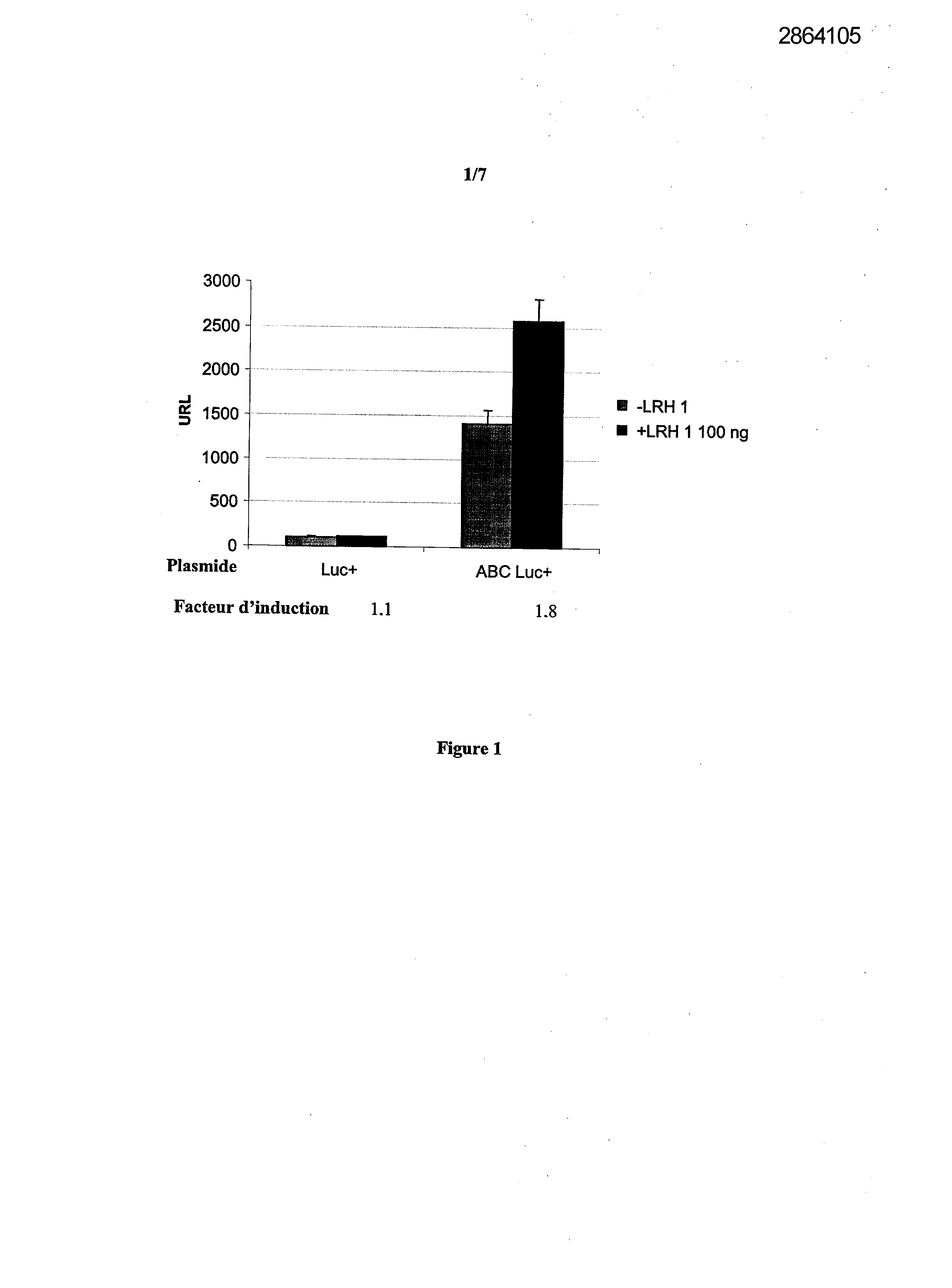
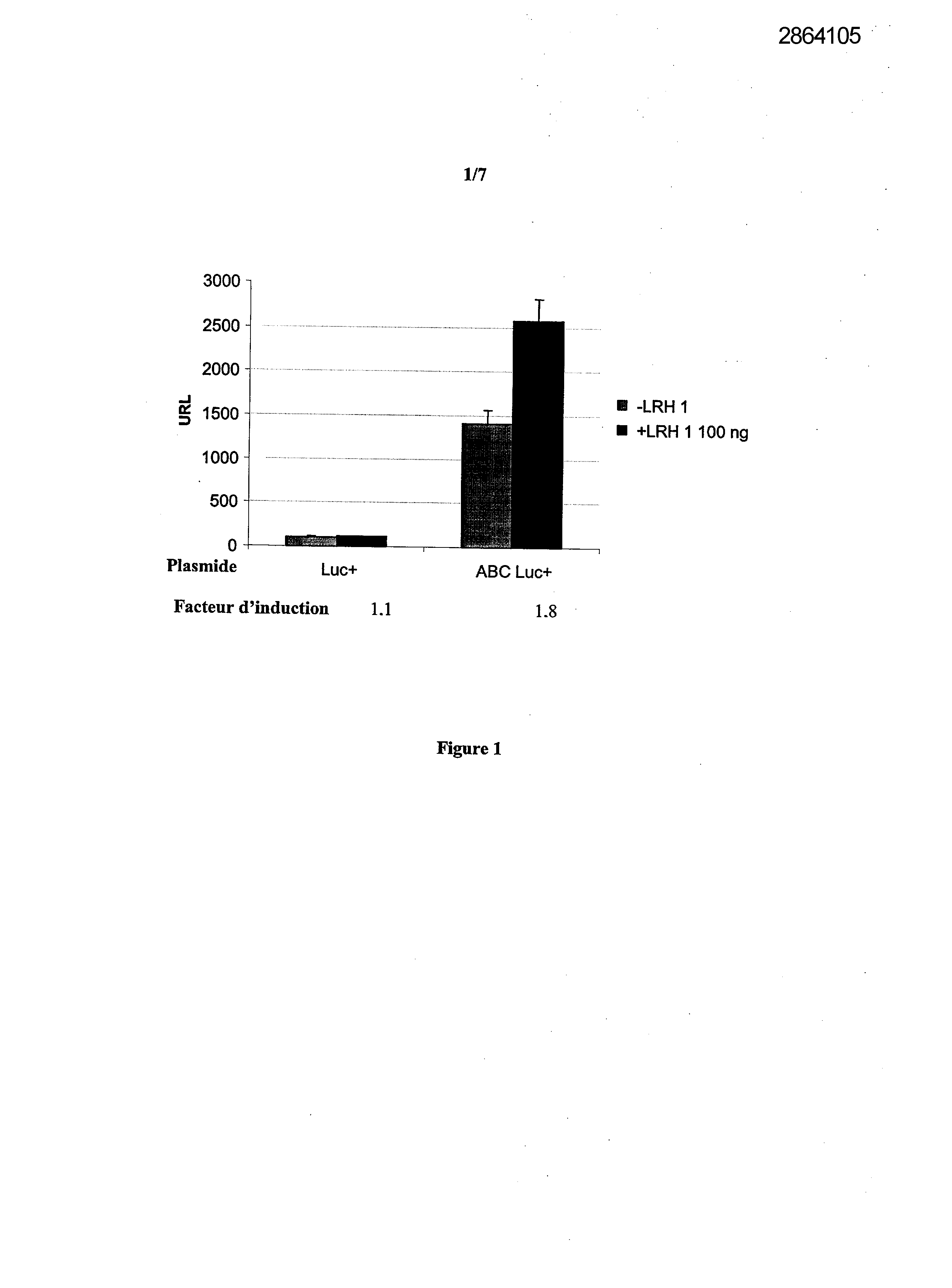
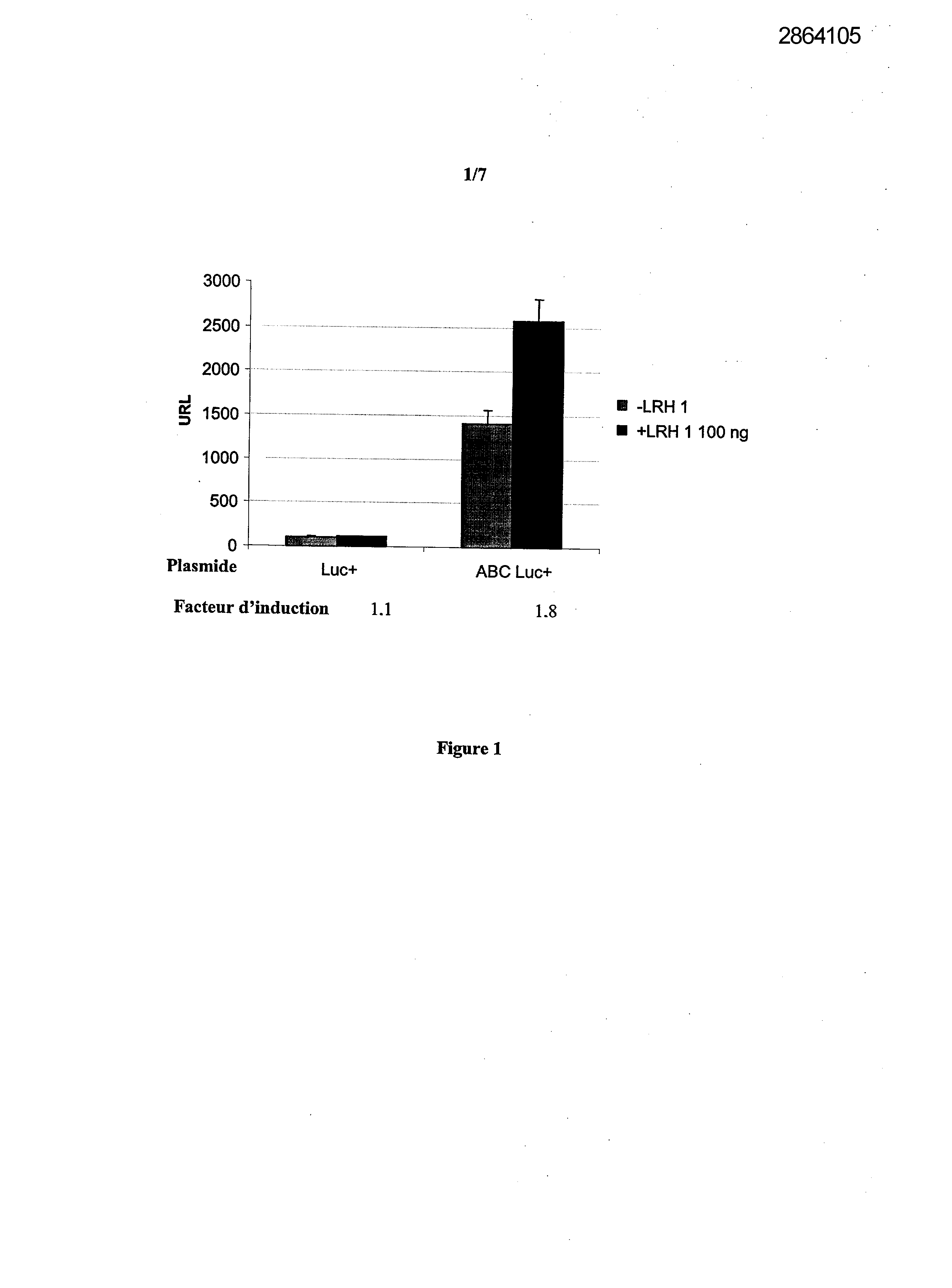
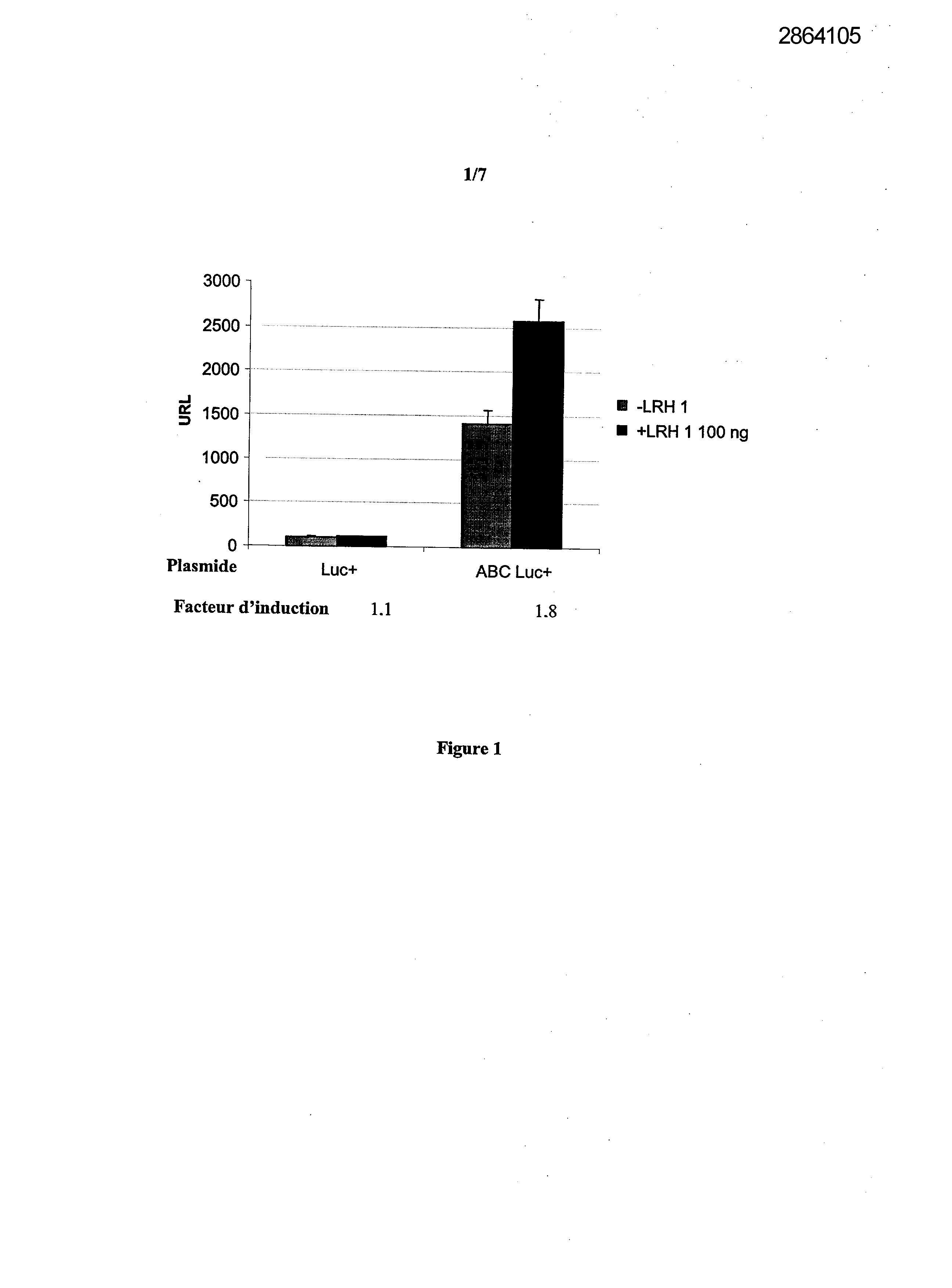
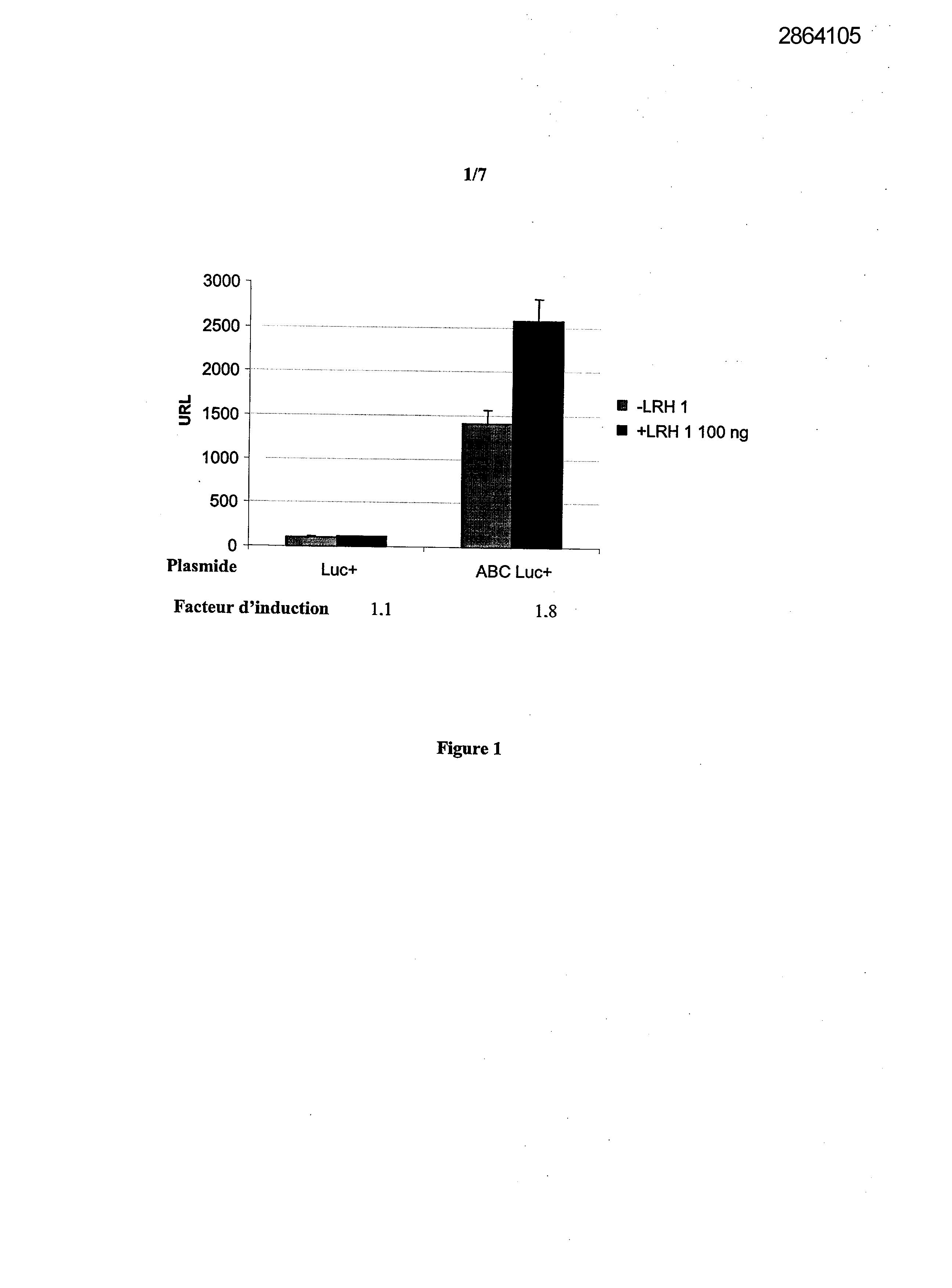
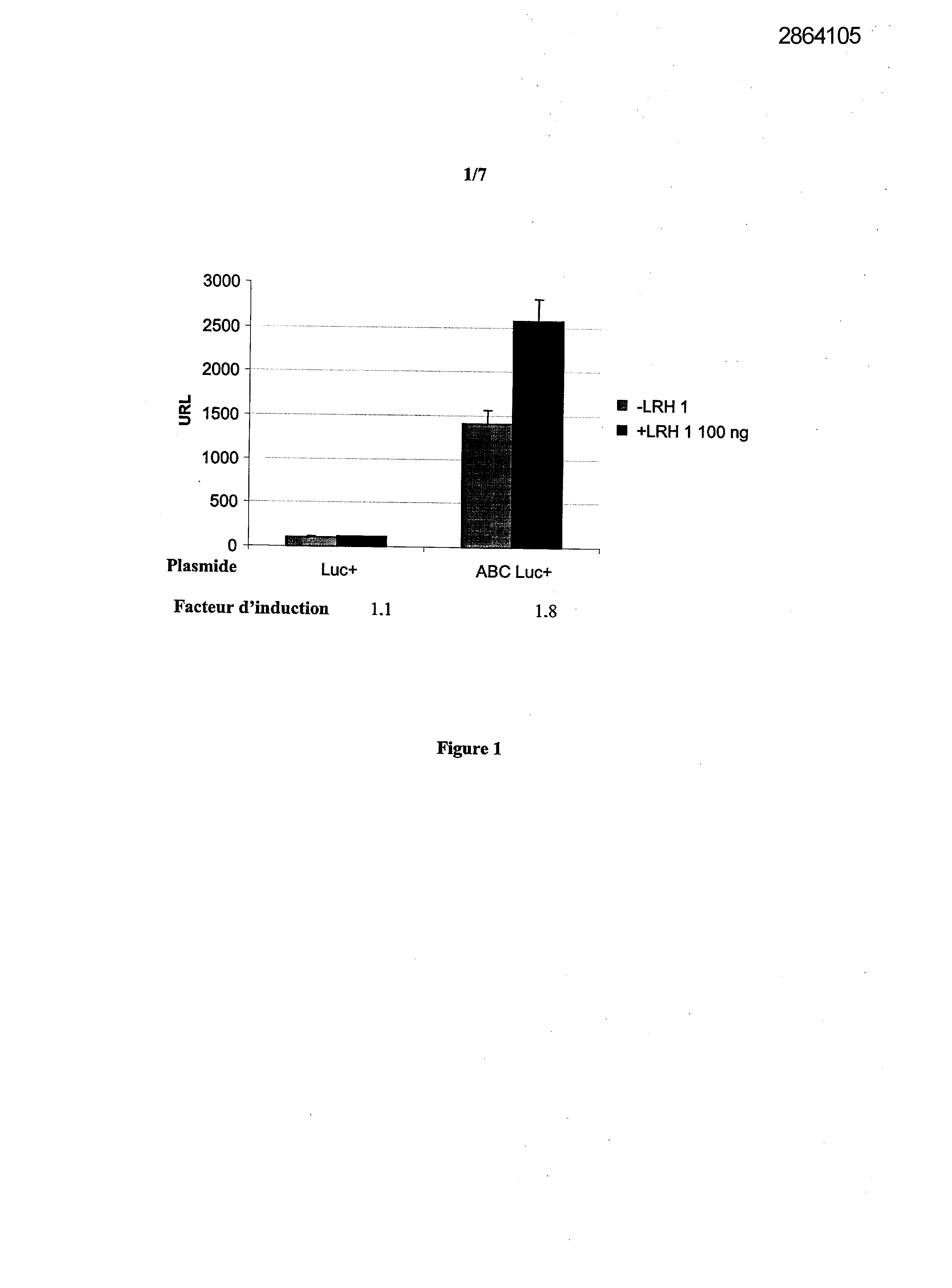
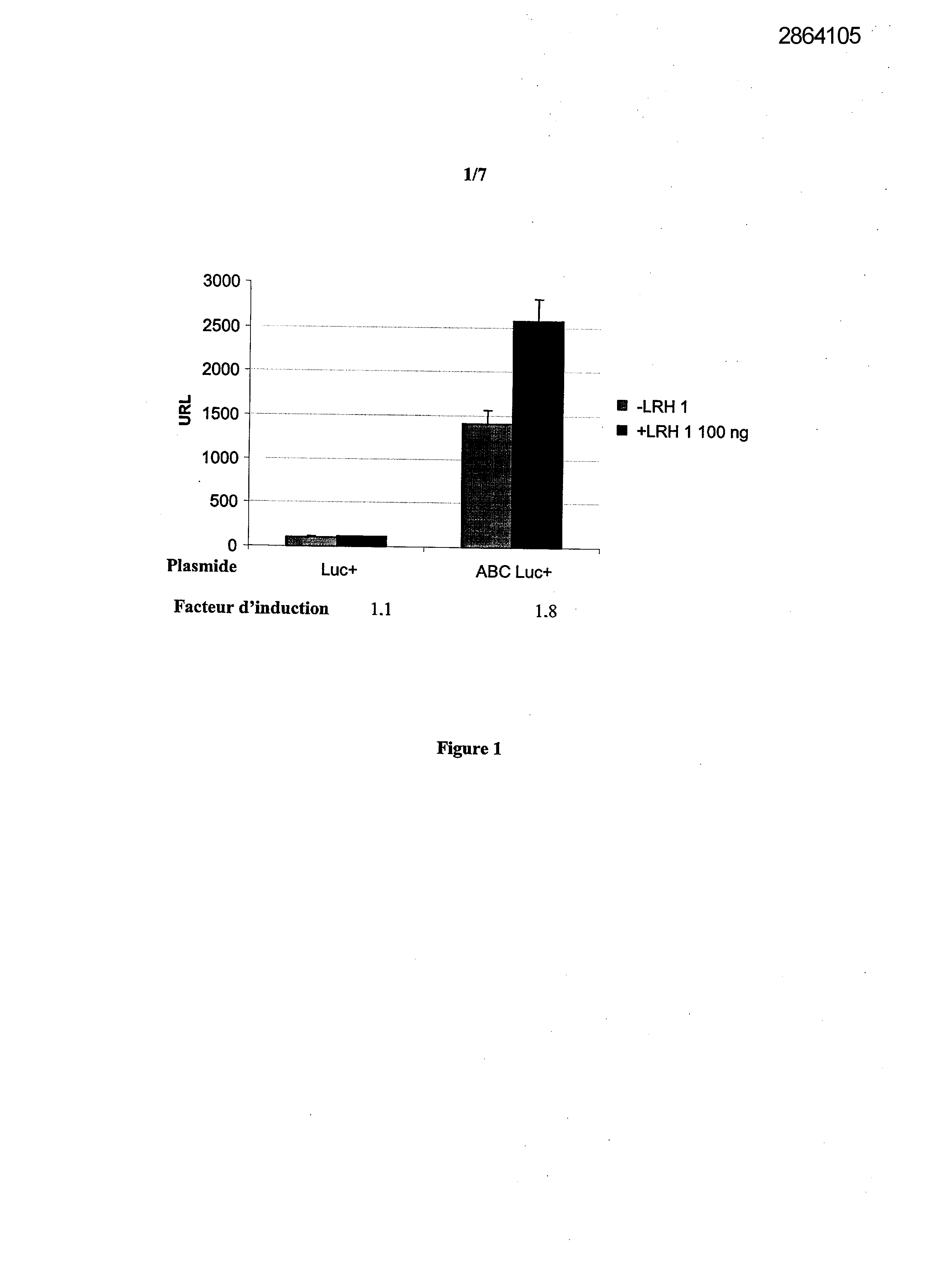
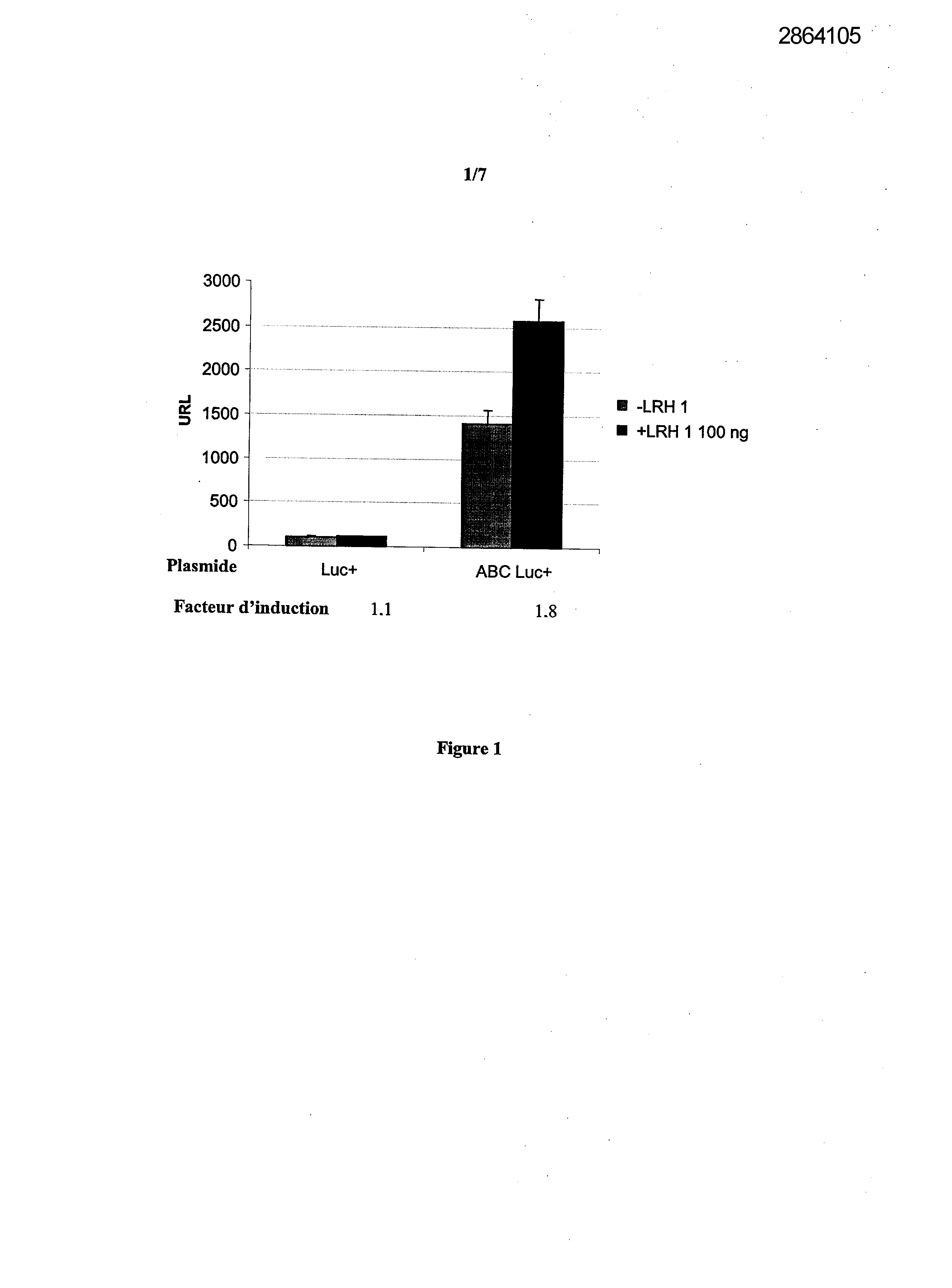
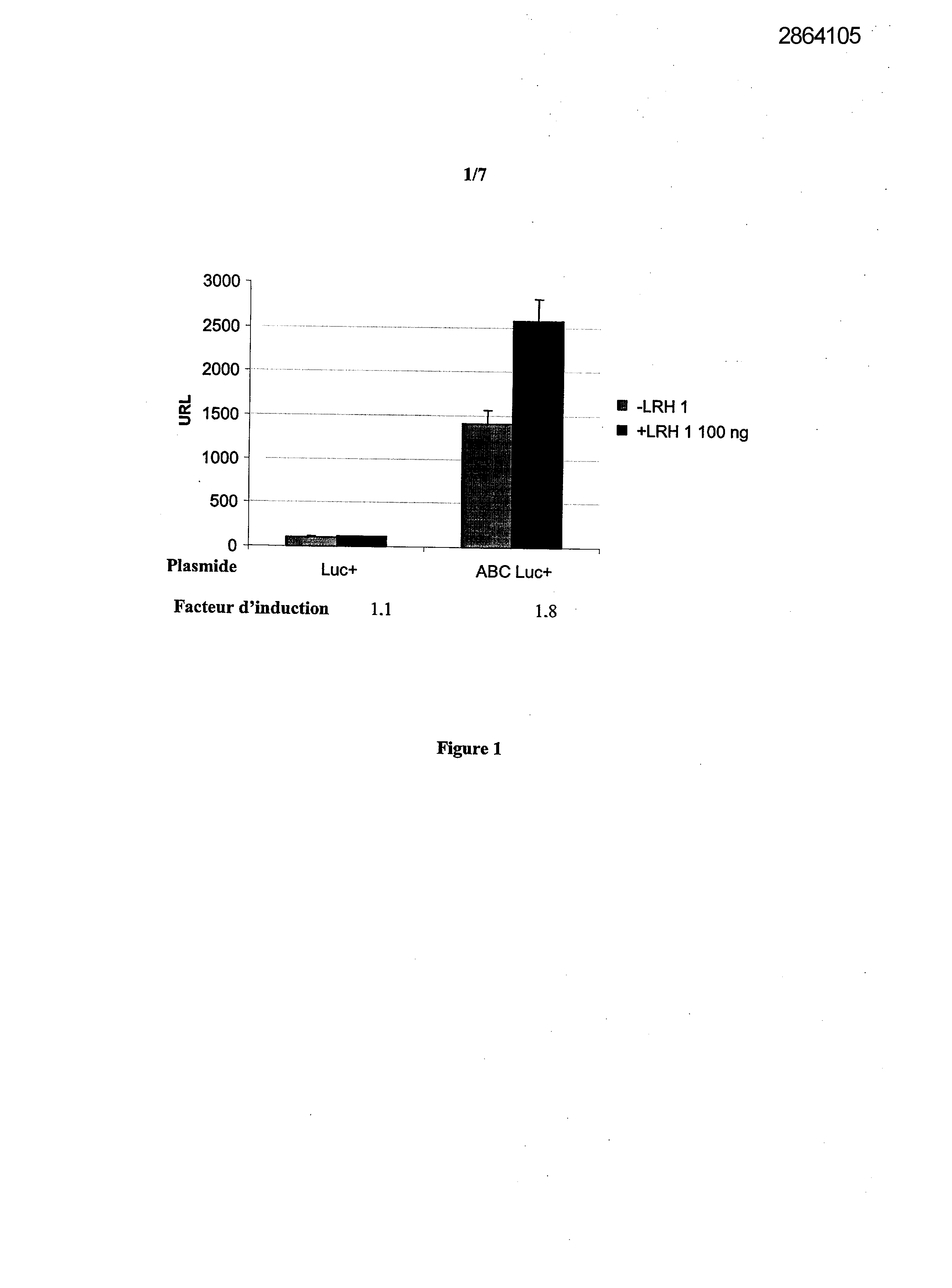
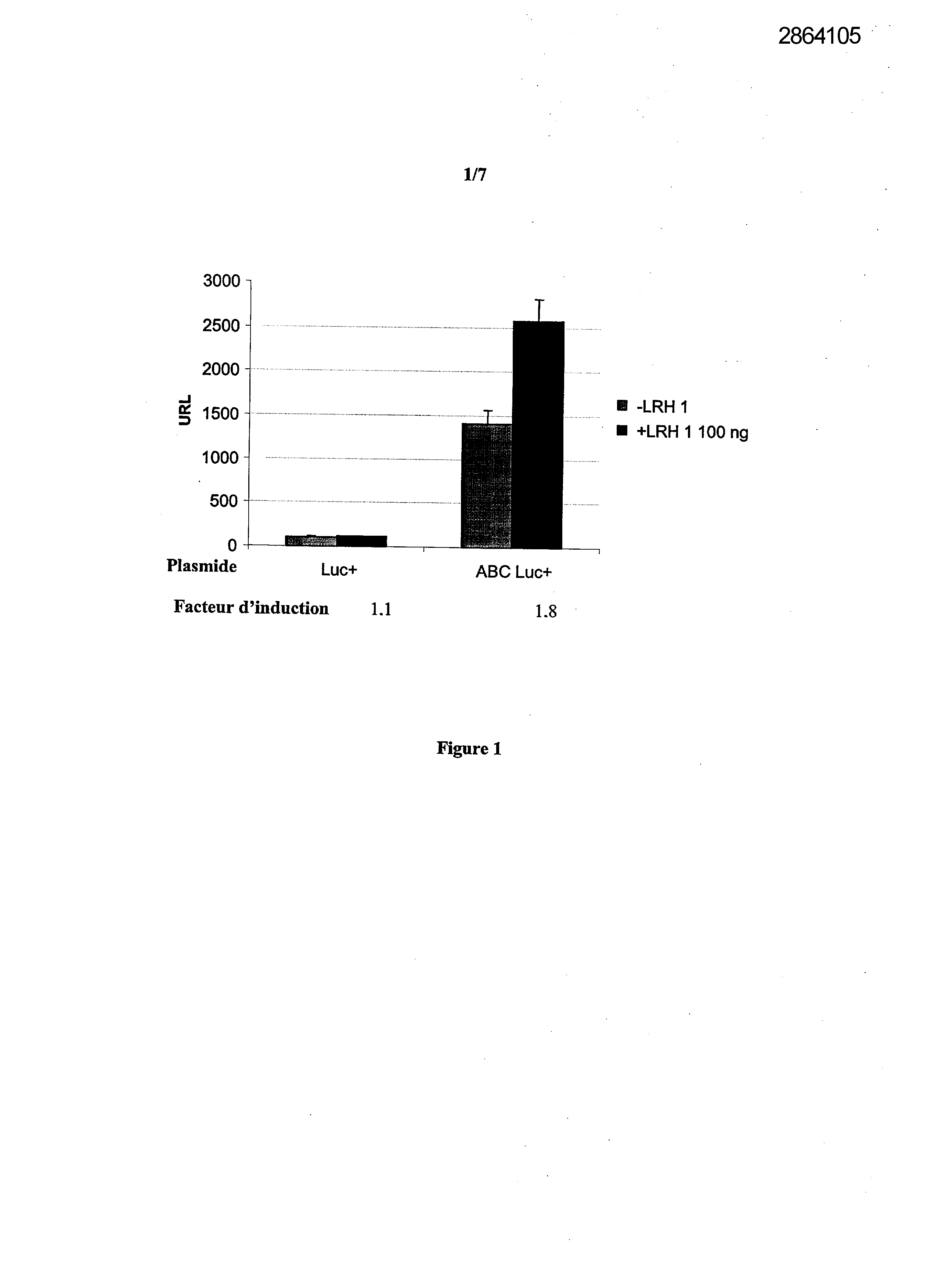
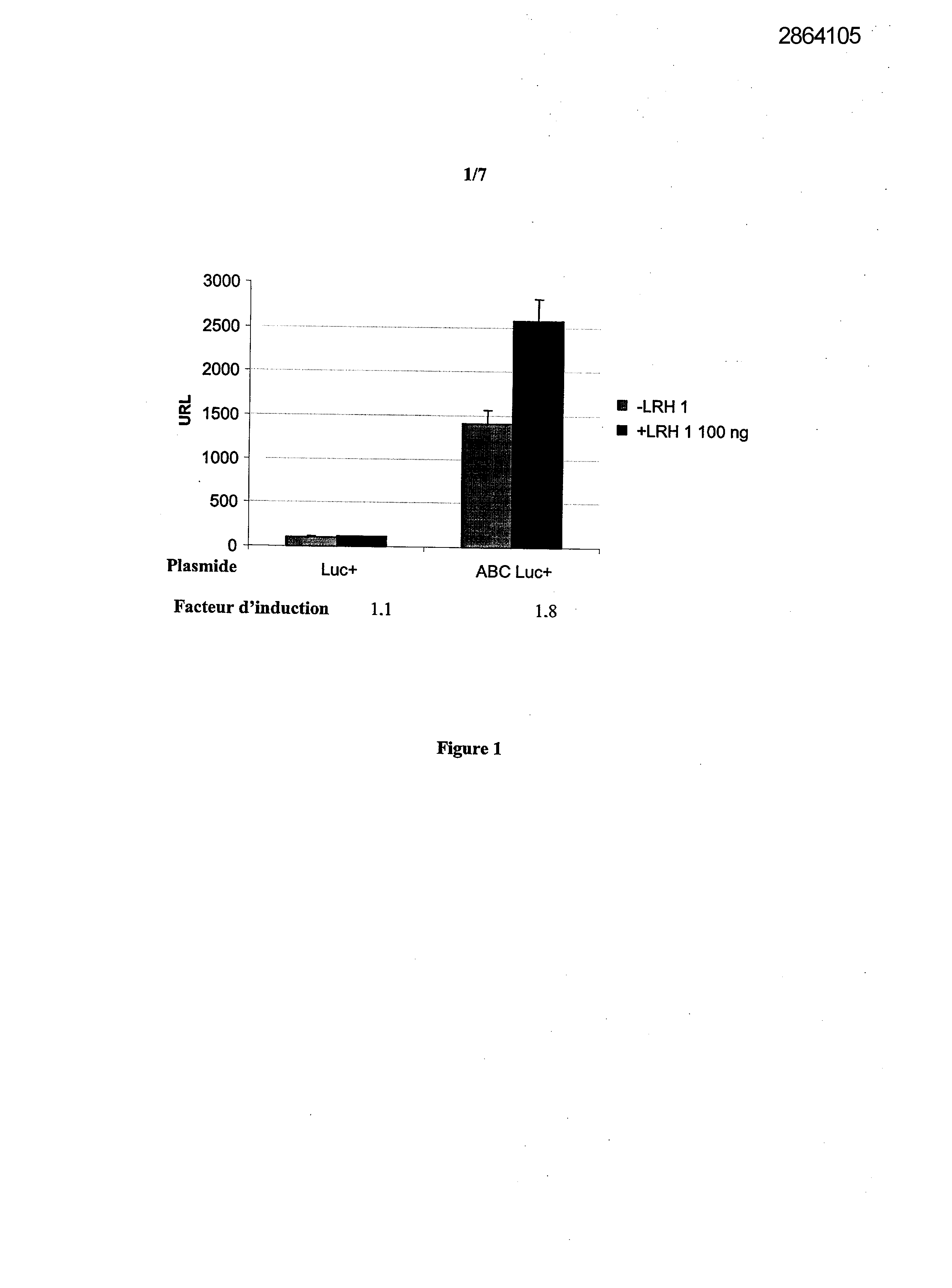
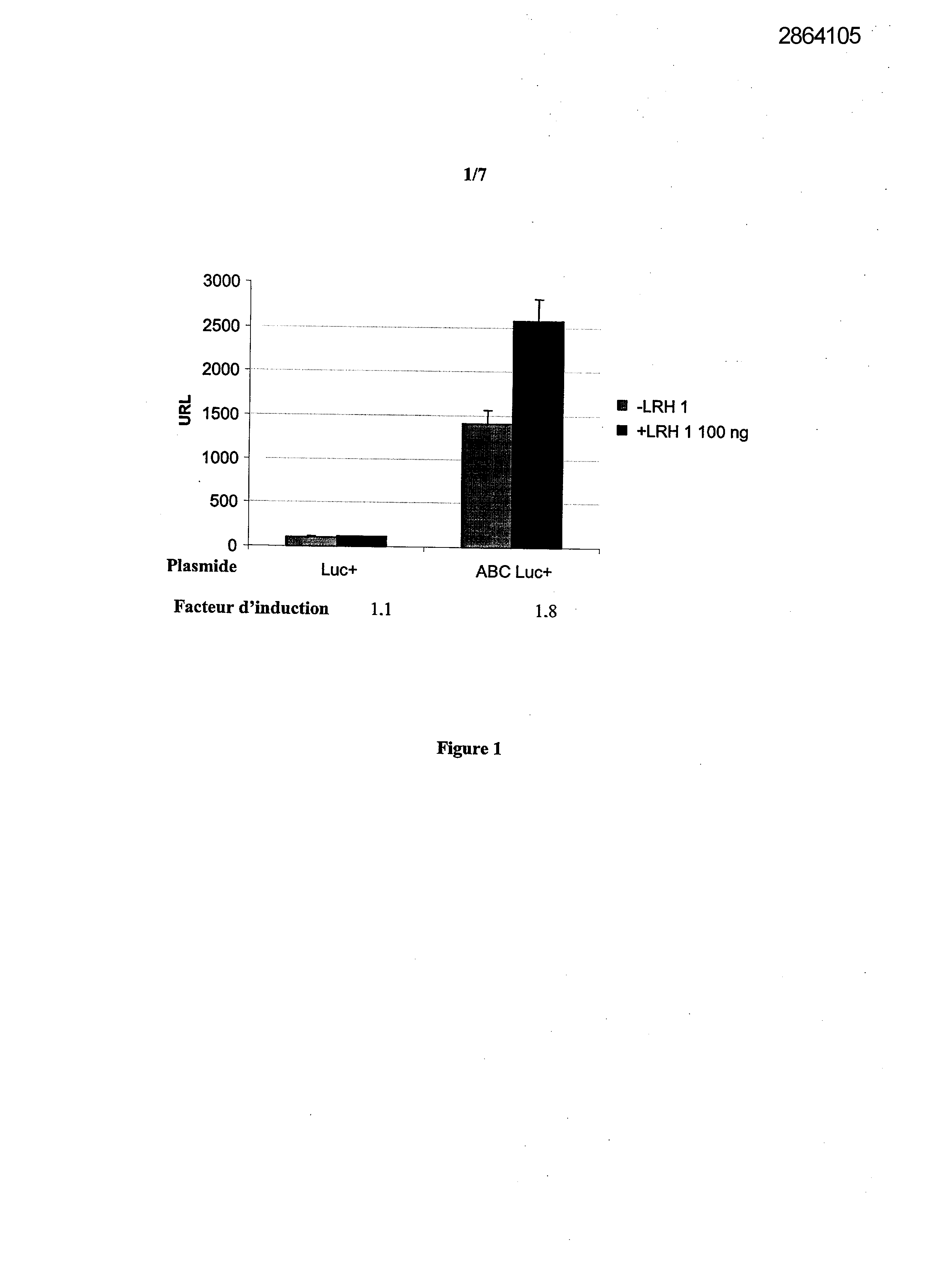
Selecting compounds that modulate reverse transport of cholesterol, useful e.g. for treating atherosclerosis, from their ability to bind to the liver receptor homolog-1 response element
Method for identifying compounds that modulate The present invention concerns methods and compounds capable of modulating reverse cholesterol transport in a mammal as well as screening methods for selecting, identifying and/or characterizing compounds capable of modulating reverse cholesterol transport. The invention further relates to cells, vectors and genetic constructs used for the use of these methods, and relates to pharmaceutical compositions for the treatment of atherosclerosis. Atherosclerosis is a major cause of morbidity, mortality, myocardial infarction, cerebral ischemia, and cardiovascular diseases of the peripheral vasculature. The cholesterol hypercholesterolaemia and overhead by macrophages, involved in vascular inflammation, are major factors that contribute to atherosclerosis. The hypercholesterolemia is currently treated by combining a diet and drug intervention with, for example, statins or bile acid sequestering agents. The development of new therapeutic strategies, it is necessary to alleviate the boundaries of the existing therapies. The reverse transport of cholesterol, implemented by the HDL ("High Density [...] The hormone nuclear receptors form a large family of transcription factors whose activity is modulated by natural ligands and/or artificial. The transcription factors control the expression of genes-targets in their binding generally to specific response elements acting in cis by recruiting proteins and accessories necessary for activation of the transcriptional machinery. The nuclear receptor LRH-1 (Liver Receptor [...] -1), also known as [...], CPF, [...], PHR or FTF is an orphan receptor ligand for which no have been identified. LRH-1 is a receptor homolog FTZ-F 1 drosophila the paralog which in humans is the receiver SF-1. At least two isoforms, from likely an alternating use of certain poly-adenylation sites, have been identified. Expression of LRH-1 is confined to the liver, the exocrine pancreas and to the intestine and the ovaries and the pre-adipocytes. LRH-1 is expressed early during embryogenesis [4,5]. LRH-1 does not form a heterodimer with RXR but binds, as a monomer, on a DNA response element sequence [...] wherein Y= C, R= G or T or A. Several target genes have been identified in the control of the synthesis or transport bile acids, steroid metabolism and lipoproteins [7,8] as well as in the transcriptional control or development. LRH-1 also appears to be involved in the development of the endodermis. The present invention is based on the observation the role of LRH-1 in the expression of the gene encoding human apolipoprotein AI (apo AI) as well as the direct interaction between LRH-1 and a fragment of the promoter of said gene. It is based also on the original observation of stimulation of activity of the promoter of the human apo Al, by the over-expression of LRH-1. It is also based on the identification of a response element functional LRH-1 at the junction regions B and C of the promoter of the gene for the human Apo AI (defined according to) and characterization of its sequence. The present invention thus demonstrates for the first time a modulating production of AI [...] LRH-1 by the nuclear receptor. The present invention thus provides novel targets and novel approaches for screening of compounds capable of regulating expression of this protein, HDL activity or reverse cholesterol transport. The invention also provides methods for increasing reverse cholesterol transport based on the use of compounds which modulate the binding of the promoter of the LRH-1 apo AI and/or the effect on the transcription of the human gene of the apo AI. The invention also provides screening methods for selecting, identifying or characterizing therapeutic substances capable of modulating the expression of the human gene of the apo AI and/or HDL activity and/or the reverse transport of cholesterol. In a particular embodiment, the assays according to the present invention include more particularly the following steps: -contacting one or more compounds with a nucleic acid construct comprising at least one response element LRH-1 of the promoter of the human gene of the apo AI or a functional variant thereof, -determining the optional bond upon the one or more elements) response, and -optionally comparing the previous measurement with a measurement carried out with the same conditions but with a nucleic acid construct comprising at least one mutated copy of a response element to LRH-1 of the promoter of the human Apo AI. Typically, said contacting is performed under conditions that could enable said compounds of binding to said response member, In a particular embodiment of the method of the invention, conditions that will allow said compounds become fixed to the member (s) comprise LRH-1 of response to the presence of the receptor LRH-1, exogenous in general, (for example as a monomer) or a functional equivalent, and determining the optional bond of said test compound on the response element LRH-1 and/or the complex formed by the binding of LRH-1 to its response element. In a specific embodiment (test transcriptional activity), comprises measuring the effect of one or more test compounds, optionally in the presence of the exogenous LRH-1 receiver or a functional equivalent thereof, on the transcriptional activity of a promoter comprising at least one response element LRH-1 according to the invention. Such testing is preferably made of cellular system, by determining the expression of a reporter gene placed under the control of such a promoter, especially in a cell comprising exogenous LRH-1 or an equivalent and/or comprising a ligand LRH-1 or a functional equivalent thereof. In another embodiment, the test is performed in a cell comprising (e. g., expressing, naturally occurring or recombinant) the receiver LRH-1 or a functional equivalent thereof. A preferred form of implementation of the invention is to use, optionally in the presence of exogenous LRH-1 or an equivalent, an expression cassette combining one or more response elements LRH-1, according to the invention, with a reporter gene. Advantageously, said reporter gene is placed under the control of a promoter comprising at least one copy of the response elements, for example, the promoter of the apo AI or variants or fragments thereof. Any gene known to those skilled in the art whose activity or presence in biological extracts is easily measurable can be used as a reporter gene for carrying out the screening method. The compounds which may be identified by the method of the invention can be of nature, structure and various origin, in particular biological compounds, nuclear factors, cofactors, chemical compounds, synthetic, etc, capable of affecting the activity of LRH-1. It may also consist in libraries, such as libraries or libraries of proteins, peptides or nucleic acids, such as clones encoding proteins or DNA-binding peptides. The methods of the invention can be used for selecting, identifying or characterizing compounds that can alter the binding of LRH-1 and/or of its cofactor one and/or the other of its response element (s) and/or modulating (i.e.. increase or decrease) the expression of the gene encoding the human Apo AI and expression of apo AI and/or modulating HDL activity and/or of modulating reverse cholesterol transport. The invention also encompasses the use of the compounds thus selected, in the preparation of a composition for modulating reverse cholesterol transport or HDL activity, and the