COMPOUND FOR ORGANIC ELECTRONIC ELEMENT, ORGANIC ELECTRONIC ELEMENT USING SAME, AND ELECTRONIC DEVICE THEREOF
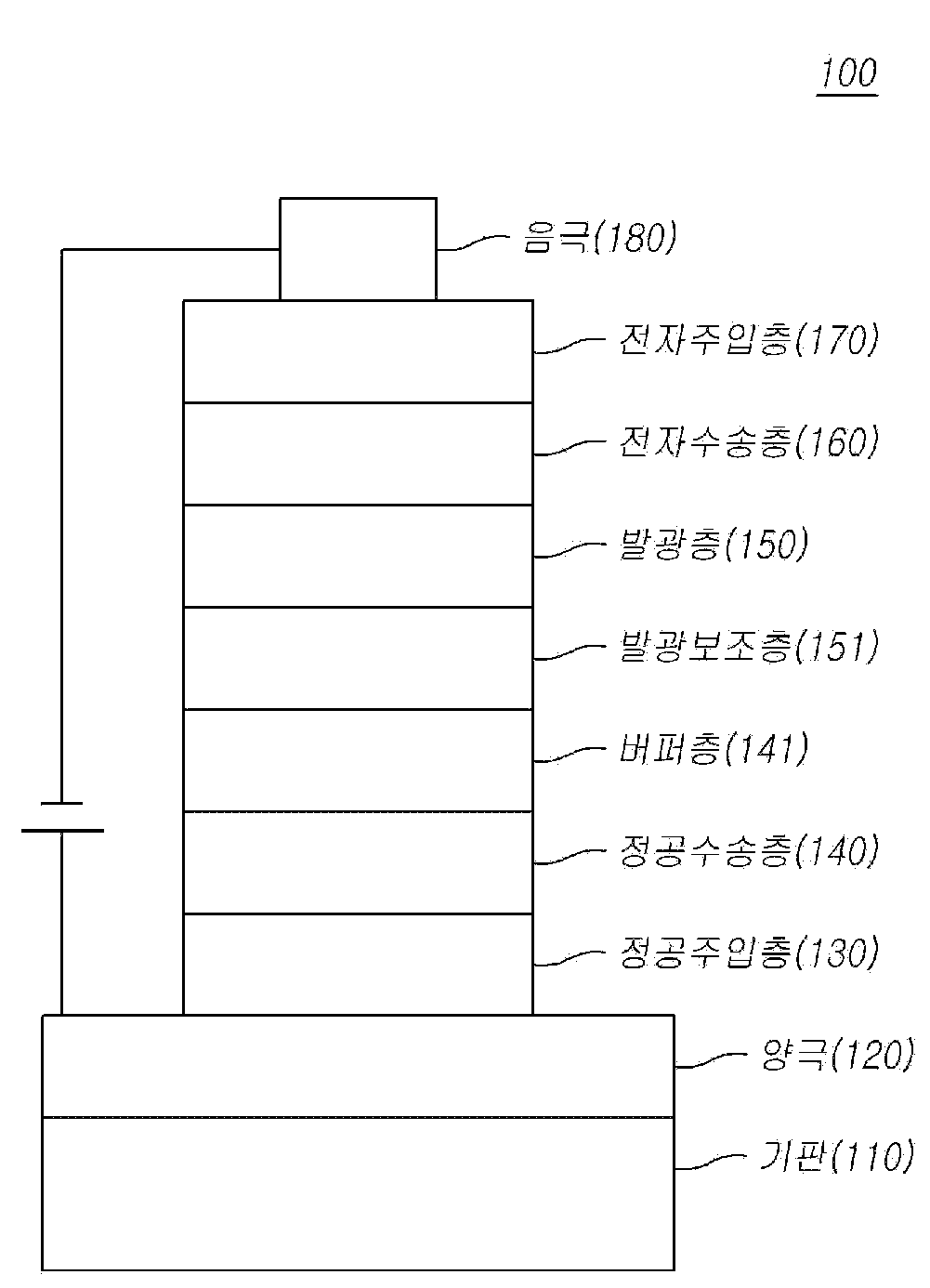
The present invention refers to organic compound, the organic electroluminescence element and electronic device using the same are disclosed. The means by which electrical energy using organic material generally organic light emitting phenomenon to light installed on the pipe body. Organic light emitting phenomena between an anode and a cathode and an organic solvent including an electrical element can have a conventional structure. The organic compound layer is formed of a material different of the electrical component in order to enhance stability and organic multilayer structure and is in many cases, e.g. hole injection layer, hole transport layer, light emitting layer, with an alkali metal-containing fullerene and can be consists of. Materials used in the layer packed in organic electrical component to other persons, light-emitting material and a charge transporting material, such as a hole injection material, hole transporting material, an electron transport material, electron injection in order to remove can be classification. Currently display the mayor of the electric service into its enlarged and, an existing thereby required in portable display power consumption greater than about power consumption etc. required. The, battery as a power source but number having the portable display discriminates whether the power consumption has been major, efficiency and life necessarily required number in addition door valuable element are disclosed. Efficiency and life, or the like may be driving voltage relative to one another, located spaced apart relatively driving voltage would increase efficiency, driving drop is generated by crystallization (Joule heating) frame has ten which it will pick up organic is applied to as a result life can be enhanced by a goniophotometer tendency. Said but simply improving efficiency by recycling a solvent that doesn't have a. However each organic layer between T1 and energy level value, the inherent properties of material (mobility, interface characteristics) in an optimal combination such as when long life and a high efficiency can be when a are disclosed. In addition, recent organic electroluminescent element is a luminous door hole number in order to solve with [song layer using light emitting light emitting layer inside the hole has to be we shall be cadence layer method, the respective light emitting layer (R, G, B) hereinafter on to reach the desired material characteristics, the respective light emitting layer emitting [...] according to the development time switch are disclosed. Generally consisting of alkali metal-containing fullerene in electronic (electron) is passed to the active layer has hole (hole) of the article to the light emitting layer in OLEDs (exciton) is generated by recombinant (recombination). But since most low HOMO value when of material employed in [song layer hole should have low T1 and thermally, thereby OLEDs (exciton) is generated in a light emitting layer toward the hole transport layer in the interface of the resulting hole next interface or hole charge imbalance (charge unbalance) at the interface between the light-emitting layer emitting hole by improving the light emission or light to be coated. When light emitting hole at the interface, resulting in shorter life of the electrical component adjusting color purity and lowering the efficiency of organic door number as well to the TFTs. The, hole HOMO energy level and light-emitting layer between the materials having a HOMO energy level must be HOMO level, high T1 values, suitable driving voltage range (full device within the range of blue device driving voltage) in at least one of the development [...] executing to 10sup16. hole mobility (hole mobility). However, this core consisting simply of light emitting [...] structural features which cannot, light emitting [...] material core and sub - substituted with cadence layer light-emitting characteristic of a hole, a light emitting layer made of high efficiency and long-lived element when suitable light emitting with cadence layer combination can be implemented or hypermetropia. On the other hand, element (Joule heating) generated ten which it will pick up even for impurity concentration, the hole injection/transport layer and light emitting [...] material having a glass transition temperature that of developed also required a state of exhaustion. A low glass transition temperature in drive hole and light emitting [...] material, so as to reduce the uniformity of the surface and the element and the rotational shafts of the thin film material can be modified, this affecting life have a great impact on wirelessly reports is coming in now. In addition, the OLED element is primarily formed by a deposition method, can withstand long deposition material, i.e. the heat resisting property is a liquid material in a database development are disclosed. I.e., an electron in order to fully exhibited features than the organic organic materials for the contact material, e.g. hole injection material, hole transport material, luminescent material, electron transport material, electron injection material, light emitting [...] stable substance or the like efficient material supporting the parking brake and one, stable and efficient development of organic layer material sufficiently cladding organic for electrical components of the possible out-a state of exhaustion. Thus, the development of novel materials off and a continuation request, in particular light emitting with cadence layer [...] hole executing the developed material etc. required. The present invention refers to said door of the existing method in order to solve number point is such as loose number, number [...] compounds having electron transporting ability and efficient electronic stop capability, device using the such compounds of high luminous efficiency, low driving voltage, high heat-resistant, compounds capable of color purity on the organic layer, the organic electric element and electronic device using the same [...] intended for a number. In one aspect, the present invention refers to compounds represented by formula for a number [...] substrate. In another aspect, the present invention refers to said formula a compounds represented by using organic electric element and electronic device number [...] substrate. According to the present invention, die [...] core type connected to amine groups, such as the number and position of the electrical component by using a certain compounds define organic material, hole transport capacity (hole transfer ability) and improved thermal stability, light emitting layer charge in the balanced energy level has a higher refractive index value and HOMO are easy to T1 for organic electroluminescence device emitting efficiency, heat resistance, etc. improvement can be life moves in the barrier layers. Figure 1 shows a organic electroluminescent device of the present invention according to example also are disclosed. Hereinafter, with reference to the attached drawing of the present invention in the embodiment detailed as follows. Each of the drawings in adding references components, although other drawing even for the same components displayed on a possible code accomplishing the same may have a significantly negative. In addition, the present invention is connected to the described, publicly known or a function of the associated specific description the subject matter of invention description if a haze can be decided to be dispensed to each other. The constitution of the invention described is provided to element, number 1, number 2, A, B, (a), (b) using terms such as can be. Other components such terms having an element discriminate between the hell of, its terms corresponding components are not limited by the nature of the or the like or sequence. Any component and other components "connected", "coupled" or "connected" are blocked when described, other components or the components thereof are connected directly but can be connected, each between components of the other components "connected", "coupled" or "connected" is a device that may be will be. In addition, layer, film, region, a component of "on" or "on" other components such as plate changed when, as well as any other components when "directly on" another intermediate components may also include a will be that it will be used. On the contrary, if any component other portions intermediate "directly on" another motor vehicle is started without understanding that it will be. The following is claimed as used in the specification and appended, not otherwise mentioned, for the meanings of terms to such as disclosed. "Halo" or "halogen" is other descriptive terms used in the specification free fluorine (F), bromine (Br), chlorine (Cl) or iodine (I) are disclosed. The present invention "alkyl" or "alkyl group" of other terms used in the description is free carbon number 1 to 60 has a single bond, linear alkyl, branched alkyl group, a cycloalkyl (alicyclic), alkyl - substituted cycloalkyl, cycloalkyl - substituted alkyl saturated aliphatic radical of the bondable functionality including big. The present invention "halo alkyl" or "halogen alkyl" of other terms used in the description are substituted with alkyl halogen-free big. The present invention "alkene diary" or "alkynyl group" of other terms used in the description is in each carbon number 2 to 60 free process for preparing has, such as caprylic or side chain chain that comprises a, one