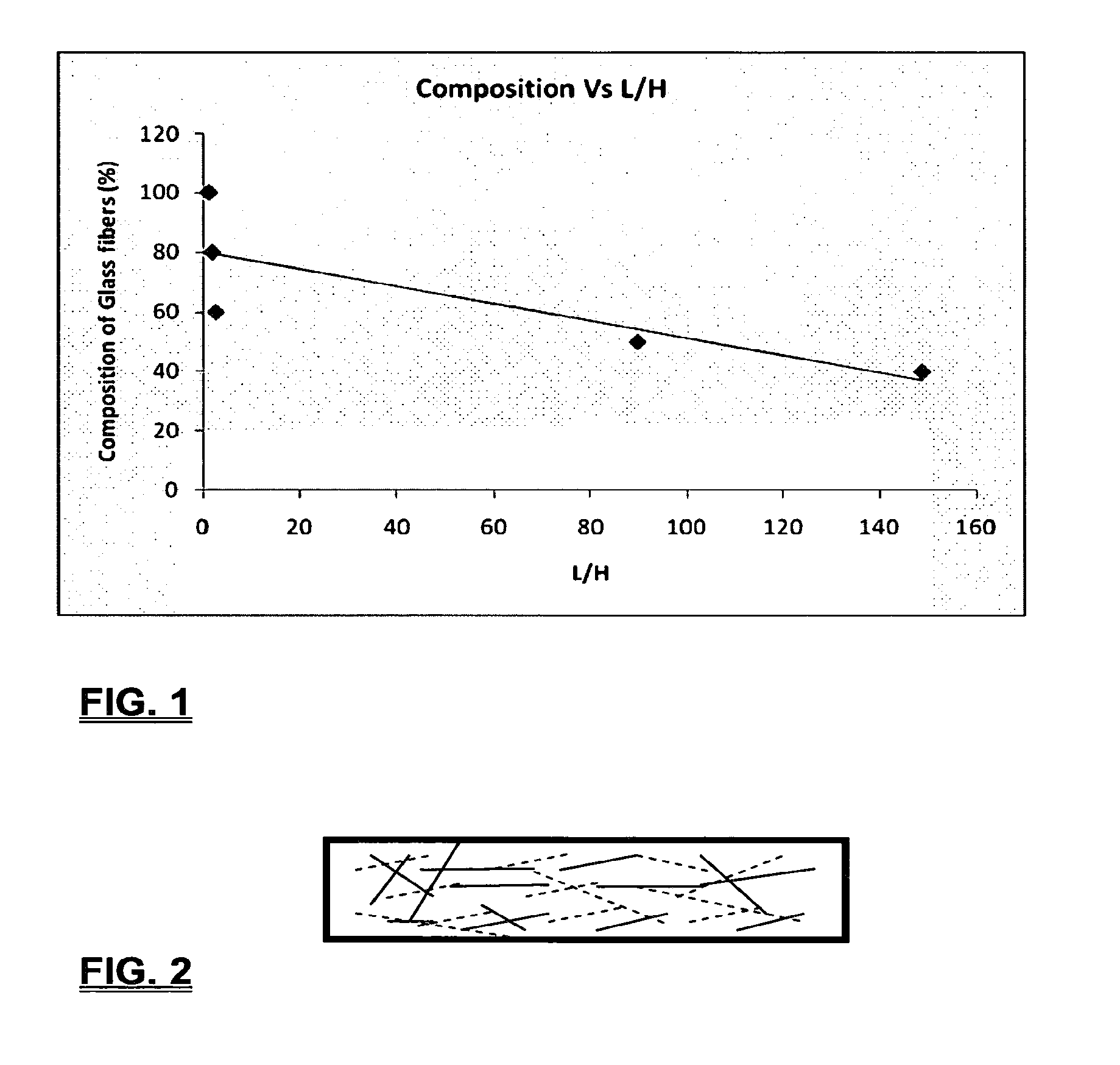
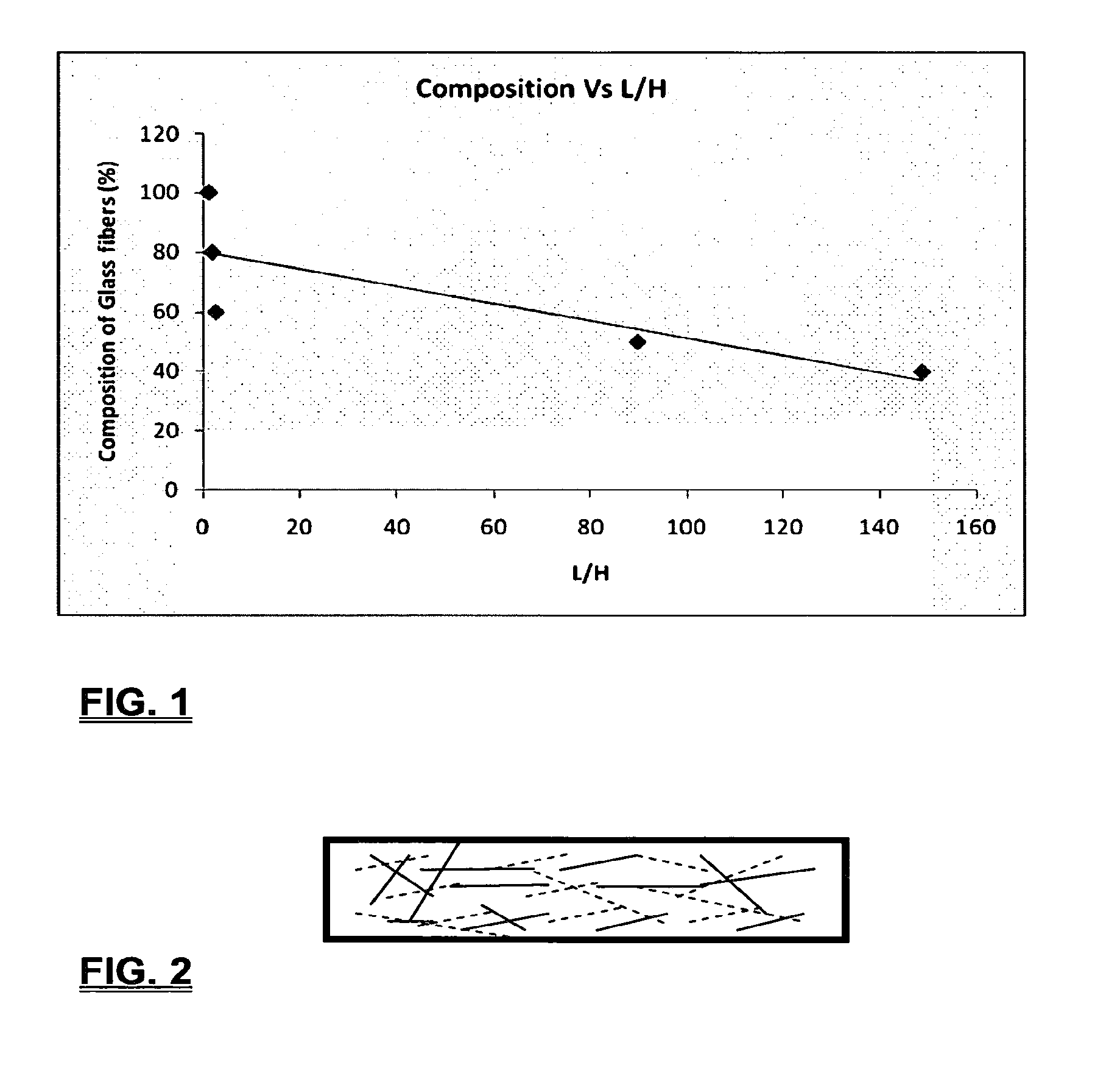
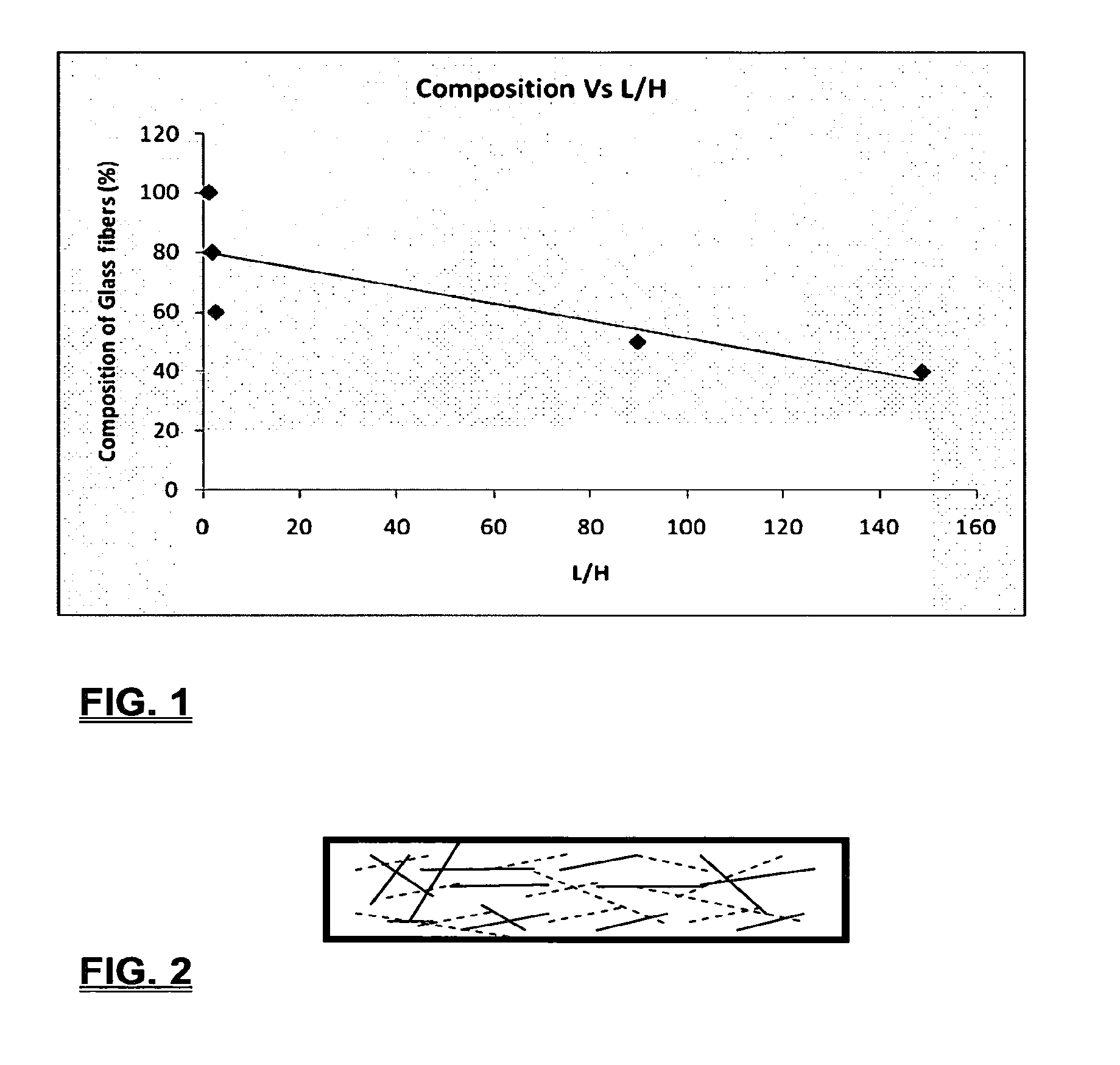
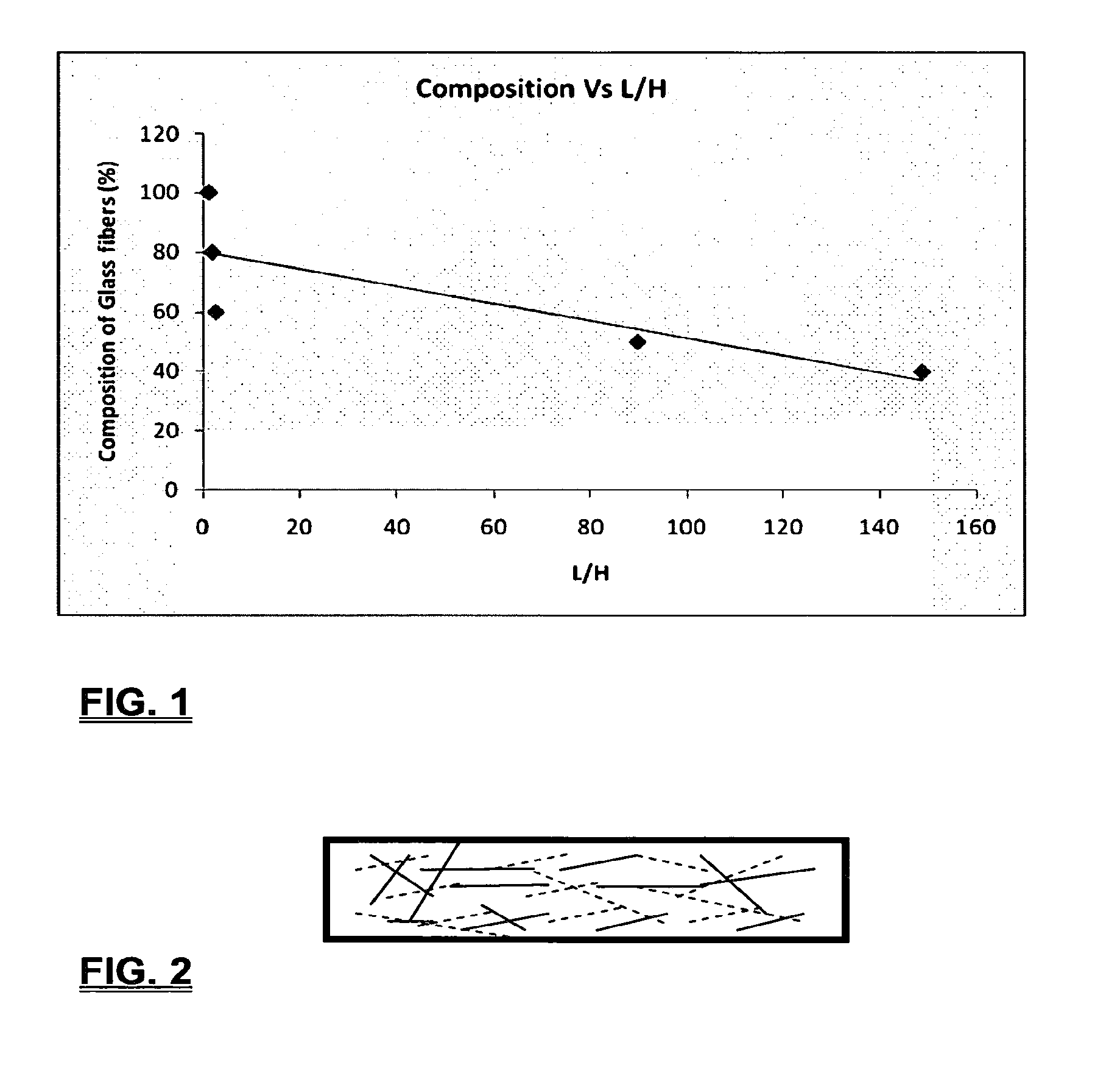
Coalescing filters are used to remove small liquid droplets from immiscible liquids and also gases. The droplets are carried into the filter by the flow of the continuous phase where the droplets collide with fine fibers. The droplets are captured on the fibers, coalesce to form larger drops, and the larger drops migrate to the exit surface of the medium. The larger drops are subsequently separated from the gas as by gravity settling. An aspect of the present invention is to develop filters that can vary with respect to the wettability values thereof by using the different micron sized fibers with hydrophilic and hydrophobic properties. Wettability of a liquid on a flat surface can be related to contact angle and surface tension (or surface energy). Liquids on a high surface energy material have low contact angles (approaching zero) and tend to spread across the surface. Low surface energy materials have high contact angles in the range of from about 90 to about 180 degrees. Polypropylene fibers were selected as hydrophobic fibers (contact angle with water) ˜104°) and micro glass fibers are the hydrophilic fibers (contact angle with water ˜0°). The filter media has been prepared with different compositions of micro glass and short cut polypropylene fibers. The filter media has been also characterized for their permeability and porosity and effect on wettability. Measurements of wettability of porous materials such as filter media are not trivial. The size and shape of the pores tend to deform droplets and hence the method of measuring contact angles does not work. Washburn describes a capillary rise method for liquid uptake in a porous medium that is a function of the wettability (surface energy) (Washburn E. W, “The dynamics of capillary flow”, The American Physical Society, Vol. XVII, No. 3, 374-375, 1921). Washburn's approach results in a measure of wettability through the Lipophilic/Hydrophilic Ratio (L/H). Small values of L/H indicate the surface prefers water to oil and visa versa for large L/H values. Mixtures of glass fibers and polypropylene fibers show that we can control the L/H value by controlling the mixture composition of glass and polypropylene fibers when constructing a filter medium. The concept of mixing fibers of different surface properties to obtain a specific L/H value to control the coalescence properties of the filter medium is an important aspect of this invention. An embodiment of the present invention relates to a process for removing an immiscible lipophilic or a hydrophilic liquid respectively from a continuous hydrophilic or a lipophilic liquid phase, comprising the steps of: 1) forming a filter comprising a specific weight ratio of hydrophobic fibers to hydrophilic fibers; 2) determining an initial slope of a weight gain take-up versus time plot of the immiscible liquid by said filter; 3) determining an initial slope of weight gain take-up versus time plot of said continuous liquid by said filter; 4) calculating an L/H ratio from said initial slope of the plot of said immiscible liquid and of said initial slope of the plot of said continuous liquid and obtaining a wettability value for each; 5) forming a plurality of filters comprising different weight ratios of said hydrophobic fibers to said hydrophilic fibers from a range of from about 90% by weight of said hydrophobic fibers to about 10% by weight, or any portion thereof, of hydrophobic fibers with the remaining weight percent being said hydrophilic fibers; repeating steps 2), 3), and 4) with respect to each weight ratio of said lipophobic fibers to said hydrophilic fibers in step 5); plotting a wettability range from said L/H wettability values obtained from said plurality of said different weight ratios of said hydrophobic fibers to said hydrophilic fibers; and utilizing a filter having a wetness value within a weight range of from about 20% to about 80% of hydrophobic fibers to hydrophilic fibers to coalesce said immiscible liquid phase within said continuous liquid phase. Another embodiment of the present invention relates to a process for removing an immiscible lipophilic or a hydrophilic liquid respectively from a continuous hydrophilic or a lipophilic liquid phase, comprising the steps of: forming a filter containing hydrophobic fibers and hydrophilic fibers; flowing said immiscible lipophilic liquid or hydrophilic liquid respectively in said continuous liquid phase hydrophilic liquid or lipophilic liquid through said filter and capturing said immiscible liquid; coalescing said captured immiscible liquid; and removing said