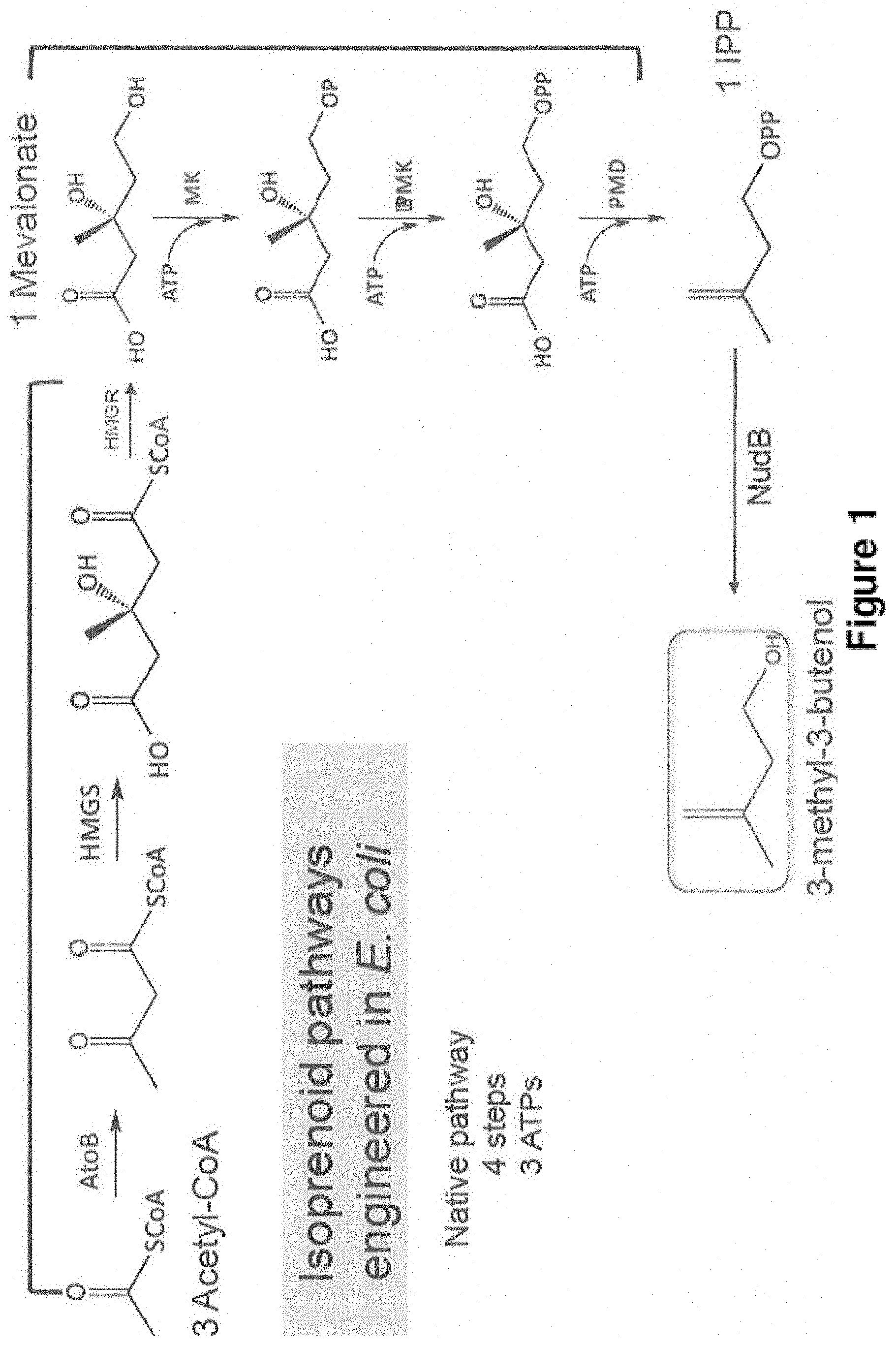
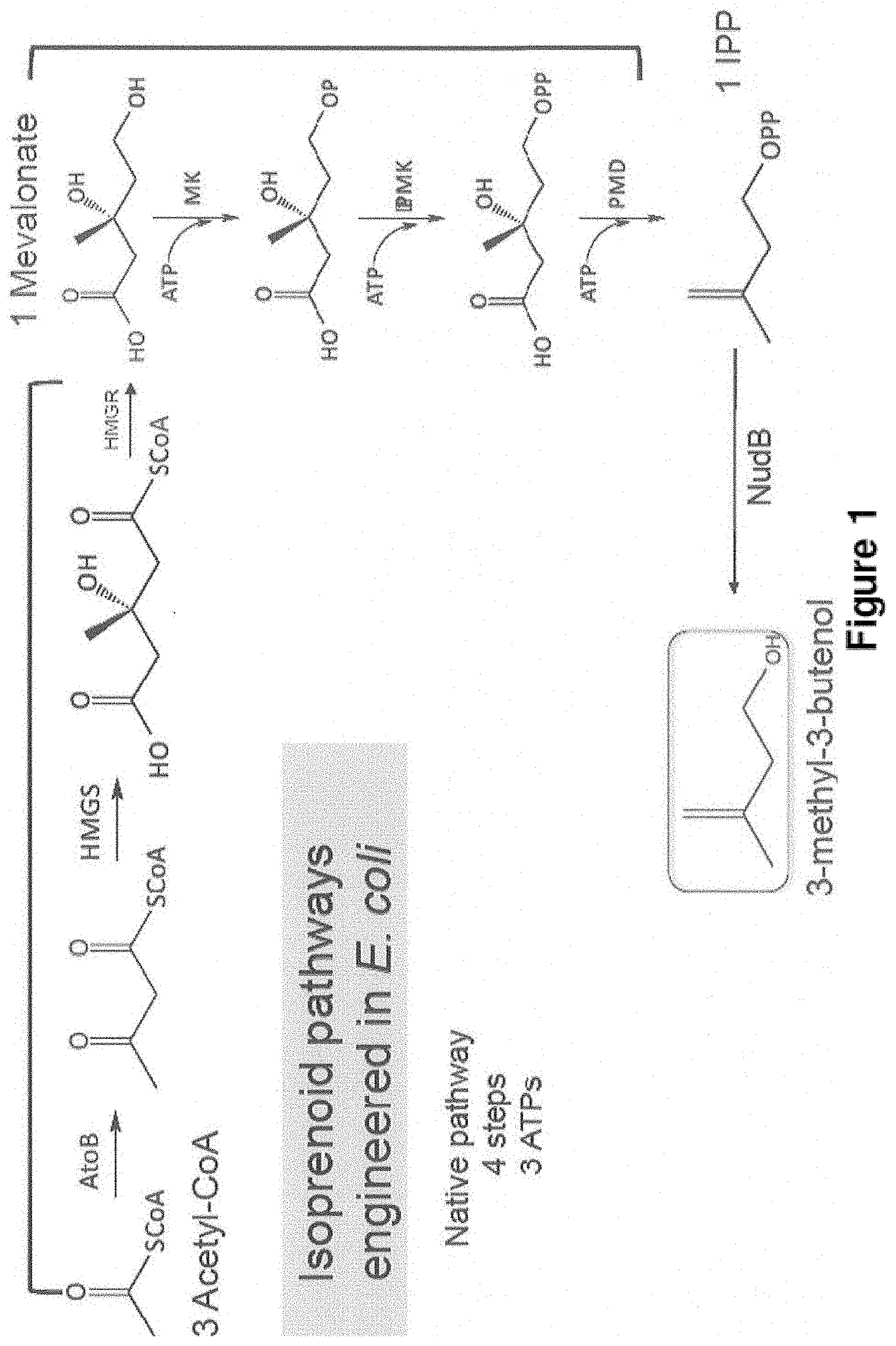
Novel Host Cells and Methods for Producing Isopentenol from Mevalonate
The application claims priority as a continuation application to U.S. patent application Ser. No. 16/388,556, filed Apr. 18, 2019, which claims priority as a continuation application to U.S. patent application Ser. No. 15/682,325, filed Aug. 21, 2017, now U.S. Pat. No. 10,273,506, issued Apr. 30, 2019, which claims priority as a continuation application to PCT International Patent Application No. PCT/US16/18984, filed Feb. 22, 2016, which claims priority to U.S. Provisional Patent Application Ser. No. 62/119,071, filed Feb. 20, 2015; all of which are incorporated herein by reference. The invention was made with government support under Contract Nos. DE-AC02-05CH11231 awarded by the U.S. Department of Energy. The government has certain rights in the invention. The present invention is in the field of producing isopentenol. The mevalonate pathway has been extensively used to produce a range of valuable chemicals via isopentenyl pyrophosphates (IPP) or dimethylallyl pyrophosphates (DMAPP) as essential intermediates for terpene synthesis. In addition to terpene-based chemicals, Chou and colleagues engineered the mevalonate pathway in To our knowledge, there has been no report that attempts to resolve the aforementioned problem in the mevalonate-derived isopentenol pathway. Although there were other pathways reported to produce isopentenols of which carbon backbones were derived from keto acids intermediates in biosynthesis pathways of valine, leucine and isoleucine (Atsumi et al. Nature 2008). However, biosynthesis of these amino acids from pyruvate requires multiple biochemical reactions, which are tightly regulated in The present invention provides for a genetically modified host cell capable of producing isopentenol and/or 3-methyl-3-butenol, comprising (a) an increased expression of phosphomevalonate decarboxylase (PMD) (b) an increased expression of a phosphatase capable of converting isopentenol into 3-methyl-3-butenol, (c) optionally the genetically modified host cell does not express, or has a decreased expression of one or more of NudB, PMK, and/or PMD, and (d) optionally one or more further enzymes capable of converting isopentenol and/or 3-methyl-3-butenol into a third compound, such as isoprene. In some embodiments, the decreased expression is a disruption of the promoter or knock out of the gene encoding the enzyme. In some embodiments, the genetically modified host cell further comprises an increased expression of one or more of AtoB, hydroxymethylglutaryl-CoA synthase (HMGS), hydroxymethylglutaryl-CoA reductase (HMGR), and/or MK. In some embodiments, one or more of the described expressed enzymes, such as PMD, phosphatase, AtoB, HMGS, HMGR, and/or MK, are encoded on one or more nucleotide sequences which are in one or more nucleic acids which are transformed into the genetically modified host cell, or host cell prior to genetic modification. In some embodiments, the nucleotide sequences encoding the one or more enzymes are operatively linked to one or more promoters capable of transcription in the genetically modified host cell. In some embodiments, each nucleic acid of the one or more nucleic acids is a vector capable of stable introduction into and/or maintenance in the host cell. The present invention provides for a method for producing isopentenol and/or 3-methyl-3-butenol and/or the third compound, comprising: (a) providing a genetically modified host cell of the present invention, (b) culturing the genetically modified host cell under a condition wherein PMD and/or phosphatase are expressed, and (c) optionally recovering the isopentenol and/or 3-methyl-3-butenol and/or the third compound. In some embodiments, the (b) culturing step further comprises expressing AtoB, HMGS, HMGR, and/or MK. In some embodiments, the (b) culturing step is under an anaerobic or microaerobic condition. In some embodiments, one or more of the enzymes, including PMD, phosphatase, AtoB, HMGS, HMGR, and MK, is an engineered enzyme, or homologous, mutant or variant enzymes having the same enzymatic activity, with an amino acid sequence having equal to or more than 70%, 80%, 90%, 95%, or 99% identity to the amino acid sequence of the corresponding wild-type enzyme, such as the specific enzymes described in Examples 1, 2, or 3. In some embodiments, one or more of the enzymes, including PMD, phosphatase, AtoB, HMGS, HMGR, and MK, is heterologous to the host cell. In some embodiments, the genetically modified host cell is capable of producing one or more compounds in titers or yields equal to or more than the titers or yields described herein. The present invention provides for a mutant or engineered enzyme described in Examples 1, 2, or 3, including, but not limited to, a PMD mutants described in Example 3. The present invention provides for PMD mutants comprising an amino acid sequence substantially identically to the amino acid sequence of a wild type PMD, such as PMDsc or PMDse, wherein the PMD mutant comprises one or more of the amino acid mutations described in Example 3. The foregoing aspects and others will be readily appreciated by the skilled artisan from the following description of illustrative embodiments when read in conjunction with the accompanying drawings. Before the invention is described in detail, it is to be understood that, unless otherwise indicated, this invention is not limited to particular sequences, expression vectors, enzymes, host microorganisms, or processes, as such may vary. It is also to be understood that the terminology used herein is for purposes of describing particular embodiments only, and is not intended to be limiting. As used in the specification and the appended claims, the singular forms “a,” “an,” and “the” include plural referents unless the context clearly dictates otherwise. Thus, for example, reference to an “enzyme” includes a single enzyme as well as a plurality of enzymes, either the same (e.g., the same molecule) or different. In this specification and in the claims that follow, reference will be made to a number of terms that shall be defined to have the following meanings: The terms “optional” or “optionally” as used herein mean that the subsequently described feature