number herein are not disclosed. "Cycloalkyl" is other descriptive terms used in the present invention to free a 3 to 60 and has an alkyl carbon a number means, number herein are not disclosed. The terms used in the present invention "alkoxy group", "alkoxy", or "alkyl jade time" means oxygen radicals and fluoroalkyl group is attached, has a carbon number of 1 to 60 other descriptive free, one number herein are not disclosed. The present invention is oxygen radicals attached to terms used in "biting jade group" or "[...]" means aryl which, has a carbon number of 6 to 60 other descriptive free, one number herein are not disclosed. The present invention "fluorene diary" or "flue [...]" of other terms used in the description in R is lower than each have battery, R 'and R "is 1 or 2 and the both are H means is functional groups," substituted fluorene "includes a substituent R, R', R" group "or" substituted the flue [...] substituted and due at least one meaning other than hydrogen, R and R ' engage one another when they are carbon with cyber comprising compound formed. The present invention "aryl" and "it will be biting, [leyn" of other terms used in the description is lower than 6 to 60 each having carbon number, the one number are not disclosed. In the present invention it will be biting and [leyn it crawls aryl group or single annular, ring assembly, bonded various ring-based, cyber compounds etc.. The present invention includes "heteroaryl group" or "hetero [...]" used terms such as "hetero it will be biting, [leyn" as well as non-aromatic ring which may contain an aromatic ring, each including one or more heteroatoms atoms to carbon atoms is lower than other descriptive 2 means the number herein one loop of 60 or not the. The term "heteroatom" used in the specification description of other free N, O, S, P or Si exhibit, hetero ring heteroatoms including single annular, ring assembly, bonded various ring-based, cyber justice e applies to compounds and the like. In addition "hetero [...]" is, instead SO carbon has an2 The system also includes a ring including can be. For example, "hetero [...]" receive a next compound substrate. In the present invention which comprises a unitary ring and multi annularity terms used in "ring", as well as hydrocarbon ring including at least one hetero atoms and heterocyclic, aromatic and nonaromatic ring substrate. In the present invention terms used in "polycyclic" is biphenyl, such as separating ring assembly (ring assemblies), bonded (fused) ring systems and cyber which various compounds, aromatic as well as non-aromatic may contain, as well as heterocyclic hydrocarbon ring including at least one hetero atoms it contains. In the present invention terms used in "ring assembly (ring assemblies)" includes two or more ring-based (single ring or bonded ring-based) single bond or double bond directly connected to each other via such ring located in a ring-based total number of directly connecting between a compound characterized by a big number 1 the dog. The same or a different ring system is single bond or double bond ring aggregates can be interconnected directly via. In the present invention includes at least two terms used in "bonded various ring-based" sharing of atoms of the annular joint (fused) and which means, two or more of hydrocarbon ring system is junction forms thereof and at least one hetero atoms including at least one hetero ring taking count of crosses etc. bonded form. These bonding of a number of ring-based aromatic ring, a heteroaromatic ring, a combination of aliphatic or ring can be. Terms used in "cyber compounds" in the present invention is' cyber (spiro union) connected ' has, the annulus is only 1 share of atoms of 2 connected cyber achieved by big connection. The, two ring the shared atoms' cyber to atoms' and as, according to a number of atoms so as to located in a cyber compounds to each 'mono cyber to -', 'dice to fine -', 'to tris fine -' compound this may be. In addition, when a designated prefix meaning substituents listed in a continuous first order means that the substrate. For example, when will be biting and the cock time which it will know which means with allyl substituted alkoxy, alkoxy substituted with alkoxy carbonyl group which when [...] means, wherein the biting carbonyl it crawls in addition when biting carbonyl crossroad substituted alkenes which means a carbonyl substituted with allyl biting carbonyl alkene diary diary residue. In addition, without explicit description that, in the present invention in the terms used in "a substituted or unsubstituted" "substituted" deuterium, halogen, amino, nitrile, nitro, C1 - C20 Alkyl, C1 - C20 Alkoxy group, C1 - C20 Alkyl hydroxybutyrolactone, C1 - C20 Alkyl [...], C6 - C20 Of mote five pen it will be biting, C2 - C20 An alkenoic diary, C2 - C20 Of alkyne diary, C3 - C20 Of cyclo-alkyl, C6 - C20 Aryl, substituted with deuterium C6 - C20 Aryl, C8 - C20 Of biting alkene diary, silane, for boron, germanium to, fluorene diary, and O, N, S, Si and P including C hetero atoms selected from the group consisting of at least one2 - C20 Heteroatoms selected from the group consisting of at least one substituted 1 ring crossroad which means, these process is provided one number are not endured. The specification of each symbol and substituted aryl group exemplified in example, it will be biting, [leyn, such as hetero [...] corresponding to 'name' is' to reflect hydrolysis of name 'section described, ' parent compound name ' disapproval form of the substrate. For example, in the case of electromagnetic wave is [...] 'phenanthrene', 'a' is 1 is of '(a) [...]' is for 2 to the 'lung difficulty [thu reel [leyn (a)' section name of hydrolysis such as charge described, regardless of the form of the substrate hydrolysis parent compound name 'phenanthrene' disapproval. Similarly, even if pyrimidine, regardless of 'pyrimidine' hydrolysis described or, if the pyrimidine anomers 1 (a), 2 (a) in the case of hydrolysis of corresponding pyrimidine [...] is such as' name of ' disapproval form of the substrate. In addition, without explicit description that, in the present invention represented by formula formula index of definitions used by substituent definitions and equal to the received signals. Wherein, a is an integer of 0 when R substituents1 In the absence which meaning, i.e. a benzene is 0 when combined with all of the dispersing medium has an carbon, the carbon bonded hydrogen or compounds of formula can be protruded. In addition, a is an integer from 1 whether substituent R1 Forming one of the benzene ring carbon which will bind to the carbon, for example when a is 2 or 3 can be coupled together under an integer, integer is 4 to 6 and in a similar manner even when a carbon which will bind to the benzene ring, R when a is 2 or more levels1 Or each other's personal persona disclosed. In the embodiment according to one example of the present invention Figure 1 shows a organic electroluminescence element also are disclosed. The reference also 1, one in the embodiment according to of the present invention organic electrical component (100) substrate (110) formed on electrode number 1 (120), number 2 electrode (180) and number 1 electrode (120) and number 2 electrode (180) between the present invention according to a solvent including compounds with each other. The, number 1 electrode (120) and the cathode (positive electrode), number 2 electrode (180) and can have a cathode (cathode), the butt style which is number 1 number 2 electrode be [...] electrode if it will be a child node. Organic compound layer is formed electrode number 1 (120) sequentially on hole injection layer (130), hole (140), a light emitting layer (150), alkali metal-containing fullerene (160) and with an (170) can be comprising. The, exposed to at least one of these layers, hole obstruction layer, electronic obstruction layer, light emitting [...] (151), electronic [...], buffer layer (141) may further include, alkali metal-containing fullerene (160) electrons are serves like peeling may be, hole (140) and alkali metal-containing fullerene (160) is 1 can be formed inside the cathode. In addition, it does but not shown, in the embodiment according to number 1 and number 2 of the present invention one surface of at least one organic layer of said organic an electrical element can electrode formed on one surface opposite further includes a protective layer or optical efficiency enhancement layers (Capping layer) can be. Said organic layer applied in the embodiment according to one of the present invention compounds are hole injection layer (130), hole (140), light emitting [...] (151), electronic [...], alkali metal-containing fullerene (160), with an (170), light emitting layer (150) made up of a host or doping or efficiency improvement can be used it will rain. For example, the light emitting layer of the present invention compound (150), hole (140) and/or a light emitting [...] (151) may be using the same are disclosed. On the other hand, even the same core which is a methyl substituent coupling along band gap (band gap), electrical characteristics, interface characteristics may change is, for selecting and coupled subsystems are coupled to core (sub)- forward and for each combination of substituents, in particular energy level between T and each organic layer1 Value, such as the inherent properties of material (mobility, interface characteristics) when a long life and a high efficiency when an optimal combination can be. As already described, generally organic electroluminescent element hole in order to solve with [song