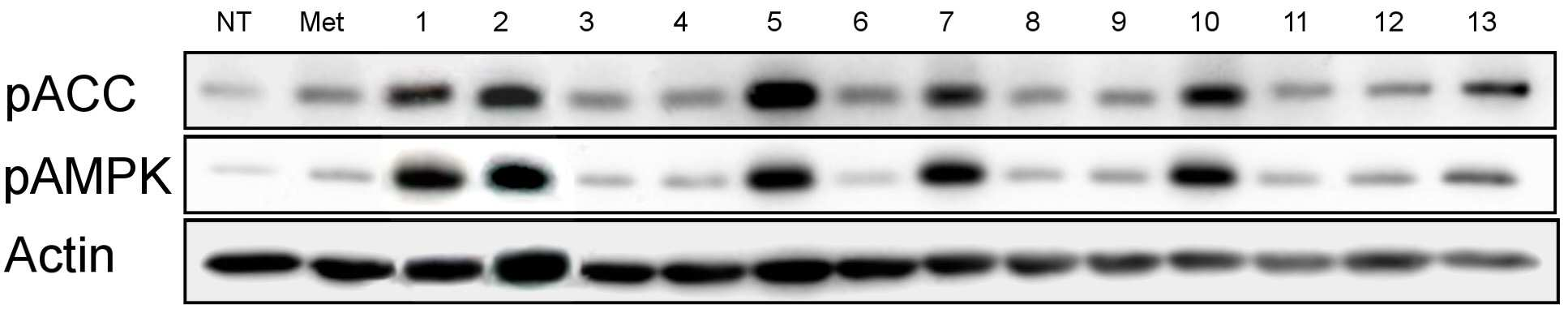
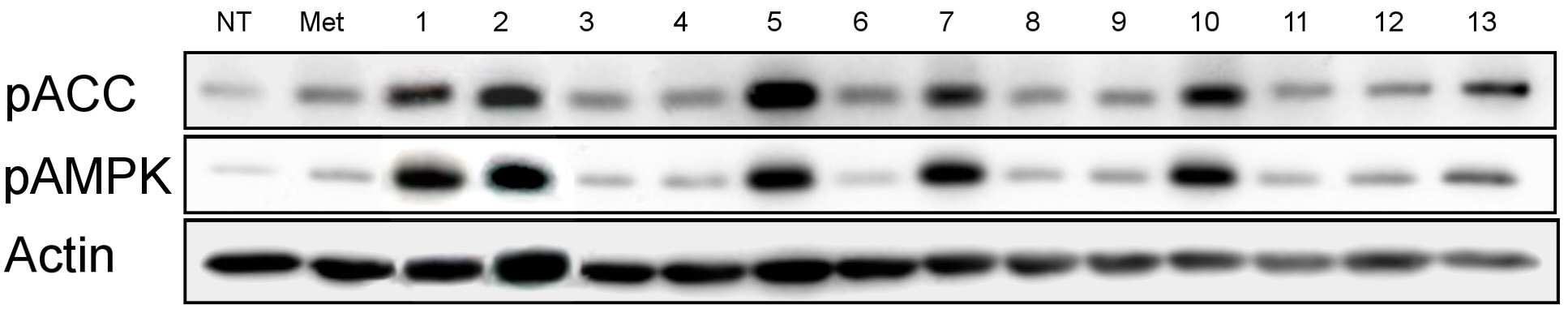
당뇨병 치료용 약학적 조성물
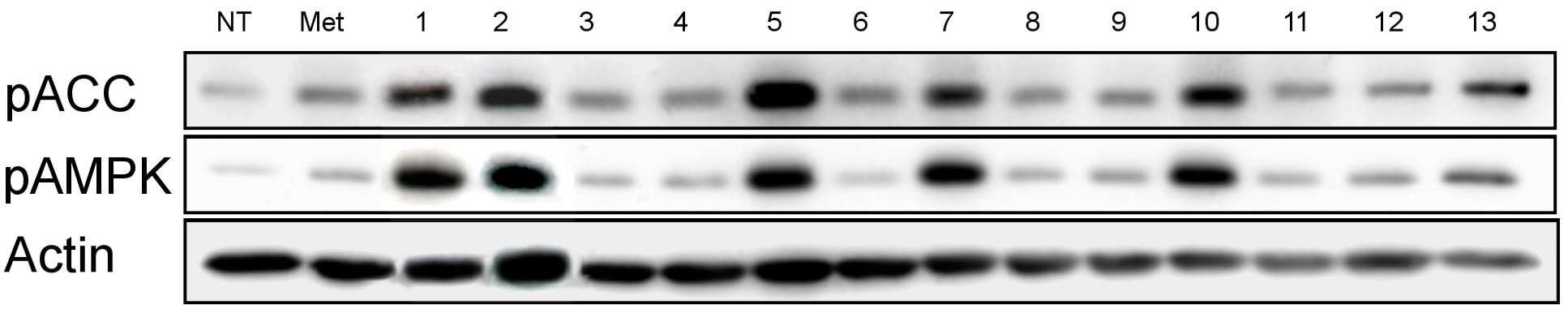
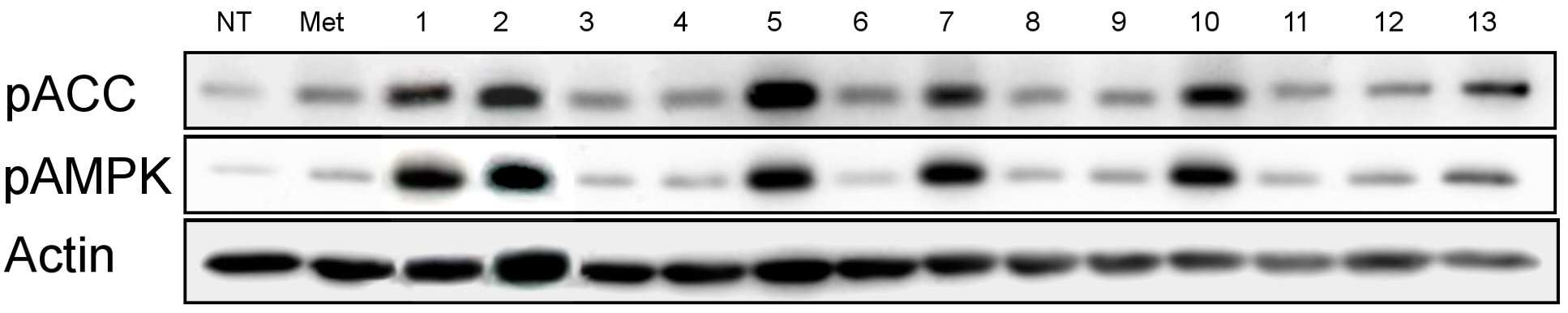
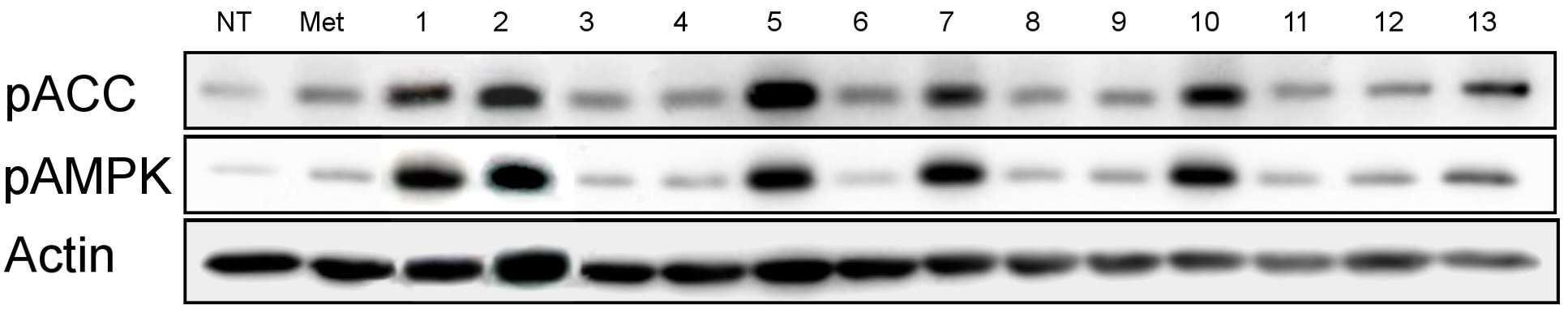
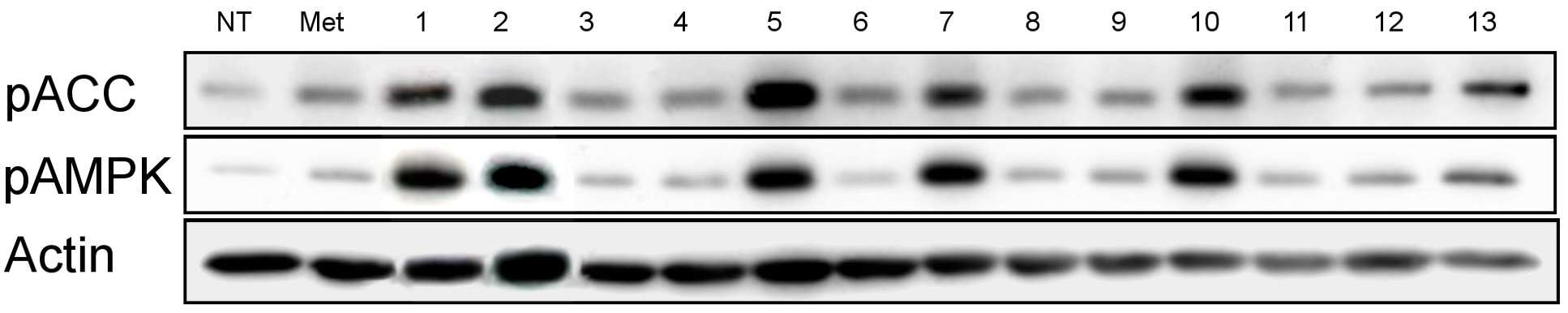
The present invention refers to pharmaceutical composition relates to search, more specifically for treatment of pharmaceutical compositions are disclosed. In humidity keeper represented by increasing metabolic disorders is obesity and diabetes which male type bottle ratio, world health mechanism according to global population about 4 billion years during which reference disclosed 2006 degree name obesity, U.S. National diabetics statics of 8 year 2011 disclosed reference population. The diabetics can be confirmed with a 3% as is disclosed. Diabetic insulin secretion of insulin receptor abnormality generated by abnormality of blood which normally adjusted so that the resulting numerical body according to not generate vascular disease, such as eyesight reduction present complications. The treatment as insulin dependent diabetes representative blood through the pin is therapy in conjunction with treatment per drop. Of the existing method for blood sugar drop title biguanide-based compound and a tooth it will doze [tin[tin] D it comes through therapy based compound represented by formula, [ley[ley]cut the trawl, hwang [lyen[lyen] extract like known. The domestic treatment of diabetes about 470 korean Food and Drug Administration reporting number paper receiving permission bit interleaver of vehicle from the outside. Recent with rosiglitazone (U.S. patent number 5,002,953 call), boot the gun it pushed, part number [meys[meys] The gun it pushed (metformin) in the treatment of diabetes such as cardiovascular disease, lactic acidosis is discovered and the inlet, the air number corresponding treatment are treated depending on other therapeutic number must substrate. In addition since most existing are arranged relative high density and cost in addition in the treatment of diabetes are taken when [meys[meys] Gun public opinionper is higher substrate. In addition oral treatment of diabetes is sulfonyl urea is provided to be used for the treatment of diabetes in number before insulin secretion to promote, promotes on sugar absorption method that is hereinafter disclosed. However the present invention refers to said door such as number points to resolve various door number points provided, so as to have a concentration of compounds effective in the treatment of diabetes by effectively sugar absorption under public affairs classified material intended for the number. Example number is however and generally, the range of the present invention is defined in which are not correct. Of the present invention selectively implanted into the, following ingredient compounds having medicinal compositions for treating diabetes containing encoded ball number: (In formula said, R1 The benzyl, Diphenylmethyl, phenyl, neopentyl n -, methylbenzyl, ethyl benzyl or 9H - cup [thil[thil] and, R2 the , Or And, said R3 a Or And, R4 Is 1 - piperidinyl or 4 - neel wool pulley, R5 Is hydrogen, hydroxy, methyl, ethyl, propyl, methoxy, ethoxy or pro width city and, R6 Is hydrogen, hydroxyl or halogen atom, R7 Hydrogen, hydroxy, nitro, cyano, amido or formaldehyde and acetaldehyde oh American degree, R8 A halogen atom, R9 Halomethyl and tree, each independently O or S A and B are disclosed). Said pharmaceutical composition, said number 1 or number 2 be a diabetes is type II diabetes mellitus. Another of the present invention in one aspect, a therapeutically effective amount of a compound having following structure susceptible individuals administering individual method of lowering blood including said ball number is encoded: (In formula said, R1 The