CONTROL OF CRUSTACEAN INFESTATION OF AQUATIC ANIMALS
CONTROL OF CRUSTACEAN INFESTATION OF AQUATIC ANIMALS FIELD OF INVENTION The present invention relates in its broadest aspect to the production of aquatic animals such as fish. In particular there is provided novel and improved means of controlling parasitic crustacean infestations of fish, including farmed fish, using juvenile hormone analogue compounds. TECHNICAL BACKGROUND AND PRIOR ART Ectoparasitic infestations constitute considerable problems in the fish farming industry as well as in wild fish. However, the problems are particularly serious in farmed fish in both fresh water and sea water environments. Damages due to ectoparasitic infestations of fish result in considerable losses and increased workloads for fish farmers. Among ectoparasites in fish and other aquatic animals, parasitic species of crustacean ectoparasites, also generally referred to as sea lice, are particularly harmful. Thus, infestation with sea lice in- eluding Lepeophtheirus salmonis and Caligus elongatus is considered to be one of the most important disease problems in farming of salmonids, especially in Atlantic salmon (Salmo salaή and rainbow trout (Oncorhynchus mykiss). In addition to the costs that are associated with treatment, lower classification ratings of slaughtered fish and reduced growth rate due to reduced feed intake contribute to the economic losses caused by sea lice. The life cycle of Lepeophtheirus salmonis is direct, meaning that no intermediate host is required for the development of the adult lice. The two nauplius stages of this species are pelagic. The infective stage, the copepodites, attach to fish, normally on their ventral surfaces and on the fins. It moults to the first chalimus stage, which is attached to its host by a frontal filament projecting from the leading edge of the parasite's carapace. All four chalimus stages are attached to the host by this filament. At the moult to the first pre-adult stage, the frontal filament is normally lost, and the parasite may move around on the skin of the host. Adult males may mate with pre-adult females, which may therefore already be pregnant when they moult to adults. The eggs are stored in egg sacs hanging from the
genital segment of the body. The completion of the life cycle normally takes about six weeks at a water temperature between 9 and 12°C. The life cycle of Caligus elongatus is similar to that of Lepeophtheirus salmonis. In addition to the damages caused in farmed fish, recent research has shown that sea lice could be the most important single cause leading to weakening of several wild salmon stocks. The emigration of salmon smolt from river systems is often coincidental with rising sea water temperatures in the fjord and coastal areas, which leads to a massive attack of copepodites (sea lice larvae) on the smolt. An attack of 40 or more sea lice on salmon smolt is fatal to fish weighing less than 25 grams, and in several regions premature return of post-smolt sea trout has been observed. The decline in the stock of salmonids that have been documented in several salmon rivers over the past few years, could be related to the fact that the amount of sea lice in connection with fish farming has increased. Up till now, the most common treatment of fish ectoparasites involves bathing or immersing the fish in a treatment solution comprising an antiparasitically active compound. This includes both skin and gill parasites. Bathing in formalin is a widespread treatment against many ectoparasites, especially in fresh water, while bathing in a solution of an organo- phosphate such as e.g. metrifonate, dichlorvos or azamethiphos, a pyrethroid compound such as pyrethrum, cypermethrin or deltamethrin (WO 92/16106), or hydrogen peroxide are the most common bath treatments against e.g. sea lice. These compounds act directly on the ectoparasites via the water and a possible absorption of the active substance into the fish itself is unimportant for the effect of the active substances on the parasite. Substances that are effective against parasitic infestations via oral administration have also been tested in fish. Substances such as the chitin synthesis inhibitors diflubenzuron and teflubenzuron, are examples of substances, which, if administered orally, can be effective against parasitic diseases in fish. In addition to the substances mentioned above, wrasse (Labridae) has been used extensively to keep sea lice infestations under control. Organophosphates and hydrogen peroxide are only effective against the pre-adult and adult stages of sea lice (the last 3 stages of the total of 8 stages which exist on the skin of salmonids), while pyrethroids and ivermectin also have a more or less well-defined effect against the other 5 stages. None of these substances protect against new infestations af- ter the treatment has been completed.
Hydrogen peroxide is corrosive, and must therefore be handled with great care. Transport of hydrogen peroxide requires certain precautionary measures, as it is defined as hazardous goods in great quantities. The organophosphates are toxic to humans and must therefore be treated with caution. The organophosphates can be absorbed through the skin and lead to poisoning. The therapeutic margin for organophosphates and hydrogen peroxide is small. Fish mortality has been reported on several occasions, due to overdosing of such drugs. Pyrethroids have, in addition to their effect against pre-adult and adult lice, also an effect against the attached stages. They are not acutely toxic to the user, but is a drug group that is toxic to fish, especially small fish. Overdosing and increased mortality of the target fish is thus possible. In addition to bath treatments, oral treatments for parasite control in fish have been developed. Two chitin synthesis inhibitors, diflubenzuron and tefiubenzuron, have been documented for use against sea lice in salmon. Diflubenzuron and tefiubenzuron act by inhibiting the synthesis of chitin which is an im- portant component of the cuticle of insects and crustaceans. At each moulting or ectdysis, a new synthesis of chitin is required for development. If this synthesis is inhibited, the development of the insect or crustacean will be halted, and the animal under development will die. In principle, chitin synthesis inhibitors will be effective against all organisms containing chitin. Fish and mammals do not contain chitin and will therefore not be affected by substances within this drug group. This is reflected in very low toxicity for fish and mammals, including humans. Diflubenzuron and tefiubenzuron are administered to the fish via the feed, absorbed and distributed to skin and mucus, where the concentration will be high enough to inhibit the development of the parasite. The disadvantages of diflubenzuron and tefiubenzuron include that these substances have no effect against adult stages of the ectoparasites which do not actively synthesise chitin. Neither do they have any effect beyond the period of treatment, as attacks by new parasites may occur within days of completing the treatment. This is due to the fact that the substances are eliminated relatively fast so that the concentration hereof rapidly ends up below therapeutically active levels in skin and mucus. The treatment must therefore be repeated if there is a continu-
ous risk of parasite infection from the surroundings which, under normal circumstances, is often the case. Ivermectin impairs the transfer of neural impulses in insects and crustaceans. This leads to paralysis and death. Mammals and fish are also affected by ivermectin. However, the same transfer mechanisms that are affected in insects, are only found in the brain of fish and mammals. Mammals have an advanced blood-brain barrier which prevents toxic effect from lower concentrations. The blood-brain barrier in fish is less developed and this causes a substantial transition to the brain of ivermectin in treated fish, and toxic symp- toms are found at relatively low concentrations. Ivermectin, however, is effective for treatment of ectoparasites in fish provided that precautions with the dosing are taken. Toxic effects and mortality may arise if the fish are overdosed. Ivermectin is often dosed 1 or 2 times a week to control sea lice infestation in salmonids. This means that the substance must be added on a continuous basis to maintain therapeutic effect and keep the fish reasonably free of lice. Ivermectin is eliminated at a slow rate which means that the effect of the treatment is maintained for 2 to 3 weeks after the treatment has been completed. This also means, however, that the treatment involves a long withdrawal period before the fish can be slaughtered and consumed. Up till now, ivermectin has not been approved for use in fish in any country. As it appears from the above summary of the state-of-the-art, there is an industrial need for improved means of controlling parasitic infestations in fish, which are antiparasitically effective and non-toxic to the fish and which are non-toxic to the end-user and environmentally friendly. The metamorphosis of arthropods (insect and crustacean species) is controlled by several hormones. In insects, such hormones include moulting hormones (MH), also referred to as ecdysones, and juvenile hormones (JH) that are terpenoid compounds. Together with the moulting hormones circulating in the bloodstream of the insects, the juvenile hor- mones that are released from the corpora allata in the insect's head play vital roles in growth, development and reproduction of insects. If insects are treated with an excess of the juvenile hormone at an early stage in their development, they remain at a juvenile stage or develop into a sterile adult insect.
