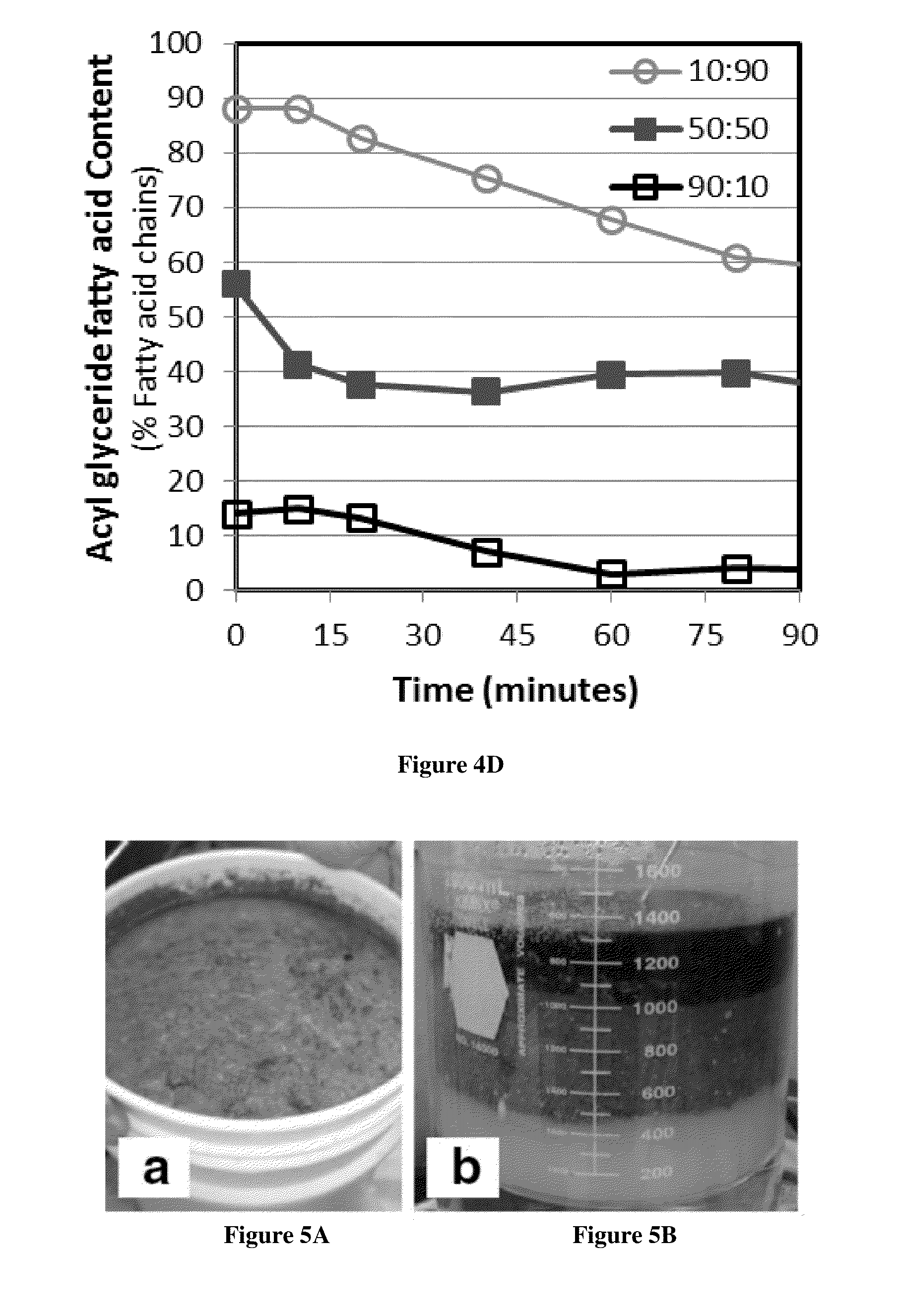
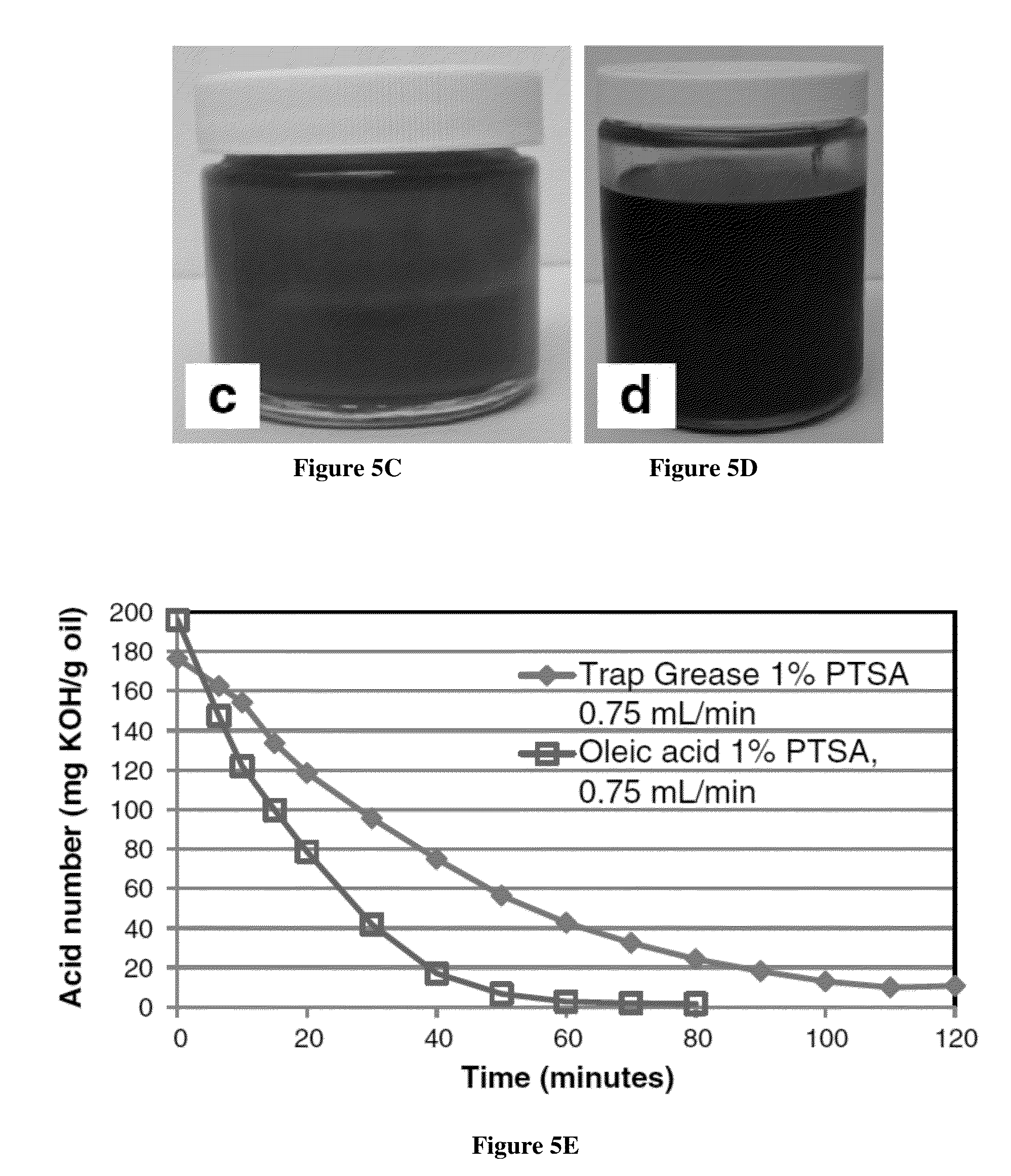
Acidic Methanol Stripping Process That Reduces Sulfur Content of Biodiesel From Waste Greases
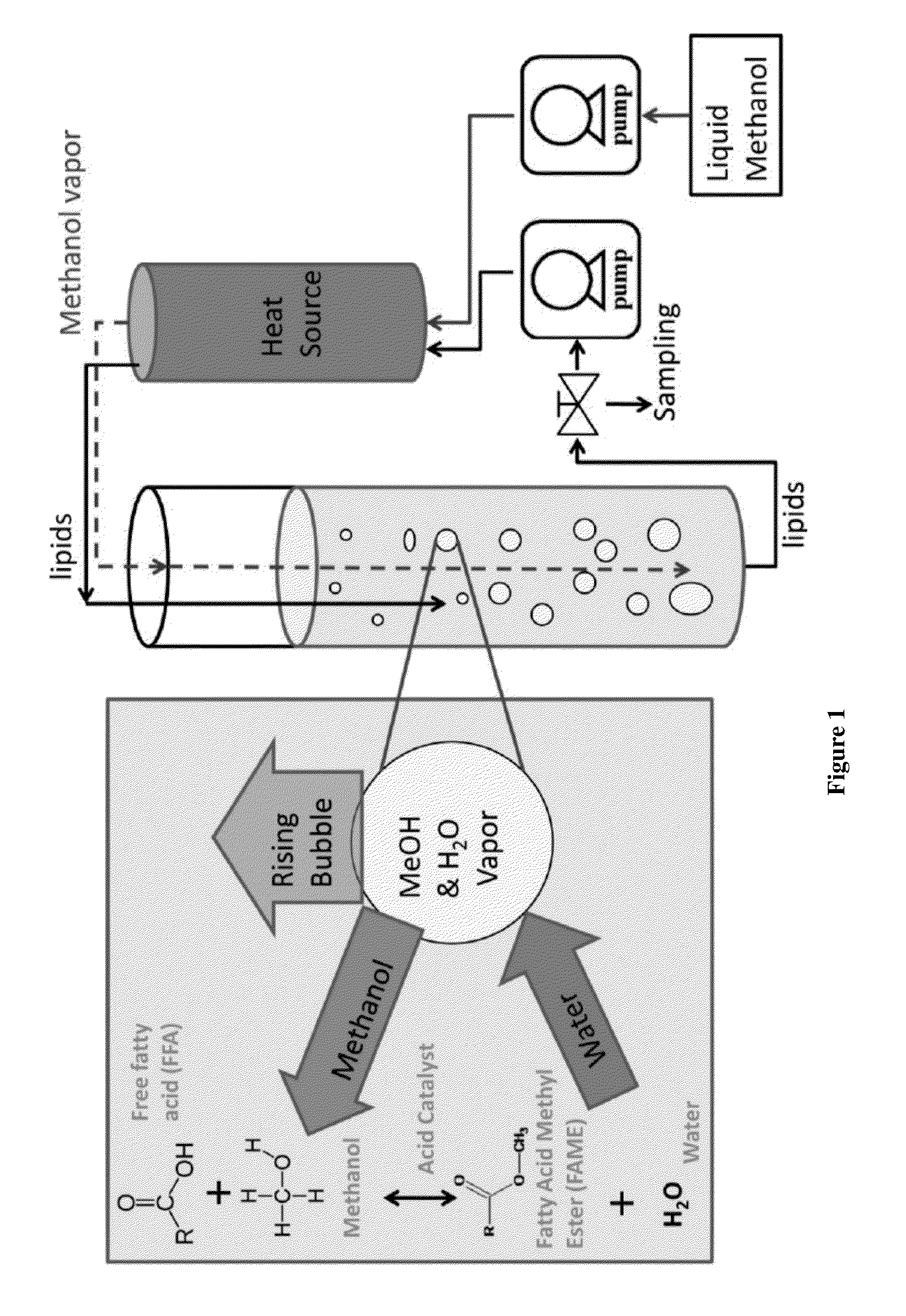
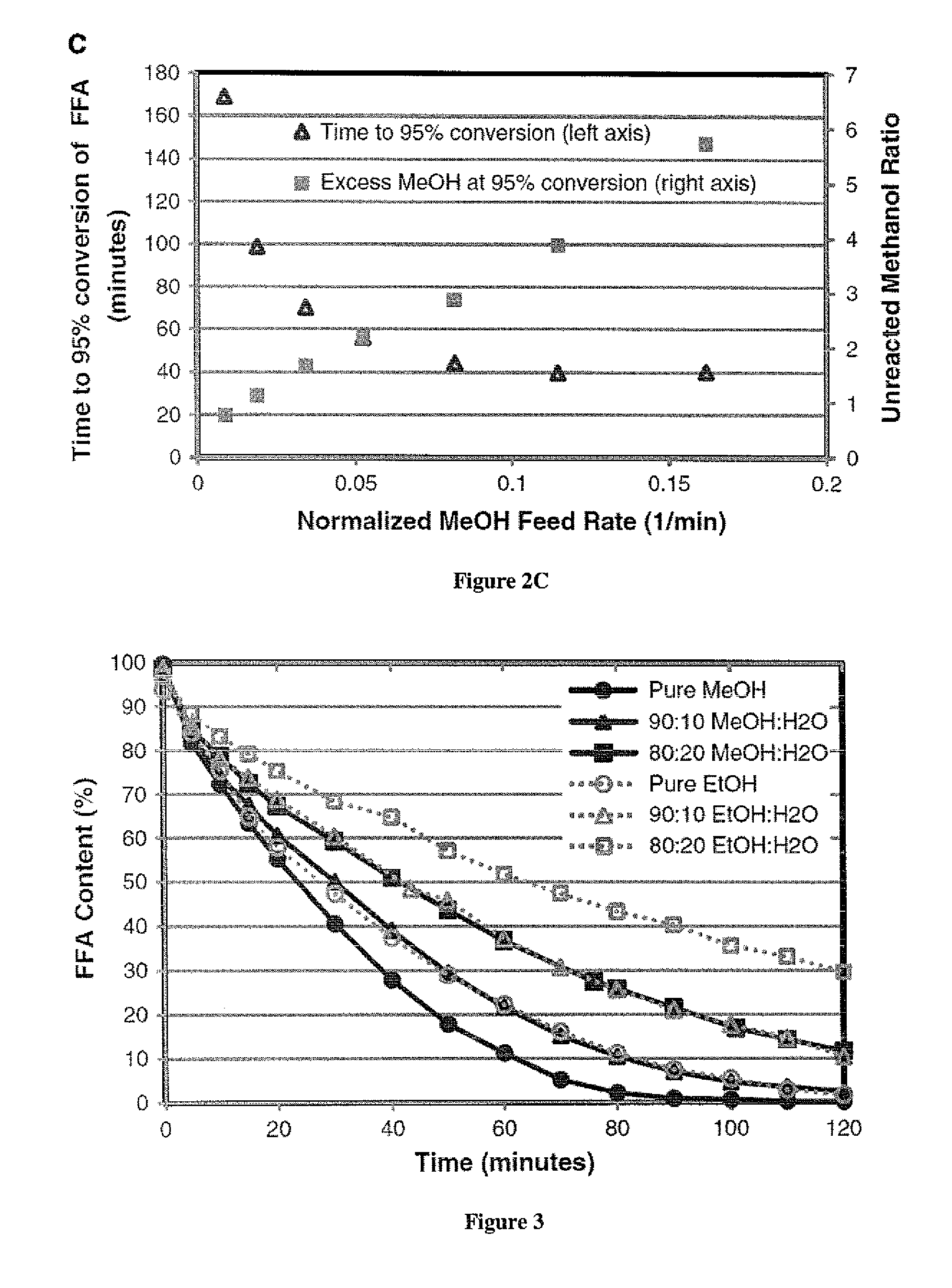
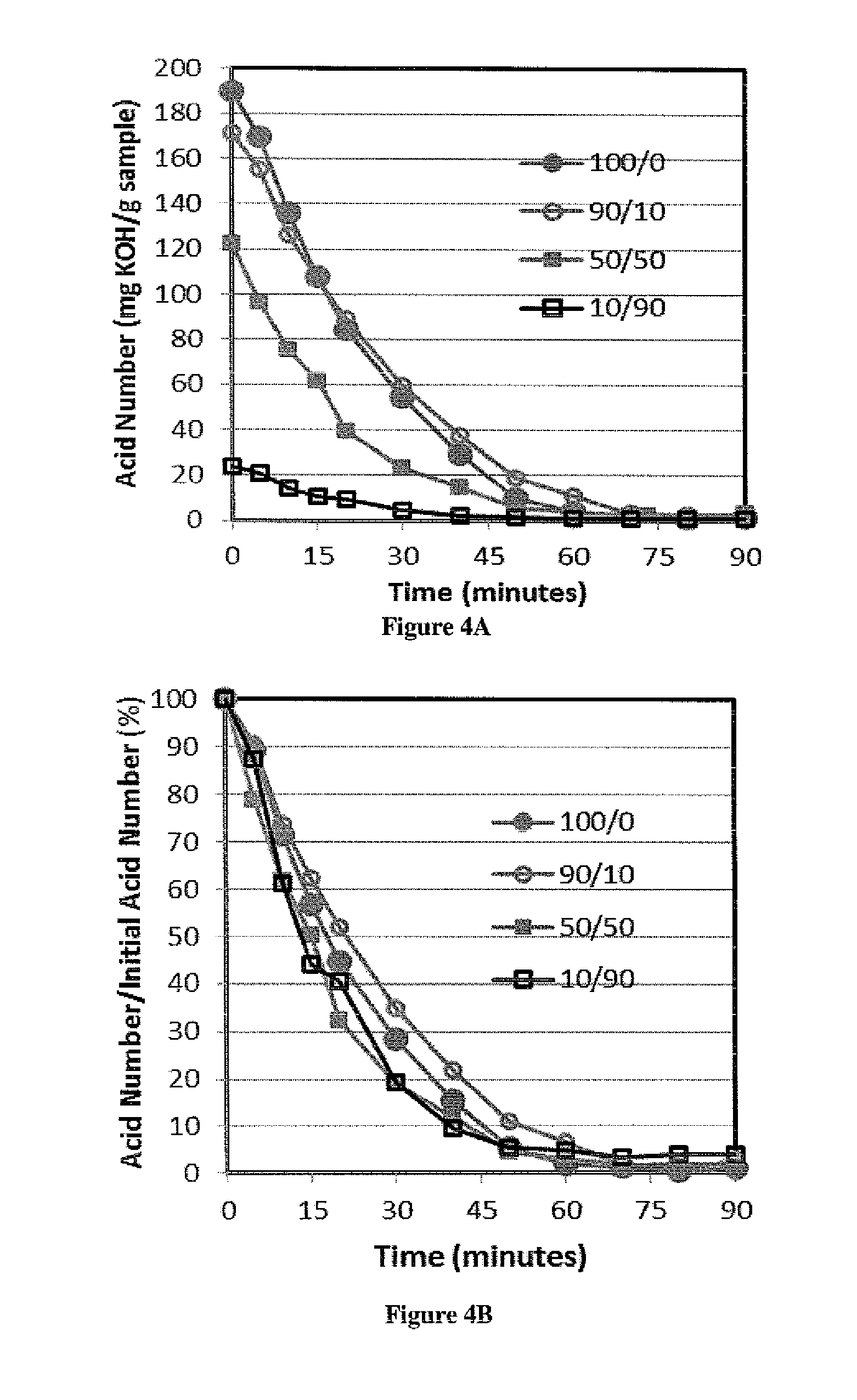
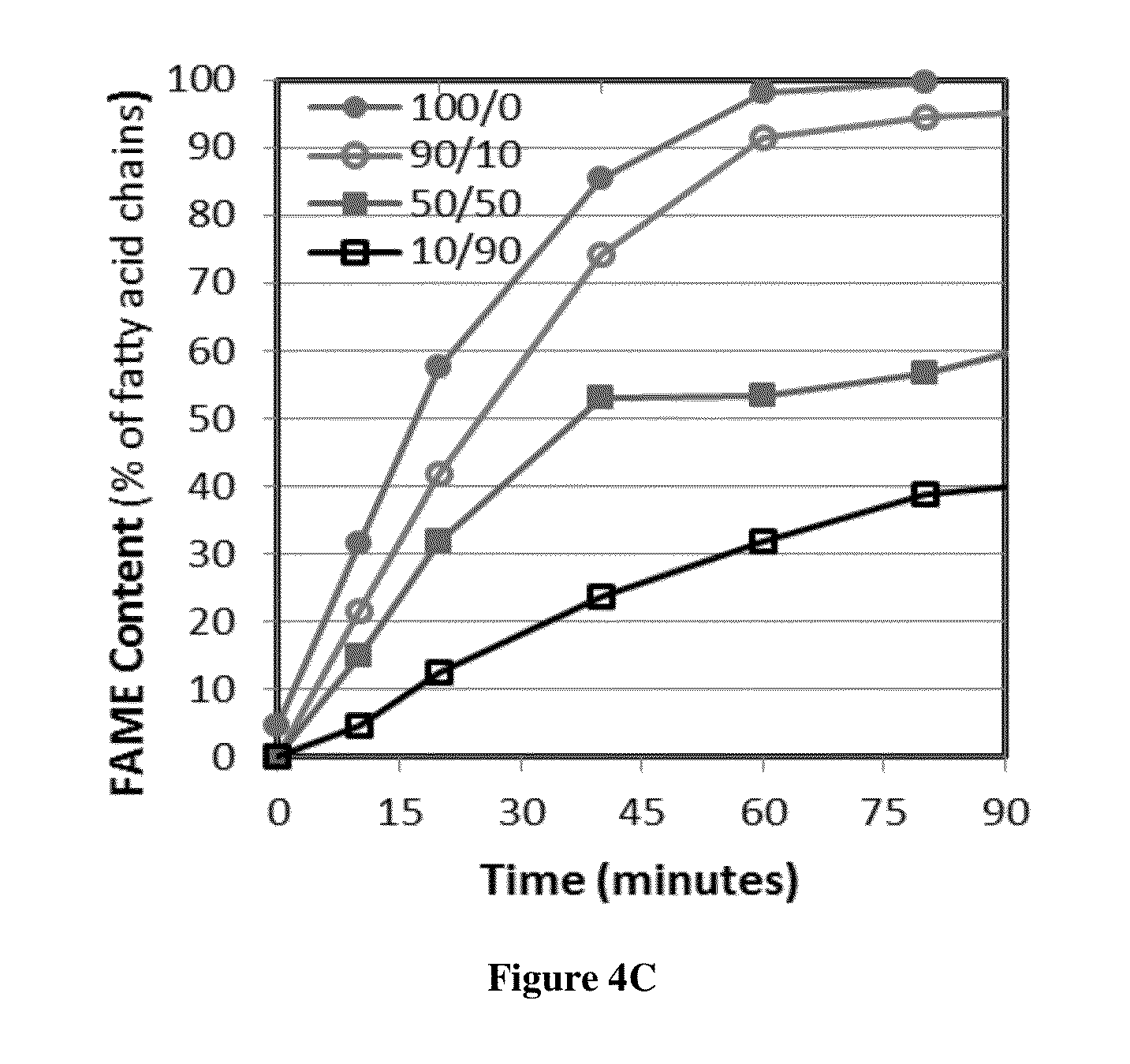
This invention was made with government support under Contract No. EP-D-14-019 awarded by the Environmental Protection Agency. The government has certain rights in the invention. 1. Field of the Invention The present invention is directed to the field of producing biodiesel from a lipid. In particular, the present invention is directed to a process using a gaseous alcohol to convert a lipid to biodiesel and strip impurities from the lipid and/or biodiesel product. 2. Description of the Related Technology There are many factors that have led to increased research into alternative fuels and renewable energy. Some of these factors include rising prices of crude petroleum, concerns about carbon dioxide emissions, worsening air quality by emissions of sulfur oxides, particle matter and other gases, as well as security of domestic energy supply coupled with limited long-term supplies of petroleum. Biodiesel is a promising renewable fuel, which contains mostly fatty acid alkyl esters. Biodiesel is typically produced chemically by reacting plant or animal derived lipids with an alcohol. The majority of biodiesels are produced by reacting lipids with methanol to produce fatty acid methyl esters (FAME). Currently, most of the lipids for biodiesel production are refined lipids that have low free fatty acid (FFA) concentrations, such as soybean oil (in USA), rapeseed oil (in Europe), and palm oil (in Asia). However, these refined lipids are agricultural crops that are relatively high-cost, because their production requires significant fertilizer and chemical inputs. Apostolakou et al. (Techno-economic analysis of a biodiesel production process from vegetable oils, The costs of lipids are related to their FFA content. Edible lipids have low FFA content and command relatively high prices. Inedible lipids tend to be high in FFA and have low prices. The high-FFA lipids are mostly waste products and have limited commercial value, while low-FFA lipids tend to be viable food sources. For example, soybean oil currently sells for about $3.52 per gallon, and yellow grease (filtered and dewatered waste cooking oil with FFA content below 15%) sells for $2.19 per gallon. Trap grease is a potential source of high-FFA lipids, because wastewater utilities charge $0.06 or more per gallon to dispose of trap grease. Lipids separated from trap grease, which may be 2%-10% of the trap grease, can have over 95% FFA. Producing biodiesel from high-FFA lipids entails low feedstock costs and is less prone to controversies associated with producing fuels from food-grade lipids (M. Canakci, The potential of restaurant waste lipids as biodiesel feedstocks, The high cost of the raw materials for biodiesel is one of the major reasons that biodiesel is not an ideal solution for the energy demand in the United States. Van Gerpen (Biodiesel processing and production, It has been proposed that high-FFA lipids such as waste lipid feedstocks can provide significant cost reduction and production capacity for biodiesel (Tyson et al., Biomass Oil Analysis: Research Needs and Recommendations, NREL, Golden Colo., 2004). There are several technologies available for converting high-FFA lipids to FAME. Acid-catalyzed esterification technology is effective for lipids over a large range of FFA concentrations. This technology is often used for pretreatment of lipids prior to base-catalyzed transesterification in a two-step process. A significant disadvantage of acid-catalyzed esterification is slower reactions. There are several ways to increase acid-catalyzed esterification reaction rates, including increasing temperature, increasing catalyst concentration, and removing by-product water. For low FFA lipids (containing 1%-10% FFA), a two-step process including low-temperature acid-catalyzed esterification followed by base catalyzed transesterification is commonly used for converting the lipid to biodiesel. For lipids containing more than 50% FFA, a process with multiple moderate-pressure reactors with intermediate removal of water are used effectively for producing biodiesel (W. W. Berry, B. J. Ratigan, Process of making alkyl esters of free fatty acids, Philadelphia Fry-o-Diesel Inc., US, 2010). Multiple, identical reactors with intermediate water removal will increase reaction speed and conversion. But the process with multiple reactors also increases the capital and operating costs significantly. In addition, to