METHODS FOR DIAGNOSIS AND TREATMENT OF CHRONIC FATIGUE SYNDROME
The present invention relates to methods for the diagnosis and treatment of chronic fatigue syndrome. Chronic fatigue syndrome (CFS), also known as Myalgic Encephalomyelitis (ME), is a term used to describe a heterogeneous, multi-systemic condition which is primarily characterised by persistent debilitating fatigue that cannot be attributed to any alternative condition. The underlying aetiology of CFS is unknown and no diagnostic test presently exists. Instead, CFS is presently diagnosed on subjective symptomology, wherein other medical conditions that may explain the symptoms have been ruled out. Specifically, the subject must have clinically-evaluated, unexplained, persistent or relapsing fatigue for six months or more, that: (1) is of new or definite onset; (2) is not the result of ongoing exertion; (3) is not substantially alleviated by rest; and (4) results in a substantial reduction in previous levels of occupational, educational, social or personal activities. Additionally, the subject must have four or more of the following symptoms that are concurrent, persistent for six months or more and which do not predate the fatigue: (1) impaired short-term memory or concentration; (2) sore throat; (3) tender cervical or axillary lymph nodes; (4) muscle pain; (5) multi-joint pain without arthritis; (6) headaches of a new type, pattern, or severity; (7) unrefreshing sleep; and (8) post-exertional malaise lasting more than 24 hours (Royal Australasian College of Physicians Working Group, 2002). A variety of biochemical factors have been associated with CFS, including depressed mitochondrial respiration and alteration in carnitine homeostasis; however, no causative links have presently been established. Carnitine is an important endogenous compound that is found in all mammalian species (Bremer, 1983), with Carnitine transports long-chain acyl groups of fatty acids across the inner mitochondrial membrane which is important for energy production in a process known as fatty acid β-oxidation, wherein fatty acids are metabolised to produce energy. As depicted in It has been suggested that due to the important role of Another study found that acylcarnitine was not decreased in CFS patients, and further that there were no significant difference in the level of total carnitine, free carnitine and 20 different acylcarnitine compounds between CFS patients and healthy controls (Soetekouw et al., 2000). However, this study utilised a lower quantification limit that was well above the levels reported for all of the medium- and long-chain acylcarnitine compounds quantified, and only about two-thirds of the individual acylcarnitines were analysed; accordingly, it is not possible to draw firm conclusions regarding the carnitine pool composition in CFS patients from that study. The present applicant has now found that the concentration of a number of individual acylcarnitines is decreased in CFS, and that others are present at an increased concentration in CFS, compared to healthy controls. Further, it has been realised that the individual acylcarnitines that are present at a modified concentration may be utilised to diagnose CFS. Moreover, it has been realised that this finding enables the rational design of novel methods for treatment of CFS. Thus, in a first aspect, the present invention provides a method of diagnosing chronic fatigue syndrome (CFS) in a test subject, said method comprising the steps of:
In a second aspect, the present invention provides a method of diagnosing chronic fatigue syndrome (CFS) in a test subject, said method comprising the steps of:
In a third aspect, the present invention provides a method of diagnosing chronic fatigue syndrome (CFS) in a test subject, said method comprising the steps of:
In a fourth aspect, the present invention provides a method of treating chronic fatigue syndrome (CFS) in a subject, said method comprising administering an effective amount of a supplement comprising:
In a fifth aspect, the present invention provides a method of treating chronic fatigue syndrome (CFS) in a subject, said method comprising the steps of:
In a sixth aspect of the present invention, the present invention provides a method of fortifying a food comprising adding to the food a supplement comprising:
In a seventh aspect, the present invention provides a method of treating chronic fatigue syndrome (CFS) in a subject, said method comprising administering an effective amount of a modulator of carnitine/acylcarnitine metabolism wherein the modulator stimulates the activity of an enzyme selected from the group consisting of carnitine palmitoyltransferase (CPT)-I, carnitine palmitoyltransferase (CPT)-II and carnitine/acylcarnitine translocase. It is thought that fatty acid metabolism may be linked to CFS; however, studies in this area have produced conflicting results. It has now been found that particular individual acylcarnitines are present in modified concentrations (ie decreased or increased concentrations) in CFS patients compared to healthy control subjects. Further, it has been realised that this finding may provide a means to diagnose CFS and/or provide the basis for the rational design of novel methods for treatment of CFS. The term “fatty acid” as used herein will be understood by persons skilled in the art as referring to a carboxylic acid, represented by the formula R—C(═O)OH, wherein the R represents an alkyl group. The alkyl group, together with the carbon atom from the carboxylic group, is referred to as a carbon chain. The carbon chain may be of variable length, for example, between 4 and 32 (or more) carbon atoms. A “short chain” is considered to be a chain with less than 6 carbon atoms but, preferably, no less than 4 carbon atoms; a “medium chain” is considered to be a chain with 6 to 11 carbon atoms; and a “long chain” is considered to be a chain with 12 or more carbon atoms. The term “very long chain” is sometimes used for chains with more than 22 carbon atoms; however, the term “long chain” is used herein when referring to any chain with 12 or more carbon atoms. The chains are generally linear, and may be branched or unbranched. The chains can be “saturated”, meaning that the carbon atoms are connected by single bonds only, or may be “unsaturated”, meaning that there is at least one double bond (or triple bond) between the carbon atoms. The “acyl group” of a fatty acid has the formula R—C(═O)—, wherein R represents an alkyl group. Different fatty acids have different alkyl groups and hence different acyl groups. There are several nomenclature systems assigned to fatty acids, for example the “trivial nomenclature” (or common name) system and the “lipid number” system, both of which are used herein. The lipid number system takes the form C:D, where C is the number of carbon atoms in the fatty acid, and D is the number of double bonds in the fatty acid. For example, oleic acid has the formula CH3(CH2)7CH═CH(CH2)7COOH. It has 18 carbon atoms, and one double bond, and so is given the lipid number 18:1. However, the lipid number system can be ambigtious as different fatty acids can have the same lipid number, if, for example, a double bond is present in a different place on a chain that has the same number of carbon atoms. The lipid number system may also utilise “DC”, wherein the DC signifies that the compound is dicarboxylic; that is, the compound has two carboxylic acid groups. The term “individual acylcarnitine” will be understood by persons skilled in the art to refer to a molecule consisting of In a first aspect, the present invention provides a method of diagnosing chronic fatigue syndrome (CFS) in a test subject, said method comprising the steps of:
Preferably, the at least one individual acylcarnitine is a medium-chain or a long-chain acylcarnitine. For example, the at least one acylcarnitine may have a carbon chain that is 6 or more carbon atoms long. Preferably, the acylcarnitine has a carbon chain that is 12 or more carbon atoms long. In an embodiment, the at least one individual acylcarnitine is selected from the group consisting of octenoyl- The method of the first aspect does not require the use of a detectably-labelled acylcarnitine, since the at least one individual acylcarnitine referred to in step (i) is endogenous; that is, the at least one individual acylcarnitine is found naturally in the subject, having arisen from A diagnosis of CFS may be made, for example, when the concentration of at least one individual acylcarnitine from the test subject is decreased compared to that of the reference concentration (or reference concentration range), wherein the at least one individual acylcarnitine is selected from the group consisting of octenoyl- However, in another example, a diagnosis of CFS may be made when the concentration of the at least one individual acylcarnitine from the test subject is increased compared to that of the reference concentration (or reference concentration range), wherein the at least one individual acylcarnitine is selected from dodecanedioyl- In an embodiment of the method of the first aspect, the method comprises the steps of:
Preferably, the two or more individual acylcarnitines are selected from those listed above; however, persons skilled in the art will appreciate that other acylcarnitine compounds may also be suitable. In some embodiments, the two or more individual acylcarnitines will be three or more, four or more, or five or more, etc, individual acylcarnitines. The concentration of the individual acylcarnitine(s) from the test subject may be compared to the concentration of the same individual acylcarnitine(s) from an equivalent body sample(s) from a healthy control subject, or, preferably, to a concentration range of the same acylcarnitine(s) from equivalent body samples from a plurality of healthy control subjects (eg 10 to 1000 healthy control subjects). The body samples may be any body sample type that can be sampled for acylcarnitine concentration. For example, the body samples may be whole blood, serum, plasma, urine or sputum. Preferably, body samples are plasma, serum or whole blood. The concentration of the individual acylcarnitine(s) in the body samples may be determined by any suitable method including those well known to persons skilled in the art including mass spectrometry (eg tandem mass spectrometry); chromatographic techniques, such as high performance liquid chromatography (eg radioisotopic exchange HPLC), gas chrorhatography and thin layer chromatography; electrochemical sensing; and chemical sensing using suitable probes, etc. It has also been realised that a diagnosis of CFS in a subject may be based upon the determination of an aberrant concentration of at least one acylcarnitine or L-carnitine present in a test body sample(s) relative to the concentration of at least one fatty acid corresponding to an acyl group of at least one acylcarnitine compound and, similarly, an aberrant relationship between the concentration of at least one acylcarnitine or L-carnitine present in a test body sample(s) and the concentration of at least one fatty acid corresponding to an acyl group of at least one acylcarnitine compound. Thus, in a second aspect, the present invention provides a method of diagnosing chronic fatigue syndrome (CFS) in a test subject, said method comprising the steps of:
Preferably, step (iii) comprises determining a ratio of the concentration of the at least one individual acylcarnitine compound to the concentration of the at least one individual fatty acid, in which case, the determination of an aberrant ratio is indicative of CFS in the test subject. An aberrant ratio in this context may, for example, constitute a fatty acid:acylcarnitine concentration ratio that differs from a reference ratio (eg a control ratio) determined from one or more healthy subjects by ≧1.5 fold, more preferably ≧two-fold, and even more preferably ≧three-fold. Alternatively, step (iii) comprises assessing a relationship between the concentration of the at least one individual acylcarnitine compound and the concentration of the at least one individual fatty acid, in which case, the assessment of an aberrant relationship is indicative of CFS in the test subject. In a third aspect, the present invention provides a method of diagnosing chronic fatigue syndrome (CFS) in a test subject, said method comprising the steps of:
Preferably, step (iii) comprises determining a ratio of the concentration of Alternatively, step (iii) comprises assessing a relationship between the concentration of In the method of the second and third aspects, the first and second body samples are preferably the same. That is, preferably, the method utilises a single sample (or aliquots of a single sample) in the determination of the concentrations mentioned in the respective steps (i) and (ii). The sample may therefore be a single sample of whole blood, serum, plasma, urine or sputum. The concentration of the individual acylcarnitine(s) and The methods of the second and third aspects do not require the use of a detectably-labelled acylcarnitine, It has been additionally realised that the modified concentration of individual acylcarnitines in CFS patients compared to healthy subjects may be at least partly associated with at least some of the symptoms of CFS, for example, a decreased concentration of an individual acylcarnitine may be associated with fatigue due to a lesser amount of the acylcarnitine being available for energy metabolism compared to healthy subjects. Accordingly, supplementing a CFS patient with an individual acylcarnitine may reduce at least some of the CFS symptoms. Alternatively or additionally, administering a patient with Thus, in a fourth aspect, the present invention provides a method of treating chronic fatigue syndrome (CFS) in a subject, said method comprising administering an effective amount of a supplement comprising:
Preferably, the carbon chain of the acylcarnitine and/or the fatty acid is 12 or more carbon atoms long. For example, the at least one acylcarnitine may be selected from the group consisting of octenoyl- Similarly, the at least one individual fatty acid may be selected from the group consisting of octenoic acid, dodecanedioic acid, myristoic acid, palmitoleic acid, stearoic acid, oleic acid, linoleic acid and hydroxyl-oleic acid. However, preferably, the at least one individual fatty acid is selected from oleic acid and linoleic acid. In an embodiment, the method of treating CFS in a subject comprises administering an effective amount of a supplement comprising two or more individual acylcarnitine compounds wherein at least one of the individual acylcarnitines is selected from medium-chain and long-chain acylcarnitines, or a supplement comprising It has also been realised that CFS patients may be deficient in a particular individual acylcarnitine if the patient has a decreased ability to convert Accordingly, in an embodiment of the method of the fourth aspect, the method further comprises administering a modulator(s) of any one or more of CPT-I, CPT-II and carnitine/acylcarnitine translocase. More preferably, the modulator(s) stimulates the activity of at least CPT-I. The modulator(s) may be a drug or a dietary supplement. For instance, Accordingly, the modulator(s) is preferably selected from the group consisting of The modulator(s) of CPT-I and/or CPT-II and/or carnitine/acylcarnitine translocase may be administered before or after the supplement, however preferably, the supplement itself comprises the CPT-I/CPT-II/carnitine/acylcarnitine translocase modulator(s). Thus, in a particular embodiment of the method of the fourth aspect, the method comprises administering an effective amount of a supplement comprising Where the supplement administered in the method of the fourth aspect comprises an acylcarnitine that may be converted within a subject to The supplement may further comprise a pharmaceutically-acceptable carrier, excipient and/or diluent. The “effective amount” of the supplement will be any amount that will elicit a beneficial or therapeutic effect in the subject. However, generally, the effective amount will be about 0.01 to about 500 mg/kg of the subject body weight per day which can be administered in single or multiple doses. Preferably, the amount will be about 0.1 to about 250 mg/kg per day; more preferably, about 0.5 to about 100 mg/kg per day. The supplement may be administered to the subject by any suitable means, for example, orally, intravenously, intramuscularly or intranasally. However, preferably, the supplement is administered orally. Accordingly, the supplement is preferably formulated in an oral dosage form such as, for example, a capsule, tablet, caplet, granules or powders (which may be suspended or dissolved in water to provide a beverage). In some embodiments, the supplement is provided to the subject in a fortified food as described in more detail below. In a fifth aspect, the present invention provides a method of treating chronic fatigue syndrome (CFS) in a subject, said method comprising the steps of:
The concentration of the individual acylcarnitine(s) from the subject may be compared to the concentration of the same individual acylcarnitine from an equivalent body sample(s) from a healthy control subject, or, preferably, from a concentration range of the same acylcarnitine(s) from equivalent body samples from healthy control subjects. The body samples may be any body sample type that can be sampled for acylcarnitine concentration. For example, the body samples may be whole blood, serum, plasma, urine or sputum. Preferably, the body samples are plasma, serum or whole blood. The method of the fifth aspect, like that of the first aspect, does not require the use of a detectably-labelled acylcarnitine. The phrase “[at least one] individual fatty acid that corresponds to the deficient [at least one] individual acylcarnitine” is intended to refer to a particular individual fatty acid that has the same acyl group as the particular individual acylcarnitine that is deficient in the CFS patient. In other words, the corresponding fatty acid is a particular individual fatty acid that could theoretically be transformed into the particular individual acylcarnitine (that has a decreased concentration in the CFS patient) by CPT-I as shown in It will be understood by persons skilled in the art that in some embodiments the supplement may comprise Preferably, the at least one individual acylcarnitine is a medium-chain or a long-chain acylcarnitine. For example, the at least one acylcarnitine may have a carbon chain that is 6 or more carbon atoms long. Preferably, the acylcarnitine has a carbon chain that is 12 or more carbon atoms long. In an embodiment, the at least one individual acylcarnitine is selected from the group consisting of octenoyl- Accordingly, the at least one individual fatty acid may be selected from the group consisting of octenoic acid, dodecanedioic acid, myristoic acid, palmitoleic acid, stearoic acid, oleic acid, linoleic acid and hydroxyl-oleic acid. Preferably, the individual fatty acid(s) is selected from oleic acid and linoleic acid. In an embodiment of the method of the fifth aspect, the method further comprises administering a modulator(s) of any one or more of CPT-I, CPT-II and carnitine/acylcarnitine translocase. More preferably, the modulator(s) stimulates the activity of at least CPT-I. The modulator(s) may, for example, be selected from the group consisting of In some embodiments, the supplement administered in the methods of the fourth and fifth aspects is provided to the subject in a fortified food. The fortified food may be any suitable food that is able to be modified to contain the supplement in a desired amount. For example, the fortified food may be bread, cake, biscuits (crackers or cookies), cereal, food bars (such as health food bars and muesli bars), drinks, etc. In a sixth aspect, the present invention provides a method of fortifying a food comprising adding to the food a supplement comprising:
In an embodiment, the method further comprises fortifying the food with a modulator(s) of any one or more of CPT-I, CPT-II and carnitine/acylcarnitine translocase. More preferably, the modulator(s) stimulates the activity of at least CPT-I. The modulator(s) may, for example, be selected from the group consisting of The supplement may be added to the food in any suitable manner, for example, the supplement may be added during the mixing process of foods, or may alternatively be added following baking of the food product, or alternatively, added prior to packaging. The invention further extends to a fortified food produced in accordance with the method of the sixth aspect. In a seventh aspect, the present invention provides a method of treating chronic fatigue syndrome (CFS) in a subject, said method comprising administering an effective amount of a modulator of carnitine/acylcarnitine metabolism, for example, a modulator of carnitine palmitoyltransferase (CPT)-I and/or carnitine palmitoyltransferase (CPT)-II and/or carnitine/acylcarnitine translocase. While not wishing to be bound by theory, it is considered that the administration of a modulator(s) which stimulates the activity of at least CPT-I will represent an effective treatment of CFS by modulating carnitine and/or fatty acid metabolism so as to increase the ratio of acylcarnitines to free fatty acids. The modulator(s) may be a drug or a dietary supplement. Preferably, the modulator(s) is selected from the group consisting of The “effective amount” of the modulator(s) will be any amount that will elicit a beneficial or therapeutic effect in the subject. However, generally, the effective amount will be about 0.01 to about 500 mg/kg of the subject body weight per day which can be administered in single or multiple doses. Preferably, the amount will be about 0.1 to about 250 mg/kg per day; more preferably, about 0.5 to about 100 mg/kg per day. The modulator(s) may be administered to the subject by any suitable means, for example, orally, intravenously, intramuscularly or intranasally. However, preferably, the modulator(s) is administered orally. Accordingly, the modulator(s) is preferably formulated in an oral dosage form such as, for example, a capsule, tablet, caplet, granules or powders (which may be suspended or dissolved in water to provide a beverage). The modulator(s) may be provided in combination with a pharmaceutically-acceptable carrier, excipient and/or diluent. The invention is hereinafter described by reference to the following non-limiting example and accompanying figures. Previous studies have predominantly used enzymatic assays for the quantification of Tandem mass spectrometry methods have been developed which are capable of quantifying individual acylcarnitine levels in human plasma (Chace et al., 1997; Chace et al., 2003), and this method has now been utilised to provide a more complete representation of the full carnitine profile. The present study examined the concentration of endogenous plasma Chronic fatigue syndrome patients (n=44) were recruited via the Chronic Fatigue Syndrome Society of South Australia (ME/CFS Society Inc). Patients had been previously diagnosed with CFS by a physician according to the standard diagnostic criteria, that is, fatigue and at least four other symptoms as described in Table 1. Age- and gender-matched healthy subjects with no significant illnesses (n=49), were recruited from the general population via advertising. Neither patients nor healthy subjects had received any carnitine supplementation in the two months prior to the assessment. On the study assessment day, each subject/patient completed a Fatigue Severity Scale questionnaire and had a single blood sample collected via venepuncture for carnitine profiling. The Fatigue Severity Scale is a validated functional measure which comprises nine items that are rated according to a Likert-type rating scale from 1 to 7, with 1 indicating no impairment and 7 indicating severe impairment (Table 2; Krupp et al., 1989). The Fatigue Severity Scale has been shown to be an appropriate and accurate measure of fatigue severity and symptomology, and is able to distinguish between individuals with chronic fatigue syndrome-like symptomology and those individuals with no of varying levels of general fatigue (Taylor et al., 2000). A single blood sample was collected from each study subject to determine the plasma concentration of various carnitine and acylcarnitine types (described below). Analysis was conducted using a MDS-SCIEX API4000 triple quadruple tandem mass spectrometer (Applied Biosystems Inc, Foster City, Calif., United States of America) with sample delivery using a 1100 HPLC system (Agilent Technologies, Santa Clara, Calif., United States of America). Aliquots (2 μL) of each plasma sample were applied to 3 mm punches of filter paper (Whatman BFC-180, Whatman Inc, Fairfield, N.J., United States of America) and allowed to dry at room temperature. Once dry, filter papers were shipped to the analytical laboratory for analysis. A solution of pure methanol containing known concentrations of stable isotopically enriched acylcarnitines was used to extract samples from the filter paper as described below. Samples were extracted from the filter paper using the solution of pure methanol containing the known concentrations of stable isotopically enriched acylcarnitines. After a 15 minute extraction period, samples were dried under nitrogen. Samples were then esterified using acidified butanol to form the butyl-ester of each acylcarnitine followed by drying under nitrogen to remove excess butanolic HCl. The butyl-esters were determined by precursor scan of 85.1 amu. The levels of acylcarnitines were determined against the respective deuterated stable isotope using Analyst® software (Applied Biosystems Inc). A single blood sample was collected from each study subject to determine the plasma concentration of the following analytes: The total acylcarnitine concentration (AcylLC) was determined as the sum of all individual acylcarnitine concentrations; and the total carnitine concentration (TC) was determined as the sum of Unless otherwise indicated, data are expressed as mean±standard deviation. Carnitine concentrations and demographic characteristics (ie age and Fatigue Severity Scale results) obtained from CFS patients were statistically compared to those obtained from healthy subjects using an analysis of variance (ANOVA). Gender distribution between the groups was compared using Pearson's Chi-Squared (χ2) cross-tabulation analysis. Significance was set at an α-level of 0.05. WinNonlin® Professional Version 5.2 (Pharsight Corporation, Mountain View, Calif., United States of America) was used for ANOVA analysis. SPSS for Windows Version 16.0 (SPSS Inc, Chicago, Ill., United States of America) was used for the Pearson's Chi-Squared (χ2) cross-tabulation analysis. Forty-four CFS patients (17 males; 27 females), with an average age of 49.9±15.0 years, participated in the study. In addition, 49 healthy subjects (20 males; 29 females), aged 45.6±11.6 years, were recruited to serve as controls. Average Fatigue Severity Scale scores for the