layer number is a luminous door hole forming light-emitting layer is preferably a light emitting cadence layer, each light emitting layer (R, G, B) according to different light emitting [...] development time switch are disclosed. On the other hand, when light emitting [...] hole and a light emitting layer (host) with the kernel of the correlation similar core used in an organic layer if used different features as described in the literature to a control of a doctorate. Thus, in the present invention formula 1 compound having a hole and/or a light emitting organic layer formed using cadence layer between each energy level and T1 Value, the inherent properties of material such as organic electroluminescence (mobility, interface characteristics) can be simultaneously improving of prolonging the life and efficiency is optimized. The organic electroluminescent device in the embodiment according to one of the present invention various deposition (deposition) number in an bath using are disclosed. PVD or CVD method can be provided on the tank which number using, for example, depositing a metal film on the substrate or conductive metal oxides or alloys thereof anodes (120) is formed, on the hole injection layer (130), hole (140), a light emitting layer (150), alkali metal-containing fullerene (160) and with an (170) including a layer is formed on the organic, for the plurality (180) by depositing a material which can be use number bath 1308. In addition, hole (140) and a light-emitting layer (150) light emitting [...] (151) is, light emitting layer (150) and alkali metal-containing fullerene (160) further can be formed between electronic transportation cadence layer. In addition, various organic compound layer is formed polymer solution or solvent (solvent process) not a material deposition process, such as spin coating process, nozzle printing, inkjet printing process, slot coating process, dip coating process, roll-to-roll process, doctor steam turbines process, screen printing process, or thermal transfer method such as number of layer number can be by means of a high pressure liquid coolant. The present invention according to ITO layers can be formed into various method first, forming method of the present invention by the number range rights are not the. In the embodiment according to materials used in the formed at each-emitting organic an electrical element can one of the present invention, can be a backlit or two-sided light emitting type. And exhibits excellent processability for high resolution achieved by the WOLED (White Organic Light Emitting Device) is hereinafter, color filter is detected by the number of LCD can be bath...copyright 2001. White backlight device to perform various structures are mainly used as blue light emission and to perish patent number etc. and stored. Typically, R (Red), G (Green), B (Blue) light emitting portions disposed in parallel each other in a way that the (side provided by a-side), R, G, B down (stacking) and luminescent layer lamination scheme, blue (B) and inorganic phosphor converts the organic electro-luminescent in luminous layer therefrom light (photo-a luminescence) material (color conversion material, CCM) such as using the gamut to the ends of the first, also be applied to the present invention refers to such WOLED S802. In addition, in the embodiment according to one of the present invention organic an electrical element can organic electroluminescence device, organic solar cells, organic photoreceptor, organic transistor, one white lighting element can be monochrome or color. In the embodiment of the present invention is device including an electrical component on the aforementioned of the present invention other organic display, the display device comprising a number plower number including electronic device can be a water level. The, current or future electronic device is wire communication terminal may be, of a mobile phone, PDA, electronic dictionary, PMP, remote control, navigation, game machine, various TV, all electronic device comprising the various personal computer or the like. Hereinafter, according to one aspect of the present invention compounds are disclosed therein. According to one aspect of the present invention represented by formula 1 is displayed to. <Formula 1> In said formula 1, which each symbol is defined as follows. L1 C is6 - C60 It will be biting, [leyn strangeness of which, preferably C6 - C30 It will be biting, [leyn of, more preferably C6 - C18 It will be biting, [leyn strangeness of which, illustratively phenyl, naphthalene, biphenyl, xylene, phenanthrene, triphenylene etc. disclosed. L2 And L3 Are each independently a single bond is; C6 - C60 It will be biting, [leyn of; the flue [...]; O, N, S, Si and at least one of the hetero atoms including C P2 - C60 A variety of [...]; and C6 - C60 C aromatic ring and3 - C60 Aliphatic ring fusion [...]; selected from the group consisting of. L2 And L3 When the [...], preferably C6 - C30 It will be biting, [leyn of, more preferably C6 - C18 It will be biting, [leyn strangeness of which, illustratively phenyl, naphthalene, biphenyl etc. disclosed. L2 And L3 When heterocyclic is due, preferably C2 - C30 A variety of [...], C2 - C10 Ring heteroatoms which, illustratively pyridine, die [...] etc. disclosed. Ar1 To Ar5 Are each independently C6 - C60 Aryl; O, N, S, Se, Si and P including C hetero atoms selected from the group consisting of at least one2 - C60 A variety of [...]; fluorene diary; C6 - C60 C aromatic ring and3 - C60 Aliphatic ring fusion [...]; C1 - C50 Alkyl; C2 - C20 An alkenoic diary; C2 - C20 Of alkyne diary; C1 - C30 The alkoxylation group; and C6 - C30 With biting jade time selected from the group consisting of. But, Ar2 And Ar3 In addition it will doze number encoded in cover. Ar1 To Ar5 When is it is an allyl, preferably C6 - C60 Aryl, more preferably C6 - C18 R of which, illustratively phenyl, naphthyl, biphenyl, xylene, phenanthrene, fluoranthene etc. disclosed. Ar1 To Ar5 When heterocyclic is due, preferably C2 - C60[...] heteroatoms, more preferably C2 - C18 Ring heteroatoms which, illustratively the offending cycle, pyridine, indole, for treatment, carbazole, indole roca it will doze, it cuts trillion [phyu column die, die [...], die it cuts trillion the leno pen which it will count etc. disclosed. Ar1 To Ar5 The flue is when they are five [leyn diaries, illustratively 9, 9 - methyl - 9H - fluorene, 9, 9 - diphenyl - 9H - fluorene, 9, 9' - with at all [phul base five [leyn cyber etc. disclosed. Ar1 To Ar5 When fusion ring, e.g. 1, 2 - disclosed hereinafter be synchronized with it will be a gap claw hit benzene. M and n are each an integer from 0 to 3. R1 And R2 Are each independently deuterium; of tritium; halogen; cyano group; alkoxy carbonyl; C6 - C60 Aryl; fluorene diary; O, N, S, Se, Si and P including C hetero atoms selected from the group consisting of at least one2 - C60 A variety of [...]; C6 - C60 C aromatic ring and3 - C60 Aliphatic ring fusion [...]; C1 - C50 Alkyl; C2 - C20 An alkenoic diary; C2 - C20 Of alkyne diary; C1 - C30 The alkoxylation group; and C6 - C30 Selected from the group consisting of with biting jade time and, when m is 2 or more integer adjacent R1 Each other forms a ring in combination can be, even when n is an integer 2 or more adjacent R2 Each other forms a ring combination can be. M is an integer of 2 or more when a plurality of R1 Are identical to each other or can be different and, n is an integer of 2 or more even when a plurality of R2 To each be the same or different. R1 And R2 When is it is an allyl, preferably C6 - C60 Aryl, more preferably C6 - C10[...] of which, illustratively phenyl, me [phu etc. disclosed. Adjacent R1 Each other and/or adjacent R2 C ring formed each in combination6 - C60 Aromatic rings, fluorene, O, N, S, Se, Si and P including C hetero atoms selected from the group consisting of at least one2 - C60 N-heterocyclic, or C6 - C60 C aromatic ring and3 - C60 Aliphatic ring fusion [...] may be like, illustratively be benzene it will be a ring. Adjacent R1 Each other and/or adjacent R2 The mold each benzene ring are bonded to each other, these combined with benzene ring with naphthalene, phenanthrene or the like can be formed. Ar1 - Ar5 , R1 , R2 , L1 - L3 , Adjacent R1 An annular each in combination, and adjacent R2 An annular each in combination is deuterated; halogen; C1 - C20 C phenyl or benzyl6 - C20 Of with allyl substituted or substituted or unsubstituted silane; C1 - C20 C phenyl or benzyl6 - C20 A substituted or unsubstituted to phosphine oxide with allyl of; a siloxane group; to boron; for germanium; cyano group; nitro; C1 - C20 Alkyl [...]; C1 - C20 The alkoxylation group; C1 - C20 Alkyl; C2 - C20 An alkenoic diary; C2 - C20 Of alkyne diary; C6 - C20 Aryl; substituted with deuterium C6 - C20 Aryl; fluorene diary; O, N, S, Se, Si and P including C hetero atoms selected from the group consisting of at least one2 - C20 A variety of [...]; C3 - C20 Of cyclo-alkyl; C7 - C20 Of aryl-alkyl; - N (Ra ) (Rb ); C8 - C20 Of biting alkene group; and Selected from the group consisting of one or more substituted can be further substituted. - N (Ra ) (Rb ) In, Ra And Rb Are each independently C6 - C60 Aryl; fluorene diary; and O, N, S, Se, Si and at least one of the hetero atoms including C P2 - C60 A variety of [...]; can be selected from the group consisting of, Ra And Rb Deuterium is; halogen; C1 - C20 C phenyl or benzyl6 - C20 With allyl substituted or substituted or unsubstituted silane of; a siloxane group; to boron; for germanium; cyano group; nitro; C1 - C20 Alkyl [...]; C1 - C20 The alkoxylation