코폴리카보네이트 수지 조성물
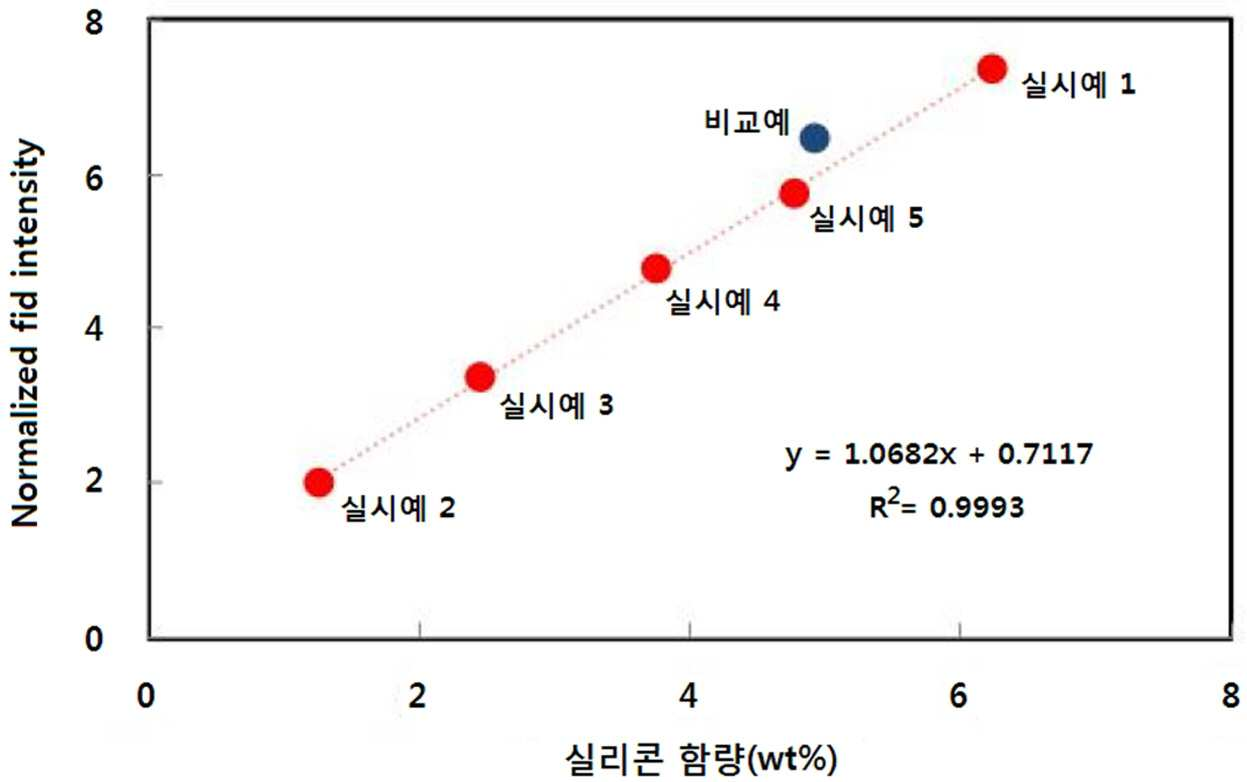
[...] excellent in various properties and reduced in the present invention refers to is directed to compositions. A bisphenol polycarbonate resin precursor directly from an aromatic diol and such as polycarbonate such as phosgene is bath number by polycondensing a precursor, excellent impact strength, dimensional stability, has heat resistance and transparency, etc., electric/electronic number article a sheathing of, automobile parts, building material, optical components or the like, unwoven fabric is 2000. Various polymeric drug delivery systems via these polycarbonate resin recently to apply the field of 2 different data structure at least one aromatic diol compound by copolymerizing a second units of polycarbonate backbone has physical properties durable desired by introducing sufficient to obtain has many attempted of wet liquid to flow down. Specially of polycarbonate backbone for the introduction of mountain-shaped structure polysiloxane be inclined progress also study, high production costs are techniques are most of, chemical resistance or impact strength, in particular low temperature impact strength such as flowable vice versa is e.g. increased the operates a key or particular mode KIPO &. Thus, order entire structure material properties chemical structures of polycarbonate, polycarbonate chain fluidity of which comprises at least three types of (mobility) should injects and the like. In the present invention TD the - NMR Fid test (time-domain) analyzing the mobility of matrix on a molecular level. The laser beam is transmitted through an important Fid signal pattern as NMR T2 relaxation time information, of molecular structures which show differences according to flexibility, the decay rate is than θ1. in Fid signal. I.e., molecules though the Fid decay rate is made faster, and is rigid, though the flexible. the slower the Fid decay rate. In the present invention the, of polycarbonate backbone polysiloxane-introduced [...] and optionally a polycarbonate for including [...] compositions for TD (time-domain) - NMR Fid analysis, various excellent in various properties and reduced in number was under trillion[...] composition. The present invention refers to a and optionally a polycarbonate [...][...] composition including under public affairs number. for. In addition, the present invention refers to said article including composition [...]under public affairs number. for. In order to solve number and said, the present invention refers to i) aromatic polycarbonate number 1 repeating units; and at least one having a siloxane bond a aromatic polycarbonate number 2 including repeating units include or copolycarbonates, or ii) including said [...] and polycarbonate, [...] composition, these compositions comprise said [...] satisfying 1 expressions, . under public affairs[...] number composition: [Expressions 1] 1. 0682 × X + 0. 51 < Y < 1. 0682 × X + 1. 2 In 1 said expressions, X the total weight of and polycarbonates [...] of a silicon content (weight %) means, The Y (time-domain) TD obtained by test Fid Fid Intensity 0 value. 1 msec in. then, a point on the normalized. Hereinafter, the present invention describes detail the, each component for classifying and of the phenylindane bisphenol, for facilitating 'A' to, represented by a 'B' the polycarbonate. [...]( A) The present invention according to (A) [...] the, mountain-shaped structure polysiloxane of polycarbonate backbone. polymer have been introduced. Specifically, said aromatic polycarbonate number 1 precursor or a diol compound aromatic the repeat unit by the reaction between the formed in an inner, preferably a. under public affairs number copolycarbonates represented by formula 1: [Formula 1] In said formula 1, R1toR4independently from each other hydrogen, C1-10alkyl, C1-10alkoxy, or halogens, Z a non-substituted or phenyl is replaced by the processing result aC1-10alkylene, or unsubstitutedC1-10alkyl substitutedC3-15alkylene cycle, O, S, SO, SO2, or is CO. Preferably, R1toR4independently from each other hydrogen, methyl, chloro, or bromo is. In addition preferably, Z a non-substituted or phenyl substituted with straight or branched chainC1-10alkyl [...] which, more preferably methylene, ethane - 1, 1 - diyl, propane - 2, 2 - diyl, butane - 2, 2 - diyl, 1 - phenyl ethane - 1, 1 - diyl, or is [...]