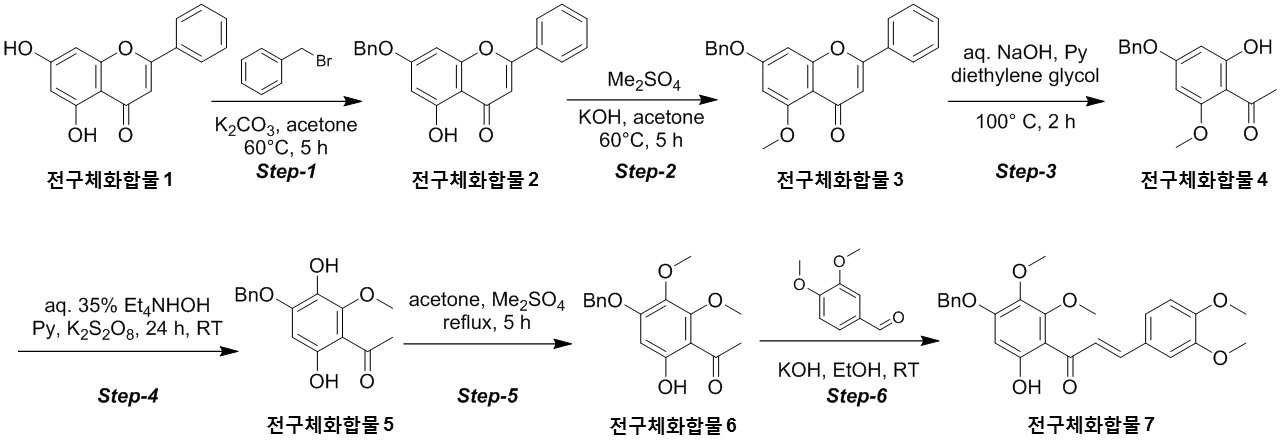
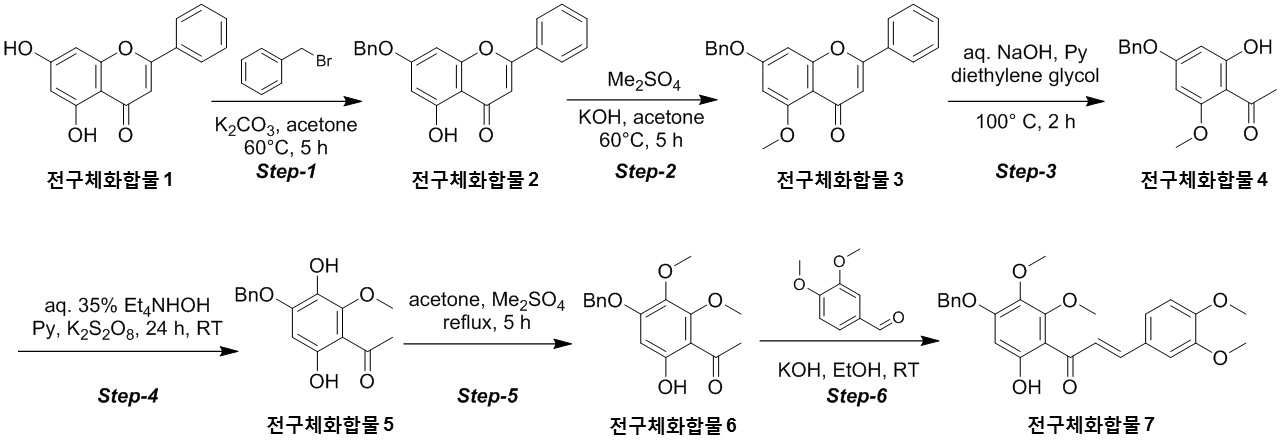
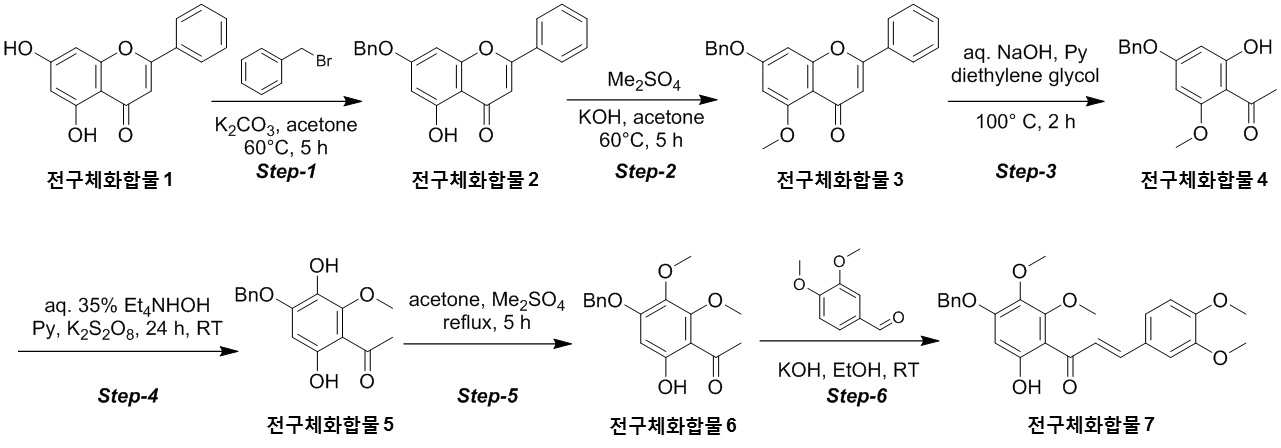
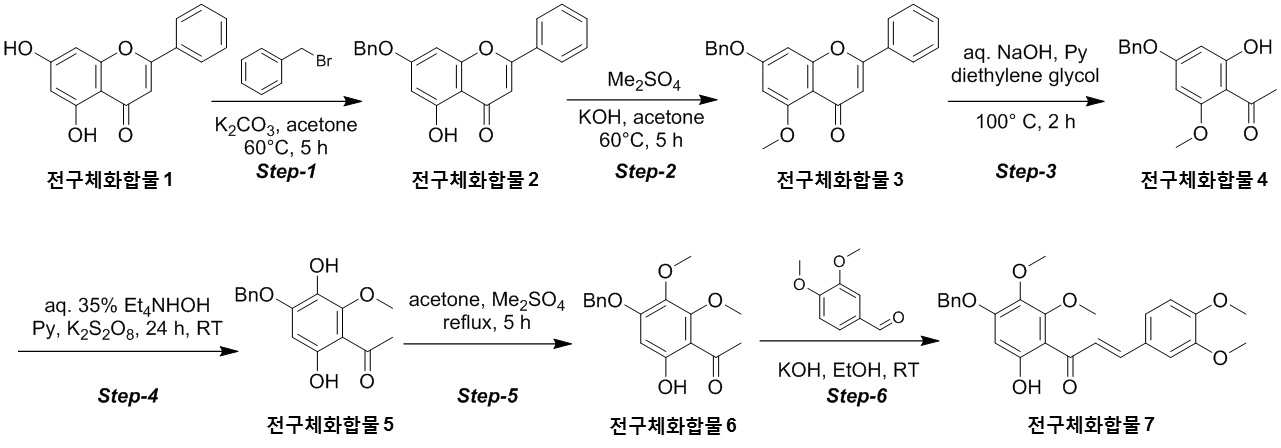
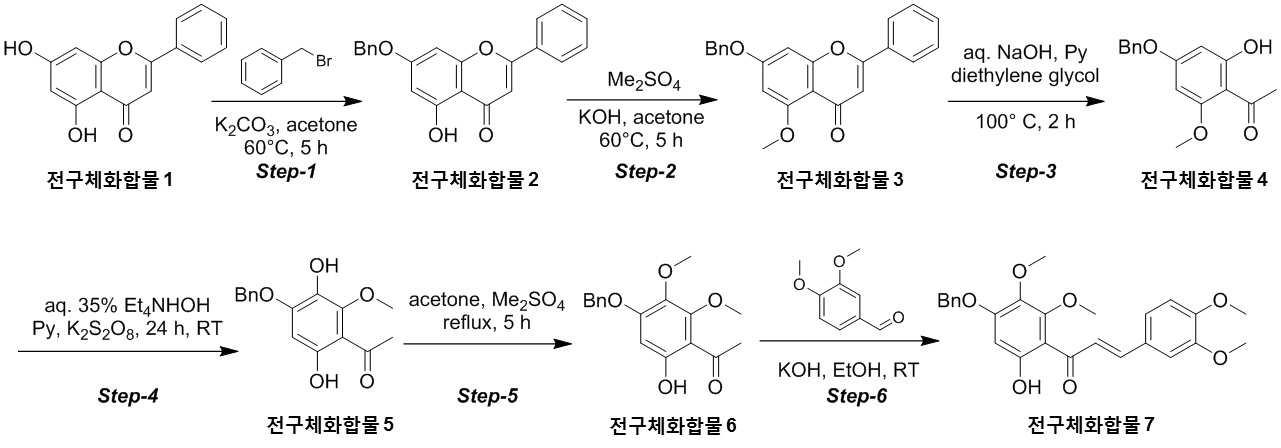
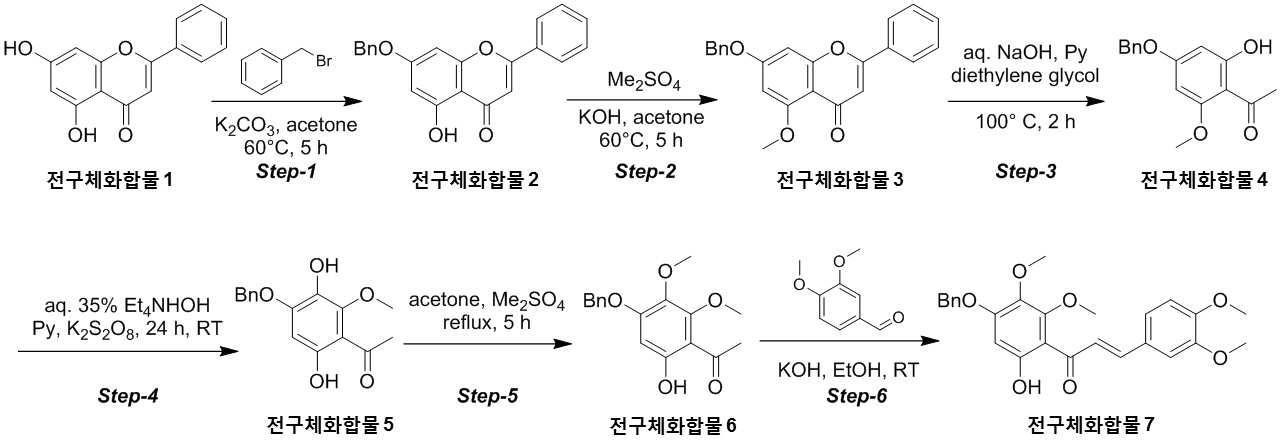
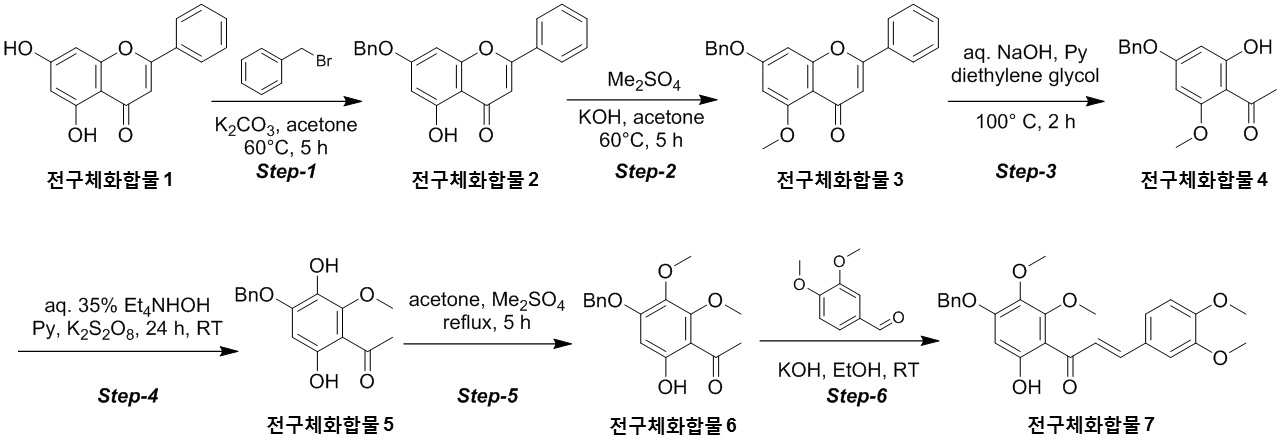
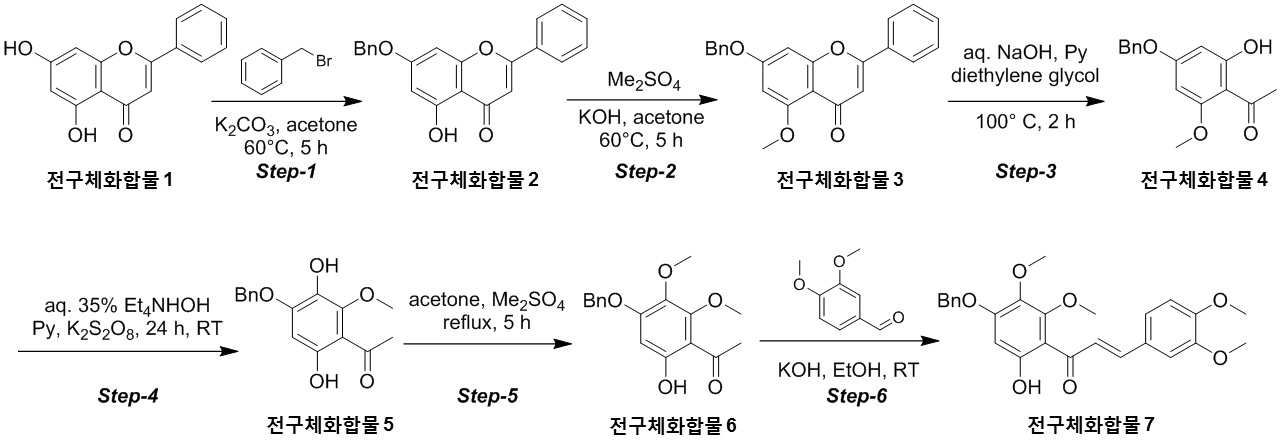
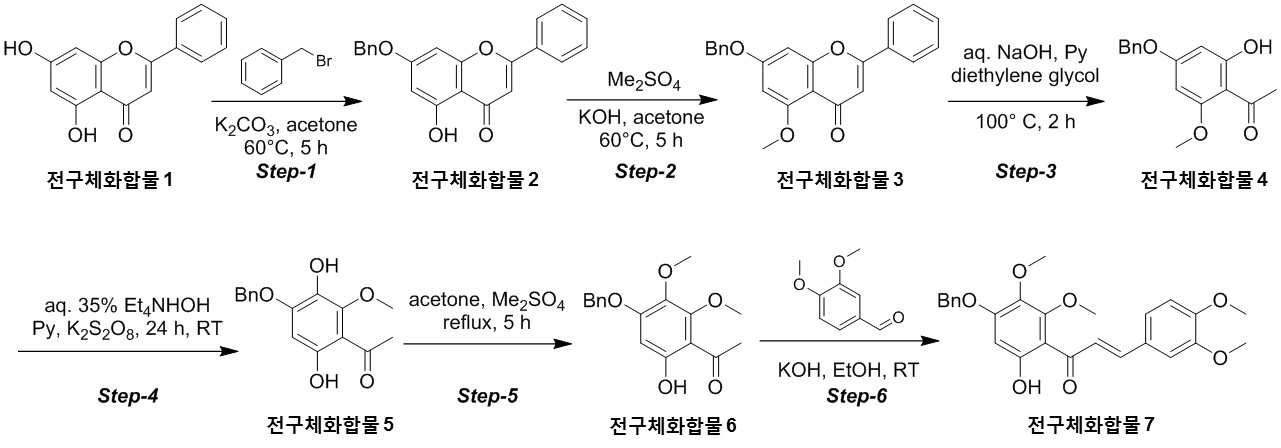
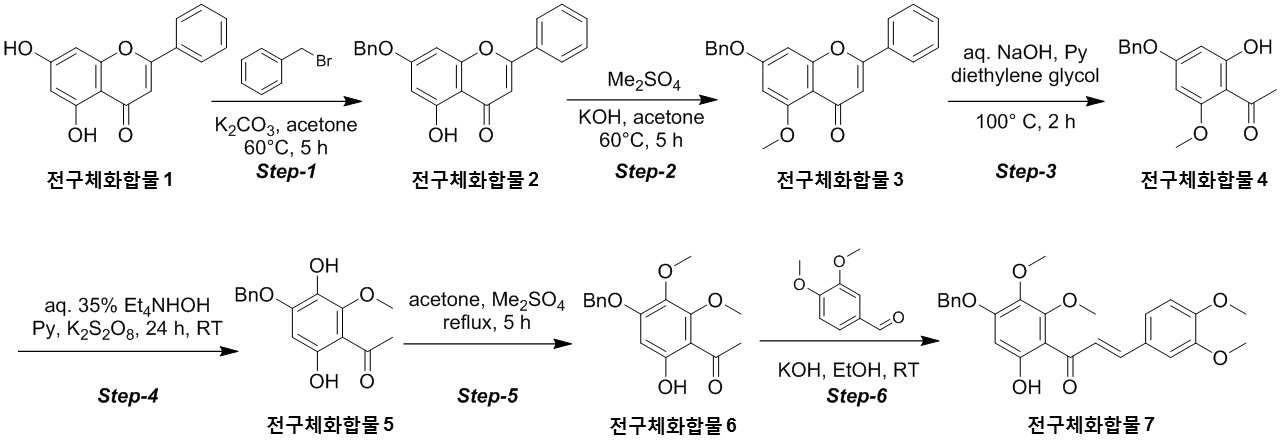
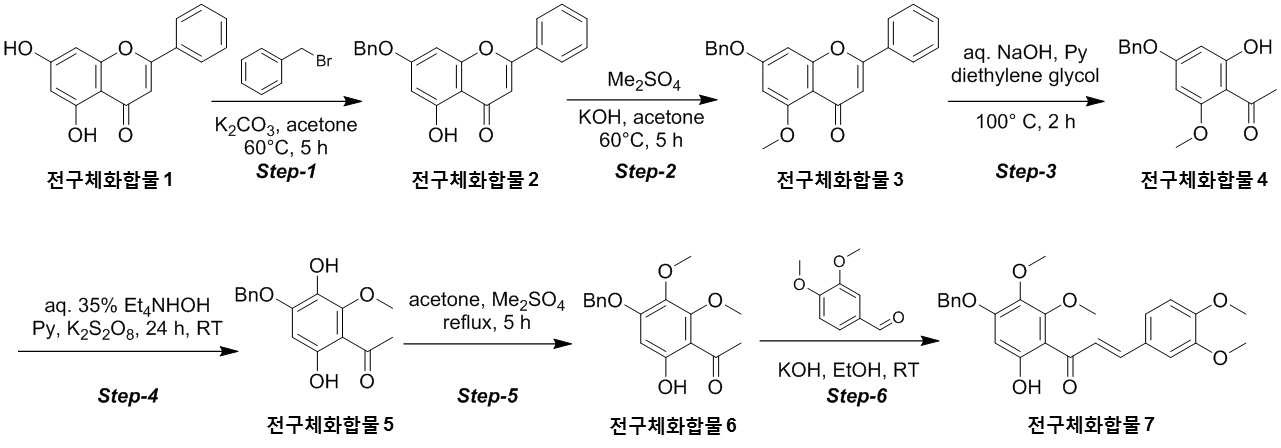
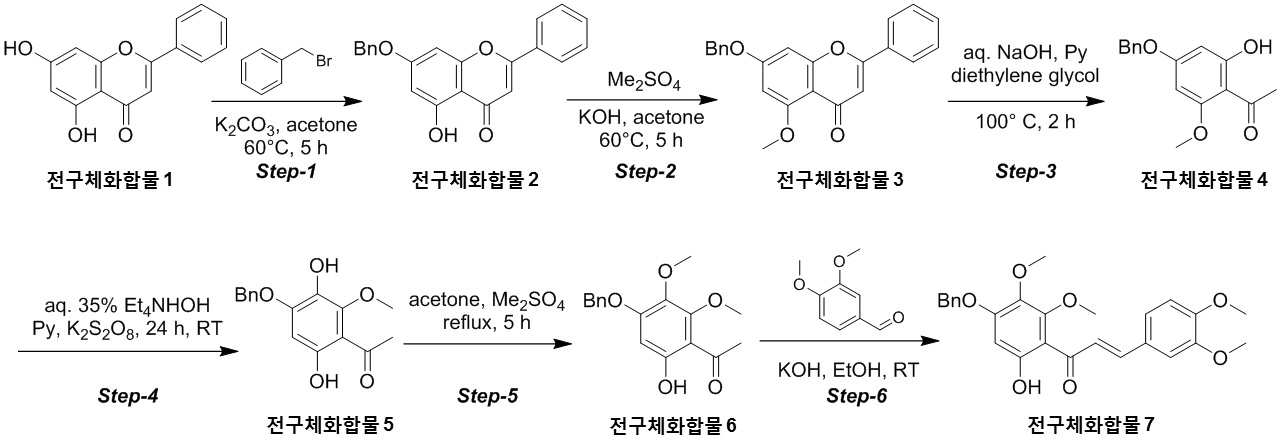
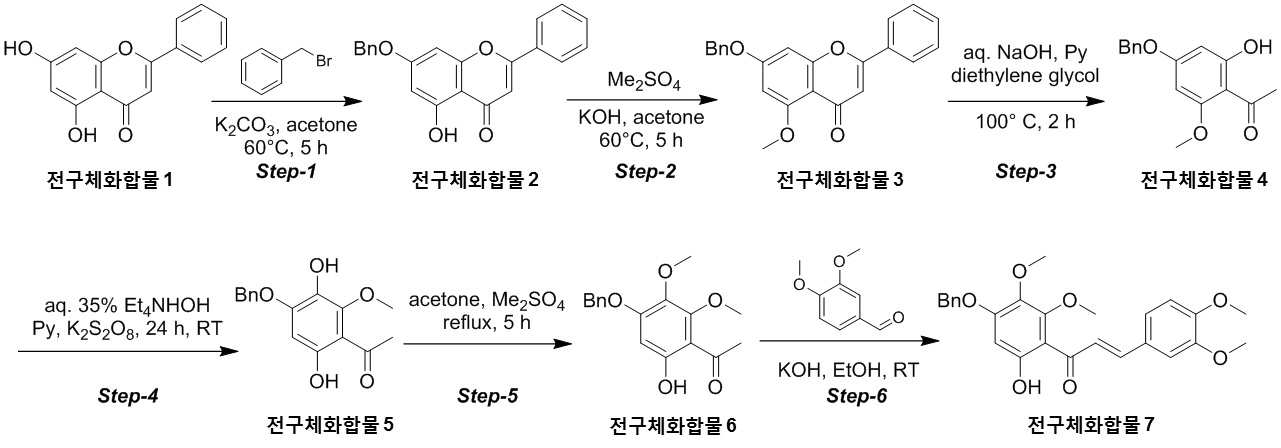
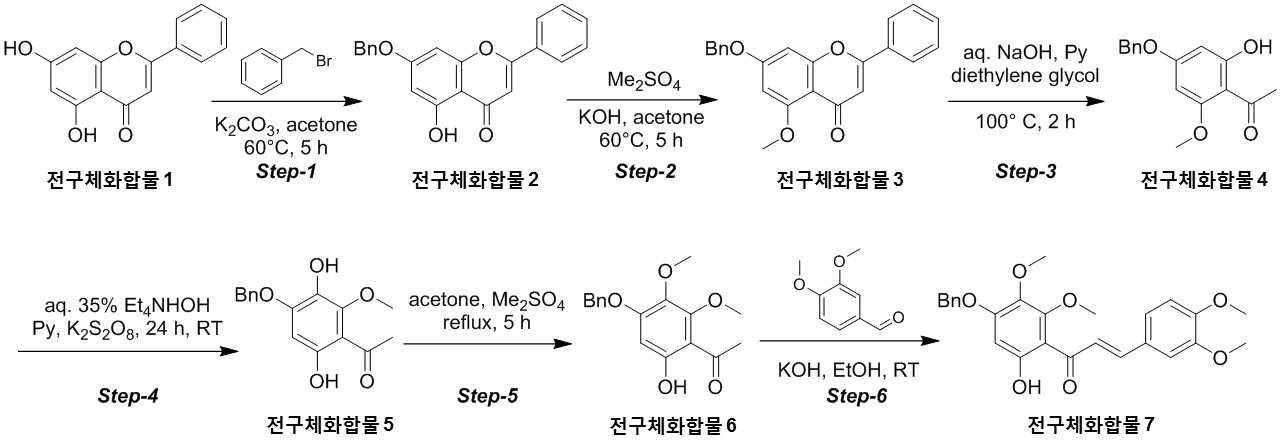
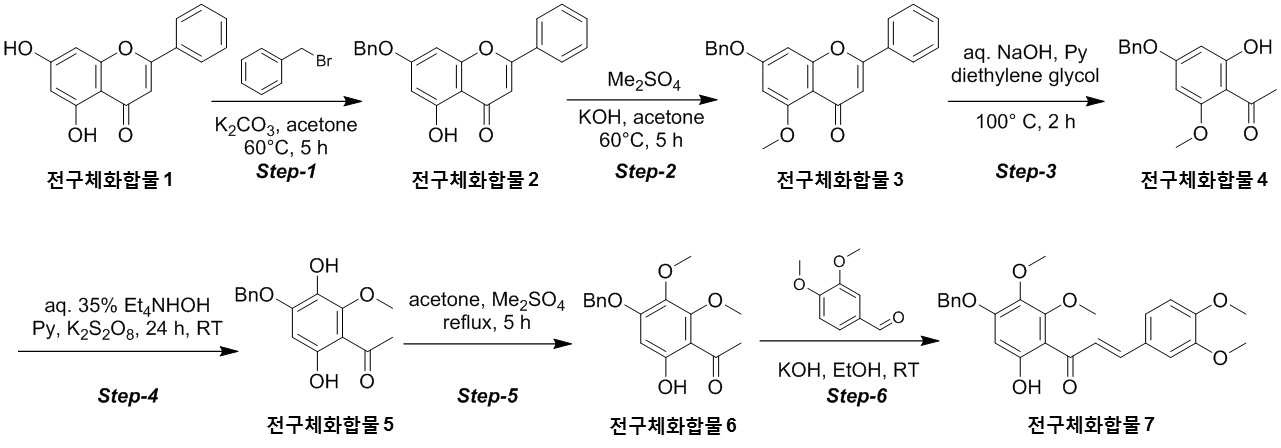
NOVEL COMPOUND AND COMPOSITION FOR PREVENTION, IMPROVEMENT, OR TREATMENT OF FIBROSIS OR NONALCOHOLIC STEATOHEPATITIS COMPRISING SAME AS ACTIVE INGREDIENT
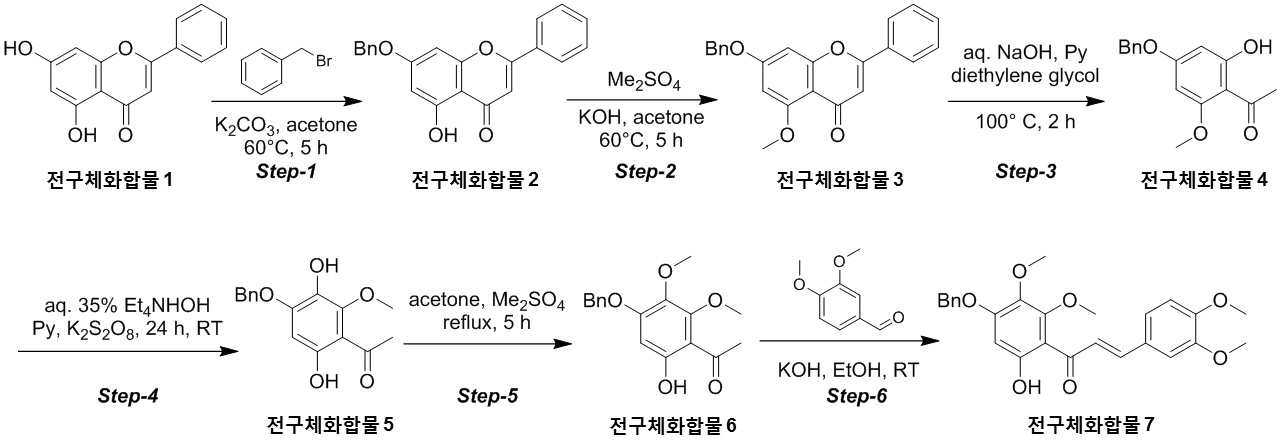
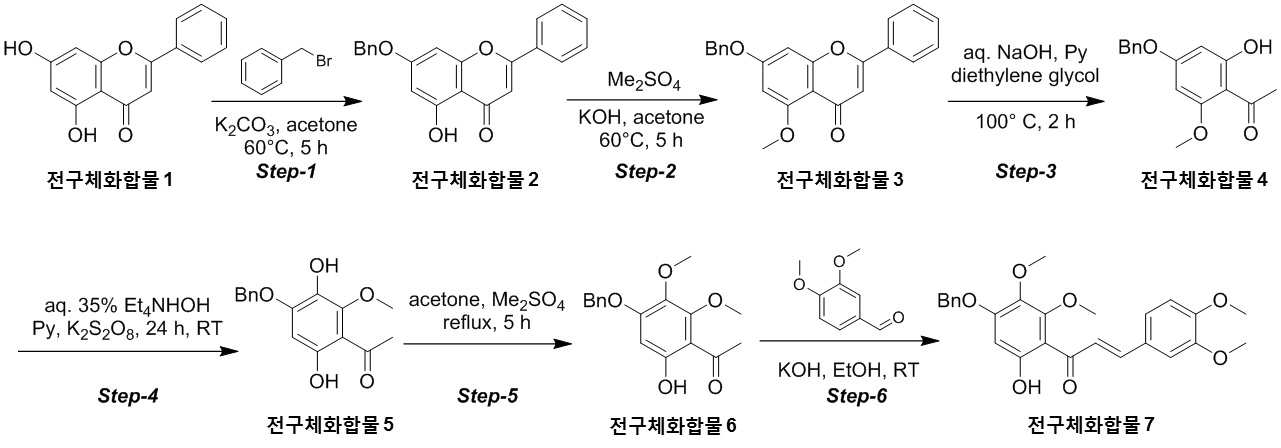
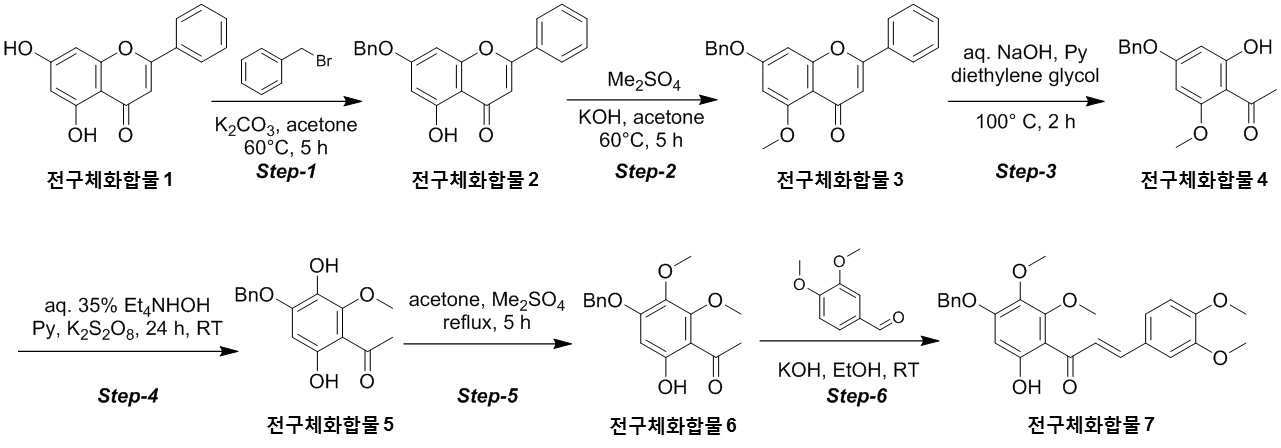
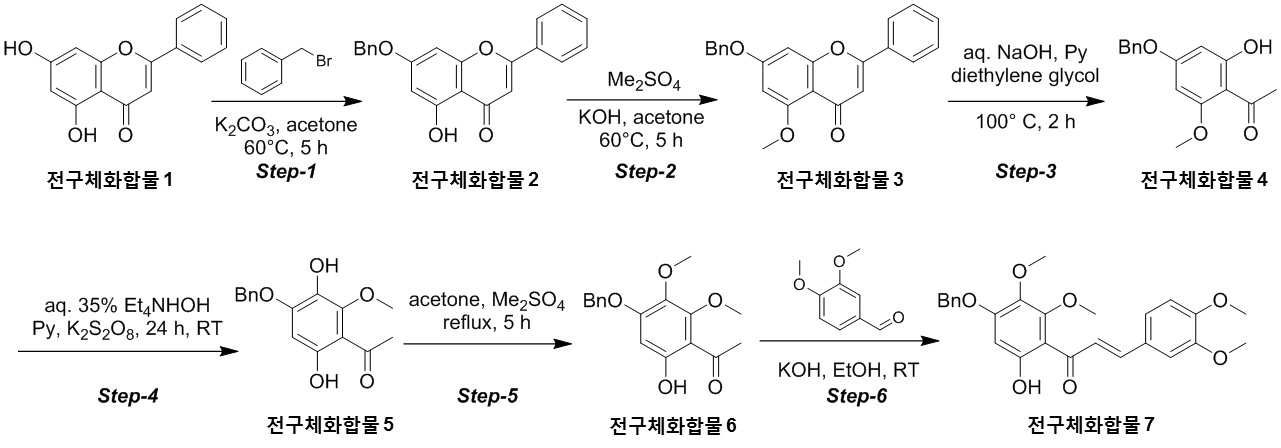
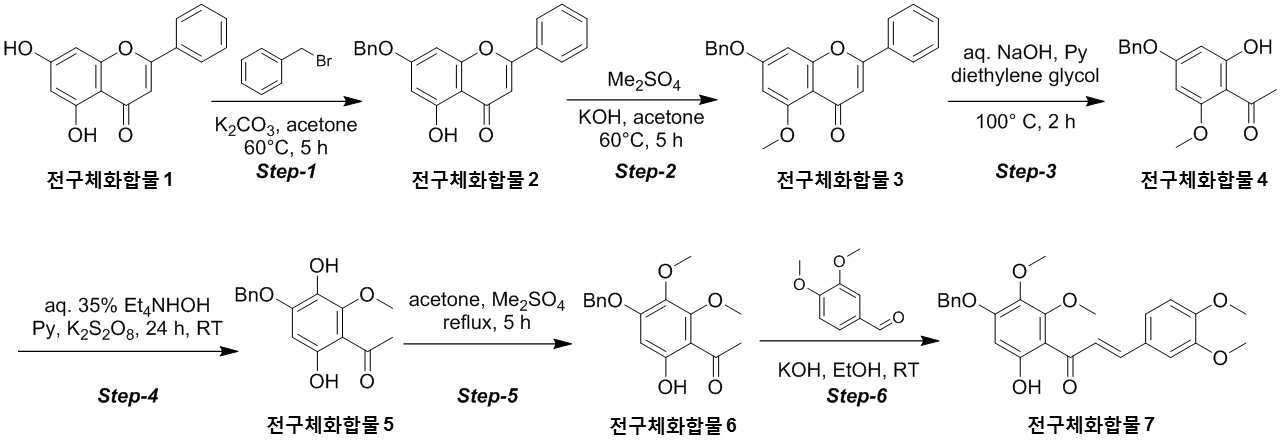
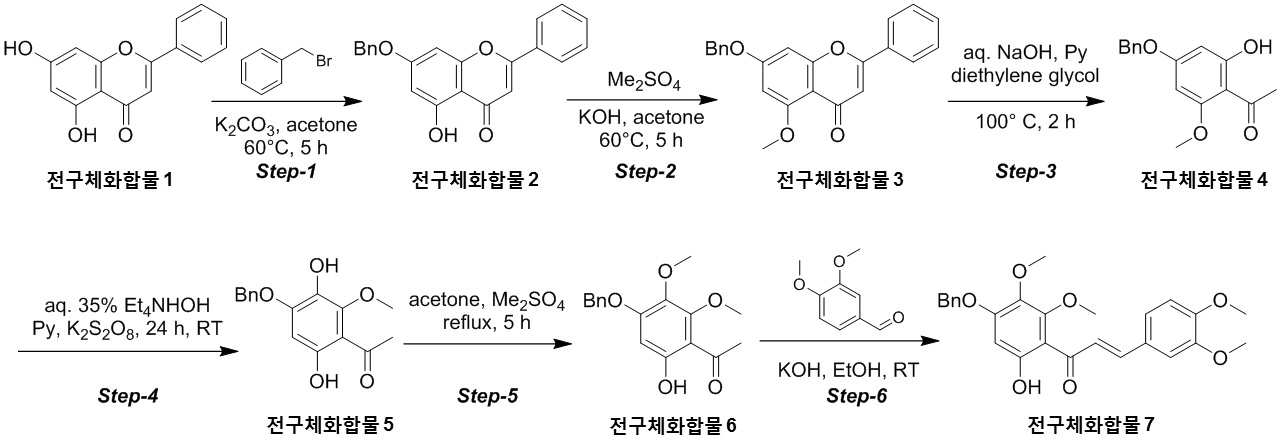
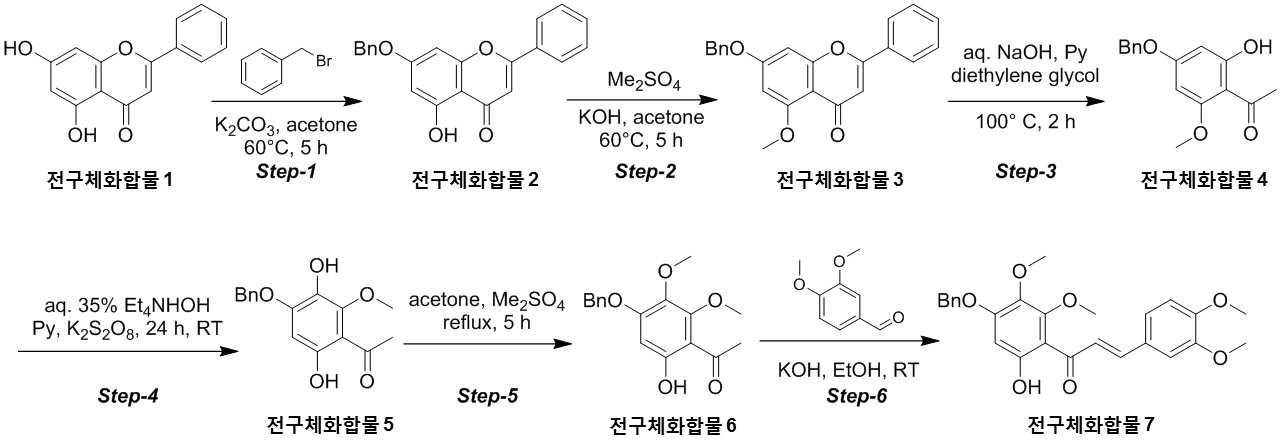
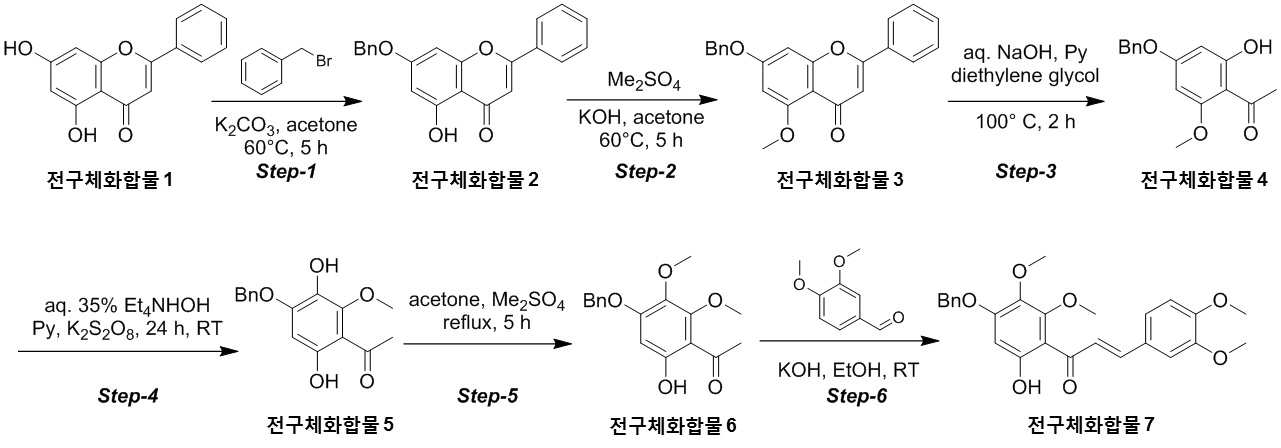
The present invention refers to novel compounds capable of containing liver fibrosis or nonalcoholic steatotic hepatitis, relates to improving or treating composition, specifically fiber useful for preventing, improving or treating novel compounds of formula 1 containing active ingredient having an effect and nonalcoholic steatotic hepatitis liver fibrosis or for preventing, improving or treating compositions are disclosed. In confidential excessive fibrosis (fibrosis) is reproduced or occurrence engine formed fibrous connective tissue disease, normal tissue such fibrous connective tissue fibers opposed of the pipe. Engine confidential fibrous connective tissue has been reduced and the inflow of a body fluid such as excessively rigid tissue in vivo sufficiently not able to perform functions to be coated. It cause injury, inflammation, Image, radiation, chemotherapy, edema methods like known. The fibrosis is formed due to door number depending on position where it is fibrous connective tissue, primarily liver, secretion engine, waste and the like subjected to damage. Fibrosis is typically such as idiopathic pulmonary fibrosis (idiopathic pulmonary fibrosis, IPF), bone marrow fibrosis (myelo fibrosis), and the pin is (liver fibrosis) fibrosis (kidney fibrosis). This fibrosis treatment to current blood [lu number (Pirfenidone, such as idiopathic pulmonary fibrosis treatment number), (Nintedanib, such as idiopathic pulmonary fibrosis treatment number), mote [nip [lwuk cow (Ruxolitinib, bone marrow fibrosis treatment number) are known but such as, and more effective development of new therapeutic number number number easily possible to human body and excellent need disclosed. Each of new treatment in the invention number associated with fibrosis studies when the insects and various attempts, in particular epithelial mesenchymal transition (Epithelial Mesenchymal Transition, EMT) (hereinafter, 'EMT' is equal to.) has been varies. A top cells EMT tumour cells than cytoskeletal changes from the vertical intermediate the upper cells migrate in the form of genetic reprogramming the mesenchymal cells (mesenchymal cell) (genetic reprogramming) pipe substrate. The expression of the protein involved in proliferation of tumor EMT billion billion can think to transition and number number, number of tumor treatment studies for developing various users associated with these EMT etc. studied. The EMT Twist modulators of include, Snail, Slug, E-a cadherin, vimentin, such as collagen 1 a 1 about about possibility hundred the dog known. The EMT and most cancer or tumor EMT studies of regulators to been progress only. However the portion of interest in the invention based on the use of association study result existing conveyor EMT maintain an EMT if so control may be provided for preventing and treating fibrosis was expected. The EMT fibrosis with association on fibrosis varies effective in preventing, improving or treating for developing novel substance can be for serving as, a compound represented by formula 1 of the present invention specification EMT fibrosis effectively by an excellent preventing, improving or treating benefits, in addition effectively improving or treating non alcoholic fatty liver due to this effect can be confirms that the salinity has been completed the present invention. The main object of the present invention fiber useful for preventing, improving or treating novel compounds having excellent number 30 to 60 seconds. It is another object of the present invention said fibers containing ingredient compounds useful for preventing, improving or treating composition number 30 to 60 seconds. Said composition containing an ingredient compounds of the present invention another object is to provide a composition for treatment improvements in or steatotic hepatitis number 30 to 60 seconds. In one aspect of the present invention according, to the present invention refers to compounds of formula 1 or a pharmaceutically acceptable salt thereof number substrate. [Formula 1] In formula said, R1 A substituted or unsubstituted C1 - 5 Linear or branched alkyl, C5 - 6 Cyclic alkyl, one or more heteroatoms O or N C atoms5 - 6 Cyclic alkyl, a substituted or unsubstituted C6 - 12 Aryl, or one or more heteroatoms O or N C atoms5 - 6 It will be biting and heteroatoms, R2 Is hydrogen, ethyl, acetyl, acetoxy, carboxyl, benzoyl oxy or 3, 4, 5 - tree , R3 To R5 Are each independently a hydrogen, hydroxyl, methyl, methoxy, acetoxy, carboxyl or benzoyl oxy among others. According to another aspect of the present invention, the present invention refers to said compound or its pharmaceutically acceptable salts as the active ingredient containing a pharmaceutical composition for the prevention or treatment of fibrosis number substrate. According to another aspect of the present invention, the present invention refers to said compound or its pharmaceutically acceptable salts as the active ingredient a food containing low number containing fibrosis for preventing or ameliorating substrate. According to another aspect of the present invention, the present invention refers to said compound or its pharmaceutically acceptable salts as the active ingredient containing pharmaceutical compositions for the treatment of nonalcoholic steatotic hepatitis (non non-alcoholic steatohepatitis, NASH) number substrate. According to another aspect of the present invention, the present invention refers to said compound or its pharmaceutically acceptable salts as the active ingredient of composition containing an steatotic hepatitis food composition number substrate. EMT (Epithelial Mesenchymal Transition, epithelial mesenchymal transition) of the present invention novel compounds are modulators of the expression of vimentin and snail be effectively regulated to control activation of the EMT, the fibrosis effective in preventing, improving or treating can. Oral administration of the present invention as well as novel compounds are very good pharmacokinetic characteristics as fast drug delivery with respect to the body, can be stable in vivo effect, without side can be large to plants. In addition of the present invention novel compounds are fibers of hepatocytes can be effectively breaking a non alcoholic fatty liver can effectively improving or treating salinity. Mm for Figure 1 of the present invention are disclosed. The synthesis of compound process according to one embodiment of the invention the present invention revealing the secret key 2 and 3 also are disclosed. 