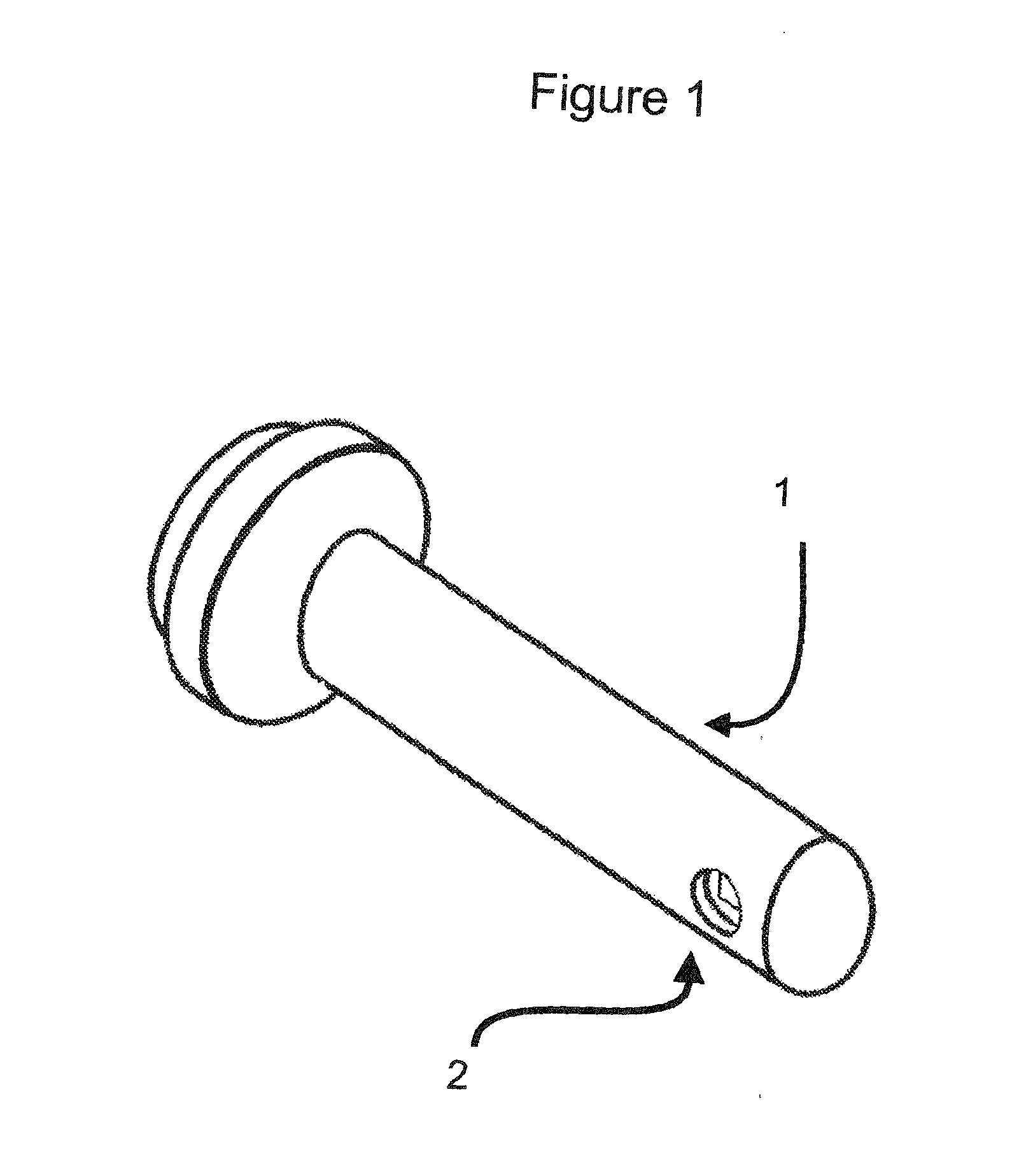
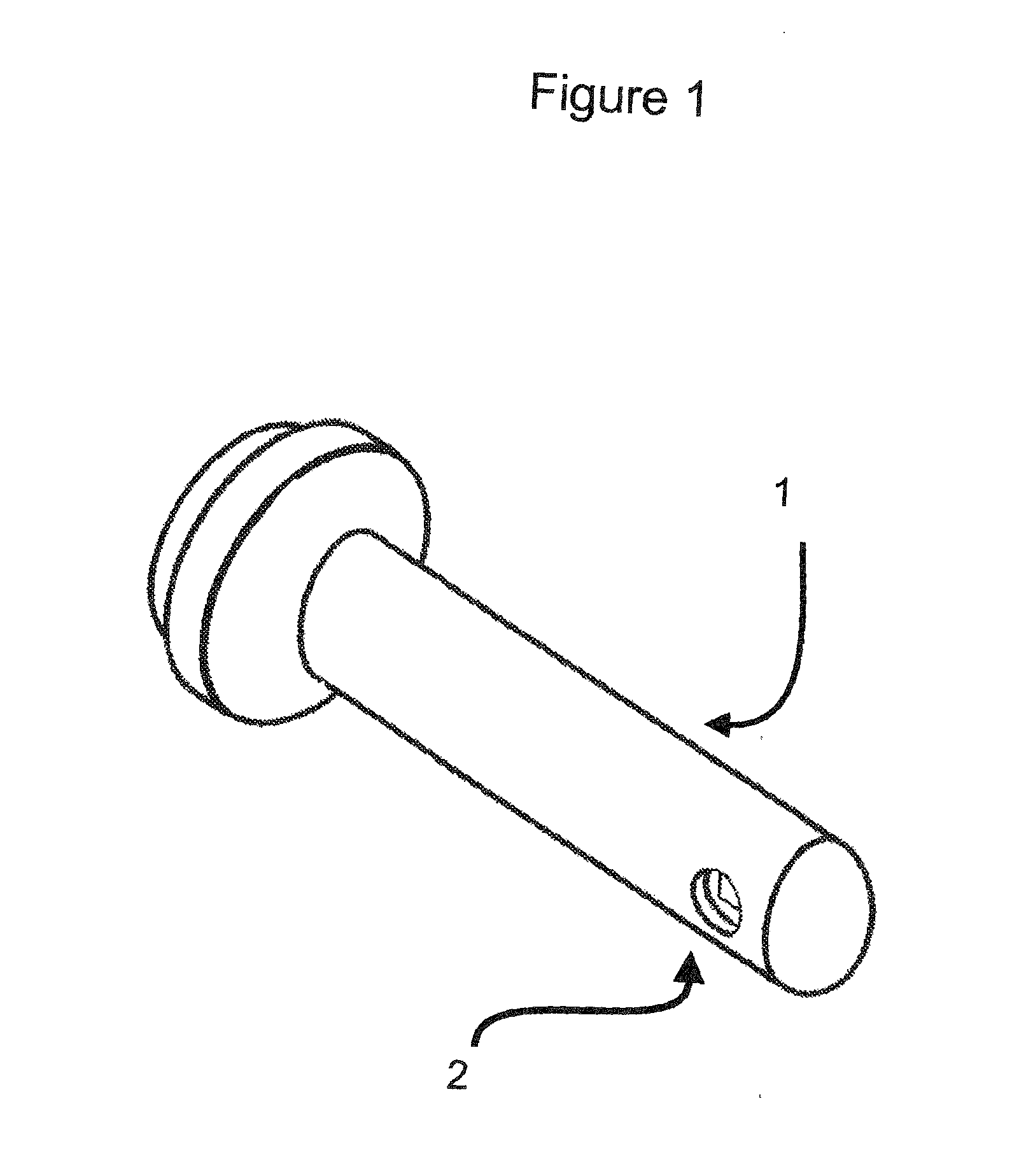
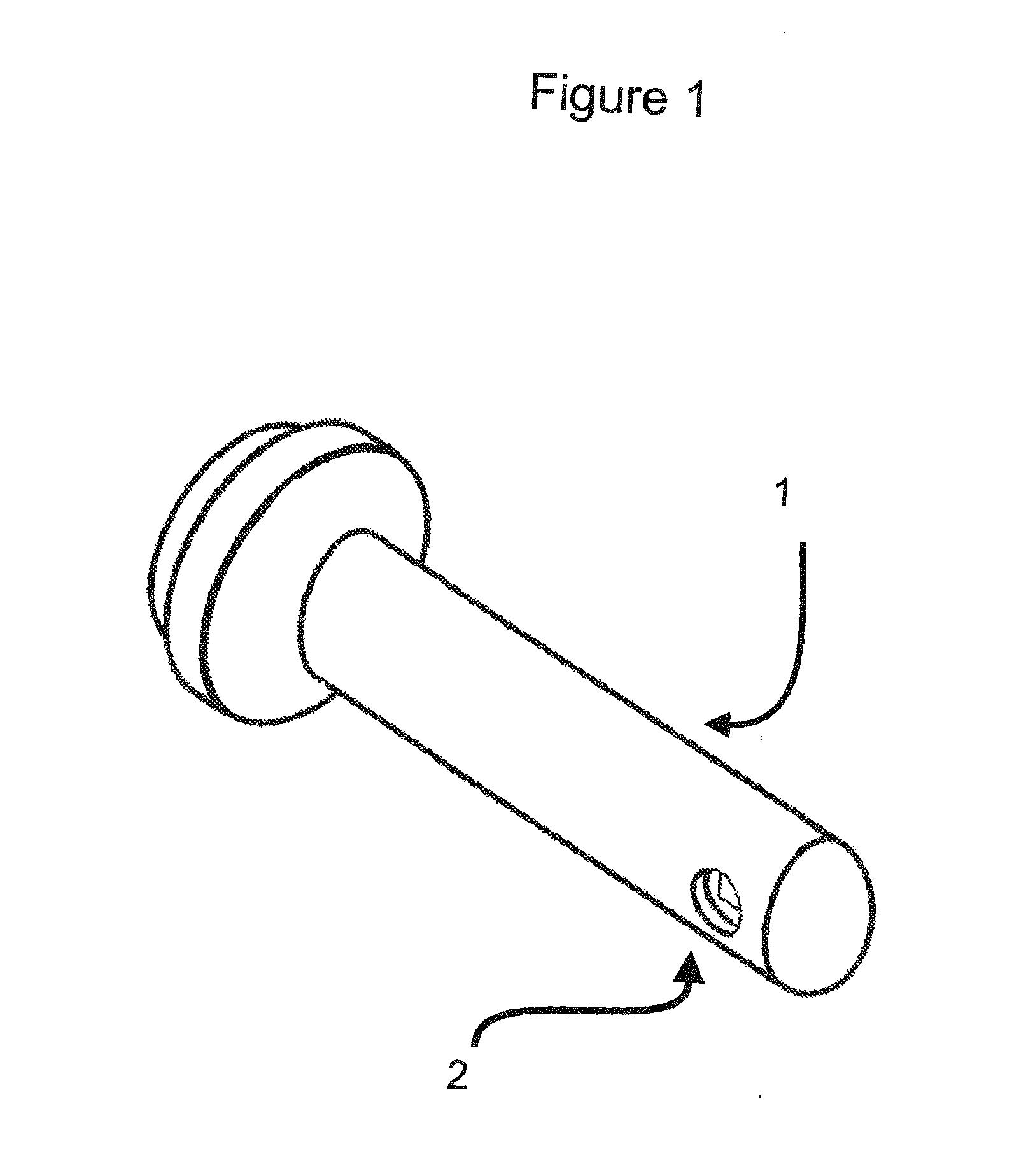
VAGINAL BIOMECHANICS ANALYZER
This application claims priority to and is a continuation-in-part of U.S. patent application Ser. No. 13/564,682, filed Aug. 1, 2012, which claims priority to U.S. Provisional Application Ser. No. 61/574,290, filed Aug. 1, 2011, the entire contents of which are incorporated herein by reference. The present invention relates in general to the field of biomechanical skin analyzers, and more particularly, to a novel biomechanical tissue analyzer. None. Without limiting the scope of the invention, its background is described in connection with pelvic organ prolapse. The present invention relates to an electro mechanical device that measures skin elasticity for assessing the viscoelastic properties of the anterior wall of the vagina. Vaginal wall tissue deterioration can cause pelvic organ prolapse (POP), a hernia of the pelvic organs to or through the vaginal opening. POP affects a large number of aging women that often necessitates surgical repair and tends to recur over time. Approximately 200,000 operations are performed yearly in the United States for POP. Although not life threatening, POP is life altering and results in significant quality of life changes in women. Medical researchers have studied vaginal wall properties in freshly excised tissue, at the time of surgery, using an Instron tensile testing machine but this is limited by its applicability, namely patients requiring surgery. Currently, evaluation of the vaginal wall is limited to physical examination and imaging modalities. There are no quantitative and practical devices that a physician can use during an office visit to measure the unique viscoelastic properties of the vagina to objectively determine tissue deterioration. The ability to measure the elasticity of the inner walls of the vagina in healthy patients for study controls, patients in less advanced degrees of POP, patients before and after surgical repair and patients on hormonal therapy will lead to a myriad of common vaginal interventions, from pelvic floor therapy to reconstructive surgery. Like the thermometer to objectively determine how sick a feverish patient is, the present invention will serve as a diagnostic resource for clinicians and researchers interested in the management of POP. Skin elasticity measurement devices include US Patent Application Publication No. US 2008/0234607 A1. In this US Patent Application, the user applies a vacuum to a chamber that is placed over an area of the skin. When the vacuum draws the skin through an opening a video camera in an adjacent chamber captures light reflected from the skin. U.S. Pat. No. 7,955,278 B1 creates a vacuum that draws the skin into a chamber until the skin reaches the vacuum tube in the chamber. The vacuum pressures are measured and pressure changes are used to calculate elasticity. U.S. Pat. No. 5,278,776 describes the use of a camera that monitors the movement of dots placed on the skin. When the vacuum is applied the skin moves into the chamber causing the dots to move. The elasticity is determined by the dot separation. The present invention is a safe, easily insertable, user-friendly, and quickly sterilizable vaginal device that would allow rapid and reproducible measurements of different areas of the vagina, in the office setting. The present invention is simple to use and extremely accurate. The probe design is small enough to be inserted in the vagina, yet precisely measure the tissue deflection and recovery under mild suction and vacuum release. The stored data for each patient can be compared to previously collected data to detect the changes in tissue elasticity. For the first time, the present invention allows for a direct in-vivo measurement of vaginal wall tissue properties. In one embodiment, the present invention includes a device for measuring skin elasticity comprising: a probe, wherein the probe comprises one or more holes, a vacuum source, a pressure sensor, and one or more proximity sensors aligned about the one or more holes; and a processor for recording the deformation of the skin using a control unit comprising a microcontroller connected to the proximity and the pressure sensors, wherein the proximity sensor and the processor is adapted to automatically initiate a test when the sensor is positioned at a pre-determined distance from the skin, wherein a vacuum in the probe is capable of pulling