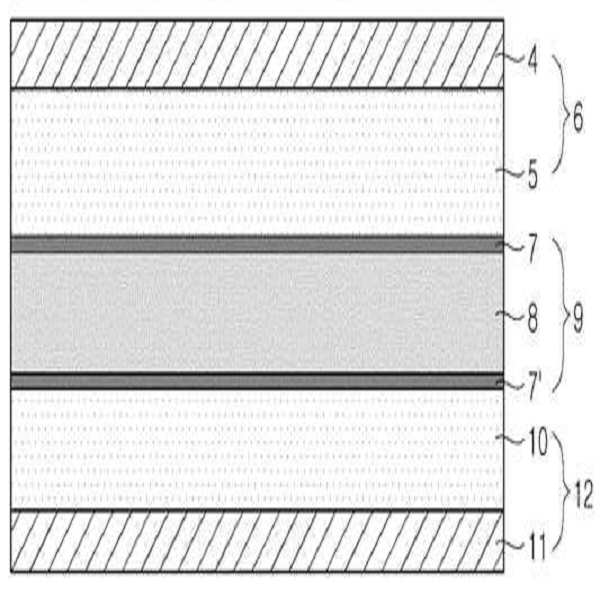
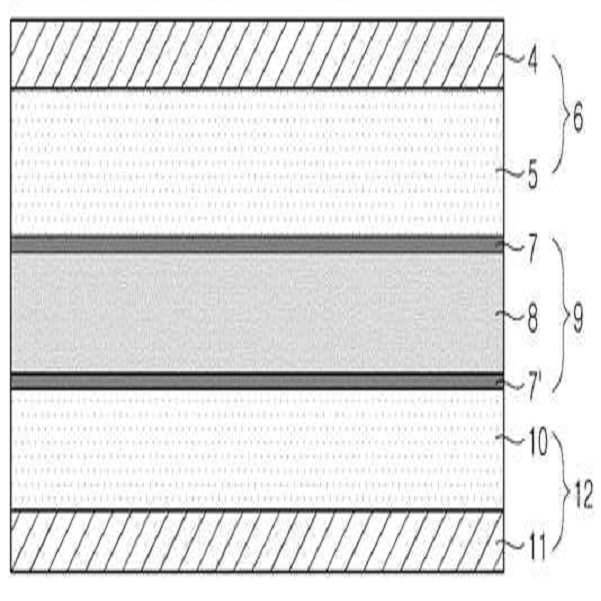
개선된 굴곡강도를 가지는 전극 조립체, 이의 제조 방법 및 이를 포함하는 전기 화학 전지
The present invention refers to flexural strength in the cell with improved electrode assembly, including electrochemical cells and number of bath method are disclosed. Generally video camera, cellular phone, portable electronic device such as portable computer by functional A1 and undergo secondary battery is used for driving the same forward more is not disclosed. This secondary battery comprising, e.g. nickel - harmful ions contained in cell, nickel - hydrogen battery, nickel - zinc, etc. is used as the lithium secondary battery. Double, and scale-up and small lithium secondary battery, high operating voltage, high energy density per unit weight advantage etc. used in simultaneously performed. The electrode assembly includes a major component of such lithium secondary battery are disclosed. However, method for forming spacer placed between the press separation film electrode layer/electrode assembly according to several large area due to increasing weight or number sample pore electrode wound between the trench former end at easy, adhesion-enhancing is required and a window layer are disclosed. In addition, the outer surface of the swell like battery is persistent, prevent changes to electrode assembly in the form of form stability are required. In this regard, floating electrode layer and porous substrate for improving heat-resistance and separating film adhesion between the woven inorganic mixed porous adhesive layer/oil flushes but (a compensation registration patent number 10 - 0775310 call), to sufficiently secure the desired adhesion separated from various size with collectively shaped to a separator subsystem to tame. The, receive RF power an electrochemical cell to control with which adhesion layer, improving stability in the form of cell electrode assembly where name is disclosed. The present invention refers to electrode assembly that is superior in adhesion between the plasticizer, form using the same electrode assembly and having improved stability number under public affairs intended for the electrochemical cell. In the embodiment of the present invention according to one, positive active material applied onto the anode, cathode active material has been applied cathode, and said separation film interposed between an anode and a cathode which, in 20 °C to 110 °C 1 seconds to 15 seconds, 1 kgf/cm2 To 30 kgf/cm2 When compression force, bending strength is 15 kgf/cm2 Number encoded at least electrode assembly and a ball. According to another in the embodiment of the present invention, said electrochemical cell including one in the embodiment according to electrode assemblies, in particular ball number encoded lithium secondary cell. Of the present invention in the embodiment according to the electrode in the assembly, electrode assembly has a plasticizer or adhesion between the SB. The process for preparing the trench electrode assembly number provides a reaction can be inserted in the second, process from long period of time. In addition, according to the electrode assembly includes a form of the present invention in the embodiment are superior stability to, long-lasting charge can be minimized even unchanged form. Through, charge and discharge characteristics may have efficiency which has cell prepared by the number, of the battery performance can be prevented from being lowered. Figure 1 shows a part of the electrode assembly of the present invention also one in the embodiment according to the internal to, said electrode assembly includes a positive electrode current collector (4) anode active material layer (5) formed anode (6); cathode current collector (11) to cathode active material layer (10) is from a (12); and said anode (6) and said cathode (12) which is arranged between the anode or cathode attached to each, porous substrate (8) on both sides of the porous substrate porous adhesive layer (7, 7') including a layer (9) comprises. Figure 2 shows a portion of the internal electrode assembly of the present invention also in the embodiment according to the other, said electrode assembly includes a positive electrode current collector (4) anode active material layer (5) formed anode (6); cathode current collector (11) to cathode active material layer (10) is from a (12); and said anode (6) and said cathode (12) which is arranged between the anode or cathode attached to each, porous substrate (8) on a porous adhesive layer formed on one surface of the porous base material (7) including a layer (9) comprises. Detailed hereinafter the present invention greater than 2000. The contents of the present invention herein described specification is not sufficiently recognize can be converted in the field of technical field or similar splicing which the surface plural description omit other. Hereinafter, with reference to the one in the embodiment according to electrode assembly of the present invention also 1 is described substrate. In the embodiment of the present invention according to one, lithium secondary battery and anode current collector including anode, cathode including a cathode active material and a cathode current collector, and said separation film disposed between an anode and a cathode which, in 20 °C to 110 °C 1 seconds to 15 seconds, 1 kgf/cm2 To 30 kgf/cm2 When compression force, bending strength is 15 kgf/cm2 Number encoded at least electrode assembly and a ball. Electrode assemblies 20 °C to 110 °C in 1 seconds to 15 seconds, 1 kgf/cm2 To 30 kgf/cm2 When compression force, bending strength is 15 kgf/cm2 Preferably the pin is related at least in the form of stability. Through, such as in the form of edges extended continuous despite changes in battery charge battery can charge and discharge characteristics to minimize efficiency may have, of the battery performance can be prevented from being lowered. The 3 point bending machine said flexural strength (3 point bending machine) (example: UTM) but using measured by ASTM D790, the one number are not disclosed. Said bending strength specifically 17 kgf/cm2 To 50 kgf/cm2 Can be in the range of, more specifically 20 kgf/cm2 To 30 kgf/cm2 Can be in the range of. The reference also 1, said in the embodiment according to the electrode assembly includes a, anode current collector (4) anode active material layer (5) formed anode (6); cathode current collector (11) to cathode active material layer (10) is from a (12); and said anode (6) and said cathode (12) which is arranged between the anode or cathode and each bonded to a layer (9) can be comprising. Said membrane (9) is porous substrate (8) and said porous substrate (8) on both sides of the porous adhesive layer (7, 7') can be comprising. The porous base material (8) includes a plurality of having pores with a conventional porous can be used for electrochemical element may be used. Porous substrate (8) include non-number limited to polyethylene, polypropylene, polyethylene terephthalate, polybutylene terephthalate, polyester, polyacetal, polyamide, polyimide, polycarbonate, polyether ether keton, polyaryl ether ketone, polyether-imide, polyamide-imide, polybenzimidazole, polyethersulfone, poly [...], cyclic olefin copolymer, polyphenylene sulfide and polyethylene naphthalene selected from the group consisting either polymer or among these heterogeneous or more polymer formed from a mixture of main disclosed. In one example, the porous base material (8) which comprises a polyolefin it will be a record, polyolefin substrate shutdown (shut down) cathode can be money results. Polyolefin substrate e.g. polyethylene film, single layer polypropylene, polyethylene/polypropylene double film, polypropylene/polyethylene/polypropylene and polyethylene/polypropylene/polyethylene triple film triple film can be selected from the group consisting. In another example, non-olefin resin or resin in addition to the polyolefin resin, olefin and a non-olefin monomers can be copolymer. The thickness of the porous substrate may be 1 to 40 micro m said micro m, 5 m to 15 m more specifically micro micro implementation being. Said base film thickness in a range of the desired, sufficient to prevent a short circuit of the internal resistance of the battery cathode formed thick so as to increase are not thick, having suitable thickness layer number can be high pressure liquid coolant. Porous adhesive layer (7, 7') is porous substrate (8) can be formed on both sides of, porous adhesive layer can be formed from the silicone composition. Said porous adhesive layer composition organic binder, and a solvent can be. Said organic binder may be an acrylic copolymer, (meth) acrylate monomer including acrylic copolymer derived from e.g. be a repeating units. In addition, repeating units derived from (meth) acrylate monomer in addition to said acrylic copolymer further comprises repeating units derived from acetate containing monomer can be. Repeating units derived from (meth) acrylate monomer binder, and/or the use of the acrylic copolymer having repeat units derived from acetate containing monomer used in the seal layer number is added to the elapsed time in the environment or secondary battery anode electrode assembly process can be fed to both the trench provides a reaction reducing the reject, process from long period of time. In addition, porous adhesive layer by retaining electrolyte electrode can be maintaining good ion conductivity, degree thereof can not inhibiting the pores of the porous substrate. Glass transition temperature (Tg) less than 100 °C said acrylic copolymer is, e.g., 20 °C to 60 °C, specifically in the range of 30 °C to 45 °C can be. The sclera in the range between said pressing at temperatures in good adhesion layer formed shape stability can be ensured. In the embodiment of the present invention derived from (meth) acrylate monomer repeating units that can be used in one, and/or acetate-containing monomer derived repeating unit the acrylic copolymer formed good adhesion at temperatures in squeezable between anode and cathode can be one number but not particularly if, for example, butyl (meth) acrylate said acrylic copolymer, propyl (meth) acrylate, ethyl (meth) acrylate and methyl (meth) acrylate selected from the group consisting of at least one (meth) acrylate monomer is polymerized 1 be a copolymer. Or, butyl (meth) acrylate said acrylic copolymer, propyl (meth) acrylate, ethyl (meth) acrylate and methyl (meth) acrylate to form at least one (meth) acrylate monomers selected from the group consisting 1, vinyl acetate and allyl acetate 1 containing at least one selected from the group consisting of acetate be a copolymer monomer is polymerized. Said repeating units of the formula 1 be a acetate-containing monomer derived from repeating units of: [Formula 1] In said formula 1, R1 Or a single bond, a linear or branched 1 to 6 carbon atoms of alkyl, R2 Is hydrogen or methyl and, respectively an integer between 1 to 100 l. For example, acetate-containing monomer derived from said one or more selected from the group consisting of repeating units of the vinyl acetate and allyl acetate acetate-containing monomer derived be a repeating units. (Meth) acrylate monomers are superposed over each other number tank or said acrylic copolymer, (meth) acrylate monomers are (meth) acrylate other than other monomer is polymerized number bath 1308. For example, acetate-containing monomer be said other monomer is present. In this case, (meth) acrylate monomer with other monomer, acetate-containing monomer mole ratio 3:7 to 7:3 specifically, specifically 4:6 to 6:4, and more particularly by about 5:5 ratio number bath polymers can be disclosed. said acrylic copolymer e.g., butyl (meth) acrylate monomer, methyl (meth) acrylate monomer, and vinyl acetate and/or allyl acetate monomer, the molar ratio of 3 to 5:0. 5 to 1. 5:4 to 6, specifically, 4:1: 5 molar ratio from 1:4 polymerized to form number bath 1308. In the embodiment of the present invention in one, said porous adhesive layer composition further comprises inorganic particles can be. In the embodiment of the present invention correspondingly in one particularly number inorganic particles used in one non-art commonly used can be inorganic particles. In the embodiment of the present invention is usable in a number of inorganic particles but examples Al one2 O3 , SiO2 , B2 O3 , Ga2 O3 , TiO2 Or SnO2 Or the like is cited. They can be mixed alone or in combination with at least one 2. In the embodiment of the present invention used in one inorganic particles include e.g., Al2 O3 (Alumina) can be used. In the embodiment of the present invention are not particularly number or size used in one non-inorganic particles, an average particle diameter of 1 nm to 2,000 nm may be, for example, 100 nm to 1,000 nm, 300 nm to 500 nm implementation being. Said inorganic particles in a size range is used, porous adhesive layer composition in liquid dispersion of inorganic particles and porous