TREATMENT OF PITUITARY CORTICOTROPH TUMORS USING R-ROSCOVITINE
This invention relates to the treatment of pituitary tumors and related disease conditions. Despite being small (< 2mm) and often undetectable by MRI, pituitary corticotroph tumors are associated with significant morbidities and mortality due to adrenal glucocorticoid (Gc) hypersecretion in response to autonomous tumor ACTH production1. The standard of care for Cushing's disease consists of transsphenoidal pituitary tumor resection, pituitary-directed radiation, adrenalectomy and/or medical suppression of adrenal gland cortisol production. While transsphenoidal ACTH-secreting tumor resection yields 30-70% surgical cure rate, adenoma recurrence rate is high2. Efficacies of other therapeutic modalities are limited by factors such as slow therapeutic response, development of pituitary insufficiency, and uncontrolled pituitary tumor growth in the face of adrenal gland resection or inhibition2,3. Effective pharmacotherapy directly targeting corticotroph tumor growth and/or ACTH production remains a major challenge4. The pituitary is highly sensitive to cell cycle disruptions5,6. Pituitary tumors acquire oncogene and tumor suppressor genetic and epigenetic alterations, which result in unrestrained proliferation, aberrant neuroendocrine regulatory signals and disrupted humoral milieu, mediated directly or indirectly by dysregulated cyclin-dependent kinases (CDKs)5,7. Although CDK gene mutations have not readily been identified in human pituitary tumors, overexpression of cyclins and dysregulation of CDK inhibitors are common features of pituitary adenomas, indicating that CDK activation has important pathological and potential therapeutic implications8,9. Small molecule CDK inhibitors are being evaluated for cancer therapy, some of which have led to clinical trials for lymphoma, lung and nasopharyngeal cancers10,11. Preclinical studies of CDK inhibitors, however, are often hampered by the requirement for large drug quantity, and prolonged duration of administration to observe potential efficacy. While the genetic spectrum of tumor associated mutations and/or their cellular context may dictate specific CDK dependence, particular CDK inhibitors may not have been tested in the most appropriate tumor types in vivo11,12. Animal models faithfully recapitulating human pituitary tumors would enable rapid and efficient testing to identify small molecule CDK inhibitors with optimal potency. Regardless of cell lineage origin, pituitary tumors almost invariably overexpress pituitary tumor transforming gene (PTTG), which encodes a securin that binds separase in the APC complex, and governs faithful chromosome segregation during mitosis13 PTTG was originally isolated from rat pituitary tumor cells14. Dysequilibrium of intracellular PTTG abundance leads to cell cycle disruption and neoplastic formation, causing chromosomal instability and aneuploidy, and also aberrant G1/S and G2/M transition by transcriptional dysregulation of cyclin expression13, 15-20. On the other hand, PTTG overexpession also triggers irreversible cell cycle arrest in pituitary growth hormone (GH)- and gonadotropin (LH, FSH)-expressing tumors by activating lineage-specific senescence pathways, contributing to the benign propensity of pituitary tumors13,21 . There remains a need in the art for alternative and/or improved methods of treating pituitary tumors and particularly, pituitary corticotroph tumors. Various embodiments of the present invention provide oleomoucine and R-roscovitine or salts thereof for use in a method of treating a pituitary corticotroph tumor, suppressing ACTH and/or corticosterone levels in a ACTH-secreting pituitary adenoma, inhibiting the growth of an ACTH-secreting pituitary adenoma, and/or treating Cushing's disease. The method can comprise: providing a composition comprising a selective CDK/cyclin inhibitor; and administering a therapeutically effective amount of the composition to a mammalian subject in need of treating for a pituitary corticotroph tumor, suppressing ACTH and/or corticosterone levels in a ACTH-secreting pituitary adenoma, inhibiting the growth of an ACTH-secreting pituitary adenoma, or treating Cushing's disease to treat the pituitary corticotroph tumor, suppress the ACTH and/or corticosterone levels in a ACTH-secreting pituitary adenoma, inhibit the growth of an ACTH-secreting pituitary adenoma, or treat Cushing's disease. In various embodiments, the mammalian subject can be in need of treating a pituitary corticotroph tumor and the pituitary corticotroph tumor is treated. In certain embodiments, the corticotroph tumor is a PTTG overexpressing corticotroph tumor. In other embodiments, the mammalian subject can be in need of suppressing ACTH and/or corticosterone levels in a ACTH-secreting pituitary adenoma and the ACTH and/or corticosterone levels in the ACTH-secreting pituitary adenoma are suppressed. In still other embodiments, the mammalian