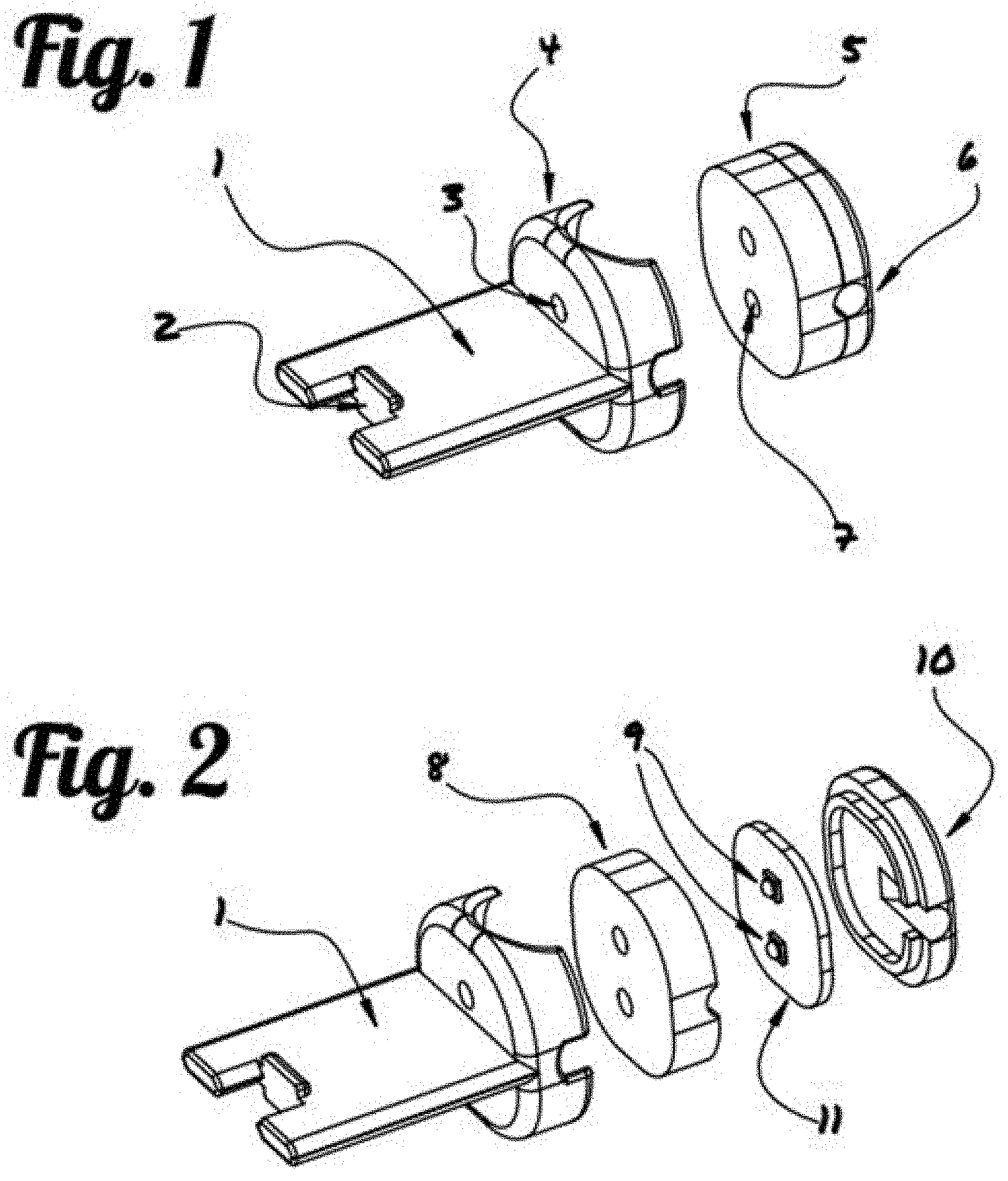
Photoplethysmography Imaging (PPGI)-Based Pulp Vitality Test
This application claims priority to U.S. Provisional Application 62/878,087, filed on Jul. 24, 2019. This invention is directed to a photoplethysmography imaging (PPGI)-based pulp vitality testing system, and the method using said system to assess the distribution of perfused endodontic tissues and evaluating the health of the pulp. Endodontic maladies, which affect the pulp of the tooth, can be particularly difficult to diagnose. A review of U.S. Marine Corps dental records between 2003 and 2006 found nearly 20% of in-theater dental emergencies were classified as endodontic, and approximately 49% of those emergencies were not predicted in previous oral examinations [2]. The development of an improved diagnostic test to assess the status of the pulp could enable dentists to better predict tooth health and inform treatment decisions prior to deployment, thereby reducing costs incurred as a result of endodontic dental emergencies. Vitality testing is an important aid in the diagnosis of pulp disease and apical periodontitis. Thermal and electric pulp sensibility tests, the current gold standard, have been used to indirectly determine the state of pulpal health by assessing the condition of the nerves within the dental pulp. Although these traditional tests offer high sensitivity, the response does not provide any information about the state of the pulp tissue, often generating false positive or negative results. To resolve this problem, radiographs are often administered in conjunction with sensibility tests. However, radiographs only provide images of the denser enamel, and dentin regions of the tooth, showing defects in the hard-tissue surrounding the pulp, but providing limited diagnostic utility for assessing the state of the soft pulp tissue. Additionally, radiographs are costly, often requiring specialized equipment, and dedicated resources to operate and maintain. Recent research efforts have explored the use of Laser Doppler flowmetry (LDF) and pulse oximetry to directly assess pulp vitality. LDF, a microvascular blood perfusion assessment technique, measures slight shifts in the wavelengths of incident, and reflected light scattered by moving red blood cells. Studies have shown that LDF laser light can be transmitted through tooth enamel and dentin, and detect blood flow within the pulp vasculature. However, the technique is susceptible to noise contamination from light reflected off periodontal tissues, and any movement of the LDF sensor relative to the tooth also changes the measured wavelength shift, producing an artifact in the measurement. Additionally, LDF is highly susceptible to external sources of error, such as interference of the blood flow in the gingiva, and obstruction of the light pathway. Pulse oximetry, which is ubiquitous in the clinical setting, uses a similar approach to LDF, but is relatively less expensive and provides a more stable measurement of regional perfusion. Pulse oximetry measures differences between the intensity of light absorbed by perfused tissue, at multiple wavelengths, to derive blood oxygenation. The amount of light blood absorbed at certain visible-red and near-infrared wavelengths fluctuates with the pulse as the proportion of oxygen-rich arterial blood changes. Unlike LDF, the light source and the detector can be positioned on the opposing sides of the perfused tissue, and by measuring light at only the specific wavelengths of interest, pulse oximetry can provide a more robust measurement less affected by sensor motion. A photodetector identifies absorbance peaks caused by pulsatile blood circulation, and thereby calculates the pulse rate and oxygen saturation level. Recent studies have shown that modified, commercially-available, finger pulse oximeters can measure blood oxygenation within the pulp vasculature, and can differentiate between vital and root-filled teeth. However, standard pulse oximeters are susceptible to noise contamination from external sources, including blood flow in periodontal tissues, such as the gingiva. Camera-based tools have recently been investigated to image blood oxygenation in soft tissues, including vasculature in the retina, and regions of ischemic tissue in diabetic wounds. It is the goal of this invention to develop a photoplethysmography imaging based diagnostic tool for dental applications, in particular, imaging the distribution of perfused pulpal tissue, and assessing pulp vitality. This assessment could allow dental professionals to draw