achieve acceptable reaction speed, temperatures above the boiling point of methanol are often used, which requires elevated pressure to maintain methanol in the liquid phase. For example, Van Gerpen reports using 240° C. and 90 bar for such a process (Biodiesel processing and production, Kocsisova et al. (High-temperature esterification of fatty acids with methanol at ambient pressure, U.S. Pat. No. 8,603,198 discloses a method for producing fatty acid alkyl esters from lipids through transesterification and/or esterification using a flow-through cavitation device for generating cavitation bubbles in a fluidic reaction medium. The fluidic medium is passed through sequential compartments in the cavitation device having varying diameters and inner surface features to create localized reductions in fluid pressure thus vaporizing volatile alcohols in the medium to create volatile alcohol-filled bubbles, which provide an increased surface area and optimized conditions for the transesterification and/or esterification to occur at the gas-liquid interface. The method can produce fatty acid alkyl esters and a glycerol, with the former being used in biodiesel. There is a need of a low cost and efficient process to convert lipids with high FFA concentration such as waste lipids to biodiesel. The present invention provides a method based on passing bubbles with an alcohol vapor through lipids with high concentrations of free fatty acids. The present invention has low energy cost and low feedstock cost, which results in producing biodiesel at a much lower cost. The low cost biodiesel will likely lead to wider applications for the biodiesel. In one aspect, the present invention provides a method of producing fatty acid alkyl esters from a lipid, comprising steps of introducing a gas comprising vapor of an alcohol selected from methanol and ethanol into the lipid in a form of bubbles to enable the bubbles to pass through the lipid and be discharged from the lipid. In another aspect, the method of the present invention further comprises recycling the alcohol in the discharged bubbles. In yet another aspect, the method of the present invention further comprises adding an acid catalyst to the lipid and the acid catalyst may be selected from sulfonic acid, para-toluene sulfonic acid, sulfuric acid, methane sulfonic acid, and hydrochloric acid. In another aspect, the method of the present invention further comprises subjecting the product of the introducing step to a transesterification process catalyzed by a base catalyst. In some embodiments, the present invention provides a method of producing fatty acid alkyl esters from a lipid, comprising steps of introducing a gas comprising vapor of an alcohol into the lipid in a form of bubbles to enable the bubbles to pass through the lipid and be discharged from the lipid. The alcohol used may be selected from alcohols that are in a gaseous state at the temperature of the lipid. In another aspect, the alcohol is selected from 1-propanol, iso-propanol and butanols. In yet another aspect, the lipid is selected from trap grease, sewage scum grease, acid oils and other waste materials having a free fatty acid (FFA) content higher than 40%. For illustrative purposes, the principles of the present disclosure are described by referencing various exemplary embodiments. Although certain embodiments are specifically described herein, one of ordinary skill in the art will readily recognize that the same principles are equally applicable to, and can be employed in other systems and methods. Before explaining the disclosed embodiments of the present disclosure in detail, it is to be understood that the disclosure is not limited in its application to the details of any particular embodiment shown. Additionally, the terminology used herein is for the purpose of description and not of limitation. Furthermore, although certain methods are described with reference to steps that are presented herein in a certain order, in many instances, these steps may be performed in any order as may be appreciated by one skilled in the art; the novel method is therefore not limited to the particular arrangement of steps disclosed herein. It must be noted that as used herein and in the appended claims, the singular forms “a”, “an”, and “the” include plural references unless the context clearly dictates otherwise. Furthermore, the terms “a” (or “an”), “one or more” and “at least one” can be used interchangeably herein. The terms “comprising”, “including”, “having” and “constructed from” can also be used interchangeably. Unless otherwise indicated, all numbers expressing quantities of ingredients, properties such as molecular weight, percent, ratio, reaction conditions, and so forth used in the