group; C1 - C20 Alkyl; C2 - C20 An alkenoic diary; C2 - C20 Of alkyne diary; C6 - C20 Aryl; substituted with deuterium C6 - C20 Aryl; fluorene diary; O, N, S, Se, Si and P including C hetero atoms selected from the group consisting of at least one2 - C20 A variety of [...]; C3 - C20 Of cyclo-alkyl; C7 - C20 Of aryl-alkyl; and C8 - C20 Of biting alkene diary; can be further substituted one or more selected from the group consisting of substituted. Preferably, represented by formula 2 may be displayed one of said formula 1 to formula 5 and, more preferably of formula 6 to purchasers to formula 10 may be displayed disclosed. <Formula 2><formula 3> <Formula 4><formula 5> <Formula 6><formula 7><formula 8> <Formula 9><formula 10> In said formula 2 to formula 10, Ar1 - Ar5 , R1 , R2 , L1 - L3 , M and n are the same and each symbol such as defined in formula 1. In said formula 1 to formula 10, L1 One of the display can be represented by formula L1 provided 1 to formula L1 provided 7. L1 provided 2><formula L1 provided 3><formula L1 provided 1><formula L1 provided 5><formula L1 provided 6><formula L1 provided 7><formula L1 provided 4><formula In said formula L1 provided 1 to formula L1 provided 7, each an integer of 0 to 4 a to c, d is an integer of 0 to 6, e is an integer of 0 to 5, f and g each integer number of 0 to 3, R3 To R5 Are each independently aryl; fluorene diary; O, N, S, Si and P including C hetero atoms selected from the group consisting of at least one2 - C60 A variety of [...]; C3 - C60 C aliphatic ring and6 - C60 An aromatic ring in a fusion [...]; C1 - C50 Alkyl; C2 - C20 An alkenoic diary; C2 - C20 Of alkyne diary; C1 - C30 The alkoxylation group; and C6 - C30 With biting jade time selected from the group consisting of, a plurality of R when 2 or more levels respectively to g3 To R5 Each be the same or different. L1 When the L1 provided 1, preferably L1 One is a structure may be, R for structure3 , Or the like defined in a L1 provided 1 such as disclosed. , , Preferably, in formula 1 to formula 10, Ar1 To Ar5 For at least one of the formula 11 can be represented. <Formula 11> In said formula 11, which each symbol can be defined as follows. Ar1 , Ar4 And Ar5 When X is at least one of the formula 11 S, Se, O, C (Rc ) (Rd ) Or N (Re ) And, Ar2 And Ar3 When X is at least one of the formula 11 S, Se, O or C (Rc ) (Rd ) Are disclosed. C (Rc ) (Rd ) And N (Re ) In, Rc To Re Are each independently C6 - C60 Aryl; fluorene diary; O, N, S, Si and P including C hetero atoms selected from the group consisting of at least one2 - C60 A variety of [...]; C3 - C60 C aliphatic ring and6 - C60 An aromatic ring in a fusion [...]; C1 - C50 Alkyl; C2 - C20 An alkenoic diary; C2 - C20 Of alkyne diary; C1 - C30 The alkoxylation group; and C6 - C30 With biting jade time selected from the group consisting of, Rc And Rd Is in combination with compound cyber carbon they can be formed. L4 A single coupling; C6 - C20 It will be biting, [leyn of; the flue [...]; O, N, S, Si and at least one of the hetero atoms including C P2 - C20 A variety of [...]; and C3 - C20 C aliphatic ring and6 - C20 Selected from the group consisting of an aromatic ring in a fusion ring crossroad. O is an integer between 0 and 3, p is integer number of 0 to 4, R6 And R7 Deuterium independently of each other; of tritium; halogen; cyano group; alkoxy carbonyl; C6 - C60 Aryl; fluorene diary; O, N, S, Si and P including C hetero atoms selected from the group consisting of at least one2 - C60 A variety of [...]; C3 - C60 C aliphatic ring and6 - C60 An aromatic ring in a fusion [...]; C1 - C50 Alkyl; C2 - C20 An alkenoic diary; C2 - C20 Of alkyne diary; C1 - C30 The alkoxylation group; and C6 - C30 Selected from the group consisting of with biting jade time and, when 2 or more levels on p o respectively, each of a plurality of R6 And R7 Each can be the same or different, when 2 or more integer p o on respectively, adjacent R6 Each other, adjacent R7 Each other forms a ring combination can be. More preferably Ar1 , Ar2 And Ar5 And at least one of the formula 11, the implementation being S is X in said formula 11. Ar1 To Ar5 When at least one of said formula 11, preferably represented by formula 1 to formula 1 - 3 can be display one of formula 1 - 1. In said formula 1 - 1 and formula 1 - 3, X is S, Se, O, C (Rc ) (Rd ) Or N (Re ) And, in said formula 1 - 2, X is S, Se, O or C (Rc ) (Rd ) And, Ar1 To Ar5 , L1 To L3 , R1 , R2 , M and n are the same as those defined in said formula 1 such as symbol which, L4 , Rc To Re , R6 , R7 , O and p is defined in said formula 11 and are the same. Specifically, one of compounds can be represented by said formula 1. Another aspect of the present invention, electrode number 1, number 2 electrode, and said number 1 and number 2 electrode formed between organic solvent including electrical elements number [...] substrate. The, said organic compound layer is formed as a raw material compound having said formula 1 to formula 10. Preferably said organic compound layer is formed hole injection layer, hole transport layer, light emitting [...], light emitting layer, electronic [...], with an alkali metal-containing fullerene and at least one layer comprising a 1, said hole injection layer, hole transport layer, light emitting [...], light emitting layer, electronic [...], said compound with an alkali metal-containing fullerene and at least 1 species alone or in admixture of at least one compound 2 1 layer is provided with a multiple myelomas are included. More preferably, light-emitting layer and said organic compound layer is formed comprising light emitting cadence layer, containing the red phosphor and said light-emitting layer, said light emitting [...] containing said compounds with each other. Another aspect of the present invention is, according to Claim 8 display device and said display device including electronic device including an electrical driving a water level a number organic [...] number which, preferably said organic an electrical element can organic electroluminescence device, organic solar cells, organic photoreceptor, organic transistor, and monochrome or white lighting element can be one. In hereinafter, the present invention according to formula 1 in the embodiment of the electrical component with respect to the synthesis of compound number for example specifically described attainments organic and positive examples but, in the embodiment of the present invention is confined to not the. Synthesis example The present invention according to formula 1 is a compound such as compound 1 (final products) and reacting Sub 1 and Sub 2 is generated, the pieces are not correct. Ar1 To Ar5 , L1 To L3 , R1 , R2 , M and n are the same as defined in formula 1 or the like. 1><reactive I. Sub 1 synthesis of 1 is a compound synthesized by the reaction of said reactive Sub 1 of 2 path but, limited to are not correct. Hal1 Is I, Br, Cl and, Hal2 Is Br, Cl are disclosed. 2><reactive In said reactive 2, amine (HN-a Ar2 Ar3 ) Reactants applicant when the Korean registration patent number 10 - 1251451 call (2013. 04. 05 user publication of registration) disclosure to synthetic method was used. Sub 1 particular embodiments belonging to synthesis of compound as follows. 1. Sub 1 - 1, Sub 1 - 83 Synthesis example 3><reactive (1) Sub 1 a-III-a 1 synthesis Diphenylamine (CAS Registry Number: 122 - 39 - 4) starting material (17. 24 g, 101. 87 mmol) in a toluene [...] (850 ml) to a seam sealing, 2, 8 a-dibromodibenzo [ (2) Sub 1 - 1 synthesis Said [...] Sub 1 a-III-a 1 obtained in the synthesis (12. 22 g, 28. 39 mmol), toluene (200 ml), (CAS Registry Number: 62 - 53 - 3) aniline (2. 91 g, 31. 23 mmol), Pd2 (Dba)3 (0. 78 g, 0. 85 mmol), 50% P ( (3) Sub 1 a-IV a-83 synthesis Said Sub 1 a-III-a 1 obtained in the synthesis (14. 59 g, 33. 90 mmol) [...] a THF (120 ml) to a seam sealing, (4 a-chlorophenyl) boronic acid (CAS Registry Number: 1679 - 18 - 1) (5. 83 g, 37. 29 mmol), Pd (PPh3 )4 (1. 57 g, 1. 36 mmol), NaOH (4. 07 g, 101. 71 mmol), water (60 ml) added with agitation-gate 80 °C. Reaction is complete CH2 Cl2 After extracting a MgSO on organic layer4 After the resulting compound and concentrated to dry silicagel column and subsequent recrystallization product 10. 34 g (yield: 66%) by Congress. (4) synthetic Sub 1 - 83 Said obtained in the synthesis Sub 1 a-IV-a 83 (10. 34 g, 22. 38 mmol) aniline (CAS Registry Number: 62 - 53 - 3) to (2. 29 g, 24. 62 mmol), Pd2 (Dba)3 (0. 61 g, 0. 67 mmol), 50% P ( 2. Sub 1 - 20 Synthesis example 4><reactive (1) Sub 1 a-I-a 20 synthesis Starting material (4 a-bromophenyl) boronic acid (CAS Registry Number: 5467 - 74 - 3) (15. 17 g, 75. 54 mmol) [...] a toluene (755 ml) to a seam sealing, (2) Sub 1 a-II-a 20 synthesis Said Sub 1 a-I-a 20 obtained in the synthesis (25. 68 g, 64. 97 mmol) [...] a THF (230 ml) to a seam sealing, 2, 4 a-dibromo-a 1 - (methylsulfinyl) benzene (CAS Registry Number: 1820757 - 87 - 6) (21. 30 g, 71. 46 mmol), Pd (PPh3 )4 (3. 00 g, 2. 60 mmol), NaOH (7. 80 g, 194. 90 mmol), water (115 ml) with agitation adding 80 °C-gate. Reaction is complete CH2 Cl2 After extracting a MgSO on organic layer4 After the resulting compound and concentrated to dry silicagel column and subsequent recrystallization product 25. 12 g (yield: 68%) by Congress. (3) Sub 1 a-III-a 20 synthesis Said Sub 1 a-II-a 20 obtained in the synthesis (25. 12 g, 44. 18 mmol) [...] a triflic acid (58. 6 ml, 662. 74 mmol) at room temperature 24 hours on specified behind it agitated, pyridine aqueous solution (775 ml, pyridine: H2 O=1:5) 30 minutes and a reflux stirring slowly enemy-gate. Reaction is complete CH2 Cl2 After extracting a MgSO on organic layer4 After the resulting compound and concentrated to dry silicagel column and subsequent recrystallization product 11. 