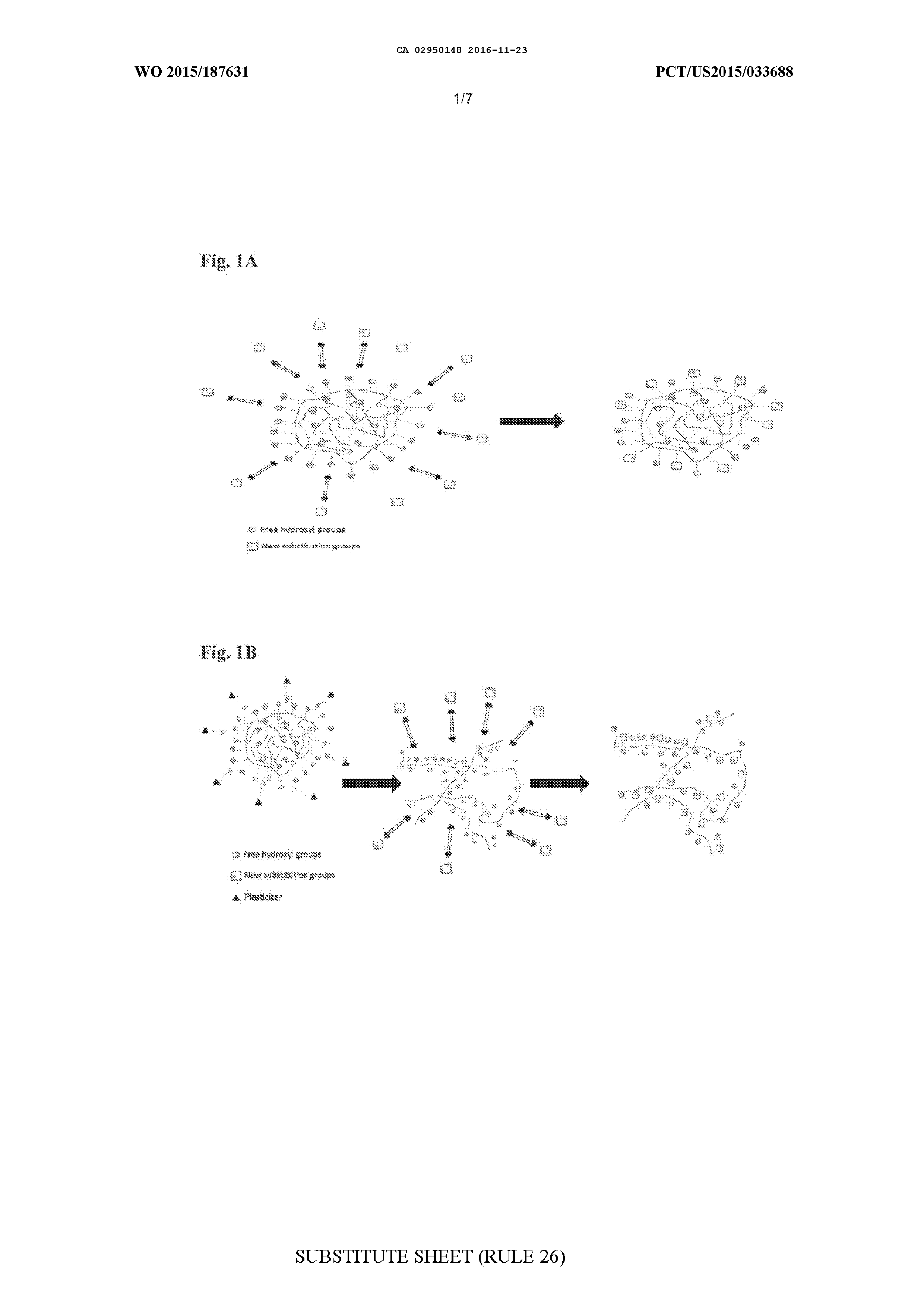
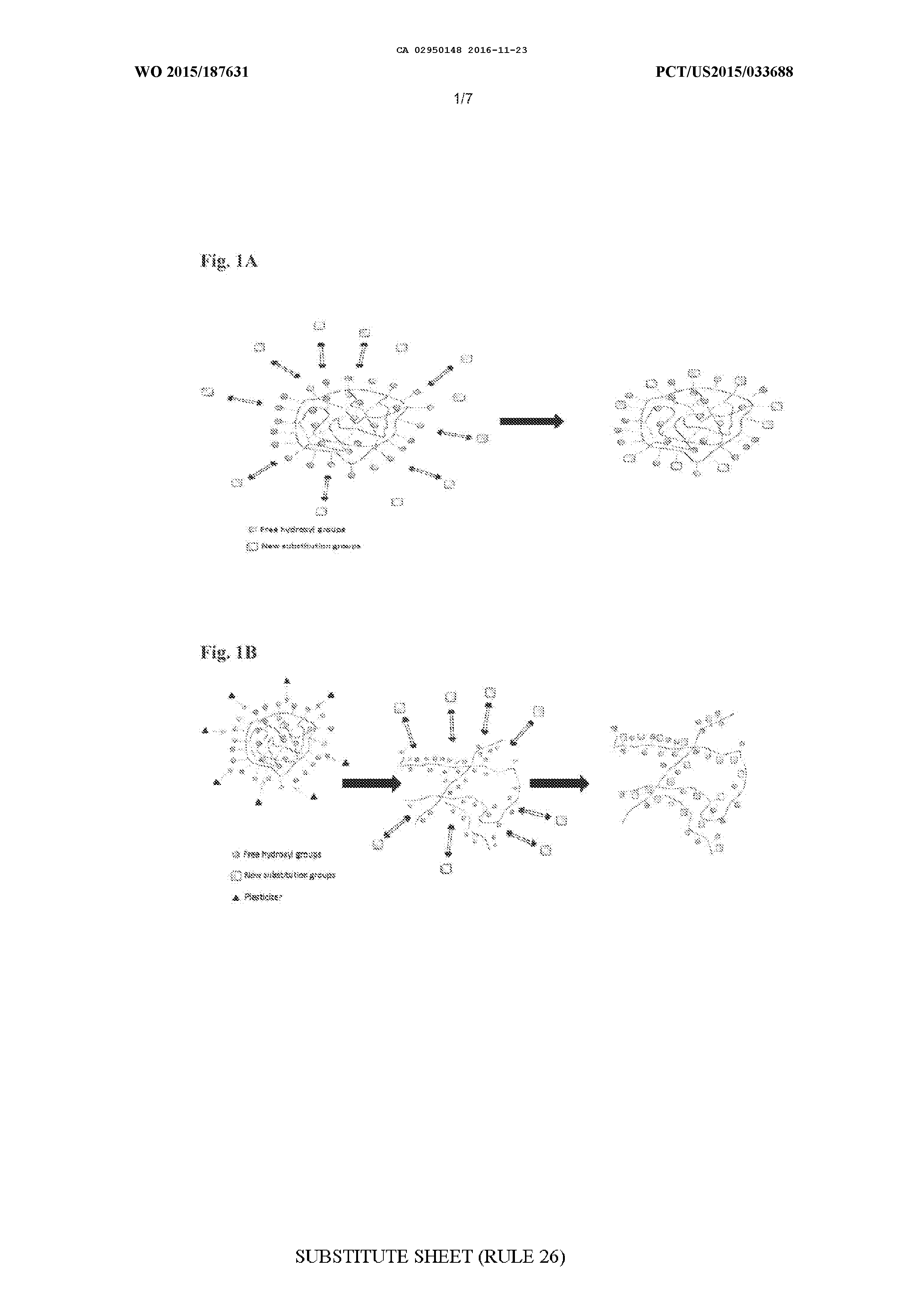
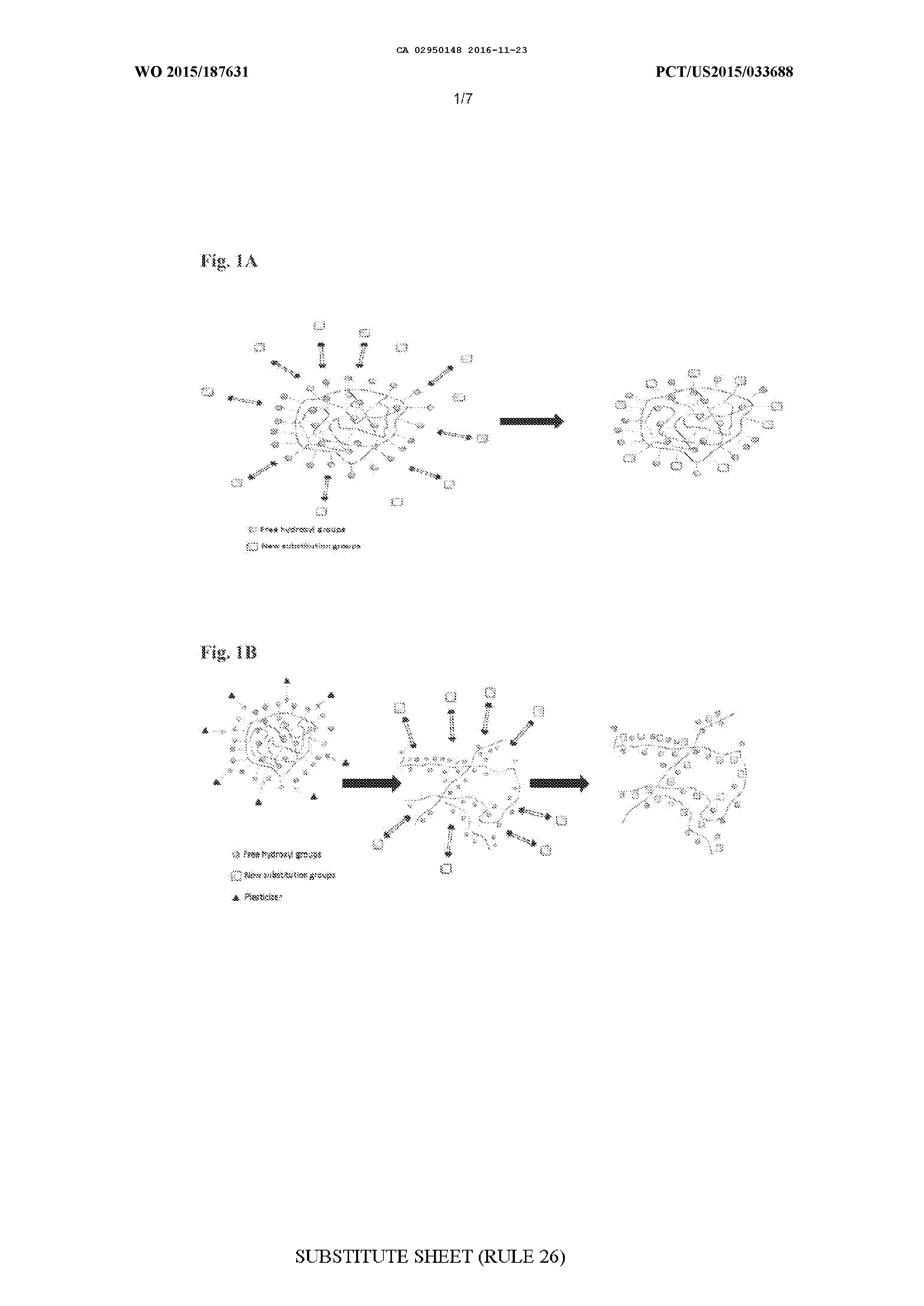
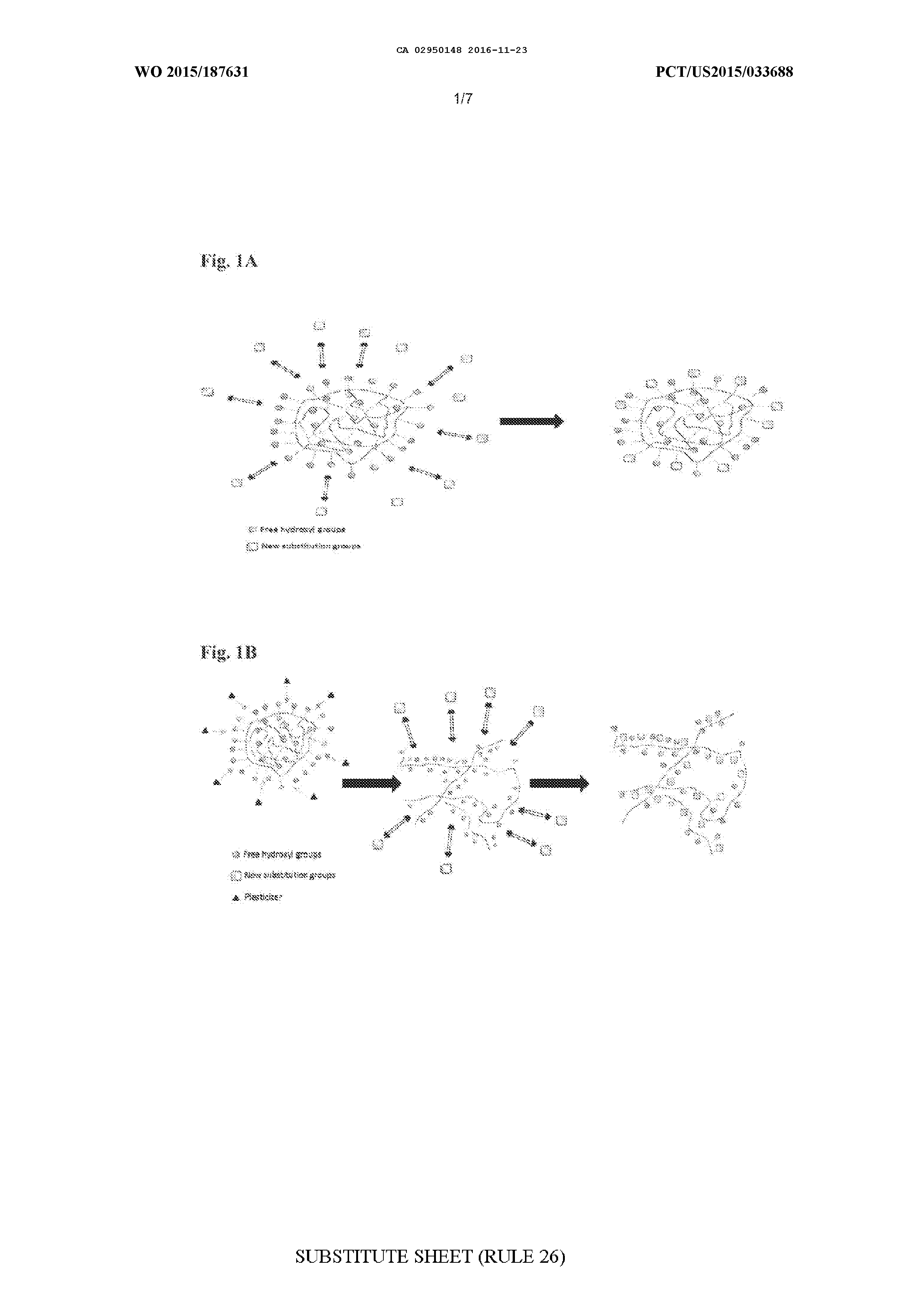
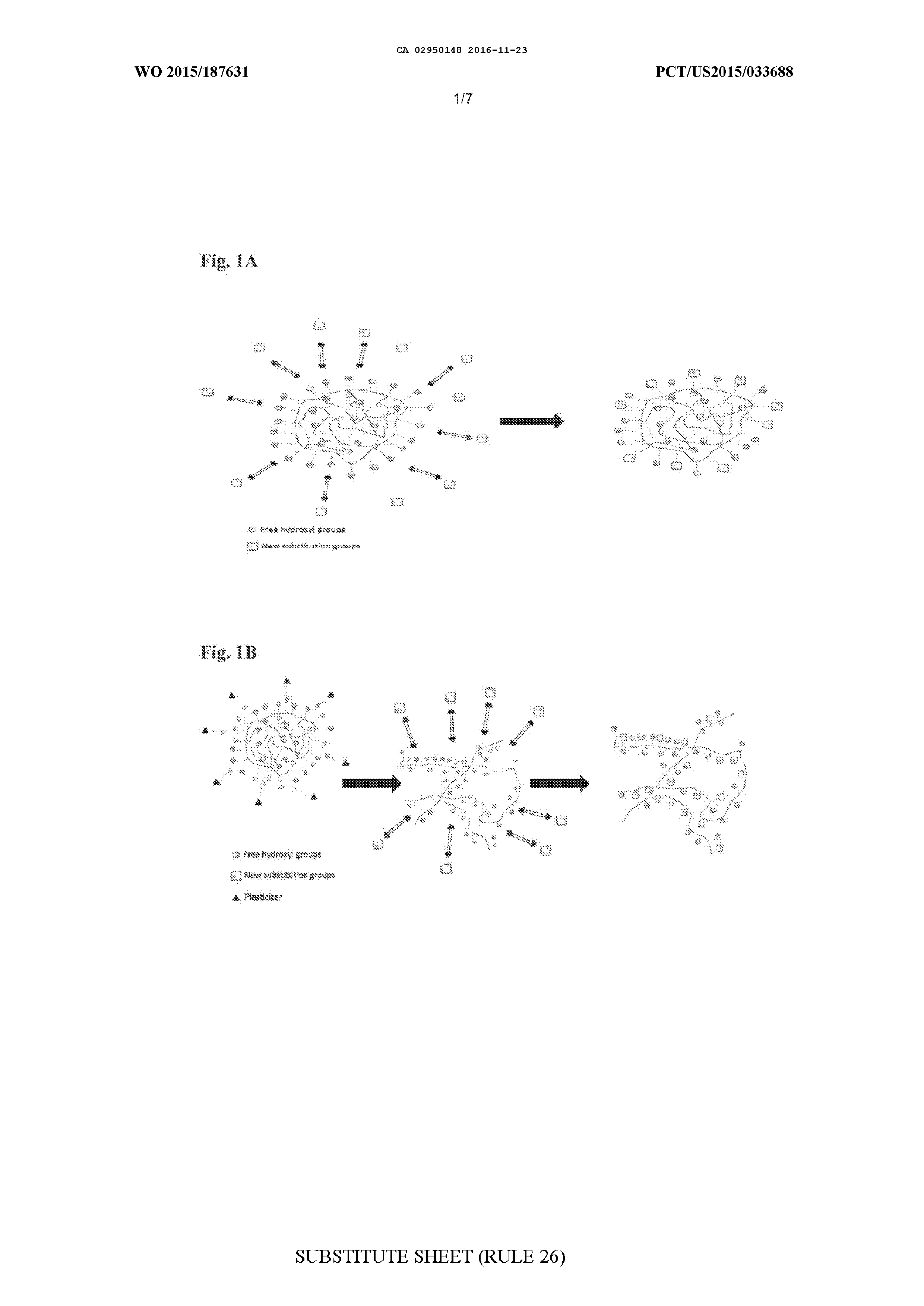
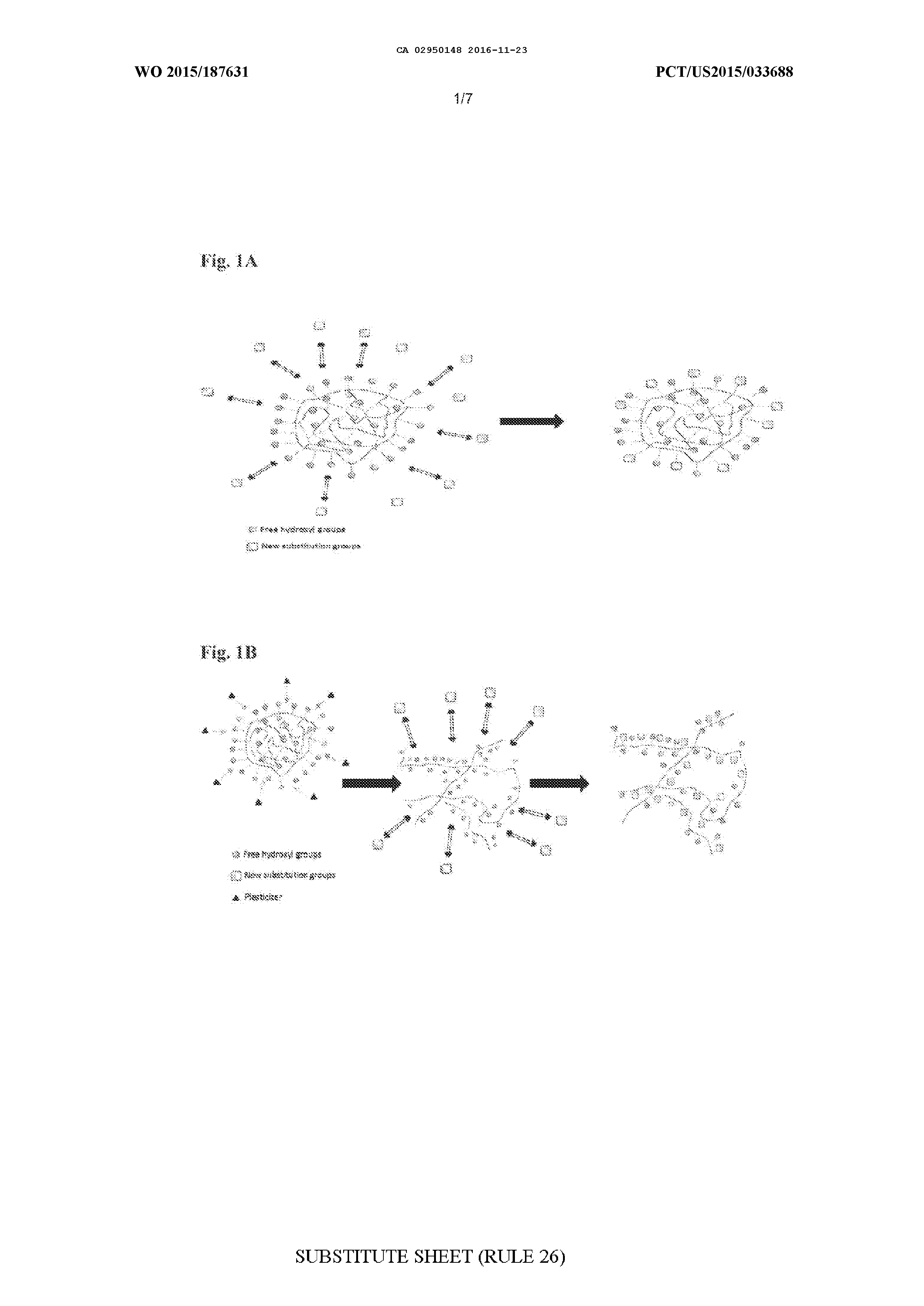
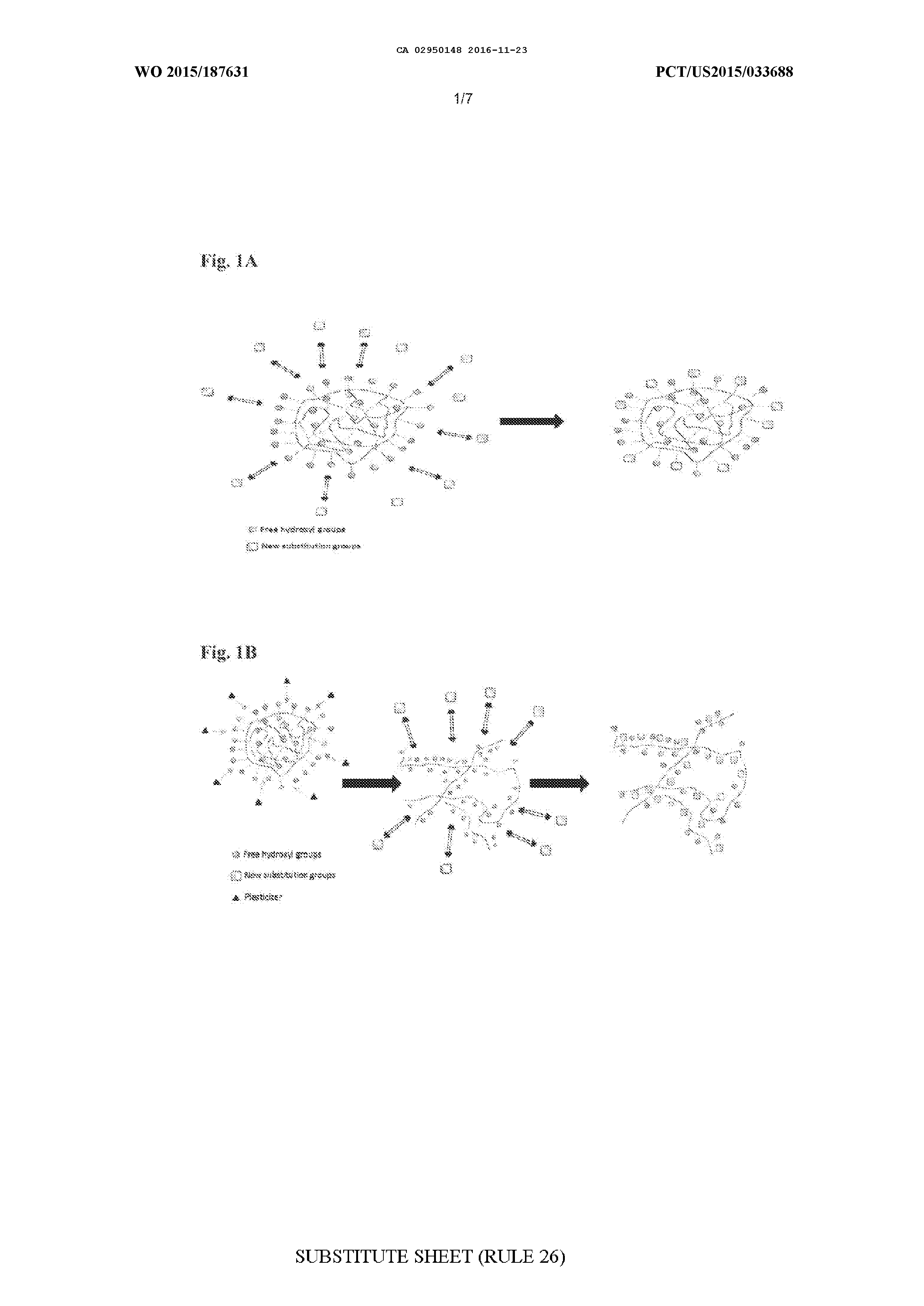
MODIFIED BIOPOLYMERS AND METHODS OF PRODUCING AND USING THE SAME
MODIFIED BIOPOLYMERS AND METHODS OF PRODUCING AND USING THE SAME This application claims the benefit of U.S. Provisional Patent Application Serial No. 62/006,317, filed June 2, 2014, the disclosure of which is incorporated herein by reference in its entirety. The present invention relates to modified biopolymers, including charge-modified biopolymers, cross-linked biopolymers, and cross-linked, charge-modified biopolymers. Methods of producing and using a modified biopolymer of the present invention are also provided. Biopolymers are of interest due to their many uses. For example, biopolymers may be useful as absorbents, such as in diapers, hygiene products, and wound dressings, and may be useful as adsorbents. In addition, biopolymers may have the advantage of providing environmentally friendly products. However, methods of forming and/or processing biopolymers into useful products, such as traditional heterogeneous wet chemistry methods, can often be complex, expensive, and inefficient. The present invention addresses previous shortcomings in the art by providing modified biopolymers and methods of producing and using the same. A first aspect of the present invention includes a method for producing a cross-linked, charge-modified biopolymer comprising: combining a biopolymer and at least one charge-modifying agent to form a homogenous reaction blend; reacting the biopolymer and the at least one charge-modifying agent in the homogenous reaction blend; and cross-linking the biopolymer in the homogeneous reaction blend to form a cross-linked, charge-modified biopolymer. The method may include reacting the biopolymer and the at least one charge-modifying agent to form a charge-modified biopolymer. In some embodiments, the charge-modified biopolymer may be cross-linked with a different biopolymer, which may optionally be charged-modified. The method may include forming a homogenous reaction blend in an extruder, optionally using a reactive extrusion process. Another aspect of the present invention includes a method for producing a cross-linked, charge-modified starch-chitosan comprising: combining starch, chitosan, at least one charge-modifying agent, a catalyst, and a plasticizer to form a homogenous reaction blend; charge-modifying the starch and chitosan to form a charge-modified starch and a charge-modified chitosan; and cross-linking the charge-modified starch and charge-modified chitosan to form a cross-linked, charge-modified starch-chitosan. In a further aspect of the present invention, a method for producing a cross-linked, charge-modified starch-chitosan comprises: combining starch, a first charge-modifying agent, and a catalyst to form a homogeneous reaction blend comprising a charge-modified starch; adding charge-modified chitosan and a plasticizer to the homogeneous reaction blend comprising the charge-modified starch; and cross-linking the charge-modified starch and charge-modified chitosan to form a cross-linked, charge-modified starch-chitosan. Another aspect of the present invention includes a method for producing a cross-linked, charge-modified starch-chitosan comprising: combining starch, a first charge-modifying agent, and a catalyst, to form a charge-modified starch; forming a homogeneous reaction blend comprising the charged-modified starch, a charged-modified chitosan, and a plasticizer; and cross-linking the charge-modified starch and charged-modified chitosan to form a cross-linked, charge-modified starch-chitosan. A further aspect of the present invention includes a cross-linked, charge-modified biopolymer prepared according to a method of the present invention. Another aspect of the present invention includes a cross-linked, charge-modified biopolymer having a charge density of at least 3 meq/g as determined by titration. A further aspect of the present invention includes a cross-linked, charge-modified biopolymer having an increased charge density and/or degree of cross-linking compared to a cross-linked, charge-modified biopolymer prepared using a conventional method. An additional aspect of the present invention includes a cross-linked, charge-modified biopolymer having an increased porosity and/or pore size compared to a cross-linked, charge-modified biopolymer prepared using a conventional method. A further aspect of the present invention includes a method of absorbing a fluid comprising contacting a cross-linked, charge-modified biopolymer of the present invention with the fluid, thereby absorbing the fluid. Another aspect of the present invention includes a cross-linked, charge-modified biopolymer having an increased salt uptake and/or metal chelation property compared to a cross-linked, charge-modified biopolymer prepared using a conventional method. Another aspect of the present invention includes a method of reducing the amount of a salt and/or metal in a solution comprising contacting the cross-linked, charge-modified biopolymer of the present invention with a solution comprising a salt and/or metal, wherein the salt and/or metal binds to the cross-linked, charge-modified biopolymer, thereby reducing the amount of the salt and/or metal in the solution. The foregoing and other aspects of the present invention will now be described in more detail with respect to other embodiments described herein. It should be appreciated that the invention can be embodied in different forms and should not be construed as limited to the embodiments set forth herein. Rather, these embodiments are provided so that this disclosure will be thorough and complete, and will fully convey the scope of the invention to those skilled in the art. Figure 1A is a schematic of a heterogeneous phase reaction. Figure IB is a schematic of a homogeneous phase reaction. Figure 2 illustrates a parallel twin screw extruder with multiple injection and reaction zones according to embodiments of the present invention. Figure 3 illustrates FTIR spectra for unmodified hemicellulose and charge-modified hemicellulose according to embodiments of the present invention. Figure 4 illustrates FTIR spectra for unmodified pectin and charge-modified pectin according to embodiments of the present invention. Figure 5 illustrates FTIR spectra for unmodified soy protein and charge-modified soy protein according to embodiments of the present invention. Figure 6 illustrates exemplary screw configurations according to embodiments of the present invention. Figures 7A-7F show SEM images of A) a commercially available cationic starch at 33χ, Β) the commercially available cationic starch at 1000χ, C) an EDS chlorine map of the commercially available cationic starch, D) a cationic starch prepared according to methods of the present invention at 33χ, Ε) the cationic starch prepared according to methods of the present invention at 1000χ, and F) an EDS chlorine map of the cationic starch prepared according to methods of the present invention. The present invention will now be described more fully hereinafter. This invention may, however, be embodied in different forms and should not be construed as limited to the embodiments set forth herein. Rather, these embodiments are provided so that this disclosure will be thorough and complete, and will fully convey the scope of the invention to those skilled in the art. The terminology used in the description of the invention herein is for the purpose of describing particular embodiments only and is not intended to be limiting of the invention. As used in the description of the invention and the appended claims, the singular forms "a", "an" and "the" are intended to include the plural forms as well, unless the context clearly indicates otherwise. Unless otherwise defined, all terms (including technical and scientific terms) used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. It will be further understood that tenus, such as those defined in commonly used dictionaries, should be interpreted as having a meaning that is consistent with their meaning in the context of the present application and relevant art and should not be interpreted in an idealized or overly formal sense unless expressly so defined herein. The terminology used in the description of the invention herein is for the purpose of describing particular embodiments only and is not intended to be limiting of the invention. All publications, patent applications, patents and other references mentioned herein are incorporated by reference in their entirety. In case of a conflict in terminology, the present specification is controlling. Also as used herein, "and/or" refers to and encompasses any and all possible combinations of one or more of the associated listed items, as well as the lack of combinations when interpreted in the alternative ("or"). Unless the context indicates otherwise, it is specifically intended that the various features of the invention described herein can be used in any combination. Moreover, the present invention also contemplates that in some embodiments of the invention, any feature or combination of features set forth herein can be excluded or omitted. To illustrate, if the specification states that a complex comprises components A, Β and C, it is specifically intended that any of A, Β or C, or a combination thereof, can be omitted and disclaimed. As used herein, the transitional phrase "consisting essentially of (and grammatical variants) is to be interpreted as encompassing the recited materials or steps "and those that do not materially affect the basic and novel characteristic(s)" of the claimed invention. The term "about," as used herein when referring to a measurable value, such as, for example, an amount or concentration and the like, is meant to refer to variations of up to ± 20% of the specified value, such as, but not limited to, ± 20%, ± 15%, ± 10%, ± 5%, ± 1%, ± 0.5%, or even ± 0.1% of the specified value, as well as the specified value. For example, "about X" where X is the measurable value, is meant to include X as well as variations of ± 20%, ± 15%, ± 10%, ± 5%, ± 1%, ± 0.5%, or even ± 0.1% of X. A range provided herein for a measureable value may include any other range and/or individual value therein. According to some embodiments of the present invention, provided herein are modified biopolymers, such as, charge-modified biopolymers, cross-linked biopolymers, and/or cross-linked, charge-modified biopolymers. The cross-linked, charged-modified biopolymers of the present invention may comprise one biopolymer that has been charge-modified and cross-linked. In some embodiments, the cross-linked, charged-modified biopolymers of the present invention may comprise two or more different biopolymers that are cross-linked and at least one of the biopolymers has been charge-modified. The two or more different biopolymers may be cross-linked with each other. In certain embodiments, a cross-linked, charge-modified biopolymer may comprise two different biopolymers that are cross-linked and both of the biopolymers may be charge-modified. A "biopolymer" as used herein refers to a polymer that has at least one free amine and/or hydroxyl group present on a majority of the monomeric units of the polymer and is a polymer produced by a living organism or a derivative thereof. In some embodiments, a free amine and/or hydroxyl group may be present on each of the monomeric units of the polymer backbone. Exemplary biopolymers include, but are not limited to, proteins and/or polysaccharides. As one of ordinary skill in the art will understand, a biopolymer may be synthetically obtained Further exemplary biopolymers include, but are not limited to, starches (including amylose and/or amylopectin), chitosans, hemicelluloses, lignins, celluloses, chitins, alginates, dextrans, pullanes, polyhydroxyalkanoates, fibrins, cyclodextrins, proteins A biopolymer used in a method of the present invention may have a moisture content of about 20% by weight or less. In some embodiments, the biopolymer may have a moisture content of about 20%, 15%, 10%, 5%, or less by weight. In certain embodiments, the biopolymer may have a moisture content in a range of about 5% to about 20% by weight or about 10% to about 15% by weight. In some embodiments, a method of the present invention utilizes a biopolymer, such as, for example, starch, having a moisture content of about 20% by weight or less, and the biopolymer may be in powder form. A biopolymer used in a method of the present invention may have a molecular weight of about 10,000 Daltons or more. In some embodiments, the biopolymer may have a molecular weight of about 10,000; 20,000; 30,000; 40,000, 50,000; 60,000; 70,000; 80,000; 90,000; 100,000; 200,000; 300,000; 400,000; 500,000; 600,000; 700,000; 800,000; 900,000; 1.000. 000; 2,000,000; 3,000,000, 4,000,000 Daltons or more. In certain embodiments, the biopolymer may have a molecular weight of about 50,000 Daltons or more. In some embodiments, the biopolymer may have a molecular weight of about 100,000 Daltons to about 4,000,000 Daltons, about 500,000 Daltons to about 3,000,000 Daltons, or about 1.000. 