. In addition preferably, Z has cyclohexane - 1, 1 - diyl, O, S, SO, SO2, or is CO. Preferably, said formula 1 bis derivative is marked as the chemical (4 - hydroxyphenyl) methane, bis (4 - hydroxyphenyl) ether, bis (4 - hydroxyphenyl) sulfone, bis (4 - hydroxyphenyl) sulfoxide, bis (4 - hydroxyphenyl) sulfide, bis (4 - hydroxyphenyl) ketone, 1, 1 - bis (4 - hydroxyphenyl) ethane, bisphenol A, 2, 2 - bis (4 - hydroxyphenyl) butane, 1, 1 - bis (4 - hydroxyphenyl) cyclohexane, 2, 2 - bis (4 - hydroxy - 3, 5 - [...]) propane, 2, 2 - bis (4 - hydroxy - 3, 5 - dichlorophenyl) propane, 2, 2 - bis 99900002 28999 (4 - hydroxy - 3 - bromo phenyl) propane, 2, 2 - bis (4 - hydroxy - 3 - chlorophenyl) propane, 2, 2 - bis (4 - hydroxy - 3 - methyl phenyl) propane, 2, 2 - bis (4 - hydroxy - 3, 5 - dimethyl-phenyl) propane, 1, 1 - bis (4 - hydroxyphenyl) - 1 - phenyl ethane, bis (4 - hydroxyphenyl) diphenylmethane, and α, ω - bis [3 - (ο - hydroxyphenyl) profile] polydimethylsiloxane selected from the group consisting of aromatic least one. may be derived from a diol compound. Said 'aromatic diol compound derived from a' a meaning of, aromatic diol compound hydroxy group by the reaction between the precursor polycarbonate and said formula 1 derivative is marked as the chemical to form a.. E.g., aromatic diol compound bisphenol A and a polycarbonate which are the precursors when polymerized [...] tree, said formula 1 formula 1 - 1 which is marked as a chemical derivative is marked as the chemical is represented by: [Formula 1 - 1] . Said polycarbonate precursor include, dimethyl carbonate, diethyl carbonate, dibutyl polycarbonate, dicyclohexyl polycarbonate, diphenyl carbonate, d toe carbonate, bis (chlorophenyl) polycarbonate, polycarbonate [...] di - m -, dinitrate, polycarbonate [...], bis (diphenyl) polycarbonate, phosgene, triphosgene, diphosgene, bromo triphosgene and bis [...] 1 selected from the group consisting of, use can be made of, at least one. Preferably, triphosgene or phosgene, use can be made of, . In addition, one or more said having a siloxane bond a aromatic polycarbonate the repeat unit number 2, one or more siloxane compound or precursor by the reaction between the formed in an inner, preferably a derivative is marked as the chemical formula 2 and a repeating unit expressed by formula 3 including copolycarbonates. under public affairs number: [Formula 2] In said formula 2, X1are each independentlyC1-10[...] alkyl, R5independently from each other hydrogen; [...] or or unsubstituted, oxy [...] substitutedC1-10alkoxy, orC6-20R4 substitutedC1-15alkyl; halogen; C1-10alkoxy; allyl; C1-10haloalkyl; orC6-20it will be biting and, The n being integers, of 10 to 200, [Formula 3] In said formula 3, X2are each independentlyC1-10[...] alkyl, Y1are each independently hydrogen, C1-6alkyl, halogen, hydroxy, C1-6alkoxy, orC6-20it will be biting and, R2independently from each other hydrogen; [...] or or unsubstituted, oxy [...] substitutedC1-10alkoxy, orC6-20R4 substitutedC1-15alkyl; halogen; C1-10alkoxy; allyl; C1-10haloalkyl; orC6-20it will be biting and, M is integer number of the 10 to 200. In said formula 2, preferably, X1independently from each otherC2-10alkyl [...], more preferablyC2-4alkyl [...], is most preferably propane - 1, 3 - diyl. In addition preferably, R5independently from each other hydrogen, methyl, ethyl, profile, 3 - phenylpropyl, 2 - phenylpropyl, 3 - (oxy [...]) profile, fluoro, chloro, bromo, mol % of a multifunctional crosslinking agent, methoxy, ethoxy, propoxy, allyl, 2, 2, 2 - trifluoroethyl, 3, 3, 3 - trifluoropropyl, phenyl, is naphthyl or. In addition preferably, R5independently from each otherC1-10alkyl, more preferablyC1-6alkyl, more preferablyC1-3alkyl, most preferably methyl, is. In addition preferably, the n 10 or more, 15 or more, 20 or more, 25 or more, 30 or more, 31 or more, or at least 32, 50 hereinafter, 45 hereinafter, 40 hereinafter, 39 hereinafter, 38 hereinafter, or is integer number of 37 hereinafter. In said formula 3, preferably, X2independently from each otherC2-10alkyl [...], more preferablyC2-6alkyl [...], most preferably is polyisobutylene. In addition preferably, Y1. is hydrogen. In addition preferably, R6independently from each other hydrogen, methyl, ethyl, profile, 3 - phenylpropyl, 2 - phenylpropyl, 3 - (oxy [...]) profile, fluoro, chloro, bromo, mol % of a multifunctional crosslinking agent, methoxy, ethoxy, propoxy, allyl, 2, 2, 2 - trifluoroethyl, 3, 3, 3 - trifluoropropyl, phenyl, is naphthyl or. In addition preferably, R6independently from each otherC1-10alkyl, more preferablyC1-6alkyl, more preferablyC1-3alkyl, most preferably methyl, is. In addition preferably, the m 40 or more, 45 or more, 50 or more, 55 or more, 56 or more, 57 or more, or at least 58, 80 hereinafter, 75 hereinafter, 70 hereinafter, 65 hereinafter, 64 hereinafter, 63 hereinafter, or is integer number of 62 hereinafter. Said formula 2 derivative is marked as the chemical units and said formula 3 to the repeat unit represented by formula 2 - 1 respectively and it and siloxane compound represented by represented by formula 3 - 1 derived from a siloxane compound. [Formula 2 - 1] In said formula 2 - 1, X1, R5and a previous definition of n as defined. [Formula 3 - 1] In said formula 3 - 1, X2, Y1, R6and a previous definition of m as defined. Said 'siloxane compound derived from a' a meaning of, said each siloxane compound hydroxy group by the reaction between the precursor polycarbonate and each said units and derivative is marked as the chemical formula 2 a repeating unit expressed by formula 3 to form a.. In addition, the