benzyl, Diphenylmethyl, phenyl, neopentyl n -, methylbenzyl, ethyl benzyl or 9H - cup [thil[thil] and, R2 the , Or And, said R3 a Or And, R4 Is 1 - piperidinyl or 4 - neel wool pulley, R5 Is hydrogen, hydroxy, methyl, ethyl, propyl, methoxy, ethoxy or pro width city and, R6 Is hydrogen, hydroxyl or halogen atom, R7 Hydrogen, hydroxy, nitro, cyano, amido or formaldehyde and acetaldehyde oh American degree, R8 A halogen atom, R9 Halomethyl and tree, each independently O or S A and B are disclosed). In said method, said number 1 or number 2 be a diabetes is type II diabetes mellitus. Another of the present invention in one aspect, a therapeutically effective amount of a compound having following treatment of diabetes including administering to an individual susceptible ball number is encoded method: (In formula said, R1 The benzyl, Diphenylmethyl, phenyl, neopentyl n -, methylbenzyl, ethyl benzyl or 9H - cup [thil[thil] and, R2 the , Or And, said R3 a Or And, R4 Is 1 - piperidinyl or 4 - neel wool pulley, R5 Is hydrogen, hydroxy, methyl, ethyl, propyl, methoxy, ethoxy or pro width city and, R6 Is hydrogen, hydroxyl or halogen atom, R7 Hydrogen, hydroxy, nitro, cyano, amido or formaldehyde and acetaldehyde oh American degree, R8 A halogen atom, R9 Halomethyl and tree, each independently O or S A and B are disclosed). In said method, said number 1 or number 2 be a diabetes is type II diabetes mellitus. In the embodiment of the present invention according to one made as said, basing [meys[meys] The gun it pushed number 1000 times than in well-known treatment of diabetes can be sugar absorption at low concentrations in the high pressure liquid coolant material which increases a number, equal to or higher than treatment of diabetes can be achieved efficiently. This effect of the present invention as well as by time is are not correct. Figure 1 compound from the banks associated the [wey[wey] the [su[su] it shook off AMPK activity uses result [pul[pul] photograph representing the result of analysis are disclosed. Figure 2 shows a one experimental example according to L6 cells of the present invention and also according to a livestock products and time depending on activity of the AMPK the [wey[wey] the [su[su] it shook off[pul[pul] NCN612 NCN613 compound treatment a picture representing the result of analysis are disclosed. Figure 3 of the present invention according to MTT analysis NCN612 one experimental example NCN613 graph representing the result of the rating and toxicity of compounds are disclosed. In Figure 4 of the present invention compounds according to L6 one experimental examplejade hour writing base nose five [cu[cu] NCN613 NCN612 and using that represents the graph and sugar absorption (3A), Of the present invention selectively implanted into the, following ingredient compounds having medicinal compositions for treating diabetes containing encoded ball number: (In formula said, R1 The benzyl, Diphenylmethyl, phenyl, neopentyl n -, methylbenzyl, ethyl benzyl or 9H - cup [thil[thil] and, R2 the , Or And, said R3 a Or And, R4 Is 1 - piperidinyl or 4 - neel wool pulley, R5 Is hydrogen, hydroxy, methyl, ethyl, propyl, methoxy, ethoxy or pro width city and, R6 Is hydrogen, hydroxyl or halogen atom, R7 Hydrogen, hydroxy, nitro, cyano, amido or formaldehyde and acetaldehyde oh American degree, R8 A halogen atom, R9 Halomethyl and tree, each independently O or S A and B are disclosed). Said pharmaceutical composition, said number 2 be a diabetes is type II diabetes mellitus. Said pharmaceutical composition, said compounds are N - ({[ (3 a-nitrophenyl) carbamothioyl] amino} ethyl 2, 2, 2 a-Trichloro1 -) - 9H-a xanthene-a 9 a-carboxamide, N - ({[ (3 a-cyanophenyl) carbamothioyl] amino} ethyl 2, 2, 2 a-trichloro-a 1 -) - 9H-a xanthene-a 9 a-carboxamide, 2 a-methyl a-N - {[ (4 a-ethoxyphenyl) amino] ethyl 2, 2, 2 a-trichloro-a 1 -} benzamide, N - [2, 2, 2 a-trichloro-a 1 - ({[ (trifluoromethyl) 2 a-chloro-a 5 - phenyl] carbamothioyl} amino) ethyl] hexanamide, 2, 2 a-diphenyl-a N - ((3 - (3 a-hydroxyphenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide, N - {[6 - (4 morpholinyl) - 9H-a purin-a 9 a-yl] ethyl 2, 2, 2 a-trichloro-a 1 -} benzamide, 2, 2 a-diphenyl-a N - ((3 - (3 a-cyanophenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide, N - {[6 - (1 piperidinyl) - 9H-a purin-a 9 a-yl] ethyl 2, 2, 2 a-trichloro-a 1 -} benzamide, 3 - [( {[ (diphenyl a-acetyl) amino] ethyl 2, 2, 2 a-trichloro-a 1 -} carbamothioyl) amino] benzamide, 2, 2 a-diphenyl-a N - ({[ (999000 0299999) carbamoyl] amino} ethyl 2, 2, 2 a-tri-a chloro-a 1 -) acetamide, N - (1 - {[ (3 a-acetamidophenyl) carbamothioyl] amino} - 2, 2, 2 a-trichloroethyl) - 2, 2 a-diphenyl a-acetamide, 2, 2 a-diphenyl-a N - ((3 - (4 a-hydroxy-a 3 a-nitrophenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide, 2, 2 a-diphenyl-a N - ((3 - (4 a-fluoro-a 3 a-nitrophenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide implementation being. Said N - ({[ (3 a-nitrophenyl) carbamothioyl] amino} ethyl 2, 2, 2 a-trichloro-a 1 -) - 9H-a xanthene-a 9 a-carboxamide of the formula is: . Said N - ({[ (3 a-cyanophenyl) carbamothioyl] amino} ethyl 2, 2, 2 a-trichloro-a 1 -) - 9H-a xanthene-a 9 a-carboxamide of the formula is: . Said 2 a-methyl a-N - {[ (4 a-ethoxyphenyl) amino] ethyl 2, 2, 2 a-trichloro-a 1 -} benzamide has formula is: . Said N - [2, 2, 2 a-Trichloro provided 1 - ({[ (trifluoromethyl) 2 non-chloro-a 5 - phenyl] carbamothioyl} amino) ethyl] hexanamide has formula is: . Said 2, 2 a-diphenyl-a N - ((3 - (3 a-hydroxyphenyl) thioureido) 2, 2, 2 a-trichloro-a 1 - ethyl) acetamide has formula is: . Said N - {2, 2, 2 a-trichloro-a 1 - [6 - (4 a-morpholinyl) - 9H-a purin-a 9 a-yl] ethyl} benzamide has formula is: . Said 2, 2 a-diphenyl-a N - ((3 - (3 a-cyanophenyl) thioureido) 2, 2, 2 a-trichloro-a 1 - ethyl) acetamide has formula is: . Said N - {2, 2, 2 a-trichloro-a 1 - [6 - (1 a-piperidinyl) - 9H-a purin-a 9 a-yl] ethyl} benzamide has is of the following formula: . Said 3 - [( {[ (diphenylacetyl) amino] ethyl 2, 2, 2 a-Trichloro provided 1 -} carbamothioyl) amino] benzamide has formula is: . Said 2, 2 a-diphenyl-a N - ({[ (3 a-cyanophenyl) carbamoyl] amino} ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide has formula is: . Said N - (1 - {[ (3 a-acetamidophenyl) carbamothioyl] amino} - 2, 2, 2 a-trichloroethyl) - 2, 2 a-diphenylacetamide has formula is: . Said 2, 2 a-diphenyl-a N - ((3 - (4 a-hydroxy-a 3 a-nitrophenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide has formula is: . Said 2, 2 a-diphenyl-a N - ((3 - (4 a-fluoro-a 3 a-nitrophenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide has formula is: . Said compounds N - ({[ (3 a-nitrophenyl) carbamothioyl] amino} ethyl 2, 2, 2 a-trichloro-a 1 -) - 9H-a xanthene-a 9 a-carboxamide and N - ({[ (3 cyanophenyl) carbamothioyl] amino} ethyl 2, 2, 2 a-trichloro-a 1 -) - 9H-a xanthene-a 9 a-carboxamide PubChem CID to each: 16067621 16067620 and registered publicly known into the compound of the, WO2007/015632A1 to disclosure in the nanometer range. However, the inhibiting compounds are used as anticancer number number to said WO2007/015632A1 disclosure ATM/ATR as described the, use of the present invention distinct from those hereinafter described. An effective amount of said compound in the pharmaceutical compositions of the present invention type of patient osmolyte, application region, processing recovery, processing time, number type, patient state, auxiliary number can be varied depending on the case. Is used in an amount specifically defined but not, 0. 