CRYOPROTECTANT FOR USE WITH A TREATMENT DEVICE FOR IMPROVED COOLING OF SUBCUTANEOUS LIPID-RICH CELLS
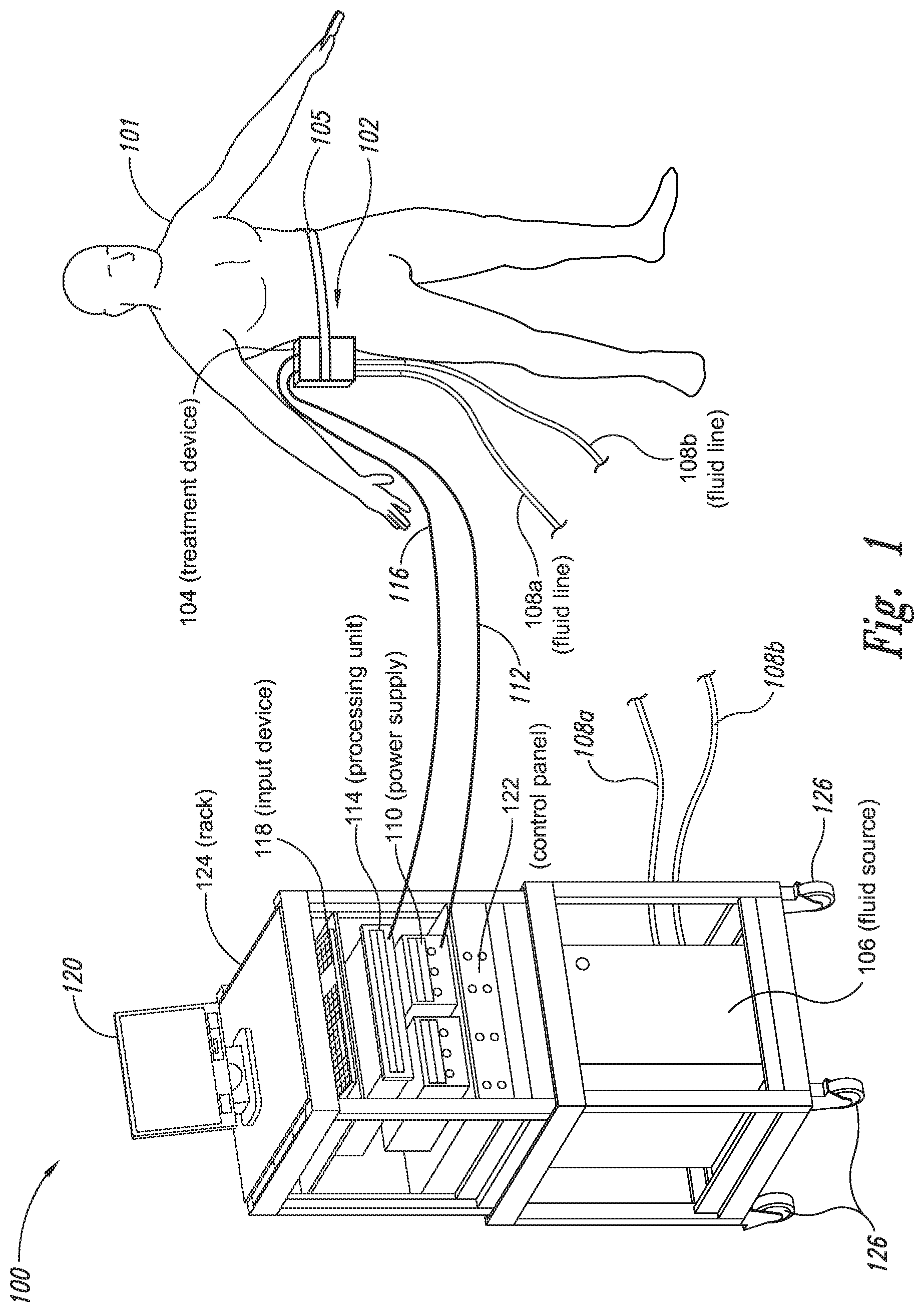
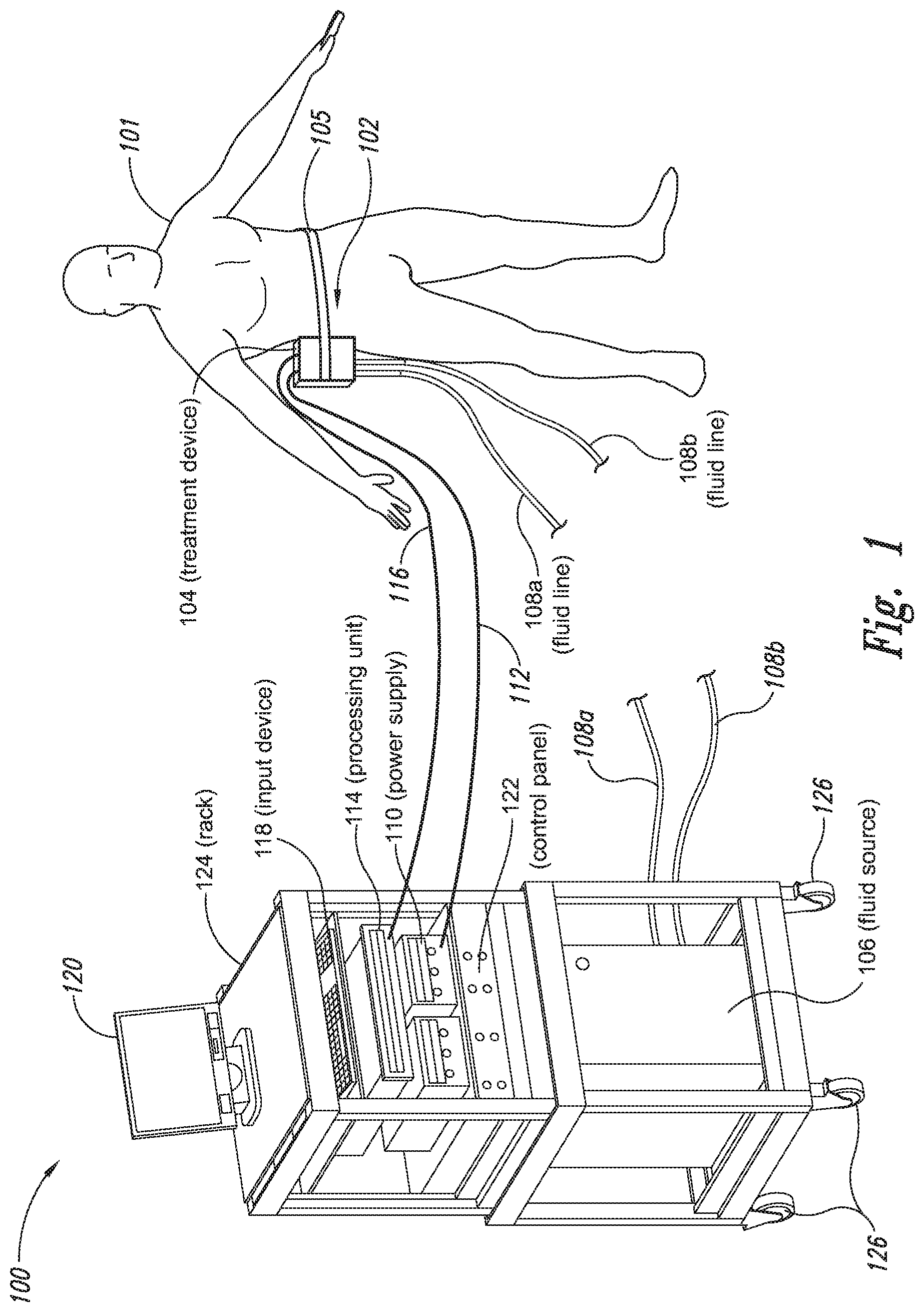
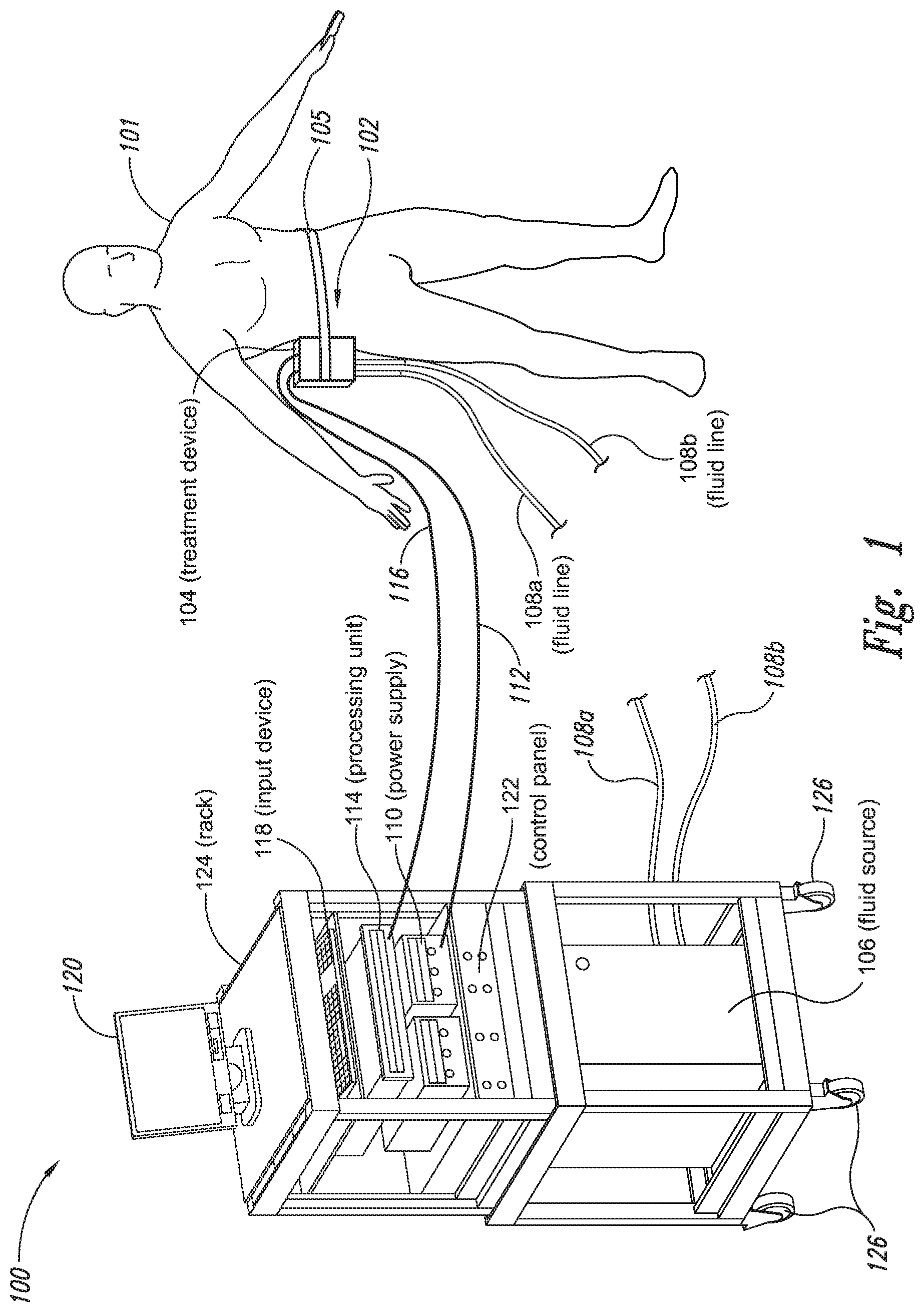
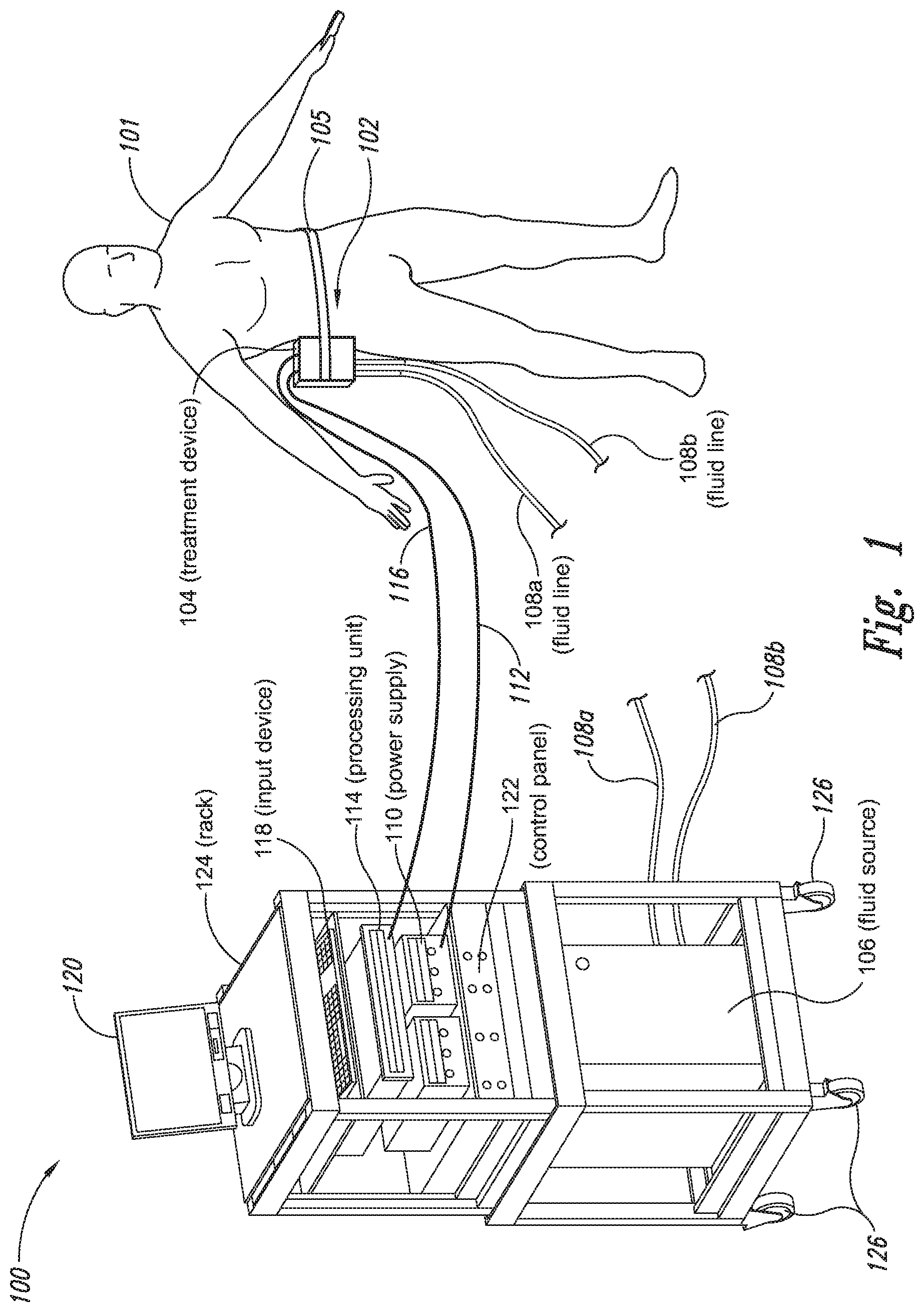
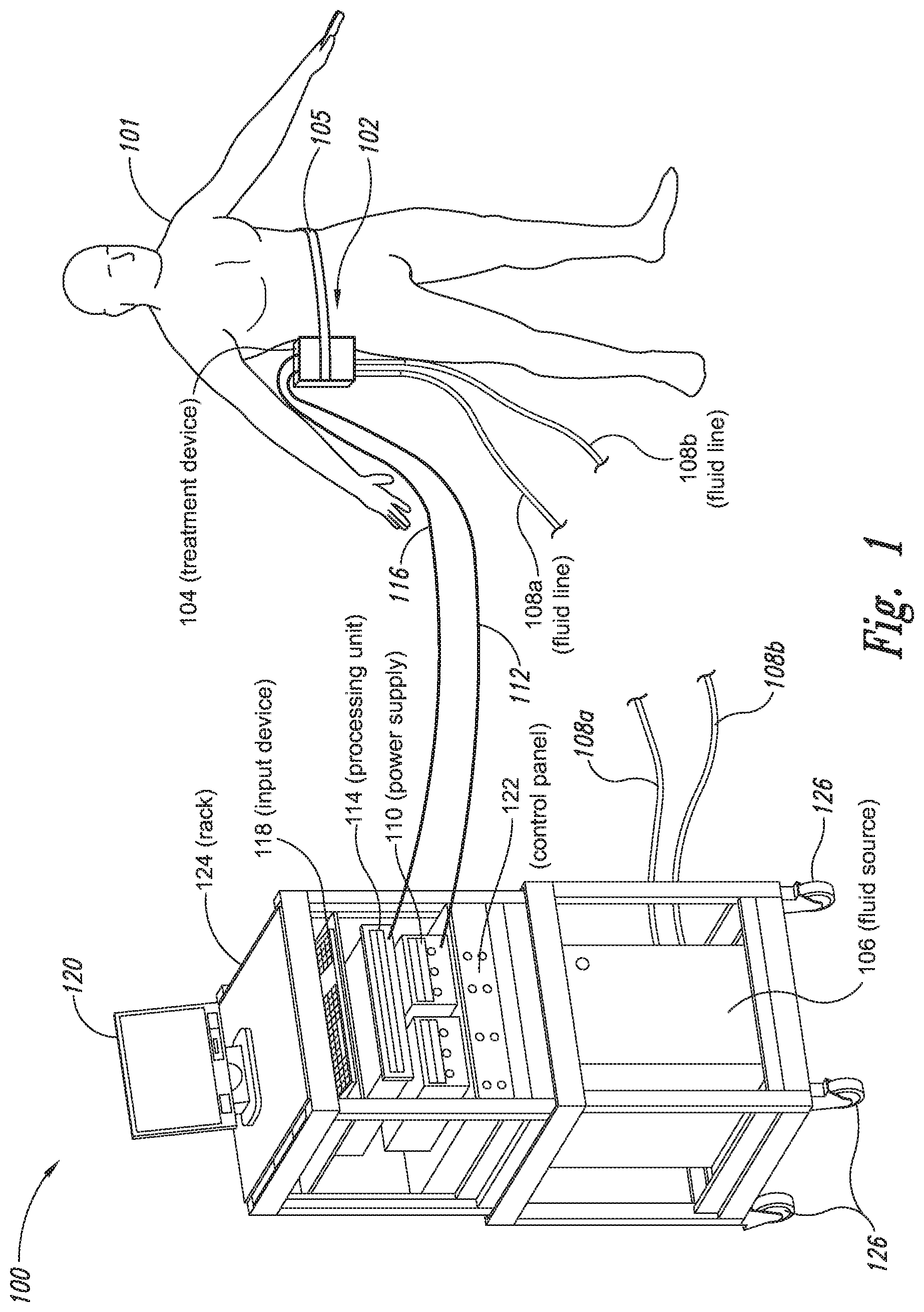
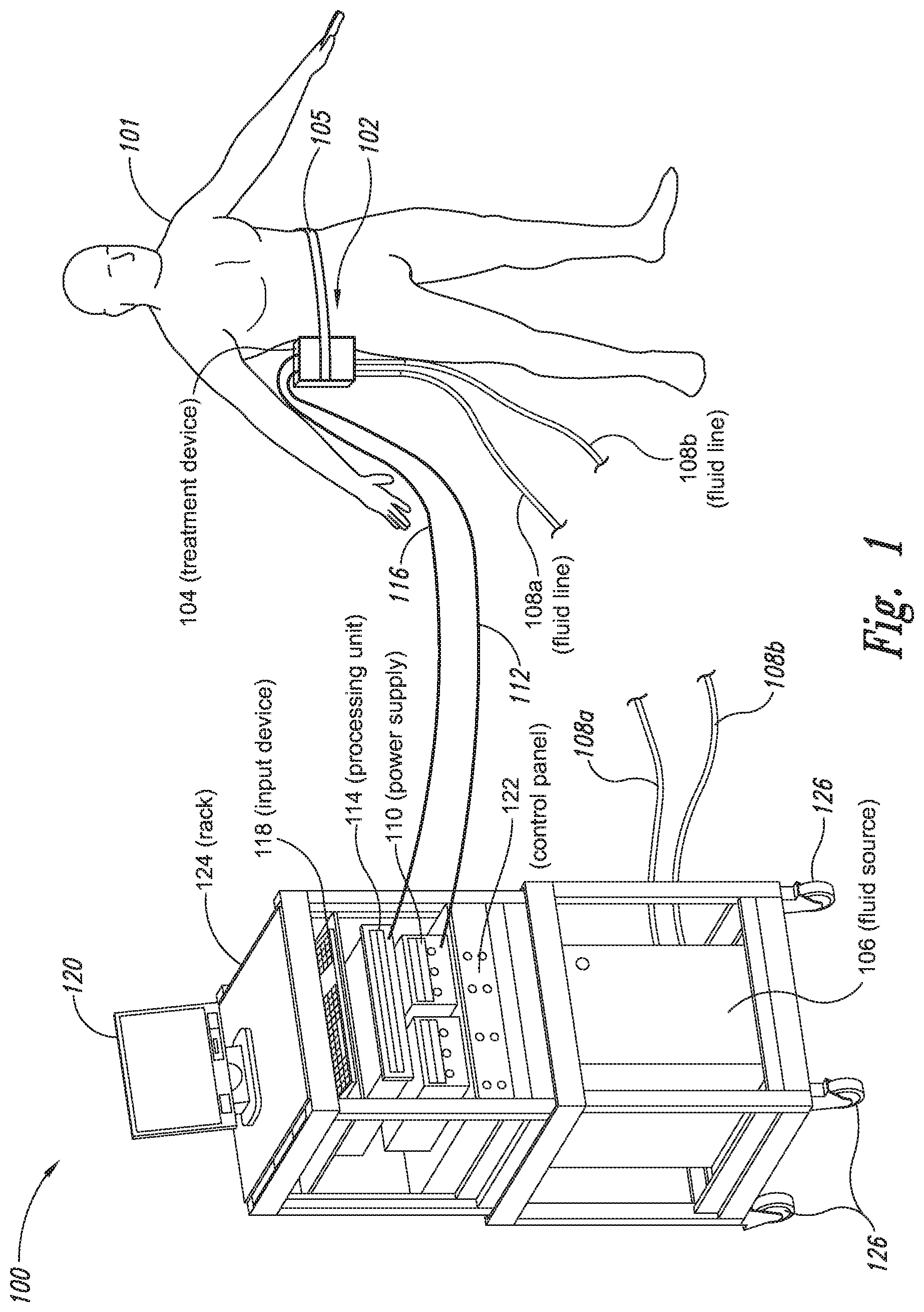
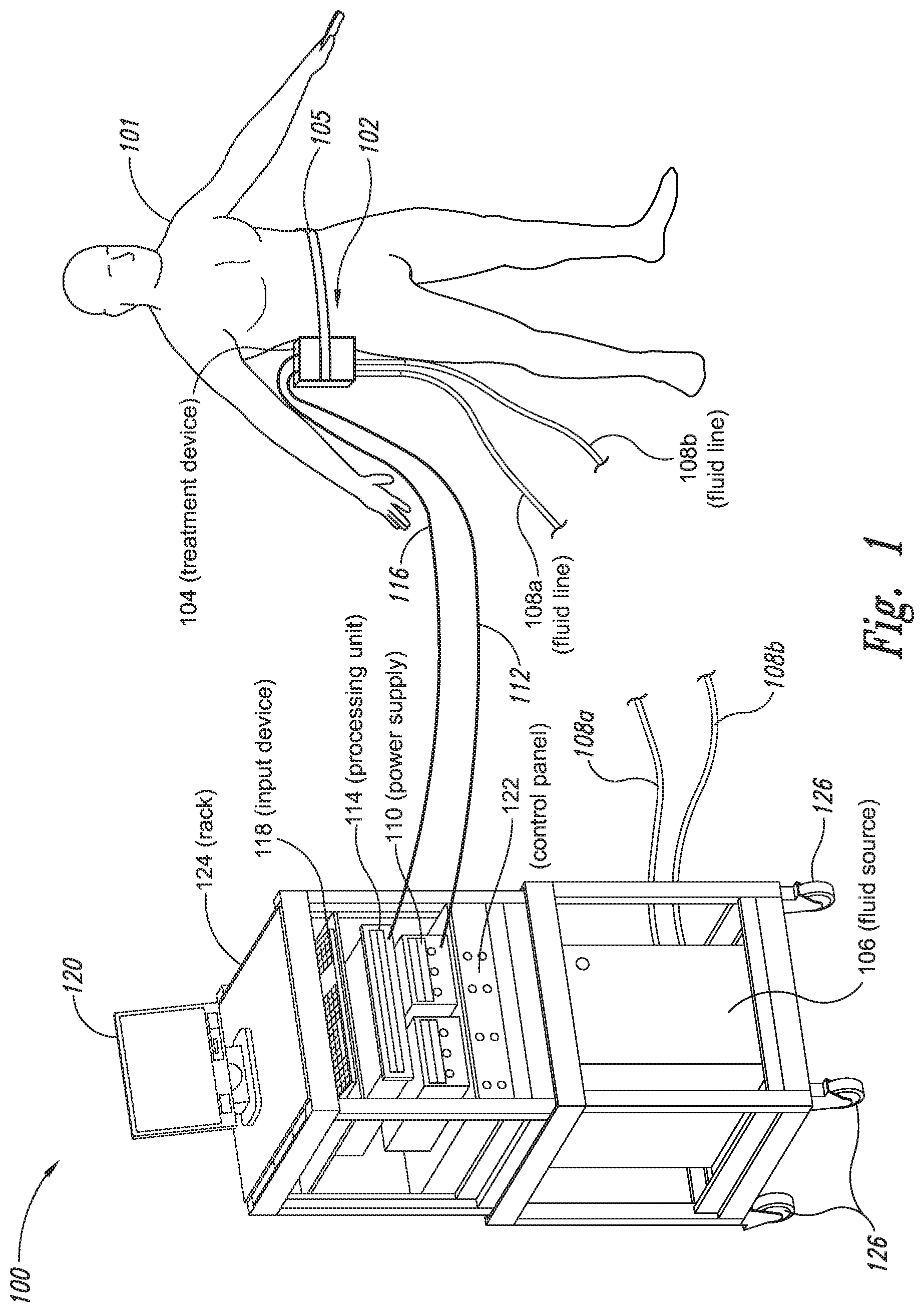
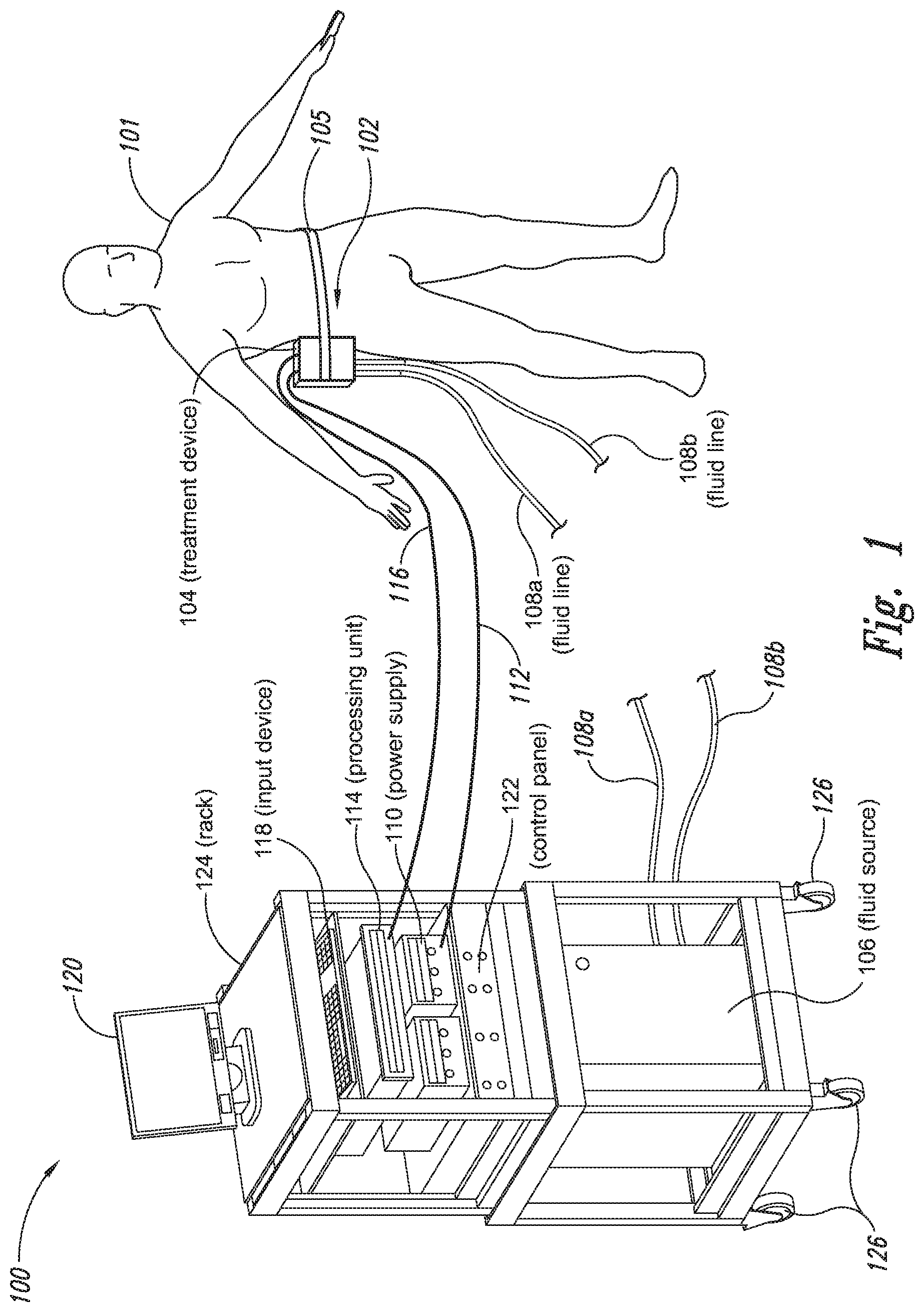
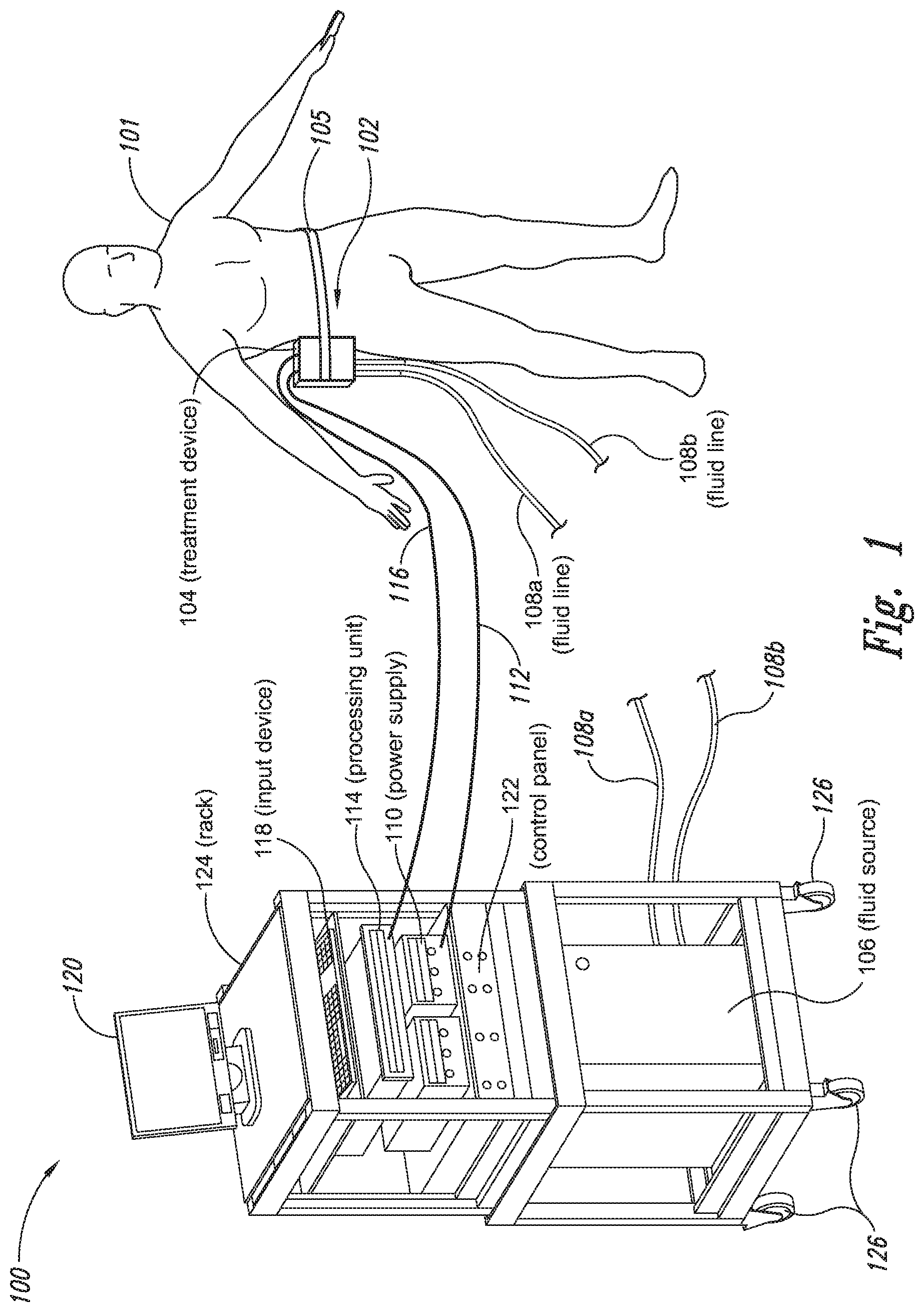
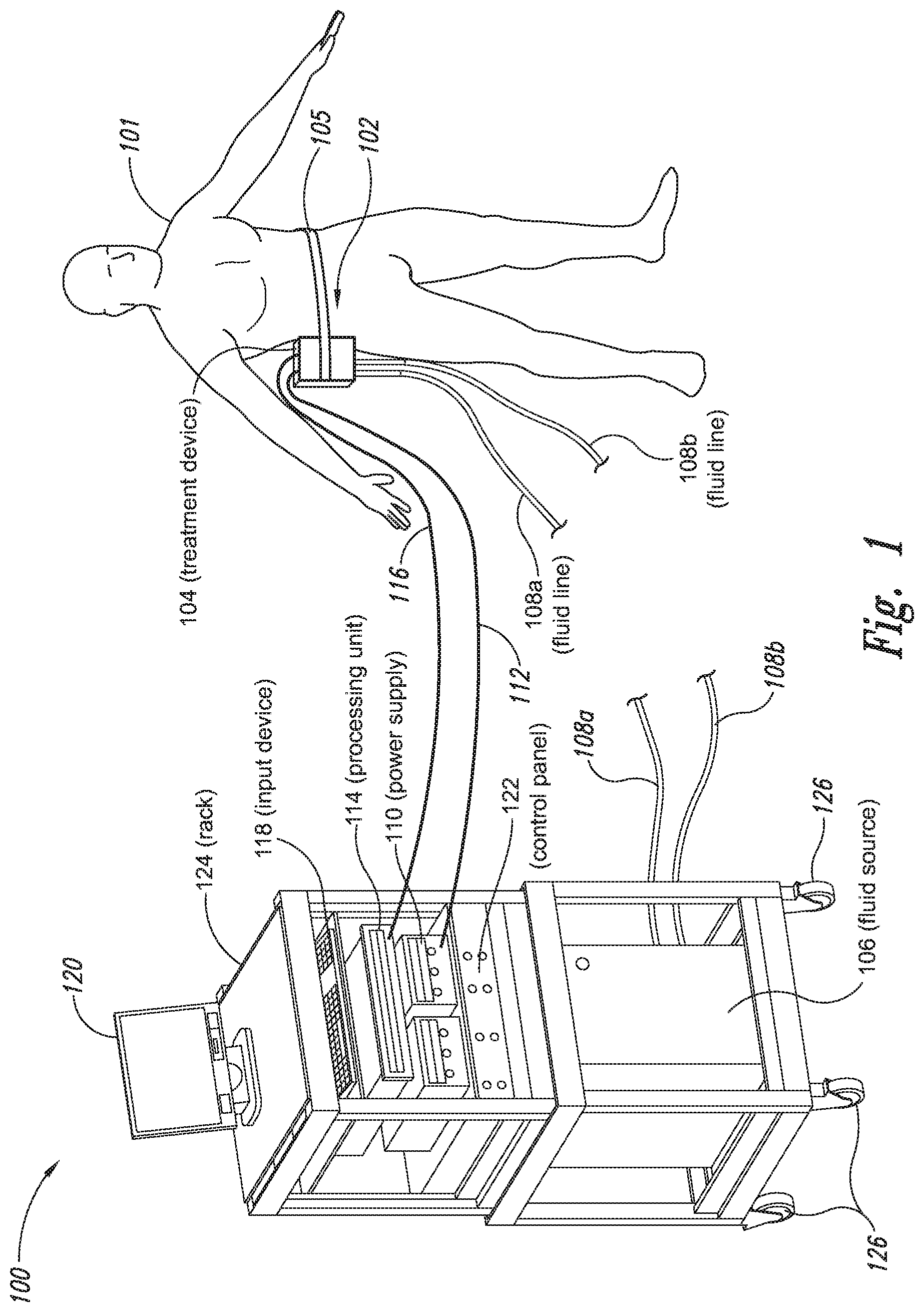
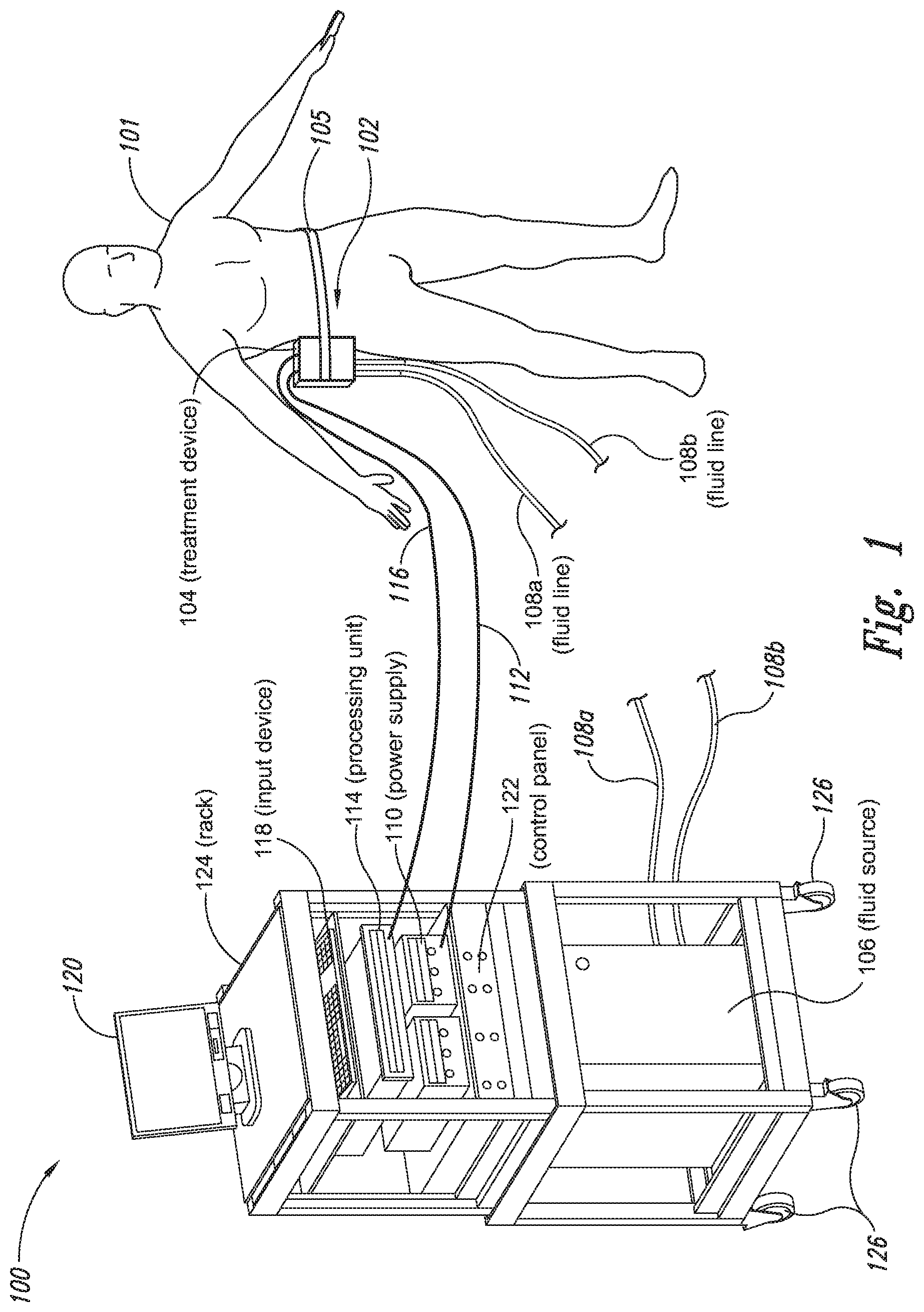
The present application relates to a cryoprotectant for use with treatment devices, systems, and methods for removing heat from subcutaneous lipid-rich cells. Excess body fat, or adipose tissue, can detract from personal appearance and athletic performance. Excess adipose tissue may be present in various locations of the body, including, for example, the thigh, buttocks, abdomen, knees, back, face, arms, and other areas. Moreover, excess adipose tissue is thought to magnify the unattractive appearance of cellulite, which forms when subcutaneous fat protrudes into the dermis and creates dimples where the skin is attached to underlying structural fibrous strands. Cellulite and excessive amounts of adipose tissue are often considered to be unappealing. Moreover, significant health risks may be associated with higher amounts of excess body fat. An effective way of controlling or removing excess body fat therefore is needed. Liposuction is a method for selectively removing adipose tissue to “sculpt” a person's body. Liposuction typically is performed by plastic surgeons or dermatologists using specialized surgical equipment that invasively removes subcutaneous adipose tissue via suction. One drawback of liposuction is that it is a surgical procedure, and the recovery may be painful and lengthy. Moreover, the procedure typically requires the injection of tumescent anesthetics, which is often associated temporary bruising. Liposuction can also have serious and occasionally even fatal complications. In addition, the cost for liposuction is usually substantial. Other emerging techniques for removal of subcutaneous adipose tissue include mesotherapy, laser-assisted liposuction, and high intensity focused ultrasound. Conventional non-invasive treatments for removing excess body fat typically include topical agents, weight-loss drugs, regular exercise, dieting, or a combination of these treatments. One drawback of these treatments is that they may not be effective or even possible under certain circumstances. For example, when a person is physically injured or ill, regular exercise may not be an option. Similarly, weight-loss drugs or topical agents are not an option when they cause an allergic or negative reaction. Furthermore, fat loss in selective areas of a person's body cannot be achieved using general or systemic weight-loss methods. Other non-invasive treatment methods include applying heat to a zone of subcutaneous lipid-rich cells. U.S. Pat. No. 5,948,011 discloses altering subcutaneous body fat and/or collagen by heating the subcutaneous fat layer with radiant energy while cooling the surface of the skin. The applied heat denatures fibrous septae made of collagen tissue and may destroy fat cells below the skin, and the cooling protects the epidermis from thermal damage. This method is less invasive than liposuction, but it still may cause thermal damage to adjacent tissue, and can also be painful and unpredictable. Another promising method of reducing subcutaneous fat cells is to cool the target cells as disclosed in U.S. Patent Publication No. 2003/0220674, the entire disclosure of which is incorporated herein. This publication discloses, among other things, reducing the temperature of lipid-rich subcutaneous fat cells to selectively affect the fat cells without damaging the cells in the epidermis. Although this publication provides promising methods and devices, several improvements for enhancing the implementation of these methods and devices would be desirable. U.S. Patent Publication No. 2003/0220674 also discloses methods for selective removal of lipid-rich cells, and avoidance of damage to other structures including dermal and epidermal cells. A method for inducing collagen compaction, remodeling, and formation is also needed for treatment of loose or sagging skin, age- or sun-damaged skin, or a variety of other skin disorders. Therefore, a method for simultaneously removing lipid-rich cells while providing beneficial collagen effects is also needed. The present disclosure describes devices, systems, and methods for cooling subcutaneous lipid-rich cells with a heat exchanging element and a thermally conductive cryoprotectant. The term “subcutaneous tissue” means tissue lying beneath the dermis and includes subcutaneous fat, or adipose tissue, which primarily is composed of lipid-rich cells, or adipocytes. It