62 g (yield: 49%) are obtained. (4) synthetic Sub 1 - 20 Said Sub 1 a-III-a 20 obtained in the synthesis (11. 62 g, 21. 66 mmol) to [1, 1' a-biphenyl] provided 4 provided amine (CAS Registry Number: 92 - 67 - 1) (4. 03 g, 23. 82 mmol), Pd2 (Dba)3 (0. 59 g, 0. 65 mmol), 50% P ( 3. Sub 1 - 28 Synthesis example 5><reactive (1) Sub 1 a-I-a 28 synthesis Starting material (3 a-bromonaphthalen provided 2 a-yl) boronic acid (CAS Registry Number: 1301205 - 62 - 8) (29. 33 g, 116. 90 mmol) diphenylamine (CAS Registry Number: 122 - 39 - 4) to (19. 78 g, 116. 90 mmol), Pd2 (Dba)3 (3. 21 g, 3. 51 mmol), 50% P ( (2) Sub 1 a-II-a 28 synthesis Said Sub 1 a-I-a 28 obtained in the synthesis (29. 34 g, 86. 50 mmol) 2, 4 a-dibromo-a 1 - (methylsulfinyl) benzene (CAS Registry Number: 1820757 - 87 - 6) to (28. 35 g, 95. 15 mmol), Pd (PPh3 )4 (4. 00 g, 3. 46 mmol), NaOH (10. 38 g, 259. 49 mmol), THF (300 ml), water (150 ml) adding said Sub 1 a-II-a 20 using synthesis product 24. 82 g (yield: 56%) by Congress. (3) Sub 1 a-III-a 28 synthesis Said Sub 1 a-II-a 28 obtained in the synthesis (24. 82 g, 48. 43 mmol) to triflic acid (64. 3 ml, 726. 48 mmol), pyridine aqueous solution (850 ml, pyridine: H2 O=1:5) adding said Sub 1 a-III-a 20 using synthesis product 11. 63 g (yield: 50%) by Congress. (4) synthetic Sub 1 - 28 Said Sub 1 a-III-a 28 obtained in the synthesis (11. 63 g, 24. 21 mmol) (CAS Registry Number: 14770 - 85 - 5) to 5 a-phenylthiophen provided 2 a-amine (4. 67 g, 26. 63 mmol), Pd2 (Dba)3 (0. 67 g, 0. 73 mmol), 50% P ( 4. Sub 1 - 44, Sub 1 - 96 Synthesis example 6><reactive (1) Sub 1 a-III-a 44 synthesis Starting material (2) synthetic Sub 1 - 44 Said Sub 1 a-III-a 44 obtained in the synthesis (10. 42 g, 19. 50 mmol) to indolo [3, 2, 1 - (3) Sub 1 a-IV-a 96 synthesis Said Sub 1 a-III-a 44 obtained in the synthesis (27. 50 g, 51. 45 mmol) to (5 a-chloropyridin provided 3 a-yl) boronic acid (CAS Registry Number: 872041 - 85 - 5) (8. 91 g, 56. 60 mmol), Pd (PPh3 )4 (2. 38 g, 2. 06 mmol), NaOH (6. 17 g, 154. 36 mmol), THF (180 ml), water (90 ml) adding said Sub 1 a-IV a-83 using synthesis product 14. 88 g (yield: 51%) by Congress. (4) synthetic Sub 1 - 96 Said obtained in the synthesis Sub 1 a-IV-a 96 (14. 88 g, 26. 24 mmol) to 3 - (pyridin-a 3 a-yl) aniline (CAS Registry Number: 57976 - 57 - 5) (4. 91 g, 28. 86 mmol), Pd2 (Dba)3 (0. 72 g, 0. 79 mmol), 50% P ( 5. Sub 1 - 46 and Sub 1 - 73 Synthesis example 7><reactive (1) Sub 1 a-I-a 46 synthesis Starting material (3 a-bromophenyl) boronic acid (CAS Registry Number: 89598 - 96 - 9) (38. 32 g, 190. 81 mmol) diphenylamine (CAS Registry Number: 122 - 39 - 4) to (32. 29 g, 190. 81 mmol), Pd2 (Dba)3 (5. 24 g, 5. 72 mmol), 50% P ( (2) Sub 1 a-II-a 46 synthesis Said Sub 1 a-I-a 46 obtained in the synthesis (50. 20 g, 173. 62 mmol) (methylsulfinyl) benzene (CAS Registry Number: 1638151 - 06 - 0) to 4 a-bromo-a 1 a-iodo-a 2 - (65. 89 g, 190. 98 mmol), Pd (PPh3 )4 (8. 03 g, 6. 94 mmol), NaOH (20. 83 g, 520. 85 mmol), THF (610 ml), water (305 ml) adding said Sub 1 a-II-a 20 using synthesis product 60. 21 g (yield: 75%) by Congress. (3) Sub 1 a-III provided 46 and Sub 1 a-III-a 73 synthesis Said Sub 1 a-II-a 46 obtained in the synthesis (60. 21 g, 130. 21 mmol) to triflic acid (115. 2 ml, 1302. 09 mmol), pyridine aqueous solution (1520 ml, pyridine: H2 O=1:5) adding said Sub 1 a-III-a 20 synthesis using product Sub 1 a-III-a 46 23. 54 g (yield: 42%) and Sub 1 a-III-a 71 20. 73 g (yield: 37%) are obtained. (4) synthetic Sub 1 - 46 Said Sub 1 a-III-a 46 obtained in the synthesis (11. 53 g, 26. 79 mmol) aniline (CAS Registry Number: 62 - 53 - 3) to (2. 74 g, 29. 47 mmol), Pd2 (Dba)3 (0. 74 g, 0. 80 mmol), 50% P ( (5) synthesis Sub 1 - 73 Said Sub 1 a-III-a 73 obtained in the synthesis (10. 16 g, 23. 61 mmol) to [1, 1' a-biphenyl] provided 3 a-amine (CAS Registry Number: 2243 - 47 - 2) (4. 39 g, 25. 97 mmol), Pd2 (Dba)3 (0. 65 g, 0. 71 mmol), 50% P ( 6. Sub 1 - 60 Synthesis example 8><reactive Said Sub 1 a-III-a 46 obtained in the synthesis (11. 74 g, 27. 28 mmol) to dibenzo [ 7. Sub 1 - 79 Synthesis example 9><reactive (1) Sub 1 a-I-a 79 synthesis Starting material (4 a-bromophenyl) boronic acid (CAS Registry Number: 5467 - 74 - 3) (22. 50 g, 112. 04 mmol) to (2) Sub 1 a-II-a 79 synthesis Said Sub 1 a-I-a 79 obtained in the synthesis (35. 34 g, 104. 19 mmol) 1, 3 non-dichloro-a 2 - (methylsulfinyl) benzene (CAS Registry Number: 122199 - 98 - 8) to (23. 96 g, 114. 60 mmol), Pd (PPh3 )4 (4. 82 g, 4. 17 mmol), NaOH (12. 50 g, 312. 56 mmol), THF (360 ml), water (180 ml) adding said Sub 1 a-II-a 20 using synthesis product 25. 36 g (yield: 52%) by Congress. (3) Sub 1 a-III-a 79 synthesis Said obtained in the synthesis Sub 1 a-II-a 79 (25. 36 g, 54. 19 mmol) to triflic acid (71. 9 ml, 812. 80 mmol), pyridine aqueous solution (950 ml, pyridine: H2 O=1:5) adding said Sub 1 a-III-a 20 using synthesis product 11. 10 g (yield: 47%) by Congress. (4) synthetic Sub 1 - 79 Said Sub 1 a-III-a 79 obtained in the synthesis (11. 10 g, 25. 46 mmol) to dibenzo [ 8. Sub 1 - 109, Sub 1 - 114, Sub 1 - 119 Synthesis example 10><reactive (1) Sub 1 a-I-a 109 synthesis Starting material (3 '- bromo - [1, 1' - biphenyl] - 3 a-yl) boronic acid (CAS Registry Number: 1048990 - 21 - 1) (73. 05 g, 263. 79 mmol) diphenylamine (CAS Registry Number: 122 - 39 - 4) to (44. 64 g, 263. 79 mmol), Pd2 (Dba)3 (7. 25 g, 7. 91 mmol), 50% P ( (2) Sub 1 a-II-a 109 synthesis Said obtained in the synthesis Sub 1 a-I-a 109 (86. 71 g, 237. 41 mmol) (methylsulfinyl) benzene (CAS Registry Number: 1638151 - 06 - 0) to 4 a-bromo-a 1 a-iodo-a 2 - (90. 09 g, 261. 15 mmol), Pd (PPh3 )4 (10. 97 g, 9. 50 mmol), NaOH (28. 49 g, 712. 22 mmol), THF (830 ml), water (415 ml) adding said Sub 1 a-II-a 20 95 using synthesis product. 88 g (yield: 75%) by Congress. (3) Sub 1 a-III-a 109 synthesis Said obtained in the synthesis Sub 1 a-II-a 109 (95. 88 g, 178. 05 mmol) to triflic acid (157. 6 ml, 1780. 50 mmol), pyridine aqueous solution (2080 ml, pyridine: H2 O=1:5) adding said Sub 1 a-III-a 20 using synthesis product 36. 97 g (yield: 41%) by Congress. (4) synthetic Sub 1 - 109 Said obtained in the synthesis Sub 1 a-III-a 109 (10. 21 g, 20. 16 mmol) to [1, 1' a-biphenyl] provided 4 provided amine (CAS Registry Number: 92 - 67 - 1) (3. 75 g, 22. 18 mmol), Pd2 (Dba)3 (0. 55 g, 0. 60 mmol), 50% P ( (5) synthesis Sub 1 - 114 Said obtained in the synthesis Sub 1 a-III-a 109 (10. 59 g, 20. 91 mmol) to dibenzo [ 9. Sub 1 - 119 Synthesis example 11><reactive (1) Sub 1 a-IV-a 119 synthesis Said obtained in the synthesis Sub 1 a-III-a 109 (15. 81 g, 31. 22 mmol) to (3 a-chlorophenyl) boronic acid (CAS Registry Number: 63503 - 60 - 6) (5. 37 g, 34. 34 mmol), Pd (PPh3 )4 (1. 44 g, 1. 25 mmol), NaOH (3. 75 g, 93. 65 mmol), THF (110 ml), water (55 ml) using 10 adding said Sub 1 a-IV a-83 synthesis product. 58 g (yield: 63%) by Congress. (2) synthetic Sub 1 - 119 Said obtained in the synthesis Sub 1 a-IV-a 119 (10. 58 g, 19. 66 mmol) dibenzo [-b, d-] furan-a 4 a-amine (CAS Registry Number: 50548 - 43 - 1) to (3. 96 g, 21. 63 mmol), Pd2 (Dba)3 (0. 54 g, 0. 59 mmol), 50% P ( Sub 1 below can be a compounds belonging to such compounds, which are not limited to, table 1 Sub 1 some compounds belonging to the FD-a MS (Field Desorption provided Mass Spectrometry) represents the value are disclosed. [Table 1] II. Sub 2 synthesis of 1 reactive 11 is synthesized by the reaction of said reactive Sub 2 of path but, limited to are not correct. Hal3 The Br or I are disclosed. 12><reactive In said reactive 11, amine (HN-a Ar4 Ar5 ) Reactants applicant when the Korean registration patent number 10 - 1251451 call (2013. 04. 05 user publication of registration) disclosure to synthetic method was used. Sub 2 synthesis of compound belonging to particular embodiments as follows. 1. Sub 2 - 1 Synthesis example 13><reactive Diphenylamine (CAS Registry Number: 122 - 39 - 4) starting material (7. 93 g, 43. 86 mmol) [...] a toluene (390 ml) after the melting, 1 a-bromo-a 3 a-iodobenzene (CAS Registry Number: 591 - 18 - 4) (19. 89 g, 70. 29 mmol), Pd2 (Dba)3 (1. 29 g, 1. 41 mmol), 50% P ( 2. Sub 2 - 3 Synthesis example 14><reactive Di - starting material 3. Sub 2 - 4 Synthesis example 15><reactive Diphenylamine (CAS Registry Number: 122 - 39 - 4) starting material (6. 31 g, 37. 29 mmol) to 3 a-bromo-a 5 a-iodo-a ' 1, 1 a-biphenyl (CAS Registry Number: 136649 - 44 - 0) (20. 08 g, 55. 93 mmol), Pd2 (Dba)3 (1. 02 g, 1. 12 mmol), 50% P ( 4. Sub 2 - 13 Synthesis example 16><reactive Starting material 5. Sub 2 - 29 Synthesis example 17><reactive Diphenylamine (CAS Registry Number: 122 - 39 - 4) starting material (4. 29 g, 25. 35 mmol) 2, 11 a-dibromotriphenylene (CAS Registry Number: 24253 - 51 - 8) to (14. 68 g, 38. 03 mmol), Pd2 (Dba)3 (0. 70 g, 0. 76 mmol), 50% P ( 6. Sub 2 - 30 Synthesis example 18><reactive Diphenylamine (CAS Registry Number: 122 - 39 - 4) starting material (7. 68 g, 45. 38 mmol) 1 a-bromo-a 4 a-iodobenzene (CAS Registry Number: 589 - 87 - 7) to (19. 26 g, 68. 07 mmol), Pd2 (Dba)3 (1. 25 g, 1. 36 mmol), 50% P ( 7. Sub 2 - 43 Synthesis example 19><reactive Diphenylamine (CAS Registry Number: 122 - 39 - 4) starting material (6. 57 g, 38. 82 mmol) to 4 a-bromo-a 4 'a-iodo-a' 1, 1 a-biphenyl (CAS Registry Number: 105946 - 82 - 5) (20. 91 g, 58. 23 mmol), Pd2 (Dba)3 (1. 07 g, 1. 