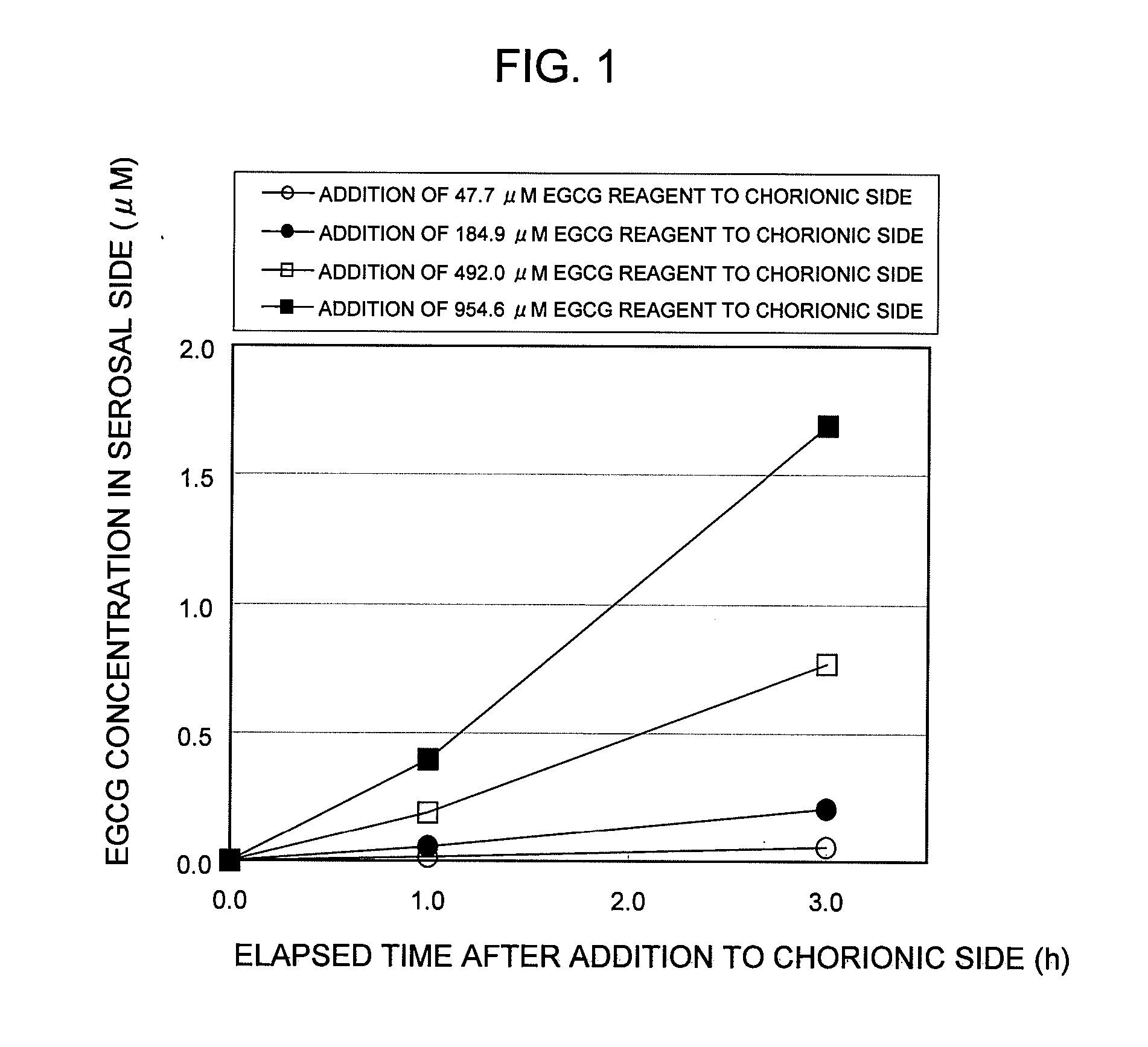
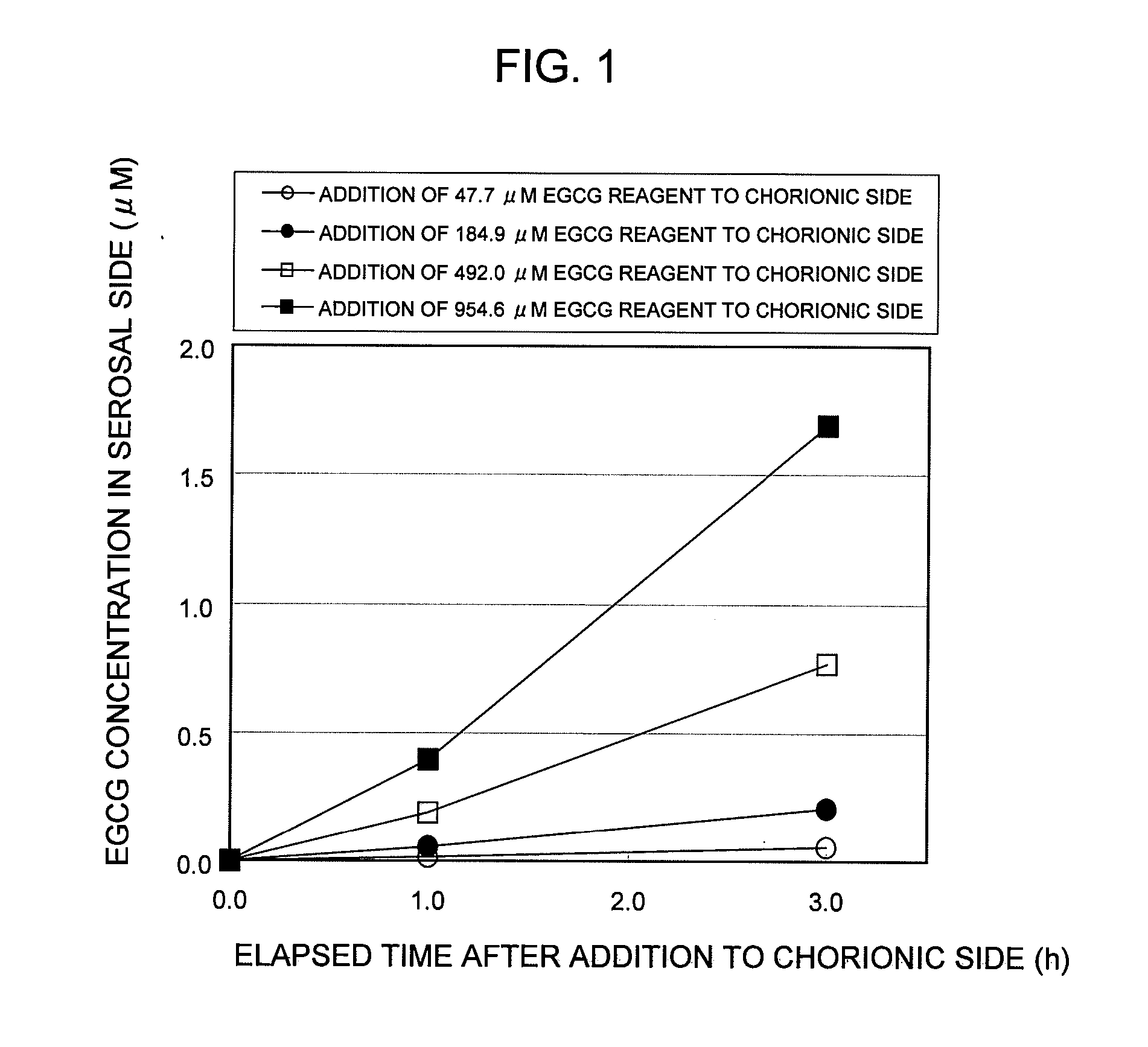
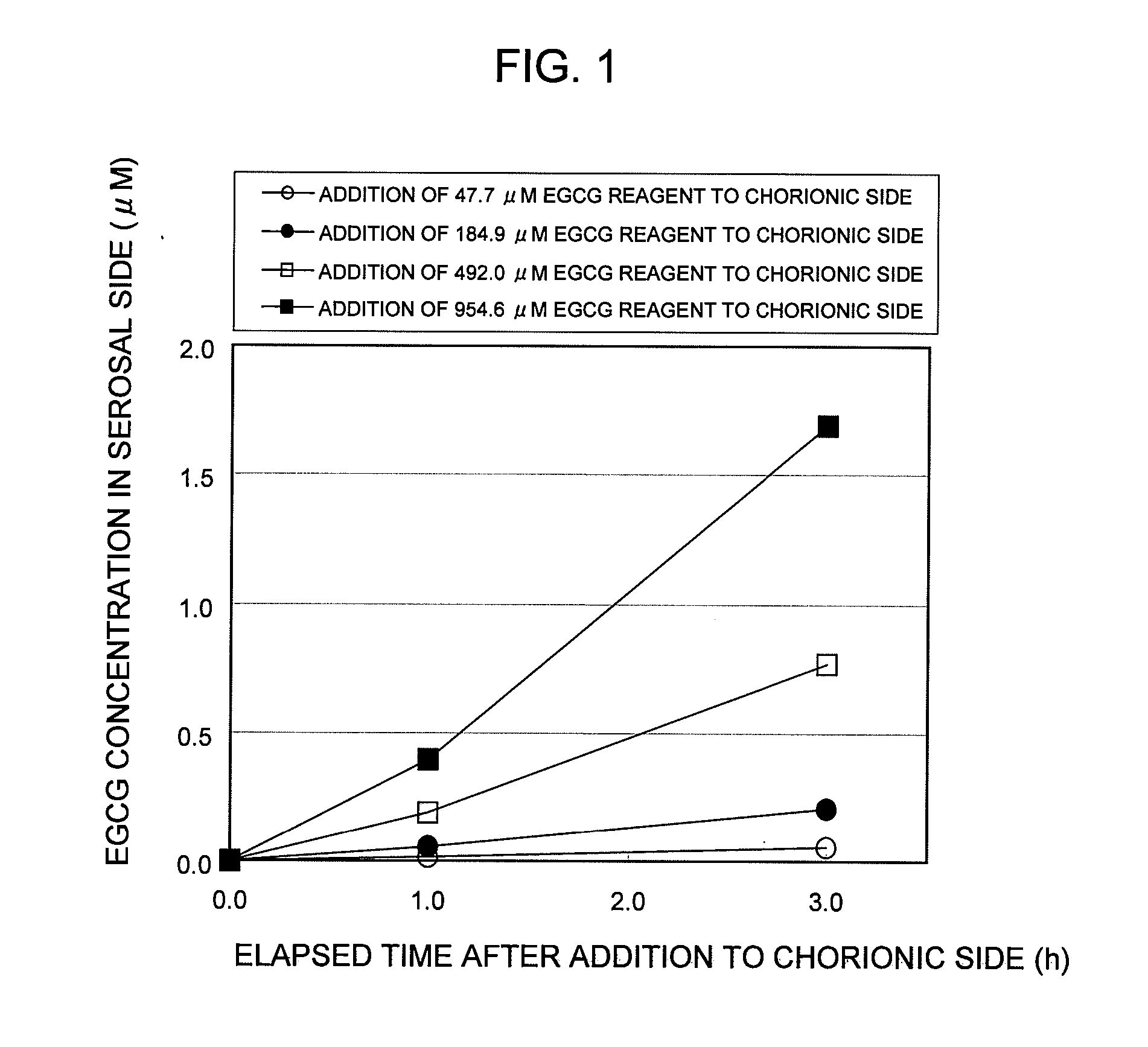
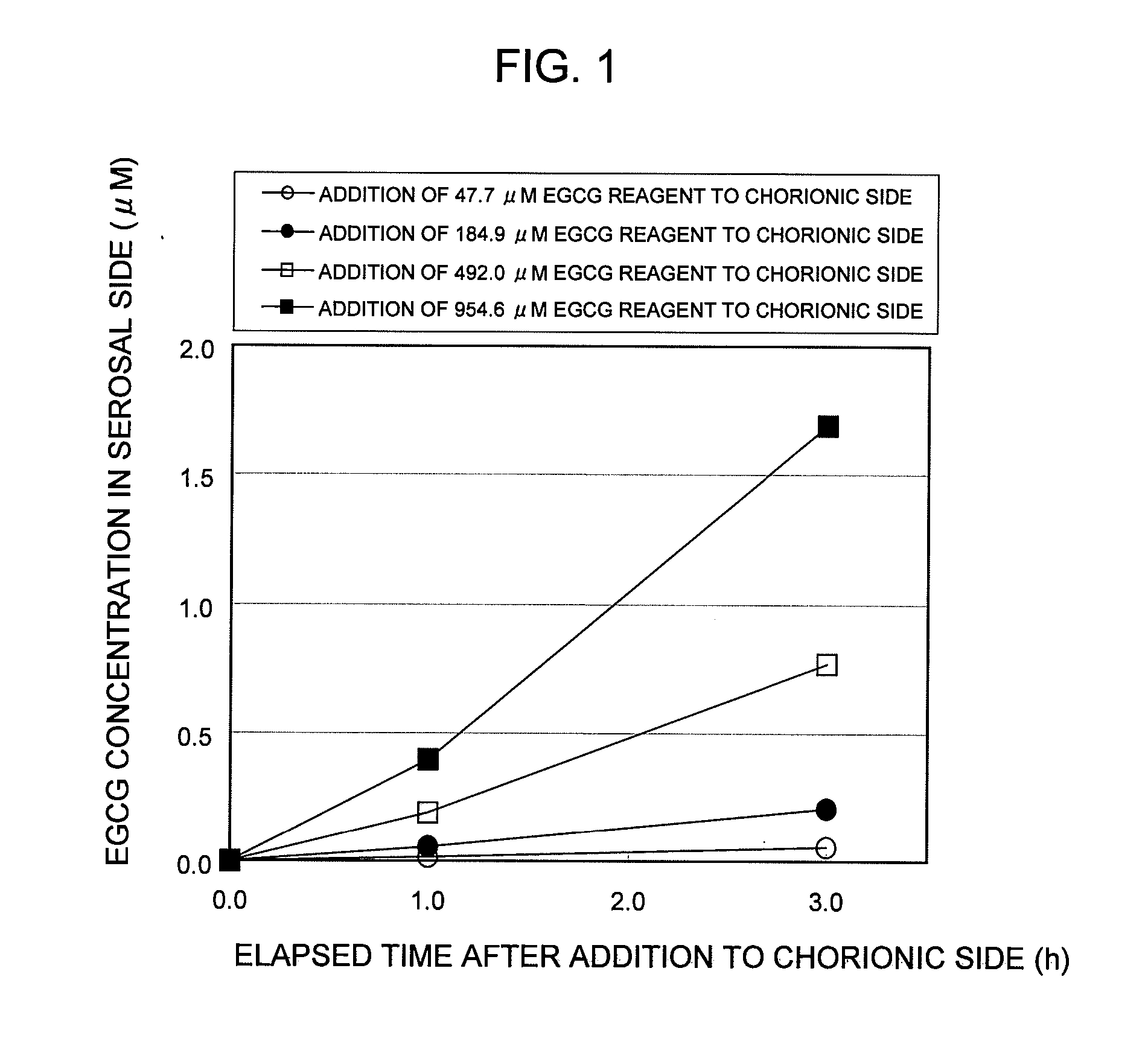
POLYPHENOL COMPOUND ABSORPTION PROMOTER AND UTILIZATION OF SAME
The present invention relates to an absorption promoter for a polyphenol compound and a use thereof. Green tea is a drink which has been familiar to Japanese through the ages, and has been drunk by very many people through widespread use of packaged green tea drinks in recent years. The green tea contains a group of catechin compounds (flavanol compounds) collectively referred to as tea catechin and is known to have a number of functions beneficial for health, such as an anti-tumor effect, an anti-platelet aggregation effect, an anti-hypertension effect, an anti-obesity effect, and an anti-oxidation effect. However, in general, even when the catechin compounds are ingested orally, only a small percentage of amounts of the compounds ingested are absorbed into the body because rates of absorption of the compounds are low. Therefore, many attempts to enhance absorbability of the catechin compounds have been made. For example, Patent Literature 1 below discloses a method of increasing an absorption amount and a blood concentration of a tea catechin by increasing a concentration of the tea catechin in a green tea drink to increase an ingestion amount of the tea catechin. However, the method has problems, for example, in that there is a limitation to the amount of the tea catechin which may be added to the green tea drink because a bitter taste or a harsh taste peculiar to the tea catechin strongly appears, and in that an economic burden increases because a large amount of the tea catechin is used as a raw material. Meanwhile, Patent Literature 2 below discloses a method of increasing a rate of absorption of a tea catechin by reducing an amount of an alcohol-precipitable substance in a drink. However, the method has a problem in that procedures such as purification and an enzymatic treatment need to be additionally performed to reduce the amount of the alcohol-precipitable substance to a certain content or less, resulting in complicated production steps and an increase in cost. Further, there is known a method of increasing a rate of absorption of a tea catechin by blending epi and non-epi forms of a tea catechin contained in a black tea drink so that contents of the forms satisfy a certain condition (see Patent Literature 3 and Patent Literature 4 below). However, requirements for the contents of the epi and non-epi forms are usually satisfied in green tea or the like which is drunk in households. Hence, the method is hardly applicable to foods and drinks other than the black tea drink among packaged drinks. Further, Patent Literature 5 below discloses a method of promoting absorption of epigallocatechin gallate by blending epigallocatechin gallate and caffeine in green tea so that contents of epigallocatechin gallate and caffeine satisfy a predetermined relationship. However, it is necessary to perform a treatment for removing caffeine by, for example, a chromatography treatment using a synthetic adsorbent column, which may impair original taste and flavor ingredients.