may be appreciated that several of the details set forth below are provided to describe the following embodiments in a manner sufficient to enable a person skilled in the relevant art to make and use the disclosed embodiments. Several of the details and advantages described below, however, may not be necessary to practice certain embodiments of the invention. Additionally, the invention may include other embodiments that are within the scope of the claims but are not described in detail with respect to the Figures. In one embodiment, the treatment device 104 is configured to cool subcutaneous lipid-rich cells of the subject 101. In such cases, the treatment system 100 may further include a fluid source 106 and fluid lines 108 The treatment device 104 may also include one or more thermoelectric elements, such as Peltier-type thermoelectric elements. In such cases, the treatment system 100 may further include a power supply 110 and a processing unit 114 operatively coupled to the treatment device 104 via electrical cables 112, 116. In one embodiment, the power supply 110 may provide a direct current voltage to the treatment device 104 remove heat from the subject 101. The processing unit 114 may monitor process parameters via sensors (not shown in The processing unit 114 may be in electrical communication with an input device 118, an output device 120, and/or a control panel 122. The input device 118 may include a keyboard, a mouse, a touch screen, a push button, a switch, a potentiometer, and any other device suitable for accepting user input. The output device 120 may include a display screen, a printer, a medium reader, an audio device, and any other device suitable for providing user feedback. The control panel 122 may include indicator lights, numerical displays, and audio devices. In the embodiment shown in As explained in more detail below, a cryoprotectant applied to the treatment device 104 may allow the treatment device 104 to be pre-cooled prior to being applied to the subject 101 for more efficient treatment. Further, the cryoprotectant can also enable the treatment device 104 to be maintained at a desired temperature while preventing ice from forming on a surface of the treatment device 104, and thus reduces the delay in reapplying the treatment device 104 to the subject. Yet another advantage is that the cryoprotectant may prevent the treatment device 104 from freezing to the skin of the subject. If the cryoprotectant is hygroscopic, it can adsorb moisture from the atmosphere and/or from the skin, which might otherwise form ice. The treatment device 104, the cryoprotectant, and/or other components of the treatment system 100 can be included in a kit (not shown) for removing heat from subcutaneous lipid rich cells of the subject 101. The cryoprotectant can have a freezing point in the range of about −40° C. to about 0° C. and be configured to be applied to an interface between the treatment device 104 and the skin of the subject 101. The kit can also include instruction documentation containing information regarding how to (a) apply the cryoprotectant to a target region and/or a heat exchanging surface of the treatment device 104 and (b) reduce a temperature of the target region such that lipid rich cells in the region are affected while preserving non-lipid rich cells proximate to the heat exchanging surface. The coupling device 502 may include a backside portion 504 proximate to the heat exchanging element 130, a front side portion 508 spaced apart from the backside portion 504, and an intermediate portion 506 between the backside portion 504 and the front side portion 508. In certain embodiments, the coupling device 502 optionally may include a protective layer (e.g., a polymeric film, not shown) attached to the front side portion 508. The protective layer may isolate the front side portion 508 from the environment and may be peeled off to expose the front side portion 508 before treatment. The backside portion 504 may be a film, a plate, a sheet, or other structure constructed from a metal, a metal alloy, ceramics, a polymeric material, or other suitable conductive material. The backside portion 504 may transfer heat between the heat exchanging element 130 and the treatment region 501. The backside portion 504 may also isolate the heat exchanging element 130 from the treatment region 501 for sanitation purposes. The intermediate portion 506 may be a reservoir constructed from a mesh, a foam material, a porous plastic and/or metal, or other materials that may at least temporarily contain a fluid and/or a gel. In one embodiment, the intermediate portion 506 contains, or is loaded with, a cryoprotectant before a treatment process begins. In another embodiment, the intermediate portion 506 may be generally empty before a treatment process begins and only loaded with cryoprotectant immediately before and/or during the treatment