repeating units of said formula 2 and 3 can be use in the formation of carbonate precursors, reticle the repeating units of formula 1 can be use in the formation of carbonate precursor taught equal. Said formula 3 - 1 and siloxane compound represented by said formula 2 - 1 represented by number acid compounds that siloxane each of known as a raw material of the method bath 1 and 2 and a equal. [Known as a raw material of the 1] Said known as a raw material of the in 1, X1'theC2-10[...], X1, R5and a previous definition of n are defined above are, [Known as a raw material of the 2] In said known as a raw material of the 2, X2'theC2-10[...], X2, Y1, R6and a previous definition of m as defined. Said known as a raw material of the reactions of 2 and reactive 1, may perform well under the metal catalyst it is preferable that the. Pt said metal catalyst it is preferable that the catalyst, bi ash catalyst Pt (Ashby) catalyst, [...] in cals (Karstedt) catalyst, ramosetron Reoviruses (Lamoreaux) catalyst, of spire, (Speier) catalyst, PtCl2 (COD), PtCl2 (cyclohexanol and its salts)2, andH2PtBr6selected from the group consisting of 1, use can be made of, at least one. Said formula 7 or 9 a metal catalyst said derivative is represented by the following 0 based on 100 parts by weight of. 001 part by weight or more of metal, 0. 005 part by weight or more of metal, or 0. 01 part by weight or more of metal and, hereinafter parts by weight 1, 0. 1 parts by weight hereinafter, or 0. 05 parts by weight, use can be made of, to hereinafter. In addition, said reaction temperature is preferably 80 to 100 °C. In addition, time, the reaction times are said preferably 1 hr to 5. In addition, the derivative is represented by the following said formula 7 or 9 [...]under trillion[...] acid catalyst of cationic exchange can be the number, of silica preferably, said n m and. capable of modulating. said reaction temperature is preferably 50 to 70 °C. In addition, said, the reaction times are preferably time 6 hr to 1. [...] in said, tetra hexamethyldisiloxane, tetra the phenyl D thread rock it buys, hexamethyldisiloxane and hexamethylenediamine the phenyl D thread rock it buys selected from the group consisting of 1, use can be made of, at least one. In addition, said [...][...], for example, may be used in, is, in octahydro methyl cycloalkyl represented cycloalkyl phenyl and represented as to the aromatic hydrocarbon KIPO &. Said [...], based on 100 parts by weight of said 0 [...]. 1 part by weight or more of metal, or 2 part by weight or more of metal and, hereinafter 10 parts by weight, or 8 parts by weight, use can be made of, to hereinafter. Said acid catalystH2SO4, HClO4, AlCl3, SbCl5, SnCl4and acidic white clay selected from the group consisting of 1, use can be made of, at least one. In addition, said [...] based on 100 parts by weight of a acid catalyst 0. 1 part by weight or more of metal, 0. 5 part by weight or more of metal, or 1 part by weight or more of metal and, hereinafter 10 parts by weight, hereinafter 5 parts by weight, or 3 parts by weight, use can be made of, to hereinafter. In particular, said formula 2 derivative is marked as the chemical and of repeating units represented by said formula 3 of silica, . capable of modulating properties. The weight ratio between said repeating units can be is 99:1 to 1:99. Preferably 3:97 to 97:3, 95:5 to 5:95, 10:90 to 90:10, or 15:85 to 85:15 and, more preferably is 80:20 to 20:80. Said siloxane compound the weight ratio of repeating units, e.g. and siloxane compound represented by said formula 2 - 1 represented by said formula 3 - 1 weight ratio of siloxane compound is corresponding to. Preferably, a derivative is marked as the chemical said formula 2, a is represented by formula 2 - 2: [Formula 2 - 2] In said formula 2 - 2, R5and n as defined sequence. Preferably, R5is the methyl. In addition preferably, the repeat unit represented by said formula 3, is represented by formula 3 - 2 a: [Formula 3 - 2] In said formula 3 - 2, R6and m as defined sequence. Preferably, R6is the methyl. In addition preferably, said phenylindane bisphenol,, said formula 1 - 1 derivative is marked as the chemical, said formula 2 - 2 derivative is marked as the chemical units and said formula 3 - 2 derivative is marked as the chemical includes both. In addition, copolycarbonates number bath method as the above-mentioned the present invention refers to, aromatic diol compound, polycarbonate precursor and at least one siloxane compound polymerizing including copolycarbonates under public affairs method bath number number of.. Said aromatic diol compound, polycarbonate precursor and at least one siloxane compound provide the taught equal. Upon polymerization said, the siloxane compound one or more said, aromatic diol compound, polycarbonate precursor and at least one siloxane compound total 0 to 100 weight %. 1 weight % or more, 0. 5 weight % or more, 1 weight % or more, or 1. 