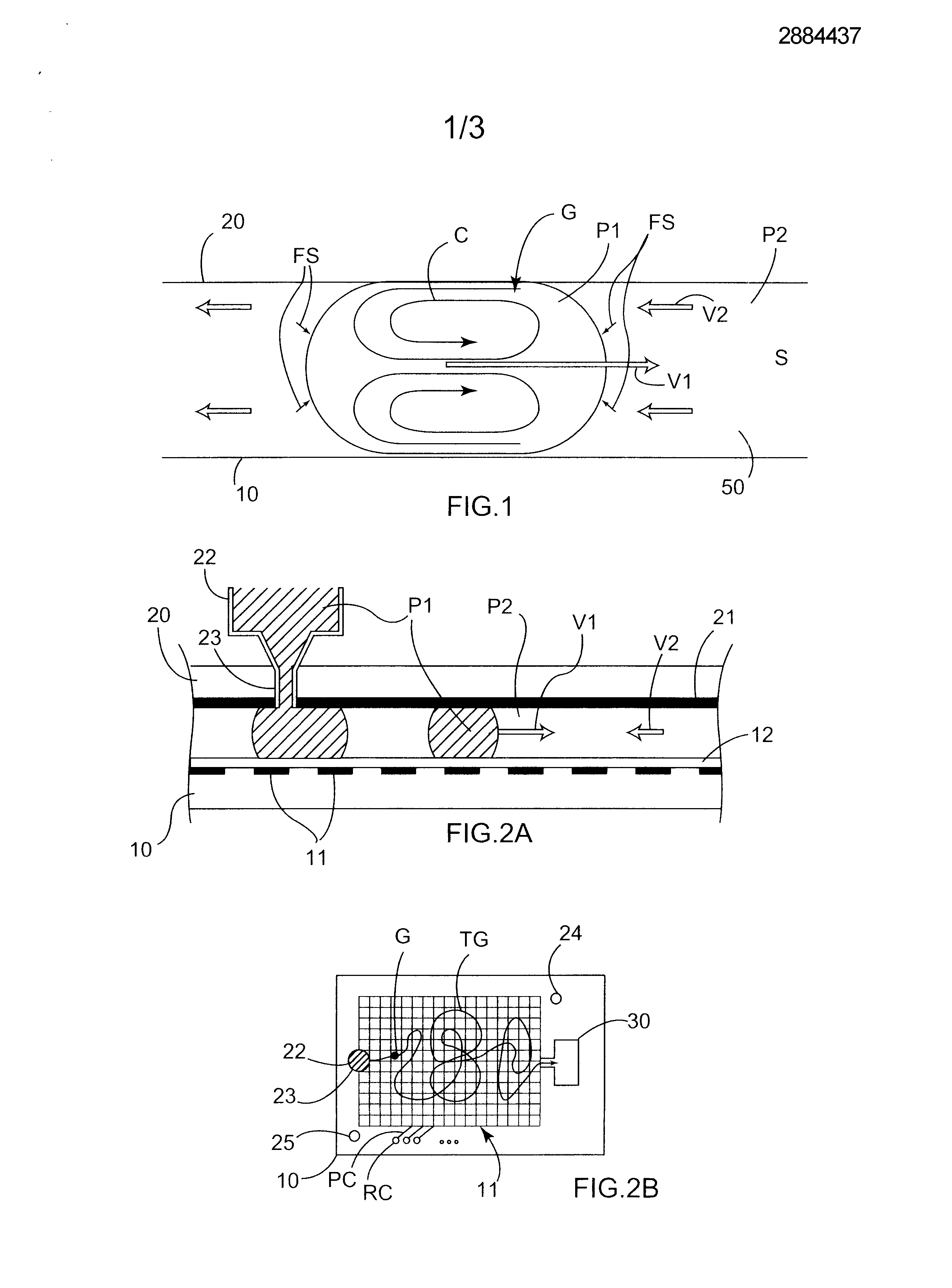
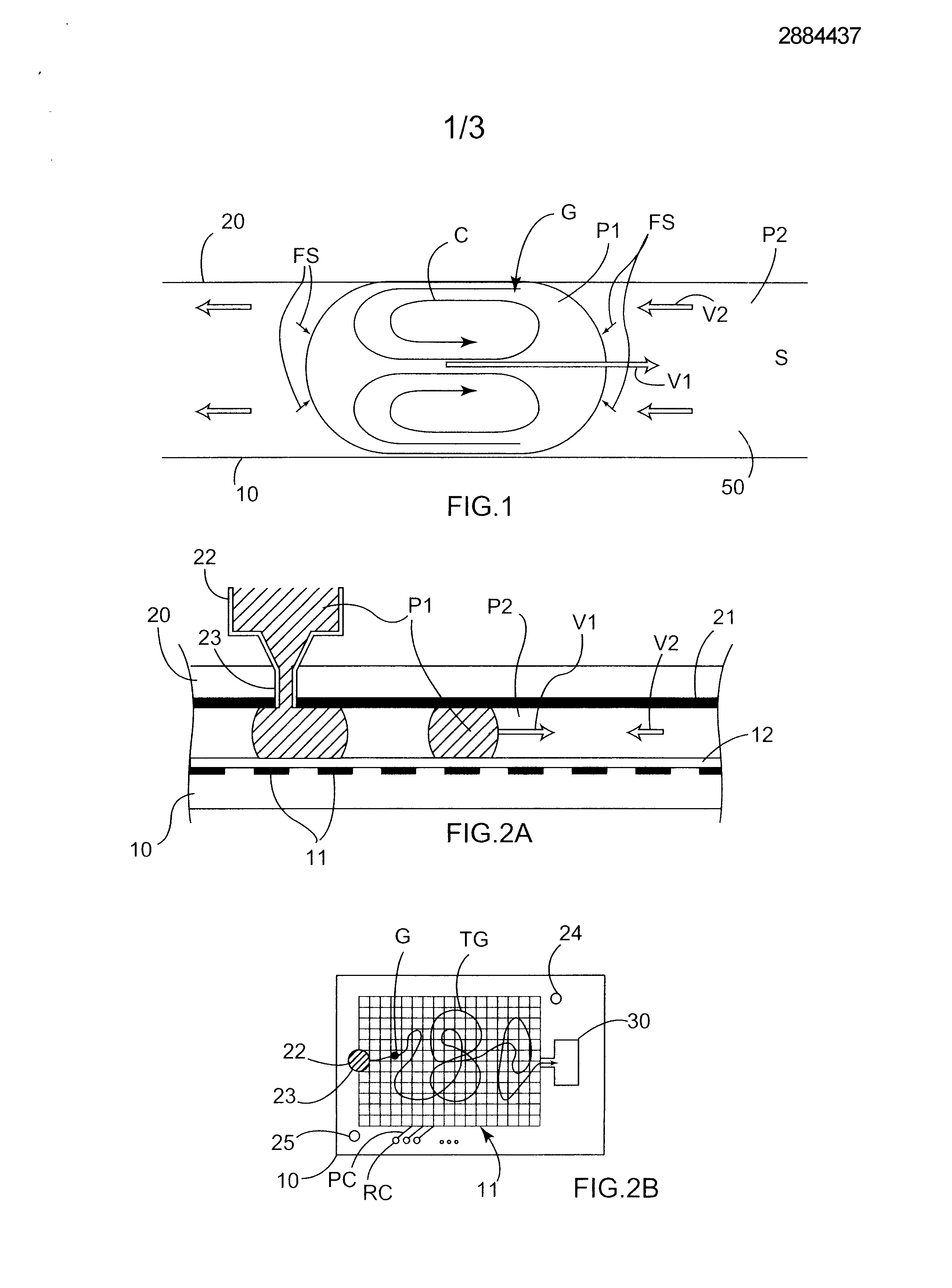
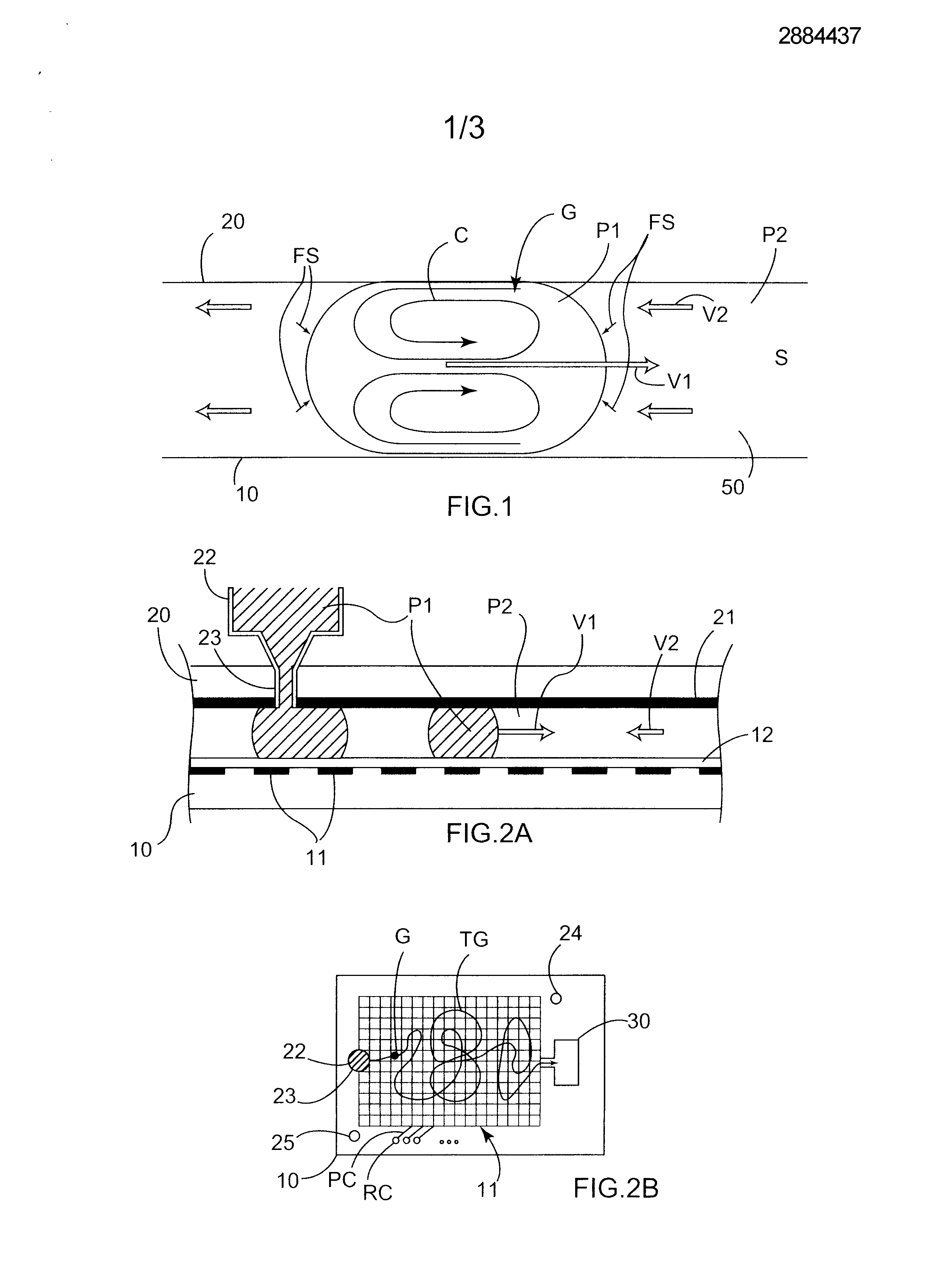
Micro-fluid unit for transferring matter between two non-miscible phases uses natural electrical forces to move at least one microdrop
A mass transfer method between two immiscible phases, and in particular liquid extraction-fluid, mainly for analytical applications. The extraction liquid-fluid is a unit operation commonly used industrial chemistry and analytical chemistry, which comprises causing a transfer of material from at least one solute between a first liquid phase and a second phase fluid (liquid or gaseous) immiscible with the first. It is well known that, to promote such transfer, it is necessary to maximize the ratio of the area of contact between phases and the volume of the phases. Typically, this is achieved by performing a dispersion of one phase in the other, for example in a beaker using a magnetic stirrer, followed by a separation by settling. WO 96/12540 The document discloses a method for performing transfer of solute between two immiscible liquid phases through a porous membrane planar. The transfer of solute from one phase to the other is through the pores of the membrane, without the aid of which the planar interface between the two fluids would be unstable. The two phases are retrieved independently since at any time they are mixed. The article " Analytical Chemistry in a drop. Solvent Extraction in a [...]" Liu and H. P. Dasgupta, Analytical Chemistry, Volume 68, [...],er 1 analytic application June 1996 discloses a liquid-liquid extraction to the scale of microlitres. In this article, a droplet of about 1 pI of chloroform is suspended within a larger drop of an aqueous solution continuously renewed; a solute passes from the aqueous phase to the organic phase and is detected by Raman spectroscopy. The method requires only minute amounts of solvents and analyte, and makes it possible to obtain a very high volume-surface. However, its implementation is complex, because it requires delicate assembly discrete microfluidic components. An object of the present invention is to provide a method of manufacturing small-scale fluid (microlitres), mainly for analytical applications. Advantageously such a process should allow maximizing between the two fluids, while being simple and rapid. The hardware to carry out the process must be simple and economical. Such a method may form a step of a microfluidic process-chemical complex: for example it may be applied to the purification of small quantities of reagents, or extraction reaction products of a droplet of solvent. Alternatively, it may be used to concentrate a solute in a solvent present in traces in a solution to be analyzed, so as to enable its detection. The idea of the present invention is to circulate a droplet (volume microliter, see tens or hundreds of nanolitres) of a first liquid phase within a larger volume (such as between ten microlitres and few millilitres) a second phase fluid-liquid or gas-using a microfluidic device type "fluidic chip"; by "microfluidic device" is meant herein a device for handling volumes of liquids less than 1 milliliter, typically between a few hundred nanolitres and some hundreds of microliters. Such devices can be made in large series at very low cost by manufacturing techniques derived from microelectronics, such as photolithography and thin film deposition. Subsequent A advantage of this solution is constituted by the fact that the chip may include integrated analysis devices. A object of the invention is therefore a method for transfer of material from at least one solute between a first liquid phase and a second phase fluid (liquid or gas), immiscible with the first, characterized in that it includes moving, in a microfluidic device and using electrical forces, of at least one droplet of said first liquid phase within a space filled with fluid by said second phase. The droplet may typically include a volume of [...] pl ni and 10 and preferably between 1 and 100 and pl. Preferably, a method according to the invention includes moving said drop using said forces of an electric nature along a path between a feed point or generation of said droplet in said microfluidic device and an extraction zone and/or analysis, said path is determined in such a way that said droplet scans of a fraction of at least 20%, preferably at least 50% and even preferably at least 80%, of said filled space by said second fluid phase. The path may be a pseudo-random path. Advantageously said second phase fluid flows inside said space of said microfluidic device, its speed is held to a value low enough to allow the displacement of said droplet of said first liquid phase with the aid of an electric nature of said forces. According to one embodiment of the invention, said first liquid