corresponding methods of treatment. To facilitate understanding of the present application, the following definitions are provided, that specify or complete their usual meaning. " [...] AI" or apo AI:AI The apolipoprotein is a protein of 243 amino acids that contains a globular amino terminus and a carboxyl terminus which is capable of binding to lipid. This protein is a major constituent and high density lipoprotein plays a fundamental role in the reverse transport of cholesterol [10.11]. The gene, cDNA and mRNA apo AI have been cloned and sequenced [12-14], and are accessible on databases Genbank® (for example, over the Internet to the address: http ://www.ncbi.nlm.nih.gov) under the accession numbers: NM_000039, and J00098 M20656 (Promoter). "High Density Lipoprotein (HDL)": The HDL particles are high density lipoprotein (l, 063-l, g/ml) have a reputation for a protective role against atherosclerosis basically due to their ability to extract cholesterol peripheral cells and to promote its return to the liver where it is eliminated from. The apo AI is the major protein component of HDL, up to 70% of the proteins. They also provide apo Ail, apo IC, apo [...], apo CIII and apo E lower proportion. "LRH-1": The receiver LRH-1 has been isolated, characterized, and sequenced human and rat. The sequence of the mRNA is also available on databases Genbank®, under the accession numbers NM_003822, and NM_021742 NM_030676 to humans, the mouse and rat, respectively. The region of LRH-1 involved in the binding to ANDS ("DNA Binding domain") is predominantly in the range of between residues [...] - [...] human protein (corresponding to 319-546 in NM_003822) or between residues [...] - [...] of the mouse protein. Expression "functional equivalent" that references the receiver LRH-1, denotes any polypeptide derived from the structure of the receiver and LRH-1 maintaining the binding capacity response element, in particular, any response element sequence SEQ ID NO:1 or functional variants thereof. The functional equivalents may be natural variants (polymorphism, splicing, etc), fragments, mutants, deletion, etc. Preferably, it is of polypeptides comprising at least one region of amino acids identical to at least 60% than that of a receiver LRH-1, preferentially at least 75% and more preferably at least 90-95%. Expression also includes the receptor fragments LRH-1, in particular the fragments containing the DNA binding site LRH-1 receptor. The term "reverse transport" is used to designate the physiological mechanism, sometimes failed, by which the excess cholesterol in the peripheral tissues is supported by high-density lipoprotein, the HDL (High Density Lipoprotein), and transported to the liver to be removed. A. identification of a response element LRH-1. The present invention shows the implication and the mechanism of action of LRH-1 in regulating expression of apo AI and, thereby, in the regulation of reverse transport of cholesterol. Overexpression of LRH-1 results in an increase in the activity of the promoter of the apo AI. Disclosed is, further, the precise sequence of the response element LRH-1, within the promoter of the gene coding for the human Apo AI. The invention also relates to particular constructions, in particular nucleic acids comprising responsive elements LRH-1, as well as cassettes, vectors and recombinant cells containing them. Thus the invention provides the sequence (SEQ ID NO:1) response element LRH-1, originally identified within the promoter of the human gene of the apo AI, responsible for an interaction between LRH-1 and the promoter apo AI and regulation by LRH-1 of the expression of Apo AI. 3 different functional regions have been identified within the promoter of the apo AI. In the present document, the functional regions of the promoter of the gene for [...] A-I are named A, B and C according to the previously defined. Therefore, the presence of the B and C (SEQ DD NO:3 and 4) causes an increase of LRH-1 the expression of a reporter gene (see: examples 1.2,5 and 6). A particular object of the invention is a nucleic acid comprising the sequence SEQ ID NO:1 following: 5 '- [...] -3', or a functional variant thereof ("LRH-1 response element"). Another object of the invention is a nucleic acid construct comprising a response element LRH-1 as defined above. Which may include an expression cassette comprising at least one copy of a response element such as defined above. The invention also relates to any artificial promoter or chimeric comprising a response element LRH-1 as defined above. Functional variants response element according to the invention, may be any derivative or fragment of the native sequence maintaining the ability to bind the receiver LRH-1. Typically, the variants retain at least 50% of the residues of the native sequence described herein. Conventionally, the variants have changes in less than 5 nucleotides in the sequence considered. Preferably, it is a sequence identical to at least 60%, preferably at least 75% and more preferably at least 90% to the native sequence described herein. The variants may comprise different types of modifications such as one or more point mutations or not, additions, deletions and/or substitutions. These changes can be introduced by conventional methods physical, chemical or molecular biology, such as, for example, site-directed mutagenesis or, more precisely, by artificial synthesis of the sequence in a synthesizer. The variants may be tested for the ability to bind to LRH-1 in different ways, and in particular: (i) by contacting the test sequence with the receiver LRH-1 (for example in a test cell-free), and detecting the formation of a complex (for example by retarding gel migration); (ii) by inserting the test sequence in an expression cassette comprising a minimal promoter and a reporter gene, placement of the cassette into a cell, and detection (optionally assay) of the expression of the reporter gene in the presence and absence of LRH-1; (iii) any known technique to those skilled in the art, for detecting the interaction between nucleic acid and protein, for example. The invention also concerns variants inactive response elements defined above, particular variants substantially incapable of binding the receiver LRH-1. Examples of such variants are particularly the sequence SEQ ID NO:2. These inactive variants can be prepared and tested under the conditions described above for the functional variants. The variants of the invention advantageously have the ability to hybridize with the sequence SEQ ID NO:1 or a portion thereof. The invention provides methods for identifying compounds that modulate (i.e., increase or decrease) the reverse transport of cholesterol. The compounds may act by manipulating the binding of LRH-1 with its ligands or corepressor and with its coactivator, etc. They can still modify, or elimination of, the binding of LRH-1 alone or LRH-1 and its cofactors, with its elements) response and thereby alter the expression of the gene of the human apo AI. Attaching LRH-1 response element present at the junction regions B and C of the promoter of the [...] AI (SEQ ID NO:3 and 4) increases the transcription of the human gene of the apo AI and stimulates the reverse transport of cholesterol. The use of compounds capable of enhancing the uptake of LRH-1 to the response element, wherein LRH-1 acts as activator therefore, improving the transcription of the human gene of the apo AI and stimulate the reverse transport of cholesterol. The invention provides novel methods for selecting, identifying or characterizing compounds capable of increasing reverse cholesterol transport. The present invention provides a method for selecting, identifying or characterizing compounds capable of modulating reverse cholesterol transport, which comprises: (i) contacting a test compound with a host cell comprising an expression cassette of a reporter gene, said cassette comprising a reporter gene placed under the control of a promoter containing at least one copy of a response element LRH-1 of the promoter of the human gene of the apo AI or a functional variant thereof, and (ii) determining the expression