process. In any of these embodiments, the intermediate portion 506 may be pressurized with the cryoprotectant or may be at a generally atmospheric pressure during treatment. The front side portion 508 may be a film constructed from a polymeric material, a plastic material, or other material that is at least partially flexible. The front side portion 508 may include one or more apertures 516 in fluid communication with the intermediate portion 506. During treatment, the aperture or apertures 516 may allow the cryoprotectant contained in the intermediate portion 506 to escape to the treatment region 501 of the subject 101 through capillary actions or other mechanisms. For example, the intermediate portion 506 may continually supply the cryoprotectant to the treatment region 501 during treatment. In certain embodiments, the intermediate portion 506 is pre-loaded with excess cryoprotectant. As a portion of the cryoprotectant escapes from the apertures 516, additional cryoprotectant may be supplied from the intermediate portion 506 to the skin of the subject during treatment. In other embodiments, the intermediate portion 506 may be constantly replenished to provide a continuous supply of the cryoprotectant. The cryoprotectant can be absorbed by the skin in the treatment region 501. The degree of cryoprotectant absorption by the skin depends on a number of factors, the most important of which are cryoprotectant concentration, duration of contact, solubility, and the physical condition of the skin. The coupling device 502 optionally may include at least one sensor 514 proximate to the front side portion 508 to measure at least one parameter of the treatment process. The sensor 514 may be a temperature sensor, a pressure sensor, a transmissivity sensor, a bio-resistance sensor, an ultrasound sensor, an optical sensor, an infrared sensor, a heat flux sensor, any other desired sensors, or any combination thereof. An operator may adjust the treatment process based on the measured parameter. In the illustrated embodiment, the treatment device 104 optionally may include a supply device 520 connected to a port 515 of the coupling device 502 by a conduit 522 for supplying and/or replenishing the cryoprotectant in the intermediate portion 506. In the illustrated embodiment, the supply device 520 is a syringe holding a volume of the cryoprotectant. In other embodiments, the supply device 520 may include a pump coupled to a cryoprotectant storage (not shown), or other suitable supply configurations. Optionally, a pressure sensor 524 (shown schematically) may be used for monitoring a cryoprotectant pressure in the intermediate portion 506. The pressure sensor 524 may be operatively coupled to the conduit 522, the intermediate portion 506, or the supply device 520. During treatment, the pressure sensor 524 may provide an electric, visual, or other signal indicating the cryoprotectant pressure in the intermediate portion 506. In one embodiment, an operator may manually adjust the output of the supply device 520 based on the indicated pressure. In another embodiment, the signal from the pressure sensor 524 may be used as a process variable to automatically control the output of the supply device 520. Several embodiments of the treatment system 100 may continually protect the skin of the subject against freezing damage. According to conventional techniques, a cryoprotectant may be topically applied to the skin before a treatment begins. The skin then absorbs the applied cryoprotectant, which dissipates over a period of time. After the cryoprotectant dissipates, in conventional techniques, the skin may be subject to freezing damage. As a result, by continually replenishing the dissipated cryoprotectant from the intermediate portion 506, the treatment system 100 may at least reduce the risk of freezing damage, or even prevent such freezing damage, during treatment. Several embodiments of the treatment system 100 may also reduce the risk of air pockets that can reduce the heat transfer efficiency between the treatment region 501 and the treatment device 104. As the cryoprotectant escapes through the aperture or apertures 516 during treatment, the pressure in the intermediate portion 506 decreases, and air pockets may form. The air pockets may interfere with the heat transfer efficiency between the treatment region 501 and the treatment device 104. As a result, maintaining the intermediate portion 506 at a constant pressure may at least reduce the risk of air pocket formation, and thus improve the efficiency of such heat transfer. Even though the coupling device 502 is illustrated as having the attachment features 510, in certain embodiments, the attachment features 510 may be omitted, and the coupling device 502 may be configured and/or incorporated into other structures. For example, The second sleeve portion 166 may also include attachment features to affix the sleeve 162 to