PHARMACEUTICAL COMPOSITION COMPRISING EXTRACT FROM CAMELLIA JAPONICA AS ACTIVE INGREDIENT FOR PREVENTION AND TREATMENT OF VIRAL INFECTION
This application is a continuation-in-part application of PCT/KR2019/006129, filed May 22, 2019, the contents of all of which are incorporated herein by reference. The Sequence Listing in an ASCII text file, named 40054_SequenceListing.txt of 1 KB, created on Oct. 19, 2021, and submitted to the United States Patent and Trademark Office via EFS-Web, is incorporated herein by reference. The present disclosure relates to a pharmaceutical composition for prevention and treatment of a viral infection and, more specifically, to a pharmaceutical composition containing a Influenza is an acute respiratory disease caused by an influenza virus infection, and in Korea, an epidemic of influenza occurs mostly in winter. Such influenza viruses are single-chain RNA viruses belonging to the Orthomyxovirus family, and influenza viruses are classified into Influenza A, B, and C viruses according to the antigen type, and of these, influenza A viruses have various mutations, causing global pandemic from which many people suffer. Retroviruses are also a type of RNA virus, and have reverse transcriptase for inserting RNA containing its own genetic information into DNA of a host cell. Due to these characteristics, most RNA viruses do not follow the central dogma. A capsid protein enclosing viral RNA is composed of a proteinaceous outer membrane for infiltrating into an adjacent host cell when releasing from the host cell. The viruses are characterized in that DNA is synthesized by using RNA as a template together with such reverse transcriptase. RNA viruses, unlike general DNA enzymes, have no ability to correct genetic errors during synthesis, resulting in various viral mutants. Like general viruses, each RNA virus itself infiltrates into a host cell to become a part of the host, and therefore, it is impossible for the immune system of the body to remove only the virus, and when a strong immune system attacks a virus-infected host cell, the host cell is also destroyed, resulting in damages to the functions of body organs. Hence, for the fundamental treatment of a viral infection, a method of primarily inhibiting a viral infection and a method of attacking and selectively destroying a virus-infected cell are effective. Acquired Immune Deficiency Syndrome (AIDS) may be caused by a human immunodeficiency virus infecting humans. In this regard, Korean Patent Publication No. 2016-0024092 discloses a pharmaceutical composition containing a However, the prior art has a problem that, due to the compound derivative, side effects such as drug resistance may occur and the antiviral effect is limited. An aspect of the present disclosure is to provide a pharmaceutical composition for prevention and treatment of a viral infection, the pharmaceutical composition containing as an active ingredient a In accordance with an aspect of the present disclosure, there is provided a pharmaceutical composition containing a According to an embodiment of the present disclosure, the production effect of a pharmaceutical composition for prevention and treatment of a viral infection, which contains as an active ingredient a As used herein, the term “ As used herein, the term “influenza virus” is a causative virus of influenza, wherein virus particles have a coat and are spherical with a diameter of 80-120 nm, but exhibit polymorphism such as filamentous particles. A pathogen of influenza was discovered in 1933, and variant thereof was discovered in 1940. Even people immune to the conventional virus are not immune to variant, which causes influenza in the event of an infection. Therefore, to distinguish between the two, the virus discovered in 1933 was sorted as Type A and the virus discovered in 1940 was sorted as Type B. In 1944, an influenza virus different from