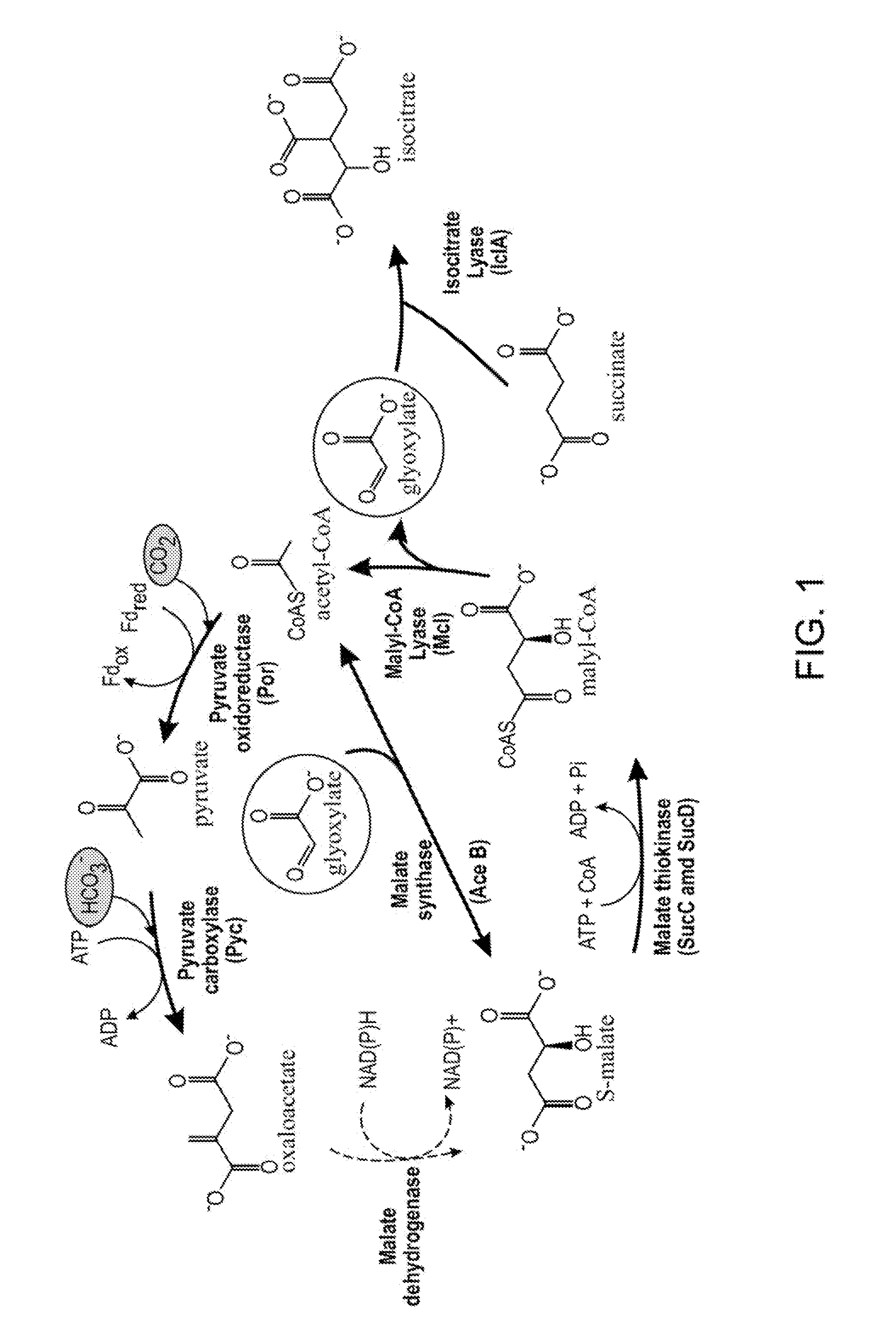
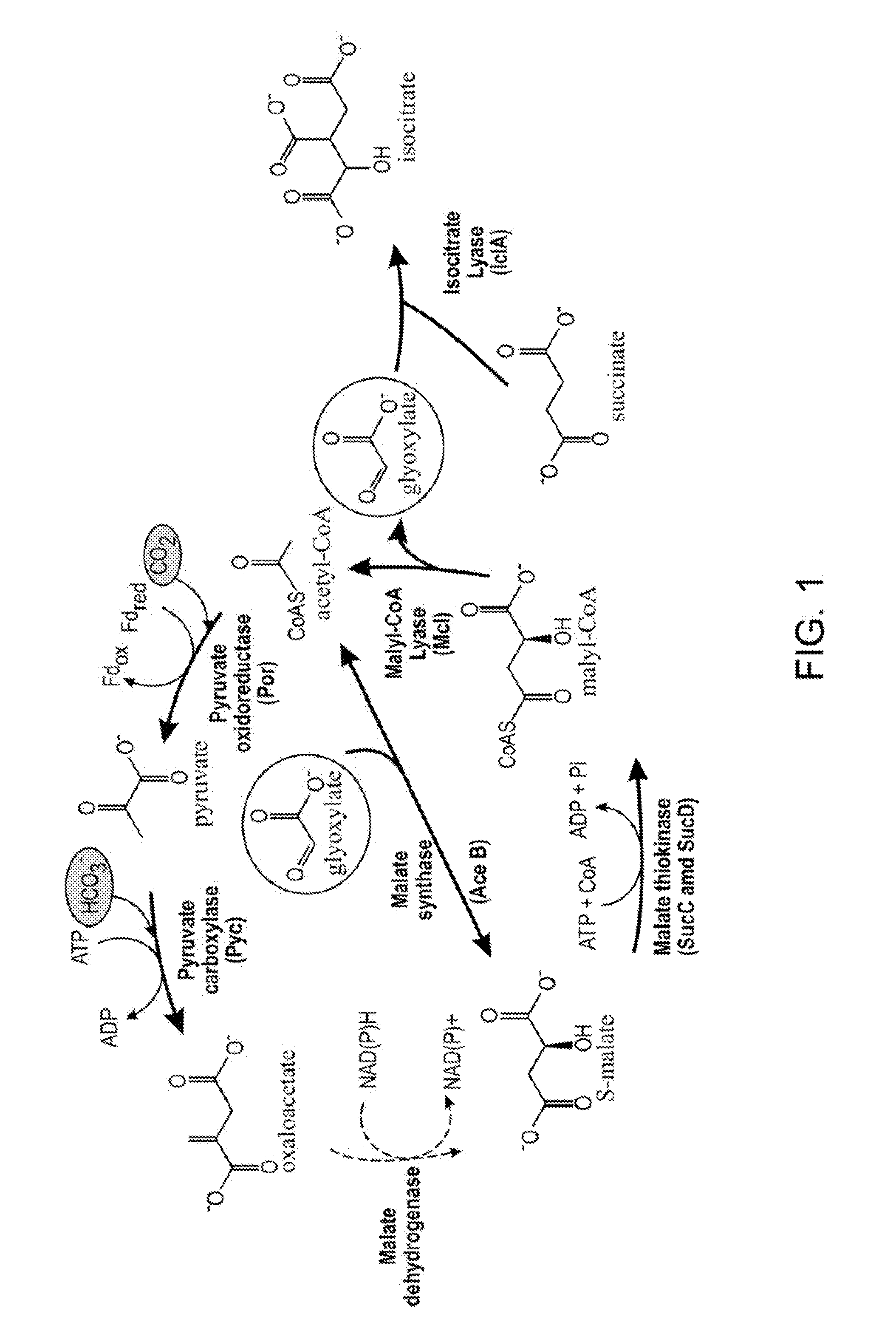
PLANTS WITH ENHANCED YIELD AND METHODS OF CONSTRUCTION
This application claims the benefit of U.S. Provisional Application No. 62/144,727, filed on Apr. 8, 2015, 62/145,757 filed on Apr. 10, 2015 and 62/190,281 filed on Jul. 9, 2015. The entire teachings of the above application(s) are incorporated herein by reference. This invention was made in part with government support under Grant Number DE-AR0000201 from the United States DOE, ARPA-e PETRO program. The government has certain rights in this invention. The world faces a major challenge in the next 35 years to meet the increased demands for food production to feed a growing global population which is expected to reach 9 billion by the year 2050 ( Over the last twenty years metabolic engineering primarily of microbial systems to improve and/or introduce entirely new metabolic pathways to increase carbon utilization or make entirely new products based on multiple enzymatic steps has advanced enormously. This technology has already demonstrated some success in plants. There are multiple known existing prokaryotic carbon fixation pathways (Fuchs, G., The present disclosure relates to methods of using novel metabolic pathways having enzymes catalyzing carboxylation reactions and/or enzymes using NADPH or NADH as a cofactor to enhance the yield of desirable crop traits including increased biomass yield, increased seed yield, increased oil content in seed, increased protein content in seed, increased starch content in seed, or increased sucrose content in stalks or seed. These modifications can be combined with other traits including herbicide resistance, pest resistance, nitrogen use efficiency, heat tolerance, drought tolerance and water use efficiency or industrial traits such as polyhydroxyalkanoate polymers or modified oil compositions. The invention is particularly relevant to reducing economic costs to farmers and increasing food production. Disclosed herein are transgenic plants and seeds of transgenic plants selected on the basis of having a higher yield in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). The metabolic pathways and enzymatic steps, which are the subject of the disclosed invention are shown in In a first embodiment of the disclosed invention, the transgenic plant comprises one or more transgenes encoding two, three, four, five, six, seven, eight or more enzymes selected from the group: an oxygen tolerant pyruvate oxidoreductase (Por); pyruvate carboxylase (Pyc); malate synthase (AceB), malate dehydrogenase (Mdh); malate thiokinase (SucC and SucD), malyl-CoA Lyase (Mcl) and isocitrate lyase (Icl) wherein the transgenic plant is selected on the basis of having a higher yield in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). In a second embodiment of the disclosed invention, the transgenic plant comprises one or more transgenes encoding an oxygen tolerant pyruvate oxidoreductase (Por) and a pyruvate carboxylase (Pyc) wherein the transgenic plant is selected on the basis of having a higher yield in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). In a third embodiment of the disclosed invention, the transgenic plant comprises two or more transgenes encoding an oxygen tolerant pyruvate oxidoreductase (Por), a pyruvate carboxylase (Pyc), and a malate synthase (AceB), wherein the transgenic plant is selected on the basis of having a higher yield in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). In a fourth embodiment of the disclosed invention, the transgenic plant comprises five or more transgenes encoding an oxygen tolerant pyruvate oxidoreductase (Por), a pyruvate carboxylase (Pyc), a malate synthase (AceB), malate thiokinase (SucC, SucD), and a malyl-CoA Lyase (Mcl), wherein the transgenic plant is selected on the basis of having a higher yield in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). In a fifth embodiment of the disclosed invention, the transgenic plant comprises six or more transgenes encoding an oxygen tolerant pyruvate oxidoreductase (Por), a pyruvate carboxylase (Pyc), a malate synthase (AceB), malate thiokinase (SucC, SucD), a malyl-CoA Lyase (Mcl), and a malate dehydrogenase (Mdh), wherein the transgenic plant is selected on the basis of having a higher yield in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). In a sixth embodiment of the disclosed invention, the transgenic plant comprises seven or more transgenes encoding an oxygen tolerant pyruvate oxidoreductase (Por), a pyruvate carboxylase (Pyc), a malate synthase (AceB), malate thiokinase (SucC, SucD), a Malyl-CoA Lyase (Mcl), a malate dehydrogenase (Mdh), and an isocitrate lyase (Id), wherein the transgenic plant is selected on the basis of having a higher yield in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). In a seventh embodiment of the disclosed invention, the transgenic plant comprises two or more transgenes encoding an oxygen tolerant pyruvate oxidoreductase (Por), a malate synthase (AceB), and one or more transgenes encoding a pyruvate carboxylase (Pyc), malate thiokinase (SucC, SucD), a Malyl-CoA Lyase (Mel), a malate dehydrogenase (Mdh), and an isocitrate lyase (Id), wherein the transgenic plant is selected on the basis of having a higher yield in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). In a eighth embodiment of the disclosed invention, the transgenic plant of embodiments one through seven further comprises an additional one or more transgenes encoding one or more additional enzymes selected from the group: NADP-malate dehydrogenase (NADP-Mdh); fumarate hydratase (FumC); NADH-dependent fumarate reductase (FRDg); aconitase hydratase 1 (AcnA); ATP-citrate lyase A-1 (AclA-1); and ATP-citrate lyase subunit B2 (AclB-2), wherein the transgenic plant is selected on the basis of having a higher yield in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). In a ninth embodiment of the disclosed invention, the transgenic plant of embodiments one through seven further comprises an additional one or more transgenes encoding an NADP-malate dehydrogenase (NADP-Mdh) enzyme or the transgenes used in embodiments one through seven are expressed in a plant which has been modified through precise genome engineering to increase the expression of an existing plant gene encoding NADP-malate dehydrogenase (NADP-Mdh) enzyme activity. In a tenth embodiment of the disclosed invention, the transgenic plant comprises one or more transgenes encoding an NADP-malate dehydrogenase (NADP-Mdh) enzyme or is a plant which has been modified through precise genome engineering to increase the expression of an existing plant gene encoding NADP-malate dehydrogenase (NADP-Mdh) enzyme activity wherein the transgenic plant or plant is selected on the basis of having a higher yield in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). In a first aspect of embodiments one through ten, the heterologous enzymes expressed from the transgenes are targeted to the plastids of the plant wherein the transgenic plant is selected on the basis of having a higher yield in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). In a second aspect of embodiments one through ten, the expression of the transgene(s) is under the control of one or more seed specific promoter(s) and the heterologous enzymes expressed from the transgenes are targeted to the plastids of the plant wherein the transgenic plant is selected on the basis of having a higher seed yield in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). In an aspect of embodiments one through ten including the first and second aspects, the transgenic plant is selected on the basis of having a yield increase of at least 5%, at least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least 60%, or at least 80%, at least 90%, at least 100% at least 120% or higher in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). In an aspect of embodiments one through ten including the first and second aspects, the transgenic plant is selected on the basis of having a seed yield increase at least 5%, at least 10%, at least 15%, at least 20%, at least 30%, at least 40%, at least 50% or higher in comparison with a corresponding plant that is not expressing the heterologous enzyme(s). In an embodiment, the transgenic plant has a seed oil content at least 1%, at least 3%, at least 5%, at least 10%, at least 15%, at least 20%, or at least 25% or higher than the oil content of a wild type plant of the same species. In an additional embodiment of the invention, the transgenic plants of all of the previous embodiments and aspects include additional transgenes encoding a bicarbonate transporter localized to the chloroplast membrane to increase the level of bicarbonate and/or carbon dioxide available for use by carbon fixation enzymes within the disclosed metabolic pathways. In an embodiment, a method of producing a transformed plant having enhanced yield comprises transforming a plant cell with the disclosed transgenes; growing a plant from the plant cell until the plant produces seed; and selecting seeds from a plant in which yield is enhanced in comparison with a corresponding plant that is not expressing the heterologous enzyme(s) is disclosed. In an additional embodiment describe methods and genetic constructs are described that minimize the number of transgenes, or transgenes plus modifications to the expression of genes present naturally in the plant, to achieve the yield change outcomes. In an embodiment methods, metabolic pathways, enzymes, and crops for enhancing the yield of food crops to support the future needs of the growing world population are disclosed. In an embodiment the transgenic plants of all of the previous embodiments and aspects include additional transgenes encoding input traits such as herbicide or pesticide tolerance, insect resistance, drought tolerance, stress tolerance, nitrogen and water use efficiency or additional enzymes or traits to further increase yield. For example, the disclosed constructs may also contain expression cassettes for one or more transgenes encoding enzymes or other proteins for enhancing the availability of substrates for the disclosed metabolic pathways and enzymes. These include for example enzymes capable of increasing photosynthesis, increasing carbon flow through the Calvin cycle in photosynthesis, and/or increasing regeneration of ribulose 1,5-bisphosphate, the acceptor molecule in the Calvin cycle that upon fixation of CO2is converted to two molecules of 3-phosphoglycerate, the key intermediate for acetyl-CoA production. Candidate enzymes include but are not limited to sedoheptulose 1,7-bisphosphatase (SBPase, EC 3.1.3.37), fructose 1,6-bisphosphatase (FBPase, EC 3.1.3.11), a bi-functional enzyme encoding both SBPase and FBPase activities, transketolase (EC 2.2.1.1), and aldolase (EC 4.1.2.13). SBPase, transketolase, and aldolase activities have been shown to have an impact on the control of carbon fixed by the Calvin cycle (Raines, 2003, Transgenes encoding proteins involved in the transport of bicarbonate in cyanobacterial