or structure may or may not be present, or that the subsequently described event or circumstance may or may not occur, and that the description includes instances where a particular feature or structure is present and instances where the feature or structure is absent, or instances where the event or circumstance occurs and instances where it does not. The terms “host cell” and “host microorganism” are used interchangeably herein to refer to a living biological cell that can be transformed via insertion of an expression vector. Thus, a host organism or cell as described herein may be a prokaryotic organism (e.g., an organism of the kingdom Eubacteria) or a eukaryotic cell. As will be appreciated by one of ordinary skill in the art, a prokaryotic cell lacks a membrane-bound nucleus, while a eukaryotic cell has a membrane-bound nucleus. The term “heterologous” as used herein refers to a composition, such as enzyme or nucleic acid, or the like, that in nature is not found together with another composition. For example, an enzyme is heterologous to a host cell, if in nature the species of the host cell does not have the enzyme. The term “heterologous DNA” as used herein refers to a polymer of nucleic acids wherein at least one of the following is true: (a) the sequence of nucleic acids is foreign to (i.e., not naturally found in) a given host microorganism; (b) the sequence may be naturally found in a given host microorganism, but in an unnatural (e.g., greater than expected) amount; or (c) the sequence of nucleic acids comprises two or more subsequences that are not found in the same relationship to each other in nature. For example, regarding instance (c), a heterologous nucleic acid sequence that is recombinantly produced will have two or more sequences from unrelated genes arranged to make a new functional nucleic acid. Specifically, the present invention describes the introduction of an expression vector into a host microorganism, wherein the expression vector contains a nucleic acid sequence coding for an enzyme that is not normally found in a host microorganism. With reference to the host microorganism's genome, then, the nucleic acid sequence that codes for the enzyme is heterologous. The term “mevalonate pathway” is used herein to refer to the pathway that converts acetyl-CoA to isopentenyl pyrophosphate through a mevalonate intermediate. The terms “expression vector” or “vector” refer to a compound and/or composition that transduces, transforms, or infects a host microorganism, thereby causing the cell to express nucleic acids and/or proteins other than those native to the cell, or in a manner not native to the cell. An “expression vector” contains a sequence of nucleic acids (ordinarily RNA or DNA) to be expressed by the host microorganism. Optionally, the expression vector also comprises materials to aid in achieving entry of the nucleic acid into the host microorganism, such as a virus, liposome, protein coating, or the like. The expression vectors contemplated for use in the present invention include those into which a nucleic acid sequence can be inserted, along with any preferred or required operational elements. Further, the expression vector must be one that can be transferred into a host microorganism and replicated therein. Preferred expression vectors are plasmids, particularly those with restriction sites that have been well documented and that contain the operational elements preferred or required for transcription of the nucleic acid sequence. Such plasmids, as well as other expression vectors, are well known to those of ordinary skill in the art. The term “transduce” as used herein refers to the transfer of a sequence of nucleic acids into a host microorganism or cell. Only when the sequence of nucleic acids becomes stably replicated by the cell does the host microorganism or cell become “transformed.” As will be appreciated by those of ordinary skill in the art, “transformation” may take place either by incorporation of the sequence of nucleic acids into the cellular genome, i.e., chromosomal integration, or by extrachromosomal integration. In contrast, an expression vector, e.g., a virus, is “infective” when it transduces a host microorganism, replicates, and (without the benefit of any complementary virus or vector) spreads progeny expression vectors, e.g., viruses, of the same type as the original transducing expression vector to other microorganisms, wherein the progeny expression vectors possess the same ability to reproduce. As used herein, the terms “nucleic acid sequence,” “sequence of nucleic acids,” and variations thereof shall be generic to polydeoxyribonucleotides (containing 2-deoxy-D-ribose), to polyribonucleotides (containing D-ribose), to any other type of polynucleotide that is an N-glycoside of a purine or pyrimidine base, and to other polymers containing nonnucleotidic backbones, provided that the polymers contain nucleobases in a configuration that allows for base pairing and base stacking, as found in DNA and RNA. Thus, these terms include known types of nucleic acid sequence modifications, for example, substitution of one or more of the naturally occurring nucleotides with an analog; intemucleotide modifications, such as, for example, those with uncharged linkages (e.g., methyl phosphonates, phosphotriesters, phosphoramidates, carbamates, etc.), with negatively charged linkages (e.g., phosphorothioates, phosphorodithioates, etc.), and with positively charged linkages (e.g., arninoalklyphosphoramidates, aminoalkylphosphotriesters); those containing pendant moieties, such as, for example, proteins (including nucleases, toxins, antibodies, signal peptides, poly-L-lysine, etc.); those with intercalators (e.g., acridine, psoralen, etc.); and those containing chelators (e.g., metals, radioactive metals, boron, oxidative metals, etc.). As used herein, the symbols for nucleotides and polynucleotides are those recommended by the IUPAC-IUB Commission of Biochemical Nomenclature (Biochem. 