LIGHT EMITTING DEVICE COMPRISING PHOSPHORESCENT MATERIALS FOR WHITE LIGHT GENERATION
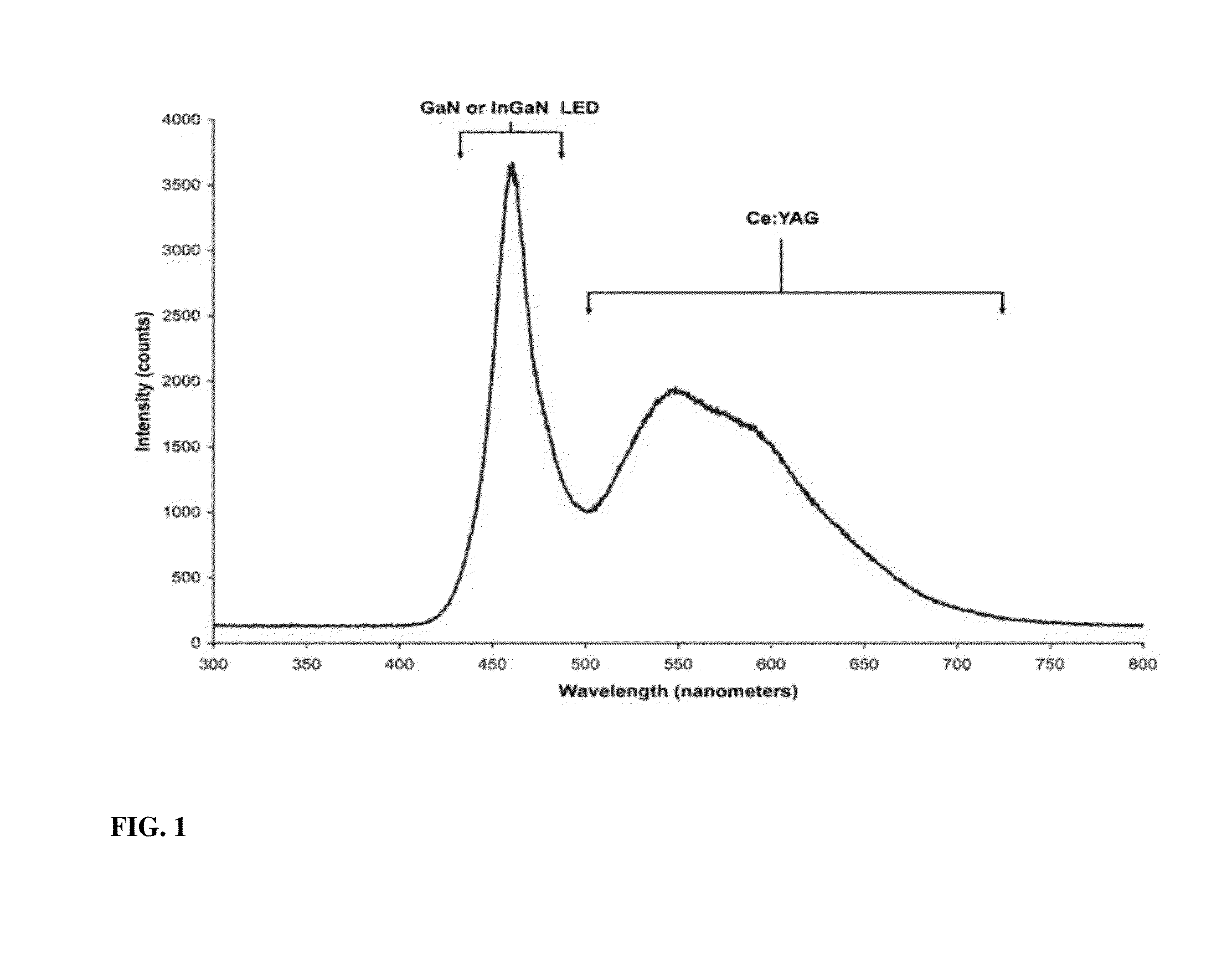
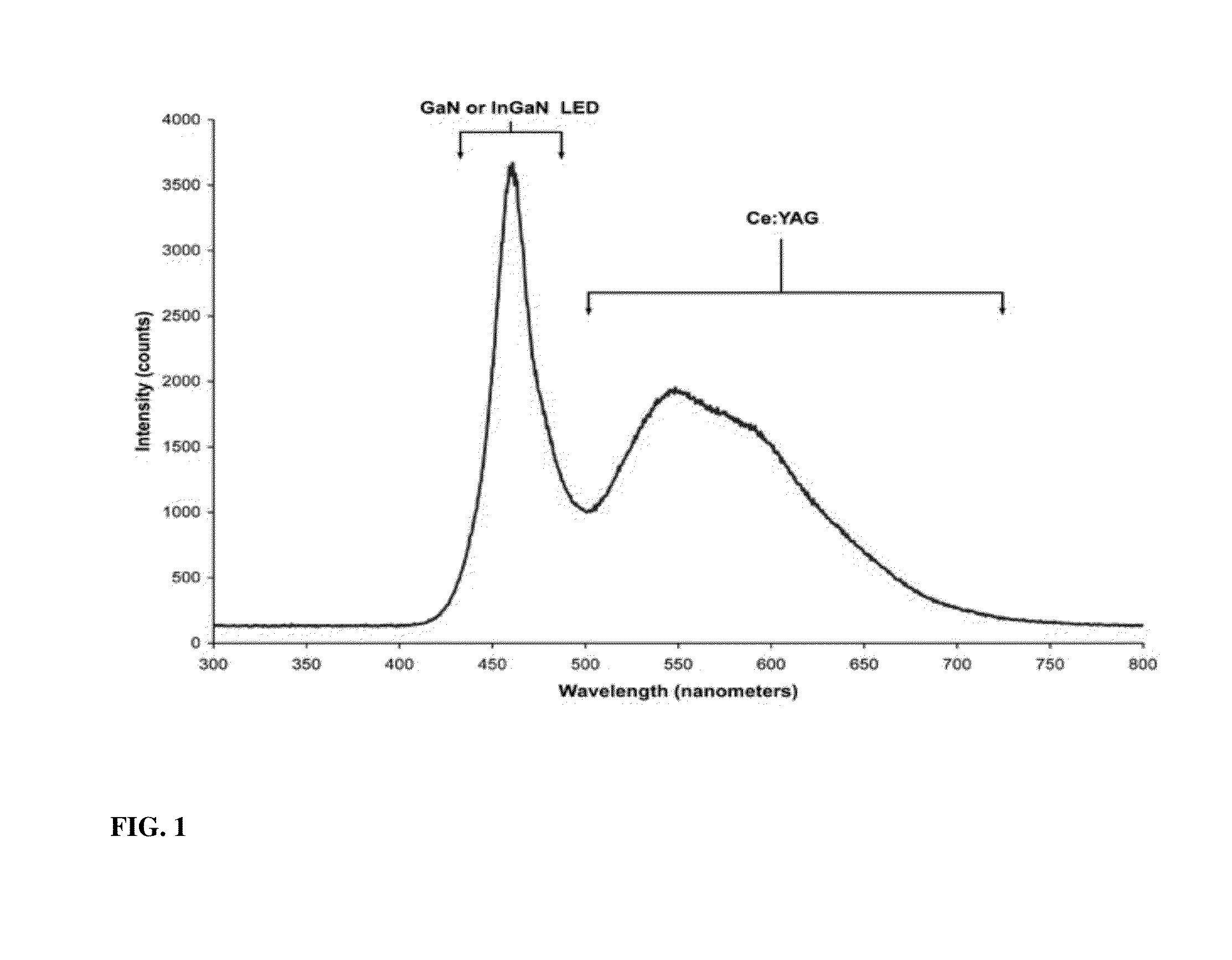
This work was supported in part by Grants de-sc0001013 the Department of Energy-Energy Frontier Research Center (DoE-EFRC). The government has certain rights in the invention. The claimed invention was made by, on behalf of, and/or in connection with one or more of the following parties to a joint university corporation research agreement: Regents of the University of Michigan, Princeton University, The University of Southern California, and the Universal Display Corporation. The agreement was in effect on and before the date the claimed invention was made, and the claimed invention was made as a result of activities undertaken within the scope of the agreement. The present invention relates to phosphors for energy downconversion of high energy light to generate a broadband light spectrum, which emit light of different emission wavelengths. In particular, the present invention relates to metal-organic and orgnanometallic phosphors. These phosphors capable of energy downcoversion are useful materials in Light emitting devices (LEDs). Light emitting diodes (LEDs) are emerging as the superior technology for lighting applications, offering energy efficient, eco friendly, robust and long-lasting alternative to traditional incandescent and fluorescent lamps. In view of global attention to energy saving the long term goal is to replace the conventional light sources with LEDs. This requires further major improvements such as cost reduction and enhanced light quality. A common approach to white light generation is the downconversion of high energy light, for example, from the UV, violet or blue end of the electromagnetic spectrum, into broadband visible light. Such an approach is used in fluorescent light sources, in both tube and compact fluorescent devices, as well as many white LEDs. The downconverting materials that are commonly used for this purpose includes mixtures of rare earth materials. Typically, these rare earth materials have high efficiencies in converting UV or violet light into visible light, and are thermally and chemically stable. Thus, they may withstand the harsh environments inside fluorescent sources. While these rare earth materials work well as downconverters for white light sources, they are expensive and in limited supply. Therefore, there exists a great deal of interest in seeking solutions for energy downconversion for lighting that does not involve rare earth metals. Some of the most efficient white light emitting inorganic LEDs (iLEDs) use a downconverting strategy, similar to fluorescent tube sources to produce their broadband emission. These sources typically rely on rare earth metals for converting high energy light into a broadband (white) spectrum of emission. While it is possible to generate white light with several different LEDs running in parallel, e.g., separate red, green and blue iLEDs, this adds cost to the system and suffers from the “green gap” problem, which is associated with the observation that green LEDs show a marked drop in efficiency when they are run at high brightness. The green portion of the light produced by multiple iLEDs is produced inefficiently, thus limiting the overall system efficiency. Such drop in efficiency is smaller for UV, violet and blue emitting devices. Therefore, devices that are able to downconvert UV/violet/blue light into the visible spectrum can be run at high brightness and high efficiency. Thus, there is a need to design and employ downcoverting materials that can be efficiently excited by light from UV, violet or blue iLEDs, to generate a broad band spectrum. The present