phase initially contains a solute and said second phase fluid has an affinity for the solute at least equal to that of the first liquid phase, resulting in a depletion of said solute droplet (extraction [...] ). In another embodiment of the invention, said second fluid phase initially contains a solute and said first liquid phase has an affinity for said solute greater than that of said second fluid phase, which leads to strengthening-solute said droplet (extraction [...] ). In one embodiment of the invention, the displacement of said droplet of said first liquid phase is carried out by electrowetting by exploiting a difference in conductivity between said first and said second liquid phase fluid phase, said phases being maintained in a space between a bottom plate having an array of electrodes and a top plate, parallel to said lower plate and having a counter electrode facing said electrode array. When the first liquid phase has a conductivity of less than that of said second fluid phase, the displacement of said droplet a position corresponding to a start electrode at a position corresponding to a destination electrode is obtained by maintaining said destination electrode at the same potential as said counter electrode while a potential difference is established between said counter electrode and the electrode and the adjacent electrodes. Conversely, when said first liquid phase has a higher conductivity than that of said second fluid phase, the displacement of said droplet a position corresponding to a start electrode at a position corresponding to a destination electrode is achieved by producing a potential difference between said electrode and said counter electrode destination, while said starting electrode and the adjacent electrodes are maintained at the same potential as said counter electrode. The difference in conductivity between said first liquid phase and said second phase fluid is typically at least a factor 10 and preferably by at least a factor of 100. In another embodiment, the displacement of said droplet from said first phase liquid is performed by dielectrophoresis by exploiting a difference magnetic permeability between said first liquid phase and said second phase fluid. Magnetic permeability Typically said difference between said first liquid phase and said second phase fluid is at least 10% and preferably at least 50%. A method according to the invention may comprise a step of transferring said droplet using the electrical forces to a chemical analysis device integrated with said microfluidic device and a step of chemical analysis of said droplet. The step of analyzing may include a spectrophotometric analysis of said droplet, and/or a step electrospray said droplet in a mass spectrometer. Another object of the invention is a device for implementing such a method comprising: a bottom plate having an array of electrodes; a counter electrode facing said array of electrodes; injection means or generation of droplets of a first liquid phase on the surface of said bottom plate; injection means on the surface of said bottom plate of a second phase fluid in which the droplets are immersed in a first liquid phase; and a control device for independently establishing a potential difference between each of said gate and said counter electrode so as to determine a movement of said droplets within the volume of said second liquid phase. In particular embodiments: The device also comprises means of chemical analysis said droplets. The device also comprises means for evacuating the second liquid phase to determine a circulation of the latter on the surface of said bottom plate. The device also includes a top plate, parallel to said lower plate, defining with it a space capable of being filled up by said second fluid phase and carrying said counter electrode. The counter electrode is constituted by at least one conductor wire immersed or stretched in at least one of said fluid phases. The controller is provided to determine a moving said droplets along a path for scanning at least 20%, preferably at least 50% and even preferably at least 80% of the volume of said second liquid phase. The invention be included at the reading of the following description, in connection with the accompanying drawings in which: figure 1 illustrates the process of mixing and mass transfer upon the implementation of a method of the invention; figures 2A and 2B show respectively a view in lateral cross section and a plan view of a microfluidic device that is suitable for carrying out the method of the invention; figures 3A and 3B show a elementary process displacement by electrowetting a droplet of conductive liquid in a medium comprised of a non-conductive fluid, in accordance with an embodiment of the present invention; figures 4A and 4B show a elementary process by electrowetting movement