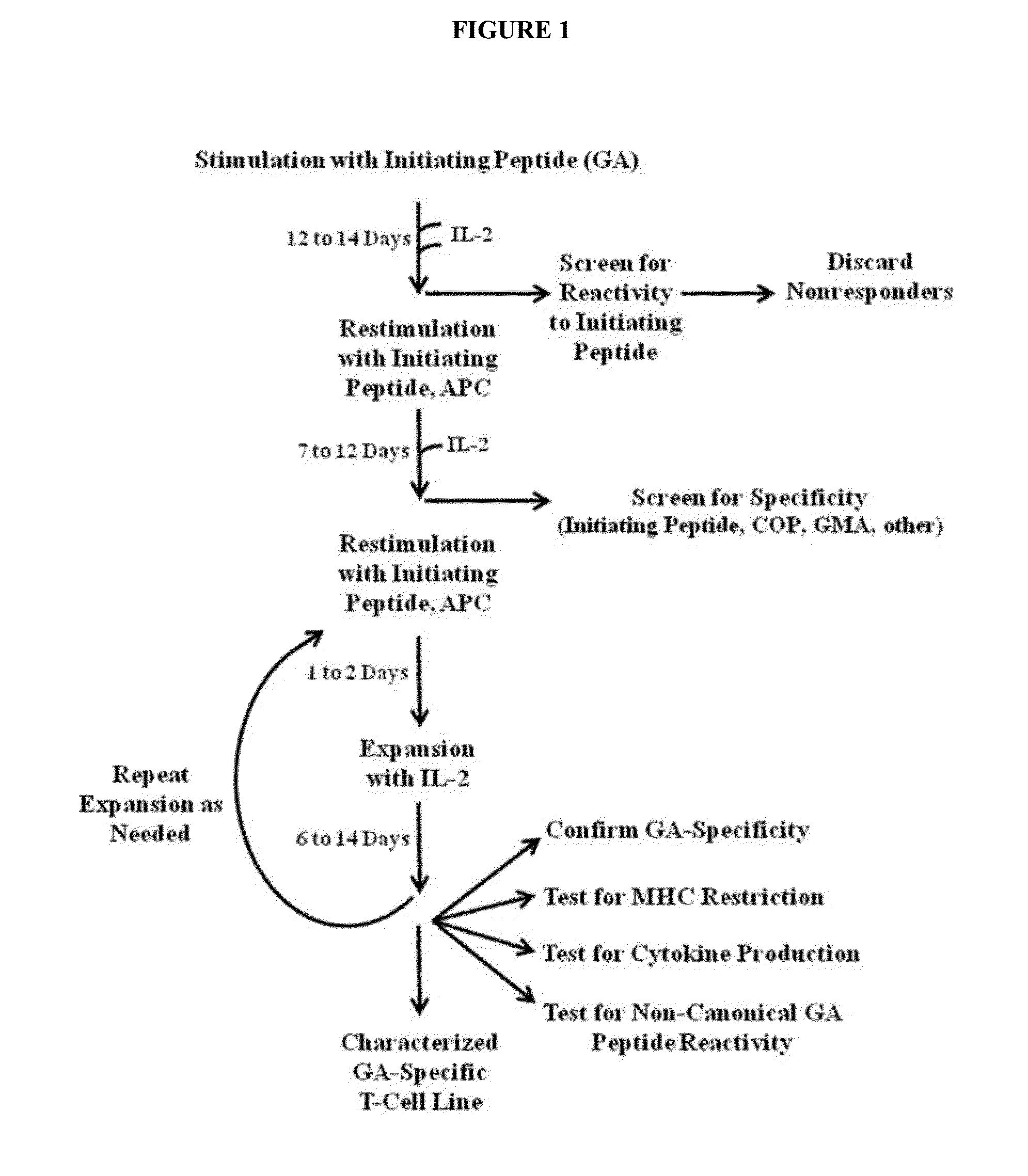
HUMAN T CELL LINE ASSAY FOR EVALUATING THE IMMUNOLOGIC IDENTITY OF GLATIRAMER ACETATE PREPARATIONS
This application claims the benefit of priority of U.S. Provisional Patent Application No. 61/895,370, filed Oct. 24, 2013; which is incorporated herein by reference in its entirety. Glatiramer acetate is a synthetic peptide drug approved for treating multiple sclerosis. It consists of the acetate salts of synthetic polypeptides containing the amino acids L-glutamic acid, L-alanine, L-tyrosine, and L-lysine. Currently sold as Copaxone®, glatiramer acetate injection is indicated for the reduction of the frequency of relapses in patients with Relapsing-Remitting Multiple Sclerosis (RRMS), including patients who have experienced a first clinical episode and have MRI features consistent with Multiple Sclerosis. Glatiramer acetate is thought to act in multiple sclerosis by modifying immune processes responsible for the pathogenesis of the disease. In particular, it is believed that the mechanism of action of Copaxone® in Multiple Sclerosis is at least in part mediated by immunomodulation of T-cell activity. During the manufacture of glatiramer acetate, reliable and cost-effective assays for interrogating glatiramer acetate preparations to demonstrate immunological identity and/or comparable potency to reference standard lots of glatiramer acetate are needed for maintaining consistency among drug lots for pharmaceutical use. The invention is based on the discovery of methods for generating and identifying glatiramer acetate (GA)-specific human T-cell lines that respond similarly to different GA preparations, but differ in responsiveness to non-canonical GA peptides, have different MHC restriction elements, and produce different cytokines in response to stimulation with GA. The invention relates to methods for obtaining GA-specific human T-cell lines that recognize different GA epitopes, and to methods for using these GA-specific human T-cell lines to determine whether GA preparations are immunologically identical. In embodiments, the invention relates to a method for determining whether a GA test preparation and a GA reference standard preparation are immunologically identical, using at least one GA-specific human T-cell line or a panel of GA-specific human T-cell lines generated using the described methods. The invention further relates to processes for preparing a drug product or pharmaceutical composition containing GA, comprising determining whether a GA test preparation and a GA reference standard are immunologically identical, and including the GA test lot in the drug product if it is determined to be immunologically identical to the GA reference standard. The invention also relates to a drug product containing GA prepared by the methods of the invention. The invention further relates to human B-cell lines appropriate for use as antigen presenting cells in the methods of the invention. The assays of the present invention can demonstrate the immunological identity or reveal non-identity of GA product preparations with an accuracy not available using existing GA batch assays. The assays of the present invention do not require animal immunization, thereby eliminating assay-to-assay variability, and they do not require animal sacrifice. In particular, the present invention relates to a method of determining whether a test preparation of GA and a GA reference standard are immunologically identical, using at least one GA-specific human T-cell line, the method comprising: a) incubating cells of the at least one GA-specific human T-cell line with appropriate antigen presenting cells (APC); b) stimulating at least one sample of the GA-specific human T-cells and appropriate APC incubated in step (a) with an amount of the test preparation of GA, and separately stimulating at least one sample of the GA-specific human T-cells and appropriate APC incubated in step (a) with the same amount of the GA reference standard; c) measuring at least one GA-elicited response of the at least one sample of cells stimulated in step (b) with the test preparation of GA, and measuring the same at least one GA-elicited response of the at least one sample of cells stimulated in step (b) with the GA reference standard; and d) comparing the measurements obtained in step (c); wherein the test preparation of GA and the GA reference standard are determined to be immunologically identical when the comparison of the measurements made in step (d) falls within an acceptable range. In embodiments, the present invention relates to a method of determining whether a test preparation of glatiramer acetate (GA) and a GA reference standard are immunologically identical, the method comprising: measuring at least one GA-elicited response of at least one GA-specific human T-cell line to stimulation with the test preparation of GA in the presence of appropriate APC to obtain a first measurement, and at least one GA-elicited response of the at least one GA-specific human T-cell line to a GA reference standard in the presence of appropriate APC to obtain a second measurement; and comparing the first and second measurements; wherein the test preparation of GA and the GA reference standard are determined to be immunologically identical when the comparison of the first and second measurements falls within an acceptable range. In the above embodiments, the appropriate APC can comprise cells selected from the group consisting of: Epstein Ban Virus-transformed (EBV-transformed) human B-cells autologous to the GA-specific human T-cells; EBV-transformed human B-cells having the DR molecule involved in antigen presentation to the GA-specific human T-cells; PBMC autologous to the GA-specific human T cells; PBMC having the DR molecule involved in antigen presentation to the GA-specific human T-cells; purified monocytes autologous to the GA-specific human T-cells; purified monocytes having the DR molecule involved in antigen presentation to the GA-specific human T-cells; purified dendritic cells autologous to the GA-specific human T-cells; and purified dendritic cells having the DR molecule involved in antigen presentation to the GA-specific human T-cells. The invention also relates to a method comprising selecting for pharmaceutical use a test preparation of GA having immunologic identity to a GA reference standard determined according to methods described. The invention relates to the above methods, wherein the acceptable range is about 80% to about 120%, or about 90% to about 110%. In embodiments of the above methods, the at least one measured GA-elicited response is selected from proliferation, production of a response biomarker, expression of a nucleic acid encoding a response biomarker, and a combination thereof. In embodiments, the at least one measured GA-elicited response is production of a response biomarker, and the response biomarker is a cytokine, a chemokine, or an activation marker. In certain embodiments, the response biomarker is a cytokine selected from: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21 IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), and IL-1b. In embodiments, the response biomarker is an activation marker selected from: CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, the response biomarker is a chemokine selected from: IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. The invention relates to the above methods, wherein the at least one measured GA-elicited response is expression of a nucleic acid encoding a response biomarker, and the response biomarker encoded by the nucleic acid is a cytokine, a chemokine, or an activation marker. In embodiments, the encoded response biomarker is a cytokine selected from: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β and IL-1b. In certain embodiments, the encoded response biomarker is an activation marker selected from: CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, the encoded response biomarker is a chemokine selected from: IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. The invention relates to the above methods, wherein at least two GA-elicited responses of the cells stimulated with the test preparation of GA are measured, and the same at least two GA-elicited responses of the cells stimulated with the GA reference standard, are measured. In these embodiments, the at least two GA-elicited responses can be selected from the expression of: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, IL-1b, CD69, CD25, CD71, CD137, CD154, CD278, CD279, HLA-DR, IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, CXCL7, a nucleic acid encoding IL-2, a nucleic acid encoding IL-4, a nucleic acid encoding IL-5, a nucleic acid encoding IL-6, a nucleic acid encoding IL-10, a nucleic acid encoding IL-13, or a nucleic acid encoding IL-17, a nucleic acid encoding IL-22, a nucleic acid encoding IFN-γ, a nucleic acid encoding TNF-α (TNF), a nucleic acid encoding TNF-β (LT), a nucleic acid encoding TGF-β, a nucleic acid encoding IL-1b, a nucleic acid encoding CD69, a nucleic acid encoding CD25, a nucleic acid encoding CD71, a nucleic acid encoding CD137, a nucleic acid encoding CD154, a nucleic acid encoding CD278, a nucleic acid encoding CD279, a nucleic acid encoding HLA-DR, a nucleic acid encoding IL-8 (CXCL8), a nucleic acid encoding RANTES (CCL5), a nucleic acid encoding CCL1, a nucleic acid encoding CXCL4, and a nucleic acid encoding CXCL7. The invention relates to the above methods, wherein the GA reference standard is Copaxone (COP) or Mylan GA (GMA). The invention relates to the above methods, wherein the appropriate APC have at least one HLA-DR restriction element capable of presenting GA peptides. In certain embodiments, the at least one HLA-DR restriction element capable of presenting GA peptides is selected from: DR-1, DR-2, DR-3, DR-4, DR-7, DR-11, DR-13, and DR-15. The invention relates to the above methods, wherein the at least one GA-specific human T-cell line incubated in step (a) is a long-term T-cell line. In embodiments, the GA-specific human T-cell line is clonal. In embodiments, the at least one long-term GA-specific human T-cell line has been maintained in culture for at least about four weeks prior to stimulation, previously restimulated at least four times, or both. In embodiments, the at least one long-term GA-specific human T-cell line has been previously restimulated at least eight times. In embodiments, the maintenance in culture of the at least one long-term GA-specific human T-cell line comprises recurrent restimulation with GA and autologous APC, in the absence of mitogen. The invention relates to the above methods, wherein the stimulation includes stimulating each of at least three samples of GA-specific human T-cells, incubated with or in the presence of APC, with one of a series of amounts of the test preparation of GA, and stimulating each of at least three samples of GA-specific human T-cells, incubated with or in the presence of APC, with one of the same series of amounts of the GA reference standard. In embodiments, the series of amounts of the test preparation of GA, and the series of amounts of the GA reference standard, comprise escalating doses of GA of about 1 ng/mL to about 1 mg/mL GA. In embodiments, the series of amounts of the test preparation of GA, and the series of amounts of the GA reference standard, are escalating doses of GA of about 1 μg/mL to about 30 μg/mL GA. In embodiments, the invention relates to a process for preparing a drug product or pharmaceutical composition containing GA, comprising: 1) reacting protected glatiramer acetate with hydrobromic acid to form trifluoroacetyl GA, treating said trifluoroacetyl copolymer-1 with aqueous piperidine solution to form a test preparation of GA, and purifying the test preparation of GA; 2) determining whether the test preparation of GA and a GA reference standard are immunologically identical, by: a) incubating cells of at least one GA-specific human T-cell line with appropriate antigen presenting cells (APC); b) stimulating at least one sample of the GA-specific human T-cells and appropriate APC incubated in step (a) with an amount of the test preparation of GA, and separately stimulating at least one sample of the GA-specific human T-cells and appropriate APC incubated in step (a) with the same amount of the GA reference standard; c) measuring at least one GA-elicited response of the at least one sample of cells stimulated in step (b) with the test preparation of GA, and measuring the same at least one GA-elicited response of the at least one sample of cells stimulated in step (b) with the GA reference standard; and d) comparing the measurements obtained in step (c); wherein the test preparation of GA and the GA reference standard are determined to be immunologically identical when the comparison of the measurements of step (d) falls within an acceptable range, and wherein the test preparation of GA is admixed in the drug product or pharmaceutical composition if it is determined to be immunologically identical to the GA reference standard. In these embodiments, the appropriate APC can comprise cells selected from the group consisting of: Epstein Barr Virus-transformed (EBV-transformed) human B-cells autologous to the GA-specific human T-cells; EBV-transformed human B-cells having the DR molecule involved in antigen presentation to the GA-specific human T-cells; PBMC autologous to the GA-specific human T cells; PBMC having the DR molecule involved in antigen presentation to the GA-specific human T-cells; purified monocytes autologous to the GA-specific human T-cells; purified monocytes having the DR molecule involved in antigen presentation to the GA-specific human T-cells; purified dendritic cells autologous to the GA-specific human T-cells; and purified dendritic cells having the DR molecule involved in antigen presentation to the GA-specific human T-cells. In embodiments, the acceptable range is about 80% to about 120%, or about 90% to about 110%. In certain embodiments of the above methods, the at least one measured GA-elicited response is selected from proliferation, production of a response biomarker, expression of a nucleic acid encoding a response biomarker, and a combination thereof. In embodiments, the at least one measured GA-elicited response is production of a response biomarker, and the response biomarker is a cytokine, a chemokine, or an activation marker. In certain embodiments, the response biomarker is a cytokine selected from: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21 IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), and IL-1b. In embodiments, the response biomarker is an activation marker selected from: CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, the response biomarker is a chemokine selected from: IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, the at least one measured GA-elicited response is expression of a nucleic acid encoding a response biomarker, and the response biomarker encoded by the nucleic acid is a cytokine, a chemokine, or an activation marker. In embodiments, the encoded response biomarker is a cytokine selected from: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β and IL-1b. In certain embodiments, the encoded response biomarker is an activation marker selected from: CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, the encoded response biomarker is a chemokine selected from: IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, wherein at least two GA-elicited responses of the cells stimulated with the test preparation of GA are measured, and the same at least two GA-elicited responses of the cells stimulated with the GA reference standard, are measured. In these embodiments, the at least two GA-elicited responses can be selected from the expression of: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, IL-1b, CD69, CD25, CD71, CD137, CD154, CD278, CD279, HLA-DR, IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, CXCL7, a nucleic acid encoding IL-2, a nucleic acid encoding IL-4, a nucleic acid encoding IL-5, a nucleic acid encoding IL-6, a nucleic acid encoding IL-10, a nucleic acid encoding IL-13, or a nucleic acid encoding IL-17, a nucleic acid encoding IL-22, a nucleic acid encoding IFN-γ, a nucleic acid encoding TNF-α (TNF), a nucleic acid encoding TNF-β (LT), a nucleic acid encoding TGF-β, a nucleic acid encoding IL-1b, a nucleic acid encoding CD69, a nucleic acid encoding CD25, a nucleic acid encoding CD71, a nucleic acid encoding CD137, a nucleic acid encoding CD154, a nucleic acid encoding CD278, a nucleic acid encoding CD279, a nucleic acid encoding HLA-DR, a nucleic acid encoding IL-8 (CXCL8), a nucleic acid encoding RANTES (CCL5), a nucleic acid encoding CCL1, a nucleic acid encoding CXCL4, and a nucleic acid encoding CXCL7. In embodiments, the GA reference standard is Copaxone or GMA. In embodiments, the appropriate APC have at least one HLA-DR restriction element capable of presenting GA peptides. In certain embodiments, the at least one HLA-DR restriction element capable of presenting GA peptides is selected from: DR-1, DR-2, DR-3, DR-4, DR-7, DR-11, DR-13, and DR-15. In embodiments, the at least one GA-specific human T-cell line incubated in step (a) is a long-term T-cell line. In embodiments, the at least one GA-specific human T-cell line is clonal. In embodiments, the at least one long-term GA-specific human T-cell line has been maintained in culture for at least about four weeks prior to stimulation, previously restimulated at least four times, or both. In embodiments, the at least one long-term GA-specific human T-cell line has been previously restimulated at least eight times. In embodiments, the maintenance in culture of the at least one long-term GA-specific human T-cell line comprises recurrent restimulation with GA and autologous APC, in the absence of mitogen. In embodiments, the stimulation includes stimulating each of at least three samples of GA-specific human T-cells, incubated with or in the presence of APC, with one of a series of amounts of the test preparation of GA, and stimulating each of at least three samples of GA-specific human T-cells, incubated with or in the presence of APC, with one of the same series of amounts of the GA reference standard. In embodiments, the series of amounts of the test preparation of GA, and the series of amounts of the GA reference standard, comprise escalating doses of GA of about 1 ng/mL to about 1 mg/mL GA. In embodiments, the series of amounts of the test preparation of GA, and the series of amounts of the GA reference standard, are escalating doses of GA of about 1 μg/mL to about 30 μg/mL GA. The invention further relates to a method for generating and identifying a GA-specific human T-cell line, the method comprising: a) obtaining a sample of cells, wherein the sample of cells comprises T-cells from a human donor and appropriate APC; b) preparing a culture from the sample of cells of step (a), wherein the culture comprises GA; c) incubating the culture of step (b) with IL-2; d) assaying the culture of step (c) by preparing at least one test culture comprising cells from the culture of step (c), appropriate APC, and a test preparation of GA, preparing at least one control culture from the culture of step (c), and (i) measuring at least one GA-elicited response of the at least one test culture; (ii) measuring the same at least one GA-elicited response of the control culture; and e) comparing the measurement of step (d)(i) with the measurement of step (d)(ii); wherein the generated GA-specific human T-cell line is identified based on an increase in the at least one GA-elicited response measured in step (d)(i) relative to the at least one GA-elicited response measured in step (d)(ii); and f) optionally expanding the culture, and repeating steps (d) and (e) using the expanded culture, to further characterize the identified GA-specific human T-cell line. In these embodiments, the at least one measured GA-elicited response can be selected from proliferation, production of a response biomarker, expression of a nucleic acid encoding a response biomarker, and a combination thereof. In related embodiments, the at least one measured GA-elicited response is production of a response biomarker, and the response biomarker is a cytokine, an activation marker, or a chemokine. In certain embodiments, the response biomarker is a cytokine selected from: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, and IL-1b. In embodiments, the response biomarker is an activation marker selected from: CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, the response biomarker is a chemokine selected from: IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In related embodiments, the at least one measured GA-elicited response is expression of a nucleic acid encoding a response biomarker, and the encoded response biomarker is a cytokine, an activation marker, or a chemokine. In certain embodiments, the encoded response biomarker is a cytokine selected from: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, and IL-1b. In embodiments, the encoded response biomarker is a chemokine selected from: IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, the encoded response biomarker is an activation marker selected from: CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments of the invention, step (e) comprises measuring at least two GA-elicited responses of the cultured T-cells to the GA, and step (f) comprises measuring the same at least two GA-elicited responses of the cultured T-cells to the control antigen. In related embodiments, the at least two GA-elicited responses are selected from the expression of: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, IL-1b, CD69, CD25, CD71, CD137, CD154, CD278, CD279, HLA-DR, IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, CXCL7, a nucleic acid encoding IL-2, a nucleic acid encoding IL-4, a nucleic acid encoding IL-5, a nucleic acid encoding IL-6, a nucleic acid encoding IL-10, a nucleic acid encoding IL-13, or a nucleic acid encoding IL-17, a nucleic acid encoding IL-21, a nucleic acid encoding IL-22, a nucleic acid encoding IFN-γ, a nucleic acid encoding TNF-α (TNF), a nucleic acid encoding TNF-β (LT), a nucleic acid encoding TGF-β, a nucleic acid encoding IL-1b, a nucleic acid encoding CD69, a nucleic acid encoding CD25, a nucleic acid encoding CD71, a nucleic acid encoding CD137, a nucleic acid encoding CD154, a nucleic acid encoding CD278, a nucleic acid encoding CD279, a nucleic acid encoding HLA-DR, a nucleic acid encoding IL-8 (CXCL8), a nucleic acid encoding RANTES (CCL5), a nucleic acid encoding CCL1, a nucleic acid encoding CXCL4, and a nucleic acid encoding CXCL7. In embodiments, the appropriate APC are autologous cells. In embodiments, the appropriate APC are autologous PBMC. In embodiments, the appropriate APC are autologous PBMC treated with a an anti-mitotic agent, e.g., mitomycin C. In embodiments, the appropriate APC have at least one HLA-DR restriction element capable of presenting GA peptides. In certain embodiments, the at least one HLA-DR restriction element capable of presenting GA peptides is selected from: DR-1, DR-2, DR-3, DR-4, DR-7, DR-11, DR-13, and DR-15. The invention also relates to a GA-specific human T-cell line obtained according to these methods. In embodiments, the generated GA-specific human T-cell line is a long-term T-cell line. In embodiments, the generated GA-specific human T-cell line is clonal. In embodiments of the invention, no mitogen is added to the culture of step (b) or (c). In embodiments, the method further comprises characterizing the identified GA-specific human T-cell line, wherein the characterization comprises one or more of: a) confirming or reconfirming the GA-specificity of the identified GA-specific human T-cell line; b) determining the MHC restriction of the identified GA-specific human T-cell line; c) determining a response biomarker profile of the identified GA-specific human T-cell line; and d) determining the reactivity of the identified GA-specific human T-cell line to at least one non-canonical GA peptide. In embodiments, the method further comprises characterizing the response of the identified GA-specific human T-cell line to a non-canonical GA peptide by: preparing at least one test culture comprising cells of the identified GA-specific human T-cell line, appropriate APC, and a non-canonical GA peptide; preparing at least one control culture comprising cells of the identified GA-specific human T-cell line; measuring at least one GA-elicited response of the at least one test culture; measuring the same at least one GA-elicited response of the control culture; and comparing the measurement of the GA-elicited responses of the test and control cultures; wherein the GA-specific human T-cell line that responds to stimulation with the non-canonical GA peptide is identified by an increase in the at least one GA-elicited response of the test culture relative to the control culture. The invention also relates to a long-term GA-specific human T-cell line, wherein the long-term GA-specific human T-cell line is capable of producing at least one measured response to stimulation with a test preparation of GA that is increased relative to the same at least one measured response to stimulation with a control antigen, said at least one measured response comprising proliferation, production of a response biomarker, expression of a nucleic acid encoding a response biomarker, or a combination thereof. In embodiments, the GA-specific human T-cell line is clonal. In embodiments, the at least one measured GA-elicited response is selected from proliferation, production of a response biomarker, expression of a nucleic acid encoding a response biomarker, and a combination thereof. In embodiments, the at least one measured GA-elicited response is production of a response biomarker, and the response biomarker is a cytokine, a chemokine, or an activation marker. In certain embodiments, the response biomarker is a cytokine selected from: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21 IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), and IL-1b. In embodiments, the response biomarker is an activation marker selected from: CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, the response biomarker is a chemokine selected from: IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, the at least one measured GA-elicited response is expression of a nucleic acid encoding a response biomarker, and the response biomarker encoded by the nucleic acid is a cytokine, a chemokine, or an activation marker. In embodiments, the encoded response biomarker is a cytokine selected from: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β and IL-1b. In certain embodiments, the encoded response biomarker is an activation marker selected from: CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, the encoded response biomarker is a chemokine selected from: IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, at least two GA-elicited responses of the cells stimulated with the test preparation of GA are measured, and the same at least two GA-elicited responses of the cells stimulated with the GA reference standard, are measured. In these embodiments, the at least two GA-elicited responses can be selected from the expression of: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, IL-1b, CD69, CD25, CD71, CD137, CD154, CD278, CD279, HLA-DR, IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, CXCL7, a nucleic acid encoding IL-2, a nucleic acid encoding IL-4, a nucleic acid encoding IL-5, a nucleic acid encoding IL-6, a nucleic acid encoding IL-10, a nucleic acid encoding IL-13, or a nucleic acid encoding IL-17, a nucleic acid encoding IL-22, a nucleic acid encoding IFN-γ, a nucleic acid encoding TNF-α (TNF), a nucleic acid encoding TNF-β (LT), a nucleic acid encoding TGF-β, a nucleic acid encoding IL-1b, a nucleic acid encoding CD69, a nucleic acid encoding CD25, a nucleic acid encoding CD71, a nucleic acid encoding CD137, a nucleic acid encoding CD154, a nucleic acid encoding CD278, a nucleic acid encoding CD279, a nucleic acid encoding HLA-DR, a nucleic acid encoding IL-8 (CXCL8), a nucleic acid encoding RANTES (CCL5), a nucleic acid encoding CCL1, a nucleic acid encoding CXCL4, and a nucleic acid encoding CXCL7. An APC line comprising antigen presenting cells capable of presenting antigen to the long-term GA-specific human T-cell line of claim 90. In embodiments, the APC line is selected from: Epstein Barr Virus-transformed (EBV-transformed) human B-cells autologous to the GA-specific human T-cells; EBV-transformed human B-cells having the DR molecule involved in antigen presentation to the GA-specific human T-cells; PBMC autologous to the GA-specific human T cells; PBMC having the DR molecule involved in antigen presentation to the GA-specific human T-cells; purified monocytes autologous to the GA-specific human T-cells; purified monocytes having the DR molecule involved in antigen presentation to the GA-specific human T-cells; purified dendritic cells autologous to the GA-specific human T-cells; and purified dendritic cells having the DR molecule involved in antigen presentation to the GA-specific human T-cells. In embodiments, the long-term GA-specific human T-cell line is generated by culturing for at least about four weeks. In embodiments, the long-term GA-specific human T-cell line is generated by stimulation with a GMA or COP preparation. The present invention also relates to an assay panel of GA-specific human T-cell lines for use in determining whether a test preparation of GA and a GA reference standard are immunologically identical, wherein the assay panel comprises the following GA-specific human T-cell lines: a) a GA-specific human T-cell line generated by culturing human T-cells in the presence of a first preparation of GA; b) a GA-specific human T-cell line generated by culturing human T-cells in the presence of a second preparation of GA; and c) a GA-specific human T-cell line that is not reactive to at least one non-canonical GA peptide. In embodiments, the GA-specific human T-cell line of (a) or (b) also is the GA-specific human T-cell line of (c). In embodiments, the non-canonical GA peptide of (c) consists of the amino acids tyrosine (Y), glutamic acid (E), alanine (A), and lysine (K) at a molar ratio that differs from the ratio of the amino acids tyrosine (Y), glutamic acid (E), alanine (A), and lysine (K) in Copaxone®. In embodiments, the assay panel of claim 105, wherein the non-canonical GA peptide of (c) consists of any one, two, or three of the amino acids tyrosine (Y), glutamic acid (E), alanine (A), and lysine (K). In embodiments, the assay panel of claim 105, further comprising a GA-specific human T-cell line that is reactive to the at least one non-canonical GA peptide. In certain embodiments, the invention relates to the above assay panel wherein the GA-specific human T-cell line of (a) is selected from the group consisting of 222-AG12, 222-BA11, 222-BC11, 165-B5G, 165-C4G, 165-C5G, 165-C8G, 165-D8G, 165-E7G, 165-E9G, 165-F10G, 165-F5G, 165-F8G, and 165-H11G; the GA-specific human T-cell line of (b) is selected from the group consisting of 222-1C5, 222-1H12, 222-2B11, 222-2B8, 222-2C1, 222-2C3, 222-2D2, 222-2D8, 222-2E1, 222-1F8, 222-2F12, 222-2G4, 222-1G8, 222-2G12, 165-B6C, 165-B10C, 165-C4C, 165-C7C, 165-D2C, 165-D3C, 165-D11C, 165-E3C, 165-F3C, 165-F6C, 205-1B4, 205-1B7, 205-1C4, 205-1D1, 205-1E1, 205-1F2, 205-1F4, 205-1H1, 205-1H3, 205-1H5, 205-1H7, 205-1H9, and 205-1H11; and the GA-specific human T-cell line of (c) is selected from the group consisting of 222-AG12, 165-B5G, 165-D8G, 165-E7G, 165-E9G, 165-F5G, 165-F10G, 222-1H12, 222-2F12, 165-B10C, 165-C4C, 165-C7C, 165-D11C, 165-E3C, 165-F3C, 165-F6C, and 205-1H7. In embodiments, the GA-specific human T-cell line of (a) is selected from the group consisting of 222-AG12, 222-AG12, 222-BA11, 222-BC11, 165-B5G, 165-C5G, 165-C8G, 165-D8G, 165-E9G, 165-F10G, 165-F5G, 165-F8G, and 165-H11G; the GA-specific human T-cell line of (b) is selected from the group consisting of 222-1C5, 222-1H12, 222-2B8, 222-2C3, 222-2E1, 222-1F8, 222-2F12, 222-2G4, 222-1G8, 165-C4C, 165-C7C, 165-D2C, 165-D3C, 205-1B4, 205-1B7, 205-1C4, and 205-1F4; and the GA-specific human T-cell line of (c) is selected from the group consisting of 222-AG12, 165-B5G, 165-D8G, 165-E7G, 165-E9G, 165-F5G, 165-F10G, 222-1H12, 222-2F12, 165-B10C, 165-C4C, 165-C7C, 165-D11C, 165-E3C, 165-F3C, 165-F6C, and 205-1H7. In certain embodiments, the GA-specific human T-cell line of (a) is selected from the group consisting of 165-B5G or 165-D8G; the GA-specific human T-cell line of (b) is selected from the group consisting of 222-1H12 or 222-2E1; and the GA-specific human T-cell line of (c) is 222-AG12. In embodiments, the assay panel comprises or further comprises at least one GA-specific human T cell line that has a first known GA response biomarker profile, and comprising or further comprising at least one GA-specific human T cell line that has a second known GA response biomarker profile that is different from the first known GA response biomarker profile. In embodiments, the assay panel comprises or further comprises at least one GA-specific human T cell line that has a first known MHC restriction, and comprising or further comprising at least one GA-specific human T cell line that has a second known MHC restriction that is different from the first known MHC restriction. In embodiments, the assay panel comprises or further comprises at least one GA-specific human T-cell line having an HLA-DR restriction element capable of presenting GA peptides. In embodiments, the first HLA-DR restriction element is selected from: DR-1, DR-2, DR-3, DR-4, DR-7, DR-11, DR-13, and DR-15; and wherein the second HLA-DR restriction element is selected from: DR-4, DR-7, DR-11, DR-13, and DR-15. In embodiments, the assay panel comprises or further comprises at least one GA-specific human T-cell line that has a first known GA biomarker response profile, and at least one GA-specific human T-cell line that has a second known GA biomarker response profile. In embodiments, the first and second known biomarker response profiles each comprise at least one cytokine, at least one chemokine, at least one activation marker, at least one nucleic acid encoding a cytokine, at least one nucleic acid encoding a chemokine, or at least one nucleic acid encoding an activation marker. In embodiments, the first and second known GA biomarker response profiles each comprise at least one cytokine selected from: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, and IL-1b. In certain embodiments, the first and second known GA biomarker response profiles each comprise an activation marker selected from: CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, the first and second known GA biomarker response profiles each comprise a chemokine selected from: IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, the first and second known GA biomarker response profiles each comprise at least one nucleic acid encoding a cytokine selected from the group consisting of: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, and IL-1b. In embodiments, the first and second known GA biomarker response profiles each comprise at least one nucleic acid encoding an activation marker selected from: CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, the first and second known GA biomarker response profiles each comprise at least one nucleic acid encoding a chemokine selected from: IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, the GA-specific human T-cell lines in the assay panel are long-term T-cell lines. In embodiments, the GA-specific human T-cell lines in the assay panel are clonal. In related embodiments, the GA-specific human T-cell lines have been cultured for at least about four weeks and/or restimulated at least four times. In embodiments, the GA-specific human T-cell lines have been restimulated at least eight times. In embodiments, the non-canonical peptide is selected from peptide 026, GLT 631, GAT 631, GAT111, LT11, GT11, GT 41, GL 14, and GA 64. In embodiments, the non-canonical peptide is peptide 026. In embodiments, the assay panel comprises or further comprises two to six GA-specific human T-cell lines not reactive to a non-canonical GA peptide, wherein each GA-specific human T-cell line is not reactive to a different non-canonical GA peptide. In embodiments, the assay panel comprises or further comprises two GA-specific human T-cell lines not reactive to a non-canonical GA peptide, wherein one of the two GA-specific human T-cell lines is not reactive to a non-canonical GA peptide selected from peptide 026, GLT 631, GAT 631, GAT111, LT11, GT11, GT 41, GL 14, GT41S, and GA 64, and the second of the two GA-specific human T-cell lines is not reactive to a non-canonical GA peptide selected from peptide 026, GLT 631, GAT 631, GAT111, LT11, GT11, GT 41, GL 14, GT 41S, and GA 64. In embodiments, one of the two GA-specific human T-cell lines is not reactive to a non-canonical GA peptide selected from peptide 026, GLT 631, GAT 631, LT11, and GL 14, and the second of the two GA-specific human T-cell lines is not reactive to a non-canonical GA peptide selected from peptide 026, GLT 631, GAT 631, LT11, and GL 14. In embodiments, one of the two GA-specific human T-cell lines is not reactive to peptide 026, and the second of the two GA-specific human T-cell lines is not reactive to GLT 631, GAT 631, LT11, or GL 14. In certain embodiments, each GA-specific human T-cell line of (a), (b), and (c) is CD4+. In related embodiments, each GA-specific human T-cell line of (a), (b), and (c) comprises more than 98% CD4+ cells. In embodiments, each GA-specific human T-cell line of (a), (b), and (c) comprises more than 99% CD4+ cells. In embodiments, each GA-specific human T-cell line of (a), (b), and (c) is not CD8+. In related embodiments, each GA-specific human T-cell line of (a), (b), and (c) comprises less than 2% CD8+ cells. In certain embodiments, each GA-specific human T-cell line of (a), (b), and (c) comprises less than 1% CD8+ cells. The invention also relates to a method of determining whether a test preparation of glatiramer acetate (GA) and a GA reference standard are immunologically identical using an assay panel, the method comprising: 1) incubating cells of each GA-specific human T-cell line in the assay panel with appropriate APC; 2) stimulating a predetermined number of cells of each of the GA-specific human T-cell lines incubated in step (1) with an amount of the test preparation of GA; 3) separately stimulating the same predetermined number of cells of each of the GA-specific human T-cell lines incubated in step (1) with the same amount of the GA reference standard; 4) measuring at least one GA-elicited response of each of the GA-specific human T-cell lines stimulated in steps (2) and (3); and 5) comparing the measurement of the at least one GA-elicited response obtained for each GA-specific human T-cell line stimulated with the test preparation of GA with the measurement of the at least one GA-elicited response obtained for the same GA-specific human T-cell line stimulated with the GA reference standard; wherein the test preparation of GA and the GA reference standard are determined to be immunologically identical when the comparison of the measurements of step (5) falls within an acceptable range. In embodiments, the appropriate APC can comprise cells selected from the group consisting of: Epstein Barr Virus-transformed (EBV-transformed) human B-cells autologous to the GA-specific human T-cells; EBV-transformed human B-cells having the DR molecule involved in antigen presentation to the GA-specific human T-cells; PBMC autologous to the GA-specific human T cells; PBMC having the DR molecule involved in antigen presentation to the GA-specific human T-cells; purified monocytes autologous to the GA-specific human T-cells; purified monocytes having the DR molecule involved in antigen presentation to the GA-specific human T-cells; purified dendritic cells autologous to the GA-specific human T-cells; and purified dendritic cells having the DR molecule involved in antigen presentation to the GA-specific human T-cells. In embodiments, the acceptable range is about 80% to about 120%, or about 90% to about 110%. In embodiments of the above methods, the at least one measured GA-elicited response is selected from proliferation, production of a response biomarker, expression of a nucleic acid encoding a response biomarker, and a combination thereof. In embodiments, the at least one measured GA-elicited response is production of a response biomarker, and the response biomarker is a cytokine, a chemokine, or an activation marker. In certain embodiments, the response biomarker is a cytokine selected from: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21 IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), and IL-1b. In embodiments, the response biomarker is an activation marker selected from: CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, the response biomarker is a chemokine selected from: IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, the at least one measured GA-elicited response is expression of a nucleic acid encoding a response biomarker, and the response biomarker encoded by the nucleic acid is a cytokine, a chemokine, or an activation marker. In embodiments, the encoded response biomarker is a cytokine selected from: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β and IL-1b. In certain embodiments, the encoded response biomarker is an activation marker selected from: CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, the encoded response biomarker is a chemokine selected from: IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, at least two GA-elicited responses of the cells stimulated with the test preparation of GA are measured, and the same at least two GA-elicited responses of the cells stimulated with the GA reference standard, are measured. In these embodiments, the at least two GA-elicited responses can be selected from the expression of: IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, IL-1b, CD69, CD25, CD71, CD137, CD154, CD278, CD279, HLA-DR, IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, CXCL7, a nucleic acid encoding IL-2, a nucleic acid encoding IL-4, a nucleic acid encoding IL-5, a nucleic acid encoding IL-6, a nucleic acid encoding IL-10, a nucleic acid encoding IL-13, or a nucleic acid encoding IL-17, a nucleic acid encoding IL-22, a nucleic acid encoding IFN-γ, a nucleic acid encoding TNF-α (TNF), a nucleic acid encoding TNF-β (LT), a nucleic acid encoding TGF-β, a nucleic acid encoding IL-1b, a nucleic acid encoding CD69, a nucleic acid encoding CD25, a nucleic acid encoding CD71, a nucleic acid encoding CD137, a nucleic acid encoding CD154, a nucleic acid encoding CD278, a nucleic acid encoding CD279, a nucleic acid encoding HLA-DR, a nucleic acid encoding IL-8 (CXCL8), a nucleic acid encoding RANTES (CCL5), a nucleic acid encoding CCL1, a nucleic acid encoding CXCL4, and a nucleic acid encoding CXCL7. In embodiments, the GA reference standard is Copaxone or GMA. In embodiments, wherein the appropriate APC have at least one HLA-DR restriction element capable of presenting GA peptides. In certain embodiments, the at least one HLA-DR restriction element capable of presenting GA peptides is selected from: DR-1, DR-2, DR-3, DR-4, DR-7, DR-11, DR-13, and DR-15. In embodiments, wherein the at least one GA-specific human T-cell line incubated in step (a) is a long-term T-cell line. In embodiments, the at least one GA-specific human T-cell line is clonal. In embodiments, the at least one long-term GA-specific human T-cell line has been maintained in culture for at least about four weeks prior to stimulation, previously restimulated at least four times, or both. In embodiments, the at least one long-term GA-specific human T-cell line has been previously restimulated at least eight times. In embodiments, the maintenance in culture of the at least one long-term GA-specific human T-cell line comprises recurrent restimulation with GA and autologous APC, in the absence of mitogen. In embodiments, the stimulation includes stimulating each of at least three samples of GA-specific human T-cells, incubated with or in the presence of APC, with one of a series of amounts of the test preparation of GA, and stimulating each of at least three samples of GA-specific human T-cells, incubated with or in the presence of APC, with one of the same series of amounts of the GA reference standard. In embodiments, the series of amounts of the test preparation of GA, and the series of amounts of the GA reference standard, comprise escalating doses of GA of about 1 ng/mL to about 1 mg/mL GA. In embodiments, the series of amounts of the test preparation of GA, and the series of amounts of the GA reference standard, are escalating doses of GA of about 1 μg/mL to about 30 μg/mL GA. The invention also relates to a drug product or pharmaceutical composition containing GA, prepared by a process comprising: 1) preparing a test preparation of GA by reacting protected glatiramer acetate with hydrobromic acid to form trifluoroacetyl GA, treating said trifluoroacetyl copolymer-1 with aqueous piperidine solution to form GA, and purifying said GA; 2) determining whether the test preparation of GA and a GA reference standard are immunologically identical, by: a) incubating cells of the at least one GA-specific human T-cell line with appropriate antigen presenting cells (APC); b) stimulating at least one sample comprising a predetermined number of cells of the at least one GA-specific human T-cell line incubated in (a) with an amount of the test preparation of GA, and separately stimulating at least one sample comprising the same predetermined number of cells of the at least one GA-specific human T-cell line incubated in step (a) with the amount of the GA reference standard; c) measuring at least one