coalesced immiscible liquid from said filter. Yet another embodiment of the present invention relates to a filter for removing an immiscible lipophilic liquid or a hydrophilic liquid respectively from a continuous hydrophilic liquid phase or a continuous lipophilic liquid phase, comprising: a plurality of hydrophobic fibers and a plurality of hydrophilic fibers, said hydrophilic fibers having a wetting value and said hydrophobic fibers having a different wetting value within a liquid system comprising the immiscible lipophilic liquid or the immiscible hydrophilic liquid and respectively the continuous hydrophilic liquid phase or the continuous lipophilic continuous liquid phase; the weight ratio amount of said hydrophobic fibers to said hydrophilic fibers being within a range of from about 80% to about 20% by weight with the remaining weight percent being said hydrophilic fibers and said fiber weight ratio amount being an effective amount to coalesce an immiscible liquid within a continuous liquid phase; and the filter being capable of removing an immiscible liquid from a continuous liquid phase. The filters of the present invention can exist in many sizes, shapes and forms. The one or more hydrophobic fibers and the one or more hydrophilic fibers can generally either be mixed, or exist in separate layers. When mixed, the hydrophilic fibers and the hydrophobic-fiber types are blended so that within a small sample volume of the filter both types of fibers are present such as indicated in An alternative method to make the filter with control over the L/H ratio is by utilizing thin layers of fibers of different types. This is shown in Numerous types of hydrophobic fibers can be utilized so long as they are inert to the solution or gas they are treating. Hydrophobic fibers generally include polymers such as polyethylene, polypropylene, nomex, polyester such as polyethylene terephthalate, halogen-containing polymers such as Teflon and poly (vinyl chloride), various rubbers including natural rubber, polyisoprene, and polymers derived from butadiene, polyurethanes, polycarbonates, and silicone polymers. Hydrophobic fibers also include various minerals such as zinc oxide, for example zinc oxide nano-rods that are superhydrophobic, and the like. Still additional hydrophobic fibers include various fibers that contain coatings thereon such as various silanes such as (3-aminopropyltriethoxysilane) APTS, (2-carboxymethylthio)ethyltrimethylsilane) CES, and (heptadecafluoro-1,1,2,3-tetrahydrodecyl)trichlorosilane FTS. Examples of hydrophilic fibers include various types of glasses including sodium glass, boron glass, phosphate glass, B-glass and the like, various minerals such as alumina, titania, and silica, various metals such as aluminum and alloys thereof, various polymers such as cellulose acetate, poly(methylmethacrylate), polyethylene oxide, nylon, and the like. In general, polymers that absorb or swell with water are examples of hydrophilic polymers. The hydrophobic fibers are generally distinguished from the hydrophilic fibers generally with regard to their wettability, that is, their ability to hold water. Various tests or methods can be utilized such as the contact angle of water located on a flat surface of the fiber composition. Contact angles less than about 90 degrees or less and generally 20 degrees or less are generally considered to be hydrophilic, whereas contact angles greater than about 90 degrees and generally at least about 120 degrees or greater are considered to be hydrophobic. The one or more hydrophobic and the one or more hydrophilic fibers, of the present invention, independently, can have various thicknesses such as diameters as from about 0.1 to about 500 microns, desirably from about 0.5 to about 50 microns, and preferably from about 1 to about 10 microns. For this application the fiber diameters are generally about the same so that the pore sizes are about the same throughout the medium. Depending upon the type of fiber, they can generally have smooth surfaces or contain some pores. In general the internal pore structure affects the fiber wettability and is characterized by its contact angle and its performance is characterized by the L/H ratio. Because we are generally characterizing the L/H ratio the characterization of the internal pore structure of the fibers is not essential. It is an important aspect of the present invention that at least one hydrophobic fiber be utilized and that at least one hydrophilic fiber be utilized. That is, the present invention is free of any filters that essentially contain only one type of fiber such as only one hydrophobic-type fiber and no hydrophilic fiber or only one hydrophilic-type of fiber and no hydrophilic