skin into the one or more holes and the proximity sensor is capable of measuring an amount of skin drawn into the one or more holes to determine an elasticity of the skin. In one aspect, the control unit further comprises a switch, an electronic control valve, and a liquid crystal display, and wherein the wand assembly is defined further as comprising a detachable handle and the probe. In another aspect, the control unit records and stores a vacuum data and a proximity sensor data. In another aspect, the proximity sensor comprises a camera capable of detecting extend and shape of skin deflection. In another aspect, the probe further comprises at least one orifice that comprises a membrane to determine the rheological properties of a liquid on or about the skin. In another aspect, the processor calculates the skin elasticity of the inner walls of the vagina. In another aspect, the one or more proximity sensors are capable of detecting and measuring the shape of a body cavity into which the probe is inserted. In another aspect, the handle further comprises a circuit board, a proximity sensor, a data cable connection, and a vacuum tube connection. In another aspect, the proximity sensor is mounted on a circuit board in the probe and attached to the handle. In another aspect, when the probe is attached to the handle, the proximity sensor fits under and aligns with a hole in the probe. In another aspect, the area surrounding the hole is polished. In another aspect, the proximity sensor is configured to measure a distance the skin recoils when the vacuum is released. In another aspect, the device is adapted to measure biomechanical measurements of normal and lesion-rich regions of the mouth (cheek, tongue, gingiva); rectum (assessment of fecal incontinence, rectal tumors, polyps); airway (trachea); or gastrointestinal tract (esophagus, stomach, duodenum, small intestine, large intestine); cardiovascular (heart, arteries or veins); or bladder (bladder wall compliance and degree of detrusor muscle wall aging). In another aspect, the device is adapted for deployment via a catheter. In another aspect, the device further comprises a memory connected to the processor and stores the data from the proximity sensor data for immediate processing or processing at a later time. Another embodiment of the present invention includes a device for analyzing a skin elasticity comprising: a wand assembly comprising a hole, a probe, a proximity sensor, and a vacuum source, wherein the probe and proximity sensor measure skin elasticity; an electronic control unit configured to record the deformation of the skin measured by the probe and proximity sensor, wherein the proximity sensor and the processor is adapted to automatically initiate a test when the sensor is positioned at a pre-determined distance from the skin; and a stand capable of positioning and supporting the wand assembly in three-dimensions to permit hands-free operation of the device. In one aspect, the electronic control unit further comprises an electronic control valve, a vacuum pump, a microcontroller, and a pressure sensor. In another aspect, the electronic control unit records and stores a vacuum data and a proximity sensor data. In another aspect, the electronic control unit further comprises a visual display. In another aspect, the wand assembly comprises the circuit board with the proximity sensor, a data cable connection, and a vacuum tube connection. In another aspect, the probe further comprises at least one orifice that comprises a membrane to determine the rheological properties of a liquid on or about the skin. In another aspect, the probe is configured to hold a vacuum when the proximity sensor measures the amount of skin being pulled through a hole in the probe. In another aspect, the area surrounding the hole is polished. In another aspect, the device is adapted to measure biomechanical measurements of normal and lesion-rich regions of the mouth (cheek, tongue, gingiva); rectum (assessment of fecal incontinence, rectal tumors, polyps); airway (trachea); or gastrointestinal tract (esophagus, stomach, duodenum, small intestine, large intestine); cardiovascular (heart, arteries or veins); or bladder (bladder wall compliance and degree of detrusor muscle wall aging). In another aspect, the device is adapted for deployment via a catheter. Yet another embodiment of the invention includes a method for analyzing skin elasticity comprising: obtaining a device for analyzing a skin elasticity comprising: a