invention provides a white light emitting device comprising an emissive layer, wherein the emissive layer comprises a molecular phosphorescent phosphor for energy downconversion to generate a broadband light spectrum, wherein the phosphor absorbs light at a first wavelength λ1of radiation, and emits light of different emission wavelengths having lower energies than the first wavelength λ1. The present invention also provides a method of generating white light comprising providing an excitation source (light source); providing a molecular phosphorescent phosphor for absorbing the light source at a first wavelength λ1of radiation; and emitting light of different emission wavelengths having lower energies than the first wavelength λ1, wherein the light emitted by the phosphor from the sum of the different emission wavelengths provides an appearance of white light. The present invention is directed to producing efficient white emitting light emitting devices (LEDs). The device employs a phosphor that absorbs energy from an excitation source in the blue, violet, or ultra violet region, and emits energy in the visible region at multiple wavelengths. The sum of the multiple emission wavelengths gives broad band (white emission) and produces an appearance of white light. Thus, the light emitted by the phosphor from the sum of the different emission wavelengths contributes to the results in the appearance of white light. The excitation source may or may not contribute to the total spectrum and produce white light. In certain embodiments, the white LED of the present invention has luminous efficacy of radiation (LER) in excess of 50 lm/W. The phosphor of the present invention refers to a molecular phosphor, which is a non-atomic emitter. For example, the phosphor of the present invention is not a lanthanide (atomic emitter). The phosphor of the present invention may comprise multiple phosphors or a single phosphor. Multiple Phosphors In certain embodiments, the phosphor comprises two or more phosphors, such as a first phosphor, a second phosphor, and so forth. In certain embodiments, the phosphor comprises two phosphors, e.g., a first phosphor and a second phosphor. In certain embodiments, the phosphor comprises three phosphors, e.g., a first phosphor, a second phosphor and a third phosphor. In certain embodiments, the phosphor comprises four phosphors, e.g., a first phosphor, a second phosphor, a third phosphor, and a forth phosphor. Suitable phosphor molecules may include metal complexes, such as copper and iridium complexes. These phosphor molecules are attractive materials for wavelength downconverters for white LEDs due to their broad-band absorption and emission and high phosphorescent quantum yields. Their photophysical properties can be fine-tuned to achieve high performance and to fulfill requirements for specific lighting applications. Other suitable phosphor molecules may include organic materials that provides good phosphorescent efficiency and do not substantially absorb their own emission. Halogenated organic compounds, such as those containing bromine or iodine, promote intersystem crossing to the triplet excited state. Certain examples of suitable organometallic copper complexes are described in WO 20011/063083, which is incorporated herein by reference in its entirety. In certain embodiments, the organometallic copper complex includes a carbine ligand coordinated to a three coordinate copper atom. In one aspect, the carbene ligand has the formula: Formula I, wherein *C is a divalent carbon atom coordinated to a monovalent copper atom Cu. X1and X2are substituents independently selected from alkyl, amine, phosphine, heteroalkyl, aryl and heteroaryl. X1and X2may be further substituted, and X1and X2are