MEDICAL SLING
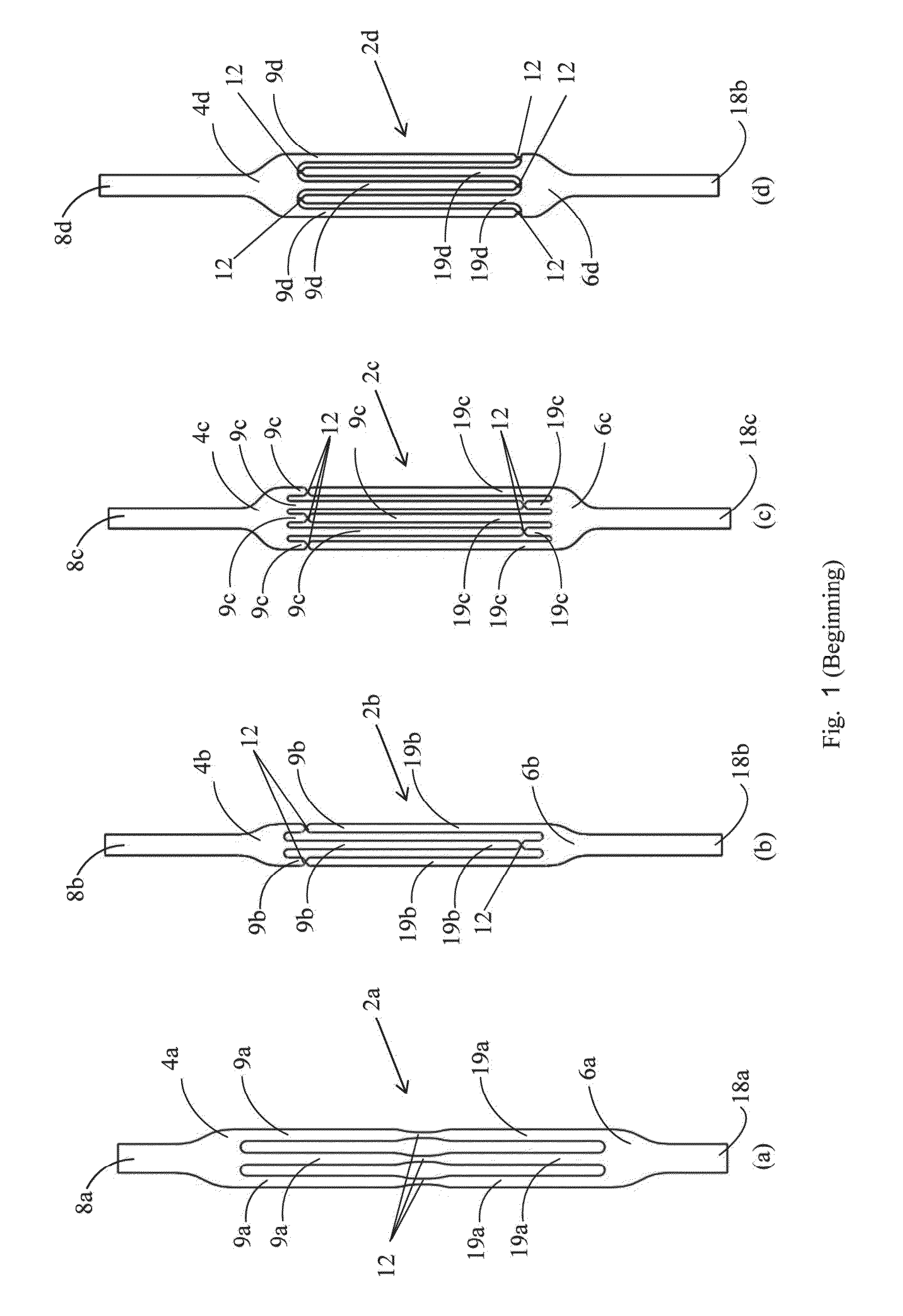
This invention relates to a removable sling for use in the treatment of various conditions such as urinary stress incontinence, fecal incontinence and pelvic organ prolapse. Stress urinary incontinence (SUI) is the unintentional urine leakage during times of abdominal stress, such as occurs during coughing, laughing, or sneezing. One cause of SUI is inadequate anatomical support of the urethra which allows the urethra to move out of the retropubic space and rotate into the vagina. This condition is known as “hypermobility of the urethra” or simply “hypermobility”. SUI may also result from insufficient closing pressure of the urethra which prevents the urethra from fully collapsing and sealing. SUI can develop in men as a result of prostate surgery during which the voluntary sphincter mechanism is damaged either partially or totally. Another medical condition suffered by women is pelvic organ prolapse (POP) which refers to prolapse of the organs (urinary bladder, intestines and uterus) normally positioned within the pelvis. Normally these organs are supported by the “pelvic floor”. Weakening of the pelvic floor allows herniation of these organs towards the vagina. Another medical condition is fecal incontinence which is the inability to control bowel movements, causing feces to leak unexpectedly from the rectum. It may be due to a weakened anal sphincter associated with aging or to damaged nerves and muscles of the rectum and anus that can occur during childbirth. Urethral slings have been used to treat hypermobility in women and postoperative incontinence in men. A urethral sling is typically implanted below the urethra to provide support under the urethra and prevent unwanted movement of the urethra towards the vagina or compress the male bulbous urethra. A variety of different slings have been developed that are implanted below the urethra to support it. In women, these slings are generally not intended to raise the urethra but to provide support for the urethra to prevent the unwanted downward urethral movement associated with hypermobility. A typical urethral sling comprises a strip of mesh that is implanted using a transvaginal approach in which opposite ends of the mesh are arranged on opposite sides (towards the sides or upwards) of the urethra so that the mesh loops under the urethra to form a sling. The mesh is typically implanted using needles that attach to the mesh of the sling so that the mesh ends can be inserted into the body and exit out of the body at a desired exit site and then are either anchored to the pubic bones or tissues, left under the skin, or in the periurethral or perivaginal space for self anchoring by tissue proliferation. Excess mesh extending out of the body can be removed and the remainder of the mesh left under the skin. During the first days after its implantation the unanchored sling is held in place by friction, then by tissue ingrowth through the interstices of the mesh. POP can be repaired either by applying sutures to the pelvic floor to reshape the vagina and return the prolapsed organ to their normal position or by placing a layer of mesh to support the prolapsed organs. Surgery either through the vagina or through the abdomen (either open or laparoscopic) is the usual method of POP repair. Fecal incontinence is treated by sphincteroplasty. Patients who are not suitable for such surgery or who have failed sphincteroplasty can be implanted a puborectal sling. Mesh slings are generally successful in treating incontinence or POP but occasionally fail. One cause of failure is improper positioning of the sling, insufficient or excessive tension of the sling during implantation and dislocation of the sling sometime after implantation. Some of the complications which may develop after mesh implantation include failure of the sling to prevent incontinence, postoperative urine retention, sling caused bladder hyperactivity, coital pain, sexual impairment and/or discomfort, infection, vaginal and urethral tissue erosion. In some of these complications there may be an indication to remove the mesh sling. After tissue ingrowth into the spaces of the mesh, complete removal of the implanted mesh is very difficult. During the surgery or immediately after or within the first days after a mesh sling implantation, adjustment of a misplaced mesh sling can only be performed by