As described above, green tea contains a group of catechin compounds (flavanol compounds) collectively referred to as tea catechin and is known to have a number of functions beneficial for health, such as an anti-tumor effect (Katiyar S K and Mukhtar H (1996) Int J Oncol 7:133-141; Bu-Tian J I et al. (1997) Int J Cancer 70:255-258; Su L J and Arab L (2002) Public Health Nutr 5:419-425), an anti-platelet aggregation effect (Duffy S J et al. (2001) Arterioscler Thromb Vasc 21:1084-1089), an anti-hypertention effect (Negishi H et al. (2005) J Nutr 134:38-42), an anti-obesity effect (Nagao T et al. (2005) Am J Clin Nutr 81:122-9; Murase T et al. (2002) Int J Obes 26:1459-64), and an anti-oxidation effect (Sano M et al. (2003) J Agric Food Chem 51:2912-2916). Green tea contains eight major catechin compounds including catechin, epicatechin, gallocatechin, epigallocatechin, catechin gallate, epicatechin gallate, gallocatechin gallate, and epigallocatechin gallate. Of those, epigallocatechin gallate, which accounts for about 50% of catechin compounds in green tea, is considered to have a high physiological activity compared with the other catechin compounds. However, in general, a rate of absorption of the catechin compounds which are polyphenol compounds is low. In particular, a rate of absorption of epigallocatechin gallate is very low, and hence only a small percentage of epigallocatechin gallate ingested is absorbed into the body (Chen L et al. (1997) Drug Metab Dispos 25:1045-1050; Zhu M et al. (2000) Planta Med 66:444-447 for research results in rats) (Warden B A et al. (2001) J Nutr 131:1731-1737; Chow et al. (2001) Cancer Epidemiol Biomarkers Prev 10:53-58; Vaidyanathan and Walle (2001) Pharm Res (NY) 18:1420-1425 for research results in humans). Therefore, an object of the present invention is to find a method of improving absorbability of a polyphenol compound to be ingested, in particular, epigallocatechin gallate having a strong physiological action, which is applicable to a wide range of foods and drinks, and to provide a food and drink having improved absorbability of a polyphenol compound, in particular, epigallocatechin gallate (hereinafter, also referred to as “EGCG”) which is a catechin compound by the method, and a food and drink material for improving the absorbability. In order to achieve the object, the inventors of the present invention have searched foods or food-related ingredients which can enhance the efficiency of EGCG absorption. As a result, the inventors have found that some of the foods or food-related ingredients have effects of significantly promoting EGCG absorption, thus completing the present invention. That is, the present invention is as described below. [1] An absorption promoter for a polyphenol compound, including, as an active ingredient, at least one kind selected from the group consisting of the following items (1) to (7): (1) serine or a salt thereof; (2) aspartic acid or a salt thereof; (3) malic acid or a salt thereof; (4) capric acid, a salt thereof, or an ester thereof; (5) lauric acid, a salt thereof, or an ester thereof; (6) grapefruit juice; and (7) a [2] An absorption promoter according to the above-mentioned item, in which the polyphenol compound includes a flavonoid compound. [3] An absorption promoter according to the above-mentioned item, in which the polyphenol compound includes a catechin compound. [4] An absorption promoter according to the above-mentioned item, in which the polyphenol compound includes a catechin compound including at least one kind selected from the group consisting of catechin, epicatechin, gallocatechin, epigallocatechin, catechin gallate, epicatechin gallate, gallocatechin gallate, epigallocatechin gallate, and methylated compounds thereof. [5] A production method for a food and drink or a food and drink material containing a polyphenol compound, the method including the step of adding at least one kind selected from the group consisting of the following items (1) to (7) to a raw material for a food and drink or a raw material for a food and drink material containing a polyphenol compound: (1) serine or a salt thereof; (2) aspartic acid or a salt thereof; (3) malic acid or a salt thereof; (4) capric acid, a salt thereof, or an ester thereof; (5) lauric acid, a salt thereof, or an ester thereof; (6) grapefruit juice; and (7) a [6] A production method for a food and drink or a food and drink material containing a polyphenol compound, the method including the step of adding a polyphenol compound to a raw material for a food and drink or a raw material for a food and drink material containing at least one kind selected from the group consisting of the following items (1) to (7): (1) serine or a salt thereof; (2) aspartic acid or a salt thereof; (3) malic acid or a salt thereof; (4) capric acid, a salt thereof, or an ester thereof; (5) lauric acid, a salt thereof, or an ester thereof; (6) grapefruit juice; and (7) a [7] A production method according to the above-mentioned item or, in which the polyphenol compound includes a flavonoid compound. [8] A production method according to the above-mentioned item or, in which the polyphenol compound includes a catechin compound. [9] A production method according to the above-mentioned item or, in which the polyphenol compound includes a catechin compound including at least one kind selected from the group consisting of catechin, epicatechin, gallocatechin, epigallocatechin, catechin gallate, epicatechin gallate, gallocatechin gallate, epigallocatechin gallate, and methylated compounds thereof. [10] A production method according to any one of the above-mentioned items to, in which the raw material for a food and drink or the raw material for a food and drink material containing a polyphenol compound includes a tea extract. [11] A production method according to the above-mentioned item, in which the tea extract includes a green tea extract. [12] A food and drink, including a [13] A food and drink according to the above-mentioned item, in which the tea extract includes a green tea extract. [14] A food and drink according to the above-mentioned item or, in which the food and drink includes one kind selected from the group consisting of: confectionery such as chocolates, biscuits, gums, candies, cookies, gummi candies, and tablet confectionery; cereals; drinks such as powder drinks, soft drinks, milk drinks, nutritional drinks, carbonated drinks, and jelly drinks; and chilled sweets such as ice creams and sherbets. [15] A food and drink according to the above-mentioned item or, in which the food and drink is a health food or a dietary supplement having one kind of form selected from the group consisting of powders, granules, capsules, syrups, tablets, and sugar-coated tablets. According to the present invention, it is possible to significantly improve the rate of absorption of a polyphenol compound, e.g., a catechin compound by ingesting at least one kind selected from the group consisting of serine or a salt thereof, aspartic acid or a salt thereof, malic acid or a salt thereof, capric acid, a salt thereof, or an ester thereof, lauric acid, a salt thereof, or an ester thereof, grapefruit juice, and a The foods or food-related ingredients each independently have an EGCG absorption promoting effect, but when the foods or food-related ingredients are used in combination of two or more thereof, larger absorption promoting effects can be obtained. For example, the Hereinafter, the present invention is described in more detail by way of preferred embodiments. In an absorption promoter for a polyphenol compound of the present invention, serine serving as an active ingredient for the absorption promotion is one kind of non-essential amino acid and is important as a raw material for phosphatidylserine which is a component of cell membranes. In most cases, serine is present in a form of a protein in foods and is a nutrient contained in many foods. L-serine has been approved as an existing additive and is widely used as a seasoning or a nutrition reinforcement. Meanwhile, a salt of serine is exemplified by, but not limited to, serine hydrochloride, serine sulfate, and serine phosphate. In the absorption promoter for a polyphenol compound of the present invention, aspartic acid serving an active ingredient for the absorption promotion is one kind of non-essential amino acid and is classified into acidic amino acids. Aspartic acid is contained in various foods, and L-aspartic acid has been approved as an existing additive and is used as a seasoning or a nutrition reinforcement. Meanwhile, a salt of aspartic acid is exemplified by, but not limited to, sodium aspartate, potassium aspartate, magnesium aspartate, and calcium aspartate. Of those, a sodium salt of L-aspartic acid has been approved as a specified additive and is used as a seasoning or a nutrition reinforcement. In the absorption promoter for a polyphenol compound of the present invention, malic acid serving an active ingredient for the absorption promotion is one kind of organic compound classified into hydroxy acids and is an ingredient contained in apple or the like. Meanwhile, a salt of malic acid is exemplified by, but not limited to, sodium malate, potassium malate, magnesium malate, and calcium malate. Of those, DL-malic acid and sodium DL-malate have been approved as specified additives and are widely used as food additives