adhesive layer protects the fairness of prevent porous adhesive layer having a thickness can be properly adjusted to reduce the property and preventing an increase of the electrical resistance can be. In addition, when properly adjusted charge produced layer of pore sizes of internal short circuit will occur probability may be copyright 2001. Porous adhesive layer composition and production number in said inorganic particles dispersed in a suitable solvent and a mixture thereof can be used as inorganic dispersion forms. Said non-number correspondingly in one particularly suitable solvent using a solvent that can be conventional in the art. A suitable solvent for distributing said inorganic particles as e.g., use acetone can be. Said special number grudge without inorganic dispersion by conventional method a number bath method can be, e.g. Al2 O3 A beads mill (Beads mill) by adding acetone vacancy content to exhibit number inorganic dispersion can be distributed in a way that reduces high pressure liquid coolant. Porous adhesive layer 70 to 95% by weight based on the total inorganic particles in said porous adhesive layer % by weight, % by weight to 90% by weight 75 specifically, 80 weight % to 90 weight % more specifically can be included. Range when said composition containing the inorganic particles, inorganic particles can be sufficiently porous substrate inside the frame so when using the same porous layer and forming an adhesive layer can be shrunk effectively billion number. In the embodiment of the present invention is usable in a number of solvent in said but include acetone, dimethyl formamide (Dimethyl formamide), dimethyl sulfoxide (Dimethyl sulfoxide), dimethylacetamide (Dimethyl acetamide), dimethyl N - methylpyrrolidone ([...] provided methylpyrrolydone) purification (Dimethyl carbonate) or the like is cited. The content of 20% by weight to 99% by weight to the weight of the porous adhesive layer composition from which the solvent may be, 50 weight % to 95 weight % can have a specifically, 70 weight % to 95 weight % more specifically implementation being. Said oxide or porous adhesive layer composition range of solvent drying operation so as to number when transmitted porous adhesive layer can be rotates. Said porous adhesive layer has a thickness may be 1 to 15 micro m micro m, specifically 1 to 10 micro m, more specifically 1 micro m to 8 micro m, 1 m to 5 micro m micro implementation being. Said porous adhesive layer thickness in the range of is used, suitable porous adhesive layer can be obtained superior thermal stability and adhesion, the thickness of the entire layer preventing excessively thickened number can increase in the internal resistance of the battery by billion. The electrode assembly includes a thickness change rate compression of 10% or more in the embodiment according to formula 1 be a. [Formula 1] The rate of change (%) compression thickness=[(20 °C press electrode assembly thickness of -100 °C press thickness of electrode assembly) thickness of electrode assembly press/20 °C] × 100 In said type 1, the thickness of the layer/anode/cathode electrode assembly press 20 °C order 1 to 10 seconds in stacked electrode assembly 20 °C, 1 to 30 kgf/cm2 Pressing the central portion of the electrode assembly has been completed pressure measured thickness and is 1, 1 to 10 seconds in the thickness of the electrode assembly press 100 °C 100 °C, 1 to 30 kgf/cm2 Pressure pressing the central portion of the electrode assembly 1 measure after time thickness are disclosed. Also if said change rate of compression thickness, excellent adhesion between the plasticizer, electrode assembly inserted in the trench provides a reaction can be reducing the reject, process from long period of time. The rate of change of compression thickness said specifically 13% or more, less than 50%, more specifically 15% or more, less than 47%, 20% or more than specifically, can be less than 45%. Compression thickness detecting unit detects can be more improved adhesion between the layer and the anode or cathode. In the embodiment of the present invention hereinafter with reference to the other electrode assembly also 2 other is described substrate. The reference 2 also, in the embodiment according to the electrode assembly is an other of the present invention, anode current collector (4) anode active material layer (5) formed anode (6); cathode current collector (11) to cathode active material layer (10) is from a (12); and said anode (6) and said cathode (12) which is arranged between the anode or cathode adhesive separation film (9) can be comprising. Said membrane (9) is porous substrate (8) and said porous substrate (8) formed on one surface of porous adhesive layer (7) can be comprising. In the embodiment according to the electrode assembly includes a layer (9) of the porous base material (8) is then formed on both sides of only porous adhesive layer on the side (7) and one in the embodiment according to the number that an electrode assembly of the present invention form under the outside other components hereinafter described is substantially the same in the V-shaped substrate. The electrode assembly includes a 20 °C to 110 °C 1 seconds to 15 seconds in the embodiment according to in, 1 kgf/cm2 To 30 kgf/cm2 When compression force, bending strength is 15 kgf/cm2 Or more, e.g., 17 kgf/cm2 To 50 kgf/cm2 , Specifically 20 kgf/cm2 To 30 kgf/cm2 Implementation being. I.e., porous adhesive layer (7) is porous substrate (8) with one side of the electrode (anode or cathode) exhibit sufficient adhesion even when formed only in a persistent even cell form transition to prevent deformation can be minimize. Hereinafter, another electrode assembly of the present invention is described as follows. The organic binder in the embodiment of the electrode assembly includes a porous adhesive layer in addition to the acrylic copolymer disclosure herein can further comprise different types of organic binder. Porous adhesive layer further comprises a number one in the embodiment of the present invention that an organic binder of the present invention or in the embodiment according to the aforementioned and other under the outside substantially electrode assembly are the same. Thus, in addition to acrylic copolymer further comprises about other binder hereinafter described as follows. The in the embodiment one of the binder can be by adhesion and further comprises a further improved heat resistance. Examples of the binder may be added in addition to the