fiber. Thus, filters that essentially contain only one type of fiber are excluded from the present invention such as filters that contain small amounts of a second fiber, for exampled less than 5% by weight of a second philic fiber, for example a hydrophilic fiber, or less than about 3% by weight, or less than about 2% by weight, or no amount of a second different type of philic fiber. The reasoning is as set forth hereinabove as well as herein below that the utilization of at least one type of hydrophilic fiber and at least one type of hydrophobic fiber has been found to yield improved and efficient results with regard to removing an immiscible phase from a continuous phase liquid solution. An important aspect of the present invention is the determination of the wettability value of the filter per se so that proper amounts of hydrophobic and hydrophilic fibers can be utilized that will result in efficient removal of the immiscible liquid or gas from the continuous liquid phase with low pressure drops since high pressure drops can result in expensive pumping cost. That is, high amounts of the immiscible fluids such as water in oil can result in high water saturation on the hydrophilic fibers that reduces porosity and permeability of the filter with the subsequent low porosity leading to excessive pressure drops. An additional disadvantage of high pressure is that high shearing forces within the filter can cause droplet breakup and re-entrainment. Also, high pressure drops result in large forces acting on the filter (pressure drop times filter area) and can cause the filter to collapse, deform, or loose integrity and hence render the filter useless. On the other hand, if the filter overall is too hydrophobic, there will be little or no coalescence of the immiscible fluid; the filter may capture solid particles but it would be ineffective for coalescing drops. It has been found that traditional contact angles are not suitable for use in the present invention because liquid drops will simultaneously be in contact with multiple fibers, fiber types, and the binder (if present), all of which affect the contact angle. If the fibers are too small in diameter, the capillary action on the immiscible liquid will be affected and not yield a true contact angle. Instead, a liquid penetration approach is used to measure the contact angles of filter media treating the pores of the media as a bundle of uniform capillaries. This method of the liquid penetration is based on the equilibrium capillary pressure and Washburn's equation. Washburn's equation is based on the capillary driving force of a liquid that penetrates a compact vertical bed of particles with small pores and the viscous drag. However, a modified Washburn equation has been found to be suitable. The modified Washburn equation is: Wherein So is the initial weight gain take-up slope of the penetrated lipophilic fluid or liquid such as oil, Sw is the initial weight gain take-up slope of the penetrated hydrophilic liquid, no is the viscosity of the lipophilic liquid whereas nw is the viscosity of the hydrophilic fluid. Both co and cw, are the same for a filter medium where c is a geometric constant that accounts for the effective pore diameter and the porosity. When experiments are conducted on the same medium with the organic and water liquids then cw equals co and cancel out of the equation whereas ρw is the density of the hydrophilic liquid such as water, and ρo is the density of the lipophilic liquid such as the oil. Yw is the surface tension of the hydrophilic liquid whereas the Yo is the surface tension of the lipophilic liquid. In order to find the L/H ratio, the slope and hence the amount of take-up of the lipophilic liquid and the hydrophobic liquid must be determined. One method is as follows. Materials and Filter Media Preparation The filter samples were made of glass fibers supplied by Hollingsworth and Vose and polypropylene fibers supplied by Minifibers Inc. The slurry of fibers with desired composition was vacuum filtered onto a fine mesh screen in a mold with a hole of inside diameter 2.54 cm. The filter samples were dried and heated in oven for 2 hrs at 100° C. The filter samples were prepared with varying compositions of glass to polypropylene (PP) fibers, i.e. glass: PP of 80:20, 60:40, 50:50, and 40:60. The reference fluids used were Viscor oil 1487 (Rock Valley Oil & Chemical Company) and water. The Viscor oil 1487 is a calibration fluid and has similar properties to that of diesel fuel. Physical properties of water and Viscor oil 1487 are presented in Table 1. Wettability Technique and Approach The setup for this work is illustrated in In The tube was descended slowly with a low speed of 1.0 mm/sec. It was carefully