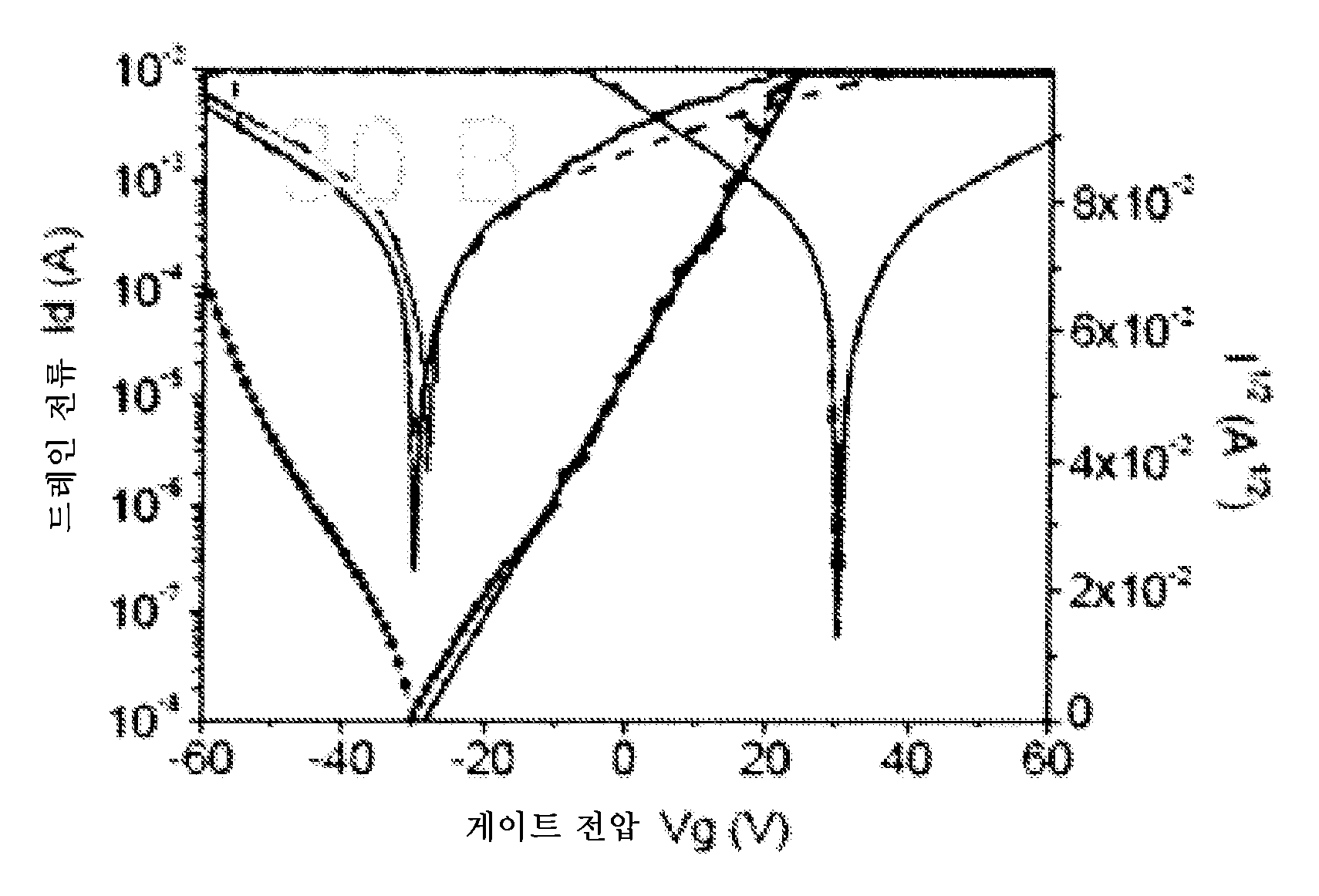
유기 전계 효과 트랜지스터에 사용하기 위한 디케토피롤로피롤 중합체
The present invention refers to, polymer including recurring structural units of formula I or III, and organic device, in particular organic field effect transistor (OFET), or diode containing device and/or organic field effect transistors in their as organic semiconductors relates to the use. Polymer in the holes of the present invention according to good solubility and excellent film - has properties. In addition, the present invention according to polymer organic field effect transistor when used for a, a high energy conversion efficiency, excellent field - effect mobility, excellent on/off current ratio (on/off) and/or the providing the stability may appear KIPO &. uS-b-6451459 (literature [B. Tieke et al. , Synth. Met. 130 (2002) 115 - 119]; [Macromol. Rapid Commun. 21 (4) (2000) 182 - 189] reference) the, including units a with toe blood roll blood roll diketone based polymer and copolymers, and their use in EL device disclosed is is. In formula said, 0 the x. 005 to 1, preferably 0. 01 to 1 is selected from range of, 0 the y. 995 to 0, preferably 0. 99 to 0 is selected from range of, and x + y=1 wherein, Ar1andAr2each independently a -idiotypic antibodies which represent an, M, n and of the 1 to 10, R1andR2each independently aH, C1-C18alkyl, -C(O)O -C1-C18alkyl, perfluoro-C1-C12alkyl, unsubstitutedC6-C12aryl, or 1 to 3 timesC1-C12alkyl, C1-C12alkoxy, or halogen substitutedC6-C12aryl, C1-C12alkyl-C6-C12aryl, orC6-C12aryl-C1-C12and alkyl, R3andR4the preferably, the hydrogen, C1-C12alkyl, C1-C12alkoxy, unsubstitutedC6-C12aryl, or 1 to 3 timesC1-C12alkyl, C1-C12alkoxy, or halogen substitutedC6-C12aryl or perfluoro-C1-C12and alkyl, R5preferablyC1-C12alkyl, C1-C12alkoxy, unsubstitutedC6-C12aryl, or 1 to 3 timesC1-C12alkyl, C1-C12alkoxy, or halogen substitutedC6-C12aryl, or perfluoro-C1-C12exhibits alkyl. Polymer a The literature [Tieke et al. , Synth. Met. 130 (2002) 115 - 119] disclosure is explicitly to. Polymer a The literature [Macromol. Rapid Commun. 21 (4) (2000) 182 - 189] disclosure is explicitly to. WO 05/049695 the, with toe blood roll blood roll diketone (DPP) based polymer, and PLED, organic integrated circuit (O-IC), organic field effect transistor (OFET), organic thin film transistor (OTFT), organic solar cells (O-SC) or organic laser diode disclosure is their use in the drug can be, polymers based on specific DPP formula I is do not go disclosure. Preferred polymers formula (I) the repeating units of repeating unit (Wherein, R1andR2each independently a 1 one or more oxygen atoms may be interposed aC1-C25alkyl,<aotran type="glo" translation="in particularC4-C12, R is an alkyl group containing, Ar1andAr2each independently a formula Group of, -Ar3-the formula Of group, wherein R6hydrogen, C1-C18alkyl orC1-C18alkoxy, R32the methyl, Cl or OMe and, R8theH, C1-C18alkyl, or E by substituted and/or a confirmation of the wafer is performed by DC1-C18alkyl, in particular - O - is interposed aC1-C18it) includes. In the embodiment 12 to, polymer a are described KIPO & number: WO 08/000664 to the formula of (repeated) unit (are) is for including polymer having: . Said in formula, Ar1andAr1'vertical direction is the same as the preferably, formula , In particular Of group, Ar2, Ar2', Ar3, Ar3', Ar4andAr4'each independently a formula Of group, -idiotypic antibodies which represent an 0, 1 or 2 the p, R3one of the same within or can be different and can, optionally E by substituted and/or a may be interposed is DC1-C25alkyl, or optionally E by substituted and/or a may be interposed is DC1-C18graphical object is selected from alkoxy, R4the optionally E by substituted and/or a may be interposed is DC6-C25alkyl, optionally G a may be substitutedC6-C14aryl, e.g. phenyl, naphthyl or biphenylyl, optionally E by substituted and/or a may be interposed is DC1-C25alkoxy, orC7-C15aralkyl and, wherein aralkyl the G (ar) optionally may be substituted and, The D-CO-, -COO-, -S-, -SO-, -SO2-, -O-, -NR25-and, whereinR25theC1-C12alkyl, such as methyl, ethyl, n - profile, ISO - profile, n - butyl, isobutyl or sec -, and butyl is and, The E-OR29; -SR29; -NR25R25; -COR28; -COOR27; -CONR25R25;or - CN and; whereinR25, R27, R28andR29each independently aC1-C12alkyl, such as methyl, ethyl, n - profile, ISO - profile, n - butyl, isobutyl, sec - butyl, ethylhexyl, octyl, or 2 - ethyl - ethylhexyl, orC6-C14aryl, e.g. phenyl, naphthyl or non-phenyl reel and, G the E the same a preferred option associated with the region is chosen or that have, orC1-C18alkyl,<aotran type="glo" translation="in particularC1-C12alkyl, such as methyl, ethyl, n - profile, ISO - profile, n - butyl, isobutyl, sec - butyl, ethylhexyl, octyl or 2 - ethylhexyl is - ethyl. In the embodiment to polymer is a disclosure is: (In the embodiment 1, homopolymers; literature [Adv. Mat. 2008, 20, 13, 2556 - 2560]); (In the embodiment 2, homopolymers; literature [Adv. Mat. 2008, 20, 11, 2217 - 2224]); (In the embodiment 3, homopolymer); (In the embodiment 4, homopolymer); (In the embodiment 5, copolymer); (In the embodiment 6, copolymer). The present invention (31. 10. 2008) prior than priority date (06. 09. 2007) but, after a disclosure of the present invention priority date (11. 03. 2009) the EP 2034537A 2, including semiconductor layer relates to thin film transistor device, enabled to operate close to the loop aligning a semiconductor layer ring represented by formula including compound: . In formula said, each X independently S, Se, NR and O "graphical object is selected from, each R" are independently the atom of hydrogen, and hydrocarbon optionally substituted - containing ground into selected and, each Z in an independently optionally substituted hydrocarbon, hetero - to containing, and halogen which is one, the d 1 or more and, the e and of 2 to 0, 1 or more may indicate the number of the a, the b may indicate the number of 20 to 0, 1 or more water exhibits and the n. Disclosure explicitly polymer is a is: . In formula said, n and of the repeat units, about 2 to about 5000 and the shaft transfers the, "' and R" R " of the same or different substituted alkyl, R3 and, wherein the substituents independently optionally substituted hydrocarbon group and is selected from the group consisting of - containing group are. The present purpose of the invention the, in the case of being employed for organic field effect transistor, a high energy conversion efficiency, excellent field - effect mobility, excellent on/off current ratio and a and/or excellent stability the recording operation. under public affairs number polymer. The aim said, one or more (repeated) unit of formula I or III (are) for including was settled by the polymer. < Formula I > < Formula III > Said in formula, The a being integers, of 1 to 5, The b being integers, of 3 to 1, The c being integers, of 3 to 1, D an integer 1, 2 or 3 and, E an integer 1, 2 or 3 and, A, b and c a total of 7 hereinafter and, Ar1, Ar1', Ar3andAr3'each independently a formula Of, or-Ar4-Ar5- [Ar6]f-group, Ar4formula a Of group, Ar5andAr6, independently one of the other areAr1has meaning of, the f 0, or water purification 1 and, Ar2formula a Of group, X1andX2N and one, and at least one other of which CH and, R1, R2, R1'andR2'the same or can be different and can, hydrogen, C1-C100alkyl,<aotran type="glo" translation="in particularC8-C36alkyl, C6-C24aryl,<aotran type="glo" translation="in particularC1-C8alkyl, C1-C8thio alkoxy and/orC1-C8alkoxy to 1 to 3 times or phenyl may be substituted 1 or 2 - naphthyl, or pentafluorophenyl graphical object is selected from; Stage, having a molecular weight of less than 10000 formula Polymers is outside number, in addition formula having a molecular weight of less than 10000 further Polymers is outside number. In one aspect of the present invention a preferred embodiment, the e is 2 or 3. Preferably d e. same. Formula I including recurring structural units of polymer including recurring structural units of formula III preferably compared to polymerization. In one aspect a preferred embodiment, the present invention refers to one or more (repeated) unit of formula II (are) relates to polymer including a. < Formula II > Said in formula, The a being integers, of 1 to 5, Ar1andAr1'each independently a formula Of group, Ar2formula a Of group, R1andR2the same or can be different and can, hydrogen, C1-C100alkyl,<aotran type="glo" translation="in particularC8-C36alkyl, C6-C24aryl,<aotran type="glo" translation="in particularC1-C8alkyl, C1-C8thio alkoxy and/orC1-C8alkoxy to 1 to 3 times or phenyl may be substituted 1 or 2 - naphthyl, or pentafluorophenyl is chosen from. Advantageously, of the present invention polymer, or of the present invention polymer including organic semiconductor material, layer OFET or elements can be used in. Highly between n - and Figure 1 p -, and has a well balanced ratio indicative of the rate of transfer curve show. Ar1, Ar1', Ar3andAr3'and can be as equal as has, but may be different from the user, . preferably identical. Ar1, Ar1', Ar3andAr3'the formula Of which alkyl, R3, formula wherein Preferably a dispatcher. Ar2formula a Of and alkyl, R3, formula wherein Of preferably a, formula Preferably further a dispatcher. A length different from each 2 is a, Ar2the formula Of may be configured into, i.e. for example formula Alkyl, R3 KIPO & of. Formula As if the represented by, The DPP basic unit is attached to and, of two ways in chain polymer . May be arranged in. Notation Must decide include the possibilities of both. Preferably a integer of 1 to 5, in particular 1 to 3 is integer number of. The b is integer number of 1 to 3. The c is integer number of 1 to 3. A, b and c is a total of 7 hereinafter. R1, R2, R1'andR2'but the may be different from the user, . preferably identical. R1, R2, R1'andR2'but may be a linear, preferably is branched. R1, R2, R1'andR2'preferablyC8-C36alkyl,<aotran type="glo" translation="in particularC12-C24alkyl, e.g. n - dodecyl, midifiers targeting tree, tetra midifiers targeting, pen hit thread, methylhexadecyl, - ethyl 2 - ethylhexyl, 2 - ethylhexyl - butyl, 2 - butyl - octyl, 2 - [...], midifiers targeting tetra - 2 - midifiers targeting, [...], midifiers targeting, eicosa chamber, [...], is nose room or nose room. C8-C36alkyl andC12-C24the alkyl may be linear or branched but, preferably is branched. In one aspect a preferred embodiment of the present invention in particular, R1, R2, R1'andR2'[...] or the 2 - 2 -. double midifiers targeting tetra - midifiers targeting. Advantageously, R1, R2, R1'andR2'the formula (Wherein, m1=n1 + 4 and, m1 + n1 ≤ 22 randomly choosing) .can be represented by. Chiral chain, e.g.R1, R2, R1'andR2'it will be a minute body or can the homochiral, morphological make polymer of (morphology) can affect the KIPO &. In one aspect a preferred embodiment, the present invention refers to a formula (Wherein, R1andR2the branchedC8-C36alkyl, in particular branchedC12-C24alkyl, e.g. 2 - tetra - 2 - or [...] midifiers targeting by double midifiers targeting) is copolymer. Said polymer is preferably a 10,000 to 100,000 Daltons, most preferably to 20,000 has a weight average molecular weight of 60,000 Daltons. Said polymer is preferably a 1. 1 to 3. 0, most preferably 1. 5 to 2. 