of the reporter gene. The present invention provides a method for selecting, identifying or characterizing compounds capable of modulating reverse cholesterol transport, which comprises: (i) contacting, in the presence of the exogenous LRH-1 receiver or a functional equivalent thereof, of a test compound with a host cell comprising an expression cassette of a reporter gene, said cassette comprising a reporter gene placed under the control of a promoter containing at least one copy of a response element LRH-1 of the promoter of the human gene of the apo AI or a functional variant thereof, and (ii) determining the effect of the presence of the test compound on the binding of LRH-1 response element or on the expression of the reporter gene. The methods of the invention, provide more specifically contacting a test compound with a nucleic acid construct or an expression cassette comprising at least one copy of a response element LRH-1 (SEQ ID NO:1). A particular object of the invention provides an expression cassette comprising at least one copy of the nucleic acid fragment SEQ ID NO:1, and a promoter associated with a reporter gene placed under the control of said promoter. Another particular object of the invention provides an expression cassette comprising at least one copy of the mutated nucleic acid fragment SEQ ID NO:1, and a promoter associated with a reporter gene placed under the control of said promoter. According to a particular embodiment of the invention the methods of the invention, there is further provided a comparing potential effects, determined by one of these methods with those, optional, determined by a method carried out with the same conditions but with a nucleic acid construct comprising at least one variant inactive (for example, a mutated copy) of a response element to LRH-1 of the promoter of the gene encoding the human Apo AI (SEQ ID NO:2) or a functional variant thereof. The methods of the invention may be implemented with different types of cells, promoters, of reporter genes, and under different conditions, as described below. Certain methods of screening, described by the invention, provide a step of contacting the test compound, optionally in the presence of the exogenous LRH-1 receiver or a functional equivalent thereof, with host cells, in particular conditions for determining the expression of a gene rapporteur and thereby obtain information about the effect of the test compound. Preferably, The receiver LRH-1 is introduced or added artificially to have at least 2 times the amount of endogenous LRH-1. It may be a LRH-1 equivalent in other words any amino acid sequence identical to at least 60% than that of a receiver LRH-1, preferentially at least 75% and more preferably at least 90-95%. Conventionally, the effect of the test compound is compared to the level of expression of the reporter gene measured in the absence of said compound (and/or with a mutated response element). These cells, in a preferred embodiment of the invention, can be mammalian cells (hepatocytes, fibroblasts, endothelial cells, muscle, etc). Even more preferably, the cells may be human cells. 11 may also be primary cultures or established lines. In another embodiment, it can also be used (bacteria) prokaryotic cells, yeast cells (Saccharomyces, Kluyveromyces, etc), plant cells, etc. The compounds may be contacted with the cells at different times, according to their effect (s) (s), their concentration, the nature of the cells and assessment technique. The contact may be performed on any suitable carrier and in particular on a plate, in a tube or a flange. Typically, the contacting is carried out in a multiwell plate which enables, in parallel, numerous and varied assays. Among the typical supports include microtiter plates and more particularly to 96 or 384 well plates (or more), easy to handle and on which the developing can be achieved by conventional pacing. According to the support and the nature of the test compound, varying amounts of cells can be used when implementing the disclosed methods. Conventionally, 103 to 106 cells are contacted with a type of test compound, in a suitable culture medium, and preferably between4 and 10 105 cells. Exemplary, in a 96 well plate,s 10 cells can be incubated in each well with a desired quantity of a test compound. In a 384 well plate,5 and less than 10 cells typically between [...]4 and 4xl04, cells are incubated in each well with the test compound. The amount (or concentration) of test compound can be adjusted by the user according to the type of compound (its toxicity, its ability to cell penetration, etc), the number of cells, the length of the incubation period, .etc Typically, the cells are exposed to quantities of test compounds which vary [...] to [...]. It is of course possible to test other concentrations without departing from the present invention. Each compound can be tested in parallel with different concentrations. Different adjuvants and/or carriers and/or products for the facilitated penetration of the compounds into cells such as liposomes, cationic lipids, polymers, of the penetratin, Tat PDT, peptides derived from adenovirus (penton or fibres) or other viruses, etc can also be used if necessary. The contact is maintained between 5 and 72 hours, generally between 12 and 48 hours. Indeed, the cells and the various reagents must preferably remain in contact for a time sufficient to allow the de novo synthesis of the expression product of the reporter gene. Preferably, the incubation lasts about 36 hours. The method proposed by the invention for selecting, identifying or characterizing compounds capable of modulating reverse cholesterol transport transformed host cells with an expression cassette of a reporter gene. The reporter gene can be any gene whose transcription or expression product can be detected or assayed in biological extracts. It can be, for example, of the gene coding for the human Apo AI itself, or the gene encoding the luciferase and more particularly for firefly luciferase or those [...], for secreted alkaline phosphatase, galactosidase, the lactamase, the [...] acetyl transferase (CAT), human growth hormone (hGH), p-glucuronidase ( [...] ) and Green fluorescent protein (GFP) etc. It is understood that the term "gene" denotes, in the broadest sense, any nucleic acid, in particular a cDNA, a gDNA, synthetic DNA, an RNA, etc. The reporter gene, any, is placed under the control of a promoter comprising at least one copy of a response element LRH-1 as defined above. The reporter gene can be placed under the control of any promoter whose sequence comprises the sequence SEQ ID NO:1 or a functional variant thereof. The particular sequence may be present in one or more copies in the promoter (preferably 1 to 10 and even more preferably 1 to 6), upstream, downstream or internally, in the same orientation or in the opposite orientation. Preferably, it is a promoter whose activity the differential in the absence and presence of LRH-1 or a functional equivalent can be detected. To form a promoter of the invention, the response element LRH-1 may be associated with a transcriptional minimal promoter. The minimal promoter is a transcriptional promoter having a low or no basal activity, and can be enhanced in the presence of a transcriptional activator (the interaction of LRH-1 with the junction regions B and C). A minimal promoter can be a promoter naturally low in mammalian cells, i.e. generating expression non-toxic and/or non-sufficient to obtain a pronounced biological effect. Advantageously, a minimal promoter is a construct prepared from a native promoter, by deletion of non-essential region (s) ^) to the transcriptional activity. Therefore, it is preferably a promoter comprising essentially a TATA box, generally of a size of less than 160 nucleotides, centered around the initiation codon of the transcription. A minimal promoter can be prepared from viral promoters, cellular, strong or weak, such as, for example, the promoter of the gene for thymidine kinase (TK) herpes virus, the CMV immediate early promoter, the PGK promoter, the promoter of the gene encoding human apolipoprotein AI, the SV40 