000 Daltons to about 2,000,000 Daltons. In some embodiments, when only one biopolymer is used to prepare a modified biopolymer of the present invention 30,000; 40,000, 50,000 Daltons or more. In certain embodiments, a modified biopolymer of the present invention In some embodiments, the biopolymer used in a method of the present invention may be a starch. Exemplary starches include, but are not limited to, potato starch, wheat starch, tapioca starch, cassava starch, rice starch, corn starch, waxy corn starch, waxy wheat starch, waxy rice starch, waxy sorghum starch, waxy cassava starch, waxy barley starch, and/or waxy potato starch. The starch may have an amylopectin content of about 70% w/w or more and an amylose content of about 30% w/w or less. In certain embodiments, the starch may have an amylopectin content of about 70%, 75%, 80%, 85%, 90%, 95% w/w or more and an amylose content of about 30%, 25%, 20%, 15%, 10%, 5% w/w or less. In some embodiments, the starch may have an amylopectin content of less than 90%, such as, for example, about 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%, 10%, etc. In some embodiments, starch may have an amylopectin content in a range of about 10% to about 85%, such as, for example, about 25% to about 85% or about 50% to about 80%. In some embodiments, the starch may be dissolvable in water ( A biopolymer used in a method of the present invention may be charge-modified according to a method described herein "Charge-modifying agent" as used herein refers to a molecule or compound comprising one moiety that may react with an amine and/or hydroxyl group of the biopolymer and a second moiety that may be positively charged or negatively charged under suitable conditions, such as, for example, at a certain pH. "Moiety" as used herein, refers to a portion of a molecule or compound having a particular functional or structural feature. For example, a moiety may comprise a functional group or a reactive portion of a compound. As those of skill in the art recognize, a strong acidic moiety -ΝΗ3ΟΗ) or a weak basic moiety (-Ν¾) may form a positively charged moiety. The charge-modifying agent may comprise at least one moiety that may be a positively charged group, such as, but not limited to, a primary amine, secondary amine, tertiary amine, quaternary ammonium, sulfonium, and/or phosphonium group. Exemplary charge-modifying agents that can have a positively charged moiety include, but are not limited to, ethylene imine, Ν-(2-hydroxy ethyl) ethylene imine, cyanamide, betamorpholinoethylchloride, beta-diethyl aminoethylchloride, S-diethyl amino 1,2-epoxypropane dimethyl aminoethyl methacrylate, epoxy 3-methyl ammonium, glycidyltrimethylammonium chloride In some embodiments, a positively charged moiety may be introduced into and/or onto a biopolymer by reacting the biopolymer and charge-modifying agent in a homogeneous reaction blend, optionally in the presence of a catalyst. This reaction may be a dry melt process and/or may be an etherification or esterification reaction. The charge-modifying agent may comprise at least one moiety that may be a negatively charged group, such as, but not limited to, a carboxyl, sulfonate, sulfate, and/or a phosphate group In some embodiments, a negatively charged moiety may be introduced into a biopolymer by reacting the biopolymer and charge-modifying agent in a homogeneous reaction blend in the presence an alkaline catalyst. In certain embodiments, the charge-modifying agent may be acrylonitrile and the reaction of the biopolymer and acrylonitrile in the presence of an alkaline catalyst may be followed by hydrolysis of the cyanoethyl groups. When the charge-modifying agent is sodium periodate, the reaction with the biopolymer may be followed by a treatment to transform the carbonyl groups into carboxyl groups, such as, but not limited to, by treating with sodium chlorite, and/or by a treatment with sodium bisulfite and/or potassium bisulfite. In certain embodiments, both carboxyl and sulfonate groups may be introduced into a biopolymer by reacting the biopolymer with an anhydride of an unsaturated acid In some embodiments, the charge-modifying agent may react with an amine and/or hydroxyl group of a biopolymer to provide a charge-modified biopolymer. The charge-modified biopolymer may be cationic (/.℮., have a net positive charge) or may be anionic A biopolymer used in a method of the present invention may be cross-linked by reacting a cross-linking agent with the biopolymer and optionally with at least one different biopolymer that may optionally be charge-modified. In some embodiments, a cross-linking agent may be reacted with at least one charge-modified biopolymer. "Cross-linking agent" as used herein refers to a compound that links two or more biopolymer chains and/or portions of the biopolymer together, the biopolymer optionally being charge-modified. The linkage may be achieved via a covalent bond or an ionic bond. In some embodiments, the linkage may be through a moiety or group of the biopolymer or different biopolymers. Exemplary cross-linking agents include, but are not limited to, epichlorohydrin, glutaraldehyde, oxalic acid, malonic acid, succinic acid, glutaric acid, adipic acid, pimelic acid, fumaric acid, maleic acid, malic acid, tartartic acid, sodium trimetaphosphate, sodium tripolyphosphate, ionic cross-linkers Without wishing to be bound to any particular theory, in some embodiments, a charge-modifying agent, such as, for example, citric acid, when heated inside an extruder may dehydrate to yield an anhydride. The free hydroxyl groups from a biopolymer, such as, for example, starch, present in the reaction mixture may react with the anhydride to form starch citrate. Further, without wishing to be bound to any particular theory, in some embodiments, additional dehydration of the biopolymer and/or charge-modified biopolymer may allow for cross-linking of the biopolymer and/or charge-modified biopolymer to occur. In some embodiments, cross-linking of the biopolymer and/or charge-modified biopolymer may be achieved due to the heat inside the extruder and/or during a post treatment process, such as, for example, a thermal post-treatment process. In some embodiments, a charge-modified biopolymer may be prepared using a ring-opening polymerization of anhydrous acids. A modified biopolymer of the present invention In some embodiments, a modified biopolymer of the present invention A modified biopolymer of the present invention A modified biopolymer of the present invention In some embodiments, a method of the present invention may provide a modified biopolymer In some embodiments, the modified biopolymer may have an increased charge density and/or degree of cross-linking compared to a modified biopolymer The modified biopolymer may have a charge density and/or degree of cross-linking that is increased by at least about 5% or more compared to a modified biopolymer prepared using a conventional method. In some embodiments, the modified biopolymer may have a charge density and/or degree of cross-linking that is increased by at least about 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 100%, 150%, 200%, or more compared to a modified biopolymer prepared using a conventional method. In some embodiments, the degree or amount of cross-linking present in a modified biopolymer of the present invention In some embodiments, a modified biopolymer of the present invention In some embodiments, a modified biopolymer of the present invention ( In some embodiments, a modified biopolymer of the present invention A modified biopolymer of the present invention In some embodiments, the modified biopolymer may have a more uniform porosity and/or pore size compared to a modified biopolymer prepared using a conventional method. A more uniform porosity may include a more uniformly or evenly dispersed number of pores or void spaces throughout the modified biopolymer, hi some embodiments, a more uniform pore size may include a more uniform diameter of the pores or void spaces throughout the modified biopolymer. In certain embodiments, the porosity and/or pore size of the modified biopolymer may be more uniform compared to the porosity and/or pore size of a modified biopolymer prepared using a conventional method, and may vary by less than about 20%, such as, for example, by about 20%, 15%, 10%, 5% or less, as determined by comparing two or more defined areas of the modified biopolymer compared to two or more defined areas of the modified biopolymer prepared using a conventional method. A modified biopolymer of the present invention ( In certain embodiments, a modified biopolymer of the present invention In some embodiments, a modified biopolymer of the present invention ( A modified biopolymer of the present invention In some embodiments, a modified biopolymer of the present invention may comprise starch and chitosan. The starch and chitosan may both be charged-modified and cross-linked with each other to form a cross-linked, charged-modified starch-chitosan biopolymer. According to some embodiments of the present invention, a method for producing a charge-modified biopolymer may be provided. The method may comprise reacting a biopolymer and at least one charge-modifying agent in a homogeneous reaction blend to form a charge-modified biopolymer. In some embodiments, the method may comprise combining the biopolymer and at least one charge-modifying agent, optionally with a plasticizer and/or catalyst, to form a homogenous reaction blend. In some embodiments, the method may comprise reacting two or more different biopolymers with a charge-modifying agent in a homogeneous reaction blend. Optionally, at least one of the two or more different biopolymers may be charge-modified According to some embodiments of the present invention, a method for producing a cross-linked biopolymer may be provided. The method may comprise reacting a biopolymer and at least one cross-linking agent in a homogeneous reaction blend to form a cross-linked biopolymer. In some embodiments, the method may comprise combining the biopolymer and at least one cross-linking agent, optionally with a plasticizer and/or catalyst, to form a homogenous reaction blend. In some embodiments, the method may comprise reacting two or more different biopolymers with a cross-linking agent in a homogeneous reaction blend. According to some embodiments of the present invention, a method for producing a cross-linked, charge-modified biopolymer may be provided. The method may comprise reacting a biopolymer and at least one charge-modifying agent in a homogenous reaction blend to form a charge-modified biopolymer and cross-linking the charge-modified biopolymer in the homogeneous reaction blend to form a cross-linked, charge-modified biopolymer. Some embodiments may include reacting two or more different biopolymers with the at least one charge-modifying agent in the homogeneous reaction blend to form at least one charge-modified biopolymer. In some embodiments, the charge-modified biopolymer may be cross-linked to one or more different biopolymers in the homogeneous reaction blend, and the one or more different biopolymers may optionally be charge-modified, such as, for example, prior to a combining, reacting, and/or cross-linking step. In some embodiments, a charge-modified biopolymer prepared according to a method of the present invention is used to prepare a cross-linked, charge-modified biopolymer of the present invention. In some embodiments, the biopolymer and the at least one charge-modifying agent may be combined to form a homogeneous reaction blend. In some embodiments, one or more steps of a method of the present invention ( In some embodiments, a method for producing a cross-linked, charge-modified biopolymer may comprise combining a first charge-modified biopolymer and a second charge-modified biopolymer that is different than the first charge-modified biopolymer, optionally with a plasticizer, cross-linking agent, and/or catalyst, to form a homogeneous reaction blend, and cross-linking the first and second charge-modified biopolymers in the homogeneous reaction blend to form a cross-linked, charge-modified biopolymer. The first and/or second charge-modified biopolymers may be charge-modified according to a method of the present invention. Some embodiments include a method for producing a cross-linked, charge-modified biopolymer comprising: combining a first biopolymer, a second biopolymer that is different than the first biopolymer, at least one charge-modifying agent, a plasticizer, and optionally a catalyst to form a homogenous reaction blend; reacting the first biopolymer and second biopolymer with the at least one charge-modifying agent to form a charge-modified first biopolymer and a charge-modified second biopolymer; and cross-linking the charge-modified first biopolymer and charge-modified second biopolymer to form a cross-linked, charge-modified biopolymer. In some embodiments, a method for producing a cross-linked, charge-modified biopolymer comprises: combining a first biopolymer, a first charge-modifying agent, and optionally a catalyst to form a homogeneous reaction blend comprising a charge-modified first biopolymer; adding a charge-modified second biopolymer and a plasticizer to the homogeneous reaction blend comprising the charge-modified first biopolymer; and cross-linking the charge-modified first biopolymer and charge-modified second biopolymer to form a cross-linked, charge-modified biopolymer. In some embodiments, the charge-modified second biopolymer is prepared according to a method of the present invention. Some embodiments include a method for producing a cross-linked, charge-modified biopolymer comprising: combining a first biopolymer, a first charge-modifying agent, and optionally a catalyst to form a charge-modified first biopolymer; forming a homogeneous reaction blend comprising the charged-modified first biopolymer, a charged-modified second biopolymer, and a plasticizer; and cross-linking the charge-modified first biopolymer and charged-modified second biopolymer to form a cross-linked, charge-modified biopolymer. In some embodiments, the charge-modified second biopolymer is prepared according to a method of the present invention. In some embodiments, a method for producing a cross-linked, charge-modified biopolymer comprises: forming a homogenous reaction blend comprising a first biopolymer, a second biopolymer that is optionally charged-modified, and at least one charge-modifying agent; reacting the first biopolymer and the at least one charge-modifying agent in the homogenous reaction blend to form a charge-modified biopolymer; and cross-linking the charge-modified biopolymer and the second biopolymer in the homogeneous reaction blend to form a cross-linked, charge-modified biopolymer. In some embodiments, the second biopolymer may be charge-modified according to a method of the present invention. In some embodiments, a method for producing a cross-linked, charge-modified biopolymer comprises: forming a first homogenous reaction blend comprising a first biopolymer and at least one charge-modifying agent; reacting the first biopolymer and the at least one charge-modifying agent in the first homogenous reaction blend to form a charge-modified biopolymer; combining the charge-modified biopolymer with a second biopolymer that is optionally charge-modified to form a second homogeneous reaction blend; and cross-linking the charge-modified biopolymer and second biopolymer in the second homogeneous reaction blend to form a cross-linked, charge-modified biopolymer. In some embodiments, the second biopolymer may be charge-modified according to a method of the present invention. In some embodiments, a method for producing a modified biopolymer of the present invention In some embodiments, a method of the present invention may comprise a continuous process followed by a non-continuous