optionally linked to form a cycle. In one aspect, the carbene ligand is monodentate. In certain embodiments, each of X1and X2independently forms a bond with *C. A first bond is formed between *C and an atom X′1in substituent X1, and a second bond is formed between *C and an atom X′2in substituent X2. X′1and X′2are independently selected from the group consisting of C, N, O, S and P. In another aspect, the carbene ligand is monodentate. In one aspect, X1and X2are not joined to form a cycle. In another aspect, X1and X2are joined to form a cycle. In one aspect, the copper complex is neutral. In another aspect, the copper complex is charged. In one aspect, the complex has the formula: Formula II, wherein Yi is independently selected from the group consisting of alkyl, alkoxy, amino, alkenyl, alkynyl, arylalkyl, heteroalkyl, aryl and heteroaryl. Yi is a monodentate ligand or a bidentate ligand. n is 1 or 2. In another aspect, the complex has the formula: Formula III, wherein Y1and Y2are substituents that are independently selected from the group consisting of alkyl, heteroalkyl, aryl and heteroaryl. Y1and Y2may be further substituted. Y1and Y2are joined. Each of Y1and Y2form a bond with Cu. A first bond is formed between Cu and an atom Y′1in substituent Y1and a second bond is formed between Cu and an atom Y′2in substituent Y2. Y′1is selected from the group consisting of N, P, *C, O, and S. Y′2is selected from the group consisting of N, P, *C, tetravalent carbon, O, and S. In certain embodiments, Y′1is N. In certain embodiments, the ring comprising Cu, Y′1and Y′2is a 5-membered or 6-membered ring Certain examples of suitable organometallic iridium complexes are described in WO 00/70655 and Organometallic Complexes for Optoelectronic Applications, Chapter 12.04, M. E. Thompson et al., which are incorporated herein by reference in its entirety. Non-limiting examples of the organometallic iridium complexes includes the following: The phosphor may include two or more phosphors chosen from the same type or different types of compounds. For example, the phosphor may include a mixture of organometallic copper complexes (e.g., two or more different organometallic copper complexes), or the phosphor may include a mixture of organometallic iridium compounds (e.g., two or more different organometallic iridium complexes), or the phosphor may include an organometallic copper complex, an organometallic iridium compound, or the phosphor may include a mixture of organometallic copper complexes and an organometallic iridium compound, etc. Single Phosphor In certain embodiments, the phosphor comprises a single phosphor. The single phosphor emits simultaneously from molecular and aggregate states. When the single phosphor emits from the molecular state, a molecular/monomer emitter is referred to, which comprises a monomer. When the single phosphor emits from the aggregate state, an aggregate emitter is referred to, which comprises two molecules (i.e., dimer). Thus, the phosphor of the present invention may comprise a first phosphor being a molecular emitter, and a second phosphor being an aggregate emitter. In this scenario, both monomer and aggregate emission are achieved from the same phosphor. When the phosphor molecules are in relatively close contact with one another, aggregate emission may be produced. When the phosphor molecules are isolated from one another, monomer emission (i.e., not aggregate emission) may be produced. White emission may result, if the relative contribution from each emissive center (i.e., monomer emission/aggregate emission) is appropriately controlled, for example, by adjusting the concentration of each emitter. To achieve well balanced monomer and aggregate emission with a single phosphor and achieve high