Whereas natural JHs have not been used commercially as insect controlling compounds, several JH analogues (JHAs), also referred to herein as juvenile hormone-like compounds, juvenoids or JH mimics, are used as insect controlling compounds. Examples of such compounds include epofenonane, triprene, methoprene, hydroprene, kinoprene, phenoxycarb and those compounds disclosed in US 4,061 ,757. Currently, typical uses of JH analogues include control of mosquitoes, mites, ants, aphids, cockroaches and fleas. It has now been discovered that compounds having juvenile hormone activity in insects are highly active in the control of crustacean infestations of aquatic animals. SUMMARY OF THE INVENTION Accordingly, the present invention pertains in a first aspect to the use of a compound having juvenile hormone activity, in the manufacturing of a medicament for controlling crustacean infestation of aquatic animals including fish such as wild and farmed fish of the Salmonidae family. In a further aspect, there are provided novel juvenile hormone analogue compounds se- lected from the group consisting of (i) a compound of the general formula I where R1 is alkyl, branched alkyl including alkenyl or alkadienyl, optionally substituted isoprenoid or alkoxy substituted alkyl, R2 is hydrogen or alkyl, X is oxygen, sulfur, methyl- ene, carbinol or carbonyl, Y is nitrogen or methine and Z is nitrogen, methine or nitrogen oxide;
(ii) a compound of general formula II where R1 is 2,2-dimethylcyclopropyl, 2,2-dihalo-3,3-dimethylcyclopropyl, 2-methyl-1- propenyl or 3-methyl-1 ,2-butadienyl, R2 is hydrogen or methoxy, R3 is alkyl, alkenyl, ace- tyl, formyl or carbomethoxy, R4 is hydrogen or R3 and R4 together is methylenedioxy and X is oxygen or sulfur; (iii) a compound of the general formula III where R is 2-methylbutyl, 2,2-dimethylcyclopropyl or 2,2-dιhalo-3,3-dimethylcyclopropyl and R2 is methyl, ethyl or isopropyl, (iv) a compound of the general formula IV where X is oxygen or sulfur and Ar is a heteroaromatic group, and (v) a compound selected from the group consisting of
55: 4-Octylmorpholine; 56: 1-Octylpiperidine; 57: 2-(3',7'-Dimethyloctylsulfanyl)thiophene; 58: 1 ,4-Dioctylpiperidine; F-1 : 3-[2'-(4"-Phenoxyphenoxy)ethoxy]pyridine; F-4: 2-[2'-(4"-Phenoxyphenoxy)ethoxy]pyridine; and F-5: 3-(4'-Phenoxybenzyloxy)pyridine. for use as a medicament for treating or preventing crustacean infestations in an aquatic animal. In a still further aspect the invention provides a method of controlling crustacean infestation of an aquatic animal, the method comprising administering to said animal or to the aquatic environment of the animal an effective amount of a juvenile hormone analogue compound including any of the above novel compounds. There is also provided a pharmaceutical composition for controlling crustacean infestation of an aquatic animal, comprising a juvenile hormone analogue compound and at least one pharmaceutically acceptable carrier, and an aquatic animal feed composition comprising a juvenile hormone analogue compound. DETAILED DISCLOSURE OF THE INVENTION A major objective of the present invention is to provide improved means for controlling crustacean ectoparasitic infestations in aquatic animals such as fish. The invention is based on the finding that a range of compounds having juvenile hormone activity generally referred to as juvenile hormone analogues (JHAs), which are known to have insect controlling activities including insecticidal activity, have a strong inhibiting effect on the metamorphosis of parasitic crustacean species resulting in a high mortality of these parasites not only in vitro but also in vivo, i.e. when the parasites are attached to the host animal, rendering such compounds potentially useful in the control of such ectoparasitic infestations in live fish.
As used herein, the term "juvenile hormone analogue" indicates a synthetic compound that is structurally and/or functionally related to the naturally occurring juvenile hormones I, II and III, terpenoid substances that are found in insects where they regulate the development from the larval stage to the imago stage of the insects. Due to this juvenile hor- mone effect, JHAs are currently used as insect controlling agents (see e.g. US 4,002,615 and 4,061 ,757). Juvenile hormone analogues are also referred to as juvenoids or juvenile hormone mimics. In one aspect of the present invention there is provided the use of a compound having ju- venile hormone activity, in the manufacturing of a medicament for controlling crustacean infestation of aquatic animals. Whereas other aquatic animals than fish may be infested by crustacean ectoparasites, the use according to the invention is particularly interesting for manufacturing medicaments for control of crustacean infestation in fish species having their natural habitat in cold, temperate or warm water environments. Thus, the medicament of the invention can be used therapeutically and/or prophylactically for controlling infestations in both wild and farmed fish including ornamental fish, occurring in freshwater, sea water or brackish water. Fish that can be treated with JHAs include any fish that can be infestated by ecto- parasitic crustaceans such as wild and farmed fish belonging to the Salmonidae family including, but not limited to Salmo salar, Salmo trutta, Salmo clarkii, Oncorhynchus gor- buscha, Oncorhynchus keta, Oncorhynchus nekra, Oncorhynchus kisutch, Oncorhynchus tshawytscha, Oncorhynchus mason, Oncorhynchus mossambicus, Oncorhynchus mykiss and Salvelinus species. Other examples of fish where the JHAs can be used include carps, whitefish, roach, rudd, chub, sole, plaice, Japanese yellowtail, sea bass, sea bream, grey mullet, po pano, gilthread seabream, Tilapia spp., Cichlidae spp., cod, halibut, wolf fish, flounder, aju and eel including Japanese eel. Presently preferred target fish for the present invention include Atlantic salmon, Pacific salmon and trout. It has been found that a large range of JHA compounds effectively control infestations by crustacean species generally referred to as sea lice such as Lepeophtheirus species. However, as the metamorphosis processes are essentially the same in any crustacean species, it is envisaged that JHAs will be effective against other ectoparasitic crustacean
species infestating aquatic animals. Such species are i.a. found in the following genera: Ergasalus, Bromolochus, Chondracaushus, genera and species belonging to the Cope- poda class including the genera Caligus and Lepeophtheirus, Dichelestinum, Lambro- glenz, Hatschekia, Legophilus, Symphodus, Ceudrolasus, Pseudocycmus, Lernaea, Ler- naeocera, Pennella, Achthares, Banasistes, Salmonicola, Brachiella, Epibrachiella, Pseu- dotracheliastes; and the families: Ergasilidae, Bromolochidae, Chondracanthidae, Caliji- dae, Dichelestiidae, Philichthyidae, Pseudocycnidae, Larnaeidae, Lernaepotidae, Sphyrii- dae, Cecorpidae, Branchiuriae (carp lice) with the family Argulidae that includes Argulus species. In this context, important target species of crustaceans include Lepeophtheirus salmonis, Caligus elongatus and Aniiocra physodes L, crustaceans of a species selected from the group consisting of an Aniiocra species, a Chymothoa species, a Lironeca species, a Meinertia species, an Olencira species and a Bopyrus species and crustaceans of the Iso- poda class including Aniiocra physodes, Chymothoa exigua, Lironeca californica, Lironeca convexa, Lironeca ovalis, Lironeca vulgaris, Meinertia oestroides, Meinertia parallela and Olencira praegustator. In accordance with the invention, any compound having juvenile hormone activity, includ- ing a juvenile hormone analogue compound, that is effective with respect to retarding or inhibiting any stage of the development of crustaceans from the first larval stage to the pre-adult stage and/or which has a biological effect on any stage of the crustaceans including the adult stage is encompassed. Thus, a compound according to the invention that is capable of retarding or inhibiting at least one stage shift from the nauplius I stage through further nauplius stages, copepodite stages, chalimus stages, the pre-adult stage to the adult stage. Such compounds include juvenile hormone mimics such as epofe- nonane, phenoxycarb, hydroprene, kinoprene, methoprene, pyriproxyfen and triprene, juvenile hormones I, II and III, and the compounds disclosed in US 4,002,615 and 4,061 ,757. It should be understood that chitin synthesis inhibitors are not included in the above definition of compounds having juvenile hormone activity. Also encompassed within the meaning of compounds having "juvenile hormone activity" are compounds which prove to be positive when tested in a standard test system for evaluating compounds for juvenile hormone activity. Such standard tests, wherein Teni-
