Methods for seamless nucleic acid assembly
17-05-2022 дата публикации
Номер:
US0011332740B2
Принадлежит: TWIST BIOSCIENCE CORPORATION
Контакты:
Номер заявки: 05-97-1687
Дата заявки: 20-05-2020









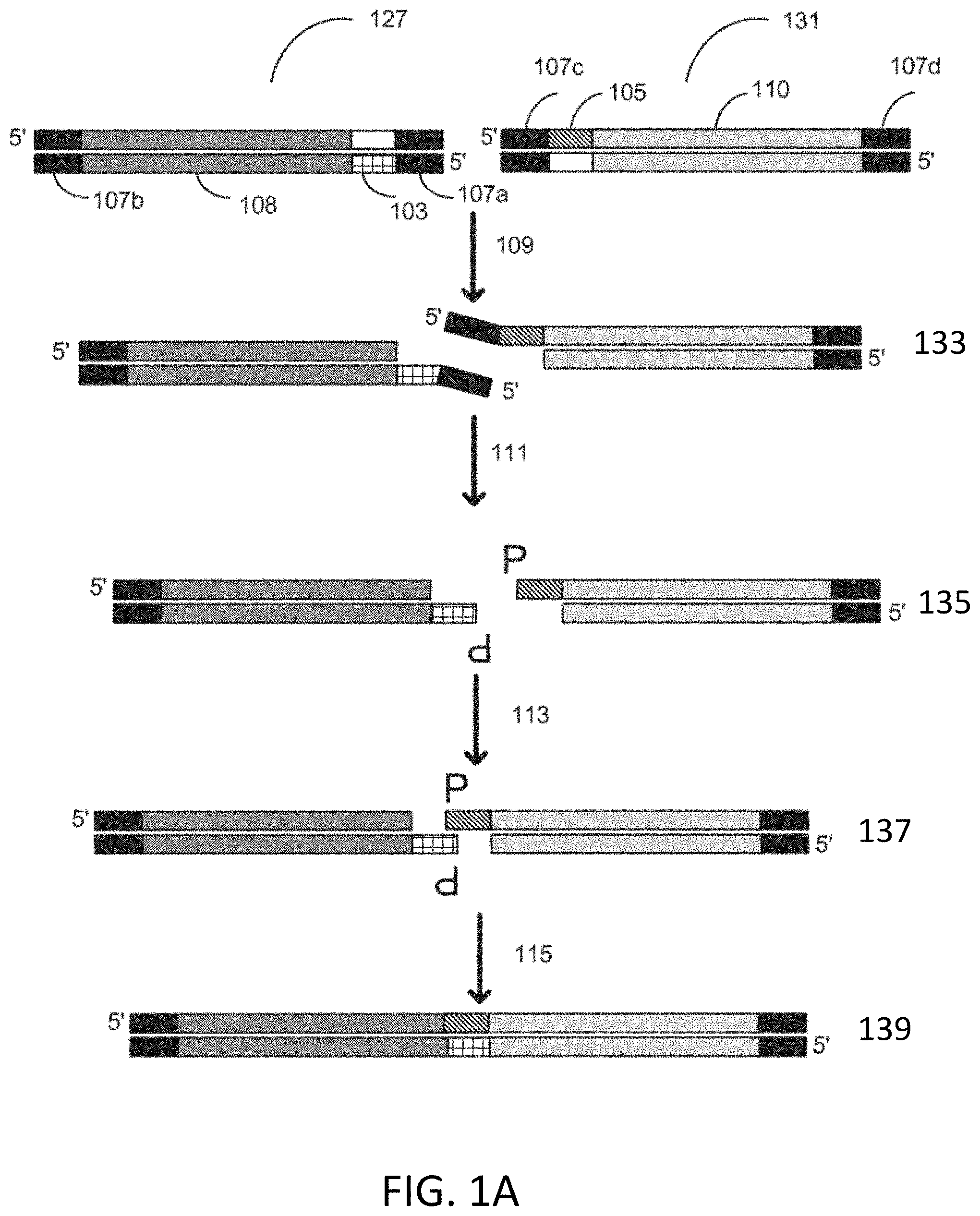







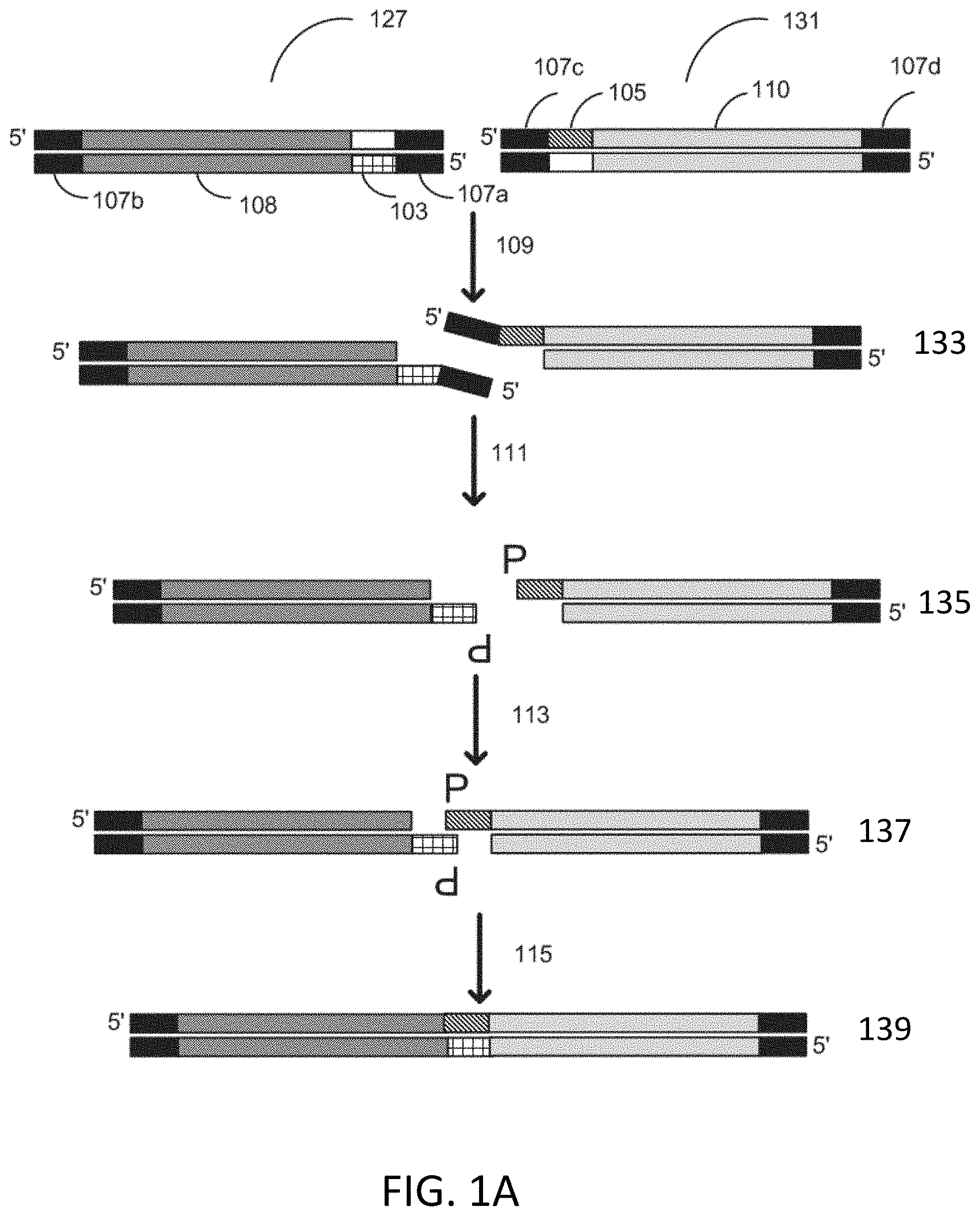








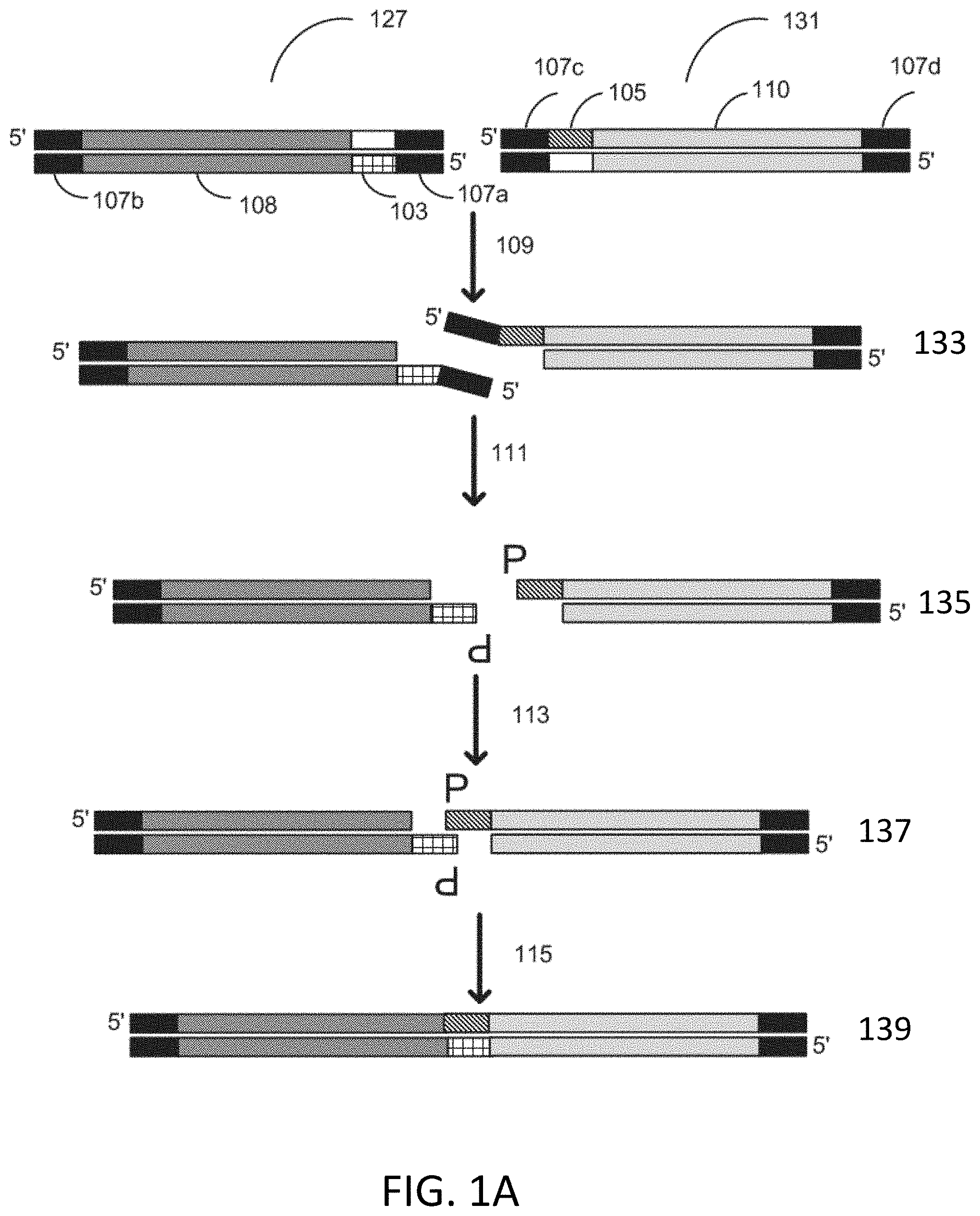






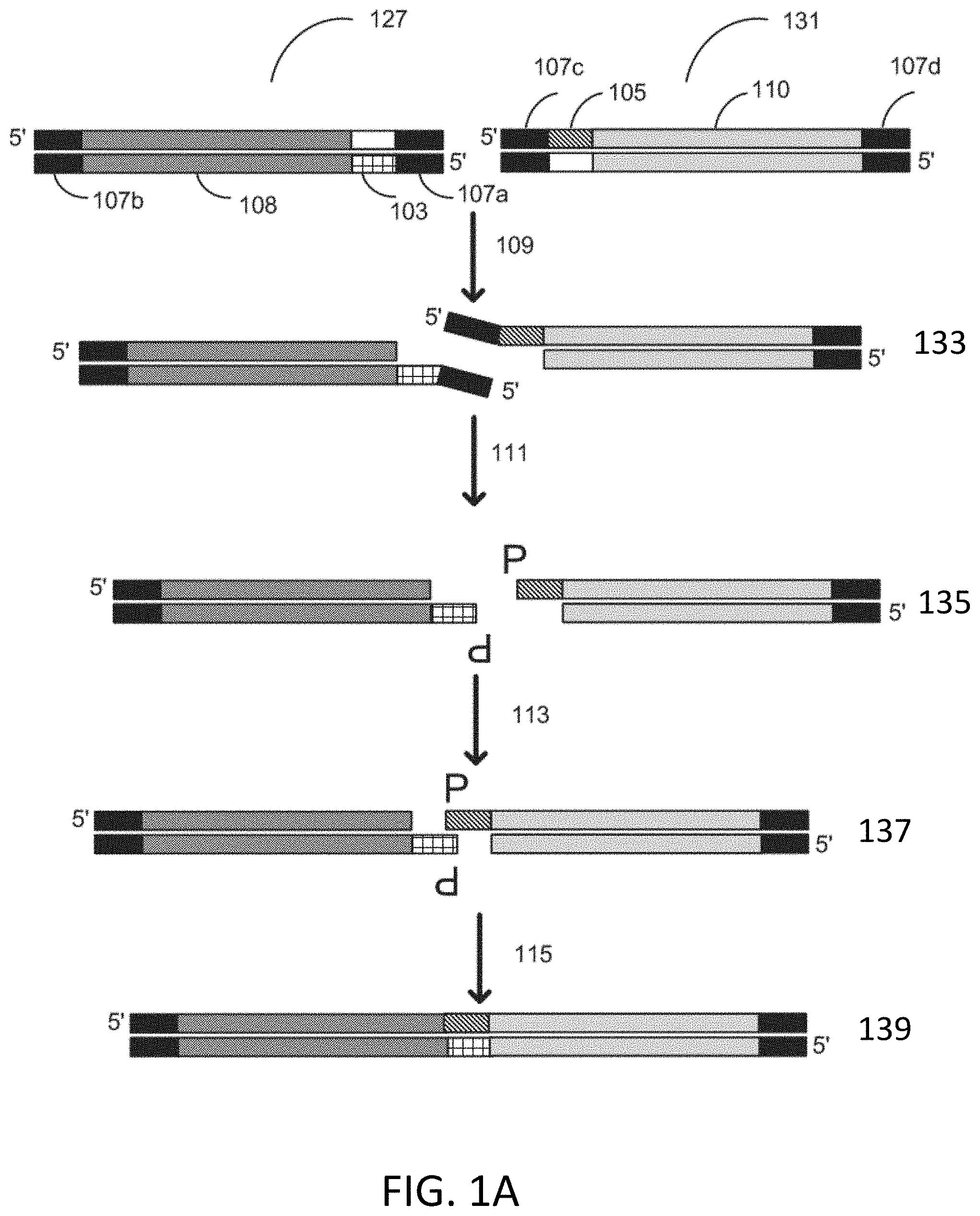


CPC - классификация
CC1C12C12NC12N1C12N15C12N15/C12N15/1C12N15/10C12N15/103C12N15/1031C12N15/109C12N15/1093C12N15/1096C12N9C12N9/C12N9/1C12N9/12C12N9/125C12N9/1252C12N9/9C12N9/93C12PC12P1C12P19C12P19/C12P19/3C12P19/34C12QC12Q1C12Q1/C12Q1/6C12Q1/68C12Q1/681C12Q1/6811C12Q1/685C12Q1/6855C12Q1/686C12Q1/6869C12Q2C12Q25C12Q252C12Q2521C12Q2521/C12Q2521/1C12Q2521/10C12Q2521/101C12Q2521/3C12Q2521/30C12Q2521/301C12Q2521/31C12Q2521/319C12Q2521/5C12Q2521/50C12Q2521/501C12Q2525C12Q2525/C12Q2525/1C12Q2525/19C12Q2525/191C12Q253C12Q2531C12Q2531/C12Q2531/1C12Q2531/10C12Q2531/101C12YC12Y2C12Y20C12Y207C12Y207/C12Y207/0C12Y207/07C12Y207/070C12Y207/0700C12Y207/07007C12Y3C12Y30C12Y301C12Y301/C12Y301/1C12Y301/11C12Y301/110C12Y301/1100C12Y301/11002IPC - классификация
AA6A61A61PA61P3A61P35A61P35/A61P35/0A61P35/00CC1C12C12NC12N1C12N15C12N15/C12N15/1C12N15/10C12N9C12N9/C12N9/0C12N9/00C12N9/1C12N9/12C12PC12P1C12P19C12P19/C12P19/3C12P19/34C12QC12Q1C12Q1/C12Q1/6C12Q1/68C12Q1/681C12Q1/6811C12Q1/685C12Q1/6855C12Q1/686C12Q1/6869Цитирование НПИ
(Novartis Institutes for Biomedical Research) Immunoglobulin Heavy Chain [Homo sapiens]. National Center for Biotechnology Information. Genbank Entry, pp. 1-2 (2018) https://www.ncbi.nlm.nih.gov/nuccore/MH975524.1ttps://https://www.ncbi.nlm.nih.gov/nuccore/MH975524.1.(Novartis Institutes for Biomedical Research) Immunoglobulin Lambda Chain [Homo sapiens]. National Center for Biotechnology Information. Genbank Entry, pp. 1-2 (2018) https://www.ncbi.nlm.nih.gov/nuccore/MH975524.1.
ATDBio. Nucleic Acid Structure, Nucleic Acids Book, 9 pages, published on Jan. 22, 2005. from: http://www.atdbio.com/content/5/Nucleic-acid-structure.
ATDBio. Solid-Phase Oligonucleotide Synthesis, Nucleic Acids Book, 20 pages, Published on Jul. 31, 2011. from: http://www.atdbio.com/content/17/Solid-phase-oligonucleotide-synthesis.
Abudayyeh et al.: C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science, available on line, Jun. 13, 2016, at: http://zlab.mit.edu/assets/reprints/Abudayyeh_OO_Science_2016.pdf, 17 pages.
Acevedo-Rocha et al.: Directed evolution of stereoselective enzymes based on genetic selection as opposed to screening systems. J. Biotechnol. 191:3-10 (2014).
Adessi et al.: Solid phase DNA amplification: characterisation of primer attachment and amplification mechanisms. Nucleic Acids Res. 28(20):E87, 2000.
Al-Housseiny et al.: Control of interfacial instabilities using flow geometry Nature Physics, 8:747-750, 2012.
Alberts et al.: Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. The Generation of Antibody Diversity. https://www.ncbi.nlm.nih.gov/books/NBK26860/.
Alexeyev et al.: Gene synthesis, bacterial expression and purification of the Rickettsia prowazekii ATP/ADP translocase, Biochimica et Biophysics Acta, 1419:299-306, 1999.
Almagro et al.: Progress and Challenges in the Design and Clinical Development of Antibodies for Cancer Therapy. Frontiers in immunology; 8, 1751 (2018) doi:10.3389/fimmu.2017.01751 https://www.frontiersin.org/articles/10.3389/fimmu.2017.01751/full.
Amblard et al.: A magnetic manipulator for studying local rheology and micromechanical properties of biological systems, Rev. Sci. Instrum., 67(3):18-827, 1996.
Andoni and Indyk. Near-Optimal Hashing Algorithms for Approximate Nearest Neighbor in High Dimensions, Communications of the ACM, 51(1):117-122, 2008.
Arand et al.: Structure of Rhodococcus erythropolis limonene-1,2-epoxide hydrolase reveals a novel active site. EMBO J. 22:2583-2592 (2003).
Arkles et al.: The Role of Polarity in the Structure of Silanes Employed in Surface Modification. Silanes and Other Coupling Agents. 5:51-64, 2009.
Arkles. Hydrophobicity, Hydrophilicity Reprinted with permission from the Oct. 2006 issue of Paint & Coatings Industry magazine, Retrieved on Mar. 19, 2016, 10 pages.