wand assembly having a vacuum source to measure skin elasticity, the wand assembly comprising one or more holes, wherein a probe, a proximity sensor and a vacuum are aligned with the one or more holes; an electronic control unit configured to record the deformation of the skin measured; inserting the device for analyzing the skin elasticity into a body cavity, wherein the proximity sensor and the processor is adapted to automatically initiate a test when the sensor is positioned at a pre-determined distance from the skin; measuring the skin elasticity using the wand assembly of the inserted device to obtain a skin elasticity data; recording the skin elasticity data using the inserted device; and analyzing the recorded skin elasticity data to determine the skin elasticity. In one aspect, the skin elasticity data further comprises measuring and recording a skin movement, a change in vacuum pressure, and an increment of time. In another aspect, the analyzing of the data recorded by the device is used to generate a visual representation of the skin elasticity. In another aspect, the step of measuring the skin elasticity using the wand assembly further comprises the steps of pulling and releasing a vacuum through the opening, and measuring the deformation between the skin and the proximity sensor with or without the vacuum over time to determine the skin elasticity. In another aspect, the probe further comprises at least one orifice that comprises a membrane to determine the rheological properties of a liquid on or about the skin. In another aspect, the skin elasticity data is further processed by at least one of: assessing a deformation of the skin under load; matching the compliance of a surgical mesh to that of the vaginal wall in order to enhance healing; or predicting the effects of remote perturbations of the pelvic organs (abdominal pressure, pelvic bending and twisting) on a vaginal configuration. In another aspect, the method comprises adapting the device to measure biomechanical measurements of normal and lesion-rich regions of the mouth (cheek, tongue, gingiva); rectum (assessment of fecal incontinence, rectal tumors, polyps); airway (trachea); or gastrointestinal tract (esophagus, stomach, duodenum, small intestine, large intestine); cardiovascular (heart, arteries or veins); or bladder (bladder wall compliance and degree of detrusor muscle wall aging). For a more complete understanding of the features and advantages of the present invention, reference is now made to the detailed description of the invention along with the accompanying figures and in which: While the making and using of various embodiments of the present invention are discussed in detail below, it should be appreciated that the present invention provides many applicable inventive concepts that can be embodied in a wide variety of specific contexts. The specific embodiments discussed herein are merely illustrative of specific ways to make and use the invention and do not delimit the scope of the invention. To facilitate the understanding of this invention, a number of terms are defined below. Terms defined herein have meanings as commonly understood by a person of ordinary skill in the areas relevant to the present invention. Terms such as “a”, “an” and “the” are not intended to refer to only a singular entity, but include the general class of which a specific example may be used for illustration. The terminology herein is used to describe specific embodiments of the invention, but their usage does not delimit the invention, except as outlined in the claims. The present invention relates to an apparatus that measures the elasticity of skin. Skin elasticity is measured to determine the effects of medications, skin creams, surgery procedures and the effects of aging. The present invention is designed to measure the skin elasticity of the inner walls of the vagina to detect changes in the integrity of connective tissues in the vagina. The present invention includes a small probe that allows a physician to easily perform elasticity measurements on patients during a regular office exam. The present invention provides the physician with a medical device to determine, among other conditions, if a woman is susceptible to prolapse, a condition that happens when the bladder falls down into the vagina. Skin elasticity is calculated from the data derived from the combination of vacuum pressure, time, the amount of skin pulled by the vacuum, the length of time the skin returns to the original