Type A or Type B was discovered and named Type C. In accordance with an aspect of the present disclosure, there is provided a pharmaceutical composition containing a The In the pharmaceutical composition, the extract may be obtained by extraction with water, a C1to C4lower alcohol, or a mixture thereof, and the active fraction may be prepared by a preparative liquid chromatography process. The preparative liquid chromatography process may be high-performance liquid chromatography (HPLC) using a concentration gradient of a mixture solution of tertiary distilled water and methanol, as a developing solvent, and the active fraction may be a fraction eluted at a methanol concentration in the range of 30% to 70%, more preferably 40% to 60%, and most preferably 45% to 55%. In the pharmaceutical composition, the virus may be an RNA virus, and the RNA virus may be a negative single stranded RNA virus, positive single stranded RNA virus, or single stranded RNA reverse-transcribing virus, and the RNA virus may be a reovirus, a picornavirus, a calicivirus, a togavirus, an arenavirus, a flavivirus, an orthomyxovirus, a paramyxovirus, a bunyavirus, a rhabdovirus, a filovirus, a coronavirus, an astrovirus, a bornavirus, a retrovirus or an arterivirus. The orthomyxovirus may be an influenza A virus, an influenza B virus, an influenza C virus, influenza D virus, an isavirus, a thogotovirus, or a quaranjavirus. In addition, the influenza A virus may be human influenza A virus or avian influenza A virus. The coronavirus may be SARS coronavirus, MERS coronavirus, or novel coronavirus (SARS-CoV-2). In accordance with another aspect of the present disclosure, there is provided a health functional food containing a In accordance with another aspect of the present disclosure, there is provided use of a In accordance with another aspect of the present disclosure, there is provided a method for treating a viral infection in a subject infected with a virus, the method including administering to the subject a therapeutically effective amount of a In the treatment method, the treatment of the viral infection may be attained by inhibiting the expression of actin in an infected cell of the subject, inhibiting the replication of a virus in an infected cell of the subject, or inhibiting the release of virions from an infected cell of the subject. In accordance with another aspect of the present disclosure, there is provided a method for preventing a viral infection in a subject, the method including administering to the subject a therapeutically effective amount of a In accordance with another aspect of the present disclosure, there is provided a method for inhibiting the expression of actin in an infected cell in a subject, the method including administering to the subject a therapeutically effective amount of a In accordance with another aspect of the present disclosure, there is provided a method for inhibiting the release of virions from an infected cell in a subject, the method including administering to the subject a therapeutically effective amount of a The pharmaceutical composition of the present disclosure can be administered through oral administration or parenteral administration, and more specifically oral administration, but is not limited thereto. As for the parenteral administration, administration can be conducted through various routes, such as intravenous injection, intranasal inhalation, intramuscular administration, intraperitoneal administration, and transdermal absorption. The pharmaceutical composition for treatment of a viral infection of the present disclosure may be administered at a dose of 0.1 mg/kg to 1 g/kg, and more preferably at a dose of 1 mg/kg to 600 mg/kg. The dose may be appropriately adjusted according to the age, sex, and severity of a patient. The pharmaceutical composition may be formulated with various formulations, for example, the