promoter, etc. The minimal promoter may have an activity sufficiently high to identify compounds that increase activation by LRH-1, via the regions B and C for example. The promoter (P), the response element LRH-1 (ER) and the reporter gene (GR) are arranged operatively in the expression cassette, i.e. so that the minimal promoter controls the expression of said gene, and activity controlled by LRH-1. Typically, these regions are therefore arranged in the following order, in the orientation 5 '-> 3': ER-P-GR. However, any other functional arrangement may be contemplated by those skilled in the art without still of the present invention. Furthermore, the different functional domains above can be bonded directly to one another, or separated by nucleotides not significantly affecting the functionality of the expression cassette or for providing improved performance characteristics or the system (amplifier, silencing, intron, splicing site, etc). The selection method, for identifying and characterizing compounds capable of modulating reverse cholesterol transport provided a step of determining the expression of the reporter gene. It may be a determination of the transcriptional activity. To this end, total RNA is extracted from the cells in culture under experimental conditions on the one hand and in a control situation on the other hand. This RNA is used as a probe to analyze, for example, changes in expression of the gene (s) rapporteurs). It can also be a developing the expression of the reporter gene using a suitable substrate. The developing can be achieved using various techniques whose nature depends on the type of reporter gene used. The measurement may, for example, correspond to an optical density, in a fluorescent emission or luminescent in the case of use as a reporter gene of the gene encoding p-galactosidase or luciferase. In one particular embodiment, the expression of the reporter gene is measured across the level of hydrolysis of a substrate of the expression product of the reporter gene. For example, many substrates may be used to assess the expression of the P-lactamase. This can involve any product containing a core and P-lactam hydrolysis of which can be controlled. Preferred substrates are those specific p-lactamase (i.e., they are not typically hydrolyzed in mammalian cells in the absence of [...] ), those that are not toxic to the cells of mammals and/or whose product of hydrolysis can be controlled easily, for example by methods based on fluorescence, radioactivity, enzymatic activity or any other method of detection. Substrates even more preferred are the ratiometric substrates. Hydrolysis of these substrates can be connected directly to the activity of the expression product of the reporter gene by the number of cells. Ratiometric A substrate specific and non-toxic for use in the present invention is the CCF2-AM. The concentration of the substrate can be adjusted by those skilled in the art depending on the number of cells, for example. The cells are generally maintained in contact with the substrate during about 60 minutes. The presence of the reporter gene product (or the product of hydrolysis of the substrate) can be determined by conventional methods known to those skilled in the art (fluorescence, D. O., luminescence, FRET (see WO 0037077), SP A, biochips, immunological methods, etc). Typically, ascertaining the activity of a test compound in a cell and this is compared to the level of activity in the absence of test compound or an average value determined in the absence of any test compound. Measuring the hydrolysis involves essentially a measure (or determining the relative amount) of the hydrolysis product contained in each reaction sample. The measurement can be carried out by various techniques known to those skilled in the art, including detection of fluorescence, of radioactivity, color, enzymatic activity, of a immune antigen-antibody complex, etc. Preferably, the product of hydrolysis is detected and quantified through a technique of fluorescence detection. The various fluorochromes can be used and controlled on samples of cells. A secondary test for validating, in the animal, selecting compounds, also can be made by determining the amount of HDL expressed or by determining a significant variation of the reverse transport of cholesterol to cells treated with said compounds as compared with untreated cells. It is also possible to measure the cholesterol level in plasma and/or to determine the expression of hepatic apo AL In a preferred embodiment of the invention, the host cell also includes a ligand LRH-1. The designation "ligand LRH-1" also applies to transcription factors, with the co-activators and co-repressors, and other polypeptides involved in the regulation of gene expression. It can be, for example, or other receptors as RXR the nuclear hormone receptors. In another preferred embodiment of the invention, are used in the methods of the invention, and as aforesaid, a host cell comprising the receiver LRH-1 or a functional equivalent. LRH-1 The presence of the receiver it is possible to reproduce a situation and physiological identifies, by the methods previously described, compounds capable of modulating interactions between LRH-1 and one and/or the other of its response element (s), such (s) (s) as disclosed by the present invention, or between LRH-1 and one or more ligands of LRH-1 (s). The methods allow the determination of the level of expression of the reporter gene, according to one of the techniques known to those skilled in the art described previously, in the presence of the test compound and/or in the absence of said compound, an increase or a decrease in the level of expression of the reporter gene indicating the capacity of the test compound to modulate reverse cholesterol transport. The invention therefore can be implemented with a construct, of a cassette or a cell according to the invention used for in vitro screening of compounds capable of modulating HDL activity. As described above, these methods permit the screening, rapid and in parallel, many test compounds on one or more cell populations (mammalian cells, human cells such as, for example, hepatocytes, prokaryotic cells, etc). The methods are predictive, automatable and adapted for selecting, the identification and characterization of the compounds. A particular form of implementation the screening method uses the conventional methods of identification of clones that express proteins that bind DNA. For example, screening cDNA expression libraries in [...] 1 or using the method termed "One Hybrid" or "phage display", or form an affinity chromatographic purification. The or the protein (s) isolated (s) are then sequenced. The invention also provides a method for selecting, identifying or characterizing compounds capable of modulating (i.e. increase or decrease) the reverse transport of cholesterol, based on the measuring the binding of a test compound to one or more response elements. The method more particularly comprises: -contacting a test compound with a nucleic acid construct comprising at least one copy of a response element LRH-1 of the promoter of the human gene of the apo AI or a functional variant thereof, and -determining the optional bond said test compound to the response element. Another method of the invention comprises: -contacting, in the presence of the exogenous LRH-1 receiver or a functional equivalent thereof, a test compound with a nucleic acid construct comprising at least one copy of a response element LRH-1 of the promoter of the human gene of the apo AI or a functional variant thereof, and determining the binding of the test compound on the and/or element (s) of the response to LRH-1 and/or the complex formed by the binding of LRH-1 at its and/or element (s) response. A preferred embodiment of the invention establishes the ability of said test compound to modulate binding of LRH-1 response element, determining the amount of LRH-1 linked in the presence of the test compound relative to the amount in the absence of the test compound. A competition test using FF (Fluorescence Polarization) known to those skilled in the