efficiencies the monomer-aggregate ratio must be achieved at an appropriate concentration of the phosphor. Different approaches that affect the nature of intermolecular interactions in the matrix, and thus the degree of monomer-aggregate emission may be used to control the monomer-aggregate emission ratio. One such approach is to vary the amount of steric bulk in the phosphor molecule. An alternative approach is to change the host matrix. Both approaches are believed to affect the degree of association of the dopant material in the emissive layer and hence the ratio of monomer and aggregate states. Generally, the monomer emitter of the single phosphor emits in the high energy (e.g., blue or green) portion of the visible spectrum, while the aggregate emitter provides a broad emission which spans the low energy portion of the visible spectrum. Typically, there is no absorption into the aggregate emitters, thus there is minimal or no energy transfer from the monomer emitter to the aggregate emitters. At appropriate concentrations, both monomer and aggregate emission may be obtained from the same phosphor. Only those phosphor molecules that are in relatively close contact with another phosphor molecule will be able to form the aggregate state. Phosphor molecules that are isolated will give monomer and not aggregate emission. If the monomer is blue emitting and the aggregate is yellow emitting, a white OLED may result, if the relative contribution from each emitter is appropriately controlled, for example, by adjusting the concentration of each emitter in the emissive layer. Forming aggregate states requires that the two phosphor molecules be in close proximity to each other, so that they can dimerize when one of them is promoted to its excited state. This suggests that there should be a strong concentration dependence in aggregate formation. A monomer emission may be observed in the photoluminescence spectrum of the thin film at a low doping level, typically less than 2%. As the doping concentration is raised, the amount of aggregate emission increases as the monomer line decreases. The ratio of monomer to aggregate emission may be close to 1:1 at a higher doping level. The appropriate doping level to achieve balanced monomer and aggregate emission to give a broadband white emission depends on the steric constraints of the emitter. A sterically encumbered emitter will require a higher doping level to achieve balanced emission than an emitter without sterically demanding groups. Emitters with a minimal amount of steric bulk can be designed to give balanced emission at a doping level of 8-10% dopant relative to host. At such doping level, some of the phosphor molecules are isolated and others are in close proximity to another phosphor molecule, leading to efficient aggregate formation. The monomer and aggregate emitters are described in WO 03/059015 A1, which is incorporated herein in its entirety. Examples of phosphors that are capable to produce monomer and aggregate emitters include organometallic transition metal complexes that have a square planar geometry and have ligands of sufficient steric bulk to hinder aggregate formation. The phosphor may absorbs light at a first wavelength λ1in the range of 300 nm to 450 nm or in the range of 300 nm and 400 nm. Upon absorption of light at the first wavelength λ1, the phosphor is promoted to a triplet state, T1. Typically, the phosphor is first promoted from the ground state, S0, to the lowest-energy singlet excited state, S1, which then decay undergo intersystem cross (ISC) to the triplet state. The phosphor emits from triplet states and generates triplet excitons. Thus, the phosphor of the present invention is a phosphorescent emitter, and is not a fluorescent emitter. The