9:4022, 1970). The term “operably linked” refers to a functional linkage between a nucleic acid expression control sequence (such as a promoter) and a second nucleic acid sequence, wherein the expression control sequence directs transcription of the nucleic acid corresponding to the second sequence. The term “substantially identical” describes an amino acid sequence that is at least 70%, 75%, 80%, 85%, 90%, 95% or 99% identical to any one of the amino acid sequences described herein. The “substantially identical” amino acid sequence may retain amino acids residues that are recognized as conserved for the enzyme, and may have non-conserved amino acid residues substituted or found to be of a different amino acid, or amino acid(s) inserted or deleted, but which does not affect or has insignificant effect its enzymatic activity, as compared to the enzyme described herein. The “substantially identical” enzyme has an enzymatic activity that is identical or essentially identical to the biological activity of the regulator or enzyme described herein. The “substantially identical” enzyme may be found in nature, i.e. naturally occurring, or be an engineered mutant thereof. The PMD is any suitable PMD, such as any PMD with an amino acid sequence substantially identical to the amino acid sequences of SEQ ID NO: 1 or 2. The substantially identical PMD comprises one or more, or all, of the conserved residues are identified in The host cell can be a eukaryote or prokaryote cell that produces acetyl-CoA. Any prokaryotic or eukaryotic host cell may be used in the present method so long as it remains viable after being transformed with a sequence of nucleic acids. Generally, although not necessarily, the host cell is bacterial. Examples of bacterial host cells include, without limitation, those species assigned to the Suitable eukaryotic cells include, but are not limited to, fungal, insect or mammalian cells. Suitable fungal cells are yeast cells, such as yeast cells of the One of the advantages of the present invention is the improvement in cost-effectiveness of isopentenol production in a host cell, such as Prior approaches to making isopentenol/chemicals via IPP (isopentenyl pyrophosphate) consume three ATPs between mevalonate and IPP. These prior approaches contain unnecessary phosphorylation-dephosphorylation steps which consume energy, are inefficient, and require expensive aeration in large tanks. For example, see In some embodiments, the host cells comprise pathways which go directly or via two-steps from mevalonate to isopentenol. For example, see The present invention provides one or more of the following advantages. (1) Improved yields (carbon and redox balances of the modified pathways are basically same as the original pathway). The method has an improved efficiency and reduced costs at the industrial scale by reduced or eliminated need for aeration during the culturing of the host cells. (2) IPP toxicity is relieved: IPP toxicity is a key factor in limiting pathway function in the original isopentenol pathway (George et al. Biotech Bioeng 2014). Modified pathways do not generate IPP. (3) Higher isopentenol production. In an experiment, a host cell with the pathway in In some embodiments, the host cell is capable of carrying out the following steps: biological hydrogenation of isopentenol to isopentanol via IPDMAP3-methyl-2-butenolisopentanol by engineered IDI and NemA. In some embodiments, the host cell is further capable of carrying out the following step: isopentenolisoprene. To decrease energy cost for production of isopentenol, we have invented two novel biosynthesis pathways that implement engineered phosphomevalonate decarboxylase (PMD). In the original pathway, PMD catalyzes the reaction that converts diphosphomevalonate to isopentenyl diphosphate, which is subsequently hydrolyzed to 3-methyl-3-butenol. However, in our first new pathways, the engineered PMD enabled First of all, the modified pathways in this invention do not improve theoretical maximum yield for isopentenol since the carbon and redox balances of the modified pathways are basically same as the original pathway. The advantages of this pathway are one or more of the following: (1) This pathway would be more favored than the original pathway not only in the aerobic but also in the anaerobic fermentation condition, since it requires less ATP which is one of the limiting factors both in aerobic and anaerobic application of mevalonate pathway. (2) Aeration is also one of the most expensive processes in industrial application, and relieving (not removing but relieving) the requirement of aeration would have a huge benefit for industrial application. (3) We can also relieve the IPP toxicity issue of the original pathway using these modified pathways. In the original pathway for isopentenol, the pathway balance has been a very important issue and IPP toxicity is one of the key factors to limit the pathway function (George et al. Biotech Bioeng 2014). One of the modified pathways of the present invention overcomes this toxicity issue since it does not generate toxic IPP from the modified pathway which in turn makes large scale/high flux production of isopentenol possible. It is to be understood that, while the invention has been described in conjunction with the preferred specific embodiments thereof, the foregoing description is intended to illustrate and not limit the scope of the invention. Other aspects, advantages, and modifications within the scope of the invention will be apparent to those skilled in the art to which the invention pertains. All patents, patent applications, and publications mentioned herein are hereby incorporated by reference in their entireties. The invention having been described, the following examples are offered to illustrate the subject invention by way of illustration, not by way of limitation. Isoprenoids are considered as one of the most promising advanced biofuels. Among the isoprenoid compounds, branched C5 alcohols (3-methyl-butanol, 3-methyl-3-butenol, and 3-methyl-2-butenol) have been tested as good biofuel compounds with favorable combustion properties and octane numbers to gasoline. A synthetic pathway for C5 alcohols production has been reported previously in Even though the MVA pathway has been known to be less efficient than the MEP pathway in carbon and redox balance as well as in energy balance, the MVA pathway has been extensively used for microbial production of a range of valuable isoprenoids due to its tractability and the high titers it can provide. However, the energy demands of this pathway and the operational cost for aeration to meet the energy demands have been a problem when