brio molitor typically is applied as test organism, are well known in the art (se e.g. Slama et al., Insect Hormones and Bioanalogues, Springer-Verlag, New York 1974, p. 93). Additionally, the below evaluation system for determining juvenile hormone activity can be applied (adapted from "Methods used to evaluate candidate materials for juvenile hormone activity", Pesticide Chemicals Research Branch, Beltsville, Maryland): Juvenile hormone activity may be determined using newly-molted (4-8 hours) Tenib o molitor pupae. The compounds to be evaluated are formulated to contain 10 μg in 1 ml of solution. Acetone is the preferred solvent and is used in both topical and vapor tests. Topical application is with a micro applicator (Isco model M) fitted with a tuberculin syringe and a 27 gauge needle. One μl of the desired solution is administered/pupa on the venter of the last three abdominal segments. Vapor action is determined by applying the candidate compound to the lower 1/3 of a 1 pint freezer type jar and then inverting the jar into a 1/2 pint container, containing 5 pupae. A dosage of 1 μl of the desired solution per pupa (5 μl/jar) is used. All pupae are held until the following molt to determine juvenile hormone activity, which is indicated by the presence of immature characters; e.g. i) retention of gin traps, ii) retention of gin traps and urogomphi, iii) retention of gin traps and urogomphi plus retention of pupal cuticle around area of treatment, and iv) 2nd pupae - retention of all pupal characters. If a perfect adult is obtained after molting, the compound has no juvenile hormone activity. Famesyl methyl ether is used as the standard for both topical and vapor tests. In the present context, particularly interesting compounds having juvenile hormone activ- ity include: (i) compounds of the general formula I
where R1 is alkyl, branched alkyl including alkenyl or alkadienyl, optionally substituted iso- prenoid or alkoxy substituted alkyl, R2 is hydrogen or alkyl, X is oxygen, sulfur, methylene, carbinol or carbonyl, Y is nitrogen or methinine and Z is nitrogen, methine or nitrogen oxide; (ii) compounds of general formula II where R1 is 2,2-dimethylcyclopropyl, 2,2-dihalo-3,3-dimethylcyclopropyl, 2-methyl-1- propenyl or 3-methyl-1 ,2-butadienyl, R2 is hydrogen or methoxy, R3 is alkyl, alkenyl, ace- tyl, formyl or carbomethoxy, R4 is hydrogen or R3 and R4 together is methylenedioxy and X is oxygen or sulfur; (iii) a compound of the general formula III where R is 2-methylbutyl, 2,2-dimethylcyclopropyl or 2,2-dihalo-3,3-dimethylcyclopropyl and R2 is methyl, ethyl or isopropyl, and
(iv) a compound of the general formula IV where X is oxygen or sulfur and Ar is a heteroaromatic group. 10 In this context, specific juvenile hormone analogue compounds (JHA ) include the following: 1 : 5-(7,-Ethoxy-3',7'-dimethyloctyloxy)-2-methylpyridine; 15 2: 5-(3',7'-Dimethyloctyloxy)-2-methylpyridine; R-2: (3'R)-5-(3'-Dimethyloctyloxy)-2-methylpyridine; S-2: (3'S)-5-(3',7'-Dimethyloctyloxy)-2-methylpyridine; 3: 2-Methyl-5-(7'-methyloctyloxy)pyridine; 4: 2-Methyl-5-octyloxypyridine; 20 5: 5-(3',7'-Dimethyloct-6'-enyloxy)-2-methylpyridine; 6: 5-(3',7'-Dimethylocta-2',6'-dienyloxy)-2-methylpyridine; 7: 5-(3',7'-Dimethylocta-2',6'-dienyloxy)pyridine; 8: 5-[5'-(2",2"-Dichloro-3",3"-dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]-2- ethylpyridine; 25 9: 5-[5'-(2",2"-Dichloro-3",3"-dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]-2- methylpyridine; 10: 5-[5'-(2",2"-Dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]-2-ethylpyridine; 11 : 5-[5'-(2",2"-Dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]-2-methylpyridine; 12: 5-[5'-(2",2"-Dimethylcyclopropyl)-3'-methylpentyioxy]-2-methylpyridine; 30 13: 5-(3',7'-Dimethyloctylsulfanyl)-2-methylpyridine; 14: 2-(3',7'-Dimethylocta-2',6'-dienyloxy)pyridine; 15: 2-(3',7'-Dimethyloctylsulfanyl)pyridine; 16: 3-(3',7'-Dimethyloctyloxy)-1 -methyipiperidine; 17: 5-(3',7'-Dimethyloctyloxy)benzo[1 ,3]dioxole; 35 18: 5-(3',7'-Dimethylocta-2',6'-dienyloxy)benzo[1 ,3]dioxole;
19: 5-[5'-(2",2"-Dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]benzo[1 ,3]dioxole; 20: 5-[5'-(2",2"-Dichloro-3",3"-dimethylcyclopropyl)-3'-methylpent-2'- enyloxy]benzo[1 ,3]dioxole; 21 : 5-[5'-(2",2"-Dibromo-3",3"-dimethylcyclopropyl)-3'-methylpent-2'- 5 enyloxy]benzo[1 ,3]dioxole; 22: 5-(3',8'-Dimethylnona-2',6',7'-trienyloxy)benzo[1 ,3]dioxole; 23: 5-(3',7'-Dimethylocta-2',6'-dienylsulfanyl)benzo[1 ,3]dioxole; 24: 1-[5'-(2",2"-Dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]-4-ethylbenzene; 25:1-[5,-(2",2"-Dichloro-3",3"-dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]-4- 10 ethylbenzene; 26: 1-[5'-(2",2"-Dibromo-3",3"-dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]-4- ethylbenzene; 27: 1-(3',8'-Dimethylnona-2',6',7'-trienyloxy)-4-ethylbenzene; 28: Methyl 4-[5'-(2",2"-dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]benzoate; 15 29: Methyl 4-[5'-(2",2"-dichloro-3",3"-dimethylcyclopropyl)-3'-methylpent-2'- enyloxyjbenzoate; 30: Methyl 4-[5'-(2",2"-dibromo-3",3"-dimethylcyclopropyl)-3'-methylpent-2'- enyloxyjbenzoate; 31 : 1 -Acetyl-4-[5'-(2",2"-dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]benzene; 20 32: 1-Acetyl-4-[5'-(2",2"-dichloro-3",3"-dimethylcyclopropyl)-3'-methylpent-2'- enyloxyjbenzene; 33: 1-Acetyl-4-[5'-(2",2"-dibromo-3",3"-dimethylcyclopropyl)-3'-methylpent-2'- enyloxyjbenzene; 34: (3,7-Dimethyloctyloxy)benzene; 25 35: 4-[5'-(2",2"-Dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]-3-methoxybenzaldehyde; 36: 4-[5'-(2",2"-Dichloro-3",3"-dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]-3- methoxybenzaldehyde; 37: 1-[5'-(2",2"-Dichloro-3",3"-dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]-2-methoxy- 4-propenylbenzene; 30 38: 4-Allyl-1-[5'-(2",2"-dichloro-3",3"-dimethylcyclopropyl)-3'-methylpent-2'-enyloxy]-2- methoxybenzene; 39: Methyl 7,11-dimethyl-dodeca-2,4-dienoate; 40: Methyl 9-(2',2'-dimethylcyclopropyl)-7-methylnona-2,4-dienoate; 41 : Ethyl 9-(2',2'-dimethylcyclopropyl)-7-methylnona-2,4-dienoate; 35 42: Isopropyl 9-(2',2'-dimethylcyclopropyl)-7-methylnona-2,4-dienoate;
43: Methyl 9-(2',2'-dichloro-3',3'-dimethylcyclopropyl)-7-methylnona-2,4-dienoate; 44: Ethyl 9-(2',2'-dichloro-3',3'-dimethylcyclopropyl)-7-methylnona-2,4-dienoate; 45: Isopropyl 9-(2',2'-dichloro-3',3'-dimethylcyclopropyl)-7-methylnona-2,4-dienoate; 46: 2-Methyl-5-nonyloxypyridine; 5 47: 2-Methyl-5-undecyloxypyridine; 48: 5-Dodecyloxy-2-methylpyridine; 49: 2-Methyl-5-(3',7', 11 '-trimethyldodeca-2',6', 10'-trienyloxy)pyridine; 50: 5-(3',7'-Dimethyloctyloxy)-2-methylpyrimidine; 51 : 3-(4',8'-Dimethylnonyl)pyridine; 10 52: 3-(4',8'-Dimethylnonyl)pyridine-N-oxide; 53: 4,8-Dimethyl-1-pyridin-3-ylnonan-1-ol; 54: 4,8-Dimethyl-1 -pyridin-3-ylnonan-1 -one; 55: 4-Octylmorpholine; 56: 1-Octylpiperidine; 15 57: 2-(3',7'-Dimethyloctylsulfanyl)thiophene; 58: 1 ,4-Dioctylpiperazine; 59: 3-(3',7'-Dimethyloctyioxy)pyridine; 60: 3-(3*,7'-Dimethyloctylsulfanyl)pyridine; 61 : (3',7'-Dimethylocta-2',6'-dienyioxy)benzene; 20 F-1 : 3-[2'-(4"-Phenoxyphenoxy)ethoxy]pyridine; F-2: 3-(7'-Ethoxy-3',7'-dimethyloctyloxy)pyridine; F-3: Ethylcarbamic acid 2-(4'-phenoxyphenoxy) ethyl ester (phenoxycarb) ; F-4: 2-[2'-(4"-Phenoxyphenoxy)ethoxy]pyridine; F-5: 3-(4'-Phenoxybenzyloxy)pyridine; 25 methoprene; and hydroprene. The fish or any other aquatic animal species can be treated orally, e.g. via their feed, or by bath treatment, e.g. in a medicinal bath where the fish are kept for a period of time 30 (minutes to several hours) that provides a sufficient contact time to inhibit the parasitic crustaceans. Alternatively, it is possible to treat the biotope of the fish temporarily or continuously, e.g. the net cages, entire ponds, aquaria, tanks or basins in which the fish are kept. It is a further suitable possibility to administer the compounds parenterally, e.g. by injection. 35
The active compounds are administered in medicament formulations which are adjusted to the applications. Formulations for oral administration include e.g. powders, granulates, solutions, emulsifiable concentrates or suspension concentrates which are mixed homogeneously as feed additives with the feed, or powders, granulates, emulsifiable concen- trates or suspension concentrates which are administered in the form of pills, the outer coat of which can consist e.g. of fish feed compositions which cover the active substance completely. Alternatively, the active substance may be applied onto the surface of feed particles such as pellets or granules, e.g. incorporated in a lipid component such as a fish oil. Useful medicament formulations for bath application or for treating the biotope of the fish include powders, granulates, solutions, emulsions, micro-emulsions, suspensions, tablets or the active substance itself. The end-user may use these formulations in dilute or undi- lute form. The active compound in any of these formulations may be used in pure form, as a solid active substance e.g. in a specific particle size, or together with at least one of the adjuvants that are conventionally used in formulation technology, such as extenders, typically solvents or solid carriers, or surface active compounds. The formulations are prepared in a manner known per se, typically by mixing, granulating and/or compacting the active compound with solid or liquid carriers, where appropriate, with the addition of further auxiliary substances such as emulsifying or dispersing agents, solubilisers, colorants, antioxidants and/or preservatives. It is also possible to use semi-solid formulations for the bath treatment. The active substance, which is suspended or dissolved in oily or fatty matrices, is washed out on administration. The release can be controlled by the choice of adjuvants, concentration of the active substance and form. Coprimates or melts of hard fats comprising the active sub- stance are also suitable for use. The diluted compositions of this invention are generally prepared by contacting the active substance with liquid and/or solid formulation assistants by stepwise mixing and/or grinding such that an optimal development of the antiparasitic activity of the formulation is achieved that conforms with the application.