art, thus, can be efficiently in this determination. A test compound capable of modulating the binding of LRH-1 response element may be subsequent testing of its ability to modulate the expression of a reporter gene and/or reverse cholesterol transport, according to any one of the methods described previously. The binding of the test compound on at least one of the response elements LRH-1 can be detected by a gel migration, by electrophoresis of the heterodimers formed as a result of implementing the method described above. Some test compounds are effectively capable of carrying a DNA binding site largely identical with that of LRH-1 and thereby exert a competition therewith. The electrophoresis directly distinguishes heterodimers LRH-1/response element LRH-1, heterodimers test compound/response element LRH-1 and responsive elements LRH-1. Other methods based on luminescence or using FRET (Fluorescence Resonance Energy Transfer) well known to those skilled in the art or technique SPA (Scintillation Proximity Assay), can be carried out in the frame of the present invention to determine the optional bond of the test compound on one and/or the other (s) of response to LRH-1. In one particular embodiment, the nucleic acid construct comprises at least 1 copy, preferably 2 to 5 copies of the sequence SEQ ID NO:1 or a functional variant thereof. The test compounds capable of activating (i.e. increase at least partially) the binding of LRH-1 on the structure make it possible to activate the expression of the reporter gene are candidates and to stimulate the reverse transport of cholesterol. The methods described before for selecting, identifying or characterizing compounds capable of modulating the expression of a reporter gene and/or reverse cholesterol transport can, according to another embodiment of the invention, be used for selecting, identifying or characterizing compounds capable of modulating HDL activity and/or expression of apo AI. D. Compounds test. The present invention can be applied to any type of test compound. Therefore, the test compound may be any product in isolated form or in mixture with other products. The compound can be defined in terms of structure and/or composition or may not be defined. The compound can, for example, be an insulated product and structurally defined, an insulated product of indefinite structure, a product mixture known and characterized or indefinite a composition comprising one or more products. One or more compounds can be tested, as a mixture or separately. Indefinite Such compositions can be, for example, tissue samples, biological fluids, cell supernatants, plant preparations, etc. The test compounds may be products inorganic or organic and in particular a polypeptide (or a protein or peptide), a nucleic acid, a lipid, a polysaccharide, a chemical or biological compound such as a nuclear factor, a cofactor or any mixture or derivative thereof. The compound can be of natural or synthetic origin and include a combinatorial library, a clone or a library of nucleic acid clones expressing one or more polypeptide (s) DNA binding, etc. The present invention is particularly suitable for selecting, identifying or characterizing a large number of compounds for. The simple and efficient screening can be accomplished within a very short period of time. Described methods may in particular be partially automated, thereby allowing for the efficient simultaneous screening of compounds and various and sundry, or in the form of a mixture or separately. E. The use of the compounds identified. The compounds identified in accordance with the invention have beneficial properties for use in therapy, in particular in the field of atherosclerosis. The invention thus provides for the use of a compound capable of modulating (i.e., increasing or decreasing) the binding of LRH-1 to the response elements of the promoter of the gene encoding the human Apo AI or a functional variant thereof, for the preparation of a composition for modulating (i.e., increasing or decreasing) reverse cholesterol transport. In another embodiment of the invention, the use may be for modulating (i.e., increasing or decreasing) HDL activity or to modulate the expression of apo AI. Another embodiment of the invention contemplates the use of a compound capable of modulating (i.e. increase or decrease) the effect of LRH-1 on the transcription of the human gene of the apo AI or a functional variant thereof, for the preparation of a composition for modulating (i.e. increase) reverse cholesterol transport and/or to modulate (i.e., increase or decrease) the activity of HDL. According to a preferred embodiment of the invention it is a chemical compound or a biological compound. In another preferred embodiment, it is a nuclear factor or a cofactor. In even more preferred, it is a clone expressing one or more polypeptide (s) DNA binding. Generally, it is any compound selected, identified or characterized according to one of the methods previously described. The invention includes the use of any compound (or derivatives thereof) selected, identified or characterized according to one of the methods previously described, in the present invention, as a target or experimental search for the manufacture of pharmaceutical compositions for increasing reverse cholesterol transport or treating hypercholesterolemia, atherosclerosis, lipid disorders and/or cardiovascular disorders, as well as said pharmaceutical compositions. Other advantages and applications of the present invention will appear when reading the examples that follow, which must be considered as purely illustrative and not restrictive. Figure 1: Effect of the over expression of LRH-1 on the activity of the promoter of the human Apo AI in HepG2 cells (URL: relative luminescence unit). Figure 2: Effect of the over expression of LRH-1 on the activity of the promoter of the human Apo AI in RK13 cells (URL: relative luminescence unit). Figure 3: Delay gel showing the identification of a response element LRH-1 included at the junction of the region and B C of the promoter of the human Apo AI. The complexes separated, appearing on the electrophoresis gel, are identified in the example 3. Figure 4 A/B: Delay gel showing the identification of a response element LRH-1 -144/-122 included in the fragment of the promoter of the human apoAI. Figure 5: Effect overexpression of LRH-1 on the activity of the promoter of the human Apo AI mutated or not in the cells [...] (URL: relative luminescence unit). Figure 6: Effect overexpression of LRH-1 on the activity of different mutants of the promoter of I ' Apo AI [...] in human cells. SEQ ID NO: 1 (element response to LRH-1 of the promoter of the gene for human AI po) 5 '-3' - [...] SEQ Π) NO:2 (element response to LRH-1 mutated gene mutated Apo AI) 5 '-3' - [...] SEQ ID NO: 3 (Region B of the promoter of the gene for the human Apo AI) [...][...] -3' SEQ ID NO:4 (Region C of the promoter of the gene for the human apoAI): 5 ' [...][...] -3' SEQ ID NO:5 (promoter apo AI-j [...] (apoAI gene) 1819-2167) 5 ' [...][...][...][...]' SEQ ID NO:6 (promoter Tk-M80483 ( [...] ) 38-204 ; J02224 (Herpes simplex) 302-462) 5 '-3' [...][...] SEQ ID NO:7 (sense sequence of [...] wt): 5 '- [...][...] -3' SEQ ID NO:8 (sequence antisense [...] wt): 5 '- [...][...][...][...] A-3 G' SEQ ID NO:9 (sense sequence [...] 7 has mut): 5 '- [...][...] -3' SEQ ID NO:10 (antisense sequence [...] 