5% weight of at least, 20 weight % hereinafter, 10 weight % hereinafter, 7 weight % hereinafter, 5 weight % hereinafter, 4 weight % hereinafter, 3 weight % hereinafter, or 2 weight % hereinafter that is capable of using optical. In addition, said aromatic diol compounds, aromatic diol compound, polycarbonate precursor and at least one siloxane compound total 100 weight % to 40 weight % or more, 50 weight % or more, or at least 55 weight %, 80 weight % hereinafter, 70 weight % hereinafter, or, use can be made of, to 65 weight % hereinafter. In addition, polycarbonate precursors said, aromatic diol compound, polycarbonate precursor and at least one siloxane compound total 100 weight % to 10 weight % or more, 20 weight % or more, or 30 weight % and, 60 weight % hereinafter, 50 weight % hereinafter, or 40 weight % hereinafter, use can be made of, to. In addition, at said polymerization method, using, for example, can be interfacially polymerized method, in this case atmospheric pressure and low polymerization at temperature reaction which are KIPO & the controlled molecular weight. it buys combination the interfacially polymerized said number and an organic solvent carried out in the presence of preferably. In addition, said interfacially polymerized, for example, for rectifies sum (pre-metal oxide, metal carbonate, silica) then charging after coupling number, again polymerisation of the Michael adducts may comprise, in this case high molecular weight material is obtained copolycarbonates. Said interface used in the polymerization of polycarbonate materials materials that may be used for the polymerization of specially if not one number, used amount of thereof can be is adjusted as necessary. it buys combination said number include, for example, sodium hydroxide, alkali metal hydroxide such as potassium hydroxide or pyridine such as use can be made of, for amine compound. Said organic solvents comprise trialkylamines polycarbonate conventional solvent used in the polymerization of a specially if not one number, in one example methylene chloride, trichlorobenzene of a halogen, such use can be made of, for hydrogen hydrocarbons. In addition, said interfacially polymerized the reaction promotes the triethylamine, tetra - n - butyl ammonium bromide, tetra - n - butyl [...] such as amine compound difference 3, 4 quaternary ammonium compounds, such as compounds and the like phosphonium difference 4 number for promoting conversion reaction of toxic further, use can be made of, . 0 to 40 °C said interfacially polymerized of the reaction temperature is,, the reaction times are 10 minutes to 5 preferably time. In addition, interface during polymerization, the pH 9 or more, or 11 preferably greater than. In addition, the controlled molecular weight the interfacially polymerized said further includes a number can be. The controlled molecular weight in a polymeric disclosure number said before, during or after polymerization disclosure disclosure polymerization. can be input. Number to said alkylphenol using the controlled molecular weight can be mono -, said mono - alkylphenol, for example then, a P-tert - butyphynol, p - [...], midifiers targeting phenol, use of Saccharomyces cerevisiae thread lung glow, midifiers targeting tetra phenol, methylhexadecyl phenol, phenol midifiers targeting, thread lung glow eicosa, [...] and nose room lung glow 1 selected from the group consisting of and at least one inert gas, preferably P-tert - butyphynol is, greater is this case molecular weight regulating effects. The number said controlled molecular weight diol compound aromatic, for example, based on 100 parts by weight of 0. 01 part by weight or more of metal, part by weight or more of metal 0, 1, or 1 part by weight or more of metal and, hereinafter 10 parts by weight, hereinafter 6 parts by weight, or 5 parts by weight and body is included to hereinafter, within this range can cause a molecular weight desired. Said phenylindane bisphenol,, weight average molecular weight of 1,000 to 100,000 g/mol, more preferably 15,000 to 35,000 g/mol is. More preferably, a weight average molecular weights in said 20,000 g/mol or more, 21,000 g/mol or more, 22,000 g/mol or more, 23,000 g/mol or more, 24,000 g/mol or more, 25,000 g/mol or more, 26,000 g/mol or more, 27,000 g/mol or more, or at least 28,000 g/mol. In addition, a weight average molecular weights in said hereinafter 34,000 g/mol, hereinafter 33,000 g/mol, or 32,000 g/mol is hereinafter. Polycarbonate (B) Polycarbonate (B) the present invention according to the, of polycarbonate be introduced in the polysiloxane backbone is not in that, said [...] is divided and a (A). Preferably, said to the polycarbonate derivative is marked as the chemical formula 4 includes: [Formula 4] In said formula 4, R'1toR'4independently from each other hydrogen, C1-10alkyl, C1-10alkoxy, or halogens, Z ' a non-substituted or phenyl is replaced by the processing result aC1-10alkylene, or unsubstitutedC1-10alkyl substitutedC3-15alkylene cycle, O, S, SO, SO2, or is CO. In addition