Formulation assistants can e.g. be solid carriers, solvents and, where appropriate, surface active substances which are non-toxic for marine fauna and flora. The bath application of the medicament compositions of the invention to the parasites to be controlled can e.g. be carried out such that the compositions are placed in the cage in the form of solutions, emulsions, micro-emulsions, suspensions, powders or tablets, where they are dissolved or dispersed by the movement of the fish and the flow of the water. Concentrated compositions can also be diluted with large volumes of water before applied to the cages. In one embodiment, micro-emulsions can advantageously be applied for bath application of the medicament compositions when using juvenile hormone analogues which are not water-soluble or have a low water-solubility. When such micro-emulsions, comprising ju- venile hormone analogues are added to the bath treatment water, a clear solution is typically seen. In one useful embodiment, the micro-emulsion comprises solvents, surfactants and stabilisers in addition to the juvenile hormone analogues. Preferably, no water should be pres- ent in the micro-emulsion. Examples of solvents which can be applied in a micro-emulsion include acetone, mono- hydric, dihydric or polyhydric alcohol (including ethanol, polyethylene glycol, propylene glycol), glycerine, mineral oil, vegetable oils or oils of animal origin, fatty acid esters, di- methyl sulphoxid (DMSO), dioxan, tetrahydrofuran (THF), 2-pyrrolidon, N-methyl-2- pyrrolidon. Examples of suitable surfactants include acetylated mono-and diglycerides with fatty acids, polyacrylic copolymers, beeswax, lecithin, fatty acids, guar gum, xanthan gum, tragacanth gum, PEG linked with fatty acids or sugars, hydrogenated and etoxylated castor oil, cellulose derivatives, compounds consisting of fatty acids esterified to sugars, N-octyl-2-pyrrolidon, N-dodecyl-2-pyrrolidon, EO/PO blockpolymers and dodecylbenzene sulfonate salts. Examples of stabilisers include antioxidants, pH regulating compounds such as citric acid, and complexing agents. In useful embodiments the micro-emulsion comprises: i) juvenile hormone analogues in an amount which is in the range of 0.1-10 wt% including the range of 0.5-5 wt%; ii) sol-
vents in the range of 20-30 wt% including the range of 22-28 wt% such as in the range of 25-28 wt%; iii) surfactants in the range of 50-80 wt% including the range of 55-75 wt% such as in the range of 60 to 70 wt%; and iv) stabilisers in the range of 0.1-5 wt% including the range of 0.5-5 wt% such as in the range of 1 to 2 wt%. One example of a specific composition of a micro-emulsion is given in the below examples. In a further aspect the invention relates to novel juvenile hormone analogue compounds of the general formula I, the general formula II, the general formula III or of the general formula IV and the compounds 55-58, F-1, F-4 and F-5 as defined above, including a compound of the general formula II having saturated side chains to the aromatic ring, for use as a medicament for treating or preventing crustacean infestations in an aquatic animal. It is also a significant objective of the invention to provide a method of controlling infesta- tions with any of the above crustacean species in aquatic animals including fish of the species as mentioned above, the method comprising administering to said animal or to the aquatic environment of the animal an effective amount of a juvenile hormone analogue compound. In the method of the invention any of the above juvenile hormone analogues including epofenonane, phenoxycarb, hydroprene, kinoprene, methoprene and triprene and the above compounds of the general formula I, the general formula II, the general formula ill or of the general formula IV as also defined above can be used as the antiparasitically active compound. In this method, the active compound is administered by any of the above routes using the compound as such or in the form of a composition that is adjusted to the selected manner of administration, including compositions for bath treatment or addition to the biotope of animals or fish, compositions for oral administration, optionally via the feed, or injectable compositions. The amount of active substance in the administration form can vary depending i.a. on the dosage form, the target parasite, the age and condition of the fish to be treated. When the compound is used as a medicament for bath treatment or as a composition that is added to the aquatic environment of the aquatic animal, the amount of the active juve-
nile hormone analogue compound in the environment of the aquatic animal depends on the manner and duration of treatment and also on the age and condition of the fish to be treated. Effective dosages of the active substance in the water is generally in the range of 1 ppb to 1 ppm such as in the range of 10 ppb to 500 ppb including the range of 100 to 5 300 ppb. In one preferred embodiment, the compound is added the aquatic environment in the form of a micro-emulsion In accordance with the invention there is also provided a pharmaceutical composition for controlling crustacean infestation of an aquatic animal such as a fish, comprising a juve- 0 nile hormone analogue compound and at least one pharmaceutically acceptable carrier. In this context, suitable carriers include, but are not limited to, solid carriers such as e.g. kaolin, talcum, bentonite, sodium chloride, calcium phosphate, carbohydrates, cellulose, cotton seed meal, polyethylene glycol ether and, if necessary, binders such as gelatine, soluble cellulose derivatives or surface active substances such as ionic or anionic dispers- 5 ing agents. As it is explained above, such a composition can e.g. be in the form of a powder, a granulate, a suspension, an emulsion, a micro-emulsion and a solution or it can for certain purposes be in the form of an injectable composition. Depending on the intended application 0 the composition of the invention may further comprise a component selected from the group consisting of a solvent, a wetting agent, an emulsifying agent, a bulking agent and a dispersing agent. Suitable solvents include aromatic hydrocarbons, alkylated naphtalenes, or tetrahydronaphtalenes, aliphatic or cycloaliphatic hydrocarbons such as paraffins or cyclohexane, alcohols such as ethanol, propanol or butanol, glycois and their esters and 5 ethers, ketones such as acetone, strongly polar solvents such as N-methyl-2-pyrrolidone, dimethyl sulfoxide, water, as well as vegetable oils and oils of animal origin such as e.g. fish oil. Depending on the type of formulation, suitable surface active compounds are nonionic, 0 cationic and/or anionic surfactants having good emulsifying, dispersing and/or wetting properties. It will be appreciated that the composition of the invention can comprise two or more juvenile hormone analogue compounds or it can comprise at least one further anti-parasitically 5 active compound of any of the conventionally used types of antiparasitic substances in-