acrylic copolymer, polyvinylidene fluoride (Polyvinylidene fluoride, PVdF) homopolymer, polyvinylidene fluoride - hexafluoropropylene copolymer (copolymer Polyvinylidene fluoride provided hexafluoropropylene, pVdF non-hFP), polyvinylidene fluoride - process for the preparation (polyvinylidene fluoride-a trichloroethylene, pVdF-a tCE), polyvinylidene - chloro with tree flow ethylene (polyvinylidenefluoride-a trifluoroethylene, pVdF non-cTFE), poly polymethylmethacrylate (polymethylmethacrylate), polyacrylonitrile (polyacrylonitrile), polyvinyl pyrrolidone (polyvinylpyrrolidone), polyvinyl acetate (polyvinylacetate), polyethylene oxide (polyethylene oxide), cellulose acetate (cellulose acetate), cellulose acetate butyl rate (cellulose acetate butyrate), cellulose acetate propionate (cellulose acetate propionate), cyano roh ethyl grass base column (cyanoethylpullulan), cyano roh ethyl pulley vinyl alcohol (cyanoethylpolyvinylalcohol), cyano roh ethyl cellulose (cyanoethylcellulose), cyano [...] (cyanoethylsucrose), or jellies (pullulan), carboxyl methyl cellulose (carboxyl methyl cellulose), and acrylonitrile (acrylonitrilestyrene-a butadiene copolymer) selected from the group consisting n [...][...] alone or a mixture of these is cited. More specifically, polyvinylidene fluoride homopolymer or polyvinylidene fluoride - hexafluoropropylene copolymer (Polyvinylidene fluoride, PVdF) (copolymer Polyvinylidene fluoride provided hexafluoropropylene, pVdF non-hFP) can be used. Said acrylic copolymer and said added binder weight ratio of 9. 9:0. 1 to 2. 5:7. 5 can be used. Specifically 9. 9:0. 1 to 5:5, more specifically 5 to 9:1. 5:4. 5 can be used. When used in said range, while maintaining sufficient adhesion layer excellent in stability and high pressure liquid coolant electrode assembly can be in the form number. Through, can be preventing performance degradation of the cell prepared by the number, cell efficiency which has high charge and discharge characteristics may have. When further including PVdF binder, binder has a weight average molecular weight (Mw) (g/mol) is 500,000 to 1,500,000 PVdF implementation being. In one embodiment, binder has a weight average molecular weight (Mw) (g/mol) is 1,000,000 to 1,500,000 PVdF implementation being. In another example, 2 different weight average molecular weight of at least one can be mixed. For example, weight average molecular weight of at least 1,000,000 g/mol 1 1 in 1,000,000 g/mol hereinafter can be at least one and at least one mixed. The use of porous adhesive layer in said molecular weight range PVdF binder enhanced adhesion between the porous base material, a porous substrate is heated and shrinks the machining can be effectively billion by number, in addition electrolyte layer having a layer number can be sufficiently improved high pressure liquid coolant system and the stresses comprises a battery capable of producing electrical output occurs efficiently. Hereinafter, manufacturing method of the present invention in the embodiment according to one electrode assembly is described substrate. In the embodiment according to the method of the present invention one bath electrode assembly number, number anode and anode current collector anode active material layer is formed by high pressure liquid coolant, cathode current collector cathode active material layer is formed a cathode and number and high pressure liquid coolant, said positive and negative electrodes between the layer comprising placing the disclosure herein as can be. In the embodiment according to the method of the present invention other number bath electrode assembly, disposed between the positive and negative electrode assembly 20 °C to 1 seconds to 10 seconds in layer 110 °C liver 1 kgf/cm2 To 30 kgf/cm2 The compression force can be further comprises. Said method such as between said positive and negative after 10 seconds to 1 in number by placing 20 °C to 110 °C liver seconds layer produced therewith, 1 kgf/cm2 To 30 kgf/cm2 The compression force, in an acrylic copolymer is in the form of anode or cathode electrode assembly to form a strong bond transformed can be improved. Said porous substrate is performed by using a pressing conditions when polyol [...] porous substrate and separating film porous adhesive layer not heat shrinkage temperature which take into account temperature adhesive, specifically ordinary temperature or 80 °C to 100 °C 1 seconds to 5 seconds in liver 5 kgf/cm2 To 10 kgf/cm2 Applying pressure to the of be a. In the embodiment according to the method of the present invention another electrode assembly number tank, arranged between the positive and negative electrode assembly 20 °C to 110 °C 1 seconds to 10 seconds in layer 1 kgf/cm2 To 30 kgf/cm2 1 pressing pressure difference, embedded with in a battery case behind, and injecting an electrolyte, in 30 seconds to 180 seconds 60 °C to 110 °C liver 1 kgf/cm2 To 30 kgf/cm2 2 pressing can further include a pressure difference. In this case, said battery case is formed aluminum pouch like number to one but are not disclosed. In the embodiment according to the above method of the present invention another electrode assembly of the present invention number tank manufacturing method in the embodiment according to another electrode assembly after said 2 difference before pressing in electrolyte, electrode assembly 6 hr to 48 time, further comprises storing the can be in a range of 10 °C to 30 °C. Said 2 difference by compression of the present disclosure acrylic copolymer is in the form of anode or cathode electrode assembly to form a strong bond than transformed can be improved. Said anode current collector and the anode has an anode current collector can be formed on said cathode active material layer. qualitative layer said positive active material for lithium secondary battery, binder and optionally comprising a conductive material can be. Said anode current collector of aluminium (Al), nickel (Ni) etc. but, is not limited. Said positive electrode active material include lithium reversibly intercalation and [...] compounds can be possible. Specifically cobalt, manganese, nickel, aluminum, iron or combinations of those metal lithium composite oxide or composite phosphate 1 can be one or more than species in cargo. More specifically, lithium cobalt oxide, lithium nickel oxide, lithium manganese oxide, lithium nickel cobalt manganese oxide, lithium nickel cobalt aluminum oxide, lithium iron phosphate cargo or a combination thereof can be used. Said binder as well as a positive electrode active material particles don't be attached to the anode current collector adheres well to serve as an anode active material, specific examples include polyvinyl alcohol, carboxymethylcellulose, hydroxypropyl cellulose, diacetyl cellulose, polyvinyl chloride, carboxylic it became luck misfire polyvinyl chloride, poly [...], ethylene oxide-containing polymer, polyvinyl pyrrolidone, polyurethane, polytetrafluoroethylene, polyvinylidene fluoride, polyethylene, polypropylene, styrene - butadiene rubber, upper part of a styrene - butadiene rubber acrylic, epoxy resin, nylon is provided to be used, is not limited. 