of a droplet of non-conductive liquid in a medium comprised of a conductive fluid, in accordance with another embodiment of the present invention; figures 5A and 5B show views over two microfluidic devices suitable for implementing two particular embodiments of a method of the invention; figure 6 illustrates schematically a method of manufacturing the microfluidic device figures 2A and 2B. The principle of the invention can be included using Figure 1. G A droplet of a first liquid phase P1, a volume of, for example, between 10 pl ni and 10, preferably between 1 and 100 and pl, is placed in the space 50 between a bottom plate 10 and a top plate 20, immersed in a second fluid phase P2, immiscible with it. For example, the first phase P1 may be formed of an organic solvent, such as chloroform or carbon tetrachloride, while the second phase P2 is an aqueous solution. The second phase P2 initially contains a solute S that has high affinity with the first phase P1; the solute S may be consisting of atoms, ions, molecules simple or complex, cells or biological entities such as viruses. The solute may also have an affinity for one of the components of the phase P1. All formation of a complex or precipitate incorporating S in the receiving phase P1. The solute S may also be an antigen to perform an antigen/antibody reaction, for example if S is a bacterium. Similarly, a chemical compound can be introduced into the phase P2 into a (complex, precipitate, ...) very soluble in the P1 so as to be transferred easily in said phase, where it may reside as is or can be again transformed. As shown by the arrows FS, a solute stream FS is produced through the interface between the two phases, of the phase to the phase P1 P2. If the two phases P1 and P2 remain stationary, the mass transfer is performed only by effect of the molecular diffusion, and equilibrium is achieved very slowly, particularly if the volume of the space filled by the 50 second phase P2 is large in relation to the droplet g. Therefore, the method of the invention includes moving said droplet (arrow V1) so as to scan said space 50. Therefore, when the solute S was extracted in a region of the microfluidic device, the droplet G leaves the depleted region and moves to areas further solute-rich. Furthermore, moving causes a mixture of the content of the droplet G (vortex C in Figure 1), homogenising its solute concentration promotes S and thus extraction. Such a homogenizing effect induced by the movement of a droplet has been described by C. J. R. Burns and Ramshaw in the article "The intensification of fast reactions in multi-phase systems in hair using slug flow", chip Lab is, 2001, pages 10-15, published on 9 August 2001 Internet. However, the article provides a very different situation, wherein the droplet is the seat of a chemical reaction without any transfer of material to or from the surrounding liquid phase. In these conditions, it may be regarded that the solute concentrations S in the droplet, C1 ; and in the second liquid phase, C2, are homogeneous, with the exception of two diffusing films, in which the concentrations vary rapidly. When a stationary state is reached, the concentrations at the interface are respectively, under the assumption of zero interfacial resistance, C2 ' and Ci-H-C2', where H is the partition coefficient of the solute S between the first and the second liquid phase (H > 1 if it is desirable to concentrate the solute in the droplet). The transfer rate of the solute of the phase to the phase P1 P2 per unit area of contact Preferably, the second phase P2 fluid circulates within the microfluidic device (arrow V2) to be continuously renewed. This can improve process kinetics, because the solute phase P2 depleted S is continuously removed; further, in this way it is possible to contact a large volume phase P2 with the droplet G while using a small-size device. The speed V2 of the phase P2 must be small enough to not cause the G or droplets of the first liquid phase P1; the maximum permissible speed V2 depends on the case in particular of the wetting of the two phases P1 and P2 on their respective surface and the intensity of the electrical forces used to move the droplet g. The flow rate of the second fluid phase P2 can be, for example, between 10 nl/min and few pl/min. Alternatively, the method of the invention can be used to purify the first liquid phase P1:in this case (extraction " [...]") the first liquid phase P1 initially contains the solute S and the second fluid phase P2 has an affinity with said solute S which is at least equal to that of said first liquid phase P1, resulting in a depletion S of said solute droplet g. As shown on Fig. 2A, a device 22 of injection of the first liquid phase P1, such as a needle connected to a reservoir, passes through an opening 23 formed in the top plate 20 and