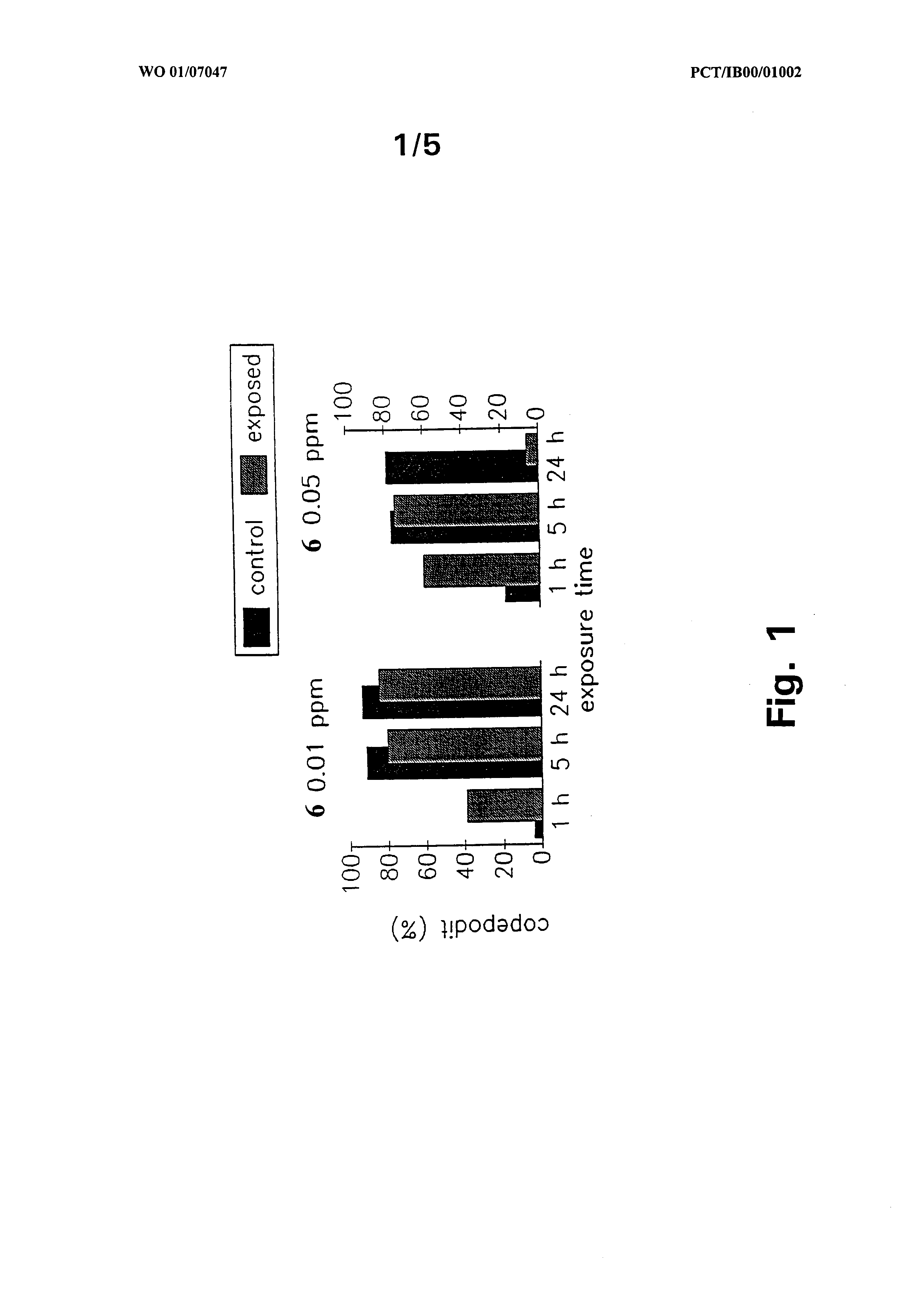
eluding formaldehyde, hydrogen peroxide, a cholinesterase inhibitor such as e.g. metri- fonate or dichlorvos, an organophosphorus compound, a carbamate compound, a chitin inhibiting compound, an avermectin compound and/or a pyrethroid compound. As it is mentioned above, one convenient manner of administering a juvenile hormone analogue compound to fish is to add the compound to the normal fish feed. Accordingly, there is provided an aquatic animal feed composition comprising a juvenile hormone analogue compound as defined above, in an amount that will provide an antiparasitically active amount of the JH analogue compound in the fish. The active compound can be in- corporated in the feed in any of the above manners. The active compound can be mixed directly with the feed ingredient as such or it can be added in the form of a more or less concentrated feed additive composition. As an example such an additive composition can consist of 1-10 wt% of active compound, 49-90 wt% of a protein carrier such as soy bean protein, 0-50 wt% of ground calcium powder, 0-2 wt% of an alcohol, hydroxypropyl cellu- lose and water ad 100 wt%. Suitable forms of "medicated" feed comprising the JH analogue compound include granulated feed, pelleted feed and feed in the form of an emulsion or micro-emulsion. The amount of the juvenile hormone analogue in feed compositions of the invention is typically in the range of 0.001 to 5 % wt% such as 0.01 to 3 wt% including 0.1 to 1 wt%. As it was described for the above composition of the invention, the feed composition of the invention can comprise two or more juvenile hormone analogue compounds or it can comprise at least one further anti-parasitically active compound of any of the conventionally used types of antiparasitic substances including formaldehyde, hydrogen peroxide, a cholinesterase inhibitor such as e.g. metrifonate or dichlorvos, an organophosphorus compound, a carbamate compound, a chitin inhibiting compound, an avermectin compound and/or a pyrethroid compound. The invention will now be further illustrated in the following non-limiting examples and in the drawing where Fig. 1 shows the percentage of copepodite larvae relative to the number of nauplius larvae exposed to compound 6 as defined herein [5-(3',7'-Dimethylocta-2',6'-dienyloxy)-2- methylpyridine] at concentrations of 0.01 ppm, 0.05 ppm, 0.3 pp and 1.0 ppm, respec- tively for 1 , 5 and 24 hours, respectively (Example 3);
Fig. 2 shows the percentage of copepodite larvae relative to the number of nauplius larvae exposed to compound 7 as defined herein [5-(3',7'-Dimethylocta-2',6'- dienyloxy)pyridine] at concentrations of 0.3 ppm and 1.0 ppm for 1 , 5 and 24 hours, re- spectively (Example 3); Fig. 3 shows the percentage of copepodite larvae relative to the number of nauplius larvae exposed to compound 59 as defined herein [3-(3',7'-Dimethyloctyloxy)pyridine;] at concentrations of 0.3 ppm and 1.0 ppm for 1 , 5 and 24 hours, respectively (Example 3); Fig. 4 shows the ratio between number of copepodites in groups of Lepeophtheirus salmonis larvae exposed compounds 6, 7 or 59 for 1 , 5 and 24 hours, respectively at concentrations of 0.01 ppm, 0.05 ppm, 0.3 ppm and 1.0 ppm, respectively (Example 3). Data for these exposure periods were combined for each concentration and the controls for each compound were combined. A ratio of 1 indicates that the same number of copepodites were developed in the exposed groups and the control groups; and Fig. 5 summarises the results of the experiments in Example 4 where Lepeophtheirus salmonis nauplius stage larvae were exposed for 24 hours to 1.0 ppm of compounds 4 [2- Methyl-5-octyloxypyridine], 2 [5-(3',7'-Dimethyloctyloxy)-2-methylpyridine, 34 [(3,7- Dimethyloctyloxy)benzene], 17 [5-(3',7'-Dimethyloctyloxy)benzo[1 ,3]dioxole] 60 [3-(3',7'- Dimethyloctylsulfanyl)pyridine] or 61 [(3',7'-Dimethylocta-2',6'-dienyloxy)benzene]. EXAMPLES In the following examples the effect of a range of juvenile hormone analogues on viability and metamorphosis of parasitic larval stages of crustacean species are reported. The following compounds were tested: 4: 2-Methyl-5-octyloxypyridine; 2: 5-(3',7'-Dimethyloctyloxy)-2-methylpyridine; 6 = B: 5-(3'.7,-Dimethylocta-2'.6'-dienyloxy)-2-methylpyridine; 7: 5-(3'.7'-Dimethylocta-2'.6'-dienyloxy)pyridine; 14: 2-(3',7'-Dimethylocta-2',6'-dienyloxy)pyridine;
34: (3,7-Dimethyloctyloxy)benzene; 17: 5-(3',7'-Dimethyloctyloxy)benzo;[1 ,3]dioxole; 60: 3-(3',7'-Dimethyioctyisulfanyl)pyridine; 57: 2-(3',7'-Dimethyloctylsulfanyl)thiopene; 1 : 5-(7'-Ethoxy-3',7'-dimethyloctyloxy)-2-methylpyridine; 51 : 3-(4',8'-Dimethylnonyl)pyridine; 50: 5-(3',7'-Dimethyloctyloxy)-2-methylpyrimidine; 59: 3-(3',7'-Dimethyloctyloxy)pyridine; 61 : (3',7'-Dimethylocta-2',6'-dienyloxy)benzene; F-1 : 3-[2'-(4"-Phenoxyphenoxy)ethoxy]pyridine; F-2: 3-(7'-Ethoxy-3',7'-dimethyloctyloxy)pyridine; F-3: Ethylcarbamic acid 2-(4'-phenoxyphenoxy) ethyl ester (phenoxycarb); F-4: 2-[2'-(4"-Phenoxyphenoxy)ethoxy]pyridine; F-5 3-(4'-Phenoxybenzyloxy)pyridine; AC = methoprene; and AD = hydroprene. EXAMPLE 1 The effect of juvenile hormone (JH) analogue compounds on Lepeophtheirus salmonis larvae Nine JH analogue compounds designated 2, 6, 7, 14, 57, 1, 51, 50 and 59 (see above) were tested according to the following protocol: Female sea lice with mature ovaries (dark pigmented) were collected in a tank containing 0.5 I sea water. Newly developed nauplius stage larvae were picked with a pipette or were separated from the water by filtration and used for further studies A large number of nauplii larvae (>100) in 300-400 ml aerated sea water were subjected to different JH analogues at a concentration of 3 ppm, and vitality and mortality of the nauplii monitored. The results of this experiment is summarized in Table 1.1.