specification and claims are to be understood as being modified in all instances by the term “about,” whether or not the term “about” is present. Accordingly, unless indicated to the contrary, the numerical parameters set forth in the specification and claims are approximations that may vary depending upon the desired properties sought to be obtained by the present disclosure. At the very least, and not as an attempt to limit the application of the doctrine of equivalents to the scope of the claims, each numerical parameter should at least be construed in light of the number of reported significant digits and by applying ordinary rounding techniques. Notwithstanding that the numerical ranges and parameters setting forth the broad scope of the disclosure are approximations, the numerical values set forth in the specific examples are reported as precisely as possible. Any numerical value, however, inherently contains certain errors necessarily resulting from the standard deviation found in their respective testing measurements. It is to be understood that each component, compound, substituent, or parameter disclosed herein is to be interpreted as being disclosed for use alone or in combination with one or more of each and every other component, compound, substituent, or parameter disclosed herein. It is also to be understood that each amount/value or range of amounts/values for each component, compound, substituent, or parameter disclosed herein is to be interpreted as also being disclosed in combination with each amount/value or range of amounts/values disclosed for any other component(s), compounds(s), substituent(s), or parameter(s) disclosed herein and that any combination of amounts/values or ranges of amounts/values for two or more component(s), compounds(s), substituent(s), or parameters disclosed herein are thus also disclosed in combination with each other for the purposes of this description. It is further understood that each lower limit of each range disclosed herein is to be interpreted as disclosed in combination with each upper limit of each range disclosed herein for the same component, compounds, substituent, or parameter. Thus, a disclosure of two ranges is to be interpreted as a disclosure of four ranges derived by combining each lower limit of each range with each upper limit of each range. A disclosure of three ranges is to be interpreted as a disclosure of nine ranges derived by combining each lower limit of each range with each upper limit of each range, etc. Furthermore, any value within a disclosed range whether explicitly mentioned or not, as well as specific amounts/values of a component, compound, substituent, or parameter disclosed in the description or an example is to be interpreted as a disclosure of either a lower or an upper limit of a range and thus can be combined with any other lower or upper limit of a range or specific amount/value for the same component, compound, substituent, or parameter disclosed elsewhere in the application to form a range for that component, compound, substituent, or parameter. The present invention provides a method for producing fatty acid alkyl esters from a lipid. Referring to In some embodiments, the alcohol may be selected from 1-propanol, iso-propanol and butanols. In some embodiments, any alcohol with a boiling temperature below the reaction temperature of the present invention may be used. To reduce cost of producing the fatty acid alkyl esters, the lipid is preferably selected from a low-value lipid obtained from non-food crops grown on marginal land (not land suitable for food crops) or from waste fats, waste greases, and waste oils. The lipid may have relatively high FFA content, for example at least about 10% FFA, or at least about 50% FFA, or at least 90% of FFA. In some embodiments, lipid from trap grease may be used as the lipid feedstock for the present invention. Trap grease is a waste by-product of the food service industry that contains water, lipids, detergents, food particles and other waste. Trap grease is collected in grease interceptors, which are storage tanks ranging from a few gallons to several thousand gallons. Grease interceptors remove grease and sediments from kitchen effluent that could otherwise enter the sewage systems and cause blockages. Grease interceptors are required by law to be emptied at specific intervals, usually by commercial grease handlers, who are paid to pump and remove trap grease and then must pay to process and dispose of the grease properly. The lipids separated from trap grease may be used by the present invention as a lipid feedstock to produce fatty acid alkyl esters, which reduces the feedstock cost for biodiesel production to just a fraction of refined soybean lipids and other