shape, and the recoil reaction of the skin. The values are collected, calculated, and stored by the microcontroller unit or MCU and then down loaded to a computer through a data port or USB port. The data can be compiled by a computer program to display tables, plot graphs, indicate changes in the vaginal wall elasticity and assist physicians to diagnose any change of elasticity and the probability of prolapse and other conditions related to vaginal disease. The present invention is not limited to the vagina skin elasticity measurement. The present invention can test elasticity of any skin on any area of the body of any living animal. The present invention will also test the elasticity of flexible materials such as rubber, vinyl, foams or other elastic materials. As discussed in greater detail in At step 41 of Additional modifications of the present invention included: a 4.3″ diagonal graphic screen that displays the graph of the skin deformation in real time. The display is programmed to show three consecutive graphs for comparing the results. Optionally, another feature assists the clinician before each test to accurately synch the initiation of a measurement when the proximity sensor indicates the minimal amount of force applied to the probe against the skin to create a vacuum seal without deforming the skin. When the force is in range the test automatically begins. The force or proximity sensor feature improved test results and reduced the overall time for testing. Finally, a new probe was design that changed the shape and reduced the size of the probe shaft. The latest version of the probe was reduced to an approximate diameter of 0.65″, which is approximately the size of a finger. The exterior of the probe can also be covered with a soft or viscoelastic material, and/or provided with one or more joints such that the probe can be flexed to provide a better fit for insertion into a body orifice or to reach a specific location with minimal discomfort or damage to underlying or adjacent tissues. The probe of the present invention allows for in vivo, reliable and reproducible, hand-free, quantitative measurements of the biomechanical properties of the human anterior vaginal wall. These properties include elastic deformation during suction, followed by visco-elastic changes during the return to baseline. Another optional feature of the present invention is that if the system for some reason loses power or the connection is lost during recording, the data is saved on a memory connected to the processor and the data can be retrieved at a later time. In addition, more memory can be added to the circuit board so that all the information collected in a day can be transferred immediately, or stored and processes all at once for analysis, or can be processed sequentially throughout the day. Yet another optional feature is polishing the surface of the area around the hole. It has been found that this greatly improved suction when just barely touching the skin. In one non-limiting example, the surface surrounding the hole is polished to the point that there are no longer any visible scratches. The anterior vaginal wall is the location most susceptible to pelvic organ prolapse (POP) compartment conditions because this is the area where increases in abdominal pressure (by coughing, straining, etc.) apply first. Generally, the top or apex of the vagina and the back wall or posterior compartment, meanwhile are more protected and therefore less subject to prolapse. The probe has been designed to create rapid deformations within one second suction time-intervals as well as longer deformations over six seconds to reproduce straining efforts. The device can also include surface markers, which serve to measure the anterior vaginal wall's biomechanical properties at different locations and the extent of insertion of the device; firstly, around 3 cm from the vaginal entrance or introitus, corresponding to the level of the bladder neck region; secondly, at 5 cm from the introitus, corresponding to the area of the bladder base. The probe can record several measurements at the same location. Each curve is composed of measurements obtained every 1/10thof a second. It was found that the probe provides an accurate and reliable vaginal biomechanics measurement device that is urgently needed to address the growing demands in the management of POP. POP affects a large number of aging women, tends to recur over time and often requires surgical repair. The severe changes in quality of