GA-elicited response of the at least one GA-specific human T-cell line stimulated in step (b) with the test preparation of GA, and measuring the same at least one GA-elicited response of the at least one GA-specific human T-cell lines stimulated in step (b) with the GA reference standard; and d) comparing the measurement of the at least one GA-elicited response of the at least one sample of cells of the at least one GA-specific human T-cell line stimulated in step (b) with the test preparation of GA, with the measurement of the same at least one GA-elicited response of the at least one sample of cells of the at least one GA-specific human T-cell line stimulated in step (b) with the GA reference standard; wherein the test preparation of GA and the GA reference standard are determined to be immunologically identical when the comparison of the measurements of step (d) falls within an acceptable range, and wherein the test preparation of GA is admixed in the drug product or pharmaceutical composition if it is determined to have immunologic identity to the GA reference standard. The invention additionally relates to a drug product or pharmaceutical composition containing GA, prepared by a process comprising: 1) preparing a test preparation of GA by reacting protected glatiramer acetate with hydrobromic acid to form trifluoroacetyl GA, treating said trifluoroacetyl copolymer-1 with aqueous piperidine solution to form GA, and purifying said GA; 2) determining whether the test preparation of GA and the GA reference standard are immunologically identical using the method of claim 1-24, 25-46, or 139-160. The invention further relates to a kit comprising an assay panel of the invention, appropriate APC for each GA-specific human T-cell line in the assay panel, and a GA reference standard. All publications, patents, and patent applications mentioned in this specification are herein incorporated by reference to the same extent as if each individual publication, patent, or patent application was specifically and individually indicated to be incorporated by reference. The novel features of the invention are set forth with particularity in the appended claims. A better understanding of the features and advantages of the present invention will be obtained by reference to the following detailed description that sets forth illustrative embodiments, in which the principles of the invention are utilized, and the accompanying drawings. The mechanism of action of Copaxone® in Multiple Sclerosis is at least in part mediated by immunomodulation of T-cell activity, and the immunologic response of T-cells to GA is a specific and sensitive measure of epitopes present in a GA preparation. Because of the unique ability of individual T-cells to distinguish between different but very similar peptides, analyzing GA-elicited responses of GA-specific human T-cells in culture can distinguish immunologically relevant differences between preparations of GA. In this regard, equivalent GA-elicited responses of a GA-specific T-cell line to a test preparation of GA and a GA reference standard can indicate that the GA reference standard and GA test preparation have immunological identity. The present invention is based on the demonstration that GA-specific human T-cell lines can be generated and identified and used to test the immunological identity of different GA preparations. Further, it has been shown that GA-specific human T-cell lines that recognize different epitopes of GA can be generated. As a result, GA-specific human T-cell lines that recognize many if not all different epitopes of GA can be generated, identified, and used in parallel in assay panels to allow determination with a high degree of confidence that compared GA preparations have immunological identity. Glatiramer acetate (GA) consists of the acetate salts of synthetic polypeptides that contain four naturally occurring amino acids: L-glutamic acid, L-alanine, L-tyrosine, and L-lysine with an average molar fraction of 0.141, 0.427, 0.095, and 0.338, respectively. The average molecular weight of GA is 5,000-9,000 daltons. Chemically, GA is designated L-glutamic acid polymer with L-alanine, L-lysine and L-tyrosine, acetate (salt). The empirical formula of glatiramer acetate is (C5H9NO4.C3H7NO2—C6H14N2O2—C9H11NO3)x.xC2H4O2(CAS-147245-92-9, Physician's Desk Reference). As used herein, “GA” refers to glatiramer acetate, including, e.g., “GMA,” glatiramer acetate produced by Mylan Pharmaceuticals, Inc., and “COP,” or “Copaxone,” glatiramer acetate produced and sold by Teva Pharmaceuticals USA, Inc. Copaxone was approved by the FDA in 1996 for the treatment of relapsing-remitting multiple sclerosis (RRMS). Its composition is described in the literature, e.g., in U.S. Pat. No. 3,849,550, “Therapeutic copolymer,” and U.S. Pat. No. 5,800,808, “Copolymer-1 improvements in compositions of copolymers,” and in the product labeling for Copaxone® (Teva Pharmaceuticals USA, Inc.), each incorporated by reference herein in its entirety. GA can be produced by known and published methods. Methods for producing Copaxone have been described in, e.g., in U.S. Pat. No. 3,849,550, “Therapeutic copolymer,” and U.S. Pat. No. 5,800,808, “Copolymer-1 improvements in compositions of copolymers.” According to U.S. Pat. No. 5,800,808, Copolymer-1 may be prepared by methods known in the art, for example, by the process disclosed in U.S. Pat. No. 3,849,550, wherein the N-carboxyanhydrides of tyrosine, alanine, y-benzyl glutamate and E-N-trifluoro-acetyllysine are polymerised at ambient temperature in anhydrous dioxane with diethylamine as initiator. The deblocking of the y-carboxyl group of the glutamic acid is effected by hydrogen bromide in glacial acetic acid and is followed by the removal of the trifluoroacetyl groups from the lysine residues by 1M piperidine. In embodiments of the methods of the present invention, a GA-elicited response of a GA-specific human T-cell line to a test or production preparation, lot, or batch of GA is compared to the same GA-elicited response of a reference standard preparation of GA. In embodiments, a test preparation of GA is any preparation, lot or batch of GA desired to be tested. In embodiments, the test preparation of GA is a newly manufactured preparation of GA. In other embodiments, the test preparation of GA is not newly manufactured but is desired to be tested nonetheless, e.g., to evaluate shelf-life of the preparation. A reference standard lot of GA, or GA reference standard, also can be any preparation, lot or batch of GA as desired, e.g., GMA or COP. In embodiments, the methods of the invention are used to compare two or more test or production preparations to GA, to each other, or to any another preparation of GA as desired. In embodiments, the GA test preparation is a preparation of GMA. In embodiments, the GA test preparation and GA reference standard are different preparations of GMA. In embodiments, multiple test preparations of GA are compared with a GA reference standard. In embodiments, the preparations of GA compared are, e.g., a preparation of GMA and a preparation of COP, two preparations of GMA, or a preparation of GA made by another manufacturer and GMA or COP. The present invention relates to GA-specific human T-cell-line assays useful for evaluating GA preparations. These assays can ensure consistency of GA preparations, e.g., during manufacturing or following changes in manufacturing processes. In embodiments, the methods of the invention are used to determine whether a test preparation of GA and a GA reference standard are immunologically identical, by comparing at least one GA-elicited response of a GA-specific human T-cell line after separately stimulating the cell line with either the test preparation of GA or the GA reference standard. Based on the results presented herein, GA-specific human T-cell lines capable of discerning subtle differences in the composition of a stimulating preparation of GA can be generated and identified. When used to stimulate the GA-specific T-cell lines, subtle differences in stimulating antigen result in measurable differences in GA-elicited responses of the GA-specific human T-cell lines. Assays of the invention also are useful for determining the relative GA potency of a test preparation of GA, e.g., the potency of the GA test preparation as compared to that of a GA reference standard. These methods are useful for ensuring consistent potency among drug preparations in the manufacture of glatiramer acetate acceptable for pharmaceutical use. In embodiments of the invention, at least one GA-elicited response is measured in an assay of a test preparation of GA and compared to the same GA-elicited response to a GA reference standard to give a desired relative potency. Desired relative potency can be expressed, e.g., as a ratio of the response to the GA test preparation to the response to the GA reference standard, or as a part, fraction, or percentage, where the percentage is 100 when the measurements are equal. In embodiments of the present invention, the relative potency of GA in a production or test preparation is determined by comparing a value representing the immunologic response of a GA-specific human T-cell line to a test preparation of GA, to a value representing the same response of the same cell line to a GA reference standard. This potency determination describes the stimulation capacity of a test preparation of GA. In embodiments, a potency assay of the present invention is used to determine whether a preparation of GA has a desired potency. Initially, a sample of cells is obtained from a normal, healthy, GA-naïve donor. Donor PBMC are collected for use, e.g., by leukapheresis or venipuncture. The collected cells are stimulated at Day 0 with an amount of initiating peptide (a preparation of GA) at a density of about 5×105to about 1×106cells/mL. In embodiments, the collected cells initially are stimulated at a density of about 5×105cells/mL, about 6×105cells/mL, about 7×105cells/mL, about 8×105cells/mL, about 9×105cells/mL, or about 1×106cells/mL, cells/mL. The stimulated cells are cultured and in embodiments periodically are treated with a growth promoter, e.g., IL-2, before screening a portion for reactivity to the initiating peptide. A remaining portion of the culture of a cell line that is reactive to the initiating peptide is restimulated with the initiating peptide and appropriate APC, and a growth promoter added as needed. A portion of the restimulated GA-reactive cell line culture then is screened for antigen specificity by comparing the cells' response to stimulation with the initiating peptide in the presence of MHC-matched APC, and appropriate controls, e.g., other preparations of GA, and other (non-relevant) antigens, e.g., tetanus toxoid, in the presence of MHC-matched APC, using a specificity assay as described herein. A line that responds to GA and not to a non-relevant antigen is identified as a GA-specific human T-cell line. The remaining cell line culture is restimulated as before with the initiating peptide and appropriate APC, and a growth promoter added as needed, e.g., for cell line expansion or in preparation for an assay to confirm GA-specificity or further characterize the cell line. Characterization assays further test an identified GA-responsive human T-cell line, e.g., for MHC-restriction, response biomarker production in response to GA stimulation, or reactivity to a non-canonical GA peptide. In certain embodiments, screening a T-cell line for GA-reactivity and screening for GA-specificity are carried out together following the initial stimulation. In these embodiments, use of both a no antigen control and a non-relevant antigen control, each in the presence of MHC-matched APC, are contemplated. In embodiments, an initial determination of GA-specificity of a human T-cell line is confirmed following restimulation of the cell line. In embodiments, an identified GA-specific human T-cell line is demonstrated to be GA-specific after multiple rounds of restimulation, e.g., after at least 2 to 10 rounds of restimulation or more. In embodiments, an identified GA-specific human T-cell line is demonstrated to be GA-specific after at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least 10, 3 to 10, 4 to 10, 5 to 10, 6 to 10, 7 to 10, 8 to 10, 9 to 10, 2 to 9, 2 to 8, 2 to 7, 2 to 6, 2 to 5, 2 to 4, 3 to 9, 3 to 8, 3 to 7, 3 to 6, 3 to 5, 4 to 9, 4 to 8, 4 to 7, 4 to 6, 5 to 9, 5 to 8, 5 to 7, 6 to 9, 6 to 8, 7 to 9, or 8 to 10 rounds of restimulation. In embodiments, the collected cells are restimulated at a density of about 1×105to about 1×106cells/mL, about 1×105cells/mL, about 1.5×105cells/mL, about 2×105cells/mL, about 2.5×105cells/mL, about 3×105cells/mL, about 3.5×105cells/mL, about 4×105cells/mL, about 4.5×105cells/mL, about 5×105cells/mL, about 6×105cells/mL, about 7×105cells/mL, about 8×105cells/mL, about 9×105cells/mL, or about 1×106cells/mL. In other embodiments of the present invention, GA-specific human T-cell lines identified by the described method, including GA-specific T-cell lines that respond to non-canonical GA peptides, are contemplated for use in the methods of the invention for determining immunological identity of GA preparations. The initiating peptide used for initial stimulation can be any GA preparation as desired, including, but not limited to, any preparation of COP or any preparation of GMA. In embodiments, the peptide used for restimulation is the same GA preparation as the initiating peptide. In embodiments, the concentration of peptide used for restimulation is the same as that used for the initial stimulation. In embodiments, the stimulating peptide (also referred to as “initiating peptide”) or restimulating peptide is added at a concentration of about 1 ng/mL to about 1 mg/mL. In embodiments, the concentration of peptide used for the initial stimulation or for restimulation is about 1 ng/mL to about 1 mg/mL, about 1 ng/mL to about 500 μg/mL, about 1 ng/mL to about 100 μg/mL, about 1 ng/mL to about 50 μg/mL, about 1 ng/mL to about 10 μg/mL, about 1 ng/mL to about 1 μg/mL, about 1 ng/mL to about 500 ng/mL, about 1 ng/mL to about 100 ng/mL, about 100 ng/mL to about 1 mg/mL, about 100 ng/mL to about 500 μg/mL, about 100 ng/mL to about 100 μg/mL, about 100 ng/mL to about 10 μg/mL, about 100 ng/mL to about 1 μg/mL, about 100 ng/mL to about 500 ng/mL, about 500 ng/mL to about 1 mg/mL, about 500 ng/mL to about 500 μg/mL, about 500 ng/mL to about 100 μg/mL, about 500 ng/mL to about 10 μg/mL, about 500 ng/mL to about 1 μg/mL, about 1 μg/mL to about 1 mg/mL, about 1 μg/mL to about 900 μg/mL, about 1 μg/mL to about 800 μg/mL, about 1 μg/mL to about 700 μg/mL, about 1 μg/mL to about 600 μg/mL, about 1 μg/mL to about 500 μg/mL, about 1 μg/mL to about 400 μg/mL, about 1 μg/mL to about 300 μg/mL, about 1 μg/mL to about 200 μg/mL, 1 μg/mL to about 100 μg/mL, 1 μg/mL to about 90 μg/mL, 1 μg/mL to about 80 μg/mL, 1 μg/mL to about 70 μg/mL, 1 μg/mL to about 60 μg/mL, 1 μg/mL to about 50 μg/mL, 1 μg/mL to about 40 μg/mL, 1 μg/mL to about 30 μg/mL, about 10 μg/mL to about 1 mg/mL, about 10 μg/mL to about 900 μg/mL, about 10 μg/mL to about 800 μg/mL, about 10 μg/mL to about 700 μg/mL, about 10 μg/mL to about 600 μg/mL, about 10 μg/mL to about 500 μg/mL, about 10 μg/mL to about 400 μg/mL, about 10 μg/mL to about 300 μg/mL, about 10 μg/mL to about 200 μg/mL, about 10 μg/mL to about 100 μg/mL, about 10 μg/mL to about 90 μg/mL, about 10 μg/mL to about 80 μg/mL, about 10 μg/mL to about 70 μg/mL, about 10 μg/mL to about 60 μg/mL, about 10 μg/mL to about 50 μg/mL, about 10 μg/mL to about 40 μg/mL, about 10 μg/mL to about 30 μg/mL, about 50 μg/mL to about 1 mg/mL, about 50 μg/mL to about 900 μg/mL, about 50 μg/mL to about 800 μg/mL, about 50 μg/mL to about 700 μg/mL, about 50 μg/mL to about 600 μg/mL, about 50 μg/mL to about 500 μg/mL, about 50 μg/mL to about 400 μg/mL, about 50 μg/mL to about 300 μg/mL, about 50 μg/mL to about 200 μg/mL, about 50 μg/mL to about 500 μg/mL, about 50 μg/mL to about 90 μg/mL, about 50 μg/mL to about 80 μg/mL, about 50 μg/mL to about 70 μg/mL, about 100 μg/mL to about 1 mg/mL, about 100 μg/mL to about 900 μg/mL, about 100 μg/mL to about 800 μg/mL, about 100 μg/mL to about 700 μg/mL, about 100 μg/mL to about 600 μg/mL, about 100 μg/mL to about 500 μg/mL, about 100 μg/mL to about 400 μg/mL, about 100 μg/mL to about 300 μg/mL, about 100 μg/mL to about 200 μg/mL, about 100 μg/mL to about 150 μg/mL, about 200 μg/mL to about 1 mg/mL, about 200 μg/mL to about 900 μg/mL, about 200 μg/mL to about 800 μg/mL, about 200 μg/mL to about 700 μg/mL, about 200 μg/mL to about 600 μg/mL, about 200 μg/mL to about 500 μg/mL, about 200 μg/mL to about 400 μg/mL, about 200 μg/mL to about 300 μg/mL, about 200 μg/mL to about 250 μg/mL, about 300 μg/mL to about 1 mg/mL, about 300 μg/mL to about 900 μg/mL, about 300 μg/mL to about 800 μg/mL, about 300 μg/mL to about 700 μg/mL, about 300 μg/mL to about 600 μg/mL, about 300 μg/mL to about 500 μg/mL, about 300 μg/mL to about 400 μg/mL, about 400 μg/mL to about 1 mg/mL, about 400 μg/mL to about 900 μg/mL, about 400 μg/mL to about 800 μg/mL, about 400 μg/mL to about 700 μg/mL, about 400 μg/mL to about 600 μg/mL, about 400 μg/mL to about 500 μg/mL, about 500 μg/mL to about 1 mg/mL, about 500 μg/mL to about 900 μg/mL, about 500 μg/mL to about 800 μg/mL, about 500 μg/mL to about 700 μg/mL, about 500 μg/mL to about 600 μg/mL, about 750 μg/mL to about 1 mg/mL, about 750 μg/mL to about 900 μg/mL, about 750 μg/mL to about 800 μg/mL, or about 800 μg/mL to about 1 mg/mL. In embodiments, initial stimulation is carried out by adding the initiating peptide at the desired concentration to a cell sample containing about 1×105cells to about 2×105cells. In embodiments, the cell sample volume is 100 to 200 μl, and the cell density in the sample is about 5×105cells/mL to about 1×106cells/mL. In embodiments, the initiating peptide is added to the cell sample at a final peptide concentration of about 1 μg/mL to about 100 μg/mL. In embodiments, one or more growth promoter is added one or more times to the cell culture or sample following initial stimulation or restimulation. In embodiments, the growth promoter is IL-2. The growth promoter can be added at regular or irregular intervals during the culturing, e.g., every 2 to 10 days as needed, depending on, e.g., the rate of cell growth. In embodiments, growth promoter is added every 2 days, every 3 days, every 4 days, every 5 days, every 6 days, every 7 days, every 8 days, every 9 days, or every 10 days. As known to one of skill in the art, the appropriate amount of growth promoter to add can be determined based on the growth characteristics of the culture, e.g., cell health as examined microscopically, and media pH as indicated by a pH indicator dye. In embodiments, about 5 to about 30 units (U) of IL-2 is added per mL of cell culture. In embodiments, about 10, about 15, about 17, about 18, about 19, about 20, about 21, about 22, about 23, about 5 to about 30, about 5 to about 25, about 5 to about 20, about 5 to about 15, about 5 to about 10, about 10 to about 30, about 10 to about 25, about 10 to about 20, about 10 to about 15, about 15 to about 30, about 15 to about 25, about 15 to about 20, about 20 to about 30, about 20 to about 25, about 25 to about 30, about 17 to about 23, about 18 to about 22, or about 19 to about 21 units of IL-2 are added to each mL of cell culture. In embodiments, about 20 U/mL IL-2 is added to the cell culture on D3 and D6 following initial stimulation. In embodiments, about 15 to about 25 U/mL IL-2 is added to the cell culture every 3 days following initial stimulation. In embodiments, about 15 to about 25 U/mL IL-2 is added to the cell culture 18 hours to 2 days following restimulation. In embodiments, about 20 U/mL IL-2 is added to the cell culture 18 hours to 24 hours following restimulation. In embodiments, the initiating peptide is added to a cell sample containing about 5×105cells/mL to about 1×106cells/mL. In embodiments, the initiating peptide is added to the cell sample at a final peptide concentration of about 1 μg/mL to about 100 μg/mL at a final concentration of about 1 μg/mL to about 100 μg/mL, and IL-2 is added to the cell sample at a final concentration of about 15 to about 25 U/mL. In embodiments, the initiating peptide is added to a cell sample containing about 5×105cells/mL to about 1×106cells/mL at a final concentration of about 1 μg/mL to about 100 μg/mL, and IL-2 is added to the cell sample at a final concentration of about 15 to about 25 U/mL at least twice after initial stimulation and before screening for reactivity. In embodiments, the initiating peptide is added to a cell sample containing about 5×105cells/mL to about 1×106cells/mL cells at a final concentration of about 1 μg/mL to about 100 μg/mL, and IL-2 is added to the cell sample at a final concentration of about 15 to about 25 U/mL at D3 and D6 following initial stimulation. In embodiments, the initiating peptide is added to a cell sample containing about 5×105cells/mL to about 1×106cells/mL at a final concentration of about 1 μg/mL to about 100 μg/mL, and IL-2 is added to the cell sample at a final concentration of about 15 to about 25 U/mL every 3 days following initial stimulation. At least about 7 to about 16 days after stimulation with the initiating peptide, as shown in The GA initiating peptide can be prepared by diluting the peptide in solution according to methods known in the art, e.g., by suspension in water and about 20 mg/mL mannitol, or in culture medium. GA preparation for bioassays is described, e.g., in U.S. Pat. No. 7,429,374, “Process for the measurement of the potency of glatiramer acetate,” incorporated by reference herein. As described and as depicted in In embodiments, proliferation of the APC prior to use is inhibited, by any known method, including exposure to γ-radiation or to an anti-mitotic agent, e.g., mitomycin C. In embodiments, a T-cell culture is screened for GA-reactivity at a cell density of about 1×105to about 5×105cells/mL, about 1×105cells/mL, about 1.5×105cells/mL, about 2×105cells/mL, about 2.5×105cells/mL, about 3×105cells/mL, about 3.5×105cells/mL, about 4×105cells/mL, about 4.5×105cells/mL, or about 5×105cells/mL. In embodiments, the APC are present at a number equal to the number of T-cells used to twice the number of T-cells used. In embodiments, autologous mitomycin-treated PBMC are present at about 2.5×105to about 5×106cells/mL. In embodiments, autologous mitomycin-treated PBMC are present at 1×106-2×106cells/mL. In embodiments, autologous mitomycin-treated PBMC are present at 2.5×106cells/mL. In embodiments, autologous mitomycin-treated B-LCL are present at about 1×105-1×106cells/mL. In embodiments, autologous mitomycin-treated B-LCL are present at about 1×105-5×105cells/mL. In embodiments, autologous mitomycin-treated B-LCL are present at about 1×105cells/mL, about 1.5×105cells/mL, about 2×105cells/mL, about 2.5×105cells/mL, or about 5×105cells/mL. In embodiments, the number of APC used in a screening or characterization assay in the methods of the invention is adjusted as needed to increase the GA-elicited response, for example, to a minimum desired level relative to a negative control. In embodiments, the minimum desired level of the GA-elicited response relative to the negative control is 1.5-fold (i.e., the response is about 50% higher) to about 1000-fold, as described below. The GA-elicited response measured is compared with the same response measured in a control, e.g., the response of the same T-cell line in a culture containing appropriate APC in the absence of antigen (GA), or in the presence of a non-relevant antigen suitable for serving as a negative control. The screening readout can be from any appropriate assay for a GA-elicited response preferred, including, but not limited to, a proliferation assay, or a response biomarker assay in which the amount of at least one cytokine, cytokine receptor, chemokine, or T-cell activation marker produced by the culture is measured. In embodiments, the GA-elicited response is the expression of a nucleic acid that encodes a response biomarker. Based on comparison of the GA-elicited response(s) measured in the T-cell lines cultured with GA and a control culture, GA-specific T-cell lines are identified. In embodiments, a GA-reactive or responsive T-cell line is identified by an increase in the at least one GA-elicited response measured in the test culture stimulated with GA that is statistically greater than the same at least one GA-elicited response measured in a no-antigen control culture or a non-relevant antigen control culture. In embodiments, a GA-reactive or responsive T-cell line is identified by an increase in a GA-elicited response of the test culture relative to the control of about 1.5-fold (i.e., the response is about 50% higher) to about 1000-fold, about 1.5 to about 2-fold, about 1.5 to about 3-fold, about 1.5 to about 4-fold, about 1.5 to about 5-fold, about 1.5 to about 6-fold, about 1.5 to about 7-fold, about 1.5 to about 8-fold, about 1.5 to about 9-fold, about 2 to about 3-fold, about 2 to about 4-fold, about 2 to about 5-fold, about 2 to about 6-fold, about 2 to about 7-fold, about 2 to about 8-fold, about 2 to about 9-fold, about 2 to about 10-fold, about 3 to about 4-fold, about 3 to about 5-fold, about 3 to about 6-fold, about 3 to about 7-fold, about 3 to about 8-fold, about 3 to about 9-fold, about 3 to about 10-fold, about 4 to about 5-fold, about 4 to about 6-fold, about 4 to about 7-fold, about 4 to about 8-fold, about 4 to about 9-fold, about 4 to about 10-fold, about 5 to about 6-fold, about 5 to about 7-fold, about 5 to about 8-fold, about 5 to about 9-fold, about 5 to about 10-fold, about 6 to about 7-fold, about 6 to about 8-fold, about 6 to about 9-fold, about 6 to about 10-fold, about 7 to about 8-fold, about 7 to about 9-fold, about 7 to about 10-fold, about 8 to about 9-fold, about 8 to about 10-fold, about 1.5-fold to about 80-fold, about 1.5-fold to about 70-fold, about 1.5-fold to about 60-fold, about 1.5-fold to about 50-fold, about 1.5-fold to about 40-fold, about 1.5-fold to about 30-fold, about 1.5-fold to about 20-fold, about 2-fold to about 80-fold, about 1.5-fold to about 70-fold, about 1.5-fold to about 60-fold, about 1.5-fold to about 50-fold, about 1.5-fold to about 40-fold, about 1.5-fold to about 30-fold, about 1.5-fold to about 20-fold, about 2-fold to about 80-fold, about 2-fold to about 70-fold, about 2-fold to about 60-fold, about 2-fold to about 50-fold, about 2-fold to about 40-fold, about 2-fold to about 30-fold, about 2-fold to about 20-fold, about 3-fold to about 80-fold, about 3-fold to about 70-fold, about 3-fold to about 60-fold, about 3-fold to about 50-fold, about 3-fold to about 40-fold, about 3-fold to about 30-fold, about 3-fold to about 20-fold, about 5-fold to about 80-fold, about 5-fold to about 70-fold, about 5-fold to about 60-fold, about 5-fold to about 50-fold, about 5-fold to about 40-fold, about 5-fold to about 30-fold, about 3-fold to about 20-fold, about 10-fold to about 80-fold, about 10-fold to about 60-fold, about 10-fold to about 40-fold, or about 10-fold to about 20-fold, at least about 1.5-fold, at least about 2-fold, at least about 2.5-fold, at least about 3-fold, at least about 3.5-fold, at least about 4-fold, at least about 4.5-fold, at least about 5-fold, at least about 6-fold, at least about 7-fold, at least about 8-fold, at least about 9-fold, or at least about 10-fold. In certain embodiments, a GA-reactive or responsive T-cell line is identified by an increase of at least 50% in proliferation of the test culture stimulated with GA, relative to proliferation of a no-antigen control culture or a non-relevant antigen control culture. In embodiments, a GA-reactive or responsive T-cell line is identified by an increase in two or more GA-elicited responses measured in the test culture stimulated with GA, relative to the same two or more GA-elicited response measured in a no-antigen control culture or a non-relevant antigen control culture. In embodiments, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more GA-elicited responses are measured. As further shown in A test of an identified GA-reactive T-cell line for GA specificity can serve as a confirmatory test for GA reactivity. In embodiments, GA-reactivity is confirmed before specificity testing. In embodiments, GA-reactivity of an identified GA-reactive or GA-specific human T-cell line is reconfirmed at any point during culturing, e.g., after thawing a frozen culture. In embodiments, when a confirmatory test for reactivity of a T-cell line to a GA reference standard is negative, the T-cell line no longer is considered GA-specific or reactive and can be discarded. In embodiments, a GA-specific human T-cell line is identified by a (mean) GA-elicited response measured in response to GA stimulation that is statistically greater than the (mean) negative control GA-elicited response. In embodiments, a GA-specific T-cell line is identified by an increase in the at least one GA-elicited response measured in the test culture stimulated with GA, relative to the same at least one GA-elicited response measured in the negative control culture. In embodiments, the increase is about 1.5 to about 10-fold (i.e., the response is about 50% higher to about 1000% higher), about 1.5 to about 2-fold, about 1.5 to about 3-fold, about 1.5 to about 4-fold, about 1.5 to about 5-fold, about 1.5 to about 6-fold, about 1.5 to about 7-fold, about 1.5 to about 8-fold, about 1.5 to about 9-fold, about 2 to about 10-fold, about 2 to about 3-fold, about 2 to about 4-fold, about 2 to about 5-fold, about 2 to about 6-fold, about 2 to about 7-fold, about 2 to about 8-fold, about 2 to about 9-fold, about 3 to about 10-fold, about 3 to about 4-fold, about 3 to about 5-fold, about 3 to about 6-fold, about 3 to about 7-fold, about 3 to about 8-fold, about 3 to about 9-fold, about 4 to about 10-fold, about 4 to about 5-fold, about 4 to about 6-fold, about 4 to about 7-fold, about 4 to about 8-fold, about 4 to about 9-fold, about 5 to about 10-fold, about 5 to about 6-fold, about 5 to about 7-fold, about 5 to about 8-fold, about 5 to about 9-fold, about 6 to about 10-fold, about 6 to about 7-fold, about 6 to about 8-fold, about 6 to about 9-fold, about 7 to about 10-fold, about 7 to about 8-fold, about 7 to about 9-fold, about 8 to about 10-fold, about 8 to about 9-fold, at least about 1.5-fold, at least about 2-fold, at least about 2.5-fold, at least about 3-fold, at least about 3.5-fold, at least about 4-fold, at least about 4.5-fold, at least about 5-fold, at least about 6-fold, at least about 7-fold, at least about 8-fold, at least about 9-fold, or at least about 10-fold. In embodiments, a GA-specific human T-cell line is identified using the following formula: GA-elicited response to test antigen minus the same response to control÷ GA-elicited response to reference antigen minus response to control=0.8 to 1.2, or 80% to 120%. The comparison is thus within acceptable range or limits. In these embodiments, the T-cell line is determined to be GA-specific based on a “single reference lot, single dose analysis.” In embodiments, the GA-specificity of a human T-cell line is identified when the single reference, single dose analysis comparison value is within the acceptable range at one GA dose, two different GA doses, three different GA doses, or more. In embodiments, the reference antigen used to obtain the denominator value of the formula above is the same antigen that was used to initiate and stimulate the T-cell line. In embodiments, the GA-elicited response observed after stimulation with the test antigen is not more than 20% above or below the value observed after stimulation with the antigen used to obtain the cell line. In embodiments, human T-cell lines are identified as GA-specific if they meet these criteria in the majority of assays run, show no major deviation at any concentration tested, and have similar dose-response curves with no major deviation in curve shape for test and reference antigen response values. Any appropriate control can be used in the above embodiments, e.g., a no antigen control or a non-relevant antigen control. In the above embodiments, the acceptable range is, e.g., about 80% to about 120%, about 75% to about 120%, about 75% to about 115%, about 75% to about 110%, about 75% to about 105%, about 75% to about 100%, about 80% to about 125%, about 85% to about 125%, about 90% to about 125%, about 95% to about 125%, about 100% to about 125%, about 80% to about 118%, about 80% to about 115%, about 80% to about 112%, about 80% to about 110%, about 80% to about 108%, about 80% to about 105%, about 80% to about 102%, about 80% to about 101%, about 80% to about 100%, about 80% to about 99%, about 80% to about 98%, about 80% to about 97%, about 80% to about 95%, about 80% to about 92%, about 80% to about 90%, about 82% to about 120%, about 82% to about 118%, about 82% to about 115%, about 82% to about 112%, about 82% to about 110%, about 82% to about 108%, about 82% to about 105%, about 82% to about 102%, about 82% to about 101%, about 82% to about 100%, about 82% to about 99%, about 82% to about 98%, about 82% to about 97%, about 82% to about 95%, about 82% to about 92%, about 82% to about 90%, about 84% to about 120%, about 84% to about 118%, about 84% to about 115%, about 84% to about 112%, about 84% to about 110%, about 84% to about 108%, about 84% to about 105%, about 84% to about 102%, about 84% to about 101%, about 84% to about 100%, about 84% to about 99%, about 84% to about 98%, about 84% to about 97%, about 84% to about 95%, about 84% to about 92%, about 84% to about 90%, about 86% to about 120%, about 86% to about 118%, about 86% to about 115%, about 86% to about 112%, about 86% to about 110%, about 86% to about 108%, about 86% to about 105%, about 86% to about 102%, about 86% to about 101%, about 86% to about 100%, about 86% to about 99%, about 86% to about 98%, about 86% to about 97%, about 86% to about 95%, about 86% to about 92%, about 88% to about 120%, about 88% to about 118%, about 88% to about 115%, about 88% to about 112%, about 88% to about 110%, about 88% to about 108%, about 88% to about 105%, about 88% to about 102%, about 88% to about 101%, about 88% to about 100%, about 88% to about 99%, about 88% to about 98%, about 88% to about 97%, about 88% to about 95%, about 90% to about 120%, about 90% to about 118%, about 90% to about 115%, about 90% to about 112%, about 90% to about 110%, about 90% to about 108%, about 90% to about 105%, about 90% to about 102%, about 90% to about 101%, about 90% to about 100%, about 90% to about 99%, about 90% to about 98%, about 90% to about 97%, about 90% to about 95%, about 92% to about 120%, about 92% to about 118%, about 92% to about 115%, about 92% to about 112%, about 92% to about 110%, about 92% to about 108%, about 92% to about 105%, about 92% to about 102%, about 92% to about 101%, about 92% to about 100%, about 92% to about 99%, about 92% to about 98%, about 95% to about 120%, about 95% to about 118%, about 95% to about 115%, about 95% to about 112%, about 95% to about 110%, about 95% to about 108%, about 95% to about 105%, about 95% to about 102%, about 95% to about 101%, about 95% to about 100%, about 97% to about 120%, about 97% to about 118%, about 97% to about 115%, about 97% to about 112%, about 97% to about 110%, about 97% to about 108%, about 97% to about 105%, about 97% to about 102%, about 98% to about 120%, about 98% to about 118%, about 98% to about 115%, about 98% to about 112%, about 98% to about 110%, about 98% to about 108%, about 98% to about 105%, about 99% to about 120%, about 99% to about 118%, about 99% to about 115%, about 99% to about 112%, about 99% to about 110%, about 99% to about 108%, about 99% to about 105%, about 100% to about 120%, about 100% to about 118%, about 100% to about 115%, about 100% to about 112%, about 100% to about 110%, about 100% to about 108%, about 100% to about 105%, about 101% to about 120%, about 101% to about 118%, about 101% to about 115%, about 101% to about 112%, about 101% to about 110%, about 101% to about 108%, about 102% to about 120%, about 102% to about 118%, about 102% to about 115%, about 102% to about 112%, about 102% to about 110%, about 102% to about 108%, about 105% to about 120%, about 105% to about 118%, about 105% to about 115%, about 105% to about 112%, about 105% to about 110%, about 110% to about 120%, about 110% to about 118%, or about 110% to about 115%. In embodiments, a GA-specific T-cell line is identified by an increase in at least 2, at least 3, at least 4, or more, different GA-elicited responses measured in the test culture stimulated with GA, relative to the same GA-elicited responses of the negative control culture. In embodiments, a GA-elicited response measured at different GA doses is used to generate a dose response curve, and GA-specificity is identified by comparison of the dose response curve obtained using the test GA lot to that obtained using the reference GA lot. In these embodiments, the GA-specificity of a human T-cell line is identified based on a “single-reference lot dose response curve analysis.” In these embodiments, the GA-specificity of a human T-cell line can be identified when the slopes in the linear range of the test GA lot and reference GA lot dose-response curves are statistically similar or identical as determined according to any appropriate statistical methods known in the art. In embodiments, to reach a determination of GA-specificity, the slope (13) of the reference lot dose response curve must meet appropriate acceptance criteria. Appropriate acceptance criteria can be predetermined by those of skill in the art. In certain embodiments, appropriate acceptance criteria are:
In embodiments, the GA-elicited response in 75% of positive control samples (e.g., cells stimulated with ConA) must be above the highest response elicited when the same cells are stimulated with any concentration of GA. In embodiments, the GA-elicited response in 75% of negative control samples (e.g., cells treated with a control peptide such as myelin basic protein, MBP) must be below or close to the lowest response elicited with any concentration of GA. Linear regression can be performed on the GA reference lot sample set, where the data points are plotted on a log-log scale. The log GA-elicited response values (by increasing concentration of analyte, shown below as IL-2) are on the Y-axis, and the log GA reference lot concentration (dose) values are on the X-axis. The best fit linear regression model used for the above data set is as follows: where Y=log10(mIL−2 concentration) and X=log10(GA concentration). Substituting X and Y variables with the appropriate representations, the above model becomes the following: In embodiments, GA-specificity is established when the slope of the test lot curve is within acceptable limits. The acceptable limits for the slope of the test lot curve are determined based on the reference lot curve using the following series of equations: The back-calculated dose value for a given log (response) is: The accuracy can be calculated by the following: Therefore, the region where the hypothesis of the equality of the slopes is to be accepted is: β* is calculated as follows: where β is the slope of the GA reference lot curve. Therefore, the acceptable range for the GA test lot slope, β*, determined by equation (6) can be displayed as: If β* is within the above acceptable limits, parallelism can be concluded. In embodiments, the coefficient of correlation is 0.90 to 0.98. In embodiments, the coefficient of correlation is greater than or equal to 0.90, greater than or equal to 0.91, greater than or equal to 0.92, greater than or equal to 0.93, greater than or equal to 0.94, greater than or equal to 0.95, greater than or equal to 0.96, greater than or equal to 0.97, greater than or equal to 0.98, 0.90 to 0.98, 0.91 to 0.98, 0.92 to 0.98, 0.93 to 0.98, 0.94 to 0.98, 0.95 to 0.98, 0.96 to 0.98, 0.90 to 0.97, 0.91 to 0.97, 0.92 to 0.97, 0.93 to 0.97, 0.94 to 0.97, 0.95 to 0.97, or 0.96 to 0.98. In embodiments, a true hypothesis test for equal slope is used. In embodiments, the GA-specific human T-cell lines are long-term GA-specific human T-cell lines, that is, they are demonstrated to maintain GA-specificity for an extended culturing period. In embodiments, the long-term GA-specific human T-cell lines of the invention are demonstrated to maintain GA-specificity after culturing for at least about 4 weeks to at least about 10 weeks, or longer, e.g., by rescreening to confirm GA-specificity. In embodiments, the GA-specific human T-cell lines of the invention are demonstrated to maintain GA-specificity after culturing at least about 4 weeks to at least about 12 weeks, or longer, not including any time spent in frozen storage. In embodiments, the GA-specific human T-cell lines of the invention are demonstrated to maintain GA-specificity after culturing at least about 4, at least about 5, at least about 6, at least about 7, at least about 8, at least about 9, at least about 10 weeks, at least about 11 weeks, at least about 12 weeks, about 4 to about 12 weeks, about 4 to about 11 weeks, about 4 to about 10 weeks, about 4 to about 9 weeks, about 4 to about 8 weeks, about 4 to about 7 weeks, about 4 to about 6 weeks, about 5 to about 12 weeks, about 5 to about 11 weeks, about 5 to about 10 weeks, about 5 to about 9 weeks, about 5 to about 8 weeks, about 5 to about 7 weeks, about 6 to about 12 weeks, about 6 to about 11 weeks, about 6 to about 10 weeks, about 6 to about 9 weeks, about 6 to about 8 weeks, about 7 to about 12 weeks, about 7 to about 11 weeks, about 7 to about 10 weeks, about 7 to about 9 weeks, about 8 to about 12 weeks, about 8 to about 11 weeks, about 8 to about 10 weeks, about 9 to about 12 weeks, or about 9 to about 11 weeks, not including any time spent in frozen storage. In embodiments, the long-term GA-specific human T-cell lines of the invention are demonstrated to maintain GA-specificity for an extended culturing period when grown in the presence of appropriate APC as described herein. In embodiments, the appropriate APC are autologous APC. In specific embodiments, the long-term GA-specific human T-cell lines of the invention are demonstrated to maintain GA-specificity long-term when grown in the absence of a mitogen. In embodiments, the mitogen that is absent is, e.g., phytohaemagglutinin (PHA), Concanavalin A (ConA), lipopolysaccharide, or Staphylococcal enterotoxin B (SEB). Long-term growth with maintenance of GA-specificity in the absence of a mitogen is a notable feature of GA-specific human T-cell lines of the present invention. In specific embodiments, the long-term GA-specific human T-cell lines of the invention are demonstrated to maintain GA-specificity in culture for at least about 4 weeks in culture or for at least 1 to 10 rounds of restimulation and expansion, when grown in the presence of GA and appropriate autologous APC, without added mitogen. In embodiments, an identified GA-specific human T-cell line is demonstrated to be GA-specific after multiple rounds of restimulation and expansion, e.g., after at least 1 to 10 rounds of restimulation and expansion or more. In embodiments, a long-term GA-specific human T-cell line is demonstrated to be GA-specific after at least 1, at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least 10, 2 to 10, 3 to 10, 4 to 10, 5 to 10, 6 to 10, 7 to 10, 8 to 10, 9 to 10, 1 to 9, 2 to 9, 3 to 9, 4 to 9, 5 to 9, 6 to 9, 7 to 9, 1 to 8, 2 to 8, 3 to 8, 4 to 8, 5 to 8, 6 to 8, 1 to 7, 2 to 7, 3 to 7, 4 to 7, 5 to 7, 1 to 6, 2 to 6, 3 to 6, 4 to 6, 1 to 5, 2 to 5, 3 to 5, 1 to 4, 2 to 4, or 1 to 3, rounds of restimulation and expansion. In embodiments, the GA-specific human T-cell lines are maintained at a cell density of about 1×105to about 1×106cells/mL, about 1×105cells/mL, about 1.5×105cells/mL, about 2×105cells/mL, about 2.5×105cells/mL, about 3×105cells/mL, about 3.5×105cells/mL, about 4×105cells/mL, about 4.5×105cells/mL, about 5×105cells/mL, about 6×105cells/mL, about 7×105cells/mL, about 8×105cells/mL, about 9×105cells/mL, or about 1×106cells/mL, during expansion. In embodiments, a GA-specific human T-cell line is determined to be GA-specific based on a comparison of the value obtained by measuring a GA-elicited response when the T-cell line is stimulated with a first preparation of GA, to the value obtained by measuring the same GA-elicited response when the T-cell line is stimulated with a second preparation of GA, wherein the comparison of the response value of the second preparation of GA to the response value of the first preparation of GA is within a predetermined acceptable range, and wherein the GA-specific human T-cell line is a long-term GA-specific human T-cell line. In embodiments, a GA-specific human T-cell line is determined to be GA-specific based on a comparison of the dose response curve obtained by measuring a GA-elicited response when the GA-specific human T-cell line is stimulated at multiple doses with a first preparation of GA, to the dose response curve obtained by measuring the same GA-elicited response when the GA-specific human T-cell line is stimulated at multiple doses with a second preparation of GA, wherein the comparison of the slope of the dose response curve of the second preparation of GA to the slope of the dose response curve of the first preparation of GA is within acceptable limits, and wherein the GA-specific human T-cell line is a long-term GA-specific human T-cell line. In either of the above two embodiments, the first preparation of GA can be the same preparation used to generate the GA-specific human T-cell line. In embodiments, the first preparation of GA is COP. In embodiments, the GA-specific human T-cell line is clonal. In embodiments, the GA-specific human T-cell line has a known MHC restriction. In embodiments, the GA-specific human T-cell line has a known MHC restriction selected from: DR-1, DR-2, DR-3, DR-4, DR-7, DR-11, DR-13, and