opens into the space 50 between said upper and lower plates 20 10,50 second space filled with the fluid phase (liquid or gaseous) P2. G The droplets may be formed using the electrical forces used for moving: the potentials of the electrodes and 11 of the counter electrode 21 are established to produce a liquid stream which extends from the needle 22, then this vein is "throttled" for separation of a droplet. This technique is described in of R.B. Fair, V. Srinivasan, H. Ren, P. Paik, V.K. [...] and M. G. Pollak " [...] -Based The-chip sample processing for Integrated [...]" IEEE International Electron Devices Meeting (IEDM) 2003. The second fluid phase P2 is circulated by injection devices (e.g., a syringe) and discharge (a pipe opening into a container) not shown. These are connected to the space 50 by capillaries 24,25 an inner diameter of the order of 100 pm. G The travel of the droplets of the first liquid phase P1 cannot be induced by a pressure differential, because this will cause a simultaneous displacement of the two fluid phases, which is not desired. Therefore, it has been selected move said droplets using electrical forces, and in particular the effect known as electrowetting. This effect is known, for example, of the article "thorough and [...] Trapping of charge: Model and Experiments", of [...][...] M.V. and J. Prins, Langmuir 1999, 15, 6616-6620. The article " [...] -based actuation of liquid [...] for microfluidic applications" of Μ. G. Pollack, R. Β. A. D. [...] Fair and discloses the movement of droplets of a conductive liquid in an insulating medium using electrowetting. The electrical actuation of the droplets is permitted, in the frame of the present invention, by a gate electrode 11 disposed on the upper surface of the bottom plate 10 and with a counter electrode 21 disposed on the bottom surface of the top plate 20. Alternatively, the counter electrode 21 can be replaced by conductive wires immersed or tensioned in at least one of the two fluid phases. In wires tensioned in parallel to the bottom plate 10 and performs a function counter electrode and a guide for the droplets G are known as "micro-catenary"; this technology is described in the article Y- [...], H. Jeanson, D. Dary, Constantin and O. C. [...]"with [...] Moving [...]", 7th International Conference is [...] Chemical and Biochemical Analysis Systems ", 5-9 October 2003, Squaw Valley, California, United States of America. In the case of a device of Figure 2A, the electrodes 11 are separated from the fluid phases Ρ1, P2 by an insulating coating 12, while the counter electrode 21 is in electrical contact with them. Alternatively, the counter electrode 21 could, also, include an insulating coating. Both the electrodes 11 that the counter electrode 21 are connected, by individual electrical connections, to a control device for independently establishing a potential difference between each electrode 11 and the corresponding counter electrode. The establishment of this potential difference allows actuation of the droplets G, which can be brought to follow a trajectory accurately determined, as it will be shown further. For sake of clarity of the representation, said electrical connections have been omitted figures. The viewed from above Figure 2B, through the top plate 20 assumed transparent, shows that the grid of electrodes 11 connects the injection device 22 to an area 30 extraction and/or analyzing droplets g. At the periphery of the electrode array 11, three times contact RC have been represented. In reality, there are as many times that contact RC of electrodes in the array 11, and each of which is connected to the corresponding electrode by a conductor track PC. For sake of simplicity, the electrodes of the array 11 have, in Figure, a square shape, but in reality it is advantageous if they have a contour with protrusions to inter-engage with each other; it is known that this facilitates the movement of droplets g. The line GT shows a sample path a droplet G of the injection device 22 to said extraction zone and/or analysis 30. TG The path is two-dimensional and pseudo-random and allows the droplet G of scanning a large part of the volume of the space filled with the 50 second phase P2 fluid; in this manner, it can be efficiently charge S solute while without the excessively long time the molecular diffusion. In this context, "a substantial portion" means a fraction of at least 20%, but preferably at least 50% and preferably even a fraction of the order of 80% or more. Instead of be pseudo-random, the path GT may also have a regular character: what is more important is that it allows the droplet effectively G scan a large portion of the space 50 filled with the second fluid phase P2. Figures 3A and 3B illustrate a step elementary G movement of a droplet of a first liquid phase P1 conductive