pulling on the ends of the sling to increase the sling tension or releasing the tension through a vaginal incision. The extent of tension readjustement is limited even after a couple of weeks due to the tissue ingrowth and may not allow proper positioning of the mesh. Mesh slings do not allow late removal by simple surgery. Some of the currently available mesh slings allow readjustment only after a few days following their implantation. United States Patent Publication 20080269547 to Hortenstine discloses an adjustable urethral mesh sling having an expansion chamber that is positioned under the urethra after implantation. A conduit in fluid communication with the expansion chamber allows remote expansion of the expansion chamber. The expanded chamber presses on the urethra and contributes to the closure of the urethral lumen during stress. Any foreign body placed in a living tissue can elicit an inflammatory reaction in the surrounding tissue. This process is usually followed by gradual development of a cocoon-like collagen shell and/or fibrous tissue as a natural barrier around the foreign body (encapsulation). Mature cross-linked collagen and other extracellular matrix proteins gradually contribute to the formation of a hypocellular dense fibrous capsule that becomes impermeable or hypopermeable to many compounds. All soft-tissue implanted devices cause such a reaction. Since non-absorbable and biocompatible, smooth surfaced implants are unaffected by the biological activities of the surrounding tissues during the encapsulation process, they do not adhere to tissues and can be pulled-out easily at anytime. i.e. smooth surfaced monofilament surgical sutures remain within a smooth surfaced capsule and they can be pulled out at anytime much easily than a comparable multifilament braided sutures that are invaded by tissue. Other factors influencing the host response include implant location, size, shape, micromotion, surface chemistry, surface roughness, and porosity. (Dee K C, Puleo D A, Bizios R. Wound healing. In: The present invention provides a medical sling that may be used, for example, as a urethral sling, a puborectal sling or a surgical mesh for POP repair. The sling of the invention comprises a first sling element and a second sling element. A first set of one or more finger like projections extend from an end of the first sling element, and a second set of one or more finger line projections extend from an end of the second sling element. The first and second sling elements are integral with each other by means of a plurality of connections between the finger like projections of the sling elements that are configured to allow the first and second sling elements to separate from each other when the first and second sling elements are pulled apart. This allows the sling to be removed from the body. The sling may be removed, for example, in cases of failure of the sling to prevent incontinence, postoperative urine retention, sling caused bladder hyperactivity, coital pain, sexual impairment, discomfort, infection, vaginal and urethral tissue erosion. The sling of the invention may be embedded between two layers of biodegradable and/or bioabsorbable mesh to facilitate fixation of the sling. The biodegradable mesh allows tissue ingrowth through its interstices and fixes the sling during the first weeks after its implantation. The mesh then disintegrates allowing the tissue to enter the longitudinal spaces along the fingers of the sling. The sling of the invention may be made from a biostable or biodegradable polymeric material that is inelastic, soft flexible, and biocompatible. The sling material may be reinforced by a mesh or filaments embedded into a polymer. The sling may be made from a bio stable knitted or woven material that can be unraveled, or from a yarn imbedded in a smooth biostable polymer layers. The knitted material or yarns may be tightly knitted or woven with a porosity below the size of living cells to prevent tissue ingrowth into the material. The sling of the invention may be inflatable, in which case one or both of the first and second sling elements is made from a fluid impervious material and is provided with 1 or 2 ports through which an inflation material is introduced into an interior of the sling element. The inflation material may be, for example, sterile saline. The sling of the invention may be introduced into the body