preferably, said polycarbonate (B) weight average molecular weight of 1,000 to 100, 0000 g/mol, more preferably is 10,000 to 35,000 g/mol. More preferably, the 11,000 or more said weight average molecular weight (g/mol), 12,000 or more, 13,000 or more, 14,000 or more, 15,000 or more, 16,000 or more, 17,000 or more, or at least 18,000. In addition, the weight average molecular weight 34,000 hereinafter (g/mol), 33,000 hereinafter, 32,000 hereinafter, 31,000 hereinafter, 30,000 hereinafter, or 29,000 is hereinafter. Said formula 4 a derivative is marked as the chemical, precursor or aromatic diol compound is formed by the reaction between the. Said can be used an aromatic diol compound or a precursor, derivative is marked as the chemical formula 1 prior taught same as.. Preferably, said formula 4 ofR'1toR'4and Z ' the, formula 1 of each reticleR1toR4Z and. the same. In addition preferably, a derivative is marked as the chemical said formula 4, a is represented by formula 4 - 1: [Formula 4 - 1] . In addition, bath number of said polycarbonate (B) the method, one or more siloxane compound using sense that it does not point a and under the outside number [...] number of said (A) equals method bath. [...] resin composition [...] resin composition the present invention according to, the above-mentioned [...] (A) and optionally a polycarbonate (B) includes. The X of said expressions [...] 1 silicone contents by resin composition (wt %) meaning, NMR analysis.. In addition, said polycarbonate (B) polysiloxane has mountain-shaped structure at this time have been introduced, [...] polycarbonate resin composition (B) controlling the content of the, .type can be control the X. The X preferably 0. 1 to 20 and, more preferably, 1 to 10 and, most preferably 1. 2 to 7. 0 is. In addition, said expressions Y value of 1, such as examples of the experiment the after alcoholic beverage it will do TD (time-domain) - NMR Fid. is provided with a bag. According to one embodiment of the present invention, the present invention according to [...] composition, while included within the scope of 1 expressions, included within the scope of is relate compared where polymer structure and fluidity of signal of the falling part or the (mobility) can be confirm that the user. In addition, these fluid (mobility) yarn to produce various the difference in is able to confirm that KIPO & impacting. More preferably, the present invention according to [...] composition, to satisfy an 1 - 1 expressions: [Expressions 1 - 1] 1. 0682 × X + 0. 60 < Y < 1. 0682 × X + 1. 0 Said expressions in 1 - 1, X and Y has a sequence as defined. The present invention according to [...] composition, preferably weight average molecular weight is 1,000 to 100,000 (g/mol) and, more preferably is 15,000 to 35,000. More preferably, a weight average molecular weights in said 20,000 or more, 21,000 or more, 22,000 or more, 23,000 or more, 24,000 or more, 25,000 or more, 26,000 or more, 27,000 or more, or at least 28,000. In addition, a weight average molecular weights in said 34,000 hereinafter, 33,000 hereinafter, or 32,000 is hereinafter. In addition, the present invention according to [...] composition, preferably ASTM D256 (1/8 inch, Notched Izod) based on the impact strength measured at 23 °C down the substrate is 1000 J/m to 750. More preferably, said room temperature impact strength (J/m) or more the 760, 770 or more, 780 or more, 790 or more, 800 or more, 810 or more, 820 or more, 830 or more, 840 or more, 850 or more, 860 or more, or at least 870. In addition, said room temperature impact strength (J/m) the value in the higher the excellent korean Bank cannot number of an upper limit, in one example 990 hereinafter, 980 hereinafter, or can be 970 hereinafter. In addition, the present invention according to [...] composition, preferably ASTM D256 (1/8 inch, Notched Izod) -30 °C based on the measured at a low temperature impact strength is 1000 J/m to 150. More preferably, said low temperature impact strength the 160 or more (J/m), 170 or more, 180 or more, 190 or more, or at least 200. In addition, said low temperature impact strength (J/m) the value in the higher the excellent korean Bank cannot number of an upper limit, in one example 990 hereinafter, 980 hereinafter, or can be 970 hereinafter. In addition, said resin composition [...], oxidation-resistant necessary number, thermal comfort number, of sunscreen number, number plasticizer, antistatic number, nuclear number, flame retardant number, bow number, number impact, whitening number, ultraviolet absorbing number, dyestuffs pigment and selected from the group consisting of any one or more of further may include. In addition, the present invention refers to said article including resin composition. under public affairs[...] number. Preferably, said article is injection-molded articles. Said number of the article the method bath, the present invention according to [...] resin composition addition to necessary number after using an a mixer, said