serving as acidulants or pH adjusters. The acceptable daily intake (ADI) has not been set, and malic acid has been recognized as a very highly safe ingredient. In the absorption promoter for a polyphenol compound of the present invention, a grapefruit which is a raw material for grapefruit juice serving as an active ingredient for the absorption promotion is a citrus fruit which is eaten as it is and used for juices, cocktails, sours, and the like and has been eaten by many people. In the absorption promoter for a polyphenol compound of the present invention, capric acid and lauric acid serving as active ingredients for the absorption promotion are saturated fatty acids having 10 carbon atoms and having 12 carbon atoms, respectively, and are contained in coconut oil or palm oil at high concentrations. Meanwhile, a salt of capric acid or lauric acid is exemplified by, but not limited to, sodium, potassium, magnesium, and calcium salts thereof. In addition, an ester of capric acid or lauric acid is exemplified by, but not limited to, esters such as a methyl ester, an ethyl ester, a glycol ester, a glycerin ester, and a sucrose ester. Of those, ethyl caprylate (also known as ethyl decanoate) has been approved as a specified additive. In the absorption promoter for a polyphenol compound of the present invention, a In the present invention, when the above-mentioned active ingredients are ingested singly or in combination in oral ingestion of a polyphenol compound, a flavonoid compound, or a catechin compound, absorption of the polyphenol compound, flavonoid compound, or catechin compound into the body can be promoted. In this case, the polyphenol compound includes phenylcarboxylic acids, lignans, curcumins, coumarins, and flavonoids. Further, the flavonoid compound includes flavones, flavonols, isoflavones, flavans, flavanols (catechins), flavanones, flavanonols, chalcones, and anthocyanidins. In addition, the catechin compound includes non-epi-form catechins such as catechin, gallocatechin, catechin gallate, and gallocatechin gallate and derivatives such as methylated compounds thereof, and epi-form catechins such as epicatechin, epigallocatechin, epicatechin gallate, and epigallocatechin gallate and derivatives such as methylated compounds thereof. The present invention is preferably used for promotion of absorption of the catechin compound out of the above-mentioned compounds into the body, more preferably used for promotion of absorption of catechin, epicatechin, gallocatechin, epigallocatechin, catechin gallate, epicatechin gallate, gallocatechin gallate, epigallocatechin gallate, and derivatives thereof such as methylated compounds into the body, particularly preferably used for promotion of absorption of epigallocatechin gallate and a derivative such as a methylated compound thereof into the body. For example, a catechin compound contained in tea or the like is preferred. Preferred ingestion amounts per day of the above-mentioned active ingredients for exhibiting the effectiveness of the absorption promoter for a polyphenol compound of the present invention, approximately calculated based on the results of test examples to be described later, are as follows: in the case of serine, preferably 0.05 to 110 mg/l kg of body weight, more preferably 10 to 90 mg/l kg of body weight, still more preferably 10 to 25 mg/l kg of body weight; in the case of aspartic acid, preferably 0.05 to 130 mg/l kg of body weight, more preferably 10 to 110 mg/l kg of body weight, still more preferably 10 to 30 mg/l kg of body weight; in the case of malic acid, preferably 0.05 to 260 mg/l kg of body weight, more preferably 10 to 220 mg/l kg of body weight, still more preferably 10 to 60 mg/l kg of body weight; in the case of capric acid, preferably 0.004 to 25 mg/l kg of body weight, more preferably 1 to 20 mg/l kg of body weight, still more preferably 1 to 10 mg/l kg of body weight; in the case of lauric acid, preferably 0.01 to 25 mg/l kg of body weight, more preferably 2 to 20 mg/l kg of body weight, still more preferably 2 to 6 mg/l kg of body weight; in the case of the grapefruit juice, preferably 5 to 10,000 mg/l kg of body weight, more preferably 1,000 to 8,000 mg/l kg of body weight, still more preferably 1,000 to 2,000 mg/l kg of body weight in terms of a solid content; and in the case of the Further, mass ratios for effectively enhancing absorption of the polyphenol compound are as follows: in the case of serine, preferably 0.01 to 30 mg, more preferably 2 to 20 mg, still more preferably 2 to 5 mg per mg of the polyphenol compound; in the case of aspartic acid, preferably 0.01 to 40 mg, more preferably 2 to 30 mg, still more preferably 2 to 6 mg per mg of the polyphenol compound; in the case of malic acid, preferably 0.01 to 70 mg, more preferably 2 to 50 mg, still more preferably 2 to 15 mg per mg of the polyphenol compound; in the case of capric acid, preferably 0.001 to 6 mg, more preferably 0.2 to 4 mg, still more preferably 0.2 to 3 mg per mg of the polyphenol compound; in the case of lauric acid, preferably 0.002 to 7 mg, more preferably 0.4 to 5 mg, still more preferably 0.4 to 2 mg per mg of the polyphenol compound; in the case of the grapefruit juice, in terms of a solid content, preferably 1 to 2,600 mg, more preferably 200 to 1,800 mg, still more preferably 200 to 500 mg per mg of the polyphenol compound; and in the case of the In the absorption promoter for a polyphenol compound of the present invention, the contents of the active ingredients with respect to the total amount are as follows: in the case of serine, the content is preferably 1.0 to 100 mass %, more preferably 4.5 to 100 mass % in terms of a solid content; in the case of aspartic acid, the content is preferably 1.0 to 100 mass %, more preferably 4.5 to 100 mass % in terms of a solid content; in the case of malic acid, the content is preferably 1.5 to 100 mass %, more preferably 4.5 to 100 mass % in terms of a solid content; in the case of capric acid, the content is preferably 1.5 to 100 mass %, more preferably 4.5 to 100 mass % in terms of a solid content; in the case of lauric acid, the content is preferably 1.0 to 100 mass %, more preferably 4.5 to 100 mass % in terms of a solid content; in the case of the grapefruit juice, the content is preferably 1.5 to 100 mass %, more preferably 4.5 to 100 mass % in terms of a solid content; and in the case of the In the case where the The raw material for the tea extract is not particularly limited, but is preferably tea leaves of a green tea cultivar. The tea extract is preferably a green tea extract from green tea prepared from the tea leaves. Further, the raw material is preferably a black tea cultivar such as Darjeeling, Assam, or Ceylon, and the tea extract may be a black tea extract from black tea prepared from the tea leaves. The extract is particularly preferably a green tea extract from tea leaves of a green tea cultivar Yabukita. Preparation of the extract is not particularly limited, and can be performed by extraction of tea leaves with a solvent such as water or hot water. There is given, for example, a method involving adding 5 to 100 parts by mass of the solvent with respect to 1 part by mass of the tea leaves, and performing extraction at a temperature of room temperature to about 100° C. for about 1 to 120 minutes. It should be noted that, in the present invention, the “tea extract” is a concept including a state included in tea leaves. Instead of the extract obtained by extraction with the solvent, dry powder of the tea leaves may be used. The absorption promoter for a polyphenol compound of the present invention may contain carbohydrates, dietary fibers, proteins, vitamins, and the like in addition to the above-mentioned fundamental ingredients. The absorption promoter for a polyphenol compound of the present invention is used in various fields of medicines, health foods, processed foods, and the like, and can be used as an active ingredient of a drug or a food raw material, for example. For example, in the case where the absorption promoter is used as a medicine, the absorption promoter may be formulated together with a pharmaceutically acceptable base or carrier to provide a pharmaceutical composition. In the pharmaceutical composition, not only the base or carrier but also an additive such as a binder, a disintegrant, a buffer, a preservative, a moisturizer, an antimicrobial agent, an antiseptic, a fragrance, a pigment, a surfactant, a stabilizer, or a solubilizing agent may be optionally blended as long as it is pharmaceutically acceptable. A form of the pharmaceutical composition is exemplified by dosage forms of a pill, a powder, a tablet, a granule, a capsule, a syrup, a liquid, a jelly, and a pastille. Further, in the case where the absorption promoter for a polyphenol compound of the present invention is added to a food and drink before ingestion, the absorption promoter may be used by being blended in a general food, a food for specified health use, a dietary supplement, a functional food, and the like. Examples of the foods include: confectionery such as chocolates, biscuits, gums, candies, cookies, gummi candies, and tablet confectionery; cereals; drinks such as powder drinks, soft drinks, milk drinks, nutritional drinks, and carbonated drinks; and chilled sweets such as ice creams and sherbets. In addition, in the cases of the food for specified health use, dietary