the treatment device 104. In the illustrated embodiment, the second sleeve portion 166 includes four brackets 172 (identified individually as 172 In another embodiment, the second sleeve portion 166 may include brackets that may engage each other. For example, the bracket 172 In addition to the expected advantages described above, one expected advantage of using the sleeve 162 is the improved sanitation of using the treatment device 104. The sleeve 162 may prevent cross-contamination between the skin of the subject and the heat exchanging element 130 because the sleeve 162 is substantially impermeable. Also, operating expense of the treatment device 104 may be reduced because the heat exchanging element 130 does not need to be sanitized after each use. The sleeve 162 may have many additional embodiments with different and/or additional features without detracting from its operation. For example, the first and second sleeve portions 164, 166 may be constructed from the same material (e.g., polyimide) or different materials. The sleeve 162 may include an adhesive layer (not shown) that binds the sleeve 162 to the treatment device 104. After a selected period of time, the treatment device may then be removed from the skin of the subject (block 18), and the process may then end (block 20). Once the treatment device is removed from the skin of the subject, the reduced temperature of the heat exchanging element optionally may be maintained at a desired temperature (block 22). In certain embodiments, the heat exchanging element optionally may be placed adjacent to another region of the skin of the subject to selectively affect lipid-rich cells in a different region of the skin of the subject (block 24). Once the heat exchanging element is placed adjacent to another region of the skin of the subject, the lipid-rich cells are affected (block 16). The treatment device may then be removed from the skin of the subject (block 18) and then the process may end (block 20). Optionally, the cryoprotectant may be reapplied to the heat exchanging element, the skin of the subject, or to an interface between the treatment device and the skin of the subject (block 28) prior to placing the heat exchanging element on another region of the skin of the subject. In another embodiment, a cryoprotectant may be applied to the heat exchanging element, the skin of the subject, or an interface between the treatment device and the skin of the subject to prevent the formation of ice (block 10) as the temperature of the heat exchanging element is reduced to a desired temperature. The heat exchanging element is placed adjacent to the skin of the subject in a desired region (block 14), and the lipid-rich cells are selectively affected (block 16). After a selected period of time, the heat exchanging element may then be removed from the skin of the subject (block 18). Optionally, the cryoprotectant is reapplied to the heat exchanging element, the skin of the subject, and/or an interface between the treatment device and the skin of the subject (block 28), and the temperature of the heat exchanging element is maintained at a desired temperature (block 22). The process of treating the selected region of the skin of the subject optionally may be repeated to selectively affect the lipid-rich cells in a region of the subject while non-lipid-rich cells in the epidermis and/or dermis are not generally affected (block 26). By cooling the subcutaneous tissues to a temperature lower than 37° C., subcutaneous lipid-rich cells may be selectively affected. In general, the epidermis and dermis of a subject have lower amounts of unsaturated fatty acids compared to the underlying lipid-rich cells forming the subcutaneous tissues. Because non-lipid-rich cells usually withstand colder temperatures better than lipid-rich cells, the subcutaneous lipid-rich cells may be selectively affected while maintaining the non-lipid-rich cells in the dermis and epidermis. For example, a range for the heat exchanging elements may be from about −20° C. to about 20° C., preferably from about −20° C. to about 10° C., more preferably from about −15° C. to about 5° C., more preferably from about −10° C. to about 0° C. The lipid-rich cells may be affected by affecting, shrinking, disabling, destroying, removing, killing, or otherwise being altered. Without being bound by theory, selectively affecting lipid-rich cells is believed to result from localized crystallization of highly saturated fatty acids at temperatures that do not induce crystallization in non-lipid-rich cells. The crystals may rupture the bi-lipid membrane of lipid-rich cells to selectively necrose these cells. Thus, damage of non-lipid-rich cells, such as dermal cells, may be avoided at temperatures that induce crystal formation in lipid-rich cells. Cooling is also believed to induce lipolysis (e.g., fat metabolism) of lipid-rich cells to further enhance the