extruding a mixture into and under trillion number into pellets by extruding a, which bear comparison with imported block said injection dried pellets may comprise an injecting a. As longitude in said, the present invention according to of polycarbonate backbone polysiloxane-introduced [...] and optionally a polycarbonate a [...] composition including, according to analysis - NMR Fid TD (time-domain) is connected to the semiconductor layer. that certain conditions are met. Figure 1, the present invention measured according to the saturated in range where R has considerable T2 relaxation is shown with codes. Hereinafter, preferred embodiment to assist in the understanding of the invention are is in number. However the in the embodiment of the present invention is exemplified for is, the present invention. are not limited to only for their. 1 memorable number: aSSOCIATED PRESS-34 Octahydro 47 represented methyl cycloalkyl. 60 g (160 mmol), tetra hexamethyldisiloxane 2. 40 g (17. 8 mmol) after mixing, said 100 represented methyl cycloalkyl octahydro mixture acidic contrast parts by weight clay (DC-A3) 3L with 1 parts by weight 4 to 60 °C multi function cap paste has better mouth feeling and reacted time. After reaction, ethyl which has dilution and the filtered buffer whose width is the same BTS plates comprises a back plate supply unit. Thus obtained end and adherent polyorganosiloxane composition repeating units (n) the1H NMRidentified as this solution is poured into a tender 34 results. Polyorganosiloxane and adherent end obtained said the reel lung glow which it will know 2 - 4. 81 g (35. 9 mmol) (Karstedt's platinum catalyst) [...] in cals and platinum catalyst 0. 01 g (50 ppm) in discharging commodities by throwing a 90 °C reacted time 3. After reaction, unreacted siloxanes have siloxane backbones 120 °C, 1 torr by [...]eBay about an article which he/she was authored in the first client by a number. Thus obtained an end modified polyorganosiloxanes' aSSOCIATED PRESS-34' was referred to as. The yearly yellow oil is aSSOCIATED PRESS-34, using Varian 500MHz1H NMRthrough repeating units (n) heterocomplexes 34 identifying the corresponding advertisement based on the shown list, longer for alternating current load did not necessary has number. 2 memorable number: MB-58 Octahydro 47 represented methyl cycloalkyl. 60 g (160 mmol), tetra hexamethyldisiloxane 1. 5 g (11 mmol) after mixing, said 100 represented methyl cycloalkyl octahydro mixture acidic contrast parts by weight clay (DC-A3) 3L with 1 parts by weight 4 to 60 °C multi function cap paste has better mouth feeling and reacted time. After reaction, ethyl which has dilution and the filtered buffer whose width is the same BTS plates comprises a back plate supply unit. Thus obtained end and adherent polyorganosiloxane composition (m) repeating units the1H NMRidentified as this solution is poured into a tender 58 results. Polyorganosiloxane and adherent end obtained said 3 - methylbut - 3 - enyl 4 - hydroxybenzoate (3-methylbut-3-enyl 4-hydroxybenzoate) 6. 13 g (29. 7 mmol) (Karstedt's platinum catalyst) [...] in cals and platinum catalyst 0. 01 g (50 ppm) in discharging commodities by throwing a 90 °C reacted time 3. After reaction, unreacted siloxanes have siloxane backbones 120 °C, 1 torr by [...]eBay about an article which he/she was authored in the first client by a number. Thus obtained an end modified polyorganosiloxanes' MB-58' was referred to as. The yearly yellow oil is MB-58, using Varian 500MHz1H NMR( m) repeating units through the 58 corresponding advertisement based on the shown list identifying randomly choosing, longer for alternating current load did not necessary has number. 3 memorable number: eU-50 Octahydro 47 represented methyl cycloalkyl. 60 g (160 mmol), tetra hexamethyldisiloxane 1. 7 g (13 mmol) after mixing, said 100 represented methyl cycloalkyl octahydro mixture acidic contrast parts by weight clay (DC-A3) 3L with 1 parts by weight 4 to 60 °C multi function cap paste has better mouth feeling and reacted time. After reaction, ethyl which has dilution and the filtered buffer whose width is the same BTS plates comprises a back plate supply unit. Thus obtained end and adherent polyorganosiloxane composition repeating units (n) the1H NMR50 results identified as this solution is poured into a tender. Methyleugenol said polyorganosiloxane and adherent end obtained (Eugenol) 6. 13 g (29. 7 mmol) (Karstedt's platinum catalyst) [...] in cals and platinum catalyst 0. 01 g (50 ppm) in discharging commodities by throwing a 90 °C reacted time 3. After reaction, unreacted siloxanes have siloxane backbones 120 °C, 1 torr by [...]eBay about an article which he/she was authored in the first client by a number. Thus obtained an end modified polyorganosiloxanes' eU-50' the designated. The yearly yellow oil is eU-50, using Varian 500MHz1H NMRthrough the 50 repeating units (n) heterocomplexes corresponding advertisement based on the shown list identifying, longer for alternating current load did not necessary has number. 