process, such as, but not limited to, a post-treatment step. In certain embodiments, a method of the present invention may comprise a continuous process, a non-continuous process A "reactive extrusion process" as used herein refers to a process in which a biopolymer is both chemically and physically modified. A reactive extrusion process may provide for a chemical modification of a biopolymer, such as, but not limited to, grafting onto the biopolymer, cross-linking of the biopolymer, functionalization of the biopolymer, and/or charge-modification of the biopolymer. In some embodiments, a reactive extrusion process may provide for polymerization and/or branching of a biopolymer. The polymerization and/or branching may be with a different biopolymer to provide a co-polymer. An exemplary physical modification may be changing the form of the biopolymer, such as, but not limited to, from a powder, particulate, and/or solid form to a molten or melted form. At least one charge-modifying agent may be present in a homogeneous reaction blend in an amount of about 5% to about 200% or more by weight of a biopolymer present in the homogeneous reaction blend. In some embodiments, at least one charge-modifying agent may be present in a homogeneous reaction blend in an amount of about 5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 100%, 110%, 120%, 130%, 140%, 150%, 160%, 170%, 180%, 190%, 200%, or more by weight of a biopolymer present in the homogeneous reaction blend. In some embodiments, at least one charge-modifying agent may be present in a homogeneous reaction blend in an amount of at least about 75% by weight of a biopolymer present in the homogeneous reaction blend. In some embodiments, a method of the present invention may include at least one charge-modifying agent present in a homogeneous reaction blend in an amount of at least about 75% by weight of the biopolymer and provide a modified biopolymer having a charge density of at least 1.5 meq/g of the modified biopolymer. A homogeneous reaction blend is a melted blend of all the components in a single phase. In some embodiments, a homogeneous reaction blend may be obtained using an extruder. In certain embodiments, a homogeneous reaction blend may be obtained using a reactive extrusion process in an extruder. The homogeneous reaction blend may be in the form of a single liquid phase. A homogeneous reaction blend may provide a uniform distribution of the components or reactants as compared to a conventional method. In some embodiments, a method of the present invention may provide a chemical reaction that occurs more uniformly and/or completely due to the formation of a homogeneous reaction blend as compared to a conventional method. In some embodiments, the biopolymer in the homogeneous reaction blend may be a melted thermoplastic. A biopolymer may react thermo-mechanically and/or chemically with one or more reagents to form a charge-modified biopolymer of the present invention, which may be thermoplastic and/or a viscoelastic material. In some embodiments, a method of the present invention removes hydrogen bonding and/or crystalline domains present in a biopolymer. This may allow for all or substantially all portions of the biopolymer to be available for chemical reaction, such as, for example, charge-modification and/or cross-linking. In some embodiments, a homogeneous reaction blend may contain a plasticized biopolymer, which may allow for greater access to moieties throughout the biopolymer. In contrast, in a heterogeneous phase reaction (for example, in which modified biopolymers are synthesized by a coating process, in a diluted suspension, or with a concentrated gel solution) there is a limited amount of moieties In some embodiments, a method of the present invention may provide a modified biopolymer In certain embodiments, a method of the present invention may provide a reaction with faster kinetics than the kinetics of the same reaction in a conventional method. The speed of at least one reaction in a method of the present invention may be increased compared to the speed of the same reaction in a conventional method. In some embodiments, a method of the present invention provides an overall greater speed of reaction to produce a modified biopolymer of the present invention compared to a conventional method. In some embodiments, a plasticizer may be present in the homogeneous reaction blend with a biopolymer and a charge-modifying agent. In some embodiments, a plasticizer may be combined with the biopolymer and the at least one charge-modifying agent to form a homogenous reaction blend. A plasticizer may be present in a homogeneous reaction blend in an amount of about 10% to about 400% or more by weight of a biopolymer present in the homogeneous reaction blend. In some embodiments, a plasticizer may be present in a homogeneous reaction blend in an amount of about 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 100%, 110%, 120%, 130%, 140%, 150%, 160%, 170%, 180%, 190%, 200%, 210%, 220%, 230%, 240%, 250%, 260%, 270%, 280%, 290%, 300%, 310%, 320%, 330%, 340%, 350%, 360%, 370%, 380%, 390%, 400%, or more by weight of a biopolymer present in the homogeneous reaction blend. In some embodiments, a plasticizer may be present in a homogeneous reaction blend in an amount of at least about 30% or more by weight of a biopolymer In some embodiments, where a reactive plasticizer is used The plasticizer may reduce the glass transition temperature (Tg). In some embodiments, the plasticizer may improve the flexibility, workability, distensibility, and/or processability of a biopolymer and may do so by lowering the glass transition temperature (Tg). In certain embodiments, a biopolymer to be extruded by a method of the present invention may not be thermoplastic. Thus, to extrude a biopolymer that is not thermoplastic, the glass transition temperature (Tg) must be lowered by addition of a plasticizer. A plasticizer may reduce the tension of deformation, hardness, density, viscosity and/or electrostatic charge of a biopolymer and at the same time may increase the biopolymer chain flexibility, resistance to fracture and/or dielectric constant. Other properties of the biopolymer may also be affected by the inclusion of a plasticizer, such as, but not limited to, the degree of crystallinity, optical clarity, electric conductivity, fire behavior and/or resistance to biological degradation. In some embodiments, a plasticizer may disrupt hydrogen bonds present in a crystalline structure of the biopolymer and this may lead to the breaking of the crystalline domains that prevent thermal processing. In some embodiments, the plasticizer may allow for the biopolymer to melt and/or become thermoplastic to provide a single phase. In some embodiments, a plasticizer may lower the Tg by solvating the inherent crystallinity of the biopolymer and disrupting hydrogen bonding. This may allow for the melt processability of biopolymers that are not traditionally melt processable. A plasticizer may be a low molecular weight non-volatile compound. Additional exemplary plasticizers include, but are not limited to, citric acid, triphenyl phosphate, camphor oil, amylacetate, allyurea, citrate esters, phthalic acid esters, dioctyl phthalate, fatty acid esters, benzoates, tartrates, chlorinated hydrocarbons, esters of adipic acid, polyols ( In some embodiments, citric acid is present in a homogeneous reaction blend and it may function as both a charge-modifying agent and a plasticizer. A catalyst may optionally be present in a homogeneous reaction blend. In some embodiments, a catalyst and/or a plasticizer may be combined with a biopolymer and at least one charge-modifying agent to form a homogenous reaction blend. A catalyst may be present in a homogeneous reaction blend in an amount of about 1 % to about 100% or more by weight of a biopolymer present in the homogeneous reaction blend. In some embodiments, a catalyst may be present in a homogeneous reaction blend in an amount of about 1%, 5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 100%, or more by weight of a biopolymer present in the homogeneous reaction blend. A catalyst may accelerate the charge-modification and/or cross-linking reaction. In some embodiments, a catalyst may adjust the pH to enhance the opening of chemical bonds. Exemplary catalysts include, but are not limited to, sodium hypophosphite, sodium bisulfate, sodium bisulfite, and/or caustics ( In some embodiments, the catalyst may be an initiator, hi some embodiments, the cross-linking step may comprise reacting the biopolymer with at least one cross-linking agent, optionally in the presence of an initiator. The biopolymer may be a charge-modified biopolymer. Exemplary initiators include, but are not limited to, peroxides such as acyl peroxides An initiator may be present in a homogeneous reaction blend in an amount of about 1% to about 100% or more by weight of a biopolymer present in the homogeneous reaction blend. In some embodiments, an initiator may be present in a homogeneous reaction blend in an amount of about 1%, 5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 100%, or more by weight of a biopolymer present in the homogeneous reaction blend. Optional additives may be used in a method of preparing a modified biopolymer. Exemplary optional additives include, but are not limited to, dyes, pigments, organic fillers, inorganic fillers, softening agents Forming a homogenous reaction blend may comprise melt blending at least one biopolymer and at least one charge-modifying agent, optionally with at least one plasticizer, a catalyst In certain embodiments, a homogeneous reaction blend may be formed comprising at least two different biopolymers. In some embodiments, a homogeneous reaction blend may be formed comprising a charge-modified biopolymer and at least one different biopolymer, which may optionally be charge-modified. When two biopolymers are present in a homogeneous reaction blend, a first biopolymer may be present in the homogeneous reaction blend in an amount of about 10% to about 200% or more by weight of a second biopolymer present in the homogeneous reaction blend. In some embodiments, a first biopolymer may be present in a homogeneous reaction blend in an amount of about 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 100%, 110%, 120%, 130%, 140%, 150%, 160%, 170%, 180%, 190%, 200%, or more by weight of a second biopolymer present in the homogeneous reaction blend. In some embodiments, a first biopolymer and a second biopolymer may be present in a homogeneous reaction blend in a ratio in a range of 0.1:1 to 4:1 (first biopolymensecond biopolymer), such as, for example, in a ratio in a range of 0.5:1 to 2:1 or 1:1 to 3:1. In certain embodiments, a first biopolymer and a second biopolymer may be present in a homogeneous reaction blend in a ratio of about 0.5:1, 1:1, or 1:0.5. In some embodiments, the reacting and/or cross-linking step(s) may be carried out and/or performed in a homogeneous reaction blend. The reacting and/or cross-linking step(s) may be carried out and/or performed using a reactive extrusion process. In some embodiments, the reacting and/or cross-linking step(s) may be carried out at a temperature in a range of about 80°C to about 200°C, such as, for example, at a temperature in a range of about 80°C to about 120°C, about 80°C to about 150°C, about 90°C to about 120°C, about 100°C to about 120°C, about 100°C to about 200°C, about 150°C to about 180°C, or about 110°C to about 130°C. In certain embodiments, the reacting and/or cross-linking step(s) may be carried out at a temperature of about 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, or 150°C. In some embodiments, the reacting and/or cross-linking step(s) may be carried out at a temperature of about 140°C or less. In some embodiments, the reacting and/or cross-linking step(s) may be carried out at a temperature that avoids degradation of a biopolymer and/or modified biopolymer. In some embodiments, increasing the temperature of the reacting and/or cross-linking steps may provide for an increased amount of charge-modification on the biopolymer if the temperature remains below the degradation temperature for the biopolymer. In some embodiments, the reacting step may be carried out and/or performed at a temperature in a range of about 100°C to about 175°C, such as, for example, about 120°C to about 140°C or about 100°C to 150°C. In some embodiments, the cross-linking steps may be earned out and/or performed at a temperature in a range of about 120°C or more, such as, for example, about 120°C to about 175°C or about 120°C to about 140°C. One or more process conditions for a method of the present invention may be modified to provide a particular modified biopolymer, such as, for example, a super absorbent, ion exchange resin, etc., and/or a particular property of a modified biopolymer, such as, for example, the degree of charge modification, cross-linking, etc. Example processing conditions for a method of the present invention include, but are not limited to, the type of extruder In some embodiments, the reacting and/or cross-linking step(s) may be carried out in an extruder. The reacting and/or cross-linking step(s) may carried out in an extruder with a residence time in a range of about 0.1 minutes to about 30 minutes, such as, for example, in a range of about 0.1 minutes to about 10 minutes, about 0.5 minutes to about 5 minutes, about 1 minute to about 10 minutes, about 1 minute to about 5 minutes, or about 1 minute to about 3 minutes. In certain embodiments, the reacting and/or cross-linking step(s) may be carried out in an extruder with a residence time of about 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30 minutes. In some embodiments, the reacting and/or cross-linking step(s) may be carried out in an extruder with a residence time of about 5 minutes. In some embodiments, increasing the residence time of the reacting and/or cross-linking steps may provide for an increased amount of charge-modification on the biopolymer. The reacting and/or cross-linking step(s) may carried out in an extruder having a screw RPM in a range of about 10 to about 500 rpm, such as, but not limited to, about 10 to about 200, about 50 to about 200, about 100 to about 200, about 125 to 250, about 100 to about 500, or about 90 to about 130. In certain embodiments, the reacting and/or cross-linking step(s) may be earned out in an extruder having a screw RPM in a range of about 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 210, 220, 230, 240, 250, 260, 270, 280, 290, 300, 310, 320, 330, 340, 350, 360, 370, 380, 390, 