7 has mut): 5 '-GG [...][...] G [...] AAG A-3' SEQ ID NO:11 ( [...] sense sequence): 5 '-3' - [...] SEQ ID NO:12 (antisense sequence [...] ): 5 '-3' - [...] SEQ Π) NO:13 ( [...] sense sequence): 5 '-3' - [...] SEQ ID NO:14 (antisense sequence [...] ): 5 '-3' - [...] AAG SEQ ID NO:15 ( [...] sense sequence): 5 '-3' - [...] SEQ ID NO:16 (antisense sequence [...] ): 5 '-3' - [...] SEQ ID NO:17 ( [...] sense sequence): 5 '-3' - [...] SEQ ID NO:18 (antisense sequence [...] ): 5 '-3' - [...] SEQ ID NO:19 (sense sequence used for mutagenesis of [...] +): 5 '-' [...] -3 SEQ ID NO:20 (antisense sequence used for mutagenesis of [...] +): 5 '-' [...] -3 The example 1 shows that the over-expression of [...] -1 increases the activity of the fragment + 254/91 (which includes the regions A, B and C) of the promoter of the apo AI, cloned upstream of the luciferase reporter gene. HepG2 cells are co-transfected by lipofection ( [...] as the provider) with 100 ng of the vector pCI- [...] -1 which allows for the over-expression of vacuum pCI LRH-1 or the vector used as negative control and 250 ng of a vector rapporteur noted [...] + which enables the expression of the luciferase reporter gene under the control of the fragment of the promoter of the 254/+91 apo AI (comprising the regions A, B, C promoter [...] Al, noted [...] +) or 250 ng of the vector rapporteur promoterless as a control (noted Luc +). These constructs are obtained by exchanging the CAT reporter gene constructs described previously with the gene of the plasmid [...] rapporteur Luciferase extract of unique (Madison, WI, USA) as described previously, The total amount of DNA transfected is set to 500ng using the plasmid pBKS +. After 3 hours of transfection, the cells are incubated in the culture medium for 36 hours. Luciferase The activity is then measured as described previously in the presence or absence of the protein LRH-1. Figure 1 shows an increase by a factor 2 Luciferase activity by the over-expression of LRH-1 when HepG2 cells are transfected with the construction [...] +. This increase is not observed when the cells are transfected with the construct Luc + free control promoter. The example 2 shows that the over-expression of [...] -1 increases the activity fragments + 254/91 (which includes the regions A, B and C) and + / - 192 21 (which includes the regions B and C) of the promoter of the apo AI but not fragments 128 + / - 91 (which comprises the region C) and + 40/91 (that includes only the minimum promoter), cloned upstream of the luciferase reporter gene. RK13 cells are co-transfected by lipofection ( [...] as the provider) with 100 ng of the vector pCI- [...] -1 which allows for the over-expression of vacuum pCI LRH-1 or the vector used as negative control and 250 ng of a vector rapporteur which enables the expression of the luciferase reporter gene under the control of the fragments -254/+91 (comprising the regions A, B and C, noted [...] +), -192/+91 (comprising the regions B and C, noted [...] +), -128/+91 (including the region C, noted [...] +) or -40 / + 91 (comprising the minimum promoter pmin, noted pmin) of the promoter of the apo AI or 250 ng of the vector rapporteur free promoter as a control (noted Luc +). These constructs are obtained by exchanging the CAT reporter gene constructs described previously with the gene of the plasmid [...] rapporteur Luciferase extract of unique (Madison, WI, USA) as described previously. The total amount of DNA transfected is set to 500 ng using the plasmid pBKS +. After 3 hours of transfection, the cells are incubated in the culture medium for 36 hours. Luciferase The activity is then measured as described previously in the presence or absence of the protein LRH-1. Figure 2 shows that in the presence of LRH-1, expression of the gene encoding the luciferase placed under the control of the promoter of the human Apo AI comprising the regions A, B, C and the minimum promoter (+ [...] -fragment -254/+91) is increased by a factor 12 in RK13 cells which do not express the receptor LRH-1 endogenously. Expression of the luciferase controlled by a construct comprising the regions B, C and the minimum promoter (+ - -192/+91 [...] fragment) of the promoter of the human Apo AI is increased by a factor 15. Expression of the luciferase controlled by a construction including the region C and the minimum promoter (+ - -128/+91 [...] fragment) of the promoter of the human Apo AI is not affected. Expression of the luciferase controlled by a construction including only the minimum promoter (pmin-fragment -40/+91) of the promoter of the human Apo AI is only slightly stimulated by a factor 2. Does not observe We also enabling the expression of luciferase when the cells RK13 are transfected with the vector rapporteur Luc + free of promoter sequence. Expression of the apoAI gene is much LRH-1 regulated by the protein. There is cis a site located at the region B gene promoter human apoAI fixing trans LRH-1 of the protein. The example 3 shows that LRH-1 -144/-122 attaches on the fragment of the promoter of the human gene of the apo AI. [...] The protein -1 is produced in vitro using the TNT-T kit 7 rabbit reticulocyte lysate of unique (ref. L4610) and the vector pCI-LRH -1. Double strand Oligonucleotides corresponding to the response element present on the gene LRH-1 Cyp7a, noted [...] wt, [ direction: 5 '- [...] -3' (SEQ ID NO:7) and antisense: 5 '- [...] -3' (SEQ ID NO:8)], to the same mutated response, has noted [...] 7 mut, [ direction: 5 ' [...] -3' (SEQ ID NO:9) and antisense: 5 ' [...] -3' (SEQ ID NO:10], the fragment -191/-171, noted [...][...] A1, [ direction: 5 ' [...] -3' (SEQ ID NO:11) and antisense: 5 ' [...] -3' (SEQ ID NO:12)], the fragment -178/-145, noted [...][...] A1, [ direction: 5 '- [...] -3' (SEQ ID NO:13) and anti-sense: 5 '- [...] -3' (SEQ ID NO:14)], the fragment -144/-122 wild, noted [...], or mutated, noted [...] mut, [ direction: 5 '- [...] -3' (SEQ ID NO:15) and anti-sense: 5 '- [...] -3' (SEQ ID NO:16)] and the fragment 180/-158, noted [...][...] A1, [ direction: 5 '- [...] -3' (SEQ ID NO:17) and anti-sense: 5 '- [...] -3' (SEQ ID NO:18)] of the promoter of the human gene of the apo AI (SEQ ID NO:5) were prepared as described previously, and labelled with [y-32 P]-ATP using the polynucleotide kinase. 2 μ1 rabbit reticulocyte lysate programmed by [...] -1 are incubated for 15 minutes at room temperature in a final volume of [...] buffer containing 10 mm HEPES, 2.5 mm [...], 10% glycerol, 2.5 mg/ml BSA, 50 mm and 0.5 mm NaCl with 2.5 TDI pg of [...] -dC and 1 μg herring sperm DNA in the presence of the oligonucleotides double tagged strands (0.5 ng). The complexes are then separated by non-denaturing gel electrophoresis in buffer TBE 0, [...]. Figure 3 shows a complex LRH-l/specific DNA LRH-1 when the protein produced in vitro is incubated in the presence of a double-stranded oligonucleotide labelled response element LRH-1 present at the gene Cyp7a ( [...] wt). In contrast, no complex is detected in the presence of the double-stranded oligonucleotide labeled corresponding response element is mutated ( [...] mut).. Figure 3 also shows that no complex DNA/LRH -1 is detected with the oligonucleotides corresponding to the double strands -191/-171 fragments ( [...] ), -178/-145 ( [...][...] ), and -180 / - 158 ( [...] ApoA1 [...] ), of the promoter of the human gene of the apo AI. In contrast, Figure 3 shows the presence of a complex DNA/LRH -1 specific when the oligonucleotide labelled double stranded corresponds to the fragment -144/-122 ( [...] ) of the promoter of the human gene of the apo AI. This fragment is located astride the regions B and C of the promoter of the human gene of the apo AI and comprises, on the antisense strand, the sequence [...][...] close to the consensus sequence of a response element to LRH-1. The element is functional in that Figure 3 shows that 1' double-stranded oligonucleotide [...] corresponding whose sequence is mutated ( [...] ) is unable to form a complex with [...] ( [...] mut). The example 4 shows that the fragment of the promoter of the human gene -144/-122 apo AI is a site to low affinity for LRH-1. [...] The protein -1 is produced in vitro using the TNT-T kit 7 rabbit reticulocyte lysate of unique (ref. L4610) and the vector pCI-LRH -1. Double strand Oligonucleotides corresponding to the response element present on the gene LRH-1 Cyp7a (noted LRH-1- [...] ) or fragment ( [...] ) wild -144/-122 of the promoter of the human gene of the apo AI (SEQ ID NO:4) have been prepared as described previously, and labelled with [y-32 P]-ATP using the polynucleotide kinase. 