pharmaceutical composition may be prepared as a decoction, which may be contained in a retort pouch, or may be formulated in the formulation of a power, a tablet, and a capsule after being dried through hot air drying or freeze drying, or may be formulated as a gel formulation by being mixed with a gelation ingredient, such as gelatin. Any known formulation that is used in the manufacture of pharmaceutical preparations may be used as needed. In addition, one or more pharmaceutically acceptable carriers may be used for the preparation of the pharmaceutical composition of the present disclosure, and examples of these carriers may include, conventional organic or inorganic carries, such as an excipient, a lubricant, a binder, and a disintegrating agent when the composition is in a solid formulation, or a solvent, a solubilizing agent, an emulsifying agent, an isotonic agent, a buffer agent, and a soothing agent may be used when the composition is in a liquid formulation. Moreover, one or more additives, such as a conventional preservative, an antioxidant, a coloring agent, a sweetening agent, an absorbent, and a wetting agent, may be used as needed. Furthermore, the heath functional food of the present disclosure may be a health functional food for an antiviral, wherein the health functional food is a preparation in the form of a capsule, a tablet, a powder, granules, a liquid, pills, flakes, a paste, a syrup, a jelly, or a bar. The health functional food may be used as various formulations suitable for a health functional food, for example, in the formation of a decoction, a drink, a powder, pills, a capsule, a tablet (a coated tablet, a sugarcoated tablet, a sublingual tablet, etc.), and a jelly. The viral infection converts to optimize the normal functions of a host cell for virus replication and virion production. Therefore, the structural change and reorganization of the intracellular actin are significantly converted to affect the entire life cycle including viral recombination and egression. Such cell transformation by viral infection is caused by Rous sarcoma viruses, avian retroviruses, simian virus 40, adenoviruses, and the like, and causes abnormal cell proliferation and morphological modification through several phases. Especially, actin plays an important role when the virus is exported out of the cell after the completion of the assembly of the virus in the host cell, and therefore, the egression of a whole virion can be inhibited by controlling the abnormal overexpression of actin. In the present disclosure, the For conventional treatment for viruses, an inhibitor for cell membrane fusion, an inhibitor for reverse transcriptase changing from RNA to DNA, a protease inhibitor that blocks the protein cleavage process of proteolytic enzymes, and the like were used, and in addition to these, an inhibitor for CCR5 receptor involved in binding at the stage of viruses fusing into host cells, an integrase inhibitor that inhibits becoming proviruses, a medicine that inhibits the final maturation stage in which newly created viruses egressed from the host cell, and the like were used as therapeutic agents. However, most of the medicines currently used, which are synthetic drugs and fail to completely suppress viruses, caused side effects, such as frequent drug resistances, reduced immunity, abnormal adipose distribution syndrome, mitochondrial toxicity, bone metabolism abnormality, and hepatotoxicity. Therefore, many studies are being conducted on the development of natural product-derived materials with few side effects and excellent antiviral effects. Since the fact that Tamiflu, which is used to treat novel swine-origin influenza A (H1N1), is derived from According to the present invention, it was confirmed that an active substance contained in the chromatographic fraction (F2) of the Hereinafter, the present disclosure will be described in