conventional sources of lipids for biodiesel production. In some embodiments, other waste greases may also be used as feedstocks. Examples of these additional feedstocks include sewage scum grease (also called wastewater fats oils and greases (FOGs) or black grease), acid oils and other waste materials having a free fatty acid (FFA) content greater than 40% or by-products from processes selected from rendering processes, animal processing, vegetable oil separation and refining processes, biodiesel production processes. In some embodiments, a bubble column reactor is used for the present invention, where the lipid is placed in the bubble column reactor and the bubbles with the alcohol pass through the lipid in the reactor, as show in As the bubbles pass through the lipid, some alcohol from the bubble may dissolve into the lipid though the solubility of the alcohol in lipids is small. The dissolved alcohol will react with the components (such as FFA and TAG) in the lipid. In addition, on the interface between bubbles and the lipid, reaction may also occur. There are two types of reactions that each can produce fatty acid alkyl esters. One is transesterification of triacyl glycerides (TAG), or triglycerides. During transesterification, a TAG molecule reacts with three alcohol molecules (such as methanol) to form three FAME molecules with by-product glycerin, as shown by the following reaction scheme: In this reaction equation, the alcohol is methanol, and R represents a long aliphatic fatty acid chain, which typically contains 8-22 carbon atoms (Samios et al., A transesterification double step process—TDSP for biodiesel preparation from fatty acids triglycerides, For the FFA in the lipids, acid-catalyzed esterification is effective for producing FAME from FFA: This esterification of FFA is catalyzed by acids like sulfuric acid. Many acids have been described previously that are capable-to catalyze esterification of FFA (Ghadge et al., Biodiesel production from mahua ( The esterification reaction produces water as a by-product. Esterification reaction is reversible and equilibrium-limited by the accumulation of the water by-product. The presence of water generally reduces the conversion rate of FFA to fatty acid alkyl ester in comparison with the reaction when water is not present. Continuously removal of water by-product from the reaction product has been shown to dramatically increase yields of the fatty acid alkyl ester (Lucena et al., Oleic acid esterification with ethanol under continuous water removal conditions, Surprisingly, the present invention can also reduce the sulfur content in the biodiesel produced using the present invention. Without being bound by theory, the alcohol (such as methanol) from the bubbles can also convert the sulfur-containing components of the lipid to intermediates that can either vaporize and be stripped from the lipid by the bubbles or are water soluble which can easily be washed away by water at a late stage. The present invention also has the advantage of tolerating sulfur impurities in the acid catalyst because the alcohol is effective in removing or helping to remove the sulfur-containing components from the reaction mixture in the bubble column reactor. The bubbles exiting the lipid may still contain some unreacted alcohol, along with water vapor. The unreacted alcohol may be recycled for use in the introducing step 100. For example, the alcohol may be collected using a condenser. Referring to A person skilled in the art will appreciate that high temperature may accelerate the transesterification reaction and/or esterification reaction between the alcohol and the lipid. In some embodiments, the lipid in the reactor is heated to a temperature of about 120° C. to shorten the reaction time. Strong acids such as sulfonic acid, sulfuric acid, methane sulfonic acid, hydrochloric acid, or p-toluene sulfonic acid are used as catalyst at a concentration in the lipid in a range of from about 0.01% (w/w) to 2.0% (w/w) of the lipid. In one embodiment, the acid at a concentration of about 0.1% (w/w) of the lipid is used. In some embodiments, the catalyst is mixed with an alcohol to form a catalyst solution. This solution may then be gradually added to the lipid by a peristaltic pump during the first 5 min of the reaction. The bubbles comprising alcohol vapor pass through the lipid to provide alcohol for the esterification and/or transesterification reactions. Higher flow rate for the alcohol affords more reactant thus accelerating the reactions. The flow rate of the alcohol through the lipid may be from about 0.20 mL/min to about 3.5 mL/min, or from about 0.43 mL/min to about 2.57 mL/min, or from about 0.75 mL/min to about 1.75 mL/min, or from about 0.75 mL/min to