Assembly manual for the POSaM: The ISB Piezoelelctric Oligonucleotide Synthesizer and Microarrayer, The Institute for Systems Biology, May 28, 2004 (50 pages).
Assi et al.: Massive-parallel adhesion and reactivity—measurements using simple and inexpensive magnetic tweezers. J. Appl. Phys. 92(9):5584-5586 (2002).
Au et al.: Gene synthesis by a LCR-based approach: high level production of Leptin-L54 using synthetic gene in Escherichia coli. Biochemical and Biophysical Research Communications 248:200-203 (1998).
Baedeker et al.: Overexpression of a designed 2.2kb gene of eukaryotic phenylalanine ammonialyase in Escherichia coli. FEBS Letters, 457:57-60, 1999.
Bai. A Novel Human scFv Library with Non-Combinatorial Synthetic CDR Diversity. PLoS One. 10(10):1-18 (2015).
Barbee et al.: Magnetic Assembly of High-Density DNA Arrays for Genomic Analyses. Anal Chem. 80(6):2149-2154, 2008.
Barton et al.: A desk electrohydrodynamic jet printing system. Mechatronics, 20:611-616, 2010.
Beaucage et al.: Advances in the synthesis of oligonucleotides by the phosphoramidite approach. Tetrahedron. 48:2223-2311, 1992.
Beaucage et al.: Deoxynucleoside phosphoramidites—A new class of key intermediates for deoxypolynucleotide synthesis. Tetrahedron Lett. 22(20):1859-1862, 1981.
Beaucage et al.: The Chemical synthesis of DNA/RNA Chapter 2 in: Encyclopedia of Cell Biology, 1:36-53, 2016.
Beaulieu et al.: PCR candidate region mismatch scanning adaptation to quantitative, high-throughput genotyping, Nucleic Acids Research, 29(5): 1114-1124, 2001.
Beigelman et al.: Base-modified phosphoramidite analogs of pyrimidine ribonucleosides for RNA structure-activity studies. Methods Enzymol. 317:39-65, 2000.
Berg: Biochemistry. 5th ED. New York (2002) 148-149.
Bethge et al.: Reverse synthesis and 3′-modification of RNA. Jan. 1, 2011, pp. 64-64, XP055353420. Retrieved from the Internet: URL:http://www.is3na.org/assets/events/Category%202-Medicinal %20Chemistry%20of%2001igonucleotides%20%2864-108%29.pdf.
Binkowski et al.: Correcting errors in synthetic DNA through consensus shuffling. Nucleic Acids Research, 33(6):e55, 8 pages, 2005.
Biswas et al.: Identification and characterization of a thermostable MutS homolog from Thennus aquaticus, The Journal of Biological Chemistry, 271(9):5040-5048, 1996.
Biswas et al.: Interaction of MutS protein with the major and minor grooves of a heteroduplex DNA, The Journal of Biological Chemistry, 272(20):1 3355-13364, 1997.
Bjornson et al.: Differential and simultaneous adenosine Di- and Triphosphate binding by MutS, The Journal of Biological Chemistry, 278(20):18557-18562, 2003.
Blanchard et al.: High-Density Oligonucleotide Arrays, Biosensors & Bioelectronics, 11(6/7):687-690, 1996.
Blanchard: Genetic Engineering, Principles and Methods, vol. 20, Ed. J. Sedlow, New York: Plenum Press, p. 111-124, 1979.
Blawat et al.: Forward error correction for DNA data storage. Procedia Computer Science, 80:1011-1022, 2016.
Bonini and Mondino, Adoptive T-cell therapy for cancer: The era of engineered T cells. European Journal of Immunology, 45:2457-2469, 2015 .
Borda et al.: Secret writing by DNA hybridization. Acta Technica Napocensis Electronics and Telecommunications. 50(2):21-24 (2008).
Bornholt et al.: A DNA-Based Archival Storage System, in International Conference on Architectural Support for Programming Languages and Operating Systems (ASPLOS), Apr. 2-6, 2016, Atlanta, GA, 2016, 637-649.
Borovkov et al.: High-quality gene assembly directly from unpurified mixtures of microassay-synthesized oligonucleotides. Nucleic Acid Research, 38(19):e180, 10 pages, 2010.
Brunet: Aims and methods of biosteganography. Journal of Biotechnology, 226:56-64, 2016.
Buermans et al.: Next Generation sequencing technology: Advances and applications, Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease, 1842:1931-1941, 2014.
Butler et al.: In situ synthesis of oligonucleotide arrays by using surface tension. J Am Chem Soc. 123(37):8887-94, 2001.
CRICK. On protein synthesis. Symp Soc Exp Biol12:138-163,1958.
Calvert. Lithographically patterned self-assembled films. In: Organic Thin Films and Surfaces: Directions for The Nineties, vol. 20, p. 109, ed. By Abraham Ulman, San Diego: Academic Press, 1995.