2 alone or they can be mixed at least one. Number of imparting electrical conductivity to said conductive electrodes, and vertically moving the natural graphite, artificial graphite, carbon black, carbon fiber, metal powder, metal fiber is provided to be used, is not limited. 2 alone or they can be mixed at least one. Said metal powder and said metal fiber has a copper, nickel, aluminum, the like can be used. Said cathode cathode current collector and said secondary cathode can be cathode active material layer on the current collector. Said cathode current collector copper (Cu), gold (Au), nickel (Ni), copper alloy etc. but, is not limited. Said negative electrode active material for negative electrode active material qualitative layer, binder and optionally comprising a conductive material can be. Said anode active material include lithium ions reversibly intercalation and [...] substance, lithium metal, lithium metal alloy, lithium dope and [...] substance, can be using transition metal oxide or combinations thereof. Said lithium ions reversibly intercalation and [...] title and the carbon-containing substance is cited, examples thereof amorphous carbon, amorphous carbon or a combination thereof is cited. Examples of said amorphous carbon is amorphous, plate, scale-like (flake), natural graphite or artificial graphite spherical or fiber-type is cited. Examples of soft carbon or hard carbon (hard carbon) (soft carbon) said amorphous carbon, carbide battery negative electrode material, mixture of calcined coke or the like is cited. Said lithium lithium metal alloy include Na, K, Rb, Cs, Fr, Be, Mg, Ca, Sr, Si, Sb, Pb, In, Zn, Ba, Ra, Ge, composed of a metal selected from the group consisting Al and Sn can be produced. Said [...] dope and lithium substance include Si, SiOx (0<x<2), si-a [...] composite, si a-[...] alloy, Sn, SnO2 , sn-a [...] composite, such as sn-a [...] cited, in addition at least one of these and SiO2 Mixed a disapproval. Said element Y include Mg, Ca, Sr, Ba, Ra, Sc, Y, Ti, Zr, Hf, Rf, V, Nb, Ta, Db, Cr, Mo, W, Sg, Tc, Re, Bh, Fe, Pb, Ru, Os, Hs, Rh, Ir, Pd, Pt, Cu, Ag, Au, Zn, Cd, B, Al, Ga, Sn, In, Tl, Ge, P, As, Sb, Bi, S, Se, Te, Po and combination thereof can be selected from the group consisting. Said transition metal oxide include vanadium oxide, lithium vanadium oxide or the like as is cited. Said conductive material of the type used in the aforementioned binder and processed anode conductive material binder such as disclosed. An anode and a cathode active material and binder and optionally a conductive material on said each solvent composition and number each active material incorporated in high pressure liquid coolant, said active material composition can be applied to a number each current collector high pressure liquid coolant. the solvent is N - methylpyrrolidone etc. but, is not limited. Said organic solvent is involved in the electrochemical reaction medium can move the ions could be bonded each other. Its specific examples, carbonate-based solvent, ester-based solvent, ether-based solvent, ketone-based solvent, alcohol solvent and aprotic solvent can be selected. Examples of said carbonate-based solvent, dimethyl carbonate (DMC), diethyl carbonate (DEC), dipropyl carbonate (DPC), methyl propyl carbonate (MPC), (EPC) ethyl propyl carbonate, ethyl methyl carbonate (EMC), ethylene carbonate (EC), propylene carbonate (PC), butylene carbonate (BC) or the like is cited. Specifically, carbonate solvent compound and an annular carbonate composition for dielectric constant viscosity number executed when small solvent bath 1308. The cyclic carbonate compound and carbonate solvent can be mixed at a volumetric ratio of 1:1 to 1:9 compounds. Examples of said ester solvent, methyl acetate, ethyl acetate, n - propyl acetate, dimethyl acetate, methyl propionate, ethyl propionate, γ - butyrolactone, the car it is yellow the id (decanolide), ballet [...], [...] (mevalonolactone), polycaprolactone (caprolactone) or the like is cited. Examples of said ether-based solvent, d butyl ether, tetra glycol Lyme, being a D writing,, [...], 2 - methyl [...], like 2 [...] is cited. Said ketone-based solvent such as cyclohexanone cited, said alcohol solvent ethyl alcohol, isopropyl alcohol is cited. Said at least one organic solvent can be either alone or by weight of 2, 2 by weight of at least one battery when desired according to the blending ratio of the performance can be properly controlled. Said lithium salt is dissolved in an organic solvent, the underlying source of lithium ions act permitting failsafe operation of an electrochemical cell and, between cathode and anode and the material that promoting the movement of lithium ions. Said examples lithium salt, LiPF6 , LiBF4 , LiSbF6 , LiAsF6 , LiN (SO3 C2 F5 )2 , LiN (CF3 SO2 )2 , LiC4 F9 SO3 , LiClO4 , LiAlO2 , LiAlCl4 , LiN (Cx F2x+ 1 SO2 ) (Cy F2y+ 1 SO2 ) (X and y is natural number), LiCl, LiI, LiB (C2 O4 )2 Or a combination thereof is cited. Said lithium salt concentration 0. 1M to 2. 