and algal systems can be added to increase the availability of CO2for the Calvin cycle and for the carboxylation enzymes present in the metabolic pathways disclosed herein. Suitable bicarbonate transporter genes can be obtained from cyanobacteria and algal species. A novel bicarbonate transporter from Other suitable examples of bicarbonate transporter genes are provided in Table 11 shown in The transgenic plants of embodiments 1-10 may have additional transgenes that provide resistance to one or more herbicides seleceted from, but not limited to, the following group: glyphosate, 2,4-D, 2,4-D choline, Liberty Link, Dicambia, glufosinate, mesotrione, isoxaflutole, tembotrione, pyroxasulfone, fluthiacet-methyl, atrazine, triazines, metolachlor, imazethapyr, fomesafen, metribuzin, and bicyclopyrone. Vectors expressing genes encoding enzymes to metabolize glyoxylate in the plastid, including plastid targeted glyoxylate carboligase and/or plastid targeted tartronic semialdehyde reductase, can be combined with the vectors described in the invention to further enhance the efficiency of the yield traits disclosed in this invention. Glyoxylate is a key intermediate in the disclosed pathways ( Alternatively, endogenous plastid localized glyoxylate reductase activity can be increased through promoter replacement, precise genome engineering, or heterologous expression of the transgene to increase the conversion of glyoxylate to glycolate. Previous researchers have suggested that cytosolic and plastid localized glyoxylate reductases in In an embodiment, transgenic plants are generated which express the gene and enzyme combinations disclosed herein and are grown through a number of planting cyles to generate homozygous lines and screened for increased seed yield and/or increased seed oil content and those lines having significantly higher seed yield and/or increased seed oil content are selected. In a method embodiments, metabolic pathways, enzymes and crops for enhancing the yield of food crops to support the future needs of the growing world population are disclosed. The disclosed invention describes the in planta expression of combinations of transgenes encoding multiple enzymes in complex metabolic pathways resulting in step changes in crop yield. An exemplary seed crop was engineered to increase its yield. Unexpected step changes in yield have been obtained by engineering seed specific expression of novel combinations of enzymes to increase carbon fixation. Furthermore, the targeting of this yield increase to the harvested product of interest was demonstrated in the embodiment of the oilseed crop, where the product of interest is the seed. Herein there is described the introduction of multiple transgenes, encoding novel metabolic pathways having enzymes catalyzing carboxylation reactions and/or enzymes using NADPH or NADH as a cofactor, into crops, screening the resulting transgenic crop lines produced for increased yield and selecting those transgenic lines having higher yield. In particular, by using plant promoters active in the developing seed and targeting each of the enzymes introduced by the transgenes to the plastids in the plant cells step changes had been demonstrated in seed yield and increased oil content. Although described and demonstrated with large numbers of transgenes, it will be obvious to those skilled in the art that it is routine experimentation to define the minimum gene sets essential to achieve the yield change outcomes demonstrated and provide the simplest system possible to facilitate regulatory approval for large scale planting. A description of example embodiments of the invention follows. For the purposes of the invention, “plant” refers to all genera and species of higher and lower plants of the Plant Kingdom. The term includes the mature plants, seeds, shoots and seedlings, and parts, propagation material, plant organ tissue, protoplasts, callus and other cultures, for example cell cultures, derived from them, and all other species of groups of plant cells giving functional or structural units. Mature plants refers to plants at any developmental stage beyond the seedling. Seedling refers to a young, immature-plant at an early developmental stage. “Plant” encompasses all annual and perennial monocotyldedonous or dicotyledonous plants and includes by way of example, but not by limitation, those of the genera Preferred plants are those from the following plant families: Amaranthaceae, Asteraceae, Brassicaceae, Carophyllaceae, Chenopodiaceae, Compositae, Cruciferae, Cucurbitaceae, Euphorbiaceae, Fabaceae, Labiatae, Leguminosae, Papilionoideae, Liliaceae, Linaceae, Malvaceae, Poaceae, Rosaceae, Rubiaceae, Saxifragaceae, Scrophulariaceae, Solanaceae, Sterculiaceae, Tetragoniaceae, Theaceae, Umbelliferae. The invention can particularly be applied advantageously to monocotyledonous or dicotyledonous plant organisms. Preferred dicotyledonous plants are selected in particular from the dicotyledonous crop plants such as, for example, Asteraceae such as sunflower, In some cases preferred crops are used for food production for animals, humans or both. In some cases, the entire crop is used, for example by animal consumption directly in the field, or harvested after the growing season and used or processed in which case it is desirable to increase the yield of the entire plant biomass. In this case, the transgenes should be expressed in the green tissue of the plant using for example constitutive or leaf-specific promoters and the enzymes encoded by the transgenes to the plastids, in particular the chloroplasts of the plants. Examples of these types of crops include forage crops such as hay, alfalfa, silage corn etc. In other cases the seed is the most valuable part of the plant harvested and the plant stems, stalks leaves etc. are left in the field. Examples of this include the majority of the major food crops including maize (corn), wheat, oats, barley, soybean, millet, sorghum, potato, pulses, beans, tomatoes, oilseeds, etc. In the case of plants used for the harvesting of seed, it is desirable to increase the yield of the seed without necessarily increasing the yield of the other parts of the plant to maximize the use of agronomic inputs such as fertilizer, water etc for the production of the seed. This can be achieved as described in the disclosed invention by using seed specific or silique specific promoters to control the expression of the transgenes in the developing seed and targeting the enzymes expressed from the transgenes to the plastid of the seed using plastid targeting signals as is well known in the art. Of particular interest for transformation are plants, which are oilseed plants. In oilseed plants of interest the oil is accumulated in the seed and can account for greater than 10%, greater than 15%, greater than 18%, greater than 25%, greater than 35%, greater than 50% by weight of the weight of dry seed. Oil crops encompass by way of example: Metabolic Enzymes and Genes Encoding them Useful for Practising the Invention Metabolic pathways and the enzymes useful for practicing the disclosed invention are illustrated in It is well known in the art that alternative genes encoding these metabolic enzymes can be identified based on nucleotide and or protein sequence homology and either isolated from their species of origin or constructed by DNA synthesis techniques. Metabolic enzyme includes metabolic enzymes homologous to the enzymes listed in Table 1 so long as the metabolic enzyme can catalyze the same enzymatic reaction shown in either As used herein, “percent homology” of two amino acid sequences or of two nucleic acid sequences is determined using the algorithm of Karlin and Altschul (1990) In addition, polynucleotides that are substantially identical to a polynucleotide encoding any of the metabolic enzymes listed in Table 1 are included. By “substantially identical” is meant a polypeptide or polynucleotide having a sequence that is at least about 85%, specifically about 90%, and more specifically about 95% or more identical to the sequence of the reference amino acid or nucleic acid sequence. For polypeptides, the length of the reference polypeptide sequence will generally be at least about 16 amino acids, or specifically at least about 20 amino acids, more specifically at least about 25 amino acids, and most specifically at least about 35 amino acids. For nucleic acids, the length of the reference nucleic acid sequence will generally be at least about 50 nucleotides, specifically at least about 60 nucleotides, more specifically at least about 75 nucleotides, and most specifically at least about 110 nucleotides. Typically, homologous sequences can be confirmed by hybridization, wherein hybridization under stringent conditions. Using the stringent hybridization [i.e., washing the nucleic acid fragments twice where each wash is at room temperature for 30 minutes with 2× sodium chloride and sodium citrate buffer (2×SSC buffer; 300 mM sodium chloride and 30 mM sodium citrate, pH 7.0) and 0.1% sodium dodecyl sulfate (SDS); followed by washing one time at 50° C. for 30 minutes with 2×SCC and 0.1% SDS; and then washing two times where each wash is at room temperature for 10 minutes with 2×SSC], homologous sequences can be identified comprising at most about 25 to about 30% base pair mismatches, or about 15 to about 25% base pair mismatches, or about 5 to about 15% base pair mismatches. The term metabolic enzymes includes polynucleotides that encode the enzyme activities listed in Table 1 including polypeptides or full-length proteins that contain substitutions, insertions, or deletions into the polypeptide backbone. Related polypeptides are aligned with the metabolic enzymes listed in Table 1 by assigning degrees of homology to various deletions, substitutions and other modifications. Homology can be determined along the entire polypeptide or polynucleotide, or along subsets of contiguous residues. The percent identity is the percentage of amino acids or nucleotides that are identical when the two sequences are compared. The percent similarity is the percentage of amino acids or nucleotides that are chemically similar when the two sequences are compared. Metabolic enzymes and homologous polypeptides are preferably greater than or equal to about 75%, preferably greater than or equal to about 80%, more preferably greater than or equal to about 90% or most preferably greater than or equal to about 95% identical. A homologous polypeptide may be produced, for example, by conventional site-directed mutagenesis of polynucleotides (which is one avenue for routinely identifying residues of the molecule that are functionally important or not), by random mutation, by chemical synthesis, or by chemical or enzymatic cleavage of the polypeptides. In the case of polypeptide sequences that are less than 100% identical to a reference sequence, the non-identical positions are preferably, but not necessarily, conservative substitutions for the reference sequence. Conservative substitutions typically include substitutions within the following groups: glycine and alanine; valine, isoleucine, and leucine; aspartic acid and glutamic acid; asparagine and glutamine; serine and threonine; lysine and arginine; and phenylalanine and tyrosine. Where a particular polypeptide is said to have a specific percent identity to a reference polypeptide of a defined length, the percent identity is relative to the reference peptide. Thus, a peptide that is 50% identical to a reference polypeptide that is 100 amino acids long can be a 50 amino acid polypeptide that is completely identical to a 50 amino acid long portion of the reference polypeptide. It might also be a 100 amino acid long polypeptide that is 50% identical to the reference polypeptide over its entire length. Of course, many other polypeptides will meet the same criteria. In some cases, for example the Pyruvate oxidoreductase enzyme it is desirable to use an enzyme which retains its enzymatic activity in the presence of oxygen for example, from Unless otherwise indicated, the disclosure encompasses all conventional techniques of plant transformation, plant breeding, microbiology, cell biology and recombinant DNA, which are within the skill of the art. See, e.g., Sambrook and Russell, Unless otherwise noted, technical terms are used according to conventional usage. Definitions of common terms in molecular biology may be found in Lewin, A number of terms used herein are defined and clarified in the following section. As used herein, a “vector” is a replicon, such as a plasmid, phage, or cosmid, into which another DNA segment may be inserted so as to bring about the replication of the inserted segment. The vectors described herein can be expression vectors. As used herein, an “expression vector” is a vector that includes one or more expression control sequences. As used herein, an “expression control sequence” is a DNA sequence that controls and regulates the transcription and/or translation of another DNA sequence. As used herein, “operably linked” means incorporated into a genetic construct so that expression control sequences effectively control expression of a coding sequence of interest. As used herein, “transformed” and “transfected” encompass the introduction of a nucleic acid (e.g., a vector) into a cell by a number of techniques known in the art. “Plasmids” are designated by a lower case “p” preceded and/or followed by capital letters and/or numbers. The term “plant” is used in its broadest sense. It includes, but is not limited to, any species of woody, ornamental or decorative, crop or cereal, fruit or vegetable plant, and photosynthetic green algae (e.g., The term “plant tissue” includes differentiated and undifferentiated tissues of plants including those present in roots, shoots, leaves, inflorescences, anthers, pollen, ovaries, seeds and tumors, as well as cells in culture (e.g., single cells, protoplasts, embryos, callus, etc.). Plant tissue may be in planta, in organ culture, tissue culture, or cell culture. The term “plant part” as used herein refers to a plant structure, a plant organ, a plant tissue or a plant cell. A “non-naturally occurring plant” refers to a plant that does not occur in nature without human intervention. Non-naturally occurring plants include transgenic plants and plants created through genetic engineering. The term “plant cell” refers to a structural and physiological unit of a plant, comprising a protoplast and a cell wall. The plant cell may be in the form of an isolated single cell or a cultured cell, or as a part of a higher organized unit such as, for example, a plant tissue, a plant organ, or a whole plant. The term plastid refers to a subcellular organelle of the plant and includes chloropolasts and plastids in developing seed. The term “plant cell culture” refers to cultures of plant units such as, for example, protoplasts, cells and cell clusters in a liquid medium or on a solid medium, cells in plant tissues and organs, microspores and pollen, pollen tubes, anthers, ovules, embryo sacs, zygotes and embryos at various stages of development. The term “plant material” refers to leaves, stems, roots, inflorescences and flowers or flower parts, fruits, pollen, anthers, egg cells, zygotes, seeds, cuttings, cell or tissue cultures, or any other part or product of a plant. A “plant organ” refers to a distinct and visibly structured and differentiated part of a plant, such as a root, stem, leaf, flower bud, inflorescence, spikelet, floret, seed or embryo. The term “non-transgenic plant” refers to a plant that has not been genetically engineered with heterologous nucleic acids. These non-transgenic plants can be the test or control plant when comparisons are made, including wild-type plants. A “corresponding non-transgenic plant” refers to the plant prior to the introduction of heterologous nucleic acids. This plant can be the test plant or control plant, including wild type plants. A “trait” refers to morphological, physiological, biochemical and physical characteristics or other distinguishing feature of a plant or a plant part or a cell or plant material. The term “trait modification” refers to a detectable change in a characteristic of a plant or a plant part or a plant cell induced by the expression of a polynucleotide or a polypeptide of the invention compared to a plant not expressing them, such as a wild type plant. Some trait modifications can be evaluated quantitatively, such as content of different metabolites, proteins, pigments, lignin, vitamins, starch, sucrose, glucose, fatty acids and other storage compounds, seed size and number, organ size and weight, total plant biomass, yield of seed and yield of genetically engineered products. Physical plant characteristics that can be modified include cell development (such as the number of trichomes), fruit and seed size and number, yields and size of plant parts such as stems, leaves and roots, the stability of the seeds during storage, characteristics of the seed pod (e.g., susceptibility to shattering), root hair length and quantity, internode distances, or the quality of seed coat. Plant growth characteristics that can be modified include growth rate, germination rate of seeds, vigor of plants and seedlings, leaf and flower senescence, male sterility, apomixis, flowering time, flower abscission, rate of nitrogen uptake, biomass or transpiration characteristics, as well as plant architecture characteristics such as apical dominance, branching patterns, number of organs, organ identity, organ shape or size. The ability to improve plant yield, plant seed yield, and plant seed oil content would be of great economic advantage to farmers worldwide and would allow for increased food production necessary to meet the demands of the growing global population. Described herein are methods of producing a transgenic plant, plant tissue, seed, or plant cell, wherein said plant, plant tissue, seed or plant cell comprises incorporated in the genome of said plant, plant tissue, seed, or plant cell: one or more polynucleotides encoding one or more transgenes encoding metabolic pathway enzymes, heterologous to the plant with DNA sequences to enable their expression or in the case of a metabolic enzyme native to that plant its increased expression or the cellular location of that enzyme. In some cases alternative regulatory sequences, homologous or heterologous to the plant can be inserted in front of a native plant gene to alter the expression of a plant enzyme and/or alter the cellular location in which the plant enzyme is functionally active. The term transgene refers to a recombinant polynucleotide or nucleic acid that comprises a coding sequence encoding a protein or RNA molecule. The transgenes encoding the specific enzymes illustrated in It was found that incorporation of combinations of genes encoding subsets of the metabolic enzymes listed in Table 1 increased the yield of the plant as determined by measuring the weight of the transgenic plant or measuring the weight of the seed produced by the transgenic plant and comparing it to a transgenic plant or plant seed containing vector sequences without the transgenes encoding the metabolic anzymes. For example, increases in the yield of seed up to two times or higher than plants not having the metabolic enzymes expressed are shown in the examples herein. In some cases, in addition to the increase in seed yield, the oil content of those seed are measurably higher. DNA constructs useful in the methods described herein include transformation vectors capable of introducing transgenes into plants. As used herein, “transgenic” refers to an organism in which a nucleic acid fragment containing a heterologous nucleotide sequence has been introduced. The transgenes in the transgenic organism are preferably stable and inheritable. The heterologous nucleic acid fragment may or may not be integrated into the host genome. Several plant transformation vector options are available, including those described in Many vectors are available for transformation using Transformation without the use of Engineered minichromosomes can also be used to express one or more genes in plant cells. Cloned telomeric repeats introduced into cells may truncate the distal portion of a chromosome by the formation of a new telomere at the integration site. Using this method, a vector for gene transfer can be prepared by trimming off the arms of a natural plant chromosome and adding an insertion site for large inserts (Yu et al., 2006 An alternative approach to chromosome engineering in plants involves in vivo assembly of autonomous plant minichromosomes (Carlson et al., 2007 Another approach useful to the described invention is Engineered Trait Loci (“ETL”) technology (U.S. Pat. No. 6,077,697; US 2006/0143732). This system targets DNA to a heterochromatic region of plant chromosomes, such as the pericentric heterochromatin, in the short arm of acrocentric chromosomes. Targeting sequences may include ribosomal DNA (rDNA) or lambda phage DNA. The pericentric rDNA region supports stable insertion, low recombination, and high levels of gene expression. This technology is also useful for stacking of multiple traits in a plant (US 2006/0246586). Zinc-finger nucleases (ZFNs) are also useful for practicing the invention in that they allow double strand DNA cleavage at specific sites in plant chromosomes such that targeted gene insertion or deletion can be performed (Shukla et al., 2009 The CRISPR/Cas9 system (Sander, J. D. and Joung, J. K., Nature Biotechnology, published online Mar. 2, 2014; doi; 10.1038/nbt.2842) is particularly useful for editing plant genomes to modulate the expression of homologous genes encoding enzymes, for example the NADP-specific malate dehydrogenase enzyme found naturally in the plant cell plastids useful for practicing the disclosed invention. Several examples of the use of this technology to edit the genomes of plants have now been reported (Belhaj et al. Plant Methods 2013, 9:39). Transformation protocols as well as protocols for introducing nucleotide sequences into plants may vary depending on the type of plant or plant cell targeted for