Cardelli. Two-Domain DNA Strand Displacement, Electron. Proc. Theor. Comput. Sci., 26:47-61, 2010.
Carlson. Time for New DNA Synthesis and Sequencing Cost Curves, 2014., Available: http://www.synthesis.cc/synthesis/2014/02/time_for_new_cost_curves_2014. 10 pages.
Carr et al.: Protein-mediated error correction for de novo DNA synthesis. Nucleic Acids Res. 32(20):e162, 9 pages, 2004.
Carter and Friedman. DNA synthesis and Biosecurity: Lessons learned and options for the future. J. Craig Venter Institute, La Jolla, CA, 28 pages, Oct. 2015.
Caruthers, The Chemical Synthesis of DNA/RNA: Our Gift to Science. J. Biol. Chem., 288(2):1420-1427, 2013.
Caruthers. Chemical synthesis of deoxyoligonucleotides by the phosphoramidite method. In Methods in Enzymology, Chapter 15, 154:287-313, 1987.
Caruthers. Gene synthesis machines: DNA chemistry and its uses. Science 230(4723):281-285 (1985).
Casmiro et al.: PCR-based gene synthesis and protein NMR spectroscopy, Structure, 5(11):1407-1412, 1997.
CeGaT. Tech Note available at https://www.cegat.de/web/wp-content/uploads/2018/06/Twist-Exome-Tech-Note.pdf (4 pgs.) (2018).
Cello et al.: Chemical synthesis of poliovirus cDNA: generation of infectious virus in the absence of natural template. Science. 297(5583):1016-8, 2000.
Chalmers et al.: Scaling up the ligase chain reaction-based approach to gene synthesis. Biotechniques. 30(2):249-52, 2001.
Chan et al.: Natural and engineered nicking endonucleases—from cleavage mechanism to engineering of strand-specificity. Nucleic Acids Res. 39(1):1-18, 2011.
Chen et al.: Chemical modification of gene silencing oligonucleotides fordrug discovery and development. Drug Discov Today. 10(8):587-93 2005.
Chen et al.: Programmable chemical controllers made from DNA, Nat. Nanotechnol., 8(10):755-762, 2013.
Cheng et al.: High throughput parallel synthesis of oligonucleotides with 1536 channel synthesizer. Nucleic Acids Res. 30(18):e93, 2002.
Chervin et al.: Design of T-cell receptor libraries with diverse binding properties to examine adoptive T-cell responses. Gene Therapy. 20(6):634-644 (2012).
Chilamakuri et al.: Performance comparison of four exome capture systems for deep sequencing. BMC Genomics 15(1):449 (2014).
Cho et al.: Capillary passive valve in microfluidic systems. NSTI-Nanotech. 2004; 1:263-266.
Chrisey et al.: Fabrication of patterned DNA surfaces Nucleic Acids Research, 24(15):3040-3047 (1996).
Chung et al.: One-step preparation of competent Escherichia coli: Transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. Apr. 1989;86(7):2172-2175.
Church et al.: Next-generation digital information storage in DNA. Science, 337:6102, 1628-1629, 2012.
Cleary et al.: Production of complex nucleic acid libraries using highly parallel in situ oligonucleotide synthesis. Nature Methods, 1(13):241-248, 2004.
Cohen et al.: Human population: The next half century. Science, 302:1172-1175, 2003.
Cruse et al.: Atlas of Immunology, Third Edition. Boca Raton:CRC Press (pp. 282-283) (2010).
Cui et al.: Information Security Technology Based on DNA Computing. International Workshop on Anti-Counterfeiting, Security and Identification (ASID); IEEE Xplore 4 pages (2007).
Cutler et al.: High-throughput variation detection and genotyping using microarrays, Genome Research, vol. 11, 1913-19 (2001).
Dahl et al.: Circle-to-circle amplification for precise and sensitive DNA analysis. Proc Natl Acad Sci U S A. Mar. 30, 2004;101(13):4548-53. Epub Mar. 15, 2004.
De Graff et al.: Glucagon-Like Peptide-1 and its Class B G Protein-Coupled Receptors: A Long March to Therapeutic Successes. Pharmacol Rev. 68(4):954-1013 (2016).
De Mesmaeker et al.: Backbone modifications in oligonucleotides and peptide nucleic acid systems. Curr Opin Struct Biol. Jun. 1995;5(3):343-55.
De Silva et al.: New Trends of Digital Data Storage in DNA. BioMed Res Int. 2016:8072463 (2016).
Deamer et al.: Characterization of nucleic acids by nanopore analysis, Ace. Cham. Res., vol. 35, No. 10, 817-825 (2002).
Deaven. The Human Genome Project: Recombinant clones for mapping and sequencing DNA. Los Alamos Science, 20:218-249, 1992.
Deng et al.: Targeted bisulfite sequencing reveals changes in DNA methylation associated with nuclear reprogramming Nature Biotechnology, 27:352-360 (2009).
Dietrich et al.: Gene assembly based on blunt-ended double-stranded DNA-modules, Biotechnology Techniques, vol. 12, No. 1, 49-54 (Jan. 1998).
Dillon et al.: Exome sequencing has higher diagnostic yield compared to simulated disease-specific panels in children with suspected monogenic disorders. Eur J Hum Genet 26(5):644-651 (2018).
Dormitzer et al.: Synthetic generation of influenza vaccine viruses for rapid response to pandemics. Sci Translational Medicine, 5(185):185ra68, 14 pages, 2013.
Doudna et al.: Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346(6213):1258096-1-1258096-9, 2014.
Douthwaite et al.: Affinity maturation of a novel antagonistic human monoclonal antibody with a long VH CDR3 targeting the Class A GPCR formyl-peptide receptor 1; mAbs, vol. 7, Iss. 1, pp. 152-166 (Jan. 1, 2015).
Dower et al.: High efficiency transformation of E.coli by high voltage electroporation. Nucleic Acids Res. 16(13):6127-45 (1988).
Dressman et al.: Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proc Natl Acad Sci U S A. Jul. 22, 2003;100(15):8817-22. Epub Jul. 11, 2003.
Drmanac et al.: Human genome sequencing using unchained base reads on self-assembling DNA nanoarrays. Science. Jan. 1, 2010;327(5961):78-81. doi: 10.1126/science.1181498. Epub Nov. 5, 2009.
Droege and Hill. The Genome Sequencer FLXTM System-Longer reads, more applications, straight forward bioinformatics and more complete data sets Journal of Biotechnology, 136:3-10, 2008.
Duffy et al.: Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal Chem. Dec. 1, 1998;70(23):4974-84. doi: 10.1021/ac980656z.
Duggan et al.: Expression profiling using cDNA microarrays. Nat Genet. Jan. 1999;21(1 Suppl):10-4.
Dvorsky. Living Bacteria Can Now Store Data. GIZMODO internet publication. Retrieved from https://gizmodo.com/living-bacteria-can-now-store-data-1781773517 (4 pgs) (Jun. 10, 2016).
Eadie et al.: Guanine modification during chemical DNA synthesis. Nucleic Acids Res. Oct. 26, 1987;15(20):8333-49.
Eisen: A phylogenomic study of the MutS family of proteins, Nucleic Acids Research, vol. 26, No. 18, 4291-4300 (1998).
El-Sagheer et al.: Biocompatible artificial DNA linker that is read through by DNA polymerases and is functional in Escherichia coli. Proc Natl Acad Sci USA. Jul. 12, 2011;108(28):11338-43. doi: 10.1073/pnas.1101519108. Epub Jun. 27, 2011.
Ellis et al.: DNA assembly for synthetic biology: from parts to pathways and beyond. Integr Biol (Camb). Feb. 2011;3(2):109-18. doi: 10.1039/c0ib00070a. Epub Jan. 19, 2011.
Elsik et al.: The Genome sequence of taurine cattle: A window of ruminant biology and evolution. Science, 324:522-528, 2009.
Elsner et al.: 172 nm excimer VUV-triggered photodegradation and micropatterning of aminosilane films, Thin Solid Films, 517:6772-6776 (2009).
Engler et al.: A one pot, one step, precision cloning method with high throughput capability. PLoS One. 2008;3(11):e3647. doi: 10.1371/journal.pone.0003647. Epub Nov. 5, 2008.
Engler et al.: Golden gate shuffling: a one-pot DNA shuffling method based on type IIs restriction enzymes. PLoS One. 2009;4(5):e5553. doi: 10.1371/journal.pone.0005553. Epub May 14, 2009.
Erlich and Zielinski. DNA fountain enables a robust and efficient storage architecture. Science, 355(6328):950-054, 2017.
Eroshenko et al.: Gene Assembly from Chip-Synthesized Oligonucleotides; Current Protocols in Chemical biology 4: 1-17 (2012).
European Patent Application No. 12827479.2 Extended European Search Report dated May 18, 2015.
European Patent Application No. 12827479.2 Partial European Search Report dated Jan. 29, 2015.
European Patent Application No. 14834665.3 Communication dated Jan. 16, 2018.
European Patent Application No. 14834665.3 Further Examination Report dated Nov. 28, 2018.
European Patent Application No. 14834665.3 Office Action dated May 2, 2018.
European Patent Application No. 14834665.3 extended European Search Report dated Apr. 28, 2017.
European Patent Application No. 16847497.1 Extended European Search Report dated Jan. 9, 2019.
European Patent Application No. 16871446.7 European Search Report dated Apr. 10, 2019.
European Patent Application No. 16871446.7 First Official Action dated Nov. 13, 2019.
European Patent Application No. 17844060.8 Extended Search Report dated Apr. 20, 2020.
European Patent Application No. 17881617.9 European Search Report and Written Opinion dated Jul. 2, 2020.
Evans et al.: DNA Repair Enzymes. Current Protocols in Molecular Biology 84:III:3.9:3.9.1-3.9.12 http://www.ncbi.nlm.nih.gov/pubmed/18972391 (Published online Oct. 1, 2008 Abstract only provided).
Fahy et al.: Self-sustained sequence replication (3SR): an isothermal transcription-based amplification system alternative to PCR. PCR Methods Appl. Aug. 1991;1(1):25-33.
Fedoryak et al.: Brominated hydroxyquinoline as a photolabile protecting group with sensitivity to multiphoton excitation, Org. Lett., vol. 4, No. 2, 3419-3422 (2002).
Fernández-Quintero et al.: Characterizing the Diversity of the CDR-H3 Loop Conformational Ensembles in Relationship to Antibody Binding Properties. Front. Immunol. 9:1-11 (2019).
Ferretti et al.: Total synthesis of a gene for bovine rhodopsin. PNAS, 83:599-603 (1986).
Finger et al.: The wonders of Flap Endonucleases: Structure, function, mechanism and regulation. Subcell Biochem., 62:301-326, 2012.
Fodor et al.: Light-directed, spatially addressable parallel chemical synthesis. Science. 251(4995):767-773 (1991).
Fogg et al.: Structural basis for uracil recognition by archaeal family B DNA polymerases. Nature Structural Biology, 9(12):922-927, 2002.
Foldesi et al.: The synthesis of deuterionucleosides. Nucleosides Nucleotides Nucleic Acids. Oct.-Dec. 2000;19(10-12):1615-56.
Frandsen et al.: Efficient four fragment cloning for the construction of vectors for targeted gene replacement in filamentous fungi. BMC Molecular Biology 2008, 9:70.
Frandsen. Experimental setup. Dec. 7, 2010, 3 pages. http://www.rasmusfrandsen.dk/experimental_setup.htm.
Frandsen. The USER Friendly technology. USER cloning. Oct. 7, 2010, 2 pages. http://www.rasmusfrandsen.dk/user_cloning.htm.
Fullwood et al.: Next-generation DNA sequencing of paired-end tags for transcriptome and genome analysis Genome Research, 19:521-532, 2009.
GE Healthcare. AKTA oligopilot plus. Data File 18-114-66 AD©. 8 pages (2006).
GE Healthcare. Robust and cost-efficient oligonucleotide synthesis. Application Note 28-4058-08 AA. 4 pages (2005).
Galka et al.: QuickLib, a method for building fully synthetic plasmid libraries by seamless cloning of degenerate oligonucleotides. Plos One, 12, e0175146:1-9 (2017).
Galka et al.: QuickLib, a method for building fully synthetic plasmid libraries by seamless cloning of degenerate oligonucleotides. Plos One, 12, e0175146:S1 Table (2017).
Galka et al.: QuickLib, a method for building fully synthetic plasmid libraries by seamless cloning of degenerate oligonucleotides. Plos One, 12, e0175146:S1 figure (2017).
Galka et al.: QuickLib, a method for building fully synthetic plasmid libraries by seamless cloning of degenerate oligonucleotides. Plos One, 12, e0175146:S2 figure (2017).