phosphors of the present invention absorb only from the excitation source (i.e., blue/violet, ultra violet light source). The phosphors of the present invention do not absorb any proportion of the excitation light generated in the visible region. Suitable excitation sources for the present invention include light sources that are produced with III-V semiconducting materials and emit a first wavelength λ1of light (e.g., in the blue, violet, or ultra violet region). Non-limiting examples of inorganic light emitting diodes (iLEDs) include GaN (gallium nitrile), or InGaN (indium gallium nitride), each of which may be coated with cerium-doped yttrium aluminum garnet (Ce 3+:YAG). The phosphors of the present invention may emit lights at different regions in the visible spectrum, such as, blue, green, red, orange, yellow, or mixtures thereof (e.g., blue-green, green-red, yellow-green, yellow-orange, etc.). The phosphors may emit lights at any ranges in the visible spectrum. The phosphor (including single and multiple phosphors) should together display emission covering the visible spectrum. For example, the first phosphor may emit light covering the lower wavelength region of the visible spectrum, the second phosphor may emit light covering the higher wavelength region of the visible spectrum, etc. The phosphor's emissions may or may not overlap one another's emissions. Specific ranges of emissions in the visible spectrum includes wavelengths between 400 nm and 500 nm, between 400 nm and 520 nm, between 400 nm and 540 nm, between 440 nm and 560 nm, between 450 nm and 550 nm, between 450 nm and 570 nm, between 500 nm and 600 nm, between 500 nm and 620 nm, between 500 nm and 640 nm, between 520 and 640 nm, between 520 and 660 nm, between 540 and 660 nm, between 550 nm and 650 nm, between 560 nm and 660 nm, between 560 and 680 nm, between 580 and 680 nm, between 580 and 70 nm, etc. In certain embodiments, a first phosphor may emit light at a blue-green region (i.e., at wavelength between 400 nm and 520 nm), and a second phosphor may emit light at a green-red region (i.e., at wavelength between 520 nm and 700 nm. For instance, referring to Referring to The phosphors should have substantial absorption at the same energy as the emission of the pumping LED (λem=365-460 nm), such that, in certain embodiments, a film of 50-100 microns will absorb >99% of the incident LED illumination. The phosphor, when excited, emits white light with a quantum efficiency of at least 40%, 50%, 60%, 70%, 80%, 90%, 95%, or 99%. Generally, the phosphor has a photoluminescent efficiency of from about 50% to about 100%, from about 60% to about 100%, or from 75% to about 100%. The white light emission produced by the present invention may have a CRI (Color Rendering Index) of about 60 or higher, or about 80 or higher. The CRI is a measure of the quality of color light, devised by the CIE, which gives an indication of how well the light source will render colors of objects it illuminates. A perfect match of a given source to the standard illuminant gives a CRI of 100. In certain embodiments, the phosphors may be dispersed at a molecular level. In other embodiments, the phosphors may be dispersed into a solid matrix, such as a polymer matrix. Examples of such polymers include polystyrene, polymethylmethacrylate, epoxy resins and other optical grade materials. In certain embodiments, the solid matrix includes carbazole biphenyl (CBP), and derivatives thereof, N,N′-dicarbazoloylbenzenes, and derivatives thereof, and N,N′,N″-1,3,5-tricarbazoloylbenzenes, and derivatives thereof. Derivatives may include the above compounds substituted with one or more alkyl, alkenyl, alkynyl, aryl, CN, CF3, CO2alkyl, C(O)alkyl, N(alkyl)2, NO2, O-alkyl, and halo. In