400, 410, 420, 430, 440, 450, 460, 470, 480, 490, or 500 rpm. In some embodiments, the reacting and/or cross-linking step(s) may be carried out in an extruder having a screw RPM of about 120 rpm. In some embodiments, the reacting and/or cross-linking step(s) may be carried out in an extruder with a Specific Mechanical Energy (SME) value of at least about 20 kJ/kg. In certain embodiments, the reacting and/or cross-linking step(s) may be carried out in an extruder with a SME value in a range of about 20 kJ/kg to about 500 kJ/kg or about 25 kJ/kg to about 250 kJ/kg. The SME value may be measured using methods known to those of skill in the art. The step of reacting a biopolymer with a charge-modifying agent and the step of cross-linking the biopolymer may occur simultaneously. Alternatively or in addition, in some embodiments, the step of reacting the biopolymer with the charge-modifying agent and the step of cross-linking the biopolymer may be done sequentially. Thus, in some embodiments, the step of reacting the biopolymer with the charge-modifying agent may be carried out first to form a charge-modified biopolymer and then cross-linking step may be earned out with the charge-modified biopolymer. Exemplary devices for carrying out a method of the present invention include, but are not limited to, co-rotational and counter rotational twin screws, thermal kinetic compounders, high shear mixers, paddle mixers, static mixers blenders, open-type mixing roll, closed Banbury mixer, kneader, single-screw extruder, vented screw extruder, and/or twin-screw extruder In some embodiments, the components or reactants for one or more steps in a method of the present invention may be dry mixed together prior addition to an extruder. Alternately or in addition, two or more feeders A method of the present invention may be performed and/or carried out as a single-stage direct extrusion process or a multi-stage extrusion process. In some embodiments, the method comprises in-line compounding. In some embodiments, the method is carried out in an extruder comprising at least two reaction zones and the at least two reaction zones are used for one or more steps in the method for preparing a modified biopolymer of the present invention In some embodiments, one or more reagents may be in powder form when added to an extruder and may not be in the form of a liquid or paste. In some embodiments, the one or more reagents in powder form may have a moisture content of about 20% by weight or less. Some embodiments include adding a biopolymer and/or charged-biopolymer to an extruder in powder form and/or adding one or more additional reagents In some embodiments, an extruder is used as one complete reaction vessel, which may allow for the reaction to occur along the entire length of the extruder. When two or more reaction zones are provided, the one or more process conditions In some embodiments, an extruder may be used as a sequential reactor. For example, in some embodiments, a mixture of a biopolymer In some embodiments, a method of the present invention may comprise foaming a modified biopolymer of the present invention A foaming agent may be a chemical agent or physical agent. Exemplary foaming agents, include, but not are limited to, supercritical nitrogen (Ν2) calcium carbonate (CaCCf), water In some embodiments, a method of the present invention may comprise treating a modified biopolymer of the present invention (/.℮., a post-treatment), such as, for example, thermally treating a charge-modified biopolymer and/or cross-linked, charge-modified biopolymer post extrusion. A post-treatment of the prevent invention may increase the degree of cross-linking present in a modified biopolymer of the present invention and/or may increase and/or improve charge density and/or charge modification of a modified biopolymer. In some embodiments, a post-treatment of the present invention may decrease the soluble gel fraction in a modified biopolymer. The modified biopolymer in solid form may be undergo a post-treatment. In some embodiments, a post-treatment of the present invention may fine tune and/or modify the properties of a modified biopolymer of the present invention. A post-treatment may comprise heating the modified biopolymer. In some embodiments, a method of the present invention may comprise heating the modified biopolymer at a temperature in a range of about 80°C to about 180°C, such as, for example, about 100°C to about 150°C or about 120°C to about 140°C, for a period of time in a range of about 0.5 minutes to about 24 hours, such as, for example, about 5 minutes to about 180 minutes, or about 30 minutes to about 90 minutes. In some embodiments, a post-treatment may comprise heating the modified biopolymer at a temperature of about 110°C to about 130°C for a period of time in a range of about 60 minutes to about 120 minutes. In some embodiments, a post-treatment may comprise heating the modified biopolymer at a temperature of about 130°C to about 150°C for a period of time in a range of about 10 minutes to about 50 minutes. In some embodiments, a method of the present invention may comprise removing unreacted reagents, soluble and/or low molecular weight species, and/or degradation products from a modified biopolymer of the present invention, such as, for example, by rinsing, dialyzing, and/or the like the modified biopolymer. Some embodiments include removing unreacted reagants from the modified biopolymer after a post-treatment. Some embodiments of the present invention may include drying the modified biopolymer ( According to some embodiments of the present invention, a method of the present invention may comprise forming a homogeneous reaction blend comprising a starch, at least one charge-modifying agent, optionally at least one plasticizer, and optionally a catalyst, and reacting the starch and the at least one charge-modifying agent to form a charged-modified starch. In some embodiments, the at least one charge-modifying agent may be an acid such as, for example, citric acid, the optional at least one plasticizer may be water and/or glycerol, and/or the optional catalyst may be sodium hypophosphite. The reacting step may comprises reacting starch and the charge-modifying agent The charge-modified starch may be cross-linked with another biopolymer, such as, for example chitosan to form a cross-linked, charge-modified starch-chitosan. In some embodiments, the chitosan is charged modified, such as, but not limited, protonated. hi certain embodiments, the method may comprise combining charged-modified starch with chitosan, at least one plasticizer, and optionally a charge-modifying agent, and cross-linking the charged-modified starch and chitosan. In some embodiments, a charge-modifying agent may be an acid A method of preparing a cross-linked, charge-modified starch-chitosan may comprise providing in an extruder starch in an amount in a range of about 5 wt% to about 50 wt% and chitosan in an amount in a range of about 5 wt% to about 50 wt% to form a homogeneous reaction blend. In some embodiments, the chitosan may be charge-modified chitosan. The homogeneous reaction blend may further comprise a charge-modifying agent According to some embodiments, a method of the present invention may comprise combining starch, chitosan, at least one charge-modifying agent, a catalyst, and a plasticizer to form a homogenous reaction blend; charge-modifying the starch and chitosan to form a charge-modified starch and a charge-modified chitosan; and cross-linking the charge-modified starch and charge-modified chitosan to form a cross-linked, charge-modified starch-chitosan. In some embodiments, the combining step may be carried out by providing, adding, feeding, injecting and/or the like all components into the extruder at substantially the same time. This may allow for the charge-modifying and cross-linking reactions to occur simultaneously. In some embodiments, a method of the present invention may comprise combining starch, a first charge-modifying agent, and a catalyst to form a homogeneous reaction blend comprising a charge-modified starch; adding chitosan, a plasticizer, and optionally a second charge-modifying agent to the homogeneous composition comprising the charge-modified starch; and cross-linking the charge-modified starch and chitosan to form a cross-linked, charge-modified starch-chitosan. The chitosan, in some embodiments, may be charged-modified and/or added to an extruder in the presence of a charge-modifying agent, such as, for example, glacial acetic acid. In some embodiments, the method may use multiple inlets of an extruder. For example, starch, the first charge-modifying agent, and the catalyst may be added at a first inlet and/or reaction zone in an extruder, and chitosan, the plasticizer, and optionally the second charge-modifying agent may be added at a second inlet and/or reaction zone in an extruder. This may allow for the charge-modifying and cross-linking reactions to occur simultaneously and/or sequentially. In certain embodiments, a method of the present invention may comprise combining starch, a first charge-modifying agent, and a catalyst to form a charge-modified starch; forming a homogeneous reaction blend comprising the charged-modified starch, chitosan, a plasticizer, and optionally a second charge-modifying agent; and cross-linking the charge-modified starch and chitosan to form a cross-linked, charge-modified starch-chitosan. This may allow for the charge-modifying and cross-linking reactions to occur sequentially. The chitosan, in some embodiments, may be charged-modified and/or added to an extruder in the presence of a charge-modifying agent, such as, for example, glacial acetic acid. In some embodiments, the charge-modified starch is prepared and/or formed by forming a homogeneous reaction blend in an extruder. The charge-modified starch may be extruded and the extrudate may optionally be ground into a powder and/or pelletized. The extrudate may then be combined with chitosan, a plasticizer, and optionally a second charge-modifying agent to form a homogeneous reaction blend. A method of the present invention may provide a formed modified biopolymer In some embodiments, a modified biopolymer of the present invention Further exemplary industries and/or uses for a modified biopolymer of the present invention include, but are not limited to, water treatment such as, for example, single-use ion exchange for water deionization packaging such as, for example, biobased packaging films and biobased structural packaging; paper such as, for example, pulp and paper strength additives and/or coatings for paper; textiles such as, for example, textile adhesives, starch ester alternative for textile manufacture, and textile non-woven thickening agents; and/or construction such as, for example, construction adhesive in wallboard. hi some embodiments, a modified biopolymer of the present invention may be useful in the paper industry, cosmetics, tissue engineering, hydrogels, drug delivery applications, photonics applications, and/or as a flocculant and/or coagulant. The present invention is explained in greater detail in the following non-limiting Examples. A twin screw conical extruder manufactured by DSM, a parallel twin screw extruder manufactured by Leistritz, and a parallel twin screw extruder manufactured by Wegner were used to prepare charge-modified starch. The extruder properties are provided in Table 1. Various extruders listed here allow for demonstration of scalability from lab scale to production-relevant scale. Furthermore, multiple extruders allow for transposition of process parameters across a range of extruder configurations and size. Additionally, the parallel twin screw extruders manufactured by Lestritz and Wegner supported multiple reactions zones, allowing for increased capabilities, including: temperature, screw, and injection profiles. Examples of temperature and injection profiles may be found in Examples 1.2, 5.1, and 7, below. In preparing the charge-modified starch, the following parameters were varied: temperature, screw RPM, and amount of citric acid using each extruder. Table 2 sets forth the ranges for the temperature, screw RPM, and amount of citric acid tested using each extruder. Starch (Native Corn Starch, Item 18321, Batory Foods, Des Plaines IL), citric acid (Item 756707, Univar, Downers Grove, IL) as a charge modifier and plasticizer, and sodium hypophosphate (SHP) (Item SI320, Spectrum Chemical, New Brunswick, NJ) as a catalyst were combined and hand mixed in powder form. Powder mixtures were loaded into custom powder injectors and input into the extruder feed port. Various amounts of citric acid were added to the mixture as provided in Table 2. The resulting mixture was added to the extruder as a powder at varying extrusion conditions as provided in Table 2. The powder mixture was melt-blended in the extruder to form a homogeneous blend reaction in which the citric acid was grafted onto the starch to form a charge-modified starch, termed starch citrate. In some runs, this charge-modified starch was utilized as a precursor polymer to subsequently crosslink to another biopolymer as described in Example 5. Select samples underwent a thermal post treatment following extrusion by way of vacuum oven at 120°C for 90 mins. Table 3 provides specific parameters tested on the DSM extruder with responses described in Table 4 and described below. Each sample was titrated to determine its charge density, and analyzed via FTIR (at wavelengths of 1720cm'1) to determine each sample’s relative carboxyl content via methods described below. Additionally, parameters such as DI uptake, and % extractables were measured as qualitative gauges of material performance. Fourier Transform Infrared Spectroscopy (FTIR) is a measure of a samples’ absorbance/transmittance of wavelengths in the IR spectrum. The intensity of absorbed IR radiation at a given wavelength can be correlated to particular covalent bonds. When data is normalized to the primary alcohol peak (-1000cm'1), relative peak intensities may be used to estimate the amount characteristic groups on the polymer, where decreasing transmittance or, inversely, increasing absorbance indicates an increased degree of reagent grafting. Bonds of interest for biopolymers modified with citric acid, such as, for example, starch citrate, include the carboxyl (R-CO2H) bond at -1713cm'1,where decreasing transmittance or, inversely, increasing absorbance indicates an increased degree of charge density. Back titration is a measure of charge density in anionic, charge-modified biopolymer samples. The results of this measurement technique scales with the FTIR data. As described here in Example 1.1, along with Examples 1.2,1.3, 2.1, 3.1, 3.2, and 3.3, 0.2-0.3g of sample was exposed to 50 ml of 0.05 Μ NaOH solution for 1 hr. One drop of phenolphthalein (Item 3241Ν80, Thomas Scientific, Swedesboro, New Jersey) was added and mixed into solution to act as a visual indication, approximating neutrality of the solution. A pH probe was used to monitor acid/alkaline nature of the solution during mixing and titration. The solution was then titrated with 0.05Μ HC1 at, -0.05 ml/second. The volume of HC1 required to reach pH neutrality was recorded and assumed to be equivalent to the number of mois needed to neutralize excess NaOH in solution. The difference between the recorded mois and initial mois was then normalized to the original sample weight to yield a mol/g or meq/g charge density unit. DI uptake is a measure of a sample’s degree of swelling (/.