Galneder et al.: Microelectrophoresis of a bilayer-coated silica bead in an optical trap: application to enzymology. Biophysical Journal, vol. 80, No. 5, 2298-2309 (May 2001).
Gao et al.: A flexible light-directed DNA chip synthesis gated by deprotection using solution photogenerated acids. Nucleic Acids Res. Nov. 15, 2001;29(22):4744-50.
Gao et al.: A method for the generation of combinatorial antibody libraries using pIX phage display. PNAS 99(20):12612-12616 (2002).
Gao et al.: Thermodynamically balanced inside-out (TBIO) PCR-based gene synthesis: a novel method of primer design for high-fidelity assembly of longer gene sequences. Nucleic Acids Res. Nov. 15, 2003;31(22):e143.
Garaj et al.: Graphene as a subnanometre trans-electrode membrane. Nature. Sep. 9, 2010;467(7312):190-3. doi: 10.1038/nature09379.
Garbow et al.: Optical tweezing electrophoresisof isolated, highly charged colloidal spheres, Colloids and Surfaces A: Physiochem. Eng. Aspects, vol. 195, 227-241 (2001).
Geetha et al.: Survey on Security Mechanisms for Public Cloud Data. 2016 International Conference on Emerging Trends in Engineering, Technology and Science (ICETETS). 8 pages (2016).
GeneArt Seamless Cloning and Assembly Kits. Life Technologies Synthetic Biology. 8 pages, available online Jun. 15, 2012.
Genomics 101. An Introduction to the Genomic Workflow. 2016 edition, 64 pages. Available at: http://www.frontlinegenomics.com/magazine/6757/genomics-101/.
Geu-Flores et al.: USER fusion: a rapid and efficient method for simultaneous fusion and cloning of multiple PCR products. Nucleic Acids Res. 2007;35(7):e55. Epub Mar. 27, 2007.
Gibson Assembly. Product Listing. Application Overview. 2 pages, available online Dec. 16, 2014.
Gibson et al.: Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science. Feb. 29, 2008;319(5867):1215-20. doi: 10.1126/science.1151721. Epub Jan. 24, 2008.
Gibson et al.: Creation of a Bacterial Cell Controlled by A Chemically Synthesized Genome. Science 329(5989):52-56 (2010).
Goldfeder et al.: Medical implications of technical accuracy in genome sequencing. Genome Med 8(1):24 (2016).
Goldman et al.: Towards practical, high-capacity, low-maintenance information storage in synthesized DNA, Nature, 494(7435):77-80, 2013.
Goodwin et al.: immunoglobulin heavy chain variable region, partial [Homo sapiens], Genbank entry (online). National Institute of Biotechnology Information. (2018) https://www.ncbi.nim.nih.gov/protein/AXA12486.1.
Gosse et al.: Magnetic tweezers: micromanipulation and force measurement at the molecular level, Biophysical Journal, vol. 8, 3314-3329 (Jun. 2002).
Grass et al.: Robust chemical preservation of digital information on DNA in silica with error-correcting codes, Angew. Chemie—Int. Ed., 54(8):2552-2555, 2015.
Greagg et al.: A read-ahead function in archaeal DNA polymerases detects promutagenic template-strand uracil. Proc. Nat. Acad. Sci. USA, 96:9045-9050, 1999.
Grovenor. Microelectronic materials. Graduate Student Series in Materials Science and Engineering. Bristol, England: Adam Hilger, 1989; p. 113-123.
Gu et al.: Depletion of abundant sequences by hybridization (DASH): using Cas9 to remove unwanted high-abundance species in sequencing libraries and molecular counting applications. Genome Biology, 17:41, 13 pages, 2016.
Haber et al.: Magnetic tweezers for DNA micromanipulation, Rev. Sci. Instrum., vol. 71, No. 12, 4561-4570 (Dec. 2000).
Han et al.: Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat Biotechnol 32(7):684-692 (2014).
Hanahan and Cold Spring Harbor Laboratory. Studies on transformation of Escherichia coli with plasmids J. Mol. Biol. 166:557-580 (1983).
Hanahan et al.: Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol, vol. 204, p. 63-113 (1991).
Harada et al.: Unexpected substrate specificity of T4 DNA ligase revealed by in vitro selection. Nucleic Acids Res. May 25, 1993; 21(10):2287-91.
Hauser et al.: Trends in GPCR drug discovery: new agents, targets and indications. Nature Reviews Drug Discovery, 16, 829-842 (2017). doi:10.1038/nrd.2017.178 https://www.nature.com/articles/nrd.2017.178.
Heckers et al.: Error analysis of chemically synthesized polynucleotides, BioTechniques, vol. 24, No. 2, 256-260 (1998).
Herzer et al.: Fabrication of patterned silane based self-assembled monolayers by photolithography and surface reactions on silicon-oxide substrates Chem. Commun., 46:5634-5652 (2010).
Hoover et al.: DNAWorks: an automated method for designing oligonucleotides for PCR-based gene synthesis, Nucleic Acids Research, vol. 30, No. 10, e43, 7 pages (2002).
Hopcroft et al.: What is the Young's Modulus of Silicon?. Journal of Microelectromechanical Systems. 19(2):229-238 (2010).
Hosu et al.: Magnetic tweezers for intracellular applications⋅, Rev. Sci. Instrum., vol. 74, No. 9, 4158-4163 (Sep. 2003).
Huang et al.: Three-dimensional cellular deformation analysis with a two-photon magnetic manipulator workstation, Biophysical Journal, vol. 8 2, No. 4, 2211-2223 (Apr. 2002).
Hudson: Matrix Assisted Synthetic Transformations: A Mosaic of Diverse Contributions. Journal of Combinatorial Chemistry. 1(6):403-457 (1999).
Hughes et al.: Expression profiling using microarrays fabricated by an ink-jet oligonucleotide synthesizer Nat Biotech 4:342-347 (2001).
Hughes et al.: Principles of early drug discovery. Br J Pharmacol 162(2):1239-1249, 2011.
Hutchison et al.: Cell-free cloning using phi29 DNA polymerase. Proc Natl Acad Sci U S A. Nov. 29, 2005;102(48):17332-6. Epub Nov. 14, 2005.
Hötzel et al.: A strategy for risk mitigation of antibodies with fast clearance. mAbs, 4(6), 753-760 (2012). doi:10.4161/mabs.22189 https://www.ncbi.nlm.nih.gov/pubmed/23778268.
Imgur: The magic of the internet. Uploaded May 10, 2012, 2 pages, retrieved from: https://imgur.com/mEWuW.
In-Fusion Cloning: Accuracy, Not Background. Cloning & Competent Cells, ClonTech Laboratories, 3 pages, available online Jul. 6, 2014.
International Application No. PCT/US2017/026232 International Preliminary Report on Patentability dated Feb. 26, 2019.
International Application No. PCT/US2017/045105 International Preliminary Report on Patentability dated Feb. 5, 2019.
International Application No. PCT/US2017/052305 International Preliminary Report on Patentability dated Apr. 30, 2019.
International Application No. PCT/US2017/062391 International Preliminary Report on Patentability dated May 21, 2019.
International Application No. PCT/US2018/019268 International Preliminary Report on Patentability dated Sep. 6, 2019.
International Application No. PCT/US2018/050511 International Search Report and Written Opinion dated Jan. 11, 2019.
International Application No. PCT/US2018/057857 International Search Report and Written Opinion dated Mar. 18, 2019.
International Application No. PCT/US2019/012218 International Search Report and Written Opinion dated Mar. 21, 2019.
International Application No. PCT/US2019/032992 International Search Report and Written Opinion dated Oct. 28, 2019.
International Application No. PCT/US2019/032992 Invitation to Pay Additional Fees dated Sep. 6, 2019.
Jackson et al.: Recognition of DNA base mismatches by a rhodium intercalator, J. Am. Chem. Soc., vol. 19, 12986-12987 (1997).
Jacobs et al.: DNAglycosylases: In DNA repairand beyond. Chromosoma 121:1-20 (2012)—http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3260424/.
Jager et al.: Simultaneous Humoral and Cellular: Immune Response against Cancer—Testis Antigen NY-ES0-1: Definition of Human Histocompatibility Leukocyte Antigen (HLA)-A2-binding Peptide Epitopes. J. Exp. Med. 187(2):265-270 (1998).
Jaiswal et al.: An architecture for creating collaborative semantically capable scientific data sharing infrastructures. Proceeding WIDM '06 Proceedings of the 8th annual ACM international workshop on Web information and data management. ACM Digital Library pp. 75-82 (2006).
Jang et al.: Characterization of T cell repertoire of blood, tumor, and ascites in ovarian cancer patients using next generation sequencing. Oncoimmunology, 4(11):e1030561:1-10 (2015).
Jinek et al.: A Programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science, 337:816-821, 2012.
Jo et al.: Engineering therapeutic antibodies targeting G-protein-coupled receptors; Experimental & Molecular Medicine; 48; 9 pages (2016).
Kalva et al.: Gibson Deletion: a novel application of isothermal in vitro recombination. Biological Procedures Online. 20(1):1-10 (2018).
Karagiannis and El-Osta. RNA interference and potential therapeutic applications of short interfering RNAs Cancer Gene Therapy, 12:787-795, 2005.
Ke et al.: Influence of neighboring base pairs on the stability of single base bulges and base pairs in a DNA fragment, Biochemistry, Vo. 34, 4593-4600 (1995).
Kelley et al.: Single-base mismatch detection based on charge transduction through DNA, Nucleic Acids Research, vol. 27, No. 24, 4830-4837 (1999).
Kim et al.: Chimeric restriction endonuclease, Proc. Natl. Acad. Sci. USA, vol. 91, 883-887 (Feb. 1994).
Kim et al.: High-resolution patterns of quantum dots formed by electrohydrodynamic jet printing for light-emitting diodes. Nano Letters, 15:969-973, 2015.
Kim et al.: Site specific cleavage of DNA-RNA hybrids by zinc finger/Fok I cleavage domain fusions Gene, vol. 203, 43-49 (1997).
Kim, The interaction between Z-ONA and the Zab domain of double-stranded RNA adenosine deaminase characterized using fusion nucleases, The Journal of Biological Chemistry, vol. 274, No. 27, 19081-19086 (1999).
Kinde et al.: Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci U S A. Jun. 7, 2011;108(23):9530-5. Epub May 17, 2011.
Kodumal et al.: Total synthesis of long DNA sequences: synthesis of a contiguous 32-kb polyketide synthase gene cluster. Proc Natl Acad Sci U S A. Nov. 2, 2004;101(44):15573-8. Epub Oct. 20, 2004.
Koike-Yusa et al.: Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nature Biotechnology, 32:267-273, 2014 (with three pages of supplemental Online Methods).
Kong et al.: Parallel gene synthesis in a microfluidic device. Nucleic Acids Res., 35(8):e61 (2007).
Kong. Microfluidic Gene Synthesis. MIT Thesis. Submitted to the program in Media Arts and Sciences, School of Architecture and Planning, in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Media Arts and Sciences at the Massachusetts Institute of Technology. 143 pages Jun. 2008.
Kopp et al.: Chemical amplification: continuous-flow PCR on a chip, Science, vol. 280, 1046-1048 (May 15, 1998).
Kosuri and Church. Large-scale de novo DNA synthesis: technologies and applications, Nature Methods, 11:499-507, 2014. Available at: http://www.nature.com/nmeth/journal/v11/n5/full/nmeth.2918.html.
Kosuri et al.: A scalable gene synthesis platform using high-fidelity DNA microchips Nat.Biotechnol., 28(12):1295-1299, 2010.
Kosuri, et al. A scalable gene synthesis by selective amplification of DNA pools from high-fidelity microchips. Nature Biotechnology. 2010; 28:1295-1299.
Krayden, Inc.: A Guide to Silane Solutions. Silane coupling agents. 7 pages. Published on May 31, 2005 at: http://krayden.com/pdf/xia_silane_chemistry.pdf.
Lagally et al.: Single-Molecule DNA Amplification and Analysis in an Integrated Microfluidic Device. Analytical Chemistry. 2001;73(3): 565-570.
Lahue et al., DNA mismatch correction in a defined system, Science, vol. 425; No. 4914, 160-164 (Jul. 14, 1989).
Lambrinakos, A. et al.: Reactivity of potassium permanganate and tetraethylammonium chloride with mismatched bases and a simple mutation detection protocol,Nucleic Acids Research, vol. 27, No. 8, 1866-1874 (1999).
Landegren et al.: A ligase-mediated gene detection technique. Science. Aug. 26, 1988;241(4869):1077-80.