certain embodiments, the host matrix materials for the emissive layer include 4,4′-N,N′-dicarbazole-biphenyl (CBP), N,N′-meta-dicarbazoloylbenzene (mCP), and N,N′,N″-1,3,5-tricarbazoloylbenzene (tCP). CBP has a number of important properties as a matrix material, such as, a high triplet energy of 2.56 eN (484 nm) and ambipolar charge transporting properties, that make it an excellent host for phosphorescent phosphors, and could be used as a matrix material for the present invention. The desired color of the emitted white light of the present invention can be controlled by adjusting the mixing weight ratio (or percent weight) of the phosphors. By doing so, the emission spectrum can be determined accurately from the extinction spectra of the different phosphors and their concentrations. Such adjusting allows the same combination of phosphors to be used in producing different white light sources with differing color temperature. The correlated color temperature (CCT) characterizes color appearance of a light source, comparing its color to color of light emitted by a theoretical black body heated to high temperatures. For example, the CCT for warm white is from 2700 K to 3500 K, and the CCT for cool white is from 5000 K to 7000 K. The percent weight of each phosphor may be independently from 10% to 90% by weight, such as from 10% to 20%, from 20% to 30%, from 30% to 40%, from 40% to 50%, from 50% to 60%, from 60% to 70%, from 70% to 80%, or from 80% to 90%, from 10% to 30%, from 20% to 40%, from 30% to 50%, from 40% to 60%, from 50% to 70%, from 60% to 80%, or from 70% to 90% of the total weight of the phosphors. For example, when the phosphor includes a first phosphor and a second phosphor, and so forth, the percent weight of the first phosphor may be from 10% to 90% and the percent weight of the second phosphor may be from 90% to 10%; and the total percent weight of the phosphor, including the first phosphor, the second phosphor and so forth, is 100% by weight of the phosphor. Typically, the concentration for an isolation of phosphors is less than 0.1 weight percent in a solid matrix, e.g., polymer. While the concentration is low, it is sufficient that films on the order or several hundred microns may have an optical density in the UV or violet part of the spectrum of greater than 1.0. Thus, the film may efficiently collect and re-emit the light from the illuminating iLED. It is understood that the various embodiments described herein are by way of example only, and are not intended to limit the scope of the invention. For example, many of the materials and structures described herein may be substituted with other materials and structures without deviating from the spirit of the invention. The present invention as claimed may therefore include variations from the particular examples and preferred embodiments described herein, as will be apparent to one of skill in the art. It is understood that various theories as to why the invention works are not intended to be limiting. All the emission spectra were generated assuming that each emitting material has the same emission efficiency when pumped with violet light and that it does not absorb its own emission, or that of the other emitters present. Films can be prepared by suspending molecular or microcrystalline phosphors in a transparent matrix. If the materials have different emission efficiencies the amounts of each material can be adjusted to achieve the desired emission ratio. This example illustrates three components mixing to achieve white light downconversion. Three iridium complexes 9, 10 and 11 are synthesized and mixed according to the emission ratio depicted in Table 1, and then dispersed in a polymer matrix. The emission efficiencies for each material