through midline vaginal incisions under the urethra and then each end of the sling is directed through its ipsilateral obturator foramine before exiting the body through a skin incision. The sling ends can have mesh-like segments for self fixation. If a trans-obturator approach is used, the mesh ends should be over the obturator fascia. If an abdominal approach is used the mesh ends should be over the rectus fascia, where the mesh tips can be reached easily to be disconnected from the removable sling, in case the sling has to be removed. For an inflatable sling, the sling is inflated after insertion and the ports of the sling may remain beneath the skin at the level of the obturator foraminae or under the skin at the suprapubic level after implantation. At any time, the amount of inflation fluid inside the inflatable elements can be changed in order to readjust the sling tension when it is determined that the urethra is not supported in a desired manner. When implanted, the sling of the invention may become encapsulated by fibrotic scar tissue. The sling of the invention may have a smooth outer surface which tens to prevent invasion by the surrounding tissue. Spacing between adjacent finger-like projections in the sling allows vascularization of the tissues covering the sling. After encapsulation by collagen, the sling may be removed from the body, leaving behind the encapsulation tissue which may function as an autologous sling. At any time after implantation, the sling may be removed from the body. For removal, the first and second sling elements are detached from each other. In some embodiments, this is accomplished by grasping the ends of the first and second sling elements and simultaneously pulling the first and second sling elements apart causing the connections between the first and second sling elements to be broken. As the two sling elements continue to be pulled apart, the sling elements become separated as they are removed from the body. In other embodiments, fingers in one sling element are stitched to the other sling element by a filament. In these embodiments, the first and second sling elements are detached from each other by removing the filament, and then the two sling elements can be separated from each other by pulling the two sling elements apart. In order to understand the invention and to see how it may be carried out in practice, embodiments will now be described, by way of non-limiting example only, with reference to the accompanying drawings, in which: The first and second sling elements are integral with each other by means of a plurality of constrictions 12. The constrictions 12 are weak points in the sling and are configured to when the first and second sling elements are pulled apart, as explained below. The slings of the invention can be made of a single layer material (non-inflatable) or double-layered (inflatable). Both the single layer and the double layer slings can be reinforced by a mesh or filaments embedded into a polymer. The slings 2 The sling of the invention can be either non-inflatable or inflatable. In the inflatable embodiment, as shown in the slings 2 The first and second sling elements are integral with each other by means of a plurality of constrictions 32. The constrictions 32 are weak points in the sling and are configured to tear when the first and second sling elements are pulled apart, as explained below. In addition, the projections on the first and second sling elements are attached together at a plurality of weak lateral connections 35, shown in greater detail in the insert to The slings 20 The slings 20 The sling 20 The inflatable unit 108 and 110 has two finger-like projections, 112 and 114, respectively. The projections 112 of the inflatable unit 108 are interdigitated with the projections 114 of the inflatable unit 110. [It's not clear what holds this thing together] The two layers of mesh 126 may be attached to one another by means of a snap-fit attachment. In one embodiment, shown in The sling 130 can be mono-layered (wider than the suburethral slings and wider fixation ends) or multi-layered. The sling 130 can be removed by pulling its anterior end. The attachments detach from the narrow posterior end which remains attached to the posterior fixation tissues. A sling of the invention, such as the sling 2 The sling ends can have mesh-like segments for self fixation. If a trans-obturator