℮., its absorbency by weight under given conditions). DI uptake was measured by inserting ~0.25g sample/cm in 33 mm diameter, of 12-14 kD dialysis tubing (Item 684219, Carolina Biological, Burlington, North Carolina). The ends of tubing were sealed and labeled, then exposed to 20 ml DI water per gram of sample for 72 hours. DI water was replaced every 2-3 hours over the course of a 72 hr period. Samples were then removed from the dialysis tubing and weighed. Changes in weight between the initial and final (wet) measurements were normalized to initial mass to grams of DI water absorbed per gram of sample (g/g). Samples were then dried using a forced air oven and/or freeze dryer. Weight loss between dried sample and initial sample weight (pre dialysis) was used to calculate extractables as a % of initial sample (inverse of yield). These extractables reflect a measure of the amount of sample that elutes upon initial contact with water. This parameter qualitatively measures the mass fraction of unreacted moieties, plasticizer, and/or degraded polymeric products in a given sample. As can be seen from Tables 3 and 4, charge-modified starch was produced in the process example of reactive extrusion described here. %Transmittance as measured via FTIR is shown to decrease significantly below that of starch (94.5%) while titration values are shown to increase significantly over that of starch (Omeq/g). Temperature and citric acid (charge modifying agent) concentration are the parameters where increasing inputs show increased charge density. Furthermore, inclusion of a thermal post treatment after extrusion was also studied and addition of a post treatment also shows increasing charge density. Relative similarity and relatively low values of DI uptake parameters across indicate a lack of crosslinking. Extractable values are indicative of excess reagent and generally trend with charge modifier concentration. FTIR transmittance values achieved ranged from approximately 35-98% while charge density values achieved ranged from approximately 1 to 6.5 meq/g. A parallel twin screw extruder with multiple injection and reaction zones manufactured by Leistritz was also used to prepare charge-modified starch citrate. These experiments were performed to determine scalability and behavior of materials through varied reaction zones. The extruder properties are provided in Table 1 above. Figure 2 illustrates the 8-zone extruder with injection ports in this configuration located prior to zone 1 and at zone 3. Raw materials were prepared in a similar manner to extrusion as described above in Example 1.1. However, samples were mixed in 1kg units and fed using gravimetric powder feeders manufactured by Brabender (Duisburg, Germany) to account for scale. Studies below utilized multiple injection and reaction zones to simulate full-scale extrusion processes. Screw profile utilized is described in Figure 6 (medium shear screw). Powder samples of each of the following components: starch, citric acid, and SHP were fed into the primary injection zone (prior to zone 1) where the mixture was allowed to react at 120°C. Without wishing to be bound to any particular theory, at this temperature the citric acid dehydrates to yield an anhydride that reacts faster with the free hydroxyl groups. Temperature profiles for each zone are detailed in Table 5 below. Extrusion and composition parameters for starch citrate were varied as described in Table 6 below. In some runs, extruded samples in solid form were post-treated by placing the charge-modified starch in an oven at 120°C for 90 minutes. Specific examples of process parameters and resulting responses are shown in Tables 7 & 8 below, respectively. Table 7: Process parameters for preparing charged-modified starch via 18mm, parallel twin-screw extruder. As can be seen from Tables 7 and 8, this work demonstrated the feasibility of producing charge-modified starch via a reactive extrusion process. %Transmittance as measured via FTIR is shown to decrease significantly below that of starch (94.5%) while titration values are shown to increase significantly over that of starch (Omeq/g). Furthermore, it should be noted that titration and FTIR values have a positive correlation. While not wishing to be bound to any particular theory, it appears that increased RPM in this method can improve the degree of charge modification as a response to increased shear. A parallel twin screw extruder manufactured by Wegner was used to prepare charged-modified starch and to further demonstrate scaling. The extruder properties are provided in Table 1.1 above. Screw profile utilized largely conforms to a purely conveying screw as described in Figure 6 (low shear screw). Raw materials for charge-modified starch were prepared in a similar manner to the extrusion processes described above. However, samples were mixed and injected in ~2kg units to account for the larger scale and continuous nature of this extruder. Extrusion and composition parameters for starch citrate were varied as described in Table 9 below. Specific examples of process parameters and resulting responses are shown respectively in Tables 10 and 11 below, respectively. In some runs, extruded samples in solid form were post-treated by placing the charge-modified starch in an oven at 120°C for 90 minutes. Table 9: Parameter ranges for charge-modified starch via 52mm, parallel twin-screw extruder. Temperature Ranges (°C) 100-125 RPM Ranges (RPM) 120-200 Citric Acid Ranges (wt % relative 50-100 to starch) Table 11: Properties of the charged-modified starch. This work demonstrated the feasibility of producing charge-modified starch via a reactive extrusion process. %Transmittance as measured via FTIR is shown to decrease significantly below that of starch (94.5%) while titration values are shown to increase significantly over that of starch (Omeq/g). Examples 1.1Ε, 1.2C and 1.3 are used to compare samples at similar processing conditions. It is concluded from the similar responses that parameters listed in these examples are transposable across a significant range of extruder sizes (representing from laboratory benchtop to commonly-used industrial sizes). In addition to citric acid, additional anionic charge modifiers are demonstrated in the example below. Starch was charged modified using maleic anhydride (Item 63200-500G-F, Sigma-Aldrich, MO, St. Louis) a catalyst (NaOH, Reagent ACS, Item 630, GFS Chemicals, Powell OH), and plasticizer to form an anionic starch. Table 12 sets forth the ranges for the temperature, screw rpm, and amount of reagent tested using a Process 11,11 mm parallel twin screw extruder as described in Example 1.1, above. Screw profile utilized is described in Figure 6 (medium shear screw). Specific examples of process parameters and resulting responses are shown in Tables 13 and 14 below, respectively. In some runs, extruded samples in solid form were post-treated by placing the charge-modified starch in an oven at 120°C for 90 minutes. In addition to charge density (measured via titration), solubility of each sample was also studied. Here, purified samples (as described in the dialysis process above) are used. 0.25g of sample is mixed into in a beaker with 25ml of DI water at 60C. Beaker with mixture is set stirring on hotplate and held at 60C for 15mins. Mixture is then centrifuged at 250g (1800 RPM & 7cm radius) for 20 mins to separate solid fraction from the liquid fraction, including dissolved solids. A pipette is then used to decant the liquid layer and discarded. Aluminum weigh pans stored in a desiccator and with predetermined weights are used to collect remaining solids. Weigh pans and solids are then dried in a forced air oven for 48hrs at 40°C. Weigh pans and samples are removed from the forced air oven and immediately weighed. Sample weights as a fraction of initial weights are recorded as a % Solubility. Table 14: Properties of the Anionic Modified Starch Data indicated that a charge-modified starch was produced via a reactive extrusion process. %Transmittance as measured via FTIR is shown to decrease significantly below that of starch (94.5%) while titration and solubility values are shown to increase significantly over that of starch (Omeq/g and 7%, respectively). Ranges of charge density varied from 1.3-6,3 meq/g, and solubility varied from 27-86%. The level of charge modification of the starch increased with increasing reagent concentration. Data are further confirmed via increasing solubility with increasing charge density. In addition to anionc charges, starch was charge-modified to form a cationic starch. The cationic charge-modified starch was prepared by varying the following parameters: temperature, screw rpm, amount of charge modifying reagent (glycidyltrimethylammonium chloride [Sigma Aldrich Item 50053- 1L]), catalyst (Sodium Hydroxide) and plasticizer content. Table 15 sets forth the ranges for the studied parameters in the Leistritz, 11 mm extruder. Starch powder was mixed with a catalyst (NaOH) in powder form. Plasticizer was then added to the mixture containing the starch and catalyst and mixed well by hand. The mixture was then input into the extruder. Table 16 provides specific parameters tested with test responses described in Table 17. Note, temperature settings were set to apply a uniform temperature for all heating zones. Although temperature profiles were utilized in other experiments, they are not detailed here. Screw profile utilized is described in Figure 6 (medium shear screw). Each sample was tested to determine its charge density (degree of substitution) via elemental analysis (measuring nitrogen). Elemental analysis may be used to measure charge density for cationic charge-modified biopolymer samples, whereas titration may be used to measure charge density for anionic charge-modified biopolymer samples. Elemental analysis was carried out by means of Perkin Elmer 2400 CHNS Analyzer: The Perkin Elmer 2400 was used to determine total elemental carbon, nitrogen, hydrogen, or sulfur by total combustion. The Degree of Substitution (DS) was determined by nitrogen and calculated according to Equation (1) below: DS = 162.15χ %N/ 1401-151.64 χ %N (1), where DS is the degree of substitution and %N is the measured nitrogen content. Furthermore, %N is nearly 0% but a non-zero number (e.g. 0.002). It is subtracted from all measurements for precision. Table 17: Properties of the cationic charged-modified starch. * Degree of substitution as measured by nitrogen content Once again, a charge-modified starch was produced in this reactive extrusion process. Degree of substitution and solubility values were significantly greater than that of starch (0 DS, and 0.4% solubility, respectively) and demonstrate charge modification of a cationic starch via reactive extrusion. A range of DS values are produced. The DS values achieved here are significantly higher than previously reported values of DS for cationic starch produced via reactive extrusion. In example 2.2D, inclusion of post treatment shows increased degree of substitution with simultaneous reduction in solubility indicating presence of cross linking as discussed in later examples. In addition to starch, additional biopolymers were utilized to demonstrate charge modification. Hemicellulose (Xylan from Beechwood >=90%, Item Χ4252, Sigma Aldrich, St. Louis MO) was charge modified with citric acid to form an anionic hemicellulose using the DSM extruder described in Example 1.1. In preparing the charge-modified hemicellulose, the following parameters were varied: temperature, screw rpm, and amount of citric acid. Table 18 sets forth the ranges for the temperature, screw rpm, and amount of citric acid tested using the twin screw conical extruder. Reagents in powder form were hand mixed in 50 g batches, loaded into the extruder using custom powder injectors, and fed into the extruder at feed rates determined to be relatively and qualitatively consistent. Table 19 provides specific parameters tested with test responses described in Table 20. The FTIR spectra for the charge-modified hemicellulose and for unmodified hemicellulose is provided in Figure 3. Charge density values are reported according to the titration method described in Example 1.1. It should be noted that in this example, charge density values of the raw materials are measured and then subtracted from measured values to show a degree of change in charge density above that of the raw biopolymer. Table 20: Properties of the charged-modified hemicellulose. A charge-modified hemicellulose was produced via reactive extrusion. FTIR analysis shows %Transmission values significantly lower than that of unmodified hemicellulose (91%) and titration values significantly greater than that of unmodified hemicellulose (Omeq/g), indicating charge modification of the hemicellulose. Pectin (Item 76282, Sigma Aldrich, St. Louis, MO) was charge modified to increase the anionic property of pectin by grafting additional carboxylic acid groups onto pectin using the DSM extruder described in Example 1.1. Experimental methods followed those in Example 3.1. Table 21 sets forth the ranges for the temperature, screw rpm, and amount of citric acid tested using the twin screw conical extruder. Table 22 provides specific parameters tested with test responses described in Table 23. If the sample underwent a post treatment, then the sample was placed in a vacuum oven at 120°C for 90 mins. Each sample was tested to determine its charge density (meq/g), and absorbance/transmittance via Fourier Transform Infrared Spectroscopy (FTIR) at 1720cm'1. The FTIR spectra for the charge-modified pectin and for unmodified pectin is provided in Figure 4. Charge density values are reported according to the