4 memorable number: PC Polymerisation reactor and to an water 1784 g, NaOH 385 g and BPA (bisphenol A) doesn't have any error frames, 232 g, N2was on the 1st layer of admixing atmosphere. PTBP herein 4 (para-tert butylphenol). 7 g of dissolved in MC (methylene chloride) is incorporated. TPG (triphosgene) MC then melt to 128 g of a pH greater than 11 1 while the discharging commodities by throwing a time reacting a 46 g (triethylamine) TEA behind the ingredient 10 extended storage and is easily carried coupling (coupling) the reaction. Total reaction time 1 time 20 then last min number triggers a pH cd1a. TEA and the lower end of a gate 4 a, distilled to 3 generated cleaning times a pH of the polymer was fitted neutral 6 - 7. The polymers thus obtained mixed solution hexane methanol and precipitating the yield in, 120 °C same final dried in obtained polycarbonate, which has no hair is provided as' PC ' same. In the embodiment 1 Polymerisation reactor and to an water 1784 g, NaOH 385 g and BPA (bisphenol A) doesn't have any error frames, 232 g, N2was on the 1st layer of admixing atmosphere. PTBP herein 4 (para-tert butylphenol). 3 g polydimethylsiloxane and 6. 57 g (under trillionaSSOCIATED PRESS-pDMS in a memorable number 1 number 5 (n=34). 91 g and memorable number 2 number mBHB-pDMS in a under trillion 0 (m=58). 66 g (90:10 weight ratio), a mixed solution prepared) dissolved in a MC (methylene chloride) is incorporated. TPG (triphosgene) MC then melt to 128 g of a pH greater than 11 1 while the discharging commodities by throwing a time reacting a 46 g (triethylamine) TEA behind the ingredient 10 extended storage and is easily carried coupling (coupling) the reaction. Total reaction time 1 time 20 then last min number triggers a pH cd1a. TEA and the lower end of a gate 4 a, distilled to 3 generated cleaning times a pH of the polymer was fitted neutral 6 - 7. The polymers thus obtained mixed solution hexane methanol and precipitating the yield in, 120 °C same final dried in obtained copolycarbonates. In the embodiment 2 [...] memorable number of said in the embodiment 1 and 20 4 polycarbonate (PC) the 80 parts by weight of a, number was under trillion[...] composition. In the embodiment 3 [...] memorable number of said in the embodiment 1 and 40 4 polycarbonate (PC) 60 parts by weight of a the, number was under trillion[...] composition. In the embodiment 4 [...] memorable number of said in the embodiment 1 and 60 4 polycarbonate (PC) 40 the parts by weight of a, number was under trillion[...] composition. In the embodiment 5 [...] memorable number of said in the embodiment 1 and 80 4 polycarbonate (PC) 20 parts by weight of a the, number was under trillion[...] composition. Compared e.g. The same method is under trillion number in said in the embodiment 1, polydimethyl siloxane under trillion a memorable number eU-50 6 3 in number. 57 g using, copolycarbonates obtained. Experiment e.g. under trillion[...] a number in said in the embodiment and comparison 1 parts by weight or [...] composition, tris (2, 4 - di - tert - isobutyl phenyl) phosphite 0. 050 parts by weight, midifiers targeting - 3 - (3, 5 - di - tert - butyl - 4 - hydroxyphenyl) propionate 0. 010 parts by weight, 0 [...]. 030 part by weight, using this axial extruder Φ 30mm attached vent, after pellet grudge, JSW (main) n-20C 300 °C cylinder temperature using aggressive injection, mold temperature 80 °C by an injection molding, of specimen was under trillion number. It was determined that properties of each method hereinafter. 1) weight average molecular weight (Mw): PC using Agilent 1200 series using the Boudouard stencil (Standard) is measured with GPC. 2) low temperature impact strength: ASTM D256 (1/8 inch, Notched Izod) based on the measured at -30 °C. 3) low temperature impact strength: ASTM D256 (1/8 inch, Notched Izod) based on the measured at -30 °C. 4) (wt %) silicone contents by: NMR analysis it was determined that silicon content. 5) - NMR Fid experiment (time-domain) TD: using the standard operational process it stands The minispec mq20 Polymer Research System "sOP-0274-0k Bruker Optics [...] surge an actuating procedure standard Minispec" fid data and setup experiment according to a obtained. Said precursor and CaO precursor to 1 table a result, TD (time-domain) - NMR Fid experiments showed even Figure 1. In Figure 1, X shaft in the composition (weight %) silicone contents by [...] means, TD shaft Y (time-domain) - NMR Fid Normalized Fid intensity measured at. mixture by the addition of an initiator. Said table 1 and 1 as demonstrated, in the case of the present invention according to in the embodiment, X and Y is expressions while satisfies 1, the first deoxygenator compared, in the event of not happy with can be confirm that the user. In addition, in particular the impact strength room temperature which differs in both could confirm it. The present invention relates to a copolycarbonate and a composition comprising the same. The copolycarbonates according to the present invention has a structure in which a specific siloxane compound is introduced in a main chain of the polycarbonate, and has small difference between an impact strength at room temperature and an impact strength at low-temperature and thereby exhibits an excellent impact resistance. I) aromatic polycarbonate number 1 repeating units; and at least one having a siloxane bond a aromatic polycarbonate number 2 including repeating units include or copolycarbonates, or ii) including said [...] and polycarbonate, [...] composition, these compositions comprise said [...] satisfies 1 expressions, [expressions 1] 1. 