about 1.16 mL/min. In some embodiments, the time period for reacting the alcohol as gas bubbles passing through the lipid is from about 6.2 minutes to about 113.5 minutes, or from about 8.7 minutes to about 52.5 minutes, or from about 12.3 minutes to about 29.1 minutes, or from about 12.3 minutes to about 19.1 minutes. In some embodiments, the reactor for producing fatter acid alkyl esters is a bubble column reactor. The bubble column reactor may be constructed as a jacketed glass column 18-in. tall with a 1-in. internal diameter. Typically, the reactor operates at a temperature of about 120° C. with about 180 mL, or 400 mL, or 1500 mL, or 4000 mL of feedstock lipids. The bubble column reactor and the alcohol-vaporizer/lipid-reheater ( In some embodiments, the lipid in the reactor may be circulated from the bottom of the reactor to the top of the reactor through an external sampling loop. Before returning to the top of the reactor, the circulating lipids passes through heat exchanger tubes immersed in hot silicone oil to maintain the temperature of the lipid. This same heat exchanger may also be used to heat and vaporize the alcohol feed, which is fed to the heat exchanger as a liquid by syringe pumps and vaporized in separate tubes in the heat exchanger before being introduced to the reactor. Two syringe pumps may be used sequentially during the process where one syringe pump is filled with alcohol while the other can discharge alcohol into the reactor through the heat exchanger. Kocsisova et al. (High-temperature esterification of fatty acids with methanol at ambient pressure, The present invention converts free fatty acids to fatty acid alkyl esters (for use as biodiesel) at a conversion rate of over 95% of free fatty acid in less than 2 h under a variety of reaction conditions. For example, with a sulfuric acid catalyst concentration of 0.1 wt %, a reaction temperature of about 120° C. and ambient pressure, a methanol to fatty acid molar ratio of less than 3:1, and bubbling methanol vapor through the reactor at a flow rate of 0.034 moles of alcohol per mole of FFA per minute into liquid, the time to 95% conversion for the FFA is about 70 min. At higher methanol flow rates, the time to 95% conversion for the FFA may decrease to about 40 min but requires a methanol to fatty acid molar ratio of 5:1 or higher, which may lead to more unreacted methanol in the bubbles exiting the lipid. The alcohol may be pure methanol or ethanol, or pure propanols or pure butanols. In some embodiments, there may be up to about 10%, or up to about 20% water in the liquid alcohol that is used to generate vapor to be introduced into the lipid. In one embodiment, the liquid alcohol contains methanol and water at about 90:10 ratio, or about 80:20 ratio. In one embodiment, the liquid alcohol contains ethanol and water at about 90:10 ratio, or about 80:20 ratio. The present invention can use low-quality alcohol feedstocks that contain water. Thus, the invention is potentially useful for the conversion of low-value lipids into biodiesel using low-quality alcohols such as ethanol produced from biomass, which can lower the feedstock costs for the biodiesel production processes. As a result, the cost of producing biodiesel from renewable resources could be lowered substantially. The present invention has several other potential advantages related to sustainability including: flexibility to varying FFA content, flexibility for alcohol feed, robustness to moisture, and reduced energy requirements. The present invention is a robust method that can handle a variety of feedstocks without additional pretreatment, which will reduce economic hurdles to construct and operate biodiesel production facilities and result in lower consumer prices of biodiesel products. The transesterification reaction is low when the catalyst is an acid catalyst, as described herein. Therefore, at the end of the introducing step 100, though almost all of FFA has been converted to fatty acid alkyl esters, the TAG in the lipid may not have achieved complete conversion. However, the product of the introducing step 100 has very low FFA Referring to The transesterification process uses an alcohol, preferably methanol or ethanol, to convert TAG to fatty acid alkyl esters and glycerin, as shown below: In this reaction equation, methanol is used. The catalyst for this transesterification process is a case catalyst, typically strong bases. The transesterification process is described in details in Samios et al., A transesterification double step process—TDSP for biodiesel preparation from fatty acids triglycerides, This two-step process can utilize any renewable lipid resource that contains FFA and TAG in any ratio. Because the present invention does not require