titration method described in Example 1.1. It should be noted that in this example, charge density values of the raw materials are measured and then subtracted from measured values to show a degree of change in charge density above that of the raw biopolymer. Table 23: Properties of the charged-modified pectin. A charge-modified pectin was produced via reactive extrusion. FTIR analysis shows %Transmission values significantly lower than that of unmodified pectin (63%) and titration values significantly greater than that of unmodified pectin (Omeq/g), indicating charge modification of the pectin. Soy protein was charge modified to form an anionic soy protein using the DSM extruder described in Example 1.1. In preparing the charge-modified soy protein, Experimental methods followed those in Example 3.1. Table 24 sets forth the ranges for the temperature, screw rpm, and amount of citric acid tested using the twin screw conical extruder. Table 25 provides specific parameters tested with test responses described in Table 26. If the sample underwent a post treatment, then the sample was placed in a vacuum oven at 120°C for 90 mins. Each sample was tested to determine its charge density (meq/g), and absorbance/transmittance via Fourier Transform Infrared Spectroscopy (FTIR) at 1720cm'1. The FTIR spectra for the charge-modified soy protein and for unmodified soy protein is provided in Figure 5. Charge density values are reported according to the titration method described in Example 1.1. It should be noted that in this example, charge density values of the raw materials are measured and then subtracted from measured values to show a degree of change in charge density above that of the raw biopolymer. Table 26: Properties of the charged-modified soy protein. A charge-modified soy protein was produced via reactive extrusion. FTIR analysis shows %Transmission values significantly lower than that of unmodified soy protein (93%) and titration values significantly greater than that of unmodified pectin (0meq/g), indicating charge modification of the soy protein. Charge modification was enhanced by thermal post treatment. In addition to charge modifiers, cross-linkers were utilized to form a cross-linked starch using the DSM extruder described in Example 1.1. In preparing the cross-linked starch, experimental methods followed those in Example 1.1. The following parameters are varied: temperature, screw rpm, and the amount of cross linker. In this example, water was used as the plasticizer at the level of 40wt% relative to starch. Cross linkers included: Epichlorohydrin (EPI, >=99% (GC), Item 45340, Sigma-Aldrich, St. Louis, MO), Polyethylene glycol) diglycidyl ether (PEDGE, Avg. ΜΝ 500, Item 475696, Sigma-Aldrich, St. Louis, MO), and Polypropylene glycol) diglycidyl ether (PPDGE, Avg. CA. 640, Item 406740, Sigma-Aldrich, MO, St. Louis) with sodium hydroxide as catalyst. Table 27 sets forth the ranges for the temperature, screw rpm, and amount of cross-linker tested using the twin screw conical extruder. Table 28 provides specific parameters tested with test responses described in Table 29. Here, cross-linked biopolymers were produced via a reactive extrusion process. Reactive extrusion of starch with cross linkers show: as cross linker chain length (molecular weight) is increased (EPI<PEDGE<PPDGE), swelling values improve beyond that of uncross-linked starch (0.4g/g) and solubility values approach that of uncross-linked starch (7%). In addition to starch, charge-modified starch were utilized to form a cross-linked, charge-modified starch using the DSM extruder described in Example 1.1. In preparing the cross-linked, charge-modified starch, experimental methods followed those in Example 4.1. Aquaflocc 330 AW, manufactured by Aquasol Corp (Rock Hill, SC) was used as the cationic starch in this example. Additional commercially-available cationic starches, as well as cationic starches as described in Example 2.2 were also utilized. The following parameters are varied: temperature, screw rpm, amount of cross linker, and plasticizer. Cross linkers included: Epichlorohydrin, Polyethylene glycol) diglycidyl ether, and Polypropylene glycol) diglycidyl ether with sodium hydroxide as catalyst. Table 30 sets forth the ranges for the temperature, screw rpm, and amount of cross-linker tested using the twin screw conical extruder. Table 31 provides specific parameters tested with test responses described in Table 32. Table 32: Properties of cross-linked cationic starch. Here, cross linked, charge modified biopolymers were created via reactive extrusion. Solubility results show values significantly lower than that of the raw material (84%). Here, decreasing solubility indicates increased degree of cross-linking. Swelling results may be higher or lower than that of the raw material (4.4 g/g) depending on degree of cross-linking. To demonstrate crosslinking two charge modified biopolymers, cross-linked, charged-modified starch citrate chitosan was prepared using a 2-step inline process using the Leistritz, 18mm extruder as described in Example 1.1. Grafting citric acid onto starch provides an anionic charge, which changes the degree of charge as can be measured using back titration (meq/g). Acetic acid may be used to protonate chitosan upon mixing, thereby providing a cationic charge on the chitosan. The charge-modified chitosan may be assumed to be partially (/.℮., 50% or more) or fully (100%) protonated due to its solubility in water. Furthermore, the extruder having multiple zones as shown in Figure 2, allows for implementation of temperature and injection profiles. Extrusion and composition parameters for preparing cross-linked, charge-modified starch citrate chitosan were varied as described in Table 33. Here, powder samples of starch, citric acid, and SHP were fed into the initial injection zone (Step 1), while chitosan (Trading Resources, Cocoa Beach, FL), acetic acid (Sigma Aldrich, Item# Α6283, St. Louis, MO), and plasticizers were simultaneously added in at injection zone 3 (Step 2) as shown in Table 34 below. Reactions zones 1-2 were used for charge modification, while reaction zones 3-8 were used for crosslinking the charge modified-starch to charge-modified chitosan. Temperature profiles for each zone are provided in Table 34 below. Screw profile utilized largely conforms to the medium shear screw as described in Figure 6 (medium shear screw). After the graft reaction of citric acid onto starch, the temperature was decreased to 100°C to allow for the injection of protonated chitosan inside the extruder in zone 3 before raising the temperature to 105°C and 110°C in zones 4 and 5, respectively, to initiate the crosslinking reaction between the starch carboxylate and the free amine groups on the backbone of chitosan. In some runs, extruded samples in solid form were post-treated by placing the charge-modified, cross-linked polymer in an oven at 120°C for 90 minutes. The simultaneous injection of two mixtures demonstrated below is defined as a 2-step, inline reaction. Specific examples of process parameters and resulting responses are shown in Tables 35 and 36 below, respectively. The methods for determining the measured responses (e.g. solubility, DI uptake, and extractables) are described in Example 1. For FTIR analysis, bonds of interest for charged-modified starch, cross linked to chitosan system include the Amide-Carbonyl (R-CO-CNH-R) stretch at -1650℮ηΤ1. Table 36: Properties of the cross-linked, charge-modified starch citrate chitosan. As described in Example 2, charge modified polymers without cross-linking show increasing solubility with increasing charge density (>5% and up to 100%). Due to the presence of charge modified starch and charge modified chitosan, solubility values <5% indicate presence of crosslinking. FTIR analysis confirms presence of the amide-carbonyl stretch where a modified and unmodified chitosan shows transmission values of 26% and modified and unmodified starch shows values of 5%. %Transmission values above 26% indicate presence of charge modified starch cross linked to charge modified chitosan, confirming the ability to form a charge modified biopolymer cross linked to another biopolymer in a 2-step, in-line method. To demonstrate a method where charged-modified biopolymers may be produced and subsequently cross linked to another biopolymer, charged-modified starches as prepared in Example 1.1 were cross-linked with chitosan by mixing powdered starch citrate (/.℮., the citric acid-modified starch) with acetic acid, chitosan, and plasticizer so that the mixture was in powdered form. To obtain the powdered charge-modified starch, the charge-modified starch was ground using a blender to sugar/starch consistency where there were no visible chunks/inconsistencies in the powder mixtures. At least one plasticizer selected from: glycerol [Item#0854, Amresco, Solon, OH], citric acid, and polyethylene glycol [molecular weights of 400, 800, 20,000, Sigma Aldrich, St. Louis, MO] was added to the mixture comprising of starch citrate, acetic acid, chitosan, and plasticizer to induce melt blending during the extrusion process. The resulting powder mixture was added to the extruder described in Example 1.1 in a method resembling the process for preparing charge-modified starch as described in Example 1.1. Extrusion parameters and compositions were modified according to Table 37 below. Completion of the reaction in two steps is defined here as a “2-step, 2-pass” reaction. Examples of process parameters for cross-linked, charge-modified starch citrate chitosan and measured responses are shown in Tables 38 and 39, respectively below. The charge-modified starch used to prepare the cross-linked, charge-modified starch citrate chitosan had previously been prepared as described in Example 1 according to parameters described in sample 1.1 A. Each sample was analyzed via FTIR to characterize chemical identity, determine its deionized water (DI) uptake, and to measure extractables (inverse of yield) following the methods described in Example 1. Table 38: Process parameters for preparing cross-linked, charge-modified starch citrate chitosan. As described in Example 5.1, a citric acid modified starch cross-linked to chitosan may show the amide-carbonyl (R-CO-CNH-R) stretch at -1650cm'1 when subjected to FTIR analysis. FTIR analysis confirms presence of the amide-carbonyl stretch where a modified and unmodified chitosan shows transmission values of 26% and modified and unmodified starch shows values of 5%. Here, %Transmission values of 59 and 62% (>26%) indicate presence of charge modified starch cross linked to charge modified chitosan and confirming the ability to form a charge modified biopolymer cross linked to another biopolymer in a 2-step, 2-pass method. To demonstrate simultaneous charge modification and cross-linking via reactive extrusion, all raw materials chitosan. As described in Example 5.1 and 5.2, a citric acid modified starch cross-linked to chitosan may show the Amide-Carbonyl (R-CO-CNH-R) stretch at ~1650cm'1 when subjected to FTIR analysis. The presence of carbonyl groups indicate citric acid charge modification on starch, and the presence of the Amide-carbonyl group indicates cross-linking. FTIR analysis confirms presence of the Amide-carbonyl stretch where a modified and unmodified chitosan shows transmission values of 26% and modified and unmodified starch shows values of 5%. Here, %Transmission values of >26% indicate simultaneous charge modification and cross linking of charge modified starch cross linked to chitosan to form a charge modified biopolymer cross linked to another biopolymer in an all-in-one method. To demonstrate ion removal capabilities of a charge modified, cross linked biopolymer, citric acid-modified starch cross-linked to chitosan were prepared according to Examples 5.1, 5.2, and 5.3. Samples were tested for their salt uptake capacity measured by conductivity and ash content post exposure to a saline solution. Ash content testing is a measure of residual inorganic material in a sample upon exposure to high temperatures. 0.3g of samples was exposed to a 10% saline (NaCl) solution for 5 minutes, then squeezed by hand to remove absorbed liquids. Samples were then transferred to clean, dry, glass vials whose weights were previously recorded. Samples were then exposed to high temperatures in a muffle furnace (Vulcan, Model 3-550), for 4 hours at 575°C following TAPPI Standard: Τ211 om-02 - “Ash in wood, pulp, paper and paperboard, combustion at 525°C”. To determine the ash content, the vial weight was subtracted from the final recorded weight comprising of vial and ash. Final ash weight was assumed to be residual captured salts where the final ash weight is divided by the initial sample weight to normalize data to a g NaCl/g sample (g/g) format. Conductivity is a measure of ionic mobility in a given solution. Reductions in conductivity may be attributed to captured ions, changes in system energy (ζ.℮., temperature, pressure, etc.), and/or potential of dissolved ions (/.