transformation. Suitable methods of introducing nucleotide sequences into plant cells and subsequent insertion into the plant genome include microinjection (Crossway et al. (1986) Methods for transformation of plastids such as chloroplasts are known in the art. See, for example, Svab et al. (1990) Recombinase technologies which are useful for producing the disclosed transgenic plants include the cre-lox, FLP/FRT and Gin systems. Methods by which these technologies can be used for the purpose described herein are described for example in (U.S. Pat. No. 5,527,695; Dale And Ow, 1991 Transformation protocols as well as protocols for introducing nucleotide sequences into plants may vary depending on the type of plant or plant cell, i.e., monocot or dicot, targeted for transformation. Suitable methods of introducing nucleotide sequences into plant cells and subsequent insertion into the plant genome are described in US 2010/0229256 A1 to Somleva & Ali and US 2012/0060413 to Somleva et al. The transformed cells are grown into plants in accordance with conventional techniques. See, for example, McCormick et al., 1986 Procedures for in planta transformation can be simple. Tissue culture manipulations and possible somaclonal variations are avoided and only a short time is required to obtain transgenic plants. However, the frequency of transformants in the progeny of such inoculated plants is relatively low and variable. At present, there are very few species that can be routinely transformed in the absence of a tissue culture-based regeneration system. Stable Following transformation by any one of the methods described above, the following procedures can be used to obtain a transformed plant expressing the transgenes: select the plant cells that have been transformed on a selective medium; regenerate the plant cells that have been transformed to produce differentiated plants; select transformed plants expressing the transgene producing the desired level of desired polypeptide(s) in the desired tissue and cellular location. The cells that have been transformed may be grown into plants in accordance with conventional techniques. See, for example, McCormick et al. In some scenarios, it may be advantageous to insert a multi-gene pathway into the plant by crossing of lines containing portions of the pathway to produce hybrid plants in which the entire pathway has been reconstructed. This is especially the case when high levels of product in a seed compromises the ability of the seed to germinate or the resulting seedling to survive under normal soil growth conditions. Hybrid lines can be created by crossing a line containing one or more genes with a line containing the other gene(s) needed to complete a biosynthetic pathway. Use of lines that possess cytoplasmic male sterility (Esser, K. et al., 2006, Progress in Botany, Springer Berlin Heidelberg. 67, 31-52) with the appropriate maintainer and restorer lines allows these hybrid lines to be produced efficiently. Cytoplasmic male sterility systems are already available for some Brassicaceae species (Esser, K. et al., 2006, Progress in Botany, Springer Berlin Heidelberg. 67, 31-52). These Brassicaceae species can be used as gene sources to produce cytoplasmic male sterility systems for other oilseeds of interest such as Transgenic plants can be produced using conventional techniques to express any genes of interest in plants or plant cells ( Plant promoters can be selected to control the expression of the transgene in different plant tissues or organelles for all of which methods are known to those skilled in the art (Gasser & Fraley, 1989 Chemical-regulated promoters can be used to modulate the expression of a gene in a plant through the application of an exogenous chemical regulator. Depending upon the objective, the promoter may be a chemical-inducible promoter, where application of the chemical induces gene expression, or a chemical-repressible promoter, where application of the chemical represses gene expression. Chemical-inducible promoters are known in the art and include, but are not limited to, the maize 1n2-2 promoter, which is activated by benzenesulfonamide herbicide safeners, the maize GST promoter, which is activated by hydrophobic electrophlic compounds that are used as pre-emergent herbicides, and the tobacco PR-1 promoter which is activated by salicylic acid. Other chemical-regulated promoters include steroid-responsive promoters [see, for example, the glucocorticoid-inducible promoter (Schena et al., 1991 A three-component osmotically inducible expression system suitable for plant metabolic engineering has recently been reported (Feng et al., 2011 Constitutive promoters include, for example, the core promoter of the Rsyn7 promoter and other constitutive promoters disclosed in WO 99/43838 and U.S. Pat. No. 6,072,050, the core CaMV 35S promoter (Odell et al., 1985 “Tissue-preferred” promoters can be used to target gene expression within a particular tissue. Compared to chemically inducible systems, developmentally and spatially regulated stimuli are less dependent on penetration of external factors into plant sells. Tissue-preferred promoters include those described by Van Ex et al., 2009 “Seed-preferred” promoters include both “seed-specific” promoters (those promoters active during seed development such as promoters of seed storage proteins) as well as “seed-germinating” promoters (those promoters active during seed germination). See Thompson et al., 1989 Leaf-specific promoters are known in the art. See, for example, WO/2011/041499 and U.S. Patent No 2011/0179511 A1 to Thilmony et al.; Yamamoto et al., 1997 Certain embodiments use transgenic plants or plant cells having multi-gene expression constructs harboring more than one promoter. The promoters can be the same or different. Any of the described promoters can be used to control the expression of one or more of the genes of the invention, their homologs and/or orthologs as well as any other genes of interest in a defined spatiotemporal manner. Nucleic acid sequences intended for expression in transgenic plants are first assembled in expression cassettes behind a suitable promoter active in plants. The expression cassettes may also include any further sequences required or selected for the expression of the transgene. Such sequences include, but are not restricted to, transcription terminators, extraneous sequences to enhance expression such as introns, vital sequences, and sequences intended for the targeting of the gene product to specific organelles and cell compartments. These expression cassettes can then be transferred to the plant transformation vectors described infra. The following is a description of various components of typical expression cassettes. A variety of transcriptional terminators are available for use in expression cassettes. These are responsible for the termination of transcription beyond the transgene and the correct polyadenylation of the transcripts. Appropriate transcriptional terminators are those that are known to function in plants and include the CaMV 35S terminator, the tm1 terminator, the nopaline synthase terminator and the pea rbcS E9 terminator. These are used in both monocotyledonous and dicotyledonous plants. The coding sequence of the selected gene may be genetically engineered by altering the coding sequence for optimal expression in the crop species of interest. Methods for modifying coding sequences to achieve optimal expression in a particular crop species are well known (Perlak et al., 1991 Individual plants within a population of transgenic plants that express a recombinant gene(s) may have different levels of gene expression. The variable gene expression is due to multiple factors including multiple copies of the recombinant gene, chromatin effects, and gene suppression. Accordingly, a phenotype of the transgenic plant may be measured as a percentage of individual plants within a population. The yield of a plant can be measured simply by weighing. The yield of seed from a plant can also be determined by weighing. The present inventors have transformed plants with recombinant DNA molecules that encode heterologous metabolic enzymes in the nuclear genome. The expressed recombinant metabolic enzymes are transported into the plastid compartments of the plant cells. Transgenic plants and plant cells expressing the recombinant metabolic enzymes are selected on the basis of having higher yield of total biomass or seed compared to wild type plants of the same species not comprising the recombinant metabolic enzymes. The transgenic plants also show increased seed yield compared to wild type plants of the same species not comprising the recombinant heterologous enzymes. In some cases the transgenic plants show increased seed yield and higher oil content as compared to wild type plants of the same species not comprising the recombinant heterologous enzymes. A recombinant DNA construct including a plant-expressible gene or other DNA of interest is inserted into the genome of a plant by a suitable method. Suitable methods include, for example, In one embodiment, the transgenic plants are grown (e.g., on soil) and harvested. In one embodiment, above ground tissue is harvested separately from below ground tissue. Suitable above ground tissues include shoots, stems, leaves, flowers, grain, and seed. Exemplary below ground tissues include tubers, roots, and root hairs. In one embodiment, whole plants are harvested and the above ground tissue is subsequently separated from the below ground tissue. Genetic constructs may encode a selectable marker to enable selection of transformation events. There are many methods that have been described for the selection of transformed plants [for review see (Miki et al., Methods of plant selection that do not use antibiotics or herbicides as a selective agent have been previously described and include expression of glucosamine-6-phosphate deaminase to inactive glucosamine in plant selection medium (U.S. Pat. No. 6,444,878) and a positive/negative system that utilizes D-amino acids (Erikson et al., Methods for positive selection using sorbitol dehydrogenase to convert sorbitol to fructose for plant growth have also been described (WO 2010/102293). Screenable marker genes include the beta-glucuronidase gene (Jefferson et al., 1987 Transformation events can also be selected through visualization of fluorescent proteins such as the fluorescent proteins from the nonbioluminescent Anthozoa species which include DsRed, a red fluorescent protein from the Visual selection can also be performed with the yellow fluorescent proteins (YFP) including the variant with accelerated maturation of the signal (Nagai, T. et al. (2002), Nat Biotech 20: 87-90), the blue fluorescent protein, the cyan fluorescent protein, and the green fluorescent protein (Sheen et al. (1995), Plant J 8: 777-84; Davis and Vierstra (1998), Plant Molecular Biology 36: 521-528). A summary of fluorescent proteins can be found in Tzfira et al. (Tzfira et al. (2005), Plant Molecular Biology 57: 503-516) and Verkhusha and Lukyanov (Verkhusha, V. V. and