5 has a polydispersity. In one aspect a preferred embodiment, the present invention refers to a formula (Wherein, R1'andR2'the branchedC8-C36alkyl, in particular branchedC12-C24alkyl, e.g. 2 - tetra - 2 - or [...] midifiers targeting by double midifiers targeting) relates to are either a homopolymer of. Said polymer is preferably a 10,000 to 100,000 Daltons, most preferably to 20,000 has a weight average molecular weight of 60,000 Daltons. Said polymer is preferably a 1. 1 to 3. 0, most preferably 1. 5 to 2. 5 has a polydispersity. Said polymer can exhibit a positive polarity. In one aspect a preferred embodiment, the present invention refers to one or more of a formula (repeated) unit (are) relates to polymer including: . Said in formula, The a 1 to 5, in particular 1 to 3 being integers, of, 2 or 3 being integers, of the b, b ' being integers, of the 2, b " being integers, of the 3, X1andX2N and one, and at least one other of which CH and, R1andR2the same or can be different and can, hydrogen orC8-C36is chosen from alkyl. One or more (repeated) unit of formula (are) of polymer is further preferably including: . In formula said, R1andR2the same or can be different and can, C8-C36is chosen from alkyl. In one aspect of the present invention a preferred embodiment, different properties of the polymer 2 includes recurring structural units of formula I. Advantageously, the repeat unit formula IIa, IIb, IIc, IId, IIe, IIf, is chosen from the repeating units of IIh and IIg. Formula IIa ' and IIa " polymer including recurring structural units of, for example excellent field effect mobility and exhibits on/off current ratio and a. According to the present invention, 1 species polymer alone (chamber in number, potential or virtual) a polymer having a derived from monomers. The polymers many complementary monomer interpolymers is bath number by the reaction. A such monomers, polymer, alone, is are either a homopolymer of the same thread that can be is considered a under public affairs number product number "potential monomer" react to a number for by under public affairs hereinafter can be visible. Some polymers, resulting polymers molecular structure consists of a large-size structure are either a homopolymer of monomer the virtual deemed to be formed by may be chemical modification of the other polymer obtained by. Thus, greater than species 1 copolymer polymers derived from monomers, for example binary copolymer, terpolymer, staff copolymer, etc.. Terms polymer containing oligomer as well as.. Of the present invention oligomers < has a weight average molecular weight of 4,000 Daltons. Of the present invention polymer is preferably a 4,000 Daltons or more, 4,000 to 2,000,000 Daltons in particular, very in particular 10,000 to 1,000,000 Daltons, more preferably 10,000 to 100,000 Daltons, most preferably to 20,000 has a weight average molecular weight of 60,000 Daltons. Using standard polystyrene molecular high temperature gel permeation chromatography, (HT1A-gPC) is measured according to. 1 polymer is preferably a of the present invention. 01 to 10, more preferably 1. 1 to 3. 0, most preferably 1. 5 to 2. 5 has a polydispersity. In one aspect of the present invention a preferred embodiment, polymer formula (VII) and copolymer including recurring structural units of, in particular wherein A formula a Of group, COM1the formula Of group, R1, R2, Ar1, Ar1', Ar2and the a is defined. Copolymers of formula VII, for example [...] (Suzuki) can be obtained by the reaction of an. Aromatic boronate [...] with an, in particular bromide condensation reaction of the carboxylic acid compound (generally, referred to as "[...] reaction" by) the, literature [N. Miyaura and A. Suzuki, Chemical Reviews, Vol. 95, pp. 457 - 2483 (1995)] reported in a wide variety of organic as presence of the. in soil or rock. Preferred catalysts [...] DC 2 - -2 ', 6' - di - palladium (II) acetate/phenyl planter alkoxy, tree - alkyl - phosphonium salts/palladium (0) derivatives and tree - alkyl phosphine/palladium (0) is derivatives. In particular preferred catalysts [...] DC 2 - -2 ', 6' - di - [...] (sPhos)/palladium (II) acetate, and - tert - butyl [...] tetrafluoroborate((t -Bu)3P*HBF4) /tris (d it cuts qualitative the acetone which is burnt) d palladium( 0)( Pd2 (dba)3)and - tert - butyl phosphine(t -Bu)3P/tris (d it cuts qualitative the acetone which is burnt) d palladium( 0)( Pd2 (dba)3)is. Preferred solvents a propanamines (THF), or is mixture of toluene and a THF. Preferred base an aqueousK2CO3or aqueousNa2CO3is. High molecular weight polymer the reaction and copolymers can be applied for bath number. Formula (VII) (Wherein, the A formula Of group, COM1the formula Group of, the a being integers, of 1 to 5, The n number molecular weight of 4,000 to 2,000,000 Daltons under public affairs and, R1, R2, Ar1, Ar1', Ar2the a and as defined in the) A polymer corresponding to number to under trillion, solvent in the, in addition in the presence of catalyst; e.g. to catalyzing the Pd and triphenyl phosphine, [...], E.g. [...] or d claw id, or d the id which comes, in particular formula Corresponding to formula [...] equivalent molar amount of Or reacting at [...] or d step theory it buys corresponding to, or formula [...] of formula equivalent molar amount of d step theory it buys or [...] reaction to corresponding to (wherein, X10a halogen, in particular Br and, X11independently, have in each case one-B(OH)2, -B(OY1)2, , -BF3Na, -BF3N (Y15)4, or-BF3Kand, whereinY1independently, have in each case oneC1-C10, R is an alkyl group containing, Y2each if theC2-C101015 per square meter, e.g.-CY3Y4-CY5Y6-, or-CY7Y8-CY9Y10-CY11Y12-and, whereinY3, Y4, Y5, Y6, Y7, Y8, Y9, Y10, Y11andY12each independently a hydrogen, orC1-C10alkyl,<aotran type="glo" translation="in particular-C(CH3)2C (CH3)2-, or-C(CH3)2CH2C (CH3)2-, -CH2C (CH3)2CH2-and, Y13andY14each independently a hydrogen, orC1-C10, R is an alkyl group containing, Y15the H, or - O - is optionally may be interposed aC1-C25fluoroalkyl silane is). Reaction is typically aromatic hydrocarbon solvent, such as toluene, xylene, not brush, trichlorobenzene, about in fluorobenzene is carried out in 0 °C to 180 °C. In addition, other solvent, e.g. dimethyl formamide, dioxane, [...], 2 - methyl polytetrahydrofuran, tetrahydrofuran and alone [...] cycloalkyl, or aromatic hydrocarbons, use can be made of, mixture with hydrogen. Most preferably is toluene THF/or THF. Aqueous base (e.g., alkali and alkaline earth hydroxide, carboxylate, carbonate, fluoride and phosphate, e.g. sodium and potassium, hydroxide, acetate, carbonate, fluorides and phosphate or in addition metal alcoholates), preferably sodium carbonate or bicarbonate, potassium phosphate, potassium carbonate or middle carbonic acid potassium used as the HBr scavenger. 0 the polymerization. 2 to 100 can be time it would take for the. Organic base, e.g. tetraalkylammonium hydroxide, and phase shift catalyst, e.g. TBAB of boron is can be effects of promoting tyrosinase activity (for example, literature [Leadbeater & Marco; Angew. Chem. Int. Ed. Eng. 42 (2003) 1407] citation and a reference literature reference). Another reaction conditions variations literature [T. I. Wallow and B. M. Novak, J. Org. Chem. 59 (1994) 5034 - 5037]; and [M. Remmers, M. Schulze, and G. Wegner, Macromol. Rapid Commun. 17 (1996) 239 - 252] by is ball number. Excess of [...] molecular weight adjustment, [...] or d step theory it buys, or chain terminated. by the use of number. Palladium catalyst present in catalyst amount to produce a in said mixture. As used herein, a "catalytic amount" as the terms, used [...][...] or d step theory it buys and a small amount is pivotally 1 equivalent, preferably used based on the equivalent [...] or d step theory it buys and [...] 0. 01 to 5 mol %, most preferably 0. 01 to ., " refers to 1 mol %. In reaction mixture is phosphine or phosphonium salts or by the amount, used [...][...] or d step theory it buys and 0 preferably based on the equivalent. 02 to 10 mol %, most preferably 0. 02 is to 2 mol %. Pd: phosphine the desired ratio is 1:2. Boron 1 per functional group. 5 but not more than of said, a reaction mixture of a base is. preferably there is also a. Single solvent or solvent mixture in the case of of polymers which can be carried out in an, polymerization when disclosure, after a given time in addition, 2 or 3 can add KIPO & class a co-solvent and, . The aim of added co-solvent such growing polymer chain during polymerization processes and maintaining it at solution is intended. In addition this, completion in from a reaction mixture and assists in the recovery of polymer, thereof improves yield of isolating a polymer. Case that the user wants, in these reactions chain - terminating number as monofunctional aryl halide or aryl boronate can be used and, a second pixel electrode is formed on a distal is will aryl. . [...] reaction monomers in feed order and by controlling the composition of such monomer unit in the resulting copolymer is in the array of can be control. After polymerization, polymer is preferably a, for example (work-up) normally for the working-up of a the reaction mixture by means of is withdrawn from, is forward number. This, a publicly known to one skilled in the art, in addition standard substrate literature can be this is achieved in method. In addition polymer of the present invention, steel (Stille) can be combined by a coupling (for example, literature [Babudri et al. , J. Mater. Chem. , 2004, 14, 11 - 34]; [J. K. Stille, Angew. Chemie Int. Ed. Engl. 1986, 25, 508] reference). A polymer corresponding to formula VII to under trillion number, [...], e.g. [...] or d claw id, in particular formula [...] corresponding to, in the presence of a catalyst comprising palladium - 200 °C to 0 °C in an inert solvent at temperatures in the range of from, formula (Wherein, X21the-SnR207R208R209group, whereinR207, R208andR209of the same or hereinafter and, H orC1-C6alkyl, wherein 2 two radical optionally common and by forming rings and, such radicals optionally is stored by liquid phase in a branched or non-branched) of a compound and a reaction to. Used here very balanced all monomer is completely displayed by following the organotin of that is guaranteed to be with halogen to function should. In addition, monofunctional polysaccharide end - by capping completion an optional number of excessive amounts of reactive groups it is advantageous to that triggers can be to exhibit. To perform process, preferably tin compound, and halogenated one compound or more inert in the organic solvent introducing a starter and, 0 to 200 °C, preferably at a temperature of 30 to 170 °C time 200 hr to 1, preferably 2000 time 150 hr to 5. A publicly known to one skilled in the art the crude product, each polymer appropriate method, for example used in the secondary oxidation is the repeated material - formed in dialysis or even can be number. Method is a a suitable organic solvent to, for example, ether, for example diethyl ether, [...], diethylene glycol dimethyl ether, tetrahydrofuran, dioxane, dioxolane, diisopropyl ether and tert - butyl ether, hydrocarbon, for example hexane, ISO hexane, heptane, cyclohexane, benzene, toluene or xylene, alcohol, for example methanol, ethanol, 1 - propanol, 2 - propanol, ethylene glycol, 1 - butanol, 2 - butanol and tert - butanol, ketone, for example acetone, ethyl methyl ketone and isobutyl methyl ketones, amide, for example dimethyl formamide (DMF), dimethyl acetamides and N - methylpyrrolidone, nitrile, for example acetonitrile, hydroxypropionitrile and phenylbutyronitrile, is and mixtures thereof. Palladium and phosphine component [...] to deformation from those described similarly should selected. Alternatively, polymer of the present invention in addition, zinc reagent( A- (ZnX22)2 (wherein, X22by halogen)), and halide or triflate( COM1- (X23)2 (wherein, X23by a halogen or triflate)) using four it crawls, (Negishi) can be synthesized by the reaction. For example, literature [E. Negishi et al. , Heterocycles 18 (1982) 117 - 22] a reference. R1and/orR2the polymers is hydrogen, after polymerization for producing a sedated number which can be saver can be obtained using the (for example, EP single-0648770, EP single-0648817, EP single-0742255, EP single-0761772, WO 98/32802, WO 98/45757, WO 98/58027, WO 99/01511, WO 00/17275, WO 00/39221, WO 00/63297 and EP single-1086984 reference). The form of pigmented their pigment precursor, conversion, for example thermally, optionally additional catalyst, for example the presence of a catalyst which is described WO 00/36210, publicly known conditions is carried out by fragmentation to. Examples of such protector, formula (Wherein, L solubility assigned as the suitable by any the desired double) of. double. Formula L is preferably , (Wherein, Z1, Z2andZ3, independently one of the other areC1-C6alkyl, Z4andZ8, independently one of the other areC1-C6alkyl, oxygen, sulfur orN (Z12)2a confirmation of the wafer is performed byC1-C6alkyl, or or unsubstitutedC1-C6alkyl-, C1-C6alkoxy -, halo -, cyano - nitro roh or and biphenyl a phenyl or substituted, Z5, Z6andZ7, independently one of the other are hydrogen orC1-C6alkyl, Z9hydrogen, C1-C6alkyl or formula Of group, Z10andZ11the other is independently hydrogen, C1-C6alkyl, C1-C6alkoxy, halogen, cyano, nitro, N (Z12)2, or unsubstituted or haloalkoxide -, cyano -, nitro-, C1-C6alkyl orC1-C6alkoxy - substituted phenyl and, Z12andZ13theC1-C6alkyl, Z14hydrogen orC1-C6alkyl, Z15hydrogen, C1-C6alkyl, or or unsubstitutedC1-C6alkyl - substituted phenyl and, A non-Q or substitutedC1-C6alkoxy, C1-C6alkylthio orC2-C12in D alkyl amino by mono or multi-substituedp, q -C2-C6alkyl [...], wherein p and q has different positions number, X nitrogen, selected from the group consisting of oxygen and sulfur hetero-atoms in a and, when the or sulfur oxygen X the m'and of 0, X is 1 when is nitrogen and of the m, L1andL2each independently a unsubstituted or mono or-C1-C12alkoxy-, -C1-C12alkylthio-, -C2-C24dialkylamino-, -C6-C12aryloxy-, -C6-C12arylthio-, -C7-C24alkyl biting amino or-C12-C24diarylamino - substitutedC1-C6alkyl or[ - (p',q' -C2-C6alkylene) -Z-]n'-C1-C6alkyl, the n'and of 1 to 1000, p 'and q' has different positions number, each Z any that different ones of the oxygen heteroatom, but independently of, sulfur orC1-C12alkyl - substituted is nitrogen and, [-C2-C6alkylene - Z -] repeated unitsC2-C6alkyl [...] can be different and can equal to or, L1andL2the 1 to 10 times and can be saturated or unsaturated, in any location (C=O) - - and-C6H4-selected from the group consisting of 1 to 10 is of and may be interposed or non-interposed, or additional substituents, halogen, an abrupt turning nitro cyano and selected from the group consisting of one further 10 to 1 which may have substituents) of. double. The most preferred L the formula Of. double. Formula The synthesis of compound of WO 05/049695, or described in WO 09/047104 and WO 08/000664, method is or can be much like an. N - aryl substituted formula The synthesis of compound of WO 03/022848 uS single-5,354,869 and method is can be much like an. Another embodiment in one aspect, the present invention refers to formula III relates of a polymer, of an. Said embodiment embodiments, one or more (repeated) unit of formula (are) polymer is preferably including a.. , In formula said, R1'andR2'the same or can be different and can, C8-C36is chosen from alkyl. Another preferred embodiment in one aspect, the present invention refers to, formula Are either a homopolymer of (IIIe), or formula Copolymer of (IIm ") (wherein, R1, R2, R1'andR2'the branchedC8-C36alkyl, in particular branchedC12-C24fluoroalkyl silane is), or 51,500 of Mw and 2. 0 polydispersity (HT1A-gPC measured by a) having formula Relates to copolymer of. Including recurring structural units of formula III polymer is preferably a, corresponding, the mole ratio of dihaloaromatic compound, e.g. Ni is 0 for polycondensation for the dehalogenation of complex (reductively coupling) (yamawool also (Yamamoto) coupling reaction) under trillion the number can be a polymer having a alone. Nickel as a source bis (cyclo jade other D n) bipyridine the nickel is, triarylphosphine or trialkyl phosphine can be and is used in combination with a. Literature for example [T. Yamamoto, et al. , Synthetic Metals (1993), 55 (2 - 3), 1214 - 20] reference a. Alternatively, such polymer, [...]A equivalent molar amount of formula Corresponding to the number by reacting [...] or d step theory it buys may be bath. Additional embodiment aspect of the present invention, a relates to compounds of formula X. <Formula X> Said in formula, D an integer 1, 2 or 3 and, E an integer 1, 2 or 3 and, Ar3andAr3'each independently a formula Of, or-Ar4-Ar5- [Ar6]f-group, Ar4formula a Of group, Ar5andAr6, independently one of the other areAr3has meaning of, the f 0, or water purification 1 and, R1'andR2'is represented by the following the same as the according to Claim 1, X12a-B(OH)2, -B(OY1)2, , -BF3Na, -BF3N (Y15)4, or-BF3Kand, whereinY1independently, have in each case oneC1-C10, R is an alkyl group containing, Y2each if theC2-C101015 per square meter, e.g.-CY3Y4-CY5Y6-, or-CY7Y8-CY9Y10-CY11Y12-and, whereinY3, Y4, Y5, Y6, Y7, Y8, Y9, Y10, Y11andY12each independently a hydrogen, orC1-C10alkyl,<aotran type="glo" translation="in particular-C(CH3)2C (CH3)2-, or-C(CH3)2CH2C (CH3)2-and, Y15the H, or O may be interposed is stopped and aC1-C25copyright 2000. The compounds of formula X for the synthesis of polymer of the present invention exhibits intermediates. Of such compounds showed to e.g. specifically: . Semiconductor device of the present invention polymer membrane can be used as a the semiconductor layer, . Thus, the present invention refers to in addition, of the present invention polymer, or organic semiconductor material, layer or components from including relates to semiconductor device. The semiconductor device is in particular organic field effect transistor (OFET). Numerous semiconductor device is present. Common for all the one or more semiconductor materials. a speaker is not formed. The semiconductor device, for example, literature [S. M. Sze, Physics of Semiconductor Devices, 2nd edition, John Wiley and Sons, New York( 1981)]are depicted in. Such device the rectifier, transistor (P-n-P, n-P-n including various types of transistor, and thin film transistor), emitting semiconductor device (for example, display applications device in organic light emitting diode, in for example a liquid crystal display or backlight), photoconductor, current number chilly, solar cell, thermistor, P-n conjugates, field --effect diodes, sort key (Schottky) comprises material. In each semiconductor device, the semiconductor material is at least one metal, metal oxide, e.g. indium tin oxide (ITO) and/or insulator combined with to form a device. The semiconductor device, for example literature [Peter Van Zant, Microchip Fabrication, Fourth Edition, mcGraw-hill, New York (2000)] to such as are discussed in by a publicly known method can be or production bath number. In particular, the organic electronic component literature [D. R. Gamota et al. , Printed Organic and Molecular Electronics, Kluver Academic Publ. , Boston, 2004] as described number can be under trillion. In particular useful transistor type device in thin film transistor (TFT) are generally, gate electrode, gate dielectric on gate electrode, gate dielectric adjacent to the source electrode and the drain electrode, and gate dielectric adjacent to and adjacent source electrode and a drain electrode layer, said layer including a semiconductor (for example, literature [S. M. Sze, Physics of Semiconductor Devices, 2nd edition, John Wiley and Sons, page 492, New York( 1981)]reference). These components in various configurations can be assembly. More specifically, have a of organic semiconductor OFET. Typically, substrate number bath, testing and/or is held a OFET during use. Optionally, substrate electrically OFET can be under public affairs number function. Useful substrate materials comprises organic and inorganic substances. For example, various substrate silicon material including Si, which Si-addition lowers the correct shape, inorganic glass, ceramic foil, polymeric material (for example, acrylic, polyester, epoxy, polyamide, polycarbonate, polyimide, polyketone, poly (oxy - 1, 4 - polyphenylene oxy - 1, 4 - polyphenylene carbonyl - 1, 4 - polyphenylene) (often poly (ether ether ketone) or PEEK as referred to), poly [...], poly phenylene oxide, poly (ethylene naphthalenedicarboxylate) (PEN), poly (ethylene terephthalate) (PET), poly (polyphenylene sulfide) (PPS)), filled polymer material (e.g., fiber - enhanced plastic (FRP)) and coated metallic foil may include a. Of the available resources in a any gate electrode can be a conductive material. For example, gate electrode doped silicon, or metal, e.g., aluminium, chromium, gold, the, nickel, palladium, platinum, tantalum and titanium may include a. Conductive oxide, e.g. indium tin oxide, or optionally containing number polymer binder, carbon black/graphite or of a colloidal the ink/paste is preferably the dispersion can be used in addition. Conductive polymer, for example polyaniline or (3, 4 - ethylenedioxythiophene)/poly (styrene sulfonate) (PEDOT: PSS) is can be used in addition. In addition, alloy of these materials, having useful multilayer metals with minimal slag production and can be. OFET in part, the same material can be under public affairs number function gate electrode, in addition substrate support function can be under public affairs number. For example, doped silicon a gate electrode and is supported with the KIPO & OFET. Gate dielectric generally is ball number on gate electrode. The gate dielectric gate from the balance OFET device electrically isolating the electrode.. The useful materials as gate dielectric is disclosed, e.g. an inorganic an electric insulating material may comprise an. The oxide gate dielectric (insulator), or may be a substance such as a nitride, or a ferroelectric insulator series (example, organic material, such as poly (vinylidene fluoride/trifluoro ethylene or ([...] xylylenediamine units m -)) or may be a substance selected from, or the, for example literature [J. Veres et al. , Chem. Mat. 2004, 16, 4543] or [A. Facchetti et al. , Adv. Mat. 2005, 17, 1705], as described an organic polymer insulator (for example, poly (methacrylate), poly (acrylate), polyimide, benzocyclobutene (BCB), parylene, polyvinyl alcohol, poly vinyl phenol (PVP), polystyrene, polyester, polycarbonate) can be. As gate dielectric is disclosed examples specifically of useful substances, PbZrxTi1-xO3 (PZT), Bi4Ti3O12, BaMgF4, Ba (Zr[a]x[/a] -xTix) O3 (BZT)including (802.11a not one number them to), [...], tantalate, titanate, zirconate titanates may be of the, aluminum oxide, silicon oxide, tantalum oxide, titanium oxide, silicon nitride, barium titanate, titanium it buys the barium strontium, barium zirconate titanate, selenate comprises zinc sulfide and anger zinc. In addition, alloy of these materials, hybrid materials (for example, polysiloxane or nano particle filled polymer) irons metals with minimal slag production and having multiple filler layers may be used in the gate dielectric. Dielectric layer has a thickness of from, for example about 10 to 1000 nm and, than to a particular and a thickness of from about 100 to 500 nm, 0. 