immersed in a second fluid phase P2 substantially non-conductive, i.e. having a conductivity at least 10 times, and preferably at least 100 times, smaller than that of said first liquid phase P1. Initially, as illustrated by the figure 3A, the droplet G is positioned in correspondence of an electrode 11 "which is held at an electric potential different from that of the counter electrode 21, while the electrodes 1T, 1T" neighboring are held, they, at the same potential as the latter; in the figure to the next and the at least one "active", having a potential difference with respect to the counter electrode 21 are represented in white, while the electrodes at the same potential as the counter electrode are represented in black. Been easily, on the basis of the laws of electrostatics, that the droplet G is in a condition of stable equilibrium. If is desired to move said droplet to the electrode 11 "', it is sufficient to exert a potential difference between it and the counter electrode 21, while the start electrode 11" is returned to the same potential as said counter electrode 21. The potential differences are typically between 10 V and several hundred volts. In these conditions, of the moving speeds of the droplets of the order of a few centimeters per second can be achieved. This method exploits the phenomenon known as electrowetting, which requires a significant difference in conductivity between the two phases Ρ1, P2. The same effect is obtained by dielectrophoresis when said two phases are all substantially insulating, and that the first liquid phase P1 has a polarizability electrically substantially greater than that of the second phase P2 fluid (for example, higher by at least 10%, preferably at least 50%). Movement of droplets by dielectrophoresis is described, for example, in the article of J. [...], Schwartz J. A., Becker F. and F. P. R. C. Gascoyne "A Programmable [...] hydraulic Processor for [...] -Based Chemistry", Micro Total Analyses Analysis Systems 2001, pages 72-74, Kluwer Academic Publishing. When the second fluid phase P2 which is electrically conductive, while the first liquid phase is substantially non-conductive, it is possible to proceed as shown by the figures 4A and 4B. Initially, the droplet G is positioned in correspondence of an electrode 11 "which is held at the same electric potential as the counter electrode 21, while the electrodes [...], 1T" neighboring have a potential difference relative thereto. As in the previous case, the droplet G is in a condition of stable equilibrium. If is desired to move said droplet to the electrode 11' ", it is sufficient to return the latter the same potential of the counter electrode 21, while a potential difference is established between said counter electrode 21 and the start electrode 11". The same effect is obtained by dielectrophoresis when said two phases are all substantially insulating, and that the second phase P2 electric polarizability fluid has a significantly greater than that of the first liquid phase P1 (for example, higher by at least 10%, preferably at least 50%). In cases shown in figures 3A to 4B the counter electrode 21 is in electrical contact with the phases P1 and P2, while the electrodes of the gate 11 are separated from them by an isolation layer 12, preferably made of a material that is not wettable by the first liquid phase P1 and having a high dielectric constant such as, for example, the2 Si0 or[...]. The person skilled in the art will understand that similar results may also be achieved by using a counter electrode that would be, it also, isolated. The use of constant potential differences has been considered, but the person skilled in the art will understand that the application of alternating signals, for example sinusoidal, to the electrodes 11 ', 11 "and 11" ' can achieve the same results. Typically will be used frequencies of a few tens of Hertz to several in electrowetting kHz and 100 kHz to 10MHz of dielectrophoresis. A mass transfer method according to the invention can be applied advantageously to chemical analyses very small scale. In this case, the second phase P2 fluid may be an aqueous solution which may contain chemical pollutants or nuclear P1 and the first liquid phase be an organic solvent having a high affinity for said pollutants. G The droplets can be loading said pollutants so as to allow easy detection. For example, it is possible to use as phase P1 drops of chloroform of 1 pl (conductivity of the chloroform: hasct, = 0.4-10 'mS 11 cm'1) and as phase P2 a bath with a volume of 100 pl to a few ml tap water solution containing metal cations Pb2 + at a concentration of 10 to 100 mg/L (conductivity: 0,3 < [...] mS < 1 cm '1). Chloroform The microdrop can be moved in the bath by electrowetting using a potential difference of about 100V at a frequency of 3 kHz. The potential difference can advantageously be applied using, instead of the counter electrode 21, a conductive wire that extends into the fluid, thereby providing a bath "open cast". Detection of Pb2 + ions concentrated in the microdrop chloroform may be by colorimetry or spectrophotometry using [...] ( [...] ) chloroform soluble, by the reaction [...] 