subject can be in need of inhibiting the growth of an ACTH-secreting pituitary adenoma and the growth of an ACTH-secreting pituitary adenoma is inhibited. In various embodiments, the mammalian subject can be in need of treating Cushing's disease and Cushing's disease is treated. Oleomoucine and R-roscovitine are selective CDK/cyclin inhibitors and 2,6,9-substituted purine analogues. In various preferred embodiments, the selective CDK/cyclin inhibitor is R-roscovitine or salts thereof. Other features and advantages of the invention will become apparent from the following detailed description, taken in conjunction with the accompanying drawings, which illustrate, by way of example, various features of embodiments of the invention. Exemplary embodiments are illustrated in referenced figures. It is intended that the embodiments and figures disclosed herein are to be considered illustrative rather than restrictive.
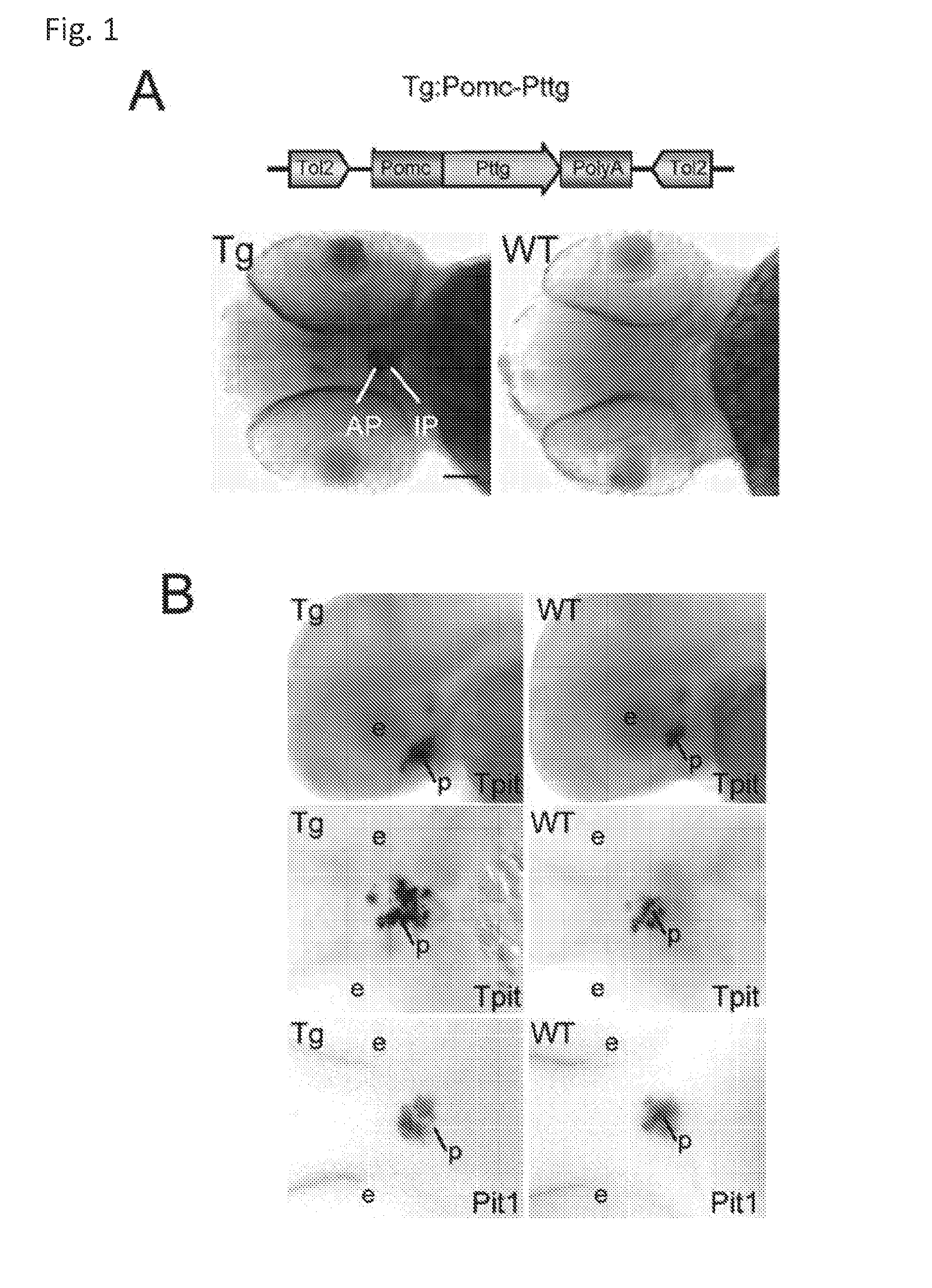
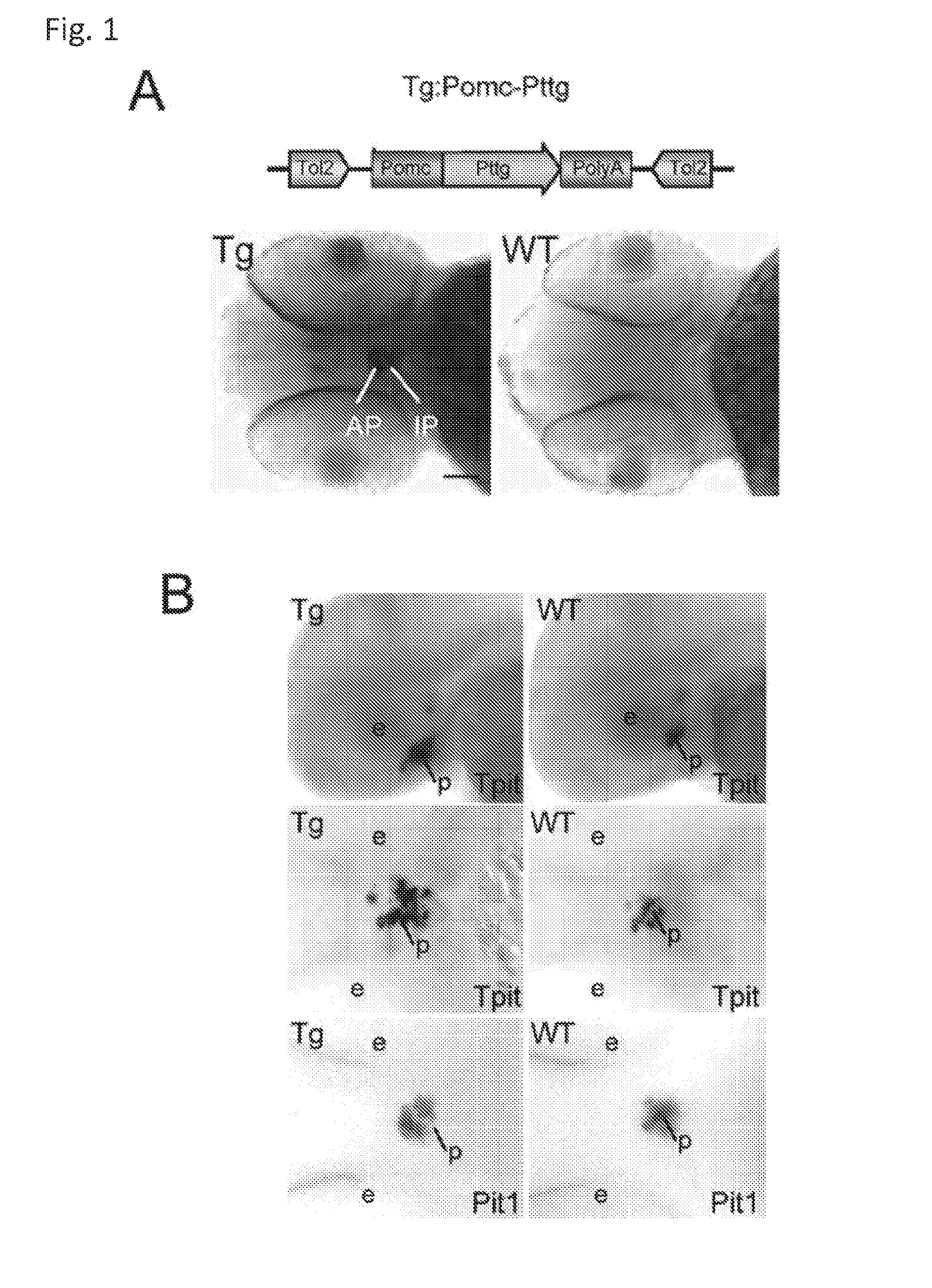
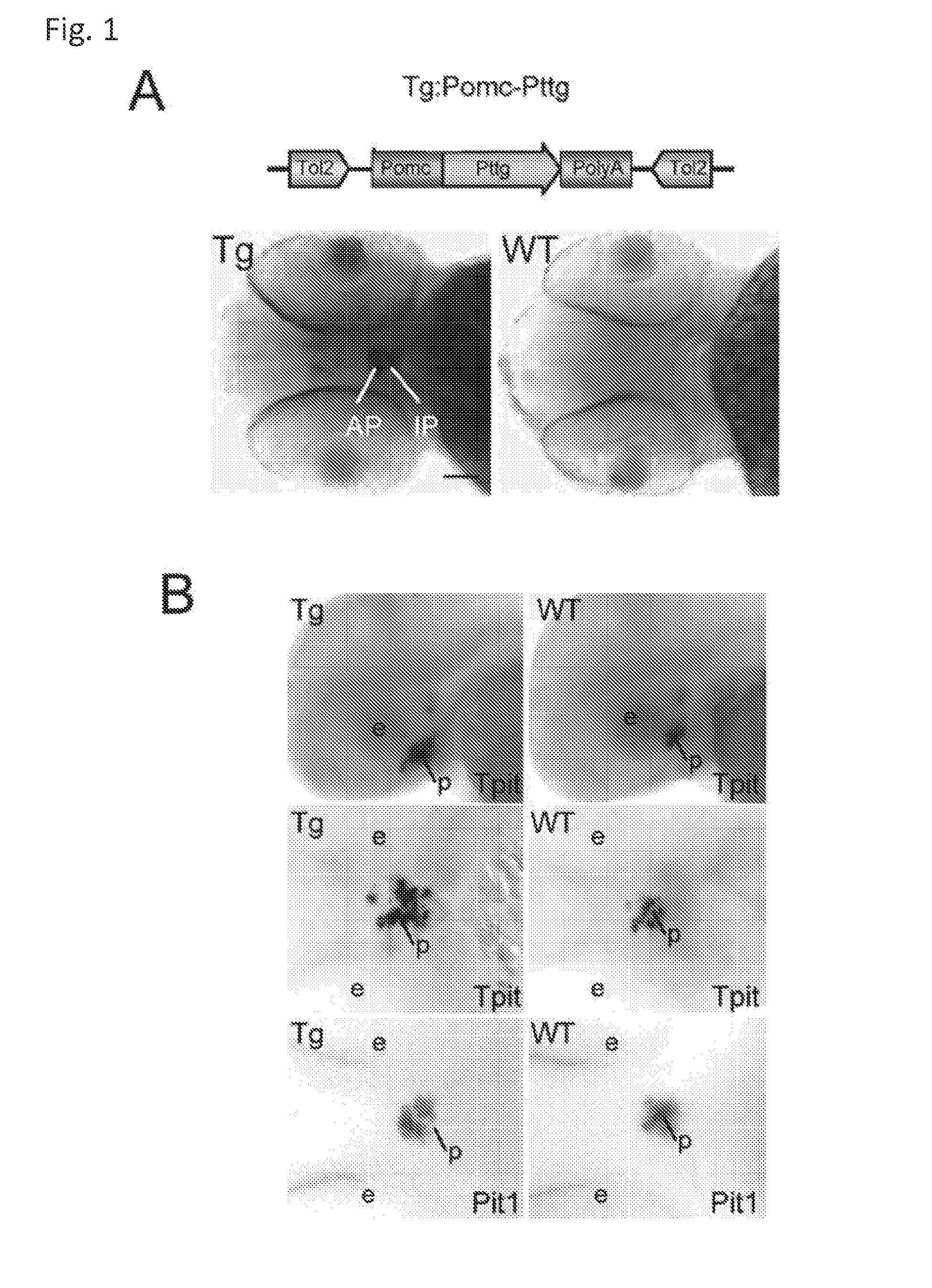
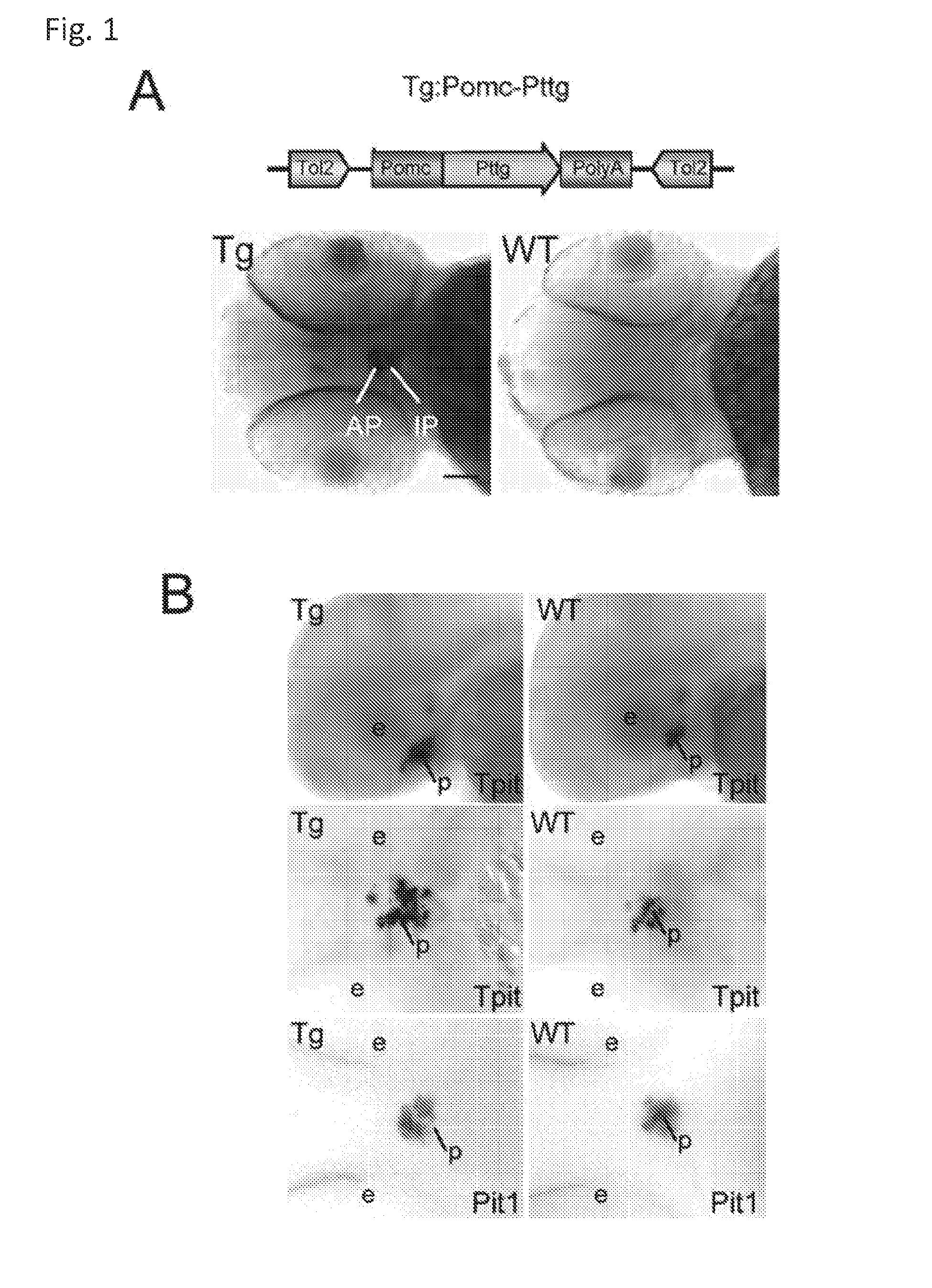
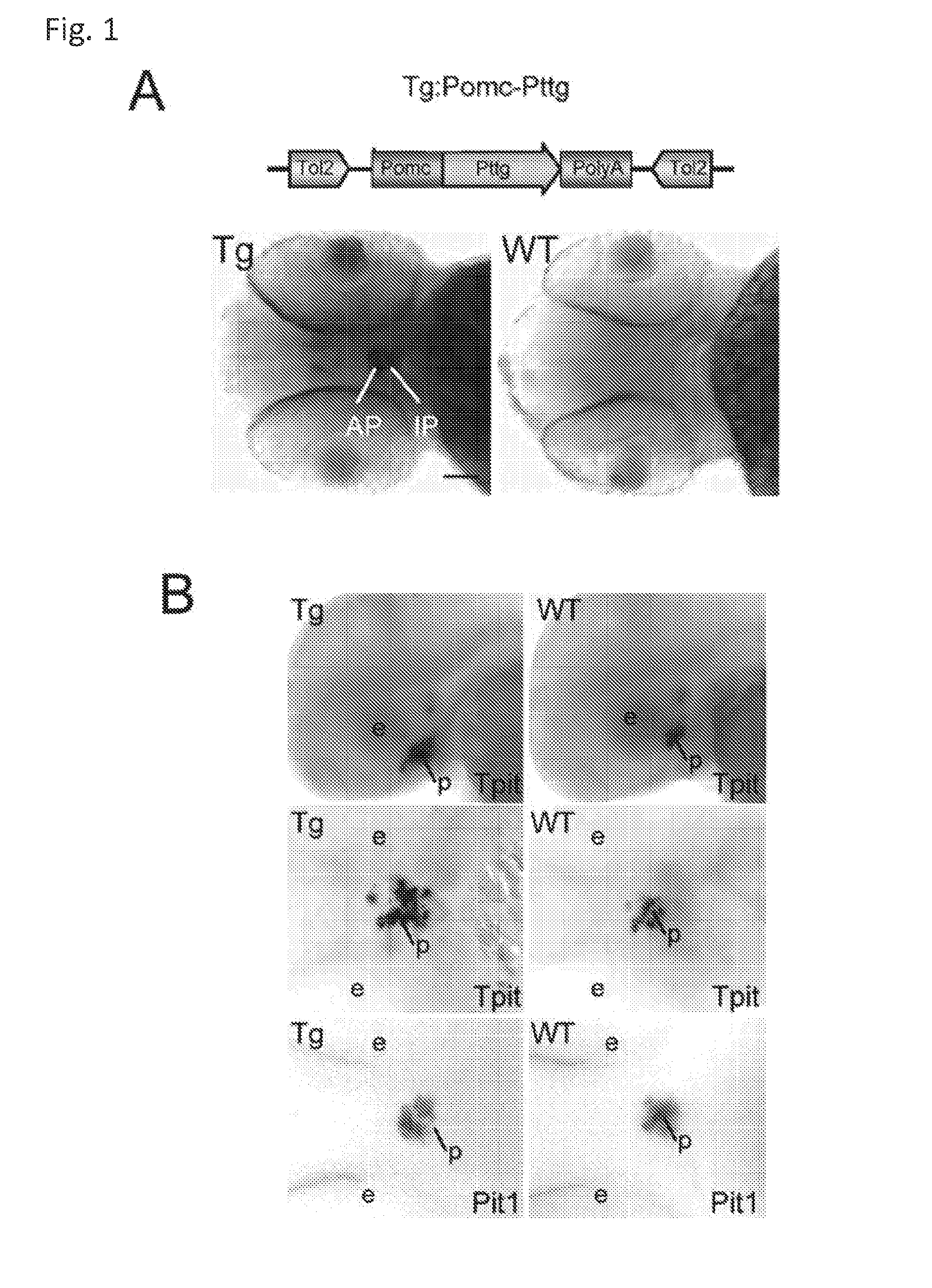
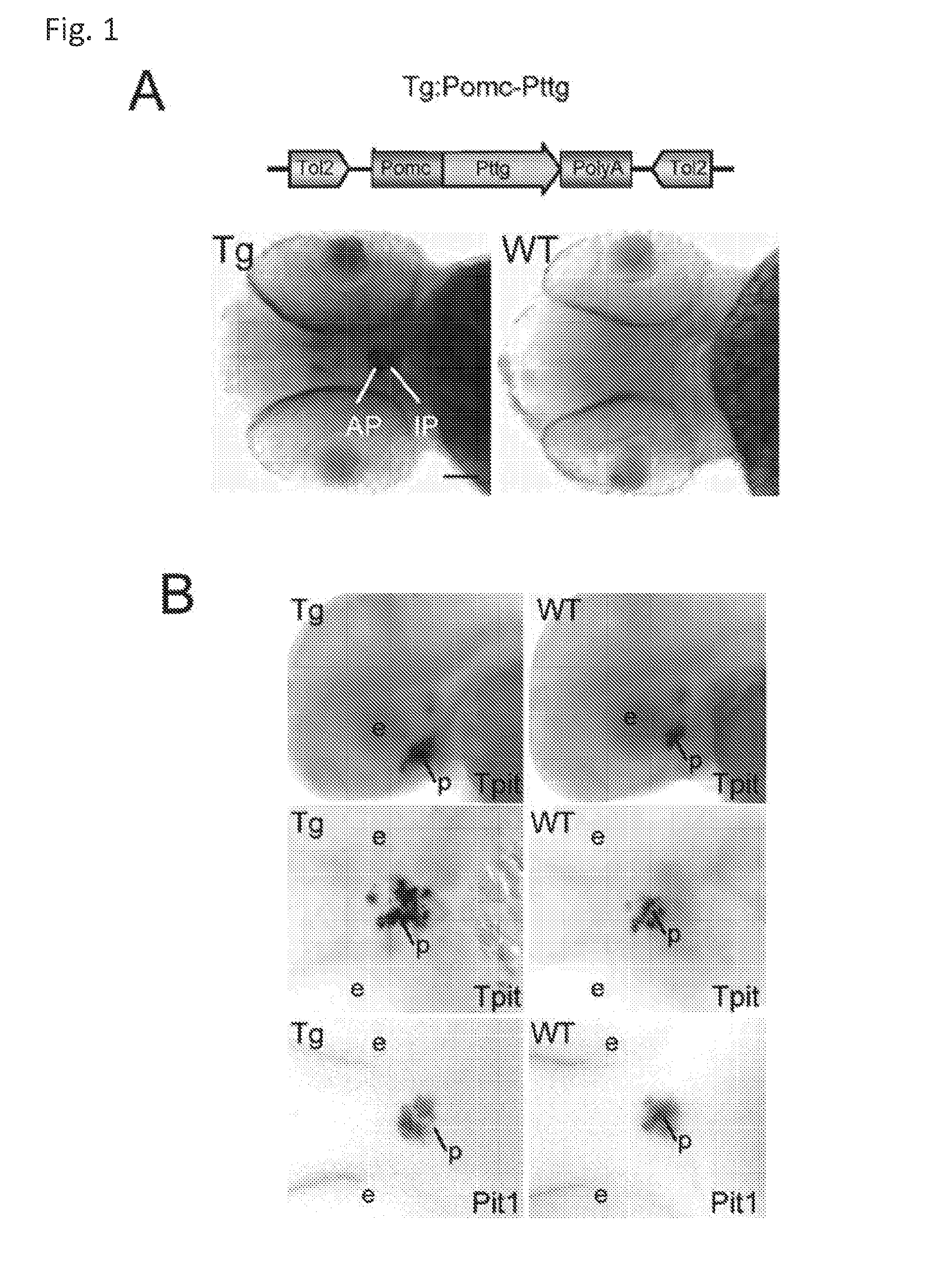
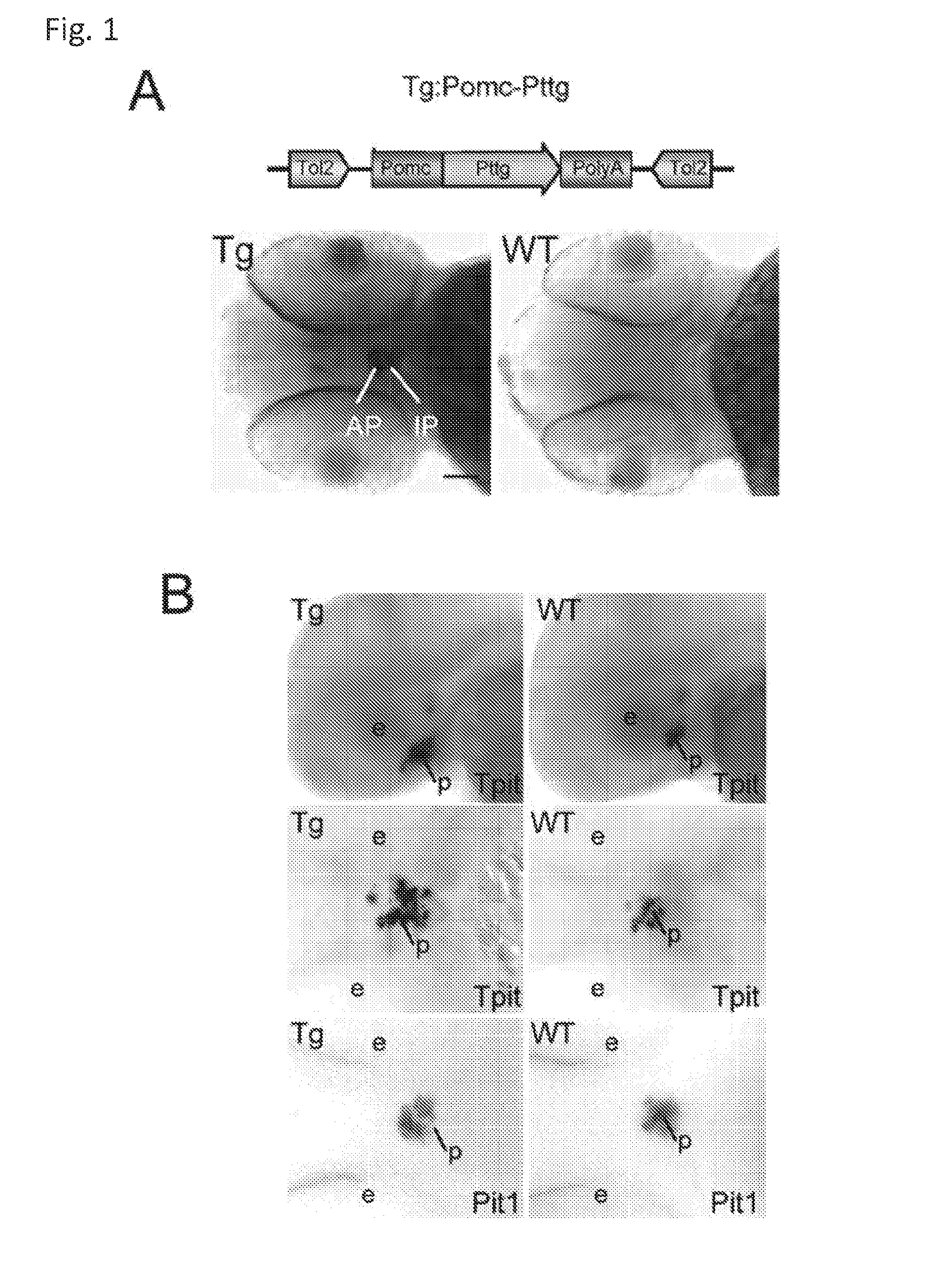
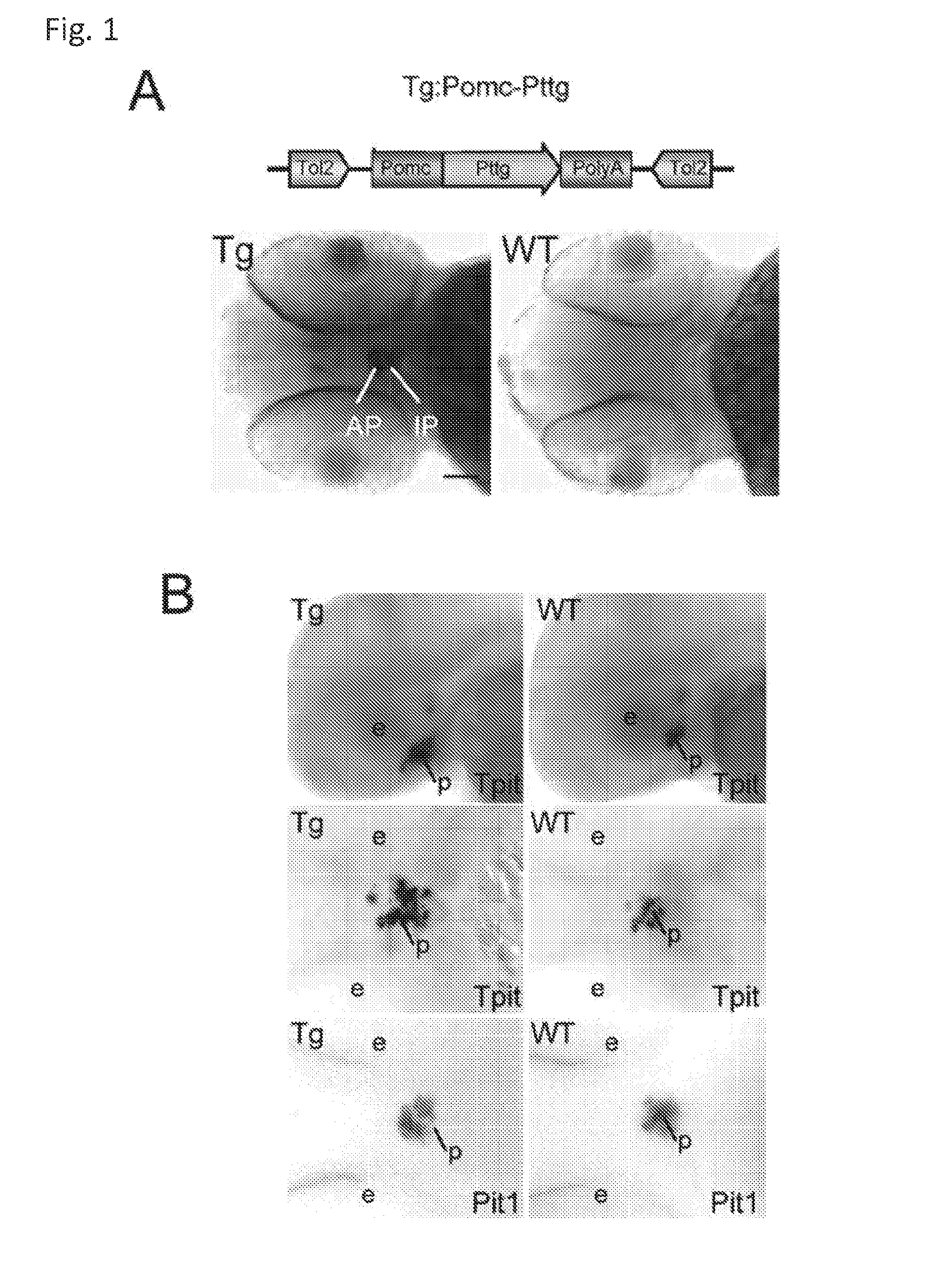
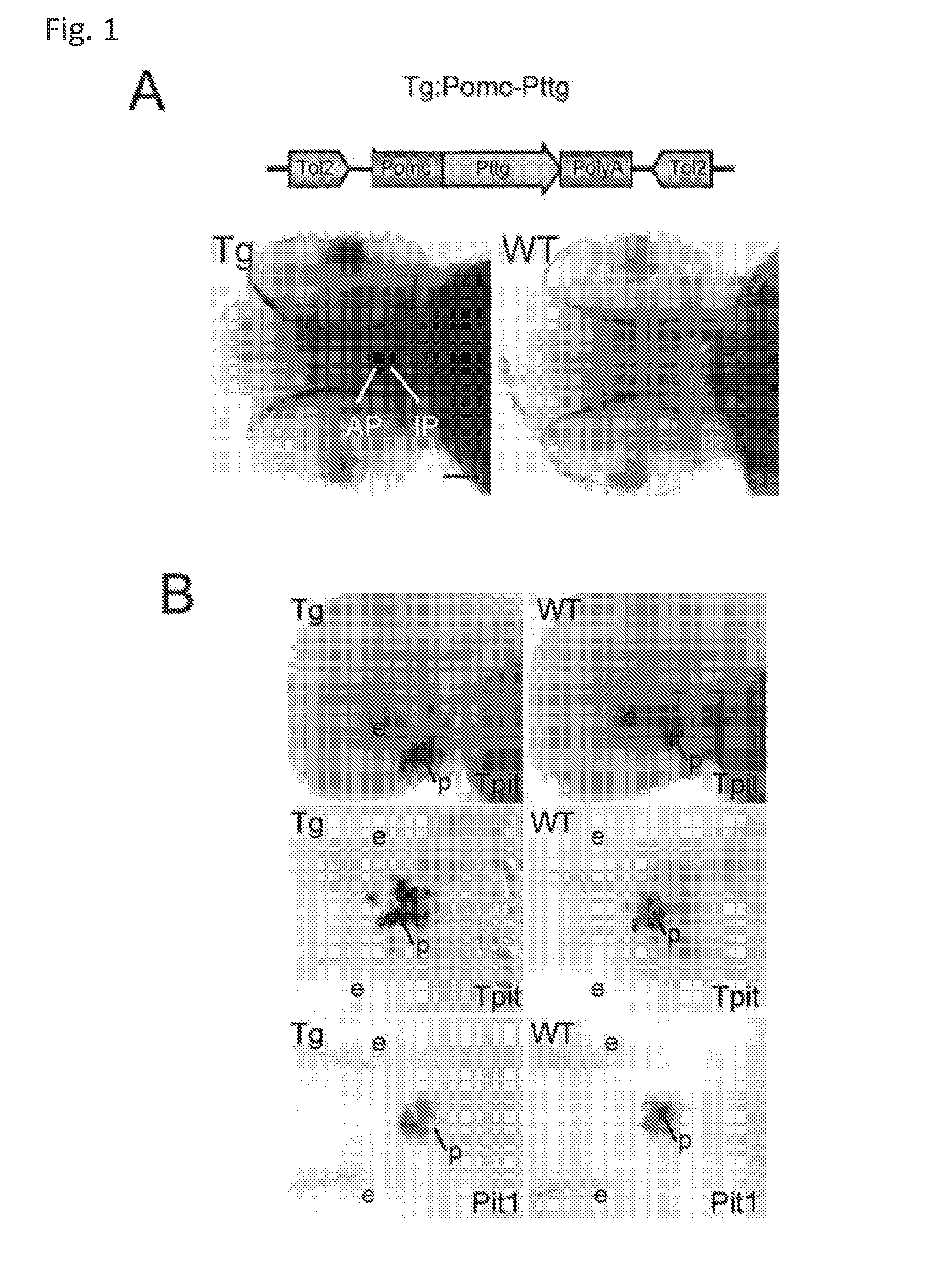
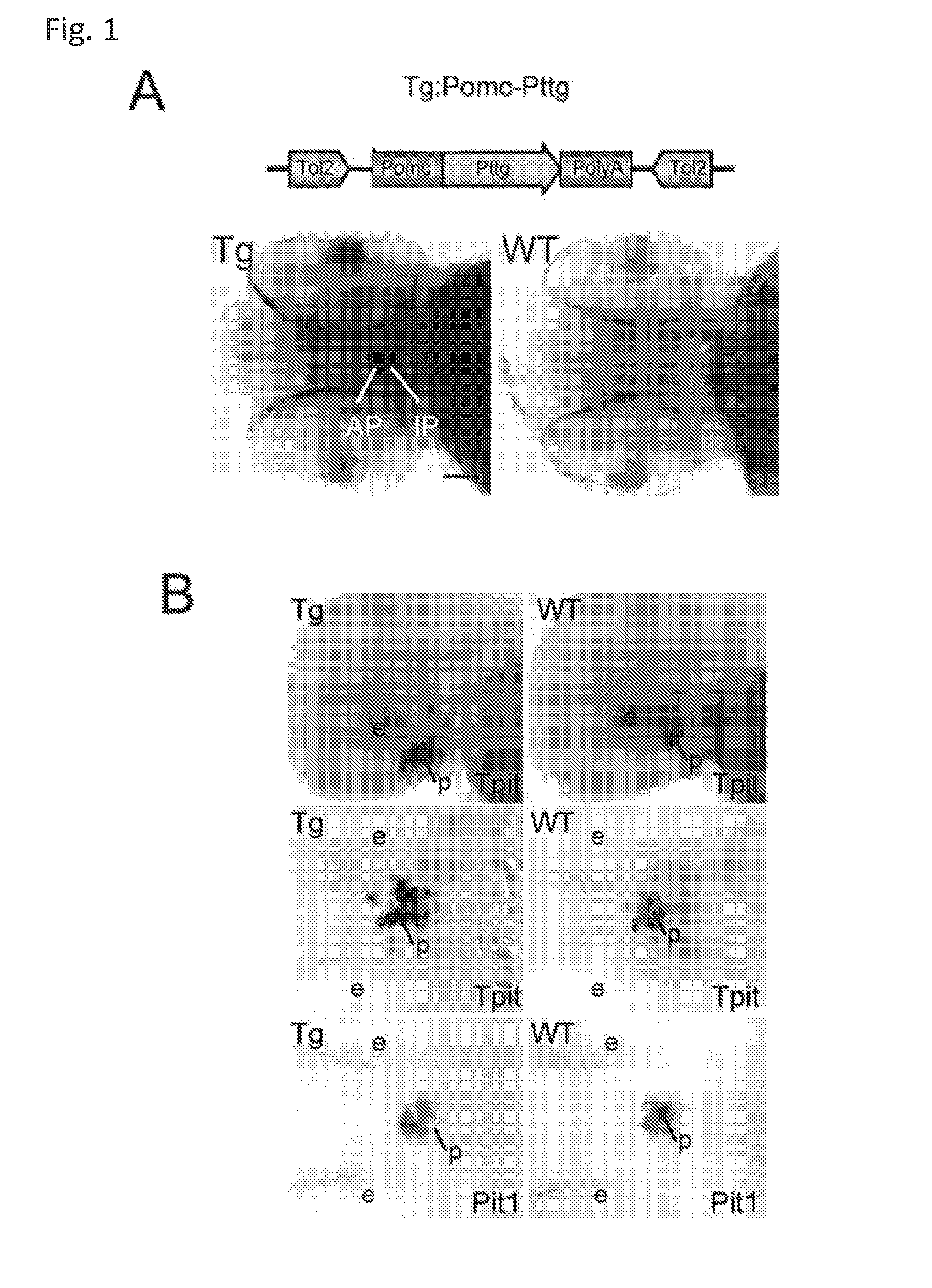
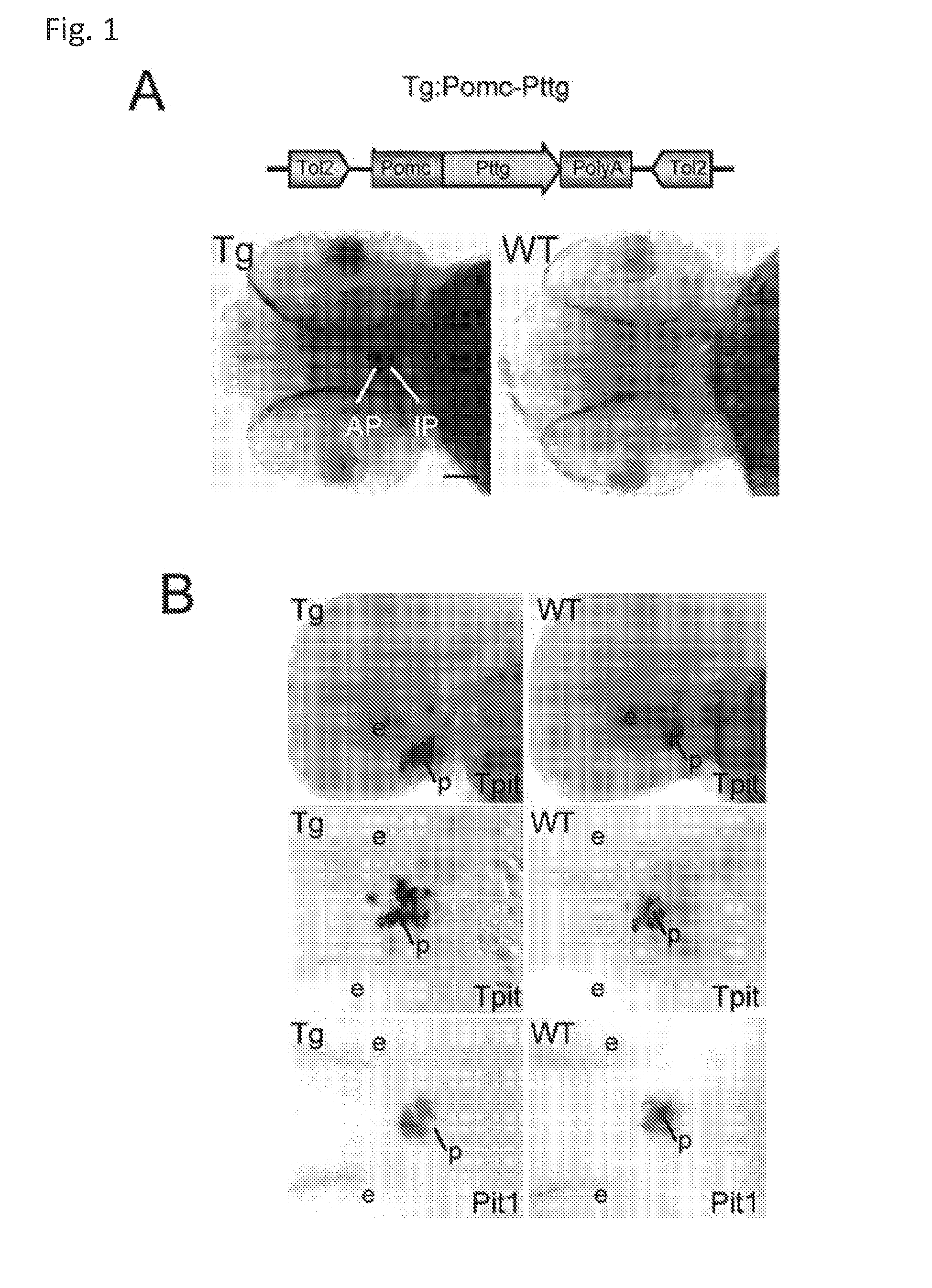
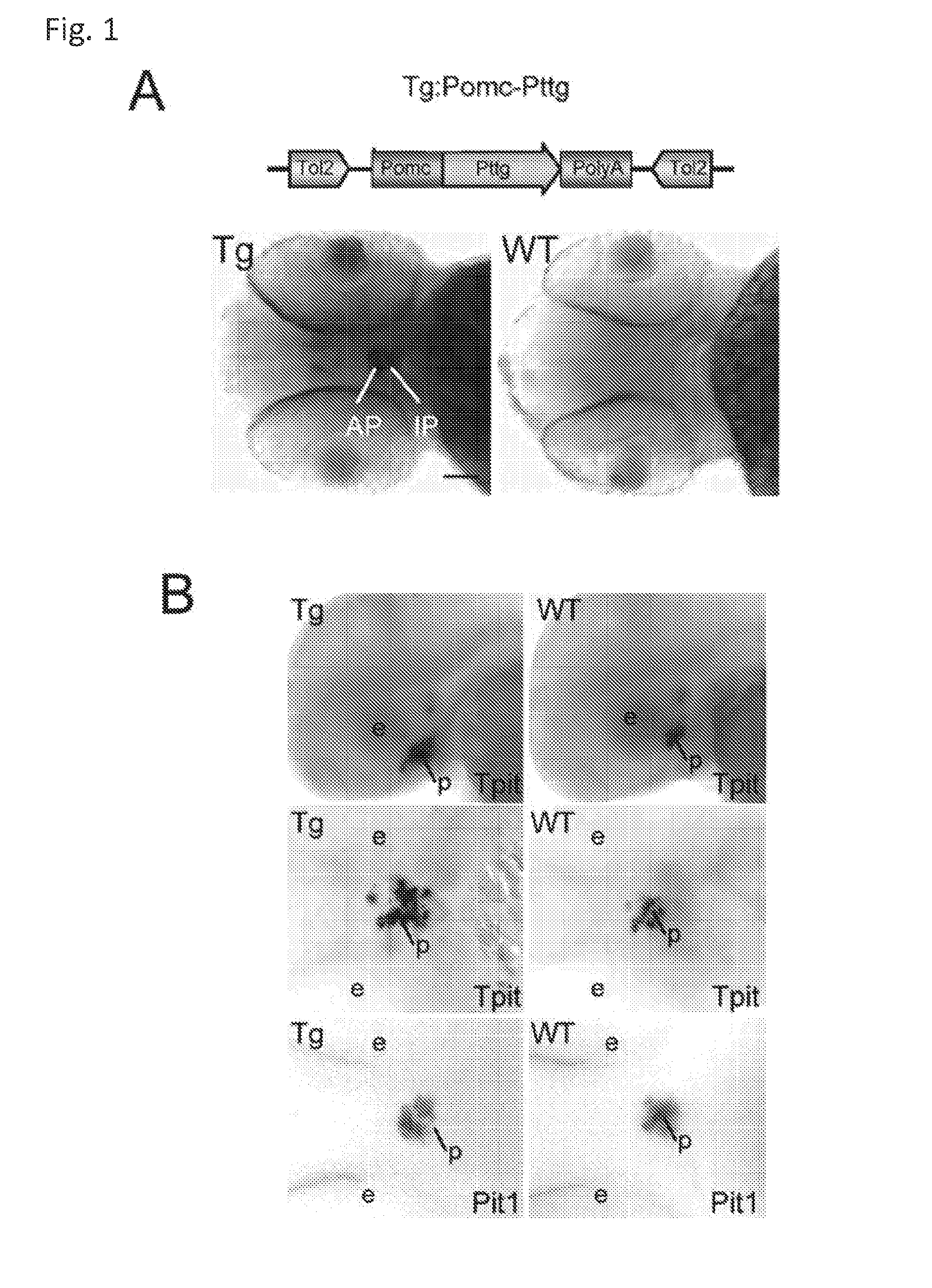
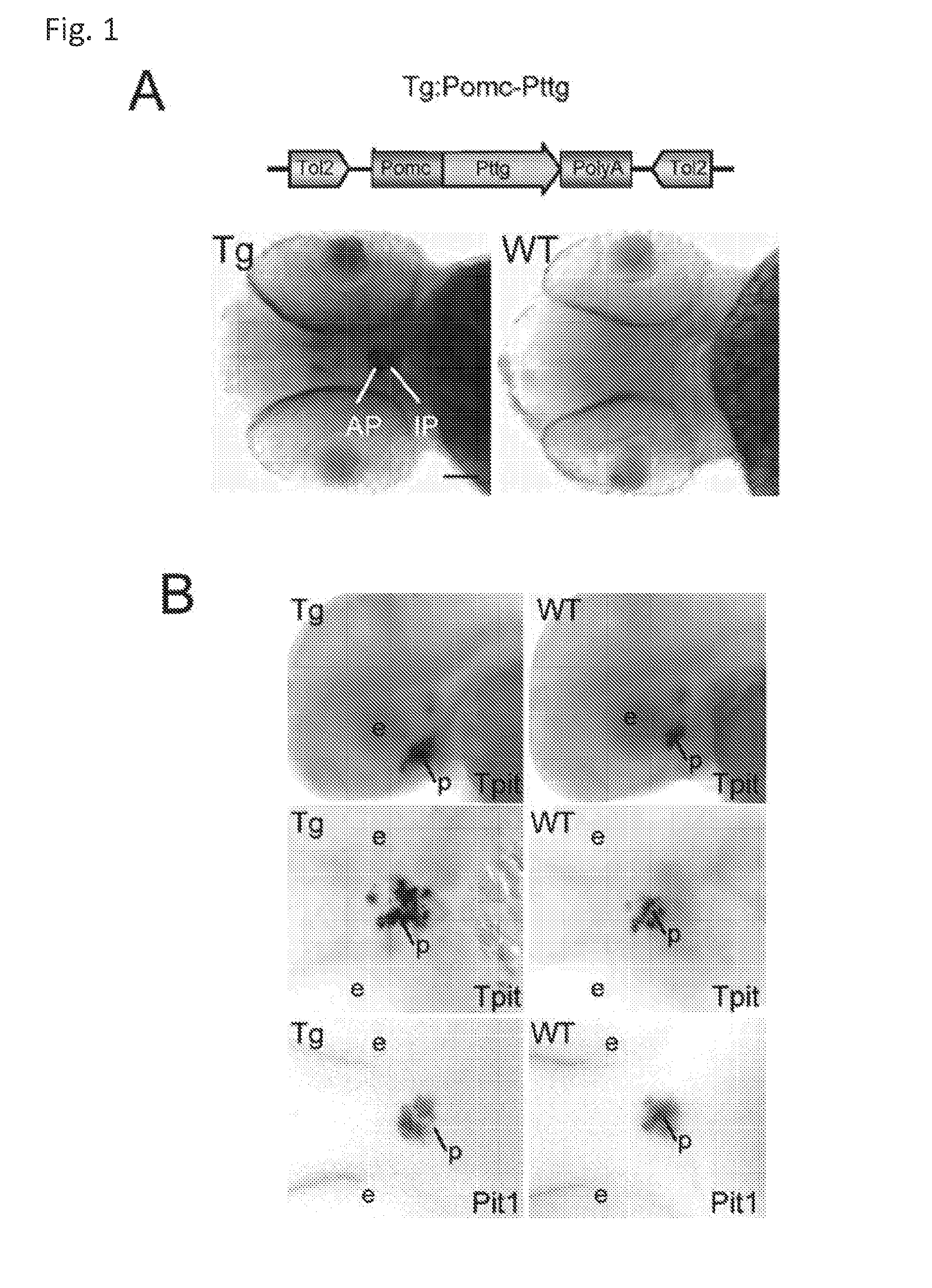
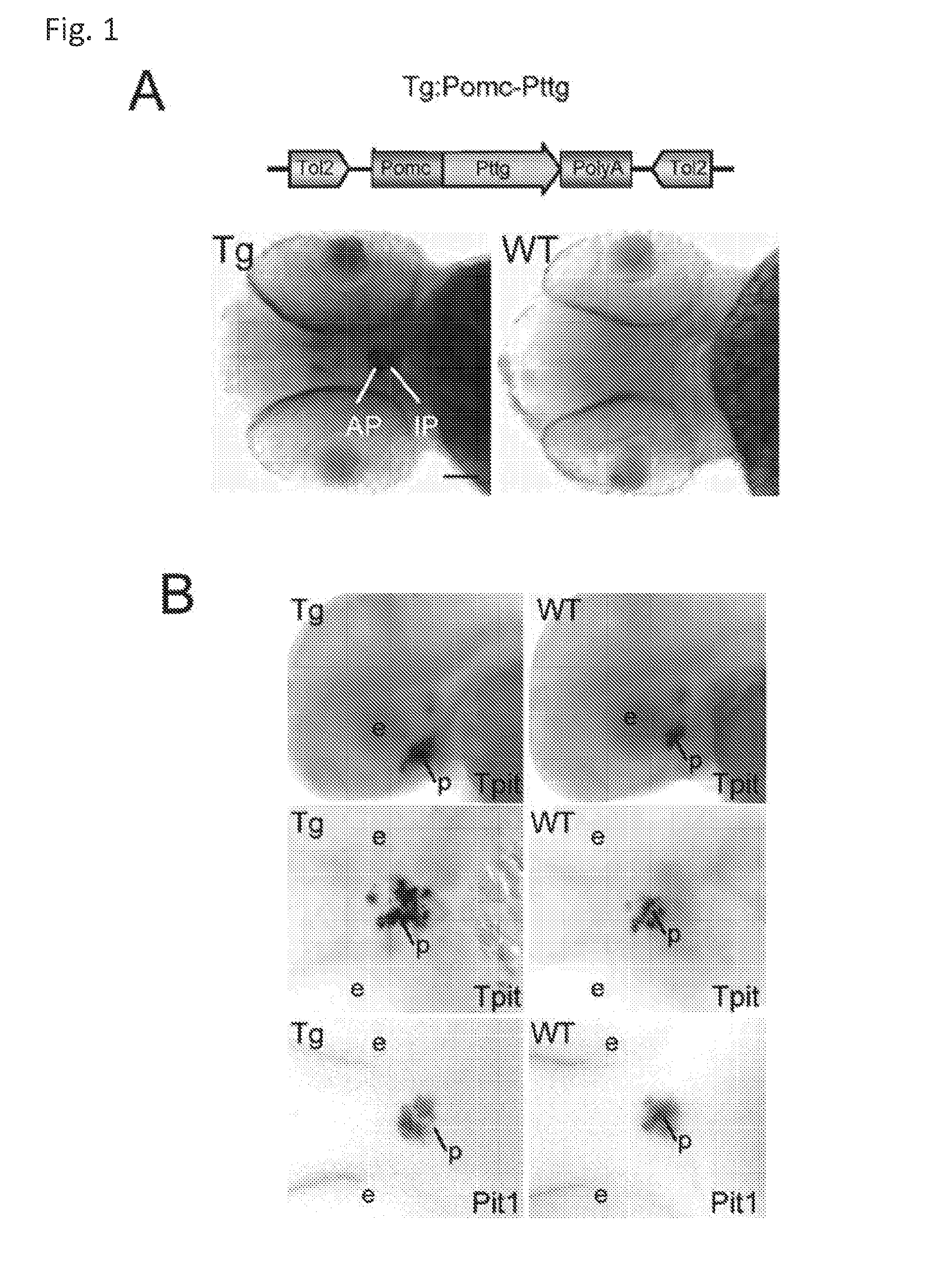
Unless defined otherwise, technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. One skilled in the art will recognize many methods and materials similar or equivalent to those described herein, which could be used in the practice of the present invention. Indeed, the present invention is in no way limited to the methods and materials described. "Pituitary tumor" as used herein includes, but is not limited to, lactotrophic adenoma or prolactinoma, ACTH-secreting adenoma, somatotrophic adenoms, corticotrophic adenoma, gonadotrophic adenoma, thyrotrophic adenoms, and null cell adenoma. "Mammal" as used herein refers to any member of the class "Therapeutically effective amount" as used herein refers to that amount which is capable of achieving beneficial results in a mammalian subject with a pituitary tumor, a pituitary corticotroph tumor, an ACTH-secreting pituitary adenoma and/or or a mammalian subject with Cushing's disease. A therapeutically effective amount can be determined on an individual basis and will be based, at least in part, on consideration of the physiological characteristics of the mammal, the type of delivery system or therapeutic technique used and the time of administration relative to the progression of the disease. Described herein, the inventors report the generation of a stable transgenic zebrafish with zPttg overexpression targeted to pituitary proopiomelanocortin (POMC) lineages (corticotrophs and melanotrophs). Tg:Pomc-Pttg larvae develop early pathologies reflective of corticotroph tumors including neoplastic corticotrophs with partial Gc resistance, and hypercortisolemia-induced metabolic disturbances in adult transgenic fish. Taking advantage of the early-observed corticotroph pathology, combined with pituitary POMC lineage-specific expression of a fluorescent reporter in live transparent larvae, small molecule CDK inhibitors were tested, which lead to identification of R-roscovitine against PTTG overexpressing corticotrophs. Inhibitory effects of R-roscovitine on corticotroph tumor cells were subsequently validated in an in vivo and in vitro mouse model, supporting use of selective CDK inhibitors as effective therapy for Cushing's disease. The average diameter of human pituitary corticotroph tumors is 6 mm37, and despite the use of petrosal sinus sampling to establish pituitary ACTH hypersecretion, up to 40% of corticotroph tumors are not visible on MRI, posing significant challenges for surgical resection 38. On the other hand, less commonly encountered large corticotroph tumors may impinge upon surrounding critical structures, thus hampering complete tumor resection. Furthermore, extensive surgical resection may cause significant damage to normal pituitary tissue leading to hypopituitarism in most of these patients. The diagnostic and therapeutic dilemma posed by Cushing's disease is further complicated by significant metabolic and cardiovascular morbidities, and mortality associated with uncontrolled chronic hypercortisolism1. Tumor-targeted drug development for Cushing's disease is a major challenge as the pathogenesis of corticotroph adenomas remains enigmatic. Pharmacological protein kinase inhibitors capable of controlling cell growth and metabolism, blocking cell-cycle progression, modulating transcription and inducing apoptosis in cancer cells have been developed for mechanism-based and non-genotoxic target tumor therapies10,11. Recently, protein kinases, e.g., epidermal growth factor receptor (HER) family and cyclin-dependent kinases have been suggested as therapeutic targets for pituitary tumors 8, 39, 40. Although tumor responses to protein kinase inhibitors are selective, and may be dictated by specific mutations and/or tumor cellular context, preclinical testing is hampered by poor predictabilities with respect to molecular pathophysiology of the tumors being assessed. Animal models that faithfully reflect molecular pathogenesis of human disease and allow rapid, non-invasive read-out of tumor-cellular inhibition would facilitate drug testing against corticotroph tumors. Here, the inventors report generation of germline transgenic zebrafish overexpressing zPttg targeted to pituitary POMC cells, as a small vertebrate animal model of Cushing's disease. While the phenotype of hypercortisolism was observed in adult Tg:Pomc-Pttg zebrafish by 3 months of age, pituitary corticotroph expansion with partial resistance to glucocorticoid negative feedback was already detected within the first 2 days of embryonic development of stable transgenic zebrafish. Furthermore, the Tg:Pomc-Pttg pituitary demonstrates a characteristic feature of human corticotroph adenomas, i.e., cyclin E up-regulation and G1/S phase disruption. The molecular features and early pathologies of corticotroph tumors in Tg:Pomc-Pttg transgenic fish allowed for insight into mechanisms underlying the disease pathogenesis, and also to test drug efficacy in vivo. Cyclin E overexpression is associated with disrupted G1/S transition contributing to development and progression of breast carcinomas, leukemia and lymphomas31. In the pituitary, cyclin E expression is preferentially up-regulated in corticotroph adenomas compared with tumors arising from other lineages, the mechanisms of which remain to be fully defined 32, 41, 42. In a subgroup of corticotroph adenomas, cyclin E up-regulation was associated with loss of Brg1 expression, suggesting the presence of additional cyclin E regulators in corticotrophs27. These results show that corticotroph PTTG overexpression induces cyclin E, while PTTG siRNA suppresses cyclin E expression in murine corticotroph tumor cells ( Corticotroph cyclin E up-regulation contributes to cell cycle reentry of differentiated corticotrophs and centrosome instability9. To investigate the clinical significance of cyclin E dysregulation in corticotroph adenomas, in vivo drug testing on Tg:Pomc-Pttg embryos was performed using known small molecule compounds with different spectra of CDK/cyclin inhibitory selectivity. These results indicated inhibition of PTTG-overexpressing corticotrophs by the 2,6,9-substituted purine analogues, olomoucine and R-roscovitine, with the latter demonstrating a higher efficacy in vivo ( These results suggest that the anti-tumor activity of R-roscovitine in corticotroph adenomas involve CDK2/cyclinE and Rb mediated pathways, independent of p53 ( The inventors have shown herein that a particular CDK/cyclin inhibitor can suppress pituitary tumor growth and hormone production (e.g., CDK2/cyclin E inhibitor against corticotroph tumor). While not wishing to be bound by any particular theory, the inventors believe that the suppression is via cell lineage and/or CDK inhibitor-specific mechanisms. Therefore, other types of CDK/cyclin inhibitor can also be capable of inhibiting a different types of pituitary tumors, such as somatotroph tumors. As such, the use of CDK/cyclin inhibitors to treat pituitary tumors is included in the embodiments of the present invention. According to the invention there is provided a composition comprising oleomoucine and R-roscovitine or salts thereof for use as a medicament for administration, in a therapeutically effective amount, to a mammalian subject in need of treating a pituitary corticotroph tumor, suppressing ACTH and/or corticosterone levels in a ACTH-secreting pituitary adenoma, inhibiting the growth of an ACTH-secreting pituitary adenoma, or treating Cushing's disease to treat the corticotroph pituitary tumor, suppress the ACTH and/or corticosterone levels in a ACTH-secreting pituitary adenoma, inhibit the growth of an ACTH-secreting pituitary adenoma, or treat Cushing's disease. These methods can comprise: providing a composition comprising a selective CDK/cyclin inhibitor and administering a therapeutically effect amount of the composition to the mammalian subject to treat the pituitary tumor, to suppress ACTH and/or corticosterone levels in an ACTH-secreting pituitary adenoma, to inhibit the growth of an ACTH-secreting pituitary adenoma, or to treat Cushing's disease. Oleomoucine and R-roscovitine are selective CDK/cyclin inhibitors. Examples of other CDK/cyclin inhibitors are provided in Tables 1-3. In various embodiments, the pituitary tumor is a pituitary corticotroph tumor. In various embodiments, the corticotroph tumor is a PTTG overexpressing corticotroph tumor. In various embodiments, the ACTH-secreting pituitary adenoma is a pituitary corticotroph