1 to 100 nano faradunder public affairs the photosensitive polymer containing the number (nF) range.. Gate dielectric the source and drain electrodes can be separated from gate electrode by, an organic semiconductor layer above or below the source electrode and. may be present. Advantageously the source and drain electrodes resistance semiconductor layer and any number of the available resources in a under public affairs ohmic contact can be a conductive material. Layer is useful, gate electrode for said material, for example, with aluminium, barium, calcium, chromium, gold, the, nickel, palladium, platinum, titanium, polyaniline, PEDOT: PSS, other conductive polymer, alloys thereof, most multilayer and their combinations thereof comprises. Some of these materials, as publicly known art, n - type semiconductor material suitable in use with, remainder p - type semiconductor material. suitable for use with. Thin film electrode (i.e., gate electrode, source electrode and a drain electrode) of the available resources in a any, e.g. physical vapor deposition (e.g., thermal deposition or sputtering) or (ink jet) printing method by may be ball number. A patterned of the electrodes a publicly known method, e.g. shadow masking (shadow masking), additional photolithography, attenuation photolithography, printing, contact printing and pattern coating can be achieved by. Further the present invention refers to, Disposed on the substrate is a plurality of electrical conductive gate electrode; Said electrically conductive having contact hole arranged on gate electrode gate insulator layer; Each said setting unit are aligned with the gate electrode disposed on said insulator layer sets of electrically conductive source electrode and a drain electrode; and Said gate electrode and substantially each region of the metal layer overlapped source electrode and a drain electrode on said insulator layer between a channels, of the present invention polymer or of the present invention including a mixture containing of a polymer organic semiconductor layer For including organic field effect transistor device. under public affairs a number. Further the present invention refers to, Plurality of electrical conductive gate electrode the deposited on a substrate; Said gate insulator layer step depositing the plurality of strands onto the electrically conductive gate electrode; Each setting unit said gate electrode on said layer are aligned with the sets of electrically conductive source electrode and a drain electrode and for depositing the step; and Of the present invention compound or of mixtures containing polymer of the present invention said layer gate electrode and substantially overlaid on each other of the present invention polymer said insulator layer on said layer by precipitating a, step bath number a thin film transistor device Including a, thin film transistor device of a number. under public affairs number bath method. The mixture comprising a polymer or polymers of the present invention, of the present invention polymer (typically 5 weight % to 99. 9999 weight %, in particular 20 to 85 weight %) and at least another material for including semi - conductive layer generates. Material other, having different molecular weights of polymers same fraction of the present invention, another of the present invention polymer, semi - conductive polymer, organic small molecule, carbon nanotube, fullerene derivative, inorganic particles (quantum dots, rod quantization, quantization tree pod, TiO2, ZnOsuch as), conductive particles (Au, such as Ag), and gate dielectric for an insulating material as are discussed in (PET, such as PS), the logical source including the device, the 802.11a not one number. Thus, the present invention refers to in addition, the present invention according to polymer including organic semiconductor material, layer relates to or components. Polymer of the present invention, for example Europe patent application number 09155919. 5 call, WO 09/047104, US 6,690,029, WO 2008107089 and WO 2007082584 blending small molecule and is may be: WO 2007082584: . WO 2008107089: . Said in formula, Y1andY2-idiotypic antibodies which represent an - CH=or=CH - one, and at least one other of which indicates the - X -, Y3andY4-idiotypic antibodies which represent an - CH=or=CH - one, and at least one other of which indicates the - X -, The X - O -, - S -, - NR or - Se "' - and, R3C - the 1 to 20 of a cyclic atoms, straight or branched chain alkyl or alkoxy groups, or 2 to 30 C - atoms of an aryl (both is perfluorinated or fluorinated optionally) and, R ' the H, F, Cl, Br, I, CN, C - atoms of 1 to 20 and optionally fluorinated or perfluoride is straight or branched chain alkyl or alkoxy groups, C - atoms of 6 to 30 optionally fluorinated or perfluorinated aryl, orCO2R "and, wherein R" the H, of 20 to 1 C - atoms optionally fluorinated alkyl, or 2 to 30 of C - it will be biting and fluorinated optionally atoms, R "' C - 1 to 10 of the H or a cyclic atoms, straight or branched chain alkyl, the y and 1 or 0, the x is 1 or 0. . At of small molecules, or 2 at least one of small molecules can compounds. Alternatively, the OFET, for example a high degree covered by a layer of a thermally grown oxide polymers to substrate silicon doped with a solution of an post-deposition, source electrode and a drain electrode number of vacuum deposited and pattern by is small. In one approach is another, the OFET, a thermally grown oxide covered by highly doped silicon substrate post-deposition the source electrode and the drain electrode, a solution of an is small number by formation of a thin film from deposition. In addition gate electrode patterned metal gate electrode and a conductive material on a substrate, can be conducting polymer e.g., the preventing liquid from coating or vacuum deposition gate electrode patterned by is coating which is applied onto a primer insulator. Any suitable a solvent dissolving the polymer of the present invention reliable peers are allowed to distribute or/which, and is inert end, conventional drying means (for example, heat applied, pressure sensitive, such as airflow) or completely part, from substrate may be for producing a sedated number. Of the present invention semiconductor processing a suitable organic solvent the aromatic or aliphatic hydrocarbon, halogenated (e.g., chlorinated or a fluorinated) hydrocarbon, ester, ether amide, e.g. chloroform, ethane benzoic acid, propanamines, toluene, tetraline, azadecalin, not brush, xylene, ethyl acetate, methyl ethyl ketone, dimethyl formamide, chloroform, trichlorobenzene, dichloro benzene, trichlorobenzene, propylene glycol monomethyl ether acetate (PGMEA), and combinations of these, including but, 802.11a not one number them to. Preferred solvents a xylene, toluene, tetraline, azadecalin, chlorine having been, e.g. chloroform, trichlorobenzene, ortho - dichloro benzene, trichlorobenzene is and mixtures thereof. Spindle or dispersion/solution and then - coating, immersion - coating, screen printing, fine contact printing, doctor blading (doctor blading) or art the art such as solution applying other publicly known method, by applying to the on a substrate, the semiconductor thin film of the low-obtained. Terms "dispersion" a flavored films typically do not completely dissolve in a solvent and including semiconductor material of the present invention any composition comprising a. Dispersion is at least of the present invention polymer, or a mixture containing polymer of the present invention, selects composition including and a solvent (wherein, polymer at room temperature solvent in the low solubility yet elevated temperature in solvent in the greater than solubility exhibits, without stirring or agitation where the temperature number 1 becoming gelatination, composition to when a strong low temperature); At least a portion of polymer at elevated temperatures - dissolving the solvent; temperature of the water within a composition at elevated temperatures in order to drive them a strong low temperature number 1;, gelation of the destroy any agitating composition (wherein, agitation composition heating of a number 1 at a low temperature lowering before certain time, to, drop and simultaneously or after strong disclosure steps); composition layer depositing the plurality of strands (wherein, composition number 2 negative at low temperatures a lower elevated temperature); layer contains at least partially dried can be thereof is performed. Dispersions (a) solvent in addition, coupled number resin, and optionally dispersion including continuous phase and a number (b) of the present invention polymer, or a mixture containing of a polymer of the present invention including a dispersed phase can be constructed. The solubility of the polymer of the present invention in a solvent, for example solubility 0% to about 20%, in particular 0% to about 5% solubility can be which may vary from. Preferably, organic semiconductor layer has a thickness of from about 5 to about 1000 nm in a range, in particular the range of and a thickness of from about 10 to about 100 nm. Semiconductor polymer of the present invention device of organic semiconductor layers may be used alone or in combination as can be. Of the available resources in a any layer, e.g. vapor deposition (in the case of materials having relatively low molecular weight) and techniques by may be ball number. In an organic solvent in polymer of the present invention may be sufficiently soluble, (for example, spin coating, dip coating, ink-jet printing, Gravia printing, flexographic, offset printing, screen printing, contact (wave)- printing, drop or zones casting (zone casting) or other publicly known to the technologies by) can be pattern deposited and solution. A plurality of polymer of the present invention as well as integrated circuit including a OTFT, for the various kinds of electronics for number may be used in the article. The such articles,, for example le radio frequency identification (RFID) tag, flexible display (e.g., personal computer, a mobile phone or portable device for use in) backplane (backplane), smart card, memory device, sensor (e.g., optical -, Image -, bio -, - chemical, mechanical or temperature sensor), in particular photodiode, or security device comprises such as. Due to fluorescent solid state thereof, material in addition organic light-emitting transistor (OLET) may be used in the. The a further aspect of the present invention, one or more of the present invention polymer including organic semiconductor material, layer is or components. A further aspect of the present invention in an organic field effect transistor (OFET). is the use of polymers or materials. The a further aspect of the present invention is including OFET polymers or materials. Typically polymer of the present invention a thin organic layer or film (preferably less than 30 micrometers thickness) may be used as organic semiconductors in the form of. Typically, the semiconductor layer of the present invention (micro m=1) thickness 1 micrometers but hereinafter, the if necessary may be thicker. A variety of electronic device