DR-15. In embodiments, the GA-specific human T-cell line comprises at least 98% CD4+ T-cells, and not more than 2% CD8+ T-cells. In embodiments, the GA-specific human T-cell line is reactive to a noncanonical GA peptide. In embodiments, the GA-specific human T-cell line is reactive to a noncanonical GA peptide selected from: Peptide 026, GLT 631, GAT 631, GAT 111, GL 14, LT 11, GA 41S, GA 64, and GT 11. In embodiments, the GA-specific human T-cell line is not reactive to a noncanonical GA peptide. In embodiments, the GA-specific human T-cell line is not reactive to a noncanonical GA peptide selected from Peptide 026, GLT 631, GAT 631, GAT 111, GL 14, LT 11, GA 41S, GA 64, and GT 11. In embodiments, a cell line used in the methods of the present invention, e.g., an initial human T-cell line (unscreened), a GA-reactive human T-cell line (shown to be GA-reactive), or an identified GA-specific human T-cell line (shown to be GA-specific), is expanded before or after one or more screening stages. Expansion increases the number of cells in the culture and can be carried out at any time, and as many times, during the GA-specific T-cell line identification and characterization process described, as desired. Typically, expansion of a human T-cell line is carried out after establishing or after confirming the GA-specificity of the line. In embodiments, a cell line is expanded prior to being frozen for storage, or after thawing. In embodiments, a human T-cell line is expanded multiple times during a process as generally outlined in Expansion of a T-cell line is carried out by restimulating the T-cell line with the initiating peptide as described above for initial stimulation, in the presence of appropriate APC, and if needed, a growth promoter, e.g., IL-2. In embodiments, IL-2 is added about 12 to about 36 hours following restimulation and the culture is incubated for about 6 to about 14 days. In embodiments, this process is repeated, and portions of the culture frozen, as desired. In embodiments, expansion of a confirmed GA-specific human T-cell line is carried out by restimulating the cells with initiating peptide at a final concentration of about 1 μg/mL to about 100 μg/mL, adding APC at an amount that is about equal to the number of T-cells used to an amount that is about twice the number of T-cells used, and after about 12 to about 36 hours adding IL-2 at a final concentration of about 10 to about 25 U/mL. In embodiments, this process is repeated multiple times at intervals of about 6 days, about 7 days, about 8 days, about 9 days, about 10 days, about 11 days, about 12 days, about 13 days, about 14 days, about 7 to about 14 days, about 7 to about 13 days, about 7 to about 12 days, about 7 to about 11 days, about 7 to about 10 days, about 7 to about 9 days, about 8 to about 14 days, about 8 to about 13 days, about 8 to about 12 days, about 8 to about 11 days, about 8 to about 10 days, about 9 to about 14 days, about 9 to about 13 days, about 9 to about 12 days, about 9 to about 11 days, about 10 to about 14 days, about 10 to about 13 days, about 10 to about 12 days, about 10 to about 11 days, about 11 to about 14 days, about 11 to about 13 days, or about 12 to about 14 days. In certain embodiments, expansion of a confirmed GA-specific human T-cell line is carried out by restimulating the cells with initiating peptide at a final concentration of about 1 μg/mL to about 100 μg/mL, adding APC at an amount that is about equal to the number of T-cells used to an amount that is about twice the number of T-cells used, and after about 12 to about 36 hours adding IL-2 at a final concentration of about 10 to about 25 U/mL, and further incubating the cells for about 7 to about 10 days. The incubation time before restimulating to initiate another cycle of expansion, if desired, can be determined by any method known to one of skill in the art of tissue culture, e.g., by evaluating pH indicator dye color in the medium, and cell characteristics. In embodiments, at the end of the incubation period the expansion process is repeated by splitting the cultures according to methods known in the art and restimulating for further expansion. In embodiments, the expanded cells are frozen for storage, restimulated and tested for GA-specificity, or used in characterization tests as described herein. Any suitable T-cell expansion medium (permissive for development of the lymphoid lineage) known in the art can be used for growing the T-cell lines according to the methods of the present invention. In embodiments, a serum-free medium is used. In embodiments, the medium used is, e.g., AIM V (Invitrogen), X-VIVO 15® Medium (Lonza), Stemline® T Cell Expansion Medium (Sigma-Aldrich). In embodiments, the medium lacks animal serum, feeder cells, and cell-based extracellular matrices. The formulations of suitable media, including serum-free media, are known and described in the art, e.g., in U.S. Pat. No. 6,733,746, “Hematopoietic cell culture nutrient supplement” and U.S. Pat. No. 8,481,315, “Methods of expanding myeloid cell populations and uses thereof” both incorporated by reference herein. The GA-specific human T-cell lines of the present invention can be characterized as described herein. Based on the characteristics, e.g., immunological characteristics, of each GA-specific human T-cell line, panels comprising cell lines having different MHC restrictions, different biomarker profiles, and different responses to non-canonical GA peptides are assembled for use in a parallel assay format for comprehensive interrogation of the GA epitopes in a GA preparation. This strategy enables determination of the immunologic identity between different GA preparations with greater accuracy than possible using any previously described assay. As described, GA consists of the acetate salts of synthetic polypeptides that contain four naturally occurring amino acids: L-glutamic acid, L-alanine, L-tyrosine, and L-lysine. These amino acids are present at an average molar fraction of 0.141, 0.427, 0.095, and 0.338, respectively, in random sequence. Multiple epitopes undoubtedly are present on a given GA molecule. The more GA epitopes interrogated in an assay for determining whether GA test and reference standard preparations have immunologic identity, the more accurately the assay can identify a GA test preparation having a comparable clinical effect to the reference standard, e.g., the FDA-approved drug, Copaxone. However, the identification of antigenic epitopes in GA has eluded researchers, primarily because GA, as a mixture of peptides having many different amino acid sequences, is difficult to characterize using conventional techniques such as X-ray crystallography. The present invention overcomes this problem by using individual T-cells' unique ability to distinguish between different but similar antigen structures. The invention is based on the discovery that GA-specific human T-cell lines that recognize different epitopes in a GA preparation can be generated and identified. Using the methods of the present invention, GA-specific human T-cell lines that recognize different GA epitopes based on the cell lines' antigen recognition properties are obtained. A panel comprising GA-specific human T-cell lines that together recognize many different GA epitopes can be assembled and used to interrogate GA preparations. Characterization can be carried out by the methods described herein, or by any other method known in the art for characterizing antigen-specific T-cell lines, for example, as described by using T cell receptor spectratyping as described by Gorski, et al., 1994 May 15, (“Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping. Correlation with immune status,” J Immunol. 152(10):5109-19), or identifying T cell receptor Vβ chain expression using PCR as described by Choi, et al., 1989 November, “Interaction of In embodiments of the present invention, GA-specific human T-cell lines that recognize different epitopes in GA are identified based on their reactivity to stimulation with non-canonical GA peptides. Non-canonical GA peptides are peptides consisting of any subset of the four GA amino acids, or all four GA amino acids, in different average molar fraction than described for COP. Examples of non-canonical GA peptides are poly-glutamic acid, poly-alanine, poly-tyrosine, and poly-lysine, peptides containing only two or only three of the four GA amino acids (in any average molar fraction), and peptides having all four of the GA amino acids in different average molar fraction than described for Copaxone. Table 1 in the Examples provides a non-limiting list of certain non-canonical GA peptides, including peptide 026, which contains all four GA amino acids but was synthesized by withholding tyrosine for the first 5 minutes of GA manufacturing. Methods for synthesizing non-canonical GA peptides useful in the methods of the invention are described in the literature. Peptide 026 contains less tyrosine than GA and an altered distribution of amino acids over the length of each polypeptide species in the preparation. Many peptides suitable for use as non-canonical GA peptides in the methods of the present invention are commercially available, e.g., from Sigma-Aldrich (St. Louis, Mo.). The epitopes in a non-canonical GA peptide are expected to be present in canonical GA. A non-canonical peptide containing fewer than the four amino acids that make up GA is expected to contain a smaller number of different epitopes than GA. A non-canonical peptide containing all four GA amino acids, in an altered molar ratio relative to GA (i.e., the ratio described for Copaxone), is expected to contain GA epitopes in different relative proportion to that found in Copaxone, depending on the alteration. The successful identification of GA-specific human T-cell lines that are not reactive to a non-canonical peptide containing all four GA amino acids, e.g., peptide 026, as well as GA-specific human T-cell lines that are reactive to the same non-canonical peptide, demonstrates that GA-specific human T-cell lines having very fine differences in specificity can be generated. These GA-specific human T-cell lines can be useful for detecting an aberrant GA preparation. Thus, characterizing GA-specific human T-cell lines based on reactivity to different non-canonical GA peptides allows the identification of GA-specific human T-cell lines that when used in parallel in an assay panel can determine accurately whether GA preparations have immunological identity. A T-cell line assay panel that includes multiple GA-specific T-cell lines that each recognize a different epitope of GA can determine with greater accuracy the immunological identity of GA preparations when compared to a test that measures reactivity to only a single epitope, or a test that cannot distinguish between reactivity to different epitopes, e.g., an assay using a primary polyclonal T-cell culture obtained from a GA-immunized animal. In embodiments, the GA-specific human T-cell lines of the present invention are derived from one clone and therefore are clonal, i.e., monoclonal. In embodiments, they are derived from multiple clones. In embodiments where the GA-specific human T-cell lines are derived from multiple clones, they are derived from a few clones and are oligoclonal. A GA-specific human T-cell line that is reactive to a non-canonical GA peptide is expected to recognize a different epitope in GA than a GA-specific human T-cell line that does not react to the same non-canonical GA peptide. Thus, a GA-specific human T-cell line that reacts to a first non-canonical GA peptide but does not react to a second non-canonical peptide, a GA-specific human T-cell line that does not react to the first non-canonical GA peptide but does react to the second non-canonical peptide, and a GA-specific human T-cell line that reacts to neither the first or second non-canonical peptide, each can be presumed to recognize a different epitope of GA. As an example, a first GA-specific human T-cell line that reacts to GL 41, poly (Glu-Tyr; 4:1) but not to GL 14 (Glu-Lys; 1:4), a second GA-specific human T-cell line that reacts to GL 14 but not GL 41, and a third GA-specific human T-cell line that does not react to either GL 41 or GL 14, each recognizes a different GA epitope. In embodiments of the present invention, a combination of two or more GA-specific human T-cell lines that each react to a different non-canonical peptide are identified and used together in an assay panel to determine whether a GA test preparation contains the epitopes recognized by the GA-specific human T-cell lines in the panel. In embodiments, the assay panel includes two or more GA-specific human T-cell lines determined not to react to the non-canonical peptides. In embodiments, use of the panel includes testing the reactivity of one or more GA-specific human T-cell lines in the panel to a non-canonical peptide, by measuring a response of the T-cell line following stimulation with the non-canonical peptide. In embodiments, the response measured is a GA-elicited response as described above, e.g., proliferation, production of a response biomarker, or expression of a nucleic acid encoding a response biomarker. In embodiments, the reactivity of a GA-specific human T-cell line to a non-canonical peptide is tested by measuring a GA-elicited response at multiple timepoints following stimulation, or after stimulation with a series of different GA concentrations, to obtain a dose-response profile or curve, as described above. Reactivity to a non-canonical peptide can be determined using the same methods for determining reactivity to to GA, that is, by measuring a GA-elicited response to stimulation with the non-canonical peptide and comparing the measurement with a negative control, e.g., a no antigen control. In embodiments, a T-cell line that is reactive to a non-canonical peptide is identified by an increase in at least one GA-elicited response measured in a test culture of the T-cell line stimulated with the non-canonical peptide that is statistically greater than the same at least one GA-elicited response measured in a no-antigen control culture or a non-relevant antigen control culture. In embodiments, a T-cell line that is reactive to a non-canonical peptide is identified by an increase in a GA-elicited response of the test culture relative to the control of about 1.5 to about 10-fold (i.e., the response is about 50% higher to about 1000% higher), about 1.5 to about 2-fold, about 1.5 to about 3-fold, about 1.5 to about 4-fold, about 1.5 to about 5-fold, about 1.5 to about 6-fold, about 1.5 to about 7-fold, about 1.5 to about 8-fold, about 1.5 to about 9-fold, about 2 to about 10-fold, about 2 to about 3-fold, about 2 to about 4-fold, about 2 to about 5-fold, about 2 to about 6-fold, about 2 to about 7-fold, about 2 to about 8-fold, about 2 to about 9-fold, about 3 to about 10-fold, about 3 to about 4-fold, about 3 to about 5-fold, about 3 to about 6-fold, about 3 to about 7-fold, about 3 to about 8-fold, about 3 to about 9-fold, about 4 to about 10-fold, about 4 to about 5-fold, about 4 to about 6-fold, about 4 to about 7-fold, about 4 to about 8-fold, about 4 to about 9-fold, about 5 to about 10-fold, about 5 to about 6-fold, about 5 to about 7-fold, about 5 to about 8-fold, about 5 to about 9-fold, about 6 to about 10-fold, about 6 to about 7-fold, about 6 to about 8-fold, about 6 to about 9-fold, about 7 to about 10-fold, about 7 to about 8-fold, about 7 to about 9-fold, about 8 to about 10-fold, about 8 to about 9-fold, at least about 1.5-fold, at least about 2-fold, at least about 2.5-fold, at least about 3-fold, at least about 3.5-fold, at least about 4-fold, at least about 4.5-fold, at least about 5-fold, at least about 6-fold, at least about 7-fold, at least about 8-fold, at least about 9-fold, or at least about 10-fold. In embodiments of the present invention, a GA-specific human T-cell line is characterized by measuring the production and/or expression of at least one GA response biomarker by the GA-specific T-cell line after it is restimulated with GA. In embodiments, the GA-specific human T-cell line is characterized by measuring the production and/or expression of a set of GA response biomarkers, to generate a response biomarker profile. As shown herein in the Examples, different GA-specific human T-cell lines were demonstrated to have different GA-response biomarker profiles, that is, different lines produced different amounts of GA response biomarkers following restimulation with GA. Such differences can reflect subtle differences in the binding specificities of GA-specific human T-cell lines that make the use of these cell lines particularly useful for recognizing a difference between a GA test preparation and a GA reference standard. In embodiments, as described herein, the GA response biomarker profile of one or more GA-specific human T-cell line is characterized and the line used in the methods and/or assay panels of the invention to determine whether a GA test preparation and a GA reference standard are immunologically identical. The expression levels of the biomarkers in the GA response biomarker profile of a GA-specific human T-cell line stimulated with a GA test preparation are compared with the respective biomarker expression levels of the same GA-specific human T-cell line stimulated with the GA reference standard. The GA test preparation and GA reference standard are determined to be immunologically identical when the comparison value, of the expression level of each biomarker by the GA-specific human T-cell line following stimulation with the GA test preparation, and the expression level of each respective biomarker by the GA-specific human T-cell line following stimulation with the GA reference standard, falls within an acceptable range. In embodiments, the acceptable range for the comparison value is about 75% to about 125%, about 80% to about 120%, or about 90% to about 110%. In embodiments, the acceptable range is about 75% to about 120%, about 75% to about 115%, about 75% to about 110%, about 75% to about 105%, about 75% to about 100%, about 80% to about 125%, about 85% to about 125%, about 90% to about 125%, about 95% to about 125%, about 100% to about 125%, about 80% to about 118%, about 80% to about 115%, about 80% to about 112%, about 80% to about 110%, about 80% to about 108%, about 80% to about 105%, about 80% to about 102%, about 80% to about 101%, about 80% to about 100%, about 80% to about 99%, about 80% to about 98%, about 80% to about 97%, about 80% to about 95%, about 80% to about 92%, about 80% to about 90%, about 82% to about 120%, about 82% to about 118%, about 82% to about 115%, about 82% to about 112%, about 82% to about 110%, about 82% to about 108%, about 82% to about 105%, about 82% to about 102%, about 82% to about 101%, about 82% to about 100%, about 82% to about 99%, about 82% to about 98%, about 82% to about 97%, about 82% to about 95%, about 82% to about 92%, about 82% to about 90%, about 84% to about 120%, about 84% to about 118%, about 84% to about 115%, about 84% to about 112%, about 84% to about 110%, about 84% to about 108%, about 84% to about 105%, about 84% to about 102%, about 84% to about 101%, about 84% to about 100%, about 84% to about 99%, about 84% to about 98%, about 84% to about 97%, about 84% to about 95%, about 84% to about 92%, about 84% to about 90%, about 86% to about 120%, about 86% to about 118%, about 86% to about 115%, about 86% to about 112%, about 86% to about 110%, about 86% to about 108%, about 86% to about 105%, about 86% to about 102%, about 86% to about 101%, about 86% to about 100%, about 86% to about 99%, about 86% to about 98%, about 86% to about 97%, about 86% to about 95%, about 86% to about 92%, about 88% to about 120%, about 88% to about 118%, about 88% to about 115%, about 88% to about 112%, about 88% to about 110%, about 88% to about 108%, about 88% to about 105%, about 88% to about 102%, about 88% to about 101%, about 88% to about 100%, about 88% to about 99%, about 88% to about 98%, about 88% to about 97%, about 88% to about 95%, about 90% to about 120%, about 90% to about 118%, about 90% to about 115%, about 90% to about 112%, about 90% to about 110%, about 90% to about 108%, about 90% to about 105%, about 90% to about 102%, about 90% to about 101%, about 90% to about 100%, about 90% to about 99%, about 90% to about 98%, about 90% to about 97%, about 90% to about 95%, about 92% to about 120%, about 92% to about 118%, about 92% to about 115%, about 92% to about 112%, about 92% to about 110%, about 92% to about 108%, about 92% to about 105%, about 92% to about 102%, about 92% to about 101%, about 92% to about 100%, about 92% to about 99%, about 92% to about 98%, about 95% to about 120%, about 95% to about 118%, about 95% to about 115%, about 95% to about 112%, about 95% to about 110%, about 95% to about 108%, about 95% to about 105%, about 95% to about 102%, about 95% to about 101%, about 95% to about 100%, about 97% to about 120%, about 97% to about 118%, about 97% to about 115%, about 97% to about 112%, about 97% to about 110%, about 97% to about 108%, about 97% to about 105%, about 97% to about 102%, about 98% to about 120%, about 98% to about 118%, about 98% to about 115%, about 98% to about 112%, about 98% to about 110%, about 98% to about 108%, about 98% to about 105%, about 99% to about 120%, about 99% to about 118%, about 99% to about 115%, about 99% to about 112%, about 99% to about 110%, about 99% to about 108%, about 99% to about 105%, about 100% to about 120%, about 100% to about 118%, about 100% to about 115%, about 100% to about 112%, about 100% to about 110%, about 100% to about 108%, about 100% to about 105%, about 101% to about 120%, about 101% to about 118%, about 101% to about 115%, about 101% to about 112%, about 101% to about 110%, about 101% to about 108%, about 102% to about 120%, about 102% to about 118%, about 102% to about 115%, about 102% to about 112%, about 102% to about 110%, about 102% to about 108%, about 105% to about 120%, about 105% to about 118%, about 105% to about 115%, about 105% to about 112%, about 105% to about 110%, about 110% to about 120%, about 110% to about 118%, or about 110% to about 115%. In embodiments, more than one GA-specific human T-cell line, each having a different GA response biomarker profile, are used in an assay panel of GA-specific human T-cell lines to determine whether a test preparation of GA and a GA reference standard are immunologically identical. A GA response biomarker measured in the characterization of a GA-specific human T-cell line is, e.g., a cytokine, cytokine receptor, chemokine, T-cell activation marker, or a nucleic acid that encodes a cytokine, cytokine receptor, chemokine, or T-cell activation marker. In embodiments, a response biomarker measured in the characterization of a GA-specific human T-cell line is a cytokine selected from, e.g., IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, and IL-1b. In embodiments, a response biomarker is an activation marker selected from, e.g., CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, a response biomarker is a chemokine, selected from, e.g., IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, a response biomarker is a Th1-associated cytokine, a Th2-associated cytokine, a Th17-associated cytokine, or a ThFH-associated cytokine. In embodiments, a response biomarker is a Th1-associated cytokine and is IFN-γ. In embodiments, a response biomarker is a Th2-associated cytokine selected from: IL-4, IL-5, and IL-13. In embodiments, a response biomarker is a Th17-associated cytokine selected from: IL-17, and IL-22. In embodiments, a response biomarker is a ThFH-associated cytokine selected from: IL-21, and TGF-β. In embodiments, a response biomarker is a key regulatory associated cytokine selected from IL-10 and TGF-β. In embodiments, a response biomarker measured in the characterization of a GA-specific human T-cell line is a nucleic acid encoding a cytokine selected from, e.g., IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, and IL-1b. In embodiments, a response biomarker is a nucleic acid encoding an activation marker selected from, e.g., CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, a response biomarker is a nucleic acid encoding chemokine, selected from, e.g., IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, a response biomarker is a nucleic acid encoding Th1-associated cytokine, a nucleic acid encoding Th2-associated cytokine, a nucleic acid encoding Th17-associated cytokine, or a nucleic acid encoding ThFH-associated cytokine. In embodiments, a response biomarker is a nucleic acid encoding Th1-associated cytokine and is IFN-γ. In embodiments, a response biomarker is a nucleic acid encoding Th2-associated cytokine selected from: IL-4, IL-5, and IL-13. In embodiments, a response biomarker is a nucleic acid encoding Th17-associated cytokine selected from: IL-17, and IL-22. In embodiments, a response biomarker is a nucleic acid encoding a ThFH-associated cytokine selected from: IL-21, and TGF-β. In embodiments, a response biomarker is a nucleic acid encoding a key regulatory associated cytokine selected from IL-10 and TGF-β. In embodiments of the present invention, a GA-specific human T-cell line is characterized by testing its MHC restriction. T-cell lines that react to the same antigen through different MHC restriction elements may recognize different epitopes. MHC restriction therefore offers another parameter for immunologically distinguishing the epitope specificity of the GA-specific human T-cell lines of the invention, and thereby for selecting an appropriate panel of GA-specific human T-cell lines for use in an assay to determine whether GA preparations are immunologically identical. MHC restriction can be tested using any method known in the art e.g., as described by Oftung, et. al. (Oftung F., et al., 1994, “Mapping of multiple HLA class II restricted T-cell epitopes of the mycobacterial 70-kilodalton heat shock protein,” Infect. Immun. 62:5411-5418), or as set forth herein in the Examples. In embodiments of the present invention, a GA-specific human T-cell line is characterized by analyzing its expression of cell surface markers. In embodiments, a GA-specific human T-cell line used in the methods of the invention is CD4+. CD4 expression can be analyzed, and a CD4+ T-cell line identified, using methods known in the art. In embodiments, CD4 is analyzed by flow cytometry using a fluorochrome-labeled monoclonal antibody having specificity for CD4. In embodiments, a GA-specific human T-cell line is determined to be CD4+ when 98% or more of the cells in a sample tested are CD4+. In embodiments, a GA-specific human T-cell line is determined to be CD4+ when 99% or more of the cells in a sample tested are CD8+. In embodiments, a GA-specific human T-cell line determined to contain 97% or more, more than 97%, 97.5% or more, more than 97.5%, 98% or more, more than 98%, 98.5% or more, more than 98.5%, 99% or more, or more than 99% CD4+ cells is included in an assay panel of the invention. In embodiments, the GA-specific human T-cell line is CD8−. As with CD4, the presence of the CD8 marker can be analyzed using methods known in the art, e.g., by flow cytometry using a fluorochrome-labeled monoclonal antibody having reactivity to CD8. In embodiments, a GA-specific human T-cell line used in the methods of the invention is CD8−. In embodiments, CD8 is analyzed by flow cytometry using a fluorochrome-labeled monoclonal antibody having specificity for CD8. In embodiments, a control using nonspecific antibodies, e.g., fluorochrome-labeled mouse isotype matched control monoclonal antibody is included. In embodiments, a GA-specific human T-cell line used in the methods of the invention is CD4+ and CD8−. In embodiments, a GA-specific human T-cell line is determined to be CD8−when 2% or fewer of the cells in a sample tested are CD8+. In embodiments, a GA-specific human T-cell line is determined to be CD8−when 1% or fewer of the cells in a sample tested are CD8+. In embodiments, a GA-specific human T-cell line determined to contain 3% or less, less than 3%, 2.5% or less, less than 2.5%, 2% or less, less than 2%, 1.5% or less, less than 1.5%, 1% or less, or less than 1% CD8+ cells is included in an assay panel of the invention. In embodiments, a control using nonspecific antibodies, e.g., fluorochrome-labeled mouse isotype matched control monoclonal antibody IgG1 antibodies, is included as described in the Examples herein. As described, in the methods of the present invention GA elicited responses are measured in the context of identifying and characterizing GA-specific human T-cell lines. Reactivity and specificity screenings of human T-cell lines are carried out by measuring at least one GA-elicited response of a human T-cell line to stimulation with GA and comparison with a measurement of the same response of an appropriate control, e.g., in the absence of antigen. Similarly, a GA-specific human T-cell line of the invention can be further characterized as reactive to a non-canonical GA peptide by measurement of a response elicited by a GA-specific human T-cell line after stimulation with the non-canonical GA peptide. “GA-elicited response” as used herein refers to a response elicited by stimulation of a GA-specific human T-cell line after stimulation with GA or a non-canonical GA peptide. A GA-elicited response measured in the context of the present invention is a response observed following treatment of a T-cell line with a stimulating peptide (in the presence of appropriate APC) that is not observed in a suitable control, e.g., the same T-cell line treated with APC and no peptide, or the same T-cell line treated with APC and a non-relevant control antigen (a negative control). Evaluation of a GA-elicited response of a GA-specific T-cell line following stimulation with GA preparations can reveal the presence of minute differences between epitopes present in the GA preparations tested. In certain methods of the invention, a GA-elicited response of an identified GA-specific human T-cell line to a test preparation of GA and a GA reference standard is measured, and the measured GA-elicited responses are compared. Based on the comparison, it is determined whether the preparations of GA are immunologically identical. In related methods, comparing a GA-elicited response of an identified GA-specific human T-cell line allows determination of the potency of a first GA preparation, e.g., a GA test preparation, relative to that of a second GA preparation, e.g., a GA reference standard. In other methods described herein, a GA-elicited response is measured in the characterization of a GA-specific human T-cell line. A GA-elicited response in the context of the present invention can be a measure of, e.g., T-cell proliferation, production of a response biomarker, or expression of a nucleic acid encoding a response biomarker. In embodiments, a GA-elicited response is measured at multiple timepoints following restimulation. In embodiments, a GA-elicited response is measured after restimulation of a T-cell line with a series of different concentrations of a GA test preparation and a GA reference standard. In embodiments, this data is used to plot a dose-response curve, and the dose-response curve obtained for each GA preparation compared. In embodiments, compared GA preparations are determined to be immunologically identical when the slopes in the linear range of their respective dose-response curves are statistically similar or identical. Proliferation of T-cell lines in response to antigen can be evaluated using any suitable method known in the art. For example, proliferation can be evaluated by measuring uptake of tritiated thymidine, BrdU uptake (using, e.g., Millipore cat. #2752), by CFSE assay (using, e.g., CellTrace™ CFSE Cell Proliferation Kit, Life Technologies, and as described by Quah, et al., 2007, “Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester,” Nature Protocols 2(9): 2049-2056), or by ATP quantification to determine viable cell number (using, e.g., CellTiter Glo® Luminescent Cell Viability Assay, Promega, catalog #G7571). Methods for evaluating antigen-specific T-cells are described in the literature, e.g., in “Techniques for Immune Function Analysis,” Application Handbook 1st Edition, 2006, Becton, Dickinson and Company. A GA-specific T-cell line can be identified based on an increase in proliferation of the T-cell line after stimulation with GA and appropriate APC, when compared to the level after stimulation with no antigen or a control antigen and appropriate APC. In embodiments, a GA-specific T-cell line is identified based on an increase in proliferation represented by an increase in cell number, DNA synthesis (e.g., in a BrdU assay), or increased dilution of an intracellular dye (e.g., in a CFSE assay), wherein the increase is about 2-fold to about 10 fold relative to a control. In embodiments, the increase is at least about 2-fold, at least about 3-fold, at least about 4-fold, at least about 5-fold, at least about 6-fold, at least about 7-fold, at least about 8-fold, at least about 9-fold, at least about 10-fold, about 2-fold to about 9-fold, about 2-fold to about 8-fold, about 2-fold to about 7-fold, about 2-fold to about 6-fold, about 2-fold to about 5-fold, about 2-fold to about 4-fold, about 2-fold to about 3-fold, about 3-fold to about 10-fold, about 3-fold to about 9-fold, about 3-fold to about 8-fold, about 3-fold to about 7-fold, about 3-fold to about 6-fold, about 3-fold to about 5-fold, about 3-fold to about 4-fold, about 4-fold to about 10-fold, about 4-fold to about 9-fold, about 4-fold to about 8-fold, about 4-fold to about 7-fold, about 4-fold to about 6-fold, about 4-fold to about 5-fold, about 5-fold to about 10-fold, about 5-fold to about 9-fold, about 5-fold to about 8-fold, about 5-fold to about 7-fold, about 5-fold to about 6-fold, about 6-fold to about 10-fold, about 6-fold to about 9-fold, about 6-fold to about 8-fold, about 6-fold to about 7-fold, about 7-fold to about 10-fold, about 7-fold to about 9-fold, about 7-fold to about 8-fold, about 8-fold to about 10-fold, about 8-fold to about 9-fold, or about 9-fold to about 10-fold. In embodiments, a test preparation of GA and a GA reference standard are demonstrated to be immunologically identical when the comparison of the measurements (expressed as, e.g., a ratio, fraction, or percentage) of proliferation of a sample of GA-specific T-cells stimulated with the test preparation of GA and a sample of GA-specific T-cells stimulated with a GA reference standard falls within an acceptable range. In embodiments of the present invention, measurement of a GA-elicited response of a GA-specific human T-cell line to a test preparation of GA, and measurement of the same GA-elicited response of the GA-specific human T-cell line to a GA reference standard are compared. Based on the comparison, the test preparation of GA and the GA reference standard are determined to be immunologically identical. In embodiments, the test preparation of GA and the GA reference standard are determined to be immunologically identical when the comparison value falls within an acceptable range. In embodiments, the comparison value is expressed as a percentage, where the percentage is 100 when the measurements are equal. In these embodiments, the acceptable range for the comparison value is about 75% to about 125%, about 80% to about 120%, or about 90% to about 110%. In embodiments, the acceptable range is about 75% to about 120%, about 75% to about 115%, about 75% to about 110%, about 75% to about 105%, about 75% to about 100%, about 80% to about 125%, about 85% to about 125%, about 90% to about 125%, about 95% to about 125%, about 100% to about 125%, about 80% to about 118%, about 80% to about 115%, about 80% to about 112%, about 80% to about 110%, about 80% to about 108%, about 80% to about 105%, about 80% to about 102%, about 80% to about 101%, about 80% to about 100%, about 80% to about 99%, about 80% to about 98%, about 80% to about 97%, about 80% to about 95%, about 80% to about 92%, about 80% to about 90%, about 82% to about 120%, about 82% to about 118%, about 82% to about 115%, about 82% to about 112%, about 82% to about 110%, about 82% to about 108%, about 82% to about 105%, about 82% to about 102%, about 82% to about 101%, about 82% to about 100%, about 82% to about 99%, about 82% to about 98%, about 82% to about 97%, about 82% to about 95%, about 82% to about 92%, about 82% to about 90%, about 84% to about 120%, about 84% to about 118%, about 84% to about 115%, about 84% to about 112%, about 84% to about 110%, about 84% to about 108%, about 84% to about 105%, about 84% to about 102%, about 84% to about 101%, about 84% to about 100%, about 84% to about 99%, about 84% to about 98%, about 84% to about 97%, about 84% to about 95%, about 84% to about 92%, about 84% to about 90%, about 86% to about 120%, about 86% to about 118%, about 86% to about 115%, about 86% to about 112%, about 86% to about 110%, about 86% to about 108%, about 86% to about 105%, about 86% to about 102%, about 86% to about 101%, about 86% to about 100%, about 86% to about 99%, about 86% to about 98%, about 86% to about 97%, about 86% to about 95%, about 86% to about 92%, about 88% to about 120%, about 88% to about 118%, about 88% to about 115%, about 88% to about 112%, about 88% to about 110%, about 88% to about 108%, about 88% to about 105%, about 88% to about 102%, about 88% to about 101%, about 88% to about 100%, about 88% to about 99%, about 88% to about 98%, about 88% to about 97%, about 88% to about 95%, about 90% to about 120%, about 90% to about 118%, about 90% to about 115%, about 90% to about 112%, about 90% to about 110%, about 90% to about 108%, about 90% to about 105%, about 90% to about 102%, about 90% to about 101%, about 90% to about 100%, about 90% to about 99%, about 90% to about 98%, about 90% to about 97%, about 90% to about 95%, about 92% to about 120%, about 92% to about 118%, about 92% to about 115%, about 92% to about 112%, about 92% to about 110%, about 92% to about 108%, about 92% to about 105%, about 92% to about 102%, about 92% to about 101%, about 92% to about 100%, about 92% to about 99%, about 92% to about 98%, about 95% to about 120%, about 95% to about 118%, about 95% to about 115%, about 95% to about 112%, about 95% to about 110%, about 95% to about 108%, about 95% to about 105%, about 95% to about 102%, about 95% to about 101%, about 95% to about 100%, about 97% to about 120%, about 97% to about 118%, about 97% to about 115%, about 97% to about 112%, about 97% to about 110%, about 97% to about 108%, about 97% to about 105%, about 97% to about 102%, about 98% to about 120%, about 98% to about 118%, about 98% to about 115%, about 98% to about 112%, about 98% to about 110%, about 98% to about 108%, about 98% to about 105%, about 99% to about 120%, about 99% to about 118%, about 99% to about 115%, about 99% to about 112%, about 99% to about 110%, about 99% to about 108%, about 99% to about 105%, about 100% to about 120%, about 100% to about 118%, about 100% to about 115%, about 100% to about 112%, about 100% to about 110%, about 100% to about 108%, about 100% to about 105%, about 101% to about 120%, about 101% to about 118%, about 101% to about 115%, about 101% to about 112%, about 101% to about 110%, about 101% to about 108%, about 102% to about 120%, about 102% to about 118%, about 102% to about 115%, about 102% to about 112%, about 102% to about 110%, about 102% to about 108%, about 105% to about 120%, about 105% to about 118%, about 105% to about 115%, about 105% to about 112%, about 105% to about 110%, about 110% to about 120%, about 110% to about 118%, or about 110% to about 115%. In related methods, the comparison value is used as a measure of the potency of a first GA preparation, e.g., a GA test preparation, relative to that of a second GA preparation, e.g., a GA reference standard. In embodiments, the GA-elicited response measured in the methods of the invention is the production of a response biomarker. In embodiments, the response biomarker is a cytokine, cytokine receptor, chemokine, T-cell activation marker, or a nucleic acid that encodes a cytokine, cytokine receptor, chemokine, or T-cell activation marker (which can be a cytokine receptor). In embodiments, a GA-response biomarker is a cytokine, cytokine receptor, chemokine, T-cell activation marker, or a nucleic acid that encodes a cytokine, cytokine receptor, chemokine, or T-cell activation marker. In embodiments, a response biomarker is a cytokine selected from, e.g., IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, and IL-1b. In embodiments, a response biomarker is an activation marker selected from, e.g., CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, a response biomarker is a chemokine, selected from, e.g., IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, a response biomarker is a Th1-associated cytokine, a Th2-associated cytokine, a Th17-associated cytokine, or a ThFH-associated cytokine. In embodiments, a response biomarker is a Th1-associated cytokine and is IFN-γ. In embodiments, a response biomarker is a Th2-associated cytokine selected from: IL-4, IL-5, and IL-13. In embodiments, a response biomarker is a Th17-associated cytokine selected from: IL-17, and IL-22. In embodiments, a response biomarker is a ThFH-associated cytokine selected from: IL-21, and TGF-β. In embodiments, a response biomarker is a key regulatory associated cytokine selected from IL-10 and TGF-β. In embodiments, a response biomarker is a nucleic acid encoding a cytokine selected from, e.g., IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, and IL-1b. In embodiments, a response biomarker is a nucleic acid encoding an activation marker selected from, e.g., CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, a response biomarker is a nucleic acid encoding chemokine, selected from, e.g., IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, a response biomarker is a nucleic acid encoding Th1-associated cytokine, a nucleic acid encoding Th2-associated cytokine, a nucleic acid encoding Th17-associated cytokine, or a nucleic acid encoding ThFH-associated cytokine. In embodiments, a response biomarker is a nucleic acid encoding Th1-associated cytokine and is IFN-γ. In embodiments, a response biomarker is a nucleic acid encoding Th2-associated cytokine selected from: IL-4, IL-5, and IL-13. In embodiments, a response biomarker is a nucleic acid encoding Th17-associated cytokine selected from: IL-17, and IL-22. In embodiments, a response biomarker is a nucleic acid encoding ThFH-associated cytokine selected from: IL-21, and TGF-β. In embodiments, a response biomarker is a nucleic acid encoding a key regulatory associated cytokine selected from IL-10 and TGF-β. The production of a response biomarker protein can be measured in according to any suitable method known in the art and published in the literature, e.g., by ELISA, Western Blot assay, flow cytometric assay, or any appropriate multiplex assay. Commercial assays are available for measuring production of cytokines, chemokines, cytokine receptors, and activation markers as called for in the methods of the present invention. For example, multiplex assays for detection of human cytokines and chemokines can be created using the BD™ Cytometric Bead Array Flex Set system (BD Biosciences, San Jose, Calif.). A GA-specific human T-cell line can be identified or characterized by comparing the production of a response biomarker after restimulation with GA in the presence of appropriate APC, to the amount of response biomarker produced in an appropriate negative control, e.g., after restimulation with no antigen or a non-relevant control antigen (in the presence of appropriate APC). In embodiments, a GA-specific human T-cell line is identified based on an increase in response biomarker production relative to the control. In embodiments, the increase observed in production of the response biomarker relative to a negative control is about 1.5-fold to about 1000-fold. response of the test culture relative to the control of about 1.5-fold (i.e., the response is about 50% higher) to about 1000-fold, about 1.5 to about 2-fold, about 1.5 to about 3-fold, about 1.5 to about 4-fold, about 1.5 to about 5-fold, about 1.5 to about 6-fold, about 1.5 to about 7-fold, about 1.5 to about 8-fold, about 1.5 to about 9-fold, about 2 to about 3-fold, about 2 to about 4-fold, about 2 to about 5-fold, about 2 to about 6-fold, about 2 to about 7-fold, about 2 to about 8-fold, about 2 to about 9-fold, about 2 to about 10-fold, about 3 to about 4-fold, about 3 to about 5-fold, about 3 to about 6-fold, about 3 to about 7-fold, about 3 to about 8-fold, about 3 to about 9-fold, about 3 to about 10-fold, about 4 to about 5-fold, about 4 to about 6-fold, about 4 to about 7-fold, about 4 to about 8-fold, about 4 to about 9-fold, about 4 to about 10-fold, about 5 to about 6-fold, about 5 to about 7-fold, about 5 to about 8-fold, about 5 to about 9-fold, about 5 to about 10-fold, about 6 to about 7-fold, about 6 to about 8-fold, about 6 to about 9-fold, about 6 to about 10-fold, about 7 to about 8-fold, about 7 to about 9-fold, about 7 to about 10-fold, about 8 to about 9-fold, about 8 to about 10-fold, about 1.5-fold to about 80-fold, about 1.5-fold to about 70-fold, about 1.5-fold to about 60-fold, about 1.5-fold to about 50-fold, about 1.5-fold to about 40-fold, about 1.5-fold to about 30-fold, about 1.5-fold to about 20-fold, about 2-fold to about 80-fold, about 1.5-fold to about 70-fold, about 1.5-fold to about 60-fold, about 1.5-fold to about 50-fold, about 1.5-fold to about 40-fold, about 1.5-fold to about 30-fold, about 1.5-fold to about 20-fold, about 2-fold to about 80-fold, about 2-fold to about 70-fold, about 2-fold to about 60-fold, about 2-fold to about 50-fold, about 2-fold to about 40-fold, about 2-fold to about 30-fold, about 2-fold to about 20-fold, about 3-fold to about 80-fold, about 3-fold to about 70-fold, about 3-fold to about 