2 μ1 reticulocyte lysate programmed by [...] -1 are incubated for 15 minutes at 4 °C [...] in a final volume of buffer containing 10 mm HEPES, 2.5 mm [...], 10% glycerol, 2.5 mg/ml BSA, 50 mm and 0.5 mm NaCl with 2.5 DTT pg of [...] -dC and 1 pg herring sperm DNA in the presence of the oligonucleotides double strand excess unlabeled (10X, 50 and 100X X) with respect to the labeled probe used (0.5 ng). The oligonucleotides (0,5ng) double tagged strands are then added to the mixture and incubated at room temperature for 15 minutes before the complexes are then separated by non-denaturing gel electrophoresis in buffer TBE 0, [...]. The figure 4A shows that a double-stranded oligonucleotide non-radioactive -144/-122 corresponding to the fragment of the promoter of the human gene of the apo AI partly displaces the complex formed between LRH-1 and a double-stranded oligonucleotide labelled response element LRH-1 Cyp7a present in the gene. However, Figure 4A show no movement of the complex formed between LRH-1 and a double-stranded oligonucleotide labelled response element LRH-1 present at the gene by a double-stranded oligonucleotide Cyp7a non-radioactive -144/-122 corresponding to the fragment of the promoter of the gene mutated human apo AI. The figure 4B shows that a double-stranded oligonucleotide corresponding to the non-radioactive response element LRH-1 present at the gene Cyp7a moves fully the complex formed between LRH-1 and a double-stranded oligonucleotide labeled -144/-122 corresponding to the fragment of the promoter of the human gene of the apo AI. However, Figure 4B show no movement of the complex formed between LRH-1 and a double-stranded oligonucleotide labeled -144/-122 corresponding to the fragment of the promoter of the human gene of the apo AI by a double-stranded oligonucleotide corresponding non-radioactive response element mutated LRH-1 Cyp7a present in the gene. Comparison of the results shown in Figures and 4A B indicates that the affinity for LRH-1 -144/-122 fragment of the promoter of the human gene of the apo AI is smaller than that of the response element LRH-1 Cyp7a present in the gene. The example 5 shows that the mutation site [...] -144/-122 present in the fragment of the promoter of the human gene of the Apo AI LRH-1 reduces the sensitivity of a construct comprises a fragment of the promoter of the human gene -254/+91 apo AI. [...] cells are co-transfected by lipofection ( [...] as the provider) with 100 ng of the vector pCI- [...] -1 which allows for the over-expression of LRH-1 or the vector pCI vacuum and used as negative control 50 ng of a vector rapporteur which enables the expression of the luciferase reporter gene under the control of the fragment -254/+91 comprising the regions A, and wild B C of the promoter of the apo AI (ABC noted Luc +), under the control of the fragment including the sites -254/+91 A, B and C of the promoter of the apo AI units [...] is mutated (noted [...] +), under the control of the fragment comprising the regions and -192/+91 B C promoter [...] AI (noted [...] +), under the control of the fragment -128/+91 C including the region of the promoter of the apo AI (noted [...] +), under the control of the fragment comprising the promoter -40/+91 minimum apo AI (noted pmin) or 50 ng of the vector rapporteur free promoter as a control (noted Luc +). The construction [...] + is obtained by site-directed mutagenesis of the wild [...] + construction, using the kit site Quick Briefs directed mutagenesis (Stratagene) corresponding to the direction and sequences SEQ ID NO 19 antisense SEQ ID NO 20. Figure 5 shows an increase by a factor of 5.8 Luciferase activity by the over-expression of LRH-1 [...] when the cells are transfected with the construction [...] and 2.6 + [...] when the cells are transfected with the construction [...] +. This increase is not or little observed when the cells are transfected with constructs comprising the sites A, B and C of the promoter of the apo AI units [...] is mutated ( [...] +), the region of the promoter of the C apo AI ( [...] +), the minimum promoter apo AI (pmin) or the construction free promoter (Luc +). The example 5 shows that the site [...] -144/-122 present in the fragment of the promoter of the human gene of the sensitized Apo AI thereof to LRH-1. The example 6 shows that the mutation site present in the fragment [...] -144/-122 LRH-1 reduces the sensitivity of a construct comprises a fragment of the promoter of the human gene -254/+91 apo AI cloned upstream of the luciferase reporter gene unlike the mutation of the FXR response element of the promoter of the human gene of the adjacent Apo AI. [...] cells are co-transfected by lipofection ( [...] as the provider) with vector [...] pCI- [...] -1 which allows for the over-expression of LRH-1 or the vector pCI vacuum and used as negative control 50 ng of a vector rapporteur which enables the expression of the luciferase reporter gene under the control of the fragment -254/+91 comprising the regions A, and wild B C of the promoter of the apo AI (noted ABC Luc + and [...] ), under the control of the fragment including the sites -254/+91 A, B and C of the promoter of the apo AI units [...] is mutated (noted + [...] see example 5), under the control of the fragment including the sites -254/+91 A, B and C of the promoter of the apo AI whose FXR response element is mutated (noted [...] ), under the control of the fragment comprising the regions and -192/+91 B C promoter [...] AI (+ noted [...] and [...] ), under the control of the fragment 192/+91 whose FXR response element is mutated (noted [...] ), under the control of the fragment -128/+91 C including the region of the promoter of the apo AI (noted [...] +), under the control of the fragment comprising the promoter -40/+91 minimum apo AI (noted pmin) or 50 ng of rapporteurs promoter-free vector as a control (noted Luc + and [...] ). The construction [...] has been obtained by subcloning the -254/+91 fragment of the promoter of the gene of the vector [...] of apoAI + (SalI/SphI digestion) in the vector of unique [...] (digested by XhoI/SphI). The construction [...] has been obtained by sub-cloning of the mutated fragment -254/+91 gene promoter of apoAI [...] vector + [...] described above (SalI/SphI digestion) in the vector [...] (digested by XhoI/SphI). The constructs [...][...] and have been obtained by partial digestion and re-ligation [...][...] and constructs, respectively. Figure 6 shows an increase of a 4.8 Luciferase factor activity by the over-expression of LRH-1 [...] when the cells are transfected with the construction [...] +, of 2.1 [...] when the cells are transfected with the construction [...] +, of 8.7 [...] when the cells are transfected with the construction [...], of 11,5 [...] when the cells are transfected with the construction [...], of 1.9 [...] when the cells are transfected with the construction [...] and 2.4 [...] when the cells are transfected with the construction [...]. This increase is not or little observed when the cells are transfected with constructs comprising the sites A, B and C of the promoter of the apo AI units [...] is mutated ( [...] +), the region of the promoter of the C apo AI ( [...] +), the minimum promoter apo AI (pmin) or free of the promoter constructs (Luc + and [...] ). The example 6 shows that the site [...] -144/-122 present in the fragment of the promoter of the human gene of the sensitized Apo AI thereof to LRH-1. The presence of the FXR response element adjacent to the responding to LRH does not appear to be required for the response to LRH. Although physically close, these response elements are therefore functionally distinct. 1. Francis, g. A., et al., 2. Zhang, C. K., et al., 3. [...], R., et al., 4. Ellinger- [...], H., et al., 5. Lin, W., et al., 6. Chen, F., et al., - 7. [...], K., et al., 8. The Goff, W., et al., 9. [...], J., et al., and function of apolipoprotein A-I 10. [...], and J. P. [...], 11. Leroy, A., J. [...], and J. [...], 12. Breslow, J., et al., [...], C. and F. [...], [...], S., et al., of human [...] A-I Widom, R., et al., Vu-Dac, Ν ., al. and, [...], [...]