0M range can be use in. When said lithium salt concentration range, suitable electrolytic solution excellent in performance in the periphery of the electrolyte conductivity and viscosity may be, lithium ions can be effectively. In the embodiment according to said electrochemical cell of the present invention are specifically directed one lithium metal secondary battery, lithium ion secondary battery, lithium polymer secondary battery or lithium ion polymer secondary battery such as be a lithium secondary battery. Hereinafter, in the embodiment, the present invention could be compared and positive examples experiment by describing the more detailed as follows. But, in the embodiment of the following, embodiments of the present invention which in one example of the present invention comparing experiments and positive examples only in limited to content interpreted WD other. Number attainments Number attainments 1: a floating number bath Butyl methacrylate (Buthyl Methacrylate, BMA), methyl methacrylate (Methyl Methacrylate, MMA), vinyl acetate (Vinyl Acetate, VAc) is polymerized acrylic copolymer binder in a molar ratio 4/1/5 (Tg: 35 °C, Mw: 600K (GPC)) using a 10% by weight acetone (acetone) dissolving agent to binder solution number 1 number 2 in time 40 °C agitator agitating his high pressure liquid coolant. The addition of a 25% by weight alumina (LS235, Japanese light metal) after 2 hours 25 °C beads mill dispersion in acetone to alumina through the dispersion number was high pressure liquid coolant. The heater and alumina and alumina binder solution number 1 to 1/5 solid binder solids percentage when the mixed dispersion, 10% by weight on the total solid component is so porous adhesive layer number by adding acetone solution was high pressure liquid coolant. In polyethylene fabric thickness on both sides of each said porous adhesive layer composition (W scope) 12 micro m 2 m m total thickness layer liquid coating thickness smaller number 16 micro degree of micro-gate. Number attainments 2: a floating number bath Butyl methacrylate (Buthyl Methacrylate, BMA), methyl methacrylate (Methyl Methacrylate, MMA), vinyl acetate (Vinyl Acetate, VAc) is polymerized acrylic copolymer binder in a molar ratio 4/1/5 (Tg: 35 °C, Mw: 600K (GPC)) using a 10% by weight acetone (acetone) dissolving agent to binder solution number 1 number 2 in time 40 °C agitator agitating his high pressure liquid coolant. Production of binder KF9300 pVdF non-hFP (inferior oblique muscle [...], Mw: 1,200,000 g/mol), DMAc mixed solvent dissolving an active layer 4 to 7 weight % solids solutions in time number number 2 binder solution was 40 °C agitating high pressure liquid coolant. The addition of a 25% by weight alumina (LS235, Japanese light metal) after 2 hours 25 °C beads mill dispersion in acetone to alumina through the dispersion number was high pressure liquid coolant. On acrylic binder so that the binder weight ratio of 8/2 pVdF non-hFP, 1/5 the heater and percentage solids alumina solid binder to number 1, number 2 binder solution and alumina dispersion when the mixing, 10% by weight on the total solid component is porous adhesive layer number by adding acetone solution was so high pressure liquid coolant. In polyethylene fabric thickness on both sides of each said porous adhesive layer composition (W scope) 12 micro m 2 m m total thickness layer liquid coating thickness smaller number 16 micro degree of micro-gate. Number attainments 3: a floating number bath In acrylic binder weight ratio of 7/3 pVdF non-hFPattainments said number 2 is the number 2 and number under the outsideattainments binder mechanical layer method identical to the high pressure liquid coolant to his number. Number attainments 4: a floating number bath In acrylic binder weight ratio is 6/4 pVdF non-hFPattainments said number 2 off number 2 number attainmentsunder the outside binder and a method identical to the layer number to his high pressure liquid coolant. Number attainments 5: a floating number bath 2 acrylic binder weight ratio is 3/7 binder in said number attainmentspVdF non-hFP off number the method identical to the number 2 and number under the outsideattainments layer high pressure liquid coolant to him. Number attainments 6: a floating number bath 1 polyethylene fabric on one surface of said porous adhesive layer in said number attainments solution coating thickness layer 14 is lowered to about 2 micro m m is a total thickness of the cassette to the number 1 and number under the outsideattainments micro method was high pressure liquid coolant into the same layer number. Comparison number attainments 1: a floating number bath 2 acrylic binder weight ratio is 1/9 binder in said number attainmentspVdF non-hFP off number the method identical to the number 2 and number under the outsideattainments layer high pressure liquid coolant to him. Comparison number attainments 2: a floating number bath 2 acrylic binder weight ratio of binder in said number attainmentspVdF non-hFP 0. 5/9. 5 off number is the method identical to the number 2 and number under the outsideattainments layer high pressure liquid coolant to him. Comparison number attainments 3: a floating number bath In acrylic copolymer binder attainments without using said number 2, number 2 and number in the range of 0.1 under the outsideattainmentspVdF non-hFP binder only a method identical to the layer number to his high pressure liquid coolant. 1 to 6 and comparison number 1 to 3 according to each layer to said number attainmentsattainments binder composition exhibits to table 1. In the embodiment In the embodiment 1: electrode assembly number bath The anode in a LCO (LiCoO 2) deflection, binder include PVdF (Polyvinylidene Fluoride) 0.50 to, an anode active material include carbon black coating composition was high pressure liquid coolant conductive number number. In this case positive active material for lithium secondary battery coating composition: binder: a cup in a weight ratio of the conductive number 94:3:3, N - methyl - 2 - pyrrole (Planetary Despa Mixer) said positive active material coating composition mixer using doctor blade thickness 94 μm aluminum foil of 14 μm thickness after dispersing a pesticidal [...] separator and very dry. After this roll to both press and vacuum drying (vacuum dryer) facility in a stand-alone embodiment number number anode coating layer moisture to his high pressure liquid coolant. A negative electrode active material into a raw material graphite (Graphite) deflection, and CMC binder include SBR (styrene provided butadiene Rubber) (Carboxy Methyl Celluose) number was negative active material coating composition using high pressure liquid coolant. In this case, cathode active material: in the combustion chamber to said SBR binder weight ratio is 1:1 and the weight ratio of 96:4 CMC toenjang. After this, 8 μm thickness copper foil is coated with a separator 120 μm under the outside number is the same as the anode and cathode and number number tub was high pressure liquid coolant. Said anodes and cathodes 100cm × 4. Each cutting and 2 cm, attainments 100cm × 4 layer prepared by the number in said number 1. After plate 4 cm, 7 cm × 4 (longitudinal) and interposed between the positive and negative electrodes. 