uses, in addition thickness may be less than about 1 micrometers thickness. For example, for use in OFET, layer thickness typically can be 100 nm hereinafter. Layer thickness of, for example layers are employed electronic device will differ depending on the requirements. For example, between drain and source of OFET active semiconductor channel may include a layer of the present invention. Preferably the present invention according to OFET device Source electrode -, - Drain electrode, - Gate electrode, - Semiconductor layer, - One or more gate insulator layer, and - Optionally substrate Includes, aligning a semiconductor of a of the present invention includes a. Source and drain electrodes are insulating layer by can be separated from gate electrode, gate electrode and insulating layer to which makes contact with both semiconductor layer, source electrode and drain electrodes are both if contact semiconductor layer and, gate OFET device, the source and drain electrodes insulating and a semiconductor layer in any sequence can be arranged. OFET preferably the number 1 number 2 side and an insulator side, of the insulator a gate electrode that is positioned on the side number 1, number 2 a that is positioned on the side of the insulator, including layer of the present invention polymer, and polymer layer includes the drain electrode and source electrode. The upper gate device or bottom gate OFET device can be device. OFET device structure and suitable number bath the method is publicly known to one skilled in the art, literature, for example WO 03/052841 are depicted in. The gate insulator layer, for example, the commercially available between top (Cytop) 809M orยฎ top 107M (asahi glassยฎ (Asahi Glass)) such as may include fluoropolymer. Preferably the gate insulator layer, for example, one or more of fluorothermoplastic at least one solvent atoms (fluorine solvent), solvent and bovine perfluoride preferably from blends including insulating material, spin coating, doctor blading, wire bar coating, solution is sprayed or dip coating or other is depositing by a publicly known method. Bovine perfluoride suitable solvent, for example FC75 (ยฎ arc loss (Acros) available from by, catalog number 12380) is. Other suitable fluoropolymer and fluorine is publicly known in prior art solvent, e.g. Teflon polymer bovine perfluoride (Teflon) AF 1600 orยฎ 2400 ([...] (DuPont)) or fluorocarbon-pel (Fluoropel) (species Nix cytometerยฎ (Cytonix)) or perfluoride bovine solvent FC 43 (arc loss, ยฎ No. 12377) is. Polymer of the present invention including semiconductor the layer comprises at least another material further may include. Material other, another of the present invention polymer, semi - conductive polymer, polymer binder number, small molecule different organic polymer and of the present invention, carbon nanotube, fullerene derivative, inorganic particles (quantum dots, rod quantization, quantization tree pod, TiO2, ZnOsuch as), conductive particles (Au, such as Ag), and gate dielectric for an insulating material as are discussed in (PET, such as PS), the logical source including the device, the 802.11a not one number. As, mentioned in the, the semiconductor layer in addition one or more of the present invention polymer and polymer binder can be comprised of a mixture of number. Polymer binder for number the ratio of polymer of the present invention can be changed to 5 to 95%. Preferably, the semicrystalline polymer number polymer binder, such as polysterene (PS), and - density polyethylene (HDPE), polypropylene, acrylates, methacrylates, an unsaturated (PP) and is (PMMA). With this technique, electrical performance can be for avoiding a deterioration (WO 2008/001123 A1 reference). P - primarily digital circuit using both reactive and n - type transistors having the same conductivity type, complementary metal oxide (CMOS) based in that. A lower power loss advantage of CMOS circuit, higher velocity, and a larger variation is change in operating characteristics and transistor, as tolerance. P - or n - such CMOS circuit transistors having the same conductivity type, having-type semiconductors can be constructed using. For example, poly [2 - methoxy - 5 - (3 ', 7' - dimethyl [...])] - p - phenylene vinylene( OC1C10-PPV)p-semiconductor and [6, 6] - phenylC61-butyric acid methyl ester (PCBM) n - type a semiconductor, transistors having the same conductivity type, each about respectively, if used as10-2 cm2/Vsexhibits mobility of the. The mobility of semiconductor howeverOC1C10-PPVPCBM and a mixture of each bipolar transistor using10-04 cm2/Vsand10-05 cm2/Vsreduces the to (literature [E. J. Meijer, et al. , Nature Materials, 2003, Vol. 2 page 678]). WO 2008/122778 the, for achieving mobility, and has a well balanced improved blend composition disclosure, however, this has a lower mobility still. Levels thereof in, an electronic mobility device, e. g. wireless frequency identification tag number seal for light the. low too to having utility. Typically required integration of a large, lateral dimension of several micrometers down organic individual small number-channel transistor p - and n very still. worth the challenge. Solution processable transistor-based more efficient circuit to design a, n - and p - single component transistor realized as the core diamond and both the actuation devices on each arm a complementary technique a dire the need present. Ideally, mobility formed,, and has a well balanced current and/or should mobility, and has a well balanced. The WO 20080246095 and US 20080099758, approximately2x10-4 cm2/Vsof holes and electronic mobility bipolar single component is disclosure and the monomer are being polymer. Literature [Adv. Material. 2008, 20, 2217 - 2224] the, various device as determined at a configured, 0. 05 cm2/Vsmaximum hole mobility to the amorphized substrate to form a (wherein electron mobility a bottom contact gold electrode has not been altered detected by using the) formula Disclosure as, for example, are either a homopolymer of is. Layer and the upper contact gold electrode in use, 0 for hole mobility. 11 cm2/Vsand 0. 04 to 0. 09 cm2/Vsis measured mobility range. A bottom gate bottom contact device of the present invention polymer in a composite structure is 5 to 10 times more electron mobility hole and excellent can exhibit. Low contact a copolymer this type owing to coating, the bipolar, for electron injection longer reactive metal of low work function, e.g. Ca, without depending on Mg, two type of carrier such unified gold contact material can be induced by. Source and drain even of the invention is to allow injection Cu and Ag as electrodes is achieved by or alloys thereof, and the. For example, polymer in the embodiment 1: To, layer and the upper contact gold electrode in use, 0 for hole mobility. 43 cm2/Vsand 0. 35 cm2/Vsis measured mobility range. Thus, the present invention refers to in addition, gate electrode, gate insulating layer, including organic active layer of the present invention polymer, including and substrate on source/drain electrode, including p - and n - type behavior, bipolar organic field effect transistor (OFET), in particular organic thin film transistor (OTFT). under public affairs a number. Preferably, the active layer consists of polymer of the present invention. Active composition of layer (semiconductor),, and the mobility of the hole are substantially equivalent to those of and any electron transfer in the while, the transport both electronic and hole, delivery of its transistor characterized in exhibiting bipolar substantially is composition. Bipolar OTFT the substrate, gate electrode, gate insulating layer, source/drain electrode and the active layer may comprise, or alternatively substrate, gate electrode, gate insulating layer, an active layer and/source can be degraded and selected from a group consisting of Cr, by a backing layer, which is exemplary embodiment aspect may not one number. Using the catalyst and polymer of the present invention to form active layer, trichlorobenzene or chloroform for including organic active layer compositions for, use can be made of, . Organic active layer for examples of these compositions and corresponding regulated so as to have a solvent, chloroform, trichlorobenzene, dichloro benzene, trichlorobenzene may include a and toluene. Organic active forming a layer examples of method, screen printing, printing, spin coating, dipped or ink jet includes can be degraded and, the 802.11a not one number. The, bipolar OTFT contained in the gate on the insulating layer, publicly known art a pair of shell members that are typically a, use can be made of, for any. These examples of the specific, Ba0. 33Sr0. 66TiO3 (BST :titanium it buys the barium strontium), Al2O3, Ta2O5, La2O5, Y2O5, orTiO2including ferroelectric insulator, PbZr0. 33Ti0. 66O3 (PZT), Bi4Ti3O12, BaMgF4, SrBi2 (TaNb)2O9, Ba (ZrTi) O3 (BZT), BaTiO3, SrTiO3, Bi4Ti3O12, SiO2, SiNx, including AlON or inorganic insulator, or poly amide, benzo cyclo butane (BCB), parylene, polyvinyl alcohol or polyvinyl phenol including organic insulator can be degraded and, the may not one number. OTFT bipolar of the present invention/gate electrode and source contained in the drain electrode, which can be used metal typically, these examples of the specific, gold (Au), the (Ag), copper (Cu), aluminum (Al), nickel (Ni) and indium tin oxide (ITO), including, but, the number one are not). Preferably, gate, source and drain electrodes material at least one of Cu, Ag, Au or alloys thereof, and the is selected from the group of. OTFT bipolar of the present invention examples of a substance for the substrate, glass, polyethylene naphthalate (PEN), polyethylene terephthalate (PET), polycarbonate, polyvinyl alcohol, polyacrylate, polyimide, poly [...] or polyether sulfone (PES) but may include, the may not one number. The present invention refers to in addition, bipolar organic field effect transistor (OFET) of the present invention, including in particular organic thin film transistor (OTFT) a electronic device. under public affairs a number. Bipolar polymer of the present invention device for improving properties of organic TFT in order to action, said polymer liquid crystal display (LCD) device, optical voltage device, organic light emitting device (OLED), sensor, and/or an integrated circuit memory device including, a number for controlling a variety of electronic device can be used thereon effective to. Small number of organic TFT bipolar the method, gate electrode, gate insulating layer, organic active layer and source/drain electrode on the substrate includes structure is formed on the, wherein the organic active layer of the present invention includes a. Organic active layer screen printing, printing, spin coating, dipped or ink PCB the metallic film can be formed.0 Ba the insulation layer. 33 Sr0. 66TiO3 (BST :titanium it buys the barium strontium), Al2O3, Ta2O5, La2O5, Y2O5, orTiO2including ferroelectric insulator, PbZr0. 33Ti0. 