reduction in subcutaneous lipid-rich cells. Lipolysis may be enhanced by local cold exposure, inducing stimulation of the sympathetic nervous system. One expected advantage of several of the embodiments described above is that the treatment device may selectively reduce subcutaneous lipid-rich cells without unacceptably affecting the dermis, epidermis, and/or other tissues. Another expected advantage is that the treatment device may simultaneously selectively reduce subcutaneous lipid-rich cells while providing beneficial effects to the dermis and/or epidermis. These effects may include: fibroplasias, neocollagenesis, collagen contraction, collagen compaction, collagen density increase, collagen remodeling, and acanthosis (epidermal thickening). Another expected advantage of several of the embodiments described above is that the heat exchanging element may be pre-cooled in advance of treatment to more efficiently treat the skin of the subject. Further, the embodiments allow the treatment device to be maintained at a temperature at or below 0° C. or at a target temperature because the cryoprotectant may prevent icing on the heat exchanging element and/or on the skin of the subject. In the illustrated embodiment, the method 80 may include applying a cryoprotectant to a treatment region of the skin of the subject (block 82). For example, applying the cryoprotectant may include spraying or smearing the cryoprotectant onto the skin using an instrument including, e.g., a spatula, a spray bottle, and/or a coupling device as shown in A heat exchanging element is subsequently placed adjacent to the skin of the subject (block 84). The heat exchanging element may cool the treatment region that is in contact with the cryoprotectant to selectively affect lipid-rich cells in the region (block 86). During treatment, the cryoprotectant may be continually supplied to the skin of the subject (block 88). The continually supplied cryoprotectant may maintain a sufficient concentration of absorbed cryoprotectant in the epidermis and/or dermis of the subject for reducing the risk of freezing damage. The cryoprotectant may be continually supplied using an absorbent (e.g., a cotton pad, a gauze, or other absorbents) pre-loaded with the cryoprotectant, or using a coupling device releasably attached to the treatment device. A decision is made to determine whether the treatment should be continued (block 90). The determination may be based on time, skin temperatures, and/or other parameters of the treatment process. If the treatment is continued, then the process returns to block 86; otherwise, the process ends. The applied cryoprotectant may at least reduce the risk of freezing damage in the epidermis and/or dermis of the subject during treatment and may even prevent such freezing damage. Without being bound by theory, it is believed that low temperatures may potentially cause damage in the epidermis and/or dermis via at least intracellular and/or extracellular ice formation. Intracellular ice formation occurs when ice forms inside a cell. The ice may expand and rupture the cell as the ice grows through the cellular wall, thus causing cell death. When extracellular ice formation occurs, extracellular water freezes to form ice. As a result, the remaining extracellular fluid becomes concentrated with solutes. The high concentration of the extracellular fluid may cause intracellular fluid to permeate through the semi-permeable cellular wall and eventually cause cell dehydration and death. The high concentration of the extracellular fluid may also interrupt electrical and/or ionic interactions among neighboring cells to cause irreversible protein damage. Applying a cryoprotectant may at least reduce the risk of intracellular and/or extracellular ice formation, or even prevent such ice formation, by reducing the freezing point of water in the body fluid affected by the cryoprotectant. It is believed that after the cryoprotectant is absorbed into the epidermis and/or dermis, the cryoprotectant dissolves in or otherwise combines with water of the intracellular and/or extracellular fluid to delay the onset of ice formation by lowering the freezing point of the solution in which it resides. For example, the cryoprotectant may reduce the freezing point of the body fluid from, e.g., about −2° C. to about −5° C., −10° C., −16° C., or other temperatures suitable for a particular treatment. In some embodiments, the cryoprotectant may have a sufficient concentration in the body fluid such that water in the body fluid does not freeze but instead vitrifies under low temperature conditions. As a result, the onset of intracellular and/or extracellular ice formation may be prevented in these embodiments. One expected advantage of several of the embodiments of the method 80 is that an operator may use lower treatment temperatures for selectively affecting lipid-rich cells of the subject without causing