temperatures or pressures as high as other esterification methods, it may prove to be a cost effective esterification step in a two-step conversion both FFA and TAG to fatty acid alkyl esters. This robustness of the method with respect to lipid feedstocks and alcohol feedstocks allows biodiesel manufacturers employing this technology to greatly diminish their feedstock costs. Because feedstock costs are a dominant expense in producing biodiesel, it could lower the price of biodiesel to a level that is more competitive with petroleum diesel. The following examples are illustrative, but not limiting, of the methods and compositions of the present disclosure. Other suitable modifications and adaptations of the variety of conditions and parameters normally encountered in the field, and which are obvious to those skilled in the art, are within the scope of the disclosure. Oleic acid was used to model a lipid feedstock with FFA for this example. Oleic acid at technical grade purity (N90%) as well as toluene and methanol with purities above 99% were purchased from Sigma Aldrich and used without further purification. Ethanol was anhydrous and denatured with 5% isopropyl alcohol and was also purchased from Sigma Aldrich. Isopropyl alcohol was purchased from Azer Scientific and was 99.99% purity. Sulfuric acid at 93% weight (66° Baume) was purchased from Fischer Scientific. Paratoluene sulfonic acid (PTSA) was purchased from Sigma-Aldrich and dissolved in methanol for use. Triglyceride samples were refined soybean oil purchased from local supermarkets. Trap grease was donated by Russell Reid waste management. The conversion of fatty acid chains to FAME was quantified using two techniques: (1) base titration and (2) and nuclear magnetic resonance (NMR). The titrant used was a 0.1 molar solution of sodium hydroxide in methanol, and samples from the reactor were dissolved in a titration solution containing equal parts toluene and isopropyl alcohol with trace phenolphthalein as an indicator; this is a titration procedure similar to AOCS Cd 3d-63 and ASTM D-664. The base titrant was prepared from a standard base concentrate (Fixanal purchased from Sigma Aldrich) and tested against an acid standard prior to experimentation. This titration determined the acid number of the sample (mg KOH/g sample). For samples where the average molecular weight of the fatty acid is known (for example, oleic acid), the molar fraction of FFA was readily determined. For experiments with partial TAG feedstocks, titration cannot completely determine the conversion rate of fatty acid to FAME. So proton NMR (H-NMR) spectroscopy was used to measure the FAME content of samples over time. The machine used for analysis of FAME samples was Drexel University's 500 MHz Inova Varian NMR. H-NMR peaks were assigned to their appropriate functional groups as shown in the supplementary documentation. A combination of NMR and titration enables determining the content of FFA, FAME, and acyl glyceride fatty acids. The experimental conditions for this example were as follows: a reactor temperature of 120° C., ambient pressure, lipid volume of 180 mL of pure oleic acid, alcohol feed at 0.75 mL of liquid methanol per minute, and 0.1% (wt. catalyst/wt. lipids) of sulfuric acid catalyst added during first 5 min of reaction. Deviations from these conditions are indicated when used. Oleic acid was used as the FFA for these experiments as an appropriate surrogate for naturally occurring FFA. The catalyst concentration of 0.1% (w/w) sulfuric acid was used for this example. Methanol was continuously fed to the bubble column reactor filled with FFA (oleic acid) by syringe pumps. The effect of methanol feed rate to the reactor on conversion of FFA to FAME is shown in In As the flow rate increased, the reaction rate increased until the reaction approached an apparently kinetically limited regime. Faster methanol flow rates created more vigorous bubbling, which leads to a reduction in mass transfer resistance and contributed to faster reaction rates and faster decrease of FFA content in the lipid as shown in Table 1 contains alternative expressions of those flow rates that may be useful for interpreting the results. The first column in Table 1 is the liquid methanol flow rates in milliliters per minute. The second column shows the flow rate of methanol in moles of methanol per minute. The third column shows the molar flow rate of methanol that is scaled by the initial moles of oleic acid in the reactor. This is a normalized flow rate expressed in inverse minutes. This normalized flow rate is useful in representing how much methanol is available to react with the oleic acid in the reactor. The normalized flow rate is also useful for scaling between reactors