66O3 (PZT), Bi4Ti3O12, BaMgF4, SrBi2 (TaNb)2O9, Ba (ZrTi) O3 (BZT), BaTiO3, SrTiO3, Bi4Ti3O12, SiO2, SiNx, including AlON or inorganic insulator, or poly amide, benzo cyclo butane (BCB), parylene, polyvinyl alcohol or polyvinyl phenol including selected from the group consisting of organic dielectric utilizing a material which can be formed. Glass, polyethylene naphthalate (PEN), polyethylene terephthalate (PET), polycarbonate, polyvinyl alcohol, polyacrylate, polyimide, poly [...], and polyehtersulfone (PES) selected from the group consisting of utilizing a material which can be formed. Gate electrode and source/drain electrode gold (Au), the (Ag), copper (Cu), aluminum (Al), nickel (Ni) and indium tin oxide (ITO) selected from the group consisting of utilizing a material which can be formed. In addition polymer of the present invention, organic optical voltage (PV) device (solar cell) may be used in the. Thus, the present invention refers to, the present invention according to. under public affairs a number including PV device polymer. A PV device, (A) cathode (cathode) (electrode), (B) optionally transfer layer, e.g. alkali halo [...], in particular lithium fluoride, (C) photoactive layers, (D) optionally smooth layer, (E) (electrode) anode (anode), and (F) substrate Comprising described below in this order. The photoactive layers of the present invention includes a. Preferably, photoactive layers polymer conjugates of the present invention as the electron donor, and electronic recipient as recipient material, e.g. fullerene, consists of in in particular functionalized fullerene PCBM. Hetero-junction solar cells, active layer is preferably applied by weight ratio of 1:3 to 1:1 of fullerene polymer and of the present invention, e.g. PCBM (=6, 6 - phenyl-C61-butyric acid methyl ester) or contain a mixture of PCBM. The present invention according to field effect transistor integrated circuit is further preferably including. In the embodiment a winding the tape around the head drum herein for purposes of illustration, includes then, the claim-executable commands for transmitting the clock not only number. Is not referred alternatively, all and is weight percentage. - Average molecular weight polydispersity (Mw/Mn=PD) (Mw) and the thermal temperature gel permeation chromatography, (HT1A-gPC) is measured by [device : polymer [...] (Polymer laboratories) ([...] treatment Britain; current into gate of the memory cell) GPC PL 220 from (refractive index (RI) from reaction obtaining a), chromatography conditions : column: of 3 "PLgel Olexis" column (polymer [...] ([...] treatment Britain)); m micro 13 average particle size (dimension 300 x 8 mm I. D.) bed: number formed in vacuum distillation is butyl [...] n (BHT, 200 mg/L) 1, 2, 4 - trichlorobenzene-solvent and, stabilized by, chromatography temperature: 150 °C; bed flow: 1 ml/ingredient; solute concentration: about 1 mg/mL; injection volume: 200 micro l; detecting: RI, molecular weight of the spherical aberration correction by small procedure : 1 '930' 000 Da - 5 '050 Da [...] polymer over a range molecular weight of ([...] treatment Britain) from polystyrene of 10 obtained by a set of correction criterion (i.e., PS 1' 930 '000, PS 1' 460 '000, PS 1' 075 '000, PS 560' 000, PS 330 '000, PS 96' 000, PS 52 '000, PS 30' 300, PS 10 '100, PS 5' 050 Da) using luminance components and color components to relatively]. Poly autonomic fault calculates a utilizing calibration in molecular weight. A number a substrate structure in the embodiment to any polymer outliers procedural polymers polymer products obtained is the display of. 2 when copolymerized each other component greater than species, according to polymerization conditions arranged in polymers can be alternate, or. < In the embodiment > In the embodiment 1 Polymer 3 starting materials for under trillion according to WO 2008000664 1 was in the embodiment 2a of a number. 3 neck multi function cap in -, dissolved in 5 ml water (arithmetic mean deviation on the predetermined) potassium phosphate( K3PO4) 1. 45 g for propanamines in 20 ml 2. 07 g compounds of 1, 0. 74 g of 2, 5 - thiophene step theory it buys bis (call arrangements) ester, 32. Treatment of 1 mg - tert - butyl [...] tetrafluoroborate((t -Bu)3P*HBF4)and 52. 2 mg of tris (d it cuts qualitative the acetone which is burnt) d palladium( 0)( Pd2 (dba)3)of arithmetic mean deviation on the added to solution. Reaction mixture is heated at a temperature reflux time was 2. Subsequently, bromo - thiophene 18 mg and 20 minutes after thiophene boronic acid - 24 mg ester call arrangements broke up is added and the polymerization is carried. Reaction mixture cooled to room temperature and, the used in the secondary oxidation is in methanol. Cyclopentane and residue (soxhlet) using heptane [...] by ligand abstraction forward number and, by extracting cyclohexane polymer then, a dark powder 1. 45 g for are obtained. Mw =39' 500, polydispersity=2. 2 (HT1A-gPC measured by a). In the embodiment 1a applied P-Si gate with bottom - gate thin film transistor (TFT) structure (10 cm), the third to eo at, and selects addresses of all lists. 300 nm thickness of high quality thermalSiO2layer capacitance per unit areaCi=32. - Gate of 6 nF/cm2 was insulator. - Gate source electrode, and a drain electrode patterns, in particular in the photolithography directly on oxide was used to. Width W=10 mm and various lengths L=4, 8, 15, 30 micro m defines channels of source/drain electrode by cylinder to have a gold. Prior to the deposition of the organic semiconductors, SiO2surface, silane the vapor of the silane coupling agent 2 in 160 °C saturation time by exposing the fluorotoluene or substrate (OTS) of trichlorosilane midifiers targeting 0. 1 m solution by 20 minutes in 60 °C polymethylsilazane dimethyl hexa was used to derivatives in (HMDS). After rinsing the propanol - isocyanate, the for drying substrates. Transistor performance in xylene Semiconductor thin film, 1% in xylene (w/w) in solution that is obtained from DPP derivatives of formula 3 in the embodiment 1 - to the spindle was under trillion number is cast or drip-coating. 0 solution prior to use. 2 m filter was are filtered. Spin coating the surrounding conditions (number of revolutions over ingredient) 800 rpm about spin rate of 20 seconds performed for all the. A device 1 in 80 °C was assessed after dried time. Transistor behavior automated transistor prober (tP-10) measured at. Saturation transfer characteristics from fitting a linearization scheme for an in product muscle number of 8. 5x105having on/off current ratio and a 2. 4x10-1 cm2/Vsof field-effect mobility were capable of is measured. The -2. 7 V was. Transistor performance in chloroform Semiconductor thin film, during chloroform 0. 5% (w/w) in solution that is obtained from DPP derivatives of formula 3 in the embodiment 1 - to the spindle was under trillion number is cast or drip-coating. Spin coating the surrounding conditions (number of revolutions over ingredient) 3000 rpm about spin rate of 20 seconds performed for all the. A device 15 minutes in 120 °C was assessed after dried. Transistor behavior automated transistor prober (tP-10) measured at. Saturation transfer characteristics from fitting a linearization scheme for an in product muscle number of 5. 7x106having on/off current ratio and a 2. 1x10-1 cm2/Vsof field-effect mobility were capable of is measured. The 2. 0 V was. Measurement of bipolar Applied in the embodiment 1b O - applications using xylene as a solvent described bipolar transistor in the embodiment 1, the gate 60 V from - 60 V by a sweep (sweeping) measured at drain bias of (+ - 30 V). Highly between n - and Figure 1 p -, and has a well balanced ratio indicative of the rate of transfer curve show. P - mobile may be even 0. 43 cm2/Vsand, 0 may be even mobile n -. 35 cm2/Vswas. Literature [Adv. Mat. 2008, 2011, 2217 - 2224] to the compared measurement disclosure, been 500 10 may be even mobile p -, identical source - drain electrode (Au) in approximately behavior type n - has proven performance equivalent to those were capable of. In the embodiment applied 1c In the embodiment 1a is repeatedly applied, gold source and drain electrodes by cylinder to have instead of the contact electrodes (1c). Showed listed in tables so that individual asset a result: In the embodiment 2 Starting material 4 - tetra midifiers targeting using iodides midifiers targeting a - number was in the embodiment 2a of under trillion according to WO 2008000664. Propanamines in 50 ml 2. 0 g compounds of 4, 0. 59 g of 2, 5 - thiophene step theory it buys bis (call arrangements) ester, 24. Treatment of 4 mg - tert - butyl [...] tetrafluoroborate((t -Bu)3P*HBF4), 48. 6 mg of tris (d it cuts qualitative the acetone which is burnt) d palladium( 0)( Pd2 (dba)3)and dissolved in 10 ml water (arithmetic mean deviation on the predetermined) potassium phosphate( K3PO4) 1. 13 g by cylinder to have. Reflux in 2 process has been completed, bromo - thiophene boronic acid - thiophene minutes after 20 and 24 mg arrangements call ester 31 mg is added and the polymerization is carried broke up. Reaction mixture cooled to room temperature and, the used in the secondary oxidation is in methanol. Using pentane residue number and forward by ligand abstraction [...], by extracting cyclohexane polymer then a dark powder 1. 67 g for are obtained. Mw=43' 300, polydispersity=1. 9 (HT1A-gPC measured by a). In the embodiment 2 applied: DPP - polymer 5 based on organic field effect transistor P-Si gate with bottom - gate thin film transistor (TFT) structure (10 cm), the third to eo at, and selects addresses of all lists. 300 nm thickness of high quality thermalSiO2layer capacitance per unit areaCi=32. - Gate of 6 nF/cm2 was insulator. - Gate source electrode, and a drain electrode patterns, in particular in the photolithography directly on oxide was used to. Width W=10 mm and various lengths L=4, 8, 15, 30 micro m defines channels of source/drain electrode by cylinder to have a gold. Prior to the deposition of the organic semiconductors, SiO2surface, silane the vapor of the silane coupling agent 2 in 160 °C saturation time by exposing the fluorotoluene or substrate (OTS) of trichlorosilane midifiers targeting 0. 1 m solution by 20 minutes in 60 °C polymethylsilazane dimethyl hexa was used to derivatives in (HMDS). After rinsing the propanol - isocyanate, the for drying substrates. Transistor performance in toluene Semiconductor thin film, fluorotoluene 0. 5% (w/w) in the embodiment 2 in solution that is obtained from DPP derivatives of formula 5 is cast or drip-coating - to the spindle was under trillion number. Spin coating the surrounding conditions (number of revolutions over ingredient) 6000 rpm about spin rate of 10 seconds performed for all the. A device 15 minutes in 100 °C was assessed after dried. Transistor behavior automated transistor prober (tP-10) measured at. Saturation transfer characteristics from fitting a linearization scheme for an in product muscle number of 4. 7x105having on/off current ratio and a 2. 8x10-2 cm2/Vsof field-effect mobility were capable of is measured. The 5. 6 V was. Transistor performance in chloroform Semiconductor thin film, during chloroform 0. 5% (w/w) in the embodiment 2 in solution that is obtained from DPP derivatives of formula 5 is cast or drip-coating - to the spindle was under trillion number. Spin coating the surrounding conditions (number of revolutions over ingredient) 3000 rpm about spin rate of 20 seconds performed for all the. Was assessed after due to the deposition of the device. Transistor behavior automated transistor prober (tP-10) measured at. Saturation transfer characteristics from fitting a linearization scheme for an in product muscle number of 3. 3x104having on/off current ratio and a 1. 0x10-2 cm2/Vsof field-effect mobility were capable of is measured. The 5. 4 V was. In the embodiment 3 7. 1 g compounds of 4, 2. 