detail with reference to examples. However, the present disclosure is not limited to the examples described below, but may be implemented in various different forms. The following examples are provided to complete the present disclosure and to fully inform a person skilled in the art the scope of the present disclosure. In order to prepare a The The gas chromatography-mass spectrometer (GC-MS) analysis and thin layer chromatography (TLC) analysis were performed on the obtained Specifically, the GC-MS analysis on the fraction and CWE having biological activity was performed using GC-MS (Agilent Technologies 5975C) equipped with CTC CombiPAL autosampler system (Palo, Alto, USA). The column used for sample analysis was HP-5 column (Agilent Technologies, 250 μm×0.25 μm×30 m), and the column was held at 50° C. for 5 minutes, and the temperature of the column was raised by 10° C. per minute, and then the column was held at 310° C. for 5 minutes. Each sample was dissolved to 10 mg/mL using a suitable solvent for each for GC-MS analysis, and then 5 μL of each was injected by splitless injection. For TLC analysis, a hydroquinone standard (Sigma-Aldrich, USA), The GC/MC analysis results identified that hydroquinone (91-93% homogeneity), which is expected to have inhibitory activity against virus and host cell membrane binding, was contained, wherein 11.9% and 5.5% of hydroquinone were contained in CWE and the F2 fraction, respectively. Although the content of hydroquinone contained in the F2 fraction was lower than that of CWE, a better effect was observed in the F2 fraction, and thus the above material was expected to a play a subsidiary role in terms of antiviral effect, but it was confirmed that the inhibition for the assembly and egression of the completed viral body (virion) essentially attributes to the antiviral effect of the active substance in the F2 fraction. Through the thin layer chromatography (TLC) analysis, hydroquinone (Rf=0.94) was confirmed at 254 nm in the F2 fraction fractionated from the The viral infection converts to optimize the normal functions of a host cell for virus replication and virion production. Above all, the structural change and reorganization of the intracellular actin are significantly converted to affect the entire life cycle including viral recombination and egression. Such cell transformation by viral infection is caused by Rous sarcoma viruses, avian retroviruses, simian virus 40, adenoviruses, and the like, and causes abnormal cell proliferation and morphological modification through several phases. Especially, actin plays an important role when the virus is exported out of the cell after the completion of the assembly of the virus in the host cell, and therefore, the egression of whole virions can be inhibited by controlling the abnormal overexpression of actin. Therefore, the effects of inhibiting the expression of cytoskeletal actin gene and inhibiting the release of virions, which were formed in host cells infected with viruses to cause secondary infection in neighbor cells, according to the treatment with the Specifically, in order to investigate whether the cytoskeletal actin gene expression was inhibited, a mouse macrophage cell line (Raw264.7) was treated with the The expression level of the actin gene was quantified through RT-PCR. For performing this, first, total RNA of the cultured cells was extracted from Raw264.7 cells by using Trizol reagent (Invitrogen, MA, USA). That is, the cells were lysed by addition of 1 mL of Trizol reagent, and left at room temperature for 5 minutes, and then 200 μL of chloroform was added, followed by centrifugation at 13,500 rpm for 15 minutes. Thereafter, the transparent supernatant (500 μL) was taken out and transferred to a new tube, and an equal amount of isopropyl alcohol was added, followed by centrifugation at 13,500 rpm for 10 minutes, thereby precipitating RNA. The