supplement, and the like, forms such as a powder, a granule, a capsule, a syrup, a tablet, and a sugar-coated tablet may be employed. Further, as shown in examples to be described later, the On the other hand, the present invention also provides a production method for a food and drink or a food and drink material containing a polyphenol compound, the method including the step of adding at least one kind selected from the group consisting of serine or a salt thereof, aspartic acid or a salt thereof, malic acid or a salt thereof, capric acid, a salt thereof, or an ester thereof, lauric acid, a salt thereof, or an ester thereof, grapefruit juice, and a The production method can be performed by adding, as a raw material or a blending ingredient, at least one kind selected from the group consisting of serine or a salt thereof, aspartic acid or a salt thereof, malic acid or a salt thereof, capric acid, a salt thereof, or an ester thereof, lauric acid, a salt thereof, or an ester thereof, grapefruit juice, and a In addition, the present invention also provides a production method for a food and drink or a food and drink material containing a polyphenol compound, the method including the step of adding a polyphenol compound to a raw material for a food and drink or a raw material for a food and drink material containing at least one kind selected from the group consisting of serine or a salt thereof, aspartic acid or a salt thereof, malic acid or a salt thereof, capric acid, a salt thereof, or an ester thereof, lauric acid, a salt thereof, or an ester thereof, grapefruit juice, and a The production method can be performed by adding, as a raw material or a blending ingredient, a polyphenol compound to a raw material for a food and drink or a raw material for a food and drink material containing at least one kind selected from the group consisting of serine or a salt thereof, aspartic acid or a salt thereof, malic acid or a salt thereof, capric acid, a salt thereof, or an ester thereof, lauric acid, a salt thereof, or an ester thereof, grapefruit juice, and a Further, as shown in examples to be described later, the Hereinafter, the present invention is described specifically by way of examples, but the scope of the present invention is not limited by the examples. An orally-ingested polyphenol compound, in particular, catechin compound is considered to be absorbed mainly in the intestinal tract, especially the small intestine in the body. Therefore, in order to easily screen a food or a food-related ingredient which promotes small intestinal absorption of the catechin compound, especially EGCG, from a wide range of targets, the inventors of the present invention have established an in vitro EGCG absorption measurement system with a small intestinal epithelial model using a Caco-2 cell line derived from human colon cancer (American Type Culture Collection). Specific methods and the results are shown below. Caco-2 cells are known to spontaneously differentiate while the cells are cultured for about 3 weeks and to exhibit many small intestinal epithelium-like features. The Caco-2 cells were cultured in the presence of humidified air containing 5% CO2in a Dulbecco's modified Eagle medium (DMEM, pH 7.4; manufactured by GIBCO) containing 10% inactivated fetal bovine serum (manufactured by HyClone), 50 U/mL penicillin-50 μg/mL streptomycin (manufactured by GIBCO), and a 1% non-essential amino acid (manufactured by GIBCO) in a plastic flask of 75 cm2. The cells were maintained by performing subculture every 5 to 8 days when the cells reached 80 to 90% confluence. An EGCG absorption experiment to be described below was performed using Caco-2 cells which had been subcultured 30 to 70 times. The EGCG absorption experiment was performed using a Caco-2 cell monolayer cultured on a Transwell with a diameter of 12 mm (polycarbonate filter culture area: 1.13 cm2, pore size: 0.4 μm; manufactured by Corning). The Caco-2 cells were inoculated onto an insert of the Transwell at a density of 1.0×105cells/cm2, and culture was continued while the medium was exchanged for a fresh one every 2 days. Transepithelial electrical resistance (hereinafter, referred to as “TEER”) was measured using a Millicell ERS meter (manufactured by Millipore) to monitor the degree of completion of a tight junction of the cell monolayer, and a monolayer which had been confirmed that formation of the tight junction had been completed was used in the experiment. Mean values of TEER reached 500 to 1,000 Ωcm24 weeks after inoculation and were maintained stably until 7 weeks after inoculation. The Caco-2 cell monolayer used in the absorption experiment was one obtained 4 to 6 weeks after inoculation. The medium of the upper part of the Transwell insert on which the Caco-2 cell monolayer was placed (chorionic side) was replaced by a Hanks' Balanced Salt Solution-like Transport Medium having a pH of 5.5 (chorionic side HBSS solution; containing 10 mM MES, 5 mM glucose, 10 mM glutamine, and 1 mM ascorbic acid), and the medium of the lower part of the insert (serosal side) was replaced by HBSS having a pH of 7.4 (serosal side HBSS solution; containing mM HEPES, 5 mM glucose, 10 mM glutamine, and 1 mM ascorbic acid). The cells were washed twice, and 0.5 mL and 1.5 mL of the respective fresh HBSS solutions were then added to the chorionic side and the serosal side, respectively, followed by incubation in the presence of 5% CO2at 37° C. for 30 minutes. After a lapse of 30 minutes, TEER was measured, and the solutions of the upper and lower parts were then replaced again by the fresh HBSS solutions. The cells were incubated for 30 minutes, and TEER was measured again. That time was defined as 0 hours after the beginning of absorption. EGCG is easily oxidized and unstable in a neutral to alkaline aqueous solution, but EGCG was maintained completely and stably during the absorption experiment period by adding 1 mM ascorbic acid to the HBSS solution of the chorionic side and maintaining the pH at 5.5. In addition, the EGCG absorption capacity of the Caco-2 cells was maintained constantly for 4 hours or more by adding 10 mM glutamine to the HBSS solutions of the chorionic side and serosal side in the measurement system. After measurement of TEER at 0 hours after the beginning of absorption, the solutions of the upper and lower parts of the insert were replaced by fresh HBSS solutions. In this procedure, EGCG (epigallocatechin gallate, Cat. No. E4143; manufactured by Sigma) was added to the HBSS solution of the chorionic side of the Caco-2 cell monolayer so that the EGCG final concentrations were 47.7 μM, 184.9 μM, 492.0 μM, and 954.6 μM, or a green tea extract (green tea hot water extract, No. 16714; manufactured by San-Ei Gen F.F.I., Inc.) was added thereto so that the EGCG final concentrations were 38.9 μM, 169.5 μM, 430.2 μM, and 826.7 μM. The cells were incubated in the presence of 5% CO2at 37° C. for 1 hour, and TEER was measured. Then, the insert was transferred to wells including a fresh HBSS solution of the serosal side without replacing the HBSS solution of the chorionic side on the insert, and the cells were incubated for an additional 2 hours. After a lapse of 2 hours, TEER was measured, and the EGCG concentrations contained in the HBSS solutions of the serosal side, obtained 0, 1, and 3 hours after the beginning of absorption, were measured by high-performance liquid chromatography (HPLC) as described below. Measurement of the EGCG concentrations contained in the HBSS solutions of the serosal side was performed by measuring peak areas of elution patterns of an absorbance at 280 nm using an HPLC apparatus (CBM-20A or SCL-10Avp; manufactured by Shimadzu Corporation) equipped with a reversed-phase column with a size of 150 mm×4.6 mm (particle size: 5 μm) (Mightysil RP-18 GP; manufactured by Kanto Chemical Co., Inc.). Measurement was performed at a column temperature of 40° C. Elution of EGCG from the column was performed by a linear gradient method using Solution A and Solution B (Solution A: water/acetonitrile/phosphoric acid=700/10/1; Solution B: solution A/methanol=2/1) at a flow rate of 0.5 mL/min. First, 400 μL of the HBSS solution of the serosal side were injected into the column, and 100% Solution A was passed through the column for 12 minutes from 0 minutes to 12 minutes, and the concentration of Solution B was increased from 0 to 80% for 43 minutes until 55 minutes. 