freezing damage to the epidermis and/or dermis of the subject. The applied cryoprotectant may lower the freezing point of the skin of the subject or body fluid in the target region to at least reduce the risk of intracellular and/or extracellular ice formation at such low treatment temperatures. Another expected advantage is that the epidermis and/or dermis of the subject may be continually protected against freezing damage. It is believed that a topically administered cryoprotectant may protect the treatment region of the skin of the subject. After the cryoprotectant is applied to the skin of the subject, the cryoprotectant is believed to enter the epidermis, the dermis, and eventually the blood stream of the subject. The subject's blood stream then may carry the cryoprotectant away from the treatment region. As a result, the cryoprotectant concentration in the treatment region drops, and the freezing point of the subject's affected body fluid increases to heighten the risk of freezing damage. Accordingly, continually supplying the cryoprotectant to the skin of the subject may at least reduce or even prevent such a risk. Another expected advantage of several of the embodiments is that cooling the skin of the subject may increase the residence time of the cryoprotectant and may reduce local and/or systemic side effects of the cryoprotectant. It is believed that the skin of the subject absorbs the cryoprotectant at a slower rate under low temperature conditions than under normal temperature (e.g., body temperature) conditions. Thus, the reduced absorption rate may increase the amount of time it takes for the subject's blood stream to remove the cryoprotectant, and thus prolong the efficacy of the cryoprotectant. It is also believed that certain cryoprotectants at certain concentration levels may be toxic to the subject by causing, for example, denaturation of proteins (e.g., enzymes). Thus, reducing the absorption rate of the cryoprotectant may reduce the cryoprotectant concentration in deeper tissues, and thus may reduce the associated local or systemic side effects. A cryoprotectant suitable to be used in the treatment system 100 of The temperature depressant may include polypropylene glycol (PPG), polyethylene glycol (PEG), propylene glycol, ethylene glycol, dimethyl sulfoxide (DMSO), or other glycols. The temperature depressant may also include ethanol, propanol, iso-propanol, butanol, and/or other suitable alcohol compounds. The temperature depressant may lower the freezing point of a solution (e.g., body fluid) to about 0° C. to −40° C., and more preferably to about −10° C. to −16° C. Certain temperature depressants (e.g., PPG, PEG, etc.) may also be used to improve smoothness of the cryoprotectant and to provide lubrication. The thickening agent may include carboxyl polyethylene polymer, hydroxyethyl xylose polymer, and/or other viscosity modifiers to provide a viscosity in the range of about 1 cP to about 10,000 cP, more preferably in the range of about 4,000 cP to about 8,000 cP, and most preferably from about 5,000 cP to about 7,000 cP. The cryoprotectant with a viscosity in this range may readily adhere to the treatment device, the skin of the subject, and/or the interface between the treatment device and the skin of the subject during treatment. The pH buffer may include cholamine chloride, cetamidoglycine, tricine, glycinamide, bicine, and/or other suitable pH buffers. The pH buffer may help the cryoprotectant to have a consistent pH of about 3.5 to about 11.5, more preferably about 5 to about 9.5, and most preferably about 6 to about 7.5. In certain embodiments, the pH of the cryoprotectant may be close to the pH of the skin of the subject. The humectant may include glycerin, alkylene glycol, polyalkylene glycol, propylene glycol, glyceryl triacetate, polyols (e.g., sorbitol and/or maltitol), polymeric polyols (e.g., polydextrose), quillaia, lactic acid, and/or urea. The humectant may promote the retention of water to prevent the cryoprotectant from drying out. The surfactant may include sodium dodecyl sulfate, ammonium lauryl sulfate, sodium lauryl sulfate, alkyl benzene sulfonate, sodium lauryl ether sulfate, and other suitable surfactants. The surfactant may promote easy spreading of the cryoprotectant when an operator applies the cryoprotectant to the treatment device, the skin of the subject, and/or the interface between the treatment device and the skin of the subject during treatment. The cryoprotectant may also include other additives in addition to or in lieu of the ingredients described above. For example, the cryoprotectant may also include a coloring agent, perfume, emulsifier, an anesthetic agent, and/or other ingredient. In a particular embodiment, the cryoprotectant may include about 30% polypropylene glycol, about 30% glycerin, and about 40% ethanol. In another embodiment, the cryoprotectant may include about 40% propylene