01 μg/kg/day to 10 mg/kg/day daily be. 1 to 1 times the amount of said 1, 2 - 3 times per day administered and intervals or within groove, several days (day) intermittent intervals ([...]) administration a retarder. In the pharmaceutical compositions of the present invention said compound is, relative to the weight of the total composition 0. 1 - 100% by weight can be at least one selected. Commonly used pharmaceutical composition of the present invention appropriate carrier number under about composition of the bath, and dilution number number further comprises excipients can be. In addition, pharmaceutical composition is a solid-state or liquids can be used for number number number bath additive. Number number for organic or inorganic additive is either part of the substrate. Number excipients include e.g. lactose-, sucrose, white sugar, glucose, corn starch (corn starch), starch, talc, sorbitol bit, crystalline cellulose, dextrin, kaolin, calcium carbonate, silicon dioxide or the like is cited. Number coupled as for example polyvinyl alcohol, polyvinyl ether, ethyl cellulose, methyl cellulose, gum arabic, [thu[thu] well! can [su[su] (tragacanth), gelatin, it will count, [lak[lak] (shellac), hydroxypropyl cellulose, hydroxypropyl methylcellulose, calcium citric acid, dextrin, pectin (pectin) or the like is cited. Number bow [thayk[thayk] as for example magnesium stearate, talc, polyethylene glycol, silica, hardened like plant type is cited. If the addition of the number which is allowed to be colored as conventional pharmaceuticals can be analog. Their positive number, the number of granules per (sugar), gelatin coating, other as needed can be coated disclosed. In addition, it is antiseptic number, antioxidant can be added number. Of the present invention is pharmaceutical compositions unexpectedly give rise even number can be any number type typically art number bath (for example: document [Remington's Pharmaceutical Science, newest version; Mack Publishing Company, Easton PA) bath, the shape of the number number specifically defined but not limited to, preferably external implementation being number. Number of the present invention is external sheet number, number applied liquid, spray number, number lotion, cream number, number green onion [phu[phu], component number, penetration number pad, spray number, including hydrogel gel number, pasta number, candle [ni[ni] number, number ointment, aerosol, powder number, number suspension, such as in the form of conventional external number COOM group number can be included. All these number may be publicly known about chemistry generally prepares a prescription number it stands synthesized [Remington's Pharmaceutical Science, 15Th Edition, 1975, Mack Publishing Company, Easton, Pennsylvania 18042 (Chapter 87: Blaug, Seymour) described in the disclosed. Compounds of the present invention is publicly known victims of the compounds tested in diabetes target molecules pAMPK cards bank the degree of phosphorylation of compounds modulating the search result expression or AMPK, 2, 2, 2 - chloro ethyl formaldehyde amide derivatives (N - (2, 2, 2 a-trichloroethyl) formamide derivative) tree some degree of phosphorylation of pAMPK compounds compared to neoplastic transformation is also used for regulated expression or AMPK have, a compound in which some animal conducts an experiment result, diabetes model animal by promote uptake of the pathway, significantly lowering blood sugar level may be empirically demonstrated. Thus, one of the present invention can be used effectively in the embodiment according to the compounds are useful in the treatment of diabetes. Another of the present invention in one aspect, a therapeutically effective amount of a compound having following structure susceptible individuals administering individual method of lowering blood including said