7 - (Benzyloxy) - 5 - hydroxy - 2 - phenyl - 4H - during synthesis of compound according to one embodiment of the invention the present invention Figure 4 the chromen - 4 - ones to result number is confirmed with a high pressure liquid coolant LCMS non-NMR are disclosed. 7 - Methoxy - 5 - (benzyloxy) - 2 - phenyl - 4H - Figure 5 the synthesis of compound according to one embodiment of the invention the present invention during the chromen - 4 - ones LCMS non-NMR results high pressure liquid coolant is confirmed with a number are disclosed. Figure 6 according to one embodiment of the invention the present invention during the synthesis of compound 1 - (4 - (benzyloxy) - 6 - hydroxy - 2 - methoxyphenyl) ethane - 1 - ones to result number is confirmed with a high pressure liquid coolant LCMS non-NMR are disclosed. Figure 7 the synthesis of compound according to one embodiment of the invention the present invention during 1 - - 1 - (4 - (benzyloxy) - 3, 6 - substituted - 2 - methoxyphenyl hereinafter disclosed agent) number 1 to ones result is confirmed with a high pressure liquid coolant LCMS non-NMR are disclosed. Figure 8 according to one embodiment of the invention the present invention during the synthesis of compound 1 - 1 - 1 - (4 - (benzyloxy) - 6 - hydroxy - 2, 3 - methoxyphenyl die) to result number is confirmed with a high pressure liquid coolant LCMS non-NMR ones are disclosed. Figure 9 according to one embodiment of the invention the present invention during the synthesis of compound (E)- 1 - (4 - (benzyloxy) - 6 - hydroxy - 2, 3 - die methoxyphenyl) - 2 - en - 1 - (3, 4 - die methoxyphenyl) - 3 - ones to be done by high pressure liquid coolant is confirmed with a number LCMS non-NMR results are disclosed. 5 - (Benzyloxy) - 2 - during synthesis of compound according to one embodiment of the invention the present invention Figure 10 a (3, 4 - die methoxyphenyl) - 6, 7 - dimethoxy - 4H - chromen - 4 - ones to result number is confirmed with a high pressure liquid coolant LCMS non-NMR are disclosed. Figure 11 according to one embodiment of the invention the present invention during the synthesis of compound 2 - (3, 4 - die methoxyphenyl) - 7 - hydroxy - 5, 6 - dimethoxy - 4H - chromen - 4 - ones to result number is confirmed with a high pressure liquid coolant LCMS non-NMR are disclosed. Figure 12 according to one embodiment of the invention the present invention during the synthesis of compound 2 - (3, 4 - die methoxyphenyl) - 5, 7 - substituted - 6 - methoxy - 4H - chromen - 4 - ones by LCMS-a NMR confirms the high pressure liquid coolant agent hereinafter disclosed result number are disclosed. Figure 13 according to one embodiment of the invention the present invention during the synthesis of compound 7 - (2 - bromo ethoxy) - 5 - hydroxy - 6 - methoxy - 2 - (3, 4 - die methoxyphenyl) - 4H - chromen - 4 - ones to result number is confirmed with a high pressure liquid coolant LCMS non-NMR are disclosed. Figure 14 according to one embodiment of the invention the present invention during the synthesis of compound 4 - (2 - ((2 - (3, 4 - die methoxyphenyl) - 5 - hydroxy - 6 - methoxy - 4 - oxo - 4H - chromen - 7 - yl) oxy) ethyl) piperazine - 2 - one (compounds of formula 2) to a high pressure liquid coolant is confirmed with a number LCMS non-NMR results are disclosed. Figure 15 liver astrocyte activation bulwark (Hepatic Stellate Cells, HSC) derived mesenchymal stem cell of the present invention compound (mesenchymal stem cell, MSC) for preparation of an aqueous dispersion effect in ONGHEPA 1 billion number are disclosed. Figure 16 ONGHEPA 1 (Epithelial Mesenchymal Transition, epithelial mesenchymal transition) of EMT in preparation of an aqueous dispersion of the representative marker for expression of the present invention effect of α-a SMA (alpha smoothe muscle actin) billion number are disclosed. Figure 17 isolated fibroblast cell lines (Diseased Human Lung Fibroblasts, DHLF) patient lung fibrosis of the present invention effect solubility number billion fibers are disclosed. Figure 18 of the present invention effect human A549 cancer sell master viscosity for fiber lines which are obsolete vessel number billion are disclosed. Figure 19 A549 cell line for expression of the present invention compounds of the representative marker Snail and Vimentin EMT in effect billion number are disclosed. Figure 20 is a metabolic stability evaluation result for the present invention compounds micro liposome are disclosed. Figure 21 of the present invention pharmacokinetic characteristics result by oral administration are disclosed. The present invention also includes a compound 22 to 24 comparison compounds used in these cells fiber irradiated result effect are disclosed. Number number Figure 25 the present invention compounds according to results of a comparison of pharmacokinetic properties are disclosed. The present invention refers to a novel compound or pharmaceutically acceptable salt thereof represented by formula 1 number substrate. [Formula 1] In formula said, R1 A substituted or unsubstituted C1 - 5 Linear or branched alkyl, C5 - 6 Cyclic alkyl, one or more heteroatoms O or N C atoms5 - 6 Cyclic alkyl, a substituted or unsubstituted C6 - 12 Aryl, or one or more heteroatoms O or N C atoms5 - 6 It will be biting and heteroatoms, R2 Is hydrogen, ethyl, acetyl, acetoxy, carboxyl, benzoyl oxy or 3, 4, 5 - tree , R3 To R5 Are each independently a hydrogen, hydroxyl, methyl, methoxy, acetoxy, carboxyl or benzoyl oxy among others. Novel compounds of the present invention or pharmaceutically acceptable salts can be used to prevent fibrosis, can thereby improving or treating. Of the present invention compound or its pharmaceutically acceptable salts (alpha smoothe muscle actin) α-a SMA, such as Snail and Vimentin EMT (Epithelial Mesenchymal Transition, epithelial mesenchymal transition) to a number of valuable factors involved in expression can number billion billion of an aqueous dispersion. To this effect the present invention compound or its pharmaceutically acceptable salts are the preparation of organs or tissues and cells generated by any causes fibrosis disease preventing, improving or treating can be overdimensioned. In addition, non-alcohol fat hepatitis (Nonalcoholic steatohepatitis, NASH) since the diseases also of hepatocytes fiber takes place, the present invention compound or its pharmaceutically acceptable salts in addition these fat alcohol can thereby improving or treating hepatitis. In particular, fibers of the present invention compound or its pharmaceutically acceptable salts are already programmed to the number of cells growth and fiberized strongly billion normal cells which can be regression, fibrosis these effects even switched to return the used for a normal state that the treatment of the present invention compound or its pharmaceutically