important conclusions about dental tissue status, including vitality and the presence of a range of endodontic maladies. In photoplethysmography imaging (PPGI)-based pulp vitality system of this invention, the image capturing device serves as an array of individual sensors to provide a spatially resolute map of tissue perfusion. The 2-dimensional spatial array allows the test to differentiate regions of interest from external sources of error. Each pixel of the digitized image serves as a discrete signal at a discrete location, together providing a spatial representation of blood oxygenation of perfused pulpal tissues. Light passing through perfused tissues generates a pulsatile signal, while light passing through non-perfused tissues remains static. By analyzing the strength of the pulsatile signals at each pixel, blood-perfused regions of tissue can be spatially differentiated from those that do not receive blood flow, allowing direct assessment of the pulpal tissue. A clearer understanding of the present invention can be achieved by studying the following detailed description of the preferred exemplary embodiment together with the drawings in which: “Transilluminate” is referred to as allowing light to pass through a sample or part of the body. It is used in this case for the tooth, and parts of the tooth such as dentin, enamel, and the pulpal tissue. A novel PPGI-pulp vitality testing system of this invention is developed to generate a distribution map of perfused pulpal tissues, and thereby determine the vitality of a dental pulp ( Illustration of the imaging hardware is shown in The light source (5) is designed to transilluminate the tooth. This light source can be set to emit light within the visible or near-infrared spectrum. In one embodiment as shown in A bitewing (1), is used to stabilize and align the light source (5) with the image capturing device (12), such as the intraoral camera (not shown in During operation as shown in The light traveled through the tooth is then captured and measured by the image capturing device (12), which is operationally connected to a computing device (15). In one embodiment, the computing device (15) is equipped with customized PPG image processing software designed to provide near real-time PPG image analysis of captured sequential images (video) by integrating spatial decomposition, temporal filtration, amplification, and mapping through a single graphical user interface. The spatial decomposition serves to reduce spatial noise, and shrink the images in the spatial domain to reduce subsequent image processing times. Temporal bandpass filtering is then done in the time domain at each discrete spatial location within the images to filter out noise, and isolate pulsatile components within the frequency range of a typical heartbeat. The PPGI system may also incorporate a heartrate monitor that to adaptively adjust the temporal bandpass filter cutoff frequencies based on the heartrate of the patient. Once the pulsatile component of the signal is isolated, it is then amplified, and mapped so that spatial regions with greater pulsatility can be differentiated from regions with less pulsatility. The spatiotemporal filtering and amplification parameters are optimized to maximize the contrast between images collected during the systolic and diastolic portions of the cardiac cycle. In an additional embodiment, the image processing software may further include the addition of an automatic pulp chamber selection tool for increased accuracy. Visual information provided by the tooth scan will also be investigated in further studies to determine the relationship of changes in pulse distribution with various pulpal conditions and tested with machine learning algorithms. In one embodiment, after the generation of a distribution map of pulpal tissue of the tooth, the dental clinician will use the Region of Interest (ROI) tool to highlight the parts of the tooth with the most movement. After choosing the RO, a pulse rate will be generated to assist the dental clinician or operator in making an assessment based on this direct measurement of vitality. Compared to the current dental pulp vitality testing technologies, the PPGI-pulp vitality test technology provides a direct, cost-effective, radiation-free, non-invasive, and reliable diagnostic test of tissue oxygenation within a dental pulp. Pulp sensibility tests are the current gold-standard for assessing pulp vitality. The test assessments are indirect and subjective in nature, often generating false positive or negative results. Radiographs only provide