2 + Pb = > Pb ( [...] )2. It is also possible to inject droplets successively in the device G of different solvents, having a different affinity with the solutes may be present in the phase P2. Therefore, it is particularly advantageous that chemical analysis means are integrated to the microfluidic device used for carrying out the mass transfer method. The figure 5A watch, for example, a partial view of a device of the same type as shown in Figure 2B, wherein the area of analysis 30 comprises a positioning piece 301 of a droplet G and a first and a second optical fiber 302,303, aligned with each other and having ends that face each other on one side and said positioning portion 301. The first optical fiber 302 is intended to be connected to a source of optical radiation, such as a laser LA, to illuminate a droplet G brought into the positioning portion 301 using electrical methods described above with reference to Figures to 4B 3A. The second optical fiber 303 is intended to be connected to a SP spectrophotometer for collecting and analyzing the optical radiation transmitted through said droplet G and/or the fluorescence radiation emitted by said droplet. Therefore it is possible to carry out an analysis of the solute contained in said droplet G by spectrophotometric methods. Optionally, the optical fibers 302 and 303 can be replaced by planar dielectric waveguides. Alternatively, as shown in Figure 5B, the area 30 may be a "spout" electrospray 310 comprises a tip projecting from the edge of the plates 10 and 20 and having a slot delimited by two electrodes 311 312 and 313, preferably elongated and converging. G A droplet, once fed to the "spout" 310 merges into a vein liquid which fills the slot 311. By applying a potential difference of the order of 2 kV between the electrodes 312,313 and a counter external electrode, it is possible to nebulize said liquid stream in the form of a gas of ions or a cloud of electrically charged droplets. If the "spout" 310 is arranged at the inlet of a mass spectrometer, mass spectrometric analysis of the first liquid phase P1 and the solute S contained therein can be obtained. It is also possible to combine several analysis devices on the same "chip": for example, there can be provided a device wherein a droplet G would be first fed to an area spectrophotometry analysis to be then [...] in a mass spectrometer. Figure 6 illustrates schematically a method of manufacturing a device for carrying out a method of the invention. The steps A1-A4 refer to the production of the bottom plate 10, the steps B1-B2 to that of the top plate 20 and the steps C1 --C2 to the assembly of the two. The manufacturing method uses derived techniques of microelectronics and commonly used in the field of microfluidics. In order to make the lower plate 10 is typically a substrate glass "Pyrex" or oxidized silicon with a thickness of the order of 500 pm (A1) on which is provided by photolithography a network 11 of electrodes, preferably gold (A2), using a titanium anchoring layer. At the same time are formed the conductor tracks PC (not shown) which connect each electrode to one of the RC times contact arranged in the peripheral part of the plate 10. At step A3, an insulation layer 12 is deposited on said electrodes: for example, said layer 12 may be made of2 Si0 and be deposited using the method known as PECVD (deposition plasma chemical vapor deposition). A step of photolithography disengaging the contact times, for permitting the electrical connection of the microfluidic device with external circuitry. (A4) Then walls 40 in thick resin (SU-8, for example) of 50-300 pm height and thickness are disposed around the electrode array 11 to define the space 50 for containing the second liquid phase P2; the volume of this space is on the order of 10 to 100 pl. The walls 40 have not been shown in Figures [...], 5A and 5B to not overload on it. The top plate 20 is made of a glass substrate "Pyrex" or plastic (polycarbonate, for example), with an opening 23 to permit insertion of a device 22 of injection of the first liquid phase P1 (step B1); then (phase B2) a counter electrode 21 is performed by photolithography. Preferably, said counter electrode will be formed of ITO (indium tin oxide), which has the advantage of being transparent and thereby enable the observation of the course of the method of the invention. Optionally it is possible to deposit an insulating layer on the counter electrode, as has been made for the bottom plate 10. The assembly of the two plates (step C1) is by screen printing adhesive resin made on said thick walls; the screen printing of adhesive is a technique that allows the spreading of an adhesive layer very thin (1-10 pm) and homogeneous. A appropriate adhesive is glue OLED- [...] 