Table 1 1 Effect on nauplius larvae of tested JH analogue compounds The effect of the above compounds 6, 7 and 59 was further investigated at different con- centrations and the effect against sea lice was monitored In the below Table 1 2 the following designations are used- +++ all nauplius larvae dead, ++ most nauplius larvae dead, + few dead nauplius larvae and - no dead nauplius larvae Table 1 2. Effect of compounds 6, 7 and 59 on nauplius larvae Among the tested compounds 6, 7 and 59 were the most effective against sea lice nauplius larvae
EXAMPLE 2 The effect of juvenile hormone analogues on the development of Lepeophtheirus salmonis from nauplius stage to copepodite stage The JH analogue compounds 51 , 1 and 50 were tested at a concentration of 0.7 ppm to determine their effect on the development from nauplius larvae to copepodites as compared to a control group not exposed to JH analogue. After 14 days of exposure the number of copepodites were calculated. Most of the nauplius larvae in the control group de- veloped into copepodites. Larvae exposed to the compounds 51 and 50 showed results that were similar to the control. However, no copepodites were present in the group exposed to the compound 1 , and only dead nauplius larvae were found. EXAMPLE 3 The effect of JH analogue compounds 6, 7 and 59 on the development of Lepeophtheirus salmonis from nauplius stage to copepodite stage The apparently most effective compounds from Example 2 were selected for further investigation. The effect of the compounds 6, 7 and 59 on the development from nauplius larvae to copepodites were tested at concentrations of 0.01 ppm, 0.05 ppm, 0.3 ppm and 1.0 ppm, respectively at the exposure periods of 1 , 5 and 24 hours, respectively. The number of nauplius and copepodite larvae were counted after 3-7 days. The experi- ments were run in triplicate at each concentration and each exposure period. Additionally, control groups exposed to equivalent amount of acetone used as solvent for the test compounds were included. The results of these experiments are summarised in Figures 1-4. It is concluded that the tested compounds showed high effect in respect of inhibiting the development from nauplius larval stage to copepodites. In particular, compound 59 showed complete inhibition at 1.0 ppm and an exposure period of 24 hours. This compound also had a significant metamorphosis inhibiting effect at concentrations less than
1.0 ppm. Thus, at 0.01 ppm, 0.05 ppm and 0.3 ppm about 40% reduction in numbers of copepodites, relative to the controls, was observed EXAMPLE 4 The effect of compounds 4, 2, 34, 17, 60 and 61 on the development of Lepeophtheirus salmonis from nauplius stage to copepodite stage The compounds 4, 2, 34, 17, 60 and 61 were tested using the protocol as in Example 1 using exposure at 1.0 ppm for 24 hours. The results of this experiment are shown in Fig. 5. All of the tested compounds except compound 61 completely inhibited the development from the nauplius to the copepodite stage. The compound 60 were tested further at 1.0 ppm using an exposure period of only 1 hour. Under these conditions, the compound had a good effect in that only 7% of the larvae exposed developed into the copepodite stage. EXAMPLE 5 The effects of compounds 6, F-1 , F-2, F-3, F-4 and F-5 on Lepeophtheirus salmonis nauplius larvae and the development of Lepeophtheirus salmonis from the nauplius stage to the copepodite stage The experiments were conducted essentially as in Example 2. 10-50 newly hatched nau- plius I larvae were placed in trays containing 50 ml of sea water (temperature 16-19°C, salinity 2.2%) to which the compound to be tested was added at 1.0 ppm and the exposure time was 24 hours following which the larvae were separated from the water and transferred to fresh sea water. After 3-5 days, the number of nauplii and copepodites were counted. Acetone was used as solvent for the compounds and the same volume of ace- tone was added to trays with control larvae. All experiments were carried out in triplicate or quadruplicate. The results are summarised in Tables 5.1. and 5.2.
Table 5.1. No. of copepodites on day 4 after the exposure The compounds F-1, F-2, F-3, F4 and F-5 were tested for their effect on nauplius stage larvae at a concentration of 1.0 ppm. The number of surviving and dead nauplii in the trays were counted immediately after exposure. Table 5.2. Effect of compounds F-1, F-2, F-3 F-4 and F-5 against nauplii and inhibition of development into the copepodite stage All of the tested compounds F-1 to F-5 showed a significant inhibition of development of nauplii into copepodites under the test conditions. Compounds F-1 and F-5 inhibited the development into copepodites completely and showed a high efficiency against nauplius larvae.
EXAMPLE 6 Screening of JHA compounds for sea lice larvicid effect The effect of 12 compounds designated A (= 59, B (= 6), C (= F-1), D (= F-3), E (= F-4) , F (= F-5), G (=propoxur) , H (=bassa), I (=resmethrin), J (=deltamethrin), K (=permethrin) and L (=agromazine), respectively on the survival of nauplius larval stages 1 and 2 of sea lice (Lepeophtheirus salmonis) was tested. The compounds designated G-L are not juvenile hormone analogues, but are included for comparison. Survival, mortality and possible deformations on the larvae were recorded for each group 6 days after exposure to the test compounds for 24 hours. Adult female sea lice with mature ovaries were collected and the ovaries were separated from the animals and incubated in running sea water at 8°C. By means of a pipette 30 newly hatched larvae at the nauplius 1 stage were transferred to 50 ml pure sea water in petri dishes. The respective test compounds dissolved in acetone were added to the dishes at a concentration of 1.0 ppm (50 μi). The larvae were exposed for 24 hours before new fresh water was supplied. The water was changed daily during the test period. The experiments were done in triplicate. To one control group of larvae 50 μl of acetone was added and another group was kept in pure sea water. A considerable variation in survival and mortality for the test groups was observed, but the control groups were relatively similar. Data for survival and mortality are summarised in Table 6.1 :
Table 6.1 The compounds B, C, F, I and J had the best larvicidal effect on the sea lice larvae. None of the larvae reached the copepodite stage during the screening period. In the groups B and C, the distribution of dead larvae were equal between nauplius stages 1 and 2. For the groups I, J and K, nauplius 1 larvae dominated among the dead larvae, whereas stage 2 nauplii dominated among the dead larvae in group F. A common observation for groups C, I, J and K was that the dead larvae had protrusions (vacuoles) at the antenna region. It is possible that this is due to the fact that the larvae grow to a size that is to large for the shell resulting in rupture of the larvae.
EXAMPLE 7 The effect of JH analogues on development and survival of sea lice (Lepeophtheirus salmonis) on sea water adapted Atlantic salmon (Salmo salar) 7.1. Materials and methods Test compounds The commercial juvenile hormone analogue compound methoprene (AC) was tested. Test fish The test fish were distributed into tanks of 500 I (45 fish per tank). Running sea water that was adjusted in respect of oxygen (at least 70% saturation in outlet water), salinity (34 promille) and temperature (11.5°C) was supplied to the tanks. The photoperiod was set at 18 hours of light and 6 hours of dark throughout the test period. Bath experiment protocol For a 14 days bath regimen 9.239 ml of pure methoprene (AC) was mixed with 1 ,700 ml of acetone which gave a stock solution at 5,000 ppm compound AC, which was dosed over 14 days. The compound was added to the water at a concentration of 0.1 ppm. Cultivation of L. salmonis Adult female sea lice with mature ovaries were collected and the ovaries were separated from the female sea lice and incubated in running sea water (salinity 34 promille) at 10°C. Newly hatched nauplius larvae were harvested daily and transferred to trays to produce infective copepodite larvae. Challenge with sea lice During exposure to the sea lice, the water supply was stopped and the tanks were ad- justed to about 100 I of water. About 2,000 two days old copepodites were distributed to
each tank. During exposure the tanks were aerated. After 2 hours the tanks were filled and the water supply was stopped. The fish were exposed to copepodites in stagnant water for 12 hours under aeration before the usual water regimen was re-established. Data recording Five days following copepodite exposure, the success of infestation was recorded on 5 fish from each tank and the medication regimen was initiated. In all tanks the number of sea lice was recorded 1 , 5 and 10 days after termination of the medication period (10 fish). The distribution of the various stages of sea lice was recorded when the fish was removed from the tanks. The recording of the various chalimus stages was simplified by recording chalimus stages 1 and 2 as juvenile chalimus (ChJ) whereas chalimus 3 and 4 were recorded as "old" chalimus (ChG). The first and second pre-adult stages and the adult stage were also recorded. 7.2. Results Fish mortality No fish mortality was recorded throughout the entire test period. Sea lice mortality: 14 days of bath treatment After 14 days of bath treatment with compound methoprene (AC), the fish had signifi- cantly less sea lice infestation than the corresponding control group (p<0.01)
EXAMPLE 8 The effect of oral administration of JH analogue 59 on development and survival of sea lice (Lepeophtheirus salmonis) on Atlantic salmon in sea water(Sa/mo salar) 5 Materials and methods Test compounds 10 The juvenile hormone analogue compound 59, 3-(3',7'-Dimethyloctyloxy)pyridine, was tested Test fish 15 400 Atlantic salmon of average weight 2.5 kg were distributed equally into two net pens (4x4x4 m) in a fish farm. The fish was deloused with NUVAN (dichlorvos) before the experiment. One group was used as a control and one group was treated with JH analogue 59, 3-(3',7'-Dimethyloctyloxy)pyridine;, by oral administration. The fish farm was heavily infested with sea lice. 20 Preparation of feed containing JH analogue 59 Ordinary fish feed for Atlantic salmon (Felleskjøpet) 12 mm was coated with JH analogue 59, 3-(3',7'-Dimethyloctyloxy)pyridine. The pure compound (liquid) was dissolved in Cape- 25 line-oil to a concentration of 5.0% and then coated on feed pellet at a concentration of 10% oil. 9.4 kg feed coated with 59 was produced. The concentration was 5.0 g of substance 59 per kg feed. Oral administration of JH analogue. 30 Feed containing 59, 3-(3',7'-Dimethyloctyloxy)pyridine, was fed on alternate days at a feeding rate of 0.5% per day. Ordinary feed was fed the days without medication and control fish was fed ordinary feed throughout the experiment. A total of 4 days of medication was used and the total amount of 59, 3-(3',7'-Dimethyloctyloxy)pyridine, applied was 35 20 gram per kg fish.