life for those afflicted demand a major improvement in the standard of care. The best tools for examination at present are qualitative, based primarily on visual inspection and finger palpation. But these measures are suboptimal in important ways because they are: (1) subject to operator variability; (2) do not allow quantitative time series histories; and thus (3) cannot provide accurate metrics. Therefore, the “intelligent finger” of the present invention is able to provide quantitative information for tracking the intrinsic properties of, e.g., the human vaginal wall over time. Role in diagnosis: The degree of vaginal wall tissue impairment cannot be judged at present. Clinicians have learned to distinguish well-vascularized, strong, thick tissues with deep rugae in young healthy nulliparous women from thin tissues with effaced rugae and atrophic changes, characteristic of post-menopausal women. Presently, these are purely descriptive observations and not consistent because as POP progresses, the vaginal tissue becomes even more lax and thinner. With aging, the coloration and degree of tissue elasticity can also change towards a more pale and stretchable vaginal wall, but there is no current technique to measure, and thereby diagnose, such changes over time from the same area of the vaginal wall. Role in surgical treatments: The present invention can also be used to reduce the risks of serious complications from trans-vaginal mesh usage for POP repair, because it can be used to test important vaginal wall tissue properties. The present invention can be used to: (1) match mesh biomechanical properties to natural vaginal wall tissue properties, in order to guide improved surgical mesh design; (2) determine suitable candidates for mesh interposition when the vaginal wall exhibits very lax parameters; (3) follow remodeling of tissue changes over time; and (4) assess the quality of surgical healing. Role in non-surgical treatments: Pelvic floor therapy as well as local vaginal wall therapy using hormonotherapy or laser vaginal rejuvenation techniques will benefit from a direct measurement to assess progress in vaginal wall tone and elasticity. The present invention can also be used to determine if the proposed intervention has reached its goal. Nomograms of the vaginal wall tissue's intrinsic properties can be provided using the present invention to determine the range of normalcy with aging changes. The present invention can be used to determine if the abnormal tissues have returned to a normal range as would be expected if the intervention was successful. The present invention can be used for long-term monitoring of the intervention's results. Role in prevention: Existing literature suggests a strong relationship between pregnancy and/or delivery mode (vaginal versus C-section), and the occurrence of POP later on in life. Using the present invention the user is able to measure from early on these intrinsic changes that arise when damage is detected in the post-partum phase. Thus, the present invention can be used to assess the status of the tissue to more specifically guide one or several interventions to consolidate existing tissues found to have incurred early damage, including: (1) straining prevention (avoiding heavy lifting, avoiding straining from constipation, maintaining proper body weight, tailoring sport activities to condition, treating coughing conditions, avoiding smoking); (2) hormonal supplementation when indicated, and (3) pelvic floor reeducation programs. The present invention can also be used for tracking the stabilization of early damage using bioabsorbable agents for pelvic tears, and for culturing and directly re-injecting vaginal smooth muscle cells for vaginal wall tone improvement. There are also populations at risk of POP, based either on race or on familial pre-disposition. These women's conditions have not been well characterized in the past due to the lack of a quantitative and qualitative tool to measure their vaginal wall tissue properties. The present invention provides a simple and non-invasive measurement device that can be part of the early stages of examination of young women post-puberty to determine their vulnerability to developing POP later on in life. Pelvic organ prolapse affects millions of women worldwide and many will need surgical repair of their vaginal wall hernias. The present invention allows for the first time a direct, rapid, simple, painless, and reproducible, office-based quantification of biomechanical vaginal wall tissue properties. These intrinsic changes will be measurable not only over time for preemptive approaches in at-risk populations, but also when damage is suspected post-partum or when guidance is needed for repair procedures later on in life to restore quality of life. The applications for use of the present invention include, but are not limited to the following:
In addition it was found that the present invention has additional advantages, namely: the probe of the present invention is small enough (size of a finger) that it does not require a speculum for insertion. The only variable is the skin movement. For each test, the vacuum pressure is generally always the same value and the vacuum duration is always the same as well. The infrared (IR) detector is located under the opening or aperture at the tip of the probe to directly measure the movement of the skin. The sync feature assures that the probe is placed properly and at the same pressure against the skin in order to standardize every test performed on the patient now and in the future. Too much pressure against the skin will stretch the skin and would skew the results. Good measurement reproducibility is a strength of the device of the present invention. The graphs are instantly displayed in real time and are a direct representation of skin movement. The present inventors have observed that the vaginal skin moves differently as it relaxes from the instant drop in vacuum pressure. The probe is ultrasensitive and can detect skin rebounds past zero (like a rubber band) once the pressure drops. The present invention can detect these minute variations. Furthermore, the present invention is sufficiently simple to be able to use a simple MCU (micro control unit) processor, as such; a complete computer is not a requirement to perform the test, which lowers the cost. The length of the device permits measurements at different locations along the vaginal wall or in other body orifices. The device can also be held by a tripod to avoid manual interference with the measurements, thus enhancing data reproducibility. Finally, the device can be calibrated to ensure reliable measurements over time. It is contemplated that any embodiment discussed in this specification can be implemented with respect to any method, kit, reagent, or composition of the invention, and vice versa. Furthermore, compositions of the invention can be used to achieve methods of the invention. It will be understood that particular embodiments described herein are shown by way of illustration and not as limitations of the invention. The principal features of this invention can be employed in various embodiments without departing from the scope of the invention. Those skilled in the art will recognize, or be able to ascertain using no more than routine experimentation, numerous equivalents to the specific procedures described herein. Such equivalents are considered to be within the scope of this invention and are covered by the claims. All publications and patent applications mentioned in the specification are indicative of the level of skill of those skilled in the art to which this invention pertains. All publications and patent applications are herein incorporated by reference to the same extent as if each individual publication or patent application was specifically and individually indicated to be incorporated by reference. The use of the word “a” or “an” when used in conjunction with the term “comprising” in the claims and/or the specification may mean “one,” but it is also consistent with the meaning of “one or more,” “at least one,” and “one or more than one.” The use of the term “or” in the claims is used to mean “and/or” unless explicitly indicated to refer to alternatives only or the alternatives are mutually exclusive, although the disclosure supports a definition that refers to only alternatives and “and/or.” Throughout this application, the term “about” is used to indicate that a value includes the inherent variation of error for the device, the method being employed to determine the value, or the variation that exists among the study subjects. As used in this specification and claim(s), the words “comprising” (and any form of comprising, such as “comprise” and “comprises”), “having” (and any form of having, such as “have” and “has”), “including” (and any form of including, such as “includes” and “include”) or “containing” (and any form of containing, such as “contains” and “contain”) are inclusive or open-ended