., al. and, Claudel, Τ ., al. and, Method for selection, identification and characterization of compounds (A) able to modulate reverse transport of cholesterol (Ch) comprises detecting any binding between test compound and a nucleic acid construct that contains at least one copy of the LRH-1 (liver receptor homolog-1) response element from the promoter of the human gene for apolipoprotein AI (apoAI), or its functional variant. - Independent claims are also included for the following: - (1) similar method in which the test compound is contacted with a cell that includes an expression cassette for a reporter gene (RG) under control of a promoter that includes at least one copy of the LRH-1 response element; - (2) nucleic acid fragment (NA) characterized by sequence (SEQ ID NO: 1) or its variants, able to bind to the LRH-1 receptor 5'-CTGATCCTTGAAC (SEQ ID NO: 1); - (3) nucleic acid construct (NAC) that includes at least one copy of NA or of its mutant that can not bind to the LRH-1 receptor; -(4) expression cassette (EC) containing at least one copy of NA, or its mutant, and a promoter that is linked to, and controls, RG; and - (5) host cell that contains the NAC or (3) or the EC of (4). - ACTIVITY - Antiarteriosclerotic; Antilipemic; Cardiovascular-Gen.. - No details of tests for these activities are given. - MECHANISM OF ACTION - Cholesterol modulator; LRH-1 modulator. 1. A method for selecting, identifying or characterizing compounds capable of modulating reverse cholesterol transport, which comprises: -contacting a test compound with a nucleic acid construct comprising at least one copy of a response element LRH-1 of the promoter of the human gene of the [...] AI or a functional variant thereof, and -determining the optional bond said test compound to the response element. 2. A method according to claim 1, characterized in that contacting is carried out in the presence of exogenous LRH-1 receptor or a functional equivalent thereof, and in determining the optional bond of said test compound on the response element LRH-1 and/or the complex formed by the binding of LRH-1 to its response element. 3. A method for selecting, identifying or characterizing compounds capable of modulating reverse cholesterol transport, which comprises: - (i) contacting a test compound with a host cell comprising an expression cassette of a reporter gene, said cassette comprising a reporter gene placed under the control of a promoter containing at least one copy of a response element LRH-1 of the promoter of the human gene of the apo AI or a functional variant thereof, and - (ii) determining the effect of the presence of the test compound on the binding of LRH-1 response element or on the expression of the reporter gene. 4. A method according to claim 3, characterized in that the host cell comprises an exogenous LRH-1 receiver or a functional equivalent thereof. 5. A method according to any one of claims 3 or 4, characterized in that the host cell comprises a ligand LRH-1. 6. A method according to any one of claims 3 to 5 comprising determining the level of expression of the reporter gene in the presence of the test compound and in the absence of said compound, an increase or a decrease in the level of expression of the reporter gene indicating the capacity of the test compound to modulate reverse cholesterol transport. 7. A method according to any one of claims 3 to 6, characterized in that the host cell is a mammalian cell. 8. A method according to claim 7, characterized in that the mammalian cell is a human cell. 9. A method according to any one of claims 1 to 8, characterized in that the response element LRH-1 comprises the sequence (SEQ ID NO:1) following: 5 '-3' - [...] or a functional variant thereof capable of binding the receiver LRH-1. 10. A method according to any one of claims 3 to 9, characterized in that the reporter gene is a gene encoding a product whose activity or presence in biological extracts can be measured, particularly one of the genes coding for luciferase, secreted alkaline phosphatase, galactosidase or lactamase. 11. A method according to any one of claims 3 to 10, characterized in that the promoter is selected from the HSV-TK promoter, the CMV immediate early promoter, the PGK promoter and the promoter of the gene encoding human apolipoprotein AI, the SV40 promoter. 12. A method according to any one of claims 1 to 11, characterized in that one or more compounds are tested, as a mixture or separately. 13. A method according to any one of claims 1 to 12, characterized in that the test compound is a combinatorial library. 14. A method according to claim 13 characterized in that the test compound is a clone or a library of nucleic acid clones expressing one or more polypeptide (s) DNA binding. 15. A method according to any one of the preceding claims, characterized in that contacting is carried out in a multiwell plate. 16. A method according to any one of the preceding claims comprising, further, the comparison of the possible effects determined by said method with those, optional, determined by a method carried out with the same conditions but with a nucleic acid construct comprising at least one mutated copy of a response element to LRH-1 of the promoter of the gene encoding the human alipoprotein AI, said mutated copy being essentially incapable of binding the receiver LRH-1. 17. A method according to any one of the preceding claims, for selecting, identifying or characterizing compounds capable of increasing reverse cholesterol transport. 18. The method of any one of claims 1 to 16, for selecting, identifying or characterizing compounds capable of modulating HDL activity. 19. The method of any one of claims 1 to 16, for selecting, identifying or characterizing compounds capable of modulating the expression of apolipoprotein AI. 20. Nucleic acid fragment characterized by the sequence (SEQ ID NO:1) following: 5 '-G CT [...] AAC -3' or a variant thereof capable of binding the receiver LRH-1. 21. A nucleic acid construct comprising at least one copy of the nucleic acid fragment according to claim 20. 22. A nucleic acid construct comprising at least one copy of the mutated nucleic acid fragment according to claim 20, said mutated copy being essentially incapable of binding the receiver LRH-1. 23. An expression cassette comprising at least one copy of the nucleic acid fragment according to claim 20, and a promoter associated with a reporter gene placed under the control of said promoter. 24. An expression cassette comprising at least one copy of the mutated nucleic acid fragment according to claim 20, and a promoter associated with a reporter gene placed under the control of said promoter. 25. The cassette according to any of claims 23 or 24, characterized in that said promoter is selected from the HSV-TK promoter, the CMV immediate early promoter, the PGK promoter, the promoter SV-40 and the promoter of the gene encoding human apolipoprotein AI. 26. The cassette according to any of claims 23 to 25, characterized in that the reporter gene is the gene coding for luciferase, secreted alkaline phosphatase, galactosidase or lactamase. 27. Host cell containing a construct or cassette according to any one of claims 21 to 26. 28. The use of a construct or cassette according to any one of claims 21 to 26 or a cell according to claim 27, for in vitro screening of compounds capable of modulating 1' activity of HDL. Reverse cholesterol transport
B. Methods of selection, for identifying and characterizing compounds that modulate reverse cholesterol transport.
1. Methods-based expression screening.
a) Contacting in compounds with the host cell
b) determining the activity of the compounds.
2. Methods based on a binding assay.
C. Active HDL and apolipoprotein AI.
Legends of Figures
Sequences
Examples
Example 1: Effect of the over expression of LRH-1 on the activity of the promoter of the human Apo AI in HepG2 cells.
Example 2 ; Effect of the over expression of LRH-1 on the activity of the promoter of the human Apo AI in RK13 cells
Example 3: identification of a binding site of LRH-1 in the promoter of the human apo AI
Example 4: -144/-122 The fragment of the promoter of the human gene of the Apo AI response element is a low affinity LRH-1
Example 5 : Effect overexpression of LRH-1 on the activity of the promoter of the human Apo AI mutated or [...] not in the cells.
Example 6 : Effect overexpression of LRH-1 on the activity of different mutants of the promoter of the human Apo AI [...] in the cells.
REFERENCES