K. A. Lukyanov (2004), Nat Biotech 22: 289-296) whose references are incorporated in entirety. Improved versions of many of the fluorescent proteins have been made for various applications. Use of the improved versions of these proteins or the use of combinations of these proteins for selection of transformants will be obvious to those skilled in the art. For plastid transformation constructs, a preferred selectable marker is the spectinomycin-resistant allele of the plastid 16S ribosomal RNA gene (Staub J M, Maliga P, Plastid targeting sequences are known in the art and include the chloroplast small subunit of ribulose-1,5-bisphosphate carboxylase (Rubisco) (de Castro Silva Filho et al. The engineered plants for increased yield may have stacked input traits that include herbicide resistance and insect tolerance, for example a plant that is tolerant to the herbicide glyphosate and that produces the Plasmids pMBXS918, pMBXS919, pMBXS994, pMBXS1022, pMBXS1023, and pMBXS1024, are derivatives of pCAMBIA binary vectors (Centre for Application of Molecular Biology to International Agriculture, Canberra, Australia) and were constructed using conventional molecular biology and cloning techniques. The transgenes encoded by these plasmids are listed in Table 2. The enzyme activities, substrates, and metabolic pathways are shown in Maps illustrating the metabolic enzyme encoding genes and plant expression elements for directing their expression in plants in the plasmid vectors pMBXS918, pMBXS919, pMBXS1022, pMBXS1023, and pMBXS1024 are shown in To construct gene expression cassettes for metabolic pathway enzymes, a DNA sequence encoding a plastid signal peptide was fused to the N-terminus of each gene to direct the encoded protein to the plastid. The plastid signal peptide consisted of DNA encoding the signal peptide from the ribulose-1,5-bisphosphatase carboxylase (Rubisco) small subunit from In preparation for plant transformation experiments, seeds of To identify Transgenic plant lines produced using the different plasmid vectors and vector combinations are shown in Table 3 together with the analysis of the yield of the T2 generation seed from each line. Seed weight yield was determined by harvesting all of the mature seeds from a plant and drying them in an oven with mechanical convection set at 22° C. for two days. The weight of the entire harvested seed was recorded. Total seed oil content and oil fatty acid profile were determined using published procedures for preparation of fatty acid methyl esters (Li et al., Phytochemistry, 67, 904) with some modifications. Briefly, 25-30 mg of mature seeds were placed in 13×100 mm screw-cap test tubes. To each tube, 1.5 mL of 2.5% (v/v) sulfuric acid in methanol (w/ 0.01% w/v BHT), 400 μL toluene, and 500 μg of a triheptadecanoin (Nu-Chek Prep, Elysian, Minn.) solution (10 mg/mL in toluene) as internal standard were added. Tubes were purged with nitrogen, capped, and heated at 90° C. for 1 h. Upon cooling, 1 mL of 1 M sodium chloride and 1 mL of heptane were added to each tube. Following mixing and centrifugation, the heptane layer containing fatty acid methyl esters was analyzed with an Agilent 7890A gas chromatograph with a 30 m×0.25 mm (inner diameter) INNOWax column (Agilent) and flame ionization detection. The oven temperature was programmed from 185° C. (1 min hold) to 235° C. (1 min hold) at a rate of 10° C./min (11 min total run time), and the front inlet pressure was 35.8 psi of He. The oil content (% of seed weight) was determined by comparison of the detector response from seed-derived fatty acid methyl esters relative to methyl heptadecanoate from the triheptadecanoin internal standard. Transgenic lines produced with either of plasmids pMBXS994 or pMBSX1022 not only had significantly higher seed yield but in addition the seed oil content was increased by up to 25% as compared to the control plants. Numeric ranges are inclusive of the numbers defining the range. It is intended that every maximum numerical limitation given throughout this specification includes every lower numerical limitation, as if such lower numerical limitations were expressly written herein. Every minimum numerical limitation given throughout this specification will include every higher numerical limitation, as if such higher numerical limitations were expressly written herein. Every numerical range given throughout this specification will include every narrower numerical range that falls within such broader numerical range, as if such narrower numerical ranges were all expressly written herein. The modifier “about” used in connection with a quantity is inclusive of the stated value and has the meaning dictated by the context (e.g., includes the degree of error associated with measurement of the particular quantity). Two single colonies of GV3101 (pMP90), each carrying a specific construct required for co-transformation, were obtained from freshly streaked plates and inoculated into two separate vials containing 5 mL LB medium. After overnight growth at 28° C., 2 mL of culture of each construct was transferred to separate 500-mL flask containing 300 mL of LB and incubated overnight at 28° C. Cells were pelleted by centrifugation (4,000 rpm, 20 min), and diluted to an OD600 of ˜0.8 with infiltration medium containing 5% sucrose. The cultures, each carrying a specific construct, were mixed 1:1 by volume and 0.05% (v/v) Silwet-L77 (Lehle Seeds, Round Rock, Tex., USA) was added. To identify co-transformed lines, GFP expressing seeds were visualized by fluorescent microscopy using a Nikon AZ100 microscope with an eGFP filter (Excitation bandpass 470/40, Emission Bandpass 525/50) and planted in soil. The presence of the bar gene on the T-DNA of pMBXS918 or pMBXS919 constructs allowed selction of co-transformants by spraying a solution of 400 mg/L of the herbicide Liberty (active ingredient 15% glufosinate-ammonium) on plantlets obtained from GFP expressing seeds. Co-transformations of select plasmid combinations were performed and transgenic plants isolated. T1 plants were grown in a greenhouse to produce T2 seed. Seed yield from select lines is shown in Table 4. T2 seeds were sown and eight individual T2 plants from lines transformed with constructs for high yield were grown in the greenhouse in a randomized complete block design. T3 seed was harvested and seed weight was recorded for the individual plants. Average T3 seed yield was calculated from 8 T2 plants per line and compared to yield data of 7 plants containing empty vector pMBXO12 (Table 5). Multiple individual plants within a line showed significantly increased yield. The highest seed yield was obtained with a plant from line 15-0406 that was co-transformed with plasmids pMBXS919 and pMBXS1024. The seed yield of this plant was 276% of the average of the vector control line JS11 and produced 13.63 grams of seed from a single plant under greenhouse growth conditions. The weight of 100 seeds from the highest yielding co-transformed lines within Table 5 was determined. Seeds from these lines were larger as determined by the increase in 100 seed weight (Table 6). The largest seeds were from a plant co-transformed with pMBXS919/p1024 which contained an average 100 seed weight of 141.53 mg, significantly higher (132%) than the control line JS11 that contained an average 100 seed weight of 106.64 mg. The T3 seed yield and 100 seed weight were used to estimate the total number of seeds per plant. Results from these calculations show that the highest yielding plants produced both heavier seeds and more seeds per plant. The approach and methods described in this example can be used to screen for and select the highest yielding lines for commercial production. The length and width of individual seeds from the highest yielding control line (JS11) and the highest yielding transgenic lines were also measured (Table 7) showing a small increase in seed size. The oil content of lines was measured for each of the plants described in Table 5 using the procedures described earlier. The oil content of replicate plants from an individual event is shown in Protein content in bulk It is well known in the art that it is desirable for plant breeding and regulatory approval purposes to reduce the number of transgenes in a line for commercial development to the minimum set while still achieving the desired outcome, which in the case of this invention is higher plant yield and/or higher plant seed yield and/or higher seed oil content. Having unequivocally demonstrated the achievement of significantly higher yield in the transgenic plants containing the different sets of metabolic enzymes alone and in combination, it is routine to now proceed to determine the optimum yield increase with the minimum set of genes. For this reason a series of additional plasmid vectors are constructed encoding the metabolic enzyme combinations as shown in Table 9. By transforming camelina as described above and determining the change in seed yield as compared to a vector control and to the highest yielding lines in Tables 3, 4, and 5, it will be routine experimentation to achieve the desired outcome. Alternate combinations of transgenes that can be used to improve seed and/or seed oil yield are listed in Table 9. In a preferred embodiment, genes sets in plasmids pMBXS1056, pMBXS1057, pMBXS1058, pMBXS1059, and pMBXS1060 are transformed into Vector pMBXS1056 ( Construct pMBXS1058 ( Construct pMBXS1059 ( Construct pMBXS1060 ( Construct pMBXS1057 ( Alternative constructs can be constructed to convert 1 molecule of pyruvate and 1 molecule of HCO3− to 1 molecule of acetyl-CoA and 1 molecule of glyoxylate as shown in Examples 1-6 describe novel sets of transgenes to increase seed and/or seed oil yield. These methods can be further enhanced by co-expression of a bicarbonate transporter localized to the chloroplast envelope since diffusion of CO2across plastid membranes is considered to be a significant limiting factor of photosynthesis (Tholen & Zhu, 2011, Plant Physiol. 