Lang, Matthew J. et al.: An automated two-dimensional optical force clamp for single molecule studies, Biophysical Journal, vol. 83, 491-501 (Jul. 2002).
Lashkari et al.: An automated multiplex oligonucleotide synthesizer: development of high-throughput, low-cost DNA synthesis. Proc Natl Acad Sci U S A. Aug. 15, 1995;92(17):7912-5.
Lausted et al.: POSaM: a fast, flexible, open-source, inkjet oligonucleotide synthesizer and microarrayer, Genome Biology, 5:R58, 17 pages, 2004. available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC507883/.
Leamon et al.: A massively parallel PicoTiterPlate based platform for discrete picoliter-scale polymerase chain reactions. Electrophoresis. Nov. 2003;24(21):3769-77.
Lebl et al.: Economical Parallel Oligonucleotide and Peptide Synthesizer—Pet Oligator. Int. J. Peptide Res. Ther. 13(1-2):367-376 (2007).
Lee et al.: A microfluidic oligonucleotide synthesizer. Nucleic Acids Research 2010 vol. 38(8):2514-2521. DOI: 10.1093/nar/gkq092.
Lee et al.: Microelectromagnets for the control of magnetic nanoparticles, Appl. Phys. Lett., vol. 79, No. 20, 3308-3310 (Nov. 12, 2001).
Lee: Covalent End-Immobilization of Oligonucleotides onto Solid Surfaces; Thesis, Massachusetts Institute of Technology, Aug. 2001 (315 pages).
Leproust et al.: Agilent's Microarray Platform: How High-Fidelity DNA Synthesis Maximizes the Dynamic Range of Gene Expression Measurements. 2008; 1-12. http://www.miltenyibiotec.eom/˜/media/Files/Navigation/Genomic%20Services/Agilent_DNA_Microarray_Platform.ashx.
Leproust et al.: Synthesis of high-quality libraries of long (150mer) oligonucleotides by a novel depurination controlled process, Nucleic Acids Research, 35(8):2522-2540, 2010.
Lesnikowski et al.: Nucleic acids and nucleosides containing carboranes. J. Organometallic Chem. 1999; 581:156-169.
Leumann. DNA analogues: from supramolecular principles to biological properties. Bioorg Med Chem. Apr. 2002;10(4):841-54.
Levene et al.: Zero-mode waveguides for single-molecule analysis at high concentrations. Science. Jan. 31, 2003;299(5607):682-6.
Lewontin and Harti, Population genetics in forensic DNA typing. Science, 254:1745-1750, 1991.
Li et al.: Beating Bias in the Directed Evolution of Proteins: Combining High-Fidelity on-Chip Solid-Phase Gene Synthesis with Efficient Gene Assembly for Combinatorial Library Construction. ChemBioChem 19:221-228 (2018).
Li et al.: Beating bias in the directed evolution of proteins: Combining high-fidelity on-chip solid-phase gene synthesis with efficient gene assembly for combinatorial library construction. First published Nov. 24, 2017, 2 pages, retrieved from: https://doi.org/10.1002/cbic.201700540.
Li et al.: Functional domains in Fok I restriction endonuclease, Proc. Natl. Acad. Sci. USA, 89:4275-4279, 1992.
Light source unit for printable patterning VUV-Aligner/ USHIO Inc., Link here: https://www.ushio.co.jp/en/products/1005.html, published Apr. 25, 2016, printed from the internet on Aug. 2, 2016, 3 pages.
Limbachiya et al.: Natural data storage: A review on sending information from now to then via Nature. ACM Journal on Emerging Technologies in Computing Systems, V(N):Article A, May 19, 2015, 17 pages.
Link Technologies. Product Guide 2010. Nov. 27, 2009, 136 pages. XP055353191. Retrieved from the Internet: URL:http://www.linktech.co.uk/documents/517/517.pdf.
Lipshutz, Robert J. et al.: High density synthetic oligonucleotide arrays, Nature Genetics Supplement, vol. 21, 20-24 (Jan. 1999).
Lishanski, Alia et al.: Mutation detection by mismatch binding protein, MutS, in amplified DNA: application to the cystic fibrosis gene, Proc. Natl. Acad. Sci. USA, vol. 91, 2674-2678 (Mar. 1994).
Liu et al.: Comparison of Next-Generation Sequencing Systems. J Biomed Biotechnol 2012: 251364 (2012).
Liu et al.: Enhanced Signals and Fast Nucleic Acid Hybridization By Microfluidic Chaotic Mixing. Angew. Chem. Int. Ed. 2006; 45:3618-3623.
Liu et al.: Rational design of CXCR4 specific antibodies with elongated CDRs. JACS, 136:10557-10560, 2014.
Lizardi et al.: Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nat Genet. Jul. 1998;19(3):225-32.
Lu et al.: Methyl-directed repair of DNA base-pair mismatches in vitro, Proc. Natl. Acad. Sci. USA, 80:4639-4643, 1983.
Lund et al.: A validated system for ligation-free uracilexcision based assembly of expression vectors for mammalian cell engineering. DTU Systems of Biology. 2011. 1 page. http://www.lepublicsystemepco.com/files/modules/gestion_rubriques/REF-B036-Lund_Anne%20Mathilde.pdf.
MLAB 2321 Molecular Diagnostics for Clinical Laboratory Science. Mar. 6, 2015.
Ma et al.: DNA synthesis, assembly and application in synthetic biology. Current Opinion in Chemical Biology. 16:260-267, 2012.
Ma et al.: Versatile surface functionalization of cyclic olefin copolymer (COC) with sputtered SiO2 thin film for potential BioMEMS applications. Journal of Materials Chemistry, 11 pages, 2009.
Mahato et al.: Modulation of gene expression by antisense and antigene oligodeoxynucleotides and small interfering RNA Expert Opin. Drug Delivery, 2(1):3-28, 2005.
Malecek et al.: Engineering improved T cell receptors using an alanine-scan guided T cell display selection system. Journal of Immunological Methods. Elsevier Science Publishers. 392(1):1-11 (2013).
Margulies et al.: Genome sequencing in open microfabricated high-density picolitre reactors. Nature. 437(7057):376-80, 2005.
Martinez-Torrecuadrada et al.: Targeting the Extracellular Domain of Fibroblast Growth Factor Receptor 3 with Human Single-Chain Fv Antibodies Inhibits Bladder Carcinoma Cell Line Proliferation; Clinical Cancer Research; vol. 11; pp. 6282-6290 (2005).
Matteucci et al.: Synthesis of deoxyoligonucleotides on a polymer support. J. Am. Chem. Soc. 103(11):3185-3191, 1981.
Matzas et al.: Next generation gene synthesis by targeted retrieval of bead-immobilized, sequence verified DNA clones from a high throughput pyrosequencing device. Nat. Biotechnol., 28(12):1291-1294, 2010.
Mazor et al.: Isolation of Full-Length IgG Antibodies from Combinatorial Libraries Expressed in Escherichia coli; Antony S. Dimitrov (ed.), Therapeutic Antibodies: Methods and Protocols, vol. 525, Chapter 11, pp. 217-239 (2009).
McBride & Caruthers. An investigation of several deoxynucleoside phosphoramidites useful for synthesizing deoxyoligonucleotides. Tetrahedron Lett. 24: 245-248, 1983.
McGall et al.: Light-directed synthesis of high-density oligonucleotide arrays using semiconductor photoresists. Proc Natl Acad Sci U S A. 93(24):13555-60, 1996.
McGall et al.: The Efficiency of Light-Directed Synthesis of DNA Arrays on Glass Substrates. J. Am. Chem. Soc. 119(22):5081-5090, 1997.
Mei et al.: Cell-free protein synthesis in microfluidic array devices Biotechnol. Prog., 23(6):1305-1311,2007.
Mendel-Hartvig. Padlock probes and rolling circle amplification. New possibilities for sensitive gene detection. Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1175. Uppsala University. 2002, 39 pages, http://www.diva-portal.org/smash/get/diva2:161926/FULLTEXT01. pdf.
Meyers and Friedland, Knowledge-based simulation of genetic regulation in bacteriophage lambda. Nucl. Acids Research, 12(1):1-16, 1984.
Meynert et al.: Quantifying single nucleotide variant detection sensitivity in exome sequencing. BMC Bioinformatics 14:195 (2013).
Meynert et al.: Variant detection sensitivity and biases in whole genome and exome sequencing. BMC Bioinformatics 15:247 (2014).
Milo and Phillips. Numbers here reflect the No. of protein coding genes and excludes tRNA and non-coding RNA. Cell Biology by the Numbers, p. 286, 2015.
Mitra et al.: In situ localized amplification and contact replication of many individual DNA molecules. Nucleic Acids Res. 27(24):e34, 1999.
Momentiv. Technical Data Sheet. Silquest A-1100. Momentiv. 1-6 (2020).
Morin et al.: Profiling the HeLa S3 transcriptome using randomly primed cDNA and massively parallel short-read sequencing. Biotechniques, 45:81-94, 2008.
Morris and Stauss. Optimizing T-cell receptor gene therapy for hematologic malignancies. Blood, 127(26):3305-3311, 2016.
Muller et al.: Protection and labelling of thymidine by a fluorescent photolabile group, Helvetica Chimica Acta, vol. 84, 3735-3741 (2001).
Mulligan. Commercial Gene Synthesis Technology PowerPoint presentation. BlueHeron® Biotechnology. Apr. 5, 2006 (48 pgs).
Nakatani et al.: Recognition of a single guanine bulge by 2-Acylamino-1 ,8-naphthyridine, J. Am. Chem. Soc., vol. 122, 2172-2177 (2000).
Neiman M.S.: Negentropy principle in information processing systems. Radiotekhnika, 1966, No. 11, p. 2-9.
Neiman M.S.: On the bases of the theory of information retrieval. Radiotekhnika, 1967, No. 5, p. 2-10.
Neiman M.S.: On the molecular memory systems and the directed mutations. Radiotekhnika, 1965, No. 6, pp. 1-8.
Neiman M.S.: On the relationships between the reliability, performance and degree of microminiaturization at the molecular-atomic level. Radiotekhnika, 1965, No. 1, pp. 1-9.
Neiman M.S.: Some fundamental issues of microminiaturization. Radiotekhnika, 1964, No. 1, pp. 3-12.
Nishikura. A short primer on RNAi: RNA-directed RNA polymerase acts as a key catalyst Cell, 107:415-418, 2001.
Nour-Eldin et al.: USER Cloning and USER Fusion: The Ideal Cloning Techniques for Small and Big Laboratories. Plant Secondary Metabolism Engineering. Methods in Molecular Biology vol. 643, 2010, pp. 185-200.
Nucleic acid thermodynamics. Wikipedia. Feb. 4, 2021.
O'Driscoll et al.: Synthetic DNA: The next generation of big data storage. Bioengineered. 4(3):123-125 (2013).
Ochman et al.: Genetic applications of an inverse polymerase chain reaction. Genetics. Nov. 1988;120(3):621-3.
Opposition to European Patent No. 3030682 filed Mar. 3, 2021.
Organick et al.: Random access in large-scale DNA data storage. Nature Biotechnology, Advance Online Publication, 8 pages, 2018.
Organick et al.: Scaling up DNA data storage and random access retrieval, bioRxiv, preprint first posted online Mar. 7, 2017, 14 pages.
PCT/IL2012/000326 International Preliminary Report on Patentability dated Dec. 5, 2013.
PCT/IL2012/000326 International Search Report dated Jan. 29, 2013.
PCT/US2014/049834 International Preliminary Report on Patentability dated Feb. 18, 2016.
PCT/US2014/049834 International Search Report and Written Opinion dated Mar. 19, 2015.
PCT/US2014/049834, Invitation to Pay Additional Fees and, where applicable, protest fee, mailed Jan. 5, 2015.
PCT/US2015/043605 International Preliminary Report on Patentability dated Feb. 16, 2017.
PCT/US2015/043605 International Search Report and Written Opinion dated Jan. 6, 2016.
PCT/US2015/043605 Invitation To Pay Additional Fees dated Oct. 28, 2015.
PCT/US2016/016459 International Preliminary Report on Patentability dated Aug. 17, 2017.
PCT/US2016/016459 International Search Report and Written Opinion dated Apr. 13, 2016.
PCT/US2016/016636 International Preliminary Report on Patentability dated Aug. 17, 2017.
PCT/US2016/016636 International Search Report and Written Opinion dated May 2, 2016.
PCT/US2016/028699 International Preliminary Report on Patentability dated Nov. 2, 2017.
PCT/US2016/028699 International Search Report and Written Opinion dated Jul. 29, 2016.
PCT/US2016/031674 International Preliminary Report on Patentability dated Nov. 23, 2017.