The fish was free of sea lice when the treatment started after the NUVAN treatment Data recording In all net pens the number of sea lice was recorded 3 months after termination of the medication period (10 fish of each group). The distribution of the various stages of sea lice was recorded when the fish was removed from the net pens. Results Fish mortality No fish mortality was recorded throughout the entire test period. Table 8.1 Number of sea lice per fish 3 months after treatment The number of sea lice at stages chalimus and adults are significantly lower in the group treated with JH analogue 59 than in the control group (α=0.05)
The total average number of all sea lice stages in controls is 98 per fish while in the group treated with JH analogue 59 this number is reduced to 61. Because salmon in neighbouring cages were heavily infested with sea lice, migration of adult and pre-adult lice to the experimental groups occurred. The significant reduction in chalimus stages in the group treated with JH analogue 59 shows that development of copepodite through the four chalimus stages is inhibited by the treatment. EXAMPLE 9 The effect of oral administration of JH analogues methoprene and hydroprene on development and survival of sea lice (Lepeophtheirus salmonis) on Atlantic salmon in sea wa- ter(Salmo salar) Materials and methods Tesf compounds The juvenile hormone analogues methoprene (AC) and hydroprene (AD) were tested. Test fish A number of 135 Atlantic salmon of average weight 117-145 grams was used in the experiment which took place in a fish disease facility. The test fish were distributed into tanks of 500 I with 45 fish per tank. Running sea water that was adjusted in respect of oxygen (at least 70% saturation in outlet water), salinity (34 promille) and temperature (11.5°C) was supplied to the tanks. The photoperiod was set at 18 hours of light and 6 hours of dark throughout the test period.
Preparation of feed containing the JH analogues methoprene (AC) and hydroprene (AD). Ordinary fish feed for Atlantic salmon (Felleskjøpet) 3 mm was coated with the JH analogues methoprene (AC) and hydroprene (AD). The pure compounds were added to the feed in a mixer and then Capeline-oil was added to a concentration of 3.3% while the mixer was running. 480 grams of medicated feed was made of each group. Two concentrations of methoprene: 10 gram per kg feed (AC/X) and 2 gram per kg feed (AC/Y) were made. Also two concentrations of hydroprene (AD) were made: 10 gram per kg feed (AD/X) and 2 gram per kg feed (AD/Y) Cultivation of L. salmonis Adult female sea lice with mature ovaries were collected and the ovaries were separated from the female sea lice and incubated in running sea water (salinity 34 promille) at 10°C. Newly hatched nauplius larvae were harvested daily and transferred to trays to produce infective copepodite larvae. Challenge with sea lice During exposure to the sea lice, the water supply was stopped and the tanks were adjusted to about 100 I of water. About 20,000 two days old copepodites were distributed to each tank. During exposure the tanks were aerated. After 2 hours the tanks were filled and the water supply was stopped. The fish were exposed to copepodites in stagnant water for 12 hours under aeration before the usual water regimen was re-established. Oral administration of JH analogue Feed containing methoprene (AC/X and AC/Y) and hydroprene (AD/X and AD/Y) were fed at a feeding rate of 0.5% per day for 14 days. Control fish was fed ordinary feed through- out the experiment. A total of 371 gram of medicated feed was distributed to each medicated fish group.
Data recording Five days following copepodite exposure, the success of infestation was recorded on 5 fish from each tank and the medication regimen was initiated. In all tanks the number of sea lice was recorded one day after termination of the medication period of 14 days (10 fish). The distribution of the various stages of sea lice was recorded. The recording of the various chalimus stages was simplified by recording chalimus stages 1 and 2 as juvenile chalimus (ChJ) whereas chalimus 3 and 4 were recorded as "old" chalimus (ChG). The first and second pre-adult stages and the adult stage were also recorded. Results Infection Due to a very high infestation rate of copepodites the number of sea lice attached to each fish was extremely high (> 100 per fish) and some mortality occurred among the fish. The mortality of fish due to sea lice in the different groups is shown in Table 9.1 : Table 9.1 Highest mortality was observed in the control fish group which also had the largest number of sea lice (146 per fish). Lowest mortality was observed in the group medicated with hydroprene at highest concentration (AD/X) and which also had the lowest number of sea lice after medication (70 per fish).
Medication It was observed that the appetite was poor during feeding of medicated feed and that lots of medicated feed was not eaten At sampling the day after medication only control fish 5 had feed pellets in the stomach The reason for less intake of medicated feed could be due to taste problems The actual dose of substance AC and AD presented for the fish is then considerably lower than the theoretical amounts This is further supported by the fact that the average weight of medicated fish dropped during medication AC/X from 142 to 123 gram, AC/Y from 145 to 117 gram, AD/X from 130 to 119 gram and AD/Y from 141 to 10 138 gram The average weight of control fish increased slightly in the same period from 117 to 128 gram An ANOVA analysis of the number of sea lice per fish before medication and one day after medication was performed, and the results are shown in the below Table 9 2 15 Table 9 2 The ANOVA analysis of the above data in Table 9 2 show that there are no significant reduction of sea lice in the control fish group and in the AD/Y group, while the groups 20 methoprene AC/X, AC/Y and hydroprene AD/X experienced a significant reduction of sea lice during medication
A dose relation is seen with hydroprene in that the largest reduction in sea lice is observed with the highest concentration of hydroprene (AD/X). The benefit of the administration of the JH analogues methoprene and hydroprene was clearly demonstrated by the lowering of the number of fish that died from sea lice infestation and by the significant reduction in sea lice numbers. EXAMPLE 10 Micro-emulsion concentrate for juvenile hormone analogues In the below Table 10.1 is given an example of a base micro-emulsion concentrate for bath treatment of fish with a juvenile hormone analogue. The micro-emulsion is prepared in a tank kept at a constant temperature of 30°C, by adding the juvenile hormone analogue to the solvents (N-metyl-2-pyrrolidon and N-octyl- 2-pyrrolidon) while stirring, and subsequently adding the remaining components (polyok- syl 35 castor oil, tetrapropylene benzenesulfonate Ca-salt and citric acid) to the mixture. In bath treatment of fish, the juvenile hormone analogue is typically applied at concentration of 0.1 to 1 ppm. I order to prepare a treatment bath of 100 m3 of water with a juvenile hormone analogue concentration of 0.1 ppm, 0.2 litre of micro-emulsion concentrate (stock solution) containing 50g juvenile hormone analogue per kg is added to the 100m3 of water. This micro-emulsion concentrate comprising the juvenile hormone analogue, can advantageously be diluted in a volume of water, e.g. 10 litres, before it is added to the aquatic environment wherein the fish to be treated is kept. A clear solution is seen when the micro-emulsion comprising the juvenile hormone analogue is added to the water.