156, 90-105). The bicarbonate transporter would increase the supply of bicarbonate (HCO3−) available to pyruvate carboxylase and, in the presence of a carbonic anhydrase as well as increase the supply of CO2to the pyruvate oxidoreductase reactions described in Examples 1-6. Carbonic anhydrases are known to be present in chloroplasts to allow the interconversion of bicarbonate and carbon dioxide as shown below: Bicarbonate transporters from cyanobacteria can be modified with a targeting signal to direct the protein to the chloroplast envelope. In a preferred embodiment, bicarbonate transporters from green algae that possess chloroplasts and whose bicarbonate transporters already localize to a chloroplast envelope can be used. In another embodiment, the bicarbonate transporter is encoded by the CCP1 gene [Accession No. XM_001692145.1] (SEQ ID NO:6) as described in WO2015103074, incorporated herein by reference in its entirety. In yet another embodiment, the bicarbonate transporter is expressed under a seed specific or constitutive promoter. In an alternative embodiment, the bicarbonate transporter is expressed under a seed specific or constitutive promoter and does not localize to the plastid, such as the CCP1 gene [Accession No. XM_001692145.1] (SEQ ID NO:6) as described in pending U.S. Provisional Patent Application No. 62/291,341. An expression cassette including a seed specific promoter sequence operably linked to a heterologous nucleotide sequence encoding a bicarbonate transporter can be co-transformed with constructs selected from those described in Examples 1-6. In the case of a cyanobacterial bicarbonate transporter, the transgene nucleotide sequence is modified with a sequence that will direct the bicarbonate transporter to the plastid envelope. Constructs described in Examples 1-7 can be transformed into other crops to increase seed yield. Transformation of Construct pMBXS1023 is modified to add the neomycin tranferase (nptII) selectable marker. Constructs pMBXS919 or pMBXS1023 are introduced into For transformation, cotyledons are excised from 4 or in some cases 5 day old seedlings so that they included ˜2 mm of petiole at the base. Individual cotyledons with the cut surface of their petioles are immersed in diluted bacterial suspension for 1 s and immediately embedded to a depth of ˜2 mm in co-cultivation medium, MS medium with 3% (w/v) sucrose and 0.7% phytagar and enriched with 20 μM benzyladenine. For co-transformation of pMBXS1023 and pMBXS919 constructs, the bacterial suspension for immersion contains a mixture of two Transformation of Explants are cocultivated with Plantlets are removed from agar, gently washed, and transferred to potting soil in pots. Plants are grown in a humid environment for a week and then transferred to the greenhouse. Transgenic lines are screened and transgenic lines having increased plant yield and/or increased seed yield and/or increased seed oil content are selected. The binary vectors provided in the invention can be used for Plant Material: Plants grown in a greenhouse are used as an explant source. Ears are harvested 9-13 d after pollination and surface sterilized with 80% ethanol. Explant Isolation, Infection and Co-Cultivation: Immature zygotic embryos (1.2-2.0 mm) are aseptically dissected from individual kernels and incubated in Callus Selection: All embryos are transferred on to the first selection medium (the resting medium described above supplemented with 1.5 mg/l bialaphos for selection of pMBXS919 and glyphosate for selection of pMBXS1023) and incubated at 28° C., in the dark for 2 weeks followed by subculture on a selection medium containing glyphosate and 3 mg/l bialaphos. Proliferating pieces of callus are propagated and maintained by subculture on the same medium every 2 weeks. Plant Regeneration and Selection: Herbicide resistant embryogenic callus lines are transferred on to regeneration medium I (MS basal medium supplemented with 60 g/l sucrose, glyphosate, 1.5 mg/l bialaphos and 100 mg/l cefotaxime and solidified with 3 g/l Gelrite) and incubated at 25° C., in the dark for 2 to 3 weeks. Mature embryos formed during this period are transferred on to regeneration medium II (the same as regeneration medium I with 3 mg/l bialaphos) for germination in the light (25° C., 80-100 μE/m2/s light intensity, 16/8-h photoperiod). Regenerated plants are ready for transfer to soil within 10-14 days. Transgenic lines are screened and transgenic lines having increased plant yield and/or increased seed yield are selected. The vectors provided in the invention can be used for sorghum transformation following a previously described procedure (Zhao, 2006, Plant Material: Plants grown under greenhouse, growth chamber or field conditions are used as an explant source. Immature panicles are harvested 9-12 d post pollination and individual kernels are surface sterilized with 50% bleach for 30 min followed by three washes with sterile distilled water. Explant Isolation, Infection and Co-Cultivation: Immature zygotic embryos (1-1.5 mm) are aseptically dissected from individual kernels and incubated in Callus Selection: Embryos are transferred on to the first selection medium PHI-U (PHI-T medium described above supplemented with 1.5 mg/l 2,4-D, 100 mg/l carbenicillin and 5 mg/l PPT without glucose and acetosyringone) and incubated at 28° C., in the dark for 2 weeks followed by subculture on a selection medium containing 10 mg/l PPT. Proliferating pieces of callus are propagated and maintained by subculture on the same medium every 2 weeks for the remainder of the callus selection process of 10 weeks. Plant Regeneration and Selection: Herbicide-resistant callus is transferred on to regeneration medium I (PHI-U medium supplemented with 0.5 mg/l kinetin) and incubated at 28° C., in the dark for 2 to 3 weeks for callus growth and embryo development. Cultures are transferred on to regeneration medium II (MS basal medium with 0.5 mg/l zeatin, 700 mg/l proline, 60 g/l sucrose and 100 mg/l carbenicillin) for shoot formation (28° C., in the dark). After 2-3 weeks, shoots are transferred on to a rooting medium (regeneration II medium supplemented with 20 g/l sucrose, 0.5 mg/l NAA and 0.5 mg/l IBA) and grown at 25° C., 270 μE/m2/s light intensity with a 16/8-h photoperiod. When the regenerated plants are 8-10 cm tall, they can be transferred to soil and grown under greenhouse conditions. Transgenic lines are screened and transgenic lines having increased plant yield and/or increased seed yield are selected. The vectors pMBXS1023 and/or pMBXS919 provided in the invention can be used for transformation of barley as described by Tingay et al., 1997, Plant J. 11: 1369-1376. For barley transformation, the visual GFP marker described in pMBXS1023 can be changed to a selectable marker that imparts resistance to hygromycin, such as the hygromycin phosphotranferase gene. Plant Material: Plants of the spring cultivar Golden Promise are grown under greenhouse or growth chamber conditions at 18° C. with a 16/8 hours photoperiod. Spikes are harvested when the zygotic embryos are 1.5-2.5 mm in length. Developing caryopses are sterilized with sodium hypochlorite (15% w/v chlorine) for 10 min and rinsed four times with sterile water. Immature zygotic embryos are aseptically dissected from individual kernels and after removal of the embryonic axes are placed scutellum side up on a callus induction medium (Gelrite-solidified MS basal medium containing 30 g/l maltose, 1.0 g/l casein hydrolysate, 0.69 g/l proline and 2.5 mg/L dicamba. Embryos are incubated at 24° C. in the dark during subsequent culture. One day after isolation, the embryos are incubated in After co-cultivation for 2-3 d, embryos are transferred on to the callus induction medium supplemented with 50 mg/L hygromycin, 3 mg/l bialaphos and 150 mg/l Timentin. Cultures are selected for about 2 months with transfers to a fresh selection medium every 2 weeks. Bialaphos and hygromycin-resistant embryogenic callus lines are transferred to a Phytagel-solidified regeneration medium containing 1 mg/l BA, 50 mg/L hygromycin, and 3 mg/l bialaphos for selection of transgenic plants and grown at 24° C. under fluorescent lights with a 16/8 h photoperiod. For root development, regenerated plants are transferred to a hormone-free callus induction medium supplemented with 50 mg/L hygromycin and 1 mg/l bialaphos. After development of a root system, plants are transferred to soil and grown in a greenhouse or a growth chamber under the conditions described above. Transgenic lines are screened and transgenic lines having increased plant yield and/or increased seed yield are selected. The binary vectors provided in the invention can be used for Plant Material: Mature seeds from Culture Transformation and Selection: Dehusked seeds are surface sterilized with 70% ethanol for 1 min and 3% sodium hypochlorite for 30 min followed by six washes with sterile distilled water. Seeds are plated embryo side up on an induction medium (Gelrite-solidified N6 basal medium supplemented with 300 mg/l casamino acids, 2.88 g/l proline, 30 g/l sucrose and 2 mg/l 2,4-D) and incubated at 32° C., under continuous light for 5 d. Germinated seeds with swelling of the scutellum are infected with For selection of transformed embryogenic tissues, whole seedlings washed with 250 mg/l cephotaxine are transferred on to N6 agar-solidified medium containing 300 mg/l casamino acids, 2.88 g/l proline, 30 g/l sucrose, 2 mg/l 2,4-D, 100 mg/l cefotaxime, 100 mg/l vancomycin and 35 mg/l G418 disulfate). Cultures are incubated at 32° C., under continuous light for 2-3 weeks. Plant Regeneration and Selection: Resistant proliferating calluses are transferred on to agar-solidified N6 medium containing 300 mg/l casamino acids, 500 mg/l proline, 30 g/l sucrose, 1 mg/l NAA, 5 mg/l ABA, 2 mg/l kinetin, 100 mg/l cefotaxime, 100 mg/l vancomycin and 20 mg/l G418 disulfate. After one week of growth at 32° C., under continuous light, the surviving calluses are transferred on to MS medium (solidified with 10 g/l agarose) supplemented with 2 g/l casamino acids, 30 g/l sucrose, 30 g/l sorbitol, 0.02 mg/l NAA, 2 mg/l kinetin, 100 mg/l cefotaxime, 100 mg/l vancomycin and 20 mg/l G418 disulfate and incubated under the same conditions for another week followed by a transfer on to the same medium with 7 g/l agarose. After 2 weeks, the emerging shoots are transferred on to Gelrite-solidified MS hormone-free medium containing 30 g/l sucrose and grown under continuous light for 1-2 weeks to promote shoot and root development. When the regenerated plants are 8-10 cm tall, they can be transferred to soil and grown under greenhouse conditions. After about 10-16 weeks, transgenic seeds are harvested. Indica rice varieties are transformed with Following transformation transgenic lines are screened and plants having increased plant yield and/or increased seed yield are selected. For transformation of sugarcane the visual GFP marker described in pMBXS1023 can be changed to a selectable marker appropriate for sugarcane transformation, such as the npt gene. Transformation of sugarcane via biolistics follows a previously described protocol (Taparia et al., 2012 Plant Material: Greenhouse-grown plants with 6-8 visible nodes are used as an explant source. Tops are collected and surface sterilized with 70% ethanol. The outermost leaves are removed under aseptic conditions and immature leaf whorl cross sections (about 2 mm) are cut from the region 1-10 cm above the apical node. Culture Initiation, Transformation and Selection: The isolated leaf sections are cultured on MS basal media supplemented with 20 g/l sucrose, 1.86 mg/l p-chlorophenoxyacetic acid (CPA), 1.86 mg/l NAA and 0.09 mg/l BA at 28° C., under 30 μmol/m2/s light intensity and a 16/8-h photoperiod for 7 d. Embryogenic cultures are subcultured to fresh medium and used for transformation. For microprojectile bombardment, leaf disks are plated on the culture initiation medium supplemented with 0.4 M sorbitol 4 hours before gene transfer. Plasmid DNA (200 ng) containing plasmids pMBXS919 and/or pMBXS1023, modified to contain the appropriate selectable marker gene, is precipitated onto 1.8 mg