acceptable salt thereof is extremely strong effect of fibrosis that supporting each other. Pharmaceutically acceptable salts of the present invention compound or different material be rapidly degraded by micro liposome is not continuous in vivo transitions to an inherent structure can be matte effect. In addition, phosphoric acid buffer commonly used in each of the number number and not a low solubility, CYP450 billion number activity for lower human body disclosed. Oral medication can be administered to rapidly absorb and ease which it also very good disclosed. Said fibers such as billion number effect, in vivo stability, human body of the present invention compound or its pharmaceutically acceptable salts such as to safety in said R1 Is methyl, ethyl, cyclopentyl, cyclohexyl, phenyl or benzyl which preferably, more preferably methyl Fresnels disclosed. In addition, R2 Is hydrogen and, R4 Is hydroxyl or maul [thok city and, R3 And R5 Are each independently a hydrogen, preferably hydroxyl or maul [thok poet. More preferably a compound having a formula 2 to 5 or a pharmaceutically acceptable salt thereof is either Fresnels disclosed. [Formula 2] [Formula 3] [Formula 4] [Formula 5] High pressure liquid coolant to said of the present invention compound is a compound such as method number 1 and 2 can be. [Compound 1] [Compound 2] In said reactive, R1 To R5 R of said formula 1 is1 To R5 Into the slide groove. The present invention refers to said compound or its pharmaceutically acceptable based on said effect such as part of a base containing a pharmaceutical composition for the prevention or treatment of fibrosis number substrate. the fibrosis such as idiopathic pulmonary fibrosis (idiopathic pulmonary fibrosis), bone marrow fibrosis (myelofibrosis), fibrosis (liver fibrosis) and selected from the group consisting of cost (kidney fibrosis) preferably. In addition the present invention refers to said compound or its pharmaceutically acceptable salts as the active ingredient a food containing low number containing fibrosis for preventing or ameliorating substrate. the fibrosis such as idiopathic pulmonary fibrosis, bone marrow fibrosis, fibrosis and preferably selected from the group consisting of cost. In addition the present invention refers to said compound or its pharmaceutically acceptable salts as the active ingredient containing pharmaceutical compositions for the treatment of nonalcoholic steatotic hepatitis (non non-alcoholic steatohepatitis, NASH) number substrate. In addition the present invention refers to said compound or its pharmaceutically acceptable salts as the active ingredient of composition containing an steatotic hepatitis food composition number substrate. Pharmaceutical compositions of the present invention compound or its pharmaceutically acceptable salts of the present invention itself, or pharmaceutically acceptable carrier mixed with sprayable compositions disclosed. The overall composition of the present invention pharmaceutical compositions of the present invention compound or its pharmaceutically acceptable salt to the weight of 0. 0001 To 100% by weight containing S. may be determined. Pharmaceutical compositions of the present invention when administered oral or parenteral administration of a clinical material which, when parenteral administration intraperitoneally scanning, scanning in colorectal cancer, hypodermic injection, intravenous injection, intramuscular injection, intrauterine epidural scanning, in the above described shortcomings in such as administration by injection or a thorax can be, may be used in form of a general pharmaceuticals number number to determine the other. Pharmaceutical compositions of the present invention alone, or surgical, radiation therapy, hormone therapy, chemotherapeutic and biological reaction control number and may be used in combination with using method to determine the other. The present invention pharmaceutical compositions of the present invention compound or its pharmaceutically acceptable composition containing daily dosage is based on the salts 1 kg weight between about 0. 0001 To 100 mg, preferably 0. 001 To 10 mg can have, but many times to 1 times per day can be administered into the body weight of patients, age, sex, health condition, dietary, administration time, administration method, excretion rate and disease severity range according to the variety of will. Under anger-number number number of clinical types of oral or parenteral administration can be, generally the filling number, a specific number, number coupled, wet number, number disintegrating, dilution number or excipients such as surfactants may number number number using tank are disclosed. A body capable of absorbing and in particular of the present invention compounds are administered orally double layers, the NMP (N-a Methyl-a 2 a-pyrrolidone), PEG400, SOLUTOL HS and water for preferably number number for the local network using the bioavailability and in vivo stability. Said NMP, PEG400, SOLUTOL HS and water ratio is 5 - 15:10 - 30:10 - 30: (v/v) is preferably 40 - 60, more preferably 8 - 12:15 - 25:15 - 25:45 - 55 (v/v) is now. Pharmaceutical compositions of the present invention compound or its pharmaceutically acceptable salts of the present invention further may contain at least one active principle 1 indicating a function same or similar it will rain. Food composition of the present invention compound or its pharmaceutically acceptable salts of the present invention itself, or food mixed with a cosmetically acceptable carrier sprayable compositions disclosed. The pharmaceutical compositions of the present invention compound or its pharmaceutically acceptable salts of content and the content of said reference dose according to a general method can be appropriately controlled. Food composition of the present invention meat workpiece, article number fish, bean curd, powder, dead, Chow mein if such as noodles, soy sauce, soybean paste, red pepper paste, such as seasoning food mixing field, source, confectionery, such as milk or cheese type manufactured goods, such as pickles or kimchi pickling, food, fruit, vegetables, soy milk, fermented beverage drink such as food form is pathogenic substrate. A cosmetically acceptable carrier said food in addition pharmaceutically acceptable carrier may also be employed S802. Hereinafter, the present invention to be incorporated in the embodiment and experiment more detailed the on-sensors other. In the embodiment for the present invention and to exemplify the embodiments these experiments only since, in the embodiment of the present invention these not be interpreted that the number range or empirical process by one. In the embodiment 1. The present invention compound number bath 1 - 1. 7 - (Benzyloxy) - 5 - hydroxy - 2 - phenyl - 4H - chromen - 4 - one (precursor compound 2) number of bath (Step-a 1) The embodied in the performing step of Figure 2 Step-a 1 generally described as follows. Acetone (700 ml) - 2 - phenyl - 4H - chromen - 4 - one (precursor compound 1) substituted 5, 7 - disclosed hereinafter to agent (75g; 0. 294Mol; 1equiv) and suspension, potassium carbonate (121. 8G; 0. 442Mol; 3. 0Equiv) and benzyl bromide (75. 5G; 0. 442Mol; 1. 