16 mmol), 50% P ( 8. Sub 2 - 52 Synthesis example 20><reactive Starting material 4 - (dibenzo [ Sub 2 below can be a compounds belonging to such compounds, which are not limited to, table 2 is obtained from some compounds belonging to FD-a MS Sub 2 (Field Desorption provided Mass Spectrometry) are disclosed. [Table 2] III. Product synthesis Sub 1 (1 equivalent) in a Toluene [...] seam sealing, Sub 2 (1 equivalent), Pd2 (Dba)3 (0. 03 equivalent), (t-a Bu)3 P (0. 06 equivalent), NaOt-a Bu (3 equivalents) added with agitation 100 °C-gate. Reaction is complete CH2 Cl2 After extracting a MgSO on organic layer4 Silicagel column and recrystallization of the final product and concentrated to dry after the resulting compound (final product) are obtained. 1. P-a 1 Synthesis example 21><reactive Said Sub 1 - 1 obtained in the synthesis (3. 96 g, 8. 95 mmol) [...] a toluene (90 ml) to a seam sealing, Sub 2 - 1 (2. 90 g, 8. 95 mmol), Pd2 (Dba)3 (0. 25 g, 0. 27 mmol), 50% P ( 2. P-a 20 Synthesis example 22><reactive Said Sub 1 - 20 obtained in the synthesis (3. 81 g, 6. 10 mmol) Sub 2 - 13 to (2. 62 g, 6. 10 mmol), Pd2 (Dba)3 (0. 17 g, 0. 18 mmol), 50% P ( 3. P-a 35 Synthesis example 23><reactive Said obtained in the synthesis Sub 1 - 28 (4. 03 g, 7. 01 mmol) to Sub 2 - 29 (3. 33 g, 7. 01 mmol), Pd2 (Dba)3 (0. 19 g, 0. 21 mmol), 50% P ( 4. P-a 40 Synthesis example 24><reactive Said Sub 1 - 44 obtained in the synthesis (4. 67 g, 6. 58 mmol) to Sub 2 - 3 (2. 32 g, 6. 58 mmol), Pd2 (Dba)3 (0. 18 g, 0. 20 mmol), 50% P ( 5. P-a 52 Synthesis example 25><reactive Said Sub 1 - 46 obtained in the synthesis (3. 54 g, 8. 00 mmol) Sub 2 - 4 to (3. 20 g, 8. 00 mmol), Pd2 (Dba)3 (0. 22 g, 0. 24 mmol), 50% P ( 6. P-a 71 Synthesis example 26><reactive Said Sub 1 - 60 obtained in the synthesis (4. 10 g, 7. 47 mmol) Sub 2 - 30 to (2. 42 g, 7. 47 mmol), Pd2 (Dba)3 (0. 21 g, 0. 22 mmol), 50% P ( 7. P-a 79 Synthesis example 27><reactive Said Sub 1 - 46 obtained in the synthesis (3. 22 g, 7. 28 mmol) Sub 2 - 43 to (2. 91 g, 7. 28 mmol), Pd2 (Dba)3 (0. 20 g, 0. 22 mmol), 50% P ( 8. P provided 85 Synthesis example 28><reactive Said obtained in the synthesis Sub 1 - 73 (4. 06 g, 7. 83 mmol) to Sub 2 - 52 (3. 84 g, 7. 83 mmol), Pd2 (Dba)3 (0. 22 g, 0. 23 mmol), 50% P ( 9. P-a 89 Synthesis example 29><reactive Said obtained in the synthesis Sub 1 - 79 (4. 23 g, 7. 06 mmol) Sub 2 - 30 to (2. 29 g, 7. 06 mmol), Pd2 (Dba)3 (0. 19 g, 0. 21 mmol), 50% P ( 10. P-a 96 Synthesis example 30><reactive Said obtained in the synthesis Sub 1 - 83 (4. 65 g, 8. 97 mmol) to Sub 2 - 1 (2. 91 g, 8. 97 mmol), Pd2 (Dba)3 (0. 25 g, 0. 27 mmol), 50% P ( 11. P-a 101 Synthesis example 31><reactive Said obtained in the synthesis Sub 1 - 96 (5. 52 g, 7. 88 mmol) to Sub 2 - 3 (2. 77 g, 7. 88 mmol), Pd2 (Dba)3 (0. 22 g, 0. 24 mmol), 50% P ( 12. P-a 119 Synthesis example 32><reactive Said obtained in the synthesis Sub 1 - 109 (5. 12 g, 8. 61 mmol) to Sub 2 - 1 (2. 79 g, 8. 61 mmol), Pd2 (Dba)3 (0. 24 g, 0. 26 mmol), 50% P ( 13. P-a 125 Synthesis example 33><reactive Said Sub 1 - 114 obtained in the synthesis (5. 36 g, 8. 58 mmol) to Sub 2 - 1 (2. 78 g, 8. 58 mmol), Pd2 (Dba)3 (0. 24 g, 0. 26 mmol), 50% P ( 14. P-a 135 Synthesis example 34><reactive Said obtained in the synthesis Sub 1 - 119 (5. 61 g, 8. 19 mmol) to Sub 2 - 1 (2. 66 g, 8. 19 mmol), Pd2 (Dba)3 (0. 23 g, 0. 25 mmol), 50% P ( Some compounds of the present invention prepared by the number according to exemplary synthesis such as FD-a MS value is said to table 3 such as disclosed. [Table 3] In an exemplary synthesis of the present invention represented by said formula 1 are described but, they all are on a Buchwald non-Hartwig cross coupling reaction, Suzuki cross-a coupling reaction, Intramolecular acid a-induced cyclization reaction ( Number of the electrical component trillion phyengs (1 phyeng=3.954 square yards) organic [In the embodiment 1] Green organic light-emitting device(Hole) According to the method of the present invention compound transport layer material using conventional organic electroluminescent device small number-gate. First, ITO layer formed on the organic substrate (anode) 4, 4', 4 "- Tris [2 a-naphthyl (phenyl) amino] triphenylamine (hereinafter" 2 a-TNATA " by being shrewd, to) 60 nm thickness by vacuum deposition of a formed hole-injecting layer, a 60 nm thickness by vacuum deposition on said hole injection of the present invention compound P-a 1 weight percent a chemical formula. Then, hole on said 4, 4 '- N, N' - dicarbazole-a biphenyl (hereinafter, "CBP" by being shrewd, to) host material, tris (2 a-phenylpyridine) - iridium (hereinafter, " Ir (ppy)3 " By being shrewd, to) dopant material in the weight ratio of 90:10 using vacuum deposition thickness 30 nm to form a luminescent layer by doping to him. Then on said light emitting layer (1, 1' - bis phenyl) - 4 - oleyl this toe) bis (2 - methyl - 8 - quinoline the [ley which comes the toe) aluminum (hereinafter "BAlq" by being shrewd, to) 10 nm thickness is formed by vacuum deposition of a hole a field oxidation, said hole obstruction layer tris (8 - quinolinol) aluminum (hereinafter " Alq3 " By being shrewd, to) electronic vacuum deposition of a 40 nm thickness transporting bed weight percent. Then, a LiF halo [ceyn anger alkali metal 0. 2 nm thickness deposited electronic-injecting layer is formed, to a thickness of 150 nm by forming a negative electrode Al then depositing organic electroluminescent device number was high pressure liquid coolant. [In the embodiment 2] to [In the embodiment 33] Green organic electroluminescent device (Hole) Compounds of the present invention a compound of the present invention P-a 1 hole material on behalf bright 4 P-a 6 to the same method in the embodiment 1 and P-a 134 each using the point number [...] small number-gate organic electroluminescent device. [Comparison example 1] to [Comparison example 6] Green organic electroluminescent device (Hole) Transport layer material of the present invention to a comparison by the comparison compound 1 to compound P-a 1 bright 4 on behalf of each using the same method the number point 6 compounds and organic electroluminescent device small number-gate [...] said in the embodiment 1. 3><comparison compound 1><comparison compound 2><comparison compounds 4><comparison compound 5><comparison compound 6><comparison compounds In the embodiment 33 of the present invention in the embodiment 1 and comparison example 1 to example 6 compared to allow the organic electroluminescent device prepared by the number by applying direct current to electroluminescent (EL) research (photoresearch) yarn is irradiated to measure characteristics when the PR-a 650, 5000 cd/m measurement2 In reference number in parts of equipment when the measuring life through T95 [...] produced therewith, represented table 4 measurement such as disclosed. [Table 4] From the result of said table 4 you will, organic electroluminescent device of the present invention compound is used as a material hole hole using a comparison compound 1 compared to compound 6 degrees of organic electroluminescent element material which has a low driving voltage, and the like that are formed as well as making sure that the life can be improved. Compounds of the present invention comparing compared compound 3 to 5 P-a 6 by solar heat, but they all have a same skeleton, core are different kind of atoms (S, C, N, O) is introduced, the core hole is different from atoms of different compound is used as a material when introduced to the outside can be informed that the color shift. The present invention S have been introduced such as core hole like when it cuts trillion be enveloped the pen thickness of the die is used as a material for organic electroluminescence device can be know that crucial. This carbazole (in the embodiment 4) or die (in the embodiment 5) heterocyclic core die it cuts trillion be enveloped the pen when it cuts trillion [phyu column instead of introducing, to open the hole deep HOMO energy level and high refractive index material from same number since it became work element rises to the luminous efficiency and high light transmittance, deep HOMO energy level mobility of holes through fixing holes and the electrons by an increasing charge balance in the emissive layer of the light emitting time such as molding and has subsided substrate. In addition, comparison example 3, example 4 comparison, comparison example 5 of the present invention in the embodiment and induced by comparing, when the atom is introduced on the same backbone heteroatoms can be remarkably improved efficiency life molecules. In particular, the present invention compound including carbon compounds such as 3 core when compared Sp3 exhibits lower thermal stability upon electroluminescent organic layer, directly between the organic layer, the organic layer metal electrode which occur among ten which it will pick up (Joule heat) heat resistance and resistance to high-temperature environment in making sure that the reduced first call request. Comparison example 2 of the present invention compound (in particular P-a 1, P-a 6) comparing on induced by using in the embodiment, of the present invention in the embodiment driving voltage is lowered in the case of command and an indirectly-a remarkable improvement in the efficiency can be. Compounds of the present invention compound P-a 1 or 2 benzene ring framework of each die comprises a die [...] compared both P-a 6 amine (- N (C6 H6 )2 ) Bonded to the substitution that provides the same or, in the case of compounds of the present invention and diphenyl amine substituted of phenyl amine substituted on one die back higher that are hereinafter disclosed. I.e., it cuts trillion be enveloped in the pen