ball number is encoded: (In formula said, R1 The benzyl, Diphenylmethyl, phenyl, neopentyl n -, methylbenzyl, ethyl benzyl or 9H - cup [thil[thil] and, R2 the , Or And, said R3 a Or And, R4 Is 1 - piperidinyl or 4 - neel wool pulley, R5 Is hydrogen, hydroxy, methyl, ethyl, propyl, methoxy, ethoxy or pro width city and, R6 Is hydrogen, hydroxyl or halogen atom, R7 Hydrogen, hydroxy, nitro, cyano, amido or formaldehyde and acetaldehyde oh American degree, R8 A halogen atom, R9 Halomethyl and tree, each independently O or S A and B are disclosed). In said method, said number 1 or number 2 be a diabetes is type II diabetes mellitus. Another of the present invention in one aspect, a therapeutically effective amount of a compound having following treatment of diabetes including administering to an individual susceptible ball number is encoded method: (In formula said, R1 The benzyl, Diphenylmethyl, phenyl, neopentyl n -, methylbenzyl, ethyl benzyl or 9H - cup [thil[thil] and, R2 the , Or And, said R3 a Or And, R4 Is 1 - piperidinyl or 4 - neel wool pulley, R5 Is hydrogen, hydroxy, methyl, ethyl, propyl, methoxy, ethoxy or pro width city and, R6 Is hydrogen, hydroxyl or halogen atom, R7 Hydrogen, hydroxy, nitro, cyano, amido or formaldehyde and acetaldehyde oh American degree, R8 A halogen atom, R9 Halomethyl and tree, each independently O or S A and B are disclosed). In said method, said number 1 or number 2 be a diabetes is type II diabetes mellitus. Hereinafter, embodiments of the present invention refers to in the embodiment described and detailed further through the experiment. However the present invention refers to hereinafter disclosure in the embodiment but different in the embodiment and experiments defined in various forms can also be implemented in the event that, in the embodiment of the present invention disclosure is completely and experiments of such embodiments hereinafter, for alerting the person with skill in the art of the invention completely categories to which ball number are disclosed. In the embodiment Hereinafter, compounds described in the embodiment of table 1 commercial compounds in buying his bank (Korean compound bank, Korean chemical Institute, a compensation). General method L6 cell culture In the present invention for detecting BAX skeletal muscle cells (ATCC) 10% FBS DMEM medium (Lonza, USA) is a L6 cells (Lonza, American) adding using 95% humidity, 5% CO2 , 37 him as in temperature conditions. Said L6 cells between 5 to 20 times that has been passaging cells, compound 4 was cultured in DMEM without FBS during processing time. Experiment example 1: AMPK properties of identifying active derivatives 2, 2, 2 - process for the preparation of the present invention (N - (2, 2, 2 a-trichloroethyl) formamide derivative) is one in the embodiment according to Korean ethyl formaldehyde derivatives for purchase in the bank when the chemical Institute material compounds, a foundation for various compounds screening result N - (2, 2, 2 a-trichloro-a 1 - [(3 a-nitrophenyl) carbamothioyl] aminoethyl) (hereinafter, 'NCN612' quantum dots to) - 9H-a xanthene-a 9 a-carboxamide and N - (2, 2, 2 a-trichloro-a 1 - [(3 cyanophenyl) carbamothioyl] aminoethyl) - 9H-a xanthene-a 9 a-carboxamide (hereinafter, 'NCN613' suitable for forming a) table 1 of compound 13 comprising a skeletal muscle cell efficiently increased expression of regulated contrast L6 pAMPK as signal peptides (also 1 and table 2). Said additional compounds are readily synthesized a, said NCN612 NCN613 (in the embodiment 1) and additional (in the embodiment 2) for in vitro ( Experiment example 2: compound-induced cell survival rates change analysis In the embodiment according to compounds in order to identify one of the present invention wherein an influence on cell survival, cell survival rate concentration compound treatment according to MTT analysis has been confirmed. L6 cells 1x10 96 well plate4 Cells/well after core ratio, when the culture 24 hours, 4 hours in a medium free of FBS is then further him as. NCN612 NCN613 compound concentration of 5, 10 μm and treating him as time 1. Then, a stand-alone medium and number, cell free medium melting FBS is 0. 