80% Solution B was passed through the column until 60 minutes, and the concentration of Solution A was raised to 100% again for 5 minutes until 65 minutes, followed by completion of the analysis of 75 minutes per cycle. The EGCG concentrations in the samples were determined using a standard line prepared from peak areas of an EGCG standard substance having known concentrations. In the case of the standard substance, the retention time of EGCG was 53 minutes, and the detection sensitivity was 10 nM. The results show that EGCG absorption from the EGCG reagent and green tea can be measured easily by using the Caco-2 cell monolayer as a small intestinal absorption model. In other words, an in vitro experiment system capable of maintaining EGCG stably and measuring EGCG absorption easily under conditions similar to those in living bodies was established. The rates of EGCG absorption in the cases where various foods or food-related ingredients shown in Table 1 below were added to the chorionic side in the EGCG absorption measurement system of Test Example 1 above were calculated as apparent permeability coefficients (Papp), and compared with the result in the case of no additive. Specifically, each of the ingredients shown in Table 1 above was added at different concentrations (2 or 3 concentrations) together with EGCG to the chorionic side of the Caco-2 cells at the time of 0 hours after the beginning of absorption, and the amount (mol) of EGCG which had permeated to the serosal side after a lapse of a certain period of time was measured by HPLC. EGCG permeation amounts, Q (mol), were plotted against time, t (sec), to calculate dQ/dt from the slope, and Papp (cm/sec) was determined by the following equation (1) from a cell surface area, A (cm2), and a concentration of the ingredient added to the chorionic side, C0(M). [Math. 1] The Papp value in the case where no food or food-related ingredient was added was defined as 1, and relative values of Papp in the cases where the respective foods or food-related ingredients were added were calculated. Of those, cases where significantly high Papp values were observed are shown in Table 2. As shown in Table 2, six ingredients including serine, aspartic acid, malic acid, capric acid, lauric acid, and grapefruit juice each independently promoted EGCG absorption. Of the six kinds of foods or food-related ingredients, which had been found to have EGCG absorption promoting effects, the five kinds ingredients including serine, aspartic acid, malic acid, grapefruit juice, lauric acid, except for capric acid belonging to the same medium chain fatty acid as lauric acid, were used to examine synergistic or additive EGCG absorption promoting effects in the combinations of two kinds thereof. Specifically, in the same manner as in Test Example 2, two kinds of foods or food-related ingredients were added in combination simultaneously with 1 mM EGCG to the chorionic side of the Caco-2 cells, and rates of EGCG absorption to the serosal side were measured and compared with the result of the case of no additive. Table 3 shows the results. As shown in Table 3, additive EGCG absorption promoting effects were obtained in the combinations of serine+aspartic acid, serine+malic acid, serine+grapefruit juice, aspartic acid+malic acid, aspartic acid+grapefruit juice, aspartic acid+lauric acid, and malic acid+grapefruit juice. Further, the EGCG absorption promoting effect in the combination of grapefruit juice+lauric acid was synergistic. Further, in this case, TEER serving as an indicator of the degree of opening and closing of an intercellular tight junction was inversely correlated with the rate of EGCG absorption calculated as the apparent permeability coefficient (Papp) of EGCG. Therefore, the intracellular pathway of the Caco-2 cells was opened by the functions of serine, aspartic acid, malic acid, grapefruit juice, lauric acid, capric acid, and the like, and EGCG was probably transferred to the serosal side through the pathway. Further, in the case where EGCG is absorbed via cells, EGCG may be conjugated with sulfuric acid or glucuronic acid. Therefore, the deconjugation treatment of EGCG which had permeated to the serosal side was attempted, but there was no EGCG absorbed in the form of a conjugate. This suggests that most of EGCG is absorbed in the form of a free body in the Caco-2 cells, and that EGCG may be absorbed via the intracellular pathway. This experiment was performed for examining whether the foods or food-related ingredients which exhibited EGCG absorption promoting effects in the measurement system using the Caco-2 cells had the same EGCG absorption promoting effects even when orally ingested by an animal. Specifically, four kinds of administration solutions were prepared by adding serine and malic acid to a green tea hot water extract (manufactured by San-Ei Gen F.F.I., Inc., hereinafter, referred to as “green tea extract”) so that the final concentrations of serine and malic acid in the green tea extraction solution were (i) no additive, (ii) 20 mM serine+40 mM malic acid, (iii) 40 mM serine+80 mM malic acid, and (iv) mM serine+160 mM malic acid, and the respective administration solutions were orally administered forcibly to rats, and EGCG concentrations in plasma obtained by blood collection performed with time after administration were measured and converted into concentration×time values (areas under curve) to compare amounts of EGCG remaining in blood. The final EGCG concentration derived from the green tea extract contained in each administration solution was adjusted to 1 mM. Rats used in this experiment (Sprague Dawley, male, 8-week-old; purchased from Japan SLC, Inc.) were subjected to indwelling surgery in advance by inserting a cannula from the jugular vein toward the heart so that the opening of the top of the cannula was located in the vicinity of the right atrial inlet, and a 1-week recuperation period was then taken until the start of the experiment. During the recuperation period, the rats were fed individually in cages equipped with floor nets and allowed to ingest CE-2 feed (manufactured by CLEA Japan, Inc.) freely. To the rats which had fasted for 16 hours from the early-evening on the previous day of the experiment, each of the administration solutions (i) to (iv) was orally administered forcibly at a single dose of 1 mL per 100 g body weight of the rats. Before administration and 0.25, 0.5, 1, 2, 3 hours after administration, heparin blood collection was performed in an amount of 0.5 mL through the cannula from each rat, and immediately after blood collection, plasma was separated and stored at −80° C. under an acidic condition until analysis. The EGCG concentration in plasma was measured as follows: Each plasma before and after deconjugation treatments with β-glucuronidase and sulfatase was extracted with ethyl acetate under an acidic condition and evaporated to dryness; and the residue was dissolved in a solution for a mobile phase of HPLC and subjected to HPLC analysis (electrochemical detection method). Separation of EGCG by HPLC was performed using an HPLC apparatus (SCL-10Avp; manufactured by Shimadzu Corporation) equipped with a reversed-phase column with a size of 2.0 mm×150 mm (particle size: 5 μm) (CAPCELL PAK C18 MGII; manufactured by Shiseido Co., Ltd.) at a column temperature of 40° C. Isocratic elution was performed at a sample injection volume to the column of 20 μL using 0.1% phosphoric acid containing 0.1 mM EDTA·2Na:acetonitrile=90:10 as the mobile phase at a flow rate of 0.4 mL/min. Detection of EGCG was performed using an electrochemical detector (NANOSPACE SI-2; manufactured by Shiseido Co., Ltd.), and a peak area was calculated from an elution pattern of a current value detected at an applied voltage of 600 mV and quantified using a standard line prepared from peak areas of known concentrations of an EGCG standard substance. The retention time of the EGCG standard substance was 8.0 minutes, and the detection sensitivity was 2 nM. The EGCG concentrations in actual administration solutions were also analyzed again, and it was confirmed that EGCG was administered to the rats in scheduled amounts. Rat plasma collected after administration of each of the administration solutions (i), (ii), (iii), and (iv) was treated and subjected to HPLC analysis as mentioned above. The results show that there were no changes in EGCG concentrations in all the solutions (i) to (iv) before and after the deconjugation treatments. In other words, it was considered that most of EGCG in blood was absorbed in blood as an unconjugated free body. First, for the time-course changes in EGCG concentrations in plasma until 3 hours after administration of the administration solutions to the rats, a multiple comparison test (Scheffe's method; level of significance: 5%) was performed by two-way factorial analysis of variance with replication based on factors, i.e., the kinds of the administration solutions and elapsed time after administration. Table 4 shows the results. As shown in Table 4, the results of the Scheffe's multiple comparison test suggest that the EGCG concentrations in plasma in the cases where (ii) 20 mM serine+40 mM malic acid, (iii) 40 mM serine+80 mM malic acid, and (iv) 80 mM serine+160 mM malic acid were added to the green tea extract, and the mixture was administered to the rats were significantly high (P<0.05) compared with (i) the case where the green tea extract containing no additive was administered, and that EGCG absorption from the green tea extract was promoted by adding, to the green tea extract, the combination of serine+malic acid at any of concentrations according to the above-mentioned items (ii), (iii), and (iv). Subsequently, in the same manner as above, a multiple comparison test (Scheffe's method; level of significance: 5%) was performed by two-way factorial analysis of variance with replication based on factors, i.e., the EGCG concentration in plasma until 3 hours after administration of the administration solutions to the rats×time (area under curve) and elapsed time after administration. Table 5 shows the results. As shown in Table 5, the results of the Scheffe's multiple comparison test suggest that the EGCG concentrations in plasma×time (areas under curve) in the cases where (ii) 20 mM serine+40 mM malic acid, (iii) 40 mM serine+80 mM malic acid, and (iv) 80 mM serine+160 mM