℮., pH changes in presence of acids/bases). Samples (0.3g) were exposed to 25ml of 10% NaCl solution where initial conductivity (Metier- Toledo conductivity instrument [model # 51302530]) was measured to be 142 mS/cm with a standard deviation of 3.7. Final conductivity measurements were assumed to be attributed to ion capture and was therefore used to calculate a percent difference in conductivity (captured salt). The uptake of salt was correlated to the resulting decrease in conductivity by the following formula: Uptake = (volume in mL * salinity * %A) / sample weight , where Here, salt uptake results of a cross linked biopolymer (cross-linked cationic starch as prepared via the method found in Example 4.2) as measured by conductivity show values of Og/g. While not wishing to be bound to any particular theory, the presence of amphoteric charge (including both cationic and anionic charge simultaneously) is expected to improve the polymer’s interaction with free ions in solution and is shown in Table 43. Data demonstrated the ability of charge modified and cross linked biopolymers to remove ions from solution at a greater rate than a cross-linked biopolymer. Salt uptake is further demonstrated through ash content measurements as shown in Table 43 above. A superabsorbent polymer was prepared using a commercially available charged cationic starch (AquaFlocc 330AW) and a catalyst on the Pll extruder described in Example 1.1. The extruder having multiple zones similar to that shown in Figure 2, allows for implementation of temperature and injection profiles. Screw profile utilized is described in Figure 6 (medium shear screw). Extrusion and composition parameters for preparing a material for super absorbent material were varied as described in Table 44. In preparing the super absorbent polymer, powder cationic starch (Aquafloc 330AW) and sodium hydroxide were fed into the initial injection zone via volumetric powder feeder (olumetric MiniTwin Process 11, Typ 567-7660, Thermo Electron / Thermo Fisher Scientific, Germany), while plasticizer (glycerol) was simultaneously added in at injection zone 2 via liquid injector and peristaltic pump (Masterflex P/S Peristaltic Pump, Model No 1300-3600-0004, Thermo Fisher Scientific, USA) with corresponding peristaltic pump head (Masterflex P/S Easy Load II, Model No 955-0000, Thermo Fisher Scientific, USA). In some runs, extruded samples in solid form were post-treated by placing the modified cationic polymer in an oven at 120°C for 90 minutes. The simultaneous injection of two mixtures demonstrated below is defined as a 2-step, inline reaction. In preparing the absorbent polymers, the following parameters were varied: temperature, screw rpm, plasticizer, plasticizer concentration, and amount of catalyst. Table 44 sets forth the ranges for the temperature, screw rpm, and amount of catalyst tested using the 11 mm, parallel twin screw extruder. Samples were tested as absorbents using the EDANA/INDA method WSP 240.2.R3: free swell capacity in saline by gravimetric determination in order to measure the fluid uptake of samples. For the gravimetric method, 0.2 g of sample was sealed in a 2”x2” teabag. The teabag/sample packet was submerged in a solution for 1 hr, then hanged to dry for 10 mins. Solutions were prepared according to an industrially relevant application (0.9% NaCl). Weight measurements were recorded pre- and-post submerging and normalized for a teabag control sample undergoing the same conditions. The calculation was as follows: where Table 45 provides specific parameters tested with specific temperature profiles shown in Table 46 and test responses described in Table 47. Each sample was tested to determine its swelling capacity according to the method described above, and solubility according to the method in example 2. Table 45: Process and formulation parameters for absorbent polymers. Sample # Sample 7Α Sample 7Β Temperature (°C) Temp Profile 7 Temp Profile 7 RPM 150 80 Post Treatment Yes Yes Plasticizer (wt % relative to cat. Glycerol (25%) Glycerol (25%) starch) NaOH (wt % relative to cat. starch) 7.5% 7.5% starch via 11 mm, parallel twin-screw extruder. Table 47: Properties of the absorbent polymers. As shown by results in Table 47, reactive extrusion is used to make a biopolymer material that is useful for absorbing liquids in industrially relevant applications. Additionally, samples as described in Example 7 were tested as absorbents for other fluids using a modified the EDANA/INDA method WSP 240.2.R3: free swell capacity in saline by gravimetric determination in order to measure the fluid uptake of samples. Here, alternative solutions are used as shown in Table 48, in place of the specified 0.9% NaCl (saline). Instant Ocean Seal Salt was used as a sea water simulant. Canola oil, conventional motor oil, and synthetic motor oil, were used as oil references. Gasoline and diesel fuel were used as fuel references, and whole bovine blood was used as a blood reference. The results demonstrated an improved performance for cross-linked, charge-modified biopolymers of the present invention relative to conventional superabsorbent materials: Sodium Polyacrylate (NaPoly, Item 432784, Sigma-Aldrich, St. Louis, MO) in Table 48 below. Table 48: Biosorbent properties of a cross-linked, charge-modified biopolymer of the present invention ("Modified Biopolymer") relative to commercially available superabsorbent polymers (NaPoly). As shown by results in Table 48, reactive extrusion is used to make a biopolymer material that is useful for absorbing liquids in a range of industrially relevant applications. A JEOL JSM-6010LA scanning electron microscopy (SEM) with a solid state EDS detector was used to characterize and compare samples. Samples were adhered to a mount using double sided carbon tape and analyzed at 20kV. Micrographs were collected along with corresponding EDS scans of the target area. Indications of homogeneity were derived from the comparison of commercially available cationic starch to an extruded cationic starch of the present invention (Example 2.2C). AquaFlocc 330AW manufactured by Aquasol Corp (Rock Hill, SC) represented the commercially available starch. It is believed that the commercially available cationic starch is modified in a dry process, which maintains starch in granular form and allows only for surface modification of the starch. In contrast, while not wishing to be bound to any particular theory, the extrusion process is believed to completely destroy the granular structure of the biopolymer ( As can be seen in Figures 7Α and 7Β, which are SEM images of commercially available starch, the commercially available starch retains the starch’s characteristic granular structure. In contrast, as can be seen in Figures 7D and 7Ε, which are SEM images of extruded cationic starch prepared according to methods of the present invention, starches extruded according to embodiments of the present invention exhibit complete destruction of the granular structure and morphology arises only from topology in sample preparation. This can be seen by comparing Figures 7Α and 7Β with Figures 7D and 7Ε. Furthermore, when exposed to water and dried, the commercially available starches showed the presence of insoluble materials. These insoluble materials indicate uncharged or lowly charged regions, which are a product of inhomogeneous processing. These results were confirmed via Energy-Dispersive X-ray Spectroscopy (EDS of EDXS), which was used to map the elemental composition of the SEM image for the commercially available starch (Figure 7C) and the extruded cationic starch prepared according to methods of the present invention (Figure 7F). As can be seen in Figure 7C, a clear/defined dark region is present where the discrete particles are imaged. This indicates that these particles are different in composition (lacking chlorine) compared to the surrounding region. In contrast, as can be seen in Figure 7F, EDS scans of the extruded starch show a gradual change in contrast towards the bottom right of the image. This change correlates to a sloping region on the SEM image towards the bottom right. However, the top left of the image in Figure 7F also shows a sloping region in the SEM image, with little change in the EDS map. Thus, it can be concluded that any contrast here is from a shadowing effect, rather than a compositional effect and the sample is therefore homogeneous. The foregoing is illustrative of the present invention, and is not to be construed as limiting thereof. The invention is defined by the following claims, with equivalents of the claims to be included therein. All publications, patent applications, patents, patent publications, and other references cited herein are incorporated by reference in their entireties for the teachings relevant to the sentence and/or paragraph in which the reference is presented. Modified biopolymers, such as, charge-modified biopolymers, cross-linked biopolymers, and cross-linked, charge-modified biopolymers are provided along with methods of producing and using the same. 1. A method for producing a cross-linked, charge-modified biopolymer comprising: combining a biopolymer and at least one charge-modifying agent to form a homogenous reaction blend; reacting the biopolymer and the at least one charge-modifying agent in the homogenous reaction blend; and cross-linking the biopolymer in the homogeneous reaction blend to form a cross-linked, charge-modified biopolymer. 2. The method of claim 1, wherein the combining step further comprises combining a plasticizer and optionally a catalyst with the biopolymer and the at least one charge-modifying agent to form the homogenous reaction blend. 3. The method of claim 2, wherein the plasticizer is citric acid, water, glycerol, polyethylene glycol, and/or sorbitol. 4. The method of any one of claims 1-3, wherein the at least one charge-modifying agent comprises a carboxyl, sulfonate, sulfate, phosphate, primary amine, secondary amine, tertiary amine, quaternary ammonium, sulfonium, and/or phosphonium group. 5. The method of any one of claims 2-4, wherein the catalyst is sodium hypophosphite, sodium bisulfate, and/or sodium bisulfite. 6. The method of any one of claims 1-5, wherein, after the reacting step, the biopolymer has a net positive charge or a net negative charge. 7. The method of any one claims 1-5, wherein, after the reacting step, the biopolymer is a polyampholyte. 8. The method of any one of claims 1-7, wherein the cross-linking step further comprises reacting the charge-modified biopolymer with at least one cross-linking agent, optionally in the presence of an initiator. 9. The method of claim 8, wherein the at least one cross-linking agent is an acid ( 10. The method of claim 8, wherein the initiator is a peroxide, peroxy ester, hydroperoxide, ketone peroxide, and/or an azo compound. 11. The method of any one of claims 1-10, wherein the reacting and cross-linking steps occur simultaneously. 12. The method of any one of claims 1-11, further comprising foaming the cross-linked, charge-modified biopolymer. 13. The method of claim 12, wherein the foaming step is carried out with a foaming agent. 14. The method of claim 13, wherein the foaming agent is supercritical carbon dioxide, water 15. The method of any one of claims 1-14, wherein the cross-linked, charge-modified biopolymer comprises a plurality of void spaces formed therein having an average diameter of about 0.1 to about 500 microns. 16. The method of any one of claims 1-15, wherein the biopolymer comprises at least two different biopolymers, optionally wherein one of the at least two different biopolymers is a charge-modified biopolymer. 17. The method of claim 16, wherein the at least two different biopolymers comprise starch and chitosan. 18. The method of any one of claims 1-17, wherein the cross-linked, charge-modified biopolymer has a net positive charge or a net negative charge. 19. The method of any one of claims 1-18, wherein the cross-linked, charge-modified biopolymer is a polyampholyte. 20. The method of any one of claims 1-19, wherein the combining step comprises melting blending the biopolymer and the at least charge-modifying agent using a reactive extrusion process. 21. The method of any one of claims 1-20, wherein the reacting and cross-linking steps are carried out using a reactive extrusion process. 22. The method of any one of claims 1-21, wherein the method is carried out in an extruder. 23. The method of claim 22, wherein the method is a single-stage direct extrusion process or a multi-stage extrusion process. 24. The method of claim 22, wherein the extruder comprises at least two reaction zones. 25. The method of 24, wherein the method comprises reacting the biopolymer and at least one charge-modifying agent at a first reaction zone and cross-linking the biopolymer at a second reaction zone. 26. The method of any one of claims 24 or 25, wherein the method comprises in-line compounding. 27. The method of any one of claims 1-26, wherein the cross-linked, charge-modified biopolymer is a formed product of a defined shape. 28. The method of any one of claims 1-27, wherein cross-linked, charge-modified biopolymer is in the form of a particle have a diameter in a range of about 10 microns to about 1000 microns. 29. The method of any one of claims 1-28, wherein the reacting and/or cross-linking step(s) is/are carried out at a temperature in a range of about 80°C to about 150°C, optionally at a temperature in a range of about 80°C to about 120°C. 30. The method of any one of claims 1-29, wherein the reacting and/or cross-linking step(s) is/are carried out in an extruder with a residence time in a range of about 0.1 minutes to about 30 minutes, optionally in a range of about 0.1 minutes to about 10 minutes. 31. The method of any one of claims 1-30, wherein the reacting and/or cross-linking step(s) is/are carried out in an extruder having a screw RPM in a range of about 10 to about 500, optionally in a range of about 90 to about 150. 32. The method of any one of claims 1-31, further comprising heating the cross-linked, charge-modified biopolymer at a temperature in a range of about 100°C to about 150°C for a period of time in a range of about 5 minutes to about 24 hours. 33. The method of any one of claims 1-32, further comprising radiating the cross-linked, charge-modified biopolymer with UV radiation for a period of time in a range of about 5 minutes to about 24 hours. 34. The method of claim 1, wherein the biopolymer is starch and the at least one charge-modifying agent is citric acid that are combined to form a homogeneous reaction blend and reacted to form a charged-modified starch. 35. The method of claim 34, wherein the reacting step comprises reacting starch and citric acid in a ratio in a range of 0.1:1 to 4:1 (citric acid:starch), optionally in a ratio in a range of 0.5:1 to 2:1. 36. The method of any one of claims 34 or 35, wherein the homogeneous reaction blend further comprises a charge-modified chitosan and a plasticizer, and the cross-linking step comprises cross-linking the charged modified chitosan with the charge-modified starch to form a cross-linked, charged modified starch-chitosan. 37. The method of claim 36, wherein the cross-linked, charged modified starch-chitosan comprises a covalent bond between a carboxyl group of starch and an amino group of chitosan. 38. The method of any one of claims 36 or 37, wherein the homogeneous reaction blend comprises starch in an amount in a range of about 5 wt% to about 30 wt%, citric acid in an amount in a range of about 5 wt% to about 30 wt%, charge-modified chitosan in an amount in a range of about 5 wt% to about 30 wt%, a catalyst in an amount in a range of about 0.1 wt% to about 5 wt%, and a plasticizer in an amount in a range of about 20 wt% to about 40 wt%. 39. The method of any one of claims 34-38, wherein the method is carried out using a reactive extrusion process in an extruder. 40. A cross-linked, charge-modified biopolymer prepared according to the method of any one of claims 1-39. 41. The cross-linked, charge-modified biopolymer of claim 40, wherein the cross-linked, charge-modified biopolymer is a biosorbent. 