RNA precipitate was washed with 0.75 mL of 70% ethanol diluted in distilled water and treated with diethyl pyrocarbonate (DEPC, Sigma-Aldrich), dried in air, and used as a sample for reverse transcription. In addition, the total RNA was treated with the As a result, it was confirmed that the expression of actin was inhibited when the cells were treated with 100 μg/ml the In order to compare the protein expression level of actin, Raw264.7 cells were treated with the F2 fraction at 1, 25, and 50 μg/mL, and then subjected to immunocytochemical staining. Specifically, for immunocytochemical analysis, the Raw264.7 cells cultured in a slide culture chamber were fixed in 4% paraformaldehyde for 15 minutes and washed with a phosphate buffer (PBS) supplemented with 100 mM glycine for 5 minutes, followed by the addition of 0.1% Triton X-100 (Sigma-Aldrich) solution as a cell membrane penetration solution, and then the cells were left at room temperature for 30 minutes. The cells were blocked by the treatment with 1% bovine serum albumin, followed by incubation at room temperature for 30 minutes, and then washed three times with a phosphate buffer. Then, the beta-actin monoclonal antibody was diluted 1:200 in the Tween 20-phosphate buffer containing 1% bovine serum albumin, followed by an antigen-antibody reaction at room temperature for 3 hours, and then washing was conducted. Thereafter, the cells were reacted with the secondary antibody conjugated with Alexafluor-594 attached thereto for 2 hours, followed by observation using a fluorescence microscope. As a result, the entire distribution of cytoskeletal actin was low, and these results mean that the actin gene control by the To analyze the viruses that were egressed from packaging cells (GP2-293) by the Specifically, a commercially constructed virus infection system (pQCXIP Retroviral Vector, Clontech, CA, USA) was used to investigate the virion release inhibitory effect of the As a result, the RNA viral vector gene of 194 bp was not amplified in both the test groups treated with CWE (100 μg/mL) and the F2 fraction (50 μg/mL), and thus secondary infection by viruses was effectively controlled in the vicinity of virus-infected cells ( To search the antiviral effect, a cytopathic effect (CPE) inhibition test and an MTT (3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay were performed using sensitive cells in vitro. The antiviral agents, amantadine (M2 protein inhibitor) and ribavirin (RNA synthesis inhibitor), were used as positive controls. The order of tests was cell adhesion, the analysis of cytotoxicity and antiviral activity against viruses (Flu A 2 types; PR8/Hong Kong, Flu B 1 type; Lee) for the cytopathic effect (CPE) test, and then the investigation of antiviral activity using the MTT assay. Madin-Darby Canine Kindey (MDCK) cells were used as sensitive cells for culturing each virus, and a minimum essential medium (MEM) containing 10% fetal bovine serum and penicillin/streptomycin/nystatin at 1% for each was used as a medium for maintaining and culturing MDCK cells. The number of seeded MDCK cells was maintained at 2.0×105cells/well, and the serum-free MEM containing a vitamin solution, diglucose, and trypsin was used as a medium for virus infection. The viruses for infection were cultured in a 75-cm2tissue culture flask. When the adhering cells fell out due to the occurrence of CPE, freezing-thawing was repeated twice, followed by centrifugation at 1,500×g for 5 minutes, and the cell residue was discarded, and then the supernatant containing viruses was dispensed into frozen vials and stored at −80° C. for use. As for the infectious titer of the viruses for infection, the 50% cell culture infection dose (CCID50) was calculated, and 30,000-fold dilutions of the crude cultures (1.2, 0.6, 3.8) of each of Flu A (PR8, Hong Kong) and Flu B (Lee) were used. As for the antiviral effect test