done with several manual practices in order to get reproducible results. It was done with the extremity of the tube just touching the reference liquids. The video camera and stop watch were turned on when the filter medium touched the reference liquid to record the change in weight with time. The decline of the glass tube was ended and the liquid rose (penetrated) through the filter until it reached the top of the medium, causing an increase in weight of the cylinder. The video recording was stopped when liquid reached top of the filter medium. The decrease in weight of the reference liquid on the balance is equal to the liquid taken by the filter media. The rate of decrease in weight of reference liquids on balance was measured until the liquid reached top of the filter. The experimental data was obtained from the recorded video. The weight of liquid raised in the filter media can be obtained for any instant of time until the liquid reaches the top of the filter. Once the weight gain take-up of the filter has been determined with regard to the lipophilic fluid or liquid, such as an oil, and once it also has been obtained with regard to the hydrophilic liquid such as water, charts of the weight take-up versus time are plotted as shown in Also plotted in The above scenario will now be discussed with regard to a specific immiscible liquid, i.e. water and a continuous phase oil, as well as specific amounts of hydrophilic fibers such as glass and hydrophobic fibers such as polypropylene. Once a specific lipophobic-hydrophilic system has been analyzed with regard to removal of an immiscible component thereof, other weight ratios of hydrophobic fibers to hydrophilic fiber systems can be analyzed in the same manner as set forth above to determine what types of hydrophobic fiber hydrophilic fiber system are the most efficient. That is, the above steps as for example set forth in For example, Table 2 sets for L/H values obtained for layers of hydrophobic/hydrophilic fiber media such as those set forth above utilizing water and Viscor Oil 1487. That is, the various hydrophobic/hydrophilic fiber systems were made containing the fiber ratios as set forth in Table 2 and the L/H values calculated. For the above-noted immiscible water-continuous oil system, a ratio of 80% by weight of glass fibers to 20% by weight of propylene fibers yielded a value of 7.021. When tested, as set forth in Table 3, this ratio gave a good efficiency of water coalescence of 0.91 and a low pressure drop of 18.34 resulting in a quality factor or 0.132 that was very good. Table 4 relates to L/H values for glass fiber filters containing a binder thereon whereas Table 5 relates to L/H values for mixed (i.e. non layered) hydrophilic/hydrophobic fiber filters. Thus, utilizing the above procedures, different immiscible liquid-continuous liquid phase systems can be tested and filters designed to yield high amount of take up of the immiscible liquid with a fairly low amount of a pressure drop. In summary, the above procedures of the present invention relate to the extraction of an immiscible liquid from a different continuous phase liquid in a filter essentially by three steps; that of capture, coalescence, and removal. As the liquid system moves through the filter, small droplets of an immiscible fluid, such as water, attach and adhere to a hydrophilic fiber such as glass. Continued flow of the liquid system results in additional water build up on the hydrophilic fibers. That is, immiscible water is coalesced into larger droplets. Finally, a droplet size is reached such that it no longer adheres to the hydrophilic fiber due to the flow of the liquid system but detaches itself. The size of the large droplet will naturally vary with regard to the immiscible fluid be it a hydrophilic liquid such as water or hydrophobic liquid such as oil, and the wettability of the droplet on the hydrophilic fiber or hydrophobic fiber as the case may be. Generally such droplet sizes can range from about 5 to about 500 microns, and desirably within a range of from about 20 to about 100 microns. Removal of the large water droplets can be achieved by a number of methods, generally non-mechanical, such as collection of the large droplets in a gravity separator. Other collection methods include hydrocyclones, membrane separators, and absorbers. Thus, the present invention preferably is free of or does not utilize mechanism collection methods such as centrifuge, etc. Putting a coalescing filter upstream of these other devices can help the other devices to be more efficient, smaller in size, and less expensive to operate. With regard to the coalescence filters of the present invention, other preparation factors include fluid velocity, fiber