4 cm (width) by winding electrode assembly number was high pressure liquid coolant. In the embodiment 2: electrode assembly number bath In said in the embodiment 1, number patterns as in the embodiment 1 and the embodiment 2 in the range of 0.1 under the outsideattainments isolation method is equal to the number of electrode assembly number to his high pressure liquid coolant in the embodiment 2. In the embodiment 3: number bath electrode assembly In said in the embodiment 1, number 3 and number under the outsideattainments patterns as in the embodiment 1 embodiment the isolation method is in the range of 0.1 in the embodiment 3 was equal to the number of electrode assemblies to high pressure liquid coolant. In the embodiment 4: electrode assembly number bath In said in the embodiment 1, the number of layer patterns as in the embodiment 1 and 4 in the range of 0.1 under the outsideattainments number equal to the number of electrode assemblies in the embodiment 4 to embodiment was high pressure liquid coolant. In the embodiment 5: electrode assembly number bath In said in the embodiment 1, the number of layer patterns as in the embodiment 1 and embodiment 5 attainmentsunder the outside number in the range of 0.1 to equal to the number of electrode assembly was high pressure liquid coolant in the embodiment 5. In the embodiment 6: electrode assembly number bath In said in the embodiment 1, and number of patterns as attainments 6 uses a separation membrane, porous adhesive layer separation film surface and replacement anode, cathode surface in the embodiment 1 and the liquid layer porous adhesive with no embodiment in the embodiment 6 equal to the number of replacement under the outside to electrode assembly number was high pressure liquid coolant. Comparison example 1: electrode assembly number bath In said in the embodiment 1, the number 1 in the embodiment 1 and comparison number patterns as attainments isolation method is in the range of 0.1 under the outside comparison embodiment example 1 was equal to the number of electrode assemblies high pressure liquid coolant. Comparison example 2: electrode assembly number bath In said in the embodiment 1, the number of layer 2 in the range of 0.1 in the embodiment 1 and comparison patterns as attainmentsunder the outside comparison embodiment example number equal to the number of electrode assemblies 2 was high pressure liquid coolant. Comparison example 3: number bath electrode assembly In said in the embodiment 1, number 3 in the embodiment 1 and comparison patterns as a number in the range of 0.1 attainments isolation method is equal to the number of electrode assemblies under the outside comparison embodiment example 3 was high pressure liquid coolant. Experiment example Said in the embodiment 1 to 6 and comparison example 1 to 3 in number prepared by the electrode assembly for measuring flexural strength and pressing the disclosure below variable ratio of thickness measurement method, the result shown in table 2. Bending strength In the embodiment 1 to 6 and comparison example 1 to 3 in number prepared by the electrode assembly 9 kgf/cm2 Each pressure 80 °C, 90 °C, 100 °C, 10 seconds was 110 °C is in compression. Then, by using the 3 point bending machine (UTM) ASTM D790, 60 mm distance by electrode assembly directs [...] MD direction right-plate is connected to the speed 2. While lowering the electrode assembly at a rate of 8 mm/min were measured flexural strength. The rate of change of compression thickness In the embodiment 1 to 6 and comparison example 1 to 3 in number in 20 °C 9 kgf/cm electrode assembly prepared by the2 3 seconds at a pressure corresponding to thickness of 15 cm steel has been completed to crush the central portion 1 liver were measured. 9 kgf/cm in 100 °C2 1 pressing the pressure 10 seconds has been completed 15 cm steel thickness corresponding to central portions of liver were measured. 20 °C using equation below 100 °C in compression thickness and in compression thickness through a variable ratio of thickness were measured. The rate of change (%) compression thickness=[(20 °C press electrode assembly thickness of -100 °C press thickness of electrode assembly) thickness of electrode assembly press/20 °C] × 100 Cycle after variable ratio of thickness Said in the embodiment 2, 3 and comparison example 3 using a high pressure liquid coolant downwardly in the battery electrode assembly prepared by the number in number after method, after cycle measuring variable ratio of thickness, for table 3 have shown to result. 9 kgf/cm 2, 3 and comparison example 3 in said in the embodiment of electrode assembly 100 °C 3 seconds2 After compression force, which can be aluminum pouch was sealing into the electrolyte. The electrolyte solution is 1. 1M concentration of ethylene carbonate (EC)/ethyl methyl carbonate (EMC)/LiPF6 is dissolved (volume ratio EC: EMC 30/70 mixture ratio) mixed organic solvent 2. 7 g was used. After this, 12 time after storage at room temperature, 100 °C 9 kgf/cm in 30 seconds2 After compression force, 12 with respect to the storage time at room temperature. After this, in insect discharge 0. 2C conditions then damage to a embodiment 1 time supplement, 15 cm steel using an electrode assembly of a center part thickness were measured. After this, the base of a stand-alone gas pouch after number 0. 7C certain interval symmetrically about an embodiment 500 times were measured thickness and central part of the electrode assembly. the reference table 2, 3, or using only acrylic copolymer, acrylic copolymer and polyvinylidene fluoride binder in weight 9. 9:0. 1 to 2. 5:7. 5 when used, the rate of change is 10% or more compression thickness appear, an excellent combination has been confirmed that the adhesion between the plasticizer. In addition flexural strength is 15 kgf/cm2 Appear above, electrode assembly in the form of excellent stability has been confirmed. The electrode assembly inserted in the trench provides a reaction can be reducing the reject, process from long period of time. Even unchanged form long-lasting charge can be minimized. This cycle has been confirmed that the thickness change rate after 0.1 through 10% hereinafter. Through, charge and discharge characteristics may have efficiency which has cell prepared by the number, of the battery performance can be prevented from being lowered. The present invention relates to a separator comprising a coating layer and a battery using the same, the separator having improved adhesive force to an electrode, thereby minimizing the rate of thickness change. More specifically, the present invention relates