of the present invention in the embodiment such as die directly or indirectly coupled amine in substituent amine (- L1 - NAr4 Ar5 ) Forms at least one hole is further substituted diamine type is used as a material when, the Pig tail and efficiency comparison example 2 molecules can be remarkably improved. This compound of the present invention substituted aryl amine compound 2 amine than comparison with substituent (- L1 - NAr4 Ar5 ) In the introduction of a bend extending to unduly number by introducing appropriate range, the most suitable HOMO [...] HOMO energy level is adjusted hole hole [song layer an organic electroluminescence light emitting layer emitting energy level differences to permit charge balance an increase in better because in made to said substrate. Comparison example 6 of the present invention in the embodiment and induced by comparing, in the case of comparison example 6 of the present invention in the embodiment emits a green light than head can be boosted. Comparison example 6 of the present invention in the embodiment and used in etched by using the compounds, compounds of the present invention and comparison compound 6 P-a 51, die it cuts trillion be enveloped in the pen P-a 58 coupled amine (- L3 - N (Ar2 ) (Ar3 )) Ar in2 Ar but have the same3 Only different structure can be know. I.e., compound 6 of the present invention compound P-a 51 all L and P-a 58 and comparison3 Single bond and, Ar2 As phenyl is equal, Ar3 While compounds of the present invention cover the induction chain which will doze compared compounds are P provided 51 is phenyl, P-a 58 on each other die [...] hereinafter disclosed. Thus, even the same backbone substituted amine groups it cuts trillion be enveloped in the pen die core having such a structure in accordance with a classification, insulating layer exhibit a different result, Ar2 And/or Ar3 Is carbazole (cover derivatives) phosphorous compound is used as a material of the present invention compound is used as a material with respect to hole hole when can be informed that a remarkable improvement in the luminous efficiency and lifespan. In particular, the present invention compound substituted amine substituted die cuts trillion is enveloped the pen to a substituted to amines used as hole material introduced P-a 58 substituted organic material a comparison compound introduced diazole cover hole 6 than at ends of the electrical component making sure that the luminous efficiency can be remarkably improved. On the other hand, the present invention compounds of the sub-amine amine linkage connection between L1 When the phenylene, insulating layer exhibits different results according to the engaged position. The present invention compound is P-a 1, P-a 6, P-a 11 can be by selecting a comparison, the present invention compound P-a 1, P-a 11 (meta) or five [lu meta phenylene amine is attached to locking position (ortho) while, P-a 6 (para) that is attached to a methyl para phenylene amine, these compounds as well as in the embodiment 1 is used as a material a hole (compound using P-a 1), and in the embodiment 6 in the embodiment 2 (compound using P-a 6) comparing (compound using P-a 11) by solar heat, efficiency and life is better in the embodiment 2 can be unworkable. The, linking group L1 When the phenylene, amine compound to mate at the para-position to male and female hole can be more suitable for removing material. While, linking group L1 When the phenylene, amine compounds are coupled to male and female mate than five [lu position meta or para-position with deep HOMO energy level a result amine compounds having five [lu derived more suitable material bonded to male and female [...] meta or position can be found. In front described characteristics (high refractive index, high thermal stability, deep HOMO energy level) induced by a classifies the type of atoms (S, C, N, O) that is incorporated into a core and core (die [...]) amine substituted structure 2 to two amine substituted amine substituted aryl (- L1 - NAr4 Ar5 ) Introduced in accordance with the kind of amine substituted and whether more band gap, electrical characteristics, characteristics largely varying in the interface that can be know, this major sidewalls of that acting can be confirmed. If light emitting layer (host) surrounds a kernel of correlation with hole [...], similar core used in an effect by the present invention according to compound is used as a material hole representing relative to conventional art NaOCl is converted (features) belonging to ill afford the even are disclosed. [In the embodiment 34] Red organic electroluminescent device (Light emitting [...]) Compounds of the present invention emitting organic electroluminescent device according to a conventional method O3 [...] small number-gate. First ITO layer (anode) formed on a glass substrate by vacuum deposition thickness 60 nm 2 provided TNATA formed hole-injecting layer, a hole injection layer on said vacuum deposition NPB 60 nm thickness by a chemical formula weight percent. Then, said compounds of the present invention has a thickness of 20 nm on the hole formed on the second P-a 1 vacuum deposition cadence layer, said light emitting [...] CBP on host material, bis - (1 a-phenylisoquinolyl) iridium (III) acetylacetonate (hereinafter " (piq)2 Ir (acac)" by being shrewd, to) and dopant materials for use with 30 nm thickness by vacuum deposition to form a luminescent layer by doping was 95:5 by weight. Then, a 10 nm thickness on said light emitting layer is formed by vacuum deposition of BAlq hole a field oxidation, said hole obstruction layer Alq on3 Electronic vacuum deposition of a 40 nm thickness transporting bed weight percent. Then, a LiF halo [ceyn anger alkali metal 0. 2 nm thickness deposited electronic-injecting layer is formed, to a thickness of 150 nm by forming a negative electrode Al then depositing organic electroluminescent device number was high pressure liquid coolant. [In the embodiment 35] to [In the embodiment 104] Red organic electroluminescent device (Light emitting [...]) Light emitting [...] material of the present invention compound 5 of the present invention compound as a bright place P-a 1 P-a 2 to the point number in the embodiment 34 identical to the method using P-a 134 and [...] small number-gate organic electroluminescent device. [Comparison example 7] Light emitting cadence layer is concentrated and do not form a small number-gate organic electroluminescent device and method identical to the number [...] said in the embodiment 34. [Comparison example 8] to [Comparison example 14] A compound of the present invention P-a 1 5 bright light emitting [...] material on behalf as compared to comparison compound 8 to compound 2 each using the point number [...] the method identical to the organic electroluminescent device and said in the embodiment 34 small number-gate. 7><comparison compound 8><comparison compounds In the embodiment 104 and comparison example 7 of the present invention in the embodiment 34 to allow the organic electroluminescent device prepared by the number by comparison to example 14 to electroluminescent (EL) to the photoresist applying direct current research (photoresearch) yarn PR provided 650 when the measurement, measurement 2500 cd/m2 In reference number in parts of equipment when the measuring life through T95 [...] produced therewith, represented table 5 measurement such as disclosed. [Table 5] From the result of said table 5 you will, compounds of the present invention emitting organic electroluminescent device using the comparison example 7 [...] material compared compared to the organic electroluminescent device of example 14 are formed on life are improved significantly. As compared to the comparison example 7 do not form light emitting cadence layer, comparison compound 2 using 8 to 14 formed on light emitting cadence layer compared to comparison compound 8 for example, the present invention compounds of the present invention in the embodiment comprises a light-emitting efficiency of light emitting organic electroluminescent device of cadence layer formed P can be sure that the, in particular of the present invention in the embodiment according to command when crystalline luminous efficiency can be boosted. Table 4 aforementioned as well as already said, core introduced (S, C, N, O) on the type of atoms, substituted amine to amine substituted structure of core 2 (die [...]) amine substituted aryl (- L1 - NAr4 Ar5 ) And whether more introduced, as well as light emitting [...] hole types of amine substituted (phosphorescent red) to form the sidewalls of even major factor, high refractive index, 220R high T1 value in deep HOMO energy level of the light hole capable of efficiently charge balance within the operations hereinafter for aggregates and build up to determine the other. In particular, the present invention compound substituted heterocyclic substituent among other things to amines such as substituents at the die [...] introducing substituents at the at least one substituted aryl or both when a to amines introduced at least one refractive index than fixed cover diazole, high Tg value and therefore is capable of making sure that the luminous efficiency and thermal stability. On the other hand, the compound of the present invention plot evaluation result of the aforementioned number desirable device characteristics are described but applied only to one hole and light emitting [...] layer, compounds of the present invention may be filled with [song layer light emitting [...] synchronous section of the hole. According to the present invention description is to exemplify the user connects to the more generally described, the present invention essentially having the properties of the present invention belonging to NaOCl consultation knowledge of various deformable inputted from deviating from a will. Thus, the specification in the embodiment the present invention is to define the disclosure but rather to explain the which, in the embodiment of the present invention by such range is defined in which event are not correct. Comprises a charging range of the present invention scope of protection must be interpreted by, the equivalent of the present invention technique is interpreted in a range including all rights should range. 