tumor. In various embodiments, the mammalian subject is a human subject. Olomoucine, and R-roscovitine may be provided as pharmaceutical compositions including a pharmaceutically acceptable excipient along with a therapeutically effective amount of a olomoucine, R-roscovitine or a salt thereof. "Pharmaceutically acceptable excipient" means an excipient that is useful in preparing a pharmaceutical composition that is generally safe, non-toxic, and desirable, and includes excipients that are acceptable for veterinary use as well as for human pharmaceutical use. Such excipients may be solid, liquid, semisolid, or, in the case of an aerosol composition, gaseous. Pharmaceutical compositions comprising olomoucine, R-roscovitine or a salt thereof may be formulated for delivery via any route of administration. "Route of administration" may refer to any administration pathway known in the art, including but not limited to aerosol, nasal, oral, transmucosal, transdermal, parenteral, or enteral. "Parenteral" refers to a route of administration that is generally associated with injection, including intraorbital, infusion, intraarterial, intracapsular, intracardiac, intradermal, intramuscular, intraperitoneal, intrapulmonary, intraspinal, intrasternal, intrathecal, intrauterine, intravenous, subarachnoid, subcapsular, subcutaneous, transmucosal, or transtracheal. Via the parenteral route, the compositions may be in the form of solutions or suspensions for infusion or for injection, or as lyophilized powders. Via the parenteral route, the compositions may be in the form of solutions or suspensions for infusion or for injection. Via the enteral route, the pharmaceutical compositions can be in the form of tablets, gel capsules, sugar-coated tablets, syrups, suspensions, solutions, powders, granules, emulsions, microspheres or nanospheres or lipid vesicles or polymer vesicles allowing controlled release. The pharmaceutical compositions can also contain any pharmaceutically acceptable carrier. "Pharmaceutically acceptable carrier" as used herein refers to a pharmaceutically acceptable material, composition, or vehicle that is involved in carrying or transporting a compound of interest from one tissue, organ, or portion of the body to another tissue, organ, or portion of the body. For example, the carrier may be a liquid or solid filler, diluent, excipient, solvent, or encapsulating material, or a combination thereof. Each component of the carrier must be "pharmaceutically acceptable" in that it must be compatible with the other ingredients of the formulation. It must also be suitable for use in contact with any tissues or organs with which it may come in contact, meaning that it must not carry a risk of toxicity, irritation, allergic response, immunogenicity, or any other complication that excessively outweighs its therapeutic benefits. The pharmaceutical compositions can also be encapsulated, tableted or prepared in an emulsion or syrup for oral administration. Pharmaceutically acceptable solid or liquid carriers may be added to enhance or stabilize the composition, or to facilitate preparation of the composition. Liquid carriers include syrup, peanut oil, olive oil, glycerin, saline, alcohols and water. Solid carriers include starch, lactose, calcium sulfate, dihydrate, terra alba, magnesium stearate or stearic acid, talc, pectin, acacia, agar or gelatin. The carrier may also include a sustained release material such as glyceryl monostearate or glyceryl distearate, alone or with a wax. The pharmaceutical preparations are made following the conventional techniques of pharmacy involving milling, mixing, granulation, and compressing, when necessary, for tablet forms; or milling, mixing and filling for hard gelatin capsule forms. When a liquid carrier is used, the preparation will be in the form of a syrup, elixir, emulsion or an aqueous or non-aqueous suspension. Such a liquid formulation may be administered directly p.o. or filled into a soft gelatin capsule. The pharmaceutical compositions may be delivered in a therapeutically effective amount. The precise therapeutically effective amount is that amount of the composition that will yield the most effective results in terms of efficacy of treatment in a given subject. This amount will vary depending upon a variety of factors, including but not limited to the characteristics of the therapeutic compound (including activity, pharmacokinetics, pharmacodynamics, and bioavailability), the physiological condition of the subject (including age, sex, disease type and stage, general physical condition, responsiveness to a given dosage, and type of medication), the nature of the pharmaceutically acceptable carrier or carriers in the formulation, and the route of administration. One skilled in the clinical and pharmacological arts will be able to determine a therapeutically effective amount through routine experimentation, for instance, by monitoring a subject's response to