0682 × X + 0. 51 < Y < 1. 0682 × X + 1. 2 said expressions in 1, X the total weight of and polycarbonates [...] of a silicon content (weight %) means, the Y (time-domain) TD obtained by test Fid Fid Intensity 0 value. 1 msec then, a point on the normalized in means, said having a siloxane bond one or more repeating units of a aromatic polycarbonate number 2, a derivative is marked as the chemical formula 2 and including a repeating unit expressed by formula 3, [...] composition: [formula 2] In said formula 2, X1are each independentlyC1-10alkyl [...], R5independently from each other hydrogen; [...] or or unsubstituted, oxy [...] substitutedC1-10alkoxy, orC6-20R4 substitutedC1-15alkyl; halogen; C1-10alkoxy; allyl; C1-10haloalkyl; orC6-20it will be biting and, the n being integers, of 10 to 200, [formula 3] In said formula 3, X2are each independentlyC1-10alkyl [...], Y1are each independently hydrogen, C1-6alkyl, halogen, hydroxy, C1-6alkoxy, orC6-20it will be biting and, R6independently from each other hydrogen; [...] or or unsubstituted, oxy [...] substitutedC1-10alkoxy, orC6-20R4 substitutedC1-15alkyl; halogen; C1-10alkoxy; allyl; C1-10haloalkyl; orC6-20it will be biting and, m is integer number of the 10 to 200. According to Claim 1, said X a 0. 1 to 20 characterized in that, [...] composition. According to Claim 1, these compositions comprise expressions [...] said 1 - 1 characterized in that that it satisfies, [...] composition: [1 - 1] 1 expressions. 0682 × X + 0. 60 < Y < 1. 0682 × X + 1. 0 said expressions in 1 - 1, the Y and X as defined according to Claim 1. According to Claim 1, said composition [...], ASTM D256 (1/8 inch, Notched Izod) based on the impact strength measured at 23 °C down the substrate-free bismuth glass characterized in that 1000 J/m to 750, [...] composition. According to Claim 1, said [...] composition, weight average molecular weight 1,000 to 100,000 (g/mol) is characterized in that, [...] composition. According to Claim 1, said aromatic polycarbonate number 1 to the repeat unit represented by formula 1 characterized in that, [...] composition: [formula 1] In said formula 1, R1toR4independently from each other hydrogen, C1-10alkyl, C1-10alkoxy, or halogens, phenyl substituted or a non-Z is replaced by the processing result aC1-10alkylene, or unsubstitutedC1-10alkyl substitutedC3-15alkylene cycle, O, S, SO, SO2, or is CO. According to Claim 1, repeating units of said aromatic polycarbonate number 1, bis (4 - hydroxyphenyl) methane, bis (4 - hydroxyphenyl) ether, bis (4 - hydroxyphenyl) sulfone, bis (4 - hydroxyphenyl) sulfoxide, bis (4 - hydroxyphenyl) sulfide, bis (4 - hydroxyphenyl) ketone, 1, 1 - bis (4 - hydroxyphenyl) ethane, bisphenol A, 2, 2 - bis (4 - hydroxyphenyl) butane, 1, 1 - bis (4 - hydroxyphenyl) cyclohexane, 2, 2 - bis (4 - hydroxy - 3, 5 - [...]) propane, 2, 2 - bis (4 - hydroxy - 3, 5 - dichlorophenyl) profile, 2, 2 - bis (4 - hydroxy - 3 - bromo phenyl) propane, 2, 2 - bis (4 - hydroxy - 3 - chlorophenyl) propane, 2, 2 - bis (4 - hydroxy - 3 - methyl phenyl) propane, 2, 2 - bis (4 - hydroxy - 3, 5 - dimethyl-phenyl) propane, 1, 1 - bis (4 - hydroxyphenyl) - 1 - phenyl ethane, bis (4 - hydroxyphenyl) diphenylmethane, and α, ω - bis [3 - (ο - hydroxyphenyl) profile] polydimethylsiloxane selected from the group consisting of at least one aromatic diol compound be decreased characterized in that the derived from, [...] composition. According to Claim 1, repeating units of said aromatic polycarbonate number 1, represented by formula 1 - 1 to characterized in that, [...] composition: [formula 1 - 1] . Number ablation According to Claim 1, said formula 2 derivative is marked as the chemical unit, represented by formula 2 - 2 to characterized in that, [...] composition: [formula 2 - 2] . According to Claim 1, the repeat unit represented by said formula 3, a represented by formula 3 - 2 characterized in that, [...] composition: [formula 3 - 2] . Mw (g/mol) Room temperature impact strength (J/m) Low temperature impact strength (J/m) Fid TD /-nMR X Y In the embodiment 1 30000 889 732 6. 23 7. 364 In the embodiment 2 24100 807 190 1. 26 2. 008 In the embodiment 3 25400 822 243 2. 45 3. 374 In the embodiment 4 27200 830 641 3. 75 4. 780 In the embodiment 5 28400 849 707 4. 77 5. 752 Compared e.g. 26100 802 679 4. 92 6. 466