malic acid were added to the green tea extract, and the mixture was administered to the rats were significantly high (P<0.05) compared with (i) the case where the green tea extract containing no additive was administered, and that the amount of EGCG absorbed from the green tea extract remaining in blood was increased by adding, to the green tea extract, the combination of serine+malic acid at any of concentrations according to the above-mentioned items (ii), (iii), and (iv). In comparison of the areas under curve until 3 hours after administration (AUC0→3h), the degrees of promotion of EGCG absorption from the green tea extract through addition of serine+malic acid in combination in the cases of (ii) addition of 20 mM serine+40 mM malic acid, (iii) addition of 40 mM serine+80 mM malic acid, and (iv) addition of 80 mM serine+160 mM malic acid were 9.9 times, 11.0 times, and 12.7 times as large as that in the case of (i) no additive, respectively. The results of the above-mentioned two kinds of multiple comparison tests show that EGCG absorption is promoted by the administration solutions (ii), (iii), and (iv) containing serine and malic acid compared with the administration solution (i) containing no additive, but there are no significant differences in the results of the above-mentioned items (ii), (iii), and (iv) although the administration solutions contain serine and malic acid at different concentrations (P≧0.05). That is, the EGCG absorption promoting effect is considered to almost reach a plateau at the concentration of (ii) 20 mM serine+40 mM malic acid. Next, in order to demonstrate that the EGCG absorption promoting effect provided by addition of serine+malic acid is effective for green tea generally, the EGCG absorption promoting effect of another kind of green tea extract was tested. Specifically, a In the same manner as in Test Example 4, two kinds of administration solutions were prepared by adding serine and malic acid to the First, for the time-course changes in EGCG concentrations in plasma until 3 hours after administration of the administration solutions to the rats, two-way factorial analysis of variance with replication based on factors, i.e., the kinds of the administration solutions and elapsed time after administration was performed. Table 6 shows the results. As shown in Table 6, there was a significant difference (P<0.05) between the kinds of the administration solutions. It was found that the EGCG concentration in plasma in the case of administration of the Subsequently, in the same manner as above, two-way factorial analysis of variance with replication based on factors, i.e., the EGCG concentrations in plasma until 3 hours after administration of the administration solutions to the rats×time (areas under curve) and elapsed time after administration was performed. Table 7 shows the results. As shown in Table 7, there was a significant difference (P<0.05) between the kinds of the administration solutions. It was found that the EGCG concentration in plasma×time (area under curve) in the case of administration of the In comparison of the (areas under curve) until 3 hours after administration (AUC0→3h), the degree of promotion of EGCG absorption from the As for an EGCG reagent replaced in green tea extracts, the EGCG absorption promoting effect provided by addition of 20 mM serine+40 mM malic acid, which is the combination of serine and malic acid at the lowest concentrations among the three kinds of combinations of serine+malic acid used in Test Example 4, was examined in the same manner as in Test Example 4. In the same manner as in Test Example 4, two kinds of administration solutions were prepared by adding serine and malic acid to the administration solution containing an EGCG reagent at 1 mM so that the final concentrations were (i) no additive and (ii) 20 mM serine+40 mM malic acid, and the respective administration solutions were orally administered forcibly to rats with a cannula in the vein, and EGCG concentrations in plasma obtained by blood collection with time after administration were measured and converted into concentration×time values (areas under curve) to compare amounts of EGCG remaining in blood. First, for the time-course changes in EGCG concentrations in plasma until 3 hours after administration of the administration solutions to the rats, two-way factorial analysis of variance with replication based on factors, i.e., the kinds of the administration solutions and elapsed time after administration was performed. Table 8 shows the results. As shown in Table 8, there was a significant difference (P<0.05) between the kinds of the administration solutions. It was found that the EGCG concentration in plasma in the case of administration of the EGCG reagent solution having added thereto (ii) 20 mM serine+40 mM malic acid to the rats was significantly higher than that in the case of administration of the EGCG reagent solution containing (i) no additive, and that EGCG absorption was promoted by adding serine+malic acid in combination to the EGCG reagent solution in the same manner as in the case of addition to the green tea extract shown in each of Test Example 4 and Test Example 5. Subsequently, in the same manner as above, two-way factorial analysis of variance with replication based on factors, i.e., the EGCG concentrations in plasma until 3 hours after administration of the administration solutions to the rats×time (areas under curve) and elapsed time after administration was performed. Table 9 shows the results. As shown in Table 9, there was a significant difference (P<0.05) between the kinds of the administration solutions. It was found that the EGCG concentration in plasma×time (area under curve) in the case of administration of the EGCG reagent solution having added thereto (ii) 20 mM serine+40 mM malic acid to the rats was significantly higher than that in the case of administration of the EGCG reagent solution containing (i) no additive, and that the amount of EGCG adsorbed remaining in blood was increased by adding serine+malic acid in combination to the EGCG reagent solution in the same manner as in the case of addition to the green tea extract shown in each of Test Example 4 and Test Example 5. In comparison of the (areas under curve) until 3 hours after administration (AUC0→3h), the degree of promotion of EGCG absorption from the EGCG reagent solution through (ii) addition of 20 mM serine+40 mM malic acid in combination was 3.1 times as large as that in the case of (i) no additive. While Test Example 4 to 6 above show the combinations of serine+malic acid as examples of the foods or food-related ingredients to be added to promote EGCG absorption from the two different kinds of green tea extracts and EGCG reagent, in consideration with the results of the in vitro tests shown in Test Examples 2 and 3 above, it is suggested that the EGCG absorption promoting effect can be achieved by adding capric acid, lauric acid, grapefruit juice, serine, aspartic acid, and malic acid singly or in combination. For More specific experimental procedures are as described below. Rats used in this experiment (Sprague Dawley, male, 8-week-old; purchased from Japan SLC, Inc.) were subjected to indwelling surgery in advance by inserting a cannula from the jugular vein toward the heart so that the opening of the top of the cannula was located in the vicinity of the right atrial inlet, and a 1-week recuperation period was taken until the start of the experiment. During the recuperation period, the rats were fed individually in cages equipped with floor nets and allowed to ingest CE-2 feed (manufactured by CLEA Japan, Inc.) freely. The numbers of samples of the administration solutions are as follows: To the rats which had fasted for 16 hours from the early-evening on the previous day of the experiment, each of the administration solutions to was orally administered forcibly at a single dose of 1 mL per 100 g body weight of the rats. Before administration and 0.25, 0.5, 1, 2, hours after administration, heparin blood collection was performed in an amount of 0.5 mL through the cannula from each rat, and immediately after blood collection, plasma was separated and stored at −80° C. under an acidic condition until analysis. The EGCG concentration in plasma was measured in the same manner as in Test Example 4. That is, the concentration was measured as follows: each plasma was extracted with ethyl acetate under an acidic condition and evaporated to dryness; and the residue was then dissolved in a solution for a mobile phase of HPLC and subjected to HPLC analysis (electrochemical detection method). Separation of EGCG by HPLC was performed using an HPLC apparatus (SCL-10Avp; manufactured by Shimadzu Corporation) equipped with a reversed-phase column with a size of 2.0 mm×150 mm (particle size: 5 μm) (CAPCELL PAK C18MGII; manufactured by Shiseido Co., Ltd.) at a column temperature of 40° C. Isocratic elution was performed at a sample injection volume to the column of 20 μL using 0.1% phosphoric acid containing 0.1 mM EDTA·2Na:acetonitrile=90:10 as the mobile phase at a flow rate of 0.4 mL/min. Detection of EGCG was performed using an electrochemical detector (NANOSPACESI-2; manufactured by Shiseido Co., Ltd.), and a peak area was calculated from an elution pattern of a current value detected at an applied voltage of 600 mV and quantified using a standard line prepared from