CFS group were 6.22±0.660, compared with scores of 3.04±1.23 for the healthy control group (p<0.0001). There were no significant differences in age or gender distribution between the groups. Endogenous plasma This study confirmed that CFS is not associated with alterations in plasma As long-chain fatty acids are the most energy-rich substrate for β-oxidation, small changes in acylcarnitine levels may have a significant impact on energy production, leading to fatigue. The deficiency in long-chain acylcarnitines observed in this study is indicative of a reduction in β-oxidation. Specifically, a lower plasma concentration of an acylcarnitine may indicate the transport of less long-chain acylcarnitines across the inner mitochondrial membrane, a corresponding reduction in the amount of acylcarnitines within the mitochondria that can undergo reverse transesterification by carnitine palmitoyltransferase II (CPT-II), and hence a reduction in long-chain fatty acid oxidation (as shown in Based on the results of this study, it is anticipated that the administration of particular individual acylcarnitines, or alternatively, L-carnitine administered in combination with particular individual fatty acids (for example, oleic acid and linoleic acid) will be beneficial for the treatment of CFS. Supplementation with L-carnitine in combination with long-chain fatty acids may provide more substrate for long-chain acylcarnitine formation and/or concurrently increasing CPT-I activity. This, in turn, is expected to increase availability of long-chain acylcarnitines within the mitochondria and hence increase substrate availability for β-oxidation. Indeed, in a previous study (Maes et al., 2005) wherein endogenous levels of fatty acids were examined in 22 chronic fatigue syndrome patients and 12 healthy controls, it was demonstrated that CFS was accompanied by increased levels of omega-6 poly-unsaturated fatty acids and mono-unsaturated fatty acids. Interestingly, of the fatty acids measured in that study, for which the corresponding acylcarnitine was quantified in the present study (ie C14, C16, C16:1, C18, C18:1, C18:2), for five of the six cases there was a significant reduction in the acylcarnitine levels and an increase in the corresponding free fatty acid levels (ie C14, C16:1, C18, C18:1, C18:2). In fact, when the present findings are considered in combination with those of Maes et al., it can be speculated that the ratio of free fatty acid to acylcarnitine for these acyl groups is approximately 2- to 3-fold higher in CFS patients than in healthy controls, indicating a substantial disruption in fatty acid/carnitine homeostasis in these patients. While not wishing to be bound by theory, this may be due to either: (1) a reduction in the activity of AcylCoA synthase required for the conversion of free fatty acid to AcylCoA; or (2) a reduction in the activity of CPT-I. As CPT-I is the rate-controlling enzyme in mitochondrial fatty acid oxidation (Leonhardt et al., 2004), it is therefore anticipated that a reduction in CPT-I activity contributes to the symptomology of CFS. In keeping with this, high levels of omega-6 fatty acids (such as C18:2 seen in the patient group of the present study) have been shown to inhibit CPT-I activity in rats (Niot et al., 1994), whereas an increase in the ratio of omega-3 to omega-6 fatty acids has been shown to increase CPT-I activity in both rats (Vamecq et al., 1993) and healthy controls (Beermann et al., 2003; Guebre-Egziabher et al., 2008). As L-carnitine is also known to increase CPT-I activity (Yoon et al., 2003), it is also anticipated that the administration of omega-3 fatty acids in combination with L-carnitine would stimulate CPT-I activity in CFS, thereby decreasing the ratio of free fatty acid to acylcarnitine and theoretically normalising mitochondrial fatty acid oxidation in these patients. Moreover, omega-3 fatty acids inhibit the production of malonyl-CoA, the major endogenous inhibitor of CPT-I, and reduce the sensitivity of CPT-I to inhibition by malonyl-CoA (Baker and Gibbons, 2000). Throughout this specification the word “comprise”, or variations such as “comprises” or “comprising”, will be understood to imply the inclusion of a stated element, integer or step, or group of elements, integers or steps, but not the exclusion of any other element, integer or step, or group of elements, integers or steps. All publications mentioned in this specification are herein incorporated by reference. Any discussion of documents, acts, materials, devices, articles or the like which has been included in the present specification is solely for the purpose of providing a context for the present invention. It is not to be taken as an admission that any or all of these matters form part of the prior art base or were common general knowledge in the field relevant to the present invention as it existed in Australia or elsewhere before the priority date of each claim of this application. It will be appreciated by persons skilled in the art that numerous variations and/or modifications may be made to the invention as shown in the specific embodiments without departing from the spirit or scope of the invention as broadly described. The present embodiments are, therefore, to be considered in all respects as illustrative and not restrictive. Methods for the diagnosis and treatment of chronic fatigue syndrome (CFS) are disclosed based upon the finding that particular individual acylcarnitines are present in modified concentrations (ie decreased or increased concentrations) in CFS patients compared to healthy control subjects. In one form of the invention, a diagnostic method comprises determining a concentration of at least one individual acylcarnitine compound (eg oleyl-L-carnitine and linoleyl-L-carnitine) in a body sample from a test subject and comparing the concentration to a reference concentration, wherein a difference in the concentration of the at least one individual acylcarnitine from the test subject compared to the reference concentration is indicative of CFS. In another form of the invention, a method of treating CFS is provided which comprises administering an effective amount of a supplement comprising: at least one acylcarnitine compound selected from short-chain, medium-chain and long-chain acylcarnitines, L-carnitine (or an acylcarnitine that may be converted within a subject to L-carnitine) in combination with at least one fatty acid selected from short-chain, medium-chain and long-chain fatty acids, or at least one acylcarnitine in combination with at least one fatty acid selected from short-chain, medium-chain and long-chain fatty acids. 1. A method of diagnosing chronic fatigue syndrome (CFS) in a test subject, said method comprising the steps of:
(i) determining a concentration of at least one individual acylcarnitine compound in a body sample from the test subject; and (ii) comparing the concentration determined in step (i) to a reference concentration of the at least one individual acylcarnitine determined from an equivalent body sample from a healthy control subject (or a reference concentration range of the at least one individual acylcarnitine determined from equivalent body samples from a plurality of healthy control subjects), wherein a difference in the concentration of the at least one individual acylcarnitine from the test subject compared to the reference concentration (or reference concentration range) is indicative of CFS in the test subject. 2. A method of diagnosing chronic fatigue syndrome (CFS) in a test subject, said method comprising the steps of:
(i) determining a concentration of at least one individual acylcarnitine compound in a first body sample from the test subject, (ii) determining a concentration of at least one individual fatty acid that corresponds to an acyl group of said at least one individual acylcarnitine compound in a second body sample from the test subject, wherein said first and second body sample may be the same, and (iii) determining a ratio of the concentration of the at least one individual acylcarnitine compound to the concentration of the at least one individual fatty acid, or
assessing a relationship between the concentration of the at least one individual acylcarnitine compound and the concentration of the at least one individual fatty acid; wherein an aberrant ratio determined in step (iii) or an aberrant relationship assessed in step (iii) is indicative of CFS in the test subject. 3. A method of diagnosing chronic fatigue syndrome (CFS) in a test subject, said method comprising the steps of:
(i) determining a concentration of (ii) determining a concentration of at least one individual fatty acid that corresponds to an acyl group of at least one individual acylcarnitine compound in a second body sample from the test subject, wherein said first and second body sample may be the same, and (iii) determining a ratio of the concentration of assessing a relationship between the concentration of the wherein an aberrant ratio determined in step (iii) or an aberrant relationship assessed in step (iii) is indicative of CFS in the test subject. 4. The method of any one of 5. The method of 6. The method of 7. The method of 8. A method of treating chronic fatigue syndrome (CFS) in a subject, said method comprising administering an effective amount of a supplement comprising:
at least one acylcarnitine compound selected from short-chain, medium-chain and long-chain acylcarnitines, at least one acylcarnitine in combination with at least one fatty acid selected from short-chain, medium-chain and long-chain fatty acids. 9. The method of 10. The method of 11. The method of 12. The method of 13. The method of 14. The method of 15. The method of 16. The method of 17. The method of 18. The method of 19. A method of treating chronic fatigue syndrome (CFS) in a subject, said method comprising the steps of:
(i) identifying in said subject a deficiency in one or more individual acylcarnitine compound(s) by
(a) determining a concentration of at least one individual acylcarnitine in a test body sample from the subject, and (b) comparing the concentration determined in (a) to a reference concentration of the at least one individual acylcarnitine determined from an equivalent body sample(s) from a healthy control subject (or a reference concentration range of the at least one individual acylcarnitine determined from equivalent body samples from a plurality of healthy control subjects), wherein a lesser concentration of the at least one individual acylcarnitine compound(s) from the subject compared to the reference concentration (or reference concentration range) indicates a deficiency in the said at least one individual acylcarnitine compound(s); and (ii) administering an effective amount of a supplement comprising the deficient at least one individual acylcarnitine compound(s), 20. The method of 21. The method of 22. The method of 23. The method of 24. The method of 25. The method of 26. The method of 27. The method of 28. The method of 29. A method of fortifying a food comprising adding to the food a supplement comprising;
at least one acylcarnitine compound selected from short-chain, medium-chain and long-chain acylcarnitines, at least one acylcarnitine in combination with at least one fatty acid selected from short-chain, medium-chain and long-chain fatty acids. 30. The method of 31. The method of 32. The method of 33. The method of 34. The method of 35. The method of 36. The method of 37. The method of 38. A method of treating chronic fatigue syndrome (CFS) in a subject, said method comprising administering an effective amount of a modulator(s) of carnitine/acylcarnitine metabolism wherein the modulator stimulates the activity of an enzyme selected from the group consisting of carnitine palmitoyltransferase (CPT)-I, carnitine palmitoyltransferase (CPT)-II and carnitine/acylcarnitine translocase. 39. The method of 40. The method of 41. The method of FIELD OF THE INVENTION
BACKGROUND OF THE INVENTION
SUMMARY OF THE INVENTION
wherein a difference in the concentration of the at least one individual acylcarnitine from the test subject compared to the reference concentration (or reference concentration range) is indicative of CFS in the test subject.
wherein an aberrant ratio determined in step (iii) or an aberrant relationship assessed in step (iii) is indicative of CFS in the test subject.
wherein an aberrant ratio determined in step (iii) or an aberrant relationship assessed in step (iii) is indicative of CFS in the test subject.
wherein a lesser concentration of the at least one individual acylcarnitine compound(s) from the subject compared to the reference concentration (or reference concentration range) indicates a deficiency in the said at least one individual acylcarnitine compound(s); and
BRIEF DESCRIPTION OF THE FIGURES
DETAILED DESCRIPTION OF THE INVENTION
wherein a difference in the concentration of the at least one individual acylcarnitine from the test subject compared to the reference concentration (or reference concentration range) is indicative of CFS in the test subject.
wherein a difference in the concentrations of the two or more individual acylcarnitines from the test subject compared to the reference concentrations (or reference concentration ranges) is indicative of CFS in the test subject.
wherein an aberrant ratio determined in step (iii) or an aberrant relationship assessed in step (iii) is indicative of CFS in the test subject.
wherein an aberrant ratio determined in step (iii) or an aberrant relationship assessed in step (iii) is indicative of CFS in the test subject.