images of the denser enamel and dentin regions of the tooth without any diagnostic utility for assessing the state of the pulp tissue. They also produce harmful ionizing radiation, often requiring specialized infrastructure and dedicated resources to operate and maintain. Thus the PPGI-based pulp vitality test would provide a valuable diagnostic test capable of directly assessing the state of the pulp. The imaging system is expected, not only to identify the presence of blood flow in the pulp, but also to identify the regional disruptions or anastomosis caused by trauma or indicative of underlying pathology. The technology would be a low-cost, accessible, non-invasive, and provide rapid diagnostics in a small package that could be implemented in a clinic or a field-setting. The technique could improve upon the accuracy and reliability of current pulp vitality tests, to more readily identify pulp pathology, and reduce the number of potentially preventable endodontic dental emergencies occurring in-theater. Imaging hardware and optics will be configured to measure light transmitted through the perfused tooth at discrete wavelengths in the visible-red and near-infrared spectrum. Image analysis software will be developed to process the images, and extract physiological characteristics associated with the pulse. The hardware configuration and imaging parameters will be optimized to maximize signal quality and resolution. A printed circuit board (PCB) located inside the bitewing was designed for production to operate the LED light source. Ex-vivo tooth model: A circulation system is designed to simulate pulp hemodynamics and oxygenation by pumping blood through the pulp chamber of extracted human molars. An oxygenator, which precisely controls blood oxygen saturation by promoting gas exchange between blood and a controlled mixture of oxygen, nitrogen, and carbon dioxide, will be used to condition reservoirs of blood representing both arterial and venous oxygenation saturation levels. Blood oxygen saturation in the arterial and venous reservoirs will be measured using blood gas analysis to confirm target the oxygenation. A dual dispensing pump will be used to alternate the flow of arterial and venous blood into the pulp to simulate changes in regional blood oxygenation during the cardiac cycle. An algorithm was developed to control the rates and volumes of the dual syringe pump, camera video acquisition, and gas flow during validation testing. The dual syringe pump control was used to draw, dispense, and circulate conditioned blood. The camera controls were used to record video from the camera and save the data in an audio video interleave (AVI) format for analysis using the PABLO algorithm. The gas controls were used before each experiment to introduce concentration of gases to the blood to condition it to appropriate arterial and venous oxygenation levels. Materials and Methods: The PABLO algorithm is used to analyze the PPGI signals to determine dental pulp pulse frequency. Both the classification of a tooth as vital or nonvital and the pulse rate determination were made by quantifying the fluctuation of light intensity transmitted through the tooth during the cardiac cycle. A step-by-step demonstration is shown in Results and Discussion: Results of the analysis (see Future developments will incorporate machine learning algorithm to automatically determine ROI. The imaging system will be optimized by testing multiple camera-light configurations, and using wavelengths of interest throughout the visible-red and near-infrared spectrum, to maximize the strength and resolution of the measured signals. Simulations will be run with the teeth illuminated from either or both lingual and occlusal surfaces. Likewise, camera-position will be tested facing the buccal or occlusal surfaces. In each configuration, a series of wavelengths will be evaluated to identify those which exhibit the greatest signal strength, maximizing the contrast between images captured during the systolic and diastolic portion of the pulsatile waveform. During the initial testing, low venous oxygen saturations will be used to maximize the contrast between arterial and venous light absorption and serve as proof-of-principal for the technique. If visualization of pulp blood flow cannot be achieved in the maximum contrast condition, additional testing will not be perused. However, if the initial proof-of-principal testing is successful, the venous oxygen saturation will be elevated to physiologically normal levels for further system optimization and testing. Future development