Table 10.1 CLAIMS 1. Use of a compound having juvenile hormone activity, in the manufacturing of a medicament for controlling crustacean infestation of aquatic animals. 2. Use according to claim 1 wherein the aquatic animal is a fish. 3. Use according to claim 2 wherein the fish is selected from the group consisting of a species of the Salmonidae family, sea brass, sea bream, cod, halibut, wolf fish, flounder, aju and eel. 4. Use according to claim 3 wherein the fish is selected from the group consisting of Salmo salar, Salmo trutta, Salmo clarkii, Oncorhynchus gorbuscha, Oncorhynchus keta, Oncorhynchus nekra, Oncorhynchus kisutch, Oncorhynchus tshawytscha, Oncorhynchus mason, Oncorhynchus mossambicus, Oncorhynchus mykiss and Salvelinus species. 5. Use according to claim 1 wherein the crustacean is a species selected from the group consisting of species belonging to the Copepoda class including a Lepeophtheirus species and a Caligus species and species belonging to the Isopoda class including Aniiocra species. 6. Use according to claim 5 wherein the crustacean is selected from the group consisting of Lepeophtheirus salmonis, Caligus elongatus and Aniiocra physodes L. 7. Use according to claim 1 wherein the crustacean is a species of the Branchiura class including an Argulus species. 8. Use according to claim 1 wherein the crustacean is of the Isopoda class. 9. Use according to claim 8 wherein the crustacean is of a species selected from the group consisting of a Aniiocra species, a Chymothoa species, a Lironeca species, a Meinertia species, a Olencira species and a Bopyrus species. 10. Use according to claim 9 wherein the crustacean is selected from the group consisting of Aniiocra physodes, Chymothoa exigua, Lironeca californica, Lironeca convexa, Liro-
neca ovalis, Lironeca vulgaris, Meinertia oestroides, Meinertia parallela and Olencira praegustator. 11. Use according to claim 1 wherein the compound is selected from the group consisting of epofenonane, fenoxycarb, hydroprene, kinoprene, methoprene and triprene. 12. Use according to claim 1 where the medicament is a medicament which is to be administered to the aquatic animal via the oral or parenteral route or by adding the compound to the environment of the animal. 13. A juvenile hormone analogue compound selected from the group consisting of (i) a compound of the general formula I where R1 is alkyl, branched alkyl including alkenyl or alkadienyl, optionally substituted iso- prenoid or alkoxy substituted alkyl, R2 is hydrogen or alkyl, X is oxygen, sulfur, methylene, carbinol or carbonyl, Y is nitrogen or methinine and Z is nitrogen, methine or nitrogen oxide; (ii) a compound of general formula II
where R1 is 2,2-dimethylcyclopropyl, 2,2-dihalo-3,3-dimethylcyclopropyl, 2-methyl-1- propenyl or 3-methyl-1 ,2-butadienyl, R2 is hydrogen or methoxy, R3 is alkyl, alkenyl, ace- tyl, formyl or carbomethoxy, R4 is hydrogen or R3 and R4 together is methylenedioxy and X is oxygen or sulfur; (iii) a compound of the general formula III 10 where R1 is 2-methylbutyl, 2,2-dimethylcyclopropyl or 2,2-dihalo-3,3-dimethylcyclopropyl and R2 is methyl, ethyl or isopropyl, (iv) a compound of the general formula IV 15 20 where X is oxygen or sulfur and Ar is a heteroaromatic group, and (v) a compound selected from the group consisting of 25 55: 4-Octylmorpholine; 56: 1-Octylpiperidine; 57: 2-(3',7'-Dimethyloctylsulfanyl)thiophene; 58: 1 ,4-Dioctylpiperazine; F-1 : 3-[2'-(4"-Phenoxyphenoxy)ethoxy]pyridine; 30 F-4: 2-[2'-(4"-Phenoxyphenoxy)ethoxy]pyridine; and F-5: 3-(4'-Phenoxybenzyloxy)pyridine,
for use as a medicament for treating or preventing crustacean infestations in an aquatic animal. 14. A compound according to claim 13 of the general formula I where R1 is alkyl. 5 15. A compound according to claim 13 of the general formula I where R1 is branched alkyl. 16. A compound according to claim 13 of the general formula I where R1 is alkoxy substi- 10 tuted alkyl. 17. A compound according to claim 13 of the general formula I where R1 is cyclopropyl substituted isoprenoid including farsenyl. 15 18. A. compound according to claim 13 of the general formula I where R1 is gem- dihalocyclopropyl substituted isoprenoid. 19. A compound according to any of claims' 13-18 of the general formula I where X is oxygen. 20 20. A compound according to any of claims 13-18 of the general formula I where X is sulfur. 21. A compound according to any of claims 13-20 of the general formula I where Y is ni- 25 trogen. 22. A compound according to any of claims 13-21 of the general formula where Z is nitrogen. 30 23. A compound according to claim 13 of the general formula II having saturated side chains to the aromatic ring. 24. Use according to claim 1 wherein the compound having juvenile hormone activity is a juvenile hormone analogue compound according to any of claims 13-23. 35
25. Use according to claim 1 wherein the compound is selected from the group consisting compounds 1 , 2, 4, 6, 7, 17, 34, 59, 60, 61 , F-1, F-2, F-4 and F-5, as defined herein. 26. A method of controlling crustacean infestation of an aquatic animal, the method com- 5 prising administering to said animal or to the aquatic environment of the animal an effective amount of a compound having juvenile hormone activity. 27. A method according to claim 25 wherein the aquatic animal is a fish. 10 28. A method according to claim 27 wherein the fish is of a species belonging to the Sal- monidae family including Salmo salar, Salmo trutta, Oncorhynchus gorbuscha, Oncorhynchus keta, Oncorhynchus nekra, Oncorhynchus kisutch, Oncorhynchus tshawytscha, Oncorhynchus mason, Oncorhynchus mossambicus, Oncorhynchus mykiss, Salvelinus species and Salmo clarkii . 15 29. A method according to claim 26 wherein the crustacean is of the Copepoda class. 30. A method according to claim 29 wherein the crustacean is of a species selected from the group consisting of a Lepeophtheirus species and a Caligus species. 20 31. A method according to claim 30 wherein the crustacean is selected from the group consisting of Lepeophtheirus salmonis and Caligus elongatus. 32. A method according to claim 26 wherein the crustacean is of the Branchiura class. 25 33. A method according to claim 32 wherein the parasitic crustacean is an Argulus species. 34. A method according to claim 26 wherein the compound having juvenile hormone ac- 30 tivity is selected from the group consisting of epofenonane, fenoxycarb, hydroprene, kino- prene, methoprene and triprene. 35. A method according to claim 26 wherein the compound having juvenile hormone activity is a juvenile hormone analogue compound selected from the group consisting of 35
(i) a compound of the general formula where R1 is alkyl, branched alkyl including alkenyl or alkadienyl, optionally substituted isoprenoid or alkoxy substituted alkyl, R2 is hydrogen or alkyl, X is oxygen, sulfur, methyl- ene, carbinol or carbonyl, Y is nitrogen or methinine and Z is nitrogen, methine or nitrogen oxide; (ii) a compound of general formula II where R1 is 2,2-dimethylcyclopropyl, 2,2-dihalo-3,3-dimethylcyclopropyl, 2-methyl-1- propenyl or 3-methyl-1 ,2-butadienyl, R2 is hydrogen or methoxy, R3 is alkyl, alkenyl, ace- tyl, formyl or carbomethoxy, R4 is hydrogen or R3 and R4 together is methylenedioxy and X is oxygen or sulfur; (iii) a compound of the general formula III
where R1 is 2-methylbutyl, 2,2-dimethylcyclopropyl or 2,2-dihalo-3,3-dimethylcyclopropyl and R2 is methyl, ethyl or isopropyl, 5 (iv) a compound of the general formula IV where X is oxygen or sulfur and Ar is a heteroaromatic group, and 15 (v) a compound selected from the group consisting of 55: 4-Octylmorpholine; 56: 1-Octylpiperidine; 57: 2-(3',7'-Dimethyloctylsulfanyl)thiophene; 20 58: 1 ,4-Dioctylpiperazine; F-1 : 3-[2'-(4"-Phenoxyphenoxy)ethoxy]pyridine; F-4: 2-[2'-(4"-Phenoxyphenoxy)ethoxy]pyridine; and F-5: 3-(4'-Phenoxybenzyloxy)pyridine. 25 36. A method according to claim 26 wherein the compound having juvenile hormone activity is a juvenile hormone analogue compound selected from the group consisting of compounds 1 , 2, 4, 6, 7, 17, 34, 59, 60 and 61 , as defined herein. 37. A method according to claim 26 wherein the compound having juvenile hormone ac- 30 tivity is administered to the aquatic animal orally or parenterally. 38. A method according to claim 37 wherein the compound having juvenile hormone activity is administered via the feed for the aquatic animal.
39. A method according to claim 26 wherein the compound having juvenile hormone activity is administered to the aquatic animal by adding an antiparasitically active amount of the compound to the aquatic environment of said animal. 5 40. A method according to claim 39 wherein the compound is added to the aquatic environment in the form of a micro-emulsion. 41. A method according to claim 39 wherein the concentration of the compound having juvenile hormone activity in the environment of the aquatic animal is in the range of 1 ppb 10 to 1 ppm. 42. A pharmaceutical composition for controlling crustacean infestation of an aquatic animal, comprising a compound having juvenile hormone activity and at least one pharmaceutically acceptable carrier. 15 43. A composition according to claim 42 which is in a form selected from the group consisting of a powder, a granulate, a suspension, an emulsion, a micro-emulsion and a solution. 20 44. A composition according to claim 42 which is injectable. 45. A composition according to claim 42 further comprising a component selected from the group consisting of a wetting agent, an emulsifying agent, a bulking agent, a dispersing agent. 25 46. A composition according to claim 42 comprising a further anti-parasitically active compound. 47. A composition according to claim 46 where the further anti-parasitically active com- 30 pound is selected from the group consisting of an organophosphate compound, a pyrethroid compound, hydrogen peroxide, formalin and a chitin synthesis inhibiting compound. 48. A composition according to claim 42 comprising as the compound having juvenile hormone activity a juvenile hormone analogue compound according to any of claims 13- 35 23.
49. An aquatic animal feed composition comprising a compound having juvenile hormone activity. 50. A feed composition according to claim 49 comprising as the compound having juvenile hormone activity a compound according to any of claims 13-23. 51. A feed composition according to claim 49 which is in a form selected from the group consisting of a granulate, a pellet and an emulsion. 52. A feed composition according to claim 49 wherein the amount of the compound having juvenile hormone activity is in the range of 0.001 to 5 % by weight. 53. A feed composition according to claim 49 comprising a further anti-parasitically active compound. 54. A composition according to claim 53 where the further anti-parasitically active compound is selected from the group consisting of an organophosphate compound, a pyrethroid compound, hydrogen peroxide, formalin and a chitin synthesis inhibiting compound.