60-fold, about 3-fold to about 50-fold, about 3-fold to about 40-fold, about 3-fold to about 30-fold, about 3-fold to about 20-fold, about 5-fold to about 80-fold, about 5-fold to about 70-fold, about 5-fold to about 60-fold, about 5-fold to about 50-fold, about 5-fold to about 40-fold, about 5-fold to about 30-fold, about 3-fold to about 20-fold, about 10-fold to about 80-fold, about 10-fold to about 60-fold, about 10-fold to about 40-fold, or about 10-fold to about 20-fold, at least about 1.5-fold, at least about 2-fold, at least about 2.5-fold, at least about 3-fold, at least about 3.5-fold, at least about 4-fold, at least about 4.5-fold, at least about 5-fold, at least about 6-fold, at least about 7-fold, at least about 8-fold, at least about 9-fold, or at least about 10-fold. A nucleic acid encoding a response biomarker can be quantitatively measured using any of a number of commercially available assay kits and systems, or according to any method described in the art. In embodiments, a response biomarker mRNA is measured by Real-Time PCR. The use of any appropriate quantitative method for measuring mRNA expression levels known in the art is contemplated. For example, mRNA can be copied by reverse transcriptase, and amplified using a appropriate PCR method, including RT-PCR, and real time reverse-transcription PCR (qRT-PCR). PCR methods for quantitating gene expression are described by, e.g., VanGuilder, et al., 2008, “Twenty-five years of quantitative PCR for gene expression analysis,” Biotechniques 44: 619-626, and Bustin, et al., 2005, “Quantitative real-time RT-PCR—a perspective,” Journal of Molecular Endocrinology, 34:597-601, each incorporated herein by reference in its entirety. Quantitative PCR of mouse cytokine mRNAs is described by, e.g., Overbergh, et al., 1999, “Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR,” Cytokine 11(4): 305-312, incorporated herein by reference, describing probes and primers for quantifying IL-1, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12, p40, IL-13, IL-15, IFN-γ, TNF-α, TGF-β and iNOS. Methods for measuring response biomarker mRNAs following ex vivo GA stimulation of mouse lymph node cells, are described in, e.g., U.S. Pat. App. Pub. No. 2014/0272987, “Glatiramer Acetate Response Biomarker mRNA Potency Assay,” incorporated by reference in its entirety herein. A GA-specific human T-cell line can be identified or characterized by comparing the production of a nucleic acid encoding a response biomarker after restimulation with GA in the presence of appropriate APC, to the amount of the nucleic acid encoding the response biomarker produced in an appropriate negative control, e.g., after restimulation with no antigen or a non-relevant control antigen (in the presence of appropriate APC). In embodiments, a GA-specific human T-cell line is identified based on an increase in production of a nucleic acid encoding a response biomarker relative to the control. In embodiments, the increase observed in production of the nucleic acid encoding the response biomarker relative to a negative control is about 1.5-fold to about 1000-fold. In embodiments, the increase is about 1.5-fold to about 100-fold, about 1.5-fold to about 50-fold, about 1.5-fold to about 45-fold, about 1.5-fold to about 40-fold, about 1.5-fold to about 35-fold, about 1.5-fold to about 30-fold, about 1.5-fold to about 25-fold, about 1.5-fold to about 22-fold, about 1.5-fold to about 20-fold, about 1.5-fold to about 15-fold, about 1.5-fold to about 12-fold, about 1.5-fold to about 10-fold, about 1.5-fold to about 9-fold, about 1.5-fold to about 8-fold, about 1.5-fold to about 7-fold, about 1.5-fold to about 6-fold, about 1.5-fold to about 5-fold, about 1.5-fold to about 4-fold, about 1.5-fold to about 3-fold, about 2-fold to about 100-fold, about 2-fold to about 50-fold, about 2-fold to about 45-fold, about 2-fold to about 40-fold, about 2-fold to about 35-fold, about 2-fold to about 30-fold, about 2-fold to about 25-fold, about 2-fold to about 22-fold, about 2-fold to about 20-fold, about 2-fold to about 15-fold, about 2-fold to about 12-fold, about 2-fold to about 10-fold, about 2-fold to about 9-fold, about 2-fold to about 8-fold, about 2-fold to about 7-fold, about 2-fold to about 6-fold, about 2-fold to about 5-fold, about 2-fold to about 4-fold, about 3-fold to about 100-fold, about 3-fold to about 50-fold, about 3-fold to about 45-fold, about 3-fold to about 40-fold, about 3-fold to about 35-fold, about 3-fold to about 30-fold, about 3-fold to about 25-fold, about 3-fold to about 22-fold, about 3-fold to about 20-fold, about 3-fold to about 15-fold, about 3-fold to about 12-fold, about 3-fold to about 10-fold, about 3-fold to about 9-fold, about 3-fold to about 8-fold, about 3-fold to about 7-fold, about 3-fold to about 6-fold, about 3-fold to about 5-fold, about 4-fold to about 100-fold, about 4-fold to about 50-fold, about 4-fold to about 45-fold, about 4-fold to about 40-fold, about 4-fold to about 35-fold, about 4-fold to about 30-fold, about 4-fold to about 25-fold, about 4-fold to about 22-fold, about 4-fold to about 20-fold, about 4-fold to about 15-fold, about 4-fold to about 12-fold, about 4-fold to about 10-fold, about 4-fold to about 9-fold, about 4-fold to about 8-fold, about 4-fold to about 7-fold, about 4-fold to about 6-fold, about 5-fold to about 100-fold, about 5-fold to about 50-fold, about 5-fold to about 45-fold, about 5-fold to about 40-fold, about 5-fold to about 35-fold, about 5-fold to about 30-fold, about 5-fold to about 25-fold, about 5-fold to about 22-fold, about 5-fold to about 20-fold, about 5-fold to about 15-fold, about 5-fold to about 12-fold, about 5-fold to about 10-fold, about 5-fold to about 9-fold, about 5-fold to about 8-fold, about 5-fold to about 7-fold, about 7-fold to about 100-fold, about 7-fold to about 50-fold, about 7-fold to about 45-fold, about 7-fold to about 40-fold, about 7-fold to about 35-fold, about 7-fold to about 30-fold, about 7-fold to about 25-fold, about 7-fold to about 22-fold, about 7-fold to about 20-fold, about 7-fold to about 15-fold, about 7-fold to about 12-fold, about 7-fold to about 10-fold, about 7-fold to about 9-fold, about 10-fold to about 100-fold, about 10-fold to about 50-fold, about 10-fold to about 45-fold, about 10-fold to about 40-fold, about 10-fold to about 35-fold, about 10-fold to about 30-fold, about 10-fold to about 25-fold, about 10-fold to about 22-fold, about 10-fold to about 20-fold, about 10-fold to about 15-fold, about 15-fold to about 100-fold, about 15-fold to about 50-fold, about 15-fold to about 45-fold, about 15-fold to about 40-fold, about 15-fold to about 35-fold, about 15-fold to about 30-fold, about 15-fold to about 25-fold, about 15-fold to about 22-fold, about 15-fold to about 20-fold, about 20-fold to about 100-fold, about 20-fold to about 50-fold, about 20-fold to about 45-fold, about 20-fold to about 40-fold, about 20-fold to about 35-fold, about 20-fold to about 30-fold, about 20-fold to about 25-fold, about 25-fold to about 100-fold, about 25-fold to about 50-fold, about 25-fold to about 45-fold, about 25-fold to about 40-fold, about 25-fold to about 35-fold, about 25-fold to about 30-fold, about 30-fold to about 100-fold, about 30-fold to about 50-fold, about 40-fold to about 100-fold, about 50-fold to about 100-fold, about 60-fold to about 100-fold, about 70-fold to about 100-fold, at least about 2-fold, at least about 3-fold, at least about 4-fold, at least about 5-fold, at least about 6-fold, at least about 7-fold, at least about 8-fold, at least about 9-fold, at least about 10-fold, at least about 11-fold, at least about 12-fold, at least about 13-fold, at least about 14-fold, at least about 15-fold, at least about 20-fold, at least about 25-fold, at least about 30-fold, at least about 35-fold, at least about 40-fold, at least about 50-fold, at least about 60-fold, at least about 70-fold, at least about 80-fold, at least about 90-fold, at least about 100-fold, at least about 250-fold, at least about 500-fold, or at least about 1000-fold. The invention relates to a process for preparing a drug product or pharmaceutical composition containing GA. In embodiments of this process, glatiramer acetate is prepared according to standard methods known in the art and described in the literature, e.g., in U.S. Pat. Nos. 3,849,550 and 5,800,808. In embodiments, a drug product or pharmaceutical composition containing GA is prepared by: reacting protected glatiramer acetate with hydrobromic acid to form trifluoroacetyl GA, treating said trifluoroacetyl copolymer-1 with aqueous piperidine solution to form the test preparation of GA, and purifying the test preparation of GA. The test preparation of GA and the GA reference standard are determined to be immunologically identical or not immunologically identical, using the methods and/or assay panels as described herein. For example, the test preparation of GA and the GA reference standard are determined to be immunologically identical or not immunologically identical, by (a) incubating cells of at least one GA-specific human T-cell line with appropriate antigen presenting cells (APC), (b) stimulating at least one sample of the GA-specific human T-cells and appropriate APC incubated in step (a) with an amount of the test preparation of GA, and separately stimulating at least one sample of the GA-specific human T-cells and appropriate APC incubated in step (a) with the same amount of the GA reference standard, (c) measuring at least one GA-elicited response of the at least one sample of cells stimulated in step (b) with the test preparation of GA, and measuring the same at least one GA-elicited response of the at least one sample of cells stimulated in step (b) with the GA reference standard, and (d) comparing the measurements obtained in step (c), wherein the test preparation of GA and the GA reference standard are determined to be immunologically identical when the comparison of the measurements of step (d) falls within an acceptable range, and wherein the test preparation of GA is admixed into the drug product or pharmaceutical composition if it is determined to be immunologically identical to the GA reference standard. In embodiments, the drug product or pharmaceutical composition additionally contains excipients and/or diluents, for example, as described in the package labeling for Copaxone®. In certain embodiments, the methods of the invention are used to determine whether a GA test preparation and a GA reference standard are immunologically identical, or have an acceptable relative potency, prior to potential addition of a GA preparation to a drug product or pharmaceutical composition after addition of the GA preparation, or at any other time as desired. In embodiments, a test preparation of GA is added to other components, e.g., a diluent, an excipient, or another active ingredient, of a GA drug product or pharmaceutical composition, only if it is determined to have immunologic identity or comparable potency to the GA reference standard. Potency assays including methods for evaluating data comparing GA preparations are described in the literature, e.g., in U.S. Pat. No. 7,429,374, “Process for the measurement of the potency of glatiramer acetate,” incorporated by reference herein in its entirety. Additional response biomarkers can be identified by comparing the amount of a potential response biomarker, present in a GA-specific T-cell culture stimulated with GA in the presence of appropriate APC, to the amount of the response biomarker present in a control culture incubated with appropriate APC but not antigen stimulated, or a control culture stimulated with a control antigen. A suitable GA response biomarker for use in the methods of the invention can be identified by comparing the amount of a potential GA response biomarker protein or a nucleic acid encoding the protein, present in a GA-specific human T-cell line stimulated with GA in the presence of appropriate APC, to the amount of the same potential GA response biomarker protein or a nucleic acid encoding the protein in an appropriate negative control. A suitable GA response biomarker is significantly modulated (increased or decreased) in a GA-specific human T-cell line stimulated with GA in the presence of appropriate APC relative to the negative control. In embodiments, a GA response biomarker for a GA-specific human T-cell line is identified based on an increase in its production after GA restimulation of the GA-specific human T-cell line of about 2-fold to about 1000-fold relative to a negative control. As described, the invention contemplates the use of at least one GA-specific human T-cell line in a method for determining the immunological identity of GA preparations, e.g., a GA test preparation and a GA reference standard. Because GA contains a number of epitopes, generating and identifying GA-specific human T-cell lines by stimulating them with GA and testing their responsiveness to GA, it was not necessarily known that GA-specific human T-cell lines having the ability to recognize different epitopes could be obtained. Based on the discovery that GA-specific human T-cell lines with distinguishable epitope binding specificities can be obtained, the present invention provides assays using multiple GA-specific human T-cell lines to determine with greater accuracy the immunological identity of GA preparations. In embodiments of the invention, an assay panel of GA-specific human T-cell lines is used to analyze GA preparations, e.g., to test for immunological identity and/or determine their degree of similarity or dissimilarity. In embodiments, the assay panel comprises multiple GA-specific human T-cell lines. In embodiments, the assay panel comprises 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, or 50 different GA-specific human T-cell lines. In embodiments, the assay panel comprises at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least 10, at least 15, at least 20, at least 25, at least 30, at least 40, at least 50, at least 60, at least 70, at least 80, at least 90, or at least 100 different GA-specific human T-cell lines. In embodiments, the assay panel comprises 2 to 100, 2 to 50, 2 to 25, 2 to 10, 5 to 100, 5 to 50, 5 to 25, 5 to 10, 10 to 100, 10 to 50, 10 to 25, or 50 to 100, different GA-specific human T-cell lines. In embodiments of the invention, the assay panel comprises clonal GA-specific human T-cell lines, oligoclonal GA-specific human T-cell lines, or a combination of both. In embodiments, the assay panel comprises a combination of clonal and oligoclonal GA-specific human T-cell lines, wherein the majority (>50%) are clonal GA-specific human T-cell lines. In embodiments, the assay panel comprises a combination of clonal and oligoclonal GA-specific human T-cell lines, wherein the panel is comprised of about 20% or greater, about 25% or greater, about 30% or greater, about 35% or greater, about 40% or greater, about 45% or greater, about 50% or greater, about 55% or greater, about 60% or greater, about 65% or greater, about 70% or greater, about 75% or greater, about 80% or greater, about 85% or greater, about 90% or greater, about 95% or greater, or about 100%, clonal GA-specific human T-cell lines. In embodiments, the assay panel comprises a combination of clonal and oligoclonal GA-specific human T-cell lines, wherein the panel is comprised of about 20% to about 100%, about 25% to about 100%, about 30% to about 100%, about 35% to about 100%, about 40% to about 100%, about 45% to about 100%, about 50% to about 100%, about 55% to about 100%, about 60% to about 100%, about 65% to about 100%, about 70% to about 100%, about 75% to about 100%, about 80% to about 100%, about 85% to about 100%, about 90% to about 100%, or about 95% to about 100%, clonal GA-specific human T-cell lines. In embodiments, the assay panel comprises a combination of clonal and oligoclonal GA-specific human T-cell lines, wherein the panel is comprised of ⅙ or greater, ⅓ or greater, ⅔ or greater, ¾ or greater, or ⅚ or greater, clonal GA-specific human T-cell lines. In embodiments, all GA-specific human T-cell lines in an assay panel are clonal. A T-cell line can be cloned from a population of T cells using any method known to those of skill in the art and described in the literature, for example, limiting dilution cloning. In limiting dilution cloning, a candidate cell line is cultured in multiple wells at limiting dilution (e.g., 0.3 cells/well, or not more than one cell/well) with antigen and APC. The resulting cells are restimulated as described herein. Methods for T-cell cloning are widely known and described in the literature, e.g., by Mariotti, S., and Nisini, R., 2009, “Generation of Human T Cell Clones,” in T Cell Protocols, Gennaro de Libero (ed.), Humana Press, Second edition, vol. 514, pages 65-93, incorporated herein by reference in its entirety. In embodiments, the limiting dilution cloning process is repeated one or more times to obtain a clonal GA-specific human T-cell line. A T-cell line also can be cloned using flow cytometry methods, e.g., as described by Lee, S-T, et al., 2008, “A Novel Strategy for Rapid and Efficient Isolation of Human Tumor-Specific CD4+ and CD8+ T-Cell Clones,” J. Imm. Meth. 331(1-2): 13-26, incorporated herein by reference in its entirety. In embodiments, clonality of a T-cell line is confirmed by T-cell receptor sequencing according to methods known to those of skill in the art and published in the literature. In embodiments, an assay panel comprises GA-specific human T-cell lines generated by culturing human T-cells in the presence of a first preparation of GA, and at least one GA-specific human T-cell line generated by culturing human T-cells in the presence of a second preparation of GA. In embodiments, the first preparation of GA is COP and the second preparation of GA is GMA. In embodiments, the first and second preparations of GA are different COP preparations or different GMA preparations. In embodiments, an assay panel of GA-specific human T-cell lines comprises at least one GA-specific human T-cell line that produces a similar or identical GA-elicited response following stimulation with a first preparation of GA as it does following stimulation with a second preparation of GA. In embodiments, the first preparation of GA is COP and the second preparation of GA is GMA. In embodiments, the first and second preparations of GA are different COP preparations or different GMA preparations. In embodiments, the panel comprises at least one GA-specific human T-cell line that responds to stimulation with a first non-canonical GA peptide. In embodiments, the panel comprises at least one GA-specific human T-cell line that responds to stimulation with a first non-canonical GA peptide, and at least one GA-specific human T-cell line that responds to stimulation with a second non-canonical GA peptide. In embodiments, the panel comprises at least two GA-specific human T-cell lines that each produce a different response biomarker profile in response to stimulation with GA. In embodiments, the assay panel comprises at least two GA-specific human T-cell lines that each has a different MHC restriction. In embodiments, the assay panel of GA-specific human T-cell lines comprises at least two GA-specific human T-cell lines, wherein at least one of the at least two GA-specific cell lines is: a) a GA-specific human T-cell line that does not respond to stimulation with a non-canonical GA peptide; b) a GA-specific human T-cell line that has a known biomarker response profile; and c) a GA-specific human T-cell line that has a known MHC restriction. In embodiments, an assay panel of the invention comprises at least one GA-specific human T-cell line generated by culturing human T-cells in the presence of a first preparation of GA, and at least one GA-specific human T-cell line generated by culturing human T-cells in the presence of a second preparation of GA. In embodiments, this assay panel further comprises at least one GA-specific human T-cell line that does not react to a non-canonical GA peptide. In embodiments, the at least one GA-specific human T-cell line generated by culturing human T-cells in the presence of a first preparation of GA is generated by culturing human T-cells in the presence of GMA. In embodiments, the GA-specific human T-cell lines in an assay panel are selected from: 1) a GA-specific human T-cell line that was generated by culturing with a first preparation of GA 2) a GA-specific human T-cell line that was generated by culturing with a second preparation of GA 3) a GA-specific human T-cell line that does not respond to stimulation with a first non-canonical GA peptide; 4) a GA-specific human T-cell line that responds to stimulation with the first non-canonical GA peptide; 5) a GA-specific human T-cell line that does not respond to stimulation with a second non-canonical GA peptide; 6) a GA-specific human T-cell line that responds to stimulation with a second non-canonical GA peptide; 7) a GA-specific human T-cell line that has a known biomarker response profile; 8) a GA-specific human T-cell line that has a known biomarker response profile different from that of the GA-specific human T-cell line of (7); 9) a GA-specific human T-cell line that has a known MHC restriction; and 10) a GA-specific human T-cell line that has a known MHC restriction different from that of the GA-specific human T-cell line of (9). In embodiments, the GA-specific human T-cell lines are long-term GA-specific human T-cell lines, and/or clonal GA-specific human T-cell lines, and/or CD4+GA-specific human T-cell lines, and/or CD8-GA-specific human T-cell lines. In embodiments, the assay panel includes one or more T-cell lines listed in Table A. In embodiments, the assay panel includes one or more T-cell lines listed in Table B. In embodiments, the assay panel comprises one or more positive or negative controls. In embodiments, when the assay panel includes one or more GA-specific human T-cell line that does not respond to stimulation with a non-canonical GA peptide, it further comprises one or more GA-specific human T-cell line that responds to stimulation with the same non-canonical GA peptide. For example, the assay panel can comprise a first characterized GA-specific human T-cell line determined to not respond to stimulation with a non-canonical GA peptide, and a second characterized GA-specific human T-cell line determined to respond to stimulation with the non-canonical GA peptide. In embodiments, the first and second characterized GA-specific human T-cell lines both respond to stimulation with the non-canonical GA peptide but each responds differently (e.g., they each produce a different GA response biomarker profile). In related embodiments, a negative control is included for any GA-specific human T-cell line determined to respond or not to respond to stimulation with a non-canonical GA peptide. In embodiments, the negative control sample contains the cell line, appropriate APC, and no antigen. In embodiments, the assay panel includes one or more GA-specific human T-cell line that has a known biomarker response profile, and one or more GA-specific human T-cell line that has a different known biomarker response profile. For example, the assay panel comprises a characterized GA-specific human T-cell line determined to have a first known biomarker response profile, and a characterized GA-specific human T-cell line determined to have a second known biomarker response profile different from the first known biomarker response profile. In embodiments, the first and second known biomarker response profiles each comprise at least one cytokine, at least one chemokine, at least one activation marker, at least one nucleic acid encoding a cytokine, at least one nucleic acid encoding a chemokine, or at least one nucleic acid encoding an activation marker. In related embodiments, a negative control is included for any GA-specific human T-cell line having a known biomarker response profile. In embodiments, the negative control sample contains the cell line, appropriate APC, and no antigen. In embodiments, the assay panel includes one or more GA-specific human T-cell line that has a known MHC restriction, and one or more GA-specific human T-cell line that has a different known MHC restriction. In embodiments, the assay panel includes one or more GA-specific human T-cell line that has a known MHC restriction wherein the HLA-DR restriction element is selected from: DR-1, DR-2, DR-3, DR-4, DR-7, DR-11, DR-13, and DR-15; and one or more GA-specific human T-cell line that has a different known MHC restriction wherein the HLA-DR restriction element is selected from: DR-4, DR-7, DR-11, DR-13, and DR-15. For example, the assay panel comprises a characterized GA-specific human T-cell line determined to have first a known MHC restriction, and a characterized GA-specific human T-cell line determined to have a second known MHC restriction different from the first known MHC restriction. In related embodiments, a negative control is included for any GA-specific human T-cell line determined to have a different known MHC restriction. In embodiments, the negative control sample contains the cell line, non-matched APC, and GA. In embodiments, the negative control sample contains the cell line, appropriate APC, and no antigen. In embodiments, an assay panel comprises at least two GA-specific human T-cell lines, wherein each of the at least two GA-specific human T-cell lines is determined to be GA-specific based on a comparison of the response obtained by measuring a GA-elicited response when the T-cell line is stimulated with a first preparation of GA, to the response obtained by measuring the same GA-elicited response when the T-cell line is stimulated with a second preparation of GA, and wherein the comparison of the response of the second preparation of GA to the response of the first preparation of GA is within acceptable range or limits, and wherein each of the at least two GA-specific human T-cell lines is a long-term GA-specific human T-cell line. In embodiments, the first preparation of GA was used to generate the GA-specific human T-cell line. In embodiments, the first preparation of GA is Copaxone. In embodiments, an assay panel comprises at least two GA-specific human T-cell lines, wherein each of the at least two GA-specific human T-cell lines is determined to be GA-specific based on a comparison of the dose response curve obtained by measuring a GA-elicited response when the GA-specific human T-cell line is stimulated at multiple doses with at least a first preparation of GA, to the dose response curve obtained by measuring the same GA-elicited response when the GA-specific human T-cell line is stimulated at multiple doses with a second preparation of GA, wherein the comparison of the slope of the dose response curve of the second preparation of GA to the slope of the dose response curve of the first preparation of GA is within acceptable range or limits, and wherein each of the at least two GA-specific human T-cell lines is a long-term GA-specific human T-cell line. In embodiments, the first preparation of GA was used to generate the GA-specific human T-cell line. In embodiments, the first preparation of GA is Copaxone. In embodiments, at least one of the at least two GA-specific human T-cell lines in the assay panel is reactive to a noncanonical GA peptide. In embodiments, at least one of the at least two GA-specific human T-cell lines in the assay panel is reactive to a noncanonical GA peptide selected from: Peptide 026, GLT 631, GAT 631, GAT 111, GL 14, LT 11, GA 41S, GA 64, and GT 11. In embodiments, at least one of the at least two GA-specific human T-cell lines in the assay panel is not reactive to a noncanonical GA peptide. In embodiments, at least one of the at least two GA-specific human T-cell lines in the assay panel is not reactive to a noncanonical GA peptide selected from Peptide 026, GLT 631, GAT 631, GAT 111, GL 14, LT 11, GA 41S, GA 64, and GT 11. In embodiments, at least one of the at least two GA-specific human T-cell lines in the assay panel is reactive to a noncanonical GA peptide and wherein at least one of the at least two GA-specific human T-cell lines in the assay panel is not reactive to the same noncanonical GA peptide. In embodiments, at least one of the at least two GA-specific human T-cell lines in the assay panel is reactive to a noncanonical GA peptide selected from Peptide 026, GLT 631, GAT 631, GAT 111, GL 14, LT 11, GA 41S, GA 64, and GT 11, and wherein at least one of the at least two GA-specific human T-cell lines in the assay panel is not reactive to the same noncanonical GA peptide. In embodiments, at least one of the at least two GA-specific human T-cell lines in the assay panel is clonal. In embodiments, all of the at least two GA-specific human T-cell lines in the assay panel are clonal. In embodiments, the at least two GA-specific human T-cell lines in the assay panel have different MHC restrictions. In embodiments, at least two GA-specific human T-cell lines in the assay panel include one or more GA-specific human T-cell line that has a known MHC restriction wherein the HLA-DR restriction element is selected from: DR-1, DR-2, DR-3, DR-4, DR-7, DR-11, DR-13, and DR-15; and one or more GA-specific human T-cell line that has a different known MHC restriction wherein the HLA-DR restriction element is selected from: DR-4, DR-7, DR-11, DR-13, and DR-15. In embodiments, the at least two GA-specific human T-cell lines in the assay panel comprise at least 98% CD4+ T-cells and not more than 2% CD8+ T-cells. In embodiments of the invention, an assay panel of the invention is used in a method of determining whether a test preparation of GA and a GA reference standard are immunologically identical. In these embodiments, cell samples are prepared using a predetermined number of cells of each GA-specific human T-cell line in the panel, appropriate APC, and an amount of the test preparation of GA. Cell samples also are prepared using the same predetermined number of cells of each GA-specific human T-cell line in the panel, appropriate APC, and an amount of the GA reference standard. In embodiments, control samples are prepared. In embodiments, a negative control is prepared using the same predetermined number of cells of each GA-specific human T-cell line in the panel, no APC, and no antigen. In embodiments, a control is prepared using the same predetermined number of cells of each GA-specific human T-cell line in the panel, appropriate APC, and a non-relevant antigen. In embodiments, a negative control is prepared using the same predetermined number of cells of each GA-specific human T-cell line in the panel, and non-matched APC. In embodiments, the same at least one to at least 25 GA-elicited responses are measured in each cell sample. In embodiments, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least 10, at least 11, at least 12, at least 13, at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23, at least 24, or at least 25, GA-elicited responses are measured in each cell sample. The measurements obtained using an assay panel of the invention can be used to determine whether a test preparation of GA and a GA reference standard are immunologically identical by comparing the measurement of a given GA-elicited response in the T-cell line sample stimulated with the GA test preparation to the same GA-elicited response in the T-cell line sample stimulated with the GA reference standard. In embodiments, these measurements are normalized to take into account any background observed in the negative control sample before the comparison is made. As described herein, the immunological identity of a GA test preparation and a GA reference standard can be determined using the methods of the invention by comparing the measurement of at least one GA-elicited response in a GA-specific T-cell line stimulated with the GA test preparation and the same least one GA-elicited response in the GA-specific T-cell line stimulated with the GA reference standard. As described elsewhere herein, the GA-elicited response measured can be proliferation, production of a response biomarker, expression of a nucleic acid encoding a response biomarker, or a combination thereof. In embodiments, wherein the GA-elicited response measured in the methods of the invention is the production of a response biomarker, the response biomarker can be, e.g., a cytokine, cytokine receptor, chemokine, T-cell activation marker, or a nucleic acid that encodes a cytokine, cytokine receptor, chemokine, or T-cell activation marker. Response biomarkers can be measured according to methods known in the art and described elsewhere herein. In embodiments, the response biomarker is a cytokine selected from, e.g., IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, and IL-1b. In embodiments, the response biomarker is an activation marker selected from, e.g., CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, the response biomarker is a chemokine, selected from, e.g., IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, the response biomarker is a Th1-associated cytokine, a Th2-associated cytokine, a Th17-associated cytokine, or a ThFH-associated cytokine. In embodiments, the response biomarker is a Th1-associated cytokine and is IFN-γ. In embodiments, the response biomarker is a Th2-associated cytokine selected from: IL-4, IL-5, and IL-13. In embodiments, the response biomarker is a Th17-associated cytokine selected from: IL-17, and IL-22. In embodiments, the response biomarker is a ThFH-associated cytokine selected from: IL-21, and TGF-β. In embodiments, the response biomarker is a key regulatory associated cytokine selected from IL-10 and TGF-β. In embodiments, the response biomarker is a nucleic acid encoding a cytokine selected from, e.g., IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, or IL-17, IL-21, IL-22, IFN-γ, TNF-α (TNF), TNF-β (LT), TGF-β, and IL-1b. In embodiments, the response biomarker is a nucleic acid encoding an activation marker selected from, e.g., CD69, CD25, CD71, CD137, CD154, CD278, CD279, and HLA-DR. In embodiments, the response biomarker is a nucleic acid encoding chemokine, selected from, e.g., IL-8 (CXCL8), RANTES (CCL5), CCL1, CXCL4, and CXCL7. In embodiments, the response biomarker is a nucleic acid encoding Th1-associated cytokine, a nucleic acid encoding Th2-associated cytokine, a nucleic acid encoding Th17-associated cytokine, or a nucleic acid encoding ThFH-associated cytokine. In embodiments, the response biomarker is a nucleic acid encoding Th1-associated cytokine and is IFN-γ. In embodiments, the response biomarker is a nucleic acid encoding Th2-associated cytokine selected from: IL-4, IL-5, and IL-13. In embodiments, the response biomarker is a nucleic acid encoding Th17-associated cytokine selected from: IL-17, and IL-22. In embodiments, the response biomarker is a nucleic acid encoding a ThFH-associated cytokine selected from: IL-21, and TGF-β. In embodiments, the response biomarker is a nucleic acid encoding a key regulatory associated cytokine selected from IL-10 and TGF-β. Additional response biomarkers can be identified according to methods described herein. In embodiments, a determination of immunological identity of the GA test preparation and the GA reference standard is made when each comparison of a respective pair of GA test preparation and GA reference standard measurements (e.g., expressed as a fraction, ratio, or percentage) falls within an acceptable range or acceptable limits. In embodiments of the invention, immunological identity between the GA preparations is determined using a panel of GA-specific human T-cell lines. In embodiments, a measurement of at least one GA-elicited response in each GA-specific human T-cell line in the panel stimulated with a GA test preparation, and the same at least one GA-elicited response in the respective GA-specific human T-cell line in the panel stimulated with a GA reference standard, are compared. As described, in certain embodiments, each GA-elicited response measurement is normalized to take into account any signal observed in a suitable negative control sample prior to the comparison. In embodiments, wherein a panel of multiple GA-specific T-cell lines is used to evaluate at least one test lot of GA, the dose response curves for any/all cell lines in the panel stimulated with the reference lot(s) must meet predetermined criteria, e.g., as described below wherein the coefficient of correlation (r) is ≧0.90, the slope is ≧0.60, the back-calculated concentration of GA standards is within ±30% of the nominal concentration, and the precision of a GA sample is ≦20% of coefficient of variation (CV). In embodiments, the dose response curve for the at least one test lot of GA must be within predetermined acceptable limits to be determined immunologically identical to the GA reference lot. In embodiments, the coefficient of correlation is 0.90 to 0.98. In embodiments, the coefficient of correlation is greater than or equal to 0.90, greater than or equal to 0.91, greater than or equal to 0.92, greater than or equal to 0.93, greater than or equal to 0.94, greater than or equal to 0.95, greater than or equal to 0.96, greater than or equal to 0.97, greater than or equal to 0.98, 0.90 to 0.98, 0.91 to 0.98, 0.92 to 0.98, 0.93 to 0.98, 0.94 to 0.98, 0.95 to 0.98, 0.96 to 0.98, 0.90 to 0.97, 0.91 to 0.97, 0.92 to 0.97, 0.93 to 0.97, 0.94 to 0.97, 0.95 to 0.97, or 0.96 to 0.98. In embodiments, a determination of immunological identity is made when the comparison (the GA-elicited response measurement in the GA-specific human T-cell line stimulated with the GA test preparation, relative to the GA-elicited response measurement in the GA-specific human T-cell line stimulated with the GA reference standard) is 75% to 125%. In embodiments, an appropriate negative control value, e.g., a no antigen control, is subtracted from the test values prior to making the comparison. In embodiments, a no antigen control value is not subtracted from the test values prior to making the comparison. In embodiments, the values are compared directly. In embodiments, the acceptable range or limits for the comparison value is about 80% to about 120%, or about 90% to about 110%. In embodiments, the acceptable range is about 75% to about 120%, about 75% to about 115%, about 75% to about 110%, about 75% to about 105%, about 75% to about 100%, about 80% to about 125%, about 85% to about 125%, about 90% to about 125%, about 95% to about 125%, about 100% to about 125%, about 80% to about 118%, about 80% to about 115%, about 80% to about 112%, about 80% to about 110%, about 80% to about 108%, about 80% to about 105%, about 80% to about 102%, about 80% to about 101%, about 80% to about 100%, about 80% to about 99%, about 80% to about 98%, about 80% to about 97%, about 80% to about 95%, about 80% to about 92%, about 80% to about 90%, about 82% to about 120%, about 82% to about 118%, about 82% to about 115%, about 82% to about 112%, about 82% to about 110%, about 82% to about 108%, about 82% to about 105%, about 82% to about 102%, about 82% to about 101%, about 82% to about 100%, about 82% to about 99%, about 82% to about 98%, about 82% to about 97%, about 82% to about 95%, about 82% to about 92%, about 82% to about 90%, about 84% to about 120%, about 84% to about 118%, about 84% to about 115%, about 84% to about 112%, about 84% to about 110%, about 84% to about 108%, about 84% to about 105%, about 84% to about 102%, about 84% to about 101%, about 84% to about 100%, about 84% to about 99%, about 84% to about 98%, about 84% to about 97%, about 84% to about 95%, about 84% to about 92%, about 84% to about 90%, about 86% to about 120%, about 86% to about 118%, about 86% to about 115%, about 86% to about 112%, about 86% to about 110%, about 86% to about 108%, about 86% to about 105%, about 86% to about 102%, about 86% to about 101%, about 86% to about 100%, about 86% to about 99%, about 86% to about 98%, about 86% to about 97%, about 86% to about 95%, about 86% to about 92%, about 88% to about 120%, about 88% to about 118%, about 88% to about 115%, about 88% to about 112%, about 88% to about 110%, about 88% to about 108%, about 88% to about 105%, about 88% to about 102%, about 88% to about 101%, about 88% to about 100%, about 88% to about 99%, about 88% to about 98%, about 88% to about 97%, about 88% to about 95%, about 90% to about 120%, about 90% to about 118%, about 90% to about 115%, about 90% to about 112%, about 90% to about 110%, about 90% to about 108%, about 90% to about 105%, about 90% to about 102%, about 90% to about 101%, about 90% to about 100%, about 90% to about 99%, about 90% to about 98%, about 90% to about 97%, about 90% to about 95%, about 92% to about 120%, about 92% to about 118%, about 92% to about 115%, about 92% to about 112%, about 92% to about 110%, about 92% to about 108%, about 92% to about 105%, about 92% to about 102%, about 92% to about 101%, about 92% to about 100%, about 92% to about 99%, about 92% to about 98%, about 95% to about 120%, about 95% to about 118%, about 95% to about 115%, about 95% to about 112%, about 95% to about 110%, about 95% to about 108%, about 95% to about 105%, about 95% to about 102%, about 95% to about 101%, about 95% to about 100%, about 97% to about 120%, about 97% to about 118%, about 97% to about 115%, about 97% to about 112%, about 97% to about 110%, about 97% to about 108%, about 97% to about 105%, about 97% to about 102%, about 98% to about 120%, about 98% to about 118%, about 98% to about 115%, about 98% to about 112%, about 98% to about 110%, about 98% to about 108%, about 98% to about 105%, about 99% to about 120%, about 99% to about 118%, about 99% to about 115%, about 99% to about 112%, about 99% to about 110%, about 99% to about 108%, about 99% to about 105%, about 100% to about 120%, about 100% to about 118%, about 100% to about 115%, about 100% to about 112%, about 100% to about 110%, about 100% to about 108%, about 100% to about 105%, about 101% to about 120%, about 101% to about 118%, about 101% to about 115%, about 101% to about 112%, about 101% to about 110%, about 101% to about 108%, about 102% to about 120%, about 102% to about 118%, about 102% to about 115%, about 102% to about 112%, about 102% to about 110%, about 102% to about 108%, about 105% to about 120%, about 105% to about 118%, about 105% to about 115%, about 105% to about 112%, about 105% to about 110%, about 110% to about 120%, about 110% to about 118%, or about 110% to about 115%. In embodiments, a determination of immunological identity of the GA test preparation and the GA reference standard using an assay panel of GA-specific T-cell lines is identified by comparing at least 2, at least 3, at least 4, or more, different GA-elicited responses measured following stimulation with the GA test and GA reference standard preparations. In embodiments, a determination of immunological identity of the GA test preparation and the GA reference standard using an assay panel of GA-specific T-cell lines is made when the comparison of each respective pair of measurements in the assay panel is within a predetermined acceptable range. In embodiments, a determination of immunological identity of the GA test preparation and the GA reference standard is made when the comparison of 80 to 100%, 85 to 100%, 90 to 100%, or 95 to 100%, 80%, 85%, 90%, 95%, or 100% of respective pairs of measurements in an assay panel are within an acceptable range. In embodiments wherein the test and reference standard GA preparations are determined to be immunologically identical based on a use of a single reference lot and comparison of a GA-elicited response at a single dose, the determination of immunological identity is made based on a “single reference lot, single dose analysis.” In embodiments, the immunological identity of two GA preparations is determined when the single reference, single dose analysis comparison value is within a predetermined acceptable range at each of one GA dose, two different GA doses, three different GA doses, or more. In embodiments, the GA-elicited response to each GA lot is measured at a series of increasing GA doses, and a dose-response curve is generated for each lot using the resulting response values. In embodiments, the lots are determined to be immunologically identical (or not immunologically identical), by comparing the slope (β*) of the reference lot dose response curve with the slope of the test lot dose response curve. In these embodiments, the GA-specificity of a human T-cell line is identified based on a “single-reference lot dose response curve analysis.” In embodiments, the test preparation of GA and the GA reference standard are confirmed as immunologically identical when the slopes in the linear range of their respective dose-response curves are statistically similar or identical. In embodiments, the immunological identity of two GA preparations can be determined when the slopes in the linear range of the test GA lot and reference GA lot dose-response curves are statistically similar or identical as determined according to any appropriate statistical methods known to those of skill in the art. In embodiments, immunological identity of the GA test preparation and the GA reference standard are determined using an EC50comparison and/or regression analysis, or another appropriate method known in the art or described in the literature. In embodiments, to reach a determination of immunological identity by comparing dose response curves, the slope (β*) of the reference lot dose response curve must meet appropriate acceptance criteria. Appropriate acceptance criteria can be predetermined by those of skill in the art. In certain embodiments, appropriate acceptance criteria are:
In embodiments, the GA-elicited response in 75% of positive control samples (e.g., cells stimulated with ConA) must be above the highest response elicited when the same cells are stimulated with any concentration of GA. In embodiments, the GA-elicited response in 75% of negative control samples (e.g., cells treated with a control peptide such as myelin basic protein, MBP) must be below or close to the lowest response elicited with any concentration of GA. Linear regression can be performed on the GA reference lot sample set, where the data points are plotted on a log-log scale. The log GA-elicited response values (by increasing concentration of analyte, shown below as IL-2) are on the Y-axis, and the log GA reference lot concentration (dose) values are on the X-axis. The best fit linear regression model used for the above data set is as follows: where Y=log10(mIL−2 concentration) and X=log10(GA concentration). Substituting X and Y variables with the appropriate representations, the above model becomes the following: In embodiments, immunological identity is established when the slope of the test lot curve is within acceptable limits. The acceptable range for the slope of the test lot curve is determined based on the reference lot curve using the following series of equations: The back-calculated dose value for a given log (response) is: The accuracy can be calculated by the following: Therefore, the region where the hypothesis of the equality of the slopes is to be accepted is: β* is calculated as follows: where β is the slope of the GA-RS curve. Therefore, the acceptable range for the GA-Batch slope, β*, determined by equation (6) can be displayed as: If β* is within the above acceptable limits, parallelism can be concluded. In embodiments, a true hypothesis test for equal slope is used. In embodiments, a calibration curve is generated using multiple assays of the reference lot (to account for assay variation), or assays of multiple reference lots (to account for variation among reference lots, e.g., multiple lots of commercially available COP). In embodiments wherein multiple COP reference lots are included in the assay, to provide multiple datasets, e.g., to account for lot-to-lot variation, the data from the multiple reference lots are used to determine acceptable variation from the reference slope. In these embodiments, the test and reference GA lots are determined to be immunologically identical based on a “multiple-reference dose-response curve analysis.” In embodiments wherein multiple samples of the same Copaxone reference lot are included in the assay, to provide repeat data, e.g., to account for assay variation, the reference lot repeat data are used to determine acceptable variation from the reference slope. In these embodiments, the test and reference GA lots are determined to be immunologically identical based on a “repeated reference dose-response curve analysis.” In embodiments, multiple GA-specific human T-cell lines with known characteristics, e.g., MHC restriction and reactivity to non-canonical peptides, are employed in a panel. In these embodiments, each GA-specific T-cell line in the panel can be stimulated with a reference GA lot or multiple reference GA lots, and one or more test lots of GA. To establish immunological identity between the reference lot(s) and a test lot of GA, the dose response curves for the reference lot(s) must meet appropriate acceptance criteria, e.g., as discussed above, and the dose response curve for the test lot of GA must be within the acceptable limits as described. In embodiments, a single GA-specific T-cell line or multiple GA-specific T-cell lines are stimulated with a reference GA lot, a non-canonical GA peptide synthesized in a manner that alters the primary amino acid composition as described elsewhere herein, and a test lot of GA. Using the approach described above the acceptable limits for β* for the reference peptide can be determined, as well as the slope generated by the dose response curve of a non-canonical peptide. Statistical comparison of the similarity or dissimilarity of the slope of the test peptide with the slopes of the dose response curves of both the standard and the non-canonical peptides simultaneously can provide a determination of the degree of similarity or dissimilarity of the test peptide. Using this comparison, the immunological identity or non-identity of a test lot of GA can be determined. A GA response biomarker produced in the context of a GA-elicited response measured in the methods of the present invention can be identified by comparing the amount of a candidate GA response biomarker, produced in a GA-specific T-cell culture stimulated with GA in the presence of appropriate APC, to the amount of the same biomarker produced in a control culture incubated with appropriate APC but not stimulated with antigen, or in a control culture stimulated with a negative control antigen. In embodiments, a GA response biomarker is identified based on an increase relative to a control following GA stimulation of about 1.5-fold (i.e., the response is about 50% higher) to about 1000-fold, about 1.5 to about 2-fold, about 1.5 to about 3-fold, about 1.5 to about 4-fold, about 1.5 to about 5-fold, about 1.5 to about 6-fold, about 1.5 to about 7-fold, about 1.5 to about 8-fold, about 1.5 to about 9-fold, about 2 to about 3-fold, about 2 to about 4-fold, about 2 to about 5-fold, about 2 to about 6-fold, about 2 to about 7-fold, about 2 to about 8-fold, about 2 to about 9-fold, about 2 to about 10-fold, about 3 to about 4-fold, about 3 to about 5-fold, about 3 to about 6-fold, about 3 to about 7-fold, about 3 to about 8-fold, about 3 to about 9-fold, about 3 to about 10-fold, about 4 to about 5-fold, about 4 to about 6-fold, about 4 to about 7-fold, about 4 to about 8-fold, about 4 to about 9-fold, about 4 to about 10-fold, about 5 to about 6-fold, about 5 to about 7-fold, about 5 to about 8-fold, about 5 to about 9-fold, about 5 to about 10-fold, about 6 to about 7-fold, about 6 to about 8-fold, about 6 to about 9-fold, about 6 to about 10-fold, about 7 to about 8-fold, about 7 to about 9-fold, about 7 to about 10-fold, about 8 to about 9-fold, about 8 to about 10-fold, about 1.5-fold to about 80-fold, about 1.5-fold to about 70-fold, about 1.5-fold to about 60-fold, about 1.5-fold to about 50-fold, about 1.5-fold to about 40-fold, about 1.5-fold to about 30-fold, about 1.5-fold to about 20-fold, about 2-fold to about 80-fold, about 1.5-fold to about 70-fold, about 1.5-fold to about 60-fold, about 1.5-fold to about 50-fold, about 1.5-fold to about 40-fold, about 1.5-fold to about 30-fold, about 1.5-fold to about 20-fold, about 2-fold to about 80-fold, about 2-fold to about 70-fold, about 2-fold to about 60-fold, about 2-fold to about 50-fold, about 2-fold to about 40-fold, about 2-fold to about 30-fold, about 2-fold to about 20-fold, about 3-fold to about 80-fold, about 3-fold to about 70-fold, about 3-fold to about 60-fold, about 3-fold to about 50-fold, about 3-fold to about 40-fold, about 3-fold to about 30-fold, about 3-fold to about 20-fold, about 5-fold to about 80-fold, about 5-fold to about 70-fold, about 5-fold to about 60-fold, about 5-fold to about 50-fold, about 5-fold to about 40-fold, about 5-fold to about 30-fold, about 3-fold to about 20-fold, about 10-fold to about 80-fold, about 10-fold to about 60-fold, about 10-fold to about 40-fold, or about 10-fold to about 20-fold, at least about 1.5-fold, at least about 2-fold, at least about 2.5-fold, at least about 3-fold, at least about 3.5-fold, at least about 4-fold, at least about 4.5-fold, at least about 5-fold, at least about 6-fold, at least about 7-fold, at least about 8-fold, at least about 9-fold, or at least about 10-fold. While preferred embodiments of the present invention have been shown and described herein, it will be obvious to those skilled in the art that such embodiments are provided by way of example only. Numerous variations, changes, and substitutions will now occur to those skilled in the art without departing from the invention. It should be understood that various alternatives to the embodiments of the invention described herein may be employed in practicing the invention. It is intended that the following claims define the scope of the invention and that methods and structures within the scope of these claims and their equivalents be covered thereby. The following methods were used in the Examples herein unless otherwise specified. Assay samples were run in triplicate. T-cell lines were isolated using a method described by Good et al, and Ota, et al. (Good, et al., 1987; Ota, et al., 1990). PBMC were collected by leukapheresis using acid citrate dextrose (ACD) as an anticoagulant, separated by Ficoll-Paque gradient centrifugation according to the manufacturer's protocol (Amersham Pharmacia Biotech, Uppsala, Sweden), and cryopreserved in PBS containing 2% human serum albumin and 10% DMSO. PBMC cultures were prepared in AIM V® medium (Invitrogen) or X-VIVO 15® medium (Lonza). On Day 1, 10 μg/mL of antigen (GMA, Mylan Pharmaceuticals, Inc., or Copaxone, Teva Pharmaceuticals USA, Inc.) was added to U-bottom 96-well plates containing 5×105to 2×106cells/mL (1-4×105cells per 200 μl well). The plates were incubated at 37° C., 6% CO2. On Day 3 or 4, 10 U/mL IL-2 (2 U/well, eBioscience cat#14-8029-63 or R&D Systems cat#202-IL-010/CF) was added. IL-2 was prepared by diluting stock IL-2 to 40 U/mL in medium and adding 50 μl of the diluted stock IL-2 (2 U) to each well. IL-2 was added again in the same amount on Day 6 or 7, and potentially again on or around Day 10 if needed based on evaluation of cell growth. On Day 12-14, the cell lines were subjected to an initial screening for reactivity to GA restimulation by assaying for proliferation in the presence of APC. Confirmatory assays were carried out following at least 3 total rounds of restimulation (at least about 31 days or longer). Proliferation was evaluated by measuring ATP content using a luminescent method (CellTiter Glo Luminescent Cell Viability Assay, Promega, Madison, Wis., cat. #G7571) or by measuring BrdU uptake by ELISA (Millipore cat. #2752), in both cases following kit instructions. A split well format was used. Cells in each well were resuspended and 65 μl transferred to the wells of each of two new U-bottom 96-well plates, to generate the replicate plates. For the initial screening, the original 96-well plate received mitomycin-treated autologous PBMC as APC plus 10 μg/mL antigen (GA). A first replicate plate received 100 μL mitomycin-treated PBMC alone (2×105), and no antigen, and a second replicate plate received 100 μL mitomycin-treated PBMC plus GA at a final concentration of 10 μg/mL. Therefore, all three plates received autologous APC (PBMC) treated with mitomycin C to prevent cell division. Each well had a total volume of 200 μL. The latter two plates were incubated for 3 days at 37° C., 6% CO2before adding CellTiterGlo or BrdU and measuring signal. IL-2 was added at 10 U/mL to the original 96-well plate for expansion on Day 17. For subsequent screenings, mitomycin-treated EBV-transformed autologous B-LCL (2×105to 5×105cells/mL) were used as APC. Potential antigen-reactive wells were identified by the increase in response relative to the corresponding well in the control (APC added, no antigen) plate. The original wells identified as antigen-reactive typically were transferred to 24-well plates 7-10 days after the screening, and restimulated/expanded with GA in the presence of mitomycin-treated autologous PBMC as described below. At the time of initial screening for GA reactivity, the original replicate plate containing one third of the cells from the original stimulation were restimulated/expanded. Cell lines in the original plate were restimulated with 10 μg/mL GA (same preparation used for initial stimulation) and mitomycin C-treated PBMC at about Day 12-14 when assay plates were restimulated. IL-2 was added at 10 U/mL on about Day 17. About 7-12 days after the initial screening (about Day 19 to 26), wells in the original plates corresponding to the positive wells were expanded/restimulated again. Cells in presumptive positive wells were collected and cultured in a 24-well plate with GA added at the initial stimulation concentration (10 μg/mL), and mitomycin-treated APC. APC were adjusted to 2.5×106per mL (if PBMC) or 5×105per mL (if B-LCL). 1 mL APC was added per well to a 24-well plate containing T-cells, and the plates were incubated for 24 hours at 37° C. with 6% CO2. IL-2 was added to each well at a final IL-2 concentration of 10 U/mL or as otherwise specified. The cultures were continued for 6-13 days, and stimulation repeated. T-cells were stimulated every 7 to 14 days during expansion. Volumes used in culture vessels for the procedures described herein: 200 μL/well of a 96-well plate; 2 mL/well of a 24-well plate; 4 mL/well of a 12-well plate; 10 mL in a T-25 flask; and 20 mL in a T-75 flask. Culture medium was removed from cultures prepared as for proliferation assay and utilized to measure cytokine production. In most cases culture medium was collected 18-24 hours after mixing T-cells, APC, and stimulating antigen. Cytokine production was measured by ELISA or bead assay. For measuring cytokine production, 100-150 μL culture medium (100 μL if culture was to be continued, otherwise 150 μL) was removed from each well. In some cases, the culture was continued 48 hours, for a total of 72 hours of culture, and the proliferative response measured. IFN-γ concentration in culture supernatants was measured by a sandwich ELISA using two anti-IFN-γ monoclonal antibodies. Clone 2G1 was used for capture, and biotinylated antibody from clone B133.5 was used for detection (both from Endogen). Recombinant IFN-γ was used to construct a standard curve ranging from 1000 to 15 pg/mL. ELISA plates were coated with capture antibody by diluting antibody to 1 μg/mL in PBS and adding 100 μl of diluted antibody per well. Plates were incubated overnight at 4° C. The coating antibody was removed and 100 μl of PBS-BSA added to all wells. The plates were incubated for one hour at room temperature. A standard curve was prepared. Standards were prepared using 1 μl of stock IFN-γ (100 μg/mL) in 1 mL of PBS-BSA (100 ng/mL final concentration) diluted in PBS-BSA (1 g BSA/100 mL PBS) as follows:
Biotinylated antibody was diluted to 0.5 μg/mL in PBS-BSA. 5 μl of antibody was diluted in 5 mL of PBS-BSA for each plate. After incubation with PBS-BSA, the plate was washed four times using a plate washer and PBS 0.05% Tween 20 as wash solution. 50 μl of biotinylated antibody was added to all wells. 50 μl of samples or standards were added to appropriate wells. Standards and samples were run in duplicate or triplicate. Plates were incubated for 90 minutes at room temperature. Streptavidin-peroxidase was diluted 1:10,000 in PBS-Tween. 1 μl of streptavidin-peroxidase in 10 mL of PBS-Tween was used for each plate. The plate was washed four times, 100 μl of streptavidin-peroxidase added to each well, and the plate incubated 30 minutes at room temperature. The plate was washed four times with PBS-Tween. 100 μl of TMB (3,3′,5,5″-tetramethylbenzidine) was added to each well, and the plate was incubated 15-30 minutes at room temperature. The reaction was stopped by adding 100 μl of 0.2N NH2SO4per well, and the plate was read at 450 nm with a 630 nm reference. The capture antibody used was monoclonal anti-human IFN-γ (Endogen catalog no. M-700A). The biotinylated anti-IFN-γ was Endogen catalog no. M-701B. The streptavidin peroxidase was Zymed catalog no. 43-4323. TMB used was Ultra TMB, Thermo Scientific cat no. 34028. Standard formulation TMB also can be used. The concentration of multiple cytokines was measured simultaneously using FlowCytoMix™ kits (eBioscience, San Diego Calif.). These are fluorescent bead based assays consisting of beads conjugated to anti cytokine antibodies. Beads specific for each cytokine are distinguished by size and fluorescence at 670 nm. Cytokine bound to each bead is detected using a phycoerythrin conjugated antibody so that the fluorescence at 585 nm reflects the concentration of cytokine in the sample. Two kits were utilized which measured different cytokine panels. The TH1/TH2 kit measures IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, TNF-α (TNF) and TNF-β (LT). The TH1/TH2/TH9/TH17/TH22 kit measures IFN-β, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-17, IL-22, and TNF-α (TNF). Both kits were used following the manufacturer's instructions. PBMC for use as APC were prepared by thawing frozen PBMC and adjusting the cell concentration to 5×106cells per mL in PBS. Mitomycin C (Sigma-Aldrich cat#M4287 or EMD Millipore cat#475820-10MG) was added to a final concentration of 25 μg/mL. Plates were incubated for 30-45 minutes at 37° C., then the cells were washed 3 times with PBS to remove the mitomycin C. The PBMC concentration was adjusted to 1-1.5×106cells per mL. Human B-cells were immortalized using Epstein-Barr virus to use as APC. PBMC were thawed according to standard protocol and the cells suspended at a concentration of 107cells per mL. Aliquots of PBMC were infected with Epstein-Ban virus (ATCC VR-1492) for use as immortalized B lymphoblastoid cell lines (B-LCL). Infection was carried out by culturing PBMC with virus in RPMI 1640 medium containing 10% fetal bovine serum and 1 μg/mL cyclosporin A (cyclosporine) to prevent the activation and proliferation of T-cells. Two mL of Epstein-Barr virus (ATCC VR-1492) were added to 2 mL of cells in a 15 mL centrifuge tube, mixed well, and incubated at 37° C. for one hour in a water bath. Eight mL of culture medium (RPMI 1640 medium with 10% fetal bovine serum) and 10 μg of cyclosporine (prepared by dissolving 1 mg of cyclosporine in 1 mL of 100% ethanol and 100 uL of Tween 80, and bringing volume to 10 mL with PBS) were added to the cells and the mixture transferred to a 25 cm2flask. The flask was incubated upright in a 37° C. incubator with 5-10% CO2, and the flask left undisturbed for 2 to 6 weeks. Successful transformation was apparent after 2-6 weeks of culture when clusters of large lymphoblasts were observed microscopically. At this point the cultures were maintained and expanded by keeping the cell concentration between 2×105and 1×106cells per mL. This typically was accomplished by diluting the cells 1:4-1:5 every 3 to 5 days. For restimulation, 5×105mitomycin-treated B-LCL or mitomycin-treated autologous PBMC were added to each well. When B-LCL were used as APC, 50 μg/mL mitomycin C was added to a cell suspension of 2×106cells per mL. Cells were incubated with mitomycin C for 45 minutes and then washed three times to remove excess mitomycin C. After the wash to remove mitomycin C, cells were adjusted to 2-5×105per mL. As described above, transformed B cells were established in the laboratory by infecting peripheral blood mononuclear cells (PBMC) with virus and adding cyclosporine to the medium (RPMI 1640 10% fetal calf serum) to prevent the activation and proliferation of T-cells. Stimulation of PBMC with autologous EBV-B cells potentially results in stimulation of any EBV specific T-cells resulting in virus-specific T-cell lines. Therefore, EBV-B cells were not used with primary cultures or early passage T-cell lines except as otherwise specified. EBV-B cells can be used to restimulate T cell lines that have been passaged several times. Because EBV-B cells grow in suspension and tend to form clumps of cells, the cell concentration was kept between 2×105/mL and 106/mL. Cultures were split 1:4-1:5 every 3 to 5 days by pipetting cells up and down to break up cell clumps and remove 80% of the suspension. An equal volume of fresh medium was added, and the culture incubated. MHC DR restriction testing of cell lines was carried out by measuring the proliferation response of the cell lines as described above in the presence of an array of APC selected based on the known HLA-DR loci of the original PBMC donor for the cell line tested, including autologous APC (B-LCL). The donors used are described in Table 2. To define the MHC molecule used to present antigen to GA specific T cell lines, GA specific responses were measured in the presence of autologous APC and in the presence of APC matching one of the T cell donor's HLA-DR alleles. Therefore at least 3 APC were used in each experiment: autologous APC, APC matching DR allele 1 but not allele 2 and a third APC matching allele 2 but not allele 1. In some experiments a fourth APC B-LCL was used. A no antigen negative control was used. APC were inactivated by incubation with 50 ug/mL mitomycin C. Following inactivation, cells were washed three times to remove mitomycin C and suspended in X-VIVO 15 medium at 1×106cells per mL. GA was added to the cell suspension to a final concentration of 20-60 μg/mL. The cells were incubated with antigen for 1 hour at 37° C. to allow antigen uptake and binding. The cell suspension was then centrifuged to pellet the cells and the cell pellet was washed once with medium. APC were adjusted to 2×105cells per mL in X-VIVO 15 medium and added to opaque 96-well plates at 100 μL per well. T-cell suspensions of 2-4×105cells per mL (unless otherwise specified) were added to the 96-well plates at 100 μL per well and the cultures incubated 4 days before measuring cell numbers using CellTiter Glo® (to evaluate proliferation). Alternatively, APC were not pulsed. Instead, each well contained 10 ug/mL antigen, 50 μl APC and 100 μL of T cells. After incubating for 4 days at 37° C. (5% CO2), 100 μL were removed from each well, combined with 100 μL CellTiterGlo reagent, and luminescence was measured using a Packard Fusion. Results were expressed in relative light units (RLU). Donor HLA typing shown in Table 2 was carried out by the Puget Sound Blood Center (Seattle, Wash.) following standards set by the American Society for Histocompatibility and Immunogenetics (ASHI) and by the Clinical Laboratory Improvement Act (CLIA). Polymerase chain amplification-based testing was used to assign HLA class I and class II alleles. Cryopreserved cells were thawed by immersing the vial in a 37° C. water bath and agitating for 2-3 minutes. As soon as the cell suspension was completely liquid it was transferred to a 15 mL centrifuge tube containing 10 mL warm X-VIVO 15 medium. The tube was inverted gently two to three times to mix. A 50 μL aliquot of the suspension was removed to obtain a cell count and the remaining suspension was centrifuged at 200×g for 10 minutes to pellet the cells. The medium was decanted and the cell pellet was resuspended in X-VIVO 15 medium at the desired cell concentration. GA preparations were Copaxone (COP, Teva Pharmaceuticals USA, Inc.) or GMA (Mylan Pharmaceuticals, Inc.) each diluted to 20 mg/mL in mannitol (40 mg/mL). Peptide 026 was prepared by withholding tyrosine for the first five minutes of synthesis. Tetanus toxoid was supplied by Astarte Biologics. The Stern peptide is described by Stern, et al., 2005, “Peptide 15-mers of defined sequence that substitute for random amino acid copolymers in amelioration of experimental autoimmune encephalomyelitis,” Proc. Nat. Acad. Sci. 102:1620-1625). Human T-cell lines were prepared from PBMC, obtained as described above from each of several different donors with no previous exposure to GA, and shown to respond specifically to GA. Characteristics of the donors used for studies described herein are summarized in Table 2. More than sixty GA-reactive T-cell lines were generated by culturing PBMC from these donors according to the methods described above, in the presence of either GMA or Copaxone. These cell lines, listed in Table 3 by donor and stimulating antigen used, were demonstrated to have reactivity to GA based on proliferation assay, GA-specificity based on proliferation or cytokine production assay, or both. GMA-responsive cell lines were obtained from Donor 1 PBMC as described above (2×105cells/well, i.e., 1×106cells/mL, plated with 10 μg/mL GMA; IL-2 added on Days 3 and 7). In a split well assay begun on Day 12 the cells were stimulated with 10 μg/mL GMA and 1×105mitomycin-treated autologous PBMC as APC. BrdU was added on Day 3, cells were incubated 4.5 hours and fixed, and uptake measured. Table 4 shows the proliferative response measured for eight presumptive GA-specific human T-cell lines obtained from Donor 1 PBMC were selected. These cell lines each produced an increase in signal of at least 50% relative to (no antigen) control. On Day 24, cells from the wells corresponding to the eight presumptive GA-specific human T-cell lines listed in Table 4 were transferred to a 24-well plate and expanded by stimulating with 10 μg/mL GMA and mitomycin-treated autologous PBMC as APC (3×106per well, i.e., 1.5×106/mL). On Day 32 four of the eight lines, 222-BA11, 222-BC11, 222-AG11, and 222-AG12, were stimulated with 0, 1, 10 and 100 μg/mL GMA and tested in a 96-well plate for a proliferative response to GMA using an ATP assay, again using autologous PBMC as APC (2×105per well, i.e., 1×106/mL). The best proliferative response relative to control was observed in the cells stimulated with 10 μg/mL GMA. Cytokine Production by Donor 1 Human T-Cell Lines Stimulated with GMA 222-AG11 and 222-AG12 vials frozen at Day 32 were thawed and the cells tested for cytokine production. The lines were stimulated with 10 μg/mL GMA, 10 μg/mL Copaxone, or 1 μg/mL Tetanus Toxoid (control antigen) in the presence of either autologous EBV-transformed B-LCL or EBV-transformed B-LCL from a donor matched at one allele (Donor 2 B-LCL). Control samples to which APC but no antigen was added were included. Culture medium was collected after 24 hours and assayed by ELISA for IFN-γ as described above. Donor 1: Cell Lines Initially Stimulated with Copaxone COP-responsive T-cell lines were obtained from Donor 1. In the initial stimulation, isolated PBMC were plated at 1×105cells per well (5×105cells/mL) with 10 μg/mL Copaxone and cultured as described above. IL-2 was added to all wells on Days 4 and 7 of culture, and the wells screened for reactivity on Day 14 using a luminescent proliferation assay in split well format. The original plate was restimulated, and IL-2 added 4 days after restimulation. Table 5 shows the proliferation assay results for the fifteen Donor 1 COP T-cell lines (producing at least twice the signal as control). T-cell line 222-2D8, generated from Donor 1 by initial stimulation with COP, was shown in at least two experiments to proliferate comparably following restimulation with GMA and COP. Table 6 shows the results of an ATP proliferation assay carried out on Day 56 of culturing following stimulation with 3 or 10 μg/mL of GMA or COP. ATP levels were measured at 48 hours after stimulation. The control in the GMA series was no antigen, and the control in the COP series was MBP. The response to GMA in comparison to COP at the 10 μg/mL dose in this assay was 103.4%. In a second ATP proliferation assay, carried out on Day 56 of culturing following stimulation with 1, 3, or 10 μg/mL of GMA or COP, the response to GMA in comparison to COP at the 10 μg/mL dose was 107.1%. The results are shown in Table 7. T-cell line 222-2D8, generated from Donor 222 by initial stimulation with COP, was shown in at least two experiments to proliferate comparably following restimulation with GMA and COP. Table 8 shows the results of an ATP proliferation assay carried out on Day 56 of culturing following stimulation with 3 or 10 μg/mL of GMA or COP. ATP levels were measured at 48 hours after stimulation. The control in the GMA series was no antigen, and the control in the COP series was MBP. The response to GMA in comparison to COP at the 10 μg/mL dose in this assay was 85.1%. On Day 56 of culturing, after six total rounds of stimulation, cell line 222-2F12 was assayed for proliferation in response to stimulation with GA and a series of non-canonical peptides. For the assay, 0, 3, 10, or 30 μg/mL of each of peptides GL 14, GLT 631, GAT 111, and GAT 631, COP, and GMA, were added to the T-cell lines at 1×105cells/mL, in the presence of mitomycin C-treated autologous B-LCL. Proliferation was measured by luminescent ATP assay. Cell line 222-2F12 responded to GL 14 at 3 μg/mL, and not to the other non-canonical GA peptides tested. The response to GMA in comparison to COP at the 10 μg/mL dose in this assay was 99.4%. The data are shown in Table 9. Based on the increases in ATP level observed (i.e., a ratio of relative luminescence in wells with antigen to control wells without antigen of two or more), fifteen presumptive GA-specific T-cell lines in the original plate were moved to a 24-well plate at Day 26 and restimulated with 10 μg/mL Copaxone in the presence of autologous EBV-transformed B-LCL (5×105per well, i.e., 2.5×105/mL). At Day 27 IL-2 was added. On Day 34, the fifteen presumptive lines were restimulated with 10 μg/mL GMA or 10 μg/mL COP in the presence of mitomycin-treated autologous B-LCL (4×104per well; 2×105per mL), or incubated with mitomycin-treated autologous B-LCL and no antigen. On Day 35 IL-2 was added to continued cultures. On Day 36 culture supernatant was collected from test cultures and IFN-γ production measured by ELISA (48 hours after restimulation). The results are shown in Table 10. IFN-γ protein concentrations were determined using a TMB standard curve. At least seven Donor 1 COP T-cell lines, 222-2D8, 222-2E1, 222-2B11, 222-2C3, 222-2B8, 222-2G12, and 222-2F12, produced I IFN-γ in response to GA, and the cell lines reacted to both Copaxone and GMA. The 222-2D8, 222-1H12, 222-1C5, and 222-2D2 Day 34 supernatants also were tested in triplicate for IFN-γ, IL-2, IL-10, IL-8, IL-5, IL-1β, TNF-α, and TNF-β secretion in response to GMA, COP, or no antigen (as a control) by immunofluorescent bead assay (FlowCytoMix™ kit). Little or no IL-4, IL-6, or IL-12 was detected. Table 11 shows the resulting cytokine secretion profiles of lines 222-2D8 and 222-1H12. Table 12 shows the resulting cytokine secretion profiles of lines 222-1C5, and 222-2D2. It is important to note that in Table 12 supernatants of T-cell line 1H12 were titrated, demonstrating that this cell line also produced interferon in response to both GMA and COP. The cultures were restimulated on days 46, 56, 67 and 75. On Day 56, cell lines 222-2D8, 222-1H12, 222-2F12, 222-2B8, 222-1C5, 222-2B11, and 222-2E1 again were tested for reactivity to stimulation with 1, 3, or 10 μg/mL of GMA or COP in a proliferation assay. ATP levels were measured in all seven cell lines at 48 hours (results in Four culture supernatants (from 222-2B8, 222-1C5, 222-2B11, and 222-2E1) taken 24 hours after the Day 56 restimulation were assayed for cytokines, using a bead-based multiplex assay (results in Tables 13-16). All cytokine values represent the mean of triplicate values in pg/mL. In both the proliferation and cytokine assay the lines showed reactivity to both GMA and COP stimulation. Line 1C5, previously identified as not making IFN-γ in response to stimulation with GA, was found, after appropriate dilution of supernatants to produce IFN-γ in response to both GMA and COP, and in comparable amounts (see Table 14). Cell lines 222-2D8, 222-1H12, and 222-2F12 were further assayed after 48 hours (on Day 56, following the 6th stimulation) for cytokine secretion by immunofluorescent bead assay (results in Donor 2: Cell Lines Stimulated with GMA PBMC were obtained from a normal, healthy, 63-year old female donor having MHC Class II HLA-DRβ1 04:01, 04:04 (Donor 2). The isolated PBMC were cultured on Day 0 at 2×105cells per well (1×106cells/mL) with 100 μg/mL GMA in AIM V medium, and cultured as described in the Methods. The cultures were screened for proliferation at Day 13 using a BrdU uptake assay after stimulation with 100 μg/mL GMA using mitomycin-treated autologous PBMC as APC. Nine presumptive GA-specific human T-cell lines were selected for expansion and further analysis based on an increase in the OD of the GA-stimulated test sample of at least 50% relative to an unstimulated control sample. Table 17 shows the BrdU proliferation data for the nine presumptive GA-specific human T-cell lines obtained from Donor 2 PBMC. It is notable that for all of these T-cell lines the difference in BrdU uptake from control was greater than two-fold. These presumptive Donor 2 T-cell lines were expanded by stimulation with 10 μg/mL GMA and mitomycin-treated autologous PBMC and later with autologous EBV-transformed B-LCL. Line 206-1A7 was switched to X-VIVO 15 medium. On Day 88 line 206-1A7 was stimulated with B-LCL and 10 μg/mL GMA or 1 μg/mL Tetanus Toxoid (TT). After four days the culture was tested for proliferation by ATP assay and supernatants were tested for IFN-γ and TNF-α secretion by ELISA. Donor 3: Cell Lines Stimulated with GMA PBMC were obtained from Donor 3, a normal, healthy, 52-year old male donor having MHC Class II HLA-DRβ1*15, *11. Cultures were stimulated at 1×105cells per well (1×106cells/mL) in a U bottom 96-well plate with 10 μg/mL GMA. IL-2 was added on Days 3 and 7, and cultures were restimulated with 10 μg/mL GMA on Day 14 in the presence of 2×104autologous B-LCL per well, and screened by ATP proliferation assay. Based on the assay data (Table 18), fourteen presumptive GA-specific T-cell lines were selected and expanded to a 24-well plate on Day 24. IL-2 was added on Day 25. For these cell lines, the ratio of proliferation in response to GMA:control (APC only) was greater than 3.0. On Day 32 these cell lines were restimulated at 1-2.5×105cells/mL with 10 μg/mL GMA in the presence of 2.5×105autologous B-LCL and rescreened by proliferation assay. For the assay, stimulation with 10 μg/mL GMA, 10 μg/mL COP, or 1 μg/mL Tetanus Toxoid (as a control) was tested. All 8 cell lines tested were responsive to GMA and COP (Table 19). Culture medium from six of the cell lines was tested in a cytokine multiplex assay, confirming reactivity and revealing production of IL-13, IL-22, IL-5 and IFN-γ (Table 20). On Day 33 the cell lines were expanded into T-25 flasks (10 mL volume with 10 U IL-2 per mL). On Day 40, 12 cell lines were again tested for reactivity using 1, 3 or 10 μg/mL GMA or COP, or 10 μg/mL MBP as a control. Eleven of the 12 cell lines (all but F2G) were responsive to both antigen preparations. The results are shown in Table 21. In an initial stimulation, isolated PBMC were plated on Day 0 at 2×105cells per well (1×106cells/mL) with 100 μg/mL COP in AIM V medium, and cultured as described above in the Methods. Similarly, PBMC were plated on Day 0 at 2×105cells per well with 100 μg/mL GMA in AIM V medium. The cultures were stimulated with 100 μg/mL of the respective antigen (COP or GMA) in the presence of mitomycin-treated autologous APC, and screened for proliferation at Day 13 using a BrdU uptake assay. None of the lines tested showed GA-specific reactivity. Significant loss of cells was observed following addition of GA, suggesting use of a lower concentration of GA. GA titration studies showed better proliferative and cytokine responses at 10 and 30 μg/mL GA than at 100 μg/mL GA. A second set of presumptive COP-responsive T-cell lines was generated by plating PBMC plated on Day 0 at 1×105cells per well with 10 μg/mL COP in X VIVO-15 medium. IL-2 was added at 2 U per well on days 3 and 7, and on Day 14 the cultures were screened for proliferation by split well assay. The lines were restimulated (in a first restimulation) with 10 μg/mL COP in the presence of 2×104autologous B-LCL as APC and a luminescent ATP assay used to measure proliferation. Fourteen wells were selected for expansion if the ratio of proliferation after stimulation with COP to proliferation in response to the APC control was greater than 3.0 (Table 22). On Day 24 the continued cultures of the lines listed in Table 22 were expanded into a 24-well plate (2 mL/well) and restimulated with 10 μg/mL COP in the presence of 5×104autologous B-LCL in a second restimulation. On Day 25, 10 U/mL IL-2 were added. On Day 32, 2-5×105T-cells from each of six of the cell lines were restimulated with 10 μg/mL COP or tetanus toxoid in the presence of 5×104autologous B-LCL for confirmatory proliferation testing by luminescent ATP proliferation assay. All six lines showed reactivity to both COP and GMA (Table 23). On Day 32, the continued cultures corresponding to the assayed cultures also were restimulated, and on Day 33 seven cell lines (165-B6C, 165-B10C, 165-D11C, 165-F6C, 165-D3C, 165-E3C, and 165-F3C) were expanded to T-25 flasks (10 mL total volume, 10 U/mL IL-2). Seven others were left in the 24-well plate for two additional days before expanding to flasks. Four of the cell lines in the 24-well plate (165-F4C, 165-C9C, 165-E9C, and 165-F9C) were lost. On Day 39, three of the cell lines (165-D2C, 165-C7C, and 165-C4C) in the 24-well plate again were tested for reactivity to three concentrations of COP and GMA, including a no antigen and an MBP control (all in the presence of 5×104autologous mitomycin-treated B-LCL as APC) by ATP proliferation assay. All three cell lines responded comparably to COP and GMA (Table 25). At Day 40, after the fourth restimulation on Day 39, the continued cultures corresponding to the assayed cell lines were expanded from the 24-well plate to T-25 flasks with IL-2 as described above. Seven Donor 3 COP lines were tested on Day 46 of culture. The cell lines were restimulated using 3 concentrations of COP and GMA, including a no antigen and an MBP control as before (all in the presence of 5×104autologous B-LCL). Proliferation was measured by ATP assay after 3 days. The results are shown below in Table 26. The continuing cell line cultures were restimulated (in the cell lines' fifth restimulation) on Day 46 by expanding to a 12-well plate at a 4 mL/well volume (each well containing 1×106T-cells, 1×106APC, with 10 μg/mL COP). The remaining cells were cryopreserved. On Day 47 the cell culture from each well of the 12-well plate was expanded into T-75 flasks (20 mL, 10 U/mL IL-2). A sixth restimulation was carried out on Day 53 by expanding into a 24-well plate (each well containing 5×105T-cells and 5×105APC per well, with 10 μg/mL COP), and remaining cells cryopreserved. On Day 54 the cell culture from each well of the 24-well plate was expanded into T-75 flasks as before. A seventh restimulation was carried out on Day 60 by expanding to into a 24-well plate (each well containing 5×105T-cells, 5×105APC, with 10 μg/mL COP), and remaining cells cryopreserved. On Day 61 the cell culture from each well of the 24-well plate was expanded into T-25 flasks. These cells were harvested and cryopreserved on Day 67, after a total of seven rounds of restimulation/expansion. Donor 4: Cell Lines Stimulated with Copaxone PBMC were obtained from a normal, healthy, 21-year old male donor having MHC Class II DRβ1*07, 13 (Donor 4). PBMC were collected by leukapheresis using ACD as an anticoagulant and separated by Ficoll-Paque gradient centrifugation. Cultures were initiated in 96-well plates at 1×105cells per well (5×105cells per mL) in X-VIVO 15 medium with 10 μg/mL COP, and cultured as described in the Methods. IL-2 was added at 10 U/mL on days 3 and 6 of culture. The cultures were screened for proliferation on Day 13 by luminescent ATP assay after restimulation with 10 μg/mL COP in the presence of 2×105mitomycin-treated autologous PBMC per well (1×106cells per mL). Fifteen wells were scored as presumptively responsive based on an ATP ratio (COP stimulated:unstimulated control) of two or greater (Table 27). T-cell line 205-1F4, generated from Donor 205 by initial stimulation with COP, was shown in at least two experiments to proliferate comparably following restimulation with GMA and COP. Table 28 shows reactivity of 205-1F4 to GMA and COP assayed on Day 50 of culture, after four restimulations. The cell lines were restimulated in the presence of autologous B-LCL using three concentrations of COP and GMA, including a no antigen control. Proliferation was measured by luminescent ATP assay. The response to GMA in comparison to COP at the 10 μg/mL dose in this assay was 102.7%. Table 29 shows reactivity of 205-1F4 to GMA and COP assayed on Day 58 of culture, after four restimulations. The cell lines were restimulated in the presence of autologous B-LCL using four concentrations of COP and GMA, including a no antigen control. Proliferation was measured by luminescent ATP assay. The response to GMA in comparison to COP at the 10 μg/mL dose in this assay was 98.5%. Cell lines 205-1H7 and 205-1H11 were stimulated on Day 59 of culture after a total of six stimulations (five restimulations). The proliferation responses of lines 205-1H7 and 205-1H11 in response to a range of antigen concentrations were measured by luminescent ATP assay. T-cells were collected from each culture, washed and added to the plates at 20,000 cells per well in a volume of 100 uL. All conditions were tested in triplicate. After 4 days incubation at 37° C., 6% CO2, 100 μL of medium was removed from each well and 100 μL of CellTiterGlo® added to each well. The results are shown in Tables 30 and 31. On Day 23 the positive wells (cell lines listed in Table 27) were transferred to a 24-well plate and restimulated with 10 μg/mL COP in the presence of mitomycin-treated autologous PBMC. IL-2 was added at Day 24. On Day 31 (after four rounds of restimulation) ten lines were restimulated with 10 μg/mL COP to confirm antigen reactivity by a cytokine secretion assay. T-cells were added at 2×104cells/well in 96-well plates in X-VIVO 15 medium. Each line was separately stimulated with 10 μg/mL GMA, and 10 μg/mL COP in the presence of (2×105cells/well, i.e., 1×106cells/mL) mitomycin-treated autologous PBMC. Culture medium was collected after 20-24 hours for cytokine assay (FlowCytomix multiplex bead assay). The supernatants were tested in triplicate for IL-12 p70, IFN-γ, IL-2, IL-10, IL-6, IL-4, IL-5, TNF-α, and TNF-β secretion. Tables 32 and 33 show the resulting cytokine secretion profiles. Most tested cell lines secreted IFN-γ, IL-5, and TNF-α in response to both GMA and COP. Secretion of IL-2, IL-10, and IL-4 varied among the lines. All samples also secreted IL-8 (data not shown). Culture medium from each of two Donor 4 cell lines was assayed for an extended panel of cytokines, showing production of IL-13 and IL-22 (Table 33). PBMC were obtained from a normal, healthy, 49-year old female donor having MHC Class II HLA-DRβ1*13, *15:01 (Donor 6). PBMC were collected by leukapheresis using ACD as an anticoagulant and separated by Ficoll-Paque gradient centrifugation. Cultures were initiated in 96-well plates at 1×105cells per well (5×105cells per mL) in X-VIVO 15 medium with 10 μg/mL GMA/001/014 (Lot 001), a lot of GA prepared by standard methods. Cells were cultured as described in the Methods. IL-2 was added at 10 U/mL on days 3 and 8 of culture. The cultures were screened for proliferation on Day 16 by luminescent ATP assay and were restimulated with 10 μg/mL GMA/001/014 (Lot 001) in the presence of 2×104mitomycin-treated autologous B-LCL per well (1×105cells per mL). Fourteen wells were scored as presumptively responsive based on an ATP ratio (COP stimulated:unstimulated control) of two or greater. The fourteen T-cell cultures were restimulated on Day 26 (second restimulation), and expanded in 10 U/ml IL-2 on Day 28. On Day 35, all 14 cultures were restimulated (third restimulation), and thirteen were assayed for proliferation in the presence of GMA Lot 001, COP, tetanus toxoid, or no antigen control. Table 34 shows the results of the proliferation assay. The reactivities of GA-specific human T-cell lines identified as described above to a series of non-canonical GA peptides (listed in Table 1) were tested. GA-specific T-cell lines identified as reactive to non-canonical GA peptides are shown in Table 35 below. These cell lines were identified based on at least a 50% increase in proliferation relative to a control following stimulation with a non-canonical GA peptide after at least five rounds of GA restimulation/expansion. Each cell line previously had been tested for reactivity to GA at least twice and in many cases more. Certain cell lines responded comparably to GA and the non-canonical peptide, as indicated. Peptide 026 is an altered GA peptide consisting of the amino acids tyrosine, glutamic acid, alanine, and lysine, made by withholding tyrosine during the first five minutes of GA synthesis. T-cell lines from Donors 3 and 4 were tested for reactivity to peptide 026. As summarized in Table 35, at least four Donor 3 and two Donor 4 T-cell lines showed reactivity to peptide 026. One Donor 4 T-cell line showed reactivity to peptide 026 comparable to that observed following stimulation with GA. On Day 54 of culture seven Donor 3 COP T-cell lines were tested for reactivity to peptide 026 (dissolved in water containing 20 mg/mL mannitol). Three of the cell lines tested (165-B6C, 165-D3C, 165-F3C, and 165-F6C) had low level reactivity to the 026/11 antigen. The remaining cell lines had no significant reactivity to Peptide 026; all cell lines responded well to both GMA and COP. The data in RLU are shown in Table 36. Donor 3 GMA T-Cell lines 165-B5G, 165-D8G, 165-E7G, and 165-F5G also were tested for reactivity to peptide 026, with negative results. On Day 56 of culturing (described in Example I), the cell lines were stimulated with COP, GMA, and peptide 026, each at 0, 1, 5, 10, and 100 μg/mL. All Donor 3 lines responded well to GMA and COP, and the GMA and COP responses were comparable. Donor 4 COP T-cell lines 205-1B4, 205-1C4, and 205-1H5 were tested for reactivity to peptide 026. peptides GAT 631, GLT, and COP also were tested at 1, 5, 10, and 30 μg/mL. Cell line 205-1C4 showed reactivity to peptide 026 comparable to its reactivity to GA, while 205-1H5 showed minimal reactivity, and 205-1B4 did not show reactivity (Table 37). Donor 4 COP cell line 205-1H11 was tested for reactivity to peptides 026, GLT 631, LT 11, GL 14, GT 41S, GAT 631 and GAT 111. This experiment was performed at the same time as the COP dose response experiment described in Example I, using the same cells and method. Cell line 205-1H11 showed reactivity to peptide 026 at 30 μg/mL of antigen (Table 38). Donor 4 COP cell line 205-1H3 was tested for reactivity to peptides GLT 631, GAT 631, GAT 111, GL 14, LT 11, GA 64, and GT 41S. Cell line 205-1H3 showed reactivity to peptide GL 14 at 1 μg/mL of antigen (Table 39). Donor 1, 3 and 4 GA-specific T-cell lines were tested for proliferation in response to stimulation with peptide GLT 631 (GLT), poly (Glu-Lys-Tyr; 6:3:1). One Donor 1 cell line, three Donor 3 T-cell lines and one Donor 4 T-cell line showed some reactivity to GLT. Two of the Donor 3 T-cell lines showed reactivity to GLT comparable to that observed following stimulation with GA. Three Donor 3 T-cell lines showed reactivity to GAT. On Day 56 of culturing (described in Example I), after five rounds of stimulation (including the initial stimulation and four rounds of restimulation), Donor 1 COP T-cell line 222-1H12 was assayed for proliferation following addition of 0, 3, 10, and 30 μg/mL of each of peptides GL 14, GLT 631, GAT 111, and GAT 631, COP, and GMA, at a T-cell concentration of 1×105cells/mL, in the presence of mitomycin C-treated autologous B-LCL. Proliferation was measured by luminescent ATP assay. Cell line 222-1H12 responded to GLT 631 and not to the other non-canonical GA peptides tested. The data in RLU are shown in Table 40. On Day 81 of culturing (described in Example I), after nine total rounds of stimulation (including the initial stimulation and eight rounds of restimulation), Donor 3 GMA T-cell lines 165-B5G and 165-E7G were assayed for proliferation. For the assay, 0, 1, 3, 10, and 30 μg/mL of each of peptides GAT 631, GLT 631, and COP were added, at a T-cell concentration of 1×105cells/mL, in the presence of mitomycin C-treated autologous B-LCL. Proliferation was measured by luminescent ATP assay. Both lines responded well to GLT 631. 