were assumed to be 100% and the percentage values here correspond to the percentage of the incident light absorbed by each phosphor. Thus, the percentage corresponds to the contribution of each phosphor's emission profile to the overall emission profile. The iridium complexes absorb violet light from a UV source, and produce white light. This example illustrates two components mixing to achieve white light downconversion. Two copper complexes 12 and 13 are synthesized and mixed at various weight percent ratio, and then dispersed in a polymer matrix. This example illustrates a single component mixing to achieve white light downconversion. Single phosphors Cu414clusters supported by two P̂N-type ligands 2-[(di-R-phosphino)methyl]pyridine (14, R=phenyl; 15, R=cyclohexyl; 16, R=tert-bytyl; 17, R=iso-propyl; 18, R=ethyl) were synthesized according to procedures described in “Cu4I4Clusters Supported by P̂N-type Ligands: New Structures with Tunable Emission Colors;” Zhiwei Liu, Peter I. Djurovich, Matthew T. Whited, and Mark E. Thompson, Emission spectra of these Cu4I4clusters are shown in The present invention relates to phosphors for energy downconversion of high energy light to generate a broadband light spectrum, which emit light of different emission wavelengths. 1. A white light emitting device comprising an emissive layer, wherein the emissive layer comprises a phosphorescent phosphor for energy downconversion to generate a broadband light spectrum, wherein the phosphor absorbs light at a first wavelength λ1of radiation, and emits light of different emission wavelengths having lower energies than the first wavelength λ1. 2. The white light emitting device of 3. The white light emitting device of 4. The white light emitting device of 5. The white light emitting device of 6. The white light emitting device of 7. The white light emitting device of 8. The white light emitting device of 9. The white light emitting device of 10. The white light emitting device of 11. The white light emitting device of 12. The white light emitting device of 13. The white light emitting device of 14. The white light emitting device of 15. The white light emitting device of 16. The white light emitting device of 17. The white light emitting device of 18. The white light emitting device of 19. The white light emitting device of 20. The white light emitting device of 21. The white light emitting device of 22. The white light emitting device of 23. The white light emitting device of 24. The white light emitting device of 25. The white light emitting device of 26. The white light emitting device of 27. A white light emitting device comprising an emissive layer, wherein the emissive layer comprises a phosphorescent phosphor for energy downconversion to generate a broadband light spectrum, wherein the phosphor absorbs light at a first wavelength λ1of radiation from an excitation source in the blue, violet, or ultra violet region, and emits light of different emission wavelengths having lower energies than the first wavelength λ1; wherein the phosphorescent comprises a copper complex, iridium compound or mixtures thereof. 28. A method of generating white light comprising:
providing an excitation source; providing a molecular phosphorescent phosphor for absorbing the UV light source at a first wavelength λ1of radiation; and emitting light of different emission wavelengths having lower energies than the first wavelength λ1. GOVERNMENT SUPPORT
FIELD OF THE INVENTION
BACKGROUND
SUMMARY OF THE INVENTION
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION
EXPERIMENTAL
Example 1
Mixture Ir-complex 9 (% contribution) Ir-complex 10 (% contribution) Ir-complex 11 (% contribution) a 25 38 38 b 33 33 34 c 20 30 50 d 15 25 60 Example 2
Example 3