administration of a compound and adjusting the dosage accordingly. For additional guidance, see Typical dosages of olomoucine, R-roscovitine or salt thereof can be in the ranges recommended by the manufacturer where known therapeutic compounds are used, and also as indicated to the skilled artisan by the The following examples are provided to better illustrate the claimed invention and are not to be interpreted as limiting the scope of the invention. To the extent that specific materials are mentioned, it is merely for purposes of illustration and is not intended to limit the invention. One skilled in the art may develop equivalent means or reactants without the exercise of inventive capacity and without departing from the scope of the invention. As an initial step toward identification of novel targets for Cushing's disease therapy, a zebrafish model of pituitary corticotroph tumors was created. Given the highly conserved zebrafish PTTG protein sequence ( To investigate the effect of zPttg overexpression on embryonic pituitary POMC lineage development, the inventors analyzed highly conserved pituitary transcription factors as markers for both non-POMC ( Pituitary corticotrophs are a critical component of the hypothalamic-pituitary-adrenal (HPA) axis that mediates the stress response via corticotropin-releasing hormone (CRH)-stimulated and subsequently pituitary ACTH stimulated adrenal gland Gc production. Gcs exert negative feedback on CRH and POMC-derived ACTH expression and secretion to restore HPA homeostasis following stress. In human corticotroph tumors, ACTH hypersecretion is partially resistant to Gc negative feedback regulation, further exacerbating uncontrolled hypercortisolism27. To investigate the integrity of the Gc negative feedback pathway in Tg:Pomc-Pttg corticotrophs, live zebrafish embryos were exposed to dexamethasone containing culture medium starting from 10 hours post fertilization (hpf). Pituitary eGFP expression was suppressed in POMC-GFP larvae exposed to 10-7 M dexamethasone by 4 days post fertilization (dpf) but not in double transgenic (Tg:Pomc-Pttg; POMC-eGFP) larvae, which only exhibited inhibition of pituitary eGFP expression in response to 10 times higher dexamethasone concentrations (10-6 M) ( In adult Tg:Pomc-Pttg fish (20 months of age), immunohistochemistry revealed overt neoplastic-appearing pituitary cells with a high nuclear/cytoplasmic ratio, distinct nucleoli and basophilic cytoplasm that stained strongly for ACTH in two of six Tg:Pomc-Pttg pituitary glands analyzed, morphologically resembling human pituitary ACTH-secreting adenomas, while none of six WT pituitary glands showed a similar phenotype ( It was tested whether the observed neoplastic corticotroph cell changes in Tg:Pomc-Pttg zebrafish lead to autonomous ACTH secretion and subsequent hypercortisolism. Because one is technically hampered from measuring plasma ACTH or serum cortisol levels by the very limited amount of blood obtainable from each adult zebrafish (∼5 (µl), the inventors measured total cortisol content in age- and weight-matched Tg:Pomc-Pttg zebrafish and their transgene-negative siblings. At 3 months of age, adult Tg:Pomc-Pttg fish showed 40% increased cortisol content vs WT siblings (1.4 ± 0.2 µg/L/mg vs. 1.0 ± 0.2 µg/L/mg, n=12 for each group, mean ± SE, p<0.01). Histological sections of zebrafish kidney were performed to identify zebrafish glucocorticoid steroidogenic cells28. Tg:Pomc-Pttg fish demonstrated increased intra-renal epithelial cell layers surrounding the posterior cardinal vein compared with WT, consistent with ACTH-stimulated adrenal hyperplasia ( To determine the metabolic impact of hypercortisolism in Tg:Pomc-Pttg zebrafish, adult Tg:Pomc-Pttg and WT fish were subjected to 16 hour fasting followed by ad libitum feeding of regular diet for one hour. Tg:Pomc-Pttg zebrafish exhibited consistently higher levels of fasting and postprandial blood glucose levels than WT zebrafish (96 ± 9 vs. 65 ± 10 mg/dL, mean ± S.D., p< 0.0001) ( Previous studies indicated that PTTG facilitates G1/S transition by acting coordinately with Sp1 to up-regulate cyclin D expression in human choriocarcinoma cells17. To understand the mechanism for zebrafish corticotroph PTTG overexpression inducing altered G1/S transition ( Zebrafish pituitary POMC cell differentiation starts at the anterior neural ridge by 20 hpf, and is completed within the mature pituitary by 48 hpf 23. Within the first few days of embryonic development, the transgenic fish shown here recapitulate hallmark features of Cushing's disease, i.e., lineage-specific corticotroph expansion with partial glucocorticoid resistance ( To determine the specificity of R-roscovitine action against zPttg-overexpressing POMC cells, another double transgenic line (Tg:Pomc-Pttg;Prl-RFP) was generated by breeding Tg:Pomc-Pttg fish with a previously generated PRL-RFP transgenic line, in which RFP was targeted to pituitary lactotrophs by a zebrafish Olomoucine