and do not exclude additional, unrecited elements or method steps. In embodiments of any of the compositions and methods provided herein, “comprising” may be replaced with “consisting essentially of” or “consisting of”. As used herein, the phrase “consisting essentially of” requires the specified integer(s) or steps as well as those that do not materially affect the character or function of the claimed invention. As used herein, the term “consisting” is used to indicate the presence of the recited integer (e.g., a feature, an element, a characteristic, a property, a method/process step or a limitation) or group of integers (e.g., feature(s), element(s), characteristic(s), propertie(s), method/process steps or limitation(s)) only. The term “or combinations thereof” as used herein refers to all permutations and combinations of the listed items preceding the term. For example, “A, B, C, or combinations thereof” is intended to include at least one of: A, B, C, AB, AC, BC, or ABC, and if order is important in a particular context, also BA, CA, CB, CBA, BCA, ACB, BAC, or CAB. Continuing with this example, expressly included are combinations that contain repeats of one or more item or term, such as BB, AAA, AB, BBC, AAABCCCC, CBBAAA, CABABB, and so forth. The skilled artisan will understand that typically there is no limit on the number of items or terms in any combination, unless otherwise apparent from the context. As used herein, words of approximation such as, without limitation, “about”, “substantial” or “substantially” refers to a condition that when so modified is understood to not necessarily be absolute or perfect but would be considered close enough to those of ordinary skill in the art to warrant designating the condition as being present. The extent to which the description may vary will depend on how great a change can be instituted and still have one of ordinary skilled in the art recognize the modified feature as still having the required characteristics and capabilities of the unmodified feature. In general, but subject to the preceding discussion, a numerical value herein that is modified by a word of approximation such as “about” may vary from the stated value by at least ±1, 2, 3, 4, 5, 6, 7, 10, 12 or 15%. Additionally, the section headings herein are provided for consistency with the suggestions under 37 CFR 1.77 or otherwise to provide organizational cues. These headings shall not limit or characterize the invention(s) set out in any claims that may issue from this disclosure. Specifically and by way of example, although the headings refer to a “Field of Invention,” such claims should not be limited by the language under this heading to describe the so-called technical field. Further, a description of technology in the “Background of the Invention” section is not to be construed as an admission that technology is prior art to any invention(s) in this disclosure. Neither is the “Summary” to be considered a characterization of the invention(s) set forth in issued claims. Furthermore, any reference in this disclosure to “invention” in the singular should not be used to argue that there is only a single point of novelty in this disclosure. Multiple inventions may be set forth according to the limitations of the multiple claims issuing from this disclosure, and such claims accordingly define the invention(s), and their equivalents, that are protected thereby. In all instances, the scope of such claims shall be considered on their own merits in light of this disclosure, but should not be constrained by the headings set forth herein. All of the compositions and/or methods disclosed and claimed herein can be made and executed without undue experimentation in light of the present disclosure. While the compositions and methods of this invention have been described in terms of preferred embodiments, it will be apparent to those of skill in the art that variations may be applied to the compositions and/or methods and in the steps or in the sequence of steps of the method described herein without departing from the concept, spirit and scope of the invention. All such similar substitutes and modifications apparent to those skilled in the art are deemed to be within the spirit, scope and concept of the invention as defined by the appended claims. The present invention includes a device and method for measuring skin elasticity that comprises: a probe, wherein the probe comprises one or more holes, a vacuum source, a pressure sensor, and one or more proximity sensors aligned about the one or more holes; and a processor for recording the deformation of the skin using a control unit comprising a microcontroller connected to the proximity and the pressure sensors, wherein the proximity sensor and the processor is adapted to automatically initiate a test when the sensor is positioned at a pre-determined distance from the skin, wherein a vacuum in the probe is capable of pulling skin into the one or more holes and the proximity sensor is capable of measuring an amount of skin drawn into the one or more holes to determine an elasticity of the skin. 1. A device for measuring skin elasticity comprising:
a probe, wherein the probe comprises one or more holes, a vacuum source, a pressure sensor, and one or more proximity sensors aligned about the one or more holes; and a processor for recording the deformation of the skin using a control unit comprising a microcontroller connected to the proximity and the pressure sensors, wherein the proximity sensor and the processor is adapted to automatically initiate a test when the sensor is positioned at a pre-determined distance from the skin, wherein a vacuum in the probe is capable of pulling skin into the one or more holes and the proximity sensor is capable of measuring an amount of skin drawn into the one or more holes to determine an elasticity of the skin. 2. The device of 3. The device of 4. The device of 5. The device of 6. The device of 7. The device of 8. The device of 9. The device of 10. The device of 11. The device of 12. The device of 13. The device of rectum (assessment of fecal incontinence, rectal tumors, polyps); airway (trachea); or gastrointestinal tract (esophagus, stomach, duodenum, small intestine, large intestine); cardiovascular (heart, arteries or veins); or bladder (bladder wall compliance and degree of detrusor muscle wall aging). 14. The device of 15. The device of 16. A device for analyzing a skin elasticity comprising:
a wand assembly comprising a hole, a probe, a proximity sensor, and a vacuum source, wherein the probe and proximity sensor measure skin elasticity; an electronic control unit configured to record the deformation of the skin measured by the probe and proximity sensor, wherein the proximity sensor and the processor is adapted to automatically initiate a test when the sensor is positioned at a pre-determined distance from the skin; and a stand capable of positioning and supporting the wand assembly in three-dimensions to permit hands-free operation of the device. 17. The device of 18. The device of 19. The device of 20. The device of 21. The device of 22. The device of 23. The device of 24. The device of rectum (assessment of fecal incontinence, rectal tumors, polyps); airway (trachea); or gastrointestinal tract (esophagus, stomach, duodenum, small intestine, large intestine); cardiovascular (heart, arteries or veins); or bladder (bladder wall compliance and degree of detrusor muscle wall aging). 25. The device of 26. A method for analyzing skin elasticity comprising:
obtaining a device for analyzing a skin elasticity comprising:
a wand assembly having a vacuum source to measure skin elasticity, the wand assembly comprising one or more holes, wherein a probe, a proximity sensor and a vacuum are aligned with the one or more holes; an electronic control unit configured to record the deformation of the skin measured; inserting the device for analyzing the skin elasticity into a body cavity, wherein the proximity sensor and the processor is adapted to automatically initiate a test when the sensor is positioned at a pre-determined distance from the skin; measuring the skin elasticity using the wand assembly of the inserted device to obtain a skin elasticity data; recording the skin elasticity data using the inserted device; and analyzing the recorded skin elasticity data to determine the skin elasticity. 27. The method of 28. The method of 29. The method of 30. The method of 31. The method of assessing a deformation of the skin under load; matching the compliance of a surgical mesh to that of the vaginal wall in order to enhance healing; or predicting the effects of remote perturbations of the pelvic organs (abdominal pressure, pelvic bending and twisting) on a vaginal configuration. 32. The method of CROSS-REFERENCE TO RELATED APPLICATIONS
TECHNICAL FIELD OF THE INVENTION
STATEMENT OF FEDERALLY FUNDED RESEARCH
BACKGROUND OF THE INVENTION
SUMMARY OF THE INVENTION
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION OF THE INVENTION