165-B5G and 165-E7G also responded to GAT 631. The data in RLU are shown in Tables 41-42. On Day 50 of culturing (described in Example I), after six total rounds of stimulation, Donor 3 GMA T-cell lines 165-H11G, 165-C5G, 165-E9G, and 165-FLOG were assayed for proliferation. Cell lines were incubated with 0, 1, 3, 10, and 30 μg/mL of each of peptides GAT 631, GLT 631, GAT 111, GMA, COP, and STERN (165-FLOG was stimulated with MBP rather than STERN), at a T-cell concentration of 1×105cells/mL, in the presence of mitomycin C-treated autologous B-LCL. Proliferation was measured by luminescent ATP assay. Cell line 165-H11G responded to GLT 631 and GAT 631. The data in RLU are shown in Tables 43-46. Table 47 below shows reactivity of 165-F6C following stimulation with 0, 1.25, 2.5, 5, and 10 μg/mL each of peptides GLT 631, GAT 631, GAT 111, LT 11, GT 11, GL 14, GT 41S, and GMA, as demonstrated by luminescent ATP proliferation assay. The assay was carried out at Day 76 of culture, after eight restimulations, at a T-cell concentration of 1×105cells/mL, in the presence of mitomycin C-treated autologous B-LCL. Line 165-F6C reacted to GL 14, and not to the other non-canonical GA peptides tested. The response to GL 14 relative to the no antigen control reached 4.4-fold at a dose of 2.5 μg/mL antigen. Donor 3 line 165-E3C did not react to GAT 631, GLT 631, GAT 111, or the STERN peptide in a separate experiment. Donor 4 COP T-cell line 205-1H7 also was shown to have reactivity to peptide GLT 631, and not to GAT 631 and GAT 611. The cell line was assayed for proliferation after seven stimulations. The assay was carried out by adding 0, 1, 10, or 30 μg/mL of peptide GLT 631, to the T-cell lines at 1×105cells/mL, in the presence of mitomycin C-treated autologous B-LCL. Proliferation was measured by luminescent ATP assay. The data in RLU are shown in Table 48. GA-specific T-cell lines from Donors 1 and 3 were tested for proliferation in response to stimulation with peptide GL 14, poly (Glu-Lys 1:4). At least one Donor 1 T-cell line and eight Donor 3 T-cell lines showed reactivity to GL at low concentrations. Two of the Donor 3 T-lines showed reactivity comparable to that observed following stimulation with GA at low concentrations. Separate experiments indicated that GL-14 causes cell death at concentrations approaching and exceeding 10 μg/mL (data not shown). On Day 56 of culturing (described in Example I), after six total rounds of stimulation, Donor 1 COP T-cell line 222-2F12 was assayed for proliferation. For the assay, 0, 3, 10, or 30 μg/mL of each of peptides GL 14, GLT 631, GAT 111, and GAT 631, COP, and GMA, were added to the T-cell lines at 1×105cells/mL, in the presence of mitomycin C-treated autologous B-LCL. Proliferation was measured by luminescent ATP assay. Cell line 222-2F12 responded to GL 14 at 3 μg/mL, and not to the other non-canonical GA peptides tested. The data are shown in Table 49. Donor 3 COP T-cell lines 165-F3C and 165-C4C were assayed for proliferation following stimulation with 0, 1, 3, and 10 μg/mL each of peptides GLT 631, GAT 631, GAT 111, LT 11, GT 11, GL 14, GT 41S, GA 64, COP, and GMA, at a T-cell concentration of 1×105cells/mL, in the presence of mitomycin C-treated autologous B-LCL. Line 165-F3C was assayed at Day 77 of culture, after nine stimulations. Line 165-C4C was assayed at Day 66 of culture, after seven stimulations. Proliferation was measured by luminescent ATP assay. These lines responded well to GL 14 and LT 11, and not to the other non-canonical GA peptides tested. The data are shown in Table 50. Donor 3 GMA T-cell lines 165-B5G and 165-E7G were assayed for proliferation at Day 55 of culturing, after five rounds of restimulation (not including the initial stimulation). Peptides GLT 631, GAT 631, GAT 111, LT 11, GT 11, GL 14, MBP, COP, and GMA, were added at 0, 1, 3, or 10 μg/mL, to T-cells at a concentration of 2×106cells/mL, in the presence of mitomycin C-treated autologous B-LCL. Proliferation was measured by luminescent ATP assay. These lines responded well to GL 14 and not to the other non-canonical GA peptides tested. The data in RLU are shown in Tables 51 and 52. Donor 3 GMA and COP T-cell lines 165-F10G, 165-C5G, 165-H11G, 165-C7C, 165-E9G, and 165-F5G were assayed for proliferation following stimulation with 0, 1, 3, and 10 μg/mL each of peptides GLT 631, GAT 631, GAT 111, LT 11, GT 11, GL 14, MBP, COP, and GMA, at a T-cell concentration of 1-2×105cells/mL, in the presence of mitomycin C-treated autologous B-LCL. Each cell line was assayed for proliferation after a minimum of 55 days in culture, following at least six stimulations. A luminescent ATP assay was used. These lines responded well to GL 14 and not to the other non-canonical GA peptides tested, except for 165-C5G, which also responded to LT11. The data in RLU are shown in Tables 53-58. Table 59 shows reactivity of 205-1F4 following stimulation with 1, 2, 5, and 10 μg/mL of peptide LT 11 and GMA, as demonstrated by luminescent ATP proliferation assay. The assay was carried out at Day 80 of culture, after seven restimulations, at a T-cell concentration of 1×105cells/mL, in the presence of mitomycin C-treated autologous B-LCL. Line 165-1F4 showed reactivity to LT 11. The response to LT 11 relative to the no antigen control reached 17.3-fold at a dose of 10 μg/mL antigen. Table 60 shows reactivity of 205-1F4 following stimulation with 0, 1, 3, and 10 μg/mL each of peptides GLT 631, GAT 631, GAT 111, LT 11, GL 14, GT 41S, GA 64, COP, and GMA, as demonstrated by luminescent ATP proliferation assay. The response to GMA in comparison to COP at the 10 μg/mL dose in this assay was 101.3%. The assay was carried out at Day 71 of culture, after six restimulations, at a T-cell concentration of 1×105 cells/mL, in the presence of mitomycin C-treated autologous B-LCL. Line 165-1F4 showed reactivity to LT 11, and not to the other non-canonical GA peptides tested. The response to LT 11 relative to the no antigen control reached 13.8-fold at a dose of 10 μg/mL antigen. Donor 1 GMA T-cell line 222-AG12 was assayed for proliferation following stimulation with 0, 1, 10, and 30 μg/mL each of peptides GLT 631, GAT 631, GAT 111, LT 11, GT 11, GL 14, COP, and GMA, at a T-cell concentration of 2×105cells/mL, in the presence of mitomycin C-treated autologous B-LCL. Line 222-AG12 was assayed after 3 rounds of restimulation (4 total stimulations). Proliferation was measured by luminescent ATP assay. This line did not respond to any of the non-canonical GA peptides tested. The data are shown in Table 61. As described in the Methods, T-cell lines were tested for MHC restriction by measuring proliferation following incubation with APC. Mitomycin-treated autologous APC, and test APC, e.g., from donors having at least one HLA-DR match with the original PBMC donor were employed. The results are summarized in Table 62. The values in the data tables below are in RLU. Donor 3 T-cell line 165-B5G was assayed for proliferation, using mitomycin-treated APC from Donors 3, 5, and 6 that had been incubated in the presence or the absence of 20 μg/mL GMA. T-cell line 165-B5G had been restimulated six times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The data, shown in Table 63 and Donor 3 T-cell line 165-C4G was assayed for proliferation, using mitomycin-treated APC from Donors 3, 5, and 7 that had been incubated in the presence or the absence of 20 μg/mL GMA. T-cell line 165-C4G had been restimulated five times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The data, shown in Table 64 and Donor 3 T-cell line 165-C5G was assayed for proliferation, using mitomycin-treated APC from Donors 3, 5, and 6 that had been incubated in the presence or the absence of 20 μg/mL GMA. T-cell line 165-C5G had been restimulated four times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The data, shown in Table 65 and Donor 3 T-cell line 165-E9G was assayed for proliferation, using mitomycin-treated APC from Donors 3, 5, and 6 that had been incubated in the presence or the absence of 20 μg/mL GMA. T-cell line 165-E9G had been restimulated four times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The data, shown in Table 66 and Donor 3 T-cell line 165-F5G was assayed for proliferation, using mitomycin-treated APC from Donors 3, 5, and 6 that had been incubated in the presence or the absence of 20 μg/mL GMA. T-cell line 165-F5G had been restimulated seven times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The data, shown in Table 67 and Donor 3 T-cell line 165-F10G was assayed for proliferation, using mitomycin-treated APC from Donors 3, 5, and 7 that had been incubated in the presence or the absence of 20 μg/mL GMA. T-cell line 165-F10G had been restimulated five times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The data, shown in Table 68 and Donor 3 T-cell line 165-B6C was assayed for proliferation, using mitomycin-treated APC from Donors 3, 5, and 6 that had been incubated in the presence or the absence of 20 μg/mL COP. T-cell line 165-B6C had been restimulated six times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The data, shown in Table 69 and Donor 3 T-cell line 165-C7C was assayed for proliferation, using mitomycin-treated APC from Donors 3, 5, and 6 that had been incubated in the presence or the absence of 20 μg/mL COP. T-cell line 165-C7C had been restimulated five times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The data, shown in Table 70 and Donor 3 T-cell line 165-D3C was assayed for proliferation, using mitomycin-treated APC from Donors 3, 5, and 7 that had been incubated in the presence or the absence of 20 μg/mL COP. T-cell line 165-D3C had been restimulated six times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The data, shown in Table 71 and Donor 3 T-cell line 165-F3C was assayed for proliferation, using mitomycin-treated APC from Donors 3, 5, and 7 that had been incubated in the presence or the absence of 20 μg/mL COP. T-cell line 165-F3C had been restimulated eight times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The data, shown in Table 72 and Donor 3 T-cell line 165-F6C was assayed for proliferation, using mitomycin-treated APC from Donors 3, 5, and 6 that had been incubated in the presence or the absence of 20 μg/mL COP. T-cell line 165-F6C had been restimulated eight times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The data, shown in Table 73 and Donor 4 T-cell line 205-1D1 was assayed for proliferation, using autologous B-LCL, and B-LCL from Donors 1, 2, and 6 that had been incubated in the presence or absence of 60 μg/mL COP. T-cell line 205-1D1 was restimulated 5 times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The results, shown in Table 74, indicate that 205-1D1 is restricted by HLA-DRβ1*13. Donor 4 T-cell line 205-1H7 was assayed for proliferation, using autologous B-LCL, and B-LCL from Donors 1, 2, and 6 that had been incubated in the presence or absence of 40 μg/mL COP. T-cell line 205-1H7 was restimulated 5 times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The results, shown in Table 75 and Donor 4 T-cell line 205-1F4 and 205-1C4 was assayed for proliferation, using autologous B-LCL, and B-LCL from Donors 1, 2, and 6 that had been incubated in the presence or absence of 40 μg/mL COP. T-cell line 205-1F4 was restimulated 7 times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. T-cell line 205-1F4 was restimulated 7 times (not including the initial stimulation) with antigen before the MHC restriction assay was carried out. The results, shown in Table 76, indicate that 205-1F4 is restricted by HLA-DRβ1*13. GA-specific T-cell lines were analyzed for surface expression of CD4 and CD8. A PE-labeled CD8 monoclonal antibody and a FITC-labeled CD4 monoclonal antibody were used for detection. As a nonspecific control, the cells were labeled with a PE-labeled mouse isotype matched control IgG1 monoclonal antibody and a FITC-labeled mouse IgG1 monoclonal antibody. For immunofluorescent staining, T-cells were suspended in PBS containing 2% fetal calf serum at a cell concentration of 1 to 5×106per mL. Cells were distributed in 12×75 mm polystyrene tubes at 100 uL per tube. Isotype matched control monoclonal antibodies were non-specific mouse IgG1 labeled with either fluorescein (FITC) or phycoerythrin (PE). FITC labeled anti CD4 and PE labeled anti CD8 were purchased as a cocktail from eBioscience, Inc. (San Diego, Calif.). Fluorochrome-labeled monoclonal antibodies were added at the manufacturer's recommended volume of 5 uL per tube. Cells were incubated with monoclonal antibodies for 15-30 minutes at 2-8° C. Cells were washed once by adding 2 mL of PBS per tube and centrifuging 5 minutes at 200×g. Buffer was decanted and 0.5 mL of PBS was added to each tube. Staining was evaluated using a FACScan flow cytometer and CellQuest software. Dead cells were excluded from analysis based upon forward and side scatter characteristics. For GA-specific T-cell line 165-E9G, 99.76% detected cells were observed to be CD4+, and none to be CD8+. For GA-specific T-cell line 165-F5G, 97.46% detected cells were observed to be CD4+, and 1.50% to be CD8+. The control flow plots for 165-E9G and 165-F5G are shown in Proliferation assays, using the panel of six GA-specific long-term human T-cell lines listed in Table 78, were carried out to compare nine GA test lots, GMA/009/14 (009), GMA/010/14 (010), GMA/011/14 (011), GMA/012/14 (012), GMA/013/14 (013), GMA/014/14 (014), GMA/017/14 (017), GMA/018/14 (018), and GMA/070/14 (070), manufactured using standard methods, to a Copaxone reference lot. Each T-cell line was restimulated using methods described above, using five concentrations of the reference antigen (0.5, 1, 2, 2.5, and 5 μg/ml Copaxone), the same five concentrations of test antigen (the nine GA test lots), and a no antigen control, in the presence of 5×104mitomycin-treated autologous B-LCL. Proliferation was measured by ATP assay after 4 days of incubation with antigen, using methods described previously. The results are shown in Tables 79-88. The percentages in the last column of each table were calculated as follows: The dose response curves for GA Lot 009 are shown in The panel of six GA-specific human T-cell lines listed in Table 78 above was used to test the immunological identity of altered GA lots to Copaxone. Manufacture of Altered GA Lots Each of nine altered GA lots was manufactured using an altered mole fraction of one or more N-carboxy anhydrides (NCAs) used to charge the polymerization, and with an altered concentration of DEA (ranging from 0.01% to 0.1%) used to initiate the polymerization reaction. In other respects, all the lots were prepared using standard processes. A summary of the conditions used to prepare these lots is provided in Table 89. Molecular weight and amino acid ratios for the negative control lots and the specification ranges for each parameter, are shown in Table 90. The shaded cells containing bold text indicate values outside of GA specification ranges, shown at the top of each column. Initiator concentration and NCA mole fraction conditions can affect the initiation of polymerization and chain propagation. Significant differences between the negative lots and control were shown by total DEA analysis, DEA/COOH analysis, adduct analysis, peptide mapping, NTS (Edman), and intact HPLC (data not shown). Comparison of Copaxone and Negative GA Lots Using a Panel of GA-Specific T-Cell Lines Proliferation assays using the panel of six GA-specific T-cell lines (Table 78) were carried out, to compare negative GA lots 051F, 054F, 055F, 057F, 058F, 059F, and 060F (test lots), to Copaxone (reference lot). Each cell line was restimulated using 5 concentrations of reference antigen (Copaxone) and test antigen (the negative GA lots), including a no antigen control (in the presence of 5×104mitomycin-treated autologous B-LCL). Proliferation was measured by ATP assay after 4 days of incubation with antigen, using methods described previously. The results are shown in Tables 91 to 107. The percentages in the last column of each table were calculated as follows: An assay panel of clonal GA-specific human T-cell lines, representing a range of MHC restrictions and non-canonical peptide reactivities, is selected and used to determine the immunological identity of GA preparations. All cell lines are tested in triplicate samples for reactivity to 1, 3, and 10 μg/mL of each of a test preparation of GA and Copaxone (as a reference standard), including a no antigen control and an MBP control (all in the presence of 2×105mitomycin-treated autologous B-LCL/mL) by ATP proliferation assay. For each cell line, the mean RLU at each concentration of the test preparation of GA is calculated and compared with the corresponding mean RLU at each concentration of the GA reference standard using the formula: Immunological identity of the test preparation of GA and the GA reference standard is determined when this formula gives a relative value of 0.80 to 1.20 (80% to 120%) at a given GA concentration. Thus, the test and reference standard GA preparations are determined to be immunologically identical based on a single reference, single dose analysis. The mean RLU are further plotted by GA concentration to obtain a dose-response curve and the slopes of the GA test and reference standard curves compared. The test preparation of GA and the GA reference standard are confirmed as immunologically identical based on a single-reference lot dose response curve analysis. An assay panel of clonal GA-specific human T-cell lines, representing a range of MHC restrictions and non-canonical peptide reactivities, is selected and used to determine the immunological identity of GA preparations. All cell lines are tested in triplicate samples for reactivity to 1, 3, and 10 μg/mL of each of a test preparation of GA and multiple Copaxone lots (as a reference standard), including a no antigen control and an MBP control (all in the presence of 2×105mitomycin-treated autologous B-LCL/mL) by ATP proliferation assay. Multiple Copaxone reference lots are included in the assay, to provide multiple datasets, e.g., to account for lot-to-lot variation. For each cell line, the mean RLU at each concentration of the test preparation of GA is calculated and compared with the corresponding mean RLU at each concentration of the GA reference standard. The mean RLU are plotted by GA concentration to obtain a dose-response curve and the slopes of the curves compared. The data from the multiple reference lots are used to determine acceptable variation from the reference slope. The test preparation of GA and the GA reference standards are confirmed to be immunologically identical based on a multiple-reference dose-response curve analysis. An assay panel of clonal GA-specific human T-cell lines, representing a range of MHC restrictions and non-canonical peptide reactivities, is selected and used to determine the immunological identity of GA preparations. All cell lines are tested in triplicate samples for reactivity to 1, 3, and 10 μg/mL of each of a test preparation of GA and a Copaxone lot (as a reference standard), including a no antigen control and an MBP control (all in the presence of 2×105mitomycin-treated autologous B-LCL/mL) by ATP proliferation assay. Multiple samples of the same Copaxone reference lot are included in the assay, to provide repeat data, e.g., to account for assay variation. For each cell line, the mean RLU at each concentration of the test preparation of GA is calculated and compared with the corresponding mean RLU at each concentration of the GA reference standard. The mean RLU are plotted by GA concentration to obtain a dose-response curve and the slopes of the curves compared. The reference lot repeat data are used to determine acceptable variation from the reference slope. The test preparation of GA and the GA reference standard are confirmed to be immunologically identical based on a repeated reference dose-response curve analysis. The invention relates to the field of medicine. The invention provides methods for generating glatiramer-acetate-specific human T-cell lines, and assays that use these T-cell lines for demonstrating immunological identity between glatiramer acetate preparations. These assays allow sensitive and accurate comparison of glatiramer acetate preparations, and find utility as lot-release assays. 1. A method of determining whether a test preparation of glatiramer acetate (GA) and a GA reference standard are immunologically identical, the method comprising:
comparing a measurement of at least one GA-elicited response of at least one GA-specific human T-cell line to stimulation with at least one amount of the test preparation of GA in the presence of appropriate APC, with a measurement of at least one GA-elicited response of the at least one GA-specific human T-cell line to at least one amount of a GA reference standard in the presence of appropriate APC; wherein the test preparation of GA and the GA reference standard are determined to be immunologically identical when the comparison of the measurement of at least one GA-elicited response of at least one GA-specific human T-cell line to stimulation with at least one amount of the test preparation of GA, to the measurement of the at least one GA-elicited response of the at least one GA-specific human T-cell line to at least one amount of a GA reference standard, is within acceptable limits. 2. A method comprising selecting for pharmaceutical use a test preparation of GA that is immunologically identical to a GA reference standard determined according to the method of 3. The method of 4. The method of 5. The method of 6. The method of 7. The method of 8. The method of 9. The method of 10. The method of 11. The method of 12. The method of 13. The method of generating dose response curves for the test and reference preparations of GA, wherein the test preparation of GA and the GA reference standard are determined to be immunologically identical when the comparison of the dose response curves is within acceptable limits. 14. A process for preparing a drug product or pharmaceutical composition containing GA, comprising:
determining whether a test preparation of GA, produced by reacting protected GA with hydrobromic acid to form trifluoroacetyl GA, treating said trifluoroacetyl copolymer-1 with aqueous piperidine solution to form a test preparation of GA, and a GA reference standard, are immunologically identical, by:
a) incubating cells of at least one GA-specific human T-cell line with appropriate antigen presenting cells (APC); b) stimulating at least one sample of the GA-specific human T-cells and appropriate APC incubated in step (a) with an amount of the test preparation of GA, and separately stimulating at least one sample of the GA-specific human T-cells and appropriate APC incubated in step (a) with the same amount of the GA reference standard; c) measuring at least one GA-elicited response of the at least one sample of cells stimulated in step (b) with the test preparation of GA, and measuring the same at least one GA-elicited response of the at least one sample of cells stimulated in step (b) with the GA reference standard; and d) determining the test preparation of GA and the GA reference standard to be immunologically identical when a comparison of the measurements obtained in step (c) is within acceptable limits; wherein the test preparation of GA is admixed in the drug product or pharmaceutical composition if it is determined to be immunologically identical to the GA reference standard. 15. The method of 16. The method of 17. The method of 18. The method of 19. The method of 20. The method of 21. A method for generating and identifying a GA-specific human T-cell line, the method comprising:
a) obtaining a sample of cells, wherein the sample of cells comprises T-cells from a human donor and appropriate APC; b) preparing a culture from the sample of cells of step (a), wherein the culture comprises GA; c) incubating the culture of step (b) with IL-2; d) assaying the culture of step (c) by preparing at least one test culture comprising cells from the culture of step (c), appropriate APC, and an amount of a test preparation of GA, preparing at least one control culture from the culture of step (c), and (i) measuring at least one GA-elicited response of the at least one test culture; (ii) measuring the same at least one GA-elicited response of the control culture; and e) comparing the measurement of step (d)(i) with the measurement of step (d)(ii); wherein the generated GA-specific human T-cell line is identified based on an increase in the at least one GA-elicited response measured in step (d)(i) relative to the at least one GA-elicited response measured in step (d)(ii) that is within acceptable limits; and f) optionally expanding the culture, and repeating steps (d) and (e) using the expanded culture, to further characterize the identified GA-specific human T-cell line. 22. The method of 23. The method of 24. A long-term GA-specific human T-cell line obtained according to the method of 25. The method of 26. The method of a) confirming or reconfirming the GA-specificity of the identified GA-specific human T-cell line; b) determining the MHC restriction of the identified GA-specific human T-cell line; c) determining a response biomarker profile of the identified GA-specific human T-cell line; and d) determining the reactivity of the identified GA-specific human T-cell line to at least one non-canonical GA peptide. 27. The method of preparing at least one test culture comprising cells of the identified GA-specific human T-cell line, appropriate APC, and a non-canonical GA peptide; preparing at least one control culture comprising cells of the identified GA-specific human T-cell line; measuring at least one GA-elicited response of the at least one test culture; measuring the same at least one GA-elicited response of the control culture; and comparing the measurement of the GA-elicited responses of the test and control cultures; wherein the GA-specific human T-cell line that responds to stimulation with the non-canonical GA peptide is identified by an increase in the at least one GA-elicited response of the test culture relative to the control culture that is within acceptable limits. 28. The method of 29. A long-term GA-specific human T-cell line, wherein the long-term GA-specific human T-cell line is capable of producing at least one measured response to stimulation with a test preparation of GA that is increased relative to the same at least one measured response to stimulation with a control antigen, said at least one measured response comprising proliferation, production of a response biomarker, expression of a nucleic acid encoding a response biomarker, or a combination thereof. 30. The long-term GA-specific human T-cell line of 31. An assay panel of at least two GA-specific human T-cell lines, for use in determining whether a test preparation of GA and a GA reference standard are immunologically identical, wherein the assay panel comprises the following GA-specific human T-cell lines:
a) a GA-specific human T-cell line generated by culturing human T-cells in the presence of a first preparation of GA; b) a GA-specific human T-cell line generated by culturing human T-cells in the presence of a second preparation of GA. 32. The assay panel of c) a GA-specific human T-cell line that is not reactive to at least one non-canonical GA peptide; and d) a GA-specific human T-cell line that is reactive to at least one non-canonical GA peptide, wherein the non-canonical GA peptide of (c) is the same as or different from the non-canonical GA peptide of (d). 33. The assay panel of 34. The assay panel of 35. The assay panel of 36. The assay panel of 37. The assay panel of a) 222-AG11, 222-AG12, 222-BA11, 222-BC11, 165-B2G, 165-B5G, 165-C4G, 165-C5G, 165-C8G, 165-D8G, 165-E7G, 165-E9G, 165-F10G, 165-F5G, 165-F8G, 165-H11G, 222-1C5; and b) 222-1H12, 222-2B8, 222-2C3, 222-2D8, 222-2E1, 222-2F12, 222-1G8, 222-2G12, 165-B6C, 165-B10C, 165-C4C, 165-C7C, 165-D2C, 165-D3C, 165-D11C, 165-E3C, 165-F3C, 165-F6C, 165-F9C, 205-1B4, 205-1B7, 205-1C4, 205-1D1, 205-1E1, 205-1F2, 205-1F4, 205-1H3, 205-1H5, 205-1H7, 205-1H9, 205-1H11 38. The assay panel of 39. The method of 40. The method of 41. The method of 42. The method of 43. The method of 44. The method of 45. The assay panel of 46. The assay panel of 47. A method of determining whether a test preparation of glatiramer acetate (GA) and a GA reference standard are immunologically identical using an assay panel of 1) incubating cells of each GA-specific human T-cell line in the assay panel with appropriate APC; 2) stimulating a predetermined number of cells of each of the GA-specific human T-cell lines incubated in step (1) with an amount of the test preparation of GA; 3) separately stimulating the same predetermined number of cells of each of the GA-specific human T-cell lines incubated in step (1) with the same amount of the GA reference standard; 4) measuring at least one GA-elicited response of each of the GA-specific human T-cell lines stimulated in steps (2) and (3); and 5) comparing the measurement of the at least one GA-elicited response obtained for each GA-specific human T-cell line stimulated with the test preparation of GA with the measurement of the at least one GA-elicited response obtained for the same GA-specific human T-cell line stimulated with the GA reference standard; wherein the test preparation of GA and the GA reference standard are determined to be immunologically identical when the comparison of the measurements of step (5) is within acceptable limits. 48. A method comprising selecting for pharmaceutical use a test preparation of GA having immunologic identity to a GA reference standard, wherein immunologic identity is determined according to the method of 49. The method of 50. The method of 51. The method of 52. The method of 53. The method of 54. The method of 55. The method of 56. The method of 57. The method of 58. The method of 59. The method of 60. The method of CROSS-REFERENCE
BACKGROUND OF THE INVENTION
SUMMARY OF THE INVENTION
INCORPORATION BY REFERENCE
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION OF THE INVENTION
Glatiramer Acetate
Production of Glatiramer Acetate
GA Test Preparations
Testing GA Preparations
Generation and Identification of GA-Specific Human T-Cell Lines
Screening for Reactivity to GA
Screening T-Cell Lines for GA-Specificity
log10(
β·(lower limit)≦β*≦β·(upper limit) (9)Expansion of GA-Specific Human T-Cell Lines
Characterization of GA-Specific Human T-Cell Lines
Characterization of GA-Specific Human T-Cell Lines Reactive to Non-Canonical GA Peptides
Characterization of GA-Specific Human T-Cells Based on Response Biomarker Production
Characterization of GA-Specific Human T-Cell Lines Based on MHC Restriction
Characterization of GA-Specific Human T-Cell Lines Based on Analysis of Surface Marker Expression
GA-Elicited Responses
T-Cell Proliferation
Comparison of GA-Elicited Responses of GA-Specific Human T-Cell Lines to Test and Reference Standard GA Preparations
GA Response Biomarker Production
Preparation of a Drug Product of GA
Identification of New Biomarkers
Assay Panels of GA-Specific Human T-Cell Lines
COP and GMA-Reactive T Cell Lines No Cell Line Donor Culturing Antigen 1 222-AG11 1 GMA 2 222-AG12 1 GMA 3 222-BA11 1 GMA 4 222-BC11 1 GMA 5 165-B2G 3 GMA 6 165-B5G 3 GMA 7 165-C4G 3 GMA 8 165-C5G 3 GMA 9 165-C8G 3 GMA 10 165-D8G 3 GMA 11 165-E7G 3 GMA 12 165-E9G 3 GMA 13 165-F10G 3 GMA 14 165-F5G 3 GMA 15 165-F8G 3 GMA 16 165-H11G 3 GMA 17 222-1C5 1 COP 18 222-1H12 1 COP 19 222-2B11 1 COP 20 222-2B8 1 COP 21 222-2C3 1 COP 22 222-2D8 1 COP 23 222-2E1 1 COP 24 222-2F12 1 COP 25 222-1G8 1 COP 26 222-2G12 1 COP 27 165-B6C 3 COP 28 165-B10C 3 COP 29 165-C4C 3 COP 30 165-C7C 3 COP 31 165-D2C 3 COP 32 165-D3C 3 COP 33 165-D11C 3 COP 34 165-E3C 3 COP 35 165-F3C 3 COP 36 165-F6C 3 COP 37 165-F9C 3 COP 38 205-1B4 4 COP 39 205-1B7 4 COP 40 205-1C4 4 COP 41 205-1D1 4 COP 42 205-1E1 4 COP 43 205-1F2 4 COP 44 205-1F4 4 COP 45 205-1H3 4 COP 46 205-1H5 4 COP 47 205-1H7 4 COP 48 205-1H9 4 COP 49 205-1H11 4 COP 50 224-D2-001 6 GMA 51 224-G11-001 6 GMA 52 224-G2-001 6 GMA 53 224-C4-001 6 GMA 54 224-F4-001 6 GMA 55 224-B6-001 6 GMA 56 224-B7-001 6 GMA 57 224-G10-001 6 GMA 58 224-D6-001 6 GMA 59 224-E7-001 6 GMA 60 224-B11-001 6 GMA 61 224-C11-001 6 GMA 62 224-E2-001 6 GMA GMA and Copaxone-Specific* T Cell Lines Non-Canonical Non-Canonical Initiating MHC Peptide Peptide Non- No. Cell Line Antigen Restriction Reactivity** Reactivity*** 1 222-AG12 GMA Peptide 026 GLT 631 GAT 631 GAT 111 GL 14 LT 11 GT 11 2 222-BC11 GMA 3 165-B5G GMA DR-11 GL 14 Peptide 026 GLT 631 GAT 631 GAT 111 LT 11 GT 11 4 165-C4G GMA DR-15 GT 11 5 165-C5G GL 14 GLT 631 LT 11 GAT 631 GAT 111 GT 11 6 165-D8G GMA Peptide 026 7 165-E7G GMA GLT 631 Peptide 026 GAT 631 GAT 111 GL 14 LT 11 GT 11 8 165-E9G GMA DR-11 GL 14 GLT 631 GAT 631 GAT 111 LT 11 GT 11 9 165-F5G GMA DR-11 GL 14 Peptide 026 GLT 631 GAT 631 GAT 111 LT 11 GT 11 10 165-F8G GMA GT 11 11 165-F10G GMA DR-15 GL 14 GLT 631 LT 11 GAT 631 GAT 111 GT 11 12 165-H11G GMA GLT 631 GAT 111 GAT 631 LT 11 GL 14 GT 11 13 222-2D8 COP 14 222-2E1 COP 15 222-2F12 COP GL 14 GLT 631 GAT 631 GAT 111 16 165-B6C COP DR-11 Peptide 026 GLT 631 GAT 631 GT 11 17 165-B10C COP Peptide 026 GT 11 18 165-C4C COP GL 14 GLT 631 LT 11 GAT 631 GAT 111 GT 11 GT41S GA64 19 165-C7C COP DR-11 GL 14 GLT 631 LT 11 GAT 631 GAT 111 GT 11 20 165-D2C COP GT 11 21 165-D3C COP DR-15 Peptide 026 GT 11 22 165-U3C COP Peptide 026 GLT 631 GAT 631 GAT 111 GT 11 23 165-F3C COP DR-11 Peptide 026 GLT 631 GL 14 GAT 631 LT 11 GAT 111 GT 11 GT 41S GA 64 24 165-F6C COP DR-15 Peptide 026 GT 11 GL 14 GLT 631 GAT 631 GAT 111 LT 11 GT 11 GT 41S GA64 25 205-1B4 COP Peptide 026 GLT 631 GAT 631 26 205-1C4 COP Peptide 026 GLT 631 GAT 631 27 205-1F2 COP 28 205-1F4 COP DR-13 LT 11 GLT 631 GAT 631 GAT 111 GL 14 GT 41S GA 64 29 205-1H3 COP GL 14 GLT 631 GAT 631 GAT 111 LT 11 GT 41S GA 64 30 205-1H5 COP Peptide 026 GLT 631 GAT 631 31 205-1H7 COP DR-13 GLT 631 GAT 631 GAT 111 32 205-1H11 COP Peptide 026 GLT 631 GAT 631 GAT 111 GL 14 LT 11 GT 11 *Identified based on proliferation response using the equation: (Proliferation response to test antigen - proliferation response to control) ÷ (proliferation response to reference antigen - proliferation response to control) = 0.8 to 1.2. **Percent proliferation of no antigen control; lines identified based on at least one proliferation value of greater than or equal to 2 times no antigen control value. ***All cell lines in this column identified based on proliferation values of less than 2 times no antigen control value. Use of an Assay Panel of GA-Specific Human T-Cell Lines
Determining Immunological Identity
log10(
β·(lower limit)≦β*≦β·(upper limit) (9)Identification of GA Response Biomarkers
EXAMPLES
Methods
1. Preparation and Initial Stimulation of Human T-Cell Lines
2. Screening of Human T-Cell Lines for Reactivity to GA by Proliferation Assay
3. Expansion/Restimulation of GA-Specific T-Cells
4. Screening T-Cell Lines for GA-Specificity
IFN-γ ELISA
Multiplex Assay
5. Preparation of Antigen Presenting Cells
6. Maintenance of EBV Transformed B Cells (B-LCL)
7. MHC DR Restriction Testing
8. Donor HLA Typing
9. Thawing Cryopreserved Cells
10. Reagents
Peptides Peptide Composition 026 YEAK polymer; tyrosine withheld for first five minutes of manufacture GLT 631 (GLT) poly (Glu-Lys-Tyr; 6:3:1) (Sigma-Aldrich, St. Louis, MO) GAT 631 poly (Glu-Ala-Tyr; 6:3:1) (Sigma-Aldrich, St. Louis, MO) GAT 111 poly (Glu-Ala-Tyr; 1:1:1) (Sigma-Aldrich, St. Louis, MO) LT 11 poly (Lys-Tyr; 1:1) (Sigma-Aldrich, St. Louis, MO) GT 11 poly (Glu-Tyr; 1:1) (Sigma-Aldrich, St. Louis, MO) GT 41 poly (Glu-Tyr; 4:1) (Sigma-Aldrich, St. Louis, MO) GL 14 poly (Glu-Lys; 1:4) (Sigma-Aldrich, St. Louis, MO) GA 64 poly (Glu-Ala; 6:4) (Sigma-Aldrich, St. Louis, MO) MBP FFKNIVTPRTPPPSQGK (Myelin basic protein peptide, residues 84-102) (AnaSpec, Fremont, CA) ST (STERN) EKPKVEAYKAAAAPA (Bachem H-6292, Torrance, CA) Example I
Generation of GA-Specific Human T-Cell Lines
Donor HLA-DR Loci MHC Class II Donor Age Gender Race HLA-DRβ1 Donor 1 (222) 25 Female Caucasian *04:01, *07:01 Donor 2 (206) 63 Female Caucasian *04:01, *04:04 Donor 3 (165) 51 Male Caucasian *15, *11 Donor 4 (205) 21 Male Caucasian *07, *13 Donor 5 (213) 23 Female Caucasian *11, *1 Donor 6 (224) 49 Female Caucasian *13, *15:01 Donor 7 (228) 30 Male Caucasian *15, *03 T Cell Lines Obtained No Cell Line Donor Culturing Antigen 1 222-AG11 1 GMA 2 222-AG12 1 GMA 3 222-BA11 1 GMA 4 222-BC11 1 GMA 5 206-1A7 2 GMA 6 165-B2G 3 GMA 7 165-B5G 3 GMA 8 165-B11G 3 GMA 9 165-C4G 3 GMA 10 165-C5G 3 GMA 11 165-C8G 3 GMA 12 165-D8G 3 GMA 13 165-E7G 3 GMA 14 165-E9G 3 GMA 15 165-F10G 3 GMA 16 165-F2G 3 GMA 17 165-F5G 3 GMA 18 165-F8G 3 GMA 19 165-H11G 3 GMA 20 222-1C5 1 COP 21 222-1H12 1 COP 22 222-2B11 1 COP 23 222-2B8 1 COP 24 222-2C1 1 COP 25 222-2C3 1 COP 26 222-2D2 1 COP 27 222-2D8 1 COP 28 222-2E1 1 COP 29 222-1F8 1 COP 30 222-2F12 1 COP 31 222-2G4 1 COP 32 222-1G8 1 COP 33 222-2G12 1 COP 34 165-B6C 3 COP 35 165-B10C 3 COP 36 165-C4C 3 COP 37 165-C7C 3 COP 38 165-C9C 3 COP 39 165-D2C 3 COP 40 165-D3C 3 COP 41 165-D11C 3 COP 42 165-E3C 3 COP 43 165-E9C 3 COP 44 165-F3C 3 COP 45 165-F4C 3 COP 46 165-F6C 3 COP 47 165-F9C 3 COP 48 165-F11C 3 COP 49 165-G7C 3 COP 50 205-1B4 4 COP 51 205-1B7 4 COP 52 205-1C4 4 COP 53 205-1D1 4 COP 54 205-1E1 4 COP 55 205-1F2 4 COP 56 205-1F4 4 COP 57 205-1G3 4 COP 58 205-1H1 4 COP 59 205-1H3 4 COP 60 205-1H4 4 COP 61 205-1H5 4 COP 62 205-1H7 4 COP 63 205-1H9 4 COP 64 205-1H11 4 COP
Donor 1: Cell Lines Initially Stimulated with GMA
BrdU Uptake by Eight Donor 1 Human T-Cell Lines after Stimulation with GMA. Donor 1 Cell Line Control (OD450) GMA (OD450) 222-AF2 0.454 1.119 222-AF5 0.606 1.133 222-AC11 0.680 1.244 222-BA4 0.370 0.603 222-AG11 0.359 0.875 222-BA11 0.255 0.783 222-AG12 0.448 0.949 222-BC11 0.499 0.921 Donor 1 Presumptive COP T Cell Lines - ATP Proliferation Assay Donor 1 Cell Line Control COP 10 μg/mL 222-1C5 7087 14906 222-1F8 2308 6519 222-1G8 4030 8308 222-1H12 8141 23836 222-2B8 5543 13600 222-2B11 6340 16460 222-2C1 8350 20166 222-2C3 6839 14045 222-2C5 4695 9577 222-2D2 7135 16271 222-2D8 13024 26495 222-2E1 8035 17064 222-2F12 9703 28022 222-2G4 2566 6812 222-2G12 3913 9899 (Data in RLU.) Antigen Reactivity of Cell Line 222-2D8 (Data from FIG. 5A) GMA COP No Antigen 2290 1999 1 μg/mL 4369 4888 3 μg/mL 7480 6392 10 μg/mL 12793 12161 Antigen Reactivity of Cell Line 222-2D8 (Day 56) GMA COP Mean Std dev Mean Std dev No Antigen 697 18 1 μg/mL 2078 50.5 7229 1968 3 μg/mL 149752 7960.4 134127 15480 10 μg/mL 156417 21302.6 146092 13377 Antigen Reactivity of Cell Line 222-2F12 (Data from FIG. 5C) GMA COP No Antigen 4442 2417 1 μg/mL 6500 5281 3 μg/mL 8520 8684 10 μg/mL 12382 11747 Reactivity of Donor 1 COP T-Cell Line 222-2F12 to Non-Canonical Peptides (Day 56) 0 μg/mL 3 μg/mL 10 μg/mL 30 μg/mL Std Std Std Std Mean dev Mean dev Mean dev Mean dev COP 5673 664 233087 4 230009 3534 118769 7210 GLT 5277 1002 4934 809 5217 1933 631 GAT 4342 187 3226 378 3071 923 631 GAT 3914 333 3590 748 2430 288 111 GL 14 17007 350 331 288 203 175 GMA 232779 451 228761 2425 125994 7400
Cytokine Production by Donor 1 Human T-Cell Lines Stimulated with Copaxone
IFN-γ Produced by Donor 1 COP T-Cell Lines in Response to Stimulation with GMA or COP. IFN-γ: T-Cells + IFN-γ: T-Cells + IFN-γ: T-Cells + IFN-γ: T-Cells Alone B-LCL GMA, B-LCL Copaxone, B-LCL Line Mean Std Dev Mean Std Dev Mean Std Dev Mean Std Dev 222-2D8 224 38 270 18 >1000 >1000 222-1C5 849 37 821 118 776 53 700 49 222-2E1 303 32 457 23 >1000 >1000 222- 943 13 >1000 >1000 >1000 1H12 222-2B11 389 20 354 8 >1000 >1000 222-2D2 25 7 68 11 131 13 133 11 222-2C3 489 32 538 32 >1000 >1000 222-2B8 686 10 599 44 >1000 >1000 222- 294 11 515 35 >1000 >1000 2G12 222-2C5 306 12 325 56 245 45 273 8 222-2F12 232 17.9 295 17.1 >1000 >1000 222-2G4 94 3.2 280 26.2 438 12.8 416 29.0 222-1F8 28 9.3 120 7.5 169 14.4 156 16.2 222-1G8 907 >1000 >1000 >1000 222-2C1 206 33 369 28 392 56 417 48 (Data in mean pg/mL of triplicate cultures.) Cytokine Production by Donor 1 COP T-Cell Lines 222-2D8, 222-1H12 IFN-γ IL-2 IL-10 IL-5 IL-1β IL-4 TNF-α TNF-β (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) 222-2D8 Control 92 0 56 22 0 0 0 0 222-2D8 GMA 2616 52 107 1501 2420 41 405 243 222-2D8 COP 3296 0 100 1587 2535 0 493 267 222-1H12 Control 785 125 72 83 0 89 20 44 222-1H12 GMA 3138 152 234 336 20 99 83 102 222-1H12 COP 3626 0 240 398 59 0 22 98 Cytokine Production by Donor 1 COP T-Cell Lines 222-1C5 and 222-2D2 IFN-γ IL-2 IL-10 IL-5 IL-8 IL-1β TNF-α TNF-β (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) 222-1C5 Control 590.55 145.76 74.93 95.43 <= 0 88.72 6.64 19.5 222-1C5 GMA 511.42 185.35 77.88 67.83 <= 0 94.12 31.35 29.94 222-1C5 COP 416.83 <= 0 50.58 56.39 <= 0 <= 0 <= 0 15.34 222-2D2 Control 9.11 24.59 35.81 14.93 <= 0 22.54 <= 0 5.79 222-2D2 GMA 53.75 55.42 26.12 29.13 <= 0 103.85 5.35 5.79 222-2D2 COP 120 210.47 45.09 38.13 0.85 130.9 30.87 23.15 Cytokine Production by Donor 1 COP T-Cell Line 222-2B8 IFN-γ IL-2 IL-10 IL-22 IL-13 IL-5 TNF-α (pg/ (pg/ (pg/ (pg/ (pg/ (pg/ (pg/ mL) mL) mL) mL) mL) mL) mL) Control 0 0 0 57.94 11.85 0 0 GMA-1 1.88 0 0 0 183.09 0 0 μg/mL GMA-3 44.52 1.6 1.8 63.98 1214.45 78.29 0 μg/mL GMA- 140.65 0 41 124.15 3162.51 400.46 96.57 10 μg/mL MBP 0 0 0 20.62 26.77 0 0 Control 0 0 0 57.94 11.85 0 0 COP-1 8.46 0 2.99 83.01 204.31 0 0 μg/mL COP-3 39.91 19.65 2.83 77.82 1028.23 30.3 0 μg/mL COP- 119.12 0 36.16 143.57 2825.8 314.77 91.29 10 μg/mL MBP 0 0 0 20.62 26.77 0 0 Cytokine Production by Donor 1 COP T-Cell Line 1C5 IFN-γ IL-2 IL-10 IL-22 IL-13 IL-5 TNF-α (pg/ (pg/ (pg/ (pg/ (pg/ (pg/ (pg/ mL) mL) mL) mL) mL) mL) mL) Control 84 80 5 105 107 0 14 GMA-1 μg/mL 181 30 11 39 154 0 3 GMA-3 μg/mL 325 83 38 101 316 0 72 GMA-10 μg/mL 664 0 55 0 942 0 80 MBP 64 0 0 36 82 0 9 Control 84 80 5 105 107 0 14 COP-1 μg/mL 158 0 11 0 129 0 11 COP-3 μg/mL 256 0 16 32 254 0 42 COP-10 μg/mL 585 40 49 42 837 0 42 MBP 64 0 0 36 82 0 9 Cytokine Production by Donor 1 COP T-Cell Line 2B11 IFN-γ IL-2 IL-10 IL-22 IL-13 IL-5 TNF-α (pg/ (pg/ (pg/ (pg/ (pg/ (pg/ (pg/ mL) mL) mL) mL) mL) mL) mL) Control 0 19.65 3.79 84.94 36.59 0 0 GMA-1 0 0 1.3 42.51 29.41 0 0 μg/mL GMA-3 0 0 0 51.87 133.55 0 0 μg/mL GMA- 37.79 0 0.4 0 1563.22 94.56 0 10 μg/mL MBP 0 0 0 0 0.83 0 0 Control 0 19.65 3.8 84.94 36.59 0 0 COP-1 0.65 63.02 0 69.37 47.69 0 0 μg/mL COP-3 0 0 0 45.67 105.51 0 0 μg/mL COP- 47.35 0 11.27 19.45 1839.63 119 13.51 10 μg/mL MBP 0 0 0 0 0.83 0 0 Cytokine Production by Donor 1 COP T-Cell Line 2E1 IFN-γ IL-2 IL-10 IL-22 IL-13 IL-5 TNF-α (pg/ (pg/ (pg/ (pg/ (pg/ (pg/ (pg/ mL) mL) mL) mL) mL) mL) mL) Control 22.54 67.91 0 75.29 54.48 0 0 GMA-1 49.11 0 0 21.77 146.02 0 9.36 μg/mL GMA-3 162.68 0 7.1 25.71 1050.77 0 65.44 μg/mL GMA- 349.76 0 16.33 0 2036.36 128.4 170.24 10 μg/mL MBP 20.69 42.43 2.28 107.54 51.1 0 13.51 Control 22.54 67.91 0 75.29 54.48 0 0 COP-1 53.73 26.46 5.3 72.54 165.79 0 25.28 μg/mL COP-3 163.61 40.39 12.19 40.37 1037.93 0 0 μg/mL COP- 405.26 0 22 60.95 2046.94 106.24 333.22 10 μg/mL MBP 20.69 42.43 2.3 107.54 51.1 0 13.51 BrdU Uptake by Donor 2 Human T-Cell Lines after GMA Stimulation. Donor 2 Cell Line Control (OD450) GMA (OD450) 206-1A6 0.220 0.563 206-1A7 0.329 0.688 206-1A11 0.151 0.452 206-1C12 0.272 0.626 206-1H1 0.109 0.447 206-1H2 0.095 0.526 206-2A1 0.259 0.548 206-2A10 0.162 0.444 206-2C3 0.146 0.358 Donor 3 Presumptive GMA Cell Lines - ATP Proliferation Assay Donor 3 Cell Line Control GMA 10 μg/mL 165-B2G 9692 28770 165-B5G 15423 63023 165-B11G 18412 56588 165-C4G 14869 62306 165-C5G 14240 76296 165-C8G 18205 79281 165-D8G 30331 136938 165-E7G 18215 66364 165-E9G 17059 52948 165-F2G 14734 44951 165-F5G 16943 100677 165-F8G 17986 72716 165-F10G 13314 42447 165-H11G 17149 52850 (Data in RLU.) 165-F2G 165-B5G 165-F8G 165-H11G Antigen Mean Std dev Mean Std dev Mean Std dev Mean Std dev None 17575 570 27019 2046 20130 954 12847 591 COP 79071 2082 51934 2971 40596 2709 59388 1477 GMA 74327 5970 51364 1200 35103 1993 54450 498 Tetanus 17519 649 20861 81 19132 1294 9511 962 165-F5G 165-D8G 165-E9G 165-F10G Antigen Mean Std dev Mean Std dev Mean Std dev Mean Std dev None 40831 2747 39276 453 17077 1992 15039 1436 COP 114287 3327 83592 4132 64509 6068 120610 1200 GMA 108326 11172 81880 2323 62461 3778 111713 3974 Tetanus 29012 1074 27814 2555 11745 2413 9121 2749 (Data in RLU.)