a production in large scale using fermentor is exploited. Presented herein are modified pathways for C5 alcohol production that will address these issues of the traditional MVA pathways. The mevalonate pathway condenses 3 molecules of acetyl-CoA to produce one molecule of isopentenyl pyrophosphate (IPP). One of the intermediate, mevalonate is phosphorylated twice by two different kinases, mevalonate kinase (MK) and phosphomevalonate kinase (PMK), and lastly diphosphomevalonate decarboxylase removes the carboxylate group resulting in isopentenol pyrophosphate. This study aims to identify and engineer an enzyme that catalyzes the decarboxylation of phosphomevalonate or mevalonate to isopentenyl monophosphate or isopentenol. One initially seeks a decarboxylation activity from diphosphomevalonate decarboxylase from In vitro assay shows that hydrolysis of isopentenyl monophosphate can be a limiting step if decarboxylation step is not the rate-limiting reaction. To identify the endogenous phosphatase, which hydrolyze the isopentenyl monophosphate producing isopentenol, 36 endogenous monophosphatase mutants of Two bypass pathways are constructed. The activity of PMD on MevP is measured in vitro assay. PMD has a promiscuous activity for MevP, but its kinetics are poor (high Km, lower Kcat). In vitro assay showed that PMD decarboxylase MevP. However, its Km for MevP is significantly higher and Kcat for MevP is significantly lower than those for MevPP. This result suggests that decarboxylation of MevP might be the limiting step in the bypass pathway 2. Since we are relying on the promiscuous activity of PMD on MevP, improved activity of PMD for MevP might increase the isopentenol productivity and yield. Two serine residues in PMD active site are essential for MevP decarboxylation, but not K22 and T209. Active site of PMD is enriched with positively charged amino residues such as arginine and lysine. While the original substrate MevPP has two phosphate resulting the net charge to −4, the alternative substrate mevP has a net charge of −2 and mevalonate has a net charge of 0. To make the active site residues to be more neutral, we have substituted K22 to methionine (neutral residue) and T209 to aspartate (negatively charged) to compensate the decreased negativity of the original substrate. Mutation of K22M and T209D do not significantly change the in vivo production titer. Production of isopentenol and growth under aerobic condition. Test of different genes from various species. The first heterologous mevalonate pathway is established in Identification of endogenous phosphatase (knock-out-in vitro experiment) and overexpression of phosphatases. NudB hydrolyzes a phosphate of IPP, producing IP. Subsequently, an endogenous phosphatase hydrolyzes another phosphate from IP, producing Isopentenol. In previous studies, Chou (2013) and George (2014) constructed and optimized the heterologous pathway for isopentenol production. Chou and colleagues determined activity and kinetics of NudB on IPP in vitro. Initially, it was assumed that NudB would hydrolyze diphosphate of IPP since the native activity of NudB is to hydrolyze DHNTP to DHNMP (Gabelli, 2007, Cell). Therefore, kinetics of NudB was determined in vitro by an assay, which includes inorganic pyrophosphatase and phosphate sensor. Since the bypass pathway 2 requires a phosphatase, we question whether the NudB hydrolyze two phosphates sequentially or the diphosphate at once. The in vitro assay results show that NudB hydrolyzed the beta phosphate of IPP, but not the alpha phosphate. Also, NudB do not show the activity of hydrolyzing IP to Isopentenol in vitro. Unexpected activity of NudB is puzzling because it has been observed that in the original pathway, isopentenol production can be improved by enhanced expression of NudB in vivo. This result indicated that there is an endogenous phosphatase that hydrolyze the alpha phosphate from IP produced by the hydrolysis reaction of NudB. AphA, an alkaline phosphatase, is found to actively hydrolyze IP to Isopentenol. To identify the endogenous phosphatase, we test IP hydrolysis activity of cell lysates of 36 monophosphatase mutants and compared its relative activity with that of wild type. All 36 mutants are retrieved from Keio collection (Baba, MSB), and cell lysates are prepared by culturing mutants in LB. We identify three mutants that produce less IP that other mutants and wild type. These three mutants are subsequently cloned to a vector for overexpression. Expression of three phosphatase are induced by 0.5 mM IPTG, and cell lysates are prepared. Among these three phosphatase, aphA expressed cell lysates show significantly higher and faster hydrolysis activity on IP. AphA overexpression in vivo do not improve the isopentenol production in the bypass pathway. Next we seek to determine whether overexpression of aphA increases the production of isopentenol in vivo. Overexpression of aphA does not significantly increase the isopentenol production. Given that aphA is expressed in vivo, this result suggests that IP hydrolysis may not be the limiting step in our bypass pathway 2. Production of isopentenol and growth under anaerobic condition. Initial run of microaerobic fermentation shows that under the oxygen-limiting conditions, glucose are consumed for producing mixed acids such as lactate, formate and acetate. Therefore, we introduce mutants where three fermentation-related genes are deleted and tested whether the bypass pathway 2 performs better under the microaerobic condition than the original pathway. The result shows that the bypass pathway in three knock out mutants produced higher mg/L isopentenol than the original pathway under microaerobic condition. In vitro assay shows that hydrolysis of isopentenyl monophosphate can be a limiting step if decarboxylation step is not the rate-limiting reaction. To identify the endogenous phosphatase, which hydrolyze the isopentenyl monophosphate producing isopentenol, we have screened 36 endogenous monophosphatase mutants of Based on our initial works, the modified pathway that doesn't have PMK and NudB produced about 900 mg/L of isopentenol after 42 hrs incubation, which is 40% higher than the titer from the original pathway under aerobic condition (640 mg/L). Also, cells with the modified pathway showed better growth giving the final