structure, fiber geometry, surface properties, fluid properties, and bed length (determines the filter efficiency). Liquid-liquid coalescence wettability of the fibers is also known to have effect on filter performance, especially when interfacial tension between phases is low. Wettability of fibers can be defined as ability of filter fibers to hold water. Wettability also depends on surface properties of fibers and porosity of the filter. An optional aspect of the present invention is to utilize coating agents on the fibers such as silanes for making hydrophobic surfaces. As above noted, Immiscible liquids include oil and water, produced water, fuels (diesel, gasoline, jet fuel) and water, Complete immiscibility is rare (some water is found in the oil phase and some oil in the water) but for the purposes of this patent it is sufficient that two or more distinctive liquid phases form. Perry's handbook (R. H. Perry, D. W. Green, J. O. Maloney, Perry's Chemical Engineer's Handbook, 6th ed, McGraw-Hill, NY 1984, pages 15-9 thru 15-13) a table of solvents used in liquid-liquid extraction gives an extensive list of two liquid phase systems (solvent A and solvent S). These tables are hereby fully incorporated by reference. However, they are also reproduced as Table 6. While in accordance with the patent statutes the best mode and preferred embodiment have been set forth, the scope of the invention is not intended to be limited thereto, but only by the scope of the attached claims. An immiscible lipophilic or hydrophilic liquid phase is separated respectively from a continuous hydrophilic or a lipophilic phase liquid. Fibers having hydrophilic and hydrophobic properties are formed into a filter. The separation mechanism involves coalescence of the small droplets into larger droplets as the immiscible liquid flows through the fiber filter, and release of the large immiscible droplets from the filter. With respect to separation of a hydrophilic immiscible fluid in a lipophilic continuous fluid, the hydrophobic fibers cause small water droplets to migrate towards the hydrophilic fibers whereby large droplets are formed on hydrophilic surface. The large droplets coalescence until they are so large that they are released and drained off of the filter. The filter media can be designed by mixing hydrophilic and hydrophobic fibers in various proportions to achieve an optimum wettability range for separation of the immiscible liquid from the continuous phase liquid. 1. A process for removing an immiscible lipophilic or a hydrophilic liquid respectively from a continuous hydrophilic or a lipophilic liquid phase, comprising the steps of:
forming a filter containing hydrophobic fibers and hydrophilic fibers; flowing said immiscible lipophilic liquid or hydrophilic liquid respectively in said continuous liquid phase hydrophilic liquid or lipophilic liquid through said filter and capturing said immiscible liquid; coalescing said captured immiscible liquid; and removing said coalesced immiscible liquid from said filter. 2. The process of 3. The process of 4. The process of 5. The process of 6. The process of wherein said hydrophilic fibers comprise one or more glasses including sodium glass, boron glass, phosphate glass, or B-glass; one or more minerals including alumina, titania, or silica; one or more metals including aluminum or aluminum alloys; and one or more polymers including cellulose acetate, poly(methylmethacrylate), polyethylene oxide, or nylon. 7. The process of 8. A filter for removing an immiscible lipophilic liquid or a hydrophilic liquid respectively from a continuous hydrophilic liquid phase or a continuous lipophilic liquid phase, comprising:
a plurality of hydrophobic fibers and a plurality of hydrophilic fibers, said hydrophilic fibers having a wetting value and said hydrophobic fibers having a different wetting value within a liquid system comprising the immiscible lipophilic liquid or the immiscible hydrophilic liquid and respectively the continuous hydrophilic liquid phase or the continuous lipophilic continuous liquid phase; the weight ratio amount of said hydrophobic fibers to said hydrophilic fibers being within a range of from about 80% to about 20% by weight with the remaining weight percent being said hydrophilic fibers and said fiber weight ratio amount being an effective amount to coalesce an immiscible liquid within a continuous liquid phase; and the filter being capable of removing an immiscible liquid from a continuous liquid phase. 9. The filter of 10. The filter of 11. The filter of 12. The filter of wherein said hydrophilic fibers comprise one or more glasses including sodium glass, boron glass, phosphate glass, or 8-glass; one or more minerals including alumina, titania, or silica; one or more metals including aluminum or aluminum alloys; and one or more polymers including cellulose acetate, poly(methylmethacrylate), polyethylene oxide, or nylon. 13. The filter of CROSS-REFERENCE TO RELATED APPLICATIONS