42. A cross-linked, charge-modified biopolymer having a charge density of at least 3 meq/g as determined by titration, 43. A cross-linked, charge-modified biopolymer having an increased charge density and/or degree of cross-linking compared to a cross-linked, charge-modified biopolymer prepared using a conventional method. 44. A cross-linked, charge-modified biopolymer having an increased porosity and/or pore size compared to a cross-linked, charge-modified biopolymer prepared using a conventional method. 45. A cross-linked, charge-modified biopolymer having an increased salt uptake compared to a cross-linked, charge-modified biopolymer prepared using a conventional method. 46. A cross-linked, charge-modified biopolymer having an increased metal chelation property compared to a cross-linked, charge-modified biopolymer prepared using a conventional method. 47. A method of absorbing a fluid comprising contacting the cross-linked, charge-modified biopolymer of claim 1 with the fluid, thereby absorbing the fluid. 48. A method of reducing the amount of a salt and/or metal in a solution comprising contacting the cross-linked, charge-modified biopolymer of claim 1 with a solution comprising a salt and/or metal, wherein the salt and/or metal binds to the cross-linked, charge-modified biopolymer, thereby reducing the amount of the salt and/or metal in the solution. 49. A method comprising combining starch, chitosan, at least one charge-modifying agent, a catalyst, and a plasticizer to form a homogenous reaction blend; charge-modifying the starch and chitosan to form a charge-modified starch and a charge-modified chitosan; and cross-linking the charge-modified starch and charge-modified chitosan to form a cross-linked, charge-modified starch-chitosan. 50. A method comprising combining starch, a first charge-modifying agent, and a catalyst to form a homogeneous reaction blend comprising a charge-modified starch; adding charge-modified chitosan and a plasticizer to the homogeneous reaction blend comprising the charge-modified starch; and cross-linking the charge-modified starch and charge-modified chitosan to form a cross-linked, charge-modified starch-chitosan. 51. The method of claim 50, wherein the homogeneous reaction blend comprising a charge-modified starch is formed at a first reaction zone in an extruder, and the charge-modified chitosan and plasticizer are added to the homogeneous reaction blend at a second reaction zone in the extruder. 52. A method comprising combining starch, a first charge-modifying agent, and a catalyst to form a charge-modified starch; forming a homogeneous reaction blend comprising the charged-modified starch, a charged-modified chitosan, and a plasticizer; and cross-linking the charge-modified starch and charged-modified chitosan to form a cross-linked, charge-modified starch-chitosan. 53. The method of any one of claims 49, 50, or 52, wherein the method is carried out using a reactive extrusion process.Related Application Data
Field
Background
Summary
Brief Description of the Drawings
Detailed Description
Examples
Example 1.1 - Extruded Charge Modified Biopolymer (Example of Citric Acid grafted on to starch at ~2mm scale)
Extruder Manufacturer DSM Thermo Fisher Leistritz Wegner Extruder Model Xplore Process 11 Ν/Α TX-52 Residence Time 0.25-10 mins 0.25-5 mins ~3mins ~3mins Screw Size (Screw Diameter) 3 cm 11 mm 18 mm 52 mm L/D 5 40 40 27 Die Size 1 -2 mm 0.5-11 mm 1mm & 4.5 mm 2-4 mm Rotation Corotating Screws Co-rotating Screws Corotating Screws Corotating Screws Throughput 0.05-0.2 Kg/hr 0.1-5 Kg/hr 0.5-8 Kg/hr 3-30 Kg/hr Type of heating Electric Electric Electric Electric Number of heating zones 1 8 8 1 Type of Die Single hole/circular Single hole/circular single holes 1 or 2 holes Additive zones One 8 3 feed ports 2 feed ports, 1 extra for foaming agent Type of cooling Water Water Air Water Parameter Range (DSM) Range (Leistritz) Range (Wegner) Temperature Ranges (°C) 90-150 100-120 100-125 RPM Ranges (RPM) 60-200 120-200 120-200 Citric Acid Ranges (rel% to starch) 50-100 50-100 50-100 Sample # Sample 1.1Α Sample 1.1Β Sample 1.1C Sample 1.1D Sample LIE Temperature (°C) 140 140 100 140 125 RPM 120 120 120 120 120 Post Treatment Yes No Yes Yes Yes Citric Acid (wt % relative to Starch) 150 150 50 50 75 SHP (wt % relative to Citric Acid) 20 20 20 20 20 Sample # FTIR (%Trans.) Titration (meq/g) DI Uptake (s/s) Extractables (%) 1.1Α 36 5.9 3.4 89 1.1Β 96 1.9 2.8 87 1.1C 59 2.8 2.7 37 1.1D 48 4.2 3.1 30 1.1Ε 49 3.8 5.4 49 Example 1.2 - Extruded Charge Modified Biopolymer (Example of Citric Acid grafted on to starch at 18mm scale)
Zone 1 2 3 4 5 6 7 8 Temperature (°C) 100 105 115 120 120 120 120 115 Injection Starch + Reagents Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α Temperature Ranges (°C) 100-120 (see Table 5) RPM Ranges (RPM) 120-200 Citric Acid Ranges (rel% to starch) 50-100 Sample # Sample 1.2 A Sample 1.2Β Sample 1.2C Sample 1.2D Temperature (°C) 100-120 (multiple zones) 100-120 (multiple zones) 100-120 (multiple zones) 100-120 (multiple zones) RPM 100 160 100 170 Post Treatment Yes Yes Yes Yes Citric Acid (wt % relative to Starch) 100 50 75 75 SHP (wt % relative to starch) 20 20 20 20 Sample # FTIR (%Trans.) Titration (meq/g) DI Uptake (g/g) Extractables (%) 1.2Α 35 6.55 1.7 32 1.2Β 53 2.10 1.1 10.5 1.2C 54 2.0 1.8 45 1.2D 43 5.8 1.3 10 Example 1.3 - Extruded Charge Modified Biopolvmer (Example of Citric Acid grafted on to starch at 52mm scale)
Sample # Sample 1.3Α Sample 1.3Β Temperature (°C) 110 120 RPM 120 100 Post Treatment Yes Yes Citric Acid (wt % relative to Starch) 66 66 SHP (wt % relative to Citric Acid) 20 20 Sample# FTIR (%Trans.) Titration (meq/g) DI Uptake (g/g) Extractables (%) 1.3 A 65 2.9 Ν/Α 64 1.3Β 69 2.4 Ν/Α 68 Example 2.1 - Extruded Charge Modified Biopolymer (Examples of additional anionic charge modifiers grafted oil to starch)
Temperature Ranges (°C) 85-140 RPM Ranges (RPM) 10-500 Maleic Anhydride Ranges (wt % relative to starch) 5-120 Catalyst (NaOH) Ranges (wt% relative to starch 2-60 Plasticizer Ranges (wt% relative to starch) Water, Glycerol, & Water/Glycerol mixes at 40% Example 2.1Α Example 2.1Β Temperature (°C) 110 110 RPM (RPM) 50 50 Post Treatment No No Maleic Anhydride Ranges (wt 30 60 Catalyst (NaOH) Ranges (wt% relative to starch 12 24 Plasticizer (wt% relative to starch) Water (40%) Water (40%) Sample# FTIR (%Trans) Titration (meq/g) Solubility (%) 2.1Α 83 3.26 76 2.IB 73 5.11 84 Example 2.2 - Extruded Charge Modified Biopolymer (Examples of cationic charge modifiers grafted on to starch)
Temperature Ranges (°C) 85-140 RPM Ranges (RPM) 10-500 glycidyltrimethylammonium chloride Ranges (wt 5-150 Catalyst (NaOH) Ranges (wt% relative to starch 2-60 Plasticizer Ranges (wt% relative to starch) Water, Glycerol, & Water/Glycerol mixes at 40% Sample # Sample 2.2Α Sample 2.2Β Sample 2.2C Sample 2.2D Temperature (°C) 90 120 90 90 Plasticizer (wt% relative to starch) Water (40%) Water (40%) Water (40%) Water (40%) RPM 100 120 50 50 Post Treatment No No No Yes glycidyltriinethylammonium chloride (wt % relative to Starch) 4 85 30 30 NaOH (wt % relative to Starch) 1.2 24 12 12 Sample # Degree of Substitution* Solubility (%) 0.035 28 2.2Β 0.12 68 2.2C 0.19 76 2.2D 0.21 13 Example 3.1 - Extruded Charge Modified Biopolymer (Demonstration of charge grafting onto hemicellulose)
Temperature Ranges (°C) 90-150 RPM Ranges (RPM) 50-200 Citric Acid Ranges (wt % relative to hemicellulose) 40-150 Sample # Sample 3.1Α Sample 3.IB Temperature (°C) 140 140 RPM 120 120 Post Treatment No Yes Citric Acid (wt % relative to Hemicellulose) 150 150 SHP (wt 20 20 Sample # FTIR (%Trans.) Titration (meq/g) 3.1Α 81.4% 1.66 3.IB 53.4% 4.68 Example 3.2 - Extruded Charge Modified Biopolymer ('Demonstration of charge grafting onto pectin)
Temperature Ranges (°C) 90-150 RPM Ranges (RPM) 50-200 Citric Acid Ranges (wt % relative to pectin) 40-150 Sample # Sample 3.2Α Sample 3.2Β Temperature (°C) 140 140 RPM 120 120 Post Τreatment No Yes Citric Acid (% relative to pectin) 150 150 SHP (% relative to Citric Acid) 20 20 Sample FTIR (%Trans.) Titration (meq/g) 3.2Α 59.1 4.96 3.2Β 26.6 5.72 Example 3.3 - Extruded Charge Modified Biopolymer (Demonstration of charge grafting onto Soy Protein)
Temperature Ranges (°C) 90-150 RPM Ranges (RPM) 50-200 Citric Acid Ranges (wt % relative to soy protein) 40-150 Sample # Sample 3.3A Sample 3.3Β Temperature (°C) 140 140 RPM 120 120 Post Treatment No Yes Citric Acid (wt % relative to Hemicellulose) 150 150 SHP (wt % relative to soy protein) 20 20 Sample # FTIR (%Trans.) Titration (meq/g) 3.3Α 68.4 1.66 3.3Β 42.8 4.68 Example 4.1 - Extruded cross linked biopolviner (Demonstration of starch modified
with a range of cross linkers)
Temperature (°C) 80-110 RPM 50-120 Crosslinker Epichlorohydrin, PEDGE, and PPDGE Crosslinker (wt%relative to starch) 0.01-0.1 NaOH (wt% relative to Starch) 0.005-0.2 Sample 4.1Α Sample 4.IB Sample 4.1C Temperature (°C) 90 90 90 RPM 120 120 120 Post-treatment No No No Crosslinker EPI PEDGE PPDGE Crosslinker (wt%relative to starch) 0.1 0.1 0.1 NaOH (wt% relative to Starch) 0.2 0.2 0.2 Plasticizer (40% relative to starch) Water Water Water Solubility (%) Swelling (g/g) Sample 4.1Α 1.6 0.35 Sample 4.1Β 2.53 2.67 Sample 4.1C 4.07 3.15 Example 4.2 - Extruded, cross linked, charge modified biopolymer (Demonstration of cationic starch modified with various cross linkers')
Temperature (°C) 80-160 RPM 10-300 Crosslinker (wt%relative to starch) 0.0001 to 10 NaOH (wt% relative to Starch) 0.001 to 20 Plasticizer (%) Water, Glycerol (20-50%) Sample 4.2Α Sample 4.2Β Sample 4.2C Temperature (°C) 90 90 90 RPM 120 120 120 Post-treatment No No No Crosslinker EPI PEDGE PPDGE Crosslinker (wt%relative to starch) 0.1 0.1 0.1 NaOH (wt% relative to Starch) 0.2 0.2 0.2 Plasticizer (% relative to starch) Water (40%) Water (40%) Water (40%) Solubility (%) Swelling (g/g) Sample 4.2Α 12.6 1.9 Sample 4.2Β 39.9 11.2 Sample 4.2C 40.3 14.7 Example 5.1 - Extruded cross linked, charge modified biopolymer (Demonstration of cross linking multiple biopolymers using 2-step, in line method)
Temperature Ranges (°C) 100-120 (see Table 34) RPM Ranges (RPM) 140-170 Chitosan Ranges (wt % relative to Starch) 100 Acetic Acid Ranges (wt % relative to Chitosan) 33 Starch Citrate Ranges (wt % relative to Chitosan) 100 Plasticizer Types Citric Acid Plasticizer Ranges (wt 90-140 Zone 1 2 3 4 5 6 7 8 Temperature (°C) 120 120 100 105 110 110 110 105 Injection Starch + Reagents Ν/Α Ν/Α Chitosan + Reagents Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α Sample # Sample 5.1Α Sample 5.1Β Sample 5.1C Temperature (°C) 100-120 (multiple zones) 100-120 (multiple zones) 100-120 (multiple zones) RPM 140 140 170 Post Treatment Yes No Yes Reaction Type 2-step inline 2-step inline 2-step inline Plasticizer Type Citric Acid Citric Acid Citric Acid Plasticizer ( wt % relative to Chitosan) 75 75 75 Starch Citrate ( wt % relative to Chitosan) 100 100 100 Acetic Acid ( wt % relative to Chitosan) 33 33 33 Sample # Solubility FTIR (%Trans) DI Uptake (g/g) Extractables (%) 5.1Α 4.1 67.5 2.6 19 5.1Β 2.4 65.4 9.5 69 5.1C 4.1 68.2 1.9 19 Example 5.2 - Extruded cross linked, charge modified biopolymer (Demonstration of cross linking multiple biopolymers using 2-step, 2-pass method)
Temperature Ranges (°C) 90-130 RPM Ranges (RPM) 60-200 Chitosan Ranges (rel% to Starch) 50-150 Acetic Acid Ranges (rel% to Chitosan) 5-100 Starch Citrate Ranges (rel% to Chitosan) 150-250 Plasticizer Types Glycerol Citric Acid, Water Plasticizer Ranges (rel% to Chitosan) 120-275 Sample # Sample 5.2Α Sample 5.2Β Extruder DSM DSM Temperature (°C) 100 110 RPM 120 120 Post Treatment No No Reaction Type 2-step, 2-pass 2-step, 2-pass Plasticizer Type Citric acid Citric Acid Plasticizer (wt % relative to Chitosan) 175 175 Starch Citrate (wt % relative to Chitosan) 100 250 Acetic Acid (wt % relative to Chitosan) 33 33 Sample # FTIR (% Trans) DI Uptake (g/g) Extractables (%) 5.2Α 59.4 2.7 73 5.2Β 62.6 1.5 61 Example 5.3 - Extruded cross linked, charge modified biopolymer (Demonstration of cross linking multiple biopolymers using all-in-one method)
Extruder DSM Wegner TX-52 Temperature Ranges (°C) 90-130 105-130 RPM Ranges (RPM) 60-200 120-250 Chitosan Ranges (wt % relative to Starch) 50-150 50-75 Acetic Acid Ranges (wt % relative to Chitosan) 5-100 Ν/Α Starch Ranges (wt % relative to Chitosan) 150-250 100 Plasticizer Types Glycerol Citric Acid, Poly Ethylene Glycol, Water Citric Acid, Water Plasticizer Ranges (wt % relative to Chitosan) 120-275 100-130 Sample # Sample 5.3Α Sample 5.3Β Sample 5.3C Sample 5.3D Extruder DSM DSM Wegner TX-52 Wegner TX-52 Temperature (°C) 100 133 120 110 RPM 120 120 120 200 Post Treatment No No No No Reaction Type All-in-one All-in-one All-in-one All-in-one Plasticizer Type Citric Acid Glycerol Citric Acid Citric Acid Plasticizer (%rel to Chitosan) 175 175 100 100 Starch (%rel to Chitosan) 150 100 150 150 Citric Acid (%rel to Starch) 66 66 Ν/Α Ν/Α SHP (%rel to Starch) 20 20 20 20 Acetic Acid (%rel to Chitosan) 33 33 Ν/Α Ν.Α Sample # FTIR (%Trans) DI Uptake (g/g) Extractables (%) 5.3Α 58.8 4.3 58 5.3Β 66.9 4.9 54 5.3C 67.2 Ν/Α 67 5.3D 65.3 Ν/Α 65 Example 6 - Example of modified biopolymer for IEX application (Demonstration of Salt/Heavy Metal Uptake)
Sample # Salt Uptake Conductivity (g/g) Salt Uptake - Ash Content (g/g) 5.1Α 0.24 0.98 5.1Β 0.22 1.1 5.1C 0.16 0.69 5.2Α 0.13 Ν/Α 5.2Β 0.13 Ν/Α 5.3Α Ν/Α 0.1 5.3Β 0.25 0.74 5.3C 0.2 Ν/Α 5.3D 0.24 Ν/Α Example 7 - Example of modified biopolymer for SAP application (Demonstration of charge modified starch (Cationic) crosslinked to form superabsorbent
Temperature Ranges (°C) 80-160 RPM Ranges (RPM) 50-200 Plasticizer Water, Glycerol, PEG Plasticizer (wt% relative to cat. Starch) 20-60% NaOH (wt % relative to cat. starch) 0-30% Sample # Zone 1 2 3 4 5 6 7 8 Die Temp Profile 7 (°C) Unheated 70 75 80 95 110 100 100 100 Injection Cat. Starch + NaOH Glycerol Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α 7Α Feed Rate (RPM) 50 5.9 Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α 7Β Feed Rate (RPM) 25 2.4 Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α Ν/Α Sample # Free Swell Capacity (g/g) Solubility (g/g) 7Α 31.6 4.5 7Β 28.8 2.0 Example 8 - Example of modified biopolymer for biosorbent application
Solution Sample Uptake (g/g) Instant Ocean (Sea Water) Modified Biopolymer 29 Na Poly 19 Canola Oil Modified Biopolymer 18 Na Poly 2.2 Motor Oil (Conventional) Modified Biopolymer 5.3 Na Poly 1.7 Motor Oil (Synthetic) Modified Biopolymer 9.2 Na Poly 2.3 Gasoline Modified Biopolymer 3.9 Na Poly 0.8 Diesel Modified Biopolymer 4.7 Na Poly 3.4 Blood Modified Biopolymer 16.6 Na Poly 1.48 Example 9 - Example of modified biopolymer showing comparative homogeneity (Homogeneity Analysis)