and roscovitine are structurally related 2,6,9-trisubstituted purines, which cause G1/S or G2/M arrest by competing for ATP binding sites on CDK1 and CDK2. The R-isomer of roscovitine (R-roscovitine, CYC202) is a more potent and selective inhibitor of CDK2/cyclinE, and murine corticotrophs are highly sensitive to disrupted CDK2/cyclin E-mediated cell cycle pathways 9. Cyclin E up-regulation leads to cell cycle reentry of differentiated POMC cells and also inactivates p27kip1, further enhancing cell cycle progression 9. In addition, p27kip1 protects differentiated pituitary POMC cells from reentering the cell cycle, whereas p57Kip2 is required for cell cycle exit of pituitary precursor cells36. Given the in vivo potency of R-roscovitine against zebrafish Pttg-overexpressing corticotrophs ( Treatment with R-roscovitine (1-2 x 10-5 M) led to decreased cell number by 24 hours ( Consistent with decreased cell viability, decreased ACTH concentrations was detected in culture medium derived from R-roscovitine-treated AtT20 cells ( To further establish R-roscovitine action on corticotroph tumors in vivo, athymic nude mice (6 ∼ 8 week-old) were injected subcutaneously with AtT20 corticotroph tumor cells (1 x105 cells). Three days after tumor cell injection, 29 of 30 mice had developed small (∼2-3 mm3) but visible subcutaneous tumors, and were randomized to receive either R-roscovitine (150 mg/kg) or vehicle via oral gavage twice daily for five days each week. After three weeks, R-roscovitine caused ∼50% weight reduction of dissected tumor xenografts (40.0 ± 4.7 mg vs. 21.0 ± 2.6 mg, mean ± S.E., n = 13-14 for each group, p < 0.02) ( Consistent with the in vitro observations, Western-blot and immunohistochemistry analysis of tumor specimens showed suppressed ACTH and PCNA protein expression by R-roscovitine ( The zebrafish Pttg EST sequence was identified by Blast searching the zebrafish genome database from Sanger Institute website (GenBank accession number XM_689974). A pair of primers corresponding to zPttg cDNA 5' and 3' coding sequences, zPttg1: 5'-AACGCTGGAC-CTTAGCGAAGACT-3' (SEQ ID NO:1) and zPttg2: 5'-TACTAGAACAGGTTTCTTTATTTTCTTGCGTG-3' (SEQ ID NO:2), were used for PCR amplification to generate a zPttg cDNA containing a complete coding sequence. A tol2 transposon cassette was used to generate the Tg:pomc-pttg transgene, and transgenic founder fish were generated as previously described23. Briefly, PCR products of the Tg:pomc-pttg transgene construct (without vector DNA) were purified using a GENECLEAN III kit (Bio 101, Vista, CA) and resuspended in 5 mM Tris, 0.5 mM EDTA, 0.1 M KCl at a final concentration of 100 µg/ml. Fertilized embryos from wild-type zebrafish were injected at the one-cell stage. Microinjections were carried out five times to generate approximately 300 surviving embryos. Injected founder fish were mated to wild-type fish and their progeny. Two lines of Tg:pomc-pttg transgenics were analyzed and showed similar metabolic features. All depicted data are derived from F2 and F3 transgenic progeny. Maintenance of zebrafish and in vivo drug treatments Zebrafish embryos were maintained and raised as described23. Dexamethasone (Sigma) was dissolved in distilled water, R-roscovitin (Selleck Chemicals), flavopiridol (Selleck Chemicals), PD0332991 (Selleck Chemicals), olomoucine (Cayman Chemical Co.), and CAY10578 (Cayman Chemical Co.) were dissolved in 0.2% DMSO at a stock concentration of 1 mM, and diluted in fish medium immediately before adding to live embryos at the stages indicated. A 1.0-kb zebrafish zPttg PCR product was subcloned into the pCR4-TOPO vector, which was subsequently linearized by NotI and transcribed with T3 polymerase to generate zPttg antisense mRNA. Pit-1 antisense mRNA was generated as described46. Identification and isolation of zebrafish Tpit by Bioinformatic search of the Zebrafish Genome Database ( Adult zebrafish heads were fixed in 4% paraformaldehyde overnight and paraffin-embedded. H&E and immunohistochemical staining were performed on 5- µM sections. The streptavidin-biotin-peroxidase complex technique was used with rabbit anti-human ACTH antibodies ( WT and transgenic embryos were examined at various developmental stages under a fluorescein isothiocyanate filter on an Axioplan-2 microscope (Carl Zeiss). Live embryo images were generated with an Axiocam video system (Carl Zeiss). Fluorescence intensity of POMC-GFP-positive cells was measured by the area of interest function in Openlab software (Improvision). For confocal imaging, images were captured in a Z-series by using a TCS SP confocal microscope (Leica Microsystems). GFP was detected at a spectral range from 507 to 550 nm, RFP from 585 to 690 nm, and DAPI from 460 to 480 nm. Images were prepared using Leica LCS lite, Volocity 5.2 (Improvision), and Photoshop 7.0 (Adobe). Pituitary GFP and RFP intensity and area were measured using Volocity 5.2 (Improvision). Lipid Staining. Liver cryosectionswere stained with