FIELD OF THE INVENTION
BACKGROUND OF THE INVENTION
SUMMARY OF THE INVENTION
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
Physical properties of reference fluids Surface Tension Density Reference Liquid (N/m) (Kg/m3) Viscosity Viscor Oil 1487 0.0285 832 0.00207 (Ns/m2) Water 0.072 998 0.001 (Ns/m2) L/H values for layered hydrophilic/hydrophobic fiber media Composition (Glass:PP) L/H G (100) 1.830 G:PP (80:20) 7.021 G:PP (60:40) 8.652 G:PP (50:50) 17.438 G:PP (40:60) 22.879 G:PP (20:80 36.981 Liquid-liquid coalescence results Steady state Composition Pressure Drop Quality Factor (Glass:PP) L/H Efficiency (kPa) (kPa−1) 100% Glass 1.83 0.84 (±0.02) 20.39 (±3.02) 0.106 (with binder) G:PP (80:20) 7.02 0.91 (±0.02) 18.34 (±3.3) 0.132 L/H values for glass fiber filters showing effect of binder Composition (Glass:PP) L/H With Binder 1.83 Binder on outside edge 1.26 Without binder 1.49 L/H values of mixed hydrophilic/hydrophobic fiber filters Composition (Glass:PP) L/H Glass 1.296 G:PP (80:20) 1.984 G:PP (60:40) 2.744 G:PP (50:50) 89.787 G:PP (40:60) 148.742 Selected List of Ternary Systems Component A = feed solvent, component B = solute, and component S = extraction solvent. K1 is the distribution coefficient in weight-fraction solute y/x for the tie line of lowest solute concentration reported. Ordinarily, K will approach unity as the solute concentration is increased. TABLE 15-5 Selected List of Ternary Systems Component A = feed solvent, component B = solute, and component S = extraction solvent. K1 is the distribution coefficient in weight-fraction solute y/x for the tie line of lowest solute concentration reported. Ordinarily, K will approach unity as the solute concentration is increased. Component B Component S Temp., ° C. K1 Ref. A = cetane Benzene Aniline 25 1.290 47 n-Heptane Aniline 25 0.0784 47 A = cottonseed oil Oleic acid Propane 85 0.150 46 93.5 0.1272 46 A = cyclohexane Benzene Furfural 25 0.630 44 Benzene Nitromethane 25 0.397 127 A = docosane 1,6-Diphenylherane Furfural 45 0.950 11 80 1.100 11 115 1.062 11 A = dodecane Methylnaphthalene β,β′-Iminodipropionitrile ca. 25 0.625 92 Methylnaphthalene β,β′-Oxydipropionitrile ca. 25 0.377 92 A = ethylbenzene Styrene Ethylene glycol 25 0.190 10 A = ethylene glycol Acetone Amyl acetate 31 1.838 86 Acetone n-Butyl acetate 31 1.940 86 Acetone Cyclohexane 27 0.508 86 Acetone Ethyl acetate 31 1.850 86 Acetone Ethyl butyrate 31 1.903 86 Acetone Ethyl propionate 31 2.32 86 A = furfural Trilimolein n-Heptane 30 47.5 15 50 21.4 15 70 19.5 15 Triolein n-Heptane 30 95 15 50 108 15 70 41.5 15 A = glycerol Ethanol Benzene 25 0.159 62 Ethanol Carbon tetrachloride 25 0.0887 63 A = n-heptane Benzene Ethylene glycol 25 0.300 50 125 0.316 50 Benzene β,β′-thiodipropionitrile 25 0.350 92 Benzene Triethylene glycol 25 0.351 89 Cyclohexane Aniline 25 0.0815 47 Cyclohexane Benzyl alcohol 0 0.107 29 15 0.267 29 Cyclohexane Dimethylformamide 20 0.1320 28 Cyclohexane Furfural 30 0.0635 78 Ethylbenzene Dipropylene glycol 25 0.329 90 Ethylbenzene β,β′-Oxydipropionitrile 25 0.180 101 Ethylbenzene β,β′-Thiodipropionitrile 25 0.100 101 Ethylbenzcne Triethylene glycol 25 0.140 89 Methylcyclohexane Aniline 25 0.057 116 Toluene Aniline 0 0.577 27 13 0.477 27 20 0.457 27 40 0.425 27 Toluene Benzyl alcohol 0 0.694 29 Toluene Dimethylformamide 0 0.667 28 20 0.514 28 Toluene Dipropylene glycol 25 0.331 90 Toluene Ethylene glycol 25 0.150 101 Toluene Propylene carbonate 20 0.732 39 Toluene β,β′-Thiodipropionitrile 25 0.150 101 Toluene Triethylene glycol 25 0.289 89 m-Xylene β,β′-Thiodipropionitrile 25 0.050 101 o-Xylene β,β′-Thiodipropionitrile 25 0.150 101 p-Xylene β,β′-Thiodipropionitrile 25 0.030 101 A = n-hexane Benzene Ethylenediamine 20 4.14 23 A = nco-hexane Cyclopentane Aniline 15 0.1259 96 25 0.311 96 A = methylcyclohexane Toluene Methylperfluorooctanoate 10 0.1297 58 25 0.200 58 A = isooctane Benzene Furfural 25 0.833 44 Cyclohexane Furfural 25 0.1076 44 n-Hexane Furfural 30 0.083 78 A = perfluoroheptane Perfluorocyclic oxide Carbon tetrachloride 30 0.1370 58 Perfluorocyclic oxide n-Heptane 30 0.329 58 A = perfluoro-n-hexane n-Hexane Benzene 30 6.22 80 n-Hexane Carbon disulfide 25 6.50 80 A = perfluorotri-n-butylamine Iso-octane Nitroethane 25 3.59 119 31.5 2.36 119 33.7 4.56 119 A = toluene Acetone Ethylene glycol 0 0.286 100 24 0.326 100 A = triethylene glycol α-Picotine Methylcyclohexane 20 3.87 14 α-Picotine Diisobutylene 20 0.445 14 α-Picotine Mixed heptanes 20 0.317 14 A = triolein Oleic acid Propane 