Cytokine Production by Donor 3 Human T-Cell Lines Stimulated with Different Concentrations of GMA
Cytokine Production by Donor 3 GMA Responsive T Cells IFN-γ IL-22 IL-13 IL-4 IL-5 (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) 165-B5G CONTROL 0 40 321 2 4 165-B5G GMA 201 248 2930 47 399 165-B5G COP 190 106 2927 17 374 165-B5G TETANUS 0 98 294 16 14 165-D8G CONTROL 0 143 172 13 0 165-D8G GMA 121 405 3326 12 309 165-D8G COP 115 365 3121 14 309 165-D8G TETANUS 0 146 186 0 0 165-F8G CONTROL 235 143 378 4 145 165-F8G GMA 889 197 1193 5 370 165-F8G COP 771 198 1017 0 297 165-F8G TETANUS 286 156 407 3 179 165-E9G CONTROL 0 126 87 0 0 165-E9G GMA 837 245 1499 6 236 165-E9G COP 638 192 1232 12 208 165-E9G TETANUS 0 96 83 5 0 165-H11G CONTROL 94 123 93 13 9 165-H11G GMA 724 201 1058 7 320 165-H11G COP 724 190 1095 9 321 165-H11G TETANUS 84 163 107 17 0 165-F10G CONTROL 7 176 263 13 63 165-F10G GMA 1108 680 10897 114 2589 165-F10G COP 1165 686 11285 122 2586 165-F10G TETANUS 2 54 248 0 44 165-B5G 165-F8G 165-F5G 165-D8G Mean Std dev Mean Std dev Mean Std dev Mean Std dev No Antigen 4422 577 4857 367 3704 517 9496 763 GMA 1 μg/mL 3866 374 4804 684 5669 324 9427 957 GMA 3 μg/mL 6591 792 6841 115 14024 943 12644 817 GMA 10 μg/mL 12367 2111 11880 1318 23774 1393 31264 529 COP 1 μg/mL 3672 567 3690 902 4332 204 8088 899 COP 3 μg/mL 3673 781 3184 527 6099 670 8722 2268 COP 10 μg/mL 14282 516 10663 880 19498 2649 28872 1737 MBP 3398 622 3366 404 2904 233 6909 320 165-C5G 165-H11G 165-E9G 165-F10G Mean Std dev Mean Std dev Mean Std dev Mean Std dev No Antigen 3398 474 2541 219 2882 301 3977 444 GMA 1 μg/mL 12236 932 10205 715 2961 273 6015 702 GMA 3 μg/mL 36288 763 27515 3713 7837 98 17900 875 GMA 10 μg/mL 45444 2719 44067 1144 24935 1588 30198 2512 COP 1 μg/mL 12667 933 9102 561 3216 928 8427 923 COP 3 μg/mL 24835 2274 15637 2647 5584 1143 31303** 2532 COP 10 μg/mL 47537 1478 43786 1906 25454 2250 29986 1994 MBP 3401 538 2925 265 2892 312 3585 542 165-B2G 165-C4G 165-E7G 165-F2G Mean Std dev Mean Std dev Mean Std dev Mean Std dev No Antigen 2149 176 2558 131 2083 147 1659 137 GMA 1 μg/mL 4034 456 3434 309 2569 221 1753 226 GMA 3 μg/mL 20994 1235 6312 527 6610 700 1422 181 GMA 10 μg/mL 38244 3194 19169 1494 14279 967 1502 116 COP 1 μg/mL 3779 446 3143 608 3794 695 1700 120 COP 3 μg/mL 11983 1946 3959 393 4147 1073 1332 86 COP 10 μg/mL 10980* 1844 3717* 308 12565 240 1554 61 MBP 3320 440 3482 404 2989 438 2345 1269 *Well contained 3 μg/mL COP. **Well contained 10 μg/mL COP.
Donor 3: Cell Lines Stimulated with COP
Donor 3 Presumptive COP Responsive T Cell Lines - ATP Proliferation Assay Donor 3 Cell Line Control COP 10 μg/mL 165-B6C 14966 77564 165-B10C 10604 54779 165-C4C 23510 108089 165-C7C 12707 63491 165-C9C 22840 113199 165-D2C 12767 54895 165-D3C 18380 119241 165-D11C 13302 82759 165-E3C 12219 69893 165-E9C 22512 98168 165-F3C 13157 93437 165-F4C 13598 96289 165-F6C 23457 135516 165-F9C 19736 145768 (Data in RLU.) 165-D3C 165-F3C 165-E3C Antigen Mean Std dev Mean Std dev Mean Std dev None 1283 32 245 32 1351 114 COP 3362 314 1141 21 3778 78 GMA 3436 125 1257 74 3723 389 Tetanus 1282 56 239 23 1205 67 165-B6C 165-F6C 165-D11C Antigen Mean Std dev Mean Std dev Mean Std dev None 259 33 563 27 220 16 COP 952 82 1484 49 525 96 GMA 1058 69 1489 94 416 16 Tetanus 211 31 510 3 178 22 (Data in RLU.) Confirmation of Antigen Reactivity of Three Donor 3 COP Cell Lines by ATP Proliferation Assay 165-D2C 165-C7C 165-C4C Mean Std dev Mean Std dev Mean Std dev No Antigen 2407 344 1702 211 1783 29 GMA 1 μg/mL 3881 159 3307 306 9367 174 GMA 3 μg/mL 7503 808 12832 1361 26279 1785 GMA 10 μg/mL 13253 2024 42739 3834 31014 3030 COP 1 μg/mL 3955 655 3670 332 11653 1386 COP 3 μg/mL 7378 934 16527 1385 28474 2184 COP 10 μg/mL 14239 315 40194 3057 28262 749 MBP 10 μg/mL 1927 489 1673 92 1631 131 165-B6C 165-B10C 165-F6C 165-F3C Mean Std dev Mean Std dev Mean Std dev Mean Std dev No antigen 4966 484 4739 280 4876 506 9937 1476 GMA 1 μg/mL 9925 1683 5592 1290 18511 5644 45592 3040 GMA 3 μg/mL 38105 2486 13012 928 43634 1648 101328 8124 GMA 10 μg/mL 25839 2638 19866 3407 34321 3367 84268 6292 COP 1 μg/mL COP 3 μg/mL 29815 930 8450 582 31382 4713 75407 5826 COP 10 μg/mL 34502 1598 19792 441 34071 279 85442 13367 MBP 10 μg/mL 3216 963 3003 165 2214 630 9849 1475 165-D3C 165-D11C 165-E3C 165-C8G Mean Std dev Mean Std dev Mean Std dev Mean Std dev No antigen 11537 1527 7630 1028 4874 386 2598 570 GMA 1 μg/mL 61535 6445 15655 758 10662 1175 5605 1712 GMA 3 μg/mL 80112 15879 17777 3266 35044 5182 5002 201 GMA 10 μg/mL 107772 19471 56609 3513 68577 2466 30200 2759 COP 1 μg/mL 1248 303 486 49 364 17 400 65 COP 3 μg/mL 79792 9655 19410 1034 23785 2625 4821 766 COP 10 μg/mL 86270 20682 42017 3161 51822 1083 22961 2140 MBP 10 μg/mL 15482 2939 8406 495 7662 3966 5279 821 (Data in RLU.) Donor 4 Presumptive COP Responsive T Cell Lines- ATP Proliferation Assay Donor 4 Cell Line Control COP 10 μg/mL 205-1B4 41950 84937 205-1B7 32281 74147 205-1C4 35104 69861 205-1D1 31270 63419 205-1E1 19150 44198 205-1F2 27423 71947 205-1F4 16172 33143 205-1G3 24326 54211 205-1H1 14255 29824 205-1H3 24304 55518 205-1H4 19145 50307 205-1H5 26809 57608 205-1H7 19483 64436 205-1H9 15320 52972 205-1H11 20205 54232 (Data in RLU.) Antigen Reactivity of Cell Line 205-1F4 (Day 50) GMA COP Mean Std dev Mean Std dev No Antigen 2076 497 2076 497 1 μg/mL 17341 4286 47254 19915 3 μg/mL 195335 4478 169021 13990 10 μg/mL 218954 7589 213196 4628 Antigen Reactivity of Cell Line 205-1F4 (Day 58) GMA COP Mean Std dev Mean Std dev No Antigen 3193 462 3 μg/mL 29921 4413 21031 1489 10 μg/mL 30998 1112 31423 1493 30 μg/mL 33915 652 31323 1133 100 μg/mL 32698 1743 31188 3568 Donor 4 T-Cell Line 205-1H7 Dose Response Ag Concentration Standard (μg/mL COP) Mean RLU Deviation 0 3382 781 1 13131 334 10 175652 14847 30 190750 8994 Donor 4 T-Cell Line 205-1H11 Dose Response Ag Concentration Standard (μg/mL COP) Mean RLU Deviation 0 6941 593 1 12243 1712 10 147464 2228 30 112673 6037
Cytokine Production by Donor 4 Human T-Cell Lines Stimulated with COP
Cytokine Production by Donor 4 COP Responsive T-Cell Lines IL-12 p70 IFN-y IL-2 IL-10 IL-6 IL-4 IL-5 TNF-α TNF-β (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) 205-1B7 6.48 29.28 <=0 <=0 11.31 <=0 0.82 8.36 16.12 control 205-1B7 44.57 828.5 10.89 182.81 187.85 187.29 6854.13 1305.37 217.5 GMA 205-1B7 18.39 935.78 27.99 210.01 195.27 200.19 7199.83 1286.09 244.68 COP 205-1G3 20.01 45.5 91.05 75.24 10.15 10.27 31.82 50.07 100.29 control 205-1G3 2.37 193.91 5.27 <=0 16.67 <=0 121.17 68.79 17.66 GMA 205-1G3 <=0 211.16 10.89 <=0 11.79 <=0 91.11 38.56 <=0 COP 205-1C4 <=0 0.04 <=0 <=0 5.42 <=0 <=0 10.54 <=0 control 205-1C4 46.89 1046.32 312.13 45.24 149.35 124.7 5434.06 1059.01 81.04 GMA 205-1C4 51.14 1212.03 478.51 50.31 159.24 199.97 6457.14 1529.22 69.07 COP 205-1115 3.18 32.31 <=0 <=0 6.61 <=0 2.65 5.47 4.73 control 205-1H5 8.78 902.95 <=0 <=0 52.4 46.82 2534.09 910.83 79.8 GMA 205-1H5 7.96 1149.77 15.14 28.45 57.31 79.67 3106.45 1260.75 88.62 COP 205-1F5 30.5 79.21 115.8 97.94 9.17 12.46 62.11 112.49 99.74 control 205-1F5 67.53 479.26 197.46 231.63 314.54 328.72 13930.97 1084.01 260.35 GMA 205-1F5 55.94 452.76 205.85 323.52 318.25 293.46 13727.89 833.39 278.68 COP 205-1H7 2.46 3.48 <=0 <=0 6.76 <=0 <=0 17.73 <=0 control 205-1H7 13.42 1275.97 176.52 <=0 64.82 58.41 96.95 552.89 34.81 GMA 205-1H7 11.11 1617.25 351.63 10.06 69.44 90.37 101.71 1924.81 49.17 COP 1H9 <=0 5.39 <=0 <=0 <=0 <=0 <=0 24.29 <=0 control 1H9 GMA <=0 182.77 <=0 <=0 74.92 <=0 <=0 161.89 <=0 1H9 COP <=0 419.21 <=0 <=0 <=0 <=0 <=0 159.6 <=0 1H11 1.19 770.12 <=0 <=0 <=0 <=0 159.49 110.13 <=0 control 1H11 2.24 929 22.78 145 28.28 <=0 3349.8 609.49 <=0 GMA 1H11 3.68 1016.21 44.94 143.84 20.11 2.09 3665.58 754.13 1.55 COP Donor 4 Presumptive COP Responsive T Cell Lines - Extended Cytokine Multiplex Assay IFN-γ IL-2 IL-10 1L-9 IL-22 IL-6 IL-13 IL-4 IL-5 TNF-α 205-1B4 CONTROL 54 38 0 7 99 15 217 10 2 133 205-1B4 GMA 713 0 25 21 478 109 13895 117 3104 868 205-1B4 COP 726 0 32 29 668 114 19288 174 3660 1336 205-1B4 TET 1 0 0 1 82 719 168 0 0 342 205-1F4 CONTROL 0 0 0 2 0 11 30 0 0 27 205-1F4 GMA 1207 76 16 7 186 73 4506 7 1459 323 205-1F4 COP 1393 110 0 10 168 77 4502 10 1440 616 205-1F4 TET 0 0 0 3 96 285 8 0 0 174
Donor 6: Cell Lines Stimulated with GMA
Donor 6 GMA-Reactive T-Cell Lines-ATP Proliferation Assay Donor 6 Cell Control GMA Lot 001 COP Tetanus Line (No Ag) 10 μg/mL 10 μg/mL Toxoid 224-D2-001 8251 25144 26027 7430 224-G11-001 13350 85685 91466 12447 224-G2-001 849 230429 227257 833 224-C4-001 1542 49779 52771 1238 224-F4-001 2243 116349 114667 2644 224-B6-001 657 75125 78357 611 224-B7-001 704 25730 20586 435 224-G10-001 3181 229901 229933 3440 224-D6-001 2276 51060 68767 4937 224-E7-001 3023 137297 137556 1777 224-B11-001 2927 29729 27861 3273 224-C11-001 13120 85787 87933 22863 224-E2-001 895 233093 233094 852 (Data in RLU.) Example II
Characterization of GA-Specific Human T-Cell Lines Based on Reactivity to Non-Canonical GA Peptides
Examples of GA-Reactive Human T-Cell Lines that React to a Non-Canonical GA Peptide Nonreactive Peptide Composition Reactive T-Cell Line T-Cell Line 026 YEAK polymer; 165-B6C (Donor 3) 222-AG12 (Donor 1) tyrosine withheld for 165-D3C (Donor 3) 165-B5G (Donor 3) first 5 minutes of 165-F3C (Donor 3) 165-D8G (Donor 3) synthesis 165-F6C (Donor 3) 165-E7G (Donor 3) 205-1C4 (Donor 4) 165-F5G (Donor 3) 205-1H5 (Donor 4) 165-B10C (Donor 3) 205-1H11 (Donor 4) 165-D11C (Donor 3) 165-E3C (Donor 3) 205-1B4 (Donor 4) GLT 631 (GLT) poly (Glu-Lys-Tyr; 222-1H12 (Donor 1) 222-AG12 (Donor 1) 6:3:1) 165-H11G (Donor 3) 222-2F12 (Donor 1) 205-1H7 (Donor 4) 165-B5G (Donor 3) 165-C5G (Donor 3) 165-C8G (Donor 3) 165-E7G (Donor 3) 165-E9G (Donor 3) 165-F5G (Donor 3) 165-F10G (Donor 3) 165-B6C (Donor 3) 165-C4C (Donor 3) 165-C7C (Donor 3) 165-E3C (Donor 3) 165-F3C (Donor 3) 165-F6C (Donor 3) 205-1B4 (Donor 4) 205-1C4 (Donor 4) 205-1F4 (Donor 4) 205-1H3 (Donor 4) 205-1H5 (Donor 4) 205-1H11 (Donor 4) GAT 631 poly (Glu-Ala-Tyr; 165-H11G (Donor 3) 222-AG12 (Donor 1) 6:3:1) 222-2F12 (Donor 1) 222-1H12 (Donor 1) 165-B5G (Donor 3) 165-C5G (Donor 3) 165-C8G (Donor 3) 165-E7G (Donor 3) 165-E9G (Donor 3) 165-F5G (Donor 3) 165-F10G (Donor 3) 165-B6C (Donor 3) 165-C4C (Donor 3) 165-C7C (Donor 3) 165-E3C (Donor 3) 165-F3C (Donor 3) 165-F6C (Donor 3) 205-1C4 (Donor 4) 205-1F4 (Donor 4) 205-1H3 (Donor 4) 205-1H5 (Donor 4) 205-1H7 (Donor 4) 205-1H11 (Donor 4) GAT 111 poly (Glu-Ala-Tyr; — 222-AG12 (Donor 1) 1:1:1) 222-2F12 (Donor 1) 222-1H12 (Donor 1) 165-B5G (Donor 3) 165-C5G (Donor 3) 165-C8G (Donor 3) 165-E7G (Donor 3) 165-E9G (Donor 3) 165-F5G (Donor 3) 165-F5G (Donor 3) 165-F10G (Donor 3) 165-H11G (Donor 3) 165-C4C (Donor 3) 165-C7C (Donor 3) 165-E3C (Donor 3) 165-F3C (Donor 3) 165-F6C (Donor 3) 205-1F4 (Donor 4) 205-1H3 (Donor 4) 205-1H7 (Donor 4) 205-1H11 (Donor 4) GL 14 poly (Glu-Lys; 1:4) 222-2F12 (Donor 1) 222-AG12 (Donor 1) 165-B5G (Donor 3) 222-1H12 (Donor 1) 165-C4C (Donor 3) 205-1F4 (Donor 4) 165-C5G (Donor 3) 205-1H11 (Donor 4) 165-C7C (Donor 3) 165-F6C (Donor 3) 165-E7G (Donor 3) 165-E9G (Donor 3) 165-F3C (Donor 3) 165-F5G (Donor 3) 165-F10G (Donor 3) 165-H11G (Donor 3) 205-1H3 (Donor 4) LT 11 poly (Lys-Tyr; 1:1) 165-C4C (Donor 3) 222-AG12 (Donor 1) 165-C7C (Donor 3) 165-B5G (Donor 3) 165-F3C (Donor 3) 165-F5G (Donor 3) 165-C5G (Donor 3) 165-E7G (Donor 3) 165-F10G (Donor 3) 165-E9G (Donor 3) 205-1F4 (Donor 4) 165-F5G (Donor 3) 165-H11G (Donor 3) 165-F6C (Donor 3) 205-1H3 (Donor 4) GT 11 poly (Glu-Tyr; 1:1) — 222-AG12 (Donor 1) 165-B2G (Donor 3) 165-B5G (Donor 3) 165-C4G (Donor 3) 165-C5G (Donor 3) 165-C8G (Donor 3) 165-E7G (Donor 3) 165-E9G (Donor 3) 165-F2G (Donor 3) 165-F5G (Donor 3) 165-F8G (Donor 3) 165-F10G (Donor 3) 165-H11G (Donor 3) 165-B6C (Donor 3) 165-B10C (Donor 3) 165-C4C (Donor 3) 165-C7C (Donor 3) 165-D2C (Donor 3) 165-D3C (Donor 3) 165-E3C (Donor 3) 165-F3C (Donor 3) 165-F6C (Donor 3) GT41S — 165-C4C (Donor 3) 165-F3C (Donor 3) 165-F6C (Donor 3) 205-1F4 (Donor 4) 205-1H3 (Donor 4) 205-1H11 (Donor 4) GA64 — 165-C4C (Donor 3) 165-F3C (Donor 3) 165-F6C (Donor 3) 205-1F4 (Donor 4) 205-1H3 (Donor 4) Reactivity of GA-Specific Human T-Cell Lines to Peptide 026
Donor 3 GA-Specific T-Cell Lines: Peptide 026
Reactivity of Donor 3 COP T-Cell Lines to Peptide 026 MBP GMA COP 026 Std Std Std Std 165-B6C Mean dev Mean dev Mean dev Mean dev No Antigen 2358 44 2358 44 2358 44 2358 44 1 μg/mL 3939 452 3878 231 2339 316 3 μg/mL 2205 97 19344 1921 21455 1153 2715 326 10 μg/mL 2268 143 29012 2065 32197 857 5989 721 100 μg/mL 2201 196 9856 1095 7969 1417 10083 911 MBP GMA COP 026 Std Std Std Std 165-B10C Mean dev Mean dev Mean dev Mean dev No Antigen 2234 295 2234 295 2234 295 2234 295 1 μg/mL 1942 155 2117 192 2663 126 3 μg/mL 2176 183 2658 76 2519 175 2548 175 10 μg/mL 2320 361 5854 547 6701 695 2667 190 100 μg/mL 2272 177 6380 382 6215 1277 992 181 MBP GMA COP 026 Std Std Std Std 165-D3C Mean dev Mean dev Mean dev Mean dev No Antigen 8319 1520 8319 1520 8319 1520 8319 1520 1 μg/mL 24131 692 22689 1733 9388 1069 3 μg/mL 8037 1559 58814 4975 50369 2258 14049 1015 10 μg/mL 8656 1373 57024 2006 52151 1613 20508 3121 100 μg/mL 8647 1235 14205 408 10140 1549 9062 2296 MBP GMA COP 026 Std Std Std Std 165-D11C Mean dev Mean dev Mean dev Mean dev No Antigen 4571 353 4571 353 4571 353 4571 353 1 μg/mL 3636 506 3410 260 4297 159 3 μg/mL 4348 443 6578 570 6618 216 4287 453 10 μg/mL 4301 373 16338 1033 15652 1327 4050 603 100 μg/mL 4020 476 14560 437 12958 1216 1931 216 MBP GMA COP 026 Std Std Std 165-E3C Mean dev Mean Std dev Mean dev Mean dev No Antigen 2797 124 2797 124 2797 124 2797 124 1 μg/mL 2954 231 2592 354 3582 648 3 μg/mL 2898 241 17065 2766 14394 3211 3888 61 10 μg/mL 2658 94 37602 2580 39495 2117 3443 216 100 μg/mL 2582 305 23437 4209 20538 3942 2256 1189 MBP GMA COP 026 Std Std Std Std 165-F3C Mean dev Mean dev Mean dev Mean dev No Antigen 3150 184 3150 184 3150 184 3150 184 1 μg/mL 9423 835 7777 52 3131 226 3 μg/mL 3369 343 37555 1427 36331 1813 3776 308 10 μg/mL 3115 266 44621 1586 44047 2194 8005 251 100 μg/mL 3017 263 12391 489 9932 1294 6667 776 MBP GMA COP 026 Std Std Std Std 165-F6C Mean dev Mean dev Mean dev Mean dev No Antigen 2189 82 2189 82 2189 82 2189 82 1 μg/mL 15671 823 13418 267 2027 57 3 μg/mL 1960 84 40274 2355 38973 1889 2816 354 10 μg/mL 1977 210 38921 1554 39560 924 10332 1043 100 μg/mL 3672 567 3690 902 4332 204 8088 899 Donor 4 GA-Specific T-Cell Lines: Peptide 026
Reactivity of Donor 4 COP T-Cell Lines 205-1B4, 205-1C4, and 205-1H5 to Non-Canonical GA Peptides COP 026 GLT 631 GAT 631 205-1B4 Mean Std dev Mean Std dev Mean Std dev Mean Std dev No Antigen 830 45 830 45 830 45 830 45 1 μg/mL 1522 110 870 75 912 72 843 70 3 μg/mL 5501 1043 1047 127 1059 160 890 24 10 μg/mL 11273 493 856 145 1387 204 845 92 30 μg/mL 1007 215 1389 188 766 221 COP 026 GLT 631 GAT 631 205-1C4 Mean Std dev Mean Std dev Mean Std dev Mean Std dev No Antigen 4464 605 4464 605 4464 605 4464 605 1 μg/mL 8859 211 7353 623 4559 355 4856 369 3 μg/mL 26612 1314 15709 243 4934 314 5584 419 10 μg/mL 41287 2529 32715 1111 5616 261 5666 523 30 μg/mL 43886 695 5782 872 5137 761 COP 026 GLT 631 GAT 631 205-1H5 Mean Std dev Mean Std dev Mean Std dev Mean Std dev No Antigen 749 9 749 9 749 9 749 9 1 μg/mL 2375 494 985 79 710 39 648 132 3 μg/mL 7182 229 1103 55 885 100 696 65 10 μg/mL 8580 731 1265 209 1153 124 698 105 30 μg/mL 1872 342 1294 187 669 21 Reactivity of Donor 4 COP T-Cell Line 205-1H11 to Non-Canonical GA Peptides 0 μg/mL 1 μg/mL 10 μg/mL 30 μg/mL Mean Std dev Mean Std dev Mean Std dev Mean Std dev COP 6941 593 12243 1712 147464 228 112673 6037 026/11 5513 232 11329 1298 78603 1155 GLT (6:3:1) 5627 331 5915 269 8542 332 GAT (6:3:1) 6244 522 6604 399 6089 484 GAT (1:1:1) 6121 304 6448 578 6253 520 LT 11 5961 406 6698 616 4303 445 GL 14 5954 532 102 83 74 50 GT 41S 5484 435 5840 828 5395 390 (Data in RLU.) Reactivity of Donor 4 COP T-Cell Line 205-1H3 to Non-Canonical GA Peptides 0 μg/mL 1 μg/mL 3 μg/mL 10 μg/mL Mean Std dev Mean Std dev Mean Std dev Mean Std dev COP 4121.0 829.9 32122.0 7174.4 49585.0 3808.6 60878.3 4768.1 GMA 37654.3 6157.2 55284.3 1312.7 60841.7 4656.5 GLT (6:3:1) 3619.3 521.5 2923.7 422.8 3264.7 624.6 GAT (6:3:1) 4271.0 360.7 4577.0 233.2 4589.0 665.3 GAT (1:1:1) 3892.7 601.5 3227.7 714.1 3704.3 502.7 GL 14 15619.0 1114.2 1053.3 107.2 61.0 3.0 LT 11 4780.3 769.3 3083.3 459.3 3381.0 512.1 GA 64 4012.0 574.6 4246.0 1209.8 2054.3 267.5 GT 41S 3773.3 274.2 3857.0 808.1 2349.0 124.7 (Data in RLU.) Reactivity of GA-Specific Human T-Cell Lines to Peptide GLT 631 and GAT 631
Donor 1 GA-Specific T-Cell Line 222-1H12: Peptides GLT 631 and GAT
Reactivity of Donor 1 COP T-Cell Line 222-1H12 to Non-Canonical Peptides 0 μg/mL 3 μg/mL 10 μg/mL 30 μg/mL Mean Std dev Mean Std dev Mean Std dev Mean Std dev COP 5974 1124 146874 10577 168163 10045 68492 10042 GLT (6:3:1) 11147 1604 7518 1515 9490 1361 GAT (6:3:1) 5267 1022 4204 736 3141 472 GAT (1:1:1) 5224 784 3555 406 3014 370 GL (1:4) 7143 805 234 211 131 95 GMA 141486 15062 167193 12648 82376 11177 Donor 3 GA-Specific T-Cell Lines: GLT and GAT
Reactivity of Donor 3 GMA T-Cell Lines 165-B5G to Peptides GLT 631 and GAT 631 1 μg/mL 3 μg/mL 10 μg/mL 30 μg/mL Mean Std dev Mean Std dev Mean Std dev Mean Std dev COP 6275 305 7499 775 10914 1331 13974 1841 GAT 631 4666 1106 5536 517 4997 646 3637 641 GLT 631 4288 675 5153 258 11847 1251 11416 1591 No Antigen Value: 2432 +/− 243 Reactivity of Donor 3 GMA T-Cell Lines 165-E7G to Peptides GLT 631 and GAT631 1 μg/mL 3 μg/mL 10 μg/mL 30 μg/mL Mean Std dev Mean Std dev Mean Std dev Mean Std dev COP 4217 1198 5366 663 7619 2756 7161 342 GAT 631 4043 600 6457 2644 5499 326 4178 905 GLT 631 3992 2391 5133 643 12006 461 12836 2611 No Antigen Value: 2145 +/− 1180 Reactivity of Donor 3 GMA T-Cell Line 165-H11G to Peptides GLT 631 and GAT631 1 μg/mL 3 μg/mL 10 μg/mL 30 μg/mL Mean Std dev Mean Std dev Mean Std dev Mean Std dev GMA 9569 380 46539 1071 49104 1175 32179 1534 COP 8610 2000 37551 4300 50044 2322 32898 2167 GAT 631 2413 332 2969 151 2995 136 3115 35 GLT 631 2763 139 2875 134 3028 333 3093 13 GAT 111 2448 599 2294 237 2102 31 1599 55 STERN 2279 335 2118 198 2418 43 2296 241 No Antigen Value: 1485 +/− 208 Reactivity of Donor 3 GMA T-Cell Line 165-05G to Peptides GLT 631 and GAT 631 1 μg/mL 3 μg/mL 10 μg/mL 30 μg/mL Mean Std dev Mean Std dev Mean Std dev MeanStd dev GMA 3999 413 35643 2850 53130 1978 55596 664 COP 6791 132 42584 2129 56893 2843 55001 2583 GAT 631 2004 59 2207 152 1928 72 1829 260 GLT 631 1871 253 2268 207 2256 113 2137 363 GAT 111 1778 112 1661 312 1569 87 1449 170 STERN 1750 48 1606 91 1650 140 1572 83 No Antigen Value : 2149 +/− 361 Reactivity of Donor 3 GMA T-Cell Line 165-E9G to Peptides GLT 631 and GAT631 1 μg/mL 3 μg/mL 10 μpg/mL 30 μg/mL Mean Std dev Mean Std dev Mean Std dev Mean Std dev GMA 1889 115 5974 911 37352 1044 39472 2920 COP 1999 109 5538 439 38813 828 38286 503 GAT 631 1482 162 1593 109 1454 59 1382 47 GLT 631 1630 208 1600 161 2341 554 2152 217 GAT 111 1528 128 1454 77 1462 77 1301 23 MBP 1527 162 1428 102 1247 81 1208 70 STERN 1673 62 1480 75 1471 81 1487 273 No Antigen Value : 1742 +/− 146 Reactivity of Donor 3 GMA T-Cell Line 165-F10G to Peptides GLT 631 and GAT 631 1 μg/mL 3 μg/mL 10 μg/mL 30 μg/mL Mean Std dev Mean Std dev Mean Std dev Mean Std dev GMA 4734 539 35371 2546 58913 459 59173 1484 COP 6350 1127 40495 1593 58726 2437 60488 1731 GAT 631 3755 652 3543 68 3702 332 3794 352 GLT 631 3363 395 3061 1076 4823 370 4568 699 GAT 111 3575 552 2989 196 2876 263 2299 248 STERN 2465 153 2389 31 2030 214 2583 552 No Antigen Value: 3752 +/− 233 Reactivity of 165-F6C to GA and Non-Canonical GA Peptides (Day 76) 1.25 μg/mL 2.5 μg/mL 5 μg/mL 10 μg/mL Std Std Std Std Mean dev Mean dev Mean dev Mean dev GMA 629 18 3,197 803 11,867 2,036 20,141 2,133 GLT 631 501 104 416 54 482 48 474 19 GAT 631 516 72 479 101 463 49 449 38 GAT 111 576 30 620 83 479 35 411 47 LT 11 431 26 518 70 490 49 736 129 GT 11 406 84 426 28 388 41 303 29 GL 14 699 10 2,267 164 114 19 33 2 GT 41S 566 42 578 6 600 40 No Antigen value: 517 +/− 51. Donor 4 GA-Specific T-Cell Line 205-1H7: GLT 631, GAT 631 and GAT 611
Reactivity of Donor 4 COP T-Cell Line 205-1H7 to Peptide GLT 631 0 μg/mL 1μg/mL 10 μg/mL 30 μg/mL std std std std Mean dev Mean dev Mean dev Mean dev COP 3382 781 13131 334 175652 14847 190750 8994 026/11 4962 319 2854 728 2989 515 GLT (6:3:1) 3859 1182 7376 1082 6770 111 GAT (6:3:1) 2949 392 3664 2027 2964 674 GAT (1:1:1) 2401 44 4111 674 1912 331 Reactivity of GA-Specific Human T-Cell Lines to GL 14 and LT11
Donor 1 GA-Specific T-Cell Lines: GL 14
Reactivity of Donor 1 COP T-Cell Line 222-2F12 to Non-Canonical Peptides 0 μg/mL 3 μg/mL 10 μg/mL 30 μg/mL Mean Std dev Mean Std dev Mean Std dev Mean Std dev COP 5673 664 233087 4 230009 3534 118769 7210 GLT (6:3:1) 5277 1002 4934 809 5217 1933 GAT (6:3:1) 4342 187 3226 378 3071 923 GAT (1:1:1) 3914 333 3590 748 2430 288 GL (1:4) 17007 350 331 288 203 175 GMA 232779 451 228761 2425 125994 7400 Donor 3 GA-Specific T-Cell Lines
Reactivity of Donor 3 COP T-Cell Lines 165-F3C and 165-C4C to Non-Canonical Peptides 0 μg/mL 1 μg/mL 3 μg/mL 10 μg/mL 165-F3C Mean Std dev Mean Std dev Mean Std dev Mean Std dev GMA 1563 207 7277 386 50400 1605 70563 1724 GLT 1563 207 2325 313 2557 123 2724 302 GAT 631 1563 207 2389 106 2249 191 2391 166 GAT 111 1563 207 2387 140 2269 360 1772 51 LT 11 1563 207 2144 118 2605 329 3608 166 GT 11 1563 207 2108 173 2152 290 1652 455 GL 14 1563 207 2542 532 7091 239 65 17 GT 41S 1563 207 1962 201 1778 34 1997 184 GA 64 1563 207 1791 122 1943 230 1870 208 COP 1563 207 5027 576 32906 2723 62888 2978 0 μg/mL 1 μg/mL 3 μg/mL 10 μg/mL 165-C4C Mean Std dev Mean Std dev Mean Std dev Mean Std dev GMA 1881 484 2668 396 16108 1188 88509 7445 GLT 1881 484 2465 140 2677 268 2939 156 GAT 631 1881 484 2489 142 2687 119 2682 163 GAT 111 1881 484 2330 405 2392 202 2233 88 LT 11 1881 484 2430 261 2701 187 4129 254 GT 11 1881 484 2664 295 2804 147 2311 323 GL 14 1881 484 2833 887 4920 594 49 4 GT 41S 1881 484 2119 317 1943 65 2193 275 GA 64 1881 484 2205 141 2229 205 2244 374 COP 1881 484 2357 201 7913 430 74558 5178 Reactivity of Donor 3 GMA T-Cell Line 165-B5G to Non-Canonical Peptides 1.25 μg/mL 2.5 μg/mL 5 μg/mL 10 μg/mL Std Std Std Std Mean dev Mean dev Mean dev Mean dev GMA 1406 11 2537 342 5205 321 11364 973 GLT 1054 130 1159 109 1687 396 3589 169 GAT 631 1133 131 1288 329 1511 62 1645 441 GAT 111 1005 122 886 187 947 176 931 132 LT 11 1497 164 1724 631 1666 320 2705 785 GT 11 2167 777 1957 811 1353 202 1191 219 GL 14 3912 685 3245 171 501 55 45 1 MBP 871 87 No Antigen Value: 846 +/− 71 Reactivity of Donor 3 GMA T-Cell Line 165-E7G to Non-Canonical Peptides 1.25 μg/mL 2.5 μg/mL 5 μg/mL 10 μg/mL Mean Std dev Mean Std dev Mean Std dev Mean Std dev GMA 8875 2958 10248 834 26934 4062 30721 255 GLT 2983 754 3783 834 5821 918 5710 737 GAT 631 2629 554 2827 193 2950 131 3877 972 GAT 111 1896 248 2001 587 1285 107 2176 575 LT 11 2785 619 5270 40 3559 351 1983 371 GT 11 2821 474 2914 726 2332 344 1848 183 GL 14 10001 457 14902 1074 4087 459 58 3 MBP 2116 233 No Antigen Value: 2777 +/− 398 Reactivity of Donor 3 GMA T-Cell Line 165-F10G to Non-Canonical Peptides 1.25 μg/mL 2.5 μg/mL 5 μg/mL 10 μg/mL Std Std Std Std Mean dev Mean dev Mean dev Mean dev GMA 5731 1930 38905 3450 66355 1411 57329 2055 GLT 1012 88 1389 172 2620 397 2505 184 GAT 631 948 133 1080 156 1024 107 944 69 GAT 111 929 61 1342 52 1091 160 1054 115 LT 11 1145 258 1270 393 1552 276 8012 1258 GT 11 1388 225 1506 27 1598 77 1294 147 GL 14 5153 813 19489 985 1990 1178 53 4 MBP 831 39 No Antigen Value: 1004 +/− 207 Reactivity of Donor 3 GMA T-Cell Line 165-C5G to Non-Canonical Peptides 1.25 μg/mL 2.5 μg/mL 5 μg/mL 10 μg/mL Std Std Std Std Mean dev Mean dev Mean dev Mean dev GMA 2511 550 26253 2744 67559 5332 74630 2713 GLT 485 45 552 13 585 157 579 111 GAT 631 435 33 465 81 504 68 423 74 GAT 111 445 35 406 41 422 71 430 64 LT 11 527 56 680 133 3010 445 34438 3148 GT 11 523 83 620 90 428 100 471 47 GL 14 1412 60 5418 618 703 13 43 1 MBP 469 11 No Antigen Value: 450 +/− 92 Reactivity of Donor 3 GMA T-Cell Line 165-H11G to Non-Canonical Peptides 1.25 μg/mL 2.5 μg/mL 5 μg/mL 10 μg/mL Std Std Std Std Mean dev Mean dev Mean dev Mean dev GMA 8 652 33793 895 70949 3504 72728 5989 GLT 29 263 344 53 448 57 435 83 GAT 631 43 253 290 31 297 15 305 10 GAT 111 49 340 320 67 304 72 336 22 LT 11 15 434 326 60 357 39 178 5 GT 11 17 352 354 10 352 2 308 76 GL 14 144 532 4416 51 118 68 40 2 MBP 323 31 No Antigen Value: 245 +/− 32 Reactivity of Donor 3 COP T-Cell Line 165-C7C to Non-Canonical Peptides 1.25 μg/mL 2.5 μg/mL 5 μg/mL 10 μg/mL Std Std Std Std Mean dev Mean dev Mean dev Mean dev GMA 610 187 2699 912 22588 1382 24029 2844 GLT 273 66 219 60 273 79 219 24 GAT 631 232 26 204 36 200 43 184 24 GAT 111 273 81 231 8 224 58 264 88 LT 11 418 73 305 16 244 23 119 33 GT 11 346 22 265 50 255 19 234 1 GL 14 274 23 1797 405 537 411 39 0 MBP 278 14 No Antigen Value: 188 +/− 21 Reactivity of Donor 3 GMA T-Cell Line 165-E9G to Non-Canonical Peptides 1.25 μg/mL 2.5 μg/mL 5 μg/mL 10 μg/mL Std Std Std Std Mean dev Mean dev Mean dev Mean dev GMA 253 31 1647 105 12964 2092 13809 961 GLT 142 35 195 40 233 26 312 1 GAT 631 122 8 175 35 207 13 179 20 GAT 111 154 10 240 35 240 13 153 55 LT 11 132 15 129 9 89 17 91 12 GT 11 110 32 119 8 103 15 96 7 GL 14 145 57 801 283 268 102 38 2 MBP 163 15 No Antigen Value: 125 +/− 30 Reactivity of Donor 3 GMA T-Cell Line 165-F5G to Non-Canonical Peptides 1.25 μg/mL 2.5 μg/mL 5 μg/mL 10 μg/mL Std Std Std Std Mean dev Mean dev Mean dev Mean dev GMA 1071 392 19012 658 45384 5165 48029 1665 GLT 631 131 30 143 45 176 48 110 40 GAT 631 118 22 160 35 106 24 130 22 GAT 111 139 29 121 45 142 31 122 33 LT 11 126 22 113 12 136 31 129 22 GT 11 160 50 124 42 124 18 158 35 GL 14 559 107 2779 485 89 8 38 1 MBP 138 12 No Antigen Value: 136 +/− 29 Donor 4 GA-Specific T-Cell Line 205-1F4
Reactivity of Donor 4 COP T-Cell Line 205-1F4 to Non-Canonical GA Peptide LT 11(Day 80) 1 μg/mL 2 μg/mL 5 μg/mL 10 μg/mL Mean Std dev Mean Std dev Mean Std dev Mean Std dev GMA 7,477 1,628 64,999 3,444 83,107 3,193 67,482 3,285 LT 11 3,675 488 4,589 827 7,626 1,261 8,853 513 GMA no antigen = 812 (SD 71); LT 11 no antigen = 850 (SD85) Reactivity of Donor 4 COP T-Cell Line 205-1F4 to Non-Canonical GA Peptides (Day 71) 0 μg/mL 1 μg/mL 3 μg/mL 10 μg/mL 205-1F4 Mean Std dev Mean Std dev Mean Std dev Mean Std dev COP 2076 497 47254 19915 169021 13990 213196 4628 GMA 17341 4286 195335 4478 218954 7589 GLT 631 1938 322 1863 46 2504 255 GAT 631 2424 143 2439 136 3035 374 GAT 111 2002 21 2163 238 2275 299 LT 11 73850 16093 154321 6238 158776 11492 GL 14 12630 1371 1423 686 158 134 GT 41S 2361 239 1805 396 1855 262 GA 64 2544 220 2261 80 2080 250 Donor 1 GA-Specific T-Cell Line 222-AG12: Lack of Reactivity to Non-Canonical Peptides
COP GLT 631 GAT 631 GAT 111 Std Std Std Std ug/mL Mean dev Mean dev Mean dev Mean dev 0 4071 186 1 10056 878 3801 772 3442 406 3391 708 10 233083 3 4002 340 3217 202 2568 162 30 227055 10049 5292 885 3548 367 2245 496 Peptide 026 LT 11 GL 14 GT 11 Std Std Std Std ug/mL Mean dev Mean dev Mean dev Mean dev 1 4155 630 3452 44 3531 205 2650 450 10 4120 653 4242 284 55 7 3285 773 30 2683 109 2347 748 43 2 2231 179 Data in RLU. Example III
Characterization of GA-Specific Human T-Cell Lines Based on MHC Restriction
MHC Restriction of T-Cell Lines Line Donor MHC Class II MHC Restriction 165-B5G (Donor 3) *15, *11 DR-11 165-C4G (Donor 3) *15, *11 DR-11 165-C5G (Donor 3) *15, *11 DR-15 165-E9G (Donor 3) *15, *11 DR-11 165-F5G (Donor 3) *15, *11 DR-11 165-F10G (Donor 3) *15, *11 DR-15 165-B6C (Donor 3) *15, *11 DR-11 165-C7C (Donor 3) *15, *11 DR-11 165-D3C (Donor 3) *15, *11 DR-15 165-F3C (Donor 3) *15, *11 DR-11 165-F6C (Donor 3) *15, *11 DR-15 205-1D1 (Donor 4) *07, *13 DR-13 205-1F4 (Donor 4) *07, *13 DR-13 205-1H7 (Donor 4) *07, *13 DR-13 Donor 3 T-Cell Line 165-B5G
MHC Restriction of 165-B5G B-LCL Donor Allele 1 Allele 2 No Ag SD GMA SD 3 DRβ1*1501 DRβ1*11 1736 449 4776 560 (Autologous) 5 DRβ1*01 DRβ1*11 939 101 5450 933 6 DRβ1*1501 DRβ1*13 1113 120 2360 354 Donor 3 T-Cell Line 165-C4G
MHC Restriction of 165-C4G B-LCL Donor Allele 1 Allele 2 No Ag SD GMA SD 3 DRβ1*1501 DRβ1*11 1657 25 48711 3406 (Autologous) 5 DRβ1*01 DRβ1*11 1695 453 41833 1884 7 DRβ1*1501 DRβ1*03 3126 376 4870 197 Donor 3 T-Cell Line 165-C5G
MHC Restriction of 165-C5G B-LCL Donor Allele 1 Allele 2 No Ag SD GMA SD 3 DRβ1*1501 DRβ1*11 2738 241 16461 1924 (Autologous) 5 DRβ1*01 DRβ1*11 3824 352 9825 798 6 DRβ1*1501 DRβ1*13 2341 108 31717 3547 Donor 3 T-Cell Line 165-E9G
MHC Restriction of 165-E9G B-LCL Donor Allele 1 Allele 2 No Ag SD GMA SD 3 DRβ1*1501 DRβ1*11 1276 295 5452 452 (Autologous) 5 DRβ1*01 DRβ1*11 1300 124 9514 460 6 DRβ1*1501 DRβ1*13 1255 85 1442 96 Donor 3 T-Cell Line 165-F5G
MHC Restriction of 165-F5G B-LCL Donor Allele 1 Allele 2 no Ag SD GMA SD 3 DRβ1*1501 DRβ1*11 881 96 2862 62 (Autologous) 5 DRβ1*01 DRβ1*11 1255 74 3971 755 6 DRβ1*1501 DRβ1*13 858 120 1424 38 Donor 3 T-Cell Line 165-F10G
MHC Restriction of 165-F10G B-LCL Donor Allele 1 Allele 2 no Ag SD GMA SD 3 DRβ1*1501 DRβ1*11 2603 107 120946 9732 (Autologous) 5 DRβ1*01 DRβ1*11 1200 60 6221 2138 7 DRβ1*1501 DRβ1*03 7709 336 151115 6439 Donor 3 T-Cell Line 165-B6C
MHC Restriction of 165-B6C B-LCL Donor Allele 1 Allele 2 no Ag SD GMA SD 3 DRβ1*1501 DRβ1*11 224 13 2237 421 (Autologous) 5 DRβ1*01 DRβ1*11 516 63 8944 1014 6 DRβ1*1501 DRβ1*13 250 16 322 20 Donor 3 T-Cell Line 165-C7C
MHC Restriction of 165-C7C B-LCL Donor Allele 1 Allele 2 no Ag SD GMA SD 3 DRβ1*1501 DRβ1*11 1130 18 13714 509 (Autologous) 5 DRβ1*01 DRβ1*11 7752 1101 94535 369 6 DRβ1*1501 DRβ1*13 767 66 1189 348 Donor 3 T-Cell Line 165-D3C
MHC Restriction of 165-D3C B-LCL Donor Allele 1 Allele 2 no Ag SD GMA SD 3 DRβ1*1501 DRβ1*11 7335 83 106840 7358 (Autologous) 5 DRβ1*01 DRβ1*11 3181 43 12173 5325 7 DRβ1*1501 DRβ1*03 18492 642 149506 2288 Donor 3 T-Cell Line 165-F3C
MHC Restriction of 165-F3C Std. Std. B-LCL Donor Allele 1 Allele 2 no Ag Dev GMA Dev 3 DRβ1*1501 DRβ1*11 2346 290 45559 4853 (Autologous) 5 DRβ1*01 DRβ1*11 3582 547 39565 3654 6 DRβ1*1501 DRβ1*3 2885 356 7869 2306