oil redO(Sigma-Aldrich) per manufacturer's protocol. Lipid staining was scored based upon area distribution (>50% positive cells, 2 points; <50% but>10%, 1 point;<10%, 0 points) and intensity [strong, large (size of cell) lipid droplets, 2 points; weak, small (smaller than cell size) lipid droplets, 1 point; negative, 0 points]. For blood glucose measurement, adult zebrafish were anesthetized in 0.04% tricaine methanesulfonate before tail section, and 2 µL tail blood applied to a glucometer test strip (OneTouch Ultra). For insulin tolerance test, adult zebrafish were given peritoneal insulin injection at a concentration of 0.1 U/100 mg body weight, followed by blood glucose measurement at different time points. Fish were snap-frozen in liquid nitrogen, stored at -80 °C, homogenized on ice with a micro grinder (Eppendorf), then extracted with 500 µL of cold ethanol. After centrifugation for 10 min at 1,000 × g at 4 °C, the supernatant was recovered and evaporated and the resultant pellet resuspended in 25 µL of zero calibrator buffer for cortisol radioimmunoassay (Siemens). Radioactivity was counted and results calculated by COBRA II Auto Gamma (Perkin-Elmer). Animal experiments were performed in accordance with Cedars-Sinai Institutional Animal Care and Use committee guidelines. Mouse corticotroph tumor AtT20 cells (∼1 × 105) were inoculated s.c. into 6-wk-old female nu/nu mice. Three days after tumor cell inoculation, animals were randomized to receive R-roscovitine 150 mg/kg twice daily or vehicle via oral gavage for 5 d each week. After 3 wk of treatment, mice were killed by CO2 inhalation, and tumors were dissected, weighted, and snapfrozen for further analysis. Mouse corticotroph tumorAtT20 cells were cultured in DMEM supplemented with 10% FBS at 37 °C in 5% CO2 for 24 h followed by treatment of R-roscovitine or vehicle (0.2%DMSO). Cells were replenished daily with R-roscovitine and maintained in medium for up to 48 h. siRNA transfections were performed in 70% to 80% confluent cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Protein samples were prepared in RIPA buffer (Sigma), separated on NuPAGE Novex Bis-Tris Gels (Invitrogen), and transferred onto PVDF membrane (Millipore) before blotting with primary antibody [cyclin E, 1:500 (Abeam); PTTG, 1:1,000 (Abcam); β-actin, 1:5,000 (Sigma); and p21, 1:1,000, p27, 1:500, p57, 1:1,000, pRb821, 1:1,000 (Santa Cruz Biotechnology)] at 4 °C overnight. After washes with 0.5% Tween-20 in Tris-buffered saline solution, membranes were incubated with horseradish peroxidaselinked secondary antibody (GE Healthcare) and developed using ECL Western blotting detection reagents (GE Healthcare). P value of total body cortisol levels were calculated by unpaired Student t test. Group differences in glucose levels were assessed by ANOVA. Various embodiments of the invention are described above in the Detailed Description. While these descriptions directly describe the above embodiments, it is understood that those skilled in the art may conceive modifications and/or variations to the specific embodiments shown and described herein. Any such modifications or variations that fall within the purview of this description are intended to be included therein as well. Unless specifically noted, it is the intention of the inventors that the words and phrases in the specification and claims be given the ordinary and accustomed meanings to those of ordinary skill in the applicable art(s). The foregoing description of various embodiments of the invention known to the applicant at this time of filing the application has been presented and is intended for the purposes of illustration and description. The present description is not intended to be exhaustive nor limit the invention to the precise form disclosed and many modifications and variations are possible in the light of the above teachings. The embodiments described serve to explain the principles of the invention and its practical application and to enable others skilled in the art to utilize the invention in various embodiments and with various modifications as are suited to the particular use contemplated. Therefore, it is intended that the invention not be limited to the particular embodiments disclosed for carrying out the invention. It will be understood by those within the art that, in general, terms used herein are generally intended as "open" terms ( A composition comprising oleomoucine or R-roscovitine or salts thereof for use as a medicament for administration, in a therapeutically effective amount, to a mammalian subject in need of treating a pituitary corticotroph tumor, suppressing ACTH and/or corticosterone levels in a ACTH-secreting pituitary adenoma, inhibiting the growth of an ACTH-secreting pituitary adenoma, or treating Cushing's disease to treat the pituitary corticotroph tumor, suppress the ACTH and/or corticosterone levels in a ACTH-secreting pituitary adenoma, inhibit the growth of an ACTH-secreting