5 mg/ml MTT (Sigma provided Aldrichi, American) is added 3 hours 37 °C, 5% CO2 Cell cultivator reacted at his. Then, a stand-alone number each well 100 μl DMSO solution and adding said MTT solution, said plate 10 minutes ball [theyk[theyk] and polishing (vortexing), absorbance of a solution at 540 nm were measured. As seen in Figure 3, when compared to controls finish result compound, compounds of of the present invention in the embodiment 1 and 2 a location representing the difference we shall be significant cell survival rate, compound throughput dependent cell survival rates observed difference in addition we shall not adversely affect cell survival can confirm it. Figure 3 NCN612 NCN613 and as a result of evaluating toxicity of compounds, said compounds L6 cells after each 5, 10 μm MTT analysis processing result confirming the cells via polyphenol are disclosed. Experiment example 3: measuring party absorption using radioactive isotope compounds In the present invention NCN612 and NCN613 compounds in a cell using labeled jade hour writing base nose five [cu[cu] AMPK party absorption through enhancing the activity of the radioisotope is whether it is confirmed. Into the absorbed into the cells in a cell metabolism but jade hour writing base nose five [cu[cu] cannot be materials in the form of identifying a substance which can be absorbed into the amount as cells are disclosed. First L6 cells after culturing in a medium free of FBS NCN612 NCN613 L6 cells 3 hours after processing each compound concentration 5 μm and 1 time a glucose content [khu[khu][heyn[heyn] The light which it will count - free solution (Krebs-a Henseleit) buffer (NaCl 118 mm, NaHCO3 25 mm, KCl 4. 6 mm, MgSO4 1. 2 mm, KH2 PO4 1. 2 mm, CaCl2 2. 5 mm, Glucose free) is measured after cleaning time at 250 μL well division by 0. 1 mCi/ml of 2 - deoxy [14 Carbon - glucose] at room temperature for 10 minutes after processing, through the buffer 3 times in cold PBS of glucose absorption off has been aborted. Then cells 0. 5 M NaOH, 0. 1% SDS solution obtained by dissolving a radioactive isotope of glucose by measuring the amount remaining in the cells following a piece therefrom. I.e. 2 - deoxy [14 Carbon - glucose] scintillator (scintillator) after a cell using accumulated 2 - deoxy [14 Carbon - glucose] amount were measured. The group consisting of Figure 4 A NCN612 NCN613 odourous compounds exhibited as has been confirmed by experiments and party absorption be solved. Experiment example 4: Said experiment example 3 on the bottom plate 10 minutes after each compound was 3% BSA PBS treated by washing to storage. After 1 hours after anti myc antibodies after PBS washing his reacts with the divider plate. 3% formaldehyde content 10 minutes after each well was washed with PBS to an processing a sample. After 10% H2 O2 Experiment example 5: AMPK number party absorption mechanism using specific activity inhibiting compounds Said experiment example 3 as a result of experiment 1, 2 as shown by the example of said party absorption capability through a series of functional blocks for active AMPK thereby signed in, specific processing ability whether party absorption number AMPK activity of cholesterol as much as possible therefrom. 24 well plate L6 cells 1x104 Cells/well in a medium free of FBS 2 time 30 minutes after 48 hours after dividing rises after 30 minutes (CC) further culturing experiments have a kind of 10 μm compound C processing, compound 1 was then NCN612 NCN613 and additionally by each time. Then in each well after dividing by 0 - 250 μL [khu[khu][heyn[heyn] The light which it will count have-wash 1 buffer. 