OD of above 3.5, while the Biological production of higher alcohols such as isopentenol has been extensively investigated, but major bottleneck of industrialization has been a cost-effectiveness of the biological production system in fermentor scale. This new pathways can be used in a fermentor scale, even under anaerobic or microanaerobic conditions to produce isopentenol in Project Goals: The Joint BioEnergy Institute (JBEI) aims to produce a chemically diverse suite of biofuels from lignocellulosic biomass. Isoprenoid-based biofuels have been of great interest due to their superb fuel properties such as low freezing temperature and high octane number. Mevalonate (MVA) pathway is one of the major biosynthetic pathways of isoprenoid fuel production, and the engineering of this pathway is a key approach to achieve higher production of these biofuels. Various engineering strategies and tools have been explored to identify the bottlenecks of the pathway and to understand the pathway enzymes better, but the intrinsic energy demands of this pathway and the operational cost for aeration to meet the energy demands have been still a problem when a production in large scale using fermentor is exploited. In this work, we present modified version of the MVA pathway that will address these issues for isoprenoid biofuel production. Isoprenoids are considered as one of the most promising advanced biofuels. Among the isoprenoid compounds, branched five carbon (C5) alcohols have been tested as good biofuel compounds with favorable combustion properties and octane numbers to gasoline. A synthetic pathway for C5alcohols production has been reported previously in Even though the MVA pathway has been known to be less efficient than the methylerythritol phosphate (MEP) pathway in carbon and redox balance as well as in energy balance, the MVA pathway has been extensively used for microbial production of a range of valuable isoprenoids due to its tractability and the high titers it can provide. However, the energy demands of this pathway and the operational cost for aeration to meet these energy demands have been a problem, especially when a production in large scale using fermentor is exploited. Modified pathways for C5alcohol production, of the present invention, address these issues of the traditional MVA pathways. Host cells with advanced pathways for C5alcohol production is engineered that reduce cellular costs for isopentenol production. One of the modified pathways showed that isopentenol could be produced via decarboxylation of mevalonate monophosphate to isopentenyl monophosphate by the promiscuous activity of the decarboxylase. The titer and the growth of the engineered strains with this modified pathway are better than those with the original pathway, and the efficiency of this modified pathway is tested under microaerobic condition. The decarboxylase enzyme engineering and the pathway optimization of this modified pathway leads to a microbial C5alcohol production more economically feasible, especially for large scale industrial application. Branched C5alcohols are promising biofuels with favorable combustion properties. A mevalonate (MVA)-based isoprenoid biosynthetic pathway for C5alcohols is constructed in Isopentenol (3-methyl-3-buten-1-ol) is a potential biofuel and important precursor for flavor compounds (prenols and isoamyl alcohol esters) and industrial chemicals such as isoprene (Chou and Keasling, 2012 and Peralta-Yahya et al., 2012). Two classes of metabolic pathways have been engineered to produce isopentenol in microbial hosts: amino acid production pathways utilizing 2-keto-acid intermediates (Connor and Liao, 2008 and Connor et al., 2010), and isoprenoid biosynthesis pathways, including both the mevalonate (MVA) (Chou and Keasling, 2012, Withers et al., 2007, George et al., 2014 and Zheng et al., 2013) and non-mevalonate pathway (methylerythritol 4-phosphate (MEP) or 1-deoxy-D-xylulose 5-phosphate (DXP) pathway) (Liu et al., 2013). A heterologous MVA pathway was constructed to produce isopentenol in A variety of engineering strategies have been applied to optimize the heterologous MVA pathway and improve isoprenoid production in Although the mechanism of IPP toxicity is unknown, the deleterious effects of its accumulation are clear. First, it has been demonstrated in various studies that accumulation of IPP inhibits cell growth (Withers et al., 2007, George et al., 2014 and Martin et al., 2003), which prevents a bioprocess from achieving enough cell biomass to maximize product titer. Even prior to affecting cell growth, it is likely that the transient accumulation of IPP induces a variety of stress responses as has previously been observed during the accumulation of FPP (Dahl et al., 2013). Responses to both generalized (e.g., RpoS-induced (Hengge, 2008)) and condition-specific stress (e.g., acid stress (Sun et al., 2011), oxidative stress (Adolfsen and Brynildsen, 2015) and osmotic stress (Cohen, 2014)) result in the recruitment of ATP-dependent defense mechanisms including DNA repair (Sun et al., 2011 and Adolfsen and Brynildsen, 2015), ATPases (Sun et al., 2011), and ABC transporters (Cohen, 2014). The ATP cost of these processes may serve to compete with the energetically-expensive MVA pathway, reducing the yield and productivity of isoprenoid production. In the case of isopentenol production, high flux to IPP has an additional detrimental impact: through the action of Isopentenol production is decoupled from IPP formation by constructing two novel “IPP-bypass” pathways. These two IPP-bypass pathways rely on decarboxylation of either MVA or MVA monophosphate (MVAP) for isopentenol production and do not produce IPP as an essential precursor for isopentenol. These optimized pathways eliminate the negative effects of IPP accumulation such as growth inhibition, energy-consuming stress responses, diverted carbon flux, and regulatory inhibition on mevalonate kinase (MK). It is envisioned that these two IPP-bypass pathways could open a new dimension of engineering the MVA pathway to produce isopentenol and isopentenol-derived valuable compounds such as isoprene. 