pituitary adenoma, or treat Cushing's disease. The composition for use according to claim 1, wherein the mammalian subject is in need of treating a pituitary corticotroph tumor and the pituitary corticotroph tumor is treated. The composition for use according to claim 1, wherein the mammalian subject is in need of suppressing ACTH and/or corticosterone levels in an ACTH-secreting pituitary adenoma and the ACTH and/or corticosterone levels in the ACTH-secreting pituitary adenoma are suppressed. The composition for use according to claim 1, wherein the mammalian subject is in need of inhibiting the growth of an ACTH-secreting pituitary adenoma and the growth of an ACTH-secreting pituitary adenoma is inhibited. The composition for use according to claim 1, wherein the mammalian subject is in need of treating Cushing's disease and Cushing's disease is treated. The composition for use according to claim 1, wherein composition comprises R-roscovitine or salts thereof.BACKGROUND
SUMMARY OF THE INVENTION
BRIEF DESCRIPTION OF THE FIGURES
DESCRIPTION OF THE INVENTION
P21 15-40 Not reported - CDK2/cyclin E P21 58-77 Not reported - CDK2/cyclin E P21 17-33 ACRRLFGPVDSEQLSRD 3 CDK2/cyclin E P21 63-77 AWE RVRGLGLPKLY 4 CDK2/cyclin E P21 141-160 KRRQTSMTDFYHSKRRLIFS 5 PCNA & CDK4/cyclin D1 P21 141-160 KRRQTSMTDFYHSKRRLIFS 6 CDK4/cyclin D1 P21 141-160 KRRQTS ATDFYHSKRRLIFS 7 CDK4/cyclin D1 P21 139-164 GRKRRQTSMTDFYHSKRRLIFSK RKP 8 CDK2/cylin E P21 141-160 KRRQTSMTDFYHSKRRLIFS 9 CDK2/cyclin E & PCNA P21 141-160 KRRATSMTDFYHSKRRLIFS 10 CDK2/cyclin E P21 141-160 KRRQTSATDFYHSKRRLIFS 11 CDK2/cyclin E P21 141-160 KRRQTSMTDFYHSKRRLIAS 12 CDK2/cyclin E P21 139-164 GRKRRQTSMTDFYHSKRRLIFSK RKP 13 CDK2/cylin E P21 139-164 GRKRRQTSMTDFYHSKRRLIFSK RKP 14 CDK2/cylin E P21 139-164 GRKRRQTSMTDFYHSKRRLIFSK RKP 15 CDK2/cylin E P21 152-159 HAKRRLIF 16 CDK2/cylin A P16 84-103 DAAREGFLDTLVVHRAGAR 17 CDK4 & CDK6 E2F 87-94 PVKRRLDL 18 Cdk2/cyclin A-E2F Rb 864-880 SNPPKPLKKRFDIE 19 CDK2/cylin A P27 Ala-Ala-Abu*-Arg-Lys-Leu-Phe-Gly** 20 CDK2/cylin A Rb2/p130 641-673 Spa 310 - CDK2 Cyclin A 285-306 TYTKKQVLRMEHLVLKVLTFDL 21 CDK2/cylin A Cyclin A 285-306 TYTKKQVLRMEHLVLKVLTFDL 22 CDK2/cylin A CDK2/cyclin A NBI1: RWIMYF-NH2 23 Cyclin A Flavopyridol* 2-(2-chlorophenyl)-5,7-dihydroxy-8-((3S,4S)-3-hydroxy-1-methylpiperidin-4-yl)-4H-chromen-4-one CDK2-4-6-9 P-276-00* Not reported CDK2-1-4 (R)-roscovitine* 6-(benzylamino)-9-isopropyl-9H-purin-2-ylamino)butan-1-ol CDK1-2-5-7-9 olomoucine Not reported NU2058 Not reported CDK2-1 SNS-032* N-(5 -((5 -tert-butyloxazol-2-yl)methylthio)thiazol-2 yl)piperidine-4-carboxamide CDK2-7-9 R-547* Not reported CDK1/cyclin B CDK2/cyclinE CDK4/cyclinD 1 PD-0332991 * 1-(2-(5-(piperazin-1-yl)pyridin-2-ylamino)-8-cyclopentyl-5-methylquinazolin-6-yl)ethanone CDK4-6 AT-7519 N* 4-(2,6-dichlorobenzamido)-N-(piperidin-4-yl)-1H-pyrazole-3-carboxamide CDK1-2-7-9 UCN-01 N Not reported CDK2, pRb Indirubin Derivatives 5,5'-substituted-indirubin-3-oxime CDK1-2 Indole-3 carbinol (1H-indol-3-yl)methanol cyclin D1, cyclin E, CDK2-4-6, p15, p21, p27 Paullone Derivatives dihydro-indolo-benzazepines CDKs (non-specific) Hymenialdisine Not reported CDKs, GSK-3, ch1 SU 9516 Not reported CDK2-1-4 BML-259 Not reported CDK5-2 Purvalanol A Not reported CDK2-5-4 Ryuvidine Not reported CDK4 AG-024322 Not reported CDK1-2-4 Fascaplysin Not reported CDK4 EXAMPLES
References
SEQUENCE LISTING
MELTED, Shlomo
LIU, Ning-Ai
<211> 23
<212> DNA
<213> Zebrafish
aacgctggac cttagcgaag act 23
<211> 32
<212> DNA
<213> Zebrafish
tactagaaca ggtttcttta ttttcttgcg tg 32
<211> 17
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 14
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 20
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 20
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 20
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 26
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 20
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 20
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 20
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 20
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 26
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 26
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 26
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 8
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 19
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 8
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 14
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 8
<212> PRT
<213> Artificial
<223> synthetic construct
<221> MISC_FEATURE
<222> (3)..(3)
<223> Xaa can be aminobutyric acid (Abu)
<211> 22
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 22
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 9
<212> PRT
<213> Artificial Sequence
<223> synthetic construct
<211> 202
<212> PRT
<213> Homo sapiens
<211> 199
<212> PRT
<213> Mouse
<211> 188
<212> PRT
<213> Xenopus
<211> 182
<212> PRT
<213> Zebrafish