gold particles (0.6 μm) following a previously described procedure (Altpeter and Sandhu, 2010 For selection, cultures are transferred on to the initiation medium supplemented with 30 mg/l geneticin and incubated for 10 d followed by another selection cycle under the same conditions. Plant Regeneration and Selection: Cultures are transferred on to the selection medium described above without CPA and grown at 28° C., under 100 μmol/m2/s light intensity with a 16/8-h photoperiod. Leaf disks with small shoots (about 0.5 cm) are plated on a hormone-free medium with 30 mg/l geneticin for shoot growth and root development. Prior to transfer to soil, roots of regenerated plants can be dipped into a commercially available root promoting powder. Transgenic lines are screened and transgenic lines having increased plant yield and/or increased sugar production are selected. The gene constructs provided in the invention can be used for wheat transformation by microprojectile bombardment following a previously described protocol (Weeks et al., 1993 Plant Material: Plants from the spring wheat cultivar Bobwhite are grown at 18-20° C. day and 14-16° C. night temperatures under a 16 h photoperiod. Spikes are collected 10-12 weeks after sowing (12-16 days post anthesis). Individual caryopses at the early-medium milk stage are sterilized with 70% ethanol for 5 min and 20% sodium hypochlorite for 15 min followed by three washes with sterile water. Culture Initiation, Transformation and Selection: Immature zygotic embryos (0.5-1.5 mm) are dissected under aseptic conditions, placed scutellum side up on a culture induction medium (Phytagel-solidified MS medium containing 20 g/l sucrose and 1.5 mg/l 2,4-D) and incubated at 27° C., in the light (43 μmol/m2/s) for 3-5 d. For microprojectile bombardment, embryo-derived calluses are plated on the culture initiation medium supplemented with 0.4 M sorbitol 4 hours before gene transfer. Plasmid DNA containing pMBXS919 and/or pMBXS1023 and the marker gene bar and hpt is precipitated onto 0.6-μm gold particles and delivered to the explants as described for sugarcane. The bombarded expants are transferred to callus selection medium (the culture initiation medium described above containing 1-2 mg/l bialaphos and 25 mg/L hygromycin and subcultured every 2 weeks. Plant Regeneration and Selection: After one-two selection cycles, cultures are transferred on to MS regeneration medium supplemented with 25 mg/L hygromycin, and 2 mg/l bialaphos. For root formation, the resulting antibiotic and herbicide-resistant shoots are transferred to hormone-free half-strength MS medium. Plants with well-developed roots are transferred to soil and acclimated to lower humidity at 21° C. with a 16-h photoperiod (300 μmol/m2/s) for about 2 weeks prior to transfer to a greenhouse. Transgenic lines are screened and transgenic lines having increased plant yield and/or increased seed yield are selected. For transformation of soybean, the visual GFP marker described in pMBXS1023 can be changed to a selectable marker appropriate for soybean transformation, such as a gene imparting resistance to hygromycin. Plant Material: Immature seeds from soybean plants grown under greenhouse or field conditions are used as an explant source. Young pods are harvested and surface sterilized with 70% 2-propanol for 30 sec and 25% Clorox for 20 min followed by three washes with sterile distilled water. Culture Transformation and Selection: Under aseptic conditions, immature seeds are removed from the pods and the cotyledons are separated from the seed coat followed by incubation in For selection of transformed tissues, cotyledons washed with 500 mg/l cephotaxine are placed abaxial side up on a medium for induction of somatic embryo formation (Gelrite-solidified MS medium medium containing 30 g/l sucrose, 40 mg/l 2,4-D, 500 mg/l cefotaxime, 3 mg/L glufosinate and 10 mg/l hygromycin) and incubated at 25° C., under a 23-h photoperiod (10-20 μE/m2/s) for 2 weeks. After another two weeks of growth under the same conditions in the presence of 6 mg/L glufosinate and 25 mg/l hygromycin, the antibiotic-resistant somatic embryos are transferred on MS medium for embryo maturation supplemented with 60 g/l maltose, 500 mg/l cefotaxime, 3 mg/L glufosinate, and 10 mg/l hygromycin and grown under the same conditions for 8 weeks with 2-week subculture intervals. Plant Regeneration and Selection: The resulting cotyledonary stage embryos are desiccated at 25° C., under a 23-h photoperiod (60-80 μE/m2/s) for 5-7 d followed by culture on MS regeneration medium containing 30 g/l sucrose and 500 mg/l cefotaxime for 4-6 weeks for shoot and root development. When the plants are 5-10 cm tall, they are transferred to soil and grown in a greenhouse after acclimatization for 7 d. Transgenic lines are screened and plants having increased plant yield or seed yield and/or oil content are selected. Microprojectile bombardment-mediated transformation of soybean The genes in constructs pMBSX919 or pMBXS1023 can be co-bombarded with hygromycin resistance gene via biolitics into embryogenic cultures of soybean to obtain transgenic plants. The transformation, selection, and plant regeneration protocols were described previously (Santarëm E R, J J Finer, 1999. In Vitro Cellular and Developmental Biology—Plant 35:451-455.) Plant Material: Immature zygotic embryos are isolated from developing pods from plants grown under greenhouse conditions or field. The cotyledons are excised and plated on MS salts and B5 vitamins, 6% sucrose, 40 mg/l 2,4-D, (pH 7.0) and 0.2% Gelrite for 3-4 weeks at 27° C. under a 16-h photoperiod (30 μE/m2/s) to induce somatic embryos. Transformation and Selection: Bright green, globular, proliferative embryos are transferred to MS salts and B5 vitamins, 3% sucrose, 5 mM asparagine, 20 mg/l 2,4-D (pH 5.7) and 0.2% Gelrite and are subcultured every 2-3 weeks. Embryogenic tissues are subcultured 3-5 days prior to bombardment on the same media. For bombardment, clusters of embryogenic tissues are placed in the center of 90 mm Petri dishes containing the media and co-bombarded, using bombardment apparatus, with a fragment containing genes in constructs pMBSX919 or pMBXS1023 and a fragment with the hygomycin gene precipitated on gold particles. For selection, after one week all tissues are transferred to MS salts with B5 vitamins, 3% sucrose, 5 mM asparagine, 20 mg/l 2,4-D, 15 mg/L Hygromycin (pH 5.7) and 0.2% Gelrite for 3-4 weeks. All resistant tissues are selected and transferred to fresh media until embryos are cream-colored and ready for desiccation. Embryo Maturation and Germination: Clones are regenerated by first placing embryos on MS salts with B5 vitamins, 6% Maltose (pH 5.7), 0.2% Gelrite and 0.5% activated charcoal for 3-4 weeks. Embryos are desiccated in a dry Petri dish sealed with parafilm and placed on the shelf for 2-5 days and germinated on growth regulator-free MS medium. Plants are transferred to soil after optimum root and shoot formation. The teachings of all patents, published applications and references cited herein are incorporated by reference in their entirety. While this invention has been particularly shown and described with references to example embodiments thereof, it will be understood by those skilled in the art that various changes in form and details may be made therein without departing from the scope of the invention encompassed by the appended claims. Transgenic plants having enhanced yield and having enhanced seed yield are disclosed. The transgenic plants are transformed with a transgenic polynucleotide encoding one or more metabolic enzymes. The metabolic enzymes can be from any biological source. The transgenic polynucleotide(s) comprises a nucleic acid sequences encoding the metabolic enzymes under the control of functional plant promoters, the one or more metabolic enzymes are targeted to the plastids by the addition of plastid targeting signals. Optionally the functional plant promoters are seed specific promoters and the metabolic enzymes are targeted to the plastids by the addition of plastid targeting peptide heterologous to the metabolic enzymes. Methods of making the transgenic plants and transgenic polynucleotides are disclosed. The magnitude of the increases in seed yield achieved with these transgenic plants are simply unprecedented. 1. A transgenic plant comprising a one or more heterologous enzymes, each heterologous enzyme encoded by a transgene,
wherein the one or more heterologous enzymes are selected from the group consisting of: an oxygen tolerant pyruvate oxidoreductase (Por), a pyruvate carboxylase (Pyc), a malate synthase (AceB), a malate dehydrogenase (Mdh), a malate thiokinase (SucC and SucD), a malyl-CoA Lyase (Mcl), isocitrate lyase (Id), fumarate hydratase (FumC), NADH-dependent fumarate reductase (FRDg), aconitase hydratase 1 (AcnA), ATP-citrate lyase A-1 (AclA), and ATP-citrate lyase subunit B2 (AclB), wherein the transgenic plant has one or more increased properties selected from yield, seed yield, and seed oil content compared to a plant of the same species not comprising the one or more heterologous enzymes. 2. The transgenic plant of an oxygen tolerant pyruvate oxidoreductase (Por), pyruvate carboxylase (Pyc), malate synthase (AceB), malate dehydrogenase (Mdh), malate thiokinase (SucC and SucD), malyl-CoA Lyase (Mcl), and isocitrate lyase (Id). 3. The transgenic plant according to a malate dehydrogenase (Mdh), a fumarate hydratase (FumC), an NADH-dependent fumarate reductase (FRDg), an aconitase hydratase 1 (AcnA), an ATP-citrate lyase A-1 (AclA), and an ATP-citrate lyase subunit B2 (AclB). 4. The transgenic plant of 5. The transgenic plant according to 6. The transgenic plant according to 7. A plant, genetically modified to increase the expression and/or enzyme activity of a NADP-malate dehydrogenase (NADP-Mdh) enzyme in the plastids where the enzyme is normally expressed, wherein the genetically modified plant has one or more increased properties selected from yield, seed yield, and seed oil content compared to a plant of the same species that was not genetically modified. 8. The plant of 9. The plant of 10. The plant of 11. A plant of 12. A plant of 13. A plant of any one of 14. A plant of any one of 15-18. (canceled) 19. A plant of 20. A method for making and selecting a plant having an increase in at least one property selected from yield, seed yield, or seed oil content compared a wild type plant, the method comprising:
providing one or more plants according to growing the one or more plants in soil; measuring the property of the one or more plants; and selecting the one or more plants that have an increase the increased property compared to a wild type control plant of the same species. 21-28. (canceled) 29. The transgenic plant according to 30. The transgenic plant of 31. The transgenic plant of 32. The transgenic plant of 33. (canceled) 34. The transgenic plant of 35. A transgenic plant of RELATED APPLICATION(S)
GOVERNMENT SUPPORT
BACKGROUND OF THE INVENTION
SUMMARY OF THE INVENTION
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION OF THE INVENTION