2.1. Strains and Plasmid Construction All strains and plasmids used in this study are listed in Table 2. Throughout Example 3, 2.2. Protein Expression and Purification A plasmid encoding a wild type mevalonate diphosphate decarboxylase from 2.3. Enzyme Characterization and Kinetics In vitro enzyme kinetics of decarboxylases are performed as described in previous studies (Barta et al., 2012 and Vannice et al., 2014). Briefly, enzymatic activity of decarboxylase is determined by a spectrophotometer assay quantifying ADP product formation, which is coupled to NADH oxidation by pyruvate kinase/lactate dehydrogenase. Assay mixtures are prepared in 50 mM HEPES-KOH (pH 7.5) containing 10 mM MgCl2, 400 μM phosphoenolpyruvate, 200 μM NADH, 4 mM ATP, and 25 U of pyruvate kinase/lactate dehydrogenase (Sigma, P0294). The reaction is initiated by addition of various concentrations of MVAP from 100 μM to 4,000 μM, and the reaction velocity is determined by monitoring OD at 340 nm in Spectramax 384plus microplate reader (Molecular Devices, USA). 2.4. Isopentenol Production in For isopentenol quantification, 250 μL of cell culture is combined with 250 μL of ethyl acetate containing 1-butanol (30 mg/L) as an internal standard. This mixture of ethyl acetate and cell culture is vigorously shaken for 15 min and subsequently centrifuged at 13,000 g for 2 min to separate ethyl acetate from the aqueous phase. 100 μL of the ethyl acetate layer was diluted 5-fold, and 1 μL is analyzed by Agilent GCMS equipped with Cyclosil-B column (Agilent, USA) or Thermo GCFID equipped with DB-WAX column (Agilent, USA) for quantitation of isopentenol. 2.5. Phosphatase Screening To identify IP-hydrolyzing endogenous phosphatases, single gene knockout mutants of 36 phosphatases, of which substrates are mostly mono-phosphorylated metabolites, are retrieved from the Keio collection (Baba et al., 2006). 1 mL overnight cultures from each mutant are concentrated in 0.5 mL of 50 mM Tris-HCl (pH 8.0) buffer containing 1 mM DTT and ˜50 mg of glass beads (<100 μm, Sigma-Aldrich, USA). Cells are lysed by bead-beating for 2 min at 6.0 M/s (MP biomedicals Fast Prep, USA). After centrifugation of cell lysates at 20,000 g for 10 min, clear supernatant is used for assay reaction containing 0.5 mM isopentenyl monophosphate (IP). An equal volume of ethyl acetate is added to 100 μL of assay reaction after incubating overnight at 30° C., and isopentenol is extracted for 10 min by vigorous mixing. Coding sequences of agp, aphA, and yqaB are amplified from BW25113 genome by PCR, and they are subsequently cloned to pBbE1a vector (Lee et al., 2011) for over-expression. Expression of these three genes are induced by addition of 0.5 mM IPTG to the cell cultures, and cell lysates of each sample are prepared with three biological replicates as described above for screening of the 36 mutants. 600 μL of assay reactions containing cell lysates, 1 mM DTT are prepared and the reaction is initiated by addition of 0.5 mM IP. At each time point (0, 1, 3, 6 and 22 h), 100 μL of the reaction mixture is sampled and combined with 100 μL of ethyl acetate to extract isopentenol. 2.6. Quantification of metabolites All metabolites are analyzed by liquid chromatography mass spectrometry (LC-MS; Agilent Technologies 1200 Series HPLC system and Agilent Technologies 6210 time-of-flight mass spectrometer) on a ZIC-HILIC column (150 mm length, 2.1-mm internal diameter, and 3.5-μm particle size). Standard chemicals (IPP and IP) are purchased from Sigma-Aldrich (USA). Metabolites are eluted isocratically with a mobile phase composition of 64% (v/v) acetonitrile containing 50 mM ammonium acetate with a flow rate of 0.15 mL/min. IPP and IP from 3.1. Design Rationale for IPP-Bypass Isopentenol Pathways The biosynthesis of IPP from MVA consists of three energy-consuming reactions: two kinases (MK and phosphomevalonate kinase (PMK)) result in the formation of diphosphomevalonate (MVAPP), which is subsequently transformed by a decarboxylase (PMD) to form IPP. The diphosphate group of IPP is essential in chain elongation to produce GPP and FPP, and in the carbocation formation to produce cyclic terpenes since the removal of the diphosphate group is thermodynamically-favorable (Degenhardt et al., 2009). In isopentenol production via the MVA pathway, the alcohol is also produced by removal of the diphosphate group of IPP. However, this reaction is different from carbocation formation and does not require the diphosphate group as an essential leaving group to drive the hydrolysis reaction. Therefore, formation of the diphosphate group and its subsequent removal make the overall MVA pathway for isopentenol inefficient by unnecessarily consuming two ATPs. To address the energetic limitations of IPP formation—and the deleterious effects of its accumulation—we designed two modified isopentenol pathways that bypass the formation of IPP ( 3.2. Engineering of IPP-Bypass Pathway I and Identification of Promiscuous Decarboxylase Activity Toward MVA and MVAP Engineering IPP-bypass pathways I and II requires a decarboxylase that converts MVA or MVAP to isopentenol or IP, respectively. Based on the chemical structures of the substrates and products ( With PMDscas a potential decarboxylase for MVA, IPP-bypass pathway I is first constructed in Structural analysis of a homologous PMD from 3.3. Engineering of IPP-Bypass Pathway II and Pathway Optimization in To verify the improved activity of PMDscfor phosphorylated substrates (MVAP), an in vitro assay is performed with both MVA and MVAP. While isopentenol is not detected in the in vitro reaction, a detectable amount of IP is produced when MVAP is used as a substrate for PMDsc. This result indicates that PMDschas higher decarboxylase activity towards MVAP than MVA and suggests that the phosphate group of MVAP does indeed enhance substrate binding and catalysis. The kcat(0.14 s−1) and K. (0.99 mM) of PMDsctoward MVAP are about 35-fold lower and 8-fold higher than the reported kat (4.9 s−1) and K. (123 μM) toward the native substrate (MVAPP), respectively (Krepkiy and Miziorko, 2004). With a confirmation of promiscuous PIVD activity for MVAP, a new IPP-bypass pathway (pathway II in 3.4. Identification of Endogenous Phosphatase for IP The successful production of isopentenol via IPP-bypass pathway II suggests that endogenous 3.5. PMD Engineering for Improved Activity Toward Mevalonate Monophosphate To engineer the active site of PMDscfor the non-native substrate MVAP, amino acid residues in PMD putatively responsible