to a separator having a coating layer on one or both surfaces of a base film, the coating layer comprising an acrylic-based copolymer having a glass transition temperature of less than or equal to 80° C. and an inorganic particle, so as to have improved adhesive force and heat resistance, thereby being applicable in electrochemical batteries of various sizes and having excellent thermal stability and dimensional stability. Liquid for anode active material layer and said anode current collector is formed anode active material including an anode; a cathode active material layer and said liquid for cathode current collector including from a negative electrode active material; and said separation film interposed between an anode and a cathode which, said porous substrate a plurality of pixels, and said porous adhesive layers formed on one or two surfaces of the porous substrate, repeating units derived from (meth) acrylate monomer and said porous adhesive layer including acrylic copolymer and polyvinylidene fluoride acetate-containing monomer derived repeating units comprising a binder, said binder weight ratio of 9 said acrylic copolymer and polyvinylidene fluoride. 9:0. 1 to 2. 5:7. 5 in electrode assembly. According to Claim 1, 1 seconds to 15 seconds in said electrode assembly includes a 20 °C to 110 °C, 1 kgf/cm2 To 30 kgf/cm2 After compression force, three point bending tester (3 point bending machine) measured using flexural strength is 15 kgf/cm2 Or more electrode assembly. Back number According to Claim 1, said (meth) acrylate monomer derived from (meth) acrylate repeating units of the methyl, ethyl (meth) acrylate, propyl (meth) acrylate and butyl (meth) acrylate to form repeating units derived from at least one monomer selected from the group consisting 1 electrode assembly units. According to Claim 1, said acetate containing monomer repeating units of the allyl acetate or vinyl acetate repeating units derived from units derived from electrode assembly. According to Claim 1, said porous adhesive layer further includes inorganic particles, said porous adhesive layer 70% by weight to 95% by weight based on the total weight wherein inorganic particles are in electrode assembly. Back number According to Claim 1, said polyvinylidene fluoride-based binder polyvinylidene fluoride - hexafluoropropylene (polyvinylidenefluoride-a hexafluoropropylene, pVDF non-hFP), polyvinylidene fluoride - process for the preparation (polyvinylidene fluoride-a trichloroethylene, pVDF-a tCE), polyvinylidene - chloro with tree flow ethylene (polyvinylidenefluoride-a trifluoroethylene, pVDF non-cTFE) 1 the yarn is of the at least one electrode assembly. Back number According to Claim 1, formula 1 thickness change rate at least 10% compression of said electrode assembly includes a electrode assembly. The rate of change (%) compression thickness [type 1]=[(20 °C press electrode assembly thickness of -100 °C press thickness of electrode assembly) thickness of electrode assembly press/20 °C] × 100 said in type 1, the thickness of the layer/anode/cathode electrode assembly press 20 °C order 1 to 10 seconds in stacked electrode assembly 20 °C, 1 to 30 kgf/cm2 Pressing the central portion of the electrode assembly has been completed pressure measured thickness and is 1, 1 to 10 seconds in the thickness of the electrode assembly press 100 °C 100 °C, 1 to 30 kgf/cm2 Pressure pressing the central portion of the electrode assembly 1 measure after time thickness are disclosed. Anti number 1, number 2 anti, anti number 4 to number 6 anti, anti number 8, and anti either as described in claim number 10, said electrode assembly including electrochemical cell. According to Claim 11, said lithium polymer secondary battery or lithium ion polymer secondary battery is an electrochemical cell includes an electrochemical cell. Anode current collector anode active material layer is formed by high pressure liquid coolant and number anode, cathode current collector cathode active material layer is formed a cathode and number and high pressure liquid coolant, and assigns a layer between said positive and negative electrodes, said anode/membrane/cathode structure 20 °C to 110 °C 1 seconds to 10 seconds in 1 kgf/cm2 To 30 kgf/cm2 Pressing the being, said porous substrate a plurality of pixels, and said porous adhesive layers formed on one or two surfaces of the porous substrate, said porous adhesive layer (meth) acrylate monomer including acrylic copolymer derived from repeating units derived from acetate containing monomer repeating units and including electrode assembly number bath method. According to Claim 13, 30 seconds to 180 seconds after pressing into said electrolyte in 60 °C to 110 °C, 1 kgf/cm2 To 30 kgf/cm2 The difference 2 further including pressing, number bath method. According to Claim 13, said electrode assembly includes a 20 °C to 110 °C 1 seconds to 15 seconds in, 1 kgf/cm2 To 30 kgf/cm2 After compression force, three point bending tester (3 point bending machine) measured using flexural strength is 15 kgf/cm2 Or more, number bath method. According to Claim 13, said (meth) acrylate monomer derived from (meth) acrylate repeating units of the methyl, ethyl (meth) acrylate, propyl (meth) acrylate and butyl (meth) acrylate to form repeating units derived from at least one monomer selected from the group consisting 1 units, number bath method. Acrylic binder pVdF non-hFP 1 attainments number 100 0 2 attainments number 80 20 3 attainments number 70 30 4 attainments number 60 40 5 attainments number 30 70 6 attainments number 100 0 1 attainments number comparison 10 90 2 attainments number comparison 5 95 3 attainments number comparison 0 100 Press temperature by bending strength (kgf/cm2 ) Compression thickness (mm) and pressing variable ratio of thickness (%) 80 °C 90 °C 100 °C 110 °C 20° Cpress 100° Cpress The rate of change (%) In the embodiment 1 43 47 50 50 6. 4 5 21. 9 In the embodiment 2 41 44 47 48 6. 4 5 21. 9 In the embodiment 3 36 39 40 40 6. 4 5 21. 9 In the embodiment 4 17 20 23 24 6. 4 5. 4 15. 6 In the embodiment 5 15 20 23 25 6. 4 5. 5 14. 1 In the embodiment 6 39 40 42 43 6. 38 5 21. 6 Comparison example 1 7 8 9 10 6. 4 5. 9 7. 8 Comparison example 2 3 5 6 6 6. 4 6. 0 6. 3 Comparison example 3 - (Not bends point, measuring section) 6. 4 6. 3 1. 6 Thickness (mm) after auxiliary 500 cycle after thickness (mm) Cycle (%) after variable ratio of thickness In the embodiment 2 3. 2 3. 5 9 In the embodiment 3 3. 2 3. 5 9 Comparison example 3 3. 2 3. 7 16