45952 company [...] provided by the. The screen printing of glue is described by example in the document WO 00/77509. Furthermore, step C2, a device 22 of injection of the first liquid phase P1 is inserted into the opening in the top plate 20, and the capillaries 23,24 (not shown) opening into the space 50 and for the supply and removal of the second liquid phase P2. Before or after assembly, the inner surfaces of the system or some of them can be treated by depositing a material non-wetting by at least the first liquid phase P1, for promoting the movement of droplets g. The material can be, for example, of the hydrophobic silane, vapor deposited, or teflon, liquid phase deposited metal. The complete device has a total area of a few square centimeters and a thickness of a few millimeters. It can be supported by a printed circuit board, whose conductor paths can be electrically connected to the contact times RC via gold wires (technique termed a "wire bonding"). Alternatively, the electrical connection may be provided by test probes, of dimensions of the order of a few hundred micrometers, mounted on springs. Such test probes are marketed by the FM Technologies company contact as "test probes [...]". In turn, the support circuitry can be inserted into a more complex circuit in which engage coaxial feeder cables. G The travel of the droplets can be controlled by software ad hoc made, e.g. using the language " [...]", National Instrumentation company. Proceeded of transfer of matter of at least an aqueous solution (S) between a first liquid phase (P1) and unedeuxième liquid phase (P2), immiscible with the first, characterized in that it comprises displacement, in a device microfluidic and using forces of electric nature (électromouillage or diélectrophorèse), of at least a droplet (G) of the aforesaid the first liquid phase (P1) inside a space (50) filled parladite second liquid phase (P2). Preferably, the displacement of the aforesaid the droplet (G) to the assistance of the aforesaid forces of electric nature esteffectué along a trajectory (TG) between a point of injection (22) of the aforesaid the droplet (G) in leditdispositif microfluidic and an analysis and/or extract range (30), the aforementioned trajectory (TG) étantdéterminée in such a way that the aforementioned droplet (G) carries out a sweeping of a significant fraction duditespace (50) filled by the aforementioned second liquid phase (P2). The process can comprise a stage of transfer of the aforesaid the droplet (G) to the assistance of the aforesaid forces denatures electric towards a device of chemical analysis (30) integrated to that the device microfluidic and uneétape of chemical analysis of the aforesaid the droplet. Device for the implementation of such a process. 1. Mass transfer process at least one solute (S) between a first liquid phase (P1) and a second fluid phase (P2), immiscible with the first, characterized in that it includes moving, in a microfluidic device and using electrical forces, of at least one droplet (G) of said first liquid phase (P1) within a space (50) filled with said second fluid phase (P2). 2. The method of claim 1 comprising moving said droplet (G) using the electrical forces along a path (GT) between a feed point (22) or generation of said droplet (G) in said microfluidic device and an extraction zone and/or analysis (30), said path (GT) being determined in such a way that said droplet (G) scans of a fraction of at least 20%, preferably at least 50% and even preferably at least 80%, said space (50) filled with said second fluid phase (P2). 3. The method of claim 2 wherein said trajectory (GT) is a pseudo-random path. 4. A method according to any preceding claim wherein said second phase (P2) fluid flows into the said space (50) of said microfluidic device, its speed (V2) being maintained at a sufficiently low value the displacement of said droplet (G) of said first liquid phase (P1) using the electrical forces. 5. A method according to any preceding claim, wherein said first liquid phase (P1) initially contains a solute (S) and said second fluid phase (P2) has an affinity for said solute (S) at least equal to that of the first liquid phase (P1), resulting in a depletion of solute (S) of said droplet (G). 6. Method according to one of claims 1 to 4 wherein said second fluid phase (P2) initially contains a solute (S) and said first liquid phase (P1) has an affinity for said solute (S) greater than that of said second fluid phase (P2), resulting in an enrichment of solute (S) of said droplet (G). 