5Equiv) 0 °C one in a drip-his knees. The reaction mixture was warmed to 60 °C 5 at ambient temperature and heating time. The reaction complete TLC (8:25) Confirmed to. Mixture cool down to the normal temperature filter then DCM (dichloromethane) filter to a stand-alone carbonic acid potassium number generated cake instead of a products until the free tea after filtering, the filtrate concentrated drying resulting solids die ethyl ether (200 ml) then to a cationizing, 7 - hydroxy - 2 - phenyl - 5 - (benzyloxy) of yellow solids filtration and suction drying - 4H - chromen - 4 - one (precursor compound 2) obtained a (the yield: 90. 0G; 88. 6%). 7 - (Benzyloxy) - 5 - hydroxy - 2 - phenyl - 4H - chromen - 4 - obtained on (precursor compound 2) according to LCMS non-NMR confirms the result of the next conditions also 4 such as disclosed. LCMS: Mass found; (345. 0; M + 1) A furnace: A - 0. 1% HCOOH in H2 O, B - ACN; Flow Rate - 1. 5 Ml/minutes; + ve mode Column: Zorbax extended C18 (50 x 4. 6 Mm, 5 μm) Rt (min): 3. 46; Area % - 97. 97 1 H-a NMR (400MHz, DMSO-a d6 ): Δ 12. 83 (S, 1H), 8. 10 - 8. 12 (M, 2H), 7. 62 - 7. 65 (M, 3H), 7. 51 - 7. 62 (M, 2H), 7. 43 - 7. 50 (M, 2H), 7. 38 - 7. 41 (M, 3H), 7. 06 (S, 1H), 6. 93 (S, 1H), 6. 51 (D, J=2. 40 Hz, 1H), 5. 27 (S, 2H). 1 - 2. 7 - Methoxy - 5 - (benzyloxy) - 2 - phenyl - 4H - chromen - 4 - one (precursor compound 3) number of bath (Step provided 2) The embodied in performing the step of Figure 2 Step-a 2 generally described as follows. Acetone (900 ml) - 5 - hydroxy - 2 - phenyl - 4H - chromen - 4 - (benzyloxy) to said 7 - on (precursor compound 2) (90g; 0. 261Mol; 1equiv) and suspended, KOH (43. 9G; 0. 784Mol; 3equiv) was added at room temperatures. The reaction mixture warmed to 60 °C die methyl sulfate (37. 1 Ml; 0. 392Mol; 1. 5Equiv) in a time-gate 60 °C drop addition of 60 °C then stirring in 5. The reaction complete TLC (1:12) To confirmed. A 10% HCl solution mixture cool down to the normal temperature and pH -2 precipitate by filtration then filter collecting degree by a user, after washing the water 7 - methoxy - 2 - phenyl - 5 - (benzyloxy) of yellow solids 12 suction drying time - 4H - chromen - 4 - one (precursor compound 3) obtained a (the yield: 90g; 96%). 7 - Methoxy - 5 - (benzyloxy) - 2 - phenyl - 4H - chromen - 4 - obtained on (precursor compound 3) according to LCMS non-NMR confirms the result of the next conditions also 5 such as disclosed. LCMS: Mass found; (359. 0; M + 1) A furnace: A - 0. 1% HCOOH in H2 O, B - ACN; Flow Rate - 1. 5 Ml/minutes; + ve mode Column: Zorbax extended C18 (50 x 4. 6 Mm, 5 μm) Rt (min): 2. 94; Area % - 97. 85 1 H-a NMR (400MHz, DMSO-a d6 ): Δ 8. 04 - 8. 06 (M, 2H), 7. 46 - 7. 58 (M, 5H), 7. 38 - 7. 44 (M, 3H), 7. 00 (D, J=2. 00 Hz, 1H), 6. 80 (D, J=1. 60 Hz, 1H), 6. 62 (D, J=2. 00 Hz, 1H), 5. 27 (S, 2H), 3. 83 (S, 3H). 1 - 3. 1 - (4 - (Benzyloxy) - 6 - hydroxy - 2 - methoxyphenyl) ethane - 1 - on (precursor compound 4) number of bath (Step-a 3) The performing step of Figure 2 Step-a 3 embodied in the generally described as follows. Aqueous sodium hydroxide (50%; 686 ml; 8. 79Mol; 35equiv) - 5 - methoxy - 2 - phenyl - 4H - chromen - 4 - (benzyloxy) to said 7 - on (precursor compound 3) (90g; 0. 251Mol; 1equiv) and suspended, pyridine (417. 1 Ml; 5. 02Mol; 20equiv) was added at ambient temperature. A mixture of dense brown strongly stirring die ethylene glycol (475 ml, 5. 02Mol, 20equiv) then a drop addition of 100 °C 2 meat and mixing and heating the mixture-gate. The reaction complete TLC (1:15) Confirmed to. 0 °C 12N hydrochloric acid aqueous solution to pH 1 by a user and a cool mixture then ethyl acetate (2 x 500 ml) extracted from the to. Saturated sodium bicarbonate aqueous solution extracted organic phase, and his knife of cleaning solution. Sodium sulfate drying, solvent pressure number been stand-alone. The resulting residue die ethyl ether (700 ml) re-dissolving, stand-alone do not dissolve with a cushion cancerous color number was. The filtrate is concentrated yellow solids wall vacuum of 1 - (4 - (benzyloxy) - 6 - hydroxy - 2 - methoxyphenyl) - 1 - 1 on a (precursor compound 4) obtained (the yield: 70g; 97%). The resulting 1 - (4 - (benzyloxy) - 6 - hydroxy - 2 - methoxyphenyl) ethane - 1 - on (precursor compound 4) according to LCMS non-NMR confirms the result of the next conditions also 6 such as disclosed. LCMS: Mass found; (273. 0; M + 1) A furnace: A - 0. 1% HCOOH in H2 O, B - ACN; Flow Rate - 1. 5 Ml/minutes; + ve mode Column: Zorbax extended C18 (50 x 4. 6 Mm, 5 μm) Rt (min): 3. 15; Area % - 93. 79 1 H-a NMR (400MHz, DMSO-a d6 ): Δ 13. 77 (S, 1H), 7. 35 - 7. 47 (M, 5H), 6. 18 - 6. 21 (D, 2H), 5. 18 (S, 2H), 3. 86 (S, 3H), 2. 51 (S, 3H). 1 - 4. 1 - (4 - (Benzyloxy) - 3, 6 -Disclosed hereinafter agent window- 2 -Methoxyphenyl) Ethane - 1 - on (5 Precursor compound) Number of bath (Step-a 4) The performing step of Figure 2 Step-a 4 to specifically described as follows. Tetra ethyl ammonium hydroxide aqueous solution (35%; 632 ml; 1. 43Mol; 13equiv) to said 1 - (4 - (benzyloxy) - 6 - hydroxy - 2 - methoxyphenyl) ethane - 1 - on (precursor compound 4) (30g; 0. 110Mol; 1equiv) and suspended, pyridine (69. 4 Ml; 0. 836Mol; 7. 6Equiv) at ambient temperature was added drop. The reaction mixture an X- solutions other. A separate cap water (1 l) to potassium it ladles, opinion pay [thu (50. 49G; 0. 187Mol; 1. 7Equiv) is added, the solutions are then seasoned with meat and mixing said aqueous reaction mixture drop-gate 24. After confirming the disappearance of material be made starting with TLC, the reaction mixture to pH 1 - 2 by adding HCl 0 °C said in a been by a user. The resulting brown viscous residue is passed through a filter, the filtrate die ethyl ether (1 x 100 ml) was washed. Separated aqueous phase sodium sulfite (11. 09G; 0. 0. 088Mol; 0. 8Equiv), HCl (110 ml) and benzene (220 ml) during delivering, the reaction mixture was heated to 95 °C 1 time. The reaction complete TLC (8:23) Confirmed to. 0 °C and cool reaction mixture into ethyl acetate (2 x 300 ml) extracted from the to. An organic phase washed and sodium sulfate drying solvent pressure of the knife of a stand-alone solution then been number. Residues silica gel (60 provided 120mesh) applying column chromatography ethyl acetate (10 - 12%) of yellow solids added intra-eluting with petroleum ether is 1 - (4 - (benzyloxy) - 3, 6 - substituted - 2 - methoxyphenyl hereinafter disclosed agent) ethane - 1 - on (precursor compound 5) obtained a (the yield: 10g; 31%). The resulting 1 - (4 - (benzyloxy) - 3, 6 - substituted - 2 - methoxyphenyl hereinafter disclosed agent) ethane - 1 - on (precursor compound 5) according to LCMS non-NMR confirms the result of the next conditions also 7 such as disclosed. LCMS: Mass found; (289. 0; M + 1) A furnace: A - 0. 1% HCOOH in H2 O, B - ACN; Flow Rate - 1. 5 Ml/minutes; + ve mode Column: Zorbax extended C18 (50 x 4. 6 Mm, 5 μm) Rt (min): 2. 68; Area % - 91. 01 1 H-a NMR (400MHz, DMSO-a d6 ): Δ 12. 76 (S, 1H), 8. 37 (S, 1H), 7. 41 - 7. 51 (M, 2H), 7. 31 - 7. 38 (M, 3H), 6. 38 (S, 1H), 5. 21 (S, 2H), 3. 84 (S, 3H), 2. 51 (S, 3H). 1 - 5. 