PCT/US2016/031674 International Search Report and Written Opinion dated Aug. 11, 2016.
PCT/US2016/052336 International Preliminary Report on Patentability dated Mar. 29, 2018.
PCT/US2016/052336 International Search Report and Written Opinion dated Dec. 7, 2016.
PCT/US2016/052916 International Preliminary Report on Patentability dated Apr. 5, 2018.
PCT/US2016/052916 International Search Report and Written Opinion dated Dec. 30, 2016.
PCT/US2016/064270 International Preliminary Report on Patentability dated Jun. 14, 2018.
PCT/US2016/064270 International Search Report and Written Opinion dated Apr. 28, 2017.
PCT/US2017/026232 International Search Report and Written Opinion dated Aug. 28, 2017.
PCT/US2017/036868 International Search Report and Written Opinion dated Aug. 11, 2017.
PCT/US2017/045105 International Search Report and Written Opinion dated Oct. 20, 2017.
PCT/US2017/052305 International Search Report and Written Opinion dated Feb. 2, 2018.
PCT/US2017/062391 International Search Report and Written Opinion dated Mar. 28, 2018.
PCT/US2017/066847 International Search Report and Written Opinion dated May 4, 2018.
PCT/US2018/022487 International Search Report and Written Opinion dated Aug. 1, 2018.
PCT/US2018/022493 International Search Report and Written Opinion dated Aug. 1, 2018.
PCT/US2018/037152 International Preliminary Report on Patentability dated Dec. 26, 2019.
PCT/US2018/037152 International Search Report and Written Opinion dated Aug. 28, 2018.
PCT/US2018/037161 International Preliminary Report on Patentability dated Dec. 17, 2019.
PCT/US2018/037161 International Search Report and Written Opinion dated Oct. 22, 2018.
PCT/US2018/037161 Invitation to Pay Additional Fees dated Aug. 27, 2018.
PCT/US2018/050511 International Preliminary Report on Patentability dated Mar. 26, 2020.
PCT/US2018/056783 International Preliminary Report on Patentability dated Apr. 30, 2020.
PCT/US2018/056783 International Search Report and Written Opinion of the International Searching Authority dated Dec. 20, 2018.
PCT/US2018/057857 International Preliminary Report on Patentability dated Apr. 28, 2020.
PCT/US2018/19268 International Search Report and Written Opinion dated Jun. 26, 2018.
PCT/US2018/19268 Invitation to Pay Additional Fees and, where applicable, protest fee dated May 2, 2018.
PCT/US2018/22487 Invitation to Pay Additional Fees and, where applicable, protest fee dated May 31, 2018.
PCT/US2018/22493 Invitation to Pay Additional Fees and, where applicable, protest fee dated May 31, 2018.
PCT/US2019/012218 International Preliminary Report on Patentability dated Jul. 16, 2020.
PCT/US2019/032992 International Preliminary Report on Patentability dated Nov. 24, 2020.
PCT/US2019/068435 International Preliminary Report on Patentability dated Jul. 8, 2021.
PCT/US2019/068435 International Search Report and Written Opinion dated Apr. 23, 2020.
PCT/US2020/019371 International Preliminary Report on Patentability dated Sep. 2, 2021.
PCT/US2020/019371 International Search Report and Written Opinion dated Jun. 25, 2020.
PCT/US2020/019986 International Preliminary Report on Patentability dated Sep. 10, 2021.
PCT/US2020/019986 International Search Report and Written Opinion dated Jul. 29, 2020.
PCT/US2020/019986 Invitation to Pay Additional Fees dated Jun. 5, 2020.
PCT/US2020/019988 International Preliminary Report on Patentability dated Sep. 10, 2021.
PCT/US2020/019988 International Search Report and Written Opinion dated Jul. 29, 2020.
PCT/US2020/019988 Invitation to Pay Additional Fees dated Jun. 8, 2020.
PCT/US2020/038679 International Search Report and Written Opinion dated Oct. 28, 2020.
PCT/US2020/052291 International Search Report and Written Opinion dated Mar. 10, 2021.
PCT/US2020/052291 Invitation to Pay Additional Fees dated Dec. 31, 2020.
PCT/US2020/052306 International Search Report and Written Opinion dated Mar. 2, 2021.
PCT/US2020/052306 Invitation to Pay Additional Fees dated Dec. 18, 2020.
PCT/US2020/064106 International Search Report and Written Opinion dated Jun. 3, 2021.
PCT/US2020/064106 Invitation to Pay Additional Fees dated Apr. 9, 2021.
Pan et al.: An approach for global scanning of single nucleotide variations. Proc Natl Acad Sci USA. Jul. 9, 2002;99(14):9346-51.
Pankiewicz. Fluorinated nucleosides. Carbohydr Res. Jul. 10, 2000;327(1-2):87-105.
Paul et al.: Acid binding and detritylation during oligonucleotide synthesis. Nucleic Acids Research. 15. pp. 3048-3052 (1996).
Pease et al.: Light-generated oligonucleotide arrays for rapid DNA sequence analysis. Proc Natl Acad Sci U S A. May 24, 1994;91(11):5022-6.
Peisajovich et al.: BBF RFC 28: A method for combinatorial multi-part assembly based on the type-lis restriction enzyme aarl. Sep. 16, 2009, 7 pages.
Pellois et al.: Individually addressable parallel peptide synthesis on microchips, Nature Biotechnology, vol. 20 , 922-926 (Sep. 2002).
Petersen et al.: LNA: a versatile tool for therapeutics and genomics. Trends Biotechnol. Feb. 2003;21(2):74-81.
Pierce and Wangh. Linear-after-the-exponential polymerase chain reaction and allied technologies Real-time detection strategies for rapid, reliable diagnosis from single cells Methods Mol. Med. 132:65-85 (2007) (Abstract only).
Pierce et al.: Linear-after-the-exponential polymerase chain reaction and allied technologies. Real-time detection strategies for rapid, reliable diagnosis from single cells. Methods Mol Med. 2007;132:65-85.
Pigott et al.: The Use of a Novel Discovery Platform to Identify Peptide-Grafted Antibodies that Activate GLP-1 Receptor Signaling. Innovative Targeting Solutions Inc. (2013) XP055327428 retrieved from the internet: http://www.innovativetargeting.com/wo-content/uploads/2013/12/Pigott-et-al-Antibody-Engineering-2013.pdf.
Pirrung. How to make a DNA chip. Angew. Chem. Int. Ed., 41:1276-1289, 2002.
Plesa et al.: Multiplexed gene synthesis in emulsions for exploring protein functional landscapes. Science, 10.1126/science.aao5167, 10 pages, 2018.
Pon. Solid-phase supports for oligonucleotide synthesis. Methods Mol Biol. 1993;20:465-96.
Ponsel. High Affinity, Developability and Functional Size: The Holy Grail of Combinatorial Antibody Library Generation. Molecules. 16:3675-3700 (2011).
Poster. Reimagine Genome Scale Research. 2016, 1 page. Available at http://www2.twistbioscience.com/Oligo_Pools_CRISPR_poster.
Powers et al.: Optimal strategies for the chemical and enzymatic synthesis of bihelical deoxyribonucleic acids. J Am Chem Soc., 97(4):875-884, 1975.
Pray. Discovery of DNA Structure and Function: Watson and Crick, Nature Education, 2008, 6 pages, available at: http://www.nature.com/scitable/topicpage/discovery-of-dna-structure-and-function-watson-397.
Prodromou et al.: Recursive PCR: a novel technique for total gene synthesis. Protein Eng. Dec. 1992;5(8):827-9.
PubChem Data Sheet Acetonitrile. Printed from website https://pubchem.ncbi.nlm.nig.gov/ pp. 1-124 (2020).
PubChem Data Sheet Dichloromethane. Printed from website https://pubchem.ncbi.nlm.nih.gov/compound/Dichloromethane (2020).
PubChem Data Sheet Methylene Chloride. Printed from website https://pubchem.ncbi.nlm.nih.gov/ pp. 1-140 (2020).
Puigbo. Optimizer: a web server for optimizing the codon usage of DNA sequences. Nucleic Acid Research, 35(14):126-131, 2007.
Qian and Winfree. Scaling up digital circuit computation with DNA strand displacement cascades. Science, 332(6034):196-1201, 2011.
Qian, et al.: Neural network computation with DNA strand displacement cascades, Nature, 475(7356):368-372, 2011.
Quan et al.: Parallel on-chip gene synthesis and application to optimization of protein expression, Nature Biotechnology, 29(5):449-452, 2011.
RF Electric discharge type excimer lamp. Products Catalog. Excimer lamp light source flat excimer, 16 pages dated Jan. 2016. From: http://www.hamamatsu.com/jp/en/product/category/1001/3026/index.html.
Rafalski and Morgante. Corn and humans: recombination and linkage disequilibrium in two genomes of similar size. Trends in Genetics, 20(2):103-111, 2004.
Raje and Murma, A Review of electrohydrodynamic-inkjet printing technology. International Journal of Emerging Technology and Advanced Engineering, 4(5):174-183, 2014.
Rajpal et al.: A general method for greatly improving the affinity of antibodies by using combinatorial libraries. Proc. Natl. Acad. Sci. 102(24):8466-8471 (2005).
Rastegari et al.: XNOR-Net: ImageNet Classification Using Binary Convolutional Neural Networks, in ECCV 2016, Part IV, LNCS 9908, p. 525-542, 2016.
Regep et al.: The H3 loop of antibodies shows unique structural characteristics. Proteins. 85(7):1311-1318 (2017).
Reimagine SequenceSpace, Reimagine Research, Twist Bioscience, Product Brochure, Published Apr. 6, 2016 online at: www2.twistbioscience.com/TB_Product_Brochure_04.2016, 8 pages.
Richmond et al.: Amplification and assembly ofchip-eluted DNA (AACED): a method for high-throughput gene synthesis. Nucleic Acids Res. Sep. 24, 2004;32(17):5011-8. Print 2004.
Roche. Restriction Enzymes from Roche Applied Science—A Tradition of Premium Quality and Scientific Support. FAQS and Ordering Guide. Roche Applied Science. Accessed Jan. 12, 2015, 37 pages.
Rogozin et al.: Origin and evolution of spliceosomal introns. Biology Direct, 7:11, 2012.
Ruminy et al.: Long-range identification of hepatocyte nuclear factor-3 (FoxA) high and low-affinity binding Sites with a chimeric nuclease, J. Mol. Bioi., vol. 310, 523-535 (2001).
Saaem et al.: In situ synthesis of DNA microarray on functionalized cyclic olefin copolymer substrate ACS Applied Materials & Interfaces, 2(2):491-497, 2010.
Saboulard et al.: High-throughput site-directed mutagenesis using oligonucleotides synthesized on DNA chips. Biotechniques. Sep. 2005;39(3):363-8.
Sacconi et al.: Three-dimensional magneto-optic trap for micro-object manipulation, Optics Letters, vol. 26, No. 17, 1359-1361 (Sep. 1, 2001).
Saiki et al.: Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature 324:163-166 (1986).
Sandhu et al.: Dual asymmetric PCR: one-step construction of synthetic genes. Biotechniques. Jan. 1992;12(1):14-6.
Sargolzaei et al.: Extent of linkage disequilibrium in Holstein cattle in North America. J.Dairy Science, 91:2106-2117, 2007.
Schaller et al.: Studies on Polynucleotides. XXV.1 The Stepwise Synthesis of Specific Deoxyribopolynucleotides (5). Further Studies on the Synthesis of Internucleotide Bond by the Carbodiimide Method. The Synthesis of Suitably Protected Dinucleotides as Intermediates in the Synthesis of Higher Oligonucleotides. J. Am. Chem. Soc. 1963; 85(23):3828-3835.
Schmalzing et al.: Microchip electrophoresis: a method for high-speed SNP detection. Nucleic Acids Res 28(9):E43 (2000).
Schmitt et al.: New strategies in engineering T-cell receptor gene-modified T cells to more effectively target malignancies. Clinical Cancer Research, 21(23):5191-5197, 2015.
Seelig et al.: Enzyme-Free Nucleic Acid Logic Circuits, Science 314(5805):1585-1588, 2006.
Sharan et al.: Recombineering: a homologous recombination-based method of genetic engineering. Nat Profile 4(2):1-37 (originally pp. 206-223) (2009).
Sharpe and Mount. Genetically modified T cells in cancer therapy: opportunities and challenges. Disease Models and Mechanisms, 8:337-350, 2015.
Shipman et al.: Molecular recordings by directed CRISPR spacer acquisition. Science. 353(6298):1-16 (2016).
Sierzchala, Agnieszka B et al.: Solid-phase oligodeoxynucleotide synthesis : a two-step cycle using peroxy anion deprotection, J. Am. Chem. Soc., vol. 125, No. 44, 13427-13441 (2003).