glycol, about 0.8% hydroxyethylcellulose, and about 59.2% water. In a further embodiment, the cryoprotectant may include about 50% polypropylene glycol, about 40% glycerin, and about 10% ethanol. The first and second heat exchanging elements 130 The first heat exchanging element 130 The first heat exchanging element 130 The treatment device 104 may further include a mounting element 136 A specific embodiment of the mounting element 136 The treatment device 104 may further include a shaft 133, and the first mounting base 134 The treatment device 104 further includes a handle 140 slidably coupled to the shaft 133 or formed as a part of the shaft 133. The handle 140 is configured to be held by a hand of an operator. For example, the handle 140 may have a grip with grooves to improve stability of the treatment device 104 when held by the operator. The handle 140 further includes an actuator 142 that operates with the shaft 133 to move the second heat exchanging element 130 In operation, an operator may hold the treatment device 104 in one hand by grasping the handle 140. Then, the heat exchanging elements 130 One expected advantage of using the treatment device 104 is that the treatment device may be applied to various regions of the subject's body because the two heat exchanging elements 130 The first and second heat exchanging elements 130 The first and second heat exchanging elements 130 The treatment device 104 is shown in a first relatively flat configuration in One advantage of the plurality of rotatable heat exchanging surfaces is that the arcuate shape of the treatment device may concentrate the heat transfer in the subcutaneous region. For example, when heat exchanging surfaces are rotated about a body contour of a subject, the arcuate shape may concentrate heat removal from the skin. The control system housing 202 may house a processing unit for controlling the treatment device 104 and/or fluid lines 108 The treatment device 104 may further include at each end of the treatment device 104 retention devices 208 The retention devices 208 As shown in The cooling assembly 308 may include a heat sink 312, a thermally conductive interface member 309, and a thermoelectric cooler 314 disposed between the heat sink 312 and the interface member 309. The thermoelectric cooler 314 may be connected to an external power supply (not shown) via connection terminals 316. In the illustrated embodiment, the heat sink 312 includes a U-shaped fluid conduit 310 at least partially embedded in a thermally conductive portion 313 of the heat sink 312. The fluid conduit 310 includes fluid ports 138 Individual retention devices 318 may include a plate 330 and a plurality of fasteners 306 extending through a plurality of apertures 332 (two are shown for illustrative purposes) of the plate 330. In the illustrated embodiment, the fasteners 306 are screws that may be received by the housing 302. In other embodiments, the fasteners 306 may include bolts, clamps, clips, nails, pins, rings, rivets, straps, and/or other suitable fasteners. During assembly, the cooling assembly 308 is first at least partially disposed in the internal space 303 of the housing 302. Then, the retention devices 318 are positioned proximate to the cooling assembly 308, and the fasteners 306 are extended through the apertures 332 of the plate 330 to engage the housing 302. The fasteners 306, the plates 330, and the housing 302 cooperate to hold the cooling assembly 308 together. By applying power to the thermoelectric cooler 314, heat may be effectively removed from the skin of the subject to a circulating fluid in the fluid conduit 310. For example, applying a current to the thermoelectric cooler 314 may achieve a temperature generally below 37° C. on the first side 315 In operation, applying electricity to the motor 325 may cause the rotating member 328 to rotate around the body axis 327 of the motor 325. The off-centered rotating member 328 causes the vibrator 322 to be off-balanced about the body axis 327, and vibration in the frame 324 and the housing 302 may result. The disclosures of U.S. patent application Ser. No. 11/741,271, U.S. patent application Ser. No. 11/750,953, and U.S. Provisional Application No. 60/795,799, are incorporated herein by reference in their entireties. The applicants conducted experiments to cool subcutaneous lipid-rich cells in a pig using a treatment device as shown in Each testing site was cleaned and shaved, and a surface thermocouple was placed on the skin of the pig to control the treatment device. A number of 3″×3″ squares of Webril® Undercast Padding #3175, supplied by Tyco Healthcare of Mansfield Mass. (“Webril”), were soaked with 8 milliliters of either cryoprotectant I or cryoprotectant II. The soaked Webril squares were then placed on the test sites for 5 minutes, and the treatment device was then applied to the Webril squares to achieve a desired surface