peak areas of an EGCG standard substance having known concentrations. The retention time of the EGCG standard substance was 8.0 minutes, and the detection sensitivity was 2 nM. The EGCG concentrations in actual administration solutions were also analyzed again, and it was confirmed that EGCG was administered to the rats in scheduled amounts. In this case, the EGCG concentrations in plasma were measured before and after the deconjugation treatment with β-glucuronidase and sulfatase, and the results show that the EGCG concentrations were not changed before and after the deconjugation treatment. Therefore, it was considered that most of EGCG in blood was present as an unconjugated free body. Accordingly, all the results to be shown below are measurement results in the case where no deconjugation treatment was performed. A multiple comparison test (Fisher's PLSD method; level of significance: 5%) was performed by two-way factorial analysis of variance with replication based on factors, i.e., the EGCG concentrations in plasma until 3 hours after administration of the administration solutions to the rats×time (areas under curve) and elapsed time after administration. Table 10 shows the results. As shown in Table 10, the results of the multiple comparison test show that there are significant differences in the areas under curve of the EGCG concentrations in plasma between the The results reveal that the Test Example 7 shows that, in general, the More specific experimental procedures are as described below. Three kinds of administration solutions, i.e., a 1 mM solution of an EGCG reagent (manufactured by Sigma; Cat. No. E4143), a mixed solution of the EGCG reagent and a green tea extract (mixed solution of [1 mM EGCG reagent solution]:[Green tea extract solution prepared so as to have an EGCG concentration of 1 mM]=1:1 (v/v)), a mixed solution of a In the same manner as in Test Example 7, the administration solutions were orally administered forcibly to rats mounted with a cannula in the vein, and EGCG concentrations in plasma obtained by blood collection performed with time after administration were measured and converted into areas under curve to compare amounts of EGCG remaining in blood. It should be noted that the numbers of samples of the administration solutions are as follows: EGCG reagent solution-administered group: n=8; EGCG reagent/green tea extract mixed solution-administered group:n=7; and A multiple comparison test (Fisher's PLSD method; level of significance: 5%) was performed by two-way factorial analysis of variance with replication based on factors, i.e., the EGCG concentrations in plasma until 3 hours after administration of the administration solutions to the rats×time (areas under curve) and elapsed time after administration. Table 11 shows the results. As shown in Table 11, the results of the multiple comparison test show that the area under curve of the EGCG concentration in plasma in the case of administration of the EGCG reagent/green tea extract mixed solution to the rats was significantly lowered compared with that in the case of administration of only the EGCG reagent, and that the amount of EGCG remaining in blood decreases by adding the green tea extract to the EGCG reagent. That is, a certain kind of ingredient contained in the green tea extract was estimated to suppress EGCG absorption. On the other hand, when a solution obtained by mixing the In comparison of the areas under curve until 3 hours after administration, the degree of the increase in the amount of EGCG remaining in blood obtained by adding the The results reveal that the effect of negating the absorption inhibitory effect due to the green tea extract itself is an effect unique to the The effect of malic acid on absorption of a catechin compound in humans was examined. Five healthy men and women which were 20 years old or more and less than 65 years old were selected as test subjects, and a test was performed on the effect of increasing the amount of the catechin compound remaining in blood by ingesting sodium malate simultaneously with the As a test substance, a drink was prepared by suspending a The test was performed by a crossover test as described below. Specifically, as the first phase, blood was collected early in the morning from each test subject who had fasted from 21:00 on the previous day of the test, and the subject ingested the test substance orally. Blood collection was performed 30 minutes, 1, 2, 3, and 4 hours after ingestion, and plasma was separated immediately. The resultant plasma was subjected to a deconjugating enzyme treatment with sulfatase and β-glucronidase, and catechin compounds in plasma were extracted with ethyl acetate. In the same manner as in Test Example 4, concentrations of the catechin compounds in plasma were measured by the HPLC-ECD method (electrochemical detection method). After that, in order to completely eliminate the effect of the catechin compounds and malic acid, each subject lived a normal life for 2 days or more. As the second phase, concentrations of the catechin compounds in plasma were measured under the same conditions as those in the first phase except that each subject ingested the control substance. It should be noted that the concentrations of the catechin compounds were determined as a total concentration of five kinds of catechin compounds including EGCG, 3″-O-methylepigallocatechin gallate, epigallocatechin, epicatechin gallate, and epicatechin. A test was performed on the effect of increasing the amount of catechin compounds remaining in blood by allowing healthy adult male test subjects to separately ingest a As a test substance, a drink was prepared by suspending a The test was performed as described below. Specifically, as the first phase, blood was collected early in the morning from each test subject who had fasted from 21:00 on the previous day of the test, and the subject ingested orally the test substance. Blood collection was performed 1, 2, and 4 hours after ingestion, and plasma was separated immediately. The resultant plasma was subjected to a deconjugating enzyme treatment with sulfatase and 13-glucronidase, and catechin compounds in plasma were extracted with ethyl acetate. In the same manner as in Test Example 4, concentrations of the catechin compounds in plasma were measured by the HPLC-ECD method (electrochemical detection method). After that, in order to completely eliminate the effect of the catechin compounds ingested, each subject took a break from the test for 7 days or more. As the second phase, concentrations of the catechin compounds in plasma were measured under the same conditions as those in the first phase except that each subject ingested the control substance. It should be noted that the concentrations of the catechin compounds were determined as a total concentration of five kinds of catechin compounds including EGCG, 3″-O-methylepigallocatechin gallate, epigallocatechin, epicatechin gallate, and epicatechin. As shown in Provided are a polyphenol compound absorption promoter which is widely applicable to foods and drinks over a wide range, and a method for producing a food, drink or food material containing a polyphenol compound. The polyphenol compound absorption promoter comprises, as the active ingredient, at least one member selected from the group consisting of serine, aspartic acid, malic acid, capric acid, lauric acid, grapefruit juice and Camellia sinensis (L.) O. Kuntze extract. The method for producing a food, drink or food material containing a polyphenol compound comprises: adding at least one member selected from the group consisting of serine, aspartic acid, malic acid, capric acid, lauric acid, grapefruit juice and Camellia sinensis (L.) O. Kuntze extract to a starting material for a food or drink or a starting material for a food material containing a polyphenol compound; or adding a polyphenol compound to a starting material for a food or drink or a starting material for a food material containing at least one member selected from the group consisting of serine, aspartic acid, malic acid, capric acid, lauric acid, grapefruit juice and Camellia sinensis (L.) O. Kuntze extract. 1. An absorption promoter for a polyphenol compound, comprising, as an active ingredient, at least one kind selected from the group consisting of the following items (1) to (7):
(1) serine or a salt thereof; (2) aspartic acid or a salt thereof; (3) malic acid or a salt thereof; (4) capric acid, a salt thereof, or an ester thereof; (5) lauric acid, a salt thereof, or an ester thereof; (6) grapefruit juice; and (7) a 2. An absorption promoter according to 3. An absorption promoter according to 4. An absorption promoter according to 5. A production method for a food and drink or a food and drink material containing a polyphenol compound, the method comprising the step of adding at least one kind selected from the group consisting of the following items (1) to (7) to a raw material for a food and drink or a raw material for a food and drink material containing a polyphenol compound:
(1) serine or a salt thereof; (2) aspartic acid or a salt thereof; (3) malic acid or a salt thereof; (4) capric acid, a salt thereof, or an ester thereof; (5) lauric acid, a salt thereof, or an ester thereof; (6) grapefruit juice; and (7) a 6. A production method for a food and drink or a food and drink material containing a polyphenol compound, the method comprising the step of adding a polyphenol compound to a raw material for a food and drink or a raw material for a food and drink material containing at least one kind selected from the group consisting of the following items (1) to (7):