85 0.138 40 A = water Acetaldehyde n-Amyl alcohol 18 1.43 74 Acetaldehyde Benzene 18 1.119 74 Acetaldehyde Furfural 16 0.007 74 Acetaldehyde Toluene 17 0.478 74 Acetaldehyde Vinyl acetate 20 0.560 81 Acetic acid Benzene 25 0.0328 43 30 0.0984 38 40 0.1022 38 50 0.0558 38 60 0.0637 38 Acetic acid 1-Butanol 26.7 1.613 102 Acetic acid Butyl acetate 30 0.705 45 0.391 67 Acetic acid Caproic acid 25 0.349 73 Acetic acid Carbon tetrachloride 27 0.1920 91 27.5 0.0549 54 Acetic acid Chloroform ca. 25 0.178 70 25 0.0865 72 56.8 0.1573 17 Acetic acid Creosote oil 34 0.706 91 Acetic acid Cyclohexanol 26.7 1.325 102 Acetic acid Diisobutyl ketone 25-26 0.284 75 Acetic acid Di-n-butyl ketone 25-26 0.379 75 Acetic acid Diisopropyl carbinol 25-26 0.800 75 Acetic acid Ethyl acetate 30 0.907 30 Acetic acid 2-Ethylbutyric acid 25 0.323 73 Acetic acid 2-Ethylhexnic acid 25 0.286 73 Acetic acid Ethylidene diacetate 25 0.85 104 Acetic acid Ethyl propionate 28 0.510 87 Acetic acid Fenchone 25-26 0.310 75 Acetic acid Furfural 26.7 0.787 102 Acetic acid Heptadecanol 25 0.312 114 50 0.1623 114 Acetic acid 3-Heptanol 25 0.828 76 Acetic add Hexane acetate 25-26 0.520 75 Acetic acid Hexane 31 0.0167 85 Acetic acid Isoamyl acetate 25-26 0.343 75 Acetic acid Isophorone 25-26 0.858 75 Acetic acid Isopropyl ether 20 0.248 31 25-26 0.429 75 Acetic acid Methyl acetate — 1.273 67 Acetic acid Methyl butyrate 30 0.690 66 Acetic acid Methyl cyclohexanone 25-26 0.930 75 Acetic acid Methylisobutyl carbinol 30 1.058 83 Acetic acid Methylisobutyl ketone 25 0.657 97 25-26 0.753 75 Acetic acid Monochlorobenzene 25 0.0435 77 Acetic acid Octyl acetate 25-26 0.1805 75 Acetic acid n-Propyl acetate — 0.638 67 Acetic acid Toluene 23 0.0644 131 Acetic acid Trichloroethylene 27 0.140 91 30 0.0549 54 Acetic acid Vinyl acetate 28 0.294 103 Acetone Amyl acetate 30 1.228 117 Acetone Benzene 15 0.940 11 30 0.862 11 45 0.725 11 Acetone n-Butyl acetate — 1.127 67 Acetone Carbon tetrachloride 30 0.238 12 Acetone Chloroform 25 1.830 43 25 1.720 3 Acetone Dibutyl ether 25.26 1.941 75 Acetone Diethyl ether 30 1.00 54 Acetone Ethyl acetate 30 1.500 117 Acetone Ethyl butyrate 30 1.278 117 Acetone Ethyl propionate 30 1.385 117 Acetone n-Heptane 25 0.274 112 Acetone n-Hexane 25 0.343 114 Acetone Methyl acetate 30 1.153 117 Acetone Methylisobutyl ketone 25-26 1.910 75 Acetone Monochlorobenzene 25-26 1.000 75 Acetone Propyl acetate 30 0.243 117 Acetone Tetrachloroethane 25-26 2.37 57 Acetone Tetrachloroethylene 30 0.237 88 Acetone 1,1,2-Trichloroethane 25 1.467 113 Acetone Toluene 25-26 0.835 75 Acetone Vinyl acetate 20 1.237 81 25 3.63 104 Acetone Xylene 25-26 0.659 75 Allyl alcohol Diallyl ether 22 0.572 32 Aniline Benzene 25 14.40 40 50 15.50 40 Aniline n-Heptane 25 1.425 40 50 2.20 40 Aniline Methylcyclohexane 25 2.05 40 50 3.41 40 Aniline Nitrobenzene 25 18.69 108 Aniline Toluene 25 12.91 107 Aniline hydrochloride Aniline 25 0.0540 98 Benzoic acid Methylisobutyl ketone 26.7 76.9* 49 iso-Butanol Benzene 25 0.989 1 iso-Butanol 1,1,2,2-Tetrachloroethane 25 1.80 38 iso-Butanol Tetrachloroethylene 25 0.0460 7 n-Butanol Benzene 25 1.263 126 35 2.12 126 n-Butanol Toluene 30 1.176 37 tert-Butanol Benzene 25 0.401 99 tert-Butanol tert-Butyl hypochlorite 0 0.1393 130 20 0.1487 130 40 0.200 129 60 0.539 129 tert-Butanol Ethyl acetate 20 1.74 5 2-Butoxyethanol Methylethyl ketone 25 3.05 68 2,3-Butylene glycol n-Butanol 26 0.597 71 50 0.893 71 2,3-Butylene glycol Butyl acetate 26 0.0222 71 50 0.0325 71 2,3-Butylene glycol Butylene glycol diacetate 26 0.1328 71 75 0.565 71 2,3-Butylene glycol Methylvinyl carbinol acetate 26 0.237 71 50 0.351 71 75 0.247 71 n-Butylamine Monochlorobenzene 25 1.391 77 t-Butyraldehyde Ethyl acetate 37.8 41.3 52 Butyric acid Methyl butyrate 30 6.75 66 Butyric acid Methylisobutyl carbinol 30 12.12 83 Cobaltous chloride Dioxane 25 0.0052 93 Cupric sulfate n-Butanol 30 0.000501 9 Cupric sulfate sec-Butanol 30 0.00702 9 Cupric sulfate Mixed pentanols 30 0.000225 9 p-Cresol Methylnaphthalene 35 9.89 62 Diacetone alcohol Ethylbenzene 25 0.335 22 Diacetone alcohol Styrene 25 0.445 22 Dichloroacetic acid Monochlorobenzene 25 0.0690 77 1,4-Dioxane Benzene 25 1.020 8 Ethanol n-Amyl alcohol 25-28 0.598 75 Ethanol Benzene 25 0.1191 13 25 0.0536 115 Ethanol n-Butanol 20 3.00 26 Ethanol Cyclohexane 25 0.0157 118 Ethanol Cyclohexene 25 0.0244 124 Ethanol Dibutyl ether 25-26 0.1458 75 Ethanol Di-n-propyl ketone 25-26 0.592 75 Ethanol Ethyl acetate 0 0.0263 5 20 0.500 5 70 0.455 41 Ethanol Ethyl isovalerate 25 0.392 13 Ethanol Heptadecanol 25 0.270 114 Ethanol a-Heptane 30 0.274 94 Ethanol 3-Heptanol 25 0.783 76 Ethanol n-Hexane 25 0.00212 111 Ethanol n-Hexanol 28 1.00 56 Ethanol sec-Octanol 28 0.825 56 Ethanol Toluene 25 0.01816 122 Ethanol