temperature. Once the desired surface temperature was achieved, the surface temperature was maintained for a treatment period of up to about 30 minutes. After the treatment period, the skin of the pig was inspected for freezing. The results of several experiments indicate that both cryoprotectant I and cryoprotectant II significantly lowered the freezing point of the skin of the pig. In particular, when the surface temperature was between about −12° C. to about −16° C., limited or no skin freezing was observed. Unless the context clearly requires otherwise, throughout the description and the claims, the words “comprise,” “comprising,” and the like are to be construed in an inclusive sense as opposed to an exclusive or exhaustive sense; that is to say, in a sense of “including, but not limited to.” Words using the singular or plural number also include the plural or singular number, respectively. When the claims use the word “or” in reference to a list of two or more items, that word covers all of the following interpretations of the word: any of the items in the list, all of the items in the list, and any combination of the items in the list. The above detailed descriptions of embodiments of the invention are not intended to be exhaustive or to limit the invention to the precise form disclosed above. While specific embodiments of, and examples for, the invention are described above for illustrative purposes, various equivalent modifications are possible within the scope of the invention, as those skilled in the relevant art may recognize. For example, while steps are presented in a given order, alternative embodiments may perform steps in a different order. The various embodiments described herein may be combined to provide further embodiments. In general, the terms used in the following claims should not be construed to limit the invention to the specific embodiments disclosed in the specification, unless the above detailed description explicitly defines such terms. While certain aspects of the invention are presented below in certain claim forms, the inventors contemplate the various aspects of the invention in any number of claim forms. Accordingly, the inventors reserve the right to add additional claims after filing the application to pursue such additional claim forms for other aspects of the invention. A cryoprotectant for use with a treatment device for improved removal of heat from subcutaneous lipid-rich cells of a subject having skin is provided. The cryoprotectant is a non-freezing liquid, gel, or paste for allowing pre-cooling of the treatment device below 0° C. while preventing the formation of ice thereon. The cryoprotectant may also prevent freezing of the treatment device to the skin or ice from forming from moisture seeping out from the skin. The cryoprotectant may further be hygroscopic, thermally conductive, and biocompatible. 1. A system for selectively destroying subcutaneous lipid rich cells of a subject with skin, comprising:
a thermoelectric treatment device having a heat exchanging element having a first side in thermal communication with a heat exchanging surface and a second side opposite the first side, the heat exchanging element being configured to reduce a temperature of the target region such that lipid rich cells in the region are affected while preserving non-lipid rich cells proximate to the heat exchanging surface; and a coupling device being releasably coupled to the treatment device and proximate to the heat exchanging surface. 2. The system of 3. The system of 4. The system of 5. The system of 6. The system of 7. The system of claim 0 further comprising a cryoprotectant applied to an interface between the heat exchanging surface and the skin of the subject. 8. The system of 9. The system of 10. A system for removing heat from subcutaneous lipid rich cells of a subject having skin, comprising:
a treatment device having a housing and a thermal mass in thermal communication with a heat exchanging surface, the thermal mass being configured to reduce a temperature of a region of the skin such that lipid rich cells in the region are affected while preserving non-lipid rich cells proximate to the heat exchanging surface; and a coupling device releasably coupled to the treatment device. 11. The system of 12. The system of 13. The system of 14. The system of 15. The system of 16. The system of 17. The system of 18. The system of TECHNICAL FIELD
BACKGROUND
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION
A. OVERVIEW
B. SYSTEM FOR SELECTIVELY REDUCING LIPID-RICH CELLS
C. COUPLING DEVICE
D. METHOD OF PRE-COOLING A TREATMENT DEVICE USING A CRYOPROTECTANT
E. METHOD OF PROTECTING THE SKIN OF A SUBJECT USING CRYOPROTECTANT
F. CRYOPROTECTANTS
G. TREATMENT DEVICES WITH ROTATABLE HEAT EXCHANGING ELEMENTS
H. TREATMENT DEVICE HAVING A PLURALITY OF COOLING ELEMENTS
I. ADDITIONAL EMBODIMENTS OF TREATMENT DEVICE
J. EXAMPLES