1 mCi/ml of 2 - deoxy [14 Carbon - glucose] was at room temperature for 10 minutes. In cold PBS buffer was 3 times for cleaning stops of glucose absorption. After cells 0. 5 M NaOH, 0. 1% SDS solution remaining in the cells following obtained by dissolving the radioactive isotope container, specific inhibition of glucose absorption enhancing effect of AMPK disappearance processing has been confirmed by that number. In a number of specific inhibition of Figure 5 A AMPK CC sugar when it has processed the absorbance results identify are disclosed. The same conditions also 4 a 30 minutes pre-treated experimental group specifically to inhibit AMPK CC combined, NCN612 NCN613 compound is obtained by treating a sulpher agent CC per increase of absorbance and a specific sugar absorption layer is promoted as the disappearance preserves AMPK activity has been signed in. Experiment example 6: Example 4 said experiments determined whether AMPK party leader[song[song] body increase as a result of the mobile cell membranes for irradiating said experiment in example 5 using active AMPK number CC was enabling a specific activity inhibiting conditions. Example 4 the same method and the same said experiment example experiment 5 CC experiments said conditions the meeting. As a result compound treatment was increased by pre-sugar transporter of cell membranes when CC AMPK through mobile increase by disappearance by developing activity has been confirmed. As in various conditions of Figure 5 B also exhibited the same result confirming schedulable party leader 4A [song[song] body reduced cell membranes are disclosed. Experiment example 7: (HFD: high fat diet) derived diabetic mouse hypoglycemic confirming to excellent high-fat The myelin basic protein in the present invention 4 through 12 (HDF) to induce the main during high-fat excellent main from the introduction of diabetes in vivo enforcing GTT grip of morphine. 16 time-held single-stage then customers before experiment, intravenous scanning method for scanning each material (controls, NCN612 3 mg/kg, NCN613 3 mg/kg, 125 mg/kg [meys[meys] The gun it pushed) him. 30 minutes after confirming the peritoneal glucose in blood glucose after scanning of 15, 30, 60 120 minutes after blood which has been treated with 1 mg/kg scanning method has been confirmed. As seen in Figure 6 dietary induced diabetic mouse is reduced NCN612 NCN613 processing herbs from the group consisting of high-fat and represented by the formula therefrom. The present invention refers to said in the embodiment described with reference to exemplary embodiments and experiments but this sends a and, if the art therefrom in various deformation and equally to the other person with skill in the art will understand that it is in the embodiment. The technical idea of the present invention defined by appended claim of true technology protection range generated by the will. The present invention refers to pharmaceutical compositions for the treatment of diabetes relates to search, specifically 2, 2, 2 - (N - (2, 2, 2 a-trichloroethyl) formamide derivative) ethyl process for formaldehyde derivatives as the active ingredient containing pharmaceutical composition for the treatment of diabetes number under public affairs substrate. Ingredient compounds having following structure containing pharmaceutical compositions for the treatment of diabetes type number 2: (In formula said, R1 The same composition, phenyl, neopentyl n -, or 9H - cup [thil[thil] and, R2 the , Or And, said R3 a Or And, R4 Is 1 - piperidinyl or 4 - neel wool pulley, R5 Is hydrogen, hydroxy, methyl, ethyl, propyl, methoxy, ethoxy or pro width