for binding the native substrate (MVAPP) are first identified. Since the only X-ray crystal structure of PMDscwas solved without a bound substrate (Bonanno et al., 2001), the coordinates of MVAPP in the active site of PMDscare predicted by aligning the crystal structure of PMDsc(PDB #: 1FI4) to that of the homologous PMD enzyme from After the bypass pathway II with PMDscis engineered, an archaeal MVAP-specific decarboxylase is identified in Based on structural analysis of these three PMDs (PMDsc, PMDscand PMDhv), four residues (K22, S155, S208 and T209) of PMDscadjacent to the β-phosphate of the MVAPP are selected for engineering. While the original substrate MVAPP has a net charge of −4, two alternative substrates, MVAP and MVA, have a net charge of −2 and 0, respectively. To compensate for this reduced negative charge, two serine residues (S155 and S208) are mutated to negatively charged glutamate (E), and the other two residues near the phosphate moiety (K22 and T209) are mutated to neutral methionine (M) and negatively charged aspartate (D), respectively. In addition, two more mutants, R74H and I145F ( After identifying two mutations in PMDscthat improve activity toward MVAP, Successful identification of PMD mutants that improve or significantly reduce isopentenol titer and productivity supports the hypothesis that the promiscuous activity of PMD toward MVAP is the current bottleneck of the IPP-bypass pathway II. Given the huge engineering space to explore various mutations that can potentially improve the activity of PMD toward MVAP, this result provides a clear opportunity to improve IPP-bypass pathway II for isopentenol production. 3.6. Effect of MVA Levels on Isopentenol Production in the IPP-Bypass Pathway II IPP-bypass MVA pathways are engineered for isopentenol production and show that pathway II can be improved by facilitating two limiting reactions: hydrolysis of IP and decarboxylation of MVAP to IP. Next, the “top” portion of the MVA pathway is targeted with engineering that would modulate pathway flux to MVA and tested how this variation affects isopentenol production in IPP-bypass pathway II. Previously, heterologous MVA pathways are constructed and tested with various combinations of HMGS and HMGR, and different pairs of HMGS-HMGR result in different levels of MVA and final isoprenoid titers (George et al., 2014, Pfleger et al., 2006, Pitera et al., 2007 and Ma et al., 2011). The MVA level is reported to affect MK activity by substrate inhibition (Ma et al., 2011), and therefore, optimizing MVA flux has been one approach to improve titers of isoprenoid products. To evaluate the effects of MVA concentration in IPP-bypass pathway II, the original and the modified pathways are reconstructed with four different pairs of HMGS and HMGR in the “top” portion of the pathway ( In accordance with the previous reports, the original IPP-dependent isopentenol pathways show different isopentenol titers depending on which pairs of HMGS and HMGR are used ( In addition, metabolite analysis shows that strains with pathway O or pathway II accumulate significantly high levels of IPP or MVAP, respectively, regardless of intracellular MVA concentrations. Interestingly, MVAP is accumulated to considerably higher concentrations than that of IPP (100˜200 mM for MVAP vs 30-60 mM for IPP) without any significant toxicity, which is consistent with the previous report that MVAP is not inhibitory to cell growth (Martin et al., 2003). 3.7. Relief of IPP-Toxicity in the Bypass Pathway II Previous studies showed that the performance of the original MVA pathway was sensitive to MK expression levels: low MK expression resulted in attenuated flux to IPP and isopentenol, but high levels led IPP accumulation and resulted in growth inhibition (George et al., 2014 and George et al., 2015). Interestingly, growth is restored when NudB is overexpressed in IPP-accumulating strain to relieve IPP-toxicity. In Example 3, it is demonstrated that NudB hydrolyzes IPP to IP, but not further to isopentenol ( Since the bypass pathway II does not produce IPP, it is hypothesized that the pathway would be insensitive to changes in MK expression and free from related toxicity. To compare growth and isopentenol production in the original and IPP-bypass pathway (pathway O and pathway II) under IPP- or IP-accumulating conditions, respectively, two modifications are made to the strains ARK1a and ARK2a. First, to achieve a moderate level of MK expression in the control strains, the promoter previous added for MK overexpression is removed in the medium copy plasmids JBEI-12056 and JBEI-9310. With this engineering, MK expresses at a moderate level as the fourth enzyme in the operon containing three enzymes for the top portion of the MVA pathway, and it results in strains ARK1e (harboring JBEI-6818 and JBEI-6833) and ARK2e (harboring JBEI-12051 and JBEI-9314). Second, to achieve very high MK expression level, an additional copy of MK is added to the high copy plasmids, JBEI-6833 and JBEI-9314, resulting ARK1f and ARK2f, respectively. Confirming the previous results (George et al., 2014), balancing flux in the upstream pathway is critical for growth and isopentenol production ( 3.8. The Effect of Limited Aeration on Isopentenol Production Via IPP-Bypass Pathway After characterizing the IPP-bypass pathway II in Isopentenol is a potential gasoline alternative and a precursor of commodity chemicals such as isoprene. Example 3 reports efforts to remove “IPP-dependency” of the original MVA pathway and to overcome limitations intrinsic to IPP accumulation and “unnecessary” consumption of ATPs for isopentenol production. By implementing two previously unidentified activities of PMDscand AphA, it is demonstrated that considerable isopentenol titers could be achieved without producing IPP via the pathway II. The IPP-bypass pathway II is shown to be a robust alternative to the original pathway (pathway O) for isopentenol production. This modified pathway is insensitive to both MVA level and MK expression level, and reduces the engineering burden to balance the upstream MVA pathway and IPP toxicity. Most significantly, the IPP-bypass pathway II is more competitive when aeration is limited, which would significantly lower operational costs for aeration in a large scale fermentation. Finally, it is found that the