L-carnitine (or an acylcarnitine that may 60 to 95 wt % be converted to L-carnitine), short-chain, medium-chain or long-chain 0.5 to 20 wt % fatty acid omega-3 fatty acid 0.5 to 20 wt %
wherein a lesser concentration of the at least one individual acylcarnitine compound(s) from the subject compared to the reference concentration (or reference concentration range) indicates a deficiency in the said at least one individual acylcarnitine compound(s); and
L-carnitine (or an acylcarnitine that may 60 to 95 wt % be converted to L-carnitine) omega-3 fatty acid 1 to 40 wt % EXAMPLE
Example 1
Methods and Materials
Study Design
Fatigue Clinically-evaluated, unexplained, persistent or relapsing fatigue persistent for six months or more, that: is of new or definite onset; is not the result of ongoing exertion; is not substantially alleviated by rest; and results in substantial reduction in previous levels of occupational, educational, social or personal activities AND Other Symptoms Four or more of the following symptoms that are concurrent, persistent for six months or more and which did not predate the fatigue: impaired short-term memory or concentration sore throat tender cervical or axillary lymph nodes muscle pain multi-joint pain without arthritis headaches of a new type, pattern, or severity unrefreshing sleep post-exertional malaise lasting more than 24 hours *Royal Australasian College of Physicians Working Group (2002) Fatigue Severity Scale
Carnitine Profiling
1. My motivation is lower when I am fatigued. 1 2 3 4 5 6 7 2. Exercise brings on my fatigue. 1 2 3 4 5 6 7 3. I am easily fatigued. 1 2 3 4 5 6 7 4. Fatigue interferes with my physical functioning. 1 2 3 4 5 6 7 5. Fatigue causes frequent problems for me. 1 2 3 4 5 6 7 6. My fatigue prevents sustained physical functioning. 1 2 3 4 5 6 7 7. Fatigue interferes with me carrying out certain 1 2 3 4 5 6 7 duties and responsibilities. 8. Fatigue is among my three most disabling symptoms. 1 2 3 4 5 6 7 9. Fatigue interferes with my work, family or social life. 1 2 3 4 5 6 7 Carnitine and Acylcarnitines Detected
Statistical Analysis
Results
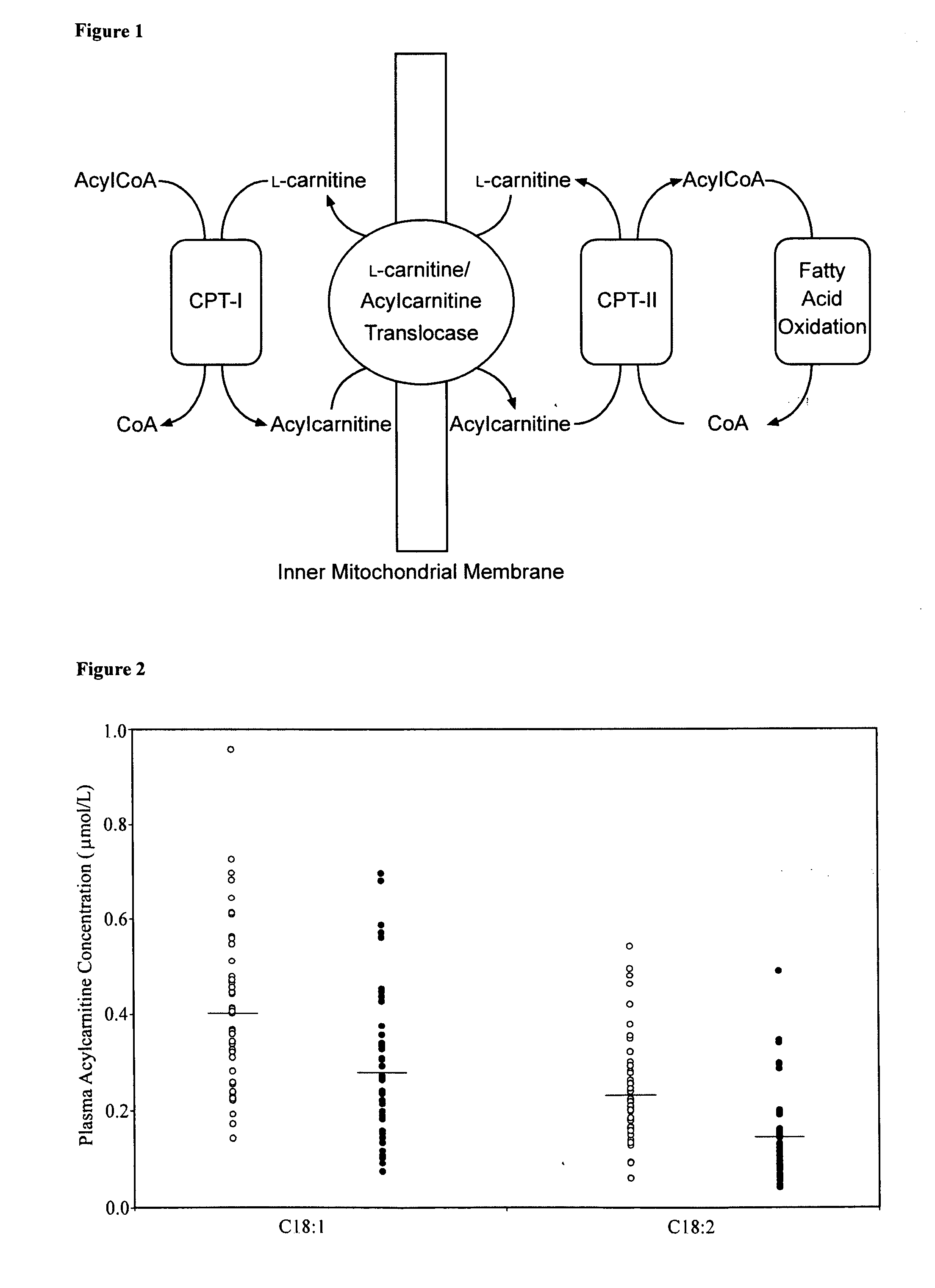