1 - (4 - (Benzyloxy) - 6 - hydroxy - 2, 3 - die methoxyphenyl) ethane - 1 - on (precursor compound 6) number of bath (Step provided 5) The embodied in performing the step of Figure 2 Step-a 5 generally described as follows. Acetone (300 ml) said 1 - to - 1 - (4 - (benzyloxy) - 3, 6 - substituted - 2 - methoxyphenyl hereinafter disclosed agent) on (precursor compound 5) ethane (28g; 0. 097Mol; 1equiv) and suspended, K2 CO3 (20G; 0. 145Mol; 1. 5Equiv) was added at room temperatures. The reaction mixture warmed to 60 °C die methyl sulfate (18. 2 Ml; 0. 145Mol; 2equiv) in a time-gate 5 to 60 °C then drop 60 °C seasoned with stirring. Reaction completion is confirmed with a TLC a mixture followed by cool down to the normal temperature and filter K2 CO3 The DCM cake after filtering a number then tea into a stand-alone, the filtrate concentrated generated then is dried to residue silica gel (60 provided 120mesh) applying column chromatography ethyl acetate (8 - 10%) of white solids added intra-eluting with petroleum ether is 1 - (4 - (benzyloxy) - 6 - hydroxy - 2, 3 - die methoxyphenyl) ethane - 1 - on (precursor compound 6) obtained a (the yield: 25g; 85%). The resulting 1 - (4 - (benzyloxy) - 6 - hydroxy - 2, 3 - die methoxyphenyl) ethane - 1 - on (precursor compound 6) according to LCMS non-NMR confirms the result of the next conditions also 8 such as disclosed. LCMS: Mass found; (303. 0; M + 1) A furnace: A - 0. 1% HCOOH in H2 O, B - ACN; Flow Rate - 1. 5 Ml/minutes; + ve mode Column: Zorbax extended C18 (50 x 4. 6 Mm, 5 μm) Rt (min): 3. 11; Area % - 99. 85 1 H-a NMR (400MHz, DMSO-a d6 ): Δ 12. 95 (S, 1H), 7. 36 - 7. 48 (M, 5H), 6. 45 (S, 1H), 5. 20 (S, 2H), 3. 96 (S, 3H), 3. 92 (S, 3H), 2. 51 (S, 3H). 1 - 6. (E)- 1 - (4 - (benzyloxy) - 6 - hydroxy - 2, 3 - die methoxyphenyl) - 2 - en - 1 - (3, 4 - die methoxyphenyl) - 3 - on (precursor compound 7) indicating the number of bath (Step provided 6) The embodied in the performing step of Figure 2 Step provided 6 generally described as follows. 3, 4 - Die the maul [thok city it cuts the [cu the high [tu which it knows (16. 4G; 0. 099Mol; 1. 2Equiv) added ethanol (200 ml) to said 1 - (4 - (benzyloxy) - 6 - hydroxy - 2, 3 - die methoxyphenyl) ethane - 1 - on (precursor compound 6) (25g; 0. 082Mol; 1equiv) and suspended, KOH (46g; 0. 0. 82Mol; 1equiv) then adding aqueous solution at ambient temperature, the reaction mixture is stirred at room temperature 24 time-gate. TLC (6:4/PE: EtOAc; Rf - 0. 4) Formed product of 70 - 75% to formate, starting materials are non-reactive distance 24 even after his time remaining. Mixture was vacuum concentrated aqueous sodium hydrogen sulfate residues and DCM to seeds. Knife of separated organic phase was washed and sodium sulfate solution and drying. Vacuum concentration generated by residues die ethyl ether (100 ml) and the filtration and suction drying of yellow solids can adopt - 1 - (E)- 3 - (4 - (benzyloxy) - 6 - hydroxy - 2, 3 - die methoxyphenyl) - 2 - en - 1 - (3, 4 - methoxyphenyl die) to be done on (precursor compound 7) obtained (the yield: 23. 0G; 39%). Obtained (E)- 1 - (4 - (benzyloxy) - 6 - hydroxy - 2, 3 - die methoxyphenyl) - 2 - en - 1 - (3, 4 - die methoxyphenyl) - 3 - on (precursor compound 7) indicating the next condition according to LCMS non-NMR confirms the result of the 9 also such as disclosed. LCMS: Mass found; (450. 9; M + 1) A furnace: A - 0. 1% HCOOH in H2 O, B - ACN; Flow Rate?? 1. 5 Ml/minutes; + ve mode Column: Zorbax extended C18 (50 x 4. 6 Mm, 5 μm) Rt (min): 3. 34; Area % - 98. 16 1 H-a NMR (400MHz, DMSO-a d6 ): Δ 11. 96 (S, 1H), 7. 49 - 7. 57 (M, 3H), 7. 49 - 7. 57 (M, 4H), 7. 29 - 7. 39 (M, 2H), 7. 03 (D, J=8. 40 Hz, 1H), 6. 47 (S, 1H), 5. 19 (S, 2H), 3. 85 (S, 3H), 3. 83 (S, 3H), 3. 77 (S, 3H), 3. 74 (S, 3H). 1 - 7. 5 - (Benzyloxy) - 2 - (3, 4 - die methoxyphenyl) - 6, 7 - dimethoxy - 4H - chromen - 4 - one (precursor compound 8) number of bath (Step provided 7) The performing step of Figure 3 Step-a 7 to specifically described as follows. ISO amyl alcohol (300 ml) to said (E)- 1 - (4 - (benzyloxy) - 6 - hydroxy - 2, 3 - die methoxyphenyl) - 2 - en - 1 - (3, 4 - die methoxyphenyl) - 3 - on (precursor compound 7) indicating (21g; 0. 0466Mol; 1equiv) and suspended, selenium dioxide (21g; 0. 466Mol; 10equiv) then added at ambient temperature, and heating the meat and mixing-gate 140 °C 7. TLC (4:6/PE: EtOAc; Rf - 0. 2) After confirming completion of the reaction, mixture cool down to the normal temperature resulting particles number 2000 light on the pad was contained in a stand-alone. The filtrate was concentrated in vacuum wall DCM pad. The resulting residue DCM (500 ml) 10 to NaHCO3 Aqueous solution, and then drying a solvent cleaning knife of a stand-alone tank and the sodium sulfate solution was number. Residues silica gel (60 provided 120mesh) applying column chromatography ethyl acetate (50 - 60%) of 5 - (benzyloxy) - 2 - eluting with petroleum ether added to intra-yellow solids (3, 4 - methoxyphenyl die) a - 6, 7 - dimethoxy - 4H - chromen - 4 - one (precursor compound 8) obtained (the yield: 16g; 76%). 5 - (Benzyloxy) - 2 - obtained (3, 4 - die methoxyphenyl) - 6, 7 - dimethoxy - 4H - chromen - 4 - one (precursor compound 8) according to LCMS non-NMR confirms the result of the next conditions also 10 such as disclosed. LCMS: Mass found; (449. 0; M + 1) A furnace: A - 0. 1% HCOOH in H2 O, B - ACN; Flow Rate - 1. 5 Ml/minutes; + ve mode Column: Zorbax extended C18 (50 x 4. 6 Mm, 5 μm) Rt (min): 2. 83; Area % - 98. 62 1 H-a NMR (400MHz, DMSO-a d6 ): Δ 7. 54 (D, J=8. 40 Hz, 1H), 7. 45 (T, J=7. 60 Hz, 2H), 7. 37 (D, J=8. 40 Hz, 2H), 7. 13 (D, J=8. 80 Hz, 2H), 6. 82 (S, 1H), 5. 30 (S, 2H), 3. 89 (S, 3H), 3. 85 (S, 3H), 3. 82 (S, 3H), 3. 77 (S, 3H). 1 - 8. 2 - (3, 4 - Die methoxyphenyl) - 7 - hydroxy - 5, 6 - dimethoxy - 4H - chromen - 4 - one (precursor compound 9) number of bath (Step provided 8) The performing step of Figure 3 Step-a 8 embodied in the generally described as follows. Chloroform (200 ml) to said 5 - (benzyloxy) - 2 - (3, 4 - die methoxyphenyl) - 6, 7 - dimethoxy - 4H - chromen - 4 - one (precursor compound 8) (19g; 0. 0424Mol; 1 equiv) and suspension, 10% Pd/C (palladium on carbon) (3. 8G) for isolating the reactants in hydrogen balloon at ambient temperature for 6 - 7 stored in the waiting time. TLC after confirming completion of the reaction, catalyst was 2000 light pad filtering. 20% MeOH in DCM and the filtrate is concentrated under vaccum pad added tea obtained scrapers dark brown. Solids ethyl acetate (80 ml) and subjected to, filtration and suction is dried to form a yellow solid of 2 - (3, 4 - die methoxyphenyl) - 7 - hydroxy - 5, 6 - dimethoxy - 4H - chromen - 4 - one (precursor compound 9) obtained a (the yield: 11. 0G; 72%). Obtained 2 - (3, 4 - die methoxyphenyl) - 7 - hydroxy - 5, 6 - dimethoxy - 4H - chromen - 4 - one (precursor compound 9) according to LCMS non-NMR confirms the result of the next conditions also 11 such as disclosed. LCMS: Mass found; (359. 0; M + 1) A furnace: A - 0. 1% HCOOH in H2 O, B - ACN; Flow Rate - 1. 5 Ml/min; + ve mode Column: Zorbax extended C18 (50 x 4. 6 Mm, 5 μm) Rt (min): 2. 07; Area % - 99. 63 1 H-a NMR (400MHz, DMSO-a d6 ): Δ 10. 69 (S, 1H), 7. 61 (D, J=8. 40 Hz, 1H), 7. 51 (S, 1H), 7. 11 (D, J=8. 40 Hz, 1H), 6. 90 (S, 1H), 6. 74 (S, 1H), 3. 