62 g of 2, 2 '- cyanothiophene BTS - 5, 5' - d step theory it buys bis (call arrangements) ester, 86. Treatment of 2 mg - tert - butyl [...] tetrafluoroborate((t -Bu)3P*HBF4), 172. 3 mg of tris (d it cuts qualitative the acetone which is burnt) d palladium( 0)( Pd2 (dba)3), 140 mLpropanamines of dissolved in 28 ml water (arithmetic mean deviation on the predetermined) potassium phosphate( K3PO4) 3. 99 g by cylinder to have. Reflux in 2 process has been completed, bromo - thiophene boronic acid - 94 mg and 20 minutes after thiophene ester call arrangements 110 mg is added and the polymerization is carried broke up. Reaction mixture cooled to room temperature and, the used in the secondary oxidation is in methanol. Residue using THF forward by ligand abstraction [...] number and, by extracting chloroform polymer then, a dark powder 5. 34 g for are obtained. Mw=54' 500, polydispersity=1. 7 (HT1A-gPC measured by a). Applied in the embodiment 3 P-Si gate with bottom - gate thin film transistor (TFT) structure (10 cm), the third to eo at, and selects addresses of all lists. 300 nm thickness of high quality thermalSiO2layer capacitance per unit areaCi=32. - Gate of 6 nF/cm2 was insulator. - Gate source electrode, and a drain electrode patterns, in particular in the photolithography directly on oxide was used to. Width W=10 mm and various lengths L=4, 8, 15, 30 micro m defines channels of source/drain electrode by cylinder to have a gold. Prior to the deposition of the organic semiconductors, SiO2surface, silane the vapor of the silane coupling agent 2 in 160 °C saturation time by exposing the fluorotoluene or substrate (OTS) of trichlorosilane midifiers targeting 0. 1 m solution by 20 minutes in 60 °C polymethylsilazane dimethyl hexa was used to derivatives in (HMDS). After rinsing the propanol - isocyanate, the for drying substrates. Transistor performance in xylene Semiconductor thin film, in the embodiment 3 in solution 1% in xylene (w/w) that is obtained from DPP derivatives of formula 7 - to the spindle was under trillion number is cast or drip-coating. 0 solution prior to use. 2 m filter was are filtered. Spin coating the surrounding conditions (number of revolutions over ingredient) 800 rpm about spin rate of 20 seconds performed for all the. A device 1 in 80 °C was assessed after dried time. Transistor behavior automated transistor prober (tP-10) measured at. Saturation transfer characteristics from fitting a linearization scheme for an in product muscle number of 8. 9x108having on/off current ratio and a 2. 5x10-1 cm2/Vsof field-effect mobility were capable of is measured. The 0. 5 V was. Transistor performance in chloroform Semiconductor thin film, during chloroform 0. 5% in the embodiment 3 (w/w) in solution that is obtained from DPP derivatives of formula 7 - to the spindle was under trillion number is cast or drip-coating. Spin coating the surrounding conditions (number of revolutions over ingredient) 3000 rpm about spin rate of 20 seconds performed for all the. A device 15 minutes in 100 °C was assessed after dried. Transistor behavior automated transistor prober (tP-10) measured at. Saturation transfer characteristics from fitting a linearization scheme for an in product muscle number of 9. 3x106having on/off current ratio and a 3. 0x10-1 cm2/Vsof field-effect mobility were capable of is measured. The 0. 8 V was. In the embodiment 4 1. 0 g of 4, 148 mg of 2, 5 - thiophene step theory it buys bis (call arrangements) ester, 185 mg 2, 2 '- cyanothiophene BTS - 5, 5' - d step theory it buys bis (call arrangements) ester, 12. Treatment of 2 mg - tert - butyl [...] tetrafluoroborate((t -Bu)3P*HBF4), 24. 3 mg of tris (d it cuts qualitative the acetone which is burnt) d palladium( 0)( Pd2 (dba)3), 25 mLpropanamines of (arithmetic mean deviation on the predetermined) dissolved in 5 ml of potassium phosphate( K3PO4) 0. 56 g by cylinder to have. Reflux in 2 process has been completed, bromo - thiophene 12 mg and 20 minutes after thiophene boronic acid - 16 mg ester call arrangements broke up is added and the polymerization is carried. Reaction mixture cooled to room temperature and, the used in the secondary oxidation is in methanol. Using pentane residue number and forward by ligand abstraction [...], by extracting cyclohexane polymer then, a dark powder 0. 83 g for are obtained. Mw=51,500, polydispersity=2. 0 (HT1A-gPC measured by a). In the embodiment applied 4: DPP - polymer 8 based on organic field effect transistor P-Si gate with bottom - gate thin film transistor (TFT) structure (10 cm), the third to eo at, and selects addresses of all lists. 300 nm thickness of high quality thermalSiO2layer capacitance per unit areaCi=32. - Gate of 6 nF/cm2 was insulator. - Gate source electrode, and a drain electrode patterns, in particular in the photolithography directly on oxide was used to. Width W=10 mm and various lengths L=4, 8, 15, 30 m of a gold defines channels by cylinder to have source/drain electrode. Prior to the deposition of the organic semiconductors, SiO2surface, silane the vapor of the silane coupling agent 2 in 160 °C saturation time by exposing the fluorotoluene or substrate (OTS) of trichlorosilane midifiers targeting 0. 1 m solution by 20 minutes in 60 °C polymethylsilazane dimethyl hexa was used to derivatives in (HMDS). After rinsing the propanol - isocyanate, the for drying substrates. Transistor performance in toluene Semiconductor thin film, fluorotoluene 0. 5% (w/w) in solution that is obtained from DPP derivatives of formula 8 in the embodiment 4 - to the spindle was under trillion number is cast or drip-coating. Spin coating the surrounding conditions (number of revolutions over ingredient) 6000 rpm about spin rate of 10 seconds performed for all the. 15 minutes in a device 130 °C was assessed after dried. Transistor behavior automated transistor prober (tP-10) measured at. Saturation transfer characteristics from fitting a linearization scheme for an in product muscle number of 1. 9x107having on/off current ratio and a 2. 1x10-1 cm2/Vsof field-effect mobility were capable of is measured. The 0. 4 V was. In the embodiment 5 A) 2 - 228 - 1 - tetra tridecanols midifiers targeting. 06 g of 47% hydrogen iodide acid 484. 51 g mixing the, mixture was recirculating a night. Product extracted to ether - butyl t -. Then, was and concentrating drying the organic phase. On column silica gels product 211 the control unit and a number. 54 g 9 compounds the aim of a are obtained (73%). b) FeCl3 30 mg, sodium 10. 27 g oh wheat berry call t - and mixture of 600 ml 30 minutes after in the heating process, the 110 °C, nitrile 30. 52 g and d - tert - [...] 24. 83 g blended mixture of adaptation. After night in 110 °C reaction mixture, same - the water to be swelled up on methanol mixture. [...] (Buechner) filtration and 33 from cleaning " end-to-end into methanol. 60 g and can cancerous blue color of 10 compounds the aim of yield 90% are obtained. MS m/z: 464; C) 33. 55 g in 110 °C of 10 compounds of DMF 1300 ml in night 12. 22 g ofK2CO3and 74. 4 g of midifiers targeting 2 - reacted and a 9 iodide midifiers targeting tetra - 1 -. Reaction mixture poured in and on ice, methylene chloride extracted. Of column chromatography on silica gels number is carried out in order to formed in, 35. 1 g 11 compounds are obtained for the aim of (42. 7%). D) 10. 00 g 11 and a perchlorate compounds of dissolving the 200 ml chloroform droplets, and cooled to 0 °C, then 1 2 equivalent of N - bromo succinimide added little by little over time. After complete, was cleaning mixture with water within. To extract the organic phase, was and concentrating drying. Then, on column silica gels compound formula 12 the control unit and a number of dark violet powder 5. 31 g are obtained of (47%). E) compounds of 300 mg - di - thiophene boronic acid arrangements 12, 78 mg of call ester, 5 mg ofPd2 (dba)3 (tris (d it cuts qualitative the acetone which is burnt) - di - palladium) and treatment of 3 mg - tert - butyl - phosphonium - 3 ml dissolved the propanamines by weight of a tetrafluoroborate. For freezing period the solution into an 3 (Ar) visitor is checked through a degassing in/pump/thaw. Then, was is heated to a temperature in the reflux reaction mixture. Then, K3PO4 149 mgwith water 0. Dissolving the 7 ml, visitor is checked through a degassing in the argon. THF aqueous solution, are added to the solution, the reflux night reaction mixture. Then, mono - 2 - thiophene boronic acid - - acid - adding an 5 mg ester - call arrangements, reflux the mixture with additional 30 minutes. Then, adding an 4 mg thiophene - bromo 2 -, reflux the mixture with additional 30 minutes. Reaction mixture cooled to room temperature and, and dilution water, then extracted in chloroform. Then, chloroform solution 1 water NaCN solution before drawing the yarn and the reflux time. Which separates water, the chloroform solution. Then, extracted [...] in propanamines residue. The aim of organic phase in order to carry out the method 224 mg of polymer 13 are obtained. In the embodiment 6 (Arithmetic mean deviation on the in the Ar) in 20 ml propanamines 1. 006 g compounds of 4, 0. 349 g of 2, 5 - thieno [3,2-b] thiophene d step theory it buys bis (call arrangements) ester (for example, toluene reflux in the arrangements using call a corresponding number by esterification d step theory it buysbecoming trillion, (literature [J. Org. Chem. , 1978, 43 (11), p 2199])), treatment of 13 mg - tert - butyl [...] tetrafluoroborate((t -Bu)3P*HBF4), 21 mgof tris (d it cuts qualitative the acetone which is burnt) d palladium( 0)( Pd2 (dba)3)(arithmetic mean deviation on the predetermined) mixture of water 1. Potassium phosphate dissolved in 7 ml( K3PO4) 0. 572 g added solution of. Reflux in 2 process has been completed, bromo - thiophene boronic acid - 4 mg and 20 minutes after thiophene ester call arrangements 5 mg is added and the polymerization is carried broke up. Reaction mixture is either cooled, the dilution in chloroform. Then, added water. Face toward a half-mirror member layer, organic layer with water was further cleaning 1 times. Then, the concentrated or depressurization of the fluid bag for organic layer. Then, chloroform solution recirculating a night with water was 1% NaCN solution. Face toward a half-mirror member layer, then organic layer with water which washes the further times 1, the concentrated or depressurization of the fluid bag for. Crude product for precipitating by the addition methanol, was filtering. Then, product by ligand abstraction [...] isolated section. A extracted propanamines the cost of disposal of waste fraction number 1, number 2 a extracted chloroform methanol fraction precipitating the by the addition, the aim of formula A polymer 76 mg of a dark powder are obtained (Mw=25' 800, polydispersity=2. 0 (HT1A-gPC measured by a)). In the embodiment 7 [...] (Schlenk) tubes, which, in 90 ml tolueneNi (COD)2 1. 13 g and bipyridine 0. 65 g solution of visitor is checked through a degassing minutes 15. Into the solution 3 g a corresponding one of the adding an 1 monomer d bromide, the same circuit, is heated to 80 °C mixture then, buck night stirring section. 3/1/1 methanol/HCl solution (4 N) and poured in on 500 ml/acetone mixture, stirring time adaptation 1. Then, precipitate, is filtered and, CHCl3dissolving the, additional 1 time ethylenedaiminetetra acetic acid tetra sodium salt (EDTA) in 60 °C together with an aqueous solution of buck stirring section. Which washes the organic phase with water, and to, the used in the secondary oxidation is in methanol. Methanol residue, using cyclohexane and 30 parts by weight of diethyl ether by ligand abstraction [...] forward number and, then polymerCHCl3by extracting, a dark powder 1. 