Endogenous plasma carnitine concentrations (μmol/L) Endogenous Plasma Carnitine Controls CFS Patients Significance LC L-carnitine 45.2 ± 9.79 45.0 ± 11.3 TC Total Carnitine 59.5 ± 12.9 58.8 ± 13.6 AcylLC Total Acylcarnitines 14.3 ± 4.13 13.8 ± 3.45 C2 Acetyl-L-carnitine 10.6 ± 3.37 10.2 ± 2.72 C3 Propionyl-L-carnitine 0.502 ± 0.153 0.489 ± 0.199 C3DC Malonyl-L-carnitine 0.0447 ± 0.0217 0.0416 ± 0.0166 C4 Butyryl-L-carnitine 0.255 ± 0.0926 0.250 ± 0.107 C4—OH Hydroxy-butyryl-L-carnitine 0.0239 ± 0.0117 0.0252 ± 0.0130 C4DC Succinyl-L-carnitine 0.0750 ± 0.0135 0.104 ± 0.135 C5 Isovaleryl-L-carnitine 0.111 ± 0.0408 0.103 ± 0.0424 C5:1 Tiglyl-L-carnitine 0.0276 ± 0.00787 0.0285 ± 0.0110 C5—OH Hydroxy-isovaleryl-L-carnitine 0.0352 ± 0.00789 0.0392 ± 0.0103 C5DC Glutaryl-L-carnitine 0.129 ± 0.0419 0.132 ± 0.0575 C6 Hexanoyl-L-carnitine 0.0602 ± 0.0249 0.0605 ± 0.0213 C6:1 Hexenoyl-L-carnitine 0.0211 ± 0.00818 0.0222 ± 0.00988 C6DC Adipyl-L-carnitine 0.0947 ± 0.0121 0.0971 ± 0.0277 C8 Octanoyl-L-carnitine 0.0994 ± 0.0655 0.0953 ± 0.0553 C8:1 Octenoyl-L-carnitine 0.178 ± 0.117 0.132 ± 0.0752 * p = 0.0201 C8DC Suberyl-L-carnitine 0.0549 ± 0.00773 0.0601 ± 0.0177 C10 Decanoyl-L-carnitine 0.161 ± 0.113 0.154 ± 0.106 C10:1 Decenoyl-L-carnitine 0.105 ± 0.0520 0.109 ± 0.0536 C10:2 Decadienoyl-L-carnitine 0.0374 ± 0.0184 0.0395 ± 0.0216 C10DC Sebacyl-L-carnitine 0.0970 ± 0.0118 0.0999 ± 0.0162 C12 Lauroyl-L-carnitine 0.0668 ± 0.0364 0.0633 ± 0.0276 C12:1 Dodecenoyl-L-carnitine 0.0681 ± 0.0424 0.0707 ± 0.0437 C12DC Dodecanedioyl-L-carnitine 0.0782 ± 0.0124 0.108 ± 0.0489 * p < 0.0001 C14 Myristoyl-L-carnitine 0.0751 ± 0.0248 0.0612 ± 0.0269 * p = 0.0023 C14:1 Myristoleyl-L-carnitine 0.0694 ± 0.0435 0.0651 ± 0.0324 C14:2 Tetradecadienoyl-L-carnitine 0.0390 ± 0.0164 0.0398 ± 0.0177 C14—OH Hydroxy-myristoyl-L-carnitine 0.0149 ± 0.00667 0.0157 ± 0.00605 C16 Palmitoyl-L-carnitine 0.352 ± 0.148 0.404 ± 0.220 C16:1 Palmitoleyl-L-carnitine 0.0586 ± 0.0276 0.0472 ± 0.0212 * p = 0.0383 C16—OH Hydroxyl-palmitoyl-L-carnitine 0.0104 ± 0.00361 0.0114 ± 0.00570 C16:1-OH Hydroxy-palmitoleyl-L-Carnitine 0.0193 ± 0.00764 0.0211 ± 0.0111 C18 Stearoyl-L-carnitine 0.103 ± 0.0350 0.0874 ± 0.0326 * p = 0.0104 C18:1 Oleyl-L-carnitine 0.401 ± 0.170 0.279 ± 0.159 * p < 0.0001 C18:2 Linoleyl-L-carnitine 0.232 ± 0.111 0.146 ± 0.0911 * p < 0.0001 C18:1-OH Hydroxy-oleyl-L-carnitine 0.0178 ± 0.00858 0.0228 ± 0.0116 * p = 0.0191 Discussion
REFERENCES