Trichloroethylene 25 0.0662 16 Ethylene glycol n-Amyl alcohol 20 0.1159 59 Ethylene glycol n-Butanol 27 0.412 85 Ethylene glycol Furfural 25 0.315 18 Ethylene glycol n-Hexanol 20 0.275 59 Ethylene glycol Methylethyl ketone 30 0.0527 85 Formic acid Chloroform 25 0.00445 72 50.9 0.0192 17 Formic acid Methylisobutyl carbinol 30 1.218 83 Furfural n-Butane 51.5 0.712 42 79.5 0.930 42 Furfural Methylisobutyl ketone 25 7.10 19 Furfural Toluene 25 5.64 53 Hydrogen chloride iso-Amyl alcohol 25 0.170 21 Hydrogen chloride 2,6-Dimethyl-4-heptanol 25 0.266 21 Hydrogen chloride 2-Ethyl-1-butanol 25 0.534 21 Hydrogen chloride Ethylbutyl ketone 25 0.01515 79 Hydrogen chloride 3-Heptanol 25 0.0250 21 Hydrogen chloride 1-Hexanol 25 0.345 21 Hydrogen chloride 2-Methyl-1-butanol 25 0.470 21 Hydrogen chloride Methylisobutyl ketone 25 0.0273 70 Hydrogen chloride 2-Methyl-1-pentanol 25 0.502 21 Hydrogen chloride 2-Methyl-2-pentanol 25 0.411 21 Hydrogen chloride Methylisopropyl ketone 25 0.0814 79 Hydrogen chloride 1-Octanol 25 0.424 21 Hydrogen chloride 2-Octanol 25 0.380 21 Hydrogen chloride 1-Pantanol 25 0.257 21 Hydrogen chloride Pentanols (mixed) 25 0.271 21 Hydrogen Buoride Methylisobutyl ketone 25 0.370 79 Lactic acid iso-Amyl alcohol 25 0.352 128 Methanol Benzene 25 0.01022 4 Methanol n-Butanol 0 0.600 65 15 0.479 65 30 0.510 65 45 1.260 65 60 0.682 65 Methanol p-Cresol 35 0.313 82 Methanol Cyclohexane 25 0.0150 125 Methanol Cyclohexane 25 0.01043 124 Methanol Ethyl acetate 0 0.0589 5 20 0.238 5 Methanol n-Hexanol 28 0.585 55 Methanol Methylnaphthalene 25 0.025 82 35 0.0223 82 Methanol sec-Octanol 28 0.584 55 Methanol Phenol 25 1.333 82 Methanol Toluene 25 0.0099 60 Methanol Trichloroethylene 27.5 0.0167 54 Methyl-n-butyl ketone n-Butanol 37.8 53.4 52 Methylethyl ketone Cyclohexane 25 1.775 48 30 3.60 85 Methylethyl ketone Casoline 25 1.686 64 Methylethyl ketone n-Heptane 25 1.548 112 Methylethyl ketone n-Hexane 25 1.775 112 37.8 2.22 52 Methylethyl ketone 2-Methyl furan 25 84.0 109 Methylethyl ketone Monochlorobenzene 25 2.36 68 Methylethyl ketone Naphtha 28.7 0.885† 6 Methylethyl ketone 1,1,2-Trichloroethane 25 3.44 68 Methylethyl ketone Trichloroethylene 25 3.87 68 Methylethyl ketone 2,2,4-Trimethylpentane 25 1.572 64 Nickelous chloride Dioxane 25 0.0017 93 Nicotine Carbon tetrachloride 25 9.50 34 Phenol Methylnaphthalene 23 7.00 82 a-Picoline Benzene 20 8.75 14 a-Picoline Diisobutylene 20 1.360 14 a-Picoline Heptanes (mixed) 20 1.378 14 a-Picoline Methylcylohoxane 20 1.00 14 iso-Propanol Benzene 25 0.276 69 iso-Propanol Carbon tetrachloride 20 1.405 25 iso-Propanol Cyclohexane 25 0.0282 123 iso-Propanol Cyclohexane 15 0.0583 124 25 0.0682 124 35 0.1875 124 iso-Propanol Diisopropyl ether 25 0.406 35 iso-Propanol Ethyl acetate 0 0.200 5 20 1.205 5 iso-Propanol Tetrachloroethylene 25 0.388 7 iso-Propanol Toluene 25 0.1296 121 n-Propanol iso-Amyl alcohol 25 3.34 20 n-Propanol Benzene 37.8 0.650 61 n-Propanol n-Butanol 37.8 3.61 61 n-Propanol Cyclohexane 25 0.1553 123 35 0.1775 123 n-Propanol Ethyl acetate 0 1.419 5 20 1.542 5 n-Propanol n-Heptane 37.8 0.540 61 n-Propanol n-Hexane 37.8 0.320 61 n-Propanol n-Propyl acetate 20 1.55 106 35 2.14 106 n-Propanol Toluene 25 0.299 2 Propionic acid Benzene 30 0.598 57 Propionic acid Cyclohexane 31 0.1955 84 Propionic acid Cyclohexene 31 0.303 84 Propionic acid Ethyl acetate 30 2.77 87 Propionic acid Ethyl butyrate 26 1.470 87 Propionic acid Ethyl propionate 28 0.510 87 Propionic acid Hexane (mixed) 31 0.186 84 Propionic acid Methyl butyrate 30 2.15 68 Propionic acid Methylisobutyl carbinol 30 3.52 83 Propionic acid Methylisobutyl ketone 26.7 1.949* 49 Propionic acid Monochlorobenzene 30 0.513 57 Propionic acid Tetrachloroethylene 31 0.167 84 Propionic acid Toluene 31 0.515 84 Propionic acid Trichloroethylene 30 0.496 57 Pyridine Benzene 15 2.19 110 25 3.00 105 25 2.73 120 45 2.49 110 60 2.10 110 Pyridine Monochlorobenzene 25 2.10 77 Pyridine Toluene 25 1.900 120 Pyridine Xylene 25 1.260 120 Sodium chloride iso-Butanol 25 0.0182 36 Sodium chloride n-Ethyl-sec-butyl amine 32 0.0583 24 Sodium chloride n-Ethyl-tert-butyl amine 40 0.1792 24 Sodium chloride 2-Ethylhexyl amine 30 0.187 24 Sodium chloride 1-Methyldiethyl amine 39.1 0.0597 24 Sodium chloride 1-Methyldodecyl amine 30 0.693 24 Sodium chloride n-Methyl-1,3-dimethylbutyl amine 30 0.0537 24 Sodium chloride 1-Methyloctyl amine 30 0.589 24 Sodium chloride tert-Nonyl amine 30 0.0318 24 Sodium chloride 1,1,3,3-Tetramethyl butyl amine 30 0.072 24 Sodium hydroxide iso-Butanol 25 0.00857 36 Sodium nitrate Dioxane 25 0.0246 95 Succinic acid Ethyl ether 15 0.220 33 20 0.193 33 25 0.1805 33 Trimethyl amine Benzene 25 0.857 51 70 2.36 51 *Concentrations in lb-moles/cu. ft. †Concentrations in volume fraction.