7 g for are obtained. Mw=37' 000, polydispersity=2. 3 (HT1A-gPC measured by a). Applied in the embodiment 5 P-Si gate with bottom - gate thin film transistor (TFT) structure (10 cm), the third to eo at, and selects addresses of all lists. 300 nm thickness of high quality thermalSiO2layer capacitance per unit areaCi=32. - Gate of 6 nF/cm2 was insulator. - Gate source electrode, and a drain electrode patterns, in particular in the photolithography directly on oxide was used to. Width W=10 mm and various lengths L=4, 8, 15, 30 micro m defines channels of source/drain electrode by cylinder to have a gold. Prior to the deposition of the organic semiconductors, SiO2surface, silane saturation time 2 in 160 °C by exposing the vapor of the silane coupling agent, about 1 minutes 800 rpm (number of revolutions over ingredient) polymethylsilazane dimethyl of hexa in spin rate of the spindle by coating the (HMDS), or substrate (OTS) of trichlorosilane midifiers targeting fluorotoluene 0. 1 M solution by 20 minutes in 60 °C derivatized with HMDS was used to. After rinsing the propanol - isocyanate, the for drying substrates. Transistor performance in chloroform Semiconductor thin film, during chloroform 0. 5% (w/w) in solution that is obtained from DPP derivatives of formula 15 in the embodiment 7 - to the spindle was under trillion number is cast or drip-coating. Spin coating the surrounding conditions (number of revolutions over ingredient) 3000 rpm about spin rate of 20 seconds performed for all the. State due to the deposition of the device, in addition 120 °C in 15 minutes was assessed after dried. Transistor behavior automated transistor prober (tP-10) measured at. DPP derivatives of formula 15 a standard device bipolar behavior showed excellent configuration. In the embodiment 8 A) in the nitrogen in -25 °C THF 30 ml in 5. 0 g - DPP enyl tape (16) and 3. 73 g of 2 - isopropoxy - 4, 4, 5, 5 - tetramethyl - 1, 3, 2 - LDA prepared by the method number newly solution d iodic step roll column solution (lithium butyl in THF 20 ml 2. 7 M 5. dee small pro the amine which will bloom and 4 ml 2. From 2 ml) for 15 factor over adaptation blended. The responses generated are in 0 °C mixture which agitates the time 1, then visitor is checked through a quenching by 1 M HCl 100 ml. Product and extraction of TBME 2 x 50 ml, brine to an organic layer combining which washes the times 2, was dried by sodium sulfate. After between the tube body and the tube, residue dissolving the 20 ml methylene chloride, 200 ml acetone agitated strongly then added slowly. Precipitate collecting and by filtration, which washes the times to acetone, thereby 40 °C in an oven to a vacuum -, Fink color violet powder 6. 3 g for are obtained. B) synthesis of polymer described in the embodiment 1 according to procedure 3, 0. 91 g compounds of 1 and 1. 004 g polymer by reacting compounds of a 15 17 are obtained. After reaction, and poured in in methanol mixture, the washing acetone, 1. 2 g polymer 15 a are obtained. In the embodiment 9 Synthesis of polymer described in the embodiment 1 according to procedure 3, 0. 5 g compounds of 0 and 17. 12 g of a polymer reaction [...] 3 are obtained. After reaction, and poured in in methanol mixture, acetone washing the, 0. 380 g of polymer 3 are obtained. Mw=20' 000, polydispersity=2. 3 (HT1A-gPC measured by a). In the embodiment 10 Synthesis of polymer described in the embodiment 1 according to procedure 3, 0. 5 g compounds of 0 and 17. 12 g of 2, 5 - 18 polymer reaction [...] for are obtained. Using pentane residue number and forward by ligand abstraction [...], by extracting cyclohexane polymer then, a dark powder 0. 26 g for are obtained. Mw=17' 700, polydispersity=2. 0 (HT1A-gPC measured by a). In the embodiment 11 Synthesis of polymer described in the embodiment 1 according to procedure 3, 0. 15 g compounds of 0 and 17. 05 g of 2, 2 '- dibromo - [5, 5'] a polymer reaction oh it will become sleepy BTS 19 are obtained. After reaction, and poured in in methanol mixture, acetone washing the, 0. 13 g polymer 19 a are obtained. In the embodiment 12 Synthesis of polymer described in the embodiment 1 according to procedure 3, 2. 3 g 1 g and 1 of compounds of 2, 5 - thieno [3,2-b] thiophene d step theory it buys bis (call arrangements) ester (for example, toluene reflux in the arrangements using call a corresponding number by esterification d step theory it buysbecoming trillion, (literature [J. Org. Chem. , 1978, 43 (11), p 2199])) polymer reacting for 20 are obtained. After reaction, and poured in in methanol mixture, the washing acetone, 2. 0 g are obtained of polymer 20. In the embodiment 13 A) three ammonium seasons( FeCl3) 5 mg, sodium 2. 6 g and t - oh wheat berry call mixture of 100 ml in the heating process, the 110 °C after 20 minutes, sol - 2 - nitrile 5 tis of formula 21. 0 g and formula 22 di - tert - amyl succinate 8. 25 g blended mixture of adaptation. 3 reaction mixture after in 110 °C time, the water to be same - methanol mixture (200 ml/100 ml) acetic acid in 8. 15 g swelled up on. Filtration and [...] 5 from cleaning " end-to-end into methanol. 2 g 1, 4 - diketone of formula 23 with toe blood roll the desired of [3,4-c] pyrrole (DPP) and can cancerous blue color derivatives are obtained. eSI-number list m/z (% int.): 303. 13 ([M + H] +, 100%). B) 1, 4 - diketone of formula 3 with toe blood roll [3,4-c] pyrrole (DPP) derivatives 4 g, KOH 2 in 3 ml water. 9 g and N - methyl - pyrrolidone (NMP) in 50 ml 1 - bromo - 2 - ethylhexyl - 18 midifiers targeting. 5 g 6 solution of the heated to 140 °C time. Which washes the mixture with water within, dichloro methane at extracted. Of column chromatography on silica gels used in the secondary oxidation is from methanol/chloroform and forward number is carried out in order to, 0. 4 g of a blue solid as the desired DPP 24 are obtained. C) in the embodiment 5d and a similarly compound 25 a are obtained. D) of polymer a and in the embodiment 5e are obtained for 26. E) of polymer 27 of similar in the embodiment 7 are obtained. In the embodiment 14 A) potassium tert - butoxide 554. 6 g, of dimethyl carbonate 424. 2 g 3 L toluene acid and acetic anhydride was heated to 100 °C wherein agitating. 1 - acetyl thiophene 300 g of adding drip a time 3, time 15 100 °C stirring section. Reaction mixture cooled to room temperature and, 4 L swelled up on for removing ice from ice-making. Separating possibility layer, extracted times 2 to 200 ml ethyl acetate. Combined organic layer, the dried on sodium sulfate, wherein the filtration of the slurry suspension, and evaporating, is dried to, 363. 7 g are obtained of compounds of 28. Then without crude product with additional forward number, the third to eo reaction stage. B) 363. 7 g compounds of 28, 322. 7 g of methyl bromo acetate, 288. 7 g of potassium carbonate, 1100 ml of acetone and 750 ml of put it in his container 1, 2 - dimethoxyethane. 20 mixture 80 °C time stirring section. After cooled to room temperature mixture, wherein the filtration of the slurry suspension same, the drying. 460 g compounds of a 29 are obtained. Then without crude product with additional forward number, the third to eo reaction stage. C) compounds of 29, 643 g 218 g, the amount of acetic acid, ammonium and 680 ml 115 °C time 3 of acetic acid of stirring section. After cooled to room temperature reaction mixture, swelled up during 3 L acetone same. And for separating solids generated, and then being washed with methanol, the drying. 99. 6 g for 30 compounds of are obtained. D) three ammonium seasons( FeCl3) 5 mg, oh wheat berry call mixture of 40 ml sodium 2 g and t - 20 minutes after in the heating process, the 110 °C, 3. 9 g of sol tis of formula 21 - 2 - nitrile and 7. 82 g for 30 compounds of added little by little. 3 reaction mixture after in 110 °C time, the water to be same - methanol mixture (100 ml/100 ml) acetic acid in 6. 3 g swelled up on. Filtration and [...] cleaning " end-to-end into methanol from the desired 1, 4 - diketone of formula 31 with toe blood roll [3,4-c] pyrrole (DPP) 4 derivatives. 5 g for cancerous blue color and can are obtained. E) in the embodiment 5c and a similarly compound 32 a are obtained. F) for 33 in the embodiment 5d and a similarly compound are obtained. G) of polymer a and in the embodiment 5e 34 a are obtained. H) a 35 of polymer similar in the embodiment 7 are obtained. In the embodiment 15 Much like an in the embodiment 7 36 of polymer from a 12 compound are obtained. In the embodiment 16 A) multi function cap in - neck 3, dissolved in 110 ml water (arithmetic mean deviation on the predetermined) potassium phosphate( K3PO4) 83. 6 g 20 g for propanamines neel step theory it buys in the mote in 350 ml, aminothiazole bromo 2 - 22. 0 g, tree - tert - butyl [...] tetrafluoroborate((t -Bu)3P*HBF4) 2. 3 g and tris (d it cuts qualitative the acetone which is burnt) d palladium( 0)( Pd2 (dba)3) 3. 6 g of arithmetic mean deviation on the added to solution. Heating the night until reflux temperature reaction mixture. Reaction mixture cooled to room temperature and, added a 100 ml water. Ethyl acetate as a starting material in the reaction mixture, are dried, organic layer,, and the vapor the depressurization of the fluid bag for measurement. Additional same silica gel of ethyl acetate/hexane on a column gradient number the forward chromatography. 8. 0 g of 2 - thiophene - 2 - one - thiazole 37 to obtain the n bit parallel data inputted, spectral data four it crawls, - cross-coupling (negishi-cross coupling) harmful by-products by using a was corresponding to are discussed in literature (literature [J. Jensen et al. , Synthesis, 2001, 1, 128]). B) publicly known literature a procedure applicable to Internet user of IP the compounds are obtained for 38 (literature [P. Chauvin et al. , Bull. Soc. Chim. Fr. 1974, 9 - 10, 2099]). C) similar to procedure publicly known literature compound 39 a are obtained (literature [A. D. Borthwick et al. ; J. Chem. Soc. , Perkin Trans 1, 1973; 2769]). D) for 40 in the embodiment 5b and a similarly compound are obtained. E) in the embodiment 5c and a similarly compound 41 a are obtained. F) in the embodiment 5d and a similarly compound 42 a are obtained. G) of polymer a and in the embodiment 5e are obtained for 43. H) of polymer 44 a similar in the embodiment 7 are obtained. WO 08/000664 to a disclosure of the present invention polymer such as a polymer - a high electric field than can exhibit mobility effect. The present invention relates to polymers comprising a repeating unit of the formula I, or III and their use as organic semiconductor in organic devices, especially an organic field effect transistor (OFET), or a device containing a diode and/or an organic field effect transistor. The polymers according to the invention have excellent solubility in organic solvents and excellent film-forming properties. In addition, high efficiency of energy conversion, excellent field-effect mobility, good on/off current ratios and/or excellent stability can be observed, when the polymers according to the invention are used in organic field effect transistors. Organic field effect transistor for use in polymer with toe blood roll blood roll diketone.