(1) serine or a salt thereof; (2) aspartic acid or a salt thereof; (3) malic acid or a salt thereof; (4) capric acid, a salt thereof, or an ester thereof; (5) lauric acid, a salt thereof, or an ester thereof; (6) grapefruit juice; and (7) a 7. A production method according to 8. A production method according to 9. A production method according to 10. A production method according to 11. A production method according to 12. A food and drink, comprising a 13. A food and drink according to 14. A food and drink according to 15. A food and drink according to TECHNICAL FIELD
BACKGROUND ART
SUMMARY OF INVENTION
Technical Problem
Solution to Problem
Effects of Invention
BRIEF DESCRIPTION OF DRAWINGS
DESCRIPTION OF EMBODIMENTS
EXAMPLES
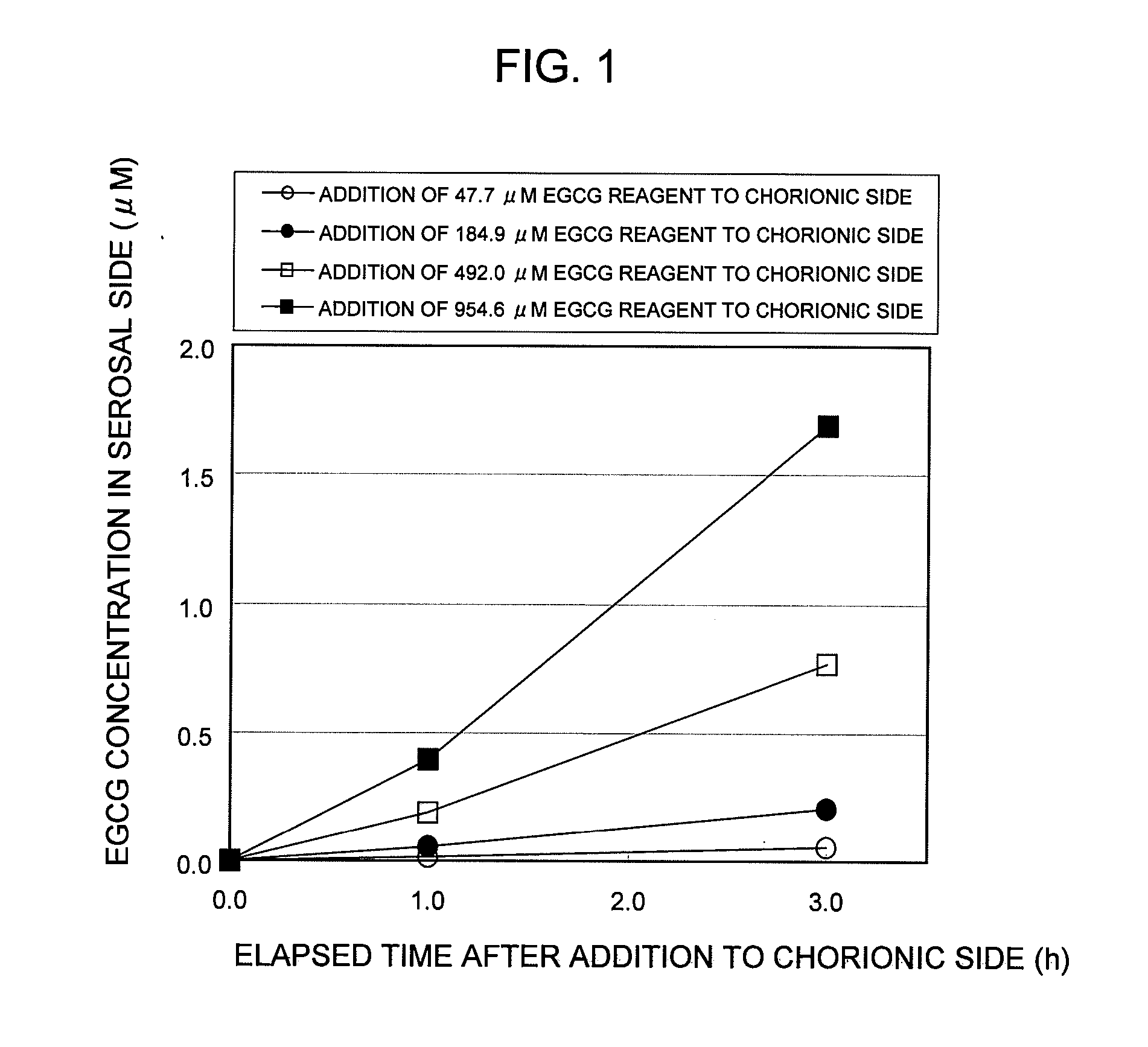
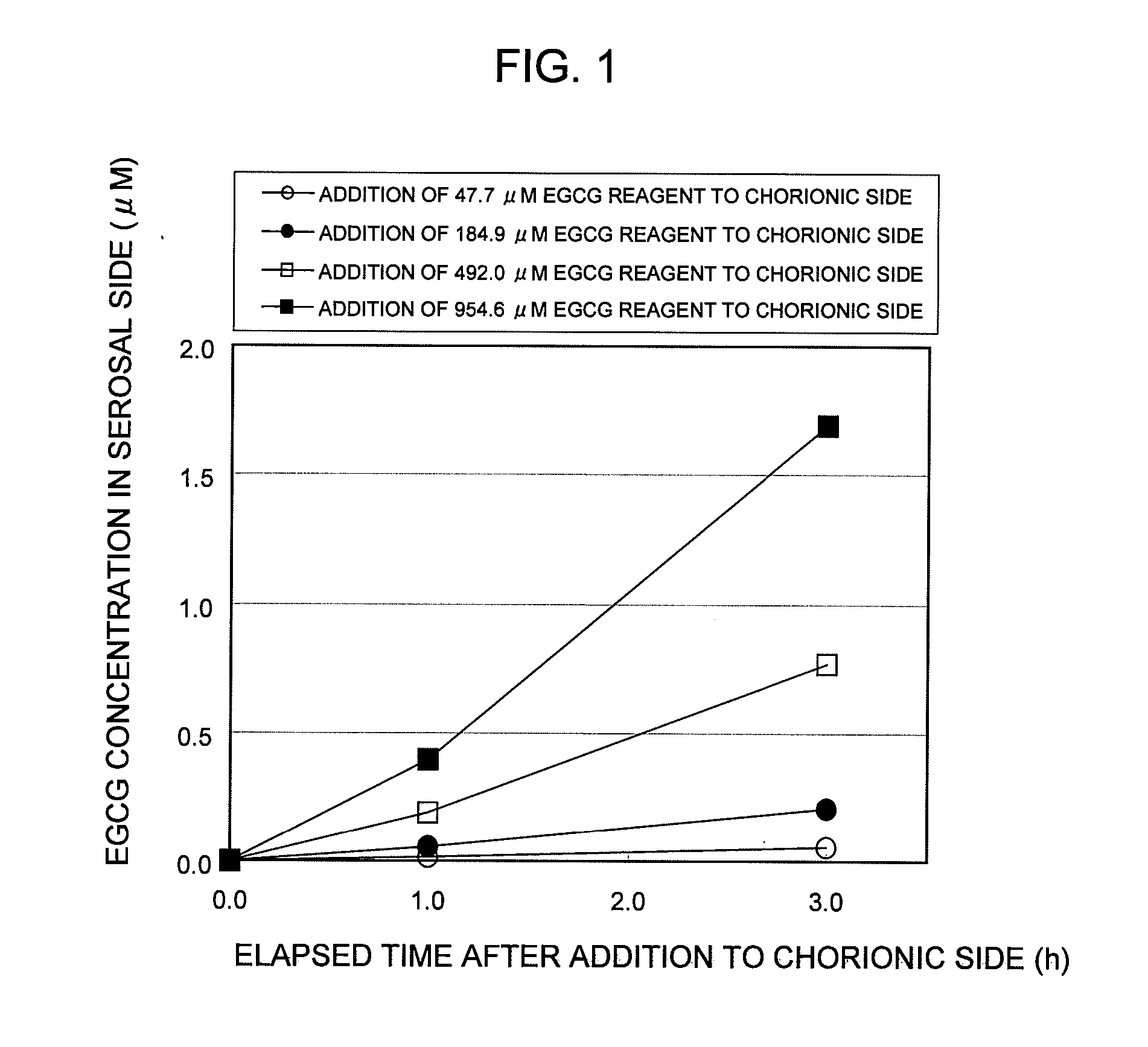
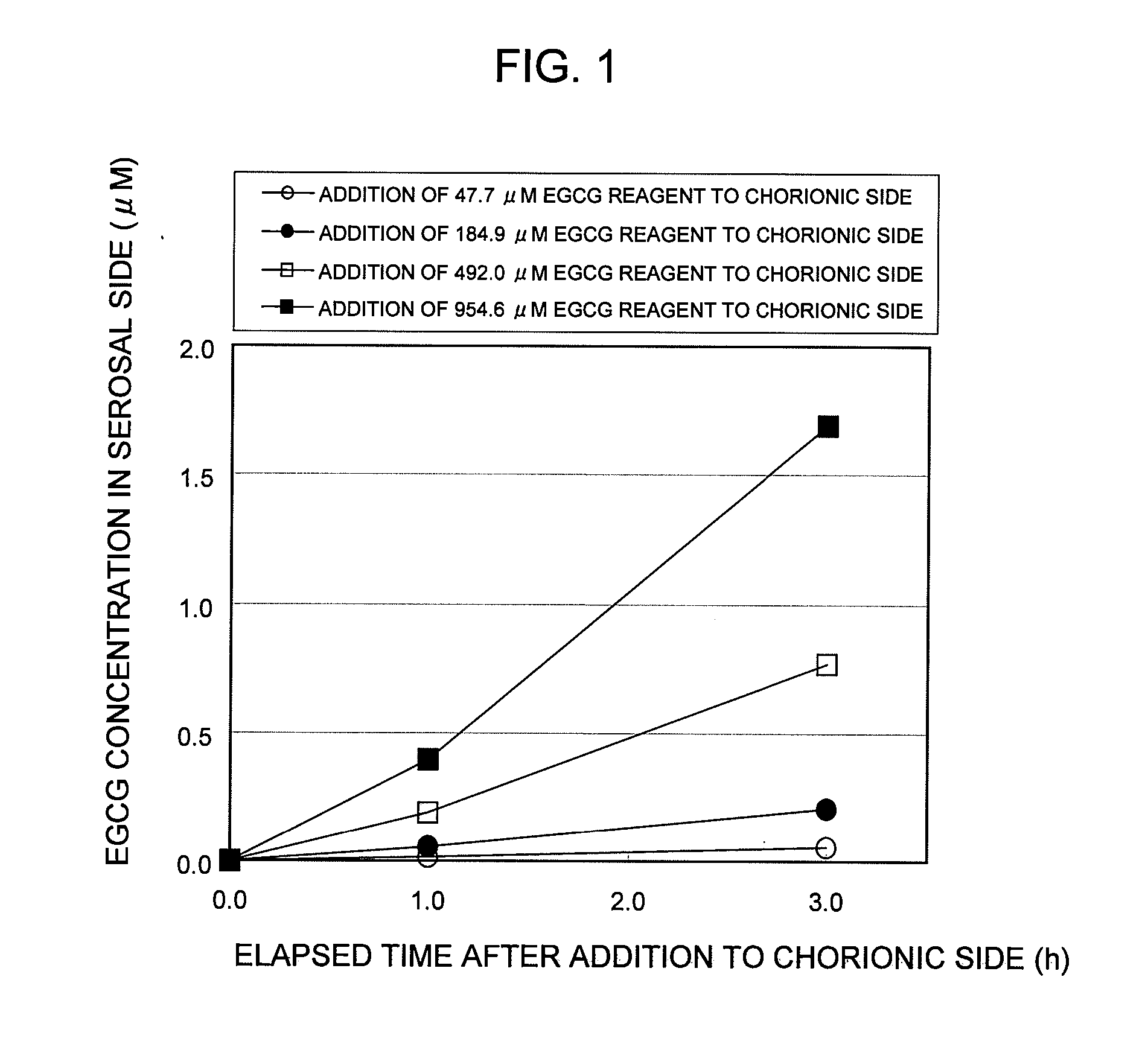
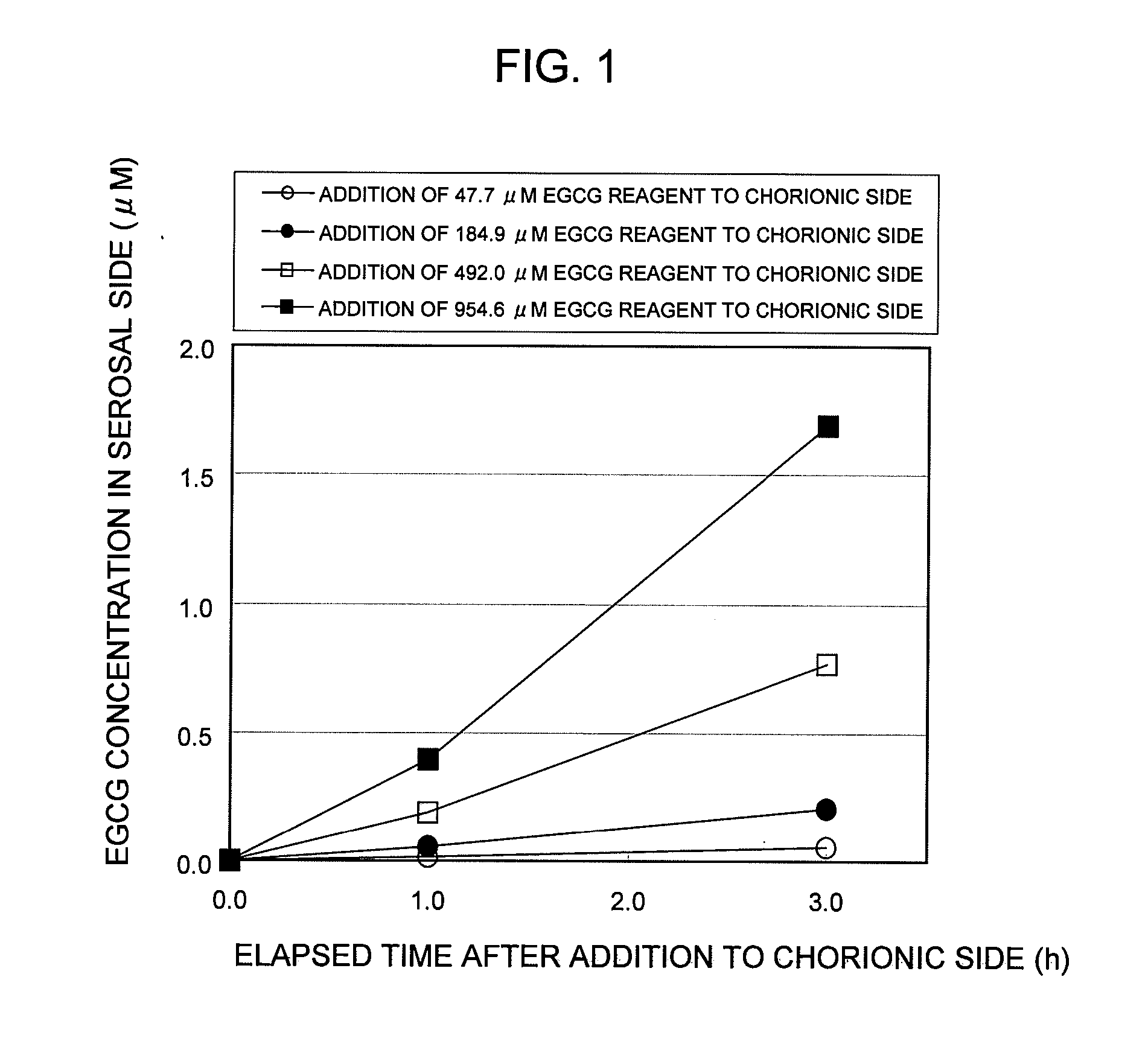
Test Example 1
Establishment of In Vitro EGCG Absorption Measurement System
Test Example 2
Screening of Food or Food-Related Ingredient which Promotes EGCG Absorption by Caco-2 Cells
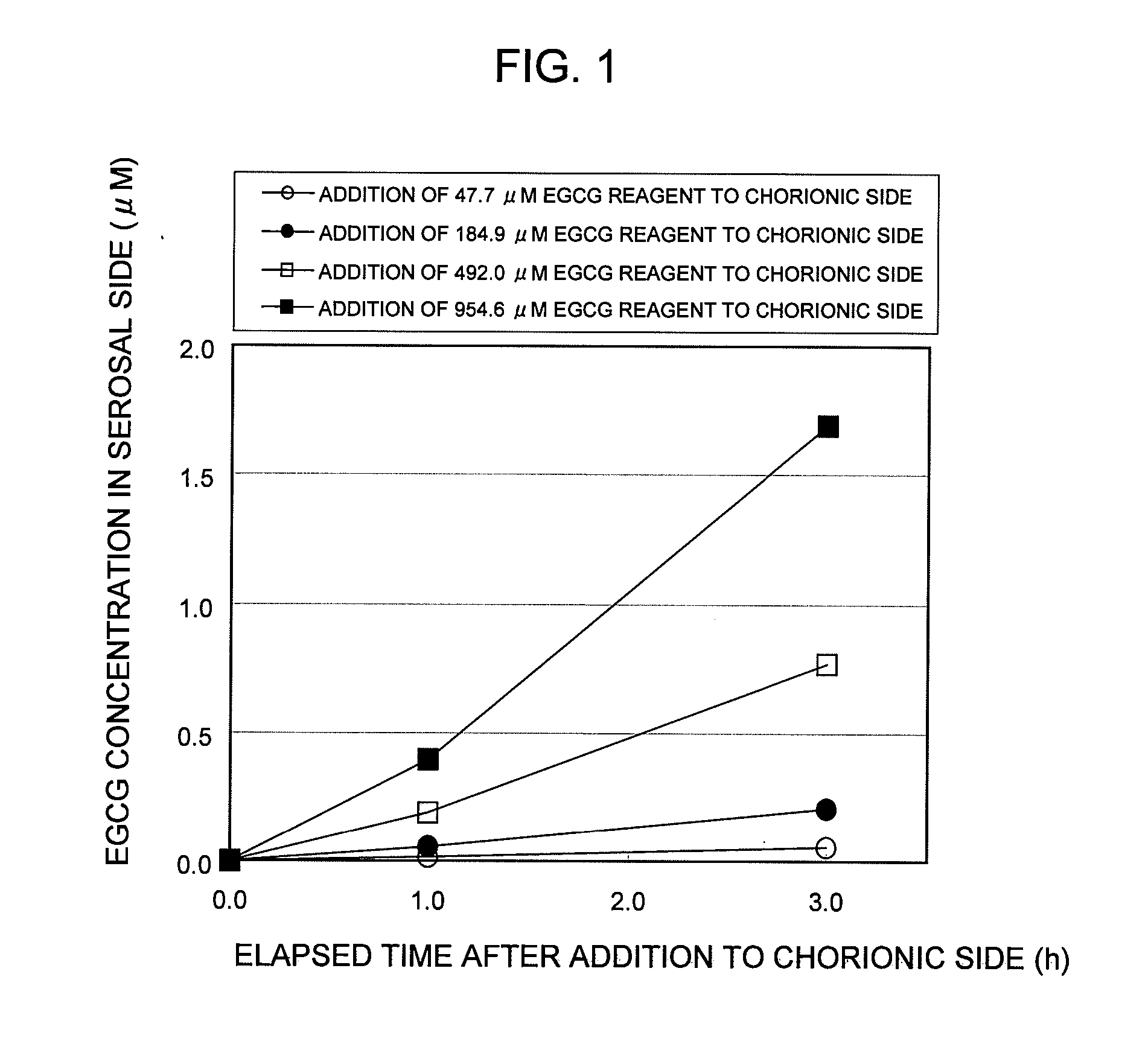
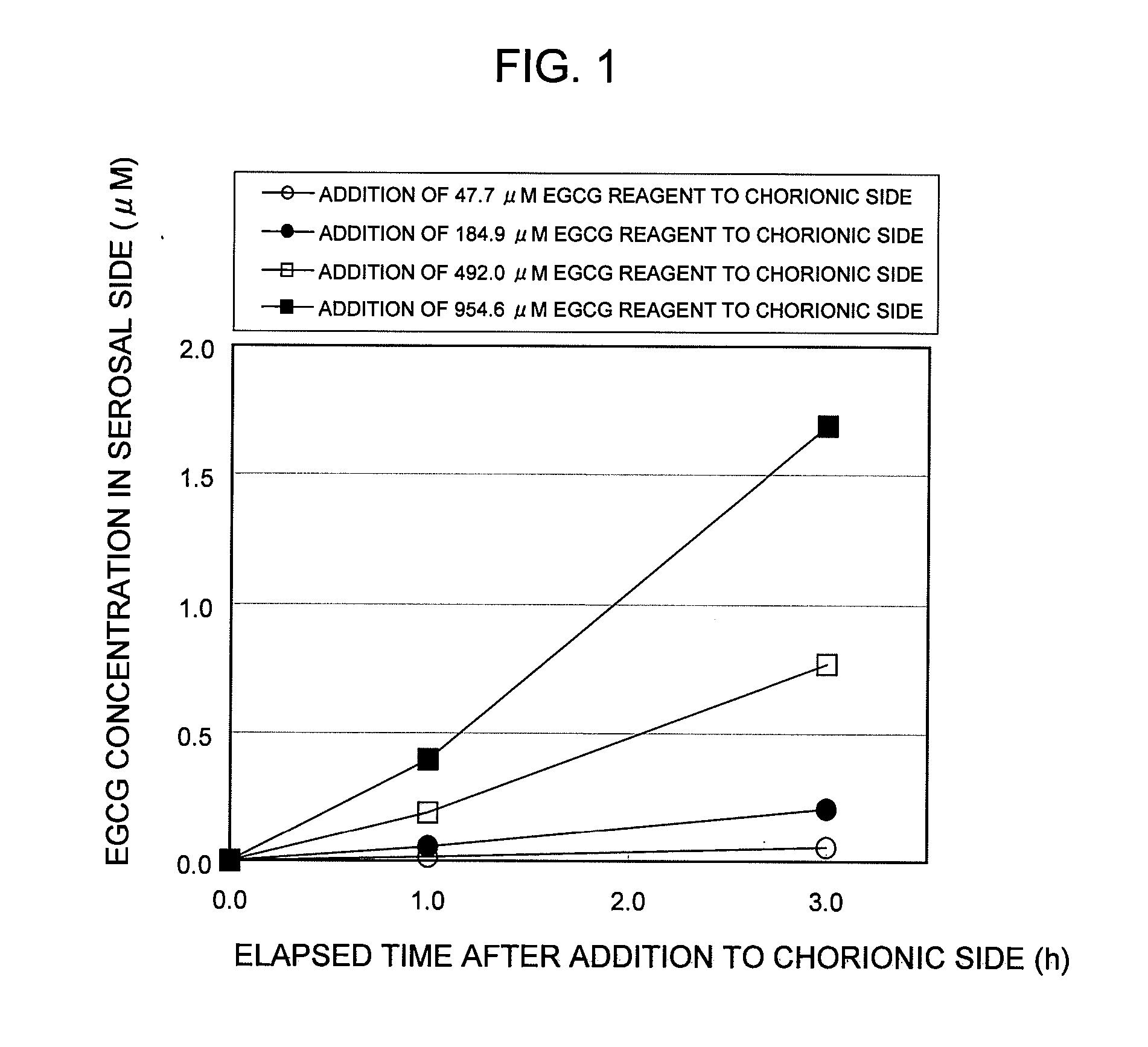
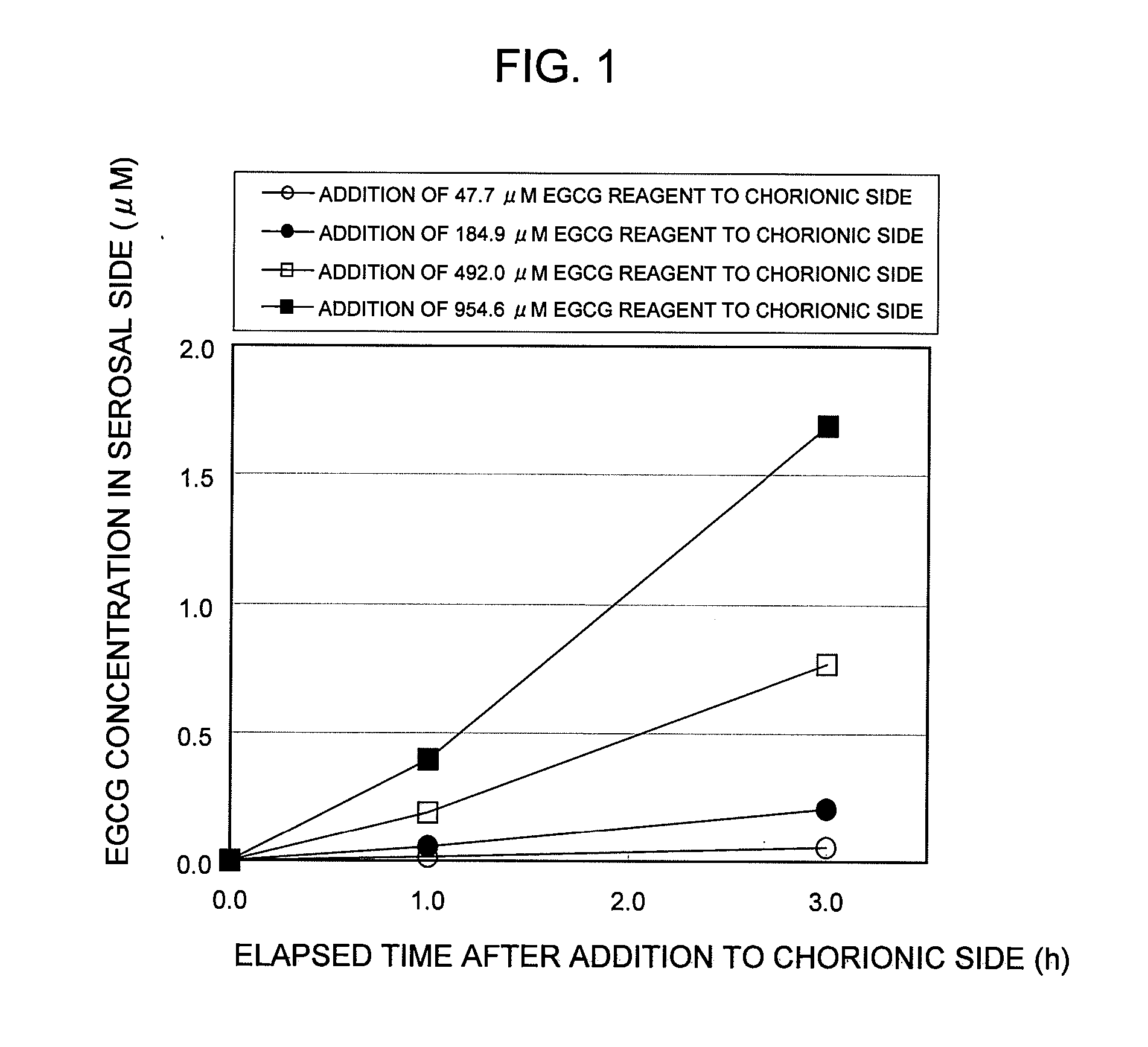
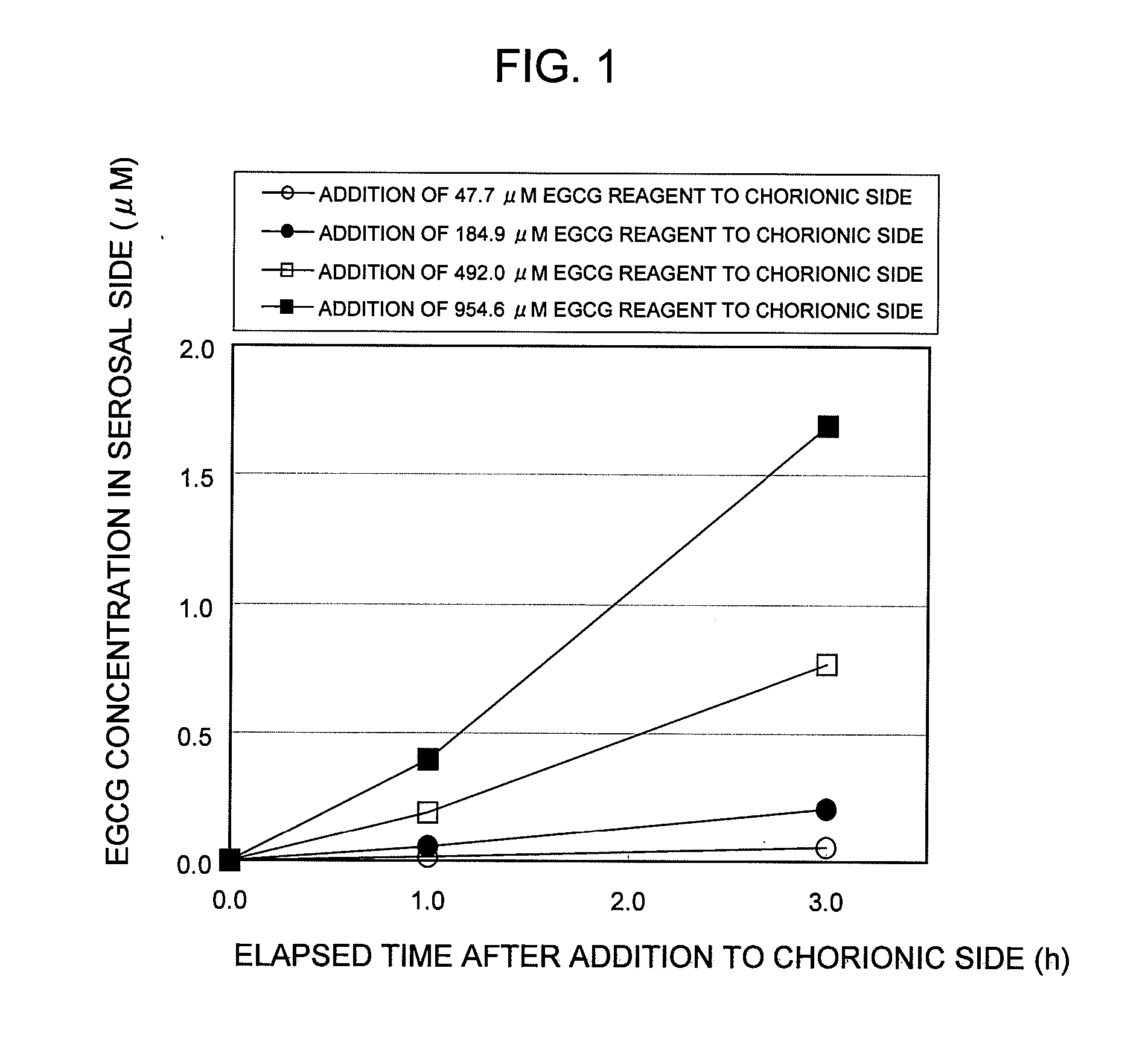
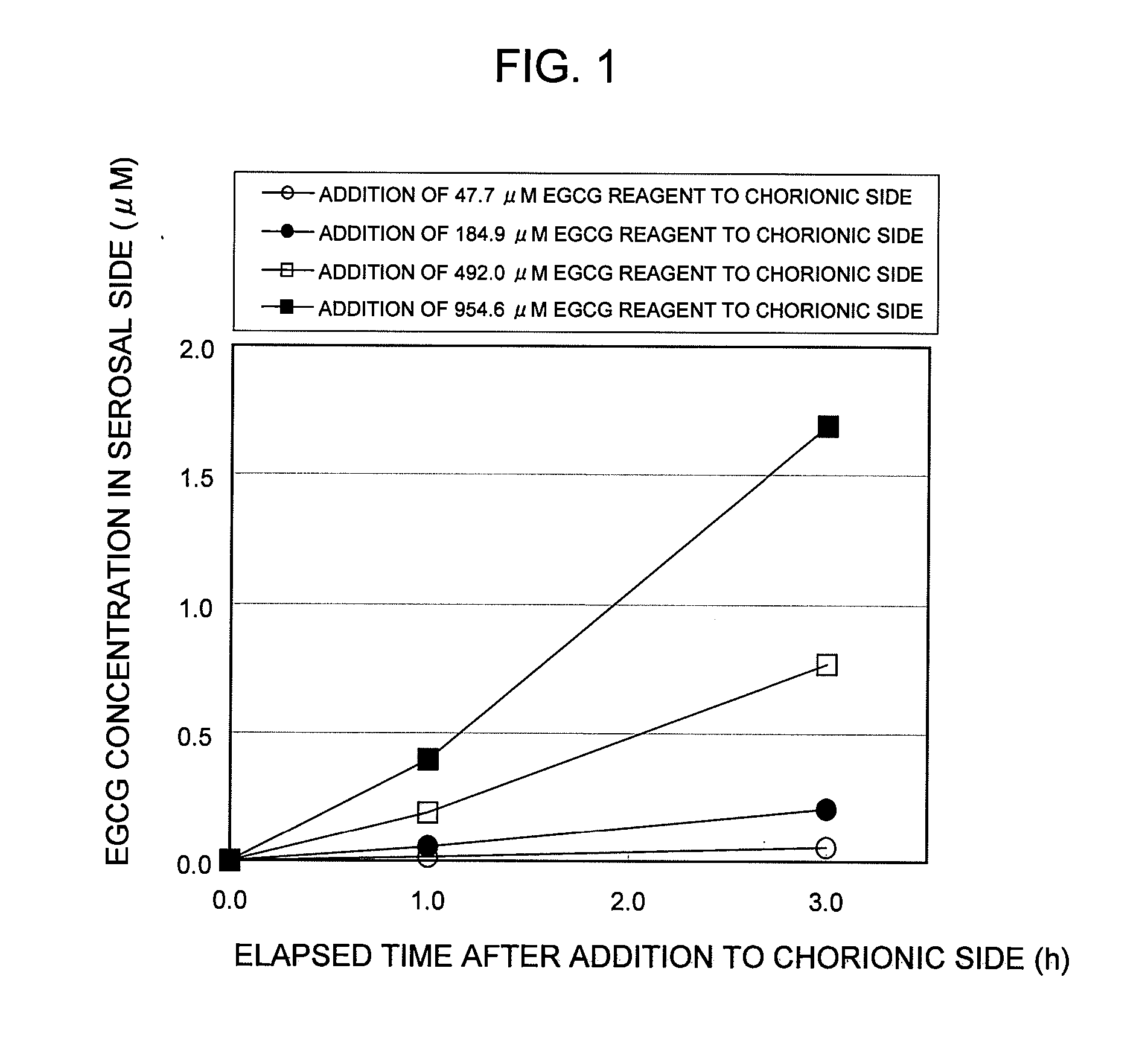
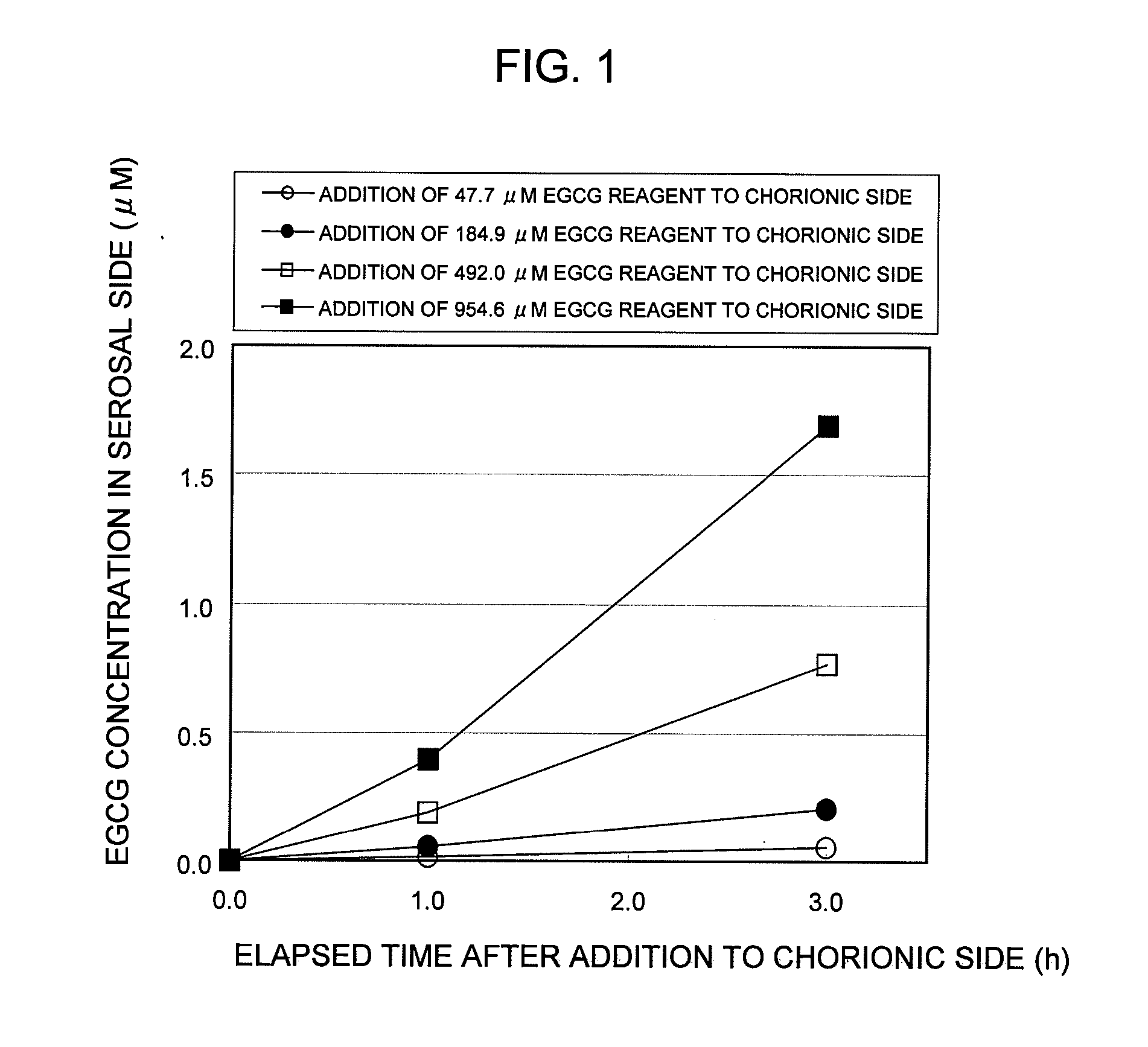
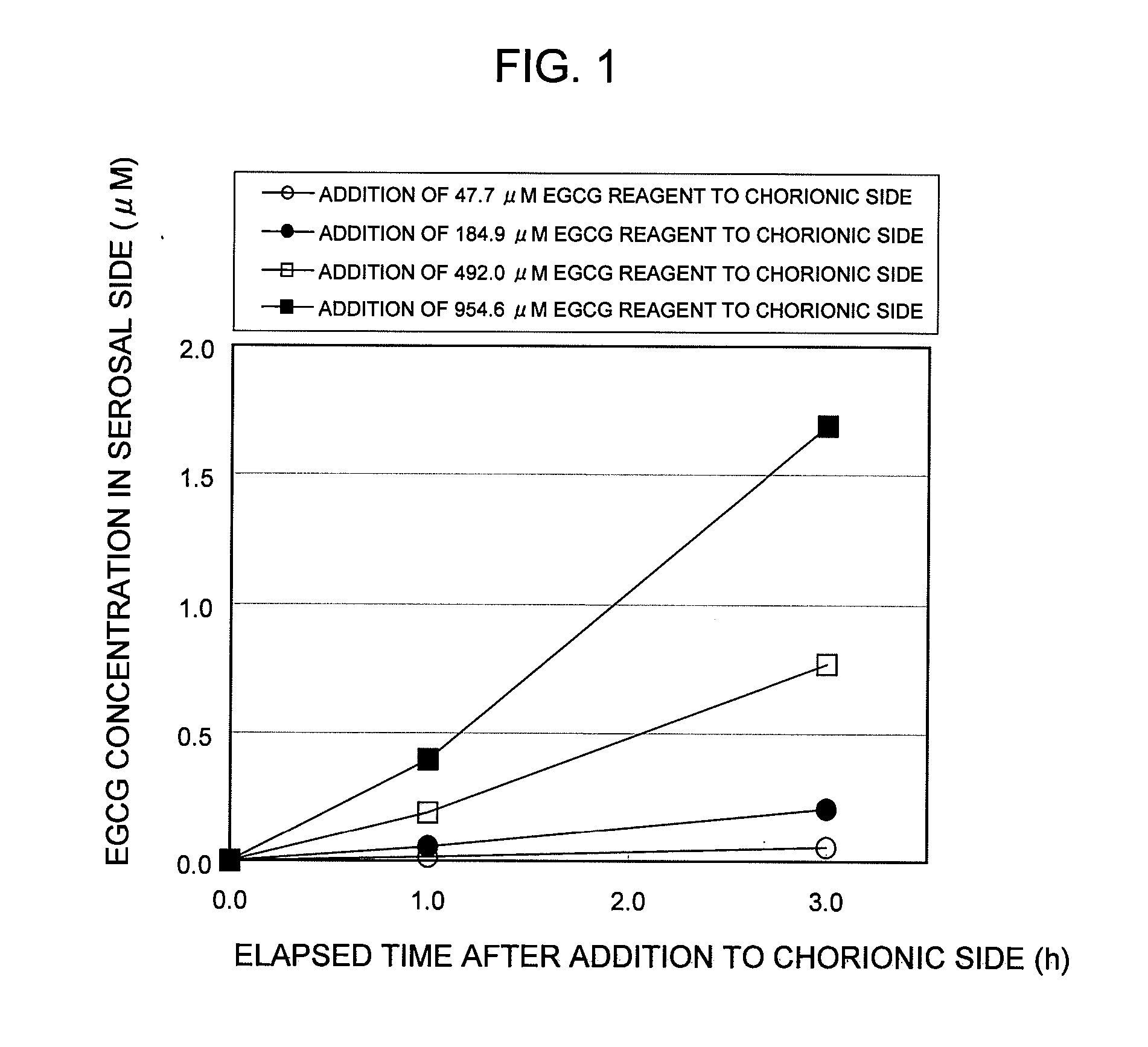
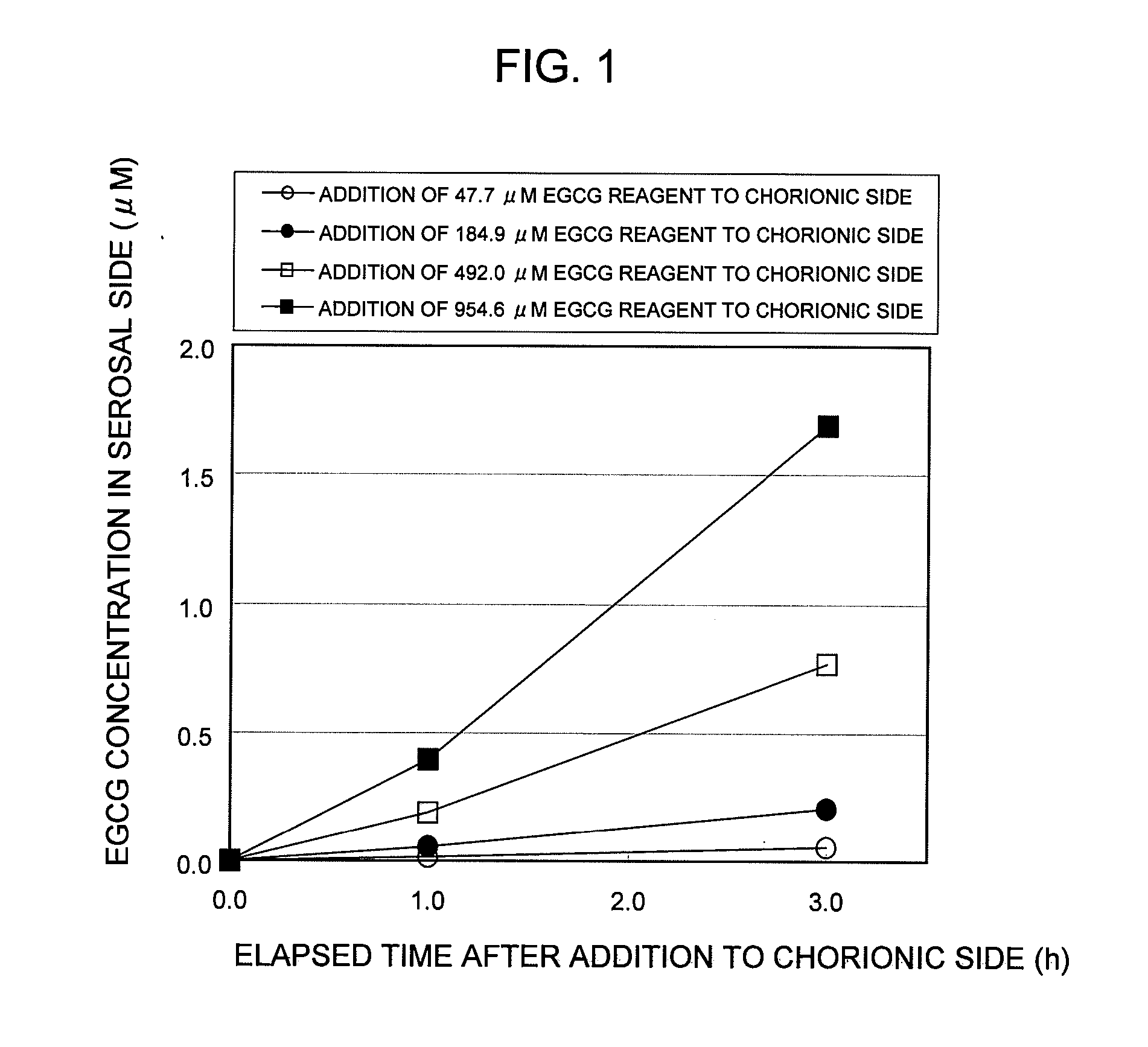
Saccharides Fructose Amino acids Theanine Polyphenols (7) Quercetin (11) (16) Sucrose GABA (including tea Rutin catechins (4)) Galactose Arginine Hesperidin Stachyose Serine Epicatechin (EC) Isomaltooligosaccharide Proline Epicatechin gallate (ECG) Raffinose Methionine Epigallocatechin (EGC) Glucose Alanine Methylated catechin (EGCG-3″Me) Fructooligosaccharide Valine Xylitol Leucine Organic acids (8) Malic acid Erythritol Phenylalanine Citric acid Glucosamine Lysine Quinic acid Glycine Tartaric acid Dietary fibers Guar gum Threonine Nicotinic acid (9) Pine fiber Glutamic acid Sodium caprylate Locust bean gum Aspartic acid Sodium laurate Xanthan gum Histidine Phytic acid Carrageenan Agar Others (10) Caffeine Glucomannan Saponin Gellan gum Thiamine Inulin Pectin Lecithin Theophylline Riboflavin Carnitine Grapefruit juice Relative rates of EGCG absorption when various foods or food- related ingredients were added to Caco-2 cells at various concentrations together with 1 mM EGCG solution Addition amount (parts by mass) of Relative food or food- Food or food- value of Papp related ingredient related based on that based on 100 parts ingredient Addition of no by mass of tea added concentration additive catechin No additive 1 Capric acid 1 mM 1.9 38 (M.W.: 172.26) 3 mM 2.1 113 6 mM 2.3 225 Lauric acid 0.5 mM 1 22 (M.W.: 200.32) 1 mM 1.4 44 3 mM 4.3 131 Grapefruit 10% 1.3 21,816 juice (100%) 20% 2.7 43,633 Serine (M.W.: 5 mM 1 115 105.09) 20 mM 3.2 459 Aspartic acid 10 mM 1.1 290 (M.W.: 133.10) 20 mM 3.2 581 Malic acid 20 mM 1.6 585 (M.W.: 134.09) 40 mM 2.6 1,170 EGCG: M.W.: 458.37 Test Example 3
EGCG Absorption Promoting Effect by Combination of Foods or Food-Related Ingredients)
Relative rates of EGCG absorption when various foods or food- related ingredients were added to Caco-2 cells at various concentrations together with 1 mM EGCG solution Combinations and concentrations Relative value of of foods or food-related Papp based on that ingredients added of no additive No additive 1.0 20 mM serine + 20 mM aspartic acid 5.3 20 mM serine + 40 mM malic acid 5.4 20 mM serine + 20% grapefruit juice 6.8 20 mM serine + 3 mM lauric acid 2.3 20 mM aspartic acid + 40 mM malic acid 3.9 20 mM aspartic acid + 20% grapefruit 5.2 juice 20 mM aspartic acid + 3 mM lauric acid 4.7 40 mM malic acid + 20% grapefruit 3.7 juice 40 mM malic acid + 3 mM lauric acid 2.1 3 mM lauric acid + 20% grapefruit 11.6 juice Test Example 4
EGCG Absorption Promoting Effects of Foods or Food-Related Ingredients in Rats
Multiple comparison test by Scheffe's method on EGCG concentrations in plasma of rats to which green tea extract solution containing 1 mM EGCG was orally administered forcibly together with serine and malic acid at various concentrations. Difference in mean Critical values value P value Green tea extract vs −0.0152 0.0070 1.7E−06 S Green tea extract + 20 mM serine + 40 mM malic acid Green tea extract vs −0.0174 0.0080 1.4E−06 S Green tea extract + 40 mM serine + 80 mM malic acid Green tea extract vs −0.0175 0.0069 2.7E−08 S Green tea extract + 80 mM serine + 160 mM malic acid Green tea extract + 20 mM −0.0022 0.0093 0.9227 serine + 40 mM malic acid vs Green tea extract + 40 mM serine + 80 mM malic acid Green tea extract + 20 mM −0.0023 0.0083 0.8836 serine + 40 mM malic acid vs Green tea extract + 80 mM serine + 160 mM malic acid Green tea extract + 40 mM −0.0001 0.0092 1.0000 serine + 80 mM malic acid vs Green tea extract + 80 mM serine + 160 mM malic acid “S” described outside the column means that the administration group has a significant difference at a level of significance of 5%. Multiple comparison test by Scheffe's method on time-course changes in EGCG concentrations in plasma of rats to which green tea extract containing 1 mM EGCG was orally administered forcibly together with serine and malic acid at various concentrations × time (areas under curve). Difference in mean Critical values value P value Green tea extract vs −0.01624 0.0090 5.8E−05 S Green tea extract + 20 mM serine + 40 mM malic acid Green tea extract vs −0.02128 0.0102 3.6E−06 S Green tea extract + 40 mM serine + 80 mM malic acid Green tea extract vs −0.02168 0.0088 6.4E−08 S Green tea extract + 80 mM serine + 160 mM malic acid Green tea extract + 20 mM −0.00504 0.0119 0.6857 serine + 40 mM malic acid vs Green tea extract + 40 mM serine + 80 mM malic acid Green tea extract + 20 mM −0.00544 0.0107 0.5448 serine + 40 mM malic acid vs Green tea extract + 80 mM serine + 160 mM malic acid Green tea extract + 40 mM −0.0004 0.0117 0.9997 serine + 80 mM malic acid vs Green tea extract + 80 mM serine + 160 mM malic acid “S” described outside the column means that the administration group has a significant difference at a level of significance of 5%. Test Example 5
Total 0.5876 37 variation Variation 0.2399 1 0.2399 21.0950 0.0001 4.1960 depending on kind of adminis- tration solution Variation 0.0091 4 0.0023 0.2003 0.9361 2.7141 depending on elapsed after time adminis- tration Interaction 0.0202 4 0.0051 0.4446 0.7754 2.7141 Error 0.3184 28 0.0114 variation Total 1.3581 37 variation Variation 0.3775 1 0.3775 15.2306 0.0005 4.1960 depending on kind of adminis- tration solution Variation 0.0940 4 0.0235 0.9485 0.4508 2.7141 depending on elapsed time after administra- tion Interaction 0.1926 4 0.0482 1.9432 0.1309 2.7141 Error 0.6939 28 0.0248 variation Test Example 6
Total 0.008576 39 variation Variation 0.001941 1 0.0019 15.7355 0.0004 4.1709 depending on kind of adminis- tration solution Variation 0.001454 4 0.0004 2.9477 0.0362 2.6896 depending on elapsed time after adminis- tration Interaction 0.001479 4 0.0004 2.9984 0.0340 2.6896 Error 0.003701 30 0.0001 variation Total 0.01025 39 variation Variation 0.00306 1 0.0031 23.7175 3.4E−05 4.1709 depending on kind of adminis- tration solution Variation 0.00250 4 0.0006 4.8531 0.0039 2.6896 depending on elapsed time after adminis- tration Interaction 0.00081 4 0.0002 1.5788 0.2055 2.6896 Error 0.00387 30 0.0001 variation Test Example 7
Multiple comparison test by Fisher's PLSD method on time-course changes in EGCG concentrations in plasma of rats to which “green tea extract” solution each containing 1 mM EGCG were ” administered × time (areas under curve) Difference in mean Critical P values value value 0.0034 0.0033 0.044 S extract vs (L.) O. Kuntze extract 0.0043 0.0034 0.014 S extract vs (L.) O. Kuntze extract 0.0083 0.0037 0.000 S extract vs “green tea extract” 0.0084 0.0039 0.000 S extract vs “green tea extract” 0.0009 0.0034 0.596 extract vs (L.) 0. Kuntze extract 0.0049 0.0037 0.009 S extract vs “green tea extract” 0.0050 0.0039 0.011 S extract vs “green tea extract” 0.0040 0.0038 0.040 S extract vs “green tea extract” 0.0041 0.0040 0.044 S extract vs “green tea extract” “green tea extract” vs 0.0001 0.0042 0.949 “green tea extract” “S” described outside the column means that the administration group has a significant difference at a level of significance of 5%. Test Example 8
Multiple comparison test by Fisher's PLSD method on time- course changes in EGCG concentrations in plasma of rats to which administration solutions each containing EGCG at 1 mM were orally administered forcibly × time (areas under curve) Difference in mean Critical P values value value [i] EGCG reagent vs EGCG 0.0052 0.0039 0.010 S reagent + “green tea extract” [i] EGCG reagent vs 0.0009 0.0041 0.657 extract + “green tea extract” [ii] EGCG reagent + “green tea −0.0042 0.0042 0.047 S extract” vs extract + “green tea extract” “S” described outside the column means that the administration group has a significant difference at a level of significance of 5%. Test Example 9
Catechin Compound Absorption Promoting Effect of Malic Acid in Humans
Test Example 10
Catechin Compound Absorption Promoting Effect of