promiscuous activity PMD is rate-limiting. The identification of PMD as the rate-limiting step in these bypass pathways provides clear engineering opportunities. Although a few PMD mutants with improved activity toward MVAP are constructed, more concerted efforts to engineer PMD promiscuity or identify homologous enzymes should yield additional increases in isopentenol yield and productivity. With further engineering, these bypass pathways will provide valuable platforms for the energetically-favored production of isopentenol, isoprene, and related C5compounds. While the present invention has been described with reference to the specific embodiments thereof, it should be understood by those skilled in the art that various changes may be made and equivalents may be substituted without departing from the true spirit and scope of the invention. In addition, many modifications may be made to adapt a particular situation, material, composition of matter, process, process step or steps, to the objective, spirit and scope of the present invention. All such modifications are intended to be within the scope of the claims appended hereto. The present invention provides for a genetically modified host cell capable of producing isopentenol and/or 3-methyl-3-butenol, comprising (a) an increased expression of phosphomevalonate decarboxylase (PMD) (b) an increased expression of a phosphatase capable of converting isopentenol into 3-methyl-3-butenol, (c) optionally the genetically modified host cell does not express, or has a decreased expression of one or more of NudB, phosphomevalonate kinase (PMK), and/or PMD, and (d) optionally one or more further enzymes capable of converting isopentenol and/or 3-methyl-3-butenol into a third compound, such as isoprene. 1. A polypeptide having a phosphomevalonate decarboxylase (PMD) enzymatic activity, and encoding an amino acid sequence comprising (a) at least 70% identity with SEQ ID NO:1, and (b) a histidine at position 74, a phenylalanine at position 145, a histidine at position 74, or a phenylalanine at position 145, corresponding to the numbering of SEQ ID NO:1. 2. The polypeptide of 3. The polypeptide of 4. The polypeptide of 5. The polypeptide of 6. The polypeptide of 7. The polypeptide of 8. A vector encoding the polypeptide of 9. A genetically modified host cell comprising the vector of 10. The genetically modified host cell of 11. The genetically modified host cell of 12. The genetically modified host cell of 13. The genetically modified host cell of 14. The genetically modified host cell of 15. The genetically modified host cell of 16. A method for producing 3-methyl-3-butenol, comprising:
(a) providing a genetically modified host cell of (b) culturing the genetically modified host cell under a condition wherein the polypeptide is expressed and 3-methyl-3-butenol is produced. 17. The method of 18. The method of 19. The method of 20. The method of 21. The method of 22. The method of 23. The method of 24. The method of 25. The method of RELATED PATENT APPLICATIONS
STATEMENT OF GOVERNMENTAL SUPPORT
FIELD OF THE INVENTION
BACKGROUND OF THE INVENTION
SUMMARY OF THE INVENTION
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION OF THE INVENTION
Example 1
A Modified MVA Pathway for Isopentenol Production
ATP 3 1 2 # of gene 7 4 6 Example 2
Advanced Pathways for Microbial Production of Branched C5Alcohols
References Cited in Example 2
Example 3
Isopentenyl Diphosphate (IPP)-Bypass Mevalonate Pathways for Isopentenol Production
1. Introduction
2. Materials and Methods
Strains Description Reference ΔaphA Keio Collection (Baba et al., 2006) Δagp Keio Collection (Baba et al., 2006) ΔyqaB Keio Collection (Baba et al., 2006) ARK1a JBEI-12056 + JBEI-9348 This study ARK1b JBEI-6824 + JBEI-9348 This study ARK1c JBEI-6831 + JBEI-9348 This study ARK1d JBEI-7575 + JBEI-9348 This study ARK1e JBEI-6818 + JBEI-6833 This study ARK1f JBEI-6818 + JBEI-6834 This study ARK2a JBEI-9310 + JBEI-9314 This study ARK2b JBEI-9309 + JBEI-9314 This study ARK2c JBEI-9312 + JBEI-9314 This study ARK2d JBEI-9311 + JBEI-9314 This study ARK2e JBEI-12051 + JBEI-9314 This study ARK2f JBEI-12051 + JBEI-12064 This study ARK2aa JBEI-12050 + JBEI-9314 This study ARK2aM1 JBEI-9310 + JBEI-12060 This study ARK2aM2 JBEI-9310 + JBEI-12061 This study ARK2aM3 JBEI-9310 + JBEI-12062 This study ARK3a JBEI-3100 + JBEI-12229 This study ARK3b JBEI-3100 + JBEI-3277 This study ARK4 JBEI-9310 + JBEI-12054 This study ARK5 JBEI-9310 + JBEI-12059 This study Plasmids Description Reference JBEI-6818 pBbA5c-MevTo-MKco-PMKco (George et al., 2014) JBEI-6824 pBbA5c-MevTco-BBa1002-pTrc-MKco-PMKco (George et al., 2014) JBEI-6831 pBbA5c-MTSA-BBa1002-pTrc-MKco-PMKco (George et al., 2014) JBEI-6833 pTrc99a-NudB-PMDsc (George et al., 2014) JBEI-6834 pTrc99a-NudB-PMDsc-Mkco (George et al., 2014) JBEI-7575 pBbA5c-MTDA-BBa1002-pTrc-MKco-PMKco Gift from Eunmi Kim JBEI-9309 pBbA5c-MevTco-BBa1002-pTrc-MKco This study JBEI-9310 pBbA5c-MevTo-BBa1002-pTrc-MKco This study JBEI-9311 pBbA5c-MTDA-BBa1002-pTrc-MKco This study JBEI-9312 pBbA5c-MTSA-BBa1002-pTrc-MKco This study JBEI-9314 pTrc99a-PMDsc This study JBEI-9348 pTrc99a-PMDsc-NudB This study JBEI-12050 pBbA5c-MevTo-BBa1002-pTrc-MKco-aphA This study JBEI-12051 pBbA5c-MevTo-MKco This study JBEI-12052 pSKB3-PMDsc This study JBEI-12053 pSKB3-PMDsc_K22M This study JBEI-12054 pTrc99a-PMDse This study JBEI-12055 pSKB3-PMDsc_T209D This study JBEI-12056 pBbA5c-MevTo-BBa1002-pTrc-MKco-PMKco This study JBEI-12057 pSKB3-PMDsc_R74H This study JBEI-12058 pSKB3-PMDsc_I145F This study JBEI-12059 pTrc99a-PMDhv This study JBEI-12060 pTrc99a-PMDsc_R74H This study JBEI-12061 pTrc99a-PMDsc_I145F This study JBEI-12062 pTrc99a-PMDsc_R74H/I145F This study JBEI-12064 pTrc99a-PMDsc-MKco This study JBEI-12229 pE1a-PMDsc This study 3. Results and Discussions
Kinetic parameters of PMD wild type, PMDscmutants, PMDse, PMDhvfrom other literature. Km kcat kcat/Km % of Name (mM) (s−1) (s−1M−1) WT Substrate Reference PMDsc WT 0.99 0.14 1.4 × 102 100% R74H 0.77 0.23 3.0 × 102 220% K22M 2.47 0.09 3.5 × 101 25% MVAP This study T209D 0.99 0.13 1.3 × 102 98% I145F 1.36 0.28 2.0 × 102 147% PMDhv 0.159 3.5 2.2 × 104 MVAP (Vannice et al., 2014) PMDsc 0.009 5.9 6.5 × 105 MVAPP (Barta et al., 2012) PMDsc 0.123 5.4 4.0 × 104 MVAPP (Krepkiy and Miziorko, 2004) 4. Conclusion
References Cited in Example 3