87 (S, 3H), 3. 84 (S, 3H), 3. 80 (S, 3H), 3. 77 (S, 3H). 1 - 9. 2 - (3, 4 - Die methoxyphenyl) - 5, 7 - substituted - 6 - methoxy - 4H - chromen - 4 - one (precursor compound 10) number of agent disclosed hereinafter (Step provided 9) bath The embodied in performing the step of Figure 3 Step provided 9 generally described as follows. Acetonitrile (100 ml) to said 2 - (3, 4 - die methoxyphenyl) - 7 - hydroxy - 5, 6 - dimethoxy - 4H - chromen - 4 - one (precursor compound 9) (11g; 0. 0307Mol; 1 equiv) and suspended, AlCl3 (20. 3G; 0. 153Mol; 5equiv) then added a little at a time at ambient temperature with respect to the mixture in 2 hours reflux 90 °C. TLC confirms that the reaction completion for vaporizing the solvent with respect to the drying. The resulting residue HCL aqueous solution (10%; 200 ml) and chloroform (200 ml) during delivering, for recirculating a clear reaction mixture has been sensed. TLC (7:3/PE: EtOAc; Rf - 0. 4) The reaction completion was separating an organic phase and cool down to the normal temperature after confirming the reaction mixture. Organic phase and aqueous phase extracted with DCM again and then drying was knife of sodium sulfate and concentrated cleaning solution. Residues silica gel (60 provided 120mesh) column chromatography eluting with DCM to applying for dissolving solid state yellow 2 - (3, 4 - die methoxyphenyl) - 5, 7 - substituted - 6 - methoxy - 4H - chromen - 4 - one (precursor compound 10) agent hereinafter disclosed a obtained (the yield: 7. 0G; 66%). Obtained 2 - (3, 4 - die methoxyphenyl) - 6 - methoxy - 4H - chromen - 4 - substituted - 5, 7 - disclosed hereinafter agent is confirmed with a result of the next conditions according to LCMS non-NMR(precursor compound 10) on 12 also such as disclosed. LCMS: Mass found; (344. 9; M + 1) A furnace: A - 0. 1% HCOOH in H2 O, B - ACN; Flow Rate - 1. 5 Ml/minutes; + ve mode Column: Zorbax extended C18 (50 x 4. 6 Mm, 5 μm) Rt (min): 2. 44; Area % - 98. 32 HPLC: 97. 64% A furnace: A - 0. 1% TFA in H2 O, B - ACN; Flow Rate - 1. 0 Ml/minutes Column: Atlatis dC provided 18 (4. 6 X 250) mm; 5u; Rt (min): 12. 68; Area % - 97. 64. 1 H-a NMR (400MHz, DMSO-a d6 ): Δ 13. 05 (S, 1H), 10. 73 (S, 1H), 7. 68 - 7. 71 (M, 1H), 7. 58 (D, J=2. 04 Hz, 1H), 7. 14 (D, J=8. 64 Hz, 1H), 6. 99 (S, 1H), 6. 65 (S, 1H), 3. 89 (S, 3H), 3. 86 (S, 3H), 3. 76 (S, 3H). 13 C non-NMR(100MHz, DMSO-a d6 ): Δ 182. 6, 163. 8, 157. 8, 153. 1, 152. 8, 152. 5, 149. 4, 131. 8, 123. 4, 120. 4, 112. 1, 109. 9, 104. 5, 103. 8, 94. 8, 60. 4, 56. 3, 56. 2. 1 - 10. 7 - (2 - Bromo ethoxy) - 5 - hydroxy - 6 - methoxy - 2 - (3, 4 - die methoxyphenyl) - 4H - chromen - 4 - one (precursor compound 11) number of bath (Step-a 10) The embodied in performing the step of Figure 3 Step provided 10 generally described as follows. THF (tetrahydrofuran) (400 ml, 40 vol.) to said 2 - (3, 4 - die methoxyphenyl) - 5, 7 - substituted - 6 - methoxy - 4H - chromen - 4 - one (precursor compound 10) hereinafter disclosed agent (10g, 29mmol, 1 equiv.) and potassium carbonate (12. 04G, 87. 1Mmol, 3. 0 equiv.) stirring mixing, 1, 2 - ethane with wool dive (27. 28G, 145. 2Mol, 5 equiv.) and TBAI (tetrabutylammonium iodide) (1. 05G, 0. 0029Mol, 0. 1 equiv.) then the resulting mixture is added with respect to the 16 hours reflux in 60 °C. TLC after confirming a pressure reaction completion number obtained C5 THF in a stand-alone. Washed with an excess of solids to solids THF and methanol of 7 - (2 - bromo ethoxy) - 5 - hydroxy - 6 - methoxy - 2 - (3, 4 - die methoxyphenyl) - 4H - chromen - 4 - one (precursor compound 11) obtained a (the yield: 8g; 61%). Was used in the next step without after a separate additional positive number. Obtained 7 - (2 - bromo ethoxy) - 5 - hydroxy - 6 - methoxy - 2 - (3, 4 - die methoxyphenyl) - 4H - chromen - 4 - one (precursor compound 11) according to LCMS non-NMR confirms the result of the next conditions also 13 such as disclosed. LCMS: Mass found; (451. 0; M + 1) A furnace: A - 0. 1% HCOOH in H2 O, B - ACN; Flow Rate - 1. 5 Ml/minutes; + ve mode Column: Atlantis dC18 (50 x 4. 6 Mm, 5 μm) Rt (min): 3. 14; Area % - 80. 41 1 H-a NMR (400MHz, DMSO-a d6 ): Δ 12. 91 (S, 1H), 7. 73 (Dd, J=5. 6, 2. 0 Hz, 1H), 7. 60 (D, J=2. 0 Hz, 1H), 7. 15 (D, J=8. 4 Hz, 1H), 7. 06 (S, 1H), 7. 02 (S, 1H), 4. 51 (T, J=5. 2 Hz, 2H), 3. 91 (S, 3H), 3. 85 (S, 3H), 3. 79 (S, 3H). 1 - 11. 4 - (2 - ((2 - (3, 4 - Die methoxyphenyl) - 5 - hydroxy - 6 - methoxy - 4 - oxo - 4H - chromen - 7 - yl) oxy) ethyl) piperazine - 2 - one (compound 1) (Step provided 11) number of bath The embodied in performing the step of Figure 3 Step provided 11 generally described as follows. Acetonitrile (80 ml) to said 7 - (2 - bromo ethoxy) - 5 - hydroxy - 6 - methoxy - 2 - (3, 4 - die methoxyphenyl) - 4H - chromen - 4 - one (precursor compound 11) (8g, 17. 7Mmol, 1. 0 equiv.) and piperazine - 2 - one (5. 33G, 53. 3Mmol, 3. 0 equiv.) added, with respect to the 12 hours reflux in nitrogen atmosphere. After confirming completion of the reaction TLC pressure been in process for a stand-alone number. Generating a viscous petroleum ether to obtain powdered C5 and DCM, methanol, THF and DCM continuous cleaning of yellow solids interlocks and dried to 4 - (2 - ((2 - (3, 4 - die methoxyphenyl) - 5 - hydroxy - 6 - methoxy - 4 - oxo - 4H - chromen - 7 - yl) oxy) ethyl) piperazine - 2 - one (compound 1) (formula 2) obtained a (the yield: 3. 2G; 41%). Obtained 4 - (2 - ((2 - (3, 4 - die methoxyphenyl) - 5 - hydroxy - 6 - methoxy - 4 - oxo - 4H - chromen - 7 - yl) oxy) ethyl) piperazine - 2 - one (compound 1) is confirmed with a result of the next conditions according to LCMS non-NMR also 14 such as disclosed. LCMS: Mass found (471. 2; M + 1) A furnace: A - 0. 1% Formic Acid in H2 O; B - ACN Column: Atlantis dC18 (50 x 4. 6 Mm, 5 μm) Rt (min): 1. 515; Area % - 95. 488 HPLC: 97. 03% A furnace: A - 0. 1% TFA in H2 O; B - Acetonitrile Column: Atlantis dC18 (50 x 4. 6 Mm, 5 μm) Rt (min): 9. 74; Area % - 97. 03 1 H NMR (400MHz, DMSO-a d6 ): Δ 12. 89 (S, 1H), 7. 75 (Br s. 1H), 7. 70 (Dd, J=8. 0, 1. 6 Hz, 1H), 7. 58 (S, 1H), 7. 13 (D, J=8. 5 Hz, 1H), 7. 02 (D, J=10. 4 Hz, 2H), 4. 28 (T, J=5. 2 Hz, 2H), 3. 89 (S, 3H), 3. 86 (S, 3H), 3. 75 (S, 3H), 3. 17 (Br s, 2H), 3. 11 (S, 2H), 2. 87 (T, J=5. 2 Hz, 2H), 2. 73 (T, J=5. 2 Hz, 2H). 13 C non-NMR(100MHz, CDCl3 ): 182. 4, 169. 2, 163. 9, 157. 5, 153. 1, 152. 9, 152. 2, 149. 1, 132. 7, 123. 4, 119. 9, 111. 0, 108. 6, 106. 1, 104. 1, 91. 4, 67. 1, 60. 7, 57. 0, 56. 0, 55. 9, 55. 5, 49. 4, 41. 1 Experiment example 1. The present invention compounds for their effectiveness against irradiation fibrosis 1 - 1. Mesenchymal stem cell applied to effect experiments The present invention compounds for irradiating the effect of fibrosis, liver astrocyte activation (Hepatic Stellate Cells, HSC) derived from first bulwark of RAT mesenchymal stem cells capable of proliferating endless ONGHEPA 1 (KCTC13086BP) (mesenchymal stem cell, MSC) using the air in the cell. The cell lines TGF-a β (Transforming growth factor beta) or PDGF (Platelet non-derived growth factor) can be derived fiber of a simple processing method. ONGHEPA 1 cells seeded onto a medium (seeding) 24 and then processing time culturing TGF-a β (5 ng/Ml) on compounds of the present invention induces the fiber or TGF-a β (50 μm) and 24 hours after culturing can be processed simultaneously, the degree of fiber, i.e. as the differentiation to the fiber (myofibroblast) degree phase difference microscope (magnification 200) was irradiated. The specific testing method of the Kim papers (Han non-Soo Kim, Jun-a Hwan Kim, Ji Yong Lee, Young-a Min Yoon, Ik non-Hwan Kim, Ho-a Sup Yoon, Byung-a Soo Youn. Small molecule-a mediated reprogramming of epithelial non-mesenchymal transition thereby blocking fibrosis. BioRxiv preprint first posted online Feb. 16, 2017; Doi: http://dx. Doi. Org/10. 