with different volumes of FFA. The fourth column is the reciprocal of the third column and is the time required for the moles of methanol fed to the reactor to be equal to the initial moles of oleic acid in the reactor. This is the theoretical minimum time for complete conversion if all methanol entering the reactor reacted with oleic acid to produce FAME. At some methanol flow rates, some methanol passed through the reactor without reacting with FFA to form FAME. The ratio of the unreacted methanol to the initial amount of FFA in the reactor is the “unreacted methanol ratio.” The unreacted methanol in bubbles exiting the reactor may be vented to a fume hood. This unreacted methanol could be collected and recycled, which is especially important for full scale production processes. The amount of unreacted methanol was calculated from the known methanol flow rate and the measured conversion of FFA to FAME: where NMeOHis the molar flow rate of methanol, NFFA(t)is the moles of FFA in the reactor at time t measured by titration, and NFFA,0is the initial number of moles of FFA. At low methanol flow rates, the unreacted methanol in the bubble exiting the reactor was close to zero, which means that most of the methanol fed to the reactor reacted with FFA to produce FAME, i.e., the methanol used is close to the stoichiometric ratio of methanol to FAME. As the methanol flow rate increased, unreacted methanol in the bubble exiting the reactor increased to over eight times the stoichiometric ratio of methanol required. In all cases, the unreacted methanol was initially lower and increases approximately linearly at long times. At long times, the esterification reaction rate approached zero. Thus nearly all of the methanol that entered the reactor passed through the lipid unreacted, leading to a linear increase in unreacted methanol. The dashed curve of The reaction profiles in Surprisingly, the conversion profile for pure ethanol feed was nearly the same as the conversion for methanol feed with 10% water; likewise, the profile for ethanol with 10% water overlaps the profile for methanol for 20% water. Hence, under these conditions, switching from methanol to ethanol had roughly the same effect on conversion time as adding 10% water by volume to the methanol feed. The use of different types of alcohol feedstock and alcohol moisture content (displayed in In addition to being robust for impure feeds, this example shows that larger alcohols (such as ethanol) can react with fats, greases, and oils for FAAE production in the bubble column. Using ethanol as the alcohol feedstock could have several advantages, including the potential to be produced from renewable feedstocks and having lower toxicity than methanol. In this example, the lipid is a mixture of FFA and TAG at different ratio (v/v %) from 100:0 to 10:90. This example is for examining the performance of the bubble column reactor for creating FAME using feedstocks containing a mixture of FFA and TAG. The FAME, FFA, and TAG content were analyzed using titration and NMR. Trap grease samples ( Other types of waste greases have also been processed by a bubble column reactor according to an embodiment of the present invention. The results of these experiments are given in Table 2 below. Each row in Table 2 corresponds to different waste grease samples that were converted to biodiesel. The first column of Table 2 indicates whether the corresponding sample was lipids extracted from grease trap waste (GTW) or sewage scum grease (SSG). The second column indicates the method used to separate the biodiesel (fatty acid methyl ester) fraction from heavy residuals by either wiped film evaporation (WFE) or rotary evaporation (rotovap). The third, fourth and fifth column related the temperature and pressure conditions used during the vacuum evaporation process. In these experiments two vacuum evaporation steps were used with an initial condensate collected at lower temperatures and a distillate fraction collected at higher temperatures, where the residue fraction did not evaporate. The last five columns of Table 2 display sulfur contents of the initial lipids, the crude FAME after reaction and washing, and the products of the vacuum evaporation purification step: the condensate collected at lower temperatures, the distillate collected at higher temperatures, and the residue sulfur which did not evaporate (remains in the heavy residuals). These results show that the sulfur content decreased by 30-75% during the esterification reactions and washing of crude FAME. The sulfur was further reduced by vacuum evaporation to produce a distillate containing between 6-80 PPM sulfur. Additional reductions in sulfur content may be achieved by optimizing the vacuum evaporation