7. A method according to any preceding claim wherein movement of said droplet (G) of said first liquid phase is carried out by electrowetting by exploiting a difference in conductivity between said first liquid phase (P1) and said second fluid phase (P2), said phases (Ρ1, P2) being maintained in contact with an array of electrodes (11) and a counter electrode (21) between which can be established a potential difference. 8. The method of claim 7 wherein said first liquid phase (P1) has a conductivity of less than that of said second fluid phase (P2) and wherein the movement of said droplet (G) a corresponding position to a start electrode (11 ") at a corresponding position to an electrode destination (11 '") is attained by holding said electrode destination (11' ") at the same potential as said counter electrode (21) while a potential difference is established between said counter electrode (21) and the start electrode (11") and the adjacent electrodes (11). 9. The method of claim 7 wherein said first liquid phase (P1) has a conductivity higher than that of said second fluid phase (P2) and wherein the movement of said droplet (G) a corresponding position to a start electrode (11 ") at a corresponding position to an electrode destination (11" ') is achieved by producing a potential difference between said electrode destination (11' ") and said counter electrode (21), while said start electrode (11") and the adjacent electrodes (11') are maintained at the same potential as said counter electrode (21). 10. A method according to any one of claims 8 or 9 wherein said difference in conductivity between said first liquid phase (P1) and said second fluid phase (P2) is at least a factor of 10 and preferably by at least a factor of 100. 11. Method according to one of claims 1 to 6 wherein the movement of said droplet (G) of said first liquid phase (P1) is effected by dielectrophoresis by exploiting a difference between said first electrical permeability liquid phase (P1) and said second fluid phase (P2), said phases (Ρ1, P2) being maintained in contact with an array of electrodes (11) and a counter electrode (21) between which can be established a potential difference. 12. The method of claim 11 wherein said difference between said first magnetic permeability liquid phase (P1) and said second fluid phase (P2) is at least 10% and preferably at least 50%. 13. A method according to any one of the preceding claims comprising the step of transferring said droplet (G) using the electrical forces to a chemical analysis device (30) integrated with said microfluidic device and a step of chemical analysis of said droplet. 14. The method of claim 13 wherein said step of analyzing comprises spectrophotometric analysis of said droplet. 15. The method of claim 13 wherein said step of analyzing includes electrospray said droplet in a mass spectrometer. 16. A method according to any preceding claim wherein said droplet (G) of said first liquid phase has a volume of [...] pl ni and 10 and preferably between 1 and 100 and pl. 17. A device for implementing the method according to one of claims 1 to 16 comprising: a bottom plate (10) comprising an array of electrodes (11); a counter electrode (21) facing said array of electrodes (21); injection means (22) or droplet creation (G) of a first liquid phase (P1) on the surface of said bottom plate (10); injection means (24) on the surface of said bottom plate (10) a second fluid phase (P2) in which are immersed the droplets (G) of said first liquid phase (P1); and a control device for independently establishing a potential difference between each of said gate (11) and said counter electrode (21) so as to determine a movement of said droplets (G) within the volume of said second liquid phase (P2). 18. Device according to claim 17 further comprising means for chemical analysis (30) said droplets (G). 19. Device according to one of claims 17 or 18 further comprising discharge means (25) of the second liquid phase (P2) to determine a circulation of the latter on the surface of said bottom plate (10). 20. Device according to one of claims 17 to 19 further comprising a top plate (20), parallel to said lower plate (10), defining with it a space (50) which can be filled with said second fluid phase (P2) and carrying said counter electrode (21). 21. Device according to one of claims 17 to 19 wherein said counter electrode is constituted by at least one conductor wire immersed or stretched in at least one of said fluid phases (Ρ1, P2). 22. Device according to one of claims 17 to 21 wherein said controller is provided to determine a moving said droplets (G) along a path (GT) their for scanning at least 20%, preferably at least 50% and even preferably at least 80% of the volume of said second liquid phase (P2). Device and method for processing thermoplastic material between two immiscible phases