Simonyan and Zisserman. Very Deep Convolutional Networks for Large-Scale Image Recognition, Published as a conference paper at Int. Conf. Learn. Represent., pp. 1-14, 2015.
Singh-Gasson. Sangeet et al.: Maskless fabrication of light-directed oligonucleotide microarrays using a digital micromirror array, Nature Biotechnology, vol. 17, 974-978 (Oct. 1999).
Skerra. Phosphorothioate primers improve the amplification of DNA sequences by DNA polymerases with proofreading activity. Nucleic Acids Res. Jul. 25, 1992; 20(14):3551-4.
Smith et al.: Direct mechanical measurements of the elasticity of single DNA molecules using magnetic beads, Science, vol. 258, 1122-1126 (Nov. 13, 1992).
Smith et al.: Generating a synthetic genome by whole genome assembly: phiX174 bacteriophage from synthetic oligonucleotides. Proc Natl Acad Sci U S A. Dec. 23, 2003;100(26):15440-5. Epub Dec. 2, 2003.
Smith et al.: Generation of cohesive ends on PCR products by UDG-mediated excision of dU, and application for cloning into restriction digest-linearized vectors. PCR Methods Appl. May 1993;2(4):328-32.
Smith et al.: Mutation detection with MutH, MutL, and MutS mismatch repair proteins, Proc. Natl. Acad. Sci. USA, vol. 93, 4374-4379 (Apr. 1996).
Smith et al.: Removal of Polymerase-Produced mutant sequences from PCR products, Proc. Natl. Acad. Sci. USA, vol. 94, 6847-6850 (Jun. 1997).
Solomon et al.: Genomics at Agilent: Driving Value in DNA Sequencing.https://www.agilent.com/labs/features/2010_genomics.html, 8 pages (Aug. 5, 2010).
Soni et al.: Progress toward ultrafast DNA sequencing using solid-state nanopores. Clin Chem. Nov. 2007;53(11):1996-2001. Epub Sep. 21, 2007.
Southern et al.: Analyzing and comparing nucleic acid sequences by hybridization to arrays of oligonucleotides: evaluation using experimental models. Genomics. Aug. 1992;13(4):1008-17.
Sproat et al.: An efficient method for the isolation and purification of oligoribonucleotides. Nucleosides & Nucleotides. 1995; 14(1&2):255-273.
Srivannavit et al.: Design and fabrication of microwell array chips for a solution-based, photogenerated acid-catalyzed parallel oligonuclotide DNA synthesis. Sensors and Actuators A, 116:150-160, 2004.
Srivastava et al.: RNA synthesis: phosphoramidites for RNA synthesis in the reverse direction. Highly efficient synthesis and application to convenient introduction of ligands, chromophores and modifications of synthetic RNA at the 3′-end, Nucleic Acids Symposium Series, 52(1):103-104, 2008.
Steel. The Flow-Thru Chip A Three-dimensional biochip platform. In: Schena, Microarray Biochip Technology, Chapter 5, Natick, MA: Eaton Publishing, 2000, 33 pages.
Stemmer et al.: Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene. Oct. 16, 1995;164(1):49-53.
Stryer. DNA Probes and genes can be synthesized by automated solid-phase methods. Biochemistry, 3rd edition, New York: W.H. Freeman and Company, 1988; 123-125.
Stutz et al.: Novel fluoride-labile nucleobase-protecting groups for the synthesis of 3′(2′)-O-amino-acylated RNA sequences. Helv. Chim. Acta. 2000; 83(9):2477-2503.
Sullivan et al.: Library construction and evaluation for site saturation mutagenesis. Enzyme Microb. Technol. 53:70-77 (2013).
Sun et al.: Structure-Guided Triple-Code Saturation Mutagenesis: Efficient Tuning of the Stereoselectivity of an Epoxide Hydrolase. ACS Catal. 6:1590-1597 (2016).
Takahashi. Cell-free cloning using multiply-primed rolling circle amplification with modified RNA primers. Biotechniques. Jul. 2009;47(1):609-15. doi: 10.2144/000113155.
Tanase et al.: Magnetic trapping of multicomponent nanowires, The Johns Hopkins University, Baltimore, Maryland, p. 1-3 (Jun. 25, 2001).
Taylor et al.: Impact of surface chemistry and blocking strategies on DNA microarrays. Nucleic Acids Research, 31(16):e87, 19 pages, 2003.
The Hood Laboratory, Beta Group. Assembly Manual for the POSaM: The ISB Piezoelelctric Oligonucleotide Synthesizer and Microarrayer, Inkjet Microarrayer Manual Version 1.2, 50 pages, May 28, 2004.
The SLIC. Gibson, OPEC and SLiCE assembly methods (and GeneArt Seamless, In-Fusion Cloning). 5 pages, available online Sep. 2, 2010.
Tian et al.: Accurate multiplex gene synthesis from programmable DNA microchips. Nature. Dec. 23, 2004;432(7020):1050-4.
Tsai et al.: Dimeric CRISPR RNA-guided Fokl nucleases for highly specific genome editing Nat. Biotechnol., 32(6):569-576, 2014.
Twist Bioscience | White Paper. DNA-Based Digital Storage. Retrieved from the internet, Twistbioscience.com, Mar. 27, 2018, 5 pages.
U.S. Appl. No. 14/241,874 Final Office Action dated Jan. 28, 2019.
U.S. Appl. No. 14/241,874 Office Action dated Feb. 27, 2017.
U.S. Appl. No. 14/241,874 Office Action dated Jul. 14, 2016.
U.S. Appl. No. 14/241,874 Office Action dated May 4, 2018.
U.S. Appl. No. 14/452,429 Notice of Allowance dated Jun. 7, 2016.
U.S. Appl. No. 14/452,429 Office Action dated Apr. 9, 2015.
U.S. Appl. No. 14/452,429 Office Action dated Oct. 21, 2015.
U.S. Appl. No. 14/452,429 Restriction Requirement dated Dec. 12, 2014.
U.S. Appl. No. 14/885,962 Notice of Allowance dated Nov. 8, 2017 and Sep. 29, 2017.
U.S. Appl. No. 14/885,962 Office Action dated Dec. 16, 2016.
U.S. Appl. No. 14/885,962 Office Action dated Sep. 8, 2016.
U.S. Appl. No. 14/885,962 Restriction Requirement dated Mar. 1, 2016.
U.S. Appl. No. 14/885,963 Notice of Allowance dated May 24, 2016.
U.S. Appl. No. 14/885,963 Office Action dated Feb. 5, 2016.
U.S. Appl. No. 14/885,965 Office Action dated Aug. 28, 2018.
U.S. Appl. No. 14/885,965 Office Action dated Aug. 30, 2017.
U.S. Appl. No. 14/885,965 Office Action dated Feb. 10, 2017.
U.S. Appl. No. 14/885,965 Office Action dated Feb. 18, 2016.
U.S. Appl. No. 14/885,965 Office Action dated Jan. 4, 2018.
U.S. Appl. No. 14/885,965 Office Action dated Jul. 7, 2016.
U.S. Appl. No. 15/015,059 Final Office Action dated Jul. 17, 2019.
U.S. Appl. No. 15/015,059 Office Action dated Aug. 19, 2019.
U.S. Appl. No. 15/015,059 Office Action dated Feb. 7, 2019.
U.S. Appl. No. 15/135,434 Notice of Allowance dated Feb. 9, 2018.
U.S. Appl. No. 15/135,434 Office Action dated Nov. 30, 2017.
U.S. Appl. No. 15/135,434 Restriction Requirement dated Jul. 12, 2017.
U.S. Appl. No. 15/151,316 Final Office Action dated Feb. 21, 2019.
U.S. Appl. No. 15/151,316 Final Office Action dated Jul. 9, 2020.
U.S. Appl. No. 15/151,316 Office Action dated Jun. 7, 2018.
U.S. Appl. No. 15/151,316 Office Action dated Oct. 4, 2019.
U.S. Appl. No. 15/154,879 Notice of Allowance dated Feb. 1, 2017.
U.S. Appl. No. 15/156,134 Final Office Action dated Aug. 18, 2021.
U.S. Appl. No. 15/156,134 Final Office Action dated Jan. 3, 2020.
U.S. Appl. No. 15/156,134 Office Action dated Apr. 4, 2019.
U.S. Appl. No. 15/156,134 Office Action dated Nov. 25, 2020.
U.S. Appl. No. 15/187,714 Final Office Action dated Sep. 17, 2019.
U.S. Appl. No. 15/187,714 Office Action dated Apr. 4, 2019.
U.S. Appl. No. 15/187,714 Restriction Requirement dated Sep. 17, 2018.
U.S. Appl. No. 15/187,721 Notice of Allowance dated Dec. 7, 2016.
U.S. Appl. No. 15/187,721 Office Action dated Oct. 14, 2016.
U.S. Appl. No. 15/233,835 Notice of Allowance dated Oct. 4, 2017.
U.S. Appl. No. 15/233,835 Office Action dated Feb. 8, 2017.
U.S. Appl. No. 15/233,835 Office Action dated Jul. 26, 2017.
U.S. Appl. No. 15/233,835 Restriction Requirement dated Nov. 4, 2016.
U.S. Appl. No. 15/245,054 Notice of Allowance dated Dec. 14, 2017.
U.S. Appl. No. 15/245,054 Office Action dated Mar. 21, 2017.
U.S. Appl. No. 15/245,054 Office Action dated Oct. 19, 2016.
U.S. Appl. No. 15/268,422 Final Office Action dated Oct. 3, 2019.
U.S. Appl. No. 15/268,422 Office Action dated Mar. 1, 2019.
U.S. Appl. No. 15/268,422 Restriction Requirement dated Oct. 4, 2018.
U.S. Appl. No. 15/272,004 Final Office Action dated Mar. 18, 2021.
U.S. Appl. No. 15/272,004 Office Action dated Jun. 12, 2020.
U.S. Appl. No. 15/377,547 Final Office Action dated Feb. 8, 2019.
U.S. Appl. No. 15/377,547 Office Action dated Jul. 27, 2018.
U.S. Appl. No. 15/377,547 Office Action dated Mar. 24, 2017.
U.S. Appl. No. 15/377,547 Office Action dated Nov. 30, 2017.
U.S. Appl. No. 15/433,909 Non-Final Office Action dated Feb. 8, 2019.
U.S. Appl. No. 15/433,909 Restriction Requirement dated Sep. 17, 2018.
U.S. Appl. No. 15/602,991 Final Office Action dated Dec. 13, 2018.
U.S. Appl. No. 15/602,991 Notice of Allowance dated Oct. 25, 2017.
U.S. Appl. No. 15/602,991 Office Action dated May 31, 2018.
U.S. Appl. No. 15/602,991 Office Action dated May 31, 2019.
U.S. Appl. No. 15/602,991 Office Action dated Sep. 21, 2017.
U.S. Appl. No. 15/603,013 Final Office Action dated Nov. 6, 2019.
U.S. Appl. No. 15/603,013 Office Action dated Jan. 30, 2018.
U.S. Appl. No. 15/603,013 Office Action dated Jul. 10, 2018.
U.S. Appl. No. 15/603,013 Office Action dated Jun. 26, 2019.
U.S. Appl. No. 15/603,013 Office Action dated Oct. 20, 2017.
U.S. Appl. No. 15/619,322 Final Office Action dated Jul. 9, 2021.
U.S. Appl. No. 15/619,322 Final Office Action dated Mar. 30, 2020.
U.S. Appl. No. 15/619,322 Office Action dated Aug. 14, 2019.
U.S. Appl. No. 15/619,322 Office Action dated Nov. 4, 2020.
U.S. Appl. No. 15/682,100 Office Action dated Jan. 2, 2018.
U.S. Appl. No. 15/682,100 Restriction Requirement dated Nov. 8, 2017.
U.S. Appl. No. 15/709,274 Notice of Allowance dated Apr. 3, 2019.
U.S. Appl. No. 15/729,564 Final Office Action dated Dec. 13, 2018.
U.S. Appl. No. 15/729,564 Office Action dated Jan. 8, 2018.
U.S. Appl. No. 15/729,564 Office Action dated Jun. 6, 2018.
U.S. Appl. No. 15/729,564 Office Action dated May 30, 2019.
U.S. Appl. No. 15/816,995 Office Action dated May 19, 2020.
U.S. Appl. No. 15/816,995 Office Action dated Sep. 20, 2019.
U.S. Appl. No. 15/816,995 Restriction Requirement dated Apr. 4, 2019.
U.S. Appl. No. 15/835,342 Final Office Action dated Sep. 8, 2020.