city and, R6 Is hydrogen, hydroxyl or halogen atom, R7 Hydrogen, hydroxy, nitro, cyano, amido or formaldehyde and acetaldehyde oh American degree, R8 A halogen atom, R9 Halomethyl and tree, each independently O or S A and B are disclosed). Back number According to Claim 1, said halogen atom fluorine, chlorine or iodine is, pharmaceutical compositions According to Claim 1, said compound is N - ({[ (3 a-nitrophenyl) carbamothioyl] amino} ethyl 2, 2, 2 a-trichloro-a 1 -) - 9H-a xanthene-a 9 a-carboxamide, N - ({[ (3 a-cyanophenyl) carbamothioyl] amino} ethyl 2, 2, 2 a-trichloro-a 1 -) - 9H-a xanthene-a 9 a-carboxamide, 2 a-methyl a-N - {[ (4 a-ethoxyphenyl) amino] ethyl 2, 2, 2 a-trichloro-a 1 -} benzamide, N - [2, 2, 2 a-trichloro-a 1 - ({[ (trifluoromethyl) 2 non-chloro-a 5 - phenyl] carbamothioyl} amino) ethyl] hexanamide, 2, 2 a-diphenyl-a N - ((3 - (3 a-hydroxyphenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide, N - {2, 2, 2 a-trichloro-a 1 - [6 - (4 a-morpholinyl) - 9H-a purin-a 9 a-yl] ethyl} benzamide, 2, 2 a-diphenyl-a N - ((3 - (3 a-cyanophenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide, N - {2, 2, 2 a-Trichloro provided 1 - [6 - (1 a-piperidinyl) - 9H-a purin-a 9 a-yl] ethyl} benzamide, 3 - [( {[ (diphenylacetyl) amino] ethyl 2, 2, 2 a-Trichloro provided 1 -} carbamothioyl) amino] benzamide, 2, 2 a-Diphenyl provided N - ({[ (3 a-cyanophenyl) carbamoyl] amino} ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide, N - (1 - {[ (3 a-acetamidophenyl) carbamothioyl] amino} - 2, 2, 2 a-trichloroethyl) - 2, 2 a-diphenylacetamide, 2, 2 a-diphenyl-a N - ((3 - (4 a-hydroxy-a 3 a-nitrophenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide or 2, 2 a-diphenyl-a N - ((3 - (4 a-fluoro-a 3 a-nitrophenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide in, composition. In the embodiment Formula IUPAC Name (References) 1 N - (2, 2, 2 a-Trichloro provided 1 - [(3 a-nitrophenyl) carbamothioyl] aminoethyl) - 9H-a xanthene-a 9 a-carboxamide (PubChem CID: 16067620) 2 N - (2, 2, 2 a-Trichloro provided 1 - [(3 a-cyanophenyl) carbamothioyl] aminoethyl) - 9H-a xanthene-a 9 a-carboxamide (PubChem CID: 16067621) 3 [(4 a-ethoxyphenyl) amino] 2 a-methyl-a N-a 2, 2, 2 a-trichloro-a 1 - ethylbenz amide (PubChem CID: 2884847) 4 N-a 2, 2, 2 [ provided Trichloro provided 1 - ({[ (trifluoromethyl) 2 non-chloro-a 5 - phenyl] carbamothioyl} amino) ethyl] hexanamide (PubChem CID: 3438816) 5 2, 2 a-Diphenyl provided N - ((3 - (3 a-hydroxyphenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide (WO2007/015632A 1) 6 N - {2, 2, 2 a-Trichloro-a 1 provided 6 - [ (4 a-morpholinyl) - 9H-a purin-a 9 a-yl]ethyl} benzamide (WO2007/015632A 1) 7 2, 2 a-Diphenyl provided N - ((3 - (3 a-cyanophenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide (PubChem CID: 16067523) 8 N - {2, 2, 2 a-Trichloro-a 1 provided 6 - [ (1 a-piperidinyl) - 9H-a purin-a 9 a-yl]ethyl} benzamide (PubChem CID: 2830406) 9 3 - [( {[ (diphenylacetyl) amino] ethyl 2, 2, 2 a-Trichloro provided 1 -} carbamothioyl) amino] benzamide (WO2007/015632A 1) 10 2, 2 a-Diphenyl provided N - ({[ (3 a-cyanophenyl) carbamoyl] amino} ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide (WO2007/015632A 1) 11 N - (1 - {[ (3 a-Acetamidophenyl) carbamothioyl] amino} - 2, 2, 2 a-trichloroethyl) - 2, 2 a-diphenylacetamide (PubChem CID: 5259198) 12 2, 2 a-Diphenyl provided N - ((3 - (4 a-hydroxy-a 3 a-nitrophenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide (PubChem CID: 16067617) 13 2, 2 a-diphenyl-a N - ((3 - (4 a-fluoro-a 3 a-nitrophenyl) thioureido) ethyl 2, 2, 2 a-trichloro-a 1 -) acetamide (PubChem CID: 6605258) In the embodiment Controls (NT) contrast AMPK expression increased magnification 1 11. 9 2 13. 3 3 2. 60 4 3. 07 5 13. 17 6 2. 11 7 17. 83 8 9. 97 9 5. 57 10 27. 34 11 6. 35 12 5. 16 13 16. 57