Donor 3 T-Cell line 165-F6C
MHC Restriction of 165-F6C B-LCL Donor Allele 1 Allele 2 No Ag SD GMA SD 3 DRβ1*1501 DRβ1*11 837 108 12562 1236 (Autologous) 5 DRβ1*01 DRβ1*11 963 87 3142 285 6 DRβ1*1501 DRβ1*13 1122 62 30293 3836 Donor 4 T-Cell Line 205-1D1
MHC Restriction of 205-1D1 B-LCL No Donor Allele 1 Allele 2 Ag SD COP SD 4 DRβ1* DRβ1* 1581.0 227.4 231113.0 3122.5 (autologous) 07 13 2 DRβ1* DRβ1* 1216.7 119.9 1203.7 546.4 04:01 04:04 1 DRβ1* DRβ1* 2546.3 251.2 1504.7 40.4 04:01 07:01 6 DRβ1* DRβ1* 13472.0 1971.9 203780.3 21756.6 15:01 13 Donor 4 T-Cell Line 205-1H7
MHC Restriction of 205-1H7 B-LCL Donor Allele 1 Allele 2 No Ag SD COP SD 4 (autologous) DRβ1*07 DRβ1*13 1956 464 32418 2996 1 DRβ1*04:01 DRβ1*07:01 2748 784 8430 2540 6 DRβ1*15:01 DRβ1*13 2729 300 30583 174 2 DRβ1*04:01 DRβ1*04:04 2471 217 2065 250 Donor 4 T-Cell Line 205-1F4
MHC Restriction of 205-1F4 205-1F4 APC donor Donor Alleles Stimulation Mean SD 4 *07, *13 no antigen 36999 2980 (autologous) COP 84094 5074 2 *04:01*04:04 no antigen 2412 140 COP 1786 197 1 *04:01, *07 no antigen 24850 1063 COP 11323 1116 6 *13, *15 no antigen 10542 297 COP 113079 4646 Example IV
Characterization of GA-Specific Human T-Cell Lines Based on Analysis of Surface Marker Expression
GMA and Copaxone Initiated T-Cell Lines- CD4+ and CD8+ Expression Culturing Cell Line Donor Antigen % CD4+ % CD8+ 222-BA11 1 GMA 99 0 165-B5G 3 GMA 99.1 0.7 165-C4G 3 GMA 99.8 165-C5G 3 GMA 99.9 165-D8G 3 GMA 97.8 1.1 165-E7G 3 GMA 99.8 165-E9G 3 GMA 99.8 165-F10G 3 GMA 99.7 165-F5G 3 GMA 97.5 1.5 165-F8G 3 GMA 99.6 165-H11G 3 GMA 99.9 222-2D8 1 COP 99.8 222-2F12 1 COP 99.2 165-B6C 3 COP 99.4 165-B10C 3 COP 99.4 165-C4C 3 COP 99.5 165-C7C 3 COP 98.6 165-D3C 3 COP 97.7 5.4 165-D11C 3 COP 99.8 165-E3C 3 COP 99.4 165-F3C 3 COP 99.4 165-F6C 3 COP 97.8 0.9 205-1C4 4 COP 98 205-1F4 4 COP 97.8 205-1H5 4 COP 95.8 205-1H7 4 COP 98.6 0 205-1H11 4 COP 96.4 Example V
GA-Specific Human T-Cell Line Panel Assay—Comparison of Nine GA Test Lots to Copaxone
Assay Panel GA-Specific Human T-Cell Lines Noncanon. Donor GA-Specificity Noncanon. Peptide T-Cell MHC Culturing MHC CD4/CD8 Maintained Peptide Non- Line Class II Antigen Restriction Expression (Restimulations) Reactivity Reactivity 222-2D8 *04:01, COP DR 4 CD4+/CD8− ≧8 *07:01 222-2F12 *04:01, COP DR 4 CD4+/CD8− ≧8 GL 14 GLT 631 *07:01 GAT 631 GAT 111 165-F3C *15,*11 COP DR 11 CD4+/CD8− ≧8 Peptide 026 GLT 631 GL 14 GAT 631 LT 11 GAT 111 GT 11 GT 41S GA 64 165-F6C *15,*11 COP DR 15 CD4+/CD8− ≧8 Peptide 026 GT 11 GL 14 GLT 631 GAT 631 GAT 111 LT 11 GT 11 GT 41S GA64 205-1C4 *07, *13 COP CD4+/CD8− ≧8 Peptide 026 GLT 631 GAT 631 205-1F4 *07, *13 COP DR 13 CD4+/CD8− ≧8 LT 11 GLT 631 GAT 631 GAT 111 GL 14 GT 41S GA 64
Proliferation response to test antigen minus proliferation response to control(no antigen)÷proliferation response to reference antigen minus proliferation response to control(no antigen)222-2D8 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 5,873 928 6,520 467 136.0% 1 14,017 3,561 15,533 1,280 115.2% 2 90,328 20,393 104,628 7,566 116.6% 2.5 129,878 3,893 142,861 6,649 110.3% 5 171,116 12,003 174,692 5,624 102.1% Background (No Antigen) value: 4,017 +/− 441 222-2F12 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 10,879 171 13,129 576 162.8% 1 27,042 508 24,268 2,645 86.0% 2 105,024 1,834 113,978 4,261 109.2% 2.5 115,241 1,764 115,441 7,433 100.2% 5 129,291 1,465 132,528 3,596 102.7% Background (No Antigen) value: 7,297 +/− 910 165-F3C Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 4,783 773 4,673 1,336 95.1% 1 22,290 2,463 16,447 3,682 70.4% 2 53,771 5,010 49,404 2,335 91.5% 2.5 54,539 2,237 51,336 1,503 93.8% 5 58,110 3,484 52,941 4,149 90.7% Background (No Antigen) value: 2,555 +/− 186 165-F6C COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 3,328 321 3,251 181 39.3% 1 4,620 594 4,618 455 99.8% 2 13,255 4,631 9,672 963 64.4% 2.5 18,726 6,563 16,212 4,933 83.8% 5 38,653 4,745 35,066 4,305 89.9% Background (No Antigen) value: 3,202 +/− 327 205-1C4 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 932 357 825 49 33.7% 1 2,481 146 1,829 335 61.9% 2 9,432 657 6,614 1,422 67.5% 2.5 13,744 1,733 12,582 4,369 91.0% 5 45,451 3,658 39,117 7,455 85.8% Background (No Antigen) value: 770 +/− 62 205-1F4 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 548 91 479 66 46.7% 1 1,906 653 1,815 712 93.9% 2 9,959 1,393 9,129 1,261 91.3% 2.5 13,589 1,245 12,785 1,362 93.9% 5 18,491 3,267 18,752 1,679 101.4% Background (No Antigen) value: 419 +/− 4 222-2D8 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 4,255 505 5,049 722 190.4% 1 10,314 1,870 10,915 2,583 108.7% 2 78,457 15,207 78,823 6,965 100.5% 2.5 97,873 6,379 122,230 10,886 125.8% 5 144,399 8,605 149,682 16,251 103.7% Background (No Antigen) value: 3,378 +/− 153 222-2F12 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 12,703 501 12,673 497 99.3% 1 23,391 2,618 21,487 1,792 87.3% 2 112,115 7,484 109,769 12,264 97.7% 2.5 120,306 5,905 124,373 1,295 103.6% 5 124,636 4,818 124,165 3,982 99.6% Background (No Antigen) value: 8,395 +/− 464 165-F3C Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 4,463 1,250 3,972 568 75.1% 1 21,816 6,712 17,145 2,862 75.8% 2 49,532 5,529 45,626 4,664 91.7% 2.5 49,651 1,323 52,096 4,000 105.2% 5 49,174 3,503 53,439 3,483 109.1% Background (No Antigen) value: 2,493 +/− 192 165-F6C COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 3,280 222 3,339 344 122.6% 1 3,964 745 3,496 400 50.6% 2 11,309 3,969 7,475 1,069 53.8% 2.5 17,679 3,758 10,730 2,294 52.6% 5 44,855 2,714 38,352 5,451 84.5% Background (No Antigen) value: 3,018 +/− 159 205-1C4 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 1,161 172 978 121 29.5% 1 2,900 683 1,868 339 48.4% 2 11,911 1,293 6,957 1,479 55.0% 2.5 22,329 3,810 11,920 4,092 51.4% 5 44,721 3,924 40,858 2,367 91.2% Background (No Antigen) value: 902 +/− 83 205-1F4 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 798 73 862 84 151.0% 1 5,066 1,752 3,707 1,629 69.1% 2 18,121 3,930 13,446 2,549 73.2% 2.5 21,921 2,364 18,853 1,841 85.6% 5 29,173 1,524 23,905 854 81.5% Background (No Antigen) value: 672 +/− 68 222-2D8 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 1,557 456 1,512 286 95.9% 1 8,703 1,362 8,397 1,693 96.3% 2 32,940 3,445 34,123 3,955 103.6% 2.5 48,525 8,931 51,422 5,676 106.0% 5 71,567 8,205 75,757 4,512 105.9% Background (No Antigen) value: 461 +/− 76 222-2F12 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 2,110 681 1,618 225 55.2% 1 10,990 1,608 8,548 999 75.5% 2 57,764 3,942 40,708 7,498 69.9% 2.5 65,033 4,308 59,306 3,142 91.1% 5 73,060 2,058 74,006 2,646 101.3% Background (No Antigen) value: 1,012 +/− 168 165-F3C Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 20,493 3,108 15,553 2,464 68.0% 1 68,598 5,356 70,805 5,415 103.5% 2 100,809 9,587 110,379 7,763 110.0% 2.5 105,322 4,191 114,694 2,804 109.3% 5 102,499 3,416 110,289 5,200 108.0% Background (No Antigen) value: 5,034 +/− 307 165-F6C COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 5,357 351 5,685 469 154.7% 1 6,511 874 6,820 283 117.6% 2 7,708 541 8,527 414 127.8% 2.5 11,862 2,882 11,399 1,492 93.5% 5 35,725 5,659 36,869 3,807 103.7% Background (No Antigen) value: 4,787 +/− 482 205-1C4 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 5,075 1,021 4,842 200 74.6% 1 17,052 4,570 10,681 3,096 50.6% 2 68,021 4,447 58,057 6,966 84.4% 2.5 78,633 4,450 79,010 4,431 100.5% 5 81,137 5,758 81,325 7,340 100.2% Background (No Antigen) value: 4,161 +/− 347 205-1F4 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 3,568 598 4,342 690 162.7% 1 69,032 22,687 69,419 12,433 100.6% 2 149,921 7,505 152,894 10,185 102.0% 2.5 163,087 9,462 168,342 4,837 103.3% 5 152,755 6,433 157,571 3,542 103.2% Background (No Antigen) value: 2,334 +/− 162 222-2D8 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 1,067 60 1,101 122 105.4% 1 8,354 2,087 6,193 733 72.7% 2 36,821 4,297 31,068 6,184 84.2% 2.5 53,029 5,449 50,894 2,421 95.9% 5 81,271 5,745 82,775 4,069 101.9% Background (No Antigen) value: 430 +/− 34 222-2F12 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 1,893 188 1,469 216 55.1% 1 10,225 2,333 7,538 1,448 71.0% 2 40,220 1,419 32,024 3,241 79.1% 2.5 57,292 3,144 53,168 5,176 92.7% 5 68,039 1,978 69,258 1,701 101.8% Background (No Antigen) value: 950 +/− 100 165-F3C Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 15,194 1,152 14,737 4,629 95.8% 1 67,378 4,756 58,886 6,743 86.5% 2 102,921 5,040 106,309 2,930 103.4% 2.5 110,400 4,921 114,473 6,296 103.8% 5 104,688 5,035 105,772 5,365 101.1% Background (No Antigen) value: 4,283 +/− 251 165-F6C COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 4,003 479 3,890 381 80.8% 1 4,700 323 4,462 400 81.5% 2 5,888 499 5,200 290 72.2% 2.5 6,324 849 5,967 1,013 87.7% 5 13,607 3,286 13,687 3,965 100.8% Background (No Antigen) value: 3,414 +/− 278 205-1C4 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 6,392 771 5,887 803 79.7% 1 19,042 4,648 11,232 3,081 48.4% 2 82,263 9,522 78,506 8,350 95.2% 2.5 95,299 6,137 94,727 2,832 99.4% 5 93,952 5,481 98,785 4,142 105.4% Background (No Antigen) value: 3,901 +/− 250 205-1F4 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 3,257 321 3,945 628 166.8% 1 68,785 10,930 44,032 14,509 62.8% 2 139,390 7,392 146,170 3,179 104.9% 2.5 160,581 5,164 155,997 7,765 97.1% 5 155,605 6,639 150,329 6,076 96.6% Background (No Antigen) value: 2,227 +/− 157 222-2D8 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 25,365 4,220 27,316 1,982 112.4% 1 77,981 4,058 99,345 4,984 131.2% 2 153,313 3,143 161,150 1,648 105.5% 2.5 168,388 4,098 170,296 5,988 101.2% 5 147,783 5,013 158,207 5,260 107.5% Background (No Antigen) value: 9,575 +/− 465 222-2F12 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 24,901 2,351 23,156 1,553 84.6% 1 55,453 7,018 66,725 4,436 126.9% 2 103,212 3,256 110,099 1,828 107.7% 2.5 108,843 4,486 111,414 3,588 102.7% 5 106,365 4,274 109,397 2,414 103.3% Background (No Antigen) value: 13,595 +/− 616 165-F3C Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 20,375 4,140 17,678 2,582 76.4% 1 54,977 6,036 48,036 9,773 84.9% 2 75,772 3,879 76,003 5,773 100.3% 2.5 71,148 8,060 76,871 5,841 109.2% 5 63,195 4,782 68,854 4,797 110.4% Background (No Antigen) value: 8,941 +/− 494 165-F6C COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 44,939 5,060 38,142 4,681 81.8% 1 66,323 6,482 58,969 7,116 87.5% 2 79,579 14,622 82,653 12,578 104.3% 2.5 76,333 11,683 81,175 6,255 107.1% 5 59,919 2,411 70,322 3,375 119.9% Background (No Antigen) value: 7,671 +/− 612 205-1C4 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 3,847 615 3,415 336 42.3% 1 7,480 929 6,177 430 70.3% 2 21,751 6,694 17,669 3,423 78.1% 2.5 24,737 3,120 29,260 3,679 120.9% 5 45,039 3,496 46,083 2,233 102.5% Background (No Antigen) value: 3,098 +/− 267 205-1F4 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 2,220 198 2,196 168 84.5% 1 11,015 1,628 9,630 1,309 84.5% 2 39,375 2,106 38,187 5,098 96.8% 2.5 42,534 1,314 37,818 2,326 88.3% 5 38,810 1,569 39,028 2,977 100.6% Background (No Antigen) value: 2,063 +/− 134 222-2D8 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 17,943 2,305 18,146 1,407 102.1% 1 49,878 1,107 75,336 10,941 161.0% 2 138,669 4,438 137,785 2,730 99.3% 2.5 148,889 5,061 145,978 4,334 97.9% 5 141,114 4,171 149,762 2,305 106.5% Background (No Antigen) value: 8,118 +/− 377 222-2F12 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 25,481 2,172 20,573 940 58.9% 1 58,460 4,482 70,075 5,596 125.9% 2 106,340 2,628 106,563 2,741 100.2% 2.5 109,948 1,984 112,155 2,215 102.3% 5 104,449 3,162 108,272 4,634 104.2% Background (No Antigen) value: 13,533 +/− 630 165-F3C Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 21,955 4,311 19,689 3,711 83.1% 1 56,707 4,625 54,835 10,988 96.1% 2 78,456 6,403 82,864 4,558 106.3% 2.5 76,247 7,609 83,752 4,254 111.1% 5 76,428 6,804 83,080 5,529 109.8% Background (No Antigen) value: 8,584 +/− 523 165-F6C COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 43,850 3,708 41,448 3,670 93.3% 1 70,054 7,287 64,182 5,576 90.5% 2 87,633 9,863 88,354 5,734 100.9% 2.5 86,427 4,647 87,136 9,302 100.9% 5 71,493 8,356 75,027 3,629 105.6% Background (No Antigen) value: 8,054 +/− 426 205-1C4 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 2,414 259 2,054 112 −3.5% 1 4,570 831 4,101 901 81.3% 2 11,006 253 9,628 1,032 84.6% 2.5 15,584 1,789 14,199 1,978 89.8% 5 32,966 2,194 30,063 3,957 90.6% Background (No Antigen) value: 2,067 +/− 117 205-1F4 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 1,164 67 1,250 138 45.6% 1 4,500 976 6,012 1,120 147.6% 2 16,304 1,271 15,354 1,092 93.7% 2.5 20,256 1,947 20,671 2,045 102.2% 5 21,762 2,639 19,090 1,673 86.9% Background (No Antigen) value: 1,323 +/− 95 222-2D8 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 31,023 5,756 31,816 10,004 102.8% 1 95,723 17,899 96,463 10,112 100.8% 2 115,013 22,227 117,909 23,892 102.6% 2.5 104,841 15,044 108,050 15,244 103.1% 5 87,827 5,622 91,643 5,449 104.5% Background (No Antigen) value: 4,044 +/− 280 222-2F12 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 41,489 10,690 37,371 4,627 88.8% 1 83,701 11,958 79,918 13,963 95.2% 2 92,174 9,955 92,618 12,040 100.5% 2.5 86,569 9,297 85,640 13,917 98.9% 5 71,287 5,525 70,707 2,740 99.1% Background (No Antigen) value: 4,810 +/− 497 165-F3C Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 78,891 4,035 80,754 4,671 102.5% 1 89,826 6,773 90,449 5,890 100.7% 2 84,724 6,869 86,489 4,049 102.2% 2.5 87,108 4,468 87,222 4,785 100.1% 5 72,178 2,119 74,019 1,948 102.8% Background (No Antigen) value: 5,663 +/− 452 165-F6C COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 6,258 781 6,729 616 115.5% 1 13,893 2,128 13,854 2,672 99.6% 2 20,516 1,860 19,304 3,305 93.0% 2.5 22,094 3,625 24,113 2,613 110.7% 5 20,991 2,302 21,941 1,849 105.3% Background (No Antigen) value: 3,218 +/− 368 205-1C4 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 12,176 1,890 9,295 377 51.8% 1 30,953 3,200 21,346 1,865 61.2% 2 64,477 3,039 57,946 3,563 88.8% 2.5 62,634 2,931 63,316 1,320 101.2% 5 63,770 2,019 66,859 4,931 105.4% Background (No Antigen) value: 6,197 +/− 373 205-1F4 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 10,197 1,212 9,640 1,510 90.9% 1 43,418 2,404 40,835 5,758 93.4% 2 61,785 4,871 61,704 2,824 99.9% 2.5 60,599 2,929 64,556 6,341 107.0% 5 50,562 2,481 51,692 3,820 102.4% Background (No Antigen) value: 4044 +/− 280 222-2D8 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 28,793 4,832 30,374 6,186 106.0% 1 95,026 17,365 98,267 9,070 103.5% 2 111,486 22,040 114,291 18,273 102.6% 2.5 100,939 19,984 109,576 13,575 108.8% 5 88,131 7,657 94,293 8,005 107.2% Background (No Antigen) value: 2,514 +/− 261 222-2F12 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 35,563 3,834 32,747 9,281 90.5% 1 88,820 7,709 85,453 7,026 95.9% 2 96,806 11,199 97,289 10,505 100.5% 2.5 89,833 8,398 93,615 9,801 104.5% 5 80,896 7,523 80,191 6,788 99.1% Background (No Antigen) value: 5,780 +/− 790 165-F3C Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 82,850 5,942 79,417 4,445 95.5% 1 90,234 9,300 88,309 7,755 97.7% 2 89,229 8,897 89,200 6,741 100.0% 2.5 88,674 4,502 88,597 6,532 99.9% 5 76,581 2,992 77,791 2,298 101.7% Background (No Antigen) value: 5,797 +/− 342 165-F6C COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 5,612 741 5,862 681 109.7% 1 13,330 826 13,964 2,477 106.2% 2 18,159 3,332 20,619 3,110 116.3% 2.5 25,240 5,226 23,195 3,489 90.8% 5 22,362 2,114 25,175 2,992 114.6% Background (No Antigen) value: 3,036 +/− 293 205-1C4 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 11,301 1,328 9,729 875 64.3% 1 37,118 3,918 21,686 3,554 48.9% 2 60,447 3,187 50,742 3,003 81.9% 2.5 62,925 2,714 59,712 3,709 94.3% 5 61,292 2,550 57,744 3,702 93.5% Background (No Antigen) value: 6,903 +/− 247 205-1F4 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 13,053 396 8,506 687 46.4% 1 50,782 4,550 44,749 3,160 86.9% 2 66,285 2,472 66,789 2,684 100.8% 2.5 63,369 3,794 64,778 1,527 102.4% 5 49,594 1,977 53,923 2,566 109.6% Background (No Antigen) value: 4,569 +/− 255 222-2D8 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 14,204 2,736 11,322 1,737 76.8% 1 67,434 14,868 64,839 8,011 96.0% 2 107,488 16,353 104,372 18,590 97.1% 2.5 112,971 13,658 107,559 10,793 95.1% 5 99,766 3,400 90,302 10,854 90.3% Background (No Antigen) value: 1,783 +/− 137 222-2F12 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 25,388 2,983 16,736 2,458 60.6% 1 87,334 7,524 82,511 4,558 94.3% 2 97,914 7,734 100,294 7,977 102.5% 2.5 104,480 4,494 99,857 6,994 95.4% 5 91,459 1,768 91,294 4,298 99.8% Background (No Antigen) value: 3,407 +/− 606 165-F3C Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 64,525 4,267 66,703 4,081 103.6% 1 73,751 6,341 74,075 5,365 100.5% 2 67,506 5,930 69,322 3,309 102.9% 2.5 71,144 4,118 71,388 4,041 100.4% 5 58,956 2,151 60,672 2,182 103.2% Background (No Antigen) value: 4,528 +/− 363 165-F6C COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 10,706 1,170 11,538 1,050 115.4% 1 23,394 3,828 23,349 5,054 99.8% 2 33,747 2,967 32,298 6,088 94.9% 2.5 37,094 6,123 38,969 1,913 105.9% 5 34,012 3,756 35,774 4,039 106.1% Background (No Antigen) value: 5,292 +/− 646 205-1C4 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 9,318 1,557 7,944 1,226 71.4% 1 15,538 872 16,767 1,979 111.1% 2 32,955 5,076 33,706 6,896 102.6% 2.5 38,475 2,726 31,322 5,682 78.9% 5 37,495 4,063 34,474 3,594 90.8% Background (No Antigen) value: 4,516 +/− 285 205-1F4 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 97,968 8,249 97,744 5,919 99.8% 1 127,580 16,207 123,254 12,623 96.5% 2 132,098 14,469 129,142 16,175 97.7% 2.5 120,168 5,213 116,750 17,156 97.1% 5 80,689 3,329 75,277 2,801 92.9% Background (No Antigen) value: 4,300 +/− 438 222-2D8 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 11,842 1,576 8,720 1,175 68.8% 1 69,696 7,696 54,975 6,608 78.3% 2 109,375 10,010 107,963 12,438 98.7% 2.5 113,924 10,927 107,193 11,487 94.0% 5 110,672 4,065 100,564 7,354 90.7% Background (No Antigen) value: 1,846 +/− 262 222-2F12 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 25,260 7,567 13,483 512 42.8% 1 87,397 7,948 74,532 8,208 84.4% 2 101,740 13,208 98,756 12,703 96.9% 2.5 98,368 6,874 96,695 6,339 98.2% 5 85,490 2,250 84,329 3,489 98.6% Background (No Antigen) value: 4,680 +/− 322 165-F3C Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 69,624 5,387 67,045 3,968 96.0% 1 74,498 8,247 73,087 6,776 98.0% 2 75,820 8,024 75,066 6,222 98.9% 2.5 75,347 4,056 75,150 6,037 99.7% 5 62,262 2,789 62,866 2,399 101.1% Background (No Antigen) value: 4,684 +/− 274 165-F6C COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 9,191 1,241 9,584 1,087 109.8% 1 21,591 1,344 24,186 2,552 115.8% 2 31,427 4,009 35,297 2,101 114.8% 2.5 43,097 4,356 38,878 4,273 88.9% 5 34,995 3,786 39,286 4,699 114.4% Background (No Antigen) value: 5,198 +/− 497 205-1C4 Dose COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 11,016 748 10,031 362 84.6% 1 15,555 1,750 17,283 1,782 115.8% 2 31,330 5,756 34,642 2,703 112.4% 2.5 37,734 7,687 37,583 6,091 99.5% 5 35,506 2,472 37,185 3,205 105.4% Background (No Antigen) value: 4,643 +/− 294 205-1F4 COP (Reference) 009 (Test) μg/mL Mean Std Dev Mean Std Dev 009/COP 0.5 92,174 13,223 90,344 9,200 97.9% 1 118,103 11,828 122,053 13,761 103.5% 2 124,021 12,956 123,294 15,746 99.4% 2.5 114,498 12,591 111,085 15,009 96.9% 5 79,373 5,584 79,935 7,122 100.7% Background (No Antigen) value: 3,664 +/− 330 Example VI
GA-Specific Human T-Cell Line Panel Assay—Comparison of Altered GA Lots to Copaxone
Altered Initiator Concentration/N-Carboxy Anhydride (NCA) Mole Fraction Negative Control Lots; Summary of Manufacturing Process Conditions Stage 1 NCA Moles Added in Charge DEA NCA Mole Fraction in Cage Sample A E K Y Conc A E K Y NC-1 (056F) Per control process 0.05 0.449 0.137 0.321 0.093 NC-2 (051F) + + − + 0.1 0.403 0.185 0.288 0.125 NC-3 (053F) + + + + 0.1 0.449 0.137 0.321 0.093 NC-4 (054F) + + − − 0.01 0.464 0.142 0.331 0.064 NC-5 (055F) + − − + 0.01 0.530 0.108 0.252 0.109 NC-6 (057F) − + − − 0.01 0.420 0.193 0.300 0.087 NC-7 (058F) − − + + 0.01 0.372 0.114 0.399 0.115 NC-8 (059F) + − − − 0.1 0.550 0.112 0.262 0.076 NC-9 (060F) − − + − 0.1 0.387 0.118 0.414 0.080 (+) Denotes NCA moles increased by 20% relative to control; (−) Denotes NCA moles decreased by 20% relative to control. Altered Initiator Concentration/NCA Mole Fraction Negative Control Lots; Molecular Weight and Amino Acid Composition Data
Proliferation response to test antigen minus proliferation response to control(no antigen)÷proliferation response to reference antigen minus proliferation response to control(no antigen).Proliferation of Assay Panel T-Cell Lines Stimulated with Altered GA Lot NC-2 (051F) GA conc. COP (Reference) 051F (Test) μg/mL Mean Std Dev Mean Std Dev 051F/COP 222-2D8 0 555 78 555 78 0.5 2,523 717 2,455 398 97% 1 11,178 1,463 8,192 1,850 72% 2 45,028 4,147 38,828 5,943 86% 2.5 66,202 9,918 60,869 5,492 92% 5 117,638 11,070 114,522 2,997 97% 222-2F12 0 1,712 263 1,712 263 0.5 4,396 844 2,958 553 46% 1 11,426 1,126 7,692 1,354 62% 2 75,298 9,861 20,827 3,809 26% 2.5 142,360 19,820 62,617 6,424 43% 5 189,730 12,428 186,767 21,203 98% 165-F3 C 0 2,475 162 2,475 162 0.5 4,299 483 3,459 75 54% 1 7,765 1,004 5,386 312 55% 2 27,428 2,286 16,581 1,247 57% 2.5 33,740 5,955 26,095 3,248 76% 5 38,127 4,705 37,496 2,126 98% 165-F6C 0 2,578 274 2,578 274 0.5 3,678 360 3,359 253 71% 1 8,745 1,126 6,068 702 57% 2 36,485 5,750 16,976 2,047 42% 2.5 53,250 4,188 34,572 4,212 63% 5 56,466 4,402 67,326 4,561 120% 205-1C4 0 5,935 390 5,935 390 0.5 12,962 2,258 13,252 647 104% 1 67,399 14,359 48,834 12,622 70% 2 120,159 4,117 133,578 4,943 112% 2.5 121,868 5,875 145,780 6,121 121% 5 107,018 3,031 122,986 1,879 116% 205-1F4 0 2,707 176 2,707 176 0.5 2,328 209 2,425 127 74% 1 4,969 750 2,890 188 8% 2 18,430 2,806 13,696 1,643 70% 2.5 20,113 3,771 21,850 823 110% 5 24,016 1,692 29,856 4,451 127% Proliferation of Assay Panel T-Cell Lines Stimulated with Altered GA Lot NC-4 (054F) GA conc. COP (Reference) 054F (Test) μg/mL Mean Std Dev Mean Std Dev 054F/COP 222-2D8 0 238 37 238 37 0.5 275 79 287 71 133% 1 1,244 191 1,346 160 110% 2 10,074 1,282 6,498 1,612 64% 2.5 16,451 3,700 18,859 3,729 115% 5 37,005 6,547 37,433 5,442 101% 222-2F12 0 312 59 312 59 0.5 239 27 225 63 119.9% 1 780 232 412 34 21.3% 2 10,284 2,467 9,652 1,065 93.7% 2.5 28,607 5,246 32,495 10,353 113.7% 5 71,541 2,085 74,514 4,545 104.2% 165-F3C 0 2,402 236 2,402 236 0.5 5,684 295 4,020 666 49% 1 10,841 1,105 7,003 1,072 55% 2 29,175 3,938 21,460 3,935 71% 2.5 33,073 1,602 32,409 3,192 98% 5 34,862 3,362 33,339 1,875 95% 165-F6C 0 2,358 140 2,358 140 0.5 3,499 200 2,783 282 37% 1 7,910 1,486 5,242 603 52% 2 30,602 3,805 16,776 1,344 51% 2.5 41,112 4,609 30,869 3,046 74% 5 56,465 4,233 43,141 2,771 75% 205-1C4 0 5,764 385 5,764 385 0.5 12,143 1,393 6,552 304 12% 1 65,518 13,711 10,840 744 8% 2 119,307 6,285 45,562 5,558 35% 2.5 120,551 2,673 76,503 10,228 62% 5 109,154 5,613 96,264 3,687 88% 205-1F4 0 2,708 137 2,708 137 47% 0.5 2,535 171 2,626 151 −3% 1 3,922 508 2,677 102 36% 2 14,322 1,834 6,927 1,231 42% 2.5 20,059 1,764 9,949 2,560 68% 5 20,471 1,465 14,846 2,149 47% Proliferation of Assay Panel T-Cell Lines Stimulated with Altered GA Lot NC-5 (055F) GA conc. COP (Reference) 055F (Test) μg/mL Mean Std Dev Mean Std Dev 055F/COP 222-2D8 0 477 36 477 36 0.5 715 56 811 120 140% 1 3,526 887 2,898 567 79% 2 34,786 4,545 27,832 5,823 80% 2.5 46,255 3,991 44,882 3,616 97% 5 70,248 3,206 68,952 7,184 98% 222-2F12 0 546 71 546 71 0.5 468 81 514 75 40% 1 1,847 261 1,324 156 60% 2 19,966 5,933 12,673 1,900 62% 2.5 41,329 7,049 36,730 5,904 89% 5 76,781 6,073 81,376 2,755 106% 165-F3C 0 3,135 247 3,135 247 0.5 8,095 3,117 7,582 1,085 90% 1 22,164 3,232 26,044 7,638 120% 2 86,801 11,964 83,923 7,426 97% 2.5 94,830 7,778 91,725 7,214 97% 5 97,824 8,015 94,559 6,454 97% 165-F6C 0 2,820 418 2,820 418 0.5 2,706 333 2,936 422 −101% 1 4,067 516 3,838 381 82% 2 12,513 1,539 8,417 1,280 58% 2.5 21,520 4,146 19,798 4,538 91% 5 42,493 4,988 43,546 2,744 103% 205-1C4 0 1,333 175 1,333 175 0.5 1,904 302 3,108 731 311% 1 6,495 1,747 7,415 1,243 118% 2 31,541 7,693 63,584 4,740 206% 2.5 50,442 4,147 73,823 1,646 148% 5 52,458 1,923 68,206 3,022 131% 205-1F4 0 570 38 570 38 0.5 598 96 778 154 725% 1 3,332 2,411 2,753 433 79% 2 22,705 4,347 23,727 5,988 105% 2.5 30,794 2,666 33,707 6,660 110% 5 37,796 3,159 47,090 4,703 125% Proliferation of Assay Panel T-Cell Lines Stimulated with Altered GA Lot NC-6 (057F) GA conc. COP (Reference) 057F (Test) μg/mL Mean Std Dev Mean Std Dev 057F/COP 222-2D8 0 549 72 549 72 0.5 926 165 602 129 14% 1 7,205 1,741 1,727 224 18% 2 34,638 2,838 17,730 4,240 50% 2.5 41,414 3,718 35,392 4,651 85% 5 63,294 7,114 60,764 3,004 96% 222-2F12 0 532 104 532 104 0.5 607 90 455 51 −103% 1 2,975 604 612 112 3% 2 36,232 6,852 5,370 558 14% 2.5 60,048 10,334 13,749 2,566 22% 5 86,151 1,958 70,458 6,145 82% 165-F3C 0 2,935 205 2,935 205 0.5 6,874 617 4,929 362 51% 1 18,867 2,046 8,433 2,404 35% 2 80,910 8,861 15,686 2,071 16% 2.5 90,295 6,532 29,419 3,540 30% 5 88,856 2,768 69,152 7,060 77% 165-F6C 0 2,215 179 2,215 179 0.5 2,827 402 2,898 148 112% 1 4,119 484 3,129 269 48% 2 20,236 4,873 5,078 204 16% 2.5 33,695 5,758 5,511 661 10% 5 41,233 4,060 18,802 3,139 43% 205-1C4 0 1,283 186 1,283 186 0.5 2,397 324 1,400 161 10% 1 7,118 1,772 2,105 224 14% 2 35,803 11,537 6,863 821 16% 2.5 47,782 9,663 17,453 6,703 35% 5 55,213 3,152 45,214 7,441 81% 205-1F4 0 404 78 404 78 0.5 444 76 436 37 80% 1 2,060 761 424 24 1% 2 13,054 3,462 3,625 1,460 25% 2.5 15,008 3,523 11,620 5,543 77% 5 26,457 3,990 22,288 7,335 84% Proliferation of Assay Panel T-Cell Lines Stimulated with Altered GA Lot NC-7 (058F) GA conc. COP (Reference) 058F (Test) μg/mL Mean Std Dev Mean Std Dev 058F/COP 222-2D8 0 1,609 201 1,609 201 0.5 27,735 6,744 11,584 3,964 38% 1 95,926 17,011 77,810 15,598 81% 2 125,126 12,315 115,411 15,617 92% 2.5 148,061 15,380 117,194 3,794 79% 5 152,649 3,418 121,044 16,413 79% 222-2F12 0 1,952 208 1,952 208 0.5 7,427 1,218 6,124 645 118% 1 50,426 5,994 46,619 9,555 99% 2 138,876 8,988 133,258 8,184 115% 2.5 138,757 3,210 141,639 2,482 104% 5 154,068 6,261 142,470 5,726 105% 165-F3C 0 1,292 112 1,292 112 0.5 3,169 486 4,223 264 156% 1 8,378 955 8,805 1,027 106% 2 31,536 8,165 26,984 3,809 85% 2.5 33,777 3,717 30,925 5,419 91% 5 38,783 3,851 34,122 1,373 88% 165-F6C 0 803 803 154 154 0.5 1,035 2,504 153 533 733% 1 2,825 12,943 457 3,070 600% 2 14,164 40,675 2,959 11,470 298% 2.5 26,862 54,895 2,679 5,730 208% 5 36,303 47,985 4,022 4,391 133% 205-1C4 0 8,068 328 8,068 328 0.5 14,583 1,835 17,074 3,008 138% 1 26,878 4,458 42,746 7,114 184% 2 148,531 16,218 158,272 9,359 107% 2.5 156,331 23,982 151,305 11,863 97% 5 148,116 12,905 123,960 9,327 83% 205-1F4 0 5,347 519 5,347 519 0.5 7,657 837 7,569 928 96% 1 17,074 2,403 17,741 2,509 106% 2 55,155 5,678 54,330 13,960 98% 2.5 64,071 11,199 67,967 14,024 107% 5 69,484 6,674 64,750 5,589 93% Proliferation of Assay Panel T-Cell Lines Stimulated with Altered GA Lot NC-8 (059F) GA conc. COP (Reference) 059F (Test) μg/mL Mean Std Dev Mean Std Dev 059F/COP 222-2D8 0 1,443 91 1,443 91 0.5 13,958 4,236 24,260 3,408 182% 1 87,807 9,513 96,343 8,132 110% 2 142,231 12,632 154,705 6,717 109% 2.5 140,804 12,189 149,503 10,927 106% 5 147,182 15,768 145,847 9,712 99% 222-2F12 0 1,353 191 1,353 191 0.5 3,580 562 3,990 604 118% 1 42,304 6,772 42,076 2,732 99% 2 108,046 9,855 123,646 4,582 115% 2.5 115,182 6,255 119,534 5,610 104% 5 123,169 9,352 129,351 8,023 105% 165-F3C 0 1,248 199 1,248 199 0.5 3,154 233 3,241 662 105% 1 9,050 1,350 7,412 2,107 79% 2 27,693 1,889 25,320 8,018 91% 2.5 28,018 5,559 26,608 4,862 95% 5 32,151 1,963 33,907 1,902 106% 165-F6C 0 658 78 658 78 0.5 863 126 808 185 73% 1 1,838 209 1,088 258 36% 2 8,163 1,400 2,593 916 26% 2.5 11,665 830 6,961 1,236 57% 5 30,422 3,878 19,251 4,424 62% 205-1C4 0 7,851 404 7,851 404 0.5 13,267 865 10,922 619 57% 1 26,848 4,312 16,622 2,503 46% 2 142,528 17,311 65,015 15,448 42% 2.5 159,785 11,573 120,442 31,287 74% 5 171,452 6,507 167,530 2,995 98% 205-1F4 0 5,118 384 5,118 384 0.5 6,984 357 6,442 644 71% 1 15,174 2,862 11,253 1,455 61% 2 46,598 6,377 32,522 6,841 66% 2.5 51,372 8,818 46,936 6,968 90% 5 61,252 3,086 62,607 8,096 102% Proliferation of Assay Panel T-Cell Lines Stimulated with Altered GA Lot NC-9 (060F) GA conc. COP (Reference) 060F (Test) μg/mL Mean Std Dev Mean Std Dev 060F/COP 222-2D8 0 1,470 126 1,470 126 0.5 2,785 401 1,838 251 28% 1 28,970 2,992 6,093 1,608 17% 2 76,089 9,113 52,562 7,512 68% 2.5 90,666 4,198 82,771 6,024 91% 5 99,491 7,485 91,463 4,473 92% 222-2F12 0 1,616 1,616 160 160 0.5 2,404 2,707 541 326 139% 1 11,359 8,807 4,259 1,616 74% 2 78,880 70,354 6,266 8,423 89% 2.5 95,825 104,397 4,400 8,044 109% 5 107,930 104,946 4,915 6,586 97% 165-F3C 0 1,428 129 1,428 129 0.5 2,577 212 2,174 223 65% 1 5,743 178 5,188 379 87% 2 16,415 1,314 15,903 1,645 97% 2.5 20,446 1,413 24,511 3,124 121% 5 32,600 2,124 33,905 3,546 104% 165-F6C 0 1,831 149 1,831 149 0.5 2,228 389 2,449 219 155% 1 4,270 486 5,413 717 147% 2 15,430 2,875 26,433 4,456 181% 2.5 28,942 3,101 46,334 3,480 164% 5 45,836 2,157 47,301 2,765 103% 205-1C4 0 8,028 680 8,028 680 0.5 11,790 1,061 10,768 629 73% 1 26,004 2,141 15,367 1,352 41% 2 102,672 11,452 65,511 8,168 61% 2.5 124,300 17,322 110,976 4,372 89% 5 129,908 3,973 117,103 9,212 89% 205-1F4 0 6,912 628 6,912 628 0.5 8,158 255 7,907 658 80% 1 17,491 3,649 11,531 931 44% 2 51,613 9,037 49,567 6,068 95% 2.5 60,244 3,025 51,070 5,174 83% 5 54,345 7,884 41,670 3,255 73% Example VII
Determination of Immunological Identity Using a Panel of GA-Specific Human T-Cell Lines
(GA-elicited response to test antigen minus the same response to control)÷(GA-elicited response to reference antigen minus response to control)Example VIII
Determination of Immunological Identity Using a Panel of GA-Specific Human T-Cell Lines: Multiple Reference Dose-Response Curve Analysis
Example IX
Determination of Immunological Identity Using a Panel of GA-Specific Human T-Cell Lines: Reference Repeat Dose-Response Curve Analysis