1101/106591.) To dried. Utilization, such as processing the controls are also 15 TGF a-β fibers while indicating the typical supply unit supplies fiber differentiation conditions, compounds of the present invention treated experimental group together normal controls differs it cannot fibers without symptoms as in the picomolar. In addition, such as epithelial mesenchymal transition (Epithelial Mesenchymal Transition, EMT) (hereinafter 'EMT' being shrewd, to) said is due in order to identify whether the influence on, each experimental group cells subject to EMT representative marker expression of α-a SMA (alpha smoothe muscle actin) immune fluorescent staining and nuclear dyeing (DAPI dyeing) obtained by dividing a visit from the police. Utilization, such as TGF-a β also 16 are processed controls are significantly higher expression of α-a SMA can be carried out while, normal processing of the present invention compound together differs regulated expression of experimental group that it cannot α-a SMA is inhibited as in the picomolar. These results indicate that compounds of the present invention strongly billion cells of subjects which effected on the EMT fiber number, the fibrosis, in particular which occur among different liver fibrosis or effective in preventing or treating nonalcoholic steatotic hepatitis (non non-alcoholic steatohepatitis, NASH) are not easily supporting each other. 1 - 2. Lung fibrosis patient fibroblast cell lines applied to effect experiments Isolated fibroblast cell lines (Diseased Human Lung Fibroblasts, DHLF) patient lung fibrosis (Lonza yarn, Swiss) inoculating the culture contains 24 and then processing time TGF-a β (5 ng/Ml) on compounds of the present invention induces the fiber or TGF-a β (50 μm) and 24, 48 or 72 hours after culturing can be processed simultaneously, fiber cells differentiation to the degree phase difference microscope (magnification 200) was irradiated. Utilization, such as RIE TGF-a β-group and 17 also are processed fiber differentiation to supply unit supplies controls are typical of an indicating conditions while, processing of the present invention compound together nearly as well as cell growth experiment group number billion in addition the symptoms of an aqueous dispersion as in the picomolar. These results indicate that compounds of the present invention already fiberized and fiberized programmed to a number of fibroblast growth of the transformed cell is captured as the strongly billion, fibrosis, such as idiopathic pulmonary fibrosis in particular is effective in preventing or treating fibrosis such as lungs are not easily supporting each other. 1 - 3. Cancer sell week obsolete vessel target effect experiments Human A549 cancer sell master contains obsolete vessel used model of lung EMT related studies which reduces processing time 24 then TGF-a β cell culture (5 ng/Ml) on or TGF-a β of the present invention compound (25 or 50 μm) and 24 or 48 hours after culturing can be processed simultaneously, cells phase difference microscope (magnification 200) was irradiated. Utilization, such as TGF-a β also 18 a supply unit supplies when it has processed fiber differentiation whereas, processing of the present invention compounds together is the experimental group phenomenon as in the picomolar billion number. In addition, such as whether the influence is effected EMT due in order to identify, each experimental group cells subject to EMT representative marker has been confirmed that the expression of aspects to Snail and Vimentin Real provided Time PCR. Utilization, such as 19 also are processed using an expression of a marker these controls are TGF-a β while, compounds of the present invention treating the experimental group significantly billion number together these of expression of biological markers as in the picomolar. These results indicate that compounds of the present invention strongly billion cells of subjects which effected on the EMT fiber number, the fibrosis, such as idiopathic pulmonary fibrosis in particular is effective in preventing or treating fibrosis such as lungs are not easily supporting each other. Experiment example 2. The present invention compound pharmacokinetic characterizing 2 - 1. Metabolic stability Compounds of the present invention is micro cotton of RAT (microsome) culturing the rewrite ratio with NADPH and then compounds the compounds integral cleaning rate (intrinsic clearance value, CLint value) was calculated. The matching group verapamil (verapamil) and oh reel glow this year (atenolol) was used. A jacket of the present invention compounds are metabolic utilization can be compared as in the picomolar (table 1 and also 20 reference) stable. 2 - 2. Buffer soluble Of the present invention compound phosphate buffer (phosphate buffer, pH 7. 4) At a concentration of 1 mg/Ml to 16 by adding the following agitated sample dissolves to analysis by HPLC-a UV filter after filtering dissolved content of and decided. The matching group (diethyl stilbesterol) caffeine (caffeine) and die ethyl steel hemp cloth [su reel roll was used. The present invention compounds soluble buffer utilization is high in comparison with steel die but is lower than the caffeine into foods (table 2 reference) ethyl. 2 - 3. CYP450 whether billion number CYP3A 4 compounds of the present invention, number for a visit from the police whether CYP2D 6 and CYP2C 9 billion. The controls to ketoconazole (ketoconazole), epilepsy and patrol lung dextromethorphan DIN (quinidine) (sulfaphenazole) was used. CYP2C 9 billion of utilization of the present invention compounds are stage number activity appears to be but relatively to low contrast of the troop sulfanyl number active billion, billion number active for CYP3A 4 CYP2D 6 and very low or no into foods (table 3 reference). * Na: not activity 2 - 4. Oral dosage by pharmacokinetic properties Mouse model for identifying pharmacokinetic properties of the compounds according administered orally using the air. Male SD by weight 250 - 300 g of mouse embryo arranged 3 per group, compounds of the present invention when administered orally to 200 mg/kg (body weight) then, plasma (plasma) elapsed time measuring the amount of a compound according to the present method by water. The oral administration of 0. 