Definitions
Plants and Plant Species Suitable for Practising the Disclosed Invention:
Metabolic Enzymes and Genes Useful for Practising the Invention EC Enzyme number Gene Source Accession Alternate genes Pyruvate EC por Y09702 oxidoreductase 1.2.7.1 amino acid homology to POR WP_012612979, 67% amino acid homology homology to POR AEI34679.1, 53% amino acid homology homology to POR Malate EC sucC WP_010960994 thiokinase1 6.2.1.9 76% amino acid homology to sucC WP_009708795.1, 60% amino acid homology to sucC Malate EC sucD WP_010960995.1 thiokinase1 6.2.1.9 WP_035525486.1, 60% amino acid homology to sucD WP_031597727.1, 60% amino acid homology to sucD Malyl-CoA EC mcl WP_011336971.1 Lyase 4.1.3.24 WP_037207860.1, 91% amino acid homology to mcl WP_008227028.1, 89% homology to Isocitrate lyase EC iclA WP_013957076.1 4.1.3.1 WP_022539987.1, 99% amino acid homology to iclA from MSMB22, 97% amino acid homology to iclA from Pyruvate EC pyc WP_003244778 carboxylase 6.4.1.1 98% amino acid homology homology to WP_041084951.1, 84% amino acid homology homology to pyc Malate synthase EC aceB EGI07962.1 2.3.3.9 WP_039061102.1, 99% amino acid homology to WP_032940912.1, 92% amino acid homology to Malate EC mdh EFJ62433.1 dehydrogenase 1.1.1.37 WP_039060497.1, 99% amino (NAD specific) acid homology to WP_012908482, 96% amino acid homology to Malate EC MDH5 XP_001696786.1 dehydrogenase 1.1.1.37 ABY61960.1, 76% homology to (NADP specific) homology to O48902.1 Fumarate EC fumC WP_032187409 hydratase class 4.2.1.2 WP_001099068.1, 99% homology II to CDZ83504.1, 94% homology to fumC Fumarate EC FRDg AAN40014.1 reductase 1.3.1.6 84% homology to FRDg of EPY23130.1, 66% homology to FRDg Aconitate EC acnA WP_045149543 hydratase 4.2.1.3 WP_000099511.1, 99% homology to CDZ83277.1, 93% homology to acnA ATP-citrate EC aclA-1 NP_172537.1 lyase 2.3.3.8 homology to CDY70177.1, 95% homology to aclA-1 ATP-citrate EC aclB-2 NP_199757 lyase 2.3.3.8 CDY00032.1, 99% homology to aclB-2 homology to 1The Methods for Producing Transgenic Plants
Methods and Transgenic Plants, Plant Tissue, Seed and Plant Cell of the Invention
Selectable Markers
Plastid Targeting Signals
Herbicide Resistance and Insect Tolerance
EXAMPLES
Example 1. Construction of Metabolic Enzyme Expression Vectors pMBXS918, pMBXS919, pMBXS994, pMBXS1022, pMBXS1023, and pMBXS1024
Summary of constructs for transformation into Camelina.1 Enzyme gene pMBXS918 pMBXS919 pMBXS994 pMBXS1022 pMBXS1023 pMBXS1024 Malate dehydrogenase (NADH) mdh ✓ Malate dehydrogenase (NADPH) Mdh5 ✓ Fumarate hydratase fumC ✓ ✓ Fumarate reductase FRDg ✓ ✓ Aconitase acnA ✓ ✓ ATP-citrate lyase subunit aclA-1 ✓ ✓ ATP citrate lyase subunit aclB-2 ✓ ✓ Pyruvate oxidoreductase Por ✓ ✓ ✓ Succinyl-CoA synthetase subunit sucC ✓ ✓ ✓ ✓ Succinyl-CoA synthetase subunit sucD ✓ ✓ ✓ ✓ Malyl-CoA lyase mcl ✓ ✓ ✓ ✓ isocitrate lyase iclA ✓ ✓ ✓ ✓ Pyruvate carboxylase pyc ✓ ✓ ✓ ✓ Malate synthase aceB ✓ ✓ ✓ Phosphinothricin acetyl transferase bar ✓ ✓ Green fluorescent protein GFP (with 355 ✓ ✓ ✓ ✓ promoter) GFP (with ✓ ✓ ✓ ) 1Co-expression of indicates data missing or illegible when filed Example 2. Generation of
Example 3. Screening of Transgenic Plants and Identification of Plants with Higher Yield
T2 Seed yield in lines of Camelina transformed with one genetic construct to enhance yield. % compared Transformed seed yield to vector Line Plasmids (g) control Wild-type1 3.02 ± 1.36 87% JS112 pMBXS0123 3.49 ± 1.30 100% 14-1721 pMBXS918 3.60 103% 14-1722 pMBXS918 2.94 84% 14-1645 pMBXS919 7.20 206% 14-1646 pMBXS919 3.23 92% 14-1686 pMBXS919 4.47 128% 14-1621 pMBXS994 6.42 184% 14-1635 pMBXS1022 6.36 182% 14-1636 pMBXS1022 6.72 192% 1Wild-type seed yield values are an average of 5 plants. 2JS11 seed yield values are an average of 18 plants. 3vectror control containing the bar gene. Example 4. Alternate Combinations of Metabolic Enzymes to Increase Seed Yield Obtained by Co-Transformation of Plasmids
T2 seed yield in co-transformed lines of Camelina. % compared Transformed seed yield to vector Line Plasmids (g) control Wild-type1 3.02 ± 1.36 87% JS112 pMBXO123 3.49 ± 1.30 100% 14-1685 pMBXS919/ 7.70 220% pMBXS994 14-1724 pMBXS919/ 3.79 108% pMBXS1022 14-1704 pMBXS919/ 2.93 84% pMBXS1023 14-1749 pMBXS918/ 4.26 122% pMBXS1024 14-1745 pMBXS919/ 3.00 86% pMBXS1024 1Wild-type seed yield values are an average of 5 plants. 2JS11 seed yield values are an average of 18 plants. 3vectror control containing the bar and gfp gene T3 seed yield in co-transformed lines of Camelina. % compared seed yield to Gene- Parental Transformed (g) per vector Line ration line Plasmids plant control JS11 (bar T3 plants — pMBXO12 4.94 ± 0.94 100% containing producing vector T4 seed control)1 15-0382 T2 plants 14-1704 pMBXS919, 12.90 261% producing pMBXS1023 T3 seed 15-0383 3.88 79% 15-0384 6.23 126% 15-0385 3.72 75% 15-0386 10.25 207% 15-0387 3.28 66% 15-0388 4.00 81% 15-0389 7.97 161% Average value of 8 plants from this event = 6.53 ± 3.55 15-0390 T2 plants 14-1724 pMBXS919, 3.04 62% producing pMBXS1022 T3 seed 15-0391 3.21 65% 15-0392 3.82 77% 15-0393 3.97 80% 15-0394 5.06 102% 15-0395 5.64 114% 15-0396 4.77 97% 15-0397 5.32 108% Average value of 8 plants from this event = 4.35 ± 0.98 15-0398 T2 plants 14-1905 pMBXS919, 10.33 209% producing pMBXS1022 T3 seed 15-0399 3.95 80% 15-0400 8.25 167% 15-0401 3.94 80% 15-0402 8.2 166% 15-0403 6.55 133% 15-0404 3.79 77% 15-0405 8.72 177% Average value of 8 plants from this event = 6.72 ± 2.55 15-0406 T2 plants 14-1745 pMBXS919, 13.63 276% producing pMBXS1024 T3 seed 15-0407 2.32 47% 15-0408 6.37 129% 15-0409 5.05 102% 15-0410 7.15 145% 15-0411 1.85 37% 15-0412 3.87 78% 15-0413 5.98 121% Average value of 8 plants from this event = 5.78 ± 3.69 1JS11 seed yield values are an average of 7 plants. Average 100 T3 seed weight produced from highest yielding T2 plants from cotransformed lines T3seed yield of highest Average seed % Calculated total # % producing weight of 100 compared of seeds produced compared Plant ID Constructs T2plant (g) seeds (mg)1 to control per plant to control 15-0417_JS11 pMBXO122 5.97 106.64 ± 2.71 100 5598 100 15-0406_LX03 pMBXS919/ 13.63 141.53 ± 3.57 132 9631 172 pMBXS1024 15-0398_LG08 pMBXS919/ 10.33 139.11 ± 2.69 130 7426 133 pMBXS1022 15-0382_LU03 pMBXS919/ 12.9 136.88 ± 1.14 128 9424 168 pMBXS1023 13 replicates of 100 seeds were weighed from a plant that produced highest seed yield in that line. 2bar and gfp gene containing vector control. Average seed length and width of seed harvested from select lines co-transformed with plasmids to increase yield1 Seed length % compared % compared Plant ID Constructs (mm) to control Seed width to control 15-0417_JS11 pMBXO122 1.86 ± 0.15 100 1.04 ± 0.12 100 15-0406_LX03 pMBXS919/ 2.04 ± 0.09 110 1.19 ± 0.11 114 pMBXS1024 15-0398_LG08 pMBXS919/ 2.14 ± 0.14 115 1.08 ± 0.12 104 pMBXS1022 15-0382_LU03 pMBXS919/ 2.10 ± 0.11 113 1.07 ± 0.09 103 pMBXS1023 1Each data set is the average and standard deviation of approximately 100 seeds. Image J software was used to calculate seed size. 2bar gene containing vector control. Example 5. Oil and Protein Content of Seed Harvested from Co-Transformed Lines
Protein content in seed harvested from the highest oil producing co-transformed lines of Camelina. Oil content Protein Parental Transformed (% seed content (% Line Generation line Plasmids weight) seed weight) JS111 T3 plants — pMBXO12 28.6 ± 1.4 33.8 ± 0.6% producing T4 seed 15-0382 T2 plants 14-1704 pMBXS919, 44.5 34.6 producing pMBXS1023 T3 seed 15-0386 45.3 34.0 15-0389 45.3 34.0 15-0398 T2 plants 14-1905 pMBXS919, 34.3 35.7 producing pMBXS1022 T3 seed 15-0400 37.9 33.5 15-0405 37.5 31.6 15-0406 T2 plants 14-1745 pMBXS919, 35.1 34.8 producing pMBXS1024 T3 seed 15-0410 37.2 34.0 15-0412 39.3 33.9 1bar containing vector control Example 6. Minimum Gene Sets Encoding Metabolic Enzymes to Increase Seed Yield
Alternate combinations of metabolic enzymes to increase seed yield Transgenes Enzymes Result of combined reactions por and pyc Pyruvate oxidoreductase and Conversion of acetyl-CoA to oxaloacetate pyruvate carboxylase with the fixation of 1 molecule of HCO3− and 1 molecule of CO2 por Pyruvate oxidoreductase Conversion of acetyl-CoA to pyruvate with the fixation of 1 molecule of CO2 pyc pyruvate carboxylase Conversion of pyruvate to oxaloacetate with the fixation of 1 molecule of HCO3− por, pyc, sucC, sucD, and Pyruvate oxidoreductase, Cycle that fixes 1 molecule of HCO3− and 1 mcl in combination with pyruvate carboxylase, malate molecule of CO2and produces glyoxylate endogenous malate thiokinase, malyl-CoA lyase, dehydrogenase activity endogenous malate dehydrogenase activity por, pyc, aceB in Pyruvate oxidoreductase, Cycle that fixes 1 molecule of HCO3− and 1 combination with pyruvate carboxylase, malate molecule of CO2and produces glyoxylate endogenous malate synthase, endogenous malate dehydrogenase activity dehydrogenase activity pyc, sucC, sucD, and mcl Pyruvate carboxylase, malate Conversion of pyruvate to acetyl-CoA and in combination with thiokinase, malyl-CoA lyase, glyoxylate with fixation of 1 molecule of endogenous malate endogenous malate HCO3− dehydrogenase activity dehydrogenase activity pyc and aceB in Pyruvate carboxylase, malate Conversion of pyruvate to acetyl-CoA and combination with synthase, and endogenous glyoxylate with fixation of 1 molecule of endogenous malate malate dehydrogenase activity HCO3− dehydrogenase activity MDH5 NADP specific malate Inter-conversion of oxaloacetate and malate, dehydrogenase balance of redox aclA-1, aclB-2, MDH5, ATP citrate lyase, malate Conversion of citrate to acetyl-CoA and fumC, FRDg dehydrogenase, fumarate succinate hydratase, fumarate reductase MDH5, fumC, FRDg malate dehydrogenase, fumarate Conversion of oxaloacetate to succinate hydratase, fumarate reductase fumC, FRDg fumarate hydratase, fumarate Conversion of malate to succinate reductase Transformation constructs for delivering enhanced yield expressing a reduced number of transgenes. Enzyme gene pMBXS1056 pMBXS1057 pMBXS1058 pMBXS1059 pMBXS1060 Malate dehydrogenase (NADH) mdh Malate dehydrogenase (NADPH) Mdh5 ✓ ✓ Fumarate hydratase fumC Fumarate reductase FRDg Aconitase acnA ATP-citrate lyase subunit aclA-1 ATP citrate lyase subunit aclB-2 Pyruvate oxidoreductase Por ✓ ✓ ✓ ✓ ✓ Succinyl-CoA synthetase subunit sucC ✓ ✓ Succinyl-CoA synthetase subunit sucD ✓ ✓ Malyl-CoA lyase mcl ✓ ✓ Isocitrate lyase iclA Pyruvate carboxylase pyc ✓ ✓ ✓ ✓ ✓ Malate synthase aceB ✓ ✓ Phosphinothricin acetyl transferase bar Green fluorescent protein GFP (with 355 ✓ ✓ ✓ ✓ ✓ promoter) GFP (with ) ✓ ✓ ✓ ✓ ✓ Co-expression of indicates data missing or illegible when filed Example 7. Co-Expression of Yield Enhancing Genes with a Nucleotide Sequence Encoding a Bicarbonate Transporter Operably Linked to a Seed Specific Promoter
[CO2+H2O←→HCO3−+H+]Example 8. Enhancing Yield in Other Crops
Transformation of Explant Isolation, Infection and Co-Cultivation:
Callus Selection:
Plant Regeneration and Selection:
Microprojectile Bombardment-Mediated Transformation of Sugarcane
Transformation of Wheat by Microprojectile Bombardment