approach is used the mesh ends should be over the obturator fascia. If an abdominal approach is used the mesh ends should be over the rectus fascia, where the mesh tips can be reached easily to be disconnected from the removable sling, in case the slings has to be removed. The sling of the invention may be provided with a muscle stimulating device 130, shown schematically in When implanted, the sling of the invention may become encapsulated by fibrous tissue. However, unlike a mesh sling, the sling 2 At any time after implantation, the sling 2 Provided is an implantable sling for supporting a body organ. The sling may be used, for example, as a urethral sling, a puborectal sling or a surgical mesh for pelvic organ prolapsed repair. The sling of the invention has a first sling element, having a first sling body and two or more slender first projections extending from the first sling body. The sling may further include a second sling element having a second sling body and two or more slender second projections extending from the second sling body, and one or more detachable connections connecting the first sling element and the second sling element. In some embodiments, the connections are configured to tear when the first and second sling elements are pulled apart. 1. An implantable sling comprising
(a) a first sling element, the first sling element comprising a first sling body and two or more slender first projections extending from the first sling body. 2. The implantable sling according to (a) a second sling element, the second sling element comprising a second sling body and at least two slender second projections extending from the second sling body; and (b) at least one detachable connection connecting the first sling element and the second sling element. 3. The sling according to 4. The sling according to 5. The sling according to 6. The sling according to 7. (canceled) 8. The sling according to 9. The sling according to 10. The sling according to (i) wherein the sling is made from an inelastic flexible material; and, (ii) wherein the sling has a smooth outer surface. 11. (canceled) 12. The sling according to 13. The sling according to 14. The sling according to 15. The sling according to 16. The sling according to 17. A system comprising a sling according to 18. The system according to 19. The system according to (a) one or more sensors that are of a type selected from a pressure sensor and a motion sensor, the one or more sensors being configured to monitor the pressure on the sling or motion of the sling; (b) one or more electrodes configured to be implanted in one or more selected from the periurethral and the pelvic muscles; and (c) a processor configured to:
(i) analyze signals from the sensors to detect a sudden increase in one or both of the pressure on the sling and a sudden increase in the motion of the sling; and (ii) when a sudden increase in one or both of the pressure on the sling and the motion of the sling is detected, activating the electrodes to cause contraction of the muscles in which the electrodes are implanted. 20. The system according to (a) generating electrical energy from natural body movements; (b) storing the electrical energy; and (c) activating the electrodes from the stored energy. 21. A method for supporting a body organ comprising:
i) providing a urethral sling comprising:
(a) an inflatable first sling element, the first sling element comprising a first sling body and two or more slender first projections extending from the first sling body; ii) attaching the first sling element to a first body structure; and iii) inflating the first sling element. 22. A method for supporting a body organ comprising:
i) providing a urethral sling comprising:
(a) a first sling element; (b) a second sling element; and (c) one or more detachable connections connecting the first sling element and the second sling element; ii) attaching the first sling element to a first body structure, the first body structure being located on a first side of the body organ; and iii) attaching the second sling element to a second body structure, the second body structure being located on a second side of the body organ, the sling passing around the body organ from the first body structure to the second body structure. FIELD OF THE INVENTION
BACKGROUND OF THE INVENTION
SUMMARY OF THE INVENTION
BRIEF DESCRIPTION OF THE DRAWINGS
DESCRIPTION OF THE INVENTION