efforts will also focus on further optimizing the image processing software to visualize the distribution of blood flow in real-time. The imaging technique will be evaluated across a range of different tooth morphologies in vivo and compared clinically against the predictive value of the gold-standard pulp sensibility tests. A long-term goal would be to use camera-based oximetry to monitor pulp disease progression over time, and identify metrics that could be used to identify the onset of underlying pathology. This novel technique could potentially provide valuable diagnostic information, enabling dentists to better assess the health of the pulp and guide endodontic treatment decisions. This invention discloses a vitality-based photoplethysmography imaging (PPGI) system capable of determining the pulse frequency within the dental pulp, allowing for direct, accurate, real-time visualization of tooth vitality. The system comprises a transilluminating bitewing, capable of aligning and stabilizing a light source and an intraoral camera, which is operationally connected to a computing device that is equipped with video stabilization and digital signal processing algorithm (Pulp Assessment by Local Observation, i.e. PABLO) to assess pulp vitality via analyzing videos captured with the intraoral camera. 1) A system for assessing vitality of a tooth and measuring oxygenation and blood flow in pulpal tissue of said tooth comprising:
(a) a light source capable of emitting light that transilluminate said tooth; (b) a bitewing; (c) an image capturing device capable of detecting a plurality of sequential images of light transmitted through said tooth, wherein said image capturing device is hold in an aligned position with said light source on opposite sides of said tooth when a patient bite onto said bitewing; (d) a computing device, which is operatively connected to said image capturing device and is equipped with an image processing software; wherein said plurality of sequential image of said tooth are received and processed by said computing device to generate an distribution map of said tooth. 2) The system of 3) The system of 4) The system of 5) The system of 6) The system of 7) The system of 8) The system of 9) The system of 10) The system of 11) The system of 12) The system of 13) The system of 14) A method for real-time visualization and analysis of the vitality of the tooth, comprising:
a) Emitting light onto a surface of a tooth; b) Capturing light passed through said tooth on the opposite surface of said tooth over a period of time to create a plurality of sequential images; c) Processing said plurality of sequential image frames at each pixel to generate an distribution map of perfused pulpal tissues of said tooth, wherein a pulsed signal at a pixel of said sequential image frames represent perfused tissue, and static signal at said pixel represent non-perfused tissue; and d) Assessing pulp vitality by analyzing displayed oxygenation distribution map of perfused pulpal tissues of said tooth. 15) The method of 16) The method of 17) A method of 18) An apparatus for taking a plurality of sequential images of a tooth for generating a distribution map of perfused tissues of a tooth, comprising a light source housing and a bitewing with a bite surface, a distal end and a proximal end, wherein said bitewing has a housing receiver in the distal end to receive a light source housing and a groove on periphery of the proximal end to position and hold an image capturing device; such that an one or more openings on the light source housing aligns with corresponding openings on the light source housing receiver to allow the passage of light from said light source housing through said tooth to said image capturing device.CROSS-REFERENCE TO RELATED APPLICATIONS
TECHNICAL FIELD
BACKGROUND
SUMMARY OF INVENTION
DESCRIPTION OF FIGURES
DETAILED DESCRIPTION OF THE INVENTION
Definition
Example 1: Construction of Prototype PPGI Based Pulp Vitality System
Example 2: Testing of the PPGI Based Pulp Vitality System Using an Ex Vivo Perfused Tooth Model
Results of the PABLO analysis. Simulated Adjacent Teeth Video Control Gumline Interference Stabilization Sensitivity 93.0% 90.0% 90.4% 100.0% Specificity 100.0% 79.4% 94.4% 100.0% SMAPE 7.3% 11.0% 9.6% 6.3%
The sensitivity, specificity, and symmetric mean absolute percent error (SMAPE) were calculated to understand how each confounding variable affected the algorithm's ability to detect and quantify pulse.
Example 3: Automatic Determination of ROI Using Machine Learning
REFERENCES
References—Updated List