100: organic electrical component 110: substrate 120: number 1 electrode 130: hole injection layer 140: hole 141: buffer layer 150: light emitting layer 151: light emitting [...] 160: alkali metal-containing fullerene 170: with an 180: number 2 electrode Disclosed are an organic electronic element which contains a compound represented by chemical formula 1, a first electrode, a second electrode, and an organic layer located between the first and second electrodes; and an electronic device including thereof. By containing the compound represented by the chemical formula 1 in the organic layer, the driving voltage of the organic electronic element can be lowered, and the luminous efficiency and lifetime can be improved. COPYRIGHT KIPO 2017 A compound represented by the formula 1:<formula 1> In said formula 1, L1 C is6 - C60 It will be biting, [leyn strangeness of which, L2 And L3 Are each independently a single bond is; C6 - C60 It will be biting, [leyn of; the flue [...]; O, N, S, Si and at least one of the hetero atoms including C P2 - C60 A variety of [...]; and C6 - C60 C aromatic ring and3 - C60 Aliphatic ring fusion [...]; selected from the group consisting of, Ar1 To Ar5 Are each independently C6 - C60 Aryl; O, N, S, Se, Si and P including C hetero atoms selected from the group consisting of at least one2 - C60 A variety of [...]; fluorene diary; C6 - C60 C aromatic ring and3 - C60 Aliphatic ring fusion [...]; C1 - C50 Alkyl; C2 - C20 An alkenoic diary; C2 - C20 Of alkyne diary; C1 - C30 The alkoxylation group; and C6 - C30 Selected from the group consisting of and with biting jade time, stage Ar2 And Ar3 In addition it will doze in cover wherein the number, m and n are each an integer of 0 to 3 and, R1 And R2 Are each independently deuterium; of tritium; halogen; cyano group; alkoxy carbonyl; C6 - C60 Aryl; fluorene diary; O, N, S, Se, Si and P including C hetero atoms selected from the group consisting of at least one2 - C60 A variety of [...]; C6 - C60 C aromatic ring and3 - C60 Aliphatic ring fusion [...]; C1 - C50 Alkyl; C2 - C20 An alkenoic diary; C2 - C20 Of alkyne diary; C1 - C30 The alkoxylation group; and C6 - C30 With biting jade time selected from the group consisting of, 2 or more integer m and n respectively when each of a plurality of R1 And R2 Hereinafter same or on and, adjacent R1 Devices or adjacent R2 Each other forms a ring in combination can be, Ar1 - Ar5 , R1 , R2 , L1 - L3 , Adjacent R1 An annular each in combination, and adjacent R2 An annular each in combination is deuterated; halogen; C1 - C20 C phenyl or benzyl6 - C20 Of with allyl substituted or substituted or unsubstituted silane; C1 - C20 C phenyl or benzyl6 - C20 A substituted or unsubstituted to phosphine oxide with allyl of; a siloxane group; to boron; for germanium; cyano group; nitro; C1 - C20 Alkyl [...]; C1 - C20 The alkoxylation group; C1 - C20 Alkyl; C2 - C20 An alkenoic diary; C2 - C20 Of alkyne diary; C6 - C20 Aryl; substituted with deuterium C6 - C20 Aryl; fluorene diary; O, N, S, Se, Si and P including C hetero atoms selected from the group consisting of at least one2 - C20 A variety of [...]; C3 - C20 Of cyclo-alkyl; C7 - C20 Of aryl-alkyl; - N (Ra ) (Rb ); C8 - C20 Of biting alkene group; and Selected from the group consisting of one or more substituted can be further substituted. According to Claim 1, said formula 1 to formula 5 formula 2 in one of the second gate is a characterized compound:<formula 2><formula 3> <Formula 4><formula 5> In said formula 2 to formula 5, Ar1 - Ar5 , R1 , R2 , L1 - L3 , M and n are the same and the defined according to Claim 1. According to Claim 1, represented by formula 6 to one of the second gate in said formula 1 formula 10 characterized compound:<formula 6><formula 7><formula 8> <Formula 9><formula 10> In said formula 6 to formula 10, Ar1 - Ar5 , R1 , R2 , L1 - L3 , M and n are the same and the defined according to Claim 1. According to Claim 1, said L1 One of the second gate is a formula L1 provided 1 to formula L1 provided 7 characterized compound:><formula L1 provided 1><formula L1 provided 2><formula L1 provided 3 L1 provided 5><formula L1 provided 6><formula L1 provided 7><formula L1 provided 4><formula In said formula L1 provided 1 to formula L1 provided 7, each an integer of 0 to 4 a to c, d is an integer of 0 to 6, e is an integer of 0 to 5, f and g each integer number of 0 to 3, R3 To R5 Are each independently aryl; fluorene diary; O, N, S, Si and P including C hetero atoms selected from the group consisting of at least one2 - C60 A variety of [...]; C3 - C60 C aliphatic ring and6 - C60 An aromatic ring in a fusion [...]; C1 - C50 Alkyl; C2 - C20 An alkenoic diary; C2 - C20 Of alkyne diary; C1 - C30 The alkoxylation group; and C6 - C30 With biting jade time selected from the group consisting of, a plurality of R when 2 or more levels respectively to g3 To R5 Each be the same or different. According to Claim 1, said Ar1 To Ar5 Characterized in that for at least one of compounds represented by formula 11:<formula 11> In said formula 11, Ar1 , Ar4 And Ar5 When X is at least one of the formula 11 S, Se, O, C (Rc ) (Rd ) Or N (Re ) And, Ar2 And Ar3 When X is at least one of the formula 11 S, Se, O or C (Rc ) (Rd ) And, C (Rc ) (Rd ) And N (Re ) In, Rc To Re Are each independently C6 - C60 Aryl; fluorene diary; O, N, S, Si and P including C hetero atoms selected from the group consisting of at least one2 - C60 A variety of [...]; C3 - C60 C aliphatic ring and6 - C60 An aromatic ring in a fusion [...]; C1 - C50 Alkyl; C2 - C20 An alkenoic diary; C2 - C20 Of alkyne diary; C1 - C30 The alkoxylation group; and C6 - C30 With biting jade time selected from the group consisting of, Rc And Rd Is in combination with compound cyber carbon they can be formed and, L4 A single coupling; C6 - C20 It will be biting, [leyn of; the flue [...]; O, N, S, Si and at least one of the hetero atoms including C P2 - C20 A variety of [...]; and C3 - C20 C aliphatic ring and6 - C20 Selected from the group consisting of an aromatic ring in a fusion ring crossroad, o is an integer 0 to 3 which, p is integer number of 0 to 4, R6 And R7 Deuterium independently of each other; of tritium; halogen; cyano group; alkoxy carbonyl; C6 - C60 Aryl; fluorene diary; O, N, S, Si and P including C hetero atoms selected from the group consisting of at least one2 - C60 A variety of [...]; C3 - C60 C aliphatic ring and6 - C60 An aromatic ring in a fusion [...]; C1 - C50 Alkyl; C2 - C20 An alkenoic diary; C2 - C20 Of alkyne diary; C1 - C30 The alkoxylation group; and C6 - C30 Selected from the group consisting of with biting jade time and, when 2 or more levels on p o respectively, each of a plurality of R6 And R7 And or different socket are hereinafter, when 2 or more integer p o on respectively, adjacent R6 Each other, adjacent R7 Each other forms a ring combination can be. According to Claim 5, Ar1 , Ar2 And Ar5 And at least one of the formula 11, the X in said formula 11 is characterized in S compounds. According to Claim 1, characterized in that said formula 1 is a compound one of compounds: Electrode number 1, number 2 electrode, and said number 1 and number 2 electrode formed between the organic electroluminescence element including a solvent, said organic compound layer is formed either containing terms compound number 1 to number 7 anti anti is characterized by electrical component. According to Claim 8, said organic compound layer is formed hole injection layer, hole transport layer, light emitting [...], light emitting layer, electronic [...], with an alkali metal-containing fullerene and at least one layer comprising a 1, said hole injection layer, hole transport layer, light emitting [...], light emitting layer, electronic [...], said compound with an alkali metal-containing fullerene and at least 1 layer is provided with at least one compound or a mixture of at least one selected species alone 1 2 characterized in that the organic electroluminescence element. According to Claim 9 said organic compound layer is formed comprising a light-emitting layer and light emitting cadence layer, containing the red phosphor and said luminescent layer, said organic electroluminescence element characterized in that said light emitting [...] compounds are contained. According to Claim 8, said organic compound layer is formed spin coating process, nozzle printing, inkjet printing process, slot coating process, formed by dip coating process or roll-to-roll process is characterized by the electrical component. Organic electroluminescence display device according to Claim 8 including element; and said display device driving control section number; a including electronic device. According to Claim 12, said organic an electrical element can organic electroluminescence device, organic solar cells, organic photoreceptor, organic transistor, and monochrome or white lighting element characterized in one of the electronic device.