conditions. The results of Example 4 are summarized in Table 3 below. Table 3 summarizes the ranges of sulfur content observed for samples at different stages of converting waste greases to biodiesel. For sewage scum grease (SSG), the average sulfur content was reduced by 55% from the waste grease to the reacted and washed FAME and by 82% from the waste grease to distilled biodiesel. For grease trap waste (GTW), the sulfur average content was reduced by 58% from the waste grease to reacted and washed FAME and by 95% from the waste grease to distilled biodiesel. These are typical results and it should be understood that the actual sulfur content depends upon many factors including the composition of the starting lipids, reaction conditions, washing processes used, and purification conditions. It is to be understood, however, that even though numerous characteristics and advantages of the present invention have been set forth in the foregoing description, together with details of the structure and function of the invention, the disclosure is illustrative only, and changes may be made in detail, especially in matters of shape, size and arrangement of parts within the principles of the invention to the full extent indicated by the broad general meaning of the terms in which the appended claims are expressed. Throughout this application, various publications are referenced. The disclosures of these publications in their entireties are hereby incorporated by reference into this application in order to more fully describe the state of the art as known to those skilled therein as of the date of the disclosure described and claimed herein. The present invention provides a method of producing fatty acid alkyl esters from a lipid, comprising steps of introducing a gas comprising vapor of an alcohol selected from methanol, ethanol, 1-propanol, iso-propanol and butanols, into the lipid in a form of bubbles to enable the bubbles to pass through the lipid and be discharged from the lipid. The product may then be subjected to a transesterification process catalyzed by a base catalyst. The present invention is robust with low quality feedstocks thus significantly reduce production cost for biodiesel. 1. A method of producing fatty acid alkyl esters from a lipid, comprising steps of:
introducing a gas comprising vapor containing an alcohol into the lipid in a bubble column reactor in a form of bubbles to enable the bubbles to pass through the lipid and be discharged from the lipid; and adding an alcohol solution of an acid catalyst to the lipid in the bubble column reactor. 2. The method of 3. The method of 4. The method of 5. The method of 6. The method of 7. (canceled) 8. The method of 9. The method of 10. (canceled) 11. The method of 12. The method of 13. The method of 14. The method of 15. The method of 16. The method of 17. The method of 18. The method of 19. The method of 20. The method of 21. The method of 22. The method of STATEMENT OF FEDERALLY SPONSORED RESEARCH
BACKGROUND OF THE INVENTION
SUMMARY OF THE INVENTION
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT(S)
EXAMPLES
Example 1
Representations for alcohol flow rates for experiments shown in FIGS. 2A-2C Volumetric flow Molar flow MeOH flow Time to rate of rate of normalized stoichiometric liquid MeOH MeOH by FFA0 delivery of MeOH (mL/mon) (mol/min) (1/min) (min) 3.5 0.0859 0.1617 6.2 2.57 0.0631 0.1146 8.7 1.75 0.0430 0.0816 12.3 1.16 0.0285 0.0523 19.1 0.75 0.0184 0.0343 29.1 0.43 0.0106 0.0190 52.5 0.2 0.0049 0.0088 113.5 Example 2
Example 3
Example 4
Results of processing different types of waste greases. Washed Starting FAME Purification Vacuum Max Temp. Max Temp. of Yield of Yield of Lipid PPM Distillate Residue PPM Grease Type Method (mBar) Condensate (° C.) Distillate (° C.) Distillate Residue PPM Sulfur Sulfur PPM Sulfur Sulfur GTW WFE 1.3 115 190 52% 22% 230 157 15 556 GTW WFE 1.3 120 190 93% 6% 96 65 10 393 SSG WFE 1.3 120 190 78% 12% 429 259 87 439 GTW WFE 1.3 120 190 81% 19% N/A N/A 27.2 N/A GTW WFE 1.3 120 190 69% 31% N/A N/A 17.6 N/A GTW WFE 1.3 120 190 92% 8% N/A N/A 15.0 N/A GTW VacEvap 4 N/A 200 76% 24% N/A 118 15.1 434 GTW Rotovap 5 N/A 195 76% 24% 409 183 N/A N/A GTW Rotovap 3 180 198 81% 17% 409 183 18.0 N/A GTW Rotovap 1 175 194 83% 10% 303 N/A 19.9 N/A GTW Rotovap 2 172 200 75% 15% 303 80 12.3 416 GTW WFE 1.3 120 190 82% 18% 303 80 26.0 1189 GTW WFE 1.3 120 160 55% 44% 409 183 5.8 224 GTW WFE 1.3 120 190 94% 5% 409 183 14.3 751 SSG Rotovap 1.3 N/A 200 33% 10% 474 143 76 474 Reduction of sulfur content by the method of the present invention. SSG Std GTW Std GTW Avg Dev Avg Dev Outlier Lipids 453 23 347 71 96 Washed FAME 201 82 146 46 65 Residue 457 25 595 339 393 Condensate 41 12 89 0 22 Distillate 82 8 17 6 10 REFERENCES