5% CMC and 1% Tween provided 80 state number elder brother anger room temperature for 24 hours. Utilization of the present invention compounds are oral dosage by which it can be rapidly absorb as in the picomolar (table 4 and also 21 reference). Average ± SD (n=3 MICE/group)* Comparing experimental example 1. Comparison of the effect of irradiation fibrosis The same method example experiment 1 - 1 said fibrosis next comparison compounds for their effectiveness against a visit from the police. Utilization, such as 22 to 24 of the present invention comparing compounds of the formula also exhibits cytotoxicity or leads to apoptosis of progress can prevent ETEC is preparation of an aqueous dispersion. Experiment example 3. According to pharmacokinetic properties of number number According to the method according to the present invention compounds effective number number number number of various oral dosage pharmacokinetic properties such as for example 2 - 4 said experiment method according to visit from the police. 5 Utilization table such as, Tween 80:0. 5% CMC aqueous solution (1:99 v/v) when using number number under anger (example number number 2) compound 1 bioavailability. 4% To low and, NMP: Ethanol: PEG200: Normal saline (5:10: 30:55 v/v) when the number number under anger (example number number 4) up to about 3% bioavailability. While, NMP: PEG400: SOLUTOL HS: water (10:20: 20:50 v/v) under anger to number number (example number number 3) when 10% bioavailability forth significantly improved ETEC. Example 1 - i * number number. V. 100% DMSO vehicle use Example 2 - Tween 80:0 * number number. 5% CMC aqueous solution (1:99 v/v) use Example 3 - NMP * number number: PEG400: SOLUTOL HS: water (10:20: 20:50 v/v) use Example 4 - NMP * number number: Ethanol: PEG200: Normal saline (5:10: 30:55 v/v) use In addition, in the case of example 3 compared to example 2 number number number number such as 25 also when, by administering compounds as well as the oral which it decays rapidly absorb on long plasma can be processed into a sustained effect is not removed and held as in the picomolar. Other method number number of attempted but even out, door number is not completely dissolve most compounds which make solution further pharmacokinetic study been number. The present invention relates to a novel compound and a composition for prevention, improvement, or treatment of fibrosis or nonalcoholic steatohepatitis comprising the same as an active ingredient. More specifically, the present invention relates to a novel compound of chemical formula 1 which has an excellent effect for prevention, improvement, or treatment of fibrosis and a composition for prevention, improvement, or treatment of fibrosis or nonalcoholic steatohepatitis comprising the same as an active ingredient. A novel compound of the present invention effectively controls expression of snail and vimentin which are a controlling element of Epithelial Mesenchymal Transition (EMT) and controls activation of EMT, and thus effectively prevents, improves, or treats fibrosis accordingly. Additionally, a novel compound of the present invention has a very excellent pharmacokinetic characteristic, and thus can perform fast drug delivery to the body through oral administration, stably displays an effect in the body, and is secure to use without a big side effect. Moreover, since a novel compound of the present invention can effectively block fibrosis of a hepatic cell, nonalcoholic steatohepatitis can effectively improved or treated. COPYRIGHT KIPO 2018 For compounds of formula 1 or a pharmaceutically acceptable salt thereof: [formula 1] In formula said, R1 Is methyl, ethyl, cyclopentyl, cyclohexyl, phenyl or benzyl and, R2 Is hydrogen, ethyl, acetyl, acetoxy, carboxyl, benzoyl oxy or 3, 4, 5 - tree , R3 To R5 Are each independently a hydrogen, hydroxyl, methyl, methoxy, acetoxy, carboxyl or benzoyl oxy among others. Back number According to Claim 1, R1 Is methyl and, R2 Is hydrogen and, R4 Which is hydroxyl or maul [thok city, R3 And R5 Are each independently a hydrogen, hydroxyl or maul [thok poet compounds or pharmaceutically acceptable salts characterized. According to Claim 1, represented by formula 2 to 5 said second gate either characterized compound or its pharmaceutically acceptable salts. [Formula 2] [Formula 3] [Formula 4] [Formula 5] Anti number 1, number 3 term and terms compound or its pharmaceutically acceptable salts either anti number 4 active ingredient for prevention or treatment of fibrosis containing pharmaceutical compositions. According to Claim 5, said fibrosis such as idiopathic pulmonary fibrosis (idiopathic pulmonary fibrosis), bone marrow fibrosis (myelofibrosis), fibrosis (liver fibrosis) and selected from the group consisting of cost (kidney fibrosis) characterized composition. Anti number 1, number 3 term and terms compound or its pharmaceutically acceptable salts either anti number 4 active ingredient containing food composition for preventing or ameliorating fibrosis. According to Claim 7, said fibrosis such as idiopathic pulmonary fibrosis (idiopathic pulmonary fibrosis), bone marrow fibrosis (myelofibrosis), fibrosis (liver fibrosis) and selected from the group consisting of cost (kidney fibrosis) characterized composition. Anti number 1, number 3 term and terms compound or its pharmaceutically acceptable salts either anti number 4 active ingredient containing pharmaceutical compositions for the treatment of nonalcoholic steatotic hepatitis (non non-alcoholic steatohepatitis, NASH). Anti number 1, number 3 term and terms compound or its pharmaceutically acceptable salts either anti number 4 active ingredient containing food composition for treatment of nonalcoholic steatotic hepatitis (non non-alcoholic steatohepatitis, NASH). Compound name Mouse liver micro cotton T1/2 (min) CL int (micro l/min/mg protein) Cleaning rate grade Compound 1 71. 43 19. 40 Intermediate Verapamil 6. 37 217. 68 Highly Oh reel glow this year 9415. 85 0. 15 Low Compound name Solubility (micro g/Ml) Caffeine 972. 68 Die ethyl steel hemp cloth [su reel roll 4. 50 Compound 1 9. 59 3A 4 provided midazolam 3A 4 a-testosterone 2D 6 2C 9 Compound name IC50 (µm) IC50 (µm) IC50 (µm) IC50 (µm) Compound 1 50> 49. 72 50> 5. 54 Ketoconazole 0. 027 0. 023 Na Na Dextromethorphan DIN Na Na 0. 047 Na Sulfanyl patrol lung Na Na Na 0. 40 PK parameters Compound 1 (200mpk, po) Cmax (ng/ml) 260. 8 ± 41. 4 Tmax (h) 1 ± 0 (H * ng/ml) AUC (inf) 426. 5 ± 67. 7 AUC (0 - 24) (h * ng/ml) 420. 1 ± 69. 3 AUC_% Extrap (obs) 1. 6 ± 0. 7 (Inf) MRT (h) 1. 7 ± 0. 2 T1/2 (h) 1. 5 ± 0. 4 PK parameters Compound 1 (1mpk, i. V) Compound 1 (10mpk, po) Compound 1 (200mpk, po) Compound 1 (10mpk, po) Compound 1 (10mpk, po) Compound 1 (10mpk, po) Example number number 1 Example number number 2 Example number number 2 Example 3 number number Example 3 number number Example 4 number number C0 (Ng/ml) 506. 4 ± 129. 7 - - - - - - - - - - Cmax (ng/ml) - - 22. 3 ± 8. 9 260. 8 ± 41. 4 107. 8 62. 2 ± 37. 5 46. 7 ± 8. 8 Tmax (h) - - 0. 4 ± 0. 1 1 ± 0 0. 25 0. 25 ± 0. 1 0. 25 ± 0. 0 AUC(Inf) (H * ng/ml) 149. 3 ± 3. 0 426. 5 ± 67. 7 225. 3 155. 2 ± 7. 5 46. 4 ± 14. 1 AUC(0 A-t) (H * ng/ml) 146. 6 ± 2. 2 20. 4 ± 6. 2 420. 1 ± 69. 3 187. 9 126. 2 ± 19. 1 42. 5 ± 16. 0 AUC_% Extrap (obs) 1. 8 ± 0. 5 - - 1. 6 ± 0. 7 16. 6 - - - - Vd (L/kg) 3. 6 ± 0. 25 - - - - - - - - - - CLp (L/hr/kg) 6. 7 ± 0. 4 - - - - - - - - - - MRT(Inf) (H) 0. 4 ± 0. 0 - - 1. 7 ± 0. 2 - - - - - - t1/2 (H) 0. 38 ± 0. 03 - - 1. 5 ± 0. 4 - - - - - - Bioavailability (%) - - 1. 4 1. 4 13 9 3