U.S. Appl. No. 15/835,342 Office Action dated Apr. 16, 2021.
U.S. Appl. No. 15/835,342 Office Action dated Dec. 2, 2019.
U.S. Appl. No. 15/835,342 Restriction Requirement dated Sep. 10, 2019.
U.S. Appl. No. 15/844,395 Office Action dated Jan. 24, 2020.
U.S. Appl. No. 15/844,395 Restriction Requirement dated May 17, 2019.
U.S. Appl. No. 15/860,445 Final Office Action dated Dec. 13, 2018.
U.S. Appl. No. 15/860,445 Office Action dated May 30, 2018.
U.S. Appl. No. 15/902,855 Restriction Requirement dated Apr. 6, 2021.
U.S. Appl. No. 15/921,479 Final Office Action dated Jun. 15, 2020.
U.S. Appl. No. 15/921,479 Office Action dated Apr. 27, 2021.
U.S. Appl. No. 15/921,479 Office Action dated Nov. 12, 2019.
U.S. Appl. No. 15/921,479 Restriction Requirement dated May 24, 2019.
U.S. Appl. No. 15/921,537 Office Action dated Apr. 1, 2020.
U.S. Appl. No. 15/960,319 Office Action dated Aug. 16, 2019.
U.S. Appl. No. 15/991,992 Office Action dated May 21, 2020.
U.S. Appl. No. 15/991,992 Restriction Requirement dated Mar. 10, 2020.
U.S. Appl. No. 16/006,581 Office Action dated Sep. 25, 2019.
U.S. Appl. No. 16/031,784 Office Action dated May 12, 2020.
U.S. Appl. No. 16/039,256 Final Office Action dated Mar. 30, 2021.
U.S. Appl. No. 16/039,256 Office Action dated Aug. 20, 2020.
U.S. Appl. No. 16/039,256 Restriction Requirement dated May 18, 2020.
U.S. Appl. No. 16/128,372 Final Office Action dated Mar. 18, 2021.
U.S. Appl. No. 16/128,372 Office Action dated Oct. 8, 2020.
U.S. Appl. No. 16/128,372 Restriction Requirement dated May 18, 2020.
U.S. Appl. No. 16/165,952 Office Action dated Mar. 12, 2020.
U.S. Appl. No. 16/239,453 Office Action dated May 11, 2020.
U.S. Appl. No. 16/239,453 Office Action dated Nov. 7, 2019.
U.S. Appl. No. 16/384,678 Final Office Action dated Oct. 15, 2020.
U.S. Appl. No. 16/384,678 Office Action dated Jan. 21, 2020.
U.S. Appl. No. 16/409,608 Office Action dated Sep. 9, 2019.
U.S. Appl. No. 16/530,717 Final Office Action dated Apr. 15, 2020.
U.S. Appl. No. 16/530,717 Office Action dated Sep. 6, 2019.
U.S. Appl. No. 16/535,777 Final Office Action dated Oct. 20, 2020.
U.S. Appl. No. 16/535,777 Office Action dated Feb. 8, 2021.
U.S. Appl. No. 16/535,777 Office Action dated Jan. 23, 2020.
U.S. Appl. No. 16/535,779 First Action Interview dated Feb. 10, 2020.
U.S. Appl. No. 16/712,678 Office Action dated Nov. 26, 2021.
U.S. Appl. No. 16/712,678 Restriction Requirement dated Aug. 25, 2021.
U.S. Appl. No. 16/737,401 Restriction Requirement dated Nov. 15, 2021.
U.S. Appl. No. 16/798,275 Final Office Action dated Aug. 30, 2021.
U.S. Appl. No. 16/798,275 Office Action dated Feb. 10, 2021.
U.S. Appl. No. 16/802,439 Restriction Requirement dated Oct. 1, 2021.
U.S. Appl. No. 16/854,719 Office Action dated Nov. 24, 2021.
U.S. Appl. No. 16/854,719 Restriction Requirement dated Jul. 28, 2021.
U.S. Appl. No. 16/906,555 Office Action dated Aug. 17, 2021.
U.S. Appl. No. 17/154,906 Office Action dated Nov. 10, 2021.
U.S. Appl. No. 17/154,906 Restriction Requirement dated Jul. 26, 2021.
Unger et al.: Monolithic microfabricated valves and pumps by multilayer soft lithography. Science. Apr. 7, 2000;288(5463):113-6.
Vaijayanthi et al.: Recent advances in oligonucleotide synthesis and their applications. Indian J Biochem Biophys. Dec. 2003;40(6):377-91.
Van Den Brulle et al.: A novel solid phase technology for high-throughput gene synthesis. Biotechniques. 2008; 45(3):340-343.
Van Der Werf et al.: Limonene-1,2-epoxide hydrolase from Rhodococcus erythropolis DCL14 belongs to a novel class of epoxide hydrolases. J. Bacteriol. 180:5052-5057 (1998).
Van Tassell et al.: SNP discovery and allele frequency estimation by deep sequencing of reduced representation libraries. Nature Methods, 5:247-252, 2008.
Van der Velde: Thesis. Finding the Strength of Glass. Delft University of Technology. 1-16 (2015).
Vargeese et al.: Efficient activation of nucleoside phosphoramidites with 4,5-dicyanoimidazole during oligonucleotide synthesis. Nucleic Acids Res. Feb. 15, 1998;26(4):1046-50.
Verma et al.: Modified oligonucleotides: synthesis and strategy for users. Annu Rev Biochem 67:99-134 (1998).
Vincent et al.: Helicase-dependent isothermal DNA amplification. EMBO Rep. Aug. 2004;5(8):795-800.
Visscher et al.: Construction of multiple-beam optical traps with nanometer-resolution position sensing, IEEE Journal of Selected Topics in Quantum Electronics, vol. 2, No. 4, 1066-1076 (Dec. 1996).
Voldmans et al.: Holding forces of single-particle dielectrophoretic traps. Biophysical Journal, vol. 80, No. 1, 531-541 (Jan. 2001).
Vos et al.: AFLP:A new technique for DNA fingerprinting. Nucleic Acids Res. Nov. 11, 1995;23(21):4407-14.
Wagner et al.: Nucleotides, Part LXV, Synthesis of 2′-Deoxyribonucleoside 5′-Phosphoramidites: New Building Blocks for the Inverse (5′-3′)-Oligonucleotide Approach. Helvetica Chimica Acta, 83(8):2023-2035, 2000.
Wah et al.: Structure of Fok I has implications for DNA cleavage, Proc. Natl. Acad. Sci. USA, vol. 95, 10564-10569 (Sep. 1998).
Wah et al.: Structure of the multimodular endonuclease Fok I bound to DNA, Nature, vol. 388, 97-100 (Jul. 1997).
Walker et al.: Strand displacement amplification—an isothermal, in vitro DNA amplification technique. Nucleic Acids Res. Apr. 11, 1992;20(7):1691-6.
Wan et al.: Deep Learning for Content-Based Image Retrieval: A comprehensive study, in Proceedings of the 22nd ACM International Conference on Multimedia—Nov. 3-7, 2014, Orlando, FL, p. 157-166, 2014.
Warr et al.: Exome Sequencing: current and future perspectives. G3: (Bethesda) 5(8):1543-1550 (2015).
Weber et al.: A modular cloning system for standardized assembly of multigene constructs. PLoS One. Feb. 1, 20118;6(2):e16765. doi: 10.1371/journal.pone.0016765.
Welz et al.: 5-(Benzylmercapto)-1H-tetrazole as activator for 2′-O-TBDMS phosphoramidite building blocks in RNA synthesis. Tetrahedron Lett. 2002; 43(5):795-797.
Westin et al.: Anchored multiplex amplification on a microelectronic chip array Nature Biotechnology, 18:199-202 (2000) (abstract only).
Whitehouse et al.: Analysis of the mismatch and insertion/deletion binding properties of Thermus thermophilus, HB8, MutS, Biochemical and Biophysical Research Communications, vol. 233, 834-837 (1997).
Wiedenheft et al.: RNA-guided genetic silencing systems in bacteria and archaea. Nature 482:331-338 (2012).
Wijshoff. Structure and fluid-dynamics in Piezo inkjet printheads. Thesis. Venio, The Netherlands, published 2008, p. 1-185.
Wirtz. Direct measurement of the transport properties of a single DNA molecule, Physical Review Letters, vol. 75, No. 12, 2436-2439 (Sep. 18, 1995).
Withers-Martinez et al.: PCR-based gene synthesis as an efficient approach for expression of the A+ T-rich malaria genome, Protein Engineering, vol. 12, No. 12, 1113-1120 (1999).
Wood et al.: Human DNA repair genes, Science, vol. 291, 1284-1289 (Feb. 16, 2001).
Wosnick et al.: Rapid construction of large synthetic genes: total chemical synthesis of two different versions of the bovine prochymosin gene. Gene. 1987;60(1):115-27.
Wright and Church. An open-source oligomicroarray standard for human and mouse. Nature Biotechnology, 20:1082-1083, 2002.
Wu et al.: An improvement of the on-line electrophoretic concentration method for capillary electrophoresis of proteins an experimental factors affecting he concentration effect, Analytical Sciences, vol. 16, 329-331 (Mar. 2000).
Wu et al.: RNA-mediated gene assembly from DNA arrays. Angew Chem Int Ed Engl. May 7, 2012;51(19):4628-32. doi: 10.1002/anie.201109058.
Wu et al.: Sequence-Specific Capture of Protein-DNA Complexes for Mass Spectrometric Protein Identification PLoS ONE. Oct. 20, 2011, vol. 6, No. 10.
Wu et al.: Specificity of the nick-closing activity of bacteriophage T4 DNA ligase. Gene. 1989;76(2):245-54.
Xiong et al.: A simple, rapid, high-fidelity and cost-effective PCR-based two-step DNA synthesis method for long gene sequences. Nucleic Acids Res. 2004, 32(12):e98.
Xiong et al.: Chemical gene synthesis: Strategies, softwares, error corrections, and applications. FEMS Microbiol. Rev., 32:522-540, 2008.
Xiong et al.: Non-polymerase-cycling-assembly-based chemical gene synthesis: Strategies, methods, and progress. Biotechnology Advances. 26(2):121-134, 2008.
Xu et al.: Coordination between the Polymerase and 5 ′-Nuclease Components of DNA Polymerase I of Escherichia coli. The Journal of Biological Chemistry. 275(27):20949-20955 (2000).
Xu et al.: Design of 240,000 orthogonal 25mer DNA barcode probes. PNAS, 106(7):2289-2294, 2009.
Yang et al.: Purification, cloning, and characterization of the CEL I nuclease, Biochemistry, 39(13):3533-35, 2000.
Yazdi et al.: A Rewritable, Random-Access DNA-Based Storage System, Scientific Reports, 5, Article No. 14138, 27 pages, 2015.
Yazdi et al.: DNA-Based Storage: Trends and Methods. IEEE Transactions on Molecular, Biological and Multi-Scale Communications. IEEE. 1(3):230-248 (2016).
Yehezkel et al.: De novo DNA synthesis using single molecule PCR Nucleic Acids Research, 36(17):e107, 2008.
Yes HMDS vapor prime process application note Prepared by UC Berkeley and University of Texas at Dallas and re-printed by Yield Engineering Systems, Inc., 6 pages (http://www.yieldengineering.com/Portals/0/HMDS%20Application%20Note.pdf (Published online Aug. 23, 2013).
Youil et al.: Detection of 81 of 81 known mouse Beta-Globin promoter mutations with T4 Endonuclease VII—The EMC Method. Genomics, 32:431-435, 1996.
Young et al.: Two-step total gene synthesis method. Nucleic Acids Res. 32(7):e59, 2004.
Zhang and Seelig. Dynamic DNA nanotechnology using strand-displacement reactions, Nat. Chem., 3(2):103-113, 2011.
Zheleznaya et al.: Nicking endonucleases. Biochemistry (Mose). 74(13):1457-66, 2009.
Zheng et al.: Manipulating the Stereoselectivity of Limonene Epoxide Hydrolase by Directed Evolution Based on Iterative Saturation Mutagenesis. J. Am. Chem. Soc. 132:15744-15751 (2010).
Zhirnov et al.: Nucleic acid memory. Nature Materials, 15:366, 2016.
Zhou et al.: Establishment and application of a loop-mediated isothermal amplification (LAMP) system for detection of cry1Ac transgenic sugarcane Scientific Reports May 9, 2014, vol. 4, No. 4912.
Zhou et al.: Microfluidic PicoArray synthesis of oligodeoxynucleotides and simultaneous assembling of multiple DNA sequences Nucleic Acids Research, 32(18):5409-5417, 2004.