for the As a result, as shown in Table 2 below, in the CC50(μg/mL) analysis, the cytotoxicity of each of the The antiviral effect on novel coronavirus (SARS-CoV-2) was investigated according to the SARS-CoV-2 Pseudovirus Assay by using a fluorescent biosensor system (Montana Molecular, USA), following the manufacturer's protocol. First, a human lung cancer cell line (A549) was seeded at 5×104cells/well in a 96-well plate, and cultured using DMEM medium containing 10% fetal bovine serum. A transduction mixture containing 3.3×108Vg/mL of Pseudo SARS-CoV-2 Green-Reporter pseudovirus and 2 mM sodium bytyrate was added to the cultured cells. To the mixture, 10 or 50 ug/mL of CWE, or 10 or 20 ug/mL of F2 was added, or chloroquine (PC, CQ) was added for a positive control. In addition, an untreated control group (Ctrl) and a virus-treated group (NC) with only virus treatment were used as negative controls. After the cells were incubated from 48 hours under conditions of 37° C. and 5% CO2, the medium was removed, and an equal amount of DMEM medium was added, followed by a fluorescence microscope (Eclipse Ti—S, Nikon, Japan) and a fluorescence microplate reader (VICTORX2, PerkinElmer, USA) used for. As a result, the CWE and F2 fraction significantly inhibited the infection with SARS-CoV-2 virus. Especially, the treatment with the 20 ug/mL F2 fraction showed an antiviral effect at the same level as the positive control, chloroquine ( In conclusion, according to the pharmaceutical composition containing the The present disclosure has been described with reference to the embodiments, but these are intended to be merely illustrative, and a person skilled in the art will understand that various modifications and other equivalent embodiments can be derived therefrom. Accordingly, the true scope of protection of the present disclosure should be defined based on the appended claims. An aspect of the present invention provides a pharmaceutical composition containing 1. A method for preventing, improving, or treating a viral infection in a subject, the method comprising administering to the subject a therapeutically effective amount of a 2. The method of 3. The method of 4. The method of 5. The method of 6. The method of 7. The method of 8. The method of 9. A method for inhibiting the expression of actin in an infected cell in a subject infected with a virus, the method comprising administering to the subject a therapeutically effective amount of a 10. A method for inhibiting the release of virions from an infected cell in a subject infected with a virus, the method comprising administering to the subject a therapeutically effective amount of a CROSS REFERENCE TO RELATED APPLICATION
INCORPORATION BY REFERENCE OF SEQUENCE LISTING
TECHNICAL FIELD
BACKGROUND ART
DISCLOSURE OF INVENTION
Technical Problem
Solution to Problem
Advantageous Effects of Invention
BRIEF DESCRIPTION OF DRAWINGS
MODE FOR CARRYING OUT THE INVENTION
Definition
DETAILED DESCRIPTION
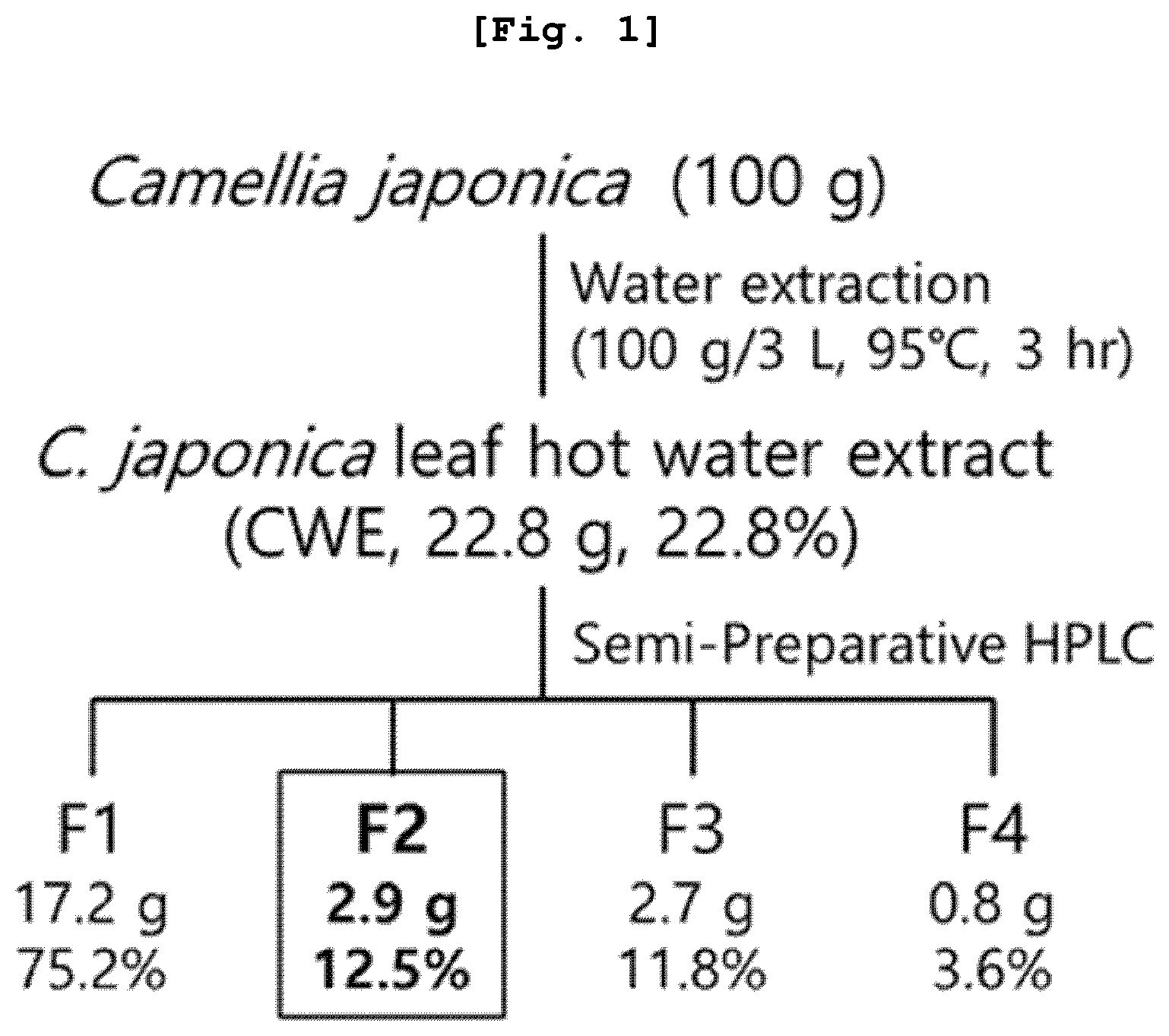
Example 1: Preparation of
Example 2: Separation and Fractionation of
Example 3: GS-MS and TLC Analysis
Example 4: Inhibition of Cytoskeletal Actin Gene Expression
Forward TACAGCTTCACCACCACAGC 1 Reverse AAGGAAGGCTGGAAAAGAGC 2 Example 5: Immunocytochemical Staining Analysis
Example 6: Inhibition of Virion Release
Example 7: Antiviral Effect Test
Antiviral activity comparison test results Antiviral activity (EC50: ug/ml) Selectivity index Toxicity FluA FluA FluB FluA FluA FluB CC50 H1N1 H3N2 — H1N1 H3N2 — NO Code (ug/ml) PR8 HongKong Lee PR8 HongKong Lee 1 CWE (μg) >2400.0 31.4 <29.6 74.0 >76.4 >81.1 >32.4 2 F2 (μg) >2400.0 <29.6 <29.6 45.7 >81.1 >81.1 >52.5 4 Solvent >42.0 >42.0 >42.0 >42.0 ND ND ND (methanol) 5 AMT (μM) >100.0 >100.0 2.3 >100.0 ND >43.5 ND (Amantadin) 6 RBV (μM) >100.0 29.9 13.4 19.6 >3.3 >7.5 >5.1 (Ribavirin) Example 8: Antiviral Effect Test on Novel Coronavirus